User login
Anti-infective update addresses SSSI choices
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
EXPERT ANALYSIS FROM ODAC 2019
Recognizing the Scale of the Problem
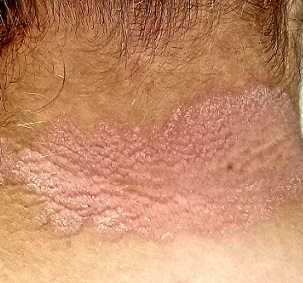
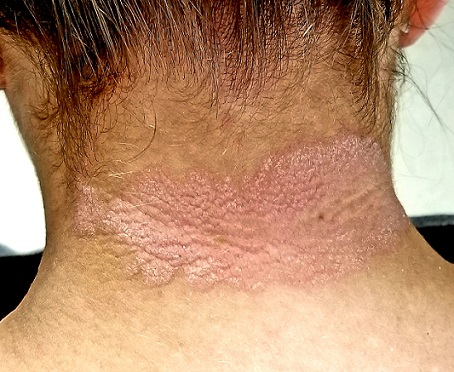
For more than 2 years, this 36-year-old woman has had a slightly itchy rash that waxes and wanes on her posterior neck. She has consulted several primary care providers and received multiple diagnoses, the most consistent of which has been fungal infection. However, despite use of a variety of antifungal creams (nystatin, clotrimazole, and combination clotrimazole/betamethasone), a 1-month course of oral terbinafine, and OTC tolnaftate, no improvement has occurred.
The patient asserts that she is otherwise in good health, with no joint pain or fever and no history of recent health crises. Family history is free of dermatologic complaints except for psoriasis in her father.
EXAMINATION
A pink plaque with white, fairly adherent scale covers most of the patient’s posterior neck/upper midline back. When a 3-mm section of scaling is peeled away, 2 tiny dots of pinpoint bleeding are immediately noted.
The rest of her scalp is free of any such changes, as are her elbows and knees. But a similar rash is seen in the upper intergluteal area, and 3 of 10 fingernails are mildly pitted.
What’s the diagnosis?
DISCUSSION
Psoriasis vulgaris (common psoriasis) affects around 3% of the white population in this country. That incidence almost doubles in northern Europe and Scandinavia.
Psoriasis is so common that you should expect to see it regularly; the important question is not “Will you see it?” but rather “Will you know it when you see it?” Sometimes the various clinical elements of psoriasis must be sought, and those dots connected, as this case demonstrates effectively.
For one thing, the nape of the neck is commonly affected, especially in women. It is pure speculation, but one imagines that the heat and sweat associated with longer hair might contribute to this predilection.
The pink color, whitish scale, and pinpoint bleeding (termed the Auspitz sign) all corroborate the diagnosis, as does the positive family history and nail pitting. The intergluteal involvement was the icing on the cake; this is seen in only 2 common conditions: psoriasis and seborrhea.
The lesson? Even though psoriasis is supposed to appear on elbows, knees, and other extensor surfaces, sometimes it breaks the rules. The posterior neck was the primary area of involvement in this case, but sometimes psoriasis is completely confined to the scalp or the palms. And, of course, there are different types of psoriasis, some of which bear scant resemblance to psoriasis vulgaris. That’s where biopsies and/or referrals prove to be useful.
It is true that this patient’s rash could have had a fungal origin. When in doubt, however, a punch or shave biopsy would most likely settle the matter, since the histologic picture is usually pathognomic.
TAKE-HOME LEARNING POINTS
- Psoriasis is often be easy to diagnose—but just as often, it takes a bit of detective work.
- This “investigation” consists of looking for and asking about findings that could corroborate the diagnosis.
- The morphology of the neck lesion, as well as the Auspitz sign, nail pitting, intergluteal involvement, and family history in this case all served quite well to establish the diagnosis of psoriasis.
- It is helpful to remember how utterly common psoriasis is, affecting around 10,000,000 Americans.
For more than 2 years, this 36-year-old woman has had a slightly itchy rash that waxes and wanes on her posterior neck. She has consulted several primary care providers and received multiple diagnoses, the most consistent of which has been fungal infection. However, despite use of a variety of antifungal creams (nystatin, clotrimazole, and combination clotrimazole/betamethasone), a 1-month course of oral terbinafine, and OTC tolnaftate, no improvement has occurred.
The patient asserts that she is otherwise in good health, with no joint pain or fever and no history of recent health crises. Family history is free of dermatologic complaints except for psoriasis in her father.

EXAMINATION
A pink plaque with white, fairly adherent scale covers most of the patient’s posterior neck/upper midline back. When a 3-mm section of scaling is peeled away, 2 tiny dots of pinpoint bleeding are immediately noted.
The rest of her scalp is free of any such changes, as are her elbows and knees. But a similar rash is seen in the upper intergluteal area, and 3 of 10 fingernails are mildly pitted.
What’s the diagnosis?
DISCUSSION
Psoriasis vulgaris (common psoriasis) affects around 3% of the white population in this country. That incidence almost doubles in northern Europe and Scandinavia.
Psoriasis is so common that you should expect to see it regularly; the important question is not “Will you see it?” but rather “Will you know it when you see it?” Sometimes the various clinical elements of psoriasis must be sought, and those dots connected, as this case demonstrates effectively.
For one thing, the nape of the neck is commonly affected, especially in women. It is pure speculation, but one imagines that the heat and sweat associated with longer hair might contribute to this predilection.
The pink color, whitish scale, and pinpoint bleeding (termed the Auspitz sign) all corroborate the diagnosis, as does the positive family history and nail pitting. The intergluteal involvement was the icing on the cake; this is seen in only 2 common conditions: psoriasis and seborrhea.
The lesson? Even though psoriasis is supposed to appear on elbows, knees, and other extensor surfaces, sometimes it breaks the rules. The posterior neck was the primary area of involvement in this case, but sometimes psoriasis is completely confined to the scalp or the palms. And, of course, there are different types of psoriasis, some of which bear scant resemblance to psoriasis vulgaris. That’s where biopsies and/or referrals prove to be useful.
It is true that this patient’s rash could have had a fungal origin. When in doubt, however, a punch or shave biopsy would most likely settle the matter, since the histologic picture is usually pathognomic.
TAKE-HOME LEARNING POINTS
- Psoriasis is often be easy to diagnose—but just as often, it takes a bit of detective work.
- This “investigation” consists of looking for and asking about findings that could corroborate the diagnosis.
- The morphology of the neck lesion, as well as the Auspitz sign, nail pitting, intergluteal involvement, and family history in this case all served quite well to establish the diagnosis of psoriasis.
- It is helpful to remember how utterly common psoriasis is, affecting around 10,000,000 Americans.
For more than 2 years, this 36-year-old woman has had a slightly itchy rash that waxes and wanes on her posterior neck. She has consulted several primary care providers and received multiple diagnoses, the most consistent of which has been fungal infection. However, despite use of a variety of antifungal creams (nystatin, clotrimazole, and combination clotrimazole/betamethasone), a 1-month course of oral terbinafine, and OTC tolnaftate, no improvement has occurred.
The patient asserts that she is otherwise in good health, with no joint pain or fever and no history of recent health crises. Family history is free of dermatologic complaints except for psoriasis in her father.

EXAMINATION
A pink plaque with white, fairly adherent scale covers most of the patient’s posterior neck/upper midline back. When a 3-mm section of scaling is peeled away, 2 tiny dots of pinpoint bleeding are immediately noted.
The rest of her scalp is free of any such changes, as are her elbows and knees. But a similar rash is seen in the upper intergluteal area, and 3 of 10 fingernails are mildly pitted.
What’s the diagnosis?
DISCUSSION
Psoriasis vulgaris (common psoriasis) affects around 3% of the white population in this country. That incidence almost doubles in northern Europe and Scandinavia.
Psoriasis is so common that you should expect to see it regularly; the important question is not “Will you see it?” but rather “Will you know it when you see it?” Sometimes the various clinical elements of psoriasis must be sought, and those dots connected, as this case demonstrates effectively.
For one thing, the nape of the neck is commonly affected, especially in women. It is pure speculation, but one imagines that the heat and sweat associated with longer hair might contribute to this predilection.
The pink color, whitish scale, and pinpoint bleeding (termed the Auspitz sign) all corroborate the diagnosis, as does the positive family history and nail pitting. The intergluteal involvement was the icing on the cake; this is seen in only 2 common conditions: psoriasis and seborrhea.
The lesson? Even though psoriasis is supposed to appear on elbows, knees, and other extensor surfaces, sometimes it breaks the rules. The posterior neck was the primary area of involvement in this case, but sometimes psoriasis is completely confined to the scalp or the palms. And, of course, there are different types of psoriasis, some of which bear scant resemblance to psoriasis vulgaris. That’s where biopsies and/or referrals prove to be useful.
It is true that this patient’s rash could have had a fungal origin. When in doubt, however, a punch or shave biopsy would most likely settle the matter, since the histologic picture is usually pathognomic.
TAKE-HOME LEARNING POINTS
- Psoriasis is often be easy to diagnose—but just as often, it takes a bit of detective work.
- This “investigation” consists of looking for and asking about findings that could corroborate the diagnosis.
- The morphology of the neck lesion, as well as the Auspitz sign, nail pitting, intergluteal involvement, and family history in this case all served quite well to establish the diagnosis of psoriasis.
- It is helpful to remember how utterly common psoriasis is, affecting around 10,000,000 Americans.
Cellulitis pearls
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
Violaceous patches on baby’s foot/leg

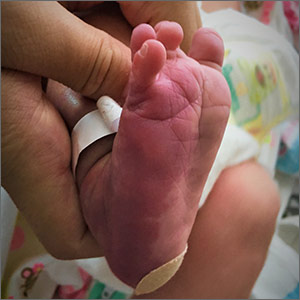
The presence of the large red to purple, well-demarcated patches with a lateral predilection led the FP to diagnose a port-wine-stain.1,2
Port-wine-stains are a type of capillary malformation that fall under the over-arching category of “simple vascular malformations.”3 Occurring in approximately 3/1000 live births, port-wine-stains have no gender predilection and can occur anywhere on the body, however, 80% of cases are associated with the head and neck.1,4 Lesions tend to be present at birth and grow in proportion with the child.1-4 While port-wine-stains may lighten during the infant’s first year of life, they tend to darken and become more nodular with time.1,3-5 Darkening of lesions is thought to be due to a lack of neural input to the capillaries, leading to poor vascular tone and dilation.5
Port-wine-stains are often isolated and benign, but their presence may indicate an underlying syndrome. Two of the more common syndromes associated with port-wine-stains include Sturge-Webber syndrome and Klippel-Trenaunay syndrome.1,4
Sturge-Webber syndrome is characterized by a port-wine-stain in the distribution of the first trigeminal division (V1), with possible involvement of the second or third trigeminal divisions (V2 and V3).1,4 Central nervous system abnormalities are also characteristic of Sturge-Webber Syndrome and can include cerebral atrophy, leptomeningeal angiomatosis, and cortical calcifications that can cause seizures, mental retardation, and hemiparesis.1,2,4
Ophthalmologic complications of Sturge-Webber syndrome can include glaucoma, and are seen in 10% to 30% of patients with a port-wine-stain in the periocular region and in 30% to 70% of patients with leptomeningeal involvement.2 A larger facial distribution of a port-wine-stain correlates to a stronger association with Sturge-Webber syndrome.2
Klippel-Trenaunay syndrome is characterized by port-wine-stains on the lower extremities with limb hypertrophy and length discrepancy, varicose veins, lymphedema, and phleboliths.1,4 Diagnosis is typically clinical and based on physical exam findings. However, an elevated d-dimer, magnetic resonance imaging (MRI), or ultrasound may aid in confirmation. The MRI or ultrasound may reveal tissue hypertrophy and the associated vascular malformations.6
The differential diagnosis for a port-wine stain includes nevus simplex, another type of capillary malformation. Nevus simplex is the most common capillary malformation, occurring in up to 82% of newborns.2 Depending on the location, nevus simplex is also referred to as a “stork bite” (lesion on nape of neck) or “angel’s kiss” (lesion on forehead).2 Nevus simplex is distinguished from a port-wine-stain by a more central location, indistinct borders, and a pale pink to red coloring.2,3 Nevus simplex lesions tend to fade as the child grows, while port-wine-stains tend to darken.2,3
Port-wine-stains also can be confused with infantile or congenital hemangiomas, which were considered in this case. Congenital hemangiomas are present at birth, while infantile hemangiomas appear within the first few weeks of life.1,2 Superficial hemangiomas can be red and macular, and often have well-defined borders, which makes distinction from port-wine-stains difficult at times.1 Hemangiomas will typically go through proliferations and involution stages making them dynamic lesions, whereas port-wine-stains grow in proportion to the child.1,2
Pulsed-dye laser (PDL) treatments are the gold standard for treatment of port-wine-stains.1,4 PDL selectively targets the vascular chromophore, which minimizes the appearance of the vascular stain but can’t completely eradicate it.1,4 Treatment is generally initiated after 6 months of life.1 In this case, the patient was referred to Dermatology for a discussion of the benefits of PDL therapy.
1. Slaughter KA, Chen T, Williams E. Vascular lesions. Facial Plast Surg Clin North Am. 2016;24:559-571.
2. Rozas-Muñoz E, Frieden IJ, Roé E1, et al. Vascular stains: proposal for a clinical classification to improve diagnosis and management. Pediatr Dermatol. 2016;33:570-584.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203-e214.
4. Lam SM, Williams EF III. Practical considerations in the treatment of capillary vascular malformations, or port wine stains. Facial Plast Surg. 2004;20:71-76.
5. Cordoro KM, Speetzen LS, Doerper MA, et al. Physiologic changes in vascular birthmarks during early infancy: mechanisms and clinical implications. J Am Acad Dermatol. 2009;60:669-675.
6. Wang, SK, Drucker NA, Gupta AK, et al. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5:587-595.

The presence of the large red to purple, well-demarcated patches with a lateral predilection led the FP to diagnose a port-wine-stain.1,2
Port-wine-stains are a type of capillary malformation that fall under the over-arching category of “simple vascular malformations.”3 Occurring in approximately 3/1000 live births, port-wine-stains have no gender predilection and can occur anywhere on the body, however, 80% of cases are associated with the head and neck.1,4 Lesions tend to be present at birth and grow in proportion with the child.1-4 While port-wine-stains may lighten during the infant’s first year of life, they tend to darken and become more nodular with time.1,3-5 Darkening of lesions is thought to be due to a lack of neural input to the capillaries, leading to poor vascular tone and dilation.5
Port-wine-stains are often isolated and benign, but their presence may indicate an underlying syndrome. Two of the more common syndromes associated with port-wine-stains include Sturge-Webber syndrome and Klippel-Trenaunay syndrome.1,4
Sturge-Webber syndrome is characterized by a port-wine-stain in the distribution of the first trigeminal division (V1), with possible involvement of the second or third trigeminal divisions (V2 and V3).1,4 Central nervous system abnormalities are also characteristic of Sturge-Webber Syndrome and can include cerebral atrophy, leptomeningeal angiomatosis, and cortical calcifications that can cause seizures, mental retardation, and hemiparesis.1,2,4
Ophthalmologic complications of Sturge-Webber syndrome can include glaucoma, and are seen in 10% to 30% of patients with a port-wine-stain in the periocular region and in 30% to 70% of patients with leptomeningeal involvement.2 A larger facial distribution of a port-wine-stain correlates to a stronger association with Sturge-Webber syndrome.2
Klippel-Trenaunay syndrome is characterized by port-wine-stains on the lower extremities with limb hypertrophy and length discrepancy, varicose veins, lymphedema, and phleboliths.1,4 Diagnosis is typically clinical and based on physical exam findings. However, an elevated d-dimer, magnetic resonance imaging (MRI), or ultrasound may aid in confirmation. The MRI or ultrasound may reveal tissue hypertrophy and the associated vascular malformations.6
The differential diagnosis for a port-wine stain includes nevus simplex, another type of capillary malformation. Nevus simplex is the most common capillary malformation, occurring in up to 82% of newborns.2 Depending on the location, nevus simplex is also referred to as a “stork bite” (lesion on nape of neck) or “angel’s kiss” (lesion on forehead).2 Nevus simplex is distinguished from a port-wine-stain by a more central location, indistinct borders, and a pale pink to red coloring.2,3 Nevus simplex lesions tend to fade as the child grows, while port-wine-stains tend to darken.2,3
Port-wine-stains also can be confused with infantile or congenital hemangiomas, which were considered in this case. Congenital hemangiomas are present at birth, while infantile hemangiomas appear within the first few weeks of life.1,2 Superficial hemangiomas can be red and macular, and often have well-defined borders, which makes distinction from port-wine-stains difficult at times.1 Hemangiomas will typically go through proliferations and involution stages making them dynamic lesions, whereas port-wine-stains grow in proportion to the child.1,2
Pulsed-dye laser (PDL) treatments are the gold standard for treatment of port-wine-stains.1,4 PDL selectively targets the vascular chromophore, which minimizes the appearance of the vascular stain but can’t completely eradicate it.1,4 Treatment is generally initiated after 6 months of life.1 In this case, the patient was referred to Dermatology for a discussion of the benefits of PDL therapy.

The presence of the large red to purple, well-demarcated patches with a lateral predilection led the FP to diagnose a port-wine-stain.1,2
Port-wine-stains are a type of capillary malformation that fall under the over-arching category of “simple vascular malformations.”3 Occurring in approximately 3/1000 live births, port-wine-stains have no gender predilection and can occur anywhere on the body, however, 80% of cases are associated with the head and neck.1,4 Lesions tend to be present at birth and grow in proportion with the child.1-4 While port-wine-stains may lighten during the infant’s first year of life, they tend to darken and become more nodular with time.1,3-5 Darkening of lesions is thought to be due to a lack of neural input to the capillaries, leading to poor vascular tone and dilation.5
Port-wine-stains are often isolated and benign, but their presence may indicate an underlying syndrome. Two of the more common syndromes associated with port-wine-stains include Sturge-Webber syndrome and Klippel-Trenaunay syndrome.1,4
Sturge-Webber syndrome is characterized by a port-wine-stain in the distribution of the first trigeminal division (V1), with possible involvement of the second or third trigeminal divisions (V2 and V3).1,4 Central nervous system abnormalities are also characteristic of Sturge-Webber Syndrome and can include cerebral atrophy, leptomeningeal angiomatosis, and cortical calcifications that can cause seizures, mental retardation, and hemiparesis.1,2,4
Ophthalmologic complications of Sturge-Webber syndrome can include glaucoma, and are seen in 10% to 30% of patients with a port-wine-stain in the periocular region and in 30% to 70% of patients with leptomeningeal involvement.2 A larger facial distribution of a port-wine-stain correlates to a stronger association with Sturge-Webber syndrome.2
Klippel-Trenaunay syndrome is characterized by port-wine-stains on the lower extremities with limb hypertrophy and length discrepancy, varicose veins, lymphedema, and phleboliths.1,4 Diagnosis is typically clinical and based on physical exam findings. However, an elevated d-dimer, magnetic resonance imaging (MRI), or ultrasound may aid in confirmation. The MRI or ultrasound may reveal tissue hypertrophy and the associated vascular malformations.6
The differential diagnosis for a port-wine stain includes nevus simplex, another type of capillary malformation. Nevus simplex is the most common capillary malformation, occurring in up to 82% of newborns.2 Depending on the location, nevus simplex is also referred to as a “stork bite” (lesion on nape of neck) or “angel’s kiss” (lesion on forehead).2 Nevus simplex is distinguished from a port-wine-stain by a more central location, indistinct borders, and a pale pink to red coloring.2,3 Nevus simplex lesions tend to fade as the child grows, while port-wine-stains tend to darken.2,3
Port-wine-stains also can be confused with infantile or congenital hemangiomas, which were considered in this case. Congenital hemangiomas are present at birth, while infantile hemangiomas appear within the first few weeks of life.1,2 Superficial hemangiomas can be red and macular, and often have well-defined borders, which makes distinction from port-wine-stains difficult at times.1 Hemangiomas will typically go through proliferations and involution stages making them dynamic lesions, whereas port-wine-stains grow in proportion to the child.1,2
Pulsed-dye laser (PDL) treatments are the gold standard for treatment of port-wine-stains.1,4 PDL selectively targets the vascular chromophore, which minimizes the appearance of the vascular stain but can’t completely eradicate it.1,4 Treatment is generally initiated after 6 months of life.1 In this case, the patient was referred to Dermatology for a discussion of the benefits of PDL therapy.
1. Slaughter KA, Chen T, Williams E. Vascular lesions. Facial Plast Surg Clin North Am. 2016;24:559-571.
2. Rozas-Muñoz E, Frieden IJ, Roé E1, et al. Vascular stains: proposal for a clinical classification to improve diagnosis and management. Pediatr Dermatol. 2016;33:570-584.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203-e214.
4. Lam SM, Williams EF III. Practical considerations in the treatment of capillary vascular malformations, or port wine stains. Facial Plast Surg. 2004;20:71-76.
5. Cordoro KM, Speetzen LS, Doerper MA, et al. Physiologic changes in vascular birthmarks during early infancy: mechanisms and clinical implications. J Am Acad Dermatol. 2009;60:669-675.
6. Wang, SK, Drucker NA, Gupta AK, et al. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5:587-595.
1. Slaughter KA, Chen T, Williams E. Vascular lesions. Facial Plast Surg Clin North Am. 2016;24:559-571.
2. Rozas-Muñoz E, Frieden IJ, Roé E1, et al. Vascular stains: proposal for a clinical classification to improve diagnosis and management. Pediatr Dermatol. 2016;33:570-584.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203-e214.
4. Lam SM, Williams EF III. Practical considerations in the treatment of capillary vascular malformations, or port wine stains. Facial Plast Surg. 2004;20:71-76.
5. Cordoro KM, Speetzen LS, Doerper MA, et al. Physiologic changes in vascular birthmarks during early infancy: mechanisms and clinical implications. J Am Acad Dermatol. 2009;60:669-675.
6. Wang, SK, Drucker NA, Gupta AK, et al. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5:587-595.
Herpes zoster risk increased with some psoriasis, psoriatic arthritis treatments
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Don’t miss baby scabies
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
How to manage infected eczema in children
WAIKOLOA, HAWAII – , according to Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at the University of California, San Diego.
It’s the breach in skin integrity that gives skin flora – most commonly Staphylococcus or Streptococcus – an opening for infection.
Dr. Eichenfield shared his treatment approach in a pearl-filled interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Many times, anti-inflammatories are going to be the effective therapy,” whether topical steroids or systemic therapies. But with a secondary infection, systemic antibiotics are in order, too, but topical ones aren’t much use, he said.
Dr. Eichenfield gets a lot of questions about steroid-sparing options for very young children, since topical calcineurin inhibitors and the like aren’t approved in children under 2 years old, and insurance coverage can be a problem. He noted, however, that various guidelines support their use even in the very young, “so I tell my physicians to fight for them ... If you need a steroid-sparing agent, push for it, because it may be the right thing for your patient,” he said.
He’s finding in his area that infected eczema often is resistant to clindamycin, which has been used heavily because of concerns about methicillin-resistant Staphylococcus aureus (MRSA). But it often will “respond to what we considered to be wimpier antibiotics in the past, such as cephalosporin ... So I’ll use cephalosporin or an extended-spectrum penicillin as my first-line agent, and then I’ll culture, depending on history, to see if I have to be concerned about methicillin resistance,” he said.
Be on the lookout for cutaneous herpes and show parents pictures of what it looks like so they recognize it and know to come in right away. It is a dangerous infection, but can be shut down quickly with oral acyclovir and similar agents, Dr. Eichenfield added.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – , according to Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at the University of California, San Diego.
It’s the breach in skin integrity that gives skin flora – most commonly Staphylococcus or Streptococcus – an opening for infection.
Dr. Eichenfield shared his treatment approach in a pearl-filled interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Many times, anti-inflammatories are going to be the effective therapy,” whether topical steroids or systemic therapies. But with a secondary infection, systemic antibiotics are in order, too, but topical ones aren’t much use, he said.
Dr. Eichenfield gets a lot of questions about steroid-sparing options for very young children, since topical calcineurin inhibitors and the like aren’t approved in children under 2 years old, and insurance coverage can be a problem. He noted, however, that various guidelines support their use even in the very young, “so I tell my physicians to fight for them ... If you need a steroid-sparing agent, push for it, because it may be the right thing for your patient,” he said.
He’s finding in his area that infected eczema often is resistant to clindamycin, which has been used heavily because of concerns about methicillin-resistant Staphylococcus aureus (MRSA). But it often will “respond to what we considered to be wimpier antibiotics in the past, such as cephalosporin ... So I’ll use cephalosporin or an extended-spectrum penicillin as my first-line agent, and then I’ll culture, depending on history, to see if I have to be concerned about methicillin resistance,” he said.
Be on the lookout for cutaneous herpes and show parents pictures of what it looks like so they recognize it and know to come in right away. It is a dangerous infection, but can be shut down quickly with oral acyclovir and similar agents, Dr. Eichenfield added.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – , according to Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at the University of California, San Diego.
It’s the breach in skin integrity that gives skin flora – most commonly Staphylococcus or Streptococcus – an opening for infection.
Dr. Eichenfield shared his treatment approach in a pearl-filled interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Many times, anti-inflammatories are going to be the effective therapy,” whether topical steroids or systemic therapies. But with a secondary infection, systemic antibiotics are in order, too, but topical ones aren’t much use, he said.
Dr. Eichenfield gets a lot of questions about steroid-sparing options for very young children, since topical calcineurin inhibitors and the like aren’t approved in children under 2 years old, and insurance coverage can be a problem. He noted, however, that various guidelines support their use even in the very young, “so I tell my physicians to fight for them ... If you need a steroid-sparing agent, push for it, because it may be the right thing for your patient,” he said.
He’s finding in his area that infected eczema often is resistant to clindamycin, which has been used heavily because of concerns about methicillin-resistant Staphylococcus aureus (MRSA). But it often will “respond to what we considered to be wimpier antibiotics in the past, such as cephalosporin ... So I’ll use cephalosporin or an extended-spectrum penicillin as my first-line agent, and then I’ll culture, depending on history, to see if I have to be concerned about methicillin resistance,” he said.
Be on the lookout for cutaneous herpes and show parents pictures of what it looks like so they recognize it and know to come in right away. It is a dangerous infection, but can be shut down quickly with oral acyclovir and similar agents, Dr. Eichenfield added.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Acne take home messages from the AAD annual meeting
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
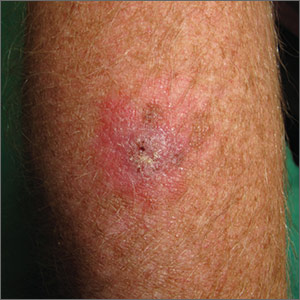
Sore on arm
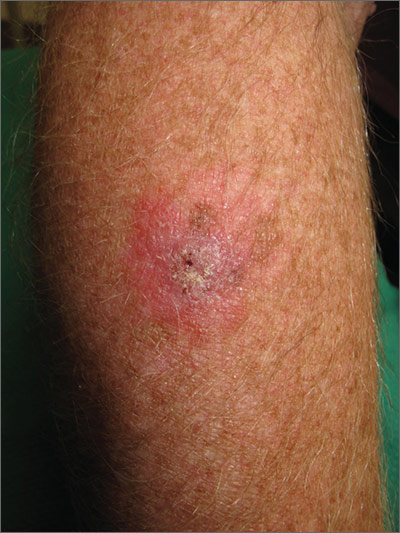
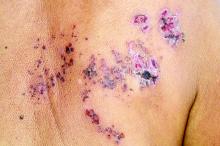
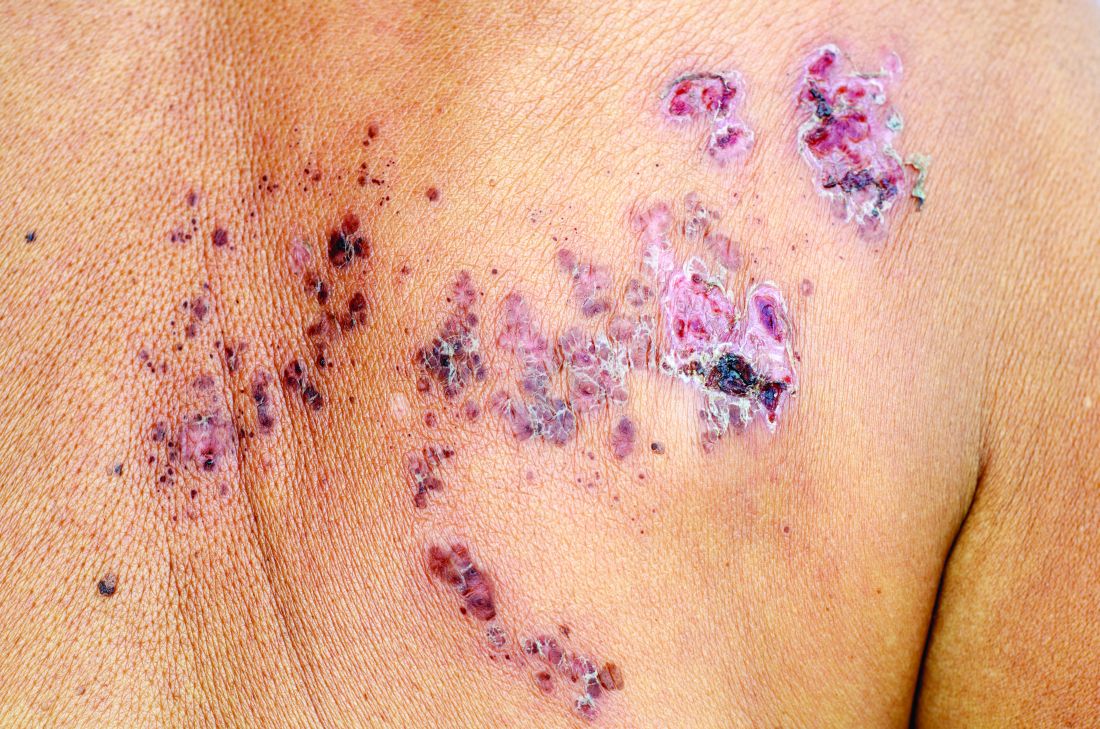
The FP suspected that this was a skin cancer with ulceration rather than a dermatitis because of the ulceration and polymorphous vessels on dermoscopy. The differential diagnosis included squamous cell carcinoma, amelanotic melanoma, and basal cell carcinoma.
The FP explained to the patient that a biopsy would be needed and suggested a broad shave biopsy because it would provide adequate tissue to the pathologist. The physician performed a broad shave biopsy with a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The biopsy report came back as amelanotic melanoma with a depth of 1.5 mm.
While most melanomas have visible pigment, some melanomas will present without pigmentation. Based on a Breslow depth of 1.5 mm, the patient was sent to Surgical Oncology for a wide excision with 1 cm margins and sentinel lymph node biopsy. Fortunately, the sentinel lymph node biopsy did not show any metastasis. The FP advised the patient that he would require regular skin surveillance and would need to take skin care precautions when exposed to the sun.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this was a skin cancer with ulceration rather than a dermatitis because of the ulceration and polymorphous vessels on dermoscopy. The differential diagnosis included squamous cell carcinoma, amelanotic melanoma, and basal cell carcinoma.
The FP explained to the patient that a biopsy would be needed and suggested a broad shave biopsy because it would provide adequate tissue to the pathologist. The physician performed a broad shave biopsy with a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The biopsy report came back as amelanotic melanoma with a depth of 1.5 mm.
While most melanomas have visible pigment, some melanomas will present without pigmentation. Based on a Breslow depth of 1.5 mm, the patient was sent to Surgical Oncology for a wide excision with 1 cm margins and sentinel lymph node biopsy. Fortunately, the sentinel lymph node biopsy did not show any metastasis. The FP advised the patient that he would require regular skin surveillance and would need to take skin care precautions when exposed to the sun.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this was a skin cancer with ulceration rather than a dermatitis because of the ulceration and polymorphous vessels on dermoscopy. The differential diagnosis included squamous cell carcinoma, amelanotic melanoma, and basal cell carcinoma.
The FP explained to the patient that a biopsy would be needed and suggested a broad shave biopsy because it would provide adequate tissue to the pathologist. The physician performed a broad shave biopsy with a DermaBlade. (See the Watch & Learn video on “Shave biopsy.”) The biopsy report came back as amelanotic melanoma with a depth of 1.5 mm.
While most melanomas have visible pigment, some melanomas will present without pigmentation. Based on a Breslow depth of 1.5 mm, the patient was sent to Surgical Oncology for a wide excision with 1 cm margins and sentinel lymph node biopsy. Fortunately, the sentinel lymph node biopsy did not show any metastasis. The FP advised the patient that he would require regular skin surveillance and would need to take skin care precautions when exposed to the sun.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
A 13-month-old, healthy black male presented with a 6-month history of dry, scaly skin on the body
Ichthyosis vulgaris
Ichthyoses describe a group of disorders of cornification in which the epidermis differentiates abnormally, leading to generalized scaling of the skin. Ichthyosis is derived from the Greek word for fish, “ichthys.” Ichthyosis vulgaris is the most common of these conditions and often presents in early childhood during the first year of life. It is inherited in an autosomal-dominant pattern. Skin is dry and scaly over the entire body, although the antecubital and popliteal fossa may be uninvolved. The scalp may be involved as well. Atopy and keratosis pilaris may be associated. By adulthood, symptoms tend to abate.
X-linked ichthyosis is an X-linked recessive trait, in which males are affected and mothers are carriers. The condition is caused by a deficiency of steroid sulfatase. This deficiency can result in low levels of estrogen during pregnancy in the mother of an affected fetus, hampering labor progression, and often requiring C-section. Children usually present before 3 months of age. Scales are large and dark. The antecubital and popliteal fossa are usually spared. The neck almost always is involved, coining the term “dirty neck disease.” Corneal opacities are present upon ophthalmologic examination. There is an increased risk of cryptorchidism and testicular cancer. Skin symptoms tend to worsen into adulthood.
Lamellar ichthyosis generally occurs at birth with a striking collodion-type membrane covering the body and underlying erythroderma, which then desquamates. Ectropion is usually present as well. Resulting scales are large and gray-brown. Lamellar ichthyosis is inherited in an autosomal recessive pattern. Mutations in transglutaminase 1 (TGM1), ALOXE3, ALOX12B, and ABCA12 genes have been implicated in this disorder.
Acquired ichthyosis can appear clinically similar to ichthyosis vulgaris. It occurs in patients with systemic diseases such as Hodgkin disease, non-Hodgkin lymphoma, mycosis fungoides, multiple myeloma, hypothyroidism, sarcoidosis, AIDS, and others.
improve hyperkeratosis. Urea-containing products can be helpful. Salicylic acid may be used but merit caution in children because of salicylate toxicity. Oral and topical retinoid can be helpful in lamellar ichthyosis.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Ichthyosis vulgaris
Ichthyoses describe a group of disorders of cornification in which the epidermis differentiates abnormally, leading to generalized scaling of the skin. Ichthyosis is derived from the Greek word for fish, “ichthys.” Ichthyosis vulgaris is the most common of these conditions and often presents in early childhood during the first year of life. It is inherited in an autosomal-dominant pattern. Skin is dry and scaly over the entire body, although the antecubital and popliteal fossa may be uninvolved. The scalp may be involved as well. Atopy and keratosis pilaris may be associated. By adulthood, symptoms tend to abate.
X-linked ichthyosis is an X-linked recessive trait, in which males are affected and mothers are carriers. The condition is caused by a deficiency of steroid sulfatase. This deficiency can result in low levels of estrogen during pregnancy in the mother of an affected fetus, hampering labor progression, and often requiring C-section. Children usually present before 3 months of age. Scales are large and dark. The antecubital and popliteal fossa are usually spared. The neck almost always is involved, coining the term “dirty neck disease.” Corneal opacities are present upon ophthalmologic examination. There is an increased risk of cryptorchidism and testicular cancer. Skin symptoms tend to worsen into adulthood.
Lamellar ichthyosis generally occurs at birth with a striking collodion-type membrane covering the body and underlying erythroderma, which then desquamates. Ectropion is usually present as well. Resulting scales are large and gray-brown. Lamellar ichthyosis is inherited in an autosomal recessive pattern. Mutations in transglutaminase 1 (TGM1), ALOXE3, ALOX12B, and ABCA12 genes have been implicated in this disorder.
Acquired ichthyosis can appear clinically similar to ichthyosis vulgaris. It occurs in patients with systemic diseases such as Hodgkin disease, non-Hodgkin lymphoma, mycosis fungoides, multiple myeloma, hypothyroidism, sarcoidosis, AIDS, and others.
improve hyperkeratosis. Urea-containing products can be helpful. Salicylic acid may be used but merit caution in children because of salicylate toxicity. Oral and topical retinoid can be helpful in lamellar ichthyosis.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Ichthyosis vulgaris
Ichthyoses describe a group of disorders of cornification in which the epidermis differentiates abnormally, leading to generalized scaling of the skin. Ichthyosis is derived from the Greek word for fish, “ichthys.” Ichthyosis vulgaris is the most common of these conditions and often presents in early childhood during the first year of life. It is inherited in an autosomal-dominant pattern. Skin is dry and scaly over the entire body, although the antecubital and popliteal fossa may be uninvolved. The scalp may be involved as well. Atopy and keratosis pilaris may be associated. By adulthood, symptoms tend to abate.
X-linked ichthyosis is an X-linked recessive trait, in which males are affected and mothers are carriers. The condition is caused by a deficiency of steroid sulfatase. This deficiency can result in low levels of estrogen during pregnancy in the mother of an affected fetus, hampering labor progression, and often requiring C-section. Children usually present before 3 months of age. Scales are large and dark. The antecubital and popliteal fossa are usually spared. The neck almost always is involved, coining the term “dirty neck disease.” Corneal opacities are present upon ophthalmologic examination. There is an increased risk of cryptorchidism and testicular cancer. Skin symptoms tend to worsen into adulthood.
Lamellar ichthyosis generally occurs at birth with a striking collodion-type membrane covering the body and underlying erythroderma, which then desquamates. Ectropion is usually present as well. Resulting scales are large and gray-brown. Lamellar ichthyosis is inherited in an autosomal recessive pattern. Mutations in transglutaminase 1 (TGM1), ALOXE3, ALOX12B, and ABCA12 genes have been implicated in this disorder.
Acquired ichthyosis can appear clinically similar to ichthyosis vulgaris. It occurs in patients with systemic diseases such as Hodgkin disease, non-Hodgkin lymphoma, mycosis fungoides, multiple myeloma, hypothyroidism, sarcoidosis, AIDS, and others.
improve hyperkeratosis. Urea-containing products can be helpful. Salicylic acid may be used but merit caution in children because of salicylate toxicity. Oral and topical retinoid can be helpful in lamellar ichthyosis.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
A 13-month-old, healthy black male presented with a 6-month history of dry, scaly skin on the body, including scalp and extremities. His neck was unaffected. His mother reports an uneventful pregnancy and natural childbirth. He had been prescribed triamcinolone in the past for eczema.