User login
Chronic blistering rash on hands
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
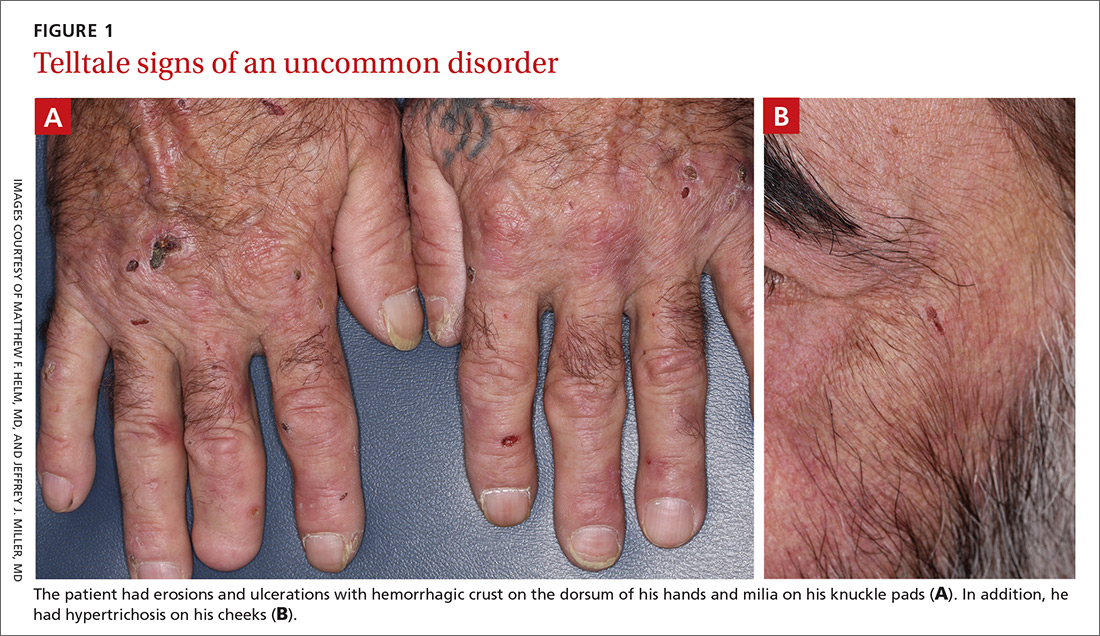
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; [email protected]
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; [email protected]
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; [email protected]
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
Melanoma incidence continues to increase, yet mortality stabilizing
LAS VEGAS – The according to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Laura Korb Ferris, MD, PhD, said that SEER data project 96,480 new cases of melanoma in 2019, as well as 7,230 deaths from the disease. In 2016, SEER projected 10,130 deaths from melanoma, “so we’re actually projecting a reduction in melanoma deaths,” said Dr. Ferris, director of clinical trials at the University of Pittsburgh Medical Center’s department of dermatology. She added that the death rate from melanoma in 2016 was 2.17 per 100,000 population, a reduction from 2.69 per 100,000 population in 2011, “so it looks like melanoma mortality may be stable,” or even reduced, despite an increase in melanoma incidence.
A study of SEER data between 1989 and 2009 found that melanoma incidence is increasing across all lesion thicknesses (J Natl Cancer Inst. 2015 Nov 12. doi: 10.1093/jnci/djv294). Specifically, the incidence increased most among thin lesions, but there was a smaller increased incidence of thick melanoma. “This suggests that the overall burden of disease is truly increasing, but it is primarily stemming from an increase in T1/T2 disease,” Dr. Ferris said. “This could be due in part to increased early detection.”
Improvements in melanoma-specific survival, she continued, are likely a combination of improved management of T4 disease, a shift toward detection of thinner T1/T2 melanoma, and increased detection of T1/T2 disease.
The SEER data also showed that the incidence of fatal cases of melanoma has decreased since 1989, but only in thick melanomas. This trend may indicate a modest improvement in the management of T4 tumors. “Optimistically, I think increased detection efforts are improving survival by early detection of thin but ultimately fatal melanomas,” Dr. Ferris said. “Hopefully we are finding disease earlier and we are preventing patients from progressing to these fatal T4 melanomas.”
Disparities in melanoma-specific survival also come into play. Men have poorer survival compared with women, whites have the highest survival, and non-Hispanic whites have a better survival than Hispanic whites, Dr. Ferris said, while lower rates of survival are seen in blacks and nonblack minorities, as well as among those in high poverty and those who are separated/nonmarried. Lesion type also matters. The highest survival is seen in those with superficial spreading melanoma, while lower survival is observed in those with nodular melanoma, and acral lentiginous melanoma.
Early detection of thin nodular melanomas has the potential to significantly impact melanoma mortality, “but we want to keep in mind that the majority of ultimately fatal melanomas are superficial spreading melanomas,” Dr. Ferris said. “That is because they are so much more prevalent. As a dermatologist, I think a lot about screening and early detection. Periodic screening is a good strategy for a slower-growing superficial spreading melanoma, but it’s not necessarily a good strategy for a rapidly growing nodular melanoma. That’s going to require better education and better access to health care.”
Self-detection of melanoma is another strategy to consider. According to Dr. Ferris, results from multiple studies suggest that about 50% of all melanomas are detected by patients, but the ones they find tend to be thicker than the ones that clinicians detect during office visits. “It would be great if we can get that number higher than 50%,” Dr. Ferris said. “If patients really understood what melanoma is, what it looks like, and when they needed to seek medical attention, perhaps we could get that over 50% and see self-detection of thinner melanomas. That’s a very low-cost intervention.”
Targeted screening efforts that stratify by risk factors and by age “makes screening more efficient and more cost-effective,” she added. She cited one analysis, which found that clinicians need to screen 606 people and conduct 25 biopsies in order to find one melanoma. “That’s very resource intensive,” she said. “However, if you only screened people 50 or older or 65 or older, the number needed to screen goes down, and because your pretest probability is higher, your number need to biopsy goes down as well. If you factor in things like a history of atypical nevi or a personal history of melanoma, those patients are at a higher risk of developing melanoma.”
Dr. Ferris closed her presentation by noting that Australia leads other countries in melanoma prevention efforts. There, the combined incidence of skin cancer is higher than the incidence of any other type of cancer. Four decades ago, Australian health officials launched SunSmart, a series of initiatives intended to reduce skin cancer. These include implementation of policies for hat wearing and shade provision in schools and at work, availability of more effective sunscreens, inclusion of sun protection items as a tax-deductible expense for outdoor workers, increased availability since the 1980s of long-sleeved sun protective swimwear, a ban on the use of indoor tanning since 2014, provision of UV forecasts in weather, and a comprehensive program of grants for community shade structures (PLoSMed. 2019 Oct 8;16[10]:e1002932).
“One approach to melanoma prevention won’t fit all,” she concluded. “We need to focus on prevention, public education to improve knowledge and self-detection.”
Dr. Ferris disclosed that she is a consultant to and an investigator for DermTech and Scibase. She is also an investigator for Castle Biosciences.
SDEF and this news organization are owned by the same parent company. Dr. Ferris spoke during a forum on cutaneous malignancies at the meeting.
LAS VEGAS – The according to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Laura Korb Ferris, MD, PhD, said that SEER data project 96,480 new cases of melanoma in 2019, as well as 7,230 deaths from the disease. In 2016, SEER projected 10,130 deaths from melanoma, “so we’re actually projecting a reduction in melanoma deaths,” said Dr. Ferris, director of clinical trials at the University of Pittsburgh Medical Center’s department of dermatology. She added that the death rate from melanoma in 2016 was 2.17 per 100,000 population, a reduction from 2.69 per 100,000 population in 2011, “so it looks like melanoma mortality may be stable,” or even reduced, despite an increase in melanoma incidence.
A study of SEER data between 1989 and 2009 found that melanoma incidence is increasing across all lesion thicknesses (J Natl Cancer Inst. 2015 Nov 12. doi: 10.1093/jnci/djv294). Specifically, the incidence increased most among thin lesions, but there was a smaller increased incidence of thick melanoma. “This suggests that the overall burden of disease is truly increasing, but it is primarily stemming from an increase in T1/T2 disease,” Dr. Ferris said. “This could be due in part to increased early detection.”
Improvements in melanoma-specific survival, she continued, are likely a combination of improved management of T4 disease, a shift toward detection of thinner T1/T2 melanoma, and increased detection of T1/T2 disease.
The SEER data also showed that the incidence of fatal cases of melanoma has decreased since 1989, but only in thick melanomas. This trend may indicate a modest improvement in the management of T4 tumors. “Optimistically, I think increased detection efforts are improving survival by early detection of thin but ultimately fatal melanomas,” Dr. Ferris said. “Hopefully we are finding disease earlier and we are preventing patients from progressing to these fatal T4 melanomas.”
Disparities in melanoma-specific survival also come into play. Men have poorer survival compared with women, whites have the highest survival, and non-Hispanic whites have a better survival than Hispanic whites, Dr. Ferris said, while lower rates of survival are seen in blacks and nonblack minorities, as well as among those in high poverty and those who are separated/nonmarried. Lesion type also matters. The highest survival is seen in those with superficial spreading melanoma, while lower survival is observed in those with nodular melanoma, and acral lentiginous melanoma.
Early detection of thin nodular melanomas has the potential to significantly impact melanoma mortality, “but we want to keep in mind that the majority of ultimately fatal melanomas are superficial spreading melanomas,” Dr. Ferris said. “That is because they are so much more prevalent. As a dermatologist, I think a lot about screening and early detection. Periodic screening is a good strategy for a slower-growing superficial spreading melanoma, but it’s not necessarily a good strategy for a rapidly growing nodular melanoma. That’s going to require better education and better access to health care.”
Self-detection of melanoma is another strategy to consider. According to Dr. Ferris, results from multiple studies suggest that about 50% of all melanomas are detected by patients, but the ones they find tend to be thicker than the ones that clinicians detect during office visits. “It would be great if we can get that number higher than 50%,” Dr. Ferris said. “If patients really understood what melanoma is, what it looks like, and when they needed to seek medical attention, perhaps we could get that over 50% and see self-detection of thinner melanomas. That’s a very low-cost intervention.”
Targeted screening efforts that stratify by risk factors and by age “makes screening more efficient and more cost-effective,” she added. She cited one analysis, which found that clinicians need to screen 606 people and conduct 25 biopsies in order to find one melanoma. “That’s very resource intensive,” she said. “However, if you only screened people 50 or older or 65 or older, the number needed to screen goes down, and because your pretest probability is higher, your number need to biopsy goes down as well. If you factor in things like a history of atypical nevi or a personal history of melanoma, those patients are at a higher risk of developing melanoma.”
Dr. Ferris closed her presentation by noting that Australia leads other countries in melanoma prevention efforts. There, the combined incidence of skin cancer is higher than the incidence of any other type of cancer. Four decades ago, Australian health officials launched SunSmart, a series of initiatives intended to reduce skin cancer. These include implementation of policies for hat wearing and shade provision in schools and at work, availability of more effective sunscreens, inclusion of sun protection items as a tax-deductible expense for outdoor workers, increased availability since the 1980s of long-sleeved sun protective swimwear, a ban on the use of indoor tanning since 2014, provision of UV forecasts in weather, and a comprehensive program of grants for community shade structures (PLoSMed. 2019 Oct 8;16[10]:e1002932).
“One approach to melanoma prevention won’t fit all,” she concluded. “We need to focus on prevention, public education to improve knowledge and self-detection.”
Dr. Ferris disclosed that she is a consultant to and an investigator for DermTech and Scibase. She is also an investigator for Castle Biosciences.
SDEF and this news organization are owned by the same parent company. Dr. Ferris spoke during a forum on cutaneous malignancies at the meeting.
LAS VEGAS – The according to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Laura Korb Ferris, MD, PhD, said that SEER data project 96,480 new cases of melanoma in 2019, as well as 7,230 deaths from the disease. In 2016, SEER projected 10,130 deaths from melanoma, “so we’re actually projecting a reduction in melanoma deaths,” said Dr. Ferris, director of clinical trials at the University of Pittsburgh Medical Center’s department of dermatology. She added that the death rate from melanoma in 2016 was 2.17 per 100,000 population, a reduction from 2.69 per 100,000 population in 2011, “so it looks like melanoma mortality may be stable,” or even reduced, despite an increase in melanoma incidence.
A study of SEER data between 1989 and 2009 found that melanoma incidence is increasing across all lesion thicknesses (J Natl Cancer Inst. 2015 Nov 12. doi: 10.1093/jnci/djv294). Specifically, the incidence increased most among thin lesions, but there was a smaller increased incidence of thick melanoma. “This suggests that the overall burden of disease is truly increasing, but it is primarily stemming from an increase in T1/T2 disease,” Dr. Ferris said. “This could be due in part to increased early detection.”
Improvements in melanoma-specific survival, she continued, are likely a combination of improved management of T4 disease, a shift toward detection of thinner T1/T2 melanoma, and increased detection of T1/T2 disease.
The SEER data also showed that the incidence of fatal cases of melanoma has decreased since 1989, but only in thick melanomas. This trend may indicate a modest improvement in the management of T4 tumors. “Optimistically, I think increased detection efforts are improving survival by early detection of thin but ultimately fatal melanomas,” Dr. Ferris said. “Hopefully we are finding disease earlier and we are preventing patients from progressing to these fatal T4 melanomas.”
Disparities in melanoma-specific survival also come into play. Men have poorer survival compared with women, whites have the highest survival, and non-Hispanic whites have a better survival than Hispanic whites, Dr. Ferris said, while lower rates of survival are seen in blacks and nonblack minorities, as well as among those in high poverty and those who are separated/nonmarried. Lesion type also matters. The highest survival is seen in those with superficial spreading melanoma, while lower survival is observed in those with nodular melanoma, and acral lentiginous melanoma.
Early detection of thin nodular melanomas has the potential to significantly impact melanoma mortality, “but we want to keep in mind that the majority of ultimately fatal melanomas are superficial spreading melanomas,” Dr. Ferris said. “That is because they are so much more prevalent. As a dermatologist, I think a lot about screening and early detection. Periodic screening is a good strategy for a slower-growing superficial spreading melanoma, but it’s not necessarily a good strategy for a rapidly growing nodular melanoma. That’s going to require better education and better access to health care.”
Self-detection of melanoma is another strategy to consider. According to Dr. Ferris, results from multiple studies suggest that about 50% of all melanomas are detected by patients, but the ones they find tend to be thicker than the ones that clinicians detect during office visits. “It would be great if we can get that number higher than 50%,” Dr. Ferris said. “If patients really understood what melanoma is, what it looks like, and when they needed to seek medical attention, perhaps we could get that over 50% and see self-detection of thinner melanomas. That’s a very low-cost intervention.”
Targeted screening efforts that stratify by risk factors and by age “makes screening more efficient and more cost-effective,” she added. She cited one analysis, which found that clinicians need to screen 606 people and conduct 25 biopsies in order to find one melanoma. “That’s very resource intensive,” she said. “However, if you only screened people 50 or older or 65 or older, the number needed to screen goes down, and because your pretest probability is higher, your number need to biopsy goes down as well. If you factor in things like a history of atypical nevi or a personal history of melanoma, those patients are at a higher risk of developing melanoma.”
Dr. Ferris closed her presentation by noting that Australia leads other countries in melanoma prevention efforts. There, the combined incidence of skin cancer is higher than the incidence of any other type of cancer. Four decades ago, Australian health officials launched SunSmart, a series of initiatives intended to reduce skin cancer. These include implementation of policies for hat wearing and shade provision in schools and at work, availability of more effective sunscreens, inclusion of sun protection items as a tax-deductible expense for outdoor workers, increased availability since the 1980s of long-sleeved sun protective swimwear, a ban on the use of indoor tanning since 2014, provision of UV forecasts in weather, and a comprehensive program of grants for community shade structures (PLoSMed. 2019 Oct 8;16[10]:e1002932).
“One approach to melanoma prevention won’t fit all,” she concluded. “We need to focus on prevention, public education to improve knowledge and self-detection.”
Dr. Ferris disclosed that she is a consultant to and an investigator for DermTech and Scibase. She is also an investigator for Castle Biosciences.
SDEF and this news organization are owned by the same parent company. Dr. Ferris spoke during a forum on cutaneous malignancies at the meeting.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
The Dog Can Stay, but the Rash Must Go
A 50-year-old man presents with a 1-year history of an itchy, bumpy rash on his chest. He denies any history of similar rash and says there have been no “extraordinary changes” in his life that could have triggered this manifestation. Despite consulting various primary care providers, he has been unable to acquire either a definitive diagnosis or effective treatment.
The patient works exclusively in a climate-controlled office. Although there were no changes to laundry detergent, body soap, deodorant, or other products that might have precipitated the rash’s manifestation, he tried alternate products to see what effect they might have. Nothing beneficial came from these experiments. Similarly, the family dogs were temporarily “banished” with no improvement to his condition.
From the outset, the rash and the associated itching have been confined to the patient’s chest. No one else in his family is similarly affected.
The patient is otherwise quite well. He takes no prescription medications and denies any recent foreign travel.
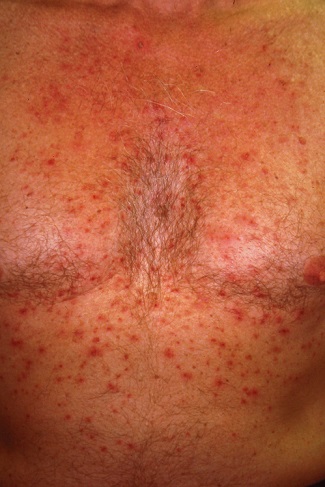
EXAMINATION
The papulovesicular rash is strikingly uniform. The patient’s entire chest is covered with tiny vesicles, many with clear fluid inside. The lesions average 1.2 to 2 mm in width, and nearly all are quite palpable. Each lesion is slightly erythematous but neither warm nor tender on palpation.
Examination of the rest of the patient’s exposed skin reveals no similar lesions. His back, hands, and genitals are notably free of any such lesions.
A shave biopsy is performed, utilizing a saucerization technique, and the specimen is submitted to pathology for routine processing. The report confirms the papulovesicular nature of the lesions—but more significantly, it shows consistent acantholysis (loss of intracellular connections between keratinocytes), along with focal lymphohistiocytic infiltrates.
What’s the diagnosis?
DISCUSSION
This is a classic presentation of Grover disease, also known as transient acantholytic dermatosis (AD). While not rare, it is seen only occasionally in dermatology practices. When it does walk through the door, it is twice as likely to be seen in a male than in a female patient and less commonly seen in those with darker skin.
AD is easy enough to diagnose clinically, without biopsy, particularly in classic cases such as this one. The distribution and morphology of the rash, as well as the gender and age of the patient, are all typical of this idiopathic condition. The biopsy results, besides being consistent with AD, did serve to rule out other items in the differential (eg, bacterial folliculitis, pemphigus, and acne).
Since AD was first described in 1974 by R.W. Grover, MD, much research has been conducted to flesh out the nature of the disease, its potential causes, and possible treatment. One certainty about so-called transient AD is that most cases are far from transient—in fact, they can last for a year or more. Attempts have been made to connect AD with internal disease (eg, occult malignancy) or even mercury exposure, but these theories have not been corroborated.
Consistent treatment success has also been elusive. Most patients achieve decent relief with the use of topical steroid creams, with or without the addition of anti-inflammatory medications (eg, doxycycline). Other options include isotretinoin and psoralen plus ultraviolet A (PUVA) photochemotherapy. Fortunately, most cases eventually clear up.
TAKE-HOME LEARNING POINTS
- Grover disease, also known as transient acantholytic dermatosis (AD), usually manifests with an acute eruption of papulovesicular lesions.
- AD lesions tend to be confined to the chest and are typically pruritic.
- Clinical diagnosis is usually adequate, although biopsy, which will reveal typical findings of acantholysis, may be necessary to rule out other items in the differential.
- Treatment with topical steroids, oral doxycycline, and “tincture of time” usually suffices, but resolution may take a year or more.
A 50-year-old man presents with a 1-year history of an itchy, bumpy rash on his chest. He denies any history of similar rash and says there have been no “extraordinary changes” in his life that could have triggered this manifestation. Despite consulting various primary care providers, he has been unable to acquire either a definitive diagnosis or effective treatment.
The patient works exclusively in a climate-controlled office. Although there were no changes to laundry detergent, body soap, deodorant, or other products that might have precipitated the rash’s manifestation, he tried alternate products to see what effect they might have. Nothing beneficial came from these experiments. Similarly, the family dogs were temporarily “banished” with no improvement to his condition.
From the outset, the rash and the associated itching have been confined to the patient’s chest. No one else in his family is similarly affected.
The patient is otherwise quite well. He takes no prescription medications and denies any recent foreign travel.

EXAMINATION
The papulovesicular rash is strikingly uniform. The patient’s entire chest is covered with tiny vesicles, many with clear fluid inside. The lesions average 1.2 to 2 mm in width, and nearly all are quite palpable. Each lesion is slightly erythematous but neither warm nor tender on palpation.
Examination of the rest of the patient’s exposed skin reveals no similar lesions. His back, hands, and genitals are notably free of any such lesions.
A shave biopsy is performed, utilizing a saucerization technique, and the specimen is submitted to pathology for routine processing. The report confirms the papulovesicular nature of the lesions—but more significantly, it shows consistent acantholysis (loss of intracellular connections between keratinocytes), along with focal lymphohistiocytic infiltrates.
What’s the diagnosis?
DISCUSSION
This is a classic presentation of Grover disease, also known as transient acantholytic dermatosis (AD). While not rare, it is seen only occasionally in dermatology practices. When it does walk through the door, it is twice as likely to be seen in a male than in a female patient and less commonly seen in those with darker skin.
AD is easy enough to diagnose clinically, without biopsy, particularly in classic cases such as this one. The distribution and morphology of the rash, as well as the gender and age of the patient, are all typical of this idiopathic condition. The biopsy results, besides being consistent with AD, did serve to rule out other items in the differential (eg, bacterial folliculitis, pemphigus, and acne).
Since AD was first described in 1974 by R.W. Grover, MD, much research has been conducted to flesh out the nature of the disease, its potential causes, and possible treatment. One certainty about so-called transient AD is that most cases are far from transient—in fact, they can last for a year or more. Attempts have been made to connect AD with internal disease (eg, occult malignancy) or even mercury exposure, but these theories have not been corroborated.
Consistent treatment success has also been elusive. Most patients achieve decent relief with the use of topical steroid creams, with or without the addition of anti-inflammatory medications (eg, doxycycline). Other options include isotretinoin and psoralen plus ultraviolet A (PUVA) photochemotherapy. Fortunately, most cases eventually clear up.
TAKE-HOME LEARNING POINTS
- Grover disease, also known as transient acantholytic dermatosis (AD), usually manifests with an acute eruption of papulovesicular lesions.
- AD lesions tend to be confined to the chest and are typically pruritic.
- Clinical diagnosis is usually adequate, although biopsy, which will reveal typical findings of acantholysis, may be necessary to rule out other items in the differential.
- Treatment with topical steroids, oral doxycycline, and “tincture of time” usually suffices, but resolution may take a year or more.
A 50-year-old man presents with a 1-year history of an itchy, bumpy rash on his chest. He denies any history of similar rash and says there have been no “extraordinary changes” in his life that could have triggered this manifestation. Despite consulting various primary care providers, he has been unable to acquire either a definitive diagnosis or effective treatment.
The patient works exclusively in a climate-controlled office. Although there were no changes to laundry detergent, body soap, deodorant, or other products that might have precipitated the rash’s manifestation, he tried alternate products to see what effect they might have. Nothing beneficial came from these experiments. Similarly, the family dogs were temporarily “banished” with no improvement to his condition.
From the outset, the rash and the associated itching have been confined to the patient’s chest. No one else in his family is similarly affected.
The patient is otherwise quite well. He takes no prescription medications and denies any recent foreign travel.

EXAMINATION
The papulovesicular rash is strikingly uniform. The patient’s entire chest is covered with tiny vesicles, many with clear fluid inside. The lesions average 1.2 to 2 mm in width, and nearly all are quite palpable. Each lesion is slightly erythematous but neither warm nor tender on palpation.
Examination of the rest of the patient’s exposed skin reveals no similar lesions. His back, hands, and genitals are notably free of any such lesions.
A shave biopsy is performed, utilizing a saucerization technique, and the specimen is submitted to pathology for routine processing. The report confirms the papulovesicular nature of the lesions—but more significantly, it shows consistent acantholysis (loss of intracellular connections between keratinocytes), along with focal lymphohistiocytic infiltrates.
What’s the diagnosis?
DISCUSSION
This is a classic presentation of Grover disease, also known as transient acantholytic dermatosis (AD). While not rare, it is seen only occasionally in dermatology practices. When it does walk through the door, it is twice as likely to be seen in a male than in a female patient and less commonly seen in those with darker skin.
AD is easy enough to diagnose clinically, without biopsy, particularly in classic cases such as this one. The distribution and morphology of the rash, as well as the gender and age of the patient, are all typical of this idiopathic condition. The biopsy results, besides being consistent with AD, did serve to rule out other items in the differential (eg, bacterial folliculitis, pemphigus, and acne).
Since AD was first described in 1974 by R.W. Grover, MD, much research has been conducted to flesh out the nature of the disease, its potential causes, and possible treatment. One certainty about so-called transient AD is that most cases are far from transient—in fact, they can last for a year or more. Attempts have been made to connect AD with internal disease (eg, occult malignancy) or even mercury exposure, but these theories have not been corroborated.
Consistent treatment success has also been elusive. Most patients achieve decent relief with the use of topical steroid creams, with or without the addition of anti-inflammatory medications (eg, doxycycline). Other options include isotretinoin and psoralen plus ultraviolet A (PUVA) photochemotherapy. Fortunately, most cases eventually clear up.
TAKE-HOME LEARNING POINTS
- Grover disease, also known as transient acantholytic dermatosis (AD), usually manifests with an acute eruption of papulovesicular lesions.
- AD lesions tend to be confined to the chest and are typically pruritic.
- Clinical diagnosis is usually adequate, although biopsy, which will reveal typical findings of acantholysis, may be necessary to rule out other items in the differential.
- Treatment with topical steroids, oral doxycycline, and “tincture of time” usually suffices, but resolution may take a year or more.
Red patches and thin plaques on feet
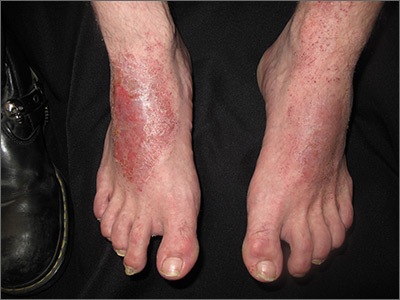
The FP conducted a physical exam and noticed bilateral dorsal foot dermatitis with occasional small vesicles and lichenified papules, which was suggestive of chronic contact or irritant dermatitis. The patient’s favorite pair of boots offered another clue as to the most likely contact allergens. (The boots were leather, and leather is treated with tanning agents and dyes.) A biopsy was not performed but would be expected to show spongiosis with some degree of lichenification (thickening of the dermis)—a sign of the acute on chronic nature of this process. The diagnosis of irritant or allergic contact dermatitis was made empirically.
The differential diagnosis for rashes on the feet can be broad and includes common tinea pedis, pitted keratolysis, stasis dermatitis, psoriasis, eczemas of various types, keratoderma, and contact dermatitis.
Many patients misconstrue that materials they use every day are exempt from becoming allergens. In counseling patients about this, point out that contact allergens often arise from repeated exposure. For example, dentists often develop dental amalgam allergies, hair professionals develop hair dye allergies, and machinists commonly develop cutting oil allergies. These reactions can and do occur years into their use.
The patient was started on topical clobetasol 0.05% ointment bid for 3 weeks, which provided quick relief and cleared his feet of the patches and plaques. He continued to wear his boots until contact allergy patch testing was performed in the office over a series of 3 days. This revealed an allergy to chromium, a common leather tanning agent. The patient was advised to avoid leather products including jackets, car upholstery, and gloves. After he carefully chose different footwear without a leather insole or tongue, the patient required no further therapy and remained clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).

The FP conducted a physical exam and noticed bilateral dorsal foot dermatitis with occasional small vesicles and lichenified papules, which was suggestive of chronic contact or irritant dermatitis. The patient’s favorite pair of boots offered another clue as to the most likely contact allergens. (The boots were leather, and leather is treated with tanning agents and dyes.) A biopsy was not performed but would be expected to show spongiosis with some degree of lichenification (thickening of the dermis)—a sign of the acute on chronic nature of this process. The diagnosis of irritant or allergic contact dermatitis was made empirically.
The differential diagnosis for rashes on the feet can be broad and includes common tinea pedis, pitted keratolysis, stasis dermatitis, psoriasis, eczemas of various types, keratoderma, and contact dermatitis.
Many patients misconstrue that materials they use every day are exempt from becoming allergens. In counseling patients about this, point out that contact allergens often arise from repeated exposure. For example, dentists often develop dental amalgam allergies, hair professionals develop hair dye allergies, and machinists commonly develop cutting oil allergies. These reactions can and do occur years into their use.
The patient was started on topical clobetasol 0.05% ointment bid for 3 weeks, which provided quick relief and cleared his feet of the patches and plaques. He continued to wear his boots until contact allergy patch testing was performed in the office over a series of 3 days. This revealed an allergy to chromium, a common leather tanning agent. The patient was advised to avoid leather products including jackets, car upholstery, and gloves. After he carefully chose different footwear without a leather insole or tongue, the patient required no further therapy and remained clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).

The FP conducted a physical exam and noticed bilateral dorsal foot dermatitis with occasional small vesicles and lichenified papules, which was suggestive of chronic contact or irritant dermatitis. The patient’s favorite pair of boots offered another clue as to the most likely contact allergens. (The boots were leather, and leather is treated with tanning agents and dyes.) A biopsy was not performed but would be expected to show spongiosis with some degree of lichenification (thickening of the dermis)—a sign of the acute on chronic nature of this process. The diagnosis of irritant or allergic contact dermatitis was made empirically.
The differential diagnosis for rashes on the feet can be broad and includes common tinea pedis, pitted keratolysis, stasis dermatitis, psoriasis, eczemas of various types, keratoderma, and contact dermatitis.
Many patients misconstrue that materials they use every day are exempt from becoming allergens. In counseling patients about this, point out that contact allergens often arise from repeated exposure. For example, dentists often develop dental amalgam allergies, hair professionals develop hair dye allergies, and machinists commonly develop cutting oil allergies. These reactions can and do occur years into their use.
The patient was started on topical clobetasol 0.05% ointment bid for 3 weeks, which provided quick relief and cleared his feet of the patches and plaques. He continued to wear his boots until contact allergy patch testing was performed in the office over a series of 3 days. This revealed an allergy to chromium, a common leather tanning agent. The patient was advised to avoid leather products including jackets, car upholstery, and gloves. After he carefully chose different footwear without a leather insole or tongue, the patient required no further therapy and remained clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).
Severe psoriasis associated with increased cancer risk, mortality
according to a meta-analysis of cohort and case-control studies.
Compared with a psoriasis-free population, having a diagnosis of severe psoriasis was associated with a 22% increase in cancer risk, Alex Trafford of the University of Manchester (England) and colleagues reported in JAMA Dermatology. The risk of cancer mortality was also increased by 22% among those with severe psoriasis.
The site-specific risks ranged from a low of 18% for colon cancer to more than a twofold increased risk for oral and esophageal cancer, according to the investigators.
Since these were associations only, any underlying mechanism is still unclear, they wrote. The chronic inflammation that drives psoriasis can also drive the development of cancer, but immunomodulatory therapies may also play a part, they suggested.
“Of particular relevance in this regard are biological therapies, which are being increasingly used for the management of psoriasis,” they added. “Although preliminary studies have suggested little to no increased risk of cancer incidence in patients with psoriasis receiving these therapies, further study allowing greater follow-up and increased power is required to properly examine the potential cancer risk, particularly for site-specific cancers.”
The analysis included 58 studies, published between 1983 and 2017. Nine of these reported risks for cancer incidence among patients with severe psoriasis, and seven reported the risk of cancer mortality among patients with all severities of psoriasis.
Overall, severe psoriasis was associated with an increased cancer risk of 22%; for all severities of psoriasis combined, the risk increase was 18%. Relative risks for specific cancer types were as follows: colon, 1.18; colorectal, 1.34; kidney, 1.58; laryngeal, 1.79; liver, 1.83; lymphoma, 1.40; non-Hodgkin lymphoma, 1.28; keratinocyte cancers, 1.71; esophageal 2.05; oral cavity, 2.80; and pancreatic, 1.41.
Overall cancer mortality risk was 22% higher in patients with severe psoriasis than the general population. Site-specific relative mortality risks included liver, 1.43; esophageal 2.53; and pancreatic, 1.31.
In light of these findings, clinicians should stress lifestyle modifications known to decrease cancer risk, the investigators said. “Although it has been noted that lifestyle behavior change is challenging for healthcare professionals to implement, the importance of a more holistic approach to psoriasis care involving lifestyle behavior change is reinforced through the results of this meta-analysis.”
Among the coauthors were Darren M. Ashcroft, PhD, the senior author, and Christopher Griffiths, MD, both of the University of Manchester. Dr. Ashcroft reported receiving research grants from AbbVie, Almirall, Celgene, Eli Lilly, Novartis, UCB, and the Leo Foundation. Dr. Griffiths reported receiving honoraria and/or research grants from AbbVie, Almirall, Bristol-Myers Squibb, Celgene, Eli Lilly, Galderma, Janssen, Leo Pharma, Novartis, Sandoz, and UCB. The lead author and the other authors had no disclosures. The Global Psoriasis Atlas (GPA) Collaborating Organizations (the International Federation of Psoriasis Associations, the International League of Dermatological Societies, and the International Psoriasis Council) were involved with funding of the study.
SOURCE: Trafford A et al. JAMA Dermatol. 2019 Oct 16. doi:10.1001/jamadermatol.2019.3056.
according to a meta-analysis of cohort and case-control studies.
Compared with a psoriasis-free population, having a diagnosis of severe psoriasis was associated with a 22% increase in cancer risk, Alex Trafford of the University of Manchester (England) and colleagues reported in JAMA Dermatology. The risk of cancer mortality was also increased by 22% among those with severe psoriasis.
The site-specific risks ranged from a low of 18% for colon cancer to more than a twofold increased risk for oral and esophageal cancer, according to the investigators.
Since these were associations only, any underlying mechanism is still unclear, they wrote. The chronic inflammation that drives psoriasis can also drive the development of cancer, but immunomodulatory therapies may also play a part, they suggested.
“Of particular relevance in this regard are biological therapies, which are being increasingly used for the management of psoriasis,” they added. “Although preliminary studies have suggested little to no increased risk of cancer incidence in patients with psoriasis receiving these therapies, further study allowing greater follow-up and increased power is required to properly examine the potential cancer risk, particularly for site-specific cancers.”
The analysis included 58 studies, published between 1983 and 2017. Nine of these reported risks for cancer incidence among patients with severe psoriasis, and seven reported the risk of cancer mortality among patients with all severities of psoriasis.
Overall, severe psoriasis was associated with an increased cancer risk of 22%; for all severities of psoriasis combined, the risk increase was 18%. Relative risks for specific cancer types were as follows: colon, 1.18; colorectal, 1.34; kidney, 1.58; laryngeal, 1.79; liver, 1.83; lymphoma, 1.40; non-Hodgkin lymphoma, 1.28; keratinocyte cancers, 1.71; esophageal 2.05; oral cavity, 2.80; and pancreatic, 1.41.
Overall cancer mortality risk was 22% higher in patients with severe psoriasis than the general population. Site-specific relative mortality risks included liver, 1.43; esophageal 2.53; and pancreatic, 1.31.
In light of these findings, clinicians should stress lifestyle modifications known to decrease cancer risk, the investigators said. “Although it has been noted that lifestyle behavior change is challenging for healthcare professionals to implement, the importance of a more holistic approach to psoriasis care involving lifestyle behavior change is reinforced through the results of this meta-analysis.”
Among the coauthors were Darren M. Ashcroft, PhD, the senior author, and Christopher Griffiths, MD, both of the University of Manchester. Dr. Ashcroft reported receiving research grants from AbbVie, Almirall, Celgene, Eli Lilly, Novartis, UCB, and the Leo Foundation. Dr. Griffiths reported receiving honoraria and/or research grants from AbbVie, Almirall, Bristol-Myers Squibb, Celgene, Eli Lilly, Galderma, Janssen, Leo Pharma, Novartis, Sandoz, and UCB. The lead author and the other authors had no disclosures. The Global Psoriasis Atlas (GPA) Collaborating Organizations (the International Federation of Psoriasis Associations, the International League of Dermatological Societies, and the International Psoriasis Council) were involved with funding of the study.
SOURCE: Trafford A et al. JAMA Dermatol. 2019 Oct 16. doi:10.1001/jamadermatol.2019.3056.
according to a meta-analysis of cohort and case-control studies.
Compared with a psoriasis-free population, having a diagnosis of severe psoriasis was associated with a 22% increase in cancer risk, Alex Trafford of the University of Manchester (England) and colleagues reported in JAMA Dermatology. The risk of cancer mortality was also increased by 22% among those with severe psoriasis.
The site-specific risks ranged from a low of 18% for colon cancer to more than a twofold increased risk for oral and esophageal cancer, according to the investigators.
Since these were associations only, any underlying mechanism is still unclear, they wrote. The chronic inflammation that drives psoriasis can also drive the development of cancer, but immunomodulatory therapies may also play a part, they suggested.
“Of particular relevance in this regard are biological therapies, which are being increasingly used for the management of psoriasis,” they added. “Although preliminary studies have suggested little to no increased risk of cancer incidence in patients with psoriasis receiving these therapies, further study allowing greater follow-up and increased power is required to properly examine the potential cancer risk, particularly for site-specific cancers.”
The analysis included 58 studies, published between 1983 and 2017. Nine of these reported risks for cancer incidence among patients with severe psoriasis, and seven reported the risk of cancer mortality among patients with all severities of psoriasis.
Overall, severe psoriasis was associated with an increased cancer risk of 22%; for all severities of psoriasis combined, the risk increase was 18%. Relative risks for specific cancer types were as follows: colon, 1.18; colorectal, 1.34; kidney, 1.58; laryngeal, 1.79; liver, 1.83; lymphoma, 1.40; non-Hodgkin lymphoma, 1.28; keratinocyte cancers, 1.71; esophageal 2.05; oral cavity, 2.80; and pancreatic, 1.41.
Overall cancer mortality risk was 22% higher in patients with severe psoriasis than the general population. Site-specific relative mortality risks included liver, 1.43; esophageal 2.53; and pancreatic, 1.31.
In light of these findings, clinicians should stress lifestyle modifications known to decrease cancer risk, the investigators said. “Although it has been noted that lifestyle behavior change is challenging for healthcare professionals to implement, the importance of a more holistic approach to psoriasis care involving lifestyle behavior change is reinforced through the results of this meta-analysis.”
Among the coauthors were Darren M. Ashcroft, PhD, the senior author, and Christopher Griffiths, MD, both of the University of Manchester. Dr. Ashcroft reported receiving research grants from AbbVie, Almirall, Celgene, Eli Lilly, Novartis, UCB, and the Leo Foundation. Dr. Griffiths reported receiving honoraria and/or research grants from AbbVie, Almirall, Bristol-Myers Squibb, Celgene, Eli Lilly, Galderma, Janssen, Leo Pharma, Novartis, Sandoz, and UCB. The lead author and the other authors had no disclosures. The Global Psoriasis Atlas (GPA) Collaborating Organizations (the International Federation of Psoriasis Associations, the International League of Dermatological Societies, and the International Psoriasis Council) were involved with funding of the study.
SOURCE: Trafford A et al. JAMA Dermatol. 2019 Oct 16. doi:10.1001/jamadermatol.2019.3056.
FROM JAMA DERMATOLOGY
Appropriate laboratory testing in Lyme disease
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
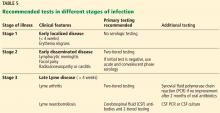
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
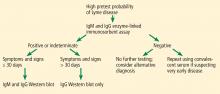
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
KEY POINTS
- Lyme disease, the most common tick-borne infection in North America, is a complex multisystem bacterial disease caused by Borrelia burgdorferi.
- Lyme disease preferably affects the skin, joints, and nervous system and presents with typical and atypical features. Certain clinical features are diagnostic. Its diagnosis is mainly clinical and epidemiologic and, when doubtful, is supported by serologic testing.
- Standard 2-tiered testing is the diagnostic testing method of choice—enzyme-linked immunoassay followed by Western blot. Interpretation of the bands depends on the duration of infection.
- When interpreting the test results, be aware of false-positives and the reasons for them.
Dark patches around the trunk
The FP noticed a lacy net-like or reticulate appearance and thin brown papules to warty plaques over the trunk and recognized this condition as confluent and reticulated papillomatosis (CARP). A potassium hydroxide (KOH) test of a skin scraping failed to reveal yeast forms or hyphae. The FP determined that a biopsy was not necessary for diagnosis due to the distinct clinical appearance and negative KOH test. However, a biopsy could have distinguished this presentation from similar appearing disorders, including acanthosis nigricans and pityriasis versicolor.
CARP is an uncommon disorder of keratinization that affects adolescents and young adults, and is more common in Caucasians. A classic presentation involves the neck, chest, and abdomen. The differential diagnosis includes acanthosis nigricans and pityriasis versicolor, as well as more rare disorders that include Darier disease and keratosis follicularis.
There appears to be an association between the disorder and weight (specifically, being overweight). In addition, some familial cases have been reported.
Most recently, Dietzia papillomatosis, a gram-positive actinomycete has been implicated as a likely cause, which supports antibiotic therapy as the first-line approach. Minocycline 50 mg bid for 6 weeks clears the papules and plaques for most patients. Azithromycin and clarithromycin are alternatives, with various dosing strategies lasting 6 to 12 weeks. Complete clearance may take months to more than a year. About 15% of patients will experience recurrence.
This patient was treated with minocycline 50 mg bid for 12 weeks, a more common strategy at the time she was diagnosed. This led to complete clearance at 3 months, and she remained clear a year after beginning treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).

The FP noticed a lacy net-like or reticulate appearance and thin brown papules to warty plaques over the trunk and recognized this condition as confluent and reticulated papillomatosis (CARP). A potassium hydroxide (KOH) test of a skin scraping failed to reveal yeast forms or hyphae. The FP determined that a biopsy was not necessary for diagnosis due to the distinct clinical appearance and negative KOH test. However, a biopsy could have distinguished this presentation from similar appearing disorders, including acanthosis nigricans and pityriasis versicolor.
CARP is an uncommon disorder of keratinization that affects adolescents and young adults, and is more common in Caucasians. A classic presentation involves the neck, chest, and abdomen. The differential diagnosis includes acanthosis nigricans and pityriasis versicolor, as well as more rare disorders that include Darier disease and keratosis follicularis.
There appears to be an association between the disorder and weight (specifically, being overweight). In addition, some familial cases have been reported.
Most recently, Dietzia papillomatosis, a gram-positive actinomycete has been implicated as a likely cause, which supports antibiotic therapy as the first-line approach. Minocycline 50 mg bid for 6 weeks clears the papules and plaques for most patients. Azithromycin and clarithromycin are alternatives, with various dosing strategies lasting 6 to 12 weeks. Complete clearance may take months to more than a year. About 15% of patients will experience recurrence.
This patient was treated with minocycline 50 mg bid for 12 weeks, a more common strategy at the time she was diagnosed. This led to complete clearance at 3 months, and she remained clear a year after beginning treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).

The FP noticed a lacy net-like or reticulate appearance and thin brown papules to warty plaques over the trunk and recognized this condition as confluent and reticulated papillomatosis (CARP). A potassium hydroxide (KOH) test of a skin scraping failed to reveal yeast forms or hyphae. The FP determined that a biopsy was not necessary for diagnosis due to the distinct clinical appearance and negative KOH test. However, a biopsy could have distinguished this presentation from similar appearing disorders, including acanthosis nigricans and pityriasis versicolor.
CARP is an uncommon disorder of keratinization that affects adolescents and young adults, and is more common in Caucasians. A classic presentation involves the neck, chest, and abdomen. The differential diagnosis includes acanthosis nigricans and pityriasis versicolor, as well as more rare disorders that include Darier disease and keratosis follicularis.
There appears to be an association between the disorder and weight (specifically, being overweight). In addition, some familial cases have been reported.
Most recently, Dietzia papillomatosis, a gram-positive actinomycete has been implicated as a likely cause, which supports antibiotic therapy as the first-line approach. Minocycline 50 mg bid for 6 weeks clears the papules and plaques for most patients. Azithromycin and clarithromycin are alternatives, with various dosing strategies lasting 6 to 12 weeks. Complete clearance may take months to more than a year. About 15% of patients will experience recurrence.
This patient was treated with minocycline 50 mg bid for 12 weeks, a more common strategy at the time she was diagnosed. This led to complete clearance at 3 months, and she remained clear a year after beginning treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).
Psoriasis risk rises with TNF inhibitor use in children with inflammatory disorders
, a retrospective cohort study has determined.
“The incidence rate and risk factors of psoriasis in children with IBD [inflammatory bowel disease], JIA [juvenile idiopathic arthritis], or CNO [chronic nonbacterial osteomyelitis] who are exposed to TNFi [tumor necrosis factor inhibitors] are unknown. Additionally, there is a well-established association between these inflammatory conditions and psoriasis development. Yet, as TNFi can both treat and trigger psoriasis, it is not clear how TNFi exposure affects this relationship,” wrote Lisa H. Buckley, MD, of Children’s Hospital at Vanderbilt, Nashville, Tenn., and colleagues. Their report is in Arthritis Care & Research.
The team examined the relationship in children who were treated for an inflammatory disorder at Children’s Hospital of Philadelphia during 2008-2018. IBD was most common at 74%, followed by JIA at 24% and CNO at 2%.
Among 4,111 children with those inflammatory disorders, the psoriasis incidence was 12.3 per 1,000 person-years in exposed children and 3.8 per 1,000 person-years in unexposed. This significant difference equated to a hazard ratio of 3.84 for developing psoriasis after TNFi exposure.
“These data reflect the established association between inflammatory conditions and psoriasis development and suggest that TNFi exposure further increases the risk of psoriasis,” Dr. Buckley and coauthors wrote.
The median duration of follow-up in this study was about 2.5 years for patients exposed to TNFi and 2 years for those unexposed. Among the entire cohort, 39% had been exposed to a TNFi, with 4,705 person-years of follow-up. Among the unexposed children (61%), there were 6,604 person-years of follow-up.
In all, 83 cases of psoriasis developed: 58 in the exposed group and 25 in the unexposed group. Psoriasis incidence varied by disorder. Exposed children with IBD had a higher incidence than did unexposed children (10.9 vs. 2.6 per 1,000 person-years; HR = 4.52). Exposed children with JIA also had a higher incidence than did unexposed children (14.7 vs. 5.5 per 1,000 person-years; HR = 2.90). Among those with CNO, incidences were similar for exposed and unexposed children (33.5 and 38.9 per 1,000 person-years).
A family history of psoriasis significantly increased the risk of psoriasis with a hazard ratio of 3.11, the authors noted. But none of the other covariates (age, sex, race, obesity, methotrexate exposure, and underlying diagnosis) exerted a significant additional risk.
The study had no outside funding source. The authors had no financial disclosures. Dr. Buckley conducted the research when she was a pediatric rheumatology fellow at Children’s Hospital of Philadelphia.
SOURCE: Buckley LH et al. Arthritis Care Res. 2019 Oct 23. doi: 10.1002/ACR.24100
, a retrospective cohort study has determined.
“The incidence rate and risk factors of psoriasis in children with IBD [inflammatory bowel disease], JIA [juvenile idiopathic arthritis], or CNO [chronic nonbacterial osteomyelitis] who are exposed to TNFi [tumor necrosis factor inhibitors] are unknown. Additionally, there is a well-established association between these inflammatory conditions and psoriasis development. Yet, as TNFi can both treat and trigger psoriasis, it is not clear how TNFi exposure affects this relationship,” wrote Lisa H. Buckley, MD, of Children’s Hospital at Vanderbilt, Nashville, Tenn., and colleagues. Their report is in Arthritis Care & Research.
The team examined the relationship in children who were treated for an inflammatory disorder at Children’s Hospital of Philadelphia during 2008-2018. IBD was most common at 74%, followed by JIA at 24% and CNO at 2%.
Among 4,111 children with those inflammatory disorders, the psoriasis incidence was 12.3 per 1,000 person-years in exposed children and 3.8 per 1,000 person-years in unexposed. This significant difference equated to a hazard ratio of 3.84 for developing psoriasis after TNFi exposure.
“These data reflect the established association between inflammatory conditions and psoriasis development and suggest that TNFi exposure further increases the risk of psoriasis,” Dr. Buckley and coauthors wrote.
The median duration of follow-up in this study was about 2.5 years for patients exposed to TNFi and 2 years for those unexposed. Among the entire cohort, 39% had been exposed to a TNFi, with 4,705 person-years of follow-up. Among the unexposed children (61%), there were 6,604 person-years of follow-up.
In all, 83 cases of psoriasis developed: 58 in the exposed group and 25 in the unexposed group. Psoriasis incidence varied by disorder. Exposed children with IBD had a higher incidence than did unexposed children (10.9 vs. 2.6 per 1,000 person-years; HR = 4.52). Exposed children with JIA also had a higher incidence than did unexposed children (14.7 vs. 5.5 per 1,000 person-years; HR = 2.90). Among those with CNO, incidences were similar for exposed and unexposed children (33.5 and 38.9 per 1,000 person-years).
A family history of psoriasis significantly increased the risk of psoriasis with a hazard ratio of 3.11, the authors noted. But none of the other covariates (age, sex, race, obesity, methotrexate exposure, and underlying diagnosis) exerted a significant additional risk.
The study had no outside funding source. The authors had no financial disclosures. Dr. Buckley conducted the research when she was a pediatric rheumatology fellow at Children’s Hospital of Philadelphia.
SOURCE: Buckley LH et al. Arthritis Care Res. 2019 Oct 23. doi: 10.1002/ACR.24100
, a retrospective cohort study has determined.
“The incidence rate and risk factors of psoriasis in children with IBD [inflammatory bowel disease], JIA [juvenile idiopathic arthritis], or CNO [chronic nonbacterial osteomyelitis] who are exposed to TNFi [tumor necrosis factor inhibitors] are unknown. Additionally, there is a well-established association between these inflammatory conditions and psoriasis development. Yet, as TNFi can both treat and trigger psoriasis, it is not clear how TNFi exposure affects this relationship,” wrote Lisa H. Buckley, MD, of Children’s Hospital at Vanderbilt, Nashville, Tenn., and colleagues. Their report is in Arthritis Care & Research.
The team examined the relationship in children who were treated for an inflammatory disorder at Children’s Hospital of Philadelphia during 2008-2018. IBD was most common at 74%, followed by JIA at 24% and CNO at 2%.
Among 4,111 children with those inflammatory disorders, the psoriasis incidence was 12.3 per 1,000 person-years in exposed children and 3.8 per 1,000 person-years in unexposed. This significant difference equated to a hazard ratio of 3.84 for developing psoriasis after TNFi exposure.
“These data reflect the established association between inflammatory conditions and psoriasis development and suggest that TNFi exposure further increases the risk of psoriasis,” Dr. Buckley and coauthors wrote.
The median duration of follow-up in this study was about 2.5 years for patients exposed to TNFi and 2 years for those unexposed. Among the entire cohort, 39% had been exposed to a TNFi, with 4,705 person-years of follow-up. Among the unexposed children (61%), there were 6,604 person-years of follow-up.
In all, 83 cases of psoriasis developed: 58 in the exposed group and 25 in the unexposed group. Psoriasis incidence varied by disorder. Exposed children with IBD had a higher incidence than did unexposed children (10.9 vs. 2.6 per 1,000 person-years; HR = 4.52). Exposed children with JIA also had a higher incidence than did unexposed children (14.7 vs. 5.5 per 1,000 person-years; HR = 2.90). Among those with CNO, incidences were similar for exposed and unexposed children (33.5 and 38.9 per 1,000 person-years).
A family history of psoriasis significantly increased the risk of psoriasis with a hazard ratio of 3.11, the authors noted. But none of the other covariates (age, sex, race, obesity, methotrexate exposure, and underlying diagnosis) exerted a significant additional risk.
The study had no outside funding source. The authors had no financial disclosures. Dr. Buckley conducted the research when she was a pediatric rheumatology fellow at Children’s Hospital of Philadelphia.
SOURCE: Buckley LH et al. Arthritis Care Res. 2019 Oct 23. doi: 10.1002/ACR.24100
FROM ARTHRITIS RESEARCH & CARE
No infection increase seen with biologics in older psoriasis patients
MADRID – Psoriasis patients aged 65 years and older are at more than twice the risk of serious bacterial and opportunistic infections, compared with younger patients, but that risk is not further elevated by being on biologic agents, Joseph F. Merola, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a large, The study implications, he said, are clear: When moderate to severe psoriasis warrants consideration of highly effective biologic therapies, that therapeutic option shouldn’t be taken off the table on the basis of a mistaken belief that biologics pose a greater infection risk just because the affected patient is over age 65 years.
“We really think that older patients should be offered treatments at the same level of disease control as all the rest of our psoriasis patients, in the context of shared decision making,” said Dr. Merola, a dermatologist and rheumatologist who is the director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston.
The study utilized longitudinal claims data from a very large U.S. database covering the years 2003-2017. Among the 185 million covered lives were 1.1 million individuals with psoriasis, including 150,000 aged 65 years or older. After excluding older psoriasis patients with comorbid cancer or autoimmune disease, the investigators were left with 11,218 older psoriasis patients initiating systemic therapy for the first time and therefore eligible for propensity score matching using a highly accurate proprietary platform. The final study population consisted of 2,795 older psoriasis patients newly initiating biologic therapy, 2,795 others newly initiating nonbiologic systemic agents, and 2,529 seniors starting phototherapy. The matching was based upon factors including age, sex, prior infections, comorbid psoriatic arthritis, diabetes, and obesity.
The primary study endpoint was the rate of serious bacterial or opportunistic infections requiring hospitalization during the first 6 months of treatment. The bottom line: The rates were closely similar across all three groups, with the most common serious infections being pneumonia and cellulitis.
In contrast, among a population of 115,047 senior psoriasis patients who never used systemic therapy, the risk of serious infection was 12.2 events per 1,000 patients over 6 months, compared with 5.3 events in 120,174 matched controls without psoriasis. That translates to a 2.24-fold increased risk.
One audience member commented that a limitation of the study was that all biologics were lumped together. He would expect that the tumor necrosis factor inhibitors, for example, would be associated with a significantly higher serious infection risk than biologics with other targets.
Dr. Merola conceded the point, adding that the investigators are trying to reanalyze the data in a more granular way to address that shortcoming. Other study limitations included an inability to access the specific doses of systemic treatments used or to stratify patients by disease severity.
Another audience member noted that dermatologists often reassure surgeons that there’s no increased risk of infection associated with psoriasis when in fact there is increased risk in older psoriasis patients, according to these new data.
“We’re not trying to send a message to surgeons to withhold a knee transplant because of a psoriasis plaque over the knee,” Dr. Merola replied. “I think we’ve all been there; we’ve all fought that battle.” Based on the data, he said, he would advise that “our patients who need to be on systemics should remain appropriately on systemics as we see fit.”
The study was entirely funded by Brigham and Women’s Hospital. Dr. Merola reported serving as a consultant to and/or recipient of research grants from nearly two dozen pharmaceutical companies.
MADRID – Psoriasis patients aged 65 years and older are at more than twice the risk of serious bacterial and opportunistic infections, compared with younger patients, but that risk is not further elevated by being on biologic agents, Joseph F. Merola, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a large, The study implications, he said, are clear: When moderate to severe psoriasis warrants consideration of highly effective biologic therapies, that therapeutic option shouldn’t be taken off the table on the basis of a mistaken belief that biologics pose a greater infection risk just because the affected patient is over age 65 years.
“We really think that older patients should be offered treatments at the same level of disease control as all the rest of our psoriasis patients, in the context of shared decision making,” said Dr. Merola, a dermatologist and rheumatologist who is the director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston.
The study utilized longitudinal claims data from a very large U.S. database covering the years 2003-2017. Among the 185 million covered lives were 1.1 million individuals with psoriasis, including 150,000 aged 65 years or older. After excluding older psoriasis patients with comorbid cancer or autoimmune disease, the investigators were left with 11,218 older psoriasis patients initiating systemic therapy for the first time and therefore eligible for propensity score matching using a highly accurate proprietary platform. The final study population consisted of 2,795 older psoriasis patients newly initiating biologic therapy, 2,795 others newly initiating nonbiologic systemic agents, and 2,529 seniors starting phototherapy. The matching was based upon factors including age, sex, prior infections, comorbid psoriatic arthritis, diabetes, and obesity.
The primary study endpoint was the rate of serious bacterial or opportunistic infections requiring hospitalization during the first 6 months of treatment. The bottom line: The rates were closely similar across all three groups, with the most common serious infections being pneumonia and cellulitis.
In contrast, among a population of 115,047 senior psoriasis patients who never used systemic therapy, the risk of serious infection was 12.2 events per 1,000 patients over 6 months, compared with 5.3 events in 120,174 matched controls without psoriasis. That translates to a 2.24-fold increased risk.
One audience member commented that a limitation of the study was that all biologics were lumped together. He would expect that the tumor necrosis factor inhibitors, for example, would be associated with a significantly higher serious infection risk than biologics with other targets.
Dr. Merola conceded the point, adding that the investigators are trying to reanalyze the data in a more granular way to address that shortcoming. Other study limitations included an inability to access the specific doses of systemic treatments used or to stratify patients by disease severity.
Another audience member noted that dermatologists often reassure surgeons that there’s no increased risk of infection associated with psoriasis when in fact there is increased risk in older psoriasis patients, according to these new data.
“We’re not trying to send a message to surgeons to withhold a knee transplant because of a psoriasis plaque over the knee,” Dr. Merola replied. “I think we’ve all been there; we’ve all fought that battle.” Based on the data, he said, he would advise that “our patients who need to be on systemics should remain appropriately on systemics as we see fit.”
The study was entirely funded by Brigham and Women’s Hospital. Dr. Merola reported serving as a consultant to and/or recipient of research grants from nearly two dozen pharmaceutical companies.
MADRID – Psoriasis patients aged 65 years and older are at more than twice the risk of serious bacterial and opportunistic infections, compared with younger patients, but that risk is not further elevated by being on biologic agents, Joseph F. Merola, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a large, The study implications, he said, are clear: When moderate to severe psoriasis warrants consideration of highly effective biologic therapies, that therapeutic option shouldn’t be taken off the table on the basis of a mistaken belief that biologics pose a greater infection risk just because the affected patient is over age 65 years.
“We really think that older patients should be offered treatments at the same level of disease control as all the rest of our psoriasis patients, in the context of shared decision making,” said Dr. Merola, a dermatologist and rheumatologist who is the director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston.
The study utilized longitudinal claims data from a very large U.S. database covering the years 2003-2017. Among the 185 million covered lives were 1.1 million individuals with psoriasis, including 150,000 aged 65 years or older. After excluding older psoriasis patients with comorbid cancer or autoimmune disease, the investigators were left with 11,218 older psoriasis patients initiating systemic therapy for the first time and therefore eligible for propensity score matching using a highly accurate proprietary platform. The final study population consisted of 2,795 older psoriasis patients newly initiating biologic therapy, 2,795 others newly initiating nonbiologic systemic agents, and 2,529 seniors starting phototherapy. The matching was based upon factors including age, sex, prior infections, comorbid psoriatic arthritis, diabetes, and obesity.
The primary study endpoint was the rate of serious bacterial or opportunistic infections requiring hospitalization during the first 6 months of treatment. The bottom line: The rates were closely similar across all three groups, with the most common serious infections being pneumonia and cellulitis.
In contrast, among a population of 115,047 senior psoriasis patients who never used systemic therapy, the risk of serious infection was 12.2 events per 1,000 patients over 6 months, compared with 5.3 events in 120,174 matched controls without psoriasis. That translates to a 2.24-fold increased risk.
One audience member commented that a limitation of the study was that all biologics were lumped together. He would expect that the tumor necrosis factor inhibitors, for example, would be associated with a significantly higher serious infection risk than biologics with other targets.
Dr. Merola conceded the point, adding that the investigators are trying to reanalyze the data in a more granular way to address that shortcoming. Other study limitations included an inability to access the specific doses of systemic treatments used or to stratify patients by disease severity.
Another audience member noted that dermatologists often reassure surgeons that there’s no increased risk of infection associated with psoriasis when in fact there is increased risk in older psoriasis patients, according to these new data.
“We’re not trying to send a message to surgeons to withhold a knee transplant because of a psoriasis plaque over the knee,” Dr. Merola replied. “I think we’ve all been there; we’ve all fought that battle.” Based on the data, he said, he would advise that “our patients who need to be on systemics should remain appropriately on systemics as we see fit.”
The study was entirely funded by Brigham and Women’s Hospital. Dr. Merola reported serving as a consultant to and/or recipient of research grants from nearly two dozen pharmaceutical companies.
REPORTING FROM EADV 2019
PASI-75 with ixekizumab approaches 90% in pediatric psoriasis study
MADRID – The interleukin-17A inhibitor , Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The results bode well for an underserved population.
“I think all of us know that there is still a vulnerable population that remains a high-risk population because of the limited number of therapies available for them, and that is children,” said Dr. Papp, a dermatologist and president of Probity Medical Research, Inc., of Waterloo, Ont.
At present, etanercept, one of the earliest biologics to become available, and a relatively less effective one, is the only biologic approved for treatment of pediatric psoriasis. However, Lilly, which sponsored the phase 3 ixekizumab study, has announced that based upon the highly positive findings the company plans to seek Food and Drug Administration approval for an expanded indication for the medication in pediatric psoriasis. The company now markets ixekizumab for the approved indications of treatment of adults with moderate to severe plaque psoriasis, active psoriatic arthritis, or active ankylosing spondylitis.
The 12-week, double-blind, multicenter phase 3 trial known as IXORA-PEDS included 115 pediatric psoriasis patients randomized to weight-based ixekizumab, 30 on weight-based etanercept, and 58 on placebo. At the 12-week mark, everyone was switched to open-label ixekizumab in a long-term extension study. Children weighing less than 25 kg received a 40-mg loading dose of ixekizumab, followed by a maintenance dose of 20 mg by subcutaneous injection every 4 weeks. Patients weighing 25-50 kg got a starting dose of 80 mg, then 40 mg for maintenance therapy. Those who weighed more than 50 kg got the usual adult dosing: a 160-mg loading dose followed by 80 mg every 4 weeks. Etanercept was dosed at 0.8 mg/kg once weekly.
The coprimary endpoints were the proportion of subjects achieving a static Physician’s Global Assessment (sPGA) of 0 or 1 – that is, clear or almost clear skin – at week 12, and the PASI 75 response rate.
An sPGA of 0 or 1 at week 12 was documented in 81% of the ixekizumab group, 11% on placebo, and 40% of etanercept-treated patients, who on average had more severe baseline disease than did the other two groups.
The PASI 75 rate was 89% with ixekizumab, 25% for placebo, and 63% on etanercept. But Dr. Papp indicated that’s too low a bar. “I don’t think PASI 75s are the standard any longer,” he said.
More revealing was the PASI 90 rate: 78% with the IL-17A inhibitor, 5% in placebo-treated controls, and 40% with etanercept.
And then there’s the PASI 100 response rate: 50% with ixekizumab, 2% for placebo, and 17% for etanercept.
“I think this is very telling. I’ll leave it as a tantalizing comment that if one looks at the slope of the curve, it doesn’t yet seem to have reached its plateau at week 12 – and this is very similar to the pattern that we see in the adult population. I don’t have the long-term extension efficacy data, but I am, like you, very interested in seeing where this PASI 100 response rate finally plateaus,” Dr. Papp said.
He did, however, have the combined safety data for the 12-week double-blind phase plus the open-label extension, which he described as essentially the same as the adult experience. Injection-site reactions occurred in 19% of pediatric patients on ixekizumab, but they were generally mild and there were few if any treatment discontinuations for that reason. There was a 2% incidence of Crohn’s disease. Candidiasis and other infections were rare.
Seventy-one percent of the ixekizumab group had at least a 4-point improvement in itch on a 10-point self-rated scale by week 12, as did 20% of placebo-treated controls. A Dermatologic Life Quality Index score of 0 or 1 at week 12, indicative of no or minimal impact of psoriasis on quality of life, was documented in 64% of the ixekizumab group and 23% of controls.
Dr. Papp reported serving as a consultant, investigator, and/or speaker for Lilly and more than three dozen other pharmaceutical companies.
SOURCE: Papp KA. EADV Late breaker.
MADRID – The interleukin-17A inhibitor , Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The results bode well for an underserved population.
“I think all of us know that there is still a vulnerable population that remains a high-risk population because of the limited number of therapies available for them, and that is children,” said Dr. Papp, a dermatologist and president of Probity Medical Research, Inc., of Waterloo, Ont.
At present, etanercept, one of the earliest biologics to become available, and a relatively less effective one, is the only biologic approved for treatment of pediatric psoriasis. However, Lilly, which sponsored the phase 3 ixekizumab study, has announced that based upon the highly positive findings the company plans to seek Food and Drug Administration approval for an expanded indication for the medication in pediatric psoriasis. The company now markets ixekizumab for the approved indications of treatment of adults with moderate to severe plaque psoriasis, active psoriatic arthritis, or active ankylosing spondylitis.
The 12-week, double-blind, multicenter phase 3 trial known as IXORA-PEDS included 115 pediatric psoriasis patients randomized to weight-based ixekizumab, 30 on weight-based etanercept, and 58 on placebo. At the 12-week mark, everyone was switched to open-label ixekizumab in a long-term extension study. Children weighing less than 25 kg received a 40-mg loading dose of ixekizumab, followed by a maintenance dose of 20 mg by subcutaneous injection every 4 weeks. Patients weighing 25-50 kg got a starting dose of 80 mg, then 40 mg for maintenance therapy. Those who weighed more than 50 kg got the usual adult dosing: a 160-mg loading dose followed by 80 mg every 4 weeks. Etanercept was dosed at 0.8 mg/kg once weekly.
The coprimary endpoints were the proportion of subjects achieving a static Physician’s Global Assessment (sPGA) of 0 or 1 – that is, clear or almost clear skin – at week 12, and the PASI 75 response rate.
An sPGA of 0 or 1 at week 12 was documented in 81% of the ixekizumab group, 11% on placebo, and 40% of etanercept-treated patients, who on average had more severe baseline disease than did the other two groups.
The PASI 75 rate was 89% with ixekizumab, 25% for placebo, and 63% on etanercept. But Dr. Papp indicated that’s too low a bar. “I don’t think PASI 75s are the standard any longer,” he said.
More revealing was the PASI 90 rate: 78% with the IL-17A inhibitor, 5% in placebo-treated controls, and 40% with etanercept.
And then there’s the PASI 100 response rate: 50% with ixekizumab, 2% for placebo, and 17% for etanercept.
“I think this is very telling. I’ll leave it as a tantalizing comment that if one looks at the slope of the curve, it doesn’t yet seem to have reached its plateau at week 12 – and this is very similar to the pattern that we see in the adult population. I don’t have the long-term extension efficacy data, but I am, like you, very interested in seeing where this PASI 100 response rate finally plateaus,” Dr. Papp said.
He did, however, have the combined safety data for the 12-week double-blind phase plus the open-label extension, which he described as essentially the same as the adult experience. Injection-site reactions occurred in 19% of pediatric patients on ixekizumab, but they were generally mild and there were few if any treatment discontinuations for that reason. There was a 2% incidence of Crohn’s disease. Candidiasis and other infections were rare.
Seventy-one percent of the ixekizumab group had at least a 4-point improvement in itch on a 10-point self-rated scale by week 12, as did 20% of placebo-treated controls. A Dermatologic Life Quality Index score of 0 or 1 at week 12, indicative of no or minimal impact of psoriasis on quality of life, was documented in 64% of the ixekizumab group and 23% of controls.
Dr. Papp reported serving as a consultant, investigator, and/or speaker for Lilly and more than three dozen other pharmaceutical companies.
SOURCE: Papp KA. EADV Late breaker.
MADRID – The interleukin-17A inhibitor , Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The results bode well for an underserved population.
“I think all of us know that there is still a vulnerable population that remains a high-risk population because of the limited number of therapies available for them, and that is children,” said Dr. Papp, a dermatologist and president of Probity Medical Research, Inc., of Waterloo, Ont.
At present, etanercept, one of the earliest biologics to become available, and a relatively less effective one, is the only biologic approved for treatment of pediatric psoriasis. However, Lilly, which sponsored the phase 3 ixekizumab study, has announced that based upon the highly positive findings the company plans to seek Food and Drug Administration approval for an expanded indication for the medication in pediatric psoriasis. The company now markets ixekizumab for the approved indications of treatment of adults with moderate to severe plaque psoriasis, active psoriatic arthritis, or active ankylosing spondylitis.
The 12-week, double-blind, multicenter phase 3 trial known as IXORA-PEDS included 115 pediatric psoriasis patients randomized to weight-based ixekizumab, 30 on weight-based etanercept, and 58 on placebo. At the 12-week mark, everyone was switched to open-label ixekizumab in a long-term extension study. Children weighing less than 25 kg received a 40-mg loading dose of ixekizumab, followed by a maintenance dose of 20 mg by subcutaneous injection every 4 weeks. Patients weighing 25-50 kg got a starting dose of 80 mg, then 40 mg for maintenance therapy. Those who weighed more than 50 kg got the usual adult dosing: a 160-mg loading dose followed by 80 mg every 4 weeks. Etanercept was dosed at 0.8 mg/kg once weekly.
The coprimary endpoints were the proportion of subjects achieving a static Physician’s Global Assessment (sPGA) of 0 or 1 – that is, clear or almost clear skin – at week 12, and the PASI 75 response rate.
An sPGA of 0 or 1 at week 12 was documented in 81% of the ixekizumab group, 11% on placebo, and 40% of etanercept-treated patients, who on average had more severe baseline disease than did the other two groups.
The PASI 75 rate was 89% with ixekizumab, 25% for placebo, and 63% on etanercept. But Dr. Papp indicated that’s too low a bar. “I don’t think PASI 75s are the standard any longer,” he said.
More revealing was the PASI 90 rate: 78% with the IL-17A inhibitor, 5% in placebo-treated controls, and 40% with etanercept.
And then there’s the PASI 100 response rate: 50% with ixekizumab, 2% for placebo, and 17% for etanercept.
“I think this is very telling. I’ll leave it as a tantalizing comment that if one looks at the slope of the curve, it doesn’t yet seem to have reached its plateau at week 12 – and this is very similar to the pattern that we see in the adult population. I don’t have the long-term extension efficacy data, but I am, like you, very interested in seeing where this PASI 100 response rate finally plateaus,” Dr. Papp said.
He did, however, have the combined safety data for the 12-week double-blind phase plus the open-label extension, which he described as essentially the same as the adult experience. Injection-site reactions occurred in 19% of pediatric patients on ixekizumab, but they were generally mild and there were few if any treatment discontinuations for that reason. There was a 2% incidence of Crohn’s disease. Candidiasis and other infections were rare.
Seventy-one percent of the ixekizumab group had at least a 4-point improvement in itch on a 10-point self-rated scale by week 12, as did 20% of placebo-treated controls. A Dermatologic Life Quality Index score of 0 or 1 at week 12, indicative of no or minimal impact of psoriasis on quality of life, was documented in 64% of the ixekizumab group and 23% of controls.
Dr. Papp reported serving as a consultant, investigator, and/or speaker for Lilly and more than three dozen other pharmaceutical companies.
SOURCE: Papp KA. EADV Late breaker.
REPORTING FROM THE EADV CONGRESS
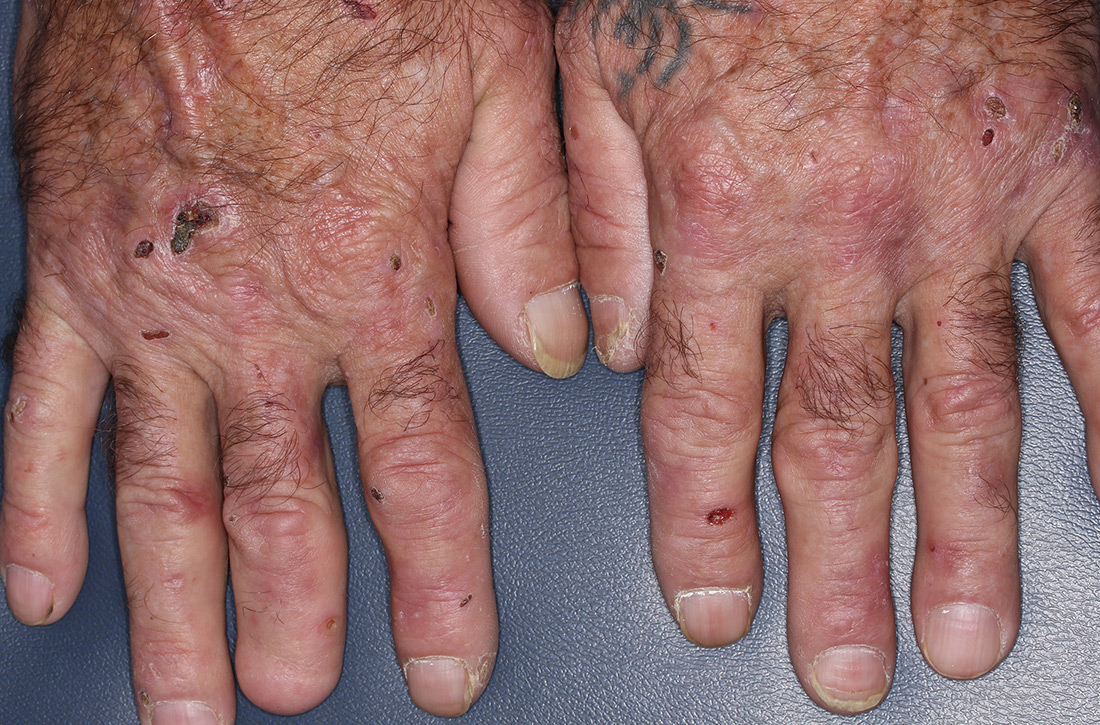


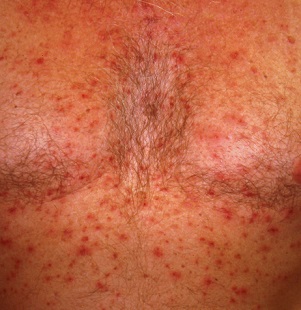
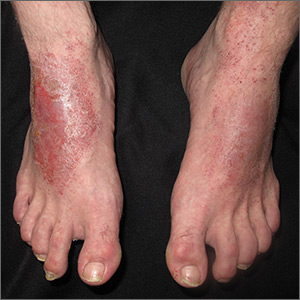
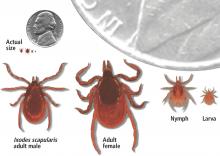



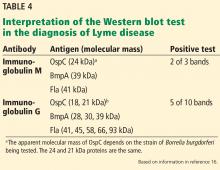
![Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto. Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto.](https://cdn.mdedge.com/files/s3fs-public/styles/medium/public/756fig2.jpg?itok=fMD7ruHJ)