User login
Predictors of HA1c Goal Attainment in Patients Treated With Insulin at a VA Pharmacist-Managed Insulin Clinic (FULL)
Showing up to appointments and adherence to treatment recommendations correlated with glycemic goal attainment for patients.
About 30.3 million Americans (9.4%) have diabetes mellitus (DM).1 Veterans are disproportionately affected—about 1 in 4 of those who receive US Department of Veterans Affairs (VA) care have DM.2 The consequences of uncontrolled DM include microvascular complications (eg, retinopathy, neuropathy, and nephropathy) and macrovascular complications (eg, cardiovascular disease).
The American Diabetes Association (ADA) recommends achieving a goal hemoglobin A1c (HbA1c) level of < 7% to prevent these complications. However, a goal of < 8% HbA1c may be more appropriate for certain patients when a more strict goal may be impractical or have the potential to cause harm.3 Furthermore, guidelines developed by the VA and the US Department of Defense suggest a target HbA1c range of 7.0% to 8.5% for patients with established microvascular or macrovascular disease, comorbid conditions, or a life expectancy of 5 to 10 years.4
Despite the existence of evidence showing the importance of glycemic control in preventing morbidity and mortality associated with DM, many patients have inadequate glycemic control. Diabetes mellitus is the seventh leading cause of death in the US. Moreover, DM is a known risk factor for heart disease, stroke, and kidney disease, which are the first, fifth, and ninth leading causes of death in the US, respectively.5
Because DM management requires ongoing and comprehensive maintenance and monitoring, the ADA supports a collaborative, multidisciplinary, and patient-centered approach to delivery of care.3 Collaborative teams involving pharmacists have been shown to improve outcomes and cost savings for chronic diseases, including DM.6-12 In 1995, the VA launched a national policy providing clinical pharmacists with prescribing privileges that would aid in the provision of coordinated medication management for patients with chronic illnesses.13 The policy created a framework for collaborative drug therapy management (CDTM) models, which grants pharmacists the ability to perform patient assessments, order laboratory tests, and modify medications within a scope of practice.
Since the initiation of these services, several examples of successful DM management services using clinical pharmacists within the VA exist in the literature.14-16 However, even with intensive chronic disease and drug therapy management, not all patients who enroll in these services successfully reach clinical goals. Although these pharmacist-driven services seem to demonstrate overall benefit and cost savings to veteran patients and the VA system, little published data exist to help determine patient behaviors that are associated with glycemic goal attainment when using these services.
At the Corporal Michael J. Crescenz VA Medical Center in (CMCVAMC) Philadelphia, Pennsylvania, where this study was performed, primary care providers may refer patients with uncontrolled DM to the pharmacist disease state management (DSM) clinic. The clinic is a form of a CDTM and receives numerous referrals per year, with many patients discharged for successfully meeting glycemic targets.
However, a percentage of patients fail to attain glycemic goals despite involvement in this clinic. We observed specific patient behaviors that delayed glycemic goal attainment. This study examined whether these behaviors correlated with prolonged glycemic goal attainment. The purpose of this study was to identify patient behaviors that led to glycemic goal attainment in insulin-treated patients referred to this pharmacist DSM clinic.
Methods
This study was performed as a single-center retrospective chart review. The protocol and data collection documents were approved by the CMCVAMC Institutional Review Board. It included patients referred to a pharmacist-led DSM clinic for insulin titration/optimization from January 1, 2011 through December 31, 2012. Data were collected through June 30, 2013, to allow for 6 months after the last referral date of December 31, 2012.
This study included patients who were on insulin therapy at the time of pharmacy consult, who attended at least 3 consecutive pharmacy DSM clinic visits, and had an HbA1c ≥ 8% at the time of initial clinic consult. Patients who failed to have 3 consecutive pharmacy DSM clinic visits, were insulin-naïve at the time of referral, aged ≥ 90, lacked at least 1 follow-up HbA1c result while enrolled in the clinic, or had HbA1c < 8% were excluded.
Among the patients who met eligibility criteria, charts within the Computerized Patient Record System (CPRS) were reviewed in a chronologic order within the respective study time frame. A convenience sample of 100 patients were enrolled in each treatment arm: the goal-attained arm or the goal-not-attained arm.
The primary study variable was HbA1c goal attainment, which was defined in this investigation as at least 1 HbA1c reading of < 8% while enrolled in the DSM clinic during the review period. Secondary variables included specific patient factors such as optimal frequency of self-monitoring of blood glucose (SMBG) testing, adherence to pharmacist’s instructions for changes to glucose-lowering medications, adherence to bringing glucose meter/glucose log book to clinic appointments, and percentage of visits attended. Definitions for each variable are provided in Table 1.
We hypothesized that patients who were more adherent to treatment plans, regularly attend clinic visits, and appropriately monitor their glucose levels were more likely to meet their glycemic goals.
Statistical Analysis
Univariate descriptive statistics described the individual variables/predictors of HbA1c goal attainment. As the study’s purpose was to identify patient factors and characteristics associated with HbA1c goal attainment, a logistic regression model framework was used for all covariates to evaluate each measured variable’s independent association with HbA1c. The univariate tests were used to compare patient characteristics between the 2 study groups: Pearson chi-square test was used for nominal data, and a paired t test (for normally distributed data) or Wilcoxon rank sum test (for non-normally distributed data) was used for continuous variables. Variables having a P value < .2 underwent a multivariate analysis stepwise logistic regression model to identify patient factors and characteristics associated with HbA1c goal attainment. A Fisher exact test was used to determine gender effect on HbA1c goal attainment, categoric variables were analyzed using Pearson chi-square test, and an unpaired t test was used for continuous data. The backward elimination approach to inclusion of variables in the model was used to build the most parsimonious and best-fitting model, and the Hosmer-Lemeshow goodness-of-fit tests was used to assess model fit. Data analyses were performed using IBM SPSS, version 18.0 (Armonk, NY).
Results
Five hundred eighty-four patient records were reviewed, and 207 patients met inclusion criteria: 102 patient records were reviewed for the goal-attained arm, and 105 patient records for the goal-not-attained arm. Most patients were excluded from the analysis due to not having 3 consecutive visits during the specified period or having an HbA1c of < 8% at the time of referral to the pharmacist DSM clinic.
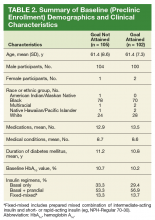
The patients in this study had type 2 diabetes for about 11 years, were overwhelmingly male (99%), were aged about 61 years, and were taking on average 13 medications at the time of referral to the pharmacist DSM clinic. Mean HbA1c at time of enrollment was slightly higher in the goal-not-attained arm vs goal-attained arm (10.7% vs 10.2%, respectively), but the difference was not statistically significant (P = .066). A little more than half the patients in both study arms were on basal + prandial insulin regimens (Table 2).
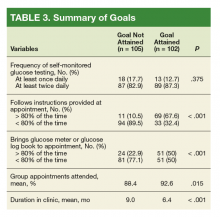
Patients who attained their goal HbA1cwere more likely to bring their glucose meter/glucose log book to at least 80% of their appointments (P < .001). Additionally, this same cohort followed insulin dosing instructions at least 80% of the time (P < .001).
Five variables were included in the multivariate analysis because they had a P value ≤ .2 in univariate analyses: (1) patient adherence to instructions (P < .001); (2) duration in clinic (P < .001); (3) patient bringingglucose meter or glucose log to appointments (P < .001); (4) percentage of scheduled appointments patient attended (P = .015); and (5) baseline HbA1c (P = .066).
Discussion
The development and constant modification of clinical practicing guidelines has made DM treatment a focus and priority.3,4 Additionally, the collaborative approach to health care and creation of VA pharmacist-driven services have demonstrated successful patient outcomes.6-16 Despite these efforts, further insight is needed to improve the management of DM. Our study identified specific behavioral factors that correlated to veteran patients to attaining their HbA1c goal of < 8% within a VA pharmacist DSM clinic. Additionally, it highlighted factors that contributed to patients not achieving their glycemic goals.
Our univariate analysis showed behaviors such as showing up for appointments and following directions regimens to correlate with glycemic goal attainment. However, following directions was the only behavioral factor that correlated to glycemic goal attainment in our multivariate analysis. Additionally, our findings indicated that factors for HbA1c goal attainment included patients who brought their glucose meter/glucose log book and attended clinic appointments at least 80% of the time, respectively.
These findings can help further refine the process for identifying patients who are most likely to achieve glycemic goals when referred to pharmacist DSM clinics or to any DM treatment program. Assessment of a patient’s motivation and ability to attend clinic appointments, bring their glucose meter/glucose log book, and to follow instructions provided at these appointments are reasonable screening questions to ask before referring that patient to a diabetes care program or service. Currently, this is not performed during the consult process to the pharmacist DSM clinic at the respective VA.
Additionally, our findings show that patients who met goal did so, on average, within 6 months of referral to the pharmacist DSM clinic. This finding may have occurred because patients who successfully reach HbA1c goal in 2 consecutive checks are discharged from the clinic. Patients who do not meet this goal continue with the clinic, thus increasing their duration of enrollment in this service. This finding could help clinical pharmacists estimate how long patients will be followed by the service, thus allowing for a more accurate estimation of workload and clinic capacity. Additionally, this finding provides insight if the patient should remain in clinic or be transferred to another program. Our findings aligned with previous studies showing the link between patient behaviors and glycemic goal attainment.17-19
Limitations
This study has a few notable limitations. First, it is limited to 1 VA medical center, so our findings may not be extrapolated easily to other institutions of the Veterans Health Administration. Ideally, future studies centered on identifying factors that lead to successful glycemic goal attainment would be helpful from multiple VA institutions. This would encourage more factors to be identified and trends to be strengthened. Ultimately, this would allow for more global changes to the consult process from primary care to pharmacist DSM clinics nationally vs at a local VA institution. Additionally, this study was limited to a specific retrospective time frame, therefore limiting its ability to identify trends. This study also relied on some subjective factors, such as the patient’s self-report of properly following the clinic instructions. Another limitation was that our investigation was not designed to characterize the specific pharmacist’s interventions that improved glycemic control. Future studies would benefit from the inclusion of specific interventions and their effect on glycemic goal attainment.
Conclusion
This retrospective study offers insight to specific patient behavioral factors that correlate with glycemic goal attainment in a VA pharmacist DSM clinic. Behavioral factors linked to HbA1c goal attainment of < 8% included appointment keeping, bringing glucose meter/glucose log book at least 80% of the time to these appointments, and following clinic instructions. This investigation also found that patients who attain glycemic goals generally do so within 6 months of enrollment. Furthermore, this study provided insight that following the clinic instructions a majority of the time strongly contributes to glycemic goal attainment. We believe that an assessment of patients’ behaviors prior to referrals to diabetes management programs will yield useful information about possible barriers to glycemic goal attainment.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Published 2017. Accessed September 25, 2018.
2. Gaspar JL, Dahlke ME, Kasper B. Efficacy of patient aligned care team pharmacist service in reaching diabetes and hyperlipidemia treatment goals. Fed Pract. 2015;32(11):42-47.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S6-S135.
4. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Published April 2017. Accessed September 7, 2018.
5. Centers for Disease Control and Prevention. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1-96.
6. Nigro SC, Garwood CL, Berlie H, et al. Clinical pharmacists as key members of the patient-centered medical home: an opinion statement of the Ambulatory Care Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(1):96-108.
7. Smith M, Bates DW, Bodenheimer T, et al. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29(5):906-913.
8. Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US Pharmacists’ effect as team members on patient care. Med Care. 2010;48(10):923-933.
9. Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 2008;28(4):421-436.
10. Touchette DR, Doloresco F, Suda KJ, et al. Economic evaluations of clinical pharmacy services: 2006-2010. Pharmacotherapy. 2014;34(8):771-793.
11. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report of the U.S. Surgeon General. American College of Clinical Pharmacy. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed September 10, 2018.
12. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc (2003). 2008;48(2):203-211.
13. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
14. Taveira TH, Friedmann PD, Cohen LB, et al. Pharmacist-led group medical appointment model in type 2 diabetes. Diabetes Educ. 2010;36(1):109-117.
15. Edwards KL, Hadley RL, Baby N, Yeary JC, Chastain LM, Brown CD. Utilizing clinical pharmacy specialists to address access to care barriers in the veteran population for the management of diabetes. J Pharm Pract. 2017;30(4):412-418.
16. Cripps RJ, Gourley ES, Johnson W, et al. An evaluation of diabetes-related measures of control after 6 months of clinical pharmacy specialist intervention. J Pharm Prac. 2011;24(3):332-338.
17. Jones H, Edwards L, Vallis TM, et al; Diabetes Stages of Change (DiSC) Study. Changes in diabetes self-care behaviors make a difference in glycemic control. Diabetes Care. 2003;26(3):732-737.
18. Schetman JM, Schorling JB, Voss JD. Appointment adherence and disparities in outcomes among patients with diabetes. J Gen Intern Med. 2008;23(10):1685-1687.
19. Rhee, MK, Slocum W, Zeimer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31(2):240-250.
Showing up to appointments and adherence to treatment recommendations correlated with glycemic goal attainment for patients.
Showing up to appointments and adherence to treatment recommendations correlated with glycemic goal attainment for patients.
About 30.3 million Americans (9.4%) have diabetes mellitus (DM).1 Veterans are disproportionately affected—about 1 in 4 of those who receive US Department of Veterans Affairs (VA) care have DM.2 The consequences of uncontrolled DM include microvascular complications (eg, retinopathy, neuropathy, and nephropathy) and macrovascular complications (eg, cardiovascular disease).
The American Diabetes Association (ADA) recommends achieving a goal hemoglobin A1c (HbA1c) level of < 7% to prevent these complications. However, a goal of < 8% HbA1c may be more appropriate for certain patients when a more strict goal may be impractical or have the potential to cause harm.3 Furthermore, guidelines developed by the VA and the US Department of Defense suggest a target HbA1c range of 7.0% to 8.5% for patients with established microvascular or macrovascular disease, comorbid conditions, or a life expectancy of 5 to 10 years.4
Despite the existence of evidence showing the importance of glycemic control in preventing morbidity and mortality associated with DM, many patients have inadequate glycemic control. Diabetes mellitus is the seventh leading cause of death in the US. Moreover, DM is a known risk factor for heart disease, stroke, and kidney disease, which are the first, fifth, and ninth leading causes of death in the US, respectively.5
Because DM management requires ongoing and comprehensive maintenance and monitoring, the ADA supports a collaborative, multidisciplinary, and patient-centered approach to delivery of care.3 Collaborative teams involving pharmacists have been shown to improve outcomes and cost savings for chronic diseases, including DM.6-12 In 1995, the VA launched a national policy providing clinical pharmacists with prescribing privileges that would aid in the provision of coordinated medication management for patients with chronic illnesses.13 The policy created a framework for collaborative drug therapy management (CDTM) models, which grants pharmacists the ability to perform patient assessments, order laboratory tests, and modify medications within a scope of practice.
Since the initiation of these services, several examples of successful DM management services using clinical pharmacists within the VA exist in the literature.14-16 However, even with intensive chronic disease and drug therapy management, not all patients who enroll in these services successfully reach clinical goals. Although these pharmacist-driven services seem to demonstrate overall benefit and cost savings to veteran patients and the VA system, little published data exist to help determine patient behaviors that are associated with glycemic goal attainment when using these services.
At the Corporal Michael J. Crescenz VA Medical Center in (CMCVAMC) Philadelphia, Pennsylvania, where this study was performed, primary care providers may refer patients with uncontrolled DM to the pharmacist disease state management (DSM) clinic. The clinic is a form of a CDTM and receives numerous referrals per year, with many patients discharged for successfully meeting glycemic targets.
However, a percentage of patients fail to attain glycemic goals despite involvement in this clinic. We observed specific patient behaviors that delayed glycemic goal attainment. This study examined whether these behaviors correlated with prolonged glycemic goal attainment. The purpose of this study was to identify patient behaviors that led to glycemic goal attainment in insulin-treated patients referred to this pharmacist DSM clinic.
Methods
This study was performed as a single-center retrospective chart review. The protocol and data collection documents were approved by the CMCVAMC Institutional Review Board. It included patients referred to a pharmacist-led DSM clinic for insulin titration/optimization from January 1, 2011 through December 31, 2012. Data were collected through June 30, 2013, to allow for 6 months after the last referral date of December 31, 2012.
This study included patients who were on insulin therapy at the time of pharmacy consult, who attended at least 3 consecutive pharmacy DSM clinic visits, and had an HbA1c ≥ 8% at the time of initial clinic consult. Patients who failed to have 3 consecutive pharmacy DSM clinic visits, were insulin-naïve at the time of referral, aged ≥ 90, lacked at least 1 follow-up HbA1c result while enrolled in the clinic, or had HbA1c < 8% were excluded.
Among the patients who met eligibility criteria, charts within the Computerized Patient Record System (CPRS) were reviewed in a chronologic order within the respective study time frame. A convenience sample of 100 patients were enrolled in each treatment arm: the goal-attained arm or the goal-not-attained arm.
The primary study variable was HbA1c goal attainment, which was defined in this investigation as at least 1 HbA1c reading of < 8% while enrolled in the DSM clinic during the review period. Secondary variables included specific patient factors such as optimal frequency of self-monitoring of blood glucose (SMBG) testing, adherence to pharmacist’s instructions for changes to glucose-lowering medications, adherence to bringing glucose meter/glucose log book to clinic appointments, and percentage of visits attended. Definitions for each variable are provided in Table 1.
We hypothesized that patients who were more adherent to treatment plans, regularly attend clinic visits, and appropriately monitor their glucose levels were more likely to meet their glycemic goals.
Statistical Analysis
Univariate descriptive statistics described the individual variables/predictors of HbA1c goal attainment. As the study’s purpose was to identify patient factors and characteristics associated with HbA1c goal attainment, a logistic regression model framework was used for all covariates to evaluate each measured variable’s independent association with HbA1c. The univariate tests were used to compare patient characteristics between the 2 study groups: Pearson chi-square test was used for nominal data, and a paired t test (for normally distributed data) or Wilcoxon rank sum test (for non-normally distributed data) was used for continuous variables. Variables having a P value < .2 underwent a multivariate analysis stepwise logistic regression model to identify patient factors and characteristics associated with HbA1c goal attainment. A Fisher exact test was used to determine gender effect on HbA1c goal attainment, categoric variables were analyzed using Pearson chi-square test, and an unpaired t test was used for continuous data. The backward elimination approach to inclusion of variables in the model was used to build the most parsimonious and best-fitting model, and the Hosmer-Lemeshow goodness-of-fit tests was used to assess model fit. Data analyses were performed using IBM SPSS, version 18.0 (Armonk, NY).
Results
Five hundred eighty-four patient records were reviewed, and 207 patients met inclusion criteria: 102 patient records were reviewed for the goal-attained arm, and 105 patient records for the goal-not-attained arm. Most patients were excluded from the analysis due to not having 3 consecutive visits during the specified period or having an HbA1c of < 8% at the time of referral to the pharmacist DSM clinic.
The patients in this study had type 2 diabetes for about 11 years, were overwhelmingly male (99%), were aged about 61 years, and were taking on average 13 medications at the time of referral to the pharmacist DSM clinic. Mean HbA1c at time of enrollment was slightly higher in the goal-not-attained arm vs goal-attained arm (10.7% vs 10.2%, respectively), but the difference was not statistically significant (P = .066). A little more than half the patients in both study arms were on basal + prandial insulin regimens (Table 2).
Patients who attained their goal HbA1cwere more likely to bring their glucose meter/glucose log book to at least 80% of their appointments (P < .001). Additionally, this same cohort followed insulin dosing instructions at least 80% of the time (P < .001).
Five variables were included in the multivariate analysis because they had a P value ≤ .2 in univariate analyses: (1) patient adherence to instructions (P < .001); (2) duration in clinic (P < .001); (3) patient bringingglucose meter or glucose log to appointments (P < .001); (4) percentage of scheduled appointments patient attended (P = .015); and (5) baseline HbA1c (P = .066).
Discussion
The development and constant modification of clinical practicing guidelines has made DM treatment a focus and priority.3,4 Additionally, the collaborative approach to health care and creation of VA pharmacist-driven services have demonstrated successful patient outcomes.6-16 Despite these efforts, further insight is needed to improve the management of DM. Our study identified specific behavioral factors that correlated to veteran patients to attaining their HbA1c goal of < 8% within a VA pharmacist DSM clinic. Additionally, it highlighted factors that contributed to patients not achieving their glycemic goals.
Our univariate analysis showed behaviors such as showing up for appointments and following directions regimens to correlate with glycemic goal attainment. However, following directions was the only behavioral factor that correlated to glycemic goal attainment in our multivariate analysis. Additionally, our findings indicated that factors for HbA1c goal attainment included patients who brought their glucose meter/glucose log book and attended clinic appointments at least 80% of the time, respectively.
These findings can help further refine the process for identifying patients who are most likely to achieve glycemic goals when referred to pharmacist DSM clinics or to any DM treatment program. Assessment of a patient’s motivation and ability to attend clinic appointments, bring their glucose meter/glucose log book, and to follow instructions provided at these appointments are reasonable screening questions to ask before referring that patient to a diabetes care program or service. Currently, this is not performed during the consult process to the pharmacist DSM clinic at the respective VA.
Additionally, our findings show that patients who met goal did so, on average, within 6 months of referral to the pharmacist DSM clinic. This finding may have occurred because patients who successfully reach HbA1c goal in 2 consecutive checks are discharged from the clinic. Patients who do not meet this goal continue with the clinic, thus increasing their duration of enrollment in this service. This finding could help clinical pharmacists estimate how long patients will be followed by the service, thus allowing for a more accurate estimation of workload and clinic capacity. Additionally, this finding provides insight if the patient should remain in clinic or be transferred to another program. Our findings aligned with previous studies showing the link between patient behaviors and glycemic goal attainment.17-19
Limitations
This study has a few notable limitations. First, it is limited to 1 VA medical center, so our findings may not be extrapolated easily to other institutions of the Veterans Health Administration. Ideally, future studies centered on identifying factors that lead to successful glycemic goal attainment would be helpful from multiple VA institutions. This would encourage more factors to be identified and trends to be strengthened. Ultimately, this would allow for more global changes to the consult process from primary care to pharmacist DSM clinics nationally vs at a local VA institution. Additionally, this study was limited to a specific retrospective time frame, therefore limiting its ability to identify trends. This study also relied on some subjective factors, such as the patient’s self-report of properly following the clinic instructions. Another limitation was that our investigation was not designed to characterize the specific pharmacist’s interventions that improved glycemic control. Future studies would benefit from the inclusion of specific interventions and their effect on glycemic goal attainment.
Conclusion
This retrospective study offers insight to specific patient behavioral factors that correlate with glycemic goal attainment in a VA pharmacist DSM clinic. Behavioral factors linked to HbA1c goal attainment of < 8% included appointment keeping, bringing glucose meter/glucose log book at least 80% of the time to these appointments, and following clinic instructions. This investigation also found that patients who attain glycemic goals generally do so within 6 months of enrollment. Furthermore, this study provided insight that following the clinic instructions a majority of the time strongly contributes to glycemic goal attainment. We believe that an assessment of patients’ behaviors prior to referrals to diabetes management programs will yield useful information about possible barriers to glycemic goal attainment.
About 30.3 million Americans (9.4%) have diabetes mellitus (DM).1 Veterans are disproportionately affected—about 1 in 4 of those who receive US Department of Veterans Affairs (VA) care have DM.2 The consequences of uncontrolled DM include microvascular complications (eg, retinopathy, neuropathy, and nephropathy) and macrovascular complications (eg, cardiovascular disease).
The American Diabetes Association (ADA) recommends achieving a goal hemoglobin A1c (HbA1c) level of < 7% to prevent these complications. However, a goal of < 8% HbA1c may be more appropriate for certain patients when a more strict goal may be impractical or have the potential to cause harm.3 Furthermore, guidelines developed by the VA and the US Department of Defense suggest a target HbA1c range of 7.0% to 8.5% for patients with established microvascular or macrovascular disease, comorbid conditions, or a life expectancy of 5 to 10 years.4
Despite the existence of evidence showing the importance of glycemic control in preventing morbidity and mortality associated with DM, many patients have inadequate glycemic control. Diabetes mellitus is the seventh leading cause of death in the US. Moreover, DM is a known risk factor for heart disease, stroke, and kidney disease, which are the first, fifth, and ninth leading causes of death in the US, respectively.5
Because DM management requires ongoing and comprehensive maintenance and monitoring, the ADA supports a collaborative, multidisciplinary, and patient-centered approach to delivery of care.3 Collaborative teams involving pharmacists have been shown to improve outcomes and cost savings for chronic diseases, including DM.6-12 In 1995, the VA launched a national policy providing clinical pharmacists with prescribing privileges that would aid in the provision of coordinated medication management for patients with chronic illnesses.13 The policy created a framework for collaborative drug therapy management (CDTM) models, which grants pharmacists the ability to perform patient assessments, order laboratory tests, and modify medications within a scope of practice.
Since the initiation of these services, several examples of successful DM management services using clinical pharmacists within the VA exist in the literature.14-16 However, even with intensive chronic disease and drug therapy management, not all patients who enroll in these services successfully reach clinical goals. Although these pharmacist-driven services seem to demonstrate overall benefit and cost savings to veteran patients and the VA system, little published data exist to help determine patient behaviors that are associated with glycemic goal attainment when using these services.
At the Corporal Michael J. Crescenz VA Medical Center in (CMCVAMC) Philadelphia, Pennsylvania, where this study was performed, primary care providers may refer patients with uncontrolled DM to the pharmacist disease state management (DSM) clinic. The clinic is a form of a CDTM and receives numerous referrals per year, with many patients discharged for successfully meeting glycemic targets.
However, a percentage of patients fail to attain glycemic goals despite involvement in this clinic. We observed specific patient behaviors that delayed glycemic goal attainment. This study examined whether these behaviors correlated with prolonged glycemic goal attainment. The purpose of this study was to identify patient behaviors that led to glycemic goal attainment in insulin-treated patients referred to this pharmacist DSM clinic.
Methods
This study was performed as a single-center retrospective chart review. The protocol and data collection documents were approved by the CMCVAMC Institutional Review Board. It included patients referred to a pharmacist-led DSM clinic for insulin titration/optimization from January 1, 2011 through December 31, 2012. Data were collected through June 30, 2013, to allow for 6 months after the last referral date of December 31, 2012.
This study included patients who were on insulin therapy at the time of pharmacy consult, who attended at least 3 consecutive pharmacy DSM clinic visits, and had an HbA1c ≥ 8% at the time of initial clinic consult. Patients who failed to have 3 consecutive pharmacy DSM clinic visits, were insulin-naïve at the time of referral, aged ≥ 90, lacked at least 1 follow-up HbA1c result while enrolled in the clinic, or had HbA1c < 8% were excluded.
Among the patients who met eligibility criteria, charts within the Computerized Patient Record System (CPRS) were reviewed in a chronologic order within the respective study time frame. A convenience sample of 100 patients were enrolled in each treatment arm: the goal-attained arm or the goal-not-attained arm.
The primary study variable was HbA1c goal attainment, which was defined in this investigation as at least 1 HbA1c reading of < 8% while enrolled in the DSM clinic during the review period. Secondary variables included specific patient factors such as optimal frequency of self-monitoring of blood glucose (SMBG) testing, adherence to pharmacist’s instructions for changes to glucose-lowering medications, adherence to bringing glucose meter/glucose log book to clinic appointments, and percentage of visits attended. Definitions for each variable are provided in Table 1.
We hypothesized that patients who were more adherent to treatment plans, regularly attend clinic visits, and appropriately monitor their glucose levels were more likely to meet their glycemic goals.
Statistical Analysis
Univariate descriptive statistics described the individual variables/predictors of HbA1c goal attainment. As the study’s purpose was to identify patient factors and characteristics associated with HbA1c goal attainment, a logistic regression model framework was used for all covariates to evaluate each measured variable’s independent association with HbA1c. The univariate tests were used to compare patient characteristics between the 2 study groups: Pearson chi-square test was used for nominal data, and a paired t test (for normally distributed data) or Wilcoxon rank sum test (for non-normally distributed data) was used for continuous variables. Variables having a P value < .2 underwent a multivariate analysis stepwise logistic regression model to identify patient factors and characteristics associated with HbA1c goal attainment. A Fisher exact test was used to determine gender effect on HbA1c goal attainment, categoric variables were analyzed using Pearson chi-square test, and an unpaired t test was used for continuous data. The backward elimination approach to inclusion of variables in the model was used to build the most parsimonious and best-fitting model, and the Hosmer-Lemeshow goodness-of-fit tests was used to assess model fit. Data analyses were performed using IBM SPSS, version 18.0 (Armonk, NY).
Results
Five hundred eighty-four patient records were reviewed, and 207 patients met inclusion criteria: 102 patient records were reviewed for the goal-attained arm, and 105 patient records for the goal-not-attained arm. Most patients were excluded from the analysis due to not having 3 consecutive visits during the specified period or having an HbA1c of < 8% at the time of referral to the pharmacist DSM clinic.
The patients in this study had type 2 diabetes for about 11 years, were overwhelmingly male (99%), were aged about 61 years, and were taking on average 13 medications at the time of referral to the pharmacist DSM clinic. Mean HbA1c at time of enrollment was slightly higher in the goal-not-attained arm vs goal-attained arm (10.7% vs 10.2%, respectively), but the difference was not statistically significant (P = .066). A little more than half the patients in both study arms were on basal + prandial insulin regimens (Table 2).
Patients who attained their goal HbA1cwere more likely to bring their glucose meter/glucose log book to at least 80% of their appointments (P < .001). Additionally, this same cohort followed insulin dosing instructions at least 80% of the time (P < .001).
Five variables were included in the multivariate analysis because they had a P value ≤ .2 in univariate analyses: (1) patient adherence to instructions (P < .001); (2) duration in clinic (P < .001); (3) patient bringingglucose meter or glucose log to appointments (P < .001); (4) percentage of scheduled appointments patient attended (P = .015); and (5) baseline HbA1c (P = .066).
Discussion
The development and constant modification of clinical practicing guidelines has made DM treatment a focus and priority.3,4 Additionally, the collaborative approach to health care and creation of VA pharmacist-driven services have demonstrated successful patient outcomes.6-16 Despite these efforts, further insight is needed to improve the management of DM. Our study identified specific behavioral factors that correlated to veteran patients to attaining their HbA1c goal of < 8% within a VA pharmacist DSM clinic. Additionally, it highlighted factors that contributed to patients not achieving their glycemic goals.
Our univariate analysis showed behaviors such as showing up for appointments and following directions regimens to correlate with glycemic goal attainment. However, following directions was the only behavioral factor that correlated to glycemic goal attainment in our multivariate analysis. Additionally, our findings indicated that factors for HbA1c goal attainment included patients who brought their glucose meter/glucose log book and attended clinic appointments at least 80% of the time, respectively.
These findings can help further refine the process for identifying patients who are most likely to achieve glycemic goals when referred to pharmacist DSM clinics or to any DM treatment program. Assessment of a patient’s motivation and ability to attend clinic appointments, bring their glucose meter/glucose log book, and to follow instructions provided at these appointments are reasonable screening questions to ask before referring that patient to a diabetes care program or service. Currently, this is not performed during the consult process to the pharmacist DSM clinic at the respective VA.
Additionally, our findings show that patients who met goal did so, on average, within 6 months of referral to the pharmacist DSM clinic. This finding may have occurred because patients who successfully reach HbA1c goal in 2 consecutive checks are discharged from the clinic. Patients who do not meet this goal continue with the clinic, thus increasing their duration of enrollment in this service. This finding could help clinical pharmacists estimate how long patients will be followed by the service, thus allowing for a more accurate estimation of workload and clinic capacity. Additionally, this finding provides insight if the patient should remain in clinic or be transferred to another program. Our findings aligned with previous studies showing the link between patient behaviors and glycemic goal attainment.17-19
Limitations
This study has a few notable limitations. First, it is limited to 1 VA medical center, so our findings may not be extrapolated easily to other institutions of the Veterans Health Administration. Ideally, future studies centered on identifying factors that lead to successful glycemic goal attainment would be helpful from multiple VA institutions. This would encourage more factors to be identified and trends to be strengthened. Ultimately, this would allow for more global changes to the consult process from primary care to pharmacist DSM clinics nationally vs at a local VA institution. Additionally, this study was limited to a specific retrospective time frame, therefore limiting its ability to identify trends. This study also relied on some subjective factors, such as the patient’s self-report of properly following the clinic instructions. Another limitation was that our investigation was not designed to characterize the specific pharmacist’s interventions that improved glycemic control. Future studies would benefit from the inclusion of specific interventions and their effect on glycemic goal attainment.
Conclusion
This retrospective study offers insight to specific patient behavioral factors that correlate with glycemic goal attainment in a VA pharmacist DSM clinic. Behavioral factors linked to HbA1c goal attainment of < 8% included appointment keeping, bringing glucose meter/glucose log book at least 80% of the time to these appointments, and following clinic instructions. This investigation also found that patients who attain glycemic goals generally do so within 6 months of enrollment. Furthermore, this study provided insight that following the clinic instructions a majority of the time strongly contributes to glycemic goal attainment. We believe that an assessment of patients’ behaviors prior to referrals to diabetes management programs will yield useful information about possible barriers to glycemic goal attainment.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Published 2017. Accessed September 25, 2018.
2. Gaspar JL, Dahlke ME, Kasper B. Efficacy of patient aligned care team pharmacist service in reaching diabetes and hyperlipidemia treatment goals. Fed Pract. 2015;32(11):42-47.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S6-S135.
4. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Published April 2017. Accessed September 7, 2018.
5. Centers for Disease Control and Prevention. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1-96.
6. Nigro SC, Garwood CL, Berlie H, et al. Clinical pharmacists as key members of the patient-centered medical home: an opinion statement of the Ambulatory Care Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(1):96-108.
7. Smith M, Bates DW, Bodenheimer T, et al. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29(5):906-913.
8. Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US Pharmacists’ effect as team members on patient care. Med Care. 2010;48(10):923-933.
9. Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 2008;28(4):421-436.
10. Touchette DR, Doloresco F, Suda KJ, et al. Economic evaluations of clinical pharmacy services: 2006-2010. Pharmacotherapy. 2014;34(8):771-793.
11. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report of the U.S. Surgeon General. American College of Clinical Pharmacy. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed September 10, 2018.
12. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc (2003). 2008;48(2):203-211.
13. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
14. Taveira TH, Friedmann PD, Cohen LB, et al. Pharmacist-led group medical appointment model in type 2 diabetes. Diabetes Educ. 2010;36(1):109-117.
15. Edwards KL, Hadley RL, Baby N, Yeary JC, Chastain LM, Brown CD. Utilizing clinical pharmacy specialists to address access to care barriers in the veteran population for the management of diabetes. J Pharm Pract. 2017;30(4):412-418.
16. Cripps RJ, Gourley ES, Johnson W, et al. An evaluation of diabetes-related measures of control after 6 months of clinical pharmacy specialist intervention. J Pharm Prac. 2011;24(3):332-338.
17. Jones H, Edwards L, Vallis TM, et al; Diabetes Stages of Change (DiSC) Study. Changes in diabetes self-care behaviors make a difference in glycemic control. Diabetes Care. 2003;26(3):732-737.
18. Schetman JM, Schorling JB, Voss JD. Appointment adherence and disparities in outcomes among patients with diabetes. J Gen Intern Med. 2008;23(10):1685-1687.
19. Rhee, MK, Slocum W, Zeimer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31(2):240-250.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Published 2017. Accessed September 25, 2018.
2. Gaspar JL, Dahlke ME, Kasper B. Efficacy of patient aligned care team pharmacist service in reaching diabetes and hyperlipidemia treatment goals. Fed Pract. 2015;32(11):42-47.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S6-S135.
4. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Published April 2017. Accessed September 7, 2018.
5. Centers for Disease Control and Prevention. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1-96.
6. Nigro SC, Garwood CL, Berlie H, et al. Clinical pharmacists as key members of the patient-centered medical home: an opinion statement of the Ambulatory Care Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(1):96-108.
7. Smith M, Bates DW, Bodenheimer T, et al. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29(5):906-913.
8. Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US Pharmacists’ effect as team members on patient care. Med Care. 2010;48(10):923-933.
9. Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 2008;28(4):421-436.
10. Touchette DR, Doloresco F, Suda KJ, et al. Economic evaluations of clinical pharmacy services: 2006-2010. Pharmacotherapy. 2014;34(8):771-793.
11. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report of the U.S. Surgeon General. American College of Clinical Pharmacy. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed September 10, 2018.
12. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc (2003). 2008;48(2):203-211.
13. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
14. Taveira TH, Friedmann PD, Cohen LB, et al. Pharmacist-led group medical appointment model in type 2 diabetes. Diabetes Educ. 2010;36(1):109-117.
15. Edwards KL, Hadley RL, Baby N, Yeary JC, Chastain LM, Brown CD. Utilizing clinical pharmacy specialists to address access to care barriers in the veteran population for the management of diabetes. J Pharm Pract. 2017;30(4):412-418.
16. Cripps RJ, Gourley ES, Johnson W, et al. An evaluation of diabetes-related measures of control after 6 months of clinical pharmacy specialist intervention. J Pharm Prac. 2011;24(3):332-338.
17. Jones H, Edwards L, Vallis TM, et al; Diabetes Stages of Change (DiSC) Study. Changes in diabetes self-care behaviors make a difference in glycemic control. Diabetes Care. 2003;26(3):732-737.
18. Schetman JM, Schorling JB, Voss JD. Appointment adherence and disparities in outcomes among patients with diabetes. J Gen Intern Med. 2008;23(10):1685-1687.
19. Rhee, MK, Slocum W, Zeimer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31(2):240-250.
New model for CKD risk draws on clinical, demographic factors
Data from more than 5 million individuals has been used to develop an equation for predicting the risk of incident chronic kidney disease (CKD) in people with or without diabetes, according to a presentation at Kidney Week 2019, sponsored by the American Society of Nephrology.
In a paper published simultaneously online in JAMA, researchers reported the outcome of an individual-level data analysis of 34 multinational cohorts involving 5,222,711 individuals – including 781,627 with diabetes – from 28 countries as part of the Chronic Kidney Disease Prognosis Consortium.
“An equation for kidney failure risk may help improve care for patients with established CKD, but relatively little work has been performed to develop predictive tools to identify those at increased risk of developing CKD – defined by reduced eGFR [estimated glomerular filtration rate], despite the high lifetime risk of CKD – which is estimated to be 59.1% in the United States,” wrote Robert G. Nelson, MD, PhD, from the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix and colleagues.
Over a mean follow-up of 4 years, 15% of individuals without diabetes and 40% of individuals with diabetes developed incident chronic kidney disease, defined as an eGFR below 60 mL/min per 1.73m2.
The key risk factors were older age, female sex, black race, hypertension, history of cardiovascular disease, lower eGFR values, and higher urine albumin to creatinine ratio. Smoking was also significantly associated with reduced eGFR but only in cohorts without diabetes. In cohorts with diabetes, elevated hemoglobin A1c and the presence and type of diabetes medication were also significantly associated with reduced eGFR.
Using this information, the researchers developed a prediction model built from weighted-average hazard ratios and validated it in nine external validation cohorts of 18 study populations involving a total of 2,253,540 individuals. They found that in 16 of the 18 study populations, the slope of observed to predicted risk ranged from 0.80 to 1.25.
Moreover, in the cohorts without diabetes, the risk equations had a median C-statistic for the 5-year predicted probability of 0.845 (interquartile range, 0.789-0.890) and of 0.801 (IQR, 0.750-0.819) in the cohorts with diabetes, the investigators reported.
“Several models have been developed for estimating the risk of prevalent and incident CKD and end-stage kidney disease, but even those with good discriminative performance have not always performed well for cohorts of people outside the original derivation cohort,” the authors wrote. They argued that their model “demonstrated high discrimination and variable calibration in diverse populations.”
However, they stressed that further study was needed to determine if use of the equations would actually lead to improvements in clinical care and patient outcomes. In an accompanying editorial, Sri Lekha Tummalapalli, MD, and Michelle M. Estrella, MD, of the Kidney Health Research Collaborative at the University of California, San Francisco, said the study and its focus on primary, rather than secondary, prevention of kidney disease is a critical step toward reducing the burden of that disease, especially given that an estimated 37 million people in the United States have chronic kidney disease.
It is also important, they added, that primary prevention of kidney disease is tailored to the individual patient’s risk because risk prediction and screening strategies are unlikely to improve outcomes if they are not paired with effective individualized interventions, such as lifestyle modification or management of blood pressure.
These risk equations could be more holistic by integrating the prediction of both elevated albuminuria and reduced eGFR because more than 40% of individuals with chronic kidney disease have increased albuminuria without reduced eGFR, they noted (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17378).
The study and CKD Prognosis Consortium were supported by the U.S. National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. One author was supported by a grant from the German Research Foundation. Nine authors declared grants, consultancies, and other support from the private sector and research organizations. No other conflicts of interest were declared. Dr. Tummalapalli and Dr. Estrella reported no conflicts of interest.
SOURCE: Nelson R et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17379.
Data from more than 5 million individuals has been used to develop an equation for predicting the risk of incident chronic kidney disease (CKD) in people with or without diabetes, according to a presentation at Kidney Week 2019, sponsored by the American Society of Nephrology.
In a paper published simultaneously online in JAMA, researchers reported the outcome of an individual-level data analysis of 34 multinational cohorts involving 5,222,711 individuals – including 781,627 with diabetes – from 28 countries as part of the Chronic Kidney Disease Prognosis Consortium.
“An equation for kidney failure risk may help improve care for patients with established CKD, but relatively little work has been performed to develop predictive tools to identify those at increased risk of developing CKD – defined by reduced eGFR [estimated glomerular filtration rate], despite the high lifetime risk of CKD – which is estimated to be 59.1% in the United States,” wrote Robert G. Nelson, MD, PhD, from the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix and colleagues.
Over a mean follow-up of 4 years, 15% of individuals without diabetes and 40% of individuals with diabetes developed incident chronic kidney disease, defined as an eGFR below 60 mL/min per 1.73m2.
The key risk factors were older age, female sex, black race, hypertension, history of cardiovascular disease, lower eGFR values, and higher urine albumin to creatinine ratio. Smoking was also significantly associated with reduced eGFR but only in cohorts without diabetes. In cohorts with diabetes, elevated hemoglobin A1c and the presence and type of diabetes medication were also significantly associated with reduced eGFR.
Using this information, the researchers developed a prediction model built from weighted-average hazard ratios and validated it in nine external validation cohorts of 18 study populations involving a total of 2,253,540 individuals. They found that in 16 of the 18 study populations, the slope of observed to predicted risk ranged from 0.80 to 1.25.
Moreover, in the cohorts without diabetes, the risk equations had a median C-statistic for the 5-year predicted probability of 0.845 (interquartile range, 0.789-0.890) and of 0.801 (IQR, 0.750-0.819) in the cohorts with diabetes, the investigators reported.
“Several models have been developed for estimating the risk of prevalent and incident CKD and end-stage kidney disease, but even those with good discriminative performance have not always performed well for cohorts of people outside the original derivation cohort,” the authors wrote. They argued that their model “demonstrated high discrimination and variable calibration in diverse populations.”
However, they stressed that further study was needed to determine if use of the equations would actually lead to improvements in clinical care and patient outcomes. In an accompanying editorial, Sri Lekha Tummalapalli, MD, and Michelle M. Estrella, MD, of the Kidney Health Research Collaborative at the University of California, San Francisco, said the study and its focus on primary, rather than secondary, prevention of kidney disease is a critical step toward reducing the burden of that disease, especially given that an estimated 37 million people in the United States have chronic kidney disease.
It is also important, they added, that primary prevention of kidney disease is tailored to the individual patient’s risk because risk prediction and screening strategies are unlikely to improve outcomes if they are not paired with effective individualized interventions, such as lifestyle modification or management of blood pressure.
These risk equations could be more holistic by integrating the prediction of both elevated albuminuria and reduced eGFR because more than 40% of individuals with chronic kidney disease have increased albuminuria without reduced eGFR, they noted (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17378).
The study and CKD Prognosis Consortium were supported by the U.S. National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. One author was supported by a grant from the German Research Foundation. Nine authors declared grants, consultancies, and other support from the private sector and research organizations. No other conflicts of interest were declared. Dr. Tummalapalli and Dr. Estrella reported no conflicts of interest.
SOURCE: Nelson R et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17379.
Data from more than 5 million individuals has been used to develop an equation for predicting the risk of incident chronic kidney disease (CKD) in people with or without diabetes, according to a presentation at Kidney Week 2019, sponsored by the American Society of Nephrology.
In a paper published simultaneously online in JAMA, researchers reported the outcome of an individual-level data analysis of 34 multinational cohorts involving 5,222,711 individuals – including 781,627 with diabetes – from 28 countries as part of the Chronic Kidney Disease Prognosis Consortium.
“An equation for kidney failure risk may help improve care for patients with established CKD, but relatively little work has been performed to develop predictive tools to identify those at increased risk of developing CKD – defined by reduced eGFR [estimated glomerular filtration rate], despite the high lifetime risk of CKD – which is estimated to be 59.1% in the United States,” wrote Robert G. Nelson, MD, PhD, from the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix and colleagues.
Over a mean follow-up of 4 years, 15% of individuals without diabetes and 40% of individuals with diabetes developed incident chronic kidney disease, defined as an eGFR below 60 mL/min per 1.73m2.
The key risk factors were older age, female sex, black race, hypertension, history of cardiovascular disease, lower eGFR values, and higher urine albumin to creatinine ratio. Smoking was also significantly associated with reduced eGFR but only in cohorts without diabetes. In cohorts with diabetes, elevated hemoglobin A1c and the presence and type of diabetes medication were also significantly associated with reduced eGFR.
Using this information, the researchers developed a prediction model built from weighted-average hazard ratios and validated it in nine external validation cohorts of 18 study populations involving a total of 2,253,540 individuals. They found that in 16 of the 18 study populations, the slope of observed to predicted risk ranged from 0.80 to 1.25.
Moreover, in the cohorts without diabetes, the risk equations had a median C-statistic for the 5-year predicted probability of 0.845 (interquartile range, 0.789-0.890) and of 0.801 (IQR, 0.750-0.819) in the cohorts with diabetes, the investigators reported.
“Several models have been developed for estimating the risk of prevalent and incident CKD and end-stage kidney disease, but even those with good discriminative performance have not always performed well for cohorts of people outside the original derivation cohort,” the authors wrote. They argued that their model “demonstrated high discrimination and variable calibration in diverse populations.”
However, they stressed that further study was needed to determine if use of the equations would actually lead to improvements in clinical care and patient outcomes. In an accompanying editorial, Sri Lekha Tummalapalli, MD, and Michelle M. Estrella, MD, of the Kidney Health Research Collaborative at the University of California, San Francisco, said the study and its focus on primary, rather than secondary, prevention of kidney disease is a critical step toward reducing the burden of that disease, especially given that an estimated 37 million people in the United States have chronic kidney disease.
It is also important, they added, that primary prevention of kidney disease is tailored to the individual patient’s risk because risk prediction and screening strategies are unlikely to improve outcomes if they are not paired with effective individualized interventions, such as lifestyle modification or management of blood pressure.
These risk equations could be more holistic by integrating the prediction of both elevated albuminuria and reduced eGFR because more than 40% of individuals with chronic kidney disease have increased albuminuria without reduced eGFR, they noted (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17378).
The study and CKD Prognosis Consortium were supported by the U.S. National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. One author was supported by a grant from the German Research Foundation. Nine authors declared grants, consultancies, and other support from the private sector and research organizations. No other conflicts of interest were declared. Dr. Tummalapalli and Dr. Estrella reported no conflicts of interest.
SOURCE: Nelson R et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17379.
REPORTING FROM KIDNEY WEEK 2019
Key clinical point:
Major finding: In the cohorts without diabetes, the risk equations had a median C-statistic for the 5-year predicted probability of 0.845 (interquartile range, 0.789-0.890), and of 0.801 (IQR, 0.750-0.819) in the cohorts with diabetes,
Study details: Analysis of cohort data from 5,222,711 individuals, including 781,627 with diabetes.
Disclosures: The study and CKD Prognosis Consortium were supported by the U.S. National Kidney Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. One author was supported by a grant from the German Research Foundation. Nine authors declared grants, consultancies, and other support from the private sector and research organizations. No other conflicts of interest were declared. Dr. Tummalapalli and Dr. Estrella reported no conflicts of interest.
Source: Nelson R et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17379.
Vitamin D, omega-3 fatty acids do not preserve kidney function in type 2 diabetes
A new study has found that neither vitamin D nor omega-3 fatty acids are significantly more beneficial than placebo for prevention and treatment of chronic kidney disease in patients with type 2 diabetes, according to Ian H. de Boer, MD, of the University of Washington, Seattle, and coauthors.
Findings of the study were presented at Kidney Week 2019, sponsored the American Society of Nephrology, and published simultaneously in JAMA.
To determine the benefits of either vitamin D or omega-3 fatty acids in regard to kidney function, the researchers conducted a randomized clinical trial of 1,312 patients with type 2 diabetes. The trial was designed to accompany the Vitamin D and Omega-3 Trial (VITAL), which enrolled 25,871 patients to test the two supplements in the prevention of cardiovascular disease and cancer.
Participants in this study – known as VITAL–Diabetic Kidney Disease, designed as an ancillary to VITAL – were assigned to one of four groups: vitamin D plus omega-3 fatty acids (n = 370), vitamin D plus placebo (n = 333), omega-3 fatty acids plus placebo (n = 289), or both placebos (n = 320). The goal was to assess changes in in glomerular filtration rate estimated from serum creatinine and cystatin C (eGFR) after 5 years.
Of the initial 1,312 participants, 934 (71%) finished the study. At 5-year follow-up, patients taking vitamin D had a mean change in eGFR of −12.3 mL/min per 1.73 m2 (95% confidence interval, −13.4 to −11.2), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −11.9) with placebo. Patients taking omega-3 fatty acids had a mean eGFR change of −12.2 mL/min per 1.73 m2 (95% CI, −13.3 to −11.1), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −12.0) with placebo.
The authors noted that the modest number of measurements collected per participant limited the evaluation and analyses. In addition, the study focused broadly on the type 2 diabetes population and not on subgroups, “who may derive more benefit from the study interventions.”
In an accompanying editorial, authors Anika Lucas, MD and Myles Wolf, MD, of Duke University in Durham, N.C., said multiple clinical trials, including this latest study from de Boer and colleagues on kidney function, have failed to reinforce the previously reported benefits of vitamin D.
“The VITAL-DKD study population had nearly normal mean 25-hydroxyvitamin D levels at baseline, leaving open the question of whether the results would have differed had recruitment been restricted to patients with moderate or severe vitamin D deficiency,” they wrote (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17302).
Nevertheless, it seems safe to conclude that the previous associations between vitamin D deficiency and adverse health were “driven by unmeasured residual confounding or reverse causality.
“Without certainty about the ideal approach to vitamin D treatment in advanced CKD, a randomized trial that compared cholecalciferol, exogenous 25-hydroxyvitamin D, and an activated vitamin D analogue vs. placebo could definitively lay to rest multiple remaining questions in the area,” they suggested.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, and equipment and supplies from various pharmaceutical companies and the National Institutes of Health. Dr. Wolf reported having served as a consultant for Akebia, AMAG, Amgen, Ardelyx, Diasorin, and Pharmacosmos. No other disclosures were reported.
SOURCE: de Boer IH et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17380.
A new study has found that neither vitamin D nor omega-3 fatty acids are significantly more beneficial than placebo for prevention and treatment of chronic kidney disease in patients with type 2 diabetes, according to Ian H. de Boer, MD, of the University of Washington, Seattle, and coauthors.
Findings of the study were presented at Kidney Week 2019, sponsored the American Society of Nephrology, and published simultaneously in JAMA.
To determine the benefits of either vitamin D or omega-3 fatty acids in regard to kidney function, the researchers conducted a randomized clinical trial of 1,312 patients with type 2 diabetes. The trial was designed to accompany the Vitamin D and Omega-3 Trial (VITAL), which enrolled 25,871 patients to test the two supplements in the prevention of cardiovascular disease and cancer.
Participants in this study – known as VITAL–Diabetic Kidney Disease, designed as an ancillary to VITAL – were assigned to one of four groups: vitamin D plus omega-3 fatty acids (n = 370), vitamin D plus placebo (n = 333), omega-3 fatty acids plus placebo (n = 289), or both placebos (n = 320). The goal was to assess changes in in glomerular filtration rate estimated from serum creatinine and cystatin C (eGFR) after 5 years.
Of the initial 1,312 participants, 934 (71%) finished the study. At 5-year follow-up, patients taking vitamin D had a mean change in eGFR of −12.3 mL/min per 1.73 m2 (95% confidence interval, −13.4 to −11.2), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −11.9) with placebo. Patients taking omega-3 fatty acids had a mean eGFR change of −12.2 mL/min per 1.73 m2 (95% CI, −13.3 to −11.1), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −12.0) with placebo.
The authors noted that the modest number of measurements collected per participant limited the evaluation and analyses. In addition, the study focused broadly on the type 2 diabetes population and not on subgroups, “who may derive more benefit from the study interventions.”
In an accompanying editorial, authors Anika Lucas, MD and Myles Wolf, MD, of Duke University in Durham, N.C., said multiple clinical trials, including this latest study from de Boer and colleagues on kidney function, have failed to reinforce the previously reported benefits of vitamin D.
“The VITAL-DKD study population had nearly normal mean 25-hydroxyvitamin D levels at baseline, leaving open the question of whether the results would have differed had recruitment been restricted to patients with moderate or severe vitamin D deficiency,” they wrote (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17302).
Nevertheless, it seems safe to conclude that the previous associations between vitamin D deficiency and adverse health were “driven by unmeasured residual confounding or reverse causality.
“Without certainty about the ideal approach to vitamin D treatment in advanced CKD, a randomized trial that compared cholecalciferol, exogenous 25-hydroxyvitamin D, and an activated vitamin D analogue vs. placebo could definitively lay to rest multiple remaining questions in the area,” they suggested.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, and equipment and supplies from various pharmaceutical companies and the National Institutes of Health. Dr. Wolf reported having served as a consultant for Akebia, AMAG, Amgen, Ardelyx, Diasorin, and Pharmacosmos. No other disclosures were reported.
SOURCE: de Boer IH et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17380.
A new study has found that neither vitamin D nor omega-3 fatty acids are significantly more beneficial than placebo for prevention and treatment of chronic kidney disease in patients with type 2 diabetes, according to Ian H. de Boer, MD, of the University of Washington, Seattle, and coauthors.
Findings of the study were presented at Kidney Week 2019, sponsored the American Society of Nephrology, and published simultaneously in JAMA.
To determine the benefits of either vitamin D or omega-3 fatty acids in regard to kidney function, the researchers conducted a randomized clinical trial of 1,312 patients with type 2 diabetes. The trial was designed to accompany the Vitamin D and Omega-3 Trial (VITAL), which enrolled 25,871 patients to test the two supplements in the prevention of cardiovascular disease and cancer.
Participants in this study – known as VITAL–Diabetic Kidney Disease, designed as an ancillary to VITAL – were assigned to one of four groups: vitamin D plus omega-3 fatty acids (n = 370), vitamin D plus placebo (n = 333), omega-3 fatty acids plus placebo (n = 289), or both placebos (n = 320). The goal was to assess changes in in glomerular filtration rate estimated from serum creatinine and cystatin C (eGFR) after 5 years.
Of the initial 1,312 participants, 934 (71%) finished the study. At 5-year follow-up, patients taking vitamin D had a mean change in eGFR of −12.3 mL/min per 1.73 m2 (95% confidence interval, −13.4 to −11.2), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −11.9) with placebo. Patients taking omega-3 fatty acids had a mean eGFR change of −12.2 mL/min per 1.73 m2 (95% CI, −13.3 to −11.1), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −12.0) with placebo.
The authors noted that the modest number of measurements collected per participant limited the evaluation and analyses. In addition, the study focused broadly on the type 2 diabetes population and not on subgroups, “who may derive more benefit from the study interventions.”
In an accompanying editorial, authors Anika Lucas, MD and Myles Wolf, MD, of Duke University in Durham, N.C., said multiple clinical trials, including this latest study from de Boer and colleagues on kidney function, have failed to reinforce the previously reported benefits of vitamin D.
“The VITAL-DKD study population had nearly normal mean 25-hydroxyvitamin D levels at baseline, leaving open the question of whether the results would have differed had recruitment been restricted to patients with moderate or severe vitamin D deficiency,” they wrote (JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17302).
Nevertheless, it seems safe to conclude that the previous associations between vitamin D deficiency and adverse health were “driven by unmeasured residual confounding or reverse causality.
“Without certainty about the ideal approach to vitamin D treatment in advanced CKD, a randomized trial that compared cholecalciferol, exogenous 25-hydroxyvitamin D, and an activated vitamin D analogue vs. placebo could definitively lay to rest multiple remaining questions in the area,” they suggested.
The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, and equipment and supplies from various pharmaceutical companies and the National Institutes of Health. Dr. Wolf reported having served as a consultant for Akebia, AMAG, Amgen, Ardelyx, Diasorin, and Pharmacosmos. No other disclosures were reported.
SOURCE: de Boer IH et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17380.
FROM KIDNEY WEEK 2019
Key clinical point:
Major finding: At 5-year follow-up, patients taking vitamin D had a mean change in eGFR of −12.3 mL/min per 1.73 m2 (95% CI, −13.4 to −11.2), compared with −13.1 mL/min per 1.73 m2 (95% CI, −14.2 to −11.9) with placebo.
Study details: A randomized clinical trial of 1,312 adults with type 2 diabetes.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases funded the study. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, and equipment and supplies from various pharmaceutical companies and the National Institutes of Health. Dr. Wolf reported having served as a consultant for Akebia, AMAG, Amgen, Ardelyx, Diasorin, and Pharmacosmos. No other disclosures were reported.
Source: de Boer IH et al. JAMA. 2019 Nov 8. doi: 10.1001/jama.2019.17380.
It’s time to get to know AI
This month’s cover story on artificial intelligence (AI) and machine learning provides a glimpse into the future of medical care. The article’s title, “An FP’s guide to AI-enabled clinical decision support” points to the fact that practical and useful applications of AI and machine learning are making inroads into medicine. However, other industries are far ahead of medicine when it comes to AI.
For example, I met with a financial advisor last week, and our discussion included a display of the likelihood that my wife and I would have sufficient funds in our retirement account based on a Monte Carlo simulation using 500 trials! In other words, our advisor used a huge database of financial information, analyzed that data with a sophisticated statistical technique, and applied the results to our personal situation. (No, we won’t run out of money—with 99% certainty.)
So as physicians, how can we further increase our certainty in the diagnoses we make and the guidance we offer our patients?
Halamka and Cerrato provide some insights. They discuss 2 clinical applications of AI and machine learning that are ready to use in primary care: screening for diabetic retinopathy and risk assessment for colon cancer. The first is an example of using AI for diagnosis and the second for risk assessment; both are core functions of primary care clinicians. These tools were developed with very sophisticated computer programs, but they are not unlike a plethora of clinical decision aids already widely used in primary care for diagnosis and risk assessment, such as the Ottawa Ankle Rules, the Gail Model for breast cancer risk, the FRAX tool for osteoporosis-related fracture risk, the ASCVD Risk Calculator for cardiovascular risk, and the CHA2DS2-VASC score for prediction of thrombosis and bleeding risk from anticoagulation therapy.
Some express concern that sophisticated AI could eventually replace primary care clinicians, similar to how automation reduces the need for routine labor in manufacturing. I think this is highly unlikely, but I do think AI will be widely deployed in clinical tools that improve our diagnostic accuracy and provide better personalized data to inform shared decision making. For example, the colon cancer risk calculator may actually help some patients decide NOT to be screened because their personal risk is so low.
It’s incumbent upon us, then, to familiarize ourselves with the potential that these AI tools offer. It’s time to get to know AI.
This month’s cover story on artificial intelligence (AI) and machine learning provides a glimpse into the future of medical care. The article’s title, “An FP’s guide to AI-enabled clinical decision support” points to the fact that practical and useful applications of AI and machine learning are making inroads into medicine. However, other industries are far ahead of medicine when it comes to AI.
For example, I met with a financial advisor last week, and our discussion included a display of the likelihood that my wife and I would have sufficient funds in our retirement account based on a Monte Carlo simulation using 500 trials! In other words, our advisor used a huge database of financial information, analyzed that data with a sophisticated statistical technique, and applied the results to our personal situation. (No, we won’t run out of money—with 99% certainty.)
So as physicians, how can we further increase our certainty in the diagnoses we make and the guidance we offer our patients?
Halamka and Cerrato provide some insights. They discuss 2 clinical applications of AI and machine learning that are ready to use in primary care: screening for diabetic retinopathy and risk assessment for colon cancer. The first is an example of using AI for diagnosis and the second for risk assessment; both are core functions of primary care clinicians. These tools were developed with very sophisticated computer programs, but they are not unlike a plethora of clinical decision aids already widely used in primary care for diagnosis and risk assessment, such as the Ottawa Ankle Rules, the Gail Model for breast cancer risk, the FRAX tool for osteoporosis-related fracture risk, the ASCVD Risk Calculator for cardiovascular risk, and the CHA2DS2-VASC score for prediction of thrombosis and bleeding risk from anticoagulation therapy.
Some express concern that sophisticated AI could eventually replace primary care clinicians, similar to how automation reduces the need for routine labor in manufacturing. I think this is highly unlikely, but I do think AI will be widely deployed in clinical tools that improve our diagnostic accuracy and provide better personalized data to inform shared decision making. For example, the colon cancer risk calculator may actually help some patients decide NOT to be screened because their personal risk is so low.
It’s incumbent upon us, then, to familiarize ourselves with the potential that these AI tools offer. It’s time to get to know AI.
This month’s cover story on artificial intelligence (AI) and machine learning provides a glimpse into the future of medical care. The article’s title, “An FP’s guide to AI-enabled clinical decision support” points to the fact that practical and useful applications of AI and machine learning are making inroads into medicine. However, other industries are far ahead of medicine when it comes to AI.
For example, I met with a financial advisor last week, and our discussion included a display of the likelihood that my wife and I would have sufficient funds in our retirement account based on a Monte Carlo simulation using 500 trials! In other words, our advisor used a huge database of financial information, analyzed that data with a sophisticated statistical technique, and applied the results to our personal situation. (No, we won’t run out of money—with 99% certainty.)
So as physicians, how can we further increase our certainty in the diagnoses we make and the guidance we offer our patients?
Halamka and Cerrato provide some insights. They discuss 2 clinical applications of AI and machine learning that are ready to use in primary care: screening for diabetic retinopathy and risk assessment for colon cancer. The first is an example of using AI for diagnosis and the second for risk assessment; both are core functions of primary care clinicians. These tools were developed with very sophisticated computer programs, but they are not unlike a plethora of clinical decision aids already widely used in primary care for diagnosis and risk assessment, such as the Ottawa Ankle Rules, the Gail Model for breast cancer risk, the FRAX tool for osteoporosis-related fracture risk, the ASCVD Risk Calculator for cardiovascular risk, and the CHA2DS2-VASC score for prediction of thrombosis and bleeding risk from anticoagulation therapy.
Some express concern that sophisticated AI could eventually replace primary care clinicians, similar to how automation reduces the need for routine labor in manufacturing. I think this is highly unlikely, but I do think AI will be widely deployed in clinical tools that improve our diagnostic accuracy and provide better personalized data to inform shared decision making. For example, the colon cancer risk calculator may actually help some patients decide NOT to be screened because their personal risk is so low.
It’s incumbent upon us, then, to familiarize ourselves with the potential that these AI tools offer. It’s time to get to know AI.
Duodenal mucosal resurfacing has metabolic effects in type 2 diabetes
BOSTON – An ablative procedure intended to promote regrowth of duodenal mucosa was safe and had disease-modifying metabolic effects in a randomized study including patients with type 2 diabetes, according to investigators.
A single duodenal mucosal resurfacing (DMR) procedure improved glycemic, hepatic, and body-weight measures at 24 weeks in the multicenter study, investigators will report at the annual meeting of the American Association for the Study of Liver Diseases.
The novel and minimally invasive endoscopic procedure treats the duodenum, which is increasingly recognized as a key metabolic signaling center, according to the study authors, including senior author Arun Sanyal, MD, professor in the gastroenterology division of the department of internal medicine at Virginia Commonwealth University, Richmond.
“Duodenal mucosal hyperplasia is a potential therapeutic target for insulin-resistance–related metabolic diseases,” Dr. Sanyal and coauthors said in a late-breaking abstract for the study published in the AASLD meeting proceedings.
In a previous international open-label, prospective, multicenter study, published in July in Gut, DMR was feasible and safe, producing durable glycemic improvement in patients with type 2 diabetes with suboptimal control on oral glucose-lowering mediation, according to investigators.
The present study, conducted at nine sites in the European Union and two in Brazil, is the first sham-controlled, double-blind, prospective study of the modality in patients with suboptimally controlled type 2 diabetes, according to Dr. Sanyal and coauthors.
A total of 39 patients in the study underwent DMR, while 36 underwent a sham procedure, according to the published abstract. The mean hemoglobin A1c for those patients was 8.3, the mean body mass index was 31.1 kg/m2, and most (77%) were male.
Median change in hemoglobin A1c from baseline to 24 weeks, one of two primary endpoints in the study, was –0.6% for DMR and –0.3% for the sham procedure (P less than 0.05), according to the study abstract.
Likewise, the primary efficacy endpoint of change in a nonalcoholic steatohepatitis biomarker favored the DMR arm. The median change in liver MRI–proton density fat fraction (MRI-PDFF) from baseline to 12 weeks was –5.4% for DMR and –2.4% for the sham procedure (P less than 0.05), according to the reported data.
Hypoglycemia rates were similar in the DMR and sham arms, and over 24 weeks of study, there were no unanticipated adverse effects attributable to the device and no serious adverse events, Dr. Sanyal and colleagues reported.
Dr. Sanyal reported disclosures related to Fractyl Laboratories, Sanyal Biotechnology, Exalenz Bioscience, Akarna Therapeutics, Genfit, Durect, Indalo, Tiziana, Novartis, Merck, Galectin Therapeutics, Janssen, and others.
BOSTON – An ablative procedure intended to promote regrowth of duodenal mucosa was safe and had disease-modifying metabolic effects in a randomized study including patients with type 2 diabetes, according to investigators.
A single duodenal mucosal resurfacing (DMR) procedure improved glycemic, hepatic, and body-weight measures at 24 weeks in the multicenter study, investigators will report at the annual meeting of the American Association for the Study of Liver Diseases.
The novel and minimally invasive endoscopic procedure treats the duodenum, which is increasingly recognized as a key metabolic signaling center, according to the study authors, including senior author Arun Sanyal, MD, professor in the gastroenterology division of the department of internal medicine at Virginia Commonwealth University, Richmond.
“Duodenal mucosal hyperplasia is a potential therapeutic target for insulin-resistance–related metabolic diseases,” Dr. Sanyal and coauthors said in a late-breaking abstract for the study published in the AASLD meeting proceedings.
In a previous international open-label, prospective, multicenter study, published in July in Gut, DMR was feasible and safe, producing durable glycemic improvement in patients with type 2 diabetes with suboptimal control on oral glucose-lowering mediation, according to investigators.
The present study, conducted at nine sites in the European Union and two in Brazil, is the first sham-controlled, double-blind, prospective study of the modality in patients with suboptimally controlled type 2 diabetes, according to Dr. Sanyal and coauthors.
A total of 39 patients in the study underwent DMR, while 36 underwent a sham procedure, according to the published abstract. The mean hemoglobin A1c for those patients was 8.3, the mean body mass index was 31.1 kg/m2, and most (77%) were male.
Median change in hemoglobin A1c from baseline to 24 weeks, one of two primary endpoints in the study, was –0.6% for DMR and –0.3% for the sham procedure (P less than 0.05), according to the study abstract.
Likewise, the primary efficacy endpoint of change in a nonalcoholic steatohepatitis biomarker favored the DMR arm. The median change in liver MRI–proton density fat fraction (MRI-PDFF) from baseline to 12 weeks was –5.4% for DMR and –2.4% for the sham procedure (P less than 0.05), according to the reported data.
Hypoglycemia rates were similar in the DMR and sham arms, and over 24 weeks of study, there were no unanticipated adverse effects attributable to the device and no serious adverse events, Dr. Sanyal and colleagues reported.
Dr. Sanyal reported disclosures related to Fractyl Laboratories, Sanyal Biotechnology, Exalenz Bioscience, Akarna Therapeutics, Genfit, Durect, Indalo, Tiziana, Novartis, Merck, Galectin Therapeutics, Janssen, and others.
BOSTON – An ablative procedure intended to promote regrowth of duodenal mucosa was safe and had disease-modifying metabolic effects in a randomized study including patients with type 2 diabetes, according to investigators.
A single duodenal mucosal resurfacing (DMR) procedure improved glycemic, hepatic, and body-weight measures at 24 weeks in the multicenter study, investigators will report at the annual meeting of the American Association for the Study of Liver Diseases.
The novel and minimally invasive endoscopic procedure treats the duodenum, which is increasingly recognized as a key metabolic signaling center, according to the study authors, including senior author Arun Sanyal, MD, professor in the gastroenterology division of the department of internal medicine at Virginia Commonwealth University, Richmond.
“Duodenal mucosal hyperplasia is a potential therapeutic target for insulin-resistance–related metabolic diseases,” Dr. Sanyal and coauthors said in a late-breaking abstract for the study published in the AASLD meeting proceedings.
In a previous international open-label, prospective, multicenter study, published in July in Gut, DMR was feasible and safe, producing durable glycemic improvement in patients with type 2 diabetes with suboptimal control on oral glucose-lowering mediation, according to investigators.
The present study, conducted at nine sites in the European Union and two in Brazil, is the first sham-controlled, double-blind, prospective study of the modality in patients with suboptimally controlled type 2 diabetes, according to Dr. Sanyal and coauthors.
A total of 39 patients in the study underwent DMR, while 36 underwent a sham procedure, according to the published abstract. The mean hemoglobin A1c for those patients was 8.3, the mean body mass index was 31.1 kg/m2, and most (77%) were male.
Median change in hemoglobin A1c from baseline to 24 weeks, one of two primary endpoints in the study, was –0.6% for DMR and –0.3% for the sham procedure (P less than 0.05), according to the study abstract.
Likewise, the primary efficacy endpoint of change in a nonalcoholic steatohepatitis biomarker favored the DMR arm. The median change in liver MRI–proton density fat fraction (MRI-PDFF) from baseline to 12 weeks was –5.4% for DMR and –2.4% for the sham procedure (P less than 0.05), according to the reported data.
Hypoglycemia rates were similar in the DMR and sham arms, and over 24 weeks of study, there were no unanticipated adverse effects attributable to the device and no serious adverse events, Dr. Sanyal and colleagues reported.
Dr. Sanyal reported disclosures related to Fractyl Laboratories, Sanyal Biotechnology, Exalenz Bioscience, Akarna Therapeutics, Genfit, Durect, Indalo, Tiziana, Novartis, Merck, Galectin Therapeutics, Janssen, and others.
REPORTING FROM THE LIVER MEETING 2019
Key clinical point: Duodenal mucosal resurfacing was safe and had disease-modifying metabolic effects in patients with type 2 diabetes.
Major finding: Results favored duodenal mucosal resurfacing over sham procedure for changes in median HbA1c (–0.6% vs. –0.3%; P less than .05) and liver MRI–proton density fat fraction (–5.4% vs. –2.4%; P less than 0.05).
Study details: A report on 75 patients treated in a randomized, sham-controlled, double-blind, prospective study.
Disclosures: Dr. Sanyal reported disclosures related to Fractyl Laboratories, Sanyal Biotechnology, Exalenz Bioscience, Akarna Therapeutics, Genfit, Durect, Indalo, Tiziana, Novartis, Merck, Galectin Therapeutics, Janssen, and others.
Source: Sanyal A et al. Liver Meeting 2019, Presentation LO2.
An FP’s guide to AI-enabled clinical decision support
Computer technology and artificial intelligence (AI) have come a long way in several decades:
- Between 1971 and 1996, access to the Medline database was primarily limited to university libraries and other institutions; in 1997, the database became universally available online as PubMed.1
- In 2004, the President of the United States issued an executive order that launched a 10-year plan to put electronic health records (EHRs) in place nationwide; EHRs are now employed in nearly 9 of 10 (85.9%) medical offices.2
Over time, numerous online resources sprouted as well, including DxPlain, UpToDate, and Clinical Key, to name a few. These digital tools were impressive for their time, but many of them are now considered “old-school” AI-enabled clinical decision support.
In the past 2 to 3 years, innovative clinicians and technologists have pushed medicine into a new era that takes advantage of machine learning (ML)-enhanced diagnostic aids, software systems that predict disease progression, and advanced clinical pathways to help individualize treatment. Enthusiastic early adopters believe these resources are transforming patient care—although skeptics remain unconvinced, cautioning that they have yet to prove their worth in everyday clinical practice.
In this review, we first analyze the strengths and weaknesses of evidence supporting these tools, then propose a potential role for them in family medicine.
Machine learning takes on retinopathy
The term “artificial intelligence” has been with us for longer than a half century.3 In the broadest sense, AI refers to any computer system capable of automating a process usually performed manually by humans. But the latest innovations in AI take advantage of a subset of AI called “machine learning”: the ability of software systems to learn new functionality or insights on their own, without additional programming from human data engineers. Case in point: A software platform has been developed that is capable of diagnosing or screening for diabetic retinopathy without the involvement of an experienced ophthalmologist.
The landmark study that started clinicians and health care executives thinking seriously about the potential role of ML in medical practice was spearheaded by Varun Gulshan, PhD, at Google, and associates from several medical schools.4 Gulshan used an artificial neural network designed to mimic the functions of the human nervous system to analyze more than 128,000 retinal images, looking for evidence of diabetic retinopathy. (See “Deciphering artificial neural networks,” for an explanation of how such networks function.5) The algorithm they employed was compared with the diagnostic skills of several board-certified ophthalmologists.
[polldaddy:10453606]
Continue to: Deciperhing artificial neural networks
Deciphering artificial neural networks
The promise of health care information technology relies heavily on statistical methods and software constructs, including logistic regression, random forest modeling, clustering, and neural networks. The machine learning-enabled image analysis used to detect diabetic retinopathy and to differentiate a malignant melanoma and a normal mole is based on neural networking.
As we discussed in the body of this article, these networks mimic the nervous system, in that they comprise computer-generated “neurons,” or nodes, and are connected by “synapses” (FIGURE5). When a node in Layer 1 is excited by pixels coming from a scanned image, it sends on that excitement, represented by a numerical value, to a second set of nodes in Layer 2, which, in turns, sends signals to the next layer— and so on.
Eventually, the software’s interpretation of the pixels of the image reaches the output layer of the network, generating a negative or positive diagnosis. The initial process results in many interpretations, which are corrected by a backward analytic process called backpropagation. The video tutorials mentioned in the main text provide a more detailed explanation of neural networking.
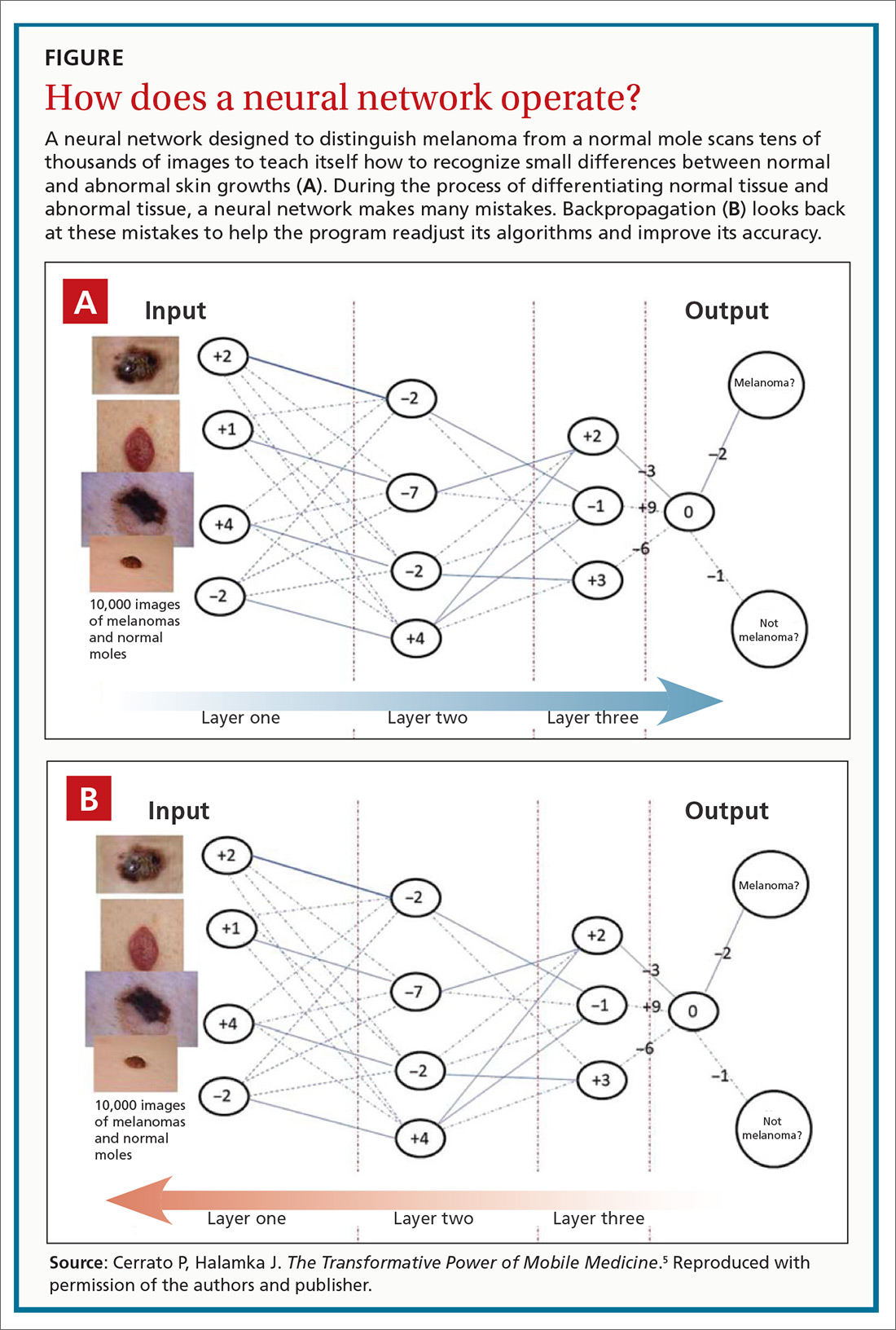
Using an area-under-the-receiver operating curve (AUROC) as a metric, and choosing an operating point for high specificity, the algorithm generated sensitivity of 87% and 90.3% and specificity of 98.1% and 98.5% for 2 validation data sets for detecting referable retinopathy, as defined by a panel of at least 7 ophthalmologists. When AUROC was set for high sensitivity, the algorithm generated sensitivity of 97.5% and 96.1% and specificity of 93.4% and 93.9% for the 2 data sets.
These results are impressive, but the researchers used a retrospective approach in their analysis. A prospective analysis would provide stronger evidence.
That shortcoming was addressed by a pivotal clinical trial that convinced the US Food and Drug Administration (FDA) to approve the technology. Michael Abramoff, MD, PhD, at the University of Iowa Department of Ophthalmology and Visual Sciences and his associates6 conducted a prospective study that compared the gold standard for detecting retinopathy, the Fundus Photograph Reading Center (of the University of Wisconsin School of Medicine and Public Health), to an ML-based algorithm, the commercialized IDx-DR. The IDx-DR is a software system that is used in combination with a fundal camera to capture retinal images. The researchers found that “the AI system exceeded all pre-specified superiority endpoints at sensitivity of 87.2% ... [and] specificity of 90.7% ....”
Continue to: The FDA clearance statement...
The FDA clearance statement for this technology7 limits its use, emphasizing that it is intended only as a screening tool, not a stand-alone diagnostic system. Because IDx-DR is being used in primary care, the FDA states that patients who have a positive result should be referred to an eye care professional. The technology is contraindicated in patients who have a history of laser treatment, surgery, or injection in the eye or who have any of the following: persistent vision loss, blurred vision, floaters, previously diagnosed macular edema, severe nonproliferative retinopathy, proliferative retinopathy, radiation retinopathy, and retinal vein occlusion. It is also not intended for pregnant patients because their eye disease often progresses rapidly.
Additional caveats to keep in mind when evaluating this new technology include that, although the software can help detect retinopathy, it does not address other key issues for this patient population, including cataracts and glaucoma. The cost of the new technology also requires attention: Software must be used in conjunction with a specific retinal camera, the Topcon TRC-NW400, which is expensive (new, as much as $20,000).
Speaking of cost: Health care providers and insurers still question whether implementing AI-enabled systems is cost-effective. It is too early to say definitively how AI and machine learning will have an impact on health care expenditures, because the most promising technological systems have yet to be fully implemented in hospitals and medical practices nationwide. Projections by Forbes suggest that private investment in health care AI will reach $6.6 billion by 2021; on a more confident note, an Accenture analysis predicts that the best possible application of AI might save the health care sector $150 billion annually by 2026.8
What role might this diabetic retinopathy technology play in family medicine? Physicians are constantly advising patients who have diabetes about the need to have a regular ophthalmic examination to check for early signs of retinopathy—advice that is often ignored. The American Academy of Ophthalmology points out that “6 out of 10 people with diabetes skip a sight-saving exam.”9 When a patient is screened with this type of device and found to be at high risk of eye disease, however, the advice to see an eye-care specialist might carry more weight.
Screening colonoscopy: Improving patient incentives
No responsible physician doubts the value of screening colonoscopy in patients 50 years and older, but many patients have yet to realize that the procedure just might save their life. Is there a way to incentivize resistant patients to have a colonoscopy performed? An ML-based software system that only requires access to a few readily available parameters might be the needed impetus for many patients.
Continue to: A large-scale validation...
A large-scale validation study performed on data from Kaiser Permanente Northwest found that it is possible to estimate a person’s risk of colorectal cancer by using age, gender, and complete blood count.10 This retrospective investigation analyzed more than 17,000 Kaiser Permanente patients, including 900 who already had colorectal cancer. The analysis generated a risk score for patients who did not have the malignancy to gauge their likelihood of developing it. The algorithms were more sensitive for detecting tumors of the cecum and ascending colon, and less sensitive for detection of tumors of the transverse and sigmoid colon and rectum.
To provide more definitive evidence to support the value of the software platform, a prospective study was subsequently conducted on more than 79,000 patients who had initially declined to undergo colorectal screening. The platform, called ColonFlag, was used to detect 688 patients at highest risk, who were then offered screening colonoscopy. In this subgroup, 254 agreed to the procedure; ColonFlag identified 19 malignancies (7.5%) among patients within the Maccabi Health System (Israel), and 15 more in patients outside that health system.11 (In the United States, the same program is known as LGI Flag and has been cleared by the FDA.)
Although ColonFlag has the potential to reduce the incidence of colorectal cancer, other evidence-based screening modalities are highlighted in US Preventive Services Task Force guidelines, including the guaiac-based fecal occult blood test and the fecal immunochemical test.12
Beyond screening to applications in managing disease
The complex etiology of sepsis makes the condition difficult to treat. That complexity has also led to disagreement on the best course of management. Using an ML algorithm called an “Artificial Intelligence Clinician,” Komorowski and associates13 extracted data from a large data set from 2 nonoverlapping intensive care unit databases collected from US adults.The researchers’ analysis suggested a list of 48 variables that likely influence sepsis outcomes, including:
- demographics,
- Elixhauser premorbid status,
- vital signs,
- clinical laboratory data,
- intravenous fluids given, and
- vasopressors administered.
Komorowski and co-workers concluded that “… mortality was lowest in patients for whom clinicians’ actual doses matched the AI decisions. Our model provides individualized and clinically interpretable treatment decisions for sepsis that could improve patient outcomes.”
A randomized clinical trial has found that an ML program that uses only 6 common clinical markers—blood pressure, heart rate, temperature, respiratory rate, peripheral capillary oxygen saturation (SpO2), and age—can improve clinical outcomes in patients with severe sepsis.14 The alerts generated by the algorithm were used to guide treatment. Average length of stay was 13 days in controls, compared with 10.3 days in those evaluated with the ML algorithm. The algorithm was also associated with a 12.4% drop in in-hospital mortality.
Continue to: Addressing challenges, tapping resources
Addressing challenges, tapping resources
Advances in the management of diabetic retinopathy, colorectal cancer, and sepsis are the tip of the AI iceberg. There are now ML programs to distinguish melanoma from benign nevi; to improve insulin dosing for patients with type 1 diabetes; to predict which hospital patients are most likely to end up in the intensive care unit; and to mitigate the opioid epidemic.
An ML Web page on the JAMA Network (https://sites.jamanetwork.com/machine-learning/) features a long list of published research studies, reviews, and opinion papers suggesting that the future of medicine is closely tied to innovative developments in this area. This Web page also addresses the potential use of ML in detecting lymph node metastases in breast cancer, the need to temper AI with human intelligence, the role of AI in clinical decision support, and more.
The JAMA Network also discusses a few of the challenges that still need to be overcome in developing ML tools for clinical medicine—challenges that you will want to be cognizant of as you evaluate new research in the field.
Black-box dilemma. A challenge that technologists face as they introduce new programs that have the potential to improve diagnosis, treatment, and prognosis is a phenomenon called the “black-box dilemma,” which refers to the complex data science, advanced statistics, and mathematical equations that underpin ML algorithms. These complexities make it difficult to explain the mechanism of action upon which software is based, which, in turn, makes many clinicians skeptical about its worth.
For example, the neural networks that are the backbone of the retinopathy algorithm discussed earlier might seem like voodoo science to those unfamiliar with the technology. It’s fortunate that several technology-savvy physicians have mastered these digital tools and have the teaching skills to explain them in plain-English tutorials. One such tutorial, “Understanding How Machine Learning Works,” is posted on the JAMA Network (https://sites.jamanetwork.com/machine-learning/#multimedia). A more basic explanation was included in a recent Public Broadcasting System “Nova” episode, viewable at www.youtube.com/watch?v=xS2G0oolHpo.
Continue to: Limited analysis
Limited analysis. Another problem that plagues many ML-based algorithms is that they have been tested on only a single data set. (Typically, a data set refers to a collection of clinical parameters from a patient population.) For example, researchers developing an algorithm might collect their data from a single health care system.
Several investigators have addressed this shortcoming by testing their software on 2 completely independent patient populations. Banda and colleagues15 recently developed a software platform to improve the detection rate in familial hypercholesterolemia, a significant cause of premature cardiovascular disease and death that affects approximately 1 of every 250 people. Despite the urgency of identifying the disorder and providing potentially lifesaving treatment, only 10% of patients receive an accurate diagnosis.16 Banda and colleagues developed a deep-learning algorithm that is far more effective than the traditional screening approach now in use.
To address the generalizability of the algorithm, it was tested on EHR data from 2 independent health care systems: Stanford Health Care and Geisinger Health System. In Stanford patients, the positive predictive value of the algorithm was 88%, with a sensitivity of 75%; it identified 84% of affected patients at the highest probability threshold. In Geisinger patients, the classifier generated a positive predictive value of 85%.
The future of these technologies
AI and ML are not panaceas that will revolutionize medicine in the near future. Likewise, the digital tools discussed in this article are not going to solve multiple complex medical problems addressed during a single office visit. But physicians who ignore mounting evidence that supports these emerging technologies will be left behind by more forward-thinking colleagues.
A recent commentary in Gastroenterology17 sums up the situation best: “It is now too conservative to suggest that CADe [computer-assisted detection] and CADx [computer-assisted diagnosis] carry the potential to revolutionize colonoscopy. The artificial intelligence revolution has already begun.”
CORRESPONDENCE
Paul Cerrato, MA, [email protected], [email protected]. John Halamka, MD, MS, [email protected].
1. Lindberg DA. Internet access to National Library of Medicine. Eff Clin Pract. 2000;3:256-260.
2. National Center for Health Statistics, Centers for Disease Control and Prevention. Electronic medical records/electronic health records (EMRs/EHRs). www.cdc.gov/nchs/fastats/electronic-medical-records.htm. Updated March 31, 2017. Accessed October 1, 2019.
3. Smith C, McGuire B, Huang T, et al. The history of artificial intelligence. University of Washington. https://courses.cs.washington.edu/courses/csep590/06au/projects/history-ai.pdf. Published December 2006. Accessed October 1, 2019.
4. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA; 2016;316:2402-2410.
5. Cerrato P, Halamka J. The Transformative Power of Mobile Medicine. Cambridge, MA: Academic Press; 2019.
6. Abràmoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
7. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. Press release. www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye. Published April 11, 2018. Accessed October 1, 2019.
8. AI and healthcare: a giant opportunity. Forbes Web site. www.forbes.com/sites/insights-intelai/2019/02/11/ai-and-healthcare-a-giant-opportunity/#5906c4014c68. Published February 11, 2019. Accessed October 25, 2019.
9. Boyd K. Six out of 10 people with diabetes skip a sight-saving exam. American Academy of Ophthalmology Website. https://www.aao.org/eye-health/news/sixty-percent-skip-diabetic-eye-exams. Published November 1, 2016. Accessed October 25, 2019.
10. Hornbrook MC, Goshen R, Choman E, et al. Early colorectal cancer detected by machine learning model using gender, age, and complete blood count data. Dig Dis Sci. 2017;62:2719-2727.
11. Goshen R, Choman E, Ran A, et al. Computer-assisted flagging of individuals at high risk of colorectal cancer in a large health maintenance organization using the ColonFlag test. JCO Clin Cancer Inform. 2018;2:1-8.
12. US Preventive Services Task Force. Final recommendation statement: colorectal cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2#tab. Published May 2019. Accessed October 1, 2019.
13. Komorowski M, Celi LA, Badawi O, et al. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24:1716-1720.
14. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4:e000234.
15. Banda J, Sarraju A, Abbasi F, et al. Finding missed cases of familial hypercholesterolemia in health systems using machine learning. NPJ Digit Med. 2019;2:23.
16. What is familial hypercholesterolemia? FH Foundation Web site. https://thefhfoundation.org/familial-hypercholesterolemia/what-is-familial-hypercholesterolemia. Accessed November 1, 2019.
17. Byrne MF, Shahidi N, Rex DK. Will computer-aided detection and diagnosis revolutionize colonoscopy? Gastroenterology. 2017;153:1460-1464.E1.
Computer technology and artificial intelligence (AI) have come a long way in several decades:
- Between 1971 and 1996, access to the Medline database was primarily limited to university libraries and other institutions; in 1997, the database became universally available online as PubMed.1
- In 2004, the President of the United States issued an executive order that launched a 10-year plan to put electronic health records (EHRs) in place nationwide; EHRs are now employed in nearly 9 of 10 (85.9%) medical offices.2
Over time, numerous online resources sprouted as well, including DxPlain, UpToDate, and Clinical Key, to name a few. These digital tools were impressive for their time, but many of them are now considered “old-school” AI-enabled clinical decision support.
In the past 2 to 3 years, innovative clinicians and technologists have pushed medicine into a new era that takes advantage of machine learning (ML)-enhanced diagnostic aids, software systems that predict disease progression, and advanced clinical pathways to help individualize treatment. Enthusiastic early adopters believe these resources are transforming patient care—although skeptics remain unconvinced, cautioning that they have yet to prove their worth in everyday clinical practice.
In this review, we first analyze the strengths and weaknesses of evidence supporting these tools, then propose a potential role for them in family medicine.
Machine learning takes on retinopathy
The term “artificial intelligence” has been with us for longer than a half century.3 In the broadest sense, AI refers to any computer system capable of automating a process usually performed manually by humans. But the latest innovations in AI take advantage of a subset of AI called “machine learning”: the ability of software systems to learn new functionality or insights on their own, without additional programming from human data engineers. Case in point: A software platform has been developed that is capable of diagnosing or screening for diabetic retinopathy without the involvement of an experienced ophthalmologist.
The landmark study that started clinicians and health care executives thinking seriously about the potential role of ML in medical practice was spearheaded by Varun Gulshan, PhD, at Google, and associates from several medical schools.4 Gulshan used an artificial neural network designed to mimic the functions of the human nervous system to analyze more than 128,000 retinal images, looking for evidence of diabetic retinopathy. (See “Deciphering artificial neural networks,” for an explanation of how such networks function.5) The algorithm they employed was compared with the diagnostic skills of several board-certified ophthalmologists.
[polldaddy:10453606]
Continue to: Deciperhing artificial neural networks
Deciphering artificial neural networks
The promise of health care information technology relies heavily on statistical methods and software constructs, including logistic regression, random forest modeling, clustering, and neural networks. The machine learning-enabled image analysis used to detect diabetic retinopathy and to differentiate a malignant melanoma and a normal mole is based on neural networking.
As we discussed in the body of this article, these networks mimic the nervous system, in that they comprise computer-generated “neurons,” or nodes, and are connected by “synapses” (FIGURE5). When a node in Layer 1 is excited by pixels coming from a scanned image, it sends on that excitement, represented by a numerical value, to a second set of nodes in Layer 2, which, in turns, sends signals to the next layer— and so on.
Eventually, the software’s interpretation of the pixels of the image reaches the output layer of the network, generating a negative or positive diagnosis. The initial process results in many interpretations, which are corrected by a backward analytic process called backpropagation. The video tutorials mentioned in the main text provide a more detailed explanation of neural networking.

Using an area-under-the-receiver operating curve (AUROC) as a metric, and choosing an operating point for high specificity, the algorithm generated sensitivity of 87% and 90.3% and specificity of 98.1% and 98.5% for 2 validation data sets for detecting referable retinopathy, as defined by a panel of at least 7 ophthalmologists. When AUROC was set for high sensitivity, the algorithm generated sensitivity of 97.5% and 96.1% and specificity of 93.4% and 93.9% for the 2 data sets.
These results are impressive, but the researchers used a retrospective approach in their analysis. A prospective analysis would provide stronger evidence.
That shortcoming was addressed by a pivotal clinical trial that convinced the US Food and Drug Administration (FDA) to approve the technology. Michael Abramoff, MD, PhD, at the University of Iowa Department of Ophthalmology and Visual Sciences and his associates6 conducted a prospective study that compared the gold standard for detecting retinopathy, the Fundus Photograph Reading Center (of the University of Wisconsin School of Medicine and Public Health), to an ML-based algorithm, the commercialized IDx-DR. The IDx-DR is a software system that is used in combination with a fundal camera to capture retinal images. The researchers found that “the AI system exceeded all pre-specified superiority endpoints at sensitivity of 87.2% ... [and] specificity of 90.7% ....”
Continue to: The FDA clearance statement...
The FDA clearance statement for this technology7 limits its use, emphasizing that it is intended only as a screening tool, not a stand-alone diagnostic system. Because IDx-DR is being used in primary care, the FDA states that patients who have a positive result should be referred to an eye care professional. The technology is contraindicated in patients who have a history of laser treatment, surgery, or injection in the eye or who have any of the following: persistent vision loss, blurred vision, floaters, previously diagnosed macular edema, severe nonproliferative retinopathy, proliferative retinopathy, radiation retinopathy, and retinal vein occlusion. It is also not intended for pregnant patients because their eye disease often progresses rapidly.
Additional caveats to keep in mind when evaluating this new technology include that, although the software can help detect retinopathy, it does not address other key issues for this patient population, including cataracts and glaucoma. The cost of the new technology also requires attention: Software must be used in conjunction with a specific retinal camera, the Topcon TRC-NW400, which is expensive (new, as much as $20,000).

Speaking of cost: Health care providers and insurers still question whether implementing AI-enabled systems is cost-effective. It is too early to say definitively how AI and machine learning will have an impact on health care expenditures, because the most promising technological systems have yet to be fully implemented in hospitals and medical practices nationwide. Projections by Forbes suggest that private investment in health care AI will reach $6.6 billion by 2021; on a more confident note, an Accenture analysis predicts that the best possible application of AI might save the health care sector $150 billion annually by 2026.8
What role might this diabetic retinopathy technology play in family medicine? Physicians are constantly advising patients who have diabetes about the need to have a regular ophthalmic examination to check for early signs of retinopathy—advice that is often ignored. The American Academy of Ophthalmology points out that “6 out of 10 people with diabetes skip a sight-saving exam.”9 When a patient is screened with this type of device and found to be at high risk of eye disease, however, the advice to see an eye-care specialist might carry more weight.
Screening colonoscopy: Improving patient incentives
No responsible physician doubts the value of screening colonoscopy in patients 50 years and older, but many patients have yet to realize that the procedure just might save their life. Is there a way to incentivize resistant patients to have a colonoscopy performed? An ML-based software system that only requires access to a few readily available parameters might be the needed impetus for many patients.
Continue to: A large-scale validation...
A large-scale validation study performed on data from Kaiser Permanente Northwest found that it is possible to estimate a person’s risk of colorectal cancer by using age, gender, and complete blood count.10 This retrospective investigation analyzed more than 17,000 Kaiser Permanente patients, including 900 who already had colorectal cancer. The analysis generated a risk score for patients who did not have the malignancy to gauge their likelihood of developing it. The algorithms were more sensitive for detecting tumors of the cecum and ascending colon, and less sensitive for detection of tumors of the transverse and sigmoid colon and rectum.
To provide more definitive evidence to support the value of the software platform, a prospective study was subsequently conducted on more than 79,000 patients who had initially declined to undergo colorectal screening. The platform, called ColonFlag, was used to detect 688 patients at highest risk, who were then offered screening colonoscopy. In this subgroup, 254 agreed to the procedure; ColonFlag identified 19 malignancies (7.5%) among patients within the Maccabi Health System (Israel), and 15 more in patients outside that health system.11 (In the United States, the same program is known as LGI Flag and has been cleared by the FDA.)
Although ColonFlag has the potential to reduce the incidence of colorectal cancer, other evidence-based screening modalities are highlighted in US Preventive Services Task Force guidelines, including the guaiac-based fecal occult blood test and the fecal immunochemical test.12
Beyond screening to applications in managing disease
The complex etiology of sepsis makes the condition difficult to treat. That complexity has also led to disagreement on the best course of management. Using an ML algorithm called an “Artificial Intelligence Clinician,” Komorowski and associates13 extracted data from a large data set from 2 nonoverlapping intensive care unit databases collected from US adults.The researchers’ analysis suggested a list of 48 variables that likely influence sepsis outcomes, including:
- demographics,
- Elixhauser premorbid status,
- vital signs,
- clinical laboratory data,
- intravenous fluids given, and
- vasopressors administered.
Komorowski and co-workers concluded that “… mortality was lowest in patients for whom clinicians’ actual doses matched the AI decisions. Our model provides individualized and clinically interpretable treatment decisions for sepsis that could improve patient outcomes.”
A randomized clinical trial has found that an ML program that uses only 6 common clinical markers—blood pressure, heart rate, temperature, respiratory rate, peripheral capillary oxygen saturation (SpO2), and age—can improve clinical outcomes in patients with severe sepsis.14 The alerts generated by the algorithm were used to guide treatment. Average length of stay was 13 days in controls, compared with 10.3 days in those evaluated with the ML algorithm. The algorithm was also associated with a 12.4% drop in in-hospital mortality.
Continue to: Addressing challenges, tapping resources
Addressing challenges, tapping resources
Advances in the management of diabetic retinopathy, colorectal cancer, and sepsis are the tip of the AI iceberg. There are now ML programs to distinguish melanoma from benign nevi; to improve insulin dosing for patients with type 1 diabetes; to predict which hospital patients are most likely to end up in the intensive care unit; and to mitigate the opioid epidemic.
An ML Web page on the JAMA Network (https://sites.jamanetwork.com/machine-learning/) features a long list of published research studies, reviews, and opinion papers suggesting that the future of medicine is closely tied to innovative developments in this area. This Web page also addresses the potential use of ML in detecting lymph node metastases in breast cancer, the need to temper AI with human intelligence, the role of AI in clinical decision support, and more.
The JAMA Network also discusses a few of the challenges that still need to be overcome in developing ML tools for clinical medicine—challenges that you will want to be cognizant of as you evaluate new research in the field.
Black-box dilemma. A challenge that technologists face as they introduce new programs that have the potential to improve diagnosis, treatment, and prognosis is a phenomenon called the “black-box dilemma,” which refers to the complex data science, advanced statistics, and mathematical equations that underpin ML algorithms. These complexities make it difficult to explain the mechanism of action upon which software is based, which, in turn, makes many clinicians skeptical about its worth.
For example, the neural networks that are the backbone of the retinopathy algorithm discussed earlier might seem like voodoo science to those unfamiliar with the technology. It’s fortunate that several technology-savvy physicians have mastered these digital tools and have the teaching skills to explain them in plain-English tutorials. One such tutorial, “Understanding How Machine Learning Works,” is posted on the JAMA Network (https://sites.jamanetwork.com/machine-learning/#multimedia). A more basic explanation was included in a recent Public Broadcasting System “Nova” episode, viewable at www.youtube.com/watch?v=xS2G0oolHpo.
Continue to: Limited analysis
Limited analysis. Another problem that plagues many ML-based algorithms is that they have been tested on only a single data set. (Typically, a data set refers to a collection of clinical parameters from a patient population.) For example, researchers developing an algorithm might collect their data from a single health care system.
Several investigators have addressed this shortcoming by testing their software on 2 completely independent patient populations. Banda and colleagues15 recently developed a software platform to improve the detection rate in familial hypercholesterolemia, a significant cause of premature cardiovascular disease and death that affects approximately 1 of every 250 people. Despite the urgency of identifying the disorder and providing potentially lifesaving treatment, only 10% of patients receive an accurate diagnosis.16 Banda and colleagues developed a deep-learning algorithm that is far more effective than the traditional screening approach now in use.
To address the generalizability of the algorithm, it was tested on EHR data from 2 independent health care systems: Stanford Health Care and Geisinger Health System. In Stanford patients, the positive predictive value of the algorithm was 88%, with a sensitivity of 75%; it identified 84% of affected patients at the highest probability threshold. In Geisinger patients, the classifier generated a positive predictive value of 85%.
The future of these technologies
AI and ML are not panaceas that will revolutionize medicine in the near future. Likewise, the digital tools discussed in this article are not going to solve multiple complex medical problems addressed during a single office visit. But physicians who ignore mounting evidence that supports these emerging technologies will be left behind by more forward-thinking colleagues.
A recent commentary in Gastroenterology17 sums up the situation best: “It is now too conservative to suggest that CADe [computer-assisted detection] and CADx [computer-assisted diagnosis] carry the potential to revolutionize colonoscopy. The artificial intelligence revolution has already begun.”
CORRESPONDENCE
Paul Cerrato, MA, [email protected], [email protected]. John Halamka, MD, MS, [email protected].
Computer technology and artificial intelligence (AI) have come a long way in several decades:
- Between 1971 and 1996, access to the Medline database was primarily limited to university libraries and other institutions; in 1997, the database became universally available online as PubMed.1
- In 2004, the President of the United States issued an executive order that launched a 10-year plan to put electronic health records (EHRs) in place nationwide; EHRs are now employed in nearly 9 of 10 (85.9%) medical offices.2
Over time, numerous online resources sprouted as well, including DxPlain, UpToDate, and Clinical Key, to name a few. These digital tools were impressive for their time, but many of them are now considered “old-school” AI-enabled clinical decision support.
In the past 2 to 3 years, innovative clinicians and technologists have pushed medicine into a new era that takes advantage of machine learning (ML)-enhanced diagnostic aids, software systems that predict disease progression, and advanced clinical pathways to help individualize treatment. Enthusiastic early adopters believe these resources are transforming patient care—although skeptics remain unconvinced, cautioning that they have yet to prove their worth in everyday clinical practice.
In this review, we first analyze the strengths and weaknesses of evidence supporting these tools, then propose a potential role for them in family medicine.
Machine learning takes on retinopathy
The term “artificial intelligence” has been with us for longer than a half century.3 In the broadest sense, AI refers to any computer system capable of automating a process usually performed manually by humans. But the latest innovations in AI take advantage of a subset of AI called “machine learning”: the ability of software systems to learn new functionality or insights on their own, without additional programming from human data engineers. Case in point: A software platform has been developed that is capable of diagnosing or screening for diabetic retinopathy without the involvement of an experienced ophthalmologist.
The landmark study that started clinicians and health care executives thinking seriously about the potential role of ML in medical practice was spearheaded by Varun Gulshan, PhD, at Google, and associates from several medical schools.4 Gulshan used an artificial neural network designed to mimic the functions of the human nervous system to analyze more than 128,000 retinal images, looking for evidence of diabetic retinopathy. (See “Deciphering artificial neural networks,” for an explanation of how such networks function.5) The algorithm they employed was compared with the diagnostic skills of several board-certified ophthalmologists.
[polldaddy:10453606]
Continue to: Deciperhing artificial neural networks
Deciphering artificial neural networks
The promise of health care information technology relies heavily on statistical methods and software constructs, including logistic regression, random forest modeling, clustering, and neural networks. The machine learning-enabled image analysis used to detect diabetic retinopathy and to differentiate a malignant melanoma and a normal mole is based on neural networking.
As we discussed in the body of this article, these networks mimic the nervous system, in that they comprise computer-generated “neurons,” or nodes, and are connected by “synapses” (FIGURE5). When a node in Layer 1 is excited by pixels coming from a scanned image, it sends on that excitement, represented by a numerical value, to a second set of nodes in Layer 2, which, in turns, sends signals to the next layer— and so on.
Eventually, the software’s interpretation of the pixels of the image reaches the output layer of the network, generating a negative or positive diagnosis. The initial process results in many interpretations, which are corrected by a backward analytic process called backpropagation. The video tutorials mentioned in the main text provide a more detailed explanation of neural networking.

Using an area-under-the-receiver operating curve (AUROC) as a metric, and choosing an operating point for high specificity, the algorithm generated sensitivity of 87% and 90.3% and specificity of 98.1% and 98.5% for 2 validation data sets for detecting referable retinopathy, as defined by a panel of at least 7 ophthalmologists. When AUROC was set for high sensitivity, the algorithm generated sensitivity of 97.5% and 96.1% and specificity of 93.4% and 93.9% for the 2 data sets.
These results are impressive, but the researchers used a retrospective approach in their analysis. A prospective analysis would provide stronger evidence.
That shortcoming was addressed by a pivotal clinical trial that convinced the US Food and Drug Administration (FDA) to approve the technology. Michael Abramoff, MD, PhD, at the University of Iowa Department of Ophthalmology and Visual Sciences and his associates6 conducted a prospective study that compared the gold standard for detecting retinopathy, the Fundus Photograph Reading Center (of the University of Wisconsin School of Medicine and Public Health), to an ML-based algorithm, the commercialized IDx-DR. The IDx-DR is a software system that is used in combination with a fundal camera to capture retinal images. The researchers found that “the AI system exceeded all pre-specified superiority endpoints at sensitivity of 87.2% ... [and] specificity of 90.7% ....”
Continue to: The FDA clearance statement...
The FDA clearance statement for this technology7 limits its use, emphasizing that it is intended only as a screening tool, not a stand-alone diagnostic system. Because IDx-DR is being used in primary care, the FDA states that patients who have a positive result should be referred to an eye care professional. The technology is contraindicated in patients who have a history of laser treatment, surgery, or injection in the eye or who have any of the following: persistent vision loss, blurred vision, floaters, previously diagnosed macular edema, severe nonproliferative retinopathy, proliferative retinopathy, radiation retinopathy, and retinal vein occlusion. It is also not intended for pregnant patients because their eye disease often progresses rapidly.
Additional caveats to keep in mind when evaluating this new technology include that, although the software can help detect retinopathy, it does not address other key issues for this patient population, including cataracts and glaucoma. The cost of the new technology also requires attention: Software must be used in conjunction with a specific retinal camera, the Topcon TRC-NW400, which is expensive (new, as much as $20,000).

Speaking of cost: Health care providers and insurers still question whether implementing AI-enabled systems is cost-effective. It is too early to say definitively how AI and machine learning will have an impact on health care expenditures, because the most promising technological systems have yet to be fully implemented in hospitals and medical practices nationwide. Projections by Forbes suggest that private investment in health care AI will reach $6.6 billion by 2021; on a more confident note, an Accenture analysis predicts that the best possible application of AI might save the health care sector $150 billion annually by 2026.8
What role might this diabetic retinopathy technology play in family medicine? Physicians are constantly advising patients who have diabetes about the need to have a regular ophthalmic examination to check for early signs of retinopathy—advice that is often ignored. The American Academy of Ophthalmology points out that “6 out of 10 people with diabetes skip a sight-saving exam.”9 When a patient is screened with this type of device and found to be at high risk of eye disease, however, the advice to see an eye-care specialist might carry more weight.
Screening colonoscopy: Improving patient incentives
No responsible physician doubts the value of screening colonoscopy in patients 50 years and older, but many patients have yet to realize that the procedure just might save their life. Is there a way to incentivize resistant patients to have a colonoscopy performed? An ML-based software system that only requires access to a few readily available parameters might be the needed impetus for many patients.
Continue to: A large-scale validation...
A large-scale validation study performed on data from Kaiser Permanente Northwest found that it is possible to estimate a person’s risk of colorectal cancer by using age, gender, and complete blood count.10 This retrospective investigation analyzed more than 17,000 Kaiser Permanente patients, including 900 who already had colorectal cancer. The analysis generated a risk score for patients who did not have the malignancy to gauge their likelihood of developing it. The algorithms were more sensitive for detecting tumors of the cecum and ascending colon, and less sensitive for detection of tumors of the transverse and sigmoid colon and rectum.
To provide more definitive evidence to support the value of the software platform, a prospective study was subsequently conducted on more than 79,000 patients who had initially declined to undergo colorectal screening. The platform, called ColonFlag, was used to detect 688 patients at highest risk, who were then offered screening colonoscopy. In this subgroup, 254 agreed to the procedure; ColonFlag identified 19 malignancies (7.5%) among patients within the Maccabi Health System (Israel), and 15 more in patients outside that health system.11 (In the United States, the same program is known as LGI Flag and has been cleared by the FDA.)
Although ColonFlag has the potential to reduce the incidence of colorectal cancer, other evidence-based screening modalities are highlighted in US Preventive Services Task Force guidelines, including the guaiac-based fecal occult blood test and the fecal immunochemical test.12
Beyond screening to applications in managing disease
The complex etiology of sepsis makes the condition difficult to treat. That complexity has also led to disagreement on the best course of management. Using an ML algorithm called an “Artificial Intelligence Clinician,” Komorowski and associates13 extracted data from a large data set from 2 nonoverlapping intensive care unit databases collected from US adults.The researchers’ analysis suggested a list of 48 variables that likely influence sepsis outcomes, including:
- demographics,
- Elixhauser premorbid status,
- vital signs,
- clinical laboratory data,
- intravenous fluids given, and
- vasopressors administered.
Komorowski and co-workers concluded that “… mortality was lowest in patients for whom clinicians’ actual doses matched the AI decisions. Our model provides individualized and clinically interpretable treatment decisions for sepsis that could improve patient outcomes.”
A randomized clinical trial has found that an ML program that uses only 6 common clinical markers—blood pressure, heart rate, temperature, respiratory rate, peripheral capillary oxygen saturation (SpO2), and age—can improve clinical outcomes in patients with severe sepsis.14 The alerts generated by the algorithm were used to guide treatment. Average length of stay was 13 days in controls, compared with 10.3 days in those evaluated with the ML algorithm. The algorithm was also associated with a 12.4% drop in in-hospital mortality.
Continue to: Addressing challenges, tapping resources
Addressing challenges, tapping resources
Advances in the management of diabetic retinopathy, colorectal cancer, and sepsis are the tip of the AI iceberg. There are now ML programs to distinguish melanoma from benign nevi; to improve insulin dosing for patients with type 1 diabetes; to predict which hospital patients are most likely to end up in the intensive care unit; and to mitigate the opioid epidemic.
An ML Web page on the JAMA Network (https://sites.jamanetwork.com/machine-learning/) features a long list of published research studies, reviews, and opinion papers suggesting that the future of medicine is closely tied to innovative developments in this area. This Web page also addresses the potential use of ML in detecting lymph node metastases in breast cancer, the need to temper AI with human intelligence, the role of AI in clinical decision support, and more.
The JAMA Network also discusses a few of the challenges that still need to be overcome in developing ML tools for clinical medicine—challenges that you will want to be cognizant of as you evaluate new research in the field.
Black-box dilemma. A challenge that technologists face as they introduce new programs that have the potential to improve diagnosis, treatment, and prognosis is a phenomenon called the “black-box dilemma,” which refers to the complex data science, advanced statistics, and mathematical equations that underpin ML algorithms. These complexities make it difficult to explain the mechanism of action upon which software is based, which, in turn, makes many clinicians skeptical about its worth.
For example, the neural networks that are the backbone of the retinopathy algorithm discussed earlier might seem like voodoo science to those unfamiliar with the technology. It’s fortunate that several technology-savvy physicians have mastered these digital tools and have the teaching skills to explain them in plain-English tutorials. One such tutorial, “Understanding How Machine Learning Works,” is posted on the JAMA Network (https://sites.jamanetwork.com/machine-learning/#multimedia). A more basic explanation was included in a recent Public Broadcasting System “Nova” episode, viewable at www.youtube.com/watch?v=xS2G0oolHpo.
Continue to: Limited analysis
Limited analysis. Another problem that plagues many ML-based algorithms is that they have been tested on only a single data set. (Typically, a data set refers to a collection of clinical parameters from a patient population.) For example, researchers developing an algorithm might collect their data from a single health care system.
Several investigators have addressed this shortcoming by testing their software on 2 completely independent patient populations. Banda and colleagues15 recently developed a software platform to improve the detection rate in familial hypercholesterolemia, a significant cause of premature cardiovascular disease and death that affects approximately 1 of every 250 people. Despite the urgency of identifying the disorder and providing potentially lifesaving treatment, only 10% of patients receive an accurate diagnosis.16 Banda and colleagues developed a deep-learning algorithm that is far more effective than the traditional screening approach now in use.
To address the generalizability of the algorithm, it was tested on EHR data from 2 independent health care systems: Stanford Health Care and Geisinger Health System. In Stanford patients, the positive predictive value of the algorithm was 88%, with a sensitivity of 75%; it identified 84% of affected patients at the highest probability threshold. In Geisinger patients, the classifier generated a positive predictive value of 85%.
The future of these technologies
AI and ML are not panaceas that will revolutionize medicine in the near future. Likewise, the digital tools discussed in this article are not going to solve multiple complex medical problems addressed during a single office visit. But physicians who ignore mounting evidence that supports these emerging technologies will be left behind by more forward-thinking colleagues.
A recent commentary in Gastroenterology17 sums up the situation best: “It is now too conservative to suggest that CADe [computer-assisted detection] and CADx [computer-assisted diagnosis] carry the potential to revolutionize colonoscopy. The artificial intelligence revolution has already begun.”
CORRESPONDENCE
Paul Cerrato, MA, [email protected], [email protected]. John Halamka, MD, MS, [email protected].
1. Lindberg DA. Internet access to National Library of Medicine. Eff Clin Pract. 2000;3:256-260.
2. National Center for Health Statistics, Centers for Disease Control and Prevention. Electronic medical records/electronic health records (EMRs/EHRs). www.cdc.gov/nchs/fastats/electronic-medical-records.htm. Updated March 31, 2017. Accessed October 1, 2019.
3. Smith C, McGuire B, Huang T, et al. The history of artificial intelligence. University of Washington. https://courses.cs.washington.edu/courses/csep590/06au/projects/history-ai.pdf. Published December 2006. Accessed October 1, 2019.
4. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA; 2016;316:2402-2410.
5. Cerrato P, Halamka J. The Transformative Power of Mobile Medicine. Cambridge, MA: Academic Press; 2019.
6. Abràmoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
7. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. Press release. www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye. Published April 11, 2018. Accessed October 1, 2019.
8. AI and healthcare: a giant opportunity. Forbes Web site. www.forbes.com/sites/insights-intelai/2019/02/11/ai-and-healthcare-a-giant-opportunity/#5906c4014c68. Published February 11, 2019. Accessed October 25, 2019.
9. Boyd K. Six out of 10 people with diabetes skip a sight-saving exam. American Academy of Ophthalmology Website. https://www.aao.org/eye-health/news/sixty-percent-skip-diabetic-eye-exams. Published November 1, 2016. Accessed October 25, 2019.
10. Hornbrook MC, Goshen R, Choman E, et al. Early colorectal cancer detected by machine learning model using gender, age, and complete blood count data. Dig Dis Sci. 2017;62:2719-2727.
11. Goshen R, Choman E, Ran A, et al. Computer-assisted flagging of individuals at high risk of colorectal cancer in a large health maintenance organization using the ColonFlag test. JCO Clin Cancer Inform. 2018;2:1-8.
12. US Preventive Services Task Force. Final recommendation statement: colorectal cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2#tab. Published May 2019. Accessed October 1, 2019.
13. Komorowski M, Celi LA, Badawi O, et al. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24:1716-1720.
14. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4:e000234.
15. Banda J, Sarraju A, Abbasi F, et al. Finding missed cases of familial hypercholesterolemia in health systems using machine learning. NPJ Digit Med. 2019;2:23.
16. What is familial hypercholesterolemia? FH Foundation Web site. https://thefhfoundation.org/familial-hypercholesterolemia/what-is-familial-hypercholesterolemia. Accessed November 1, 2019.
17. Byrne MF, Shahidi N, Rex DK. Will computer-aided detection and diagnosis revolutionize colonoscopy? Gastroenterology. 2017;153:1460-1464.E1.
1. Lindberg DA. Internet access to National Library of Medicine. Eff Clin Pract. 2000;3:256-260.
2. National Center for Health Statistics, Centers for Disease Control and Prevention. Electronic medical records/electronic health records (EMRs/EHRs). www.cdc.gov/nchs/fastats/electronic-medical-records.htm. Updated March 31, 2017. Accessed October 1, 2019.
3. Smith C, McGuire B, Huang T, et al. The history of artificial intelligence. University of Washington. https://courses.cs.washington.edu/courses/csep590/06au/projects/history-ai.pdf. Published December 2006. Accessed October 1, 2019.
4. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA; 2016;316:2402-2410.
5. Cerrato P, Halamka J. The Transformative Power of Mobile Medicine. Cambridge, MA: Academic Press; 2019.
6. Abràmoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
7. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. Press release. www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye. Published April 11, 2018. Accessed October 1, 2019.
8. AI and healthcare: a giant opportunity. Forbes Web site. www.forbes.com/sites/insights-intelai/2019/02/11/ai-and-healthcare-a-giant-opportunity/#5906c4014c68. Published February 11, 2019. Accessed October 25, 2019.
9. Boyd K. Six out of 10 people with diabetes skip a sight-saving exam. American Academy of Ophthalmology Website. https://www.aao.org/eye-health/news/sixty-percent-skip-diabetic-eye-exams. Published November 1, 2016. Accessed October 25, 2019.
10. Hornbrook MC, Goshen R, Choman E, et al. Early colorectal cancer detected by machine learning model using gender, age, and complete blood count data. Dig Dis Sci. 2017;62:2719-2727.
11. Goshen R, Choman E, Ran A, et al. Computer-assisted flagging of individuals at high risk of colorectal cancer in a large health maintenance organization using the ColonFlag test. JCO Clin Cancer Inform. 2018;2:1-8.
12. US Preventive Services Task Force. Final recommendation statement: colorectal cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2#tab. Published May 2019. Accessed October 1, 2019.
13. Komorowski M, Celi LA, Badawi O, et al. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24:1716-1720.
14. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4:e000234.
15. Banda J, Sarraju A, Abbasi F, et al. Finding missed cases of familial hypercholesterolemia in health systems using machine learning. NPJ Digit Med. 2019;2:23.
16. What is familial hypercholesterolemia? FH Foundation Web site. https://thefhfoundation.org/familial-hypercholesterolemia/what-is-familial-hypercholesterolemia. Accessed November 1, 2019.
17. Byrne MF, Shahidi N, Rex DK. Will computer-aided detection and diagnosis revolutionize colonoscopy? Gastroenterology. 2017;153:1460-1464.E1.
PRACTICE RECOMMENDATIONS
› Encourage patients with diabetes who are unwilling to have a regular eye exam to have an artificial intelligence-based retinal scan that can detect retinopathy. B
› Consider using a machine learning-based algorithm to help evaluate the risk of colorectal cancer in patients who are resistant to screening colonoscopy. B
› Question the effectiveness of any artificial intelligence-based software algorithm that has not been validated by at least 2 independent data sets derived from clinical parameters. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Cardiometabolic risk burden is high in under-50s with type 2 diabetes
BARCELONA – People diagnosed with type 2 diabetes when they are 18-39 years old have significantly higher cardiometabolic risk burden, compared with older people, according to the results of a large study from the United Kingdom presented at the annual meeting of the European Association for the Study of Diabetes.
Patients in that younger age group were found to have higher glycated hemoglobin (HbA1c) levels, along with higher levels of low-density lipoprotein cholesterol and higher body weight.
“We wanted to evaluate the population-level trend in the incidence of young-onset type 2 diabetes in the United Kingdom, compared with later-onset diabetes,” said senior study author Sanjoy Paul, PhD, the director of the Melbourne EpiCentre at the University of Melbourne at a press briefing during the meeting.
Other aims of the study were to compare temporal trends in the incidence of atherosclerotic cardiovascular disease in younger and older patients with type 2 diabetes, and to see how being “high risk” at diagnosis affected patients’ risk of ASCVD and subsequent risk of death.
High-risk status was defined as having at least two of the risk factors for ASCVD – smoking, high systolic blood pressure, high low-density lipoprotein cholesterol, or chronic kidney disease.
The investigators searched a nationally representative sample of primary care electronic medical records from The Health Improvement Network (THIN) database to find incident cases of type 2 diabetes that occurred between 2000 and 2017, with a total of 370,854 cases identified.
At diagnosis of type 2 diabetes, 8% of the sample (n = 29,678) was aged 18-39 years; 15% (n = 56,798), 40-49 years; 25% (n = 93,698), 50-59 years; 29% (n = 107,261), 60-69 years; and 23% (n = 83,419), 70-79 years. Follow-up was just more than 6 years.
Baseline HbA1c in the respective age groups was 8.6%, 8.4%, 8.1%, 7.8%, and 7.6%, with more than 55% of patients in the two youngest age groups having an HbA1c of 7.5% or higher, compared with 34%-47% in the three oldest age groups.
The percentage of patients with a high LDL cholesterol value (2.6 mmol/L or higher in those without ASCVD, and 1.8 or higher in those with ASCVD) was 71%, 75%, 74%, 69%, and 65%, from the youngest to oldest age groups. A respective 71%, 70%, 66%, 57%, and 44% of the patients had a body mass index of 35 kg/m2 or higher.
Few younger patients had ASCVD at diagnosis (2% of the 18-39 age group; 6% of the 40-49 group), with higher rates in the older age groups (13% of the 50-59 group; 23% of the 60-69 group; and 33% of the 70-79 group).
The percentage of patients considered to be at high risk of ASCVD at diagnosis was 23%, 37%, 45%, 50%, and 53%, respectively, across the five age groups.
Although high systolic blood pressure (SBP; 130 mmHg in those with ASCVD, 140 mmHg in those without) was more common in the older age groups (52% at 50-59 years; 60% at 60-69 years, and 64% at 70-79 years,) a substantial proportion of the younger patients also had a high SBP (27% at 18-39 years and 41% at 40-49 years).
Digsu Koye, PhD, also of the Melbourne EpiCentre, presented the main findings of the study during the meeting, noting that the proportion of people diagnosed when they were younger than 50 years remained stable between 2000 and 2017, with a marginal increase in those diagnosed when they were aged 50-59 years, and a decline in those diagnosed when they were older than 70 years.
In the youngest and oldest age groups, equal numbers of men and women were diagnosed with type 2 diabetes, and more women than men were diagnosed in the 60-69 age group, Dr. Koye said. However, for the 40-49 and 50-59 age groups, there were more men than women diagnosed with type 2 diabetes.
Patients were followed for an average of just more than 6 years. “The rate of atherosclerotic cardiovascular disease was declining in all age categories during 2000-2006, but after that, we saw a stable and consistent pattern for all age categories after 2007,” Dr. Koye observed.
In regard to all-cause mortality, there was a 30% decline in the oldest age group (70-79 years), and a 20% decline in the 60-69 age group, but there was no significant decline in the younger age groups, he added.
The investigators determined the average time to event (ASCVD or all-cause mortality) by high-risk status at type 2 diabetes diagnosis for each age group. These analyses showed that there was little difference between the high- and low-risk groups for the average time to ASCVD or all-cause mortality in the youngest age group, with wider differences in the older patients of 1-2 years for ASCVD and 0.5-2 years for all-cause mortality.
Dr. Koye noted that people with young-onset type 2 diabetes had a risk of ASCVD or all-cause mortality that was similar to that of older people, irrespective of whether or not they were considered to be at high or low risk of events. “So we need a more focused treatment strategy for the youngest age group, irrespective of the cardiometabolic risk level at diagnosis,” he said.
Dr. Paul and Dr. Koye reported having no conflicts of interest.
SOURCE: Koye D et al. EASD 2019, Abstract 82.
BARCELONA – People diagnosed with type 2 diabetes when they are 18-39 years old have significantly higher cardiometabolic risk burden, compared with older people, according to the results of a large study from the United Kingdom presented at the annual meeting of the European Association for the Study of Diabetes.
Patients in that younger age group were found to have higher glycated hemoglobin (HbA1c) levels, along with higher levels of low-density lipoprotein cholesterol and higher body weight.
“We wanted to evaluate the population-level trend in the incidence of young-onset type 2 diabetes in the United Kingdom, compared with later-onset diabetes,” said senior study author Sanjoy Paul, PhD, the director of the Melbourne EpiCentre at the University of Melbourne at a press briefing during the meeting.
Other aims of the study were to compare temporal trends in the incidence of atherosclerotic cardiovascular disease in younger and older patients with type 2 diabetes, and to see how being “high risk” at diagnosis affected patients’ risk of ASCVD and subsequent risk of death.
High-risk status was defined as having at least two of the risk factors for ASCVD – smoking, high systolic blood pressure, high low-density lipoprotein cholesterol, or chronic kidney disease.
The investigators searched a nationally representative sample of primary care electronic medical records from The Health Improvement Network (THIN) database to find incident cases of type 2 diabetes that occurred between 2000 and 2017, with a total of 370,854 cases identified.
At diagnosis of type 2 diabetes, 8% of the sample (n = 29,678) was aged 18-39 years; 15% (n = 56,798), 40-49 years; 25% (n = 93,698), 50-59 years; 29% (n = 107,261), 60-69 years; and 23% (n = 83,419), 70-79 years. Follow-up was just more than 6 years.
Baseline HbA1c in the respective age groups was 8.6%, 8.4%, 8.1%, 7.8%, and 7.6%, with more than 55% of patients in the two youngest age groups having an HbA1c of 7.5% or higher, compared with 34%-47% in the three oldest age groups.
The percentage of patients with a high LDL cholesterol value (2.6 mmol/L or higher in those without ASCVD, and 1.8 or higher in those with ASCVD) was 71%, 75%, 74%, 69%, and 65%, from the youngest to oldest age groups. A respective 71%, 70%, 66%, 57%, and 44% of the patients had a body mass index of 35 kg/m2 or higher.
Few younger patients had ASCVD at diagnosis (2% of the 18-39 age group; 6% of the 40-49 group), with higher rates in the older age groups (13% of the 50-59 group; 23% of the 60-69 group; and 33% of the 70-79 group).
The percentage of patients considered to be at high risk of ASCVD at diagnosis was 23%, 37%, 45%, 50%, and 53%, respectively, across the five age groups.
Although high systolic blood pressure (SBP; 130 mmHg in those with ASCVD, 140 mmHg in those without) was more common in the older age groups (52% at 50-59 years; 60% at 60-69 years, and 64% at 70-79 years,) a substantial proportion of the younger patients also had a high SBP (27% at 18-39 years and 41% at 40-49 years).
Digsu Koye, PhD, also of the Melbourne EpiCentre, presented the main findings of the study during the meeting, noting that the proportion of people diagnosed when they were younger than 50 years remained stable between 2000 and 2017, with a marginal increase in those diagnosed when they were aged 50-59 years, and a decline in those diagnosed when they were older than 70 years.
In the youngest and oldest age groups, equal numbers of men and women were diagnosed with type 2 diabetes, and more women than men were diagnosed in the 60-69 age group, Dr. Koye said. However, for the 40-49 and 50-59 age groups, there were more men than women diagnosed with type 2 diabetes.
Patients were followed for an average of just more than 6 years. “The rate of atherosclerotic cardiovascular disease was declining in all age categories during 2000-2006, but after that, we saw a stable and consistent pattern for all age categories after 2007,” Dr. Koye observed.
In regard to all-cause mortality, there was a 30% decline in the oldest age group (70-79 years), and a 20% decline in the 60-69 age group, but there was no significant decline in the younger age groups, he added.
The investigators determined the average time to event (ASCVD or all-cause mortality) by high-risk status at type 2 diabetes diagnosis for each age group. These analyses showed that there was little difference between the high- and low-risk groups for the average time to ASCVD or all-cause mortality in the youngest age group, with wider differences in the older patients of 1-2 years for ASCVD and 0.5-2 years for all-cause mortality.
Dr. Koye noted that people with young-onset type 2 diabetes had a risk of ASCVD or all-cause mortality that was similar to that of older people, irrespective of whether or not they were considered to be at high or low risk of events. “So we need a more focused treatment strategy for the youngest age group, irrespective of the cardiometabolic risk level at diagnosis,” he said.
Dr. Paul and Dr. Koye reported having no conflicts of interest.
SOURCE: Koye D et al. EASD 2019, Abstract 82.
BARCELONA – People diagnosed with type 2 diabetes when they are 18-39 years old have significantly higher cardiometabolic risk burden, compared with older people, according to the results of a large study from the United Kingdom presented at the annual meeting of the European Association for the Study of Diabetes.
Patients in that younger age group were found to have higher glycated hemoglobin (HbA1c) levels, along with higher levels of low-density lipoprotein cholesterol and higher body weight.
“We wanted to evaluate the population-level trend in the incidence of young-onset type 2 diabetes in the United Kingdom, compared with later-onset diabetes,” said senior study author Sanjoy Paul, PhD, the director of the Melbourne EpiCentre at the University of Melbourne at a press briefing during the meeting.
Other aims of the study were to compare temporal trends in the incidence of atherosclerotic cardiovascular disease in younger and older patients with type 2 diabetes, and to see how being “high risk” at diagnosis affected patients’ risk of ASCVD and subsequent risk of death.
High-risk status was defined as having at least two of the risk factors for ASCVD – smoking, high systolic blood pressure, high low-density lipoprotein cholesterol, or chronic kidney disease.
The investigators searched a nationally representative sample of primary care electronic medical records from The Health Improvement Network (THIN) database to find incident cases of type 2 diabetes that occurred between 2000 and 2017, with a total of 370,854 cases identified.
At diagnosis of type 2 diabetes, 8% of the sample (n = 29,678) was aged 18-39 years; 15% (n = 56,798), 40-49 years; 25% (n = 93,698), 50-59 years; 29% (n = 107,261), 60-69 years; and 23% (n = 83,419), 70-79 years. Follow-up was just more than 6 years.
Baseline HbA1c in the respective age groups was 8.6%, 8.4%, 8.1%, 7.8%, and 7.6%, with more than 55% of patients in the two youngest age groups having an HbA1c of 7.5% or higher, compared with 34%-47% in the three oldest age groups.
The percentage of patients with a high LDL cholesterol value (2.6 mmol/L or higher in those without ASCVD, and 1.8 or higher in those with ASCVD) was 71%, 75%, 74%, 69%, and 65%, from the youngest to oldest age groups. A respective 71%, 70%, 66%, 57%, and 44% of the patients had a body mass index of 35 kg/m2 or higher.
Few younger patients had ASCVD at diagnosis (2% of the 18-39 age group; 6% of the 40-49 group), with higher rates in the older age groups (13% of the 50-59 group; 23% of the 60-69 group; and 33% of the 70-79 group).
The percentage of patients considered to be at high risk of ASCVD at diagnosis was 23%, 37%, 45%, 50%, and 53%, respectively, across the five age groups.
Although high systolic blood pressure (SBP; 130 mmHg in those with ASCVD, 140 mmHg in those without) was more common in the older age groups (52% at 50-59 years; 60% at 60-69 years, and 64% at 70-79 years,) a substantial proportion of the younger patients also had a high SBP (27% at 18-39 years and 41% at 40-49 years).
Digsu Koye, PhD, also of the Melbourne EpiCentre, presented the main findings of the study during the meeting, noting that the proportion of people diagnosed when they were younger than 50 years remained stable between 2000 and 2017, with a marginal increase in those diagnosed when they were aged 50-59 years, and a decline in those diagnosed when they were older than 70 years.
In the youngest and oldest age groups, equal numbers of men and women were diagnosed with type 2 diabetes, and more women than men were diagnosed in the 60-69 age group, Dr. Koye said. However, for the 40-49 and 50-59 age groups, there were more men than women diagnosed with type 2 diabetes.
Patients were followed for an average of just more than 6 years. “The rate of atherosclerotic cardiovascular disease was declining in all age categories during 2000-2006, but after that, we saw a stable and consistent pattern for all age categories after 2007,” Dr. Koye observed.
In regard to all-cause mortality, there was a 30% decline in the oldest age group (70-79 years), and a 20% decline in the 60-69 age group, but there was no significant decline in the younger age groups, he added.
The investigators determined the average time to event (ASCVD or all-cause mortality) by high-risk status at type 2 diabetes diagnosis for each age group. These analyses showed that there was little difference between the high- and low-risk groups for the average time to ASCVD or all-cause mortality in the youngest age group, with wider differences in the older patients of 1-2 years for ASCVD and 0.5-2 years for all-cause mortality.
Dr. Koye noted that people with young-onset type 2 diabetes had a risk of ASCVD or all-cause mortality that was similar to that of older people, irrespective of whether or not they were considered to be at high or low risk of events. “So we need a more focused treatment strategy for the youngest age group, irrespective of the cardiometabolic risk level at diagnosis,” he said.
Dr. Paul and Dr. Koye reported having no conflicts of interest.
SOURCE: Koye D et al. EASD 2019, Abstract 82.
REPORTING FROM EASD 2019
How to use type 2 diabetes meds to lower CV disease risk
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4
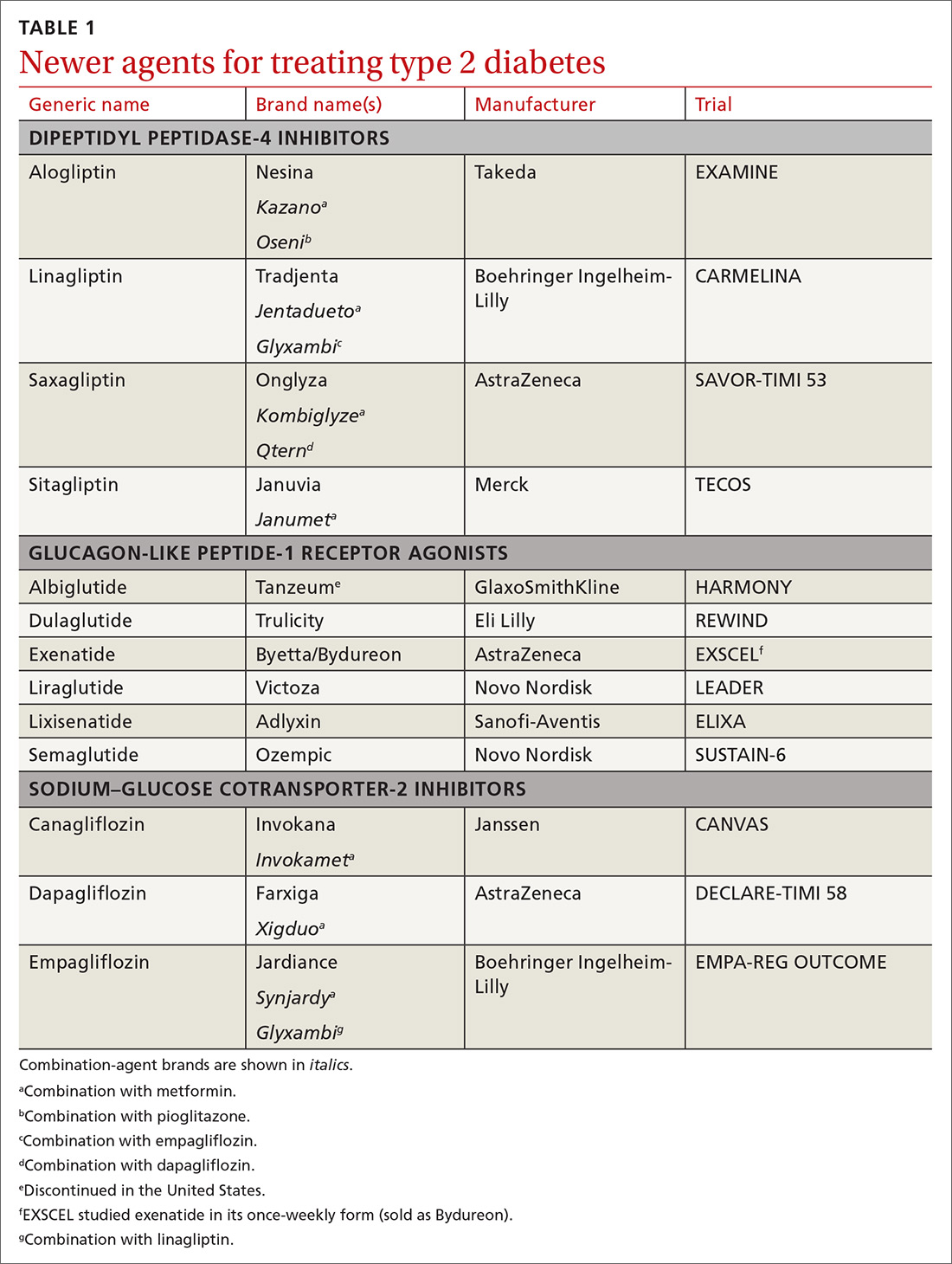
Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).
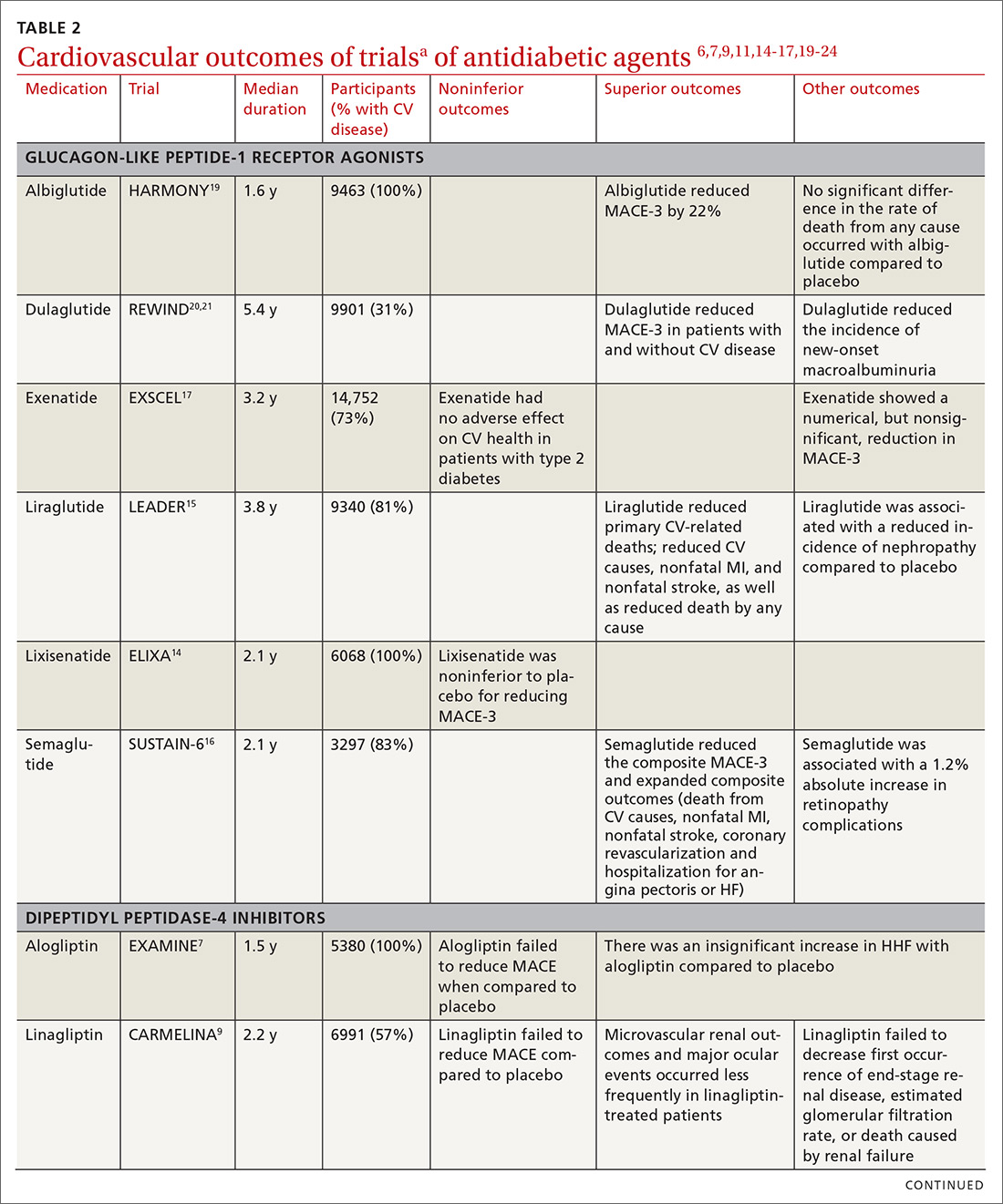
Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32
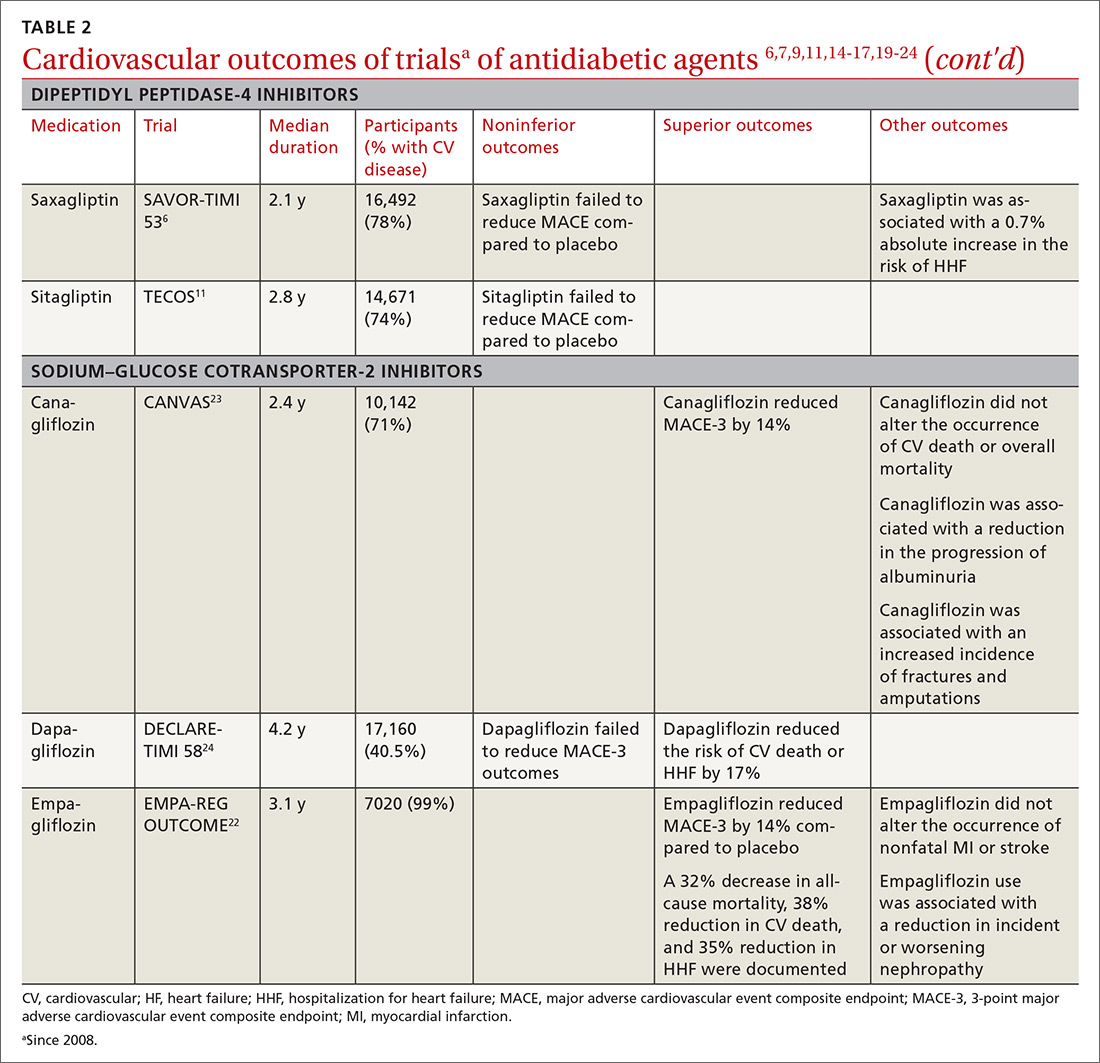
See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.
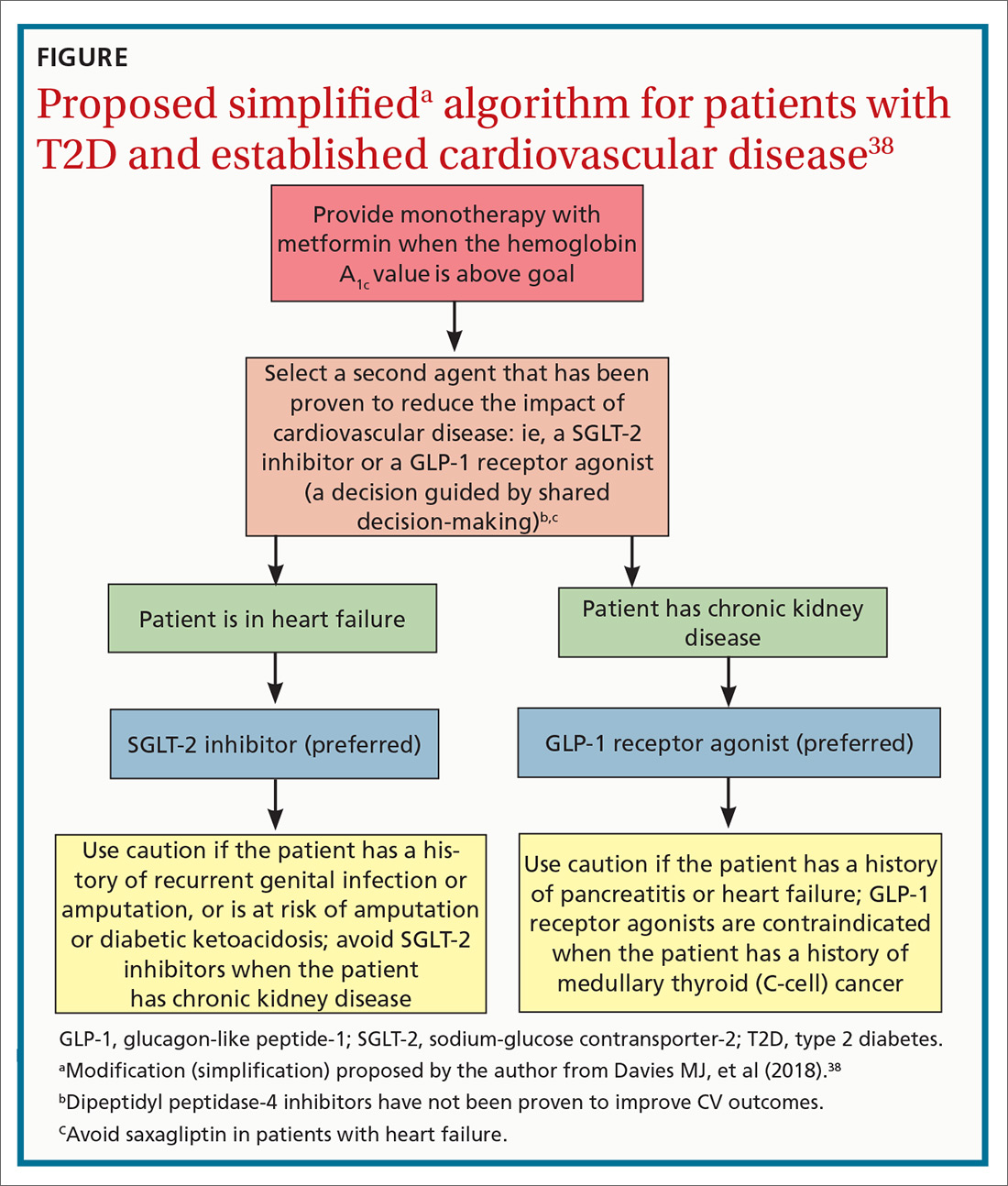
ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; [email protected].
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4

Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).

Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32

See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.

ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; [email protected].
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4

Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).

Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32

See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.

ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; [email protected].
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
PRACTICE RECOMMENDATIONS
› Consider American Diabetes Association (ADA) guidance and prescribe a sodium–glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide- 1 (GLP-1) receptor agonist that has demonstrated cardiovascular (CV) disease benefit for your patients who have type 2 diabetes (T2D) and established atherosclerotic CV disease. A
› Consider ADA’s recommendation for preferred therapy and prescribe an SGLT-2 inhibitor for your patients with T2D who have atherosclerotic CV disease and are at high risk of heart failure or in whom heart failure coexists. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Ketoacidosis is on the rise in children with type 1 diabetes
BARCELONA – As many as 40%-60% of children have diabetic ketoacidosis (DKA) at the time of being diagnosed with type 1 diabetes, according to data from two U.S. analyses – and the figures have been rising for the past 10 years.
Between 2010 and 2017, the prevalence of DKA at diagnosis in children who were followed up at the Barbara Davies Cancer Center in Denver (n = 2,429) went from 41% to 59%, with a 7% annual rise, Arleta Rewers, MD, PhD, of Children’s Hospital Colorado, Denver, reported at the annual meeting of the European Association for the Study of Diabetes.
Meanwhile, in another analysis that included multiple U.S. centers and about 7,600 cases of youth-onset type 1 diabetes, the overall prevalence of DKA at diagnosis was 38.5% between 2010 and 2016. However, the prevalence had increased from 35% in 2010 to 40.6% in 2016, according to Elizabeth T. Jensen, MPH, PhD, of Wake Forest University, Winston-Salem, N.C. The annual increase in prevalence of DKA at diagnosis of type 1 disease was 2%, adjusted for sociodemographic factors.
Rising prevalence
“DKA occurs most commonly at the time of type 1 diabetes diagnosis,” observed Dr. Jensen, who noted that “in the United States, among children, it’s younger children, uninsured or underinsured children, and children from minority racial or ethnic groups, who are at greatest risk.”
Dr. Jensen and colleagues had previously shown that the prevalence of DKA at diagnosis was around 30% between 2002 and 2010, with no significant change in its prevalence. However, more recent reports from referral-based, single-center studies had suggested there was an increase, and that led her and her colleagues to take a closer look at the data.
To characterize the risk factors for DKA and the prevalence of DKA over time, Dr. Jensen and her team used the SEARCH for Diabetes in Youth database, which, she said, was “uniquely suited” for this purpose. SEARCH is a population-based, multicenter study conducted in centers in five U.S. states: South Carolina, Ohio, Colorado, California, and Washington.
A diagnosis of DKA was based on blood bicarbonate levels of less than 15 mmol/L, a venous pH of less than 7.25 or arterial or capillary pH of less than 7.3, or if there was any documentation of a DKA diagnosis.
As expected, the prevalence of DKA was highest in the youngest age group (0-4 years), Dr. Jensen said, but the increase in prevalence in that group was no different from the increases seen over time in the other age groups (5-9 years, 10-14 years, and 15 years or older).
There were no differences in the prevalence of DKA between the sexes, although there was a general increase over time. Similar trends were seen in DKA prevalence by race or ethnicity and by season, or time of year.
Of note, higher rates of DKA were seen in children who were covered by public health insurance, than in those covered by private insurance, although there was no difference in the rate of increase in DKA prevalence between the two groups. Dr. Jensen noted that only 64% of this study population had private insurance.
She said that future research in this area would need to look at the economic drivers and the “changing landscape of health insurance coverage in the United States.”
Expansion in health coverage
In presenting the findings of a study showing an increase in the prevalence of DKA at diagnosis of type 1 diabetes in children in Colorado from 2010 to 2017, Dr. Rewers said that the increase “paradoxically occurred” at a time of increasing health insurance coverage, a reference to the expansion of Medicaid during 2008-2012 and implementation in 2013 of the Affordable Care Act.
“Our group in Colorado has followed the frequency of DKA for almost 2 decades,” Dr. Rewers said. It’s important to study DKA as it is linked to worse glycemic control – with children with DKA having an HbA1c level of around 1% higher than those without DKA – and the potential for future, long-term complications.
Dr. Rewers noted that the increase in DKA at diagnosis of type 1 diabetes was more rapid in the children who had private rather than public health insurance. Of 1,187 patients with DKA, 57% had private health insurance, and 37% had public insurance, compared with 66% and 28%, respectively, in those without DKA. In 2010, the prevalence of DKA at diagnosis was 35.3% in those who were privately insured and 52.2% of those with public health insurance, but by 2017, a similar percentage of DKA was seen in the privately and publicly insured children (59.6% and 58.5%, respectively).
She said one possible explanation for that might be that “increased enrollment in high-deductible insurance plans could discourage families with private insurance from seeking timely care.”
Another explanation is that there is a low awareness of type 1 diabetes in the general population, she added. “Educational campaigns and autoimmunity screening have been shown to reduce DKA at diabetes diagnosis, but unfortunately they are not used widely at this point.”
Identifying at-risk children
“Diabetic ketoacidosis is a serious complication of diabetes [and] is difficult to diagnose because of the variability of the symptoms, said Angela Ibald-Mulli, PhD, who presented the findings of a retrospective cohort study in which she and her colleagues used a “discovery algorithm” called Q-Finder to identify the predictive factors for DKA in youth with type 1 diabetes, based on data from the Diabetes Prospective Follow-up Registry (DPV).
“The better we know the risk factors, the better we can care for our patients,” she emphasized.
The investigators obtained data on 108,223 patients with a diagnosis of type 1 disease and with more than two visits related to diabetes. The prevalence of DKA – defined as a pH of less than 7.3 during hospitalization occurring at least 10 days after the onset of type 1 diabetes – was 5.2%, said Dr. Ibald-Mulli, head of Medical Evidence Generation Primary Care at Sanofi, Paris.
A total of 129 different features were considered for their association with DKA – including comorbidities, sociodemographic factors, laboratory values, and concomitant medications – and were then used to identify, test, and the validate likely risk profiles.
After comparing the characteristics of patients with and without DKA, eight significant factors, all of which have been reported previously in the DPV cohort, were seen: younger age, lower body weight, higher HbA1c, younger age at onset of T1D; shorter disease duration; having a migration background; being less active; and having had more medical visits.
The investigators used the algorithm, and found 11 distinct profiles associated with DKA: an HbA1c higher than 8.87%; being aged 6-10 years; being aged 11-15 years; a diagnosis of nephropathy; DKA being present at onset; a prevalence of hypoglycemia with coma; a diagnosis of thyroiditis; a standardized body mass index lower than 16.9; not using short-acting insulin; younger than age 15 years; and not using continuous glucose monitoring.
Almost two-thirds of patients (64.7%) belonged to at least one of these risk profiles, Dr. Ibald-Mulli observed, with 7.1% of them having DKA, compared with 1.6% who belonged to none of the profiles.
Dr. Ibald-Mulli said it was important to note that the DKA risk profiles could overlap. “The more profiles a patient belongs to, the higher is the risk of having DKA,” she emphasized, adding that most patients (88.8%) with DKA belonged to just one profile, and fewer than 5% belonged to three or more profiles.
“Overall, the results of the algorithm confirmed known risk-factor profiles that had been previously identified by conventional statistical methods,” she concluded. It also provided “additional insights that can be further explored.”
SEARCH is funded by the Centers for Disease and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases. The DPV Registry is funded by multiple sponsors, including the European Federation for the Study of Diabetes and other academic institutions with the support of several commercial partners. Sanofi sponsored the study presented by Dr. Ibald-Mulli. Dr. Rewers made no disclosures, and Dr. Jensen did not have any conflicts of interest to declare. Dr. Ibald-Mulli is an employee of Sanofi.
SOURCE: Rewers A et al. EASD 2019, Abstract 115; Jensen E et al. EASD 2019, Abstract 116; Ibald-Mulli A et al. EASD 2019, Abstract 117.
BARCELONA – As many as 40%-60% of children have diabetic ketoacidosis (DKA) at the time of being diagnosed with type 1 diabetes, according to data from two U.S. analyses – and the figures have been rising for the past 10 years.
Between 2010 and 2017, the prevalence of DKA at diagnosis in children who were followed up at the Barbara Davies Cancer Center in Denver (n = 2,429) went from 41% to 59%, with a 7% annual rise, Arleta Rewers, MD, PhD, of Children’s Hospital Colorado, Denver, reported at the annual meeting of the European Association for the Study of Diabetes.
Meanwhile, in another analysis that included multiple U.S. centers and about 7,600 cases of youth-onset type 1 diabetes, the overall prevalence of DKA at diagnosis was 38.5% between 2010 and 2016. However, the prevalence had increased from 35% in 2010 to 40.6% in 2016, according to Elizabeth T. Jensen, MPH, PhD, of Wake Forest University, Winston-Salem, N.C. The annual increase in prevalence of DKA at diagnosis of type 1 disease was 2%, adjusted for sociodemographic factors.
Rising prevalence
“DKA occurs most commonly at the time of type 1 diabetes diagnosis,” observed Dr. Jensen, who noted that “in the United States, among children, it’s younger children, uninsured or underinsured children, and children from minority racial or ethnic groups, who are at greatest risk.”
Dr. Jensen and colleagues had previously shown that the prevalence of DKA at diagnosis was around 30% between 2002 and 2010, with no significant change in its prevalence. However, more recent reports from referral-based, single-center studies had suggested there was an increase, and that led her and her colleagues to take a closer look at the data.
To characterize the risk factors for DKA and the prevalence of DKA over time, Dr. Jensen and her team used the SEARCH for Diabetes in Youth database, which, she said, was “uniquely suited” for this purpose. SEARCH is a population-based, multicenter study conducted in centers in five U.S. states: South Carolina, Ohio, Colorado, California, and Washington.
A diagnosis of DKA was based on blood bicarbonate levels of less than 15 mmol/L, a venous pH of less than 7.25 or arterial or capillary pH of less than 7.3, or if there was any documentation of a DKA diagnosis.
As expected, the prevalence of DKA was highest in the youngest age group (0-4 years), Dr. Jensen said, but the increase in prevalence in that group was no different from the increases seen over time in the other age groups (5-9 years, 10-14 years, and 15 years or older).
There were no differences in the prevalence of DKA between the sexes, although there was a general increase over time. Similar trends were seen in DKA prevalence by race or ethnicity and by season, or time of year.
Of note, higher rates of DKA were seen in children who were covered by public health insurance, than in those covered by private insurance, although there was no difference in the rate of increase in DKA prevalence between the two groups. Dr. Jensen noted that only 64% of this study population had private insurance.
She said that future research in this area would need to look at the economic drivers and the “changing landscape of health insurance coverage in the United States.”
Expansion in health coverage
In presenting the findings of a study showing an increase in the prevalence of DKA at diagnosis of type 1 diabetes in children in Colorado from 2010 to 2017, Dr. Rewers said that the increase “paradoxically occurred” at a time of increasing health insurance coverage, a reference to the expansion of Medicaid during 2008-2012 and implementation in 2013 of the Affordable Care Act.
“Our group in Colorado has followed the frequency of DKA for almost 2 decades,” Dr. Rewers said. It’s important to study DKA as it is linked to worse glycemic control – with children with DKA having an HbA1c level of around 1% higher than those without DKA – and the potential for future, long-term complications.
Dr. Rewers noted that the increase in DKA at diagnosis of type 1 diabetes was more rapid in the children who had private rather than public health insurance. Of 1,187 patients with DKA, 57% had private health insurance, and 37% had public insurance, compared with 66% and 28%, respectively, in those without DKA. In 2010, the prevalence of DKA at diagnosis was 35.3% in those who were privately insured and 52.2% of those with public health insurance, but by 2017, a similar percentage of DKA was seen in the privately and publicly insured children (59.6% and 58.5%, respectively).
She said one possible explanation for that might be that “increased enrollment in high-deductible insurance plans could discourage families with private insurance from seeking timely care.”
Another explanation is that there is a low awareness of type 1 diabetes in the general population, she added. “Educational campaigns and autoimmunity screening have been shown to reduce DKA at diabetes diagnosis, but unfortunately they are not used widely at this point.”
Identifying at-risk children
“Diabetic ketoacidosis is a serious complication of diabetes [and] is difficult to diagnose because of the variability of the symptoms, said Angela Ibald-Mulli, PhD, who presented the findings of a retrospective cohort study in which she and her colleagues used a “discovery algorithm” called Q-Finder to identify the predictive factors for DKA in youth with type 1 diabetes, based on data from the Diabetes Prospective Follow-up Registry (DPV).
“The better we know the risk factors, the better we can care for our patients,” she emphasized.
The investigators obtained data on 108,223 patients with a diagnosis of type 1 disease and with more than two visits related to diabetes. The prevalence of DKA – defined as a pH of less than 7.3 during hospitalization occurring at least 10 days after the onset of type 1 diabetes – was 5.2%, said Dr. Ibald-Mulli, head of Medical Evidence Generation Primary Care at Sanofi, Paris.
A total of 129 different features were considered for their association with DKA – including comorbidities, sociodemographic factors, laboratory values, and concomitant medications – and were then used to identify, test, and the validate likely risk profiles.
After comparing the characteristics of patients with and without DKA, eight significant factors, all of which have been reported previously in the DPV cohort, were seen: younger age, lower body weight, higher HbA1c, younger age at onset of T1D; shorter disease duration; having a migration background; being less active; and having had more medical visits.
The investigators used the algorithm, and found 11 distinct profiles associated with DKA: an HbA1c higher than 8.87%; being aged 6-10 years; being aged 11-15 years; a diagnosis of nephropathy; DKA being present at onset; a prevalence of hypoglycemia with coma; a diagnosis of thyroiditis; a standardized body mass index lower than 16.9; not using short-acting insulin; younger than age 15 years; and not using continuous glucose monitoring.
Almost two-thirds of patients (64.7%) belonged to at least one of these risk profiles, Dr. Ibald-Mulli observed, with 7.1% of them having DKA, compared with 1.6% who belonged to none of the profiles.
Dr. Ibald-Mulli said it was important to note that the DKA risk profiles could overlap. “The more profiles a patient belongs to, the higher is the risk of having DKA,” she emphasized, adding that most patients (88.8%) with DKA belonged to just one profile, and fewer than 5% belonged to three or more profiles.
“Overall, the results of the algorithm confirmed known risk-factor profiles that had been previously identified by conventional statistical methods,” she concluded. It also provided “additional insights that can be further explored.”
SEARCH is funded by the Centers for Disease and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases. The DPV Registry is funded by multiple sponsors, including the European Federation for the Study of Diabetes and other academic institutions with the support of several commercial partners. Sanofi sponsored the study presented by Dr. Ibald-Mulli. Dr. Rewers made no disclosures, and Dr. Jensen did not have any conflicts of interest to declare. Dr. Ibald-Mulli is an employee of Sanofi.
SOURCE: Rewers A et al. EASD 2019, Abstract 115; Jensen E et al. EASD 2019, Abstract 116; Ibald-Mulli A et al. EASD 2019, Abstract 117.
BARCELONA – As many as 40%-60% of children have diabetic ketoacidosis (DKA) at the time of being diagnosed with type 1 diabetes, according to data from two U.S. analyses – and the figures have been rising for the past 10 years.
Between 2010 and 2017, the prevalence of DKA at diagnosis in children who were followed up at the Barbara Davies Cancer Center in Denver (n = 2,429) went from 41% to 59%, with a 7% annual rise, Arleta Rewers, MD, PhD, of Children’s Hospital Colorado, Denver, reported at the annual meeting of the European Association for the Study of Diabetes.
Meanwhile, in another analysis that included multiple U.S. centers and about 7,600 cases of youth-onset type 1 diabetes, the overall prevalence of DKA at diagnosis was 38.5% between 2010 and 2016. However, the prevalence had increased from 35% in 2010 to 40.6% in 2016, according to Elizabeth T. Jensen, MPH, PhD, of Wake Forest University, Winston-Salem, N.C. The annual increase in prevalence of DKA at diagnosis of type 1 disease was 2%, adjusted for sociodemographic factors.
Rising prevalence
“DKA occurs most commonly at the time of type 1 diabetes diagnosis,” observed Dr. Jensen, who noted that “in the United States, among children, it’s younger children, uninsured or underinsured children, and children from minority racial or ethnic groups, who are at greatest risk.”
Dr. Jensen and colleagues had previously shown that the prevalence of DKA at diagnosis was around 30% between 2002 and 2010, with no significant change in its prevalence. However, more recent reports from referral-based, single-center studies had suggested there was an increase, and that led her and her colleagues to take a closer look at the data.
To characterize the risk factors for DKA and the prevalence of DKA over time, Dr. Jensen and her team used the SEARCH for Diabetes in Youth database, which, she said, was “uniquely suited” for this purpose. SEARCH is a population-based, multicenter study conducted in centers in five U.S. states: South Carolina, Ohio, Colorado, California, and Washington.
A diagnosis of DKA was based on blood bicarbonate levels of less than 15 mmol/L, a venous pH of less than 7.25 or arterial or capillary pH of less than 7.3, or if there was any documentation of a DKA diagnosis.
As expected, the prevalence of DKA was highest in the youngest age group (0-4 years), Dr. Jensen said, but the increase in prevalence in that group was no different from the increases seen over time in the other age groups (5-9 years, 10-14 years, and 15 years or older).
There were no differences in the prevalence of DKA between the sexes, although there was a general increase over time. Similar trends were seen in DKA prevalence by race or ethnicity and by season, or time of year.
Of note, higher rates of DKA were seen in children who were covered by public health insurance, than in those covered by private insurance, although there was no difference in the rate of increase in DKA prevalence between the two groups. Dr. Jensen noted that only 64% of this study population had private insurance.
She said that future research in this area would need to look at the economic drivers and the “changing landscape of health insurance coverage in the United States.”
Expansion in health coverage
In presenting the findings of a study showing an increase in the prevalence of DKA at diagnosis of type 1 diabetes in children in Colorado from 2010 to 2017, Dr. Rewers said that the increase “paradoxically occurred” at a time of increasing health insurance coverage, a reference to the expansion of Medicaid during 2008-2012 and implementation in 2013 of the Affordable Care Act.
“Our group in Colorado has followed the frequency of DKA for almost 2 decades,” Dr. Rewers said. It’s important to study DKA as it is linked to worse glycemic control – with children with DKA having an HbA1c level of around 1% higher than those without DKA – and the potential for future, long-term complications.
Dr. Rewers noted that the increase in DKA at diagnosis of type 1 diabetes was more rapid in the children who had private rather than public health insurance. Of 1,187 patients with DKA, 57% had private health insurance, and 37% had public insurance, compared with 66% and 28%, respectively, in those without DKA. In 2010, the prevalence of DKA at diagnosis was 35.3% in those who were privately insured and 52.2% of those with public health insurance, but by 2017, a similar percentage of DKA was seen in the privately and publicly insured children (59.6% and 58.5%, respectively).
She said one possible explanation for that might be that “increased enrollment in high-deductible insurance plans could discourage families with private insurance from seeking timely care.”
Another explanation is that there is a low awareness of type 1 diabetes in the general population, she added. “Educational campaigns and autoimmunity screening have been shown to reduce DKA at diabetes diagnosis, but unfortunately they are not used widely at this point.”
Identifying at-risk children
“Diabetic ketoacidosis is a serious complication of diabetes [and] is difficult to diagnose because of the variability of the symptoms, said Angela Ibald-Mulli, PhD, who presented the findings of a retrospective cohort study in which she and her colleagues used a “discovery algorithm” called Q-Finder to identify the predictive factors for DKA in youth with type 1 diabetes, based on data from the Diabetes Prospective Follow-up Registry (DPV).
“The better we know the risk factors, the better we can care for our patients,” she emphasized.
The investigators obtained data on 108,223 patients with a diagnosis of type 1 disease and with more than two visits related to diabetes. The prevalence of DKA – defined as a pH of less than 7.3 during hospitalization occurring at least 10 days after the onset of type 1 diabetes – was 5.2%, said Dr. Ibald-Mulli, head of Medical Evidence Generation Primary Care at Sanofi, Paris.
A total of 129 different features were considered for their association with DKA – including comorbidities, sociodemographic factors, laboratory values, and concomitant medications – and were then used to identify, test, and the validate likely risk profiles.
After comparing the characteristics of patients with and without DKA, eight significant factors, all of which have been reported previously in the DPV cohort, were seen: younger age, lower body weight, higher HbA1c, younger age at onset of T1D; shorter disease duration; having a migration background; being less active; and having had more medical visits.
The investigators used the algorithm, and found 11 distinct profiles associated with DKA: an HbA1c higher than 8.87%; being aged 6-10 years; being aged 11-15 years; a diagnosis of nephropathy; DKA being present at onset; a prevalence of hypoglycemia with coma; a diagnosis of thyroiditis; a standardized body mass index lower than 16.9; not using short-acting insulin; younger than age 15 years; and not using continuous glucose monitoring.
Almost two-thirds of patients (64.7%) belonged to at least one of these risk profiles, Dr. Ibald-Mulli observed, with 7.1% of them having DKA, compared with 1.6% who belonged to none of the profiles.
Dr. Ibald-Mulli said it was important to note that the DKA risk profiles could overlap. “The more profiles a patient belongs to, the higher is the risk of having DKA,” she emphasized, adding that most patients (88.8%) with DKA belonged to just one profile, and fewer than 5% belonged to three or more profiles.
“Overall, the results of the algorithm confirmed known risk-factor profiles that had been previously identified by conventional statistical methods,” she concluded. It also provided “additional insights that can be further explored.”
SEARCH is funded by the Centers for Disease and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases. The DPV Registry is funded by multiple sponsors, including the European Federation for the Study of Diabetes and other academic institutions with the support of several commercial partners. Sanofi sponsored the study presented by Dr. Ibald-Mulli. Dr. Rewers made no disclosures, and Dr. Jensen did not have any conflicts of interest to declare. Dr. Ibald-Mulli is an employee of Sanofi.
SOURCE: Rewers A et al. EASD 2019, Abstract 115; Jensen E et al. EASD 2019, Abstract 116; Ibald-Mulli A et al. EASD 2019, Abstract 117.
REPORTING FROM EASD 2019
Open Clinical Trials for Native Americans With Diabetes Mellitus(FULL)
Providing access to clinical trials for patients with diabetes mellitus can be a challenge, but a significant number of trials are now recruiting patients. The clinical trials listed below are all open as of October 31, 2019; and are focused on diabetes mellitus-related treatments for American Indians. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Cross-Sectional and Longitudinal Studies of “Pre-Diabetes” in the Pima Indians
The Pima Indians of Arizona have the highest prevalence and incidence of type 2 diabetes of any population in the world. Prospective analyses in this population have identified insulin resistance and a defect in early insulin secretion as risk factors for the development of the disease. To identify the genetic and environmental determinants of diabetes we plan to study Pima Indian families to determine: (1) if there are genes that segregate with metabolic risk factors for diabetes which might therefore be genetic markers for type 2 diabetes; and (2) the mechanisms mediating genetic and environmental determinants of insulin resistance and impaired insulin secretion.
ID: NCT00340132
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Contact: Clifton Bogardus, MD, [email protected]
Location: NIDDK, Phoenix, AZ
Empaglifozin in Early Diabetic Kidney Disease
Diabetes is common among American Indian people and diabetic kidney disease is a common complication. Kidney disease caused by diabetes can lead to the need for kidney replacement, by dialysis or kidney transplant, and is also associated with higher risk of early death. A new diabetes medicine called empagliflozin may slow kidney disease from type 2 diabetes. Researchers want to learn if it protects the kidneys when used in very early stages of diabetic kidney disease.
ID: NCT03173963
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Contact: Helen C Looker, [email protected]
Location: NIDDK, Phoenix, AZ
Family Investigation of Nephropathy and Diabetes
The Family Investigation of Nephropathy and Diabetes (FIND) is a multicenter study designed to identify genetic determinants of diabetic kidney disease. FIND will be conducted in 11 centers and in many ethnic groups throughout the United States. Two different strategies will be used to localize genes predisposing to kidney disease: a family-based genetic linkage study and a case-control study that utilizes admixture linkage disequilibrium. The center will conduct family-based linkage studies among American Indian populations in the southwestern United States.
ID: NCT00342927
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Contact: William C Knowler, MD, [email protected]
Location: NIDDK, Phoenix, AZ
Look AHEAD: Action for Health in Diabetes
The Look AHEAD study is a multi-center, randomized clinical trial to examine the long-term effects of a lifestyle intervention designed to achieve and maintain weight loss. The study will investigate the effects of the intervention on heart attacks, stroke and cardiovascular-related death in individuals with type 2 diabetes who are also overweight or obese.
ID: NCT00017953
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Location: Southwestern American Indian Center, Phoenix, AZ
Vitamin D and Type 2 Diabetes Study
The goal of the Vitamin D and type 2 diabetes (D2d) study is to determine if vitamin D supplementation works to delay the onset of type 2 diabetes in people at risk for the disease and to gain a better understand how vitamin D affects glucose (sugar) metabolism.
ID: NCT01942694
Sponsor: Tufts Medical Center
Locations: Southwest American Indian Center; Phoenix, AZ; Orlando VA Medical Center, FL; Atlanta VA Medical Center, Decatur, GA; Omaha VA Medical Center, NE
Reducing Diabetes Risk Factors in American Indian Children: Tribal Turning Point (TTP)
This study will evaluate a behavioral intervention designed to reduce risk factors for type 2 diabetes in American Indian youth aged 7-10 years.
ID: NCT03573856
Sponsor: University of Colorado, Denver
Contact: Katherine Sauder, PhD, [email protected]; Dana Dabelea, MD, PhD, [email protected]
Location: Childrens Hospital Colorado, Aurora
Strong Men, Strong Communities Diabetes Risk Reduction in American Indian Men (SMSC)
SMSC will inform the design and implementation of culturally informed, community-based lifestyle interventions for diabetes prevention in AI men in our partner communities and elsewhere, as well as in men of other minority groups who experience a heavy burden of diabetes.
ID: NCT02953977
Sponsor: Washington State University
Contact: Kaimi Sinclair, PhD, MPH, [email protected] Location: IREACH, Seattle, WA
Growing Resilience in Wind River Indian Reservation (GR)
The Growing Resilience research leverages reservation-based assets of land, family, culture, and front-line tribal health organizations to develop and evaluate home food gardens as a family-based health promotion intervention to reduce disparities suffered by Native Americans in nearly every measure of health. Home gardening interventions show great promise for enabling families to improve their health, and this study aims to fulfill that promise with university and Wind River Indian Reservation partners. The investigators will develop an empowering, scalable, and sustainable family-based health promotion intervention with, by, and for Native American families and conduct the first randomized controlled trial to assess the health impacts of home gardens.
ID: NCT02672748
Sponsor: University of Wyoming
Location: University of Wyoming, Laramie
A Comparative Effectiveness Study of Major Glycemia-lowering Medications for Treatment of Type 2 Diabetes (GRADE)
The GRADE Study is a pragmatic, unmasked clinical trial that will compare commonly used diabetes medications, when combined with metformin, on glycemia-lowering effectiveness and patient-centered outcomes.
ID: NCT01794143
Sponsor: GRADE Study Group
Location: Southwestern American Indian Center, Phoenix, AZ
Home-Based Kidney Care in Native Americans of New Mexico (HBKC)
New Mexico American Indians are experiencing an epidemic of chronic kidney disease due primarily to the high rates of obesity and diabetes. The present study entitled Home-Based Kidney Care is designed to delay / reduce rates of end stage renal disease by early interventions in chronic kidney disease (CKD). Investigators propose to assess the safety and efficacy of conducting a full-scale study to determine if home based care delivered by a collaborative team composed of community health workers, the Albuquerque Area Indian Health Board and University of New Mexico faculty will decrease the risk for the development and the progression of CKD.
ID: NCT03179085
Sponsor: University of New Mexico
Contact: Vallabh Shah, PhD, [email protected]; Kevin English, PhD, [email protected]
Location: University of New Mexico, Albuquerque
Home-based Prediabetes Care in Acoma Pueblo - Study 1
Our major goal of implementing educational interventions to slow the current rate of increase in diabetes in Native communities is aligned with the National Institute of Health (NIGMS) and New Mexico INBRE’s vision in reducing health disparity using innovative interventions. The investigators propose following aims: (1) Recruit and Screen 300 community members in Acoma Pueblo, New Mexico to identify incident cases of pre-diabetes for the proposed study of Home Based Diabetes Care (HBDC); (2) Enroll 150 Acoma Natives aged 21-70 years, at risk for type 2 diabetes mellitus and conduct HBDC for a 16-week lifestyle intervention in a longitudinal cohort study.
ID: NCT04029298
Sponsor: University of New Mexico
Contact: Matthew Bouchonville, MD, [email protected]; Vallabh Shah, PhD, [email protected]
Providing access to clinical trials for patients with diabetes mellitus can be a challenge, but a significant number of trials are now recruiting patients. The clinical trials listed below are all open as of October 31, 2019; and are focused on diabetes mellitus-related treatments for American Indians. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Cross-Sectional and Longitudinal Studies of “Pre-Diabetes” in the Pima Indians
The Pima Indians of Arizona have the highest prevalence and incidence of type 2 diabetes of any population in the world. Prospective analyses in this population have identified insulin resistance and a defect in early insulin secretion as risk factors for the development of the disease. To identify the genetic and environmental determinants of diabetes we plan to study Pima Indian families to determine: (1) if there are genes that segregate with metabolic risk factors for diabetes which might therefore be genetic markers for type 2 diabetes; and (2) the mechanisms mediating genetic and environmental determinants of insulin resistance and impaired insulin secretion.
ID: NCT00340132
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Contact: Clifton Bogardus, MD, [email protected]
Location: NIDDK, Phoenix, AZ
Empaglifozin in Early Diabetic Kidney Disease
Diabetes is common among American Indian people and diabetic kidney disease is a common complication. Kidney disease caused by diabetes can lead to the need for kidney replacement, by dialysis or kidney transplant, and is also associated with higher risk of early death. A new diabetes medicine called empagliflozin may slow kidney disease from type 2 diabetes. Researchers want to learn if it protects the kidneys when used in very early stages of diabetic kidney disease.
ID: NCT03173963
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Contact: Helen C Looker, [email protected]
Location: NIDDK, Phoenix, AZ
Family Investigation of Nephropathy and Diabetes
The Family Investigation of Nephropathy and Diabetes (FIND) is a multicenter study designed to identify genetic determinants of diabetic kidney disease. FIND will be conducted in 11 centers and in many ethnic groups throughout the United States. Two different strategies will be used to localize genes predisposing to kidney disease: a family-based genetic linkage study and a case-control study that utilizes admixture linkage disequilibrium. The center will conduct family-based linkage studies among American Indian populations in the southwestern United States.
ID: NCT00342927
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Contact: William C Knowler, MD, [email protected]
Location: NIDDK, Phoenix, AZ
Look AHEAD: Action for Health in Diabetes
The Look AHEAD study is a multi-center, randomized clinical trial to examine the long-term effects of a lifestyle intervention designed to achieve and maintain weight loss. The study will investigate the effects of the intervention on heart attacks, stroke and cardiovascular-related death in individuals with type 2 diabetes who are also overweight or obese.
ID: NCT00017953
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Location: Southwestern American Indian Center, Phoenix, AZ
Vitamin D and Type 2 Diabetes Study
The goal of the Vitamin D and type 2 diabetes (D2d) study is to determine if vitamin D supplementation works to delay the onset of type 2 diabetes in people at risk for the disease and to gain a better understand how vitamin D affects glucose (sugar) metabolism.
ID: NCT01942694
Sponsor: Tufts Medical Center
Locations: Southwest American Indian Center; Phoenix, AZ; Orlando VA Medical Center, FL; Atlanta VA Medical Center, Decatur, GA; Omaha VA Medical Center, NE
Reducing Diabetes Risk Factors in American Indian Children: Tribal Turning Point (TTP)
This study will evaluate a behavioral intervention designed to reduce risk factors for type 2 diabetes in American Indian youth aged 7-10 years.
ID: NCT03573856
Sponsor: University of Colorado, Denver
Contact: Katherine Sauder, PhD, [email protected]; Dana Dabelea, MD, PhD, [email protected]
Location: Childrens Hospital Colorado, Aurora
Strong Men, Strong Communities Diabetes Risk Reduction in American Indian Men (SMSC)
SMSC will inform the design and implementation of culturally informed, community-based lifestyle interventions for diabetes prevention in AI men in our partner communities and elsewhere, as well as in men of other minority groups who experience a heavy burden of diabetes.
ID: NCT02953977
Sponsor: Washington State University
Contact: Kaimi Sinclair, PhD, MPH, [email protected] Location: IREACH, Seattle, WA
Growing Resilience in Wind River Indian Reservation (GR)
The Growing Resilience research leverages reservation-based assets of land, family, culture, and front-line tribal health organizations to develop and evaluate home food gardens as a family-based health promotion intervention to reduce disparities suffered by Native Americans in nearly every measure of health. Home gardening interventions show great promise for enabling families to improve their health, and this study aims to fulfill that promise with university and Wind River Indian Reservation partners. The investigators will develop an empowering, scalable, and sustainable family-based health promotion intervention with, by, and for Native American families and conduct the first randomized controlled trial to assess the health impacts of home gardens.
ID: NCT02672748
Sponsor: University of Wyoming
Location: University of Wyoming, Laramie
A Comparative Effectiveness Study of Major Glycemia-lowering Medications for Treatment of Type 2 Diabetes (GRADE)
The GRADE Study is a pragmatic, unmasked clinical trial that will compare commonly used diabetes medications, when combined with metformin, on glycemia-lowering effectiveness and patient-centered outcomes.
ID: NCT01794143
Sponsor: GRADE Study Group
Location: Southwestern American Indian Center, Phoenix, AZ
Home-Based Kidney Care in Native Americans of New Mexico (HBKC)
New Mexico American Indians are experiencing an epidemic of chronic kidney disease due primarily to the high rates of obesity and diabetes. The present study entitled Home-Based Kidney Care is designed to delay / reduce rates of end stage renal disease by early interventions in chronic kidney disease (CKD). Investigators propose to assess the safety and efficacy of conducting a full-scale study to determine if home based care delivered by a collaborative team composed of community health workers, the Albuquerque Area Indian Health Board and University of New Mexico faculty will decrease the risk for the development and the progression of CKD.
ID: NCT03179085
Sponsor: University of New Mexico
Contact: Vallabh Shah, PhD, [email protected]; Kevin English, PhD, [email protected]
Location: University of New Mexico, Albuquerque
Home-based Prediabetes Care in Acoma Pueblo - Study 1
Our major goal of implementing educational interventions to slow the current rate of increase in diabetes in Native communities is aligned with the National Institute of Health (NIGMS) and New Mexico INBRE’s vision in reducing health disparity using innovative interventions. The investigators propose following aims: (1) Recruit and Screen 300 community members in Acoma Pueblo, New Mexico to identify incident cases of pre-diabetes for the proposed study of Home Based Diabetes Care (HBDC); (2) Enroll 150 Acoma Natives aged 21-70 years, at risk for type 2 diabetes mellitus and conduct HBDC for a 16-week lifestyle intervention in a longitudinal cohort study.
ID: NCT04029298
Sponsor: University of New Mexico
Contact: Matthew Bouchonville, MD, [email protected]; Vallabh Shah, PhD, [email protected]
Providing access to clinical trials for patients with diabetes mellitus can be a challenge, but a significant number of trials are now recruiting patients. The clinical trials listed below are all open as of October 31, 2019; and are focused on diabetes mellitus-related treatments for American Indians. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Cross-Sectional and Longitudinal Studies of “Pre-Diabetes” in the Pima Indians
The Pima Indians of Arizona have the highest prevalence and incidence of type 2 diabetes of any population in the world. Prospective analyses in this population have identified insulin resistance and a defect in early insulin secretion as risk factors for the development of the disease. To identify the genetic and environmental determinants of diabetes we plan to study Pima Indian families to determine: (1) if there are genes that segregate with metabolic risk factors for diabetes which might therefore be genetic markers for type 2 diabetes; and (2) the mechanisms mediating genetic and environmental determinants of insulin resistance and impaired insulin secretion.
ID: NCT00340132
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Contact: Clifton Bogardus, MD, [email protected]
Location: NIDDK, Phoenix, AZ
Empaglifozin in Early Diabetic Kidney Disease
Diabetes is common among American Indian people and diabetic kidney disease is a common complication. Kidney disease caused by diabetes can lead to the need for kidney replacement, by dialysis or kidney transplant, and is also associated with higher risk of early death. A new diabetes medicine called empagliflozin may slow kidney disease from type 2 diabetes. Researchers want to learn if it protects the kidneys when used in very early stages of diabetic kidney disease.
ID: NCT03173963
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Contact: Helen C Looker, [email protected]
Location: NIDDK, Phoenix, AZ
Family Investigation of Nephropathy and Diabetes
The Family Investigation of Nephropathy and Diabetes (FIND) is a multicenter study designed to identify genetic determinants of diabetic kidney disease. FIND will be conducted in 11 centers and in many ethnic groups throughout the United States. Two different strategies will be used to localize genes predisposing to kidney disease: a family-based genetic linkage study and a case-control study that utilizes admixture linkage disequilibrium. The center will conduct family-based linkage studies among American Indian populations in the southwestern United States.
ID: NCT00342927
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Contact: William C Knowler, MD, [email protected]
Location: NIDDK, Phoenix, AZ
Look AHEAD: Action for Health in Diabetes
The Look AHEAD study is a multi-center, randomized clinical trial to examine the long-term effects of a lifestyle intervention designed to achieve and maintain weight loss. The study will investigate the effects of the intervention on heart attacks, stroke and cardiovascular-related death in individuals with type 2 diabetes who are also overweight or obese.
ID: NCT00017953
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Location: Southwestern American Indian Center, Phoenix, AZ
Vitamin D and Type 2 Diabetes Study
The goal of the Vitamin D and type 2 diabetes (D2d) study is to determine if vitamin D supplementation works to delay the onset of type 2 diabetes in people at risk for the disease and to gain a better understand how vitamin D affects glucose (sugar) metabolism.
ID: NCT01942694
Sponsor: Tufts Medical Center
Locations: Southwest American Indian Center; Phoenix, AZ; Orlando VA Medical Center, FL; Atlanta VA Medical Center, Decatur, GA; Omaha VA Medical Center, NE
Reducing Diabetes Risk Factors in American Indian Children: Tribal Turning Point (TTP)
This study will evaluate a behavioral intervention designed to reduce risk factors for type 2 diabetes in American Indian youth aged 7-10 years.
ID: NCT03573856
Sponsor: University of Colorado, Denver
Contact: Katherine Sauder, PhD, [email protected]; Dana Dabelea, MD, PhD, [email protected]
Location: Childrens Hospital Colorado, Aurora
Strong Men, Strong Communities Diabetes Risk Reduction in American Indian Men (SMSC)
SMSC will inform the design and implementation of culturally informed, community-based lifestyle interventions for diabetes prevention in AI men in our partner communities and elsewhere, as well as in men of other minority groups who experience a heavy burden of diabetes.
ID: NCT02953977
Sponsor: Washington State University
Contact: Kaimi Sinclair, PhD, MPH, [email protected] Location: IREACH, Seattle, WA
Growing Resilience in Wind River Indian Reservation (GR)
The Growing Resilience research leverages reservation-based assets of land, family, culture, and front-line tribal health organizations to develop and evaluate home food gardens as a family-based health promotion intervention to reduce disparities suffered by Native Americans in nearly every measure of health. Home gardening interventions show great promise for enabling families to improve their health, and this study aims to fulfill that promise with university and Wind River Indian Reservation partners. The investigators will develop an empowering, scalable, and sustainable family-based health promotion intervention with, by, and for Native American families and conduct the first randomized controlled trial to assess the health impacts of home gardens.
ID: NCT02672748
Sponsor: University of Wyoming
Location: University of Wyoming, Laramie
A Comparative Effectiveness Study of Major Glycemia-lowering Medications for Treatment of Type 2 Diabetes (GRADE)
The GRADE Study is a pragmatic, unmasked clinical trial that will compare commonly used diabetes medications, when combined with metformin, on glycemia-lowering effectiveness and patient-centered outcomes.
ID: NCT01794143
Sponsor: GRADE Study Group
Location: Southwestern American Indian Center, Phoenix, AZ
Home-Based Kidney Care in Native Americans of New Mexico (HBKC)
New Mexico American Indians are experiencing an epidemic of chronic kidney disease due primarily to the high rates of obesity and diabetes. The present study entitled Home-Based Kidney Care is designed to delay / reduce rates of end stage renal disease by early interventions in chronic kidney disease (CKD). Investigators propose to assess the safety and efficacy of conducting a full-scale study to determine if home based care delivered by a collaborative team composed of community health workers, the Albuquerque Area Indian Health Board and University of New Mexico faculty will decrease the risk for the development and the progression of CKD.
ID: NCT03179085
Sponsor: University of New Mexico
Contact: Vallabh Shah, PhD, [email protected]; Kevin English, PhD, [email protected]
Location: University of New Mexico, Albuquerque
Home-based Prediabetes Care in Acoma Pueblo - Study 1
Our major goal of implementing educational interventions to slow the current rate of increase in diabetes in Native communities is aligned with the National Institute of Health (NIGMS) and New Mexico INBRE’s vision in reducing health disparity using innovative interventions. The investigators propose following aims: (1) Recruit and Screen 300 community members in Acoma Pueblo, New Mexico to identify incident cases of pre-diabetes for the proposed study of Home Based Diabetes Care (HBDC); (2) Enroll 150 Acoma Natives aged 21-70 years, at risk for type 2 diabetes mellitus and conduct HBDC for a 16-week lifestyle intervention in a longitudinal cohort study.
ID: NCT04029298
Sponsor: University of New Mexico
Contact: Matthew Bouchonville, MD, [email protected]; Vallabh Shah, PhD, [email protected]