User login
VIDEO: One in five hospital patients get health care–acquired infection
PHOENIX – If you happen to believe that the impact of health care–acquired infections is insignificant, think again. According to Dr. Kevin W. Lobdell, health care–acquired infections (HAIs) cause more deaths each year in the United States than breast cancer, lung cancer, and AIDS combined.
“If you look at hospitalized patients, one in five will acquire a health care–acquired infection,” Dr. Lobdell of the Sanger Heart and Vascular Institute at Carolinas Health System, Charlotte, N.C., said in a video interview at the annual meeting of the Society of Thoracic Surgeons. “With respect to length of stay, that goes from 5 days on average for a normal, uninfected patient, to 22 days if they’ve had an infection. The mortality rate can be as high as 6% in those people that have developed infections, so that in itself is an enormous burden.”
He went on to discuss the most common HAIs in the hospital setting and noted that combating them involves strategies that consider people, the environment, and technology. He predicted that in coming years clinicians will have a better “analytic capability to understand what we’ve done in the past and what correlates with success in the future, and then be able to implement and learn from that.”
Dr. Lobdell reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – If you happen to believe that the impact of health care–acquired infections is insignificant, think again. According to Dr. Kevin W. Lobdell, health care–acquired infections (HAIs) cause more deaths each year in the United States than breast cancer, lung cancer, and AIDS combined.
“If you look at hospitalized patients, one in five will acquire a health care–acquired infection,” Dr. Lobdell of the Sanger Heart and Vascular Institute at Carolinas Health System, Charlotte, N.C., said in a video interview at the annual meeting of the Society of Thoracic Surgeons. “With respect to length of stay, that goes from 5 days on average for a normal, uninfected patient, to 22 days if they’ve had an infection. The mortality rate can be as high as 6% in those people that have developed infections, so that in itself is an enormous burden.”
He went on to discuss the most common HAIs in the hospital setting and noted that combating them involves strategies that consider people, the environment, and technology. He predicted that in coming years clinicians will have a better “analytic capability to understand what we’ve done in the past and what correlates with success in the future, and then be able to implement and learn from that.”
Dr. Lobdell reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – If you happen to believe that the impact of health care–acquired infections is insignificant, think again. According to Dr. Kevin W. Lobdell, health care–acquired infections (HAIs) cause more deaths each year in the United States than breast cancer, lung cancer, and AIDS combined.
“If you look at hospitalized patients, one in five will acquire a health care–acquired infection,” Dr. Lobdell of the Sanger Heart and Vascular Institute at Carolinas Health System, Charlotte, N.C., said in a video interview at the annual meeting of the Society of Thoracic Surgeons. “With respect to length of stay, that goes from 5 days on average for a normal, uninfected patient, to 22 days if they’ve had an infection. The mortality rate can be as high as 6% in those people that have developed infections, so that in itself is an enormous burden.”
He went on to discuss the most common HAIs in the hospital setting and noted that combating them involves strategies that consider people, the environment, and technology. He predicted that in coming years clinicians will have a better “analytic capability to understand what we’ve done in the past and what correlates with success in the future, and then be able to implement and learn from that.”
Dr. Lobdell reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING
FDA approves new biomaterial for hernia repair
The Food and Drug Administration has granted 510K clearance to Gore Medical for a new biomaterial for hernia repair, according to a Gore statement.
Gore describes the product – GORE SYNECOR Biomaterial – as having a “unique structure,” which prevents surgeons from having to choose between using a “permanent material” and an “absorbable, nonpermanent material” when deciding which biomaterial to use for a hernia repair.
GORE SYNECOR Biomaterial is composed of the following:
• Dense monofilament polytetrafluoroethylene macroporous knit.
• GORE BIO-A Web, “a tissue scaffold with proven outcomes in contaminated hernia repair. providing rapid vascularization and ingrowth in complex repairs.”
• Nonporous PGA/TMC film.
No precertification is required for surgeons to implant GORE SYNECOR Biomaterial.
For more information about the new product, visit www.goremedical.com.
The Food and Drug Administration has granted 510K clearance to Gore Medical for a new biomaterial for hernia repair, according to a Gore statement.
Gore describes the product – GORE SYNECOR Biomaterial – as having a “unique structure,” which prevents surgeons from having to choose between using a “permanent material” and an “absorbable, nonpermanent material” when deciding which biomaterial to use for a hernia repair.
GORE SYNECOR Biomaterial is composed of the following:
• Dense monofilament polytetrafluoroethylene macroporous knit.
• GORE BIO-A Web, “a tissue scaffold with proven outcomes in contaminated hernia repair. providing rapid vascularization and ingrowth in complex repairs.”
• Nonporous PGA/TMC film.
No precertification is required for surgeons to implant GORE SYNECOR Biomaterial.
For more information about the new product, visit www.goremedical.com.
The Food and Drug Administration has granted 510K clearance to Gore Medical for a new biomaterial for hernia repair, according to a Gore statement.
Gore describes the product – GORE SYNECOR Biomaterial – as having a “unique structure,” which prevents surgeons from having to choose between using a “permanent material” and an “absorbable, nonpermanent material” when deciding which biomaterial to use for a hernia repair.
GORE SYNECOR Biomaterial is composed of the following:
• Dense monofilament polytetrafluoroethylene macroporous knit.
• GORE BIO-A Web, “a tissue scaffold with proven outcomes in contaminated hernia repair. providing rapid vascularization and ingrowth in complex repairs.”
• Nonporous PGA/TMC film.
No precertification is required for surgeons to implant GORE SYNECOR Biomaterial.
For more information about the new product, visit www.goremedical.com.
Lymphedema microsurgery gaining momentum
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
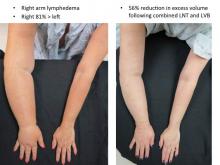
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
EXPERT ANALYSIS AT THE NORTHWESTERN VASCULAR SYMPOSIUM
AHA: Bariatric surgery slashes heart failure exacerbations
ORLANDO – Bariatric surgery in obese patients with heart failure was associated with a marked decrease in the subsequent rate of ED visits and hospitalizations for heart failure in a large, real-world, case-control study presented at the American Heart Association scientific sessions.
“This decline in the rate of heart failure morbidity was rapid in onset and sustained for at least 2 years after bariatric surgery,” according to Dr. Yuichi J. Shimada of Massachusetts General Hospital, Boston.
In a separate study, however, he found that bariatric surgery for obesity in patients with atrial fibrillation didn’t produce a reduction in ED visits and hospitalizations for the arrhythmia.
The heart failure study was a case-control study of 1,664 consecutive obese patients with heart failure who underwent a single bariatric surgical procedure in California, Florida, or Nebraska. Their median age was 49 years. Women accounted for 70% of the participants. Drawing upon federal Healthcare Cost and Utility Project databases on ED visits and hospital admissions in those three states, Dr. Shimada and coinvestigators compared the group’s rates of ED visits and hospitalizations for heart failure for 2 years before and 2 years after bariatric surgery. Thus, the subjects served as their own controls.
During the reference period, which lasted from months 13-24 presurgery, the group’s combined rate of ED visits and hospital admission for heart failure exacerbation was 14.4%. The rate wasn’t significantly different during the 12 months immediately prior to surgery, at 13.3%.
The rate dropped to 8.7% during the first 12 months after bariatric surgery and remained rock solid at 8.7% during months 13-24 postsurgery. In a logistic regression analysis, this translated to a 44% reduction in the risk of ED visits or hospital admission for heart failure during the first 2 years following bariatric surgery.
These findings are consistent with previous work by other investigators showing a link between obesity and heart failure exacerbations. The new data advance the field by providing the best evidence to date of the effectiveness of substantial weight loss on heart failure morbidity, Dr. Shimada observed.
Nonbariatric surgeries such as hysterectomy or cholecysectomy in the study population had no effect on the rate of heart failure exacerbations.
Dr. Shimada’s atrial fibrillation study was structured in the same way. It included 1,056 patients with atrial fibrillation who underwent bariatric surgery for obesity in the same three states. The rate of ED visits or hospitalization for heart failure was 12.1% in months 13-24 prior to bariatric surgery, 12.6% in presurgical months 1-12, 14.2% in the first 12 months post-bariatric surgery, and 13.4% during postsurgical months 13-24. These rates weren’t statistically different.
Dr. Shimada reported having no financial conflicts of interest regarding the two studies.
ORLANDO – Bariatric surgery in obese patients with heart failure was associated with a marked decrease in the subsequent rate of ED visits and hospitalizations for heart failure in a large, real-world, case-control study presented at the American Heart Association scientific sessions.
“This decline in the rate of heart failure morbidity was rapid in onset and sustained for at least 2 years after bariatric surgery,” according to Dr. Yuichi J. Shimada of Massachusetts General Hospital, Boston.
In a separate study, however, he found that bariatric surgery for obesity in patients with atrial fibrillation didn’t produce a reduction in ED visits and hospitalizations for the arrhythmia.
The heart failure study was a case-control study of 1,664 consecutive obese patients with heart failure who underwent a single bariatric surgical procedure in California, Florida, or Nebraska. Their median age was 49 years. Women accounted for 70% of the participants. Drawing upon federal Healthcare Cost and Utility Project databases on ED visits and hospital admissions in those three states, Dr. Shimada and coinvestigators compared the group’s rates of ED visits and hospitalizations for heart failure for 2 years before and 2 years after bariatric surgery. Thus, the subjects served as their own controls.
During the reference period, which lasted from months 13-24 presurgery, the group’s combined rate of ED visits and hospital admission for heart failure exacerbation was 14.4%. The rate wasn’t significantly different during the 12 months immediately prior to surgery, at 13.3%.
The rate dropped to 8.7% during the first 12 months after bariatric surgery and remained rock solid at 8.7% during months 13-24 postsurgery. In a logistic regression analysis, this translated to a 44% reduction in the risk of ED visits or hospital admission for heart failure during the first 2 years following bariatric surgery.
These findings are consistent with previous work by other investigators showing a link between obesity and heart failure exacerbations. The new data advance the field by providing the best evidence to date of the effectiveness of substantial weight loss on heart failure morbidity, Dr. Shimada observed.
Nonbariatric surgeries such as hysterectomy or cholecysectomy in the study population had no effect on the rate of heart failure exacerbations.
Dr. Shimada’s atrial fibrillation study was structured in the same way. It included 1,056 patients with atrial fibrillation who underwent bariatric surgery for obesity in the same three states. The rate of ED visits or hospitalization for heart failure was 12.1% in months 13-24 prior to bariatric surgery, 12.6% in presurgical months 1-12, 14.2% in the first 12 months post-bariatric surgery, and 13.4% during postsurgical months 13-24. These rates weren’t statistically different.
Dr. Shimada reported having no financial conflicts of interest regarding the two studies.
ORLANDO – Bariatric surgery in obese patients with heart failure was associated with a marked decrease in the subsequent rate of ED visits and hospitalizations for heart failure in a large, real-world, case-control study presented at the American Heart Association scientific sessions.
“This decline in the rate of heart failure morbidity was rapid in onset and sustained for at least 2 years after bariatric surgery,” according to Dr. Yuichi J. Shimada of Massachusetts General Hospital, Boston.
In a separate study, however, he found that bariatric surgery for obesity in patients with atrial fibrillation didn’t produce a reduction in ED visits and hospitalizations for the arrhythmia.
The heart failure study was a case-control study of 1,664 consecutive obese patients with heart failure who underwent a single bariatric surgical procedure in California, Florida, or Nebraska. Their median age was 49 years. Women accounted for 70% of the participants. Drawing upon federal Healthcare Cost and Utility Project databases on ED visits and hospital admissions in those three states, Dr. Shimada and coinvestigators compared the group’s rates of ED visits and hospitalizations for heart failure for 2 years before and 2 years after bariatric surgery. Thus, the subjects served as their own controls.
During the reference period, which lasted from months 13-24 presurgery, the group’s combined rate of ED visits and hospital admission for heart failure exacerbation was 14.4%. The rate wasn’t significantly different during the 12 months immediately prior to surgery, at 13.3%.
The rate dropped to 8.7% during the first 12 months after bariatric surgery and remained rock solid at 8.7% during months 13-24 postsurgery. In a logistic regression analysis, this translated to a 44% reduction in the risk of ED visits or hospital admission for heart failure during the first 2 years following bariatric surgery.
These findings are consistent with previous work by other investigators showing a link between obesity and heart failure exacerbations. The new data advance the field by providing the best evidence to date of the effectiveness of substantial weight loss on heart failure morbidity, Dr. Shimada observed.
Nonbariatric surgeries such as hysterectomy or cholecysectomy in the study population had no effect on the rate of heart failure exacerbations.
Dr. Shimada’s atrial fibrillation study was structured in the same way. It included 1,056 patients with atrial fibrillation who underwent bariatric surgery for obesity in the same three states. The rate of ED visits or hospitalization for heart failure was 12.1% in months 13-24 prior to bariatric surgery, 12.6% in presurgical months 1-12, 14.2% in the first 12 months post-bariatric surgery, and 13.4% during postsurgical months 13-24. These rates weren’t statistically different.
Dr. Shimada reported having no financial conflicts of interest regarding the two studies.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Bariatric surgery in obese patients with heart failure results in a dramatic reduction in ED visits and hospital admission for heart failure.
Major finding: The combined rate of ED visits and hospital admissions for heart failure dropped by 44% during the 2 years after a large group of patients with heart failure underwent bariatric surgery for obesity.
Data source: This case-control study compared the rates of ED visits and hospital admissions for worsening heart failure in 1,664 patients with heart failure during the 2 years before and 2 years after they underwent bariatric surgery for obesity.
Disclosures: The presenter reported having no financial conflicts of interest regarding the study, which utilized publicly available patient data.
West African Ebola–virus transmission ends, then resumes
A day after the World Health Organization declared on Jan. 14 that no known cases of Ebola-virus transmission or active infection had been identified in West Africa for more than 42 days, the agency revealed the existence of a new, single case of Ebola-virus infection in Sierra Leone.
The appearance of the new case highlighted the challenges that remain in fully stamping out the West African Ebola–virus outbreak. The crisis began in December 2013, raged throughout 2014, and only wound down toward a sputtering halt near the end of 2015.
When Dr. Rick Brennan, the World Health Organization (WHO) director for Emergency Risk Management and Humanitarian Response, announced during a press conference on Jan. 14, “the end of Ebola-virus transmission in West Africa” and “the first time since 2014 that all know chains of transmission have stopped in Guinea, Liberia, and Sierra Leone,” he and his colleague, Dr. Peter Graaff, also underscored the ongoing risk for sporadic, isolated cases. The main threat for new cases comes from men who survived Ebola-virus infection but harbored significant quantities of infectious virus in various body fluids and tissues, including their semen, for a period of about 1 year following their infection.
During the press conference, both Dr. Brennan and Dr. Graaff spoke of a new, strengthened infrastructure that WHO had placed in West Africa to better monitor and react quickly to these flare-up cases – the mechanism that led to quick identification of the new Sierra Leone case.
“I think you’ll see a more responsive and effective WHO,” said Dr. Graaff, WHO’s director of Ebola response. “We will use the Ebola experience to be more agile, more operational, and better able to react more quickly to whatever future problems come our way,” including infectious-disease outbreaks by pathogens other than Ebola virus.
The 42-day period without new infections that had led to the announcement of an end to Ebola-virus transmission in West Africa represents two 21-day incubation cycles for the virus. Until the Sierra Leone case reported on Jan. 15, the last confirmed West African case had been identified in Liberia in mid-November. Liberia had previously been declared Ebola free in last May, but four new, isolated infections appeared subsequent to that transmitted by Ebola-infection survivors who still harbored infectious virus. Before the Jan. 15 case, an additional three such flare-ups occurred in Sierra Leone and another three in Guinea since last March, Dr. Brennan said.
The current tally on the West African Ebola outbreak stands at roughly 28,500 confirmed cases identified, and about 11,300 deaths. Nearly three-quarters of infections occurred during 2014. The case fatality rate at the start of the outbreak ran about 80%, noted Dr. Graaff, but gradually fell as infected patients began receiving better care. Near the outbreak’s end, the case fatality rate stood at roughly 35%, when most patients were receiving optimal clinical care.
WHO staffers are maintaining close surveillance of people who recently survived Ebola-virus infection so that they can identify and isolate any new infections. WHO personnel have also counseled these survivors on the risk they pose for potential transmission, primarily by unprotected sex by men for up to 12 months following the end of active infection. The WHO also plans to launch an investigational protocol to vaccinate sexual partners and other close contacts of adult male survivors with an as-yet unapproved Ebola vaccine to further reduce risk of new infections.
“The WHO and world community reacted slowly at the start of the Ebola outbreak” in early 2014, admitted Dr. Brennan. “Without question, the disease got away from us. In retrospect, there were a number of things that we should have done better and sooner,” he said. “We have done a lot of soul-searching and we [at WHO] have identified a number of weaknesses in our structures and systems. We have already taken several important steps, and we are undergoing major reforms of our emergency operations. I think you’ll see a much more effective WHO in the future.”
Reform of WHO systems for infection prevention and control “will be much better able to detect new cases, not only of Ebola, but of other important diseases,” said Dr. Graaff.
The United States is also strengthening its health screening and monitoring system for travelers coming from affected countries and improving its hospital preparedness to manage cases, said Alice C. Hill, senior adviser for preparedness and resilience at the National Security Council and special assistant to President Obama. Hill, who spoke Jan. 6 at the Public Health Emergency Medical Countermeasures Enterprise stakeholders workshop, acknowledged that challenges still exist that must be addressed, including the development of safe and effective diagnostics, treatments, and vaccines for existing infectious diseases such as influenza and more emerging threats such as Ebola or MERS (Middle East Respiratory Syndrome), developing accurate disease forecasting capabilities to predict what will be the next threat, building rapid clinical trial networks and manufacturing capabilities, and the abilities for mass dispensing of medical countermeasures.
Gregory Twachtman also contributed to this story.
On Twitter @mitchelzoler
A day after the World Health Organization declared on Jan. 14 that no known cases of Ebola-virus transmission or active infection had been identified in West Africa for more than 42 days, the agency revealed the existence of a new, single case of Ebola-virus infection in Sierra Leone.
The appearance of the new case highlighted the challenges that remain in fully stamping out the West African Ebola–virus outbreak. The crisis began in December 2013, raged throughout 2014, and only wound down toward a sputtering halt near the end of 2015.
When Dr. Rick Brennan, the World Health Organization (WHO) director for Emergency Risk Management and Humanitarian Response, announced during a press conference on Jan. 14, “the end of Ebola-virus transmission in West Africa” and “the first time since 2014 that all know chains of transmission have stopped in Guinea, Liberia, and Sierra Leone,” he and his colleague, Dr. Peter Graaff, also underscored the ongoing risk for sporadic, isolated cases. The main threat for new cases comes from men who survived Ebola-virus infection but harbored significant quantities of infectious virus in various body fluids and tissues, including their semen, for a period of about 1 year following their infection.
During the press conference, both Dr. Brennan and Dr. Graaff spoke of a new, strengthened infrastructure that WHO had placed in West Africa to better monitor and react quickly to these flare-up cases – the mechanism that led to quick identification of the new Sierra Leone case.
“I think you’ll see a more responsive and effective WHO,” said Dr. Graaff, WHO’s director of Ebola response. “We will use the Ebola experience to be more agile, more operational, and better able to react more quickly to whatever future problems come our way,” including infectious-disease outbreaks by pathogens other than Ebola virus.
The 42-day period without new infections that had led to the announcement of an end to Ebola-virus transmission in West Africa represents two 21-day incubation cycles for the virus. Until the Sierra Leone case reported on Jan. 15, the last confirmed West African case had been identified in Liberia in mid-November. Liberia had previously been declared Ebola free in last May, but four new, isolated infections appeared subsequent to that transmitted by Ebola-infection survivors who still harbored infectious virus. Before the Jan. 15 case, an additional three such flare-ups occurred in Sierra Leone and another three in Guinea since last March, Dr. Brennan said.
The current tally on the West African Ebola outbreak stands at roughly 28,500 confirmed cases identified, and about 11,300 deaths. Nearly three-quarters of infections occurred during 2014. The case fatality rate at the start of the outbreak ran about 80%, noted Dr. Graaff, but gradually fell as infected patients began receiving better care. Near the outbreak’s end, the case fatality rate stood at roughly 35%, when most patients were receiving optimal clinical care.
WHO staffers are maintaining close surveillance of people who recently survived Ebola-virus infection so that they can identify and isolate any new infections. WHO personnel have also counseled these survivors on the risk they pose for potential transmission, primarily by unprotected sex by men for up to 12 months following the end of active infection. The WHO also plans to launch an investigational protocol to vaccinate sexual partners and other close contacts of adult male survivors with an as-yet unapproved Ebola vaccine to further reduce risk of new infections.
“The WHO and world community reacted slowly at the start of the Ebola outbreak” in early 2014, admitted Dr. Brennan. “Without question, the disease got away from us. In retrospect, there were a number of things that we should have done better and sooner,” he said. “We have done a lot of soul-searching and we [at WHO] have identified a number of weaknesses in our structures and systems. We have already taken several important steps, and we are undergoing major reforms of our emergency operations. I think you’ll see a much more effective WHO in the future.”
Reform of WHO systems for infection prevention and control “will be much better able to detect new cases, not only of Ebola, but of other important diseases,” said Dr. Graaff.
The United States is also strengthening its health screening and monitoring system for travelers coming from affected countries and improving its hospital preparedness to manage cases, said Alice C. Hill, senior adviser for preparedness and resilience at the National Security Council and special assistant to President Obama. Hill, who spoke Jan. 6 at the Public Health Emergency Medical Countermeasures Enterprise stakeholders workshop, acknowledged that challenges still exist that must be addressed, including the development of safe and effective diagnostics, treatments, and vaccines for existing infectious diseases such as influenza and more emerging threats such as Ebola or MERS (Middle East Respiratory Syndrome), developing accurate disease forecasting capabilities to predict what will be the next threat, building rapid clinical trial networks and manufacturing capabilities, and the abilities for mass dispensing of medical countermeasures.
Gregory Twachtman also contributed to this story.
On Twitter @mitchelzoler
A day after the World Health Organization declared on Jan. 14 that no known cases of Ebola-virus transmission or active infection had been identified in West Africa for more than 42 days, the agency revealed the existence of a new, single case of Ebola-virus infection in Sierra Leone.
The appearance of the new case highlighted the challenges that remain in fully stamping out the West African Ebola–virus outbreak. The crisis began in December 2013, raged throughout 2014, and only wound down toward a sputtering halt near the end of 2015.
When Dr. Rick Brennan, the World Health Organization (WHO) director for Emergency Risk Management and Humanitarian Response, announced during a press conference on Jan. 14, “the end of Ebola-virus transmission in West Africa” and “the first time since 2014 that all know chains of transmission have stopped in Guinea, Liberia, and Sierra Leone,” he and his colleague, Dr. Peter Graaff, also underscored the ongoing risk for sporadic, isolated cases. The main threat for new cases comes from men who survived Ebola-virus infection but harbored significant quantities of infectious virus in various body fluids and tissues, including their semen, for a period of about 1 year following their infection.
During the press conference, both Dr. Brennan and Dr. Graaff spoke of a new, strengthened infrastructure that WHO had placed in West Africa to better monitor and react quickly to these flare-up cases – the mechanism that led to quick identification of the new Sierra Leone case.
“I think you’ll see a more responsive and effective WHO,” said Dr. Graaff, WHO’s director of Ebola response. “We will use the Ebola experience to be more agile, more operational, and better able to react more quickly to whatever future problems come our way,” including infectious-disease outbreaks by pathogens other than Ebola virus.
The 42-day period without new infections that had led to the announcement of an end to Ebola-virus transmission in West Africa represents two 21-day incubation cycles for the virus. Until the Sierra Leone case reported on Jan. 15, the last confirmed West African case had been identified in Liberia in mid-November. Liberia had previously been declared Ebola free in last May, but four new, isolated infections appeared subsequent to that transmitted by Ebola-infection survivors who still harbored infectious virus. Before the Jan. 15 case, an additional three such flare-ups occurred in Sierra Leone and another three in Guinea since last March, Dr. Brennan said.
The current tally on the West African Ebola outbreak stands at roughly 28,500 confirmed cases identified, and about 11,300 deaths. Nearly three-quarters of infections occurred during 2014. The case fatality rate at the start of the outbreak ran about 80%, noted Dr. Graaff, but gradually fell as infected patients began receiving better care. Near the outbreak’s end, the case fatality rate stood at roughly 35%, when most patients were receiving optimal clinical care.
WHO staffers are maintaining close surveillance of people who recently survived Ebola-virus infection so that they can identify and isolate any new infections. WHO personnel have also counseled these survivors on the risk they pose for potential transmission, primarily by unprotected sex by men for up to 12 months following the end of active infection. The WHO also plans to launch an investigational protocol to vaccinate sexual partners and other close contacts of adult male survivors with an as-yet unapproved Ebola vaccine to further reduce risk of new infections.
“The WHO and world community reacted slowly at the start of the Ebola outbreak” in early 2014, admitted Dr. Brennan. “Without question, the disease got away from us. In retrospect, there were a number of things that we should have done better and sooner,” he said. “We have done a lot of soul-searching and we [at WHO] have identified a number of weaknesses in our structures and systems. We have already taken several important steps, and we are undergoing major reforms of our emergency operations. I think you’ll see a much more effective WHO in the future.”
Reform of WHO systems for infection prevention and control “will be much better able to detect new cases, not only of Ebola, but of other important diseases,” said Dr. Graaff.
The United States is also strengthening its health screening and monitoring system for travelers coming from affected countries and improving its hospital preparedness to manage cases, said Alice C. Hill, senior adviser for preparedness and resilience at the National Security Council and special assistant to President Obama. Hill, who spoke Jan. 6 at the Public Health Emergency Medical Countermeasures Enterprise stakeholders workshop, acknowledged that challenges still exist that must be addressed, including the development of safe and effective diagnostics, treatments, and vaccines for existing infectious diseases such as influenza and more emerging threats such as Ebola or MERS (Middle East Respiratory Syndrome), developing accurate disease forecasting capabilities to predict what will be the next threat, building rapid clinical trial networks and manufacturing capabilities, and the abilities for mass dispensing of medical countermeasures.
Gregory Twachtman also contributed to this story.
On Twitter @mitchelzoler
Consider pyoderma gangrenosum for nonhealing wounds
LAS VEGAS – Though pyoderma gangrenosum and other neutrophilic skin disorders are rare, clinicians should include them in their differential, especially for nonhealing surgical wounds or skin “infections.”
Since these painful areas of ulceration need corticosteroid treatment, not antibiotics, for resolution, accurate diagnosis is critical for healing, Dr. J. Mark Jackson said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In a review of pyoderma gangrenosum (PG) and its cousins at the meeting, Dr. Jackson noted that the etiology of PG is unknown, but disordered neutrophilic chemotaxis is thought to be a factor. The many different manifestations of this disease are now collectively called the “neutrophilic dermatoses,” he said.
“Pyoderma gangrenosum is a very important diagnosis to consider in the differential diagnosis for nonhealing ulcerations, as suspicion and early recognition of this debilitating condition can prevent long-term sequelae such as pain, scarring, and long-term immunosuppressive medications,” said Dr. Jackson of the department of dermatology at the University of Louisville (Ky.).
The diagnosis should be suspected in the setting of a painful cutaneous ulcer with necrolysis. The border is typically irregular, violaceous, and undermined, he said, adding that this classic undermined border is caused by the sheets of neutrophils that characterize the disease.
Noting that half of patients with PG have underlying associated conditions such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and hematologic malignancies, Dr. Jackson emphasized that systemic disease associated with PG should heighten suspicion. “Histopathologic findings may be consistent with but not diagnostic of PG,” and can include a sterile dermal neutrophilia, with or without mixed inflammation and a lymphocytic vasculitis.
“Where you biopsy is important,” he continued, emphasizing that the biopsy must capture the margin of ulceration, where the sheets of neutrophils characteristic of PG will be seen on pathology.
Therapy consists of corticosteroids, with or without an immunosuppressive agent, and cessation of treatments that may continue to provoke pathergy.
Other diseases should also be considered in the differential diagnosis, including dangerous infectious causes, such as atypical mycobacteria, deep fungal infections, and staphylococcal and streptococcal infections. Squamous cell carcinoma, lymphoma, and leukemia may also present with similar lesions, as may metastatic Crohn’s disease, Dr. Jackson said. Several vasculitic and vasculopathic inflammatory conditions can also have similar appearances, including Wegener’s granulomatosis and vasoocclusive disorders such as peripheral vascular disease and cryoglobulinemia.
Classically, PG presents as painful ulcerated areas, most often on the lower extremities, that have a typical undermined border, caused by the sheets of neutrophils that characterize PG, he pointed out. PG may be mistaken for venous stasis ulcers, pressure ulcers, and cellulitis, but it doesn’t improve with antibiotics and mechanical manipulation from exfoliative dressings – and debridement may worsen the condition.
For susceptible individuals, surgery may provoke a pathergic response and trigger PG at the site of the surgical wound, and dogged attempts at conventional wound care may cause continued pathergy and begin a vicious cycle, Dr. Jackson said.
Peristomal pyoderma gangrenosum is a disease subcategory that may be seen in patients whose inflammatory bowel disease has been surgically treated and who have a stoma. Patients will have ulcerating lesions around their stoma site that are often misdiagnosed and treated as infections. Some wound care therapies, such as debridement, may continue to provoke the pathergic response and worsen peristomal PG, he said.
Though associated disease is seen in up to 50% of individuals with PG, there’s no predictable timeline linking the development of PG with the course of the associated disorder. In classic PG, usually occurring on the legs, autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis or another inflammatory arthritis, and paraproteinemia may be seen. Atypical PG, occurring more commonly on the upper extremities and face, is associated with myelogenous leukemia and preleukemic states, Dr. Jackson said.
Pyoderma gangrenosum lesions improve with corticosteroid administration. Depending on disease severity and location, topical, intralesional, or systemic steroids may be used.
Adjunctive treatments for PG and other neutrophilic dermatoses can include antibiotics with anti-inflammatory properties, such as minocycline or doxycycline, dapsone, and metronidazole. Immunosuppressives such as cyclosporine, azathioprine, and mycophenolate mofetil may also help speed resolution. In some cases, skin grafts may be necessary.
PG patients with Crohn’s disease or rheumatoid arthritis who are prescribed tumor necrosis factor–alpha (TNF-alpha) inhibitors for their systemic disease may also see improvement in PG lesions, Dr. Jackson said.
Other rare categories of neutrophilic dermatoses include Sweet’s syndrome, an acute febrile neutrophilic dermatosis, and neutrophilic dermatosis of the dorsum of the hand.
Neutrophilic invasion can also occur in other organs. “These extracutaneous lesions are also ‘sterile’ neutrophilic abscesses, which are often misdiagnosed as infections,” Dr. Jackson said. The most common site of extracutaneous neutrophilic infiltration is the lungs, though any organ system may be affected.
Dr. Jackson disclosed that he has received research support, honoraria, consulting fees, and other support from Abbvie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
The Skin Disease Education Foundation and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Though pyoderma gangrenosum and other neutrophilic skin disorders are rare, clinicians should include them in their differential, especially for nonhealing surgical wounds or skin “infections.”
Since these painful areas of ulceration need corticosteroid treatment, not antibiotics, for resolution, accurate diagnosis is critical for healing, Dr. J. Mark Jackson said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In a review of pyoderma gangrenosum (PG) and its cousins at the meeting, Dr. Jackson noted that the etiology of PG is unknown, but disordered neutrophilic chemotaxis is thought to be a factor. The many different manifestations of this disease are now collectively called the “neutrophilic dermatoses,” he said.
“Pyoderma gangrenosum is a very important diagnosis to consider in the differential diagnosis for nonhealing ulcerations, as suspicion and early recognition of this debilitating condition can prevent long-term sequelae such as pain, scarring, and long-term immunosuppressive medications,” said Dr. Jackson of the department of dermatology at the University of Louisville (Ky.).
The diagnosis should be suspected in the setting of a painful cutaneous ulcer with necrolysis. The border is typically irregular, violaceous, and undermined, he said, adding that this classic undermined border is caused by the sheets of neutrophils that characterize the disease.
Noting that half of patients with PG have underlying associated conditions such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and hematologic malignancies, Dr. Jackson emphasized that systemic disease associated with PG should heighten suspicion. “Histopathologic findings may be consistent with but not diagnostic of PG,” and can include a sterile dermal neutrophilia, with or without mixed inflammation and a lymphocytic vasculitis.
“Where you biopsy is important,” he continued, emphasizing that the biopsy must capture the margin of ulceration, where the sheets of neutrophils characteristic of PG will be seen on pathology.
Therapy consists of corticosteroids, with or without an immunosuppressive agent, and cessation of treatments that may continue to provoke pathergy.
Other diseases should also be considered in the differential diagnosis, including dangerous infectious causes, such as atypical mycobacteria, deep fungal infections, and staphylococcal and streptococcal infections. Squamous cell carcinoma, lymphoma, and leukemia may also present with similar lesions, as may metastatic Crohn’s disease, Dr. Jackson said. Several vasculitic and vasculopathic inflammatory conditions can also have similar appearances, including Wegener’s granulomatosis and vasoocclusive disorders such as peripheral vascular disease and cryoglobulinemia.
Classically, PG presents as painful ulcerated areas, most often on the lower extremities, that have a typical undermined border, caused by the sheets of neutrophils that characterize PG, he pointed out. PG may be mistaken for venous stasis ulcers, pressure ulcers, and cellulitis, but it doesn’t improve with antibiotics and mechanical manipulation from exfoliative dressings – and debridement may worsen the condition.
For susceptible individuals, surgery may provoke a pathergic response and trigger PG at the site of the surgical wound, and dogged attempts at conventional wound care may cause continued pathergy and begin a vicious cycle, Dr. Jackson said.
Peristomal pyoderma gangrenosum is a disease subcategory that may be seen in patients whose inflammatory bowel disease has been surgically treated and who have a stoma. Patients will have ulcerating lesions around their stoma site that are often misdiagnosed and treated as infections. Some wound care therapies, such as debridement, may continue to provoke the pathergic response and worsen peristomal PG, he said.
Though associated disease is seen in up to 50% of individuals with PG, there’s no predictable timeline linking the development of PG with the course of the associated disorder. In classic PG, usually occurring on the legs, autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis or another inflammatory arthritis, and paraproteinemia may be seen. Atypical PG, occurring more commonly on the upper extremities and face, is associated with myelogenous leukemia and preleukemic states, Dr. Jackson said.
Pyoderma gangrenosum lesions improve with corticosteroid administration. Depending on disease severity and location, topical, intralesional, or systemic steroids may be used.
Adjunctive treatments for PG and other neutrophilic dermatoses can include antibiotics with anti-inflammatory properties, such as minocycline or doxycycline, dapsone, and metronidazole. Immunosuppressives such as cyclosporine, azathioprine, and mycophenolate mofetil may also help speed resolution. In some cases, skin grafts may be necessary.
PG patients with Crohn’s disease or rheumatoid arthritis who are prescribed tumor necrosis factor–alpha (TNF-alpha) inhibitors for their systemic disease may also see improvement in PG lesions, Dr. Jackson said.
Other rare categories of neutrophilic dermatoses include Sweet’s syndrome, an acute febrile neutrophilic dermatosis, and neutrophilic dermatosis of the dorsum of the hand.
Neutrophilic invasion can also occur in other organs. “These extracutaneous lesions are also ‘sterile’ neutrophilic abscesses, which are often misdiagnosed as infections,” Dr. Jackson said. The most common site of extracutaneous neutrophilic infiltration is the lungs, though any organ system may be affected.
Dr. Jackson disclosed that he has received research support, honoraria, consulting fees, and other support from Abbvie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
The Skin Disease Education Foundation and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Though pyoderma gangrenosum and other neutrophilic skin disorders are rare, clinicians should include them in their differential, especially for nonhealing surgical wounds or skin “infections.”
Since these painful areas of ulceration need corticosteroid treatment, not antibiotics, for resolution, accurate diagnosis is critical for healing, Dr. J. Mark Jackson said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In a review of pyoderma gangrenosum (PG) and its cousins at the meeting, Dr. Jackson noted that the etiology of PG is unknown, but disordered neutrophilic chemotaxis is thought to be a factor. The many different manifestations of this disease are now collectively called the “neutrophilic dermatoses,” he said.
“Pyoderma gangrenosum is a very important diagnosis to consider in the differential diagnosis for nonhealing ulcerations, as suspicion and early recognition of this debilitating condition can prevent long-term sequelae such as pain, scarring, and long-term immunosuppressive medications,” said Dr. Jackson of the department of dermatology at the University of Louisville (Ky.).
The diagnosis should be suspected in the setting of a painful cutaneous ulcer with necrolysis. The border is typically irregular, violaceous, and undermined, he said, adding that this classic undermined border is caused by the sheets of neutrophils that characterize the disease.
Noting that half of patients with PG have underlying associated conditions such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and hematologic malignancies, Dr. Jackson emphasized that systemic disease associated with PG should heighten suspicion. “Histopathologic findings may be consistent with but not diagnostic of PG,” and can include a sterile dermal neutrophilia, with or without mixed inflammation and a lymphocytic vasculitis.
“Where you biopsy is important,” he continued, emphasizing that the biopsy must capture the margin of ulceration, where the sheets of neutrophils characteristic of PG will be seen on pathology.
Therapy consists of corticosteroids, with or without an immunosuppressive agent, and cessation of treatments that may continue to provoke pathergy.
Other diseases should also be considered in the differential diagnosis, including dangerous infectious causes, such as atypical mycobacteria, deep fungal infections, and staphylococcal and streptococcal infections. Squamous cell carcinoma, lymphoma, and leukemia may also present with similar lesions, as may metastatic Crohn’s disease, Dr. Jackson said. Several vasculitic and vasculopathic inflammatory conditions can also have similar appearances, including Wegener’s granulomatosis and vasoocclusive disorders such as peripheral vascular disease and cryoglobulinemia.
Classically, PG presents as painful ulcerated areas, most often on the lower extremities, that have a typical undermined border, caused by the sheets of neutrophils that characterize PG, he pointed out. PG may be mistaken for venous stasis ulcers, pressure ulcers, and cellulitis, but it doesn’t improve with antibiotics and mechanical manipulation from exfoliative dressings – and debridement may worsen the condition.
For susceptible individuals, surgery may provoke a pathergic response and trigger PG at the site of the surgical wound, and dogged attempts at conventional wound care may cause continued pathergy and begin a vicious cycle, Dr. Jackson said.
Peristomal pyoderma gangrenosum is a disease subcategory that may be seen in patients whose inflammatory bowel disease has been surgically treated and who have a stoma. Patients will have ulcerating lesions around their stoma site that are often misdiagnosed and treated as infections. Some wound care therapies, such as debridement, may continue to provoke the pathergic response and worsen peristomal PG, he said.
Though associated disease is seen in up to 50% of individuals with PG, there’s no predictable timeline linking the development of PG with the course of the associated disorder. In classic PG, usually occurring on the legs, autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis or another inflammatory arthritis, and paraproteinemia may be seen. Atypical PG, occurring more commonly on the upper extremities and face, is associated with myelogenous leukemia and preleukemic states, Dr. Jackson said.
Pyoderma gangrenosum lesions improve with corticosteroid administration. Depending on disease severity and location, topical, intralesional, or systemic steroids may be used.
Adjunctive treatments for PG and other neutrophilic dermatoses can include antibiotics with anti-inflammatory properties, such as minocycline or doxycycline, dapsone, and metronidazole. Immunosuppressives such as cyclosporine, azathioprine, and mycophenolate mofetil may also help speed resolution. In some cases, skin grafts may be necessary.
PG patients with Crohn’s disease or rheumatoid arthritis who are prescribed tumor necrosis factor–alpha (TNF-alpha) inhibitors for their systemic disease may also see improvement in PG lesions, Dr. Jackson said.
Other rare categories of neutrophilic dermatoses include Sweet’s syndrome, an acute febrile neutrophilic dermatosis, and neutrophilic dermatosis of the dorsum of the hand.
Neutrophilic invasion can also occur in other organs. “These extracutaneous lesions are also ‘sterile’ neutrophilic abscesses, which are often misdiagnosed as infections,” Dr. Jackson said. The most common site of extracutaneous neutrophilic infiltration is the lungs, though any organ system may be affected.
Dr. Jackson disclosed that he has received research support, honoraria, consulting fees, and other support from Abbvie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
The Skin Disease Education Foundation and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
The Right Choice? Kindness and Surgical Ethics: Reflections on a Friend and Mentor
As I sit down to write this column, I reflect on the news that my mentor and friend, Norman W. Thompson, M.D, FACS, passed away yesterday. I had the good fortune to spend 1 year as an endocrine surgery fellow with Dr. Thompson at the University of Michigan in 1995-96. That year was certainly the most significant of my training in terms of defining my professional life as an endocrine surgeon. However, as I think back on my time with Dr. Thompson, I am struck by how much more I learned from him than how to take out a thyroid or a parathyroid or manage multiple endocrine neoplasia.
Dr. Thompson was an excellent technical surgeon, and he would have had a tremendous career helping thousands of patients if that was all that he had done. However, he was much more than an excellent technician. He was also a great doctor. In order for a surgeon to be a great doctor, it is necessary to be technically excellent, but that alone is not sufficient. I believe that what makes a surgeon a great doctor is the combination of technical mastery with outstanding interpersonal skills and ethically sound clinical judgment. Dr. Thompson had all of that, and he was exceptionally kind.
Kindness is not a word that we commonly use in describing surgeons today. In an era of surgeons being pressured to see more patients and generate more RVUs [relative value units], it is unusual to hear kindness mentioned as an essential attribute of a great surgeon. However, Dr. Thompson’s kindness was immediately apparent to all who spent time with him. He treated each patient as a unique individual. In addition, he treated his trainees and his colleagues in Ann Arbor and around the world with respect and incredible humility. He was generous with his time and was always approachable no matter how inexperienced the surgeon asking him a question. Dr. Thompson was kind to all of us and made us feel that he valued spending time with us.
What does kindness have to do with a column that traditionally focuses on ethical issues in the practice of surgery? Although acting with kindness is not the same as acting in an ethical manner, I believe that there is more overlap of the terms than we often imagine. The kind surgeon is the one who treats people – whether they are patients or colleagues – as though they matter. The ethical surgeon respects the patient’s wishes and acts to benefit the patient as much as possible in all circumstances. I am certain that I have met ethical surgeons who were not kind, but I have met very few kind surgeons who are not ethical.
As someone who has spent significant time and energy in the last 19 years as a surgery faculty member trying to teach ethics, I am also struck by a clear truth. Actions always speak louder than words. It may be valuable to talk about the ethical principles that may come to play in a particularly difficult surgical case. Defining the competing interests and assessing the patient’s wishes are important components of the ethical practice of surgery. However, no amount of discussion of these issues can substitute for the value of behavior. Treating patients and colleagues with kindness and respect is modeling the behaviors of an ethical surgeon – perhaps learned from a wise and thoughtful mentor.
Dr. Thompson was an excellent role model for me and so many others in how he treated patients and everyone around him. As I see patients and perform surgery, I still hear myself saying many of the same things that he said many years ago. His genuine expressions of optimism before difficult operations, honesty in communicating, and sadness when things did not go well were tremendous examples to me of how a great doctor treats those around him. These lessons that I learned from Dr. Thompson have influenced my practice significantly, and I am grateful for the opportunity to try to model them on a daily basis.
Although I remain convinced that formal curricula in ethics and professionalism remain important in the education of today’s surgeons, it is valuable to remember the impact that the behaviors of those we respect have on us. Perhaps we surgeons more than other physicians are molded by the people who train us, but there is no question that the ethical behaviors of our teachers and mentors will have a greater impact than any lecture or manuscript. I want to acknowledge and commemorate the kindness and ethical behaviors that Dr. Thompson modeled daily for all who were fortunate enough to work with him.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
As I sit down to write this column, I reflect on the news that my mentor and friend, Norman W. Thompson, M.D, FACS, passed away yesterday. I had the good fortune to spend 1 year as an endocrine surgery fellow with Dr. Thompson at the University of Michigan in 1995-96. That year was certainly the most significant of my training in terms of defining my professional life as an endocrine surgeon. However, as I think back on my time with Dr. Thompson, I am struck by how much more I learned from him than how to take out a thyroid or a parathyroid or manage multiple endocrine neoplasia.
Dr. Thompson was an excellent technical surgeon, and he would have had a tremendous career helping thousands of patients if that was all that he had done. However, he was much more than an excellent technician. He was also a great doctor. In order for a surgeon to be a great doctor, it is necessary to be technically excellent, but that alone is not sufficient. I believe that what makes a surgeon a great doctor is the combination of technical mastery with outstanding interpersonal skills and ethically sound clinical judgment. Dr. Thompson had all of that, and he was exceptionally kind.
Kindness is not a word that we commonly use in describing surgeons today. In an era of surgeons being pressured to see more patients and generate more RVUs [relative value units], it is unusual to hear kindness mentioned as an essential attribute of a great surgeon. However, Dr. Thompson’s kindness was immediately apparent to all who spent time with him. He treated each patient as a unique individual. In addition, he treated his trainees and his colleagues in Ann Arbor and around the world with respect and incredible humility. He was generous with his time and was always approachable no matter how inexperienced the surgeon asking him a question. Dr. Thompson was kind to all of us and made us feel that he valued spending time with us.
What does kindness have to do with a column that traditionally focuses on ethical issues in the practice of surgery? Although acting with kindness is not the same as acting in an ethical manner, I believe that there is more overlap of the terms than we often imagine. The kind surgeon is the one who treats people – whether they are patients or colleagues – as though they matter. The ethical surgeon respects the patient’s wishes and acts to benefit the patient as much as possible in all circumstances. I am certain that I have met ethical surgeons who were not kind, but I have met very few kind surgeons who are not ethical.
As someone who has spent significant time and energy in the last 19 years as a surgery faculty member trying to teach ethics, I am also struck by a clear truth. Actions always speak louder than words. It may be valuable to talk about the ethical principles that may come to play in a particularly difficult surgical case. Defining the competing interests and assessing the patient’s wishes are important components of the ethical practice of surgery. However, no amount of discussion of these issues can substitute for the value of behavior. Treating patients and colleagues with kindness and respect is modeling the behaviors of an ethical surgeon – perhaps learned from a wise and thoughtful mentor.
Dr. Thompson was an excellent role model for me and so many others in how he treated patients and everyone around him. As I see patients and perform surgery, I still hear myself saying many of the same things that he said many years ago. His genuine expressions of optimism before difficult operations, honesty in communicating, and sadness when things did not go well were tremendous examples to me of how a great doctor treats those around him. These lessons that I learned from Dr. Thompson have influenced my practice significantly, and I am grateful for the opportunity to try to model them on a daily basis.
Although I remain convinced that formal curricula in ethics and professionalism remain important in the education of today’s surgeons, it is valuable to remember the impact that the behaviors of those we respect have on us. Perhaps we surgeons more than other physicians are molded by the people who train us, but there is no question that the ethical behaviors of our teachers and mentors will have a greater impact than any lecture or manuscript. I want to acknowledge and commemorate the kindness and ethical behaviors that Dr. Thompson modeled daily for all who were fortunate enough to work with him.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
As I sit down to write this column, I reflect on the news that my mentor and friend, Norman W. Thompson, M.D, FACS, passed away yesterday. I had the good fortune to spend 1 year as an endocrine surgery fellow with Dr. Thompson at the University of Michigan in 1995-96. That year was certainly the most significant of my training in terms of defining my professional life as an endocrine surgeon. However, as I think back on my time with Dr. Thompson, I am struck by how much more I learned from him than how to take out a thyroid or a parathyroid or manage multiple endocrine neoplasia.
Dr. Thompson was an excellent technical surgeon, and he would have had a tremendous career helping thousands of patients if that was all that he had done. However, he was much more than an excellent technician. He was also a great doctor. In order for a surgeon to be a great doctor, it is necessary to be technically excellent, but that alone is not sufficient. I believe that what makes a surgeon a great doctor is the combination of technical mastery with outstanding interpersonal skills and ethically sound clinical judgment. Dr. Thompson had all of that, and he was exceptionally kind.
Kindness is not a word that we commonly use in describing surgeons today. In an era of surgeons being pressured to see more patients and generate more RVUs [relative value units], it is unusual to hear kindness mentioned as an essential attribute of a great surgeon. However, Dr. Thompson’s kindness was immediately apparent to all who spent time with him. He treated each patient as a unique individual. In addition, he treated his trainees and his colleagues in Ann Arbor and around the world with respect and incredible humility. He was generous with his time and was always approachable no matter how inexperienced the surgeon asking him a question. Dr. Thompson was kind to all of us and made us feel that he valued spending time with us.
What does kindness have to do with a column that traditionally focuses on ethical issues in the practice of surgery? Although acting with kindness is not the same as acting in an ethical manner, I believe that there is more overlap of the terms than we often imagine. The kind surgeon is the one who treats people – whether they are patients or colleagues – as though they matter. The ethical surgeon respects the patient’s wishes and acts to benefit the patient as much as possible in all circumstances. I am certain that I have met ethical surgeons who were not kind, but I have met very few kind surgeons who are not ethical.
As someone who has spent significant time and energy in the last 19 years as a surgery faculty member trying to teach ethics, I am also struck by a clear truth. Actions always speak louder than words. It may be valuable to talk about the ethical principles that may come to play in a particularly difficult surgical case. Defining the competing interests and assessing the patient’s wishes are important components of the ethical practice of surgery. However, no amount of discussion of these issues can substitute for the value of behavior. Treating patients and colleagues with kindness and respect is modeling the behaviors of an ethical surgeon – perhaps learned from a wise and thoughtful mentor.
Dr. Thompson was an excellent role model for me and so many others in how he treated patients and everyone around him. As I see patients and perform surgery, I still hear myself saying many of the same things that he said many years ago. His genuine expressions of optimism before difficult operations, honesty in communicating, and sadness when things did not go well were tremendous examples to me of how a great doctor treats those around him. These lessons that I learned from Dr. Thompson have influenced my practice significantly, and I am grateful for the opportunity to try to model them on a daily basis.
Although I remain convinced that formal curricula in ethics and professionalism remain important in the education of today’s surgeons, it is valuable to remember the impact that the behaviors of those we respect have on us. Perhaps we surgeons more than other physicians are molded by the people who train us, but there is no question that the ethical behaviors of our teachers and mentors will have a greater impact than any lecture or manuscript. I want to acknowledge and commemorate the kindness and ethical behaviors that Dr. Thompson modeled daily for all who were fortunate enough to work with him.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Candor laws growing, but are they effective?
Iowa is the latest state to launch a unique strategy that aims to reduce medical malpractice lawsuits and bolster doctor-patient communication after poor outcomes, while encouraging swift resolution. The Communication and Optimal Resolution (CANDOR) law, which took effect in July, permits privileged discussions between Iowa physicians and patients after medical errors and allows for compensation offers, when appropriate.
After years of failing to enact traditional tort reform, the alternative method is hoped to decrease litigation costs and keep doctors and patients out of a flawed court system, said Dr. Michael McCoy, chair of the Iowa Medical Society Ad Hoc Tort Reform Task Force and a West Burlington obstetrician-gynecologist.
“If there’s an untoward outcome, the current system creates isolation,” he said. “The patients get alienated, and the doctors get stressed. That’s the exact opposite of what should happen. You should be able to talk to patients and feel protection. That’s what this law does.”
About 36 states have so-called “apology laws” that prohibit certain statements, expressions, or other evidence from being admissible in a malpractice lawsuit, according to data from Sorry Works!, an advocacy group that tracks apology statutes. Most apology laws cover expressions of empathy or sympathy, while some statutes protect admissions of fault. At least three states – including Iowa – go a step further, laying the foundation for early disclosure of errors, communication between physicians and patients, and negotiation of potential resolutions. Oregon’s Early Discussion and Resolution (EDR) law, which passed in 2013, allows both providers and patients to initiate conversations after adverse events, discuss what happened, and resolve the issue outside of court. Massachusetts’ 2012 disclosure, apology, and offer legislation includes a 182-day waiting period before lawsuits can be filed to allow for the process. Other states, including Washington, Texas, California, and Utah have expressed interest in enacting similar laws.
Efforts in Iowa, Oregon, and Massachusetts were driven by the successes of similar programs at the University of Illinois Hospital and Health Sciences System, Chicago and at the University of Michigan Health System, Ann Arbor, among others. Since the University of Michigan began its CANDOR program in 2001, average legal expenses per case have been cut in half, according to the UM data. The health system’s presuit claims have gone from 260 annually to about 100 per year. Pilot efforts by the University of Illinois (Chicago) Hospital and Health Sciences System since 2006 have increased adverse event reports from 1,500 per year to 10,000 while decreasing malpractice premiums by $15 million dollars since 2010.
But questions remain as to whether such approaches are successful when implemented on a state level. So far, the majority of data on early discussion, apology, and offer programs have come from closed systems, such as academic medical centers.
“We’re starting to look at how this works in an open system when you try to disseminate it across a state,” said Dr. Alan C. Woodward, chair of the Massachusetts Medical Society Committee on Professional Liability and cofounder of the Massachusetts Alliance for Communication and Resolution following Medical Injury (MACRMI). “If we can show this is an effective model in a statewide distribution, the hope is that multiple other states will follow suit, because this is clearly a better alternative for patients and providers and for patient safety improvement efforts.”
Massachusetts program showing promise
In Massachusetts, the state’s CANDOR law enabled MACRMI to roll out its Communication, Apology, and Resolution (CARe) model at six hospital pilot sites. MACRMI was formed as part of a research initiative led by Beth Israel Deaconess Medical Center, Boston, and the Massachusetts Medical Society, Waltham, and funded by a 2010 grant from the Federal Agency for Health Care Research and Quality.
Under the CARe model, participating health providers communicate with patients and families after an unanticipated adverse outcome, investigate and explain what happened, and where appropriate, apologize and offer fair financial compensation. Data collection in the 3-year study trial closed in December 2015, and the alliance plans to publish its findings in spring 2016. The model has greatly expedited case resolutions, Dr. Woodward said. A case in the CARe program can be resolved in 6 months or less, compared with a lawsuit that can take up to 5 years to resolve. By April 2015, the alliance had screened 856 cases. In three-quarters of cases, the care provided was deemed appropriate, Dr. Woodward said. Of these cases, 615 were closed after full-disclosure meetings with patients. Of the total cases screened, 122 cases were referred to an insurer for resolution, and 93 cases were awaiting further evaluation.
“It’s doing extraordinarily well,” Dr. Woodward said. “We’re resolving cases very efficiently in a short period of time, rather than using litigation.”
Outside the pilot program, health providers in Massachusetts are benefiting from the state’s 182-day waiting period before malpractice lawsuits can be filed, said Elizabeth A. Cushing, vice president of claims for CRICO, a medical liability insurer for the Harvard University medical community. The law allows physicians and insurers the opportunity to review a case and decide whether the complaint requires compensation. In cases that are not considered malpractice, explaining the underlying reasoning to patients or plaintiffs’ attorneys prevents some lawsuits from being filed, she said.
However, Ms. Cushing notes that not every case fits smoothly into the alternative model. Cases in which a poor outcome is immediately known, such as a surgical mishap, lend themselves to quicker investigation, disclosure, and remedy, she said. Claims of misdiagnosed cancer for instance, where the alleged mistake occurred years before, are more challenging.
“A lot of what we are seeing are alleged failures to diagnose things sooner, so it’s 2 years later and someone says, ‘You should have picked up on the fact that I had lung cancer 2 years ago,’ Ms. Cushing said. “Those situations get tricky. What did you know, when? Those cases are not as easily amenable to this process.”
Oregon program gaining speed
Meanwhile, Oregon officials are hoping that more doctors will soon participate in the state’s Early Discussion and Resolution program. In 2014 – its first year in operation – no individual physicians initiated participation in the program. Because EDR is voluntary, both parties must agree to participate for EDR to begin and either party can choose to stop participating at any time.
In 2014, Oregon patients and health providers filed 29 EDR requests to participate in the program, according to data from the Oregon Patient Safety Commission. Patients filed 21 notices and health care professionals filed eight notices. A majority of the eight notices filed by health professionals were issued by hospital representatives. No requests were filed by individual health providers. In 9 of the 21 patient-filed notices, at least one involved health professional accepted the patient’s request to participate in EDR. In the remaining 12 patient-filed notices, the involved health provider(s) declined the request. Data is not yet available on resolution time or case outcomes.
EDR leaders are hopeful that more health professionals and patients will participate in the coming years, said Melissa Parkerton, director of the Early Discussion and Resolution program for the Oregon Patient Safety Commission.
“Widespread adoption of a new approach like EDR requires not just a new process, but a new mindset,” she said. “This kind of cultural shift takes time. In Oregon, our fervent hope is that EDR will be embraced by every health care organization and professional.”
Efforts to raise awareness about the program are ongoing, adds Dr. Robert Dannenhoffer, a Roseburg, Ore., pediatrician and past president of the Oregon Medical Association.
“It’s a little hard to get the word out,” he said in an interview. “Most [doctors] are not going to be dealing with this on a regular basis. It’s a slow uptake. We’re working on better educating physicians.”
A practical model for independent docs?
Questions remain about whether disclosure, apology, and offer models work for independent physicians in small practices.
Dr. Thad L. Anderson, an ob.gyn in private practice in Dubuque, Iowa, strongly supports his state’s law, but he does not foresee the process having much impact on his practice. Larger health systems are generally more situated to utilize the process, he said.
“A hospital system that employees physicians is probably better suited to take advantage of this law,” he said. Independent doctors “don’t have the infrastructure. You don’t have the in-house lawyer. It’s probably a little more problematic for an individual to take advantage of the process compared to a bigger system.”
With this issue in mind, MACRMI in Massachusetts recently added an 800-physician, multispecialty outpatient group to its program analysis. The goal is to evaluate how the process operates within this type of setting and identify challenges and impediments, Dr. Woodward said. In addition, the alliance is encouraging commercial insurers to assist independent physicians in participating in the CARe model.
“The independent or small practice all benefit from the statute we passed – they have the [182-day waiting period] – but if they don’t have an internal risk management structure, than we’ve been working with insurers to set up a support structure,” he said. That way, “when a patient is unhappy with their care, there is a process that can support even a small practice in going through this and working through it with a patient.”
*This story was updated 1/26/2016.
On Twitter @legal_med
Iowa is the latest state to launch a unique strategy that aims to reduce medical malpractice lawsuits and bolster doctor-patient communication after poor outcomes, while encouraging swift resolution. The Communication and Optimal Resolution (CANDOR) law, which took effect in July, permits privileged discussions between Iowa physicians and patients after medical errors and allows for compensation offers, when appropriate.
After years of failing to enact traditional tort reform, the alternative method is hoped to decrease litigation costs and keep doctors and patients out of a flawed court system, said Dr. Michael McCoy, chair of the Iowa Medical Society Ad Hoc Tort Reform Task Force and a West Burlington obstetrician-gynecologist.
“If there’s an untoward outcome, the current system creates isolation,” he said. “The patients get alienated, and the doctors get stressed. That’s the exact opposite of what should happen. You should be able to talk to patients and feel protection. That’s what this law does.”
About 36 states have so-called “apology laws” that prohibit certain statements, expressions, or other evidence from being admissible in a malpractice lawsuit, according to data from Sorry Works!, an advocacy group that tracks apology statutes. Most apology laws cover expressions of empathy or sympathy, while some statutes protect admissions of fault. At least three states – including Iowa – go a step further, laying the foundation for early disclosure of errors, communication between physicians and patients, and negotiation of potential resolutions. Oregon’s Early Discussion and Resolution (EDR) law, which passed in 2013, allows both providers and patients to initiate conversations after adverse events, discuss what happened, and resolve the issue outside of court. Massachusetts’ 2012 disclosure, apology, and offer legislation includes a 182-day waiting period before lawsuits can be filed to allow for the process. Other states, including Washington, Texas, California, and Utah have expressed interest in enacting similar laws.
Efforts in Iowa, Oregon, and Massachusetts were driven by the successes of similar programs at the University of Illinois Hospital and Health Sciences System, Chicago and at the University of Michigan Health System, Ann Arbor, among others. Since the University of Michigan began its CANDOR program in 2001, average legal expenses per case have been cut in half, according to the UM data. The health system’s presuit claims have gone from 260 annually to about 100 per year. Pilot efforts by the University of Illinois (Chicago) Hospital and Health Sciences System since 2006 have increased adverse event reports from 1,500 per year to 10,000 while decreasing malpractice premiums by $15 million dollars since 2010.
But questions remain as to whether such approaches are successful when implemented on a state level. So far, the majority of data on early discussion, apology, and offer programs have come from closed systems, such as academic medical centers.
“We’re starting to look at how this works in an open system when you try to disseminate it across a state,” said Dr. Alan C. Woodward, chair of the Massachusetts Medical Society Committee on Professional Liability and cofounder of the Massachusetts Alliance for Communication and Resolution following Medical Injury (MACRMI). “If we can show this is an effective model in a statewide distribution, the hope is that multiple other states will follow suit, because this is clearly a better alternative for patients and providers and for patient safety improvement efforts.”
Massachusetts program showing promise
In Massachusetts, the state’s CANDOR law enabled MACRMI to roll out its Communication, Apology, and Resolution (CARe) model at six hospital pilot sites. MACRMI was formed as part of a research initiative led by Beth Israel Deaconess Medical Center, Boston, and the Massachusetts Medical Society, Waltham, and funded by a 2010 grant from the Federal Agency for Health Care Research and Quality.
Under the CARe model, participating health providers communicate with patients and families after an unanticipated adverse outcome, investigate and explain what happened, and where appropriate, apologize and offer fair financial compensation. Data collection in the 3-year study trial closed in December 2015, and the alliance plans to publish its findings in spring 2016. The model has greatly expedited case resolutions, Dr. Woodward said. A case in the CARe program can be resolved in 6 months or less, compared with a lawsuit that can take up to 5 years to resolve. By April 2015, the alliance had screened 856 cases. In three-quarters of cases, the care provided was deemed appropriate, Dr. Woodward said. Of these cases, 615 were closed after full-disclosure meetings with patients. Of the total cases screened, 122 cases were referred to an insurer for resolution, and 93 cases were awaiting further evaluation.
“It’s doing extraordinarily well,” Dr. Woodward said. “We’re resolving cases very efficiently in a short period of time, rather than using litigation.”
Outside the pilot program, health providers in Massachusetts are benefiting from the state’s 182-day waiting period before malpractice lawsuits can be filed, said Elizabeth A. Cushing, vice president of claims for CRICO, a medical liability insurer for the Harvard University medical community. The law allows physicians and insurers the opportunity to review a case and decide whether the complaint requires compensation. In cases that are not considered malpractice, explaining the underlying reasoning to patients or plaintiffs’ attorneys prevents some lawsuits from being filed, she said.
However, Ms. Cushing notes that not every case fits smoothly into the alternative model. Cases in which a poor outcome is immediately known, such as a surgical mishap, lend themselves to quicker investigation, disclosure, and remedy, she said. Claims of misdiagnosed cancer for instance, where the alleged mistake occurred years before, are more challenging.
“A lot of what we are seeing are alleged failures to diagnose things sooner, so it’s 2 years later and someone says, ‘You should have picked up on the fact that I had lung cancer 2 years ago,’ Ms. Cushing said. “Those situations get tricky. What did you know, when? Those cases are not as easily amenable to this process.”
Oregon program gaining speed
Meanwhile, Oregon officials are hoping that more doctors will soon participate in the state’s Early Discussion and Resolution program. In 2014 – its first year in operation – no individual physicians initiated participation in the program. Because EDR is voluntary, both parties must agree to participate for EDR to begin and either party can choose to stop participating at any time.
In 2014, Oregon patients and health providers filed 29 EDR requests to participate in the program, according to data from the Oregon Patient Safety Commission. Patients filed 21 notices and health care professionals filed eight notices. A majority of the eight notices filed by health professionals were issued by hospital representatives. No requests were filed by individual health providers. In 9 of the 21 patient-filed notices, at least one involved health professional accepted the patient’s request to participate in EDR. In the remaining 12 patient-filed notices, the involved health provider(s) declined the request. Data is not yet available on resolution time or case outcomes.
EDR leaders are hopeful that more health professionals and patients will participate in the coming years, said Melissa Parkerton, director of the Early Discussion and Resolution program for the Oregon Patient Safety Commission.
“Widespread adoption of a new approach like EDR requires not just a new process, but a new mindset,” she said. “This kind of cultural shift takes time. In Oregon, our fervent hope is that EDR will be embraced by every health care organization and professional.”
Efforts to raise awareness about the program are ongoing, adds Dr. Robert Dannenhoffer, a Roseburg, Ore., pediatrician and past president of the Oregon Medical Association.
“It’s a little hard to get the word out,” he said in an interview. “Most [doctors] are not going to be dealing with this on a regular basis. It’s a slow uptake. We’re working on better educating physicians.”
A practical model for independent docs?
Questions remain about whether disclosure, apology, and offer models work for independent physicians in small practices.
Dr. Thad L. Anderson, an ob.gyn in private practice in Dubuque, Iowa, strongly supports his state’s law, but he does not foresee the process having much impact on his practice. Larger health systems are generally more situated to utilize the process, he said.
“A hospital system that employees physicians is probably better suited to take advantage of this law,” he said. Independent doctors “don’t have the infrastructure. You don’t have the in-house lawyer. It’s probably a little more problematic for an individual to take advantage of the process compared to a bigger system.”
With this issue in mind, MACRMI in Massachusetts recently added an 800-physician, multispecialty outpatient group to its program analysis. The goal is to evaluate how the process operates within this type of setting and identify challenges and impediments, Dr. Woodward said. In addition, the alliance is encouraging commercial insurers to assist independent physicians in participating in the CARe model.
“The independent or small practice all benefit from the statute we passed – they have the [182-day waiting period] – but if they don’t have an internal risk management structure, than we’ve been working with insurers to set up a support structure,” he said. That way, “when a patient is unhappy with their care, there is a process that can support even a small practice in going through this and working through it with a patient.”
*This story was updated 1/26/2016.
On Twitter @legal_med
Iowa is the latest state to launch a unique strategy that aims to reduce medical malpractice lawsuits and bolster doctor-patient communication after poor outcomes, while encouraging swift resolution. The Communication and Optimal Resolution (CANDOR) law, which took effect in July, permits privileged discussions between Iowa physicians and patients after medical errors and allows for compensation offers, when appropriate.
After years of failing to enact traditional tort reform, the alternative method is hoped to decrease litigation costs and keep doctors and patients out of a flawed court system, said Dr. Michael McCoy, chair of the Iowa Medical Society Ad Hoc Tort Reform Task Force and a West Burlington obstetrician-gynecologist.
“If there’s an untoward outcome, the current system creates isolation,” he said. “The patients get alienated, and the doctors get stressed. That’s the exact opposite of what should happen. You should be able to talk to patients and feel protection. That’s what this law does.”
About 36 states have so-called “apology laws” that prohibit certain statements, expressions, or other evidence from being admissible in a malpractice lawsuit, according to data from Sorry Works!, an advocacy group that tracks apology statutes. Most apology laws cover expressions of empathy or sympathy, while some statutes protect admissions of fault. At least three states – including Iowa – go a step further, laying the foundation for early disclosure of errors, communication between physicians and patients, and negotiation of potential resolutions. Oregon’s Early Discussion and Resolution (EDR) law, which passed in 2013, allows both providers and patients to initiate conversations after adverse events, discuss what happened, and resolve the issue outside of court. Massachusetts’ 2012 disclosure, apology, and offer legislation includes a 182-day waiting period before lawsuits can be filed to allow for the process. Other states, including Washington, Texas, California, and Utah have expressed interest in enacting similar laws.
Efforts in Iowa, Oregon, and Massachusetts were driven by the successes of similar programs at the University of Illinois Hospital and Health Sciences System, Chicago and at the University of Michigan Health System, Ann Arbor, among others. Since the University of Michigan began its CANDOR program in 2001, average legal expenses per case have been cut in half, according to the UM data. The health system’s presuit claims have gone from 260 annually to about 100 per year. Pilot efforts by the University of Illinois (Chicago) Hospital and Health Sciences System since 2006 have increased adverse event reports from 1,500 per year to 10,000 while decreasing malpractice premiums by $15 million dollars since 2010.
But questions remain as to whether such approaches are successful when implemented on a state level. So far, the majority of data on early discussion, apology, and offer programs have come from closed systems, such as academic medical centers.
“We’re starting to look at how this works in an open system when you try to disseminate it across a state,” said Dr. Alan C. Woodward, chair of the Massachusetts Medical Society Committee on Professional Liability and cofounder of the Massachusetts Alliance for Communication and Resolution following Medical Injury (MACRMI). “If we can show this is an effective model in a statewide distribution, the hope is that multiple other states will follow suit, because this is clearly a better alternative for patients and providers and for patient safety improvement efforts.”
Massachusetts program showing promise
In Massachusetts, the state’s CANDOR law enabled MACRMI to roll out its Communication, Apology, and Resolution (CARe) model at six hospital pilot sites. MACRMI was formed as part of a research initiative led by Beth Israel Deaconess Medical Center, Boston, and the Massachusetts Medical Society, Waltham, and funded by a 2010 grant from the Federal Agency for Health Care Research and Quality.
Under the CARe model, participating health providers communicate with patients and families after an unanticipated adverse outcome, investigate and explain what happened, and where appropriate, apologize and offer fair financial compensation. Data collection in the 3-year study trial closed in December 2015, and the alliance plans to publish its findings in spring 2016. The model has greatly expedited case resolutions, Dr. Woodward said. A case in the CARe program can be resolved in 6 months or less, compared with a lawsuit that can take up to 5 years to resolve. By April 2015, the alliance had screened 856 cases. In three-quarters of cases, the care provided was deemed appropriate, Dr. Woodward said. Of these cases, 615 were closed after full-disclosure meetings with patients. Of the total cases screened, 122 cases were referred to an insurer for resolution, and 93 cases were awaiting further evaluation.
“It’s doing extraordinarily well,” Dr. Woodward said. “We’re resolving cases very efficiently in a short period of time, rather than using litigation.”
Outside the pilot program, health providers in Massachusetts are benefiting from the state’s 182-day waiting period before malpractice lawsuits can be filed, said Elizabeth A. Cushing, vice president of claims for CRICO, a medical liability insurer for the Harvard University medical community. The law allows physicians and insurers the opportunity to review a case and decide whether the complaint requires compensation. In cases that are not considered malpractice, explaining the underlying reasoning to patients or plaintiffs’ attorneys prevents some lawsuits from being filed, she said.
However, Ms. Cushing notes that not every case fits smoothly into the alternative model. Cases in which a poor outcome is immediately known, such as a surgical mishap, lend themselves to quicker investigation, disclosure, and remedy, she said. Claims of misdiagnosed cancer for instance, where the alleged mistake occurred years before, are more challenging.
“A lot of what we are seeing are alleged failures to diagnose things sooner, so it’s 2 years later and someone says, ‘You should have picked up on the fact that I had lung cancer 2 years ago,’ Ms. Cushing said. “Those situations get tricky. What did you know, when? Those cases are not as easily amenable to this process.”
Oregon program gaining speed
Meanwhile, Oregon officials are hoping that more doctors will soon participate in the state’s Early Discussion and Resolution program. In 2014 – its first year in operation – no individual physicians initiated participation in the program. Because EDR is voluntary, both parties must agree to participate for EDR to begin and either party can choose to stop participating at any time.
In 2014, Oregon patients and health providers filed 29 EDR requests to participate in the program, according to data from the Oregon Patient Safety Commission. Patients filed 21 notices and health care professionals filed eight notices. A majority of the eight notices filed by health professionals were issued by hospital representatives. No requests were filed by individual health providers. In 9 of the 21 patient-filed notices, at least one involved health professional accepted the patient’s request to participate in EDR. In the remaining 12 patient-filed notices, the involved health provider(s) declined the request. Data is not yet available on resolution time or case outcomes.
EDR leaders are hopeful that more health professionals and patients will participate in the coming years, said Melissa Parkerton, director of the Early Discussion and Resolution program for the Oregon Patient Safety Commission.
“Widespread adoption of a new approach like EDR requires not just a new process, but a new mindset,” she said. “This kind of cultural shift takes time. In Oregon, our fervent hope is that EDR will be embraced by every health care organization and professional.”
Efforts to raise awareness about the program are ongoing, adds Dr. Robert Dannenhoffer, a Roseburg, Ore., pediatrician and past president of the Oregon Medical Association.
“It’s a little hard to get the word out,” he said in an interview. “Most [doctors] are not going to be dealing with this on a regular basis. It’s a slow uptake. We’re working on better educating physicians.”
A practical model for independent docs?
Questions remain about whether disclosure, apology, and offer models work for independent physicians in small practices.
Dr. Thad L. Anderson, an ob.gyn in private practice in Dubuque, Iowa, strongly supports his state’s law, but he does not foresee the process having much impact on his practice. Larger health systems are generally more situated to utilize the process, he said.
“A hospital system that employees physicians is probably better suited to take advantage of this law,” he said. Independent doctors “don’t have the infrastructure. You don’t have the in-house lawyer. It’s probably a little more problematic for an individual to take advantage of the process compared to a bigger system.”
With this issue in mind, MACRMI in Massachusetts recently added an 800-physician, multispecialty outpatient group to its program analysis. The goal is to evaluate how the process operates within this type of setting and identify challenges and impediments, Dr. Woodward said. In addition, the alliance is encouraging commercial insurers to assist independent physicians in participating in the CARe model.
“The independent or small practice all benefit from the statute we passed – they have the [182-day waiting period] – but if they don’t have an internal risk management structure, than we’ve been working with insurers to set up a support structure,” he said. That way, “when a patient is unhappy with their care, there is a process that can support even a small practice in going through this and working through it with a patient.”
*This story was updated 1/26/2016.
On Twitter @legal_med
Jury still out on appendectomy vs. antibiotics-first approach
Despite a growing movement toward the antibiotics-first approach instead of surgical intervention for uncomplicated appendicitis, a new review of existing literature shows that the jury is still out on how to advise patients about their choices, according to Dr. Anne P. Ehlers and her colleagues who undertook the study.
The findings show that surgical intervention should continue to be considered a viable option and that appendectomy is not necessarily any better or worse in terms of long-term complication rates and length of hospital stay (J Am Coll Surg. 2015. doi:10.1016/j.jamcollsurg.2015.11.009).
“What we found is that treatment of acute, uncomplicated appendicitis with antibiotics first is probably a safe approach, but that there are many questions that need to be answered before we can fully inform our patients about the long-term outcomes of this treatment strategy,” Dr. Ehlers of the department of surgery at the University of Washington, Seattle, said in an interview.
Dr. Ehlers and her coinvestigators combed the PubMed and EMBASE databases for all English-language randomized controlled trials involving comparisons of antibiotic and appendectomy-based treatments for acute appendicitis. Studies were excluded if they lacked adult population investigation.
“Our study is a critical review of the literature [available] on this topic to understand the current state of evidence,” explained Dr. Ehlers, adding that the study’s main goal was to answer the most common questions she and her coauthors encountered when talking to physicians and patients about the benefits and risks of antibiotics-first over appendectomy; namely, “Is my appendicitis going to come back?” “Am I going to have a lot of extra trips to the hospital?” and “What’s my quality of life going to be?”
Ultimately, six trials, comprising 1,720 patients, were selected for inclusion. These studies were led by Dr. S. Eriksson (40 subjects), Dr. J. Styrud (252 subjects), Dr. A.N. Turhan (290 subjects), Dr. J. Hansson (369 subjects), Dr. C. Vons (239 subjects), and Dr. P. Salminen (530 subjects). The Styrud study did not enroll any women, and no study enrolled subjects older than 75 years of age, but the average age of each study’s patients ranged from 26 to 38 years.
Length-of-stay comparisons between appendectomy and antibiotics-first cohorts varied among the studies, but most showed the same or longer LOS for antibiotics-first subjects. Mean LOS was 3.3 for surgical patients vs. 3.1 for antibiotics (Eriksson), 2.6 surgical vs. 3.0 antibiotics (Styrud), 2.4 surgical vs. 3.14 antibiotics (Turhan), 3.0 for both (Hansson), 3.04 surgical vs. 3.96 antibiotics (Vons), and 3.0 for both (Salminen).
Rates of appendectomy among patients treated with an antibiotics-first approach varied as well, with a 35% rate in the Eriksson study (7 out of the 20 subjects treated with antibiotics-first) and a 24% rate in the Styrud and Turhan studies. The Hansson trial reported an unusually high crossover between cohorts of 60%, the investigators noted.
Two studies – those led by Turhan and Vons – noted higher rates of complications in antibiotics-first patients versus those who received surgical intervention: 4.7% vs. 4.4%, and 2.5% vs. less than 1%, respectively. All other studies had high rates of complications in the surgical cohorts, with the Eriksson study reporting no complications whatsoever among antibiotics-first subjects.
These findings, Dr. Ehlers stressed, are just a first step. This review’s relatively low sample size and limiting factors require that further studies be done to more firmly ascertain which option for appendicitis treatment is the most beneficial. To that end, Dr. Ehlers said that she and her coinvestigators are working on that next step.
“Our group is currently working on a study called the CODA [Comparing Outcomes of Drugs in Appendectomy] Study,” she said, with the goal of answering “questions about long-term patient-centered outcomes related to the antibiotics-first approach.” These questions include patients’ quality of life, whether patients have decisional regret about choosing one treatment strategy over another, if they develop long-term anxiety any time they experience even a small abdominal pain, how much time they miss from school or work by choosing one treatment option over another, and so on.
The goal of this study is that physicians “will be able to give [patients] a more informed view of what each treatment entails,” she said. Funded by the Patient-Centered Outcomes Research Institute, the study has no firm date of publication.
Dr. Ehlers disclosed receiving support via a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. No other conflicts of interest were reported.
Despite a growing movement toward the antibiotics-first approach instead of surgical intervention for uncomplicated appendicitis, a new review of existing literature shows that the jury is still out on how to advise patients about their choices, according to Dr. Anne P. Ehlers and her colleagues who undertook the study.
The findings show that surgical intervention should continue to be considered a viable option and that appendectomy is not necessarily any better or worse in terms of long-term complication rates and length of hospital stay (J Am Coll Surg. 2015. doi:10.1016/j.jamcollsurg.2015.11.009).
“What we found is that treatment of acute, uncomplicated appendicitis with antibiotics first is probably a safe approach, but that there are many questions that need to be answered before we can fully inform our patients about the long-term outcomes of this treatment strategy,” Dr. Ehlers of the department of surgery at the University of Washington, Seattle, said in an interview.
Dr. Ehlers and her coinvestigators combed the PubMed and EMBASE databases for all English-language randomized controlled trials involving comparisons of antibiotic and appendectomy-based treatments for acute appendicitis. Studies were excluded if they lacked adult population investigation.
“Our study is a critical review of the literature [available] on this topic to understand the current state of evidence,” explained Dr. Ehlers, adding that the study’s main goal was to answer the most common questions she and her coauthors encountered when talking to physicians and patients about the benefits and risks of antibiotics-first over appendectomy; namely, “Is my appendicitis going to come back?” “Am I going to have a lot of extra trips to the hospital?” and “What’s my quality of life going to be?”
Ultimately, six trials, comprising 1,720 patients, were selected for inclusion. These studies were led by Dr. S. Eriksson (40 subjects), Dr. J. Styrud (252 subjects), Dr. A.N. Turhan (290 subjects), Dr. J. Hansson (369 subjects), Dr. C. Vons (239 subjects), and Dr. P. Salminen (530 subjects). The Styrud study did not enroll any women, and no study enrolled subjects older than 75 years of age, but the average age of each study’s patients ranged from 26 to 38 years.
Length-of-stay comparisons between appendectomy and antibiotics-first cohorts varied among the studies, but most showed the same or longer LOS for antibiotics-first subjects. Mean LOS was 3.3 for surgical patients vs. 3.1 for antibiotics (Eriksson), 2.6 surgical vs. 3.0 antibiotics (Styrud), 2.4 surgical vs. 3.14 antibiotics (Turhan), 3.0 for both (Hansson), 3.04 surgical vs. 3.96 antibiotics (Vons), and 3.0 for both (Salminen).
Rates of appendectomy among patients treated with an antibiotics-first approach varied as well, with a 35% rate in the Eriksson study (7 out of the 20 subjects treated with antibiotics-first) and a 24% rate in the Styrud and Turhan studies. The Hansson trial reported an unusually high crossover between cohorts of 60%, the investigators noted.
Two studies – those led by Turhan and Vons – noted higher rates of complications in antibiotics-first patients versus those who received surgical intervention: 4.7% vs. 4.4%, and 2.5% vs. less than 1%, respectively. All other studies had high rates of complications in the surgical cohorts, with the Eriksson study reporting no complications whatsoever among antibiotics-first subjects.
These findings, Dr. Ehlers stressed, are just a first step. This review’s relatively low sample size and limiting factors require that further studies be done to more firmly ascertain which option for appendicitis treatment is the most beneficial. To that end, Dr. Ehlers said that she and her coinvestigators are working on that next step.
“Our group is currently working on a study called the CODA [Comparing Outcomes of Drugs in Appendectomy] Study,” she said, with the goal of answering “questions about long-term patient-centered outcomes related to the antibiotics-first approach.” These questions include patients’ quality of life, whether patients have decisional regret about choosing one treatment strategy over another, if they develop long-term anxiety any time they experience even a small abdominal pain, how much time they miss from school or work by choosing one treatment option over another, and so on.
The goal of this study is that physicians “will be able to give [patients] a more informed view of what each treatment entails,” she said. Funded by the Patient-Centered Outcomes Research Institute, the study has no firm date of publication.
Dr. Ehlers disclosed receiving support via a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. No other conflicts of interest were reported.
Despite a growing movement toward the antibiotics-first approach instead of surgical intervention for uncomplicated appendicitis, a new review of existing literature shows that the jury is still out on how to advise patients about their choices, according to Dr. Anne P. Ehlers and her colleagues who undertook the study.
The findings show that surgical intervention should continue to be considered a viable option and that appendectomy is not necessarily any better or worse in terms of long-term complication rates and length of hospital stay (J Am Coll Surg. 2015. doi:10.1016/j.jamcollsurg.2015.11.009).
“What we found is that treatment of acute, uncomplicated appendicitis with antibiotics first is probably a safe approach, but that there are many questions that need to be answered before we can fully inform our patients about the long-term outcomes of this treatment strategy,” Dr. Ehlers of the department of surgery at the University of Washington, Seattle, said in an interview.
Dr. Ehlers and her coinvestigators combed the PubMed and EMBASE databases for all English-language randomized controlled trials involving comparisons of antibiotic and appendectomy-based treatments for acute appendicitis. Studies were excluded if they lacked adult population investigation.
“Our study is a critical review of the literature [available] on this topic to understand the current state of evidence,” explained Dr. Ehlers, adding that the study’s main goal was to answer the most common questions she and her coauthors encountered when talking to physicians and patients about the benefits and risks of antibiotics-first over appendectomy; namely, “Is my appendicitis going to come back?” “Am I going to have a lot of extra trips to the hospital?” and “What’s my quality of life going to be?”
Ultimately, six trials, comprising 1,720 patients, were selected for inclusion. These studies were led by Dr. S. Eriksson (40 subjects), Dr. J. Styrud (252 subjects), Dr. A.N. Turhan (290 subjects), Dr. J. Hansson (369 subjects), Dr. C. Vons (239 subjects), and Dr. P. Salminen (530 subjects). The Styrud study did not enroll any women, and no study enrolled subjects older than 75 years of age, but the average age of each study’s patients ranged from 26 to 38 years.
Length-of-stay comparisons between appendectomy and antibiotics-first cohorts varied among the studies, but most showed the same or longer LOS for antibiotics-first subjects. Mean LOS was 3.3 for surgical patients vs. 3.1 for antibiotics (Eriksson), 2.6 surgical vs. 3.0 antibiotics (Styrud), 2.4 surgical vs. 3.14 antibiotics (Turhan), 3.0 for both (Hansson), 3.04 surgical vs. 3.96 antibiotics (Vons), and 3.0 for both (Salminen).
Rates of appendectomy among patients treated with an antibiotics-first approach varied as well, with a 35% rate in the Eriksson study (7 out of the 20 subjects treated with antibiotics-first) and a 24% rate in the Styrud and Turhan studies. The Hansson trial reported an unusually high crossover between cohorts of 60%, the investigators noted.
Two studies – those led by Turhan and Vons – noted higher rates of complications in antibiotics-first patients versus those who received surgical intervention: 4.7% vs. 4.4%, and 2.5% vs. less than 1%, respectively. All other studies had high rates of complications in the surgical cohorts, with the Eriksson study reporting no complications whatsoever among antibiotics-first subjects.
These findings, Dr. Ehlers stressed, are just a first step. This review’s relatively low sample size and limiting factors require that further studies be done to more firmly ascertain which option for appendicitis treatment is the most beneficial. To that end, Dr. Ehlers said that she and her coinvestigators are working on that next step.
“Our group is currently working on a study called the CODA [Comparing Outcomes of Drugs in Appendectomy] Study,” she said, with the goal of answering “questions about long-term patient-centered outcomes related to the antibiotics-first approach.” These questions include patients’ quality of life, whether patients have decisional regret about choosing one treatment strategy over another, if they develop long-term anxiety any time they experience even a small abdominal pain, how much time they miss from school or work by choosing one treatment option over another, and so on.
The goal of this study is that physicians “will be able to give [patients] a more informed view of what each treatment entails,” she said. Funded by the Patient-Centered Outcomes Research Institute, the study has no firm date of publication.
Dr. Ehlers disclosed receiving support via a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. No other conflicts of interest were reported.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: While the antibiotics-first approach to uncomplicated appendicitis has become increasingly popular in recent years, existing evidence shows that appendectomy is not inferior in terms of hospital stay and rates of complications.
Major finding: Length-of-stay and complication rates vary among all six included studies between antibiotics-first and surgical cohorts, indicating that neither one is definitively better or worse than the other.
Data source: Literature review of 1,720 appendicitis patients across six studies selected from PubMed and EMBASE databases.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases supported the study. No other conflicts of interest were reported.
New definition for massive ventral hernia?
Massive ventral hernias should be defined as those with a length or width of at least 15 cm and/or an overall area of 150 cm2, according to a report published in the Journal of American Surgery.
Until now, there has been no standardized definition of these defects. Defining massive ventral hernias is the first step in collecting and analyzing data on outcomes so that the best-practice methods for managing these cases can be determined, said Dr. Samuel W. Ross and his associates in the division of gastrointestinal and minimally invasive surgery, Carolinas Medical Center, Charlotte, N.C.
Their results suggest that patients with a massive ventral hernias “can be expected to stay in hospital for approximately 8 days, have an approximately 25% wound complication rate, have an 80% rate of activity limitation, and have a 100% rate of symptomatic pain at 1 month after surgery. This not only requires a long hospital course, with incumbent morbidity, but also the subsequent follow-up and management of wound and other complications,” the investigators said.
As expected, patients with massive ventral hernias had more complications and lower postop quality of life in terms of pain, activity limitation, and “mesh sensation,” compared with patients having regular hernia.
Large hernia defects are defined in the literature as up to 10 cm in size, but Dr. Ross and his colleagues hypothesized that massive hernia needed a more accurate cutoff size. The goal of this study was to define massive ventral hernia as 15 cm or more intraoperative defect size in any dimension or area 150 cm2 or more, and validate it by comparing ventral hernia repair patients’ outcomes using an international, multicenter, prospectively collected database.
To validate that their definition correlated with patient outcomes, the investigators analyzed information in the International Hernia Mesh Registry, a prospectively collected database covering consecutive inguinal, umbilical, and ventral hernia repairs at 45 medical centers in 10 countries. They focused on 878 cases of ventral hernia treated during a 6-year period. A total of 158 cases (18%) met their criteria for massive ventral hernia.
Hernia repairs were nearly equally divided between laparoscopic (50.3%) and open (49.7%) surgeries. Approximately 18% of each group were massive ventral hernias.
Overall, there were 118 wound complications, including seromas (affecting 9.6% of patients), hematomas (2%), and superficial and deep surgical site infections (2%). Other complications included deep vein thrombosis (0.5%), pneumonia (1%), cardiac events (0.5%), and problems requiring reoperation (4.2%). At 2-year follow-up, 1.5% of the study participants had died and 5.1% had ventral hernia recurrences.
As expected, compared with patients who had regular-sized hernias, those with massive hernias were significantly more obese, had a higher percentage of previous hernia surgeries, and had larger defect sizes. In general, their outcomes were not as good as those of patients with regular-sized hernias.
In the laparoscopic group, patients with massive hernias required a longer length of hospital stay than did those with regular-sized hernias (4.8 vs. 2.6 days), but their rates of wound complications, deep vein thrombosis, cardiac events, reoperation, and recurrence were similar. They had significantly increased pain and limitation of activity for the first postoperative month, but thereafter these factors were comparable.
In the open-surgery group, patients with massive hernias required a longer length of stay and had significantly more hematomas, deep infections at the surgical site, other wound complications, and cases of pneumonia. But their rates of superficial surgical site infection, seroma, cardiac events, hernia recurrence, and death were comparable with those in patients with regular-size hernias. They had significantly increased pain and limitation of activity during the first postoperative month, but thereafter, these factors were comparable (Amer J Surg. 2015;210[5]:801-13).
Interestingly, massive hernias were associated with “increased mesh sensation” at both 1-year and 2-year follow-up only in the laparoscopic cohort. “This finding may be due to the lack of closure of the musculofascial abdominal wall during these laparoscopic repairs, which may allow the patients to directly appreciate the mesh underneath their subcutaneous tissue.” In contrast, open surgery allowed more complete closure of the abdominal wall, “which may have impacted the patients’ perception of the mesh in their abdomens,” Dr. Ross and his associates wrote.
The study had no outside funding. Dr. Ross reported having no relevant financial disclosures. His associates reported ties to W.L. Gore, Ethicon, Novadaq, Bard/Davol, and LifeCell; one associate reported holding a patent for a free mobile app predicting the rate and cost of wound complications after ventral hernia repair, for which he receives no financial gain.
Massive ventral hernias should be defined as those with a length or width of at least 15 cm and/or an overall area of 150 cm2, according to a report published in the Journal of American Surgery.
Until now, there has been no standardized definition of these defects. Defining massive ventral hernias is the first step in collecting and analyzing data on outcomes so that the best-practice methods for managing these cases can be determined, said Dr. Samuel W. Ross and his associates in the division of gastrointestinal and minimally invasive surgery, Carolinas Medical Center, Charlotte, N.C.
Their results suggest that patients with a massive ventral hernias “can be expected to stay in hospital for approximately 8 days, have an approximately 25% wound complication rate, have an 80% rate of activity limitation, and have a 100% rate of symptomatic pain at 1 month after surgery. This not only requires a long hospital course, with incumbent morbidity, but also the subsequent follow-up and management of wound and other complications,” the investigators said.
As expected, patients with massive ventral hernias had more complications and lower postop quality of life in terms of pain, activity limitation, and “mesh sensation,” compared with patients having regular hernia.
Large hernia defects are defined in the literature as up to 10 cm in size, but Dr. Ross and his colleagues hypothesized that massive hernia needed a more accurate cutoff size. The goal of this study was to define massive ventral hernia as 15 cm or more intraoperative defect size in any dimension or area 150 cm2 or more, and validate it by comparing ventral hernia repair patients’ outcomes using an international, multicenter, prospectively collected database.
To validate that their definition correlated with patient outcomes, the investigators analyzed information in the International Hernia Mesh Registry, a prospectively collected database covering consecutive inguinal, umbilical, and ventral hernia repairs at 45 medical centers in 10 countries. They focused on 878 cases of ventral hernia treated during a 6-year period. A total of 158 cases (18%) met their criteria for massive ventral hernia.
Hernia repairs were nearly equally divided between laparoscopic (50.3%) and open (49.7%) surgeries. Approximately 18% of each group were massive ventral hernias.
Overall, there were 118 wound complications, including seromas (affecting 9.6% of patients), hematomas (2%), and superficial and deep surgical site infections (2%). Other complications included deep vein thrombosis (0.5%), pneumonia (1%), cardiac events (0.5%), and problems requiring reoperation (4.2%). At 2-year follow-up, 1.5% of the study participants had died and 5.1% had ventral hernia recurrences.
As expected, compared with patients who had regular-sized hernias, those with massive hernias were significantly more obese, had a higher percentage of previous hernia surgeries, and had larger defect sizes. In general, their outcomes were not as good as those of patients with regular-sized hernias.
In the laparoscopic group, patients with massive hernias required a longer length of hospital stay than did those with regular-sized hernias (4.8 vs. 2.6 days), but their rates of wound complications, deep vein thrombosis, cardiac events, reoperation, and recurrence were similar. They had significantly increased pain and limitation of activity for the first postoperative month, but thereafter these factors were comparable.
In the open-surgery group, patients with massive hernias required a longer length of stay and had significantly more hematomas, deep infections at the surgical site, other wound complications, and cases of pneumonia. But their rates of superficial surgical site infection, seroma, cardiac events, hernia recurrence, and death were comparable with those in patients with regular-size hernias. They had significantly increased pain and limitation of activity during the first postoperative month, but thereafter, these factors were comparable (Amer J Surg. 2015;210[5]:801-13).
Interestingly, massive hernias were associated with “increased mesh sensation” at both 1-year and 2-year follow-up only in the laparoscopic cohort. “This finding may be due to the lack of closure of the musculofascial abdominal wall during these laparoscopic repairs, which may allow the patients to directly appreciate the mesh underneath their subcutaneous tissue.” In contrast, open surgery allowed more complete closure of the abdominal wall, “which may have impacted the patients’ perception of the mesh in their abdomens,” Dr. Ross and his associates wrote.
The study had no outside funding. Dr. Ross reported having no relevant financial disclosures. His associates reported ties to W.L. Gore, Ethicon, Novadaq, Bard/Davol, and LifeCell; one associate reported holding a patent for a free mobile app predicting the rate and cost of wound complications after ventral hernia repair, for which he receives no financial gain.
Massive ventral hernias should be defined as those with a length or width of at least 15 cm and/or an overall area of 150 cm2, according to a report published in the Journal of American Surgery.
Until now, there has been no standardized definition of these defects. Defining massive ventral hernias is the first step in collecting and analyzing data on outcomes so that the best-practice methods for managing these cases can be determined, said Dr. Samuel W. Ross and his associates in the division of gastrointestinal and minimally invasive surgery, Carolinas Medical Center, Charlotte, N.C.
Their results suggest that patients with a massive ventral hernias “can be expected to stay in hospital for approximately 8 days, have an approximately 25% wound complication rate, have an 80% rate of activity limitation, and have a 100% rate of symptomatic pain at 1 month after surgery. This not only requires a long hospital course, with incumbent morbidity, but also the subsequent follow-up and management of wound and other complications,” the investigators said.
As expected, patients with massive ventral hernias had more complications and lower postop quality of life in terms of pain, activity limitation, and “mesh sensation,” compared with patients having regular hernia.
Large hernia defects are defined in the literature as up to 10 cm in size, but Dr. Ross and his colleagues hypothesized that massive hernia needed a more accurate cutoff size. The goal of this study was to define massive ventral hernia as 15 cm or more intraoperative defect size in any dimension or area 150 cm2 or more, and validate it by comparing ventral hernia repair patients’ outcomes using an international, multicenter, prospectively collected database.
To validate that their definition correlated with patient outcomes, the investigators analyzed information in the International Hernia Mesh Registry, a prospectively collected database covering consecutive inguinal, umbilical, and ventral hernia repairs at 45 medical centers in 10 countries. They focused on 878 cases of ventral hernia treated during a 6-year period. A total of 158 cases (18%) met their criteria for massive ventral hernia.
Hernia repairs were nearly equally divided between laparoscopic (50.3%) and open (49.7%) surgeries. Approximately 18% of each group were massive ventral hernias.
Overall, there were 118 wound complications, including seromas (affecting 9.6% of patients), hematomas (2%), and superficial and deep surgical site infections (2%). Other complications included deep vein thrombosis (0.5%), pneumonia (1%), cardiac events (0.5%), and problems requiring reoperation (4.2%). At 2-year follow-up, 1.5% of the study participants had died and 5.1% had ventral hernia recurrences.
As expected, compared with patients who had regular-sized hernias, those with massive hernias were significantly more obese, had a higher percentage of previous hernia surgeries, and had larger defect sizes. In general, their outcomes were not as good as those of patients with regular-sized hernias.
In the laparoscopic group, patients with massive hernias required a longer length of hospital stay than did those with regular-sized hernias (4.8 vs. 2.6 days), but their rates of wound complications, deep vein thrombosis, cardiac events, reoperation, and recurrence were similar. They had significantly increased pain and limitation of activity for the first postoperative month, but thereafter these factors were comparable.
In the open-surgery group, patients with massive hernias required a longer length of stay and had significantly more hematomas, deep infections at the surgical site, other wound complications, and cases of pneumonia. But their rates of superficial surgical site infection, seroma, cardiac events, hernia recurrence, and death were comparable with those in patients with regular-size hernias. They had significantly increased pain and limitation of activity during the first postoperative month, but thereafter, these factors were comparable (Amer J Surg. 2015;210[5]:801-13).
Interestingly, massive hernias were associated with “increased mesh sensation” at both 1-year and 2-year follow-up only in the laparoscopic cohort. “This finding may be due to the lack of closure of the musculofascial abdominal wall during these laparoscopic repairs, which may allow the patients to directly appreciate the mesh underneath their subcutaneous tissue.” In contrast, open surgery allowed more complete closure of the abdominal wall, “which may have impacted the patients’ perception of the mesh in their abdomens,” Dr. Ross and his associates wrote.
The study had no outside funding. Dr. Ross reported having no relevant financial disclosures. His associates reported ties to W.L. Gore, Ethicon, Novadaq, Bard/Davol, and LifeCell; one associate reported holding a patent for a free mobile app predicting the rate and cost of wound complications after ventral hernia repair, for which he receives no financial gain.
FROM THE AMERICAN JOURNAL OF SURGERY
Key clinical point: Massive ventral hernias should be defined as having a length or width of at least 15 cm or an overall area of 150 cm2.
Major finding: Patients with a massive ventral hernias can be expected to stay in hospital for approximately 8 days, have an approximately 25% wound complication rate, have an 80% rate of activity limitation, and have a 100% rate of symptomatic pain at 1 month.
Data source: An analysis of data concerning 878 hernia cases in an international registry during a 6-year period.
Disclosures: This study has no outside funding. Dr. Ross reported having no relevant financial disclosures. His associates reported ties to W.L. Gore, Ethicon, Novadaq, Bard/Davol, and LifeCell; one associate reported holding a patent for a free mobile app predicting the rate and cost of wound complications after ventral hernia repair, for which he receives no financial gain.