User login
She Needs A-cyst-ance
This woman, now 44, first developed subcutaneous “bumps” on her neck, arms, and chest at puberty. They were initially diagnosed as acne, but treatment for that condition failed to help.
Later, she consulted a dermatologist, who suggested they were cysts and actually removed one to send for pathologic examination. The report indicated “a type of cyst,” the name of which the patient has long since forgotten.
Over the years, she has developed additional lesions, which are not only unsightly but also painful at times. Although the patient is not in distress, she is upset.
The patient has type IV skin and is of African-American ancestry. Further history-taking reveals that she is reasonably healthy, with no other skin problems. She does report that the presenting complaint “runs in the family,” on her father’s side.
EXAMINATION
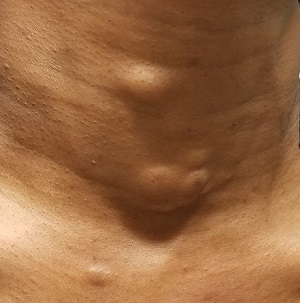
The lesions—subcutaneous, doughy, cystic-feeling papules and nodules—are widely distributed on the patient’s anterior neck, arms, and chest. They range in size from 5 mm to 3 cm. None are inflamed, and no puncta can be seen on their surfaces. Palpation provokes no reaction of pain or discomfort.
With the patient’s permission, she is anesthetized and one lesion is removed. The sample clearly establishes a cystic nature, although the contents are neither cheesy nor grumous as would be seen with an ordinary epidermal cyst. Rather, they are an oily, odorless, thick liquid surrounded by an organized cyst wall. This is removed as well and sent for pathologic examination.
What’s the diagnosis?
DISCUSSION
The pathology report confirmed the lesions to be steatocystoma—in this case, part of an autosomal dominantly inherited condition called steatocystoma multiplex (SM). When these manifest as solitary lesions, they are known as steatocystoma simplex—a true sebaceous cyst, quite different from the common epidermal cyst that contains cheesy, odoriferous material and is frequently misnamed “sebaceous cyst.”
Steatocystoma can develop spontaneously, without any genetic predisposition. SM, however, is quite unusual (if not rare) and results from a defect in keratin 17 that allows the accumulation of sebum at the base of the follicle. It has no other pathologic implication.
However, in a case such as this, SM presents a real problem, because the only effective treatment is complete excision. This not only leaves a scar, but also, in those with skin of color, has the potential to produce hypertrophic scarring or even keloid formation. Worse, in many cases, the patient keeps developing cysts in new locations.
TAKE-HOME LEARNING POINTS
- Steatocystoma multiplex (SM) is an autosomal dominant condition in which the patient, usually at puberty, develops sebum-filled cysts.
- These cysts can occur as solitary lesions (steatocystoma simplex) but more often manifest in multiples on the neck, face, chest, and arms.
- SM cysts are full of clear or yellowish sebum, unlike common epidermal cysts, which are filled with cheesy, often odoriferous material.
- The only effective treatment for SM cysts is complete excision.
This woman, now 44, first developed subcutaneous “bumps” on her neck, arms, and chest at puberty. They were initially diagnosed as acne, but treatment for that condition failed to help.
Later, she consulted a dermatologist, who suggested they were cysts and actually removed one to send for pathologic examination. The report indicated “a type of cyst,” the name of which the patient has long since forgotten.
Over the years, she has developed additional lesions, which are not only unsightly but also painful at times. Although the patient is not in distress, she is upset.
The patient has type IV skin and is of African-American ancestry. Further history-taking reveals that she is reasonably healthy, with no other skin problems. She does report that the presenting complaint “runs in the family,” on her father’s side.

EXAMINATION
The lesions—subcutaneous, doughy, cystic-feeling papules and nodules—are widely distributed on the patient’s anterior neck, arms, and chest. They range in size from 5 mm to 3 cm. None are inflamed, and no puncta can be seen on their surfaces. Palpation provokes no reaction of pain or discomfort.
With the patient’s permission, she is anesthetized and one lesion is removed. The sample clearly establishes a cystic nature, although the contents are neither cheesy nor grumous as would be seen with an ordinary epidermal cyst. Rather, they are an oily, odorless, thick liquid surrounded by an organized cyst wall. This is removed as well and sent for pathologic examination.
What’s the diagnosis?
DISCUSSION
The pathology report confirmed the lesions to be steatocystoma—in this case, part of an autosomal dominantly inherited condition called steatocystoma multiplex (SM). When these manifest as solitary lesions, they are known as steatocystoma simplex—a true sebaceous cyst, quite different from the common epidermal cyst that contains cheesy, odoriferous material and is frequently misnamed “sebaceous cyst.”
Steatocystoma can develop spontaneously, without any genetic predisposition. SM, however, is quite unusual (if not rare) and results from a defect in keratin 17 that allows the accumulation of sebum at the base of the follicle. It has no other pathologic implication.
However, in a case such as this, SM presents a real problem, because the only effective treatment is complete excision. This not only leaves a scar, but also, in those with skin of color, has the potential to produce hypertrophic scarring or even keloid formation. Worse, in many cases, the patient keeps developing cysts in new locations.
TAKE-HOME LEARNING POINTS
- Steatocystoma multiplex (SM) is an autosomal dominant condition in which the patient, usually at puberty, develops sebum-filled cysts.
- These cysts can occur as solitary lesions (steatocystoma simplex) but more often manifest in multiples on the neck, face, chest, and arms.
- SM cysts are full of clear or yellowish sebum, unlike common epidermal cysts, which are filled with cheesy, often odoriferous material.
- The only effective treatment for SM cysts is complete excision.
This woman, now 44, first developed subcutaneous “bumps” on her neck, arms, and chest at puberty. They were initially diagnosed as acne, but treatment for that condition failed to help.
Later, she consulted a dermatologist, who suggested they were cysts and actually removed one to send for pathologic examination. The report indicated “a type of cyst,” the name of which the patient has long since forgotten.
Over the years, she has developed additional lesions, which are not only unsightly but also painful at times. Although the patient is not in distress, she is upset.
The patient has type IV skin and is of African-American ancestry. Further history-taking reveals that she is reasonably healthy, with no other skin problems. She does report that the presenting complaint “runs in the family,” on her father’s side.

EXAMINATION
The lesions—subcutaneous, doughy, cystic-feeling papules and nodules—are widely distributed on the patient’s anterior neck, arms, and chest. They range in size from 5 mm to 3 cm. None are inflamed, and no puncta can be seen on their surfaces. Palpation provokes no reaction of pain or discomfort.
With the patient’s permission, she is anesthetized and one lesion is removed. The sample clearly establishes a cystic nature, although the contents are neither cheesy nor grumous as would be seen with an ordinary epidermal cyst. Rather, they are an oily, odorless, thick liquid surrounded by an organized cyst wall. This is removed as well and sent for pathologic examination.
What’s the diagnosis?
DISCUSSION
The pathology report confirmed the lesions to be steatocystoma—in this case, part of an autosomal dominantly inherited condition called steatocystoma multiplex (SM). When these manifest as solitary lesions, they are known as steatocystoma simplex—a true sebaceous cyst, quite different from the common epidermal cyst that contains cheesy, odoriferous material and is frequently misnamed “sebaceous cyst.”
Steatocystoma can develop spontaneously, without any genetic predisposition. SM, however, is quite unusual (if not rare) and results from a defect in keratin 17 that allows the accumulation of sebum at the base of the follicle. It has no other pathologic implication.
However, in a case such as this, SM presents a real problem, because the only effective treatment is complete excision. This not only leaves a scar, but also, in those with skin of color, has the potential to produce hypertrophic scarring or even keloid formation. Worse, in many cases, the patient keeps developing cysts in new locations.
TAKE-HOME LEARNING POINTS
- Steatocystoma multiplex (SM) is an autosomal dominant condition in which the patient, usually at puberty, develops sebum-filled cysts.
- These cysts can occur as solitary lesions (steatocystoma simplex) but more often manifest in multiples on the neck, face, chest, and arms.
- SM cysts are full of clear or yellowish sebum, unlike common epidermal cysts, which are filled with cheesy, often odoriferous material.
- The only effective treatment for SM cysts is complete excision.
Multiple skin ulcers
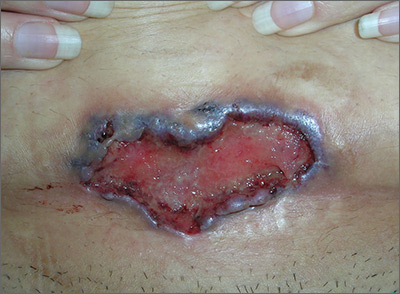
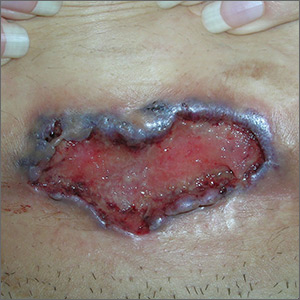
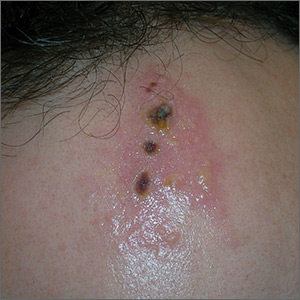
The FP noted the deep ulcers with gun-metal (violet blue coloration) undermined borders. The edge of the upper left corner of the suprapubic ulcer also had a cribriform pattern (pierced with holes like swiss cheese). The FP’s differential diagnosis included pyoderma gangrenosum (PG) and a deep fungal infection.
The FP was aware that it could take months before the patient could be seen be a dermatologist, so he offered to perform a 4-mm punch biopsy at the edge of the ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). (See the Watch & Learn video on “Punch biopsy.”)
The pathologist found a dense neutrophilic infiltrate and stated that this supported the diagnosis of PG. No fungal elements were seen with a Periodic acid–Schiff (PAS) stain. PG is a rare neutrophilic dermatosis, without a known cause, that is sometimes seen with inflammatory bowel disease.
The FP called a local dermatologist, and they decided to start the patient on oral prednisone until she could be seen in the dermatologist’s office. The dermatologist stated that she would be considering oral cyclosporine, oral dapsone, or injectable biologic agents as steroid sparing agents to treat the PG.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP noted the deep ulcers with gun-metal (violet blue coloration) undermined borders. The edge of the upper left corner of the suprapubic ulcer also had a cribriform pattern (pierced with holes like swiss cheese). The FP’s differential diagnosis included pyoderma gangrenosum (PG) and a deep fungal infection.
The FP was aware that it could take months before the patient could be seen be a dermatologist, so he offered to perform a 4-mm punch biopsy at the edge of the ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). (See the Watch & Learn video on “Punch biopsy.”)
The pathologist found a dense neutrophilic infiltrate and stated that this supported the diagnosis of PG. No fungal elements were seen with a Periodic acid–Schiff (PAS) stain. PG is a rare neutrophilic dermatosis, without a known cause, that is sometimes seen with inflammatory bowel disease.
The FP called a local dermatologist, and they decided to start the patient on oral prednisone until she could be seen in the dermatologist’s office. The dermatologist stated that she would be considering oral cyclosporine, oral dapsone, or injectable biologic agents as steroid sparing agents to treat the PG.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP noted the deep ulcers with gun-metal (violet blue coloration) undermined borders. The edge of the upper left corner of the suprapubic ulcer also had a cribriform pattern (pierced with holes like swiss cheese). The FP’s differential diagnosis included pyoderma gangrenosum (PG) and a deep fungal infection.
The FP was aware that it could take months before the patient could be seen be a dermatologist, so he offered to perform a 4-mm punch biopsy at the edge of the ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). (See the Watch & Learn video on “Punch biopsy.”)
The pathologist found a dense neutrophilic infiltrate and stated that this supported the diagnosis of PG. No fungal elements were seen with a Periodic acid–Schiff (PAS) stain. PG is a rare neutrophilic dermatosis, without a known cause, that is sometimes seen with inflammatory bowel disease.
The FP called a local dermatologist, and they decided to start the patient on oral prednisone until she could be seen in the dermatologist’s office. The dermatologist stated that she would be considering oral cyclosporine, oral dapsone, or injectable biologic agents as steroid sparing agents to treat the PG.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
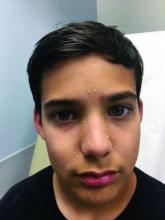
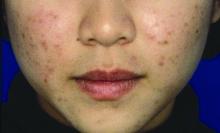
A healthy 8-year-old boy presents with several skin-colored, round 1-3 mm papules on the nose, forehead, and cheeks
A shave biopsy of one of the lesions was performed that showed a proliferation of nests of basaloid cells on the dermis with palisading and rare vacuolated clear cell change. A rare ductal structure with luminal proteinaceous contents was noted. The findings were consistent with a trichoepithelioma.
Trichoepitheliomas are rare, benign, adnexal skin tumors that can start in early childhood or during puberty. The lesions are most commonly seen in girls as skin color papules on the face, and sometimes on the trunk and the neck. Trichoepitheliomas can appear as a benign single lesion nonfamilial form or as a familial form with multiple lesions.1 Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant condition where affected individuals have multiple trichoepitheliomas, cylindromas, and spiradenomas. Depending on the predominant type of lesion, phenotypic variants include multiple familial trichoepithelioma type 1 and familial cylindromatosis.2 BSS is caused by mutations within CYLD, a tumor-suppressor gene located on chromosome 16q12-q13.3 Our patient presented only with trichoepitheliomas with no other lesions on the scalp, neck, or torso.
Multiple trichoepitheliomas also can be seen in other syndromes including Rombo syndrome, which is characterized by basal cell carcinomas, milia, hypotrichosis, distal vasodilation, and atrophoderma vermiculata; none seen in our patient. Bazex-Dupré-Christol syndrome is an X-linked dominant condition in which affected individuals can present with multiple trichoepitheliomas, as well as milia, hypotrichosis, follicular atrophoderma, and basal cell carcinomas.
The differential diagnosis of skin color papules on the central face on a child should include acne, flat warts, and angiofibromas seen in tuberous sclerosis. Our patient’s lesions were monomorphous, and there were no comedones, pustules, or inflammatory papules characteristic of acne.
He had warts on his hands which could make it suspicious for the face lesions to be verrucous in nature. Flat warts also present as skin color papules, but characteristically are flat, not round and shiny as our patient’s lesions were. Angiofibromas, as seen in individuals with tuberous sclerosis, also can start at an early age in the same location as trichoepitheliomas in BSS, but clinically the lesions are pinker and redder rather than the skin-color, round shape papules characteristic of trichoepitheliomas. Patients may have other findings suggestive of tuberous sclerosis including confetti hypopigmentation, ash leaf spots, shagreen patch, and a history of seizures or developmental delay – none of which were present in our patient. Children with basal cell nevus syndrome can present with skin color to shiny telangiectatic papules (basal cell carcinomas) that can be single or multiple on the face, chest, and back. The lesions usually are not seen in clusters around the nose and central face as seen in patients with BSS. Patients with basal cell nevus syndrome can develop jaw bone cysts, brain tumors (medulloblastoma), and fibromas on the heart or ovaries, palmar pits and be macrocephalic.4
Trichoepitheliomas usually are treated surgically but other nonsurgical removing techniques include laser resurfacing, curettage, and electrocautery.5 Malignant transformation can occur in 5%-10% of the individuals and should be managed by a multidisciplinary team. Topical treatment with sirolimus previously has been reported to be effective in young patients.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Acta Dermatovenerol Croat. 2018 Jun;26(2):162-5.
2. Eur J Med Genet. 2015;58(5):271-8.
3. Am J Dermatopathol. 2014;36(11):868-74.
4. Int J Dermatol. 2016 Apr;55(4):367-75.
5. Int J Dermatol. 2007;46(6):583-6.
6. Dermatol Ther. 2017 Mar. doi: 10.1111/dth.12458.
A shave biopsy of one of the lesions was performed that showed a proliferation of nests of basaloid cells on the dermis with palisading and rare vacuolated clear cell change. A rare ductal structure with luminal proteinaceous contents was noted. The findings were consistent with a trichoepithelioma.
Trichoepitheliomas are rare, benign, adnexal skin tumors that can start in early childhood or during puberty. The lesions are most commonly seen in girls as skin color papules on the face, and sometimes on the trunk and the neck. Trichoepitheliomas can appear as a benign single lesion nonfamilial form or as a familial form with multiple lesions.1 Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant condition where affected individuals have multiple trichoepitheliomas, cylindromas, and spiradenomas. Depending on the predominant type of lesion, phenotypic variants include multiple familial trichoepithelioma type 1 and familial cylindromatosis.2 BSS is caused by mutations within CYLD, a tumor-suppressor gene located on chromosome 16q12-q13.3 Our patient presented only with trichoepitheliomas with no other lesions on the scalp, neck, or torso.
Multiple trichoepitheliomas also can be seen in other syndromes including Rombo syndrome, which is characterized by basal cell carcinomas, milia, hypotrichosis, distal vasodilation, and atrophoderma vermiculata; none seen in our patient. Bazex-Dupré-Christol syndrome is an X-linked dominant condition in which affected individuals can present with multiple trichoepitheliomas, as well as milia, hypotrichosis, follicular atrophoderma, and basal cell carcinomas.
The differential diagnosis of skin color papules on the central face on a child should include acne, flat warts, and angiofibromas seen in tuberous sclerosis. Our patient’s lesions were monomorphous, and there were no comedones, pustules, or inflammatory papules characteristic of acne.
He had warts on his hands which could make it suspicious for the face lesions to be verrucous in nature. Flat warts also present as skin color papules, but characteristically are flat, not round and shiny as our patient’s lesions were. Angiofibromas, as seen in individuals with tuberous sclerosis, also can start at an early age in the same location as trichoepitheliomas in BSS, but clinically the lesions are pinker and redder rather than the skin-color, round shape papules characteristic of trichoepitheliomas. Patients may have other findings suggestive of tuberous sclerosis including confetti hypopigmentation, ash leaf spots, shagreen patch, and a history of seizures or developmental delay – none of which were present in our patient. Children with basal cell nevus syndrome can present with skin color to shiny telangiectatic papules (basal cell carcinomas) that can be single or multiple on the face, chest, and back. The lesions usually are not seen in clusters around the nose and central face as seen in patients with BSS. Patients with basal cell nevus syndrome can develop jaw bone cysts, brain tumors (medulloblastoma), and fibromas on the heart or ovaries, palmar pits and be macrocephalic.4
Trichoepitheliomas usually are treated surgically but other nonsurgical removing techniques include laser resurfacing, curettage, and electrocautery.5 Malignant transformation can occur in 5%-10% of the individuals and should be managed by a multidisciplinary team. Topical treatment with sirolimus previously has been reported to be effective in young patients.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Acta Dermatovenerol Croat. 2018 Jun;26(2):162-5.
2. Eur J Med Genet. 2015;58(5):271-8.
3. Am J Dermatopathol. 2014;36(11):868-74.
4. Int J Dermatol. 2016 Apr;55(4):367-75.
5. Int J Dermatol. 2007;46(6):583-6.
6. Dermatol Ther. 2017 Mar. doi: 10.1111/dth.12458.
A shave biopsy of one of the lesions was performed that showed a proliferation of nests of basaloid cells on the dermis with palisading and rare vacuolated clear cell change. A rare ductal structure with luminal proteinaceous contents was noted. The findings were consistent with a trichoepithelioma.
Trichoepitheliomas are rare, benign, adnexal skin tumors that can start in early childhood or during puberty. The lesions are most commonly seen in girls as skin color papules on the face, and sometimes on the trunk and the neck. Trichoepitheliomas can appear as a benign single lesion nonfamilial form or as a familial form with multiple lesions.1 Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant condition where affected individuals have multiple trichoepitheliomas, cylindromas, and spiradenomas. Depending on the predominant type of lesion, phenotypic variants include multiple familial trichoepithelioma type 1 and familial cylindromatosis.2 BSS is caused by mutations within CYLD, a tumor-suppressor gene located on chromosome 16q12-q13.3 Our patient presented only with trichoepitheliomas with no other lesions on the scalp, neck, or torso.
Multiple trichoepitheliomas also can be seen in other syndromes including Rombo syndrome, which is characterized by basal cell carcinomas, milia, hypotrichosis, distal vasodilation, and atrophoderma vermiculata; none seen in our patient. Bazex-Dupré-Christol syndrome is an X-linked dominant condition in which affected individuals can present with multiple trichoepitheliomas, as well as milia, hypotrichosis, follicular atrophoderma, and basal cell carcinomas.
The differential diagnosis of skin color papules on the central face on a child should include acne, flat warts, and angiofibromas seen in tuberous sclerosis. Our patient’s lesions were monomorphous, and there were no comedones, pustules, or inflammatory papules characteristic of acne.
He had warts on his hands which could make it suspicious for the face lesions to be verrucous in nature. Flat warts also present as skin color papules, but characteristically are flat, not round and shiny as our patient’s lesions were. Angiofibromas, as seen in individuals with tuberous sclerosis, also can start at an early age in the same location as trichoepitheliomas in BSS, but clinically the lesions are pinker and redder rather than the skin-color, round shape papules characteristic of trichoepitheliomas. Patients may have other findings suggestive of tuberous sclerosis including confetti hypopigmentation, ash leaf spots, shagreen patch, and a history of seizures or developmental delay – none of which were present in our patient. Children with basal cell nevus syndrome can present with skin color to shiny telangiectatic papules (basal cell carcinomas) that can be single or multiple on the face, chest, and back. The lesions usually are not seen in clusters around the nose and central face as seen in patients with BSS. Patients with basal cell nevus syndrome can develop jaw bone cysts, brain tumors (medulloblastoma), and fibromas on the heart or ovaries, palmar pits and be macrocephalic.4
Trichoepitheliomas usually are treated surgically but other nonsurgical removing techniques include laser resurfacing, curettage, and electrocautery.5 Malignant transformation can occur in 5%-10% of the individuals and should be managed by a multidisciplinary team. Topical treatment with sirolimus previously has been reported to be effective in young patients.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Acta Dermatovenerol Croat. 2018 Jun;26(2):162-5.
2. Eur J Med Genet. 2015;58(5):271-8.
3. Am J Dermatopathol. 2014;36(11):868-74.
4. Int J Dermatol. 2016 Apr;55(4):367-75.
5. Int J Dermatol. 2007;46(6):583-6.
6. Dermatol Ther. 2017 Mar. doi: 10.1111/dth.12458.
A white 8-year-old boy comes to our pediatric dermatology clinic with his mother for evaluation of acne. The lesions started about a year ago on his nose and now have spread to his cheeks. The bumps are not symptomatic. He has been applying over the counter salicylic acid and benzoyl peroxide gels with no help. The mother reports he has been growing well, denies any growth spurt, no axillary or genital hair or body odor noted.
None of the family members have a history of acne. The mother cannot recall any family members with similar lesions on the face. He has had some warts on his fingers for years and has been treated with over the counter salicylic acid. There is no family history of skin cancer.
On physical exam, he is a healthy young boy with several skin color, round papules 1-3 mm on the nose, forehead, and cheeks. There are no lesions on the scalp. He has abundant brown hair. He has few verrucous papules on the fingers. Axillary and genital hair is not noted. There is no body odor and he is Tanner stage I.
U.S. travelers to Europe need up to date measles immunization
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
FROM PEDIATRICS
Ovarian reserve markers fall on isotretinoin, but rebound after stopping treatment
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
REPORTING FROM WCD2019
Ixekizumab surpasses adalimumab in PsA head-to-head study
MADRID – The interleukin-17A inhibitor ixekizumab surpassed the tumor necrosis factor inhibitor adalimumab for treatment of patients with psoriatic arthritis in a multicenter, randomized study with 566 enrolled patients, the first reported results from a head-to-head comparison for this disease of two different classes of biological drugs.
The results showed that a standard, 24-week regimen with each of these agents, both of which already have regulatory approval for treating psoriatic arthritis (PsA), led to achievement of the primary endpoint in 36% of patients treated with ixekizumab (Taltz) and 28% of patients treated with adalimumab (Humira), a statistically significant difference, Philip J. Mease, MD, said at the European Congress of Rheumatology.
“Ixekizumab was superior to adalimumab for improving signs and symptoms of active PsA, as measured by simultaneous achievement of ACR50 [American College of Rheumatology] and PASI 100 [Psoriasis Area and Severity Index],” the study’s primary endpoint that combined a measure of joint disease activity with a measure of skin involvement, said Dr. Mease, a rheumatologist at Swedish Medical Center in Seattle.
This unconventional primary endpoint for testing drugs that treat PsA was called out during discussion of the report for having an inherent bias favoring ixekizumab by its inclusion of a skin outcome that received equal weight with an assessment tool that focused on joint responses. “This was a very unusual primary endpoint that favored ixekizumab,” Roy M. Fleischmann, MD, a Dallas rheumatologist, commented during the discussion.
Dr. Mease readily admitted that the study’s design stacked the deck in favor of ixekizumab, but he added that this decision reflected a desire by the researchers who ran the study to choose a primary endpoint that represented both of the prominent pathologies seen in patients with PsA.
The primary endpoint used in the study “looks at PsA more holistically,” Dr. Mease said in an interview. “It forced clinicians to look beyond just the joints,” in PsA patients. “That has been a limitation of prior PsA treatment assessments,” which until this study have uniformly used single primary outcomes that focus on joint responses, most commonly the ACR20 measure of joint disease activity.
The SPIRIT-H2H (A Study of Ixekizumab [LY2439821] Versus Adalimumab in Participants With Psoriatic Arthritis) study enrolled adults with active PsA who had never before received treatment with a biological drug. Enrolled patients had to have both active disease in their joints and active plaque psoriasis, with an inadequate response to at least one conventional synthetic disease-modifying antirheumatic drug. The 566 patients randomized in the study averaged about 48 years old, a bit more than half were men, and patients averaged about 6 years with diagnosed PsA and about 15 years diagnosed with psoriasis. Just over two-thirds of the patients were on concurrent treatment with a conventional synthetic agent, most often methotrexate.
The two components of the primary endpoint each showed the anticipated result. Among the 269 patients treated with adalimumab for the full 24 weeks, 47% had an ACR50 response, as did 51% of the 262 patients who completed their full course of ixekizumab, a between-group difference that was not statistically significant. In contrast, the PASI 100 measure of complete skin resolution occurred in 47% of the adalimumab-treated patients and 60% of those on ixekizumab, a statistically significant difference.
Dr. Mease reported results for several other efficacy measures, and what was notable was statistically significant superiority for ixekizumab in a measure of entheses disease activity, the SPARCC [Spondyloarthritis Research Consortium of Canada] Enthesitis Index, which fell to zero in 57% of the ixekizumab patients and 45% of those on adalimumab. “It makes you wonder whether there is something special about interleukin-17 in enthesitis,” Dr. Mease said.
The safety results of the study were consistent with the known adverse effect profiles of both drugs.
The impact of these findings on practice remains to be seen, and will likely depend on both cost considerations as well as clinicians trying to tailor drug choices to individual patients. The relatively new drug, ixekizumab, is consequentially more expensive than the older adalimumab, which has had its price depressed by the recent introduction of a biosimilar agent as well as long-standing competition from multiple TNF inhibitors.
“I think in the United States insurers will continue to steer patients toward whichever drug is cheapest among the highly-effective options,” but this result should lead to more use of interleukin-17 inhibitors as second-line agents for PsA, and it might push some clinicians to prescribe it as the first-line treatment, Dr. Mease said. He was confident that the efficacy profile shown by ixekizumab in SPIRIT-H2H was likely a class effect. Economics aside, the impetus to prescribe ixekizumab or another interleukin-17 inhibitor will be greatest when a patient has more extensive skin involvement, while for patients with little or no skin symptoms clinicians will likely stick with the more established TNF inhibitors as the first drug class to prescribe for PsA.
SPIRIT-H2H was sponsored by Eli Lilly, the company that markets ixekizumab (Taltz). Dr. Mease has been a consultant to, speaker for, and received research funding from Eli Lilly and from several other companies. Dr. Fleischmann has been a consultant to and has received research funding from several companies including Eli Lilly.
SOURCE: Mease PJ et al. Ann Rheum Dis. 2019 Jun. doi: 10.1136/annrheumdis-2019-eular.8709.
The results from SPIRIT-H2H confirm with rigorously-collected, prospective data what we had already seen during our routine use of ixekizumab for treating patients with psoriatic arthritis: it does a better job of resolving skin manifestations than any tumor necrosis factor (TNF) inhibitor. For joint symptoms, the two drugs are similar, but for skin ixekizumab has substantial superiority.
I had been hopeful that inhibition of interleukin-17 would surpass TNF inhibition for resolution of joint symptoms, but it looks like they are similar. That’s a little below expectations. But working well for improving skin symptoms is important because it’s something that many patients care about, especially those with more substantial skin symptoms. When matching the best drug to each PsA patient other considerations also exist, such as ease of use. These drugs are delivered by different devices, and ease of administration also matters to patients.
I think it would be premature to presume that the effects shown by ixekizumab extrapolate to all the other interleukin-17 inhibitors. Some of these drugs act via different mechanisms, and so the SPIRIT-H2H results may very well not reflect a class effect.
Thomas Dörner, MD, is professor of rheumatology at Charité University Hospital in Berlin. He has been a consultant to Eli Lilly, as well as to AbbVie, Celgene, Novartis, Pfizer, and Roche, he has been a speaker on behalf of Amgen, Biogen, and Celgene, and he has received research funding from Chugai, Janssen, Roche, and Sanofi. He made these comments in an interview.
The results from SPIRIT-H2H confirm with rigorously-collected, prospective data what we had already seen during our routine use of ixekizumab for treating patients with psoriatic arthritis: it does a better job of resolving skin manifestations than any tumor necrosis factor (TNF) inhibitor. For joint symptoms, the two drugs are similar, but for skin ixekizumab has substantial superiority.
I had been hopeful that inhibition of interleukin-17 would surpass TNF inhibition for resolution of joint symptoms, but it looks like they are similar. That’s a little below expectations. But working well for improving skin symptoms is important because it’s something that many patients care about, especially those with more substantial skin symptoms. When matching the best drug to each PsA patient other considerations also exist, such as ease of use. These drugs are delivered by different devices, and ease of administration also matters to patients.
I think it would be premature to presume that the effects shown by ixekizumab extrapolate to all the other interleukin-17 inhibitors. Some of these drugs act via different mechanisms, and so the SPIRIT-H2H results may very well not reflect a class effect.
Thomas Dörner, MD, is professor of rheumatology at Charité University Hospital in Berlin. He has been a consultant to Eli Lilly, as well as to AbbVie, Celgene, Novartis, Pfizer, and Roche, he has been a speaker on behalf of Amgen, Biogen, and Celgene, and he has received research funding from Chugai, Janssen, Roche, and Sanofi. He made these comments in an interview.
The results from SPIRIT-H2H confirm with rigorously-collected, prospective data what we had already seen during our routine use of ixekizumab for treating patients with psoriatic arthritis: it does a better job of resolving skin manifestations than any tumor necrosis factor (TNF) inhibitor. For joint symptoms, the two drugs are similar, but for skin ixekizumab has substantial superiority.
I had been hopeful that inhibition of interleukin-17 would surpass TNF inhibition for resolution of joint symptoms, but it looks like they are similar. That’s a little below expectations. But working well for improving skin symptoms is important because it’s something that many patients care about, especially those with more substantial skin symptoms. When matching the best drug to each PsA patient other considerations also exist, such as ease of use. These drugs are delivered by different devices, and ease of administration also matters to patients.
I think it would be premature to presume that the effects shown by ixekizumab extrapolate to all the other interleukin-17 inhibitors. Some of these drugs act via different mechanisms, and so the SPIRIT-H2H results may very well not reflect a class effect.
Thomas Dörner, MD, is professor of rheumatology at Charité University Hospital in Berlin. He has been a consultant to Eli Lilly, as well as to AbbVie, Celgene, Novartis, Pfizer, and Roche, he has been a speaker on behalf of Amgen, Biogen, and Celgene, and he has received research funding from Chugai, Janssen, Roche, and Sanofi. He made these comments in an interview.
MADRID – The interleukin-17A inhibitor ixekizumab surpassed the tumor necrosis factor inhibitor adalimumab for treatment of patients with psoriatic arthritis in a multicenter, randomized study with 566 enrolled patients, the first reported results from a head-to-head comparison for this disease of two different classes of biological drugs.
The results showed that a standard, 24-week regimen with each of these agents, both of which already have regulatory approval for treating psoriatic arthritis (PsA), led to achievement of the primary endpoint in 36% of patients treated with ixekizumab (Taltz) and 28% of patients treated with adalimumab (Humira), a statistically significant difference, Philip J. Mease, MD, said at the European Congress of Rheumatology.
“Ixekizumab was superior to adalimumab for improving signs and symptoms of active PsA, as measured by simultaneous achievement of ACR50 [American College of Rheumatology] and PASI 100 [Psoriasis Area and Severity Index],” the study’s primary endpoint that combined a measure of joint disease activity with a measure of skin involvement, said Dr. Mease, a rheumatologist at Swedish Medical Center in Seattle.
This unconventional primary endpoint for testing drugs that treat PsA was called out during discussion of the report for having an inherent bias favoring ixekizumab by its inclusion of a skin outcome that received equal weight with an assessment tool that focused on joint responses. “This was a very unusual primary endpoint that favored ixekizumab,” Roy M. Fleischmann, MD, a Dallas rheumatologist, commented during the discussion.
Dr. Mease readily admitted that the study’s design stacked the deck in favor of ixekizumab, but he added that this decision reflected a desire by the researchers who ran the study to choose a primary endpoint that represented both of the prominent pathologies seen in patients with PsA.
The primary endpoint used in the study “looks at PsA more holistically,” Dr. Mease said in an interview. “It forced clinicians to look beyond just the joints,” in PsA patients. “That has been a limitation of prior PsA treatment assessments,” which until this study have uniformly used single primary outcomes that focus on joint responses, most commonly the ACR20 measure of joint disease activity.
The SPIRIT-H2H (A Study of Ixekizumab [LY2439821] Versus Adalimumab in Participants With Psoriatic Arthritis) study enrolled adults with active PsA who had never before received treatment with a biological drug. Enrolled patients had to have both active disease in their joints and active plaque psoriasis, with an inadequate response to at least one conventional synthetic disease-modifying antirheumatic drug. The 566 patients randomized in the study averaged about 48 years old, a bit more than half were men, and patients averaged about 6 years with diagnosed PsA and about 15 years diagnosed with psoriasis. Just over two-thirds of the patients were on concurrent treatment with a conventional synthetic agent, most often methotrexate.
The two components of the primary endpoint each showed the anticipated result. Among the 269 patients treated with adalimumab for the full 24 weeks, 47% had an ACR50 response, as did 51% of the 262 patients who completed their full course of ixekizumab, a between-group difference that was not statistically significant. In contrast, the PASI 100 measure of complete skin resolution occurred in 47% of the adalimumab-treated patients and 60% of those on ixekizumab, a statistically significant difference.
Dr. Mease reported results for several other efficacy measures, and what was notable was statistically significant superiority for ixekizumab in a measure of entheses disease activity, the SPARCC [Spondyloarthritis Research Consortium of Canada] Enthesitis Index, which fell to zero in 57% of the ixekizumab patients and 45% of those on adalimumab. “It makes you wonder whether there is something special about interleukin-17 in enthesitis,” Dr. Mease said.
The safety results of the study were consistent with the known adverse effect profiles of both drugs.
The impact of these findings on practice remains to be seen, and will likely depend on both cost considerations as well as clinicians trying to tailor drug choices to individual patients. The relatively new drug, ixekizumab, is consequentially more expensive than the older adalimumab, which has had its price depressed by the recent introduction of a biosimilar agent as well as long-standing competition from multiple TNF inhibitors.
“I think in the United States insurers will continue to steer patients toward whichever drug is cheapest among the highly-effective options,” but this result should lead to more use of interleukin-17 inhibitors as second-line agents for PsA, and it might push some clinicians to prescribe it as the first-line treatment, Dr. Mease said. He was confident that the efficacy profile shown by ixekizumab in SPIRIT-H2H was likely a class effect. Economics aside, the impetus to prescribe ixekizumab or another interleukin-17 inhibitor will be greatest when a patient has more extensive skin involvement, while for patients with little or no skin symptoms clinicians will likely stick with the more established TNF inhibitors as the first drug class to prescribe for PsA.
SPIRIT-H2H was sponsored by Eli Lilly, the company that markets ixekizumab (Taltz). Dr. Mease has been a consultant to, speaker for, and received research funding from Eli Lilly and from several other companies. Dr. Fleischmann has been a consultant to and has received research funding from several companies including Eli Lilly.
SOURCE: Mease PJ et al. Ann Rheum Dis. 2019 Jun. doi: 10.1136/annrheumdis-2019-eular.8709.
MADRID – The interleukin-17A inhibitor ixekizumab surpassed the tumor necrosis factor inhibitor adalimumab for treatment of patients with psoriatic arthritis in a multicenter, randomized study with 566 enrolled patients, the first reported results from a head-to-head comparison for this disease of two different classes of biological drugs.
The results showed that a standard, 24-week regimen with each of these agents, both of which already have regulatory approval for treating psoriatic arthritis (PsA), led to achievement of the primary endpoint in 36% of patients treated with ixekizumab (Taltz) and 28% of patients treated with adalimumab (Humira), a statistically significant difference, Philip J. Mease, MD, said at the European Congress of Rheumatology.
“Ixekizumab was superior to adalimumab for improving signs and symptoms of active PsA, as measured by simultaneous achievement of ACR50 [American College of Rheumatology] and PASI 100 [Psoriasis Area and Severity Index],” the study’s primary endpoint that combined a measure of joint disease activity with a measure of skin involvement, said Dr. Mease, a rheumatologist at Swedish Medical Center in Seattle.
This unconventional primary endpoint for testing drugs that treat PsA was called out during discussion of the report for having an inherent bias favoring ixekizumab by its inclusion of a skin outcome that received equal weight with an assessment tool that focused on joint responses. “This was a very unusual primary endpoint that favored ixekizumab,” Roy M. Fleischmann, MD, a Dallas rheumatologist, commented during the discussion.
Dr. Mease readily admitted that the study’s design stacked the deck in favor of ixekizumab, but he added that this decision reflected a desire by the researchers who ran the study to choose a primary endpoint that represented both of the prominent pathologies seen in patients with PsA.
The primary endpoint used in the study “looks at PsA more holistically,” Dr. Mease said in an interview. “It forced clinicians to look beyond just the joints,” in PsA patients. “That has been a limitation of prior PsA treatment assessments,” which until this study have uniformly used single primary outcomes that focus on joint responses, most commonly the ACR20 measure of joint disease activity.
The SPIRIT-H2H (A Study of Ixekizumab [LY2439821] Versus Adalimumab in Participants With Psoriatic Arthritis) study enrolled adults with active PsA who had never before received treatment with a biological drug. Enrolled patients had to have both active disease in their joints and active plaque psoriasis, with an inadequate response to at least one conventional synthetic disease-modifying antirheumatic drug. The 566 patients randomized in the study averaged about 48 years old, a bit more than half were men, and patients averaged about 6 years with diagnosed PsA and about 15 years diagnosed with psoriasis. Just over two-thirds of the patients were on concurrent treatment with a conventional synthetic agent, most often methotrexate.
The two components of the primary endpoint each showed the anticipated result. Among the 269 patients treated with adalimumab for the full 24 weeks, 47% had an ACR50 response, as did 51% of the 262 patients who completed their full course of ixekizumab, a between-group difference that was not statistically significant. In contrast, the PASI 100 measure of complete skin resolution occurred in 47% of the adalimumab-treated patients and 60% of those on ixekizumab, a statistically significant difference.
Dr. Mease reported results for several other efficacy measures, and what was notable was statistically significant superiority for ixekizumab in a measure of entheses disease activity, the SPARCC [Spondyloarthritis Research Consortium of Canada] Enthesitis Index, which fell to zero in 57% of the ixekizumab patients and 45% of those on adalimumab. “It makes you wonder whether there is something special about interleukin-17 in enthesitis,” Dr. Mease said.
The safety results of the study were consistent with the known adverse effect profiles of both drugs.
The impact of these findings on practice remains to be seen, and will likely depend on both cost considerations as well as clinicians trying to tailor drug choices to individual patients. The relatively new drug, ixekizumab, is consequentially more expensive than the older adalimumab, which has had its price depressed by the recent introduction of a biosimilar agent as well as long-standing competition from multiple TNF inhibitors.
“I think in the United States insurers will continue to steer patients toward whichever drug is cheapest among the highly-effective options,” but this result should lead to more use of interleukin-17 inhibitors as second-line agents for PsA, and it might push some clinicians to prescribe it as the first-line treatment, Dr. Mease said. He was confident that the efficacy profile shown by ixekizumab in SPIRIT-H2H was likely a class effect. Economics aside, the impetus to prescribe ixekizumab or another interleukin-17 inhibitor will be greatest when a patient has more extensive skin involvement, while for patients with little or no skin symptoms clinicians will likely stick with the more established TNF inhibitors as the first drug class to prescribe for PsA.
SPIRIT-H2H was sponsored by Eli Lilly, the company that markets ixekizumab (Taltz). Dr. Mease has been a consultant to, speaker for, and received research funding from Eli Lilly and from several other companies. Dr. Fleischmann has been a consultant to and has received research funding from several companies including Eli Lilly.
SOURCE: Mease PJ et al. Ann Rheum Dis. 2019 Jun. doi: 10.1136/annrheumdis-2019-eular.8709.
REPORTING FROM THE EULAR 2019 CONGRESS
Teletriage connects uninsured with timely dermatologist care
MILAN – and optimized primary care physicians’ care of nonreferred patients, Cory Simpson, MD, PhD, reported at the World Congress of Dermatology.
With implementation of teledermatology, patient wait times for specialist input dropped from 13.9 days to 1.6 days (P less than .00001).
By allowing dermatologists to evaluate photographs of lesions and perform their own triage of referrals from primary care physicians (PCPs), the teletriage pilot program reduced the number of patients for whom dermatology consults were deemed necessary and also allowed optimal management for the nonreferred patients, said Dr. Simpson, of the University of Pennsylvania, Philadelphia.
“Teledermatology has the potential to increase access to dermatologist-level care, especially for underserved patients,” he commented. “It allows us to educate primary care physicians in resource-limited settings, and it also allows us to avoid suboptimal care of skin disease by nonspecialists – especially the more judicious use of antimicrobial agents and corticosteroids.”
Dr. Simpson explained to the international audience that, for many in the United States, access to a dermatologist requires a lengthy wait that can extend to months.
In Philadelphia, University of Pennsylvania dermatology residents and attending physicians volunteer in an outreach program that serves an uninsured population of primarily Latino immigrants. Operating 1 or 2 evenings a month, the medical and surgical dermatology clinics can accommodate from 8-12 appointments per clinic.
The clinic had been overwhelmed with referrals from PCPs, but Dr. Simpson and his colleagues realized that many of the conditions they were seeing – verruca vulgaris, hand dermatitis, and psoriasis, for example – did not necessarily need a face-to-face dermatologic evaluation.
The AccessDerm app, available at no cost by the American Academy of Dermatology, allows PCPs and dermatologists to communicate and collaborate. “This is a store-and-forward program, meaning the primary physician takes the photos and sends them to an off-site dermatologist who can then review them at his or her convenience,” Dr. Simpson said. “It’s a smartphone-based app, so actually, while I was at this conference, even though I’m thousands of miles from Philadelphia, I got through three consults this morning on my smartphone. It’s a very convenient way to be a volunteer.”
The consultation is between the PCP and the dermatologist, he added. “It’s the dermatologist talking to the PCP, and the patient receives the care recommendations from their primary doctor – so there’s no direct communication with the patient.”
Using the app, PCPs photographed skin lesions and completed simple history and physical exam modules within the app. Then, Dr. Simpson and his dermatology colleagues reviewed the photos and pertinent information.
If diagnostic uncertainty persisted after the teledermatology review, or if Dr. Simpson and his colleagues judged that a procedure such as a biopsy or lesion destruction was required, then the patient was scheduled for an appointment, with an interim plan put in place. Otherwise, patients were managed by teledermatology alone.
Of the 131 patients involved in the pilot study, 48 (37%) were female; the average patient age was 31.7 years (range, 1-92 years).
About 40% of patients were seen for inflammatory conditions, and another 20% for nonpigmented neoplasms. Almost 18% were seen for infectious reasons, with the remainder divided between pigmented neoplasms, hair disorders, and other conditions.
It turned out, said Dr. Simpson, that about two-thirds (65%) of the teletriage consultations ended in a definitive plan not requiring a face-to-face dermatology appointment. About a quarter (23%) were deferred to an in-person dermatology appointment, and the remaining 12% had an interim plan while more information was gathered.
Of the 32 neoplasms addressed by the teletriage strategy, 21 (66%) were deferred to an in-person visit. By contrast, 24 of the 95 non–neoplastic teletriage encounters were deferred to an in-person visit (P less than .001).
Overall, the strategy opened up 18% more appointment slots for new patients, Dr. Simpson said.
As part of the teletriage process, PCPs provided their proposed plan of care before receiving a dermatologist’s advice. When comparing the PCP’s plan to the dermatologist’s final plan, he and his colleagues found that there was a complete change of plan for three-quarters of visits (76%). A partial change happened 14% of the time, and only one in ten patients had no change in treatment plan as a result of the teledermatology consult. “This indicates again that specialist input matters,” he noted.
“This also gives us an opportunity to educate primary care physicians,” Dr. Simpson said, pointing out that in replies, he and his dermatologist colleagues included information about common diagnoses, including first-line treatments and “worrisome features they should be thinking about.”
He and his collaborators found that proper treatment would have been provided 30% of the time without a teledermatology consult, but that patients would have been undertreated 27% of the time. Overtreatment would have occurred at a rate of 11%, and care would have been unnecessarily delayed for about one in four patients. Unnecessary ED visits were averted for 6% of patients with the teletriage approach.
Examples of undertreatment included use of a weak topical steroid, missing infections or the need for referral, and using a suboptimal acne regimen. On the other hand, Dr. Simpson said, overtreatment with unnecessary antibiotics, antifungals, and antivirals also was averted; on some occasions, the PCP plan for an oral corticosteroid or an overly potent topical steroid was shifted to a more appropriate plan by teledermatology.
In sum, said Dr. Simpson, “teletriage via AccessDerm allowed us to reduce by tenfold the wait time for specialist input in dermatology cases. We were able to remove almost two-thirds of people from the queue ... waiting for dermatology appointments, which was very helpful to our clinic.”
And most importantly, he added, “this allowed us to allocate the limited number of in-person appointments that we had at this volunteer clinic to those that were more complicated cases.”
Dr. Simpson reported that he had no relevant disclosures. The project was funded by Penn Medicine and the American Academy of Dermatology.
MILAN – and optimized primary care physicians’ care of nonreferred patients, Cory Simpson, MD, PhD, reported at the World Congress of Dermatology.
With implementation of teledermatology, patient wait times for specialist input dropped from 13.9 days to 1.6 days (P less than .00001).
By allowing dermatologists to evaluate photographs of lesions and perform their own triage of referrals from primary care physicians (PCPs), the teletriage pilot program reduced the number of patients for whom dermatology consults were deemed necessary and also allowed optimal management for the nonreferred patients, said Dr. Simpson, of the University of Pennsylvania, Philadelphia.
“Teledermatology has the potential to increase access to dermatologist-level care, especially for underserved patients,” he commented. “It allows us to educate primary care physicians in resource-limited settings, and it also allows us to avoid suboptimal care of skin disease by nonspecialists – especially the more judicious use of antimicrobial agents and corticosteroids.”
Dr. Simpson explained to the international audience that, for many in the United States, access to a dermatologist requires a lengthy wait that can extend to months.
In Philadelphia, University of Pennsylvania dermatology residents and attending physicians volunteer in an outreach program that serves an uninsured population of primarily Latino immigrants. Operating 1 or 2 evenings a month, the medical and surgical dermatology clinics can accommodate from 8-12 appointments per clinic.
The clinic had been overwhelmed with referrals from PCPs, but Dr. Simpson and his colleagues realized that many of the conditions they were seeing – verruca vulgaris, hand dermatitis, and psoriasis, for example – did not necessarily need a face-to-face dermatologic evaluation.
The AccessDerm app, available at no cost by the American Academy of Dermatology, allows PCPs and dermatologists to communicate and collaborate. “This is a store-and-forward program, meaning the primary physician takes the photos and sends them to an off-site dermatologist who can then review them at his or her convenience,” Dr. Simpson said. “It’s a smartphone-based app, so actually, while I was at this conference, even though I’m thousands of miles from Philadelphia, I got through three consults this morning on my smartphone. It’s a very convenient way to be a volunteer.”
The consultation is between the PCP and the dermatologist, he added. “It’s the dermatologist talking to the PCP, and the patient receives the care recommendations from their primary doctor – so there’s no direct communication with the patient.”
Using the app, PCPs photographed skin lesions and completed simple history and physical exam modules within the app. Then, Dr. Simpson and his dermatology colleagues reviewed the photos and pertinent information.
If diagnostic uncertainty persisted after the teledermatology review, or if Dr. Simpson and his colleagues judged that a procedure such as a biopsy or lesion destruction was required, then the patient was scheduled for an appointment, with an interim plan put in place. Otherwise, patients were managed by teledermatology alone.
Of the 131 patients involved in the pilot study, 48 (37%) were female; the average patient age was 31.7 years (range, 1-92 years).
About 40% of patients were seen for inflammatory conditions, and another 20% for nonpigmented neoplasms. Almost 18% were seen for infectious reasons, with the remainder divided between pigmented neoplasms, hair disorders, and other conditions.
It turned out, said Dr. Simpson, that about two-thirds (65%) of the teletriage consultations ended in a definitive plan not requiring a face-to-face dermatology appointment. About a quarter (23%) were deferred to an in-person dermatology appointment, and the remaining 12% had an interim plan while more information was gathered.
Of the 32 neoplasms addressed by the teletriage strategy, 21 (66%) were deferred to an in-person visit. By contrast, 24 of the 95 non–neoplastic teletriage encounters were deferred to an in-person visit (P less than .001).
Overall, the strategy opened up 18% more appointment slots for new patients, Dr. Simpson said.
As part of the teletriage process, PCPs provided their proposed plan of care before receiving a dermatologist’s advice. When comparing the PCP’s plan to the dermatologist’s final plan, he and his colleagues found that there was a complete change of plan for three-quarters of visits (76%). A partial change happened 14% of the time, and only one in ten patients had no change in treatment plan as a result of the teledermatology consult. “This indicates again that specialist input matters,” he noted.
“This also gives us an opportunity to educate primary care physicians,” Dr. Simpson said, pointing out that in replies, he and his dermatologist colleagues included information about common diagnoses, including first-line treatments and “worrisome features they should be thinking about.”
He and his collaborators found that proper treatment would have been provided 30% of the time without a teledermatology consult, but that patients would have been undertreated 27% of the time. Overtreatment would have occurred at a rate of 11%, and care would have been unnecessarily delayed for about one in four patients. Unnecessary ED visits were averted for 6% of patients with the teletriage approach.
Examples of undertreatment included use of a weak topical steroid, missing infections or the need for referral, and using a suboptimal acne regimen. On the other hand, Dr. Simpson said, overtreatment with unnecessary antibiotics, antifungals, and antivirals also was averted; on some occasions, the PCP plan for an oral corticosteroid or an overly potent topical steroid was shifted to a more appropriate plan by teledermatology.
In sum, said Dr. Simpson, “teletriage via AccessDerm allowed us to reduce by tenfold the wait time for specialist input in dermatology cases. We were able to remove almost two-thirds of people from the queue ... waiting for dermatology appointments, which was very helpful to our clinic.”
And most importantly, he added, “this allowed us to allocate the limited number of in-person appointments that we had at this volunteer clinic to those that were more complicated cases.”
Dr. Simpson reported that he had no relevant disclosures. The project was funded by Penn Medicine and the American Academy of Dermatology.
MILAN – and optimized primary care physicians’ care of nonreferred patients, Cory Simpson, MD, PhD, reported at the World Congress of Dermatology.
With implementation of teledermatology, patient wait times for specialist input dropped from 13.9 days to 1.6 days (P less than .00001).
By allowing dermatologists to evaluate photographs of lesions and perform their own triage of referrals from primary care physicians (PCPs), the teletriage pilot program reduced the number of patients for whom dermatology consults were deemed necessary and also allowed optimal management for the nonreferred patients, said Dr. Simpson, of the University of Pennsylvania, Philadelphia.
“Teledermatology has the potential to increase access to dermatologist-level care, especially for underserved patients,” he commented. “It allows us to educate primary care physicians in resource-limited settings, and it also allows us to avoid suboptimal care of skin disease by nonspecialists – especially the more judicious use of antimicrobial agents and corticosteroids.”
Dr. Simpson explained to the international audience that, for many in the United States, access to a dermatologist requires a lengthy wait that can extend to months.
In Philadelphia, University of Pennsylvania dermatology residents and attending physicians volunteer in an outreach program that serves an uninsured population of primarily Latino immigrants. Operating 1 or 2 evenings a month, the medical and surgical dermatology clinics can accommodate from 8-12 appointments per clinic.
The clinic had been overwhelmed with referrals from PCPs, but Dr. Simpson and his colleagues realized that many of the conditions they were seeing – verruca vulgaris, hand dermatitis, and psoriasis, for example – did not necessarily need a face-to-face dermatologic evaluation.
The AccessDerm app, available at no cost by the American Academy of Dermatology, allows PCPs and dermatologists to communicate and collaborate. “This is a store-and-forward program, meaning the primary physician takes the photos and sends them to an off-site dermatologist who can then review them at his or her convenience,” Dr. Simpson said. “It’s a smartphone-based app, so actually, while I was at this conference, even though I’m thousands of miles from Philadelphia, I got through three consults this morning on my smartphone. It’s a very convenient way to be a volunteer.”
The consultation is between the PCP and the dermatologist, he added. “It’s the dermatologist talking to the PCP, and the patient receives the care recommendations from their primary doctor – so there’s no direct communication with the patient.”
Using the app, PCPs photographed skin lesions and completed simple history and physical exam modules within the app. Then, Dr. Simpson and his dermatology colleagues reviewed the photos and pertinent information.
If diagnostic uncertainty persisted after the teledermatology review, or if Dr. Simpson and his colleagues judged that a procedure such as a biopsy or lesion destruction was required, then the patient was scheduled for an appointment, with an interim plan put in place. Otherwise, patients were managed by teledermatology alone.
Of the 131 patients involved in the pilot study, 48 (37%) were female; the average patient age was 31.7 years (range, 1-92 years).
About 40% of patients were seen for inflammatory conditions, and another 20% for nonpigmented neoplasms. Almost 18% were seen for infectious reasons, with the remainder divided between pigmented neoplasms, hair disorders, and other conditions.
It turned out, said Dr. Simpson, that about two-thirds (65%) of the teletriage consultations ended in a definitive plan not requiring a face-to-face dermatology appointment. About a quarter (23%) were deferred to an in-person dermatology appointment, and the remaining 12% had an interim plan while more information was gathered.
Of the 32 neoplasms addressed by the teletriage strategy, 21 (66%) were deferred to an in-person visit. By contrast, 24 of the 95 non–neoplastic teletriage encounters were deferred to an in-person visit (P less than .001).
Overall, the strategy opened up 18% more appointment slots for new patients, Dr. Simpson said.
As part of the teletriage process, PCPs provided their proposed plan of care before receiving a dermatologist’s advice. When comparing the PCP’s plan to the dermatologist’s final plan, he and his colleagues found that there was a complete change of plan for three-quarters of visits (76%). A partial change happened 14% of the time, and only one in ten patients had no change in treatment plan as a result of the teledermatology consult. “This indicates again that specialist input matters,” he noted.
“This also gives us an opportunity to educate primary care physicians,” Dr. Simpson said, pointing out that in replies, he and his dermatologist colleagues included information about common diagnoses, including first-line treatments and “worrisome features they should be thinking about.”
He and his collaborators found that proper treatment would have been provided 30% of the time without a teledermatology consult, but that patients would have been undertreated 27% of the time. Overtreatment would have occurred at a rate of 11%, and care would have been unnecessarily delayed for about one in four patients. Unnecessary ED visits were averted for 6% of patients with the teletriage approach.
Examples of undertreatment included use of a weak topical steroid, missing infections or the need for referral, and using a suboptimal acne regimen. On the other hand, Dr. Simpson said, overtreatment with unnecessary antibiotics, antifungals, and antivirals also was averted; on some occasions, the PCP plan for an oral corticosteroid or an overly potent topical steroid was shifted to a more appropriate plan by teledermatology.
In sum, said Dr. Simpson, “teletriage via AccessDerm allowed us to reduce by tenfold the wait time for specialist input in dermatology cases. We were able to remove almost two-thirds of people from the queue ... waiting for dermatology appointments, which was very helpful to our clinic.”
And most importantly, he added, “this allowed us to allocate the limited number of in-person appointments that we had at this volunteer clinic to those that were more complicated cases.”
Dr. Simpson reported that he had no relevant disclosures. The project was funded by Penn Medicine and the American Academy of Dermatology.
REPORTING FROM WCD2019
Scabies rates plummeted with community mass drug administration
MILAN – In a region where scabies is endemic, a , findings that may have implications for future treatment of scabies or other infestations in other regions, dermatologist Margot Whitfield, MD, said at the World Congress of Dermatology.
“Mass drug administration is highly effective and safe in the treatment of endemic scabies,” she said.
Using a strategy of directly observed treatment (DOT) with oral ivermectin or topical permethrin for all residents of two separate island groups in Fiji, Dr. Whitfield, together with epidemiologist Lucia Romani, PhD, both of the University of New South Wales, Sydney, and coinvestigators, demonstrated large and sustained decreases in the rates of scabies and impetigo (N Engl J Med. 2015 Dec 10;373[24]:2305-13).
Across study arms, which included a usual care arm, the baseline rate for scabies ranged from 30% to 40%. With usual care, the rate dropped from 36.6% to 18.8% at the end of 12 months, a relative reduction of 49%. However, the 15.8% prevalence rate 12 months after permethrin DOT (from 41.7%), and the 1.9% rate 12 months after ivermectin DOT (from 32.1%) – reductions of 62% and 94%, respectively – represented much larger decreases, “especially since these reductions were seen without any further interventions,” Dr. Whitfield said. “This was extremely exciting, and a game-changer as far as the management of endemic scabies is concerned.”
At baseline, impetigo rates hovered around 20%-25%, and usual care resulted in a 32% reduction at 12 months. With permethrin DOT, the impetigo rate dropped by 54%; with ivermectin DOT, the impetigo rate dropped by 67%. “The community level of impetigo went down, purely as a result of treating the scabies,” Dr. Whitfield said.
The outcomes of this study, she noted, “have contributed to the global discussion of the treatment of scabies.”
Two years after the mass drug administration (MDA) campaign, scabies prevalence remained much lower than at baseline, with clinical scabies diagnosed in 15.2% of the usual care group, 13.5% of the permethrin group, and just 3.6% of the ivermectin group. “The exciting thing for us was that these levels ... were able to be sustained at 2 years,” Dr. Whitfield noted.
The islands that had received ivermectin saw a continued decline in impetigo prevalence as well: By 24 months, impetigo was seen in 2.6% of participants in that arm.
Scabies is a neglected – but highly treatable – tropical disease, she noted. It is associated with intense pruritus, which results in reduced quality of life, and excoriations predispose those affected to bacterial superinfections, commonly impetigo in the young.
In Fiji, the scabies mite infests nearly 40% of those aged 5-9 years, and over one-third of those younger than 5 years. Rates drop steeply with increasing age and then climb again for the elderly; still, prevalence tops 10% for all Fijian age groups, Dr. Whitfield pointed out. Overall, scabies prevalence is 23% in Fiji, with resultant impetigo affecting 19% of the population.
Providing more details about the study, she said that she and her collaborators – working in conjunction with the Fijian Ministry of Health – took advantage of the geography of the island country, whose 850,000 residents live on 300 islands, to compare mass drug treatment with either ivermectin or permethrin with usual care. “We actually didn’t look for ‘infected scabies,’ ” she explained. “We looked for scabies as one outcome, and infection as another.”
The study was designed to take advantage of lessons from previous public health work addressing filariasis and soil-transmitted helminths, and addressed the following question: In Fiji, could a single round of MDA for scabies control lead to sustained reductions in scabies and impetigo prevalence 12 months later, compared with standard care?
The study applied standard-of-care scabies treatment to residents of one island; here, all residents of the island were assessed for scabies, and those who received a clinical diagnosis of scabies, along with family members and close contacts, were treated. Another group of three small islands received permethrin MDA. A third pair of neighboring islands received ivermectin MDA.
For one MDA arm, island residents received oral ivermectin via DOT. A second DOT dose was administered for those with clinically diagnosed scabies. For pregnant and breastfeeding women, children weighing less than 15 kg, and those with ivermectin hypersensitivity, permethrin was used, Dr. Whitfield said.
The individuals in the permethrin MDA arm received one topical dose via DOT, with a second round of topical permethrin for those with topical scabies.
In all, 803 Fijians were assigned to receive standard of care, 532 permethrin MDA, and 716 ivermectin MDA. Of these, 623 received ivermectin DOT, and 93 received permethrin. In all, DOT was achieved for 96% of those receiving the first dose. At baseline, 230 patients had scabies, with 200 receiving ivermectin and 30 permethrin; the DOT rate was 100% for the second dose.
For the permethrin arm, just 307 of 532 participants (58%) had DOT, though all were given permethrin. Scabies was present at baseline for 222 participants, and of these, 181 had DOT. “It’s much easier to do the direct observed therapy with an oral medication than with a cream,” Dr. Whitfield said. Data were not collected for the Fijians who received usual care at community health centers.
Outcomes were clinically determined via the child skin assessment algorithm of the World Health Organization’s International Management of Childhood Illness (IMCI) guidelines.
Dr. Whitfield acknowledged that the study was not a true cluster-randomized trial, and differences existed between the communities studies. Also, “dermatoscopy was not a practical option” for this real-world trial in a resource-limited setting, but validated clinical criteria were used, she said.
Going forward, she and her colleagues are continuing to track durability of reduced scabies rates, as well as downstream sequelae such as impetigo and septicemia. Also, “we need to see whether this community- and island-based project could be scaled up to a national or regional level,” she said.
The burden of disease from scabies globally is probably underestimated, and changing migration patterns may bring endemic scabies to the doorsteps of more developed nations, prompting consideration of MDA as a strategy in expanded circumstances.
Dr. Whitfield reported that she had no relevant conflicts of interest.
MILAN – In a region where scabies is endemic, a , findings that may have implications for future treatment of scabies or other infestations in other regions, dermatologist Margot Whitfield, MD, said at the World Congress of Dermatology.
“Mass drug administration is highly effective and safe in the treatment of endemic scabies,” she said.
Using a strategy of directly observed treatment (DOT) with oral ivermectin or topical permethrin for all residents of two separate island groups in Fiji, Dr. Whitfield, together with epidemiologist Lucia Romani, PhD, both of the University of New South Wales, Sydney, and coinvestigators, demonstrated large and sustained decreases in the rates of scabies and impetigo (N Engl J Med. 2015 Dec 10;373[24]:2305-13).
Across study arms, which included a usual care arm, the baseline rate for scabies ranged from 30% to 40%. With usual care, the rate dropped from 36.6% to 18.8% at the end of 12 months, a relative reduction of 49%. However, the 15.8% prevalence rate 12 months after permethrin DOT (from 41.7%), and the 1.9% rate 12 months after ivermectin DOT (from 32.1%) – reductions of 62% and 94%, respectively – represented much larger decreases, “especially since these reductions were seen without any further interventions,” Dr. Whitfield said. “This was extremely exciting, and a game-changer as far as the management of endemic scabies is concerned.”
At baseline, impetigo rates hovered around 20%-25%, and usual care resulted in a 32% reduction at 12 months. With permethrin DOT, the impetigo rate dropped by 54%; with ivermectin DOT, the impetigo rate dropped by 67%. “The community level of impetigo went down, purely as a result of treating the scabies,” Dr. Whitfield said.
The outcomes of this study, she noted, “have contributed to the global discussion of the treatment of scabies.”
Two years after the mass drug administration (MDA) campaign, scabies prevalence remained much lower than at baseline, with clinical scabies diagnosed in 15.2% of the usual care group, 13.5% of the permethrin group, and just 3.6% of the ivermectin group. “The exciting thing for us was that these levels ... were able to be sustained at 2 years,” Dr. Whitfield noted.
The islands that had received ivermectin saw a continued decline in impetigo prevalence as well: By 24 months, impetigo was seen in 2.6% of participants in that arm.
Scabies is a neglected – but highly treatable – tropical disease, she noted. It is associated with intense pruritus, which results in reduced quality of life, and excoriations predispose those affected to bacterial superinfections, commonly impetigo in the young.
In Fiji, the scabies mite infests nearly 40% of those aged 5-9 years, and over one-third of those younger than 5 years. Rates drop steeply with increasing age and then climb again for the elderly; still, prevalence tops 10% for all Fijian age groups, Dr. Whitfield pointed out. Overall, scabies prevalence is 23% in Fiji, with resultant impetigo affecting 19% of the population.
Providing more details about the study, she said that she and her collaborators – working in conjunction with the Fijian Ministry of Health – took advantage of the geography of the island country, whose 850,000 residents live on 300 islands, to compare mass drug treatment with either ivermectin or permethrin with usual care. “We actually didn’t look for ‘infected scabies,’ ” she explained. “We looked for scabies as one outcome, and infection as another.”
The study was designed to take advantage of lessons from previous public health work addressing filariasis and soil-transmitted helminths, and addressed the following question: In Fiji, could a single round of MDA for scabies control lead to sustained reductions in scabies and impetigo prevalence 12 months later, compared with standard care?
The study applied standard-of-care scabies treatment to residents of one island; here, all residents of the island were assessed for scabies, and those who received a clinical diagnosis of scabies, along with family members and close contacts, were treated. Another group of three small islands received permethrin MDA. A third pair of neighboring islands received ivermectin MDA.
For one MDA arm, island residents received oral ivermectin via DOT. A second DOT dose was administered for those with clinically diagnosed scabies. For pregnant and breastfeeding women, children weighing less than 15 kg, and those with ivermectin hypersensitivity, permethrin was used, Dr. Whitfield said.
The individuals in the permethrin MDA arm received one topical dose via DOT, with a second round of topical permethrin for those with topical scabies.
In all, 803 Fijians were assigned to receive standard of care, 532 permethrin MDA, and 716 ivermectin MDA. Of these, 623 received ivermectin DOT, and 93 received permethrin. In all, DOT was achieved for 96% of those receiving the first dose. At baseline, 230 patients had scabies, with 200 receiving ivermectin and 30 permethrin; the DOT rate was 100% for the second dose.
For the permethrin arm, just 307 of 532 participants (58%) had DOT, though all were given permethrin. Scabies was present at baseline for 222 participants, and of these, 181 had DOT. “It’s much easier to do the direct observed therapy with an oral medication than with a cream,” Dr. Whitfield said. Data were not collected for the Fijians who received usual care at community health centers.
Outcomes were clinically determined via the child skin assessment algorithm of the World Health Organization’s International Management of Childhood Illness (IMCI) guidelines.
Dr. Whitfield acknowledged that the study was not a true cluster-randomized trial, and differences existed between the communities studies. Also, “dermatoscopy was not a practical option” for this real-world trial in a resource-limited setting, but validated clinical criteria were used, she said.
Going forward, she and her colleagues are continuing to track durability of reduced scabies rates, as well as downstream sequelae such as impetigo and septicemia. Also, “we need to see whether this community- and island-based project could be scaled up to a national or regional level,” she said.
The burden of disease from scabies globally is probably underestimated, and changing migration patterns may bring endemic scabies to the doorsteps of more developed nations, prompting consideration of MDA as a strategy in expanded circumstances.
Dr. Whitfield reported that she had no relevant conflicts of interest.
MILAN – In a region where scabies is endemic, a , findings that may have implications for future treatment of scabies or other infestations in other regions, dermatologist Margot Whitfield, MD, said at the World Congress of Dermatology.
“Mass drug administration is highly effective and safe in the treatment of endemic scabies,” she said.
Using a strategy of directly observed treatment (DOT) with oral ivermectin or topical permethrin for all residents of two separate island groups in Fiji, Dr. Whitfield, together with epidemiologist Lucia Romani, PhD, both of the University of New South Wales, Sydney, and coinvestigators, demonstrated large and sustained decreases in the rates of scabies and impetigo (N Engl J Med. 2015 Dec 10;373[24]:2305-13).
Across study arms, which included a usual care arm, the baseline rate for scabies ranged from 30% to 40%. With usual care, the rate dropped from 36.6% to 18.8% at the end of 12 months, a relative reduction of 49%. However, the 15.8% prevalence rate 12 months after permethrin DOT (from 41.7%), and the 1.9% rate 12 months after ivermectin DOT (from 32.1%) – reductions of 62% and 94%, respectively – represented much larger decreases, “especially since these reductions were seen without any further interventions,” Dr. Whitfield said. “This was extremely exciting, and a game-changer as far as the management of endemic scabies is concerned.”
At baseline, impetigo rates hovered around 20%-25%, and usual care resulted in a 32% reduction at 12 months. With permethrin DOT, the impetigo rate dropped by 54%; with ivermectin DOT, the impetigo rate dropped by 67%. “The community level of impetigo went down, purely as a result of treating the scabies,” Dr. Whitfield said.
The outcomes of this study, she noted, “have contributed to the global discussion of the treatment of scabies.”
Two years after the mass drug administration (MDA) campaign, scabies prevalence remained much lower than at baseline, with clinical scabies diagnosed in 15.2% of the usual care group, 13.5% of the permethrin group, and just 3.6% of the ivermectin group. “The exciting thing for us was that these levels ... were able to be sustained at 2 years,” Dr. Whitfield noted.
The islands that had received ivermectin saw a continued decline in impetigo prevalence as well: By 24 months, impetigo was seen in 2.6% of participants in that arm.
Scabies is a neglected – but highly treatable – tropical disease, she noted. It is associated with intense pruritus, which results in reduced quality of life, and excoriations predispose those affected to bacterial superinfections, commonly impetigo in the young.
In Fiji, the scabies mite infests nearly 40% of those aged 5-9 years, and over one-third of those younger than 5 years. Rates drop steeply with increasing age and then climb again for the elderly; still, prevalence tops 10% for all Fijian age groups, Dr. Whitfield pointed out. Overall, scabies prevalence is 23% in Fiji, with resultant impetigo affecting 19% of the population.
Providing more details about the study, she said that she and her collaborators – working in conjunction with the Fijian Ministry of Health – took advantage of the geography of the island country, whose 850,000 residents live on 300 islands, to compare mass drug treatment with either ivermectin or permethrin with usual care. “We actually didn’t look for ‘infected scabies,’ ” she explained. “We looked for scabies as one outcome, and infection as another.”
The study was designed to take advantage of lessons from previous public health work addressing filariasis and soil-transmitted helminths, and addressed the following question: In Fiji, could a single round of MDA for scabies control lead to sustained reductions in scabies and impetigo prevalence 12 months later, compared with standard care?
The study applied standard-of-care scabies treatment to residents of one island; here, all residents of the island were assessed for scabies, and those who received a clinical diagnosis of scabies, along with family members and close contacts, were treated. Another group of three small islands received permethrin MDA. A third pair of neighboring islands received ivermectin MDA.
For one MDA arm, island residents received oral ivermectin via DOT. A second DOT dose was administered for those with clinically diagnosed scabies. For pregnant and breastfeeding women, children weighing less than 15 kg, and those with ivermectin hypersensitivity, permethrin was used, Dr. Whitfield said.
The individuals in the permethrin MDA arm received one topical dose via DOT, with a second round of topical permethrin for those with topical scabies.
In all, 803 Fijians were assigned to receive standard of care, 532 permethrin MDA, and 716 ivermectin MDA. Of these, 623 received ivermectin DOT, and 93 received permethrin. In all, DOT was achieved for 96% of those receiving the first dose. At baseline, 230 patients had scabies, with 200 receiving ivermectin and 30 permethrin; the DOT rate was 100% for the second dose.
For the permethrin arm, just 307 of 532 participants (58%) had DOT, though all were given permethrin. Scabies was present at baseline for 222 participants, and of these, 181 had DOT. “It’s much easier to do the direct observed therapy with an oral medication than with a cream,” Dr. Whitfield said. Data were not collected for the Fijians who received usual care at community health centers.
Outcomes were clinically determined via the child skin assessment algorithm of the World Health Organization’s International Management of Childhood Illness (IMCI) guidelines.
Dr. Whitfield acknowledged that the study was not a true cluster-randomized trial, and differences existed between the communities studies. Also, “dermatoscopy was not a practical option” for this real-world trial in a resource-limited setting, but validated clinical criteria were used, she said.
Going forward, she and her colleagues are continuing to track durability of reduced scabies rates, as well as downstream sequelae such as impetigo and septicemia. Also, “we need to see whether this community- and island-based project could be scaled up to a national or regional level,” she said.
The burden of disease from scabies globally is probably underestimated, and changing migration patterns may bring endemic scabies to the doorsteps of more developed nations, prompting consideration of MDA as a strategy in expanded circumstances.
Dr. Whitfield reported that she had no relevant conflicts of interest.
EXPERT ANALYSIS FROM WCD2019
Painful ulcers on forehead
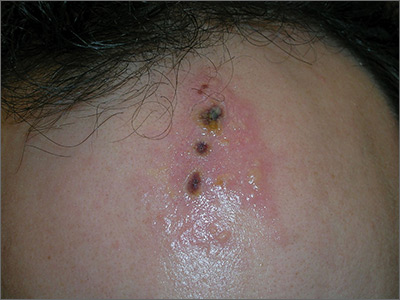
Based on the areas of necrosis, the FP suspected that a brown recluse spider bite caused the lesions. He suspected that the whole area of swelling and erythema was the bite reaction, and the 3 ulcerated areas were the regions of necrosis (often there is only 1 central area). The FP considered cellulitis as part of the differential diagnosis and debated whether to prescribe an antibiotic.
While the patient and FP believed that the most likely diagnosis was a spider bite, they were not completely certain of this diagnosis because the patient never saw the spider. Therefore, the patient requested an antibiotic in case this was a bacterial infection. A bacterial culture of the ulcer was performed and the patient was given a 5-day course of oral cephalexin 500 mg 4 times a day.
The FP recommended over-the-counter oral diphenhydramine to be taken around the clock as tolerated along with ibuprofen at mealtimes. The FP kept in contact with the patient by phone and improvement was noted daily. The bacterial culture came back negative, and the lesions all healed with time.
Spider bites can be difficult to diagnose as many lesions are blamed on spiders without evidence of a bite or spider. Conversely, spider bites may be missed as the spider is not typically available for easy identification. To this day, the FP and patient believe these lesions were the work of a brown recluse spider.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Based on the areas of necrosis, the FP suspected that a brown recluse spider bite caused the lesions. He suspected that the whole area of swelling and erythema was the bite reaction, and the 3 ulcerated areas were the regions of necrosis (often there is only 1 central area). The FP considered cellulitis as part of the differential diagnosis and debated whether to prescribe an antibiotic.
While the patient and FP believed that the most likely diagnosis was a spider bite, they were not completely certain of this diagnosis because the patient never saw the spider. Therefore, the patient requested an antibiotic in case this was a bacterial infection. A bacterial culture of the ulcer was performed and the patient was given a 5-day course of oral cephalexin 500 mg 4 times a day.
The FP recommended over-the-counter oral diphenhydramine to be taken around the clock as tolerated along with ibuprofen at mealtimes. The FP kept in contact with the patient by phone and improvement was noted daily. The bacterial culture came back negative, and the lesions all healed with time.
Spider bites can be difficult to diagnose as many lesions are blamed on spiders without evidence of a bite or spider. Conversely, spider bites may be missed as the spider is not typically available for easy identification. To this day, the FP and patient believe these lesions were the work of a brown recluse spider.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Based on the areas of necrosis, the FP suspected that a brown recluse spider bite caused the lesions. He suspected that the whole area of swelling and erythema was the bite reaction, and the 3 ulcerated areas were the regions of necrosis (often there is only 1 central area). The FP considered cellulitis as part of the differential diagnosis and debated whether to prescribe an antibiotic.
While the patient and FP believed that the most likely diagnosis was a spider bite, they were not completely certain of this diagnosis because the patient never saw the spider. Therefore, the patient requested an antibiotic in case this was a bacterial infection. A bacterial culture of the ulcer was performed and the patient was given a 5-day course of oral cephalexin 500 mg 4 times a day.
The FP recommended over-the-counter oral diphenhydramine to be taken around the clock as tolerated along with ibuprofen at mealtimes. The FP kept in contact with the patient by phone and improvement was noted daily. The bacterial culture came back negative, and the lesions all healed with time.
Spider bites can be difficult to diagnose as many lesions are blamed on spiders without evidence of a bite or spider. Conversely, spider bites may be missed as the spider is not typically available for easy identification. To this day, the FP and patient believe these lesions were the work of a brown recluse spider.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Acute Graft-vs-host Disease Following Liver Transplantation
Acute graft-vs-host disease (GVHD) is a T-cell mediated immunogenic response in which T lymphocytes from a donor regard host tissue as foreign and attack it in the setting of immunosuppression.1 The most common cause of acute GVHD is allogeneic stem cell transplantation, with solid-organ transplantation being a much less common cause.2 The incidence of acute GVHD following orthotopic liver transplantation (OLT) is 0.1%, as reported by the United Network for Organ Sharing, compared to an incidence of 40% to 60% in hematopoietic stem cell transplant recipients.3,4
Early recognition and treatment of acute GVHD following liver transplantation is imperative, as the mortality rate is 85% to 90%.2 We present a case of acute GVHD in a liver transplantation patient, with a focus on diagnostic criteria and comparison to acute GVHD following hematopoietic stem cell transplantation.
Case Report
A 68-year-old woman with a history of hepatitis C virus infection, hepatocellular carcinoma, and OLT 1 month prior presented to the hospital with fever and abdominal cellulitis in close proximity to the surgical site of 1 week’s duration. The patient was started on vancomycin and cefepime; pan cultures were performed.
At 10 days of hospitalization, the patient developed a pruritic, nontender, erythematous rash on the abdomen, with extension onto the chest and legs. The rash was associated with low-grade fever but not with diarrhea. Physical examination was notable for a few erythematous macules and scattered papules over the neck and chest and a large erythematous plaque with multiple ecchymoses over the lower abdomen (Figure 1A). Erythematous macules and papules coalescing into plaques were present on the lower back (Figure 1B) and proximal thighs. Oral, ocular, and genital lesions were absent.
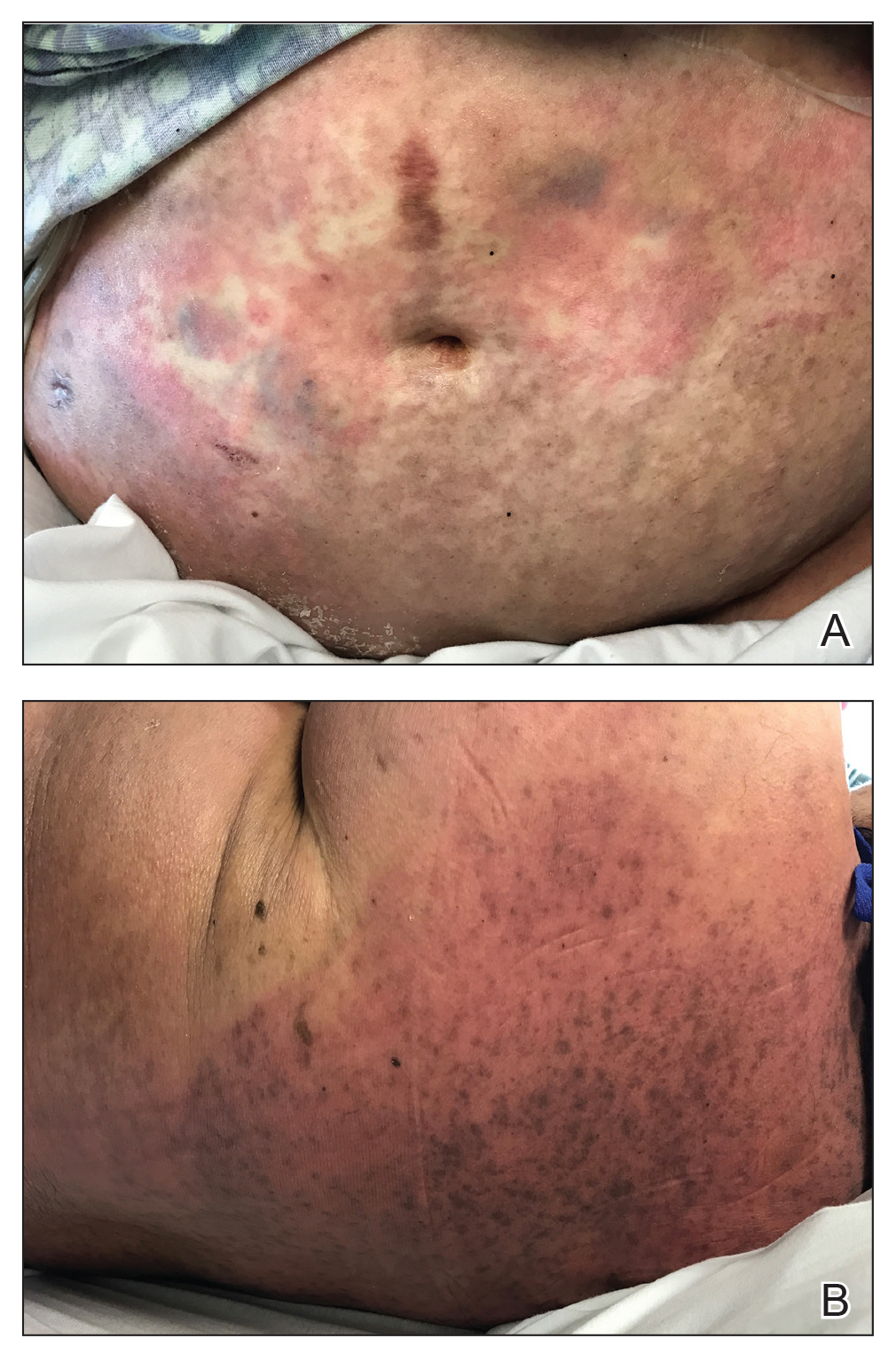
The differential diagnosis included drug reaction, viral infection, and acute GVHD. A skin biopsy was performed from the left side of the chest. Cefepime and vancomycin were discontinued; triamcinolone ointment 0.1% twice daily and antihistamines as needed for itching were started.
Over a 2-day period, the rash progressed to diffuse erythematous papules over the chest (Figure 2A) and bilateral arms (Figure 2B) including the palms. The patient also developed erythematous papules over the jawline and forehead as well as confluent erythematous plaques over the back with extension of the rash to involve the legs. She also had erythema and swelling bilaterally over the ears. She reported diarrhea. The low-grade fever resolved.
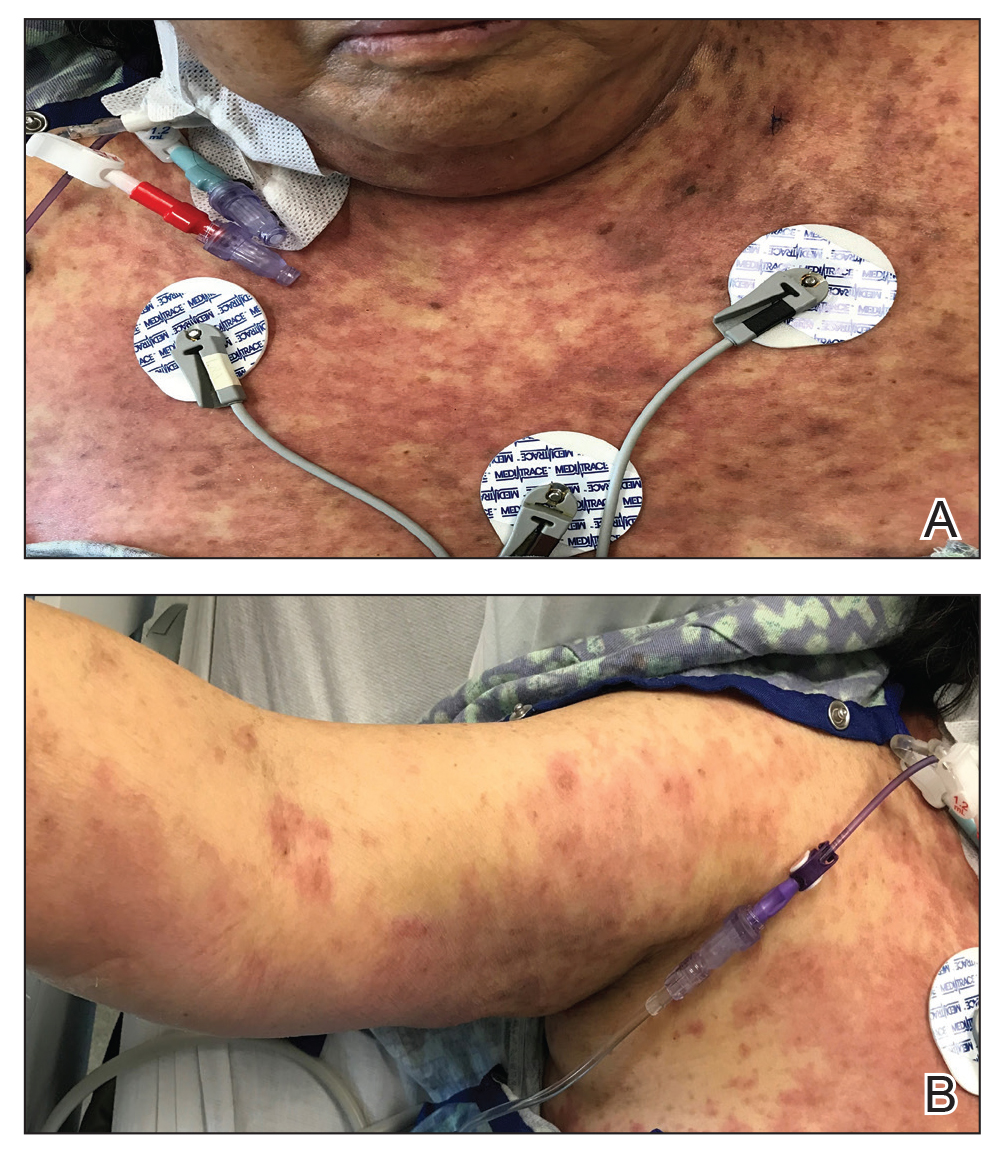
Laboratory review showed new-onset pancytopenia, normal liver function, and an elevated creatinine level of 2.3 mg/dL (reference range, 0.6–1.2 mg/dL), consistent with the patient’s baseline of stage 3 chronic kidney disease. Polymerase chain reaction analysis for cytomegalovirus was negative. Histology revealed vacuolar interface dermatitis with apoptotic keratinocytes, consistent with grade I GVHD (Figure 3). Duodenal biopsy revealed rare patchy glands with increased apoptosis, compatible with grade I GVHD.
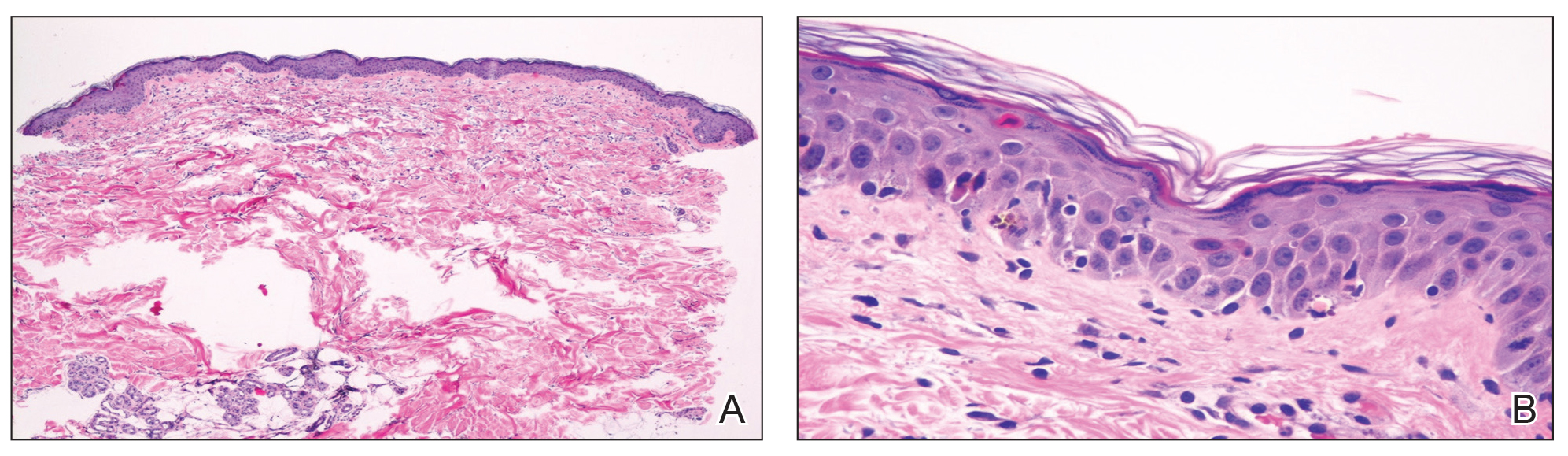
The patient was started on intravenous methylprednisolone 1 mg/kg for 3 days, then transitioned to an oral steroid taper, with improvement of the rash and other systemic symptoms.
Comment
GVHD Subtypes
The 2 types of GVHD are humoral and cellular.5 The humoral type results from ABO blood type incompatibility between donor and recipient and causes mild hemolytic anemia and fever. The cellular type is directed against major histocompatibility complexes and is associated with high morbidity and mortality.
Presentation of GVHD
Acute GVHD following OLT usually occurs 3 to 5 weeks after transplantation,6 as in our patient. Symptoms include rash, fever, pancytopenia, and diarrhea.2 Skin is the most commonly involved organ in acute GVHD; rash is the earliest manifestation.1 The rash can be asymptomatic or associated with pain and pruritus. Initial cutaneous manifestations include palmar erythema and erythematous to violaceous discoloration of the face and ears. A diffuse maculopapular rash can develop, involving the face, abdomen, and trunk. The rash may progress to formation of bullae or skin sloughing, resembling Stevens-Johnson syndrome or toxic epidermal necrolysis.1 The skin manifestation of acute GVHD following OLT is similar to hematopoietic stem cell transplantation (Table).7,8
Pancytopenia is a common manifestation of GVHD following liver transplantation and is rarely seen following hematopoietic stem cell transplantation.7 Donor lymphocytes engraft and proliferate in the bone marrow, attacking recipient hematopoietic stem cells. It is important to note that more common causes of cytopenia following liver transplantation, including infection and drug-induced bone marrow suppression, should be ruled out before diagnosing acute GVHD.6
Acute GVHD can affect the gastrointestinal tract, causing diarrhea; however, other infectious and medication-induced causes of diarrhea also should be considered.6 In contrast to hematopoietic stem cell transplantation, in which the liver is usually involved,1 the liver is spared in acute GVHD following liver transplantation.5
Diagnosis of GVHD
The diagnosis of acute GVHD following liver transplantation can be challenging because the clinical manifestations can be caused by a drug reaction or viral infection, such as cytomegalovirus infection.2 Patients who are older than 50 years and glucose intolerant are at a higher risk of acute GVHD following OLT. The combination of younger donor age and the presence of an HLA class I match also increases the risk of acute GVHD.6 The diagnosis of acute GVHD is confirmed with biopsy of the skin or gastrointestinal tract.
Morbidity and Mortality of GVHD
Because of the high morbidity and mortality associated with acute GVHD following liver transplantation, early diagnosis and treatment are crucial.5 Death in patients with acute GVHD following OLT is mainly attributable to sepsis, multiorgan failure, and gastrointestinal tract bleeding.6 It remains unclear whether this high mortality is associated with delayed diagnosis due to nonspecific signs of acute GVHD following OLT or to the lack of appropriate treatment guidelines.6
Treatment Options
Because of the low incidence of acute GVHD following OLT, most treatment modalities are extrapolated from the literature on acute GVHD following stem cell transplantation.5 The most commonly used therapies include high-dose systemic steroids and anti–thymocyte globulin that attacks activated donor T cells.6 Other treatment modalities, including anti–tumor necrosis factor agents and antibodies to CD20, have been reported to be effective in steroid-refractory GVHD.2 The major drawback of systemic steroids is an increase in the risk for sepsis and infection; therefore, these patients should be diligently screened for infection and covered with antibiotics and antifungals. Extracorporeal photopheresis is another treatment modality that does not cause generalized immunosuppression but is not well studied in the setting of acute GVHD following OLT.6
Prevention
Acute GVHD following OLT can be prevented by eliminating donor T lymphocytes from the liver before transplantation. However, because the incidence of acute GVHD following OLT is very low, this approach is not routinely taken.2
Conclusion
Acute GVHD following liver transplantation is a rare complication; however, it has high mortality, necessitating further research regarding treatment and prevention. Early recognition and treatment of this condition can improve outcomes. Dermatologists should be familiar with the skin manifestations of acute GVHD following liver transplantation due to the rising number of cases of solid-organ transplantation.
- Hu SW, Cotliar J. Acute graft-versus-host disease following hematopoietic stem-cell transplantation. Dermatol Ther. 2011;24:411-423.
- Akbulut S, Yilmaz M, Yilmaz S. Graft-versus-host disease after liver transplantation: a comprehensive literature review. World J Gastroenterol. 2012;18:5240-5248.
- Taylor AL, Gibbs P, Bradley JA. Acute graft versus host disease following liver transplantation: the enemy within. Am J Transplant. 2004;4:466-474.
- Jagasia M, Arora M, Flowers ME, et al. Risk factor for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296-307.
- Kang WH, Hwang S, Song GW, et al. Acute graft-vs-host disease after liver transplantation: experience at a high-volume liver transplantation center in Korea. Transplant Proc. 2016;48:3368-3372.
- Murali AR, Chandra S, Stewart Z, et al. Graft versus host disease after liver transplantation in adults: a case series, review of literature, and an approach to management. Transplantation. 2016;100:2661-2670.
- Chaib E, Silva FD, Figueira ER, et al. Graft-versus-host disease after liver transplantation. Clinics (Sao Paulo). 2011;66:1115-1118.
- Barton-Burke M, Dwinell DM, Kafkas L, et al. Graft-versus-host disease: a complex long-term side effect of hematopoietic stem cell transplant. Oncology (Williston Park). 2008;22(11 Suppl Nurse Ed):31-45.
Acute graft-vs-host disease (GVHD) is a T-cell mediated immunogenic response in which T lymphocytes from a donor regard host tissue as foreign and attack it in the setting of immunosuppression.1 The most common cause of acute GVHD is allogeneic stem cell transplantation, with solid-organ transplantation being a much less common cause.2 The incidence of acute GVHD following orthotopic liver transplantation (OLT) is 0.1%, as reported by the United Network for Organ Sharing, compared to an incidence of 40% to 60% in hematopoietic stem cell transplant recipients.3,4
Early recognition and treatment of acute GVHD following liver transplantation is imperative, as the mortality rate is 85% to 90%.2 We present a case of acute GVHD in a liver transplantation patient, with a focus on diagnostic criteria and comparison to acute GVHD following hematopoietic stem cell transplantation.
Case Report
A 68-year-old woman with a history of hepatitis C virus infection, hepatocellular carcinoma, and OLT 1 month prior presented to the hospital with fever and abdominal cellulitis in close proximity to the surgical site of 1 week’s duration. The patient was started on vancomycin and cefepime; pan cultures were performed.
At 10 days of hospitalization, the patient developed a pruritic, nontender, erythematous rash on the abdomen, with extension onto the chest and legs. The rash was associated with low-grade fever but not with diarrhea. Physical examination was notable for a few erythematous macules and scattered papules over the neck and chest and a large erythematous plaque with multiple ecchymoses over the lower abdomen (Figure 1A). Erythematous macules and papules coalescing into plaques were present on the lower back (Figure 1B) and proximal thighs. Oral, ocular, and genital lesions were absent.

The differential diagnosis included drug reaction, viral infection, and acute GVHD. A skin biopsy was performed from the left side of the chest. Cefepime and vancomycin were discontinued; triamcinolone ointment 0.1% twice daily and antihistamines as needed for itching were started.
Over a 2-day period, the rash progressed to diffuse erythematous papules over the chest (Figure 2A) and bilateral arms (Figure 2B) including the palms. The patient also developed erythematous papules over the jawline and forehead as well as confluent erythematous plaques over the back with extension of the rash to involve the legs. She also had erythema and swelling bilaterally over the ears. She reported diarrhea. The low-grade fever resolved.

Laboratory review showed new-onset pancytopenia, normal liver function, and an elevated creatinine level of 2.3 mg/dL (reference range, 0.6–1.2 mg/dL), consistent with the patient’s baseline of stage 3 chronic kidney disease. Polymerase chain reaction analysis for cytomegalovirus was negative. Histology revealed vacuolar interface dermatitis with apoptotic keratinocytes, consistent with grade I GVHD (Figure 3). Duodenal biopsy revealed rare patchy glands with increased apoptosis, compatible with grade I GVHD.

The patient was started on intravenous methylprednisolone 1 mg/kg for 3 days, then transitioned to an oral steroid taper, with improvement of the rash and other systemic symptoms.
Comment
GVHD Subtypes
The 2 types of GVHD are humoral and cellular.5 The humoral type results from ABO blood type incompatibility between donor and recipient and causes mild hemolytic anemia and fever. The cellular type is directed against major histocompatibility complexes and is associated with high morbidity and mortality.
Presentation of GVHD
Acute GVHD following OLT usually occurs 3 to 5 weeks after transplantation,6 as in our patient. Symptoms include rash, fever, pancytopenia, and diarrhea.2 Skin is the most commonly involved organ in acute GVHD; rash is the earliest manifestation.1 The rash can be asymptomatic or associated with pain and pruritus. Initial cutaneous manifestations include palmar erythema and erythematous to violaceous discoloration of the face and ears. A diffuse maculopapular rash can develop, involving the face, abdomen, and trunk. The rash may progress to formation of bullae or skin sloughing, resembling Stevens-Johnson syndrome or toxic epidermal necrolysis.1 The skin manifestation of acute GVHD following OLT is similar to hematopoietic stem cell transplantation (Table).7,8

Pancytopenia is a common manifestation of GVHD following liver transplantation and is rarely seen following hematopoietic stem cell transplantation.7 Donor lymphocytes engraft and proliferate in the bone marrow, attacking recipient hematopoietic stem cells. It is important to note that more common causes of cytopenia following liver transplantation, including infection and drug-induced bone marrow suppression, should be ruled out before diagnosing acute GVHD.6
Acute GVHD can affect the gastrointestinal tract, causing diarrhea; however, other infectious and medication-induced causes of diarrhea also should be considered.6 In contrast to hematopoietic stem cell transplantation, in which the liver is usually involved,1 the liver is spared in acute GVHD following liver transplantation.5
Diagnosis of GVHD
The diagnosis of acute GVHD following liver transplantation can be challenging because the clinical manifestations can be caused by a drug reaction or viral infection, such as cytomegalovirus infection.2 Patients who are older than 50 years and glucose intolerant are at a higher risk of acute GVHD following OLT. The combination of younger donor age and the presence of an HLA class I match also increases the risk of acute GVHD.6 The diagnosis of acute GVHD is confirmed with biopsy of the skin or gastrointestinal tract.
Morbidity and Mortality of GVHD
Because of the high morbidity and mortality associated with acute GVHD following liver transplantation, early diagnosis and treatment are crucial.5 Death in patients with acute GVHD following OLT is mainly attributable to sepsis, multiorgan failure, and gastrointestinal tract bleeding.6 It remains unclear whether this high mortality is associated with delayed diagnosis due to nonspecific signs of acute GVHD following OLT or to the lack of appropriate treatment guidelines.6
Treatment Options
Because of the low incidence of acute GVHD following OLT, most treatment modalities are extrapolated from the literature on acute GVHD following stem cell transplantation.5 The most commonly used therapies include high-dose systemic steroids and anti–thymocyte globulin that attacks activated donor T cells.6 Other treatment modalities, including anti–tumor necrosis factor agents and antibodies to CD20, have been reported to be effective in steroid-refractory GVHD.2 The major drawback of systemic steroids is an increase in the risk for sepsis and infection; therefore, these patients should be diligently screened for infection and covered with antibiotics and antifungals. Extracorporeal photopheresis is another treatment modality that does not cause generalized immunosuppression but is not well studied in the setting of acute GVHD following OLT.6
Prevention
Acute GVHD following OLT can be prevented by eliminating donor T lymphocytes from the liver before transplantation. However, because the incidence of acute GVHD following OLT is very low, this approach is not routinely taken.2
Conclusion
Acute GVHD following liver transplantation is a rare complication; however, it has high mortality, necessitating further research regarding treatment and prevention. Early recognition and treatment of this condition can improve outcomes. Dermatologists should be familiar with the skin manifestations of acute GVHD following liver transplantation due to the rising number of cases of solid-organ transplantation.
Acute graft-vs-host disease (GVHD) is a T-cell mediated immunogenic response in which T lymphocytes from a donor regard host tissue as foreign and attack it in the setting of immunosuppression.1 The most common cause of acute GVHD is allogeneic stem cell transplantation, with solid-organ transplantation being a much less common cause.2 The incidence of acute GVHD following orthotopic liver transplantation (OLT) is 0.1%, as reported by the United Network for Organ Sharing, compared to an incidence of 40% to 60% in hematopoietic stem cell transplant recipients.3,4
Early recognition and treatment of acute GVHD following liver transplantation is imperative, as the mortality rate is 85% to 90%.2 We present a case of acute GVHD in a liver transplantation patient, with a focus on diagnostic criteria and comparison to acute GVHD following hematopoietic stem cell transplantation.
Case Report
A 68-year-old woman with a history of hepatitis C virus infection, hepatocellular carcinoma, and OLT 1 month prior presented to the hospital with fever and abdominal cellulitis in close proximity to the surgical site of 1 week’s duration. The patient was started on vancomycin and cefepime; pan cultures were performed.
At 10 days of hospitalization, the patient developed a pruritic, nontender, erythematous rash on the abdomen, with extension onto the chest and legs. The rash was associated with low-grade fever but not with diarrhea. Physical examination was notable for a few erythematous macules and scattered papules over the neck and chest and a large erythematous plaque with multiple ecchymoses over the lower abdomen (Figure 1A). Erythematous macules and papules coalescing into plaques were present on the lower back (Figure 1B) and proximal thighs. Oral, ocular, and genital lesions were absent.

The differential diagnosis included drug reaction, viral infection, and acute GVHD. A skin biopsy was performed from the left side of the chest. Cefepime and vancomycin were discontinued; triamcinolone ointment 0.1% twice daily and antihistamines as needed for itching were started.
Over a 2-day period, the rash progressed to diffuse erythematous papules over the chest (Figure 2A) and bilateral arms (Figure 2B) including the palms. The patient also developed erythematous papules over the jawline and forehead as well as confluent erythematous plaques over the back with extension of the rash to involve the legs. She also had erythema and swelling bilaterally over the ears. She reported diarrhea. The low-grade fever resolved.

Laboratory review showed new-onset pancytopenia, normal liver function, and an elevated creatinine level of 2.3 mg/dL (reference range, 0.6–1.2 mg/dL), consistent with the patient’s baseline of stage 3 chronic kidney disease. Polymerase chain reaction analysis for cytomegalovirus was negative. Histology revealed vacuolar interface dermatitis with apoptotic keratinocytes, consistent with grade I GVHD (Figure 3). Duodenal biopsy revealed rare patchy glands with increased apoptosis, compatible with grade I GVHD.

The patient was started on intravenous methylprednisolone 1 mg/kg for 3 days, then transitioned to an oral steroid taper, with improvement of the rash and other systemic symptoms.
Comment
GVHD Subtypes
The 2 types of GVHD are humoral and cellular.5 The humoral type results from ABO blood type incompatibility between donor and recipient and causes mild hemolytic anemia and fever. The cellular type is directed against major histocompatibility complexes and is associated with high morbidity and mortality.
Presentation of GVHD
Acute GVHD following OLT usually occurs 3 to 5 weeks after transplantation,6 as in our patient. Symptoms include rash, fever, pancytopenia, and diarrhea.2 Skin is the most commonly involved organ in acute GVHD; rash is the earliest manifestation.1 The rash can be asymptomatic or associated with pain and pruritus. Initial cutaneous manifestations include palmar erythema and erythematous to violaceous discoloration of the face and ears. A diffuse maculopapular rash can develop, involving the face, abdomen, and trunk. The rash may progress to formation of bullae or skin sloughing, resembling Stevens-Johnson syndrome or toxic epidermal necrolysis.1 The skin manifestation of acute GVHD following OLT is similar to hematopoietic stem cell transplantation (Table).7,8

Pancytopenia is a common manifestation of GVHD following liver transplantation and is rarely seen following hematopoietic stem cell transplantation.7 Donor lymphocytes engraft and proliferate in the bone marrow, attacking recipient hematopoietic stem cells. It is important to note that more common causes of cytopenia following liver transplantation, including infection and drug-induced bone marrow suppression, should be ruled out before diagnosing acute GVHD.6
Acute GVHD can affect the gastrointestinal tract, causing diarrhea; however, other infectious and medication-induced causes of diarrhea also should be considered.6 In contrast to hematopoietic stem cell transplantation, in which the liver is usually involved,1 the liver is spared in acute GVHD following liver transplantation.5
Diagnosis of GVHD
The diagnosis of acute GVHD following liver transplantation can be challenging because the clinical manifestations can be caused by a drug reaction or viral infection, such as cytomegalovirus infection.2 Patients who are older than 50 years and glucose intolerant are at a higher risk of acute GVHD following OLT. The combination of younger donor age and the presence of an HLA class I match also increases the risk of acute GVHD.6 The diagnosis of acute GVHD is confirmed with biopsy of the skin or gastrointestinal tract.
Morbidity and Mortality of GVHD
Because of the high morbidity and mortality associated with acute GVHD following liver transplantation, early diagnosis and treatment are crucial.5 Death in patients with acute GVHD following OLT is mainly attributable to sepsis, multiorgan failure, and gastrointestinal tract bleeding.6 It remains unclear whether this high mortality is associated with delayed diagnosis due to nonspecific signs of acute GVHD following OLT or to the lack of appropriate treatment guidelines.6
Treatment Options
Because of the low incidence of acute GVHD following OLT, most treatment modalities are extrapolated from the literature on acute GVHD following stem cell transplantation.5 The most commonly used therapies include high-dose systemic steroids and anti–thymocyte globulin that attacks activated donor T cells.6 Other treatment modalities, including anti–tumor necrosis factor agents and antibodies to CD20, have been reported to be effective in steroid-refractory GVHD.2 The major drawback of systemic steroids is an increase in the risk for sepsis and infection; therefore, these patients should be diligently screened for infection and covered with antibiotics and antifungals. Extracorporeal photopheresis is another treatment modality that does not cause generalized immunosuppression but is not well studied in the setting of acute GVHD following OLT.6
Prevention
Acute GVHD following OLT can be prevented by eliminating donor T lymphocytes from the liver before transplantation. However, because the incidence of acute GVHD following OLT is very low, this approach is not routinely taken.2
Conclusion
Acute GVHD following liver transplantation is a rare complication; however, it has high mortality, necessitating further research regarding treatment and prevention. Early recognition and treatment of this condition can improve outcomes. Dermatologists should be familiar with the skin manifestations of acute GVHD following liver transplantation due to the rising number of cases of solid-organ transplantation.
- Hu SW, Cotliar J. Acute graft-versus-host disease following hematopoietic stem-cell transplantation. Dermatol Ther. 2011;24:411-423.
- Akbulut S, Yilmaz M, Yilmaz S. Graft-versus-host disease after liver transplantation: a comprehensive literature review. World J Gastroenterol. 2012;18:5240-5248.
- Taylor AL, Gibbs P, Bradley JA. Acute graft versus host disease following liver transplantation: the enemy within. Am J Transplant. 2004;4:466-474.
- Jagasia M, Arora M, Flowers ME, et al. Risk factor for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296-307.
- Kang WH, Hwang S, Song GW, et al. Acute graft-vs-host disease after liver transplantation: experience at a high-volume liver transplantation center in Korea. Transplant Proc. 2016;48:3368-3372.
- Murali AR, Chandra S, Stewart Z, et al. Graft versus host disease after liver transplantation in adults: a case series, review of literature, and an approach to management. Transplantation. 2016;100:2661-2670.
- Chaib E, Silva FD, Figueira ER, et al. Graft-versus-host disease after liver transplantation. Clinics (Sao Paulo). 2011;66:1115-1118.
- Barton-Burke M, Dwinell DM, Kafkas L, et al. Graft-versus-host disease: a complex long-term side effect of hematopoietic stem cell transplant. Oncology (Williston Park). 2008;22(11 Suppl Nurse Ed):31-45.
- Hu SW, Cotliar J. Acute graft-versus-host disease following hematopoietic stem-cell transplantation. Dermatol Ther. 2011;24:411-423.
- Akbulut S, Yilmaz M, Yilmaz S. Graft-versus-host disease after liver transplantation: a comprehensive literature review. World J Gastroenterol. 2012;18:5240-5248.
- Taylor AL, Gibbs P, Bradley JA. Acute graft versus host disease following liver transplantation: the enemy within. Am J Transplant. 2004;4:466-474.
- Jagasia M, Arora M, Flowers ME, et al. Risk factor for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296-307.
- Kang WH, Hwang S, Song GW, et al. Acute graft-vs-host disease after liver transplantation: experience at a high-volume liver transplantation center in Korea. Transplant Proc. 2016;48:3368-3372.
- Murali AR, Chandra S, Stewart Z, et al. Graft versus host disease after liver transplantation in adults: a case series, review of literature, and an approach to management. Transplantation. 2016;100:2661-2670.
- Chaib E, Silva FD, Figueira ER, et al. Graft-versus-host disease after liver transplantation. Clinics (Sao Paulo). 2011;66:1115-1118.
- Barton-Burke M, Dwinell DM, Kafkas L, et al. Graft-versus-host disease: a complex long-term side effect of hematopoietic stem cell transplant. Oncology (Williston Park). 2008;22(11 Suppl Nurse Ed):31-45.
Practice Points
- Acute graft-vs-host disease (GVHD) is a T cell–mediated reaction in which donor T lymphocytes attack host tissue in the setting of immunosuppression.
- Acute GVHD is more common in allogeneic stem cell transplantation but can occur in the setting of solid organ transplantation.
- Symptoms of acute GVHD include rash with or without pruritus, fever, pancytopenia, and diarrhea.
- Early recognition and treatment with systemic steroids can improve mortality.