User login
Analysis reveals recent acne prescribing trends
While.
Notably, isotretinoin prescribing among men and women decreased slightly during the study period, “which may reflect ongoing administrative burdens associated with iPLEDGE,” study author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, told this news organization.
For the cross-sectional study, which was published online as a research letter in JAMA Dermatology, Dr. Barbieri drew from the Truven Health MarketScan Commercial Claims Database from Jan. 1, 2017, to Dec. 31, 2020, to identify individuals with an encounter for acne, prescriptions for oral tetracycline antibiotics (doxycycline, minocycline), other commonly prescribed oral antibiotics (trimethoprim-sulfamethoxazole, amoxicillin, cephalexin), spironolactone, and isotretinoin. Only drug courses greater than 28 days were included in the analysis, and Dr. Barbieri stratified them according to clinician type (dermatologist, nondermatology physician, and nurse-practitioner or physician assistant). To normalize prescribing rates (to address possible changes in the number of patients treated for acne over time), the number of treatment courses prescribed each year was standardized to the number of encounters for acne with that clinician type during the same calendar year.
The study period included a mean of 1.9 million acne encounters per year.
Dr. Barbieri found that dermatologists prescribed more oral antibiotics per clinician for acne than any other major medical specialty and that oral antibiotics remained frequently prescribed for treating acne by both dermatologists and nondermatologists. “Among oral antibiotics, minocycline and trimethoprim-sulfamethoxazole remain relatively commonly prescribed, despite potential safety concerns and a lack of evidence that they are any more effective than doxycycline,” he said in an interview.
“Patient outcomes could likely be improved by reducing use of minocycline and particularly trimethoprim-sulfamethoxazole given its high risk of serious side effects such as SJS/TEN [Stevens-Johnson syndrome/toxic epidermal necrolysis] and acute respiratory failure,” he added.
Dr. Barbieri noted that there are likely opportunities to consider nonantibiotic alternatives such as hormonal therapy (spironolactone, combined oral contraceptives) and isotretinoin. “There is also a need for continued research to identify nonantibiotic treatment options for patients with acne,” he said.
The analysis revealed that for women with acne prescriptions for spironolactone increased about three- to fourfold during the study period among all clinician types. In 2017, oral antibiotics were prescribed about two- to threefold more often than spironolactone, but by 2020 they were being prescribed at about the same frequency. “Given spironolactone may have similar effectiveness to oral antibiotics in the treatment of acne, this shift in practice has the potential to improve outcomes for patients by reducing the risk of antibiotic-associated complications,” Dr. Barbieri wrote. Still, in 2020, oral antibiotics were still slightly more commonly prescribed than spironolactone by nondermatology physicians and NP or PAs.
In other findings, isotretinoin prescribing decreased slightly among male and female patients during the study period. Among antibiotic prescriptions, prescribing for doxycycline increased at a higher rate than prescribing for minocycline, especially among dermatologists and NPs or PAs.
In the interview, Dr. Barbieri acknowledged certain limitations of the study, including the fact that the dataset “does not allow for evaluation of severity of acne and it is not possible to directly link prescriptions to diagnoses, so some prescriptions might not be for acne and others that are for acne might not have been included.”
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the results, said that, while a course of antibiotic therapy was tied to an office visit in the analysis, the duration of each course of therapy was unclear. It would be interesting to see if antibiotic courses became shorter during the time period analyzed, such as 1-3 months versus 4 or more months, he added, “as this should reduce risks associated with long-term use of oral antibiotics.”
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
While.
Notably, isotretinoin prescribing among men and women decreased slightly during the study period, “which may reflect ongoing administrative burdens associated with iPLEDGE,” study author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, told this news organization.
For the cross-sectional study, which was published online as a research letter in JAMA Dermatology, Dr. Barbieri drew from the Truven Health MarketScan Commercial Claims Database from Jan. 1, 2017, to Dec. 31, 2020, to identify individuals with an encounter for acne, prescriptions for oral tetracycline antibiotics (doxycycline, minocycline), other commonly prescribed oral antibiotics (trimethoprim-sulfamethoxazole, amoxicillin, cephalexin), spironolactone, and isotretinoin. Only drug courses greater than 28 days were included in the analysis, and Dr. Barbieri stratified them according to clinician type (dermatologist, nondermatology physician, and nurse-practitioner or physician assistant). To normalize prescribing rates (to address possible changes in the number of patients treated for acne over time), the number of treatment courses prescribed each year was standardized to the number of encounters for acne with that clinician type during the same calendar year.
The study period included a mean of 1.9 million acne encounters per year.
Dr. Barbieri found that dermatologists prescribed more oral antibiotics per clinician for acne than any other major medical specialty and that oral antibiotics remained frequently prescribed for treating acne by both dermatologists and nondermatologists. “Among oral antibiotics, minocycline and trimethoprim-sulfamethoxazole remain relatively commonly prescribed, despite potential safety concerns and a lack of evidence that they are any more effective than doxycycline,” he said in an interview.
“Patient outcomes could likely be improved by reducing use of minocycline and particularly trimethoprim-sulfamethoxazole given its high risk of serious side effects such as SJS/TEN [Stevens-Johnson syndrome/toxic epidermal necrolysis] and acute respiratory failure,” he added.
Dr. Barbieri noted that there are likely opportunities to consider nonantibiotic alternatives such as hormonal therapy (spironolactone, combined oral contraceptives) and isotretinoin. “There is also a need for continued research to identify nonantibiotic treatment options for patients with acne,” he said.
The analysis revealed that for women with acne prescriptions for spironolactone increased about three- to fourfold during the study period among all clinician types. In 2017, oral antibiotics were prescribed about two- to threefold more often than spironolactone, but by 2020 they were being prescribed at about the same frequency. “Given spironolactone may have similar effectiveness to oral antibiotics in the treatment of acne, this shift in practice has the potential to improve outcomes for patients by reducing the risk of antibiotic-associated complications,” Dr. Barbieri wrote. Still, in 2020, oral antibiotics were still slightly more commonly prescribed than spironolactone by nondermatology physicians and NP or PAs.
In other findings, isotretinoin prescribing decreased slightly among male and female patients during the study period. Among antibiotic prescriptions, prescribing for doxycycline increased at a higher rate than prescribing for minocycline, especially among dermatologists and NPs or PAs.
In the interview, Dr. Barbieri acknowledged certain limitations of the study, including the fact that the dataset “does not allow for evaluation of severity of acne and it is not possible to directly link prescriptions to diagnoses, so some prescriptions might not be for acne and others that are for acne might not have been included.”
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the results, said that, while a course of antibiotic therapy was tied to an office visit in the analysis, the duration of each course of therapy was unclear. It would be interesting to see if antibiotic courses became shorter during the time period analyzed, such as 1-3 months versus 4 or more months, he added, “as this should reduce risks associated with long-term use of oral antibiotics.”
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
While.
Notably, isotretinoin prescribing among men and women decreased slightly during the study period, “which may reflect ongoing administrative burdens associated with iPLEDGE,” study author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, told this news organization.
For the cross-sectional study, which was published online as a research letter in JAMA Dermatology, Dr. Barbieri drew from the Truven Health MarketScan Commercial Claims Database from Jan. 1, 2017, to Dec. 31, 2020, to identify individuals with an encounter for acne, prescriptions for oral tetracycline antibiotics (doxycycline, minocycline), other commonly prescribed oral antibiotics (trimethoprim-sulfamethoxazole, amoxicillin, cephalexin), spironolactone, and isotretinoin. Only drug courses greater than 28 days were included in the analysis, and Dr. Barbieri stratified them according to clinician type (dermatologist, nondermatology physician, and nurse-practitioner or physician assistant). To normalize prescribing rates (to address possible changes in the number of patients treated for acne over time), the number of treatment courses prescribed each year was standardized to the number of encounters for acne with that clinician type during the same calendar year.
The study period included a mean of 1.9 million acne encounters per year.
Dr. Barbieri found that dermatologists prescribed more oral antibiotics per clinician for acne than any other major medical specialty and that oral antibiotics remained frequently prescribed for treating acne by both dermatologists and nondermatologists. “Among oral antibiotics, minocycline and trimethoprim-sulfamethoxazole remain relatively commonly prescribed, despite potential safety concerns and a lack of evidence that they are any more effective than doxycycline,” he said in an interview.
“Patient outcomes could likely be improved by reducing use of minocycline and particularly trimethoprim-sulfamethoxazole given its high risk of serious side effects such as SJS/TEN [Stevens-Johnson syndrome/toxic epidermal necrolysis] and acute respiratory failure,” he added.
Dr. Barbieri noted that there are likely opportunities to consider nonantibiotic alternatives such as hormonal therapy (spironolactone, combined oral contraceptives) and isotretinoin. “There is also a need for continued research to identify nonantibiotic treatment options for patients with acne,” he said.
The analysis revealed that for women with acne prescriptions for spironolactone increased about three- to fourfold during the study period among all clinician types. In 2017, oral antibiotics were prescribed about two- to threefold more often than spironolactone, but by 2020 they were being prescribed at about the same frequency. “Given spironolactone may have similar effectiveness to oral antibiotics in the treatment of acne, this shift in practice has the potential to improve outcomes for patients by reducing the risk of antibiotic-associated complications,” Dr. Barbieri wrote. Still, in 2020, oral antibiotics were still slightly more commonly prescribed than spironolactone by nondermatology physicians and NP or PAs.
In other findings, isotretinoin prescribing decreased slightly among male and female patients during the study period. Among antibiotic prescriptions, prescribing for doxycycline increased at a higher rate than prescribing for minocycline, especially among dermatologists and NPs or PAs.
In the interview, Dr. Barbieri acknowledged certain limitations of the study, including the fact that the dataset “does not allow for evaluation of severity of acne and it is not possible to directly link prescriptions to diagnoses, so some prescriptions might not be for acne and others that are for acne might not have been included.”
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the results, said that, while a course of antibiotic therapy was tied to an office visit in the analysis, the duration of each course of therapy was unclear. It would be interesting to see if antibiotic courses became shorter during the time period analyzed, such as 1-3 months versus 4 or more months, he added, “as this should reduce risks associated with long-term use of oral antibiotics.”
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM JAMA DERMATOLOGY
New evidence suggests genetic risk factors in hidradenitis suppurativa
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with lesions that include deep-seated nodules and abscesses, draining tracts, and fibrotic scars, and has been reported to be highly heritable.
A genomewide association study (GWAS) that involved a meta-analysis of data from three large biobanks (UK Biobank, FinnGen, and BioVU) identified one significant locus and two suggestive loci. The researchers were able to replicate the association with HS for two loci near the SOX9 and KLF5 genes in BioVU.
In addition, they also looked at genetic correlations between HS and autoimmune and inflammatory diseases, and results suggested a positive association with inflammatory bowel disease, psoriasis, and type 2 diabetes.
However, while plausible, the variant associations near candidate genes do not prove a causal effect of these variants or genes on disease risk, and further study is needed.
“It’s possible that these genes aren’t the ones affected by the variations we describe, but both are very strong candidates,” said study author Christopher J. Sayed, MD, associate professor of dermatology, University of North Carolina at Chapel Hill. “This improves our understanding of HS as a disease that is likely related to genetic changes that result in dysregulated hair follicle maintenance, inflammation, and wound healing.”
In turn, this will allow clinicians to educate patients about the underlying genetic influences on HS, he explained, as opposed to stigmatizing misconceptions focusing only on weight, smoking, and hygiene.
“Larger studies are underway and will be needed to find variants that may predict things like disease severity and response to treatment, but this is a big first step in the right direction,” Dr. Sayed added.
The study was published online in JAMA Dermatology.
Loci identified
GWASs have been limited in HS, and previous research has not identified significant risk loci. In the current study, Dr. Sayed and colleagues sought to identify underlying genes and genetic mechanisms that may underlie pathogenesis in HS.
They performed a GWAS in a cohort of 720 patients who were part of the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the UNC department of dermatology, and controls from the National Longitudinal Study of Adolescent to Adult Health (Add Health) study, a U.S.-based study following adolescents through adulthood.
The UK Biobank (UKB) is a prospective biobank with about 500,000 individuals aged 40-69 years, and FinnGen collects and analyzes genome and health data from Finnish biobank participants. To increase power to detect associations, a GWAS was performed using participants from the UKB (247 HS cases, 453,048 controls). The HS ProCARE GWAS results were meta-analyzed with UKB, along with data from FinnGen (673 HS cases, 297,544 controls). This three-way meta-analysis revealed one genomewide significant locus and two suggestive loci.
The authors found that the most strongly associated variant, rs10512572 located on chromosome 17, showed the strongest association in FinnGen; at the second locus, the most strongly associated variant was rs17090189 located on chromosome 13 and also showed the strongest association in FinnGen; and at the third locus, the most strongly associated variant, rs5792315, located on chromosome 11, showed the strongest association in HS ProCARE.
Next, they tested the 10 most strongly associated variants at the three loci in the BioVU biobank, which has 290 HS cases, including 189 individuals of European ancestry and 101 individuals of African ancestry, with 64,234 and 12,105 controls, respectively. The locus on chromosome 17 was replicated in BioVU in the same direction of effect, while one variant at the chromosome 13 locus showed nominal evidence of association in the same direction.
In a four-way meta-analysis of BioVU combined with the other three studies, the chromosome locus became more significant and the chromosome 13 locus exceeded the genomewide significance threshold. In contrast, the chromosome 11 locus was not replicated in BioVU (P = .27).
The authors pointed out that variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, which play a role in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
Finally, they looked to see if there were shared genetic components between HS and autoimmune and inflammatory diseases. A nominally significant genetic correlation was observed between HS and inflammatory bowel disease (P = .04), psoriasis (P = .03), and type 2 diabetes (P = .04), although none reached significance.
Different manifestation with genetic variant
In a related study, researchers assessed the prevalence of the NCSTN:c.671_682del variant among individuals with HS in Malta, the island country in the Mediterranean, which has a high prevalence of HS and its associated risk factors, particularly obesity.
Led by Dillon Mintoff, MD, of the department of pathology at the University of Malta, Msida, they conducted a cross-sectional study of 113 adults with HS. In this group, 14 (12.39%) were found to be heterozygous for the NCSTN:c.671_682del variant. Individuals who had this variant were more likely to develop symptoms earlier and to manifest them in atypical skin sites, including the scalp, neck, torso, and antecubital fossae. Additionally, even though their symptoms weren’t more severe than those without the variant, patients with the variant were more likely to require treatment with biologic agents.
Studies move genetics in HS forward
Writing in an accompanying editorial, Atlas Khan, PhD, and Lynn Petukhova, PhD, from Columbia University, New York, noted that both of these HS genetic studies “set a solid foundation for future studies aimed at understanding the biological and clinical relevance of new HS genetic evidence.”
“Each study suggests a series of experiments that will allow us to gain new knowledge about HS,” they wrote, including coming closer to providing patients with a genetic diagnosis.
In addition, evidence from the GWAS paper suggests that with “larger HS GWASs we will be able to better prioritize drug-repurposing studies,” concluded the editorialists. “Both of these goals will help to improve health outcomes for patients with HS and their family members.”
Dr. Sayed reported grants and/or personal fees from AbbVie, Novartis, UCB, Incyte, InflaRx, Alumis, and ChemoCentryx outside the submitted work; and serving as a volunteer member of the board of the HS Foundation and member of the European HS Foundation. The study in Malta was funded by the Government of Malta’s Tertiary Education Scholarship Scheme and Institutional Funds from the University of Malta. Dr. Mintoff reported grants from the Government of Malta Ministry for Education, Sport, Youth, Research and Innovation during the conduct of the study. Dr. Khan reported receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases. Dr. Petukhova reported receiving grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Columbia University’s Precision Medicine Initiative, Herbert Irving Comprehensive Cancer Center, and Data Science Institute.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with lesions that include deep-seated nodules and abscesses, draining tracts, and fibrotic scars, and has been reported to be highly heritable.
A genomewide association study (GWAS) that involved a meta-analysis of data from three large biobanks (UK Biobank, FinnGen, and BioVU) identified one significant locus and two suggestive loci. The researchers were able to replicate the association with HS for two loci near the SOX9 and KLF5 genes in BioVU.
In addition, they also looked at genetic correlations between HS and autoimmune and inflammatory diseases, and results suggested a positive association with inflammatory bowel disease, psoriasis, and type 2 diabetes.
However, while plausible, the variant associations near candidate genes do not prove a causal effect of these variants or genes on disease risk, and further study is needed.
“It’s possible that these genes aren’t the ones affected by the variations we describe, but both are very strong candidates,” said study author Christopher J. Sayed, MD, associate professor of dermatology, University of North Carolina at Chapel Hill. “This improves our understanding of HS as a disease that is likely related to genetic changes that result in dysregulated hair follicle maintenance, inflammation, and wound healing.”
In turn, this will allow clinicians to educate patients about the underlying genetic influences on HS, he explained, as opposed to stigmatizing misconceptions focusing only on weight, smoking, and hygiene.
“Larger studies are underway and will be needed to find variants that may predict things like disease severity and response to treatment, but this is a big first step in the right direction,” Dr. Sayed added.
The study was published online in JAMA Dermatology.
Loci identified
GWASs have been limited in HS, and previous research has not identified significant risk loci. In the current study, Dr. Sayed and colleagues sought to identify underlying genes and genetic mechanisms that may underlie pathogenesis in HS.
They performed a GWAS in a cohort of 720 patients who were part of the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the UNC department of dermatology, and controls from the National Longitudinal Study of Adolescent to Adult Health (Add Health) study, a U.S.-based study following adolescents through adulthood.
The UK Biobank (UKB) is a prospective biobank with about 500,000 individuals aged 40-69 years, and FinnGen collects and analyzes genome and health data from Finnish biobank participants. To increase power to detect associations, a GWAS was performed using participants from the UKB (247 HS cases, 453,048 controls). The HS ProCARE GWAS results were meta-analyzed with UKB, along with data from FinnGen (673 HS cases, 297,544 controls). This three-way meta-analysis revealed one genomewide significant locus and two suggestive loci.
The authors found that the most strongly associated variant, rs10512572 located on chromosome 17, showed the strongest association in FinnGen; at the second locus, the most strongly associated variant was rs17090189 located on chromosome 13 and also showed the strongest association in FinnGen; and at the third locus, the most strongly associated variant, rs5792315, located on chromosome 11, showed the strongest association in HS ProCARE.
Next, they tested the 10 most strongly associated variants at the three loci in the BioVU biobank, which has 290 HS cases, including 189 individuals of European ancestry and 101 individuals of African ancestry, with 64,234 and 12,105 controls, respectively. The locus on chromosome 17 was replicated in BioVU in the same direction of effect, while one variant at the chromosome 13 locus showed nominal evidence of association in the same direction.
In a four-way meta-analysis of BioVU combined with the other three studies, the chromosome locus became more significant and the chromosome 13 locus exceeded the genomewide significance threshold. In contrast, the chromosome 11 locus was not replicated in BioVU (P = .27).
The authors pointed out that variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, which play a role in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
Finally, they looked to see if there were shared genetic components between HS and autoimmune and inflammatory diseases. A nominally significant genetic correlation was observed between HS and inflammatory bowel disease (P = .04), psoriasis (P = .03), and type 2 diabetes (P = .04), although none reached significance.
Different manifestation with genetic variant
In a related study, researchers assessed the prevalence of the NCSTN:c.671_682del variant among individuals with HS in Malta, the island country in the Mediterranean, which has a high prevalence of HS and its associated risk factors, particularly obesity.
Led by Dillon Mintoff, MD, of the department of pathology at the University of Malta, Msida, they conducted a cross-sectional study of 113 adults with HS. In this group, 14 (12.39%) were found to be heterozygous for the NCSTN:c.671_682del variant. Individuals who had this variant were more likely to develop symptoms earlier and to manifest them in atypical skin sites, including the scalp, neck, torso, and antecubital fossae. Additionally, even though their symptoms weren’t more severe than those without the variant, patients with the variant were more likely to require treatment with biologic agents.
Studies move genetics in HS forward
Writing in an accompanying editorial, Atlas Khan, PhD, and Lynn Petukhova, PhD, from Columbia University, New York, noted that both of these HS genetic studies “set a solid foundation for future studies aimed at understanding the biological and clinical relevance of new HS genetic evidence.”
“Each study suggests a series of experiments that will allow us to gain new knowledge about HS,” they wrote, including coming closer to providing patients with a genetic diagnosis.
In addition, evidence from the GWAS paper suggests that with “larger HS GWASs we will be able to better prioritize drug-repurposing studies,” concluded the editorialists. “Both of these goals will help to improve health outcomes for patients with HS and their family members.”
Dr. Sayed reported grants and/or personal fees from AbbVie, Novartis, UCB, Incyte, InflaRx, Alumis, and ChemoCentryx outside the submitted work; and serving as a volunteer member of the board of the HS Foundation and member of the European HS Foundation. The study in Malta was funded by the Government of Malta’s Tertiary Education Scholarship Scheme and Institutional Funds from the University of Malta. Dr. Mintoff reported grants from the Government of Malta Ministry for Education, Sport, Youth, Research and Innovation during the conduct of the study. Dr. Khan reported receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases. Dr. Petukhova reported receiving grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Columbia University’s Precision Medicine Initiative, Herbert Irving Comprehensive Cancer Center, and Data Science Institute.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with lesions that include deep-seated nodules and abscesses, draining tracts, and fibrotic scars, and has been reported to be highly heritable.
A genomewide association study (GWAS) that involved a meta-analysis of data from three large biobanks (UK Biobank, FinnGen, and BioVU) identified one significant locus and two suggestive loci. The researchers were able to replicate the association with HS for two loci near the SOX9 and KLF5 genes in BioVU.
In addition, they also looked at genetic correlations between HS and autoimmune and inflammatory diseases, and results suggested a positive association with inflammatory bowel disease, psoriasis, and type 2 diabetes.
However, while plausible, the variant associations near candidate genes do not prove a causal effect of these variants or genes on disease risk, and further study is needed.
“It’s possible that these genes aren’t the ones affected by the variations we describe, but both are very strong candidates,” said study author Christopher J. Sayed, MD, associate professor of dermatology, University of North Carolina at Chapel Hill. “This improves our understanding of HS as a disease that is likely related to genetic changes that result in dysregulated hair follicle maintenance, inflammation, and wound healing.”
In turn, this will allow clinicians to educate patients about the underlying genetic influences on HS, he explained, as opposed to stigmatizing misconceptions focusing only on weight, smoking, and hygiene.
“Larger studies are underway and will be needed to find variants that may predict things like disease severity and response to treatment, but this is a big first step in the right direction,” Dr. Sayed added.
The study was published online in JAMA Dermatology.
Loci identified
GWASs have been limited in HS, and previous research has not identified significant risk loci. In the current study, Dr. Sayed and colleagues sought to identify underlying genes and genetic mechanisms that may underlie pathogenesis in HS.
They performed a GWAS in a cohort of 720 patients who were part of the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the UNC department of dermatology, and controls from the National Longitudinal Study of Adolescent to Adult Health (Add Health) study, a U.S.-based study following adolescents through adulthood.
The UK Biobank (UKB) is a prospective biobank with about 500,000 individuals aged 40-69 years, and FinnGen collects and analyzes genome and health data from Finnish biobank participants. To increase power to detect associations, a GWAS was performed using participants from the UKB (247 HS cases, 453,048 controls). The HS ProCARE GWAS results were meta-analyzed with UKB, along with data from FinnGen (673 HS cases, 297,544 controls). This three-way meta-analysis revealed one genomewide significant locus and two suggestive loci.
The authors found that the most strongly associated variant, rs10512572 located on chromosome 17, showed the strongest association in FinnGen; at the second locus, the most strongly associated variant was rs17090189 located on chromosome 13 and also showed the strongest association in FinnGen; and at the third locus, the most strongly associated variant, rs5792315, located on chromosome 11, showed the strongest association in HS ProCARE.
Next, they tested the 10 most strongly associated variants at the three loci in the BioVU biobank, which has 290 HS cases, including 189 individuals of European ancestry and 101 individuals of African ancestry, with 64,234 and 12,105 controls, respectively. The locus on chromosome 17 was replicated in BioVU in the same direction of effect, while one variant at the chromosome 13 locus showed nominal evidence of association in the same direction.
In a four-way meta-analysis of BioVU combined with the other three studies, the chromosome locus became more significant and the chromosome 13 locus exceeded the genomewide significance threshold. In contrast, the chromosome 11 locus was not replicated in BioVU (P = .27).
The authors pointed out that variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, which play a role in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
Finally, they looked to see if there were shared genetic components between HS and autoimmune and inflammatory diseases. A nominally significant genetic correlation was observed between HS and inflammatory bowel disease (P = .04), psoriasis (P = .03), and type 2 diabetes (P = .04), although none reached significance.
Different manifestation with genetic variant
In a related study, researchers assessed the prevalence of the NCSTN:c.671_682del variant among individuals with HS in Malta, the island country in the Mediterranean, which has a high prevalence of HS and its associated risk factors, particularly obesity.
Led by Dillon Mintoff, MD, of the department of pathology at the University of Malta, Msida, they conducted a cross-sectional study of 113 adults with HS. In this group, 14 (12.39%) were found to be heterozygous for the NCSTN:c.671_682del variant. Individuals who had this variant were more likely to develop symptoms earlier and to manifest them in atypical skin sites, including the scalp, neck, torso, and antecubital fossae. Additionally, even though their symptoms weren’t more severe than those without the variant, patients with the variant were more likely to require treatment with biologic agents.
Studies move genetics in HS forward
Writing in an accompanying editorial, Atlas Khan, PhD, and Lynn Petukhova, PhD, from Columbia University, New York, noted that both of these HS genetic studies “set a solid foundation for future studies aimed at understanding the biological and clinical relevance of new HS genetic evidence.”
“Each study suggests a series of experiments that will allow us to gain new knowledge about HS,” they wrote, including coming closer to providing patients with a genetic diagnosis.
In addition, evidence from the GWAS paper suggests that with “larger HS GWASs we will be able to better prioritize drug-repurposing studies,” concluded the editorialists. “Both of these goals will help to improve health outcomes for patients with HS and their family members.”
Dr. Sayed reported grants and/or personal fees from AbbVie, Novartis, UCB, Incyte, InflaRx, Alumis, and ChemoCentryx outside the submitted work; and serving as a volunteer member of the board of the HS Foundation and member of the European HS Foundation. The study in Malta was funded by the Government of Malta’s Tertiary Education Scholarship Scheme and Institutional Funds from the University of Malta. Dr. Mintoff reported grants from the Government of Malta Ministry for Education, Sport, Youth, Research and Innovation during the conduct of the study. Dr. Khan reported receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases. Dr. Petukhova reported receiving grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Columbia University’s Precision Medicine Initiative, Herbert Irving Comprehensive Cancer Center, and Data Science Institute.
FROM JAMA DERMATOLOGY
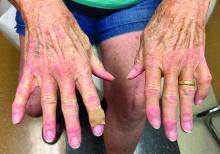
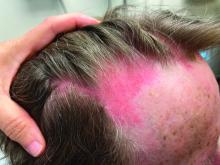
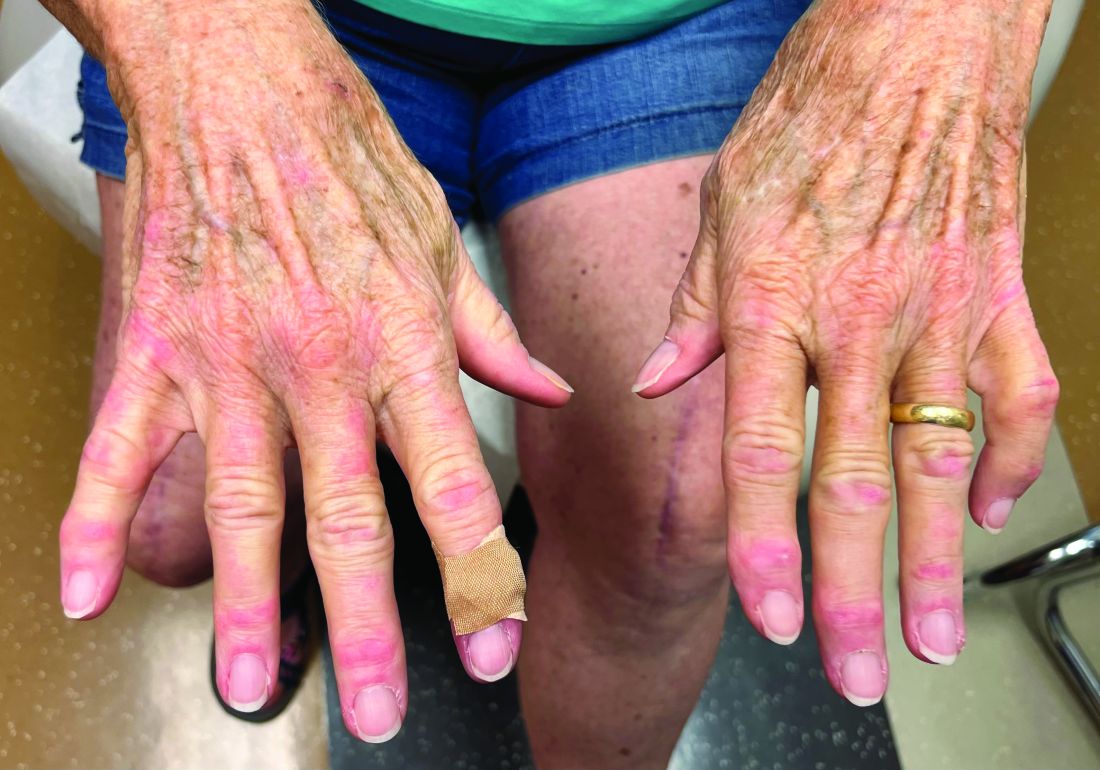
A 75-year-old White woman presented with diffuse erythema, scale, and pruritus on her scalp
The classical presentation includes symmetric proximal muscle weakness and underlying malignancy and is very common in adult patients. The etiology is unknown, however.
Some studies suggest people with certain HLA subtypes are at higher risk, and various infectious and pharmacological triggers are suspected to play a role in the pathogenesis of dermatomyositis. Infectious causes include Coxsackie B, enterovirus, and parvovirus. Drugs such as antineoplastic agents, antibiotics, and NSAIDs have been found to be triggers.
The pathogenesis of dermatomyositis involves immune-mediated damage to muscle capillaries and the endothelium of arterioles. In the typical humoral immune response, complement activation occurs. One mechanism of damage in dermatomyositis occurs when the membrane attack complex formed at the end of the complement process deposits in blood vessels, causing inflammation. B cells, autoantibodies, and interferon overexpression may also play a role in damaging the vasculature and muscle fibers. Hypoxia leads to muscular atrophy, resulting in degeneration and death of the fibers. On muscle biopsy, a perivascular and perimysial inflammatory infiltrate, perifascicular atrophy, and microangiopathy may be present. Skin histology reveals vacuolar changes in the basal layer, a lymphocytic infiltrate, and increased mucin production in the dermis.
On clinical examination, patients will have proximal muscle weakness and a skin rash that may include Gottron’s papules, heliotrope erythema, V-sign, shawl sign, holster sign, scalp erythema, midfacial erythema, and photosensitivity. Scalp erythema in dermatomyositis is highly linked to pruritus, alopecia, and telogen effluvium. Patients may experience small fiber neuropathy in dermatomyositis.
Serologies for this patient, who had previously been diagnosed and treated for dermatomyositis, were significant for a positive ANA 1:2560. Anti-Jo-1 antibody was negative. Her liver function tests, aldolase, creatinine kinase, sedimentation rate, C-reactive protein, and serum protein electrophoresis were normal. Imaging revealed mild chronic interstitial lung disease. A malignancy workup was negative.
Treatment of dermatomyositis involves lifestyle changes and pharmacologic therapy. Because of the intense photosensitivity, patients should be diligent with their sun protection. Methotrexate, azathioprine, and mycophenolate mofetil are considered first-line therapies for dermatomyositis. Therapies such as cyclophosphamide, rituximab, IVIg, and plasmapheresis may also be indicated in severe or refractory cases. Additionally, patients with pulmonary involvement should be given systemic steroids. The side effects of these drugs must be considered in the context of the patient’s demographics, comorbidities and lifestyle.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Natalie Y. Nasser, MD, of Kaiser Permanente Riverside Medical Center, Riverside, Calif. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Qudsiya Z and Waseem M. Dermatomyositis, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2023 Jan.
2. Kamperman RG et al. Int J Mol Sci. 2022 Apr 13;23(8):4301.
3. Kassamali B et al. Int J WomensDermatol. 2021 Sep 24;7(5Part A):576-82.
4. Vázquez-Herrera NE et al. Skin Appendage Disord. 2018 Aug;4(3):187-99.
The classical presentation includes symmetric proximal muscle weakness and underlying malignancy and is very common in adult patients. The etiology is unknown, however.
Some studies suggest people with certain HLA subtypes are at higher risk, and various infectious and pharmacological triggers are suspected to play a role in the pathogenesis of dermatomyositis. Infectious causes include Coxsackie B, enterovirus, and parvovirus. Drugs such as antineoplastic agents, antibiotics, and NSAIDs have been found to be triggers.
The pathogenesis of dermatomyositis involves immune-mediated damage to muscle capillaries and the endothelium of arterioles. In the typical humoral immune response, complement activation occurs. One mechanism of damage in dermatomyositis occurs when the membrane attack complex formed at the end of the complement process deposits in blood vessels, causing inflammation. B cells, autoantibodies, and interferon overexpression may also play a role in damaging the vasculature and muscle fibers. Hypoxia leads to muscular atrophy, resulting in degeneration and death of the fibers. On muscle biopsy, a perivascular and perimysial inflammatory infiltrate, perifascicular atrophy, and microangiopathy may be present. Skin histology reveals vacuolar changes in the basal layer, a lymphocytic infiltrate, and increased mucin production in the dermis.
On clinical examination, patients will have proximal muscle weakness and a skin rash that may include Gottron’s papules, heliotrope erythema, V-sign, shawl sign, holster sign, scalp erythema, midfacial erythema, and photosensitivity. Scalp erythema in dermatomyositis is highly linked to pruritus, alopecia, and telogen effluvium. Patients may experience small fiber neuropathy in dermatomyositis.
Serologies for this patient, who had previously been diagnosed and treated for dermatomyositis, were significant for a positive ANA 1:2560. Anti-Jo-1 antibody was negative. Her liver function tests, aldolase, creatinine kinase, sedimentation rate, C-reactive protein, and serum protein electrophoresis were normal. Imaging revealed mild chronic interstitial lung disease. A malignancy workup was negative.
Treatment of dermatomyositis involves lifestyle changes and pharmacologic therapy. Because of the intense photosensitivity, patients should be diligent with their sun protection. Methotrexate, azathioprine, and mycophenolate mofetil are considered first-line therapies for dermatomyositis. Therapies such as cyclophosphamide, rituximab, IVIg, and plasmapheresis may also be indicated in severe or refractory cases. Additionally, patients with pulmonary involvement should be given systemic steroids. The side effects of these drugs must be considered in the context of the patient’s demographics, comorbidities and lifestyle.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Natalie Y. Nasser, MD, of Kaiser Permanente Riverside Medical Center, Riverside, Calif. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Qudsiya Z and Waseem M. Dermatomyositis, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2023 Jan.
2. Kamperman RG et al. Int J Mol Sci. 2022 Apr 13;23(8):4301.
3. Kassamali B et al. Int J WomensDermatol. 2021 Sep 24;7(5Part A):576-82.
4. Vázquez-Herrera NE et al. Skin Appendage Disord. 2018 Aug;4(3):187-99.
The classical presentation includes symmetric proximal muscle weakness and underlying malignancy and is very common in adult patients. The etiology is unknown, however.
Some studies suggest people with certain HLA subtypes are at higher risk, and various infectious and pharmacological triggers are suspected to play a role in the pathogenesis of dermatomyositis. Infectious causes include Coxsackie B, enterovirus, and parvovirus. Drugs such as antineoplastic agents, antibiotics, and NSAIDs have been found to be triggers.
The pathogenesis of dermatomyositis involves immune-mediated damage to muscle capillaries and the endothelium of arterioles. In the typical humoral immune response, complement activation occurs. One mechanism of damage in dermatomyositis occurs when the membrane attack complex formed at the end of the complement process deposits in blood vessels, causing inflammation. B cells, autoantibodies, and interferon overexpression may also play a role in damaging the vasculature and muscle fibers. Hypoxia leads to muscular atrophy, resulting in degeneration and death of the fibers. On muscle biopsy, a perivascular and perimysial inflammatory infiltrate, perifascicular atrophy, and microangiopathy may be present. Skin histology reveals vacuolar changes in the basal layer, a lymphocytic infiltrate, and increased mucin production in the dermis.
On clinical examination, patients will have proximal muscle weakness and a skin rash that may include Gottron’s papules, heliotrope erythema, V-sign, shawl sign, holster sign, scalp erythema, midfacial erythema, and photosensitivity. Scalp erythema in dermatomyositis is highly linked to pruritus, alopecia, and telogen effluvium. Patients may experience small fiber neuropathy in dermatomyositis.
Serologies for this patient, who had previously been diagnosed and treated for dermatomyositis, were significant for a positive ANA 1:2560. Anti-Jo-1 antibody was negative. Her liver function tests, aldolase, creatinine kinase, sedimentation rate, C-reactive protein, and serum protein electrophoresis were normal. Imaging revealed mild chronic interstitial lung disease. A malignancy workup was negative.
Treatment of dermatomyositis involves lifestyle changes and pharmacologic therapy. Because of the intense photosensitivity, patients should be diligent with their sun protection. Methotrexate, azathioprine, and mycophenolate mofetil are considered first-line therapies for dermatomyositis. Therapies such as cyclophosphamide, rituximab, IVIg, and plasmapheresis may also be indicated in severe or refractory cases. Additionally, patients with pulmonary involvement should be given systemic steroids. The side effects of these drugs must be considered in the context of the patient’s demographics, comorbidities and lifestyle.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Natalie Y. Nasser, MD, of Kaiser Permanente Riverside Medical Center, Riverside, Calif. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Qudsiya Z and Waseem M. Dermatomyositis, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2023 Jan.
2. Kamperman RG et al. Int J Mol Sci. 2022 Apr 13;23(8):4301.
3. Kassamali B et al. Int J WomensDermatol. 2021 Sep 24;7(5Part A):576-82.
4. Vázquez-Herrera NE et al. Skin Appendage Disord. 2018 Aug;4(3):187-99.
Study aims to better elucidate CCCA in men
, and the most common symptom was scalp pruritus.
Researchers retrospectively reviewed the medical records of 17 male patients with a clinical diagnosis of CCCA who were seen at University of Pennsylvania outpatient clinics between 2012 and 2022. They excluded patients who had no scalp biopsy or if the scalp biopsy features limited characterization. Temitayo Ogunleye, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, led the study, published in the Journal of the American Academy of Dermatology.
CCCA, a type of scarring alopecia, most often affects women of African descent, and published data on the demographics, clinical findings, and medical histories of CCCA in men are limited, according to the authors.
The average age of the men was 43 years and 88.2% were Black, similar to women with CCCA, who tend to be middle-aged and Black. The four most common symptoms were scalp pruritus (58.8%), lesions (29.4%), pain or tenderness (23.5%), and hair thinning (23.5%). None of the men had type 2 diabetes (considered a possible CCCA risk factor), but 47.1% had a family history of alopecia. The four most common CCCA distributions were classic (47.1%), occipital (17.6%), patchy (11.8%), and posterior vertex (11.8%).
“Larger studies are needed to fully elucidate these relationships and explore etiology in males with CCCA,” the researchers wrote. “Nonetheless, we hope the data will prompt clinicians to assess for CCCA and risk factors in adult males with scarring alopecia.”
Limitations of the study included the retrospective, single-center design, and small sample size.
The researchers reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and the most common symptom was scalp pruritus.
Researchers retrospectively reviewed the medical records of 17 male patients with a clinical diagnosis of CCCA who were seen at University of Pennsylvania outpatient clinics between 2012 and 2022. They excluded patients who had no scalp biopsy or if the scalp biopsy features limited characterization. Temitayo Ogunleye, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, led the study, published in the Journal of the American Academy of Dermatology.
CCCA, a type of scarring alopecia, most often affects women of African descent, and published data on the demographics, clinical findings, and medical histories of CCCA in men are limited, according to the authors.
The average age of the men was 43 years and 88.2% were Black, similar to women with CCCA, who tend to be middle-aged and Black. The four most common symptoms were scalp pruritus (58.8%), lesions (29.4%), pain or tenderness (23.5%), and hair thinning (23.5%). None of the men had type 2 diabetes (considered a possible CCCA risk factor), but 47.1% had a family history of alopecia. The four most common CCCA distributions were classic (47.1%), occipital (17.6%), patchy (11.8%), and posterior vertex (11.8%).
“Larger studies are needed to fully elucidate these relationships and explore etiology in males with CCCA,” the researchers wrote. “Nonetheless, we hope the data will prompt clinicians to assess for CCCA and risk factors in adult males with scarring alopecia.”
Limitations of the study included the retrospective, single-center design, and small sample size.
The researchers reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and the most common symptom was scalp pruritus.
Researchers retrospectively reviewed the medical records of 17 male patients with a clinical diagnosis of CCCA who were seen at University of Pennsylvania outpatient clinics between 2012 and 2022. They excluded patients who had no scalp biopsy or if the scalp biopsy features limited characterization. Temitayo Ogunleye, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, led the study, published in the Journal of the American Academy of Dermatology.
CCCA, a type of scarring alopecia, most often affects women of African descent, and published data on the demographics, clinical findings, and medical histories of CCCA in men are limited, according to the authors.
The average age of the men was 43 years and 88.2% were Black, similar to women with CCCA, who tend to be middle-aged and Black. The four most common symptoms were scalp pruritus (58.8%), lesions (29.4%), pain or tenderness (23.5%), and hair thinning (23.5%). None of the men had type 2 diabetes (considered a possible CCCA risk factor), but 47.1% had a family history of alopecia. The four most common CCCA distributions were classic (47.1%), occipital (17.6%), patchy (11.8%), and posterior vertex (11.8%).
“Larger studies are needed to fully elucidate these relationships and explore etiology in males with CCCA,” the researchers wrote. “Nonetheless, we hope the data will prompt clinicians to assess for CCCA and risk factors in adult males with scarring alopecia.”
Limitations of the study included the retrospective, single-center design, and small sample size.
The researchers reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
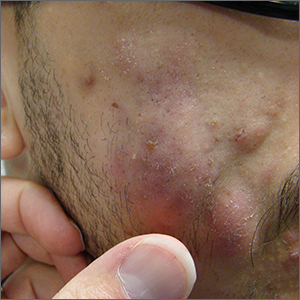
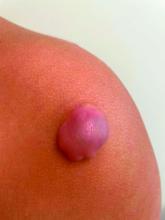
Fluctuant facial lesions
This patient had more than cystic acne; he had acne conglobata. AC is a severe form of inflammatory acne leading to coalescing lesions with purulent sinus tracts under the skin. It can be seen as part of the follicular tetrad syndrome of cystic acne, hidradenitis suppurativa, dissecting cellulitis, and pilonidal disease. AC is thought to be an elevated tumor necrosis factor (TNF)-alpha response to Propionibacterium acnes (now known as Cutibacterium acnes) that leads to excessive inflammation and sterile abscesses.1 Acne fulminans (AF) can also manifest as a purulent form of acne, but AF has associated systemic signs and symptoms that include fevers, chills, and malaise.
Due to the depth of the inflammation, AC is treated with systemic medications, most commonly isotretinoin. Isotretinoin can be started at 0.5 mg/kg (divided twice daily to enhance tolerability) and then increased to 1 mg/kg (divided twice daily) for 5 months. There is some variation in dosing regimens in practice; the target goal is 120 to 150 mg/kg over the course of treatment. In AF, the patient is pretreated with systemic steroids, and in AC, some clinicians will even prescribe systemic steroids (prednisone 0.5 mg/kg daily for the first month) along with isotretinoin.
Second-line medications include dapsone (50-150 mg/d).2 Case reports describe the successful use of the TNF-alpha antagonist adalimumab, although this is not a usual practice in AC treatment.1 Note that all of these medications have the potential for severe adverse effects and require laboratory evaluation prior to initiation.
This patient was counseled, prescribed isotretinoin (dose as above), and enrolled in the IPledge prescribing and monitoring system for isotretinoin. At 20 weeks of use, the purulent drainage ceased. The pus-filled sinus tracts and redness had resolved, although he still had thickened tissue and scarring where the tracts had been. In time, the scars will usually get flatter and softer.
If the patient’s AC were to flare, another 20-week course of isotretinoin could be prescribed after a 2-month hiatus or he could be switched to a second-line medication. Referral for any cosmetic therapy is typically delayed for another 6 months in case there is a need to treat a recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Yiu ZZ, Madan V, Griffiths CE. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. 2015;40:383-386. doi: 10.1111/ced.12540
2. Hafsi W, Arnold DL, Kassardjian M. Acne Conglobata. StatPearls Publishing; 2023.

This patient had more than cystic acne; he had acne conglobata. AC is a severe form of inflammatory acne leading to coalescing lesions with purulent sinus tracts under the skin. It can be seen as part of the follicular tetrad syndrome of cystic acne, hidradenitis suppurativa, dissecting cellulitis, and pilonidal disease. AC is thought to be an elevated tumor necrosis factor (TNF)-alpha response to Propionibacterium acnes (now known as Cutibacterium acnes) that leads to excessive inflammation and sterile abscesses.1 Acne fulminans (AF) can also manifest as a purulent form of acne, but AF has associated systemic signs and symptoms that include fevers, chills, and malaise.
Due to the depth of the inflammation, AC is treated with systemic medications, most commonly isotretinoin. Isotretinoin can be started at 0.5 mg/kg (divided twice daily to enhance tolerability) and then increased to 1 mg/kg (divided twice daily) for 5 months. There is some variation in dosing regimens in practice; the target goal is 120 to 150 mg/kg over the course of treatment. In AF, the patient is pretreated with systemic steroids, and in AC, some clinicians will even prescribe systemic steroids (prednisone 0.5 mg/kg daily for the first month) along with isotretinoin.
Second-line medications include dapsone (50-150 mg/d).2 Case reports describe the successful use of the TNF-alpha antagonist adalimumab, although this is not a usual practice in AC treatment.1 Note that all of these medications have the potential for severe adverse effects and require laboratory evaluation prior to initiation.
This patient was counseled, prescribed isotretinoin (dose as above), and enrolled in the IPledge prescribing and monitoring system for isotretinoin. At 20 weeks of use, the purulent drainage ceased. The pus-filled sinus tracts and redness had resolved, although he still had thickened tissue and scarring where the tracts had been. In time, the scars will usually get flatter and softer.
If the patient’s AC were to flare, another 20-week course of isotretinoin could be prescribed after a 2-month hiatus or he could be switched to a second-line medication. Referral for any cosmetic therapy is typically delayed for another 6 months in case there is a need to treat a recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient had more than cystic acne; he had acne conglobata. AC is a severe form of inflammatory acne leading to coalescing lesions with purulent sinus tracts under the skin. It can be seen as part of the follicular tetrad syndrome of cystic acne, hidradenitis suppurativa, dissecting cellulitis, and pilonidal disease. AC is thought to be an elevated tumor necrosis factor (TNF)-alpha response to Propionibacterium acnes (now known as Cutibacterium acnes) that leads to excessive inflammation and sterile abscesses.1 Acne fulminans (AF) can also manifest as a purulent form of acne, but AF has associated systemic signs and symptoms that include fevers, chills, and malaise.
Due to the depth of the inflammation, AC is treated with systemic medications, most commonly isotretinoin. Isotretinoin can be started at 0.5 mg/kg (divided twice daily to enhance tolerability) and then increased to 1 mg/kg (divided twice daily) for 5 months. There is some variation in dosing regimens in practice; the target goal is 120 to 150 mg/kg over the course of treatment. In AF, the patient is pretreated with systemic steroids, and in AC, some clinicians will even prescribe systemic steroids (prednisone 0.5 mg/kg daily for the first month) along with isotretinoin.
Second-line medications include dapsone (50-150 mg/d).2 Case reports describe the successful use of the TNF-alpha antagonist adalimumab, although this is not a usual practice in AC treatment.1 Note that all of these medications have the potential for severe adverse effects and require laboratory evaluation prior to initiation.
This patient was counseled, prescribed isotretinoin (dose as above), and enrolled in the IPledge prescribing and monitoring system for isotretinoin. At 20 weeks of use, the purulent drainage ceased. The pus-filled sinus tracts and redness had resolved, although he still had thickened tissue and scarring where the tracts had been. In time, the scars will usually get flatter and softer.
If the patient’s AC were to flare, another 20-week course of isotretinoin could be prescribed after a 2-month hiatus or he could be switched to a second-line medication. Referral for any cosmetic therapy is typically delayed for another 6 months in case there is a need to treat a recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Yiu ZZ, Madan V, Griffiths CE. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. 2015;40:383-386. doi: 10.1111/ced.12540
2. Hafsi W, Arnold DL, Kassardjian M. Acne Conglobata. StatPearls Publishing; 2023.
1. Yiu ZZ, Madan V, Griffiths CE. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. 2015;40:383-386. doi: 10.1111/ced.12540
2. Hafsi W, Arnold DL, Kassardjian M. Acne Conglobata. StatPearls Publishing; 2023.
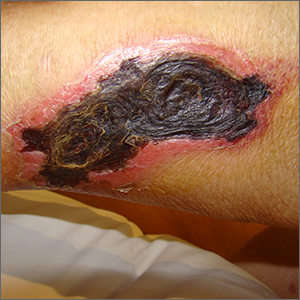
Leathery plaque on thigh
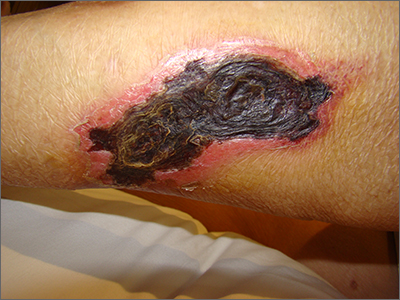
The necrotic eschar on this patient’s thigh is calciphylaxis, also known as calcific uremic arteriolopathy (CUA). Most cases are seen in ESRD and start as painful erythematous, firm lesions that progress to necrotic eschars. Up to 4% of patients with ESRD who are on dialysis develop CUA.1
The exact pathology of CUA is unknown. Calcification of the arterioles leads to ischemia and necrosis of tissue, which is not limited to the skin and can affect tissue elsewhere (eg, muscles, central nervous system, internal organs).2
Morbidity and mortality of CUA is often due to bacterial infections and sepsis related to the necrotic tissue. CUA can be treated with sodium thiosulfate (25 g in 100 mL of normal saline) infused intravenously during the last 30 minutes of dialysis treatment 3 times per week.3 Sodium thiosulfate (which acts as a calcium binder) and cinacalcet (a calcimimetic that leads to lower parathyroid hormone levels) have been used, but evidence of efficacy is limited. In a multicenter observational study involving 89 patients with chronic kidney disease and CUA, 17% of patients experienced complete wound healing, while 56% died over a median follow-up period of 5.8 months.1 (No cause of death data were available; sodium thiosulfate and a calcimimetic were the most widely used treatment strategies.) This extrapolated to a mortality rate of 72 patients per 100 individuals over the course of 1 year (the 100 patient-years rate).1
This patient continued her dialysis regimen and general care. She was seen by the wound care team and treated with topical wound care, including moist dressings for her open lesions. The eschars were not debrided because they showed no sign of active infection. Unfortunately, she was in extremely frail condition and died 1 month after evaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Chinnadurai R, Huckle A, Hegarty J, et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34:1537-1545. doi: 10.1007/s40620-020-00908-9
2. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
3. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034

The necrotic eschar on this patient’s thigh is calciphylaxis, also known as calcific uremic arteriolopathy (CUA). Most cases are seen in ESRD and start as painful erythematous, firm lesions that progress to necrotic eschars. Up to 4% of patients with ESRD who are on dialysis develop CUA.1
The exact pathology of CUA is unknown. Calcification of the arterioles leads to ischemia and necrosis of tissue, which is not limited to the skin and can affect tissue elsewhere (eg, muscles, central nervous system, internal organs).2
Morbidity and mortality of CUA is often due to bacterial infections and sepsis related to the necrotic tissue. CUA can be treated with sodium thiosulfate (25 g in 100 mL of normal saline) infused intravenously during the last 30 minutes of dialysis treatment 3 times per week.3 Sodium thiosulfate (which acts as a calcium binder) and cinacalcet (a calcimimetic that leads to lower parathyroid hormone levels) have been used, but evidence of efficacy is limited. In a multicenter observational study involving 89 patients with chronic kidney disease and CUA, 17% of patients experienced complete wound healing, while 56% died over a median follow-up period of 5.8 months.1 (No cause of death data were available; sodium thiosulfate and a calcimimetic were the most widely used treatment strategies.) This extrapolated to a mortality rate of 72 patients per 100 individuals over the course of 1 year (the 100 patient-years rate).1
This patient continued her dialysis regimen and general care. She was seen by the wound care team and treated with topical wound care, including moist dressings for her open lesions. The eschars were not debrided because they showed no sign of active infection. Unfortunately, she was in extremely frail condition and died 1 month after evaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The necrotic eschar on this patient’s thigh is calciphylaxis, also known as calcific uremic arteriolopathy (CUA). Most cases are seen in ESRD and start as painful erythematous, firm lesions that progress to necrotic eschars. Up to 4% of patients with ESRD who are on dialysis develop CUA.1
The exact pathology of CUA is unknown. Calcification of the arterioles leads to ischemia and necrosis of tissue, which is not limited to the skin and can affect tissue elsewhere (eg, muscles, central nervous system, internal organs).2
Morbidity and mortality of CUA is often due to bacterial infections and sepsis related to the necrotic tissue. CUA can be treated with sodium thiosulfate (25 g in 100 mL of normal saline) infused intravenously during the last 30 minutes of dialysis treatment 3 times per week.3 Sodium thiosulfate (which acts as a calcium binder) and cinacalcet (a calcimimetic that leads to lower parathyroid hormone levels) have been used, but evidence of efficacy is limited. In a multicenter observational study involving 89 patients with chronic kidney disease and CUA, 17% of patients experienced complete wound healing, while 56% died over a median follow-up period of 5.8 months.1 (No cause of death data were available; sodium thiosulfate and a calcimimetic were the most widely used treatment strategies.) This extrapolated to a mortality rate of 72 patients per 100 individuals over the course of 1 year (the 100 patient-years rate).1
This patient continued her dialysis regimen and general care. She was seen by the wound care team and treated with topical wound care, including moist dressings for her open lesions. The eschars were not debrided because they showed no sign of active infection. Unfortunately, she was in extremely frail condition and died 1 month after evaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Chinnadurai R, Huckle A, Hegarty J, et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34:1537-1545. doi: 10.1007/s40620-020-00908-9
2. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
3. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
1. Chinnadurai R, Huckle A, Hegarty J, et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34:1537-1545. doi: 10.1007/s40620-020-00908-9
2. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
3. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
Despite recent uptick in cases, leprosy is very rare, expert says
, according to Jose A. Lucar, MD.
“Contrary to historical beliefs, leprosy is not highly contagious,” Dr. Lucar, an infectious disease physician and associate professor of medicine at George Washington University, Washington, said in an interview. “For reasons that have to do with the makeup of genes that affect their immune system, most people are not susceptible to acquire leprosy. It’s really a small percentage of the population. It does require prolonged contact with someone with untreated leprosy – over several months – to acquire an infection. So, the risk from any type of casual contact is low.”
According to a research letter published in the CDC’s Emerging Infectious Diseases, the number of reported leprosy cases has more than doubled in the past decade. Of the 159 new cases reported nationwide in 2020, Florida accounted for about one-fifth of cases, with most limited to the central part of the state. “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born," and currently, about one-third of leprosy cases are in individuals born in the United States, he noted.
The research letter described a case of leprosy in a 54-year-old man who worked in landscaping, who sought treatment at a dermatology clinic in Central Florida in 2022 for a painful and progressive erythematous rash. The lesions began on his distal extensor extremities and progressed to involve his trunk and face. According to the report, the man denied any domestic or foreign travel, exposure to armadillos (a known source of transmission), prolonged contact with immigrants from leprosy-endemic countries, or connections with someone known to have leprosy. The authors concluded that the case “adds to the growing body of literature suggesting that central Florida represents an endemic location for leprosy. Travel to this area, even in the absence of other risk factors, should prompt consideration of leprosy in the appropriate clinical context.”
Dr. Lucar said that the mechanism of leprosy transmission is not fully understood, but armadillos, which typically traverse the southern United States, are naturally infected with the bacteria that causes leprosy. “It’s possible that they can spread it to people,” he said. “People who have occupations or hobbies that put them in potential contact with wildlife should avoid any close contact with armadillos. There’s also a discussion of whether [the spike in leprosy cases] may have to do with climate change. That is not yet confirmed. It’s not entirely clear why there’s been a recent rise. It remains an area of investigation.”
Meanwhile, clinicians should keep a high level of suspicion in patients who present with skin lesions compatible with leprosy. “These are typically discolored or numb patches on the skin,” Dr. Lucar said. “This can range from a single or a few lesions to very extensive involvement of the skin. The diminished sensation or loss of sensation within those skin patches is an important sign. There’s a loss of skin color but sometimes they can be reddish.” He emphasized that leprosy “does not spread easily from person to person; casual contact will not spread leprosy. It’s important for the public to understand that.”
Dr. Lucar reported no disclosures.
, according to Jose A. Lucar, MD.
“Contrary to historical beliefs, leprosy is not highly contagious,” Dr. Lucar, an infectious disease physician and associate professor of medicine at George Washington University, Washington, said in an interview. “For reasons that have to do with the makeup of genes that affect their immune system, most people are not susceptible to acquire leprosy. It’s really a small percentage of the population. It does require prolonged contact with someone with untreated leprosy – over several months – to acquire an infection. So, the risk from any type of casual contact is low.”
According to a research letter published in the CDC’s Emerging Infectious Diseases, the number of reported leprosy cases has more than doubled in the past decade. Of the 159 new cases reported nationwide in 2020, Florida accounted for about one-fifth of cases, with most limited to the central part of the state. “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born," and currently, about one-third of leprosy cases are in individuals born in the United States, he noted.
The research letter described a case of leprosy in a 54-year-old man who worked in landscaping, who sought treatment at a dermatology clinic in Central Florida in 2022 for a painful and progressive erythematous rash. The lesions began on his distal extensor extremities and progressed to involve his trunk and face. According to the report, the man denied any domestic or foreign travel, exposure to armadillos (a known source of transmission), prolonged contact with immigrants from leprosy-endemic countries, or connections with someone known to have leprosy. The authors concluded that the case “adds to the growing body of literature suggesting that central Florida represents an endemic location for leprosy. Travel to this area, even in the absence of other risk factors, should prompt consideration of leprosy in the appropriate clinical context.”
Dr. Lucar said that the mechanism of leprosy transmission is not fully understood, but armadillos, which typically traverse the southern United States, are naturally infected with the bacteria that causes leprosy. “It’s possible that they can spread it to people,” he said. “People who have occupations or hobbies that put them in potential contact with wildlife should avoid any close contact with armadillos. There’s also a discussion of whether [the spike in leprosy cases] may have to do with climate change. That is not yet confirmed. It’s not entirely clear why there’s been a recent rise. It remains an area of investigation.”
Meanwhile, clinicians should keep a high level of suspicion in patients who present with skin lesions compatible with leprosy. “These are typically discolored or numb patches on the skin,” Dr. Lucar said. “This can range from a single or a few lesions to very extensive involvement of the skin. The diminished sensation or loss of sensation within those skin patches is an important sign. There’s a loss of skin color but sometimes they can be reddish.” He emphasized that leprosy “does not spread easily from person to person; casual contact will not spread leprosy. It’s important for the public to understand that.”
Dr. Lucar reported no disclosures.
, according to Jose A. Lucar, MD.
“Contrary to historical beliefs, leprosy is not highly contagious,” Dr. Lucar, an infectious disease physician and associate professor of medicine at George Washington University, Washington, said in an interview. “For reasons that have to do with the makeup of genes that affect their immune system, most people are not susceptible to acquire leprosy. It’s really a small percentage of the population. It does require prolonged contact with someone with untreated leprosy – over several months – to acquire an infection. So, the risk from any type of casual contact is low.”
According to a research letter published in the CDC’s Emerging Infectious Diseases, the number of reported leprosy cases has more than doubled in the past decade. Of the 159 new cases reported nationwide in 2020, Florida accounted for about one-fifth of cases, with most limited to the central part of the state. “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born “In the U.S., there have been 150-250 cases reported each year over the past several years,” said Dr. Lucar, who was not affiliated with the research letter. “What seems to have changed is that since 2015, there has been a rise in cases in people who are U.S.-born," and currently, about one-third of leprosy cases are in individuals born in the United States, he noted.
The research letter described a case of leprosy in a 54-year-old man who worked in landscaping, who sought treatment at a dermatology clinic in Central Florida in 2022 for a painful and progressive erythematous rash. The lesions began on his distal extensor extremities and progressed to involve his trunk and face. According to the report, the man denied any domestic or foreign travel, exposure to armadillos (a known source of transmission), prolonged contact with immigrants from leprosy-endemic countries, or connections with someone known to have leprosy. The authors concluded that the case “adds to the growing body of literature suggesting that central Florida represents an endemic location for leprosy. Travel to this area, even in the absence of other risk factors, should prompt consideration of leprosy in the appropriate clinical context.”
Dr. Lucar said that the mechanism of leprosy transmission is not fully understood, but armadillos, which typically traverse the southern United States, are naturally infected with the bacteria that causes leprosy. “It’s possible that they can spread it to people,” he said. “People who have occupations or hobbies that put them in potential contact with wildlife should avoid any close contact with armadillos. There’s also a discussion of whether [the spike in leprosy cases] may have to do with climate change. That is not yet confirmed. It’s not entirely clear why there’s been a recent rise. It remains an area of investigation.”
Meanwhile, clinicians should keep a high level of suspicion in patients who present with skin lesions compatible with leprosy. “These are typically discolored or numb patches on the skin,” Dr. Lucar said. “This can range from a single or a few lesions to very extensive involvement of the skin. The diminished sensation or loss of sensation within those skin patches is an important sign. There’s a loss of skin color but sometimes they can be reddish.” He emphasized that leprosy “does not spread easily from person to person; casual contact will not spread leprosy. It’s important for the public to understand that.”
Dr. Lucar reported no disclosures.
Study validates use of new psoriatic arthritis prediction tool
Though it requires further validation, researchers led by rheumatologist Lihi Eder, MD, PhD, of the Women’s College Research Institute at Women’s College Hospital, Toronto, characterized the development and validation of PRESTO as “an important first step in the development and testing of interventional strategies that may ultimately halt disease progression,” they wrote in their study of the tool, which published in Arthritis & Rheumatology. Dr. Eder presented a summary of progress on the effort at the 2023 annual meeting of the Canadian Rheumatology Association.
To develop and validate the tool, the researchers evaluated 635 patients from the University of Toronto Psoriasis Cohort, which was launched in 2006 as a prospective longitudinal cohort study to examine risk factors for the development of PsA among patients with psoriasis. Patients enrolled in the cohort have a dermatologist-confirmed diagnosis of psoriasis and are assessed by a rheumatologist prior to enrollment to exclude those with inflammatory arthritis in the past or at the time of assessment.
To develop prediction models for PsA, Dr. Eder and colleagues used information from the patient cohort demographics, psoriasis characteristics, comorbidities, medications, and musculoskeletal symptoms. Next, they used multivariable logistic regression models adjusting for covariates, duration of psoriasis, and the log duration at risk to estimate the probability of developing PsA within 1-year and 5-year time windows from consecutive study visits.
The mean age of the study participants was 47 years, 76% were White, and 57% were male; and they had psoriasis for a mean of 16 years. The researchers found that 51 patients developed PsA during the 1-year follow-up, and 71 developed PsA during the 5-year follow-up. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, joint stiffness, use of biologic medications, patient global health, and pain severity (area under the curve, 72.3).
In addition, the risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesions, psoriasis severity, fatigue, pain, and use of systemic non-biologic medication or phototherapy (AUC, 74.9). Calibration plots showed reasonable agreement between predicted and observed probabilities.
“Interestingly, several previously reported risk factors for PsA, such as HLA-B27, family history of PsA, uveitis, and flexural psoriasis, were not included in the risk prediction model due to their scarcity in our cohort,” the researchers wrote. “This finding may be due to immortal time bias which can complicate the development of risk prediction models for PsA. Genetic factors or their surrogates (e.g., family history of PsA) are associated with the development of PsA concurrently or shortly after the onset of psoriasis.”
They acknowledged certain limitations of the study, including its relatively small sample size and questionable generalizability of the study findings, “as most of the patients were recruited from dermatology clinics leading to overrepresentation of moderate-severe psoriasis. Therefore, PRESTO will require an external validation to assess its performance in other populations of psoriasis patients with different characteristics.”
Saakshi Khattri, MD, a board-certified dermatologist, rheumatologist, and internist at the Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study and was asked to comment on the results, characterized the PRESTO tool as “an interesting step in the right direction, but it’s the first step.”
Since dermatologists are usually the first point of contact for psoriasis patients, she added, “a risk calculator can be helpful, but the question remains: When do we refer them to a rheumatologist? If the risk comes to 5%, is that a low risk that doesn’t need referral to rheumatology? I don’t think those questions have been answered here. From a rheumatology perspective, does the risk calculator help me decide when to intervene? At present, I’m not sure it does. Perhaps a higher score might make us intervene sooner if our clinical exam doesn’t show swollen or tender joints.”
Clinical exam findings and history she considers as a rheumatologist before making treatment recommendations include the following: Are there swollen and tender joints? Does the patient report morning stiffness for upwards of 30 minutes? Do they have enthesitis or dactylitis? Is there axial involvement? “Imaging can help if there isn’t anything on clinical exam and the history is compelling and/or the patient has risk factors for PsA,” she said.
The study’s finding of biologic use being associated with risk of developing PsA at year 1 but not at year 5 is “confusing,” Dr. Khattri added. “My concern is, will that now dissuade our moderate to severe psoriasis patients from using biologics to clear their psoriasis? We know that biologics are indicated for moderate to severe psoriasis. We also know psoriasis is associated with increased cardiovascular risk and there’s data to suggest that treatment with biologics with its resultant decrease in systemic inflammation can decrease cardiovascular risk.”
The study was supported by a New Investigator Grant from the Physician Services Incorporated Foundation. Dr. Eder disclosed that she is supported by the Canada Research Chair in Inflammatory Rheumatic Diseases. Dr. Khattri reported that she is a member of the advisory board for UCB, Janssen, AbbVie, Regeneron, Sanofi, Lilly, Argenx, and Arcutis. She has also received research funds from Incyte, AbbVie, Leo, Galderma, Pfizer, and Acelyrin.
Though it requires further validation, researchers led by rheumatologist Lihi Eder, MD, PhD, of the Women’s College Research Institute at Women’s College Hospital, Toronto, characterized the development and validation of PRESTO as “an important first step in the development and testing of interventional strategies that may ultimately halt disease progression,” they wrote in their study of the tool, which published in Arthritis & Rheumatology. Dr. Eder presented a summary of progress on the effort at the 2023 annual meeting of the Canadian Rheumatology Association.
To develop and validate the tool, the researchers evaluated 635 patients from the University of Toronto Psoriasis Cohort, which was launched in 2006 as a prospective longitudinal cohort study to examine risk factors for the development of PsA among patients with psoriasis. Patients enrolled in the cohort have a dermatologist-confirmed diagnosis of psoriasis and are assessed by a rheumatologist prior to enrollment to exclude those with inflammatory arthritis in the past or at the time of assessment.
To develop prediction models for PsA, Dr. Eder and colleagues used information from the patient cohort demographics, psoriasis characteristics, comorbidities, medications, and musculoskeletal symptoms. Next, they used multivariable logistic regression models adjusting for covariates, duration of psoriasis, and the log duration at risk to estimate the probability of developing PsA within 1-year and 5-year time windows from consecutive study visits.
The mean age of the study participants was 47 years, 76% were White, and 57% were male; and they had psoriasis for a mean of 16 years. The researchers found that 51 patients developed PsA during the 1-year follow-up, and 71 developed PsA during the 5-year follow-up. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, joint stiffness, use of biologic medications, patient global health, and pain severity (area under the curve, 72.3).
In addition, the risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesions, psoriasis severity, fatigue, pain, and use of systemic non-biologic medication or phototherapy (AUC, 74.9). Calibration plots showed reasonable agreement between predicted and observed probabilities.
“Interestingly, several previously reported risk factors for PsA, such as HLA-B27, family history of PsA, uveitis, and flexural psoriasis, were not included in the risk prediction model due to their scarcity in our cohort,” the researchers wrote. “This finding may be due to immortal time bias which can complicate the development of risk prediction models for PsA. Genetic factors or their surrogates (e.g., family history of PsA) are associated with the development of PsA concurrently or shortly after the onset of psoriasis.”
They acknowledged certain limitations of the study, including its relatively small sample size and questionable generalizability of the study findings, “as most of the patients were recruited from dermatology clinics leading to overrepresentation of moderate-severe psoriasis. Therefore, PRESTO will require an external validation to assess its performance in other populations of psoriasis patients with different characteristics.”
Saakshi Khattri, MD, a board-certified dermatologist, rheumatologist, and internist at the Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study and was asked to comment on the results, characterized the PRESTO tool as “an interesting step in the right direction, but it’s the first step.”
Since dermatologists are usually the first point of contact for psoriasis patients, she added, “a risk calculator can be helpful, but the question remains: When do we refer them to a rheumatologist? If the risk comes to 5%, is that a low risk that doesn’t need referral to rheumatology? I don’t think those questions have been answered here. From a rheumatology perspective, does the risk calculator help me decide when to intervene? At present, I’m not sure it does. Perhaps a higher score might make us intervene sooner if our clinical exam doesn’t show swollen or tender joints.”
Clinical exam findings and history she considers as a rheumatologist before making treatment recommendations include the following: Are there swollen and tender joints? Does the patient report morning stiffness for upwards of 30 minutes? Do they have enthesitis or dactylitis? Is there axial involvement? “Imaging can help if there isn’t anything on clinical exam and the history is compelling and/or the patient has risk factors for PsA,” she said.
The study’s finding of biologic use being associated with risk of developing PsA at year 1 but not at year 5 is “confusing,” Dr. Khattri added. “My concern is, will that now dissuade our moderate to severe psoriasis patients from using biologics to clear their psoriasis? We know that biologics are indicated for moderate to severe psoriasis. We also know psoriasis is associated with increased cardiovascular risk and there’s data to suggest that treatment with biologics with its resultant decrease in systemic inflammation can decrease cardiovascular risk.”
The study was supported by a New Investigator Grant from the Physician Services Incorporated Foundation. Dr. Eder disclosed that she is supported by the Canada Research Chair in Inflammatory Rheumatic Diseases. Dr. Khattri reported that she is a member of the advisory board for UCB, Janssen, AbbVie, Regeneron, Sanofi, Lilly, Argenx, and Arcutis. She has also received research funds from Incyte, AbbVie, Leo, Galderma, Pfizer, and Acelyrin.
Though it requires further validation, researchers led by rheumatologist Lihi Eder, MD, PhD, of the Women’s College Research Institute at Women’s College Hospital, Toronto, characterized the development and validation of PRESTO as “an important first step in the development and testing of interventional strategies that may ultimately halt disease progression,” they wrote in their study of the tool, which published in Arthritis & Rheumatology. Dr. Eder presented a summary of progress on the effort at the 2023 annual meeting of the Canadian Rheumatology Association.
To develop and validate the tool, the researchers evaluated 635 patients from the University of Toronto Psoriasis Cohort, which was launched in 2006 as a prospective longitudinal cohort study to examine risk factors for the development of PsA among patients with psoriasis. Patients enrolled in the cohort have a dermatologist-confirmed diagnosis of psoriasis and are assessed by a rheumatologist prior to enrollment to exclude those with inflammatory arthritis in the past or at the time of assessment.
To develop prediction models for PsA, Dr. Eder and colleagues used information from the patient cohort demographics, psoriasis characteristics, comorbidities, medications, and musculoskeletal symptoms. Next, they used multivariable logistic regression models adjusting for covariates, duration of psoriasis, and the log duration at risk to estimate the probability of developing PsA within 1-year and 5-year time windows from consecutive study visits.
The mean age of the study participants was 47 years, 76% were White, and 57% were male; and they had psoriasis for a mean of 16 years. The researchers found that 51 patients developed PsA during the 1-year follow-up, and 71 developed PsA during the 5-year follow-up. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, joint stiffness, use of biologic medications, patient global health, and pain severity (area under the curve, 72.3).
In addition, the risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesions, psoriasis severity, fatigue, pain, and use of systemic non-biologic medication or phototherapy (AUC, 74.9). Calibration plots showed reasonable agreement between predicted and observed probabilities.
“Interestingly, several previously reported risk factors for PsA, such as HLA-B27, family history of PsA, uveitis, and flexural psoriasis, were not included in the risk prediction model due to their scarcity in our cohort,” the researchers wrote. “This finding may be due to immortal time bias which can complicate the development of risk prediction models for PsA. Genetic factors or their surrogates (e.g., family history of PsA) are associated with the development of PsA concurrently or shortly after the onset of psoriasis.”
They acknowledged certain limitations of the study, including its relatively small sample size and questionable generalizability of the study findings, “as most of the patients were recruited from dermatology clinics leading to overrepresentation of moderate-severe psoriasis. Therefore, PRESTO will require an external validation to assess its performance in other populations of psoriasis patients with different characteristics.”
Saakshi Khattri, MD, a board-certified dermatologist, rheumatologist, and internist at the Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study and was asked to comment on the results, characterized the PRESTO tool as “an interesting step in the right direction, but it’s the first step.”
Since dermatologists are usually the first point of contact for psoriasis patients, she added, “a risk calculator can be helpful, but the question remains: When do we refer them to a rheumatologist? If the risk comes to 5%, is that a low risk that doesn’t need referral to rheumatology? I don’t think those questions have been answered here. From a rheumatology perspective, does the risk calculator help me decide when to intervene? At present, I’m not sure it does. Perhaps a higher score might make us intervene sooner if our clinical exam doesn’t show swollen or tender joints.”
Clinical exam findings and history she considers as a rheumatologist before making treatment recommendations include the following: Are there swollen and tender joints? Does the patient report morning stiffness for upwards of 30 minutes? Do they have enthesitis or dactylitis? Is there axial involvement? “Imaging can help if there isn’t anything on clinical exam and the history is compelling and/or the patient has risk factors for PsA,” she said.
The study’s finding of biologic use being associated with risk of developing PsA at year 1 but not at year 5 is “confusing,” Dr. Khattri added. “My concern is, will that now dissuade our moderate to severe psoriasis patients from using biologics to clear their psoriasis? We know that biologics are indicated for moderate to severe psoriasis. We also know psoriasis is associated with increased cardiovascular risk and there’s data to suggest that treatment with biologics with its resultant decrease in systemic inflammation can decrease cardiovascular risk.”
The study was supported by a New Investigator Grant from the Physician Services Incorporated Foundation. Dr. Eder disclosed that she is supported by the Canada Research Chair in Inflammatory Rheumatic Diseases. Dr. Khattri reported that she is a member of the advisory board for UCB, Janssen, AbbVie, Regeneron, Sanofi, Lilly, Argenx, and Arcutis. She has also received research funds from Incyte, AbbVie, Leo, Galderma, Pfizer, and Acelyrin.
FROM ARTHRITIS AND RHEUMATOLOGY
What's the diagnosis?
Given the characteristic clinical presentation, the most likely diagnosis is pilomatrixoma.
Pilomatrixomas are benign adnexal tumors that arise from immature matrix cells of the hair follicles located on dermal or subcutaneous tissue.
The cause of pilomatrixoma remains unclear. Recent studies have suggested that the development of pilomatrixoma are related to mutations in the Wnt signaling pathway, where beta-catenin gene (CTNNB1) mutation is the most frequently reported.1-4
Pilomatrixomas are more common in children and often present before 10 years of age.1,2,5 They commonly appear in head and neck, as well as upper extremities, trunk, and lower extremities.2,6
The clinical manifestations of pilomatrixomas are diverse and according to their appearance five classic clinical types are described: mass, pigmented, mixed, ulcerated, and keloid-like.2,3 The mass type is the predominant form, where it generally presents as a hard and freely mobile nodule covered by skin that may present a firm calcified protruding nodule. Other less common types include: lymphangiectasic, anetodermic, perforating, and bullous.2,6,7
Pilomatrixomas are mostly solitary, whereas multiple forms are reported to be associated with familial inheritance or syndromic conditions, such as myotonic dystrophy, Gardner syndrome, Turner’s syndrome, and Rubinstein-Taybi syndrome.2-4 However, children and adolescents occasionally present with multiple pilomatricomas with no associated syndrome.
On physical exam a helpful features for the diagnosis is the “teeter-totter sign,” which can be illustrated by pressing on one edge of the lesion that will cause the opposite edge to protrude from the skin. Another helpful tool is to use a light to transilluminate and the calcification produces a bluish opaque hue,8 as light cannot transmit through the calcification, often differentiating it from epidermal inclusion cysts or other noncalcified lesions.
What is the differential diagnosis?
Because of the diverse clinical presentations, pilomatrixomas are frequently misdiagnosed. The percentage of correct preoperative diagnosis reported is low, varying from 16% to 43% in different series.1,9-11 They most frequently are misdiagnosed as other types of cysts such as epidermal, dermoid, or sebaceous.2,3,5,12,13 Rapidly growing pilomatrixomas can be also be misdiagnosed as malignant soft-tissue tumors, cutaneous lymphoma, or sarcomas.5,13
When presenting with a classic history and physical features, diagnosis is clinical, and no further studies are recommended.14 To improve diagnostic accuracy when encountering unusual subtypes, imaging is recommended, including ultrasound. Ultrasound adds a high positive predictive value (95.56%).2 Generally, on ultrasound a pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing.2 The definitive diagnosis is, however, made by histopathologic examination.
Pilomatrixomas do not spontaneously regress, therefore complete surgical resection is the standard treatment. During the follow-up period, very low recurrence rates have been reported, varying from 1.5% to 2% which generally occurs because of incomplete resection.2,3
Plexiform neurofibromas are usually congenital tumors of peripheral nerve sheath associated with neurofibromatosis type 1, often with a “bag of worms” feel on palpation. Epidermoid cysts generally present as dermal nodules often with a visible puncture, mobile on soft and mobile on palpation. Dermatofibromas present as firm, usually hyperpigmented papule or nodules that are fixed to subcutaneous tissue, thus often “dimpling” when pitched. Dermatofibrosarcoma protuberans is a rare soft-tissue sarcoma which presents as a firm, slow growing indurated plaques growing over months to years.
Conclusion
Pilomatrixomas are a benign adnexal tumor that sometimes can present as atypical forms such as this case. Diagnosis is usually based on clinical diagnosis, and transillumination can be a bedside clue. When the clinical diagnosis remains obscure an ultrasound can be helpful. The main aim of this case is to improve awareness of the variable presentations of pilomatrixomas and the importance of high level of suspicion supported by careful clinical evaluation.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Guelfand is a visiting dermatology resident in the division of pediaric and adolescent dermatology, University of Califonia, San Diego.
References
1. Jones CD et al. Am J Dermatopathol. 2018;40:631-41.
2. Hu JL et al. Arch Craniofac Surg. 2020;21(5):288-93.
3. Adhikari G and Jadhav GS. Cureus. 2022;14(2):22228.
4. Cóbar JP et al. J Surg Case Rep. 2023;2023(4):rjad182.
5. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
6. Kose D et al. J Cancer Res Ther. 2014;10(3):549-51.
7. Sabater-Abad J et al. Dermatol Online J. 2020;26(8):13030/qt4h16s45w.
8. Alkatan HM et al. Int J Surg Case Rep. 2021;84:106068.
9. Pant I et al. Indian J Dermatol. 2010;55:390-2.
10. Kaddu S et al. Am J Dermatopathol. 1996;18(4):333-8
11. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
12. Wang YN et al. Chin Med J (Engl). 2021;134(16):2011-2.
13. Yannoutsos A et al. Am J Dermatopathol. 2018;40(9):690-3.
14. Zhao A et al. Ear Nose Throat J. 2021;1455613211044778.
Given the characteristic clinical presentation, the most likely diagnosis is pilomatrixoma.
Pilomatrixomas are benign adnexal tumors that arise from immature matrix cells of the hair follicles located on dermal or subcutaneous tissue.
The cause of pilomatrixoma remains unclear. Recent studies have suggested that the development of pilomatrixoma are related to mutations in the Wnt signaling pathway, where beta-catenin gene (CTNNB1) mutation is the most frequently reported.1-4
Pilomatrixomas are more common in children and often present before 10 years of age.1,2,5 They commonly appear in head and neck, as well as upper extremities, trunk, and lower extremities.2,6
The clinical manifestations of pilomatrixomas are diverse and according to their appearance five classic clinical types are described: mass, pigmented, mixed, ulcerated, and keloid-like.2,3 The mass type is the predominant form, where it generally presents as a hard and freely mobile nodule covered by skin that may present a firm calcified protruding nodule. Other less common types include: lymphangiectasic, anetodermic, perforating, and bullous.2,6,7
Pilomatrixomas are mostly solitary, whereas multiple forms are reported to be associated with familial inheritance or syndromic conditions, such as myotonic dystrophy, Gardner syndrome, Turner’s syndrome, and Rubinstein-Taybi syndrome.2-4 However, children and adolescents occasionally present with multiple pilomatricomas with no associated syndrome.
On physical exam a helpful features for the diagnosis is the “teeter-totter sign,” which can be illustrated by pressing on one edge of the lesion that will cause the opposite edge to protrude from the skin. Another helpful tool is to use a light to transilluminate and the calcification produces a bluish opaque hue,8 as light cannot transmit through the calcification, often differentiating it from epidermal inclusion cysts or other noncalcified lesions.
What is the differential diagnosis?
Because of the diverse clinical presentations, pilomatrixomas are frequently misdiagnosed. The percentage of correct preoperative diagnosis reported is low, varying from 16% to 43% in different series.1,9-11 They most frequently are misdiagnosed as other types of cysts such as epidermal, dermoid, or sebaceous.2,3,5,12,13 Rapidly growing pilomatrixomas can be also be misdiagnosed as malignant soft-tissue tumors, cutaneous lymphoma, or sarcomas.5,13
When presenting with a classic history and physical features, diagnosis is clinical, and no further studies are recommended.14 To improve diagnostic accuracy when encountering unusual subtypes, imaging is recommended, including ultrasound. Ultrasound adds a high positive predictive value (95.56%).2 Generally, on ultrasound a pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing.2 The definitive diagnosis is, however, made by histopathologic examination.
Pilomatrixomas do not spontaneously regress, therefore complete surgical resection is the standard treatment. During the follow-up period, very low recurrence rates have been reported, varying from 1.5% to 2% which generally occurs because of incomplete resection.2,3
Plexiform neurofibromas are usually congenital tumors of peripheral nerve sheath associated with neurofibromatosis type 1, often with a “bag of worms” feel on palpation. Epidermoid cysts generally present as dermal nodules often with a visible puncture, mobile on soft and mobile on palpation. Dermatofibromas present as firm, usually hyperpigmented papule or nodules that are fixed to subcutaneous tissue, thus often “dimpling” when pitched. Dermatofibrosarcoma protuberans is a rare soft-tissue sarcoma which presents as a firm, slow growing indurated plaques growing over months to years.
Conclusion
Pilomatrixomas are a benign adnexal tumor that sometimes can present as atypical forms such as this case. Diagnosis is usually based on clinical diagnosis, and transillumination can be a bedside clue. When the clinical diagnosis remains obscure an ultrasound can be helpful. The main aim of this case is to improve awareness of the variable presentations of pilomatrixomas and the importance of high level of suspicion supported by careful clinical evaluation.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Guelfand is a visiting dermatology resident in the division of pediaric and adolescent dermatology, University of Califonia, San Diego.
References
1. Jones CD et al. Am J Dermatopathol. 2018;40:631-41.
2. Hu JL et al. Arch Craniofac Surg. 2020;21(5):288-93.
3. Adhikari G and Jadhav GS. Cureus. 2022;14(2):22228.
4. Cóbar JP et al. J Surg Case Rep. 2023;2023(4):rjad182.
5. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
6. Kose D et al. J Cancer Res Ther. 2014;10(3):549-51.
7. Sabater-Abad J et al. Dermatol Online J. 2020;26(8):13030/qt4h16s45w.
8. Alkatan HM et al. Int J Surg Case Rep. 2021;84:106068.
9. Pant I et al. Indian J Dermatol. 2010;55:390-2.
10. Kaddu S et al. Am J Dermatopathol. 1996;18(4):333-8
11. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
12. Wang YN et al. Chin Med J (Engl). 2021;134(16):2011-2.
13. Yannoutsos A et al. Am J Dermatopathol. 2018;40(9):690-3.
14. Zhao A et al. Ear Nose Throat J. 2021;1455613211044778.
Given the characteristic clinical presentation, the most likely diagnosis is pilomatrixoma.
Pilomatrixomas are benign adnexal tumors that arise from immature matrix cells of the hair follicles located on dermal or subcutaneous tissue.
The cause of pilomatrixoma remains unclear. Recent studies have suggested that the development of pilomatrixoma are related to mutations in the Wnt signaling pathway, where beta-catenin gene (CTNNB1) mutation is the most frequently reported.1-4
Pilomatrixomas are more common in children and often present before 10 years of age.1,2,5 They commonly appear in head and neck, as well as upper extremities, trunk, and lower extremities.2,6
The clinical manifestations of pilomatrixomas are diverse and according to their appearance five classic clinical types are described: mass, pigmented, mixed, ulcerated, and keloid-like.2,3 The mass type is the predominant form, where it generally presents as a hard and freely mobile nodule covered by skin that may present a firm calcified protruding nodule. Other less common types include: lymphangiectasic, anetodermic, perforating, and bullous.2,6,7
Pilomatrixomas are mostly solitary, whereas multiple forms are reported to be associated with familial inheritance or syndromic conditions, such as myotonic dystrophy, Gardner syndrome, Turner’s syndrome, and Rubinstein-Taybi syndrome.2-4 However, children and adolescents occasionally present with multiple pilomatricomas with no associated syndrome.
On physical exam a helpful features for the diagnosis is the “teeter-totter sign,” which can be illustrated by pressing on one edge of the lesion that will cause the opposite edge to protrude from the skin. Another helpful tool is to use a light to transilluminate and the calcification produces a bluish opaque hue,8 as light cannot transmit through the calcification, often differentiating it from epidermal inclusion cysts or other noncalcified lesions.
What is the differential diagnosis?
Because of the diverse clinical presentations, pilomatrixomas are frequently misdiagnosed. The percentage of correct preoperative diagnosis reported is low, varying from 16% to 43% in different series.1,9-11 They most frequently are misdiagnosed as other types of cysts such as epidermal, dermoid, or sebaceous.2,3,5,12,13 Rapidly growing pilomatrixomas can be also be misdiagnosed as malignant soft-tissue tumors, cutaneous lymphoma, or sarcomas.5,13
When presenting with a classic history and physical features, diagnosis is clinical, and no further studies are recommended.14 To improve diagnostic accuracy when encountering unusual subtypes, imaging is recommended, including ultrasound. Ultrasound adds a high positive predictive value (95.56%).2 Generally, on ultrasound a pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing.2 The definitive diagnosis is, however, made by histopathologic examination.
Pilomatrixomas do not spontaneously regress, therefore complete surgical resection is the standard treatment. During the follow-up period, very low recurrence rates have been reported, varying from 1.5% to 2% which generally occurs because of incomplete resection.2,3
Plexiform neurofibromas are usually congenital tumors of peripheral nerve sheath associated with neurofibromatosis type 1, often with a “bag of worms” feel on palpation. Epidermoid cysts generally present as dermal nodules often with a visible puncture, mobile on soft and mobile on palpation. Dermatofibromas present as firm, usually hyperpigmented papule or nodules that are fixed to subcutaneous tissue, thus often “dimpling” when pitched. Dermatofibrosarcoma protuberans is a rare soft-tissue sarcoma which presents as a firm, slow growing indurated plaques growing over months to years.
Conclusion
Pilomatrixomas are a benign adnexal tumor that sometimes can present as atypical forms such as this case. Diagnosis is usually based on clinical diagnosis, and transillumination can be a bedside clue. When the clinical diagnosis remains obscure an ultrasound can be helpful. The main aim of this case is to improve awareness of the variable presentations of pilomatrixomas and the importance of high level of suspicion supported by careful clinical evaluation.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Guelfand is a visiting dermatology resident in the division of pediaric and adolescent dermatology, University of Califonia, San Diego.
References
1. Jones CD et al. Am J Dermatopathol. 2018;40:631-41.
2. Hu JL et al. Arch Craniofac Surg. 2020;21(5):288-93.
3. Adhikari G and Jadhav GS. Cureus. 2022;14(2):22228.
4. Cóbar JP et al. J Surg Case Rep. 2023;2023(4):rjad182.
5. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
6. Kose D et al. J Cancer Res Ther. 2014;10(3):549-51.
7. Sabater-Abad J et al. Dermatol Online J. 2020;26(8):13030/qt4h16s45w.
8. Alkatan HM et al. Int J Surg Case Rep. 2021;84:106068.
9. Pant I et al. Indian J Dermatol. 2010;55:390-2.
10. Kaddu S et al. Am J Dermatopathol. 1996;18(4):333-8
11. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
12. Wang YN et al. Chin Med J (Engl). 2021;134(16):2011-2.
13. Yannoutsos A et al. Am J Dermatopathol. 2018;40(9):690-3.
14. Zhao A et al. Ear Nose Throat J. 2021;1455613211044778.
On physical exam there was a well-circumscribed skin-colored nodule measuring 3.1 x 3 cm that was tender on palpation. The nodule was mobile, with a firm, stony feel, and no punctum was visualized. Transillumination revealed a subtle bluish hue within the nodule.
Study highlights diagnostic challenges of differentiating lichen sclerosus from vitiligo
of cases.
Researchers who tallied symptoms and physical exam findings observed fewer statistically significant differences between LS and vitiligo patients than expected, and LS and vitiligo were sometimes misdiagnosed as each other.
“LS must be treated aggressively to prevent long-term sequelae such as permanent scarring and vulvar squamous cell carcinoma, making an accurate diagnosis crucial,” the authors write in a poster they presented at the annual meeting of the Society for Pediatric Dermatology.
LS is symptomatic and has multiple exam findings, but once treated or quiescent, the discoloration can persist and create diagnostic uncertainty, lead study author Kaiane Habeshian, MD, a pediatric dermatologist at Children’s National Hospital, Washington, told this news organization following the SPD meeting.
The diagnostic uncertainty is especially true in patients with darker skin tones, who may have vitiligoid LS, an LS variant that has overlapping features of both LS and vitiligo.
Vitiligoid LS “presents clinically as a depigmented symmetric white vulvar and perianal white patch, often with minimal signs of inflammation, but is symptomatic and appears consistent with LS on histopathology,” Dr. Habeshian said.
“In our experience, in patients with medium to dark skin tones, there is a variable amount of repigmentation after treating LS,” she added. “After use of high potency topical corticosteroids, some patients almost completely repigment, while others have minimal repigmentation, and this can fluctuate over time, sometimes independent of other signs or symptoms of a flare up. This can lead to diagnostic confusion. For example, if an LS patient is examined after treatment, and their symptoms have resolved, they may subsequently be given a diagnosis of vitiligo.”
In the study, Dr. Habeshian and her coauthors aimed to characterize differences in LS and vitiligo based on history, physical exam, and demographic findings at the time of the initial clinic visit. She and her colleagues extracted and reviewed the medical records of 98 patients with a diagnosis of LS or vitiligo who were seen at a joint pediatric dermatology-gynecology vulvar clinic over 6.8 years. The median and mean age of the study population at diagnosis was about 6 years, with ages ranging from 2 to 20. The team used descriptive statistics and Z tests for data analysis.
The researchers found that pruritus, constipation, and dysuria were the most common symptoms experienced by both LS and vitiligo patients. All were experienced more frequently by LS patients, but only pruritus reached statistical significance (P = .040). Other symptoms experienced only by LS patients included vulvar pain, bleeding, and pain with defecation.
Meanwhile, apart from hypopigmentation and erythema, all physical exam findings were more frequent in LS patients, compared with vitiligo patients, including fissures and purpura/petechiae, but only epidermal atrophy and figure-of-8 distribution of hypopigmentation reached statistical significance (P values of .047 and .036, respectively).
In other findings, LS and vitiligo were misdiagnosed as each other 15 times. Nearly half of the misdiagnoses (46.7%) were made in Black patients, who composed 38.8% of all patients in the study.
“I suspect that some vitiligo cases that were previously ‘misdiagnosed’ as LS were actually LS that just didn’t repigment and then were labeled as vitiligo in the chart,” Dr. Habeshian said.
“And some of those LS cases that previously were misdiagnosed as vitiligo likely had other more subtle LS findings that were missed (shininess and wrinkling of the skin, small fissures, constipation) or that were attributed to comorbid irritant contact dermatitis or another condition,” she said. “It was interesting to see that even in a vulvar dermatology clinic there can be confusion between these diagnoses because the literature on pediatric LS in darker skin tones is so sparse.”
She emphasized that a close exam and detailed history are needed to properly diagnose patients with anogenital skin conditions.
“Don’t forget to ask about constipation and urinary symptoms as well as psychosocial and, in the appropriate patient, sexual and reproductive function,” Dr. Habeshian said. “Based on my experience, pediatric LS is much more common in our community than the literature would suggest. Its psychosocial impact is tremendous but not well documented, particularly in pediatric patients. In my experience, the longer LS is misdiagnosed or mistreated, the more challenging it becomes to manage. You don’t want to miss LS.”
She acknowledged certain limitations of the study, including the fact that photographs were not available for review for many of the earlier years of the clinic. “Therefore, we had to depend on the diagnosis given at the time of the visit,” she said. “This likely accounts in part for the smaller number than expected of significant exam and history findings between LS and vitiligo. We need further studies utilizing a standardized approach to accurate diagnosis.”
Her coauthors were Nikita Menta, Aneka Khilnani, MS, and Tazim Dowlut-McElroy, MD. The researchers reported having no financial disclosures.
of cases.
Researchers who tallied symptoms and physical exam findings observed fewer statistically significant differences between LS and vitiligo patients than expected, and LS and vitiligo were sometimes misdiagnosed as each other.
“LS must be treated aggressively to prevent long-term sequelae such as permanent scarring and vulvar squamous cell carcinoma, making an accurate diagnosis crucial,” the authors write in a poster they presented at the annual meeting of the Society for Pediatric Dermatology.
LS is symptomatic and has multiple exam findings, but once treated or quiescent, the discoloration can persist and create diagnostic uncertainty, lead study author Kaiane Habeshian, MD, a pediatric dermatologist at Children’s National Hospital, Washington, told this news organization following the SPD meeting.
The diagnostic uncertainty is especially true in patients with darker skin tones, who may have vitiligoid LS, an LS variant that has overlapping features of both LS and vitiligo.
Vitiligoid LS “presents clinically as a depigmented symmetric white vulvar and perianal white patch, often with minimal signs of inflammation, but is symptomatic and appears consistent with LS on histopathology,” Dr. Habeshian said.
“In our experience, in patients with medium to dark skin tones, there is a variable amount of repigmentation after treating LS,” she added. “After use of high potency topical corticosteroids, some patients almost completely repigment, while others have minimal repigmentation, and this can fluctuate over time, sometimes independent of other signs or symptoms of a flare up. This can lead to diagnostic confusion. For example, if an LS patient is examined after treatment, and their symptoms have resolved, they may subsequently be given a diagnosis of vitiligo.”
In the study, Dr. Habeshian and her coauthors aimed to characterize differences in LS and vitiligo based on history, physical exam, and demographic findings at the time of the initial clinic visit. She and her colleagues extracted and reviewed the medical records of 98 patients with a diagnosis of LS or vitiligo who were seen at a joint pediatric dermatology-gynecology vulvar clinic over 6.8 years. The median and mean age of the study population at diagnosis was about 6 years, with ages ranging from 2 to 20. The team used descriptive statistics and Z tests for data analysis.
The researchers found that pruritus, constipation, and dysuria were the most common symptoms experienced by both LS and vitiligo patients. All were experienced more frequently by LS patients, but only pruritus reached statistical significance (P = .040). Other symptoms experienced only by LS patients included vulvar pain, bleeding, and pain with defecation.
Meanwhile, apart from hypopigmentation and erythema, all physical exam findings were more frequent in LS patients, compared with vitiligo patients, including fissures and purpura/petechiae, but only epidermal atrophy and figure-of-8 distribution of hypopigmentation reached statistical significance (P values of .047 and .036, respectively).
In other findings, LS and vitiligo were misdiagnosed as each other 15 times. Nearly half of the misdiagnoses (46.7%) were made in Black patients, who composed 38.8% of all patients in the study.
“I suspect that some vitiligo cases that were previously ‘misdiagnosed’ as LS were actually LS that just didn’t repigment and then were labeled as vitiligo in the chart,” Dr. Habeshian said.
“And some of those LS cases that previously were misdiagnosed as vitiligo likely had other more subtle LS findings that were missed (shininess and wrinkling of the skin, small fissures, constipation) or that were attributed to comorbid irritant contact dermatitis or another condition,” she said. “It was interesting to see that even in a vulvar dermatology clinic there can be confusion between these diagnoses because the literature on pediatric LS in darker skin tones is so sparse.”
She emphasized that a close exam and detailed history are needed to properly diagnose patients with anogenital skin conditions.
“Don’t forget to ask about constipation and urinary symptoms as well as psychosocial and, in the appropriate patient, sexual and reproductive function,” Dr. Habeshian said. “Based on my experience, pediatric LS is much more common in our community than the literature would suggest. Its psychosocial impact is tremendous but not well documented, particularly in pediatric patients. In my experience, the longer LS is misdiagnosed or mistreated, the more challenging it becomes to manage. You don’t want to miss LS.”
She acknowledged certain limitations of the study, including the fact that photographs were not available for review for many of the earlier years of the clinic. “Therefore, we had to depend on the diagnosis given at the time of the visit,” she said. “This likely accounts in part for the smaller number than expected of significant exam and history findings between LS and vitiligo. We need further studies utilizing a standardized approach to accurate diagnosis.”
Her coauthors were Nikita Menta, Aneka Khilnani, MS, and Tazim Dowlut-McElroy, MD. The researchers reported having no financial disclosures.
of cases.
Researchers who tallied symptoms and physical exam findings observed fewer statistically significant differences between LS and vitiligo patients than expected, and LS and vitiligo were sometimes misdiagnosed as each other.
“LS must be treated aggressively to prevent long-term sequelae such as permanent scarring and vulvar squamous cell carcinoma, making an accurate diagnosis crucial,” the authors write in a poster they presented at the annual meeting of the Society for Pediatric Dermatology.
LS is symptomatic and has multiple exam findings, but once treated or quiescent, the discoloration can persist and create diagnostic uncertainty, lead study author Kaiane Habeshian, MD, a pediatric dermatologist at Children’s National Hospital, Washington, told this news organization following the SPD meeting.
The diagnostic uncertainty is especially true in patients with darker skin tones, who may have vitiligoid LS, an LS variant that has overlapping features of both LS and vitiligo.
Vitiligoid LS “presents clinically as a depigmented symmetric white vulvar and perianal white patch, often with minimal signs of inflammation, but is symptomatic and appears consistent with LS on histopathology,” Dr. Habeshian said.
“In our experience, in patients with medium to dark skin tones, there is a variable amount of repigmentation after treating LS,” she added. “After use of high potency topical corticosteroids, some patients almost completely repigment, while others have minimal repigmentation, and this can fluctuate over time, sometimes independent of other signs or symptoms of a flare up. This can lead to diagnostic confusion. For example, if an LS patient is examined after treatment, and their symptoms have resolved, they may subsequently be given a diagnosis of vitiligo.”
In the study, Dr. Habeshian and her coauthors aimed to characterize differences in LS and vitiligo based on history, physical exam, and demographic findings at the time of the initial clinic visit. She and her colleagues extracted and reviewed the medical records of 98 patients with a diagnosis of LS or vitiligo who were seen at a joint pediatric dermatology-gynecology vulvar clinic over 6.8 years. The median and mean age of the study population at diagnosis was about 6 years, with ages ranging from 2 to 20. The team used descriptive statistics and Z tests for data analysis.
The researchers found that pruritus, constipation, and dysuria were the most common symptoms experienced by both LS and vitiligo patients. All were experienced more frequently by LS patients, but only pruritus reached statistical significance (P = .040). Other symptoms experienced only by LS patients included vulvar pain, bleeding, and pain with defecation.
Meanwhile, apart from hypopigmentation and erythema, all physical exam findings were more frequent in LS patients, compared with vitiligo patients, including fissures and purpura/petechiae, but only epidermal atrophy and figure-of-8 distribution of hypopigmentation reached statistical significance (P values of .047 and .036, respectively).
In other findings, LS and vitiligo were misdiagnosed as each other 15 times. Nearly half of the misdiagnoses (46.7%) were made in Black patients, who composed 38.8% of all patients in the study.
“I suspect that some vitiligo cases that were previously ‘misdiagnosed’ as LS were actually LS that just didn’t repigment and then were labeled as vitiligo in the chart,” Dr. Habeshian said.
“And some of those LS cases that previously were misdiagnosed as vitiligo likely had other more subtle LS findings that were missed (shininess and wrinkling of the skin, small fissures, constipation) or that were attributed to comorbid irritant contact dermatitis or another condition,” she said. “It was interesting to see that even in a vulvar dermatology clinic there can be confusion between these diagnoses because the literature on pediatric LS in darker skin tones is so sparse.”
She emphasized that a close exam and detailed history are needed to properly diagnose patients with anogenital skin conditions.
“Don’t forget to ask about constipation and urinary symptoms as well as psychosocial and, in the appropriate patient, sexual and reproductive function,” Dr. Habeshian said. “Based on my experience, pediatric LS is much more common in our community than the literature would suggest. Its psychosocial impact is tremendous but not well documented, particularly in pediatric patients. In my experience, the longer LS is misdiagnosed or mistreated, the more challenging it becomes to manage. You don’t want to miss LS.”
She acknowledged certain limitations of the study, including the fact that photographs were not available for review for many of the earlier years of the clinic. “Therefore, we had to depend on the diagnosis given at the time of the visit,” she said. “This likely accounts in part for the smaller number than expected of significant exam and history findings between LS and vitiligo. We need further studies utilizing a standardized approach to accurate diagnosis.”
Her coauthors were Nikita Menta, Aneka Khilnani, MS, and Tazim Dowlut-McElroy, MD. The researchers reported having no financial disclosures.
FROM SPD 2023