User login
Residents’ Forum: Docs not at par on post-call days
SAN DIEGO – If you feel sleepy and out of sorts on a post-call day, compared with a normal work-day, you’re not alone.
Anesthesiology faculty reported significant increases in feeling irritable, jittery, and sleepy, along with significant decreases in feeling confident, energetic, and talkative following an on-call period, according to a study presented at the annual meeting of the American Society of Anesthesiologists.
To date, most studies of partial sleep deprivation in health care settings have focused on residents and interns, and less on medical faculty, said lead study author Dr. Haleh Saadat of the department of anesthesiology and pain medicine at Nationwide Children’s Hospital in Columbus, Ohio. “Our call is 17 hours, from 3 p.m. to 7 a.m.; but the call period at most hospitals is 24 hours, and even longer at some private practices,” she said in an interview.
To examine the effects of partial sleep deprivation on reaction time, simple cognitive skills, and mood status in 21 anesthesiologists, Dr. Saadat and her associates obtained verbal consent from the study participants and measured reaction time, mood states, and eight subjective behavioral characteristics at two different time points: between 6:30 a.m. and 8 a.m. on a regular noncall day of work, and between 6:30 a.m. and 8 a.m. after an overnight call (a shift that runs from 3 p.m. to 7 a.m.). The behavioral characteristics included feeling alert, energetic, anxious, confident, irritable, jittery/nervous, sleepy, and talkative, and the researchers used paired t-tests to compare variable means between regular sleep days and post-call days.
Reaction time decreased in all 21 subjects after night call, indicating worse performance (P = .047), while total mood disturbance was significantly higher on post-call days, relative to noncall days (P less than .001).
Of the 21 anesthesiologists, 19 completed all simple cognitive task questions at both time points and reported significant increases in several of these parameters on post-call days, compared with normal work-days.
Post-call observations found participants feeling more irritable, confident, energetic, sleepy (P less than .001), feeling more jittery (P = .003), and feeling less talkative (P less than .001) than on normal work–days.
Coping strategies used to address their sleep deprivation were measured as well, with “most of our subjects using problem solving, followed by seeking social support and avoidance,” Dr. Saadat noted. “People who used avoidance had greater declines in reaction time on post–call days, compared with the rest of the study participants. It didn’t matter whether you were male, female, younger, or older.”
Dr. Saadat called for additional studies to evaluate the neurocognitive impact of partial sleep deprivation on physicians’ on-call duties.
“I would like to see if we can replicate the results in bigger centers,” she said. “If this is what is happening, we may need to pay more attention to faculty’s work hours in both academic and private practice settings – not only among anesthesiologists, but also in other specialties. These observations require a closer look at the potential implications for patients’ and professionals’ safety.”
The researchers reported no financial disclosures.
As a surgical resident, I have experienced firsthand the “drunk-tired” phenomenon, and to be honest, I do not believe it to be such a rare occurrence. “Drunk-tired” may be eloquently defined as being so tired you start behaving like you’re drunk, without actually consuming any alcohol of course.
The first manuscript relating fatigue amongst shift workers to performance impairment was published in 1996 by Dawson et al. demonstrating that moderate levels of fatigue actually produce more impairment than being legally intoxicated (Nature 1997;388:235). It didn’t take much of a leap to translate these observations to health care workers who work long hours, do shift work, and are on-call at times for more than 24 hours at a time. Recently, at the annual meeting of the American Society of Anesthesiologists in San Diego, Dr. Haleh Saadat from Ohio presented her study on the effects of partial sleep deprivation in staff anaesthesiologists leading to significantly decreased reaction times, cognitive skills, and mood disturbances on post-call days, compared with normal work days. No surprise there, as this is in line with what Dawson and his colleagues published nearly two decades ago. This study can certainly be translated to medical students, residents, fellows and staff from the breadth of specialties in medicine. In my opinion, what’s the point? I can already foresee what these studies are going to demonstrate, namely a clean sweep of all forms of cognitive and motor impairments when a subject is sleep deprived. The question becomes how we are translating all of this information into action that changes the lives of health care professionals and more importantly improves patient safety. Understandably, this is a loaded question and I am simply too exhausted to wrap my head around it.
So, next time you’re post call, feeling irritable, discoordinated, and inhibited, just remember: you’re as good as drunk and you should probably sleep it off.
Dr. Laura Drudi is the resident medical editor for Vascular Specialist.
As a surgical resident, I have experienced firsthand the “drunk-tired” phenomenon, and to be honest, I do not believe it to be such a rare occurrence. “Drunk-tired” may be eloquently defined as being so tired you start behaving like you’re drunk, without actually consuming any alcohol of course.
The first manuscript relating fatigue amongst shift workers to performance impairment was published in 1996 by Dawson et al. demonstrating that moderate levels of fatigue actually produce more impairment than being legally intoxicated (Nature 1997;388:235). It didn’t take much of a leap to translate these observations to health care workers who work long hours, do shift work, and are on-call at times for more than 24 hours at a time. Recently, at the annual meeting of the American Society of Anesthesiologists in San Diego, Dr. Haleh Saadat from Ohio presented her study on the effects of partial sleep deprivation in staff anaesthesiologists leading to significantly decreased reaction times, cognitive skills, and mood disturbances on post-call days, compared with normal work days. No surprise there, as this is in line with what Dawson and his colleagues published nearly two decades ago. This study can certainly be translated to medical students, residents, fellows and staff from the breadth of specialties in medicine. In my opinion, what’s the point? I can already foresee what these studies are going to demonstrate, namely a clean sweep of all forms of cognitive and motor impairments when a subject is sleep deprived. The question becomes how we are translating all of this information into action that changes the lives of health care professionals and more importantly improves patient safety. Understandably, this is a loaded question and I am simply too exhausted to wrap my head around it.
So, next time you’re post call, feeling irritable, discoordinated, and inhibited, just remember: you’re as good as drunk and you should probably sleep it off.
Dr. Laura Drudi is the resident medical editor for Vascular Specialist.
As a surgical resident, I have experienced firsthand the “drunk-tired” phenomenon, and to be honest, I do not believe it to be such a rare occurrence. “Drunk-tired” may be eloquently defined as being so tired you start behaving like you’re drunk, without actually consuming any alcohol of course.
The first manuscript relating fatigue amongst shift workers to performance impairment was published in 1996 by Dawson et al. demonstrating that moderate levels of fatigue actually produce more impairment than being legally intoxicated (Nature 1997;388:235). It didn’t take much of a leap to translate these observations to health care workers who work long hours, do shift work, and are on-call at times for more than 24 hours at a time. Recently, at the annual meeting of the American Society of Anesthesiologists in San Diego, Dr. Haleh Saadat from Ohio presented her study on the effects of partial sleep deprivation in staff anaesthesiologists leading to significantly decreased reaction times, cognitive skills, and mood disturbances on post-call days, compared with normal work days. No surprise there, as this is in line with what Dawson and his colleagues published nearly two decades ago. This study can certainly be translated to medical students, residents, fellows and staff from the breadth of specialties in medicine. In my opinion, what’s the point? I can already foresee what these studies are going to demonstrate, namely a clean sweep of all forms of cognitive and motor impairments when a subject is sleep deprived. The question becomes how we are translating all of this information into action that changes the lives of health care professionals and more importantly improves patient safety. Understandably, this is a loaded question and I am simply too exhausted to wrap my head around it.
So, next time you’re post call, feeling irritable, discoordinated, and inhibited, just remember: you’re as good as drunk and you should probably sleep it off.
Dr. Laura Drudi is the resident medical editor for Vascular Specialist.
SAN DIEGO – If you feel sleepy and out of sorts on a post-call day, compared with a normal work-day, you’re not alone.
Anesthesiology faculty reported significant increases in feeling irritable, jittery, and sleepy, along with significant decreases in feeling confident, energetic, and talkative following an on-call period, according to a study presented at the annual meeting of the American Society of Anesthesiologists.
To date, most studies of partial sleep deprivation in health care settings have focused on residents and interns, and less on medical faculty, said lead study author Dr. Haleh Saadat of the department of anesthesiology and pain medicine at Nationwide Children’s Hospital in Columbus, Ohio. “Our call is 17 hours, from 3 p.m. to 7 a.m.; but the call period at most hospitals is 24 hours, and even longer at some private practices,” she said in an interview.
To examine the effects of partial sleep deprivation on reaction time, simple cognitive skills, and mood status in 21 anesthesiologists, Dr. Saadat and her associates obtained verbal consent from the study participants and measured reaction time, mood states, and eight subjective behavioral characteristics at two different time points: between 6:30 a.m. and 8 a.m. on a regular noncall day of work, and between 6:30 a.m. and 8 a.m. after an overnight call (a shift that runs from 3 p.m. to 7 a.m.). The behavioral characteristics included feeling alert, energetic, anxious, confident, irritable, jittery/nervous, sleepy, and talkative, and the researchers used paired t-tests to compare variable means between regular sleep days and post-call days.
Reaction time decreased in all 21 subjects after night call, indicating worse performance (P = .047), while total mood disturbance was significantly higher on post-call days, relative to noncall days (P less than .001).
Of the 21 anesthesiologists, 19 completed all simple cognitive task questions at both time points and reported significant increases in several of these parameters on post-call days, compared with normal work-days.
Post-call observations found participants feeling more irritable, confident, energetic, sleepy (P less than .001), feeling more jittery (P = .003), and feeling less talkative (P less than .001) than on normal work–days.
Coping strategies used to address their sleep deprivation were measured as well, with “most of our subjects using problem solving, followed by seeking social support and avoidance,” Dr. Saadat noted. “People who used avoidance had greater declines in reaction time on post–call days, compared with the rest of the study participants. It didn’t matter whether you were male, female, younger, or older.”
Dr. Saadat called for additional studies to evaluate the neurocognitive impact of partial sleep deprivation on physicians’ on-call duties.
“I would like to see if we can replicate the results in bigger centers,” she said. “If this is what is happening, we may need to pay more attention to faculty’s work hours in both academic and private practice settings – not only among anesthesiologists, but also in other specialties. These observations require a closer look at the potential implications for patients’ and professionals’ safety.”
The researchers reported no financial disclosures.
SAN DIEGO – If you feel sleepy and out of sorts on a post-call day, compared with a normal work-day, you’re not alone.
Anesthesiology faculty reported significant increases in feeling irritable, jittery, and sleepy, along with significant decreases in feeling confident, energetic, and talkative following an on-call period, according to a study presented at the annual meeting of the American Society of Anesthesiologists.
To date, most studies of partial sleep deprivation in health care settings have focused on residents and interns, and less on medical faculty, said lead study author Dr. Haleh Saadat of the department of anesthesiology and pain medicine at Nationwide Children’s Hospital in Columbus, Ohio. “Our call is 17 hours, from 3 p.m. to 7 a.m.; but the call period at most hospitals is 24 hours, and even longer at some private practices,” she said in an interview.
To examine the effects of partial sleep deprivation on reaction time, simple cognitive skills, and mood status in 21 anesthesiologists, Dr. Saadat and her associates obtained verbal consent from the study participants and measured reaction time, mood states, and eight subjective behavioral characteristics at two different time points: between 6:30 a.m. and 8 a.m. on a regular noncall day of work, and between 6:30 a.m. and 8 a.m. after an overnight call (a shift that runs from 3 p.m. to 7 a.m.). The behavioral characteristics included feeling alert, energetic, anxious, confident, irritable, jittery/nervous, sleepy, and talkative, and the researchers used paired t-tests to compare variable means between regular sleep days and post-call days.
Reaction time decreased in all 21 subjects after night call, indicating worse performance (P = .047), while total mood disturbance was significantly higher on post-call days, relative to noncall days (P less than .001).
Of the 21 anesthesiologists, 19 completed all simple cognitive task questions at both time points and reported significant increases in several of these parameters on post-call days, compared with normal work-days.
Post-call observations found participants feeling more irritable, confident, energetic, sleepy (P less than .001), feeling more jittery (P = .003), and feeling less talkative (P less than .001) than on normal work–days.
Coping strategies used to address their sleep deprivation were measured as well, with “most of our subjects using problem solving, followed by seeking social support and avoidance,” Dr. Saadat noted. “People who used avoidance had greater declines in reaction time on post–call days, compared with the rest of the study participants. It didn’t matter whether you were male, female, younger, or older.”
Dr. Saadat called for additional studies to evaluate the neurocognitive impact of partial sleep deprivation on physicians’ on-call duties.
“I would like to see if we can replicate the results in bigger centers,” she said. “If this is what is happening, we may need to pay more attention to faculty’s work hours in both academic and private practice settings – not only among anesthesiologists, but also in other specialties. These observations require a closer look at the potential implications for patients’ and professionals’ safety.”
The researchers reported no financial disclosures.
FDA finalizes 1-year blood donor deferral for gay, bisexual men
The Food and Drug Administration announced finalized blood-donor deferral guidance that includes cutting the deferral for men who have sex with men from indefinitely to 1 year from the most recent sexual contact of this type.
The finalized guidance, which had been in development since its initial announcement in December 2014, contains 10 recommended questions that the blood industry should pose to potential donors to exclude those at greatest risk for potentially transmitting HIV in their blood.
In addition to reducing the deferral period for men who have had sex with men, the finalized guidance also cuts the deferral period for a woman who has had sex with a man who had sex with men from indefinitely to 12 months, and also reduces the deferral period for any person who has had sex with someone with a history of a positive test for HIV from indefinitely to 12 months.
The new guidance continues to recommend indefinite deferral for people who have ever tested positive for HIV, those who have exchanged sex for money or drugs, and those with a history of nonprescription injection drug use.
The FDA’s guidance document highlights that these are recommended steps for the blood products industry to take and are not required by law or legally enforceable.
The finalized guidance, which followed a public comment period on essentially unchanged draft guidance issued last May, received quick support in a statement from Dr. Andrew W. Gurman, president-elect of the American Medical Association.
“The American Medical Association commends the U.S. Food and Drug Administration for ending the lifetime ban that prohibits men who have had sex with men from donating blood,” Dr. Gurman said. “The AMA has been a strong advocate for eliminating public policies that do not align with scientific evidence and best ethical practices in public policy. The FDA’s final guidance takes important steps to improve the balance among ensuring health equity, engaging with high-risk populations, and protecting the safety of the national blood supply.”
However, a highly critical comment came from Daniel Bruner, senior director of policy for Whitman-Walker Health, a Washington-based health center focused on the LGBT community and HIV care.
“Although some may argue that a 12-month ban is better than a grossly outdated lifetime ban, the updated policy is still discriminatory and not rooted in the reality of HIV testing today,” Mr. Bruner said in a statement. “As we called for in our recommendations, the deferral period should be no longer than 30 days, given that with current testing technology an HIV infection can be detected in donated blood within several weeks of exposure.
“And even then, those that would be subject to the deferral period should be able to donate blood if they agree to return for an HIV test 30 days after donating,” Mr. Bruner said. “Sadly, and also not in line with modern science, the new guidelines continue the discriminatory lifetime bans on individuals who have ever engaged in sex work or ever used nonprescription injection drugs.”
Mr. Bruner added, “We are also disheartened that the FDA has failed to give clear guidance to prevent discrimination against transgender individuals, which occurs too often at blood donation centers. The FDA must do better than this slow chipping away at antiquated bans.”
On Twitter @mitchelzoler
The Food and Drug Administration announced finalized blood-donor deferral guidance that includes cutting the deferral for men who have sex with men from indefinitely to 1 year from the most recent sexual contact of this type.
The finalized guidance, which had been in development since its initial announcement in December 2014, contains 10 recommended questions that the blood industry should pose to potential donors to exclude those at greatest risk for potentially transmitting HIV in their blood.
In addition to reducing the deferral period for men who have had sex with men, the finalized guidance also cuts the deferral period for a woman who has had sex with a man who had sex with men from indefinitely to 12 months, and also reduces the deferral period for any person who has had sex with someone with a history of a positive test for HIV from indefinitely to 12 months.
The new guidance continues to recommend indefinite deferral for people who have ever tested positive for HIV, those who have exchanged sex for money or drugs, and those with a history of nonprescription injection drug use.
The FDA’s guidance document highlights that these are recommended steps for the blood products industry to take and are not required by law or legally enforceable.
The finalized guidance, which followed a public comment period on essentially unchanged draft guidance issued last May, received quick support in a statement from Dr. Andrew W. Gurman, president-elect of the American Medical Association.
“The American Medical Association commends the U.S. Food and Drug Administration for ending the lifetime ban that prohibits men who have had sex with men from donating blood,” Dr. Gurman said. “The AMA has been a strong advocate for eliminating public policies that do not align with scientific evidence and best ethical practices in public policy. The FDA’s final guidance takes important steps to improve the balance among ensuring health equity, engaging with high-risk populations, and protecting the safety of the national blood supply.”
However, a highly critical comment came from Daniel Bruner, senior director of policy for Whitman-Walker Health, a Washington-based health center focused on the LGBT community and HIV care.
“Although some may argue that a 12-month ban is better than a grossly outdated lifetime ban, the updated policy is still discriminatory and not rooted in the reality of HIV testing today,” Mr. Bruner said in a statement. “As we called for in our recommendations, the deferral period should be no longer than 30 days, given that with current testing technology an HIV infection can be detected in donated blood within several weeks of exposure.
“And even then, those that would be subject to the deferral period should be able to donate blood if they agree to return for an HIV test 30 days after donating,” Mr. Bruner said. “Sadly, and also not in line with modern science, the new guidelines continue the discriminatory lifetime bans on individuals who have ever engaged in sex work or ever used nonprescription injection drugs.”
Mr. Bruner added, “We are also disheartened that the FDA has failed to give clear guidance to prevent discrimination against transgender individuals, which occurs too often at blood donation centers. The FDA must do better than this slow chipping away at antiquated bans.”
On Twitter @mitchelzoler
The Food and Drug Administration announced finalized blood-donor deferral guidance that includes cutting the deferral for men who have sex with men from indefinitely to 1 year from the most recent sexual contact of this type.
The finalized guidance, which had been in development since its initial announcement in December 2014, contains 10 recommended questions that the blood industry should pose to potential donors to exclude those at greatest risk for potentially transmitting HIV in their blood.
In addition to reducing the deferral period for men who have had sex with men, the finalized guidance also cuts the deferral period for a woman who has had sex with a man who had sex with men from indefinitely to 12 months, and also reduces the deferral period for any person who has had sex with someone with a history of a positive test for HIV from indefinitely to 12 months.
The new guidance continues to recommend indefinite deferral for people who have ever tested positive for HIV, those who have exchanged sex for money or drugs, and those with a history of nonprescription injection drug use.
The FDA’s guidance document highlights that these are recommended steps for the blood products industry to take and are not required by law or legally enforceable.
The finalized guidance, which followed a public comment period on essentially unchanged draft guidance issued last May, received quick support in a statement from Dr. Andrew W. Gurman, president-elect of the American Medical Association.
“The American Medical Association commends the U.S. Food and Drug Administration for ending the lifetime ban that prohibits men who have had sex with men from donating blood,” Dr. Gurman said. “The AMA has been a strong advocate for eliminating public policies that do not align with scientific evidence and best ethical practices in public policy. The FDA’s final guidance takes important steps to improve the balance among ensuring health equity, engaging with high-risk populations, and protecting the safety of the national blood supply.”
However, a highly critical comment came from Daniel Bruner, senior director of policy for Whitman-Walker Health, a Washington-based health center focused on the LGBT community and HIV care.
“Although some may argue that a 12-month ban is better than a grossly outdated lifetime ban, the updated policy is still discriminatory and not rooted in the reality of HIV testing today,” Mr. Bruner said in a statement. “As we called for in our recommendations, the deferral period should be no longer than 30 days, given that with current testing technology an HIV infection can be detected in donated blood within several weeks of exposure.
“And even then, those that would be subject to the deferral period should be able to donate blood if they agree to return for an HIV test 30 days after donating,” Mr. Bruner said. “Sadly, and also not in line with modern science, the new guidelines continue the discriminatory lifetime bans on individuals who have ever engaged in sex work or ever used nonprescription injection drugs.”
Mr. Bruner added, “We are also disheartened that the FDA has failed to give clear guidance to prevent discrimination against transgender individuals, which occurs too often at blood donation centers. The FDA must do better than this slow chipping away at antiquated bans.”
On Twitter @mitchelzoler
ACS Surgery News December digital issue is available
The December ACS Surgery News digital issue is online - use the mobile app to download or view as a pdf.
This month features a review of the American Cancer Society revised mammogram guidelines, news on what gives your patient with an infected hernia the best chance for salvage, and an interview with ACS Operation Giving Back Medical Director, Dr. Girma Tefera.
Don't miss Dr. Peter Angelos' tribute to his late mentor, Dr. Norman W. Thompson.
The December ACS Surgery News digital issue is online - use the mobile app to download or view as a pdf.
This month features a review of the American Cancer Society revised mammogram guidelines, news on what gives your patient with an infected hernia the best chance for salvage, and an interview with ACS Operation Giving Back Medical Director, Dr. Girma Tefera.
Don't miss Dr. Peter Angelos' tribute to his late mentor, Dr. Norman W. Thompson.
The December ACS Surgery News digital issue is online - use the mobile app to download or view as a pdf.
This month features a review of the American Cancer Society revised mammogram guidelines, news on what gives your patient with an infected hernia the best chance for salvage, and an interview with ACS Operation Giving Back Medical Director, Dr. Girma Tefera.
Don't miss Dr. Peter Angelos' tribute to his late mentor, Dr. Norman W. Thompson.
Sugammadex OK’d to reverse neuromuscular blockade during surgery
The Food and Drug Administration approved on Dec. 15 Merck’s sugammadex (Bridion) injection to reverse the effects of neuromuscular blockade induced by rocuronium bromide and vecuronium bromide during surgery.
The safety and efficacy of sugammadex were evaluated in three phase III trials involving 456 participants; most recovered within 5 minutes. An FDA review of the drug found that there was less residual neuromuscular blockade with sugammadex compared to neostigmine, and a 4-minute time savings to extubation and operating room discharge.
“Bridion provides a new treatment option that may help patients recover sooner from medications used for intubation or ventilation during surgery. This drug enables medical personnel to reverse the effects of neuromuscular blocking drugs and restore spontaneous breathing after surgery,” Dr. Sharon Hertz, director of the FDA’s Division of Anesthesia, Analgesia, and Addiction Products, said in a statement.
Although approved in other countries, sugammadex has been in the FDA’s review process since 2007, previously rejected and held up by concerns over anaphylaxis and other issues.
Because of that, sugammadex was further evaluated in a randomized, double-blind, parallel-group, repeat-dose trial. Of the 299 participants treated with Bridion, one person had an anaphylactic reaction. “Clinicians should be aware of the possibility of a hypersensitivity reaction or anaphylaxis and should intervene as appropriate,” the agency said in its statement.
Cases of marked bradycardia, some of which have resulted in cardiac arrest, have also been observed within minutes after the administration of Bridion. Tachycardia and bradycardia have been associated with cases of anaphylaxis. “Patients should be closely monitored for hemodynamic changes during and after reversal of neuromuscular blockade, and treatment with anticholinergic agents, such as atropine, should be administered if clinically significant bradycardia is observed,” the agency said.
The most common side effects reported in trials were vomiting, hypotension, pain, headache, and nausea. “Doctors should also advise women using hormonal contraceptives that Bridion may temporarily reduce the contraceptive effect so they must use an alternate method of birth control for a period of time,” the agency said.
Rocuronium bromide and vecuronium bromide are used to paralyze the vocal cords for tracheal intubation, as well as to paralyze patients under general anesthesia and prevent spontaneous breathing during ventilation. Sugammadex is a new molecular entity of the gamma-cyclodextrin class, designed to bind rocuronium and vecuronium.
The Food and Drug Administration approved on Dec. 15 Merck’s sugammadex (Bridion) injection to reverse the effects of neuromuscular blockade induced by rocuronium bromide and vecuronium bromide during surgery.
The safety and efficacy of sugammadex were evaluated in three phase III trials involving 456 participants; most recovered within 5 minutes. An FDA review of the drug found that there was less residual neuromuscular blockade with sugammadex compared to neostigmine, and a 4-minute time savings to extubation and operating room discharge.
“Bridion provides a new treatment option that may help patients recover sooner from medications used for intubation or ventilation during surgery. This drug enables medical personnel to reverse the effects of neuromuscular blocking drugs and restore spontaneous breathing after surgery,” Dr. Sharon Hertz, director of the FDA’s Division of Anesthesia, Analgesia, and Addiction Products, said in a statement.
Although approved in other countries, sugammadex has been in the FDA’s review process since 2007, previously rejected and held up by concerns over anaphylaxis and other issues.
Because of that, sugammadex was further evaluated in a randomized, double-blind, parallel-group, repeat-dose trial. Of the 299 participants treated with Bridion, one person had an anaphylactic reaction. “Clinicians should be aware of the possibility of a hypersensitivity reaction or anaphylaxis and should intervene as appropriate,” the agency said in its statement.
Cases of marked bradycardia, some of which have resulted in cardiac arrest, have also been observed within minutes after the administration of Bridion. Tachycardia and bradycardia have been associated with cases of anaphylaxis. “Patients should be closely monitored for hemodynamic changes during and after reversal of neuromuscular blockade, and treatment with anticholinergic agents, such as atropine, should be administered if clinically significant bradycardia is observed,” the agency said.
The most common side effects reported in trials were vomiting, hypotension, pain, headache, and nausea. “Doctors should also advise women using hormonal contraceptives that Bridion may temporarily reduce the contraceptive effect so they must use an alternate method of birth control for a period of time,” the agency said.
Rocuronium bromide and vecuronium bromide are used to paralyze the vocal cords for tracheal intubation, as well as to paralyze patients under general anesthesia and prevent spontaneous breathing during ventilation. Sugammadex is a new molecular entity of the gamma-cyclodextrin class, designed to bind rocuronium and vecuronium.
The Food and Drug Administration approved on Dec. 15 Merck’s sugammadex (Bridion) injection to reverse the effects of neuromuscular blockade induced by rocuronium bromide and vecuronium bromide during surgery.
The safety and efficacy of sugammadex were evaluated in three phase III trials involving 456 participants; most recovered within 5 minutes. An FDA review of the drug found that there was less residual neuromuscular blockade with sugammadex compared to neostigmine, and a 4-minute time savings to extubation and operating room discharge.
“Bridion provides a new treatment option that may help patients recover sooner from medications used for intubation or ventilation during surgery. This drug enables medical personnel to reverse the effects of neuromuscular blocking drugs and restore spontaneous breathing after surgery,” Dr. Sharon Hertz, director of the FDA’s Division of Anesthesia, Analgesia, and Addiction Products, said in a statement.
Although approved in other countries, sugammadex has been in the FDA’s review process since 2007, previously rejected and held up by concerns over anaphylaxis and other issues.
Because of that, sugammadex was further evaluated in a randomized, double-blind, parallel-group, repeat-dose trial. Of the 299 participants treated with Bridion, one person had an anaphylactic reaction. “Clinicians should be aware of the possibility of a hypersensitivity reaction or anaphylaxis and should intervene as appropriate,” the agency said in its statement.
Cases of marked bradycardia, some of which have resulted in cardiac arrest, have also been observed within minutes after the administration of Bridion. Tachycardia and bradycardia have been associated with cases of anaphylaxis. “Patients should be closely monitored for hemodynamic changes during and after reversal of neuromuscular blockade, and treatment with anticholinergic agents, such as atropine, should be administered if clinically significant bradycardia is observed,” the agency said.
The most common side effects reported in trials were vomiting, hypotension, pain, headache, and nausea. “Doctors should also advise women using hormonal contraceptives that Bridion may temporarily reduce the contraceptive effect so they must use an alternate method of birth control for a period of time,” the agency said.
Rocuronium bromide and vecuronium bromide are used to paralyze the vocal cords for tracheal intubation, as well as to paralyze patients under general anesthesia and prevent spontaneous breathing during ventilation. Sugammadex is a new molecular entity of the gamma-cyclodextrin class, designed to bind rocuronium and vecuronium.
Core needle biopsy proves sensitive for first-line thyroid nodule diagnosis
LAKE BUENA VISTA, FLA. – The nondiagnostic result rate was significantly lower with core needle biopsy than with fine needle aspiration as a first-line biopsy method for newly detected thyroid nodules in a comparative study.
In 631 pairs of initially detected thyroid nodules that were matched based on propensity score analysis, the nondiagnostic result rate was 1.4% when core needle biopsy (CNB) was used, compared with 8.1% with ultrasound-guided fine-needle aspiration. The indeterminate result rate was 5.1% vs. 8.1% with the two approaches, respectively, and the differences between the groups were statistically significant, Dr. Hyun Kyung Lim of Soonchunhyang University Seoul Hospital, Korea, reported at the International Thyroid Congress.
The nondiagnostic rate with CNB was also significantly lower than with fine-needle aspiration for nodules with calcifications, posterior location, or diameter less than 1 cm, Dr. Lim said at the meeting at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
No difference was seen between the groups with respect to diagnostic performance based on degree of clinician experience.
The complication rate was higher with CNB than with fine -needle aspiration (3.6% vs. 1.6%), but complications were minor, Dr. Lim said.
Core needle biopsy has been suggested as a complementary tool for the diagnosis of thyroid nodules when the results of fine-needle aspiration are inconclusive, and the approach has been shown to be both safe and accurate for biopsy. However, its role as a first-line approach for thyroid nodule biopsy has been controversial and few studies have evaluated it as a first-line tool, she noted.
The current findings suggest that CNB is a safe and highly sensitive first-line biopsy method for such nodules, she concluded.
Dr. Lim reported having no disclosures.
LAKE BUENA VISTA, FLA. – The nondiagnostic result rate was significantly lower with core needle biopsy than with fine needle aspiration as a first-line biopsy method for newly detected thyroid nodules in a comparative study.
In 631 pairs of initially detected thyroid nodules that were matched based on propensity score analysis, the nondiagnostic result rate was 1.4% when core needle biopsy (CNB) was used, compared with 8.1% with ultrasound-guided fine-needle aspiration. The indeterminate result rate was 5.1% vs. 8.1% with the two approaches, respectively, and the differences between the groups were statistically significant, Dr. Hyun Kyung Lim of Soonchunhyang University Seoul Hospital, Korea, reported at the International Thyroid Congress.
The nondiagnostic rate with CNB was also significantly lower than with fine-needle aspiration for nodules with calcifications, posterior location, or diameter less than 1 cm, Dr. Lim said at the meeting at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
No difference was seen between the groups with respect to diagnostic performance based on degree of clinician experience.
The complication rate was higher with CNB than with fine -needle aspiration (3.6% vs. 1.6%), but complications were minor, Dr. Lim said.
Core needle biopsy has been suggested as a complementary tool for the diagnosis of thyroid nodules when the results of fine-needle aspiration are inconclusive, and the approach has been shown to be both safe and accurate for biopsy. However, its role as a first-line approach for thyroid nodule biopsy has been controversial and few studies have evaluated it as a first-line tool, she noted.
The current findings suggest that CNB is a safe and highly sensitive first-line biopsy method for such nodules, she concluded.
Dr. Lim reported having no disclosures.
LAKE BUENA VISTA, FLA. – The nondiagnostic result rate was significantly lower with core needle biopsy than with fine needle aspiration as a first-line biopsy method for newly detected thyroid nodules in a comparative study.
In 631 pairs of initially detected thyroid nodules that were matched based on propensity score analysis, the nondiagnostic result rate was 1.4% when core needle biopsy (CNB) was used, compared with 8.1% with ultrasound-guided fine-needle aspiration. The indeterminate result rate was 5.1% vs. 8.1% with the two approaches, respectively, and the differences between the groups were statistically significant, Dr. Hyun Kyung Lim of Soonchunhyang University Seoul Hospital, Korea, reported at the International Thyroid Congress.
The nondiagnostic rate with CNB was also significantly lower than with fine-needle aspiration for nodules with calcifications, posterior location, or diameter less than 1 cm, Dr. Lim said at the meeting at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
No difference was seen between the groups with respect to diagnostic performance based on degree of clinician experience.
The complication rate was higher with CNB than with fine -needle aspiration (3.6% vs. 1.6%), but complications were minor, Dr. Lim said.
Core needle biopsy has been suggested as a complementary tool for the diagnosis of thyroid nodules when the results of fine-needle aspiration are inconclusive, and the approach has been shown to be both safe and accurate for biopsy. However, its role as a first-line approach for thyroid nodule biopsy has been controversial and few studies have evaluated it as a first-line tool, she noted.
The current findings suggest that CNB is a safe and highly sensitive first-line biopsy method for such nodules, she concluded.
Dr. Lim reported having no disclosures.
AT THE INTERNATIONAL THYROID CONGRESS
Key clinical point: The nondiagnostic result rate was significantly lower with core needle biopsy than with fine-needle aspiration as a first-line biopsy method for newly detected thyroid nodules in a comparative study.
Major finding: The nondiagnostic result rate was 1.4% with core needle biopsy vs. 8.1% with fine-needle aspiration.
Data source: A comparative study in 631 propensity score–matched pairs of thyroid nodules.
Disclosures: Dr. Lim reported having no disclosures.
Self-reported poor functional status predicts perioperative morbidity
SAN DIEGO – Among patients with pulmonary hypertension presenting for elective surgery, self-reported poor functional status is associated with multiple comorbidities and is independently predictive of longer hospital length of stay, results from an ongoing single-center study suggest.
“Patients with pulmonary hypertension (PHTN) presenting for elective surgery are at significantly higher risk for adverse perioperative outcomes, including increased hospital length of stay, right ventricular failure, cardiac arrhythmia, persistent postoperative hypoxemia, coronary ischemia and death,” researchers led by Dr. Aalap C. Shah wrote in an abstract presented at the at the annual meeting of the American Society of Anesthesiologists. “The diagnosis of PHTN is based on costly echocardiographic examination and right heart catheterization and should be reserved for high-risk patients. No studies have assessed the role of self-reported functional classification on PHTN severity stratification, and few studies have achieved a sufficiently large patient sample size.”
In an effort to evaluate the predictive value of self-reported exercise tolerance on echocardiogram findings, outcomes, and length of stay (LOS) after noncardiac, nonobstetric surgery, the researchers queried the University of Washington database for all PHTN seen in preoperative anesthesia clinic for noncardiac, nonobstetric procedures from April 2007 through September 2013. Inclusion criteria required an echocardiogram less than 1 year prior to the procedure and available patient-reported functional status, which was defined as less than four metabolic equivalents (METS) in exercise testing or four METS or greater. Dr. Shah, formerly a resident in the University of Washington’s department of anesthesiology and pain medicine, and his associates used univariate analyses to compare functional status with echocardiographic findings, complication rates, and length of stay (LOS). At the meeting he presented results from 294 patients evaluated to date: 143 with normal functional status and 151 with poor functional status. Their mean age was 62 years, and 51% of patients were female.
Compared with their counterparts with normal functional status, patients with poor functional status trended toward a higher complication rate at hospital discharge (14.6% vs. 7%, respectively; P = .041) and had a higher cumulative rate of complications (33 vs. 15; P = .035). However, no association between functional status and complications was observed 30 days postoperatively.
Patients with poor functional status had a significantly longer average LOS, compared with patients with normal functional status (7.21 vs. 4.73 days; P = .047). Open surgical approach was also an independent predictor of increased LOS (odds ratio 2.39; P = .005). No significant independent predictors of complications were observed at discharge or 30 days postoperatively.
“Going forward, the goal is to use these data to create a risk stratification algorithm to figure out: Does a patient with good functional status and pulmonary hypertension undergoing toe surgery, for example, really need an echocardiogram before getting surgery?” said Dr. Shah said, who is now an anesthesiology fellow at Boston Children’s Hospital. “Hopefully we can show that using these risk stratification algorithms can decrease the costs and decrease the time to actually getting surgery.”
The researchers reported having no financial disclosures.
SAN DIEGO – Among patients with pulmonary hypertension presenting for elective surgery, self-reported poor functional status is associated with multiple comorbidities and is independently predictive of longer hospital length of stay, results from an ongoing single-center study suggest.
“Patients with pulmonary hypertension (PHTN) presenting for elective surgery are at significantly higher risk for adverse perioperative outcomes, including increased hospital length of stay, right ventricular failure, cardiac arrhythmia, persistent postoperative hypoxemia, coronary ischemia and death,” researchers led by Dr. Aalap C. Shah wrote in an abstract presented at the at the annual meeting of the American Society of Anesthesiologists. “The diagnosis of PHTN is based on costly echocardiographic examination and right heart catheterization and should be reserved for high-risk patients. No studies have assessed the role of self-reported functional classification on PHTN severity stratification, and few studies have achieved a sufficiently large patient sample size.”
In an effort to evaluate the predictive value of self-reported exercise tolerance on echocardiogram findings, outcomes, and length of stay (LOS) after noncardiac, nonobstetric surgery, the researchers queried the University of Washington database for all PHTN seen in preoperative anesthesia clinic for noncardiac, nonobstetric procedures from April 2007 through September 2013. Inclusion criteria required an echocardiogram less than 1 year prior to the procedure and available patient-reported functional status, which was defined as less than four metabolic equivalents (METS) in exercise testing or four METS or greater. Dr. Shah, formerly a resident in the University of Washington’s department of anesthesiology and pain medicine, and his associates used univariate analyses to compare functional status with echocardiographic findings, complication rates, and length of stay (LOS). At the meeting he presented results from 294 patients evaluated to date: 143 with normal functional status and 151 with poor functional status. Their mean age was 62 years, and 51% of patients were female.
Compared with their counterparts with normal functional status, patients with poor functional status trended toward a higher complication rate at hospital discharge (14.6% vs. 7%, respectively; P = .041) and had a higher cumulative rate of complications (33 vs. 15; P = .035). However, no association between functional status and complications was observed 30 days postoperatively.
Patients with poor functional status had a significantly longer average LOS, compared with patients with normal functional status (7.21 vs. 4.73 days; P = .047). Open surgical approach was also an independent predictor of increased LOS (odds ratio 2.39; P = .005). No significant independent predictors of complications were observed at discharge or 30 days postoperatively.
“Going forward, the goal is to use these data to create a risk stratification algorithm to figure out: Does a patient with good functional status and pulmonary hypertension undergoing toe surgery, for example, really need an echocardiogram before getting surgery?” said Dr. Shah said, who is now an anesthesiology fellow at Boston Children’s Hospital. “Hopefully we can show that using these risk stratification algorithms can decrease the costs and decrease the time to actually getting surgery.”
The researchers reported having no financial disclosures.
SAN DIEGO – Among patients with pulmonary hypertension presenting for elective surgery, self-reported poor functional status is associated with multiple comorbidities and is independently predictive of longer hospital length of stay, results from an ongoing single-center study suggest.
“Patients with pulmonary hypertension (PHTN) presenting for elective surgery are at significantly higher risk for adverse perioperative outcomes, including increased hospital length of stay, right ventricular failure, cardiac arrhythmia, persistent postoperative hypoxemia, coronary ischemia and death,” researchers led by Dr. Aalap C. Shah wrote in an abstract presented at the at the annual meeting of the American Society of Anesthesiologists. “The diagnosis of PHTN is based on costly echocardiographic examination and right heart catheterization and should be reserved for high-risk patients. No studies have assessed the role of self-reported functional classification on PHTN severity stratification, and few studies have achieved a sufficiently large patient sample size.”
In an effort to evaluate the predictive value of self-reported exercise tolerance on echocardiogram findings, outcomes, and length of stay (LOS) after noncardiac, nonobstetric surgery, the researchers queried the University of Washington database for all PHTN seen in preoperative anesthesia clinic for noncardiac, nonobstetric procedures from April 2007 through September 2013. Inclusion criteria required an echocardiogram less than 1 year prior to the procedure and available patient-reported functional status, which was defined as less than four metabolic equivalents (METS) in exercise testing or four METS or greater. Dr. Shah, formerly a resident in the University of Washington’s department of anesthesiology and pain medicine, and his associates used univariate analyses to compare functional status with echocardiographic findings, complication rates, and length of stay (LOS). At the meeting he presented results from 294 patients evaluated to date: 143 with normal functional status and 151 with poor functional status. Their mean age was 62 years, and 51% of patients were female.
Compared with their counterparts with normal functional status, patients with poor functional status trended toward a higher complication rate at hospital discharge (14.6% vs. 7%, respectively; P = .041) and had a higher cumulative rate of complications (33 vs. 15; P = .035). However, no association between functional status and complications was observed 30 days postoperatively.
Patients with poor functional status had a significantly longer average LOS, compared with patients with normal functional status (7.21 vs. 4.73 days; P = .047). Open surgical approach was also an independent predictor of increased LOS (odds ratio 2.39; P = .005). No significant independent predictors of complications were observed at discharge or 30 days postoperatively.
“Going forward, the goal is to use these data to create a risk stratification algorithm to figure out: Does a patient with good functional status and pulmonary hypertension undergoing toe surgery, for example, really need an echocardiogram before getting surgery?” said Dr. Shah said, who is now an anesthesiology fellow at Boston Children’s Hospital. “Hopefully we can show that using these risk stratification algorithms can decrease the costs and decrease the time to actually getting surgery.”
The researchers reported having no financial disclosures.
AT THE ASA ANNUAL MEETING
Key clinical point:Poor self-reported exercise tolerance by patients with pulmonary hypertension is associated with multiple comorbidities and increased hospital length of stay.
Major finding: Compared with their counterparts with normal functional status, patients with poor functional status trended toward a higher complication rate at hospital discharge (14.6% vs. 7%, respectively; P = .041) and had a higher cumulative rate of complications (33 vs. 15; P = .035).
Data source: A study 294 PHTN patients seen in preoperative anesthesia clinic at the University of Washington for non-cardiac, nonobstetric procedures from April 2007 through September 2013.
Disclosures: The researchers reported having no financial disclosures.
FDA advisory committees support changing codeine contraindications for children
SILVER SPRING, MD. – A joint meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee (PADAC) and the Drug Safety and Risk Management Advisory Committee (DSaRM) on Dec. 10 voted overwhelmingly to support expanding the current contraindication for codeine to preclude its use for any pain management in all children under age 18 years.
Twenty members of the joint advisory panel voted for the aforementioned contraindication, while six members elected to contraindicate for any pain management in children younger than 12 years old, and another two members voted only to contraindicate for children younger than age 6 years. Only one member – Dr. Maureen Finnegan of the DSaRM – voted not to make any changes to the current contraindications for codeine.
The joint advisory panel also voted to contraindicate the use of codeine for the treatment of cough in all children younger than age 18 years by a similarly robust margin: 20 members voted for contraindicating in all pediatric patients, five voted to contraindicate only in patients younger than age 12 years, one voted to contraindicate in children younger than age 6 years, and three members voted not to make any changes at all.
The final voting question, asking whether to remove codeine from the FDA monograph for over-the-counter use in treating cough in children, was almost unanimously supported by the voting members of both committees. Only one member – Dr. Lorraine J. Gudas, a temporary voting member – supported removing codeine from the monograph only for children under age 2 years. Dr. Finnegan abstained from voting on this charge, telling the committee that “this is totally out of my wheelhouse.”
The decision to vote on approving amendments to the contraindications for codeine use – which would affect not just the monogram, but labeling as well – comes on the heels of the FDA announcing this summer that they would be investigating the safety of codeine-containing drugs in children, asking health care providers and patients to report any adverse events associated with the drug.
The joint advisory panel cited reports of respiratory depression and death in pediatric patients, variability of codeine metabolism based upon CYP2D6 activity, and the fact that “some regulatory agencies have restricted use of codeine for both cough and analgesia in pediatric patients” as their key reasons for considering the changes to current contraindications, according to Dr. Sally Seymour, the FDA’s Deputy Director for Safety.
In a presentation on codeine use for pediatric analgesia, FDA Medical Officer Dr. Timothy Jiang cited several studies detailing adverse events in children taking codeine-containing drugs. One 2007 study by Voronov et al involved a 29-month-old boy of North African heritage who took codeine/acetaminophen following adenotonsillectomy for “recurrent tonsillitis and mild-moderate sleep apnea;” the boy was found unresponsive the day after the operation, but was later resuscitated.
In another study – a 2009 study in the New England Journal of Medicine by Catherine Ciszkowski and her associates – cites a similar situation of a 2-year-old boy receiving codeine after adenotonsillectomy, only to be found unresponsive; in this case, however, the boy died 2 days after the operation. Additionally, Dr. Jiang cited a 2012 search of the Adverse Event Reporting System, looking at data from 1969 through May 1, 2012, which found that six additional cases of death, as well as seven literature cases that mentioned patients’ CYPD26 status as possibly contributing.
The FDA is not required to follow the advice of its advisory panels, but often does. No members of the panel reported any relevant financial conflicts of interest.
SILVER SPRING, MD. – A joint meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee (PADAC) and the Drug Safety and Risk Management Advisory Committee (DSaRM) on Dec. 10 voted overwhelmingly to support expanding the current contraindication for codeine to preclude its use for any pain management in all children under age 18 years.
Twenty members of the joint advisory panel voted for the aforementioned contraindication, while six members elected to contraindicate for any pain management in children younger than 12 years old, and another two members voted only to contraindicate for children younger than age 6 years. Only one member – Dr. Maureen Finnegan of the DSaRM – voted not to make any changes to the current contraindications for codeine.
The joint advisory panel also voted to contraindicate the use of codeine for the treatment of cough in all children younger than age 18 years by a similarly robust margin: 20 members voted for contraindicating in all pediatric patients, five voted to contraindicate only in patients younger than age 12 years, one voted to contraindicate in children younger than age 6 years, and three members voted not to make any changes at all.
The final voting question, asking whether to remove codeine from the FDA monograph for over-the-counter use in treating cough in children, was almost unanimously supported by the voting members of both committees. Only one member – Dr. Lorraine J. Gudas, a temporary voting member – supported removing codeine from the monograph only for children under age 2 years. Dr. Finnegan abstained from voting on this charge, telling the committee that “this is totally out of my wheelhouse.”
The decision to vote on approving amendments to the contraindications for codeine use – which would affect not just the monogram, but labeling as well – comes on the heels of the FDA announcing this summer that they would be investigating the safety of codeine-containing drugs in children, asking health care providers and patients to report any adverse events associated with the drug.
The joint advisory panel cited reports of respiratory depression and death in pediatric patients, variability of codeine metabolism based upon CYP2D6 activity, and the fact that “some regulatory agencies have restricted use of codeine for both cough and analgesia in pediatric patients” as their key reasons for considering the changes to current contraindications, according to Dr. Sally Seymour, the FDA’s Deputy Director for Safety.
In a presentation on codeine use for pediatric analgesia, FDA Medical Officer Dr. Timothy Jiang cited several studies detailing adverse events in children taking codeine-containing drugs. One 2007 study by Voronov et al involved a 29-month-old boy of North African heritage who took codeine/acetaminophen following adenotonsillectomy for “recurrent tonsillitis and mild-moderate sleep apnea;” the boy was found unresponsive the day after the operation, but was later resuscitated.
In another study – a 2009 study in the New England Journal of Medicine by Catherine Ciszkowski and her associates – cites a similar situation of a 2-year-old boy receiving codeine after adenotonsillectomy, only to be found unresponsive; in this case, however, the boy died 2 days after the operation. Additionally, Dr. Jiang cited a 2012 search of the Adverse Event Reporting System, looking at data from 1969 through May 1, 2012, which found that six additional cases of death, as well as seven literature cases that mentioned patients’ CYPD26 status as possibly contributing.
The FDA is not required to follow the advice of its advisory panels, but often does. No members of the panel reported any relevant financial conflicts of interest.
SILVER SPRING, MD. – A joint meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee (PADAC) and the Drug Safety and Risk Management Advisory Committee (DSaRM) on Dec. 10 voted overwhelmingly to support expanding the current contraindication for codeine to preclude its use for any pain management in all children under age 18 years.
Twenty members of the joint advisory panel voted for the aforementioned contraindication, while six members elected to contraindicate for any pain management in children younger than 12 years old, and another two members voted only to contraindicate for children younger than age 6 years. Only one member – Dr. Maureen Finnegan of the DSaRM – voted not to make any changes to the current contraindications for codeine.
The joint advisory panel also voted to contraindicate the use of codeine for the treatment of cough in all children younger than age 18 years by a similarly robust margin: 20 members voted for contraindicating in all pediatric patients, five voted to contraindicate only in patients younger than age 12 years, one voted to contraindicate in children younger than age 6 years, and three members voted not to make any changes at all.
The final voting question, asking whether to remove codeine from the FDA monograph for over-the-counter use in treating cough in children, was almost unanimously supported by the voting members of both committees. Only one member – Dr. Lorraine J. Gudas, a temporary voting member – supported removing codeine from the monograph only for children under age 2 years. Dr. Finnegan abstained from voting on this charge, telling the committee that “this is totally out of my wheelhouse.”
The decision to vote on approving amendments to the contraindications for codeine use – which would affect not just the monogram, but labeling as well – comes on the heels of the FDA announcing this summer that they would be investigating the safety of codeine-containing drugs in children, asking health care providers and patients to report any adverse events associated with the drug.
The joint advisory panel cited reports of respiratory depression and death in pediatric patients, variability of codeine metabolism based upon CYP2D6 activity, and the fact that “some regulatory agencies have restricted use of codeine for both cough and analgesia in pediatric patients” as their key reasons for considering the changes to current contraindications, according to Dr. Sally Seymour, the FDA’s Deputy Director for Safety.
In a presentation on codeine use for pediatric analgesia, FDA Medical Officer Dr. Timothy Jiang cited several studies detailing adverse events in children taking codeine-containing drugs. One 2007 study by Voronov et al involved a 29-month-old boy of North African heritage who took codeine/acetaminophen following adenotonsillectomy for “recurrent tonsillitis and mild-moderate sleep apnea;” the boy was found unresponsive the day after the operation, but was later resuscitated.
In another study – a 2009 study in the New England Journal of Medicine by Catherine Ciszkowski and her associates – cites a similar situation of a 2-year-old boy receiving codeine after adenotonsillectomy, only to be found unresponsive; in this case, however, the boy died 2 days after the operation. Additionally, Dr. Jiang cited a 2012 search of the Adverse Event Reporting System, looking at data from 1969 through May 1, 2012, which found that six additional cases of death, as well as seven literature cases that mentioned patients’ CYPD26 status as possibly contributing.
The FDA is not required to follow the advice of its advisory panels, but often does. No members of the panel reported any relevant financial conflicts of interest.
AT AN FDA ADVISORY COMMITTEE MEETING
Postop C. diff infection associated with presurgical antibiotics
A hospital’s rate of postoperative Clostridium difficile infection is related to the number of preoperative antibiotics patients have taken, the complexity of their procedures, and the complexity of the hospital’s surgical program, in addition to known risk factors for the infection, according to a report published online in JAMA Surgery.
Several risk factors for postoperative C. difficile infection have already been identified, including advanced age and comorbidity. To examine known risk factors and identify possible new ones, researchers analyzed information from the Veterans Affairs Surgical Quality Improvement Program’s database, which documents all noncardiac operations at 134 VA medical centers each year.
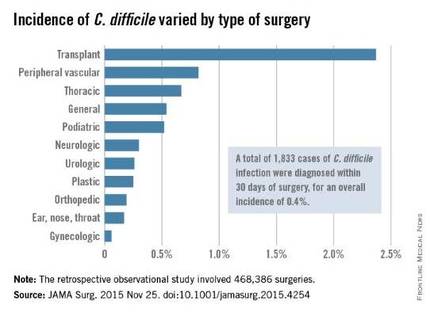
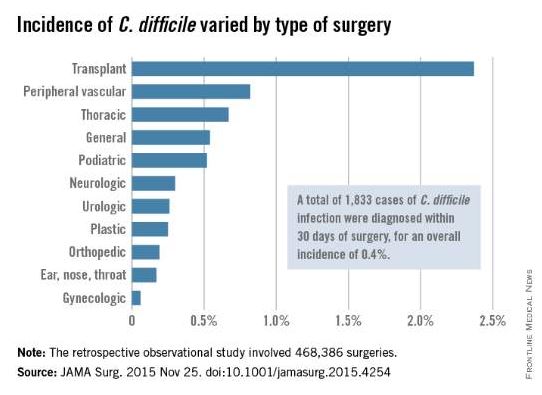
The investigators focused on 468,386 procedures performed during a 4-year period. A total of 1,833 cases of C. difficile infection were diagnosed within 30 days of surgery, for an overall incidence of 0.4% in this predominantly male, elderly population, said Xinli Li, Ph.D., of the Veterans Health Administration, Washington, and associates.
As expected, patients who developed postoperative C. difficile infection were significantly older than those who didn’t (mean age, 67.4 vs. 60.6 years) and were significantly more likely to have comorbidities such as impaired functional status, heart failure, chronic obstructive pulmonary disease, ascites, renal failure, bleeding disorders, wound infection, and recent weight loss.
Unexpectedly, the number of different antibiotics taken during the 60 days preceding surgery also was significantly associated with C. difficile infection. Patients who had taken three or more antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics, the investigators reported (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4263).In addition, patients who underwent more complex surgical procedures were at increased risk of this complication, as were patients at hospitals that frequently handled complex procedures. “These factors reflect the illness of patients, duration of operation, and hospital setting; each is an established risk factor for C. difficile infection,” Dr. Li and associates wrote.
Patients with C. difficile infection had higher rates of postoperative other morbidity (86.0% vs. 7.1%) and 30-day mortality (5.3% vs. 1.0%) and longer postoperative hospital stays (17.9 days vs. 3.6 days).
Contrary to previous studies, this study did not show a temporal increase in C. difficile infection. The overall incidence, as well as the incidences at individual hospitals, remained constant during the entire 4-year study period, the investigators added.
The incidence of C. difficile varied substantially among the 134 VA medical centers, from 0% to 1.35% of all surgical patients. “Surgical administrators and clinical teams may consider the results of this study to target interventions for specific patients undergoing high-risk procedures. Such interventions include selective antibiotic administration, early testing of at-risk patients, hand hygiene with nonalcohol agents, early contact precautions, and specific environmental cleaning protocols,” Dr. Li and associates wrote.
This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
The most important finding to highlight in the report by Li et al. is the 12-fold increase in morbidity and 5-fold increase in mortality among patients who developed postoperative C. difficile infection.
The study results underscore the importance of infection control and prevention efforts. They also show how important it is to develop prophylactic strategies, expeditious recognition of C. difficile, adequate supportive care, and improved therapies.
Dr. Paul K. Waltz and Dr. Brian S. Zuckerbraun are at the VA Pittsburgh Healthcare System and the University of Pennsylvania, Pittsburgh. They made these remarks in an invited commentary accompanying Dr. Li’s report (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4254).
The most important finding to highlight in the report by Li et al. is the 12-fold increase in morbidity and 5-fold increase in mortality among patients who developed postoperative C. difficile infection.
The study results underscore the importance of infection control and prevention efforts. They also show how important it is to develop prophylactic strategies, expeditious recognition of C. difficile, adequate supportive care, and improved therapies.
Dr. Paul K. Waltz and Dr. Brian S. Zuckerbraun are at the VA Pittsburgh Healthcare System and the University of Pennsylvania, Pittsburgh. They made these remarks in an invited commentary accompanying Dr. Li’s report (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4254).
The most important finding to highlight in the report by Li et al. is the 12-fold increase in morbidity and 5-fold increase in mortality among patients who developed postoperative C. difficile infection.
The study results underscore the importance of infection control and prevention efforts. They also show how important it is to develop prophylactic strategies, expeditious recognition of C. difficile, adequate supportive care, and improved therapies.
Dr. Paul K. Waltz and Dr. Brian S. Zuckerbraun are at the VA Pittsburgh Healthcare System and the University of Pennsylvania, Pittsburgh. They made these remarks in an invited commentary accompanying Dr. Li’s report (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4254).
A hospital’s rate of postoperative Clostridium difficile infection is related to the number of preoperative antibiotics patients have taken, the complexity of their procedures, and the complexity of the hospital’s surgical program, in addition to known risk factors for the infection, according to a report published online in JAMA Surgery.
Several risk factors for postoperative C. difficile infection have already been identified, including advanced age and comorbidity. To examine known risk factors and identify possible new ones, researchers analyzed information from the Veterans Affairs Surgical Quality Improvement Program’s database, which documents all noncardiac operations at 134 VA medical centers each year.

The investigators focused on 468,386 procedures performed during a 4-year period. A total of 1,833 cases of C. difficile infection were diagnosed within 30 days of surgery, for an overall incidence of 0.4% in this predominantly male, elderly population, said Xinli Li, Ph.D., of the Veterans Health Administration, Washington, and associates.
As expected, patients who developed postoperative C. difficile infection were significantly older than those who didn’t (mean age, 67.4 vs. 60.6 years) and were significantly more likely to have comorbidities such as impaired functional status, heart failure, chronic obstructive pulmonary disease, ascites, renal failure, bleeding disorders, wound infection, and recent weight loss.
Unexpectedly, the number of different antibiotics taken during the 60 days preceding surgery also was significantly associated with C. difficile infection. Patients who had taken three or more antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics, the investigators reported (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4263).In addition, patients who underwent more complex surgical procedures were at increased risk of this complication, as were patients at hospitals that frequently handled complex procedures. “These factors reflect the illness of patients, duration of operation, and hospital setting; each is an established risk factor for C. difficile infection,” Dr. Li and associates wrote.
Patients with C. difficile infection had higher rates of postoperative other morbidity (86.0% vs. 7.1%) and 30-day mortality (5.3% vs. 1.0%) and longer postoperative hospital stays (17.9 days vs. 3.6 days).
Contrary to previous studies, this study did not show a temporal increase in C. difficile infection. The overall incidence, as well as the incidences at individual hospitals, remained constant during the entire 4-year study period, the investigators added.
The incidence of C. difficile varied substantially among the 134 VA medical centers, from 0% to 1.35% of all surgical patients. “Surgical administrators and clinical teams may consider the results of this study to target interventions for specific patients undergoing high-risk procedures. Such interventions include selective antibiotic administration, early testing of at-risk patients, hand hygiene with nonalcohol agents, early contact precautions, and specific environmental cleaning protocols,” Dr. Li and associates wrote.
This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
A hospital’s rate of postoperative Clostridium difficile infection is related to the number of preoperative antibiotics patients have taken, the complexity of their procedures, and the complexity of the hospital’s surgical program, in addition to known risk factors for the infection, according to a report published online in JAMA Surgery.
Several risk factors for postoperative C. difficile infection have already been identified, including advanced age and comorbidity. To examine known risk factors and identify possible new ones, researchers analyzed information from the Veterans Affairs Surgical Quality Improvement Program’s database, which documents all noncardiac operations at 134 VA medical centers each year.

The investigators focused on 468,386 procedures performed during a 4-year period. A total of 1,833 cases of C. difficile infection were diagnosed within 30 days of surgery, for an overall incidence of 0.4% in this predominantly male, elderly population, said Xinli Li, Ph.D., of the Veterans Health Administration, Washington, and associates.
As expected, patients who developed postoperative C. difficile infection were significantly older than those who didn’t (mean age, 67.4 vs. 60.6 years) and were significantly more likely to have comorbidities such as impaired functional status, heart failure, chronic obstructive pulmonary disease, ascites, renal failure, bleeding disorders, wound infection, and recent weight loss.
Unexpectedly, the number of different antibiotics taken during the 60 days preceding surgery also was significantly associated with C. difficile infection. Patients who had taken three or more antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics, the investigators reported (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4263).In addition, patients who underwent more complex surgical procedures were at increased risk of this complication, as were patients at hospitals that frequently handled complex procedures. “These factors reflect the illness of patients, duration of operation, and hospital setting; each is an established risk factor for C. difficile infection,” Dr. Li and associates wrote.
Patients with C. difficile infection had higher rates of postoperative other morbidity (86.0% vs. 7.1%) and 30-day mortality (5.3% vs. 1.0%) and longer postoperative hospital stays (17.9 days vs. 3.6 days).
Contrary to previous studies, this study did not show a temporal increase in C. difficile infection. The overall incidence, as well as the incidences at individual hospitals, remained constant during the entire 4-year study period, the investigators added.
The incidence of C. difficile varied substantially among the 134 VA medical centers, from 0% to 1.35% of all surgical patients. “Surgical administrators and clinical teams may consider the results of this study to target interventions for specific patients undergoing high-risk procedures. Such interventions include selective antibiotic administration, early testing of at-risk patients, hand hygiene with nonalcohol agents, early contact precautions, and specific environmental cleaning protocols,” Dr. Li and associates wrote.
This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point: A hospital’s rate of postoperative C. difficile infection is related to the number of preoperative antibiotics patients took and the complexity of their surgeries.
Major finding: Patients who had taken three or more preoperative antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics.
Data source: A retrospective observational study involving 468,386 surgeries at 134 VA medical centers during a 4-year period.
Disclosures: This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
Roux-en-Y improves NASH in obese patients
SAN FRANCISCO – Bariatric surgery is an effective treatment for nonalcoholic steatohepatitis (NASH) in obese patients, a Markov model suggests.
Patients with all classes of obesity, including, mild, moderate, and severe, with all stages of fibrosis, experienced gains in life years following laparoscopic Roux-en-Y gastric bypass, compared with standard management and intensive lifestyle changes, based on the model, Dr. Kathleen Corey reported at the annual meeting of the American Association for the Study of Liver Diseases.
Surgery also increased quality-adjusted life years (QALY) in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis, said Dr Corey of Massachusetts General Hospital, Boston.
The number of F3 patients needed to treat to prevent one liver-related death was favorable across all body mass indexes for those with stage F3 fibrosis. The number needed to treat to prevent one case of cirrhosis in these patients was six to seven, and to prevent one liver-related death was eight to 11, she said.
A cost-effectiveness analysis, with a willingness-to-pay threshold of less than $100,000, also showed that surgery was cost effective for all fibrosis stages for those with severe and moderate obesity.
“The [incremental cost-effectiveness ratio] for BMI of 35-39.9 [kg/m2] was $34,000, for those with a BMI of 40 or greater it was $26,000, and we found that with overweight patients with F3 fibrosis, the ratio was also favorable at $59,000,” she said.
More than 78 million American adults suffer from obesity, and the prevalence of obesity is rising nationwide, she said, adding that obesity results in annual medical costs exceeding $147 billion. Treatment options for obesity are limited, and weight regain after weight loss is frequent, Dr. Corey noted.
“However, bariatric surgery has been shown to be a very effective treatment for obesity. Surgery has been shown to significantly reduce mortality in patients, and reduces progression of many comorbidities, including diabetes,” she said.
Guidelines suggest that bariatric surgery is appropriate for patients with a body mass index of 40 or greater and for those with a BMI of 35 or greater if obesity-related comorbidities such as diabetes or obstructive sleep apnea are present, and data suggest that surgery is also of benefit in those with BMI below 35 with diabetes.
The question remains, though, whether nonalcoholic fatty liver disease (NAFLD) – the most common cause of liver disease in the United States – is an indication in itself for bariatric surgery.
NAFLD is strongly associated with obesity, and its progressive form – NASH – can lead to cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.
“However, there are no FDA-approved therapies for NASH and no randomized controlled trials have been conducted to evaluate the role of bariatric surgery in NASH ... and appropriate BMI cutoffs in those with NASH have not been established,” Dr. Corey said.
Several nonrandomized prospective trials have, however, evaluated bariatric surgery in NASH patients with BMIs of 35 or greater with comorbidities, and in patients with BMIs of 40 or greater, and these have shown that NASH resolution can occur in up to 85% of patients at 1-5 years with fibrosis reduction seen in nearly a third, she said, adding that the risk-benefit ratio for surgery is unknown, as is the value of surgery in NASH patients with BMIs less than 35.
The current study looked at three treatment strategies, including standard management with lifestyle counseling; intensive lifestyle intervention based on a diabetes prevention program with lifestyle, nutrition, and exercise counseling; and bariatric Roux-en-Y gastric bypass in a hypothetical simulated cohort of 45-year-olds with NASH, F0-F3 fibrosis, and varying BMI values.
The findings, though limited by a lack of data on the impact of surgery on patients with a BMI of less than 35, and by evaluation of only one type of surgery, showed that bariatric surgery increased incremental life years at all weight and fibrosis stages vs. standard management and intensive lifestyle intervention, and that surgery increased QALY in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis.
“We found that surgery was cost effective for F3 fibrosis in those with moderate and severe obesity and those with overweight. Randomized trials, though, are needed to assess the management of weight loss surgery for NASH before this can be recommended, certainly in patients with a BMI of less than 35,” she concluded.
Dr. Corey reported serving on an advisory committee or review panel for Gilead, and serving in a speaking or teaching role for Synageva.
SAN FRANCISCO – Bariatric surgery is an effective treatment for nonalcoholic steatohepatitis (NASH) in obese patients, a Markov model suggests.
Patients with all classes of obesity, including, mild, moderate, and severe, with all stages of fibrosis, experienced gains in life years following laparoscopic Roux-en-Y gastric bypass, compared with standard management and intensive lifestyle changes, based on the model, Dr. Kathleen Corey reported at the annual meeting of the American Association for the Study of Liver Diseases.
Surgery also increased quality-adjusted life years (QALY) in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis, said Dr Corey of Massachusetts General Hospital, Boston.
The number of F3 patients needed to treat to prevent one liver-related death was favorable across all body mass indexes for those with stage F3 fibrosis. The number needed to treat to prevent one case of cirrhosis in these patients was six to seven, and to prevent one liver-related death was eight to 11, she said.
A cost-effectiveness analysis, with a willingness-to-pay threshold of less than $100,000, also showed that surgery was cost effective for all fibrosis stages for those with severe and moderate obesity.
“The [incremental cost-effectiveness ratio] for BMI of 35-39.9 [kg/m2] was $34,000, for those with a BMI of 40 or greater it was $26,000, and we found that with overweight patients with F3 fibrosis, the ratio was also favorable at $59,000,” she said.
More than 78 million American adults suffer from obesity, and the prevalence of obesity is rising nationwide, she said, adding that obesity results in annual medical costs exceeding $147 billion. Treatment options for obesity are limited, and weight regain after weight loss is frequent, Dr. Corey noted.
“However, bariatric surgery has been shown to be a very effective treatment for obesity. Surgery has been shown to significantly reduce mortality in patients, and reduces progression of many comorbidities, including diabetes,” she said.
Guidelines suggest that bariatric surgery is appropriate for patients with a body mass index of 40 or greater and for those with a BMI of 35 or greater if obesity-related comorbidities such as diabetes or obstructive sleep apnea are present, and data suggest that surgery is also of benefit in those with BMI below 35 with diabetes.
The question remains, though, whether nonalcoholic fatty liver disease (NAFLD) – the most common cause of liver disease in the United States – is an indication in itself for bariatric surgery.
NAFLD is strongly associated with obesity, and its progressive form – NASH – can lead to cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.
“However, there are no FDA-approved therapies for NASH and no randomized controlled trials have been conducted to evaluate the role of bariatric surgery in NASH ... and appropriate BMI cutoffs in those with NASH have not been established,” Dr. Corey said.
Several nonrandomized prospective trials have, however, evaluated bariatric surgery in NASH patients with BMIs of 35 or greater with comorbidities, and in patients with BMIs of 40 or greater, and these have shown that NASH resolution can occur in up to 85% of patients at 1-5 years with fibrosis reduction seen in nearly a third, she said, adding that the risk-benefit ratio for surgery is unknown, as is the value of surgery in NASH patients with BMIs less than 35.
The current study looked at three treatment strategies, including standard management with lifestyle counseling; intensive lifestyle intervention based on a diabetes prevention program with lifestyle, nutrition, and exercise counseling; and bariatric Roux-en-Y gastric bypass in a hypothetical simulated cohort of 45-year-olds with NASH, F0-F3 fibrosis, and varying BMI values.
The findings, though limited by a lack of data on the impact of surgery on patients with a BMI of less than 35, and by evaluation of only one type of surgery, showed that bariatric surgery increased incremental life years at all weight and fibrosis stages vs. standard management and intensive lifestyle intervention, and that surgery increased QALY in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis.
“We found that surgery was cost effective for F3 fibrosis in those with moderate and severe obesity and those with overweight. Randomized trials, though, are needed to assess the management of weight loss surgery for NASH before this can be recommended, certainly in patients with a BMI of less than 35,” she concluded.
Dr. Corey reported serving on an advisory committee or review panel for Gilead, and serving in a speaking or teaching role for Synageva.
SAN FRANCISCO – Bariatric surgery is an effective treatment for nonalcoholic steatohepatitis (NASH) in obese patients, a Markov model suggests.
Patients with all classes of obesity, including, mild, moderate, and severe, with all stages of fibrosis, experienced gains in life years following laparoscopic Roux-en-Y gastric bypass, compared with standard management and intensive lifestyle changes, based on the model, Dr. Kathleen Corey reported at the annual meeting of the American Association for the Study of Liver Diseases.
Surgery also increased quality-adjusted life years (QALY) in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis, said Dr Corey of Massachusetts General Hospital, Boston.
The number of F3 patients needed to treat to prevent one liver-related death was favorable across all body mass indexes for those with stage F3 fibrosis. The number needed to treat to prevent one case of cirrhosis in these patients was six to seven, and to prevent one liver-related death was eight to 11, she said.
A cost-effectiveness analysis, with a willingness-to-pay threshold of less than $100,000, also showed that surgery was cost effective for all fibrosis stages for those with severe and moderate obesity.
“The [incremental cost-effectiveness ratio] for BMI of 35-39.9 [kg/m2] was $34,000, for those with a BMI of 40 or greater it was $26,000, and we found that with overweight patients with F3 fibrosis, the ratio was also favorable at $59,000,” she said.
More than 78 million American adults suffer from obesity, and the prevalence of obesity is rising nationwide, she said, adding that obesity results in annual medical costs exceeding $147 billion. Treatment options for obesity are limited, and weight regain after weight loss is frequent, Dr. Corey noted.
“However, bariatric surgery has been shown to be a very effective treatment for obesity. Surgery has been shown to significantly reduce mortality in patients, and reduces progression of many comorbidities, including diabetes,” she said.
Guidelines suggest that bariatric surgery is appropriate for patients with a body mass index of 40 or greater and for those with a BMI of 35 or greater if obesity-related comorbidities such as diabetes or obstructive sleep apnea are present, and data suggest that surgery is also of benefit in those with BMI below 35 with diabetes.
The question remains, though, whether nonalcoholic fatty liver disease (NAFLD) – the most common cause of liver disease in the United States – is an indication in itself for bariatric surgery.
NAFLD is strongly associated with obesity, and its progressive form – NASH – can lead to cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.
“However, there are no FDA-approved therapies for NASH and no randomized controlled trials have been conducted to evaluate the role of bariatric surgery in NASH ... and appropriate BMI cutoffs in those with NASH have not been established,” Dr. Corey said.
Several nonrandomized prospective trials have, however, evaluated bariatric surgery in NASH patients with BMIs of 35 or greater with comorbidities, and in patients with BMIs of 40 or greater, and these have shown that NASH resolution can occur in up to 85% of patients at 1-5 years with fibrosis reduction seen in nearly a third, she said, adding that the risk-benefit ratio for surgery is unknown, as is the value of surgery in NASH patients with BMIs less than 35.
The current study looked at three treatment strategies, including standard management with lifestyle counseling; intensive lifestyle intervention based on a diabetes prevention program with lifestyle, nutrition, and exercise counseling; and bariatric Roux-en-Y gastric bypass in a hypothetical simulated cohort of 45-year-olds with NASH, F0-F3 fibrosis, and varying BMI values.
The findings, though limited by a lack of data on the impact of surgery on patients with a BMI of less than 35, and by evaluation of only one type of surgery, showed that bariatric surgery increased incremental life years at all weight and fibrosis stages vs. standard management and intensive lifestyle intervention, and that surgery increased QALY in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis.
“We found that surgery was cost effective for F3 fibrosis in those with moderate and severe obesity and those with overweight. Randomized trials, though, are needed to assess the management of weight loss surgery for NASH before this can be recommended, certainly in patients with a BMI of less than 35,” she concluded.
Dr. Corey reported serving on an advisory committee or review panel for Gilead, and serving in a speaking or teaching role for Synageva.
AT THE LIVER MEETING 2015
Key clinical point: Bariatric surgery is an effective treatment for nonalcoholic steatohepatitis (NASH) in obese patients, a Markov model suggests.
Major finding: The number needed to treat to prevent 1 case of cirrhosis was 6-7; to prevent 1 liver-related death, it was 8-11.
Data source: A Markov modeling study involving a hypothetical simulated cohort of patients.
Disclosures: Dr. Corey reported serving on an advisory committee or review panel for Gilead, and serving in a speaking or teaching role for Synageva.
Morcellation warning drives down minimally invasive gyn surgeries
Minimally invasive myomectomies dropped nearly 20% following the Food and Drug Administration’s warning to avoid power morcellation in the treatment of uterine fibroids, and minimally invasive hysterectomies fell nearly 6%, according to a time-series analysis of a Florida health system.
But the decline in the use of these techniques varied by subspecialty, with ob.gyns. and minimally invasive gynecologic specialists being more likely to shy away from minimally invasive hysterectomy, while reproductive endocrinologists and minimally invasive gynecologists were the subspecialists most likely not to perform minimally invasive myomectomy after the FDA warning, according to the researchers (Obstet Gynecol. 2015;126:1174-80).
On April 17, 2014, the FDA issued a warning against the use of power morcellators in the surgical treatment of uterine leiomyomas because of the risk of iatrogenic dissemination of malignant tissue in the event of an unsuspected sarcoma. In the aftermath of that warning, many hospitals have banned the use of power morcellators for myoma surgery.
Dr. Kenneth I. Barron of Florida Hospital Medical Group, Orlando, and his colleagues performed a time-series, retrospective analysis of 3,573 patients who underwent a hysterectomy or myomectomy between Aug. 1, 2013, and Dec. 31, 2014, to gauge the impact of the warning on gynecologic practice. They compared surgical interventions performed during the 8 months before the FDA announcement with surgical interventions performed during the 8 months after the announcement, quantifying the proportion of minimally invasive vaginal, laparoscopic, or robotic-assisted surgery cases for each study period.
In the pre-FDA warning arm, 1,694 hysterectomies and 102 myomectomies met inclusion criteria; in the post-FDA warning cohort, 1,690 hysterectomies and 87 myomectomies met inclusion criteria. Subjects were drawn from the surgical case logs of the operating room documentation system of the Florida Hospital System, where the use of power morcellation was suspended immediately following the FDA warning. The analysis included six hospitals and 98 surgeons.
During the study period, minimally invasive myomectomies decreased by 19%. The percentage of myomectomies performed with minimally invasive techniques fell from 62.7% before the FDA warning to 43.7% after (P = .009).
Minimally invasive hysterectomies decreased by 5.8%, dropping from 85.7% to 79.9% (P less than .001) following the FDA warning statement. The drop in minimally invasive hysterectomies were due largely to a 60% drop in laparoscopic supracervical hysterectomies, according to the study.
The findings were subspecialty specific. For instance, the researchers observed that there was an 8.7% decrease in minimally invasive hysterectomies when gynecologic oncology cases were excluded from the sample (P less than .001). Ob.gyns. and minimally invasive gynecologic specialists had the greatest decreases in minimally invasive hysterectomies. Significant decreases were not observed with urogynecologists or oncologists.
In terms of minimally invasive myomectomies, there was a drop among reproductive endocrinologists (P = .006) and minimally invasive gynecologic specialists (P = .01), but not among ob.gyns.
“Caution should be taken in interpreting this data to mean there has been an irreversible backslide toward open surgery,” the researchers wrote. “Our results reflect the immediate change in surgical practice after a policy change, and this might be neutralized once newer techniques for removing myomas and myomatous uteri by minimally invasive measures are adopted more broadly.”
Minimally invasive myomectomies dropped nearly 20% following the Food and Drug Administration’s warning to avoid power morcellation in the treatment of uterine fibroids, and minimally invasive hysterectomies fell nearly 6%, according to a time-series analysis of a Florida health system.
But the decline in the use of these techniques varied by subspecialty, with ob.gyns. and minimally invasive gynecologic specialists being more likely to shy away from minimally invasive hysterectomy, while reproductive endocrinologists and minimally invasive gynecologists were the subspecialists most likely not to perform minimally invasive myomectomy after the FDA warning, according to the researchers (Obstet Gynecol. 2015;126:1174-80).
On April 17, 2014, the FDA issued a warning against the use of power morcellators in the surgical treatment of uterine leiomyomas because of the risk of iatrogenic dissemination of malignant tissue in the event of an unsuspected sarcoma. In the aftermath of that warning, many hospitals have banned the use of power morcellators for myoma surgery.
Dr. Kenneth I. Barron of Florida Hospital Medical Group, Orlando, and his colleagues performed a time-series, retrospective analysis of 3,573 patients who underwent a hysterectomy or myomectomy between Aug. 1, 2013, and Dec. 31, 2014, to gauge the impact of the warning on gynecologic practice. They compared surgical interventions performed during the 8 months before the FDA announcement with surgical interventions performed during the 8 months after the announcement, quantifying the proportion of minimally invasive vaginal, laparoscopic, or robotic-assisted surgery cases for each study period.
In the pre-FDA warning arm, 1,694 hysterectomies and 102 myomectomies met inclusion criteria; in the post-FDA warning cohort, 1,690 hysterectomies and 87 myomectomies met inclusion criteria. Subjects were drawn from the surgical case logs of the operating room documentation system of the Florida Hospital System, where the use of power morcellation was suspended immediately following the FDA warning. The analysis included six hospitals and 98 surgeons.
During the study period, minimally invasive myomectomies decreased by 19%. The percentage of myomectomies performed with minimally invasive techniques fell from 62.7% before the FDA warning to 43.7% after (P = .009).
Minimally invasive hysterectomies decreased by 5.8%, dropping from 85.7% to 79.9% (P less than .001) following the FDA warning statement. The drop in minimally invasive hysterectomies were due largely to a 60% drop in laparoscopic supracervical hysterectomies, according to the study.
The findings were subspecialty specific. For instance, the researchers observed that there was an 8.7% decrease in minimally invasive hysterectomies when gynecologic oncology cases were excluded from the sample (P less than .001). Ob.gyns. and minimally invasive gynecologic specialists had the greatest decreases in minimally invasive hysterectomies. Significant decreases were not observed with urogynecologists or oncologists.
In terms of minimally invasive myomectomies, there was a drop among reproductive endocrinologists (P = .006) and minimally invasive gynecologic specialists (P = .01), but not among ob.gyns.
“Caution should be taken in interpreting this data to mean there has been an irreversible backslide toward open surgery,” the researchers wrote. “Our results reflect the immediate change in surgical practice after a policy change, and this might be neutralized once newer techniques for removing myomas and myomatous uteri by minimally invasive measures are adopted more broadly.”
Minimally invasive myomectomies dropped nearly 20% following the Food and Drug Administration’s warning to avoid power morcellation in the treatment of uterine fibroids, and minimally invasive hysterectomies fell nearly 6%, according to a time-series analysis of a Florida health system.
But the decline in the use of these techniques varied by subspecialty, with ob.gyns. and minimally invasive gynecologic specialists being more likely to shy away from minimally invasive hysterectomy, while reproductive endocrinologists and minimally invasive gynecologists were the subspecialists most likely not to perform minimally invasive myomectomy after the FDA warning, according to the researchers (Obstet Gynecol. 2015;126:1174-80).
On April 17, 2014, the FDA issued a warning against the use of power morcellators in the surgical treatment of uterine leiomyomas because of the risk of iatrogenic dissemination of malignant tissue in the event of an unsuspected sarcoma. In the aftermath of that warning, many hospitals have banned the use of power morcellators for myoma surgery.
Dr. Kenneth I. Barron of Florida Hospital Medical Group, Orlando, and his colleagues performed a time-series, retrospective analysis of 3,573 patients who underwent a hysterectomy or myomectomy between Aug. 1, 2013, and Dec. 31, 2014, to gauge the impact of the warning on gynecologic practice. They compared surgical interventions performed during the 8 months before the FDA announcement with surgical interventions performed during the 8 months after the announcement, quantifying the proportion of minimally invasive vaginal, laparoscopic, or robotic-assisted surgery cases for each study period.
In the pre-FDA warning arm, 1,694 hysterectomies and 102 myomectomies met inclusion criteria; in the post-FDA warning cohort, 1,690 hysterectomies and 87 myomectomies met inclusion criteria. Subjects were drawn from the surgical case logs of the operating room documentation system of the Florida Hospital System, where the use of power morcellation was suspended immediately following the FDA warning. The analysis included six hospitals and 98 surgeons.
During the study period, minimally invasive myomectomies decreased by 19%. The percentage of myomectomies performed with minimally invasive techniques fell from 62.7% before the FDA warning to 43.7% after (P = .009).
Minimally invasive hysterectomies decreased by 5.8%, dropping from 85.7% to 79.9% (P less than .001) following the FDA warning statement. The drop in minimally invasive hysterectomies were due largely to a 60% drop in laparoscopic supracervical hysterectomies, according to the study.
The findings were subspecialty specific. For instance, the researchers observed that there was an 8.7% decrease in minimally invasive hysterectomies when gynecologic oncology cases were excluded from the sample (P less than .001). Ob.gyns. and minimally invasive gynecologic specialists had the greatest decreases in minimally invasive hysterectomies. Significant decreases were not observed with urogynecologists or oncologists.
In terms of minimally invasive myomectomies, there was a drop among reproductive endocrinologists (P = .006) and minimally invasive gynecologic specialists (P = .01), but not among ob.gyns.
“Caution should be taken in interpreting this data to mean there has been an irreversible backslide toward open surgery,” the researchers wrote. “Our results reflect the immediate change in surgical practice after a policy change, and this might be neutralized once newer techniques for removing myomas and myomatous uteri by minimally invasive measures are adopted more broadly.”
FROM OBSTETRICS AND GYNECOLOGY
Key clinical point: A 2014 FDA warning about morcellation of uterine leiomyomas has significantly affected gynecologic surgery practice patterns.
Major finding: There was a 19% decrease in the percentage of minimally invasive myomectomies performed in the 8 months after the FDA’s morcellation warning compared with the prior 8 months. Minimally invasive hysterectomies decreased by 5.8%.
Data source: A time-series, retrospective analysis of 3,573 patients who underwent a hysterectomy or myomectomy between Aug. 1, 2013, and Dec. 31, 2014.
Disclosures: The researchers reported having no financial disclosures.