User login
Risk Stratification May Work Well for FIT-Based CRC Screening in Elderly
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
In particular, interval CRC risk can vary substantially based on the fecal hemoglobin (f-Hb) concentration in the patient’s last fecal immunochemical test (FIT), as well as the number of prior screening rounds.
“Less is known about what happens after the upper age limit has been reached and individuals are not invited to participate in more screening rounds. This is important as life expectancy is increasing, and it is increasingly important to consider the most efficient way of screening the elderly,” said lead author Brenda van Stigt, a PhD candidate focused on cancer screening at Erasmus University Medical Center in Rotterdam, the Netherlands.
In the Netherlands, adults between ages 55 and 75 are invited to participate in stool-based CRC screening every 2 years. Based on a fecal immunochemical testing (FIT) threshold of 47 μg Hb/g, those who test positive are referred to colonoscopy, and those who test negative are invited to participate again after a 2-year period.
FIT can play a major role in risk stratification, Ms. van Stigt noted, along with other factors that influence CRC risk, such as age, sex, and CRC screening history. Although this is documented for ages 55-75, she and colleagues wanted to know more about what happens after age 75.
Ms. Van Stigt and colleagues conducted a population-based study by analyzing Dutch national cancer registry data and FIT results around the final screening at age 75, looking at those who were diagnosed with CRC within 24 months of their last negative FIT. The researchers assessed interval CRC risk and cancer stage, accounting for sex, last f-Hb concentration, and the number of screening rounds.
Among 305,761 people with a complete 24-month follow-up after a negative FIT, 661 patients were diagnosed with interval CRC, indicating an overall interval CRC risk of 21.6 per 10,000 individuals with a negative FIT. There were no significant differences by sex.
However, there were differences by screening rounds, with those who had participated in three or four screening rounds having a lower risk than those who participated only once (HR, .49).
In addition, those with detectable f-Hb (>0 μg Hb/g) in their last screening round had a much higher interval CRC risk (HR, 4.87), at 65.8 per 10,000 negative FITs, compared with 13.8 per 10,000 among those without detectable f-Hb. Interval CRC risk also increased over time for those with detectable f-Hb.
About 15% of the total population had detectable f-Hb, whereas 46% of those with interval CRC had detectable f-Hb, Ms. van Stigt said, meaning that nearly half of patients who were diagnosed with interval CRC already had detectable f-Hb in their prior FIT.
In a survival analysis, there was no association between interval CRC risk and sex. However, those who participated in three or four screening rounds were half as likely to be diagnosed than those who participated once or twice, and those with detectable f-Hb were five times as likely to be diagnosed.
For late-stage CRC, there was no association with sex or the number of screening rounds. Detectable f-Hb was associated with not only a higher risk of interval CRC but also a late-stage diagnosis.
“These findings indicate that one uniform age to stop screening is suboptimal,” Ms. van Stigt said. “Personalized screening strategies should, therefore, also ideally incorporate a risk-stratified age to stop screening.”
The US Preventive Services Task Force recommends that clinicians personalize screening for ages 76-85, accounting for overall health, prior screening history, and patient preferences.
“But we have no clear guidance on how to quantify or weigh these factors. This interesting study highlights how one of these factors (prior screening history) and fecal hemoglobin level (an emerging factor) are powerful stratifiers of subsequent colorectal cancer risk,” said Sameer D. Saini, MD, AGAF, director and research investigator at the VA Ann Arbor Healthcare System’s Center for Clinical Management Research. Dr. Saini wasn’t involved with the study.
At the clinical level, Dr. Saini said, sophisticated modeling is needed to understand the interaction with competing risks and identify the optimal screening strategies for patients at varying levels of cancer risk and life expectancy. Models could also help to quantify the population benefits and cost-effectiveness of personalized screening.
“Finally, it is important to note that, in many health systems, access to quantitative FIT may be limited,” he said. “These data may be less informative if colonoscopy is the primary mode of screening.”
Ms. van Stigt and Dr. Saini reported no relevant disclosures.
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
In particular, interval CRC risk can vary substantially based on the fecal hemoglobin (f-Hb) concentration in the patient’s last fecal immunochemical test (FIT), as well as the number of prior screening rounds.
“Less is known about what happens after the upper age limit has been reached and individuals are not invited to participate in more screening rounds. This is important as life expectancy is increasing, and it is increasingly important to consider the most efficient way of screening the elderly,” said lead author Brenda van Stigt, a PhD candidate focused on cancer screening at Erasmus University Medical Center in Rotterdam, the Netherlands.
In the Netherlands, adults between ages 55 and 75 are invited to participate in stool-based CRC screening every 2 years. Based on a fecal immunochemical testing (FIT) threshold of 47 μg Hb/g, those who test positive are referred to colonoscopy, and those who test negative are invited to participate again after a 2-year period.
FIT can play a major role in risk stratification, Ms. van Stigt noted, along with other factors that influence CRC risk, such as age, sex, and CRC screening history. Although this is documented for ages 55-75, she and colleagues wanted to know more about what happens after age 75.
Ms. Van Stigt and colleagues conducted a population-based study by analyzing Dutch national cancer registry data and FIT results around the final screening at age 75, looking at those who were diagnosed with CRC within 24 months of their last negative FIT. The researchers assessed interval CRC risk and cancer stage, accounting for sex, last f-Hb concentration, and the number of screening rounds.
Among 305,761 people with a complete 24-month follow-up after a negative FIT, 661 patients were diagnosed with interval CRC, indicating an overall interval CRC risk of 21.6 per 10,000 individuals with a negative FIT. There were no significant differences by sex.
However, there were differences by screening rounds, with those who had participated in three or four screening rounds having a lower risk than those who participated only once (HR, .49).
In addition, those with detectable f-Hb (>0 μg Hb/g) in their last screening round had a much higher interval CRC risk (HR, 4.87), at 65.8 per 10,000 negative FITs, compared with 13.8 per 10,000 among those without detectable f-Hb. Interval CRC risk also increased over time for those with detectable f-Hb.
About 15% of the total population had detectable f-Hb, whereas 46% of those with interval CRC had detectable f-Hb, Ms. van Stigt said, meaning that nearly half of patients who were diagnosed with interval CRC already had detectable f-Hb in their prior FIT.
In a survival analysis, there was no association between interval CRC risk and sex. However, those who participated in three or four screening rounds were half as likely to be diagnosed than those who participated once or twice, and those with detectable f-Hb were five times as likely to be diagnosed.
For late-stage CRC, there was no association with sex or the number of screening rounds. Detectable f-Hb was associated with not only a higher risk of interval CRC but also a late-stage diagnosis.
“These findings indicate that one uniform age to stop screening is suboptimal,” Ms. van Stigt said. “Personalized screening strategies should, therefore, also ideally incorporate a risk-stratified age to stop screening.”
The US Preventive Services Task Force recommends that clinicians personalize screening for ages 76-85, accounting for overall health, prior screening history, and patient preferences.
“But we have no clear guidance on how to quantify or weigh these factors. This interesting study highlights how one of these factors (prior screening history) and fecal hemoglobin level (an emerging factor) are powerful stratifiers of subsequent colorectal cancer risk,” said Sameer D. Saini, MD, AGAF, director and research investigator at the VA Ann Arbor Healthcare System’s Center for Clinical Management Research. Dr. Saini wasn’t involved with the study.
At the clinical level, Dr. Saini said, sophisticated modeling is needed to understand the interaction with competing risks and identify the optimal screening strategies for patients at varying levels of cancer risk and life expectancy. Models could also help to quantify the population benefits and cost-effectiveness of personalized screening.
“Finally, it is important to note that, in many health systems, access to quantitative FIT may be limited,” he said. “These data may be less informative if colonoscopy is the primary mode of screening.”
Ms. van Stigt and Dr. Saini reported no relevant disclosures.
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
In particular, interval CRC risk can vary substantially based on the fecal hemoglobin (f-Hb) concentration in the patient’s last fecal immunochemical test (FIT), as well as the number of prior screening rounds.
“Less is known about what happens after the upper age limit has been reached and individuals are not invited to participate in more screening rounds. This is important as life expectancy is increasing, and it is increasingly important to consider the most efficient way of screening the elderly,” said lead author Brenda van Stigt, a PhD candidate focused on cancer screening at Erasmus University Medical Center in Rotterdam, the Netherlands.
In the Netherlands, adults between ages 55 and 75 are invited to participate in stool-based CRC screening every 2 years. Based on a fecal immunochemical testing (FIT) threshold of 47 μg Hb/g, those who test positive are referred to colonoscopy, and those who test negative are invited to participate again after a 2-year period.
FIT can play a major role in risk stratification, Ms. van Stigt noted, along with other factors that influence CRC risk, such as age, sex, and CRC screening history. Although this is documented for ages 55-75, she and colleagues wanted to know more about what happens after age 75.
Ms. Van Stigt and colleagues conducted a population-based study by analyzing Dutch national cancer registry data and FIT results around the final screening at age 75, looking at those who were diagnosed with CRC within 24 months of their last negative FIT. The researchers assessed interval CRC risk and cancer stage, accounting for sex, last f-Hb concentration, and the number of screening rounds.
Among 305,761 people with a complete 24-month follow-up after a negative FIT, 661 patients were diagnosed with interval CRC, indicating an overall interval CRC risk of 21.6 per 10,000 individuals with a negative FIT. There were no significant differences by sex.
However, there were differences by screening rounds, with those who had participated in three or four screening rounds having a lower risk than those who participated only once (HR, .49).
In addition, those with detectable f-Hb (>0 μg Hb/g) in their last screening round had a much higher interval CRC risk (HR, 4.87), at 65.8 per 10,000 negative FITs, compared with 13.8 per 10,000 among those without detectable f-Hb. Interval CRC risk also increased over time for those with detectable f-Hb.
About 15% of the total population had detectable f-Hb, whereas 46% of those with interval CRC had detectable f-Hb, Ms. van Stigt said, meaning that nearly half of patients who were diagnosed with interval CRC already had detectable f-Hb in their prior FIT.
In a survival analysis, there was no association between interval CRC risk and sex. However, those who participated in three or four screening rounds were half as likely to be diagnosed than those who participated once or twice, and those with detectable f-Hb were five times as likely to be diagnosed.
For late-stage CRC, there was no association with sex or the number of screening rounds. Detectable f-Hb was associated with not only a higher risk of interval CRC but also a late-stage diagnosis.
“These findings indicate that one uniform age to stop screening is suboptimal,” Ms. van Stigt said. “Personalized screening strategies should, therefore, also ideally incorporate a risk-stratified age to stop screening.”
The US Preventive Services Task Force recommends that clinicians personalize screening for ages 76-85, accounting for overall health, prior screening history, and patient preferences.
“But we have no clear guidance on how to quantify or weigh these factors. This interesting study highlights how one of these factors (prior screening history) and fecal hemoglobin level (an emerging factor) are powerful stratifiers of subsequent colorectal cancer risk,” said Sameer D. Saini, MD, AGAF, director and research investigator at the VA Ann Arbor Healthcare System’s Center for Clinical Management Research. Dr. Saini wasn’t involved with the study.
At the clinical level, Dr. Saini said, sophisticated modeling is needed to understand the interaction with competing risks and identify the optimal screening strategies for patients at varying levels of cancer risk and life expectancy. Models could also help to quantify the population benefits and cost-effectiveness of personalized screening.
“Finally, it is important to note that, in many health systems, access to quantitative FIT may be limited,” he said. “These data may be less informative if colonoscopy is the primary mode of screening.”
Ms. van Stigt and Dr. Saini reported no relevant disclosures.
FROM DDW 2024
Statins, Vitamin D, and Exercise in Older Adults
In this article, I will review several recently published articles and guidelines relevant to the care of older adults in primary care. The articles of interest address statins for primary prevention, vitamin D supplementation and testing, and physical activity for healthy aging.
Statins for Primary Prevention of Cardiovascular Disease
A common conundrum in primary care is whether an older adult should be on a statin for primary prevention. This question has been difficult to answer because of the underrepresentation of older adults in clinical trials that examine the effect of statins for primary prevention. A recent study by Xu et al. published in Annals of Internal Medicine sought to address this gap in knowledge, investigating the risks and benefits of statins for primary prevention for older adults.1
This study stratified participants by “old” (aged 75-84 years) and “very old” (85 years or older). In this study, older adults who had an indication for statins were initiated on statins and studied over a 5-year period and compared with age-matched cohorts not initiated on statin therapy. Participants with known cardiovascular disease at baseline were excluded. The outcomes of interest were major cardiovascular disease (CVD) (a composite of myocardial infarction, stroke, or heart failure), all-cause mortality, and adverse effect of drug therapy (myopathy or liver dysfunction).
The study found that among older adults aged 75-84, initiation of statin therapy led to a 1.2% risk reduction in major CVD over a 5-year period. For older adults aged 85 and greater, initiation of statins had an even larger impact, leading to a 4.4% risk reduction in major CVD over a 5-year period. The study found that there was no significant difference in adverse effects including myopathy or liver dysfunction in both age groups.
Statins, the study suggests, are appropriate and safe to initiate for primary prevention in older adults and can lead to substantial benefits in reduction of CVD. While time to benefit was not explicitly examined in this study, a prior study by Yourman et al. suggested that the time to benefit for statins for primary prevention in adults aged 50-75 would be least 2.5 years.2
My takeaway from these findings is to discuss statin initiation for primary prevention for older patients who are focused on longevity, have good functional status (often used in geriatrics as a proxy for prognosis), and are willing to accept more medications.
Empiric Vitamin D Supplementation in Adults over 75 Years
Vitamin D is one of the most common supplements taken by older adults but evidence supporting vitamin D supplementation is variable in published literature, as most data comes from observational trials. New guidelines from the Endocrine Society focused on developing recommendations for healthy individuals with data obtained from randomized controlled trials (RCTs) and large longitudinal observational trials with comparison groups if RCTs were not available. These guidelines recommend against empiric supplementation of vitamin D for healthy adults aged 18-74, excluding pregnant women and patients with high-risk diabetes.3
For older adults aged 75 or greater, empiric vitamin D supplementation is recommended because of the possible reduction of risk in all-cause mortality in this population. Of note, this was a grade 2 recommendation by the panel, indicating that the benefits of the treatment probably outweigh the risks. The panel stated that vitamin D supplementation could be delivered through fortified foods, multivitamins with vitamin D, or as a separate vitamin D supplement.
The dosage should remain within the recommended daily allowance outlined by the Institute of Medicine of 800 IU daily for adults over 70, and the panel recommends low-dose daily vitamin D supplementation over high-dose interval supplementation. The panel noted that routine screening of vitamin D levels should not be used to guide decision-making on whether to start supplementation, but vitamin D levels should be obtained for patients who have an indication for evaluation.
The reviewers highlight that these guidelines were developed for healthy individuals and are not applicable to those with conditions that warrant vitamin D evaluation. In my clinical practice, many of my patients have bone-mineral conditions and cognitive impairment that warrant evaluation. Based on these guidelines, I will consider empiric vitamin D supplementation more often for healthy patients aged 75 and older.
Sedentary Behaviors and Healthy Aging
Engaging inactive older adults in regular physical activity can be challenging, particularly as the pandemic has led to more pervasive social isolation and affected the availability of in-person exercise activities in the community. Physical activity is a key component of healthy aging and cognition, and its benefits should be a part of routine counseling for older adults.
An interesting recent study published in JAMA Network Open by Shi et al. evaluated the association of health behaviors and aging in female US nurses over a 20-year period.4 Surveys were administered to capture time spent in each behavior, such as being sedentary (TV watching, sitting at home or at work), light activity (walking around the house or at work), and moderate to vigorous activity (walking for exercise, lawn mowing). “Healthy aging” was defined by the absence of chronic conditions such as heart failure, and lack of physical, mental, and cognitive impairment.
The study found that participants who were more sedentary were less likely to age healthfully, with each additional 2 hours of TV watching per day associated with a 12% reduction in likelihood of healthy aging. Light physical activity was associated with a significant increase in healthy aging, with a 6% increase in the likelihood of healthy aging for each additional 2 hours of light activity. Each additional 1 hour of moderate to vigorous activity was associated with a 14% increase in the likelihood of healthy aging. These findings support discussions with patients that behavior change, even in small increments, can be beneficial in healthy aging.
References
1. Xu W et al. Ann Intern Med. 2024 Jun;177(6):701-10.
2. Yourman LC et al. JAMA Intern Med. 2021;181:179-85.
3. Demay MB et al. J Clin Endocrinol Metab. August 2024;109(8):1907-47.
4. Shi H et al. JAMA Netw Open. 2024;7(6):e2416300.
In this article, I will review several recently published articles and guidelines relevant to the care of older adults in primary care. The articles of interest address statins for primary prevention, vitamin D supplementation and testing, and physical activity for healthy aging.
Statins for Primary Prevention of Cardiovascular Disease
A common conundrum in primary care is whether an older adult should be on a statin for primary prevention. This question has been difficult to answer because of the underrepresentation of older adults in clinical trials that examine the effect of statins for primary prevention. A recent study by Xu et al. published in Annals of Internal Medicine sought to address this gap in knowledge, investigating the risks and benefits of statins for primary prevention for older adults.1
This study stratified participants by “old” (aged 75-84 years) and “very old” (85 years or older). In this study, older adults who had an indication for statins were initiated on statins and studied over a 5-year period and compared with age-matched cohorts not initiated on statin therapy. Participants with known cardiovascular disease at baseline were excluded. The outcomes of interest were major cardiovascular disease (CVD) (a composite of myocardial infarction, stroke, or heart failure), all-cause mortality, and adverse effect of drug therapy (myopathy or liver dysfunction).
The study found that among older adults aged 75-84, initiation of statin therapy led to a 1.2% risk reduction in major CVD over a 5-year period. For older adults aged 85 and greater, initiation of statins had an even larger impact, leading to a 4.4% risk reduction in major CVD over a 5-year period. The study found that there was no significant difference in adverse effects including myopathy or liver dysfunction in both age groups.
Statins, the study suggests, are appropriate and safe to initiate for primary prevention in older adults and can lead to substantial benefits in reduction of CVD. While time to benefit was not explicitly examined in this study, a prior study by Yourman et al. suggested that the time to benefit for statins for primary prevention in adults aged 50-75 would be least 2.5 years.2
My takeaway from these findings is to discuss statin initiation for primary prevention for older patients who are focused on longevity, have good functional status (often used in geriatrics as a proxy for prognosis), and are willing to accept more medications.
Empiric Vitamin D Supplementation in Adults over 75 Years
Vitamin D is one of the most common supplements taken by older adults but evidence supporting vitamin D supplementation is variable in published literature, as most data comes from observational trials. New guidelines from the Endocrine Society focused on developing recommendations for healthy individuals with data obtained from randomized controlled trials (RCTs) and large longitudinal observational trials with comparison groups if RCTs were not available. These guidelines recommend against empiric supplementation of vitamin D for healthy adults aged 18-74, excluding pregnant women and patients with high-risk diabetes.3
For older adults aged 75 or greater, empiric vitamin D supplementation is recommended because of the possible reduction of risk in all-cause mortality in this population. Of note, this was a grade 2 recommendation by the panel, indicating that the benefits of the treatment probably outweigh the risks. The panel stated that vitamin D supplementation could be delivered through fortified foods, multivitamins with vitamin D, or as a separate vitamin D supplement.
The dosage should remain within the recommended daily allowance outlined by the Institute of Medicine of 800 IU daily for adults over 70, and the panel recommends low-dose daily vitamin D supplementation over high-dose interval supplementation. The panel noted that routine screening of vitamin D levels should not be used to guide decision-making on whether to start supplementation, but vitamin D levels should be obtained for patients who have an indication for evaluation.
The reviewers highlight that these guidelines were developed for healthy individuals and are not applicable to those with conditions that warrant vitamin D evaluation. In my clinical practice, many of my patients have bone-mineral conditions and cognitive impairment that warrant evaluation. Based on these guidelines, I will consider empiric vitamin D supplementation more often for healthy patients aged 75 and older.
Sedentary Behaviors and Healthy Aging
Engaging inactive older adults in regular physical activity can be challenging, particularly as the pandemic has led to more pervasive social isolation and affected the availability of in-person exercise activities in the community. Physical activity is a key component of healthy aging and cognition, and its benefits should be a part of routine counseling for older adults.
An interesting recent study published in JAMA Network Open by Shi et al. evaluated the association of health behaviors and aging in female US nurses over a 20-year period.4 Surveys were administered to capture time spent in each behavior, such as being sedentary (TV watching, sitting at home or at work), light activity (walking around the house or at work), and moderate to vigorous activity (walking for exercise, lawn mowing). “Healthy aging” was defined by the absence of chronic conditions such as heart failure, and lack of physical, mental, and cognitive impairment.
The study found that participants who were more sedentary were less likely to age healthfully, with each additional 2 hours of TV watching per day associated with a 12% reduction in likelihood of healthy aging. Light physical activity was associated with a significant increase in healthy aging, with a 6% increase in the likelihood of healthy aging for each additional 2 hours of light activity. Each additional 1 hour of moderate to vigorous activity was associated with a 14% increase in the likelihood of healthy aging. These findings support discussions with patients that behavior change, even in small increments, can be beneficial in healthy aging.
References
1. Xu W et al. Ann Intern Med. 2024 Jun;177(6):701-10.
2. Yourman LC et al. JAMA Intern Med. 2021;181:179-85.
3. Demay MB et al. J Clin Endocrinol Metab. August 2024;109(8):1907-47.
4. Shi H et al. JAMA Netw Open. 2024;7(6):e2416300.
In this article, I will review several recently published articles and guidelines relevant to the care of older adults in primary care. The articles of interest address statins for primary prevention, vitamin D supplementation and testing, and physical activity for healthy aging.
Statins for Primary Prevention of Cardiovascular Disease
A common conundrum in primary care is whether an older adult should be on a statin for primary prevention. This question has been difficult to answer because of the underrepresentation of older adults in clinical trials that examine the effect of statins for primary prevention. A recent study by Xu et al. published in Annals of Internal Medicine sought to address this gap in knowledge, investigating the risks and benefits of statins for primary prevention for older adults.1
This study stratified participants by “old” (aged 75-84 years) and “very old” (85 years or older). In this study, older adults who had an indication for statins were initiated on statins and studied over a 5-year period and compared with age-matched cohorts not initiated on statin therapy. Participants with known cardiovascular disease at baseline were excluded. The outcomes of interest were major cardiovascular disease (CVD) (a composite of myocardial infarction, stroke, or heart failure), all-cause mortality, and adverse effect of drug therapy (myopathy or liver dysfunction).
The study found that among older adults aged 75-84, initiation of statin therapy led to a 1.2% risk reduction in major CVD over a 5-year period. For older adults aged 85 and greater, initiation of statins had an even larger impact, leading to a 4.4% risk reduction in major CVD over a 5-year period. The study found that there was no significant difference in adverse effects including myopathy or liver dysfunction in both age groups.
Statins, the study suggests, are appropriate and safe to initiate for primary prevention in older adults and can lead to substantial benefits in reduction of CVD. While time to benefit was not explicitly examined in this study, a prior study by Yourman et al. suggested that the time to benefit for statins for primary prevention in adults aged 50-75 would be least 2.5 years.2
My takeaway from these findings is to discuss statin initiation for primary prevention for older patients who are focused on longevity, have good functional status (often used in geriatrics as a proxy for prognosis), and are willing to accept more medications.
Empiric Vitamin D Supplementation in Adults over 75 Years
Vitamin D is one of the most common supplements taken by older adults but evidence supporting vitamin D supplementation is variable in published literature, as most data comes from observational trials. New guidelines from the Endocrine Society focused on developing recommendations for healthy individuals with data obtained from randomized controlled trials (RCTs) and large longitudinal observational trials with comparison groups if RCTs were not available. These guidelines recommend against empiric supplementation of vitamin D for healthy adults aged 18-74, excluding pregnant women and patients with high-risk diabetes.3
For older adults aged 75 or greater, empiric vitamin D supplementation is recommended because of the possible reduction of risk in all-cause mortality in this population. Of note, this was a grade 2 recommendation by the panel, indicating that the benefits of the treatment probably outweigh the risks. The panel stated that vitamin D supplementation could be delivered through fortified foods, multivitamins with vitamin D, or as a separate vitamin D supplement.
The dosage should remain within the recommended daily allowance outlined by the Institute of Medicine of 800 IU daily for adults over 70, and the panel recommends low-dose daily vitamin D supplementation over high-dose interval supplementation. The panel noted that routine screening of vitamin D levels should not be used to guide decision-making on whether to start supplementation, but vitamin D levels should be obtained for patients who have an indication for evaluation.
The reviewers highlight that these guidelines were developed for healthy individuals and are not applicable to those with conditions that warrant vitamin D evaluation. In my clinical practice, many of my patients have bone-mineral conditions and cognitive impairment that warrant evaluation. Based on these guidelines, I will consider empiric vitamin D supplementation more often for healthy patients aged 75 and older.
Sedentary Behaviors and Healthy Aging
Engaging inactive older adults in regular physical activity can be challenging, particularly as the pandemic has led to more pervasive social isolation and affected the availability of in-person exercise activities in the community. Physical activity is a key component of healthy aging and cognition, and its benefits should be a part of routine counseling for older adults.
An interesting recent study published in JAMA Network Open by Shi et al. evaluated the association of health behaviors and aging in female US nurses over a 20-year period.4 Surveys were administered to capture time spent in each behavior, such as being sedentary (TV watching, sitting at home or at work), light activity (walking around the house or at work), and moderate to vigorous activity (walking for exercise, lawn mowing). “Healthy aging” was defined by the absence of chronic conditions such as heart failure, and lack of physical, mental, and cognitive impairment.
The study found that participants who were more sedentary were less likely to age healthfully, with each additional 2 hours of TV watching per day associated with a 12% reduction in likelihood of healthy aging. Light physical activity was associated with a significant increase in healthy aging, with a 6% increase in the likelihood of healthy aging for each additional 2 hours of light activity. Each additional 1 hour of moderate to vigorous activity was associated with a 14% increase in the likelihood of healthy aging. These findings support discussions with patients that behavior change, even in small increments, can be beneficial in healthy aging.
References
1. Xu W et al. Ann Intern Med. 2024 Jun;177(6):701-10.
2. Yourman LC et al. JAMA Intern Med. 2021;181:179-85.
3. Demay MB et al. J Clin Endocrinol Metab. August 2024;109(8):1907-47.
4. Shi H et al. JAMA Netw Open. 2024;7(6):e2416300.
Dermatoporosis in Older Adults: A Condition That Requires Holistic, Creative Management
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
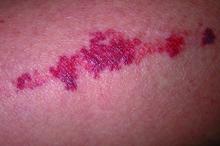
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Managing Atopic Dermatitis in Older Adults: A Common, Unique Challenge
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Over-the-Counter Hearing Aids as Effective as Traditional Models
Nearly 2 years ago, over-the-counter (OTC) hearing aids became available without a prescription. Audiologists and consumers have doubted their effectiveness, but OTC hearing aids can be as good as — and sometimes better — than traditional aids at half the cost.
A new study published in JAMA Otolaryngology–Head & Neck Surgery showed OTC hearing aids to be as or more effective in treating mild to moderate hearing loss.
“This means consumers with mild to moderate hearing loss can now access cost-effective devices without compromising on long-term benefits,” said De Wet Swanepoel, PhD, professor in the Department of Speech-Language Pathology and Audiology at the University of Pretoria in South Africa, and an author of the study.
Approximately 30% of people over the age of 70 who could benefit from hearing aids actually use them. In 2022, the US Food and Drug Administration (FDA) allowed the sale of nonprescription devices. But a year later, just 2% of people with hearing difficulty had purchased OTC hearing aids. Impaired hearing can increase the risk of developing dementia and decrease quality of life.
Dr. Swanepoel and his colleagues enrolled 44 individuals in the comparative effectiveness study, which was an extension of an initial randomized control trial lasting 6 weeks. Participants were tracked over an 8-month period, with about half using self-fitted OTC devices and the remaining with audiologist-fitted models. On the basis of users’ self-reported surveys, the results showed no clinically meaningful difference in effectiveness. The OTC hearing aids showed better satisfaction scores among users.
The typical pair of audiologist-fitted hearing aids costs $2000. OTC hearing aids, including the Lexi Lumen model used in the latest study and available in the United States, cost around $799.
“The cost savings combined with the effective performance of self-fit hearing aids make them a promising option for individuals with mild to moderate hearing loss,” Dr. Swanepoel said.
But many audiologists have reported they do not believe the nonprescription devices would provide the same benefit as a hearing aid provided by specialists, according to one survey in 2023.
OTC hearing aids may change the role of the primary care clinician, who may instead of referring patients to an audiologist, suggest a nonprescription version. They may also field questions from patients on which types are better, which Sharon Horesh Bergquist, MD, an internal medicine physician at Emory University in Atlanta, said she is already doing.
“Primary care physicians already evaluate patients with hearing loss to identify underlying causes and provide referrals to audiologists or ear, nose, and throat specialists; with the availability of OTC hearing aids, they can further support patients by informing them about these accessible options and help them understand when OTC aids may be appropriate,” Dr. Bergquist said.
When selecting a model, Dr. Bergquist recommends patients try them before buying. Certain models permit returns, but she advises patients to check the terms as not all models are the same.
“By removing the requirement to see an audiologist or ear, nose, and throat specialist, OTC hearing aids can increase use among individuals who might otherwise forgo them without eliminating the need for professional care,” Dr. Bergquist said.
She does refer some patients to visit an audiologist first to understand their type and cause of hearing impairment, which can help users select the best OTC model for them.
The study received funding from the hearX Pty Ltd Group and the National Institutes of Health. Various authors reported receiving personal fees from the hearX Group and Care Research Manchester Biomedical Research Centre.
A version of this article first appeared on Medscape.com.
Nearly 2 years ago, over-the-counter (OTC) hearing aids became available without a prescription. Audiologists and consumers have doubted their effectiveness, but OTC hearing aids can be as good as — and sometimes better — than traditional aids at half the cost.
A new study published in JAMA Otolaryngology–Head & Neck Surgery showed OTC hearing aids to be as or more effective in treating mild to moderate hearing loss.
“This means consumers with mild to moderate hearing loss can now access cost-effective devices without compromising on long-term benefits,” said De Wet Swanepoel, PhD, professor in the Department of Speech-Language Pathology and Audiology at the University of Pretoria in South Africa, and an author of the study.
Approximately 30% of people over the age of 70 who could benefit from hearing aids actually use them. In 2022, the US Food and Drug Administration (FDA) allowed the sale of nonprescription devices. But a year later, just 2% of people with hearing difficulty had purchased OTC hearing aids. Impaired hearing can increase the risk of developing dementia and decrease quality of life.
Dr. Swanepoel and his colleagues enrolled 44 individuals in the comparative effectiveness study, which was an extension of an initial randomized control trial lasting 6 weeks. Participants were tracked over an 8-month period, with about half using self-fitted OTC devices and the remaining with audiologist-fitted models. On the basis of users’ self-reported surveys, the results showed no clinically meaningful difference in effectiveness. The OTC hearing aids showed better satisfaction scores among users.
The typical pair of audiologist-fitted hearing aids costs $2000. OTC hearing aids, including the Lexi Lumen model used in the latest study and available in the United States, cost around $799.
“The cost savings combined with the effective performance of self-fit hearing aids make them a promising option for individuals with mild to moderate hearing loss,” Dr. Swanepoel said.
But many audiologists have reported they do not believe the nonprescription devices would provide the same benefit as a hearing aid provided by specialists, according to one survey in 2023.
OTC hearing aids may change the role of the primary care clinician, who may instead of referring patients to an audiologist, suggest a nonprescription version. They may also field questions from patients on which types are better, which Sharon Horesh Bergquist, MD, an internal medicine physician at Emory University in Atlanta, said she is already doing.
“Primary care physicians already evaluate patients with hearing loss to identify underlying causes and provide referrals to audiologists or ear, nose, and throat specialists; with the availability of OTC hearing aids, they can further support patients by informing them about these accessible options and help them understand when OTC aids may be appropriate,” Dr. Bergquist said.
When selecting a model, Dr. Bergquist recommends patients try them before buying. Certain models permit returns, but she advises patients to check the terms as not all models are the same.
“By removing the requirement to see an audiologist or ear, nose, and throat specialist, OTC hearing aids can increase use among individuals who might otherwise forgo them without eliminating the need for professional care,” Dr. Bergquist said.
She does refer some patients to visit an audiologist first to understand their type and cause of hearing impairment, which can help users select the best OTC model for them.
The study received funding from the hearX Pty Ltd Group and the National Institutes of Health. Various authors reported receiving personal fees from the hearX Group and Care Research Manchester Biomedical Research Centre.
A version of this article first appeared on Medscape.com.
Nearly 2 years ago, over-the-counter (OTC) hearing aids became available without a prescription. Audiologists and consumers have doubted their effectiveness, but OTC hearing aids can be as good as — and sometimes better — than traditional aids at half the cost.
A new study published in JAMA Otolaryngology–Head & Neck Surgery showed OTC hearing aids to be as or more effective in treating mild to moderate hearing loss.
“This means consumers with mild to moderate hearing loss can now access cost-effective devices without compromising on long-term benefits,” said De Wet Swanepoel, PhD, professor in the Department of Speech-Language Pathology and Audiology at the University of Pretoria in South Africa, and an author of the study.
Approximately 30% of people over the age of 70 who could benefit from hearing aids actually use them. In 2022, the US Food and Drug Administration (FDA) allowed the sale of nonprescription devices. But a year later, just 2% of people with hearing difficulty had purchased OTC hearing aids. Impaired hearing can increase the risk of developing dementia and decrease quality of life.
Dr. Swanepoel and his colleagues enrolled 44 individuals in the comparative effectiveness study, which was an extension of an initial randomized control trial lasting 6 weeks. Participants were tracked over an 8-month period, with about half using self-fitted OTC devices and the remaining with audiologist-fitted models. On the basis of users’ self-reported surveys, the results showed no clinically meaningful difference in effectiveness. The OTC hearing aids showed better satisfaction scores among users.
The typical pair of audiologist-fitted hearing aids costs $2000. OTC hearing aids, including the Lexi Lumen model used in the latest study and available in the United States, cost around $799.
“The cost savings combined with the effective performance of self-fit hearing aids make them a promising option for individuals with mild to moderate hearing loss,” Dr. Swanepoel said.
But many audiologists have reported they do not believe the nonprescription devices would provide the same benefit as a hearing aid provided by specialists, according to one survey in 2023.
OTC hearing aids may change the role of the primary care clinician, who may instead of referring patients to an audiologist, suggest a nonprescription version. They may also field questions from patients on which types are better, which Sharon Horesh Bergquist, MD, an internal medicine physician at Emory University in Atlanta, said she is already doing.
“Primary care physicians already evaluate patients with hearing loss to identify underlying causes and provide referrals to audiologists or ear, nose, and throat specialists; with the availability of OTC hearing aids, they can further support patients by informing them about these accessible options and help them understand when OTC aids may be appropriate,” Dr. Bergquist said.
When selecting a model, Dr. Bergquist recommends patients try them before buying. Certain models permit returns, but she advises patients to check the terms as not all models are the same.
“By removing the requirement to see an audiologist or ear, nose, and throat specialist, OTC hearing aids can increase use among individuals who might otherwise forgo them without eliminating the need for professional care,” Dr. Bergquist said.
She does refer some patients to visit an audiologist first to understand their type and cause of hearing impairment, which can help users select the best OTC model for them.
The study received funding from the hearX Pty Ltd Group and the National Institutes of Health. Various authors reported receiving personal fees from the hearX Group and Care Research Manchester Biomedical Research Centre.
A version of this article first appeared on Medscape.com.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
What Are the Ethics of Sex and Romance for Older Adults in Nursing Homes?
This transcript has been edited for clarity.
I had a case a couple years ago in which I found myself completely at odds with the person complaining. A daughter came to me and said [paraphrasing], look, my dad is in a nursing home, and he’s just there for care that he needs, but he’s mentally competent. He’s enjoying watching television, playing games. He plays bridge and does many things. The nursing home is letting him have a romantic relationship with a woman who’s also in the nursing home. I think you, ethicist, should both intervene and try to stop that, and write more about the immorality of facilities like nursing homes or other long-term care settings permitting romance or sexual relations to take place.
I was reminded of that case because a report recently appeared that sexually transmitted diseases are on the rise among the elderly, both in nursing homes and in other settings. This obviously is linked up to another technological advance: the erectile dysfunction drugs.
I’m sure there are many men who, at one point in their lives, could not engage in sexual activity due to impotence. We have found a treatment for erectile dysfunction. Loads and loads of men are using it, and we forget that some of them are going to be older. The rate of impotence goes up directly with aging. If you’re in a nursing home, home care, or wherever you are, you may find yourself able to engage in sex in a way that your dad or your granddad may not have been.
We also know — and I found this out when I was tracking sales of erectile dysfunction drugs — that some of these older men are going to visit prostitutes. That’s another route, unsafe sex, for sexual diseases to be spreading into various older communities.
Morally, I think every individual who is competent and wishes to engage in a romantic or sexual relationship should be able to do so. If they’re within a marriage and they want to resume sexual activity because they get better or they can use these drugs, well, that’s great. If they’re single and they’re just living with others and they form an interesting romantic relationship, why shouldn’t they be allowed to engage in sex?
It is not only something that I didn’t agree with the complaining daughter about, but also I think some of these facilities should make more rooms for privacy and more opportunity for intimacy. It’s not like we should tell granddad that he’s living in a college dorm and try to make sure that his roommate doesn’t come in if he’s going to have his girlfriend over.
Are there ethical issues? Sure. Obviously, we should remember, if we have older patients, to talk to them about sexually transmitted diseases as part of a discussion of their sex life. We shouldn’t presume that they’re not doing something. We should presume that they might be, and then remind them about safe sex, particularly if they’re going to use third parties like prostitutes.
Competency becomes important. It’s one thing to have a mutually agreed upon romantic relationship. It’s another thing if somebody is taking advantage of someone who has Alzheimer’s or severe mental dysfunction and they’re not consenting.
How do we determine that and how do we manage that? I think people who are incompetent need to be protected from sexual advances unless they have a relative or someone who says they can engage if they enjoy it and it brings them pleasure. I wouldn’t just have people who are vulnerable, exploited, or acting in a predatory way toward others.
As I said, we need to rethink the design of where older people are living, whether it’s assisted living, nursing home living, or wherever, just to give them the opportunity to have a full life, as any individual would have once they’re past the age of majority, no matter who they want to have romance with and what they want to do in terms of how far that intimacy goes.
Sadly, I didn’t agree with the daughter who came to me and asked me to stop it. I wouldn’t stop it nor would I publish against it. There are risks that we ought to be aware of, including exploiting vulnerable people if they can’t consent, and the danger of transmission of disease, as would be true in any group that might engage in high-risk behavior.
Another risk may be injury if someone is frail and can’t physically sustain sexual intimacy because they’re just too frail to do it. We also need to be sure to address the issue of sexuality with patients to make sure they know what’s going on, what risks there are, what rights they have, and so on.
At the end of the day, I’m not in the camp that says, “Just say no” when it comes to sex among the elderly.
Dr. Caplan is director, Division of Medical Ethics, New York University Langone Medical Center, New York. He has served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); he also serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I had a case a couple years ago in which I found myself completely at odds with the person complaining. A daughter came to me and said [paraphrasing], look, my dad is in a nursing home, and he’s just there for care that he needs, but he’s mentally competent. He’s enjoying watching television, playing games. He plays bridge and does many things. The nursing home is letting him have a romantic relationship with a woman who’s also in the nursing home. I think you, ethicist, should both intervene and try to stop that, and write more about the immorality of facilities like nursing homes or other long-term care settings permitting romance or sexual relations to take place.
I was reminded of that case because a report recently appeared that sexually transmitted diseases are on the rise among the elderly, both in nursing homes and in other settings. This obviously is linked up to another technological advance: the erectile dysfunction drugs.
I’m sure there are many men who, at one point in their lives, could not engage in sexual activity due to impotence. We have found a treatment for erectile dysfunction. Loads and loads of men are using it, and we forget that some of them are going to be older. The rate of impotence goes up directly with aging. If you’re in a nursing home, home care, or wherever you are, you may find yourself able to engage in sex in a way that your dad or your granddad may not have been.
We also know — and I found this out when I was tracking sales of erectile dysfunction drugs — that some of these older men are going to visit prostitutes. That’s another route, unsafe sex, for sexual diseases to be spreading into various older communities.
Morally, I think every individual who is competent and wishes to engage in a romantic or sexual relationship should be able to do so. If they’re within a marriage and they want to resume sexual activity because they get better or they can use these drugs, well, that’s great. If they’re single and they’re just living with others and they form an interesting romantic relationship, why shouldn’t they be allowed to engage in sex?
It is not only something that I didn’t agree with the complaining daughter about, but also I think some of these facilities should make more rooms for privacy and more opportunity for intimacy. It’s not like we should tell granddad that he’s living in a college dorm and try to make sure that his roommate doesn’t come in if he’s going to have his girlfriend over.
Are there ethical issues? Sure. Obviously, we should remember, if we have older patients, to talk to them about sexually transmitted diseases as part of a discussion of their sex life. We shouldn’t presume that they’re not doing something. We should presume that they might be, and then remind them about safe sex, particularly if they’re going to use third parties like prostitutes.
Competency becomes important. It’s one thing to have a mutually agreed upon romantic relationship. It’s another thing if somebody is taking advantage of someone who has Alzheimer’s or severe mental dysfunction and they’re not consenting.
How do we determine that and how do we manage that? I think people who are incompetent need to be protected from sexual advances unless they have a relative or someone who says they can engage if they enjoy it and it brings them pleasure. I wouldn’t just have people who are vulnerable, exploited, or acting in a predatory way toward others.
As I said, we need to rethink the design of where older people are living, whether it’s assisted living, nursing home living, or wherever, just to give them the opportunity to have a full life, as any individual would have once they’re past the age of majority, no matter who they want to have romance with and what they want to do in terms of how far that intimacy goes.
Sadly, I didn’t agree with the daughter who came to me and asked me to stop it. I wouldn’t stop it nor would I publish against it. There are risks that we ought to be aware of, including exploiting vulnerable people if they can’t consent, and the danger of transmission of disease, as would be true in any group that might engage in high-risk behavior.
Another risk may be injury if someone is frail and can’t physically sustain sexual intimacy because they’re just too frail to do it. We also need to be sure to address the issue of sexuality with patients to make sure they know what’s going on, what risks there are, what rights they have, and so on.
At the end of the day, I’m not in the camp that says, “Just say no” when it comes to sex among the elderly.
Dr. Caplan is director, Division of Medical Ethics, New York University Langone Medical Center, New York. He has served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); he also serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I had a case a couple years ago in which I found myself completely at odds with the person complaining. A daughter came to me and said [paraphrasing], look, my dad is in a nursing home, and he’s just there for care that he needs, but he’s mentally competent. He’s enjoying watching television, playing games. He plays bridge and does many things. The nursing home is letting him have a romantic relationship with a woman who’s also in the nursing home. I think you, ethicist, should both intervene and try to stop that, and write more about the immorality of facilities like nursing homes or other long-term care settings permitting romance or sexual relations to take place.
I was reminded of that case because a report recently appeared that sexually transmitted diseases are on the rise among the elderly, both in nursing homes and in other settings. This obviously is linked up to another technological advance: the erectile dysfunction drugs.
I’m sure there are many men who, at one point in their lives, could not engage in sexual activity due to impotence. We have found a treatment for erectile dysfunction. Loads and loads of men are using it, and we forget that some of them are going to be older. The rate of impotence goes up directly with aging. If you’re in a nursing home, home care, or wherever you are, you may find yourself able to engage in sex in a way that your dad or your granddad may not have been.
We also know — and I found this out when I was tracking sales of erectile dysfunction drugs — that some of these older men are going to visit prostitutes. That’s another route, unsafe sex, for sexual diseases to be spreading into various older communities.
Morally, I think every individual who is competent and wishes to engage in a romantic or sexual relationship should be able to do so. If they’re within a marriage and they want to resume sexual activity because they get better or they can use these drugs, well, that’s great. If they’re single and they’re just living with others and they form an interesting romantic relationship, why shouldn’t they be allowed to engage in sex?
It is not only something that I didn’t agree with the complaining daughter about, but also I think some of these facilities should make more rooms for privacy and more opportunity for intimacy. It’s not like we should tell granddad that he’s living in a college dorm and try to make sure that his roommate doesn’t come in if he’s going to have his girlfriend over.
Are there ethical issues? Sure. Obviously, we should remember, if we have older patients, to talk to them about sexually transmitted diseases as part of a discussion of their sex life. We shouldn’t presume that they’re not doing something. We should presume that they might be, and then remind them about safe sex, particularly if they’re going to use third parties like prostitutes.
Competency becomes important. It’s one thing to have a mutually agreed upon romantic relationship. It’s another thing if somebody is taking advantage of someone who has Alzheimer’s or severe mental dysfunction and they’re not consenting.
How do we determine that and how do we manage that? I think people who are incompetent need to be protected from sexual advances unless they have a relative or someone who says they can engage if they enjoy it and it brings them pleasure. I wouldn’t just have people who are vulnerable, exploited, or acting in a predatory way toward others.
As I said, we need to rethink the design of where older people are living, whether it’s assisted living, nursing home living, or wherever, just to give them the opportunity to have a full life, as any individual would have once they’re past the age of majority, no matter who they want to have romance with and what they want to do in terms of how far that intimacy goes.
Sadly, I didn’t agree with the daughter who came to me and asked me to stop it. I wouldn’t stop it nor would I publish against it. There are risks that we ought to be aware of, including exploiting vulnerable people if they can’t consent, and the danger of transmission of disease, as would be true in any group that might engage in high-risk behavior.
Another risk may be injury if someone is frail and can’t physically sustain sexual intimacy because they’re just too frail to do it. We also need to be sure to address the issue of sexuality with patients to make sure they know what’s going on, what risks there are, what rights they have, and so on.
At the end of the day, I’m not in the camp that says, “Just say no” when it comes to sex among the elderly.
Dr. Caplan is director, Division of Medical Ethics, New York University Langone Medical Center, New York. He has served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); he also serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
Guidance on How Best to Manage Opioid Risks in Older Adults
Polypharmacy and slow metabolism of drugs create a high risk among older adults for substance use disorder, raising the odds of intentional and unintentional overdoses. However, screening, assessment, and treatment for substance use disorder occurs less often in younger adults.
Rates of overdose from opioids increased the most among people aged 65 years and older from 2021 to 2022, compared with among younger age groups. Meanwhile, recent data show less than half older adults with opioid use disorder (OUD) receive care for the condition.
“Nobody is immune to developing some kind of use disorder, so don’t just assume that because someone’s 80 years old that there’s no way that they have a problem,” said Sara Meyer, PharmD, a medication safety pharmacist at Novant Health in Winston-Salem, North Carolina. “You never know who’s going to potentially have an issue.”
in an effort to reduce addiction and overdoses.
Older Adults Have Unique Needs
A major challenge of treating older adults is their high incidence of chronic pain and multiple complex chronic conditions. As a result, some of the nonopioid medications clinicians might otherwise prescribe, like nonsteroidal anti-inflammatory drugs, cannot be used, according to Caroline Goldzweig, MD, chief medical officer of the Cedars-Sinai Medical Network in Los Angeles, California.
“Before you know it, the only thing left is an opiate, so you can sometimes be between a rock and a hard place,” she said.
But for adults older than 65 years, opioids can carry problematic side effects, including sedation, cognitive impairment, falls, and fractures.
With those factors in mind, part of a yearly checkup or wellness visit should include time to discuss how a patient is managing their chronic pain, according to Timothy Anderson, MD, an assistant professor of medicine at the University of Pittsburgh, Pittsburgh, Pennsylvania, and codirector of the Prescribing Wisely Lab, a research collaboration between that institution and Beth Israel Deaconess Medical Center in Boston.
When considering a prescription for pain medication, Dr. Anderson said he evaluates the potential worst, best, and average outcomes for a patient. Nonopioid options should always be considered first-line treatment. Patients and physicians often struggle with balancing an option that meets a patient’s goals for pain relief but does not put them at a risk for adverse outcomes, he said.
Greater Risk
Older adults experience neurophysiologic effects different from younger people, said Benjamin Han, MD, a geriatrician and addiction medicine specialist at the University of California, San Diego.
Seniors also absorb, metabolize, and excrete drugs differently, sometimes affected by decreased production of gastric acid, lean body mass, and renal function. Coupled with complications of other chronic conditions or medications, diagnosing problematic opioid use or OUD can be one of the most challenging experiences in geriatrics, Dr. Han said.
As a result, OUD is often underdiagnosed in these patients, he said. Single-item screening tools like the TAPS and OWLS can be used to assess if the benefits of an opioid outweigh a patient’s risk for addiction.
Dr. Han finds medications like buprenorphine to be relatively safe and effective, along with nonpharmacologic interventions like physical therapy. He also advised clinicians to provide patients with opioid-overdose reversal agents.
“Naloxone is only used for reversing opioid withdrawal, but it is important to ensure that any patient at risk for an overdose, including being on chronic opioids, is provided naloxone and educated on preventing opioid overdoses,” he said.
Steroid injections and medications that target specific pathways, such as neuropathic pain, can be helpful in primary care for these older patients, according to Pooja Lagisetty, MD, an internal medicine physician at Michigan Medicine and a research scientist at VA Ann Arbor Health Care, Ann Arbor, Michigan.
She often recommends to her patients online programs that help them maintain strength and mobility, as well as low-impact exercises like tai chi, for pain management.
“This will ensure a much more balanced, patient-centered conversation with whatever decisions you and your patient come to,” Dr. Lagisetty said.
New Protocols for Pain Management in Older Adults
At the health system level, clinicians can use treatment agreements for patients taking opioids. At Novant, patients must attest they agree to take the medications only as prescribed and from a specified pharmacy. They promise not to seek opioids from other sources, to submit to random drug screenings, and to communicate regularly with their clinician about any health issues.
If a patient violates any part of this agreement, their clinician can stop the treatment. The system encourages clinicians to help patients find additional care for substance abuse disorder or pain management if it occurs.
Over the past 2 years, Novant also developed an AI prediction model, which generates a score for the risk a patient has in developing substance use disorder or experiencing an overdose within a year of initial opioid prescription. The model was validated by an internal team at the system but has not been independently certified.
If a patient has a high-risk score, their clinician considers additional risk mitigation strategies, such as seeing the patient more frequently or using an abuse deterrent formulation of an opioid. They also have the option of referring the patient to specialists in addiction medicine or neurology. Opioids are not necessarily withheld, according to Dr. Meyer. The tool is now used by clinicians during Medicare annual wellness visits.
And coming later this year are new protocols for pain management in patients aged 80 years and older. Clinicians will target a 50% dose reduction, compared with what a younger patient might receive to account for physiologic differences.
“We know that especially with some opioids like morphine, they’re not going to metabolize that the same way a young person with a young kidney will, so we’re trying to set the clinician up to select a lower starting dose for patients that are older,” Dr. Meyer said.
In 2017, the system implemented a program to reduce prescription of opioids to less than 350 morphine milligram equivalents (MME) per order following any kind of surgery. The health system compared numbers of prescriptions written among surgical colleagues and met with them to discuss alternative approaches. Novant said it continues to monitor the data and follow-up with surgeons who are not in alignment with the goal.
Between 2017 and 2019, patients switching to lower doses after surgeries rose by 20%.
Across the country at Cedars-Sinai Medical Network, leadership in 2016 made the move to deprescribe opioids or lower doses of the drugs to less than 90 MME per day, in accordance with Centers for Disease Control and Prevention guidelines established that year. Patients were referred to their pain program for support and for nonopioid interventions. Pharmacists worked closely with clinicians on safely tapering these medications in patients taking high doses.
The program worked, according to Dr. Goldzweig. Dr. Goldzweig could only find two patients currently taking high-dose opioids in the system’s database out of more than 7000 patients with Medicare Advantage insurance coverage.
“There will always be some patients who have no alternative than opioids, but we established some discipline with urine tox screens and pain agreements, and over time, we’ve been able to reduce the number of high-risk opioid prescriptions,” she said.
A version of this article first appeared on Medscape.com.
Polypharmacy and slow metabolism of drugs create a high risk among older adults for substance use disorder, raising the odds of intentional and unintentional overdoses. However, screening, assessment, and treatment for substance use disorder occurs less often in younger adults.
Rates of overdose from opioids increased the most among people aged 65 years and older from 2021 to 2022, compared with among younger age groups. Meanwhile, recent data show less than half older adults with opioid use disorder (OUD) receive care for the condition.
“Nobody is immune to developing some kind of use disorder, so don’t just assume that because someone’s 80 years old that there’s no way that they have a problem,” said Sara Meyer, PharmD, a medication safety pharmacist at Novant Health in Winston-Salem, North Carolina. “You never know who’s going to potentially have an issue.”
in an effort to reduce addiction and overdoses.
Older Adults Have Unique Needs
A major challenge of treating older adults is their high incidence of chronic pain and multiple complex chronic conditions. As a result, some of the nonopioid medications clinicians might otherwise prescribe, like nonsteroidal anti-inflammatory drugs, cannot be used, according to Caroline Goldzweig, MD, chief medical officer of the Cedars-Sinai Medical Network in Los Angeles, California.
“Before you know it, the only thing left is an opiate, so you can sometimes be between a rock and a hard place,” she said.
But for adults older than 65 years, opioids can carry problematic side effects, including sedation, cognitive impairment, falls, and fractures.
With those factors in mind, part of a yearly checkup or wellness visit should include time to discuss how a patient is managing their chronic pain, according to Timothy Anderson, MD, an assistant professor of medicine at the University of Pittsburgh, Pittsburgh, Pennsylvania, and codirector of the Prescribing Wisely Lab, a research collaboration between that institution and Beth Israel Deaconess Medical Center in Boston.
When considering a prescription for pain medication, Dr. Anderson said he evaluates the potential worst, best, and average outcomes for a patient. Nonopioid options should always be considered first-line treatment. Patients and physicians often struggle with balancing an option that meets a patient’s goals for pain relief but does not put them at a risk for adverse outcomes, he said.
Greater Risk
Older adults experience neurophysiologic effects different from younger people, said Benjamin Han, MD, a geriatrician and addiction medicine specialist at the University of California, San Diego.
Seniors also absorb, metabolize, and excrete drugs differently, sometimes affected by decreased production of gastric acid, lean body mass, and renal function. Coupled with complications of other chronic conditions or medications, diagnosing problematic opioid use or OUD can be one of the most challenging experiences in geriatrics, Dr. Han said.
As a result, OUD is often underdiagnosed in these patients, he said. Single-item screening tools like the TAPS and OWLS can be used to assess if the benefits of an opioid outweigh a patient’s risk for addiction.
Dr. Han finds medications like buprenorphine to be relatively safe and effective, along with nonpharmacologic interventions like physical therapy. He also advised clinicians to provide patients with opioid-overdose reversal agents.
“Naloxone is only used for reversing opioid withdrawal, but it is important to ensure that any patient at risk for an overdose, including being on chronic opioids, is provided naloxone and educated on preventing opioid overdoses,” he said.
Steroid injections and medications that target specific pathways, such as neuropathic pain, can be helpful in primary care for these older patients, according to Pooja Lagisetty, MD, an internal medicine physician at Michigan Medicine and a research scientist at VA Ann Arbor Health Care, Ann Arbor, Michigan.
She often recommends to her patients online programs that help them maintain strength and mobility, as well as low-impact exercises like tai chi, for pain management.
“This will ensure a much more balanced, patient-centered conversation with whatever decisions you and your patient come to,” Dr. Lagisetty said.
New Protocols for Pain Management in Older Adults
At the health system level, clinicians can use treatment agreements for patients taking opioids. At Novant, patients must attest they agree to take the medications only as prescribed and from a specified pharmacy. They promise not to seek opioids from other sources, to submit to random drug screenings, and to communicate regularly with their clinician about any health issues.
If a patient violates any part of this agreement, their clinician can stop the treatment. The system encourages clinicians to help patients find additional care for substance abuse disorder or pain management if it occurs.
Over the past 2 years, Novant also developed an AI prediction model, which generates a score for the risk a patient has in developing substance use disorder or experiencing an overdose within a year of initial opioid prescription. The model was validated by an internal team at the system but has not been independently certified.
If a patient has a high-risk score, their clinician considers additional risk mitigation strategies, such as seeing the patient more frequently or using an abuse deterrent formulation of an opioid. They also have the option of referring the patient to specialists in addiction medicine or neurology. Opioids are not necessarily withheld, according to Dr. Meyer. The tool is now used by clinicians during Medicare annual wellness visits.
And coming later this year are new protocols for pain management in patients aged 80 years and older. Clinicians will target a 50% dose reduction, compared with what a younger patient might receive to account for physiologic differences.
“We know that especially with some opioids like morphine, they’re not going to metabolize that the same way a young person with a young kidney will, so we’re trying to set the clinician up to select a lower starting dose for patients that are older,” Dr. Meyer said.
In 2017, the system implemented a program to reduce prescription of opioids to less than 350 morphine milligram equivalents (MME) per order following any kind of surgery. The health system compared numbers of prescriptions written among surgical colleagues and met with them to discuss alternative approaches. Novant said it continues to monitor the data and follow-up with surgeons who are not in alignment with the goal.
Between 2017 and 2019, patients switching to lower doses after surgeries rose by 20%.
Across the country at Cedars-Sinai Medical Network, leadership in 2016 made the move to deprescribe opioids or lower doses of the drugs to less than 90 MME per day, in accordance with Centers for Disease Control and Prevention guidelines established that year. Patients were referred to their pain program for support and for nonopioid interventions. Pharmacists worked closely with clinicians on safely tapering these medications in patients taking high doses.
The program worked, according to Dr. Goldzweig. Dr. Goldzweig could only find two patients currently taking high-dose opioids in the system’s database out of more than 7000 patients with Medicare Advantage insurance coverage.
“There will always be some patients who have no alternative than opioids, but we established some discipline with urine tox screens and pain agreements, and over time, we’ve been able to reduce the number of high-risk opioid prescriptions,” she said.
A version of this article first appeared on Medscape.com.
Polypharmacy and slow metabolism of drugs create a high risk among older adults for substance use disorder, raising the odds of intentional and unintentional overdoses. However, screening, assessment, and treatment for substance use disorder occurs less often in younger adults.
Rates of overdose from opioids increased the most among people aged 65 years and older from 2021 to 2022, compared with among younger age groups. Meanwhile, recent data show less than half older adults with opioid use disorder (OUD) receive care for the condition.
“Nobody is immune to developing some kind of use disorder, so don’t just assume that because someone’s 80 years old that there’s no way that they have a problem,” said Sara Meyer, PharmD, a medication safety pharmacist at Novant Health in Winston-Salem, North Carolina. “You never know who’s going to potentially have an issue.”
in an effort to reduce addiction and overdoses.
Older Adults Have Unique Needs
A major challenge of treating older adults is their high incidence of chronic pain and multiple complex chronic conditions. As a result, some of the nonopioid medications clinicians might otherwise prescribe, like nonsteroidal anti-inflammatory drugs, cannot be used, according to Caroline Goldzweig, MD, chief medical officer of the Cedars-Sinai Medical Network in Los Angeles, California.
“Before you know it, the only thing left is an opiate, so you can sometimes be between a rock and a hard place,” she said.
But for adults older than 65 years, opioids can carry problematic side effects, including sedation, cognitive impairment, falls, and fractures.
With those factors in mind, part of a yearly checkup or wellness visit should include time to discuss how a patient is managing their chronic pain, according to Timothy Anderson, MD, an assistant professor of medicine at the University of Pittsburgh, Pittsburgh, Pennsylvania, and codirector of the Prescribing Wisely Lab, a research collaboration between that institution and Beth Israel Deaconess Medical Center in Boston.
When considering a prescription for pain medication, Dr. Anderson said he evaluates the potential worst, best, and average outcomes for a patient. Nonopioid options should always be considered first-line treatment. Patients and physicians often struggle with balancing an option that meets a patient’s goals for pain relief but does not put them at a risk for adverse outcomes, he said.
Greater Risk
Older adults experience neurophysiologic effects different from younger people, said Benjamin Han, MD, a geriatrician and addiction medicine specialist at the University of California, San Diego.
Seniors also absorb, metabolize, and excrete drugs differently, sometimes affected by decreased production of gastric acid, lean body mass, and renal function. Coupled with complications of other chronic conditions or medications, diagnosing problematic opioid use or OUD can be one of the most challenging experiences in geriatrics, Dr. Han said.
As a result, OUD is often underdiagnosed in these patients, he said. Single-item screening tools like the TAPS and OWLS can be used to assess if the benefits of an opioid outweigh a patient’s risk for addiction.
Dr. Han finds medications like buprenorphine to be relatively safe and effective, along with nonpharmacologic interventions like physical therapy. He also advised clinicians to provide patients with opioid-overdose reversal agents.
“Naloxone is only used for reversing opioid withdrawal, but it is important to ensure that any patient at risk for an overdose, including being on chronic opioids, is provided naloxone and educated on preventing opioid overdoses,” he said.
Steroid injections and medications that target specific pathways, such as neuropathic pain, can be helpful in primary care for these older patients, according to Pooja Lagisetty, MD, an internal medicine physician at Michigan Medicine and a research scientist at VA Ann Arbor Health Care, Ann Arbor, Michigan.
She often recommends to her patients online programs that help them maintain strength and mobility, as well as low-impact exercises like tai chi, for pain management.
“This will ensure a much more balanced, patient-centered conversation with whatever decisions you and your patient come to,” Dr. Lagisetty said.
New Protocols for Pain Management in Older Adults
At the health system level, clinicians can use treatment agreements for patients taking opioids. At Novant, patients must attest they agree to take the medications only as prescribed and from a specified pharmacy. They promise not to seek opioids from other sources, to submit to random drug screenings, and to communicate regularly with their clinician about any health issues.
If a patient violates any part of this agreement, their clinician can stop the treatment. The system encourages clinicians to help patients find additional care for substance abuse disorder or pain management if it occurs.
Over the past 2 years, Novant also developed an AI prediction model, which generates a score for the risk a patient has in developing substance use disorder or experiencing an overdose within a year of initial opioid prescription. The model was validated by an internal team at the system but has not been independently certified.
If a patient has a high-risk score, their clinician considers additional risk mitigation strategies, such as seeing the patient more frequently or using an abuse deterrent formulation of an opioid. They also have the option of referring the patient to specialists in addiction medicine or neurology. Opioids are not necessarily withheld, according to Dr. Meyer. The tool is now used by clinicians during Medicare annual wellness visits.
And coming later this year are new protocols for pain management in patients aged 80 years and older. Clinicians will target a 50% dose reduction, compared with what a younger patient might receive to account for physiologic differences.
“We know that especially with some opioids like morphine, they’re not going to metabolize that the same way a young person with a young kidney will, so we’re trying to set the clinician up to select a lower starting dose for patients that are older,” Dr. Meyer said.
In 2017, the system implemented a program to reduce prescription of opioids to less than 350 morphine milligram equivalents (MME) per order following any kind of surgery. The health system compared numbers of prescriptions written among surgical colleagues and met with them to discuss alternative approaches. Novant said it continues to monitor the data and follow-up with surgeons who are not in alignment with the goal.
Between 2017 and 2019, patients switching to lower doses after surgeries rose by 20%.
Across the country at Cedars-Sinai Medical Network, leadership in 2016 made the move to deprescribe opioids or lower doses of the drugs to less than 90 MME per day, in accordance with Centers for Disease Control and Prevention guidelines established that year. Patients were referred to their pain program for support and for nonopioid interventions. Pharmacists worked closely with clinicians on safely tapering these medications in patients taking high doses.
The program worked, according to Dr. Goldzweig. Dr. Goldzweig could only find two patients currently taking high-dose opioids in the system’s database out of more than 7000 patients with Medicare Advantage insurance coverage.
“There will always be some patients who have no alternative than opioids, but we established some discipline with urine tox screens and pain agreements, and over time, we’ve been able to reduce the number of high-risk opioid prescriptions,” she said.
A version of this article first appeared on Medscape.com.
Buprenorphine One of Many Options For Pain Relief In Oldest Adults
Some degree of pain is inevitable in older individuals, and as people pass 80 years of age, the harms of medications used to control chronic pain increase. Pain-reducing medication use in this age group may cause inflammation, gastric bleeding, kidney damage, or constipation.
These risks may lead some clinicians to avoid aggressive pain treatment in their eldest patients, resulting in unnecessary suffering.
“Pain causes harm beyond just the physical suffering associated with it,” said Diane Meier, MD, a geriatrician and palliative care specialist at Mount Sinai Medicine in New York City who treats many people in their 80s and 90s.
Downstream effects of untreated pain could include a loss of mobility and isolation, Dr. Meier said. And, as these harms are mounting, some clinicians may avoid using an analgesic that could bring great relief: buprenorphine.
“People think about buprenorphine like they think about methadone,” Dr. Meier said, as something prescribed to treat substance use disorder. In reality, it is an effective analgesic in other situations.
Buprenorphine is better at treating chronic pain than other opioids that carry a higher addiction risk and often cause constipation in elderly patients. Buprenorphine is easier on the kidneys and has a lower addiction risk than opioids like oxycodone.
The transdermal patch form of buprenorphine (Butrans, PurduePharma) is changed weekly and starts at low doses.
“There’s an adage in geriatrics: start low and go slow,” said Jessica Merlin, MD, PhD, a palliative care and addiction medicine physician at the University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania.
Dr. Merlin recommends beginning elderly patients with chronic pain on a 10-microgram/hour dose of Butrans, among the lowest doses available. Physicians could monitor side effects, which will generally be mild, with the aim of never increasing the dose if pain is managed.
Nonpharmacologic Remedies, Drug Considerations
“Nonpharmacologic therapy is very underutilized,” Dr. Merlin said, even though multiple alternatives to medications can improve chronic pain symptoms at any age.
Cognitive-behavioral therapy or acceptance and commitment therapy can both help people reduce the impact of pain, Dr. Merlin said. And for people who can do so, physical therapy programs, yoga, or tai chi are all ways to strengthen the body’s defenses against pain, Dr. Merlin added.
Sometimes medication is necessary, however.
“You can’t get an older person to participate in rehab if they are in severe pain,” Dr. Meier said, adding that judicious use of medications should go hand in hand with nonpharmacologic treatment.
When medications are unavoidable, internist Douglas S. Paauw, MD, starts with topical injections at the site of the pain — a troublesome joint, for example — rather than systemic medications that affect multiple organs and the brain.
“We try not to flood their body with meds” for localized problems, Dr. Paauw said, whose goal when treating elderly patients with pain is to improve their daily functioning and quality of life.
Dr. Paauw works at the University of Washington in Seattle and treats people who are approaching 100 years old. As some of his patients have grown older, Dr. Paauw’s interest in effective pain management has grown; he thinks that all internists and family medicine physician need to know how to manage chronic pain in their eldest patients.
“Were you able to play with your grandkid? Were you able to go grocery shopping? Were you able to take a walk outside?” These are the kinds of improvements Dr. Paauw hopes to see in older patients, recognizing that the wear and tear of life — orthopedic stresses or healed fractures that cause lingering pain — make it impossible for many older people to be pain free.
Pain is often spread throughout the body rather than focusing at one point, which requires systemic medications if physical therapy and similar approaches have not reduced pain. Per American Geriatrics Society (AGS) guidelines, in this situation Dr. Paauw starts with acetaminophen (Tylenol) as the lowest-risk systemic pain treatment.
Dr. Pauuw often counsels older patients to begin with 2 grams/day of acetaminophen and then progress to 3 grams if the lower dose has manageable side effects, rather than the standard dose of 4 grams that he feels is geared toward younger patients.
When acetaminophen doesn’t reduce pain sufficiently, or aggravates inflammation, Dr. Paauw may use the nerve pain medication pregabalin, or the antidepressant duloxetine — especially if the pain appears to be neuropathic.
Tricyclic antidepressants used to be recommended for neuropathic pain in older adults, but are now on the AGS’s Beers Criteria of drugs to avoid in elderly patients due to risk of causing dizziness or cardiac stress. Dr. Paauw might still use a tricyclic, but only after a careful risk-benefit analysis.
Nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen (Motrin) or naproxen (Aleve) could work in short bursts, Dr. Paauw said, although they may cause stomach bleeding or kidney damage in older patients.
This is why NSAIDs are not recommended by the AGS for chronic pain management. And opioids like oxycodone don’t work long at low doses, often leading to dose escalation and addiction.
“The American Geriatrics Society really puts opioids down at the bottom of the list,” Dr. Paauw said, to be used “judiciously and rarely.”
Opioids may interact with other drugs to increase risk of a fall, Dr. Meier added, making them inadvisable for older patients who live alone.
“That’s why knowing something about buprenorphine is so important,” Dr. Meier said.
Dr. Meier and Dr. Paauw are on the editorial board for Internal Medicine News. Dr. Merlin is a trainer for the Center to Advance Palliative Care, which Dr. Meier founded.
Some degree of pain is inevitable in older individuals, and as people pass 80 years of age, the harms of medications used to control chronic pain increase. Pain-reducing medication use in this age group may cause inflammation, gastric bleeding, kidney damage, or constipation.
These risks may lead some clinicians to avoid aggressive pain treatment in their eldest patients, resulting in unnecessary suffering.
“Pain causes harm beyond just the physical suffering associated with it,” said Diane Meier, MD, a geriatrician and palliative care specialist at Mount Sinai Medicine in New York City who treats many people in their 80s and 90s.
Downstream effects of untreated pain could include a loss of mobility and isolation, Dr. Meier said. And, as these harms are mounting, some clinicians may avoid using an analgesic that could bring great relief: buprenorphine.
“People think about buprenorphine like they think about methadone,” Dr. Meier said, as something prescribed to treat substance use disorder. In reality, it is an effective analgesic in other situations.
Buprenorphine is better at treating chronic pain than other opioids that carry a higher addiction risk and often cause constipation in elderly patients. Buprenorphine is easier on the kidneys and has a lower addiction risk than opioids like oxycodone.
The transdermal patch form of buprenorphine (Butrans, PurduePharma) is changed weekly and starts at low doses.
“There’s an adage in geriatrics: start low and go slow,” said Jessica Merlin, MD, PhD, a palliative care and addiction medicine physician at the University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania.
Dr. Merlin recommends beginning elderly patients with chronic pain on a 10-microgram/hour dose of Butrans, among the lowest doses available. Physicians could monitor side effects, which will generally be mild, with the aim of never increasing the dose if pain is managed.
Nonpharmacologic Remedies, Drug Considerations
“Nonpharmacologic therapy is very underutilized,” Dr. Merlin said, even though multiple alternatives to medications can improve chronic pain symptoms at any age.
Cognitive-behavioral therapy or acceptance and commitment therapy can both help people reduce the impact of pain, Dr. Merlin said. And for people who can do so, physical therapy programs, yoga, or tai chi are all ways to strengthen the body’s defenses against pain, Dr. Merlin added.
Sometimes medication is necessary, however.
“You can’t get an older person to participate in rehab if they are in severe pain,” Dr. Meier said, adding that judicious use of medications should go hand in hand with nonpharmacologic treatment.
When medications are unavoidable, internist Douglas S. Paauw, MD, starts with topical injections at the site of the pain — a troublesome joint, for example — rather than systemic medications that affect multiple organs and the brain.
“We try not to flood their body with meds” for localized problems, Dr. Paauw said, whose goal when treating elderly patients with pain is to improve their daily functioning and quality of life.
Dr. Paauw works at the University of Washington in Seattle and treats people who are approaching 100 years old. As some of his patients have grown older, Dr. Paauw’s interest in effective pain management has grown; he thinks that all internists and family medicine physician need to know how to manage chronic pain in their eldest patients.
“Were you able to play with your grandkid? Were you able to go grocery shopping? Were you able to take a walk outside?” These are the kinds of improvements Dr. Paauw hopes to see in older patients, recognizing that the wear and tear of life — orthopedic stresses or healed fractures that cause lingering pain — make it impossible for many older people to be pain free.
Pain is often spread throughout the body rather than focusing at one point, which requires systemic medications if physical therapy and similar approaches have not reduced pain. Per American Geriatrics Society (AGS) guidelines, in this situation Dr. Paauw starts with acetaminophen (Tylenol) as the lowest-risk systemic pain treatment.
Dr. Pauuw often counsels older patients to begin with 2 grams/day of acetaminophen and then progress to 3 grams if the lower dose has manageable side effects, rather than the standard dose of 4 grams that he feels is geared toward younger patients.
When acetaminophen doesn’t reduce pain sufficiently, or aggravates inflammation, Dr. Paauw may use the nerve pain medication pregabalin, or the antidepressant duloxetine — especially if the pain appears to be neuropathic.
Tricyclic antidepressants used to be recommended for neuropathic pain in older adults, but are now on the AGS’s Beers Criteria of drugs to avoid in elderly patients due to risk of causing dizziness or cardiac stress. Dr. Paauw might still use a tricyclic, but only after a careful risk-benefit analysis.
Nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen (Motrin) or naproxen (Aleve) could work in short bursts, Dr. Paauw said, although they may cause stomach bleeding or kidney damage in older patients.
This is why NSAIDs are not recommended by the AGS for chronic pain management. And opioids like oxycodone don’t work long at low doses, often leading to dose escalation and addiction.
“The American Geriatrics Society really puts opioids down at the bottom of the list,” Dr. Paauw said, to be used “judiciously and rarely.”
Opioids may interact with other drugs to increase risk of a fall, Dr. Meier added, making them inadvisable for older patients who live alone.
“That’s why knowing something about buprenorphine is so important,” Dr. Meier said.
Dr. Meier and Dr. Paauw are on the editorial board for Internal Medicine News. Dr. Merlin is a trainer for the Center to Advance Palliative Care, which Dr. Meier founded.
Some degree of pain is inevitable in older individuals, and as people pass 80 years of age, the harms of medications used to control chronic pain increase. Pain-reducing medication use in this age group may cause inflammation, gastric bleeding, kidney damage, or constipation.
These risks may lead some clinicians to avoid aggressive pain treatment in their eldest patients, resulting in unnecessary suffering.
“Pain causes harm beyond just the physical suffering associated with it,” said Diane Meier, MD, a geriatrician and palliative care specialist at Mount Sinai Medicine in New York City who treats many people in their 80s and 90s.
Downstream effects of untreated pain could include a loss of mobility and isolation, Dr. Meier said. And, as these harms are mounting, some clinicians may avoid using an analgesic that could bring great relief: buprenorphine.
“People think about buprenorphine like they think about methadone,” Dr. Meier said, as something prescribed to treat substance use disorder. In reality, it is an effective analgesic in other situations.
Buprenorphine is better at treating chronic pain than other opioids that carry a higher addiction risk and often cause constipation in elderly patients. Buprenorphine is easier on the kidneys and has a lower addiction risk than opioids like oxycodone.
The transdermal patch form of buprenorphine (Butrans, PurduePharma) is changed weekly and starts at low doses.
“There’s an adage in geriatrics: start low and go slow,” said Jessica Merlin, MD, PhD, a palliative care and addiction medicine physician at the University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania.
Dr. Merlin recommends beginning elderly patients with chronic pain on a 10-microgram/hour dose of Butrans, among the lowest doses available. Physicians could monitor side effects, which will generally be mild, with the aim of never increasing the dose if pain is managed.
Nonpharmacologic Remedies, Drug Considerations
“Nonpharmacologic therapy is very underutilized,” Dr. Merlin said, even though multiple alternatives to medications can improve chronic pain symptoms at any age.
Cognitive-behavioral therapy or acceptance and commitment therapy can both help people reduce the impact of pain, Dr. Merlin said. And for people who can do so, physical therapy programs, yoga, or tai chi are all ways to strengthen the body’s defenses against pain, Dr. Merlin added.
Sometimes medication is necessary, however.
“You can’t get an older person to participate in rehab if they are in severe pain,” Dr. Meier said, adding that judicious use of medications should go hand in hand with nonpharmacologic treatment.
When medications are unavoidable, internist Douglas S. Paauw, MD, starts with topical injections at the site of the pain — a troublesome joint, for example — rather than systemic medications that affect multiple organs and the brain.
“We try not to flood their body with meds” for localized problems, Dr. Paauw said, whose goal when treating elderly patients with pain is to improve their daily functioning and quality of life.
Dr. Paauw works at the University of Washington in Seattle and treats people who are approaching 100 years old. As some of his patients have grown older, Dr. Paauw’s interest in effective pain management has grown; he thinks that all internists and family medicine physician need to know how to manage chronic pain in their eldest patients.
“Were you able to play with your grandkid? Were you able to go grocery shopping? Were you able to take a walk outside?” These are the kinds of improvements Dr. Paauw hopes to see in older patients, recognizing that the wear and tear of life — orthopedic stresses or healed fractures that cause lingering pain — make it impossible for many older people to be pain free.
Pain is often spread throughout the body rather than focusing at one point, which requires systemic medications if physical therapy and similar approaches have not reduced pain. Per American Geriatrics Society (AGS) guidelines, in this situation Dr. Paauw starts with acetaminophen (Tylenol) as the lowest-risk systemic pain treatment.
Dr. Pauuw often counsels older patients to begin with 2 grams/day of acetaminophen and then progress to 3 grams if the lower dose has manageable side effects, rather than the standard dose of 4 grams that he feels is geared toward younger patients.
When acetaminophen doesn’t reduce pain sufficiently, or aggravates inflammation, Dr. Paauw may use the nerve pain medication pregabalin, or the antidepressant duloxetine — especially if the pain appears to be neuropathic.
Tricyclic antidepressants used to be recommended for neuropathic pain in older adults, but are now on the AGS’s Beers Criteria of drugs to avoid in elderly patients due to risk of causing dizziness or cardiac stress. Dr. Paauw might still use a tricyclic, but only after a careful risk-benefit analysis.
Nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen (Motrin) or naproxen (Aleve) could work in short bursts, Dr. Paauw said, although they may cause stomach bleeding or kidney damage in older patients.
This is why NSAIDs are not recommended by the AGS for chronic pain management. And opioids like oxycodone don’t work long at low doses, often leading to dose escalation and addiction.
“The American Geriatrics Society really puts opioids down at the bottom of the list,” Dr. Paauw said, to be used “judiciously and rarely.”
Opioids may interact with other drugs to increase risk of a fall, Dr. Meier added, making them inadvisable for older patients who live alone.
“That’s why knowing something about buprenorphine is so important,” Dr. Meier said.
Dr. Meier and Dr. Paauw are on the editorial board for Internal Medicine News. Dr. Merlin is a trainer for the Center to Advance Palliative Care, which Dr. Meier founded.
Managing Agitation in Alzheimer’s Disease: Five Things to Know
Agitation is a neuropsychiatric symptom in patients with Alzheimer’s disease (AD), the most common form of dementia. The prevalence of this symptom is about 40%-65%, with the higher end of the range applying to patients who have moderate to severe dementia. . The DICE approach is a collaborative process for managing behavioral symptoms in dementia, wherein the caregiver describes the behaviors, the provider investigates the etiology, the provider and caregiver create a treatment plan, and the provider evaluates the outcome of the interventions. We use this widely adopted approach as the framework for discussing recent advances in the management of agitation.
Here are five things to know about managing agitation in AD.
1. There is a new operational definition for agitation in dementia.
Agitation in dementia is a syndrome that encompasses specific behaviors across all dementia types. The 2023 operational definition of agitation in dementia by the International Psychogeriatric Association (IPA) includes three domains: excessive motor activity (including pacing, rocking, restlessness, and performing repetitious mannerisms), verbal aggression (including using profanity, screaming, and shouting), and physical aggression (including interpersonal aggression and mishandling or destruction of property). These behaviors must be persistent or recurrent for at least 2 weeks or represent a dramatic change from the person’s baseline behavior, must be associated with excessive distress or disability beyond what is caused by the cognitive impairment itself, and result in significant impairment in at least one of the three specified functional domains. Behavioral symptoms in dementia frequently co-occur, which affects treatment and prognosis. For instance, the risk for stroke associated with antipsychotic treatments appears to be higher in dementia-related psychosis without agitation than in agitation alone or in psychosis with agitation. Therefore, the use of a rating scale such as the Neuropsychiatric Inventory–Questionnaire (NPI-Q), which takes 5 minutes or less to administer, is recommended to identify and track behavioral symptoms and caregiver distress.
2. The etiology of agitation in dementia may be multifactorial.
It is important in every case to identify all underlying etiologies so that presumed causal and/or exacerbating factors are not inadvertently missed. Agitation may be a means of communicating distress owing to unmet needs or a patient-environment mismatch (function-focused approach) or may be a direct consequence of the dementia itself (behavioral-symptom approach). These approaches are not mutually exclusive. A patient can present with agitation as a direct consequence of dementia and inadequately treated pain concurrently.
The new IPA definition specifies several exclusion criteria for agitation in dementia, including underlying medical conditions, delirium, substance use, and suboptimal care conditions. It is especially crucial to accurately identify delirium because dementia is an independent risk factor for delirium, which in turn may accelerate the progression of cognitive and functional decline. Even subsyndromal delirium in older adults leads to a higher 3-year mortality rate that is comparable to that seen in delirium. Older adults with acute-onset agitation in the context of dementia should undergo a comprehensive assessment for delirium, as agitation may be the only indication of a serious underlying medical condition.
3. Nonpharmacologic interventions should be used whenever possible.
The wider adoption of nonpharmacologic interventions in clinical practice has been greatly limited by the heterogeneity in study protocols, including in selection of participants, in the types of dementias included, and in defining and applying the intervention strategies. Nevertheless, there is general consensus that individualized behavioral strategies that build on the patients’ interests and preserved abilities are more effective, at least in the short term. Patients best suited for these interventions are those with less cognitive decline, better communication skills, less impairment in activities of daily living, and higher responsiveness. A systematic review of systematic reviews found music therapy to be the most effective intervention for reducing agitation and aggression in dementia, along with behavioral management techniques when supervised by healthcare professionals. On the other hand, physical restraints are best avoided, as their use in hospitalized patients has been associated with longer stays, higher costs, lower odds of being discharged to home, and in long-term care patients with longer stays, with increased risk for medical complications and functional decline.
4. Antidepressants are not all equally safe or efficacious in managing agitation.
In a network meta-analysis that looked at the effects of several antidepressants on agitation in dementia, citalopram had just under 95% probability of efficacy and was the only antidepressant that was significantly more efficacious than placebo. In the multicenter CitAD trial, citalopram was efficacious and well tolerated for the treatment of agitation in AD, but the mean dose of citalopram used, 30 mg/d, was higher than the maximum dose of 20 mg/d recommended by the US Food and Drug Administration (FDA) in those aged 60 years or above. The optimal candidates for citalopram were those under the age of 85 with mild to moderate AD and mild to moderate nonpsychotic agitation, and it took up to 9 weeks for it to be fully effective. Due to the risk for dose-dependent QTc prolongation with citalopram, a baseline ECG must be done, and a second ECG is recommended if a clinical decision is made to exceed the recommended maximum daily dose. In the CitAD trial, 66% of patients in the citalopram arm received cholinesterase inhibitors concurrently while 44% received memantine, so these symptomatic treatments for AD should not be stopped solely for initiating a citalopram trial.
The antiagitation effect of citalopram may well be a class effect of all selective serotonin reuptake inhibitors (SSRIs), given that there is also evidence favoring the use of sertraline and escitalopram. The S-CitAD trial, the first large, randomized controlled study of escitalopram for the treatment of agitation in dementia, is expected to announce its top-line results sometime this year. However, not all antidepressant classes appear to be equally efficacious or safe. In the large, 12-week randomized placebo-controlled trial SYMBAD, mirtazapine was not only ineffective in treating nonpsychotic agitation in AD but was also associated with a higher mortality rate that just missed statistical significance. Trazodone is also often used for treating agitation, but there is insufficient evidence regarding efficacy and a high probability of adverse effects, even at low doses.
5. Antipsychotics may be effective drugs for treating severe dementia-related agitation.
The CATIE-AD study found that the small beneficial effects of antipsychotics for treating agitation and psychosis in AD were offset by their adverse effects and high discontinuation rates, and the FDA-imposed boxed warnings in 2005 and 2008 cautioned against the use of both first- and second-generation antipsychotics to manage dementia-related psychosis owing to an increased risk for death. Subsequently, the quest for safer and more effective alternatives culminated in the FDA approval of brexpiprazole in 2023 for the treatment of agitation in AD, but the black box warning was left in place. Three randomized controlled trials found brexpiprazole to be relatively safe, with statistically significant improvement in agitation. It was especially efficacious for severe agitation, but there is controversy about whether such improvement is clinically meaningful and whether brexpiprazole is truly superior to other antipsychotics for treating dementia-related agitation. As in the previously mentioned citalopram studies, most patients in the brexpiprazole studies received the drug as an add-on to memantine and/or a cholinesterase inhibitor, and it was proven effective over a period of up to 12 weeks across the three trials. Regarding other antipsychotics, aripiprazole and risperidone have been shown to be effective in treating agitation in patients with mixed dementia, but risperidone has also been associated with the highest risk for strokes (about 80% probability). Unfortunately, an unintended consequence of the boxed warnings on antipsychotics has been an increase in off-label substitution of psychotropic drugs with unproven efficacy and a questionable safety profile, such as valproic acid preparations, that have been linked to an increased short-term risk for accelerated brain volume loss and rapid cognitive decline, as well as a higher risk for mortality.
Lisa M. Wise, assistant professor, Psychiatry, at Oregon Health & Science University, and staff psychiatrist, Department of Psychiatry, Portland VA Medical Center, Portland, Oregon, and Vimal M. Aga, adjunct assistant professor, Department of Neurology, Oregon Health & Science University, and geriatric psychiatrist, Layton Aging and Alzheimer’s Disease Center, Portland, Oregon, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Agitation is a neuropsychiatric symptom in patients with Alzheimer’s disease (AD), the most common form of dementia. The prevalence of this symptom is about 40%-65%, with the higher end of the range applying to patients who have moderate to severe dementia. . The DICE approach is a collaborative process for managing behavioral symptoms in dementia, wherein the caregiver describes the behaviors, the provider investigates the etiology, the provider and caregiver create a treatment plan, and the provider evaluates the outcome of the interventions. We use this widely adopted approach as the framework for discussing recent advances in the management of agitation.
Here are five things to know about managing agitation in AD.
1. There is a new operational definition for agitation in dementia.
Agitation in dementia is a syndrome that encompasses specific behaviors across all dementia types. The 2023 operational definition of agitation in dementia by the International Psychogeriatric Association (IPA) includes three domains: excessive motor activity (including pacing, rocking, restlessness, and performing repetitious mannerisms), verbal aggression (including using profanity, screaming, and shouting), and physical aggression (including interpersonal aggression and mishandling or destruction of property). These behaviors must be persistent or recurrent for at least 2 weeks or represent a dramatic change from the person’s baseline behavior, must be associated with excessive distress or disability beyond what is caused by the cognitive impairment itself, and result in significant impairment in at least one of the three specified functional domains. Behavioral symptoms in dementia frequently co-occur, which affects treatment and prognosis. For instance, the risk for stroke associated with antipsychotic treatments appears to be higher in dementia-related psychosis without agitation than in agitation alone or in psychosis with agitation. Therefore, the use of a rating scale such as the Neuropsychiatric Inventory–Questionnaire (NPI-Q), which takes 5 minutes or less to administer, is recommended to identify and track behavioral symptoms and caregiver distress.
2. The etiology of agitation in dementia may be multifactorial.
It is important in every case to identify all underlying etiologies so that presumed causal and/or exacerbating factors are not inadvertently missed. Agitation may be a means of communicating distress owing to unmet needs or a patient-environment mismatch (function-focused approach) or may be a direct consequence of the dementia itself (behavioral-symptom approach). These approaches are not mutually exclusive. A patient can present with agitation as a direct consequence of dementia and inadequately treated pain concurrently.
The new IPA definition specifies several exclusion criteria for agitation in dementia, including underlying medical conditions, delirium, substance use, and suboptimal care conditions. It is especially crucial to accurately identify delirium because dementia is an independent risk factor for delirium, which in turn may accelerate the progression of cognitive and functional decline. Even subsyndromal delirium in older adults leads to a higher 3-year mortality rate that is comparable to that seen in delirium. Older adults with acute-onset agitation in the context of dementia should undergo a comprehensive assessment for delirium, as agitation may be the only indication of a serious underlying medical condition.
3. Nonpharmacologic interventions should be used whenever possible.
The wider adoption of nonpharmacologic interventions in clinical practice has been greatly limited by the heterogeneity in study protocols, including in selection of participants, in the types of dementias included, and in defining and applying the intervention strategies. Nevertheless, there is general consensus that individualized behavioral strategies that build on the patients’ interests and preserved abilities are more effective, at least in the short term. Patients best suited for these interventions are those with less cognitive decline, better communication skills, less impairment in activities of daily living, and higher responsiveness. A systematic review of systematic reviews found music therapy to be the most effective intervention for reducing agitation and aggression in dementia, along with behavioral management techniques when supervised by healthcare professionals. On the other hand, physical restraints are best avoided, as their use in hospitalized patients has been associated with longer stays, higher costs, lower odds of being discharged to home, and in long-term care patients with longer stays, with increased risk for medical complications and functional decline.
4. Antidepressants are not all equally safe or efficacious in managing agitation.
In a network meta-analysis that looked at the effects of several antidepressants on agitation in dementia, citalopram had just under 95% probability of efficacy and was the only antidepressant that was significantly more efficacious than placebo. In the multicenter CitAD trial, citalopram was efficacious and well tolerated for the treatment of agitation in AD, but the mean dose of citalopram used, 30 mg/d, was higher than the maximum dose of 20 mg/d recommended by the US Food and Drug Administration (FDA) in those aged 60 years or above. The optimal candidates for citalopram were those under the age of 85 with mild to moderate AD and mild to moderate nonpsychotic agitation, and it took up to 9 weeks for it to be fully effective. Due to the risk for dose-dependent QTc prolongation with citalopram, a baseline ECG must be done, and a second ECG is recommended if a clinical decision is made to exceed the recommended maximum daily dose. In the CitAD trial, 66% of patients in the citalopram arm received cholinesterase inhibitors concurrently while 44% received memantine, so these symptomatic treatments for AD should not be stopped solely for initiating a citalopram trial.
The antiagitation effect of citalopram may well be a class effect of all selective serotonin reuptake inhibitors (SSRIs), given that there is also evidence favoring the use of sertraline and escitalopram. The S-CitAD trial, the first large, randomized controlled study of escitalopram for the treatment of agitation in dementia, is expected to announce its top-line results sometime this year. However, not all antidepressant classes appear to be equally efficacious or safe. In the large, 12-week randomized placebo-controlled trial SYMBAD, mirtazapine was not only ineffective in treating nonpsychotic agitation in AD but was also associated with a higher mortality rate that just missed statistical significance. Trazodone is also often used for treating agitation, but there is insufficient evidence regarding efficacy and a high probability of adverse effects, even at low doses.
5. Antipsychotics may be effective drugs for treating severe dementia-related agitation.
The CATIE-AD study found that the small beneficial effects of antipsychotics for treating agitation and psychosis in AD were offset by their adverse effects and high discontinuation rates, and the FDA-imposed boxed warnings in 2005 and 2008 cautioned against the use of both first- and second-generation antipsychotics to manage dementia-related psychosis owing to an increased risk for death. Subsequently, the quest for safer and more effective alternatives culminated in the FDA approval of brexpiprazole in 2023 for the treatment of agitation in AD, but the black box warning was left in place. Three randomized controlled trials found brexpiprazole to be relatively safe, with statistically significant improvement in agitation. It was especially efficacious for severe agitation, but there is controversy about whether such improvement is clinically meaningful and whether brexpiprazole is truly superior to other antipsychotics for treating dementia-related agitation. As in the previously mentioned citalopram studies, most patients in the brexpiprazole studies received the drug as an add-on to memantine and/or a cholinesterase inhibitor, and it was proven effective over a period of up to 12 weeks across the three trials. Regarding other antipsychotics, aripiprazole and risperidone have been shown to be effective in treating agitation in patients with mixed dementia, but risperidone has also been associated with the highest risk for strokes (about 80% probability). Unfortunately, an unintended consequence of the boxed warnings on antipsychotics has been an increase in off-label substitution of psychotropic drugs with unproven efficacy and a questionable safety profile, such as valproic acid preparations, that have been linked to an increased short-term risk for accelerated brain volume loss and rapid cognitive decline, as well as a higher risk for mortality.
Lisa M. Wise, assistant professor, Psychiatry, at Oregon Health & Science University, and staff psychiatrist, Department of Psychiatry, Portland VA Medical Center, Portland, Oregon, and Vimal M. Aga, adjunct assistant professor, Department of Neurology, Oregon Health & Science University, and geriatric psychiatrist, Layton Aging and Alzheimer’s Disease Center, Portland, Oregon, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Agitation is a neuropsychiatric symptom in patients with Alzheimer’s disease (AD), the most common form of dementia. The prevalence of this symptom is about 40%-65%, with the higher end of the range applying to patients who have moderate to severe dementia. . The DICE approach is a collaborative process for managing behavioral symptoms in dementia, wherein the caregiver describes the behaviors, the provider investigates the etiology, the provider and caregiver create a treatment plan, and the provider evaluates the outcome of the interventions. We use this widely adopted approach as the framework for discussing recent advances in the management of agitation.
Here are five things to know about managing agitation in AD.
1. There is a new operational definition for agitation in dementia.
Agitation in dementia is a syndrome that encompasses specific behaviors across all dementia types. The 2023 operational definition of agitation in dementia by the International Psychogeriatric Association (IPA) includes three domains: excessive motor activity (including pacing, rocking, restlessness, and performing repetitious mannerisms), verbal aggression (including using profanity, screaming, and shouting), and physical aggression (including interpersonal aggression and mishandling or destruction of property). These behaviors must be persistent or recurrent for at least 2 weeks or represent a dramatic change from the person’s baseline behavior, must be associated with excessive distress or disability beyond what is caused by the cognitive impairment itself, and result in significant impairment in at least one of the three specified functional domains. Behavioral symptoms in dementia frequently co-occur, which affects treatment and prognosis. For instance, the risk for stroke associated with antipsychotic treatments appears to be higher in dementia-related psychosis without agitation than in agitation alone or in psychosis with agitation. Therefore, the use of a rating scale such as the Neuropsychiatric Inventory–Questionnaire (NPI-Q), which takes 5 minutes or less to administer, is recommended to identify and track behavioral symptoms and caregiver distress.
2. The etiology of agitation in dementia may be multifactorial.
It is important in every case to identify all underlying etiologies so that presumed causal and/or exacerbating factors are not inadvertently missed. Agitation may be a means of communicating distress owing to unmet needs or a patient-environment mismatch (function-focused approach) or may be a direct consequence of the dementia itself (behavioral-symptom approach). These approaches are not mutually exclusive. A patient can present with agitation as a direct consequence of dementia and inadequately treated pain concurrently.
The new IPA definition specifies several exclusion criteria for agitation in dementia, including underlying medical conditions, delirium, substance use, and suboptimal care conditions. It is especially crucial to accurately identify delirium because dementia is an independent risk factor for delirium, which in turn may accelerate the progression of cognitive and functional decline. Even subsyndromal delirium in older adults leads to a higher 3-year mortality rate that is comparable to that seen in delirium. Older adults with acute-onset agitation in the context of dementia should undergo a comprehensive assessment for delirium, as agitation may be the only indication of a serious underlying medical condition.
3. Nonpharmacologic interventions should be used whenever possible.
The wider adoption of nonpharmacologic interventions in clinical practice has been greatly limited by the heterogeneity in study protocols, including in selection of participants, in the types of dementias included, and in defining and applying the intervention strategies. Nevertheless, there is general consensus that individualized behavioral strategies that build on the patients’ interests and preserved abilities are more effective, at least in the short term. Patients best suited for these interventions are those with less cognitive decline, better communication skills, less impairment in activities of daily living, and higher responsiveness. A systematic review of systematic reviews found music therapy to be the most effective intervention for reducing agitation and aggression in dementia, along with behavioral management techniques when supervised by healthcare professionals. On the other hand, physical restraints are best avoided, as their use in hospitalized patients has been associated with longer stays, higher costs, lower odds of being discharged to home, and in long-term care patients with longer stays, with increased risk for medical complications and functional decline.
4. Antidepressants are not all equally safe or efficacious in managing agitation.
In a network meta-analysis that looked at the effects of several antidepressants on agitation in dementia, citalopram had just under 95% probability of efficacy and was the only antidepressant that was significantly more efficacious than placebo. In the multicenter CitAD trial, citalopram was efficacious and well tolerated for the treatment of agitation in AD, but the mean dose of citalopram used, 30 mg/d, was higher than the maximum dose of 20 mg/d recommended by the US Food and Drug Administration (FDA) in those aged 60 years or above. The optimal candidates for citalopram were those under the age of 85 with mild to moderate AD and mild to moderate nonpsychotic agitation, and it took up to 9 weeks for it to be fully effective. Due to the risk for dose-dependent QTc prolongation with citalopram, a baseline ECG must be done, and a second ECG is recommended if a clinical decision is made to exceed the recommended maximum daily dose. In the CitAD trial, 66% of patients in the citalopram arm received cholinesterase inhibitors concurrently while 44% received memantine, so these symptomatic treatments for AD should not be stopped solely for initiating a citalopram trial.
The antiagitation effect of citalopram may well be a class effect of all selective serotonin reuptake inhibitors (SSRIs), given that there is also evidence favoring the use of sertraline and escitalopram. The S-CitAD trial, the first large, randomized controlled study of escitalopram for the treatment of agitation in dementia, is expected to announce its top-line results sometime this year. However, not all antidepressant classes appear to be equally efficacious or safe. In the large, 12-week randomized placebo-controlled trial SYMBAD, mirtazapine was not only ineffective in treating nonpsychotic agitation in AD but was also associated with a higher mortality rate that just missed statistical significance. Trazodone is also often used for treating agitation, but there is insufficient evidence regarding efficacy and a high probability of adverse effects, even at low doses.
5. Antipsychotics may be effective drugs for treating severe dementia-related agitation.
The CATIE-AD study found that the small beneficial effects of antipsychotics for treating agitation and psychosis in AD were offset by their adverse effects and high discontinuation rates, and the FDA-imposed boxed warnings in 2005 and 2008 cautioned against the use of both first- and second-generation antipsychotics to manage dementia-related psychosis owing to an increased risk for death. Subsequently, the quest for safer and more effective alternatives culminated in the FDA approval of brexpiprazole in 2023 for the treatment of agitation in AD, but the black box warning was left in place. Three randomized controlled trials found brexpiprazole to be relatively safe, with statistically significant improvement in agitation. It was especially efficacious for severe agitation, but there is controversy about whether such improvement is clinically meaningful and whether brexpiprazole is truly superior to other antipsychotics for treating dementia-related agitation. As in the previously mentioned citalopram studies, most patients in the brexpiprazole studies received the drug as an add-on to memantine and/or a cholinesterase inhibitor, and it was proven effective over a period of up to 12 weeks across the three trials. Regarding other antipsychotics, aripiprazole and risperidone have been shown to be effective in treating agitation in patients with mixed dementia, but risperidone has also been associated with the highest risk for strokes (about 80% probability). Unfortunately, an unintended consequence of the boxed warnings on antipsychotics has been an increase in off-label substitution of psychotropic drugs with unproven efficacy and a questionable safety profile, such as valproic acid preparations, that have been linked to an increased short-term risk for accelerated brain volume loss and rapid cognitive decline, as well as a higher risk for mortality.
Lisa M. Wise, assistant professor, Psychiatry, at Oregon Health & Science University, and staff psychiatrist, Department of Psychiatry, Portland VA Medical Center, Portland, Oregon, and Vimal M. Aga, adjunct assistant professor, Department of Neurology, Oregon Health & Science University, and geriatric psychiatrist, Layton Aging and Alzheimer’s Disease Center, Portland, Oregon, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Trading TV Time for Physical Activity Boosts Healthy Aging
TOPLINE:
, but substituting it with any physical activity — or even sleeping, in case of women with inadequate sleep — may lead to better overall health.
METHODOLOGY:
- Previous studies have shown that replacing sedentary behavior with physical activity may improve mortality outcomes, but whether this increased lifespan is accompanied by better overall health remains an unanswered question.
- To understand the impact of sedentary behavior and physical activity on healthy aging, researchers analyzed data from the prospective cohort Nurses’ Health Study.
- They included 45,176 women aged > 50 years in 1992 (mean age, 59.2 years) who were free of major chronic diseases and were followed up for 20 years.
- In 1992, validated questionnaires were used to record exposure to sedentary behavior, different levels of physical activity, and sleep. The time spent watching television was the primary exposure in the sedentary behavior category.
- The main outcome was healthy aging, defined as survival to ≥ 70 years of age and maintenance of four domains of health — being free of 11 main chronic diseases and having no impairment of subjective memory, physical function, or mental health.
TAKEAWAY:
- At 20 years of follow-up, 8.6% of the women achieved healthy aging, while 41.4% had none of the 11 chronic diseases, 16.1% had no physical function impairment, 44.1% had no mental health limitation, and 51.9% reported no memory impairment.
- For each increase of 2 hours per day spent sitting and watching television, the odds of healthy aging dropped by 12% (95% confidence interval [CI], 7%-17%).
- Conversely, every additional 2 hours per day of low-level physical activity at work upped the odds of healthy aging by 6% (95% CI, 3%-9%); furthermore, each extra hour per day of standardized moderate to vigorous physical activity (normal pace walking or the equivalent) was associated with 14% higher odds (95% CI, 11%-16%) of healthy aging.
- In a theoretical modeling analysis, individuals could increase their odds of healthy aging by replacing 1 hour of television time per day with low levels of physical activity at home and work or with moderate to vigorous levels of physical activity — or even sleeping, for those who slept for ≤ 7 hours.
IN PRACTICE:
“These findings expand on the literature reporting that replacing sedentary behavior with light or moderate to vigorous physical activity is associated with decreased mortality by suggesting that this increased lifespan might be accompanied by better overall health,” the authors wrote.
SOURCE:
Hongying Shi, PhD, Department of Epidemiology and Health Statistics, School of Public Health, Wenzhou Medical University, Wenzhou, China, led this study, which was published online in JAMA Network Open.
LIMITATIONS:
The measures of different behaviors were self-reported and may, therefore, be less accurate than objective measurement methods. Measurement error may have attenuated the effect of low levels of physical activity. The single exposure assessment at baseline may not reflect the long-term pattern of these activities.
DISCLOSURES:
The lead author was supported by the National Social Science Foundation Project of China and the Zhejiang Provincial Philosophy and Social Sciences Planning Project. A co-author and the Nurses’ Health Study were supported by the US National Institutes of Health. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, but substituting it with any physical activity — or even sleeping, in case of women with inadequate sleep — may lead to better overall health.
METHODOLOGY:
- Previous studies have shown that replacing sedentary behavior with physical activity may improve mortality outcomes, but whether this increased lifespan is accompanied by better overall health remains an unanswered question.
- To understand the impact of sedentary behavior and physical activity on healthy aging, researchers analyzed data from the prospective cohort Nurses’ Health Study.
- They included 45,176 women aged > 50 years in 1992 (mean age, 59.2 years) who were free of major chronic diseases and were followed up for 20 years.
- In 1992, validated questionnaires were used to record exposure to sedentary behavior, different levels of physical activity, and sleep. The time spent watching television was the primary exposure in the sedentary behavior category.
- The main outcome was healthy aging, defined as survival to ≥ 70 years of age and maintenance of four domains of health — being free of 11 main chronic diseases and having no impairment of subjective memory, physical function, or mental health.
TAKEAWAY:
- At 20 years of follow-up, 8.6% of the women achieved healthy aging, while 41.4% had none of the 11 chronic diseases, 16.1% had no physical function impairment, 44.1% had no mental health limitation, and 51.9% reported no memory impairment.
- For each increase of 2 hours per day spent sitting and watching television, the odds of healthy aging dropped by 12% (95% confidence interval [CI], 7%-17%).
- Conversely, every additional 2 hours per day of low-level physical activity at work upped the odds of healthy aging by 6% (95% CI, 3%-9%); furthermore, each extra hour per day of standardized moderate to vigorous physical activity (normal pace walking or the equivalent) was associated with 14% higher odds (95% CI, 11%-16%) of healthy aging.
- In a theoretical modeling analysis, individuals could increase their odds of healthy aging by replacing 1 hour of television time per day with low levels of physical activity at home and work or with moderate to vigorous levels of physical activity — or even sleeping, for those who slept for ≤ 7 hours.
IN PRACTICE:
“These findings expand on the literature reporting that replacing sedentary behavior with light or moderate to vigorous physical activity is associated with decreased mortality by suggesting that this increased lifespan might be accompanied by better overall health,” the authors wrote.
SOURCE:
Hongying Shi, PhD, Department of Epidemiology and Health Statistics, School of Public Health, Wenzhou Medical University, Wenzhou, China, led this study, which was published online in JAMA Network Open.
LIMITATIONS:
The measures of different behaviors were self-reported and may, therefore, be less accurate than objective measurement methods. Measurement error may have attenuated the effect of low levels of physical activity. The single exposure assessment at baseline may not reflect the long-term pattern of these activities.
DISCLOSURES:
The lead author was supported by the National Social Science Foundation Project of China and the Zhejiang Provincial Philosophy and Social Sciences Planning Project. A co-author and the Nurses’ Health Study were supported by the US National Institutes of Health. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, but substituting it with any physical activity — or even sleeping, in case of women with inadequate sleep — may lead to better overall health.
METHODOLOGY:
- Previous studies have shown that replacing sedentary behavior with physical activity may improve mortality outcomes, but whether this increased lifespan is accompanied by better overall health remains an unanswered question.
- To understand the impact of sedentary behavior and physical activity on healthy aging, researchers analyzed data from the prospective cohort Nurses’ Health Study.
- They included 45,176 women aged > 50 years in 1992 (mean age, 59.2 years) who were free of major chronic diseases and were followed up for 20 years.
- In 1992, validated questionnaires were used to record exposure to sedentary behavior, different levels of physical activity, and sleep. The time spent watching television was the primary exposure in the sedentary behavior category.
- The main outcome was healthy aging, defined as survival to ≥ 70 years of age and maintenance of four domains of health — being free of 11 main chronic diseases and having no impairment of subjective memory, physical function, or mental health.
TAKEAWAY:
- At 20 years of follow-up, 8.6% of the women achieved healthy aging, while 41.4% had none of the 11 chronic diseases, 16.1% had no physical function impairment, 44.1% had no mental health limitation, and 51.9% reported no memory impairment.
- For each increase of 2 hours per day spent sitting and watching television, the odds of healthy aging dropped by 12% (95% confidence interval [CI], 7%-17%).
- Conversely, every additional 2 hours per day of low-level physical activity at work upped the odds of healthy aging by 6% (95% CI, 3%-9%); furthermore, each extra hour per day of standardized moderate to vigorous physical activity (normal pace walking or the equivalent) was associated with 14% higher odds (95% CI, 11%-16%) of healthy aging.
- In a theoretical modeling analysis, individuals could increase their odds of healthy aging by replacing 1 hour of television time per day with low levels of physical activity at home and work or with moderate to vigorous levels of physical activity — or even sleeping, for those who slept for ≤ 7 hours.
IN PRACTICE:
“These findings expand on the literature reporting that replacing sedentary behavior with light or moderate to vigorous physical activity is associated with decreased mortality by suggesting that this increased lifespan might be accompanied by better overall health,” the authors wrote.
SOURCE:
Hongying Shi, PhD, Department of Epidemiology and Health Statistics, School of Public Health, Wenzhou Medical University, Wenzhou, China, led this study, which was published online in JAMA Network Open.
LIMITATIONS:
The measures of different behaviors were self-reported and may, therefore, be less accurate than objective measurement methods. Measurement error may have attenuated the effect of low levels of physical activity. The single exposure assessment at baseline may not reflect the long-term pattern of these activities.
DISCLOSURES:
The lead author was supported by the National Social Science Foundation Project of China and the Zhejiang Provincial Philosophy and Social Sciences Planning Project. A co-author and the Nurses’ Health Study were supported by the US National Institutes of Health. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.