User login
‘We Will Rock You’ Into Real-time Diabetes Control
, reveals a series of experiments.
The research was published in The Lancet Diabetes & Endocrinology.
After developing a cell line in which music-sensitive calcium channels triggered the release of insulin-containing vesicles, the researchers conducted a series of studies identifying the optimal frequency, pitch, and volume of sounds for triggering release.
After settling on low-bass heavy popular music, they tested their system on mice with type 1 diabetes that had the insulin-releasing cells implanted in their abdomen. Applying the music directly at 60 dB led to near wild-type levels of insulin in the blood within 15 minutes.
“With only 4 hours required for a full refill, [the system] can provide several therapeutic doses a day,” says Martin Fussenegger, PhD, professor of biotechnology and bioengineering, Department of Biosystems Science and Engineering, ETH Zurich, Basel, Switzerland, and colleagues.
“This would match the typical needs of people with type 2 diabetes consuming three meals a day, and for whom administration of prandial insulin is an established treatment option, as they do not have capability for early postprandial insulin secretion from preformed insulin.”
As the system requires nothing more than portable battery-powered commercially available loudspeakers, the multiple daily dosing of biopharmaceuticals becomes “straightforward in the absence of medical infrastructure or staff, simply by having the patient listen to the prescribed music.”
It therefore “could be an interesting option for cell-based therapies, especially where the need for frequent dosing raises compliance issues.”
It is a “very exciting piece of work, no doubt,” said Anandwardhan A. Hardikar, PhD, group leader, Diabetes and Islet Biology Group, Translational Health Research Institute, Western Sydney University, Penrith NSW, Australia.
He pointed out that the concept of using music to drive gene expression “is something we’ve known for the last 20 years,” but bringing the different strands of research together to generate cells that can be implanted into mice is “an amazing idea.”
Dr. Hardikar, who was not involved in the study, said, however, the publication of the study as a correspondence “does not allow for a lot of the detail that I would have expected as an academic,” and consequently some questions remain.
The most important is whether the music itself is required to trigger the insulin release, as opposed simply to sounds in general.
Is Music or Sound the “Trigger?”
Music is “frequency, it’s the amplitude of the waveform, and it’s the duration for which those waveforms are present,” he noted, but the same profile can be achieved by cutting up and editing the melody so it becomes a jumble of sounds.
For Dr. Hardikar, the “best control” for the study would be to have no music as well as the edited song, with “bits of pieces” played randomly so “it sounds like it’s the same frequency and amplitude.”
Then it would be clear whether the effect is owing to the “noise, or we have to appreciate the melody.”
The other outstanding question is whether the results “can directly translate to larger animals,” such as humans, Dr. Hardikar said.
The authors point out that when translated into mechanical vibrations in the middle ear, the acoustic waves of music activate mechanosensitive ion channels, a form of trigger that is seen across the animal kingdom.
They go on to highlight that while gene switches have been developed for use in next-generation cell-based therapies for a range of conditions, small-molecular trigger compounds face a number of challenges and may cause adverse effects.
With “traceless triggers” such as light, ultrasound, magnetic fields, radio waves, electricity, and heat also facing issues, there is a “need for new switching modalities.”
The researchers therefore developed a music-inducible cellular control (MUSIC) system, which leverages the known intracellular calcium surge in response to music, via calcium-permeable mechanosensitive channels, to drive the release of biopharmaceuticals from vesicles.
They then generated MUSIC-controlled insulin-releasing cell lines, finding that, using a customized box containing off-the-shelf loudspeakers, they could induce channel activation and insulin release with 60 dB at 50 Hz, which is “within the safe range for the human ear.”
Further experiments revealed that insulin release was greatest at 50-100 Hz, and higher than that seen with potassium chloride, the “gold-standard” depolarization control for calcium channels.
The researchers then showed that with optimal stimulation at 50 Hz and 60 dB, channel activation and subsequent insulin release required at least 3 seconds of continuous music, “which might protect the cellular device from inadvertent activation during everyday activities.”
Next, they examined the impact of different musical genres on insulin release, finding that low-bass heavy popular music and movie soundtracks induced maximum release, while the responses were more diverse to classical and guitar-based music.
Specifically, “We Will Rock You,” by the British rock band Queen, induced the release of 70% of available insulin within 5 minutes and 100% within 15 minutes. This, the team notes, is “similar to the dynamics of glucose-triggered insulin release by human pancreatic islets.”
Exposing the cells to a second music session at different intervals revealed that full insulin refill was achieved within 4 hours, which “would be appropriate to attenuate glycemic excursions associated with typical dietary habits.”
Finally, the researchers tested the system in vivo, constructing a box with two off-the-shelf loudspeakers that focuses acoustic waves, via deflectors, onto the abdomens of mice with type 1 diabetes.
Exposing the mice, which had been implanted with microencapsulated MUSIC cells in the peritoneum, to low-bass acoustic waves at 60 dB (50 m/s2) for 15 minutes allowed them to achieve near wild-type levels of insulin in the blood and restored normoglycemia.
Moreover, “Queen’s song ‘We Will Rock You’ generated sufficient insulin to rapidly attenuate postprandial glycemic excursions during glucose tolerance tests,” the team says.
In contrast, animals without implants, or those that had implants but did not have music immersion, remained severely hyperglycemic, they add.
They also note that the effect was seen only when the sound waves “directly impinge on the skin just above the implantation site” for at least 15 minutes, with no increase in insulin release observed with commercially available headphones or ear plugs, such as Apple AirPods, or with loud environmental noises.
Consequently, “therapeutic MUSIC sessions would still be compatible with listening to other types of music or listening to all types of music via headphones,” the researchers write, and are “compatible with standard drug administration schemes.”
The study was supported by a European Research Council advanced grant and in part by the Swiss National Science Foundation NCCR Molecular Systems Engineering. One author acknowledges the support of the Chinese Scholarship Council.
No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
, reveals a series of experiments.
The research was published in The Lancet Diabetes & Endocrinology.
After developing a cell line in which music-sensitive calcium channels triggered the release of insulin-containing vesicles, the researchers conducted a series of studies identifying the optimal frequency, pitch, and volume of sounds for triggering release.
After settling on low-bass heavy popular music, they tested their system on mice with type 1 diabetes that had the insulin-releasing cells implanted in their abdomen. Applying the music directly at 60 dB led to near wild-type levels of insulin in the blood within 15 minutes.
“With only 4 hours required for a full refill, [the system] can provide several therapeutic doses a day,” says Martin Fussenegger, PhD, professor of biotechnology and bioengineering, Department of Biosystems Science and Engineering, ETH Zurich, Basel, Switzerland, and colleagues.
“This would match the typical needs of people with type 2 diabetes consuming three meals a day, and for whom administration of prandial insulin is an established treatment option, as they do not have capability for early postprandial insulin secretion from preformed insulin.”
As the system requires nothing more than portable battery-powered commercially available loudspeakers, the multiple daily dosing of biopharmaceuticals becomes “straightforward in the absence of medical infrastructure or staff, simply by having the patient listen to the prescribed music.”
It therefore “could be an interesting option for cell-based therapies, especially where the need for frequent dosing raises compliance issues.”
It is a “very exciting piece of work, no doubt,” said Anandwardhan A. Hardikar, PhD, group leader, Diabetes and Islet Biology Group, Translational Health Research Institute, Western Sydney University, Penrith NSW, Australia.
He pointed out that the concept of using music to drive gene expression “is something we’ve known for the last 20 years,” but bringing the different strands of research together to generate cells that can be implanted into mice is “an amazing idea.”
Dr. Hardikar, who was not involved in the study, said, however, the publication of the study as a correspondence “does not allow for a lot of the detail that I would have expected as an academic,” and consequently some questions remain.
The most important is whether the music itself is required to trigger the insulin release, as opposed simply to sounds in general.
Is Music or Sound the “Trigger?”
Music is “frequency, it’s the amplitude of the waveform, and it’s the duration for which those waveforms are present,” he noted, but the same profile can be achieved by cutting up and editing the melody so it becomes a jumble of sounds.
For Dr. Hardikar, the “best control” for the study would be to have no music as well as the edited song, with “bits of pieces” played randomly so “it sounds like it’s the same frequency and amplitude.”
Then it would be clear whether the effect is owing to the “noise, or we have to appreciate the melody.”
The other outstanding question is whether the results “can directly translate to larger animals,” such as humans, Dr. Hardikar said.
The authors point out that when translated into mechanical vibrations in the middle ear, the acoustic waves of music activate mechanosensitive ion channels, a form of trigger that is seen across the animal kingdom.
They go on to highlight that while gene switches have been developed for use in next-generation cell-based therapies for a range of conditions, small-molecular trigger compounds face a number of challenges and may cause adverse effects.
With “traceless triggers” such as light, ultrasound, magnetic fields, radio waves, electricity, and heat also facing issues, there is a “need for new switching modalities.”
The researchers therefore developed a music-inducible cellular control (MUSIC) system, which leverages the known intracellular calcium surge in response to music, via calcium-permeable mechanosensitive channels, to drive the release of biopharmaceuticals from vesicles.
They then generated MUSIC-controlled insulin-releasing cell lines, finding that, using a customized box containing off-the-shelf loudspeakers, they could induce channel activation and insulin release with 60 dB at 50 Hz, which is “within the safe range for the human ear.”
Further experiments revealed that insulin release was greatest at 50-100 Hz, and higher than that seen with potassium chloride, the “gold-standard” depolarization control for calcium channels.
The researchers then showed that with optimal stimulation at 50 Hz and 60 dB, channel activation and subsequent insulin release required at least 3 seconds of continuous music, “which might protect the cellular device from inadvertent activation during everyday activities.”
Next, they examined the impact of different musical genres on insulin release, finding that low-bass heavy popular music and movie soundtracks induced maximum release, while the responses were more diverse to classical and guitar-based music.
Specifically, “We Will Rock You,” by the British rock band Queen, induced the release of 70% of available insulin within 5 minutes and 100% within 15 minutes. This, the team notes, is “similar to the dynamics of glucose-triggered insulin release by human pancreatic islets.”
Exposing the cells to a second music session at different intervals revealed that full insulin refill was achieved within 4 hours, which “would be appropriate to attenuate glycemic excursions associated with typical dietary habits.”
Finally, the researchers tested the system in vivo, constructing a box with two off-the-shelf loudspeakers that focuses acoustic waves, via deflectors, onto the abdomens of mice with type 1 diabetes.
Exposing the mice, which had been implanted with microencapsulated MUSIC cells in the peritoneum, to low-bass acoustic waves at 60 dB (50 m/s2) for 15 minutes allowed them to achieve near wild-type levels of insulin in the blood and restored normoglycemia.
Moreover, “Queen’s song ‘We Will Rock You’ generated sufficient insulin to rapidly attenuate postprandial glycemic excursions during glucose tolerance tests,” the team says.
In contrast, animals without implants, or those that had implants but did not have music immersion, remained severely hyperglycemic, they add.
They also note that the effect was seen only when the sound waves “directly impinge on the skin just above the implantation site” for at least 15 minutes, with no increase in insulin release observed with commercially available headphones or ear plugs, such as Apple AirPods, or with loud environmental noises.
Consequently, “therapeutic MUSIC sessions would still be compatible with listening to other types of music or listening to all types of music via headphones,” the researchers write, and are “compatible with standard drug administration schemes.”
The study was supported by a European Research Council advanced grant and in part by the Swiss National Science Foundation NCCR Molecular Systems Engineering. One author acknowledges the support of the Chinese Scholarship Council.
No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
, reveals a series of experiments.
The research was published in The Lancet Diabetes & Endocrinology.
After developing a cell line in which music-sensitive calcium channels triggered the release of insulin-containing vesicles, the researchers conducted a series of studies identifying the optimal frequency, pitch, and volume of sounds for triggering release.
After settling on low-bass heavy popular music, they tested their system on mice with type 1 diabetes that had the insulin-releasing cells implanted in their abdomen. Applying the music directly at 60 dB led to near wild-type levels of insulin in the blood within 15 minutes.
“With only 4 hours required for a full refill, [the system] can provide several therapeutic doses a day,” says Martin Fussenegger, PhD, professor of biotechnology and bioengineering, Department of Biosystems Science and Engineering, ETH Zurich, Basel, Switzerland, and colleagues.
“This would match the typical needs of people with type 2 diabetes consuming three meals a day, and for whom administration of prandial insulin is an established treatment option, as they do not have capability for early postprandial insulin secretion from preformed insulin.”
As the system requires nothing more than portable battery-powered commercially available loudspeakers, the multiple daily dosing of biopharmaceuticals becomes “straightforward in the absence of medical infrastructure or staff, simply by having the patient listen to the prescribed music.”
It therefore “could be an interesting option for cell-based therapies, especially where the need for frequent dosing raises compliance issues.”
It is a “very exciting piece of work, no doubt,” said Anandwardhan A. Hardikar, PhD, group leader, Diabetes and Islet Biology Group, Translational Health Research Institute, Western Sydney University, Penrith NSW, Australia.
He pointed out that the concept of using music to drive gene expression “is something we’ve known for the last 20 years,” but bringing the different strands of research together to generate cells that can be implanted into mice is “an amazing idea.”
Dr. Hardikar, who was not involved in the study, said, however, the publication of the study as a correspondence “does not allow for a lot of the detail that I would have expected as an academic,” and consequently some questions remain.
The most important is whether the music itself is required to trigger the insulin release, as opposed simply to sounds in general.
Is Music or Sound the “Trigger?”
Music is “frequency, it’s the amplitude of the waveform, and it’s the duration for which those waveforms are present,” he noted, but the same profile can be achieved by cutting up and editing the melody so it becomes a jumble of sounds.
For Dr. Hardikar, the “best control” for the study would be to have no music as well as the edited song, with “bits of pieces” played randomly so “it sounds like it’s the same frequency and amplitude.”
Then it would be clear whether the effect is owing to the “noise, or we have to appreciate the melody.”
The other outstanding question is whether the results “can directly translate to larger animals,” such as humans, Dr. Hardikar said.
The authors point out that when translated into mechanical vibrations in the middle ear, the acoustic waves of music activate mechanosensitive ion channels, a form of trigger that is seen across the animal kingdom.
They go on to highlight that while gene switches have been developed for use in next-generation cell-based therapies for a range of conditions, small-molecular trigger compounds face a number of challenges and may cause adverse effects.
With “traceless triggers” such as light, ultrasound, magnetic fields, radio waves, electricity, and heat also facing issues, there is a “need for new switching modalities.”
The researchers therefore developed a music-inducible cellular control (MUSIC) system, which leverages the known intracellular calcium surge in response to music, via calcium-permeable mechanosensitive channels, to drive the release of biopharmaceuticals from vesicles.
They then generated MUSIC-controlled insulin-releasing cell lines, finding that, using a customized box containing off-the-shelf loudspeakers, they could induce channel activation and insulin release with 60 dB at 50 Hz, which is “within the safe range for the human ear.”
Further experiments revealed that insulin release was greatest at 50-100 Hz, and higher than that seen with potassium chloride, the “gold-standard” depolarization control for calcium channels.
The researchers then showed that with optimal stimulation at 50 Hz and 60 dB, channel activation and subsequent insulin release required at least 3 seconds of continuous music, “which might protect the cellular device from inadvertent activation during everyday activities.”
Next, they examined the impact of different musical genres on insulin release, finding that low-bass heavy popular music and movie soundtracks induced maximum release, while the responses were more diverse to classical and guitar-based music.
Specifically, “We Will Rock You,” by the British rock band Queen, induced the release of 70% of available insulin within 5 minutes and 100% within 15 minutes. This, the team notes, is “similar to the dynamics of glucose-triggered insulin release by human pancreatic islets.”
Exposing the cells to a second music session at different intervals revealed that full insulin refill was achieved within 4 hours, which “would be appropriate to attenuate glycemic excursions associated with typical dietary habits.”
Finally, the researchers tested the system in vivo, constructing a box with two off-the-shelf loudspeakers that focuses acoustic waves, via deflectors, onto the abdomens of mice with type 1 diabetes.
Exposing the mice, which had been implanted with microencapsulated MUSIC cells in the peritoneum, to low-bass acoustic waves at 60 dB (50 m/s2) for 15 minutes allowed them to achieve near wild-type levels of insulin in the blood and restored normoglycemia.
Moreover, “Queen’s song ‘We Will Rock You’ generated sufficient insulin to rapidly attenuate postprandial glycemic excursions during glucose tolerance tests,” the team says.
In contrast, animals without implants, or those that had implants but did not have music immersion, remained severely hyperglycemic, they add.
They also note that the effect was seen only when the sound waves “directly impinge on the skin just above the implantation site” for at least 15 minutes, with no increase in insulin release observed with commercially available headphones or ear plugs, such as Apple AirPods, or with loud environmental noises.
Consequently, “therapeutic MUSIC sessions would still be compatible with listening to other types of music or listening to all types of music via headphones,” the researchers write, and are “compatible with standard drug administration schemes.”
The study was supported by a European Research Council advanced grant and in part by the Swiss National Science Foundation NCCR Molecular Systems Engineering. One author acknowledges the support of the Chinese Scholarship Council.
No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
Erectile Dysfunction Rx: Give It a Shot
This transcript has been edited for clarity.
I’m Dr Rachel Rubin. I am a urologist with fellowship training in sexual medicine. Today I’m going to explain why I may recommend that your patients put a needle directly into their penises for help with erectile dysfunction (ED).
I know that sounds crazy, but in a recent video when I talked about erection hardness, I acknowledged that it may not be easy to talk with patients about their penises, but it’s important.
ED can be a marker for cardiovascular disease, with 50% of our 50-year-old patients having ED. As physicians, we must do a better job of talking to our patients about ED and letting them know that it’s a marker for overall health.
How do we treat ED? Primary care doctors can do a great deal for patients with ED, and there are other things that urologists can do when you run out of options in your own toolbox.
What’s important for a healthy erection? You need three things: healthy muscle, healthy nerves, and healthy arteries. If anything goes wrong with muscles, nerves, or arteries, this is what leads to ED. Think through the algorithm of your patient’s medical history: Do they have diabetes, which can affect their nerves? Do they have high blood pressure, which can affect their arteries? Do they have problems with testosterone, which can affect the smooth muscles of the penis? Understanding your patient’s history can be really helpful when you figure out what is the best treatment strategy for your patient.
For the penis to work, those smooth muscles have to relax; therefore, your brain has to be relaxed, along with your pelvic floor muscles. The smooth muscle of the penis has to be relaxed so it can fill with blood, increase in girth and size, and hold that erection in place.
To treat ED, we have a biopsychosocial toolbox. Biology refers to the muscles, arteries, and nerves. The psychosocial component is stress: If your brain is stressed, you have a lot of adrenaline around that can tighten those smooth muscles and cause you to lose an erection.
So, what are these treatments? I’ll start with lifestyle. A healthy heart means a healthy penis, so, all of the things you already recommend for lifestyle changes can really help with ED. Sleep is important. Does your patient need a sleep study? Do they have sleep apnea? Are they exercising? Recent data show that exercise may be just as effective, if not more effective, than Viagra. How about a good diet? The Mediterranean diet seems to be the most helpful. So, encourage your patients to make dietary, exercise, sleep, and other lifestyle changes if they want to improve erectile function.
What about sex education? Most physicians didn’t get great education about sex in medical school, but it’s very important to our patients who likewise have had inadequate sex education. Ask questions, talk to them, explain what is normal.
I can’t stress enough how important mental health is to a great sex life. Everyone would benefit from sex therapy and becoming better at sex. We need to get better at communicating and educating patients and their partners to maximize their quality of life. If you need to refer to a specialist, we recommend going to psychologytoday.com or aasect.org to find a local sex therapist. Call them and use them in your referral networks.
In the “bio” component of the biopsychosocial approach, we can do a lot to treat ED with medications and hormones. Testosterone has been shown to help with low libido and erectile function. Checking the patient’s testosterone level can be very helpful. Pills — we are familiar with Viagra, Cialis, Levitra, and Stendra. The oral PDE-5 inhibitors have been around since the late 1990s and they work quite well for many people with ED. Viagra and Cialis are generic now and patients can get them fairly inexpensively with discount coupons from GoodRx or Cost Plus Drugs. They may not even have to worry about insurance coverage.
Pills relax the smooth muscle of the penis so that it fills with blood and becomes erect, but they don’t work for everybody. If pills stop working, we often talk about synergistic treatments — combining pills and devices. Devices for ED should be discussed more often, and clinicians should consider prescribing them. We commonly discuss eyeglasses and wheelchairs, but we don’t talk about the sexual health devices that could help patients have more success and fun in the bedroom.
What are the various types of devices for ED? One common device is a vacuum pump, which can be very effective. This is how they work: The penis is lubricated and placed into the pump. A button on the pump creates suction that brings blood into the penis. The patient then applies a constriction band around the base of the penis to hold that erection in place.
“Sex tech” has really expanded to help patients with ED with devices that vibrate and hold the erection in place. Vibrating devices allow for a better orgasm. We even have devices that monitor erectile fitness (like a Fitbit for the penis), gathering data to help patients understand the firmness of their erections.
Devices are helpful adjuncts, but they don’t always do enough to achieve an erect penis that’s hard enough for penetration. In those cases, we can recommend injections that increase smooth muscle relaxation of the penis. I know it sounds crazy. If the muscles, arteries, and nerves of the penis aren’t functioning well, additional smooth muscle relaxation can be achieved by injecting alprostadil (prostaglandin E1) directly into the penis. It’s a tiny needle. It doesn’t hurt. These injections can be quite helpful for our patients, and we often recommend them.
But what happens when your patient doesn’t even respond to injections or any of the synergistic treatments? They’ve tried everything. Urologists may suggest a surgical option, the penile implant. Penile implants contain a pump inside the scrotum that fills with fluid, allowing a rigid erection. Penile implants are wonderful for patients who can no longer get erections. Talking to a urologist about the pros and the cons and the risks and benefits of surgically placed implants is very important.
Finally, ED is a marker for cardiovascular disease. These patients may need a cardiology workup. They need to improve their general health. We have to ask our patients about their goals and what they care about, and find a toolbox that makes sense for each patient and couple to maximize their sexual health and quality of life. Don’t give up. If you have questions, let us know.
Rachel S. Rubin, MD, is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, Rachel Rubin MD PLLC, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr Rachel Rubin. I am a urologist with fellowship training in sexual medicine. Today I’m going to explain why I may recommend that your patients put a needle directly into their penises for help with erectile dysfunction (ED).
I know that sounds crazy, but in a recent video when I talked about erection hardness, I acknowledged that it may not be easy to talk with patients about their penises, but it’s important.
ED can be a marker for cardiovascular disease, with 50% of our 50-year-old patients having ED. As physicians, we must do a better job of talking to our patients about ED and letting them know that it’s a marker for overall health.
How do we treat ED? Primary care doctors can do a great deal for patients with ED, and there are other things that urologists can do when you run out of options in your own toolbox.
What’s important for a healthy erection? You need three things: healthy muscle, healthy nerves, and healthy arteries. If anything goes wrong with muscles, nerves, or arteries, this is what leads to ED. Think through the algorithm of your patient’s medical history: Do they have diabetes, which can affect their nerves? Do they have high blood pressure, which can affect their arteries? Do they have problems with testosterone, which can affect the smooth muscles of the penis? Understanding your patient’s history can be really helpful when you figure out what is the best treatment strategy for your patient.
For the penis to work, those smooth muscles have to relax; therefore, your brain has to be relaxed, along with your pelvic floor muscles. The smooth muscle of the penis has to be relaxed so it can fill with blood, increase in girth and size, and hold that erection in place.
To treat ED, we have a biopsychosocial toolbox. Biology refers to the muscles, arteries, and nerves. The psychosocial component is stress: If your brain is stressed, you have a lot of adrenaline around that can tighten those smooth muscles and cause you to lose an erection.
So, what are these treatments? I’ll start with lifestyle. A healthy heart means a healthy penis, so, all of the things you already recommend for lifestyle changes can really help with ED. Sleep is important. Does your patient need a sleep study? Do they have sleep apnea? Are they exercising? Recent data show that exercise may be just as effective, if not more effective, than Viagra. How about a good diet? The Mediterranean diet seems to be the most helpful. So, encourage your patients to make dietary, exercise, sleep, and other lifestyle changes if they want to improve erectile function.
What about sex education? Most physicians didn’t get great education about sex in medical school, but it’s very important to our patients who likewise have had inadequate sex education. Ask questions, talk to them, explain what is normal.
I can’t stress enough how important mental health is to a great sex life. Everyone would benefit from sex therapy and becoming better at sex. We need to get better at communicating and educating patients and their partners to maximize their quality of life. If you need to refer to a specialist, we recommend going to psychologytoday.com or aasect.org to find a local sex therapist. Call them and use them in your referral networks.
In the “bio” component of the biopsychosocial approach, we can do a lot to treat ED with medications and hormones. Testosterone has been shown to help with low libido and erectile function. Checking the patient’s testosterone level can be very helpful. Pills — we are familiar with Viagra, Cialis, Levitra, and Stendra. The oral PDE-5 inhibitors have been around since the late 1990s and they work quite well for many people with ED. Viagra and Cialis are generic now and patients can get them fairly inexpensively with discount coupons from GoodRx or Cost Plus Drugs. They may not even have to worry about insurance coverage.
Pills relax the smooth muscle of the penis so that it fills with blood and becomes erect, but they don’t work for everybody. If pills stop working, we often talk about synergistic treatments — combining pills and devices. Devices for ED should be discussed more often, and clinicians should consider prescribing them. We commonly discuss eyeglasses and wheelchairs, but we don’t talk about the sexual health devices that could help patients have more success and fun in the bedroom.
What are the various types of devices for ED? One common device is a vacuum pump, which can be very effective. This is how they work: The penis is lubricated and placed into the pump. A button on the pump creates suction that brings blood into the penis. The patient then applies a constriction band around the base of the penis to hold that erection in place.
“Sex tech” has really expanded to help patients with ED with devices that vibrate and hold the erection in place. Vibrating devices allow for a better orgasm. We even have devices that monitor erectile fitness (like a Fitbit for the penis), gathering data to help patients understand the firmness of their erections.
Devices are helpful adjuncts, but they don’t always do enough to achieve an erect penis that’s hard enough for penetration. In those cases, we can recommend injections that increase smooth muscle relaxation of the penis. I know it sounds crazy. If the muscles, arteries, and nerves of the penis aren’t functioning well, additional smooth muscle relaxation can be achieved by injecting alprostadil (prostaglandin E1) directly into the penis. It’s a tiny needle. It doesn’t hurt. These injections can be quite helpful for our patients, and we often recommend them.
But what happens when your patient doesn’t even respond to injections or any of the synergistic treatments? They’ve tried everything. Urologists may suggest a surgical option, the penile implant. Penile implants contain a pump inside the scrotum that fills with fluid, allowing a rigid erection. Penile implants are wonderful for patients who can no longer get erections. Talking to a urologist about the pros and the cons and the risks and benefits of surgically placed implants is very important.
Finally, ED is a marker for cardiovascular disease. These patients may need a cardiology workup. They need to improve their general health. We have to ask our patients about their goals and what they care about, and find a toolbox that makes sense for each patient and couple to maximize their sexual health and quality of life. Don’t give up. If you have questions, let us know.
Rachel S. Rubin, MD, is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, Rachel Rubin MD PLLC, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr Rachel Rubin. I am a urologist with fellowship training in sexual medicine. Today I’m going to explain why I may recommend that your patients put a needle directly into their penises for help with erectile dysfunction (ED).
I know that sounds crazy, but in a recent video when I talked about erection hardness, I acknowledged that it may not be easy to talk with patients about their penises, but it’s important.
ED can be a marker for cardiovascular disease, with 50% of our 50-year-old patients having ED. As physicians, we must do a better job of talking to our patients about ED and letting them know that it’s a marker for overall health.
How do we treat ED? Primary care doctors can do a great deal for patients with ED, and there are other things that urologists can do when you run out of options in your own toolbox.
What’s important for a healthy erection? You need three things: healthy muscle, healthy nerves, and healthy arteries. If anything goes wrong with muscles, nerves, or arteries, this is what leads to ED. Think through the algorithm of your patient’s medical history: Do they have diabetes, which can affect their nerves? Do they have high blood pressure, which can affect their arteries? Do they have problems with testosterone, which can affect the smooth muscles of the penis? Understanding your patient’s history can be really helpful when you figure out what is the best treatment strategy for your patient.
For the penis to work, those smooth muscles have to relax; therefore, your brain has to be relaxed, along with your pelvic floor muscles. The smooth muscle of the penis has to be relaxed so it can fill with blood, increase in girth and size, and hold that erection in place.
To treat ED, we have a biopsychosocial toolbox. Biology refers to the muscles, arteries, and nerves. The psychosocial component is stress: If your brain is stressed, you have a lot of adrenaline around that can tighten those smooth muscles and cause you to lose an erection.
So, what are these treatments? I’ll start with lifestyle. A healthy heart means a healthy penis, so, all of the things you already recommend for lifestyle changes can really help with ED. Sleep is important. Does your patient need a sleep study? Do they have sleep apnea? Are they exercising? Recent data show that exercise may be just as effective, if not more effective, than Viagra. How about a good diet? The Mediterranean diet seems to be the most helpful. So, encourage your patients to make dietary, exercise, sleep, and other lifestyle changes if they want to improve erectile function.
What about sex education? Most physicians didn’t get great education about sex in medical school, but it’s very important to our patients who likewise have had inadequate sex education. Ask questions, talk to them, explain what is normal.
I can’t stress enough how important mental health is to a great sex life. Everyone would benefit from sex therapy and becoming better at sex. We need to get better at communicating and educating patients and their partners to maximize their quality of life. If you need to refer to a specialist, we recommend going to psychologytoday.com or aasect.org to find a local sex therapist. Call them and use them in your referral networks.
In the “bio” component of the biopsychosocial approach, we can do a lot to treat ED with medications and hormones. Testosterone has been shown to help with low libido and erectile function. Checking the patient’s testosterone level can be very helpful. Pills — we are familiar with Viagra, Cialis, Levitra, and Stendra. The oral PDE-5 inhibitors have been around since the late 1990s and they work quite well for many people with ED. Viagra and Cialis are generic now and patients can get them fairly inexpensively with discount coupons from GoodRx or Cost Plus Drugs. They may not even have to worry about insurance coverage.
Pills relax the smooth muscle of the penis so that it fills with blood and becomes erect, but they don’t work for everybody. If pills stop working, we often talk about synergistic treatments — combining pills and devices. Devices for ED should be discussed more often, and clinicians should consider prescribing them. We commonly discuss eyeglasses and wheelchairs, but we don’t talk about the sexual health devices that could help patients have more success and fun in the bedroom.
What are the various types of devices for ED? One common device is a vacuum pump, which can be very effective. This is how they work: The penis is lubricated and placed into the pump. A button on the pump creates suction that brings blood into the penis. The patient then applies a constriction band around the base of the penis to hold that erection in place.
“Sex tech” has really expanded to help patients with ED with devices that vibrate and hold the erection in place. Vibrating devices allow for a better orgasm. We even have devices that monitor erectile fitness (like a Fitbit for the penis), gathering data to help patients understand the firmness of their erections.
Devices are helpful adjuncts, but they don’t always do enough to achieve an erect penis that’s hard enough for penetration. In those cases, we can recommend injections that increase smooth muscle relaxation of the penis. I know it sounds crazy. If the muscles, arteries, and nerves of the penis aren’t functioning well, additional smooth muscle relaxation can be achieved by injecting alprostadil (prostaglandin E1) directly into the penis. It’s a tiny needle. It doesn’t hurt. These injections can be quite helpful for our patients, and we often recommend them.
But what happens when your patient doesn’t even respond to injections or any of the synergistic treatments? They’ve tried everything. Urologists may suggest a surgical option, the penile implant. Penile implants contain a pump inside the scrotum that fills with fluid, allowing a rigid erection. Penile implants are wonderful for patients who can no longer get erections. Talking to a urologist about the pros and the cons and the risks and benefits of surgically placed implants is very important.
Finally, ED is a marker for cardiovascular disease. These patients may need a cardiology workup. They need to improve their general health. We have to ask our patients about their goals and what they care about, and find a toolbox that makes sense for each patient and couple to maximize their sexual health and quality of life. Don’t give up. If you have questions, let us know.
Rachel S. Rubin, MD, is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, Rachel Rubin MD PLLC, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
Report: CKD Severity Linked to Thinning of Retina, Choroid Layers
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
Changes in tissue thickness in the back of the eye can correlate with worsening or improvement of renal problems and could help predict who will have worsening of kidney function, a new analysis report finds.
The research, published in the journal Nature Communications, is the first to show an association between chronic kidney disease (CKD) and the thickness of the retinal and choroidal layers in the back of the eye as measured by optical coherence tomography (OCT), a noninvasive imaging technology commonly used to evaluate eye diseases such as age-related macular degeneration (AMD), diabetic eye disease, and retinal detachments.
“These are common scans that people get at the opticians and now in many hospitals,” said Neeraj Dhaun, MD, PhD, a professor of nephrology at the University of Edinburgh, Scotland. (Opticians in the United Kingdom are the equivalent of optometrists in North America.)
CKD Severity Equals Thinner Retinas
“We scanned the back of eye of healthy people as well as patients with various types and degrees of kidney disease, and we found that two layers in the back of eye, the retina and the choroid, were thinner in patients with kidney disease compared to people who are healthy, and that the extent of this thinning predicts whether kidney function would decline going forward over a period of 2 or 3 years,” Dr. Dhaun, the corresponding author of the new paper, said.
The publication is a report of four different studies. The first study measured OCT metrics in 112 patients with CKD, 92 patients with a functional kidney transplant, and 86 control volunteers. The researchers found the retina was 5% thinner in patients with CKD than in healthy controls. They also found that patients with CKD had reduced macular volume: 8.44 ± .44 mm3 vs 8.73 ± .36 mm3 (P < .001). The choroid was also found to be thinner at each of three macular locations measured in patients with CKD vs control volunteers. At baseline, CKD and transplant patients had significantly lower estimated glomerular filtration rate (eGFR) at 55 ± 27 and 55 ± 24 mL/min/1.73 m2 compared with control volunteers at 97 ± 14 mL/min/1.73 m2.
The second study reported on OCT measurements and kidney histologic injury in 50 patients who had a kidney biopsy within 30 days of their OCT. It found that choroidal thinning at all three macular locations was independently associated with more extensive kidney scarring.
The third study focused on 25 patients with kidney failure who had a kidney transplant. Their eGFR improved from 8 ± 3 to 58 ± 21 mL/min/1.73 m2 in the first week after the transplant. The choroid in these patients thickened about 5% at 1 week and by about 10% at 1 month posttransplant. OCT of 22 kidney donors showed thickening of the choroid a week after nephrectomy before a tendency to thinning over the next year.
The fourth study found that for patients with stable CKD, every 1 mm3 decrease in macular volume correlated to an increased odds of a decline in eGFR by more than 10% at 1 year (2.48; 95% CI, 1.26-5.08; P = .01) and by more than 20% at 2 years (3.75; 95% CI, 1.26-5.08; P = .004).
Exploring the Kidney-Eye Connection
The potential explanation for the correlation between retinal and choroidal thickness and kidney function is unclear, Dr. Dhaun said.
“We don’t know the exact mechanisms, and these are difficult to define from studies in patients, which is why we are doing more work in animal models of kidney disease to see if we can establish the pathways that lead to the changes in the eye,” he said.
“However,” Dr. Dhaun added, “what we do know is that kidney disease affects the whole body. For example, kidney disease can lead to high blood pressure and heart disease, as well as diseases in the brain, and it is these effects of kidney disease on the body as whole that we are probably picking up in the back of the eye.”
OCT has the potential to make the monitoring of patients with CKD and kidney transplant more convenient than it is now, Dr. Dhaun said. “These scanners are available in the community, and what would be ideal at some point in the future is to be able to do a patient’s kidney health check in the community potentially incorporating OCT scanning alongside blood-pressure monitoring and other healthcare measures,” he said.
“The findings provide an exciting example of how noninvasive retinal imaging using OCT can provide quantitative biomarkers of systemic disease,” Amir Kashani, MD, PhD, the Boone Pickens Professor of Ophthalmology and Biomedical Engineering at the Wilmer Eye Institute of Johns Hopkins University in Baltimore, told this news organization. “It is striking that their findings demonstrate some potential of reversible changes in choroidal perfusion after kidney transplantation.”
The finding that choroidal thickness changes in CKD are at least partly reversible with kidney transplantation is a revelation, Dr. Kashani said, and may point to a greater role for ophthalmologists in managing systemic disease.
“Ophthalmologists can and should use their unique experience and understanding of the eye to help monitor and manage systemic conditions in collaboration with our medicine colleagues,” he said. “There are many systemic diseases that can impact the eye and ophthalmologist are uniquely positioned to help interpret those findings.”
Dr. Kashani noted that a particular strength of the report was the comparison of choroidal measurements in patients who had kidney transplantation and those that had a nephrectomy. “The consistent direction of changes in these two groups suggests the study findings are real and meaningful,” he said.
The study was independently supported. Dr. Dhaun and co-authors report no relevant financial relationships. Dr. Kashani disclosed a financial relationship with Carl Zeiss Meditec.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
What if a single GLP-1 shot could last for months?
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
FROM CELL REPORTS MEDICINE
How to prescribe Zepbound
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 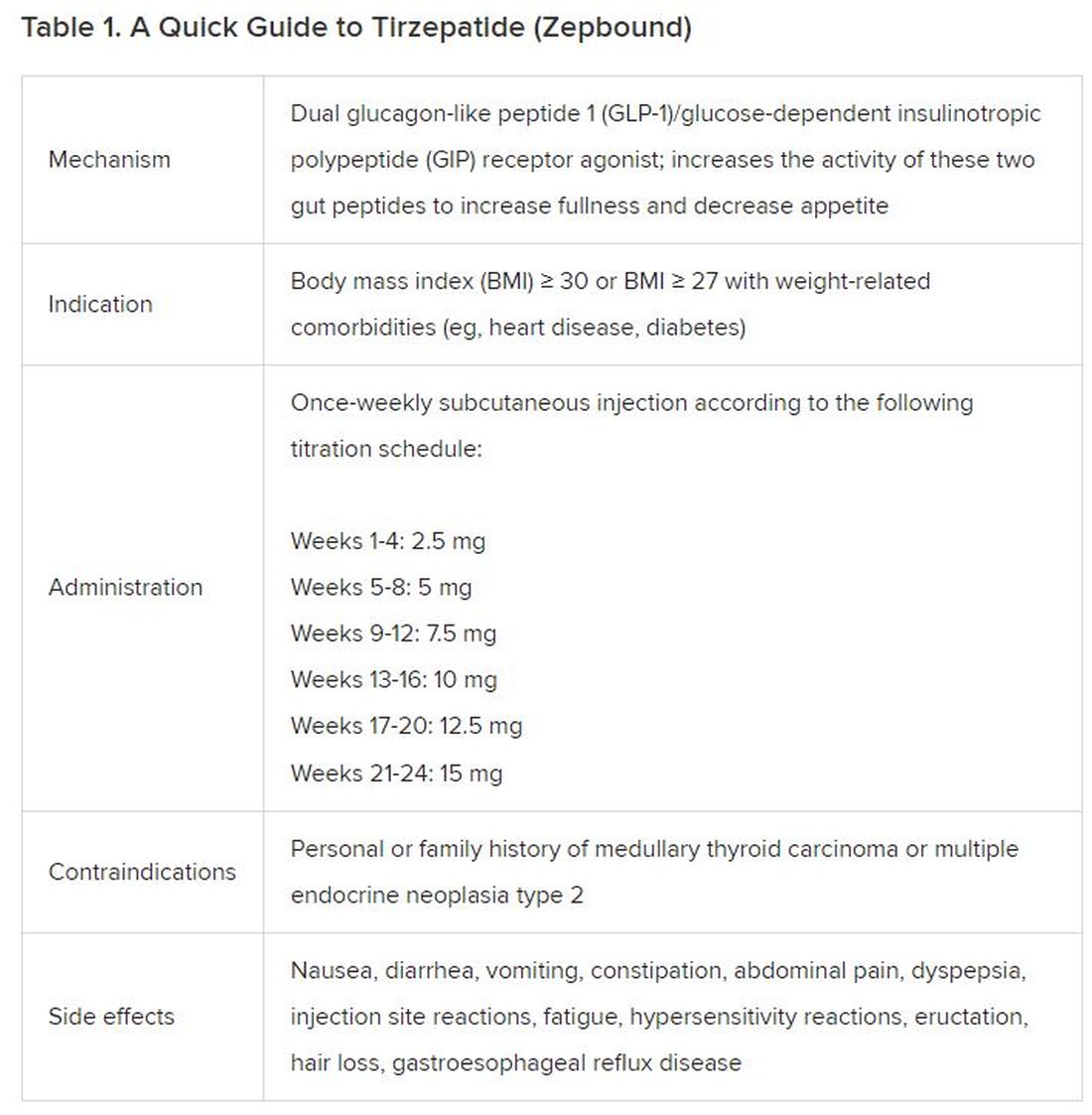
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).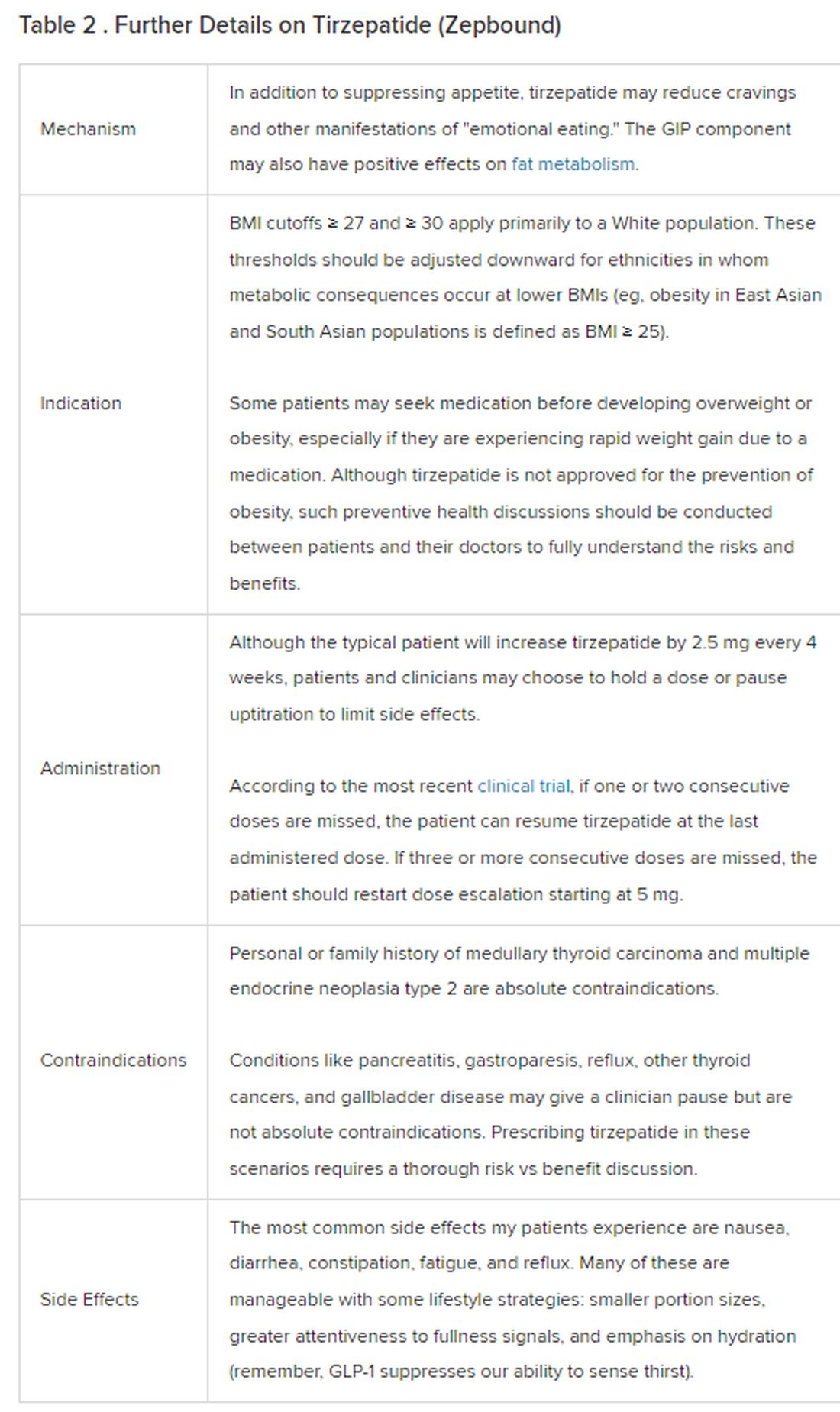
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
New KDIGO guideline encourages use of HCV-positive kidneys for HCV-negative recipients
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
ADA issues new screening, obesity management recommendations
for 2024.
“The Standards of Care are essentially the global guidelines for the care of individuals with diabetes and those at risk,” ADA chief scientific and medical officer Robert Gabbay, MD, PhD, said during a briefing announcing the new Standards.
The document was developed via a scientific literature review by the ADA’s Professional Practice Committee. The panel comprises 21 professionals, including physicians from many specialties, nurse practitioners, certified diabetes care and education specialists, dietitians, and pharmacists. The chair is Nuha A. El Sayed, MD, ADA’s senior vice president of healthcare improvement.
Specific sections of the 2024 document have been endorsed by the American College of Cardiology, the American Society of Bone and Mineral Research, and the Obesity Society. It was published on December 11, 2023, as a supplement in Diabetes Care.
An introductory section summarizing the changes for 2024 spans six pages. Those addressed during the briefing included the following:
Heart Failure Screening: Two new recommendations have been added to include screening of adults with diabetes for asymptomatic heart failure by measuring natriuretic peptide levels to facilitate the prevention or progression to symptomatic stages of heart failure.
“This is a really important and exciting area. We know that people with type 2 diabetes in particular are at high risk for heart failure,” Dr. Gabbay said, adding that these recommendations “are to really more aggressively screen those at high risk for heart failure with a simple blood test and, based on those values, then be able to move on to further evaluation and echocardiography, for example. The recommendations are really to screen a broad number of individuals with type 2 diabetes because many are at risk, [particularly] those without symptoms.”
PAD Screening: A new strong recommendation is to screen for PAD with ankle-brachial index testing in asymptomatic people with diabetes who are aged ≥ 50 years and have microvascular disease in any location, foot complications, or any end-organ damage from diabetes. The document also advises consideration of PAD screening for all individuals who have had diabetes for ≥ 10 years.
Dr. Gabbay commented, “We know that amputation rates are rising, unlike many other complications. We know that there are incredible health disparities. Blacks are two to four times more likely than Whites to have an amputation.”
Dr. El Sayed added, “Many patients don’t show the common symptoms of peripheral arterial disease. Screening is the most important way to find out if they have it or not because it can be a very devastating disease.”
Type 1 Diabetes Screening: This involves several new recommendations, including a framework for investigating suspected type 1 diabetes in newly diagnosed adults using islet autoantibody tests and diagnostic criteria for preclinical stages based on the recent approval of teplizumab for delaying the onset of type 1 diabetes.
“Screening and capturing disease earlier so that we can intervene is really an important consideration here. That includes screening for type 1 diabetes and thinking about therapeutic options to delay the development of frank type 1 diabetes,” Dr. Gabbay said.
Screening first-degree relatives of people with type 1 diabetes is a high priority because they’re at an elevated risk, he added.
Obesity Management: New recommendations here include the use of anthropomorphic measurements beyond body mass index to include waist circumference and waist:hip ratio and individual assessment of body fat mass and distribution.
Individualization of obesity management including behavioral, pharmacologic, and surgical approaches is encouraged. The use of a glucagon-like peptide-1 (GLP-1) receptor agonist or a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist with greater weight loss efficacy is preferred for obesity management in people with diabetes.
“Obesity management is one of the biggest changes over this last year,” Dr. Gabbay commented.
Other New Recommendations: Among the many other revisions in the 2024 document are new recommendations about regular evaluation and treatment for bone health, assessment of disability and guidance for referral, and alignment of guidance for liver disease screening and management with those of other professional societies. Regarding the last item, Dr. Gabbay noted, “I don’t think it’s gotten the attention it deserves. Diabetes and obesity are becoming the leading causes of liver disease.”
Clinicians can also download the Standards of Care app on their smartphones. “That can be really helpful when questions come up since you can’t remember everything in there. Here you can look it up in a matter of seconds,” Dr. Gabbay said.
Dr. El Sayed added that asking patients about their priorities is also important. “If they aren’t brought up during the visit, it’s unlikely to be as fruitful as it should be.”
Dr. El Sayed has no disclosures. Dr. Gabbay serves as a consultant and/or advisor for HealthReveal, Lark Technologies, Onduo, StartUp Health, Sweetech, and Vida Health.
A version of this article appeared on Medscape.com.
for 2024.
“The Standards of Care are essentially the global guidelines for the care of individuals with diabetes and those at risk,” ADA chief scientific and medical officer Robert Gabbay, MD, PhD, said during a briefing announcing the new Standards.
The document was developed via a scientific literature review by the ADA’s Professional Practice Committee. The panel comprises 21 professionals, including physicians from many specialties, nurse practitioners, certified diabetes care and education specialists, dietitians, and pharmacists. The chair is Nuha A. El Sayed, MD, ADA’s senior vice president of healthcare improvement.
Specific sections of the 2024 document have been endorsed by the American College of Cardiology, the American Society of Bone and Mineral Research, and the Obesity Society. It was published on December 11, 2023, as a supplement in Diabetes Care.
An introductory section summarizing the changes for 2024 spans six pages. Those addressed during the briefing included the following:
Heart Failure Screening: Two new recommendations have been added to include screening of adults with diabetes for asymptomatic heart failure by measuring natriuretic peptide levels to facilitate the prevention or progression to symptomatic stages of heart failure.
“This is a really important and exciting area. We know that people with type 2 diabetes in particular are at high risk for heart failure,” Dr. Gabbay said, adding that these recommendations “are to really more aggressively screen those at high risk for heart failure with a simple blood test and, based on those values, then be able to move on to further evaluation and echocardiography, for example. The recommendations are really to screen a broad number of individuals with type 2 diabetes because many are at risk, [particularly] those without symptoms.”
PAD Screening: A new strong recommendation is to screen for PAD with ankle-brachial index testing in asymptomatic people with diabetes who are aged ≥ 50 years and have microvascular disease in any location, foot complications, or any end-organ damage from diabetes. The document also advises consideration of PAD screening for all individuals who have had diabetes for ≥ 10 years.
Dr. Gabbay commented, “We know that amputation rates are rising, unlike many other complications. We know that there are incredible health disparities. Blacks are two to four times more likely than Whites to have an amputation.”
Dr. El Sayed added, “Many patients don’t show the common symptoms of peripheral arterial disease. Screening is the most important way to find out if they have it or not because it can be a very devastating disease.”
Type 1 Diabetes Screening: This involves several new recommendations, including a framework for investigating suspected type 1 diabetes in newly diagnosed adults using islet autoantibody tests and diagnostic criteria for preclinical stages based on the recent approval of teplizumab for delaying the onset of type 1 diabetes.
“Screening and capturing disease earlier so that we can intervene is really an important consideration here. That includes screening for type 1 diabetes and thinking about therapeutic options to delay the development of frank type 1 diabetes,” Dr. Gabbay said.
Screening first-degree relatives of people with type 1 diabetes is a high priority because they’re at an elevated risk, he added.
Obesity Management: New recommendations here include the use of anthropomorphic measurements beyond body mass index to include waist circumference and waist:hip ratio and individual assessment of body fat mass and distribution.
Individualization of obesity management including behavioral, pharmacologic, and surgical approaches is encouraged. The use of a glucagon-like peptide-1 (GLP-1) receptor agonist or a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist with greater weight loss efficacy is preferred for obesity management in people with diabetes.
“Obesity management is one of the biggest changes over this last year,” Dr. Gabbay commented.
Other New Recommendations: Among the many other revisions in the 2024 document are new recommendations about regular evaluation and treatment for bone health, assessment of disability and guidance for referral, and alignment of guidance for liver disease screening and management with those of other professional societies. Regarding the last item, Dr. Gabbay noted, “I don’t think it’s gotten the attention it deserves. Diabetes and obesity are becoming the leading causes of liver disease.”
Clinicians can also download the Standards of Care app on their smartphones. “That can be really helpful when questions come up since you can’t remember everything in there. Here you can look it up in a matter of seconds,” Dr. Gabbay said.
Dr. El Sayed added that asking patients about their priorities is also important. “If they aren’t brought up during the visit, it’s unlikely to be as fruitful as it should be.”
Dr. El Sayed has no disclosures. Dr. Gabbay serves as a consultant and/or advisor for HealthReveal, Lark Technologies, Onduo, StartUp Health, Sweetech, and Vida Health.
A version of this article appeared on Medscape.com.
for 2024.
“The Standards of Care are essentially the global guidelines for the care of individuals with diabetes and those at risk,” ADA chief scientific and medical officer Robert Gabbay, MD, PhD, said during a briefing announcing the new Standards.
The document was developed via a scientific literature review by the ADA’s Professional Practice Committee. The panel comprises 21 professionals, including physicians from many specialties, nurse practitioners, certified diabetes care and education specialists, dietitians, and pharmacists. The chair is Nuha A. El Sayed, MD, ADA’s senior vice president of healthcare improvement.
Specific sections of the 2024 document have been endorsed by the American College of Cardiology, the American Society of Bone and Mineral Research, and the Obesity Society. It was published on December 11, 2023, as a supplement in Diabetes Care.
An introductory section summarizing the changes for 2024 spans six pages. Those addressed during the briefing included the following:
Heart Failure Screening: Two new recommendations have been added to include screening of adults with diabetes for asymptomatic heart failure by measuring natriuretic peptide levels to facilitate the prevention or progression to symptomatic stages of heart failure.
“This is a really important and exciting area. We know that people with type 2 diabetes in particular are at high risk for heart failure,” Dr. Gabbay said, adding that these recommendations “are to really more aggressively screen those at high risk for heart failure with a simple blood test and, based on those values, then be able to move on to further evaluation and echocardiography, for example. The recommendations are really to screen a broad number of individuals with type 2 diabetes because many are at risk, [particularly] those without symptoms.”
PAD Screening: A new strong recommendation is to screen for PAD with ankle-brachial index testing in asymptomatic people with diabetes who are aged ≥ 50 years and have microvascular disease in any location, foot complications, or any end-organ damage from diabetes. The document also advises consideration of PAD screening for all individuals who have had diabetes for ≥ 10 years.
Dr. Gabbay commented, “We know that amputation rates are rising, unlike many other complications. We know that there are incredible health disparities. Blacks are two to four times more likely than Whites to have an amputation.”
Dr. El Sayed added, “Many patients don’t show the common symptoms of peripheral arterial disease. Screening is the most important way to find out if they have it or not because it can be a very devastating disease.”
Type 1 Diabetes Screening: This involves several new recommendations, including a framework for investigating suspected type 1 diabetes in newly diagnosed adults using islet autoantibody tests and diagnostic criteria for preclinical stages based on the recent approval of teplizumab for delaying the onset of type 1 diabetes.
“Screening and capturing disease earlier so that we can intervene is really an important consideration here. That includes screening for type 1 diabetes and thinking about therapeutic options to delay the development of frank type 1 diabetes,” Dr. Gabbay said.
Screening first-degree relatives of people with type 1 diabetes is a high priority because they’re at an elevated risk, he added.
Obesity Management: New recommendations here include the use of anthropomorphic measurements beyond body mass index to include waist circumference and waist:hip ratio and individual assessment of body fat mass and distribution.
Individualization of obesity management including behavioral, pharmacologic, and surgical approaches is encouraged. The use of a glucagon-like peptide-1 (GLP-1) receptor agonist or a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist with greater weight loss efficacy is preferred for obesity management in people with diabetes.
“Obesity management is one of the biggest changes over this last year,” Dr. Gabbay commented.
Other New Recommendations: Among the many other revisions in the 2024 document are new recommendations about regular evaluation and treatment for bone health, assessment of disability and guidance for referral, and alignment of guidance for liver disease screening and management with those of other professional societies. Regarding the last item, Dr. Gabbay noted, “I don’t think it’s gotten the attention it deserves. Diabetes and obesity are becoming the leading causes of liver disease.”
Clinicians can also download the Standards of Care app on their smartphones. “That can be really helpful when questions come up since you can’t remember everything in there. Here you can look it up in a matter of seconds,” Dr. Gabbay said.
Dr. El Sayed added that asking patients about their priorities is also important. “If they aren’t brought up during the visit, it’s unlikely to be as fruitful as it should be.”
Dr. El Sayed has no disclosures. Dr. Gabbay serves as a consultant and/or advisor for HealthReveal, Lark Technologies, Onduo, StartUp Health, Sweetech, and Vida Health.
A version of this article appeared on Medscape.com.
Telemedicine in diabetes care associated with worse outcomes
TOPLINE:
Adult patients with type 2 diabetes and complex care needs receiving endocrinology treatment through telemedicine alone show worse glycemic outcomes compared with those receiving treatment either in-person or in mixed-care models.
The findings contrast with some previous studies showing similar glycemic outcomes with telemedicine care vs in-person care for type 2 diabetes management.
The study is believed to be the first to examine telemedicine care outcomes specifically in the endocrinology setting and based on clinical factors that affect treatment complexity.
METHODOLOGY:
- The retrospective cohort study included 3778 adults with type 2 diabetes in a single, large integrated US health system who had received either telemedicine-only, in-person, or a mix of telemedicine and in-person care between May and October 2020.
- Patients were followed up through May 2022 and evaluated for estimated A1c change after 12 months within each treatment cohort, as well as factors associated with any changes.
- Of the patients, 1182 received telemedicine-only, 1049 received in-person, and 1547 received mixed care. Mean ages in the groups ranged from 57 to 63 years, and women made up between 55% and 63%.
TAKEAWAY:
- Over the 12-month evaluation period, patients receiving telemedicine-only care had no significant changes or improvements in adjusted A1c (−0.06; P = .55), those receiving in-person care had an improvement of 0.37% (P < .001), and those receiving mixed care had an improvement of 0.22% (P = .004).
- The glycemic outcome patterns were similar among patients with a baseline A1c of 8% or higher.
- Of those prescribed multiple daily injections vs no insulin, estimated changes in A1c were 0.25% higher for those receiving telemedicine than for those receiving in-person care (P = .03).
- No associations were observed between changes in A1c and comorbidities.
- Regarding reasons for the differences, the authors noted that “the strategies to support glycemic improvement that are available during in-person appointments have not consistently been translated to telemedicine care.”
- Essential components of telemedicine such as self-management education support may not currently be routinely available through telemedicine or at the point-of-care during telemedicine visits, they added.
- “In our prior work in this care setting, practitioners described how inferior availability of glucose data limited their ability to intensify treatment through telemedicine.”
- “Implementation of approaches to overcome these differences, such as team-based virtual care and technological tools to automate blood glucose data sharing, are needed to ensure all patients receive high-quality diabetes care regardless of care modality.”
IN PRACTICE:
“These findings suggest that patients with type 2 diabetes who rely on telemedicine alone to access endocrinology care may require additional support to achieve glycemic goals,” the authors reported.
“Since some patients with barriers to in-person endocrinology care will continue to rely on telemedicine to access care, structured approaches to ensure routine delivery of high-quality team-based diabetes care are needed,” they asserted.
“Translation of successful strategies from clinical trials into routine telemedicine care, especially targeted toward adults with more complex diabetes, is critical to improve clinical outcomes for patients who rely on this care modality.”
SOURCE:
The study was conducted by first author Margaret F. Zupa, MD, of the division of endocrinology and metabolism, University of Pittsburgh School of Medicine, Pennsylvania, and colleagues.
It was published in JAMA Network Open.
LIMITATIONS:
While demographic differences between the groups were included as covariates, the treatment modality cohorts were not balanced based on baseline characteristics that could be confounders.
Various factors, such as treatment complexity, glycemic control, and transportation barriers, could have affected whether patients received care with telemedicine; therefore, causal associations could not be established.
DISCLOSURES:
The study received funding from the National Center for Advancing Translational Sciences, National Institutes of Health, Pittsburgh Foundation, and Fraternal Order of the Eagles Charity Foundation Diabetes Fund. The authors’ disclosures are detailed in the study.
A version of this article appeared on Medscape.com.
TOPLINE:
Adult patients with type 2 diabetes and complex care needs receiving endocrinology treatment through telemedicine alone show worse glycemic outcomes compared with those receiving treatment either in-person or in mixed-care models.
The findings contrast with some previous studies showing similar glycemic outcomes with telemedicine care vs in-person care for type 2 diabetes management.
The study is believed to be the first to examine telemedicine care outcomes specifically in the endocrinology setting and based on clinical factors that affect treatment complexity.
METHODOLOGY:
- The retrospective cohort study included 3778 adults with type 2 diabetes in a single, large integrated US health system who had received either telemedicine-only, in-person, or a mix of telemedicine and in-person care between May and October 2020.
- Patients were followed up through May 2022 and evaluated for estimated A1c change after 12 months within each treatment cohort, as well as factors associated with any changes.
- Of the patients, 1182 received telemedicine-only, 1049 received in-person, and 1547 received mixed care. Mean ages in the groups ranged from 57 to 63 years, and women made up between 55% and 63%.
TAKEAWAY:
- Over the 12-month evaluation period, patients receiving telemedicine-only care had no significant changes or improvements in adjusted A1c (−0.06; P = .55), those receiving in-person care had an improvement of 0.37% (P < .001), and those receiving mixed care had an improvement of 0.22% (P = .004).
- The glycemic outcome patterns were similar among patients with a baseline A1c of 8% or higher.
- Of those prescribed multiple daily injections vs no insulin, estimated changes in A1c were 0.25% higher for those receiving telemedicine than for those receiving in-person care (P = .03).
- No associations were observed between changes in A1c and comorbidities.
- Regarding reasons for the differences, the authors noted that “the strategies to support glycemic improvement that are available during in-person appointments have not consistently been translated to telemedicine care.”
- Essential components of telemedicine such as self-management education support may not currently be routinely available through telemedicine or at the point-of-care during telemedicine visits, they added.
- “In our prior work in this care setting, practitioners described how inferior availability of glucose data limited their ability to intensify treatment through telemedicine.”
- “Implementation of approaches to overcome these differences, such as team-based virtual care and technological tools to automate blood glucose data sharing, are needed to ensure all patients receive high-quality diabetes care regardless of care modality.”
IN PRACTICE:
“These findings suggest that patients with type 2 diabetes who rely on telemedicine alone to access endocrinology care may require additional support to achieve glycemic goals,” the authors reported.
“Since some patients with barriers to in-person endocrinology care will continue to rely on telemedicine to access care, structured approaches to ensure routine delivery of high-quality team-based diabetes care are needed,” they asserted.
“Translation of successful strategies from clinical trials into routine telemedicine care, especially targeted toward adults with more complex diabetes, is critical to improve clinical outcomes for patients who rely on this care modality.”
SOURCE:
The study was conducted by first author Margaret F. Zupa, MD, of the division of endocrinology and metabolism, University of Pittsburgh School of Medicine, Pennsylvania, and colleagues.
It was published in JAMA Network Open.
LIMITATIONS:
While demographic differences between the groups were included as covariates, the treatment modality cohorts were not balanced based on baseline characteristics that could be confounders.
Various factors, such as treatment complexity, glycemic control, and transportation barriers, could have affected whether patients received care with telemedicine; therefore, causal associations could not be established.
DISCLOSURES:
The study received funding from the National Center for Advancing Translational Sciences, National Institutes of Health, Pittsburgh Foundation, and Fraternal Order of the Eagles Charity Foundation Diabetes Fund. The authors’ disclosures are detailed in the study.
A version of this article appeared on Medscape.com.
TOPLINE:
Adult patients with type 2 diabetes and complex care needs receiving endocrinology treatment through telemedicine alone show worse glycemic outcomes compared with those receiving treatment either in-person or in mixed-care models.
The findings contrast with some previous studies showing similar glycemic outcomes with telemedicine care vs in-person care for type 2 diabetes management.
The study is believed to be the first to examine telemedicine care outcomes specifically in the endocrinology setting and based on clinical factors that affect treatment complexity.
METHODOLOGY:
- The retrospective cohort study included 3778 adults with type 2 diabetes in a single, large integrated US health system who had received either telemedicine-only, in-person, or a mix of telemedicine and in-person care between May and October 2020.
- Patients were followed up through May 2022 and evaluated for estimated A1c change after 12 months within each treatment cohort, as well as factors associated with any changes.
- Of the patients, 1182 received telemedicine-only, 1049 received in-person, and 1547 received mixed care. Mean ages in the groups ranged from 57 to 63 years, and women made up between 55% and 63%.
TAKEAWAY:
- Over the 12-month evaluation period, patients receiving telemedicine-only care had no significant changes or improvements in adjusted A1c (−0.06; P = .55), those receiving in-person care had an improvement of 0.37% (P < .001), and those receiving mixed care had an improvement of 0.22% (P = .004).
- The glycemic outcome patterns were similar among patients with a baseline A1c of 8% or higher.
- Of those prescribed multiple daily injections vs no insulin, estimated changes in A1c were 0.25% higher for those receiving telemedicine than for those receiving in-person care (P = .03).
- No associations were observed between changes in A1c and comorbidities.
- Regarding reasons for the differences, the authors noted that “the strategies to support glycemic improvement that are available during in-person appointments have not consistently been translated to telemedicine care.”
- Essential components of telemedicine such as self-management education support may not currently be routinely available through telemedicine or at the point-of-care during telemedicine visits, they added.
- “In our prior work in this care setting, practitioners described how inferior availability of glucose data limited their ability to intensify treatment through telemedicine.”
- “Implementation of approaches to overcome these differences, such as team-based virtual care and technological tools to automate blood glucose data sharing, are needed to ensure all patients receive high-quality diabetes care regardless of care modality.”
IN PRACTICE:
“These findings suggest that patients with type 2 diabetes who rely on telemedicine alone to access endocrinology care may require additional support to achieve glycemic goals,” the authors reported.
“Since some patients with barriers to in-person endocrinology care will continue to rely on telemedicine to access care, structured approaches to ensure routine delivery of high-quality team-based diabetes care are needed,” they asserted.
“Translation of successful strategies from clinical trials into routine telemedicine care, especially targeted toward adults with more complex diabetes, is critical to improve clinical outcomes for patients who rely on this care modality.”
SOURCE:
The study was conducted by first author Margaret F. Zupa, MD, of the division of endocrinology and metabolism, University of Pittsburgh School of Medicine, Pennsylvania, and colleagues.
It was published in JAMA Network Open.
LIMITATIONS:
While demographic differences between the groups were included as covariates, the treatment modality cohorts were not balanced based on baseline characteristics that could be confounders.
Various factors, such as treatment complexity, glycemic control, and transportation barriers, could have affected whether patients received care with telemedicine; therefore, causal associations could not be established.
DISCLOSURES:
The study received funding from the National Center for Advancing Translational Sciences, National Institutes of Health, Pittsburgh Foundation, and Fraternal Order of the Eagles Charity Foundation Diabetes Fund. The authors’ disclosures are detailed in the study.
A version of this article appeared on Medscape.com.
Personalized nutrition therapy promotes diabetes remission
LEIPZIG, GERMANY — For patients newly diagnosed with type 2 diabetes, nutrition therapy is highly effective at achieving remission. “The greater the reduction in body weight, the higher the chances that blood sugar levels will normalize,” Diana Rubin, MD, said at the fall press conference of the German Diabetes Society (DDG). Dr. Rubin is conference president and chief physician of the Center for Nutritional Medicine and Diabetology at Vivantes Humboldt Hospital and the Spandau Hospital, Berlin, Germany.
Because of the development of modern medicines, nutrition therapy has increasingly been pushed into the background over the past 50 years. However, nutrition therapy and weight reduction can effectively delay diabetes for years, said Dr. Rubin. The patients are healthy, without being healed.
Nevertheless, the remission is rarely permanent. Most of the patients develop type 2 diabetes again after 5 years.
Personalized Nutrition Therapy
It is not just developments in medicine that have pushed nutrition therapy into the background. Another contributing factor is that statutory health insurance companies do not cover personalized nutrition counseling as standard, said Dr. Rubin.
Modern research in nutrition therapy has shown that patients with diabetes should receive personalized treatment. However, this idea is not taken into consideration in current diabetes training programs, which are the only forms of nutrition therapy covered by statutory health insurance companies.
Instead, nutrition information is mostly conveyed through group training sessions. Individuals do not necessarily find each other again. What’s more, these sessions are seldom led by nutrition experts. “It is rarely helpful to use a ‘one size fits all’ approach, as is often the case with these group training sessions,” said Dr. Rubin.
The DiRECT study, in which patients reduced their weight by 15 kg and achieved remission rates of almost 90%, is an example of how nutrition therapy can be highly effective. This is especially true if the aims and methods are determined on an individual basis and if there is frequent contact with a therapist. German and international guidelines, including the DDG’s best practice guides from 2022, highlight the importance of personalized nutrition therapy.
Telemedicine Encourages Adherence
“It is very important to consider the current living situation of the person concerned,” said Dr. Rubin. “It is important to set small objectives that can also be implemented in everyday life.” This can only succeed with a professional face-to-face consultation. “Achieving this objective then also becomes realistic — i.e., losing 10% to 15% body weight and maintaining this loss,” she said. “Long-term monitoring is needed to maintain this weight.”
Weight reduction methods should generally be determined according to the preferences of the person concerned, since dietary habits and environments are personal. For example, reducing the intake of carbohydrates and fats, intermittent fasting, or using meal replacement drinks can all be considered.
New data also show that digital apps available on prescription (DiGA) can be helpful for support; this idea is reflected in the DDG’s nutrition best practice guides for patients with type 2 diabetes.
“Studies show that adherence is highly dependent on the amount of contact with therapists and the long-term nature of the treatment,” said Dr. Rubin. She referred to the need for long-term monitoring, during which the patient can be repeatedly reminded of the therapeutic objective. “In this respect, I see a lot of potential in digital apps, and also in telemedicine, to cater to the short-term contact with the person concerned.”
A 2015 meta-analysis of 92 studies revealed a significant reduction in A1c for patients with type 1 or type 2 diabetes when using telemedicine nutrition therapy. Dr. Rubin frequently prescribes DiGAs, which are approved for obesity, “simply because I can recognize it makes it easier for many patients to stick to their goals.”
Dr. Rubin also recommends connecting with sport groups and self-help groups. “Maintaining the weight is a long-term project.”
Abdominal Fat Decisive
Prediabetes is a precursor to type 2 diabetes and entails an increased risk of heart attack, kidney and eye diseases, and various kinds of cancer. To date, physicians have tried to delay the onset of type 2 diabetes by aiming to reduce the weight of patients with prediabetes. However, scientists at the German Center for Diabetes Research showed with the Prediabetes Lifestyle Intervention Study that abdominal fat plays an important role in the remission of prediabetes.
The 1-year program with a healthy diet and increased physical activity was followed by 1105 patients with prediabetes. When every subject lost at least 5% of their weight, it turned out that some achieved remission, and others did not.
People who achieved remission exhibited better insulin sensitivity and had lost more visceral abdominal fat. Visceral abdominal fat can influence insulin sensitivity, not least by an inflammatory reaction in the fatty tissue.
Reducing visceral abdominal fat is clearly crucially important in achieving prediabetes remission. Subjects who achieved remission in the study had a strongly reduced risk for type 2 diabetes for up to 2 years after the end of the program. They had improved kidney function, and their blood vessels were in better condition.
Waist Circumference
According to the new results, the chances of remission increase if body weight is reduced by 5% and waist circumference is reduced by around 4 cm in women and 7 cm in men.
“Based on the new data, remission should be the new therapeutic objective in people with prediabetes. This could potentially change clinical practice and minimize the complication rate for our patients, both male and female,” said author Reiner Jumpertz-von Schwartzenberg, MD, a researcher at the Tübingen University Hospital in Germany.
Prediabetes remission can be assumed if the fasting blood glucose falls below 100 mg/dL (5.6 mmol/L), the 2-hour glucose below 140 mg/dL (7.8 mmol/L), and the A1c value below 5.7%. From the new findings, it can be seen that the chances of remission increase the more the body weight decreases.
Dr. Jumpertz-von Schwartzenberg and his colleagues want to investigate whether this strategy is cost-effective so that the support of payers can also be ensured.
This article was translated from the Medscape German edition.
A version of this article appeared on Medscape.com.
LEIPZIG, GERMANY — For patients newly diagnosed with type 2 diabetes, nutrition therapy is highly effective at achieving remission. “The greater the reduction in body weight, the higher the chances that blood sugar levels will normalize,” Diana Rubin, MD, said at the fall press conference of the German Diabetes Society (DDG). Dr. Rubin is conference president and chief physician of the Center for Nutritional Medicine and Diabetology at Vivantes Humboldt Hospital and the Spandau Hospital, Berlin, Germany.
Because of the development of modern medicines, nutrition therapy has increasingly been pushed into the background over the past 50 years. However, nutrition therapy and weight reduction can effectively delay diabetes for years, said Dr. Rubin. The patients are healthy, without being healed.
Nevertheless, the remission is rarely permanent. Most of the patients develop type 2 diabetes again after 5 years.
Personalized Nutrition Therapy
It is not just developments in medicine that have pushed nutrition therapy into the background. Another contributing factor is that statutory health insurance companies do not cover personalized nutrition counseling as standard, said Dr. Rubin.
Modern research in nutrition therapy has shown that patients with diabetes should receive personalized treatment. However, this idea is not taken into consideration in current diabetes training programs, which are the only forms of nutrition therapy covered by statutory health insurance companies.
Instead, nutrition information is mostly conveyed through group training sessions. Individuals do not necessarily find each other again. What’s more, these sessions are seldom led by nutrition experts. “It is rarely helpful to use a ‘one size fits all’ approach, as is often the case with these group training sessions,” said Dr. Rubin.
The DiRECT study, in which patients reduced their weight by 15 kg and achieved remission rates of almost 90%, is an example of how nutrition therapy can be highly effective. This is especially true if the aims and methods are determined on an individual basis and if there is frequent contact with a therapist. German and international guidelines, including the DDG’s best practice guides from 2022, highlight the importance of personalized nutrition therapy.
Telemedicine Encourages Adherence
“It is very important to consider the current living situation of the person concerned,” said Dr. Rubin. “It is important to set small objectives that can also be implemented in everyday life.” This can only succeed with a professional face-to-face consultation. “Achieving this objective then also becomes realistic — i.e., losing 10% to 15% body weight and maintaining this loss,” she said. “Long-term monitoring is needed to maintain this weight.”
Weight reduction methods should generally be determined according to the preferences of the person concerned, since dietary habits and environments are personal. For example, reducing the intake of carbohydrates and fats, intermittent fasting, or using meal replacement drinks can all be considered.
New data also show that digital apps available on prescription (DiGA) can be helpful for support; this idea is reflected in the DDG’s nutrition best practice guides for patients with type 2 diabetes.
“Studies show that adherence is highly dependent on the amount of contact with therapists and the long-term nature of the treatment,” said Dr. Rubin. She referred to the need for long-term monitoring, during which the patient can be repeatedly reminded of the therapeutic objective. “In this respect, I see a lot of potential in digital apps, and also in telemedicine, to cater to the short-term contact with the person concerned.”
A 2015 meta-analysis of 92 studies revealed a significant reduction in A1c for patients with type 1 or type 2 diabetes when using telemedicine nutrition therapy. Dr. Rubin frequently prescribes DiGAs, which are approved for obesity, “simply because I can recognize it makes it easier for many patients to stick to their goals.”
Dr. Rubin also recommends connecting with sport groups and self-help groups. “Maintaining the weight is a long-term project.”
Abdominal Fat Decisive
Prediabetes is a precursor to type 2 diabetes and entails an increased risk of heart attack, kidney and eye diseases, and various kinds of cancer. To date, physicians have tried to delay the onset of type 2 diabetes by aiming to reduce the weight of patients with prediabetes. However, scientists at the German Center for Diabetes Research showed with the Prediabetes Lifestyle Intervention Study that abdominal fat plays an important role in the remission of prediabetes.
The 1-year program with a healthy diet and increased physical activity was followed by 1105 patients with prediabetes. When every subject lost at least 5% of their weight, it turned out that some achieved remission, and others did not.
People who achieved remission exhibited better insulin sensitivity and had lost more visceral abdominal fat. Visceral abdominal fat can influence insulin sensitivity, not least by an inflammatory reaction in the fatty tissue.
Reducing visceral abdominal fat is clearly crucially important in achieving prediabetes remission. Subjects who achieved remission in the study had a strongly reduced risk for type 2 diabetes for up to 2 years after the end of the program. They had improved kidney function, and their blood vessels were in better condition.
Waist Circumference
According to the new results, the chances of remission increase if body weight is reduced by 5% and waist circumference is reduced by around 4 cm in women and 7 cm in men.
“Based on the new data, remission should be the new therapeutic objective in people with prediabetes. This could potentially change clinical practice and minimize the complication rate for our patients, both male and female,” said author Reiner Jumpertz-von Schwartzenberg, MD, a researcher at the Tübingen University Hospital in Germany.
Prediabetes remission can be assumed if the fasting blood glucose falls below 100 mg/dL (5.6 mmol/L), the 2-hour glucose below 140 mg/dL (7.8 mmol/L), and the A1c value below 5.7%. From the new findings, it can be seen that the chances of remission increase the more the body weight decreases.
Dr. Jumpertz-von Schwartzenberg and his colleagues want to investigate whether this strategy is cost-effective so that the support of payers can also be ensured.
This article was translated from the Medscape German edition.
A version of this article appeared on Medscape.com.
LEIPZIG, GERMANY — For patients newly diagnosed with type 2 diabetes, nutrition therapy is highly effective at achieving remission. “The greater the reduction in body weight, the higher the chances that blood sugar levels will normalize,” Diana Rubin, MD, said at the fall press conference of the German Diabetes Society (DDG). Dr. Rubin is conference president and chief physician of the Center for Nutritional Medicine and Diabetology at Vivantes Humboldt Hospital and the Spandau Hospital, Berlin, Germany.
Because of the development of modern medicines, nutrition therapy has increasingly been pushed into the background over the past 50 years. However, nutrition therapy and weight reduction can effectively delay diabetes for years, said Dr. Rubin. The patients are healthy, without being healed.
Nevertheless, the remission is rarely permanent. Most of the patients develop type 2 diabetes again after 5 years.
Personalized Nutrition Therapy
It is not just developments in medicine that have pushed nutrition therapy into the background. Another contributing factor is that statutory health insurance companies do not cover personalized nutrition counseling as standard, said Dr. Rubin.
Modern research in nutrition therapy has shown that patients with diabetes should receive personalized treatment. However, this idea is not taken into consideration in current diabetes training programs, which are the only forms of nutrition therapy covered by statutory health insurance companies.
Instead, nutrition information is mostly conveyed through group training sessions. Individuals do not necessarily find each other again. What’s more, these sessions are seldom led by nutrition experts. “It is rarely helpful to use a ‘one size fits all’ approach, as is often the case with these group training sessions,” said Dr. Rubin.
The DiRECT study, in which patients reduced their weight by 15 kg and achieved remission rates of almost 90%, is an example of how nutrition therapy can be highly effective. This is especially true if the aims and methods are determined on an individual basis and if there is frequent contact with a therapist. German and international guidelines, including the DDG’s best practice guides from 2022, highlight the importance of personalized nutrition therapy.
Telemedicine Encourages Adherence
“It is very important to consider the current living situation of the person concerned,” said Dr. Rubin. “It is important to set small objectives that can also be implemented in everyday life.” This can only succeed with a professional face-to-face consultation. “Achieving this objective then also becomes realistic — i.e., losing 10% to 15% body weight and maintaining this loss,” she said. “Long-term monitoring is needed to maintain this weight.”
Weight reduction methods should generally be determined according to the preferences of the person concerned, since dietary habits and environments are personal. For example, reducing the intake of carbohydrates and fats, intermittent fasting, or using meal replacement drinks can all be considered.
New data also show that digital apps available on prescription (DiGA) can be helpful for support; this idea is reflected in the DDG’s nutrition best practice guides for patients with type 2 diabetes.
“Studies show that adherence is highly dependent on the amount of contact with therapists and the long-term nature of the treatment,” said Dr. Rubin. She referred to the need for long-term monitoring, during which the patient can be repeatedly reminded of the therapeutic objective. “In this respect, I see a lot of potential in digital apps, and also in telemedicine, to cater to the short-term contact with the person concerned.”
A 2015 meta-analysis of 92 studies revealed a significant reduction in A1c for patients with type 1 or type 2 diabetes when using telemedicine nutrition therapy. Dr. Rubin frequently prescribes DiGAs, which are approved for obesity, “simply because I can recognize it makes it easier for many patients to stick to their goals.”
Dr. Rubin also recommends connecting with sport groups and self-help groups. “Maintaining the weight is a long-term project.”
Abdominal Fat Decisive
Prediabetes is a precursor to type 2 diabetes and entails an increased risk of heart attack, kidney and eye diseases, and various kinds of cancer. To date, physicians have tried to delay the onset of type 2 diabetes by aiming to reduce the weight of patients with prediabetes. However, scientists at the German Center for Diabetes Research showed with the Prediabetes Lifestyle Intervention Study that abdominal fat plays an important role in the remission of prediabetes.
The 1-year program with a healthy diet and increased physical activity was followed by 1105 patients with prediabetes. When every subject lost at least 5% of their weight, it turned out that some achieved remission, and others did not.
People who achieved remission exhibited better insulin sensitivity and had lost more visceral abdominal fat. Visceral abdominal fat can influence insulin sensitivity, not least by an inflammatory reaction in the fatty tissue.
Reducing visceral abdominal fat is clearly crucially important in achieving prediabetes remission. Subjects who achieved remission in the study had a strongly reduced risk for type 2 diabetes for up to 2 years after the end of the program. They had improved kidney function, and their blood vessels were in better condition.
Waist Circumference
According to the new results, the chances of remission increase if body weight is reduced by 5% and waist circumference is reduced by around 4 cm in women and 7 cm in men.
“Based on the new data, remission should be the new therapeutic objective in people with prediabetes. This could potentially change clinical practice and minimize the complication rate for our patients, both male and female,” said author Reiner Jumpertz-von Schwartzenberg, MD, a researcher at the Tübingen University Hospital in Germany.
Prediabetes remission can be assumed if the fasting blood glucose falls below 100 mg/dL (5.6 mmol/L), the 2-hour glucose below 140 mg/dL (7.8 mmol/L), and the A1c value below 5.7%. From the new findings, it can be seen that the chances of remission increase the more the body weight decreases.
Dr. Jumpertz-von Schwartzenberg and his colleagues want to investigate whether this strategy is cost-effective so that the support of payers can also be ensured.
This article was translated from the Medscape German edition.
A version of this article appeared on Medscape.com.
Meet the newest acronym in primary care: CKM
The advisory, published recently in Circulation introduces the concept of CKM health and reevaluates the relationships between obesity, diabetes, kidney disease, and cardiovascular disease (CVD).
“This approach not only raises awareness, it also empowers PCPs to diagnose and treat these conditions more holistically,” Salim Hayek, MD, associate professor of cardiovascular disease and internal medicine, and medical director of the Frankel Cardiovascular Center Clinics at the University of Michigan in Ann Arbor, said in an interview.
New CKM Staging, Testing, and Care Strategies
The advisory introduces a new scoring system that ranges from stage 0 (patients with no risk factors for CKM) through stage 4 (patients with clinical CVD in CKM syndrome). Each stage requires specific management strategies and may include screening starting at age 30 years for diabetes, hypertension, and heart failure.
“Stage 0 CKM is usually found in young people, and CKM risk factors and scores typically increase as people age,” said Sean M. Drake, MD, a primary care physician at Henry Ford Health in Sterling Heights, Michigan.
Dr. Drake advised PCPs to encourage patients who are at stage 0 to maintain ideal cardiovascular health and to monitor those at risk of progressing through the stages.
While PCPs already perform many of the tests the advisory recommends, the conditions overlap and an abnormality in one system should prompt more testing for other conditions. Additional tests, such as urine albumin-creatinine ratio, and more frequent glomerular filtration rate and lipid profile are advised, according to Dr. Drake.
“There also appears to be a role for additional cardiac testing, including echocardiograms and coronary CT scans, and for liver fibrosis screening,” Dr. Drake said. “Medications such as SGLT2 inhibitors, GLP-1 receptor agonists, and ACE inhibitors, beyond current routine use, are emphasized.”
To better characterize body composition and help diagnose metabolic syndrome, the advisory also recommends measuring waist circumference, which is not routine practice, noted Joshua J. Joseph, MD, MPH, an associate professor of endocrinology, diabetes, and metabolism at The Ohio State University Wexner Medical Center in Columbus, and a co-author of the advisory.
Recognizing the interconnected nature of cardiac, kidney, and metabolic diseases encourages a shift in mindset for clinicians, according to Neha Pagidipati, MD, MPH, a cardiologist at Duke Health in Durham, North Carolina.
“We have often been trained to focus on the specific problem in front of us,” Dr. Pagidipati said. “We need to be hyper-aware that many patients we see are at risk for multiple CKM entities. We need to be proactive about screening for and treating these when appropriate.”
The advisory emphasizes the need for CKM coordinators to support teams of clinicians from primary care, cardiology, endocrinology, nephrology, nursing, and pharmacy, as well as social workers, care navigators, or community health workers, Dr. Joseph said.
“The advisory repositions the PCP at the forefront of CKM care coordination, marking a departure from the traditional model where subspecialists primarily manage complications,” Dr. Hayek added.
Changes to Payment
The new recommendations are consistent with current management guidelines for obesity, hypertriglyceridemia, hypertension, type 2 diabetes, and chronic kidney disease.
“The advisory provides integrated algorithms for cardiovascular prevention and management, with specific therapeutic guidance tied to CKM stages, bringing together the current evidence for best practices from the various guidelines and filling gaps in a unified approach,” Dr. Joseph said.
In addition, the advisory draws attention to the care of younger patients, who may be at increased risk for cardiovascular disease due to lifestyle factors, according to Nishant Shah, MD, assistant professor of medicine at Duke.
“It considers barriers to care that prevent people from optimizing their cardiovascular health,” Dr. Shah said.
Although the advisory does not specify proposed payment changes to support the new care model, the move towards value-based care may require billing practices that accommodate integrated care as well as more frequent and more specialized testing, Dr. Hayek said.
“The advisory is an empowering tool for PCPs, underscoring their critical role in healthcare,” Dr. Hayek said. “It encourages PCPs to advocate for integrated care within their practices and to consider workflow adjustments that enhance the identification and initiation of preventive care for at-risk patients.”
Funding information was not provided.
Dr. Joseph reports no relevant financial involvements; several advisory co-authors report financial involvements with pharmaceutical companies. Dr. Pagidipati reports relevant financial involvement with pharmaceutical companies. Dr. Hayek, Dr. Drake, and Dr. Shah report no relevant financial involvements. Dr. Joseph is an author of the advisory. Dr. Pagidipati, Dr. Hayek, Dr. Drake, and Dr. Shah were not involved in the writing of the advisory.
A version of this article appeared on Medscape.com.
The advisory, published recently in Circulation introduces the concept of CKM health and reevaluates the relationships between obesity, diabetes, kidney disease, and cardiovascular disease (CVD).
“This approach not only raises awareness, it also empowers PCPs to diagnose and treat these conditions more holistically,” Salim Hayek, MD, associate professor of cardiovascular disease and internal medicine, and medical director of the Frankel Cardiovascular Center Clinics at the University of Michigan in Ann Arbor, said in an interview.
New CKM Staging, Testing, and Care Strategies
The advisory introduces a new scoring system that ranges from stage 0 (patients with no risk factors for CKM) through stage 4 (patients with clinical CVD in CKM syndrome). Each stage requires specific management strategies and may include screening starting at age 30 years for diabetes, hypertension, and heart failure.
“Stage 0 CKM is usually found in young people, and CKM risk factors and scores typically increase as people age,” said Sean M. Drake, MD, a primary care physician at Henry Ford Health in Sterling Heights, Michigan.
Dr. Drake advised PCPs to encourage patients who are at stage 0 to maintain ideal cardiovascular health and to monitor those at risk of progressing through the stages.
While PCPs already perform many of the tests the advisory recommends, the conditions overlap and an abnormality in one system should prompt more testing for other conditions. Additional tests, such as urine albumin-creatinine ratio, and more frequent glomerular filtration rate and lipid profile are advised, according to Dr. Drake.
“There also appears to be a role for additional cardiac testing, including echocardiograms and coronary CT scans, and for liver fibrosis screening,” Dr. Drake said. “Medications such as SGLT2 inhibitors, GLP-1 receptor agonists, and ACE inhibitors, beyond current routine use, are emphasized.”
To better characterize body composition and help diagnose metabolic syndrome, the advisory also recommends measuring waist circumference, which is not routine practice, noted Joshua J. Joseph, MD, MPH, an associate professor of endocrinology, diabetes, and metabolism at The Ohio State University Wexner Medical Center in Columbus, and a co-author of the advisory.
Recognizing the interconnected nature of cardiac, kidney, and metabolic diseases encourages a shift in mindset for clinicians, according to Neha Pagidipati, MD, MPH, a cardiologist at Duke Health in Durham, North Carolina.
“We have often been trained to focus on the specific problem in front of us,” Dr. Pagidipati said. “We need to be hyper-aware that many patients we see are at risk for multiple CKM entities. We need to be proactive about screening for and treating these when appropriate.”
The advisory emphasizes the need for CKM coordinators to support teams of clinicians from primary care, cardiology, endocrinology, nephrology, nursing, and pharmacy, as well as social workers, care navigators, or community health workers, Dr. Joseph said.
“The advisory repositions the PCP at the forefront of CKM care coordination, marking a departure from the traditional model where subspecialists primarily manage complications,” Dr. Hayek added.
Changes to Payment
The new recommendations are consistent with current management guidelines for obesity, hypertriglyceridemia, hypertension, type 2 diabetes, and chronic kidney disease.
“The advisory provides integrated algorithms for cardiovascular prevention and management, with specific therapeutic guidance tied to CKM stages, bringing together the current evidence for best practices from the various guidelines and filling gaps in a unified approach,” Dr. Joseph said.
In addition, the advisory draws attention to the care of younger patients, who may be at increased risk for cardiovascular disease due to lifestyle factors, according to Nishant Shah, MD, assistant professor of medicine at Duke.
“It considers barriers to care that prevent people from optimizing their cardiovascular health,” Dr. Shah said.
Although the advisory does not specify proposed payment changes to support the new care model, the move towards value-based care may require billing practices that accommodate integrated care as well as more frequent and more specialized testing, Dr. Hayek said.
“The advisory is an empowering tool for PCPs, underscoring their critical role in healthcare,” Dr. Hayek said. “It encourages PCPs to advocate for integrated care within their practices and to consider workflow adjustments that enhance the identification and initiation of preventive care for at-risk patients.”
Funding information was not provided.
Dr. Joseph reports no relevant financial involvements; several advisory co-authors report financial involvements with pharmaceutical companies. Dr. Pagidipati reports relevant financial involvement with pharmaceutical companies. Dr. Hayek, Dr. Drake, and Dr. Shah report no relevant financial involvements. Dr. Joseph is an author of the advisory. Dr. Pagidipati, Dr. Hayek, Dr. Drake, and Dr. Shah were not involved in the writing of the advisory.
A version of this article appeared on Medscape.com.
The advisory, published recently in Circulation introduces the concept of CKM health and reevaluates the relationships between obesity, diabetes, kidney disease, and cardiovascular disease (CVD).
“This approach not only raises awareness, it also empowers PCPs to diagnose and treat these conditions more holistically,” Salim Hayek, MD, associate professor of cardiovascular disease and internal medicine, and medical director of the Frankel Cardiovascular Center Clinics at the University of Michigan in Ann Arbor, said in an interview.
New CKM Staging, Testing, and Care Strategies
The advisory introduces a new scoring system that ranges from stage 0 (patients with no risk factors for CKM) through stage 4 (patients with clinical CVD in CKM syndrome). Each stage requires specific management strategies and may include screening starting at age 30 years for diabetes, hypertension, and heart failure.
“Stage 0 CKM is usually found in young people, and CKM risk factors and scores typically increase as people age,” said Sean M. Drake, MD, a primary care physician at Henry Ford Health in Sterling Heights, Michigan.
Dr. Drake advised PCPs to encourage patients who are at stage 0 to maintain ideal cardiovascular health and to monitor those at risk of progressing through the stages.
While PCPs already perform many of the tests the advisory recommends, the conditions overlap and an abnormality in one system should prompt more testing for other conditions. Additional tests, such as urine albumin-creatinine ratio, and more frequent glomerular filtration rate and lipid profile are advised, according to Dr. Drake.
“There also appears to be a role for additional cardiac testing, including echocardiograms and coronary CT scans, and for liver fibrosis screening,” Dr. Drake said. “Medications such as SGLT2 inhibitors, GLP-1 receptor agonists, and ACE inhibitors, beyond current routine use, are emphasized.”
To better characterize body composition and help diagnose metabolic syndrome, the advisory also recommends measuring waist circumference, which is not routine practice, noted Joshua J. Joseph, MD, MPH, an associate professor of endocrinology, diabetes, and metabolism at The Ohio State University Wexner Medical Center in Columbus, and a co-author of the advisory.
Recognizing the interconnected nature of cardiac, kidney, and metabolic diseases encourages a shift in mindset for clinicians, according to Neha Pagidipati, MD, MPH, a cardiologist at Duke Health in Durham, North Carolina.
“We have often been trained to focus on the specific problem in front of us,” Dr. Pagidipati said. “We need to be hyper-aware that many patients we see are at risk for multiple CKM entities. We need to be proactive about screening for and treating these when appropriate.”
The advisory emphasizes the need for CKM coordinators to support teams of clinicians from primary care, cardiology, endocrinology, nephrology, nursing, and pharmacy, as well as social workers, care navigators, or community health workers, Dr. Joseph said.
“The advisory repositions the PCP at the forefront of CKM care coordination, marking a departure from the traditional model where subspecialists primarily manage complications,” Dr. Hayek added.
Changes to Payment
The new recommendations are consistent with current management guidelines for obesity, hypertriglyceridemia, hypertension, type 2 diabetes, and chronic kidney disease.
“The advisory provides integrated algorithms for cardiovascular prevention and management, with specific therapeutic guidance tied to CKM stages, bringing together the current evidence for best practices from the various guidelines and filling gaps in a unified approach,” Dr. Joseph said.
In addition, the advisory draws attention to the care of younger patients, who may be at increased risk for cardiovascular disease due to lifestyle factors, according to Nishant Shah, MD, assistant professor of medicine at Duke.
“It considers barriers to care that prevent people from optimizing their cardiovascular health,” Dr. Shah said.
Although the advisory does not specify proposed payment changes to support the new care model, the move towards value-based care may require billing practices that accommodate integrated care as well as more frequent and more specialized testing, Dr. Hayek said.
“The advisory is an empowering tool for PCPs, underscoring their critical role in healthcare,” Dr. Hayek said. “It encourages PCPs to advocate for integrated care within their practices and to consider workflow adjustments that enhance the identification and initiation of preventive care for at-risk patients.”
Funding information was not provided.
Dr. Joseph reports no relevant financial involvements; several advisory co-authors report financial involvements with pharmaceutical companies. Dr. Pagidipati reports relevant financial involvement with pharmaceutical companies. Dr. Hayek, Dr. Drake, and Dr. Shah report no relevant financial involvements. Dr. Joseph is an author of the advisory. Dr. Pagidipati, Dr. Hayek, Dr. Drake, and Dr. Shah were not involved in the writing of the advisory.
A version of this article appeared on Medscape.com.
FROM CIRCULATION