User login
Weekend Botox training: Shortcut to cash or risky business?
This transcript has been edited for clarity.
Dr. Patel: A friend recently joked with me and said, “I wish you were a dermatologist so you could hook me up with Botox and fillers.” Well, little does this friend know that I could be a certified cosmetic injector just after a weekend course. Botox parties, here I come?
I can’t blame any health care professional for having a side hustle. People are burned out, want to supplement their income, or scale back clinical hours. According to one Medscape survey, almost 40% of physicians do have some form of a side hustle, whether it is consulting, speaking engagements, being an expert witness, or moonlighting. I know plenty of doctors and nurses who have taken on Botox injecting as a way to make some extra cash.
Now, going back to me and smoothing out wrinkles. I’m a pediatric hospitalist. I’ve never injected an aesthetic product in anyone’s face. When it comes to sharp objects and faces, I’ve sewn lacerations and drained abscesses. In my world, when we talk about botulinum toxin, we’re usually talking about botulism or the therapeutic treatment of migraines and muscle spasms – pathology.
The National Laser Institute has a 2-day Botox and dermal filler training. “Our 2-day Botox and filler course will also teach you how to build a practice and capitalize on the enormous Botox and dermal filler market that exists in the United States.” That’s a lot to cover in 2 days. They even have lunch breaks.
Just from a quick search, I even found an online video course for $1,500. For an additional fee, you can have a live, hands-on component. There are so many trainings out there, including one that’s only 8 hours long, offered by Empire Medical. I also went and spoke with an employee at Empire Medical who told me that because I’m an MD, if I do the course, I can use my certificate and go directly to a manufacturer, buy Botox, and start injecting right away.
Now, is this training actually sufficient for me to go and get good results while minimizing adverse effects like brow ptosis, dry eyes, and asymmetry? I have no idea. According to a review from the Journal of Cosmetic Dermatology, it’s crucial to understand anatomic landmarks, muscle function, baseline asymmetry, potential migration of the toxin, and site-specific precautions.
Okay, that sounds really intimidating, but people still do it. I saw a Business Insider article about a hospitalist who took a 2-day Botox course and then, to her credit, she trained under supervision for an additional 6 months. She then started hosting Botox parties and each time was making $3,500 to upwards of $20,000.
Let’s do some quick mental math. If I were to go online and buy Botox for $3-$6 a unit and then charge patients $15 a unit, and then I consider that in areas like the forehead or in between the eyes – I read that could take 25-50 units – and I repeat this for multiple patients, I can make a few thousand dollars. Well, I may have to adjust my prices according to the market, obviously, because I did see some Groupons advertising $10 per unit.
Who can get in on some Botox cosmetic cash action? Well, physicians can right away. For other health care professionals, it depends on the state. For example, in California, dentists cannot get Botox solely for cosmetic purposes, whereas in Arizona, they can. Generally speaking, NPs and PAs require some type of physician oversight or supervision, but again, it depends on the state.
Oh, and fun fact: Connecticut outright banned Botox parties and said that Botox must be performed “in a medical spa or licensed health care facility and by a Connecticut-licensed health care provider within his or her scope of practice.”
It definitely worries me that someone could go online or go overseas, buy Botox, claim to be a health care professional, and literally commit fraud. I found stories out there such as a couple in San Jose who are giving out Botox from their home without a license. They got arrested. Also, a woman in Alabama who lied about being a licensed dermatologist and did the same, or another woman in Los Angeles who got arrested after selling counterfeit Botox to undercover law enforcement. Surely, there are plenty more cases out there like this.
I asked Dr. Jacqueline Watchmaker, a board-certified dermatologist at U.S. Dermatology Partners in Arizona who has an expertise in cosmetic procedures, what she thought about the booming med spa industry and what, if any, regulatory changes she wanted to see.
Jacqueline Watchmaker, MD: I do think the fact that people can just go to a 1- or 2-day injection course and inject filler and Botox is concerning. I think the lack of regulation surrounding this topic is also very concerning.
There’s so much that goes into being a skilled injector. It’s an intricate knowledge of facial anatomy, which takes weeks, if not months, to really master. There’s actually injection technique, which can be very complex depending on the part of the face that you’re injecting. Even more important, it’s how to prevent complications, but also how to deal with complications if they do occur. There’s no way that these weekend injection courses are able to cover those topics in a thorough and satisfactory manner.
I see complications from med spas all the time, and I think it’s people going to injectors who are not skilled. They don’t know their anatomy, they don’t know the appropriate filler to use, and then heaven forbid there is a complication, they don’t know how to manage the complication – and then those patients get sent to me.
I think patients sometimes forget that these cosmetic procedures are true medical procedures. You need sterile technique. Again, you need to know the anatomy. It can look easy on social media, but there’s a large amount of thought behind it. I think there needs to be more regulation around this topic.
Dr. Patel: In one study, out of 400 people who received a cosmetic procedure, 50 reported an adverse event, such as discoloration or burns, and these adverse events were more likely to occur if a nonphysician was doing the procedure. Granted, this was a small study. You can’t make a generalization out of it, but this does add to the argument that there needs to be more regulation and oversight.
Let’s be real. The cosmetic injection side hustle is alive and well, but I’m good. I’m not going there. Maybe there should be some more quality control. At Botox parties, do people even ask if their injectors are certified or where they bought their vials?
You might be thinking that this isn’t a big deal because it’s just Botox. Let me ask you all a question: If you or your family member were going to go get Botox or another cosmetic injection, would it still not be a big deal?
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He reported conflicts of interest with Medumo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. Patel: A friend recently joked with me and said, “I wish you were a dermatologist so you could hook me up with Botox and fillers.” Well, little does this friend know that I could be a certified cosmetic injector just after a weekend course. Botox parties, here I come?
I can’t blame any health care professional for having a side hustle. People are burned out, want to supplement their income, or scale back clinical hours. According to one Medscape survey, almost 40% of physicians do have some form of a side hustle, whether it is consulting, speaking engagements, being an expert witness, or moonlighting. I know plenty of doctors and nurses who have taken on Botox injecting as a way to make some extra cash.
Now, going back to me and smoothing out wrinkles. I’m a pediatric hospitalist. I’ve never injected an aesthetic product in anyone’s face. When it comes to sharp objects and faces, I’ve sewn lacerations and drained abscesses. In my world, when we talk about botulinum toxin, we’re usually talking about botulism or the therapeutic treatment of migraines and muscle spasms – pathology.
The National Laser Institute has a 2-day Botox and dermal filler training. “Our 2-day Botox and filler course will also teach you how to build a practice and capitalize on the enormous Botox and dermal filler market that exists in the United States.” That’s a lot to cover in 2 days. They even have lunch breaks.
Just from a quick search, I even found an online video course for $1,500. For an additional fee, you can have a live, hands-on component. There are so many trainings out there, including one that’s only 8 hours long, offered by Empire Medical. I also went and spoke with an employee at Empire Medical who told me that because I’m an MD, if I do the course, I can use my certificate and go directly to a manufacturer, buy Botox, and start injecting right away.
Now, is this training actually sufficient for me to go and get good results while minimizing adverse effects like brow ptosis, dry eyes, and asymmetry? I have no idea. According to a review from the Journal of Cosmetic Dermatology, it’s crucial to understand anatomic landmarks, muscle function, baseline asymmetry, potential migration of the toxin, and site-specific precautions.
Okay, that sounds really intimidating, but people still do it. I saw a Business Insider article about a hospitalist who took a 2-day Botox course and then, to her credit, she trained under supervision for an additional 6 months. She then started hosting Botox parties and each time was making $3,500 to upwards of $20,000.
Let’s do some quick mental math. If I were to go online and buy Botox for $3-$6 a unit and then charge patients $15 a unit, and then I consider that in areas like the forehead or in between the eyes – I read that could take 25-50 units – and I repeat this for multiple patients, I can make a few thousand dollars. Well, I may have to adjust my prices according to the market, obviously, because I did see some Groupons advertising $10 per unit.
Who can get in on some Botox cosmetic cash action? Well, physicians can right away. For other health care professionals, it depends on the state. For example, in California, dentists cannot get Botox solely for cosmetic purposes, whereas in Arizona, they can. Generally speaking, NPs and PAs require some type of physician oversight or supervision, but again, it depends on the state.
Oh, and fun fact: Connecticut outright banned Botox parties and said that Botox must be performed “in a medical spa or licensed health care facility and by a Connecticut-licensed health care provider within his or her scope of practice.”
It definitely worries me that someone could go online or go overseas, buy Botox, claim to be a health care professional, and literally commit fraud. I found stories out there such as a couple in San Jose who are giving out Botox from their home without a license. They got arrested. Also, a woman in Alabama who lied about being a licensed dermatologist and did the same, or another woman in Los Angeles who got arrested after selling counterfeit Botox to undercover law enforcement. Surely, there are plenty more cases out there like this.
I asked Dr. Jacqueline Watchmaker, a board-certified dermatologist at U.S. Dermatology Partners in Arizona who has an expertise in cosmetic procedures, what she thought about the booming med spa industry and what, if any, regulatory changes she wanted to see.
Jacqueline Watchmaker, MD: I do think the fact that people can just go to a 1- or 2-day injection course and inject filler and Botox is concerning. I think the lack of regulation surrounding this topic is also very concerning.
There’s so much that goes into being a skilled injector. It’s an intricate knowledge of facial anatomy, which takes weeks, if not months, to really master. There’s actually injection technique, which can be very complex depending on the part of the face that you’re injecting. Even more important, it’s how to prevent complications, but also how to deal with complications if they do occur. There’s no way that these weekend injection courses are able to cover those topics in a thorough and satisfactory manner.
I see complications from med spas all the time, and I think it’s people going to injectors who are not skilled. They don’t know their anatomy, they don’t know the appropriate filler to use, and then heaven forbid there is a complication, they don’t know how to manage the complication – and then those patients get sent to me.
I think patients sometimes forget that these cosmetic procedures are true medical procedures. You need sterile technique. Again, you need to know the anatomy. It can look easy on social media, but there’s a large amount of thought behind it. I think there needs to be more regulation around this topic.
Dr. Patel: In one study, out of 400 people who received a cosmetic procedure, 50 reported an adverse event, such as discoloration or burns, and these adverse events were more likely to occur if a nonphysician was doing the procedure. Granted, this was a small study. You can’t make a generalization out of it, but this does add to the argument that there needs to be more regulation and oversight.
Let’s be real. The cosmetic injection side hustle is alive and well, but I’m good. I’m not going there. Maybe there should be some more quality control. At Botox parties, do people even ask if their injectors are certified or where they bought their vials?
You might be thinking that this isn’t a big deal because it’s just Botox. Let me ask you all a question: If you or your family member were going to go get Botox or another cosmetic injection, would it still not be a big deal?
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He reported conflicts of interest with Medumo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. Patel: A friend recently joked with me and said, “I wish you were a dermatologist so you could hook me up with Botox and fillers.” Well, little does this friend know that I could be a certified cosmetic injector just after a weekend course. Botox parties, here I come?
I can’t blame any health care professional for having a side hustle. People are burned out, want to supplement their income, or scale back clinical hours. According to one Medscape survey, almost 40% of physicians do have some form of a side hustle, whether it is consulting, speaking engagements, being an expert witness, or moonlighting. I know plenty of doctors and nurses who have taken on Botox injecting as a way to make some extra cash.
Now, going back to me and smoothing out wrinkles. I’m a pediatric hospitalist. I’ve never injected an aesthetic product in anyone’s face. When it comes to sharp objects and faces, I’ve sewn lacerations and drained abscesses. In my world, when we talk about botulinum toxin, we’re usually talking about botulism or the therapeutic treatment of migraines and muscle spasms – pathology.
The National Laser Institute has a 2-day Botox and dermal filler training. “Our 2-day Botox and filler course will also teach you how to build a practice and capitalize on the enormous Botox and dermal filler market that exists in the United States.” That’s a lot to cover in 2 days. They even have lunch breaks.
Just from a quick search, I even found an online video course for $1,500. For an additional fee, you can have a live, hands-on component. There are so many trainings out there, including one that’s only 8 hours long, offered by Empire Medical. I also went and spoke with an employee at Empire Medical who told me that because I’m an MD, if I do the course, I can use my certificate and go directly to a manufacturer, buy Botox, and start injecting right away.
Now, is this training actually sufficient for me to go and get good results while minimizing adverse effects like brow ptosis, dry eyes, and asymmetry? I have no idea. According to a review from the Journal of Cosmetic Dermatology, it’s crucial to understand anatomic landmarks, muscle function, baseline asymmetry, potential migration of the toxin, and site-specific precautions.
Okay, that sounds really intimidating, but people still do it. I saw a Business Insider article about a hospitalist who took a 2-day Botox course and then, to her credit, she trained under supervision for an additional 6 months. She then started hosting Botox parties and each time was making $3,500 to upwards of $20,000.
Let’s do some quick mental math. If I were to go online and buy Botox for $3-$6 a unit and then charge patients $15 a unit, and then I consider that in areas like the forehead or in between the eyes – I read that could take 25-50 units – and I repeat this for multiple patients, I can make a few thousand dollars. Well, I may have to adjust my prices according to the market, obviously, because I did see some Groupons advertising $10 per unit.
Who can get in on some Botox cosmetic cash action? Well, physicians can right away. For other health care professionals, it depends on the state. For example, in California, dentists cannot get Botox solely for cosmetic purposes, whereas in Arizona, they can. Generally speaking, NPs and PAs require some type of physician oversight or supervision, but again, it depends on the state.
Oh, and fun fact: Connecticut outright banned Botox parties and said that Botox must be performed “in a medical spa or licensed health care facility and by a Connecticut-licensed health care provider within his or her scope of practice.”
It definitely worries me that someone could go online or go overseas, buy Botox, claim to be a health care professional, and literally commit fraud. I found stories out there such as a couple in San Jose who are giving out Botox from their home without a license. They got arrested. Also, a woman in Alabama who lied about being a licensed dermatologist and did the same, or another woman in Los Angeles who got arrested after selling counterfeit Botox to undercover law enforcement. Surely, there are plenty more cases out there like this.
I asked Dr. Jacqueline Watchmaker, a board-certified dermatologist at U.S. Dermatology Partners in Arizona who has an expertise in cosmetic procedures, what she thought about the booming med spa industry and what, if any, regulatory changes she wanted to see.
Jacqueline Watchmaker, MD: I do think the fact that people can just go to a 1- or 2-day injection course and inject filler and Botox is concerning. I think the lack of regulation surrounding this topic is also very concerning.
There’s so much that goes into being a skilled injector. It’s an intricate knowledge of facial anatomy, which takes weeks, if not months, to really master. There’s actually injection technique, which can be very complex depending on the part of the face that you’re injecting. Even more important, it’s how to prevent complications, but also how to deal with complications if they do occur. There’s no way that these weekend injection courses are able to cover those topics in a thorough and satisfactory manner.
I see complications from med spas all the time, and I think it’s people going to injectors who are not skilled. They don’t know their anatomy, they don’t know the appropriate filler to use, and then heaven forbid there is a complication, they don’t know how to manage the complication – and then those patients get sent to me.
I think patients sometimes forget that these cosmetic procedures are true medical procedures. You need sterile technique. Again, you need to know the anatomy. It can look easy on social media, but there’s a large amount of thought behind it. I think there needs to be more regulation around this topic.
Dr. Patel: In one study, out of 400 people who received a cosmetic procedure, 50 reported an adverse event, such as discoloration or burns, and these adverse events were more likely to occur if a nonphysician was doing the procedure. Granted, this was a small study. You can’t make a generalization out of it, but this does add to the argument that there needs to be more regulation and oversight.
Let’s be real. The cosmetic injection side hustle is alive and well, but I’m good. I’m not going there. Maybe there should be some more quality control. At Botox parties, do people even ask if their injectors are certified or where they bought their vials?
You might be thinking that this isn’t a big deal because it’s just Botox. Let me ask you all a question: If you or your family member were going to go get Botox or another cosmetic injection, would it still not be a big deal?
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He reported conflicts of interest with Medumo.
A version of this article first appeared on Medscape.com.
Free teledermatology clinic helps underserved patients initiate AD care
A in other underserved areas in the United States.
Washington, D.C., has “staggering health disparities that are among the largest in the country,” and Ward 8 and surrounding areas in the southeastern part of the city are “dermatology deserts,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who started the program in 2021 with a pilot project. Dr. Friedman spoke about the project, which has since been expanded to include alopecia areata, at the Revolutionizing Atopic Dermatitis conference in April and in an interview after the meeting.
Patients who attend the clinics – held at the Temple of Praise Church in a residential area of Ward 8, a predominantly Black community with a 30% poverty rate – are entered into the GW Medical Faculty Associates medical records system and educated on telemedicine best practices (such as not having light behind them during a session) and how to use telemedicine with their own device.
Those with AD who participate learn about the condition through an image-rich poster showing how it appears in various skin tones, handouts, National Eczema Association films, and discussion with medical students who staff the clinics under Dr. Friedman’s on-site supervision. Participants with alopecia areata similarly can view a poster and converse about the condition.
Patients then have a free 20-minute telehealth visit with a GWU dermatology resident in a private room, and a medical student volunteer nearby to assist with the technology if needed. They leave with a treatment plan, which often includes prescriptions, and a follow-up telemedicine appointment.
The program “is meant to be a stepping point for initiating care ... to set someone up for success for recurrent telehealth visits in the future” and for treatment before symptoms become too severe, Dr. Friedman said in an interview. “We want to demystify telemedicine and educate on the disease state and dispel myths ... so the patient understands why it’s happening” and how it can be treated.
The pilot project, funded with a grant from Pfizer, involved five 2-hour clinics held on Mondays from 4 p.m. to 6 p.m., that together served almost 50 adult and pediatric patients. Grants from Pfizer and Eli Lilly enabled additional clinics in the spring of 2023 and into the summer. And in June, GWU and Pfizer announced a $1 million national grant program focused on broad implementation of what they’ve coined the “Teledermatology Help Desk Clinic” model.
Practices or organizations that secure grants will utilize GWU’s experience and meet with an advisory council of experts in dermatology telemedicine and community advocacy. Having a “long-term plan” and commitment to sustainability is an important element of the model, said Dr. Friedman, who is chairing the grant program.
Patients deem clinic ‘extremely’ helpful
As one of the most prevalent skin disorders – and one with a documented history of elevated risk for specific populations – AD was a good starting point for the teledermatology clinic program. Patients who identify as Black have a higher incidence and prevalence of AD than those who identify as White and Hispanic, and they tend to have more severe disease. Yet they account for fewer visits to dermatologists for AD.
One cross-sectional study of about 3,500 adults in the United States with AD documented that racial/ethnic and socioeconomic disparities reduce outpatient utilization of AD care and increase urgent care and hospital utilization. And in a longitudinal cohort study of children in the United States with AD, Black children with poorly controlled AD were significantly less likely than White children to see a dermatologist.
Like other programs, the GWU department of dermatology had pivoted to telehealth in 2020, and a published survey of patients who attended telehealth appointments during the early part of the pandemic showed that it was generally well liked – and not only for social distancing, but for time efficiency and because transportation was not needed. Only 10% of the 168 patients who completed the survey (out of 894 asked) reported they were unlikely to undertake another telehealth visit. For 10%, eczema was the reason for the visit.
However, only 1% of the survey respondents were from Ward 8, which “begged the question, did those who really need access know this was an option?” Dr. Friedman said at the RAD meeting. He wondered whether there was not only a dermatology desert in Ward 8, but a “technology desert” as well.
Findings from a patient satisfaction survey taken at the end of the pilot program are encouraging, Dr. Friedman said. While data on follow-up visits has not been collected yet, “what I do now have a sense of” is that “the entry point [afforded by the clinics] changed the course in terms of patients’ understanding of the disease and how they feel about its management.”
About 94% of survey respondents indicated the clinic was “extremely” helpful and the remainder said it was “very” helpful; 90% said telehealth significantly changed how they will manage their condition; and 97% said it is “extremely” important to continue the clinics. The majority of patients – 70% – indicated they did not have a dermatologist.
Education about AD at the clinics covers moisturizers/emollients, bathing habits, soaps and detergents, trigger avoidance, and the role of stress and environmental factors in disease exacerbation. Trade samples of moisturizers, mild cleansers, and other products have increasingly been available.
For prescriptions of topical steroids and other commonly prescribed medications, Dr. Friedman and associates combed GoodRx for coupons and surveyed local pharmacies for self-pay pricing to identify least expensive options. Patients with AD who were deemed likely candidates for more advanced therapies in the future were educated about these possibilities.
Alopecia areata
The addition of alopecia areata drew patients with other forms of hair loss as well, but “we weren’t going to turn anyone away who did not have that specific autoimmune form of hair loss,” Dr. Friedman said. Depending on the diagnosis, prescriptions were written for minoxidil and 5-alpha reductase inhibitors.
Important for follow-up is GWU’s acceptance of Medicaid and the availability of both a sliding scale for self-pay and services that assist patients in registering for Medicaid and, if eligible, other insurance plans.
Building partnerships, earning trust
Establishment of the teledermatology clinic program took legwork and relationship building. “You can’t just show up. That’s not enough,” said Dr. Friedman, who also directs the dermatology residency program at GWU. “You have to show through action and through investment of time and energy that you are legitimate, that you’re really there for the long haul.”
Dr. Friedman had assistance from the Rodham Institute, which was established at GWU (and until recently was housed there) and has a history of engagement with local stakeholders such as community centers, church leadership, politicians, and others in the Washington area. He was put in touch with Bishop Deborah Webb at the Temple of Praise Church, a community pillar in Ward 8, and from there “it was a courtship,” he said, with trust to be built and logistics to be worked out. (Budgets for the clinics, he noted, have included compensation to the church and gift cards for church volunteers who are present at the clinics.)
In the meantime, medical student volunteers from GWU, Howard University, and Georgetown University were trained in telemedicine and attended a “boot camp” on AD “so they’d be able to talk with anyone about it,” Dr. Friedman said.
Advertising “was a learning experience,” he said, and was ultimately multipronged, involving church service announcements, flyers, and, most importantly, Facebook and Instagram advertisements. (People were asked to call a dedicated phone line to schedule an appointment and were invited to register in the GW Medical Faculty Associates records system, though walk-ins to the clinics were still welcomed.)
In a comment, Misty Eleryan, MD, MS, a Mohs micrographic surgeon and dermatologist in Santa Monica, Calif., said dermatology deserts are often found in rural areas and/or areas “with a higher population of marginalized communities, such as Black, Brown, or poorer individuals” – communities that tend to rely on care from urgent care or ED physicians who are unaware of how skin conditions present on darker skin tones.
Programs that educate patients about various presentations of skin conditions are helpful not only for the patients themselves, but could also enable them to help friends, family members, and colleagues, said Dr. Eleryan, who did her residency training at GWU.
“Access,” she noted, is more than just physical access to a person, place, or thing. Referring to a “five A’s” framework described several decades ago, Dr. Eleryan said access to care is characterized by affordability, availability (extent to which the physician has the requisite resources, such as personnel and technology, to meet the patient’s needs), accessibility (geographic), accommodation (extent to which the physician can meet the patient’s constraints and preferences – such as hours of operation, how communications are handled, ability to receive care without prior appointments), and acceptability (extent to which the patient is comfortable with the “more immutable characteristics” of the physician and vice versa).
The GWU program, she said, “is a great start.”
Dr. Friedman said he’s fully invested. There has long been a perception, “rightfully so, that underserved communities are overlooked especially by large institutions. One attendee told me she never expected in her lifetime to see something like this clinic and someone who looked like me caring about her community. ... It certainly says a great deal about the work we need to put in to repair longstanding injury.”
Dr. Friedman disclosed that, in addition to being a recipient of grants from Pfizer and Lilly, he is a speaker for Lilly. Dr. Eleryan said she has no relevant disclosures.
A in other underserved areas in the United States.
Washington, D.C., has “staggering health disparities that are among the largest in the country,” and Ward 8 and surrounding areas in the southeastern part of the city are “dermatology deserts,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who started the program in 2021 with a pilot project. Dr. Friedman spoke about the project, which has since been expanded to include alopecia areata, at the Revolutionizing Atopic Dermatitis conference in April and in an interview after the meeting.
Patients who attend the clinics – held at the Temple of Praise Church in a residential area of Ward 8, a predominantly Black community with a 30% poverty rate – are entered into the GW Medical Faculty Associates medical records system and educated on telemedicine best practices (such as not having light behind them during a session) and how to use telemedicine with their own device.
Those with AD who participate learn about the condition through an image-rich poster showing how it appears in various skin tones, handouts, National Eczema Association films, and discussion with medical students who staff the clinics under Dr. Friedman’s on-site supervision. Participants with alopecia areata similarly can view a poster and converse about the condition.
Patients then have a free 20-minute telehealth visit with a GWU dermatology resident in a private room, and a medical student volunteer nearby to assist with the technology if needed. They leave with a treatment plan, which often includes prescriptions, and a follow-up telemedicine appointment.
The program “is meant to be a stepping point for initiating care ... to set someone up for success for recurrent telehealth visits in the future” and for treatment before symptoms become too severe, Dr. Friedman said in an interview. “We want to demystify telemedicine and educate on the disease state and dispel myths ... so the patient understands why it’s happening” and how it can be treated.
The pilot project, funded with a grant from Pfizer, involved five 2-hour clinics held on Mondays from 4 p.m. to 6 p.m., that together served almost 50 adult and pediatric patients. Grants from Pfizer and Eli Lilly enabled additional clinics in the spring of 2023 and into the summer. And in June, GWU and Pfizer announced a $1 million national grant program focused on broad implementation of what they’ve coined the “Teledermatology Help Desk Clinic” model.
Practices or organizations that secure grants will utilize GWU’s experience and meet with an advisory council of experts in dermatology telemedicine and community advocacy. Having a “long-term plan” and commitment to sustainability is an important element of the model, said Dr. Friedman, who is chairing the grant program.
Patients deem clinic ‘extremely’ helpful
As one of the most prevalent skin disorders – and one with a documented history of elevated risk for specific populations – AD was a good starting point for the teledermatology clinic program. Patients who identify as Black have a higher incidence and prevalence of AD than those who identify as White and Hispanic, and they tend to have more severe disease. Yet they account for fewer visits to dermatologists for AD.
One cross-sectional study of about 3,500 adults in the United States with AD documented that racial/ethnic and socioeconomic disparities reduce outpatient utilization of AD care and increase urgent care and hospital utilization. And in a longitudinal cohort study of children in the United States with AD, Black children with poorly controlled AD were significantly less likely than White children to see a dermatologist.
Like other programs, the GWU department of dermatology had pivoted to telehealth in 2020, and a published survey of patients who attended telehealth appointments during the early part of the pandemic showed that it was generally well liked – and not only for social distancing, but for time efficiency and because transportation was not needed. Only 10% of the 168 patients who completed the survey (out of 894 asked) reported they were unlikely to undertake another telehealth visit. For 10%, eczema was the reason for the visit.
However, only 1% of the survey respondents were from Ward 8, which “begged the question, did those who really need access know this was an option?” Dr. Friedman said at the RAD meeting. He wondered whether there was not only a dermatology desert in Ward 8, but a “technology desert” as well.
Findings from a patient satisfaction survey taken at the end of the pilot program are encouraging, Dr. Friedman said. While data on follow-up visits has not been collected yet, “what I do now have a sense of” is that “the entry point [afforded by the clinics] changed the course in terms of patients’ understanding of the disease and how they feel about its management.”
About 94% of survey respondents indicated the clinic was “extremely” helpful and the remainder said it was “very” helpful; 90% said telehealth significantly changed how they will manage their condition; and 97% said it is “extremely” important to continue the clinics. The majority of patients – 70% – indicated they did not have a dermatologist.
Education about AD at the clinics covers moisturizers/emollients, bathing habits, soaps and detergents, trigger avoidance, and the role of stress and environmental factors in disease exacerbation. Trade samples of moisturizers, mild cleansers, and other products have increasingly been available.
For prescriptions of topical steroids and other commonly prescribed medications, Dr. Friedman and associates combed GoodRx for coupons and surveyed local pharmacies for self-pay pricing to identify least expensive options. Patients with AD who were deemed likely candidates for more advanced therapies in the future were educated about these possibilities.
Alopecia areata
The addition of alopecia areata drew patients with other forms of hair loss as well, but “we weren’t going to turn anyone away who did not have that specific autoimmune form of hair loss,” Dr. Friedman said. Depending on the diagnosis, prescriptions were written for minoxidil and 5-alpha reductase inhibitors.
Important for follow-up is GWU’s acceptance of Medicaid and the availability of both a sliding scale for self-pay and services that assist patients in registering for Medicaid and, if eligible, other insurance plans.
Building partnerships, earning trust
Establishment of the teledermatology clinic program took legwork and relationship building. “You can’t just show up. That’s not enough,” said Dr. Friedman, who also directs the dermatology residency program at GWU. “You have to show through action and through investment of time and energy that you are legitimate, that you’re really there for the long haul.”
Dr. Friedman had assistance from the Rodham Institute, which was established at GWU (and until recently was housed there) and has a history of engagement with local stakeholders such as community centers, church leadership, politicians, and others in the Washington area. He was put in touch with Bishop Deborah Webb at the Temple of Praise Church, a community pillar in Ward 8, and from there “it was a courtship,” he said, with trust to be built and logistics to be worked out. (Budgets for the clinics, he noted, have included compensation to the church and gift cards for church volunteers who are present at the clinics.)
In the meantime, medical student volunteers from GWU, Howard University, and Georgetown University were trained in telemedicine and attended a “boot camp” on AD “so they’d be able to talk with anyone about it,” Dr. Friedman said.
Advertising “was a learning experience,” he said, and was ultimately multipronged, involving church service announcements, flyers, and, most importantly, Facebook and Instagram advertisements. (People were asked to call a dedicated phone line to schedule an appointment and were invited to register in the GW Medical Faculty Associates records system, though walk-ins to the clinics were still welcomed.)
In a comment, Misty Eleryan, MD, MS, a Mohs micrographic surgeon and dermatologist in Santa Monica, Calif., said dermatology deserts are often found in rural areas and/or areas “with a higher population of marginalized communities, such as Black, Brown, or poorer individuals” – communities that tend to rely on care from urgent care or ED physicians who are unaware of how skin conditions present on darker skin tones.
Programs that educate patients about various presentations of skin conditions are helpful not only for the patients themselves, but could also enable them to help friends, family members, and colleagues, said Dr. Eleryan, who did her residency training at GWU.
“Access,” she noted, is more than just physical access to a person, place, or thing. Referring to a “five A’s” framework described several decades ago, Dr. Eleryan said access to care is characterized by affordability, availability (extent to which the physician has the requisite resources, such as personnel and technology, to meet the patient’s needs), accessibility (geographic), accommodation (extent to which the physician can meet the patient’s constraints and preferences – such as hours of operation, how communications are handled, ability to receive care without prior appointments), and acceptability (extent to which the patient is comfortable with the “more immutable characteristics” of the physician and vice versa).
The GWU program, she said, “is a great start.”
Dr. Friedman said he’s fully invested. There has long been a perception, “rightfully so, that underserved communities are overlooked especially by large institutions. One attendee told me she never expected in her lifetime to see something like this clinic and someone who looked like me caring about her community. ... It certainly says a great deal about the work we need to put in to repair longstanding injury.”
Dr. Friedman disclosed that, in addition to being a recipient of grants from Pfizer and Lilly, he is a speaker for Lilly. Dr. Eleryan said she has no relevant disclosures.
A in other underserved areas in the United States.
Washington, D.C., has “staggering health disparities that are among the largest in the country,” and Ward 8 and surrounding areas in the southeastern part of the city are “dermatology deserts,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who started the program in 2021 with a pilot project. Dr. Friedman spoke about the project, which has since been expanded to include alopecia areata, at the Revolutionizing Atopic Dermatitis conference in April and in an interview after the meeting.
Patients who attend the clinics – held at the Temple of Praise Church in a residential area of Ward 8, a predominantly Black community with a 30% poverty rate – are entered into the GW Medical Faculty Associates medical records system and educated on telemedicine best practices (such as not having light behind them during a session) and how to use telemedicine with their own device.
Those with AD who participate learn about the condition through an image-rich poster showing how it appears in various skin tones, handouts, National Eczema Association films, and discussion with medical students who staff the clinics under Dr. Friedman’s on-site supervision. Participants with alopecia areata similarly can view a poster and converse about the condition.
Patients then have a free 20-minute telehealth visit with a GWU dermatology resident in a private room, and a medical student volunteer nearby to assist with the technology if needed. They leave with a treatment plan, which often includes prescriptions, and a follow-up telemedicine appointment.
The program “is meant to be a stepping point for initiating care ... to set someone up for success for recurrent telehealth visits in the future” and for treatment before symptoms become too severe, Dr. Friedman said in an interview. “We want to demystify telemedicine and educate on the disease state and dispel myths ... so the patient understands why it’s happening” and how it can be treated.
The pilot project, funded with a grant from Pfizer, involved five 2-hour clinics held on Mondays from 4 p.m. to 6 p.m., that together served almost 50 adult and pediatric patients. Grants from Pfizer and Eli Lilly enabled additional clinics in the spring of 2023 and into the summer. And in June, GWU and Pfizer announced a $1 million national grant program focused on broad implementation of what they’ve coined the “Teledermatology Help Desk Clinic” model.
Practices or organizations that secure grants will utilize GWU’s experience and meet with an advisory council of experts in dermatology telemedicine and community advocacy. Having a “long-term plan” and commitment to sustainability is an important element of the model, said Dr. Friedman, who is chairing the grant program.
Patients deem clinic ‘extremely’ helpful
As one of the most prevalent skin disorders – and one with a documented history of elevated risk for specific populations – AD was a good starting point for the teledermatology clinic program. Patients who identify as Black have a higher incidence and prevalence of AD than those who identify as White and Hispanic, and they tend to have more severe disease. Yet they account for fewer visits to dermatologists for AD.
One cross-sectional study of about 3,500 adults in the United States with AD documented that racial/ethnic and socioeconomic disparities reduce outpatient utilization of AD care and increase urgent care and hospital utilization. And in a longitudinal cohort study of children in the United States with AD, Black children with poorly controlled AD were significantly less likely than White children to see a dermatologist.
Like other programs, the GWU department of dermatology had pivoted to telehealth in 2020, and a published survey of patients who attended telehealth appointments during the early part of the pandemic showed that it was generally well liked – and not only for social distancing, but for time efficiency and because transportation was not needed. Only 10% of the 168 patients who completed the survey (out of 894 asked) reported they were unlikely to undertake another telehealth visit. For 10%, eczema was the reason for the visit.
However, only 1% of the survey respondents were from Ward 8, which “begged the question, did those who really need access know this was an option?” Dr. Friedman said at the RAD meeting. He wondered whether there was not only a dermatology desert in Ward 8, but a “technology desert” as well.
Findings from a patient satisfaction survey taken at the end of the pilot program are encouraging, Dr. Friedman said. While data on follow-up visits has not been collected yet, “what I do now have a sense of” is that “the entry point [afforded by the clinics] changed the course in terms of patients’ understanding of the disease and how they feel about its management.”
About 94% of survey respondents indicated the clinic was “extremely” helpful and the remainder said it was “very” helpful; 90% said telehealth significantly changed how they will manage their condition; and 97% said it is “extremely” important to continue the clinics. The majority of patients – 70% – indicated they did not have a dermatologist.
Education about AD at the clinics covers moisturizers/emollients, bathing habits, soaps and detergents, trigger avoidance, and the role of stress and environmental factors in disease exacerbation. Trade samples of moisturizers, mild cleansers, and other products have increasingly been available.
For prescriptions of topical steroids and other commonly prescribed medications, Dr. Friedman and associates combed GoodRx for coupons and surveyed local pharmacies for self-pay pricing to identify least expensive options. Patients with AD who were deemed likely candidates for more advanced therapies in the future were educated about these possibilities.
Alopecia areata
The addition of alopecia areata drew patients with other forms of hair loss as well, but “we weren’t going to turn anyone away who did not have that specific autoimmune form of hair loss,” Dr. Friedman said. Depending on the diagnosis, prescriptions were written for minoxidil and 5-alpha reductase inhibitors.
Important for follow-up is GWU’s acceptance of Medicaid and the availability of both a sliding scale for self-pay and services that assist patients in registering for Medicaid and, if eligible, other insurance plans.
Building partnerships, earning trust
Establishment of the teledermatology clinic program took legwork and relationship building. “You can’t just show up. That’s not enough,” said Dr. Friedman, who also directs the dermatology residency program at GWU. “You have to show through action and through investment of time and energy that you are legitimate, that you’re really there for the long haul.”
Dr. Friedman had assistance from the Rodham Institute, which was established at GWU (and until recently was housed there) and has a history of engagement with local stakeholders such as community centers, church leadership, politicians, and others in the Washington area. He was put in touch with Bishop Deborah Webb at the Temple of Praise Church, a community pillar in Ward 8, and from there “it was a courtship,” he said, with trust to be built and logistics to be worked out. (Budgets for the clinics, he noted, have included compensation to the church and gift cards for church volunteers who are present at the clinics.)
In the meantime, medical student volunteers from GWU, Howard University, and Georgetown University were trained in telemedicine and attended a “boot camp” on AD “so they’d be able to talk with anyone about it,” Dr. Friedman said.
Advertising “was a learning experience,” he said, and was ultimately multipronged, involving church service announcements, flyers, and, most importantly, Facebook and Instagram advertisements. (People were asked to call a dedicated phone line to schedule an appointment and were invited to register in the GW Medical Faculty Associates records system, though walk-ins to the clinics were still welcomed.)
In a comment, Misty Eleryan, MD, MS, a Mohs micrographic surgeon and dermatologist in Santa Monica, Calif., said dermatology deserts are often found in rural areas and/or areas “with a higher population of marginalized communities, such as Black, Brown, or poorer individuals” – communities that tend to rely on care from urgent care or ED physicians who are unaware of how skin conditions present on darker skin tones.
Programs that educate patients about various presentations of skin conditions are helpful not only for the patients themselves, but could also enable them to help friends, family members, and colleagues, said Dr. Eleryan, who did her residency training at GWU.
“Access,” she noted, is more than just physical access to a person, place, or thing. Referring to a “five A’s” framework described several decades ago, Dr. Eleryan said access to care is characterized by affordability, availability (extent to which the physician has the requisite resources, such as personnel and technology, to meet the patient’s needs), accessibility (geographic), accommodation (extent to which the physician can meet the patient’s constraints and preferences – such as hours of operation, how communications are handled, ability to receive care without prior appointments), and acceptability (extent to which the patient is comfortable with the “more immutable characteristics” of the physician and vice versa).
The GWU program, she said, “is a great start.”
Dr. Friedman said he’s fully invested. There has long been a perception, “rightfully so, that underserved communities are overlooked especially by large institutions. One attendee told me she never expected in her lifetime to see something like this clinic and someone who looked like me caring about her community. ... It certainly says a great deal about the work we need to put in to repair longstanding injury.”
Dr. Friedman disclosed that, in addition to being a recipient of grants from Pfizer and Lilly, he is a speaker for Lilly. Dr. Eleryan said she has no relevant disclosures.
Foot rash during self-treatment
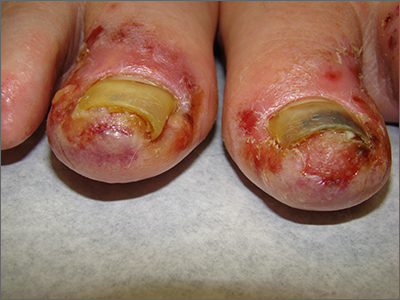
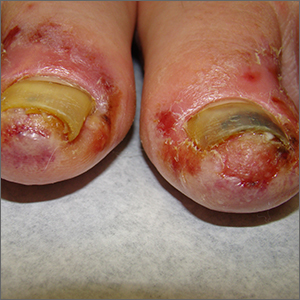
The patient’s toenail thickening appeared consistent with possible onychomycosis—but in addition, there was a marked inflammatory and vesicular eruption consistent with an allergic contact dermatitis.
TTO, also known as melaleuca oil, is a popular product used to treat many disorders including alopecia, seborrheic dermatitis, and onychomycosis.1 Unfortunately, it is a complex compound, and the rate of positive reactions to patch testing ranges from 0.1% to 3.5%.2
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis results from an irritating or relatively caustic substance causing direct damage and inflammation to the skin. In allergic contact dermatitis, as occurred here, there is sensitization to a substance that causes a type IV delayed cell-mediated immune response. Although radioallergosorbent blood testing will usually show immunoglobulin E antibodies to the inciting substance, patch testing is more specific and will show a reaction to the imputed substance on direct skin application. This usually is performed as a panel of antigens tested at the same time.
The mainstay of treatment is to identify, stop use of, and then avoid the sensitizing substance. Topical steroids (triamcinolone 0.1% ointment or clobetasol 0.05% ointment twice daily) are helpful in most cases. If the condition is severe or does not respond to initial therapy, systemic steroids (prednisone 40 mg/d for 5 days for most cases or a 2- to 3-week taper for Rhus dermatitis [eg, poison ivy]) are often effective.3
This patient was instructed to stop using TTO and counseled to avoid it in the future. She was told that her nails might fall off due to the inflammation, which might cure her onychomycosis, and that it takes 12 to 18 months to grow new toenails. She was advised to return for evaluation if the new nails developed any abnormalities or if her onychomycosis recurred. Oral terbinafine 250 mg/d for 90 days is usually a safe and effective therapy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790. doi: 10.1111/j.1365-4632.2012.05654.x
2. de Groot AC, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143. doi: 10.1111/cod.12591
3. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.

The patient’s toenail thickening appeared consistent with possible onychomycosis—but in addition, there was a marked inflammatory and vesicular eruption consistent with an allergic contact dermatitis.
TTO, also known as melaleuca oil, is a popular product used to treat many disorders including alopecia, seborrheic dermatitis, and onychomycosis.1 Unfortunately, it is a complex compound, and the rate of positive reactions to patch testing ranges from 0.1% to 3.5%.2
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis results from an irritating or relatively caustic substance causing direct damage and inflammation to the skin. In allergic contact dermatitis, as occurred here, there is sensitization to a substance that causes a type IV delayed cell-mediated immune response. Although radioallergosorbent blood testing will usually show immunoglobulin E antibodies to the inciting substance, patch testing is more specific and will show a reaction to the imputed substance on direct skin application. This usually is performed as a panel of antigens tested at the same time.
The mainstay of treatment is to identify, stop use of, and then avoid the sensitizing substance. Topical steroids (triamcinolone 0.1% ointment or clobetasol 0.05% ointment twice daily) are helpful in most cases. If the condition is severe or does not respond to initial therapy, systemic steroids (prednisone 40 mg/d for 5 days for most cases or a 2- to 3-week taper for Rhus dermatitis [eg, poison ivy]) are often effective.3
This patient was instructed to stop using TTO and counseled to avoid it in the future. She was told that her nails might fall off due to the inflammation, which might cure her onychomycosis, and that it takes 12 to 18 months to grow new toenails. She was advised to return for evaluation if the new nails developed any abnormalities or if her onychomycosis recurred. Oral terbinafine 250 mg/d for 90 days is usually a safe and effective therapy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The patient’s toenail thickening appeared consistent with possible onychomycosis—but in addition, there was a marked inflammatory and vesicular eruption consistent with an allergic contact dermatitis.
TTO, also known as melaleuca oil, is a popular product used to treat many disorders including alopecia, seborrheic dermatitis, and onychomycosis.1 Unfortunately, it is a complex compound, and the rate of positive reactions to patch testing ranges from 0.1% to 3.5%.2
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis results from an irritating or relatively caustic substance causing direct damage and inflammation to the skin. In allergic contact dermatitis, as occurred here, there is sensitization to a substance that causes a type IV delayed cell-mediated immune response. Although radioallergosorbent blood testing will usually show immunoglobulin E antibodies to the inciting substance, patch testing is more specific and will show a reaction to the imputed substance on direct skin application. This usually is performed as a panel of antigens tested at the same time.
The mainstay of treatment is to identify, stop use of, and then avoid the sensitizing substance. Topical steroids (triamcinolone 0.1% ointment or clobetasol 0.05% ointment twice daily) are helpful in most cases. If the condition is severe or does not respond to initial therapy, systemic steroids (prednisone 40 mg/d for 5 days for most cases or a 2- to 3-week taper for Rhus dermatitis [eg, poison ivy]) are often effective.3
This patient was instructed to stop using TTO and counseled to avoid it in the future. She was told that her nails might fall off due to the inflammation, which might cure her onychomycosis, and that it takes 12 to 18 months to grow new toenails. She was advised to return for evaluation if the new nails developed any abnormalities or if her onychomycosis recurred. Oral terbinafine 250 mg/d for 90 days is usually a safe and effective therapy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790. doi: 10.1111/j.1365-4632.2012.05654.x
2. de Groot AC, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143. doi: 10.1111/cod.12591
3. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
1. Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790. doi: 10.1111/j.1365-4632.2012.05654.x
2. de Groot AC, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143. doi: 10.1111/cod.12591
3. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
Case series supports targeted drugs in treatment of alopecia in children with AD
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
New guidelines for laser treatment of cutaneous vascular anomalies
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
Progress seen on five fronts for substantially improving treatment of epidermolysis bullosa
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
AT SPD 2023
Vitamin D deficiency linked to psoriasis severity
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
FROM NUTRITION 2023
Pediatric dermatologists encouraged to counter misinformation on TikTok, other social media sites
ASHEVILLE, N.C. – , warned an expert at the annual meeting of the Society for Pediatric Dermatology.
“If we don’t get involved, we are basically letting misinformation win. We need to be there,” said Angelo Landriscina, MD, director of dermatology at a Mount Sinai Doctors Clinic in New York.
Most of the content currently available on medical topics, including dermatology and pediatric dermatology, is not created by health care professionals, Dr. Landriscina noted. Not surprisingly, given that much of the content is based on personal opinion from individuals who have no expertise in medical care, he described the information as being of “low quality” when not fully erroneous.
Dr. Landriscina has been active on social media, including TikTok, for several years. Most of his posts involve responses to misinformation. When he sets the record straight on the basis of existing evidence, he often supports his counterargument with references.
He acknowledged that when he became involved in social media he faced criticism from colleagues about participating on an entertainment platform that many considered unworthy of providing objective information. If that was ever true, he argued, it is no longer the case.
“TikTok has adopted a new strategy. The goal is to unseat Google as a search tool, and it’s working,” he said. He explained that many people now use TikTok and other social media sites as their primary source of information on essentially every topic, from where to eat to whether to be screened for cancer.
The particular problem with TikTok – one of the most popular social media outlets – is that there is no mechanism for vetting the source of information. YouTube, by contrast, now requires some sort of validation for anyone who claims to have a medical degree or any other verifiable qualification, according to Dr. Landriscina. TikTok, like many other platforms, has no such requirement.
“Anyone can buy a pair of scrubs [implying expertise] and then post a video,” Dr. Landriscina said.
Even if information from one content provider is more valid than information from others, the TikTok algorithm is specifically designed to emphasize content that has the potential for going viral, which means it favors videos that are provocative over those that are not.
“The algorithm favors any content that is more controversial, more surprising, and keeps viewers engaged,” Dr. Landriscina pointed out.
This does not mean that objective and factual information is ignored, but the algorithm is indifferent to the validity of information, meaning that it allows videos to be posted without regard to whether the content is true, untrue, purposefully misleading, or utter nonsense. For that reason, it is often easier to attract attention by responding to a post that has already gone viral. Information that is clear and digestible can attract viewers and therefore is distributed more widely with the TikTok algorithm.
Parents are on Tiktok too
There is a misperception that the TikTok audience is younger, according to Dr. Landriscina. While peak use in the United States fell among people between the ages of 25 and 34 years in 2022, he said the number of users falls off relatively slowly with subsequent 10-year increments in age. In 2022, there were nearly 20 million users in the peak 10-year age range, but 7.5 million users were 55 years of age or older.
“Pediatric dermatologists should recognize that it is not just kids who are looking for information about their skin diseases, but also their parents,” Dr. Landriscina said.
The top three dermatology topics searched on TikTok in a recent period were acne, alopecia, and cysts. But top searches are very fluid and are extremely hard to quantify, because the basis of the algorithm, which is a proprietary secret, is not only unknown but produces different results for every user.
“The second you touch the app, it changes,” Dr. Landriscina said. He explained that an inquiry about any subject, including those that are medically related, yields content that is different, or at least ordered differently, “depending on how you behaved on the app in the past.”
The phenomenon that drives social media predates this technology. Dr. Landriscina cited a study in 1956 that described the “parasocial interaction theory.” The theory was based on the observation that those who consume media, such as television, which was relatively new in 1956, believed that they had a personal relationship with media figures.
“The users begin to trust influencers as a source, like a friend providing them advice,” Dr. Landriscina said. As an example, he suggested that a fan of the television show Friends who follows actor Jennifer Aniston on social media platforms may begin to think of her as a trusted source of information on any topic, including those for which she may not have expertise.
The reason that he urges medical professionals to become active on TikTok and other social media platforms is that they have a potentially critical role in responding to information that is not just wrong but harmful.
On TikTok and other social media platforms, “there is a lot of interest in content about dermatologic conditions in children. There is a real need for accurate information,” he said,
In the question-and-answer session following his presentation, Dr. Landriscina’s message was not uniformly embraced. One risk, according to an audience member, is that medical professionals will begin to express their own personal opinions rather than rely on evidence, with the result that they will “just add to the sea of misinformation.”
However, this opinion appeared to be the minority view. Most of those who commented took a “that-ship-has-sailed” stance, recognizing the irreversible ascendancy of social media.
“Whether you like it or not, social media is here to stay. We cannot fight it. Rather, we need to embrace it in a responsible way,” said Dakara R. Wright, MD, a dermatologist at the Mid-Atlantic Kaiser Permanente Group, Halethorpe, Md. She, like others, reported that she has come to recognize that social media is a major source of medical information for her patients.
“We need to be a presence on these platforms for the benefit of our patients and their parents,” she said. She acknowledged that she has not been active in posting on social media in the past but said that she has been speaking with administrators in her organization about how to become involved in a responsible way that can be useful to patients.
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, has been active on social media for several years, posting content on her own account, which is not related to her academic affiliation. She posts for many reasons, not least of which is drawing attention to her expertise.
Like Dr. Landriscina, she recognizes that users of these platforms are guided by the content to make decisions about health care. She also agreed that physicians should not ignore this phenomenon.
Tips on providing content
Given the fact that the algorithm is intended to produce posts that go viral, Dr. Landriscina urged clinicians to make their content easy to watch. He said it is not necessary to overthink content beyond providing accurate information, but he advised that videos be made with attention to adequate lighting and other simple factors to promote visual quality. He said that accurate information is not necessarily dull.
“Some facts can actually be surprising to patients,” he said. He noted that a calm, coherent video can be particularly effective in attracting an audience when it is in reaction to information that has gone viral but is misleading or patently incorrect.
Dr. Landriscina has been an influencer associated with multiple social media platforms, including TikTok. He has in the past been paid for consulting work for TikTok. Dr. Wright and Dr. Heath reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , warned an expert at the annual meeting of the Society for Pediatric Dermatology.
“If we don’t get involved, we are basically letting misinformation win. We need to be there,” said Angelo Landriscina, MD, director of dermatology at a Mount Sinai Doctors Clinic in New York.
Most of the content currently available on medical topics, including dermatology and pediatric dermatology, is not created by health care professionals, Dr. Landriscina noted. Not surprisingly, given that much of the content is based on personal opinion from individuals who have no expertise in medical care, he described the information as being of “low quality” when not fully erroneous.
Dr. Landriscina has been active on social media, including TikTok, for several years. Most of his posts involve responses to misinformation. When he sets the record straight on the basis of existing evidence, he often supports his counterargument with references.
He acknowledged that when he became involved in social media he faced criticism from colleagues about participating on an entertainment platform that many considered unworthy of providing objective information. If that was ever true, he argued, it is no longer the case.
“TikTok has adopted a new strategy. The goal is to unseat Google as a search tool, and it’s working,” he said. He explained that many people now use TikTok and other social media sites as their primary source of information on essentially every topic, from where to eat to whether to be screened for cancer.
The particular problem with TikTok – one of the most popular social media outlets – is that there is no mechanism for vetting the source of information. YouTube, by contrast, now requires some sort of validation for anyone who claims to have a medical degree or any other verifiable qualification, according to Dr. Landriscina. TikTok, like many other platforms, has no such requirement.
“Anyone can buy a pair of scrubs [implying expertise] and then post a video,” Dr. Landriscina said.
Even if information from one content provider is more valid than information from others, the TikTok algorithm is specifically designed to emphasize content that has the potential for going viral, which means it favors videos that are provocative over those that are not.
“The algorithm favors any content that is more controversial, more surprising, and keeps viewers engaged,” Dr. Landriscina pointed out.
This does not mean that objective and factual information is ignored, but the algorithm is indifferent to the validity of information, meaning that it allows videos to be posted without regard to whether the content is true, untrue, purposefully misleading, or utter nonsense. For that reason, it is often easier to attract attention by responding to a post that has already gone viral. Information that is clear and digestible can attract viewers and therefore is distributed more widely with the TikTok algorithm.
Parents are on Tiktok too
There is a misperception that the TikTok audience is younger, according to Dr. Landriscina. While peak use in the United States fell among people between the ages of 25 and 34 years in 2022, he said the number of users falls off relatively slowly with subsequent 10-year increments in age. In 2022, there were nearly 20 million users in the peak 10-year age range, but 7.5 million users were 55 years of age or older.
“Pediatric dermatologists should recognize that it is not just kids who are looking for information about their skin diseases, but also their parents,” Dr. Landriscina said.
The top three dermatology topics searched on TikTok in a recent period were acne, alopecia, and cysts. But top searches are very fluid and are extremely hard to quantify, because the basis of the algorithm, which is a proprietary secret, is not only unknown but produces different results for every user.
“The second you touch the app, it changes,” Dr. Landriscina said. He explained that an inquiry about any subject, including those that are medically related, yields content that is different, or at least ordered differently, “depending on how you behaved on the app in the past.”
The phenomenon that drives social media predates this technology. Dr. Landriscina cited a study in 1956 that described the “parasocial interaction theory.” The theory was based on the observation that those who consume media, such as television, which was relatively new in 1956, believed that they had a personal relationship with media figures.
“The users begin to trust influencers as a source, like a friend providing them advice,” Dr. Landriscina said. As an example, he suggested that a fan of the television show Friends who follows actor Jennifer Aniston on social media platforms may begin to think of her as a trusted source of information on any topic, including those for which she may not have expertise.
The reason that he urges medical professionals to become active on TikTok and other social media platforms is that they have a potentially critical role in responding to information that is not just wrong but harmful.
On TikTok and other social media platforms, “there is a lot of interest in content about dermatologic conditions in children. There is a real need for accurate information,” he said,
In the question-and-answer session following his presentation, Dr. Landriscina’s message was not uniformly embraced. One risk, according to an audience member, is that medical professionals will begin to express their own personal opinions rather than rely on evidence, with the result that they will “just add to the sea of misinformation.”
However, this opinion appeared to be the minority view. Most of those who commented took a “that-ship-has-sailed” stance, recognizing the irreversible ascendancy of social media.
“Whether you like it or not, social media is here to stay. We cannot fight it. Rather, we need to embrace it in a responsible way,” said Dakara R. Wright, MD, a dermatologist at the Mid-Atlantic Kaiser Permanente Group, Halethorpe, Md. She, like others, reported that she has come to recognize that social media is a major source of medical information for her patients.
“We need to be a presence on these platforms for the benefit of our patients and their parents,” she said. She acknowledged that she has not been active in posting on social media in the past but said that she has been speaking with administrators in her organization about how to become involved in a responsible way that can be useful to patients.
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, has been active on social media for several years, posting content on her own account, which is not related to her academic affiliation. She posts for many reasons, not least of which is drawing attention to her expertise.
Like Dr. Landriscina, she recognizes that users of these platforms are guided by the content to make decisions about health care. She also agreed that physicians should not ignore this phenomenon.
Tips on providing content
Given the fact that the algorithm is intended to produce posts that go viral, Dr. Landriscina urged clinicians to make their content easy to watch. He said it is not necessary to overthink content beyond providing accurate information, but he advised that videos be made with attention to adequate lighting and other simple factors to promote visual quality. He said that accurate information is not necessarily dull.
“Some facts can actually be surprising to patients,” he said. He noted that a calm, coherent video can be particularly effective in attracting an audience when it is in reaction to information that has gone viral but is misleading or patently incorrect.
Dr. Landriscina has been an influencer associated with multiple social media platforms, including TikTok. He has in the past been paid for consulting work for TikTok. Dr. Wright and Dr. Heath reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , warned an expert at the annual meeting of the Society for Pediatric Dermatology.
“If we don’t get involved, we are basically letting misinformation win. We need to be there,” said Angelo Landriscina, MD, director of dermatology at a Mount Sinai Doctors Clinic in New York.
Most of the content currently available on medical topics, including dermatology and pediatric dermatology, is not created by health care professionals, Dr. Landriscina noted. Not surprisingly, given that much of the content is based on personal opinion from individuals who have no expertise in medical care, he described the information as being of “low quality” when not fully erroneous.
Dr. Landriscina has been active on social media, including TikTok, for several years. Most of his posts involve responses to misinformation. When he sets the record straight on the basis of existing evidence, he often supports his counterargument with references.
He acknowledged that when he became involved in social media he faced criticism from colleagues about participating on an entertainment platform that many considered unworthy of providing objective information. If that was ever true, he argued, it is no longer the case.
“TikTok has adopted a new strategy. The goal is to unseat Google as a search tool, and it’s working,” he said. He explained that many people now use TikTok and other social media sites as their primary source of information on essentially every topic, from where to eat to whether to be screened for cancer.
The particular problem with TikTok – one of the most popular social media outlets – is that there is no mechanism for vetting the source of information. YouTube, by contrast, now requires some sort of validation for anyone who claims to have a medical degree or any other verifiable qualification, according to Dr. Landriscina. TikTok, like many other platforms, has no such requirement.
“Anyone can buy a pair of scrubs [implying expertise] and then post a video,” Dr. Landriscina said.
Even if information from one content provider is more valid than information from others, the TikTok algorithm is specifically designed to emphasize content that has the potential for going viral, which means it favors videos that are provocative over those that are not.
“The algorithm favors any content that is more controversial, more surprising, and keeps viewers engaged,” Dr. Landriscina pointed out.
This does not mean that objective and factual information is ignored, but the algorithm is indifferent to the validity of information, meaning that it allows videos to be posted without regard to whether the content is true, untrue, purposefully misleading, or utter nonsense. For that reason, it is often easier to attract attention by responding to a post that has already gone viral. Information that is clear and digestible can attract viewers and therefore is distributed more widely with the TikTok algorithm.
Parents are on Tiktok too
There is a misperception that the TikTok audience is younger, according to Dr. Landriscina. While peak use in the United States fell among people between the ages of 25 and 34 years in 2022, he said the number of users falls off relatively slowly with subsequent 10-year increments in age. In 2022, there were nearly 20 million users in the peak 10-year age range, but 7.5 million users were 55 years of age or older.
“Pediatric dermatologists should recognize that it is not just kids who are looking for information about their skin diseases, but also their parents,” Dr. Landriscina said.
The top three dermatology topics searched on TikTok in a recent period were acne, alopecia, and cysts. But top searches are very fluid and are extremely hard to quantify, because the basis of the algorithm, which is a proprietary secret, is not only unknown but produces different results for every user.
“The second you touch the app, it changes,” Dr. Landriscina said. He explained that an inquiry about any subject, including those that are medically related, yields content that is different, or at least ordered differently, “depending on how you behaved on the app in the past.”
The phenomenon that drives social media predates this technology. Dr. Landriscina cited a study in 1956 that described the “parasocial interaction theory.” The theory was based on the observation that those who consume media, such as television, which was relatively new in 1956, believed that they had a personal relationship with media figures.
“The users begin to trust influencers as a source, like a friend providing them advice,” Dr. Landriscina said. As an example, he suggested that a fan of the television show Friends who follows actor Jennifer Aniston on social media platforms may begin to think of her as a trusted source of information on any topic, including those for which she may not have expertise.
The reason that he urges medical professionals to become active on TikTok and other social media platforms is that they have a potentially critical role in responding to information that is not just wrong but harmful.
On TikTok and other social media platforms, “there is a lot of interest in content about dermatologic conditions in children. There is a real need for accurate information,” he said,
In the question-and-answer session following his presentation, Dr. Landriscina’s message was not uniformly embraced. One risk, according to an audience member, is that medical professionals will begin to express their own personal opinions rather than rely on evidence, with the result that they will “just add to the sea of misinformation.”
However, this opinion appeared to be the minority view. Most of those who commented took a “that-ship-has-sailed” stance, recognizing the irreversible ascendancy of social media.
“Whether you like it or not, social media is here to stay. We cannot fight it. Rather, we need to embrace it in a responsible way,” said Dakara R. Wright, MD, a dermatologist at the Mid-Atlantic Kaiser Permanente Group, Halethorpe, Md. She, like others, reported that she has come to recognize that social media is a major source of medical information for her patients.
“We need to be a presence on these platforms for the benefit of our patients and their parents,” she said. She acknowledged that she has not been active in posting on social media in the past but said that she has been speaking with administrators in her organization about how to become involved in a responsible way that can be useful to patients.
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, has been active on social media for several years, posting content on her own account, which is not related to her academic affiliation. She posts for many reasons, not least of which is drawing attention to her expertise.
Like Dr. Landriscina, she recognizes that users of these platforms are guided by the content to make decisions about health care. She also agreed that physicians should not ignore this phenomenon.
Tips on providing content
Given the fact that the algorithm is intended to produce posts that go viral, Dr. Landriscina urged clinicians to make their content easy to watch. He said it is not necessary to overthink content beyond providing accurate information, but he advised that videos be made with attention to adequate lighting and other simple factors to promote visual quality. He said that accurate information is not necessarily dull.
“Some facts can actually be surprising to patients,” he said. He noted that a calm, coherent video can be particularly effective in attracting an audience when it is in reaction to information that has gone viral but is misleading or patently incorrect.
Dr. Landriscina has been an influencer associated with multiple social media platforms, including TikTok. He has in the past been paid for consulting work for TikTok. Dr. Wright and Dr. Heath reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
AT SPD 2023
Squamous cell carcinoma
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (FIGURE A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (FIGURE B). SCC is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3
Epidemiology
SCC is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N = 413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see highrisk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17
Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 SCC arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (FIGURE C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
SCC is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
Continue to: The risk for SCC...
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
ACKNOWLEDGMENT
The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
1. Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi: 10.1097/ DSS.0000000000000292
2. Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi: 10.1002/1096-9071(200007)61:3<289::aidjmv2> 3.0.co;2-z
3. Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. doi: 10.1111/ijd.12553.
4. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public J Am Acad Dermatol. 2014;70:748-762. doi: 10.1016/j.jaad.2013.11.038
5. Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
6. Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
7. Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. doi: 10.1016/ j.ijwd.2021.01.017
8. Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
9. Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi: 10.1001/jamadermatol.2016.3328
10. Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi: 10.1093/jnci/ djj092
11. Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173:17-21. doi: 10.1111/bjd.13380
12. Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi: 10.1016/ s1011-1344(01)00198-1
13. Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
14. Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi: 10.1016/s0190-9622 (81)70113-0
15. Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi: 10.5826/dpc.0902a09
16. Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
17. Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635 (03)00085-8
18. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
19. Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009; 61:426-432.
20. Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
21. Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. Part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. doi: 10.1016/j.jaad.2021. 12.062
22. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137- 151. doi: 10.1007/s40257-021-00662-z
23. Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi: 10.1046/j.1365- 2230.2003.01210.x
24. Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi: 10.1200/ GO.20.00094
25. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi: 10.1016/j.det.2019.05.009
26. Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
27. Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (FIGURE A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (FIGURE B). SCC is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
SCC is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N = 413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see highrisk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17
Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 SCC arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (FIGURE C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
SCC is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
Continue to: The risk for SCC...
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
ACKNOWLEDGMENT
The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (FIGURE A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (FIGURE B). SCC is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
SCC is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N = 413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see highrisk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17
Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 SCC arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (FIGURE C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
SCC is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
Continue to: The risk for SCC...
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
ACKNOWLEDGMENT
The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
1. Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi: 10.1097/ DSS.0000000000000292
2. Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi: 10.1002/1096-9071(200007)61:3<289::aidjmv2> 3.0.co;2-z
3. Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. doi: 10.1111/ijd.12553.
4. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public J Am Acad Dermatol. 2014;70:748-762. doi: 10.1016/j.jaad.2013.11.038
5. Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
6. Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
7. Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. doi: 10.1016/ j.ijwd.2021.01.017
8. Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
9. Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi: 10.1001/jamadermatol.2016.3328
10. Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi: 10.1093/jnci/ djj092
11. Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173:17-21. doi: 10.1111/bjd.13380
12. Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi: 10.1016/ s1011-1344(01)00198-1
13. Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
14. Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi: 10.1016/s0190-9622 (81)70113-0
15. Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi: 10.5826/dpc.0902a09
16. Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
17. Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635 (03)00085-8
18. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
19. Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009; 61:426-432.
20. Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
21. Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. Part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. doi: 10.1016/j.jaad.2021. 12.062
22. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137- 151. doi: 10.1007/s40257-021-00662-z
23. Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi: 10.1046/j.1365- 2230.2003.01210.x
24. Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi: 10.1200/ GO.20.00094
25. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi: 10.1016/j.det.2019.05.009
26. Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
27. Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
1. Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi: 10.1097/ DSS.0000000000000292
2. Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi: 10.1002/1096-9071(200007)61:3<289::aidjmv2> 3.0.co;2-z
3. Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. doi: 10.1111/ijd.12553.
4. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public J Am Acad Dermatol. 2014;70:748-762. doi: 10.1016/j.jaad.2013.11.038
5. Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
6. Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
7. Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. doi: 10.1016/ j.ijwd.2021.01.017
8. Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
9. Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi: 10.1001/jamadermatol.2016.3328
10. Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi: 10.1093/jnci/ djj092
11. Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173:17-21. doi: 10.1111/bjd.13380
12. Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi: 10.1016/ s1011-1344(01)00198-1
13. Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
14. Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi: 10.1016/s0190-9622 (81)70113-0
15. Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi: 10.5826/dpc.0902a09
16. Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
17. Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635 (03)00085-8
18. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
19. Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009; 61:426-432.
20. Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
21. Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. Part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. doi: 10.1016/j.jaad.2021. 12.062
22. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137- 151. doi: 10.1007/s40257-021-00662-z
23. Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi: 10.1046/j.1365- 2230.2003.01210.x
24. Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi: 10.1200/ GO.20.00094
25. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi: 10.1016/j.det.2019.05.009
26. Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
27. Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Study examines burden of vitiligo in the U.S.
To investigate the incidence and prevalence of diagnosed vitiligo in the United States, researchers used a 15% random sample of electronic medical records from the IBM Explorys database. Two cohorts were included: 2,980,778 patients diagnosed with vitiligo between Jan. 1, 2015, and Dec. 31, 2019 (incidence analysis), and 1,057,534 patients diagnosed with vitiligo between Jan. 1 and Dec. 31, 2019 (prevalence analysis).The main outcomes were incidence (per 100,000 person-years) and prevalence of diagnosed vitiligo overall and by age, race/ethnicity, and sex. Amit Garg, MD, a dermatologist with Northwell Health, New Hyde Park, N.Y., led the study, which was published in JAMA Dermatology.
The age- and sex-adjusted overall incidence rate of diagnosed vitiligo was 22.6 per 100,000 person-years, and the prevalence was 0.16%, the authors reported. The sex-adjusted IR was highest among patients aged 60-69 years (25.3 per 100,000 person-years); prevalence was highest among patients aged 70 years or older (0.21%).
The highest age-adjusted IR was among Asian American patients (41.2 per 100,000 person-years), followed by Hispanic/Latino patients (37.3 per 100,000 PY), those reporting other or multiple races (31.1 per 100,000), Black patients (29.6 per 100,000 person-years), and White patients (18.7 per 100,000 person-years). The highest age-adjusted prevalence was among Hispanic/Latino patients (0.29%), followed by Asian American patients (0.27%), those reporting other or multiple races (0.24%), Black patients (0.22%), and White patients (0.13%).
The burden of vitiligo in the United States is poorly understood, and the findings “may support improving awareness of vitiligo disease burden in medical and public sectors, informing research agendas, improving enrollment of racial and ethnic minority populations in trials, and developing health policies,” the authors wrote.
Limitations of the study included that the analysis only captured patients who sought care in health systems included in the database, and there was the potential for underreporting, “since not all patients with vitiligo seek care,” the authors noted.
Dr. Garg reported being an adviser for and receiving honoraria from many pharmaceutical companies. He has also received research grants from AbbVie, UCB, the National Psoriasis Foundation, and the CHORD COUSIN Collaboration. No other disclosures were reported.
A version of this article first appeared on Medscape.com .
To investigate the incidence and prevalence of diagnosed vitiligo in the United States, researchers used a 15% random sample of electronic medical records from the IBM Explorys database. Two cohorts were included: 2,980,778 patients diagnosed with vitiligo between Jan. 1, 2015, and Dec. 31, 2019 (incidence analysis), and 1,057,534 patients diagnosed with vitiligo between Jan. 1 and Dec. 31, 2019 (prevalence analysis).The main outcomes were incidence (per 100,000 person-years) and prevalence of diagnosed vitiligo overall and by age, race/ethnicity, and sex. Amit Garg, MD, a dermatologist with Northwell Health, New Hyde Park, N.Y., led the study, which was published in JAMA Dermatology.
The age- and sex-adjusted overall incidence rate of diagnosed vitiligo was 22.6 per 100,000 person-years, and the prevalence was 0.16%, the authors reported. The sex-adjusted IR was highest among patients aged 60-69 years (25.3 per 100,000 person-years); prevalence was highest among patients aged 70 years or older (0.21%).
The highest age-adjusted IR was among Asian American patients (41.2 per 100,000 person-years), followed by Hispanic/Latino patients (37.3 per 100,000 PY), those reporting other or multiple races (31.1 per 100,000), Black patients (29.6 per 100,000 person-years), and White patients (18.7 per 100,000 person-years). The highest age-adjusted prevalence was among Hispanic/Latino patients (0.29%), followed by Asian American patients (0.27%), those reporting other or multiple races (0.24%), Black patients (0.22%), and White patients (0.13%).
The burden of vitiligo in the United States is poorly understood, and the findings “may support improving awareness of vitiligo disease burden in medical and public sectors, informing research agendas, improving enrollment of racial and ethnic minority populations in trials, and developing health policies,” the authors wrote.
Limitations of the study included that the analysis only captured patients who sought care in health systems included in the database, and there was the potential for underreporting, “since not all patients with vitiligo seek care,” the authors noted.
Dr. Garg reported being an adviser for and receiving honoraria from many pharmaceutical companies. He has also received research grants from AbbVie, UCB, the National Psoriasis Foundation, and the CHORD COUSIN Collaboration. No other disclosures were reported.
A version of this article first appeared on Medscape.com .
To investigate the incidence and prevalence of diagnosed vitiligo in the United States, researchers used a 15% random sample of electronic medical records from the IBM Explorys database. Two cohorts were included: 2,980,778 patients diagnosed with vitiligo between Jan. 1, 2015, and Dec. 31, 2019 (incidence analysis), and 1,057,534 patients diagnosed with vitiligo between Jan. 1 and Dec. 31, 2019 (prevalence analysis).The main outcomes were incidence (per 100,000 person-years) and prevalence of diagnosed vitiligo overall and by age, race/ethnicity, and sex. Amit Garg, MD, a dermatologist with Northwell Health, New Hyde Park, N.Y., led the study, which was published in JAMA Dermatology.
The age- and sex-adjusted overall incidence rate of diagnosed vitiligo was 22.6 per 100,000 person-years, and the prevalence was 0.16%, the authors reported. The sex-adjusted IR was highest among patients aged 60-69 years (25.3 per 100,000 person-years); prevalence was highest among patients aged 70 years or older (0.21%).
The highest age-adjusted IR was among Asian American patients (41.2 per 100,000 person-years), followed by Hispanic/Latino patients (37.3 per 100,000 PY), those reporting other or multiple races (31.1 per 100,000), Black patients (29.6 per 100,000 person-years), and White patients (18.7 per 100,000 person-years). The highest age-adjusted prevalence was among Hispanic/Latino patients (0.29%), followed by Asian American patients (0.27%), those reporting other or multiple races (0.24%), Black patients (0.22%), and White patients (0.13%).
The burden of vitiligo in the United States is poorly understood, and the findings “may support improving awareness of vitiligo disease burden in medical and public sectors, informing research agendas, improving enrollment of racial and ethnic minority populations in trials, and developing health policies,” the authors wrote.
Limitations of the study included that the analysis only captured patients who sought care in health systems included in the database, and there was the potential for underreporting, “since not all patients with vitiligo seek care,” the authors noted.
Dr. Garg reported being an adviser for and receiving honoraria from many pharmaceutical companies. He has also received research grants from AbbVie, UCB, the National Psoriasis Foundation, and the CHORD COUSIN Collaboration. No other disclosures were reported.
A version of this article first appeared on Medscape.com .
FROM JAMA DERMATOLOGY