User login
Round 1: Biologics, JAK inhibitors training up for atopic dermatitis ‘boxing match’
MILAN – Get ready for a “boxing match” (AD), Thomas Bieber, MD, PhD, said in an oral presentation at the World Congress of Dermatology.
“In one corner of the ring you will have the biologics, and in the other corner of the ring, you will have the Janus kinase (JAK) inhibitors and some other small molecules,” said Dr. Bieber, medical director of dermatology and allergy, at University Hospital Bonn, Germany.
There will be no winner-take-all scenario, since AD is a heterogeneous disease for which a variety of treatments will be needed, Dr. Bieber noted. However, he said, there are clear differences in mode of action, safety, mode of application, price, and more that will be important as dermatologists weigh the choice of biologics versus JAK inhibitors for a specific patient.
“Currently, dupilumab is the only one that is on the market (in the European Union) and this molecule is really a revolution for us,” Dr. Bieber said at the meeting. “But we will see in the next years, many, many other molecules coming up.”
Beyond dupilumab (Dupixent), the interleukin-4 (IL-4) receptor alpha antagonist approved by the Food and Drug Administration in 2017, biologics under development include tralokinumab, lebrikizumab, and nemolizumab, among others. Development on the small molecule side includes agents such as ZPL-389, a histamine H4 receptor agonist, but is dominated by JAK inhibitors such as baricitinib(Olumiant), approved by the FDA in 2018 for rheumatoid arthritis; upadacitinib; and abrocitinib, according to Dr. Bieber.
There are advantages and disadvantages to each class of molecule, he said. For example, JAK inhibitors act very rapidly to control itch and improve exacerbations, while biologics are “much slower” and provide delayed control of inflammation, he said. In terms of safety, by contrast, biologics probably offer a “better” benefit-to-risk ratio, with no risk for drug-drug interactions and no monitoring needed, while JAK inhibitors appear to have a “narrow” therapeutic window, with a risk for drug-drug interactions and a need for some monitoring, he said.
JAK inhibitors come in a tablet formulation, which, from the patient perspective, may be more convenient and acceptable compared with repeated injections of biologics every 2 or 4 weeks, Dr. Bieber noted. Another point potentially in favor of JAK inhibitors is price: “I think the current situation tells us clearly that the biologics are the more expensive drugs, while the small molecules typically are much less expensive,” he said.
One theoretical advantage of biologics, he said, is the potential for disease modification, while the disease-modifying potential of JAK inhibitors is unclear, as of yet. “We have not had enough experience with all these molecules in order to understand how we can do that particular job, in terms of switching off the disease,” he said.
In terms of the need for patient stratification in the future, it’s a draw between biologics and JAK inhibitors, Dr. Bieber said. “You will not see any of these drugs doing 100 percent of the job in 100 percent of the patients, so to me, currently from my understanding of this disease, there is no one-size-fits-all molecule.”
Toward that end, machine learning and artificial intelligence may aid in evaluating large amounts of data to better understand AD phenotypes. “That’s really something that is a particular kind of challenge in the next years, in order to improve at-the-end drug development, and the management of our patients,” he concluded.
Dr. Bieber reported disclosures related to LEO Pharmaceuticals, Regeneron, and Sanofi.
MILAN – Get ready for a “boxing match” (AD), Thomas Bieber, MD, PhD, said in an oral presentation at the World Congress of Dermatology.
“In one corner of the ring you will have the biologics, and in the other corner of the ring, you will have the Janus kinase (JAK) inhibitors and some other small molecules,” said Dr. Bieber, medical director of dermatology and allergy, at University Hospital Bonn, Germany.
There will be no winner-take-all scenario, since AD is a heterogeneous disease for which a variety of treatments will be needed, Dr. Bieber noted. However, he said, there are clear differences in mode of action, safety, mode of application, price, and more that will be important as dermatologists weigh the choice of biologics versus JAK inhibitors for a specific patient.
“Currently, dupilumab is the only one that is on the market (in the European Union) and this molecule is really a revolution for us,” Dr. Bieber said at the meeting. “But we will see in the next years, many, many other molecules coming up.”
Beyond dupilumab (Dupixent), the interleukin-4 (IL-4) receptor alpha antagonist approved by the Food and Drug Administration in 2017, biologics under development include tralokinumab, lebrikizumab, and nemolizumab, among others. Development on the small molecule side includes agents such as ZPL-389, a histamine H4 receptor agonist, but is dominated by JAK inhibitors such as baricitinib(Olumiant), approved by the FDA in 2018 for rheumatoid arthritis; upadacitinib; and abrocitinib, according to Dr. Bieber.
There are advantages and disadvantages to each class of molecule, he said. For example, JAK inhibitors act very rapidly to control itch and improve exacerbations, while biologics are “much slower” and provide delayed control of inflammation, he said. In terms of safety, by contrast, biologics probably offer a “better” benefit-to-risk ratio, with no risk for drug-drug interactions and no monitoring needed, while JAK inhibitors appear to have a “narrow” therapeutic window, with a risk for drug-drug interactions and a need for some monitoring, he said.
JAK inhibitors come in a tablet formulation, which, from the patient perspective, may be more convenient and acceptable compared with repeated injections of biologics every 2 or 4 weeks, Dr. Bieber noted. Another point potentially in favor of JAK inhibitors is price: “I think the current situation tells us clearly that the biologics are the more expensive drugs, while the small molecules typically are much less expensive,” he said.
One theoretical advantage of biologics, he said, is the potential for disease modification, while the disease-modifying potential of JAK inhibitors is unclear, as of yet. “We have not had enough experience with all these molecules in order to understand how we can do that particular job, in terms of switching off the disease,” he said.
In terms of the need for patient stratification in the future, it’s a draw between biologics and JAK inhibitors, Dr. Bieber said. “You will not see any of these drugs doing 100 percent of the job in 100 percent of the patients, so to me, currently from my understanding of this disease, there is no one-size-fits-all molecule.”
Toward that end, machine learning and artificial intelligence may aid in evaluating large amounts of data to better understand AD phenotypes. “That’s really something that is a particular kind of challenge in the next years, in order to improve at-the-end drug development, and the management of our patients,” he concluded.
Dr. Bieber reported disclosures related to LEO Pharmaceuticals, Regeneron, and Sanofi.
MILAN – Get ready for a “boxing match” (AD), Thomas Bieber, MD, PhD, said in an oral presentation at the World Congress of Dermatology.
“In one corner of the ring you will have the biologics, and in the other corner of the ring, you will have the Janus kinase (JAK) inhibitors and some other small molecules,” said Dr. Bieber, medical director of dermatology and allergy, at University Hospital Bonn, Germany.
There will be no winner-take-all scenario, since AD is a heterogeneous disease for which a variety of treatments will be needed, Dr. Bieber noted. However, he said, there are clear differences in mode of action, safety, mode of application, price, and more that will be important as dermatologists weigh the choice of biologics versus JAK inhibitors for a specific patient.
“Currently, dupilumab is the only one that is on the market (in the European Union) and this molecule is really a revolution for us,” Dr. Bieber said at the meeting. “But we will see in the next years, many, many other molecules coming up.”
Beyond dupilumab (Dupixent), the interleukin-4 (IL-4) receptor alpha antagonist approved by the Food and Drug Administration in 2017, biologics under development include tralokinumab, lebrikizumab, and nemolizumab, among others. Development on the small molecule side includes agents such as ZPL-389, a histamine H4 receptor agonist, but is dominated by JAK inhibitors such as baricitinib(Olumiant), approved by the FDA in 2018 for rheumatoid arthritis; upadacitinib; and abrocitinib, according to Dr. Bieber.
There are advantages and disadvantages to each class of molecule, he said. For example, JAK inhibitors act very rapidly to control itch and improve exacerbations, while biologics are “much slower” and provide delayed control of inflammation, he said. In terms of safety, by contrast, biologics probably offer a “better” benefit-to-risk ratio, with no risk for drug-drug interactions and no monitoring needed, while JAK inhibitors appear to have a “narrow” therapeutic window, with a risk for drug-drug interactions and a need for some monitoring, he said.
JAK inhibitors come in a tablet formulation, which, from the patient perspective, may be more convenient and acceptable compared with repeated injections of biologics every 2 or 4 weeks, Dr. Bieber noted. Another point potentially in favor of JAK inhibitors is price: “I think the current situation tells us clearly that the biologics are the more expensive drugs, while the small molecules typically are much less expensive,” he said.
One theoretical advantage of biologics, he said, is the potential for disease modification, while the disease-modifying potential of JAK inhibitors is unclear, as of yet. “We have not had enough experience with all these molecules in order to understand how we can do that particular job, in terms of switching off the disease,” he said.
In terms of the need for patient stratification in the future, it’s a draw between biologics and JAK inhibitors, Dr. Bieber said. “You will not see any of these drugs doing 100 percent of the job in 100 percent of the patients, so to me, currently from my understanding of this disease, there is no one-size-fits-all molecule.”
Toward that end, machine learning and artificial intelligence may aid in evaluating large amounts of data to better understand AD phenotypes. “That’s really something that is a particular kind of challenge in the next years, in order to improve at-the-end drug development, and the management of our patients,” he concluded.
Dr. Bieber reported disclosures related to LEO Pharmaceuticals, Regeneron, and Sanofi.
EXPERT ANALYSIS FROM WCD2019
Acne in women: What new insights tell us
NEWPORT BEACH, CALIF. – When it comes to acne in adult women, look past the jawline, beyond traditional medications, and toward greater control. That’s the message of a dermatologist who spoke at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“We should be aiming to get our patients to clear or almost clear, and we have the tools necessary to help that happen,” said Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.
she noted. Acne appears to affect 51% of women aged 20-29 years, she said, and prevalence dips to 15% in women older than 50 years.
About 80% of cases continue from adolescence, compared with about 20% that are new-onset during adulthood, she said. According to studies, she added, “most adult women have acne on multiple different areas of their face, not just the jawline. It’s similar to what we see in the adolescent population.”
Dr. Stein Gold offered these tips about treatment in this group of patients:
Inflammation
Researchers now consider that “all acne is inflammatory acne.” Be aggressive with anti-inflammatory treatment, and “continue even after the lesion is resolved” if needed to prevent scarring.
Oral contraceptives (OCs)
OCs can be helpful, but “we have to proceed with caution,” she said. A 2012 Cochrane Library review of 31 trials found that six combination OCs (COCs) “evaluated in placebo-controlled trials are effective in reducing inflammatory and noninflammatory facial acne lesions. Few important and consistent differences were found between COC types in their effectiveness for treating acne,” the review concluded (Cochrane Database Syst Rev. 2012 Jul 11;[7]:CD004425).
Results take time, however, and it “can take 3 months to see an effect, and 6 months for full effect,” Dr. Stein Gold noted.
There are multiple contraindications to the use of OCs, and they’ve been linked – controversially – to an increased risk of blood clots and breast cancer. However, risk of thrombosis also spikes – to significantly higher levels than with OC use – during pregnancy and the postpartum period, she said.
Spironolactone
This antihypertensive drug can be helpful, Dr. Stein Gold noted, although the one study in a 2009 Cochrane review that had acne as an outcome failed to find evidence of efficacy versus placebo (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD000194). Be aware of the boxed warning about links to cancer in rat studies, and consider the risk of potassium elevation in certain populations, she added. Watch the dose: fewer side effects are seen at 50-100 mg daily, although they’re still common, and it can take 3 months or more for improvements to appear, she said.
Truncal acne
Patients may be hesitant to mention they have acne on their chest and back. “They may not tell you about it, and you may not ask about it but [some patients] expect you to know about it and treat it,” Dr. Stein Gold said. She referred to trifarotene, a topical retinoid cream that, although not yet approved, appears to be safe and effective in treating acne on the face and trunk in phase 3 studies.
“Some people will say the trunk will get too irritated if you put a retinoid on it. But it absolutely can be used on the chest and back. The first thing I say to my patients is to expect to have redness and scaling for first 2 weeks. People pay money for that. It’s a chemical peel! It’s okay to have some sloughing; use an oil-free moisturizer.”
Dr. Stein Gold disclosed relationships with Galderma, Foamix, and Sol Gel (investigator, consultant); Valeant (consultant, speaker); and Dermira (investigator, speaker).
SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – When it comes to acne in adult women, look past the jawline, beyond traditional medications, and toward greater control. That’s the message of a dermatologist who spoke at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“We should be aiming to get our patients to clear or almost clear, and we have the tools necessary to help that happen,” said Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.
she noted. Acne appears to affect 51% of women aged 20-29 years, she said, and prevalence dips to 15% in women older than 50 years.
About 80% of cases continue from adolescence, compared with about 20% that are new-onset during adulthood, she said. According to studies, she added, “most adult women have acne on multiple different areas of their face, not just the jawline. It’s similar to what we see in the adolescent population.”
Dr. Stein Gold offered these tips about treatment in this group of patients:
Inflammation
Researchers now consider that “all acne is inflammatory acne.” Be aggressive with anti-inflammatory treatment, and “continue even after the lesion is resolved” if needed to prevent scarring.
Oral contraceptives (OCs)
OCs can be helpful, but “we have to proceed with caution,” she said. A 2012 Cochrane Library review of 31 trials found that six combination OCs (COCs) “evaluated in placebo-controlled trials are effective in reducing inflammatory and noninflammatory facial acne lesions. Few important and consistent differences were found between COC types in their effectiveness for treating acne,” the review concluded (Cochrane Database Syst Rev. 2012 Jul 11;[7]:CD004425).
Results take time, however, and it “can take 3 months to see an effect, and 6 months for full effect,” Dr. Stein Gold noted.
There are multiple contraindications to the use of OCs, and they’ve been linked – controversially – to an increased risk of blood clots and breast cancer. However, risk of thrombosis also spikes – to significantly higher levels than with OC use – during pregnancy and the postpartum period, she said.
Spironolactone
This antihypertensive drug can be helpful, Dr. Stein Gold noted, although the one study in a 2009 Cochrane review that had acne as an outcome failed to find evidence of efficacy versus placebo (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD000194). Be aware of the boxed warning about links to cancer in rat studies, and consider the risk of potassium elevation in certain populations, she added. Watch the dose: fewer side effects are seen at 50-100 mg daily, although they’re still common, and it can take 3 months or more for improvements to appear, she said.
Truncal acne
Patients may be hesitant to mention they have acne on their chest and back. “They may not tell you about it, and you may not ask about it but [some patients] expect you to know about it and treat it,” Dr. Stein Gold said. She referred to trifarotene, a topical retinoid cream that, although not yet approved, appears to be safe and effective in treating acne on the face and trunk in phase 3 studies.
“Some people will say the trunk will get too irritated if you put a retinoid on it. But it absolutely can be used on the chest and back. The first thing I say to my patients is to expect to have redness and scaling for first 2 weeks. People pay money for that. It’s a chemical peel! It’s okay to have some sloughing; use an oil-free moisturizer.”
Dr. Stein Gold disclosed relationships with Galderma, Foamix, and Sol Gel (investigator, consultant); Valeant (consultant, speaker); and Dermira (investigator, speaker).
SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – When it comes to acne in adult women, look past the jawline, beyond traditional medications, and toward greater control. That’s the message of a dermatologist who spoke at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“We should be aiming to get our patients to clear or almost clear, and we have the tools necessary to help that happen,” said Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.
she noted. Acne appears to affect 51% of women aged 20-29 years, she said, and prevalence dips to 15% in women older than 50 years.
About 80% of cases continue from adolescence, compared with about 20% that are new-onset during adulthood, she said. According to studies, she added, “most adult women have acne on multiple different areas of their face, not just the jawline. It’s similar to what we see in the adolescent population.”
Dr. Stein Gold offered these tips about treatment in this group of patients:
Inflammation
Researchers now consider that “all acne is inflammatory acne.” Be aggressive with anti-inflammatory treatment, and “continue even after the lesion is resolved” if needed to prevent scarring.
Oral contraceptives (OCs)
OCs can be helpful, but “we have to proceed with caution,” she said. A 2012 Cochrane Library review of 31 trials found that six combination OCs (COCs) “evaluated in placebo-controlled trials are effective in reducing inflammatory and noninflammatory facial acne lesions. Few important and consistent differences were found between COC types in their effectiveness for treating acne,” the review concluded (Cochrane Database Syst Rev. 2012 Jul 11;[7]:CD004425).
Results take time, however, and it “can take 3 months to see an effect, and 6 months for full effect,” Dr. Stein Gold noted.
There are multiple contraindications to the use of OCs, and they’ve been linked – controversially – to an increased risk of blood clots and breast cancer. However, risk of thrombosis also spikes – to significantly higher levels than with OC use – during pregnancy and the postpartum period, she said.
Spironolactone
This antihypertensive drug can be helpful, Dr. Stein Gold noted, although the one study in a 2009 Cochrane review that had acne as an outcome failed to find evidence of efficacy versus placebo (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD000194). Be aware of the boxed warning about links to cancer in rat studies, and consider the risk of potassium elevation in certain populations, she added. Watch the dose: fewer side effects are seen at 50-100 mg daily, although they’re still common, and it can take 3 months or more for improvements to appear, she said.
Truncal acne
Patients may be hesitant to mention they have acne on their chest and back. “They may not tell you about it, and you may not ask about it but [some patients] expect you to know about it and treat it,” Dr. Stein Gold said. She referred to trifarotene, a topical retinoid cream that, although not yet approved, appears to be safe and effective in treating acne on the face and trunk in phase 3 studies.
“Some people will say the trunk will get too irritated if you put a retinoid on it. But it absolutely can be used on the chest and back. The first thing I say to my patients is to expect to have redness and scaling for first 2 weeks. People pay money for that. It’s a chemical peel! It’s okay to have some sloughing; use an oil-free moisturizer.”
Dr. Stein Gold disclosed relationships with Galderma, Foamix, and Sol Gel (investigator, consultant); Valeant (consultant, speaker); and Dermira (investigator, speaker).
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Consider cutaneous endometriosis in women with umbilical lesions
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
No increased risk of psychiatric problems tied to isotretinoin
Isotretinoin use may increase vulnerability to psychiatric conditions, but available evidence does not support a causal relationship, on the basis of data from a retrospective study of 17,829 psychiatric adverse events reported to the Food and Drug Administration over 2 decades.
“Although one study highlighted consistent reporting of depression and suicide in patients taking isotretinoin in the United States from 1982 to 2000, few studies have examined reports of psychiatric adverse events at the national level since 2000,” wrote Sean Singer of Harvard University, Boston, and his colleagues.
In a study published in JAMA Dermatology, the researchers reviewed data from the FDA’s Adverse Event Reporting System between 1997 and 2017.
A total of 17,829 psychiatric adverse events in which isotretinoin was the primary suspect drug were reported during the study period. The researchers classified the events into 12 categories; the most common were depressive disorders (42%), emotional lability (17%), and anxiety (14%). The number of reported psychiatric adverse events was similar between men and women (8,936 and 8,362 events, respectively).
The researchers also identified 2,278 reports of suicidal ideation, 602 reports of attempted suicide, and 368 reports of completed suicide.
In addition, the researchers examined data from the iPLEDGE program and found completed suicide rates of 8.4 per 100,000 patients in 2009 and 5.6 per 100,000 patients in 2010. However, these rates were lower than national suicide rates in the general population of 11.8 per 100,000 people in 2009 and 12.1 per 100,000 people in 2010.
Patient age was available for 13,553 adverse event reports, and patients aged 10-19 years accounted for 53% of the reports overall and 58% of completed suicides for which age was reported.
The high number of psychiatric adverse events in the youngest age group “could reflect more isotretinoin prescriptions in this age group or may suggest that teenagers are particularly vulnerable to psychiatric adverse events while taking isotretinoin,” the researchers said.
The findings were limited by several factors, including the reliance on proper clinician reports to the Adverse Event Reporting System database and the separation of some psychiatric terms into categories that may reflect symptoms of other psychiatric diagnoses, the researchers said.
However, “Our data showed high numbers of reports of emotional lability, anxiety disorders, insomnia, self-injurious behavior, and psychotic disorders with isotretinoin as the primary suspect drug,” they noted.
“Although no causal link has been established between isotretinoin and psychiatric adverse events, it is important to recognize that there are data that suggest patients using this drug may be vulnerable to a number of psychiatric conditions” and that monthly iPLEDGE visits are an opportunity to screen patients for these conditions, they said.
They also stressed that “the risk of psychiatric adverse events in patients taking isotretinoin must be considered in the context of a known increased risk of suicidal ideation in patients with acne independent of isotretinoin therapy.”
Mr. Singer had no financial conflicts to disclose. Study coauthor John S. Barbieri, MD, disclosed partial salary support from Pfizer and grand support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and Arash Mostaghimi, MD, disclosed personal fees from Pfizer.
SOURCE: Singer S et al. JAMA Dermatol. 2019. Jul 3. doi: 10.1001/jamadermatol.2019.1416.
Isotretinoin use may increase vulnerability to psychiatric conditions, but available evidence does not support a causal relationship, on the basis of data from a retrospective study of 17,829 psychiatric adverse events reported to the Food and Drug Administration over 2 decades.
“Although one study highlighted consistent reporting of depression and suicide in patients taking isotretinoin in the United States from 1982 to 2000, few studies have examined reports of psychiatric adverse events at the national level since 2000,” wrote Sean Singer of Harvard University, Boston, and his colleagues.
In a study published in JAMA Dermatology, the researchers reviewed data from the FDA’s Adverse Event Reporting System between 1997 and 2017.
A total of 17,829 psychiatric adverse events in which isotretinoin was the primary suspect drug were reported during the study period. The researchers classified the events into 12 categories; the most common were depressive disorders (42%), emotional lability (17%), and anxiety (14%). The number of reported psychiatric adverse events was similar between men and women (8,936 and 8,362 events, respectively).
The researchers also identified 2,278 reports of suicidal ideation, 602 reports of attempted suicide, and 368 reports of completed suicide.
In addition, the researchers examined data from the iPLEDGE program and found completed suicide rates of 8.4 per 100,000 patients in 2009 and 5.6 per 100,000 patients in 2010. However, these rates were lower than national suicide rates in the general population of 11.8 per 100,000 people in 2009 and 12.1 per 100,000 people in 2010.
Patient age was available for 13,553 adverse event reports, and patients aged 10-19 years accounted for 53% of the reports overall and 58% of completed suicides for which age was reported.
The high number of psychiatric adverse events in the youngest age group “could reflect more isotretinoin prescriptions in this age group or may suggest that teenagers are particularly vulnerable to psychiatric adverse events while taking isotretinoin,” the researchers said.
The findings were limited by several factors, including the reliance on proper clinician reports to the Adverse Event Reporting System database and the separation of some psychiatric terms into categories that may reflect symptoms of other psychiatric diagnoses, the researchers said.
However, “Our data showed high numbers of reports of emotional lability, anxiety disorders, insomnia, self-injurious behavior, and psychotic disorders with isotretinoin as the primary suspect drug,” they noted.
“Although no causal link has been established between isotretinoin and psychiatric adverse events, it is important to recognize that there are data that suggest patients using this drug may be vulnerable to a number of psychiatric conditions” and that monthly iPLEDGE visits are an opportunity to screen patients for these conditions, they said.
They also stressed that “the risk of psychiatric adverse events in patients taking isotretinoin must be considered in the context of a known increased risk of suicidal ideation in patients with acne independent of isotretinoin therapy.”
Mr. Singer had no financial conflicts to disclose. Study coauthor John S. Barbieri, MD, disclosed partial salary support from Pfizer and grand support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and Arash Mostaghimi, MD, disclosed personal fees from Pfizer.
SOURCE: Singer S et al. JAMA Dermatol. 2019. Jul 3. doi: 10.1001/jamadermatol.2019.1416.
Isotretinoin use may increase vulnerability to psychiatric conditions, but available evidence does not support a causal relationship, on the basis of data from a retrospective study of 17,829 psychiatric adverse events reported to the Food and Drug Administration over 2 decades.
“Although one study highlighted consistent reporting of depression and suicide in patients taking isotretinoin in the United States from 1982 to 2000, few studies have examined reports of psychiatric adverse events at the national level since 2000,” wrote Sean Singer of Harvard University, Boston, and his colleagues.
In a study published in JAMA Dermatology, the researchers reviewed data from the FDA’s Adverse Event Reporting System between 1997 and 2017.
A total of 17,829 psychiatric adverse events in which isotretinoin was the primary suspect drug were reported during the study period. The researchers classified the events into 12 categories; the most common were depressive disorders (42%), emotional lability (17%), and anxiety (14%). The number of reported psychiatric adverse events was similar between men and women (8,936 and 8,362 events, respectively).
The researchers also identified 2,278 reports of suicidal ideation, 602 reports of attempted suicide, and 368 reports of completed suicide.
In addition, the researchers examined data from the iPLEDGE program and found completed suicide rates of 8.4 per 100,000 patients in 2009 and 5.6 per 100,000 patients in 2010. However, these rates were lower than national suicide rates in the general population of 11.8 per 100,000 people in 2009 and 12.1 per 100,000 people in 2010.
Patient age was available for 13,553 adverse event reports, and patients aged 10-19 years accounted for 53% of the reports overall and 58% of completed suicides for which age was reported.
The high number of psychiatric adverse events in the youngest age group “could reflect more isotretinoin prescriptions in this age group or may suggest that teenagers are particularly vulnerable to psychiatric adverse events while taking isotretinoin,” the researchers said.
The findings were limited by several factors, including the reliance on proper clinician reports to the Adverse Event Reporting System database and the separation of some psychiatric terms into categories that may reflect symptoms of other psychiatric diagnoses, the researchers said.
However, “Our data showed high numbers of reports of emotional lability, anxiety disorders, insomnia, self-injurious behavior, and psychotic disorders with isotretinoin as the primary suspect drug,” they noted.
“Although no causal link has been established between isotretinoin and psychiatric adverse events, it is important to recognize that there are data that suggest patients using this drug may be vulnerable to a number of psychiatric conditions” and that monthly iPLEDGE visits are an opportunity to screen patients for these conditions, they said.
They also stressed that “the risk of psychiatric adverse events in patients taking isotretinoin must be considered in the context of a known increased risk of suicidal ideation in patients with acne independent of isotretinoin therapy.”
Mr. Singer had no financial conflicts to disclose. Study coauthor John S. Barbieri, MD, disclosed partial salary support from Pfizer and grand support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and Arash Mostaghimi, MD, disclosed personal fees from Pfizer.
SOURCE: Singer S et al. JAMA Dermatol. 2019. Jul 3. doi: 10.1001/jamadermatol.2019.1416.
FROM JAMA DERMATOLOGY
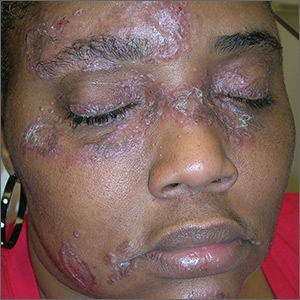
Lesions on face, arms, and legs
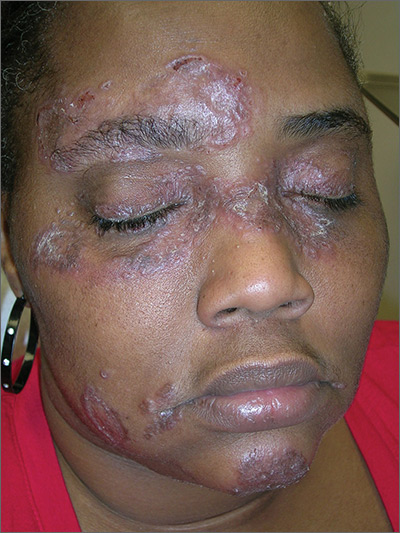
The FP suspected that the patient had sarcoidosis, based on the infiltrated plaques and their distribution on her face. The appearance of the lesions was similar to images he’d seen online of lupus pernio, a pathognomonic finding of sarcoidosis. The FP recommended a punch biopsy of one of the lesions, and the patient consented.
(See the Watch & Learn video on “Punch biopsy.”)
The FP also sent the patient for a chest x-ray, which showed bilateral hilar adenopathy consistent with sarcoidosis. Histopathology of the biopsy also showed sarcoidosis. Chest radiographic involvement is seen in almost 90% of patients with sarcoidosis and is used in staging the disease. Stage I disease shows bilateral hilar lymphadenopathy (BHL). Stage II disease shows BHL plus pulmonary infiltrates. Stage III disease shows pulmonary infiltrates without BHL. Stage IV disease shows pulmonary fibrosis. In this case, the patient had stage I disease.
The FP prescribed topical 0.1% triamcinolone ointment in an 80 g tube and instructed the patient to apply it to all of the areas involved. Although this medication typically comes in a 15 g or 30 g tube, the FP knew that these quantities would be insufficient. He had also read that a mid-potency steroid would be permissible on the face for this diagnosis. Not having any experience with sarcoidosis, the FP referred the patient to Dermatology and Pulmonology.
The dermatologist started the patient on oral prednisone 40 mg/d and awaited the completion of the patient’s baseline labs before beginning a steroid sparing agent such as methotrexate. Treatments for sarcoidosis include topical steroids, systemic steroids, methotrexate, azathioprine, and biologics (with adalimumab and infliximab having the greatest evidence for effectiveness).
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that the patient had sarcoidosis, based on the infiltrated plaques and their distribution on her face. The appearance of the lesions was similar to images he’d seen online of lupus pernio, a pathognomonic finding of sarcoidosis. The FP recommended a punch biopsy of one of the lesions, and the patient consented.
(See the Watch & Learn video on “Punch biopsy.”)
The FP also sent the patient for a chest x-ray, which showed bilateral hilar adenopathy consistent with sarcoidosis. Histopathology of the biopsy also showed sarcoidosis. Chest radiographic involvement is seen in almost 90% of patients with sarcoidosis and is used in staging the disease. Stage I disease shows bilateral hilar lymphadenopathy (BHL). Stage II disease shows BHL plus pulmonary infiltrates. Stage III disease shows pulmonary infiltrates without BHL. Stage IV disease shows pulmonary fibrosis. In this case, the patient had stage I disease.
The FP prescribed topical 0.1% triamcinolone ointment in an 80 g tube and instructed the patient to apply it to all of the areas involved. Although this medication typically comes in a 15 g or 30 g tube, the FP knew that these quantities would be insufficient. He had also read that a mid-potency steroid would be permissible on the face for this diagnosis. Not having any experience with sarcoidosis, the FP referred the patient to Dermatology and Pulmonology.
The dermatologist started the patient on oral prednisone 40 mg/d and awaited the completion of the patient’s baseline labs before beginning a steroid sparing agent such as methotrexate. Treatments for sarcoidosis include topical steroids, systemic steroids, methotrexate, azathioprine, and biologics (with adalimumab and infliximab having the greatest evidence for effectiveness).
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that the patient had sarcoidosis, based on the infiltrated plaques and their distribution on her face. The appearance of the lesions was similar to images he’d seen online of lupus pernio, a pathognomonic finding of sarcoidosis. The FP recommended a punch biopsy of one of the lesions, and the patient consented.
(See the Watch & Learn video on “Punch biopsy.”)
The FP also sent the patient for a chest x-ray, which showed bilateral hilar adenopathy consistent with sarcoidosis. Histopathology of the biopsy also showed sarcoidosis. Chest radiographic involvement is seen in almost 90% of patients with sarcoidosis and is used in staging the disease. Stage I disease shows bilateral hilar lymphadenopathy (BHL). Stage II disease shows BHL plus pulmonary infiltrates. Stage III disease shows pulmonary infiltrates without BHL. Stage IV disease shows pulmonary fibrosis. In this case, the patient had stage I disease.
The FP prescribed topical 0.1% triamcinolone ointment in an 80 g tube and instructed the patient to apply it to all of the areas involved. Although this medication typically comes in a 15 g or 30 g tube, the FP knew that these quantities would be insufficient. He had also read that a mid-potency steroid would be permissible on the face for this diagnosis. Not having any experience with sarcoidosis, the FP referred the patient to Dermatology and Pulmonology.
The dermatologist started the patient on oral prednisone 40 mg/d and awaited the completion of the patient’s baseline labs before beginning a steroid sparing agent such as methotrexate. Treatments for sarcoidosis include topical steroids, systemic steroids, methotrexate, azathioprine, and biologics (with adalimumab and infliximab having the greatest evidence for effectiveness).
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
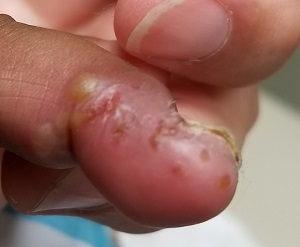
Can You Put Your Finger on the Diagnosis?
An 8-year-old boy is brought in for evaluation of a collection of blisters on his finger, near the nail. The problem manifested about 6 days ago. The affected area is tender to touch. The child reportedly feels well, with no fever or malaise.
The patient has an extensive personal and family history of atopy. Since birth, he has had dry, sensitive skin and has experienced episodes of eczema, seasonal allergies, and asthma. Three months ago, he was admitted to the hospital with eczema herpeticum and successfully treated with IV acyclovir.
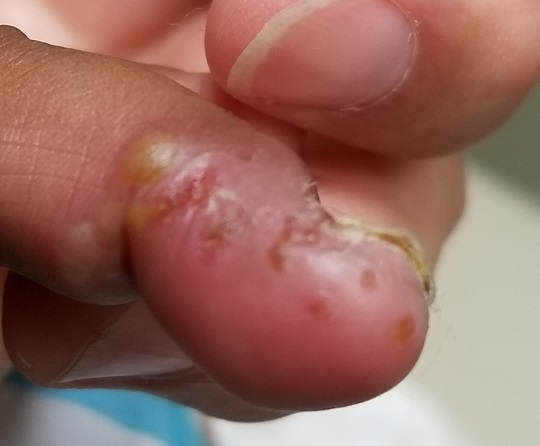
EXAMINATION
A cluster of vesicles is seen in the lateral perionychial area of the left third finger. Very modest erythema surrounds the vesicles, which contain cloudy yellow fluid suggestive of pus. There is a palpable lymph node in the left epitrochlear area.
The child is afebrile and in no distress. Patches of mild eczema are seen on the extremities and trunk.
What’s the diagnosis?
DISCUSSION
The lesion on this child’s finger is a herpetic whitlow. Patients with atopy are often susceptible to all types of skin infections: bacterial, fungal, and viral. In fact, human papillomavirus infection manifesting as multiple warts is not uncommon in this population. Nor is herpes simplex virus (HSV) infection, of which this case represents 1 manifestation.
A culture could have been done to confirm the diagnosis, but that would entail opening a vesicle to collect the fluid and then waiting at least 2 weeks for the results. By then, this whitlow would have long since resolved.
As with all HSV infections in the immunocompetent, treatment with acyclovir must be started in the first 2 to 3 days to have any effect—so such treatment in this case would be useless. If the herpetic whitlow were to recur in the same location, prompt treatment could be initiated, which would likely shorten the disease course and reduce symptoms.
Another HSV infection seen almost exclusively in atopic patients is eczema herpeticum (also known as Kaposi varicelliform eruption). This diffuse infection comprises dozens of tiny papulovesicular lesions, mostly concentrated on the face but often spilling down onto the chest. Patients with Darier disease or seborrheic dermatitis can also acquire it.
TAKE-HOME LEARNING POINTS
- Patients with atopy, especially children, are susceptible to all kinds of skin infections—fungal, bacterial, and viral.
- Herpes simplex virus (HSV) can appear in almost any location, including on fingers, but can also manifest as diffuse papulovesicular lesions on the face and chest of atopic patients.
- The blisters/vesicles of HSV are often pus-filled and usually provoke regional adenopathy.
- If diagnosed early enough, herpetic whitlows can be successfully treated with oral acyclovir; this doesn’t provide a cure but does stop the particular episode.
An 8-year-old boy is brought in for evaluation of a collection of blisters on his finger, near the nail. The problem manifested about 6 days ago. The affected area is tender to touch. The child reportedly feels well, with no fever or malaise.
The patient has an extensive personal and family history of atopy. Since birth, he has had dry, sensitive skin and has experienced episodes of eczema, seasonal allergies, and asthma. Three months ago, he was admitted to the hospital with eczema herpeticum and successfully treated with IV acyclovir.

EXAMINATION
A cluster of vesicles is seen in the lateral perionychial area of the left third finger. Very modest erythema surrounds the vesicles, which contain cloudy yellow fluid suggestive of pus. There is a palpable lymph node in the left epitrochlear area.
The child is afebrile and in no distress. Patches of mild eczema are seen on the extremities and trunk.
What’s the diagnosis?
DISCUSSION
The lesion on this child’s finger is a herpetic whitlow. Patients with atopy are often susceptible to all types of skin infections: bacterial, fungal, and viral. In fact, human papillomavirus infection manifesting as multiple warts is not uncommon in this population. Nor is herpes simplex virus (HSV) infection, of which this case represents 1 manifestation.
A culture could have been done to confirm the diagnosis, but that would entail opening a vesicle to collect the fluid and then waiting at least 2 weeks for the results. By then, this whitlow would have long since resolved.
As with all HSV infections in the immunocompetent, treatment with acyclovir must be started in the first 2 to 3 days to have any effect—so such treatment in this case would be useless. If the herpetic whitlow were to recur in the same location, prompt treatment could be initiated, which would likely shorten the disease course and reduce symptoms.
Another HSV infection seen almost exclusively in atopic patients is eczema herpeticum (also known as Kaposi varicelliform eruption). This diffuse infection comprises dozens of tiny papulovesicular lesions, mostly concentrated on the face but often spilling down onto the chest. Patients with Darier disease or seborrheic dermatitis can also acquire it.
TAKE-HOME LEARNING POINTS
- Patients with atopy, especially children, are susceptible to all kinds of skin infections—fungal, bacterial, and viral.
- Herpes simplex virus (HSV) can appear in almost any location, including on fingers, but can also manifest as diffuse papulovesicular lesions on the face and chest of atopic patients.
- The blisters/vesicles of HSV are often pus-filled and usually provoke regional adenopathy.
- If diagnosed early enough, herpetic whitlows can be successfully treated with oral acyclovir; this doesn’t provide a cure but does stop the particular episode.
An 8-year-old boy is brought in for evaluation of a collection of blisters on his finger, near the nail. The problem manifested about 6 days ago. The affected area is tender to touch. The child reportedly feels well, with no fever or malaise.
The patient has an extensive personal and family history of atopy. Since birth, he has had dry, sensitive skin and has experienced episodes of eczema, seasonal allergies, and asthma. Three months ago, he was admitted to the hospital with eczema herpeticum and successfully treated with IV acyclovir.

EXAMINATION
A cluster of vesicles is seen in the lateral perionychial area of the left third finger. Very modest erythema surrounds the vesicles, which contain cloudy yellow fluid suggestive of pus. There is a palpable lymph node in the left epitrochlear area.
The child is afebrile and in no distress. Patches of mild eczema are seen on the extremities and trunk.
What’s the diagnosis?
DISCUSSION
The lesion on this child’s finger is a herpetic whitlow. Patients with atopy are often susceptible to all types of skin infections: bacterial, fungal, and viral. In fact, human papillomavirus infection manifesting as multiple warts is not uncommon in this population. Nor is herpes simplex virus (HSV) infection, of which this case represents 1 manifestation.
A culture could have been done to confirm the diagnosis, but that would entail opening a vesicle to collect the fluid and then waiting at least 2 weeks for the results. By then, this whitlow would have long since resolved.
As with all HSV infections in the immunocompetent, treatment with acyclovir must be started in the first 2 to 3 days to have any effect—so such treatment in this case would be useless. If the herpetic whitlow were to recur in the same location, prompt treatment could be initiated, which would likely shorten the disease course and reduce symptoms.
Another HSV infection seen almost exclusively in atopic patients is eczema herpeticum (also known as Kaposi varicelliform eruption). This diffuse infection comprises dozens of tiny papulovesicular lesions, mostly concentrated on the face but often spilling down onto the chest. Patients with Darier disease or seborrheic dermatitis can also acquire it.
TAKE-HOME LEARNING POINTS
- Patients with atopy, especially children, are susceptible to all kinds of skin infections—fungal, bacterial, and viral.
- Herpes simplex virus (HSV) can appear in almost any location, including on fingers, but can also manifest as diffuse papulovesicular lesions on the face and chest of atopic patients.
- The blisters/vesicles of HSV are often pus-filled and usually provoke regional adenopathy.
- If diagnosed early enough, herpetic whitlows can be successfully treated with oral acyclovir; this doesn’t provide a cure but does stop the particular episode.
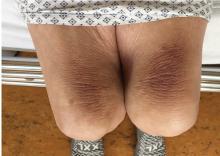
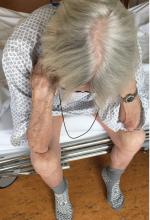
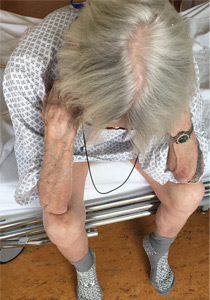
Thinker’s sign
See Mangione and Aronowitz editorials
Mechanical pressure induced by friction of the elbows on the thighs may result in proliferation of the stratum corneum and the release of hemosiderin from erythrocytes, resulting in the skin changes seen in this patient, which because of the tripod positioning are known as “thinker’s sign,” a term coined in 1963 by Rothenberg1 to describe findings in patients with chronic pulmonary disease and advanced respiratory insufficiency. It is also referred to as the Dahl sign, based on a report by Dahl of similar findings in patients with emphysema.2
- Rothenberg HJ. The thinker's sign. JAMA 1963; 184:902–903. pmid:13975358
- Dahl MV. Emphysema. Arch Dermatol 1970; 101(1):117. pmid:5416788
See Mangione and Aronowitz editorials
Mechanical pressure induced by friction of the elbows on the thighs may result in proliferation of the stratum corneum and the release of hemosiderin from erythrocytes, resulting in the skin changes seen in this patient, which because of the tripod positioning are known as “thinker’s sign,” a term coined in 1963 by Rothenberg1 to describe findings in patients with chronic pulmonary disease and advanced respiratory insufficiency. It is also referred to as the Dahl sign, based on a report by Dahl of similar findings in patients with emphysema.2
See Mangione and Aronowitz editorials
Mechanical pressure induced by friction of the elbows on the thighs may result in proliferation of the stratum corneum and the release of hemosiderin from erythrocytes, resulting in the skin changes seen in this patient, which because of the tripod positioning are known as “thinker’s sign,” a term coined in 1963 by Rothenberg1 to describe findings in patients with chronic pulmonary disease and advanced respiratory insufficiency. It is also referred to as the Dahl sign, based on a report by Dahl of similar findings in patients with emphysema.2
- Rothenberg HJ. The thinker's sign. JAMA 1963; 184:902–903. pmid:13975358
- Dahl MV. Emphysema. Arch Dermatol 1970; 101(1):117. pmid:5416788
- Rothenberg HJ. The thinker's sign. JAMA 1963; 184:902–903. pmid:13975358
- Dahl MV. Emphysema. Arch Dermatol 1970; 101(1):117. pmid:5416788
Severity, itch improvements remain steady with ruxolitinib for atopic dermatitis
MILAN – A in the 4-week open-label period of a 16-week randomized phase 2 study of adults with mild to moderate atopic dermatitis (AD), Leon H. Kircik, MD, said at the World Congress of Dermatology.
Improvements in disease severity and itch in patients receiving 1.5% ruxolitinib cream twice daily were sustained over the open-label period, said Dr. Kircik, a dermatologist in Louisville, Ky., affiliated with Mount Sinai Medical Center, New York.
Patients who switched from vehicle or 0.1% triamcinolone cream to the JAK1/2 selective inhibitor in the open-label period also experienced rapid improvements in disease severity and itch.
“This is a novel treatment that’s a topical JAK inhibitor, which so far we don’t have any in the market for atopic dermatitis, and it does have a very good efficacy and safety profile,” Dr. Kircik said during an oral presentation at the meeting.
Janus kinases modulate inflammatory cytokines implicated in AD, and may also directly modulate itch, Dr. Kircik noted.
The study comprised 307 adults with mild to moderate AD (Investigator’s Global Assessment [IGA] score of 2 or 3) and body surface area involvement of 3%-20%. They were randomized equally to six arms, including vehicle, triamcinolone cream, and ruxolitinib at dosages of 0.15%, 0.5%, 1.5% once daily, or the target dose level of 1.5% twice daily.
After an 8-week double-blind period, there was a 4-week open-label period during which patients randomized to vehicle or triamcinolone were switched to ruxolitinib, and then a 4-week follow-up period during which no treatment was given, Dr. Kircik said.
The mean age of the patients was 35 years, 54% were female, and the median duration of disease was 20.8 years.
In the double-blind period, 1.5% ruxolitinib cream twice daily significantly improved Eczema Area and Severity Index (EASI) score versus vehicle, Dr. Kircik said.
The mean change in EASI scores at weeks 2, 4, and 6 were 52.7%, 71.6%, and 78.5% for ruxolitinib, versus 4.8%, 15.5%, and 26.9% for vehicle (P less than .001 for all comparisons), according to Dr. Kircik.
The patients on the target ruxolitinib dose maintained the improvements in EASI score throughout the open label period, with mean improvement from baseline reaching 81.4% by week 10 and 84.9% by week 12.
Meanwhile, there was a sharp increase in mean EASI score improvement in patients switched to ruxolitinib, according to Dr. Kircik. In the vehicle arm, mean improvement leapt from 26.9% at week 8 to 78.4% by week 12.
Significant reductions in itch scores were seen within 36 hours of starting the 1.5% ruxolitinib cream, with itch Numeric Rating Scale (NRS) scores of –1.8 versus –0.2 for vehicle at that time point (P less than .0001), he added.
Reduction in itch score was similarly maintained in the ruxolitinib target dose group, and rapidly fell to similar levels for patients switched over to that treatment in the open-label period, Dr. Kircik said.
The target ruxolitinib dose was also noninferior to triamcinolone cream, for which mean change in EASI scores at weeks 2 and 4 were 40.0% and 59.8%, respectively.
Recruitment of patients in phase 3 studies of ruxolitinib cream for AD has just started, Dr. Kircik said.
The TRuE AD1 and TRuE AD2 studies are set to enroll 1,200 adolescents and adults with AD who will be randomized to ruxolitinib cream or vehicle, according to listings on ClinicalTrials.gov.
Dr. Kircik disclosed ties to several companies including Incyte, which was the sponsor of the phase 2 study and the phase 3 studies.
MILAN – A in the 4-week open-label period of a 16-week randomized phase 2 study of adults with mild to moderate atopic dermatitis (AD), Leon H. Kircik, MD, said at the World Congress of Dermatology.
Improvements in disease severity and itch in patients receiving 1.5% ruxolitinib cream twice daily were sustained over the open-label period, said Dr. Kircik, a dermatologist in Louisville, Ky., affiliated with Mount Sinai Medical Center, New York.
Patients who switched from vehicle or 0.1% triamcinolone cream to the JAK1/2 selective inhibitor in the open-label period also experienced rapid improvements in disease severity and itch.
“This is a novel treatment that’s a topical JAK inhibitor, which so far we don’t have any in the market for atopic dermatitis, and it does have a very good efficacy and safety profile,” Dr. Kircik said during an oral presentation at the meeting.
Janus kinases modulate inflammatory cytokines implicated in AD, and may also directly modulate itch, Dr. Kircik noted.
The study comprised 307 adults with mild to moderate AD (Investigator’s Global Assessment [IGA] score of 2 or 3) and body surface area involvement of 3%-20%. They were randomized equally to six arms, including vehicle, triamcinolone cream, and ruxolitinib at dosages of 0.15%, 0.5%, 1.5% once daily, or the target dose level of 1.5% twice daily.
After an 8-week double-blind period, there was a 4-week open-label period during which patients randomized to vehicle or triamcinolone were switched to ruxolitinib, and then a 4-week follow-up period during which no treatment was given, Dr. Kircik said.
The mean age of the patients was 35 years, 54% were female, and the median duration of disease was 20.8 years.
In the double-blind period, 1.5% ruxolitinib cream twice daily significantly improved Eczema Area and Severity Index (EASI) score versus vehicle, Dr. Kircik said.
The mean change in EASI scores at weeks 2, 4, and 6 were 52.7%, 71.6%, and 78.5% for ruxolitinib, versus 4.8%, 15.5%, and 26.9% for vehicle (P less than .001 for all comparisons), according to Dr. Kircik.
The patients on the target ruxolitinib dose maintained the improvements in EASI score throughout the open label period, with mean improvement from baseline reaching 81.4% by week 10 and 84.9% by week 12.
Meanwhile, there was a sharp increase in mean EASI score improvement in patients switched to ruxolitinib, according to Dr. Kircik. In the vehicle arm, mean improvement leapt from 26.9% at week 8 to 78.4% by week 12.
Significant reductions in itch scores were seen within 36 hours of starting the 1.5% ruxolitinib cream, with itch Numeric Rating Scale (NRS) scores of –1.8 versus –0.2 for vehicle at that time point (P less than .0001), he added.
Reduction in itch score was similarly maintained in the ruxolitinib target dose group, and rapidly fell to similar levels for patients switched over to that treatment in the open-label period, Dr. Kircik said.
The target ruxolitinib dose was also noninferior to triamcinolone cream, for which mean change in EASI scores at weeks 2 and 4 were 40.0% and 59.8%, respectively.
Recruitment of patients in phase 3 studies of ruxolitinib cream for AD has just started, Dr. Kircik said.
The TRuE AD1 and TRuE AD2 studies are set to enroll 1,200 adolescents and adults with AD who will be randomized to ruxolitinib cream or vehicle, according to listings on ClinicalTrials.gov.
Dr. Kircik disclosed ties to several companies including Incyte, which was the sponsor of the phase 2 study and the phase 3 studies.
MILAN – A in the 4-week open-label period of a 16-week randomized phase 2 study of adults with mild to moderate atopic dermatitis (AD), Leon H. Kircik, MD, said at the World Congress of Dermatology.
Improvements in disease severity and itch in patients receiving 1.5% ruxolitinib cream twice daily were sustained over the open-label period, said Dr. Kircik, a dermatologist in Louisville, Ky., affiliated with Mount Sinai Medical Center, New York.
Patients who switched from vehicle or 0.1% triamcinolone cream to the JAK1/2 selective inhibitor in the open-label period also experienced rapid improvements in disease severity and itch.
“This is a novel treatment that’s a topical JAK inhibitor, which so far we don’t have any in the market for atopic dermatitis, and it does have a very good efficacy and safety profile,” Dr. Kircik said during an oral presentation at the meeting.
Janus kinases modulate inflammatory cytokines implicated in AD, and may also directly modulate itch, Dr. Kircik noted.
The study comprised 307 adults with mild to moderate AD (Investigator’s Global Assessment [IGA] score of 2 or 3) and body surface area involvement of 3%-20%. They were randomized equally to six arms, including vehicle, triamcinolone cream, and ruxolitinib at dosages of 0.15%, 0.5%, 1.5% once daily, or the target dose level of 1.5% twice daily.
After an 8-week double-blind period, there was a 4-week open-label period during which patients randomized to vehicle or triamcinolone were switched to ruxolitinib, and then a 4-week follow-up period during which no treatment was given, Dr. Kircik said.
The mean age of the patients was 35 years, 54% were female, and the median duration of disease was 20.8 years.
In the double-blind period, 1.5% ruxolitinib cream twice daily significantly improved Eczema Area and Severity Index (EASI) score versus vehicle, Dr. Kircik said.
The mean change in EASI scores at weeks 2, 4, and 6 were 52.7%, 71.6%, and 78.5% for ruxolitinib, versus 4.8%, 15.5%, and 26.9% for vehicle (P less than .001 for all comparisons), according to Dr. Kircik.
The patients on the target ruxolitinib dose maintained the improvements in EASI score throughout the open label period, with mean improvement from baseline reaching 81.4% by week 10 and 84.9% by week 12.
Meanwhile, there was a sharp increase in mean EASI score improvement in patients switched to ruxolitinib, according to Dr. Kircik. In the vehicle arm, mean improvement leapt from 26.9% at week 8 to 78.4% by week 12.
Significant reductions in itch scores were seen within 36 hours of starting the 1.5% ruxolitinib cream, with itch Numeric Rating Scale (NRS) scores of –1.8 versus –0.2 for vehicle at that time point (P less than .0001), he added.
Reduction in itch score was similarly maintained in the ruxolitinib target dose group, and rapidly fell to similar levels for patients switched over to that treatment in the open-label period, Dr. Kircik said.
The target ruxolitinib dose was also noninferior to triamcinolone cream, for which mean change in EASI scores at weeks 2 and 4 were 40.0% and 59.8%, respectively.
Recruitment of patients in phase 3 studies of ruxolitinib cream for AD has just started, Dr. Kircik said.
The TRuE AD1 and TRuE AD2 studies are set to enroll 1,200 adolescents and adults with AD who will be randomized to ruxolitinib cream or vehicle, according to listings on ClinicalTrials.gov.
Dr. Kircik disclosed ties to several companies including Incyte, which was the sponsor of the phase 2 study and the phase 3 studies.
REPORTING FROM WCD2019
Atopic dermatitis patients achieved freedom from itch on JAK inhibitor upadacitinib
MILAN – according to a report presented at the World Congress of Dermatology.
Compared with those in the placebo group, more patients receiving the selective Janus kinase 1 (JAK1) inhibitor achieved an itch-free state and maintained it over the 16 weeks of the phase 2b trial, said investigator Kristian Reich, MD, professor of translational research in inflammatory skin diseases at the University Medical Center Hamburg-Eppendorf (Germany).
These improvements in pruritus occurred early with upadacitinib and were pronounced at the highest dose studied, 30 mg daily, he commented. Treatment with upadacitinib also rapidly and significantly improved clinical signs of AD versus placebo, as previously reported primary endpoint data show.
“It’s a drug that works in eczema,” Dr. Reich said in an oral presentation. “We still do not fully understand what the exact relationship between itch and eczema is. Is there a neurogenic inflammation? Is there an epidermal pathology? But clearly with this drug, it does seem to reduce the itch, it does reduce the eczema, it does this early on, and the 30 mg does seem to be the right dose.”
Upadacitinib is a selective inhibitor of JAK1, a member of the signal transduction cascade for many cytokines implicated in AD, including interleukin-4, IL-13, IL-22, and others, Dr. Reich told attendees at the meeting.
In the phase 2b study, 167 patients with moderate to severe AD were randomized to placebo or upadacitinib at 7.5 mg, 15 mg, or 30 mg daily over a 16-week, double-blind period, followed by a 72-week, blinded extension. The mean age across these groups ranged from 39 to 42 years, and the mean time since onset of symptoms was 24-34 years.
Significantly improvements in Eczema Area and Severity Index (EASI) scores were seen as early as 2 weeks and were maintained throughout the 16-week, double-blind period, as previously shown. By 16 weeks, the mean percentage improvement in EASI score was 74.4% for upadacitinib 30 mg daily versus 23.0% for placebo (P less than .001).
In this more recent post hoc analysis of itch, the percentage of patients with a weekly rolling average pruritus Numerical Rating Scale (NRS) score of 0-1 was significantly higher in the upadacitinib groups, Dr. Reich said.
The placebo-adjusted difference in average pruritus NRS scores of 0-1 was highest in the 30-mg daily group, at 37.7% by week 16 (P less than .001).
Those itch scores correlated with the Patient Global Impression of Severity results, in that almost all patients rating their disease as absent or minimal by that scale also had a pruritus NRS score of 0 (81.6%) or 1 (10.5%), he said.
That link shows the important contribution of itch to the overall rating of disease severity by the patient. “Patients want to be able to say, ‘I have only minimal or absent disease,’ ” he said. “This will likely require that you really get the itch down, for example, to 0 or 1, using this pruritus numerical rating scale.”
Pruritus improvements in favor of upadacitinib were also seen when using Scoring AD itch and Patient-Oriented Eczema Measure (POEM) itch measures, Dr. Reich said. With POEM, 0% of placebo-treated patients had 0 days of itch in the past week, compared with 28.6% in the upadacitinib 30-mg daily group.
The risk-to-benefit profile of upadacitinib supports proceeding to phase 3 trials in patients with AD, according to Dr. Reich and coinvestigators.
Phase 3 trials of upadacitinib are underway in AD, psoriatic arthritis, Crohn’s disease, and ulcerative colitis, according to a recent AbbVie press release. The Food and Drug Administration accepted a New Drug Application Accepted For Priority Review for upadacitinib treatment of moderate to severe RA, based on a phase 3 program including more than 4,900 patients, the company announced in February.
Support for the study was provided by AbbVie. Dr. Reich reported disclosures related to AbbVie, Affibody, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen-Cilag, Leo Pharma, Eli Lilly, Medac Pharma, Merck, Novartis, Pfizer, Regeneron, Takeda, UCB, and XenoPort.
MILAN – according to a report presented at the World Congress of Dermatology.
Compared with those in the placebo group, more patients receiving the selective Janus kinase 1 (JAK1) inhibitor achieved an itch-free state and maintained it over the 16 weeks of the phase 2b trial, said investigator Kristian Reich, MD, professor of translational research in inflammatory skin diseases at the University Medical Center Hamburg-Eppendorf (Germany).
These improvements in pruritus occurred early with upadacitinib and were pronounced at the highest dose studied, 30 mg daily, he commented. Treatment with upadacitinib also rapidly and significantly improved clinical signs of AD versus placebo, as previously reported primary endpoint data show.
“It’s a drug that works in eczema,” Dr. Reich said in an oral presentation. “We still do not fully understand what the exact relationship between itch and eczema is. Is there a neurogenic inflammation? Is there an epidermal pathology? But clearly with this drug, it does seem to reduce the itch, it does reduce the eczema, it does this early on, and the 30 mg does seem to be the right dose.”
Upadacitinib is a selective inhibitor of JAK1, a member of the signal transduction cascade for many cytokines implicated in AD, including interleukin-4, IL-13, IL-22, and others, Dr. Reich told attendees at the meeting.
In the phase 2b study, 167 patients with moderate to severe AD were randomized to placebo or upadacitinib at 7.5 mg, 15 mg, or 30 mg daily over a 16-week, double-blind period, followed by a 72-week, blinded extension. The mean age across these groups ranged from 39 to 42 years, and the mean time since onset of symptoms was 24-34 years.
Significantly improvements in Eczema Area and Severity Index (EASI) scores were seen as early as 2 weeks and were maintained throughout the 16-week, double-blind period, as previously shown. By 16 weeks, the mean percentage improvement in EASI score was 74.4% for upadacitinib 30 mg daily versus 23.0% for placebo (P less than .001).
In this more recent post hoc analysis of itch, the percentage of patients with a weekly rolling average pruritus Numerical Rating Scale (NRS) score of 0-1 was significantly higher in the upadacitinib groups, Dr. Reich said.
The placebo-adjusted difference in average pruritus NRS scores of 0-1 was highest in the 30-mg daily group, at 37.7% by week 16 (P less than .001).
Those itch scores correlated with the Patient Global Impression of Severity results, in that almost all patients rating their disease as absent or minimal by that scale also had a pruritus NRS score of 0 (81.6%) or 1 (10.5%), he said.
That link shows the important contribution of itch to the overall rating of disease severity by the patient. “Patients want to be able to say, ‘I have only minimal or absent disease,’ ” he said. “This will likely require that you really get the itch down, for example, to 0 or 1, using this pruritus numerical rating scale.”
Pruritus improvements in favor of upadacitinib were also seen when using Scoring AD itch and Patient-Oriented Eczema Measure (POEM) itch measures, Dr. Reich said. With POEM, 0% of placebo-treated patients had 0 days of itch in the past week, compared with 28.6% in the upadacitinib 30-mg daily group.
The risk-to-benefit profile of upadacitinib supports proceeding to phase 3 trials in patients with AD, according to Dr. Reich and coinvestigators.
Phase 3 trials of upadacitinib are underway in AD, psoriatic arthritis, Crohn’s disease, and ulcerative colitis, according to a recent AbbVie press release. The Food and Drug Administration accepted a New Drug Application Accepted For Priority Review for upadacitinib treatment of moderate to severe RA, based on a phase 3 program including more than 4,900 patients, the company announced in February.
Support for the study was provided by AbbVie. Dr. Reich reported disclosures related to AbbVie, Affibody, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen-Cilag, Leo Pharma, Eli Lilly, Medac Pharma, Merck, Novartis, Pfizer, Regeneron, Takeda, UCB, and XenoPort.
MILAN – according to a report presented at the World Congress of Dermatology.
Compared with those in the placebo group, more patients receiving the selective Janus kinase 1 (JAK1) inhibitor achieved an itch-free state and maintained it over the 16 weeks of the phase 2b trial, said investigator Kristian Reich, MD, professor of translational research in inflammatory skin diseases at the University Medical Center Hamburg-Eppendorf (Germany).
These improvements in pruritus occurred early with upadacitinib and were pronounced at the highest dose studied, 30 mg daily, he commented. Treatment with upadacitinib also rapidly and significantly improved clinical signs of AD versus placebo, as previously reported primary endpoint data show.
“It’s a drug that works in eczema,” Dr. Reich said in an oral presentation. “We still do not fully understand what the exact relationship between itch and eczema is. Is there a neurogenic inflammation? Is there an epidermal pathology? But clearly with this drug, it does seem to reduce the itch, it does reduce the eczema, it does this early on, and the 30 mg does seem to be the right dose.”
Upadacitinib is a selective inhibitor of JAK1, a member of the signal transduction cascade for many cytokines implicated in AD, including interleukin-4, IL-13, IL-22, and others, Dr. Reich told attendees at the meeting.
In the phase 2b study, 167 patients with moderate to severe AD were randomized to placebo or upadacitinib at 7.5 mg, 15 mg, or 30 mg daily over a 16-week, double-blind period, followed by a 72-week, blinded extension. The mean age across these groups ranged from 39 to 42 years, and the mean time since onset of symptoms was 24-34 years.
Significantly improvements in Eczema Area and Severity Index (EASI) scores were seen as early as 2 weeks and were maintained throughout the 16-week, double-blind period, as previously shown. By 16 weeks, the mean percentage improvement in EASI score was 74.4% for upadacitinib 30 mg daily versus 23.0% for placebo (P less than .001).
In this more recent post hoc analysis of itch, the percentage of patients with a weekly rolling average pruritus Numerical Rating Scale (NRS) score of 0-1 was significantly higher in the upadacitinib groups, Dr. Reich said.
The placebo-adjusted difference in average pruritus NRS scores of 0-1 was highest in the 30-mg daily group, at 37.7% by week 16 (P less than .001).
Those itch scores correlated with the Patient Global Impression of Severity results, in that almost all patients rating their disease as absent or minimal by that scale also had a pruritus NRS score of 0 (81.6%) or 1 (10.5%), he said.
That link shows the important contribution of itch to the overall rating of disease severity by the patient. “Patients want to be able to say, ‘I have only minimal or absent disease,’ ” he said. “This will likely require that you really get the itch down, for example, to 0 or 1, using this pruritus numerical rating scale.”
Pruritus improvements in favor of upadacitinib were also seen when using Scoring AD itch and Patient-Oriented Eczema Measure (POEM) itch measures, Dr. Reich said. With POEM, 0% of placebo-treated patients had 0 days of itch in the past week, compared with 28.6% in the upadacitinib 30-mg daily group.
The risk-to-benefit profile of upadacitinib supports proceeding to phase 3 trials in patients with AD, according to Dr. Reich and coinvestigators.
Phase 3 trials of upadacitinib are underway in AD, psoriatic arthritis, Crohn’s disease, and ulcerative colitis, according to a recent AbbVie press release. The Food and Drug Administration accepted a New Drug Application Accepted For Priority Review for upadacitinib treatment of moderate to severe RA, based on a phase 3 program including more than 4,900 patients, the company announced in February.
Support for the study was provided by AbbVie. Dr. Reich reported disclosures related to AbbVie, Affibody, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen-Cilag, Leo Pharma, Eli Lilly, Medac Pharma, Merck, Novartis, Pfizer, Regeneron, Takeda, UCB, and XenoPort.
REPORTING FROM WCD2019
Sores on left arm
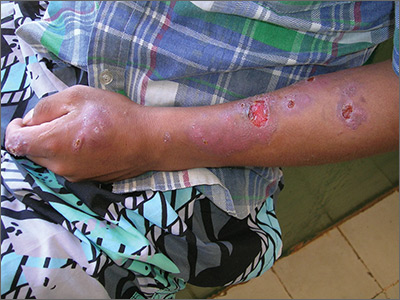
The FP noted that a pattern seemed to start on the patient’s second finger and spread up his arm. He considered that this skin disease might be secondary to sporotrichosis (a deep fungal infection, also referred to as rose gardener’s disease).
Sporotrichosis typically spreads up the arm from an inoculation of the hand from a scratch of a rose thorn. The ulcers partially resemble pyoderma gangrenosum, but the edges are neither undermined nor the color of gun metal. While sporotrichosis may be spread to humans through injuries while working with rose bushes, many other plants and animals can carry the organism Sporothrix schenckii.
The FP decided to offer a definitive diagnosis with a fungal culture since sporotrichosis treatment would require months of an oral antifungal agent. Obtaining a fungal culture would require a punch biopsy because the Sporothrix schenckii grows deeply in the tissue and is not reliably found on the skin surface. The mother and patient consented to the procedure and the FP performed a 4-mm punch biopsy on the edge of the largest ulcer on the arm. The specimen was placed in a sterile urine culture cup on sterile gauze with some saline (preservative free). (See the Watch & Learn video on “Punch biopsy.”)
It is important to note that that if the specimen had been sent in standard formalin, a culture could not be performed and histology could miss the dead organism. Clinical suspicion for sporotrichosis was so high in this case that the FP prescribed oral itraconazole 200 mg daily for the next 2 weeks while awaiting the fungal culture result.
The fungal culture grew out Sporothrix schenckii. The FP prescribed itraconazole 200 mg daily for 3 months and planned to continue the therapy until at least 2 to 4 weeks after the lesions had healed. With monthly follow-up visits, the itraconazole treatment lasted 5 months.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP noted that a pattern seemed to start on the patient’s second finger and spread up his arm. He considered that this skin disease might be secondary to sporotrichosis (a deep fungal infection, also referred to as rose gardener’s disease).
Sporotrichosis typically spreads up the arm from an inoculation of the hand from a scratch of a rose thorn. The ulcers partially resemble pyoderma gangrenosum, but the edges are neither undermined nor the color of gun metal. While sporotrichosis may be spread to humans through injuries while working with rose bushes, many other plants and animals can carry the organism Sporothrix schenckii.
The FP decided to offer a definitive diagnosis with a fungal culture since sporotrichosis treatment would require months of an oral antifungal agent. Obtaining a fungal culture would require a punch biopsy because the Sporothrix schenckii grows deeply in the tissue and is not reliably found on the skin surface. The mother and patient consented to the procedure and the FP performed a 4-mm punch biopsy on the edge of the largest ulcer on the arm. The specimen was placed in a sterile urine culture cup on sterile gauze with some saline (preservative free). (See the Watch & Learn video on “Punch biopsy.”)
It is important to note that that if the specimen had been sent in standard formalin, a culture could not be performed and histology could miss the dead organism. Clinical suspicion for sporotrichosis was so high in this case that the FP prescribed oral itraconazole 200 mg daily for the next 2 weeks while awaiting the fungal culture result.
The fungal culture grew out Sporothrix schenckii. The FP prescribed itraconazole 200 mg daily for 3 months and planned to continue the therapy until at least 2 to 4 weeks after the lesions had healed. With monthly follow-up visits, the itraconazole treatment lasted 5 months.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP noted that a pattern seemed to start on the patient’s second finger and spread up his arm. He considered that this skin disease might be secondary to sporotrichosis (a deep fungal infection, also referred to as rose gardener’s disease).
Sporotrichosis typically spreads up the arm from an inoculation of the hand from a scratch of a rose thorn. The ulcers partially resemble pyoderma gangrenosum, but the edges are neither undermined nor the color of gun metal. While sporotrichosis may be spread to humans through injuries while working with rose bushes, many other plants and animals can carry the organism Sporothrix schenckii.
The FP decided to offer a definitive diagnosis with a fungal culture since sporotrichosis treatment would require months of an oral antifungal agent. Obtaining a fungal culture would require a punch biopsy because the Sporothrix schenckii grows deeply in the tissue and is not reliably found on the skin surface. The mother and patient consented to the procedure and the FP performed a 4-mm punch biopsy on the edge of the largest ulcer on the arm. The specimen was placed in a sterile urine culture cup on sterile gauze with some saline (preservative free). (See the Watch & Learn video on “Punch biopsy.”)
It is important to note that that if the specimen had been sent in standard formalin, a culture could not be performed and histology could miss the dead organism. Clinical suspicion for sporotrichosis was so high in this case that the FP prescribed oral itraconazole 200 mg daily for the next 2 weeks while awaiting the fungal culture result.
The fungal culture grew out Sporothrix schenckii. The FP prescribed itraconazole 200 mg daily for 3 months and planned to continue the therapy until at least 2 to 4 weeks after the lesions had healed. With monthly follow-up visits, the itraconazole treatment lasted 5 months.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com