User login
Reintubation avoided by majority of patients on noninvasive ventilation therapy, high-flow oxygen
Extubated patients who either received noninvasive ventilation (NIV) therapy or high-flow nasal cannula oxygen had a lower risk of reintubation, compared with extubated patients who received some form of standard oxygen therapy, according to the results of two multicenter, randomized clinical trials published online in JAMA.
Participants in one of the studies, which included abdominal surgery patients diagnosed with respiratory failure within 7 days following surgery, either received NIV or standard oxygen therapy for 30 days or until ICU discharge, whichever came first. While NIV has been effectively used to treat nonsurgical patients with acute exacerbations of chronic obstructive pulmonary disease and cardiogenic pulmonary edema, there is no evidence to support the use of NIV in surgical patients with hypoxemic acute respiratory failure after abdominal surgery, according to Dr. Samir Jaber of the Saint Eloi University Hospital and Montpellier School of Medicine, both in Montpellier, France, and his colleagues (JAMA. 2016 Apr 5;315[13]:1345-53).
The second study included adult patients who had received mechanical ventilation for more than 12 hours and who met criteria for being considered at low risk for reintubation. Patients were administered either high-flow oxygen therapy through nasal cannula immediately after extubation or continuous conventional oxygen therapy through nasal cannula or nonrebreather facemask; the patients were observed for 72 hours. High-flow therapy has been shown to improve oxygenation and survival in clinical studies of critically ill patients in the acute phase of respiratory failure. “[A study by S.M. Maggiore and his colleagues (Am J Respir Crit Care Med. 2014;190(3):282-8)] suggested that high-flow therapy after planned extubation decreased the reintubation rate in a general population of critical patients, but the benefits might be mainly attributable to improvements in high-risk patients,” said Dr. Gonzalo Hernandez, of the Hospital Virgen de la Salud, Toledo, Spain, and his colleagues (JAMA. 2016 Apr 5;315[13]:1354-61).
In the first study, 148 patients received NIV and 145 patients received standard oxygen therapy only. NIV was administered through a facemask connected to an ICU- or a NIV-dedicated ventilator, using either a heated humidifier or heat and moisture exchanger to warm and humidify inspired gases. Patients were encouraged to use NIV for 6 hours during the first 24 hours of the study and received standard oxygen therapy at a rate of up to 15 L/minute to maintain an arterial oxygen saturation estimate (SpO2) of at least 94% in between NIV sessions. NIV was started at an inspiratory positive airway pressure of 5 cm H2O, increasing to a maximum inspiratory pressure of 15 cm H2O, aiming to achieve an expiratory tidal volume between 6 and 8 mL/kg of predicted body weight and a respiratory rate lower than 25/min. The patients in this study’s control group only received the standard oxygen therapy.
In the other study, 263 patients received conventional therapy, with the oxygen flow having been adjusted to maintain an arterial oxygen saturation estimate of greater than 92%. This study’s other 264 patients received high-flow oxygen therapy, with the flow having been initially set at 10 L/min and titrated upward in 5-L/min steps until patients experienced discomfort. The high-flow therapy was stopped after 24 hours and was followed by conventional oxygen therapy, when needed.
The primary outcome measure in the study involving NIV was cause for reintubation within 7 days of randomization.
Secondary outcome measures included gas exchange, healthcare-associated infection rate within 30 days, number of ventilator-free days between days 1 and 30, antibiotic use duration, ICU and in-hospital length of stay, and 30- and 90-day mortality.
Reintubation occurred in 49 patients in the NIV group and 66 patients in the standard oxygen therapy group, a significant difference (P = .03). Among the reintubated patients, those who had received NIV spent less time under invasive mechanical ventilation as did the patients given standard oxygen therapy. The interquartile ranges of days of invasive mechanical ventilation were 0-3 for patients in the NIV group and 0-5 for patients in the standard oxygen therapy group (P = .05). At 30 days, NIV was associated with significantly more ventilator-free days than standard oxygen therapy (25.4 vs. 23.2; P = .04). At 90 days, 22 patients in the NIV group and 31 patients in the standard oxygen therapy group had died (P = .15).
“Recent high-impact trials have demonstrated the benefits in nonsurgical hypoxemic respiratory failure or equivalence of high-flow nasal cannula compared with NIV in patients after cardiothoracic surgery with moderate to severe hypoxemia. Future studies comparing use of high-flow oxygen cannula vs standard oxygen therapy and NIV for patients after abdominal surgery as preventive (prophylactic) or curative applications are needed,” according to Dr. Jaber and his colleagues.
The primary outcome measure for the study of patients receiving high-flow oxygen therapy was reintubation within 72 hours after extubation; this occurred in fewer patients in the high-flow oxygen group than in the conventional therapy group (13 or 4.9% vs. 32 or 12.2%.) This statistically significant difference was mainly attributable to a lower incidence of respiratory-related reintubation in the high-flow group, compared with the conventional therapy group (1.5% vs. 8.7%), said Dr. Hernandez and his colleagues.
Secondary outcome measures included postextubation respiratory failure, respiratory infection, sepsis, multiorgan failure, ICU and hospital length of stay and mortality, time to reintubation, and adverse effects. Postintubation respiratory failure was less common in the high-flow therapy group than in the conventional therapy group (22 patients or 8.3% vs. 38 or 14.4%). Differences between the two groups in other secondary outcomes were not statistically significant.
“The main finding of this study was that high-flow oxygen significantly reduced the reintubation rate in critically ill patients at low risk for extubation failure ... High-flow therapy improves oxygenation, and the lower rate of reintubation secondary to hypoxia in the high-flow group corroborates this finding. High-flow oxygen also seems to reduce other causes of respiratory failure such as increased work of breathing and respiratory muscle fatigue, which are frequently associated with reintubation secondary to hypoxia. Another way in which high-flow therapy improves extubation outcome is by conditioning the inspired gas,” said Dr. Hernandez and his colleagues.
No adverse events were reported in either study.
Dr. Hernandez and his colleagues reported no conflicts of interest. Dr. Jaber and his colleagues disclosed no potential conflicts of interest with their study’s sponsors, Montpellier (France) University Hospital and the APARD Foundation.
Dr. Eric Gartman, FCCP, comments: These two studies augment a growing body of literature supporting the use of adjunctive therapies immediately following extubation to prevent reintubation for respiratory failure.
It has been known for several years that the use of noninvasive ventilation (NIV) immediately after extubation in COPD patients prevents reintubation rates, and these new data demonstrate efficacy in an expanded population. Further, the use of high-flow humidified oxygen therapy in acute respiratory failure has been shown to prevent progression to initial intubation, and now these data expand potential use to prevent reintubation, as well.
While not studied, if high-flow oxygen therapy is found to be equivalent to NIV to prevent reintubation (similar to the previously-published prevention of intubation studies), that would be clinically important since there is a significant difference in tolerance to these two therapies. Across these trials, the very important point to remember is that these therapies were found to be effective if put on directly after extubation, and one cannot wait to apply them at the point where the patient shows signs of respiratory decline.
Dr. Eric Gartman, FCCP, comments: These two studies augment a growing body of literature supporting the use of adjunctive therapies immediately following extubation to prevent reintubation for respiratory failure.
It has been known for several years that the use of noninvasive ventilation (NIV) immediately after extubation in COPD patients prevents reintubation rates, and these new data demonstrate efficacy in an expanded population. Further, the use of high-flow humidified oxygen therapy in acute respiratory failure has been shown to prevent progression to initial intubation, and now these data expand potential use to prevent reintubation, as well.
While not studied, if high-flow oxygen therapy is found to be equivalent to NIV to prevent reintubation (similar to the previously-published prevention of intubation studies), that would be clinically important since there is a significant difference in tolerance to these two therapies. Across these trials, the very important point to remember is that these therapies were found to be effective if put on directly after extubation, and one cannot wait to apply them at the point where the patient shows signs of respiratory decline.
Dr. Eric Gartman, FCCP, comments: These two studies augment a growing body of literature supporting the use of adjunctive therapies immediately following extubation to prevent reintubation for respiratory failure.
It has been known for several years that the use of noninvasive ventilation (NIV) immediately after extubation in COPD patients prevents reintubation rates, and these new data demonstrate efficacy in an expanded population. Further, the use of high-flow humidified oxygen therapy in acute respiratory failure has been shown to prevent progression to initial intubation, and now these data expand potential use to prevent reintubation, as well.
While not studied, if high-flow oxygen therapy is found to be equivalent to NIV to prevent reintubation (similar to the previously-published prevention of intubation studies), that would be clinically important since there is a significant difference in tolerance to these two therapies. Across these trials, the very important point to remember is that these therapies were found to be effective if put on directly after extubation, and one cannot wait to apply them at the point where the patient shows signs of respiratory decline.
Extubated patients who either received noninvasive ventilation (NIV) therapy or high-flow nasal cannula oxygen had a lower risk of reintubation, compared with extubated patients who received some form of standard oxygen therapy, according to the results of two multicenter, randomized clinical trials published online in JAMA.
Participants in one of the studies, which included abdominal surgery patients diagnosed with respiratory failure within 7 days following surgery, either received NIV or standard oxygen therapy for 30 days or until ICU discharge, whichever came first. While NIV has been effectively used to treat nonsurgical patients with acute exacerbations of chronic obstructive pulmonary disease and cardiogenic pulmonary edema, there is no evidence to support the use of NIV in surgical patients with hypoxemic acute respiratory failure after abdominal surgery, according to Dr. Samir Jaber of the Saint Eloi University Hospital and Montpellier School of Medicine, both in Montpellier, France, and his colleagues (JAMA. 2016 Apr 5;315[13]:1345-53).
The second study included adult patients who had received mechanical ventilation for more than 12 hours and who met criteria for being considered at low risk for reintubation. Patients were administered either high-flow oxygen therapy through nasal cannula immediately after extubation or continuous conventional oxygen therapy through nasal cannula or nonrebreather facemask; the patients were observed for 72 hours. High-flow therapy has been shown to improve oxygenation and survival in clinical studies of critically ill patients in the acute phase of respiratory failure. “[A study by S.M. Maggiore and his colleagues (Am J Respir Crit Care Med. 2014;190(3):282-8)] suggested that high-flow therapy after planned extubation decreased the reintubation rate in a general population of critical patients, but the benefits might be mainly attributable to improvements in high-risk patients,” said Dr. Gonzalo Hernandez, of the Hospital Virgen de la Salud, Toledo, Spain, and his colleagues (JAMA. 2016 Apr 5;315[13]:1354-61).
In the first study, 148 patients received NIV and 145 patients received standard oxygen therapy only. NIV was administered through a facemask connected to an ICU- or a NIV-dedicated ventilator, using either a heated humidifier or heat and moisture exchanger to warm and humidify inspired gases. Patients were encouraged to use NIV for 6 hours during the first 24 hours of the study and received standard oxygen therapy at a rate of up to 15 L/minute to maintain an arterial oxygen saturation estimate (SpO2) of at least 94% in between NIV sessions. NIV was started at an inspiratory positive airway pressure of 5 cm H2O, increasing to a maximum inspiratory pressure of 15 cm H2O, aiming to achieve an expiratory tidal volume between 6 and 8 mL/kg of predicted body weight and a respiratory rate lower than 25/min. The patients in this study’s control group only received the standard oxygen therapy.
In the other study, 263 patients received conventional therapy, with the oxygen flow having been adjusted to maintain an arterial oxygen saturation estimate of greater than 92%. This study’s other 264 patients received high-flow oxygen therapy, with the flow having been initially set at 10 L/min and titrated upward in 5-L/min steps until patients experienced discomfort. The high-flow therapy was stopped after 24 hours and was followed by conventional oxygen therapy, when needed.
The primary outcome measure in the study involving NIV was cause for reintubation within 7 days of randomization.
Secondary outcome measures included gas exchange, healthcare-associated infection rate within 30 days, number of ventilator-free days between days 1 and 30, antibiotic use duration, ICU and in-hospital length of stay, and 30- and 90-day mortality.
Reintubation occurred in 49 patients in the NIV group and 66 patients in the standard oxygen therapy group, a significant difference (P = .03). Among the reintubated patients, those who had received NIV spent less time under invasive mechanical ventilation as did the patients given standard oxygen therapy. The interquartile ranges of days of invasive mechanical ventilation were 0-3 for patients in the NIV group and 0-5 for patients in the standard oxygen therapy group (P = .05). At 30 days, NIV was associated with significantly more ventilator-free days than standard oxygen therapy (25.4 vs. 23.2; P = .04). At 90 days, 22 patients in the NIV group and 31 patients in the standard oxygen therapy group had died (P = .15).
“Recent high-impact trials have demonstrated the benefits in nonsurgical hypoxemic respiratory failure or equivalence of high-flow nasal cannula compared with NIV in patients after cardiothoracic surgery with moderate to severe hypoxemia. Future studies comparing use of high-flow oxygen cannula vs standard oxygen therapy and NIV for patients after abdominal surgery as preventive (prophylactic) or curative applications are needed,” according to Dr. Jaber and his colleagues.
The primary outcome measure for the study of patients receiving high-flow oxygen therapy was reintubation within 72 hours after extubation; this occurred in fewer patients in the high-flow oxygen group than in the conventional therapy group (13 or 4.9% vs. 32 or 12.2%.) This statistically significant difference was mainly attributable to a lower incidence of respiratory-related reintubation in the high-flow group, compared with the conventional therapy group (1.5% vs. 8.7%), said Dr. Hernandez and his colleagues.
Secondary outcome measures included postextubation respiratory failure, respiratory infection, sepsis, multiorgan failure, ICU and hospital length of stay and mortality, time to reintubation, and adverse effects. Postintubation respiratory failure was less common in the high-flow therapy group than in the conventional therapy group (22 patients or 8.3% vs. 38 or 14.4%). Differences between the two groups in other secondary outcomes were not statistically significant.
“The main finding of this study was that high-flow oxygen significantly reduced the reintubation rate in critically ill patients at low risk for extubation failure ... High-flow therapy improves oxygenation, and the lower rate of reintubation secondary to hypoxia in the high-flow group corroborates this finding. High-flow oxygen also seems to reduce other causes of respiratory failure such as increased work of breathing and respiratory muscle fatigue, which are frequently associated with reintubation secondary to hypoxia. Another way in which high-flow therapy improves extubation outcome is by conditioning the inspired gas,” said Dr. Hernandez and his colleagues.
No adverse events were reported in either study.
Dr. Hernandez and his colleagues reported no conflicts of interest. Dr. Jaber and his colleagues disclosed no potential conflicts of interest with their study’s sponsors, Montpellier (France) University Hospital and the APARD Foundation.
Extubated patients who either received noninvasive ventilation (NIV) therapy or high-flow nasal cannula oxygen had a lower risk of reintubation, compared with extubated patients who received some form of standard oxygen therapy, according to the results of two multicenter, randomized clinical trials published online in JAMA.
Participants in one of the studies, which included abdominal surgery patients diagnosed with respiratory failure within 7 days following surgery, either received NIV or standard oxygen therapy for 30 days or until ICU discharge, whichever came first. While NIV has been effectively used to treat nonsurgical patients with acute exacerbations of chronic obstructive pulmonary disease and cardiogenic pulmonary edema, there is no evidence to support the use of NIV in surgical patients with hypoxemic acute respiratory failure after abdominal surgery, according to Dr. Samir Jaber of the Saint Eloi University Hospital and Montpellier School of Medicine, both in Montpellier, France, and his colleagues (JAMA. 2016 Apr 5;315[13]:1345-53).
The second study included adult patients who had received mechanical ventilation for more than 12 hours and who met criteria for being considered at low risk for reintubation. Patients were administered either high-flow oxygen therapy through nasal cannula immediately after extubation or continuous conventional oxygen therapy through nasal cannula or nonrebreather facemask; the patients were observed for 72 hours. High-flow therapy has been shown to improve oxygenation and survival in clinical studies of critically ill patients in the acute phase of respiratory failure. “[A study by S.M. Maggiore and his colleagues (Am J Respir Crit Care Med. 2014;190(3):282-8)] suggested that high-flow therapy after planned extubation decreased the reintubation rate in a general population of critical patients, but the benefits might be mainly attributable to improvements in high-risk patients,” said Dr. Gonzalo Hernandez, of the Hospital Virgen de la Salud, Toledo, Spain, and his colleagues (JAMA. 2016 Apr 5;315[13]:1354-61).
In the first study, 148 patients received NIV and 145 patients received standard oxygen therapy only. NIV was administered through a facemask connected to an ICU- or a NIV-dedicated ventilator, using either a heated humidifier or heat and moisture exchanger to warm and humidify inspired gases. Patients were encouraged to use NIV for 6 hours during the first 24 hours of the study and received standard oxygen therapy at a rate of up to 15 L/minute to maintain an arterial oxygen saturation estimate (SpO2) of at least 94% in between NIV sessions. NIV was started at an inspiratory positive airway pressure of 5 cm H2O, increasing to a maximum inspiratory pressure of 15 cm H2O, aiming to achieve an expiratory tidal volume between 6 and 8 mL/kg of predicted body weight and a respiratory rate lower than 25/min. The patients in this study’s control group only received the standard oxygen therapy.
In the other study, 263 patients received conventional therapy, with the oxygen flow having been adjusted to maintain an arterial oxygen saturation estimate of greater than 92%. This study’s other 264 patients received high-flow oxygen therapy, with the flow having been initially set at 10 L/min and titrated upward in 5-L/min steps until patients experienced discomfort. The high-flow therapy was stopped after 24 hours and was followed by conventional oxygen therapy, when needed.
The primary outcome measure in the study involving NIV was cause for reintubation within 7 days of randomization.
Secondary outcome measures included gas exchange, healthcare-associated infection rate within 30 days, number of ventilator-free days between days 1 and 30, antibiotic use duration, ICU and in-hospital length of stay, and 30- and 90-day mortality.
Reintubation occurred in 49 patients in the NIV group and 66 patients in the standard oxygen therapy group, a significant difference (P = .03). Among the reintubated patients, those who had received NIV spent less time under invasive mechanical ventilation as did the patients given standard oxygen therapy. The interquartile ranges of days of invasive mechanical ventilation were 0-3 for patients in the NIV group and 0-5 for patients in the standard oxygen therapy group (P = .05). At 30 days, NIV was associated with significantly more ventilator-free days than standard oxygen therapy (25.4 vs. 23.2; P = .04). At 90 days, 22 patients in the NIV group and 31 patients in the standard oxygen therapy group had died (P = .15).
“Recent high-impact trials have demonstrated the benefits in nonsurgical hypoxemic respiratory failure or equivalence of high-flow nasal cannula compared with NIV in patients after cardiothoracic surgery with moderate to severe hypoxemia. Future studies comparing use of high-flow oxygen cannula vs standard oxygen therapy and NIV for patients after abdominal surgery as preventive (prophylactic) or curative applications are needed,” according to Dr. Jaber and his colleagues.
The primary outcome measure for the study of patients receiving high-flow oxygen therapy was reintubation within 72 hours after extubation; this occurred in fewer patients in the high-flow oxygen group than in the conventional therapy group (13 or 4.9% vs. 32 or 12.2%.) This statistically significant difference was mainly attributable to a lower incidence of respiratory-related reintubation in the high-flow group, compared with the conventional therapy group (1.5% vs. 8.7%), said Dr. Hernandez and his colleagues.
Secondary outcome measures included postextubation respiratory failure, respiratory infection, sepsis, multiorgan failure, ICU and hospital length of stay and mortality, time to reintubation, and adverse effects. Postintubation respiratory failure was less common in the high-flow therapy group than in the conventional therapy group (22 patients or 8.3% vs. 38 or 14.4%). Differences between the two groups in other secondary outcomes were not statistically significant.
“The main finding of this study was that high-flow oxygen significantly reduced the reintubation rate in critically ill patients at low risk for extubation failure ... High-flow therapy improves oxygenation, and the lower rate of reintubation secondary to hypoxia in the high-flow group corroborates this finding. High-flow oxygen also seems to reduce other causes of respiratory failure such as increased work of breathing and respiratory muscle fatigue, which are frequently associated with reintubation secondary to hypoxia. Another way in which high-flow therapy improves extubation outcome is by conditioning the inspired gas,” said Dr. Hernandez and his colleagues.
No adverse events were reported in either study.
Dr. Hernandez and his colleagues reported no conflicts of interest. Dr. Jaber and his colleagues disclosed no potential conflicts of interest with their study’s sponsors, Montpellier (France) University Hospital and the APARD Foundation.
FROM JAMA
Key clinical point: Extubated patients who either received noninvasive ventilation (NIV) therapy or high-flow nasal cannula oxygen reduced their risk of reintubation, compared with patients who received some form of standard oxygen therapy.
Major finding: In one study, significantly fewer of the patients who received NIV needed to be reintubated than the patients who received standard oxygen therapy.
Data source: Two multicenter, randomized clinical trials published online in JAMA.
Disclosures: Dr. Hernandez and his colleagues reported no conflicts of interest. Dr. Jaber and his colleagues disclosed no potential conflicts of interest with Montpellier (France) University Hospital and the APARD Foundation, who funded their study.
Negative sestamibi scan for primary hyperparathyroidism can mean no referral or surgery
BALTIMORE – In the treatment of primary hyperparathyroidism, clinical guidelines recommend using sestamibi scan for localizing adenoma, but increasingly endocrinologists are using sestamibi results to determine whether or not to refer a patient for parathyroidectomy surgery, while surgeons are using the scans as a factor in deciding whether to perform the operation.
That was the conclusion of a paper Dr. Susana Wu presented at the American Association of Endocrine Surgeons annual meeting. Dr. Wu reported on behalf of her colleagues at Kaiser Permanente Los Angeles Medical Center and at Scripps Clinic in San Diego.
“This study suggests that negative sestamibi scan (SS) results influence management of patients with primary hyperparathyroidism,” Dr. Wu said. “Endocrinologists were less likely to refer to surgeons and surgeons were less likely to offer parathyroidectomy to a patient with a negative sestamibi scan.”
The study involved a retrospective chart review of all 539 patients with primary hyperparathyroidism in the Kaiser Permanente Southern California database from December 2011 to December 2013, 452 of whom were seen by 63 endocrinologists at 14 centers. Among these patients, 260 had SS – 120 negative and 140 positive. The study identified statistically significant variations in how both endocrinologists and surgeons managed patients depending on SS results. The researchers used Kaiser Permanente’s electronic referral system to track referrals.
“The most significant negative predictor for endocrinologists referring to surgeons was a negative sestamibi scan, with an odds ratio of 0.36,” Dr. Wu said.
Endocrinologists referred 86% of patients with positive SS to surgeons, but only 68% of those with negative SS. Surgeons exhibited a similar practice pattern. “Surgeons were less likely to recommend parathyroidectomy for patients with a negative sestamibi scan, with an odds ratio of 0.20,” Dr. Wu said. Surgeons operated on 87% of patients with a negative SS scan but 96% with a positive SS.
In an interview, study coauthor Dr. Philip Haigh explained that parathyroidectomy when the SS is negative is a more difficult operation for the surgeon, and that might make some physicians hesitate before going forward with surgery. “It has been previously shown by other studies that it is a more difficult operation when the sestamibi scan is negative because you have to look at four glands instead of removing just one, but if the surgeon is experienced, it should achieve a high success rate,” Dr. Haigh said. He said that parathyroidectomy in sestamibi-negative hyperparathyroidism had a cure rate as high as 98% in the study presented.
He offered two thoughts on how clinicians should use the study results. “To the endocrinologist, if you’re going to order a sestamibi scan, don’t change your referral practice depending on the result,” Dr. Haigh said. “To the surgeon, if you’re not comfortable operating on a patient with a negative sestamibi scan, then find someone who is.”
The study had a few limitations, Dr. Wu said. Along with its retrospective nature, the study also did not account for potential disparity in radiological vs. surgeon interpretation of the scans.
During the discussion, Dr. Samuel Snyder, of Baylor Scott & White Health, Temple, Tex., said he concurred with the results Dr. Wu reported. “It really worries me about what is happening to patients who have negative scans,” he said. “What I’ve seen in patients referred for surgery is a lot of variation in how the sestamibi scan is done.” He asked if the study accounted for the different types of sestamibi scans and how they were performed, but Dr. Wu said it did not.
Dr. Christopher McHenry of MetroHealth Medical Center, Cleveland, also concurred. “I think this is a phenomenon that occurs more often than we think or we’re aware of,” he said. “I continue to be amazed with how clinicians equate a negative sestamibi scan with not having primary hyperparathyroidism. I think it needs to reemphasized that the sestamibi scan is not diagnostic; it’s for localization.”
He asked Dr. Wu, “How do we change behavior to deal with this problem?”
Dr. Wu said her institution is developing a safety-net program that would aim to increase the identification and chart coding of patients with primary hyperparathyroidism, automate essential labs to be ordered in patients with high calcium, and automate referral to endocrinologists. The study and its findings will be disseminated to endocrinologists in the region.
The study authors had no disclosures.
BALTIMORE – In the treatment of primary hyperparathyroidism, clinical guidelines recommend using sestamibi scan for localizing adenoma, but increasingly endocrinologists are using sestamibi results to determine whether or not to refer a patient for parathyroidectomy surgery, while surgeons are using the scans as a factor in deciding whether to perform the operation.
That was the conclusion of a paper Dr. Susana Wu presented at the American Association of Endocrine Surgeons annual meeting. Dr. Wu reported on behalf of her colleagues at Kaiser Permanente Los Angeles Medical Center and at Scripps Clinic in San Diego.
“This study suggests that negative sestamibi scan (SS) results influence management of patients with primary hyperparathyroidism,” Dr. Wu said. “Endocrinologists were less likely to refer to surgeons and surgeons were less likely to offer parathyroidectomy to a patient with a negative sestamibi scan.”
The study involved a retrospective chart review of all 539 patients with primary hyperparathyroidism in the Kaiser Permanente Southern California database from December 2011 to December 2013, 452 of whom were seen by 63 endocrinologists at 14 centers. Among these patients, 260 had SS – 120 negative and 140 positive. The study identified statistically significant variations in how both endocrinologists and surgeons managed patients depending on SS results. The researchers used Kaiser Permanente’s electronic referral system to track referrals.
“The most significant negative predictor for endocrinologists referring to surgeons was a negative sestamibi scan, with an odds ratio of 0.36,” Dr. Wu said.
Endocrinologists referred 86% of patients with positive SS to surgeons, but only 68% of those with negative SS. Surgeons exhibited a similar practice pattern. “Surgeons were less likely to recommend parathyroidectomy for patients with a negative sestamibi scan, with an odds ratio of 0.20,” Dr. Wu said. Surgeons operated on 87% of patients with a negative SS scan but 96% with a positive SS.
In an interview, study coauthor Dr. Philip Haigh explained that parathyroidectomy when the SS is negative is a more difficult operation for the surgeon, and that might make some physicians hesitate before going forward with surgery. “It has been previously shown by other studies that it is a more difficult operation when the sestamibi scan is negative because you have to look at four glands instead of removing just one, but if the surgeon is experienced, it should achieve a high success rate,” Dr. Haigh said. He said that parathyroidectomy in sestamibi-negative hyperparathyroidism had a cure rate as high as 98% in the study presented.
He offered two thoughts on how clinicians should use the study results. “To the endocrinologist, if you’re going to order a sestamibi scan, don’t change your referral practice depending on the result,” Dr. Haigh said. “To the surgeon, if you’re not comfortable operating on a patient with a negative sestamibi scan, then find someone who is.”
The study had a few limitations, Dr. Wu said. Along with its retrospective nature, the study also did not account for potential disparity in radiological vs. surgeon interpretation of the scans.
During the discussion, Dr. Samuel Snyder, of Baylor Scott & White Health, Temple, Tex., said he concurred with the results Dr. Wu reported. “It really worries me about what is happening to patients who have negative scans,” he said. “What I’ve seen in patients referred for surgery is a lot of variation in how the sestamibi scan is done.” He asked if the study accounted for the different types of sestamibi scans and how they were performed, but Dr. Wu said it did not.
Dr. Christopher McHenry of MetroHealth Medical Center, Cleveland, also concurred. “I think this is a phenomenon that occurs more often than we think or we’re aware of,” he said. “I continue to be amazed with how clinicians equate a negative sestamibi scan with not having primary hyperparathyroidism. I think it needs to reemphasized that the sestamibi scan is not diagnostic; it’s for localization.”
He asked Dr. Wu, “How do we change behavior to deal with this problem?”
Dr. Wu said her institution is developing a safety-net program that would aim to increase the identification and chart coding of patients with primary hyperparathyroidism, automate essential labs to be ordered in patients with high calcium, and automate referral to endocrinologists. The study and its findings will be disseminated to endocrinologists in the region.
The study authors had no disclosures.
BALTIMORE – In the treatment of primary hyperparathyroidism, clinical guidelines recommend using sestamibi scan for localizing adenoma, but increasingly endocrinologists are using sestamibi results to determine whether or not to refer a patient for parathyroidectomy surgery, while surgeons are using the scans as a factor in deciding whether to perform the operation.
That was the conclusion of a paper Dr. Susana Wu presented at the American Association of Endocrine Surgeons annual meeting. Dr. Wu reported on behalf of her colleagues at Kaiser Permanente Los Angeles Medical Center and at Scripps Clinic in San Diego.
“This study suggests that negative sestamibi scan (SS) results influence management of patients with primary hyperparathyroidism,” Dr. Wu said. “Endocrinologists were less likely to refer to surgeons and surgeons were less likely to offer parathyroidectomy to a patient with a negative sestamibi scan.”
The study involved a retrospective chart review of all 539 patients with primary hyperparathyroidism in the Kaiser Permanente Southern California database from December 2011 to December 2013, 452 of whom were seen by 63 endocrinologists at 14 centers. Among these patients, 260 had SS – 120 negative and 140 positive. The study identified statistically significant variations in how both endocrinologists and surgeons managed patients depending on SS results. The researchers used Kaiser Permanente’s electronic referral system to track referrals.
“The most significant negative predictor for endocrinologists referring to surgeons was a negative sestamibi scan, with an odds ratio of 0.36,” Dr. Wu said.
Endocrinologists referred 86% of patients with positive SS to surgeons, but only 68% of those with negative SS. Surgeons exhibited a similar practice pattern. “Surgeons were less likely to recommend parathyroidectomy for patients with a negative sestamibi scan, with an odds ratio of 0.20,” Dr. Wu said. Surgeons operated on 87% of patients with a negative SS scan but 96% with a positive SS.
In an interview, study coauthor Dr. Philip Haigh explained that parathyroidectomy when the SS is negative is a more difficult operation for the surgeon, and that might make some physicians hesitate before going forward with surgery. “It has been previously shown by other studies that it is a more difficult operation when the sestamibi scan is negative because you have to look at four glands instead of removing just one, but if the surgeon is experienced, it should achieve a high success rate,” Dr. Haigh said. He said that parathyroidectomy in sestamibi-negative hyperparathyroidism had a cure rate as high as 98% in the study presented.
He offered two thoughts on how clinicians should use the study results. “To the endocrinologist, if you’re going to order a sestamibi scan, don’t change your referral practice depending on the result,” Dr. Haigh said. “To the surgeon, if you’re not comfortable operating on a patient with a negative sestamibi scan, then find someone who is.”
The study had a few limitations, Dr. Wu said. Along with its retrospective nature, the study also did not account for potential disparity in radiological vs. surgeon interpretation of the scans.
During the discussion, Dr. Samuel Snyder, of Baylor Scott & White Health, Temple, Tex., said he concurred with the results Dr. Wu reported. “It really worries me about what is happening to patients who have negative scans,” he said. “What I’ve seen in patients referred for surgery is a lot of variation in how the sestamibi scan is done.” He asked if the study accounted for the different types of sestamibi scans and how they were performed, but Dr. Wu said it did not.
Dr. Christopher McHenry of MetroHealth Medical Center, Cleveland, also concurred. “I think this is a phenomenon that occurs more often than we think or we’re aware of,” he said. “I continue to be amazed with how clinicians equate a negative sestamibi scan with not having primary hyperparathyroidism. I think it needs to reemphasized that the sestamibi scan is not diagnostic; it’s for localization.”
He asked Dr. Wu, “How do we change behavior to deal with this problem?”
Dr. Wu said her institution is developing a safety-net program that would aim to increase the identification and chart coding of patients with primary hyperparathyroidism, automate essential labs to be ordered in patients with high calcium, and automate referral to endocrinologists. The study and its findings will be disseminated to endocrinologists in the region.
The study authors had no disclosures.
AT AAES 2016
Key clinical point: Endocrinologists and surgeons are less likely to order surgery when patients with primary hyperparathyroidism have negative sestamibi scan (SS) results.
Major finding: Endocrinologists referred 86% of patients with positive SS to surgeons, but only 68% of those with negative SS.
Data source: A retrospective chart review of all 539 patients with primary hyperparathyroidism in the Kaiser Permanente Southern California database over a 2-year period.
Disclosures: Dr. Wu and her coauthors reported having no financial disclosures.
Fresh Press: ACS Surgery News April digital issue is available
The April issue of ACS Surgery News is available online. Use the mobile app to download or view as a pdf.
This month’s issue features a story on an underrecognized problem among surgeons: chronic pain in the hands, neck, and back due to long hours of operating. In a related story, the AAOS has issued guidelines on, and rated the strength of, evidence for treatment of carpal tunnel syndrome.
Don’t miss the commentary from Dr. Layton F. Rikkers, Editor-in-Chief of ACS Surgery News, on the value of input from the quiet or introverted members of a team, and how to elicit their participation.
The April issue of ACS Surgery News is available online. Use the mobile app to download or view as a pdf.
This month’s issue features a story on an underrecognized problem among surgeons: chronic pain in the hands, neck, and back due to long hours of operating. In a related story, the AAOS has issued guidelines on, and rated the strength of, evidence for treatment of carpal tunnel syndrome.
Don’t miss the commentary from Dr. Layton F. Rikkers, Editor-in-Chief of ACS Surgery News, on the value of input from the quiet or introverted members of a team, and how to elicit their participation.
The April issue of ACS Surgery News is available online. Use the mobile app to download or view as a pdf.
This month’s issue features a story on an underrecognized problem among surgeons: chronic pain in the hands, neck, and back due to long hours of operating. In a related story, the AAOS has issued guidelines on, and rated the strength of, evidence for treatment of carpal tunnel syndrome.
Don’t miss the commentary from Dr. Layton F. Rikkers, Editor-in-Chief of ACS Surgery News, on the value of input from the quiet or introverted members of a team, and how to elicit their participation.
STAMPEDE: Metabolic surgery bests medical therapy long term
CHICAGO – The superiority of metabolic surgery over intensive medical therapy for achieving glycemic control in patients with type 2 diabetes was largely maintained at the final 5-year follow-up evaluation in the randomized, controlled STAMPEDE trial.
The 150 subjects, who had “fairly severe diabetes” with an average disease duration of 8 years, were randomized to receive intensive medical therapy alone, or intensive medical therapy with Roux-en-Y gastric bypass surgery or sleeve gastrectomy surgery. The primary endpoint of hemoglobin A1c less than 6% was achieved in 5%, 29%, and 23% of patients in the groups, respectively. The difference was statistically significant in favor of both types of surgery, Dr. Philip Raymond Schauer reported at the annual meeting of the American College of Cardiology.
Furthermore, patients in the surgery groups fared better than those in the intensive medical therapy group on several other measures, including disease remission (defied as HbA1c less than 6% without diabetes medication), HbA1c less than 7% (the American Diabetes Association target for therapy), change in fasting plasma glucose from baseline, and changes in high- and low-density lipoprotein cholesterol levels, said Dr. Schauer, director of the Cleveland Clinic Bariatric and Metabolic Institute.
Patients in the surgery groups also experienced a significantly greater reduction in the use of antihypertensive medications and lipid-lowering agents, he added.
The “very dramatic drop” in HbA1c seen early on in the surgical patients was, for the most part, sustained out to 5 years, he said.
The results for both surgeries were significantly better than those for intensive medical therapy, but the results with gastric bypass were more effective at 5 years than were those for sleeve gastrectomy, he added, noting that the surgery patients had better quality of life, compared with the intensive medical therapy patients.
As for adverse events in the surgery groups, no perioperative deaths occurred, and while there were some surgical complications, none resulted in long-term disability, Dr. Schauer said.
Anemia was more common in the surgery patients, but was fairly mild. The most common complication was weight gain in 20% of patients, and the overall reoperation rate was 7%.
Of note, patients in the study had body mass index ranging from 27 to 43 kg/m2, and those with BMI less than 35 had similar benefits as those with more severe obesity. This is important, as many insurance companies won’t cover metabolic surgery for patients with BMI less than 35, he explained.
These findings represent the longest follow-up to date comparing the efficacy of the two most common metabolic surgery procedures with medical treatment of type 2 diabetes for maintaining glycemic control or reducing end-organ complications. Three-year outcomes of STAMPEDE (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently) were reported in 2014 (N Engl J Med. 2014;370:2002-13).
The participants ranged in age from 20 to 60 years. The average HbA1c was about 9%, the average BMI was 36, and most were on at least three antidiabetic medications at baseline. Half were on insulin.
The findings are important, because of the roughly 25 million Americans with type 2 diabetes, only about half have good glycemic control on their current medical treatment strategies, Dr. Schauer said.
Though limited by the single-center study design, the STAMPEDE findings show that metabolic surgery is more effective long term than intensive medical therapy in patients with uncontrolled type 2 diabetes and should be considered a treatment option in this population, he concluded, adding that multicenter studies would be helpful for determining the generalizability of the findings.
Dr. Schauer reported receiving consulting fees/honoraria from Ethicon Endosurgery and The Medicines Company, and having ownership interest in Surgical Excellence.
CHICAGO – The superiority of metabolic surgery over intensive medical therapy for achieving glycemic control in patients with type 2 diabetes was largely maintained at the final 5-year follow-up evaluation in the randomized, controlled STAMPEDE trial.
The 150 subjects, who had “fairly severe diabetes” with an average disease duration of 8 years, were randomized to receive intensive medical therapy alone, or intensive medical therapy with Roux-en-Y gastric bypass surgery or sleeve gastrectomy surgery. The primary endpoint of hemoglobin A1c less than 6% was achieved in 5%, 29%, and 23% of patients in the groups, respectively. The difference was statistically significant in favor of both types of surgery, Dr. Philip Raymond Schauer reported at the annual meeting of the American College of Cardiology.
Furthermore, patients in the surgery groups fared better than those in the intensive medical therapy group on several other measures, including disease remission (defied as HbA1c less than 6% without diabetes medication), HbA1c less than 7% (the American Diabetes Association target for therapy), change in fasting plasma glucose from baseline, and changes in high- and low-density lipoprotein cholesterol levels, said Dr. Schauer, director of the Cleveland Clinic Bariatric and Metabolic Institute.
Patients in the surgery groups also experienced a significantly greater reduction in the use of antihypertensive medications and lipid-lowering agents, he added.
The “very dramatic drop” in HbA1c seen early on in the surgical patients was, for the most part, sustained out to 5 years, he said.
The results for both surgeries were significantly better than those for intensive medical therapy, but the results with gastric bypass were more effective at 5 years than were those for sleeve gastrectomy, he added, noting that the surgery patients had better quality of life, compared with the intensive medical therapy patients.
As for adverse events in the surgery groups, no perioperative deaths occurred, and while there were some surgical complications, none resulted in long-term disability, Dr. Schauer said.
Anemia was more common in the surgery patients, but was fairly mild. The most common complication was weight gain in 20% of patients, and the overall reoperation rate was 7%.
Of note, patients in the study had body mass index ranging from 27 to 43 kg/m2, and those with BMI less than 35 had similar benefits as those with more severe obesity. This is important, as many insurance companies won’t cover metabolic surgery for patients with BMI less than 35, he explained.
These findings represent the longest follow-up to date comparing the efficacy of the two most common metabolic surgery procedures with medical treatment of type 2 diabetes for maintaining glycemic control or reducing end-organ complications. Three-year outcomes of STAMPEDE (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently) were reported in 2014 (N Engl J Med. 2014;370:2002-13).
The participants ranged in age from 20 to 60 years. The average HbA1c was about 9%, the average BMI was 36, and most were on at least three antidiabetic medications at baseline. Half were on insulin.
The findings are important, because of the roughly 25 million Americans with type 2 diabetes, only about half have good glycemic control on their current medical treatment strategies, Dr. Schauer said.
Though limited by the single-center study design, the STAMPEDE findings show that metabolic surgery is more effective long term than intensive medical therapy in patients with uncontrolled type 2 diabetes and should be considered a treatment option in this population, he concluded, adding that multicenter studies would be helpful for determining the generalizability of the findings.
Dr. Schauer reported receiving consulting fees/honoraria from Ethicon Endosurgery and The Medicines Company, and having ownership interest in Surgical Excellence.
CHICAGO – The superiority of metabolic surgery over intensive medical therapy for achieving glycemic control in patients with type 2 diabetes was largely maintained at the final 5-year follow-up evaluation in the randomized, controlled STAMPEDE trial.
The 150 subjects, who had “fairly severe diabetes” with an average disease duration of 8 years, were randomized to receive intensive medical therapy alone, or intensive medical therapy with Roux-en-Y gastric bypass surgery or sleeve gastrectomy surgery. The primary endpoint of hemoglobin A1c less than 6% was achieved in 5%, 29%, and 23% of patients in the groups, respectively. The difference was statistically significant in favor of both types of surgery, Dr. Philip Raymond Schauer reported at the annual meeting of the American College of Cardiology.
Furthermore, patients in the surgery groups fared better than those in the intensive medical therapy group on several other measures, including disease remission (defied as HbA1c less than 6% without diabetes medication), HbA1c less than 7% (the American Diabetes Association target for therapy), change in fasting plasma glucose from baseline, and changes in high- and low-density lipoprotein cholesterol levels, said Dr. Schauer, director of the Cleveland Clinic Bariatric and Metabolic Institute.
Patients in the surgery groups also experienced a significantly greater reduction in the use of antihypertensive medications and lipid-lowering agents, he added.
The “very dramatic drop” in HbA1c seen early on in the surgical patients was, for the most part, sustained out to 5 years, he said.
The results for both surgeries were significantly better than those for intensive medical therapy, but the results with gastric bypass were more effective at 5 years than were those for sleeve gastrectomy, he added, noting that the surgery patients had better quality of life, compared with the intensive medical therapy patients.
As for adverse events in the surgery groups, no perioperative deaths occurred, and while there were some surgical complications, none resulted in long-term disability, Dr. Schauer said.
Anemia was more common in the surgery patients, but was fairly mild. The most common complication was weight gain in 20% of patients, and the overall reoperation rate was 7%.
Of note, patients in the study had body mass index ranging from 27 to 43 kg/m2, and those with BMI less than 35 had similar benefits as those with more severe obesity. This is important, as many insurance companies won’t cover metabolic surgery for patients with BMI less than 35, he explained.
These findings represent the longest follow-up to date comparing the efficacy of the two most common metabolic surgery procedures with medical treatment of type 2 diabetes for maintaining glycemic control or reducing end-organ complications. Three-year outcomes of STAMPEDE (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently) were reported in 2014 (N Engl J Med. 2014;370:2002-13).
The participants ranged in age from 20 to 60 years. The average HbA1c was about 9%, the average BMI was 36, and most were on at least three antidiabetic medications at baseline. Half were on insulin.
The findings are important, because of the roughly 25 million Americans with type 2 diabetes, only about half have good glycemic control on their current medical treatment strategies, Dr. Schauer said.
Though limited by the single-center study design, the STAMPEDE findings show that metabolic surgery is more effective long term than intensive medical therapy in patients with uncontrolled type 2 diabetes and should be considered a treatment option in this population, he concluded, adding that multicenter studies would be helpful for determining the generalizability of the findings.
Dr. Schauer reported receiving consulting fees/honoraria from Ethicon Endosurgery and The Medicines Company, and having ownership interest in Surgical Excellence.
AT ACC 16
Key clinical point: The superiority of metabolic surgery over intensive medical therapy for achieving glycemic control among patients with type 2 diabetes in the randomized, controlled STAMPEDE persisted at the final 5-year follow-up evaluation.
Major finding: The primary endpoint of HbA1c less than 6% was achieved in 5%, 29%, and 23% of patients in the medication and medication plus Roux-en-Y or sleeve gastrectomy groups, respectively.
Data source: The randomized, controlled STAMPEDE trial in 150 subjects.
Disclosures: Dr. Schauer reported receiving consulting fees/honoraria from Ethicon Endosurgery and The Medicines Company, and having ownership interest in Surgical Excellence.
Earlier bariatric surgery may improve cardiovascular outcomes
CHICAGO – Sooner may be better than later when it comes to the timing of bariatric surgery in patients with morbid obesity.
Of 828 patients with body mass index of at least 35 kg/m2 who underwent laparoscopic adjustable gastric banding performed by a single surgeon and were followed for up to 11 years (mean of 10 years), 423 were aged 45 years or younger, and 405 were over age 45 years at the time of surgery. A comparison of outcomes between the two age groups showed that older age at the time of surgery was an independent predictor of cardiovascular events (hazard ratio, 1.8), Maharaj Singh, Ph.D., a biostatistician at the Aurora Research Institute, Milwaukee, reported in a poster at the annual meeting of the American College of Cardiology.
Despite a similar reduction in body weight after gastric banding surgery, the older patients experienced more cardiovascular events: myocardial infarction occurred in 0.2% and 1.7% of patients in the younger and older age groups, respectively, pulmonary embolism occurred in 0.7% and 4.3%, congestive heart failure occurred in 2.8% and 7.8%, and stroke occurred in 3.7% and 7.6%, Dr. Singh said.
“Although the older group had more comorbidities, these were accounted for by multivariate analysis and age over 45 years remained an independent predictor of poor cardiovascular outcomes,” senior coauthor Dr. Arshad Jahangir, professor of medicine at the University of Wisconsin–Madison, said in an interview.
Other independent predictors of adverse cardiovascular outcomes in the study were sleep apnea (hazard ratio, 4), history of hypertension (HR, 1.9), and depression, (HR, 1.8), Dr. Jahangir said.
“Gender, race, and diabetes mellitus did not independently predict cardiovascular events,” he said.
Weight loss after bariatric surgery has been shown to reduce the risk of adverse cardiovascular events, but it has remained unclear whether the reduction in risk varies based on age at the time of surgery, he said.
The current findings suggest that the effects of laparoscopic adjustable gastric banding–induced weight loss on cardiovascular outcomes are greater in patients who undergo the surgery at a younger age, he said, adding that the findings also “raise important questions about whether better control of sleep apnea, hypertension, and depression could help further reduce cardiovascular events in morbidly obese individuals undergoing bariatric surgery and should be addressed in a prospective study of these patients.”
The authors reported having no disclosures.
CHICAGO – Sooner may be better than later when it comes to the timing of bariatric surgery in patients with morbid obesity.
Of 828 patients with body mass index of at least 35 kg/m2 who underwent laparoscopic adjustable gastric banding performed by a single surgeon and were followed for up to 11 years (mean of 10 years), 423 were aged 45 years or younger, and 405 were over age 45 years at the time of surgery. A comparison of outcomes between the two age groups showed that older age at the time of surgery was an independent predictor of cardiovascular events (hazard ratio, 1.8), Maharaj Singh, Ph.D., a biostatistician at the Aurora Research Institute, Milwaukee, reported in a poster at the annual meeting of the American College of Cardiology.
Despite a similar reduction in body weight after gastric banding surgery, the older patients experienced more cardiovascular events: myocardial infarction occurred in 0.2% and 1.7% of patients in the younger and older age groups, respectively, pulmonary embolism occurred in 0.7% and 4.3%, congestive heart failure occurred in 2.8% and 7.8%, and stroke occurred in 3.7% and 7.6%, Dr. Singh said.
“Although the older group had more comorbidities, these were accounted for by multivariate analysis and age over 45 years remained an independent predictor of poor cardiovascular outcomes,” senior coauthor Dr. Arshad Jahangir, professor of medicine at the University of Wisconsin–Madison, said in an interview.
Other independent predictors of adverse cardiovascular outcomes in the study were sleep apnea (hazard ratio, 4), history of hypertension (HR, 1.9), and depression, (HR, 1.8), Dr. Jahangir said.
“Gender, race, and diabetes mellitus did not independently predict cardiovascular events,” he said.
Weight loss after bariatric surgery has been shown to reduce the risk of adverse cardiovascular events, but it has remained unclear whether the reduction in risk varies based on age at the time of surgery, he said.
The current findings suggest that the effects of laparoscopic adjustable gastric banding–induced weight loss on cardiovascular outcomes are greater in patients who undergo the surgery at a younger age, he said, adding that the findings also “raise important questions about whether better control of sleep apnea, hypertension, and depression could help further reduce cardiovascular events in morbidly obese individuals undergoing bariatric surgery and should be addressed in a prospective study of these patients.”
The authors reported having no disclosures.
CHICAGO – Sooner may be better than later when it comes to the timing of bariatric surgery in patients with morbid obesity.
Of 828 patients with body mass index of at least 35 kg/m2 who underwent laparoscopic adjustable gastric banding performed by a single surgeon and were followed for up to 11 years (mean of 10 years), 423 were aged 45 years or younger, and 405 were over age 45 years at the time of surgery. A comparison of outcomes between the two age groups showed that older age at the time of surgery was an independent predictor of cardiovascular events (hazard ratio, 1.8), Maharaj Singh, Ph.D., a biostatistician at the Aurora Research Institute, Milwaukee, reported in a poster at the annual meeting of the American College of Cardiology.
Despite a similar reduction in body weight after gastric banding surgery, the older patients experienced more cardiovascular events: myocardial infarction occurred in 0.2% and 1.7% of patients in the younger and older age groups, respectively, pulmonary embolism occurred in 0.7% and 4.3%, congestive heart failure occurred in 2.8% and 7.8%, and stroke occurred in 3.7% and 7.6%, Dr. Singh said.
“Although the older group had more comorbidities, these were accounted for by multivariate analysis and age over 45 years remained an independent predictor of poor cardiovascular outcomes,” senior coauthor Dr. Arshad Jahangir, professor of medicine at the University of Wisconsin–Madison, said in an interview.
Other independent predictors of adverse cardiovascular outcomes in the study were sleep apnea (hazard ratio, 4), history of hypertension (HR, 1.9), and depression, (HR, 1.8), Dr. Jahangir said.
“Gender, race, and diabetes mellitus did not independently predict cardiovascular events,” he said.
Weight loss after bariatric surgery has been shown to reduce the risk of adverse cardiovascular events, but it has remained unclear whether the reduction in risk varies based on age at the time of surgery, he said.
The current findings suggest that the effects of laparoscopic adjustable gastric banding–induced weight loss on cardiovascular outcomes are greater in patients who undergo the surgery at a younger age, he said, adding that the findings also “raise important questions about whether better control of sleep apnea, hypertension, and depression could help further reduce cardiovascular events in morbidly obese individuals undergoing bariatric surgery and should be addressed in a prospective study of these patients.”
The authors reported having no disclosures.
AT ACC 16
Key clinical point: Morbidly obese patients who underwent bariatric surgery before age 45 years had a reduced risk of adverse cardiovascular outcomes vs. those aged 45 or older at the time of surgery, despite similar weight loss.
Major finding: Older vs. younger age at the time of surgery was an independent predictor of cardiovascular events (hazard ratio, 1.8).
Data source: A review of outcomes in 828 laparoscopic adjustable gastric banding patients.
Disclosures: The authors reported having no disclosures.
Drug-eluting stent recipients can safely have surgery sooner
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
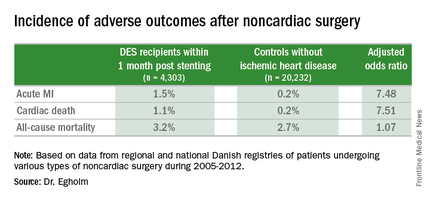
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.
Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.

Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.

Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
AT ACC 16
Key clinical point: The risk of noncardiac surgery is elevated only when the operation occurs during the first month after stenting.
Major finding: Danish drug-eluting stent recipients who underwent noncardiac surgery within 1 month after stent placement were at 7.5-fold increased risks of acute MI and cardiac death, but surgery performed 2-12 months post stenting carried no increased risks.
Data source: This retrospective observational study based upon large Danish patient registries compared outcomes of noncardiac surgery performed within 12 months after drug-eluting stent placement in 4,303 patients with 20,232 matched controls without ischemic heart disease who underwent the same operations.
Disclosures: The study was supported by Danish research funds. The presenter reported having no financial conflicts of interest.
Surgeons commonly off the mark in estimating blood loss
MONTREAL – Surgeons, nurses, and anesthesia providers were all pretty bad at estimating surgical blood loss in a small study. And more experience doesn’t improve accuracy, though experienced providers were more confident in their estimates.
These were the findings from a study that simulated operating room scenarios and asked providers to estimate blood loss. “Estimation of blood loss is inaccurate and unreliable,” Dr. Luke Rothermel said at the Central Surgical Association’s annual meeting.
Dr. Rothermel, a resident at Case Western Reserve University, Cleveland, noted that although the Joint Commission requires operative notes to contain estimated blood loss, “no study in the United States has compared the characteristics of operating room personnel or conditions associated with improved accuracy or reliability of blood loss estimation.”
Beyond the required reporting, estimating blood loss (EBL) also provides important guidance in perioperative care. Still, said Dr. Rothermel, previous studies have shown that EBL is typically inaccurate.
To assess providers’ ability to be accurate and reliable in estimating blood loss, Dr. Rothermel and his collaborator, Dr. Jeremy Lipman, assistant residency director at MetroHealth, Cleveland, designed a study to simulate three different operating room scenarios, involving high, medium, and low blood loss volumes. The materials used, such as blood-soaked sponges and suction canisters, were identical to what’s actually used in the operating room (porcine blood was used in the simulations).
Before the study, Dr. Rothermel said that he and Dr. Lipman hypothesized that those providers who had more experience and those who were working at the operating field would be more accurate in estimating blood loss. They also hypothesized that estimations in procedures with lower volumes of blood loss would be more accurate.
The study recruited providers from the surgery, anesthesia, and nursing services at an urban level 1 trauma center. Each scenario included a written description of the procedure performed and the course of surgery, and participants could handle study materials for each scenario under the supervision of study staff.
A total of 60 participants (22 from surgery, 17 from anesthesia, and 21 from nursing) participated; they had an average of 12.8 years of experience. The surgical participants included surgical scrub techs, trainees, and attending physicians. Anesthesia participants included anesthesia assistants, CRNAs, trainees, and attending physicians. Nursing participants were all RNs.
The findings? All over the board: “There was no association between specialty, years of experience, or confidence in ability with the consistency or accuracy of estimated blood loss,” said Dr. Rothermel.
Most participants were far shy of the mark, with just 5% of study participants overall able to come within 25% accuracy in judging EBL in all scenarios. Just over a quarter were consistent in over- or underestimating blood loss.
These findings held true across scenarios, across disciplines, and regardless of the number of years of experience. “Increased years of experience trended toward increased error,” said Dr. Rothermel, though the difference was not statistically significant. However, those with more years of experience tended to be more confident of their judgments.
Dr. Rothermel noted the small study size and single institution studied as limitations. Also, “this model was not a high fidelity representation of the OR experience, “ he said, explaining that during surgery, caregivers continually assess intraoperative blood loss and may form an estimate in a different – and potentially more accurate – manner than occurs when presented with the contrived presentation of a scenario.
The study calls into question the validity of using EBL as a quality indicator in assessing physician performance and patient outcomes, said Dr. Rothermel, who had no financial disclosures.
On Twitter @karioakes
MONTREAL – Surgeons, nurses, and anesthesia providers were all pretty bad at estimating surgical blood loss in a small study. And more experience doesn’t improve accuracy, though experienced providers were more confident in their estimates.
These were the findings from a study that simulated operating room scenarios and asked providers to estimate blood loss. “Estimation of blood loss is inaccurate and unreliable,” Dr. Luke Rothermel said at the Central Surgical Association’s annual meeting.
Dr. Rothermel, a resident at Case Western Reserve University, Cleveland, noted that although the Joint Commission requires operative notes to contain estimated blood loss, “no study in the United States has compared the characteristics of operating room personnel or conditions associated with improved accuracy or reliability of blood loss estimation.”
Beyond the required reporting, estimating blood loss (EBL) also provides important guidance in perioperative care. Still, said Dr. Rothermel, previous studies have shown that EBL is typically inaccurate.
To assess providers’ ability to be accurate and reliable in estimating blood loss, Dr. Rothermel and his collaborator, Dr. Jeremy Lipman, assistant residency director at MetroHealth, Cleveland, designed a study to simulate three different operating room scenarios, involving high, medium, and low blood loss volumes. The materials used, such as blood-soaked sponges and suction canisters, were identical to what’s actually used in the operating room (porcine blood was used in the simulations).
Before the study, Dr. Rothermel said that he and Dr. Lipman hypothesized that those providers who had more experience and those who were working at the operating field would be more accurate in estimating blood loss. They also hypothesized that estimations in procedures with lower volumes of blood loss would be more accurate.
The study recruited providers from the surgery, anesthesia, and nursing services at an urban level 1 trauma center. Each scenario included a written description of the procedure performed and the course of surgery, and participants could handle study materials for each scenario under the supervision of study staff.
A total of 60 participants (22 from surgery, 17 from anesthesia, and 21 from nursing) participated; they had an average of 12.8 years of experience. The surgical participants included surgical scrub techs, trainees, and attending physicians. Anesthesia participants included anesthesia assistants, CRNAs, trainees, and attending physicians. Nursing participants were all RNs.
The findings? All over the board: “There was no association between specialty, years of experience, or confidence in ability with the consistency or accuracy of estimated blood loss,” said Dr. Rothermel.
Most participants were far shy of the mark, with just 5% of study participants overall able to come within 25% accuracy in judging EBL in all scenarios. Just over a quarter were consistent in over- or underestimating blood loss.
These findings held true across scenarios, across disciplines, and regardless of the number of years of experience. “Increased years of experience trended toward increased error,” said Dr. Rothermel, though the difference was not statistically significant. However, those with more years of experience tended to be more confident of their judgments.
Dr. Rothermel noted the small study size and single institution studied as limitations. Also, “this model was not a high fidelity representation of the OR experience, “ he said, explaining that during surgery, caregivers continually assess intraoperative blood loss and may form an estimate in a different – and potentially more accurate – manner than occurs when presented with the contrived presentation of a scenario.
The study calls into question the validity of using EBL as a quality indicator in assessing physician performance and patient outcomes, said Dr. Rothermel, who had no financial disclosures.
On Twitter @karioakes
MONTREAL – Surgeons, nurses, and anesthesia providers were all pretty bad at estimating surgical blood loss in a small study. And more experience doesn’t improve accuracy, though experienced providers were more confident in their estimates.
These were the findings from a study that simulated operating room scenarios and asked providers to estimate blood loss. “Estimation of blood loss is inaccurate and unreliable,” Dr. Luke Rothermel said at the Central Surgical Association’s annual meeting.
Dr. Rothermel, a resident at Case Western Reserve University, Cleveland, noted that although the Joint Commission requires operative notes to contain estimated blood loss, “no study in the United States has compared the characteristics of operating room personnel or conditions associated with improved accuracy or reliability of blood loss estimation.”
Beyond the required reporting, estimating blood loss (EBL) also provides important guidance in perioperative care. Still, said Dr. Rothermel, previous studies have shown that EBL is typically inaccurate.
To assess providers’ ability to be accurate and reliable in estimating blood loss, Dr. Rothermel and his collaborator, Dr. Jeremy Lipman, assistant residency director at MetroHealth, Cleveland, designed a study to simulate three different operating room scenarios, involving high, medium, and low blood loss volumes. The materials used, such as blood-soaked sponges and suction canisters, were identical to what’s actually used in the operating room (porcine blood was used in the simulations).
Before the study, Dr. Rothermel said that he and Dr. Lipman hypothesized that those providers who had more experience and those who were working at the operating field would be more accurate in estimating blood loss. They also hypothesized that estimations in procedures with lower volumes of blood loss would be more accurate.
The study recruited providers from the surgery, anesthesia, and nursing services at an urban level 1 trauma center. Each scenario included a written description of the procedure performed and the course of surgery, and participants could handle study materials for each scenario under the supervision of study staff.
A total of 60 participants (22 from surgery, 17 from anesthesia, and 21 from nursing) participated; they had an average of 12.8 years of experience. The surgical participants included surgical scrub techs, trainees, and attending physicians. Anesthesia participants included anesthesia assistants, CRNAs, trainees, and attending physicians. Nursing participants were all RNs.
The findings? All over the board: “There was no association between specialty, years of experience, or confidence in ability with the consistency or accuracy of estimated blood loss,” said Dr. Rothermel.
Most participants were far shy of the mark, with just 5% of study participants overall able to come within 25% accuracy in judging EBL in all scenarios. Just over a quarter were consistent in over- or underestimating blood loss.
These findings held true across scenarios, across disciplines, and regardless of the number of years of experience. “Increased years of experience trended toward increased error,” said Dr. Rothermel, though the difference was not statistically significant. However, those with more years of experience tended to be more confident of their judgments.
Dr. Rothermel noted the small study size and single institution studied as limitations. Also, “this model was not a high fidelity representation of the OR experience, “ he said, explaining that during surgery, caregivers continually assess intraoperative blood loss and may form an estimate in a different – and potentially more accurate – manner than occurs when presented with the contrived presentation of a scenario.
The study calls into question the validity of using EBL as a quality indicator in assessing physician performance and patient outcomes, said Dr. Rothermel, who had no financial disclosures.
On Twitter @karioakes
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Surgery, anesthesia, and nursing providers were inaccurate and unreliable in estimating surgical blood loss.
Major finding: Only 5% of providers could come within 25% accuracy of simulated surgical blood loss.
Data source: Simulations of surgical scenarios depicting varying amounts of blood loss using porcine blood, presented to 60 providers.
Disclosures: The study authors reported no relevant disclosures.
VIDEO: More routine use of unilateral thyroidectomy advocated for papillary thyroid microcarcinoma
BOSTON – A study of over 60 years of patient data from the Mayo Clinic suggests a reconsideration of the routine use of unilateral thyroid lobectomy (UL) as the initial treatment for papillary thyroid microcarcinoma.
“Papillary thyroid microcarcinoma [PTM] patients have a normal life expectancy and typically are cured by adequate tumor resection. More than 99% of PTM patients are not at risk of either distant spread or mortality from cancer,” said Dr. Ian D. Hay of the Mayo Clinic, Rochester, Minn. Unilateral thyroid lobectomy is one treatment option for papillary thyroid microcarcinoma along with conventional bilateral nodal resection approaches of near-total thyroidectomy (NT) or total thyroidectomy (TT), or selective radioactive iodine remnant ablation (RRA).
Awareness of PTM is not new; examination of thyroid glands at autopsy going back decades has revealed their presence in 6%-36% of samples. A more recent development is the use of high-resolution ultrasound-guided biopsies of papillary thyroid carcinoma (PTC) lesions as small as 3 cm. For example, at the Mayo Clinic the diagnosis of PTM was about one annually from 1935 to 1944, while from 2005 to 2014 the average was close to one per day. “At Mayo, 34% of PTCs seen since 1995 are PTMs,” Dr. Hay said at the annual meeting of the Endocrine Society.
The best initial management of PTMs is disputed, with observation favored by some, TT and RRA favored by others, and ethanol ablation having been found to be effective by institutions including the Mayo Clinic. UL has been deemphasized, despite the 2015 American Thyroid Association Guidelines recommendation of UL as the usual surgical procedure for adults with PTM.
Dr. Hay and his colleagues sought to provide some clarity to the issue by taking advantage of the institute’s database of adult (18+ years) PTM patients who were consecutively treated from 1935 to 2014. The decades of data allowed a long-term look at patient outcomes. They examined data from 1,345 patients, 954 women and 391 men with a median age at surgery of 48 years. The mean follow-up was 15.4 years, representing almost 21,000 patient years. Data on tumor recurrence and cause-specific mortality were derived from a data base of over 4,300 PTC patients representing over 66,000 patient-years of observation.
Median tumor size was 7 mm (range, 0.08-1.0 cm). Extrathyroid invasion was evident in 18 (1.3%) cases and 298 tumors (26%) were multifocal. There were 399 (30%) node-positive tumors at diagnosis and 4 (0.3%) cases featuring initial distant metastases.
The mean MACIS (metastasis, age at presentation, completeness of surgical resection, invasion [extrathyroidal], size) score was 4.25 with little variation in score over time. Almost all (96%) patients had a MACIS score of under 6. Bilateral lobar resection was done in 1,132 (95%) patients, with NT or TT comprising 80% of the cases. UL was done in only 202 (15%) cases. The use of TT skyrocketed from 3% of the cases done in the first 2 decades to 40% in the last 2 decades. Regional nodes were removed at surgery in 743 (55%) cases, either by “node picking” (23%) or compartmental dissection (32%).
Overall survival following surgery in PTM patients was similar to age- and gender-matched controls (397 deaths observed, 431 deaths expected; P = .16). Only four (0.3%) patients died of PTM. The rates of locoregional recurrence were similar for the unilateral and bilateral approaches (P = .90). In 1,148 patients with potentially curable PTM, defined as the absence of metastasis at diagnosis and no gross residual disease, the rates of tumor recurrence 10, 20, and 40 years after surgery were 6%, 7%, and 10%, respectively. In these 1,148 patients, the 30-year locoregional recurrence rates after UL alone were similar to those seen after NT or TT followed by RRA (P = .99).
UL did not result in permanent unilateral vocal cord paresis or permanent hypoparathyroidism. These adversities were more likely to develop following bilateral lobectomy.
“Since [UL] produces comparable recurrence results when compared to bilateral surgery and is not associated with either cord paresis or hypoparathyroidism, then perhaps it is overdue for institutions like Mayo to individualize our treatment policies and more often employ UL when surgery, and not observation or ultrasound-guided percutaneous ethanol ablation, is chosen to treat PTM,” said Dr. Hay.
Dr. Hay was adamant on the overuse of ultrasound in the detection of small-diameter carcinomas in the decision for bilateral surgery. “It’s embarrassing how much we are wasting resources and doing too much ultrasound too often,” he said in an interview.
Dr. Hay had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – A study of over 60 years of patient data from the Mayo Clinic suggests a reconsideration of the routine use of unilateral thyroid lobectomy (UL) as the initial treatment for papillary thyroid microcarcinoma.
“Papillary thyroid microcarcinoma [PTM] patients have a normal life expectancy and typically are cured by adequate tumor resection. More than 99% of PTM patients are not at risk of either distant spread or mortality from cancer,” said Dr. Ian D. Hay of the Mayo Clinic, Rochester, Minn. Unilateral thyroid lobectomy is one treatment option for papillary thyroid microcarcinoma along with conventional bilateral nodal resection approaches of near-total thyroidectomy (NT) or total thyroidectomy (TT), or selective radioactive iodine remnant ablation (RRA).
Awareness of PTM is not new; examination of thyroid glands at autopsy going back decades has revealed their presence in 6%-36% of samples. A more recent development is the use of high-resolution ultrasound-guided biopsies of papillary thyroid carcinoma (PTC) lesions as small as 3 cm. For example, at the Mayo Clinic the diagnosis of PTM was about one annually from 1935 to 1944, while from 2005 to 2014 the average was close to one per day. “At Mayo, 34% of PTCs seen since 1995 are PTMs,” Dr. Hay said at the annual meeting of the Endocrine Society.
The best initial management of PTMs is disputed, with observation favored by some, TT and RRA favored by others, and ethanol ablation having been found to be effective by institutions including the Mayo Clinic. UL has been deemphasized, despite the 2015 American Thyroid Association Guidelines recommendation of UL as the usual surgical procedure for adults with PTM.
Dr. Hay and his colleagues sought to provide some clarity to the issue by taking advantage of the institute’s database of adult (18+ years) PTM patients who were consecutively treated from 1935 to 2014. The decades of data allowed a long-term look at patient outcomes. They examined data from 1,345 patients, 954 women and 391 men with a median age at surgery of 48 years. The mean follow-up was 15.4 years, representing almost 21,000 patient years. Data on tumor recurrence and cause-specific mortality were derived from a data base of over 4,300 PTC patients representing over 66,000 patient-years of observation.
Median tumor size was 7 mm (range, 0.08-1.0 cm). Extrathyroid invasion was evident in 18 (1.3%) cases and 298 tumors (26%) were multifocal. There were 399 (30%) node-positive tumors at diagnosis and 4 (0.3%) cases featuring initial distant metastases.
The mean MACIS (metastasis, age at presentation, completeness of surgical resection, invasion [extrathyroidal], size) score was 4.25 with little variation in score over time. Almost all (96%) patients had a MACIS score of under 6. Bilateral lobar resection was done in 1,132 (95%) patients, with NT or TT comprising 80% of the cases. UL was done in only 202 (15%) cases. The use of TT skyrocketed from 3% of the cases done in the first 2 decades to 40% in the last 2 decades. Regional nodes were removed at surgery in 743 (55%) cases, either by “node picking” (23%) or compartmental dissection (32%).
Overall survival following surgery in PTM patients was similar to age- and gender-matched controls (397 deaths observed, 431 deaths expected; P = .16). Only four (0.3%) patients died of PTM. The rates of locoregional recurrence were similar for the unilateral and bilateral approaches (P = .90). In 1,148 patients with potentially curable PTM, defined as the absence of metastasis at diagnosis and no gross residual disease, the rates of tumor recurrence 10, 20, and 40 years after surgery were 6%, 7%, and 10%, respectively. In these 1,148 patients, the 30-year locoregional recurrence rates after UL alone were similar to those seen after NT or TT followed by RRA (P = .99).
UL did not result in permanent unilateral vocal cord paresis or permanent hypoparathyroidism. These adversities were more likely to develop following bilateral lobectomy.
“Since [UL] produces comparable recurrence results when compared to bilateral surgery and is not associated with either cord paresis or hypoparathyroidism, then perhaps it is overdue for institutions like Mayo to individualize our treatment policies and more often employ UL when surgery, and not observation or ultrasound-guided percutaneous ethanol ablation, is chosen to treat PTM,” said Dr. Hay.
Dr. Hay was adamant on the overuse of ultrasound in the detection of small-diameter carcinomas in the decision for bilateral surgery. “It’s embarrassing how much we are wasting resources and doing too much ultrasound too often,” he said in an interview.
Dr. Hay had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – A study of over 60 years of patient data from the Mayo Clinic suggests a reconsideration of the routine use of unilateral thyroid lobectomy (UL) as the initial treatment for papillary thyroid microcarcinoma.
“Papillary thyroid microcarcinoma [PTM] patients have a normal life expectancy and typically are cured by adequate tumor resection. More than 99% of PTM patients are not at risk of either distant spread or mortality from cancer,” said Dr. Ian D. Hay of the Mayo Clinic, Rochester, Minn. Unilateral thyroid lobectomy is one treatment option for papillary thyroid microcarcinoma along with conventional bilateral nodal resection approaches of near-total thyroidectomy (NT) or total thyroidectomy (TT), or selective radioactive iodine remnant ablation (RRA).
Awareness of PTM is not new; examination of thyroid glands at autopsy going back decades has revealed their presence in 6%-36% of samples. A more recent development is the use of high-resolution ultrasound-guided biopsies of papillary thyroid carcinoma (PTC) lesions as small as 3 cm. For example, at the Mayo Clinic the diagnosis of PTM was about one annually from 1935 to 1944, while from 2005 to 2014 the average was close to one per day. “At Mayo, 34% of PTCs seen since 1995 are PTMs,” Dr. Hay said at the annual meeting of the Endocrine Society.
The best initial management of PTMs is disputed, with observation favored by some, TT and RRA favored by others, and ethanol ablation having been found to be effective by institutions including the Mayo Clinic. UL has been deemphasized, despite the 2015 American Thyroid Association Guidelines recommendation of UL as the usual surgical procedure for adults with PTM.
Dr. Hay and his colleagues sought to provide some clarity to the issue by taking advantage of the institute’s database of adult (18+ years) PTM patients who were consecutively treated from 1935 to 2014. The decades of data allowed a long-term look at patient outcomes. They examined data from 1,345 patients, 954 women and 391 men with a median age at surgery of 48 years. The mean follow-up was 15.4 years, representing almost 21,000 patient years. Data on tumor recurrence and cause-specific mortality were derived from a data base of over 4,300 PTC patients representing over 66,000 patient-years of observation.
Median tumor size was 7 mm (range, 0.08-1.0 cm). Extrathyroid invasion was evident in 18 (1.3%) cases and 298 tumors (26%) were multifocal. There were 399 (30%) node-positive tumors at diagnosis and 4 (0.3%) cases featuring initial distant metastases.
The mean MACIS (metastasis, age at presentation, completeness of surgical resection, invasion [extrathyroidal], size) score was 4.25 with little variation in score over time. Almost all (96%) patients had a MACIS score of under 6. Bilateral lobar resection was done in 1,132 (95%) patients, with NT or TT comprising 80% of the cases. UL was done in only 202 (15%) cases. The use of TT skyrocketed from 3% of the cases done in the first 2 decades to 40% in the last 2 decades. Regional nodes were removed at surgery in 743 (55%) cases, either by “node picking” (23%) or compartmental dissection (32%).
Overall survival following surgery in PTM patients was similar to age- and gender-matched controls (397 deaths observed, 431 deaths expected; P = .16). Only four (0.3%) patients died of PTM. The rates of locoregional recurrence were similar for the unilateral and bilateral approaches (P = .90). In 1,148 patients with potentially curable PTM, defined as the absence of metastasis at diagnosis and no gross residual disease, the rates of tumor recurrence 10, 20, and 40 years after surgery were 6%, 7%, and 10%, respectively. In these 1,148 patients, the 30-year locoregional recurrence rates after UL alone were similar to those seen after NT or TT followed by RRA (P = .99).
UL did not result in permanent unilateral vocal cord paresis or permanent hypoparathyroidism. These adversities were more likely to develop following bilateral lobectomy.
“Since [UL] produces comparable recurrence results when compared to bilateral surgery and is not associated with either cord paresis or hypoparathyroidism, then perhaps it is overdue for institutions like Mayo to individualize our treatment policies and more often employ UL when surgery, and not observation or ultrasound-guided percutaneous ethanol ablation, is chosen to treat PTM,” said Dr. Hay.
Dr. Hay was adamant on the overuse of ultrasound in the detection of small-diameter carcinomas in the decision for bilateral surgery. “It’s embarrassing how much we are wasting resources and doing too much ultrasound too often,” he said in an interview.
Dr. Hay had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ENDO 2016
Key clinical point: Unilateral thryoidectomy should be reconsidered as a routine strategy in treatment of papillary thyroid microcarcinoma.
Major finding: Data compiled from over 80 years at a single institution indicates the value of unilateral thyroidectomy in terms of recurrence and morbidity.
Data source: Retrospective analysis of data from 1,153 adult patients.
Disclosures: Dr. Hay had no disclosures.
VIDEO: Anesthesia services during colonoscopy increase risk of near-term complications
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Using anesthesia services on individuals receiving colonoscopy increases the overall risk of complications associated with the procedure.
Major finding: Colonoscopy patients who received anesthesia had a 13% higher risk of complication within 30 days, including perforation, hemorrhage, abdominal pain, and stroke.
Data source: A prospective cohort study of claims data from 3,168,228 colonoscopy procedures in the Truven Health MarketScan Research Databases from 2008 to 2011.
Disclosures: Funding provided by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Wernli and her coauthors did not report any relevant financial disclosures.
Lies, damn lies, and research: Improving reproducibility in biomedical science
The issue of scientific reproducibility has come to the fore in the past several years, driven by noteworthy failures to replicate critical findings in several much-publicized reports coupled to a series of scandals calling into question the role of journals and granting agencies in maintaining quality and oversight.
In a special Nature online collection, the journal assembled articles and perspectives from 2011 to the present dealing with this issue of research reproducibility in science and medicine. These articles were supplemented with current editorial comment.
Seeing these broad spectrum concerns pulled together in one place makes it difficult not to be pessimistic about the current state of research investigations across the board. The saving grace, however, is that these same reports show that a lot of people realize that there is a problem – people who are trying to make changes and who are in a position to be effective.
According to the reports presented in the collection, the problems in research accountability and reproducibility have grown to an alarming extent. In one estimate, irreproducibility ends up costing biomedical research some $28 billion wasted dollars per year (Nature. 2015 Jun 9. doi: 10.1038/nature.2015.17711).
A litany of concerns
In 2012, scientists at AMGEN (Thousand Oaks, Calif.) reported that, even cooperating closely with the original investigators, they were able to reproduce only 6 of 53 studies considered to be benchmarks of cancer research (Nature. 2016 Feb 4. doi: 10.1038/nature.2016.19269).
Scientists at Bayer HealthCare reported in Nature Reviews Drug Discovery that they could successfully reproduce results in only a quarter of 67 so-called seminal studies (2011 Sep. doi: 10.1038/nrd3439-c1).
According to a 2013 report in The Economist, Dr. John Ioannidis, an expert in the field of scientific reproducibility, argued that in his field, “epidemiology, you might expect one in ten hypotheses to be true. In exploratory disciplines like genomics, which rely on combing through vast troves of data about genes and proteins for interesting relationships, you might expect just one in a thousand to prove correct.”
This increasing litany of irreproducibility has raised alarm in the scientific community and has led to a search for answers, as so many preclinical studies form the precursor data for eventual human trials.
Despite the concerns raised, human clinical trials seem to be less at risk for irreproducibility, according to an editorial by Dr. Francis S. Collins, director, and Dr. Lawrence A. Tabak, principal deputy director of the U.S. National Institutes of Health, “because they are already governed by various regulations that stipulate rigorous design and independent oversight – including randomization, blinding, power estimates, pre-registration of outcome measures in standardized, public databases such as ClinicalTrials.gov and oversight by institutional review boards and data safety monitoring boards. Furthermore, the clinical trials community has taken important steps toward adopting standard reporting elements,” (Nature. 2014 Jan. doi: 10.1038/505612a).
The paucity of P
Today, the P-value, .05 or less, is all too often considered the sine qua non of scientific proof. “Most statisticians consider this appalling, as the P value was never intended to be used as a strong indicator of certainty as it too often is today. Most scientists would look at [a] P value of .01 and say that there was just a 1% chance of [the] result being a false alarm. But they would be wrong.” The 2014 report goes on to state how, according to one widely used calculation by authentic statisticians, a P value of .01 corresponds to a false-alarm probability of at least 11%, depending on the underlying probability that there is a true effect; a P value of .05 raises that chance of a false alarm to at least 29% (Nature. 2014 Feb. doi: 10.1038/506150a).
Beyond this assessment problem, P values may allow for considerable researcher bias, conscious and unconscious, even to the extent of encouraging “P-hacking”: one of the few statistical terms to ever make it into the Urban Dictionary. “P-hacking is trying multiple things until you get the desired result” – even unconsciously, according to one researcher quoted.
In addition, “unless statistical power is very high (and much higher than in most experiments), the P value should be interpreted tentatively at best” (Nat Methods. 2015 Feb 26. doi: 10.1038/nmeth.3288).
So bad is the problem that “misuse of the P value – a common test for judging the strength of scientific evidence – is contributing to the number of research findings that cannot be reproduced,” the American Statistical Association warns in a statement released in March, adding that the P value cannot be used to determine whether a hypothesis is true or even whether results are important (Nature. 2016 Mar 7. doi: 10.1038/nature.2016.19503).
And none of this even remotely addresses those instances where researchers report findings that “trend towards significance” when they can’t even meet the magical P threshold.
A muddling of mice (and more)
Fundamental to biological research is the vast array of preliminary animal studies that must be performed before clinical testing can begin.
Animal-based research has been under intense scrutiny due to a variety of perceived flaws and omissions that have been found to be all too common. For example, in a report in PLoS Biology, Dr. Ulrich Dirnagl of the Charité Medical University in Berlin reviewed 100 reports published between 2000 and 2013, which included 522 experiments using rodents to test cancer and stroke treatments. Around two-thirds of the experiments did not report whether any animals had been dropped from the final analysis, and of the 30% that did report rodents dropped from analysis, only 14 explained why (2016 Jan 4. doi: 10.1371/journal.pbio.1002331). Similarly, Dr. John Ioannidis and his colleagues assessed a random sample of 268 biomedical papers listed in PubMed published between 2000 and 2014 and found that only one contained sufficient details to replicate the work (Nature. 2016 Jan 5. doi: 10.1038/nature.2015.19101).
A multitude of genetic and environmental factors have also been found influential in animal research. For example, the gut microbiome (which has been found to influence many aspects of mouse health and metabolism) varies widely in the same species of mice fed on different diets or obtained from different vendors. And there can be differences in physiology and behavior based on circadian rhythms, and even variations in cage design (Nature. 2016 Feb 16. doi: 10.1038/530254a).
But things are looking brighter. By the beginning of 2016, more than 600 journals had signed up for the voluntary ARRIVE (Animals in Research: Reporting of In Vivo Experiments) guidelines designed to improve the reporting of animal experiments. The guidelines include a checklist of elements to be included in any reporting of animal research, including animal strain, sex, and adverse events (Nature. 2016 Feb 1. doi: 10.1038/nature.2016.19274).
Problems have also been reported in the use of cell lines and antibodies in biomedical research. For example, a report in Nature indicated that too many biomedical researchers are lax in checking for impostor cell lines when they perform their research (Nature. 2015 Oct 12. doi: 10.1038/nature.2015.18544). And recent studies have shown that improper or misused antibodies are a significant source of false findings and irreproducibility in the modern literature (Nature. 2015 May 19. doi: 10.1038/521274a).
Reviewer, view thyself
The editorial in The Economist also discussed some of the failures of the peer-reviewed scientific literature, usually considered the final gateway of quality control, to provide appropriate review and correction of research errors. The editorial cites a damning test of lower-tier research publications by Dr. John Bohannon, a biologist at Harvard, who submitted a pseudonymous paper on the effects of a chemical derived from lichen cells to 304 journals describing themselves as using peer review. The paper was concocted wholesale with manifold and obvious errors in study design, analysis, and interpretation of results, according to Dr. Bohannon. This fictitious paper from a fictitious researcher based at a fictitious university was accepted for publication by an alarming 147 of the journals.
The problem is not new. In 1998, Dr. Fiona Godlee, editor of the British Medical Journal, sent an article with eight deliberate mistakes in study design, analysis, and interpretation to more than 200 of the journal’s regular reviewers. None of the reviewers found all the mistakes, and on average, they spotted fewer than two. And another study by the BMJ showed that experience was an issue, not in improving quality of reviewers, but quite the opposite. Over a 14-year period assessed, 1,500 referees, as rated by editors at leading journals, showed a slow but steady drop in their scores.
Such studies prompted a profound reassessment by the journals, in part pushed by some major granting agencies, including the National Institutes of Health.
Not taking grants for granted
The National Institutes for Health are advancing efforts to expand scientific rigor and reproducibility in their grants projects.
“As part of an increasing drive to boost the reliability of research, the NIH will require applicants to explain the scientific premise behind their proposals and defend the quality of their experimental designs. They must also account for biological variables (for example, by including both male and female mice in planned studies) and describe how they will authenticate experimental materials such as cell lines and antibodies.”
Whether current efforts by scientists, societies, granting organizations, and journals can lead to authentic reform and a vast and relatively quick improvement in reproducibility of scientific results is still an open question. In discussing a 2015 report on the subject by the biomedical research community in the United Kingdom, neurophysiologist Dr. Dorothy Bishop had this to say: “I feel quite upbeat about it. ... Now that we’re aware of it, we have all sorts of ideas about how to deal with it. These are doable things. I feel that the mood is one of making science a much better thing. It might lead to slightly slower science. That could be better” (Nature. 2015 Oct 29. doi: 10.1038/nature.2015.18684).
In the recent Nature editorial, “Repetitive flaws,” comments are offered regarding the new NIH guidelines that require grant proposals to account for biological variables and describe how experimental materials may be authenticated (2016 Jan 21. doi: 10.1038/529256a). It is proposed that these requirements will attempt to improve the quality and reproducibility of research. Many concerns regarding scientific reproducibility have been raised in the past few years. As the editorial states, the NIH guidelines “can help to make researchers aspire to the values that produced them” and they can “inspire researchers to uphold their identity and integrity.”
To those investigators who strive to report only their best results following exhaustive and sincere confirmation, these guidelines will not seem threatening. Providing experimental details of one’s work is helpful in many ways (you can personally reproduce them with new and different lab personnel or after a lapse of time, you will have excellent experimental records, you will have excellent documentation when it comes time to write another grant, and so on), and I have personally been frustrated when my laboratory cannot duplicate published work of others. However, questions raised include who will pay for reproducing the work of others and how will the sacrifice of additional animals or subjects be justified? Many laboratories are already financially strapped due to current funding challenges and time is also extremely valuable. In addition, junior researchers are on tenure and promotion timelines that provide stress and need for publications to establish independence and credibility, and established investigators must document continued productivity to be judged adequate to obtain continued funding.
The quality of peer review of research publications has also been challenged recently, adding to the concern over the veracity of published research. Many journals now have mandatory statistical review prior to acceptance. This also delays time to publication. In addition, the generous reviewers who perform peer review often do so at the cost of their valuable, uncompensated time.
Despite these hurdles and questions, those who perform valuable and needed research to improve the lives and care of our patients must continue to strive to produce the highest level of evidence.
Dr. Jennifer S. Lawton is a professor of surgery at the division of cardiothoracic surgery, Washington University, St. Louis. She is also an associate medical editor for Thoracic Surgery News.
In the recent Nature editorial, “Repetitive flaws,” comments are offered regarding the new NIH guidelines that require grant proposals to account for biological variables and describe how experimental materials may be authenticated (2016 Jan 21. doi: 10.1038/529256a). It is proposed that these requirements will attempt to improve the quality and reproducibility of research. Many concerns regarding scientific reproducibility have been raised in the past few years. As the editorial states, the NIH guidelines “can help to make researchers aspire to the values that produced them” and they can “inspire researchers to uphold their identity and integrity.”
To those investigators who strive to report only their best results following exhaustive and sincere confirmation, these guidelines will not seem threatening. Providing experimental details of one’s work is helpful in many ways (you can personally reproduce them with new and different lab personnel or after a lapse of time, you will have excellent experimental records, you will have excellent documentation when it comes time to write another grant, and so on), and I have personally been frustrated when my laboratory cannot duplicate published work of others. However, questions raised include who will pay for reproducing the work of others and how will the sacrifice of additional animals or subjects be justified? Many laboratories are already financially strapped due to current funding challenges and time is also extremely valuable. In addition, junior researchers are on tenure and promotion timelines that provide stress and need for publications to establish independence and credibility, and established investigators must document continued productivity to be judged adequate to obtain continued funding.
The quality of peer review of research publications has also been challenged recently, adding to the concern over the veracity of published research. Many journals now have mandatory statistical review prior to acceptance. This also delays time to publication. In addition, the generous reviewers who perform peer review often do so at the cost of their valuable, uncompensated time.
Despite these hurdles and questions, those who perform valuable and needed research to improve the lives and care of our patients must continue to strive to produce the highest level of evidence.
Dr. Jennifer S. Lawton is a professor of surgery at the division of cardiothoracic surgery, Washington University, St. Louis. She is also an associate medical editor for Thoracic Surgery News.
In the recent Nature editorial, “Repetitive flaws,” comments are offered regarding the new NIH guidelines that require grant proposals to account for biological variables and describe how experimental materials may be authenticated (2016 Jan 21. doi: 10.1038/529256a). It is proposed that these requirements will attempt to improve the quality and reproducibility of research. Many concerns regarding scientific reproducibility have been raised in the past few years. As the editorial states, the NIH guidelines “can help to make researchers aspire to the values that produced them” and they can “inspire researchers to uphold their identity and integrity.”
To those investigators who strive to report only their best results following exhaustive and sincere confirmation, these guidelines will not seem threatening. Providing experimental details of one’s work is helpful in many ways (you can personally reproduce them with new and different lab personnel or after a lapse of time, you will have excellent experimental records, you will have excellent documentation when it comes time to write another grant, and so on), and I have personally been frustrated when my laboratory cannot duplicate published work of others. However, questions raised include who will pay for reproducing the work of others and how will the sacrifice of additional animals or subjects be justified? Many laboratories are already financially strapped due to current funding challenges and time is also extremely valuable. In addition, junior researchers are on tenure and promotion timelines that provide stress and need for publications to establish independence and credibility, and established investigators must document continued productivity to be judged adequate to obtain continued funding.
The quality of peer review of research publications has also been challenged recently, adding to the concern over the veracity of published research. Many journals now have mandatory statistical review prior to acceptance. This also delays time to publication. In addition, the generous reviewers who perform peer review often do so at the cost of their valuable, uncompensated time.
Despite these hurdles and questions, those who perform valuable and needed research to improve the lives and care of our patients must continue to strive to produce the highest level of evidence.
Dr. Jennifer S. Lawton is a professor of surgery at the division of cardiothoracic surgery, Washington University, St. Louis. She is also an associate medical editor for Thoracic Surgery News.
The issue of scientific reproducibility has come to the fore in the past several years, driven by noteworthy failures to replicate critical findings in several much-publicized reports coupled to a series of scandals calling into question the role of journals and granting agencies in maintaining quality and oversight.
In a special Nature online collection, the journal assembled articles and perspectives from 2011 to the present dealing with this issue of research reproducibility in science and medicine. These articles were supplemented with current editorial comment.
Seeing these broad spectrum concerns pulled together in one place makes it difficult not to be pessimistic about the current state of research investigations across the board. The saving grace, however, is that these same reports show that a lot of people realize that there is a problem – people who are trying to make changes and who are in a position to be effective.
According to the reports presented in the collection, the problems in research accountability and reproducibility have grown to an alarming extent. In one estimate, irreproducibility ends up costing biomedical research some $28 billion wasted dollars per year (Nature. 2015 Jun 9. doi: 10.1038/nature.2015.17711).
A litany of concerns
In 2012, scientists at AMGEN (Thousand Oaks, Calif.) reported that, even cooperating closely with the original investigators, they were able to reproduce only 6 of 53 studies considered to be benchmarks of cancer research (Nature. 2016 Feb 4. doi: 10.1038/nature.2016.19269).
Scientists at Bayer HealthCare reported in Nature Reviews Drug Discovery that they could successfully reproduce results in only a quarter of 67 so-called seminal studies (2011 Sep. doi: 10.1038/nrd3439-c1).
According to a 2013 report in The Economist, Dr. John Ioannidis, an expert in the field of scientific reproducibility, argued that in his field, “epidemiology, you might expect one in ten hypotheses to be true. In exploratory disciplines like genomics, which rely on combing through vast troves of data about genes and proteins for interesting relationships, you might expect just one in a thousand to prove correct.”
This increasing litany of irreproducibility has raised alarm in the scientific community and has led to a search for answers, as so many preclinical studies form the precursor data for eventual human trials.
Despite the concerns raised, human clinical trials seem to be less at risk for irreproducibility, according to an editorial by Dr. Francis S. Collins, director, and Dr. Lawrence A. Tabak, principal deputy director of the U.S. National Institutes of Health, “because they are already governed by various regulations that stipulate rigorous design and independent oversight – including randomization, blinding, power estimates, pre-registration of outcome measures in standardized, public databases such as ClinicalTrials.gov and oversight by institutional review boards and data safety monitoring boards. Furthermore, the clinical trials community has taken important steps toward adopting standard reporting elements,” (Nature. 2014 Jan. doi: 10.1038/505612a).
The paucity of P
Today, the P-value, .05 or less, is all too often considered the sine qua non of scientific proof. “Most statisticians consider this appalling, as the P value was never intended to be used as a strong indicator of certainty as it too often is today. Most scientists would look at [a] P value of .01 and say that there was just a 1% chance of [the] result being a false alarm. But they would be wrong.” The 2014 report goes on to state how, according to one widely used calculation by authentic statisticians, a P value of .01 corresponds to a false-alarm probability of at least 11%, depending on the underlying probability that there is a true effect; a P value of .05 raises that chance of a false alarm to at least 29% (Nature. 2014 Feb. doi: 10.1038/506150a).
Beyond this assessment problem, P values may allow for considerable researcher bias, conscious and unconscious, even to the extent of encouraging “P-hacking”: one of the few statistical terms to ever make it into the Urban Dictionary. “P-hacking is trying multiple things until you get the desired result” – even unconsciously, according to one researcher quoted.
In addition, “unless statistical power is very high (and much higher than in most experiments), the P value should be interpreted tentatively at best” (Nat Methods. 2015 Feb 26. doi: 10.1038/nmeth.3288).
So bad is the problem that “misuse of the P value – a common test for judging the strength of scientific evidence – is contributing to the number of research findings that cannot be reproduced,” the American Statistical Association warns in a statement released in March, adding that the P value cannot be used to determine whether a hypothesis is true or even whether results are important (Nature. 2016 Mar 7. doi: 10.1038/nature.2016.19503).
And none of this even remotely addresses those instances where researchers report findings that “trend towards significance” when they can’t even meet the magical P threshold.
A muddling of mice (and more)
Fundamental to biological research is the vast array of preliminary animal studies that must be performed before clinical testing can begin.
Animal-based research has been under intense scrutiny due to a variety of perceived flaws and omissions that have been found to be all too common. For example, in a report in PLoS Biology, Dr. Ulrich Dirnagl of the Charité Medical University in Berlin reviewed 100 reports published between 2000 and 2013, which included 522 experiments using rodents to test cancer and stroke treatments. Around two-thirds of the experiments did not report whether any animals had been dropped from the final analysis, and of the 30% that did report rodents dropped from analysis, only 14 explained why (2016 Jan 4. doi: 10.1371/journal.pbio.1002331). Similarly, Dr. John Ioannidis and his colleagues assessed a random sample of 268 biomedical papers listed in PubMed published between 2000 and 2014 and found that only one contained sufficient details to replicate the work (Nature. 2016 Jan 5. doi: 10.1038/nature.2015.19101).
A multitude of genetic and environmental factors have also been found influential in animal research. For example, the gut microbiome (which has been found to influence many aspects of mouse health and metabolism) varies widely in the same species of mice fed on different diets or obtained from different vendors. And there can be differences in physiology and behavior based on circadian rhythms, and even variations in cage design (Nature. 2016 Feb 16. doi: 10.1038/530254a).
But things are looking brighter. By the beginning of 2016, more than 600 journals had signed up for the voluntary ARRIVE (Animals in Research: Reporting of In Vivo Experiments) guidelines designed to improve the reporting of animal experiments. The guidelines include a checklist of elements to be included in any reporting of animal research, including animal strain, sex, and adverse events (Nature. 2016 Feb 1. doi: 10.1038/nature.2016.19274).
Problems have also been reported in the use of cell lines and antibodies in biomedical research. For example, a report in Nature indicated that too many biomedical researchers are lax in checking for impostor cell lines when they perform their research (Nature. 2015 Oct 12. doi: 10.1038/nature.2015.18544). And recent studies have shown that improper or misused antibodies are a significant source of false findings and irreproducibility in the modern literature (Nature. 2015 May 19. doi: 10.1038/521274a).
Reviewer, view thyself
The editorial in The Economist also discussed some of the failures of the peer-reviewed scientific literature, usually considered the final gateway of quality control, to provide appropriate review and correction of research errors. The editorial cites a damning test of lower-tier research publications by Dr. John Bohannon, a biologist at Harvard, who submitted a pseudonymous paper on the effects of a chemical derived from lichen cells to 304 journals describing themselves as using peer review. The paper was concocted wholesale with manifold and obvious errors in study design, analysis, and interpretation of results, according to Dr. Bohannon. This fictitious paper from a fictitious researcher based at a fictitious university was accepted for publication by an alarming 147 of the journals.
The problem is not new. In 1998, Dr. Fiona Godlee, editor of the British Medical Journal, sent an article with eight deliberate mistakes in study design, analysis, and interpretation to more than 200 of the journal’s regular reviewers. None of the reviewers found all the mistakes, and on average, they spotted fewer than two. And another study by the BMJ showed that experience was an issue, not in improving quality of reviewers, but quite the opposite. Over a 14-year period assessed, 1,500 referees, as rated by editors at leading journals, showed a slow but steady drop in their scores.
Such studies prompted a profound reassessment by the journals, in part pushed by some major granting agencies, including the National Institutes of Health.
Not taking grants for granted
The National Institutes for Health are advancing efforts to expand scientific rigor and reproducibility in their grants projects.
“As part of an increasing drive to boost the reliability of research, the NIH will require applicants to explain the scientific premise behind their proposals and defend the quality of their experimental designs. They must also account for biological variables (for example, by including both male and female mice in planned studies) and describe how they will authenticate experimental materials such as cell lines and antibodies.”
Whether current efforts by scientists, societies, granting organizations, and journals can lead to authentic reform and a vast and relatively quick improvement in reproducibility of scientific results is still an open question. In discussing a 2015 report on the subject by the biomedical research community in the United Kingdom, neurophysiologist Dr. Dorothy Bishop had this to say: “I feel quite upbeat about it. ... Now that we’re aware of it, we have all sorts of ideas about how to deal with it. These are doable things. I feel that the mood is one of making science a much better thing. It might lead to slightly slower science. That could be better” (Nature. 2015 Oct 29. doi: 10.1038/nature.2015.18684).
The issue of scientific reproducibility has come to the fore in the past several years, driven by noteworthy failures to replicate critical findings in several much-publicized reports coupled to a series of scandals calling into question the role of journals and granting agencies in maintaining quality and oversight.
In a special Nature online collection, the journal assembled articles and perspectives from 2011 to the present dealing with this issue of research reproducibility in science and medicine. These articles were supplemented with current editorial comment.
Seeing these broad spectrum concerns pulled together in one place makes it difficult not to be pessimistic about the current state of research investigations across the board. The saving grace, however, is that these same reports show that a lot of people realize that there is a problem – people who are trying to make changes and who are in a position to be effective.
According to the reports presented in the collection, the problems in research accountability and reproducibility have grown to an alarming extent. In one estimate, irreproducibility ends up costing biomedical research some $28 billion wasted dollars per year (Nature. 2015 Jun 9. doi: 10.1038/nature.2015.17711).
A litany of concerns
In 2012, scientists at AMGEN (Thousand Oaks, Calif.) reported that, even cooperating closely with the original investigators, they were able to reproduce only 6 of 53 studies considered to be benchmarks of cancer research (Nature. 2016 Feb 4. doi: 10.1038/nature.2016.19269).
Scientists at Bayer HealthCare reported in Nature Reviews Drug Discovery that they could successfully reproduce results in only a quarter of 67 so-called seminal studies (2011 Sep. doi: 10.1038/nrd3439-c1).
According to a 2013 report in The Economist, Dr. John Ioannidis, an expert in the field of scientific reproducibility, argued that in his field, “epidemiology, you might expect one in ten hypotheses to be true. In exploratory disciplines like genomics, which rely on combing through vast troves of data about genes and proteins for interesting relationships, you might expect just one in a thousand to prove correct.”
This increasing litany of irreproducibility has raised alarm in the scientific community and has led to a search for answers, as so many preclinical studies form the precursor data for eventual human trials.
Despite the concerns raised, human clinical trials seem to be less at risk for irreproducibility, according to an editorial by Dr. Francis S. Collins, director, and Dr. Lawrence A. Tabak, principal deputy director of the U.S. National Institutes of Health, “because they are already governed by various regulations that stipulate rigorous design and independent oversight – including randomization, blinding, power estimates, pre-registration of outcome measures in standardized, public databases such as ClinicalTrials.gov and oversight by institutional review boards and data safety monitoring boards. Furthermore, the clinical trials community has taken important steps toward adopting standard reporting elements,” (Nature. 2014 Jan. doi: 10.1038/505612a).
The paucity of P
Today, the P-value, .05 or less, is all too often considered the sine qua non of scientific proof. “Most statisticians consider this appalling, as the P value was never intended to be used as a strong indicator of certainty as it too often is today. Most scientists would look at [a] P value of .01 and say that there was just a 1% chance of [the] result being a false alarm. But they would be wrong.” The 2014 report goes on to state how, according to one widely used calculation by authentic statisticians, a P value of .01 corresponds to a false-alarm probability of at least 11%, depending on the underlying probability that there is a true effect; a P value of .05 raises that chance of a false alarm to at least 29% (Nature. 2014 Feb. doi: 10.1038/506150a).
Beyond this assessment problem, P values may allow for considerable researcher bias, conscious and unconscious, even to the extent of encouraging “P-hacking”: one of the few statistical terms to ever make it into the Urban Dictionary. “P-hacking is trying multiple things until you get the desired result” – even unconsciously, according to one researcher quoted.
In addition, “unless statistical power is very high (and much higher than in most experiments), the P value should be interpreted tentatively at best” (Nat Methods. 2015 Feb 26. doi: 10.1038/nmeth.3288).
So bad is the problem that “misuse of the P value – a common test for judging the strength of scientific evidence – is contributing to the number of research findings that cannot be reproduced,” the American Statistical Association warns in a statement released in March, adding that the P value cannot be used to determine whether a hypothesis is true or even whether results are important (Nature. 2016 Mar 7. doi: 10.1038/nature.2016.19503).
And none of this even remotely addresses those instances where researchers report findings that “trend towards significance” when they can’t even meet the magical P threshold.
A muddling of mice (and more)
Fundamental to biological research is the vast array of preliminary animal studies that must be performed before clinical testing can begin.
Animal-based research has been under intense scrutiny due to a variety of perceived flaws and omissions that have been found to be all too common. For example, in a report in PLoS Biology, Dr. Ulrich Dirnagl of the Charité Medical University in Berlin reviewed 100 reports published between 2000 and 2013, which included 522 experiments using rodents to test cancer and stroke treatments. Around two-thirds of the experiments did not report whether any animals had been dropped from the final analysis, and of the 30% that did report rodents dropped from analysis, only 14 explained why (2016 Jan 4. doi: 10.1371/journal.pbio.1002331). Similarly, Dr. John Ioannidis and his colleagues assessed a random sample of 268 biomedical papers listed in PubMed published between 2000 and 2014 and found that only one contained sufficient details to replicate the work (Nature. 2016 Jan 5. doi: 10.1038/nature.2015.19101).
A multitude of genetic and environmental factors have also been found influential in animal research. For example, the gut microbiome (which has been found to influence many aspects of mouse health and metabolism) varies widely in the same species of mice fed on different diets or obtained from different vendors. And there can be differences in physiology and behavior based on circadian rhythms, and even variations in cage design (Nature. 2016 Feb 16. doi: 10.1038/530254a).
But things are looking brighter. By the beginning of 2016, more than 600 journals had signed up for the voluntary ARRIVE (Animals in Research: Reporting of In Vivo Experiments) guidelines designed to improve the reporting of animal experiments. The guidelines include a checklist of elements to be included in any reporting of animal research, including animal strain, sex, and adverse events (Nature. 2016 Feb 1. doi: 10.1038/nature.2016.19274).
Problems have also been reported in the use of cell lines and antibodies in biomedical research. For example, a report in Nature indicated that too many biomedical researchers are lax in checking for impostor cell lines when they perform their research (Nature. 2015 Oct 12. doi: 10.1038/nature.2015.18544). And recent studies have shown that improper or misused antibodies are a significant source of false findings and irreproducibility in the modern literature (Nature. 2015 May 19. doi: 10.1038/521274a).
Reviewer, view thyself
The editorial in The Economist also discussed some of the failures of the peer-reviewed scientific literature, usually considered the final gateway of quality control, to provide appropriate review and correction of research errors. The editorial cites a damning test of lower-tier research publications by Dr. John Bohannon, a biologist at Harvard, who submitted a pseudonymous paper on the effects of a chemical derived from lichen cells to 304 journals describing themselves as using peer review. The paper was concocted wholesale with manifold and obvious errors in study design, analysis, and interpretation of results, according to Dr. Bohannon. This fictitious paper from a fictitious researcher based at a fictitious university was accepted for publication by an alarming 147 of the journals.
The problem is not new. In 1998, Dr. Fiona Godlee, editor of the British Medical Journal, sent an article with eight deliberate mistakes in study design, analysis, and interpretation to more than 200 of the journal’s regular reviewers. None of the reviewers found all the mistakes, and on average, they spotted fewer than two. And another study by the BMJ showed that experience was an issue, not in improving quality of reviewers, but quite the opposite. Over a 14-year period assessed, 1,500 referees, as rated by editors at leading journals, showed a slow but steady drop in their scores.
Such studies prompted a profound reassessment by the journals, in part pushed by some major granting agencies, including the National Institutes of Health.
Not taking grants for granted
The National Institutes for Health are advancing efforts to expand scientific rigor and reproducibility in their grants projects.
“As part of an increasing drive to boost the reliability of research, the NIH will require applicants to explain the scientific premise behind their proposals and defend the quality of their experimental designs. They must also account for biological variables (for example, by including both male and female mice in planned studies) and describe how they will authenticate experimental materials such as cell lines and antibodies.”
Whether current efforts by scientists, societies, granting organizations, and journals can lead to authentic reform and a vast and relatively quick improvement in reproducibility of scientific results is still an open question. In discussing a 2015 report on the subject by the biomedical research community in the United Kingdom, neurophysiologist Dr. Dorothy Bishop had this to say: “I feel quite upbeat about it. ... Now that we’re aware of it, we have all sorts of ideas about how to deal with it. These are doable things. I feel that the mood is one of making science a much better thing. It might lead to slightly slower science. That could be better” (Nature. 2015 Oct 29. doi: 10.1038/nature.2015.18684).