User login
At 52 weeks, complete hair regrowth rates still climbing on deuruxolitinib
BERLIN – at the annual congress of the European Academy of Dermatology and Venereology.
With response curves still climbing at follow-up to date, the results are “truly, truly remarkable,” said Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
Deuruxolitinib is a JAK inhibitor that has specificity for the 1 and 2 subtypes. At 24 weeks in the phase 3 THRIVE-AA1 and THRIVE-AA2 trials, presented at the American Academy of Dermatology annual meeting earlier this year, about 40% of those on the 12-mg twice-daily dose and 32% of those on the 8-mg twice-daily dose achieved a Severity of Alopecia Tool (SALT) score of ≤ 20%, signifying 80% or greater hair regrowth at 24 weeks. The placebo response was 0%.
By 52 weeks, the proportion had climbed to 62% among those on continuous deuruxolitinib whether maintained on the 8-mg or 12-mg twice daily doses. Among patients on placebo, 58.4% reached this endpoint after being switched at 24 weeks to the 12-mg twice daily dose. Of the patients on placebo switched to 8 mg twice daily, the 52-week response was 45.2%, according to Dr. King.
There were 741 patients available at 52 weeks for this on-going analysis. The mean SALT scores at entry exceeded 80%, meaning complete or near complete hair loss. The substantial proportion of patients who met the primary endpoint of SALT ≤ 20 at the end of the blinded period was encouraging, but Dr. King said that the 52-week results are important, not only showing the response was sustained, but that greater regrowth occurs over time.
“Alopecia takes time to treat,” said Dr. King, summarizing the lesson from these data. Moreover, he added that the long-term data are likely to under represent the absolute benefit even if no further growth is achieved with even longer follow-up. One reason is that missing long-term data were accounted for with a last-observation-carried-forward approach.
In other words, “this is the floor when considering response at 52 weeks,” Dr. King said. “In the real world, where adjunctive measures such as intralesional Kenalog [triamcinolone acetonide] or topical treatments are added, we are likely to do even better,” he added.
Adverse events remained low
Treatment-emergent adverse events remained low with “nothing particularly surprising,” Dr. King said. The rate of serious adverse events over 52 weeks was less than 2% on either dose of deuruxolitinib. The proportion of patients who discontinued treatment because of an adverse event was 0.7% in the 8-mg twice-daily arm and 1.1% in the 12-mg twice-daily arm.
Most approved oral JAK inhibitors carry a boxed warning based on a trial conducted with the relatively nonspecific tofacitinib. The trial enrolled older patients with rheumatoid arthritis at risk for thrombotic events, raising questions about its relevance to selective JAK inhibitors employed for other indications. There was only one thrombosis observed in the 52-week alopecia areata follow-up in a patient on deuruxolitinib. Dr. King noted that this patient, who was obese and was on the higher of the two doses, had multiple comorbidities, including systemic lupus erythematosus.
There were no major adverse cardiac events reported in long-term follow-up or cases of tuberculosis. The rate of opportunistic infections was 0.1% in the 8-mg twice-daily arm and 0.2% in the 12-mg twice-daily arm. Serious infections were observed in 0.6% and 0.4% of these two arms, respectively. There were four malignancies (0.5%) in each of the two study arms.
Of the side effects likely to be related to deuruxolitinib, acne was observed in about 10% of patients on either dose. The mechanism is unclear, but Dr. King reported this has been commonly observed with other JAK inhibitors.
Asked his opinion about the optimal starting dose of deuruxolitinib, Dr. King said, “in my mind, the efficacy of 8 mg is so impressive that I would not struggle at all in starting there,” noting that the higher dose could be considered with a slow or inadequate response.
Two JAK inhibitors are already approved
If approved for alopecia areata, deuruxolitinib will be the third JAK inhibitor available for this indication, following the recent approvals of baricitinib and ritlecitinib.
Calling JAK inhibitors “a major advance in the treatment of alopecia areata, particularly for those patients with severe, refractory disease,” Lynne Goldberg, MD, professor of dermatology at Boston University, and director of the hair clinic, Boston Medical Center, said that the proportion of patients with SALT scores ≤ 20 at 52-weeks is “huge.”
She is generally comfortable with the safety of the JAK inhibitors for alopecia areata.
“I believe that, in general, these medications are well tolerated in the alopecia areata population, particularly in otherwise healthy, young patients,” she said, indicating the benefit-to-risk ratio is particularly acceptable when disease is severe.
“This disease has tremendous emotional and functional implications, and many patients with severe or recurrent disease are willing to chance the side effects to live with a full head of hair,” she said. She added that well-informed patients can “make their own, individual assessment.”
Dr. King has financial relationships with approximately 20 pharmaceutical companies, including Concert Pharmaceuticals, which makes deuruxolitinib and provided funding for this study. Dr. Goldberg reports no financial conflicts relevant to this topic.
BERLIN – at the annual congress of the European Academy of Dermatology and Venereology.
With response curves still climbing at follow-up to date, the results are “truly, truly remarkable,” said Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
Deuruxolitinib is a JAK inhibitor that has specificity for the 1 and 2 subtypes. At 24 weeks in the phase 3 THRIVE-AA1 and THRIVE-AA2 trials, presented at the American Academy of Dermatology annual meeting earlier this year, about 40% of those on the 12-mg twice-daily dose and 32% of those on the 8-mg twice-daily dose achieved a Severity of Alopecia Tool (SALT) score of ≤ 20%, signifying 80% or greater hair regrowth at 24 weeks. The placebo response was 0%.
By 52 weeks, the proportion had climbed to 62% among those on continuous deuruxolitinib whether maintained on the 8-mg or 12-mg twice daily doses. Among patients on placebo, 58.4% reached this endpoint after being switched at 24 weeks to the 12-mg twice daily dose. Of the patients on placebo switched to 8 mg twice daily, the 52-week response was 45.2%, according to Dr. King.
There were 741 patients available at 52 weeks for this on-going analysis. The mean SALT scores at entry exceeded 80%, meaning complete or near complete hair loss. The substantial proportion of patients who met the primary endpoint of SALT ≤ 20 at the end of the blinded period was encouraging, but Dr. King said that the 52-week results are important, not only showing the response was sustained, but that greater regrowth occurs over time.
“Alopecia takes time to treat,” said Dr. King, summarizing the lesson from these data. Moreover, he added that the long-term data are likely to under represent the absolute benefit even if no further growth is achieved with even longer follow-up. One reason is that missing long-term data were accounted for with a last-observation-carried-forward approach.
In other words, “this is the floor when considering response at 52 weeks,” Dr. King said. “In the real world, where adjunctive measures such as intralesional Kenalog [triamcinolone acetonide] or topical treatments are added, we are likely to do even better,” he added.
Adverse events remained low
Treatment-emergent adverse events remained low with “nothing particularly surprising,” Dr. King said. The rate of serious adverse events over 52 weeks was less than 2% on either dose of deuruxolitinib. The proportion of patients who discontinued treatment because of an adverse event was 0.7% in the 8-mg twice-daily arm and 1.1% in the 12-mg twice-daily arm.
Most approved oral JAK inhibitors carry a boxed warning based on a trial conducted with the relatively nonspecific tofacitinib. The trial enrolled older patients with rheumatoid arthritis at risk for thrombotic events, raising questions about its relevance to selective JAK inhibitors employed for other indications. There was only one thrombosis observed in the 52-week alopecia areata follow-up in a patient on deuruxolitinib. Dr. King noted that this patient, who was obese and was on the higher of the two doses, had multiple comorbidities, including systemic lupus erythematosus.
There were no major adverse cardiac events reported in long-term follow-up or cases of tuberculosis. The rate of opportunistic infections was 0.1% in the 8-mg twice-daily arm and 0.2% in the 12-mg twice-daily arm. Serious infections were observed in 0.6% and 0.4% of these two arms, respectively. There were four malignancies (0.5%) in each of the two study arms.
Of the side effects likely to be related to deuruxolitinib, acne was observed in about 10% of patients on either dose. The mechanism is unclear, but Dr. King reported this has been commonly observed with other JAK inhibitors.
Asked his opinion about the optimal starting dose of deuruxolitinib, Dr. King said, “in my mind, the efficacy of 8 mg is so impressive that I would not struggle at all in starting there,” noting that the higher dose could be considered with a slow or inadequate response.
Two JAK inhibitors are already approved
If approved for alopecia areata, deuruxolitinib will be the third JAK inhibitor available for this indication, following the recent approvals of baricitinib and ritlecitinib.
Calling JAK inhibitors “a major advance in the treatment of alopecia areata, particularly for those patients with severe, refractory disease,” Lynne Goldberg, MD, professor of dermatology at Boston University, and director of the hair clinic, Boston Medical Center, said that the proportion of patients with SALT scores ≤ 20 at 52-weeks is “huge.”
She is generally comfortable with the safety of the JAK inhibitors for alopecia areata.
“I believe that, in general, these medications are well tolerated in the alopecia areata population, particularly in otherwise healthy, young patients,” she said, indicating the benefit-to-risk ratio is particularly acceptable when disease is severe.
“This disease has tremendous emotional and functional implications, and many patients with severe or recurrent disease are willing to chance the side effects to live with a full head of hair,” she said. She added that well-informed patients can “make their own, individual assessment.”
Dr. King has financial relationships with approximately 20 pharmaceutical companies, including Concert Pharmaceuticals, which makes deuruxolitinib and provided funding for this study. Dr. Goldberg reports no financial conflicts relevant to this topic.
BERLIN – at the annual congress of the European Academy of Dermatology and Venereology.
With response curves still climbing at follow-up to date, the results are “truly, truly remarkable,” said Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
Deuruxolitinib is a JAK inhibitor that has specificity for the 1 and 2 subtypes. At 24 weeks in the phase 3 THRIVE-AA1 and THRIVE-AA2 trials, presented at the American Academy of Dermatology annual meeting earlier this year, about 40% of those on the 12-mg twice-daily dose and 32% of those on the 8-mg twice-daily dose achieved a Severity of Alopecia Tool (SALT) score of ≤ 20%, signifying 80% or greater hair regrowth at 24 weeks. The placebo response was 0%.
By 52 weeks, the proportion had climbed to 62% among those on continuous deuruxolitinib whether maintained on the 8-mg or 12-mg twice daily doses. Among patients on placebo, 58.4% reached this endpoint after being switched at 24 weeks to the 12-mg twice daily dose. Of the patients on placebo switched to 8 mg twice daily, the 52-week response was 45.2%, according to Dr. King.
There were 741 patients available at 52 weeks for this on-going analysis. The mean SALT scores at entry exceeded 80%, meaning complete or near complete hair loss. The substantial proportion of patients who met the primary endpoint of SALT ≤ 20 at the end of the blinded period was encouraging, but Dr. King said that the 52-week results are important, not only showing the response was sustained, but that greater regrowth occurs over time.
“Alopecia takes time to treat,” said Dr. King, summarizing the lesson from these data. Moreover, he added that the long-term data are likely to under represent the absolute benefit even if no further growth is achieved with even longer follow-up. One reason is that missing long-term data were accounted for with a last-observation-carried-forward approach.
In other words, “this is the floor when considering response at 52 weeks,” Dr. King said. “In the real world, where adjunctive measures such as intralesional Kenalog [triamcinolone acetonide] or topical treatments are added, we are likely to do even better,” he added.
Adverse events remained low
Treatment-emergent adverse events remained low with “nothing particularly surprising,” Dr. King said. The rate of serious adverse events over 52 weeks was less than 2% on either dose of deuruxolitinib. The proportion of patients who discontinued treatment because of an adverse event was 0.7% in the 8-mg twice-daily arm and 1.1% in the 12-mg twice-daily arm.
Most approved oral JAK inhibitors carry a boxed warning based on a trial conducted with the relatively nonspecific tofacitinib. The trial enrolled older patients with rheumatoid arthritis at risk for thrombotic events, raising questions about its relevance to selective JAK inhibitors employed for other indications. There was only one thrombosis observed in the 52-week alopecia areata follow-up in a patient on deuruxolitinib. Dr. King noted that this patient, who was obese and was on the higher of the two doses, had multiple comorbidities, including systemic lupus erythematosus.
There were no major adverse cardiac events reported in long-term follow-up or cases of tuberculosis. The rate of opportunistic infections was 0.1% in the 8-mg twice-daily arm and 0.2% in the 12-mg twice-daily arm. Serious infections were observed in 0.6% and 0.4% of these two arms, respectively. There were four malignancies (0.5%) in each of the two study arms.
Of the side effects likely to be related to deuruxolitinib, acne was observed in about 10% of patients on either dose. The mechanism is unclear, but Dr. King reported this has been commonly observed with other JAK inhibitors.
Asked his opinion about the optimal starting dose of deuruxolitinib, Dr. King said, “in my mind, the efficacy of 8 mg is so impressive that I would not struggle at all in starting there,” noting that the higher dose could be considered with a slow or inadequate response.
Two JAK inhibitors are already approved
If approved for alopecia areata, deuruxolitinib will be the third JAK inhibitor available for this indication, following the recent approvals of baricitinib and ritlecitinib.
Calling JAK inhibitors “a major advance in the treatment of alopecia areata, particularly for those patients with severe, refractory disease,” Lynne Goldberg, MD, professor of dermatology at Boston University, and director of the hair clinic, Boston Medical Center, said that the proportion of patients with SALT scores ≤ 20 at 52-weeks is “huge.”
She is generally comfortable with the safety of the JAK inhibitors for alopecia areata.
“I believe that, in general, these medications are well tolerated in the alopecia areata population, particularly in otherwise healthy, young patients,” she said, indicating the benefit-to-risk ratio is particularly acceptable when disease is severe.
“This disease has tremendous emotional and functional implications, and many patients with severe or recurrent disease are willing to chance the side effects to live with a full head of hair,” she said. She added that well-informed patients can “make their own, individual assessment.”
Dr. King has financial relationships with approximately 20 pharmaceutical companies, including Concert Pharmaceuticals, which makes deuruxolitinib and provided funding for this study. Dr. Goldberg reports no financial conflicts relevant to this topic.
At THE EADV CONGRESS
Tapinarof effective for AD in patients as young as 2 years
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
AT THE EADV CONGRESS
Hidradenitis suppurativa: Two anti-IL17A/F therapies yield positive results
BERLIN – In separate trials conducted in patients with hidradenitis suppurativa (HS), two biologics that inhibit the activity of interleukin-17A (IL-17A) and IL-17F were associated with highly encouraging rates of control.
One of the trials evaluated a nanobody inhibitor, sonelokimab, a molecule with a substantially smaller size than traditional monoclonal antibodies (40 kilodaltons vs. 150 kilodaltons). After 24 weeks of treatment, the most effective of the two study doses almost doubled the proportion of patients with complete resolution of draining tunnels (41.1% vs. 23.8%; P < .05) relative to placebo.
“I think the size of sonelokimab is important,” Brian Kirby, MD, a consultant dermatologist at St. Vincent’s Hospital, Dublin, said at the annual congress of the European Academy of Dermatology and Venereology. “We think the smaller size results in better penetration of inflamed tissue,” he added, noting that penetration of abscesses, fistulae, and tunnels has been recognized in the past as a potential weakness of the larger monoclonal antibodies.
The other set of anti-17-A/F set of data were generated by a pooled 48-week maintenance from the BE HEARD I and II trials with bimekizumab. The 16-week data from these two trials were presented at the annual meeting of the American Academy of Dermatology earlier this year.
IL-17A/F trials
Both the
In the sonelokimab trial, called MIRA, 234 adults with HS were randomized in a 2:2:2:1 ratio to one of the two experimental arms, placebo, or a reference arm with the tumor necrosis factor (TNF) inhibitor adalimumab. Nearly 64% had Hurley stage II HS.
The primary endpoint was a 75% or greater reduction in total abscesses and nodules with no increase in draining tunnel count (HiSCR75) from baseline. Dr. Kirby said that this is more rigorous than the HiSCR50 endpoint more commonly used in HS clinical trials. Treatments were administered every 2 weeks for the first 8 weeks of a planned follow-up of 24 weeks and then every 4 weeks thereafter.
At 16 weeks, according to the data Dr. Kirby presented, both doses of sonelokimab were more active than placebo, but Dr. Kirby reported that the lower dose performed better for most objective endpoints.
For example, the HiSCR75 was reached by 43.3% of those randomized to the 120-mg dose (P < .001 vs. placebo), 34.8% of those randomized to the 240-mg dose (P <.01), and 14.7% of those randomized to placebo.
For HiSCR50, response rates were 65.7%, 53.0%, and 27.9%, for the 120-mg, 240-mg, and placebo arms, respectively. Again, both the lower dose (P < .001) and the higher dose (P < .01) were significantly superior to placebo.
On the International Hidradenitis Suppurativa Severity Score System (IHS4), which counts nodules and abscesses, score reductions were 19.3, 14.5, and 7.9 for the lower dose, higher dose, and placebo, respectively, with a greater statistical advantage for the lower relative to the higher dose over placebo (P <.001 vs. P <.01).
However, patient-focused outcomes were not necessarily greater for the lower dose. For the patient-completed measure, the Numerical Rating Scale 50% reduction in skin pain (NRS50), the proportion of patients responding at 12 weeks was numerically greater for the 240-mg dose (41.3%) than with the 120-mg dose (32.0%), although both reached the same statistical advantage (P < .001) over the 4.3% who reached this level of response on placebo.
For the Dermatology Life Quality Index (DLQI) and the Patient Global Impression of Severity (PGI-S), improvements from baseline were similar for the lower and higher dose, although there was a modest numerical and statistical advantage for the higher dose over placebo (P < .001 vs. P <.01).
The HiSCR50 (57.6%) and HiSCR75 (36.4%) responses were both lower for those randomized to the TNF inhibitor adalimumab relative to sonelokimab, but the smaller number of patients in this arm prohibited a statistical comparison.
Although oral candidiasis was more common among patients receiving either dose of sonelokimab than placebo, these were of mild to moderate severity. Dr. Kirby said that there were no unexpected safety issues, and sonelokimab was generally well tolerated.
The results are encouraging, but Dr. Kirby acknowledged that data are now needed to confirm that resolution of tunnels and fistulae is greater with a nanobody inhibitor of IL-17A/F than other targeted therapies. Even if this is validated, he said studies are needed to prove that the small relative molecule size is the reason behind the benefits.
Forty-eight–week bimekizumab data
From the pooled BE HEARD I and BE HEARD II maintenance data, the major message is that the robust responses observed at 16 weeks versus placebo were maintained at 48 weeks. More than 75% of patients retained a HiSCR50 response and more than 55% achieved a HiSCR75 response at the 48-week follow-up. The durable response was also reflected in other measures, according to Christos C. Zouboulis, MD, PhD, director of the department of dermatology, Brandenburg Medical School, Neuruppin, Germany.
“Improvements in disease severity were seen over time,” Dr. Zouboulis reported. “The majority of patients with severe HS at baseline shifted to mild to moderate disease according to the IHS4 classification.”
To the degree that both sonelokimab and bimekizumab target IL-17A/F, these data are mutually reinforcing. Dr. Kirby said that there is a sizable body of data implicating IL-17A/F in driving HS, and the activity of inhibitors in support the clinical value of IL-17A/F suppression.
On Oct. 18, shortly after the EADV meeting concluded, the Food and Drug Administration approved bimekizumab for treating moderate to severe plaque psoriasis, the first approved indication in the United States. In the European Union, it was approved for psoriasis in 2021, and for psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Kirby has financial relationships with more than 10 pharmaceutical companies, including MoonLake, which is developing sonelokimab and sponsored the MIRA trial. Dr. Christos, president of the European HS Foundation, has financial relationships with multiple pharmaceutical companies, including UCB, which makes bimekizumab and provided funding for the BE HEARD I and II trials.
BERLIN – In separate trials conducted in patients with hidradenitis suppurativa (HS), two biologics that inhibit the activity of interleukin-17A (IL-17A) and IL-17F were associated with highly encouraging rates of control.
One of the trials evaluated a nanobody inhibitor, sonelokimab, a molecule with a substantially smaller size than traditional monoclonal antibodies (40 kilodaltons vs. 150 kilodaltons). After 24 weeks of treatment, the most effective of the two study doses almost doubled the proportion of patients with complete resolution of draining tunnels (41.1% vs. 23.8%; P < .05) relative to placebo.
“I think the size of sonelokimab is important,” Brian Kirby, MD, a consultant dermatologist at St. Vincent’s Hospital, Dublin, said at the annual congress of the European Academy of Dermatology and Venereology. “We think the smaller size results in better penetration of inflamed tissue,” he added, noting that penetration of abscesses, fistulae, and tunnels has been recognized in the past as a potential weakness of the larger monoclonal antibodies.
The other set of anti-17-A/F set of data were generated by a pooled 48-week maintenance from the BE HEARD I and II trials with bimekizumab. The 16-week data from these two trials were presented at the annual meeting of the American Academy of Dermatology earlier this year.
IL-17A/F trials
Both the
In the sonelokimab trial, called MIRA, 234 adults with HS were randomized in a 2:2:2:1 ratio to one of the two experimental arms, placebo, or a reference arm with the tumor necrosis factor (TNF) inhibitor adalimumab. Nearly 64% had Hurley stage II HS.
The primary endpoint was a 75% or greater reduction in total abscesses and nodules with no increase in draining tunnel count (HiSCR75) from baseline. Dr. Kirby said that this is more rigorous than the HiSCR50 endpoint more commonly used in HS clinical trials. Treatments were administered every 2 weeks for the first 8 weeks of a planned follow-up of 24 weeks and then every 4 weeks thereafter.
At 16 weeks, according to the data Dr. Kirby presented, both doses of sonelokimab were more active than placebo, but Dr. Kirby reported that the lower dose performed better for most objective endpoints.
For example, the HiSCR75 was reached by 43.3% of those randomized to the 120-mg dose (P < .001 vs. placebo), 34.8% of those randomized to the 240-mg dose (P <.01), and 14.7% of those randomized to placebo.
For HiSCR50, response rates were 65.7%, 53.0%, and 27.9%, for the 120-mg, 240-mg, and placebo arms, respectively. Again, both the lower dose (P < .001) and the higher dose (P < .01) were significantly superior to placebo.
On the International Hidradenitis Suppurativa Severity Score System (IHS4), which counts nodules and abscesses, score reductions were 19.3, 14.5, and 7.9 for the lower dose, higher dose, and placebo, respectively, with a greater statistical advantage for the lower relative to the higher dose over placebo (P <.001 vs. P <.01).
However, patient-focused outcomes were not necessarily greater for the lower dose. For the patient-completed measure, the Numerical Rating Scale 50% reduction in skin pain (NRS50), the proportion of patients responding at 12 weeks was numerically greater for the 240-mg dose (41.3%) than with the 120-mg dose (32.0%), although both reached the same statistical advantage (P < .001) over the 4.3% who reached this level of response on placebo.
For the Dermatology Life Quality Index (DLQI) and the Patient Global Impression of Severity (PGI-S), improvements from baseline were similar for the lower and higher dose, although there was a modest numerical and statistical advantage for the higher dose over placebo (P < .001 vs. P <.01).
The HiSCR50 (57.6%) and HiSCR75 (36.4%) responses were both lower for those randomized to the TNF inhibitor adalimumab relative to sonelokimab, but the smaller number of patients in this arm prohibited a statistical comparison.
Although oral candidiasis was more common among patients receiving either dose of sonelokimab than placebo, these were of mild to moderate severity. Dr. Kirby said that there were no unexpected safety issues, and sonelokimab was generally well tolerated.
The results are encouraging, but Dr. Kirby acknowledged that data are now needed to confirm that resolution of tunnels and fistulae is greater with a nanobody inhibitor of IL-17A/F than other targeted therapies. Even if this is validated, he said studies are needed to prove that the small relative molecule size is the reason behind the benefits.
Forty-eight–week bimekizumab data
From the pooled BE HEARD I and BE HEARD II maintenance data, the major message is that the robust responses observed at 16 weeks versus placebo were maintained at 48 weeks. More than 75% of patients retained a HiSCR50 response and more than 55% achieved a HiSCR75 response at the 48-week follow-up. The durable response was also reflected in other measures, according to Christos C. Zouboulis, MD, PhD, director of the department of dermatology, Brandenburg Medical School, Neuruppin, Germany.
“Improvements in disease severity were seen over time,” Dr. Zouboulis reported. “The majority of patients with severe HS at baseline shifted to mild to moderate disease according to the IHS4 classification.”
To the degree that both sonelokimab and bimekizumab target IL-17A/F, these data are mutually reinforcing. Dr. Kirby said that there is a sizable body of data implicating IL-17A/F in driving HS, and the activity of inhibitors in support the clinical value of IL-17A/F suppression.
On Oct. 18, shortly after the EADV meeting concluded, the Food and Drug Administration approved bimekizumab for treating moderate to severe plaque psoriasis, the first approved indication in the United States. In the European Union, it was approved for psoriasis in 2021, and for psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Kirby has financial relationships with more than 10 pharmaceutical companies, including MoonLake, which is developing sonelokimab and sponsored the MIRA trial. Dr. Christos, president of the European HS Foundation, has financial relationships with multiple pharmaceutical companies, including UCB, which makes bimekizumab and provided funding for the BE HEARD I and II trials.
BERLIN – In separate trials conducted in patients with hidradenitis suppurativa (HS), two biologics that inhibit the activity of interleukin-17A (IL-17A) and IL-17F were associated with highly encouraging rates of control.
One of the trials evaluated a nanobody inhibitor, sonelokimab, a molecule with a substantially smaller size than traditional monoclonal antibodies (40 kilodaltons vs. 150 kilodaltons). After 24 weeks of treatment, the most effective of the two study doses almost doubled the proportion of patients with complete resolution of draining tunnels (41.1% vs. 23.8%; P < .05) relative to placebo.
“I think the size of sonelokimab is important,” Brian Kirby, MD, a consultant dermatologist at St. Vincent’s Hospital, Dublin, said at the annual congress of the European Academy of Dermatology and Venereology. “We think the smaller size results in better penetration of inflamed tissue,” he added, noting that penetration of abscesses, fistulae, and tunnels has been recognized in the past as a potential weakness of the larger monoclonal antibodies.
The other set of anti-17-A/F set of data were generated by a pooled 48-week maintenance from the BE HEARD I and II trials with bimekizumab. The 16-week data from these two trials were presented at the annual meeting of the American Academy of Dermatology earlier this year.
IL-17A/F trials
Both the
In the sonelokimab trial, called MIRA, 234 adults with HS were randomized in a 2:2:2:1 ratio to one of the two experimental arms, placebo, or a reference arm with the tumor necrosis factor (TNF) inhibitor adalimumab. Nearly 64% had Hurley stage II HS.
The primary endpoint was a 75% or greater reduction in total abscesses and nodules with no increase in draining tunnel count (HiSCR75) from baseline. Dr. Kirby said that this is more rigorous than the HiSCR50 endpoint more commonly used in HS clinical trials. Treatments were administered every 2 weeks for the first 8 weeks of a planned follow-up of 24 weeks and then every 4 weeks thereafter.
At 16 weeks, according to the data Dr. Kirby presented, both doses of sonelokimab were more active than placebo, but Dr. Kirby reported that the lower dose performed better for most objective endpoints.
For example, the HiSCR75 was reached by 43.3% of those randomized to the 120-mg dose (P < .001 vs. placebo), 34.8% of those randomized to the 240-mg dose (P <.01), and 14.7% of those randomized to placebo.
For HiSCR50, response rates were 65.7%, 53.0%, and 27.9%, for the 120-mg, 240-mg, and placebo arms, respectively. Again, both the lower dose (P < .001) and the higher dose (P < .01) were significantly superior to placebo.
On the International Hidradenitis Suppurativa Severity Score System (IHS4), which counts nodules and abscesses, score reductions were 19.3, 14.5, and 7.9 for the lower dose, higher dose, and placebo, respectively, with a greater statistical advantage for the lower relative to the higher dose over placebo (P <.001 vs. P <.01).
However, patient-focused outcomes were not necessarily greater for the lower dose. For the patient-completed measure, the Numerical Rating Scale 50% reduction in skin pain (NRS50), the proportion of patients responding at 12 weeks was numerically greater for the 240-mg dose (41.3%) than with the 120-mg dose (32.0%), although both reached the same statistical advantage (P < .001) over the 4.3% who reached this level of response on placebo.
For the Dermatology Life Quality Index (DLQI) and the Patient Global Impression of Severity (PGI-S), improvements from baseline were similar for the lower and higher dose, although there was a modest numerical and statistical advantage for the higher dose over placebo (P < .001 vs. P <.01).
The HiSCR50 (57.6%) and HiSCR75 (36.4%) responses were both lower for those randomized to the TNF inhibitor adalimumab relative to sonelokimab, but the smaller number of patients in this arm prohibited a statistical comparison.
Although oral candidiasis was more common among patients receiving either dose of sonelokimab than placebo, these were of mild to moderate severity. Dr. Kirby said that there were no unexpected safety issues, and sonelokimab was generally well tolerated.
The results are encouraging, but Dr. Kirby acknowledged that data are now needed to confirm that resolution of tunnels and fistulae is greater with a nanobody inhibitor of IL-17A/F than other targeted therapies. Even if this is validated, he said studies are needed to prove that the small relative molecule size is the reason behind the benefits.
Forty-eight–week bimekizumab data
From the pooled BE HEARD I and BE HEARD II maintenance data, the major message is that the robust responses observed at 16 weeks versus placebo were maintained at 48 weeks. More than 75% of patients retained a HiSCR50 response and more than 55% achieved a HiSCR75 response at the 48-week follow-up. The durable response was also reflected in other measures, according to Christos C. Zouboulis, MD, PhD, director of the department of dermatology, Brandenburg Medical School, Neuruppin, Germany.
“Improvements in disease severity were seen over time,” Dr. Zouboulis reported. “The majority of patients with severe HS at baseline shifted to mild to moderate disease according to the IHS4 classification.”
To the degree that both sonelokimab and bimekizumab target IL-17A/F, these data are mutually reinforcing. Dr. Kirby said that there is a sizable body of data implicating IL-17A/F in driving HS, and the activity of inhibitors in support the clinical value of IL-17A/F suppression.
On Oct. 18, shortly after the EADV meeting concluded, the Food and Drug Administration approved bimekizumab for treating moderate to severe plaque psoriasis, the first approved indication in the United States. In the European Union, it was approved for psoriasis in 2021, and for psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Kirby has financial relationships with more than 10 pharmaceutical companies, including MoonLake, which is developing sonelokimab and sponsored the MIRA trial. Dr. Christos, president of the European HS Foundation, has financial relationships with multiple pharmaceutical companies, including UCB, which makes bimekizumab and provided funding for the BE HEARD I and II trials.
AT THE EADV CONGRESS
Parent concerns a factor when treating eczema in children with darker skin types
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
AT SOC 2023
Bilateral facial swelling
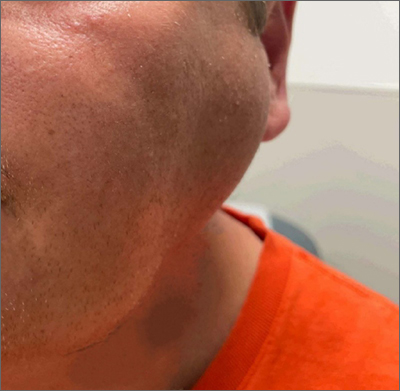
The patient was given a diagnosis of sialadenosis (also known as sialosis), a noninflammatory, non-neoplastic enlargement of the parotid glands. It can often manifest as fatty degeneration of the parotid glands, which may be associated with underlying conditions such as hypertriglyceridemia, diabetes, and metabolic syndrome.1-3
Ultrasonography and a subsequent computed tomography with contrast demonstrated fatty hypertrophy of the parotid glands without any concerning parotid mass or enlarged cervical lymph nodes. No abnormalities of the ductal system (eg, stricture or obstruction with stone) were noted, so sialography and sialendoscopy were not indicated.
Evaluation for inflammatory, autoimmune, and granulomatous diseases was negative, including negative anti-Ro/SSA and anti-La/SSB antibodies and negative HIV screen. However, our patient had an elevated serum triglyceride level of 589 mg/dL (reference range, < 150 mg/dL), while serum total cholesterol was within the reference range (< 200 mg/dL). (Interestingly, his triglycerides were normal a year earlier.) The patient’s A1c level was normal.
The differential diagnosis for this patient included Sjögren syndrome, abscess, viral infection (eg, mumps, HIV sialopathy), Kimura disease, sarcoidosis, masseter hypertrophy, and tumors of the parotid gland (eg, Warthin tumor and pleomorphic adenoma). Drug-induced sialadenitis was another possibility, as several drugs may be associated with salivary gland enlargement.4 However, no association was found for our patient.
Primary management is focused on treating the underlying disorder. The application of heat, massage, and sialagogues (eg, pilocarpine 5 mg orally tid) can be used to stimulate salivation, which may help reduce the swelling. Bilateral parotid gland swelling in patients with increased triglyceride levels often resolves after treatment of hypertriglyceridemia.3,5 Less common modalities include botulinum neurotoxin injection, tympanic neurectomy, and parotidectomy.6
The treatment plan for this patient included aggressive dietary modification and increasing his current dosage of atorvastatin from 20 mg to 80 mg at bedtime. Increasing the dosage of statin was preferred over adding another agent (such as fibrates) to decrease the risk of myopathy. Fine-needle aspiration biopsy may be considered if the swelling does not resolve after correction of lipid abnormalities, which can take between 6 months and 3 years.3
Photo courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University, Homer Stryker, MD School of Medicine, Kalamazoo.
1. Garcia DS, Bussoloti Filho I. Fat deposition of parotid glands. Braz J Otorhinolaryngol. 2013;79:173-176
2. Hida A, Akahoshi M, Takagi Y, et al. Lipid infiltration in the parotid glands: a clinical manifestation of metabolic syndrome. Exp Clin Endocrinol Diabetes. 2012;120:110-115. doi: 10.1055/s-0031-1291315
3. Sheikh JS, Sharma M, Kunath A, et al. Reversible parotid enlargement and pseudo-Sjögren's syndrome secondary to hypertriglyceridemia. J Rheumatol. 1996;23:1288-1291
4. Vinayak V, Annigeri RG, Patel HA, et al. Adverse effects of drugs on saliva and salivary glands. J Orofac Sci. 2013;5:15-20. doi: 10.4103/0975-8844.113684
5. Kaltreider HB, Talal N. Bilateral parotid gland enlargement and hyperlipoproteinemia. JAMA. 1969;210:2067-2070. doi:10.1001/jama.1969.03160370051010
6. Davis AB, Hoffman HT. Management options for sialadenosis. Otolaryngol Clin North Am. 2021;54:605-611. doi: 10.1016/j.otc.2021.02.005

The patient was given a diagnosis of sialadenosis (also known as sialosis), a noninflammatory, non-neoplastic enlargement of the parotid glands. It can often manifest as fatty degeneration of the parotid glands, which may be associated with underlying conditions such as hypertriglyceridemia, diabetes, and metabolic syndrome.1-3
Ultrasonography and a subsequent computed tomography with contrast demonstrated fatty hypertrophy of the parotid glands without any concerning parotid mass or enlarged cervical lymph nodes. No abnormalities of the ductal system (eg, stricture or obstruction with stone) were noted, so sialography and sialendoscopy were not indicated.
Evaluation for inflammatory, autoimmune, and granulomatous diseases was negative, including negative anti-Ro/SSA and anti-La/SSB antibodies and negative HIV screen. However, our patient had an elevated serum triglyceride level of 589 mg/dL (reference range, < 150 mg/dL), while serum total cholesterol was within the reference range (< 200 mg/dL). (Interestingly, his triglycerides were normal a year earlier.) The patient’s A1c level was normal.
The differential diagnosis for this patient included Sjögren syndrome, abscess, viral infection (eg, mumps, HIV sialopathy), Kimura disease, sarcoidosis, masseter hypertrophy, and tumors of the parotid gland (eg, Warthin tumor and pleomorphic adenoma). Drug-induced sialadenitis was another possibility, as several drugs may be associated with salivary gland enlargement.4 However, no association was found for our patient.
Primary management is focused on treating the underlying disorder. The application of heat, massage, and sialagogues (eg, pilocarpine 5 mg orally tid) can be used to stimulate salivation, which may help reduce the swelling. Bilateral parotid gland swelling in patients with increased triglyceride levels often resolves after treatment of hypertriglyceridemia.3,5 Less common modalities include botulinum neurotoxin injection, tympanic neurectomy, and parotidectomy.6
The treatment plan for this patient included aggressive dietary modification and increasing his current dosage of atorvastatin from 20 mg to 80 mg at bedtime. Increasing the dosage of statin was preferred over adding another agent (such as fibrates) to decrease the risk of myopathy. Fine-needle aspiration biopsy may be considered if the swelling does not resolve after correction of lipid abnormalities, which can take between 6 months and 3 years.3
Photo courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University, Homer Stryker, MD School of Medicine, Kalamazoo.

The patient was given a diagnosis of sialadenosis (also known as sialosis), a noninflammatory, non-neoplastic enlargement of the parotid glands. It can often manifest as fatty degeneration of the parotid glands, which may be associated with underlying conditions such as hypertriglyceridemia, diabetes, and metabolic syndrome.1-3
Ultrasonography and a subsequent computed tomography with contrast demonstrated fatty hypertrophy of the parotid glands without any concerning parotid mass or enlarged cervical lymph nodes. No abnormalities of the ductal system (eg, stricture or obstruction with stone) were noted, so sialography and sialendoscopy were not indicated.
Evaluation for inflammatory, autoimmune, and granulomatous diseases was negative, including negative anti-Ro/SSA and anti-La/SSB antibodies and negative HIV screen. However, our patient had an elevated serum triglyceride level of 589 mg/dL (reference range, < 150 mg/dL), while serum total cholesterol was within the reference range (< 200 mg/dL). (Interestingly, his triglycerides were normal a year earlier.) The patient’s A1c level was normal.
The differential diagnosis for this patient included Sjögren syndrome, abscess, viral infection (eg, mumps, HIV sialopathy), Kimura disease, sarcoidosis, masseter hypertrophy, and tumors of the parotid gland (eg, Warthin tumor and pleomorphic adenoma). Drug-induced sialadenitis was another possibility, as several drugs may be associated with salivary gland enlargement.4 However, no association was found for our patient.
Primary management is focused on treating the underlying disorder. The application of heat, massage, and sialagogues (eg, pilocarpine 5 mg orally tid) can be used to stimulate salivation, which may help reduce the swelling. Bilateral parotid gland swelling in patients with increased triglyceride levels often resolves after treatment of hypertriglyceridemia.3,5 Less common modalities include botulinum neurotoxin injection, tympanic neurectomy, and parotidectomy.6
The treatment plan for this patient included aggressive dietary modification and increasing his current dosage of atorvastatin from 20 mg to 80 mg at bedtime. Increasing the dosage of statin was preferred over adding another agent (such as fibrates) to decrease the risk of myopathy. Fine-needle aspiration biopsy may be considered if the swelling does not resolve after correction of lipid abnormalities, which can take between 6 months and 3 years.3
Photo courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University, Homer Stryker, MD School of Medicine, Kalamazoo.
1. Garcia DS, Bussoloti Filho I. Fat deposition of parotid glands. Braz J Otorhinolaryngol. 2013;79:173-176
2. Hida A, Akahoshi M, Takagi Y, et al. Lipid infiltration in the parotid glands: a clinical manifestation of metabolic syndrome. Exp Clin Endocrinol Diabetes. 2012;120:110-115. doi: 10.1055/s-0031-1291315
3. Sheikh JS, Sharma M, Kunath A, et al. Reversible parotid enlargement and pseudo-Sjögren's syndrome secondary to hypertriglyceridemia. J Rheumatol. 1996;23:1288-1291
4. Vinayak V, Annigeri RG, Patel HA, et al. Adverse effects of drugs on saliva and salivary glands. J Orofac Sci. 2013;5:15-20. doi: 10.4103/0975-8844.113684
5. Kaltreider HB, Talal N. Bilateral parotid gland enlargement and hyperlipoproteinemia. JAMA. 1969;210:2067-2070. doi:10.1001/jama.1969.03160370051010
6. Davis AB, Hoffman HT. Management options for sialadenosis. Otolaryngol Clin North Am. 2021;54:605-611. doi: 10.1016/j.otc.2021.02.005
1. Garcia DS, Bussoloti Filho I. Fat deposition of parotid glands. Braz J Otorhinolaryngol. 2013;79:173-176
2. Hida A, Akahoshi M, Takagi Y, et al. Lipid infiltration in the parotid glands: a clinical manifestation of metabolic syndrome. Exp Clin Endocrinol Diabetes. 2012;120:110-115. doi: 10.1055/s-0031-1291315
3. Sheikh JS, Sharma M, Kunath A, et al. Reversible parotid enlargement and pseudo-Sjögren's syndrome secondary to hypertriglyceridemia. J Rheumatol. 1996;23:1288-1291
4. Vinayak V, Annigeri RG, Patel HA, et al. Adverse effects of drugs on saliva and salivary glands. J Orofac Sci. 2013;5:15-20. doi: 10.4103/0975-8844.113684
5. Kaltreider HB, Talal N. Bilateral parotid gland enlargement and hyperlipoproteinemia. JAMA. 1969;210:2067-2070. doi:10.1001/jama.1969.03160370051010
6. Davis AB, Hoffman HT. Management options for sialadenosis. Otolaryngol Clin North Am. 2021;54:605-611. doi: 10.1016/j.otc.2021.02.005
Lebrikizumab gets European nod for treating moderate-to-severe atopic dermatitis
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
Diagnosing patients with sarcoidosis
A 40-year-old women is evaluated for liver abnormalities. She had elevated transaminases and alkaline phosphatase. A liver ultrasound showed multiple lesions. She underwent liver biopsy, which showed granulomas. What test results, if abnormal, would be most suggestive of sarcoidosis?
A. Erythrocyte sedimentation rate
B. C-reactive protein
C. Lymphocyte count
D. Antinuclear antibodies
The correct answer here is lymphocyte count. Sarcoidosis is in just about every differential diagnosis, as it can involve every organ system. I will share with you a few pearls I have learned over 30 years of taking care of patients with sarcoidosis. Lymphocyte counts drop with active sarcoidosis. Sarcoidosis should always be part of the differential when you see lymphopenia. El Jammal et al. studied 90 patients referred for possible granulomatous hepatitis.1 Seventy-three patients had a final diagnosis of granulomatous hepatitis, and 38 of those patients had sarcoidosis. Lymphopenia had a high specificity (85.7%) for the diagnosis of sarcoidosis, with a specificity of 100% in the patients under 50 years old.
Morell and colleagues looked at whether low lymphocyte counts and low lymphocyte percentage were markers of active sarcoidosis.2 Forty patients with biopsy-proven sarcoidosis were prospectively evaluated every 6 months. A low lymphocyte count and a low lymphocyte percentage (< 20%) were detected more frequently in patients with active sarcoidosis than in the patients with asymptomatic sarcoidosis (P < .02 and P < .0001).
Jones et al. looked at lymphopenia as a marker of sarcoidosis in patients presenting with uveitis.3 The study was a retrospective case-control study (112 patients with sarcoidosis-associated uveitis and 398 controls with other forms of uveitis). The mean lymphocyte count for patients with sarcoidosis was 1.43 vs. 2.04 for other causes of uveitis (P ≤ .0001).
Patients with sarcoidosis are at risk of hypercalciuria, hypercalcemia, and kidney stones. These are common in patients with sarcoidosis, with up to 50% of such patients having hypercalciuria. This is because in sarcoidosis patients 25(OH) vitamin D is converted in granulomas by activated macrophages to 1,25(OH)2 vitamin D, which is the active form of vitamin D.
Several studies have looked at the diagnostic utility of 1,25(OH)2 vitamin D levels in patients with suspected sarcoidosis. Rohmer and colleagues looked at whether 1,25(OH)2 vitamin D levels could help with the diagnosis of sarcoidosis as the cause of uveitis.4 They found that the level of 25(OH) vitamin D in sarcoidosis patients with uveitis was lower than in patients with uveitis without sarcoidosis, 34 vs. 43 nmol/mL (P < .02), whereas the 1,25(OH)2 vitamin D level was higher in patients with sarcoidosis than in those with uveitis without sarcoidosis, 132 vs. 108 pmol/L (P = .02). They looked at the 1,25(OH)2D/25(OH)D ratio; a ratio > 3.5 was strongly associated with an abnormal chest CT-scan (OR = 5.7, P = .003) and granulomas on bronchial biopsy (OR = 14.7, P = .007).
Kavathia et al. looked at whether elevated 1,25(OH)2 vitamin D levels predicted chronicity of sarcoidosis.5 A total of 59 sarcoidosis patients were recruited for the study. Higher serum 1,25(OH)2 vitamin D levels were associated with patients requiring repeated systemic immunosuppressive therapy or > 1 year of therapy. Increasing quartiles of serum 1,25(OH)2 vitamin D level were associated with increased odds of patients having chronic sarcoidosis (OR = 1.82; 95% CI, 1.11-2.99, P = .019).
Because of the higher activated vitamin D levels in sarcoidosis patients, they are at risk for problems with vitamin D supplementation. I have seen two patients develop large numbers of kidney stones after receiving high-dose vitamin D. Sodhi and Aldrich reported on a cohort of 196 sarcoidosis patients who had received vitamin D and compared them with 196 control patients with sarcoidosis who were not receiving vitamin D.6 Hypercalcemia was more frequent in the group that received vitamin D (42.3%) than in the group that did not (18.3%, P < .0001). In this study, only a minority (23%) of patients receiving vitamin D had their 1,25(OH)2 vitamin D level checked.
Pearl: Lymphocyte count and 1,25(OH)2 vitamin D levels can be helpful tests in assessing sarcoidosis activity. Patients with sarcoidosis who receive vitamin D should have their 1.25(OH)2 vitamin D levels monitored.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. El Jammal et al. Sarcoidosis Vasc Diffuse Lung Dis. 2023 Sep 13;40(3):e2023031.
2. Morell F et al. Chest. 2002 Apr;121(4):1239-44.
3. Jones NP et al. Br J Ophthalmol. 2016 Oct;100(10):1393-6.
4. Rohmer J et al. Ocul Immunol Inflamm. 2020 Apr 2;28(3):341-7.
5. Kavathia D et al. Respir Med. 2010 Apr;104(4):564–70.
6. Sodhi A and Aldrich T. Am J Med Sci. 2016 Sep;352(3):252-7.
A 40-year-old women is evaluated for liver abnormalities. She had elevated transaminases and alkaline phosphatase. A liver ultrasound showed multiple lesions. She underwent liver biopsy, which showed granulomas. What test results, if abnormal, would be most suggestive of sarcoidosis?
A. Erythrocyte sedimentation rate
B. C-reactive protein
C. Lymphocyte count
D. Antinuclear antibodies
The correct answer here is lymphocyte count. Sarcoidosis is in just about every differential diagnosis, as it can involve every organ system. I will share with you a few pearls I have learned over 30 years of taking care of patients with sarcoidosis. Lymphocyte counts drop with active sarcoidosis. Sarcoidosis should always be part of the differential when you see lymphopenia. El Jammal et al. studied 90 patients referred for possible granulomatous hepatitis.1 Seventy-three patients had a final diagnosis of granulomatous hepatitis, and 38 of those patients had sarcoidosis. Lymphopenia had a high specificity (85.7%) for the diagnosis of sarcoidosis, with a specificity of 100% in the patients under 50 years old.
Morell and colleagues looked at whether low lymphocyte counts and low lymphocyte percentage were markers of active sarcoidosis.2 Forty patients with biopsy-proven sarcoidosis were prospectively evaluated every 6 months. A low lymphocyte count and a low lymphocyte percentage (< 20%) were detected more frequently in patients with active sarcoidosis than in the patients with asymptomatic sarcoidosis (P < .02 and P < .0001).
Jones et al. looked at lymphopenia as a marker of sarcoidosis in patients presenting with uveitis.3 The study was a retrospective case-control study (112 patients with sarcoidosis-associated uveitis and 398 controls with other forms of uveitis). The mean lymphocyte count for patients with sarcoidosis was 1.43 vs. 2.04 for other causes of uveitis (P ≤ .0001).
Patients with sarcoidosis are at risk of hypercalciuria, hypercalcemia, and kidney stones. These are common in patients with sarcoidosis, with up to 50% of such patients having hypercalciuria. This is because in sarcoidosis patients 25(OH) vitamin D is converted in granulomas by activated macrophages to 1,25(OH)2 vitamin D, which is the active form of vitamin D.
Several studies have looked at the diagnostic utility of 1,25(OH)2 vitamin D levels in patients with suspected sarcoidosis. Rohmer and colleagues looked at whether 1,25(OH)2 vitamin D levels could help with the diagnosis of sarcoidosis as the cause of uveitis.4 They found that the level of 25(OH) vitamin D in sarcoidosis patients with uveitis was lower than in patients with uveitis without sarcoidosis, 34 vs. 43 nmol/mL (P < .02), whereas the 1,25(OH)2 vitamin D level was higher in patients with sarcoidosis than in those with uveitis without sarcoidosis, 132 vs. 108 pmol/L (P = .02). They looked at the 1,25(OH)2D/25(OH)D ratio; a ratio > 3.5 was strongly associated with an abnormal chest CT-scan (OR = 5.7, P = .003) and granulomas on bronchial biopsy (OR = 14.7, P = .007).
Kavathia et al. looked at whether elevated 1,25(OH)2 vitamin D levels predicted chronicity of sarcoidosis.5 A total of 59 sarcoidosis patients were recruited for the study. Higher serum 1,25(OH)2 vitamin D levels were associated with patients requiring repeated systemic immunosuppressive therapy or > 1 year of therapy. Increasing quartiles of serum 1,25(OH)2 vitamin D level were associated with increased odds of patients having chronic sarcoidosis (OR = 1.82; 95% CI, 1.11-2.99, P = .019).
Because of the higher activated vitamin D levels in sarcoidosis patients, they are at risk for problems with vitamin D supplementation. I have seen two patients develop large numbers of kidney stones after receiving high-dose vitamin D. Sodhi and Aldrich reported on a cohort of 196 sarcoidosis patients who had received vitamin D and compared them with 196 control patients with sarcoidosis who were not receiving vitamin D.6 Hypercalcemia was more frequent in the group that received vitamin D (42.3%) than in the group that did not (18.3%, P < .0001). In this study, only a minority (23%) of patients receiving vitamin D had their 1,25(OH)2 vitamin D level checked.
Pearl: Lymphocyte count and 1,25(OH)2 vitamin D levels can be helpful tests in assessing sarcoidosis activity. Patients with sarcoidosis who receive vitamin D should have their 1.25(OH)2 vitamin D levels monitored.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. El Jammal et al. Sarcoidosis Vasc Diffuse Lung Dis. 2023 Sep 13;40(3):e2023031.
2. Morell F et al. Chest. 2002 Apr;121(4):1239-44.
3. Jones NP et al. Br J Ophthalmol. 2016 Oct;100(10):1393-6.
4. Rohmer J et al. Ocul Immunol Inflamm. 2020 Apr 2;28(3):341-7.
5. Kavathia D et al. Respir Med. 2010 Apr;104(4):564–70.
6. Sodhi A and Aldrich T. Am J Med Sci. 2016 Sep;352(3):252-7.
A 40-year-old women is evaluated for liver abnormalities. She had elevated transaminases and alkaline phosphatase. A liver ultrasound showed multiple lesions. She underwent liver biopsy, which showed granulomas. What test results, if abnormal, would be most suggestive of sarcoidosis?
A. Erythrocyte sedimentation rate
B. C-reactive protein
C. Lymphocyte count
D. Antinuclear antibodies
The correct answer here is lymphocyte count. Sarcoidosis is in just about every differential diagnosis, as it can involve every organ system. I will share with you a few pearls I have learned over 30 years of taking care of patients with sarcoidosis. Lymphocyte counts drop with active sarcoidosis. Sarcoidosis should always be part of the differential when you see lymphopenia. El Jammal et al. studied 90 patients referred for possible granulomatous hepatitis.1 Seventy-three patients had a final diagnosis of granulomatous hepatitis, and 38 of those patients had sarcoidosis. Lymphopenia had a high specificity (85.7%) for the diagnosis of sarcoidosis, with a specificity of 100% in the patients under 50 years old.
Morell and colleagues looked at whether low lymphocyte counts and low lymphocyte percentage were markers of active sarcoidosis.2 Forty patients with biopsy-proven sarcoidosis were prospectively evaluated every 6 months. A low lymphocyte count and a low lymphocyte percentage (< 20%) were detected more frequently in patients with active sarcoidosis than in the patients with asymptomatic sarcoidosis (P < .02 and P < .0001).
Jones et al. looked at lymphopenia as a marker of sarcoidosis in patients presenting with uveitis.3 The study was a retrospective case-control study (112 patients with sarcoidosis-associated uveitis and 398 controls with other forms of uveitis). The mean lymphocyte count for patients with sarcoidosis was 1.43 vs. 2.04 for other causes of uveitis (P ≤ .0001).
Patients with sarcoidosis are at risk of hypercalciuria, hypercalcemia, and kidney stones. These are common in patients with sarcoidosis, with up to 50% of such patients having hypercalciuria. This is because in sarcoidosis patients 25(OH) vitamin D is converted in granulomas by activated macrophages to 1,25(OH)2 vitamin D, which is the active form of vitamin D.
Several studies have looked at the diagnostic utility of 1,25(OH)2 vitamin D levels in patients with suspected sarcoidosis. Rohmer and colleagues looked at whether 1,25(OH)2 vitamin D levels could help with the diagnosis of sarcoidosis as the cause of uveitis.4 They found that the level of 25(OH) vitamin D in sarcoidosis patients with uveitis was lower than in patients with uveitis without sarcoidosis, 34 vs. 43 nmol/mL (P < .02), whereas the 1,25(OH)2 vitamin D level was higher in patients with sarcoidosis than in those with uveitis without sarcoidosis, 132 vs. 108 pmol/L (P = .02). They looked at the 1,25(OH)2D/25(OH)D ratio; a ratio > 3.5 was strongly associated with an abnormal chest CT-scan (OR = 5.7, P = .003) and granulomas on bronchial biopsy (OR = 14.7, P = .007).
Kavathia et al. looked at whether elevated 1,25(OH)2 vitamin D levels predicted chronicity of sarcoidosis.5 A total of 59 sarcoidosis patients were recruited for the study. Higher serum 1,25(OH)2 vitamin D levels were associated with patients requiring repeated systemic immunosuppressive therapy or > 1 year of therapy. Increasing quartiles of serum 1,25(OH)2 vitamin D level were associated with increased odds of patients having chronic sarcoidosis (OR = 1.82; 95% CI, 1.11-2.99, P = .019).
Because of the higher activated vitamin D levels in sarcoidosis patients, they are at risk for problems with vitamin D supplementation. I have seen two patients develop large numbers of kidney stones after receiving high-dose vitamin D. Sodhi and Aldrich reported on a cohort of 196 sarcoidosis patients who had received vitamin D and compared them with 196 control patients with sarcoidosis who were not receiving vitamin D.6 Hypercalcemia was more frequent in the group that received vitamin D (42.3%) than in the group that did not (18.3%, P < .0001). In this study, only a minority (23%) of patients receiving vitamin D had their 1,25(OH)2 vitamin D level checked.
Pearl: Lymphocyte count and 1,25(OH)2 vitamin D levels can be helpful tests in assessing sarcoidosis activity. Patients with sarcoidosis who receive vitamin D should have their 1.25(OH)2 vitamin D levels monitored.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. El Jammal et al. Sarcoidosis Vasc Diffuse Lung Dis. 2023 Sep 13;40(3):e2023031.
2. Morell F et al. Chest. 2002 Apr;121(4):1239-44.
3. Jones NP et al. Br J Ophthalmol. 2016 Oct;100(10):1393-6.
4. Rohmer J et al. Ocul Immunol Inflamm. 2020 Apr 2;28(3):341-7.
5. Kavathia D et al. Respir Med. 2010 Apr;104(4):564–70.
6. Sodhi A and Aldrich T. Am J Med Sci. 2016 Sep;352(3):252-7.
Lower-extremity lymphedema associated with more skin cancer risk
TOPLINE:
.
METHODOLOGY:
- In the retrospective cohort study, researchers reviewed reports at Mayo Clinic for all patients who had LE lymphedema, limiting the review to those who had an ICD code for lymphedema.
- 4,437 patients with the ICD code from 2000 to 2020 were compared with 4,437 matched controls.
- The records of patients with skin cancer diagnoses were reviewed manually to determine whether the skin cancer, its management, or both were a cause of lymphedema; cancers that caused secondary lymphedema were excluded.
- This is the first large-scale study evaluating the association between LE lymphedema and LE skin cancer.
TAKEAWAY:
- 211 patients (4.6%) in the LE lymphedema group had any ICD code for LE skin cancer, compared with 89 (2%) in the control group.
- Among those with LE lymphedema, the risk for skin cancer was 1.98 times greater compared with those without lymphedema (95% confidence interval, 1.43-2.74; P < .001). Cases included all types of skin cancer.
- Nineteen of 24 patients with unilateral LE lymphedema had a history of immunosuppression.
- In the group of 24 patients with unilateral LE lymphedema, the lymphedematous LE was more likely to have one or more skin cancers than were the unaffected LE (87.5% vs. 33.3%; P < .05), and skin cancer was 2.65 times more likely to develop on the affected LE than in the unaffected LE (95% CI, 1.17-5.99; P = .02).
IN PRACTICE:
“Our findings suggest the need for a relatively high degree of suspicion of skin cancer at sites with lymphedema,” senior author, Afsaneh Alavi, MD, professor of dermatology at the Mayo Clinic, said in a Mayo Clinic press release reporting the results.
SOURCE:
The study was conducted by researchers at the Mayo Clinic and Meharry Medical College, Nashville. It was published in the November 2023 Mayo Clinic Proceedings.
LIMITATIONS:
This was a single-center retrospective study, and patients with LE lymphedema may be overdiagnosed with LE skin cancer because they have a greater number of examinations.
DISCLOSURES:
Dr. Alavi reports having been a consultant for AbbVie, Boehringer Ingelheim, InflaRx, Novartis, and UCB SA and an investigator for Processa Pharmaceuticals and Boehringer Ingelheim. The other authors had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- In the retrospective cohort study, researchers reviewed reports at Mayo Clinic for all patients who had LE lymphedema, limiting the review to those who had an ICD code for lymphedema.
- 4,437 patients with the ICD code from 2000 to 2020 were compared with 4,437 matched controls.
- The records of patients with skin cancer diagnoses were reviewed manually to determine whether the skin cancer, its management, or both were a cause of lymphedema; cancers that caused secondary lymphedema were excluded.
- This is the first large-scale study evaluating the association between LE lymphedema and LE skin cancer.
TAKEAWAY:
- 211 patients (4.6%) in the LE lymphedema group had any ICD code for LE skin cancer, compared with 89 (2%) in the control group.
- Among those with LE lymphedema, the risk for skin cancer was 1.98 times greater compared with those without lymphedema (95% confidence interval, 1.43-2.74; P < .001). Cases included all types of skin cancer.
- Nineteen of 24 patients with unilateral LE lymphedema had a history of immunosuppression.
- In the group of 24 patients with unilateral LE lymphedema, the lymphedematous LE was more likely to have one or more skin cancers than were the unaffected LE (87.5% vs. 33.3%; P < .05), and skin cancer was 2.65 times more likely to develop on the affected LE than in the unaffected LE (95% CI, 1.17-5.99; P = .02).
IN PRACTICE:
“Our findings suggest the need for a relatively high degree of suspicion of skin cancer at sites with lymphedema,” senior author, Afsaneh Alavi, MD, professor of dermatology at the Mayo Clinic, said in a Mayo Clinic press release reporting the results.
SOURCE:
The study was conducted by researchers at the Mayo Clinic and Meharry Medical College, Nashville. It was published in the November 2023 Mayo Clinic Proceedings.
LIMITATIONS:
This was a single-center retrospective study, and patients with LE lymphedema may be overdiagnosed with LE skin cancer because they have a greater number of examinations.
DISCLOSURES:
Dr. Alavi reports having been a consultant for AbbVie, Boehringer Ingelheim, InflaRx, Novartis, and UCB SA and an investigator for Processa Pharmaceuticals and Boehringer Ingelheim. The other authors had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- In the retrospective cohort study, researchers reviewed reports at Mayo Clinic for all patients who had LE lymphedema, limiting the review to those who had an ICD code for lymphedema.
- 4,437 patients with the ICD code from 2000 to 2020 were compared with 4,437 matched controls.
- The records of patients with skin cancer diagnoses were reviewed manually to determine whether the skin cancer, its management, or both were a cause of lymphedema; cancers that caused secondary lymphedema were excluded.
- This is the first large-scale study evaluating the association between LE lymphedema and LE skin cancer.
TAKEAWAY:
- 211 patients (4.6%) in the LE lymphedema group had any ICD code for LE skin cancer, compared with 89 (2%) in the control group.
- Among those with LE lymphedema, the risk for skin cancer was 1.98 times greater compared with those without lymphedema (95% confidence interval, 1.43-2.74; P < .001). Cases included all types of skin cancer.
- Nineteen of 24 patients with unilateral LE lymphedema had a history of immunosuppression.
- In the group of 24 patients with unilateral LE lymphedema, the lymphedematous LE was more likely to have one or more skin cancers than were the unaffected LE (87.5% vs. 33.3%; P < .05), and skin cancer was 2.65 times more likely to develop on the affected LE than in the unaffected LE (95% CI, 1.17-5.99; P = .02).
IN PRACTICE:
“Our findings suggest the need for a relatively high degree of suspicion of skin cancer at sites with lymphedema,” senior author, Afsaneh Alavi, MD, professor of dermatology at the Mayo Clinic, said in a Mayo Clinic press release reporting the results.
SOURCE:
The study was conducted by researchers at the Mayo Clinic and Meharry Medical College, Nashville. It was published in the November 2023 Mayo Clinic Proceedings.
LIMITATIONS:
This was a single-center retrospective study, and patients with LE lymphedema may be overdiagnosed with LE skin cancer because they have a greater number of examinations.
DISCLOSURES:
Dr. Alavi reports having been a consultant for AbbVie, Boehringer Ingelheim, InflaRx, Novartis, and UCB SA and an investigator for Processa Pharmaceuticals and Boehringer Ingelheim. The other authors had no disclosures.
A version of this article first appeared on Medscape.com.
TNF blockers not associated with poorer pregnancy outcomes
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
AT ACR 2023
Actinic keratoses may predict skin cancers in older adults
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.














