User login
Autoantibody against enteric nervous system protein linked to GI dysfunction in systemic sclerosis
(SSc), new research suggests. Researchers also found that gephyrin is expressed in the patient’s enteric nervous system (ENS), which regulates gut motility.
“While there are many antibodies that are helpful in identifying patients at risk for extraintestinal complications of this disease, markers that identify patients at higher risk for gastrointestinal complications are limited. Furthermore, the biological mechanisms that cause and perpetuate the progression of gastrointestinal disease in scleroderma are not well understood, making it challenging to distinguish between patients whose gastrointestinal disease will progress from those whose GI disease will remain stable/mild,” Zsuzsanna H. McMahan, MD, MHS, told this news organization in an email. Dr. McMahan is co–first author on the study along with Subhash Kulkarni, PhD. They conducted the research with colleagues when they both worked at Johns Hopkins University in Baltimore, Md.
When asked for comment, Kimberly Lakin, MD, MS, assistant professor of medicine at Weill Cornell Medicine and a rheumatologist at Hospital for Special Surgery, New York, called the study “interesting and novel.”
“Not only did [antigephyrin antibodies] correlate with the presence of lower GI symptoms, but also higher levels of antibodies correlated with worse lower GI symptoms. This suggests that not only could this antibody be used to predict who may have constipation and potentially need more aggressive GI interventions, but it may also be useful in quantifying GI severity in systemic sclerosis, although more research is still needed,” said Dr. Lakin, who was not involved with the research.
The study was published online in Arthritis & Rheumatology.
In the cross-sectional study, researchers identified gephyrin as an autoantigen in sera from a single patient with SSc by isolating it from immunoprecipitations performed with murine myenteric plexus neuron lysates, and then characterizing it by mass spectrometry and validating it in further assays. That patient had GI dysfunction but no defined SSc-associated autoantibodies.
Dr. McMahan and colleagues then investigated the prevalence of the autoantibody by screening the sera of 188 patients with SSc who presented consecutively to the Johns Hopkins Scleroderma Center between April 2016 and August 2017, as well as 40 controls, and compared GI symptom severity between antibody-positive and antibody-negative patients with SSc.
A total of 16 (8.5%) of the 188 patients with SSc had antigephyrin antibodies, compared with none of the controls. Of these 16 patients, 4 had no other defined SSc antibodies. In the SSc cohort, severe constipation was more common in antigephyrin antibody–positive patients, compared with antibody-negative patients (46% vs. 15%). Antibody-positive patients also had higher constipation scores, and severe distension and bloating occurred in the antibody-positive group more than twice as often (54% vs. 25%).
Patients with severe constipation, distention, and bloating had higher antigephyrin antibody levels. After adjusting for confounders such as disease duration, patients with severe constipation were nearly five times as likely (odds ratio, 4.74; P = .010) to be antigephyrin antibody–positive, and patients with severe distention and bloating were nearly four times as likely (OR, 3.71; P = .027) to be antibody-positive.
Last, the authors showed via immunohistochemistry that gephyrin is expressed in the myenteric ganglia of human GI tissue.
“Gastrointestinal function is highly regulated by the ENS, so it is interesting that antibodies that target a protein expressed by ENS cells (gephyrin) were identified in patients with scleroderma who have severe lower bowel dysfunction,” said Dr. McMahan, who is associate professor in the division of rheumatology and codirector of the scleroderma program at the University of Texas Health Science Center at Houston. “Gephyrin is a key mediator of normal communications between nerves in the gut, so it is tantalizing to speculate that autoimmune-mediated disruption (e.g., an inhibitory or blocking antibody) in neural (ENS) communications in the gut might lead to impaired bowel transit and prominent constipation.”
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and other NIH grants, as well as the Scleroderma Research Foundation, Rheumatology Research Foundation, Jerome L. Greene Foundation, Martha McCrory Professorship, and Chresanthe Stauraluakis Memorial Discovery Fund. The study authors and Dr. Lakin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SSc), new research suggests. Researchers also found that gephyrin is expressed in the patient’s enteric nervous system (ENS), which regulates gut motility.
“While there are many antibodies that are helpful in identifying patients at risk for extraintestinal complications of this disease, markers that identify patients at higher risk for gastrointestinal complications are limited. Furthermore, the biological mechanisms that cause and perpetuate the progression of gastrointestinal disease in scleroderma are not well understood, making it challenging to distinguish between patients whose gastrointestinal disease will progress from those whose GI disease will remain stable/mild,” Zsuzsanna H. McMahan, MD, MHS, told this news organization in an email. Dr. McMahan is co–first author on the study along with Subhash Kulkarni, PhD. They conducted the research with colleagues when they both worked at Johns Hopkins University in Baltimore, Md.
When asked for comment, Kimberly Lakin, MD, MS, assistant professor of medicine at Weill Cornell Medicine and a rheumatologist at Hospital for Special Surgery, New York, called the study “interesting and novel.”
“Not only did [antigephyrin antibodies] correlate with the presence of lower GI symptoms, but also higher levels of antibodies correlated with worse lower GI symptoms. This suggests that not only could this antibody be used to predict who may have constipation and potentially need more aggressive GI interventions, but it may also be useful in quantifying GI severity in systemic sclerosis, although more research is still needed,” said Dr. Lakin, who was not involved with the research.
The study was published online in Arthritis & Rheumatology.
In the cross-sectional study, researchers identified gephyrin as an autoantigen in sera from a single patient with SSc by isolating it from immunoprecipitations performed with murine myenteric plexus neuron lysates, and then characterizing it by mass spectrometry and validating it in further assays. That patient had GI dysfunction but no defined SSc-associated autoantibodies.
Dr. McMahan and colleagues then investigated the prevalence of the autoantibody by screening the sera of 188 patients with SSc who presented consecutively to the Johns Hopkins Scleroderma Center between April 2016 and August 2017, as well as 40 controls, and compared GI symptom severity between antibody-positive and antibody-negative patients with SSc.
A total of 16 (8.5%) of the 188 patients with SSc had antigephyrin antibodies, compared with none of the controls. Of these 16 patients, 4 had no other defined SSc antibodies. In the SSc cohort, severe constipation was more common in antigephyrin antibody–positive patients, compared with antibody-negative patients (46% vs. 15%). Antibody-positive patients also had higher constipation scores, and severe distension and bloating occurred in the antibody-positive group more than twice as often (54% vs. 25%).
Patients with severe constipation, distention, and bloating had higher antigephyrin antibody levels. After adjusting for confounders such as disease duration, patients with severe constipation were nearly five times as likely (odds ratio, 4.74; P = .010) to be antigephyrin antibody–positive, and patients with severe distention and bloating were nearly four times as likely (OR, 3.71; P = .027) to be antibody-positive.
Last, the authors showed via immunohistochemistry that gephyrin is expressed in the myenteric ganglia of human GI tissue.
“Gastrointestinal function is highly regulated by the ENS, so it is interesting that antibodies that target a protein expressed by ENS cells (gephyrin) were identified in patients with scleroderma who have severe lower bowel dysfunction,” said Dr. McMahan, who is associate professor in the division of rheumatology and codirector of the scleroderma program at the University of Texas Health Science Center at Houston. “Gephyrin is a key mediator of normal communications between nerves in the gut, so it is tantalizing to speculate that autoimmune-mediated disruption (e.g., an inhibitory or blocking antibody) in neural (ENS) communications in the gut might lead to impaired bowel transit and prominent constipation.”
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and other NIH grants, as well as the Scleroderma Research Foundation, Rheumatology Research Foundation, Jerome L. Greene Foundation, Martha McCrory Professorship, and Chresanthe Stauraluakis Memorial Discovery Fund. The study authors and Dr. Lakin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SSc), new research suggests. Researchers also found that gephyrin is expressed in the patient’s enteric nervous system (ENS), which regulates gut motility.
“While there are many antibodies that are helpful in identifying patients at risk for extraintestinal complications of this disease, markers that identify patients at higher risk for gastrointestinal complications are limited. Furthermore, the biological mechanisms that cause and perpetuate the progression of gastrointestinal disease in scleroderma are not well understood, making it challenging to distinguish between patients whose gastrointestinal disease will progress from those whose GI disease will remain stable/mild,” Zsuzsanna H. McMahan, MD, MHS, told this news organization in an email. Dr. McMahan is co–first author on the study along with Subhash Kulkarni, PhD. They conducted the research with colleagues when they both worked at Johns Hopkins University in Baltimore, Md.
When asked for comment, Kimberly Lakin, MD, MS, assistant professor of medicine at Weill Cornell Medicine and a rheumatologist at Hospital for Special Surgery, New York, called the study “interesting and novel.”
“Not only did [antigephyrin antibodies] correlate with the presence of lower GI symptoms, but also higher levels of antibodies correlated with worse lower GI symptoms. This suggests that not only could this antibody be used to predict who may have constipation and potentially need more aggressive GI interventions, but it may also be useful in quantifying GI severity in systemic sclerosis, although more research is still needed,” said Dr. Lakin, who was not involved with the research.
The study was published online in Arthritis & Rheumatology.
In the cross-sectional study, researchers identified gephyrin as an autoantigen in sera from a single patient with SSc by isolating it from immunoprecipitations performed with murine myenteric plexus neuron lysates, and then characterizing it by mass spectrometry and validating it in further assays. That patient had GI dysfunction but no defined SSc-associated autoantibodies.
Dr. McMahan and colleagues then investigated the prevalence of the autoantibody by screening the sera of 188 patients with SSc who presented consecutively to the Johns Hopkins Scleroderma Center between April 2016 and August 2017, as well as 40 controls, and compared GI symptom severity between antibody-positive and antibody-negative patients with SSc.
A total of 16 (8.5%) of the 188 patients with SSc had antigephyrin antibodies, compared with none of the controls. Of these 16 patients, 4 had no other defined SSc antibodies. In the SSc cohort, severe constipation was more common in antigephyrin antibody–positive patients, compared with antibody-negative patients (46% vs. 15%). Antibody-positive patients also had higher constipation scores, and severe distension and bloating occurred in the antibody-positive group more than twice as often (54% vs. 25%).
Patients with severe constipation, distention, and bloating had higher antigephyrin antibody levels. After adjusting for confounders such as disease duration, patients with severe constipation were nearly five times as likely (odds ratio, 4.74; P = .010) to be antigephyrin antibody–positive, and patients with severe distention and bloating were nearly four times as likely (OR, 3.71; P = .027) to be antibody-positive.
Last, the authors showed via immunohistochemistry that gephyrin is expressed in the myenteric ganglia of human GI tissue.
“Gastrointestinal function is highly regulated by the ENS, so it is interesting that antibodies that target a protein expressed by ENS cells (gephyrin) were identified in patients with scleroderma who have severe lower bowel dysfunction,” said Dr. McMahan, who is associate professor in the division of rheumatology and codirector of the scleroderma program at the University of Texas Health Science Center at Houston. “Gephyrin is a key mediator of normal communications between nerves in the gut, so it is tantalizing to speculate that autoimmune-mediated disruption (e.g., an inhibitory or blocking antibody) in neural (ENS) communications in the gut might lead to impaired bowel transit and prominent constipation.”
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and other NIH grants, as well as the Scleroderma Research Foundation, Rheumatology Research Foundation, Jerome L. Greene Foundation, Martha McCrory Professorship, and Chresanthe Stauraluakis Memorial Discovery Fund. The study authors and Dr. Lakin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
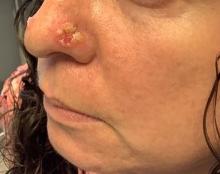
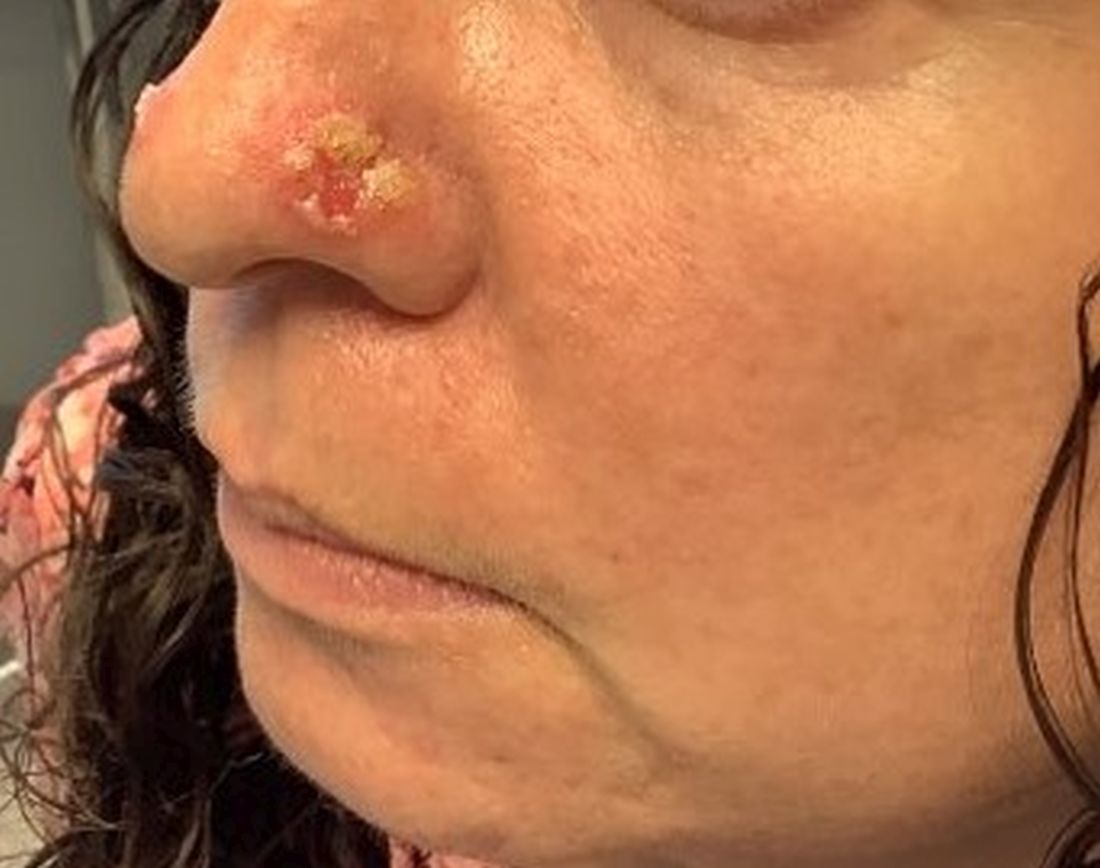
A 45-year-old White woman with no significant medical history presented with a 1-month history of lesions on the nose and right cheek
Cultures for bacteria, varicella zoster virus, herpes simplex virus, and mpox virus were all negative. A biopsy revealed suprabasilar acantholysis with follicular involvement in association with blister formation and inflammation. Direct immunofluorescence was positive for suprabasilar IgG and C3 deposition, consistent with pemphigus vulgaris (PV).
. There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, flaccid blistering lesions are present that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions may involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
Treatment is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid-sparing agent such as mycophenolate mofetil. Other steroid-sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
This case and the photos are from Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Cultures for bacteria, varicella zoster virus, herpes simplex virus, and mpox virus were all negative. A biopsy revealed suprabasilar acantholysis with follicular involvement in association with blister formation and inflammation. Direct immunofluorescence was positive for suprabasilar IgG and C3 deposition, consistent with pemphigus vulgaris (PV).
. There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, flaccid blistering lesions are present that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions may involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
Treatment is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid-sparing agent such as mycophenolate mofetil. Other steroid-sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
This case and the photos are from Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Cultures for bacteria, varicella zoster virus, herpes simplex virus, and mpox virus were all negative. A biopsy revealed suprabasilar acantholysis with follicular involvement in association with blister formation and inflammation. Direct immunofluorescence was positive for suprabasilar IgG and C3 deposition, consistent with pemphigus vulgaris (PV).
. There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, flaccid blistering lesions are present that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions may involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
Treatment is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid-sparing agent such as mycophenolate mofetil. Other steroid-sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
This case and the photos are from Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Black women weigh emerging risks of ‘creamy crack’ hair straighteners
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Many users of skin-lightening product unaware of risks
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
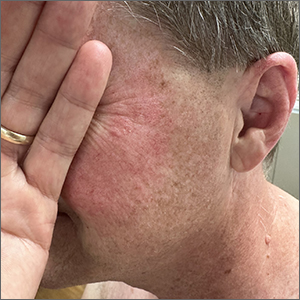
Rosacea look-alike
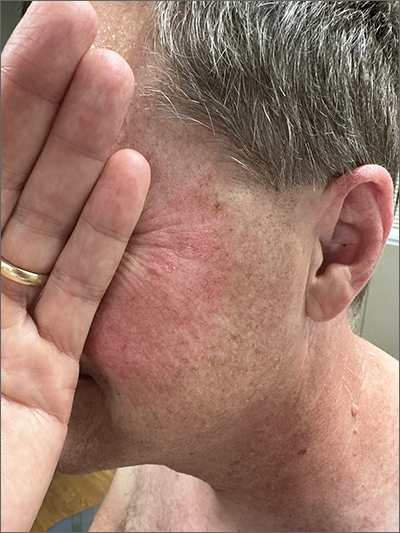
Although it’s easy to jump to the conclusion that facial erythema is rosacea, there are multiple other conditions that can lead to reddening of the face. In this case, excessive sun exposure had resulted in a diffuse actinic change of the malar and lateral aspects of this patient’s face. The palpably rough lesions were actinic keratoses.
Actinic keratoses are caused by exposure to ultraviolet radiation. These lesions are premalignant and common. Areas of the body at greatest risk include those not typically covered by clothing (eg, face, hands, arms, ears, forehead, and top of the scalp—especially in individuals with hair loss). There is a range of estimates regarding the percentage of actinic keratoses that will progress to squamous cell carcinoma in situ, and then invasive squamous cell carcinoma. One study determined that 10% of actinic keratoses progress to squamous cell carcinoma over the course of 2 years.1
In patients with broad areas of multiple clinically palpable lesions with rough sandpapery texture or visible white scale, there are likely preclinical lesions in the same areas. With so many lesions, field therapy of the entire region is often performed instead of treating the lesions 1 at a time.
There are multiple topical agents for field therapy, including 5-fluorouracil, diclofenac gel, and imiquimod gel.2 Since significant erythema and inflammation usually follow application of the topical agent, clinicians may want to have patients treat in segments to make the process more tolerable.
5-fluorouracil has a complete clearance rate (CCR) of 75% to 90% and is usually applied twice daily for 2 weeks, although there are multiple different protocols. Diclofenac has a CCR of 58% over a 60- to 90-day course, and imiquimod has a CCR of 54% after a 120-day course. Photodynamic therapy (PDT) has the advantage of a single treatment but a CCR of 38%. PDT may be advantageous for a patient who has difficulty applying topical medication over a period of weeks.
Niacinamide has been shown to help with skin repair and reduce the risk of additional nonmelanoma skin cancers (NMSC) by 23% and additional actinic keratoses by about 15% in individuals with a history of actinic keratoses or NMSC.3 In contrast to niacin, niacinamide does not cause flushing. Niacinamide is used long term; if discontinued, it no longer confers benefit in helping the skin repair itself.
The patient in this case was prescribed topical 5% fluorouracil cream to be applied twice daily to the malar regions bilaterally for 2 weeks and, if not inflamed by 2 weeks, to extend the treatment until there is robust inflammation (but not to exceed 3 weeks). He was scheduled to follow up in 3 months for reexamination. He was also advised to start taking niacinamide 500 mg twice daily to reduce his risk of additional precancerous and cancerous skin lesions and counseled on the importance of sunscreen, hats, and sun-protective clothing.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099-1101. doi: 10.1111/j.1524-4725.2007.33224.x
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
- Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.

Although it’s easy to jump to the conclusion that facial erythema is rosacea, there are multiple other conditions that can lead to reddening of the face. In this case, excessive sun exposure had resulted in a diffuse actinic change of the malar and lateral aspects of this patient’s face. The palpably rough lesions were actinic keratoses.
Actinic keratoses are caused by exposure to ultraviolet radiation. These lesions are premalignant and common. Areas of the body at greatest risk include those not typically covered by clothing (eg, face, hands, arms, ears, forehead, and top of the scalp—especially in individuals with hair loss). There is a range of estimates regarding the percentage of actinic keratoses that will progress to squamous cell carcinoma in situ, and then invasive squamous cell carcinoma. One study determined that 10% of actinic keratoses progress to squamous cell carcinoma over the course of 2 years.1
In patients with broad areas of multiple clinically palpable lesions with rough sandpapery texture or visible white scale, there are likely preclinical lesions in the same areas. With so many lesions, field therapy of the entire region is often performed instead of treating the lesions 1 at a time.
There are multiple topical agents for field therapy, including 5-fluorouracil, diclofenac gel, and imiquimod gel.2 Since significant erythema and inflammation usually follow application of the topical agent, clinicians may want to have patients treat in segments to make the process more tolerable.
5-fluorouracil has a complete clearance rate (CCR) of 75% to 90% and is usually applied twice daily for 2 weeks, although there are multiple different protocols. Diclofenac has a CCR of 58% over a 60- to 90-day course, and imiquimod has a CCR of 54% after a 120-day course. Photodynamic therapy (PDT) has the advantage of a single treatment but a CCR of 38%. PDT may be advantageous for a patient who has difficulty applying topical medication over a period of weeks.
Niacinamide has been shown to help with skin repair and reduce the risk of additional nonmelanoma skin cancers (NMSC) by 23% and additional actinic keratoses by about 15% in individuals with a history of actinic keratoses or NMSC.3 In contrast to niacin, niacinamide does not cause flushing. Niacinamide is used long term; if discontinued, it no longer confers benefit in helping the skin repair itself.
The patient in this case was prescribed topical 5% fluorouracil cream to be applied twice daily to the malar regions bilaterally for 2 weeks and, if not inflamed by 2 weeks, to extend the treatment until there is robust inflammation (but not to exceed 3 weeks). He was scheduled to follow up in 3 months for reexamination. He was also advised to start taking niacinamide 500 mg twice daily to reduce his risk of additional precancerous and cancerous skin lesions and counseled on the importance of sunscreen, hats, and sun-protective clothing.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Although it’s easy to jump to the conclusion that facial erythema is rosacea, there are multiple other conditions that can lead to reddening of the face. In this case, excessive sun exposure had resulted in a diffuse actinic change of the malar and lateral aspects of this patient’s face. The palpably rough lesions were actinic keratoses.
Actinic keratoses are caused by exposure to ultraviolet radiation. These lesions are premalignant and common. Areas of the body at greatest risk include those not typically covered by clothing (eg, face, hands, arms, ears, forehead, and top of the scalp—especially in individuals with hair loss). There is a range of estimates regarding the percentage of actinic keratoses that will progress to squamous cell carcinoma in situ, and then invasive squamous cell carcinoma. One study determined that 10% of actinic keratoses progress to squamous cell carcinoma over the course of 2 years.1
In patients with broad areas of multiple clinically palpable lesions with rough sandpapery texture or visible white scale, there are likely preclinical lesions in the same areas. With so many lesions, field therapy of the entire region is often performed instead of treating the lesions 1 at a time.
There are multiple topical agents for field therapy, including 5-fluorouracil, diclofenac gel, and imiquimod gel.2 Since significant erythema and inflammation usually follow application of the topical agent, clinicians may want to have patients treat in segments to make the process more tolerable.
5-fluorouracil has a complete clearance rate (CCR) of 75% to 90% and is usually applied twice daily for 2 weeks, although there are multiple different protocols. Diclofenac has a CCR of 58% over a 60- to 90-day course, and imiquimod has a CCR of 54% after a 120-day course. Photodynamic therapy (PDT) has the advantage of a single treatment but a CCR of 38%. PDT may be advantageous for a patient who has difficulty applying topical medication over a period of weeks.
Niacinamide has been shown to help with skin repair and reduce the risk of additional nonmelanoma skin cancers (NMSC) by 23% and additional actinic keratoses by about 15% in individuals with a history of actinic keratoses or NMSC.3 In contrast to niacin, niacinamide does not cause flushing. Niacinamide is used long term; if discontinued, it no longer confers benefit in helping the skin repair itself.
The patient in this case was prescribed topical 5% fluorouracil cream to be applied twice daily to the malar regions bilaterally for 2 weeks and, if not inflamed by 2 weeks, to extend the treatment until there is robust inflammation (but not to exceed 3 weeks). He was scheduled to follow up in 3 months for reexamination. He was also advised to start taking niacinamide 500 mg twice daily to reduce his risk of additional precancerous and cancerous skin lesions and counseled on the importance of sunscreen, hats, and sun-protective clothing.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099-1101. doi: 10.1111/j.1524-4725.2007.33224.x
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
- Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099-1101. doi: 10.1111/j.1524-4725.2007.33224.x
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
- Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
Skin reactions common at insulin pump infusion sites
new research suggests.
Insulin pump use is increasingly common, but many patients experience infusion-site failure that in some cases leads to discontinuation. In a novel investigation, researchers at the University of Washington, Seattle, used biopsies and noninvasive imaging to compare insulin pump sites with control sites in 30 patients. Several differences were found at pump sites in comparison with control sites, including fibrosis, inflammation, eosinophils, and increased vessel density.
“These findings support allergic sensitization as a potentially common reaction at [insulin pump] sites. The leading candidates causing this include insulin preservatives, plastic materials, and adhesive glue used in device manufacturing,” wrote Andrea Kalus, MD, of the university’s dermatology division, and colleagues. The findings were published recently in Diabetes Care.
The inflammatory response, they wrote, “may result in tissue changes responsible for the infusion-site failures seen frequently in clinical practice.”
Such infusion site problems represent an “Achilles heel” of these otherwise highly beneficial devices, lead author Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology, and nutrition, said in a statement. “It doesn’t really matter how good the technology is. We still don’t understand what is happening with the infusion sites, much less to [be able to] fix it.”
Significant differences between pump and nonpump sites
In the cross-sectional study, Dr. Kalus and colleagues used noninvasive optical coherence tomography (OCT) immediately prior to performing punch biopsies at three sites: the site currently in active use, the “recovery site” used 3-5 days prior to the procedures, and control sites never used for pump infusion. Punch biopsies were also performed at those sites.
The mean age of the patients was 48.3 years, the mean diabetes duration was 30.4 years, and the mean duration of pump use was 15.8 years. Nearly all patients (93.3%) reported itchiness at the site, and 76.7% reported skin redness.
Of the 25 patients for whom OCT imaging was successful, statistical analysis showed significant differences in vascular area density and the optical attenuation coefficient, a surrogate for skin inflammation, between the pump and control sites and between recovery sites and current pump sites. The greater vessel density is likely a result of injury and repair related to catheter insertion, the authors said.
In the biopsy samples, both current and recovery sites showed increased fibrosis, fibrin, inflammation, fat necrosis, vascularity, and eosinophils, compared with the control sites, but no significant differences were found between current and recovery sites.
Eosinophils: ‘The most surprising histologic finding’
Eosinophils were found in 73% of skin biopsy specimens from current sites and in 75% of specimens from recovery sites, compared with none from the control sites (for both, P < .01). In all study participants, eosinophils were found in at least one current and/or recovery infusion site deep in the dermis near the interface with fat. The number of eosinophils ranged from 0 to 31 per high-power field, with a median of 4.
The number of eosinophils didn’t vary by type of insulin or brand of pump, but higher counts were seen in those who had used pumps for less than 10 years, compared with more than 20 years (P = .02).
The prevalence and degree of eosinophils were “the most surprising histologic finding,” the authors wrote, adding that “eosinophils are not typically present as a component of resident inflammatory cells in the skin.”
While eosinophils may be present in normal wound healing, “the absolute number and density of eosinophil in these samples support a delayed-type hypersensitivity response, which is typically observed between 2 and 7 days after exposure to an allergen. ... Eosinophils are often correlated with symptoms of itchiness and likely explain the high percentage of participants who reported itchiness in this study,” Dr. Kalus and colleagues wrote.
Correlation found between inflammation and glycemic control
All participants used the Dexcom G6 continuous glucose monitor as part of their usual care. Inflammation scores were positively correlated with insulin dose (P = .009) and were negatively correlated with time in range (P = .01).
No other OCT or biopsy findings differed by duration of pump use, previous use of animal insulin, or type of insulin.
The reason for these findings is unclear, Dr. Hirsch said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives, or is this because of the insulin pump itself? All these questions need to be answered in future studies. ... The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dr. Hirsch reported grants and contracts from Insulet, Medtronic, and Dexcom outside the submitted work; consulting fees from Abbott Diabetes Care, Lifescan, and Hagar outside the submitted work; and honoraria for lectures, presentations, participation on speaker’s bureaus, manuscript writing, or educational events as section editor for UpToDate outside the submitted work. Dr. Kalus has no disclosures.
A version of this article first appeared on Medscape.com.
new research suggests.
Insulin pump use is increasingly common, but many patients experience infusion-site failure that in some cases leads to discontinuation. In a novel investigation, researchers at the University of Washington, Seattle, used biopsies and noninvasive imaging to compare insulin pump sites with control sites in 30 patients. Several differences were found at pump sites in comparison with control sites, including fibrosis, inflammation, eosinophils, and increased vessel density.
“These findings support allergic sensitization as a potentially common reaction at [insulin pump] sites. The leading candidates causing this include insulin preservatives, plastic materials, and adhesive glue used in device manufacturing,” wrote Andrea Kalus, MD, of the university’s dermatology division, and colleagues. The findings were published recently in Diabetes Care.
The inflammatory response, they wrote, “may result in tissue changes responsible for the infusion-site failures seen frequently in clinical practice.”
Such infusion site problems represent an “Achilles heel” of these otherwise highly beneficial devices, lead author Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology, and nutrition, said in a statement. “It doesn’t really matter how good the technology is. We still don’t understand what is happening with the infusion sites, much less to [be able to] fix it.”
Significant differences between pump and nonpump sites
In the cross-sectional study, Dr. Kalus and colleagues used noninvasive optical coherence tomography (OCT) immediately prior to performing punch biopsies at three sites: the site currently in active use, the “recovery site” used 3-5 days prior to the procedures, and control sites never used for pump infusion. Punch biopsies were also performed at those sites.
The mean age of the patients was 48.3 years, the mean diabetes duration was 30.4 years, and the mean duration of pump use was 15.8 years. Nearly all patients (93.3%) reported itchiness at the site, and 76.7% reported skin redness.
Of the 25 patients for whom OCT imaging was successful, statistical analysis showed significant differences in vascular area density and the optical attenuation coefficient, a surrogate for skin inflammation, between the pump and control sites and between recovery sites and current pump sites. The greater vessel density is likely a result of injury and repair related to catheter insertion, the authors said.
In the biopsy samples, both current and recovery sites showed increased fibrosis, fibrin, inflammation, fat necrosis, vascularity, and eosinophils, compared with the control sites, but no significant differences were found between current and recovery sites.
Eosinophils: ‘The most surprising histologic finding’
Eosinophils were found in 73% of skin biopsy specimens from current sites and in 75% of specimens from recovery sites, compared with none from the control sites (for both, P < .01). In all study participants, eosinophils were found in at least one current and/or recovery infusion site deep in the dermis near the interface with fat. The number of eosinophils ranged from 0 to 31 per high-power field, with a median of 4.
The number of eosinophils didn’t vary by type of insulin or brand of pump, but higher counts were seen in those who had used pumps for less than 10 years, compared with more than 20 years (P = .02).
The prevalence and degree of eosinophils were “the most surprising histologic finding,” the authors wrote, adding that “eosinophils are not typically present as a component of resident inflammatory cells in the skin.”
While eosinophils may be present in normal wound healing, “the absolute number and density of eosinophil in these samples support a delayed-type hypersensitivity response, which is typically observed between 2 and 7 days after exposure to an allergen. ... Eosinophils are often correlated with symptoms of itchiness and likely explain the high percentage of participants who reported itchiness in this study,” Dr. Kalus and colleagues wrote.
Correlation found between inflammation and glycemic control
All participants used the Dexcom G6 continuous glucose monitor as part of their usual care. Inflammation scores were positively correlated with insulin dose (P = .009) and were negatively correlated with time in range (P = .01).
No other OCT or biopsy findings differed by duration of pump use, previous use of animal insulin, or type of insulin.
The reason for these findings is unclear, Dr. Hirsch said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives, or is this because of the insulin pump itself? All these questions need to be answered in future studies. ... The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dr. Hirsch reported grants and contracts from Insulet, Medtronic, and Dexcom outside the submitted work; consulting fees from Abbott Diabetes Care, Lifescan, and Hagar outside the submitted work; and honoraria for lectures, presentations, participation on speaker’s bureaus, manuscript writing, or educational events as section editor for UpToDate outside the submitted work. Dr. Kalus has no disclosures.
A version of this article first appeared on Medscape.com.
new research suggests.
Insulin pump use is increasingly common, but many patients experience infusion-site failure that in some cases leads to discontinuation. In a novel investigation, researchers at the University of Washington, Seattle, used biopsies and noninvasive imaging to compare insulin pump sites with control sites in 30 patients. Several differences were found at pump sites in comparison with control sites, including fibrosis, inflammation, eosinophils, and increased vessel density.
“These findings support allergic sensitization as a potentially common reaction at [insulin pump] sites. The leading candidates causing this include insulin preservatives, plastic materials, and adhesive glue used in device manufacturing,” wrote Andrea Kalus, MD, of the university’s dermatology division, and colleagues. The findings were published recently in Diabetes Care.
The inflammatory response, they wrote, “may result in tissue changes responsible for the infusion-site failures seen frequently in clinical practice.”
Such infusion site problems represent an “Achilles heel” of these otherwise highly beneficial devices, lead author Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology, and nutrition, said in a statement. “It doesn’t really matter how good the technology is. We still don’t understand what is happening with the infusion sites, much less to [be able to] fix it.”
Significant differences between pump and nonpump sites
In the cross-sectional study, Dr. Kalus and colleagues used noninvasive optical coherence tomography (OCT) immediately prior to performing punch biopsies at three sites: the site currently in active use, the “recovery site” used 3-5 days prior to the procedures, and control sites never used for pump infusion. Punch biopsies were also performed at those sites.
The mean age of the patients was 48.3 years, the mean diabetes duration was 30.4 years, and the mean duration of pump use was 15.8 years. Nearly all patients (93.3%) reported itchiness at the site, and 76.7% reported skin redness.
Of the 25 patients for whom OCT imaging was successful, statistical analysis showed significant differences in vascular area density and the optical attenuation coefficient, a surrogate for skin inflammation, between the pump and control sites and between recovery sites and current pump sites. The greater vessel density is likely a result of injury and repair related to catheter insertion, the authors said.
In the biopsy samples, both current and recovery sites showed increased fibrosis, fibrin, inflammation, fat necrosis, vascularity, and eosinophils, compared with the control sites, but no significant differences were found between current and recovery sites.
Eosinophils: ‘The most surprising histologic finding’
Eosinophils were found in 73% of skin biopsy specimens from current sites and in 75% of specimens from recovery sites, compared with none from the control sites (for both, P < .01). In all study participants, eosinophils were found in at least one current and/or recovery infusion site deep in the dermis near the interface with fat. The number of eosinophils ranged from 0 to 31 per high-power field, with a median of 4.
The number of eosinophils didn’t vary by type of insulin or brand of pump, but higher counts were seen in those who had used pumps for less than 10 years, compared with more than 20 years (P = .02).
The prevalence and degree of eosinophils were “the most surprising histologic finding,” the authors wrote, adding that “eosinophils are not typically present as a component of resident inflammatory cells in the skin.”
While eosinophils may be present in normal wound healing, “the absolute number and density of eosinophil in these samples support a delayed-type hypersensitivity response, which is typically observed between 2 and 7 days after exposure to an allergen. ... Eosinophils are often correlated with symptoms of itchiness and likely explain the high percentage of participants who reported itchiness in this study,” Dr. Kalus and colleagues wrote.
Correlation found between inflammation and glycemic control
All participants used the Dexcom G6 continuous glucose monitor as part of their usual care. Inflammation scores were positively correlated with insulin dose (P = .009) and were negatively correlated with time in range (P = .01).
No other OCT or biopsy findings differed by duration of pump use, previous use of animal insulin, or type of insulin.
The reason for these findings is unclear, Dr. Hirsch said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives, or is this because of the insulin pump itself? All these questions need to be answered in future studies. ... The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dr. Hirsch reported grants and contracts from Insulet, Medtronic, and Dexcom outside the submitted work; consulting fees from Abbott Diabetes Care, Lifescan, and Hagar outside the submitted work; and honoraria for lectures, presentations, participation on speaker’s bureaus, manuscript writing, or educational events as section editor for UpToDate outside the submitted work. Dr. Kalus has no disclosures.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Roflumilast cream appears safe, effective for children with psoriasis, researchers report
In patients aged 2-11 years, roflumilast cream was well tolerated and improved signs and symptoms of psoriasis over 4 weeks, according to results from a pair of phase two studies.
“Limited topical treatments are approved for children younger than 12 years old with psoriasis,” researchers led by Adelaide A. Hebert, MD, wrote in their abstract. The results were presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
Roflumilast cream 0.3% (Zoryve) is a once-daily, topical nonsteroidal treatment from Arcutis Biotherapeutics. A phosphodiesterase-4 inhibitor, it was approved by the Food and Drug Administration in 2022 for mild, moderate and severe psoriasis in individuals aged 12 and older, including intertriginous psoriasis.
For the analysis, Dr. Hebert, chief of pediatric dermatology at the University of Texas, Houston, and colleagues conducted two 4-week, phase 2, open-label safety studies of roflumilast cream 0.3%.
One, study 216, enrolled 10 children aged 2-5, and all but one were Black. The other, study 215, enrolled 20 children aged 6-11, and half were Black and nearly half were White. At baseline, patients had 2% or greater body surface area (BSA) involvement and an Investigator Global Assessment (IGA) score of at least mild.
Caregivers applied roflumilast cream to all affected areas once daily for 28 days. The researchers collected pharmacokinetic samples at week 2 and week 4. The primary endpoints were pharmacokinetic, safety, and tolerability.
Efficacy was evaluated as exploratory endpoints: An IGA of clear or almost clear plus a 2-grade or more improvement from baseline, a 50% or greater improvement and a 75% or greater improvement on the Psoriasis Area and Severity Index (PASI-50 and PASI-75), a 4-point or greater reduction in the Worst Itch–Numeric Rating Scale (WI-NRS) in patients with a baseline score of 4 or greater, a mean change from baseline in BSA, and improvement in the Children’s Dermatology Life Quality Index (CDLQI).
At baseline, the mean BSA was similar for patients enrolled in studies 216 and 215 (9.6% and 8.8%, respectively), and 80% of all patients had baseline IGA of moderate. By week 2, the mean roflumilast and N-oxide predose plasma concentrations among patients in the younger group were 2.15 and 22.4 ng/mL, compared with 3.15 and 28.9 ng/mL among those in the older group. At week 4, the mean roflumilast and N-oxide predose concentrations were 2.04 and 15.8 ng/mL in the younger group (study 216), compared with 1.68 and 15.7 ng/mL in the older group (study 215).
As for efficacy, 90% and 40% of patients in studies 216 and 215 achieved IGA success at week 4, respectively, while 90% and 50% achieved PASI-75, 90% and 40% achieved WI-NRS success, and the mean BSA reductions at week 4 were 79.1% and 44.4%. Meanwhile, one younger patient in study 216 reported a treatment-emergent adverse event (TEAE) of headache, which was considered mild, while four older patients in study 215 reported 8 TEAEs, which were considered mild and ranged from back pain to nasal congestion.
“The rapid onset of action was surprising but exceedingly rewarding for the subjects enrolled in the study,” Dr. Hebert told this news organization after the meeting. “The PASI scores and itch scores were markedly improved at the end of the 4-week clinical trial. Patient and parents alike were pleased to use a steroid-free option with once-daily application and rapid onset of action to help control plaque psoriasis.”
In the poster abstract, she and her coauthors concluded that “under maximal use conditions in children aged 2-11 years, roflumilast cream 0.3% was well tolerated and improved signs and symptoms of psoriasis with measured improvements in IGA score, PASI score, BSA involvement, CDLQI, and WI-NRS. Overall, pharmacokinetics, safety, tolerability, and efficacy in patients aged 2-11 years were consistent with prior results in adults and adolescents.”
The study was funded by Arcutis Biotherapeutics. Dr. Hebert reported that she is an investigator for Arcutis. About half the coauthors are employees of Arcutis, and the other half disclosed grants, research funding and/or honoraria from the company. Research grants from the company for this study were paid to the McGovern Medical School at the University of Texas.
In patients aged 2-11 years, roflumilast cream was well tolerated and improved signs and symptoms of psoriasis over 4 weeks, according to results from a pair of phase two studies.
“Limited topical treatments are approved for children younger than 12 years old with psoriasis,” researchers led by Adelaide A. Hebert, MD, wrote in their abstract. The results were presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
Roflumilast cream 0.3% (Zoryve) is a once-daily, topical nonsteroidal treatment from Arcutis Biotherapeutics. A phosphodiesterase-4 inhibitor, it was approved by the Food and Drug Administration in 2022 for mild, moderate and severe psoriasis in individuals aged 12 and older, including intertriginous psoriasis.
For the analysis, Dr. Hebert, chief of pediatric dermatology at the University of Texas, Houston, and colleagues conducted two 4-week, phase 2, open-label safety studies of roflumilast cream 0.3%.
One, study 216, enrolled 10 children aged 2-5, and all but one were Black. The other, study 215, enrolled 20 children aged 6-11, and half were Black and nearly half were White. At baseline, patients had 2% or greater body surface area (BSA) involvement and an Investigator Global Assessment (IGA) score of at least mild.
Caregivers applied roflumilast cream to all affected areas once daily for 28 days. The researchers collected pharmacokinetic samples at week 2 and week 4. The primary endpoints were pharmacokinetic, safety, and tolerability.
Efficacy was evaluated as exploratory endpoints: An IGA of clear or almost clear plus a 2-grade or more improvement from baseline, a 50% or greater improvement and a 75% or greater improvement on the Psoriasis Area and Severity Index (PASI-50 and PASI-75), a 4-point or greater reduction in the Worst Itch–Numeric Rating Scale (WI-NRS) in patients with a baseline score of 4 or greater, a mean change from baseline in BSA, and improvement in the Children’s Dermatology Life Quality Index (CDLQI).
At baseline, the mean BSA was similar for patients enrolled in studies 216 and 215 (9.6% and 8.8%, respectively), and 80% of all patients had baseline IGA of moderate. By week 2, the mean roflumilast and N-oxide predose plasma concentrations among patients in the younger group were 2.15 and 22.4 ng/mL, compared with 3.15 and 28.9 ng/mL among those in the older group. At week 4, the mean roflumilast and N-oxide predose concentrations were 2.04 and 15.8 ng/mL in the younger group (study 216), compared with 1.68 and 15.7 ng/mL in the older group (study 215).
As for efficacy, 90% and 40% of patients in studies 216 and 215 achieved IGA success at week 4, respectively, while 90% and 50% achieved PASI-75, 90% and 40% achieved WI-NRS success, and the mean BSA reductions at week 4 were 79.1% and 44.4%. Meanwhile, one younger patient in study 216 reported a treatment-emergent adverse event (TEAE) of headache, which was considered mild, while four older patients in study 215 reported 8 TEAEs, which were considered mild and ranged from back pain to nasal congestion.
“The rapid onset of action was surprising but exceedingly rewarding for the subjects enrolled in the study,” Dr. Hebert told this news organization after the meeting. “The PASI scores and itch scores were markedly improved at the end of the 4-week clinical trial. Patient and parents alike were pleased to use a steroid-free option with once-daily application and rapid onset of action to help control plaque psoriasis.”
In the poster abstract, she and her coauthors concluded that “under maximal use conditions in children aged 2-11 years, roflumilast cream 0.3% was well tolerated and improved signs and symptoms of psoriasis with measured improvements in IGA score, PASI score, BSA involvement, CDLQI, and WI-NRS. Overall, pharmacokinetics, safety, tolerability, and efficacy in patients aged 2-11 years were consistent with prior results in adults and adolescents.”
The study was funded by Arcutis Biotherapeutics. Dr. Hebert reported that she is an investigator for Arcutis. About half the coauthors are employees of Arcutis, and the other half disclosed grants, research funding and/or honoraria from the company. Research grants from the company for this study were paid to the McGovern Medical School at the University of Texas.
In patients aged 2-11 years, roflumilast cream was well tolerated and improved signs and symptoms of psoriasis over 4 weeks, according to results from a pair of phase two studies.
“Limited topical treatments are approved for children younger than 12 years old with psoriasis,” researchers led by Adelaide A. Hebert, MD, wrote in their abstract. The results were presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
Roflumilast cream 0.3% (Zoryve) is a once-daily, topical nonsteroidal treatment from Arcutis Biotherapeutics. A phosphodiesterase-4 inhibitor, it was approved by the Food and Drug Administration in 2022 for mild, moderate and severe psoriasis in individuals aged 12 and older, including intertriginous psoriasis.
For the analysis, Dr. Hebert, chief of pediatric dermatology at the University of Texas, Houston, and colleagues conducted two 4-week, phase 2, open-label safety studies of roflumilast cream 0.3%.
One, study 216, enrolled 10 children aged 2-5, and all but one were Black. The other, study 215, enrolled 20 children aged 6-11, and half were Black and nearly half were White. At baseline, patients had 2% or greater body surface area (BSA) involvement and an Investigator Global Assessment (IGA) score of at least mild.
Caregivers applied roflumilast cream to all affected areas once daily for 28 days. The researchers collected pharmacokinetic samples at week 2 and week 4. The primary endpoints were pharmacokinetic, safety, and tolerability.
Efficacy was evaluated as exploratory endpoints: An IGA of clear or almost clear plus a 2-grade or more improvement from baseline, a 50% or greater improvement and a 75% or greater improvement on the Psoriasis Area and Severity Index (PASI-50 and PASI-75), a 4-point or greater reduction in the Worst Itch–Numeric Rating Scale (WI-NRS) in patients with a baseline score of 4 or greater, a mean change from baseline in BSA, and improvement in the Children’s Dermatology Life Quality Index (CDLQI).
At baseline, the mean BSA was similar for patients enrolled in studies 216 and 215 (9.6% and 8.8%, respectively), and 80% of all patients had baseline IGA of moderate. By week 2, the mean roflumilast and N-oxide predose plasma concentrations among patients in the younger group were 2.15 and 22.4 ng/mL, compared with 3.15 and 28.9 ng/mL among those in the older group. At week 4, the mean roflumilast and N-oxide predose concentrations were 2.04 and 15.8 ng/mL in the younger group (study 216), compared with 1.68 and 15.7 ng/mL in the older group (study 215).
As for efficacy, 90% and 40% of patients in studies 216 and 215 achieved IGA success at week 4, respectively, while 90% and 50% achieved PASI-75, 90% and 40% achieved WI-NRS success, and the mean BSA reductions at week 4 were 79.1% and 44.4%. Meanwhile, one younger patient in study 216 reported a treatment-emergent adverse event (TEAE) of headache, which was considered mild, while four older patients in study 215 reported 8 TEAEs, which were considered mild and ranged from back pain to nasal congestion.
“The rapid onset of action was surprising but exceedingly rewarding for the subjects enrolled in the study,” Dr. Hebert told this news organization after the meeting. “The PASI scores and itch scores were markedly improved at the end of the 4-week clinical trial. Patient and parents alike were pleased to use a steroid-free option with once-daily application and rapid onset of action to help control plaque psoriasis.”
In the poster abstract, she and her coauthors concluded that “under maximal use conditions in children aged 2-11 years, roflumilast cream 0.3% was well tolerated and improved signs and symptoms of psoriasis with measured improvements in IGA score, PASI score, BSA involvement, CDLQI, and WI-NRS. Overall, pharmacokinetics, safety, tolerability, and efficacy in patients aged 2-11 years were consistent with prior results in adults and adolescents.”
The study was funded by Arcutis Biotherapeutics. Dr. Hebert reported that she is an investigator for Arcutis. About half the coauthors are employees of Arcutis, and the other half disclosed grants, research funding and/or honoraria from the company. Research grants from the company for this study were paid to the McGovern Medical School at the University of Texas.
FROM SPD 2023
Are AI-powered skin-check tools on the horizon for dermatologists, PCPs?
.
Given that about 6.3 billion smartphones would soon be in use, this AI approach could provide a gateway for “low-cost universal access to vital diagnostic care,” wrote Justin M. Ko, MD, MBA, a dermatologist, and colleagues from Stanford (Calif.) University that included other dermatologists and engineers.
Dr. Ko and his coauthors described how they trained a computer system to identify both benign and cancerous skin lesions. They used an approach known as a convolutional neural network, often deployed for projects seeking to train computers to “see” through image analysis. They said that their test of this system found it to be on par with the performance of 21 board-certified dermatologists.
“This fast, scalable method is deployable on mobile devices and holds the potential for substantial clinical impact, including broadening the scope of primary care practice and augmenting clinical decision-making for dermatology specialists,” they wrote in their paper.
More than 6 years later, there are signs that companies are making progress toward moving skin checks using this technology into U.S. primary care settings – but only with devices that employ special tools.
It may prove tougher for companies to eventually secure the sign-off of the U.S. Food and Drug Administration for mobile apps intended to let consumers handle this task with smartphones.
Such tools would need to be proven highly accurate before release, because too many false positives mean that people would be needlessly exposed to biopsies, said Sancy A. Leachman, MD, PhD, director of the melanoma research program and chair of the department of dermatology at Oregon Health & Science University, Portland.
And false-negative readings would allow melanoma to advance and even be fatal, Dr. Leachman told this news organization.
Roxana Daneshjou, MD, PhD, a dermatologist at Stanford who has studied the promise and the pitfalls of AI in medicine, said that developers of a consumer skin-check app would need to know how people would react to their readings. That includes a good sense of how often they would appropriately seek medical care for a concerning reading. (She was not an author of the previously cited Nature paper but has published widely on AI.)
“The direct-to-consumer diagnostic space makes me nervous,” Dr. Daneshjou said in an interview. “In order to do it, you really need to have good studies in consumer populations prior to release. You need to show how effective it is with follow up.”
FDA shows interest – and reservations
As of July, the FDA had not yet given its okay for marketing of any consumer apps intended to help people detect signs of skin cancer, an agency spokesperson told this news organization.
To date, the agency has only cleared two AI-based products for this task, both meant to be used by dermatologists. And only one of these two products, Scibase’s Nevisense, remains in use in the United States. The other, MelaFind, has been discontinued. In 2017, Strata Skin Sciences said that the product did not win “a significant enough level of acceptance by dermatologists to justify the continued investment” in it. And the company said it notified the 90 owners of MelaFind devices in the United States that it would no longer support the device.
But another company, DermaSensor, said in a 2021 press release that it expects its AI-powered tool, also named DermaSensor, to be the “first ever FDA cleared or approved skin cancer detection device for primary care providers.”
The Miami-based firm said that the FDA had granted its product a “breakthrough” device designation. A breakthrough designation means that agency staff will offer extra help and guidance to companies in developing a product, because of its expected benefit for patients.
In a 2020 press release, 3Derm Systems, now owned by Digital Diagnostics, made a similar announcement about winning FDA breakthrough designation for an AI-powered tool intended to allow skin checks in primary care settings.
(The FDA generally does not comment on its reviews of experimental drugs and devices, but companies can do so. Several other companies have announced FDA breakthrough designations for AI-driven products intended to check for skin lesions, but these might be used in settings other than primary care.)
Both DermaSensor and Digital Diagnostics have chairs with notable track records for winning FDA approvals of other devices. DermaSensor’s Maurice Ferre, MD, also is the chairman of Insightec, which in 2016 won the first FDA approval for a device with a breakthrough designation device that uses ultrasound to treat tremors.
In 2018, the FDA allowed Digital Diagnostics, then called IDx, to introduce in the United States the first medical device using AI in primary care offices to check for signs of diabetic retinopathy. This product also had an FDA breakthrough designation. The executive chairman and founder of Digital Diagnostics is Michael Abramoff, MD, PhD, professor of engineering and ophthalmology at the University of Iowa, Iowa City. Dr. Abramoff and the team behind the AI tool for retinopathy, now called the LumineticsCore system, also scored a notable win with Medicare, which agreed to cover use of the product through a dedicated CPT code.
FDA draft guidance
The FDA has acknowledged the interest in broadening access to skin checks via AI.
This was a topic of discussion at a 2-day advisory committee meeting the FDA held last year. In April 2023, the FDA outlined some of its expectations for future regulation of skin-analyzing tools as part of a wide-ranging draft guidance document intended to aid companies in their efforts to develop products using a form of AI known as machine learning.
In the document, the FDA described how it might approach applications for “hypothetical” devices using this kind of AI, such as a special tool to help primary care clinicians identify lesions in need of further investigation. Such a product would use a specific camera for gathering data for its initial clearance, in the FDA’s hypothetical scenario.
The FDA staff offered technical suggestions about what the developer of this hypothetical device would have to do to extend its use to smartphones and tablets while keeping clinicians as the intended users.
Some of these expanded uses could fall within the bounds of the FDA’s initial clearance and thus not trigger a need for a new marketing submission, the agency said. But seeking to shift this hypothetical product to “patient-facing” use would require a new marketing submission to the FDA, the agency said.
In this scenario, a company would expect people to follow up with a dermatologist after receiving a report suggesting cancer. Thus, this kind of a change could expose patients to “many new, unconsidered risks,” the FDA said.
Reality check?
The state of current efforts to develop consumer apps for checking for skin cancer seems to be summarized well on the website for the MoleMapper. The app was developed by researchers at OHSU to help people track how their moles change over time.
“Mole Mapper is NOT designed to provide medical advice, professional diagnosis, opinion, or treatment. Currently, there is not enough data to develop an app that can diagnose melanoma, but if enough data is collected through Mole Mapper and shared with researchers, it may be possible in the future,” the app’s website says.
OHSU released MoleMapper as an iPhone app in 2015. The aim of this project was to help people track the moles on their skin while also fostering an experiment in “citizen science,” OHSU’s Dr. Leachman told this news organization.
OHSU researchers hoped that the digital images taken by members of the public on cell phones could one day be used to develop diagnostic algorithms for melanoma.
But around 2017, the MoleMapper team realized that they would not be able to create a diagnostic app at this time, Dr. Leachman explained. They could not collect enough data of adequate quality.
And by 2021, it was clear that they could not even develop a successful app to triage patients to assess who needs to be seen quickly. The amount of data required was, at this point, beyond what the team could collect, Dr. Leachman said in an interview.
That was a disappointment because the team had successfully completed the difficult task of creating a confidential pathway for collecting these images via both iPhones and smartphones run on Android.
“We thought if we built it, people would come, but that’s not what happened,” Dr. Leachman said. Many patients didn’t want their images used for research or would fail to follow up with details of biopsy reports. Sometimes images were not captured well enough to be of use.
“You need at least hundreds of thousands, if not millions, of data points that have been verified with pathologies, and nobody was giving us back that data. That was the reality,” Dr. Leachman said.
There were valuable lessons in that setback. The OHSU team now has a better grasp of the challenges of trying to build a data-collection system that could prove helpful in assessing skin lesions.
“If you don’t build it, you don’t know” what can go wrong, she said.
Dr. Leachman said other scientists who have worked on similar projects to build skin-analyzing apps have probably encountered the same difficulties, although they may not reveal these issues. “I think that a lot of people build these things and then they try to make it into something that it’s not,” she said.
In addition to the challenges with gathering images, dermatologists frequently need to rely on touch and other clues from in-person visits when diagnosing a suspicious lesion. “There’s something about seeing and feeling the skin in person that can’t be captured completely with an image,” Dr. Leachman said.
Public demand
Still, regulators must face the strong and immediate interest consumers have in using AI to check on moles and skin conditions, despite continuing questions about how well this approach might work.
In June, Google announced in a blog post that its Google Lens tool can help people research skin conditions.
“Just take a picture or upload a photo through Lens, and you’ll find visual matches to inform your search,” Google said in a blog post. “This feature also works if you’re not sure how to describe something else on your body, like a bump on your lip, a line on your nails or hair loss on your head. This feature is currently available in the U.S.”
Google also continues work on DermAssist, an app that’s intended to help people get personalized information about skin concerns using three photos. It is not currently publicly available, a Google spokesperson told this news organization.
Several skin-analyzing apps are already available in the Apple and Google Play stores. The British Association of Dermatologists last year issued a press release warning consumers that these apps may not be safe or effective and thus may put patients at risk for misdiagnosis.
“Unfortunately, AI-based apps which do not appear to meet regulatory requirements crop up more often than we would like,” the association said. “Additionally, the evidence to support the use of AI to diagnose skin conditions is weak which means that when it is used, it may not be safe or effective and it is possible that AI is putting patients at risk of misdiagnosis.”
Delicate and difficult balancing act
At this time, regulators, entrepreneurs, and the medical community face a delicate balancing act in considering how best to deploy AI in skin care, Dr. Ko said in an interview. (In addition to being one of the authors on the widely cited 2017 Nature paper mentioned above, Dr. Ko served until March as the initial chair of the American Academy of Dermatology’s Augmented Intelligence Committee.)
There are many solid reasons why there hasn’t been speedy progress to deploy AI in dermatology, as many envisioned a few years ago, Dr. Ko said.
Some of those reasons are specific to dermatology; this field doesn’t have a ready set of robust data from which to build AI-driven tools. In this aspect, dermatology is decades behind specialties like radiology, pathology, and ophthalmology, where clinicians have long been accumulating and storing images and other data in more standardized ways, Dr. Ko said.
“If you went to most dermatology practices and said, ‘Hey, let me learn from the data accumulated over the course of your 30-year practice to help us develop new tools,’” there may not be a whole lot there,” Dr. Ko said.
Beyond the start-up hurdles is the larger concern Dr. Ko shares with other dermatologists who work in this field, such as Dr. Daneshjou and Dr. Leachman. What would clinicians without much dermatology training and patients do with the readings from AI-driven tools and apps?
There would need to be significant research to show that such products actually help get people treated for skin diseases, including skin cancer.
Dr. Ko praised Google for being open about the stumbles with its efforts to use its AI tool for identifying diabetic retinopathy in a test in Thailand. Real-world hitches included poor Internet connections and poor image quality.
Developing reliable systems, processes, and workflows will be paramount for eventual widespread use of AI-driven tools, Dr. Ko said.
“It’s all those hidden things that are not sexy,” as are announcements about algorithms working about as well as clinicians in diagnosis, Dr. Ko said. “They don’t get the media attention, but they’re going to be make or break for AI, not just in our field but [for] AI in general.”
But he added that there also needs to be a recognition that AI-driven tools and products, even if somewhat imperfect, can help people get access to care.
In many cases, shortages of specialists prevent people from getting screened for treatable conditions such as skin cancer and retinopathy. The challenge is setting an appropriate standard to make sure that AI-driven products would help most patients in practice, without raising it so high that no such products emerge.
“There’s a risk of holding too high of a bar,” Dr. Ko said. “There is harm in not moving forward as well.”
A version of this article first appeared on Medscape.com.
.
Given that about 6.3 billion smartphones would soon be in use, this AI approach could provide a gateway for “low-cost universal access to vital diagnostic care,” wrote Justin M. Ko, MD, MBA, a dermatologist, and colleagues from Stanford (Calif.) University that included other dermatologists and engineers.
Dr. Ko and his coauthors described how they trained a computer system to identify both benign and cancerous skin lesions. They used an approach known as a convolutional neural network, often deployed for projects seeking to train computers to “see” through image analysis. They said that their test of this system found it to be on par with the performance of 21 board-certified dermatologists.
“This fast, scalable method is deployable on mobile devices and holds the potential for substantial clinical impact, including broadening the scope of primary care practice and augmenting clinical decision-making for dermatology specialists,” they wrote in their paper.
More than 6 years later, there are signs that companies are making progress toward moving skin checks using this technology into U.S. primary care settings – but only with devices that employ special tools.
It may prove tougher for companies to eventually secure the sign-off of the U.S. Food and Drug Administration for mobile apps intended to let consumers handle this task with smartphones.
Such tools would need to be proven highly accurate before release, because too many false positives mean that people would be needlessly exposed to biopsies, said Sancy A. Leachman, MD, PhD, director of the melanoma research program and chair of the department of dermatology at Oregon Health & Science University, Portland.
And false-negative readings would allow melanoma to advance and even be fatal, Dr. Leachman told this news organization.
Roxana Daneshjou, MD, PhD, a dermatologist at Stanford who has studied the promise and the pitfalls of AI in medicine, said that developers of a consumer skin-check app would need to know how people would react to their readings. That includes a good sense of how often they would appropriately seek medical care for a concerning reading. (She was not an author of the previously cited Nature paper but has published widely on AI.)
“The direct-to-consumer diagnostic space makes me nervous,” Dr. Daneshjou said in an interview. “In order to do it, you really need to have good studies in consumer populations prior to release. You need to show how effective it is with follow up.”
FDA shows interest – and reservations
As of July, the FDA had not yet given its okay for marketing of any consumer apps intended to help people detect signs of skin cancer, an agency spokesperson told this news organization.
To date, the agency has only cleared two AI-based products for this task, both meant to be used by dermatologists. And only one of these two products, Scibase’s Nevisense, remains in use in the United States. The other, MelaFind, has been discontinued. In 2017, Strata Skin Sciences said that the product did not win “a significant enough level of acceptance by dermatologists to justify the continued investment” in it. And the company said it notified the 90 owners of MelaFind devices in the United States that it would no longer support the device.
But another company, DermaSensor, said in a 2021 press release that it expects its AI-powered tool, also named DermaSensor, to be the “first ever FDA cleared or approved skin cancer detection device for primary care providers.”
The Miami-based firm said that the FDA had granted its product a “breakthrough” device designation. A breakthrough designation means that agency staff will offer extra help and guidance to companies in developing a product, because of its expected benefit for patients.
In a 2020 press release, 3Derm Systems, now owned by Digital Diagnostics, made a similar announcement about winning FDA breakthrough designation for an AI-powered tool intended to allow skin checks in primary care settings.
(The FDA generally does not comment on its reviews of experimental drugs and devices, but companies can do so. Several other companies have announced FDA breakthrough designations for AI-driven products intended to check for skin lesions, but these might be used in settings other than primary care.)
Both DermaSensor and Digital Diagnostics have chairs with notable track records for winning FDA approvals of other devices. DermaSensor’s Maurice Ferre, MD, also is the chairman of Insightec, which in 2016 won the first FDA approval for a device with a breakthrough designation device that uses ultrasound to treat tremors.
In 2018, the FDA allowed Digital Diagnostics, then called IDx, to introduce in the United States the first medical device using AI in primary care offices to check for signs of diabetic retinopathy. This product also had an FDA breakthrough designation. The executive chairman and founder of Digital Diagnostics is Michael Abramoff, MD, PhD, professor of engineering and ophthalmology at the University of Iowa, Iowa City. Dr. Abramoff and the team behind the AI tool for retinopathy, now called the LumineticsCore system, also scored a notable win with Medicare, which agreed to cover use of the product through a dedicated CPT code.
FDA draft guidance
The FDA has acknowledged the interest in broadening access to skin checks via AI.
This was a topic of discussion at a 2-day advisory committee meeting the FDA held last year. In April 2023, the FDA outlined some of its expectations for future regulation of skin-analyzing tools as part of a wide-ranging draft guidance document intended to aid companies in their efforts to develop products using a form of AI known as machine learning.
In the document, the FDA described how it might approach applications for “hypothetical” devices using this kind of AI, such as a special tool to help primary care clinicians identify lesions in need of further investigation. Such a product would use a specific camera for gathering data for its initial clearance, in the FDA’s hypothetical scenario.
The FDA staff offered technical suggestions about what the developer of this hypothetical device would have to do to extend its use to smartphones and tablets while keeping clinicians as the intended users.
Some of these expanded uses could fall within the bounds of the FDA’s initial clearance and thus not trigger a need for a new marketing submission, the agency said. But seeking to shift this hypothetical product to “patient-facing” use would require a new marketing submission to the FDA, the agency said.
In this scenario, a company would expect people to follow up with a dermatologist after receiving a report suggesting cancer. Thus, this kind of a change could expose patients to “many new, unconsidered risks,” the FDA said.
Reality check?
The state of current efforts to develop consumer apps for checking for skin cancer seems to be summarized well on the website for the MoleMapper. The app was developed by researchers at OHSU to help people track how their moles change over time.
“Mole Mapper is NOT designed to provide medical advice, professional diagnosis, opinion, or treatment. Currently, there is not enough data to develop an app that can diagnose melanoma, but if enough data is collected through Mole Mapper and shared with researchers, it may be possible in the future,” the app’s website says.
OHSU released MoleMapper as an iPhone app in 2015. The aim of this project was to help people track the moles on their skin while also fostering an experiment in “citizen science,” OHSU’s Dr. Leachman told this news organization.
OHSU researchers hoped that the digital images taken by members of the public on cell phones could one day be used to develop diagnostic algorithms for melanoma.
But around 2017, the MoleMapper team realized that they would not be able to create a diagnostic app at this time, Dr. Leachman explained. They could not collect enough data of adequate quality.
And by 2021, it was clear that they could not even develop a successful app to triage patients to assess who needs to be seen quickly. The amount of data required was, at this point, beyond what the team could collect, Dr. Leachman said in an interview.
That was a disappointment because the team had successfully completed the difficult task of creating a confidential pathway for collecting these images via both iPhones and smartphones run on Android.
“We thought if we built it, people would come, but that’s not what happened,” Dr. Leachman said. Many patients didn’t want their images used for research or would fail to follow up with details of biopsy reports. Sometimes images were not captured well enough to be of use.
“You need at least hundreds of thousands, if not millions, of data points that have been verified with pathologies, and nobody was giving us back that data. That was the reality,” Dr. Leachman said.
There were valuable lessons in that setback. The OHSU team now has a better grasp of the challenges of trying to build a data-collection system that could prove helpful in assessing skin lesions.
“If you don’t build it, you don’t know” what can go wrong, she said.
Dr. Leachman said other scientists who have worked on similar projects to build skin-analyzing apps have probably encountered the same difficulties, although they may not reveal these issues. “I think that a lot of people build these things and then they try to make it into something that it’s not,” she said.
In addition to the challenges with gathering images, dermatologists frequently need to rely on touch and other clues from in-person visits when diagnosing a suspicious lesion. “There’s something about seeing and feeling the skin in person that can’t be captured completely with an image,” Dr. Leachman said.
Public demand
Still, regulators must face the strong and immediate interest consumers have in using AI to check on moles and skin conditions, despite continuing questions about how well this approach might work.
In June, Google announced in a blog post that its Google Lens tool can help people research skin conditions.
“Just take a picture or upload a photo through Lens, and you’ll find visual matches to inform your search,” Google said in a blog post. “This feature also works if you’re not sure how to describe something else on your body, like a bump on your lip, a line on your nails or hair loss on your head. This feature is currently available in the U.S.”
Google also continues work on DermAssist, an app that’s intended to help people get personalized information about skin concerns using three photos. It is not currently publicly available, a Google spokesperson told this news organization.
Several skin-analyzing apps are already available in the Apple and Google Play stores. The British Association of Dermatologists last year issued a press release warning consumers that these apps may not be safe or effective and thus may put patients at risk for misdiagnosis.
“Unfortunately, AI-based apps which do not appear to meet regulatory requirements crop up more often than we would like,” the association said. “Additionally, the evidence to support the use of AI to diagnose skin conditions is weak which means that when it is used, it may not be safe or effective and it is possible that AI is putting patients at risk of misdiagnosis.”
Delicate and difficult balancing act
At this time, regulators, entrepreneurs, and the medical community face a delicate balancing act in considering how best to deploy AI in skin care, Dr. Ko said in an interview. (In addition to being one of the authors on the widely cited 2017 Nature paper mentioned above, Dr. Ko served until March as the initial chair of the American Academy of Dermatology’s Augmented Intelligence Committee.)
There are many solid reasons why there hasn’t been speedy progress to deploy AI in dermatology, as many envisioned a few years ago, Dr. Ko said.
Some of those reasons are specific to dermatology; this field doesn’t have a ready set of robust data from which to build AI-driven tools. In this aspect, dermatology is decades behind specialties like radiology, pathology, and ophthalmology, where clinicians have long been accumulating and storing images and other data in more standardized ways, Dr. Ko said.
“If you went to most dermatology practices and said, ‘Hey, let me learn from the data accumulated over the course of your 30-year practice to help us develop new tools,’” there may not be a whole lot there,” Dr. Ko said.
Beyond the start-up hurdles is the larger concern Dr. Ko shares with other dermatologists who work in this field, such as Dr. Daneshjou and Dr. Leachman. What would clinicians without much dermatology training and patients do with the readings from AI-driven tools and apps?
There would need to be significant research to show that such products actually help get people treated for skin diseases, including skin cancer.
Dr. Ko praised Google for being open about the stumbles with its efforts to use its AI tool for identifying diabetic retinopathy in a test in Thailand. Real-world hitches included poor Internet connections and poor image quality.
Developing reliable systems, processes, and workflows will be paramount for eventual widespread use of AI-driven tools, Dr. Ko said.
“It’s all those hidden things that are not sexy,” as are announcements about algorithms working about as well as clinicians in diagnosis, Dr. Ko said. “They don’t get the media attention, but they’re going to be make or break for AI, not just in our field but [for] AI in general.”
But he added that there also needs to be a recognition that AI-driven tools and products, even if somewhat imperfect, can help people get access to care.
In many cases, shortages of specialists prevent people from getting screened for treatable conditions such as skin cancer and retinopathy. The challenge is setting an appropriate standard to make sure that AI-driven products would help most patients in practice, without raising it so high that no such products emerge.
“There’s a risk of holding too high of a bar,” Dr. Ko said. “There is harm in not moving forward as well.”
A version of this article first appeared on Medscape.com.
.
Given that about 6.3 billion smartphones would soon be in use, this AI approach could provide a gateway for “low-cost universal access to vital diagnostic care,” wrote Justin M. Ko, MD, MBA, a dermatologist, and colleagues from Stanford (Calif.) University that included other dermatologists and engineers.
Dr. Ko and his coauthors described how they trained a computer system to identify both benign and cancerous skin lesions. They used an approach known as a convolutional neural network, often deployed for projects seeking to train computers to “see” through image analysis. They said that their test of this system found it to be on par with the performance of 21 board-certified dermatologists.
“This fast, scalable method is deployable on mobile devices and holds the potential for substantial clinical impact, including broadening the scope of primary care practice and augmenting clinical decision-making for dermatology specialists,” they wrote in their paper.
More than 6 years later, there are signs that companies are making progress toward moving skin checks using this technology into U.S. primary care settings – but only with devices that employ special tools.
It may prove tougher for companies to eventually secure the sign-off of the U.S. Food and Drug Administration for mobile apps intended to let consumers handle this task with smartphones.
Such tools would need to be proven highly accurate before release, because too many false positives mean that people would be needlessly exposed to biopsies, said Sancy A. Leachman, MD, PhD, director of the melanoma research program and chair of the department of dermatology at Oregon Health & Science University, Portland.
And false-negative readings would allow melanoma to advance and even be fatal, Dr. Leachman told this news organization.
Roxana Daneshjou, MD, PhD, a dermatologist at Stanford who has studied the promise and the pitfalls of AI in medicine, said that developers of a consumer skin-check app would need to know how people would react to their readings. That includes a good sense of how often they would appropriately seek medical care for a concerning reading. (She was not an author of the previously cited Nature paper but has published widely on AI.)
“The direct-to-consumer diagnostic space makes me nervous,” Dr. Daneshjou said in an interview. “In order to do it, you really need to have good studies in consumer populations prior to release. You need to show how effective it is with follow up.”
FDA shows interest – and reservations
As of July, the FDA had not yet given its okay for marketing of any consumer apps intended to help people detect signs of skin cancer, an agency spokesperson told this news organization.
To date, the agency has only cleared two AI-based products for this task, both meant to be used by dermatologists. And only one of these two products, Scibase’s Nevisense, remains in use in the United States. The other, MelaFind, has been discontinued. In 2017, Strata Skin Sciences said that the product did not win “a significant enough level of acceptance by dermatologists to justify the continued investment” in it. And the company said it notified the 90 owners of MelaFind devices in the United States that it would no longer support the device.
But another company, DermaSensor, said in a 2021 press release that it expects its AI-powered tool, also named DermaSensor, to be the “first ever FDA cleared or approved skin cancer detection device for primary care providers.”
The Miami-based firm said that the FDA had granted its product a “breakthrough” device designation. A breakthrough designation means that agency staff will offer extra help and guidance to companies in developing a product, because of its expected benefit for patients.
In a 2020 press release, 3Derm Systems, now owned by Digital Diagnostics, made a similar announcement about winning FDA breakthrough designation for an AI-powered tool intended to allow skin checks in primary care settings.
(The FDA generally does not comment on its reviews of experimental drugs and devices, but companies can do so. Several other companies have announced FDA breakthrough designations for AI-driven products intended to check for skin lesions, but these might be used in settings other than primary care.)
Both DermaSensor and Digital Diagnostics have chairs with notable track records for winning FDA approvals of other devices. DermaSensor’s Maurice Ferre, MD, also is the chairman of Insightec, which in 2016 won the first FDA approval for a device with a breakthrough designation device that uses ultrasound to treat tremors.
In 2018, the FDA allowed Digital Diagnostics, then called IDx, to introduce in the United States the first medical device using AI in primary care offices to check for signs of diabetic retinopathy. This product also had an FDA breakthrough designation. The executive chairman and founder of Digital Diagnostics is Michael Abramoff, MD, PhD, professor of engineering and ophthalmology at the University of Iowa, Iowa City. Dr. Abramoff and the team behind the AI tool for retinopathy, now called the LumineticsCore system, also scored a notable win with Medicare, which agreed to cover use of the product through a dedicated CPT code.
FDA draft guidance
The FDA has acknowledged the interest in broadening access to skin checks via AI.
This was a topic of discussion at a 2-day advisory committee meeting the FDA held last year. In April 2023, the FDA outlined some of its expectations for future regulation of skin-analyzing tools as part of a wide-ranging draft guidance document intended to aid companies in their efforts to develop products using a form of AI known as machine learning.
In the document, the FDA described how it might approach applications for “hypothetical” devices using this kind of AI, such as a special tool to help primary care clinicians identify lesions in need of further investigation. Such a product would use a specific camera for gathering data for its initial clearance, in the FDA’s hypothetical scenario.
The FDA staff offered technical suggestions about what the developer of this hypothetical device would have to do to extend its use to smartphones and tablets while keeping clinicians as the intended users.
Some of these expanded uses could fall within the bounds of the FDA’s initial clearance and thus not trigger a need for a new marketing submission, the agency said. But seeking to shift this hypothetical product to “patient-facing” use would require a new marketing submission to the FDA, the agency said.
In this scenario, a company would expect people to follow up with a dermatologist after receiving a report suggesting cancer. Thus, this kind of a change could expose patients to “many new, unconsidered risks,” the FDA said.
Reality check?
The state of current efforts to develop consumer apps for checking for skin cancer seems to be summarized well on the website for the MoleMapper. The app was developed by researchers at OHSU to help people track how their moles change over time.
“Mole Mapper is NOT designed to provide medical advice, professional diagnosis, opinion, or treatment. Currently, there is not enough data to develop an app that can diagnose melanoma, but if enough data is collected through Mole Mapper and shared with researchers, it may be possible in the future,” the app’s website says.
OHSU released MoleMapper as an iPhone app in 2015. The aim of this project was to help people track the moles on their skin while also fostering an experiment in “citizen science,” OHSU’s Dr. Leachman told this news organization.
OHSU researchers hoped that the digital images taken by members of the public on cell phones could one day be used to develop diagnostic algorithms for melanoma.
But around 2017, the MoleMapper team realized that they would not be able to create a diagnostic app at this time, Dr. Leachman explained. They could not collect enough data of adequate quality.
And by 2021, it was clear that they could not even develop a successful app to triage patients to assess who needs to be seen quickly. The amount of data required was, at this point, beyond what the team could collect, Dr. Leachman said in an interview.
That was a disappointment because the team had successfully completed the difficult task of creating a confidential pathway for collecting these images via both iPhones and smartphones run on Android.
“We thought if we built it, people would come, but that’s not what happened,” Dr. Leachman said. Many patients didn’t want their images used for research or would fail to follow up with details of biopsy reports. Sometimes images were not captured well enough to be of use.
“You need at least hundreds of thousands, if not millions, of data points that have been verified with pathologies, and nobody was giving us back that data. That was the reality,” Dr. Leachman said.
There were valuable lessons in that setback. The OHSU team now has a better grasp of the challenges of trying to build a data-collection system that could prove helpful in assessing skin lesions.
“If you don’t build it, you don’t know” what can go wrong, she said.
Dr. Leachman said other scientists who have worked on similar projects to build skin-analyzing apps have probably encountered the same difficulties, although they may not reveal these issues. “I think that a lot of people build these things and then they try to make it into something that it’s not,” she said.
In addition to the challenges with gathering images, dermatologists frequently need to rely on touch and other clues from in-person visits when diagnosing a suspicious lesion. “There’s something about seeing and feeling the skin in person that can’t be captured completely with an image,” Dr. Leachman said.
Public demand
Still, regulators must face the strong and immediate interest consumers have in using AI to check on moles and skin conditions, despite continuing questions about how well this approach might work.
In June, Google announced in a blog post that its Google Lens tool can help people research skin conditions.
“Just take a picture or upload a photo through Lens, and you’ll find visual matches to inform your search,” Google said in a blog post. “This feature also works if you’re not sure how to describe something else on your body, like a bump on your lip, a line on your nails or hair loss on your head. This feature is currently available in the U.S.”
Google also continues work on DermAssist, an app that’s intended to help people get personalized information about skin concerns using three photos. It is not currently publicly available, a Google spokesperson told this news organization.
Several skin-analyzing apps are already available in the Apple and Google Play stores. The British Association of Dermatologists last year issued a press release warning consumers that these apps may not be safe or effective and thus may put patients at risk for misdiagnosis.
“Unfortunately, AI-based apps which do not appear to meet regulatory requirements crop up more often than we would like,” the association said. “Additionally, the evidence to support the use of AI to diagnose skin conditions is weak which means that when it is used, it may not be safe or effective and it is possible that AI is putting patients at risk of misdiagnosis.”
Delicate and difficult balancing act
At this time, regulators, entrepreneurs, and the medical community face a delicate balancing act in considering how best to deploy AI in skin care, Dr. Ko said in an interview. (In addition to being one of the authors on the widely cited 2017 Nature paper mentioned above, Dr. Ko served until March as the initial chair of the American Academy of Dermatology’s Augmented Intelligence Committee.)
There are many solid reasons why there hasn’t been speedy progress to deploy AI in dermatology, as many envisioned a few years ago, Dr. Ko said.
Some of those reasons are specific to dermatology; this field doesn’t have a ready set of robust data from which to build AI-driven tools. In this aspect, dermatology is decades behind specialties like radiology, pathology, and ophthalmology, where clinicians have long been accumulating and storing images and other data in more standardized ways, Dr. Ko said.
“If you went to most dermatology practices and said, ‘Hey, let me learn from the data accumulated over the course of your 30-year practice to help us develop new tools,’” there may not be a whole lot there,” Dr. Ko said.
Beyond the start-up hurdles is the larger concern Dr. Ko shares with other dermatologists who work in this field, such as Dr. Daneshjou and Dr. Leachman. What would clinicians without much dermatology training and patients do with the readings from AI-driven tools and apps?
There would need to be significant research to show that such products actually help get people treated for skin diseases, including skin cancer.
Dr. Ko praised Google for being open about the stumbles with its efforts to use its AI tool for identifying diabetic retinopathy in a test in Thailand. Real-world hitches included poor Internet connections and poor image quality.
Developing reliable systems, processes, and workflows will be paramount for eventual widespread use of AI-driven tools, Dr. Ko said.
“It’s all those hidden things that are not sexy,” as are announcements about algorithms working about as well as clinicians in diagnosis, Dr. Ko said. “They don’t get the media attention, but they’re going to be make or break for AI, not just in our field but [for] AI in general.”
But he added that there also needs to be a recognition that AI-driven tools and products, even if somewhat imperfect, can help people get access to care.
In many cases, shortages of specialists prevent people from getting screened for treatable conditions such as skin cancer and retinopathy. The challenge is setting an appropriate standard to make sure that AI-driven products would help most patients in practice, without raising it so high that no such products emerge.
“There’s a risk of holding too high of a bar,” Dr. Ko said. “There is harm in not moving forward as well.”
A version of this article first appeared on Medscape.com.
Study evaluating in utero treatment for hypohidrotic ectodermal dysplasia seeks enrollees
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
What factors cause multiple biologic failure in psoriasis?
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY