User login
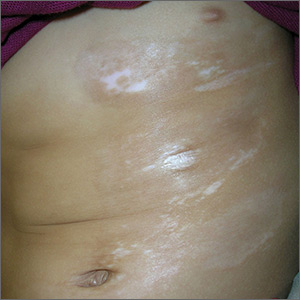
Skin changes on abdomen
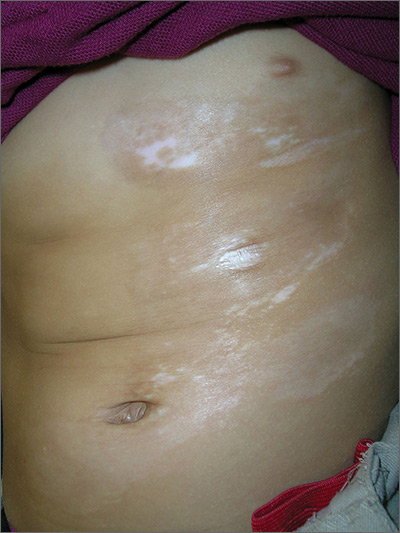
The FP suspected that the child had morphea because the skin was somewhat firm and thickened, and there was hypo- and hyperpigmentation.
Morphea is a localized type of scleroderma and may be seen in children. Fortunately, it does not involve the internal organs. There are no blood tests needed for the diagnosis and antinuclear antibodies should be normal. While a punch biopsy could be considered in less obvious cases in older children, it is probably unnecessary to put a 5-year-old child through the trauma of a biopsy.
The FP referred the child to a dermatologist to confirm the diagnosis and initiate treatment. Typical treatments include topical mid- to high-potency steroids and/or topical calcineurin inhibitors.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H. eds. Color Atlas and Synopsis of Family Medicine, 3rd Edition. New York, NY: McGraw-Hill; 2019:1204-1212.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected that the child had morphea because the skin was somewhat firm and thickened, and there was hypo- and hyperpigmentation.
Morphea is a localized type of scleroderma and may be seen in children. Fortunately, it does not involve the internal organs. There are no blood tests needed for the diagnosis and antinuclear antibodies should be normal. While a punch biopsy could be considered in less obvious cases in older children, it is probably unnecessary to put a 5-year-old child through the trauma of a biopsy.
The FP referred the child to a dermatologist to confirm the diagnosis and initiate treatment. Typical treatments include topical mid- to high-potency steroids and/or topical calcineurin inhibitors.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H. eds. Color Atlas and Synopsis of Family Medicine, 3rd Edition. New York, NY: McGraw-Hill; 2019:1204-1212.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected that the child had morphea because the skin was somewhat firm and thickened, and there was hypo- and hyperpigmentation.
Morphea is a localized type of scleroderma and may be seen in children. Fortunately, it does not involve the internal organs. There are no blood tests needed for the diagnosis and antinuclear antibodies should be normal. While a punch biopsy could be considered in less obvious cases in older children, it is probably unnecessary to put a 5-year-old child through the trauma of a biopsy.
The FP referred the child to a dermatologist to confirm the diagnosis and initiate treatment. Typical treatments include topical mid- to high-potency steroids and/or topical calcineurin inhibitors.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, Chumley H. eds. Color Atlas and Synopsis of Family Medicine, 3rd Edition. New York, NY: McGraw-Hill; 2019:1204-1212.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Boy, Is My Face Red!
A 17-year-old boy was born with rough skin on his face, arms, legs, thighs, and posterior shoulders. Over the years, his face, especially the posterior lateral aspects, has become progressively redder, while the roughness has increased. The redness is amplified with heat, exertion, anger, or embarrassment. Regarding the latter, mere mention of the condition by his siblings results in worsening of the erythema. Additionally, the skin in his eyebrows is now turning red and scaly.
The patient denies a history of dandruff. His parents, who have accompanied him to the clinic, report a family history of similar skin changes on triceps and thighs, but not on faces. There is no family history of cardiac anomalies or other congenital abnormalities. The boy’s health is otherwise excellent.
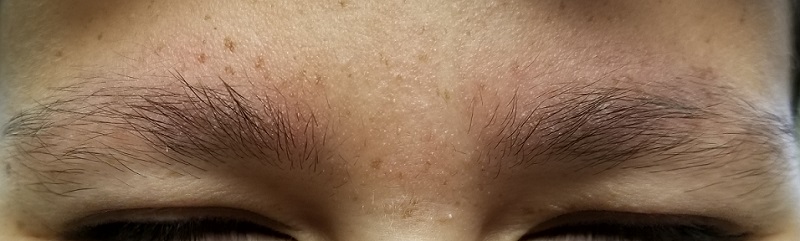
EXAMINATION
The patient’s bilateral triceps are covered with fine, rough, follicular papules, which create a faintly erythematous look. Similar lesions are visible on his posterior shoulders and anterior thighs. The skin beneath his eyebrows is faintly erythematous and scaly.
The posterior sides of his face are bright red and covered with the same type of papules. The erythema grows redder as it approaches the immediate preauricular areas, where it ends abruptly, creating a sharp demarcation with the white skin closer to the ears. The visual effect is almost clownish, as if bright red makeup had been applied.
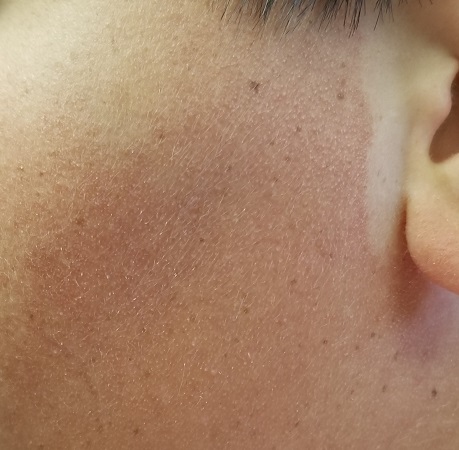
What’s the diagnosis?
DISCUSSION
This young man has all the signs of an extremely rare variant of keratosis pilaris (KP) called ulerythema ophryogenes (UO). In the United States, about 40% of adults have ordinary KP, which usually manifests in childhood (with about 80% of cases occurring in adolescent girls). KP is inherited through autosomal dominance, with highly variable penetrance, though no specific gene has been identified. In this form, KP is considered by most experts to be a minor diagnostic criterion for atopic dermatitis.
However, UO is not merely a variant of KP. Over the decades, it has been connected with more serious conditions, such as cardiofaciocutaneous syndrome, Rubinstein-Taybi syndrome, and Cornelia de Lange disease. Although these conditions are not common, they should be considered when UO is seen.
For this patient, the main concern was his appearance, especially the pronounced erythema around the periphery of his face. This aspect of the problem was addressed with a referral to a provider who can, using an assortment of lasers, try to even out his skin color and hopefully erase the sharp border at the periphery of the affected area.
For the physical discomfort caused by UO, the patient was instructed either to use general moisturizers to reduce dryness or to consider using moisturizers containing salicylic acid, which should help to reduce the prominence of the papules. For the erythema in his brows, he is using 2.5% hydrocortisone ointment two to three times a week.
TAKE-HOME LEARNING POINTS
- Ulerythema ophryogenes is a rarely encountered variant of keratosis pilaris—a condition inherited by autosomal dominance with highly variable penetrance.
- Its main significance, beyond cosmetic concerns, is the possible connection with syndromes that involve heart and structural defects (eg, cardiofaciocutaneous syndrome).
- Treatment options include heavy emollients to soften the scaly papules and laser therapy to reduce the extreme redness seen on the periphery of the face.
A 17-year-old boy was born with rough skin on his face, arms, legs, thighs, and posterior shoulders. Over the years, his face, especially the posterior lateral aspects, has become progressively redder, while the roughness has increased. The redness is amplified with heat, exertion, anger, or embarrassment. Regarding the latter, mere mention of the condition by his siblings results in worsening of the erythema. Additionally, the skin in his eyebrows is now turning red and scaly.
The patient denies a history of dandruff. His parents, who have accompanied him to the clinic, report a family history of similar skin changes on triceps and thighs, but not on faces. There is no family history of cardiac anomalies or other congenital abnormalities. The boy’s health is otherwise excellent.

EXAMINATION
The patient’s bilateral triceps are covered with fine, rough, follicular papules, which create a faintly erythematous look. Similar lesions are visible on his posterior shoulders and anterior thighs. The skin beneath his eyebrows is faintly erythematous and scaly.
The posterior sides of his face are bright red and covered with the same type of papules. The erythema grows redder as it approaches the immediate preauricular areas, where it ends abruptly, creating a sharp demarcation with the white skin closer to the ears. The visual effect is almost clownish, as if bright red makeup had been applied.

What’s the diagnosis?
DISCUSSION
This young man has all the signs of an extremely rare variant of keratosis pilaris (KP) called ulerythema ophryogenes (UO). In the United States, about 40% of adults have ordinary KP, which usually manifests in childhood (with about 80% of cases occurring in adolescent girls). KP is inherited through autosomal dominance, with highly variable penetrance, though no specific gene has been identified. In this form, KP is considered by most experts to be a minor diagnostic criterion for atopic dermatitis.
However, UO is not merely a variant of KP. Over the decades, it has been connected with more serious conditions, such as cardiofaciocutaneous syndrome, Rubinstein-Taybi syndrome, and Cornelia de Lange disease. Although these conditions are not common, they should be considered when UO is seen.
For this patient, the main concern was his appearance, especially the pronounced erythema around the periphery of his face. This aspect of the problem was addressed with a referral to a provider who can, using an assortment of lasers, try to even out his skin color and hopefully erase the sharp border at the periphery of the affected area.
For the physical discomfort caused by UO, the patient was instructed either to use general moisturizers to reduce dryness or to consider using moisturizers containing salicylic acid, which should help to reduce the prominence of the papules. For the erythema in his brows, he is using 2.5% hydrocortisone ointment two to three times a week.
TAKE-HOME LEARNING POINTS
- Ulerythema ophryogenes is a rarely encountered variant of keratosis pilaris—a condition inherited by autosomal dominance with highly variable penetrance.
- Its main significance, beyond cosmetic concerns, is the possible connection with syndromes that involve heart and structural defects (eg, cardiofaciocutaneous syndrome).
- Treatment options include heavy emollients to soften the scaly papules and laser therapy to reduce the extreme redness seen on the periphery of the face.
A 17-year-old boy was born with rough skin on his face, arms, legs, thighs, and posterior shoulders. Over the years, his face, especially the posterior lateral aspects, has become progressively redder, while the roughness has increased. The redness is amplified with heat, exertion, anger, or embarrassment. Regarding the latter, mere mention of the condition by his siblings results in worsening of the erythema. Additionally, the skin in his eyebrows is now turning red and scaly.
The patient denies a history of dandruff. His parents, who have accompanied him to the clinic, report a family history of similar skin changes on triceps and thighs, but not on faces. There is no family history of cardiac anomalies or other congenital abnormalities. The boy’s health is otherwise excellent.

EXAMINATION
The patient’s bilateral triceps are covered with fine, rough, follicular papules, which create a faintly erythematous look. Similar lesions are visible on his posterior shoulders and anterior thighs. The skin beneath his eyebrows is faintly erythematous and scaly.
The posterior sides of his face are bright red and covered with the same type of papules. The erythema grows redder as it approaches the immediate preauricular areas, where it ends abruptly, creating a sharp demarcation with the white skin closer to the ears. The visual effect is almost clownish, as if bright red makeup had been applied.

What’s the diagnosis?
DISCUSSION
This young man has all the signs of an extremely rare variant of keratosis pilaris (KP) called ulerythema ophryogenes (UO). In the United States, about 40% of adults have ordinary KP, which usually manifests in childhood (with about 80% of cases occurring in adolescent girls). KP is inherited through autosomal dominance, with highly variable penetrance, though no specific gene has been identified. In this form, KP is considered by most experts to be a minor diagnostic criterion for atopic dermatitis.
However, UO is not merely a variant of KP. Over the decades, it has been connected with more serious conditions, such as cardiofaciocutaneous syndrome, Rubinstein-Taybi syndrome, and Cornelia de Lange disease. Although these conditions are not common, they should be considered when UO is seen.
For this patient, the main concern was his appearance, especially the pronounced erythema around the periphery of his face. This aspect of the problem was addressed with a referral to a provider who can, using an assortment of lasers, try to even out his skin color and hopefully erase the sharp border at the periphery of the affected area.
For the physical discomfort caused by UO, the patient was instructed either to use general moisturizers to reduce dryness or to consider using moisturizers containing salicylic acid, which should help to reduce the prominence of the papules. For the erythema in his brows, he is using 2.5% hydrocortisone ointment two to three times a week.
TAKE-HOME LEARNING POINTS
- Ulerythema ophryogenes is a rarely encountered variant of keratosis pilaris—a condition inherited by autosomal dominance with highly variable penetrance.
- Its main significance, beyond cosmetic concerns, is the possible connection with syndromes that involve heart and structural defects (eg, cardiofaciocutaneous syndrome).
- Treatment options include heavy emollients to soften the scaly papules and laser therapy to reduce the extreme redness seen on the periphery of the face.
No tacrolimus/cancer link in atopic dermatitis in 10-year study
MADRID – participating in the large, prospective, observational APPLES study, Regina Folster-Holst, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
With nearly 45,000 person-years of follow-up in APPLES (A Prospective Pediatric Longitudinal Evaluation Study), there were no lymphomas and just a single case of skin cancer. That’s highly reassuring, since those were the two types of malignancies singled out as being of particular concern in the boxed warnings for the topical calcineurin inhibitors tacrolimus and pimecrolimus mandated by U.S. and European regulatory agencies in 2005, noted Dr. Folster-Holst, professor of dermatology at Christian Albrechts University of Kiel (Germany).
APPLES included 7,954 children with moderate or severe AD who were a median of 6 years old at enrollment in the study, conducted at 314 sites in the United States, Canada, and seven European countries. This was a naturalistic study in which patients used the topical calcineurin inhibitor as needed, with no restrictions.
A total of six cancers were diagnosed in six individuals during 44,629 person-years of prospective follow-up: one case each of chronic myeloid leukemia, alveolar rhabdomyosarcoma, malignant paraganglioma, carcinoid tumor of the appendix, spinal cord neoplasm, and Spitzoid melanoma. None of those malignancies are classically associated with immunosuppressive therapy.
The primary outcome in APPLES was the standardized incidence ratio of observed cancers to the expected number based upon extrapolation from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, as well as national cancer registries in the other countries where the study was carried out. The expected number of cancers was 5.95, yielding a standardized incidence ratio of 1.01.
Only 27% of patients completed the study. Investigators had anticipated a substantial attrition rate and recalculated their statistics based upon a range of hypothetically increased cancer rates among the dropouts. Even if the cancer rate was 2.5-fold higher in dropouts than in those who remained in the study – a far-fetched possibility – the standardized incidence ratio would not be significantly affected, according to Dr. Folster-Holst.
The new APPLES findings were preceded by a favorable report on long-term use of topical pimecrolimus from the Pediatric Eczema Elective Registry (PEER). The study included 7,457 pimecrolimus-using children with AD followed for 26,792 person-years. The standardized incidence ratio for all cancers was not significantly increased at 1.2. The investigators concluded “it seems unlikely” that topical pimecrolimus as generally used for treatment of AD is associated with an increased risk of malignancy (JAMA Dermatol. 2015 Jun;151[6]:594-9).
The boxed warnings for the topical calcineurin inhibitors have been the source of enormous frustration for dermatologists. The warnings were ordered because of regulatory concern about an increased risk of malignancy in organ transplant recipients on systemic calcineurin inhibitors for immunosuppression, even though the topical agents – unlike the systemic versions – are used intermittently, their systemic absorption is low to nil, and no plausible mechanism by which they could cause cancer has been put forth. Many physicians believe these drugs are probably safer than topical corticosteroids, so the first question put to Dr. Folster-Holst from the audience was, When will the boxed warnings be removed?
“That’s a good question,” she replied. “Patients and parents are afraid. But I think we have now a good argument to move forward with topically applied calcineurin inhibitors.”
Dr. Folster-Holst reported having no financial conflicts of interest regarding the APPLES study, funded by LEO Pharma.
MADRID – participating in the large, prospective, observational APPLES study, Regina Folster-Holst, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
With nearly 45,000 person-years of follow-up in APPLES (A Prospective Pediatric Longitudinal Evaluation Study), there were no lymphomas and just a single case of skin cancer. That’s highly reassuring, since those were the two types of malignancies singled out as being of particular concern in the boxed warnings for the topical calcineurin inhibitors tacrolimus and pimecrolimus mandated by U.S. and European regulatory agencies in 2005, noted Dr. Folster-Holst, professor of dermatology at Christian Albrechts University of Kiel (Germany).
APPLES included 7,954 children with moderate or severe AD who were a median of 6 years old at enrollment in the study, conducted at 314 sites in the United States, Canada, and seven European countries. This was a naturalistic study in which patients used the topical calcineurin inhibitor as needed, with no restrictions.
A total of six cancers were diagnosed in six individuals during 44,629 person-years of prospective follow-up: one case each of chronic myeloid leukemia, alveolar rhabdomyosarcoma, malignant paraganglioma, carcinoid tumor of the appendix, spinal cord neoplasm, and Spitzoid melanoma. None of those malignancies are classically associated with immunosuppressive therapy.
The primary outcome in APPLES was the standardized incidence ratio of observed cancers to the expected number based upon extrapolation from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, as well as national cancer registries in the other countries where the study was carried out. The expected number of cancers was 5.95, yielding a standardized incidence ratio of 1.01.
Only 27% of patients completed the study. Investigators had anticipated a substantial attrition rate and recalculated their statistics based upon a range of hypothetically increased cancer rates among the dropouts. Even if the cancer rate was 2.5-fold higher in dropouts than in those who remained in the study – a far-fetched possibility – the standardized incidence ratio would not be significantly affected, according to Dr. Folster-Holst.
The new APPLES findings were preceded by a favorable report on long-term use of topical pimecrolimus from the Pediatric Eczema Elective Registry (PEER). The study included 7,457 pimecrolimus-using children with AD followed for 26,792 person-years. The standardized incidence ratio for all cancers was not significantly increased at 1.2. The investigators concluded “it seems unlikely” that topical pimecrolimus as generally used for treatment of AD is associated with an increased risk of malignancy (JAMA Dermatol. 2015 Jun;151[6]:594-9).
The boxed warnings for the topical calcineurin inhibitors have been the source of enormous frustration for dermatologists. The warnings were ordered because of regulatory concern about an increased risk of malignancy in organ transplant recipients on systemic calcineurin inhibitors for immunosuppression, even though the topical agents – unlike the systemic versions – are used intermittently, their systemic absorption is low to nil, and no plausible mechanism by which they could cause cancer has been put forth. Many physicians believe these drugs are probably safer than topical corticosteroids, so the first question put to Dr. Folster-Holst from the audience was, When will the boxed warnings be removed?
“That’s a good question,” she replied. “Patients and parents are afraid. But I think we have now a good argument to move forward with topically applied calcineurin inhibitors.”
Dr. Folster-Holst reported having no financial conflicts of interest regarding the APPLES study, funded by LEO Pharma.
MADRID – participating in the large, prospective, observational APPLES study, Regina Folster-Holst, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
With nearly 45,000 person-years of follow-up in APPLES (A Prospective Pediatric Longitudinal Evaluation Study), there were no lymphomas and just a single case of skin cancer. That’s highly reassuring, since those were the two types of malignancies singled out as being of particular concern in the boxed warnings for the topical calcineurin inhibitors tacrolimus and pimecrolimus mandated by U.S. and European regulatory agencies in 2005, noted Dr. Folster-Holst, professor of dermatology at Christian Albrechts University of Kiel (Germany).
APPLES included 7,954 children with moderate or severe AD who were a median of 6 years old at enrollment in the study, conducted at 314 sites in the United States, Canada, and seven European countries. This was a naturalistic study in which patients used the topical calcineurin inhibitor as needed, with no restrictions.
A total of six cancers were diagnosed in six individuals during 44,629 person-years of prospective follow-up: one case each of chronic myeloid leukemia, alveolar rhabdomyosarcoma, malignant paraganglioma, carcinoid tumor of the appendix, spinal cord neoplasm, and Spitzoid melanoma. None of those malignancies are classically associated with immunosuppressive therapy.
The primary outcome in APPLES was the standardized incidence ratio of observed cancers to the expected number based upon extrapolation from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, as well as national cancer registries in the other countries where the study was carried out. The expected number of cancers was 5.95, yielding a standardized incidence ratio of 1.01.
Only 27% of patients completed the study. Investigators had anticipated a substantial attrition rate and recalculated their statistics based upon a range of hypothetically increased cancer rates among the dropouts. Even if the cancer rate was 2.5-fold higher in dropouts than in those who remained in the study – a far-fetched possibility – the standardized incidence ratio would not be significantly affected, according to Dr. Folster-Holst.
The new APPLES findings were preceded by a favorable report on long-term use of topical pimecrolimus from the Pediatric Eczema Elective Registry (PEER). The study included 7,457 pimecrolimus-using children with AD followed for 26,792 person-years. The standardized incidence ratio for all cancers was not significantly increased at 1.2. The investigators concluded “it seems unlikely” that topical pimecrolimus as generally used for treatment of AD is associated with an increased risk of malignancy (JAMA Dermatol. 2015 Jun;151[6]:594-9).
The boxed warnings for the topical calcineurin inhibitors have been the source of enormous frustration for dermatologists. The warnings were ordered because of regulatory concern about an increased risk of malignancy in organ transplant recipients on systemic calcineurin inhibitors for immunosuppression, even though the topical agents – unlike the systemic versions – are used intermittently, their systemic absorption is low to nil, and no plausible mechanism by which they could cause cancer has been put forth. Many physicians believe these drugs are probably safer than topical corticosteroids, so the first question put to Dr. Folster-Holst from the audience was, When will the boxed warnings be removed?
“That’s a good question,” she replied. “Patients and parents are afraid. But I think we have now a good argument to move forward with topically applied calcineurin inhibitors.”
Dr. Folster-Holst reported having no financial conflicts of interest regarding the APPLES study, funded by LEO Pharma.
REPORTING FROM THE EADV CONGRESS
Atopic Dermatitis
FDA approves minocycline foam for moderate, severe acne
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
Acute Palmar and Plantar Rash in a 52-Year-Old Woman
A 52-year-old woman presented to a primary care clinic with a 3-week history of rash on her feet that had spread to her hands in the previous week. She described the rash as painful, burning, and itching with no drainage. She denied any recent illness, fever, chills, medication changes, or environmental exposures. Home treatments included Epsom salt baths and lotion with no improvement.
Past medical history included hypertension. She was a smoker with a 30-pack-year history and drank alcohol on a daily basis. Her medications included losartan and atorvastatin.
On examination, multiple papular and scabbed lesions were present with mild scaling. Additional review of systems and physical exam were benign. A KOH prep showed hyphae. The patient was diagnosed with tinea pedis and prescribed fluconazole (150-mg tablet once per week for 2 weeks).
Two weeks later, after completing the antifungal therapy, the patient returned with pain limiting her ability to bear weight or grasp objects. Clinical examination showed well-demarcated erythematous scaly and hyperkeratotic plaques with scattered papular and pustular lesions on bilateral palmar and medial aspects of plantar surfaces (see Figures 1 and 2). A repeat KOH was not completed. The patient was diagnosed with palmoplantar pustulosis (PPP).
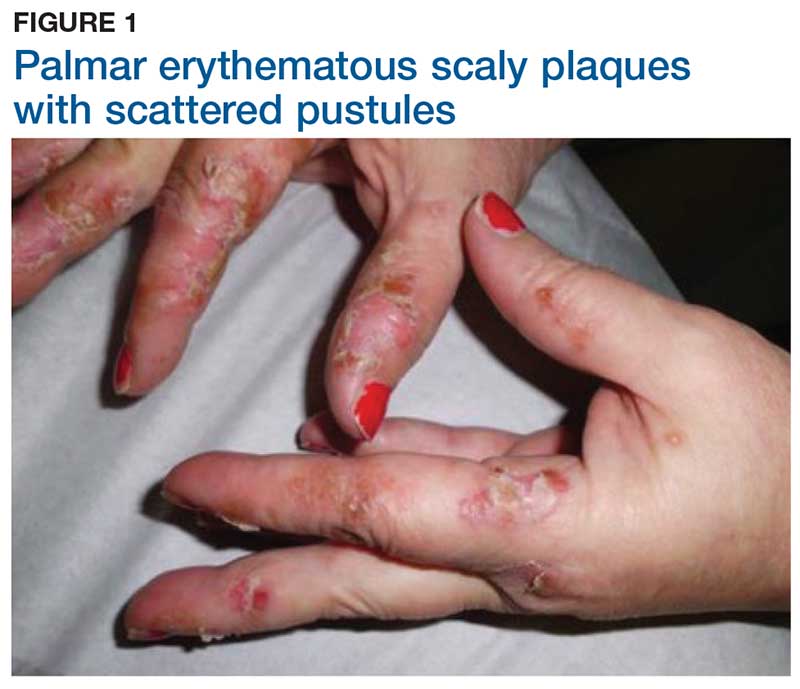
DISCUSSION
PPP is a chronic, relapsing, inflammatory skin condition that results in painful lesions on the palms and the soles.1,2 There is debate as to whether PPP is a variant of psoriasis or a separate condition; depending on physical manifestations, one can be diagnosed with palmoplantar plaque psoriasis, PPP, or a combination of the two.3,4
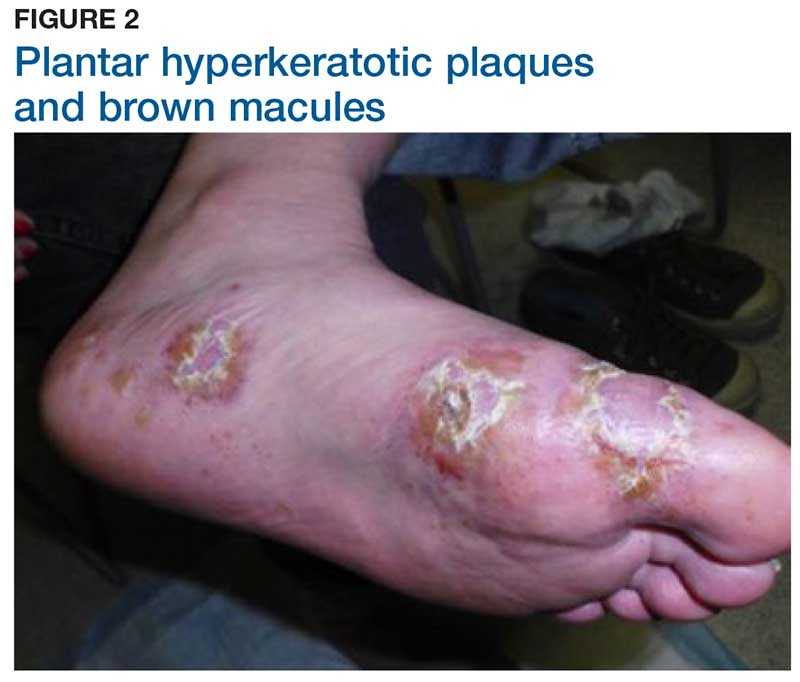
The exact cause of PPP is unknown; however, increased levels of inflammatory cytokines interleukin (IL)-17 and IL-22 may be involved in the pathogenesis of the disease.5 Additional genetic and environmental factors, most significantly smoking, play an important role in its development.2,6
Clinical presentation
Inflammation associated with PPP typically manifests in the classic features of pustules that coalesce and resolve over several days, resulting in brown macules, hyperkeratosis, fissures, and debilitating pain.4,7 Some patients may have co-occurring onycholysis resulting from nail dystrophy and destruction or plaque psoriasis elsewhere on their body.8 PPP often persists for years with periods of exacerbation and remission, and it significantly affects the patient’s ability to perform activities of daily living without pain.8,9 It is exceedingly rare and most commonly affects middle-aged women with a smoking history or current smoking status.7
Continue to: Laboratory diagnosis
Laboratory diagnosis
The diagnosis of PPP is based on clinical presentation and physical exam. Laboratory testing, such as KOH prep, may assist in ruling out dermatophyte infection; a complete blood count may assist in eliminating a bacterial infection as the cause. Skin biopsy is not necessary unless diagnosis is uncertain or prolonged treatment has not produced a response.
Differential diagnosis
The differential diagnosis of PPP includes skin conditions that involve the palms and/or the soles and may have fungal, allergic, or bacterial origins.
Fungal. Tinea manuum (palms) and tinea pedis (soles) result from dermatophyte infection and manifest with erythema and/or scaling and pruritis. A positive KOH examination can confirm diagnosis. On examination, fungal infections are commonly unilateral and asymmetric.8 Treatment with an antifungal agent should result in resolution of symptoms.
Allergic. Contact with an allergen can result in skin erythema, pruritis, and pain at the exposed area. Contact dermatitis can result from an inflammatory response to an allergen or irritant, and it is often localized and well demarcated. This is an acute condition that resolves over time with antihistamines and avoidance of irritants.
Dyshidrotic eczema results in small, pruritic blisters on the palms and the soles and can be recurrent and related to seasonal allergen exposure. Diagnosis is made from history and physical exam. Treatment often consists of emollients and occasionally topical steroids, depending on the severity.
Continue to: Bacterial
Bacterial. A primary bacterial cause of bilateral skin lesions on the palms and the soles is uncommon. However, any open skin lesion can result in secondary bacterial infection. The pustules of PPP are often sterile and do not require bacterial culture; however, additional symptoms of fever, purulence, warmth, and worsening of symptoms may prompt further evaluation for a bacterial origin or complication.
Management
Due to limited quality data on treatment recommendations, the treatment options for PPP vary greatly. Most studies recommend topical versus systemic therapy for initial management.1-2,8,10-11 Firstline therapy often consists of topical corticosteroids and occlusive dressings, followed by oral retinoids (acitretin, alitretinoin) or photochemotherapy.1,8 Third-line therapy can include immunosuppressants (ciclosporin, methotrexate) or biologics (secukinumab).1,12 Recent data have shown positive results with vitamin D3 analogs (maxacalcitol, betamethasone butyrate propionate) as monotherapy or in combination with corticosteroids.10-11 Duration of therapy ranges from 4 to 8 weeks throughout the literature, depending on severity; however, many patients see improvement in the first few weeks.
Conservative measures to maintain remission include smoking cessation, skin emollients, and avoidance of irritants. It is important to educate patients about the chronicity of the disease and early treatment to prevent secondary infection or significant impact on quality of life.
OUTCOME FOR THE CASE PATIENT
The patient was prescribed triamcinolone acetonide (0.5% ointment applied bid), to be used until symptoms improved. After 1 week of treatment, she confirmed (verbally) that symptoms had resolved. She declined a follow-up visit or referral to dermatology.
CONCLUSION
Although PPP is fairly uncommon, it is important for clinicians to consider this diagnosis in patients presenting with localized rash on their palms and soles. This debilitating condition greatly affects a patient’s quality of life and, although it is chronic in nature, available treatments described in the literature have shown success in both acute resolution and ongoing remission of the disease.
1. Sevrain M, Richard M-A, Barnetche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(suppl 5):13-16.
2. Olazagasti JM, Ma JE, Wetter DA. Clinical features, etiological factors, associated disorders, and treatment of palmoplantar pustulosis: the Mayo Clinic experience, 1996-2013. Mayo Clin Proc. 2017;92(9):1351-1358.
3. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11(5):1-11.
4. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349-358.
5. Bissonnette R, Fuentes-Duculan J, Mashiko S, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
6. Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38-44.
7. Brunasso AMG, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243-1251.
8. Engin B, As¸kın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017; 35(1):19-27.
9. Chung J, Callas Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared to moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623-632.
10. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2016;43(3):288-293.
11. Muro M, Kawakami H, Matsumoto Y, et al. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
12. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70-80.
A 52-year-old woman presented to a primary care clinic with a 3-week history of rash on her feet that had spread to her hands in the previous week. She described the rash as painful, burning, and itching with no drainage. She denied any recent illness, fever, chills, medication changes, or environmental exposures. Home treatments included Epsom salt baths and lotion with no improvement.
Past medical history included hypertension. She was a smoker with a 30-pack-year history and drank alcohol on a daily basis. Her medications included losartan and atorvastatin.
On examination, multiple papular and scabbed lesions were present with mild scaling. Additional review of systems and physical exam were benign. A KOH prep showed hyphae. The patient was diagnosed with tinea pedis and prescribed fluconazole (150-mg tablet once per week for 2 weeks).
Two weeks later, after completing the antifungal therapy, the patient returned with pain limiting her ability to bear weight or grasp objects. Clinical examination showed well-demarcated erythematous scaly and hyperkeratotic plaques with scattered papular and pustular lesions on bilateral palmar and medial aspects of plantar surfaces (see Figures 1 and 2). A repeat KOH was not completed. The patient was diagnosed with palmoplantar pustulosis (PPP).

DISCUSSION
PPP is a chronic, relapsing, inflammatory skin condition that results in painful lesions on the palms and the soles.1,2 There is debate as to whether PPP is a variant of psoriasis or a separate condition; depending on physical manifestations, one can be diagnosed with palmoplantar plaque psoriasis, PPP, or a combination of the two.3,4

The exact cause of PPP is unknown; however, increased levels of inflammatory cytokines interleukin (IL)-17 and IL-22 may be involved in the pathogenesis of the disease.5 Additional genetic and environmental factors, most significantly smoking, play an important role in its development.2,6
Clinical presentation
Inflammation associated with PPP typically manifests in the classic features of pustules that coalesce and resolve over several days, resulting in brown macules, hyperkeratosis, fissures, and debilitating pain.4,7 Some patients may have co-occurring onycholysis resulting from nail dystrophy and destruction or plaque psoriasis elsewhere on their body.8 PPP often persists for years with periods of exacerbation and remission, and it significantly affects the patient’s ability to perform activities of daily living without pain.8,9 It is exceedingly rare and most commonly affects middle-aged women with a smoking history or current smoking status.7
Continue to: Laboratory diagnosis
Laboratory diagnosis
The diagnosis of PPP is based on clinical presentation and physical exam. Laboratory testing, such as KOH prep, may assist in ruling out dermatophyte infection; a complete blood count may assist in eliminating a bacterial infection as the cause. Skin biopsy is not necessary unless diagnosis is uncertain or prolonged treatment has not produced a response.
Differential diagnosis
The differential diagnosis of PPP includes skin conditions that involve the palms and/or the soles and may have fungal, allergic, or bacterial origins.
Fungal. Tinea manuum (palms) and tinea pedis (soles) result from dermatophyte infection and manifest with erythema and/or scaling and pruritis. A positive KOH examination can confirm diagnosis. On examination, fungal infections are commonly unilateral and asymmetric.8 Treatment with an antifungal agent should result in resolution of symptoms.
Allergic. Contact with an allergen can result in skin erythema, pruritis, and pain at the exposed area. Contact dermatitis can result from an inflammatory response to an allergen or irritant, and it is often localized and well demarcated. This is an acute condition that resolves over time with antihistamines and avoidance of irritants.
Dyshidrotic eczema results in small, pruritic blisters on the palms and the soles and can be recurrent and related to seasonal allergen exposure. Diagnosis is made from history and physical exam. Treatment often consists of emollients and occasionally topical steroids, depending on the severity.
Continue to: Bacterial
Bacterial. A primary bacterial cause of bilateral skin lesions on the palms and the soles is uncommon. However, any open skin lesion can result in secondary bacterial infection. The pustules of PPP are often sterile and do not require bacterial culture; however, additional symptoms of fever, purulence, warmth, and worsening of symptoms may prompt further evaluation for a bacterial origin or complication.
Management
Due to limited quality data on treatment recommendations, the treatment options for PPP vary greatly. Most studies recommend topical versus systemic therapy for initial management.1-2,8,10-11 Firstline therapy often consists of topical corticosteroids and occlusive dressings, followed by oral retinoids (acitretin, alitretinoin) or photochemotherapy.1,8 Third-line therapy can include immunosuppressants (ciclosporin, methotrexate) or biologics (secukinumab).1,12 Recent data have shown positive results with vitamin D3 analogs (maxacalcitol, betamethasone butyrate propionate) as monotherapy or in combination with corticosteroids.10-11 Duration of therapy ranges from 4 to 8 weeks throughout the literature, depending on severity; however, many patients see improvement in the first few weeks.
Conservative measures to maintain remission include smoking cessation, skin emollients, and avoidance of irritants. It is important to educate patients about the chronicity of the disease and early treatment to prevent secondary infection or significant impact on quality of life.
OUTCOME FOR THE CASE PATIENT
The patient was prescribed triamcinolone acetonide (0.5% ointment applied bid), to be used until symptoms improved. After 1 week of treatment, she confirmed (verbally) that symptoms had resolved. She declined a follow-up visit or referral to dermatology.
CONCLUSION
Although PPP is fairly uncommon, it is important for clinicians to consider this diagnosis in patients presenting with localized rash on their palms and soles. This debilitating condition greatly affects a patient’s quality of life and, although it is chronic in nature, available treatments described in the literature have shown success in both acute resolution and ongoing remission of the disease.
A 52-year-old woman presented to a primary care clinic with a 3-week history of rash on her feet that had spread to her hands in the previous week. She described the rash as painful, burning, and itching with no drainage. She denied any recent illness, fever, chills, medication changes, or environmental exposures. Home treatments included Epsom salt baths and lotion with no improvement.
Past medical history included hypertension. She was a smoker with a 30-pack-year history and drank alcohol on a daily basis. Her medications included losartan and atorvastatin.
On examination, multiple papular and scabbed lesions were present with mild scaling. Additional review of systems and physical exam were benign. A KOH prep showed hyphae. The patient was diagnosed with tinea pedis and prescribed fluconazole (150-mg tablet once per week for 2 weeks).
Two weeks later, after completing the antifungal therapy, the patient returned with pain limiting her ability to bear weight or grasp objects. Clinical examination showed well-demarcated erythematous scaly and hyperkeratotic plaques with scattered papular and pustular lesions on bilateral palmar and medial aspects of plantar surfaces (see Figures 1 and 2). A repeat KOH was not completed. The patient was diagnosed with palmoplantar pustulosis (PPP).

DISCUSSION
PPP is a chronic, relapsing, inflammatory skin condition that results in painful lesions on the palms and the soles.1,2 There is debate as to whether PPP is a variant of psoriasis or a separate condition; depending on physical manifestations, one can be diagnosed with palmoplantar plaque psoriasis, PPP, or a combination of the two.3,4

The exact cause of PPP is unknown; however, increased levels of inflammatory cytokines interleukin (IL)-17 and IL-22 may be involved in the pathogenesis of the disease.5 Additional genetic and environmental factors, most significantly smoking, play an important role in its development.2,6
Clinical presentation
Inflammation associated with PPP typically manifests in the classic features of pustules that coalesce and resolve over several days, resulting in brown macules, hyperkeratosis, fissures, and debilitating pain.4,7 Some patients may have co-occurring onycholysis resulting from nail dystrophy and destruction or plaque psoriasis elsewhere on their body.8 PPP often persists for years with periods of exacerbation and remission, and it significantly affects the patient’s ability to perform activities of daily living without pain.8,9 It is exceedingly rare and most commonly affects middle-aged women with a smoking history or current smoking status.7
Continue to: Laboratory diagnosis
Laboratory diagnosis
The diagnosis of PPP is based on clinical presentation and physical exam. Laboratory testing, such as KOH prep, may assist in ruling out dermatophyte infection; a complete blood count may assist in eliminating a bacterial infection as the cause. Skin biopsy is not necessary unless diagnosis is uncertain or prolonged treatment has not produced a response.
Differential diagnosis
The differential diagnosis of PPP includes skin conditions that involve the palms and/or the soles and may have fungal, allergic, or bacterial origins.
Fungal. Tinea manuum (palms) and tinea pedis (soles) result from dermatophyte infection and manifest with erythema and/or scaling and pruritis. A positive KOH examination can confirm diagnosis. On examination, fungal infections are commonly unilateral and asymmetric.8 Treatment with an antifungal agent should result in resolution of symptoms.
Allergic. Contact with an allergen can result in skin erythema, pruritis, and pain at the exposed area. Contact dermatitis can result from an inflammatory response to an allergen or irritant, and it is often localized and well demarcated. This is an acute condition that resolves over time with antihistamines and avoidance of irritants.
Dyshidrotic eczema results in small, pruritic blisters on the palms and the soles and can be recurrent and related to seasonal allergen exposure. Diagnosis is made from history and physical exam. Treatment often consists of emollients and occasionally topical steroids, depending on the severity.
Continue to: Bacterial
Bacterial. A primary bacterial cause of bilateral skin lesions on the palms and the soles is uncommon. However, any open skin lesion can result in secondary bacterial infection. The pustules of PPP are often sterile and do not require bacterial culture; however, additional symptoms of fever, purulence, warmth, and worsening of symptoms may prompt further evaluation for a bacterial origin or complication.
Management
Due to limited quality data on treatment recommendations, the treatment options for PPP vary greatly. Most studies recommend topical versus systemic therapy for initial management.1-2,8,10-11 Firstline therapy often consists of topical corticosteroids and occlusive dressings, followed by oral retinoids (acitretin, alitretinoin) or photochemotherapy.1,8 Third-line therapy can include immunosuppressants (ciclosporin, methotrexate) or biologics (secukinumab).1,12 Recent data have shown positive results with vitamin D3 analogs (maxacalcitol, betamethasone butyrate propionate) as monotherapy or in combination with corticosteroids.10-11 Duration of therapy ranges from 4 to 8 weeks throughout the literature, depending on severity; however, many patients see improvement in the first few weeks.
Conservative measures to maintain remission include smoking cessation, skin emollients, and avoidance of irritants. It is important to educate patients about the chronicity of the disease and early treatment to prevent secondary infection or significant impact on quality of life.
OUTCOME FOR THE CASE PATIENT
The patient was prescribed triamcinolone acetonide (0.5% ointment applied bid), to be used until symptoms improved. After 1 week of treatment, she confirmed (verbally) that symptoms had resolved. She declined a follow-up visit or referral to dermatology.
CONCLUSION
Although PPP is fairly uncommon, it is important for clinicians to consider this diagnosis in patients presenting with localized rash on their palms and soles. This debilitating condition greatly affects a patient’s quality of life and, although it is chronic in nature, available treatments described in the literature have shown success in both acute resolution and ongoing remission of the disease.
1. Sevrain M, Richard M-A, Barnetche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(suppl 5):13-16.
2. Olazagasti JM, Ma JE, Wetter DA. Clinical features, etiological factors, associated disorders, and treatment of palmoplantar pustulosis: the Mayo Clinic experience, 1996-2013. Mayo Clin Proc. 2017;92(9):1351-1358.
3. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11(5):1-11.
4. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349-358.
5. Bissonnette R, Fuentes-Duculan J, Mashiko S, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
6. Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38-44.
7. Brunasso AMG, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243-1251.
8. Engin B, As¸kın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017; 35(1):19-27.
9. Chung J, Callas Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared to moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623-632.
10. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2016;43(3):288-293.
11. Muro M, Kawakami H, Matsumoto Y, et al. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
12. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70-80.
1. Sevrain M, Richard M-A, Barnetche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(suppl 5):13-16.
2. Olazagasti JM, Ma JE, Wetter DA. Clinical features, etiological factors, associated disorders, and treatment of palmoplantar pustulosis: the Mayo Clinic experience, 1996-2013. Mayo Clin Proc. 2017;92(9):1351-1358.
3. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11(5):1-11.
4. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349-358.
5. Bissonnette R, Fuentes-Duculan J, Mashiko S, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
6. Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38-44.
7. Brunasso AMG, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243-1251.
8. Engin B, As¸kın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017; 35(1):19-27.
9. Chung J, Callas Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared to moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623-632.
10. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2016;43(3):288-293.
11. Muro M, Kawakami H, Matsumoto Y, et al. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
12. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70-80.
New mechanisms, therapies for acne considered
SEATTLE – It used to be thought that acne begins with microcomedones, which go on to develop either inflammatory lesions or noninflammatory lesions, but more recent evidence has changed that perception, according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital, Detroit.
Dr. Stein Gold said at the annual Coastal Dermatology Symposium. “All acne is inflammation acne,” and inflammation also persists, she added. Biopsies of scarred lesions, once considered postinflammatory, also have revealed persistent inflammation, she noted.
One study found that persistent scars can evolve from closed comedones, papules, and pustules, but the most common was a papule that turned into a postinflammatory lesion (J Drugs Dermatol 2017 Jun 1;16[6]:566-72). “So when patients come in and they have these red spots on their face, it’s not over. There’s still time to be aggressive because those inflammatory lesions are more likely to lead to scars than anything else,” Dr. Stein Gold said. “And we also know that papules that develop into scars do so because they’re there for a longer period of time. Those that develop scars are present about 10.5 days, compared with 6.6 days for those that don’t develop into scars.”
She went on to review some of the new treatments for acne that can be brought to bear in such cases. These include developments with topical retinoids that are aimed at improving delivery and reducing skin irritation.
A new topical retinoid, trifarotene cream, 0.005%, showed efficacy and tolerability for both the face and trunk in a recent phase 3 trial of patients with moderate facial and truncal acne and was recently approved for patients aged 9 years and older. In the study, about 30%-40% of people aged 9 years and older treated with once-daily trifarotene cream (Aklief) achieved clear or almost-clear status of the face at 12 weeks, vs. about 20% and 26%, of those on the vehicle cream (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9).
The drug can also treat papules and pustules, nearly as well as it treats blackheads and whiteheads, according to Dr. Stein Gold. Like other retinoids, it produces some redness and scaling and rather than letting these adverse events discourage patients, she leans in. “I tell patients they’re going to have some sloughing of the skin the first 2 weeks. I tell them that people pay money for that. It’s called a chemical peel,” said Dr. Stein Gold, noting that patients respond well to this information.
If patients find the treatments too irritating, she advises them to avoid applying it to wet skin. They can also apply it every other night, or even less frequently, and then work up to more frequent use, she said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
Tazarotene is another topical retinoid that can be very irritating. A new lotion formulation of tazarotene 0.045% contains a lower dose than the 0.1% typically used in creams, and has similar efficacy but reduced irritation, Dr. Stein Gold said. In August, the manufacturer submitted an application for approval with the Food and Drug Administration for treatment of acne.
Dr. Stein Gold also talked about using retinoids to minimize scarring, referring to a study of patients with moderate and severe facial acne, and atrophic acne scars, comparing adapalene 0.3% plus benzoyl peroxide 2.5% gel on one side of the face and vehicle on the other side of the face for 24 weeks, followed by application of the active treatment to both sides of the face for 24 weeks. Treatment was associated with a reduction of atrophic acne scars at 24 weeks, which was maintained for up to 48 weeks (Am J Clin Dermatol. 2019 Oct[5];20:725-32).
“We can now say to patients, ‘Not only can I help you with your acne, but I can potentially even improve your atrophic scarring,’ ” she said.
Finally, she discussed clascoterone, a novel androgen receptor antagonist, which inhibits sebum production and prevents colonization by Cutibacterium acnes (formerly called Propionibacterium acnes) and subsequent inflammation. “It does a lot of good things in terms of the pathogenesis of acne, but more importantly, it is one of the first drugs that topically has been shown to decrease the production of sebum,” Dr. Stein Gold said. A 1% cream formulation is being studied for acne.
Dr. Stein Gold is a consultant, investigator, and/or speaker for Galderma, Ortho Derm, Sol Gel, Foamix, Cassiopea, and Almirall.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – It used to be thought that acne begins with microcomedones, which go on to develop either inflammatory lesions or noninflammatory lesions, but more recent evidence has changed that perception, according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital, Detroit.
Dr. Stein Gold said at the annual Coastal Dermatology Symposium. “All acne is inflammation acne,” and inflammation also persists, she added. Biopsies of scarred lesions, once considered postinflammatory, also have revealed persistent inflammation, she noted.
One study found that persistent scars can evolve from closed comedones, papules, and pustules, but the most common was a papule that turned into a postinflammatory lesion (J Drugs Dermatol 2017 Jun 1;16[6]:566-72). “So when patients come in and they have these red spots on their face, it’s not over. There’s still time to be aggressive because those inflammatory lesions are more likely to lead to scars than anything else,” Dr. Stein Gold said. “And we also know that papules that develop into scars do so because they’re there for a longer period of time. Those that develop scars are present about 10.5 days, compared with 6.6 days for those that don’t develop into scars.”
She went on to review some of the new treatments for acne that can be brought to bear in such cases. These include developments with topical retinoids that are aimed at improving delivery and reducing skin irritation.
A new topical retinoid, trifarotene cream, 0.005%, showed efficacy and tolerability for both the face and trunk in a recent phase 3 trial of patients with moderate facial and truncal acne and was recently approved for patients aged 9 years and older. In the study, about 30%-40% of people aged 9 years and older treated with once-daily trifarotene cream (Aklief) achieved clear or almost-clear status of the face at 12 weeks, vs. about 20% and 26%, of those on the vehicle cream (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9).
The drug can also treat papules and pustules, nearly as well as it treats blackheads and whiteheads, according to Dr. Stein Gold. Like other retinoids, it produces some redness and scaling and rather than letting these adverse events discourage patients, she leans in. “I tell patients they’re going to have some sloughing of the skin the first 2 weeks. I tell them that people pay money for that. It’s called a chemical peel,” said Dr. Stein Gold, noting that patients respond well to this information.
If patients find the treatments too irritating, she advises them to avoid applying it to wet skin. They can also apply it every other night, or even less frequently, and then work up to more frequent use, she said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
Tazarotene is another topical retinoid that can be very irritating. A new lotion formulation of tazarotene 0.045% contains a lower dose than the 0.1% typically used in creams, and has similar efficacy but reduced irritation, Dr. Stein Gold said. In August, the manufacturer submitted an application for approval with the Food and Drug Administration for treatment of acne.
Dr. Stein Gold also talked about using retinoids to minimize scarring, referring to a study of patients with moderate and severe facial acne, and atrophic acne scars, comparing adapalene 0.3% plus benzoyl peroxide 2.5% gel on one side of the face and vehicle on the other side of the face for 24 weeks, followed by application of the active treatment to both sides of the face for 24 weeks. Treatment was associated with a reduction of atrophic acne scars at 24 weeks, which was maintained for up to 48 weeks (Am J Clin Dermatol. 2019 Oct[5];20:725-32).
“We can now say to patients, ‘Not only can I help you with your acne, but I can potentially even improve your atrophic scarring,’ ” she said.
Finally, she discussed clascoterone, a novel androgen receptor antagonist, which inhibits sebum production and prevents colonization by Cutibacterium acnes (formerly called Propionibacterium acnes) and subsequent inflammation. “It does a lot of good things in terms of the pathogenesis of acne, but more importantly, it is one of the first drugs that topically has been shown to decrease the production of sebum,” Dr. Stein Gold said. A 1% cream formulation is being studied for acne.
Dr. Stein Gold is a consultant, investigator, and/or speaker for Galderma, Ortho Derm, Sol Gel, Foamix, Cassiopea, and Almirall.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – It used to be thought that acne begins with microcomedones, which go on to develop either inflammatory lesions or noninflammatory lesions, but more recent evidence has changed that perception, according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital, Detroit.
Dr. Stein Gold said at the annual Coastal Dermatology Symposium. “All acne is inflammation acne,” and inflammation also persists, she added. Biopsies of scarred lesions, once considered postinflammatory, also have revealed persistent inflammation, she noted.
One study found that persistent scars can evolve from closed comedones, papules, and pustules, but the most common was a papule that turned into a postinflammatory lesion (J Drugs Dermatol 2017 Jun 1;16[6]:566-72). “So when patients come in and they have these red spots on their face, it’s not over. There’s still time to be aggressive because those inflammatory lesions are more likely to lead to scars than anything else,” Dr. Stein Gold said. “And we also know that papules that develop into scars do so because they’re there for a longer period of time. Those that develop scars are present about 10.5 days, compared with 6.6 days for those that don’t develop into scars.”
She went on to review some of the new treatments for acne that can be brought to bear in such cases. These include developments with topical retinoids that are aimed at improving delivery and reducing skin irritation.
A new topical retinoid, trifarotene cream, 0.005%, showed efficacy and tolerability for both the face and trunk in a recent phase 3 trial of patients with moderate facial and truncal acne and was recently approved for patients aged 9 years and older. In the study, about 30%-40% of people aged 9 years and older treated with once-daily trifarotene cream (Aklief) achieved clear or almost-clear status of the face at 12 weeks, vs. about 20% and 26%, of those on the vehicle cream (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9).
The drug can also treat papules and pustules, nearly as well as it treats blackheads and whiteheads, according to Dr. Stein Gold. Like other retinoids, it produces some redness and scaling and rather than letting these adverse events discourage patients, she leans in. “I tell patients they’re going to have some sloughing of the skin the first 2 weeks. I tell them that people pay money for that. It’s called a chemical peel,” said Dr. Stein Gold, noting that patients respond well to this information.
If patients find the treatments too irritating, she advises them to avoid applying it to wet skin. They can also apply it every other night, or even less frequently, and then work up to more frequent use, she said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
Tazarotene is another topical retinoid that can be very irritating. A new lotion formulation of tazarotene 0.045% contains a lower dose than the 0.1% typically used in creams, and has similar efficacy but reduced irritation, Dr. Stein Gold said. In August, the manufacturer submitted an application for approval with the Food and Drug Administration for treatment of acne.
Dr. Stein Gold also talked about using retinoids to minimize scarring, referring to a study of patients with moderate and severe facial acne, and atrophic acne scars, comparing adapalene 0.3% plus benzoyl peroxide 2.5% gel on one side of the face and vehicle on the other side of the face for 24 weeks, followed by application of the active treatment to both sides of the face for 24 weeks. Treatment was associated with a reduction of atrophic acne scars at 24 weeks, which was maintained for up to 48 weeks (Am J Clin Dermatol. 2019 Oct[5];20:725-32).
“We can now say to patients, ‘Not only can I help you with your acne, but I can potentially even improve your atrophic scarring,’ ” she said.
Finally, she discussed clascoterone, a novel androgen receptor antagonist, which inhibits sebum production and prevents colonization by Cutibacterium acnes (formerly called Propionibacterium acnes) and subsequent inflammation. “It does a lot of good things in terms of the pathogenesis of acne, but more importantly, it is one of the first drugs that topically has been shown to decrease the production of sebum,” Dr. Stein Gold said. A 1% cream formulation is being studied for acne.
Dr. Stein Gold is a consultant, investigator, and/or speaker for Galderma, Ortho Derm, Sol Gel, Foamix, Cassiopea, and Almirall.
This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
Adjunctive therapy is among the roles for topical agents in psoriasis
SEATTLE – is not dead,” Linda Stein Gold, MD, said at the annual Coastal Dermatology Symposium.
“We have to remember when we think back to our practice, how many topical prescriptions do we write, compared to preventive prescriptions? Probably most are topical,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Hospital Center, Detroit.
Topical agents have a place when a patient is doing well on treatment with a biologic but is not responding completely, she noted. One open-label, single-arm study looked at adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% (Enstilar) foam, applied once daily for 4 week, then twice a week on consecutive days for 12 weeks in 25 patients with psoriasis who had a mean body surface area (BSA) of less than 5% but significant remaining disease despite treatment with biologics.
At week 4, 76% achieved a BSA of 1% or less and Physician’s Global Assessment score of 1 or less at week 4, as did 68% at week 16. This was compared with 12% and 4%, respectively (J Drugs Dermatol. 2018 Aug 1;17[8]:845-50). “They found that a good potent topical on top of a biologic does really well. That can really kick up the last part of the efficacy to get the patients almost to clear,” she observed.
At the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education, Dr. Stein Gold also discussed tazarotene, a topical retinoid approved by the Food and Drug Administration for treating psoriasis and is available as a 0.1% and 0.05% cream and gel. About 10%-30% of patients experience side effects with tazarotene, such as pruritus, stinging, and burning. Topical corticosteroids can help, which prompted development of a combined product, she noted.
She referred to a phase 2 study of patients with moderate to severe plaque psoriasis, which compared the fixed combination lotion formulation of tazarotene plus halobetasol propionate to the two components alone. The investigators found almost a 9% rate of treatment success with tazarotene alone, versus about 23% with halobetasol propionate alone and about 43% with the combined product. The combined individual effect of the two drugs was about 32%, so the 43% efficacy of the combined product had an absolute synergistic effect of about 11%, Dr. Stein Gold pointed out.
Two phase 3 trials of adults with moderate to severe psoriasis supported the phase 2 results of the combined lotion formulation (halobetasol 0.01% with tazarotene 0.045%), said Dr. Stein Gold, the first author (J Am Acad Dermatol. 2018 Aug;79[2]:287-93). Treatment success was defined as at least a 2-grade Investigator’s Global Assessment score and improvement from baseline and a score of “clear” or “almost clear.” In one of the studies, 36% of those on the combination versus 7% of those on the vehicle met this endpoint at week 8, as did 45% versus 13%, respectively, in the second study (P less than .001 for both studies).
Patients also had less itching, drying, and stinging than typically seen with tazarotene alone, Dr. Stein Gold said. In the studies, contact dermatitis was the most common side effect associated with treatment, reported in 6.3%
Dr. Stein Gold has received research support from Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, and Foamix. She has been a consultant for Sol-gel, Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promis, Anacor, and Medimetriks. She has been on the speakers bureau of Galderma, Leo, Valeant, Novartis, Celgene, and Allergan. She has been a member of scientific advisory boards for Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, and Promius.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – is not dead,” Linda Stein Gold, MD, said at the annual Coastal Dermatology Symposium.
“We have to remember when we think back to our practice, how many topical prescriptions do we write, compared to preventive prescriptions? Probably most are topical,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Hospital Center, Detroit.
Topical agents have a place when a patient is doing well on treatment with a biologic but is not responding completely, she noted. One open-label, single-arm study looked at adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% (Enstilar) foam, applied once daily for 4 week, then twice a week on consecutive days for 12 weeks in 25 patients with psoriasis who had a mean body surface area (BSA) of less than 5% but significant remaining disease despite treatment with biologics.
At week 4, 76% achieved a BSA of 1% or less and Physician’s Global Assessment score of 1 or less at week 4, as did 68% at week 16. This was compared with 12% and 4%, respectively (J Drugs Dermatol. 2018 Aug 1;17[8]:845-50). “They found that a good potent topical on top of a biologic does really well. That can really kick up the last part of the efficacy to get the patients almost to clear,” she observed.
At the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education, Dr. Stein Gold also discussed tazarotene, a topical retinoid approved by the Food and Drug Administration for treating psoriasis and is available as a 0.1% and 0.05% cream and gel. About 10%-30% of patients experience side effects with tazarotene, such as pruritus, stinging, and burning. Topical corticosteroids can help, which prompted development of a combined product, she noted.
She referred to a phase 2 study of patients with moderate to severe plaque psoriasis, which compared the fixed combination lotion formulation of tazarotene plus halobetasol propionate to the two components alone. The investigators found almost a 9% rate of treatment success with tazarotene alone, versus about 23% with halobetasol propionate alone and about 43% with the combined product. The combined individual effect of the two drugs was about 32%, so the 43% efficacy of the combined product had an absolute synergistic effect of about 11%, Dr. Stein Gold pointed out.
Two phase 3 trials of adults with moderate to severe psoriasis supported the phase 2 results of the combined lotion formulation (halobetasol 0.01% with tazarotene 0.045%), said Dr. Stein Gold, the first author (J Am Acad Dermatol. 2018 Aug;79[2]:287-93). Treatment success was defined as at least a 2-grade Investigator’s Global Assessment score and improvement from baseline and a score of “clear” or “almost clear.” In one of the studies, 36% of those on the combination versus 7% of those on the vehicle met this endpoint at week 8, as did 45% versus 13%, respectively, in the second study (P less than .001 for both studies).
Patients also had less itching, drying, and stinging than typically seen with tazarotene alone, Dr. Stein Gold said. In the studies, contact dermatitis was the most common side effect associated with treatment, reported in 6.3%
Dr. Stein Gold has received research support from Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, and Foamix. She has been a consultant for Sol-gel, Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promis, Anacor, and Medimetriks. She has been on the speakers bureau of Galderma, Leo, Valeant, Novartis, Celgene, and Allergan. She has been a member of scientific advisory boards for Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, and Promius.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – is not dead,” Linda Stein Gold, MD, said at the annual Coastal Dermatology Symposium.
“We have to remember when we think back to our practice, how many topical prescriptions do we write, compared to preventive prescriptions? Probably most are topical,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Hospital Center, Detroit.
Topical agents have a place when a patient is doing well on treatment with a biologic but is not responding completely, she noted. One open-label, single-arm study looked at adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% (Enstilar) foam, applied once daily for 4 week, then twice a week on consecutive days for 12 weeks in 25 patients with psoriasis who had a mean body surface area (BSA) of less than 5% but significant remaining disease despite treatment with biologics.
At week 4, 76% achieved a BSA of 1% or less and Physician’s Global Assessment score of 1 or less at week 4, as did 68% at week 16. This was compared with 12% and 4%, respectively (J Drugs Dermatol. 2018 Aug 1;17[8]:845-50). “They found that a good potent topical on top of a biologic does really well. That can really kick up the last part of the efficacy to get the patients almost to clear,” she observed.
At the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education, Dr. Stein Gold also discussed tazarotene, a topical retinoid approved by the Food and Drug Administration for treating psoriasis and is available as a 0.1% and 0.05% cream and gel. About 10%-30% of patients experience side effects with tazarotene, such as pruritus, stinging, and burning. Topical corticosteroids can help, which prompted development of a combined product, she noted.
She referred to a phase 2 study of patients with moderate to severe plaque psoriasis, which compared the fixed combination lotion formulation of tazarotene plus halobetasol propionate to the two components alone. The investigators found almost a 9% rate of treatment success with tazarotene alone, versus about 23% with halobetasol propionate alone and about 43% with the combined product. The combined individual effect of the two drugs was about 32%, so the 43% efficacy of the combined product had an absolute synergistic effect of about 11%, Dr. Stein Gold pointed out.
Two phase 3 trials of adults with moderate to severe psoriasis supported the phase 2 results of the combined lotion formulation (halobetasol 0.01% with tazarotene 0.045%), said Dr. Stein Gold, the first author (J Am Acad Dermatol. 2018 Aug;79[2]:287-93). Treatment success was defined as at least a 2-grade Investigator’s Global Assessment score and improvement from baseline and a score of “clear” or “almost clear.” In one of the studies, 36% of those on the combination versus 7% of those on the vehicle met this endpoint at week 8, as did 45% versus 13%, respectively, in the second study (P less than .001 for both studies).
Patients also had less itching, drying, and stinging than typically seen with tazarotene alone, Dr. Stein Gold said. In the studies, contact dermatitis was the most common side effect associated with treatment, reported in 6.3%
Dr. Stein Gold has received research support from Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, and Foamix. She has been a consultant for Sol-gel, Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promis, Anacor, and Medimetriks. She has been on the speakers bureau of Galderma, Leo, Valeant, Novartis, Celgene, and Allergan. She has been a member of scientific advisory boards for Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, and Promius.
This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
Once-daily oral JAK inhibitor for atopic dermatitis effective in phase 3 study
MADRID – (AD) on the strength of its knockout performance in the pivotal phase 3 JADE MONO-1 trial, presented at the annual congress of the European Academy of Dermatology and Venereology.
“The IGA [Investigator Global Assessment] and EASI [Eczema Area and Severity Index]-75 responses for both doses of abrocitinib were significantly greater than placebo as early as week 2 and continued to increase until week 12 with no plateau. This study stopped at week 12. I would have liked to see what happened at 16 weeks or further. I don’t know where this is going to end up maxing out,” commented principal investigator Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland.
JADE MONO-1 (JAK1 Atopic Dermatitis Efficacy and Safety Monotherapy–1) was a 12-week, double-blind, multicenter study which included 387 adolescents and adults with moderate or severe AD randomized 2:2:1 to abrocitinib at 200 mg or 100 mg once daily, or to placebo. Overall, this was a fairly severely affected population, with a mean baseline EASI (Eczema Area and Severity Index) score of about 30, a peak pruritus numeric rating scale score of 7 out of 10, and a mean Dermatology Quality Life Index score greater than 14. Nonetheless, this was a rigorous abrocitinib monotherapy trial, as participants were not allowed to take even a single dose of topical corticosteroids.
The coprimary endpoints in JADE MONO-1 were achievement of an IGA score of 0 or 1 – that is, clear or almost clear – at week 12 plus at least a 2-point improvement, compared with baseline on the 0-4 scale, and an EASI-75 response as defined by a 75% or greater improvement from baseline.
A clear-cut dose response was evident: the IGA response rate was 43.8% in the group on abrocitinib at 200 mg/day, 23.7% with 100 mg, and 7.9% with placebo. The EASI-75 response rates were 62.7%, 39.7%, and 11.8%, respectively, with a statistically significant separation from placebo by week 2.
Key secondary endpoints included the rigorous EASI-90 response rate: 38.6% with the higher dose of abrocitinib, 18.6% at 100 mg per day, and 5.3% with placebo. And again, that’s without resort to topical steroids, Dr. Simpson noted.
Another important secondary endpoint was the proportion of patients who achieved at least a 4-point improvement in itch on the pruritus self-rating scale by week 12: 57.2%, 37.7%, and 15.3%. The rapidity of the improvement was notable: By week 2, this endpoint was achieved in 45.6% of patients on abrocitinib on the 200-mg dose, 20.4% on the 100-mg dose, and 2.7% of placebo-treated controls.
“By day 2, 1 day after taking the first pill, you can see really nice reductions in itch at both doses, compared with placebo,” the dermatologist said.
Study dropout rates were low despite the inability to utilize topical steroids: 11% in the higher-dose abrocitinib group and 13.5% with abrocitinib at 100 mg/day, both of which were lower than in controls.
Turning to safety results, Dr. Simpson noted that the 9.1% rate of discontinuations because of adverse events in the placebo group was significantly higher than the 5.8% rates in each of the active treatment arms. The serious adverse event rate was 3.2% in each of the abrocitinib groups and 1.9% with placebo. Among the serious adverse events in abrocitinib-treated patients Dr. Simpson considered worthy of special mention were a single case of inflammatory bowel disease, which resolved after halting treatment; one case of peritonsillitis; and a case of pancreatitis in an alcoholic patient. No deaths, major adverse cardiovascular events, or malignancies occurred in this brief 12-week trial. Nor were there any cases of deep vein thrombosis or pulmonary embolism, which has been an issue with some other JAK inhibitors.
“We need long-term safety data, of course,” he said.
Laboratory findings were generally unremarkable, with no clinically significant changes. Platelet counts dropped to a nadir at about 4 weeks while staying within normal range, then came back up. Mean LDL cholesterol levels rose by about 10%, an unwelcome event that was counterbalanced by a favorable 20% rise in HDL cholesterol.
Asked about the efficacy rates in the 20%-plus adolescent study participants, compared with the adults, Dr. Simpson replied that a detailed analysis is planned, adding: “I can say that things are looking pretty similar.”
Abrocitinib is selective for inhibition of JAK1, with resultant modulation of interleukins-4, -13, and -31, as well as interferon-gamma, all of which are cytokines involved in the pathophysiology of AD.
Of note, Pfizer, the drug’s developer, has announced positive results from the sister pivotal phase 3 trial, JADE MONO-2, with full details forthcoming in early 2020. Results of a phase 2b study of abrocitinib were also recently published (JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2855).
Dr. Simpson reported receiving research grants from and serving as a consultant to Pfizer, the study sponsor. He has similar financial relationships with close to a dozen other pharmaceutical companies.
MADRID – (AD) on the strength of its knockout performance in the pivotal phase 3 JADE MONO-1 trial, presented at the annual congress of the European Academy of Dermatology and Venereology.
“The IGA [Investigator Global Assessment] and EASI [Eczema Area and Severity Index]-75 responses for both doses of abrocitinib were significantly greater than placebo as early as week 2 and continued to increase until week 12 with no plateau. This study stopped at week 12. I would have liked to see what happened at 16 weeks or further. I don’t know where this is going to end up maxing out,” commented principal investigator Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland.
JADE MONO-1 (JAK1 Atopic Dermatitis Efficacy and Safety Monotherapy–1) was a 12-week, double-blind, multicenter study which included 387 adolescents and adults with moderate or severe AD randomized 2:2:1 to abrocitinib at 200 mg or 100 mg once daily, or to placebo. Overall, this was a fairly severely affected population, with a mean baseline EASI (Eczema Area and Severity Index) score of about 30, a peak pruritus numeric rating scale score of 7 out of 10, and a mean Dermatology Quality Life Index score greater than 14. Nonetheless, this was a rigorous abrocitinib monotherapy trial, as participants were not allowed to take even a single dose of topical corticosteroids.
The coprimary endpoints in JADE MONO-1 were achievement of an IGA score of 0 or 1 – that is, clear or almost clear – at week 12 plus at least a 2-point improvement, compared with baseline on the 0-4 scale, and an EASI-75 response as defined by a 75% or greater improvement from baseline.
A clear-cut dose response was evident: the IGA response rate was 43.8% in the group on abrocitinib at 200 mg/day, 23.7% with 100 mg, and 7.9% with placebo. The EASI-75 response rates were 62.7%, 39.7%, and 11.8%, respectively, with a statistically significant separation from placebo by week 2.
Key secondary endpoints included the rigorous EASI-90 response rate: 38.6% with the higher dose of abrocitinib, 18.6% at 100 mg per day, and 5.3% with placebo. And again, that’s without resort to topical steroids, Dr. Simpson noted.
Another important secondary endpoint was the proportion of patients who achieved at least a 4-point improvement in itch on the pruritus self-rating scale by week 12: 57.2%, 37.7%, and 15.3%. The rapidity of the improvement was notable: By week 2, this endpoint was achieved in 45.6% of patients on abrocitinib on the 200-mg dose, 20.4% on the 100-mg dose, and 2.7% of placebo-treated controls.
“By day 2, 1 day after taking the first pill, you can see really nice reductions in itch at both doses, compared with placebo,” the dermatologist said.
Study dropout rates were low despite the inability to utilize topical steroids: 11% in the higher-dose abrocitinib group and 13.5% with abrocitinib at 100 mg/day, both of which were lower than in controls.
Turning to safety results, Dr. Simpson noted that the 9.1% rate of discontinuations because of adverse events in the placebo group was significantly higher than the 5.8% rates in each of the active treatment arms. The serious adverse event rate was 3.2% in each of the abrocitinib groups and 1.9% with placebo. Among the serious adverse events in abrocitinib-treated patients Dr. Simpson considered worthy of special mention were a single case of inflammatory bowel disease, which resolved after halting treatment; one case of peritonsillitis; and a case of pancreatitis in an alcoholic patient. No deaths, major adverse cardiovascular events, or malignancies occurred in this brief 12-week trial. Nor were there any cases of deep vein thrombosis or pulmonary embolism, which has been an issue with some other JAK inhibitors.
“We need long-term safety data, of course,” he said.
Laboratory findings were generally unremarkable, with no clinically significant changes. Platelet counts dropped to a nadir at about 4 weeks while staying within normal range, then came back up. Mean LDL cholesterol levels rose by about 10%, an unwelcome event that was counterbalanced by a favorable 20% rise in HDL cholesterol.
Asked about the efficacy rates in the 20%-plus adolescent study participants, compared with the adults, Dr. Simpson replied that a detailed analysis is planned, adding: “I can say that things are looking pretty similar.”
Abrocitinib is selective for inhibition of JAK1, with resultant modulation of interleukins-4, -13, and -31, as well as interferon-gamma, all of which are cytokines involved in the pathophysiology of AD.
Of note, Pfizer, the drug’s developer, has announced positive results from the sister pivotal phase 3 trial, JADE MONO-2, with full details forthcoming in early 2020. Results of a phase 2b study of abrocitinib were also recently published (JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2855).
Dr. Simpson reported receiving research grants from and serving as a consultant to Pfizer, the study sponsor. He has similar financial relationships with close to a dozen other pharmaceutical companies.
MADRID – (AD) on the strength of its knockout performance in the pivotal phase 3 JADE MONO-1 trial, presented at the annual congress of the European Academy of Dermatology and Venereology.
“The IGA [Investigator Global Assessment] and EASI [Eczema Area and Severity Index]-75 responses for both doses of abrocitinib were significantly greater than placebo as early as week 2 and continued to increase until week 12 with no plateau. This study stopped at week 12. I would have liked to see what happened at 16 weeks or further. I don’t know where this is going to end up maxing out,” commented principal investigator Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland.
JADE MONO-1 (JAK1 Atopic Dermatitis Efficacy and Safety Monotherapy–1) was a 12-week, double-blind, multicenter study which included 387 adolescents and adults with moderate or severe AD randomized 2:2:1 to abrocitinib at 200 mg or 100 mg once daily, or to placebo. Overall, this was a fairly severely affected population, with a mean baseline EASI (Eczema Area and Severity Index) score of about 30, a peak pruritus numeric rating scale score of 7 out of 10, and a mean Dermatology Quality Life Index score greater than 14. Nonetheless, this was a rigorous abrocitinib monotherapy trial, as participants were not allowed to take even a single dose of topical corticosteroids.
The coprimary endpoints in JADE MONO-1 were achievement of an IGA score of 0 or 1 – that is, clear or almost clear – at week 12 plus at least a 2-point improvement, compared with baseline on the 0-4 scale, and an EASI-75 response as defined by a 75% or greater improvement from baseline.
A clear-cut dose response was evident: the IGA response rate was 43.8% in the group on abrocitinib at 200 mg/day, 23.7% with 100 mg, and 7.9% with placebo. The EASI-75 response rates were 62.7%, 39.7%, and 11.8%, respectively, with a statistically significant separation from placebo by week 2.
Key secondary endpoints included the rigorous EASI-90 response rate: 38.6% with the higher dose of abrocitinib, 18.6% at 100 mg per day, and 5.3% with placebo. And again, that’s without resort to topical steroids, Dr. Simpson noted.
Another important secondary endpoint was the proportion of patients who achieved at least a 4-point improvement in itch on the pruritus self-rating scale by week 12: 57.2%, 37.7%, and 15.3%. The rapidity of the improvement was notable: By week 2, this endpoint was achieved in 45.6% of patients on abrocitinib on the 200-mg dose, 20.4% on the 100-mg dose, and 2.7% of placebo-treated controls.
“By day 2, 1 day after taking the first pill, you can see really nice reductions in itch at both doses, compared with placebo,” the dermatologist said.
Study dropout rates were low despite the inability to utilize topical steroids: 11% in the higher-dose abrocitinib group and 13.5% with abrocitinib at 100 mg/day, both of which were lower than in controls.
Turning to safety results, Dr. Simpson noted that the 9.1% rate of discontinuations because of adverse events in the placebo group was significantly higher than the 5.8% rates in each of the active treatment arms. The serious adverse event rate was 3.2% in each of the abrocitinib groups and 1.9% with placebo. Among the serious adverse events in abrocitinib-treated patients Dr. Simpson considered worthy of special mention were a single case of inflammatory bowel disease, which resolved after halting treatment; one case of peritonsillitis; and a case of pancreatitis in an alcoholic patient. No deaths, major adverse cardiovascular events, or malignancies occurred in this brief 12-week trial. Nor were there any cases of deep vein thrombosis or pulmonary embolism, which has been an issue with some other JAK inhibitors.
“We need long-term safety data, of course,” he said.
Laboratory findings were generally unremarkable, with no clinically significant changes. Platelet counts dropped to a nadir at about 4 weeks while staying within normal range, then came back up. Mean LDL cholesterol levels rose by about 10%, an unwelcome event that was counterbalanced by a favorable 20% rise in HDL cholesterol.
Asked about the efficacy rates in the 20%-plus adolescent study participants, compared with the adults, Dr. Simpson replied that a detailed analysis is planned, adding: “I can say that things are looking pretty similar.”
Abrocitinib is selective for inhibition of JAK1, with resultant modulation of interleukins-4, -13, and -31, as well as interferon-gamma, all of which are cytokines involved in the pathophysiology of AD.
Of note, Pfizer, the drug’s developer, has announced positive results from the sister pivotal phase 3 trial, JADE MONO-2, with full details forthcoming in early 2020. Results of a phase 2b study of abrocitinib were also recently published (JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2855).
Dr. Simpson reported receiving research grants from and serving as a consultant to Pfizer, the study sponsor. He has similar financial relationships with close to a dozen other pharmaceutical companies.
REPORTING FROM THE EADV CONGRESS
Rituximab bests mycophenolate in pemphigus vulgaris
MADRID – Pascal Joly, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Not only did rituximab prove superior in terms of efficacy in the PEMPHIX trial, with a five times greater likelihood of achieving a complete remission lasting for at least 16 weeks while off oral corticosteroids than with mycophenolate mofetil in the 52-week study, but the total number of disease flares in the mycophenolate mofetil group was five times higher. Moreover, rituximab-treated patients received a markedly lower cumulative dose of prednisone as well.
“Rituximab has a superior overall benefit-risk profile, compared to mycophenolate mofetil, in patients with moderate to severe pemphigus vulgaris,” concluded Dr. Joly, professor of dermatology at the University of Rouen (France) and president of the French Society of Dermatology.
The study was undertaken because mycophenolate mofetil is commonly used as a corticosteroid-sparing drug in patients with pemphigus vulgaris, even though its efficacy for the treatment of this rare, severe autoimmune blistering disease is unproven, he explained.
In contrast, rituximab was approved by the Food and Drug Administration and European regulators for treatment of pemphigus vulgaris on the strength of the pivotal phase 3 Ritux 3 trial – also led by Dr. Joly – which demonstrated the superiority of this intravenously administered anti-CD20 monoclonal antibody plus short-term prednisone over high-dose corticosteroid monotherapy, which for decades had been the standard treatment despite its considerable toxicity burden (Lancet. 2017 May 20;389[10083]:2031-40).
An independently conducted analysis of Ritux 3 recently concluded that rituximab plus short-term prednisone was more effective than prednisone alone, with a lower risk of life-threatening, corticosteroid-related adverse events and less cumulative corticosteroid exposure (Br J Dermatol. 2019 Sep 5. doi: 10.1111/bjd.18482).
Also, an international panel of 93 pemphigus experts has declared that rituximab should be considered an evidence-based first-line therapy for moderate to-severe pemphigus (J Am Acad Dermatol. 2018 Feb 10. doi: 10.1016/j.jaad.2018.02.021).
The phase 3, placebo-controlled PEMPHIX trial randomized 135 patients with moderate or severe pemphigus at 49 academic medical centers in the United States and nine other countries to double-blind rituximab or mycophenolate mofetil on top of background oral prednisone at 1.0-1.5 mg/kg per day, with the steroid to be tapered and discontinued within 4-6 months.
The primary endpoint of the study was the proportion of patients in each study arm at week 52 who had achieved a sustained complete remission lasting for at least 16 weeks while off prednisone. The rate was 40.3% in the rituximab group and 9.5% with mycophenolate mofetil, for a 383% increased likelihood of sustained complete remission in the rituximab group.
In addition, all of the study’s secondary endpoints significantly favored rituximab. The median cumulative dose of corticosteroid was 2.7 g through 52 weeks in the rituximab arm, compared with 4 g with mycophenolate mofetil. The total number of disease flares over 52 weeks was 6 in the rituximab group and 44 in the mycophenolate arm. Five rituximab-treated patients experienced disease flares, as did 26 on mycophenolate. Thus, the likelihood of a flare was seven times lower with rituximab.
Scores on the Dermatology Life Quality Index improved by an average of 8.87 points from baseline to week 52 in the rituximab group versus 6 points with mycophenolate. And 62.7% of rituximab-treated patients had a week-52 Dermatology Life Quality Index score of 0, meaning no impact of the disease on their quality of life, compared with 25% of the mycophenolate group.
Dr. Joly characterized the safety profile of rituximab as “manageable, with acceptable tolerability.” About 9% of the rituximab group and 7.4% of mycophenolate-treated patients had one or more treatment-related adverse events, a nonsignificant difference. The rate of treatment-related serious infections was 3.0% with rituximab and 2.9% with mycophenolate. Serious infusion reactions leading to study withdrawal occurred in three patients on rituximab and one on mycophenolate. The rate of grade 3 or worse corticosteroid-related adverse events was 1.5% with rituximab and significantly greater at 7.4% with mycophenolate.
An additional 48-week safety assessment beyond the 52-week primary outcome is ongoing.
Asked what future role he sees for mycophenolate in pemphigus vulgaris, Dr. Joly replied that the only study in the literature that shows the drug outperforms placebo was seriously flawed. “In the future, it’s very likely that the indications for use of mycophenolate in pemphigus vulgaris will be fewer and fewer,” the dermatologist added.
In reply to a question about the merits of routine antibiotic prophylaxis against pneumocystis carinii pneumonia in patients taking rituximab for pemphigus vulgaris, Dr. Joly said the incidence isn’t sufficiently high to justify such practice. After all, he noted, there were no cases of pneumocystis carinii pneumonia in rituximab-treated patients in PEMPHIX and only one in Ritux 3.
EADV Scientific Programming Committee Chair Brigitte Dreno, MD, PhD, professor and head of dermatology at University Hospital in Nantes, France, inquired as to whether there’s a role for maintenance therapy in a potent rituximab-based treatment strategy such as utilized in PEMPHIX.
Definitely, Dr. Joly replied. However, further study is required to work out the best maintenance program.
“There are many arguments for maintenance therapy in these patients. For one, the frequency of relapses increases with the length of follow-up. Also, anti–desmoglein-specific T cells can still be detected after rituximab therapy, even in patients in complete remission. So there is a need for maintenance therapy, perhaps at months 6, 12, and 18, but the optimal regimen isn’t determined yet,” according to Dr. Joly.
PEMPHIX was sponsored by F. Hoffmann-La Roche. Dr. Joly reported serving as a consultant to Roche, Amgen, Principia Biopharma, and Argenx.
MADRID – Pascal Joly, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Not only did rituximab prove superior in terms of efficacy in the PEMPHIX trial, with a five times greater likelihood of achieving a complete remission lasting for at least 16 weeks while off oral corticosteroids than with mycophenolate mofetil in the 52-week study, but the total number of disease flares in the mycophenolate mofetil group was five times higher. Moreover, rituximab-treated patients received a markedly lower cumulative dose of prednisone as well.
“Rituximab has a superior overall benefit-risk profile, compared to mycophenolate mofetil, in patients with moderate to severe pemphigus vulgaris,” concluded Dr. Joly, professor of dermatology at the University of Rouen (France) and president of the French Society of Dermatology.
The study was undertaken because mycophenolate mofetil is commonly used as a corticosteroid-sparing drug in patients with pemphigus vulgaris, even though its efficacy for the treatment of this rare, severe autoimmune blistering disease is unproven, he explained.
In contrast, rituximab was approved by the Food and Drug Administration and European regulators for treatment of pemphigus vulgaris on the strength of the pivotal phase 3 Ritux 3 trial – also led by Dr. Joly – which demonstrated the superiority of this intravenously administered anti-CD20 monoclonal antibody plus short-term prednisone over high-dose corticosteroid monotherapy, which for decades had been the standard treatment despite its considerable toxicity burden (Lancet. 2017 May 20;389[10083]:2031-40).
An independently conducted analysis of Ritux 3 recently concluded that rituximab plus short-term prednisone was more effective than prednisone alone, with a lower risk of life-threatening, corticosteroid-related adverse events and less cumulative corticosteroid exposure (Br J Dermatol. 2019 Sep 5. doi: 10.1111/bjd.18482).
Also, an international panel of 93 pemphigus experts has declared that rituximab should be considered an evidence-based first-line therapy for moderate to-severe pemphigus (J Am Acad Dermatol. 2018 Feb 10. doi: 10.1016/j.jaad.2018.02.021).
The phase 3, placebo-controlled PEMPHIX trial randomized 135 patients with moderate or severe pemphigus at 49 academic medical centers in the United States and nine other countries to double-blind rituximab or mycophenolate mofetil on top of background oral prednisone at 1.0-1.5 mg/kg per day, with the steroid to be tapered and discontinued within 4-6 months.
The primary endpoint of the study was the proportion of patients in each study arm at week 52 who had achieved a sustained complete remission lasting for at least 16 weeks while off prednisone. The rate was 40.3% in the rituximab group and 9.5% with mycophenolate mofetil, for a 383% increased likelihood of sustained complete remission in the rituximab group.
In addition, all of the study’s secondary endpoints significantly favored rituximab. The median cumulative dose of corticosteroid was 2.7 g through 52 weeks in the rituximab arm, compared with 4 g with mycophenolate mofetil. The total number of disease flares over 52 weeks was 6 in the rituximab group and 44 in the mycophenolate arm. Five rituximab-treated patients experienced disease flares, as did 26 on mycophenolate. Thus, the likelihood of a flare was seven times lower with rituximab.
Scores on the Dermatology Life Quality Index improved by an average of 8.87 points from baseline to week 52 in the rituximab group versus 6 points with mycophenolate. And 62.7% of rituximab-treated patients had a week-52 Dermatology Life Quality Index score of 0, meaning no impact of the disease on their quality of life, compared with 25% of the mycophenolate group.
Dr. Joly characterized the safety profile of rituximab as “manageable, with acceptable tolerability.” About 9% of the rituximab group and 7.4% of mycophenolate-treated patients had one or more treatment-related adverse events, a nonsignificant difference. The rate of treatment-related serious infections was 3.0% with rituximab and 2.9% with mycophenolate. Serious infusion reactions leading to study withdrawal occurred in three patients on rituximab and one on mycophenolate. The rate of grade 3 or worse corticosteroid-related adverse events was 1.5% with rituximab and significantly greater at 7.4% with mycophenolate.
An additional 48-week safety assessment beyond the 52-week primary outcome is ongoing.
Asked what future role he sees for mycophenolate in pemphigus vulgaris, Dr. Joly replied that the only study in the literature that shows the drug outperforms placebo was seriously flawed. “In the future, it’s very likely that the indications for use of mycophenolate in pemphigus vulgaris will be fewer and fewer,” the dermatologist added.
In reply to a question about the merits of routine antibiotic prophylaxis against pneumocystis carinii pneumonia in patients taking rituximab for pemphigus vulgaris, Dr. Joly said the incidence isn’t sufficiently high to justify such practice. After all, he noted, there were no cases of pneumocystis carinii pneumonia in rituximab-treated patients in PEMPHIX and only one in Ritux 3.
EADV Scientific Programming Committee Chair Brigitte Dreno, MD, PhD, professor and head of dermatology at University Hospital in Nantes, France, inquired as to whether there’s a role for maintenance therapy in a potent rituximab-based treatment strategy such as utilized in PEMPHIX.
Definitely, Dr. Joly replied. However, further study is required to work out the best maintenance program.
“There are many arguments for maintenance therapy in these patients. For one, the frequency of relapses increases with the length of follow-up. Also, anti–desmoglein-specific T cells can still be detected after rituximab therapy, even in patients in complete remission. So there is a need for maintenance therapy, perhaps at months 6, 12, and 18, but the optimal regimen isn’t determined yet,” according to Dr. Joly.
PEMPHIX was sponsored by F. Hoffmann-La Roche. Dr. Joly reported serving as a consultant to Roche, Amgen, Principia Biopharma, and Argenx.
MADRID – Pascal Joly, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Not only did rituximab prove superior in terms of efficacy in the PEMPHIX trial, with a five times greater likelihood of achieving a complete remission lasting for at least 16 weeks while off oral corticosteroids than with mycophenolate mofetil in the 52-week study, but the total number of disease flares in the mycophenolate mofetil group was five times higher. Moreover, rituximab-treated patients received a markedly lower cumulative dose of prednisone as well.
“Rituximab has a superior overall benefit-risk profile, compared to mycophenolate mofetil, in patients with moderate to severe pemphigus vulgaris,” concluded Dr. Joly, professor of dermatology at the University of Rouen (France) and president of the French Society of Dermatology.
The study was undertaken because mycophenolate mofetil is commonly used as a corticosteroid-sparing drug in patients with pemphigus vulgaris, even though its efficacy for the treatment of this rare, severe autoimmune blistering disease is unproven, he explained.
In contrast, rituximab was approved by the Food and Drug Administration and European regulators for treatment of pemphigus vulgaris on the strength of the pivotal phase 3 Ritux 3 trial – also led by Dr. Joly – which demonstrated the superiority of this intravenously administered anti-CD20 monoclonal antibody plus short-term prednisone over high-dose corticosteroid monotherapy, which for decades had been the standard treatment despite its considerable toxicity burden (Lancet. 2017 May 20;389[10083]:2031-40).
An independently conducted analysis of Ritux 3 recently concluded that rituximab plus short-term prednisone was more effective than prednisone alone, with a lower risk of life-threatening, corticosteroid-related adverse events and less cumulative corticosteroid exposure (Br J Dermatol. 2019 Sep 5. doi: 10.1111/bjd.18482).
Also, an international panel of 93 pemphigus experts has declared that rituximab should be considered an evidence-based first-line therapy for moderate to-severe pemphigus (J Am Acad Dermatol. 2018 Feb 10. doi: 10.1016/j.jaad.2018.02.021).
The phase 3, placebo-controlled PEMPHIX trial randomized 135 patients with moderate or severe pemphigus at 49 academic medical centers in the United States and nine other countries to double-blind rituximab or mycophenolate mofetil on top of background oral prednisone at 1.0-1.5 mg/kg per day, with the steroid to be tapered and discontinued within 4-6 months.
The primary endpoint of the study was the proportion of patients in each study arm at week 52 who had achieved a sustained complete remission lasting for at least 16 weeks while off prednisone. The rate was 40.3% in the rituximab group and 9.5% with mycophenolate mofetil, for a 383% increased likelihood of sustained complete remission in the rituximab group.
In addition, all of the study’s secondary endpoints significantly favored rituximab. The median cumulative dose of corticosteroid was 2.7 g through 52 weeks in the rituximab arm, compared with 4 g with mycophenolate mofetil. The total number of disease flares over 52 weeks was 6 in the rituximab group and 44 in the mycophenolate arm. Five rituximab-treated patients experienced disease flares, as did 26 on mycophenolate. Thus, the likelihood of a flare was seven times lower with rituximab.
Scores on the Dermatology Life Quality Index improved by an average of 8.87 points from baseline to week 52 in the rituximab group versus 6 points with mycophenolate. And 62.7% of rituximab-treated patients had a week-52 Dermatology Life Quality Index score of 0, meaning no impact of the disease on their quality of life, compared with 25% of the mycophenolate group.
Dr. Joly characterized the safety profile of rituximab as “manageable, with acceptable tolerability.” About 9% of the rituximab group and 7.4% of mycophenolate-treated patients had one or more treatment-related adverse events, a nonsignificant difference. The rate of treatment-related serious infections was 3.0% with rituximab and 2.9% with mycophenolate. Serious infusion reactions leading to study withdrawal occurred in three patients on rituximab and one on mycophenolate. The rate of grade 3 or worse corticosteroid-related adverse events was 1.5% with rituximab and significantly greater at 7.4% with mycophenolate.
An additional 48-week safety assessment beyond the 52-week primary outcome is ongoing.
Asked what future role he sees for mycophenolate in pemphigus vulgaris, Dr. Joly replied that the only study in the literature that shows the drug outperforms placebo was seriously flawed. “In the future, it’s very likely that the indications for use of mycophenolate in pemphigus vulgaris will be fewer and fewer,” the dermatologist added.
In reply to a question about the merits of routine antibiotic prophylaxis against pneumocystis carinii pneumonia in patients taking rituximab for pemphigus vulgaris, Dr. Joly said the incidence isn’t sufficiently high to justify such practice. After all, he noted, there were no cases of pneumocystis carinii pneumonia in rituximab-treated patients in PEMPHIX and only one in Ritux 3.
EADV Scientific Programming Committee Chair Brigitte Dreno, MD, PhD, professor and head of dermatology at University Hospital in Nantes, France, inquired as to whether there’s a role for maintenance therapy in a potent rituximab-based treatment strategy such as utilized in PEMPHIX.
Definitely, Dr. Joly replied. However, further study is required to work out the best maintenance program.
“There are many arguments for maintenance therapy in these patients. For one, the frequency of relapses increases with the length of follow-up. Also, anti–desmoglein-specific T cells can still be detected after rituximab therapy, even in patients in complete remission. So there is a need for maintenance therapy, perhaps at months 6, 12, and 18, but the optimal regimen isn’t determined yet,” according to Dr. Joly.
PEMPHIX was sponsored by F. Hoffmann-La Roche. Dr. Joly reported serving as a consultant to Roche, Amgen, Principia Biopharma, and Argenx.
REPORTING FROM THE EADV CONGRESS