User login
Rapidly growing lesions on the forehead
A 97-year-old woman with a history of atrial fibrillation and nonmelanoma skin cancer presented to our clinic from an assisted living facility with a several-month history of rapidly growing forehead lesions. She denied symptoms, other than some bleeding and crusting, but was concerned about their appearance. She reported a notable history of sun exposure.
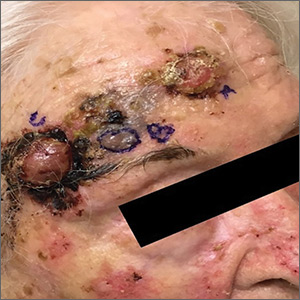
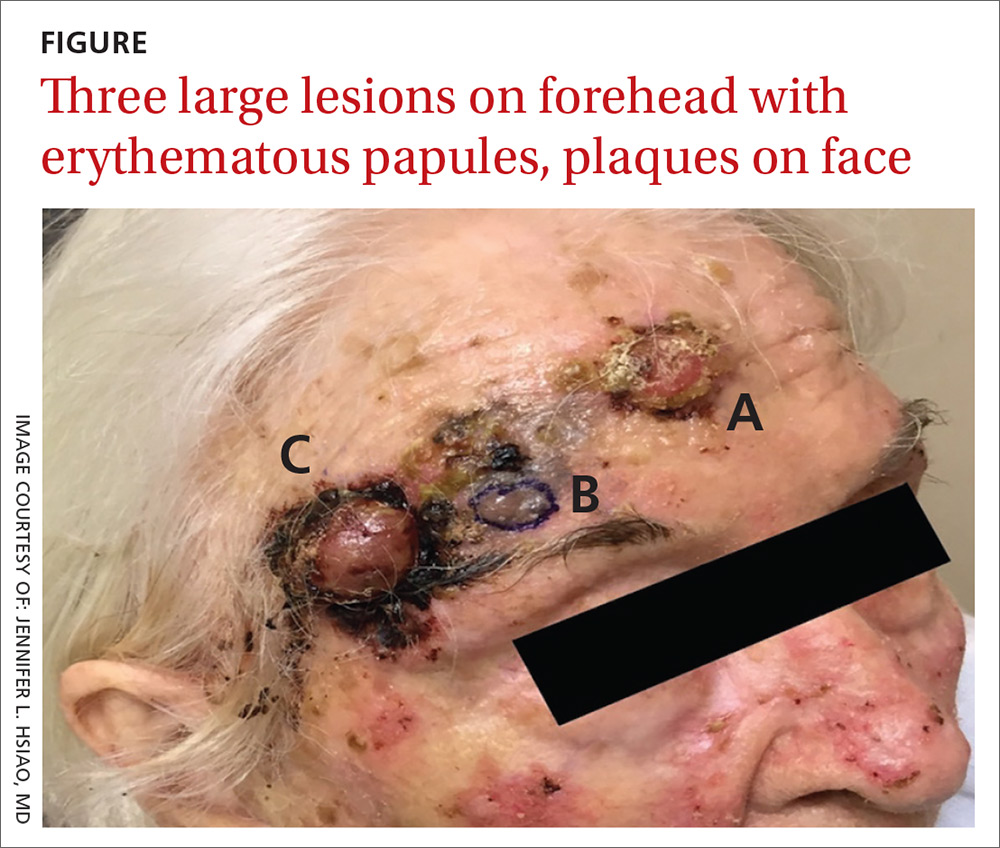
The patient had 3 confluent, but distinct, lesions on her forehead: an erythematous crateriform nodule with overlying hyperkeratotic scale (FIGURE, Lesion A); a nodular hyperpigmented plaque with irregular color and borders (Lesion B); and a pearly well-vascularized erythematous nodule with surrounding hemorrhagic crust (Lesion C).
She also had scattered, thin, gritty pink papules and plaques on the face that were thought to be actinic keratosis and nonmelanoma skin cancers based on clinical morphology; however, the patient deferred workup and treatment of these lesions to focus on the forehead lesions. The decision was made to biopsy all 3 clinical morphologies seen. The risks and benefits of biopsy were reviewed with the patient and her daughter, and they opted to proceed. The areas were anesthetized with an injection of 1% lidocaine and epinephrine 1:100,000; 3 shave biopsies were performed. Hemostasis was obtained with electrodesiccation.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Skin cancer
A histopathology report revealed that Lesion A was squamous cell carcinoma (SCC), Lesion B was a melanoma with a Breslow depth of at least 1.2 mm, and Lesion C was basal cell carcinoma (BCC). It is unusual to have a patient present with BCC, SCC, and melanoma concurrently in the same anatomic region.
Two of the lesions were nonmelanoma skin cancers (NMSC). BCC is the most common NMSC in the United States, affecting more than 3.3 million people per year.1 Although there are several subtypes of BCC with varying clinical presentations, the most classic appearance is a pearly papule with or without surface telangiectasias.2
SCC has an incidence of 200,000 to 400,000 cases per year in the United States and the lifetime risk is 9% to 14% in men and 4% to 9% in women.3 SCC most commonly presents as a hyperkeratotic papule or plaque.2 Lesions suspicious for SCC and BCC should be biopsied and the diagnosis confirmed by histopathologic analysis. These NMSCs are locally destructive, but rarely metastatic with a generally good prognosis. The standard treatment for both is surgical excision with consideration for other treatment modalities, such as topical therapies, chemotherapy, and radiation, depending on tumor characteristics as well as whether the patient is a good surgical candidate.1,3
Melanoma is rising in incidence each year, with nearly 100,000 new cases expected in the United States this year.4 It is the leading cause of skin cancer related mortality.5 The most common suspicious lesions are variably pigmented macules with irregular borders. Biopsy and subsequent histopathologic analysis will confirm the diagnosis.
When a lesion is clinically suspicious for melanoma, it is particularly important to consider an excisional biopsy to allow for proper staging.5 Examples of appropriate excisional biopsies include elliptical excisions, punch biopsies, and deep shave biopsies.5 Definitive treatment involves a wider and deeper excision with histologically confirmed clear margins.5
Continue to: This case required a multidisciplinary team
This case required a multidisciplinary team
The patient was cleared for surgery; however, after the patient held her warfarin in preparation for the resection, she suffered a left frontal operculum infarction. At this point, she was re-evaluated by her head and neck physician, cardiologist, and anesthesiologist. Consensus was reached that the patient was at high perioperative risk for morbidity and mortality, and surgical intervention was no longer considered a viable option.
The patient then opted for palliative radiation therapy to all 3 lesions, with the understanding that the local control offered by radiotherapy would be inferior to what resection would provide for the melanoma lesion. Although not curative, radiotherapy was expected to provide local symptom relief for the melanoma, consistent with the patient’s palliative goals of care. In the past, melanoma was thought to be resistant to radiation, but recent evidence suggests that it may be at least partially susceptible to hypofractionated courses of radiation.6
Radiation oncology recommended a 6 to 15 fraction regimen and she had a good clinical response with > 50% decrease in the size of all 3 lesions along with cessation of bleeding.
The take-home lesson. The findings in this case serve as an important reminder to biopsy lesions with varying morphologies—even when they are in close proximity to one another. Foregoing any of the biopsies in this case would have led to a missed diagnosis, which has implications for optimal management and treatment.
CORRESPONDENCE
Jennifer L. Hsiao, MD, 2020 Santa Monica Boulevard, Suite 510, Santa Monica, CA 90404; [email protected]
1. Kim JYS, Kozlow JH, Mittal B, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559.
2. Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161-168.
3. Kim JYS, Kozlow JH, Mittal B, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
5. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208-250.
6. Vuong W, Lin J, Wei RL. Palliative radiotherapy for skin malignancies. Ann Palliat Med. 2017;6:165-172.
A 97-year-old woman with a history of atrial fibrillation and nonmelanoma skin cancer presented to our clinic from an assisted living facility with a several-month history of rapidly growing forehead lesions. She denied symptoms, other than some bleeding and crusting, but was concerned about their appearance. She reported a notable history of sun exposure.
The patient had 3 confluent, but distinct, lesions on her forehead: an erythematous crateriform nodule with overlying hyperkeratotic scale (FIGURE, Lesion A); a nodular hyperpigmented plaque with irregular color and borders (Lesion B); and a pearly well-vascularized erythematous nodule with surrounding hemorrhagic crust (Lesion C).

She also had scattered, thin, gritty pink papules and plaques on the face that were thought to be actinic keratosis and nonmelanoma skin cancers based on clinical morphology; however, the patient deferred workup and treatment of these lesions to focus on the forehead lesions. The decision was made to biopsy all 3 clinical morphologies seen. The risks and benefits of biopsy were reviewed with the patient and her daughter, and they opted to proceed. The areas were anesthetized with an injection of 1% lidocaine and epinephrine 1:100,000; 3 shave biopsies were performed. Hemostasis was obtained with electrodesiccation.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Skin cancer
A histopathology report revealed that Lesion A was squamous cell carcinoma (SCC), Lesion B was a melanoma with a Breslow depth of at least 1.2 mm, and Lesion C was basal cell carcinoma (BCC). It is unusual to have a patient present with BCC, SCC, and melanoma concurrently in the same anatomic region.
Two of the lesions were nonmelanoma skin cancers (NMSC). BCC is the most common NMSC in the United States, affecting more than 3.3 million people per year.1 Although there are several subtypes of BCC with varying clinical presentations, the most classic appearance is a pearly papule with or without surface telangiectasias.2
SCC has an incidence of 200,000 to 400,000 cases per year in the United States and the lifetime risk is 9% to 14% in men and 4% to 9% in women.3 SCC most commonly presents as a hyperkeratotic papule or plaque.2 Lesions suspicious for SCC and BCC should be biopsied and the diagnosis confirmed by histopathologic analysis. These NMSCs are locally destructive, but rarely metastatic with a generally good prognosis. The standard treatment for both is surgical excision with consideration for other treatment modalities, such as topical therapies, chemotherapy, and radiation, depending on tumor characteristics as well as whether the patient is a good surgical candidate.1,3
Melanoma is rising in incidence each year, with nearly 100,000 new cases expected in the United States this year.4 It is the leading cause of skin cancer related mortality.5 The most common suspicious lesions are variably pigmented macules with irregular borders. Biopsy and subsequent histopathologic analysis will confirm the diagnosis.
When a lesion is clinically suspicious for melanoma, it is particularly important to consider an excisional biopsy to allow for proper staging.5 Examples of appropriate excisional biopsies include elliptical excisions, punch biopsies, and deep shave biopsies.5 Definitive treatment involves a wider and deeper excision with histologically confirmed clear margins.5
Continue to: This case required a multidisciplinary team
This case required a multidisciplinary team
The patient was cleared for surgery; however, after the patient held her warfarin in preparation for the resection, she suffered a left frontal operculum infarction. At this point, she was re-evaluated by her head and neck physician, cardiologist, and anesthesiologist. Consensus was reached that the patient was at high perioperative risk for morbidity and mortality, and surgical intervention was no longer considered a viable option.
The patient then opted for palliative radiation therapy to all 3 lesions, with the understanding that the local control offered by radiotherapy would be inferior to what resection would provide for the melanoma lesion. Although not curative, radiotherapy was expected to provide local symptom relief for the melanoma, consistent with the patient’s palliative goals of care. In the past, melanoma was thought to be resistant to radiation, but recent evidence suggests that it may be at least partially susceptible to hypofractionated courses of radiation.6
Radiation oncology recommended a 6 to 15 fraction regimen and she had a good clinical response with > 50% decrease in the size of all 3 lesions along with cessation of bleeding.
The take-home lesson. The findings in this case serve as an important reminder to biopsy lesions with varying morphologies—even when they are in close proximity to one another. Foregoing any of the biopsies in this case would have led to a missed diagnosis, which has implications for optimal management and treatment.
CORRESPONDENCE
Jennifer L. Hsiao, MD, 2020 Santa Monica Boulevard, Suite 510, Santa Monica, CA 90404; [email protected]
A 97-year-old woman with a history of atrial fibrillation and nonmelanoma skin cancer presented to our clinic from an assisted living facility with a several-month history of rapidly growing forehead lesions. She denied symptoms, other than some bleeding and crusting, but was concerned about their appearance. She reported a notable history of sun exposure.
The patient had 3 confluent, but distinct, lesions on her forehead: an erythematous crateriform nodule with overlying hyperkeratotic scale (FIGURE, Lesion A); a nodular hyperpigmented plaque with irregular color and borders (Lesion B); and a pearly well-vascularized erythematous nodule with surrounding hemorrhagic crust (Lesion C).

She also had scattered, thin, gritty pink papules and plaques on the face that were thought to be actinic keratosis and nonmelanoma skin cancers based on clinical morphology; however, the patient deferred workup and treatment of these lesions to focus on the forehead lesions. The decision was made to biopsy all 3 clinical morphologies seen. The risks and benefits of biopsy were reviewed with the patient and her daughter, and they opted to proceed. The areas were anesthetized with an injection of 1% lidocaine and epinephrine 1:100,000; 3 shave biopsies were performed. Hemostasis was obtained with electrodesiccation.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Skin cancer
A histopathology report revealed that Lesion A was squamous cell carcinoma (SCC), Lesion B was a melanoma with a Breslow depth of at least 1.2 mm, and Lesion C was basal cell carcinoma (BCC). It is unusual to have a patient present with BCC, SCC, and melanoma concurrently in the same anatomic region.
Two of the lesions were nonmelanoma skin cancers (NMSC). BCC is the most common NMSC in the United States, affecting more than 3.3 million people per year.1 Although there are several subtypes of BCC with varying clinical presentations, the most classic appearance is a pearly papule with or without surface telangiectasias.2
SCC has an incidence of 200,000 to 400,000 cases per year in the United States and the lifetime risk is 9% to 14% in men and 4% to 9% in women.3 SCC most commonly presents as a hyperkeratotic papule or plaque.2 Lesions suspicious for SCC and BCC should be biopsied and the diagnosis confirmed by histopathologic analysis. These NMSCs are locally destructive, but rarely metastatic with a generally good prognosis. The standard treatment for both is surgical excision with consideration for other treatment modalities, such as topical therapies, chemotherapy, and radiation, depending on tumor characteristics as well as whether the patient is a good surgical candidate.1,3
Melanoma is rising in incidence each year, with nearly 100,000 new cases expected in the United States this year.4 It is the leading cause of skin cancer related mortality.5 The most common suspicious lesions are variably pigmented macules with irregular borders. Biopsy and subsequent histopathologic analysis will confirm the diagnosis.
When a lesion is clinically suspicious for melanoma, it is particularly important to consider an excisional biopsy to allow for proper staging.5 Examples of appropriate excisional biopsies include elliptical excisions, punch biopsies, and deep shave biopsies.5 Definitive treatment involves a wider and deeper excision with histologically confirmed clear margins.5
Continue to: This case required a multidisciplinary team
This case required a multidisciplinary team
The patient was cleared for surgery; however, after the patient held her warfarin in preparation for the resection, she suffered a left frontal operculum infarction. At this point, she was re-evaluated by her head and neck physician, cardiologist, and anesthesiologist. Consensus was reached that the patient was at high perioperative risk for morbidity and mortality, and surgical intervention was no longer considered a viable option.
The patient then opted for palliative radiation therapy to all 3 lesions, with the understanding that the local control offered by radiotherapy would be inferior to what resection would provide for the melanoma lesion. Although not curative, radiotherapy was expected to provide local symptom relief for the melanoma, consistent with the patient’s palliative goals of care. In the past, melanoma was thought to be resistant to radiation, but recent evidence suggests that it may be at least partially susceptible to hypofractionated courses of radiation.6
Radiation oncology recommended a 6 to 15 fraction regimen and she had a good clinical response with > 50% decrease in the size of all 3 lesions along with cessation of bleeding.
The take-home lesson. The findings in this case serve as an important reminder to biopsy lesions with varying morphologies—even when they are in close proximity to one another. Foregoing any of the biopsies in this case would have led to a missed diagnosis, which has implications for optimal management and treatment.
CORRESPONDENCE
Jennifer L. Hsiao, MD, 2020 Santa Monica Boulevard, Suite 510, Santa Monica, CA 90404; [email protected]
1. Kim JYS, Kozlow JH, Mittal B, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559.
2. Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161-168.
3. Kim JYS, Kozlow JH, Mittal B, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
5. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208-250.
6. Vuong W, Lin J, Wei RL. Palliative radiotherapy for skin malignancies. Ann Palliat Med. 2017;6:165-172.
1. Kim JYS, Kozlow JH, Mittal B, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559.
2. Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161-168.
3. Kim JYS, Kozlow JH, Mittal B, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
5. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208-250.
6. Vuong W, Lin J, Wei RL. Palliative radiotherapy for skin malignancies. Ann Palliat Med. 2017;6:165-172.
Ulcers on lower leg

The FP recognized this as classic pyoderma gangrenosum (PG)—a challenging condition to treat and not within the typical scope of practice for an FP. Nonhealing, well-defined leg ulcers in a person with Crohn's disease (or any type of inflammatory bowel disease) are often seen with PG. Pathergy—the development of an exaggerated injury following minor trauma—is known to occur with PG.
The FP also noted the violet-blue coloration around the borders of the ulcers, which is referred to as a “gun-metal border.” He considered doing a biopsy on the edge of the ulcer to rule out other conditions and to see if there was a neutrophilic infiltrate that is typically seen with PG. However, the FP realized that pathergy could be stimulated by a biopsy, so he decided to refer the patient to Dermatology.
Knowing that the patient might have to wait a few months to see a dermatologist, the FP consulted online sources and prescribed topical clobetasol ointment to be applied twice daily as an initial therapy. This was not successful, so after a phone consult with the dermatologist, the FP added oral prednisone 40 mg/d for the next 2 weeks until the dermatologist could see the patient.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP recognized this as classic pyoderma gangrenosum (PG)—a challenging condition to treat and not within the typical scope of practice for an FP. Nonhealing, well-defined leg ulcers in a person with Crohn's disease (or any type of inflammatory bowel disease) are often seen with PG. Pathergy—the development of an exaggerated injury following minor trauma—is known to occur with PG.
The FP also noted the violet-blue coloration around the borders of the ulcers, which is referred to as a “gun-metal border.” He considered doing a biopsy on the edge of the ulcer to rule out other conditions and to see if there was a neutrophilic infiltrate that is typically seen with PG. However, the FP realized that pathergy could be stimulated by a biopsy, so he decided to refer the patient to Dermatology.
Knowing that the patient might have to wait a few months to see a dermatologist, the FP consulted online sources and prescribed topical clobetasol ointment to be applied twice daily as an initial therapy. This was not successful, so after a phone consult with the dermatologist, the FP added oral prednisone 40 mg/d for the next 2 weeks until the dermatologist could see the patient.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP recognized this as classic pyoderma gangrenosum (PG)—a challenging condition to treat and not within the typical scope of practice for an FP. Nonhealing, well-defined leg ulcers in a person with Crohn's disease (or any type of inflammatory bowel disease) are often seen with PG. Pathergy—the development of an exaggerated injury following minor trauma—is known to occur with PG.
The FP also noted the violet-blue coloration around the borders of the ulcers, which is referred to as a “gun-metal border.” He considered doing a biopsy on the edge of the ulcer to rule out other conditions and to see if there was a neutrophilic infiltrate that is typically seen with PG. However, the FP realized that pathergy could be stimulated by a biopsy, so he decided to refer the patient to Dermatology.
Knowing that the patient might have to wait a few months to see a dermatologist, the FP consulted online sources and prescribed topical clobetasol ointment to be applied twice daily as an initial therapy. This was not successful, so after a phone consult with the dermatologist, the FP added oral prednisone 40 mg/d for the next 2 weeks until the dermatologist could see the patient.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
When the Right Medication Has the Wrong Effect
For several years, this 16-year-old boy has had severe acne on his chest and back. The condition has steadily worsened despite use of OTC medications, including benzoyl peroxide–containing topical products. He has never received prescription treatment. His primary care provider, concerned that something more than acne could be involved, refers him to dermatology for evaluation and treatment.
The boy’s health is reportedly otherwise excellent. Family history is positive for severe acne on both sides of his family; two of his older siblings have had similar problems.
EXAMINATION
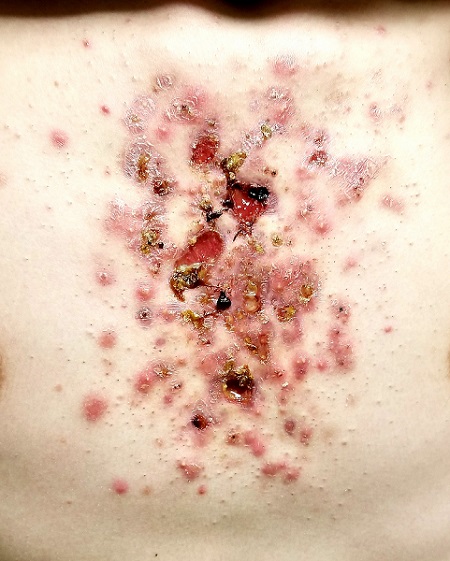
The central portion of the boy’s chest is covered by an impressive collection of discrete and confluent crusts and erosions. Some are quite deep, and the patient admits to picking at them. Little if any erythema surrounds the lesions.
Very little acne is seen on the boy’s face, but fairly dense acne vulgaris is observed on his upper back.
Following a brief but thorough discussion, the decision is made to start therapy with low-dose isotretinoin (20 mg/d), after the appropriate bloodwork is obtained. Within a week, the patient presents to the emergency department (ED) for worsening of his chest acne, along with pain and bleeding from some of the lesions.
What’s the diagnosis?
DISCUSSION
The risk for granulation tissue formation is a well-known though rare adverse effect of isotretinoin use—one that had been discussed thoroughly with the patient and his parents prior to prescription. There is no unanimity of opinion regarding the mechanism whereby this response occurs, but it is real. It can affect such areas as seen in this patient’s case but also causes a similar problem in the perionychial tissues, creating a condition quite similar to an ingrown toenail (cryptonychia). This author has also seen the same phenomenon in fingernails.
In this patient’s case, after discharge from the ED, he presented immediately to his primary care provider, who in turn called the dermatology clinic. When we saw him, there was clear evidence of inappropriate granulation formation—far worse than the initial acne had been.
The patient was given an intramuscular injection of 40 mg triamcinolone and prescribed minocycline 100 mg bid. He had already stopped taking the isotretinoin and was strongly advised not to restart it for the foreseeable future. In hindsight, he probably should have been started on minocycline and a 3-week prednisone taper to calm down his chest acne before the isotretinoin was started.
At a follow-up appointment 3 weeks later, his condition was much improved (at least back to baseline). But he will almost certainly develop hypertrophic scarring on his chest—and given the severity of his acne and the strong family history, he may well have a suboptimal response to isotretinoin when and if we return to that medication.
TAKE-HOME LEARNING POINTS
- In rare instances, isotretinoin therapy can trigger the formation of inappropriate granulation tissue on the face, back, chest, and even perionychial areas.
- There is evidence that the more inflamed the acne, the greater the likelihood of this adverse effect.
- With severely inflamed acne, consideration should be given to using small initial doses of isotretinoin or decreasing the inflammation through use of systemic steroids or doxycycline or minocycline.
For several years, this 16-year-old boy has had severe acne on his chest and back. The condition has steadily worsened despite use of OTC medications, including benzoyl peroxide–containing topical products. He has never received prescription treatment. His primary care provider, concerned that something more than acne could be involved, refers him to dermatology for evaluation and treatment.
The boy’s health is reportedly otherwise excellent. Family history is positive for severe acne on both sides of his family; two of his older siblings have had similar problems.

EXAMINATION
The central portion of the boy’s chest is covered by an impressive collection of discrete and confluent crusts and erosions. Some are quite deep, and the patient admits to picking at them. Little if any erythema surrounds the lesions.
Very little acne is seen on the boy’s face, but fairly dense acne vulgaris is observed on his upper back.
Following a brief but thorough discussion, the decision is made to start therapy with low-dose isotretinoin (20 mg/d), after the appropriate bloodwork is obtained. Within a week, the patient presents to the emergency department (ED) for worsening of his chest acne, along with pain and bleeding from some of the lesions.
What’s the diagnosis?
DISCUSSION
The risk for granulation tissue formation is a well-known though rare adverse effect of isotretinoin use—one that had been discussed thoroughly with the patient and his parents prior to prescription. There is no unanimity of opinion regarding the mechanism whereby this response occurs, but it is real. It can affect such areas as seen in this patient’s case but also causes a similar problem in the perionychial tissues, creating a condition quite similar to an ingrown toenail (cryptonychia). This author has also seen the same phenomenon in fingernails.
In this patient’s case, after discharge from the ED, he presented immediately to his primary care provider, who in turn called the dermatology clinic. When we saw him, there was clear evidence of inappropriate granulation formation—far worse than the initial acne had been.
The patient was given an intramuscular injection of 40 mg triamcinolone and prescribed minocycline 100 mg bid. He had already stopped taking the isotretinoin and was strongly advised not to restart it for the foreseeable future. In hindsight, he probably should have been started on minocycline and a 3-week prednisone taper to calm down his chest acne before the isotretinoin was started.
At a follow-up appointment 3 weeks later, his condition was much improved (at least back to baseline). But he will almost certainly develop hypertrophic scarring on his chest—and given the severity of his acne and the strong family history, he may well have a suboptimal response to isotretinoin when and if we return to that medication.
TAKE-HOME LEARNING POINTS
- In rare instances, isotretinoin therapy can trigger the formation of inappropriate granulation tissue on the face, back, chest, and even perionychial areas.
- There is evidence that the more inflamed the acne, the greater the likelihood of this adverse effect.
- With severely inflamed acne, consideration should be given to using small initial doses of isotretinoin or decreasing the inflammation through use of systemic steroids or doxycycline or minocycline.
For several years, this 16-year-old boy has had severe acne on his chest and back. The condition has steadily worsened despite use of OTC medications, including benzoyl peroxide–containing topical products. He has never received prescription treatment. His primary care provider, concerned that something more than acne could be involved, refers him to dermatology for evaluation and treatment.
The boy’s health is reportedly otherwise excellent. Family history is positive for severe acne on both sides of his family; two of his older siblings have had similar problems.

EXAMINATION
The central portion of the boy’s chest is covered by an impressive collection of discrete and confluent crusts and erosions. Some are quite deep, and the patient admits to picking at them. Little if any erythema surrounds the lesions.
Very little acne is seen on the boy’s face, but fairly dense acne vulgaris is observed on his upper back.
Following a brief but thorough discussion, the decision is made to start therapy with low-dose isotretinoin (20 mg/d), after the appropriate bloodwork is obtained. Within a week, the patient presents to the emergency department (ED) for worsening of his chest acne, along with pain and bleeding from some of the lesions.
What’s the diagnosis?
DISCUSSION
The risk for granulation tissue formation is a well-known though rare adverse effect of isotretinoin use—one that had been discussed thoroughly with the patient and his parents prior to prescription. There is no unanimity of opinion regarding the mechanism whereby this response occurs, but it is real. It can affect such areas as seen in this patient’s case but also causes a similar problem in the perionychial tissues, creating a condition quite similar to an ingrown toenail (cryptonychia). This author has also seen the same phenomenon in fingernails.
In this patient’s case, after discharge from the ED, he presented immediately to his primary care provider, who in turn called the dermatology clinic. When we saw him, there was clear evidence of inappropriate granulation formation—far worse than the initial acne had been.
The patient was given an intramuscular injection of 40 mg triamcinolone and prescribed minocycline 100 mg bid. He had already stopped taking the isotretinoin and was strongly advised not to restart it for the foreseeable future. In hindsight, he probably should have been started on minocycline and a 3-week prednisone taper to calm down his chest acne before the isotretinoin was started.
At a follow-up appointment 3 weeks later, his condition was much improved (at least back to baseline). But he will almost certainly develop hypertrophic scarring on his chest—and given the severity of his acne and the strong family history, he may well have a suboptimal response to isotretinoin when and if we return to that medication.
TAKE-HOME LEARNING POINTS
- In rare instances, isotretinoin therapy can trigger the formation of inappropriate granulation tissue on the face, back, chest, and even perionychial areas.
- There is evidence that the more inflamed the acne, the greater the likelihood of this adverse effect.
- With severely inflamed acne, consideration should be given to using small initial doses of isotretinoin or decreasing the inflammation through use of systemic steroids or doxycycline or minocycline.
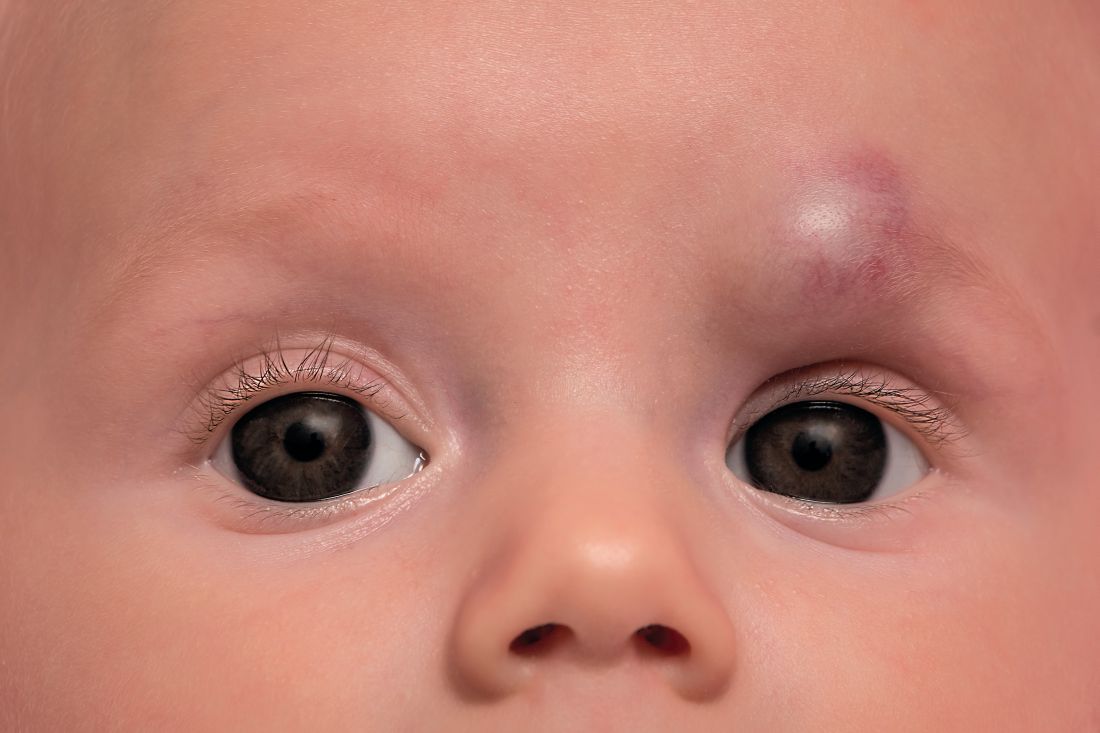
Timolol shortens propranolol use in infantile hemangioma
according to a study published in Pediatric Dermatology.
Diana B. Mannschreck, BSN, of Johns Hopkins University, Baltimore, and colleagues performed a retrospective chart review of 559 patients with infantile hemangioma seen in the dermatology clinic at Johns Hopkins between December 2008 and January 2018. Patients received any of five courses of treatment, including oral propranolol followed by topical timolol, propranolol only, and timolol only. Of the courses evaluated, propranolol followed by timolol had the shortest duration of propranolol therapy – a median of 2.2 months shorter than propranolol-only therapy (P = .0006). This sequential regimen also was associated with no reinitiations of propranolol therapy following tapering, whereas 13% of those receiving propranolol alone had to reinitiate it after tapering.
This is of interest because oral beta-blockers, including propranolol, have been associated with rare but serious adverse events, such as bronchospasm, hypotension, and hypoglycemia.
Limitations of the study include its retrospective and single-center nature. There was no funding or disclosure information given.
SOURCE: Mannschreck DB et al. Pediatr Dermatol. 2019 Apr 9. doi: 10.1111/pde.13816.
according to a study published in Pediatric Dermatology.
Diana B. Mannschreck, BSN, of Johns Hopkins University, Baltimore, and colleagues performed a retrospective chart review of 559 patients with infantile hemangioma seen in the dermatology clinic at Johns Hopkins between December 2008 and January 2018. Patients received any of five courses of treatment, including oral propranolol followed by topical timolol, propranolol only, and timolol only. Of the courses evaluated, propranolol followed by timolol had the shortest duration of propranolol therapy – a median of 2.2 months shorter than propranolol-only therapy (P = .0006). This sequential regimen also was associated with no reinitiations of propranolol therapy following tapering, whereas 13% of those receiving propranolol alone had to reinitiate it after tapering.
This is of interest because oral beta-blockers, including propranolol, have been associated with rare but serious adverse events, such as bronchospasm, hypotension, and hypoglycemia.
Limitations of the study include its retrospective and single-center nature. There was no funding or disclosure information given.
SOURCE: Mannschreck DB et al. Pediatr Dermatol. 2019 Apr 9. doi: 10.1111/pde.13816.
according to a study published in Pediatric Dermatology.
Diana B. Mannschreck, BSN, of Johns Hopkins University, Baltimore, and colleagues performed a retrospective chart review of 559 patients with infantile hemangioma seen in the dermatology clinic at Johns Hopkins between December 2008 and January 2018. Patients received any of five courses of treatment, including oral propranolol followed by topical timolol, propranolol only, and timolol only. Of the courses evaluated, propranolol followed by timolol had the shortest duration of propranolol therapy – a median of 2.2 months shorter than propranolol-only therapy (P = .0006). This sequential regimen also was associated with no reinitiations of propranolol therapy following tapering, whereas 13% of those receiving propranolol alone had to reinitiate it after tapering.
This is of interest because oral beta-blockers, including propranolol, have been associated with rare but serious adverse events, such as bronchospasm, hypotension, and hypoglycemia.
Limitations of the study include its retrospective and single-center nature. There was no funding or disclosure information given.
SOURCE: Mannschreck DB et al. Pediatr Dermatol. 2019 Apr 9. doi: 10.1111/pde.13816.
FROM PEDIATRIC DERMATOLOGY
Some “slime”-related contact dermatitis is allergic
The viscous homemade children’s plaything known as “slime” has been associated with allergic, as well as irritant, contact dermatitis of the hands thanks to an array of possible compounds with which it can be made, according to a case report in Pediatric Dermatology. The report details many possible compounds causing the dermatitis reactions seen by health care professionals.
In the case, which was reported by L. Elizabeth Anderson, MD, of the Children’s Hospital of Philadelphia and colleagues, an 11-year-old girl with a history of atopic dermatitis presented with hand dermatitis that was suspected to be related to playing with slime. After her dermatitis failed to respond to strong topical steroids, she was referred for patch testing, with positivity for methylchloroisothiazolinone/methylisothiazolinone (MCI/MI). After all contact with any products containing MCI/MI was eliminated, her hand dermatitis cleared, and bodywide atopic dermatitis improved some as well.
MCI/MI and MI are among the most commonly suspected culprits in cases of slime-related contact dermatitis. Although most cases are irritant contact dermatitis, some are allergic and can be detected using patch tests. MCI/MI is included in the T.R.U.E. Test, but according to the case report, 37% of patients with allergy to MI alone will not have positive response with the T.R.U.E. Test because of the low concentrations of MI in that test. The authors of this case report also listed many other the potential allergens in popular slime recipes; however, many are not included in the T.R.U.E. Test.
“While the T.R.U.E. Test does not capture most of the potential allergens in popular slime recipes, the recently published Pediatric Baseline Patch Test Series by Yu et al. [Dermatitis. 2018;29:206-12] does and is recommended for use in patients suspected of having dermatitis secondary to slime,” Dr. Anderson and associates wrote.
SOURCE: Anderson LE et al. Pediatr Dermatol. 2019 Mar 13. doi: 10.1111/pde.13792.
The viscous homemade children’s plaything known as “slime” has been associated with allergic, as well as irritant, contact dermatitis of the hands thanks to an array of possible compounds with which it can be made, according to a case report in Pediatric Dermatology. The report details many possible compounds causing the dermatitis reactions seen by health care professionals.
In the case, which was reported by L. Elizabeth Anderson, MD, of the Children’s Hospital of Philadelphia and colleagues, an 11-year-old girl with a history of atopic dermatitis presented with hand dermatitis that was suspected to be related to playing with slime. After her dermatitis failed to respond to strong topical steroids, she was referred for patch testing, with positivity for methylchloroisothiazolinone/methylisothiazolinone (MCI/MI). After all contact with any products containing MCI/MI was eliminated, her hand dermatitis cleared, and bodywide atopic dermatitis improved some as well.
MCI/MI and MI are among the most commonly suspected culprits in cases of slime-related contact dermatitis. Although most cases are irritant contact dermatitis, some are allergic and can be detected using patch tests. MCI/MI is included in the T.R.U.E. Test, but according to the case report, 37% of patients with allergy to MI alone will not have positive response with the T.R.U.E. Test because of the low concentrations of MI in that test. The authors of this case report also listed many other the potential allergens in popular slime recipes; however, many are not included in the T.R.U.E. Test.
“While the T.R.U.E. Test does not capture most of the potential allergens in popular slime recipes, the recently published Pediatric Baseline Patch Test Series by Yu et al. [Dermatitis. 2018;29:206-12] does and is recommended for use in patients suspected of having dermatitis secondary to slime,” Dr. Anderson and associates wrote.
SOURCE: Anderson LE et al. Pediatr Dermatol. 2019 Mar 13. doi: 10.1111/pde.13792.
The viscous homemade children’s plaything known as “slime” has been associated with allergic, as well as irritant, contact dermatitis of the hands thanks to an array of possible compounds with which it can be made, according to a case report in Pediatric Dermatology. The report details many possible compounds causing the dermatitis reactions seen by health care professionals.
In the case, which was reported by L. Elizabeth Anderson, MD, of the Children’s Hospital of Philadelphia and colleagues, an 11-year-old girl with a history of atopic dermatitis presented with hand dermatitis that was suspected to be related to playing with slime. After her dermatitis failed to respond to strong topical steroids, she was referred for patch testing, with positivity for methylchloroisothiazolinone/methylisothiazolinone (MCI/MI). After all contact with any products containing MCI/MI was eliminated, her hand dermatitis cleared, and bodywide atopic dermatitis improved some as well.
MCI/MI and MI are among the most commonly suspected culprits in cases of slime-related contact dermatitis. Although most cases are irritant contact dermatitis, some are allergic and can be detected using patch tests. MCI/MI is included in the T.R.U.E. Test, but according to the case report, 37% of patients with allergy to MI alone will not have positive response with the T.R.U.E. Test because of the low concentrations of MI in that test. The authors of this case report also listed many other the potential allergens in popular slime recipes; however, many are not included in the T.R.U.E. Test.
“While the T.R.U.E. Test does not capture most of the potential allergens in popular slime recipes, the recently published Pediatric Baseline Patch Test Series by Yu et al. [Dermatitis. 2018;29:206-12] does and is recommended for use in patients suspected of having dermatitis secondary to slime,” Dr. Anderson and associates wrote.
SOURCE: Anderson LE et al. Pediatr Dermatol. 2019 Mar 13. doi: 10.1111/pde.13792.
FROM PEDIATRIC DERMATOLOGY
Systematic review indicates cutaneous laser therapy may be safe during pregnancy
according to the results of a systematic review of 22 studies.
Among 380 women in all trimesters of pregnancy who were treated with various laser wavelengths, the only clinically significant event was a case of premature rupture of membranes (PROM) “without further morbidity,” wrote Eric C. Wilkerson, MD, of Skin Laser & Surgery Specialists of NY and NJ in New York, and associates. In that case, the cause was not clear, there was no further morbidity, “and it was uncertain whether this was related to the laser procedure.”
However, only 22 studies were identified between 1960 and 2017, all of which were case reports or series, published from 1994 to 2015. “[Thus far,] the best evidence exists for the safety of the carbon dioxide laser, particularly in the treatment of condyloma,” they wrote in Dermatologic Surgery.
Elective laser treatments are usually not recommended during pregnancy, but no evidence supports this, Dr. Wilkerson and coauthors wrote. Therefore, they searched for studies indexed in PubMed, Google Scholar, the Cochrane Library, or the EBSCO CINAHL Plus Database from 1960 to 2017. They also searched LexisNexis for relevant legal cases, but found none.
The women in the 22 case reports and series were aged 14-41 years and received laser therapy for cervical adenocarcinoma, urolithiasis, condyloma acuminata, cervical carcinoma in situ, cutaneous scarring, Buschke-Löwenstein tumor, verrucous carcinoma, and acne vulgaris. Modalities included 504-nm pulsed-dye laser, 532-nm potassium titanyl phosphate, 1,064-nm neodymium:YAG, 2,100-nm holmium:YAG, and 10,600-nm CO2.
Apart from the case of PROM, there were no instances of fetal morbidity or mortality, premature labor or preterm birth, or detectable fetal stress, the authors wrote. The case of PROM occurred at 35 weeks, 4 days after the mother had received CO2 laser therapy for condyloma acuminata. She delivered normally approximately 1 week later. There also were several cases of premature contractions without true labor, all of which responded to tocolytic therapy. (In the same study, there also were two cases of PROM in women 7 and 10 weeks after the same procedure, but were thought to be unrelated.)
The thickness of the pregnant abdomen, uterus, and amniotic fluid makes it “very unlikely” that clinically significant amounts of laser energy would reach the fetus during cutaneous laser therapy, the authors noted. Certain topical anesthetics, such as lidocaine and prilocaine, also appear safe during pregnancy “and may potentially decrease concern for fetal stress secondary to maternal stress or pain during the procedure,” they added. “Appropriate safety measures including eye protection and laser plume management should continue to be used during laser treatment.”
The authors reported no funding sources or conflicts of interest.
SOURCE: Wilkerson EJ et al. Dermatol Surg. 2019 Jun;45(6):818-28.
according to the results of a systematic review of 22 studies.
Among 380 women in all trimesters of pregnancy who were treated with various laser wavelengths, the only clinically significant event was a case of premature rupture of membranes (PROM) “without further morbidity,” wrote Eric C. Wilkerson, MD, of Skin Laser & Surgery Specialists of NY and NJ in New York, and associates. In that case, the cause was not clear, there was no further morbidity, “and it was uncertain whether this was related to the laser procedure.”
However, only 22 studies were identified between 1960 and 2017, all of which were case reports or series, published from 1994 to 2015. “[Thus far,] the best evidence exists for the safety of the carbon dioxide laser, particularly in the treatment of condyloma,” they wrote in Dermatologic Surgery.
Elective laser treatments are usually not recommended during pregnancy, but no evidence supports this, Dr. Wilkerson and coauthors wrote. Therefore, they searched for studies indexed in PubMed, Google Scholar, the Cochrane Library, or the EBSCO CINAHL Plus Database from 1960 to 2017. They also searched LexisNexis for relevant legal cases, but found none.
The women in the 22 case reports and series were aged 14-41 years and received laser therapy for cervical adenocarcinoma, urolithiasis, condyloma acuminata, cervical carcinoma in situ, cutaneous scarring, Buschke-Löwenstein tumor, verrucous carcinoma, and acne vulgaris. Modalities included 504-nm pulsed-dye laser, 532-nm potassium titanyl phosphate, 1,064-nm neodymium:YAG, 2,100-nm holmium:YAG, and 10,600-nm CO2.
Apart from the case of PROM, there were no instances of fetal morbidity or mortality, premature labor or preterm birth, or detectable fetal stress, the authors wrote. The case of PROM occurred at 35 weeks, 4 days after the mother had received CO2 laser therapy for condyloma acuminata. She delivered normally approximately 1 week later. There also were several cases of premature contractions without true labor, all of which responded to tocolytic therapy. (In the same study, there also were two cases of PROM in women 7 and 10 weeks after the same procedure, but were thought to be unrelated.)
The thickness of the pregnant abdomen, uterus, and amniotic fluid makes it “very unlikely” that clinically significant amounts of laser energy would reach the fetus during cutaneous laser therapy, the authors noted. Certain topical anesthetics, such as lidocaine and prilocaine, also appear safe during pregnancy “and may potentially decrease concern for fetal stress secondary to maternal stress or pain during the procedure,” they added. “Appropriate safety measures including eye protection and laser plume management should continue to be used during laser treatment.”
The authors reported no funding sources or conflicts of interest.
SOURCE: Wilkerson EJ et al. Dermatol Surg. 2019 Jun;45(6):818-28.
according to the results of a systematic review of 22 studies.
Among 380 women in all trimesters of pregnancy who were treated with various laser wavelengths, the only clinically significant event was a case of premature rupture of membranes (PROM) “without further morbidity,” wrote Eric C. Wilkerson, MD, of Skin Laser & Surgery Specialists of NY and NJ in New York, and associates. In that case, the cause was not clear, there was no further morbidity, “and it was uncertain whether this was related to the laser procedure.”
However, only 22 studies were identified between 1960 and 2017, all of which were case reports or series, published from 1994 to 2015. “[Thus far,] the best evidence exists for the safety of the carbon dioxide laser, particularly in the treatment of condyloma,” they wrote in Dermatologic Surgery.
Elective laser treatments are usually not recommended during pregnancy, but no evidence supports this, Dr. Wilkerson and coauthors wrote. Therefore, they searched for studies indexed in PubMed, Google Scholar, the Cochrane Library, or the EBSCO CINAHL Plus Database from 1960 to 2017. They also searched LexisNexis for relevant legal cases, but found none.
The women in the 22 case reports and series were aged 14-41 years and received laser therapy for cervical adenocarcinoma, urolithiasis, condyloma acuminata, cervical carcinoma in situ, cutaneous scarring, Buschke-Löwenstein tumor, verrucous carcinoma, and acne vulgaris. Modalities included 504-nm pulsed-dye laser, 532-nm potassium titanyl phosphate, 1,064-nm neodymium:YAG, 2,100-nm holmium:YAG, and 10,600-nm CO2.
Apart from the case of PROM, there were no instances of fetal morbidity or mortality, premature labor or preterm birth, or detectable fetal stress, the authors wrote. The case of PROM occurred at 35 weeks, 4 days after the mother had received CO2 laser therapy for condyloma acuminata. She delivered normally approximately 1 week later. There also were several cases of premature contractions without true labor, all of which responded to tocolytic therapy. (In the same study, there also were two cases of PROM in women 7 and 10 weeks after the same procedure, but were thought to be unrelated.)
The thickness of the pregnant abdomen, uterus, and amniotic fluid makes it “very unlikely” that clinically significant amounts of laser energy would reach the fetus during cutaneous laser therapy, the authors noted. Certain topical anesthetics, such as lidocaine and prilocaine, also appear safe during pregnancy “and may potentially decrease concern for fetal stress secondary to maternal stress or pain during the procedure,” they added. “Appropriate safety measures including eye protection and laser plume management should continue to be used during laser treatment.”
The authors reported no funding sources or conflicts of interest.
SOURCE: Wilkerson EJ et al. Dermatol Surg. 2019 Jun;45(6):818-28.
FROM DERMATOLOGIC SURGERY
Generalized rash following ankle ulceration
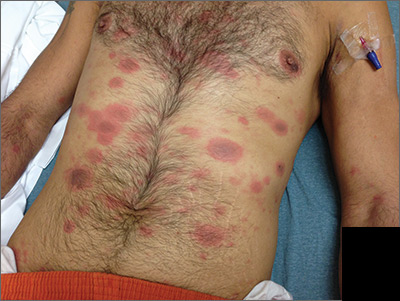
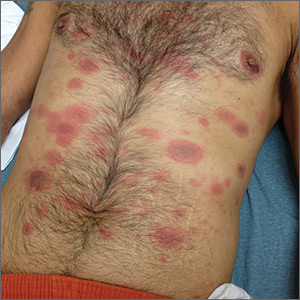
At the hospital, the physicians noted that there were no lesions on the mucous membranes of the patient’s eyes, ears, nose, mouth or anus and his vital signs were within normal limits. He was empirically treated with 1 dose of methylprednisolone (125 mg intravenous [IV]) and started on IV piperacillin-tazobactam and vancomycin. The patient subsequently revealed that he’d had a similar experience a year earlier after being treated with TMP-SMX for cellulitis. During the previous episode, he said the lesions were located on the exact same areas of his glans penis and chin. Based on the morphologic characteristics of the eruption and the history of similar lesions that appeared following previous treatment with TMP-SMX, the physician diagnosed disseminated fixed-drug eruption in this patient.
A fixed-drug eruption is an adverse cutaneous reaction to a drug that is defined by a dusky red or violaceous macule, which evolves into a patch, and eventually, an edematous plaque. Fixed-drug eruptions are typically solitary, but may be generalized (as was the case with this patient). The pathophysiology of the disease involves resident intra-epidermal CD8+ T-cells resembling effector memory T-cells. These T-cells are increased in number at the dermoepidermal junction of normal appearing skin; their aberrant activation leads to an inflammatory response, stimulating tissue destruction and formation of the classic fixed-drug lesion.
This diagnosis usually is made based on a history of similar lesions recurring at the same location in response to a specific drug and the classic physical exam findings of well-demarcated, edematous, and violaceous plaques. A skin biopsy may be performed to confirm a fixed-drug eruption in the case of clinical equipoise.
Fixed-drug eruptions occasionally exhibit bullae and erosions and must be differentiated from more serious generalized bullous diseases, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The differential diagnosis also includes erythema multiforme, early bullous drug eruption, and bullous arthropod assault, which may leave similar hyperpigmented patches.
Management of a disseminated fixed-drug eruption requires a thorough history to identify the causative agent (including over-the-counter drugs, herbals, topicals, and eye drops). Most patients are asymptomatic, but some (like this patient) are symptomatic and experience generalized pruritus, cutaneous burning, and/or pain. Symptomatic therapy includes oral antihistamines and potent topical glucocorticoid ointment for non-eroded lesions. Additionally, if not medically contraindicated, oral steroids may be used for generalized or extremely painful mucosal lesions at a dose of 0.5 mg/kg daily for 3 to 5 days.
Local wound care of eroded lesions includes keeping the site moist with a bland emollient and bandaging. The inciting agent must be added to the patient’s allergy list and avoided in the future. In equivocal cases, it is prudent to admit the patient for observation to ensure that the eruption is not a nascent SJS or TEN eruption.
In this case, the patient was admitted to the observation unit overnight to monitor for the appearance of systemic symptoms and to assess the evolution of the rash for further mucosal involvement that could have indicated SJS. Upon reassessment the next day, his older lesions had evolved into vesiculated and necrotic areas as per the natural history of severe fixed-drug eruption. He was prescribed prednisone 40 mg/d for 3 days to help with local inflammation, pain, and itching. TMP-SMX was added to his allergy list and he was given local wound care instructions. He was told to return if he developed any systemic symptoms.
This case was adapted from: Bucher J, Rahnama-Moghadam S, Osswald S. Generalized rash follows ankle ulceration. J Fam Pract. 2016;65:489-491.

At the hospital, the physicians noted that there were no lesions on the mucous membranes of the patient’s eyes, ears, nose, mouth or anus and his vital signs were within normal limits. He was empirically treated with 1 dose of methylprednisolone (125 mg intravenous [IV]) and started on IV piperacillin-tazobactam and vancomycin. The patient subsequently revealed that he’d had a similar experience a year earlier after being treated with TMP-SMX for cellulitis. During the previous episode, he said the lesions were located on the exact same areas of his glans penis and chin. Based on the morphologic characteristics of the eruption and the history of similar lesions that appeared following previous treatment with TMP-SMX, the physician diagnosed disseminated fixed-drug eruption in this patient.
A fixed-drug eruption is an adverse cutaneous reaction to a drug that is defined by a dusky red or violaceous macule, which evolves into a patch, and eventually, an edematous plaque. Fixed-drug eruptions are typically solitary, but may be generalized (as was the case with this patient). The pathophysiology of the disease involves resident intra-epidermal CD8+ T-cells resembling effector memory T-cells. These T-cells are increased in number at the dermoepidermal junction of normal appearing skin; their aberrant activation leads to an inflammatory response, stimulating tissue destruction and formation of the classic fixed-drug lesion.
This diagnosis usually is made based on a history of similar lesions recurring at the same location in response to a specific drug and the classic physical exam findings of well-demarcated, edematous, and violaceous plaques. A skin biopsy may be performed to confirm a fixed-drug eruption in the case of clinical equipoise.
Fixed-drug eruptions occasionally exhibit bullae and erosions and must be differentiated from more serious generalized bullous diseases, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The differential diagnosis also includes erythema multiforme, early bullous drug eruption, and bullous arthropod assault, which may leave similar hyperpigmented patches.
Management of a disseminated fixed-drug eruption requires a thorough history to identify the causative agent (including over-the-counter drugs, herbals, topicals, and eye drops). Most patients are asymptomatic, but some (like this patient) are symptomatic and experience generalized pruritus, cutaneous burning, and/or pain. Symptomatic therapy includes oral antihistamines and potent topical glucocorticoid ointment for non-eroded lesions. Additionally, if not medically contraindicated, oral steroids may be used for generalized or extremely painful mucosal lesions at a dose of 0.5 mg/kg daily for 3 to 5 days.
Local wound care of eroded lesions includes keeping the site moist with a bland emollient and bandaging. The inciting agent must be added to the patient’s allergy list and avoided in the future. In equivocal cases, it is prudent to admit the patient for observation to ensure that the eruption is not a nascent SJS or TEN eruption.
In this case, the patient was admitted to the observation unit overnight to monitor for the appearance of systemic symptoms and to assess the evolution of the rash for further mucosal involvement that could have indicated SJS. Upon reassessment the next day, his older lesions had evolved into vesiculated and necrotic areas as per the natural history of severe fixed-drug eruption. He was prescribed prednisone 40 mg/d for 3 days to help with local inflammation, pain, and itching. TMP-SMX was added to his allergy list and he was given local wound care instructions. He was told to return if he developed any systemic symptoms.
This case was adapted from: Bucher J, Rahnama-Moghadam S, Osswald S. Generalized rash follows ankle ulceration. J Fam Pract. 2016;65:489-491.

At the hospital, the physicians noted that there were no lesions on the mucous membranes of the patient’s eyes, ears, nose, mouth or anus and his vital signs were within normal limits. He was empirically treated with 1 dose of methylprednisolone (125 mg intravenous [IV]) and started on IV piperacillin-tazobactam and vancomycin. The patient subsequently revealed that he’d had a similar experience a year earlier after being treated with TMP-SMX for cellulitis. During the previous episode, he said the lesions were located on the exact same areas of his glans penis and chin. Based on the morphologic characteristics of the eruption and the history of similar lesions that appeared following previous treatment with TMP-SMX, the physician diagnosed disseminated fixed-drug eruption in this patient.
A fixed-drug eruption is an adverse cutaneous reaction to a drug that is defined by a dusky red or violaceous macule, which evolves into a patch, and eventually, an edematous plaque. Fixed-drug eruptions are typically solitary, but may be generalized (as was the case with this patient). The pathophysiology of the disease involves resident intra-epidermal CD8+ T-cells resembling effector memory T-cells. These T-cells are increased in number at the dermoepidermal junction of normal appearing skin; their aberrant activation leads to an inflammatory response, stimulating tissue destruction and formation of the classic fixed-drug lesion.
This diagnosis usually is made based on a history of similar lesions recurring at the same location in response to a specific drug and the classic physical exam findings of well-demarcated, edematous, and violaceous plaques. A skin biopsy may be performed to confirm a fixed-drug eruption in the case of clinical equipoise.
Fixed-drug eruptions occasionally exhibit bullae and erosions and must be differentiated from more serious generalized bullous diseases, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The differential diagnosis also includes erythema multiforme, early bullous drug eruption, and bullous arthropod assault, which may leave similar hyperpigmented patches.
Management of a disseminated fixed-drug eruption requires a thorough history to identify the causative agent (including over-the-counter drugs, herbals, topicals, and eye drops). Most patients are asymptomatic, but some (like this patient) are symptomatic and experience generalized pruritus, cutaneous burning, and/or pain. Symptomatic therapy includes oral antihistamines and potent topical glucocorticoid ointment for non-eroded lesions. Additionally, if not medically contraindicated, oral steroids may be used for generalized or extremely painful mucosal lesions at a dose of 0.5 mg/kg daily for 3 to 5 days.
Local wound care of eroded lesions includes keeping the site moist with a bland emollient and bandaging. The inciting agent must be added to the patient’s allergy list and avoided in the future. In equivocal cases, it is prudent to admit the patient for observation to ensure that the eruption is not a nascent SJS or TEN eruption.
In this case, the patient was admitted to the observation unit overnight to monitor for the appearance of systemic symptoms and to assess the evolution of the rash for further mucosal involvement that could have indicated SJS. Upon reassessment the next day, his older lesions had evolved into vesiculated and necrotic areas as per the natural history of severe fixed-drug eruption. He was prescribed prednisone 40 mg/d for 3 days to help with local inflammation, pain, and itching. TMP-SMX was added to his allergy list and he was given local wound care instructions. He was told to return if he developed any systemic symptoms.
This case was adapted from: Bucher J, Rahnama-Moghadam S, Osswald S. Generalized rash follows ankle ulceration. J Fam Pract. 2016;65:489-491.
Severe respiratory failure strikes healthy teens on trimethoprim-sulfamethoxazole
TMP-SMX, a frequently prescribed antibiotic, has been associated with “idiosyncratic adverse drug reactions, including cutaneous reactions and hypersensitivity syndromes,” but pulmonary complications are rare, especially in children, wrote Jenna O. Miller, MD, of the University of Missouri–Kansas City and colleagues.
In a case series published in Pediatrics, the researchers described the patients, who were aged 13-18 years; the 18-year-old was male, the others were female. Four of the patients (three females, one male) were taking TMP-SMX for acne vulgaris. One of these patients, a 13-year-old girl, underwent a bilateral lung and heart transplant after developing interstitial lung disease and died as a result of solid organ transplant complications. The other death occurred in a 15-year-old girl who was taking TMP-SMX to treat a urinary tract infection. This patient developed interstitial lung disease and died of complications from the disease while awaiting a lung transplant.
“In all cases, patients were transferred to academic medical facilities, and pediatric pulmonologists and infectious diseases specialists performed extensive evaluations,” the researchers wrote. The patients did not improve when the drug was discontinued, and four of the five were considered or listed for organ transplants. The spectrum of disease was varied among the patients, and the pathophysiology remains poorly understood.
Although no clinical test could confirm causality between TMP-SMX and ARDS in the five teens, “the extensive negative workup, paired with recent TMP-SMX exposure and similarity among these cases, raises the possibility that the observed ARDS was TMP-SMX triggered,” they wrote.
The researchers had no financial conflicts to disclose.
SOURCE: Miller JO et al. Pediatrics. 2019 May 29. doi: 10.1542/peds.2018.3242.
TMP-SMX, a frequently prescribed antibiotic, has been associated with “idiosyncratic adverse drug reactions, including cutaneous reactions and hypersensitivity syndromes,” but pulmonary complications are rare, especially in children, wrote Jenna O. Miller, MD, of the University of Missouri–Kansas City and colleagues.
In a case series published in Pediatrics, the researchers described the patients, who were aged 13-18 years; the 18-year-old was male, the others were female. Four of the patients (three females, one male) were taking TMP-SMX for acne vulgaris. One of these patients, a 13-year-old girl, underwent a bilateral lung and heart transplant after developing interstitial lung disease and died as a result of solid organ transplant complications. The other death occurred in a 15-year-old girl who was taking TMP-SMX to treat a urinary tract infection. This patient developed interstitial lung disease and died of complications from the disease while awaiting a lung transplant.
“In all cases, patients were transferred to academic medical facilities, and pediatric pulmonologists and infectious diseases specialists performed extensive evaluations,” the researchers wrote. The patients did not improve when the drug was discontinued, and four of the five were considered or listed for organ transplants. The spectrum of disease was varied among the patients, and the pathophysiology remains poorly understood.
Although no clinical test could confirm causality between TMP-SMX and ARDS in the five teens, “the extensive negative workup, paired with recent TMP-SMX exposure and similarity among these cases, raises the possibility that the observed ARDS was TMP-SMX triggered,” they wrote.
The researchers had no financial conflicts to disclose.
SOURCE: Miller JO et al. Pediatrics. 2019 May 29. doi: 10.1542/peds.2018.3242.
TMP-SMX, a frequently prescribed antibiotic, has been associated with “idiosyncratic adverse drug reactions, including cutaneous reactions and hypersensitivity syndromes,” but pulmonary complications are rare, especially in children, wrote Jenna O. Miller, MD, of the University of Missouri–Kansas City and colleagues.
In a case series published in Pediatrics, the researchers described the patients, who were aged 13-18 years; the 18-year-old was male, the others were female. Four of the patients (three females, one male) were taking TMP-SMX for acne vulgaris. One of these patients, a 13-year-old girl, underwent a bilateral lung and heart transplant after developing interstitial lung disease and died as a result of solid organ transplant complications. The other death occurred in a 15-year-old girl who was taking TMP-SMX to treat a urinary tract infection. This patient developed interstitial lung disease and died of complications from the disease while awaiting a lung transplant.
“In all cases, patients were transferred to academic medical facilities, and pediatric pulmonologists and infectious diseases specialists performed extensive evaluations,” the researchers wrote. The patients did not improve when the drug was discontinued, and four of the five were considered or listed for organ transplants. The spectrum of disease was varied among the patients, and the pathophysiology remains poorly understood.
Although no clinical test could confirm causality between TMP-SMX and ARDS in the five teens, “the extensive negative workup, paired with recent TMP-SMX exposure and similarity among these cases, raises the possibility that the observed ARDS was TMP-SMX triggered,” they wrote.
The researchers had no financial conflicts to disclose.
SOURCE: Miller JO et al. Pediatrics. 2019 May 29. doi: 10.1542/peds.2018.3242.
FROM PEDIATRICS
Study finds inconsistent links with aspirin, nonaspirin NSAIDs and reduced skin cancer risk
Use of aspirin or nonaspirin NSAIDs was not associated with a reduced risk of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), in a large, prospective cohort study of Australian residents.
“Overall, we observed weak and inconsistent inverse associations between use of these medications and incidence of either BCC or SCC,” wrote Nirmala Pandeya, PhD, of the University of Queensland (Australia) and coauthors. “While we did observe a modest reduction in use,” they added. The study was published in the British Journal of Dermatology.
While reviews of observational studies have suggested that NSAIDs may have “a potential benefit” in reducing the incidence of BCC and SCC, the results have varied, they noted.
To investigate the potential chemopreventive effects of NSAID use on skin cancer, the investigators used data from the QSkin Sun and Health Study, a prospective cohort of 43,764 residents of Queensland, Australia. Those eligible for the study had a white ethnic background and no history of melanoma; 34,630 participants were available for analysis, their median age was 57 years, and 55% were women
Almost 15,600 (45%) were classified as “high risk” because they had had at least one skin cancer excision or more than five actinic lesions treated; 18,828 participants were classified as “average to low risk;” and data were unavailable for 206 participants. One‐third of the participants in the high-risk group (5,398) used aspirin; of these individuals, 39% (2,132) used aspirin more than once a week (defined as “frequent” users). Also, 60% (9,236) used NSAIDs, and of those, 24% (2,229) were frequent users.
During a median follow-up of 3 years, 3,421 of those in the study (10%) developed one or more BCC, and 1,470 (4%) developed one or more SCC.
Compared with never users, frequent NSAID use in the high-risk group was modestly associated with a reduced risk of BCC (hazard ratio, 0.84; 95% confidence interval, 0.71-0.99), but not with SCC. Aspirin use was weakly associated with a reduced risk of SCC (HR, 0.77; 95% CI, 0.64-0.93) but only among infrequent users and was not associated with BCC risk. In the average- to low-risk group, there was no association with either NSAIDs or aspirin and BCC or SCC occurrence.
The authors noted limitations of their study, including its reliance on self-reported NSAID use and a lack of detail in regard to usage dose and duration. In addition, though the investigators controlled for all likely confounders, “the possibility of some residual confounding cannot be excluded.”
The QSkin Study was funded by a grant from the National Health and Medical Research Council of Australia (NHMRC). The authors declared no conflicts of interest.
SOURCE: Pandeya N et al. Br J Dermatol. 2019 Mar 28. doi: 10.1111/bjd.17938.
Use of aspirin or nonaspirin NSAIDs was not associated with a reduced risk of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), in a large, prospective cohort study of Australian residents.
“Overall, we observed weak and inconsistent inverse associations between use of these medications and incidence of either BCC or SCC,” wrote Nirmala Pandeya, PhD, of the University of Queensland (Australia) and coauthors. “While we did observe a modest reduction in use,” they added. The study was published in the British Journal of Dermatology.
While reviews of observational studies have suggested that NSAIDs may have “a potential benefit” in reducing the incidence of BCC and SCC, the results have varied, they noted.
To investigate the potential chemopreventive effects of NSAID use on skin cancer, the investigators used data from the QSkin Sun and Health Study, a prospective cohort of 43,764 residents of Queensland, Australia. Those eligible for the study had a white ethnic background and no history of melanoma; 34,630 participants were available for analysis, their median age was 57 years, and 55% were women
Almost 15,600 (45%) were classified as “high risk” because they had had at least one skin cancer excision or more than five actinic lesions treated; 18,828 participants were classified as “average to low risk;” and data were unavailable for 206 participants. One‐third of the participants in the high-risk group (5,398) used aspirin; of these individuals, 39% (2,132) used aspirin more than once a week (defined as “frequent” users). Also, 60% (9,236) used NSAIDs, and of those, 24% (2,229) were frequent users.
During a median follow-up of 3 years, 3,421 of those in the study (10%) developed one or more BCC, and 1,470 (4%) developed one or more SCC.
Compared with never users, frequent NSAID use in the high-risk group was modestly associated with a reduced risk of BCC (hazard ratio, 0.84; 95% confidence interval, 0.71-0.99), but not with SCC. Aspirin use was weakly associated with a reduced risk of SCC (HR, 0.77; 95% CI, 0.64-0.93) but only among infrequent users and was not associated with BCC risk. In the average- to low-risk group, there was no association with either NSAIDs or aspirin and BCC or SCC occurrence.
The authors noted limitations of their study, including its reliance on self-reported NSAID use and a lack of detail in regard to usage dose and duration. In addition, though the investigators controlled for all likely confounders, “the possibility of some residual confounding cannot be excluded.”
The QSkin Study was funded by a grant from the National Health and Medical Research Council of Australia (NHMRC). The authors declared no conflicts of interest.
SOURCE: Pandeya N et al. Br J Dermatol. 2019 Mar 28. doi: 10.1111/bjd.17938.
Use of aspirin or nonaspirin NSAIDs was not associated with a reduced risk of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), in a large, prospective cohort study of Australian residents.
“Overall, we observed weak and inconsistent inverse associations between use of these medications and incidence of either BCC or SCC,” wrote Nirmala Pandeya, PhD, of the University of Queensland (Australia) and coauthors. “While we did observe a modest reduction in use,” they added. The study was published in the British Journal of Dermatology.
While reviews of observational studies have suggested that NSAIDs may have “a potential benefit” in reducing the incidence of BCC and SCC, the results have varied, they noted.
To investigate the potential chemopreventive effects of NSAID use on skin cancer, the investigators used data from the QSkin Sun and Health Study, a prospective cohort of 43,764 residents of Queensland, Australia. Those eligible for the study had a white ethnic background and no history of melanoma; 34,630 participants were available for analysis, their median age was 57 years, and 55% were women
Almost 15,600 (45%) were classified as “high risk” because they had had at least one skin cancer excision or more than five actinic lesions treated; 18,828 participants were classified as “average to low risk;” and data were unavailable for 206 participants. One‐third of the participants in the high-risk group (5,398) used aspirin; of these individuals, 39% (2,132) used aspirin more than once a week (defined as “frequent” users). Also, 60% (9,236) used NSAIDs, and of those, 24% (2,229) were frequent users.
During a median follow-up of 3 years, 3,421 of those in the study (10%) developed one or more BCC, and 1,470 (4%) developed one or more SCC.
Compared with never users, frequent NSAID use in the high-risk group was modestly associated with a reduced risk of BCC (hazard ratio, 0.84; 95% confidence interval, 0.71-0.99), but not with SCC. Aspirin use was weakly associated with a reduced risk of SCC (HR, 0.77; 95% CI, 0.64-0.93) but only among infrequent users and was not associated with BCC risk. In the average- to low-risk group, there was no association with either NSAIDs or aspirin and BCC or SCC occurrence.
The authors noted limitations of their study, including its reliance on self-reported NSAID use and a lack of detail in regard to usage dose and duration. In addition, though the investigators controlled for all likely confounders, “the possibility of some residual confounding cannot be excluded.”
The QSkin Study was funded by a grant from the National Health and Medical Research Council of Australia (NHMRC). The authors declared no conflicts of interest.
SOURCE: Pandeya N et al. Br J Dermatol. 2019 Mar 28. doi: 10.1111/bjd.17938.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
By the numbers: Readmissions for skin conditions
Almost 10% of patients
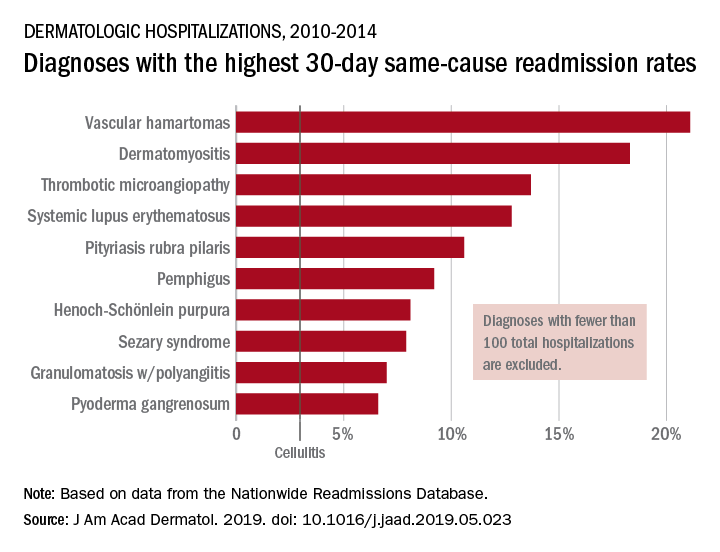
Data from the Nationwide Readmissions Database also showed that the same-cause readmission rate was 3.3% after 30 days and 7.8% within the calendar year (CY) over the 5-year study period of 2010-2014, Myron Zhang, MD, of the department of dermatology at Weill Cornell Medicine, New York, and his associates reported in the Journal of the American Academy of Dermatology.
The total cost of the CY readmissions was $2.54 billion, which works out to $508 million per year or $8,995 per visit. The most common dermatologic diagnosis – cellulitis made up 83.6% of all hospitalizations – was also the most expensive in terms of readmissions, resulting in $1.9 billion in CY costs, Dr. Zhang and associates wrote.
Overall readmission rates for cellulitis were not provided, but annual rates ranged from 9.1% to 9.3% (30-day all cause), from 7.7% to 8.1% (CY same cause), and from 3.1% to 3.3% (30-day same cause), they wrote.
The dermatologic diagnosis with the highest 30-day same-cause readmission rate was vascular hamartomas at 21.1%, followed by dermatomyositis (18.3%) and thrombotic microangiopathy (13.7%). Dermatomyositis had the highest CY same-cause readmission rate (30.8%) and mycosis fungoides had the highest 30-day all-cause rate (32.3%), according to the investigators.
“Diseases, characteristics, and comorbidities associated with high readmission rates should trigger hospitals to consider dermatology consultation, coordinate outpatient follow-up, and support underinsured outpatient access. These measures have been shown to reduce readmissions or hospital visits in general dermatologic settings, but outcomes in individual diseases are not well studied,” Dr. Zhang and associates wrote. They noted that there have been “very few prior studies of readmissions for skin diseases.”
[email protected]
SOURCE: Zhang M et al. J Am Acad. Dermatol. 2019. doi: 10.1016/j.jaad.2019.05.023. .
Almost 10% of patients

Data from the Nationwide Readmissions Database also showed that the same-cause readmission rate was 3.3% after 30 days and 7.8% within the calendar year (CY) over the 5-year study period of 2010-2014, Myron Zhang, MD, of the department of dermatology at Weill Cornell Medicine, New York, and his associates reported in the Journal of the American Academy of Dermatology.
The total cost of the CY readmissions was $2.54 billion, which works out to $508 million per year or $8,995 per visit. The most common dermatologic diagnosis – cellulitis made up 83.6% of all hospitalizations – was also the most expensive in terms of readmissions, resulting in $1.9 billion in CY costs, Dr. Zhang and associates wrote.
Overall readmission rates for cellulitis were not provided, but annual rates ranged from 9.1% to 9.3% (30-day all cause), from 7.7% to 8.1% (CY same cause), and from 3.1% to 3.3% (30-day same cause), they wrote.
The dermatologic diagnosis with the highest 30-day same-cause readmission rate was vascular hamartomas at 21.1%, followed by dermatomyositis (18.3%) and thrombotic microangiopathy (13.7%). Dermatomyositis had the highest CY same-cause readmission rate (30.8%) and mycosis fungoides had the highest 30-day all-cause rate (32.3%), according to the investigators.
“Diseases, characteristics, and comorbidities associated with high readmission rates should trigger hospitals to consider dermatology consultation, coordinate outpatient follow-up, and support underinsured outpatient access. These measures have been shown to reduce readmissions or hospital visits in general dermatologic settings, but outcomes in individual diseases are not well studied,” Dr. Zhang and associates wrote. They noted that there have been “very few prior studies of readmissions for skin diseases.”
[email protected]
SOURCE: Zhang M et al. J Am Acad. Dermatol. 2019. doi: 10.1016/j.jaad.2019.05.023. .
Almost 10% of patients

Data from the Nationwide Readmissions Database also showed that the same-cause readmission rate was 3.3% after 30 days and 7.8% within the calendar year (CY) over the 5-year study period of 2010-2014, Myron Zhang, MD, of the department of dermatology at Weill Cornell Medicine, New York, and his associates reported in the Journal of the American Academy of Dermatology.
The total cost of the CY readmissions was $2.54 billion, which works out to $508 million per year or $8,995 per visit. The most common dermatologic diagnosis – cellulitis made up 83.6% of all hospitalizations – was also the most expensive in terms of readmissions, resulting in $1.9 billion in CY costs, Dr. Zhang and associates wrote.
Overall readmission rates for cellulitis were not provided, but annual rates ranged from 9.1% to 9.3% (30-day all cause), from 7.7% to 8.1% (CY same cause), and from 3.1% to 3.3% (30-day same cause), they wrote.
The dermatologic diagnosis with the highest 30-day same-cause readmission rate was vascular hamartomas at 21.1%, followed by dermatomyositis (18.3%) and thrombotic microangiopathy (13.7%). Dermatomyositis had the highest CY same-cause readmission rate (30.8%) and mycosis fungoides had the highest 30-day all-cause rate (32.3%), according to the investigators.
“Diseases, characteristics, and comorbidities associated with high readmission rates should trigger hospitals to consider dermatology consultation, coordinate outpatient follow-up, and support underinsured outpatient access. These measures have been shown to reduce readmissions or hospital visits in general dermatologic settings, but outcomes in individual diseases are not well studied,” Dr. Zhang and associates wrote. They noted that there have been “very few prior studies of readmissions for skin diseases.”
[email protected]
SOURCE: Zhang M et al. J Am Acad. Dermatol. 2019. doi: 10.1016/j.jaad.2019.05.023. .
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY