User login
Paradoxical Eczema Risk Low With Biologic Psoriasis Treatments
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
FROM JAMA DERMATOLOGY
How to Reduce Cardiovascular Morbidity and Mortality in Psoriasis and PsA
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.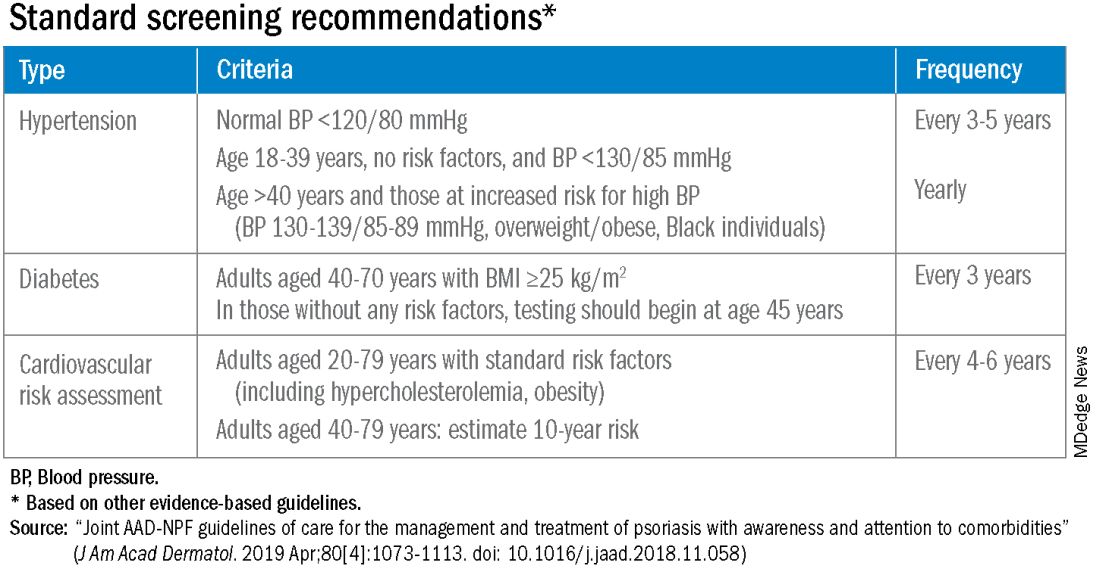
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Roflumilast foam gets nod as new option for seborrheic dermatitis
The in a press release.
The 0.3% foam, marketed as Zoryve, applied once-daily, is indicated for patients aged 9 years and older with seborrheic dermatitis, and can be used anywhere on the body, including areas with hair, with no limits on duration of use, according to the company, Arcutis. A 0.3% cream formulation of roflumilast was previously approved by the FDA for the topical treatment of plaque psoriasis in patients aged 6 years and older.
Approval was based on data from the phase 3 STRATUM trial and an accompanying phase 2 study known as Trial 203. These studies included a total of 683 adults and youth aged 9 years and older with seborrheic dermatitis. Participants were randomized to roflumilast or a placebo.
At 8 weeks, 79.5 % of patients on roflumilast met the primary efficacy endpoint of Investigator Global Assessment (IGA) scores of 0 or 1 (clear or almost clear) compared with 58.0% of patients on placebo (P < .001); the results were similar in the phase 2 Trial 203 (73.1% vs. 40.8%, respectively; P < .001). Overall, more than 50% of the patients on roflumilast achieved a clear score.
Patients in the roflumilast group also showed significant improvement in all secondary endpoints, including itching, scaling, and erythema, according to the company.
In the STRATUM study, 62.8% of roflumilast-treated patients and 40.6% of placebo patients achieved a 4-point or more reduction in itch based on the Worst Itch Numerical Rating Score (P =.0001), and 28% of roflumilast-treated patients reported significant itch improvement within the first 48 hours of use, compared with 13% of placebo patients (P = .0024).
Over a treatment period of up to 1 year, no treatment-related severe adverse events were reported in the phase 2 and 3 studies. The incidence of treatment emergent adverse events was similar between the treatment and placebo groups, and the most common adverse events (occurring in 1% of more of patients) across both studies were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast foam is scheduled to be available by the end of January 2024, according to the company. The product is for topical use only, and contraindicated for individuals with severe liver impairment.
The in a press release.
The 0.3% foam, marketed as Zoryve, applied once-daily, is indicated for patients aged 9 years and older with seborrheic dermatitis, and can be used anywhere on the body, including areas with hair, with no limits on duration of use, according to the company, Arcutis. A 0.3% cream formulation of roflumilast was previously approved by the FDA for the topical treatment of plaque psoriasis in patients aged 6 years and older.
Approval was based on data from the phase 3 STRATUM trial and an accompanying phase 2 study known as Trial 203. These studies included a total of 683 adults and youth aged 9 years and older with seborrheic dermatitis. Participants were randomized to roflumilast or a placebo.
At 8 weeks, 79.5 % of patients on roflumilast met the primary efficacy endpoint of Investigator Global Assessment (IGA) scores of 0 or 1 (clear or almost clear) compared with 58.0% of patients on placebo (P < .001); the results were similar in the phase 2 Trial 203 (73.1% vs. 40.8%, respectively; P < .001). Overall, more than 50% of the patients on roflumilast achieved a clear score.
Patients in the roflumilast group also showed significant improvement in all secondary endpoints, including itching, scaling, and erythema, according to the company.
In the STRATUM study, 62.8% of roflumilast-treated patients and 40.6% of placebo patients achieved a 4-point or more reduction in itch based on the Worst Itch Numerical Rating Score (P =.0001), and 28% of roflumilast-treated patients reported significant itch improvement within the first 48 hours of use, compared with 13% of placebo patients (P = .0024).
Over a treatment period of up to 1 year, no treatment-related severe adverse events were reported in the phase 2 and 3 studies. The incidence of treatment emergent adverse events was similar between the treatment and placebo groups, and the most common adverse events (occurring in 1% of more of patients) across both studies were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast foam is scheduled to be available by the end of January 2024, according to the company. The product is for topical use only, and contraindicated for individuals with severe liver impairment.
The in a press release.
The 0.3% foam, marketed as Zoryve, applied once-daily, is indicated for patients aged 9 years and older with seborrheic dermatitis, and can be used anywhere on the body, including areas with hair, with no limits on duration of use, according to the company, Arcutis. A 0.3% cream formulation of roflumilast was previously approved by the FDA for the topical treatment of plaque psoriasis in patients aged 6 years and older.
Approval was based on data from the phase 3 STRATUM trial and an accompanying phase 2 study known as Trial 203. These studies included a total of 683 adults and youth aged 9 years and older with seborrheic dermatitis. Participants were randomized to roflumilast or a placebo.
At 8 weeks, 79.5 % of patients on roflumilast met the primary efficacy endpoint of Investigator Global Assessment (IGA) scores of 0 or 1 (clear or almost clear) compared with 58.0% of patients on placebo (P < .001); the results were similar in the phase 2 Trial 203 (73.1% vs. 40.8%, respectively; P < .001). Overall, more than 50% of the patients on roflumilast achieved a clear score.
Patients in the roflumilast group also showed significant improvement in all secondary endpoints, including itching, scaling, and erythema, according to the company.
In the STRATUM study, 62.8% of roflumilast-treated patients and 40.6% of placebo patients achieved a 4-point or more reduction in itch based on the Worst Itch Numerical Rating Score (P =.0001), and 28% of roflumilast-treated patients reported significant itch improvement within the first 48 hours of use, compared with 13% of placebo patients (P = .0024).
Over a treatment period of up to 1 year, no treatment-related severe adverse events were reported in the phase 2 and 3 studies. The incidence of treatment emergent adverse events was similar between the treatment and placebo groups, and the most common adverse events (occurring in 1% of more of patients) across both studies were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast foam is scheduled to be available by the end of January 2024, according to the company. The product is for topical use only, and contraindicated for individuals with severe liver impairment.
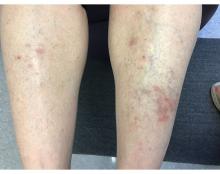
A 55-year-old female presented a with few years' history of pruritic plaques on her shins and wrists
. Lesions may have a covering of scale. HLP commonly affects middle aged men and women. Lesions are most commonly located bilaterally on the shins and ankles and can be painful or pruritic. The differential diagnosis for the condition includes lichen simplex chronicus, connective tissue disease, and other skin disorders that cause hyperkeratosis. This wide differential makes histopathological analysis a useful tool in confirming the diagnosis of HLP.
A definitive diagnosis can be made via skin biopsy. Histopathology reveals hyperkeratosis, acanthosis, and a band-like lymphocytic infiltrate in the dermis. An eosinophilic infiltrate may be present. Other common features include saw tooth rete ridges and Civatte bodies, which are apoptotic keratinocytes. The lymphocytic infiltrate may indicate an autoimmune etiology in which the body’s immune system erroneously attacks itself. However, the exact cause is not known and genetic and environmental factors may play a role.
The treatment of HLP includes symptomatic management and control of inflammation. Topical steroids can be prescribed to manage the inflammation and associated pruritus, and emollient creams and moisturizers are helpful in controlling the dryness. Oral steroids, immunosuppressant medications, or retinoids may be necessary in more severe cases. In addition, psoralen plus ultraviolet A (PUVA) light therapy has been found to be beneficial in some cases. Squamous cell carcinoma may arise in lesions.
This case and photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD; Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Arnold DL, Krishnamurthy K. Lichen Planus. [Updated 2023 Jun 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526126/
Jaime TJ et al. An Bras Dermatol. 2011 Jul-Aug;86(4 Suppl 1):S96-9.
Mirchandani S et al. Med Pharm Rep. 2020 Apr;93(2):210-2. .
Whittington CP et al. Arch Pathol Lab Med. 2023 Jun 19. doi: 10.5858/arpa.2022-0515-RA.
. Lesions may have a covering of scale. HLP commonly affects middle aged men and women. Lesions are most commonly located bilaterally on the shins and ankles and can be painful or pruritic. The differential diagnosis for the condition includes lichen simplex chronicus, connective tissue disease, and other skin disorders that cause hyperkeratosis. This wide differential makes histopathological analysis a useful tool in confirming the diagnosis of HLP.
A definitive diagnosis can be made via skin biopsy. Histopathology reveals hyperkeratosis, acanthosis, and a band-like lymphocytic infiltrate in the dermis. An eosinophilic infiltrate may be present. Other common features include saw tooth rete ridges and Civatte bodies, which are apoptotic keratinocytes. The lymphocytic infiltrate may indicate an autoimmune etiology in which the body’s immune system erroneously attacks itself. However, the exact cause is not known and genetic and environmental factors may play a role.
The treatment of HLP includes symptomatic management and control of inflammation. Topical steroids can be prescribed to manage the inflammation and associated pruritus, and emollient creams and moisturizers are helpful in controlling the dryness. Oral steroids, immunosuppressant medications, or retinoids may be necessary in more severe cases. In addition, psoralen plus ultraviolet A (PUVA) light therapy has been found to be beneficial in some cases. Squamous cell carcinoma may arise in lesions.
This case and photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD; Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Arnold DL, Krishnamurthy K. Lichen Planus. [Updated 2023 Jun 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526126/
Jaime TJ et al. An Bras Dermatol. 2011 Jul-Aug;86(4 Suppl 1):S96-9.
Mirchandani S et al. Med Pharm Rep. 2020 Apr;93(2):210-2. .
Whittington CP et al. Arch Pathol Lab Med. 2023 Jun 19. doi: 10.5858/arpa.2022-0515-RA.
. Lesions may have a covering of scale. HLP commonly affects middle aged men and women. Lesions are most commonly located bilaterally on the shins and ankles and can be painful or pruritic. The differential diagnosis for the condition includes lichen simplex chronicus, connective tissue disease, and other skin disorders that cause hyperkeratosis. This wide differential makes histopathological analysis a useful tool in confirming the diagnosis of HLP.
A definitive diagnosis can be made via skin biopsy. Histopathology reveals hyperkeratosis, acanthosis, and a band-like lymphocytic infiltrate in the dermis. An eosinophilic infiltrate may be present. Other common features include saw tooth rete ridges and Civatte bodies, which are apoptotic keratinocytes. The lymphocytic infiltrate may indicate an autoimmune etiology in which the body’s immune system erroneously attacks itself. However, the exact cause is not known and genetic and environmental factors may play a role.
The treatment of HLP includes symptomatic management and control of inflammation. Topical steroids can be prescribed to manage the inflammation and associated pruritus, and emollient creams and moisturizers are helpful in controlling the dryness. Oral steroids, immunosuppressant medications, or retinoids may be necessary in more severe cases. In addition, psoralen plus ultraviolet A (PUVA) light therapy has been found to be beneficial in some cases. Squamous cell carcinoma may arise in lesions.
This case and photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD; Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Arnold DL, Krishnamurthy K. Lichen Planus. [Updated 2023 Jun 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526126/
Jaime TJ et al. An Bras Dermatol. 2011 Jul-Aug;86(4 Suppl 1):S96-9.
Mirchandani S et al. Med Pharm Rep. 2020 Apr;93(2):210-2. .
Whittington CP et al. Arch Pathol Lab Med. 2023 Jun 19. doi: 10.5858/arpa.2022-0515-RA.
How does lebrikizumab perform across different racial and ethnic subgroups?
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
FROM RAD 2023
Pilot study educates barbers about pseudofolliculitis barbae
A .
The results were published in a research letter in JAMA Dermatology. “Educating barbers on dermatologic conditions that disproportionately affect Black males and establishing referral services between barbers and dermatologists could serve as plausible interventions,” the authors wrote.
PFB — or “razor bumps” in layman’s terms — is a chronic, inflammatory follicular disorder, which can occur in any racial group, but primarily affects Black men, noted the corresponding author of the study, Xavier Rice, MD, a dermatology resident at Washington University in Saint Louis, Missouri. PFB manifests as bumps and pustules or nodules along the beard line and are painful, he said in an interview. “They tend to leave scars once they resolve,” and impair the ability to shave, he noted.
In some communities, Black men may see their barbers more often than primary care doctors or dermatologists, “so if you equip the barbers with the knowledge to recognize the disease, make recommendations on how to prevent and to treat, and also form some allyship with barbers and dermatologists, then we can get referrals for people, especially the ones with severe disease,” he said. A lot of the barbers in the study said that “they didn’t receive much education on how to properly address it [PFB] and they had a lot of miseducation about what actually caused it,” added Dr. Rice, who was a medical student at the University of Texas Medical Branch, Galveston, when the study was conducted.
Study involved 40 barbers
For the study, Dr. Rice and his coauthors surveyed 40 barbers in the Houston, Texas, area; 39 were Black and one was Hispanic; 75% were men and 25% were women. Most (90%) said that at least 60% of their clients were Black. Between January and April 2022, the barbers received questionnaires before and after participating in a session that involved a review of a comprehensive educational brochure with information on the recognition, cause, prevention, and treatment of PFB, which they then kept for reference and to provide to clients as needed. “Common myths and nuanced home remedies from barber experience were also addressed,” the authors wrote.
No more than 2 weeks after the information session, each barber completed a posttest questionnaire.
Based on their responses to pretest questions, 39 of the 40 barbers understood that Black men were the group most impacted by PFB and that a person with severe PFB should see a physician. In the pretest survey, 12 barbers (30%) correctly recognized a photo of PFB, which increased to 39 (97.5%) in the posttest survey. In the pretest survey, two barbers (5%) identified laser hair removal as the most effective treatment for PFB, compared with 37 (92.5%) in the posttest survey.
Overall, the mean percentage of correct scores out of 20 questions was 54.8% in the pretest survey, increasing to 91% in the posttest survey (P <.001).
Limitations of the studies included heterogeneity in the survey response options that potentially could have introduced bias, the authors wrote. Another was that since there is a lack of evidence for ideal treatment strategies for PFB, there may have been some uncertainty among the correct answers for the survey that might have contributed to variability in responses. “Further research and implementation of these interventions are needed in efforts to improve health outcomes,” they added.
“Barbers can serve as allies in referral services,” Dr. Rice said in the interview. “They can be the first line for a number of diseases that are related to hair.”
Part of his role as a dermatologist, he added, includes going into a community with “boots on the ground” and talking to people who will see these patients “because access to care, presentation to big hospital systems can be challenging.”
Dr. Rice and the other study authors had no not report any financial disclosures.
A .
The results were published in a research letter in JAMA Dermatology. “Educating barbers on dermatologic conditions that disproportionately affect Black males and establishing referral services between barbers and dermatologists could serve as plausible interventions,” the authors wrote.
PFB — or “razor bumps” in layman’s terms — is a chronic, inflammatory follicular disorder, which can occur in any racial group, but primarily affects Black men, noted the corresponding author of the study, Xavier Rice, MD, a dermatology resident at Washington University in Saint Louis, Missouri. PFB manifests as bumps and pustules or nodules along the beard line and are painful, he said in an interview. “They tend to leave scars once they resolve,” and impair the ability to shave, he noted.
In some communities, Black men may see their barbers more often than primary care doctors or dermatologists, “so if you equip the barbers with the knowledge to recognize the disease, make recommendations on how to prevent and to treat, and also form some allyship with barbers and dermatologists, then we can get referrals for people, especially the ones with severe disease,” he said. A lot of the barbers in the study said that “they didn’t receive much education on how to properly address it [PFB] and they had a lot of miseducation about what actually caused it,” added Dr. Rice, who was a medical student at the University of Texas Medical Branch, Galveston, when the study was conducted.
Study involved 40 barbers
For the study, Dr. Rice and his coauthors surveyed 40 barbers in the Houston, Texas, area; 39 were Black and one was Hispanic; 75% were men and 25% were women. Most (90%) said that at least 60% of their clients were Black. Between January and April 2022, the barbers received questionnaires before and after participating in a session that involved a review of a comprehensive educational brochure with information on the recognition, cause, prevention, and treatment of PFB, which they then kept for reference and to provide to clients as needed. “Common myths and nuanced home remedies from barber experience were also addressed,” the authors wrote.
No more than 2 weeks after the information session, each barber completed a posttest questionnaire.
Based on their responses to pretest questions, 39 of the 40 barbers understood that Black men were the group most impacted by PFB and that a person with severe PFB should see a physician. In the pretest survey, 12 barbers (30%) correctly recognized a photo of PFB, which increased to 39 (97.5%) in the posttest survey. In the pretest survey, two barbers (5%) identified laser hair removal as the most effective treatment for PFB, compared with 37 (92.5%) in the posttest survey.
Overall, the mean percentage of correct scores out of 20 questions was 54.8% in the pretest survey, increasing to 91% in the posttest survey (P <.001).
Limitations of the studies included heterogeneity in the survey response options that potentially could have introduced bias, the authors wrote. Another was that since there is a lack of evidence for ideal treatment strategies for PFB, there may have been some uncertainty among the correct answers for the survey that might have contributed to variability in responses. “Further research and implementation of these interventions are needed in efforts to improve health outcomes,” they added.
“Barbers can serve as allies in referral services,” Dr. Rice said in the interview. “They can be the first line for a number of diseases that are related to hair.”
Part of his role as a dermatologist, he added, includes going into a community with “boots on the ground” and talking to people who will see these patients “because access to care, presentation to big hospital systems can be challenging.”
Dr. Rice and the other study authors had no not report any financial disclosures.
A .
The results were published in a research letter in JAMA Dermatology. “Educating barbers on dermatologic conditions that disproportionately affect Black males and establishing referral services between barbers and dermatologists could serve as plausible interventions,” the authors wrote.
PFB — or “razor bumps” in layman’s terms — is a chronic, inflammatory follicular disorder, which can occur in any racial group, but primarily affects Black men, noted the corresponding author of the study, Xavier Rice, MD, a dermatology resident at Washington University in Saint Louis, Missouri. PFB manifests as bumps and pustules or nodules along the beard line and are painful, he said in an interview. “They tend to leave scars once they resolve,” and impair the ability to shave, he noted.
In some communities, Black men may see their barbers more often than primary care doctors or dermatologists, “so if you equip the barbers with the knowledge to recognize the disease, make recommendations on how to prevent and to treat, and also form some allyship with barbers and dermatologists, then we can get referrals for people, especially the ones with severe disease,” he said. A lot of the barbers in the study said that “they didn’t receive much education on how to properly address it [PFB] and they had a lot of miseducation about what actually caused it,” added Dr. Rice, who was a medical student at the University of Texas Medical Branch, Galveston, when the study was conducted.
Study involved 40 barbers
For the study, Dr. Rice and his coauthors surveyed 40 barbers in the Houston, Texas, area; 39 were Black and one was Hispanic; 75% were men and 25% were women. Most (90%) said that at least 60% of their clients were Black. Between January and April 2022, the barbers received questionnaires before and after participating in a session that involved a review of a comprehensive educational brochure with information on the recognition, cause, prevention, and treatment of PFB, which they then kept for reference and to provide to clients as needed. “Common myths and nuanced home remedies from barber experience were also addressed,” the authors wrote.
No more than 2 weeks after the information session, each barber completed a posttest questionnaire.
Based on their responses to pretest questions, 39 of the 40 barbers understood that Black men were the group most impacted by PFB and that a person with severe PFB should see a physician. In the pretest survey, 12 barbers (30%) correctly recognized a photo of PFB, which increased to 39 (97.5%) in the posttest survey. In the pretest survey, two barbers (5%) identified laser hair removal as the most effective treatment for PFB, compared with 37 (92.5%) in the posttest survey.
Overall, the mean percentage of correct scores out of 20 questions was 54.8% in the pretest survey, increasing to 91% in the posttest survey (P <.001).
Limitations of the studies included heterogeneity in the survey response options that potentially could have introduced bias, the authors wrote. Another was that since there is a lack of evidence for ideal treatment strategies for PFB, there may have been some uncertainty among the correct answers for the survey that might have contributed to variability in responses. “Further research and implementation of these interventions are needed in efforts to improve health outcomes,” they added.
“Barbers can serve as allies in referral services,” Dr. Rice said in the interview. “They can be the first line for a number of diseases that are related to hair.”
Part of his role as a dermatologist, he added, includes going into a community with “boots on the ground” and talking to people who will see these patients “because access to care, presentation to big hospital systems can be challenging.”
Dr. Rice and the other study authors had no not report any financial disclosures.
FROM JAMA DERMATOLOGY
GVHD raises vitiligo risk in transplant recipients
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
FROM JAMA DERMATOLOGY
Adequate disease control elusive for many patients on systemic AD therapies, study finds
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
FROM RAD 2023
Acne stigma persists across social and professional settings
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
FROM JAMA DERMATOLOGY
An 18-month-old male presents with a red mark on the forehead and nose
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
On examination, a faint pink patch was observed on the right forehead, frontal scalp, and nose. The lesion paled under pressure, with small areas of hair loss on the scalp. No atrophy was noted.