User login
Thyroid surgery access and acceptance varies along racial lines
BOSTON – Access to and acceptance of thyroid cancer surgery varies by race, with black patients in particular appearing to be disadvantaged, compared with whites, investigators reported.
A review of data on nearly 138,000 patients diagnosed with thyroid cancer showed that blacks were significantly less likely than were whites to be offered surgery – despite its generally excellent outcomes and low rates of morbidity and mortality, reported Dr. Herbert Castillo Valladares and his colleagues from the department of surgery at the Yale University in New Haven, Conn.
American Indians/Alaskan natives and Asian/Pacific Islanders were significantly more likely to refuse surgery than were whites, the investigators also reported in a poster session at the Society of Surgical Oncology annual cancer symposium.
“In this project, we wanted to focus on the provider-level factors that might be perpetuating these racial disparities, and it appears that we need to educate some providers about the recommendation of surgery or how to educate patients who refuse thyroid cancer surgery,” Dr. Valladares said in an interview.
The investigators noted that although incidence and prevalence rates of thyroid cancer are similar among various racial groups, survival differs by race, and they wanted to find out why. To do so, they polled the Surveillance, Epidemiology, and End Results (SEER) registry to identify 137,483 patients diagnosed with thyroid cancer during 1988-2012. Results were stratified by thyroid cancer type, either papillary, medullary, follicular, or anaplastic.
In all, 82% of the sample were white, 75% were female, 87% had a diagnosis of papillary thyroid cancer, and 95% underwent thyroid cancer surgery.
In logistic regression analysis that controlled for race, the investigators found that blacks, Asian/Pacific Islanders, and persons of unknown race were significantly less likely than whites were to have thyroid cancer surgery (odds ratios, 0.7, 0.82, and 0.34, respectively; P for each less than .0001).
Similarly, surgery was more frequently not recommended for blacks (OR, 1.34; P less than .0001), Asian/Pacific Islanders (OR, 1.2; P = .004) and those of unknown race (OR, 3.06; P less than .0001).
American Indians/Alaskan natives and Asian/Pacific Islanders were also significantly more likely than were whites to refuse surgery (OR, 4.45; P = .0001; OR, 2.96; P less than .0001, respectively).
Compared with whites, blacks – but not other races – had significantly worse 5-year survival (hazard ratio, 1.14; P = .0002).
In an analysis by cancer type, the investigators saw that race was not a predictor for surgery recommendation or refusal of surgery by patients with medullary or anaplastic cancer. However, among patients with papillary thyroid cancer, the most common type, surgery was recommended less often for blacks (OR, 1.2), Asian/Pacific Islanders (OR, 1.3), and patients of unknown race (OR, 3.1; all comparisons significant by 95% confidence interval).
Among patients with follicular histology, patients of unknown race were significantly less likely than were whites to have the surgery recommended (OR, 2.7; significant by 95% CI).
Dr. Valladares explained that the SEER data set does not include information about provider type, such as those in community based versus academic settings, so the next step will be to find a method for analyzing factors at both the patient level and the provider level that might influence recommendations for surgery or patient refusals to accept surgery.
The study was supported by the Paul H. Lavietes, M.D., Summer Research Fellowship of Yale University. The investigators reported no relevant conflicts of interest.
BOSTON – Access to and acceptance of thyroid cancer surgery varies by race, with black patients in particular appearing to be disadvantaged, compared with whites, investigators reported.
A review of data on nearly 138,000 patients diagnosed with thyroid cancer showed that blacks were significantly less likely than were whites to be offered surgery – despite its generally excellent outcomes and low rates of morbidity and mortality, reported Dr. Herbert Castillo Valladares and his colleagues from the department of surgery at the Yale University in New Haven, Conn.
American Indians/Alaskan natives and Asian/Pacific Islanders were significantly more likely to refuse surgery than were whites, the investigators also reported in a poster session at the Society of Surgical Oncology annual cancer symposium.
“In this project, we wanted to focus on the provider-level factors that might be perpetuating these racial disparities, and it appears that we need to educate some providers about the recommendation of surgery or how to educate patients who refuse thyroid cancer surgery,” Dr. Valladares said in an interview.
The investigators noted that although incidence and prevalence rates of thyroid cancer are similar among various racial groups, survival differs by race, and they wanted to find out why. To do so, they polled the Surveillance, Epidemiology, and End Results (SEER) registry to identify 137,483 patients diagnosed with thyroid cancer during 1988-2012. Results were stratified by thyroid cancer type, either papillary, medullary, follicular, or anaplastic.
In all, 82% of the sample were white, 75% were female, 87% had a diagnosis of papillary thyroid cancer, and 95% underwent thyroid cancer surgery.
In logistic regression analysis that controlled for race, the investigators found that blacks, Asian/Pacific Islanders, and persons of unknown race were significantly less likely than whites were to have thyroid cancer surgery (odds ratios, 0.7, 0.82, and 0.34, respectively; P for each less than .0001).
Similarly, surgery was more frequently not recommended for blacks (OR, 1.34; P less than .0001), Asian/Pacific Islanders (OR, 1.2; P = .004) and those of unknown race (OR, 3.06; P less than .0001).
American Indians/Alaskan natives and Asian/Pacific Islanders were also significantly more likely than were whites to refuse surgery (OR, 4.45; P = .0001; OR, 2.96; P less than .0001, respectively).
Compared with whites, blacks – but not other races – had significantly worse 5-year survival (hazard ratio, 1.14; P = .0002).
In an analysis by cancer type, the investigators saw that race was not a predictor for surgery recommendation or refusal of surgery by patients with medullary or anaplastic cancer. However, among patients with papillary thyroid cancer, the most common type, surgery was recommended less often for blacks (OR, 1.2), Asian/Pacific Islanders (OR, 1.3), and patients of unknown race (OR, 3.1; all comparisons significant by 95% confidence interval).
Among patients with follicular histology, patients of unknown race were significantly less likely than were whites to have the surgery recommended (OR, 2.7; significant by 95% CI).
Dr. Valladares explained that the SEER data set does not include information about provider type, such as those in community based versus academic settings, so the next step will be to find a method for analyzing factors at both the patient level and the provider level that might influence recommendations for surgery or patient refusals to accept surgery.
The study was supported by the Paul H. Lavietes, M.D., Summer Research Fellowship of Yale University. The investigators reported no relevant conflicts of interest.
BOSTON – Access to and acceptance of thyroid cancer surgery varies by race, with black patients in particular appearing to be disadvantaged, compared with whites, investigators reported.
A review of data on nearly 138,000 patients diagnosed with thyroid cancer showed that blacks were significantly less likely than were whites to be offered surgery – despite its generally excellent outcomes and low rates of morbidity and mortality, reported Dr. Herbert Castillo Valladares and his colleagues from the department of surgery at the Yale University in New Haven, Conn.
American Indians/Alaskan natives and Asian/Pacific Islanders were significantly more likely to refuse surgery than were whites, the investigators also reported in a poster session at the Society of Surgical Oncology annual cancer symposium.
“In this project, we wanted to focus on the provider-level factors that might be perpetuating these racial disparities, and it appears that we need to educate some providers about the recommendation of surgery or how to educate patients who refuse thyroid cancer surgery,” Dr. Valladares said in an interview.
The investigators noted that although incidence and prevalence rates of thyroid cancer are similar among various racial groups, survival differs by race, and they wanted to find out why. To do so, they polled the Surveillance, Epidemiology, and End Results (SEER) registry to identify 137,483 patients diagnosed with thyroid cancer during 1988-2012. Results were stratified by thyroid cancer type, either papillary, medullary, follicular, or anaplastic.
In all, 82% of the sample were white, 75% were female, 87% had a diagnosis of papillary thyroid cancer, and 95% underwent thyroid cancer surgery.
In logistic regression analysis that controlled for race, the investigators found that blacks, Asian/Pacific Islanders, and persons of unknown race were significantly less likely than whites were to have thyroid cancer surgery (odds ratios, 0.7, 0.82, and 0.34, respectively; P for each less than .0001).
Similarly, surgery was more frequently not recommended for blacks (OR, 1.34; P less than .0001), Asian/Pacific Islanders (OR, 1.2; P = .004) and those of unknown race (OR, 3.06; P less than .0001).
American Indians/Alaskan natives and Asian/Pacific Islanders were also significantly more likely than were whites to refuse surgery (OR, 4.45; P = .0001; OR, 2.96; P less than .0001, respectively).
Compared with whites, blacks – but not other races – had significantly worse 5-year survival (hazard ratio, 1.14; P = .0002).
In an analysis by cancer type, the investigators saw that race was not a predictor for surgery recommendation or refusal of surgery by patients with medullary or anaplastic cancer. However, among patients with papillary thyroid cancer, the most common type, surgery was recommended less often for blacks (OR, 1.2), Asian/Pacific Islanders (OR, 1.3), and patients of unknown race (OR, 3.1; all comparisons significant by 95% confidence interval).
Among patients with follicular histology, patients of unknown race were significantly less likely than were whites to have the surgery recommended (OR, 2.7; significant by 95% CI).
Dr. Valladares explained that the SEER data set does not include information about provider type, such as those in community based versus academic settings, so the next step will be to find a method for analyzing factors at both the patient level and the provider level that might influence recommendations for surgery or patient refusals to accept surgery.
The study was supported by the Paul H. Lavietes, M.D., Summer Research Fellowship of Yale University. The investigators reported no relevant conflicts of interest.
FROM SSO 2016
Key clinical point: Compared with whites, blacks, Asian/Pacific Islanders and persons of unknown race were significantly less likely than were whites to have thyroid cancer surgery.
Major finding: Asian/Pacific Islanders and persons of unknown race were significantly less likely than were whites to have thyroid cancer surgery (OR, 0.7, 0.82, and 0.34, respectively; P for each less than .0001).
Data source: SEER data on 137,483 patients with thyroid cancer during 1988-2012.
Disclosures: The study was supported by a Paul H. Lavietes, M.D., Summer Research Fellowship at Yale University. The investigators reported no relevant conflicts of interest.
Fresh Press: ACS Surgery News March digital issue is available
The March issue of ACS Surgery News is available online. Use the mobile app to download or view as a pdf.
This month, Dr. David B. Hoyt, ACS Executive Director, offers our readers a review of ACS initiatives in 2015 to support surgeons in training and in practice. An international surgical program participant, Dr. Michael L. Bentz, describes the experience as a “partnership among friends.” And the American Pain Society has published a new postop pain guideline that surgeons should find informative.
Don’t miss the commentary by Dr. Peter Angelos on the complications surrounding a surgeon’s recommendation not to operate.
The March issue of ACS Surgery News is available online. Use the mobile app to download or view as a pdf.
This month, Dr. David B. Hoyt, ACS Executive Director, offers our readers a review of ACS initiatives in 2015 to support surgeons in training and in practice. An international surgical program participant, Dr. Michael L. Bentz, describes the experience as a “partnership among friends.” And the American Pain Society has published a new postop pain guideline that surgeons should find informative.
Don’t miss the commentary by Dr. Peter Angelos on the complications surrounding a surgeon’s recommendation not to operate.
The March issue of ACS Surgery News is available online. Use the mobile app to download or view as a pdf.
This month, Dr. David B. Hoyt, ACS Executive Director, offers our readers a review of ACS initiatives in 2015 to support surgeons in training and in practice. An international surgical program participant, Dr. Michael L. Bentz, describes the experience as a “partnership among friends.” And the American Pain Society has published a new postop pain guideline that surgeons should find informative.
Don’t miss the commentary by Dr. Peter Angelos on the complications surrounding a surgeon’s recommendation not to operate.
New CDC opioid guideline targets overprescribing for chronic pain
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
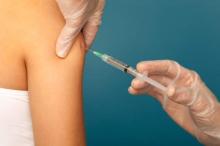
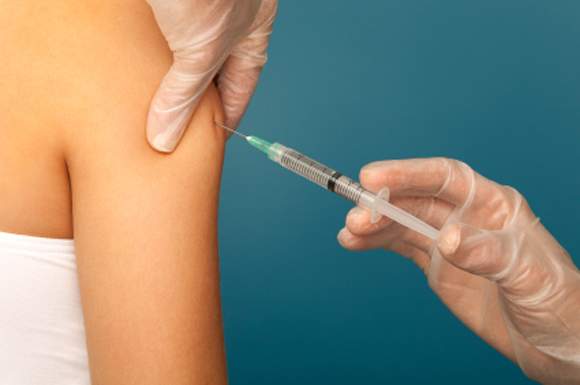
Flu vaccination found safe in surgical patients
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Immunizing surgical patients against seasonal influenza before they leave the hospital appears safe.
Major finding: Patients received a flu vaccine in only 6,420 hospital stays for surgery, comprising only 15% of the patient hospitalizations that were eligible.
Data source: A retrospective cohort study involving 81,647 surgeries at 14 California hospitals during three consecutive flu seasons.
Disclosures: This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Outcomes in major surgery unchanged by continuing clopidogrel
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Outcomes were similar whether patients did or didn’t stop clopidogrel before major surgery.
Major finding: No significant differences in blood product use, adverse events, or death were seen with continuing clopidogrel.
Data source: Retrospective, single-center review of 200 patients undergoing major elective or emergent surgery and taking clopidogrel.
Disclosures: The study authors reported no relevant disclosures.
Global Surgery: ‘Partnership Among Friends’
Surgery volunteerism has been on the rise for several decades. The American College of Surgeons is increasing its role in organizing and facilitating these programs via Operation Giving Back (OGB). And many ACS members are prominent participants in this endeavor.
A leader in global surgery is Michael L. Bentz, M.D., FAAP, FACS, professor of surgery, pediatrics, and neurosurgery, and chairman of the Division of Plastic and Reconstructive Surgery at the University of Wisconsin School of Medicine and Public Health. Dr. Bentz has led international missions in many countries of the world over nearly 20 years and has helped a team develop a long-term program of clinical care and training in Nicaragua. We talked with him about his experiences.
Q: You have been involved in international surgical missions for many years. Can you tell us something about your early projects?
I was first exposed to international work at the University of Pittsburgh. My mentor J. William Futrell, M.D., FACS, was a veteran of over 30 international surgical trips. I went on the first trip with him to Vietnam in the 1997 and have been going ever since. For that initial trip, we worked with a nonprofit organization called Interplast. I went with a large group of 20 people from the University that included plastic surgery attendings, plastic surgery residents, pediatric attendings, pediatric residents, and nursing and anesthesia staff.
In those days, many trips were based predominantly on clinical care – adult care and pediatric care. Teams would do a certain number of operations and then go home. We did cleft lip repairs, cleft palate repairs, burn reconstruction, congenital hand deformity surgery, and tumor management.
That would result in good outcomes for those who actually had a procedure done. But in any place I have ever worked overseas – Vietnam, China, Russia, Nicaragua – the need is overwhelming. The need far outstripped what surgical missions can provide in isolated, single trips back and forth.
Q: The years have brought changes to these missions. What are the most significant changes over the years in how these missions are conducted?
The scope and direction of global health is moving toward sustainable, long-term, and longitudinal education. In those earlier trips where there was an emphasis on doing as many operations as possible, people meant well – we meant well! But the real impact comes with the longitudinal education investment.
I have never been anywhere around the world where there weren’t interested, very capable, excellent surgeons committed to taking care of their patients who only need some support and facilitation.
If you compare the cases we are able to do on a trip with our partners with the cases they are able to do independently, it’s a logarithmic curve – they are far more productive than we could ever be on any number of trips. There is a multiplier effect that allows many more patients to be taken care of.
Q: Your institution has a long-term relationship with a hospital in Nicaragua. How does this work and what is the role of your team in the program?
The University of Wisconsin Division of Plastic Surgery and the Eduplast Foundation has a team of about 10 that goes to Nicaragua twice a year. Most importantly, we support a residency program in there. We move residents through a 3-year modular program much like programs in the U.S. and then examine them. We facilitate this educational process with trips there and we bring them to our institution in the U.S.
Over the past 10 years, we have been doing a weekly live webcast of our Plastic Surgery Grand Rounds which is received on several continents. This creates a very valuable bidirectional, and even tridirectional conversation. This webcast is simple, incredibly inexpensive, and has provided hundreds of hours of education over the years in addition to the on-site work we do.
There can be a language barrier in some cases, but we broadcast in English, with occasional translation support. In addition to Nicaragua, our webcast has been received in institutions in Thailand, China, Ecuador and across the United States. We keep records of cases performed. Our plastic surgery residents can get credit for the cases they do under faculty supervision at our international sites if we meet specific criteria set by our Resident Review Committee.
It is important to note that we take care of the patients in our partner institutions in Nicaragua exactly as we would care for patients in our institution in Wisconsin. There is no “practicing” as all operations are done by surgeons appropriately credentialed and trained for the task.
Q: Do you find that there is a cultural gap that you must bridge in working with colleagues and patients in Nicaragua?
Our program has an orientation session for team participants in advance of each trip, where we talk about the mechanics of the trip – safety, medical issues. We also talk about cultural considerations of each site. It is very important that the residents embed in the culture in which we are working. They also need to know the cultural norms of how to communicate with patients, parents, and children. Some of it is simply good manners – acting like your mother taught you!
The team can reside in a local hotel, but often stays in the homes of local hosts, and this can be a beautiful opportunity to learn about local norms and communication.
Q: What is your favorite part of these missions?
I have so many favorite parts! I like caring for people who otherwise might not receive medical care. This is “giving back” and I think all of the participants would agree that we come home feeling like we received much more than we gave. These experiences remind you of why you went to medical school. It is an opportunity to provide something in return for all the investment that has been made in us for our education. In working with colleagues from other countries, I learn as much as I teach. I come back a better surgeon.
The benefits to residents from our institution are many. They learn how to operate in a resource-limited setting, and they return with a greater appreciation for the equipment and supplies we have available at our institution in Madison. The cultural competence and awareness they also learn is an invaluable life skill.
I want to stress that the friendships with our fellow surgeons are what makes this work. We achieve a degree of continuity and even watch our pediatric patients grow up over the years because of our long-term relationship with the hospital in León and our dedicated colleagues there. This is a truly a partnership among friends.
Q: Do you have some advice for a surgeon interested in participating in an international program?
For those surgeons who were not exposed to these programs during residency, finding a mentor or mentoring organization is the way to begin. A beginner should consider making the first couple of trips with someone who knows the ropes in terms of understanding cultural competency, practical issues of safety, and relevant clinical issues. Almost every surgery discipline has an organization with the capability of identifying volunteer surgery groups in their specialty. ACS’ Operation Giving Back is a particularly important resource for helping Fellows find the right international program.
If you would like to learn more about global surgery programs, contact Operation Giving Back at [email protected]. Or if you would like to share your experiences as an international surgical volunteer, please email this publication at [email protected].
Surgery volunteerism has been on the rise for several decades. The American College of Surgeons is increasing its role in organizing and facilitating these programs via Operation Giving Back (OGB). And many ACS members are prominent participants in this endeavor.
A leader in global surgery is Michael L. Bentz, M.D., FAAP, FACS, professor of surgery, pediatrics, and neurosurgery, and chairman of the Division of Plastic and Reconstructive Surgery at the University of Wisconsin School of Medicine and Public Health. Dr. Bentz has led international missions in many countries of the world over nearly 20 years and has helped a team develop a long-term program of clinical care and training in Nicaragua. We talked with him about his experiences.
Q: You have been involved in international surgical missions for many years. Can you tell us something about your early projects?
I was first exposed to international work at the University of Pittsburgh. My mentor J. William Futrell, M.D., FACS, was a veteran of over 30 international surgical trips. I went on the first trip with him to Vietnam in the 1997 and have been going ever since. For that initial trip, we worked with a nonprofit organization called Interplast. I went with a large group of 20 people from the University that included plastic surgery attendings, plastic surgery residents, pediatric attendings, pediatric residents, and nursing and anesthesia staff.
In those days, many trips were based predominantly on clinical care – adult care and pediatric care. Teams would do a certain number of operations and then go home. We did cleft lip repairs, cleft palate repairs, burn reconstruction, congenital hand deformity surgery, and tumor management.
That would result in good outcomes for those who actually had a procedure done. But in any place I have ever worked overseas – Vietnam, China, Russia, Nicaragua – the need is overwhelming. The need far outstripped what surgical missions can provide in isolated, single trips back and forth.
Q: The years have brought changes to these missions. What are the most significant changes over the years in how these missions are conducted?
The scope and direction of global health is moving toward sustainable, long-term, and longitudinal education. In those earlier trips where there was an emphasis on doing as many operations as possible, people meant well – we meant well! But the real impact comes with the longitudinal education investment.
I have never been anywhere around the world where there weren’t interested, very capable, excellent surgeons committed to taking care of their patients who only need some support and facilitation.
If you compare the cases we are able to do on a trip with our partners with the cases they are able to do independently, it’s a logarithmic curve – they are far more productive than we could ever be on any number of trips. There is a multiplier effect that allows many more patients to be taken care of.
Q: Your institution has a long-term relationship with a hospital in Nicaragua. How does this work and what is the role of your team in the program?
The University of Wisconsin Division of Plastic Surgery and the Eduplast Foundation has a team of about 10 that goes to Nicaragua twice a year. Most importantly, we support a residency program in there. We move residents through a 3-year modular program much like programs in the U.S. and then examine them. We facilitate this educational process with trips there and we bring them to our institution in the U.S.
Over the past 10 years, we have been doing a weekly live webcast of our Plastic Surgery Grand Rounds which is received on several continents. This creates a very valuable bidirectional, and even tridirectional conversation. This webcast is simple, incredibly inexpensive, and has provided hundreds of hours of education over the years in addition to the on-site work we do.
There can be a language barrier in some cases, but we broadcast in English, with occasional translation support. In addition to Nicaragua, our webcast has been received in institutions in Thailand, China, Ecuador and across the United States. We keep records of cases performed. Our plastic surgery residents can get credit for the cases they do under faculty supervision at our international sites if we meet specific criteria set by our Resident Review Committee.
It is important to note that we take care of the patients in our partner institutions in Nicaragua exactly as we would care for patients in our institution in Wisconsin. There is no “practicing” as all operations are done by surgeons appropriately credentialed and trained for the task.
Q: Do you find that there is a cultural gap that you must bridge in working with colleagues and patients in Nicaragua?
Our program has an orientation session for team participants in advance of each trip, where we talk about the mechanics of the trip – safety, medical issues. We also talk about cultural considerations of each site. It is very important that the residents embed in the culture in which we are working. They also need to know the cultural norms of how to communicate with patients, parents, and children. Some of it is simply good manners – acting like your mother taught you!
The team can reside in a local hotel, but often stays in the homes of local hosts, and this can be a beautiful opportunity to learn about local norms and communication.
Q: What is your favorite part of these missions?
I have so many favorite parts! I like caring for people who otherwise might not receive medical care. This is “giving back” and I think all of the participants would agree that we come home feeling like we received much more than we gave. These experiences remind you of why you went to medical school. It is an opportunity to provide something in return for all the investment that has been made in us for our education. In working with colleagues from other countries, I learn as much as I teach. I come back a better surgeon.
The benefits to residents from our institution are many. They learn how to operate in a resource-limited setting, and they return with a greater appreciation for the equipment and supplies we have available at our institution in Madison. The cultural competence and awareness they also learn is an invaluable life skill.
I want to stress that the friendships with our fellow surgeons are what makes this work. We achieve a degree of continuity and even watch our pediatric patients grow up over the years because of our long-term relationship with the hospital in León and our dedicated colleagues there. This is a truly a partnership among friends.
Q: Do you have some advice for a surgeon interested in participating in an international program?
For those surgeons who were not exposed to these programs during residency, finding a mentor or mentoring organization is the way to begin. A beginner should consider making the first couple of trips with someone who knows the ropes in terms of understanding cultural competency, practical issues of safety, and relevant clinical issues. Almost every surgery discipline has an organization with the capability of identifying volunteer surgery groups in their specialty. ACS’ Operation Giving Back is a particularly important resource for helping Fellows find the right international program.
If you would like to learn more about global surgery programs, contact Operation Giving Back at [email protected]. Or if you would like to share your experiences as an international surgical volunteer, please email this publication at [email protected].
Surgery volunteerism has been on the rise for several decades. The American College of Surgeons is increasing its role in organizing and facilitating these programs via Operation Giving Back (OGB). And many ACS members are prominent participants in this endeavor.
A leader in global surgery is Michael L. Bentz, M.D., FAAP, FACS, professor of surgery, pediatrics, and neurosurgery, and chairman of the Division of Plastic and Reconstructive Surgery at the University of Wisconsin School of Medicine and Public Health. Dr. Bentz has led international missions in many countries of the world over nearly 20 years and has helped a team develop a long-term program of clinical care and training in Nicaragua. We talked with him about his experiences.
Q: You have been involved in international surgical missions for many years. Can you tell us something about your early projects?
I was first exposed to international work at the University of Pittsburgh. My mentor J. William Futrell, M.D., FACS, was a veteran of over 30 international surgical trips. I went on the first trip with him to Vietnam in the 1997 and have been going ever since. For that initial trip, we worked with a nonprofit organization called Interplast. I went with a large group of 20 people from the University that included plastic surgery attendings, plastic surgery residents, pediatric attendings, pediatric residents, and nursing and anesthesia staff.
In those days, many trips were based predominantly on clinical care – adult care and pediatric care. Teams would do a certain number of operations and then go home. We did cleft lip repairs, cleft palate repairs, burn reconstruction, congenital hand deformity surgery, and tumor management.
That would result in good outcomes for those who actually had a procedure done. But in any place I have ever worked overseas – Vietnam, China, Russia, Nicaragua – the need is overwhelming. The need far outstripped what surgical missions can provide in isolated, single trips back and forth.
Q: The years have brought changes to these missions. What are the most significant changes over the years in how these missions are conducted?
The scope and direction of global health is moving toward sustainable, long-term, and longitudinal education. In those earlier trips where there was an emphasis on doing as many operations as possible, people meant well – we meant well! But the real impact comes with the longitudinal education investment.
I have never been anywhere around the world where there weren’t interested, very capable, excellent surgeons committed to taking care of their patients who only need some support and facilitation.
If you compare the cases we are able to do on a trip with our partners with the cases they are able to do independently, it’s a logarithmic curve – they are far more productive than we could ever be on any number of trips. There is a multiplier effect that allows many more patients to be taken care of.
Q: Your institution has a long-term relationship with a hospital in Nicaragua. How does this work and what is the role of your team in the program?
The University of Wisconsin Division of Plastic Surgery and the Eduplast Foundation has a team of about 10 that goes to Nicaragua twice a year. Most importantly, we support a residency program in there. We move residents through a 3-year modular program much like programs in the U.S. and then examine them. We facilitate this educational process with trips there and we bring them to our institution in the U.S.
Over the past 10 years, we have been doing a weekly live webcast of our Plastic Surgery Grand Rounds which is received on several continents. This creates a very valuable bidirectional, and even tridirectional conversation. This webcast is simple, incredibly inexpensive, and has provided hundreds of hours of education over the years in addition to the on-site work we do.
There can be a language barrier in some cases, but we broadcast in English, with occasional translation support. In addition to Nicaragua, our webcast has been received in institutions in Thailand, China, Ecuador and across the United States. We keep records of cases performed. Our plastic surgery residents can get credit for the cases they do under faculty supervision at our international sites if we meet specific criteria set by our Resident Review Committee.
It is important to note that we take care of the patients in our partner institutions in Nicaragua exactly as we would care for patients in our institution in Wisconsin. There is no “practicing” as all operations are done by surgeons appropriately credentialed and trained for the task.
Q: Do you find that there is a cultural gap that you must bridge in working with colleagues and patients in Nicaragua?
Our program has an orientation session for team participants in advance of each trip, where we talk about the mechanics of the trip – safety, medical issues. We also talk about cultural considerations of each site. It is very important that the residents embed in the culture in which we are working. They also need to know the cultural norms of how to communicate with patients, parents, and children. Some of it is simply good manners – acting like your mother taught you!
The team can reside in a local hotel, but often stays in the homes of local hosts, and this can be a beautiful opportunity to learn about local norms and communication.
Q: What is your favorite part of these missions?
I have so many favorite parts! I like caring for people who otherwise might not receive medical care. This is “giving back” and I think all of the participants would agree that we come home feeling like we received much more than we gave. These experiences remind you of why you went to medical school. It is an opportunity to provide something in return for all the investment that has been made in us for our education. In working with colleagues from other countries, I learn as much as I teach. I come back a better surgeon.
The benefits to residents from our institution are many. They learn how to operate in a resource-limited setting, and they return with a greater appreciation for the equipment and supplies we have available at our institution in Madison. The cultural competence and awareness they also learn is an invaluable life skill.
I want to stress that the friendships with our fellow surgeons are what makes this work. We achieve a degree of continuity and even watch our pediatric patients grow up over the years because of our long-term relationship with the hospital in León and our dedicated colleagues there. This is a truly a partnership among friends.
Q: Do you have some advice for a surgeon interested in participating in an international program?
For those surgeons who were not exposed to these programs during residency, finding a mentor or mentoring organization is the way to begin. A beginner should consider making the first couple of trips with someone who knows the ropes in terms of understanding cultural competency, practical issues of safety, and relevant clinical issues. Almost every surgery discipline has an organization with the capability of identifying volunteer surgery groups in their specialty. ACS’ Operation Giving Back is a particularly important resource for helping Fellows find the right international program.
If you would like to learn more about global surgery programs, contact Operation Giving Back at [email protected]. Or if you would like to share your experiences as an international surgical volunteer, please email this publication at [email protected].
The Right Choice? When surgery is not the right choice
“A chance to cut is a chance to cure.” This common saying summarizes much of the philosophy of surgery. If we have an opportunity to do an operation and fix something, surgeons find it easy to recommend surgery. There is an immediacy about surgery and a surgical cure that is appealing to most surgeons.
As I think back to medical school, I remember that my decision to become a surgeon was greatly influenced by my subinternship in medicine. Whenever one of my patients needed an operation, I was always anxious to know what the surgeons were finding in the operating room. Having been a surgeon for many years now, I never tire of the opportunity to do something in the operating room that solves a patient’s problem. It is not surprising that when there is a surgical option for a problem, many surgeons find themselves recommending surgery. While it may be true that there is often an economic incentive for surgeons to recommend surgery, there are also many situations in which thoughtful surgeons recommend against surgery.
There are many cases in which the risks of the operation outweigh the benefits. If, for example, a patient with widely metastatic colon cancer presents for surgical evaluation, the recommendation will most commonly be against surgery. In such a case, the goal of cure or increased longevity may not be met by an operation to remove a portion of the colon when there will be a significant burden of disease that cannot be removed. In addition to cases of unresectable cancer, there are many situations in which the patient’s comorbidities make the risks of surgery far higher than the benefits. In such cases, surgeons commonly recommend against surgery or, in some cases, actually do not offer surgery as an option. Most often in such situations, the surgeon is consulted for an opinion and once surgery is deemed not to be an option, the surgeon generally steps aside to allow other doctors to provide care for the patient whether it be medical therapies, palliative care, or a combination of both.
In recent years, surgeons have increasingly been involved in nonoperative management strategies. For example, some thyroid cancer patients with small, presumed indolent cancers are entered into clinical trials where observation is one of the arms of the trial. Perhaps most well established is the recommendation that patients with early stage (Gleason 6) prostate cancer consider “active surveillance” as an option to be considered along with surgery and radiation. What is particularly notable in the case of prostate cancer is that even when the recommendation is made for active surveillance, most of these patients continue to follow up with the urologist. In this scenario, even though surgery may not have been recommended by the urologist or chosen by the patient, it is the urologist who maintains the ongoing surveillance with the patient.
In an era in which surgeons often complain about being treated purely as technicians, the role of surgeons in active surveillance should be seen as a breath of fresh air. Here, the surgeons are recommending a course of action that is much less financially beneficial than an operation. Having surgeons involved in such nonoperative strategies clearly expresses the belief that surgeons have in these approaches.
There is another important reason why surgeons should become increasingly engaged in nonoperative treatment strategies. The credibility of the recommendation to consider NOT having surgery is exponentially higher if the recommendation is made by a surgeon. Patients know that surgeons like to operate, are trained to operate, and, in many cases, are paid to operate. In this setting, the recommendation to forgo or, at least, postpone an operation is particularly influential.
There is widespread public acceptance of Abraham Maslow’s statement, “if all you have is a hammer, everything looks like a nail.” When a surgeon recommends something other than surgery it is a much stronger endorsement of the nonoperative treatment than if a nonsurgeon had made the same recommendation. Perhaps equally important is that a recommendation against surgery with ongoing engagement by the surgeon is an illustration of the surgeon’s acting in the patient’s best interests rather than in the surgeon’s best interests. Even though it may always be more difficult not to offer an operation to a patient, surgeons should not shy away from recommending nonoperative strategies when there is clear evidence that such strategies may be better for patients. Although not every patient will be comfortable with a nonoperative approach, surgeons should seek every opportunity to participate fully in such decisions when nonoperative treatments may be in the patients’ best interests.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
“A chance to cut is a chance to cure.” This common saying summarizes much of the philosophy of surgery. If we have an opportunity to do an operation and fix something, surgeons find it easy to recommend surgery. There is an immediacy about surgery and a surgical cure that is appealing to most surgeons.
As I think back to medical school, I remember that my decision to become a surgeon was greatly influenced by my subinternship in medicine. Whenever one of my patients needed an operation, I was always anxious to know what the surgeons were finding in the operating room. Having been a surgeon for many years now, I never tire of the opportunity to do something in the operating room that solves a patient’s problem. It is not surprising that when there is a surgical option for a problem, many surgeons find themselves recommending surgery. While it may be true that there is often an economic incentive for surgeons to recommend surgery, there are also many situations in which thoughtful surgeons recommend against surgery.
There are many cases in which the risks of the operation outweigh the benefits. If, for example, a patient with widely metastatic colon cancer presents for surgical evaluation, the recommendation will most commonly be against surgery. In such a case, the goal of cure or increased longevity may not be met by an operation to remove a portion of the colon when there will be a significant burden of disease that cannot be removed. In addition to cases of unresectable cancer, there are many situations in which the patient’s comorbidities make the risks of surgery far higher than the benefits. In such cases, surgeons commonly recommend against surgery or, in some cases, actually do not offer surgery as an option. Most often in such situations, the surgeon is consulted for an opinion and once surgery is deemed not to be an option, the surgeon generally steps aside to allow other doctors to provide care for the patient whether it be medical therapies, palliative care, or a combination of both.
In recent years, surgeons have increasingly been involved in nonoperative management strategies. For example, some thyroid cancer patients with small, presumed indolent cancers are entered into clinical trials where observation is one of the arms of the trial. Perhaps most well established is the recommendation that patients with early stage (Gleason 6) prostate cancer consider “active surveillance” as an option to be considered along with surgery and radiation. What is particularly notable in the case of prostate cancer is that even when the recommendation is made for active surveillance, most of these patients continue to follow up with the urologist. In this scenario, even though surgery may not have been recommended by the urologist or chosen by the patient, it is the urologist who maintains the ongoing surveillance with the patient.
In an era in which surgeons often complain about being treated purely as technicians, the role of surgeons in active surveillance should be seen as a breath of fresh air. Here, the surgeons are recommending a course of action that is much less financially beneficial than an operation. Having surgeons involved in such nonoperative strategies clearly expresses the belief that surgeons have in these approaches.
There is another important reason why surgeons should become increasingly engaged in nonoperative treatment strategies. The credibility of the recommendation to consider NOT having surgery is exponentially higher if the recommendation is made by a surgeon. Patients know that surgeons like to operate, are trained to operate, and, in many cases, are paid to operate. In this setting, the recommendation to forgo or, at least, postpone an operation is particularly influential.
There is widespread public acceptance of Abraham Maslow’s statement, “if all you have is a hammer, everything looks like a nail.” When a surgeon recommends something other than surgery it is a much stronger endorsement of the nonoperative treatment than if a nonsurgeon had made the same recommendation. Perhaps equally important is that a recommendation against surgery with ongoing engagement by the surgeon is an illustration of the surgeon’s acting in the patient’s best interests rather than in the surgeon’s best interests. Even though it may always be more difficult not to offer an operation to a patient, surgeons should not shy away from recommending nonoperative strategies when there is clear evidence that such strategies may be better for patients. Although not every patient will be comfortable with a nonoperative approach, surgeons should seek every opportunity to participate fully in such decisions when nonoperative treatments may be in the patients’ best interests.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
“A chance to cut is a chance to cure.” This common saying summarizes much of the philosophy of surgery. If we have an opportunity to do an operation and fix something, surgeons find it easy to recommend surgery. There is an immediacy about surgery and a surgical cure that is appealing to most surgeons.
As I think back to medical school, I remember that my decision to become a surgeon was greatly influenced by my subinternship in medicine. Whenever one of my patients needed an operation, I was always anxious to know what the surgeons were finding in the operating room. Having been a surgeon for many years now, I never tire of the opportunity to do something in the operating room that solves a patient’s problem. It is not surprising that when there is a surgical option for a problem, many surgeons find themselves recommending surgery. While it may be true that there is often an economic incentive for surgeons to recommend surgery, there are also many situations in which thoughtful surgeons recommend against surgery.
There are many cases in which the risks of the operation outweigh the benefits. If, for example, a patient with widely metastatic colon cancer presents for surgical evaluation, the recommendation will most commonly be against surgery. In such a case, the goal of cure or increased longevity may not be met by an operation to remove a portion of the colon when there will be a significant burden of disease that cannot be removed. In addition to cases of unresectable cancer, there are many situations in which the patient’s comorbidities make the risks of surgery far higher than the benefits. In such cases, surgeons commonly recommend against surgery or, in some cases, actually do not offer surgery as an option. Most often in such situations, the surgeon is consulted for an opinion and once surgery is deemed not to be an option, the surgeon generally steps aside to allow other doctors to provide care for the patient whether it be medical therapies, palliative care, or a combination of both.
In recent years, surgeons have increasingly been involved in nonoperative management strategies. For example, some thyroid cancer patients with small, presumed indolent cancers are entered into clinical trials where observation is one of the arms of the trial. Perhaps most well established is the recommendation that patients with early stage (Gleason 6) prostate cancer consider “active surveillance” as an option to be considered along with surgery and radiation. What is particularly notable in the case of prostate cancer is that even when the recommendation is made for active surveillance, most of these patients continue to follow up with the urologist. In this scenario, even though surgery may not have been recommended by the urologist or chosen by the patient, it is the urologist who maintains the ongoing surveillance with the patient.
In an era in which surgeons often complain about being treated purely as technicians, the role of surgeons in active surveillance should be seen as a breath of fresh air. Here, the surgeons are recommending a course of action that is much less financially beneficial than an operation. Having surgeons involved in such nonoperative strategies clearly expresses the belief that surgeons have in these approaches.
There is another important reason why surgeons should become increasingly engaged in nonoperative treatment strategies. The credibility of the recommendation to consider NOT having surgery is exponentially higher if the recommendation is made by a surgeon. Patients know that surgeons like to operate, are trained to operate, and, in many cases, are paid to operate. In this setting, the recommendation to forgo or, at least, postpone an operation is particularly influential.
There is widespread public acceptance of Abraham Maslow’s statement, “if all you have is a hammer, everything looks like a nail.” When a surgeon recommends something other than surgery it is a much stronger endorsement of the nonoperative treatment than if a nonsurgeon had made the same recommendation. Perhaps equally important is that a recommendation against surgery with ongoing engagement by the surgeon is an illustration of the surgeon’s acting in the patient’s best interests rather than in the surgeon’s best interests. Even though it may always be more difficult not to offer an operation to a patient, surgeons should not shy away from recommending nonoperative strategies when there is clear evidence that such strategies may be better for patients. Although not every patient will be comfortable with a nonoperative approach, surgeons should seek every opportunity to participate fully in such decisions when nonoperative treatments may be in the patients’ best interests.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Ultrasound bested tomosynthesis for screening dense breast tissue
Ultrasound was about 1.8 times more sensitive than tomosynthesis for the incremental detection of breast cancer in women with radiologically dense breasts and negative two-dimensional mammography screening, according to interim results from the first prospective trial to directly compare the two modalities.
“However, future application of adjunct screening should consider that tomosynthesis detected more than 50% of the additional breast cancers in these women, and could potentially be [a] primary screening modality,” wrote Dr. Alberto Tagliafico of the University of Genoa (Italy) and his associates. The study was published online March 9 in the Journal of Clinical Oncology and presented simultaneously at the European Breast Cancer Conference.
Radiologically dense breast tissue undermines the sensitivity of mammography and is itself an independent risk factor for breast cancer. Recently, many states began requiring that women be informed of their breast density and adjunct screening measures, such as ultrasound. But estimates of the sensitivity of ultrasound have ranged from about 1.9 to 4.2 cancers for every 1,000 screens, said the researchers. This variance, combined with costs and concerns about false-positive recalls, have fueled debates about the value of adjunct measures in breast cancer screening, they added. To help clarify these issues, the multicenter ASTOUND (Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts) study compared physician-performed ultrasound and tomosynthesis results for 3,231 asymptomatic women aged 44 to 71 years, whose median age was 51 years (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.63.4147).
In all, the researchers detected 24 additional breast cancers, 23 of which were invasive. Thus, ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 screens for tomosynthesis (95% CI, 1.8-6.2; P = .006). Only one cancer was detected by tomosynthesis alone. The rate of false-positive recalls was similar for the two modalities – 53 cases for tomosynthesis, versus 63 for ultrasound (P = .26). Rates of false-positive recalls leading to biopsy also were similar. Needle biopsies usually sufficed in recalled cases, but two women underwent surgical biopsies, both of which revealed radial scars.
If the final results of ASTOUND confirm these interim data, “it could be argued that breast tomosynthesis has little value in a setting where adjunct ultrasound is frequently used for screening women with mammography-dense breasts,” said the researchers. But tomosynthesis may have a role as a primary screening modality in other setting, especially because tomosynthesis acquisitions that also provide reconstructed 2D mammography are now available, lessening concerns about unjustified radiation exposure, they added.
The “modest” number of cancers in the interim report led to relatively wide confidence intervals, the investigators noted. Biomarker data were not available for all cancers, and both prevalent and incident ultrasound data were compared with prevalent tomosynthesis data, which might bias false-positive recall results in favor of ultrasound, they added.
Currently, 24 American states have laws requiring that women receive some level of notification about breast density with their mammography results. Dense breast tissue can hide cancer on mammography, especially when the cancer lacks calcifications, resulting in delayed diagnosis and worse outcomes. Moreover, dense breast tissue is an independent risk factor for developing breast cancer.
Because the primary goal of screening is detection of early breast cancer, ultrasound would seem the clear choice, compared with tomosynthesis. Given comparable false-positive rates in ASTOUND, the estimated cost per cancer detected would be similar or more favorable for ultrasound than tomosynthesis. Ultrasound equipment is becoming much less expensive, requires no ionizing radiation, and it is easy to guide needle biopsy of lesions seen only on ultrasound.
Preliminary results from ASTOUND are extremely important in helping to inform personalized screening choices for women with dense breasts. Guidelines on these issues are planned, but often limit recommendations to those based on evidence from randomized trials with mortality as an end point. Our knowledge of the natural history of breast cancer and results from randomized trials of mammography should inform guidelines for supplemental screening.
Dr. Wendie A. Berg is at Magee-Womens Hospital of University of Pittsburgh Medical Center. She reported serving in a consulting or advisory role with SuperSonic Imagine. These comments were taken from her accompanying editorial (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.65.8674).
Currently, 24 American states have laws requiring that women receive some level of notification about breast density with their mammography results. Dense breast tissue can hide cancer on mammography, especially when the cancer lacks calcifications, resulting in delayed diagnosis and worse outcomes. Moreover, dense breast tissue is an independent risk factor for developing breast cancer.
Because the primary goal of screening is detection of early breast cancer, ultrasound would seem the clear choice, compared with tomosynthesis. Given comparable false-positive rates in ASTOUND, the estimated cost per cancer detected would be similar or more favorable for ultrasound than tomosynthesis. Ultrasound equipment is becoming much less expensive, requires no ionizing radiation, and it is easy to guide needle biopsy of lesions seen only on ultrasound.
Preliminary results from ASTOUND are extremely important in helping to inform personalized screening choices for women with dense breasts. Guidelines on these issues are planned, but often limit recommendations to those based on evidence from randomized trials with mortality as an end point. Our knowledge of the natural history of breast cancer and results from randomized trials of mammography should inform guidelines for supplemental screening.
Dr. Wendie A. Berg is at Magee-Womens Hospital of University of Pittsburgh Medical Center. She reported serving in a consulting or advisory role with SuperSonic Imagine. These comments were taken from her accompanying editorial (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.65.8674).
Currently, 24 American states have laws requiring that women receive some level of notification about breast density with their mammography results. Dense breast tissue can hide cancer on mammography, especially when the cancer lacks calcifications, resulting in delayed diagnosis and worse outcomes. Moreover, dense breast tissue is an independent risk factor for developing breast cancer.
Because the primary goal of screening is detection of early breast cancer, ultrasound would seem the clear choice, compared with tomosynthesis. Given comparable false-positive rates in ASTOUND, the estimated cost per cancer detected would be similar or more favorable for ultrasound than tomosynthesis. Ultrasound equipment is becoming much less expensive, requires no ionizing radiation, and it is easy to guide needle biopsy of lesions seen only on ultrasound.
Preliminary results from ASTOUND are extremely important in helping to inform personalized screening choices for women with dense breasts. Guidelines on these issues are planned, but often limit recommendations to those based on evidence from randomized trials with mortality as an end point. Our knowledge of the natural history of breast cancer and results from randomized trials of mammography should inform guidelines for supplemental screening.
Dr. Wendie A. Berg is at Magee-Womens Hospital of University of Pittsburgh Medical Center. She reported serving in a consulting or advisory role with SuperSonic Imagine. These comments were taken from her accompanying editorial (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.65.8674).
Ultrasound was about 1.8 times more sensitive than tomosynthesis for the incremental detection of breast cancer in women with radiologically dense breasts and negative two-dimensional mammography screening, according to interim results from the first prospective trial to directly compare the two modalities.
“However, future application of adjunct screening should consider that tomosynthesis detected more than 50% of the additional breast cancers in these women, and could potentially be [a] primary screening modality,” wrote Dr. Alberto Tagliafico of the University of Genoa (Italy) and his associates. The study was published online March 9 in the Journal of Clinical Oncology and presented simultaneously at the European Breast Cancer Conference.
Radiologically dense breast tissue undermines the sensitivity of mammography and is itself an independent risk factor for breast cancer. Recently, many states began requiring that women be informed of their breast density and adjunct screening measures, such as ultrasound. But estimates of the sensitivity of ultrasound have ranged from about 1.9 to 4.2 cancers for every 1,000 screens, said the researchers. This variance, combined with costs and concerns about false-positive recalls, have fueled debates about the value of adjunct measures in breast cancer screening, they added. To help clarify these issues, the multicenter ASTOUND (Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts) study compared physician-performed ultrasound and tomosynthesis results for 3,231 asymptomatic women aged 44 to 71 years, whose median age was 51 years (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.63.4147).
In all, the researchers detected 24 additional breast cancers, 23 of which were invasive. Thus, ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 screens for tomosynthesis (95% CI, 1.8-6.2; P = .006). Only one cancer was detected by tomosynthesis alone. The rate of false-positive recalls was similar for the two modalities – 53 cases for tomosynthesis, versus 63 for ultrasound (P = .26). Rates of false-positive recalls leading to biopsy also were similar. Needle biopsies usually sufficed in recalled cases, but two women underwent surgical biopsies, both of which revealed radial scars.
If the final results of ASTOUND confirm these interim data, “it could be argued that breast tomosynthesis has little value in a setting where adjunct ultrasound is frequently used for screening women with mammography-dense breasts,” said the researchers. But tomosynthesis may have a role as a primary screening modality in other setting, especially because tomosynthesis acquisitions that also provide reconstructed 2D mammography are now available, lessening concerns about unjustified radiation exposure, they added.
The “modest” number of cancers in the interim report led to relatively wide confidence intervals, the investigators noted. Biomarker data were not available for all cancers, and both prevalent and incident ultrasound data were compared with prevalent tomosynthesis data, which might bias false-positive recall results in favor of ultrasound, they added.
Ultrasound was about 1.8 times more sensitive than tomosynthesis for the incremental detection of breast cancer in women with radiologically dense breasts and negative two-dimensional mammography screening, according to interim results from the first prospective trial to directly compare the two modalities.
“However, future application of adjunct screening should consider that tomosynthesis detected more than 50% of the additional breast cancers in these women, and could potentially be [a] primary screening modality,” wrote Dr. Alberto Tagliafico of the University of Genoa (Italy) and his associates. The study was published online March 9 in the Journal of Clinical Oncology and presented simultaneously at the European Breast Cancer Conference.
Radiologically dense breast tissue undermines the sensitivity of mammography and is itself an independent risk factor for breast cancer. Recently, many states began requiring that women be informed of their breast density and adjunct screening measures, such as ultrasound. But estimates of the sensitivity of ultrasound have ranged from about 1.9 to 4.2 cancers for every 1,000 screens, said the researchers. This variance, combined with costs and concerns about false-positive recalls, have fueled debates about the value of adjunct measures in breast cancer screening, they added. To help clarify these issues, the multicenter ASTOUND (Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts) study compared physician-performed ultrasound and tomosynthesis results for 3,231 asymptomatic women aged 44 to 71 years, whose median age was 51 years (J Clin Oncol. 2016 Mar 9. doi: 10.1200/JCO.2015.63.4147).
In all, the researchers detected 24 additional breast cancers, 23 of which were invasive. Thus, ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 screens for tomosynthesis (95% CI, 1.8-6.2; P = .006). Only one cancer was detected by tomosynthesis alone. The rate of false-positive recalls was similar for the two modalities – 53 cases for tomosynthesis, versus 63 for ultrasound (P = .26). Rates of false-positive recalls leading to biopsy also were similar. Needle biopsies usually sufficed in recalled cases, but two women underwent surgical biopsies, both of which revealed radial scars.
If the final results of ASTOUND confirm these interim data, “it could be argued that breast tomosynthesis has little value in a setting where adjunct ultrasound is frequently used for screening women with mammography-dense breasts,” said the researchers. But tomosynthesis may have a role as a primary screening modality in other setting, especially because tomosynthesis acquisitions that also provide reconstructed 2D mammography are now available, lessening concerns about unjustified radiation exposure, they added.
The “modest” number of cancers in the interim report led to relatively wide confidence intervals, the investigators noted. Biomarker data were not available for all cancers, and both prevalent and incident ultrasound data were compared with prevalent tomosynthesis data, which might bias false-positive recall results in favor of ultrasound, they added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Ultrasound outperformed tomosynthesis for the incremental detection of breast cancer in women with negative mammograms and radiologically dense breast tissue.
Major finding: Ultrasound detected about 7.1 additional cancers for every 1,000 screens (95% confidence interval, 4.2-10), compared with 4.0 additional cancers per 1,000 tomosynthesis screens (95% CI, 1.8-6.2; P = .006).
Data source: A multicenter, prospective trial comparing the two modalities in 3,231 women.
Disclosures: The University of Genoa sponsors the ASTOUND study. Dr. Tagliafico disclosed honoraria from Esaote-Philips, patents, royalties, or other intellectual property from Springer, and travel, accommodations, and expense support from Hologic and Technologic. Four coinvestigators reported financial relationships with several device and pharmaceutical companies. The senior author and the other seven coinvestigators had no disclosures.
Antibiotic-resistant infections remain a persistent threat
One in every seven infections in acute care hospitals related to catheters and surgeries was caused by antibiotic-resistant bacteria. In long-term acute care hospitals, that number increased to one in four.
Those are key findings from a study published March 3 in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report that is the first to combine national data on antibiotic-resistant (AR) bacteria threats with progress on health care–associated infections (HAIs).
“Antibiotic resistance threatens to return us to a time when a simple infection could kill,” CDC Director Thomas Frieden said during a March 3 telebriefing. “The more people who get infected with resistant bacteria, the more people who suffer complications, the more who, tragically, may die from preventable infections. On any given day about one in 25 hospitalized patients has at least one health care–associated infection that they didn’t come in with. No one should get sick when they’re trying to get well.”
For the study, researchers led by Dr. Clifford McDonald of the CDC’s Division of Healthcare Quality Promotion, collected data on specific infections that were reported to the National Healthcare Safety Network in 2014 by approximately 4,000 short-term acute care hospitals, 501 long-term acute care hospitals, and 1,135 inpatient rehabilitation facilities in all 50 states (MMWR. 2016 Mar 3. doi: 10.15585/mmwr.mm6509e1er). Next, they determined the proportions of AR pathogens and HAIs caused by any of six resistant bacteria highlighted by the CDC in 2013 as urgent or serious threats: CRE (carbapenem-resistant Enterobacteriaceae), MRSA (methicillin-resistant Staphylococcus aureus), ESBL-producing Enterobacteriaceae (extended-spectrum beta-lactamases), VRE (vancomycin-resistant enterococci), multidrug-resistant pseudomonas, and multidrug-resistant Acinetobacter.
The researchers found that, compared with historical data from 5-8 years earlier, central line–associated bloodstream infections decreased by 50% and surgical site infections (SSIs) by 17% in 2014.
“There is encouraging news here,” Dr. Frieden said. “Doctors, nurses, hospitals, health care systems and other partners have made progress preventing some health care–associated infections.” However, the study found that one in six remaining central line-associated bloodstream infections were caused by urgent or serious antibiotic-resistant bacteria, while one in seven remaining surgical site infections were caused by urgent or serious antibiotic-resistant bacteria.
While catheter-associated urinary tract infections appear unchanged from baseline, there have been recent decreases, according to the study. In addition, C. difficile infections in hospitals decreased 8% between 2011 and 2014.
Dr. McDonald and his associates determined that in 2014, one in seven infections in acute care hospitals related to catheters and surgeries was caused by one of the six antibiotic-resistance threat bacteria, “which is deeply concerning,” Dr. Frieden said. That number increased to one in four infections in long-term acute care hospitals, a proportion that he characterized as “chilling.”
The CDC recommends three strategies that doctors, nurses, and other health care providers should take with every patient, to prevent HAIs and stop the spread of antibiotic resistance:
• Prevent the spread of bacteria between patients. Dr. Peter Pronovost, who participated in the telebriefing, said that he and his associates at Johns Hopkins University in Baltimore “do this by practicing good hand hygiene techniques by wearing sterile equipment when inserting lines.”
• Prevent surgery-related infections and/or placement of a catheter. “Check catheters frequently and remove them when you no longer need them,” advised Dr. Pronovost, director of the Armstrong Institute for Patient Safety and Quality at Johns Hopkins. “Ask if you actually need them before you even place them.”
• Improve antibiotic use through stewardship. This means using “the right antibiotics for the right duration,” Dr. Pronovost said. “Antibiotics could be lifesaving and are necessary for critically ill patients, especially those with septic shock. But these antibiotics need to be adjusted based on lab results and new information about the organisms that are causing these infections. Forty-eight hours after antibiotics are initiated, take a ‘time out.’ Perform a brief but focused assessment to determine if antibiotic therapy is still needed, or if it should be refined. A common mistake we make is to continue vancomycin when there is no presence of MRSA. We often tell our staff at Johns Hopkins, ‘if it doesn’t grow, let it go.’ ”
Dr. Frieden concluded his remarks by noting that physicians and other clinicians on the front lines “need support of their facility leadership,” to prevent HAIs. “Health care facilities, CEOs, and administrators are a major part of the solution. It’s important that they make a priority of infection prevention, sepsis prevention, and antibiotic stewardship. Know your facility’s data and target prevention efforts to ensure improvements in patient safety.”
One in every seven infections in acute care hospitals related to catheters and surgeries was caused by antibiotic-resistant bacteria. In long-term acute care hospitals, that number increased to one in four.
Those are key findings from a study published March 3 in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report that is the first to combine national data on antibiotic-resistant (AR) bacteria threats with progress on health care–associated infections (HAIs).
“Antibiotic resistance threatens to return us to a time when a simple infection could kill,” CDC Director Thomas Frieden said during a March 3 telebriefing. “The more people who get infected with resistant bacteria, the more people who suffer complications, the more who, tragically, may die from preventable infections. On any given day about one in 25 hospitalized patients has at least one health care–associated infection that they didn’t come in with. No one should get sick when they’re trying to get well.”
For the study, researchers led by Dr. Clifford McDonald of the CDC’s Division of Healthcare Quality Promotion, collected data on specific infections that were reported to the National Healthcare Safety Network in 2014 by approximately 4,000 short-term acute care hospitals, 501 long-term acute care hospitals, and 1,135 inpatient rehabilitation facilities in all 50 states (MMWR. 2016 Mar 3. doi: 10.15585/mmwr.mm6509e1er). Next, they determined the proportions of AR pathogens and HAIs caused by any of six resistant bacteria highlighted by the CDC in 2013 as urgent or serious threats: CRE (carbapenem-resistant Enterobacteriaceae), MRSA (methicillin-resistant Staphylococcus aureus), ESBL-producing Enterobacteriaceae (extended-spectrum beta-lactamases), VRE (vancomycin-resistant enterococci), multidrug-resistant pseudomonas, and multidrug-resistant Acinetobacter.
The researchers found that, compared with historical data from 5-8 years earlier, central line–associated bloodstream infections decreased by 50% and surgical site infections (SSIs) by 17% in 2014.
“There is encouraging news here,” Dr. Frieden said. “Doctors, nurses, hospitals, health care systems and other partners have made progress preventing some health care–associated infections.” However, the study found that one in six remaining central line-associated bloodstream infections were caused by urgent or serious antibiotic-resistant bacteria, while one in seven remaining surgical site infections were caused by urgent or serious antibiotic-resistant bacteria.
While catheter-associated urinary tract infections appear unchanged from baseline, there have been recent decreases, according to the study. In addition, C. difficile infections in hospitals decreased 8% between 2011 and 2014.
Dr. McDonald and his associates determined that in 2014, one in seven infections in acute care hospitals related to catheters and surgeries was caused by one of the six antibiotic-resistance threat bacteria, “which is deeply concerning,” Dr. Frieden said. That number increased to one in four infections in long-term acute care hospitals, a proportion that he characterized as “chilling.”
The CDC recommends three strategies that doctors, nurses, and other health care providers should take with every patient, to prevent HAIs and stop the spread of antibiotic resistance:
• Prevent the spread of bacteria between patients. Dr. Peter Pronovost, who participated in the telebriefing, said that he and his associates at Johns Hopkins University in Baltimore “do this by practicing good hand hygiene techniques by wearing sterile equipment when inserting lines.”
• Prevent surgery-related infections and/or placement of a catheter. “Check catheters frequently and remove them when you no longer need them,” advised Dr. Pronovost, director of the Armstrong Institute for Patient Safety and Quality at Johns Hopkins. “Ask if you actually need them before you even place them.”
• Improve antibiotic use through stewardship. This means using “the right antibiotics for the right duration,” Dr. Pronovost said. “Antibiotics could be lifesaving and are necessary for critically ill patients, especially those with septic shock. But these antibiotics need to be adjusted based on lab results and new information about the organisms that are causing these infections. Forty-eight hours after antibiotics are initiated, take a ‘time out.’ Perform a brief but focused assessment to determine if antibiotic therapy is still needed, or if it should be refined. A common mistake we make is to continue vancomycin when there is no presence of MRSA. We often tell our staff at Johns Hopkins, ‘if it doesn’t grow, let it go.’ ”
Dr. Frieden concluded his remarks by noting that physicians and other clinicians on the front lines “need support of their facility leadership,” to prevent HAIs. “Health care facilities, CEOs, and administrators are a major part of the solution. It’s important that they make a priority of infection prevention, sepsis prevention, and antibiotic stewardship. Know your facility’s data and target prevention efforts to ensure improvements in patient safety.”
One in every seven infections in acute care hospitals related to catheters and surgeries was caused by antibiotic-resistant bacteria. In long-term acute care hospitals, that number increased to one in four.
Those are key findings from a study published March 3 in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report that is the first to combine national data on antibiotic-resistant (AR) bacteria threats with progress on health care–associated infections (HAIs).
“Antibiotic resistance threatens to return us to a time when a simple infection could kill,” CDC Director Thomas Frieden said during a March 3 telebriefing. “The more people who get infected with resistant bacteria, the more people who suffer complications, the more who, tragically, may die from preventable infections. On any given day about one in 25 hospitalized patients has at least one health care–associated infection that they didn’t come in with. No one should get sick when they’re trying to get well.”
For the study, researchers led by Dr. Clifford McDonald of the CDC’s Division of Healthcare Quality Promotion, collected data on specific infections that were reported to the National Healthcare Safety Network in 2014 by approximately 4,000 short-term acute care hospitals, 501 long-term acute care hospitals, and 1,135 inpatient rehabilitation facilities in all 50 states (MMWR. 2016 Mar 3. doi: 10.15585/mmwr.mm6509e1er). Next, they determined the proportions of AR pathogens and HAIs caused by any of six resistant bacteria highlighted by the CDC in 2013 as urgent or serious threats: CRE (carbapenem-resistant Enterobacteriaceae), MRSA (methicillin-resistant Staphylococcus aureus), ESBL-producing Enterobacteriaceae (extended-spectrum beta-lactamases), VRE (vancomycin-resistant enterococci), multidrug-resistant pseudomonas, and multidrug-resistant Acinetobacter.
The researchers found that, compared with historical data from 5-8 years earlier, central line–associated bloodstream infections decreased by 50% and surgical site infections (SSIs) by 17% in 2014.
“There is encouraging news here,” Dr. Frieden said. “Doctors, nurses, hospitals, health care systems and other partners have made progress preventing some health care–associated infections.” However, the study found that one in six remaining central line-associated bloodstream infections were caused by urgent or serious antibiotic-resistant bacteria, while one in seven remaining surgical site infections were caused by urgent or serious antibiotic-resistant bacteria.
While catheter-associated urinary tract infections appear unchanged from baseline, there have been recent decreases, according to the study. In addition, C. difficile infections in hospitals decreased 8% between 2011 and 2014.
Dr. McDonald and his associates determined that in 2014, one in seven infections in acute care hospitals related to catheters and surgeries was caused by one of the six antibiotic-resistance threat bacteria, “which is deeply concerning,” Dr. Frieden said. That number increased to one in four infections in long-term acute care hospitals, a proportion that he characterized as “chilling.”
The CDC recommends three strategies that doctors, nurses, and other health care providers should take with every patient, to prevent HAIs and stop the spread of antibiotic resistance:
• Prevent the spread of bacteria between patients. Dr. Peter Pronovost, who participated in the telebriefing, said that he and his associates at Johns Hopkins University in Baltimore “do this by practicing good hand hygiene techniques by wearing sterile equipment when inserting lines.”
• Prevent surgery-related infections and/or placement of a catheter. “Check catheters frequently and remove them when you no longer need them,” advised Dr. Pronovost, director of the Armstrong Institute for Patient Safety and Quality at Johns Hopkins. “Ask if you actually need them before you even place them.”
• Improve antibiotic use through stewardship. This means using “the right antibiotics for the right duration,” Dr. Pronovost said. “Antibiotics could be lifesaving and are necessary for critically ill patients, especially those with septic shock. But these antibiotics need to be adjusted based on lab results and new information about the organisms that are causing these infections. Forty-eight hours after antibiotics are initiated, take a ‘time out.’ Perform a brief but focused assessment to determine if antibiotic therapy is still needed, or if it should be refined. A common mistake we make is to continue vancomycin when there is no presence of MRSA. We often tell our staff at Johns Hopkins, ‘if it doesn’t grow, let it go.’ ”
Dr. Frieden concluded his remarks by noting that physicians and other clinicians on the front lines “need support of their facility leadership,” to prevent HAIs. “Health care facilities, CEOs, and administrators are a major part of the solution. It’s important that they make a priority of infection prevention, sepsis prevention, and antibiotic stewardship. Know your facility’s data and target prevention efforts to ensure improvements in patient safety.”
FROM MMWR
What Matters: What’s the magic behind successful bariatric patients?
A fair number of my patients have had or are undergoing bariatric surgery. Disconcertingly, a not insignificant number of them are regaining the weight after surgery. Weight regain will occur in 20% of patients undergoing bariatric surgery after initial weight loss.
When this occurs, not only do we have a patient with an altered gut putting them at risk for nutritional deficiencies if we are not fastidious in our follow-up, but they are discouraged and overweight again.
Add this to the concern that bariatric surgery has been associated with an increase in suicides (2.33-3.63 per 1000 patient-years), and we may have some cause for alarm.
So, what predicts success – and can we facilitate it?
Several factors have been shown to predict successful weight loss after bariatric surgery. An “active coping style” (that is, planning vs. denial) and adherence to follow-up after bariatric surgery have both been shown to be associated with a higher percentage of excess weight loss. Interestingly, psychological burden and motivation have not been associated with weight loss.
In a recent article, Lori Liebl, Ph.D., and her colleagues conducted a qualitative study of the experiences of adults who successfully maintained weight loss after bariatric surgery (J Clin Nurs. 2016 Feb 23. doi: 10.1111/jocn.13129). Success was defined as 50% or more of the excessive weight loss 24 months after bariatric surgery.
The voice of the successful bariatric patient is an interesting and important one. Several themes were identified: 1) taking life back (“I did it for myself”); 2) a new lease on life (“There are things I can do now that I am not exhausted”); 3) the importance of social support; 4) avoiding the negative (terminating unhealthy relationships in which “food is love”); 5) the void (food addiction and sense of loss); 6) fighting food demons; 7) finding the happy weight; and 8) a ripple effect (that is, if you don’t eat it, the rest of family doesn’t, either).
I was left wondering how I can best help my patients using this information.
First, I think the themes can mature our empathy for the struggles that these patients face, and perhaps help us combat bias. Second, I think this knowledge can inform early discussions around what sorts of things need to be lined up for after the procedure, such as social support.
Finally, I think the themes can be universalized and help us counsel patients who may be struggling with weight, but who are otherwise not candidates for bariatric surgery.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
A fair number of my patients have had or are undergoing bariatric surgery. Disconcertingly, a not insignificant number of them are regaining the weight after surgery. Weight regain will occur in 20% of patients undergoing bariatric surgery after initial weight loss.
When this occurs, not only do we have a patient with an altered gut putting them at risk for nutritional deficiencies if we are not fastidious in our follow-up, but they are discouraged and overweight again.
Add this to the concern that bariatric surgery has been associated with an increase in suicides (2.33-3.63 per 1000 patient-years), and we may have some cause for alarm.
So, what predicts success – and can we facilitate it?
Several factors have been shown to predict successful weight loss after bariatric surgery. An “active coping style” (that is, planning vs. denial) and adherence to follow-up after bariatric surgery have both been shown to be associated with a higher percentage of excess weight loss. Interestingly, psychological burden and motivation have not been associated with weight loss.
In a recent article, Lori Liebl, Ph.D., and her colleagues conducted a qualitative study of the experiences of adults who successfully maintained weight loss after bariatric surgery (J Clin Nurs. 2016 Feb 23. doi: 10.1111/jocn.13129). Success was defined as 50% or more of the excessive weight loss 24 months after bariatric surgery.
The voice of the successful bariatric patient is an interesting and important one. Several themes were identified: 1) taking life back (“I did it for myself”); 2) a new lease on life (“There are things I can do now that I am not exhausted”); 3) the importance of social support; 4) avoiding the negative (terminating unhealthy relationships in which “food is love”); 5) the void (food addiction and sense of loss); 6) fighting food demons; 7) finding the happy weight; and 8) a ripple effect (that is, if you don’t eat it, the rest of family doesn’t, either).
I was left wondering how I can best help my patients using this information.
First, I think the themes can mature our empathy for the struggles that these patients face, and perhaps help us combat bias. Second, I think this knowledge can inform early discussions around what sorts of things need to be lined up for after the procedure, such as social support.
Finally, I think the themes can be universalized and help us counsel patients who may be struggling with weight, but who are otherwise not candidates for bariatric surgery.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
A fair number of my patients have had or are undergoing bariatric surgery. Disconcertingly, a not insignificant number of them are regaining the weight after surgery. Weight regain will occur in 20% of patients undergoing bariatric surgery after initial weight loss.
When this occurs, not only do we have a patient with an altered gut putting them at risk for nutritional deficiencies if we are not fastidious in our follow-up, but they are discouraged and overweight again.
Add this to the concern that bariatric surgery has been associated with an increase in suicides (2.33-3.63 per 1000 patient-years), and we may have some cause for alarm.
So, what predicts success – and can we facilitate it?
Several factors have been shown to predict successful weight loss after bariatric surgery. An “active coping style” (that is, planning vs. denial) and adherence to follow-up after bariatric surgery have both been shown to be associated with a higher percentage of excess weight loss. Interestingly, psychological burden and motivation have not been associated with weight loss.
In a recent article, Lori Liebl, Ph.D., and her colleagues conducted a qualitative study of the experiences of adults who successfully maintained weight loss after bariatric surgery (J Clin Nurs. 2016 Feb 23. doi: 10.1111/jocn.13129). Success was defined as 50% or more of the excessive weight loss 24 months after bariatric surgery.
The voice of the successful bariatric patient is an interesting and important one. Several themes were identified: 1) taking life back (“I did it for myself”); 2) a new lease on life (“There are things I can do now that I am not exhausted”); 3) the importance of social support; 4) avoiding the negative (terminating unhealthy relationships in which “food is love”); 5) the void (food addiction and sense of loss); 6) fighting food demons; 7) finding the happy weight; and 8) a ripple effect (that is, if you don’t eat it, the rest of family doesn’t, either).
I was left wondering how I can best help my patients using this information.
First, I think the themes can mature our empathy for the struggles that these patients face, and perhaps help us combat bias. Second, I think this knowledge can inform early discussions around what sorts of things need to be lined up for after the procedure, such as social support.
Finally, I think the themes can be universalized and help us counsel patients who may be struggling with weight, but who are otherwise not candidates for bariatric surgery.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.