User login
DAPA-HF: Dapagliflozin’s HFrEF efficacy confirmed in nondiabetics
PHILADELPHIA – The primary outcome results from the practice-changing DAPA-HF trial gave clinicians strong evidence that the diabetes drug dapagliflozin was equally effective at reducing cardiovascular death and acute exacerbations in patients with heart failure with reduced ejection fraction, whether or not they also had type 2 diabetes. More detailed findings from the 2,605 enrolled patients in DAPA-HF who lacked diabetes (55% of the total study population) have now sealed the deal.
“The relative and absolute reductions in cardiovascular death and hospitalizations or urgent visits for heart failure were substantial, clinically important, and consistent in patients with or without type 2 diabetes,” John McMurray, MD, declared at the American Heart Association scientific sessions as he summarized new trial results that confirmed the initial finding he reported previously.
While the initial report of the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) by the study’s lead investigator, Dr. McMurray, was limited to the finding that the relative risk reduction for the study’s primary endpoint was a highly statistically significant 25% in heart failure patients with diabetes and an equally strongly significant 27% relative cut among patients without diabetes (N Engl J Med. 2019 Sep 19;doi: 10.1056/NEJMoa1911303), the new data showed that same consistency across the range of outcomes studied in the trial as well as across the range of glycosylated hemoglobin levels that patients had at study entry.
In an analysis that divided the entire study population of 4,744 patients with heart failure with reduced ejection fraction (HFrEF) into tertiles based on their entry blood level of hemoglobin A1c, patients with a normal level at or below 5.6% had a 26% relative reduction in the study’s primary endpoint, essentially the same response as the 29% relative cut in adverse events in the tertile of patients with a glycosylated hemoglobin level of 5.7%-5.9% and the relative 28% relative reduction in events in patients diagnosed with type 2 diabetes and having a hemoglobin A1c of 6.0% or greater, reported Dr. McMurray, professor of cardiology at the University of Glasgow. The results also showed a very benign safety profile in the patients without diabetes, similar to patients with diabetes and to placebo, and with no episodes of major hypoglycemia or diabetic ketoacidosis.
“It’s quite impressive that the result was consistent regardless of the level of hemoglobin A1c,” commented Larry A. Allen, MD, professor of medicine at the University of Colorado in Aurora and designated discussant for the report. Even though the patients without diabetes constituted just over half of the full DAPA-HF enrollment, the comparison of the effect of dapagliflozin in patients with or without diabetes was prespecified in a trial that enrolled a relatively large number of patients into each of the two subgroups by diabetes status. “I think there a good chance dapagliflozin will get an indication” for treating HFrEF patients without diabetes, Dr. Allen suggested in a video interview.
If the DAPA-HF results persuade the U.S. Food and Drug Administration to grant a supplemental indication to dapagliflozin for use in cutting cardiovascular deaths and acute heart failure exacerbations in patients without diabetes, it would pave the way for health insurers to pay for the drug. Right now, even though Dr. Allen and other heart failure physicians have been impressed by the DAPA-HF findings and are eager to add the drug to the list of agents that HFrEF patients routinely receive, he’s been stymied so far by patients’ out-of pocket cost for using dapagliflozin off-label, roughly $500 a month.
“The DAPA-HF results suggest there is strong reason to consider dapagliflozin for patients without diabetes, and for payers to pay for it. I’m not prescribing dapagliflozin to HFrEF patients without diabetes right now; not because of the data, but because of noncoverage. Payers have not yet caught up with the data,” he said, and they likely will continue to not pay for the drug when used by patients without diabetes until a new labeled indication appears for those patients.
The immediate availability of dapagliflozin (Farxiga) and the two other approved members of the sodium-glucose co-transporter 2 inhibitor class of drugs, empagliflozin (Jardiance) and canagliflozin (Invokana), to treat patients with HFrEF, and the prospect of soon having dapagliflozin and possibly the other drugs in this class to treat patients with HFrEF but without diabetes also raises issues of drug sequencing in these patients and the overall number of drugs that HFrEF patients must now take to be on optimized medical therapy, Dr. Allen noted.
The already-existing lineup of medications for HFrEF patients includes starting on an ACE inhibitor or angiotensin receptor blocker and adding a beta-blocker, a mineralocorticoid receptor antagonist, then swapping out the initial renin-angiotensin system inhibitor for sacubitril/valsartan, and then, on top of all this, adding dapagliflozin or another drug in the same class. It raises questions of what is objectively the best way to introduce all these drugs into patients, and how to do it without subjecting patients to “financial toxicity,” Dr. Allen said during his discussion of the trial’s results.
DAPA-HF was sponsored by AstraZeneca, which markets dapagliflozin (Farxiga). The University of Glasgow received payment from AstraZeneca to compensate for the time Dr. McMurray spent running the study. Dr. Allen has been a consultant to ACI Clinical, Boston Scientific, and Janssen.
SOURCE: McMurray JJV. AHA 19, Late-Breaking Science 1.
A labeling change for dapagliflozin that says the drug is approved for use in patients with heart failure with reduced ejection fraction (HFrEF) and without diabetes is critical so that payers will get on board with this new and important treatment. The evidence for efficacy and safety in patients without diabetes was so strong in the DAPA-HF trial that I don’t think a second trial will be needed for the Food and Drug Administration to add this indication to dapagliflozin’s label.
For patients with type 2 diabetes as well as HFrEF, it’s already full steam ahead to use dapagliflozin or another drug from the class of sodium glucose co-transporter 2 (SGLT2) inhibitors, empagliflozin and canagliflozin. However, so far these drugs are not being widely prescribed by clinicians to patients with HFrEF but without diabetes. We need to build up the familiarity of clinicians with the SGLT2 inhibitor drugs so that primary care physicians will feel comfortable starting HFrEF patients on them. It’s relatively easy to start patients on the drugs in this class because of their good safety and no signal of problems when using them with other HFrEF medications.
The growing list of key drugs to use on patients with HFrEF means that we need to become smarter on how we start patients on these agents. Currently it’s done without evidence for which order of introduction works best. We also need to confirm that all five types of drugs that now appear indicated for HFrEF patients are all truly additive: an angiotensin receptor blocker coupled with the angiotensin receptor neprilysin inhibitor sacubitril, a beta-blocker, a mineralocorticoid receptor antagonist, and now an SGLT2 inhibitor. I propose that researchers run studies that systematically stop one of these drugs to see whether the overall benefit to HFrEF patients remains unchanged, thereby identifying an agent that could be dropped from what is a growing list of drug classes, with possibly more classes to follow depending on results from studies now underway.
Christopher M. O’Connor, MD, is a heart failure physician and president of the Inova Heart and Vascular Institute in Falls Church, Va. He has been a consultant to Arena, Bayer, Bristol-Meyers Squibb, Merck, and Windtree Therapeutics. He made these comments in an interview.
A labeling change for dapagliflozin that says the drug is approved for use in patients with heart failure with reduced ejection fraction (HFrEF) and without diabetes is critical so that payers will get on board with this new and important treatment. The evidence for efficacy and safety in patients without diabetes was so strong in the DAPA-HF trial that I don’t think a second trial will be needed for the Food and Drug Administration to add this indication to dapagliflozin’s label.
For patients with type 2 diabetes as well as HFrEF, it’s already full steam ahead to use dapagliflozin or another drug from the class of sodium glucose co-transporter 2 (SGLT2) inhibitors, empagliflozin and canagliflozin. However, so far these drugs are not being widely prescribed by clinicians to patients with HFrEF but without diabetes. We need to build up the familiarity of clinicians with the SGLT2 inhibitor drugs so that primary care physicians will feel comfortable starting HFrEF patients on them. It’s relatively easy to start patients on the drugs in this class because of their good safety and no signal of problems when using them with other HFrEF medications.
The growing list of key drugs to use on patients with HFrEF means that we need to become smarter on how we start patients on these agents. Currently it’s done without evidence for which order of introduction works best. We also need to confirm that all five types of drugs that now appear indicated for HFrEF patients are all truly additive: an angiotensin receptor blocker coupled with the angiotensin receptor neprilysin inhibitor sacubitril, a beta-blocker, a mineralocorticoid receptor antagonist, and now an SGLT2 inhibitor. I propose that researchers run studies that systematically stop one of these drugs to see whether the overall benefit to HFrEF patients remains unchanged, thereby identifying an agent that could be dropped from what is a growing list of drug classes, with possibly more classes to follow depending on results from studies now underway.
Christopher M. O’Connor, MD, is a heart failure physician and president of the Inova Heart and Vascular Institute in Falls Church, Va. He has been a consultant to Arena, Bayer, Bristol-Meyers Squibb, Merck, and Windtree Therapeutics. He made these comments in an interview.
A labeling change for dapagliflozin that says the drug is approved for use in patients with heart failure with reduced ejection fraction (HFrEF) and without diabetes is critical so that payers will get on board with this new and important treatment. The evidence for efficacy and safety in patients without diabetes was so strong in the DAPA-HF trial that I don’t think a second trial will be needed for the Food and Drug Administration to add this indication to dapagliflozin’s label.
For patients with type 2 diabetes as well as HFrEF, it’s already full steam ahead to use dapagliflozin or another drug from the class of sodium glucose co-transporter 2 (SGLT2) inhibitors, empagliflozin and canagliflozin. However, so far these drugs are not being widely prescribed by clinicians to patients with HFrEF but without diabetes. We need to build up the familiarity of clinicians with the SGLT2 inhibitor drugs so that primary care physicians will feel comfortable starting HFrEF patients on them. It’s relatively easy to start patients on the drugs in this class because of their good safety and no signal of problems when using them with other HFrEF medications.
The growing list of key drugs to use on patients with HFrEF means that we need to become smarter on how we start patients on these agents. Currently it’s done without evidence for which order of introduction works best. We also need to confirm that all five types of drugs that now appear indicated for HFrEF patients are all truly additive: an angiotensin receptor blocker coupled with the angiotensin receptor neprilysin inhibitor sacubitril, a beta-blocker, a mineralocorticoid receptor antagonist, and now an SGLT2 inhibitor. I propose that researchers run studies that systematically stop one of these drugs to see whether the overall benefit to HFrEF patients remains unchanged, thereby identifying an agent that could be dropped from what is a growing list of drug classes, with possibly more classes to follow depending on results from studies now underway.
Christopher M. O’Connor, MD, is a heart failure physician and president of the Inova Heart and Vascular Institute in Falls Church, Va. He has been a consultant to Arena, Bayer, Bristol-Meyers Squibb, Merck, and Windtree Therapeutics. He made these comments in an interview.
PHILADELPHIA – The primary outcome results from the practice-changing DAPA-HF trial gave clinicians strong evidence that the diabetes drug dapagliflozin was equally effective at reducing cardiovascular death and acute exacerbations in patients with heart failure with reduced ejection fraction, whether or not they also had type 2 diabetes. More detailed findings from the 2,605 enrolled patients in DAPA-HF who lacked diabetes (55% of the total study population) have now sealed the deal.
“The relative and absolute reductions in cardiovascular death and hospitalizations or urgent visits for heart failure were substantial, clinically important, and consistent in patients with or without type 2 diabetes,” John McMurray, MD, declared at the American Heart Association scientific sessions as he summarized new trial results that confirmed the initial finding he reported previously.
While the initial report of the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) by the study’s lead investigator, Dr. McMurray, was limited to the finding that the relative risk reduction for the study’s primary endpoint was a highly statistically significant 25% in heart failure patients with diabetes and an equally strongly significant 27% relative cut among patients without diabetes (N Engl J Med. 2019 Sep 19;doi: 10.1056/NEJMoa1911303), the new data showed that same consistency across the range of outcomes studied in the trial as well as across the range of glycosylated hemoglobin levels that patients had at study entry.
In an analysis that divided the entire study population of 4,744 patients with heart failure with reduced ejection fraction (HFrEF) into tertiles based on their entry blood level of hemoglobin A1c, patients with a normal level at or below 5.6% had a 26% relative reduction in the study’s primary endpoint, essentially the same response as the 29% relative cut in adverse events in the tertile of patients with a glycosylated hemoglobin level of 5.7%-5.9% and the relative 28% relative reduction in events in patients diagnosed with type 2 diabetes and having a hemoglobin A1c of 6.0% or greater, reported Dr. McMurray, professor of cardiology at the University of Glasgow. The results also showed a very benign safety profile in the patients without diabetes, similar to patients with diabetes and to placebo, and with no episodes of major hypoglycemia or diabetic ketoacidosis.
“It’s quite impressive that the result was consistent regardless of the level of hemoglobin A1c,” commented Larry A. Allen, MD, professor of medicine at the University of Colorado in Aurora and designated discussant for the report. Even though the patients without diabetes constituted just over half of the full DAPA-HF enrollment, the comparison of the effect of dapagliflozin in patients with or without diabetes was prespecified in a trial that enrolled a relatively large number of patients into each of the two subgroups by diabetes status. “I think there a good chance dapagliflozin will get an indication” for treating HFrEF patients without diabetes, Dr. Allen suggested in a video interview.
If the DAPA-HF results persuade the U.S. Food and Drug Administration to grant a supplemental indication to dapagliflozin for use in cutting cardiovascular deaths and acute heart failure exacerbations in patients without diabetes, it would pave the way for health insurers to pay for the drug. Right now, even though Dr. Allen and other heart failure physicians have been impressed by the DAPA-HF findings and are eager to add the drug to the list of agents that HFrEF patients routinely receive, he’s been stymied so far by patients’ out-of pocket cost for using dapagliflozin off-label, roughly $500 a month.
“The DAPA-HF results suggest there is strong reason to consider dapagliflozin for patients without diabetes, and for payers to pay for it. I’m not prescribing dapagliflozin to HFrEF patients without diabetes right now; not because of the data, but because of noncoverage. Payers have not yet caught up with the data,” he said, and they likely will continue to not pay for the drug when used by patients without diabetes until a new labeled indication appears for those patients.
The immediate availability of dapagliflozin (Farxiga) and the two other approved members of the sodium-glucose co-transporter 2 inhibitor class of drugs, empagliflozin (Jardiance) and canagliflozin (Invokana), to treat patients with HFrEF, and the prospect of soon having dapagliflozin and possibly the other drugs in this class to treat patients with HFrEF but without diabetes also raises issues of drug sequencing in these patients and the overall number of drugs that HFrEF patients must now take to be on optimized medical therapy, Dr. Allen noted.
The already-existing lineup of medications for HFrEF patients includes starting on an ACE inhibitor or angiotensin receptor blocker and adding a beta-blocker, a mineralocorticoid receptor antagonist, then swapping out the initial renin-angiotensin system inhibitor for sacubitril/valsartan, and then, on top of all this, adding dapagliflozin or another drug in the same class. It raises questions of what is objectively the best way to introduce all these drugs into patients, and how to do it without subjecting patients to “financial toxicity,” Dr. Allen said during his discussion of the trial’s results.
DAPA-HF was sponsored by AstraZeneca, which markets dapagliflozin (Farxiga). The University of Glasgow received payment from AstraZeneca to compensate for the time Dr. McMurray spent running the study. Dr. Allen has been a consultant to ACI Clinical, Boston Scientific, and Janssen.
SOURCE: McMurray JJV. AHA 19, Late-Breaking Science 1.
PHILADELPHIA – The primary outcome results from the practice-changing DAPA-HF trial gave clinicians strong evidence that the diabetes drug dapagliflozin was equally effective at reducing cardiovascular death and acute exacerbations in patients with heart failure with reduced ejection fraction, whether or not they also had type 2 diabetes. More detailed findings from the 2,605 enrolled patients in DAPA-HF who lacked diabetes (55% of the total study population) have now sealed the deal.
“The relative and absolute reductions in cardiovascular death and hospitalizations or urgent visits for heart failure were substantial, clinically important, and consistent in patients with or without type 2 diabetes,” John McMurray, MD, declared at the American Heart Association scientific sessions as he summarized new trial results that confirmed the initial finding he reported previously.
While the initial report of the DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) by the study’s lead investigator, Dr. McMurray, was limited to the finding that the relative risk reduction for the study’s primary endpoint was a highly statistically significant 25% in heart failure patients with diabetes and an equally strongly significant 27% relative cut among patients without diabetes (N Engl J Med. 2019 Sep 19;doi: 10.1056/NEJMoa1911303), the new data showed that same consistency across the range of outcomes studied in the trial as well as across the range of glycosylated hemoglobin levels that patients had at study entry.
In an analysis that divided the entire study population of 4,744 patients with heart failure with reduced ejection fraction (HFrEF) into tertiles based on their entry blood level of hemoglobin A1c, patients with a normal level at or below 5.6% had a 26% relative reduction in the study’s primary endpoint, essentially the same response as the 29% relative cut in adverse events in the tertile of patients with a glycosylated hemoglobin level of 5.7%-5.9% and the relative 28% relative reduction in events in patients diagnosed with type 2 diabetes and having a hemoglobin A1c of 6.0% or greater, reported Dr. McMurray, professor of cardiology at the University of Glasgow. The results also showed a very benign safety profile in the patients without diabetes, similar to patients with diabetes and to placebo, and with no episodes of major hypoglycemia or diabetic ketoacidosis.
“It’s quite impressive that the result was consistent regardless of the level of hemoglobin A1c,” commented Larry A. Allen, MD, professor of medicine at the University of Colorado in Aurora and designated discussant for the report. Even though the patients without diabetes constituted just over half of the full DAPA-HF enrollment, the comparison of the effect of dapagliflozin in patients with or without diabetes was prespecified in a trial that enrolled a relatively large number of patients into each of the two subgroups by diabetes status. “I think there a good chance dapagliflozin will get an indication” for treating HFrEF patients without diabetes, Dr. Allen suggested in a video interview.
If the DAPA-HF results persuade the U.S. Food and Drug Administration to grant a supplemental indication to dapagliflozin for use in cutting cardiovascular deaths and acute heart failure exacerbations in patients without diabetes, it would pave the way for health insurers to pay for the drug. Right now, even though Dr. Allen and other heart failure physicians have been impressed by the DAPA-HF findings and are eager to add the drug to the list of agents that HFrEF patients routinely receive, he’s been stymied so far by patients’ out-of pocket cost for using dapagliflozin off-label, roughly $500 a month.
“The DAPA-HF results suggest there is strong reason to consider dapagliflozin for patients without diabetes, and for payers to pay for it. I’m not prescribing dapagliflozin to HFrEF patients without diabetes right now; not because of the data, but because of noncoverage. Payers have not yet caught up with the data,” he said, and they likely will continue to not pay for the drug when used by patients without diabetes until a new labeled indication appears for those patients.
The immediate availability of dapagliflozin (Farxiga) and the two other approved members of the sodium-glucose co-transporter 2 inhibitor class of drugs, empagliflozin (Jardiance) and canagliflozin (Invokana), to treat patients with HFrEF, and the prospect of soon having dapagliflozin and possibly the other drugs in this class to treat patients with HFrEF but without diabetes also raises issues of drug sequencing in these patients and the overall number of drugs that HFrEF patients must now take to be on optimized medical therapy, Dr. Allen noted.
The already-existing lineup of medications for HFrEF patients includes starting on an ACE inhibitor or angiotensin receptor blocker and adding a beta-blocker, a mineralocorticoid receptor antagonist, then swapping out the initial renin-angiotensin system inhibitor for sacubitril/valsartan, and then, on top of all this, adding dapagliflozin or another drug in the same class. It raises questions of what is objectively the best way to introduce all these drugs into patients, and how to do it without subjecting patients to “financial toxicity,” Dr. Allen said during his discussion of the trial’s results.
DAPA-HF was sponsored by AstraZeneca, which markets dapagliflozin (Farxiga). The University of Glasgow received payment from AstraZeneca to compensate for the time Dr. McMurray spent running the study. Dr. Allen has been a consultant to ACI Clinical, Boston Scientific, and Janssen.
SOURCE: McMurray JJV. AHA 19, Late-Breaking Science 1.
REPORTING FROM AHA 2019
Key clinical point: Dapaglifozin produced as much benefit in HFrEF patients without diabetes as it did in those with type 2 diabetes.
Major finding: The relative risk reduction with dapagliflozin was 26% in patients with a hemoglobin A1c of 5.6% or less.
Study details: DAPA-HF is a multicenter, randomized trial involving 4,744 patients with heart failure with reduced ejection fraction.
Disclosures: DAPA-HF was sponsored by AstraZeneca, which markets dapagliflozin (Farxiga). The University of Glasgow received payment from AstraZeneca to compensate for the time Dr. McMurray spent running the study. Dr. Allen has been a consultant to ACI Clinical, Boston Scientific, and Janssen.
Source: McMurray JJV et al. AHA 19, Late-Breaking Science 1.
Bariatric Surgery Improves Long-Term Health—but Not Long-Term Health Care Costs
Veterans have some of the highest rates of overweight and obesity in the country: 78%, compared with 35% of American adults overall. So bariatric surgery can be a boon to many veterans. But while it improves health for those with severe obesity, does it also translate into lower health care costs?
Researchers from the Durham VA Medical Center (VAMC) say no. In a study funded by VA Health Services Research and Development and the National Institute on Drug Abuse, they analyzed data on 2,498 veterans who underwent bariatric surgery between January 2000 and September 2011, and 7,456 patients (also severely obese) who did not have surgery. The researchers compared the 2 groups’ outpatient, inpatient, and pharmacy expenditures from 3 years before surgery to 10 years after surgery.
Mean total expenditures for the surgery cohort were $5,093 at 7 to 12 months before surgery, $1,400 higher than costs for the nonsurgery group. The numbers rose to $7,448 at 6 months after surgery—$3,000 higher than in the nonsurgery group. Postsurgical expenditures dropped to $6,692 at 5 years, then gradually increased to $8,495 at 10 years. Outpatient pharmacy expenditures were significantly lower among the surgery cohort throughout the follow-up, but the cost reductions were offset by higher inpatient and outpatient expenditures.
Total expenditures were higher in the surgery cohort than the nonsurgery cohort during the 3 years before and the first 2 years after surgery, but the numbers of the 2 groups converged 5 to 10 years after surgery.
The researchers offer some possible reasons that the surgery did not lower health care costs. For instance, despite better overall health, patients may still need to be treated for short-term complications of bariatric surgery, such as nausea, anemia, and vitamin deficiencies. The surgery patients also may have needed additional procedures, such as removal of excess skin. They might have become eligible for knee or hip replacement after having lost weight.
Finally, the researchers point out, many conditions linked to obesity, such as diabetes, do not necessarily go away when the patient loses weight.
The study authors noted that “few health care treatments are required to be cost saving or even cost-effective to be widely available, so requiring cost savings of bariatric surgery imposes an unfair standard.”
Veterans have some of the highest rates of overweight and obesity in the country: 78%, compared with 35% of American adults overall. So bariatric surgery can be a boon to many veterans. But while it improves health for those with severe obesity, does it also translate into lower health care costs?
Researchers from the Durham VA Medical Center (VAMC) say no. In a study funded by VA Health Services Research and Development and the National Institute on Drug Abuse, they analyzed data on 2,498 veterans who underwent bariatric surgery between January 2000 and September 2011, and 7,456 patients (also severely obese) who did not have surgery. The researchers compared the 2 groups’ outpatient, inpatient, and pharmacy expenditures from 3 years before surgery to 10 years after surgery.
Mean total expenditures for the surgery cohort were $5,093 at 7 to 12 months before surgery, $1,400 higher than costs for the nonsurgery group. The numbers rose to $7,448 at 6 months after surgery—$3,000 higher than in the nonsurgery group. Postsurgical expenditures dropped to $6,692 at 5 years, then gradually increased to $8,495 at 10 years. Outpatient pharmacy expenditures were significantly lower among the surgery cohort throughout the follow-up, but the cost reductions were offset by higher inpatient and outpatient expenditures.
Total expenditures were higher in the surgery cohort than the nonsurgery cohort during the 3 years before and the first 2 years after surgery, but the numbers of the 2 groups converged 5 to 10 years after surgery.
The researchers offer some possible reasons that the surgery did not lower health care costs. For instance, despite better overall health, patients may still need to be treated for short-term complications of bariatric surgery, such as nausea, anemia, and vitamin deficiencies. The surgery patients also may have needed additional procedures, such as removal of excess skin. They might have become eligible for knee or hip replacement after having lost weight.
Finally, the researchers point out, many conditions linked to obesity, such as diabetes, do not necessarily go away when the patient loses weight.
The study authors noted that “few health care treatments are required to be cost saving or even cost-effective to be widely available, so requiring cost savings of bariatric surgery imposes an unfair standard.”
Veterans have some of the highest rates of overweight and obesity in the country: 78%, compared with 35% of American adults overall. So bariatric surgery can be a boon to many veterans. But while it improves health for those with severe obesity, does it also translate into lower health care costs?
Researchers from the Durham VA Medical Center (VAMC) say no. In a study funded by VA Health Services Research and Development and the National Institute on Drug Abuse, they analyzed data on 2,498 veterans who underwent bariatric surgery between January 2000 and September 2011, and 7,456 patients (also severely obese) who did not have surgery. The researchers compared the 2 groups’ outpatient, inpatient, and pharmacy expenditures from 3 years before surgery to 10 years after surgery.
Mean total expenditures for the surgery cohort were $5,093 at 7 to 12 months before surgery, $1,400 higher than costs for the nonsurgery group. The numbers rose to $7,448 at 6 months after surgery—$3,000 higher than in the nonsurgery group. Postsurgical expenditures dropped to $6,692 at 5 years, then gradually increased to $8,495 at 10 years. Outpatient pharmacy expenditures were significantly lower among the surgery cohort throughout the follow-up, but the cost reductions were offset by higher inpatient and outpatient expenditures.
Total expenditures were higher in the surgery cohort than the nonsurgery cohort during the 3 years before and the first 2 years after surgery, but the numbers of the 2 groups converged 5 to 10 years after surgery.
The researchers offer some possible reasons that the surgery did not lower health care costs. For instance, despite better overall health, patients may still need to be treated for short-term complications of bariatric surgery, such as nausea, anemia, and vitamin deficiencies. The surgery patients also may have needed additional procedures, such as removal of excess skin. They might have become eligible for knee or hip replacement after having lost weight.
Finally, the researchers point out, many conditions linked to obesity, such as diabetes, do not necessarily go away when the patient loses weight.
The study authors noted that “few health care treatments are required to be cost saving or even cost-effective to be widely available, so requiring cost savings of bariatric surgery imposes an unfair standard.”
FDA panel rejects new empagliflozin indication for type 1 diabetes
A Food and Drug Administration advisory panel voted 14-2 against recommending approval of a supplemental New Drug Application for empagliflozin (Jardiance) as an adjunct to insulin therapy to improve glycemic control in adults with type 1 diabetes. The drug is already is approved for people with type 2 diabetes.
Members of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee cited persisting concerns about the risk for diabetic ketoacidosis (DKA) seen with the drug, as well as the limited pool of evidence Boehringer Ingelheim presented. Patients with type 1 diabetes are at increased risk for DKA.
The agency said it typically gets two major studies to support applications for drug approvals, but the application reviewed Nov. 13 rested largely on a single phase 3 trial, in which 241 people with type 1 diabetes took a low dose (2.5 mg) of empagliflozin for about 6 months. Panelists repeatedly objected to the paucity of data they had to consider this expanded approval.
“We owe it to patients with type 1 diabetes to do this right,” said Brendan M. Everett, MD, MPH, of Harvard Medical School, Boston, who served as a panelist. “It’s out of respect for them that I voted no.”
Boehringer Ingelheim, and members of the public argued that people with type 1 diabetes want access to new medicines such as empagliflozin that already are available for people with type 2 diabetes. The agency approved the drug in 2014 at doses of 10 mg and 25 mg as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. In 2016, it approved a new indication for empagliflozin for reducing risk of cardiovascular death in people with type 2 disease.
The panelists said they were sympathetic to that point of view, but stressed the need for further larger studies of empagliflozin in people with type 1 diabetes.
“I do think this is promising,” said panelist Michael Blaha, MD, MPH, of Johns Hopkins University, Baltimore. “But I’m not sure we are here to evaluate promising things. We’re here to evaluate proven things.”
The FDA advisers also said there was a need for greater clarity about reporting of adverse events in testing of this drug. The FDA reviewers reported disagreements with Boehringer about some cases classified as “unlikely ketoacidosis but ketosis” in the study.
“Some events adjudicated as ‘unlikely ketoacidosis but ketosis’ were clinically significant serious events requiring hospitalizations and prompt intervention, such as discontinuation of study drug,” wrote Mahtab Niyyati, MD, an FDA clinical reviewer, in her slides for the meeting.
The FDA is not obliged to accept the suggestions of its advisory panels, but it often does.
The agency did not ask the panel to weigh in directly on whether to approve the drug. Instead, the question put to the panel for a vote was whether the available data suggest that the benefits outweigh the risks of empagliflozin and support approval of a 2.5-mg dose as an adjunct to insulin for people with type 1 diabetes.
Empagliflozin is part of the sodium-glucose cotransporter 2 (SGLT2) inhibitor class of medicines, already known to have a risk for DKA, the agency noted in its briefing document for the meeting. DKA occurs as a result of insulin deficiency and subsequent ketogenesis.
There are no SGLT2 inhibitors approved for type 1 diabetes, the staff said in the review. The agency has rebuffed recent bids by makers of other SGLT2 drugs for people with type 1 diabetes. In July, AstraZeneca said the FDA had not approved its application for use of dapagliflozin (Farxiga) as an adjunct treatment to insulin to improve glycemic control in adult patients with type1 diabetes, when insulin alone does not provide adequate glycemic control. AstraZeneca said it was working with the agency on issues raised in the response letter it received.
In March, the FDA blocked a bid by Sanofi for approval of its investigational SGLT1/2 inhibitor, sotagliflozin (Zynquista) for use in people with type 1 diabetes. A panel had voted 8-8 in January on a question about the additional approval for this drug.
At the Nov. 13 meeting, panel members offered comments about the potential design of a new test for empagliflozin in type 1 diabetes, including a suggestion for a 2-year trial.
Anna McCollister-Slipp, the consumer representative on the FDA panel, cast one of the two votes in favor of use of the drug for people with type 1 diabetes. She said the agency needed to press for more research in this field but also argued that patients can manage the risks of treatments they find valuable. She cited, as an example, how she has stuck with an insulin pump to manage her own type 1 diabetes, despite having setbacks with the device that sent her to the emergency department.
The other vote in support of the empagliflozin application came from panelist Kashif M. Munir, MD, medical director of the University of Maryland Center for Diabetes and Endrocrinology, Baltimore. In explaining his vote, Dr. Munir noted that he and other physicians already are prescribing medications such as empagliflozin for people with type 1 diabetes, even though it is an off-label use. Boehringer’s strategy of using a lower dose of the drug for this group of patients would mean a reduction in effectiveness but also would lower the risk for side effects.
“Some of us do use existing medications” and have patients take partial doses, Dr. Munir said, adding that the current off-label use of the drug persuaded him to vote in favor of expanded approval, despite his concerns about the data.
Empagliflozin given at 2.5 mg resulted in a statistically significant difference of 0.26% in change in hemoglobin HbA1c at week 26, compared with placebo, said Roberto Crackel, PhD, an FDA mathematical statistician, during the presentation. There was a numerically small benefit in body weight and systolic blood pressure, but no benefit in reducing hypoglycemic events, he said.
In concluding, the agency’s presentation, Dr. Niyyati presented a slide depicting potential risk and benefit for empagliflozin with 6 months and then 6.5 years of follow-up. It showed that in terms of the benefit of HbA1c control, there is a potential but undemonstrated reduction in the risk of microvascular complications at 6 months and an estimated 2.8% reduction in microvascular complications after 6.5 years.
In terms of risk of DKA, there are limited data with unstable estimates, ranging to perhaps as many as 468 additional patients-with-events per 10,000 patients at the 6-month point. By the 6.5-year mark, treating 10,000 patients could result in 1,494 additional events.
In a statement issued after the panel’s vote, Boehringer and its partner on empagliflozin, Eli Lilly, stressed the benefit seen with the drug, a statistically significant reduction in HbA1c (0.28%), compared with insulin given with a matched placebo in adults with type 1 diabetes. Secondary endpoints of the trial demonstrated reductions in weight (1.8 kg) and systolic blood pressure (2.1 mm Hg), compared with insulin plus placebo, the companies said.
“We continue to believe the totality of data from the EASE [Empagliflozin as Adjunctive to Insulin Therapy] program indicates a favorable benefit-risk profile for empagliflozin 2.5 mg in adults with type 1 diabetes and look forward to continuing to work with the FDA in this review process,” said Mohamed Eid, MD, MPH, vice president, clinical development & medical affairs, cardiometabolism & respiratory medicine, Boehringer Ingelheim, in a statement.
Speaking as a member of the public, Kelly L. Close, founder of the diaTribe Foundation, urged the FDA to “think creatively” about approval of the drug.” Many people already are using empagliflozin off label, she said. An FDA approval would help physicians and their patients manage the risks of this medicine. Without such help, patients may be needlessly exposed to harm, she argued.
“That’s what happens with popular unregulated drugs that payers cover, and we know that many, many payers are covering this drug for people with type 1,” she said.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen’s health research group, made the opposing argument during the public session. The trial, in which 241 took the low dose of the drug and 241 on placebo, was “underpowered” in Dr. Wolfe’s view. He also stressed the issue that the FDA had raised about adjudication of the cases of side effects.
An FDA approval of the 2.5-mg dose would send “a dangerous false green signal to those doctors who are already prescribing off-label” drugs in SGLT2 inhibitor class, he said.
That would foster a misleading perception “that we have found the sweet spot” balancing safety and risk, he added. “I can’t see how the FDA or the advisory committee would suggest approval” of empagliflozin for type 1 diabetes.”
A Food and Drug Administration advisory panel voted 14-2 against recommending approval of a supplemental New Drug Application for empagliflozin (Jardiance) as an adjunct to insulin therapy to improve glycemic control in adults with type 1 diabetes. The drug is already is approved for people with type 2 diabetes.
Members of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee cited persisting concerns about the risk for diabetic ketoacidosis (DKA) seen with the drug, as well as the limited pool of evidence Boehringer Ingelheim presented. Patients with type 1 diabetes are at increased risk for DKA.
The agency said it typically gets two major studies to support applications for drug approvals, but the application reviewed Nov. 13 rested largely on a single phase 3 trial, in which 241 people with type 1 diabetes took a low dose (2.5 mg) of empagliflozin for about 6 months. Panelists repeatedly objected to the paucity of data they had to consider this expanded approval.
“We owe it to patients with type 1 diabetes to do this right,” said Brendan M. Everett, MD, MPH, of Harvard Medical School, Boston, who served as a panelist. “It’s out of respect for them that I voted no.”
Boehringer Ingelheim, and members of the public argued that people with type 1 diabetes want access to new medicines such as empagliflozin that already are available for people with type 2 diabetes. The agency approved the drug in 2014 at doses of 10 mg and 25 mg as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. In 2016, it approved a new indication for empagliflozin for reducing risk of cardiovascular death in people with type 2 disease.
The panelists said they were sympathetic to that point of view, but stressed the need for further larger studies of empagliflozin in people with type 1 diabetes.
“I do think this is promising,” said panelist Michael Blaha, MD, MPH, of Johns Hopkins University, Baltimore. “But I’m not sure we are here to evaluate promising things. We’re here to evaluate proven things.”
The FDA advisers also said there was a need for greater clarity about reporting of adverse events in testing of this drug. The FDA reviewers reported disagreements with Boehringer about some cases classified as “unlikely ketoacidosis but ketosis” in the study.
“Some events adjudicated as ‘unlikely ketoacidosis but ketosis’ were clinically significant serious events requiring hospitalizations and prompt intervention, such as discontinuation of study drug,” wrote Mahtab Niyyati, MD, an FDA clinical reviewer, in her slides for the meeting.
The FDA is not obliged to accept the suggestions of its advisory panels, but it often does.
The agency did not ask the panel to weigh in directly on whether to approve the drug. Instead, the question put to the panel for a vote was whether the available data suggest that the benefits outweigh the risks of empagliflozin and support approval of a 2.5-mg dose as an adjunct to insulin for people with type 1 diabetes.
Empagliflozin is part of the sodium-glucose cotransporter 2 (SGLT2) inhibitor class of medicines, already known to have a risk for DKA, the agency noted in its briefing document for the meeting. DKA occurs as a result of insulin deficiency and subsequent ketogenesis.
There are no SGLT2 inhibitors approved for type 1 diabetes, the staff said in the review. The agency has rebuffed recent bids by makers of other SGLT2 drugs for people with type 1 diabetes. In July, AstraZeneca said the FDA had not approved its application for use of dapagliflozin (Farxiga) as an adjunct treatment to insulin to improve glycemic control in adult patients with type1 diabetes, when insulin alone does not provide adequate glycemic control. AstraZeneca said it was working with the agency on issues raised in the response letter it received.
In March, the FDA blocked a bid by Sanofi for approval of its investigational SGLT1/2 inhibitor, sotagliflozin (Zynquista) for use in people with type 1 diabetes. A panel had voted 8-8 in January on a question about the additional approval for this drug.
At the Nov. 13 meeting, panel members offered comments about the potential design of a new test for empagliflozin in type 1 diabetes, including a suggestion for a 2-year trial.
Anna McCollister-Slipp, the consumer representative on the FDA panel, cast one of the two votes in favor of use of the drug for people with type 1 diabetes. She said the agency needed to press for more research in this field but also argued that patients can manage the risks of treatments they find valuable. She cited, as an example, how she has stuck with an insulin pump to manage her own type 1 diabetes, despite having setbacks with the device that sent her to the emergency department.
The other vote in support of the empagliflozin application came from panelist Kashif M. Munir, MD, medical director of the University of Maryland Center for Diabetes and Endrocrinology, Baltimore. In explaining his vote, Dr. Munir noted that he and other physicians already are prescribing medications such as empagliflozin for people with type 1 diabetes, even though it is an off-label use. Boehringer’s strategy of using a lower dose of the drug for this group of patients would mean a reduction in effectiveness but also would lower the risk for side effects.
“Some of us do use existing medications” and have patients take partial doses, Dr. Munir said, adding that the current off-label use of the drug persuaded him to vote in favor of expanded approval, despite his concerns about the data.
Empagliflozin given at 2.5 mg resulted in a statistically significant difference of 0.26% in change in hemoglobin HbA1c at week 26, compared with placebo, said Roberto Crackel, PhD, an FDA mathematical statistician, during the presentation. There was a numerically small benefit in body weight and systolic blood pressure, but no benefit in reducing hypoglycemic events, he said.
In concluding, the agency’s presentation, Dr. Niyyati presented a slide depicting potential risk and benefit for empagliflozin with 6 months and then 6.5 years of follow-up. It showed that in terms of the benefit of HbA1c control, there is a potential but undemonstrated reduction in the risk of microvascular complications at 6 months and an estimated 2.8% reduction in microvascular complications after 6.5 years.
In terms of risk of DKA, there are limited data with unstable estimates, ranging to perhaps as many as 468 additional patients-with-events per 10,000 patients at the 6-month point. By the 6.5-year mark, treating 10,000 patients could result in 1,494 additional events.
In a statement issued after the panel’s vote, Boehringer and its partner on empagliflozin, Eli Lilly, stressed the benefit seen with the drug, a statistically significant reduction in HbA1c (0.28%), compared with insulin given with a matched placebo in adults with type 1 diabetes. Secondary endpoints of the trial demonstrated reductions in weight (1.8 kg) and systolic blood pressure (2.1 mm Hg), compared with insulin plus placebo, the companies said.
“We continue to believe the totality of data from the EASE [Empagliflozin as Adjunctive to Insulin Therapy] program indicates a favorable benefit-risk profile for empagliflozin 2.5 mg in adults with type 1 diabetes and look forward to continuing to work with the FDA in this review process,” said Mohamed Eid, MD, MPH, vice president, clinical development & medical affairs, cardiometabolism & respiratory medicine, Boehringer Ingelheim, in a statement.
Speaking as a member of the public, Kelly L. Close, founder of the diaTribe Foundation, urged the FDA to “think creatively” about approval of the drug.” Many people already are using empagliflozin off label, she said. An FDA approval would help physicians and their patients manage the risks of this medicine. Without such help, patients may be needlessly exposed to harm, she argued.
“That’s what happens with popular unregulated drugs that payers cover, and we know that many, many payers are covering this drug for people with type 1,” she said.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen’s health research group, made the opposing argument during the public session. The trial, in which 241 took the low dose of the drug and 241 on placebo, was “underpowered” in Dr. Wolfe’s view. He also stressed the issue that the FDA had raised about adjudication of the cases of side effects.
An FDA approval of the 2.5-mg dose would send “a dangerous false green signal to those doctors who are already prescribing off-label” drugs in SGLT2 inhibitor class, he said.
That would foster a misleading perception “that we have found the sweet spot” balancing safety and risk, he added. “I can’t see how the FDA or the advisory committee would suggest approval” of empagliflozin for type 1 diabetes.”
A Food and Drug Administration advisory panel voted 14-2 against recommending approval of a supplemental New Drug Application for empagliflozin (Jardiance) as an adjunct to insulin therapy to improve glycemic control in adults with type 1 diabetes. The drug is already is approved for people with type 2 diabetes.
Members of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee cited persisting concerns about the risk for diabetic ketoacidosis (DKA) seen with the drug, as well as the limited pool of evidence Boehringer Ingelheim presented. Patients with type 1 diabetes are at increased risk for DKA.
The agency said it typically gets two major studies to support applications for drug approvals, but the application reviewed Nov. 13 rested largely on a single phase 3 trial, in which 241 people with type 1 diabetes took a low dose (2.5 mg) of empagliflozin for about 6 months. Panelists repeatedly objected to the paucity of data they had to consider this expanded approval.
“We owe it to patients with type 1 diabetes to do this right,” said Brendan M. Everett, MD, MPH, of Harvard Medical School, Boston, who served as a panelist. “It’s out of respect for them that I voted no.”
Boehringer Ingelheim, and members of the public argued that people with type 1 diabetes want access to new medicines such as empagliflozin that already are available for people with type 2 diabetes. The agency approved the drug in 2014 at doses of 10 mg and 25 mg as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. In 2016, it approved a new indication for empagliflozin for reducing risk of cardiovascular death in people with type 2 disease.
The panelists said they were sympathetic to that point of view, but stressed the need for further larger studies of empagliflozin in people with type 1 diabetes.
“I do think this is promising,” said panelist Michael Blaha, MD, MPH, of Johns Hopkins University, Baltimore. “But I’m not sure we are here to evaluate promising things. We’re here to evaluate proven things.”
The FDA advisers also said there was a need for greater clarity about reporting of adverse events in testing of this drug. The FDA reviewers reported disagreements with Boehringer about some cases classified as “unlikely ketoacidosis but ketosis” in the study.
“Some events adjudicated as ‘unlikely ketoacidosis but ketosis’ were clinically significant serious events requiring hospitalizations and prompt intervention, such as discontinuation of study drug,” wrote Mahtab Niyyati, MD, an FDA clinical reviewer, in her slides for the meeting.
The FDA is not obliged to accept the suggestions of its advisory panels, but it often does.
The agency did not ask the panel to weigh in directly on whether to approve the drug. Instead, the question put to the panel for a vote was whether the available data suggest that the benefits outweigh the risks of empagliflozin and support approval of a 2.5-mg dose as an adjunct to insulin for people with type 1 diabetes.
Empagliflozin is part of the sodium-glucose cotransporter 2 (SGLT2) inhibitor class of medicines, already known to have a risk for DKA, the agency noted in its briefing document for the meeting. DKA occurs as a result of insulin deficiency and subsequent ketogenesis.
There are no SGLT2 inhibitors approved for type 1 diabetes, the staff said in the review. The agency has rebuffed recent bids by makers of other SGLT2 drugs for people with type 1 diabetes. In July, AstraZeneca said the FDA had not approved its application for use of dapagliflozin (Farxiga) as an adjunct treatment to insulin to improve glycemic control in adult patients with type1 diabetes, when insulin alone does not provide adequate glycemic control. AstraZeneca said it was working with the agency on issues raised in the response letter it received.
In March, the FDA blocked a bid by Sanofi for approval of its investigational SGLT1/2 inhibitor, sotagliflozin (Zynquista) for use in people with type 1 diabetes. A panel had voted 8-8 in January on a question about the additional approval for this drug.
At the Nov. 13 meeting, panel members offered comments about the potential design of a new test for empagliflozin in type 1 diabetes, including a suggestion for a 2-year trial.
Anna McCollister-Slipp, the consumer representative on the FDA panel, cast one of the two votes in favor of use of the drug for people with type 1 diabetes. She said the agency needed to press for more research in this field but also argued that patients can manage the risks of treatments they find valuable. She cited, as an example, how she has stuck with an insulin pump to manage her own type 1 diabetes, despite having setbacks with the device that sent her to the emergency department.
The other vote in support of the empagliflozin application came from panelist Kashif M. Munir, MD, medical director of the University of Maryland Center for Diabetes and Endrocrinology, Baltimore. In explaining his vote, Dr. Munir noted that he and other physicians already are prescribing medications such as empagliflozin for people with type 1 diabetes, even though it is an off-label use. Boehringer’s strategy of using a lower dose of the drug for this group of patients would mean a reduction in effectiveness but also would lower the risk for side effects.
“Some of us do use existing medications” and have patients take partial doses, Dr. Munir said, adding that the current off-label use of the drug persuaded him to vote in favor of expanded approval, despite his concerns about the data.
Empagliflozin given at 2.5 mg resulted in a statistically significant difference of 0.26% in change in hemoglobin HbA1c at week 26, compared with placebo, said Roberto Crackel, PhD, an FDA mathematical statistician, during the presentation. There was a numerically small benefit in body weight and systolic blood pressure, but no benefit in reducing hypoglycemic events, he said.
In concluding, the agency’s presentation, Dr. Niyyati presented a slide depicting potential risk and benefit for empagliflozin with 6 months and then 6.5 years of follow-up. It showed that in terms of the benefit of HbA1c control, there is a potential but undemonstrated reduction in the risk of microvascular complications at 6 months and an estimated 2.8% reduction in microvascular complications after 6.5 years.
In terms of risk of DKA, there are limited data with unstable estimates, ranging to perhaps as many as 468 additional patients-with-events per 10,000 patients at the 6-month point. By the 6.5-year mark, treating 10,000 patients could result in 1,494 additional events.
In a statement issued after the panel’s vote, Boehringer and its partner on empagliflozin, Eli Lilly, stressed the benefit seen with the drug, a statistically significant reduction in HbA1c (0.28%), compared with insulin given with a matched placebo in adults with type 1 diabetes. Secondary endpoints of the trial demonstrated reductions in weight (1.8 kg) and systolic blood pressure (2.1 mm Hg), compared with insulin plus placebo, the companies said.
“We continue to believe the totality of data from the EASE [Empagliflozin as Adjunctive to Insulin Therapy] program indicates a favorable benefit-risk profile for empagliflozin 2.5 mg in adults with type 1 diabetes and look forward to continuing to work with the FDA in this review process,” said Mohamed Eid, MD, MPH, vice president, clinical development & medical affairs, cardiometabolism & respiratory medicine, Boehringer Ingelheim, in a statement.
Speaking as a member of the public, Kelly L. Close, founder of the diaTribe Foundation, urged the FDA to “think creatively” about approval of the drug.” Many people already are using empagliflozin off label, she said. An FDA approval would help physicians and their patients manage the risks of this medicine. Without such help, patients may be needlessly exposed to harm, she argued.
“That’s what happens with popular unregulated drugs that payers cover, and we know that many, many payers are covering this drug for people with type 1,” she said.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen’s health research group, made the opposing argument during the public session. The trial, in which 241 took the low dose of the drug and 241 on placebo, was “underpowered” in Dr. Wolfe’s view. He also stressed the issue that the FDA had raised about adjudication of the cases of side effects.
An FDA approval of the 2.5-mg dose would send “a dangerous false green signal to those doctors who are already prescribing off-label” drugs in SGLT2 inhibitor class, he said.
That would foster a misleading perception “that we have found the sweet spot” balancing safety and risk, he added. “I can’t see how the FDA or the advisory committee would suggest approval” of empagliflozin for type 1 diabetes.”
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
Hypoglycemia Safety Initiative: Working With PACT Clinical Pharmacy Specialists to Individualize HbA1c Goals (FULL)
Clinical pharmacy specialist interventions after patient consultation resulted in statistically significant increases in HbA1c levels in patients at risk for hypoglycemia who relaxed their therapy.
Intensive glycemic lowering for the treatment for type 2 diabetes mellitus (T2DM) has been shown to decrease microvascular and macrovascular outcomes in the UK Prospective Diabetes Study (UKPDS) without any risk of increased harm.1,2 Over the past decade, evidence has shown that the outcomes and risk do not hold true in an older population with additional comorbidities and longer duration of DM. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) trials showed no decreased incidence of macrovascular or microvascular complications of DM with intensive glucose lowering but an additional risk of hypoglycemia and even death.2-4
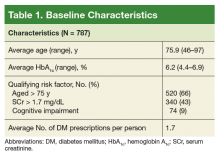
Patient-specific risk factors, such as age, impaired renal function, and cognitive impairment, have been shown to lead to an increased risk of hypoglycemia independent of hemoglobin A1c (HbA1c). Dementia and cognitive impairment are associated with a 2.42 and 1.72 times greater risk of hypoglycemia, respectively, compared with a patient without dementia or cognitive impairment.5 A post-hoc analysis of the ACCORD trial that analyzed the risk of hypoglycemia in subgroup populations showed an increased risk of hypoglycemia in those with a serum creatinine (SCr) level > 1.3 mg/dL (hazard ratio, 1.66, P < .01) and increasing age. Risk of hypoglycemia was highest in those aged ≥ 75 years but increased by 3% for every subsequent year (P < .01).6 These risk factors should be addressed and considered in individual patients with DM to safely guide therapy.
The evidence from these landmark trials has led to increased HbA1c goals for specific patient populations in the most recent 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of Type 2 Diabetes Mellitus in Primary Care.7 The majority of patients with T2DM now qualify for HBA1c goals > 7.0%. According to the 2017 VA/DoD CPG, younger patients with the absence of a major comorbidity and life expectancy of > 10 to 15 years with mild or absent microvascular complications is the only group of patients who should be treated to an A1c goal of 6.0 to 7.0%.7 The use of shared decision making and patient education to set glycemic goals based on “patient capabilities, needs, goals, prior treatment experience, and preferences” also should be used to increase patient education and satisfaction.7
In December 2014, the VA introduced the Hypoglycemia Safety Initiative (HSI). The goal of the HSI is to “enable veterans living with diabetes to work more closely with their VA clinicians to personalize health care goals and improve self-management of the disease.”8 This goal also aligns with the US Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. One of 3 initial targets of this plan includes DM agents and the prevention of hypoglycemia.9
To combat the risk of hypoglycemia and potentially negative outcomes, as part of the HSI, the VA is implementing a clinical reminder within the Computerized Patient Record System (CPRS) that will prompt the primary care team to screen select patients at risk for hypoglycemia. The purpose of this project was to identify patients at high risk of hypoglycemia, individualize HbA1c goals, and consider de-escalation in therapy, using shared decision making.
Methods
This quality improvement project, conducted at the Fayetteville VA Medical Center (FVAMC), consisted of outpatient services provided at 2 health care centers and 6 community-based outpatient clinics. The project was exempt from institutional review board approval as the protocol met national VA criteria as a quality assurance project.
Patients were identified using the HSI Corporate Data Warehouse (CDR) reports. Once patients were identified, a list was distributed to the appropriate clinical pharmacy specialist (CPS), according to patient aligned care teams (PACTs). The CPS contacted the patient via telephone or in person to conduct hypoglycemia screening. Patients on a sulfonylurea or insulin and an HbA1c < 7% plus 1 risk factor for hypoglycemia (aged ≥ 75 years, serum creatinine[SCr] ≥1.7 mg/dL, diagnosis of cognitive impairment, or prescribed a cholinesterase inhibitor) were included. These risk factors were chosen to align with the future clinical reminder, which is based on an increased risk of hypoglycemia seen in these patient populations.
Patients were included if they were receiving antidiabetic medications through the FVAMC or outside of the VA and/or prescribed by a non-VA provider. Medications and doses prescribed by a non-VA provider were verified with the patient verbally during the initial interview. Once contacted by the CPS, any patients who no longer met inclusion criteria were excluded.
The CPS used a national VA hypoglycemia screening note template to ask the patient about frequency and severity of hypoglycemia. Hypoglycemia was defined as a self-monitored blood glucose < 70 mg/dL with or without symptoms. An additional definition consisted of typical hypoglycemia symptoms as reported by the patient even if self-monitored blood glucose was not obtained while exhibiting symptoms. Using shared decision making between the CPS and veteran, antidiabetic therapy was either relaxed or continued. Relaxing therapy was defined as decreasing doses or discontinuation of antidiabetic medications that are known for potentiating hypoglycemia (ie, sulfonylurea and insulin).
The CPS had autonomy in deciding how much to lower dose(s) or when to discontinue medication(s). Additional counseling in proper medication administration, including appropriate timing of medication administration, also could have been the sole intervention needed for a given patient who experienced hypoglycemia. Counseling would have been considered continuation of therapy. For example, if a patient was experiencing hypoglycemia while taking a sulfonylurea twice daily, the CPS would provide counseling on proper timing of medication administration 20 to 30 minutes before morning and evening meals rather than the patient’s perceived administration of twice daily without regard to meals. Even in patients who met inclusion criteria but who did not experience any hypoglycemia symptoms, the CPS and patient could use shared decision making with emphasis on appropriate HbA1c goals to determine whether relaxation in therapy was appropriate.
Data Collection
Baseline demographics, including prespecified risk factors for hypoglycemia, were collected. Data were imported into the HSI CDW from the national VA hypoglycemia screening note template completed by the CPS. From the data CDW, frequency and severity of hypoglycemia were recorded. The CPS interventions were also quantified; HbA1c data were obtained in patients in whom therapy was relaxed 3- to 6-months postintervention.
Statistical Analysis
Descriptive statistics (mean, range) were used for analyzing results. A t test with a 1-tailed distribution was used to analyze the change in HbA1c after CPS intervention (α = .05).
Results
On August 17, 2017, 839 patients were identified across all FVAMC facilities from the HSI data CDW. Patients were contacted through February 16, 2018. A total of 52 patients were excluded as they no longer met inclusion criteria or were deceased at time of review.
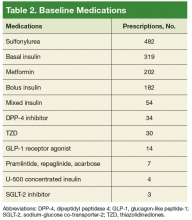
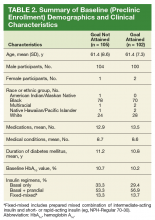
The most commonly prescribed antidiabetic prescription was a sulfonylurea (482 prescriptions) followed by basal insulin (319 prescriptions; Table 2).
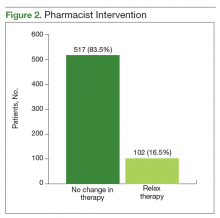
The CPS used shared decision making to relax antidiabetic therapy in 102 (16.5%) of the total number of patients contacted (Figure 2). Lab orders were entered for the patient to obtain an HbA1c in 3 to 6 months in those in whom therapy was relaxed.
Discussion
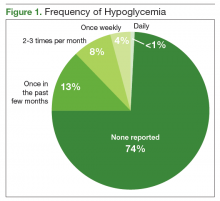
The primary objective of this project was to identify patients at risk for hypoglycemia. Approximately 1 in 4 patients reported any incidence of hypoglycemia, which shows that the prespecified inclusion criteria was an appropriate guide for hypoglycemia screening. The episodes of hypoglycemia were typically infrequent, occurring only once every few months. This could have contributed to a lower rate of therapy changes compared with the rate of hypoglycemia. Overall, hypoglycemia was not severe; 83% of patients did not report any symptoms of faintness. Pharmacists were able to intervene and relax therapy in 102 patients to try to prevent episodes of hypoglycemia and negative outcomes. These interventions led to a statistically significant increase in average HbA1c in these patients. Throughout these encounters with the CPS and patient, there were also innumerable outcomes secondary to the use of shared decision making. Regardless of medication changes, there was increased patient education concerning hypoglycemia treatment, medication administration times, and HbA1c goals.
This project’s strengths included the large sample size, appropriate inclusion criteria that identified patients at risk for hypoglycemia, and the use of shared decision making. It was also beneficial to obtain HbA1c levels after a relaxation in therapy for objective outcomes. The increase in HbA1c levels showed a statistically significant gain, which led to more patients having an HbA1c closer to a CPG-recommended goal range, given their risk factors for hypoglycemia. This pharmacy initiative fostered increased communication between providers and CPS within the PACT team. The pharmacist was not consulted by the provider for management of these patients with DM, so therapy relaxation was documented in CPRS and was addressed at the patient’s next primary care appointment. Some changes also required discussion with the primary care provider prior to relaxation in therapy. By initiating these discussions with providers, opportunities arose for additional education on appropriate HbA1c goals and why therapy should be relaxed in select patient populations.
Limitations
Some limitations to this project were the use of telephone encounters and interpharmacist variability. Patients who were contacted via telephone by a pharmacist who was unknown to them were more hesitant to make changes. Patients managed for DM by non-VA providers or patients receiving medications at a non-VA pharmacy were also reluctant to implement changes. Education was the major intervention for these patients. Pharmacists were instructed to use their clinical judgment in addition to shared decision making with the patient when relaxing therapy. There was no protocol for medication changes. Although interpharmacist variability is identified as a weakness, it could be considered more representative of daily practice.
Additionally, despite a statistically significant increase in HbA1c, which would presumably lead to fewer episodes of hypoglycemia, patients were not contacted again after the intervention to inquire whether hypoglycemia had decreased. Studies targeted at the impact of less frequent hypoglycemia events, including fewer emergency department visits, hospital admissions, or primary care walk-in appointments, would improve the clinical significance of these data. As the HSI is implemented nationally within the VA, more data will be available to better evaluate the applicability of this clinical reminder. Locally, the criteria for the clinical reminder has proved to capture a significant number of patients experiencing hypoglycemia. Using national data will also help to guide the frequency of screening needed in this population.
Conclusion
The implementation of the HSI led to increased provider and patient awareness of hypoglycemia. The CPS interventions have resulted in statistically significant increases in HbA1c levels, which would seemingly decrease the patient’s risk of adverse outcomes as shown in the ACCORD and VADT trials.
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
2. Kirkman MS, Mahmud H, Korytkowski MT. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47(1):81-96.
3. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
4. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
5. Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263-2272.
6. Miller ME, Bonds DE, Gerstein HC, et al; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
7. US Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDD MCPGFinal508.pdf. Published 2017. Accessed September 28, 2018.
8. US Department of Veterans Affairs. VA implements national hypoglycemic safety initiative. https://www.qualityandsafety.va.gov/docs/HSI-Clinician-PressRelease2014.pdf. Published December 10, 2014. Accessed September 28, 2018.
9. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. https://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. Published 2014. Accessed September 28, 2018.
Clinical pharmacy specialist interventions after patient consultation resulted in statistically significant increases in HbA1c levels in patients at risk for hypoglycemia who relaxed their therapy.
Clinical pharmacy specialist interventions after patient consultation resulted in statistically significant increases in HbA1c levels in patients at risk for hypoglycemia who relaxed their therapy.
Intensive glycemic lowering for the treatment for type 2 diabetes mellitus (T2DM) has been shown to decrease microvascular and macrovascular outcomes in the UK Prospective Diabetes Study (UKPDS) without any risk of increased harm.1,2 Over the past decade, evidence has shown that the outcomes and risk do not hold true in an older population with additional comorbidities and longer duration of DM. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) trials showed no decreased incidence of macrovascular or microvascular complications of DM with intensive glucose lowering but an additional risk of hypoglycemia and even death.2-4
Patient-specific risk factors, such as age, impaired renal function, and cognitive impairment, have been shown to lead to an increased risk of hypoglycemia independent of hemoglobin A1c (HbA1c). Dementia and cognitive impairment are associated with a 2.42 and 1.72 times greater risk of hypoglycemia, respectively, compared with a patient without dementia or cognitive impairment.5 A post-hoc analysis of the ACCORD trial that analyzed the risk of hypoglycemia in subgroup populations showed an increased risk of hypoglycemia in those with a serum creatinine (SCr) level > 1.3 mg/dL (hazard ratio, 1.66, P < .01) and increasing age. Risk of hypoglycemia was highest in those aged ≥ 75 years but increased by 3% for every subsequent year (P < .01).6 These risk factors should be addressed and considered in individual patients with DM to safely guide therapy.
The evidence from these landmark trials has led to increased HbA1c goals for specific patient populations in the most recent 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of Type 2 Diabetes Mellitus in Primary Care.7 The majority of patients with T2DM now qualify for HBA1c goals > 7.0%. According to the 2017 VA/DoD CPG, younger patients with the absence of a major comorbidity and life expectancy of > 10 to 15 years with mild or absent microvascular complications is the only group of patients who should be treated to an A1c goal of 6.0 to 7.0%.7 The use of shared decision making and patient education to set glycemic goals based on “patient capabilities, needs, goals, prior treatment experience, and preferences” also should be used to increase patient education and satisfaction.7
In December 2014, the VA introduced the Hypoglycemia Safety Initiative (HSI). The goal of the HSI is to “enable veterans living with diabetes to work more closely with their VA clinicians to personalize health care goals and improve self-management of the disease.”8 This goal also aligns with the US Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. One of 3 initial targets of this plan includes DM agents and the prevention of hypoglycemia.9
To combat the risk of hypoglycemia and potentially negative outcomes, as part of the HSI, the VA is implementing a clinical reminder within the Computerized Patient Record System (CPRS) that will prompt the primary care team to screen select patients at risk for hypoglycemia. The purpose of this project was to identify patients at high risk of hypoglycemia, individualize HbA1c goals, and consider de-escalation in therapy, using shared decision making.
Methods
This quality improvement project, conducted at the Fayetteville VA Medical Center (FVAMC), consisted of outpatient services provided at 2 health care centers and 6 community-based outpatient clinics. The project was exempt from institutional review board approval as the protocol met national VA criteria as a quality assurance project.
Patients were identified using the HSI Corporate Data Warehouse (CDR) reports. Once patients were identified, a list was distributed to the appropriate clinical pharmacy specialist (CPS), according to patient aligned care teams (PACTs). The CPS contacted the patient via telephone or in person to conduct hypoglycemia screening. Patients on a sulfonylurea or insulin and an HbA1c < 7% plus 1 risk factor for hypoglycemia (aged ≥ 75 years, serum creatinine[SCr] ≥1.7 mg/dL, diagnosis of cognitive impairment, or prescribed a cholinesterase inhibitor) were included. These risk factors were chosen to align with the future clinical reminder, which is based on an increased risk of hypoglycemia seen in these patient populations.
Patients were included if they were receiving antidiabetic medications through the FVAMC or outside of the VA and/or prescribed by a non-VA provider. Medications and doses prescribed by a non-VA provider were verified with the patient verbally during the initial interview. Once contacted by the CPS, any patients who no longer met inclusion criteria were excluded.
The CPS used a national VA hypoglycemia screening note template to ask the patient about frequency and severity of hypoglycemia. Hypoglycemia was defined as a self-monitored blood glucose < 70 mg/dL with or without symptoms. An additional definition consisted of typical hypoglycemia symptoms as reported by the patient even if self-monitored blood glucose was not obtained while exhibiting symptoms. Using shared decision making between the CPS and veteran, antidiabetic therapy was either relaxed or continued. Relaxing therapy was defined as decreasing doses or discontinuation of antidiabetic medications that are known for potentiating hypoglycemia (ie, sulfonylurea and insulin).
The CPS had autonomy in deciding how much to lower dose(s) or when to discontinue medication(s). Additional counseling in proper medication administration, including appropriate timing of medication administration, also could have been the sole intervention needed for a given patient who experienced hypoglycemia. Counseling would have been considered continuation of therapy. For example, if a patient was experiencing hypoglycemia while taking a sulfonylurea twice daily, the CPS would provide counseling on proper timing of medication administration 20 to 30 minutes before morning and evening meals rather than the patient’s perceived administration of twice daily without regard to meals. Even in patients who met inclusion criteria but who did not experience any hypoglycemia symptoms, the CPS and patient could use shared decision making with emphasis on appropriate HbA1c goals to determine whether relaxation in therapy was appropriate.
Data Collection
Baseline demographics, including prespecified risk factors for hypoglycemia, were collected. Data were imported into the HSI CDW from the national VA hypoglycemia screening note template completed by the CPS. From the data CDW, frequency and severity of hypoglycemia were recorded. The CPS interventions were also quantified; HbA1c data were obtained in patients in whom therapy was relaxed 3- to 6-months postintervention.
Statistical Analysis
Descriptive statistics (mean, range) were used for analyzing results. A t test with a 1-tailed distribution was used to analyze the change in HbA1c after CPS intervention (α = .05).
Results
On August 17, 2017, 839 patients were identified across all FVAMC facilities from the HSI data CDW. Patients were contacted through February 16, 2018. A total of 52 patients were excluded as they no longer met inclusion criteria or were deceased at time of review.
The most commonly prescribed antidiabetic prescription was a sulfonylurea (482 prescriptions) followed by basal insulin (319 prescriptions; Table 2).
The CPS used shared decision making to relax antidiabetic therapy in 102 (16.5%) of the total number of patients contacted (Figure 2). Lab orders were entered for the patient to obtain an HbA1c in 3 to 6 months in those in whom therapy was relaxed.
Discussion
The primary objective of this project was to identify patients at risk for hypoglycemia. Approximately 1 in 4 patients reported any incidence of hypoglycemia, which shows that the prespecified inclusion criteria was an appropriate guide for hypoglycemia screening. The episodes of hypoglycemia were typically infrequent, occurring only once every few months. This could have contributed to a lower rate of therapy changes compared with the rate of hypoglycemia. Overall, hypoglycemia was not severe; 83% of patients did not report any symptoms of faintness. Pharmacists were able to intervene and relax therapy in 102 patients to try to prevent episodes of hypoglycemia and negative outcomes. These interventions led to a statistically significant increase in average HbA1c in these patients. Throughout these encounters with the CPS and patient, there were also innumerable outcomes secondary to the use of shared decision making. Regardless of medication changes, there was increased patient education concerning hypoglycemia treatment, medication administration times, and HbA1c goals.
This project’s strengths included the large sample size, appropriate inclusion criteria that identified patients at risk for hypoglycemia, and the use of shared decision making. It was also beneficial to obtain HbA1c levels after a relaxation in therapy for objective outcomes. The increase in HbA1c levels showed a statistically significant gain, which led to more patients having an HbA1c closer to a CPG-recommended goal range, given their risk factors for hypoglycemia. This pharmacy initiative fostered increased communication between providers and CPS within the PACT team. The pharmacist was not consulted by the provider for management of these patients with DM, so therapy relaxation was documented in CPRS and was addressed at the patient’s next primary care appointment. Some changes also required discussion with the primary care provider prior to relaxation in therapy. By initiating these discussions with providers, opportunities arose for additional education on appropriate HbA1c goals and why therapy should be relaxed in select patient populations.
Limitations
Some limitations to this project were the use of telephone encounters and interpharmacist variability. Patients who were contacted via telephone by a pharmacist who was unknown to them were more hesitant to make changes. Patients managed for DM by non-VA providers or patients receiving medications at a non-VA pharmacy were also reluctant to implement changes. Education was the major intervention for these patients. Pharmacists were instructed to use their clinical judgment in addition to shared decision making with the patient when relaxing therapy. There was no protocol for medication changes. Although interpharmacist variability is identified as a weakness, it could be considered more representative of daily practice.
Additionally, despite a statistically significant increase in HbA1c, which would presumably lead to fewer episodes of hypoglycemia, patients were not contacted again after the intervention to inquire whether hypoglycemia had decreased. Studies targeted at the impact of less frequent hypoglycemia events, including fewer emergency department visits, hospital admissions, or primary care walk-in appointments, would improve the clinical significance of these data. As the HSI is implemented nationally within the VA, more data will be available to better evaluate the applicability of this clinical reminder. Locally, the criteria for the clinical reminder has proved to capture a significant number of patients experiencing hypoglycemia. Using national data will also help to guide the frequency of screening needed in this population.
Conclusion
The implementation of the HSI led to increased provider and patient awareness of hypoglycemia. The CPS interventions have resulted in statistically significant increases in HbA1c levels, which would seemingly decrease the patient’s risk of adverse outcomes as shown in the ACCORD and VADT trials.
Intensive glycemic lowering for the treatment for type 2 diabetes mellitus (T2DM) has been shown to decrease microvascular and macrovascular outcomes in the UK Prospective Diabetes Study (UKPDS) without any risk of increased harm.1,2 Over the past decade, evidence has shown that the outcomes and risk do not hold true in an older population with additional comorbidities and longer duration of DM. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) trials showed no decreased incidence of macrovascular or microvascular complications of DM with intensive glucose lowering but an additional risk of hypoglycemia and even death.2-4
Patient-specific risk factors, such as age, impaired renal function, and cognitive impairment, have been shown to lead to an increased risk of hypoglycemia independent of hemoglobin A1c (HbA1c). Dementia and cognitive impairment are associated with a 2.42 and 1.72 times greater risk of hypoglycemia, respectively, compared with a patient without dementia or cognitive impairment.5 A post-hoc analysis of the ACCORD trial that analyzed the risk of hypoglycemia in subgroup populations showed an increased risk of hypoglycemia in those with a serum creatinine (SCr) level > 1.3 mg/dL (hazard ratio, 1.66, P < .01) and increasing age. Risk of hypoglycemia was highest in those aged ≥ 75 years but increased by 3% for every subsequent year (P < .01).6 These risk factors should be addressed and considered in individual patients with DM to safely guide therapy.
The evidence from these landmark trials has led to increased HbA1c goals for specific patient populations in the most recent 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of Type 2 Diabetes Mellitus in Primary Care.7 The majority of patients with T2DM now qualify for HBA1c goals > 7.0%. According to the 2017 VA/DoD CPG, younger patients with the absence of a major comorbidity and life expectancy of > 10 to 15 years with mild or absent microvascular complications is the only group of patients who should be treated to an A1c goal of 6.0 to 7.0%.7 The use of shared decision making and patient education to set glycemic goals based on “patient capabilities, needs, goals, prior treatment experience, and preferences” also should be used to increase patient education and satisfaction.7
In December 2014, the VA introduced the Hypoglycemia Safety Initiative (HSI). The goal of the HSI is to “enable veterans living with diabetes to work more closely with their VA clinicians to personalize health care goals and improve self-management of the disease.”8 This goal also aligns with the US Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. One of 3 initial targets of this plan includes DM agents and the prevention of hypoglycemia.9
To combat the risk of hypoglycemia and potentially negative outcomes, as part of the HSI, the VA is implementing a clinical reminder within the Computerized Patient Record System (CPRS) that will prompt the primary care team to screen select patients at risk for hypoglycemia. The purpose of this project was to identify patients at high risk of hypoglycemia, individualize HbA1c goals, and consider de-escalation in therapy, using shared decision making.
Methods
This quality improvement project, conducted at the Fayetteville VA Medical Center (FVAMC), consisted of outpatient services provided at 2 health care centers and 6 community-based outpatient clinics. The project was exempt from institutional review board approval as the protocol met national VA criteria as a quality assurance project.
Patients were identified using the HSI Corporate Data Warehouse (CDR) reports. Once patients were identified, a list was distributed to the appropriate clinical pharmacy specialist (CPS), according to patient aligned care teams (PACTs). The CPS contacted the patient via telephone or in person to conduct hypoglycemia screening. Patients on a sulfonylurea or insulin and an HbA1c < 7% plus 1 risk factor for hypoglycemia (aged ≥ 75 years, serum creatinine[SCr] ≥1.7 mg/dL, diagnosis of cognitive impairment, or prescribed a cholinesterase inhibitor) were included. These risk factors were chosen to align with the future clinical reminder, which is based on an increased risk of hypoglycemia seen in these patient populations.
Patients were included if they were receiving antidiabetic medications through the FVAMC or outside of the VA and/or prescribed by a non-VA provider. Medications and doses prescribed by a non-VA provider were verified with the patient verbally during the initial interview. Once contacted by the CPS, any patients who no longer met inclusion criteria were excluded.
The CPS used a national VA hypoglycemia screening note template to ask the patient about frequency and severity of hypoglycemia. Hypoglycemia was defined as a self-monitored blood glucose < 70 mg/dL with or without symptoms. An additional definition consisted of typical hypoglycemia symptoms as reported by the patient even if self-monitored blood glucose was not obtained while exhibiting symptoms. Using shared decision making between the CPS and veteran, antidiabetic therapy was either relaxed or continued. Relaxing therapy was defined as decreasing doses or discontinuation of antidiabetic medications that are known for potentiating hypoglycemia (ie, sulfonylurea and insulin).
The CPS had autonomy in deciding how much to lower dose(s) or when to discontinue medication(s). Additional counseling in proper medication administration, including appropriate timing of medication administration, also could have been the sole intervention needed for a given patient who experienced hypoglycemia. Counseling would have been considered continuation of therapy. For example, if a patient was experiencing hypoglycemia while taking a sulfonylurea twice daily, the CPS would provide counseling on proper timing of medication administration 20 to 30 minutes before morning and evening meals rather than the patient’s perceived administration of twice daily without regard to meals. Even in patients who met inclusion criteria but who did not experience any hypoglycemia symptoms, the CPS and patient could use shared decision making with emphasis on appropriate HbA1c goals to determine whether relaxation in therapy was appropriate.
Data Collection
Baseline demographics, including prespecified risk factors for hypoglycemia, were collected. Data were imported into the HSI CDW from the national VA hypoglycemia screening note template completed by the CPS. From the data CDW, frequency and severity of hypoglycemia were recorded. The CPS interventions were also quantified; HbA1c data were obtained in patients in whom therapy was relaxed 3- to 6-months postintervention.
Statistical Analysis
Descriptive statistics (mean, range) were used for analyzing results. A t test with a 1-tailed distribution was used to analyze the change in HbA1c after CPS intervention (α = .05).
Results
On August 17, 2017, 839 patients were identified across all FVAMC facilities from the HSI data CDW. Patients were contacted through February 16, 2018. A total of 52 patients were excluded as they no longer met inclusion criteria or were deceased at time of review.
The most commonly prescribed antidiabetic prescription was a sulfonylurea (482 prescriptions) followed by basal insulin (319 prescriptions; Table 2).
The CPS used shared decision making to relax antidiabetic therapy in 102 (16.5%) of the total number of patients contacted (Figure 2). Lab orders were entered for the patient to obtain an HbA1c in 3 to 6 months in those in whom therapy was relaxed.
Discussion
The primary objective of this project was to identify patients at risk for hypoglycemia. Approximately 1 in 4 patients reported any incidence of hypoglycemia, which shows that the prespecified inclusion criteria was an appropriate guide for hypoglycemia screening. The episodes of hypoglycemia were typically infrequent, occurring only once every few months. This could have contributed to a lower rate of therapy changes compared with the rate of hypoglycemia. Overall, hypoglycemia was not severe; 83% of patients did not report any symptoms of faintness. Pharmacists were able to intervene and relax therapy in 102 patients to try to prevent episodes of hypoglycemia and negative outcomes. These interventions led to a statistically significant increase in average HbA1c in these patients. Throughout these encounters with the CPS and patient, there were also innumerable outcomes secondary to the use of shared decision making. Regardless of medication changes, there was increased patient education concerning hypoglycemia treatment, medication administration times, and HbA1c goals.
This project’s strengths included the large sample size, appropriate inclusion criteria that identified patients at risk for hypoglycemia, and the use of shared decision making. It was also beneficial to obtain HbA1c levels after a relaxation in therapy for objective outcomes. The increase in HbA1c levels showed a statistically significant gain, which led to more patients having an HbA1c closer to a CPG-recommended goal range, given their risk factors for hypoglycemia. This pharmacy initiative fostered increased communication between providers and CPS within the PACT team. The pharmacist was not consulted by the provider for management of these patients with DM, so therapy relaxation was documented in CPRS and was addressed at the patient’s next primary care appointment. Some changes also required discussion with the primary care provider prior to relaxation in therapy. By initiating these discussions with providers, opportunities arose for additional education on appropriate HbA1c goals and why therapy should be relaxed in select patient populations.
Limitations
Some limitations to this project were the use of telephone encounters and interpharmacist variability. Patients who were contacted via telephone by a pharmacist who was unknown to them were more hesitant to make changes. Patients managed for DM by non-VA providers or patients receiving medications at a non-VA pharmacy were also reluctant to implement changes. Education was the major intervention for these patients. Pharmacists were instructed to use their clinical judgment in addition to shared decision making with the patient when relaxing therapy. There was no protocol for medication changes. Although interpharmacist variability is identified as a weakness, it could be considered more representative of daily practice.
Additionally, despite a statistically significant increase in HbA1c, which would presumably lead to fewer episodes of hypoglycemia, patients were not contacted again after the intervention to inquire whether hypoglycemia had decreased. Studies targeted at the impact of less frequent hypoglycemia events, including fewer emergency department visits, hospital admissions, or primary care walk-in appointments, would improve the clinical significance of these data. As the HSI is implemented nationally within the VA, more data will be available to better evaluate the applicability of this clinical reminder. Locally, the criteria for the clinical reminder has proved to capture a significant number of patients experiencing hypoglycemia. Using national data will also help to guide the frequency of screening needed in this population.
Conclusion
The implementation of the HSI led to increased provider and patient awareness of hypoglycemia. The CPS interventions have resulted in statistically significant increases in HbA1c levels, which would seemingly decrease the patient’s risk of adverse outcomes as shown in the ACCORD and VADT trials.
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
2. Kirkman MS, Mahmud H, Korytkowski MT. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47(1):81-96.
3. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
4. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
5. Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263-2272.
6. Miller ME, Bonds DE, Gerstein HC, et al; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
7. US Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDD MCPGFinal508.pdf. Published 2017. Accessed September 28, 2018.
8. US Department of Veterans Affairs. VA implements national hypoglycemic safety initiative. https://www.qualityandsafety.va.gov/docs/HSI-Clinician-PressRelease2014.pdf. Published December 10, 2014. Accessed September 28, 2018.
9. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. https://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. Published 2014. Accessed September 28, 2018.
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
2. Kirkman MS, Mahmud H, Korytkowski MT. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47(1):81-96.
3. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
4. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
5. Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263-2272.
6. Miller ME, Bonds DE, Gerstein HC, et al; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
7. US Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDD MCPGFinal508.pdf. Published 2017. Accessed September 28, 2018.
8. US Department of Veterans Affairs. VA implements national hypoglycemic safety initiative. https://www.qualityandsafety.va.gov/docs/HSI-Clinician-PressRelease2014.pdf. Published December 10, 2014. Accessed September 28, 2018.
9. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. https://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. Published 2014. Accessed September 28, 2018.
Glycemic Control eQUIPS yields success at Dignity Health Sequoia Hospital
Glucometrics database aids tracking, trending
In honor of Diabetes Awareness Month, The Hospitalist spoke recently with Stephanie Dizon, PharmD, BCPS, director of pharmacy at Dignity Health Sequoia Hospital in Redwood City, Calif. Dr. Dizon was the project lead for Dignity Health Sequoia’s participation in the Society of Hospital Medicine’s Glycemic Control eQUIPS program. The Northern California hospital was recognized as a top performer in the program.
SHM’s eQUIPS offers a virtual library of resources, including a step-by-step implementation guide, that addresses various issues that range from subcutaneous insulin protocols to care coordination and good hypoglycemia management. In addition, the program offers access to a data center for performance tracking and benchmarking.
Dr. Dizon shared her experience as a participant in the program, and explained its impact on glycemic control at Dignity Health Sequoia Hospital.
Could you tell us about your personal involvement with SHM?
I started as the quality lead for glycemic control for Sequoia Hospital in 2017 while serving in the role as the clinical pharmacy manager. Currently, I am the director of pharmacy.
What inspired your institution to enroll in the GC eQUIPS program? What were the challenges it helped you address?
Sequoia Hospital started in this journey to improve overall glycemic control in a collaborative with eight other Dignity Health hospitals in 2011. At Sequoia Hospital, this effort was led by Karen Harrison, RN, MSN, CCRN. At the time, Dignity Health saw variations in insulin management and adverse events, and it inspired this group to review their practices and try to find a better way to standardize them. The hope was that sharing information and making efforts to standardize practices would lead to better glycemic control.
Enrollment in the GC eQUIPS program helped Sequoia Hospital efficiently analyze data that would otherwise be too large to manage. In addition, by tracking and trending these large data sets, it helped us not only to see where the hospital’s greatest challenges are in glycemic control but also observe what the impact is when making changes. We were part of a nine-site study that proved the effectiveness of GC eQUIPS and highlighted the collective success across the health system.
What did you find most useful in the suite of resources included in eQUIPS?
The benchmarking webinars and informational webinars that have been provided by Greg Maynard, MD, over the years have been especially helpful. They have broadened my understanding of glycemic control. The glucometrics database is especially helpful for tracking and trending – we share these reports on a monthly basis with nursing and provider leadership. In addition, being able to benchmark ourselves with other hospitals pushes us to improve and keep an eye on glycemic control.
Are there any other highlights from your participation– and your institution’s – in the program that you feel would be beneficial to others who may be considering enrollment?
Having access to the tools available in the GC eQUIPS program is very powerful for data analysis and benchmarking. As a result, it allows the people at an institution to focus on the day-to-day tasks, clinical initiatives, and building a culture that can make a program successful instead of focusing on data collection.
For more information on SHM’s Glycemic Control resources or to enroll in eQUIPS, visit hospitalmedicine.org/gc.
Glucometrics database aids tracking, trending
Glucometrics database aids tracking, trending
In honor of Diabetes Awareness Month, The Hospitalist spoke recently with Stephanie Dizon, PharmD, BCPS, director of pharmacy at Dignity Health Sequoia Hospital in Redwood City, Calif. Dr. Dizon was the project lead for Dignity Health Sequoia’s participation in the Society of Hospital Medicine’s Glycemic Control eQUIPS program. The Northern California hospital was recognized as a top performer in the program.
SHM’s eQUIPS offers a virtual library of resources, including a step-by-step implementation guide, that addresses various issues that range from subcutaneous insulin protocols to care coordination and good hypoglycemia management. In addition, the program offers access to a data center for performance tracking and benchmarking.
Dr. Dizon shared her experience as a participant in the program, and explained its impact on glycemic control at Dignity Health Sequoia Hospital.
Could you tell us about your personal involvement with SHM?
I started as the quality lead for glycemic control for Sequoia Hospital in 2017 while serving in the role as the clinical pharmacy manager. Currently, I am the director of pharmacy.
What inspired your institution to enroll in the GC eQUIPS program? What were the challenges it helped you address?
Sequoia Hospital started in this journey to improve overall glycemic control in a collaborative with eight other Dignity Health hospitals in 2011. At Sequoia Hospital, this effort was led by Karen Harrison, RN, MSN, CCRN. At the time, Dignity Health saw variations in insulin management and adverse events, and it inspired this group to review their practices and try to find a better way to standardize them. The hope was that sharing information and making efforts to standardize practices would lead to better glycemic control.
Enrollment in the GC eQUIPS program helped Sequoia Hospital efficiently analyze data that would otherwise be too large to manage. In addition, by tracking and trending these large data sets, it helped us not only to see where the hospital’s greatest challenges are in glycemic control but also observe what the impact is when making changes. We were part of a nine-site study that proved the effectiveness of GC eQUIPS and highlighted the collective success across the health system.
What did you find most useful in the suite of resources included in eQUIPS?
The benchmarking webinars and informational webinars that have been provided by Greg Maynard, MD, over the years have been especially helpful. They have broadened my understanding of glycemic control. The glucometrics database is especially helpful for tracking and trending – we share these reports on a monthly basis with nursing and provider leadership. In addition, being able to benchmark ourselves with other hospitals pushes us to improve and keep an eye on glycemic control.
Are there any other highlights from your participation– and your institution’s – in the program that you feel would be beneficial to others who may be considering enrollment?
Having access to the tools available in the GC eQUIPS program is very powerful for data analysis and benchmarking. As a result, it allows the people at an institution to focus on the day-to-day tasks, clinical initiatives, and building a culture that can make a program successful instead of focusing on data collection.
For more information on SHM’s Glycemic Control resources or to enroll in eQUIPS, visit hospitalmedicine.org/gc.
In honor of Diabetes Awareness Month, The Hospitalist spoke recently with Stephanie Dizon, PharmD, BCPS, director of pharmacy at Dignity Health Sequoia Hospital in Redwood City, Calif. Dr. Dizon was the project lead for Dignity Health Sequoia’s participation in the Society of Hospital Medicine’s Glycemic Control eQUIPS program. The Northern California hospital was recognized as a top performer in the program.
SHM’s eQUIPS offers a virtual library of resources, including a step-by-step implementation guide, that addresses various issues that range from subcutaneous insulin protocols to care coordination and good hypoglycemia management. In addition, the program offers access to a data center for performance tracking and benchmarking.
Dr. Dizon shared her experience as a participant in the program, and explained its impact on glycemic control at Dignity Health Sequoia Hospital.
Could you tell us about your personal involvement with SHM?
I started as the quality lead for glycemic control for Sequoia Hospital in 2017 while serving in the role as the clinical pharmacy manager. Currently, I am the director of pharmacy.
What inspired your institution to enroll in the GC eQUIPS program? What were the challenges it helped you address?
Sequoia Hospital started in this journey to improve overall glycemic control in a collaborative with eight other Dignity Health hospitals in 2011. At Sequoia Hospital, this effort was led by Karen Harrison, RN, MSN, CCRN. At the time, Dignity Health saw variations in insulin management and adverse events, and it inspired this group to review their practices and try to find a better way to standardize them. The hope was that sharing information and making efforts to standardize practices would lead to better glycemic control.
Enrollment in the GC eQUIPS program helped Sequoia Hospital efficiently analyze data that would otherwise be too large to manage. In addition, by tracking and trending these large data sets, it helped us not only to see where the hospital’s greatest challenges are in glycemic control but also observe what the impact is when making changes. We were part of a nine-site study that proved the effectiveness of GC eQUIPS and highlighted the collective success across the health system.
What did you find most useful in the suite of resources included in eQUIPS?
The benchmarking webinars and informational webinars that have been provided by Greg Maynard, MD, over the years have been especially helpful. They have broadened my understanding of glycemic control. The glucometrics database is especially helpful for tracking and trending – we share these reports on a monthly basis with nursing and provider leadership. In addition, being able to benchmark ourselves with other hospitals pushes us to improve and keep an eye on glycemic control.
Are there any other highlights from your participation– and your institution’s – in the program that you feel would be beneficial to others who may be considering enrollment?
Having access to the tools available in the GC eQUIPS program is very powerful for data analysis and benchmarking. As a result, it allows the people at an institution to focus on the day-to-day tasks, clinical initiatives, and building a culture that can make a program successful instead of focusing on data collection.
For more information on SHM’s Glycemic Control resources or to enroll in eQUIPS, visit hospitalmedicine.org/gc.
Predictors of HA1c Goal Attainment in Patients Treated With Insulin at a VA Pharmacist-Managed Insulin Clinic (FULL)
Showing up to appointments and adherence to treatment recommendations correlated with glycemic goal attainment for patients.
About 30.3 million Americans (9.4%) have diabetes mellitus (DM).1 Veterans are disproportionately affected—about 1 in 4 of those who receive US Department of Veterans Affairs (VA) care have DM.2 The consequences of uncontrolled DM include microvascular complications (eg, retinopathy, neuropathy, and nephropathy) and macrovascular complications (eg, cardiovascular disease).
The American Diabetes Association (ADA) recommends achieving a goal hemoglobin A1c (HbA1c) level of < 7% to prevent these complications. However, a goal of < 8% HbA1c may be more appropriate for certain patients when a more strict goal may be impractical or have the potential to cause harm.3 Furthermore, guidelines developed by the VA and the US Department of Defense suggest a target HbA1c range of 7.0% to 8.5% for patients with established microvascular or macrovascular disease, comorbid conditions, or a life expectancy of 5 to 10 years.4
Despite the existence of evidence showing the importance of glycemic control in preventing morbidity and mortality associated with DM, many patients have inadequate glycemic control. Diabetes mellitus is the seventh leading cause of death in the US. Moreover, DM is a known risk factor for heart disease, stroke, and kidney disease, which are the first, fifth, and ninth leading causes of death in the US, respectively.5
Because DM management requires ongoing and comprehensive maintenance and monitoring, the ADA supports a collaborative, multidisciplinary, and patient-centered approach to delivery of care.3 Collaborative teams involving pharmacists have been shown to improve outcomes and cost savings for chronic diseases, including DM.6-12 In 1995, the VA launched a national policy providing clinical pharmacists with prescribing privileges that would aid in the provision of coordinated medication management for patients with chronic illnesses.13 The policy created a framework for collaborative drug therapy management (CDTM) models, which grants pharmacists the ability to perform patient assessments, order laboratory tests, and modify medications within a scope of practice.
Since the initiation of these services, several examples of successful DM management services using clinical pharmacists within the VA exist in the literature.14-16 However, even with intensive chronic disease and drug therapy management, not all patients who enroll in these services successfully reach clinical goals. Although these pharmacist-driven services seem to demonstrate overall benefit and cost savings to veteran patients and the VA system, little published data exist to help determine patient behaviors that are associated with glycemic goal attainment when using these services.
At the Corporal Michael J. Crescenz VA Medical Center in (CMCVAMC) Philadelphia, Pennsylvania, where this study was performed, primary care providers may refer patients with uncontrolled DM to the pharmacist disease state management (DSM) clinic. The clinic is a form of a CDTM and receives numerous referrals per year, with many patients discharged for successfully meeting glycemic targets.
However, a percentage of patients fail to attain glycemic goals despite involvement in this clinic. We observed specific patient behaviors that delayed glycemic goal attainment. This study examined whether these behaviors correlated with prolonged glycemic goal attainment. The purpose of this study was to identify patient behaviors that led to glycemic goal attainment in insulin-treated patients referred to this pharmacist DSM clinic.
Methods
This study was performed as a single-center retrospective chart review. The protocol and data collection documents were approved by the CMCVAMC Institutional Review Board. It included patients referred to a pharmacist-led DSM clinic for insulin titration/optimization from January 1, 2011 through December 31, 2012. Data were collected through June 30, 2013, to allow for 6 months after the last referral date of December 31, 2012.
This study included patients who were on insulin therapy at the time of pharmacy consult, who attended at least 3 consecutive pharmacy DSM clinic visits, and had an HbA1c ≥ 8% at the time of initial clinic consult. Patients who failed to have 3 consecutive pharmacy DSM clinic visits, were insulin-naïve at the time of referral, aged ≥ 90, lacked at least 1 follow-up HbA1c result while enrolled in the clinic, or had HbA1c < 8% were excluded.
Among the patients who met eligibility criteria, charts within the Computerized Patient Record System (CPRS) were reviewed in a chronologic order within the respective study time frame. A convenience sample of 100 patients were enrolled in each treatment arm: the goal-attained arm or the goal-not-attained arm.
The primary study variable was HbA1c goal attainment, which was defined in this investigation as at least 1 HbA1c reading of < 8% while enrolled in the DSM clinic during the review period. Secondary variables included specific patient factors such as optimal frequency of self-monitoring of blood glucose (SMBG) testing, adherence to pharmacist’s instructions for changes to glucose-lowering medications, adherence to bringing glucose meter/glucose log book to clinic appointments, and percentage of visits attended. Definitions for each variable are provided in Table 1.
We hypothesized that patients who were more adherent to treatment plans, regularly attend clinic visits, and appropriately monitor their glucose levels were more likely to meet their glycemic goals.
Statistical Analysis
Univariate descriptive statistics described the individual variables/predictors of HbA1c goal attainment. As the study’s purpose was to identify patient factors and characteristics associated with HbA1c goal attainment, a logistic regression model framework was used for all covariates to evaluate each measured variable’s independent association with HbA1c. The univariate tests were used to compare patient characteristics between the 2 study groups: Pearson chi-square test was used for nominal data, and a paired t test (for normally distributed data) or Wilcoxon rank sum test (for non-normally distributed data) was used for continuous variables. Variables having a P value < .2 underwent a multivariate analysis stepwise logistic regression model to identify patient factors and characteristics associated with HbA1c goal attainment. A Fisher exact test was used to determine gender effect on HbA1c goal attainment, categoric variables were analyzed using Pearson chi-square test, and an unpaired t test was used for continuous data. The backward elimination approach to inclusion of variables in the model was used to build the most parsimonious and best-fitting model, and the Hosmer-Lemeshow goodness-of-fit tests was used to assess model fit. Data analyses were performed using IBM SPSS, version 18.0 (Armonk, NY).
Results
Five hundred eighty-four patient records were reviewed, and 207 patients met inclusion criteria: 102 patient records were reviewed for the goal-attained arm, and 105 patient records for the goal-not-attained arm. Most patients were excluded from the analysis due to not having 3 consecutive visits during the specified period or having an HbA1c of < 8% at the time of referral to the pharmacist DSM clinic.
The patients in this study had type 2 diabetes for about 11 years, were overwhelmingly male (99%), were aged about 61 years, and were taking on average 13 medications at the time of referral to the pharmacist DSM clinic. Mean HbA1c at time of enrollment was slightly higher in the goal-not-attained arm vs goal-attained arm (10.7% vs 10.2%, respectively), but the difference was not statistically significant (P = .066). A little more than half the patients in both study arms were on basal + prandial insulin regimens (Table 2).
Patients who attained their goal HbA1cwere more likely to bring their glucose meter/glucose log book to at least 80% of their appointments (P < .001). Additionally, this same cohort followed insulin dosing instructions at least 80% of the time (P < .001).
Five variables were included in the multivariate analysis because they had a P value ≤ .2 in univariate analyses: (1) patient adherence to instructions (P < .001); (2) duration in clinic (P < .001); (3) patient bringingglucose meter or glucose log to appointments (P < .001); (4) percentage of scheduled appointments patient attended (P = .015); and (5) baseline HbA1c (P = .066).
Discussion
The development and constant modification of clinical practicing guidelines has made DM treatment a focus and priority.3,4 Additionally, the collaborative approach to health care and creation of VA pharmacist-driven services have demonstrated successful patient outcomes.6-16 Despite these efforts, further insight is needed to improve the management of DM. Our study identified specific behavioral factors that correlated to veteran patients to attaining their HbA1c goal of < 8% within a VA pharmacist DSM clinic. Additionally, it highlighted factors that contributed to patients not achieving their glycemic goals.
Our univariate analysis showed behaviors such as showing up for appointments and following directions regimens to correlate with glycemic goal attainment. However, following directions was the only behavioral factor that correlated to glycemic goal attainment in our multivariate analysis. Additionally, our findings indicated that factors for HbA1c goal attainment included patients who brought their glucose meter/glucose log book and attended clinic appointments at least 80% of the time, respectively.
These findings can help further refine the process for identifying patients who are most likely to achieve glycemic goals when referred to pharmacist DSM clinics or to any DM treatment program. Assessment of a patient’s motivation and ability to attend clinic appointments, bring their glucose meter/glucose log book, and to follow instructions provided at these appointments are reasonable screening questions to ask before referring that patient to a diabetes care program or service. Currently, this is not performed during the consult process to the pharmacist DSM clinic at the respective VA.
Additionally, our findings show that patients who met goal did so, on average, within 6 months of referral to the pharmacist DSM clinic. This finding may have occurred because patients who successfully reach HbA1c goal in 2 consecutive checks are discharged from the clinic. Patients who do not meet this goal continue with the clinic, thus increasing their duration of enrollment in this service. This finding could help clinical pharmacists estimate how long patients will be followed by the service, thus allowing for a more accurate estimation of workload and clinic capacity. Additionally, this finding provides insight if the patient should remain in clinic or be transferred to another program. Our findings aligned with previous studies showing the link between patient behaviors and glycemic goal attainment.17-19
Limitations
This study has a few notable limitations. First, it is limited to 1 VA medical center, so our findings may not be extrapolated easily to other institutions of the Veterans Health Administration. Ideally, future studies centered on identifying factors that lead to successful glycemic goal attainment would be helpful from multiple VA institutions. This would encourage more factors to be identified and trends to be strengthened. Ultimately, this would allow for more global changes to the consult process from primary care to pharmacist DSM clinics nationally vs at a local VA institution. Additionally, this study was limited to a specific retrospective time frame, therefore limiting its ability to identify trends. This study also relied on some subjective factors, such as the patient’s self-report of properly following the clinic instructions. Another limitation was that our investigation was not designed to characterize the specific pharmacist’s interventions that improved glycemic control. Future studies would benefit from the inclusion of specific interventions and their effect on glycemic goal attainment.
Conclusion
This retrospective study offers insight to specific patient behavioral factors that correlate with glycemic goal attainment in a VA pharmacist DSM clinic. Behavioral factors linked to HbA1c goal attainment of < 8% included appointment keeping, bringing glucose meter/glucose log book at least 80% of the time to these appointments, and following clinic instructions. This investigation also found that patients who attain glycemic goals generally do so within 6 months of enrollment. Furthermore, this study provided insight that following the clinic instructions a majority of the time strongly contributes to glycemic goal attainment. We believe that an assessment of patients’ behaviors prior to referrals to diabetes management programs will yield useful information about possible barriers to glycemic goal attainment.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Published 2017. Accessed September 25, 2018.
2. Gaspar JL, Dahlke ME, Kasper B. Efficacy of patient aligned care team pharmacist service in reaching diabetes and hyperlipidemia treatment goals. Fed Pract. 2015;32(11):42-47.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S6-S135.
4. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Published April 2017. Accessed September 7, 2018.
5. Centers for Disease Control and Prevention. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1-96.
6. Nigro SC, Garwood CL, Berlie H, et al. Clinical pharmacists as key members of the patient-centered medical home: an opinion statement of the Ambulatory Care Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(1):96-108.
7. Smith M, Bates DW, Bodenheimer T, et al. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29(5):906-913.
8. Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US Pharmacists’ effect as team members on patient care. Med Care. 2010;48(10):923-933.
9. Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 2008;28(4):421-436.
10. Touchette DR, Doloresco F, Suda KJ, et al. Economic evaluations of clinical pharmacy services: 2006-2010. Pharmacotherapy. 2014;34(8):771-793.
11. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report of the U.S. Surgeon General. American College of Clinical Pharmacy. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed September 10, 2018.
12. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc (2003). 2008;48(2):203-211.
13. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
14. Taveira TH, Friedmann PD, Cohen LB, et al. Pharmacist-led group medical appointment model in type 2 diabetes. Diabetes Educ. 2010;36(1):109-117.
15. Edwards KL, Hadley RL, Baby N, Yeary JC, Chastain LM, Brown CD. Utilizing clinical pharmacy specialists to address access to care barriers in the veteran population for the management of diabetes. J Pharm Pract. 2017;30(4):412-418.
16. Cripps RJ, Gourley ES, Johnson W, et al. An evaluation of diabetes-related measures of control after 6 months of clinical pharmacy specialist intervention. J Pharm Prac. 2011;24(3):332-338.
17. Jones H, Edwards L, Vallis TM, et al; Diabetes Stages of Change (DiSC) Study. Changes in diabetes self-care behaviors make a difference in glycemic control. Diabetes Care. 2003;26(3):732-737.
18. Schetman JM, Schorling JB, Voss JD. Appointment adherence and disparities in outcomes among patients with diabetes. J Gen Intern Med. 2008;23(10):1685-1687.
19. Rhee, MK, Slocum W, Zeimer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31(2):240-250.
Showing up to appointments and adherence to treatment recommendations correlated with glycemic goal attainment for patients.
Showing up to appointments and adherence to treatment recommendations correlated with glycemic goal attainment for patients.
About 30.3 million Americans (9.4%) have diabetes mellitus (DM).1 Veterans are disproportionately affected—about 1 in 4 of those who receive US Department of Veterans Affairs (VA) care have DM.2 The consequences of uncontrolled DM include microvascular complications (eg, retinopathy, neuropathy, and nephropathy) and macrovascular complications (eg, cardiovascular disease).
The American Diabetes Association (ADA) recommends achieving a goal hemoglobin A1c (HbA1c) level of < 7% to prevent these complications. However, a goal of < 8% HbA1c may be more appropriate for certain patients when a more strict goal may be impractical or have the potential to cause harm.3 Furthermore, guidelines developed by the VA and the US Department of Defense suggest a target HbA1c range of 7.0% to 8.5% for patients with established microvascular or macrovascular disease, comorbid conditions, or a life expectancy of 5 to 10 years.4
Despite the existence of evidence showing the importance of glycemic control in preventing morbidity and mortality associated with DM, many patients have inadequate glycemic control. Diabetes mellitus is the seventh leading cause of death in the US. Moreover, DM is a known risk factor for heart disease, stroke, and kidney disease, which are the first, fifth, and ninth leading causes of death in the US, respectively.5
Because DM management requires ongoing and comprehensive maintenance and monitoring, the ADA supports a collaborative, multidisciplinary, and patient-centered approach to delivery of care.3 Collaborative teams involving pharmacists have been shown to improve outcomes and cost savings for chronic diseases, including DM.6-12 In 1995, the VA launched a national policy providing clinical pharmacists with prescribing privileges that would aid in the provision of coordinated medication management for patients with chronic illnesses.13 The policy created a framework for collaborative drug therapy management (CDTM) models, which grants pharmacists the ability to perform patient assessments, order laboratory tests, and modify medications within a scope of practice.
Since the initiation of these services, several examples of successful DM management services using clinical pharmacists within the VA exist in the literature.14-16 However, even with intensive chronic disease and drug therapy management, not all patients who enroll in these services successfully reach clinical goals. Although these pharmacist-driven services seem to demonstrate overall benefit and cost savings to veteran patients and the VA system, little published data exist to help determine patient behaviors that are associated with glycemic goal attainment when using these services.
At the Corporal Michael J. Crescenz VA Medical Center in (CMCVAMC) Philadelphia, Pennsylvania, where this study was performed, primary care providers may refer patients with uncontrolled DM to the pharmacist disease state management (DSM) clinic. The clinic is a form of a CDTM and receives numerous referrals per year, with many patients discharged for successfully meeting glycemic targets.
However, a percentage of patients fail to attain glycemic goals despite involvement in this clinic. We observed specific patient behaviors that delayed glycemic goal attainment. This study examined whether these behaviors correlated with prolonged glycemic goal attainment. The purpose of this study was to identify patient behaviors that led to glycemic goal attainment in insulin-treated patients referred to this pharmacist DSM clinic.
Methods
This study was performed as a single-center retrospective chart review. The protocol and data collection documents were approved by the CMCVAMC Institutional Review Board. It included patients referred to a pharmacist-led DSM clinic for insulin titration/optimization from January 1, 2011 through December 31, 2012. Data were collected through June 30, 2013, to allow for 6 months after the last referral date of December 31, 2012.
This study included patients who were on insulin therapy at the time of pharmacy consult, who attended at least 3 consecutive pharmacy DSM clinic visits, and had an HbA1c ≥ 8% at the time of initial clinic consult. Patients who failed to have 3 consecutive pharmacy DSM clinic visits, were insulin-naïve at the time of referral, aged ≥ 90, lacked at least 1 follow-up HbA1c result while enrolled in the clinic, or had HbA1c < 8% were excluded.
Among the patients who met eligibility criteria, charts within the Computerized Patient Record System (CPRS) were reviewed in a chronologic order within the respective study time frame. A convenience sample of 100 patients were enrolled in each treatment arm: the goal-attained arm or the goal-not-attained arm.
The primary study variable was HbA1c goal attainment, which was defined in this investigation as at least 1 HbA1c reading of < 8% while enrolled in the DSM clinic during the review period. Secondary variables included specific patient factors such as optimal frequency of self-monitoring of blood glucose (SMBG) testing, adherence to pharmacist’s instructions for changes to glucose-lowering medications, adherence to bringing glucose meter/glucose log book to clinic appointments, and percentage of visits attended. Definitions for each variable are provided in Table 1.
We hypothesized that patients who were more adherent to treatment plans, regularly attend clinic visits, and appropriately monitor their glucose levels were more likely to meet their glycemic goals.
Statistical Analysis
Univariate descriptive statistics described the individual variables/predictors of HbA1c goal attainment. As the study’s purpose was to identify patient factors and characteristics associated with HbA1c goal attainment, a logistic regression model framework was used for all covariates to evaluate each measured variable’s independent association with HbA1c. The univariate tests were used to compare patient characteristics between the 2 study groups: Pearson chi-square test was used for nominal data, and a paired t test (for normally distributed data) or Wilcoxon rank sum test (for non-normally distributed data) was used for continuous variables. Variables having a P value < .2 underwent a multivariate analysis stepwise logistic regression model to identify patient factors and characteristics associated with HbA1c goal attainment. A Fisher exact test was used to determine gender effect on HbA1c goal attainment, categoric variables were analyzed using Pearson chi-square test, and an unpaired t test was used for continuous data. The backward elimination approach to inclusion of variables in the model was used to build the most parsimonious and best-fitting model, and the Hosmer-Lemeshow goodness-of-fit tests was used to assess model fit. Data analyses were performed using IBM SPSS, version 18.0 (Armonk, NY).
Results
Five hundred eighty-four patient records were reviewed, and 207 patients met inclusion criteria: 102 patient records were reviewed for the goal-attained arm, and 105 patient records for the goal-not-attained arm. Most patients were excluded from the analysis due to not having 3 consecutive visits during the specified period or having an HbA1c of < 8% at the time of referral to the pharmacist DSM clinic.
The patients in this study had type 2 diabetes for about 11 years, were overwhelmingly male (99%), were aged about 61 years, and were taking on average 13 medications at the time of referral to the pharmacist DSM clinic. Mean HbA1c at time of enrollment was slightly higher in the goal-not-attained arm vs goal-attained arm (10.7% vs 10.2%, respectively), but the difference was not statistically significant (P = .066). A little more than half the patients in both study arms were on basal + prandial insulin regimens (Table 2).
Patients who attained their goal HbA1cwere more likely to bring their glucose meter/glucose log book to at least 80% of their appointments (P < .001). Additionally, this same cohort followed insulin dosing instructions at least 80% of the time (P < .001).
Five variables were included in the multivariate analysis because they had a P value ≤ .2 in univariate analyses: (1) patient adherence to instructions (P < .001); (2) duration in clinic (P < .001); (3) patient bringingglucose meter or glucose log to appointments (P < .001); (4) percentage of scheduled appointments patient attended (P = .015); and (5) baseline HbA1c (P = .066).
Discussion
The development and constant modification of clinical practicing guidelines has made DM treatment a focus and priority.3,4 Additionally, the collaborative approach to health care and creation of VA pharmacist-driven services have demonstrated successful patient outcomes.6-16 Despite these efforts, further insight is needed to improve the management of DM. Our study identified specific behavioral factors that correlated to veteran patients to attaining their HbA1c goal of < 8% within a VA pharmacist DSM clinic. Additionally, it highlighted factors that contributed to patients not achieving their glycemic goals.
Our univariate analysis showed behaviors such as showing up for appointments and following directions regimens to correlate with glycemic goal attainment. However, following directions was the only behavioral factor that correlated to glycemic goal attainment in our multivariate analysis. Additionally, our findings indicated that factors for HbA1c goal attainment included patients who brought their glucose meter/glucose log book and attended clinic appointments at least 80% of the time, respectively.
These findings can help further refine the process for identifying patients who are most likely to achieve glycemic goals when referred to pharmacist DSM clinics or to any DM treatment program. Assessment of a patient’s motivation and ability to attend clinic appointments, bring their glucose meter/glucose log book, and to follow instructions provided at these appointments are reasonable screening questions to ask before referring that patient to a diabetes care program or service. Currently, this is not performed during the consult process to the pharmacist DSM clinic at the respective VA.
Additionally, our findings show that patients who met goal did so, on average, within 6 months of referral to the pharmacist DSM clinic. This finding may have occurred because patients who successfully reach HbA1c goal in 2 consecutive checks are discharged from the clinic. Patients who do not meet this goal continue with the clinic, thus increasing their duration of enrollment in this service. This finding could help clinical pharmacists estimate how long patients will be followed by the service, thus allowing for a more accurate estimation of workload and clinic capacity. Additionally, this finding provides insight if the patient should remain in clinic or be transferred to another program. Our findings aligned with previous studies showing the link between patient behaviors and glycemic goal attainment.17-19
Limitations
This study has a few notable limitations. First, it is limited to 1 VA medical center, so our findings may not be extrapolated easily to other institutions of the Veterans Health Administration. Ideally, future studies centered on identifying factors that lead to successful glycemic goal attainment would be helpful from multiple VA institutions. This would encourage more factors to be identified and trends to be strengthened. Ultimately, this would allow for more global changes to the consult process from primary care to pharmacist DSM clinics nationally vs at a local VA institution. Additionally, this study was limited to a specific retrospective time frame, therefore limiting its ability to identify trends. This study also relied on some subjective factors, such as the patient’s self-report of properly following the clinic instructions. Another limitation was that our investigation was not designed to characterize the specific pharmacist’s interventions that improved glycemic control. Future studies would benefit from the inclusion of specific interventions and their effect on glycemic goal attainment.
Conclusion
This retrospective study offers insight to specific patient behavioral factors that correlate with glycemic goal attainment in a VA pharmacist DSM clinic. Behavioral factors linked to HbA1c goal attainment of < 8% included appointment keeping, bringing glucose meter/glucose log book at least 80% of the time to these appointments, and following clinic instructions. This investigation also found that patients who attain glycemic goals generally do so within 6 months of enrollment. Furthermore, this study provided insight that following the clinic instructions a majority of the time strongly contributes to glycemic goal attainment. We believe that an assessment of patients’ behaviors prior to referrals to diabetes management programs will yield useful information about possible barriers to glycemic goal attainment.
About 30.3 million Americans (9.4%) have diabetes mellitus (DM).1 Veterans are disproportionately affected—about 1 in 4 of those who receive US Department of Veterans Affairs (VA) care have DM.2 The consequences of uncontrolled DM include microvascular complications (eg, retinopathy, neuropathy, and nephropathy) and macrovascular complications (eg, cardiovascular disease).
The American Diabetes Association (ADA) recommends achieving a goal hemoglobin A1c (HbA1c) level of < 7% to prevent these complications. However, a goal of < 8% HbA1c may be more appropriate for certain patients when a more strict goal may be impractical or have the potential to cause harm.3 Furthermore, guidelines developed by the VA and the US Department of Defense suggest a target HbA1c range of 7.0% to 8.5% for patients with established microvascular or macrovascular disease, comorbid conditions, or a life expectancy of 5 to 10 years.4
Despite the existence of evidence showing the importance of glycemic control in preventing morbidity and mortality associated with DM, many patients have inadequate glycemic control. Diabetes mellitus is the seventh leading cause of death in the US. Moreover, DM is a known risk factor for heart disease, stroke, and kidney disease, which are the first, fifth, and ninth leading causes of death in the US, respectively.5
Because DM management requires ongoing and comprehensive maintenance and monitoring, the ADA supports a collaborative, multidisciplinary, and patient-centered approach to delivery of care.3 Collaborative teams involving pharmacists have been shown to improve outcomes and cost savings for chronic diseases, including DM.6-12 In 1995, the VA launched a national policy providing clinical pharmacists with prescribing privileges that would aid in the provision of coordinated medication management for patients with chronic illnesses.13 The policy created a framework for collaborative drug therapy management (CDTM) models, which grants pharmacists the ability to perform patient assessments, order laboratory tests, and modify medications within a scope of practice.
Since the initiation of these services, several examples of successful DM management services using clinical pharmacists within the VA exist in the literature.14-16 However, even with intensive chronic disease and drug therapy management, not all patients who enroll in these services successfully reach clinical goals. Although these pharmacist-driven services seem to demonstrate overall benefit and cost savings to veteran patients and the VA system, little published data exist to help determine patient behaviors that are associated with glycemic goal attainment when using these services.
At the Corporal Michael J. Crescenz VA Medical Center in (CMCVAMC) Philadelphia, Pennsylvania, where this study was performed, primary care providers may refer patients with uncontrolled DM to the pharmacist disease state management (DSM) clinic. The clinic is a form of a CDTM and receives numerous referrals per year, with many patients discharged for successfully meeting glycemic targets.
However, a percentage of patients fail to attain glycemic goals despite involvement in this clinic. We observed specific patient behaviors that delayed glycemic goal attainment. This study examined whether these behaviors correlated with prolonged glycemic goal attainment. The purpose of this study was to identify patient behaviors that led to glycemic goal attainment in insulin-treated patients referred to this pharmacist DSM clinic.
Methods
This study was performed as a single-center retrospective chart review. The protocol and data collection documents were approved by the CMCVAMC Institutional Review Board. It included patients referred to a pharmacist-led DSM clinic for insulin titration/optimization from January 1, 2011 through December 31, 2012. Data were collected through June 30, 2013, to allow for 6 months after the last referral date of December 31, 2012.
This study included patients who were on insulin therapy at the time of pharmacy consult, who attended at least 3 consecutive pharmacy DSM clinic visits, and had an HbA1c ≥ 8% at the time of initial clinic consult. Patients who failed to have 3 consecutive pharmacy DSM clinic visits, were insulin-naïve at the time of referral, aged ≥ 90, lacked at least 1 follow-up HbA1c result while enrolled in the clinic, or had HbA1c < 8% were excluded.
Among the patients who met eligibility criteria, charts within the Computerized Patient Record System (CPRS) were reviewed in a chronologic order within the respective study time frame. A convenience sample of 100 patients were enrolled in each treatment arm: the goal-attained arm or the goal-not-attained arm.
The primary study variable was HbA1c goal attainment, which was defined in this investigation as at least 1 HbA1c reading of < 8% while enrolled in the DSM clinic during the review period. Secondary variables included specific patient factors such as optimal frequency of self-monitoring of blood glucose (SMBG) testing, adherence to pharmacist’s instructions for changes to glucose-lowering medications, adherence to bringing glucose meter/glucose log book to clinic appointments, and percentage of visits attended. Definitions for each variable are provided in Table 1.
We hypothesized that patients who were more adherent to treatment plans, regularly attend clinic visits, and appropriately monitor their glucose levels were more likely to meet their glycemic goals.
Statistical Analysis
Univariate descriptive statistics described the individual variables/predictors of HbA1c goal attainment. As the study’s purpose was to identify patient factors and characteristics associated with HbA1c goal attainment, a logistic regression model framework was used for all covariates to evaluate each measured variable’s independent association with HbA1c. The univariate tests were used to compare patient characteristics between the 2 study groups: Pearson chi-square test was used for nominal data, and a paired t test (for normally distributed data) or Wilcoxon rank sum test (for non-normally distributed data) was used for continuous variables. Variables having a P value < .2 underwent a multivariate analysis stepwise logistic regression model to identify patient factors and characteristics associated with HbA1c goal attainment. A Fisher exact test was used to determine gender effect on HbA1c goal attainment, categoric variables were analyzed using Pearson chi-square test, and an unpaired t test was used for continuous data. The backward elimination approach to inclusion of variables in the model was used to build the most parsimonious and best-fitting model, and the Hosmer-Lemeshow goodness-of-fit tests was used to assess model fit. Data analyses were performed using IBM SPSS, version 18.0 (Armonk, NY).
Results
Five hundred eighty-four patient records were reviewed, and 207 patients met inclusion criteria: 102 patient records were reviewed for the goal-attained arm, and 105 patient records for the goal-not-attained arm. Most patients were excluded from the analysis due to not having 3 consecutive visits during the specified period or having an HbA1c of < 8% at the time of referral to the pharmacist DSM clinic.
The patients in this study had type 2 diabetes for about 11 years, were overwhelmingly male (99%), were aged about 61 years, and were taking on average 13 medications at the time of referral to the pharmacist DSM clinic. Mean HbA1c at time of enrollment was slightly higher in the goal-not-attained arm vs goal-attained arm (10.7% vs 10.2%, respectively), but the difference was not statistically significant (P = .066). A little more than half the patients in both study arms were on basal + prandial insulin regimens (Table 2).
Patients who attained their goal HbA1cwere more likely to bring their glucose meter/glucose log book to at least 80% of their appointments (P < .001). Additionally, this same cohort followed insulin dosing instructions at least 80% of the time (P < .001).
Five variables were included in the multivariate analysis because they had a P value ≤ .2 in univariate analyses: (1) patient adherence to instructions (P < .001); (2) duration in clinic (P < .001); (3) patient bringingglucose meter or glucose log to appointments (P < .001); (4) percentage of scheduled appointments patient attended (P = .015); and (5) baseline HbA1c (P = .066).
Discussion
The development and constant modification of clinical practicing guidelines has made DM treatment a focus and priority.3,4 Additionally, the collaborative approach to health care and creation of VA pharmacist-driven services have demonstrated successful patient outcomes.6-16 Despite these efforts, further insight is needed to improve the management of DM. Our study identified specific behavioral factors that correlated to veteran patients to attaining their HbA1c goal of < 8% within a VA pharmacist DSM clinic. Additionally, it highlighted factors that contributed to patients not achieving their glycemic goals.
Our univariate analysis showed behaviors such as showing up for appointments and following directions regimens to correlate with glycemic goal attainment. However, following directions was the only behavioral factor that correlated to glycemic goal attainment in our multivariate analysis. Additionally, our findings indicated that factors for HbA1c goal attainment included patients who brought their glucose meter/glucose log book and attended clinic appointments at least 80% of the time, respectively.
These findings can help further refine the process for identifying patients who are most likely to achieve glycemic goals when referred to pharmacist DSM clinics or to any DM treatment program. Assessment of a patient’s motivation and ability to attend clinic appointments, bring their glucose meter/glucose log book, and to follow instructions provided at these appointments are reasonable screening questions to ask before referring that patient to a diabetes care program or service. Currently, this is not performed during the consult process to the pharmacist DSM clinic at the respective VA.
Additionally, our findings show that patients who met goal did so, on average, within 6 months of referral to the pharmacist DSM clinic. This finding may have occurred because patients who successfully reach HbA1c goal in 2 consecutive checks are discharged from the clinic. Patients who do not meet this goal continue with the clinic, thus increasing their duration of enrollment in this service. This finding could help clinical pharmacists estimate how long patients will be followed by the service, thus allowing for a more accurate estimation of workload and clinic capacity. Additionally, this finding provides insight if the patient should remain in clinic or be transferred to another program. Our findings aligned with previous studies showing the link between patient behaviors and glycemic goal attainment.17-19
Limitations
This study has a few notable limitations. First, it is limited to 1 VA medical center, so our findings may not be extrapolated easily to other institutions of the Veterans Health Administration. Ideally, future studies centered on identifying factors that lead to successful glycemic goal attainment would be helpful from multiple VA institutions. This would encourage more factors to be identified and trends to be strengthened. Ultimately, this would allow for more global changes to the consult process from primary care to pharmacist DSM clinics nationally vs at a local VA institution. Additionally, this study was limited to a specific retrospective time frame, therefore limiting its ability to identify trends. This study also relied on some subjective factors, such as the patient’s self-report of properly following the clinic instructions. Another limitation was that our investigation was not designed to characterize the specific pharmacist’s interventions that improved glycemic control. Future studies would benefit from the inclusion of specific interventions and their effect on glycemic goal attainment.
Conclusion
This retrospective study offers insight to specific patient behavioral factors that correlate with glycemic goal attainment in a VA pharmacist DSM clinic. Behavioral factors linked to HbA1c goal attainment of < 8% included appointment keeping, bringing glucose meter/glucose log book at least 80% of the time to these appointments, and following clinic instructions. This investigation also found that patients who attain glycemic goals generally do so within 6 months of enrollment. Furthermore, this study provided insight that following the clinic instructions a majority of the time strongly contributes to glycemic goal attainment. We believe that an assessment of patients’ behaviors prior to referrals to diabetes management programs will yield useful information about possible barriers to glycemic goal attainment.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Published 2017. Accessed September 25, 2018.
2. Gaspar JL, Dahlke ME, Kasper B. Efficacy of patient aligned care team pharmacist service in reaching diabetes and hyperlipidemia treatment goals. Fed Pract. 2015;32(11):42-47.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S6-S135.
4. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Published April 2017. Accessed September 7, 2018.
5. Centers for Disease Control and Prevention. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1-96.
6. Nigro SC, Garwood CL, Berlie H, et al. Clinical pharmacists as key members of the patient-centered medical home: an opinion statement of the Ambulatory Care Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(1):96-108.
7. Smith M, Bates DW, Bodenheimer T, et al. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29(5):906-913.
8. Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US Pharmacists’ effect as team members on patient care. Med Care. 2010;48(10):923-933.
9. Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 2008;28(4):421-436.
10. Touchette DR, Doloresco F, Suda KJ, et al. Economic evaluations of clinical pharmacy services: 2006-2010. Pharmacotherapy. 2014;34(8):771-793.
11. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report of the U.S. Surgeon General. American College of Clinical Pharmacy. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed September 10, 2018.
12. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc (2003). 2008;48(2):203-211.
13. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
14. Taveira TH, Friedmann PD, Cohen LB, et al. Pharmacist-led group medical appointment model in type 2 diabetes. Diabetes Educ. 2010;36(1):109-117.
15. Edwards KL, Hadley RL, Baby N, Yeary JC, Chastain LM, Brown CD. Utilizing clinical pharmacy specialists to address access to care barriers in the veteran population for the management of diabetes. J Pharm Pract. 2017;30(4):412-418.
16. Cripps RJ, Gourley ES, Johnson W, et al. An evaluation of diabetes-related measures of control after 6 months of clinical pharmacy specialist intervention. J Pharm Prac. 2011;24(3):332-338.
17. Jones H, Edwards L, Vallis TM, et al; Diabetes Stages of Change (DiSC) Study. Changes in diabetes self-care behaviors make a difference in glycemic control. Diabetes Care. 2003;26(3):732-737.
18. Schetman JM, Schorling JB, Voss JD. Appointment adherence and disparities in outcomes among patients with diabetes. J Gen Intern Med. 2008;23(10):1685-1687.
19. Rhee, MK, Slocum W, Zeimer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31(2):240-250.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Published 2017. Accessed September 25, 2018.
2. Gaspar JL, Dahlke ME, Kasper B. Efficacy of patient aligned care team pharmacist service in reaching diabetes and hyperlipidemia treatment goals. Fed Pract. 2015;32(11):42-47.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S6-S135.
4. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Published April 2017. Accessed September 7, 2018.
5. Centers for Disease Control and Prevention. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1-96.
6. Nigro SC, Garwood CL, Berlie H, et al. Clinical pharmacists as key members of the patient-centered medical home: an opinion statement of the Ambulatory Care Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(1):96-108.
7. Smith M, Bates DW, Bodenheimer T, et al. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29(5):906-913.
8. Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US Pharmacists’ effect as team members on patient care. Med Care. 2010;48(10):923-933.
9. Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 2008;28(4):421-436.
10. Touchette DR, Doloresco F, Suda KJ, et al. Economic evaluations of clinical pharmacy services: 2006-2010. Pharmacotherapy. 2014;34(8):771-793.
11. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report of the U.S. Surgeon General. American College of Clinical Pharmacy. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed September 10, 2018.
12. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc (2003). 2008;48(2):203-211.
13. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
14. Taveira TH, Friedmann PD, Cohen LB, et al. Pharmacist-led group medical appointment model in type 2 diabetes. Diabetes Educ. 2010;36(1):109-117.
15. Edwards KL, Hadley RL, Baby N, Yeary JC, Chastain LM, Brown CD. Utilizing clinical pharmacy specialists to address access to care barriers in the veteran population for the management of diabetes. J Pharm Pract. 2017;30(4):412-418.
16. Cripps RJ, Gourley ES, Johnson W, et al. An evaluation of diabetes-related measures of control after 6 months of clinical pharmacy specialist intervention. J Pharm Prac. 2011;24(3):332-338.
17. Jones H, Edwards L, Vallis TM, et al; Diabetes Stages of Change (DiSC) Study. Changes in diabetes self-care behaviors make a difference in glycemic control. Diabetes Care. 2003;26(3):732-737.
18. Schetman JM, Schorling JB, Voss JD. Appointment adherence and disparities in outcomes among patients with diabetes. J Gen Intern Med. 2008;23(10):1685-1687.
19. Rhee, MK, Slocum W, Zeimer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31(2):240-250.
New model for CKD risk draws on clinical, demographic factors
Data from more than 5 million individuals has been used to develop an equation for predicting the risk of incident chronic kidney disease (CKD) in people with or without diabetes, according to a presentation at Kidney Week 2019, sponsored by the American Society of Nephrology.
In a paper published simultaneously online in JAMA, researchers reported the outcome of an individual-level data analysis of 34 multinational cohorts involving 5,222,711 individuals – including 781,627 with diabetes – from 28 countries as part of the Chronic Kidney Disease Prognosis Consortium.
“An equation for kidney failure risk may help improve care for patients with established CKD, but relatively little work has been performed to develop predictive tools to identify those at increased risk of developing CKD – defined by reduced eGFR [estimated glomerular filtration rate], despite the high lifetime risk of CKD – which is estimated to be 59.1% in the United States,” wrote Robert G. Nelson, MD, PhD, from the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix and colleagues.
Over a mean follow-up of 4 years, 15% of individuals without diabetes and 40% of individuals with diabetes developed incident chronic kidney disease, defined as an eGFR below 60 mL/min per 1.73m2.
The key risk factors were older age, female sex, black race, hypertension, history of cardiovascular disease, lower eGFR values, and higher urine albumin to creatinine ratio. Smoking was also significantly associated with reduced eGFR but only in cohorts without diabetes. In cohorts with diabetes, elevated hemoglobin A1c and the presence and type of diabetes medication were also significantly associated with reduced eGFR.
Using this information, the researchers developed a prediction model built from weighted-average hazard ratios and validated it in nine external validation cohorts of 18 study populations involving a total of 2,253,540 individuals. They found that in 16 of the 18 study populations, the slope of observed to predicted risk ranged from 0.80 to 1.25.
Moreover, in the cohorts without diabetes, the risk equations had a median C-statistic for the 5-year predicted probability of 0.845 (interquartile range, 0.789-0.890) and of 0.801 (IQR, 0.750-0.819) in the cohorts with diabetes, the investigators reported.
“Several models have been developed for estimating the risk of prevalent and incident CKD and end-stage kidney disease, but even those with good discriminative performance have not always performed well for cohorts of people outside the original derivation cohort,” the authors wrote. They argued that their model “demonstrated high discrimination and variable calibration in diverse populations.”
However, they stressed that further study was needed to determine if use of the equations would actually lead to improvements in clinical care and patient outcomes. In an accompanying editorial, Sri Lekha Tummalapalli, MD, and Michelle M. Estrella, MD, of the Kidney Health Research Collaborative at the University of California, San Francisco, said the study and its focus on primary, rather than secondary, prevention of kidney disease is a critical step toward reducing the burden of that disease, especially given that an estimated 37 million people in the United States have chronic kidney disease.
It is also important, they added, that primary prevention of kidney disease is tailored to the individual patient’s risk because risk prediction and screening strategies are unlikely to improve outcomes if they are not paired with effective individualized interventions, such as lifestyle modification or management of blood pressure.
These risk equations could be more holistic by integrating the prediction of both elevated albuminuria and reduced eGFR because more than 40% of individuals with chronic kidney disease have increased albuminuria without reduced eGFR, they noted (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17378).
The study and CKD Prognosis Consortium were supported by the U.S. National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. One author was supported by a grant from the German Research Foundation. Nine authors declared grants, consultancies, and other support from the private sector and research organizations. No other conflicts of interest were declared. Dr. Tummalapalli and Dr. Estrella reported no conflicts of interest.
SOURCE: Nelson R et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17379.
Data from more than 5 million individuals has been used to develop an equation for predicting the risk of incident chronic kidney disease (CKD) in people with or without diabetes, according to a presentation at Kidney Week 2019, sponsored by the American Society of Nephrology.
In a paper published simultaneously online in JAMA, researchers reported the outcome of an individual-level data analysis of 34 multinational cohorts involving 5,222,711 individuals – including 781,627 with diabetes – from 28 countries as part of the Chronic Kidney Disease Prognosis Consortium.
“An equation for kidney failure risk may help improve care for patients with established CKD, but relatively little work has been performed to develop predictive tools to identify those at increased risk of developing CKD – defined by reduced eGFR [estimated glomerular filtration rate], despite the high lifetime risk of CKD – which is estimated to be 59.1% in the United States,” wrote Robert G. Nelson, MD, PhD, from the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix and colleagues.
Over a mean follow-up of 4 years, 15% of individuals without diabetes and 40% of individuals with diabetes developed incident chronic kidney disease, defined as an eGFR below 60 mL/min per 1.73m2.
The key risk factors were older age, female sex, black race, hypertension, history of cardiovascular disease, lower eGFR values, and higher urine albumin to creatinine ratio. Smoking was also significantly associated with reduced eGFR but only in cohorts without diabetes. In cohorts with diabetes, elevated hemoglobin A1c and the presence and type of diabetes medication were also significantly associated with reduced eGFR.
Using this information, the researchers developed a prediction model built from weighted-average hazard ratios and validated it in nine external validation cohorts of 18 study populations involving a total of 2,253,540 individuals. They found that in 16 of the 18 study populations, the slope of observed to predicted risk ranged from 0.80 to 1.25.
Moreover, in the cohorts without diabetes, the risk equations had a median C-statistic for the 5-year predicted probability of 0.845 (interquartile range, 0.789-0.890) and of 0.801 (IQR, 0.750-0.819) in the cohorts with diabetes, the investigators reported.
“Several models have been developed for estimating the risk of prevalent and incident CKD and end-stage kidney disease, but even those with good discriminative performance have not always performed well for cohorts of people outside the original derivation cohort,” the authors wrote. They argued that their model “demonstrated high discrimination and variable calibration in diverse populations.”
However, they stressed that further study was needed to determine if use of the equations would actually lead to improvements in clinical care and patient outcomes. In an accompanying editorial, Sri Lekha Tummalapalli, MD, and Michelle M. Estrella, MD, of the Kidney Health Research Collaborative at the University of California, San Francisco, said the study and its focus on primary, rather than secondary, prevention of kidney disease is a critical step toward reducing the burden of that disease, especially given that an estimated 37 million people in the United States have chronic kidney disease.
It is also important, they added, that primary prevention of kidney disease is tailored to the individual patient’s risk because risk prediction and screening strategies are unlikely to improve outcomes if they are not paired with effective individualized interventions, such as lifestyle modification or management of blood pressure.
These risk equations could be more holistic by integrating the prediction of both elevated albuminuria and reduced eGFR because more than 40% of individuals with chronic kidney disease have increased albuminuria without reduced eGFR, they noted (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17378).
The study and CKD Prognosis Consortium were supported by the U.S. National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. One author was supported by a grant from the German Research Foundation. Nine authors declared grants, consultancies, and other support from the private sector and research organizations. No other conflicts of interest were declared. Dr. Tummalapalli and Dr. Estrella reported no conflicts of interest.
SOURCE: Nelson R et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17379.
Data from more than 5 million individuals has been used to develop an equation for predicting the risk of incident chronic kidney disease (CKD) in people with or without diabetes, according to a presentation at Kidney Week 2019, sponsored by the American Society of Nephrology.
In a paper published simultaneously online in JAMA, researchers reported the outcome of an individual-level data analysis of 34 multinational cohorts involving 5,222,711 individuals – including 781,627 with diabetes – from 28 countries as part of the Chronic Kidney Disease Prognosis Consortium.
“An equation for kidney failure risk may help improve care for patients with established CKD, but relatively little work has been performed to develop predictive tools to identify those at increased risk of developing CKD – defined by reduced eGFR [estimated glomerular filtration rate], despite the high lifetime risk of CKD – which is estimated to be 59.1% in the United States,” wrote Robert G. Nelson, MD, PhD, from the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix and colleagues.
Over a mean follow-up of 4 years, 15% of individuals without diabetes and 40% of individuals with diabetes developed incident chronic kidney disease, defined as an eGFR below 60 mL/min per 1.73m2.
The key risk factors were older age, female sex, black race, hypertension, history of cardiovascular disease, lower eGFR values, and higher urine albumin to creatinine ratio. Smoking was also significantly associated with reduced eGFR but only in cohorts without diabetes. In cohorts with diabetes, elevated hemoglobin A1c and the presence and type of diabetes medication were also significantly associated with reduced eGFR.
Using this information, the researchers developed a prediction model built from weighted-average hazard ratios and validated it in nine external validation cohorts of 18 study populations involving a total of 2,253,540 individuals. They found that in 16 of the 18 study populations, the slope of observed to predicted risk ranged from 0.80 to 1.25.
Moreover, in the cohorts without diabetes, the risk equations had a median C-statistic for the 5-year predicted probability of 0.845 (interquartile range, 0.789-0.890) and of 0.801 (IQR, 0.750-0.819) in the cohorts with diabetes, the investigators reported.
“Several models have been developed for estimating the risk of prevalent and incident CKD and end-stage kidney disease, but even those with good discriminative performance have not always performed well for cohorts of people outside the original derivation cohort,” the authors wrote. They argued that their model “demonstrated high discrimination and variable calibration in diverse populations.”
However, they stressed that further study was needed to determine if use of the equations would actually lead to improvements in clinical care and patient outcomes. In an accompanying editorial, Sri Lekha Tummalapalli, MD, and Michelle M. Estrella, MD, of the Kidney Health Research Collaborative at the University of California, San Francisco, said the study and its focus on primary, rather than secondary, prevention of kidney disease is a critical step toward reducing the burden of that disease, especially given that an estimated 37 million people in the United States have chronic kidney disease.
It is also important, they added, that primary prevention of kidney disease is tailored to the individual patient’s risk because risk prediction and screening strategies are unlikely to improve outcomes if they are not paired with effective individualized interventions, such as lifestyle modification or management of blood pressure.
These risk equations could be more holistic by integrating the prediction of both elevated albuminuria and reduced eGFR because more than 40% of individuals with chronic kidney disease have increased albuminuria without reduced eGFR, they noted (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17378).
The study and CKD Prognosis Consortium were supported by the U.S. National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. One author was supported by a grant from the German Research Foundation. Nine authors declared grants, consultancies, and other support from the private sector and research organizations. No other conflicts of interest were declared. Dr. Tummalapalli and Dr. Estrella reported no conflicts of interest.
SOURCE: Nelson R et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17379.
REPORTING FROM KIDNEY WEEK 2019
Key clinical point:
Major finding: In the cohorts without diabetes, the risk equations had a median C-statistic for the 5-year predicted probability of 0.845 (interquartile range, 0.789-0.890), and of 0.801 (IQR, 0.750-0.819) in the cohorts with diabetes,
Study details: Analysis of cohort data from 5,222,711 individuals, including 781,627 with diabetes.
Disclosures: The study and CKD Prognosis Consortium were supported by the U.S. National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. One author was supported by a grant from the German Research Foundation. Nine authors declared grants, consultancies, and other support from the private sector and research organizations. No other conflicts of interest were declared. Dr. Tummalapalli and Dr. Estrella reported no conflicts of interest.
Source: Nelson R et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17379.
Vitamin D, omega-3 fatty acids do not preserve kidney function in type 2 diabetes
A new study has found that neither vitamin D nor omega-3 fatty acids are significantly more beneficial than placebo for prevention and treatment of chronic kidney disease in patients with type 2 diabetes, according to Ian H. de Boer, MD, of the University of Washington, Seattle, and coauthors.
Findings of the study were presented at Kidney Week 2019, sponsored the American Society of Nephrology, and published simultaneously in JAMA.
To determine the benefits of either vitamin D or omega-3 fatty acids in regard to kidney function, the researchers conducted a randomized clinical trial of 1,312 patients with type 2 diabetes. The trial was designed to accompany the Vitamin D and Omega-3 Trial (VITAL), which enrolled 25,871 patients to test the two supplements in the prevention of cardiovascular disease and cancer.
Participants in this study – known as VITAL–Diabetic Kidney Disease, designed as an ancillary to VITAL – were assigned to one of four groups: vitamin D plus omega-3 fatty acids (n = 370), vitamin D plus placebo (n = 333), omega-3 fatty acids plus placebo (n = 289), or both placebos (n = 320). The goal was to assess changes in in glomerular filtration rate estimated from serum creatinine and cystatin C (eGFR) after 5 years.
Of the initial 1,312 participants, 934 (71%) finished the study. At 5-year follow-up, patients taking vitamin D had a mean change in eGFR of −12.3 mL/min per 1.73 m2 (95% confidence interval, −13.4 to −11.2), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −11.9) with placebo. Patients taking omega-3 fatty acids had a mean eGFR change of −12.2 mL/min per 1.73 m2 (95% CI, −13.3 to −11.1), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −12.0) with placebo.
The authors noted that the modest number of measurements collected per participant limited the evaluation and analyses. In addition, the study focused broadly on the type 2 diabetes population and not on subgroups, “who may derive more benefit from the study interventions.”
In an accompanying editorial, authors Anika Lucas, MD and Myles Wolf, MD, of Duke University in Durham, N.C., said multiple clinical trials, including this latest study from de Boer and colleagues on kidney function, have failed to reinforce the previously reported benefits of vitamin D.
“The VITAL-DKD study population had nearly normal mean 25-hydroxyvitamin D levels at baseline, leaving open the question of whether the results would have differed had recruitment been restricted to patients with moderate or severe vitamin D deficiency,” they wrote (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17302).
Nevertheless, it seems safe to conclude that the previous associations between vitamin D deficiency and adverse health were “driven by unmeasured residual confounding or reverse causality.
“Without certainty about the ideal approach to vitamin D treatment in advanced CKD, a randomized trial that compared cholecalciferol, exogenous 25-hydroxyvitamin D, and an activated vitamin D analogue vs. placebo could definitively lay to rest multiple remaining questions in the area,” they suggested.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, and equipment and supplies from various pharmaceutical companies and the National Institutes of Health. Dr. Wolf reported having served as a consultant for Akebia, AMAG, Amgen, Ardelyx, Diasorin, and Pharmacosmos. No other disclosures were reported.
SOURCE: de Boer IH et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17380.
A new study has found that neither vitamin D nor omega-3 fatty acids are significantly more beneficial than placebo for prevention and treatment of chronic kidney disease in patients with type 2 diabetes, according to Ian H. de Boer, MD, of the University of Washington, Seattle, and coauthors.
Findings of the study were presented at Kidney Week 2019, sponsored the American Society of Nephrology, and published simultaneously in JAMA.
To determine the benefits of either vitamin D or omega-3 fatty acids in regard to kidney function, the researchers conducted a randomized clinical trial of 1,312 patients with type 2 diabetes. The trial was designed to accompany the Vitamin D and Omega-3 Trial (VITAL), which enrolled 25,871 patients to test the two supplements in the prevention of cardiovascular disease and cancer.
Participants in this study – known as VITAL–Diabetic Kidney Disease, designed as an ancillary to VITAL – were assigned to one of four groups: vitamin D plus omega-3 fatty acids (n = 370), vitamin D plus placebo (n = 333), omega-3 fatty acids plus placebo (n = 289), or both placebos (n = 320). The goal was to assess changes in in glomerular filtration rate estimated from serum creatinine and cystatin C (eGFR) after 5 years.
Of the initial 1,312 participants, 934 (71%) finished the study. At 5-year follow-up, patients taking vitamin D had a mean change in eGFR of −12.3 mL/min per 1.73 m2 (95% confidence interval, −13.4 to −11.2), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −11.9) with placebo. Patients taking omega-3 fatty acids had a mean eGFR change of −12.2 mL/min per 1.73 m2 (95% CI, −13.3 to −11.1), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −12.0) with placebo.
The authors noted that the modest number of measurements collected per participant limited the evaluation and analyses. In addition, the study focused broadly on the type 2 diabetes population and not on subgroups, “who may derive more benefit from the study interventions.”
In an accompanying editorial, authors Anika Lucas, MD and Myles Wolf, MD, of Duke University in Durham, N.C., said multiple clinical trials, including this latest study from de Boer and colleagues on kidney function, have failed to reinforce the previously reported benefits of vitamin D.
“The VITAL-DKD study population had nearly normal mean 25-hydroxyvitamin D levels at baseline, leaving open the question of whether the results would have differed had recruitment been restricted to patients with moderate or severe vitamin D deficiency,” they wrote (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17302).
Nevertheless, it seems safe to conclude that the previous associations between vitamin D deficiency and adverse health were “driven by unmeasured residual confounding or reverse causality.
“Without certainty about the ideal approach to vitamin D treatment in advanced CKD, a randomized trial that compared cholecalciferol, exogenous 25-hydroxyvitamin D, and an activated vitamin D analogue vs. placebo could definitively lay to rest multiple remaining questions in the area,” they suggested.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, and equipment and supplies from various pharmaceutical companies and the National Institutes of Health. Dr. Wolf reported having served as a consultant for Akebia, AMAG, Amgen, Ardelyx, Diasorin, and Pharmacosmos. No other disclosures were reported.
SOURCE: de Boer IH et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17380.
A new study has found that neither vitamin D nor omega-3 fatty acids are significantly more beneficial than placebo for prevention and treatment of chronic kidney disease in patients with type 2 diabetes, according to Ian H. de Boer, MD, of the University of Washington, Seattle, and coauthors.
Findings of the study were presented at Kidney Week 2019, sponsored the American Society of Nephrology, and published simultaneously in JAMA.
To determine the benefits of either vitamin D or omega-3 fatty acids in regard to kidney function, the researchers conducted a randomized clinical trial of 1,312 patients with type 2 diabetes. The trial was designed to accompany the Vitamin D and Omega-3 Trial (VITAL), which enrolled 25,871 patients to test the two supplements in the prevention of cardiovascular disease and cancer.
Participants in this study – known as VITAL–Diabetic Kidney Disease, designed as an ancillary to VITAL – were assigned to one of four groups: vitamin D plus omega-3 fatty acids (n = 370), vitamin D plus placebo (n = 333), omega-3 fatty acids plus placebo (n = 289), or both placebos (n = 320). The goal was to assess changes in in glomerular filtration rate estimated from serum creatinine and cystatin C (eGFR) after 5 years.
Of the initial 1,312 participants, 934 (71%) finished the study. At 5-year follow-up, patients taking vitamin D had a mean change in eGFR of −12.3 mL/min per 1.73 m2 (95% confidence interval, −13.4 to −11.2), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −11.9) with placebo. Patients taking omega-3 fatty acids had a mean eGFR change of −12.2 mL/min per 1.73 m2 (95% CI, −13.3 to −11.1), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −12.0) with placebo.
The authors noted that the modest number of measurements collected per participant limited the evaluation and analyses. In addition, the study focused broadly on the type 2 diabetes population and not on subgroups, “who may derive more benefit from the study interventions.”
In an accompanying editorial, authors Anika Lucas, MD and Myles Wolf, MD, of Duke University in Durham, N.C., said multiple clinical trials, including this latest study from de Boer and colleagues on kidney function, have failed to reinforce the previously reported benefits of vitamin D.
“The VITAL-DKD study population had nearly normal mean 25-hydroxyvitamin D levels at baseline, leaving open the question of whether the results would have differed had recruitment been restricted to patients with moderate or severe vitamin D deficiency,” they wrote (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17302).
Nevertheless, it seems safe to conclude that the previous associations between vitamin D deficiency and adverse health were “driven by unmeasured residual confounding or reverse causality.
“Without certainty about the ideal approach to vitamin D treatment in advanced CKD, a randomized trial that compared cholecalciferol, exogenous 25-hydroxyvitamin D, and an activated vitamin D analogue vs. placebo could definitively lay to rest multiple remaining questions in the area,” they suggested.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, and equipment and supplies from various pharmaceutical companies and the National Institutes of Health. Dr. Wolf reported having served as a consultant for Akebia, AMAG, Amgen, Ardelyx, Diasorin, and Pharmacosmos. No other disclosures were reported.
SOURCE: de Boer IH et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17380.
FROM KIDNEY WEEK 2019
Key clinical point:
Major finding: At 5-year follow-up, patients taking vitamin D had a mean change in eGFR of −12.3 mL/min per 1.73 m2 (95% CI, −13.4 to −11.2), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −11.9) with placebo.
Study details: A randomized clinical trial of 1,312 adults with type 2 diabetes.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, and equipment and supplies from various pharmaceutical companies and the National Institutes of Health. Dr. Wolf reported having served as a consultant for Akebia, AMAG, Amgen, Ardelyx, Diasorin, and Pharmacosmos. No other disclosures were reported.
Source: de Boer IH et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17380.
It’s time to get to know AI
This month’s cover story on artificial intelligence (AI) and machine learning provides a glimpse into the future of medical care. The article’s title, “An FP’s guide to AI-enabled clinical decision support” points to the fact that practical and useful applications of AI and machine learning are making inroads into medicine. However, other industries are far ahead of medicine when it comes to AI.
For example, I met with a financial advisor last week, and our discussion included a display of the likelihood that my wife and I would have sufficient funds in our retirement account based on a Monte Carlo simulation using 500 trials! In other words, our advisor used a huge database of financial information, analyzed that data with a sophisticated statistical technique, and applied the results to our personal situation. (No, we won’t run out of money—with 99% certainty.)
So as physicians, how can we further increase our certainty in the diagnoses we make and the guidance we offer our patients?
Halamka and Cerrato provide some insights. They discuss 2 clinical applications of AI and machine learning that are ready to use in primary care: screening for diabetic retinopathy and risk assessment for colon cancer. The first is an example of using AI for diagnosis and the second for risk assessment; both are core functions of primary care clinicians. These tools were developed with very sophisticated computer programs, but they are not unlike a plethora of clinical decision aids already widely used in primary care for diagnosis and risk assessment, such as the Ottawa Ankle Rules, the Gail Model for breast cancer risk, the FRAX tool for osteoporosis-related fracture risk, the ASCVD Risk Calculator for cardiovascular risk, and the CHA2DS2-VASC score for prediction of thrombosis and bleeding risk from anticoagulation therapy.
Some express concern that sophisticated AI could eventually replace primary care clinicians, similar to how automation reduces the need for routine labor in manufacturing. I think this is highly unlikely, but I do think AI will be widely deployed in clinical tools that improve our diagnostic accuracy and provide better personalized data to inform shared decision making. For example, the colon cancer risk calculator may actually help some patients decide NOT to be screened because their personal risk is so low.
It’s incumbent upon us, then, to familiarize ourselves with the potential that these AI tools offer. It’s time to get to know AI.
This month’s cover story on artificial intelligence (AI) and machine learning provides a glimpse into the future of medical care. The article’s title, “An FP’s guide to AI-enabled clinical decision support” points to the fact that practical and useful applications of AI and machine learning are making inroads into medicine. However, other industries are far ahead of medicine when it comes to AI.
For example, I met with a financial advisor last week, and our discussion included a display of the likelihood that my wife and I would have sufficient funds in our retirement account based on a Monte Carlo simulation using 500 trials! In other words, our advisor used a huge database of financial information, analyzed that data with a sophisticated statistical technique, and applied the results to our personal situation. (No, we won’t run out of money—with 99% certainty.)
So as physicians, how can we further increase our certainty in the diagnoses we make and the guidance we offer our patients?
Halamka and Cerrato provide some insights. They discuss 2 clinical applications of AI and machine learning that are ready to use in primary care: screening for diabetic retinopathy and risk assessment for colon cancer. The first is an example of using AI for diagnosis and the second for risk assessment; both are core functions of primary care clinicians. These tools were developed with very sophisticated computer programs, but they are not unlike a plethora of clinical decision aids already widely used in primary care for diagnosis and risk assessment, such as the Ottawa Ankle Rules, the Gail Model for breast cancer risk, the FRAX tool for osteoporosis-related fracture risk, the ASCVD Risk Calculator for cardiovascular risk, and the CHA2DS2-VASC score for prediction of thrombosis and bleeding risk from anticoagulation therapy.
Some express concern that sophisticated AI could eventually replace primary care clinicians, similar to how automation reduces the need for routine labor in manufacturing. I think this is highly unlikely, but I do think AI will be widely deployed in clinical tools that improve our diagnostic accuracy and provide better personalized data to inform shared decision making. For example, the colon cancer risk calculator may actually help some patients decide NOT to be screened because their personal risk is so low.
It’s incumbent upon us, then, to familiarize ourselves with the potential that these AI tools offer. It’s time to get to know AI.
This month’s cover story on artificial intelligence (AI) and machine learning provides a glimpse into the future of medical care. The article’s title, “An FP’s guide to AI-enabled clinical decision support” points to the fact that practical and useful applications of AI and machine learning are making inroads into medicine. However, other industries are far ahead of medicine when it comes to AI.
For example, I met with a financial advisor last week, and our discussion included a display of the likelihood that my wife and I would have sufficient funds in our retirement account based on a Monte Carlo simulation using 500 trials! In other words, our advisor used a huge database of financial information, analyzed that data with a sophisticated statistical technique, and applied the results to our personal situation. (No, we won’t run out of money—with 99% certainty.)
So as physicians, how can we further increase our certainty in the diagnoses we make and the guidance we offer our patients?
Halamka and Cerrato provide some insights. They discuss 2 clinical applications of AI and machine learning that are ready to use in primary care: screening for diabetic retinopathy and risk assessment for colon cancer. The first is an example of using AI for diagnosis and the second for risk assessment; both are core functions of primary care clinicians. These tools were developed with very sophisticated computer programs, but they are not unlike a plethora of clinical decision aids already widely used in primary care for diagnosis and risk assessment, such as the Ottawa Ankle Rules, the Gail Model for breast cancer risk, the FRAX tool for osteoporosis-related fracture risk, the ASCVD Risk Calculator for cardiovascular risk, and the CHA2DS2-VASC score for prediction of thrombosis and bleeding risk from anticoagulation therapy.
Some express concern that sophisticated AI could eventually replace primary care clinicians, similar to how automation reduces the need for routine labor in manufacturing. I think this is highly unlikely, but I do think AI will be widely deployed in clinical tools that improve our diagnostic accuracy and provide better personalized data to inform shared decision making. For example, the colon cancer risk calculator may actually help some patients decide NOT to be screened because their personal risk is so low.
It’s incumbent upon us, then, to familiarize ourselves with the potential that these AI tools offer. It’s time to get to know AI.
Duodenal mucosal resurfacing has metabolic effects in type 2 diabetes
BOSTON – An ablative procedure intended to promote regrowth of duodenal mucosa was safe and had disease-modifying metabolic effects in a randomized study including patients with type 2 diabetes, according to investigators.
A single duodenal mucosal resurfacing (DMR) procedure improved glycemic, hepatic, and body-weight measures at 24 weeks in the multicenter study, investigators will report at the annual meeting of the American Association for the Study of Liver Diseases.
The novel and minimally invasive endoscopic procedure treats the duodenum, which is increasingly recognized as a key metabolic signaling center, according to the study authors, including senior author Arun Sanyal, MD, professor in the gastroenterology division of the department of internal medicine at Virginia Commonwealth University, Richmond.
“Duodenal mucosal hyperplasia is a potential therapeutic target for insulin-resistance–related metabolic diseases,” Dr. Sanyal and coauthors said in a late-breaking abstract for the study published in the AASLD meeting proceedings.
In a previous international open-label, prospective, multicenter study, published in July in Gut, DMR was feasible and safe, producing durable glycemic improvement in patients with type 2 diabetes with suboptimal control on oral glucose-lowering mediation, according to investigators.
The present study, conducted at nine sites in the European Union and two in Brazil, is the first sham-controlled, double-blind, prospective study of the modality in patients with suboptimally controlled type 2 diabetes, according to Dr. Sanyal and coauthors.
A total of 39 patients in the study underwent DMR, while 36 underwent a sham procedure, according to the published abstract. The mean hemoglobin A1c for those patients was 8.3, the mean body mass index was 31.1 kg/m2, and most (77%) were male.
Median change in hemoglobin A1c from baseline to 24 weeks, one of two primary endpoints in the study, was –0.6% for DMR and –0.3% for the sham procedure (P less than 0.05), according to the study abstract.
Likewise, the primary efficacy endpoint of change in a nonalcoholic steatohepatitis biomarker favored the DMR arm. The median change in liver MRI–proton density fat fraction (MRI-PDFF) from baseline to 12 weeks was –5.4% for DMR and –2.4% for the sham procedure (P less than 0.05), according to the reported data.
Hypoglycemia rates were similar in the DMR and sham arms, and over 24 weeks of study, there were no unanticipated adverse effects attributable to the device and no serious adverse events, Dr. Sanyal and colleagues reported.
Dr. Sanyal reported disclosures related to Fractyl Laboratories, Sanyal Biotechnology, Exalenz Bioscience, Akarna Therapeutics, Genfit, Durect, Indalo, Tiziana, Novartis, Merck, Galectin Therapeutics, Janssen, and others.
BOSTON – An ablative procedure intended to promote regrowth of duodenal mucosa was safe and had disease-modifying metabolic effects in a randomized study including patients with type 2 diabetes, according to investigators.
A single duodenal mucosal resurfacing (DMR) procedure improved glycemic, hepatic, and body-weight measures at 24 weeks in the multicenter study, investigators will report at the annual meeting of the American Association for the Study of Liver Diseases.
The novel and minimally invasive endoscopic procedure treats the duodenum, which is increasingly recognized as a key metabolic signaling center, according to the study authors, including senior author Arun Sanyal, MD, professor in the gastroenterology division of the department of internal medicine at Virginia Commonwealth University, Richmond.
“Duodenal mucosal hyperplasia is a potential therapeutic target for insulin-resistance–related metabolic diseases,” Dr. Sanyal and coauthors said in a late-breaking abstract for the study published in the AASLD meeting proceedings.
In a previous international open-label, prospective, multicenter study, published in July in Gut, DMR was feasible and safe, producing durable glycemic improvement in patients with type 2 diabetes with suboptimal control on oral glucose-lowering mediation, according to investigators.
The present study, conducted at nine sites in the European Union and two in Brazil, is the first sham-controlled, double-blind, prospective study of the modality in patients with suboptimally controlled type 2 diabetes, according to Dr. Sanyal and coauthors.
A total of 39 patients in the study underwent DMR, while 36 underwent a sham procedure, according to the published abstract. The mean hemoglobin A1c for those patients was 8.3, the mean body mass index was 31.1 kg/m2, and most (77%) were male.
Median change in hemoglobin A1c from baseline to 24 weeks, one of two primary endpoints in the study, was –0.6% for DMR and –0.3% for the sham procedure (P less than 0.05), according to the study abstract.
Likewise, the primary efficacy endpoint of change in a nonalcoholic steatohepatitis biomarker favored the DMR arm. The median change in liver MRI–proton density fat fraction (MRI-PDFF) from baseline to 12 weeks was –5.4% for DMR and –2.4% for the sham procedure (P less than 0.05), according to the reported data.
Hypoglycemia rates were similar in the DMR and sham arms, and over 24 weeks of study, there were no unanticipated adverse effects attributable to the device and no serious adverse events, Dr. Sanyal and colleagues reported.
Dr. Sanyal reported disclosures related to Fractyl Laboratories, Sanyal Biotechnology, Exalenz Bioscience, Akarna Therapeutics, Genfit, Durect, Indalo, Tiziana, Novartis, Merck, Galectin Therapeutics, Janssen, and others.
BOSTON – An ablative procedure intended to promote regrowth of duodenal mucosa was safe and had disease-modifying metabolic effects in a randomized study including patients with type 2 diabetes, according to investigators.
A single duodenal mucosal resurfacing (DMR) procedure improved glycemic, hepatic, and body-weight measures at 24 weeks in the multicenter study, investigators will report at the annual meeting of the American Association for the Study of Liver Diseases.
The novel and minimally invasive endoscopic procedure treats the duodenum, which is increasingly recognized as a key metabolic signaling center, according to the study authors, including senior author Arun Sanyal, MD, professor in the gastroenterology division of the department of internal medicine at Virginia Commonwealth University, Richmond.
“Duodenal mucosal hyperplasia is a potential therapeutic target for insulin-resistance–related metabolic diseases,” Dr. Sanyal and coauthors said in a late-breaking abstract for the study published in the AASLD meeting proceedings.
In a previous international open-label, prospective, multicenter study, published in July in Gut, DMR was feasible and safe, producing durable glycemic improvement in patients with type 2 diabetes with suboptimal control on oral glucose-lowering mediation, according to investigators.
The present study, conducted at nine sites in the European Union and two in Brazil, is the first sham-controlled, double-blind, prospective study of the modality in patients with suboptimally controlled type 2 diabetes, according to Dr. Sanyal and coauthors.
A total of 39 patients in the study underwent DMR, while 36 underwent a sham procedure, according to the published abstract. The mean hemoglobin A1c for those patients was 8.3, the mean body mass index was 31.1 kg/m2, and most (77%) were male.
Median change in hemoglobin A1c from baseline to 24 weeks, one of two primary endpoints in the study, was –0.6% for DMR and –0.3% for the sham procedure (P less than 0.05), according to the study abstract.
Likewise, the primary efficacy endpoint of change in a nonalcoholic steatohepatitis biomarker favored the DMR arm. The median change in liver MRI–proton density fat fraction (MRI-PDFF) from baseline to 12 weeks was –5.4% for DMR and –2.4% for the sham procedure (P less than 0.05), according to the reported data.
Hypoglycemia rates were similar in the DMR and sham arms, and over 24 weeks of study, there were no unanticipated adverse effects attributable to the device and no serious adverse events, Dr. Sanyal and colleagues reported.
Dr. Sanyal reported disclosures related to Fractyl Laboratories, Sanyal Biotechnology, Exalenz Bioscience, Akarna Therapeutics, Genfit, Durect, Indalo, Tiziana, Novartis, Merck, Galectin Therapeutics, Janssen, and others.
REPORTING FROM THE LIVER MEETING 2019
Key clinical point: Duodenal mucosal resurfacing was safe and had disease-modifying metabolic effects in patients with type 2 diabetes.
Major finding: Results favored duodenal mucosal resurfacing over sham procedure for changes in median HbA1c (–0.6% vs. –0.3%; P less than .05) and liver MRI–proton density fat fraction (–5.4% vs. –2.4%; P less than 0.05).
Study details: A report on 75 patients treated in a randomized, sham-controlled, double-blind, prospective study.
Disclosures: Dr. Sanyal reported disclosures related to Fractyl Laboratories, Sanyal Biotechnology, Exalenz Bioscience, Akarna Therapeutics, Genfit, Durect, Indalo, Tiziana, Novartis, Merck, Galectin Therapeutics, Janssen, and others.
Source: Sanyal A et al. Liver Meeting 2019, Presentation LO2.