User login
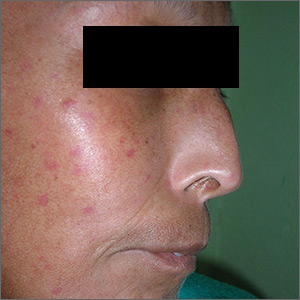
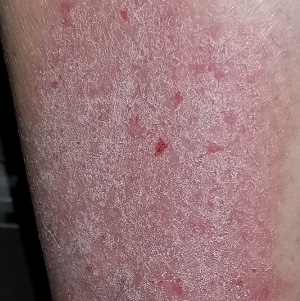
Telangiectasias with tight skin
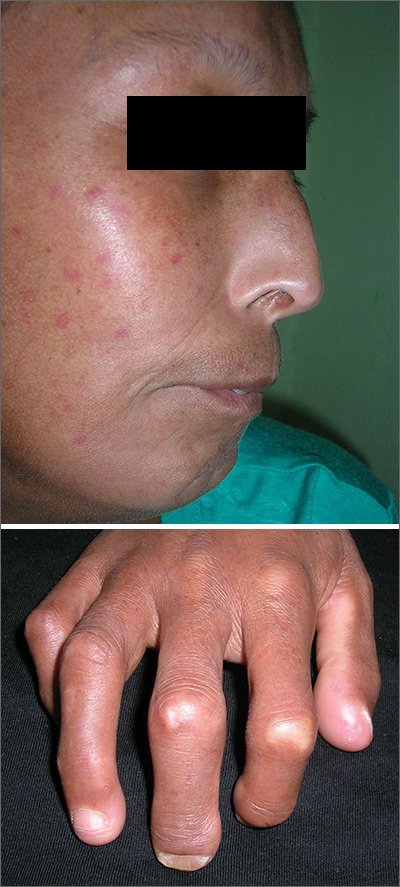
The FP suspected that the patient had CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasias) syndrome also known as limited cutaneous systemic sclerosis (LcSSc). He examined her arms and found that she had firm nodules around the elbows consistent with calcinosis. Further history revealed Raynaud's phenomenon. This clinched the diagnosis of CREST syndrome. The FP ordered blood tests, a chest x-ray (CXR), and pulmonary function tests to determine if there was pulmonary involvement and to learn more about possible systemic effects of her disease.
Systemic sclerosis (scleroderma) is characterized by skin induration and thickening accompanied by variable tissue fibrosis and inflammatory infiltration in numerous visceral organs. Systemic sclerosis can be diffuse or limited to the skin and adjacent tissues (LcSSc). Patients with LcSSc usually have skin sclerosis that is restricted to the hands and, to a lesser extent, the face and neck.
The patient’s antinuclear antibody test was positive with a speckled nucleolar staining pattern, which is a common finding in systemic sclerosis. Her CXR showed evidence of interstitial lung disease with a ground glass pattern. Her pulmonary function test showed a diminished diffusion capacity. Pulmonary disease is seen in all types of systemic sclerosis and not diagnostic for one type. The patient was referred to Rheumatology for further diagnosis and management.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1204-1212.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected that the patient had CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasias) syndrome also known as limited cutaneous systemic sclerosis (LcSSc). He examined her arms and found that she had firm nodules around the elbows consistent with calcinosis. Further history revealed Raynaud's phenomenon. This clinched the diagnosis of CREST syndrome. The FP ordered blood tests, a chest x-ray (CXR), and pulmonary function tests to determine if there was pulmonary involvement and to learn more about possible systemic effects of her disease.
Systemic sclerosis (scleroderma) is characterized by skin induration and thickening accompanied by variable tissue fibrosis and inflammatory infiltration in numerous visceral organs. Systemic sclerosis can be diffuse or limited to the skin and adjacent tissues (LcSSc). Patients with LcSSc usually have skin sclerosis that is restricted to the hands and, to a lesser extent, the face and neck.
The patient’s antinuclear antibody test was positive with a speckled nucleolar staining pattern, which is a common finding in systemic sclerosis. Her CXR showed evidence of interstitial lung disease with a ground glass pattern. Her pulmonary function test showed a diminished diffusion capacity. Pulmonary disease is seen in all types of systemic sclerosis and not diagnostic for one type. The patient was referred to Rheumatology for further diagnosis and management.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1204-1212.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected that the patient had CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasias) syndrome also known as limited cutaneous systemic sclerosis (LcSSc). He examined her arms and found that she had firm nodules around the elbows consistent with calcinosis. Further history revealed Raynaud's phenomenon. This clinched the diagnosis of CREST syndrome. The FP ordered blood tests, a chest x-ray (CXR), and pulmonary function tests to determine if there was pulmonary involvement and to learn more about possible systemic effects of her disease.
Systemic sclerosis (scleroderma) is characterized by skin induration and thickening accompanied by variable tissue fibrosis and inflammatory infiltration in numerous visceral organs. Systemic sclerosis can be diffuse or limited to the skin and adjacent tissues (LcSSc). Patients with LcSSc usually have skin sclerosis that is restricted to the hands and, to a lesser extent, the face and neck.
The patient’s antinuclear antibody test was positive with a speckled nucleolar staining pattern, which is a common finding in systemic sclerosis. Her CXR showed evidence of interstitial lung disease with a ground glass pattern. Her pulmonary function test showed a diminished diffusion capacity. Pulmonary disease is seen in all types of systemic sclerosis and not diagnostic for one type. The patient was referred to Rheumatology for further diagnosis and management.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1204-1212.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Hyperhidrosis treatment options update
SEATTLE – , David Pariser, MD, said at the annual Coastal Dermatology Symposium.
Hyperhidrosis is among the dermatological conditions that have the greatest impact on quality of life, and it can be particularly concerning to teens, said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. He referred to some new developments for an old, often misunderstood, standby: antiperspirants. “I am amazed that many people do not know the difference between an antiperspirant and a deodorant,” he said, pointing out that antiperspirants contain active ingredients – aluminum and zirconium salts – that block sweat glands, while deodorants contain a masking fragrance.
There are new-generation topical antiperspirants available over the counter, with descriptions that include “clinical strength” or “clinical protection” on the labels; they come in a box and cost about twice as much as standard products. “But they are better, and they work just as well as some of the commercial preparations,” Dr. Pariser said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
One issue he highlighted was that antiperspirants are often misapplied. They shouldn’t be applied on wet skin because they react with water to create hydrochloric acid, which can irritate the skin, he said. The best time to apply an antiperspirant is right before bedtime, since it gives the salts time to clog sweat pores before sweat or water can interfere. “The plugs last for a couple of days,” so there’s no need to worry about rinsing the product off during a morning shower, he noted.
Additional therapeutic options include agents like oral glycopyrrolate, starting at a low dose (1 mg twice per day), increasing by 1 mg/day weekly until efficacy is achieved or limited by adverse events. There is also a glycopyrrolate oral solution 1mg/5ml (Cuvposa) that can be used in children.
A topical version of the anticholinergic glycopyrronium tosylate, applied using an infused cloth, was approved for treating axillary hyperhidrosis in June2018 and offers the potential for an enhanced local anticholinergic effect. Dr. Pariser, one of the authors, discussed the recently published results of two pivotal studies that found good improvement in a specially-designed quality of life endpoint (J Am Acad Derm. 2019; Jan;80[1]:128-138.e2).
Efficacy in a subanalysis of 44 pediatric subjects (ages 9-16 years) was similar as those reported in adults, and the rate of those reporting dry mouth (24% in both age groups) was similar. Of concern was a 16% rate of mydriasis in the pediatric group, compared with 6% in the older group. One patient even wound up in the emergency room for a stroke work-up as a result, said Dr. Pariser, who is confident that the problem was caused by inadvertent exposure to the eye during application. He advises patients to avoid contact with the eyes.
Other approaches to treatment of hyperhidrosis include oxybutynin, iontophoresis, an microwave thermolysis (which may also reduce odor and hair). Endoscopic thoracic sympathectomy is effective but is the most invasive option; botulinum toxin is a minimally invasive alternative to surgery.
For those who sweat when they experience anxiety, propranolol 5-10 mg taken about 1 hour before an event that could cause hyperhydrosis can be effective, said Dr. Pariser, who recommends a test dose. “I don’t normally tell patients to try something at home. But they should try this at home” before using it prior to an important event, he added.
Dr. Pariser is a consultant and/or investigator for Dermira, Brickell Biotech, TheraVida, Atacama, TDI Surgitech, Dermavant, and Revance Therapeutics. He has not done commercial speaking, has not been on speaker’s bureaus, and has no stock or options in any company.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – , David Pariser, MD, said at the annual Coastal Dermatology Symposium.
Hyperhidrosis is among the dermatological conditions that have the greatest impact on quality of life, and it can be particularly concerning to teens, said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. He referred to some new developments for an old, often misunderstood, standby: antiperspirants. “I am amazed that many people do not know the difference between an antiperspirant and a deodorant,” he said, pointing out that antiperspirants contain active ingredients – aluminum and zirconium salts – that block sweat glands, while deodorants contain a masking fragrance.
There are new-generation topical antiperspirants available over the counter, with descriptions that include “clinical strength” or “clinical protection” on the labels; they come in a box and cost about twice as much as standard products. “But they are better, and they work just as well as some of the commercial preparations,” Dr. Pariser said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
One issue he highlighted was that antiperspirants are often misapplied. They shouldn’t be applied on wet skin because they react with water to create hydrochloric acid, which can irritate the skin, he said. The best time to apply an antiperspirant is right before bedtime, since it gives the salts time to clog sweat pores before sweat or water can interfere. “The plugs last for a couple of days,” so there’s no need to worry about rinsing the product off during a morning shower, he noted.
Additional therapeutic options include agents like oral glycopyrrolate, starting at a low dose (1 mg twice per day), increasing by 1 mg/day weekly until efficacy is achieved or limited by adverse events. There is also a glycopyrrolate oral solution 1mg/5ml (Cuvposa) that can be used in children.
A topical version of the anticholinergic glycopyrronium tosylate, applied using an infused cloth, was approved for treating axillary hyperhidrosis in June2018 and offers the potential for an enhanced local anticholinergic effect. Dr. Pariser, one of the authors, discussed the recently published results of two pivotal studies that found good improvement in a specially-designed quality of life endpoint (J Am Acad Derm. 2019; Jan;80[1]:128-138.e2).
Efficacy in a subanalysis of 44 pediatric subjects (ages 9-16 years) was similar as those reported in adults, and the rate of those reporting dry mouth (24% in both age groups) was similar. Of concern was a 16% rate of mydriasis in the pediatric group, compared with 6% in the older group. One patient even wound up in the emergency room for a stroke work-up as a result, said Dr. Pariser, who is confident that the problem was caused by inadvertent exposure to the eye during application. He advises patients to avoid contact with the eyes.
Other approaches to treatment of hyperhidrosis include oxybutynin, iontophoresis, an microwave thermolysis (which may also reduce odor and hair). Endoscopic thoracic sympathectomy is effective but is the most invasive option; botulinum toxin is a minimally invasive alternative to surgery.
For those who sweat when they experience anxiety, propranolol 5-10 mg taken about 1 hour before an event that could cause hyperhydrosis can be effective, said Dr. Pariser, who recommends a test dose. “I don’t normally tell patients to try something at home. But they should try this at home” before using it prior to an important event, he added.
Dr. Pariser is a consultant and/or investigator for Dermira, Brickell Biotech, TheraVida, Atacama, TDI Surgitech, Dermavant, and Revance Therapeutics. He has not done commercial speaking, has not been on speaker’s bureaus, and has no stock or options in any company.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – , David Pariser, MD, said at the annual Coastal Dermatology Symposium.
Hyperhidrosis is among the dermatological conditions that have the greatest impact on quality of life, and it can be particularly concerning to teens, said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. He referred to some new developments for an old, often misunderstood, standby: antiperspirants. “I am amazed that many people do not know the difference between an antiperspirant and a deodorant,” he said, pointing out that antiperspirants contain active ingredients – aluminum and zirconium salts – that block sweat glands, while deodorants contain a masking fragrance.
There are new-generation topical antiperspirants available over the counter, with descriptions that include “clinical strength” or “clinical protection” on the labels; they come in a box and cost about twice as much as standard products. “But they are better, and they work just as well as some of the commercial preparations,” Dr. Pariser said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
One issue he highlighted was that antiperspirants are often misapplied. They shouldn’t be applied on wet skin because they react with water to create hydrochloric acid, which can irritate the skin, he said. The best time to apply an antiperspirant is right before bedtime, since it gives the salts time to clog sweat pores before sweat or water can interfere. “The plugs last for a couple of days,” so there’s no need to worry about rinsing the product off during a morning shower, he noted.
Additional therapeutic options include agents like oral glycopyrrolate, starting at a low dose (1 mg twice per day), increasing by 1 mg/day weekly until efficacy is achieved or limited by adverse events. There is also a glycopyrrolate oral solution 1mg/5ml (Cuvposa) that can be used in children.
A topical version of the anticholinergic glycopyrronium tosylate, applied using an infused cloth, was approved for treating axillary hyperhidrosis in June2018 and offers the potential for an enhanced local anticholinergic effect. Dr. Pariser, one of the authors, discussed the recently published results of two pivotal studies that found good improvement in a specially-designed quality of life endpoint (J Am Acad Derm. 2019; Jan;80[1]:128-138.e2).
Efficacy in a subanalysis of 44 pediatric subjects (ages 9-16 years) was similar as those reported in adults, and the rate of those reporting dry mouth (24% in both age groups) was similar. Of concern was a 16% rate of mydriasis in the pediatric group, compared with 6% in the older group. One patient even wound up in the emergency room for a stroke work-up as a result, said Dr. Pariser, who is confident that the problem was caused by inadvertent exposure to the eye during application. He advises patients to avoid contact with the eyes.
Other approaches to treatment of hyperhidrosis include oxybutynin, iontophoresis, an microwave thermolysis (which may also reduce odor and hair). Endoscopic thoracic sympathectomy is effective but is the most invasive option; botulinum toxin is a minimally invasive alternative to surgery.
For those who sweat when they experience anxiety, propranolol 5-10 mg taken about 1 hour before an event that could cause hyperhydrosis can be effective, said Dr. Pariser, who recommends a test dose. “I don’t normally tell patients to try something at home. But they should try this at home” before using it prior to an important event, he added.
Dr. Pariser is a consultant and/or investigator for Dermira, Brickell Biotech, TheraVida, Atacama, TDI Surgitech, Dermavant, and Revance Therapeutics. He has not done commercial speaking, has not been on speaker’s bureaus, and has no stock or options in any company.
This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
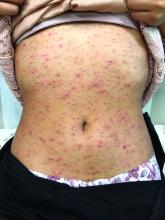
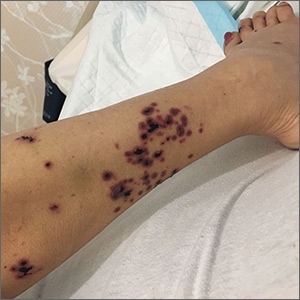
Pruritic, pink to violaceous, scaly papules
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
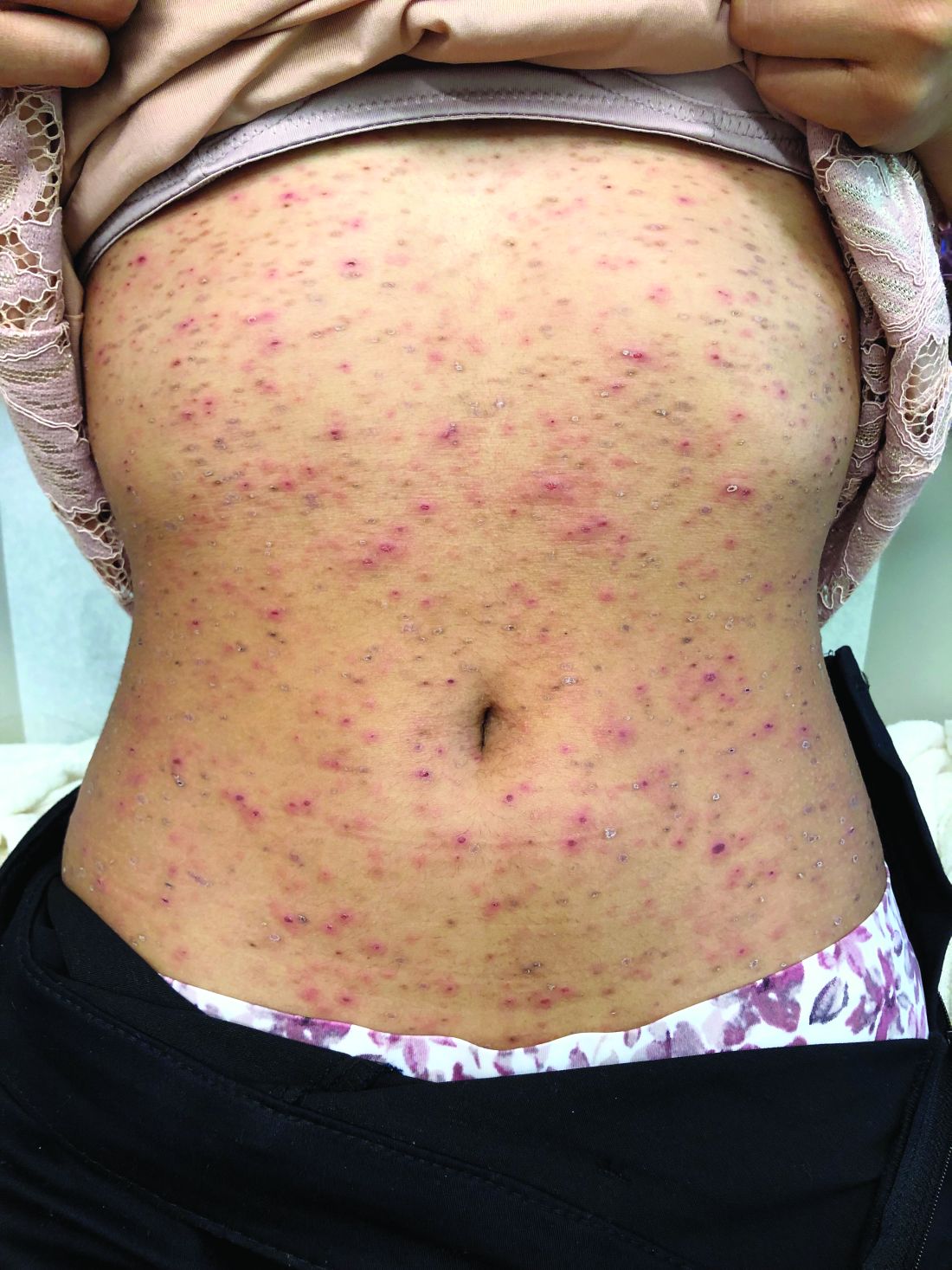
A healthy 14-year-old female was referred urgently by her pediatrician to our pediatric dermatology clinic for evaluation of a rash. The rash had been present for 4 weeks on her torso and proximal extremities, and had been spreading. She had been very itchy. She denied any fevers, chills, joint pain, oral or genital lesions.
She was visiting some family members in Washington State during the summer. The rash started 1 month after this visit.
The adolescent had been treated with acyclovir, trimethoprim/sulfamethoxazole, and intramuscular triamcinolone without improvement. She had been taking children's multivitamins occasionally. Her vaccinations were up-to-date. She denied any history of varicella or herpes infection. Her mom has a history of cold sores. The teen is not sexually active.
On physical examination, the girl was not in acute distress. Her vital signs were stable. She was not febrile. She had pink, scaly, and hyperpigmented papules and plaques, some of which were crusted with violaceous centers on the trunk and proximal extremities. There were no lesions on the mouth, palms, or soles. She had no lymphadenopathy or hepatosplenomegaly.
Short-term statin use linked to risk of skin and soft tissue infections
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
FROM THE BRITISH JOURNAL OF CLINICAL PHARMACOLOGY
Tape strips useful to identify biomarkers in skin of young children with atopic dermatitis
Adhesive according to a study published online on October 9 in JAMA Dermatology.
“Minimally invasive approaches that accurately capture key immune and barrier biomarkers in the skin of patients with early-onset pediatric AD are needed,” wrote Emma Guttman-Yassky, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Because tissue biopsies are considered the criterion standard for evaluating dysregulation in AD lesional and nonlesional skin, it is crucial to understand whether tape-strip profiling can accurately yield key AD-related biomarkers.”
In their cross-sectional study, researchers used large D-Squame tape strips to collect skin samples from 51 children under the age of 5 years (mean, 1.7-1.8 years), including 21 with moderate to severe AD and 30 controls who did not have AD. Samples were collected from lesional skin inside the crook of the elbow and nonlesional skin, on the same arm, then subjected to gene- and protein-expression analysis to identify skin biomarkers of disease.
The participants tolerated the tape stripping well, and there were no clinical effects of the procedure. The authors were able to detect mRNA in 70 of 71 samples.
They then analyzed a panel of 15 cellular markers that assessed markers of monocytes and macrophages, T cells, activated TH2 cells, dendritic cells and dendritic-cell subsets, and Langerhans cells. They found that most showed significant differences between lesional AD skin and normal skin.
They also found that levels of OX40 ligand receptor, a marker associated with atopic dendritic cells, the inducible T-cell costimulatory activation marker, CD209, CD123, and langerin protein, were also significantly higher in nonlesional AD skin.
When comparing lesional and nonlesional skin samples in the AD patients, the authors saw significant differences only in levels of colony-stimulating factor 1 and 2.
The authors noted that some of the mediators detected from the tape-strip samples had not been detected or evaluated in previous studies of the use of tape strips in AD. These included measures of cellular infiltrates, atopic dendritic cells, and key inflammatory markers.
“The novel epidermal cytokines IL [interleukin]–33 and IL-17C, which are currently targeted in clinical trials of patients with AD, were also highlighted as novel tape-strip biomarkers and demonstrated significant correlations with AD severity,” they wrote.
“Because tape stripping is painless, nonscarring, and allows repeated sampling, it may be associated with benefits for longitudinal pediatric studies and clinical trials, in which serial measures are needed to identify predictors of response, course, and comorbidities,” the authors concluded.
The study was supported by the Northwestern University Skin Disease Research Center and the Northwestern University Clinical and Translational Sciences Institute, and partly by a grant to two authors from Regeneron and Sanofi. Dr. Guttman-Yassky reported receiving grants from Regeneron during the study, and had other disclosures related to multiple pharmaceutical companies. Another author also received grants from Regeneron during the study, and another author had disclosures related to various manufacturers; no disclosures were reported for the remaining authors.
SOURCE: Guttman-Yassky E et al. JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2983.
Skin biomarkers of atopic dermatitis (AD) are not well studied in children despite the fact that the disease largely affects this age group. Part of the challenge is the difficulty obtaining samples from children because phlebotomy and skin biopsies can cause trauma and anxiety both in children and their guardians. Better, noninvasive sampling techniques are needed.
This and another recent study show that tape stripping achieves skin samples that can provide clinically relevant AD DNA-expression levels and biomarkers that have been shown in multiple other studies – including some AD biomarkers not previously reported. Importantly, these biomarkers distinguish between children with AD and those without, and even between lesional and nonlesional skin.
While it remains to be seen if these biomarkers can predict disease outcomes or response to medication, this study shows that tape stripping in children with AD is a viable and useful method for future studies.
Leslie Castelo-Soccio, MD, PhD, is with the department of dermatology at the Children’s Hospital of Philadelphia. These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2792). No conflicts of interest were reported.
Skin biomarkers of atopic dermatitis (AD) are not well studied in children despite the fact that the disease largely affects this age group. Part of the challenge is the difficulty obtaining samples from children because phlebotomy and skin biopsies can cause trauma and anxiety both in children and their guardians. Better, noninvasive sampling techniques are needed.
This and another recent study show that tape stripping achieves skin samples that can provide clinically relevant AD DNA-expression levels and biomarkers that have been shown in multiple other studies – including some AD biomarkers not previously reported. Importantly, these biomarkers distinguish between children with AD and those without, and even between lesional and nonlesional skin.
While it remains to be seen if these biomarkers can predict disease outcomes or response to medication, this study shows that tape stripping in children with AD is a viable and useful method for future studies.
Leslie Castelo-Soccio, MD, PhD, is with the department of dermatology at the Children’s Hospital of Philadelphia. These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2792). No conflicts of interest were reported.
Skin biomarkers of atopic dermatitis (AD) are not well studied in children despite the fact that the disease largely affects this age group. Part of the challenge is the difficulty obtaining samples from children because phlebotomy and skin biopsies can cause trauma and anxiety both in children and their guardians. Better, noninvasive sampling techniques are needed.
This and another recent study show that tape stripping achieves skin samples that can provide clinically relevant AD DNA-expression levels and biomarkers that have been shown in multiple other studies – including some AD biomarkers not previously reported. Importantly, these biomarkers distinguish between children with AD and those without, and even between lesional and nonlesional skin.
While it remains to be seen if these biomarkers can predict disease outcomes or response to medication, this study shows that tape stripping in children with AD is a viable and useful method for future studies.
Leslie Castelo-Soccio, MD, PhD, is with the department of dermatology at the Children’s Hospital of Philadelphia. These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2792). No conflicts of interest were reported.
Adhesive according to a study published online on October 9 in JAMA Dermatology.
“Minimally invasive approaches that accurately capture key immune and barrier biomarkers in the skin of patients with early-onset pediatric AD are needed,” wrote Emma Guttman-Yassky, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Because tissue biopsies are considered the criterion standard for evaluating dysregulation in AD lesional and nonlesional skin, it is crucial to understand whether tape-strip profiling can accurately yield key AD-related biomarkers.”
In their cross-sectional study, researchers used large D-Squame tape strips to collect skin samples from 51 children under the age of 5 years (mean, 1.7-1.8 years), including 21 with moderate to severe AD and 30 controls who did not have AD. Samples were collected from lesional skin inside the crook of the elbow and nonlesional skin, on the same arm, then subjected to gene- and protein-expression analysis to identify skin biomarkers of disease.
The participants tolerated the tape stripping well, and there were no clinical effects of the procedure. The authors were able to detect mRNA in 70 of 71 samples.
They then analyzed a panel of 15 cellular markers that assessed markers of monocytes and macrophages, T cells, activated TH2 cells, dendritic cells and dendritic-cell subsets, and Langerhans cells. They found that most showed significant differences between lesional AD skin and normal skin.
They also found that levels of OX40 ligand receptor, a marker associated with atopic dendritic cells, the inducible T-cell costimulatory activation marker, CD209, CD123, and langerin protein, were also significantly higher in nonlesional AD skin.
When comparing lesional and nonlesional skin samples in the AD patients, the authors saw significant differences only in levels of colony-stimulating factor 1 and 2.
The authors noted that some of the mediators detected from the tape-strip samples had not been detected or evaluated in previous studies of the use of tape strips in AD. These included measures of cellular infiltrates, atopic dendritic cells, and key inflammatory markers.
“The novel epidermal cytokines IL [interleukin]–33 and IL-17C, which are currently targeted in clinical trials of patients with AD, were also highlighted as novel tape-strip biomarkers and demonstrated significant correlations with AD severity,” they wrote.
“Because tape stripping is painless, nonscarring, and allows repeated sampling, it may be associated with benefits for longitudinal pediatric studies and clinical trials, in which serial measures are needed to identify predictors of response, course, and comorbidities,” the authors concluded.
The study was supported by the Northwestern University Skin Disease Research Center and the Northwestern University Clinical and Translational Sciences Institute, and partly by a grant to two authors from Regeneron and Sanofi. Dr. Guttman-Yassky reported receiving grants from Regeneron during the study, and had other disclosures related to multiple pharmaceutical companies. Another author also received grants from Regeneron during the study, and another author had disclosures related to various manufacturers; no disclosures were reported for the remaining authors.
SOURCE: Guttman-Yassky E et al. JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2983.
Adhesive according to a study published online on October 9 in JAMA Dermatology.
“Minimally invasive approaches that accurately capture key immune and barrier biomarkers in the skin of patients with early-onset pediatric AD are needed,” wrote Emma Guttman-Yassky, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Because tissue biopsies are considered the criterion standard for evaluating dysregulation in AD lesional and nonlesional skin, it is crucial to understand whether tape-strip profiling can accurately yield key AD-related biomarkers.”
In their cross-sectional study, researchers used large D-Squame tape strips to collect skin samples from 51 children under the age of 5 years (mean, 1.7-1.8 years), including 21 with moderate to severe AD and 30 controls who did not have AD. Samples were collected from lesional skin inside the crook of the elbow and nonlesional skin, on the same arm, then subjected to gene- and protein-expression analysis to identify skin biomarkers of disease.
The participants tolerated the tape stripping well, and there were no clinical effects of the procedure. The authors were able to detect mRNA in 70 of 71 samples.
They then analyzed a panel of 15 cellular markers that assessed markers of monocytes and macrophages, T cells, activated TH2 cells, dendritic cells and dendritic-cell subsets, and Langerhans cells. They found that most showed significant differences between lesional AD skin and normal skin.
They also found that levels of OX40 ligand receptor, a marker associated with atopic dendritic cells, the inducible T-cell costimulatory activation marker, CD209, CD123, and langerin protein, were also significantly higher in nonlesional AD skin.
When comparing lesional and nonlesional skin samples in the AD patients, the authors saw significant differences only in levels of colony-stimulating factor 1 and 2.
The authors noted that some of the mediators detected from the tape-strip samples had not been detected or evaluated in previous studies of the use of tape strips in AD. These included measures of cellular infiltrates, atopic dendritic cells, and key inflammatory markers.
“The novel epidermal cytokines IL [interleukin]–33 and IL-17C, which are currently targeted in clinical trials of patients with AD, were also highlighted as novel tape-strip biomarkers and demonstrated significant correlations with AD severity,” they wrote.
“Because tape stripping is painless, nonscarring, and allows repeated sampling, it may be associated with benefits for longitudinal pediatric studies and clinical trials, in which serial measures are needed to identify predictors of response, course, and comorbidities,” the authors concluded.
The study was supported by the Northwestern University Skin Disease Research Center and the Northwestern University Clinical and Translational Sciences Institute, and partly by a grant to two authors from Regeneron and Sanofi. Dr. Guttman-Yassky reported receiving grants from Regeneron during the study, and had other disclosures related to multiple pharmaceutical companies. Another author also received grants from Regeneron during the study, and another author had disclosures related to various manufacturers; no disclosures were reported for the remaining authors.
SOURCE: Guttman-Yassky E et al. JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2983.
FROM JAMA DERMATOLOGY
Start From Scratch
A 50-year-old man presents with complaints of a rash on his right leg that manifested 20 years ago. Although the rash is worrisome, he says the associated pruritus is worse. During the workday, he is able to ignore the itching—but the minute he gets home, he begins to scratch.
He knows the scratching is counterproductive in the long run, but the urge to do it is quite compelling. Sometimes he uses a wet washcloth; other times, he will actually use a hair brush to satiate the itching. The relief is intensely satisfying albeit short lived.
The rash has persisted despite multiple treatment attempts. Tried products include OTC moisturizers and antifungal creams, as well as prescription antifungal creams. None has had an effect.
The patient denies any other skin problems. He does recall having eczema as a child. Although that has long since resolved, he remains quite allergy prone and is particularly sensitive to airborne allergens—a trait that runs strongly in his family.
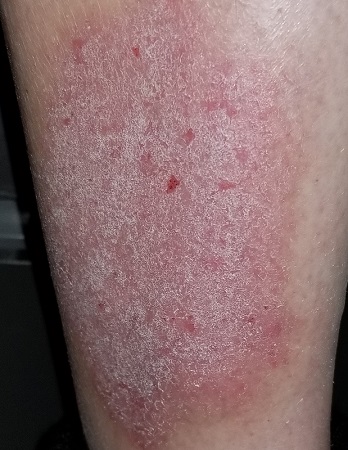
EXAMINATION
A pink, oval rash covers most of the patient’s right lateral calf. It has a thickened, faintly scaly surface that is uniform and sharply circumscribed. There is no increased warmth or tenderness on palpation. No lymph nodes can be felt in the right groin. A check of the patient’s knees, elbows, scalp, nails, and trunk show no sign of rash or other changes.
What’s the diagnosis?
DISCUSSION
Lichen simplex chronicus (LSC), previously known as neurodermatitis, is quite common but frequently misdiagnosed. Patients often report that their condition started with a bug bite or poison ivy—a provocation that gets the patient in the habit of scratching, which continues long after the initial outbreak has subsided. Thus, LSC is often associated with significant chronicity, as typified by this case.
What patients seldom understand is their own role in the perpetuation of their condition. The urge to scratch is so unbearable that few can resist it. Over time, the scratching creates more nerves that have a lower threshold for itching, and thus the itch-scratch-itch cycle is born. Many LSC patients are atopic, which predisposes them to itching in general and to xerosis especially.
The literature asserts that LSC affects the genders equally, but this ignores the fact that it can present significantly differently in men and women. This patient’s area of involvement is quite typical for men, most of whom never moisturize and for whom the lateral calf is readily accessible. In the author’s experience, the most common location for LSC in women is the nuchal scalp, where heat and sweat appear to play a role, along with ready accessibility to fingernails or the sharp end of a pencil. Other common areas of involvement include the dorsal forearms and the scrotum or vulvae.
Biopsy is seldom necessary, but if performed, it will show a marked thickening of the epidermis, orthokeratosis (normal keratinocytes about to shed), and compacted, elongated rete ridges. These and other changes effectively rule out other items in the differential (eg, psoriasis, simple eczema, fungal infection).
Stopping the itch-scratch-itch cycle with mid-strength topical steroid creams or foams is the first step in treating LSC. But then the patient must be convinced of his contribution to the treatment: leaving the affected sites alone. Truth be told, after 20 years of scratching, the best this patient can look forward to is some relief—not only from the itching, but also from concern about all the terrible things he now knows he doesn’t have.
TAKE-HOME LEARNING POINTS
- Lichen simplex chronicus is quite common in both genders and is typified by longstanding severe itching, usually confined to one area.
- Atopy, xerosis, and stress all appear to contribute to the problem.
- Stopping the itch-scratch-itch cycle with topical steroids is a key component of treatment.
- Patient education—on the nature of the problem and the patient’s role in controlling it—is just as important as any prescribed medication.
A 50-year-old man presents with complaints of a rash on his right leg that manifested 20 years ago. Although the rash is worrisome, he says the associated pruritus is worse. During the workday, he is able to ignore the itching—but the minute he gets home, he begins to scratch.
He knows the scratching is counterproductive in the long run, but the urge to do it is quite compelling. Sometimes he uses a wet washcloth; other times, he will actually use a hair brush to satiate the itching. The relief is intensely satisfying albeit short lived.
The rash has persisted despite multiple treatment attempts. Tried products include OTC moisturizers and antifungal creams, as well as prescription antifungal creams. None has had an effect.
The patient denies any other skin problems. He does recall having eczema as a child. Although that has long since resolved, he remains quite allergy prone and is particularly sensitive to airborne allergens—a trait that runs strongly in his family.

EXAMINATION
A pink, oval rash covers most of the patient’s right lateral calf. It has a thickened, faintly scaly surface that is uniform and sharply circumscribed. There is no increased warmth or tenderness on palpation. No lymph nodes can be felt in the right groin. A check of the patient’s knees, elbows, scalp, nails, and trunk show no sign of rash or other changes.
What’s the diagnosis?
DISCUSSION
Lichen simplex chronicus (LSC), previously known as neurodermatitis, is quite common but frequently misdiagnosed. Patients often report that their condition started with a bug bite or poison ivy—a provocation that gets the patient in the habit of scratching, which continues long after the initial outbreak has subsided. Thus, LSC is often associated with significant chronicity, as typified by this case.
What patients seldom understand is their own role in the perpetuation of their condition. The urge to scratch is so unbearable that few can resist it. Over time, the scratching creates more nerves that have a lower threshold for itching, and thus the itch-scratch-itch cycle is born. Many LSC patients are atopic, which predisposes them to itching in general and to xerosis especially.
The literature asserts that LSC affects the genders equally, but this ignores the fact that it can present significantly differently in men and women. This patient’s area of involvement is quite typical for men, most of whom never moisturize and for whom the lateral calf is readily accessible. In the author’s experience, the most common location for LSC in women is the nuchal scalp, where heat and sweat appear to play a role, along with ready accessibility to fingernails or the sharp end of a pencil. Other common areas of involvement include the dorsal forearms and the scrotum or vulvae.
Biopsy is seldom necessary, but if performed, it will show a marked thickening of the epidermis, orthokeratosis (normal keratinocytes about to shed), and compacted, elongated rete ridges. These and other changes effectively rule out other items in the differential (eg, psoriasis, simple eczema, fungal infection).
Stopping the itch-scratch-itch cycle with mid-strength topical steroid creams or foams is the first step in treating LSC. But then the patient must be convinced of his contribution to the treatment: leaving the affected sites alone. Truth be told, after 20 years of scratching, the best this patient can look forward to is some relief—not only from the itching, but also from concern about all the terrible things he now knows he doesn’t have.
TAKE-HOME LEARNING POINTS
- Lichen simplex chronicus is quite common in both genders and is typified by longstanding severe itching, usually confined to one area.
- Atopy, xerosis, and stress all appear to contribute to the problem.
- Stopping the itch-scratch-itch cycle with topical steroids is a key component of treatment.
- Patient education—on the nature of the problem and the patient’s role in controlling it—is just as important as any prescribed medication.
A 50-year-old man presents with complaints of a rash on his right leg that manifested 20 years ago. Although the rash is worrisome, he says the associated pruritus is worse. During the workday, he is able to ignore the itching—but the minute he gets home, he begins to scratch.
He knows the scratching is counterproductive in the long run, but the urge to do it is quite compelling. Sometimes he uses a wet washcloth; other times, he will actually use a hair brush to satiate the itching. The relief is intensely satisfying albeit short lived.
The rash has persisted despite multiple treatment attempts. Tried products include OTC moisturizers and antifungal creams, as well as prescription antifungal creams. None has had an effect.
The patient denies any other skin problems. He does recall having eczema as a child. Although that has long since resolved, he remains quite allergy prone and is particularly sensitive to airborne allergens—a trait that runs strongly in his family.

EXAMINATION
A pink, oval rash covers most of the patient’s right lateral calf. It has a thickened, faintly scaly surface that is uniform and sharply circumscribed. There is no increased warmth or tenderness on palpation. No lymph nodes can be felt in the right groin. A check of the patient’s knees, elbows, scalp, nails, and trunk show no sign of rash or other changes.
What’s the diagnosis?
DISCUSSION
Lichen simplex chronicus (LSC), previously known as neurodermatitis, is quite common but frequently misdiagnosed. Patients often report that their condition started with a bug bite or poison ivy—a provocation that gets the patient in the habit of scratching, which continues long after the initial outbreak has subsided. Thus, LSC is often associated with significant chronicity, as typified by this case.
What patients seldom understand is their own role in the perpetuation of their condition. The urge to scratch is so unbearable that few can resist it. Over time, the scratching creates more nerves that have a lower threshold for itching, and thus the itch-scratch-itch cycle is born. Many LSC patients are atopic, which predisposes them to itching in general and to xerosis especially.
The literature asserts that LSC affects the genders equally, but this ignores the fact that it can present significantly differently in men and women. This patient’s area of involvement is quite typical for men, most of whom never moisturize and for whom the lateral calf is readily accessible. In the author’s experience, the most common location for LSC in women is the nuchal scalp, where heat and sweat appear to play a role, along with ready accessibility to fingernails or the sharp end of a pencil. Other common areas of involvement include the dorsal forearms and the scrotum or vulvae.
Biopsy is seldom necessary, but if performed, it will show a marked thickening of the epidermis, orthokeratosis (normal keratinocytes about to shed), and compacted, elongated rete ridges. These and other changes effectively rule out other items in the differential (eg, psoriasis, simple eczema, fungal infection).
Stopping the itch-scratch-itch cycle with mid-strength topical steroid creams or foams is the first step in treating LSC. But then the patient must be convinced of his contribution to the treatment: leaving the affected sites alone. Truth be told, after 20 years of scratching, the best this patient can look forward to is some relief—not only from the itching, but also from concern about all the terrible things he now knows he doesn’t have.
TAKE-HOME LEARNING POINTS
- Lichen simplex chronicus is quite common in both genders and is typified by longstanding severe itching, usually confined to one area.
- Atopy, xerosis, and stress all appear to contribute to the problem.
- Stopping the itch-scratch-itch cycle with topical steroids is a key component of treatment.
- Patient education—on the nature of the problem and the patient’s role in controlling it—is just as important as any prescribed medication.
One-year data support dupilumab’s efficacy and safety in adolescents with AD
A study of and continued evidence of efficacy for up to 52 weeks, reported the authors of the study, published online Oct. 9 in the British Journal of Dermatology.
The phase 2a open-label, ascending-dose cohort study of dupilumab in 40 adolescents with moderate to severe AD was followed by a 48-week phase 3 open-label extension study in 36 of those participants. Dupilumab is a monoclonal antibody that inhibits signaling of interleukin (IL)-4 and IL-13.
In the phase 2a study, participants were treated with a single subcutaneous dose of dupilumab – either 2 mg/kg or 4 mg/kg – and had 8 weeks of pharmacokinetic sampling. They subsequently received that same dose weekly for 4 weeks, with an 8-week-long safety follow-up period. Those who participated in the open-label extension continued their weekly dose to a maximum of 300 mg. per kg
The most common treatment-emergent adverse events (a primary endpoint) seen in both the phase 2a and phase 3 studies were nasopharyngitis and exacerbation of AD – in the phase 2a study, exacerbations were seen in the period when patients weren’t taking the treatment. In the 2-mg and 4-mg groups, the incidence of skin infections was 29% and 42%, respectively, and the incidence of injection site reactions – which were mostly mild – were 18% and 11%, respectively. Researchers also noted conjunctivitis in 18% and 16% of the patients in the 2-mg and 4-mg groups, respectively, but none of the cases were considered serious and all resolved over the course of the study. In the phase 2a study, 50% of patients on the 2-mg/kg dose and 65% of those on the 4-mg/kg dose experienced an adverse event, while in the open-label extension all reported at least one adverse event.
There was one case of suicidal behavior and one case of systemic or severe hypersensitivity reported in the 2-mg/kg groups, both of which were considered adverse events of special interest. There were no deaths.
However none of the serious adverse events – which included infected AD, palpitations, patent ductus arteriosus, and food allergy – were linked to the study treatment, and no adverse events led to study discontinuation, the authors reported.
By week 12, 70% of participants in the 2-mg/kg group and 75% in the 4-mg/kg group had achieved a 50% or greater improvement in their Eczema Area and Severity Index (EASI) scores, which was a secondary outcome. By week 52, that had increased to 100% and 89% respectively.
More than half the patients (55%) in the 2-mg/kg group, and 40% of those in the 4-mg/kg group achieved a 75% or more improvement in their EASI scores by week 12, which increased to 88% and 78%, respectively, by week 52 in the open label phase.
“The results from these studies support use of dupilumab for the long-term management of moderate to severe AD in adolescents,” wrote Michael J. Cork, MD, professor of dermatology, University of Sheffield, England, and coauthors. No new safety signals were identified, “compared with the known safety profile of dupilumab in adults with moderate to severe AD,” and “the PK profile was characterized by nonlinear, target-mediated kinetics, consistent with the profile in adults with moderate to severe AD,” they added.
Dupilumab was approved in the United States in March 2019 for adolescents with moderate to severe AD whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The study was sponsored by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals, which market dupilumab as Dupixent in the United States. Dr. Cork disclosures included those related to Sanofi Genzyme and Regeneron; other authors included employees of the companies.
SOURCE: Cork M et al. Br J Dermatol. 2019 Oct 9. doi: 10.1111/bjd.18476.
A study of and continued evidence of efficacy for up to 52 weeks, reported the authors of the study, published online Oct. 9 in the British Journal of Dermatology.
The phase 2a open-label, ascending-dose cohort study of dupilumab in 40 adolescents with moderate to severe AD was followed by a 48-week phase 3 open-label extension study in 36 of those participants. Dupilumab is a monoclonal antibody that inhibits signaling of interleukin (IL)-4 and IL-13.
In the phase 2a study, participants were treated with a single subcutaneous dose of dupilumab – either 2 mg/kg or 4 mg/kg – and had 8 weeks of pharmacokinetic sampling. They subsequently received that same dose weekly for 4 weeks, with an 8-week-long safety follow-up period. Those who participated in the open-label extension continued their weekly dose to a maximum of 300 mg. per kg
The most common treatment-emergent adverse events (a primary endpoint) seen in both the phase 2a and phase 3 studies were nasopharyngitis and exacerbation of AD – in the phase 2a study, exacerbations were seen in the period when patients weren’t taking the treatment. In the 2-mg and 4-mg groups, the incidence of skin infections was 29% and 42%, respectively, and the incidence of injection site reactions – which were mostly mild – were 18% and 11%, respectively. Researchers also noted conjunctivitis in 18% and 16% of the patients in the 2-mg and 4-mg groups, respectively, but none of the cases were considered serious and all resolved over the course of the study. In the phase 2a study, 50% of patients on the 2-mg/kg dose and 65% of those on the 4-mg/kg dose experienced an adverse event, while in the open-label extension all reported at least one adverse event.
There was one case of suicidal behavior and one case of systemic or severe hypersensitivity reported in the 2-mg/kg groups, both of which were considered adverse events of special interest. There were no deaths.
However none of the serious adverse events – which included infected AD, palpitations, patent ductus arteriosus, and food allergy – were linked to the study treatment, and no adverse events led to study discontinuation, the authors reported.
By week 12, 70% of participants in the 2-mg/kg group and 75% in the 4-mg/kg group had achieved a 50% or greater improvement in their Eczema Area and Severity Index (EASI) scores, which was a secondary outcome. By week 52, that had increased to 100% and 89% respectively.
More than half the patients (55%) in the 2-mg/kg group, and 40% of those in the 4-mg/kg group achieved a 75% or more improvement in their EASI scores by week 12, which increased to 88% and 78%, respectively, by week 52 in the open label phase.
“The results from these studies support use of dupilumab for the long-term management of moderate to severe AD in adolescents,” wrote Michael J. Cork, MD, professor of dermatology, University of Sheffield, England, and coauthors. No new safety signals were identified, “compared with the known safety profile of dupilumab in adults with moderate to severe AD,” and “the PK profile was characterized by nonlinear, target-mediated kinetics, consistent with the profile in adults with moderate to severe AD,” they added.
Dupilumab was approved in the United States in March 2019 for adolescents with moderate to severe AD whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The study was sponsored by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals, which market dupilumab as Dupixent in the United States. Dr. Cork disclosures included those related to Sanofi Genzyme and Regeneron; other authors included employees of the companies.
SOURCE: Cork M et al. Br J Dermatol. 2019 Oct 9. doi: 10.1111/bjd.18476.
A study of and continued evidence of efficacy for up to 52 weeks, reported the authors of the study, published online Oct. 9 in the British Journal of Dermatology.
The phase 2a open-label, ascending-dose cohort study of dupilumab in 40 adolescents with moderate to severe AD was followed by a 48-week phase 3 open-label extension study in 36 of those participants. Dupilumab is a monoclonal antibody that inhibits signaling of interleukin (IL)-4 and IL-13.
In the phase 2a study, participants were treated with a single subcutaneous dose of dupilumab – either 2 mg/kg or 4 mg/kg – and had 8 weeks of pharmacokinetic sampling. They subsequently received that same dose weekly for 4 weeks, with an 8-week-long safety follow-up period. Those who participated in the open-label extension continued their weekly dose to a maximum of 300 mg. per kg
The most common treatment-emergent adverse events (a primary endpoint) seen in both the phase 2a and phase 3 studies were nasopharyngitis and exacerbation of AD – in the phase 2a study, exacerbations were seen in the period when patients weren’t taking the treatment. In the 2-mg and 4-mg groups, the incidence of skin infections was 29% and 42%, respectively, and the incidence of injection site reactions – which were mostly mild – were 18% and 11%, respectively. Researchers also noted conjunctivitis in 18% and 16% of the patients in the 2-mg and 4-mg groups, respectively, but none of the cases were considered serious and all resolved over the course of the study. In the phase 2a study, 50% of patients on the 2-mg/kg dose and 65% of those on the 4-mg/kg dose experienced an adverse event, while in the open-label extension all reported at least one adverse event.
There was one case of suicidal behavior and one case of systemic or severe hypersensitivity reported in the 2-mg/kg groups, both of which were considered adverse events of special interest. There were no deaths.
However none of the serious adverse events – which included infected AD, palpitations, patent ductus arteriosus, and food allergy – were linked to the study treatment, and no adverse events led to study discontinuation, the authors reported.
By week 12, 70% of participants in the 2-mg/kg group and 75% in the 4-mg/kg group had achieved a 50% or greater improvement in their Eczema Area and Severity Index (EASI) scores, which was a secondary outcome. By week 52, that had increased to 100% and 89% respectively.
More than half the patients (55%) in the 2-mg/kg group, and 40% of those in the 4-mg/kg group achieved a 75% or more improvement in their EASI scores by week 12, which increased to 88% and 78%, respectively, by week 52 in the open label phase.
“The results from these studies support use of dupilumab for the long-term management of moderate to severe AD in adolescents,” wrote Michael J. Cork, MD, professor of dermatology, University of Sheffield, England, and coauthors. No new safety signals were identified, “compared with the known safety profile of dupilumab in adults with moderate to severe AD,” and “the PK profile was characterized by nonlinear, target-mediated kinetics, consistent with the profile in adults with moderate to severe AD,” they added.
Dupilumab was approved in the United States in March 2019 for adolescents with moderate to severe AD whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The study was sponsored by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals, which market dupilumab as Dupixent in the United States. Dr. Cork disclosures included those related to Sanofi Genzyme and Regeneron; other authors included employees of the companies.
SOURCE: Cork M et al. Br J Dermatol. 2019 Oct 9. doi: 10.1111/bjd.18476.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
FDA approves afamelanotide for treatment of rare condition with light-induced pain
The Food and Drug Administration has approved , a rare condition that causes extremely painful reactions when skin is exposed to light, according to an FDA announcement.
This is the first treatment approved to help patients with this condition increase their exposure to light, according to the release.
Afamelanotide, administered in a subcutaneous implant, is a melanocortin-1 receptor (MC1-R) agonist, which “increases the production of eumelanin in the skin independent of exposure to sunlight or artificial light sources,” the release says.
Approval is based on a pair of parallel-group clinical trials that compared the number of hours spent in sunlight in the treatment and placebo groups. The first trial enrolled 93 patients; 48 received afamelanotide. The treated patients spent a median of 61 hours in total over 180 days in direct sunlight between 10 a.m. and 6 p.m. on days with no pain, compared with 41 hours for patients taking placebo.
The second trial assessed the total number of hours over 270 days spent outdoors between 10 a.m. and 3 p.m. on days with no pain for which “most of the day” was spent in direct sunlight. In this study, 38 patients treated with afamelanotide spent a median total of 6 hours, compared with 0.75 hours among the remaining 36 who were taking a placebo.
The most common side effects include implant site reaction, nausea, and oropharyngeal pain. The implant should be administered only by trained professionals. Because afamelanotide may cause skin darkening, it’s recommended that patients should undergo twice-yearly skin examinations. Patients are also encouraged to maintain sun protection measures to help prevent phototoxic reactions.
“Today’s approval is one example of the FDA’s ongoing commitment to encourage industry innovation of therapies to treat rare diseases, and work with drug developers to make promising new therapies available to patients as safely and efficiently as possible,” said Julie Beitz, MD, director of FDA’s Center for Drug Evaluation and Research Office of Drug Evaluation III in the FDA release.
The Food and Drug Administration has approved , a rare condition that causes extremely painful reactions when skin is exposed to light, according to an FDA announcement.
This is the first treatment approved to help patients with this condition increase their exposure to light, according to the release.
Afamelanotide, administered in a subcutaneous implant, is a melanocortin-1 receptor (MC1-R) agonist, which “increases the production of eumelanin in the skin independent of exposure to sunlight or artificial light sources,” the release says.
Approval is based on a pair of parallel-group clinical trials that compared the number of hours spent in sunlight in the treatment and placebo groups. The first trial enrolled 93 patients; 48 received afamelanotide. The treated patients spent a median of 61 hours in total over 180 days in direct sunlight between 10 a.m. and 6 p.m. on days with no pain, compared with 41 hours for patients taking placebo.
The second trial assessed the total number of hours over 270 days spent outdoors between 10 a.m. and 3 p.m. on days with no pain for which “most of the day” was spent in direct sunlight. In this study, 38 patients treated with afamelanotide spent a median total of 6 hours, compared with 0.75 hours among the remaining 36 who were taking a placebo.
The most common side effects include implant site reaction, nausea, and oropharyngeal pain. The implant should be administered only by trained professionals. Because afamelanotide may cause skin darkening, it’s recommended that patients should undergo twice-yearly skin examinations. Patients are also encouraged to maintain sun protection measures to help prevent phototoxic reactions.
“Today’s approval is one example of the FDA’s ongoing commitment to encourage industry innovation of therapies to treat rare diseases, and work with drug developers to make promising new therapies available to patients as safely and efficiently as possible,” said Julie Beitz, MD, director of FDA’s Center for Drug Evaluation and Research Office of Drug Evaluation III in the FDA release.
The Food and Drug Administration has approved , a rare condition that causes extremely painful reactions when skin is exposed to light, according to an FDA announcement.
This is the first treatment approved to help patients with this condition increase their exposure to light, according to the release.
Afamelanotide, administered in a subcutaneous implant, is a melanocortin-1 receptor (MC1-R) agonist, which “increases the production of eumelanin in the skin independent of exposure to sunlight or artificial light sources,” the release says.
Approval is based on a pair of parallel-group clinical trials that compared the number of hours spent in sunlight in the treatment and placebo groups. The first trial enrolled 93 patients; 48 received afamelanotide. The treated patients spent a median of 61 hours in total over 180 days in direct sunlight between 10 a.m. and 6 p.m. on days with no pain, compared with 41 hours for patients taking placebo.
The second trial assessed the total number of hours over 270 days spent outdoors between 10 a.m. and 3 p.m. on days with no pain for which “most of the day” was spent in direct sunlight. In this study, 38 patients treated with afamelanotide spent a median total of 6 hours, compared with 0.75 hours among the remaining 36 who were taking a placebo.
The most common side effects include implant site reaction, nausea, and oropharyngeal pain. The implant should be administered only by trained professionals. Because afamelanotide may cause skin darkening, it’s recommended that patients should undergo twice-yearly skin examinations. Patients are also encouraged to maintain sun protection measures to help prevent phototoxic reactions.
“Today’s approval is one example of the FDA’s ongoing commitment to encourage industry innovation of therapies to treat rare diseases, and work with drug developers to make promising new therapies available to patients as safely and efficiently as possible,” said Julie Beitz, MD, director of FDA’s Center for Drug Evaluation and Research Office of Drug Evaluation III in the FDA release.
Persistent rash on the sole
A 52-year-old Chinese woman presented to a tertiary hospital in Singapore with a 3-month history of persistent and intermittently painful rashes over her right calf and foot (FIGURE). The patient had pancytopenia due to ongoing chemotherapy for metastatic nasopharyngeal carcinoma. She was systemically well and denied other dermatoses. Examination demonstrated scattered crops of tense hemorrhagic vesicles, each surrounded by a livid purpuric base, over the right plantar aspect of the foot, with areas of eschar over the right medial hallux. No allodynia, hyperaesthesia, or lymphadenopathy was noted.
A punch biopsy of an intact vesicle was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis:
Herpes zoster
Histopathologic examination showed full-thickness epidermal necrosis with ballooning degeneration resulting in an intra-epidermal blister. Multinucleated keratinocytes with nuclear moulding were seen within the blister cavity. Grocott-Gomori methenamine-silver (GMS), acid-fast, and Gram stains were negative. Granular immunoglobulin (Ig) G, IgM, and C3 were seen intramurally. DNA analysis of vesicular fluid was positive for varicella zoster virus (VZV). A diagnosis of herpes zoster (HZ) of the right S1 dermatome with primary obliterative vasculitis was established.
Immunocompromised people—those who have impaired T-cell immunity (eg, recipients of organ or hematopoietic stem-cell transplants), take immunosuppressive therapy, or have lymphoma, leukemia, or human immunodeficiency virus (HIV) infection—have an increased risk for HZ. For example, in patients with acquired immunodeficiency syndrome (AIDS), HZ uniquely manifests as recurrent shingles. An estimated 20% to 30% of HIV-infected patients will have more than 1 episode of HZ, which may involve the same or different dermatomes.1,2 Furthermore, HZ in this population is more commonly associated with atypical presentations.3
What an atypical presentation may look like
In immunocompromised patients, HZ may present with atypical cutaneous manifestations or with atypical generalized symptoms.
Atypical cutaneous manifestations, as in disseminated zoster, manifest with multiple hyperkeratotic papules (3-20 mm in diameter) that follow no dermatomal pattern. These lesions may be chronic, persisting for months or years, and may be associated with acyclovir-resistant strains of VZV.2,3 Another dermatologic variant is ecthymatous VZV, which manifests with multiple large (10-30 mm) punched-out ulcerations with a central black eschar and a peripheral rim of vesicles.4 Viral folliculitis—in which infection is limited to the hair follicle, with no associated blisters—has also been reported in atypical HZ.5
Our patient presented with hemorrhagic vesicles mimicking vasculitic lesions, which had persisted over a 3-month period with intermittent localized pain. It has been proposed that in atypical presentations, the reactivated VZV spreads transaxonally from adjacent nerves to the outermost adventitial layer of the arterial wall, leading to a vasculitic appearance of the vesicles.6 Viral-induced vasculitis may also result either directly from infection of the blood vessels or secondary to vascular damage from an inflammatory immune complex–mediated reaction, cell-mediated hypersensitivity, or inflammation due to immune dysregulation.7,8
Continue to: Differential includes vesiculobullous conditions
Differential includes vesiculobullous conditions
There are several important items to consider in the differential.
Cutaneous vasculitis, in severe cases, may manifest with vesicles or bullae that resemble the lesions seen in HZ. However, its unilateral nature and distribution distinguish it.
Angioinvasive fungal infections in immunocompromised patients may manifest with scattered ulceronecrotic lesions to purpuric vesiculobullous dermatoses.9 However, no fungal organisms were seen on GMS staining of the biopsied tissue.
Atypical hand-foot-and-mouth disease tends to affect adults and is associated with Coxsackievirus A6 infection.10 It may manifest as generalized vesiculobullous exanthem resembling varicella. The chronic nature and restricted extent of the patient’s rash made this diagnosis unlikely.
Successful management depends on timely identification
Although most cases of HZ can be diagnosed clinically, atypical rashes may require a biopsy and direct immunofluorescence assay for VZV antigen or a polymerase-chain-reaction (PCR) assay for VZV DNA in cells from the base of blisters. Therefore, it is important to consider the diagnosis of HZ in immunocompromised patients presenting with an atypical rash to avoid misdiagnosis and costly testing.
Continue to: Our patient was treated...
Our patient was treated with oral acyclovir 800 mg 5 times/day for 10 days, with prompt resolution of her rash.
CORRESPONDENCE
Joel Hua-Liang Lim, MBBS, MRCP, MMed, 1 Mandalay Road, Singapore 308205; [email protected]
1. LeBoit PE, Limova M, Yen TS, et al. Chronic verrucous varicella-zoster virus infection in patients with the acquired immunodeficiency syndrome (AIDS): histologic and molecular biologic findings. Am J Dermatopathol. 1992;14:1-7.
2. Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
3. Weinberg JM, Mysliwiec A, Turiansky GW, et al. Viral folliculitis: atypical presentations of herpes simplex, herpes zoster, and molluscum contagiosum. Arch Dermatol. 1997;133:983-986.
4. Gilson IH, Barnett JH, Conant MA, et al. Disseminated ecthymatous herpes varicella zoster virus infection in patients with acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20:637-642.
5. Løkke BJ, Weismann K, Mathiesen L, et al. Atypical varicella-zoster infection in AIDS. Acta Derm Venereol. 1993;73:123-125.
6. Uhoda I, Piérard-Franchimont C, Piérard GE. Varicella-zoster virus vasculitis: a case of recurrent varicella without epidermal involvement. Dermatology. 2000;200:173-175.
7. Teng GG, Chatham WW. Vasculitis related to viral and other microbial agents. Best Pract Res Clin Rheumatol. 2015;29:226-243.
8. Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16:12.
9. Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1-53.
10. Lott JP, Liu K, Landry M-L, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
A 52-year-old Chinese woman presented to a tertiary hospital in Singapore with a 3-month history of persistent and intermittently painful rashes over her right calf and foot (FIGURE). The patient had pancytopenia due to ongoing chemotherapy for metastatic nasopharyngeal carcinoma. She was systemically well and denied other dermatoses. Examination demonstrated scattered crops of tense hemorrhagic vesicles, each surrounded by a livid purpuric base, over the right plantar aspect of the foot, with areas of eschar over the right medial hallux. No allodynia, hyperaesthesia, or lymphadenopathy was noted.
A punch biopsy of an intact vesicle was performed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis:
Herpes zoster
Histopathologic examination showed full-thickness epidermal necrosis with ballooning degeneration resulting in an intra-epidermal blister. Multinucleated keratinocytes with nuclear moulding were seen within the blister cavity. Grocott-Gomori methenamine-silver (GMS), acid-fast, and Gram stains were negative. Granular immunoglobulin (Ig) G, IgM, and C3 were seen intramurally. DNA analysis of vesicular fluid was positive for varicella zoster virus (VZV). A diagnosis of herpes zoster (HZ) of the right S1 dermatome with primary obliterative vasculitis was established.
Immunocompromised people—those who have impaired T-cell immunity (eg, recipients of organ or hematopoietic stem-cell transplants), take immunosuppressive therapy, or have lymphoma, leukemia, or human immunodeficiency virus (HIV) infection—have an increased risk for HZ. For example, in patients with acquired immunodeficiency syndrome (AIDS), HZ uniquely manifests as recurrent shingles. An estimated 20% to 30% of HIV-infected patients will have more than 1 episode of HZ, which may involve the same or different dermatomes.1,2 Furthermore, HZ in this population is more commonly associated with atypical presentations.3
What an atypical presentation may look like
In immunocompromised patients, HZ may present with atypical cutaneous manifestations or with atypical generalized symptoms.
Atypical cutaneous manifestations, as in disseminated zoster, manifest with multiple hyperkeratotic papules (3-20 mm in diameter) that follow no dermatomal pattern. These lesions may be chronic, persisting for months or years, and may be associated with acyclovir-resistant strains of VZV.2,3 Another dermatologic variant is ecthymatous VZV, which manifests with multiple large (10-30 mm) punched-out ulcerations with a central black eschar and a peripheral rim of vesicles.4 Viral folliculitis—in which infection is limited to the hair follicle, with no associated blisters—has also been reported in atypical HZ.5
Our patient presented with hemorrhagic vesicles mimicking vasculitic lesions, which had persisted over a 3-month period with intermittent localized pain. It has been proposed that in atypical presentations, the reactivated VZV spreads transaxonally from adjacent nerves to the outermost adventitial layer of the arterial wall, leading to a vasculitic appearance of the vesicles.6 Viral-induced vasculitis may also result either directly from infection of the blood vessels or secondary to vascular damage from an inflammatory immune complex–mediated reaction, cell-mediated hypersensitivity, or inflammation due to immune dysregulation.7,8
Continue to: Differential includes vesiculobullous conditions
Differential includes vesiculobullous conditions
There are several important items to consider in the differential.
Cutaneous vasculitis, in severe cases, may manifest with vesicles or bullae that resemble the lesions seen in HZ. However, its unilateral nature and distribution distinguish it.
Angioinvasive fungal infections in immunocompromised patients may manifest with scattered ulceronecrotic lesions to purpuric vesiculobullous dermatoses.9 However, no fungal organisms were seen on GMS staining of the biopsied tissue.
Atypical hand-foot-and-mouth disease tends to affect adults and is associated with Coxsackievirus A6 infection.10 It may manifest as generalized vesiculobullous exanthem resembling varicella. The chronic nature and restricted extent of the patient’s rash made this diagnosis unlikely.
Successful management depends on timely identification
Although most cases of HZ can be diagnosed clinically, atypical rashes may require a biopsy and direct immunofluorescence assay for VZV antigen or a polymerase-chain-reaction (PCR) assay for VZV DNA in cells from the base of blisters. Therefore, it is important to consider the diagnosis of HZ in immunocompromised patients presenting with an atypical rash to avoid misdiagnosis and costly testing.
Continue to: Our patient was treated...
Our patient was treated with oral acyclovir 800 mg 5 times/day for 10 days, with prompt resolution of her rash.
CORRESPONDENCE
Joel Hua-Liang Lim, MBBS, MRCP, MMed, 1 Mandalay Road, Singapore 308205; [email protected]
A 52-year-old Chinese woman presented to a tertiary hospital in Singapore with a 3-month history of persistent and intermittently painful rashes over her right calf and foot (FIGURE). The patient had pancytopenia due to ongoing chemotherapy for metastatic nasopharyngeal carcinoma. She was systemically well and denied other dermatoses. Examination demonstrated scattered crops of tense hemorrhagic vesicles, each surrounded by a livid purpuric base, over the right plantar aspect of the foot, with areas of eschar over the right medial hallux. No allodynia, hyperaesthesia, or lymphadenopathy was noted.
A punch biopsy of an intact vesicle was performed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis:
Herpes zoster
Histopathologic examination showed full-thickness epidermal necrosis with ballooning degeneration resulting in an intra-epidermal blister. Multinucleated keratinocytes with nuclear moulding were seen within the blister cavity. Grocott-Gomori methenamine-silver (GMS), acid-fast, and Gram stains were negative. Granular immunoglobulin (Ig) G, IgM, and C3 were seen intramurally. DNA analysis of vesicular fluid was positive for varicella zoster virus (VZV). A diagnosis of herpes zoster (HZ) of the right S1 dermatome with primary obliterative vasculitis was established.
Immunocompromised people—those who have impaired T-cell immunity (eg, recipients of organ or hematopoietic stem-cell transplants), take immunosuppressive therapy, or have lymphoma, leukemia, or human immunodeficiency virus (HIV) infection—have an increased risk for HZ. For example, in patients with acquired immunodeficiency syndrome (AIDS), HZ uniquely manifests as recurrent shingles. An estimated 20% to 30% of HIV-infected patients will have more than 1 episode of HZ, which may involve the same or different dermatomes.1,2 Furthermore, HZ in this population is more commonly associated with atypical presentations.3
What an atypical presentation may look like
In immunocompromised patients, HZ may present with atypical cutaneous manifestations or with atypical generalized symptoms.
Atypical cutaneous manifestations, as in disseminated zoster, manifest with multiple hyperkeratotic papules (3-20 mm in diameter) that follow no dermatomal pattern. These lesions may be chronic, persisting for months or years, and may be associated with acyclovir-resistant strains of VZV.2,3 Another dermatologic variant is ecthymatous VZV, which manifests with multiple large (10-30 mm) punched-out ulcerations with a central black eschar and a peripheral rim of vesicles.4 Viral folliculitis—in which infection is limited to the hair follicle, with no associated blisters—has also been reported in atypical HZ.5
Our patient presented with hemorrhagic vesicles mimicking vasculitic lesions, which had persisted over a 3-month period with intermittent localized pain. It has been proposed that in atypical presentations, the reactivated VZV spreads transaxonally from adjacent nerves to the outermost adventitial layer of the arterial wall, leading to a vasculitic appearance of the vesicles.6 Viral-induced vasculitis may also result either directly from infection of the blood vessels or secondary to vascular damage from an inflammatory immune complex–mediated reaction, cell-mediated hypersensitivity, or inflammation due to immune dysregulation.7,8
Continue to: Differential includes vesiculobullous conditions
Differential includes vesiculobullous conditions
There are several important items to consider in the differential.
Cutaneous vasculitis, in severe cases, may manifest with vesicles or bullae that resemble the lesions seen in HZ. However, its unilateral nature and distribution distinguish it.
Angioinvasive fungal infections in immunocompromised patients may manifest with scattered ulceronecrotic lesions to purpuric vesiculobullous dermatoses.9 However, no fungal organisms were seen on GMS staining of the biopsied tissue.
Atypical hand-foot-and-mouth disease tends to affect adults and is associated with Coxsackievirus A6 infection.10 It may manifest as generalized vesiculobullous exanthem resembling varicella. The chronic nature and restricted extent of the patient’s rash made this diagnosis unlikely.
Successful management depends on timely identification
Although most cases of HZ can be diagnosed clinically, atypical rashes may require a biopsy and direct immunofluorescence assay for VZV antigen or a polymerase-chain-reaction (PCR) assay for VZV DNA in cells from the base of blisters. Therefore, it is important to consider the diagnosis of HZ in immunocompromised patients presenting with an atypical rash to avoid misdiagnosis and costly testing.
Continue to: Our patient was treated...
Our patient was treated with oral acyclovir 800 mg 5 times/day for 10 days, with prompt resolution of her rash.
CORRESPONDENCE
Joel Hua-Liang Lim, MBBS, MRCP, MMed, 1 Mandalay Road, Singapore 308205; [email protected]
1. LeBoit PE, Limova M, Yen TS, et al. Chronic verrucous varicella-zoster virus infection in patients with the acquired immunodeficiency syndrome (AIDS): histologic and molecular biologic findings. Am J Dermatopathol. 1992;14:1-7.
2. Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
3. Weinberg JM, Mysliwiec A, Turiansky GW, et al. Viral folliculitis: atypical presentations of herpes simplex, herpes zoster, and molluscum contagiosum. Arch Dermatol. 1997;133:983-986.
4. Gilson IH, Barnett JH, Conant MA, et al. Disseminated ecthymatous herpes varicella zoster virus infection in patients with acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20:637-642.
5. Løkke BJ, Weismann K, Mathiesen L, et al. Atypical varicella-zoster infection in AIDS. Acta Derm Venereol. 1993;73:123-125.
6. Uhoda I, Piérard-Franchimont C, Piérard GE. Varicella-zoster virus vasculitis: a case of recurrent varicella without epidermal involvement. Dermatology. 2000;200:173-175.
7. Teng GG, Chatham WW. Vasculitis related to viral and other microbial agents. Best Pract Res Clin Rheumatol. 2015;29:226-243.
8. Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16:12.
9. Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1-53.
10. Lott JP, Liu K, Landry M-L, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
1. LeBoit PE, Limova M, Yen TS, et al. Chronic verrucous varicella-zoster virus infection in patients with the acquired immunodeficiency syndrome (AIDS): histologic and molecular biologic findings. Am J Dermatopathol. 1992;14:1-7.
2. Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
3. Weinberg JM, Mysliwiec A, Turiansky GW, et al. Viral folliculitis: atypical presentations of herpes simplex, herpes zoster, and molluscum contagiosum. Arch Dermatol. 1997;133:983-986.
4. Gilson IH, Barnett JH, Conant MA, et al. Disseminated ecthymatous herpes varicella zoster virus infection in patients with acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20:637-642.
5. Løkke BJ, Weismann K, Mathiesen L, et al. Atypical varicella-zoster infection in AIDS. Acta Derm Venereol. 1993;73:123-125.
6. Uhoda I, Piérard-Franchimont C, Piérard GE. Varicella-zoster virus vasculitis: a case of recurrent varicella without epidermal involvement. Dermatology. 2000;200:173-175.
7. Teng GG, Chatham WW. Vasculitis related to viral and other microbial agents. Best Pract Res Clin Rheumatol. 2015;29:226-243.
8. Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16:12.
9. Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1-53.
10. Lott JP, Liu K, Landry M-L, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
Painful ulcers on gingiva, tongue, and buccal mucosa
A 29-year-old man with no prior history of mouth sores abruptly developed many 1- to 1.5-mm blisters on the gingiva (FIGURE 1A),tongue (FIGURE 1B), and buccal mucosa (FIGURE 1C), which evolved into small erosions accompanied by a low-grade fever 5 days prior to presentation. The patient had no history of any dermatologic conditions or systemic illnesses and was taking no medication.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute primary herpetic gingivostomatitis
Herpes simplex virus (HSV) is the causative agent for acute primary herpetic gingivostomatitis.1 HSV-1 is primarily responsible for oral mucosal infections, while HSV-2 is implicated in most genital and cutaneous lower body lesions.1 Herpetic gingivostomatitis often presents as a sudden vesiculoulcerative eruption anywhere in the mouth, including the perioral skin, vermillion border, gingiva, tongue, or buccal mucosa.2 Associated symptoms include malaise, headache, fever, and cervical lymphadenopathy; however, most occurrences are subclinical or asymptomatic.2
A diagnosis that’s more common in children. Primary HSV occurs in people who have not previously been exposed to the virus. While it is an infection that classically presents in childhood, it is not limited to this group. Manifestations often are more severe in adults.1
Following an incubation period of a few days to 3 weeks, the primary infection typically lasts 10 to 14 days.1,2 Recurrence is highly variable and generally less severe than primary infection, with grouped vesicles often recurring in the same spot with each recurrence on the vermillion border of the lip. Triggers for reactivation include immunosuppression, pregnancy, fever, UV radiation, or trauma.1,2
Differential includes other conditions with mucosal lesions
Acute herpetic gingivostomatitis must be distinguished from other disease processes that cause ulcerative mucosal lesions.
Aphthous stomatitis (canker sores) is the most common ulcerative disease of the oral mucosa.3 It presents as painful, punched-out, shallow ulcers with a yellowish gray pseudomembranous center and surrounding erythema.3 No definitive etiology has been established; however, aphthae often occur after trauma.
Continue to: Herpangina...
Herpangina is caused by coxsackie A virus and primarily is seen in infants and children younger than 5.4 The papulovesicular lesions primarily affect the posterior oral cavity, including the soft palate, anterior tonsillar pillars, and uvula.4
Allergic contact dermatitis is precipitated by contact with an allergen and presents with pain or pruritus. Lesions are erythematous with vesicles, erosions, ulcers, or hyperkeratosis that gradually resolve after withdrawal of the causative allergen.5
Pemphigus vulgaris. Oral ulcerations of the buccal mucosa and gingiva are the first manifestation of pemphigus vulgaris in the majority of patients, with skin blisters occurring months to years later over areas exposed to frictional stress.6 Skin sloughs may be seen in response to frictional stress (Nikolsky sign).6
The new Dx gold standard is PCR
Acute herpetic gingivostomatitis usually is diagnosed by history and hallmark clinical signs and symptoms.1 In this case, our patient presented with a sudden eruption of painful blisters on multiple areas of the oral mucosa associated with fever. The diagnosis can be confirmed by viral culture, serology with anti-HSV IgM and IgG, Tzanck preparation, immunofluorescence, and polymerase chain reaction (PCR).1 Viral culture has been the gold standard for mucosal HSV diagnosis; however, PCR is emerging as the new gold standard because of its unrivaled sensitivity, specificity, and rapid turnaround time.7,8 Specimens for PCR are submitted using a swab of infected cells placed in the same viral transport medium used for HSV cultures.
Our patient’s culture was positive for HSV-1.
Continue to: Prompt use of antivirals is key
Prompt use of antivirals is key
Treatment of acute HSV gingivostomatitis involves symptomatic management with topical anesthetics, oral analgesics, and normal saline rinses.1 Acyclovir is an established therapy; however, it has poor bioavailability and gastrointestinal absorption.1 Valacyclovir has improved bioavailability and is well tolerated.1 For primary herpes gingivostomatitis, we favor 1 g twice daily for 7 days.1 Our patient responded well to this valacyclovir regimen and healed completely in 1 week.
CORRESPONDENCE
Robert T. Brodell, MD, 2500 N State St, Jackson, MS 39216; [email protected]
1. Ajar AH, Chauvin PJ. Acute herpetic gingivostomatitis in adults: a review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68:247-251.
2. George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: report of a case. J Int Oral Health. 2014;6:99-102.
3. Akintoye SO, Greenburg MS. Recurrent aphthous stomatitis. Dent Clin N Am. 2014;58:281-297.
4. Scott LA, Stone MS. Viral exanthems. Dermatol Online J. 2003;9:4.
5. Feller L, Wood NH, Khammissa RA, et al. Review: allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-565.
6. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain C, et al. Oral manifestations of pemphigus vulgaris: clinical presentation, differential diagnosis and management. J Clin Exp Dermatol Res. 2010;1:112.
7. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
8. Centers for Disease Control and Prevention. Genital HSV infections. www.cdc.gov/std/tg2015/herpes.htm. Updated June 4, 2015. Accessed September 26, 2019.
A 29-year-old man with no prior history of mouth sores abruptly developed many 1- to 1.5-mm blisters on the gingiva (FIGURE 1A),tongue (FIGURE 1B), and buccal mucosa (FIGURE 1C), which evolved into small erosions accompanied by a low-grade fever 5 days prior to presentation. The patient had no history of any dermatologic conditions or systemic illnesses and was taking no medication.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute primary herpetic gingivostomatitis
Herpes simplex virus (HSV) is the causative agent for acute primary herpetic gingivostomatitis.1 HSV-1 is primarily responsible for oral mucosal infections, while HSV-2 is implicated in most genital and cutaneous lower body lesions.1 Herpetic gingivostomatitis often presents as a sudden vesiculoulcerative eruption anywhere in the mouth, including the perioral skin, vermillion border, gingiva, tongue, or buccal mucosa.2 Associated symptoms include malaise, headache, fever, and cervical lymphadenopathy; however, most occurrences are subclinical or asymptomatic.2
A diagnosis that’s more common in children. Primary HSV occurs in people who have not previously been exposed to the virus. While it is an infection that classically presents in childhood, it is not limited to this group. Manifestations often are more severe in adults.1
Following an incubation period of a few days to 3 weeks, the primary infection typically lasts 10 to 14 days.1,2 Recurrence is highly variable and generally less severe than primary infection, with grouped vesicles often recurring in the same spot with each recurrence on the vermillion border of the lip. Triggers for reactivation include immunosuppression, pregnancy, fever, UV radiation, or trauma.1,2
Differential includes other conditions with mucosal lesions
Acute herpetic gingivostomatitis must be distinguished from other disease processes that cause ulcerative mucosal lesions.
Aphthous stomatitis (canker sores) is the most common ulcerative disease of the oral mucosa.3 It presents as painful, punched-out, shallow ulcers with a yellowish gray pseudomembranous center and surrounding erythema.3 No definitive etiology has been established; however, aphthae often occur after trauma.
Continue to: Herpangina...
Herpangina is caused by coxsackie A virus and primarily is seen in infants and children younger than 5.4 The papulovesicular lesions primarily affect the posterior oral cavity, including the soft palate, anterior tonsillar pillars, and uvula.4
Allergic contact dermatitis is precipitated by contact with an allergen and presents with pain or pruritus. Lesions are erythematous with vesicles, erosions, ulcers, or hyperkeratosis that gradually resolve after withdrawal of the causative allergen.5
Pemphigus vulgaris. Oral ulcerations of the buccal mucosa and gingiva are the first manifestation of pemphigus vulgaris in the majority of patients, with skin blisters occurring months to years later over areas exposed to frictional stress.6 Skin sloughs may be seen in response to frictional stress (Nikolsky sign).6
The new Dx gold standard is PCR
Acute herpetic gingivostomatitis usually is diagnosed by history and hallmark clinical signs and symptoms.1 In this case, our patient presented with a sudden eruption of painful blisters on multiple areas of the oral mucosa associated with fever. The diagnosis can be confirmed by viral culture, serology with anti-HSV IgM and IgG, Tzanck preparation, immunofluorescence, and polymerase chain reaction (PCR).1 Viral culture has been the gold standard for mucosal HSV diagnosis; however, PCR is emerging as the new gold standard because of its unrivaled sensitivity, specificity, and rapid turnaround time.7,8 Specimens for PCR are submitted using a swab of infected cells placed in the same viral transport medium used for HSV cultures.
Our patient’s culture was positive for HSV-1.
Continue to: Prompt use of antivirals is key
Prompt use of antivirals is key
Treatment of acute HSV gingivostomatitis involves symptomatic management with topical anesthetics, oral analgesics, and normal saline rinses.1 Acyclovir is an established therapy; however, it has poor bioavailability and gastrointestinal absorption.1 Valacyclovir has improved bioavailability and is well tolerated.1 For primary herpes gingivostomatitis, we favor 1 g twice daily for 7 days.1 Our patient responded well to this valacyclovir regimen and healed completely in 1 week.
CORRESPONDENCE
Robert T. Brodell, MD, 2500 N State St, Jackson, MS 39216; [email protected]
A 29-year-old man with no prior history of mouth sores abruptly developed many 1- to 1.5-mm blisters on the gingiva (FIGURE 1A),tongue (FIGURE 1B), and buccal mucosa (FIGURE 1C), which evolved into small erosions accompanied by a low-grade fever 5 days prior to presentation. The patient had no history of any dermatologic conditions or systemic illnesses and was taking no medication.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute primary herpetic gingivostomatitis
Herpes simplex virus (HSV) is the causative agent for acute primary herpetic gingivostomatitis.1 HSV-1 is primarily responsible for oral mucosal infections, while HSV-2 is implicated in most genital and cutaneous lower body lesions.1 Herpetic gingivostomatitis often presents as a sudden vesiculoulcerative eruption anywhere in the mouth, including the perioral skin, vermillion border, gingiva, tongue, or buccal mucosa.2 Associated symptoms include malaise, headache, fever, and cervical lymphadenopathy; however, most occurrences are subclinical or asymptomatic.2
A diagnosis that’s more common in children. Primary HSV occurs in people who have not previously been exposed to the virus. While it is an infection that classically presents in childhood, it is not limited to this group. Manifestations often are more severe in adults.1
Following an incubation period of a few days to 3 weeks, the primary infection typically lasts 10 to 14 days.1,2 Recurrence is highly variable and generally less severe than primary infection, with grouped vesicles often recurring in the same spot with each recurrence on the vermillion border of the lip. Triggers for reactivation include immunosuppression, pregnancy, fever, UV radiation, or trauma.1,2
Differential includes other conditions with mucosal lesions
Acute herpetic gingivostomatitis must be distinguished from other disease processes that cause ulcerative mucosal lesions.
Aphthous stomatitis (canker sores) is the most common ulcerative disease of the oral mucosa.3 It presents as painful, punched-out, shallow ulcers with a yellowish gray pseudomembranous center and surrounding erythema.3 No definitive etiology has been established; however, aphthae often occur after trauma.
Continue to: Herpangina...
Herpangina is caused by coxsackie A virus and primarily is seen in infants and children younger than 5.4 The papulovesicular lesions primarily affect the posterior oral cavity, including the soft palate, anterior tonsillar pillars, and uvula.4
Allergic contact dermatitis is precipitated by contact with an allergen and presents with pain or pruritus. Lesions are erythematous with vesicles, erosions, ulcers, or hyperkeratosis that gradually resolve after withdrawal of the causative allergen.5
Pemphigus vulgaris. Oral ulcerations of the buccal mucosa and gingiva are the first manifestation of pemphigus vulgaris in the majority of patients, with skin blisters occurring months to years later over areas exposed to frictional stress.6 Skin sloughs may be seen in response to frictional stress (Nikolsky sign).6
The new Dx gold standard is PCR
Acute herpetic gingivostomatitis usually is diagnosed by history and hallmark clinical signs and symptoms.1 In this case, our patient presented with a sudden eruption of painful blisters on multiple areas of the oral mucosa associated with fever. The diagnosis can be confirmed by viral culture, serology with anti-HSV IgM and IgG, Tzanck preparation, immunofluorescence, and polymerase chain reaction (PCR).1 Viral culture has been the gold standard for mucosal HSV diagnosis; however, PCR is emerging as the new gold standard because of its unrivaled sensitivity, specificity, and rapid turnaround time.7,8 Specimens for PCR are submitted using a swab of infected cells placed in the same viral transport medium used for HSV cultures.
Our patient’s culture was positive for HSV-1.
Continue to: Prompt use of antivirals is key
Prompt use of antivirals is key
Treatment of acute HSV gingivostomatitis involves symptomatic management with topical anesthetics, oral analgesics, and normal saline rinses.1 Acyclovir is an established therapy; however, it has poor bioavailability and gastrointestinal absorption.1 Valacyclovir has improved bioavailability and is well tolerated.1 For primary herpes gingivostomatitis, we favor 1 g twice daily for 7 days.1 Our patient responded well to this valacyclovir regimen and healed completely in 1 week.
CORRESPONDENCE
Robert T. Brodell, MD, 2500 N State St, Jackson, MS 39216; [email protected]
1. Ajar AH, Chauvin PJ. Acute herpetic gingivostomatitis in adults: a review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68:247-251.
2. George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: report of a case. J Int Oral Health. 2014;6:99-102.
3. Akintoye SO, Greenburg MS. Recurrent aphthous stomatitis. Dent Clin N Am. 2014;58:281-297.
4. Scott LA, Stone MS. Viral exanthems. Dermatol Online J. 2003;9:4.
5. Feller L, Wood NH, Khammissa RA, et al. Review: allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-565.
6. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain C, et al. Oral manifestations of pemphigus vulgaris: clinical presentation, differential diagnosis and management. J Clin Exp Dermatol Res. 2010;1:112.
7. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
8. Centers for Disease Control and Prevention. Genital HSV infections. www.cdc.gov/std/tg2015/herpes.htm. Updated June 4, 2015. Accessed September 26, 2019.
1. Ajar AH, Chauvin PJ. Acute herpetic gingivostomatitis in adults: a review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68:247-251.
2. George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: report of a case. J Int Oral Health. 2014;6:99-102.
3. Akintoye SO, Greenburg MS. Recurrent aphthous stomatitis. Dent Clin N Am. 2014;58:281-297.
4. Scott LA, Stone MS. Viral exanthems. Dermatol Online J. 2003;9:4.
5. Feller L, Wood NH, Khammissa RA, et al. Review: allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-565.
6. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain C, et al. Oral manifestations of pemphigus vulgaris: clinical presentation, differential diagnosis and management. J Clin Exp Dermatol Res. 2010;1:112.
7. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
8. Centers for Disease Control and Prevention. Genital HSV infections. www.cdc.gov/std/tg2015/herpes.htm. Updated June 4, 2015. Accessed September 26, 2019.