User login
Recurrence of Extranodal Natural Killer/T-cell Lymphoma Presenting as Tarsal Tunnel Syndrome
ABSTRACT
This case report is a rare form of lymphoma recurrence which presented as tarsal tunnel syndrome. The patient had been previously treated for the malignancy and was presumed to be in remission; however, standard radiology imaging protocols failed to include the distal extremities on these scans. The patient presented to the orthopedic clinic with tarsal tunnel symptoms and a mass in the tarsal tunnel. A complete evaluation resulted in a diagnosis of recurrence of the malignancy. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body fluorodeoxyglucose positron emission tomography computed tomography when evaluating for recurrence in patients.
Nasal-type, extranodal natural killer/T-cell lymphoma (ENKTL) is a rare form of non-Hodgkin lymphoma (NHL). Malignancies account for only 10% of NHL in Asian and South American populations. However, in Caucasians, it represents <1% of all cases. In addition, at 3:1 male to female ratio, the disease most commonly affects male patients who are 50 to 59 years old.1-3 The etiology of this malignancy is strongly related to prior infection with Epstein-Barr virus (EBV) as EBV-encoded early small ribonucleic acid on in situ hybridization of lymphoma cells is positive in 95% of cases.4-6
Typical sites of involvement include the nasal cavity, nasopharynx, and sinuses, causing patients to present with nasal obstruction, chronic sinusitis, or epistaxis. Additionally, ENKTL can occur primarily in the skin, gastrointestinal tract, spleen, and testis, whereas the bone marrow may be involved in 10% of cases. Although rare, unusual sites, including muscle, adrenals, and ovaries, have been published.7,8
Staging is best performed using the T-staging system, which accounts for the extent of local tumor involvement. Higher stages, such as T3 /T4, equate to locally advanced disease and imply a worse prognosis.9,10 Computed tomography (CT) and magnetic resonance imaging (MRI) help define local soft tissues and bony involvement. Furthermore, CT of the chest, abdomen, and pelvis as well as bone marrow biopsy are performed as part of the staging process. Lastly, fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) is often used to detect extranodal spread, define the extent of involvement, differentiate between lymphoma and inflammatory masses, and monitor for recurrence.11
Treatment for local ENKTL involves concurrent chemoradiotherapy followed by 3 cycles of etoposide, ifosfamide, cisplatin, and dexamethasone, which results in a complete response rate of 80%, and is the most favorable when comparing treatment modalities.12 Unfortunately, recurrence rates reach as high as 50%, whereas the 5-year survival rate is 59%.13,14 For recurrent or disseminated disease, high-dose chemotherapy and hematopoietic stem cell transplantation remain as alternative treatments for patients who have undergone 2 complete remissions and can be curative in some instances.13,15
Continue to: In summary, ENKTL is a rare form...
In summary, ENKTL is a rare form of NHL which classically presents in the nasal cavity; however, this type of lymphoma may present in a variety of extranodal sites.7,8 Despite the numerous published reports on ENKTL, no study has reported either primary or recurrent ENKTL in the feet or hands. To our knowledge, this is one of the first published cases of a patient who developed a rare and recurring ENKTL in the foot and ankle. The patient provided written informed consent for print and electronic publication of this case report.
CASE
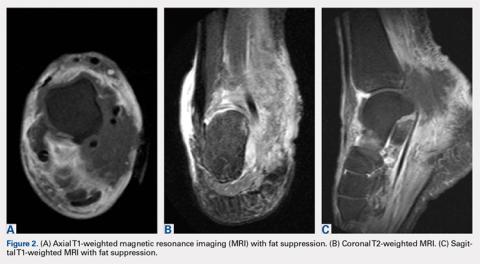
A 59-year-old Caucasian woman was referred to the orthopedic foot and ankle clinic by her primary care physician for right medial ankle pain, skin ulceration, and numbness over the plantar aspect of her right foot. Upon questioning, the patient noted that the pain and numbness were present for almost 6 months. She denied trauma to the concerned area. Previously, the patient was observed and treated elsewhere for plantar fasciitis and was prescribed a brace before being immobilized in a controlled ankle motion (CAM) boot for 6 weeks. At follow-up with her outside provider, the patient had developed skin breakdown over the medial aspect of the right ankle, and this condition was presumed to be caused by the boot. After local wound care failed to improve her skin ulceration, she returned to her primary care physician, who ordered an MRI of the area and referred her to our specialty clinic.
Upon review, the patient’s past medical history included a diagnosis of nasal-type ENKTL. Her malignancy was treated with chemoradiotherapy 2 years prior to her consultation with the foot and ankle clinic.
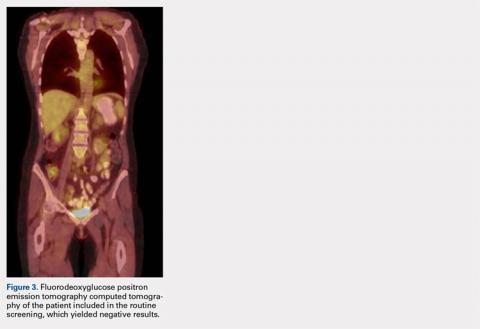
The patient was noted by her medical oncologist and interventional radiologist to be in complete stage 4 remission since being treated. She underwent routine MRI and CT scans of the head and neck at 6-month intervals and FDG PET-CT scans at 3-month intervals, as per institutional protocol. The examinations showed no evidence of malignancy or metabolically active disease. The last imaging study occurred 2 months prior to admission to the foot and ankle clinic.
The patient consulted her medical oncologist 1 month prior to presenting to our clinic and was noted to exhibit an “excellent response to chemoradiotherapy” and “continues to remain disease free at 2 years.” She was instructed to continue routine follow-up. However, the office notes mentioned no ankle pain and non-healing wounds.
During physical examination, the patient presented an antalgic gait on the right side. Inspection demonstrated an increased circumference of the right ankle compared with the left, with a soft, palpable mass over the medial aspect of her right ankle. A 3 cm × 2 cm, grade 2 abrasion of the skin was observed over the medial mass just posterior to her medial malleolus. Range of motion was within normal limits. The patient exhibited a palpable posterior tibial artery pulse and full strength upon muscle testing of the lower extremities. She featured a positive Tinel’s sign and discomfort over the mass itself, with the pain radiating down to the plantar aspect of her foot and diffuse numbness over the plantar aspect of the foot.
Continue to: Review of her plain radiographs...
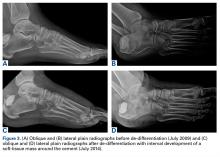
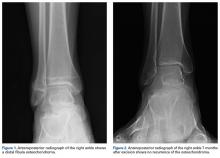
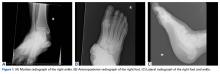
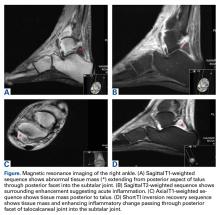
Review of her plain radiographs demonstrated no bony abnormalities, fractures, nor visible deformity (Figures 1A, 1B).
At presentation, our differential diagnosis included recurrence of the malignancy, secondary malignancy, infection, and inflammatory disease. After a lengthy discussion with the patient and consultation with our institution’s musculoskeletal oncologist, the decision was made to perform a right-ankle mass biopsy and marginal excision with wound irrigation and débridement and tarsal tunnel release.
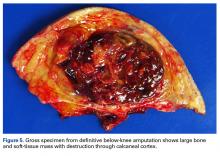
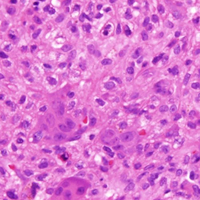
The patient was placed in the supine position with standard prepping and draping. The medial eschar was excised in an elliptical fashion, and a curvilinear, longitudinal approach was performed within the compartment to access the mass along the posteromedial aspect of the ankle. Although no evidence of infection was observed, the tissue was thickened with areas of necrosis down to the flexor retinaculum. Once the flexor retinaculum was opened, a fibrous, plaque-like mass was observed, and it was encased with flexor tendons and neurovascular structures of the tarsal tunnel. After mass excision, a complete tarsal tunnel release was performed until the neurovascular bundle was free. Irrigation and débridement of the ulcer were performed along with complicated wound closure, and the patient was placed in a well-padded postoperative splint.
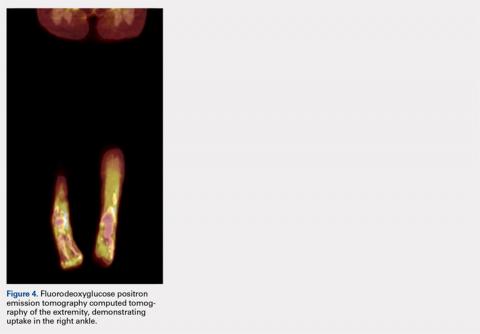
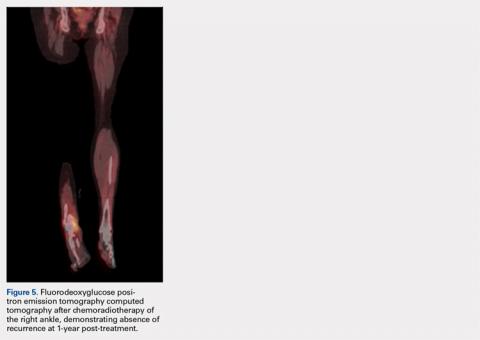
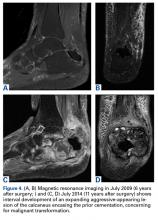
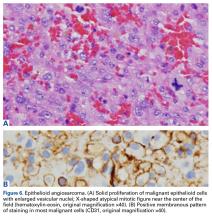
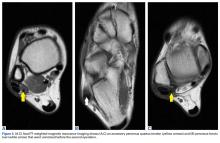
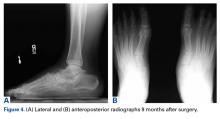
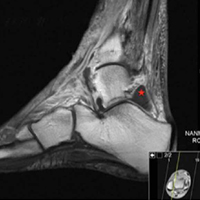
Pathology was finalized as a recurrent, EBV-positive, and nasal-type ENKTL. The patient underwent bone marrow biopsy, which yielded negative results. CT of the chest, abdomen, and pelvis were negative for the disease. FDG PET-CT, which included the extremities, was performed and demonstrated increased uptake in the right ankle, consistent with the malignancy (Figure 4).
DISCUSSION
ENKTL is an uncommon form of lymphoma and is exceedingly rare in Caucasian females.1-3 Although the patient’s primary occurrence was in the nasal cavity, recurrence in the foot and ankle must still be described.7,8 To our knowledge, this article is one of the first published cases of a patient who developed a rare-recurrence ENKTL about the foot and ankle. Occurrence in extremities is extremely rare that the staging protocol does not include FDG PET-CT of these areas. The patient’s “negative” scans led many providers to neglect the symptoms in her right ankle until the lesion had ulcerated through the skin. If one would have relied on imaging reports and outside records alone, the diagnosis would have been delayed longer or missed all together. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body FDG PET-CT when evaluating for recurrence in patients.
1. Quintanilla-Martinez L, Kremer M, Keller G, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095-2105. doi:10.1016/S0002-9440(10)63061-1.
2. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206-214. doi:10.1093/annonc/mdi037.
3. Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918.
4. Medeiros LJ, Peiper SC, Elwood L, Yano T, Raffeld M, Jaffe ES. Angiocentric immunoproliferative lesions: a molecular analysis of eight cases. Hum Pathol. 1991;22(11):1150-1157. doi:10.1016/0046-8177(91)90269-U.
5. Ho FC, Srivastava G, Loke SL, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990;8(5):271-281. doi:10.1002/hon.2900080505.
6. Gelb AB, van de Rijn M, Regula DP Jr, et al. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum Pathol. 1994;25(9):953-960. doi:10.1016/0046-8177(94)90018-3.
7. Petrella T, Delfau-Larue MH, Caillot D, et al. Nasopharyngeal lymphomas: further evidence for a natural killer cell origin. Hum Pathol. 1996;27(8):827-833. doi:10.1016/S0046-8177(96)90457-8.
8. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127(6):860-868. doi:10.1309/2F39NX1AL3L54WU8.
9. Robbins KT, Fuller LM, Vlasak M. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985;56(4):814-819. doi:10.1002/1097-0142(19850815)56.
10. Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. Am J Roentgenol. 2000;174(4):1141-1145. doi:10.2214/ajr.174.4.1741141.
11. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621. doi:10.1007/s00277-008-0494-8.
12. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027-6032. doi:10.1200/JCO.2009.23.8592.
13. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194. doi:10.1038/sj.leu.2403955.
14. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13-21. doi:10.1111/j.1365-2141.2009.07802.x.
15. Yokoyama H, Yamamoto J, Tohmiya Y, et al. Allogeneic hematopoietic stem cell transplant following chemotherapy containing l-asparaginase as a promising treatment for patients with relapsed or refractory extranodal natural killer/T cell lymphoma, nasal type. Leuk Lymphoma. 2010;51(8):1509-1512. doi:10.3109/10428194.2010.487958.
ABSTRACT
This case report is a rare form of lymphoma recurrence which presented as tarsal tunnel syndrome. The patient had been previously treated for the malignancy and was presumed to be in remission; however, standard radiology imaging protocols failed to include the distal extremities on these scans. The patient presented to the orthopedic clinic with tarsal tunnel symptoms and a mass in the tarsal tunnel. A complete evaluation resulted in a diagnosis of recurrence of the malignancy. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body fluorodeoxyglucose positron emission tomography computed tomography when evaluating for recurrence in patients.
Nasal-type, extranodal natural killer/T-cell lymphoma (ENKTL) is a rare form of non-Hodgkin lymphoma (NHL). Malignancies account for only 10% of NHL in Asian and South American populations. However, in Caucasians, it represents <1% of all cases. In addition, at 3:1 male to female ratio, the disease most commonly affects male patients who are 50 to 59 years old.1-3 The etiology of this malignancy is strongly related to prior infection with Epstein-Barr virus (EBV) as EBV-encoded early small ribonucleic acid on in situ hybridization of lymphoma cells is positive in 95% of cases.4-6
Typical sites of involvement include the nasal cavity, nasopharynx, and sinuses, causing patients to present with nasal obstruction, chronic sinusitis, or epistaxis. Additionally, ENKTL can occur primarily in the skin, gastrointestinal tract, spleen, and testis, whereas the bone marrow may be involved in 10% of cases. Although rare, unusual sites, including muscle, adrenals, and ovaries, have been published.7,8
Staging is best performed using the T-staging system, which accounts for the extent of local tumor involvement. Higher stages, such as T3 /T4, equate to locally advanced disease and imply a worse prognosis.9,10 Computed tomography (CT) and magnetic resonance imaging (MRI) help define local soft tissues and bony involvement. Furthermore, CT of the chest, abdomen, and pelvis as well as bone marrow biopsy are performed as part of the staging process. Lastly, fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) is often used to detect extranodal spread, define the extent of involvement, differentiate between lymphoma and inflammatory masses, and monitor for recurrence.11
Treatment for local ENKTL involves concurrent chemoradiotherapy followed by 3 cycles of etoposide, ifosfamide, cisplatin, and dexamethasone, which results in a complete response rate of 80%, and is the most favorable when comparing treatment modalities.12 Unfortunately, recurrence rates reach as high as 50%, whereas the 5-year survival rate is 59%.13,14 For recurrent or disseminated disease, high-dose chemotherapy and hematopoietic stem cell transplantation remain as alternative treatments for patients who have undergone 2 complete remissions and can be curative in some instances.13,15
Continue to: In summary, ENKTL is a rare form...
In summary, ENKTL is a rare form of NHL which classically presents in the nasal cavity; however, this type of lymphoma may present in a variety of extranodal sites.7,8 Despite the numerous published reports on ENKTL, no study has reported either primary or recurrent ENKTL in the feet or hands. To our knowledge, this is one of the first published cases of a patient who developed a rare and recurring ENKTL in the foot and ankle. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 59-year-old Caucasian woman was referred to the orthopedic foot and ankle clinic by her primary care physician for right medial ankle pain, skin ulceration, and numbness over the plantar aspect of her right foot. Upon questioning, the patient noted that the pain and numbness were present for almost 6 months. She denied trauma to the concerned area. Previously, the patient was observed and treated elsewhere for plantar fasciitis and was prescribed a brace before being immobilized in a controlled ankle motion (CAM) boot for 6 weeks. At follow-up with her outside provider, the patient had developed skin breakdown over the medial aspect of the right ankle, and this condition was presumed to be caused by the boot. After local wound care failed to improve her skin ulceration, she returned to her primary care physician, who ordered an MRI of the area and referred her to our specialty clinic.
Upon review, the patient’s past medical history included a diagnosis of nasal-type ENKTL. Her malignancy was treated with chemoradiotherapy 2 years prior to her consultation with the foot and ankle clinic.
The patient was noted by her medical oncologist and interventional radiologist to be in complete stage 4 remission since being treated. She underwent routine MRI and CT scans of the head and neck at 6-month intervals and FDG PET-CT scans at 3-month intervals, as per institutional protocol. The examinations showed no evidence of malignancy or metabolically active disease. The last imaging study occurred 2 months prior to admission to the foot and ankle clinic.
The patient consulted her medical oncologist 1 month prior to presenting to our clinic and was noted to exhibit an “excellent response to chemoradiotherapy” and “continues to remain disease free at 2 years.” She was instructed to continue routine follow-up. However, the office notes mentioned no ankle pain and non-healing wounds.
During physical examination, the patient presented an antalgic gait on the right side. Inspection demonstrated an increased circumference of the right ankle compared with the left, with a soft, palpable mass over the medial aspect of her right ankle. A 3 cm × 2 cm, grade 2 abrasion of the skin was observed over the medial mass just posterior to her medial malleolus. Range of motion was within normal limits. The patient exhibited a palpable posterior tibial artery pulse and full strength upon muscle testing of the lower extremities. She featured a positive Tinel’s sign and discomfort over the mass itself, with the pain radiating down to the plantar aspect of her foot and diffuse numbness over the plantar aspect of the foot.
Continue to: Review of her plain radiographs...
Review of her plain radiographs demonstrated no bony abnormalities, fractures, nor visible deformity (Figures 1A, 1B).
At presentation, our differential diagnosis included recurrence of the malignancy, secondary malignancy, infection, and inflammatory disease. After a lengthy discussion with the patient and consultation with our institution’s musculoskeletal oncologist, the decision was made to perform a right-ankle mass biopsy and marginal excision with wound irrigation and débridement and tarsal tunnel release.
The patient was placed in the supine position with standard prepping and draping. The medial eschar was excised in an elliptical fashion, and a curvilinear, longitudinal approach was performed within the compartment to access the mass along the posteromedial aspect of the ankle. Although no evidence of infection was observed, the tissue was thickened with areas of necrosis down to the flexor retinaculum. Once the flexor retinaculum was opened, a fibrous, plaque-like mass was observed, and it was encased with flexor tendons and neurovascular structures of the tarsal tunnel. After mass excision, a complete tarsal tunnel release was performed until the neurovascular bundle was free. Irrigation and débridement of the ulcer were performed along with complicated wound closure, and the patient was placed in a well-padded postoperative splint.
Pathology was finalized as a recurrent, EBV-positive, and nasal-type ENKTL. The patient underwent bone marrow biopsy, which yielded negative results. CT of the chest, abdomen, and pelvis were negative for the disease. FDG PET-CT, which included the extremities, was performed and demonstrated increased uptake in the right ankle, consistent with the malignancy (Figure 4).
DISCUSSION
ENKTL is an uncommon form of lymphoma and is exceedingly rare in Caucasian females.1-3 Although the patient’s primary occurrence was in the nasal cavity, recurrence in the foot and ankle must still be described.7,8 To our knowledge, this article is one of the first published cases of a patient who developed a rare-recurrence ENKTL about the foot and ankle. Occurrence in extremities is extremely rare that the staging protocol does not include FDG PET-CT of these areas. The patient’s “negative” scans led many providers to neglect the symptoms in her right ankle until the lesion had ulcerated through the skin. If one would have relied on imaging reports and outside records alone, the diagnosis would have been delayed longer or missed all together. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body FDG PET-CT when evaluating for recurrence in patients.
ABSTRACT
This case report is a rare form of lymphoma recurrence which presented as tarsal tunnel syndrome. The patient had been previously treated for the malignancy and was presumed to be in remission; however, standard radiology imaging protocols failed to include the distal extremities on these scans. The patient presented to the orthopedic clinic with tarsal tunnel symptoms and a mass in the tarsal tunnel. A complete evaluation resulted in a diagnosis of recurrence of the malignancy. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body fluorodeoxyglucose positron emission tomography computed tomography when evaluating for recurrence in patients.
Nasal-type, extranodal natural killer/T-cell lymphoma (ENKTL) is a rare form of non-Hodgkin lymphoma (NHL). Malignancies account for only 10% of NHL in Asian and South American populations. However, in Caucasians, it represents <1% of all cases. In addition, at 3:1 male to female ratio, the disease most commonly affects male patients who are 50 to 59 years old.1-3 The etiology of this malignancy is strongly related to prior infection with Epstein-Barr virus (EBV) as EBV-encoded early small ribonucleic acid on in situ hybridization of lymphoma cells is positive in 95% of cases.4-6
Typical sites of involvement include the nasal cavity, nasopharynx, and sinuses, causing patients to present with nasal obstruction, chronic sinusitis, or epistaxis. Additionally, ENKTL can occur primarily in the skin, gastrointestinal tract, spleen, and testis, whereas the bone marrow may be involved in 10% of cases. Although rare, unusual sites, including muscle, adrenals, and ovaries, have been published.7,8
Staging is best performed using the T-staging system, which accounts for the extent of local tumor involvement. Higher stages, such as T3 /T4, equate to locally advanced disease and imply a worse prognosis.9,10 Computed tomography (CT) and magnetic resonance imaging (MRI) help define local soft tissues and bony involvement. Furthermore, CT of the chest, abdomen, and pelvis as well as bone marrow biopsy are performed as part of the staging process. Lastly, fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) is often used to detect extranodal spread, define the extent of involvement, differentiate between lymphoma and inflammatory masses, and monitor for recurrence.11
Treatment for local ENKTL involves concurrent chemoradiotherapy followed by 3 cycles of etoposide, ifosfamide, cisplatin, and dexamethasone, which results in a complete response rate of 80%, and is the most favorable when comparing treatment modalities.12 Unfortunately, recurrence rates reach as high as 50%, whereas the 5-year survival rate is 59%.13,14 For recurrent or disseminated disease, high-dose chemotherapy and hematopoietic stem cell transplantation remain as alternative treatments for patients who have undergone 2 complete remissions and can be curative in some instances.13,15
Continue to: In summary, ENKTL is a rare form...
In summary, ENKTL is a rare form of NHL which classically presents in the nasal cavity; however, this type of lymphoma may present in a variety of extranodal sites.7,8 Despite the numerous published reports on ENKTL, no study has reported either primary or recurrent ENKTL in the feet or hands. To our knowledge, this is one of the first published cases of a patient who developed a rare and recurring ENKTL in the foot and ankle. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 59-year-old Caucasian woman was referred to the orthopedic foot and ankle clinic by her primary care physician for right medial ankle pain, skin ulceration, and numbness over the plantar aspect of her right foot. Upon questioning, the patient noted that the pain and numbness were present for almost 6 months. She denied trauma to the concerned area. Previously, the patient was observed and treated elsewhere for plantar fasciitis and was prescribed a brace before being immobilized in a controlled ankle motion (CAM) boot for 6 weeks. At follow-up with her outside provider, the patient had developed skin breakdown over the medial aspect of the right ankle, and this condition was presumed to be caused by the boot. After local wound care failed to improve her skin ulceration, she returned to her primary care physician, who ordered an MRI of the area and referred her to our specialty clinic.
Upon review, the patient’s past medical history included a diagnosis of nasal-type ENKTL. Her malignancy was treated with chemoradiotherapy 2 years prior to her consultation with the foot and ankle clinic.
The patient was noted by her medical oncologist and interventional radiologist to be in complete stage 4 remission since being treated. She underwent routine MRI and CT scans of the head and neck at 6-month intervals and FDG PET-CT scans at 3-month intervals, as per institutional protocol. The examinations showed no evidence of malignancy or metabolically active disease. The last imaging study occurred 2 months prior to admission to the foot and ankle clinic.
The patient consulted her medical oncologist 1 month prior to presenting to our clinic and was noted to exhibit an “excellent response to chemoradiotherapy” and “continues to remain disease free at 2 years.” She was instructed to continue routine follow-up. However, the office notes mentioned no ankle pain and non-healing wounds.
During physical examination, the patient presented an antalgic gait on the right side. Inspection demonstrated an increased circumference of the right ankle compared with the left, with a soft, palpable mass over the medial aspect of her right ankle. A 3 cm × 2 cm, grade 2 abrasion of the skin was observed over the medial mass just posterior to her medial malleolus. Range of motion was within normal limits. The patient exhibited a palpable posterior tibial artery pulse and full strength upon muscle testing of the lower extremities. She featured a positive Tinel’s sign and discomfort over the mass itself, with the pain radiating down to the plantar aspect of her foot and diffuse numbness over the plantar aspect of the foot.
Continue to: Review of her plain radiographs...
Review of her plain radiographs demonstrated no bony abnormalities, fractures, nor visible deformity (Figures 1A, 1B).
At presentation, our differential diagnosis included recurrence of the malignancy, secondary malignancy, infection, and inflammatory disease. After a lengthy discussion with the patient and consultation with our institution’s musculoskeletal oncologist, the decision was made to perform a right-ankle mass biopsy and marginal excision with wound irrigation and débridement and tarsal tunnel release.
The patient was placed in the supine position with standard prepping and draping. The medial eschar was excised in an elliptical fashion, and a curvilinear, longitudinal approach was performed within the compartment to access the mass along the posteromedial aspect of the ankle. Although no evidence of infection was observed, the tissue was thickened with areas of necrosis down to the flexor retinaculum. Once the flexor retinaculum was opened, a fibrous, plaque-like mass was observed, and it was encased with flexor tendons and neurovascular structures of the tarsal tunnel. After mass excision, a complete tarsal tunnel release was performed until the neurovascular bundle was free. Irrigation and débridement of the ulcer were performed along with complicated wound closure, and the patient was placed in a well-padded postoperative splint.
Pathology was finalized as a recurrent, EBV-positive, and nasal-type ENKTL. The patient underwent bone marrow biopsy, which yielded negative results. CT of the chest, abdomen, and pelvis were negative for the disease. FDG PET-CT, which included the extremities, was performed and demonstrated increased uptake in the right ankle, consistent with the malignancy (Figure 4).
DISCUSSION
ENKTL is an uncommon form of lymphoma and is exceedingly rare in Caucasian females.1-3 Although the patient’s primary occurrence was in the nasal cavity, recurrence in the foot and ankle must still be described.7,8 To our knowledge, this article is one of the first published cases of a patient who developed a rare-recurrence ENKTL about the foot and ankle. Occurrence in extremities is extremely rare that the staging protocol does not include FDG PET-CT of these areas. The patient’s “negative” scans led many providers to neglect the symptoms in her right ankle until the lesion had ulcerated through the skin. If one would have relied on imaging reports and outside records alone, the diagnosis would have been delayed longer or missed all together. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body FDG PET-CT when evaluating for recurrence in patients.
1. Quintanilla-Martinez L, Kremer M, Keller G, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095-2105. doi:10.1016/S0002-9440(10)63061-1.
2. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206-214. doi:10.1093/annonc/mdi037.
3. Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918.
4. Medeiros LJ, Peiper SC, Elwood L, Yano T, Raffeld M, Jaffe ES. Angiocentric immunoproliferative lesions: a molecular analysis of eight cases. Hum Pathol. 1991;22(11):1150-1157. doi:10.1016/0046-8177(91)90269-U.
5. Ho FC, Srivastava G, Loke SL, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990;8(5):271-281. doi:10.1002/hon.2900080505.
6. Gelb AB, van de Rijn M, Regula DP Jr, et al. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum Pathol. 1994;25(9):953-960. doi:10.1016/0046-8177(94)90018-3.
7. Petrella T, Delfau-Larue MH, Caillot D, et al. Nasopharyngeal lymphomas: further evidence for a natural killer cell origin. Hum Pathol. 1996;27(8):827-833. doi:10.1016/S0046-8177(96)90457-8.
8. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127(6):860-868. doi:10.1309/2F39NX1AL3L54WU8.
9. Robbins KT, Fuller LM, Vlasak M. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985;56(4):814-819. doi:10.1002/1097-0142(19850815)56.
10. Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. Am J Roentgenol. 2000;174(4):1141-1145. doi:10.2214/ajr.174.4.1741141.
11. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621. doi:10.1007/s00277-008-0494-8.
12. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027-6032. doi:10.1200/JCO.2009.23.8592.
13. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194. doi:10.1038/sj.leu.2403955.
14. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13-21. doi:10.1111/j.1365-2141.2009.07802.x.
15. Yokoyama H, Yamamoto J, Tohmiya Y, et al. Allogeneic hematopoietic stem cell transplant following chemotherapy containing l-asparaginase as a promising treatment for patients with relapsed or refractory extranodal natural killer/T cell lymphoma, nasal type. Leuk Lymphoma. 2010;51(8):1509-1512. doi:10.3109/10428194.2010.487958.
1. Quintanilla-Martinez L, Kremer M, Keller G, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095-2105. doi:10.1016/S0002-9440(10)63061-1.
2. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206-214. doi:10.1093/annonc/mdi037.
3. Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918.
4. Medeiros LJ, Peiper SC, Elwood L, Yano T, Raffeld M, Jaffe ES. Angiocentric immunoproliferative lesions: a molecular analysis of eight cases. Hum Pathol. 1991;22(11):1150-1157. doi:10.1016/0046-8177(91)90269-U.
5. Ho FC, Srivastava G, Loke SL, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990;8(5):271-281. doi:10.1002/hon.2900080505.
6. Gelb AB, van de Rijn M, Regula DP Jr, et al. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum Pathol. 1994;25(9):953-960. doi:10.1016/0046-8177(94)90018-3.
7. Petrella T, Delfau-Larue MH, Caillot D, et al. Nasopharyngeal lymphomas: further evidence for a natural killer cell origin. Hum Pathol. 1996;27(8):827-833. doi:10.1016/S0046-8177(96)90457-8.
8. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127(6):860-868. doi:10.1309/2F39NX1AL3L54WU8.
9. Robbins KT, Fuller LM, Vlasak M. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985;56(4):814-819. doi:10.1002/1097-0142(19850815)56.
10. Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. Am J Roentgenol. 2000;174(4):1141-1145. doi:10.2214/ajr.174.4.1741141.
11. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621. doi:10.1007/s00277-008-0494-8.
12. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027-6032. doi:10.1200/JCO.2009.23.8592.
13. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194. doi:10.1038/sj.leu.2403955.
14. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13-21. doi:10.1111/j.1365-2141.2009.07802.x.
15. Yokoyama H, Yamamoto J, Tohmiya Y, et al. Allogeneic hematopoietic stem cell transplant following chemotherapy containing l-asparaginase as a promising treatment for patients with relapsed or refractory extranodal natural killer/T cell lymphoma, nasal type. Leuk Lymphoma. 2010;51(8):1509-1512. doi:10.3109/10428194.2010.487958.
TAKE-HOME POINTS
- A thorough review of systems, physical examination, and personal review of a patient’s advanced imaging is critical to avoid missed diagnosis or delays in diagnosis.
- Any mass lesion encountered in clinical practice, no matter how benign appearing, should be presumed malignant until proven otherwise.
- Fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) should include whole-body scans when evaluating patients for recurrence of malignancy.
Arthroscopic Anterior Ankle Decompression Is Successful in National Football League Players
ABSTRACT
Anterior ankle impingement is a frequent cause of pain and disability in athletes with impingement of soft-tissue or osseous structures along the anterior margin of the tibiotalar joint during dorsiflexion.
In this study, we hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and range of motion (ROM), and would allow National Football League (NFL) players to return to their preoperative level of play.
We reviewed 29 arthroscopic ankle débridements performed by a single surgeon. Each NFL player underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to return to play (RTP), events missed secondary to surgery, and complications were recorded.
All athletes returned to the same level of NFL play at a mean (SD) of 8.4 (4.1) weeks after surgery and continued playing for a mean (SD) of 3.43 (2.57) years after surgery. Mean (SD) VAS pain scores decreased significantly (P < .001), to 0.38 (0.89) from 4.21 (1.52). Mean (SD) active ankle dorsiflexion increased significantly (P < .001), to 18.86° (2.62°) from 8.28° (4.14°). Mean (SD) AOFAS hindfoot scores increased significantly (P < .001), to 97.45 (4.72) from 70.62 (10.39). Degree of arthritis (r = 0.305) and age (r = 0.106) were poorly correlated to time to RTP.
In all cases, arthroscopic débridement of anterior ankle impingement resulted in RTP at the same level at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM.
Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
Continue to: Anterior ankle impingement...
Anterior ankle impingement is a frequent cause of disability in athletes.1 This condition results from repetitive trauma over time, which leads to osseous and soft-tissue impingement, pain, and decreased ankle range of motion (ROM).
First termed footballer’s ankle, this condition is linked to repeated, forceful plantarflexion,2 though later studies attributed the phenomenon to repeated dorsiflexion resulting in periosteal hemorrhage.3 Both osseous and soft-tissue structures can cause impingement at the tibiotalar joint, often with osteophytes anteromedially at the tibial talar joint. Soft-tissue structures, including hypertrophic synovium, meniscoid lesions, and a thickened anterior talofibular ligament, more often cause anterolateral impingement.4-6 This process results in pain in extreme dorsiflexion, which comes into play in almost all football maneuvers, including sprinting, back-peddling, and offensive and defensive stances. Therefore, maintenance of pain-free dorsiflexion is required for high-level football. Decreased ROM can lead to decreased ability to perform these high-level athletic functions and can limit performance.
Arthroscopic débridement improves functional outcomes and functional motion in both athletes and nonathletes.7,8 In addition, findings of a recent systematic review provide support for arthroscopic treatment of ankle impingement.9 Although arthroscopic treatment is effective in nonathletes and recreational athletes,10 there is a paucity of data on the efficacy of this procedure and on time to return to play (RTP) in professional football players.
We conducted a study to evaluate the outcomes (pain, ROM, RTP) of arthroscopic débridement for anterior ankle impingement in National Football League (NFL) players. We hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and ROM, and would allow NFL players to return to their preoperative level of play.
METHODS
After this study was granted Institutional Review Board approval, we retrospectively reviewed a consecutive series of arthroscopically treated anterior ankle impingement athletes by a single surgeon (JPB). Indications for surgery were anterior ankle impingement resulting in ankle pain and decreased ROM that interfered with sport. Active NFL players who underwent ankle arthroscopy for symptomatic anterior ankle impingement were included. Excluded were players who underwent surgery after retirement or who retired before returning to play for reasons unrelated to the ankle. Medical records, operative reports, and rehabilitation reports were reviewed.
Continue to: Preoperative and postoperative...
Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to RTP, events missed secondary to surgery, and complications were recorded. These preoperative and postoperative variables were compared with paired Student 2-way t tests for continuous variables. Pearson correlation coefficients were calculated.
PROCEDURE
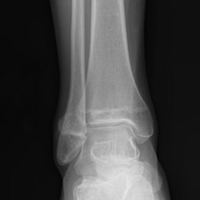
Ankle arthroscopy was performed with the patient supine after spinal or general anesthesia was induced. Prophylactic antibiotics were given in each case. Arthroscopy was performed with standard anterolateral and anteromedial portals. First, an incision was made through skin only, followed by blunt subcutaneous dissection down to the ankle capsule. A capsulotomy was then made bluntly. Care was taken to avoid all neurovascular structures. Posterior portals were not used. A 2.7-mm arthroscope was inserted and alternated between the anteromedial and anterolateral portals to maximally visualize the ankle joint. Diagnostic arthroscopy was performed to document synovitis, chondral injury, osseous, and soft-tissue impingement and any other noted pathology (Figures 1A-1C).
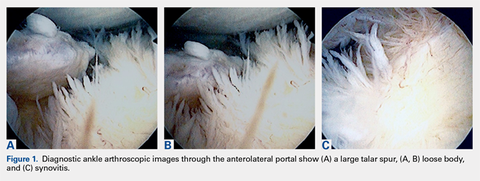
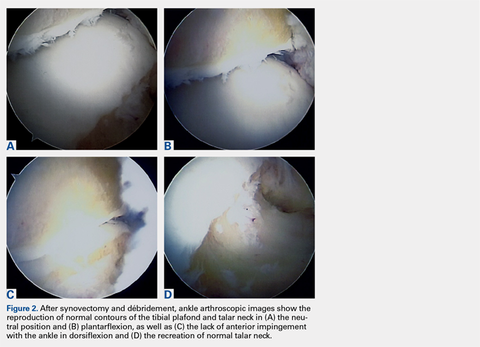
A full radius resector was then used to perform a synovectomy and débridement of impinging soft tissue from the anterior talofibular ligament or anterior inferior talofibular ligament. All patients underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. A small burr was used to débride and remove the osteophytes on the talus and/or tibia. Soft-tissue and osseous structures were resected until the contours of the talus and tibia were normal. Any unstable articular defects were débrided and loose bodies were removed. Ankle ROM was checked to confirm complete resolution of impingement (Figures 2A-2D). Patients were not immobilized and were allowed progressive weight-bearing as tolerated. Crutches were used for assisted ambulation the first 3 to 5 postoperative days.
Physical therapy progressed through 3 phases: (1) inflammation control and ROM restoration, (2) initiation of ankle strengthening, including eversion and inversion, and (3) agility, proprioception, and functional rehabilitation.
RESULTS
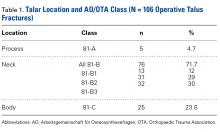
Twenty-five NFL players (29 surgeries) were included in the study. Two players were excluded because they had retired at the end of the season before the surgery for reasons unrelated to the operative ankle. Mean (SD) age was 28.1 (2.9) years. Six included players had a history of ankle sprains, 1 had a history of ipsilateral ankle fracture, and 1 had a history of ipsilateral ankle dislocation. Table 1 lists the positions of players who underwent ankle arthroscopic decompression.
Table 1. Positions of National Football League Players Who Underwent Ankle Arthroscopic Decompression for Anterior Ankle Impingement
Position | Surgeries, n |
| Offensive line | 8 |
| Defensive line | 8 |
| Wide receiver | 4 |
| Running back | 4 |
| Linebacker | 3 |
| Quarterback | 1 |
| Defensive back | 1 |
Continue to: During diagnostic arthroscopy...
During diagnostic arthroscopy, changes to the articular cartilage were noted: grade 0 in 38% of patients, grade 1 in 17%, grade 2 in 21%, grade 3 in 21%, and grade 4 in 3%. Four patients had an osteochondral lesion (<1 cm in each case), which was treated with chondroplasty without microfracture.
Each included patient returned to NFL play. Mean (SD) time to RTP without restrictions was 8.4 (4.1) weeks after surgery (range, 2-20 weeks). There was a poor correlation between degree of chondrosis and time to RTP (r = 0.305). In addition, there was a poor correlation between age and time to RTP (r = 0.106).
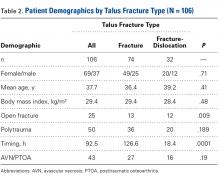
Dorsiflexion improved significantly (P < .001), patients had significantly less pain after surgery (P < .001), and AOFAS hindfoot scores improved significantly (P < .001) (Table 2).
Table 2. Preoperative and Postoperative Dorsiflexion, Pain, and AOFAS Score Before and After Arthroscopic Débridement of Anterior Ankle Impingementa
| Mean (SD) | ||
|---|---|---|
| Preoperative | Postoperative | |
| Dorsiflexion | 8.28º (4.14º) | 18.86° (2.62°) |
| VAS pain score | 4.21 (1.52) | 0.38 (0.89) |
| AOFAS score | 70.62 (10.39) | 97.45 (4.72) |
aAll values were significantly improved after surgery (P < .001).
Abbreviations: AOFAS, American Orthopaedic Foot and Ankle Society; VAS, visual analog scale.
The athletes played in the NFL for a mean (SD) of 3.43 (2.57) years after surgery (range, 1-10 seasons). These players included 6 who were still active at time of publication. No patient required revision surgery or additional surgery on the ipsilateral ankle. The one patient who was treated for superficial thrombophlebitis after surgery reported symptoms before surgery as well.
DISCUSSION
Arthroscopic decompression of anterior ankle impingement is safe and significantly improves pain and ROM in professional American football players. The procedure results in reliable RTP at an elite level, with durable results over the time remaining in their NFL careers.
Continue to: before the 1988 study by Hawkins...
Before the 1988 study by Hawkins,11 ankle spurs were removed with open procedures. Hawkins11 used arthroscopy for better and safer visualization of the ankle joint and used a burr for less painful removal of spurs from the tibia and the talus. In 2002, a series of 105 patients (median age, 35 years) had reduced pain and improved function a minimum of 2 years after arthroscopic débridement.12 These patients had a mix of pathology, including soft-tissue impingement, bony impingement, chondral lesions, loose bodies, and osteoarthritis.
For many elite athletes, anterior ankle impingement can cause significant limitation. Reduced ankle dorsiflexion can alter all limb mechanics and predispose athletes to injury.13 In addition, because NFL players’ ankle ROM often approaches or exceeds normal physiologic limits,14 an ankle ROM limitation will often hinder their performance.
Miyamoto and colleagues15 studied a series of 9 professional athletes (6 soccer players, 1 baseball pitcher, 1 mixed martial artist, 1 golfer) who underwent decompression of both anterior and posterior impingement. With regard to anterior impingement, they found anterior osteophytes in all the ankles, as was seen in the present study. Furthermore, they noted that mean dorsiflexion improved from 10° before surgery to 15° after surgery and that their athletes returned to play 12 to 15 weeks after surgery. Their results are similar to ours, though we noted more improvement in dorsiflexion, from 8.28° before surgery to 18.86° after surgery.
One of the most important metrics in evaluating treatment options for professional athletes is time from surgery to RTP without restrictions. Mean time to full RTP was shorter in our study (8.4 weeks) than in the study by Miyamoto and colleagues15 (up to 20 weeks). However, many of their procedures were performed during the off-season, when there was no need to expeditiously clear patients for full sports participation. In addition, the patients in their study had both anterior and posterior pathology.
Faster return to high-level athletics was supported in a study of 11 elite ballet dancers,16 whose pain and dance performance improved after arthroscopic débridement. Of the 11 patients, 9 returned to dance at a mean of 7 weeks after surgery; the other 2 required reoperation. Although the pathology differed in their study of elite professional soccer players, Calder and colleagues17 found that mean time to RTP after ankle arthroscopy for posterior impingement was 5 weeks.
Continue to: For the NFL players in our study...
For the NFL players in our study, RTP at their elite level was 100% after arthroscopic débridement of anterior ankle impingement. In the literature, time to RTP varies. Table 3 lists RTP rates for recreational athletes in published studies.18-27 In their recent systematic literature review, Zwiers and colleagues10 noted that 24% to 96.4% of recreational athletes returned to play after arthroscopic treatment for anterior ankle impingement. The percentage was significantly higher for the professional athletes in our study. Historical comparison supports an evolution in the indications and techniques for this procedure, with more recent literature suggesting a RTP rate much higher than earlier rates. In addition, compared with recreational athletes, professional athletes have strong financial incentives to return to their sports. Furthermore, our professional cohort was significantly younger than the recreational cohorts in those studies.
Table 3. Frequency of Recreational Athletes’ Return to Play After Arthroscopic Débridement of Anterior Ankle Impingement, as Reported in the Literature
| Study | Year | Journal | Return to Play | |
|---|---|---|---|---|
| n/N | % | |||
| Akseki et al18 | 1999 | Acta Orthop Scand | 10/11 | 91 |
| Baums et al19 | 2006 | Knee Surg Sports Traumatol Arthrosc | 25/26 | 96 |
| Branca et al20 | 1997 | Foot Ankle Int | 13/27 | 48 |
| Di Palma et al21 | 1999 | J Sports Traumatol Relat Res | 21/32 | 66 |
| Ferkel et al22 | 1991 | Am J Sports Med | 27/31 | 87.1 |
| Hassan23 | 2007 | Knee Surg Sports Traumatol Arthrosc | 9/11 | 82 |
| Jerosch et al24 | 1994 | Knee Surg Sports Traumatol Arthrosc | 9/38 | 24 |
| Murawski & Kennedy25 | 2010 | Am J Sports Med | 27/28 | 96.4 |
| Ogilvie-Harris et al26 | 1993 | J Bone Joint Surg Br | 21/28 | 75 |
| Rouvillain et al27 | 2014 | Eur J Orthop Surg Traumatol | 10/11 | 90 |
Total | 172/243 | 70 | ||
Current recommendations for recreational athletes include initial conservative treatment with rest, ankle bracing, and avoidance of jumping and other repetitive dorsiflexing activities. Physical therapy should include joint mobilization and work along the entire kinetic chain. Night splints or a removable walking boot can be used temporarily, as can a single intra-articular corticosteroid injection to reduce inflammation and evaluate improvement in more refractory cases.28 Commonly, conservative treatments fail if patients remain active, and soft tissue and/or osteophytes can be resected, though resection typically is reserved for recreational athletes for whom nonoperative treatments have been exhausted.29,30
This study had several limitations, including its retrospective nature and lack of control group. In addition, follow-up was relatively short, and we did not use more recently described outcome measures, such as the Sports subscale of the Foot and Ankle Ability Measure, which may be more sensitive in describing function in elite athletes. However, many of the cases in our study predated these measures, but the rate of RTP at the NFL level requires a very high degree of postoperative ankle function, making this outcome the most meaningful. In the context of professional athletes, specifically the length of their careers, our study results provide valuable information regarding expectations about RTP and the durability of arthroscopic débridement of anterior ankle impingement in a high-demand setting.
CONCLUSION
For all the NFL players in this study, arthroscopic débridement of anterior ankle impingement resulted in return to preoperative level of play at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM. Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
1. Lubowitz JH. Editorial commentary: ankle anterior impingement is common in athletes and could be under-recognized. Arthroscopy. 2015;31(8):1597.
2. Mcdougall A. Footballer’s ankle. Lancet. 1955;269(6902):1219-1220.
3. Kleiger B. Anterior tibiotalar impingement syndromes in dancers. Foot Ankle. 1982;3(2):69-73.
4. Bassett FH 3rd, Gates HS 3rd, Billys JB, Morris HB, Nikolaou PK. Talar impingement by the anteroinferior tibiofibular ligament. A cause of chronic pain in the ankle after inversion sprain. J Bone Joint Surg Am. 1990;72(1):55-59.
5. Liu SH, Raskin A, Osti L, et al. Arthroscopic treatment of anterolateral ankle impingement. Arthroscopy. 1994;10(2):215-218.
6. Thein R, Eichenblat M. Arthroscopic treatment of sports-related synovitis of the ankle. Am J Sports Med. 1992;20(5):496-498.
7. Arnold H. Posttraumatic impingement syndrome of the ankle—indication and results of arthroscopic therapy. Foot Ankle Surg. 2011;17(2):85-88.
8. Walsh SJ, Twaddle BC, Rosenfeldt MP, Boyle MJ. Arthroscopic treatment of anterior ankle impingement: a prospective study of 46 patients with 5-year follow-up. Am J Sports Med. 2014;42(11):2722-2726.
9. Glazebrook MA, Ganapathy V, Bridge MA, Stone JW, Allard JP. Evidence-based indications for ankle arthroscopy. Arthroscopy. 2009;25(12):1478-1490.
10. Zwiers R, Wiegerinck JI, Murawski CD, Fraser EJ, Kennedy JG, van Dijk CN. Arthroscopic treatment for anterior ankle impingement: a systematic review of the current literature. Arthroscopy. 2015;31(8):1585-1596.
11. Hawkins RB. Arthroscopic treatment of sports-related anterior osteophytes in the ankle. Foot Ankle. 1988;9(2):87-90.
12. Rasmussen S, Hjorth Jensen C. Arthroscopic treatment of impingement of the ankle reduces pain and enhances function. Scand J Med Sci Sports. 2002;12(2):69-72.
13. Mason-Mackay AR, Whatman C, Reid D. The effect of reduced ankle dorsiflexion on lower extremity mechanics during landing: a systematic review. J Sci Med Sport. 2017;20(5):451-458.
14. Riley PO, Kent RW, Dierks TA, Lievers WB, Frimenko RE, Crandall JR. Foot kinematics and loading of professional athletes in American football-specific tasks. Gait Posture. 2013;38(4):563-569.
15. Miyamoto W, Takao M, Matsui K, Matsushita T. Simultaneous ankle arthroscopy and hindfoot endoscopy for combined anterior and posterior ankle impingement syndrome in professional athletes. J Orthop Sci. 2015;20(4):642-648.
16. Nihal A, Rose DJ, Trepman E. Arthroscopic treatment of anterior ankle impingement syndrome in dancers. Foot Ankle Int. 2005;26(11):908-912.
17. Calder JD, Sexton SA, Pearce CJ. Return to training and playing after posterior ankle arthroscopy for posterior impingement in elite professional soccer. Am J Sports Med. 2010;38(1):120-124.
18. Akseki D, Pinar H, Bozkurt M, Yaldiz K, Arag S. The distal fascicle of the anterior inferior tibiofibular ligament as a cause of anterolateral ankle impingement: results of arthroscopic resection. Acta Orthop Scand. 1999;70(5):478-482.
19. Baums MH, Kahl E, Schultz W, Klinger HM. Clinical outcome of the arthroscopic management of sports-related “anterior ankle pain”: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):482-486.
20. Branca A, Di Palma L, Bucca C, Visconti CS, Di Mille M. Arthroscopic treatment of anterior ankle impingement. Foot Ankle Int. 1997;18(7):418-423.
21. Di Palma L, Bucca C, Di Mille M, Branca A. Diagnosis and arthroscopic treatment of fibrous impingement of the ankle. J Sports Traumatol Relat Res. 1999;21:170-177.
22. Ferkel RD, Karzel RP, Del Pizzo W, Friedman MJ, Fischer SP. Arthroscopic treatment of anterolateral impingement of the ankle. Am J Sports Med. 1991;19(5):440-446.
23. Hassan AH. Treatment of anterolateral impingements of the ankle joint by arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1150-1154.
24. Jerosch J, Steinbeck J, Schröder M, Halm H. Arthroscopic treatment of anterior synovitis of the ankle in athletes. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):176-181.
25. Murawski CD, Kennedy JG. Anteromedial impingement in the ankle joint: outcomes following arthroscopy. Am J Sports Med. 2010;38(10):2017-2024.
26. Ogilvie-Harris DJ, Mahomed N, Demazière A. Anterior impingement of the ankle treated by arthroscopic removal of bony spurs. J Bone Joint Surg Br. 1993;75(3):437-440.
27. Rouvillain JL, Daoud W, Donica A, Garron E, Uzel AP. Distraction-free ankle arthroscopy for anterolateral impingement. Eur J Orthop Surg Traumatol. 2014;24(6):1019-1023.
28. O’Kane JW, Kadel N. Anterior impingement syndrome in dancers. Curr Rev Musculoskelet Med. 2008;1(1):12-16.
29. Lavery KP, McHale KJ, Rossy WH, Theodore G. Ankle impingement. J Orthop Surg Res. 2016;11(1):97.
30. Talusan PG, Toy J, Perez JL, Milewski MD, Reach JS. Anterior ankle impingement: diagnosis and treatment. J Am Acad Orthop Surg. 2014;22(5):333-339.
ABSTRACT
Anterior ankle impingement is a frequent cause of pain and disability in athletes with impingement of soft-tissue or osseous structures along the anterior margin of the tibiotalar joint during dorsiflexion.
In this study, we hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and range of motion (ROM), and would allow National Football League (NFL) players to return to their preoperative level of play.
We reviewed 29 arthroscopic ankle débridements performed by a single surgeon. Each NFL player underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to return to play (RTP), events missed secondary to surgery, and complications were recorded.
All athletes returned to the same level of NFL play at a mean (SD) of 8.4 (4.1) weeks after surgery and continued playing for a mean (SD) of 3.43 (2.57) years after surgery. Mean (SD) VAS pain scores decreased significantly (P < .001), to 0.38 (0.89) from 4.21 (1.52). Mean (SD) active ankle dorsiflexion increased significantly (P < .001), to 18.86° (2.62°) from 8.28° (4.14°). Mean (SD) AOFAS hindfoot scores increased significantly (P < .001), to 97.45 (4.72) from 70.62 (10.39). Degree of arthritis (r = 0.305) and age (r = 0.106) were poorly correlated to time to RTP.
In all cases, arthroscopic débridement of anterior ankle impingement resulted in RTP at the same level at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM.
Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
Continue to: Anterior ankle impingement...
Anterior ankle impingement is a frequent cause of disability in athletes.1 This condition results from repetitive trauma over time, which leads to osseous and soft-tissue impingement, pain, and decreased ankle range of motion (ROM).
First termed footballer’s ankle, this condition is linked to repeated, forceful plantarflexion,2 though later studies attributed the phenomenon to repeated dorsiflexion resulting in periosteal hemorrhage.3 Both osseous and soft-tissue structures can cause impingement at the tibiotalar joint, often with osteophytes anteromedially at the tibial talar joint. Soft-tissue structures, including hypertrophic synovium, meniscoid lesions, and a thickened anterior talofibular ligament, more often cause anterolateral impingement.4-6 This process results in pain in extreme dorsiflexion, which comes into play in almost all football maneuvers, including sprinting, back-peddling, and offensive and defensive stances. Therefore, maintenance of pain-free dorsiflexion is required for high-level football. Decreased ROM can lead to decreased ability to perform these high-level athletic functions and can limit performance.
Arthroscopic débridement improves functional outcomes and functional motion in both athletes and nonathletes.7,8 In addition, findings of a recent systematic review provide support for arthroscopic treatment of ankle impingement.9 Although arthroscopic treatment is effective in nonathletes and recreational athletes,10 there is a paucity of data on the efficacy of this procedure and on time to return to play (RTP) in professional football players.
We conducted a study to evaluate the outcomes (pain, ROM, RTP) of arthroscopic débridement for anterior ankle impingement in National Football League (NFL) players. We hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and ROM, and would allow NFL players to return to their preoperative level of play.
METHODS
After this study was granted Institutional Review Board approval, we retrospectively reviewed a consecutive series of arthroscopically treated anterior ankle impingement athletes by a single surgeon (JPB). Indications for surgery were anterior ankle impingement resulting in ankle pain and decreased ROM that interfered with sport. Active NFL players who underwent ankle arthroscopy for symptomatic anterior ankle impingement were included. Excluded were players who underwent surgery after retirement or who retired before returning to play for reasons unrelated to the ankle. Medical records, operative reports, and rehabilitation reports were reviewed.
Continue to: Preoperative and postoperative...
Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to RTP, events missed secondary to surgery, and complications were recorded. These preoperative and postoperative variables were compared with paired Student 2-way t tests for continuous variables. Pearson correlation coefficients were calculated.
PROCEDURE
Ankle arthroscopy was performed with the patient supine after spinal or general anesthesia was induced. Prophylactic antibiotics were given in each case. Arthroscopy was performed with standard anterolateral and anteromedial portals. First, an incision was made through skin only, followed by blunt subcutaneous dissection down to the ankle capsule. A capsulotomy was then made bluntly. Care was taken to avoid all neurovascular structures. Posterior portals were not used. A 2.7-mm arthroscope was inserted and alternated between the anteromedial and anterolateral portals to maximally visualize the ankle joint. Diagnostic arthroscopy was performed to document synovitis, chondral injury, osseous, and soft-tissue impingement and any other noted pathology (Figures 1A-1C).

A full radius resector was then used to perform a synovectomy and débridement of impinging soft tissue from the anterior talofibular ligament or anterior inferior talofibular ligament. All patients underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. A small burr was used to débride and remove the osteophytes on the talus and/or tibia. Soft-tissue and osseous structures were resected until the contours of the talus and tibia were normal. Any unstable articular defects were débrided and loose bodies were removed. Ankle ROM was checked to confirm complete resolution of impingement (Figures 2A-2D). Patients were not immobilized and were allowed progressive weight-bearing as tolerated. Crutches were used for assisted ambulation the first 3 to 5 postoperative days.

Physical therapy progressed through 3 phases: (1) inflammation control and ROM restoration, (2) initiation of ankle strengthening, including eversion and inversion, and (3) agility, proprioception, and functional rehabilitation.
RESULTS
Twenty-five NFL players (29 surgeries) were included in the study. Two players were excluded because they had retired at the end of the season before the surgery for reasons unrelated to the operative ankle. Mean (SD) age was 28.1 (2.9) years. Six included players had a history of ankle sprains, 1 had a history of ipsilateral ankle fracture, and 1 had a history of ipsilateral ankle dislocation. Table 1 lists the positions of players who underwent ankle arthroscopic decompression.
Table 1. Positions of National Football League Players Who Underwent Ankle Arthroscopic Decompression for Anterior Ankle Impingement
Position | Surgeries, n |
| Offensive line | 8 |
| Defensive line | 8 |
| Wide receiver | 4 |
| Running back | 4 |
| Linebacker | 3 |
| Quarterback | 1 |
| Defensive back | 1 |
Continue to: During diagnostic arthroscopy...
During diagnostic arthroscopy, changes to the articular cartilage were noted: grade 0 in 38% of patients, grade 1 in 17%, grade 2 in 21%, grade 3 in 21%, and grade 4 in 3%. Four patients had an osteochondral lesion (<1 cm in each case), which was treated with chondroplasty without microfracture.
Each included patient returned to NFL play. Mean (SD) time to RTP without restrictions was 8.4 (4.1) weeks after surgery (range, 2-20 weeks). There was a poor correlation between degree of chondrosis and time to RTP (r = 0.305). In addition, there was a poor correlation between age and time to RTP (r = 0.106).
Dorsiflexion improved significantly (P < .001), patients had significantly less pain after surgery (P < .001), and AOFAS hindfoot scores improved significantly (P < .001) (Table 2).
Table 2. Preoperative and Postoperative Dorsiflexion, Pain, and AOFAS Score Before and After Arthroscopic Débridement of Anterior Ankle Impingementa
| Mean (SD) | ||
|---|---|---|
| Preoperative | Postoperative | |
| Dorsiflexion | 8.28º (4.14º) | 18.86° (2.62°) |
| VAS pain score | 4.21 (1.52) | 0.38 (0.89) |
| AOFAS score | 70.62 (10.39) | 97.45 (4.72) |
aAll values were significantly improved after surgery (P < .001).
Abbreviations: AOFAS, American Orthopaedic Foot and Ankle Society; VAS, visual analog scale.
The athletes played in the NFL for a mean (SD) of 3.43 (2.57) years after surgery (range, 1-10 seasons). These players included 6 who were still active at time of publication. No patient required revision surgery or additional surgery on the ipsilateral ankle. The one patient who was treated for superficial thrombophlebitis after surgery reported symptoms before surgery as well.
DISCUSSION
Arthroscopic decompression of anterior ankle impingement is safe and significantly improves pain and ROM in professional American football players. The procedure results in reliable RTP at an elite level, with durable results over the time remaining in their NFL careers.
Continue to: before the 1988 study by Hawkins...
Before the 1988 study by Hawkins,11 ankle spurs were removed with open procedures. Hawkins11 used arthroscopy for better and safer visualization of the ankle joint and used a burr for less painful removal of spurs from the tibia and the talus. In 2002, a series of 105 patients (median age, 35 years) had reduced pain and improved function a minimum of 2 years after arthroscopic débridement.12 These patients had a mix of pathology, including soft-tissue impingement, bony impingement, chondral lesions, loose bodies, and osteoarthritis.
For many elite athletes, anterior ankle impingement can cause significant limitation. Reduced ankle dorsiflexion can alter all limb mechanics and predispose athletes to injury.13 In addition, because NFL players’ ankle ROM often approaches or exceeds normal physiologic limits,14 an ankle ROM limitation will often hinder their performance.
Miyamoto and colleagues15 studied a series of 9 professional athletes (6 soccer players, 1 baseball pitcher, 1 mixed martial artist, 1 golfer) who underwent decompression of both anterior and posterior impingement. With regard to anterior impingement, they found anterior osteophytes in all the ankles, as was seen in the present study. Furthermore, they noted that mean dorsiflexion improved from 10° before surgery to 15° after surgery and that their athletes returned to play 12 to 15 weeks after surgery. Their results are similar to ours, though we noted more improvement in dorsiflexion, from 8.28° before surgery to 18.86° after surgery.
One of the most important metrics in evaluating treatment options for professional athletes is time from surgery to RTP without restrictions. Mean time to full RTP was shorter in our study (8.4 weeks) than in the study by Miyamoto and colleagues15 (up to 20 weeks). However, many of their procedures were performed during the off-season, when there was no need to expeditiously clear patients for full sports participation. In addition, the patients in their study had both anterior and posterior pathology.
Faster return to high-level athletics was supported in a study of 11 elite ballet dancers,16 whose pain and dance performance improved after arthroscopic débridement. Of the 11 patients, 9 returned to dance at a mean of 7 weeks after surgery; the other 2 required reoperation. Although the pathology differed in their study of elite professional soccer players, Calder and colleagues17 found that mean time to RTP after ankle arthroscopy for posterior impingement was 5 weeks.
Continue to: For the NFL players in our study...
For the NFL players in our study, RTP at their elite level was 100% after arthroscopic débridement of anterior ankle impingement. In the literature, time to RTP varies. Table 3 lists RTP rates for recreational athletes in published studies.18-27 In their recent systematic literature review, Zwiers and colleagues10 noted that 24% to 96.4% of recreational athletes returned to play after arthroscopic treatment for anterior ankle impingement. The percentage was significantly higher for the professional athletes in our study. Historical comparison supports an evolution in the indications and techniques for this procedure, with more recent literature suggesting a RTP rate much higher than earlier rates. In addition, compared with recreational athletes, professional athletes have strong financial incentives to return to their sports. Furthermore, our professional cohort was significantly younger than the recreational cohorts in those studies.
Table 3. Frequency of Recreational Athletes’ Return to Play After Arthroscopic Débridement of Anterior Ankle Impingement, as Reported in the Literature
| Study | Year | Journal | Return to Play | |
|---|---|---|---|---|
| n/N | % | |||
| Akseki et al18 | 1999 | Acta Orthop Scand | 10/11 | 91 |
| Baums et al19 | 2006 | Knee Surg Sports Traumatol Arthrosc | 25/26 | 96 |
| Branca et al20 | 1997 | Foot Ankle Int | 13/27 | 48 |
| Di Palma et al21 | 1999 | J Sports Traumatol Relat Res | 21/32 | 66 |
| Ferkel et al22 | 1991 | Am J Sports Med | 27/31 | 87.1 |
| Hassan23 | 2007 | Knee Surg Sports Traumatol Arthrosc | 9/11 | 82 |
| Jerosch et al24 | 1994 | Knee Surg Sports Traumatol Arthrosc | 9/38 | 24 |
| Murawski & Kennedy25 | 2010 | Am J Sports Med | 27/28 | 96.4 |
| Ogilvie-Harris et al26 | 1993 | J Bone Joint Surg Br | 21/28 | 75 |
| Rouvillain et al27 | 2014 | Eur J Orthop Surg Traumatol | 10/11 | 90 |
Total | 172/243 | 70 | ||
Current recommendations for recreational athletes include initial conservative treatment with rest, ankle bracing, and avoidance of jumping and other repetitive dorsiflexing activities. Physical therapy should include joint mobilization and work along the entire kinetic chain. Night splints or a removable walking boot can be used temporarily, as can a single intra-articular corticosteroid injection to reduce inflammation and evaluate improvement in more refractory cases.28 Commonly, conservative treatments fail if patients remain active, and soft tissue and/or osteophytes can be resected, though resection typically is reserved for recreational athletes for whom nonoperative treatments have been exhausted.29,30
This study had several limitations, including its retrospective nature and lack of control group. In addition, follow-up was relatively short, and we did not use more recently described outcome measures, such as the Sports subscale of the Foot and Ankle Ability Measure, which may be more sensitive in describing function in elite athletes. However, many of the cases in our study predated these measures, but the rate of RTP at the NFL level requires a very high degree of postoperative ankle function, making this outcome the most meaningful. In the context of professional athletes, specifically the length of their careers, our study results provide valuable information regarding expectations about RTP and the durability of arthroscopic débridement of anterior ankle impingement in a high-demand setting.
CONCLUSION
For all the NFL players in this study, arthroscopic débridement of anterior ankle impingement resulted in return to preoperative level of play at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM. Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
ABSTRACT
Anterior ankle impingement is a frequent cause of pain and disability in athletes with impingement of soft-tissue or osseous structures along the anterior margin of the tibiotalar joint during dorsiflexion.
In this study, we hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and range of motion (ROM), and would allow National Football League (NFL) players to return to their preoperative level of play.
We reviewed 29 arthroscopic ankle débridements performed by a single surgeon. Each NFL player underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to return to play (RTP), events missed secondary to surgery, and complications were recorded.
All athletes returned to the same level of NFL play at a mean (SD) of 8.4 (4.1) weeks after surgery and continued playing for a mean (SD) of 3.43 (2.57) years after surgery. Mean (SD) VAS pain scores decreased significantly (P < .001), to 0.38 (0.89) from 4.21 (1.52). Mean (SD) active ankle dorsiflexion increased significantly (P < .001), to 18.86° (2.62°) from 8.28° (4.14°). Mean (SD) AOFAS hindfoot scores increased significantly (P < .001), to 97.45 (4.72) from 70.62 (10.39). Degree of arthritis (r = 0.305) and age (r = 0.106) were poorly correlated to time to RTP.
In all cases, arthroscopic débridement of anterior ankle impingement resulted in RTP at the same level at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM.
Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
Continue to: Anterior ankle impingement...
Anterior ankle impingement is a frequent cause of disability in athletes.1 This condition results from repetitive trauma over time, which leads to osseous and soft-tissue impingement, pain, and decreased ankle range of motion (ROM).
First termed footballer’s ankle, this condition is linked to repeated, forceful plantarflexion,2 though later studies attributed the phenomenon to repeated dorsiflexion resulting in periosteal hemorrhage.3 Both osseous and soft-tissue structures can cause impingement at the tibiotalar joint, often with osteophytes anteromedially at the tibial talar joint. Soft-tissue structures, including hypertrophic synovium, meniscoid lesions, and a thickened anterior talofibular ligament, more often cause anterolateral impingement.4-6 This process results in pain in extreme dorsiflexion, which comes into play in almost all football maneuvers, including sprinting, back-peddling, and offensive and defensive stances. Therefore, maintenance of pain-free dorsiflexion is required for high-level football. Decreased ROM can lead to decreased ability to perform these high-level athletic functions and can limit performance.
Arthroscopic débridement improves functional outcomes and functional motion in both athletes and nonathletes.7,8 In addition, findings of a recent systematic review provide support for arthroscopic treatment of ankle impingement.9 Although arthroscopic treatment is effective in nonathletes and recreational athletes,10 there is a paucity of data on the efficacy of this procedure and on time to return to play (RTP) in professional football players.
We conducted a study to evaluate the outcomes (pain, ROM, RTP) of arthroscopic débridement for anterior ankle impingement in National Football League (NFL) players. We hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and ROM, and would allow NFL players to return to their preoperative level of play.
METHODS
After this study was granted Institutional Review Board approval, we retrospectively reviewed a consecutive series of arthroscopically treated anterior ankle impingement athletes by a single surgeon (JPB). Indications for surgery were anterior ankle impingement resulting in ankle pain and decreased ROM that interfered with sport. Active NFL players who underwent ankle arthroscopy for symptomatic anterior ankle impingement were included. Excluded were players who underwent surgery after retirement or who retired before returning to play for reasons unrelated to the ankle. Medical records, operative reports, and rehabilitation reports were reviewed.
Continue to: Preoperative and postoperative...
Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to RTP, events missed secondary to surgery, and complications were recorded. These preoperative and postoperative variables were compared with paired Student 2-way t tests for continuous variables. Pearson correlation coefficients were calculated.
PROCEDURE
Ankle arthroscopy was performed with the patient supine after spinal or general anesthesia was induced. Prophylactic antibiotics were given in each case. Arthroscopy was performed with standard anterolateral and anteromedial portals. First, an incision was made through skin only, followed by blunt subcutaneous dissection down to the ankle capsule. A capsulotomy was then made bluntly. Care was taken to avoid all neurovascular structures. Posterior portals were not used. A 2.7-mm arthroscope was inserted and alternated between the anteromedial and anterolateral portals to maximally visualize the ankle joint. Diagnostic arthroscopy was performed to document synovitis, chondral injury, osseous, and soft-tissue impingement and any other noted pathology (Figures 1A-1C).

A full radius resector was then used to perform a synovectomy and débridement of impinging soft tissue from the anterior talofibular ligament or anterior inferior talofibular ligament. All patients underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. A small burr was used to débride and remove the osteophytes on the talus and/or tibia. Soft-tissue and osseous structures were resected until the contours of the talus and tibia were normal. Any unstable articular defects were débrided and loose bodies were removed. Ankle ROM was checked to confirm complete resolution of impingement (Figures 2A-2D). Patients were not immobilized and were allowed progressive weight-bearing as tolerated. Crutches were used for assisted ambulation the first 3 to 5 postoperative days.

Physical therapy progressed through 3 phases: (1) inflammation control and ROM restoration, (2) initiation of ankle strengthening, including eversion and inversion, and (3) agility, proprioception, and functional rehabilitation.
RESULTS
Twenty-five NFL players (29 surgeries) were included in the study. Two players were excluded because they had retired at the end of the season before the surgery for reasons unrelated to the operative ankle. Mean (SD) age was 28.1 (2.9) years. Six included players had a history of ankle sprains, 1 had a history of ipsilateral ankle fracture, and 1 had a history of ipsilateral ankle dislocation. Table 1 lists the positions of players who underwent ankle arthroscopic decompression.
Table 1. Positions of National Football League Players Who Underwent Ankle Arthroscopic Decompression for Anterior Ankle Impingement
Position | Surgeries, n |
| Offensive line | 8 |
| Defensive line | 8 |
| Wide receiver | 4 |
| Running back | 4 |
| Linebacker | 3 |
| Quarterback | 1 |
| Defensive back | 1 |
Continue to: During diagnostic arthroscopy...
During diagnostic arthroscopy, changes to the articular cartilage were noted: grade 0 in 38% of patients, grade 1 in 17%, grade 2 in 21%, grade 3 in 21%, and grade 4 in 3%. Four patients had an osteochondral lesion (<1 cm in each case), which was treated with chondroplasty without microfracture.
Each included patient returned to NFL play. Mean (SD) time to RTP without restrictions was 8.4 (4.1) weeks after surgery (range, 2-20 weeks). There was a poor correlation between degree of chondrosis and time to RTP (r = 0.305). In addition, there was a poor correlation between age and time to RTP (r = 0.106).
Dorsiflexion improved significantly (P < .001), patients had significantly less pain after surgery (P < .001), and AOFAS hindfoot scores improved significantly (P < .001) (Table 2).
Table 2. Preoperative and Postoperative Dorsiflexion, Pain, and AOFAS Score Before and After Arthroscopic Débridement of Anterior Ankle Impingementa
| Mean (SD) | ||
|---|---|---|
| Preoperative | Postoperative | |
| Dorsiflexion | 8.28º (4.14º) | 18.86° (2.62°) |
| VAS pain score | 4.21 (1.52) | 0.38 (0.89) |
| AOFAS score | 70.62 (10.39) | 97.45 (4.72) |
aAll values were significantly improved after surgery (P < .001).
Abbreviations: AOFAS, American Orthopaedic Foot and Ankle Society; VAS, visual analog scale.
The athletes played in the NFL for a mean (SD) of 3.43 (2.57) years after surgery (range, 1-10 seasons). These players included 6 who were still active at time of publication. No patient required revision surgery or additional surgery on the ipsilateral ankle. The one patient who was treated for superficial thrombophlebitis after surgery reported symptoms before surgery as well.
DISCUSSION
Arthroscopic decompression of anterior ankle impingement is safe and significantly improves pain and ROM in professional American football players. The procedure results in reliable RTP at an elite level, with durable results over the time remaining in their NFL careers.
Continue to: before the 1988 study by Hawkins...
Before the 1988 study by Hawkins,11 ankle spurs were removed with open procedures. Hawkins11 used arthroscopy for better and safer visualization of the ankle joint and used a burr for less painful removal of spurs from the tibia and the talus. In 2002, a series of 105 patients (median age, 35 years) had reduced pain and improved function a minimum of 2 years after arthroscopic débridement.12 These patients had a mix of pathology, including soft-tissue impingement, bony impingement, chondral lesions, loose bodies, and osteoarthritis.
For many elite athletes, anterior ankle impingement can cause significant limitation. Reduced ankle dorsiflexion can alter all limb mechanics and predispose athletes to injury.13 In addition, because NFL players’ ankle ROM often approaches or exceeds normal physiologic limits,14 an ankle ROM limitation will often hinder their performance.
Miyamoto and colleagues15 studied a series of 9 professional athletes (6 soccer players, 1 baseball pitcher, 1 mixed martial artist, 1 golfer) who underwent decompression of both anterior and posterior impingement. With regard to anterior impingement, they found anterior osteophytes in all the ankles, as was seen in the present study. Furthermore, they noted that mean dorsiflexion improved from 10° before surgery to 15° after surgery and that their athletes returned to play 12 to 15 weeks after surgery. Their results are similar to ours, though we noted more improvement in dorsiflexion, from 8.28° before surgery to 18.86° after surgery.
One of the most important metrics in evaluating treatment options for professional athletes is time from surgery to RTP without restrictions. Mean time to full RTP was shorter in our study (8.4 weeks) than in the study by Miyamoto and colleagues15 (up to 20 weeks). However, many of their procedures were performed during the off-season, when there was no need to expeditiously clear patients for full sports participation. In addition, the patients in their study had both anterior and posterior pathology.
Faster return to high-level athletics was supported in a study of 11 elite ballet dancers,16 whose pain and dance performance improved after arthroscopic débridement. Of the 11 patients, 9 returned to dance at a mean of 7 weeks after surgery; the other 2 required reoperation. Although the pathology differed in their study of elite professional soccer players, Calder and colleagues17 found that mean time to RTP after ankle arthroscopy for posterior impingement was 5 weeks.
Continue to: For the NFL players in our study...
For the NFL players in our study, RTP at their elite level was 100% after arthroscopic débridement of anterior ankle impingement. In the literature, time to RTP varies. Table 3 lists RTP rates for recreational athletes in published studies.18-27 In their recent systematic literature review, Zwiers and colleagues10 noted that 24% to 96.4% of recreational athletes returned to play after arthroscopic treatment for anterior ankle impingement. The percentage was significantly higher for the professional athletes in our study. Historical comparison supports an evolution in the indications and techniques for this procedure, with more recent literature suggesting a RTP rate much higher than earlier rates. In addition, compared with recreational athletes, professional athletes have strong financial incentives to return to their sports. Furthermore, our professional cohort was significantly younger than the recreational cohorts in those studies.
Table 3. Frequency of Recreational Athletes’ Return to Play After Arthroscopic Débridement of Anterior Ankle Impingement, as Reported in the Literature
| Study | Year | Journal | Return to Play | |
|---|---|---|---|---|
| n/N | % | |||
| Akseki et al18 | 1999 | Acta Orthop Scand | 10/11 | 91 |
| Baums et al19 | 2006 | Knee Surg Sports Traumatol Arthrosc | 25/26 | 96 |
| Branca et al20 | 1997 | Foot Ankle Int | 13/27 | 48 |
| Di Palma et al21 | 1999 | J Sports Traumatol Relat Res | 21/32 | 66 |
| Ferkel et al22 | 1991 | Am J Sports Med | 27/31 | 87.1 |
| Hassan23 | 2007 | Knee Surg Sports Traumatol Arthrosc | 9/11 | 82 |
| Jerosch et al24 | 1994 | Knee Surg Sports Traumatol Arthrosc | 9/38 | 24 |
| Murawski & Kennedy25 | 2010 | Am J Sports Med | 27/28 | 96.4 |
| Ogilvie-Harris et al26 | 1993 | J Bone Joint Surg Br | 21/28 | 75 |
| Rouvillain et al27 | 2014 | Eur J Orthop Surg Traumatol | 10/11 | 90 |
Total | 172/243 | 70 | ||
Current recommendations for recreational athletes include initial conservative treatment with rest, ankle bracing, and avoidance of jumping and other repetitive dorsiflexing activities. Physical therapy should include joint mobilization and work along the entire kinetic chain. Night splints or a removable walking boot can be used temporarily, as can a single intra-articular corticosteroid injection to reduce inflammation and evaluate improvement in more refractory cases.28 Commonly, conservative treatments fail if patients remain active, and soft tissue and/or osteophytes can be resected, though resection typically is reserved for recreational athletes for whom nonoperative treatments have been exhausted.29,30
This study had several limitations, including its retrospective nature and lack of control group. In addition, follow-up was relatively short, and we did not use more recently described outcome measures, such as the Sports subscale of the Foot and Ankle Ability Measure, which may be more sensitive in describing function in elite athletes. However, many of the cases in our study predated these measures, but the rate of RTP at the NFL level requires a very high degree of postoperative ankle function, making this outcome the most meaningful. In the context of professional athletes, specifically the length of their careers, our study results provide valuable information regarding expectations about RTP and the durability of arthroscopic débridement of anterior ankle impingement in a high-demand setting.
CONCLUSION
For all the NFL players in this study, arthroscopic débridement of anterior ankle impingement resulted in return to preoperative level of play at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM. Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
1. Lubowitz JH. Editorial commentary: ankle anterior impingement is common in athletes and could be under-recognized. Arthroscopy. 2015;31(8):1597.
2. Mcdougall A. Footballer’s ankle. Lancet. 1955;269(6902):1219-1220.
3. Kleiger B. Anterior tibiotalar impingement syndromes in dancers. Foot Ankle. 1982;3(2):69-73.
4. Bassett FH 3rd, Gates HS 3rd, Billys JB, Morris HB, Nikolaou PK. Talar impingement by the anteroinferior tibiofibular ligament. A cause of chronic pain in the ankle after inversion sprain. J Bone Joint Surg Am. 1990;72(1):55-59.
5. Liu SH, Raskin A, Osti L, et al. Arthroscopic treatment of anterolateral ankle impingement. Arthroscopy. 1994;10(2):215-218.
6. Thein R, Eichenblat M. Arthroscopic treatment of sports-related synovitis of the ankle. Am J Sports Med. 1992;20(5):496-498.
7. Arnold H. Posttraumatic impingement syndrome of the ankle—indication and results of arthroscopic therapy. Foot Ankle Surg. 2011;17(2):85-88.
8. Walsh SJ, Twaddle BC, Rosenfeldt MP, Boyle MJ. Arthroscopic treatment of anterior ankle impingement: a prospective study of 46 patients with 5-year follow-up. Am J Sports Med. 2014;42(11):2722-2726.
9. Glazebrook MA, Ganapathy V, Bridge MA, Stone JW, Allard JP. Evidence-based indications for ankle arthroscopy. Arthroscopy. 2009;25(12):1478-1490.
10. Zwiers R, Wiegerinck JI, Murawski CD, Fraser EJ, Kennedy JG, van Dijk CN. Arthroscopic treatment for anterior ankle impingement: a systematic review of the current literature. Arthroscopy. 2015;31(8):1585-1596.
11. Hawkins RB. Arthroscopic treatment of sports-related anterior osteophytes in the ankle. Foot Ankle. 1988;9(2):87-90.
12. Rasmussen S, Hjorth Jensen C. Arthroscopic treatment of impingement of the ankle reduces pain and enhances function. Scand J Med Sci Sports. 2002;12(2):69-72.
13. Mason-Mackay AR, Whatman C, Reid D. The effect of reduced ankle dorsiflexion on lower extremity mechanics during landing: a systematic review. J Sci Med Sport. 2017;20(5):451-458.
14. Riley PO, Kent RW, Dierks TA, Lievers WB, Frimenko RE, Crandall JR. Foot kinematics and loading of professional athletes in American football-specific tasks. Gait Posture. 2013;38(4):563-569.
15. Miyamoto W, Takao M, Matsui K, Matsushita T. Simultaneous ankle arthroscopy and hindfoot endoscopy for combined anterior and posterior ankle impingement syndrome in professional athletes. J Orthop Sci. 2015;20(4):642-648.
16. Nihal A, Rose DJ, Trepman E. Arthroscopic treatment of anterior ankle impingement syndrome in dancers. Foot Ankle Int. 2005;26(11):908-912.
17. Calder JD, Sexton SA, Pearce CJ. Return to training and playing after posterior ankle arthroscopy for posterior impingement in elite professional soccer. Am J Sports Med. 2010;38(1):120-124.
18. Akseki D, Pinar H, Bozkurt M, Yaldiz K, Arag S. The distal fascicle of the anterior inferior tibiofibular ligament as a cause of anterolateral ankle impingement: results of arthroscopic resection. Acta Orthop Scand. 1999;70(5):478-482.
19. Baums MH, Kahl E, Schultz W, Klinger HM. Clinical outcome of the arthroscopic management of sports-related “anterior ankle pain”: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):482-486.
20. Branca A, Di Palma L, Bucca C, Visconti CS, Di Mille M. Arthroscopic treatment of anterior ankle impingement. Foot Ankle Int. 1997;18(7):418-423.
21. Di Palma L, Bucca C, Di Mille M, Branca A. Diagnosis and arthroscopic treatment of fibrous impingement of the ankle. J Sports Traumatol Relat Res. 1999;21:170-177.
22. Ferkel RD, Karzel RP, Del Pizzo W, Friedman MJ, Fischer SP. Arthroscopic treatment of anterolateral impingement of the ankle. Am J Sports Med. 1991;19(5):440-446.
23. Hassan AH. Treatment of anterolateral impingements of the ankle joint by arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1150-1154.
24. Jerosch J, Steinbeck J, Schröder M, Halm H. Arthroscopic treatment of anterior synovitis of the ankle in athletes. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):176-181.
25. Murawski CD, Kennedy JG. Anteromedial impingement in the ankle joint: outcomes following arthroscopy. Am J Sports Med. 2010;38(10):2017-2024.
26. Ogilvie-Harris DJ, Mahomed N, Demazière A. Anterior impingement of the ankle treated by arthroscopic removal of bony spurs. J Bone Joint Surg Br. 1993;75(3):437-440.
27. Rouvillain JL, Daoud W, Donica A, Garron E, Uzel AP. Distraction-free ankle arthroscopy for anterolateral impingement. Eur J Orthop Surg Traumatol. 2014;24(6):1019-1023.
28. O’Kane JW, Kadel N. Anterior impingement syndrome in dancers. Curr Rev Musculoskelet Med. 2008;1(1):12-16.
29. Lavery KP, McHale KJ, Rossy WH, Theodore G. Ankle impingement. J Orthop Surg Res. 2016;11(1):97.
30. Talusan PG, Toy J, Perez JL, Milewski MD, Reach JS. Anterior ankle impingement: diagnosis and treatment. J Am Acad Orthop Surg. 2014;22(5):333-339.
1. Lubowitz JH. Editorial commentary: ankle anterior impingement is common in athletes and could be under-recognized. Arthroscopy. 2015;31(8):1597.
2. Mcdougall A. Footballer’s ankle. Lancet. 1955;269(6902):1219-1220.
3. Kleiger B. Anterior tibiotalar impingement syndromes in dancers. Foot Ankle. 1982;3(2):69-73.
4. Bassett FH 3rd, Gates HS 3rd, Billys JB, Morris HB, Nikolaou PK. Talar impingement by the anteroinferior tibiofibular ligament. A cause of chronic pain in the ankle after inversion sprain. J Bone Joint Surg Am. 1990;72(1):55-59.
5. Liu SH, Raskin A, Osti L, et al. Arthroscopic treatment of anterolateral ankle impingement. Arthroscopy. 1994;10(2):215-218.
6. Thein R, Eichenblat M. Arthroscopic treatment of sports-related synovitis of the ankle. Am J Sports Med. 1992;20(5):496-498.
7. Arnold H. Posttraumatic impingement syndrome of the ankle—indication and results of arthroscopic therapy. Foot Ankle Surg. 2011;17(2):85-88.
8. Walsh SJ, Twaddle BC, Rosenfeldt MP, Boyle MJ. Arthroscopic treatment of anterior ankle impingement: a prospective study of 46 patients with 5-year follow-up. Am J Sports Med. 2014;42(11):2722-2726.
9. Glazebrook MA, Ganapathy V, Bridge MA, Stone JW, Allard JP. Evidence-based indications for ankle arthroscopy. Arthroscopy. 2009;25(12):1478-1490.
10. Zwiers R, Wiegerinck JI, Murawski CD, Fraser EJ, Kennedy JG, van Dijk CN. Arthroscopic treatment for anterior ankle impingement: a systematic review of the current literature. Arthroscopy. 2015;31(8):1585-1596.
11. Hawkins RB. Arthroscopic treatment of sports-related anterior osteophytes in the ankle. Foot Ankle. 1988;9(2):87-90.
12. Rasmussen S, Hjorth Jensen C. Arthroscopic treatment of impingement of the ankle reduces pain and enhances function. Scand J Med Sci Sports. 2002;12(2):69-72.
13. Mason-Mackay AR, Whatman C, Reid D. The effect of reduced ankle dorsiflexion on lower extremity mechanics during landing: a systematic review. J Sci Med Sport. 2017;20(5):451-458.
14. Riley PO, Kent RW, Dierks TA, Lievers WB, Frimenko RE, Crandall JR. Foot kinematics and loading of professional athletes in American football-specific tasks. Gait Posture. 2013;38(4):563-569.
15. Miyamoto W, Takao M, Matsui K, Matsushita T. Simultaneous ankle arthroscopy and hindfoot endoscopy for combined anterior and posterior ankle impingement syndrome in professional athletes. J Orthop Sci. 2015;20(4):642-648.
16. Nihal A, Rose DJ, Trepman E. Arthroscopic treatment of anterior ankle impingement syndrome in dancers. Foot Ankle Int. 2005;26(11):908-912.
17. Calder JD, Sexton SA, Pearce CJ. Return to training and playing after posterior ankle arthroscopy for posterior impingement in elite professional soccer. Am J Sports Med. 2010;38(1):120-124.
18. Akseki D, Pinar H, Bozkurt M, Yaldiz K, Arag S. The distal fascicle of the anterior inferior tibiofibular ligament as a cause of anterolateral ankle impingement: results of arthroscopic resection. Acta Orthop Scand. 1999;70(5):478-482.
19. Baums MH, Kahl E, Schultz W, Klinger HM. Clinical outcome of the arthroscopic management of sports-related “anterior ankle pain”: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):482-486.
20. Branca A, Di Palma L, Bucca C, Visconti CS, Di Mille M. Arthroscopic treatment of anterior ankle impingement. Foot Ankle Int. 1997;18(7):418-423.
21. Di Palma L, Bucca C, Di Mille M, Branca A. Diagnosis and arthroscopic treatment of fibrous impingement of the ankle. J Sports Traumatol Relat Res. 1999;21:170-177.
22. Ferkel RD, Karzel RP, Del Pizzo W, Friedman MJ, Fischer SP. Arthroscopic treatment of anterolateral impingement of the ankle. Am J Sports Med. 1991;19(5):440-446.
23. Hassan AH. Treatment of anterolateral impingements of the ankle joint by arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1150-1154.
24. Jerosch J, Steinbeck J, Schröder M, Halm H. Arthroscopic treatment of anterior synovitis of the ankle in athletes. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):176-181.
25. Murawski CD, Kennedy JG. Anteromedial impingement in the ankle joint: outcomes following arthroscopy. Am J Sports Med. 2010;38(10):2017-2024.
26. Ogilvie-Harris DJ, Mahomed N, Demazière A. Anterior impingement of the ankle treated by arthroscopic removal of bony spurs. J Bone Joint Surg Br. 1993;75(3):437-440.
27. Rouvillain JL, Daoud W, Donica A, Garron E, Uzel AP. Distraction-free ankle arthroscopy for anterolateral impingement. Eur J Orthop Surg Traumatol. 2014;24(6):1019-1023.
28. O’Kane JW, Kadel N. Anterior impingement syndrome in dancers. Curr Rev Musculoskelet Med. 2008;1(1):12-16.
29. Lavery KP, McHale KJ, Rossy WH, Theodore G. Ankle impingement. J Orthop Surg Res. 2016;11(1):97.
30. Talusan PG, Toy J, Perez JL, Milewski MD, Reach JS. Anterior ankle impingement: diagnosis and treatment. J Am Acad Orthop Surg. 2014;22(5):333-339.
TAKE-HOME POINTS
- Anterior ankle impingement can be very debilitating in elite athletes and may lead to significantly decreased performance.
- First line treatment for anterior ankle impingement is conservative which includes rest, ankle bracing, and avoidance of repetitive dorsiflexing activities such as jumping.
- Arthroscopic débridement of anterior ankle impingement reliably relieves pain, and restores ROM and function.
- Arthroscopic débridement of anterior ankle impingement results in reliable RTP in professional football players.
- RTP after arthroscopic anterior ankle débridement for impingement averaged 2 months in professional football players.
Transformation of Benign Giant Cell Tumor of Bone Into Epithelioid Angiosarcoma
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
Peroneus Quartus Muscle
Take-Home Points
- PQ is easily mistaken for a PB tear.
- PQ is best identified on MRI, but commonly missed.
- For symptomatic cases, excision is the best treatment.
- Consider PQ in patients with chronic ankle pain, swelling, and/or instability.
The peroneus quartus (PQ) is an accessory muscle arising from the leg’s lateral compartment, which typically contains the peroneus longus (PL) and the peroneus brevis (PB). The many cadaveric studies that have been conducted indicate a general population prevalence ranging from 6.6% to 23%.1 Radiographic studies, including magnetic resonance imaging (MRI) and ultrasonography, have shown a similar prevalence.2 Although the PQ is asymptomatic in most cases, it may compromise the space of the superior peroneal tunnel and cause problems, including ankle pain, PB tear, subluxation of peroneal tendons, tendinous calcification, painful hypertrophy of retrotrochlear eminence, and recurrent hematomas.1,3-5 Given its differing anatomy, the PQ variously has been referred to as peroneocalcaneus externum, peroneocuboideus, long peroneal tendon, and peroneoperoneolongus.1
Although the PQ’s origin and insertion differ between subjects, the most common origin is the muscle fibers of the PB, and the most common insertion is the retrotrochlear eminence of the calcaneum.3
We report a case of peroneal tendon pathology that was initially thought to be caused primarily by impingement of a large osteochondroma on the tendons, but was later thought to be caused in part by a PQ and a split PB tendon seen only at the time of the second operation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 16-year-old boy with an osteochondroma of the right distal fibula presented to clinic with the chief complaint of lateral right ankle pain. A “sharp” pain accompanied by audible “popping” occurred with ankle motion. Medical history was significant only for non-Hodgkin lymphoma treated with bone marrow transplantation and whole body radiation at a young age. Physical examination revealed a palpable exostosis of the distal right fibula and associated ankle swelling.
One year after surgery, the patient returned with recurring right ankle pain and audible popping during ankle movement. There was no appreciable peroneal tendon subluxation on physical examination. Repeat imaging of the ankle showed no recurrence of the osteochondroma (Figure 2).
Discussion
Absence of a PQ muscle in simian and prosimian species suggests that the PQ represents an evolutionary adaptation to evert the lateral foot and improve bipedal gait. Although the 3 peroneal (PL, PB, peroneus tertius [PT]) muscles evert the middle part of the lateral border of the foot, the PQ inserts on the retrotrochlear eminence, which everts the posterior part of the lateral border of the foot.1,6 The PQ has often been described as a variation of the PB. The PQ may also stabilize the ankle and reduce the energy required for walking. A similar functional adaptation has been proposed for the PT, which dorsiflexes at the ankle. Although presence of a PT also varies in the population, its occurrence does not correlate with presence of a PQ. In people with PQ muscles, there is an 83% to 95% incidence of also having PT muscles.7
PQ prevalence has ranged from 6.6% to 23% in cadaver studies2 and from 7% to 17% in radiologic studies.1 To better evaluate prevalence, Yammine2 performed a meta-analysis of data from 46 studies (cadaveric dissection, MRI, ultrasonography) and 3928 legs and found an overall incidence of 10.2% and a higher incidence in the Indian population than in other races. Another study found no correlation between PQ presence and sex.7
MRI is the best imaging modality for assessing for PQ but must be performed specifically for this anatomical variation. Axial images may show a fat pad separating the PQ muscle from the PB muscle.8 On imaging, a PQ muscle can be mistaken for a peroneal tendon tear. A feature that helps in distinguishing the 2 is location; the PQ typically is found posterior and medial to the PL and PB tendons, whereas PB tears are anterior to the retromalleolar groove.2 Presence of a PQ muscle may be missed on initial MRI, as occurred in our patient’s case. Zammit and Singh3 reviewed 80 leg MRIs and found 6 PQs. Only 1 of the 6 reports described the PQ as an “atypical appearance of peroneus brevis [that] appears to be made up of more than one tendon.”
Surgical excision is often adequate treatment for a symptomatic PQ. If the PQ muscle is small and symptomatic from pressure to the muscle mass, a short fasciotomy may be performed.9 More commonly, complete excision of the accessory muscle is required. Although the PQ muscle is usually asymptomatic, it should be considered in cases of chronic ankle pain, swelling, or instability; recurrent hematomas; and peroneal tendon subluxation or tears.5,7
Our patient’s diagnosis was initially overlooked because of an osteochondroma in the region of interest. It remains unclear whether his pain was caused by the PQ itself or, more likely, from the PB tear. It is thought that the accessory muscle adds bulk to the peroneal tunnel, predisposing to peroneal pathology, such as muscle tears and tendon subluxation. Regardless, advanced imaging performed before the index procedure, along with a general understanding of the PQ and its classic MRI findings, may have prevented the repeat operation in this case.
The PQ muscle is a rare but sometimes missed potential etiology of ankle pain and tendon subluxation. In our patient’s case, the most obvious abnormality, an osteochondroma, may have masked the true cause.
1. Athavale SA, Gupta V, Kotgirwar S, Singh V. The peroneus quartus muscle: clinical correlation with evolutionary importance. Anat Sci Int. 2012;87(2):106-110.
2. Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat. 2015;37(6):617-627.
3. Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br. 2003;85(8):1134-1137.
4. Kulshreshtha R, Kadri S, Rajan DT. A case of unusual combination of injuries around the lateral malleolus. Foot. 2006;16(1):51-53.
5. Donley BG, Leyes M. Peroneus quartus muscle. A rare cause of chronic lateral ankle pain. Am J Sports Med. 2001;29(3):373-375.
6. Hecker P. Study on the peroneus of the tarsus. Anat Rec. 1923;26(1):79-82.
7. Rios Nascimento SR, Watanabe Costa R, Ruiz CR, Wafae N. Analysis on the incidence of the fibularis quartus muscle using magnetic resonance imaging. Anat Res Int. 2012;(2012):485149.
8. Wang XT, Rosenberg ZS, Mechlin MB, Schweitzer ME. Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. Radiographics. 2005;25(3):587-602.
9. Martinelli B, Bernobi S. Peroneus quartus muscle and ankle pain. Foot Ankle Surg. 2002;8(3):223-225.
Take-Home Points
- PQ is easily mistaken for a PB tear.
- PQ is best identified on MRI, but commonly missed.
- For symptomatic cases, excision is the best treatment.
- Consider PQ in patients with chronic ankle pain, swelling, and/or instability.
The peroneus quartus (PQ) is an accessory muscle arising from the leg’s lateral compartment, which typically contains the peroneus longus (PL) and the peroneus brevis (PB). The many cadaveric studies that have been conducted indicate a general population prevalence ranging from 6.6% to 23%.1 Radiographic studies, including magnetic resonance imaging (MRI) and ultrasonography, have shown a similar prevalence.2 Although the PQ is asymptomatic in most cases, it may compromise the space of the superior peroneal tunnel and cause problems, including ankle pain, PB tear, subluxation of peroneal tendons, tendinous calcification, painful hypertrophy of retrotrochlear eminence, and recurrent hematomas.1,3-5 Given its differing anatomy, the PQ variously has been referred to as peroneocalcaneus externum, peroneocuboideus, long peroneal tendon, and peroneoperoneolongus.1
Although the PQ’s origin and insertion differ between subjects, the most common origin is the muscle fibers of the PB, and the most common insertion is the retrotrochlear eminence of the calcaneum.3
We report a case of peroneal tendon pathology that was initially thought to be caused primarily by impingement of a large osteochondroma on the tendons, but was later thought to be caused in part by a PQ and a split PB tendon seen only at the time of the second operation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 16-year-old boy with an osteochondroma of the right distal fibula presented to clinic with the chief complaint of lateral right ankle pain. A “sharp” pain accompanied by audible “popping” occurred with ankle motion. Medical history was significant only for non-Hodgkin lymphoma treated with bone marrow transplantation and whole body radiation at a young age. Physical examination revealed a palpable exostosis of the distal right fibula and associated ankle swelling.
One year after surgery, the patient returned with recurring right ankle pain and audible popping during ankle movement. There was no appreciable peroneal tendon subluxation on physical examination. Repeat imaging of the ankle showed no recurrence of the osteochondroma (Figure 2).
Discussion
Absence of a PQ muscle in simian and prosimian species suggests that the PQ represents an evolutionary adaptation to evert the lateral foot and improve bipedal gait. Although the 3 peroneal (PL, PB, peroneus tertius [PT]) muscles evert the middle part of the lateral border of the foot, the PQ inserts on the retrotrochlear eminence, which everts the posterior part of the lateral border of the foot.1,6 The PQ has often been described as a variation of the PB. The PQ may also stabilize the ankle and reduce the energy required for walking. A similar functional adaptation has been proposed for the PT, which dorsiflexes at the ankle. Although presence of a PT also varies in the population, its occurrence does not correlate with presence of a PQ. In people with PQ muscles, there is an 83% to 95% incidence of also having PT muscles.7
PQ prevalence has ranged from 6.6% to 23% in cadaver studies2 and from 7% to 17% in radiologic studies.1 To better evaluate prevalence, Yammine2 performed a meta-analysis of data from 46 studies (cadaveric dissection, MRI, ultrasonography) and 3928 legs and found an overall incidence of 10.2% and a higher incidence in the Indian population than in other races. Another study found no correlation between PQ presence and sex.7
MRI is the best imaging modality for assessing for PQ but must be performed specifically for this anatomical variation. Axial images may show a fat pad separating the PQ muscle from the PB muscle.8 On imaging, a PQ muscle can be mistaken for a peroneal tendon tear. A feature that helps in distinguishing the 2 is location; the PQ typically is found posterior and medial to the PL and PB tendons, whereas PB tears are anterior to the retromalleolar groove.2 Presence of a PQ muscle may be missed on initial MRI, as occurred in our patient’s case. Zammit and Singh3 reviewed 80 leg MRIs and found 6 PQs. Only 1 of the 6 reports described the PQ as an “atypical appearance of peroneus brevis [that] appears to be made up of more than one tendon.”
Surgical excision is often adequate treatment for a symptomatic PQ. If the PQ muscle is small and symptomatic from pressure to the muscle mass, a short fasciotomy may be performed.9 More commonly, complete excision of the accessory muscle is required. Although the PQ muscle is usually asymptomatic, it should be considered in cases of chronic ankle pain, swelling, or instability; recurrent hematomas; and peroneal tendon subluxation or tears.5,7
Our patient’s diagnosis was initially overlooked because of an osteochondroma in the region of interest. It remains unclear whether his pain was caused by the PQ itself or, more likely, from the PB tear. It is thought that the accessory muscle adds bulk to the peroneal tunnel, predisposing to peroneal pathology, such as muscle tears and tendon subluxation. Regardless, advanced imaging performed before the index procedure, along with a general understanding of the PQ and its classic MRI findings, may have prevented the repeat operation in this case.
The PQ muscle is a rare but sometimes missed potential etiology of ankle pain and tendon subluxation. In our patient’s case, the most obvious abnormality, an osteochondroma, may have masked the true cause.
Take-Home Points
- PQ is easily mistaken for a PB tear.
- PQ is best identified on MRI, but commonly missed.
- For symptomatic cases, excision is the best treatment.
- Consider PQ in patients with chronic ankle pain, swelling, and/or instability.
The peroneus quartus (PQ) is an accessory muscle arising from the leg’s lateral compartment, which typically contains the peroneus longus (PL) and the peroneus brevis (PB). The many cadaveric studies that have been conducted indicate a general population prevalence ranging from 6.6% to 23%.1 Radiographic studies, including magnetic resonance imaging (MRI) and ultrasonography, have shown a similar prevalence.2 Although the PQ is asymptomatic in most cases, it may compromise the space of the superior peroneal tunnel and cause problems, including ankle pain, PB tear, subluxation of peroneal tendons, tendinous calcification, painful hypertrophy of retrotrochlear eminence, and recurrent hematomas.1,3-5 Given its differing anatomy, the PQ variously has been referred to as peroneocalcaneus externum, peroneocuboideus, long peroneal tendon, and peroneoperoneolongus.1
Although the PQ’s origin and insertion differ between subjects, the most common origin is the muscle fibers of the PB, and the most common insertion is the retrotrochlear eminence of the calcaneum.3
We report a case of peroneal tendon pathology that was initially thought to be caused primarily by impingement of a large osteochondroma on the tendons, but was later thought to be caused in part by a PQ and a split PB tendon seen only at the time of the second operation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 16-year-old boy with an osteochondroma of the right distal fibula presented to clinic with the chief complaint of lateral right ankle pain. A “sharp” pain accompanied by audible “popping” occurred with ankle motion. Medical history was significant only for non-Hodgkin lymphoma treated with bone marrow transplantation and whole body radiation at a young age. Physical examination revealed a palpable exostosis of the distal right fibula and associated ankle swelling.
One year after surgery, the patient returned with recurring right ankle pain and audible popping during ankle movement. There was no appreciable peroneal tendon subluxation on physical examination. Repeat imaging of the ankle showed no recurrence of the osteochondroma (Figure 2).
Discussion
Absence of a PQ muscle in simian and prosimian species suggests that the PQ represents an evolutionary adaptation to evert the lateral foot and improve bipedal gait. Although the 3 peroneal (PL, PB, peroneus tertius [PT]) muscles evert the middle part of the lateral border of the foot, the PQ inserts on the retrotrochlear eminence, which everts the posterior part of the lateral border of the foot.1,6 The PQ has often been described as a variation of the PB. The PQ may also stabilize the ankle and reduce the energy required for walking. A similar functional adaptation has been proposed for the PT, which dorsiflexes at the ankle. Although presence of a PT also varies in the population, its occurrence does not correlate with presence of a PQ. In people with PQ muscles, there is an 83% to 95% incidence of also having PT muscles.7
PQ prevalence has ranged from 6.6% to 23% in cadaver studies2 and from 7% to 17% in radiologic studies.1 To better evaluate prevalence, Yammine2 performed a meta-analysis of data from 46 studies (cadaveric dissection, MRI, ultrasonography) and 3928 legs and found an overall incidence of 10.2% and a higher incidence in the Indian population than in other races. Another study found no correlation between PQ presence and sex.7
MRI is the best imaging modality for assessing for PQ but must be performed specifically for this anatomical variation. Axial images may show a fat pad separating the PQ muscle from the PB muscle.8 On imaging, a PQ muscle can be mistaken for a peroneal tendon tear. A feature that helps in distinguishing the 2 is location; the PQ typically is found posterior and medial to the PL and PB tendons, whereas PB tears are anterior to the retromalleolar groove.2 Presence of a PQ muscle may be missed on initial MRI, as occurred in our patient’s case. Zammit and Singh3 reviewed 80 leg MRIs and found 6 PQs. Only 1 of the 6 reports described the PQ as an “atypical appearance of peroneus brevis [that] appears to be made up of more than one tendon.”
Surgical excision is often adequate treatment for a symptomatic PQ. If the PQ muscle is small and symptomatic from pressure to the muscle mass, a short fasciotomy may be performed.9 More commonly, complete excision of the accessory muscle is required. Although the PQ muscle is usually asymptomatic, it should be considered in cases of chronic ankle pain, swelling, or instability; recurrent hematomas; and peroneal tendon subluxation or tears.5,7
Our patient’s diagnosis was initially overlooked because of an osteochondroma in the region of interest. It remains unclear whether his pain was caused by the PQ itself or, more likely, from the PB tear. It is thought that the accessory muscle adds bulk to the peroneal tunnel, predisposing to peroneal pathology, such as muscle tears and tendon subluxation. Regardless, advanced imaging performed before the index procedure, along with a general understanding of the PQ and its classic MRI findings, may have prevented the repeat operation in this case.
The PQ muscle is a rare but sometimes missed potential etiology of ankle pain and tendon subluxation. In our patient’s case, the most obvious abnormality, an osteochondroma, may have masked the true cause.
1. Athavale SA, Gupta V, Kotgirwar S, Singh V. The peroneus quartus muscle: clinical correlation with evolutionary importance. Anat Sci Int. 2012;87(2):106-110.
2. Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat. 2015;37(6):617-627.
3. Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br. 2003;85(8):1134-1137.
4. Kulshreshtha R, Kadri S, Rajan DT. A case of unusual combination of injuries around the lateral malleolus. Foot. 2006;16(1):51-53.
5. Donley BG, Leyes M. Peroneus quartus muscle. A rare cause of chronic lateral ankle pain. Am J Sports Med. 2001;29(3):373-375.
6. Hecker P. Study on the peroneus of the tarsus. Anat Rec. 1923;26(1):79-82.
7. Rios Nascimento SR, Watanabe Costa R, Ruiz CR, Wafae N. Analysis on the incidence of the fibularis quartus muscle using magnetic resonance imaging. Anat Res Int. 2012;(2012):485149.
8. Wang XT, Rosenberg ZS, Mechlin MB, Schweitzer ME. Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. Radiographics. 2005;25(3):587-602.
9. Martinelli B, Bernobi S. Peroneus quartus muscle and ankle pain. Foot Ankle Surg. 2002;8(3):223-225.
1. Athavale SA, Gupta V, Kotgirwar S, Singh V. The peroneus quartus muscle: clinical correlation with evolutionary importance. Anat Sci Int. 2012;87(2):106-110.
2. Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat. 2015;37(6):617-627.
3. Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br. 2003;85(8):1134-1137.
4. Kulshreshtha R, Kadri S, Rajan DT. A case of unusual combination of injuries around the lateral malleolus. Foot. 2006;16(1):51-53.
5. Donley BG, Leyes M. Peroneus quartus muscle. A rare cause of chronic lateral ankle pain. Am J Sports Med. 2001;29(3):373-375.
6. Hecker P. Study on the peroneus of the tarsus. Anat Rec. 1923;26(1):79-82.
7. Rios Nascimento SR, Watanabe Costa R, Ruiz CR, Wafae N. Analysis on the incidence of the fibularis quartus muscle using magnetic resonance imaging. Anat Res Int. 2012;(2012):485149.
8. Wang XT, Rosenberg ZS, Mechlin MB, Schweitzer ME. Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. Radiographics. 2005;25(3):587-602.
9. Martinelli B, Bernobi S. Peroneus quartus muscle and ankle pain. Foot Ankle Surg. 2002;8(3):223-225.
Timing of Surgical Reduction and Stabilization of Talus Fracture-Dislocations
Take-Home Points
- There is a 41% rate of AVN or PTOA after operatively managed talus fracture.
- Surgical timing does not affect development of AVN or PTOA.
- Open fractures are associated with development of AVN and PTOA.
- Quality of reduction is likely more important than timing of reduction.
- Urgent surgical treatment is necessary for threatened soft tissue or neurovascular compromise.
Talus fractures are rare injuries that present a significant treatment dilemma.1-12 These fractures represent <1% of all fractures4 and are second only to calcaneus fractures in fractures of the hindfoot. Talus fractures with associated dislocations are even rarer and may provide treating surgeons with a significant surgical quandary.6,13-16
Talus fractures historically have been characterized by their anatomical location: head, neck, or body. Two systems are commonly used to classify talus fractures: Hawkins and AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association). The first, developed by Hawkins7 and modified by Canale and Kelly2 and Vallier and colleagues,1 identifies 4 basic fracture types with associated dislocations. The other system, published in 199617 and republished in 2007,18 uses the combined methods of AO and OTA to systematically describe talus fractures. Although these classification systems accurately describe talus fractures with associated dislocation, both have difficulty predicting clinical outcomes.1,19,20
Talus fractures commonly result in avascular necrosis (AVN) of the talus and posttraumatic osteoarthritis (PTOA) of the tibiotalar and subtalar joints.3,8,9,12,14-16 Hawkins7 initially described subchondral lucency as indicating revascularization of the talus after injury. AVN and PTOA rates traditionally have been thought to be related to a blood supply disruption, given the prognostic value of the Hawkins sign.1,7,12,21 New methods, including a dual-incision approach and expedited transfer to foot and ankle surgeons or orthopedic traumatologists, have improved reduction quality21-24 but not patient outcomes.3,5,8,9,12,14
Recently, time from injury to surgical intervention has been a topic of much discussion, and there have been studies on the specific effects of timing with respect to outcome.1,15,16 Vallier and colleagues,1 who wanted to identify injury characteristics predictive of osteonecrosis, found that delaying reduction and surgical fixation did not increase the risk of AVN. Another study found that urgent reduction of fracture-dislocation with delayed open reduction and internal fixation (ORIF) using a dual approach may improve clinical outcomes.21
In this vein, we conducted a study to evaluate the effect of time to surgical reduction of talus fractures and talus fracture-dislocations on the development of AVN and PTOA. We hypothesized that time to surgical reduction of talus fracture-dislocation as classified with the AO/OTA system would have no effect of the development of AVN/PTOA.
Methods
After this study received Institutional Review Board approval, we retrospectively reviewed the records on talus fractures surgically managed at a level I trauma center during the 10-year period 2003 to 2013. Of the 119 potential cases identified using Current Procedural Terminology code 28445 (ORIF of talus), 13 were excluded (12 for inaccurate coding or missing documentation, 1 for being a pediatric case), leaving 106 for analysis. Using the Hawkins and AO/OTA systems, 3 independent reviewers classified the injuries on plain radiographs.
Injury dates and times were obtained from the medical records. Operating room start times were also obtained. Surgical timing was defined as time from injury to operating room start. For cases without an injury time, time of presentation to emergency department was used.
Open fracture-dislocations were managed with intravenous antibiotics, urgent surgical irrigation, débridement, and immediate fixation or temporizing external fixation after reduction. All fractures were definitively managed with standard ORIF with an anteromedial, anterolateral, or dual approach and mini-fragment implants. After fixation, weight-bearing typically was restricted for 6 to 12 weeks.
Follow-up radiographs were evaluated. Presence or absence of Hawkins sign7 was noted on radiographs at 6 or 8 weeks, and all follow-up radiographs were evaluated for AVN as defined by increased radiographic density within the talar dome or collapse of the articular surface. All radiographs were evaluated for PTOA as defined by loss of joint space within the tibiotalar, subtalar, or talonavicular joint on follow-up radiographs.
Clinical outcomes were analyzed for development of AVN, PTOA, or secondary corrective surgery or arthrodesis. Continuous variables were evaluated with the t test, and the χ2 test was used to compare distributions of categorical variables. The Wilcoxon rank sum test was used to compare non-normally distributed variables. Significance was set at P < .05.
Results
Classification Analysis (Table 1)
Subject Analysis (Table 2)
The mechanisms of injury were motor vehicle accident (70/106; 66%), fall from height (25; 24%), misstep (4), sports related (2), object falling on ankle (2), and not reported (3).
Of the 106 patients, 45 (42%) had isolated talus injuries, 35 had concomitant ipsilateral lower extremity injuries, 25 had concomitant contralateral lower extremity injuries, and 1 had a concomitant upper extremity injury.
Smoking status was everyday (14 patients), past (10), never (34), and unreported (48). Five patients reported a history of alcohol abuse, and 4 patients reported illicit drug use. Two had a history of atrial fibrillation, 9 had hypertension, 3 had hyperlipidemia, 3 had renal disease, 3 had heart disease, 4 had diabetes, 3 had lung disease, and 1 had a history of lung cancer.
Overall Analysis of AVN/PTOA (Table 3)
Analysis of AVN/PTOA in 81-B3 Fracture-Dislocations (Table 4)
Analysis of AVN/PTOA in All Other Talus Fractures (Table 5)
Discussion
Our results showed that time from talus fracture-dislocation to surgical reduction had no effect on development of AVN/PTOA. The findings in this largest series to date agree with earlier findings1,8,15,16,24 and add to the volume of literature suggesting that time to surgical reduction of talus fractures and talus fracture-dislocations does not markedly affect outcome.
Talus fractures continue to present a significant treatment dilemma. Despite recent improvements in surgical techniques and overall management of these injuries, rates of AVN and PTOA have not significantly decreased.1,16,23 At most treating facilities, talus fracture-dislocations are considered surgical emergencies/urgencies, and every effort is made to reduce and surgically address these injuries as soon as possible.1,13
In this study, rates of AVN/PTOA were 41% (all talus fractures) and 50% (displaced talar neck fractures), and the difference was not significant (Table 3). These rates are higher but consistent with previously reported rates (range, 14%-49%).1,2,7-9,12,14,24 There was no difference in surgical timing for development of AVN/PTOA. We analyzed the cases of all patients who had talus fractures and developed AVN/PTOA (43/106). Within this group, there were no significant differences in surgical timing, age, sex, polytrauma, or BMI between patients who developed AVN/PTOA and those who did not. Compared with patients who did not develop AVN/PTOA, those who developed AVN/PTOA were significantly more likely to have open injuries. This finding, consistent with those in other reports9,12,13 (Table 3), indicates outcome is more likely related to injury severity and not necessarily injury class.
We retrospectively analyzed talus fractures and talus fracture-dislocations to determine if urgent surgical management affects outcomes. Current practice at our institution is to routinely reduce and surgically address these fractures urgently, often during the middle of the night, when orthopedic resources are reduced. Our study found a significant difference in surgical timing for patients with talus fracture-dislocations and patients with talus fractures without dislocations (Table 2). Given our findings, urgent surgical reduction and fixation are not indicated to preserve the talus blood supply and prevent AVN/PTOA, though we still recommend urgent surgical management in the setting of an open wound, skin necrosis, or soft-tissue/neurovascular compromise.
This study had several limitations, primarily related to its retrospective nature. Surgical timing was defined as time from injury, as noted in the medical record, to operating room start. In some instances, time of injury was not noted in the medical record, and time of presentation to emergency room was used instead. Thus, surgical timing for these patients may have been longer than identified. In addition, given the rare injury pattern and the retrospective design, this study was susceptible to type II error and may have been underpowered to detect whether time to surgical reduction predicted complications. Also, the study did not address functional outcome as measured by validated outcome scores. Outcome measures were obtained in many but not all cases, making functional outcome measurement difficult. Similarly, the quality of the anatomical reductions was not assessed, potentially affecting complication rates. Postoperative reduction assessment, possibly performed with computed tomography, is an avenue of further study.
Strengths of this study include its large sample size (this was one of the largest studies of talus fractures), long follow-up (mean, 150 weeks), and novel use of AO/OTA classification.
We postulate that development of AVN/PTOA is not necessarily related to the urgency or timing of surgical reduction and fixation and is more likely related to injury severity. This idea is supported by the finding that development of AVN/PTOA was significantly correlated to open injuries in all talus fractures, including talus fracture-dislocations and isolated talus fractures.
Conclusion
Talus fracture-dislocations are devastating injuries with high rates of complications. In this study, open talus fractures, and fractures with associated tibiotalar or subtalar dislocations, had higher complication rates. Given the evidence presented, we recommend basing surgical timing on injury severity, not necessarily for AVN/PTOA prevention. Specifically, in the absence of an open wound, skin necrosis, or soft-tissue/neurovascular compromise, talus fracture-dislocations can be surgically reduced and stabilized when optimal resources are available.
1. Vallier HA, Reichard SG, Boyd AJ, Moore TA. A new look at the Hawkins classification for talar neck fractures: which features of injury and treatment are predictive of osteonecrosis? J Bone Joint Surg Am. 2014;96(3):192-197.
2. Canale ST, Kelly FB Jr. Fractures of the neck of the talus. Long-term evaluation of seventy-one cases. J Bone Joint Surg Am. 1978;60(2):143-156.
3. Ebraheim NA, Patil V, Owens C, Kandimalla Y. Clinical outcome of fractures of the talar body. Int Orthop. 2008;32(6):773-777.
4. Fortin PT, Balazsy JE. Talus fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(2):114-127.
5. Fournier A, Barba N, Steiger V, et al. Total talar fracture—long-term results of internal fixation of talar fractures. A multicentric study of 114 cases. Orthop Traumatol Surg Res. 2012;98(4 suppl):S48-S55.
6. Grob D, Simpson LA, Weber BG, Bray T. Operative treatment of displaced talus fractures. Clin Orthop Relat Res. 1985;(199):88-96.
7. Hawkins LG. Fractures of the neck of the talus. J Bone Joint Surg Am. 1970;52(5):991-1002.
8. Lindvall E, Haidukewych G, DiPasquale T, Herscovici D Jr, Sanders R. Open reduction and stable fixation of isolated, displaced talar neck and body fractures. J Bone Joint Surg Am. 2004;86(10):2229-2234.
9. Ohl X, Harisboure A, Hemery X, Dehoux E. Long-term follow-up after surgical treatment of talar fractures: twenty cases with an average follow-up of 7.5 years. Int Orthop. 2011;35(1):93-99.
10. Rammelt S, Zwipp H. Talar neck and body fractures. Injury. 2009;40(2):120-135.
11. Schulze W, Richter J, Russe O, Ingelfinger P, Muhr G. Surgical treatment of talus fractures: a retrospective study of 80 cases followed for 1-15 years. Acta Orthop Scand. 2002;73(3):344-351.
12. Vallier HA, Nork SE, Barei DP, Benirschke SK, Sangeorzan BJ. Talar neck fractures: results and outcomes. J Bone Joint Surg Am. 2004;86(8):1616-1624.
13. Patel R, Van Bergeyk A, Pinney S. Are displaced talar neck fractures surgical emergencies? A survey of orthopaedic trauma experts. Foot Ankle Int. 2005;26(5):378-381.
14. Sanders DW, Busam M, Hattwick E, Edwards JR, McAndrew MP, Johnson KD. Functional outcomes following displaced talar neck fractures. J Orthop Trauma. 2004;18(5):265-270.
15. Elgafy H, Ebraheim NA, Tile M, Stephen D, Kase J. Fractures of the talus: experience of two level 1 trauma centers. Foot Ankle Int. 2000;21(12):1023-1029.
16 Frawley PA, Hart JA, Young DA. Treatment outcome of major fractures of the talus. Foot Ankle Int. 1995;16(6):339-345.
17. Fracture and dislocation compendium. Orthopaedic Trauma Association committee for coding and classification. J Orthop Trauma. 1996;10(suppl 1):v-ix, 1-154.
18. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
19. Williams T, Barba N, Noailles T, et al. Total talar fracture—inter- and intra-observer reproducibility of two classification systems (Hawkins and AO) for central talar fractures. Orthop Traumatol Surg Res. 2012;98(4 suppl):S56-S65.
20. Zwipp H, Baumgart F, Cronier P, et al. Integral classification of injuries (ICI) to the bones, joints, and ligaments—application to injuries of the foot. Injury. 2004;35(suppl 2):SB3-SB9.
21. Xue Y, Zhang H, Pei F, et al. Treatment of displaced talar neck fractures using delayed procedures of plate fixation through dual approaches. Int Orthop. 2014;38(1):149-154.
22. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2003;85(9):1716-1724.
23. Fleuriau Chateau PB, Brokaw DS, Jelen BA, Scheid DK, Weber TG. Plate fixation of talar neck fractures: preliminary review of a new technique in twenty-three patients. J Orthop Trauma. 2002;16(4):213-219.
24. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2004;86(suppl 1, pt 2):180-192.
Take-Home Points
- There is a 41% rate of AVN or PTOA after operatively managed talus fracture.
- Surgical timing does not affect development of AVN or PTOA.
- Open fractures are associated with development of AVN and PTOA.
- Quality of reduction is likely more important than timing of reduction.
- Urgent surgical treatment is necessary for threatened soft tissue or neurovascular compromise.
Talus fractures are rare injuries that present a significant treatment dilemma.1-12 These fractures represent <1% of all fractures4 and are second only to calcaneus fractures in fractures of the hindfoot. Talus fractures with associated dislocations are even rarer and may provide treating surgeons with a significant surgical quandary.6,13-16
Talus fractures historically have been characterized by their anatomical location: head, neck, or body. Two systems are commonly used to classify talus fractures: Hawkins and AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association). The first, developed by Hawkins7 and modified by Canale and Kelly2 and Vallier and colleagues,1 identifies 4 basic fracture types with associated dislocations. The other system, published in 199617 and republished in 2007,18 uses the combined methods of AO and OTA to systematically describe talus fractures. Although these classification systems accurately describe talus fractures with associated dislocation, both have difficulty predicting clinical outcomes.1,19,20
Talus fractures commonly result in avascular necrosis (AVN) of the talus and posttraumatic osteoarthritis (PTOA) of the tibiotalar and subtalar joints.3,8,9,12,14-16 Hawkins7 initially described subchondral lucency as indicating revascularization of the talus after injury. AVN and PTOA rates traditionally have been thought to be related to a blood supply disruption, given the prognostic value of the Hawkins sign.1,7,12,21 New methods, including a dual-incision approach and expedited transfer to foot and ankle surgeons or orthopedic traumatologists, have improved reduction quality21-24 but not patient outcomes.3,5,8,9,12,14
Recently, time from injury to surgical intervention has been a topic of much discussion, and there have been studies on the specific effects of timing with respect to outcome.1,15,16 Vallier and colleagues,1 who wanted to identify injury characteristics predictive of osteonecrosis, found that delaying reduction and surgical fixation did not increase the risk of AVN. Another study found that urgent reduction of fracture-dislocation with delayed open reduction and internal fixation (ORIF) using a dual approach may improve clinical outcomes.21
In this vein, we conducted a study to evaluate the effect of time to surgical reduction of talus fractures and talus fracture-dislocations on the development of AVN and PTOA. We hypothesized that time to surgical reduction of talus fracture-dislocation as classified with the AO/OTA system would have no effect of the development of AVN/PTOA.
Methods
After this study received Institutional Review Board approval, we retrospectively reviewed the records on talus fractures surgically managed at a level I trauma center during the 10-year period 2003 to 2013. Of the 119 potential cases identified using Current Procedural Terminology code 28445 (ORIF of talus), 13 were excluded (12 for inaccurate coding or missing documentation, 1 for being a pediatric case), leaving 106 for analysis. Using the Hawkins and AO/OTA systems, 3 independent reviewers classified the injuries on plain radiographs.
Injury dates and times were obtained from the medical records. Operating room start times were also obtained. Surgical timing was defined as time from injury to operating room start. For cases without an injury time, time of presentation to emergency department was used.
Open fracture-dislocations were managed with intravenous antibiotics, urgent surgical irrigation, débridement, and immediate fixation or temporizing external fixation after reduction. All fractures were definitively managed with standard ORIF with an anteromedial, anterolateral, or dual approach and mini-fragment implants. After fixation, weight-bearing typically was restricted for 6 to 12 weeks.
Follow-up radiographs were evaluated. Presence or absence of Hawkins sign7 was noted on radiographs at 6 or 8 weeks, and all follow-up radiographs were evaluated for AVN as defined by increased radiographic density within the talar dome or collapse of the articular surface. All radiographs were evaluated for PTOA as defined by loss of joint space within the tibiotalar, subtalar, or talonavicular joint on follow-up radiographs.
Clinical outcomes were analyzed for development of AVN, PTOA, or secondary corrective surgery or arthrodesis. Continuous variables were evaluated with the t test, and the χ2 test was used to compare distributions of categorical variables. The Wilcoxon rank sum test was used to compare non-normally distributed variables. Significance was set at P < .05.
Results
Classification Analysis (Table 1)
Subject Analysis (Table 2)
The mechanisms of injury were motor vehicle accident (70/106; 66%), fall from height (25; 24%), misstep (4), sports related (2), object falling on ankle (2), and not reported (3).
Of the 106 patients, 45 (42%) had isolated talus injuries, 35 had concomitant ipsilateral lower extremity injuries, 25 had concomitant contralateral lower extremity injuries, and 1 had a concomitant upper extremity injury.
Smoking status was everyday (14 patients), past (10), never (34), and unreported (48). Five patients reported a history of alcohol abuse, and 4 patients reported illicit drug use. Two had a history of atrial fibrillation, 9 had hypertension, 3 had hyperlipidemia, 3 had renal disease, 3 had heart disease, 4 had diabetes, 3 had lung disease, and 1 had a history of lung cancer.
Overall Analysis of AVN/PTOA (Table 3)
Analysis of AVN/PTOA in 81-B3 Fracture-Dislocations (Table 4)
Analysis of AVN/PTOA in All Other Talus Fractures (Table 5)
Discussion
Our results showed that time from talus fracture-dislocation to surgical reduction had no effect on development of AVN/PTOA. The findings in this largest series to date agree with earlier findings1,8,15,16,24 and add to the volume of literature suggesting that time to surgical reduction of talus fractures and talus fracture-dislocations does not markedly affect outcome.
Talus fractures continue to present a significant treatment dilemma. Despite recent improvements in surgical techniques and overall management of these injuries, rates of AVN and PTOA have not significantly decreased.1,16,23 At most treating facilities, talus fracture-dislocations are considered surgical emergencies/urgencies, and every effort is made to reduce and surgically address these injuries as soon as possible.1,13
In this study, rates of AVN/PTOA were 41% (all talus fractures) and 50% (displaced talar neck fractures), and the difference was not significant (Table 3). These rates are higher but consistent with previously reported rates (range, 14%-49%).1,2,7-9,12,14,24 There was no difference in surgical timing for development of AVN/PTOA. We analyzed the cases of all patients who had talus fractures and developed AVN/PTOA (43/106). Within this group, there were no significant differences in surgical timing, age, sex, polytrauma, or BMI between patients who developed AVN/PTOA and those who did not. Compared with patients who did not develop AVN/PTOA, those who developed AVN/PTOA were significantly more likely to have open injuries. This finding, consistent with those in other reports9,12,13 (Table 3), indicates outcome is more likely related to injury severity and not necessarily injury class.
We retrospectively analyzed talus fractures and talus fracture-dislocations to determine if urgent surgical management affects outcomes. Current practice at our institution is to routinely reduce and surgically address these fractures urgently, often during the middle of the night, when orthopedic resources are reduced. Our study found a significant difference in surgical timing for patients with talus fracture-dislocations and patients with talus fractures without dislocations (Table 2). Given our findings, urgent surgical reduction and fixation are not indicated to preserve the talus blood supply and prevent AVN/PTOA, though we still recommend urgent surgical management in the setting of an open wound, skin necrosis, or soft-tissue/neurovascular compromise.
This study had several limitations, primarily related to its retrospective nature. Surgical timing was defined as time from injury, as noted in the medical record, to operating room start. In some instances, time of injury was not noted in the medical record, and time of presentation to emergency room was used instead. Thus, surgical timing for these patients may have been longer than identified. In addition, given the rare injury pattern and the retrospective design, this study was susceptible to type II error and may have been underpowered to detect whether time to surgical reduction predicted complications. Also, the study did not address functional outcome as measured by validated outcome scores. Outcome measures were obtained in many but not all cases, making functional outcome measurement difficult. Similarly, the quality of the anatomical reductions was not assessed, potentially affecting complication rates. Postoperative reduction assessment, possibly performed with computed tomography, is an avenue of further study.
Strengths of this study include its large sample size (this was one of the largest studies of talus fractures), long follow-up (mean, 150 weeks), and novel use of AO/OTA classification.
We postulate that development of AVN/PTOA is not necessarily related to the urgency or timing of surgical reduction and fixation and is more likely related to injury severity. This idea is supported by the finding that development of AVN/PTOA was significantly correlated to open injuries in all talus fractures, including talus fracture-dislocations and isolated talus fractures.
Conclusion
Talus fracture-dislocations are devastating injuries with high rates of complications. In this study, open talus fractures, and fractures with associated tibiotalar or subtalar dislocations, had higher complication rates. Given the evidence presented, we recommend basing surgical timing on injury severity, not necessarily for AVN/PTOA prevention. Specifically, in the absence of an open wound, skin necrosis, or soft-tissue/neurovascular compromise, talus fracture-dislocations can be surgically reduced and stabilized when optimal resources are available.
Take-Home Points
- There is a 41% rate of AVN or PTOA after operatively managed talus fracture.
- Surgical timing does not affect development of AVN or PTOA.
- Open fractures are associated with development of AVN and PTOA.
- Quality of reduction is likely more important than timing of reduction.
- Urgent surgical treatment is necessary for threatened soft tissue or neurovascular compromise.
Talus fractures are rare injuries that present a significant treatment dilemma.1-12 These fractures represent <1% of all fractures4 and are second only to calcaneus fractures in fractures of the hindfoot. Talus fractures with associated dislocations are even rarer and may provide treating surgeons with a significant surgical quandary.6,13-16
Talus fractures historically have been characterized by their anatomical location: head, neck, or body. Two systems are commonly used to classify talus fractures: Hawkins and AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association). The first, developed by Hawkins7 and modified by Canale and Kelly2 and Vallier and colleagues,1 identifies 4 basic fracture types with associated dislocations. The other system, published in 199617 and republished in 2007,18 uses the combined methods of AO and OTA to systematically describe talus fractures. Although these classification systems accurately describe talus fractures with associated dislocation, both have difficulty predicting clinical outcomes.1,19,20
Talus fractures commonly result in avascular necrosis (AVN) of the talus and posttraumatic osteoarthritis (PTOA) of the tibiotalar and subtalar joints.3,8,9,12,14-16 Hawkins7 initially described subchondral lucency as indicating revascularization of the talus after injury. AVN and PTOA rates traditionally have been thought to be related to a blood supply disruption, given the prognostic value of the Hawkins sign.1,7,12,21 New methods, including a dual-incision approach and expedited transfer to foot and ankle surgeons or orthopedic traumatologists, have improved reduction quality21-24 but not patient outcomes.3,5,8,9,12,14
Recently, time from injury to surgical intervention has been a topic of much discussion, and there have been studies on the specific effects of timing with respect to outcome.1,15,16 Vallier and colleagues,1 who wanted to identify injury characteristics predictive of osteonecrosis, found that delaying reduction and surgical fixation did not increase the risk of AVN. Another study found that urgent reduction of fracture-dislocation with delayed open reduction and internal fixation (ORIF) using a dual approach may improve clinical outcomes.21
In this vein, we conducted a study to evaluate the effect of time to surgical reduction of talus fractures and talus fracture-dislocations on the development of AVN and PTOA. We hypothesized that time to surgical reduction of talus fracture-dislocation as classified with the AO/OTA system would have no effect of the development of AVN/PTOA.
Methods
After this study received Institutional Review Board approval, we retrospectively reviewed the records on talus fractures surgically managed at a level I trauma center during the 10-year period 2003 to 2013. Of the 119 potential cases identified using Current Procedural Terminology code 28445 (ORIF of talus), 13 were excluded (12 for inaccurate coding or missing documentation, 1 for being a pediatric case), leaving 106 for analysis. Using the Hawkins and AO/OTA systems, 3 independent reviewers classified the injuries on plain radiographs.
Injury dates and times were obtained from the medical records. Operating room start times were also obtained. Surgical timing was defined as time from injury to operating room start. For cases without an injury time, time of presentation to emergency department was used.
Open fracture-dislocations were managed with intravenous antibiotics, urgent surgical irrigation, débridement, and immediate fixation or temporizing external fixation after reduction. All fractures were definitively managed with standard ORIF with an anteromedial, anterolateral, or dual approach and mini-fragment implants. After fixation, weight-bearing typically was restricted for 6 to 12 weeks.
Follow-up radiographs were evaluated. Presence or absence of Hawkins sign7 was noted on radiographs at 6 or 8 weeks, and all follow-up radiographs were evaluated for AVN as defined by increased radiographic density within the talar dome or collapse of the articular surface. All radiographs were evaluated for PTOA as defined by loss of joint space within the tibiotalar, subtalar, or talonavicular joint on follow-up radiographs.
Clinical outcomes were analyzed for development of AVN, PTOA, or secondary corrective surgery or arthrodesis. Continuous variables were evaluated with the t test, and the χ2 test was used to compare distributions of categorical variables. The Wilcoxon rank sum test was used to compare non-normally distributed variables. Significance was set at P < .05.
Results
Classification Analysis (Table 1)
Subject Analysis (Table 2)
The mechanisms of injury were motor vehicle accident (70/106; 66%), fall from height (25; 24%), misstep (4), sports related (2), object falling on ankle (2), and not reported (3).
Of the 106 patients, 45 (42%) had isolated talus injuries, 35 had concomitant ipsilateral lower extremity injuries, 25 had concomitant contralateral lower extremity injuries, and 1 had a concomitant upper extremity injury.
Smoking status was everyday (14 patients), past (10), never (34), and unreported (48). Five patients reported a history of alcohol abuse, and 4 patients reported illicit drug use. Two had a history of atrial fibrillation, 9 had hypertension, 3 had hyperlipidemia, 3 had renal disease, 3 had heart disease, 4 had diabetes, 3 had lung disease, and 1 had a history of lung cancer.
Overall Analysis of AVN/PTOA (Table 3)
Analysis of AVN/PTOA in 81-B3 Fracture-Dislocations (Table 4)
Analysis of AVN/PTOA in All Other Talus Fractures (Table 5)
Discussion
Our results showed that time from talus fracture-dislocation to surgical reduction had no effect on development of AVN/PTOA. The findings in this largest series to date agree with earlier findings1,8,15,16,24 and add to the volume of literature suggesting that time to surgical reduction of talus fractures and talus fracture-dislocations does not markedly affect outcome.
Talus fractures continue to present a significant treatment dilemma. Despite recent improvements in surgical techniques and overall management of these injuries, rates of AVN and PTOA have not significantly decreased.1,16,23 At most treating facilities, talus fracture-dislocations are considered surgical emergencies/urgencies, and every effort is made to reduce and surgically address these injuries as soon as possible.1,13
In this study, rates of AVN/PTOA were 41% (all talus fractures) and 50% (displaced talar neck fractures), and the difference was not significant (Table 3). These rates are higher but consistent with previously reported rates (range, 14%-49%).1,2,7-9,12,14,24 There was no difference in surgical timing for development of AVN/PTOA. We analyzed the cases of all patients who had talus fractures and developed AVN/PTOA (43/106). Within this group, there were no significant differences in surgical timing, age, sex, polytrauma, or BMI between patients who developed AVN/PTOA and those who did not. Compared with patients who did not develop AVN/PTOA, those who developed AVN/PTOA were significantly more likely to have open injuries. This finding, consistent with those in other reports9,12,13 (Table 3), indicates outcome is more likely related to injury severity and not necessarily injury class.
We retrospectively analyzed talus fractures and talus fracture-dislocations to determine if urgent surgical management affects outcomes. Current practice at our institution is to routinely reduce and surgically address these fractures urgently, often during the middle of the night, when orthopedic resources are reduced. Our study found a significant difference in surgical timing for patients with talus fracture-dislocations and patients with talus fractures without dislocations (Table 2). Given our findings, urgent surgical reduction and fixation are not indicated to preserve the talus blood supply and prevent AVN/PTOA, though we still recommend urgent surgical management in the setting of an open wound, skin necrosis, or soft-tissue/neurovascular compromise.
This study had several limitations, primarily related to its retrospective nature. Surgical timing was defined as time from injury, as noted in the medical record, to operating room start. In some instances, time of injury was not noted in the medical record, and time of presentation to emergency room was used instead. Thus, surgical timing for these patients may have been longer than identified. In addition, given the rare injury pattern and the retrospective design, this study was susceptible to type II error and may have been underpowered to detect whether time to surgical reduction predicted complications. Also, the study did not address functional outcome as measured by validated outcome scores. Outcome measures were obtained in many but not all cases, making functional outcome measurement difficult. Similarly, the quality of the anatomical reductions was not assessed, potentially affecting complication rates. Postoperative reduction assessment, possibly performed with computed tomography, is an avenue of further study.
Strengths of this study include its large sample size (this was one of the largest studies of talus fractures), long follow-up (mean, 150 weeks), and novel use of AO/OTA classification.
We postulate that development of AVN/PTOA is not necessarily related to the urgency or timing of surgical reduction and fixation and is more likely related to injury severity. This idea is supported by the finding that development of AVN/PTOA was significantly correlated to open injuries in all talus fractures, including talus fracture-dislocations and isolated talus fractures.
Conclusion
Talus fracture-dislocations are devastating injuries with high rates of complications. In this study, open talus fractures, and fractures with associated tibiotalar or subtalar dislocations, had higher complication rates. Given the evidence presented, we recommend basing surgical timing on injury severity, not necessarily for AVN/PTOA prevention. Specifically, in the absence of an open wound, skin necrosis, or soft-tissue/neurovascular compromise, talus fracture-dislocations can be surgically reduced and stabilized when optimal resources are available.
1. Vallier HA, Reichard SG, Boyd AJ, Moore TA. A new look at the Hawkins classification for talar neck fractures: which features of injury and treatment are predictive of osteonecrosis? J Bone Joint Surg Am. 2014;96(3):192-197.
2. Canale ST, Kelly FB Jr. Fractures of the neck of the talus. Long-term evaluation of seventy-one cases. J Bone Joint Surg Am. 1978;60(2):143-156.
3. Ebraheim NA, Patil V, Owens C, Kandimalla Y. Clinical outcome of fractures of the talar body. Int Orthop. 2008;32(6):773-777.
4. Fortin PT, Balazsy JE. Talus fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(2):114-127.
5. Fournier A, Barba N, Steiger V, et al. Total talar fracture—long-term results of internal fixation of talar fractures. A multicentric study of 114 cases. Orthop Traumatol Surg Res. 2012;98(4 suppl):S48-S55.
6. Grob D, Simpson LA, Weber BG, Bray T. Operative treatment of displaced talus fractures. Clin Orthop Relat Res. 1985;(199):88-96.
7. Hawkins LG. Fractures of the neck of the talus. J Bone Joint Surg Am. 1970;52(5):991-1002.
8. Lindvall E, Haidukewych G, DiPasquale T, Herscovici D Jr, Sanders R. Open reduction and stable fixation of isolated, displaced talar neck and body fractures. J Bone Joint Surg Am. 2004;86(10):2229-2234.
9. Ohl X, Harisboure A, Hemery X, Dehoux E. Long-term follow-up after surgical treatment of talar fractures: twenty cases with an average follow-up of 7.5 years. Int Orthop. 2011;35(1):93-99.
10. Rammelt S, Zwipp H. Talar neck and body fractures. Injury. 2009;40(2):120-135.
11. Schulze W, Richter J, Russe O, Ingelfinger P, Muhr G. Surgical treatment of talus fractures: a retrospective study of 80 cases followed for 1-15 years. Acta Orthop Scand. 2002;73(3):344-351.
12. Vallier HA, Nork SE, Barei DP, Benirschke SK, Sangeorzan BJ. Talar neck fractures: results and outcomes. J Bone Joint Surg Am. 2004;86(8):1616-1624.
13. Patel R, Van Bergeyk A, Pinney S. Are displaced talar neck fractures surgical emergencies? A survey of orthopaedic trauma experts. Foot Ankle Int. 2005;26(5):378-381.
14. Sanders DW, Busam M, Hattwick E, Edwards JR, McAndrew MP, Johnson KD. Functional outcomes following displaced talar neck fractures. J Orthop Trauma. 2004;18(5):265-270.
15. Elgafy H, Ebraheim NA, Tile M, Stephen D, Kase J. Fractures of the talus: experience of two level 1 trauma centers. Foot Ankle Int. 2000;21(12):1023-1029.
16 Frawley PA, Hart JA, Young DA. Treatment outcome of major fractures of the talus. Foot Ankle Int. 1995;16(6):339-345.
17. Fracture and dislocation compendium. Orthopaedic Trauma Association committee for coding and classification. J Orthop Trauma. 1996;10(suppl 1):v-ix, 1-154.
18. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
19. Williams T, Barba N, Noailles T, et al. Total talar fracture—inter- and intra-observer reproducibility of two classification systems (Hawkins and AO) for central talar fractures. Orthop Traumatol Surg Res. 2012;98(4 suppl):S56-S65.
20. Zwipp H, Baumgart F, Cronier P, et al. Integral classification of injuries (ICI) to the bones, joints, and ligaments—application to injuries of the foot. Injury. 2004;35(suppl 2):SB3-SB9.
21. Xue Y, Zhang H, Pei F, et al. Treatment of displaced talar neck fractures using delayed procedures of plate fixation through dual approaches. Int Orthop. 2014;38(1):149-154.
22. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2003;85(9):1716-1724.
23. Fleuriau Chateau PB, Brokaw DS, Jelen BA, Scheid DK, Weber TG. Plate fixation of talar neck fractures: preliminary review of a new technique in twenty-three patients. J Orthop Trauma. 2002;16(4):213-219.
24. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2004;86(suppl 1, pt 2):180-192.
1. Vallier HA, Reichard SG, Boyd AJ, Moore TA. A new look at the Hawkins classification for talar neck fractures: which features of injury and treatment are predictive of osteonecrosis? J Bone Joint Surg Am. 2014;96(3):192-197.
2. Canale ST, Kelly FB Jr. Fractures of the neck of the talus. Long-term evaluation of seventy-one cases. J Bone Joint Surg Am. 1978;60(2):143-156.
3. Ebraheim NA, Patil V, Owens C, Kandimalla Y. Clinical outcome of fractures of the talar body. Int Orthop. 2008;32(6):773-777.
4. Fortin PT, Balazsy JE. Talus fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(2):114-127.
5. Fournier A, Barba N, Steiger V, et al. Total talar fracture—long-term results of internal fixation of talar fractures. A multicentric study of 114 cases. Orthop Traumatol Surg Res. 2012;98(4 suppl):S48-S55.
6. Grob D, Simpson LA, Weber BG, Bray T. Operative treatment of displaced talus fractures. Clin Orthop Relat Res. 1985;(199):88-96.
7. Hawkins LG. Fractures of the neck of the talus. J Bone Joint Surg Am. 1970;52(5):991-1002.
8. Lindvall E, Haidukewych G, DiPasquale T, Herscovici D Jr, Sanders R. Open reduction and stable fixation of isolated, displaced talar neck and body fractures. J Bone Joint Surg Am. 2004;86(10):2229-2234.
9. Ohl X, Harisboure A, Hemery X, Dehoux E. Long-term follow-up after surgical treatment of talar fractures: twenty cases with an average follow-up of 7.5 years. Int Orthop. 2011;35(1):93-99.
10. Rammelt S, Zwipp H. Talar neck and body fractures. Injury. 2009;40(2):120-135.
11. Schulze W, Richter J, Russe O, Ingelfinger P, Muhr G. Surgical treatment of talus fractures: a retrospective study of 80 cases followed for 1-15 years. Acta Orthop Scand. 2002;73(3):344-351.
12. Vallier HA, Nork SE, Barei DP, Benirschke SK, Sangeorzan BJ. Talar neck fractures: results and outcomes. J Bone Joint Surg Am. 2004;86(8):1616-1624.
13. Patel R, Van Bergeyk A, Pinney S. Are displaced talar neck fractures surgical emergencies? A survey of orthopaedic trauma experts. Foot Ankle Int. 2005;26(5):378-381.
14. Sanders DW, Busam M, Hattwick E, Edwards JR, McAndrew MP, Johnson KD. Functional outcomes following displaced talar neck fractures. J Orthop Trauma. 2004;18(5):265-270.
15. Elgafy H, Ebraheim NA, Tile M, Stephen D, Kase J. Fractures of the talus: experience of two level 1 trauma centers. Foot Ankle Int. 2000;21(12):1023-1029.
16 Frawley PA, Hart JA, Young DA. Treatment outcome of major fractures of the talus. Foot Ankle Int. 1995;16(6):339-345.
17. Fracture and dislocation compendium. Orthopaedic Trauma Association committee for coding and classification. J Orthop Trauma. 1996;10(suppl 1):v-ix, 1-154.
18. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
19. Williams T, Barba N, Noailles T, et al. Total talar fracture—inter- and intra-observer reproducibility of two classification systems (Hawkins and AO) for central talar fractures. Orthop Traumatol Surg Res. 2012;98(4 suppl):S56-S65.
20. Zwipp H, Baumgart F, Cronier P, et al. Integral classification of injuries (ICI) to the bones, joints, and ligaments—application to injuries of the foot. Injury. 2004;35(suppl 2):SB3-SB9.
21. Xue Y, Zhang H, Pei F, et al. Treatment of displaced talar neck fractures using delayed procedures of plate fixation through dual approaches. Int Orthop. 2014;38(1):149-154.
22. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2003;85(9):1716-1724.
23. Fleuriau Chateau PB, Brokaw DS, Jelen BA, Scheid DK, Weber TG. Plate fixation of talar neck fractures: preliminary review of a new technique in twenty-three patients. J Orthop Trauma. 2002;16(4):213-219.
24. Vallier HA, Nork SE, Benirschke SK, Sangeorzan BJ. Surgical treatment of talar body fractures. J Bone Joint Surg Am. 2004;86(suppl 1, pt 2):180-192.
For women with RA, small-joint surgery rate nearly twice that of men
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
AT ACR 2017
Key clinical point: Women with RA had a higher rate of small-joint surgery, compared with men.
Major finding: The cumulative incidence of small-joint surgery was significantly higher among women, compared with men (14.4% vs. 7.6%, respectively), but there were no differences between sexes in the rates of large-joint surgery.
Study details: A retrospective, population-based study of 1,077 patients with RA.
Disclosures: The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
Open Navicular Dislocation With Midfoot Dissociation in a 45-Year-Old Man
Take-Home Points
- Stability of the foot is dependent on both the medial and lateral longitudinal columns; injuries to a single column alone are extremely rare.
- Midfoot fractures that are recognized and treated early have generally favorable outcomes compared to those identified in a delayed fashion.
- The most frequent complication of navicular dislocation is AVN, which is said to occur in as many as 25% of cases.
- Many specialists agree that navicular dislocations are best treated with open reduction.
- Ultimately, the goals of surgical intervention are to minimize pain and to establish stability of the plantigrade foot.
Traumatic dislocation of the tarsal navicular (especially without a navicular body fracture) is uncommon.1 The regional anatomy and ligamentous architecture confer stability to the midfoot.2-6 Navicular dislocation is part of a complex disruption involving structures in the adjacent column.6
Navicular dislocation has been associated with several bony and soft-tissue injury patterns, including comminuted intra-articular fracture of the calcaneus and associated calcaneocuboid joint subluxation; fracture and subluxation of the calcaneocuboid joint; fracture-dislocation of the calcaneocuboid joint with fractures of the third and fourth metatarsals; and a combination of fractures of the intermediate cuneiform, the second through fourth metatarsals, and the cuboid.4–11 In this article, we report a case of open complete navicular dislocation with talar head fracture and associated subtalar and calcaneocuboid subluxations in a 45-year-old man. The injury was managed with open reduction and stabilization with Kirschner wires (K-wires), which later required naviculocuneiform and intercuneiform fusion for posttraumatic avascular necrosis (AVN). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 45-year-old man sustained blunt trauma to the right foot in a high-speed head-on collision. He was hemodynamically stable with isolated complaints of pain in the foot. Physical examination revealed a grossly open 10-cm wound extending from the heel pad medially to the dorsal surface of the navicular. The navicular was clearly visible through the wound.
Plain radiographs of the foot showed complete medial dislocation of the navicular with complete disruption of all 3 naviculocuneiform joints (Figures 1A-1C).
On day of presentation, the patient was taken to the operating room for irrigation, débridement, reduction of the joints, and primary closure of the right foot wound. Minimal contamination was noted. Attempted gentle reduction maneuvers included distraction, adduction, and pronation of the forefoot with concomitant lateral pressure on the navicular.
An especially prominent medial navicular was noted on postreduction films. Initially, this suggested inadequate reduction of the naviculocuneiform joints, but, on close radiographic examination of each naviculocuneiform joint and imaging of the contralateral foot, we determined that the prominence represented a type III accessory navicular, also known as a cornuate navicular. Contralateral imaging showed an identical and asymptomatic medial prominence.
After surgery, the patient was made non-weight-bearing in a splint, received intravenous antibiotics for 48 hours, and was discharged shortly thereafter. Radiographs at 3 and 6 weeks after injury showed maintenance of the reduction. K-wires were removed at 6 weeks. The patient was advanced to partial weight-bearing at 6 weeks and to full weight-bearing at 3 months.
Over succeeding months, the patient developed pain accompanied by significant midfoot deformity and was found to have navicular collapse consistent with AVN and posttraumatic arthritis (Figures 4A, 4B).
Twenty-four months after fusion, the patient was fully ambulatory with no significant discomfort or disability.
Discussion
The naviculocuneiform joints are important for the dissipation of loading stresses on the midfoot but provide little motion. The plantar and dorsal ligaments are thick structures that stabilize these joints, predisposing the navicular to fracture rather than isolated dislocation. The stability of the foot is dependent on both the medial and lateral longitudinal columns, and it is thought impossible to injure one column without disrupting the other.6 Several patterns of associated lateral column disruptions have been documented, including 3 cases similar to our patient’s, involving navicular dislocation with associated calcaneocuboid joint injuries.5,6,10
Authors have proposed several mechanisms accounting for navicular dislocations. In the setting of acute trauma, the navicular displaces dorsally as the result of forefoot plantar flexion and axial loading.4 A severe abduction/pronation injury leading to a midtarsal dislocation followed by a spontaneous reduction can force the navicular to dislocate medially.6 This disruption of the naviculocuneiform joint and concurrent “nutcracker” injury to the lateral column can produce an associated disruption of the calcaneocuboid joint.6 Depending on the direction of the deforming force, the forefoot can dislocate superolaterally if the force is plantar or inferolaterally if the force is dorsal. The remaining soft-tissue attachments help determine the position of the navicular. A third postulated mechanism involves a complex wringing injury to the forefoot.10Most specialists agree that navicular dislocations are best treated with open reduction.4,6 The goal of surgical intervention is to establish a stable plantigrade foot and to minimize pain. The current literature supports using either wires or screws to maintain reduction of midfoot injuries. Wires can be used for both talonavicular and naviculocuneiform fixation. Screws can be placed across the naviculocuneiform joints, as there is little normal physiologic motion through these joints.4 The talonavicular joint and the cuboid-metatarsal joints provide most of the motion in the midfoot and should not be readily fused.5 Stabilization of both columns is considered necessary to avoid complications such as subluxation and midfoot deformity.Given the postreduction stability of the lateral column in the present case, bicolumnar stabilization was not considered necessary. It is possible that subsequent collapse of the midfoot may have been attenuated in the presence of lateral fixation, but this would not necessarily have prevented complications of AVN.
Midfoot fractures that are recognized and treated early have generally favorable outcomes,5-11 though chronic pain and subsequent deformity are not uncommon. Perhaps the most frequently reported complication of navicular dislocation is AVN, which is thought to occur in approximately 25% of cases.12 AVN is a well-recognized complication of hindfoot and midfoot trauma. In the tarsal navicular, blood supply to the central-third watershed region is marginal. Small branches of the posterior tibial and dorsalis pedis arteries that supply the medial and lateral areas are readily injured. Not surprisingly, the risk for AVN is high when the dislocated bone is severely displaced.6 In some circumstances, the shared blood supply of the posterior tibialis may be the only remaining osseous supply. The tendon and its soft-tissue attachments should therefore be carefully monitored during dissection and reduction.6 In most cases, AVN of the foot manifests clinically within the first 10 months after injury, as was the case with our patient.13 AVN can result in the Charcot-like collapse of the medial column, leading to progressive midfoot plantar deformities.4 Variations of midfoot fusion are often required.4,6AVN may be difficult to differentiate from posttraumatic arthritis. These conditions can have similar clinical presentations and appearances on plain radiographs. In such situations, magnetic resonance imaging or bone scintigraphy may determine the diagnosis. Damage to the articular surface at time of injury and residual articular displacement, instability, and joint subluxation after injury are considered risk factors for the development of posttraumatic arthritis in the foot and ankle.14 Reports suggest that the severity of the damage to the articular surface is directly proportional to the degree of arthritis.14 Such damage may not be initially visible, especially in axial impaction injuries, but latent deterioration of the articular surface can occur.15 For patients with significant dislocations of the naviculocuneiform joints, some authors advocate primary and early fusion15 instead of the more conservative approach used here. Primary fusions are argued to have minimal deleterious effects on function, secondary to the absence of normal physiologic motion through the affected joints.15 However, there is relatively little published evidence on long-term outcomes in primary versus secondary naviculocuneiform fusions.
Successful treatment of midfoot fractures and dislocations requires intimate knowledge of foot and ankle anatomy and mechanics. Surgeons must be able to anticipate, identify, and counsel patients about acute and delayed complications in these already challenging injuries.
Am J Orthop. 2017;46(3):E186-E189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Main BJ, Jowett RL. Injuries of the midtarsal joint. J Bone Jt Surg Br. 1975;57(1):89-97.
2. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001;32(1):21-33.
3. Vaishya R, Patrick JH. Isolated dorsal fracture-dislocation of tarsal navicular. Injury. 1991;22(1):47-48.
4. Early JS. Fractures and dislocations of the midfoot and forefoot. In: Bucholz WB, Heckman JD, Court-Brown C, et al, eds. Rockwood & Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott; 2005:2337-2401.
5. Rao H. Complete open dislocation of the navicular: a case report. J Foot Ankle Surg. 2012;51(2):209-211.
6. Dhillon MS, Nagi ON. Total dislocation of the navicular: are they ever isolated injuries? J Bone Joint Surg Br. 1999;81(5):881-885.
7. Kollmannsberger A, De Boer P. Isolated calcaneo-cuboid dislocation: brief report. J Bone Joint Surg Br. 1989;71(2):323.
8. Randall RL, Hall RJ, Slabaugh P. An unusual midfoot dislocation: a case report. Am J Orthop. 1997;26(7):494-496.
9. Ruthman JC, Meyn NP. Isolated plantar midtarsal dislocation. Am J Emerg Med. 1988;6(6):599-601.
10. Pathria MN, Rosenstein A, Bjorkengren AG, Gershuni D, Resnick D. Isolated dislocation of the tarsal navicular: a case report. Foot Ankle. 1988;9(3):146-149.
11. Puente CA, Alaez JP, Marti DG. Tarsal fracture dislocation with plantar dislocation of the navicular: a case study. Foot Ankle Int. 1996;17(2):111-113.
12. Davis AT, Dann A, Kuldjanov D. Complete medial dislocation of the tarsal navicular without fracture: report of a rare injury. J Foot Ankle Surg. 2013;52(3):393-396.
13. Buchan CA, Pearce DH, Lau J, White LW. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192-204.
14. Olson SA, Furman B, Guilak F. Joint injury and post-traumatic arthritis. HSS J. 2012;8(1):23-25.
15. Grambart S, Patel S, Schuberth JM. Naviculocuneiform dislocations treated with immediate arthrodesis: a report of 2 cases. J Foot Ankle Surg. 2005;44(3):228-235.
Take-Home Points
- Stability of the foot is dependent on both the medial and lateral longitudinal columns; injuries to a single column alone are extremely rare.
- Midfoot fractures that are recognized and treated early have generally favorable outcomes compared to those identified in a delayed fashion.
- The most frequent complication of navicular dislocation is AVN, which is said to occur in as many as 25% of cases.
- Many specialists agree that navicular dislocations are best treated with open reduction.
- Ultimately, the goals of surgical intervention are to minimize pain and to establish stability of the plantigrade foot.
Traumatic dislocation of the tarsal navicular (especially without a navicular body fracture) is uncommon.1 The regional anatomy and ligamentous architecture confer stability to the midfoot.2-6 Navicular dislocation is part of a complex disruption involving structures in the adjacent column.6
Navicular dislocation has been associated with several bony and soft-tissue injury patterns, including comminuted intra-articular fracture of the calcaneus and associated calcaneocuboid joint subluxation; fracture and subluxation of the calcaneocuboid joint; fracture-dislocation of the calcaneocuboid joint with fractures of the third and fourth metatarsals; and a combination of fractures of the intermediate cuneiform, the second through fourth metatarsals, and the cuboid.4–11 In this article, we report a case of open complete navicular dislocation with talar head fracture and associated subtalar and calcaneocuboid subluxations in a 45-year-old man. The injury was managed with open reduction and stabilization with Kirschner wires (K-wires), which later required naviculocuneiform and intercuneiform fusion for posttraumatic avascular necrosis (AVN). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 45-year-old man sustained blunt trauma to the right foot in a high-speed head-on collision. He was hemodynamically stable with isolated complaints of pain in the foot. Physical examination revealed a grossly open 10-cm wound extending from the heel pad medially to the dorsal surface of the navicular. The navicular was clearly visible through the wound.
Plain radiographs of the foot showed complete medial dislocation of the navicular with complete disruption of all 3 naviculocuneiform joints (Figures 1A-1C).
On day of presentation, the patient was taken to the operating room for irrigation, débridement, reduction of the joints, and primary closure of the right foot wound. Minimal contamination was noted. Attempted gentle reduction maneuvers included distraction, adduction, and pronation of the forefoot with concomitant lateral pressure on the navicular.
An especially prominent medial navicular was noted on postreduction films. Initially, this suggested inadequate reduction of the naviculocuneiform joints, but, on close radiographic examination of each naviculocuneiform joint and imaging of the contralateral foot, we determined that the prominence represented a type III accessory navicular, also known as a cornuate navicular. Contralateral imaging showed an identical and asymptomatic medial prominence.
After surgery, the patient was made non-weight-bearing in a splint, received intravenous antibiotics for 48 hours, and was discharged shortly thereafter. Radiographs at 3 and 6 weeks after injury showed maintenance of the reduction. K-wires were removed at 6 weeks. The patient was advanced to partial weight-bearing at 6 weeks and to full weight-bearing at 3 months.
Over succeeding months, the patient developed pain accompanied by significant midfoot deformity and was found to have navicular collapse consistent with AVN and posttraumatic arthritis (Figures 4A, 4B).
Twenty-four months after fusion, the patient was fully ambulatory with no significant discomfort or disability.
Discussion
The naviculocuneiform joints are important for the dissipation of loading stresses on the midfoot but provide little motion. The plantar and dorsal ligaments are thick structures that stabilize these joints, predisposing the navicular to fracture rather than isolated dislocation. The stability of the foot is dependent on both the medial and lateral longitudinal columns, and it is thought impossible to injure one column without disrupting the other.6 Several patterns of associated lateral column disruptions have been documented, including 3 cases similar to our patient’s, involving navicular dislocation with associated calcaneocuboid joint injuries.5,6,10
Authors have proposed several mechanisms accounting for navicular dislocations. In the setting of acute trauma, the navicular displaces dorsally as the result of forefoot plantar flexion and axial loading.4 A severe abduction/pronation injury leading to a midtarsal dislocation followed by a spontaneous reduction can force the navicular to dislocate medially.6 This disruption of the naviculocuneiform joint and concurrent “nutcracker” injury to the lateral column can produce an associated disruption of the calcaneocuboid joint.6 Depending on the direction of the deforming force, the forefoot can dislocate superolaterally if the force is plantar or inferolaterally if the force is dorsal. The remaining soft-tissue attachments help determine the position of the navicular. A third postulated mechanism involves a complex wringing injury to the forefoot.10Most specialists agree that navicular dislocations are best treated with open reduction.4,6 The goal of surgical intervention is to establish a stable plantigrade foot and to minimize pain. The current literature supports using either wires or screws to maintain reduction of midfoot injuries. Wires can be used for both talonavicular and naviculocuneiform fixation. Screws can be placed across the naviculocuneiform joints, as there is little normal physiologic motion through these joints.4 The talonavicular joint and the cuboid-metatarsal joints provide most of the motion in the midfoot and should not be readily fused.5 Stabilization of both columns is considered necessary to avoid complications such as subluxation and midfoot deformity.Given the postreduction stability of the lateral column in the present case, bicolumnar stabilization was not considered necessary. It is possible that subsequent collapse of the midfoot may have been attenuated in the presence of lateral fixation, but this would not necessarily have prevented complications of AVN.
Midfoot fractures that are recognized and treated early have generally favorable outcomes,5-11 though chronic pain and subsequent deformity are not uncommon. Perhaps the most frequently reported complication of navicular dislocation is AVN, which is thought to occur in approximately 25% of cases.12 AVN is a well-recognized complication of hindfoot and midfoot trauma. In the tarsal navicular, blood supply to the central-third watershed region is marginal. Small branches of the posterior tibial and dorsalis pedis arteries that supply the medial and lateral areas are readily injured. Not surprisingly, the risk for AVN is high when the dislocated bone is severely displaced.6 In some circumstances, the shared blood supply of the posterior tibialis may be the only remaining osseous supply. The tendon and its soft-tissue attachments should therefore be carefully monitored during dissection and reduction.6 In most cases, AVN of the foot manifests clinically within the first 10 months after injury, as was the case with our patient.13 AVN can result in the Charcot-like collapse of the medial column, leading to progressive midfoot plantar deformities.4 Variations of midfoot fusion are often required.4,6AVN may be difficult to differentiate from posttraumatic arthritis. These conditions can have similar clinical presentations and appearances on plain radiographs. In such situations, magnetic resonance imaging or bone scintigraphy may determine the diagnosis. Damage to the articular surface at time of injury and residual articular displacement, instability, and joint subluxation after injury are considered risk factors for the development of posttraumatic arthritis in the foot and ankle.14 Reports suggest that the severity of the damage to the articular surface is directly proportional to the degree of arthritis.14 Such damage may not be initially visible, especially in axial impaction injuries, but latent deterioration of the articular surface can occur.15 For patients with significant dislocations of the naviculocuneiform joints, some authors advocate primary and early fusion15 instead of the more conservative approach used here. Primary fusions are argued to have minimal deleterious effects on function, secondary to the absence of normal physiologic motion through the affected joints.15 However, there is relatively little published evidence on long-term outcomes in primary versus secondary naviculocuneiform fusions.
Successful treatment of midfoot fractures and dislocations requires intimate knowledge of foot and ankle anatomy and mechanics. Surgeons must be able to anticipate, identify, and counsel patients about acute and delayed complications in these already challenging injuries.
Am J Orthop. 2017;46(3):E186-E189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Stability of the foot is dependent on both the medial and lateral longitudinal columns; injuries to a single column alone are extremely rare.
- Midfoot fractures that are recognized and treated early have generally favorable outcomes compared to those identified in a delayed fashion.
- The most frequent complication of navicular dislocation is AVN, which is said to occur in as many as 25% of cases.
- Many specialists agree that navicular dislocations are best treated with open reduction.
- Ultimately, the goals of surgical intervention are to minimize pain and to establish stability of the plantigrade foot.
Traumatic dislocation of the tarsal navicular (especially without a navicular body fracture) is uncommon.1 The regional anatomy and ligamentous architecture confer stability to the midfoot.2-6 Navicular dislocation is part of a complex disruption involving structures in the adjacent column.6
Navicular dislocation has been associated with several bony and soft-tissue injury patterns, including comminuted intra-articular fracture of the calcaneus and associated calcaneocuboid joint subluxation; fracture and subluxation of the calcaneocuboid joint; fracture-dislocation of the calcaneocuboid joint with fractures of the third and fourth metatarsals; and a combination of fractures of the intermediate cuneiform, the second through fourth metatarsals, and the cuboid.4–11 In this article, we report a case of open complete navicular dislocation with talar head fracture and associated subtalar and calcaneocuboid subluxations in a 45-year-old man. The injury was managed with open reduction and stabilization with Kirschner wires (K-wires), which later required naviculocuneiform and intercuneiform fusion for posttraumatic avascular necrosis (AVN). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 45-year-old man sustained blunt trauma to the right foot in a high-speed head-on collision. He was hemodynamically stable with isolated complaints of pain in the foot. Physical examination revealed a grossly open 10-cm wound extending from the heel pad medially to the dorsal surface of the navicular. The navicular was clearly visible through the wound.
Plain radiographs of the foot showed complete medial dislocation of the navicular with complete disruption of all 3 naviculocuneiform joints (Figures 1A-1C).
On day of presentation, the patient was taken to the operating room for irrigation, débridement, reduction of the joints, and primary closure of the right foot wound. Minimal contamination was noted. Attempted gentle reduction maneuvers included distraction, adduction, and pronation of the forefoot with concomitant lateral pressure on the navicular.
An especially prominent medial navicular was noted on postreduction films. Initially, this suggested inadequate reduction of the naviculocuneiform joints, but, on close radiographic examination of each naviculocuneiform joint and imaging of the contralateral foot, we determined that the prominence represented a type III accessory navicular, also known as a cornuate navicular. Contralateral imaging showed an identical and asymptomatic medial prominence.
After surgery, the patient was made non-weight-bearing in a splint, received intravenous antibiotics for 48 hours, and was discharged shortly thereafter. Radiographs at 3 and 6 weeks after injury showed maintenance of the reduction. K-wires were removed at 6 weeks. The patient was advanced to partial weight-bearing at 6 weeks and to full weight-bearing at 3 months.
Over succeeding months, the patient developed pain accompanied by significant midfoot deformity and was found to have navicular collapse consistent with AVN and posttraumatic arthritis (Figures 4A, 4B).
Twenty-four months after fusion, the patient was fully ambulatory with no significant discomfort or disability.
Discussion
The naviculocuneiform joints are important for the dissipation of loading stresses on the midfoot but provide little motion. The plantar and dorsal ligaments are thick structures that stabilize these joints, predisposing the navicular to fracture rather than isolated dislocation. The stability of the foot is dependent on both the medial and lateral longitudinal columns, and it is thought impossible to injure one column without disrupting the other.6 Several patterns of associated lateral column disruptions have been documented, including 3 cases similar to our patient’s, involving navicular dislocation with associated calcaneocuboid joint injuries.5,6,10
Authors have proposed several mechanisms accounting for navicular dislocations. In the setting of acute trauma, the navicular displaces dorsally as the result of forefoot plantar flexion and axial loading.4 A severe abduction/pronation injury leading to a midtarsal dislocation followed by a spontaneous reduction can force the navicular to dislocate medially.6 This disruption of the naviculocuneiform joint and concurrent “nutcracker” injury to the lateral column can produce an associated disruption of the calcaneocuboid joint.6 Depending on the direction of the deforming force, the forefoot can dislocate superolaterally if the force is plantar or inferolaterally if the force is dorsal. The remaining soft-tissue attachments help determine the position of the navicular. A third postulated mechanism involves a complex wringing injury to the forefoot.10Most specialists agree that navicular dislocations are best treated with open reduction.4,6 The goal of surgical intervention is to establish a stable plantigrade foot and to minimize pain. The current literature supports using either wires or screws to maintain reduction of midfoot injuries. Wires can be used for both talonavicular and naviculocuneiform fixation. Screws can be placed across the naviculocuneiform joints, as there is little normal physiologic motion through these joints.4 The talonavicular joint and the cuboid-metatarsal joints provide most of the motion in the midfoot and should not be readily fused.5 Stabilization of both columns is considered necessary to avoid complications such as subluxation and midfoot deformity.Given the postreduction stability of the lateral column in the present case, bicolumnar stabilization was not considered necessary. It is possible that subsequent collapse of the midfoot may have been attenuated in the presence of lateral fixation, but this would not necessarily have prevented complications of AVN.
Midfoot fractures that are recognized and treated early have generally favorable outcomes,5-11 though chronic pain and subsequent deformity are not uncommon. Perhaps the most frequently reported complication of navicular dislocation is AVN, which is thought to occur in approximately 25% of cases.12 AVN is a well-recognized complication of hindfoot and midfoot trauma. In the tarsal navicular, blood supply to the central-third watershed region is marginal. Small branches of the posterior tibial and dorsalis pedis arteries that supply the medial and lateral areas are readily injured. Not surprisingly, the risk for AVN is high when the dislocated bone is severely displaced.6 In some circumstances, the shared blood supply of the posterior tibialis may be the only remaining osseous supply. The tendon and its soft-tissue attachments should therefore be carefully monitored during dissection and reduction.6 In most cases, AVN of the foot manifests clinically within the first 10 months after injury, as was the case with our patient.13 AVN can result in the Charcot-like collapse of the medial column, leading to progressive midfoot plantar deformities.4 Variations of midfoot fusion are often required.4,6AVN may be difficult to differentiate from posttraumatic arthritis. These conditions can have similar clinical presentations and appearances on plain radiographs. In such situations, magnetic resonance imaging or bone scintigraphy may determine the diagnosis. Damage to the articular surface at time of injury and residual articular displacement, instability, and joint subluxation after injury are considered risk factors for the development of posttraumatic arthritis in the foot and ankle.14 Reports suggest that the severity of the damage to the articular surface is directly proportional to the degree of arthritis.14 Such damage may not be initially visible, especially in axial impaction injuries, but latent deterioration of the articular surface can occur.15 For patients with significant dislocations of the naviculocuneiform joints, some authors advocate primary and early fusion15 instead of the more conservative approach used here. Primary fusions are argued to have minimal deleterious effects on function, secondary to the absence of normal physiologic motion through the affected joints.15 However, there is relatively little published evidence on long-term outcomes in primary versus secondary naviculocuneiform fusions.
Successful treatment of midfoot fractures and dislocations requires intimate knowledge of foot and ankle anatomy and mechanics. Surgeons must be able to anticipate, identify, and counsel patients about acute and delayed complications in these already challenging injuries.
Am J Orthop. 2017;46(3):E186-E189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Main BJ, Jowett RL. Injuries of the midtarsal joint. J Bone Jt Surg Br. 1975;57(1):89-97.
2. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001;32(1):21-33.
3. Vaishya R, Patrick JH. Isolated dorsal fracture-dislocation of tarsal navicular. Injury. 1991;22(1):47-48.
4. Early JS. Fractures and dislocations of the midfoot and forefoot. In: Bucholz WB, Heckman JD, Court-Brown C, et al, eds. Rockwood & Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott; 2005:2337-2401.
5. Rao H. Complete open dislocation of the navicular: a case report. J Foot Ankle Surg. 2012;51(2):209-211.
6. Dhillon MS, Nagi ON. Total dislocation of the navicular: are they ever isolated injuries? J Bone Joint Surg Br. 1999;81(5):881-885.
7. Kollmannsberger A, De Boer P. Isolated calcaneo-cuboid dislocation: brief report. J Bone Joint Surg Br. 1989;71(2):323.
8. Randall RL, Hall RJ, Slabaugh P. An unusual midfoot dislocation: a case report. Am J Orthop. 1997;26(7):494-496.
9. Ruthman JC, Meyn NP. Isolated plantar midtarsal dislocation. Am J Emerg Med. 1988;6(6):599-601.
10. Pathria MN, Rosenstein A, Bjorkengren AG, Gershuni D, Resnick D. Isolated dislocation of the tarsal navicular: a case report. Foot Ankle. 1988;9(3):146-149.
11. Puente CA, Alaez JP, Marti DG. Tarsal fracture dislocation with plantar dislocation of the navicular: a case study. Foot Ankle Int. 1996;17(2):111-113.
12. Davis AT, Dann A, Kuldjanov D. Complete medial dislocation of the tarsal navicular without fracture: report of a rare injury. J Foot Ankle Surg. 2013;52(3):393-396.
13. Buchan CA, Pearce DH, Lau J, White LW. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192-204.
14. Olson SA, Furman B, Guilak F. Joint injury and post-traumatic arthritis. HSS J. 2012;8(1):23-25.
15. Grambart S, Patel S, Schuberth JM. Naviculocuneiform dislocations treated with immediate arthrodesis: a report of 2 cases. J Foot Ankle Surg. 2005;44(3):228-235.
1. Main BJ, Jowett RL. Injuries of the midtarsal joint. J Bone Jt Surg Br. 1975;57(1):89-97.
2. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001;32(1):21-33.
3. Vaishya R, Patrick JH. Isolated dorsal fracture-dislocation of tarsal navicular. Injury. 1991;22(1):47-48.
4. Early JS. Fractures and dislocations of the midfoot and forefoot. In: Bucholz WB, Heckman JD, Court-Brown C, et al, eds. Rockwood & Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott; 2005:2337-2401.
5. Rao H. Complete open dislocation of the navicular: a case report. J Foot Ankle Surg. 2012;51(2):209-211.
6. Dhillon MS, Nagi ON. Total dislocation of the navicular: are they ever isolated injuries? J Bone Joint Surg Br. 1999;81(5):881-885.
7. Kollmannsberger A, De Boer P. Isolated calcaneo-cuboid dislocation: brief report. J Bone Joint Surg Br. 1989;71(2):323.
8. Randall RL, Hall RJ, Slabaugh P. An unusual midfoot dislocation: a case report. Am J Orthop. 1997;26(7):494-496.
9. Ruthman JC, Meyn NP. Isolated plantar midtarsal dislocation. Am J Emerg Med. 1988;6(6):599-601.
10. Pathria MN, Rosenstein A, Bjorkengren AG, Gershuni D, Resnick D. Isolated dislocation of the tarsal navicular: a case report. Foot Ankle. 1988;9(3):146-149.
11. Puente CA, Alaez JP, Marti DG. Tarsal fracture dislocation with plantar dislocation of the navicular: a case study. Foot Ankle Int. 1996;17(2):111-113.
12. Davis AT, Dann A, Kuldjanov D. Complete medial dislocation of the tarsal navicular without fracture: report of a rare injury. J Foot Ankle Surg. 2013;52(3):393-396.
13. Buchan CA, Pearce DH, Lau J, White LW. Imaging of postoperative avascular necrosis of the ankle and foot. Semin Musculoskelet Radiol. 2012;16(3):192-204.
14. Olson SA, Furman B, Guilak F. Joint injury and post-traumatic arthritis. HSS J. 2012;8(1):23-25.
15. Grambart S, Patel S, Schuberth JM. Naviculocuneiform dislocations treated with immediate arthrodesis: a report of 2 cases. J Foot Ankle Surg. 2005;44(3):228-235.
For bone and joint infections, oral antibiotics match IV, cost less
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
[email protected]
On Twitter @alz_gal
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
[email protected]
On Twitter @alz_gal
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
[email protected]
On Twitter @alz_gal
AT ECCMID 2017
Key clinical point:
Major finding: At 1 year, cure rates were identical, but oral treatment cost about $3,500 less than IV treatment.
Data source: The study randomized 1,054 patients.
Disclosures: OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
An Update on Management of Syndesmosis Injury: A National US Database Study
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Acute ankle injuries are common problems treated by orthopedic surgeons. In the United States, nearly 2 million ankle sprains occur each year,1 and ankle fractures account for 9% to 18% of all fractures treated in emergency departments.2,3 Ankle injuries that involve the syndesmotic ligaments may result in instability and require specific treatment beyond fixation of the malleolar fractures.
The usual mechanism of syndesmotic injury is external rotation of the ankle with hyperdorsiflexion of a pronated or supinated foot.4,5 Syndesmotic injuries are estimated to occur in up to 10% of ankle sprains6 and up to 23% of all ankle fractures.7 Overall US incidence of syndesmotic injury is estimated at 6445 injuries per year.8 Syndesmotic injury occurs in 39% to 45% of supination-external rotation IV ankle fractures.9,10 Pronation-external rotation ankle fractures have the highest rate of syndesmotic injury. Syndesmotic injury may be less common in other types of malleolar fracture, but the exact incidence has not been reliably reported.
Traditionally, isolated nondisplaced syndesmotic injuries are treated nonoperatively, and syndesmotic injuries with concomitant malleolar fractures are treated surgically. Various options are available for syndesmotic fixation. The gold standard is syndesmotic screw placement from the lateral aspect of the fibula through the tibia. Fixation may be achieved with screws in a variety of configurations and formats. However, fixation with two 4.5-mm screws is stronger.11,12 Functional outcomes are similar, regardless of screw material,13-16 number of cortices,17 or number of screws.18 Disadvantages specific to screw fixation include altered ankle biomechanics,19,20 potential for screw breakage,21 and need for implant removal.3Alternatively, suture button fixation is said to be equally as effective as screw fixation in achieving syndesmotic reduction, and their functional outcomes are similar.22,23 The initial cost of suture button fixation is higher than that of screw fixation, but the difference may be offset by potential elimination of a second surgery for syndesmotic screw removal.24 Soft-tissue irritation caused by the suture material and local osteolysis are reported complications of suture button fixation.25-27
Regardless of fixation method used, achieving anatomical reduction of the syndesmosis is considered the most important factor in optimizing functional outcomes.28-31 However, achieving and verifying anatomical reduction of the syndesmosis during surgery can be quite challenging.30,32-34 Various methods of lowering the malreduction risk, including direct visualization of the tibiofibular joint during reduction30,35 and intraoperative 3-dimensional imaging,33,36 have been proposed.
In the study reported here, we used a US insurance database to determine the incidence and rate of syndesmotic stabilization within various ankle injuries and fracture patterns.
Materials and Methods
All data for this study were obtained from a publicly available for-fee healthcare database, the PearlDiver Patient Records Database, which includes procedural volumes and demographic information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the study were derived from 2 databases within PearlDiver: a private-payer database, which has its largest contribution (>30 million individual patient records for 2007-2011) from United HealthCare, and a Medicare database (>50 million patient records for 2007-2011). Access to the database was granted by PearlDiver Technologies for the purpose of academic research. The database was stored on a password-protected server maintained by PearlDiver.
We searched the database for cases of ankle fracture fixation, including fixation of isolated lateral malleolus (CPT 27792), bimalleolar (CPT 27814), and trimalleolar (CPTs 27822 and 27823) fractures. CPT 27829 was used to search for syndesmotic fixation, and CPT 20680 for implant removal. These codes were used individually and in combination.
Overall procedural volume data are reported as number of patients with the given CPT(s) in the database output and as incidence, calculated as number of patients with the CPT of interest normalized to total number of patients in the database for that particular subgroup. Results of age group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who had the CPT procedure of interest that year. As United HealthCare is the largest contributor to the private-payer portion of the database and is represented most prominently in the southern region, data for the regional analysis are presented only as incidence. This incidence was calculated as number of patients in a particular region and year normalized to total number of patients in the database for that region or year. The regions were Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MI, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-square linear-by-linear association analysis was used to determine the statistical significance of time trends in procedural volume, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
Number of open reduction and internal fixation (ORIF) procedures increased for all ankle fracture types over the period 2007 to 2011 (Table 1).
ORIF was performed for an ankle injury in 54,767 patients during the period 2007 to 2011, resulting in a cumulative incidence of 64.2 per 1000 patients (Table 2).
More ankle ORIF procedures were performed in females (33,565) than in males (21,202); incidence of ankle ORIF procedures was higher in females (68.6/1000 patients) than in males (58.4/1000 patients) (Table 2); percentages of bimalleolar and trimalleolar fractures were higher in females (bi, 40.6%; tri, 27.8%) than in males (bi, 34.6%; tri, 15.2%); and percentage of lateral malleolus fractures was higher in males (50.2%) than in females (31.6%).
Incidence of ankle ORIF procedures was similar in the South (69.6/1000 patients), Midwest (69.4/100 patients), and West (65.1/1000 patients) but lower in the Northeast (43.3/1000 patients) (Table 2). Lateral malleolus fractures were the most common ankle fractures in the Midwest (40.7%) and West (41.3%), followed by bimalleolar fractures (Midwest, 36.3%; West 36.0%) and trimalleolar fractures (Midwest, 23.0%; West, 22.7%). Bimalleolar fractures were most common in the Northeast (40.2%) and South (39.8%), followed by lateral malleolus fractures (Northeast, 34.4%; South, 38.0%) and trimalleolar fractures (Northeast, 25.4%; South, 22.3%).
Discussion
The present study found no significant change in number of lateral malleolus, bimalleolar, and trimalleolar ankle fracture ORIF procedures performed over the period 2007 to 2011. However, over the same period, incidence of syndesmosis fixation increased significantly in patients with isolated syndesmotic injuries and in patients with concomitant ankle fracture and syndesmotic injury. The largest percentage change was found in the bimalleolar ORIF group, which showed nearly a doubling of syndesmotic fixation over the 4-year study period, followed by a 38.1% increase in syndesmotic fixation in the trimalleolar ORIF group. Both groups had a syndesmotic fixation percentage change about twice that seen in the isolated lateral malleolus group.
There are several explanations for these trends. First, bimalleolar and trimalleolar fractures are more severe ankle fractures that tend to result from a more forceful mechanism, allowing for a higher rate of syndesmotic injury. Second, these trends likely do not reflect a true increase in the rate of syndesmosis injury but, rather, increased recognition of syndesmotic injury. Third, the data likely reflect a well-established approach to ankle fracture fixation and an increase in thinking that syndesmotic injuries should be stabilized in the setting of ankle fixation.
Incidence of syndesmotic injury as indicated by stabilization procedures can be compared with the data of Vosseller and colleagues,8 who reported an incidence of 6445 syndesmotic injuries per year in the United States. Our data showed fewer syndesmotic injuries, which may be related to use of CPT codes rather than ICD-9 codes for database searches, such that only operative syndesmotic injuries are represented in our data. Population differences between the 2 studies could also account for some of the differences in syndesmotic injury incidence.
We also found a significant change in the rate of hardware removal after syndesmosis ORIF. Across all treatment groups, incidence of screw removal decreased—a trend likely reflecting a change in attitude about the need for routine screw removal. Studies have shown that patients have favorable outcomes in the setting of syndesmotic screw loosening and screw breakage.37 Some authors have suggested that screw breakage or removal could be advantageous, as it allows the syndesmosis to settle into a more anatomical position after imperfect reduction.38 In addition, the trend of decreased syndesmotic screw removal could also have resulted from increased suture button fixation, which may less frequently require implant removal. Regardless, the overall trend is that routine syndesmotic implant removal has become less common.
This study had several limitations. First are the many limitations inherent to all studies that use large administrative databases, such as PearlDiver. The power of analysis depends on data quality; potential sources of error include accuracy of billing codes and physicians’ miscoding or noncoding. Although we tried to accurately represent a large population of interest through use of this database, we cannot be sure that the database represents a true cross-section of the United States. In addition, as we could not determine the method of syndesmotic fixation—the same CPT code is used for both suture button fixation and screw fixation—we could not establish trends for the rate of each method. More research is needed to establish these trends, and this research likely will require analysis of data from a large trauma center or from multiple centers.
Potential regional differences are another limitation. In the PearlDiver database, the South and Midwest are highly represented, the Northeast and West much less so. The South, Midwest, and West (but not the Northeast) had similar overall incidence and subgroup incidence of ankle ORIF. However, any regional differences in the rate of syndesmotic fixation could have skewed our data.
Ankle fractures and associated syndesmotic injuries remain a common problem. Although the prevalence of ankle fracture fixation has been relatively constant, the rate of syndesmosis stabilization has increased significantly. Young adults have the highest incidence of ankle fracture and associated syndesmotic fixation, but more ankle fractures occur in the large and growing elderly population. Increased awareness of syndesmotic injury likely has contributed to the recent rise in syndesmosis fixation seen in the present study. Given this trend, we recommend further analysis of outcome data and to establish treatment guidelines.
Am J Orthop. 2016;45(7):E472-E477. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
1. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279-2284.
2. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
3. Miller AN, Paul O, Boraiah S, Parker RJ, Helfet DL, Lorich DG. Functional outcomes after syndesmotic screw fixation and removal. J Orthop Trauma. 2010;24(1):12-16.
4. Edwards GS Jr, DeLee JC. Ankle diastasis without fracture. Foot Ankle. 1984;4(6):305-312.
5. Norkus SA, Floyd RT. The anatomy and mechanisms of syndesmotic ankle sprains. J Athl Train. 2001;36(1):68-73.
6. Brosky T, Nyland J, Nitz A, Caborn DN. The ankle ligaments: consideration of syndesmotic injury and implications for rehabilitation. J Orthop Sports Phys Ther. 1995;21(4):197-205.
7. Purvis GD. Displaced, unstable ankle fractures: classification, incidence, and management of a consecutive series. Clin Orthop Relat Res. 1982;(165):91-98.
8. Vosseller JT, Karl JW, Greisberg JK. Incidence of syndesmotic injury. Orthopedics. 2014;37(3):e226-e229.
9. Stark E, Tornetta P 3rd, Creevy WR. Syndesmotic instability in Weber B ankle fractures: a clinical evaluation. J Orthop Trauma. 2007;21(9):643-646.
10. Tornetta P 3rd, Axelrad TW, Sibai TA, Creevy WR. Treatment of the stress positive ligamentous SE4 ankle fracture: incidence of syndesmotic injury and clinical decision making. J Orthop Trauma. 2012;26(11):659-661.
11. Xenos JS, Hopkinson WJ, Mulligan ME, Olson EJ, Popovic NA. The tibiofibular syndesmosis. Evaluation of the ligamentous structures, methods of fixation, and radiographic assessment. J Bone Joint Surg Am. 1995;77(6):847-856.
12. Ebraheim NA, Lu J, Yang H, Mekhail AO, Yeasting RA. Radiographic and CT evaluation of tibiofibular syndesmotic diastasis: a cadaver study. Foot Ankle Int. 1997;18(11):693-698.
13. Ahmad J, Raikin SM, Pour AE, Haytmanek C. Bioabsorbable screw fixation of the syndesmosis in unstable ankle injuries. Foot Ankle Int. 2009;30(2):99-105.
14. Hovis WD, Kaiser BW, Watson JT, Bucholz RW. Treatment of syndesmotic disruptions of the ankle with bioabsorbable screw fixation. J Bone Joint Surg Am. 2002;84(1):26-31.
15. Kaukonen JP, Lamberg T, Korkala O, Pajarinen J. Fixation of syndesmotic ruptures in 38 patients with a malleolar fracture: a randomized study comparing a metallic and a bioabsorbable screw. J Orthop Trauma. 2005;19(6):392-395.
16. Thordarson DB, Samuelson M, Shepherd LE, Merkle PF, Lee J. Bioabsorbable versus stainless steel screw fixation of the syndesmosis in pronation-lateral rotation ankle fractures: a prospective randomized trial. Foot Ankle Int. 2001;22(4):335-338.
17. Moore JA Jr, Shank JR, Morgan SJ, Smith WR. Syndesmosis fixation: a comparison of three and four cortices of screw fixation without hardware removal. Foot Ankle Int. 2006;27(8):567-572.
18. Høiness P, Strømsøe K. Tricortical versus quadricortical syndesmosis fixation in ankle fractures: a prospective, randomized study comparing two methods of syndesmosis fixation. J Orthop Trauma. 2004;18(6):331-337.
19. Huber T, Schmoelz W, Bölderl A. Motion of the fibula relative to the tibia and its alterations with syndesmosis screws: a cadaver study. Foot Ankle Surg. 2012;18(3):203-209.
20. Needleman RL, Skrade DA, Stiehl JB. Effect of the syndesmotic screw on ankle motion. Foot Ankle. 1989;10(1):17-24.
21. Mendelsohn ES, Hoshino CM, Harris TG, Zinar DM. The effect of obesity on early failure after operative syndesmosis injuries. J Orthop Trauma. 2013;27(4):201-206.
22. Schepers T. Acute distal tibiofibular syndesmosis injury: a systematic review of suture-button versus syndesmotic screw repair. Int Orthop. 2012;36(6):1199-1206.
23. Cottom JM, Hyer CF, Philbin TM, Berlet GC. Transosseous fixation of the distal tibiofibular syndesmosis: comparison of an interosseous suture and Endobutton to traditional screw fixation in 50 cases. J Foot Ankle Surg. 2009;48(6):620-630.
24. Thornes B, Shannon F, Guiney AM, Hession P, Masterson E. Suture-button syndesmosis fixation: accelerated rehabilitation and improved outcomes. Clin Orthop Relat Res. 2005;(431):207-212.
25. Willmott HJ, Singh B, David LA. Outcome and complications of treatment of ankle diastasis with tightrope fixation. Injury. 2009;40(11):1204-1206.
26. Qamar F, Kadakia A, Venkateswaran B. An anatomical way of treating ankle syndesmotic injuries. J Foot Ankle Surg. 2011;50(6):762-765.
27. Degroot H, Al-Omari AA, El Ghazaly SA. Outcomes of suture button repair of the distal tibiofibular syndesmosis. Foot Ankle Int. 2011;32(3):250-256.
28. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357.
29. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
30. Sagi HC, Shah AR, Sanders RW. The functional consequence of syndesmotic joint malreduction at a minimum 2-year follow-up. J Orthop Trauma. 2012;26(7):439-443.
31. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Franke J, von Recum J, Suda AJ, Grützner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am. 2012;94(15):1386-1390.
34. Gardner MJ, Demetrakopoulos D, Briggs SM, Helfet DL, Lorich DG. Malreduction of the tibiofibular syndesmosis in ankle fractures. Foot Ankle Int. 2006;27(10):788-792.
35. Miller AN, Carroll EA, Parker RJ, Boraiah S, Helfet DL, Lorich DG. Direct visualization for syndesmotic stabilization of ankle fractures. Foot Ankle Int. 2009;30(5):419-426.
36. Ruan Z, Luo C, Shi Z, Zhang B, Zeng B, Zhang C. Intraoperative reduction of distal tibiofibular joint aided by three-dimensional fluoroscopy. Technol Health Care. 2011;19(3):161-166.
37. Hamid N, Loeffler BJ, Braddy W, Kellam JF, Cohen BE, Bosse MJ. Outcome after fixation of ankle fractures with an injury to the syndesmosis: the effect of the syndesmosis screw. J Bone Joint Surg Br. 2009;91(8):1069-1073.
38. Song DJ, Lanzi JT, Groth AT, et al. The effect of syndesmosis screw removal on the reduction of the distal tibiofibular joint: a prospective radiographic study. Foot Ankle Int. 2014;35(6):543-548.
Mycotic Septic Arthritis of the Ankle Joint
Septic arthritis is a common orthopedic emergency. The most common causative organism is Staphylococcus aureus. Mycotic infections, such as those involving Candida organisms, are much less common but just as debilitating. Delayed diagnosis of septic arthritis caused by Candida infection may result in increased morbidity, making treatment more challenging. Here we report a case of Candida albicans septic arthritis of the ankle and subtalar joint in a patient with diabetes mellitus (DM) and rheumatoid arthritis (RA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 52-year-old woman with type 2 DM (requiring subcutaneous insulin analogue therapy) and RA presented to a local emergency department with a 3-day history of right ankle pain after having the subtalar joint injected with steroid by a rheumatologist 4 weeks earlier. For about 2 weeks, there was purulent discharge from the peroneal sheath. The patient’s RA was being treated with prednisolone (maintenance therapy). Physical examination revealed low-grade pyrexia (37.8°C) and difficulty bearing full weight on the ankle. Clinically, the joint was not erythematous, but active and passive movements were painful. Blood tests revealed a C-reactive protein level of 98 mg/dL and a white blood cell (WBC) count of 11.3 × 109/L. Erythrocyte sedimentation rate (ESR) was not checked. The ankle underwent magnetic resonance imaging (Figures A-D).
Mycotic screening of the fluid was positive for C albicans. The patient was referred to the orthopedic team, which performed urgent arthroscopic surgical débridement, biopsy, and washout of the subtalar joint. After surgery, a 6-week course of antifungal therapy with anidulafungin was started, per specialist microbiology advice.
The septic ankle was successfully managed with arthroscopic surgical débridement followed by treatment with anidulafungin. The patient continued to make good progress and was weight-bearing when discharged home from the orthopedic unit.
Discussion
Worldwide, about 1 in 6 people has arthritis, which affects daily lifestyle and reduces quality of life. Degenerative, inflammatory, and septic arthritis each has its management challenges.1
Septic arthritis is an acute infection of the joint, usually of bacterial etiology. It can present as a polyarticular arthropathy (~15% of cases),2,3 but a monoarthropathy of the hip, knee, or ankle is more common.4The Kocher criteria are often applied to cases of suspected septic arthritis of joints, even though they were initially used to distinguish septic arthritis from transient synovitis in pediatric hip joints.5 Kocher and colleagues5 reported 4 key clinical criteria: inability to bear weight, WBC count over 12 × 109/L, ESR over 40 mm/h, and temperature over 38.5°C. When all 4 criteria are met, the predictive value is 99.6%. These criteria are now widely applied to adult joints, and not only the hips.
In septic arthritis, the most common causative pathogen is S aureus.3,6Streptococcus, Neisseria, and Pseudomonas also are common.7 Although much rarer, Candida variants and other mycotic pathogens have been implicated as well.8C albicans is a well-known fungus that colonizes mucosal surfaces. Research indicates increased oral C albicans colonization in rheumatoid patients.9 Although most Candida septic arthritis cases are caused by C albicans, there is no large body of data showing the true incidence of fungal pathogens in septic arthritis.
Our literature search yielded 2 case reports on Candida septic arthritis involving the ankle, but the causative organisms were Candida parapsilosis and Candida glabrata.9,10 Cases of Candida septic arthritis involving the knee or shoulder have also been reported.11-15 Case reports demonstrate that Candida fungal arthritis is extremely rare.9 Etiology reportedly includes direct intra-articular inoculation by surgery or secondary to hematogenous seeding, particularly in immunocompromised patients.10 Risk factors include immunosuppression and joint suppression. DM and RA are common comorbidities in patients with septic arthritis.6,16 The pathophysiology of RA is inflammatory pannus formation of the periarticular surface with subsequent articular cartilage destruction and erosion, as well as progressive deformity and functional debilitation.1Patients with DM are at increased risk for developing fungal and other infections. Factors increasing this risk include disruption of skin-barrier integrity; reduced peripheral oxygen and blood supply, which also disrupts antibiotic delivery; and hyperglycemia-induced reduction in antibody function and disruption of phagocytosis and chemotaxis.17Fungi are eukaryotic, and infections caused by these organisms are difficult to treat.18 As fungal infections are more prevalent among immunosuppressed patients, they often result in prolonged treatment without guarantee of eradication, as spores may persist subclinically.
Literature on C albicans septic arthritis is lacking in general but especially in rheumatoid patients. Delayed diagnosis and suboptimal treatment may result in fungal osteomyelitis. There is little evidence on treating this rare fungal complication, and outcomes historically have been poor.19In an animal model, Marijnissen and colleagues20 found that C albicans infection can increase destruction in an arthritic joint by cytokine environment modification. The result was advanced destruction of the joint and debilitation. For disease management, the authors considered these essential: early diagnosis, prompt treatment, and, as indicated, surgical débridement.
Treatment of Candida septic arthritis largely involves use of antifungal medication, either with surgical débridement, as in our patient’s case, or without. Which antifungal medication to use should be based on sensitivities, identified from wound aspirate, and microbiology advice about treatment duration. The antibiotic should be a broad-spectrum antifungal cover, in keeping with local antibiotic prescribing guidelines, which can be refined once definitive organism culture and sensitivity results are known. However, early aggressive treatment is essential. Periprosthetic fungal infection is rarely resolved without implant removal.21
Conclusion
This case reflects the complexities of septic arthritis caused by atypical pathogens and highlights the need for clinical vigilance in the setting of comorbidities, such as DM and RA. Failure to consider the diagnosis early on might result in delayed and inadequate treatment, increased joint destruction, and, potentially, osteomyelitis with subsequent increased morbidity. Early diagnosis (based on joint aspirate findings), surgical débridement, and prolonged aggressive treatment with antifungal medication are the mainstays of treatment.
Am J Orthop. 2016;45(7):E478-E480. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Auday BC, Buratovich MA, Marrocco, GF, Moglia P, eds. Magill’s Medical Guide. 7th ed. Ipswich, MA: Salem Press; 2014.
2. Dhaliwal S, LeBel ME. Rapidly progressing polyarticular septic arthritis in a patient with rheumatoid arthritis. Am J Orthop. 2012;41(7):E100-E101.
3. Mateo Soria L, Olivé Marqués A, García Casares E, García Melchor E, Holgado Pérez S, Tena Marsà X. Polyarticular septic arthritis: analysis of 19 cases [in Spanish]. Reumatol Clin. 2009;5(1):18-22.
4. Caksen H, Oztürk MK, Uzüm K, Yüksel S, Ustünbaş HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000;42(5):534-540.
5. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662-1670.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. 2014;42(2):385-391.
7. Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol. 2014;20(2):74-78.
8. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
9. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans–specific Th17 responses. Arthritis Res Ther. 2014;16(1):R50.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
11. Sung J, Chun K. Candida parapsilosis arthritis involving the ankle in a diabetes patient. J Korean Soc Radiol. 2011;64:587-591.
12. Marmor L, Peter JB. Candida arthritis of the knee joint. Clin Orthop Relat Res. 1976;(118):133-135.
13. Turgut B, Vural O, Demir M, Kaldir M. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol. 2002;81(9):529-531.
14. Christensson B, Ryd L, Dahlberg L, Lohmander S. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand. 1993;64(6):695-698.
15. Jeong YM, Cho HY, Lee SW, Hwang YM, Kim YK. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465-469.
16. Favero M, Schiavon R, Riato L, Carraro V, Punzi L. Septic arthritis: a 12 years retrospective study in a rheumatological university clinic [in Italian]. Reumatismo. 2008;60(4):260-267.
17. Leslie D, Lansang C, Coppack S, Kennedy L. Diabetes: Clinician’s Desk Reference. Boca Raton, FL: CRC Press; 2012.
18. Silva PM, Gonçalves S, Santos NC. Defensins: antifungal lesions from eukaryotes. Front Microbiol. 2014;5:97.
19. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390-401.
20. Marijnissen RJ, Koenders MI, van de Veerdonk FL, et al. Exposure to Candida albicans polarizes a T-cell driven arthritis model towards Th17 responses, resulting in a more destructive arthritis. PLoS One. 2012;7(6):e38889.
21. International Consensus on Periprosthetic Joint Infection. Musculoskeletal Infection Society website. http://www.msis-na.org/international-consensus. Published August 1, 2013. Accessed October 16, 2016.
Septic arthritis is a common orthopedic emergency. The most common causative organism is Staphylococcus aureus. Mycotic infections, such as those involving Candida organisms, are much less common but just as debilitating. Delayed diagnosis of septic arthritis caused by Candida infection may result in increased morbidity, making treatment more challenging. Here we report a case of Candida albicans septic arthritis of the ankle and subtalar joint in a patient with diabetes mellitus (DM) and rheumatoid arthritis (RA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 52-year-old woman with type 2 DM (requiring subcutaneous insulin analogue therapy) and RA presented to a local emergency department with a 3-day history of right ankle pain after having the subtalar joint injected with steroid by a rheumatologist 4 weeks earlier. For about 2 weeks, there was purulent discharge from the peroneal sheath. The patient’s RA was being treated with prednisolone (maintenance therapy). Physical examination revealed low-grade pyrexia (37.8°C) and difficulty bearing full weight on the ankle. Clinically, the joint was not erythematous, but active and passive movements were painful. Blood tests revealed a C-reactive protein level of 98 mg/dL and a white blood cell (WBC) count of 11.3 × 109/L. Erythrocyte sedimentation rate (ESR) was not checked. The ankle underwent magnetic resonance imaging (Figures A-D).
Mycotic screening of the fluid was positive for C albicans. The patient was referred to the orthopedic team, which performed urgent arthroscopic surgical débridement, biopsy, and washout of the subtalar joint. After surgery, a 6-week course of antifungal therapy with anidulafungin was started, per specialist microbiology advice.
The septic ankle was successfully managed with arthroscopic surgical débridement followed by treatment with anidulafungin. The patient continued to make good progress and was weight-bearing when discharged home from the orthopedic unit.
Discussion
Worldwide, about 1 in 6 people has arthritis, which affects daily lifestyle and reduces quality of life. Degenerative, inflammatory, and septic arthritis each has its management challenges.1
Septic arthritis is an acute infection of the joint, usually of bacterial etiology. It can present as a polyarticular arthropathy (~15% of cases),2,3 but a monoarthropathy of the hip, knee, or ankle is more common.4The Kocher criteria are often applied to cases of suspected septic arthritis of joints, even though they were initially used to distinguish septic arthritis from transient synovitis in pediatric hip joints.5 Kocher and colleagues5 reported 4 key clinical criteria: inability to bear weight, WBC count over 12 × 109/L, ESR over 40 mm/h, and temperature over 38.5°C. When all 4 criteria are met, the predictive value is 99.6%. These criteria are now widely applied to adult joints, and not only the hips.
In septic arthritis, the most common causative pathogen is S aureus.3,6Streptococcus, Neisseria, and Pseudomonas also are common.7 Although much rarer, Candida variants and other mycotic pathogens have been implicated as well.8C albicans is a well-known fungus that colonizes mucosal surfaces. Research indicates increased oral C albicans colonization in rheumatoid patients.9 Although most Candida septic arthritis cases are caused by C albicans, there is no large body of data showing the true incidence of fungal pathogens in septic arthritis.
Our literature search yielded 2 case reports on Candida septic arthritis involving the ankle, but the causative organisms were Candida parapsilosis and Candida glabrata.9,10 Cases of Candida septic arthritis involving the knee or shoulder have also been reported.11-15 Case reports demonstrate that Candida fungal arthritis is extremely rare.9 Etiology reportedly includes direct intra-articular inoculation by surgery or secondary to hematogenous seeding, particularly in immunocompromised patients.10 Risk factors include immunosuppression and joint suppression. DM and RA are common comorbidities in patients with septic arthritis.6,16 The pathophysiology of RA is inflammatory pannus formation of the periarticular surface with subsequent articular cartilage destruction and erosion, as well as progressive deformity and functional debilitation.1Patients with DM are at increased risk for developing fungal and other infections. Factors increasing this risk include disruption of skin-barrier integrity; reduced peripheral oxygen and blood supply, which also disrupts antibiotic delivery; and hyperglycemia-induced reduction in antibody function and disruption of phagocytosis and chemotaxis.17Fungi are eukaryotic, and infections caused by these organisms are difficult to treat.18 As fungal infections are more prevalent among immunosuppressed patients, they often result in prolonged treatment without guarantee of eradication, as spores may persist subclinically.
Literature on C albicans septic arthritis is lacking in general but especially in rheumatoid patients. Delayed diagnosis and suboptimal treatment may result in fungal osteomyelitis. There is little evidence on treating this rare fungal complication, and outcomes historically have been poor.19In an animal model, Marijnissen and colleagues20 found that C albicans infection can increase destruction in an arthritic joint by cytokine environment modification. The result was advanced destruction of the joint and debilitation. For disease management, the authors considered these essential: early diagnosis, prompt treatment, and, as indicated, surgical débridement.
Treatment of Candida septic arthritis largely involves use of antifungal medication, either with surgical débridement, as in our patient’s case, or without. Which antifungal medication to use should be based on sensitivities, identified from wound aspirate, and microbiology advice about treatment duration. The antibiotic should be a broad-spectrum antifungal cover, in keeping with local antibiotic prescribing guidelines, which can be refined once definitive organism culture and sensitivity results are known. However, early aggressive treatment is essential. Periprosthetic fungal infection is rarely resolved without implant removal.21
Conclusion
This case reflects the complexities of septic arthritis caused by atypical pathogens and highlights the need for clinical vigilance in the setting of comorbidities, such as DM and RA. Failure to consider the diagnosis early on might result in delayed and inadequate treatment, increased joint destruction, and, potentially, osteomyelitis with subsequent increased morbidity. Early diagnosis (based on joint aspirate findings), surgical débridement, and prolonged aggressive treatment with antifungal medication are the mainstays of treatment.
Am J Orthop. 2016;45(7):E478-E480. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Septic arthritis is a common orthopedic emergency. The most common causative organism is Staphylococcus aureus. Mycotic infections, such as those involving Candida organisms, are much less common but just as debilitating. Delayed diagnosis of septic arthritis caused by Candida infection may result in increased morbidity, making treatment more challenging. Here we report a case of Candida albicans septic arthritis of the ankle and subtalar joint in a patient with diabetes mellitus (DM) and rheumatoid arthritis (RA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 52-year-old woman with type 2 DM (requiring subcutaneous insulin analogue therapy) and RA presented to a local emergency department with a 3-day history of right ankle pain after having the subtalar joint injected with steroid by a rheumatologist 4 weeks earlier. For about 2 weeks, there was purulent discharge from the peroneal sheath. The patient’s RA was being treated with prednisolone (maintenance therapy). Physical examination revealed low-grade pyrexia (37.8°C) and difficulty bearing full weight on the ankle. Clinically, the joint was not erythematous, but active and passive movements were painful. Blood tests revealed a C-reactive protein level of 98 mg/dL and a white blood cell (WBC) count of 11.3 × 109/L. Erythrocyte sedimentation rate (ESR) was not checked. The ankle underwent magnetic resonance imaging (Figures A-D).
Mycotic screening of the fluid was positive for C albicans. The patient was referred to the orthopedic team, which performed urgent arthroscopic surgical débridement, biopsy, and washout of the subtalar joint. After surgery, a 6-week course of antifungal therapy with anidulafungin was started, per specialist microbiology advice.
The septic ankle was successfully managed with arthroscopic surgical débridement followed by treatment with anidulafungin. The patient continued to make good progress and was weight-bearing when discharged home from the orthopedic unit.
Discussion
Worldwide, about 1 in 6 people has arthritis, which affects daily lifestyle and reduces quality of life. Degenerative, inflammatory, and septic arthritis each has its management challenges.1
Septic arthritis is an acute infection of the joint, usually of bacterial etiology. It can present as a polyarticular arthropathy (~15% of cases),2,3 but a monoarthropathy of the hip, knee, or ankle is more common.4The Kocher criteria are often applied to cases of suspected septic arthritis of joints, even though they were initially used to distinguish septic arthritis from transient synovitis in pediatric hip joints.5 Kocher and colleagues5 reported 4 key clinical criteria: inability to bear weight, WBC count over 12 × 109/L, ESR over 40 mm/h, and temperature over 38.5°C. When all 4 criteria are met, the predictive value is 99.6%. These criteria are now widely applied to adult joints, and not only the hips.
In septic arthritis, the most common causative pathogen is S aureus.3,6Streptococcus, Neisseria, and Pseudomonas also are common.7 Although much rarer, Candida variants and other mycotic pathogens have been implicated as well.8C albicans is a well-known fungus that colonizes mucosal surfaces. Research indicates increased oral C albicans colonization in rheumatoid patients.9 Although most Candida septic arthritis cases are caused by C albicans, there is no large body of data showing the true incidence of fungal pathogens in septic arthritis.
Our literature search yielded 2 case reports on Candida septic arthritis involving the ankle, but the causative organisms were Candida parapsilosis and Candida glabrata.9,10 Cases of Candida septic arthritis involving the knee or shoulder have also been reported.11-15 Case reports demonstrate that Candida fungal arthritis is extremely rare.9 Etiology reportedly includes direct intra-articular inoculation by surgery or secondary to hematogenous seeding, particularly in immunocompromised patients.10 Risk factors include immunosuppression and joint suppression. DM and RA are common comorbidities in patients with septic arthritis.6,16 The pathophysiology of RA is inflammatory pannus formation of the periarticular surface with subsequent articular cartilage destruction and erosion, as well as progressive deformity and functional debilitation.1Patients with DM are at increased risk for developing fungal and other infections. Factors increasing this risk include disruption of skin-barrier integrity; reduced peripheral oxygen and blood supply, which also disrupts antibiotic delivery; and hyperglycemia-induced reduction in antibody function and disruption of phagocytosis and chemotaxis.17Fungi are eukaryotic, and infections caused by these organisms are difficult to treat.18 As fungal infections are more prevalent among immunosuppressed patients, they often result in prolonged treatment without guarantee of eradication, as spores may persist subclinically.
Literature on C albicans septic arthritis is lacking in general but especially in rheumatoid patients. Delayed diagnosis and suboptimal treatment may result in fungal osteomyelitis. There is little evidence on treating this rare fungal complication, and outcomes historically have been poor.19In an animal model, Marijnissen and colleagues20 found that C albicans infection can increase destruction in an arthritic joint by cytokine environment modification. The result was advanced destruction of the joint and debilitation. For disease management, the authors considered these essential: early diagnosis, prompt treatment, and, as indicated, surgical débridement.
Treatment of Candida septic arthritis largely involves use of antifungal medication, either with surgical débridement, as in our patient’s case, or without. Which antifungal medication to use should be based on sensitivities, identified from wound aspirate, and microbiology advice about treatment duration. The antibiotic should be a broad-spectrum antifungal cover, in keeping with local antibiotic prescribing guidelines, which can be refined once definitive organism culture and sensitivity results are known. However, early aggressive treatment is essential. Periprosthetic fungal infection is rarely resolved without implant removal.21
Conclusion
This case reflects the complexities of septic arthritis caused by atypical pathogens and highlights the need for clinical vigilance in the setting of comorbidities, such as DM and RA. Failure to consider the diagnosis early on might result in delayed and inadequate treatment, increased joint destruction, and, potentially, osteomyelitis with subsequent increased morbidity. Early diagnosis (based on joint aspirate findings), surgical débridement, and prolonged aggressive treatment with antifungal medication are the mainstays of treatment.
Am J Orthop. 2016;45(7):E478-E480. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Auday BC, Buratovich MA, Marrocco, GF, Moglia P, eds. Magill’s Medical Guide. 7th ed. Ipswich, MA: Salem Press; 2014.
2. Dhaliwal S, LeBel ME. Rapidly progressing polyarticular septic arthritis in a patient with rheumatoid arthritis. Am J Orthop. 2012;41(7):E100-E101.
3. Mateo Soria L, Olivé Marqués A, García Casares E, García Melchor E, Holgado Pérez S, Tena Marsà X. Polyarticular septic arthritis: analysis of 19 cases [in Spanish]. Reumatol Clin. 2009;5(1):18-22.
4. Caksen H, Oztürk MK, Uzüm K, Yüksel S, Ustünbaş HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000;42(5):534-540.
5. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662-1670.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. 2014;42(2):385-391.
7. Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol. 2014;20(2):74-78.
8. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
9. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans–specific Th17 responses. Arthritis Res Ther. 2014;16(1):R50.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
11. Sung J, Chun K. Candida parapsilosis arthritis involving the ankle in a diabetes patient. J Korean Soc Radiol. 2011;64:587-591.
12. Marmor L, Peter JB. Candida arthritis of the knee joint. Clin Orthop Relat Res. 1976;(118):133-135.
13. Turgut B, Vural O, Demir M, Kaldir M. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol. 2002;81(9):529-531.
14. Christensson B, Ryd L, Dahlberg L, Lohmander S. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand. 1993;64(6):695-698.
15. Jeong YM, Cho HY, Lee SW, Hwang YM, Kim YK. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465-469.
16. Favero M, Schiavon R, Riato L, Carraro V, Punzi L. Septic arthritis: a 12 years retrospective study in a rheumatological university clinic [in Italian]. Reumatismo. 2008;60(4):260-267.
17. Leslie D, Lansang C, Coppack S, Kennedy L. Diabetes: Clinician’s Desk Reference. Boca Raton, FL: CRC Press; 2012.
18. Silva PM, Gonçalves S, Santos NC. Defensins: antifungal lesions from eukaryotes. Front Microbiol. 2014;5:97.
19. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390-401.
20. Marijnissen RJ, Koenders MI, van de Veerdonk FL, et al. Exposure to Candida albicans polarizes a T-cell driven arthritis model towards Th17 responses, resulting in a more destructive arthritis. PLoS One. 2012;7(6):e38889.
21. International Consensus on Periprosthetic Joint Infection. Musculoskeletal Infection Society website. http://www.msis-na.org/international-consensus. Published August 1, 2013. Accessed October 16, 2016.
1. Auday BC, Buratovich MA, Marrocco, GF, Moglia P, eds. Magill’s Medical Guide. 7th ed. Ipswich, MA: Salem Press; 2014.
2. Dhaliwal S, LeBel ME. Rapidly progressing polyarticular septic arthritis in a patient with rheumatoid arthritis. Am J Orthop. 2012;41(7):E100-E101.
3. Mateo Soria L, Olivé Marqués A, García Casares E, García Melchor E, Holgado Pérez S, Tena Marsà X. Polyarticular septic arthritis: analysis of 19 cases [in Spanish]. Reumatol Clin. 2009;5(1):18-22.
4. Caksen H, Oztürk MK, Uzüm K, Yüksel S, Ustünbaş HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000;42(5):534-540.
5. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662-1670.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. 2014;42(2):385-391.
7. Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol. 2014;20(2):74-78.
8. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
9. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans–specific Th17 responses. Arthritis Res Ther. 2014;16(1):R50.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
11. Sung J, Chun K. Candida parapsilosis arthritis involving the ankle in a diabetes patient. J Korean Soc Radiol. 2011;64:587-591.
12. Marmor L, Peter JB. Candida arthritis of the knee joint. Clin Orthop Relat Res. 1976;(118):133-135.
13. Turgut B, Vural O, Demir M, Kaldir M. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol. 2002;81(9):529-531.
14. Christensson B, Ryd L, Dahlberg L, Lohmander S. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand. 1993;64(6):695-698.
15. Jeong YM, Cho HY, Lee SW, Hwang YM, Kim YK. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465-469.
16. Favero M, Schiavon R, Riato L, Carraro V, Punzi L. Septic arthritis: a 12 years retrospective study in a rheumatological university clinic [in Italian]. Reumatismo. 2008;60(4):260-267.
17. Leslie D, Lansang C, Coppack S, Kennedy L. Diabetes: Clinician’s Desk Reference. Boca Raton, FL: CRC Press; 2012.
18. Silva PM, Gonçalves S, Santos NC. Defensins: antifungal lesions from eukaryotes. Front Microbiol. 2014;5:97.
19. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390-401.
20. Marijnissen RJ, Koenders MI, van de Veerdonk FL, et al. Exposure to Candida albicans polarizes a T-cell driven arthritis model towards Th17 responses, resulting in a more destructive arthritis. PLoS One. 2012;7(6):e38889.
21. International Consensus on Periprosthetic Joint Infection. Musculoskeletal Infection Society website. http://www.msis-na.org/international-consensus. Published August 1, 2013. Accessed October 16, 2016.