User login
Goodbye CHADSVASc: Sex Complicates Stroke Risk Scoring in AF
The European Society of Cardiology (ESC) caused a stir when they recommended in their latest atrial fibrillation (AF) management guideline that gender no longer be included in the decision to initiate oral anticoagulation therapy.
The move aims to level the playing field between men and women and follows a more nuanced understanding of stroke risk in patients with AF, said experts. It also acknowledges the lack of evidence in people receiving cross-sex hormone therapy.
In any case, the guidelines, developed in collaboration with the European Association for Cardio-Thoracic Surgery and published by the European Heart Journal on August 30, simply follow 2023’s US recommendations, they added.
One Size Does Not Fit All
So, what to the ESC guidelines actually say?
They underline that, if left untreated, the risk for ischemic stroke is increased fivefold in patients with AF, and the “default approach should therefore be to provide oral anticoagulation to all eligible AF patients, except those at low risk for incident stroke or thromboembolism.”
However, the authors note that there is a lack of strong evidence on how to apply the current risk scores to help inform that decision in real-world patients.
Dipak Kotecha, MBChB, PhD, Professor of Cardiology at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, and senior author of the ESC guidelines, said in an interview that “the available scores have a relatively poor ability to accurately predict which patients will have a stroke or thromboembolic event.”
Instead, he said “a much better approach is for healthcare professionals to look at each patient’s individual risk factors, using the risk scores to identify those patients that might not benefit from oral anticoagulant therapy.”
For these guidelines, the authors therefore wanted to “move away from a one-size-fits-all” approach, Kotecha said, and instead ensure that more patients can benefit from the new range of direct oral anticoagulants (DOACs) that are easier to take and with much lower chance of side effects or major bleeding.
To achieve this, they separated their clinical recommendations from any particular risk score, and instead focused on the practicalities of implementation.
Risk Modifier Vs Risk Factor
To explain their decision the authors highlight that “the most popular risk score” is the CHA2DS2–VASc, which gives a point for female sex, alongside factors such as congestive heart failure, hypertension, and diabetes mellitus, and a sliding scale of points for increasing age.
Kotecha pointed out the score was developed before the DOACs were available and may not account for how risk factors have changed in recent decades.
The result is that CHA2DS2–VASc gives the same number of points to an individual with heart failure or prior transient ischemic attack as to a woman aged less than 65 years, “but the magnitude of increased risk is not the same,” Usha Beth Tedrow, MD, Associate Professor of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, said in an interview.
As far back as 2018, it was known that “female sex is a risk modifier, rather than a risk factor for stroke in atrial fibrillation,” noted Jose Joglar, MD, lead author of the 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation said in an interview.
A Danish national registry study involving 239,671 AF patients treated between 1997 and 2015, nearly half of whom were women, showed that, at a CHA2DS2–VASc score of 0, the “risk of stroke between men and women is absolutely the same,” he said.
“It is not until after a CHA2DS2–VASc score of 2 that the curves start to separate,” Joglar, Program Director, Clinical Cardiac Electrophysiology Fellowship Program, The University of Texas Southwestern Medical Center, Dallas, continued, “but by then you have already made the decision to anticoagulate.”
More recently, Kotecha and colleagues conducted a population cohort study of the electronic healthcare records of UK primary care patients treated between 2005 and 2020, and identified 78,852 with AF; more than a third were women.
Their analysis, published on September 1, showed that women had a lower adjusted rate of the primary composite outcome of all-cause mortality, ischemic stroke, or arterial thromboembolism, driven by a reduced mortality rate.
“Removal of gender from clinical risk scoring could simplify the approach to which patients with AF should be offered oral anticoagulation,” Kotecha and colleagues concluded.
Joglar clarified that “women are at increased risk for stroke than men” overall, but by the time that risk “becomes manifest, other risk factors have come into play, and they have already met the criteria for anticoagulation.”
The authors of the latest ESC guideline therefore concluded that the “inclusion of gender complicates clinical practice both for healthcare professionals and patients.” Their solution was to remove the question of gender for decisions over initiating oral anticoagulant therapy in clinical practice altogether.
This includes individuals who identify as transgender or are undergoing sex hormone therapy, as all the experts interviewed by Medscape Medical News agreed that there is currently insufficient evidence to know if that affects stroke risk.
Instead, guidelines state that the drugs are “recommended in those with a CHA2DS2-VA score of 2 or more and should be considered in those with a CHA2DS2-VA score of 1, following a patient-centered and shared care approach.”
“Dropping the gender part of the risk score is not really a substantial change” from previous ESC or other guidelines, as different points were required in the past to recommend anticoagulants for women and men, Kotecha said, adding that “making the approach easier for clinicians may avoid penalizing women as well as nonbinary and transgender patients.”
Anne B. Curtis, MD, SUNY Distinguished Professor, Department of Medicine, Jacobs School of Medicine & Biomedical Sciences, University at Buffalo in New York, agreed.
Putting aside the question of female sex, she said that there are not a lot of people under the age of 65 years with “absolutely no risk factors,” and so, “if the only reason you would anticoagulate” someone of that age is because they are a woman that “doesn’t make a lot of sense to me.”
The ESC guidelines are “trying to say, ‘look at the other risk factors, and if anything is there, go ahead and anticoagulate,” Curtis said in an interview.
“It’s actually a very thoughtful decision,” Tedrow said, and not “intended to discount risk in women.” Rather, it’s a statement that acknowledges the problem of recommending anticoagulation therapy in women “for whom it is not appropriate.”
Joglar pointed out that that recommendation, although not characterized in the same way, was in fact included in the 2023 US guidelines.
“We wanted to use a more nuanced approach,” he said, and move away from using CHA2DS2–VASc as the prime determinant of whether to start oral anticoagulation and towards a magnitude risk assessment, in which female sex is seen as a risk modifier.
“The Europeans and the Americans are looking at the same data, so we often reach the same conclusions,” Joglar said, although “we sometimes use different wordings.”
Overall, Kotecha expressed the hope that the move “will lead to better implementation of guidelines, at the end of the day.”
“That’s all we can hope for: Patients will be offered a more individualized approach, leading to more appropriate use of treatment in the right patients.”
The newer direct oral anticoagulation is “a much simpler therapy,” he added. “There is very little monitoring, a similar risk of bleeding as aspirin, and yet the ability to largely prevent the high rate of stroke and thromboembolism associated with atrial fibrillation.”
“So, it’s a big ticket item for our communities and public health, particularly as atrial fibrillation is expected to double in prevalence in the next few decades and evidence is building that it can lead to vascular dementia in the long-term.”
No funding was declared. Kotecha declares relationships with Bayer, Protherics Medicines Development, Boston Scientific, Daiichi Sankyo, Boehringer Ingelheim, BMS-Pfizer Alliance, Amomed, MyoKardia. Curtis declared relationships with Janssen Pharmaceuticals, Medtronic, Abbott. Joglar declared no relevant relationships. Tedrow declared no relevant relationships.
A version of this article appeared on Medscape.com.
The European Society of Cardiology (ESC) caused a stir when they recommended in their latest atrial fibrillation (AF) management guideline that gender no longer be included in the decision to initiate oral anticoagulation therapy.
The move aims to level the playing field between men and women and follows a more nuanced understanding of stroke risk in patients with AF, said experts. It also acknowledges the lack of evidence in people receiving cross-sex hormone therapy.
In any case, the guidelines, developed in collaboration with the European Association for Cardio-Thoracic Surgery and published by the European Heart Journal on August 30, simply follow 2023’s US recommendations, they added.
One Size Does Not Fit All
So, what to the ESC guidelines actually say?
They underline that, if left untreated, the risk for ischemic stroke is increased fivefold in patients with AF, and the “default approach should therefore be to provide oral anticoagulation to all eligible AF patients, except those at low risk for incident stroke or thromboembolism.”
However, the authors note that there is a lack of strong evidence on how to apply the current risk scores to help inform that decision in real-world patients.
Dipak Kotecha, MBChB, PhD, Professor of Cardiology at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, and senior author of the ESC guidelines, said in an interview that “the available scores have a relatively poor ability to accurately predict which patients will have a stroke or thromboembolic event.”
Instead, he said “a much better approach is for healthcare professionals to look at each patient’s individual risk factors, using the risk scores to identify those patients that might not benefit from oral anticoagulant therapy.”
For these guidelines, the authors therefore wanted to “move away from a one-size-fits-all” approach, Kotecha said, and instead ensure that more patients can benefit from the new range of direct oral anticoagulants (DOACs) that are easier to take and with much lower chance of side effects or major bleeding.
To achieve this, they separated their clinical recommendations from any particular risk score, and instead focused on the practicalities of implementation.
Risk Modifier Vs Risk Factor
To explain their decision the authors highlight that “the most popular risk score” is the CHA2DS2–VASc, which gives a point for female sex, alongside factors such as congestive heart failure, hypertension, and diabetes mellitus, and a sliding scale of points for increasing age.
Kotecha pointed out the score was developed before the DOACs were available and may not account for how risk factors have changed in recent decades.
The result is that CHA2DS2–VASc gives the same number of points to an individual with heart failure or prior transient ischemic attack as to a woman aged less than 65 years, “but the magnitude of increased risk is not the same,” Usha Beth Tedrow, MD, Associate Professor of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, said in an interview.
As far back as 2018, it was known that “female sex is a risk modifier, rather than a risk factor for stroke in atrial fibrillation,” noted Jose Joglar, MD, lead author of the 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation said in an interview.
A Danish national registry study involving 239,671 AF patients treated between 1997 and 2015, nearly half of whom were women, showed that, at a CHA2DS2–VASc score of 0, the “risk of stroke between men and women is absolutely the same,” he said.
“It is not until after a CHA2DS2–VASc score of 2 that the curves start to separate,” Joglar, Program Director, Clinical Cardiac Electrophysiology Fellowship Program, The University of Texas Southwestern Medical Center, Dallas, continued, “but by then you have already made the decision to anticoagulate.”
More recently, Kotecha and colleagues conducted a population cohort study of the electronic healthcare records of UK primary care patients treated between 2005 and 2020, and identified 78,852 with AF; more than a third were women.
Their analysis, published on September 1, showed that women had a lower adjusted rate of the primary composite outcome of all-cause mortality, ischemic stroke, or arterial thromboembolism, driven by a reduced mortality rate.
“Removal of gender from clinical risk scoring could simplify the approach to which patients with AF should be offered oral anticoagulation,” Kotecha and colleagues concluded.
Joglar clarified that “women are at increased risk for stroke than men” overall, but by the time that risk “becomes manifest, other risk factors have come into play, and they have already met the criteria for anticoagulation.”
The authors of the latest ESC guideline therefore concluded that the “inclusion of gender complicates clinical practice both for healthcare professionals and patients.” Their solution was to remove the question of gender for decisions over initiating oral anticoagulant therapy in clinical practice altogether.
This includes individuals who identify as transgender or are undergoing sex hormone therapy, as all the experts interviewed by Medscape Medical News agreed that there is currently insufficient evidence to know if that affects stroke risk.
Instead, guidelines state that the drugs are “recommended in those with a CHA2DS2-VA score of 2 or more and should be considered in those with a CHA2DS2-VA score of 1, following a patient-centered and shared care approach.”
“Dropping the gender part of the risk score is not really a substantial change” from previous ESC or other guidelines, as different points were required in the past to recommend anticoagulants for women and men, Kotecha said, adding that “making the approach easier for clinicians may avoid penalizing women as well as nonbinary and transgender patients.”
Anne B. Curtis, MD, SUNY Distinguished Professor, Department of Medicine, Jacobs School of Medicine & Biomedical Sciences, University at Buffalo in New York, agreed.
Putting aside the question of female sex, she said that there are not a lot of people under the age of 65 years with “absolutely no risk factors,” and so, “if the only reason you would anticoagulate” someone of that age is because they are a woman that “doesn’t make a lot of sense to me.”
The ESC guidelines are “trying to say, ‘look at the other risk factors, and if anything is there, go ahead and anticoagulate,” Curtis said in an interview.
“It’s actually a very thoughtful decision,” Tedrow said, and not “intended to discount risk in women.” Rather, it’s a statement that acknowledges the problem of recommending anticoagulation therapy in women “for whom it is not appropriate.”
Joglar pointed out that that recommendation, although not characterized in the same way, was in fact included in the 2023 US guidelines.
“We wanted to use a more nuanced approach,” he said, and move away from using CHA2DS2–VASc as the prime determinant of whether to start oral anticoagulation and towards a magnitude risk assessment, in which female sex is seen as a risk modifier.
“The Europeans and the Americans are looking at the same data, so we often reach the same conclusions,” Joglar said, although “we sometimes use different wordings.”
Overall, Kotecha expressed the hope that the move “will lead to better implementation of guidelines, at the end of the day.”
“That’s all we can hope for: Patients will be offered a more individualized approach, leading to more appropriate use of treatment in the right patients.”
The newer direct oral anticoagulation is “a much simpler therapy,” he added. “There is very little monitoring, a similar risk of bleeding as aspirin, and yet the ability to largely prevent the high rate of stroke and thromboembolism associated with atrial fibrillation.”
“So, it’s a big ticket item for our communities and public health, particularly as atrial fibrillation is expected to double in prevalence in the next few decades and evidence is building that it can lead to vascular dementia in the long-term.”
No funding was declared. Kotecha declares relationships with Bayer, Protherics Medicines Development, Boston Scientific, Daiichi Sankyo, Boehringer Ingelheim, BMS-Pfizer Alliance, Amomed, MyoKardia. Curtis declared relationships with Janssen Pharmaceuticals, Medtronic, Abbott. Joglar declared no relevant relationships. Tedrow declared no relevant relationships.
A version of this article appeared on Medscape.com.
The European Society of Cardiology (ESC) caused a stir when they recommended in their latest atrial fibrillation (AF) management guideline that gender no longer be included in the decision to initiate oral anticoagulation therapy.
The move aims to level the playing field between men and women and follows a more nuanced understanding of stroke risk in patients with AF, said experts. It also acknowledges the lack of evidence in people receiving cross-sex hormone therapy.
In any case, the guidelines, developed in collaboration with the European Association for Cardio-Thoracic Surgery and published by the European Heart Journal on August 30, simply follow 2023’s US recommendations, they added.
One Size Does Not Fit All
So, what to the ESC guidelines actually say?
They underline that, if left untreated, the risk for ischemic stroke is increased fivefold in patients with AF, and the “default approach should therefore be to provide oral anticoagulation to all eligible AF patients, except those at low risk for incident stroke or thromboembolism.”
However, the authors note that there is a lack of strong evidence on how to apply the current risk scores to help inform that decision in real-world patients.
Dipak Kotecha, MBChB, PhD, Professor of Cardiology at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, and senior author of the ESC guidelines, said in an interview that “the available scores have a relatively poor ability to accurately predict which patients will have a stroke or thromboembolic event.”
Instead, he said “a much better approach is for healthcare professionals to look at each patient’s individual risk factors, using the risk scores to identify those patients that might not benefit from oral anticoagulant therapy.”
For these guidelines, the authors therefore wanted to “move away from a one-size-fits-all” approach, Kotecha said, and instead ensure that more patients can benefit from the new range of direct oral anticoagulants (DOACs) that are easier to take and with much lower chance of side effects or major bleeding.
To achieve this, they separated their clinical recommendations from any particular risk score, and instead focused on the practicalities of implementation.
Risk Modifier Vs Risk Factor
To explain their decision the authors highlight that “the most popular risk score” is the CHA2DS2–VASc, which gives a point for female sex, alongside factors such as congestive heart failure, hypertension, and diabetes mellitus, and a sliding scale of points for increasing age.
Kotecha pointed out the score was developed before the DOACs were available and may not account for how risk factors have changed in recent decades.
The result is that CHA2DS2–VASc gives the same number of points to an individual with heart failure or prior transient ischemic attack as to a woman aged less than 65 years, “but the magnitude of increased risk is not the same,” Usha Beth Tedrow, MD, Associate Professor of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, said in an interview.
As far back as 2018, it was known that “female sex is a risk modifier, rather than a risk factor for stroke in atrial fibrillation,” noted Jose Joglar, MD, lead author of the 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation said in an interview.
A Danish national registry study involving 239,671 AF patients treated between 1997 and 2015, nearly half of whom were women, showed that, at a CHA2DS2–VASc score of 0, the “risk of stroke between men and women is absolutely the same,” he said.
“It is not until after a CHA2DS2–VASc score of 2 that the curves start to separate,” Joglar, Program Director, Clinical Cardiac Electrophysiology Fellowship Program, The University of Texas Southwestern Medical Center, Dallas, continued, “but by then you have already made the decision to anticoagulate.”
More recently, Kotecha and colleagues conducted a population cohort study of the electronic healthcare records of UK primary care patients treated between 2005 and 2020, and identified 78,852 with AF; more than a third were women.
Their analysis, published on September 1, showed that women had a lower adjusted rate of the primary composite outcome of all-cause mortality, ischemic stroke, or arterial thromboembolism, driven by a reduced mortality rate.
“Removal of gender from clinical risk scoring could simplify the approach to which patients with AF should be offered oral anticoagulation,” Kotecha and colleagues concluded.
Joglar clarified that “women are at increased risk for stroke than men” overall, but by the time that risk “becomes manifest, other risk factors have come into play, and they have already met the criteria for anticoagulation.”
The authors of the latest ESC guideline therefore concluded that the “inclusion of gender complicates clinical practice both for healthcare professionals and patients.” Their solution was to remove the question of gender for decisions over initiating oral anticoagulant therapy in clinical practice altogether.
This includes individuals who identify as transgender or are undergoing sex hormone therapy, as all the experts interviewed by Medscape Medical News agreed that there is currently insufficient evidence to know if that affects stroke risk.
Instead, guidelines state that the drugs are “recommended in those with a CHA2DS2-VA score of 2 or more and should be considered in those with a CHA2DS2-VA score of 1, following a patient-centered and shared care approach.”
“Dropping the gender part of the risk score is not really a substantial change” from previous ESC or other guidelines, as different points were required in the past to recommend anticoagulants for women and men, Kotecha said, adding that “making the approach easier for clinicians may avoid penalizing women as well as nonbinary and transgender patients.”
Anne B. Curtis, MD, SUNY Distinguished Professor, Department of Medicine, Jacobs School of Medicine & Biomedical Sciences, University at Buffalo in New York, agreed.
Putting aside the question of female sex, she said that there are not a lot of people under the age of 65 years with “absolutely no risk factors,” and so, “if the only reason you would anticoagulate” someone of that age is because they are a woman that “doesn’t make a lot of sense to me.”
The ESC guidelines are “trying to say, ‘look at the other risk factors, and if anything is there, go ahead and anticoagulate,” Curtis said in an interview.
“It’s actually a very thoughtful decision,” Tedrow said, and not “intended to discount risk in women.” Rather, it’s a statement that acknowledges the problem of recommending anticoagulation therapy in women “for whom it is not appropriate.”
Joglar pointed out that that recommendation, although not characterized in the same way, was in fact included in the 2023 US guidelines.
“We wanted to use a more nuanced approach,” he said, and move away from using CHA2DS2–VASc as the prime determinant of whether to start oral anticoagulation and towards a magnitude risk assessment, in which female sex is seen as a risk modifier.
“The Europeans and the Americans are looking at the same data, so we often reach the same conclusions,” Joglar said, although “we sometimes use different wordings.”
Overall, Kotecha expressed the hope that the move “will lead to better implementation of guidelines, at the end of the day.”
“That’s all we can hope for: Patients will be offered a more individualized approach, leading to more appropriate use of treatment in the right patients.”
The newer direct oral anticoagulation is “a much simpler therapy,” he added. “There is very little monitoring, a similar risk of bleeding as aspirin, and yet the ability to largely prevent the high rate of stroke and thromboembolism associated with atrial fibrillation.”
“So, it’s a big ticket item for our communities and public health, particularly as atrial fibrillation is expected to double in prevalence in the next few decades and evidence is building that it can lead to vascular dementia in the long-term.”
No funding was declared. Kotecha declares relationships with Bayer, Protherics Medicines Development, Boston Scientific, Daiichi Sankyo, Boehringer Ingelheim, BMS-Pfizer Alliance, Amomed, MyoKardia. Curtis declared relationships with Janssen Pharmaceuticals, Medtronic, Abbott. Joglar declared no relevant relationships. Tedrow declared no relevant relationships.
A version of this article appeared on Medscape.com.
Vitamin K Supplementation Reduces Nocturnal Leg Cramps in Older Adults
TOPLINE:
Vitamin K supplementation significantly reduced the frequency, intensity, and duration of nocturnal leg cramps in older adults. No adverse events related to vitamin K were identified.
METHODOLOGY:
- Researchers conducted a multicenter, double-blind, placebo-controlled randomized clinical trial in China from September 2022 to December 2023.
- A total of 199 participants aged ≥ 65 years with at least two documented episodes of nocturnal leg cramps during a 2-week screening period were included.
- Participants were randomized in a 1:1 ratio to receive either 180 μg of vitamin K (menaquinone 7) or a placebo daily for 8 weeks.
- The primary outcome was the mean number of nocturnal leg cramps per week, while secondary outcomes were the duration and severity of muscle cramps.
- The ethics committees of Third People’s Hospital of Chengdu and Affiliated Hospital of North Sichuan Medical College approved the study, and all participants provided written informed consent.
TAKEAWAY:
- Vitamin K group experienced a significant reduction in the mean weekly frequency of cramps (mean difference, 2.60 [SD, 0.81] to 0.96 [SD, 1.41]) compared with the placebo group, which maintained a mean weekly frequency of 3.63 (SD, 2.20) (P < .001).
- The severity of nocturnal leg cramps decreased more in the vitamin K group (mean difference, −2.55 [SD, 2.12] points) than in the placebo group (mean difference, −1.24 [SD, 1.16] points).
- The duration of nocturnal leg cramps also decreased more in the vitamin K group (mean difference, −0.90 [SD, 0.88] minutes) than in the placebo group (mean difference, −0.32 [SD, 0.78] minutes).
- No adverse events related to vitamin K use were identified, indicating a good safety profile for the supplementation.
IN PRACTICE:
“Given the generally benign characteristics of NLCs, treatment modality must be both effective and safe, thus minimizing the risk of iatrogenic harm,” the study authors wrote.
SOURCE:
This study was led by Jing Tan, MD, the Third People’s Hospital of Chengdu in Chengdu, China. It was published online on October 28 in JAMA Internal Medicine.
LIMITATIONS:
This study did not investigate the quality of life or sleep, which could have provided additional insights into the impact of vitamin K on nocturnal leg cramps. The relatively mild nature of nocturnal leg cramps experienced by the participants may limit the generalizability of the findings to populations with more severe symptoms.
DISCLOSURES:
This study was supported by grants from China Health Promotion Foundation and the Third People’s Hospital of Chengdu Scientific Research Project. Tan disclosed receiving personal fees from BeiGene, AbbVie, Pfizer, Xian Janssen Pharmaceutical, and Takeda Pharmaceutical outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Vitamin K supplementation significantly reduced the frequency, intensity, and duration of nocturnal leg cramps in older adults. No adverse events related to vitamin K were identified.
METHODOLOGY:
- Researchers conducted a multicenter, double-blind, placebo-controlled randomized clinical trial in China from September 2022 to December 2023.
- A total of 199 participants aged ≥ 65 years with at least two documented episodes of nocturnal leg cramps during a 2-week screening period were included.
- Participants were randomized in a 1:1 ratio to receive either 180 μg of vitamin K (menaquinone 7) or a placebo daily for 8 weeks.
- The primary outcome was the mean number of nocturnal leg cramps per week, while secondary outcomes were the duration and severity of muscle cramps.
- The ethics committees of Third People’s Hospital of Chengdu and Affiliated Hospital of North Sichuan Medical College approved the study, and all participants provided written informed consent.
TAKEAWAY:
- Vitamin K group experienced a significant reduction in the mean weekly frequency of cramps (mean difference, 2.60 [SD, 0.81] to 0.96 [SD, 1.41]) compared with the placebo group, which maintained a mean weekly frequency of 3.63 (SD, 2.20) (P < .001).
- The severity of nocturnal leg cramps decreased more in the vitamin K group (mean difference, −2.55 [SD, 2.12] points) than in the placebo group (mean difference, −1.24 [SD, 1.16] points).
- The duration of nocturnal leg cramps also decreased more in the vitamin K group (mean difference, −0.90 [SD, 0.88] minutes) than in the placebo group (mean difference, −0.32 [SD, 0.78] minutes).
- No adverse events related to vitamin K use were identified, indicating a good safety profile for the supplementation.
IN PRACTICE:
“Given the generally benign characteristics of NLCs, treatment modality must be both effective and safe, thus minimizing the risk of iatrogenic harm,” the study authors wrote.
SOURCE:
This study was led by Jing Tan, MD, the Third People’s Hospital of Chengdu in Chengdu, China. It was published online on October 28 in JAMA Internal Medicine.
LIMITATIONS:
This study did not investigate the quality of life or sleep, which could have provided additional insights into the impact of vitamin K on nocturnal leg cramps. The relatively mild nature of nocturnal leg cramps experienced by the participants may limit the generalizability of the findings to populations with more severe symptoms.
DISCLOSURES:
This study was supported by grants from China Health Promotion Foundation and the Third People’s Hospital of Chengdu Scientific Research Project. Tan disclosed receiving personal fees from BeiGene, AbbVie, Pfizer, Xian Janssen Pharmaceutical, and Takeda Pharmaceutical outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Vitamin K supplementation significantly reduced the frequency, intensity, and duration of nocturnal leg cramps in older adults. No adverse events related to vitamin K were identified.
METHODOLOGY:
- Researchers conducted a multicenter, double-blind, placebo-controlled randomized clinical trial in China from September 2022 to December 2023.
- A total of 199 participants aged ≥ 65 years with at least two documented episodes of nocturnal leg cramps during a 2-week screening period were included.
- Participants were randomized in a 1:1 ratio to receive either 180 μg of vitamin K (menaquinone 7) or a placebo daily for 8 weeks.
- The primary outcome was the mean number of nocturnal leg cramps per week, while secondary outcomes were the duration and severity of muscle cramps.
- The ethics committees of Third People’s Hospital of Chengdu and Affiliated Hospital of North Sichuan Medical College approved the study, and all participants provided written informed consent.
TAKEAWAY:
- Vitamin K group experienced a significant reduction in the mean weekly frequency of cramps (mean difference, 2.60 [SD, 0.81] to 0.96 [SD, 1.41]) compared with the placebo group, which maintained a mean weekly frequency of 3.63 (SD, 2.20) (P < .001).
- The severity of nocturnal leg cramps decreased more in the vitamin K group (mean difference, −2.55 [SD, 2.12] points) than in the placebo group (mean difference, −1.24 [SD, 1.16] points).
- The duration of nocturnal leg cramps also decreased more in the vitamin K group (mean difference, −0.90 [SD, 0.88] minutes) than in the placebo group (mean difference, −0.32 [SD, 0.78] minutes).
- No adverse events related to vitamin K use were identified, indicating a good safety profile for the supplementation.
IN PRACTICE:
“Given the generally benign characteristics of NLCs, treatment modality must be both effective and safe, thus minimizing the risk of iatrogenic harm,” the study authors wrote.
SOURCE:
This study was led by Jing Tan, MD, the Third People’s Hospital of Chengdu in Chengdu, China. It was published online on October 28 in JAMA Internal Medicine.
LIMITATIONS:
This study did not investigate the quality of life or sleep, which could have provided additional insights into the impact of vitamin K on nocturnal leg cramps. The relatively mild nature of nocturnal leg cramps experienced by the participants may limit the generalizability of the findings to populations with more severe symptoms.
DISCLOSURES:
This study was supported by grants from China Health Promotion Foundation and the Third People’s Hospital of Chengdu Scientific Research Project. Tan disclosed receiving personal fees from BeiGene, AbbVie, Pfizer, Xian Janssen Pharmaceutical, and Takeda Pharmaceutical outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Heat Waves Pose Significant Health Risks for Dually Eligible Older Individuals
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
On Second Thought: Aspirin for Primary Prevention — What We Really Know
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Primary Care Physicians Underutilize Nonantibiotic Prophylaxis for Recurrent UTIs
While primary care physicians are generally comfortable prescribing vaginal estrogen therapy for recurrent urinary tract infections (UTIs), other nonantibiotic prophylactic options remain significantly underutilized, according to new research that highlights a crucial gap in antibiotic stewardship practices among primary care physicians.
UTIs are the most common bacterial infection in women of all ages, and an estimated 30%-40% of women will experience reinfection within 6 months. Recurrent UTI is typically defined as two or more infections within 6 months or a greater number of infections within a year, according to the American Academy of Family Physicians.
Antibiotics are the first line of defense in preventing and treating recurrent UTIs, but repeated and prolonged use could lead to antibiotic resistance.
Researchers at the University of North Carolina surveyed 40 primary care physicians at one academic medical center and found that 96% of primary care physicians prescribe vaginal estrogen therapy for recurrent UTI prevention, with 58% doing so “often.” Estrogen deficiency and urinary retention are strong contributors to infection.
However, 78% of physicians surveyed said they had never prescribed methenamine hippurate, and 85% said they had never prescribed D-mannose.
Physicians with specialized training in menopausal care felt more at ease prescribing vaginal estrogen therapy to patients with complex medical histories, such as those with a family history of breast cancer or endometrial cancer. This suggests that enhanced education could play a vital role in increasing comfort levels among general practitioners, said Lauren Tholemeier, MD, a urogynecology fellow at the University of North Carolina at Chapel Hill.
“Primary care physicians are the front line of managing patients with recurrent UTI,” said Tholemeier.
“There’s an opportunity for further education on, and even awareness of, methenamine hippurate and D-mannose as an option that has data behind it and can be included as a tool” for patient care, she said.
Indeed, physicians who saw six or more recurrent patients with UTI each month were more likely to prescribe methenamine hippurate, the study found, suggesting that familiarity with recurrent UTI cases can lead to greater confidence in employing alternative prophylactic strategies.
Tholemeier presented her research at the American Urogynecologic Society’s PFD Week in Washington, DC.
Expanding physician knowledge and utilization of all available nonantibiotic therapies can help them better care for patients who don’t necessarily have access to a subspecialist, Tholemeier said.
According to the American Urogynecologic Society’s best practice guidelines, there is limited evidence supporting routine use of D-mannose to prevent recurrent UTI. Methenamine hippurate, however, may be effective for short-term UTI prevention, according to the group.
By broadening the use of vaginal estrogen therapy, methenamine hippurate, and D-mannose, primary care physicians can help reduce reliance on antibiotics for recurrent UTI prevention — a practice that may contribute to growing antibiotic resistance, said Tholemeier.
“The end goal isn’t going to be to say that we should never prescribe antibiotics for UTI infection,” said Tholemeier, adding that, in some cases, physicians can consider using these other medications in conjunction with antibiotics.
“But it’s knowing they [clinicians] have some other options in their toolbox,” she said.
A version of this article first appeared on Medscape.com.
While primary care physicians are generally comfortable prescribing vaginal estrogen therapy for recurrent urinary tract infections (UTIs), other nonantibiotic prophylactic options remain significantly underutilized, according to new research that highlights a crucial gap in antibiotic stewardship practices among primary care physicians.
UTIs are the most common bacterial infection in women of all ages, and an estimated 30%-40% of women will experience reinfection within 6 months. Recurrent UTI is typically defined as two or more infections within 6 months or a greater number of infections within a year, according to the American Academy of Family Physicians.
Antibiotics are the first line of defense in preventing and treating recurrent UTIs, but repeated and prolonged use could lead to antibiotic resistance.
Researchers at the University of North Carolina surveyed 40 primary care physicians at one academic medical center and found that 96% of primary care physicians prescribe vaginal estrogen therapy for recurrent UTI prevention, with 58% doing so “often.” Estrogen deficiency and urinary retention are strong contributors to infection.
However, 78% of physicians surveyed said they had never prescribed methenamine hippurate, and 85% said they had never prescribed D-mannose.
Physicians with specialized training in menopausal care felt more at ease prescribing vaginal estrogen therapy to patients with complex medical histories, such as those with a family history of breast cancer or endometrial cancer. This suggests that enhanced education could play a vital role in increasing comfort levels among general practitioners, said Lauren Tholemeier, MD, a urogynecology fellow at the University of North Carolina at Chapel Hill.
“Primary care physicians are the front line of managing patients with recurrent UTI,” said Tholemeier.
“There’s an opportunity for further education on, and even awareness of, methenamine hippurate and D-mannose as an option that has data behind it and can be included as a tool” for patient care, she said.
Indeed, physicians who saw six or more recurrent patients with UTI each month were more likely to prescribe methenamine hippurate, the study found, suggesting that familiarity with recurrent UTI cases can lead to greater confidence in employing alternative prophylactic strategies.
Tholemeier presented her research at the American Urogynecologic Society’s PFD Week in Washington, DC.
Expanding physician knowledge and utilization of all available nonantibiotic therapies can help them better care for patients who don’t necessarily have access to a subspecialist, Tholemeier said.
According to the American Urogynecologic Society’s best practice guidelines, there is limited evidence supporting routine use of D-mannose to prevent recurrent UTI. Methenamine hippurate, however, may be effective for short-term UTI prevention, according to the group.
By broadening the use of vaginal estrogen therapy, methenamine hippurate, and D-mannose, primary care physicians can help reduce reliance on antibiotics for recurrent UTI prevention — a practice that may contribute to growing antibiotic resistance, said Tholemeier.
“The end goal isn’t going to be to say that we should never prescribe antibiotics for UTI infection,” said Tholemeier, adding that, in some cases, physicians can consider using these other medications in conjunction with antibiotics.
“But it’s knowing they [clinicians] have some other options in their toolbox,” she said.
A version of this article first appeared on Medscape.com.
While primary care physicians are generally comfortable prescribing vaginal estrogen therapy for recurrent urinary tract infections (UTIs), other nonantibiotic prophylactic options remain significantly underutilized, according to new research that highlights a crucial gap in antibiotic stewardship practices among primary care physicians.
UTIs are the most common bacterial infection in women of all ages, and an estimated 30%-40% of women will experience reinfection within 6 months. Recurrent UTI is typically defined as two or more infections within 6 months or a greater number of infections within a year, according to the American Academy of Family Physicians.
Antibiotics are the first line of defense in preventing and treating recurrent UTIs, but repeated and prolonged use could lead to antibiotic resistance.
Researchers at the University of North Carolina surveyed 40 primary care physicians at one academic medical center and found that 96% of primary care physicians prescribe vaginal estrogen therapy for recurrent UTI prevention, with 58% doing so “often.” Estrogen deficiency and urinary retention are strong contributors to infection.
However, 78% of physicians surveyed said they had never prescribed methenamine hippurate, and 85% said they had never prescribed D-mannose.
Physicians with specialized training in menopausal care felt more at ease prescribing vaginal estrogen therapy to patients with complex medical histories, such as those with a family history of breast cancer or endometrial cancer. This suggests that enhanced education could play a vital role in increasing comfort levels among general practitioners, said Lauren Tholemeier, MD, a urogynecology fellow at the University of North Carolina at Chapel Hill.
“Primary care physicians are the front line of managing patients with recurrent UTI,” said Tholemeier.
“There’s an opportunity for further education on, and even awareness of, methenamine hippurate and D-mannose as an option that has data behind it and can be included as a tool” for patient care, she said.
Indeed, physicians who saw six or more recurrent patients with UTI each month were more likely to prescribe methenamine hippurate, the study found, suggesting that familiarity with recurrent UTI cases can lead to greater confidence in employing alternative prophylactic strategies.
Tholemeier presented her research at the American Urogynecologic Society’s PFD Week in Washington, DC.
Expanding physician knowledge and utilization of all available nonantibiotic therapies can help them better care for patients who don’t necessarily have access to a subspecialist, Tholemeier said.
According to the American Urogynecologic Society’s best practice guidelines, there is limited evidence supporting routine use of D-mannose to prevent recurrent UTI. Methenamine hippurate, however, may be effective for short-term UTI prevention, according to the group.
By broadening the use of vaginal estrogen therapy, methenamine hippurate, and D-mannose, primary care physicians can help reduce reliance on antibiotics for recurrent UTI prevention — a practice that may contribute to growing antibiotic resistance, said Tholemeier.
“The end goal isn’t going to be to say that we should never prescribe antibiotics for UTI infection,” said Tholemeier, adding that, in some cases, physicians can consider using these other medications in conjunction with antibiotics.
“But it’s knowing they [clinicians] have some other options in their toolbox,” she said.
A version of this article first appeared on Medscape.com.
FROM PFD WEEK 2024
VHA Support for Home Health Agency Staff and Patients During Natural Disasters
As large-scale natural disasters become more common, health care coalitions and the engagement of health systems with local, state, and federal public health departments have effectively bolstered communities’ resilience via collective sharing and distribution of resources.1 These resources may include supplies and the dissemination of emergency information, education, and training.2 The COVID-19 pandemic demonstrated that larger health care systems including hospital networks and nursing homes are better connected to health care coalition resources than smaller, independent systems, such as community home health agencies.3 This leaves some organizations on their own to meet requirements that maintain continuity of care and support their patients and staff throughout a natural disaster.
Home health care workers play important roles in the care of older adults.4 Older adults experience high levels of disability and comorbidities that put them at risk during emergencies; they often require support from paid, family, and neighborhood caregivers to live independently.5 More than 9.3 million US adults receive paid care from 2.6 million home health care workers (eg, home health aides and personal care assistants).6 Many of these individuals are hired through small independent home health agencies (HHAs), while others may work directly for an individual. When neighborhood resources and family caregiving are disrupted during emergencies, the critical services these workers administer become even more essential to ensuring continued access to medical care and social services.
The importance of these services was underscored by the Centers for Medicare and Medicaid Services 2017 inclusion of HHAs in federal emergency preparedness guidelines.7,8 The fractured and decentralized nature of the home health care industry means many HHAs struggle to maintain continuous care during emergencies and protect their staff. HHAs, and health care workers in the home, are often isolated, under-resourced, and disconnected from broader emergency planning efforts. Additionally, home care jobs are largely part-time, unstable, and low paying, making the workers themselves vulnerable during emergencies.3,9-13
This is a significant issue for the Veterans Health Administration (VHA), which annually purchases 10.5 million home health care worker visits for 150,000 veterans from community-based HHAs to enable those individuals to live independently. Figure 1 illustrates the existing structure of directly provided and contracted VHA services for community-dwelling veterans, highlighting the circle of care around the veteran.8,9 Home health care workers anchored health care teams during the COVID-19 pandemic, observing and reporting on patients’ well-being to family caregivers, primary care practitioners, and HHAs. They also provided critical emotional support and companionship to patients isolated from family and friends.9 These workers also exposed themselves and their families to considerable risk and often lacked the protection afforded by personal protective equipment (PPE) in accordance with infection prevention guidance.3,12

Abbreviations: HBPC, home based primary care; HHA, home health agency; VHA, Veterans Health Administration.
aAdapted with permission from Wyte-Lake and Franzosa.8,9
Through a combination of its national and local health care networks, the VHA has a robust and well-positioned emergency infrastructure to supportcommunity-dwelling older adults during disasters.14 This network is supported by the VHA Office of Emergency Management, which shares resources and guidance with local emergency managers at each facility as well as individual programs such as the VHA Home Based Primary Care (HBPC) program, which provides 38,000 seriously ill veterans with home medical visits.15 Working closely with their local and national hospital networks and emergency managers, individual VHA HBPC programs were able to maintain the safety of staff and continuity of care for patients enrolled in HBPC by rapidly administering COVID-19 vaccines to patients, caregivers, and staff, and providing emergency assistance during the 2017 hurricane season.16,17 These efforts were successful because HBPC practitioners and their patients, had access to a level of emergency-related information, resources, and technology that are often out of reach for individual community-based health care practitioners (HCPs). The US Department of Veterans Affairs (VA) also supports local communities through its Fourth Mission, which provides emergency resources to non-VHA health care facilities (ie, hospitals and nursing homes) during national emergencies and natural disasters.17 Although there has been an expansion in the definition of shared resources, such as extending behavioral health support to local communities, the VHA has not historically provided these resources to HHAs.14
This study examines opportunities to leverage VHA emergency management resources to support contracted HHAs and inform other large health system emergency planning efforts. The findings from the exploratory phase are described in this article. We interviewed VHA emergency managers, HBPC and VA staff who coordinate home health care worker services, as well as administrators at contracted HHAs within a Veterans Integrated Services Network (VISN). These findings will inform the second (single-site pilot study) and third (feasibility study) phases. Our intent was to (1) better understand the relationships between VA medical centers (VAMCs) and their contracted HHAs; (2) identify existing VHA emergency protocols to support community-dwelling older adults; and (3) determine opportunities to build on existing infrastructure and relationships to better support contracted HHAs and their staff in emergencies.
Methods
The 18 VISNs act as regional systems of care that are loosely connected to better meet local health needs and maximize access to care. This study was conducted at 6 of 9 VAMCs within VISN 2, the New York/New Jersey VHA Health Care Network.18 VAMCs that serve urban, rural, and mixed urban/rural catchment areas were included.
Each VAMC has an emergency management program led by an emergency manager, an HBPC program led by a program director and medical director, and a community care or purchased care office that has a liaison who manages contracted home health care worker services. The studyfocused on HBPC programs because they are most likely to interact with veterans’ home health care workers in the home and care for community-dwelling veterans during emergencies. Each VHA also contracts with a series of local HHAs that generally have a dedicated staff member who interfaces with the VHA liaison. Our goal was to interview ≥ 1 emergency manager, ≥ 1 HBPC team member, ≥ 1 community care staff person, and ≥ 1 contracted home health agency administrator at each site to gain multiple perspectives from the range of HCPs serving veterans in the community.
Recruitment and Data Collection
The 6 sites were selected in consultation with VISN 2 leadership for their strong HBPC and emergency management programs. To recruit respondents, we contacted VISN and VAMC leads and used our professional networks to identify a sample of multidisciplinary individuals who represent both community care and HBPC programs who were contacted via email.
Since each VAMC is organized differently, we utilized a snowball sampling approach to identify the appropriate contacts.19 At the completion of each interview, we asked the participant to suggest additional contacts and introduce us to any remaining stakeholders (eg, the emergency manager) at that site or colleagues at other VISN facilities. Because roles vary among VAMCs, we contacted the person who most closely resembled the identified role and asked them to direct us to a more appropriate contact, if necessary. We asked community care managers to identify 1 to 2 agencies serving the highest volume of patients who are veterans at their site and requested interviews with those liaisons. This resulted in the recruitment of key stakeholders from 4 teams across the 6 sites (Table).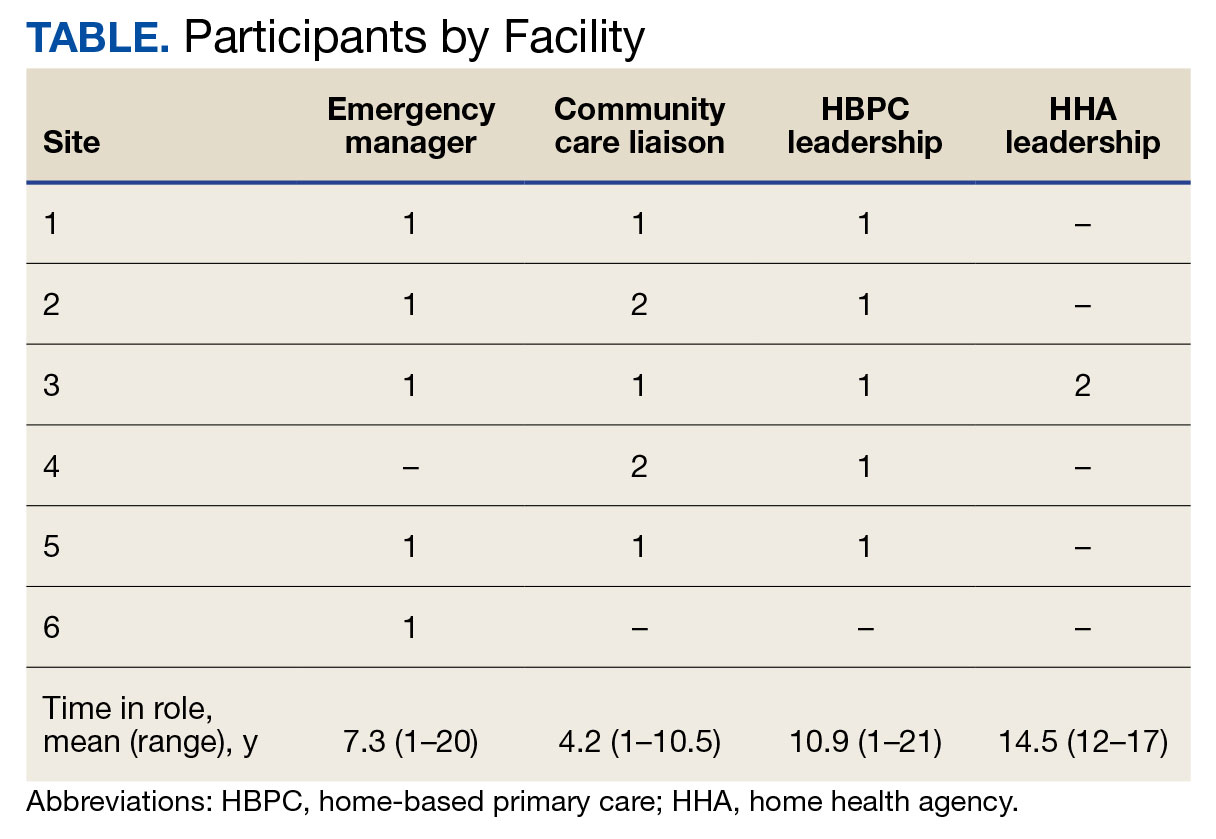
A semistructured interview guide was jointly developed based on constructs of interest, including relationships within VAMCs and between VAMCs and HHAs; existing emergency protocols and experience during disasters; and suggestions and opportunities for supporting agencies during emergencies and potential barriers. Two researchers (TWL and EF) who were trained in qualitative methods jointly conducted interviews using the interview guide, with 1 researcher leading and another taking notes and asking clarifying questions.
Interviews were conducted virtually via Microsoft Teams with respondents at their work locations between September 2022 and January 2023. Interviews were audio recorded and transcribed and 2 authors (TWL and ESO) reviewed transcripts for accuracy. Interviews averaged 47 minutes in length (range, 20-59).
The study was reviewed and determined to be exempt by institutional review boards at the James J. Peters VAMC and Greater Los Angeles VAMC. We asked participants for verbal consent to participate and preserved their confidentiality.
Analysis
Data were analyzed via an inductive approach, which involves drawing salient themes rather than imposing preconceived theories.20 Three researchers (TWL, EF, and ES) listened to and discussed 2 staff interviews and tagged text with specific codes (eg, communication between the VHA and HHA, internal communication, and barriers to case fulfillment) so the team could selectively return to the interview text for deeper analysis, allowing for the development of a final codebook. The project team synthesized the findings to identify higher-level themes, drawing comparisons across and within the respondent groups, including within and between health care systems. Throughout the analysis, we maintained analytic memos, documented discussions, and engaged in analyst triangulation to ensure trustworthiness.21,22 To ensure the analysis accurately reflected the participants’ understanding, we held 2 virtual member-checking sessions with participants to share preliminary findings and conclusions and solicit feedback. Analysis was conducted using ATLAS.ti version 20.
Results
VHA-based participants described internal emergency management systems that are deployed during a disaster to support patients and staff. Agency participants described their own internal emergency management protocols. Respondents discussed how and when the 2 intersected, as well as opportunities for future mutual support. The analysis identified several themes: (1) relationships between VAMC teams; (2) relationships between VHA and HHAs; (3) VHA and agencies responses during emergencies; (4) receptivity and opportunities for extending VHA resources into the community; and (5) barriers and facilitators to deeper engagement.
Relationships Within VHA (n = 17)
Staff at all VHA sites described close relationships between the internal emergency management and HBPC teams. HBPC teams identified patients who were most at risk during emergencies to triage those with the highest medical needs (eg, patients dependent on home infusion, oxygen, or electronic medical devices) and worked alongside emergency managers to develop plans to continue care during an emergency. HBPC representatives were part of their facilities’ local emergency response committees. Due to this close collaboration, VHA emergency managers were familiar with the needs of homebound veterans and caregivers. “I invite our [HBPC] program manager to attend [committee] meetings and … they’re part of the EOC [emergency operations center]," an emergency manager said. “We work together and I’m constantly in contact with that individual, especially during natural disasters and so forth, to ensure that everybody’s prepared in the community.”
On the other hand, community caremanagers—who described frequent interactions with HBPC teams, largely around coordinating and managing non-VHA home care services—were less likely to have direct relationships with their facility emergency managers. For example, when asked if they had a relationship with their emergency manager, a community care manager admitted, “I [only] know who he is.” They also did not report having structured protocols for veteran outreach during emergencies, “because all those veterans who are receiving [home health care worker] services also belong to a primary care team,” and considered the outreach to be the responsibility of the primary care team and HHA.
Relationships Between the VHA and HHAs (n = 17)
Communication between VAMCs and contracted agencies primarily went through community care managers, who described established long-term relationships with agency administrators. Communication was commonly restricted to operational activities, such as processing referrals and occasional troubleshooting. According to a community care manager most communication is “why haven’t you signed my orders?” There was a general sense from participants that communication was promptly answered, problems were addressed, and professional collegiality existed between the agencies as patients were referred and placed for services. One community care manager reported meeting with agencies regularly, noting, “I talk to them pretty much daily.”
If problems arose, community care managers described themselves as “the liaison” between agencies and VHA HCPs who ordered the referrals. This is particularly the case if the agency needed help finding a VHA clinician or addressing differences in care delivery protocols.
Responding During Emergencies (n = 19)
During emergencies, VHA and agency staff described following their own organization’s protocols and communicating with each other only on a case-by-case basis rather than through formal or systematic channels and had little knowledge of their counterpart’s emergency protocols. Beyond patient care, there was no evidence of information sharing between VHA and agency staff. Regarding sharing information with their local community, an HBPC Program Director said, “it’s almost like the VHA had become siloed” and operated on its own without engaging with community health systems or emergency managers.
Beyond the guidance provided by state departments of public health, HHAs described collaborating with other agencies in their network and relying on their informal professional network to manage the volume of information and updates they followed during emergencies like the COVID-19 pandemic. One agency administrator did not frequently communicate with VHA partners during the pandemic but explained that the local public health department helped work through challenges. However, “we realized pretty quickly they were overloaded and there was only so much they could do.” The agency administrator turned to a “sister agency” and local hospitals, noting, “Wherever you have connections in the field or in the industry, you know you’re going to reach out to people for guidance on policies and… protocol.”
Opportunities for Extending VHA Resources to the Community (n = 16)
All VHA emergency managers were receptive to extending support to community-based HCPS and, in some cases, felt strongly that they were an essential part of veterans’ care networks. Emergency managers offered examples for how they supportedcommunity-based HCPs, such as helping those in the VAMC medical foster home program develop and evaluate emergency plans. Many said they had not explicitly considered HHAs before (Appendix).
Emergency managers also described how supporting community-based HCPs could be considered within the scope of the VHA role and mission, specifically the Fourth Mission. “I think that we should be making our best effort to make sure that we’re also providing that same level [of protection] to the people taking care of the veteran [as our VHA staff],” an emergency manager said. “It’s our responsibility to provide the best for the staff that are going into those homes to take care of that patient.”
In many cases, emergency managers had already developed practical tools that could be easily shared outside the VHA, including weather alerts, trainings, emergency plan templates, and lists of community resources and shelters (Figure 2). A number of these examples built on existing communication channels. One emergency manager said that the extension of resources could be an opportunity to decrease the perceived isolation of home health care workers through regular

Abbreviations: PPE, personal protective equipment; VA, US Department of Veterans Affairs.
On the agency side, participants noted that some HHAs could benefit more from support than others. While some agencies are well staffed and have good protocols and keep up to date, “There are smaller agencies, agencies that are starting up that may not have the resources to just disseminate all the information. Those are the agencies [that] could well benefit from the VHA,” an HBPC medical director explained. Agency administrators suggested several areas where they would welcome support, including a deeper understanding of available community resources and access to PPE for staff. Regarding informational resources, an administrator said, “Anytime we can get information, it’s good to have it come to you and not always have to go out searching for it.”
Barriers and Facilitators to Partnering With Community Agencies (n = 16)
A primary barrier regarding resource sharing was potential misalignment between each organization’s policies. HHAs followed state and federal public health guidelines, which sometimes differed from VHA policies. Given that agencies care for both VHA and non-VHA clients, questions also arose around how agencies would prioritize information from the VHA, if they were already receiving information from other sources. When asked about information sharing, both VHA staff and agencies agreed staff time to support any additional activities should be weighed against the value of the information gained.
Six participants also shared that education around emergency preparedness could be an opportunity to bridge gaps between VAMCs and their surrounding communities.
Two emergency managers noted the need to be sensitive in the way they engaged with partners, respecting and building on the work that agencies were already doing in this area to ensure VHA was seen as a trusted partner and resource rather than trying to impose new policies or rules on community-based HCPs. “I know that like all leadership in various organizations, there’s a little bit of bristling going on when other people try and tell them what to do,” an HBPC medical director said. “However, if it is established that as a sort of greater level like a state level or a federal level, that VHA can be a resource. I think that as long as that’s recognized by their own professional organizations within each state, then I think that that would be a tremendous advantage to many agencies.”
In terms of sharing physical resources, emergency managers raised concerns around potential liability, although they also acknowledged this issue was important enough to think about potential workarounds. As one emergency manager said, “I want to know that my PPE is not compromised in any way shape or form and that I am in charge of that PPE, so to rely upon going to a home and hoping that [the PPE] wasn’t compromised … would kind of make me a little uneasy.” This emergency manager suggested possible solutions, such as creating a sealed PPE package to give directly to an aide.
Discussion
As the prevalence of climate-related disasters increases, the need to ensure the safety and independence of older adults during emergencies grows more urgent. Health systems must think beyond the direct services they provide and consider the community resources upon which their patients rely. While relationships did not formally exist between VHA emergency managers and community home health HCPs in the sample analyzed in this article, there is precedent and interest in supporting contracted home health agencies caring for veterans in the community. Although not historically part of the VA Fourth Mission, creating a pipeline of support for contracted HHAs by leveraging existing relationships and resources can potentially strengthen its mission to protect older veterans in emergencies, help them age safely in place, and provide a model for health systems to collaborate with community-based HCPs around emergency planning and response (Figure 3).23

Existing research on the value of health care coalitions highlights the need for established and growing partnerships with a focus on ensuring they are value-added, which echoes concerns we heard in interviews.24 Investment in community partnerships not only includes sharing supplies but also relying on bidirectional support that can be a trusted form of timely information.1,25 The findings in this study exhibit strong communication practices within the VHA during periods of nonemergency and underscore the untapped value of the pre-existing relationship between VAMCs and their contracted HHAs as an area of potential growth for health care coalitions.
Sharing resources in a way that does not put new demands on partners contributes to the sustainability and value-added nature of coalitions. Examples include establishing new low-investment practices (ie, information sharing) that support capacity and compliance with existing requirements rather than create new responsibilities for either member of the coalition. The relationship between the VHA emergency managers and the VHA HBPC program can act as a guide. The emergency managers interviewed for this study are currently engaged with HBPC programs and therefore understand the needs of homebound older adults and their caregivers. Extending the information already available to the HBPC teams via existing channels strengthens workforce practices and increased security for the shared patient, even without direct relationships between emergency managers and agencies. It is important to understand the limitations of these practices, including concerns around conflicting federal and state mandates, legal concerns around the liability of sharing physical resources (such as PPE), and awareness that the objective is not for the VHA to increase burdens (eg, increasing compliance requirements) but rather to serve as a resource for a mutual population in a shared community.
Offering training and practical resources to HHA home health care workers can help them meet disaster preparedness requirements. This is particularly important considering the growing home care workforce shortages, a topic mentioned by all HBPC and community care participants interviewed for this study.26,27 Home health care workers report feeling underprepared and isolated while on the job in normal conditions, a sentiment exacerbated by the COVID-19 pandemic.3,10 Supporting these individuals may help them feel more prepared and connected to their work, improving stability and quality of care.
While these issues are priorities within the VHA, there is growing recognition at the state and federal level of the importance of including older adults and their HCPs in disaster preparedness and response.5,28 The US Department of Health and Human Services, for example, includes older adults and organizations that serve them on its National Advisory Committee on Seniors and Disasters. The Senate version of the 2023 reauthorization of the Pandemic and All-Hazards Preparedness and Response Act included specific provisions to support community-dwelling older adults and people with disabilities, incorporating funding for community organizations to support continuity of services and avoid institutionalization in an emergency.29 Other proposed legislation includes the Real Emergency Access for Aging and Disability Inclusion for Disasters Act, which would ensure the needs of older adults and people with disabilities are explicitly included in all phases of emergency planning and response.30
The VHA expansion of the its VEText program to include disaster response is an effort to more efficiently extend outreach to older and vulnerable patients who are veterans.31 Given these growing efforts, the VHA and other health systems have an opportunity to expand internal emergency preparedness efforts to ensure the health and safety of individuals living in the community.
Limitations
VISN 2 has been a target of terrorism and other disasters. In addition to the sites being initially recruited for their strong emergency management protocols, this context may have biased respondents who are favorable to extending their resources into the community. At the time of recruitment, contracted HHAs were still experiencing staff shortages due to the COVID-19 pandemic, which limited the ability of agency staff to participate in interviews. Additionally, while the comprehensive exploration of VISN 2 facilities allows for confidence of the organizational structures described, the qualitative research design and small study sample, the study findings cannot be immediately generalized to all VISNs.
Conclusions
Many older veterans increasingly rely on home health care workers to age safely. The VHA, as a large national health care system and leader in emergency preparedness, could play an important role in supporting home health care workers and ameliorating their sense of isolation during emergencies and natural disasters. Leveraging existing resources and relationships may be a low-cost, low-effort opportunity to build higher-level interventions that support the needs of patients. Future research and work in this field, including the authors’ ongoing work, will expand agency participation and engage agency staff in conceptualizing pilot projects to ensure they are viable and feasible for the field.

- Barnett DJ, Knieser L, Errett NA, Rosenblum AJ, Seshamani M, Kirsch TD. Reexamining health-care coalitions in light of COVID-19. Disaster Med public Health Prep. 2022;16(3):859-863. doi:10.1017/dmp.2020.431
- Wulff K, Donato D, Lurie N. What is health resilience and how can we build it? Annu Rev Public Health. 2015;36:361-374. doi:10.1146/annurev-publhealth-031914-122829
- Franzosa E, Wyte-Lake T, Tsui EK, Reckrey JM, Sterling MR. Essential but excluded: building disaster preparedness capacity for home health care workers and home care agencies. J Am Med Dir Assoc. 2022;23(12):1990-1996. doi:10.1016/j.jamda.2022.09.012
- Miner S, Masci L, Chimenti C, Rin N, Mann A, Noonan B. An outreach phone call project: using home health to reach isolated community dwelling adults during the COVID 19 lockdown. J Community Health. 2022;47(2):266-272. doi:10.1007/s10900-021-01044-6
- National Institute on Aging. Protecting older adults from the effects of natural disasters and extreme weather. October 18, 2022. Accessed August 19, 2024. https://www.nia.nih.gov/news/protecting-older-adults-effects-natural-disasters-and-extreme-weather
- PHI. Direct Care Workers in the United States: Key Facts. September 7, 2021. Accessed August 19, 2024. https://www.phinational.org/resource/direct-care-workers-in-the-united-states-key-facts-2/
- Centers for Medicare & Medicaid Services. Emergency Preparedness Rule. September 8, 2016. Updated September 6, 2023. Accessed August 19, 2024. https://www.cms.gov/medicare/health-safety-standards/quality-safety-oversight-emergency-preparedness/emergency-preparedness-rule
- Wyte-Lake T, Claver M, Tubbesing S, Davis D, Dobalian A. Development of a home health patient assessment tool for disaster planning. Gerontology. 2019;65(4):353-361. doi:10.1159/000494971
- Franzosa E, Judon KM, Gottesman EM, et al. Home health aides’ increased role in supporting older veterans and primary healthcare teams during COVID-19: a qualitative analysis. J Gen Intern Med. 2022;37(8):1830-1837. doi:10.1007/s11606-021-07271-w
- Franzosa E, Tsui EK, Baron S. “Who’s caring for us?”: understanding and addressing the effects of emotional labor on home health aides’ well-being. Gerontologist. 2019;59(6):1055-1064. doi:10.1093/geront/gny099
- Osakwe ZT, Osborne JC, Samuel T, et al. All alone: a qualitative study of home health aides’ experiences during the COVID-19 pandemic in New York. Am J Infect Control. 2021;49(11):1362-1368. doi:10.1016/j.ajic.2021.08.004
- Feldman PH, Russell D, Onorato N, et al. Ensuring the safety of the home health aide workforce and the continuation of essential patient care through sustainable pandemic preparedness. July 2022. Accessed August 19, 2024. https://www.vnshealth.org/wp-content/uploads/2022/08/Pandemic_Preparedness_IB_07_21_22.pdf
- Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Internal Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
- Wyte-Lake T, Schmitz S, Kornegay RJ, Acevedo F, Dobalian A. Three case studies of community behavioral health support from the US Department of Veterans Affairs after disasters. BMC Public Health. 2021;21(1):639. doi:10.1186/s12889-021-10650-x
- Beales JL, Edes T. Veteran’s affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-ix. doi:10.1016/j.cger.2008.11.002
- Wyte-Lake T, Manheim C, Gillespie SM, Dobalian A, Haverhals LM. COVID-19 vaccination in VA home based primary care: experience of interdisciplinary team members. J Am Med Dir Assoc. 2022;23(6):917-922. doi:10.1016/j.jamda.2022.03.014
- Wyte-Lake T, Schmitz S, Cosme Torres-Sabater R, Dobalian A. Case study of VA Caribbean Healthcare System’s community response to Hurricane Maria. J Emerg Manag. 2022;19(8):189-199. doi:10.5055/jem.0536
- US Department of Veterans Affairs. New York/New Jersey VA Health Care Network, VISN 2 Locations. Updated January 3, 2024. Accessed August 19, 2024. https://www.visn2.va.gov/visn2/facilities.asp
- Noy C. Sampling knowledge: the hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327-344. doi:10.1080/13645570701401305
- Ritchie J, Lewis J, Nicholls CM, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd ed. Sage; 2013.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol. 2005;52(2):250-260. doi:10.1037/0022-0167.52.2.250
- Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. doi:10.1111/j.1365-2648.2006.03727.x
- Schmitz S, Wyte-Lake T, Dobalian A. Facilitators and barriers to preparedness partnerships: a veterans affairs medical center perspective. Disaster Med Public Health Prep. 2018;12(4):431-436. doi:10.1017/dmp.2017.92
- Koch AE, Bohn J, Corvin JA, Seaberg J. Maturing into high-functioning health-care coalitions: a qualitative Nationwide study of emergency preparedness and response leadership. Disaster Med Public Health Prep. 2022;17:e111. doi:10.1017/dmp.2022.13
- Lin JS, Webber EM, Bean SI, Martin AM, Davies MC. Rapid evidence review: policy actions for the integration of public health and health care in the United States. Front Public Health. 2023;11:1098431. doi:10.3389/fpubh.2023.1098431
- Watts MOM, Burns A, Ammula M. Ongoing impacts of the pandemic on medicaid home & community-based services (HCBS) programs: findings from a 50-state survey. November 28, 2022. Accessed August 19, 2024. https://www.kff.org/medicaid/issue-brief/ongoing-impacts-of-the-pandemic-on-medicaid-home-community-based-services-hcbs-programs-findings-from-a-50-state-survey/
- Kreider AR, Werner RM. The home care workforce has not kept pace with growth in home and community-based services. Health Aff (Millwood). 2023;42(5):650-657. doi:10.1377/hlthaff.2022.01351
- FEMA introduces disaster preparedness guide for older adults. News release. FEMA. September 20, 2023. Accessed August 19, 2024. https://www.fema.gov/press-release/20230920/fema-introduces-disaster-preparedness-guide-older-adults
- Pandemic and All-Hazards Preparedness and Response Act, S 2333, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/senate-bill/2333/text
- REAADI for Disasters Act, HR 2371, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/house-bill/2371
- Wyte-Lake T, Brewster P, Hubert T, Gin J, Davis D, Dobalian A. VA’s experience building capability to conduct outreach to vulnerable patients during emergencies. Innov Aging. 2023;7(suppl 1):209. doi:10.1093/geroni/igad104.0690
As large-scale natural disasters become more common, health care coalitions and the engagement of health systems with local, state, and federal public health departments have effectively bolstered communities’ resilience via collective sharing and distribution of resources.1 These resources may include supplies and the dissemination of emergency information, education, and training.2 The COVID-19 pandemic demonstrated that larger health care systems including hospital networks and nursing homes are better connected to health care coalition resources than smaller, independent systems, such as community home health agencies.3 This leaves some organizations on their own to meet requirements that maintain continuity of care and support their patients and staff throughout a natural disaster.
Home health care workers play important roles in the care of older adults.4 Older adults experience high levels of disability and comorbidities that put them at risk during emergencies; they often require support from paid, family, and neighborhood caregivers to live independently.5 More than 9.3 million US adults receive paid care from 2.6 million home health care workers (eg, home health aides and personal care assistants).6 Many of these individuals are hired through small independent home health agencies (HHAs), while others may work directly for an individual. When neighborhood resources and family caregiving are disrupted during emergencies, the critical services these workers administer become even more essential to ensuring continued access to medical care and social services.
The importance of these services was underscored by the Centers for Medicare and Medicaid Services 2017 inclusion of HHAs in federal emergency preparedness guidelines.7,8 The fractured and decentralized nature of the home health care industry means many HHAs struggle to maintain continuous care during emergencies and protect their staff. HHAs, and health care workers in the home, are often isolated, under-resourced, and disconnected from broader emergency planning efforts. Additionally, home care jobs are largely part-time, unstable, and low paying, making the workers themselves vulnerable during emergencies.3,9-13
This is a significant issue for the Veterans Health Administration (VHA), which annually purchases 10.5 million home health care worker visits for 150,000 veterans from community-based HHAs to enable those individuals to live independently. Figure 1 illustrates the existing structure of directly provided and contracted VHA services for community-dwelling veterans, highlighting the circle of care around the veteran.8,9 Home health care workers anchored health care teams during the COVID-19 pandemic, observing and reporting on patients’ well-being to family caregivers, primary care practitioners, and HHAs. They also provided critical emotional support and companionship to patients isolated from family and friends.9 These workers also exposed themselves and their families to considerable risk and often lacked the protection afforded by personal protective equipment (PPE) in accordance with infection prevention guidance.3,12

Abbreviations: HBPC, home based primary care; HHA, home health agency; VHA, Veterans Health Administration.
aAdapted with permission from Wyte-Lake and Franzosa.8,9
Through a combination of its national and local health care networks, the VHA has a robust and well-positioned emergency infrastructure to supportcommunity-dwelling older adults during disasters.14 This network is supported by the VHA Office of Emergency Management, which shares resources and guidance with local emergency managers at each facility as well as individual programs such as the VHA Home Based Primary Care (HBPC) program, which provides 38,000 seriously ill veterans with home medical visits.15 Working closely with their local and national hospital networks and emergency managers, individual VHA HBPC programs were able to maintain the safety of staff and continuity of care for patients enrolled in HBPC by rapidly administering COVID-19 vaccines to patients, caregivers, and staff, and providing emergency assistance during the 2017 hurricane season.16,17 These efforts were successful because HBPC practitioners and their patients, had access to a level of emergency-related information, resources, and technology that are often out of reach for individual community-based health care practitioners (HCPs). The US Department of Veterans Affairs (VA) also supports local communities through its Fourth Mission, which provides emergency resources to non-VHA health care facilities (ie, hospitals and nursing homes) during national emergencies and natural disasters.17 Although there has been an expansion in the definition of shared resources, such as extending behavioral health support to local communities, the VHA has not historically provided these resources to HHAs.14
This study examines opportunities to leverage VHA emergency management resources to support contracted HHAs and inform other large health system emergency planning efforts. The findings from the exploratory phase are described in this article. We interviewed VHA emergency managers, HBPC and VA staff who coordinate home health care worker services, as well as administrators at contracted HHAs within a Veterans Integrated Services Network (VISN). These findings will inform the second (single-site pilot study) and third (feasibility study) phases. Our intent was to (1) better understand the relationships between VA medical centers (VAMCs) and their contracted HHAs; (2) identify existing VHA emergency protocols to support community-dwelling older adults; and (3) determine opportunities to build on existing infrastructure and relationships to better support contracted HHAs and their staff in emergencies.
Methods
The 18 VISNs act as regional systems of care that are loosely connected to better meet local health needs and maximize access to care. This study was conducted at 6 of 9 VAMCs within VISN 2, the New York/New Jersey VHA Health Care Network.18 VAMCs that serve urban, rural, and mixed urban/rural catchment areas were included.
Each VAMC has an emergency management program led by an emergency manager, an HBPC program led by a program director and medical director, and a community care or purchased care office that has a liaison who manages contracted home health care worker services. The studyfocused on HBPC programs because they are most likely to interact with veterans’ home health care workers in the home and care for community-dwelling veterans during emergencies. Each VHA also contracts with a series of local HHAs that generally have a dedicated staff member who interfaces with the VHA liaison. Our goal was to interview ≥ 1 emergency manager, ≥ 1 HBPC team member, ≥ 1 community care staff person, and ≥ 1 contracted home health agency administrator at each site to gain multiple perspectives from the range of HCPs serving veterans in the community.
Recruitment and Data Collection
The 6 sites were selected in consultation with VISN 2 leadership for their strong HBPC and emergency management programs. To recruit respondents, we contacted VISN and VAMC leads and used our professional networks to identify a sample of multidisciplinary individuals who represent both community care and HBPC programs who were contacted via email.
Since each VAMC is organized differently, we utilized a snowball sampling approach to identify the appropriate contacts.19 At the completion of each interview, we asked the participant to suggest additional contacts and introduce us to any remaining stakeholders (eg, the emergency manager) at that site or colleagues at other VISN facilities. Because roles vary among VAMCs, we contacted the person who most closely resembled the identified role and asked them to direct us to a more appropriate contact, if necessary. We asked community care managers to identify 1 to 2 agencies serving the highest volume of patients who are veterans at their site and requested interviews with those liaisons. This resulted in the recruitment of key stakeholders from 4 teams across the 6 sites (Table).
A semistructured interview guide was jointly developed based on constructs of interest, including relationships within VAMCs and between VAMCs and HHAs; existing emergency protocols and experience during disasters; and suggestions and opportunities for supporting agencies during emergencies and potential barriers. Two researchers (TWL and EF) who were trained in qualitative methods jointly conducted interviews using the interview guide, with 1 researcher leading and another taking notes and asking clarifying questions.
Interviews were conducted virtually via Microsoft Teams with respondents at their work locations between September 2022 and January 2023. Interviews were audio recorded and transcribed and 2 authors (TWL and ESO) reviewed transcripts for accuracy. Interviews averaged 47 minutes in length (range, 20-59).
The study was reviewed and determined to be exempt by institutional review boards at the James J. Peters VAMC and Greater Los Angeles VAMC. We asked participants for verbal consent to participate and preserved their confidentiality.
Analysis
Data were analyzed via an inductive approach, which involves drawing salient themes rather than imposing preconceived theories.20 Three researchers (TWL, EF, and ES) listened to and discussed 2 staff interviews and tagged text with specific codes (eg, communication between the VHA and HHA, internal communication, and barriers to case fulfillment) so the team could selectively return to the interview text for deeper analysis, allowing for the development of a final codebook. The project team synthesized the findings to identify higher-level themes, drawing comparisons across and within the respondent groups, including within and between health care systems. Throughout the analysis, we maintained analytic memos, documented discussions, and engaged in analyst triangulation to ensure trustworthiness.21,22 To ensure the analysis accurately reflected the participants’ understanding, we held 2 virtual member-checking sessions with participants to share preliminary findings and conclusions and solicit feedback. Analysis was conducted using ATLAS.ti version 20.
Results
VHA-based participants described internal emergency management systems that are deployed during a disaster to support patients and staff. Agency participants described their own internal emergency management protocols. Respondents discussed how and when the 2 intersected, as well as opportunities for future mutual support. The analysis identified several themes: (1) relationships between VAMC teams; (2) relationships between VHA and HHAs; (3) VHA and agencies responses during emergencies; (4) receptivity and opportunities for extending VHA resources into the community; and (5) barriers and facilitators to deeper engagement.
Relationships Within VHA (n = 17)
Staff at all VHA sites described close relationships between the internal emergency management and HBPC teams. HBPC teams identified patients who were most at risk during emergencies to triage those with the highest medical needs (eg, patients dependent on home infusion, oxygen, or electronic medical devices) and worked alongside emergency managers to develop plans to continue care during an emergency. HBPC representatives were part of their facilities’ local emergency response committees. Due to this close collaboration, VHA emergency managers were familiar with the needs of homebound veterans and caregivers. “I invite our [HBPC] program manager to attend [committee] meetings and … they’re part of the EOC [emergency operations center]," an emergency manager said. “We work together and I’m constantly in contact with that individual, especially during natural disasters and so forth, to ensure that everybody’s prepared in the community.”
On the other hand, community caremanagers—who described frequent interactions with HBPC teams, largely around coordinating and managing non-VHA home care services—were less likely to have direct relationships with their facility emergency managers. For example, when asked if they had a relationship with their emergency manager, a community care manager admitted, “I [only] know who he is.” They also did not report having structured protocols for veteran outreach during emergencies, “because all those veterans who are receiving [home health care worker] services also belong to a primary care team,” and considered the outreach to be the responsibility of the primary care team and HHA.
Relationships Between the VHA and HHAs (n = 17)
Communication between VAMCs and contracted agencies primarily went through community care managers, who described established long-term relationships with agency administrators. Communication was commonly restricted to operational activities, such as processing referrals and occasional troubleshooting. According to a community care manager most communication is “why haven’t you signed my orders?” There was a general sense from participants that communication was promptly answered, problems were addressed, and professional collegiality existed between the agencies as patients were referred and placed for services. One community care manager reported meeting with agencies regularly, noting, “I talk to them pretty much daily.”
If problems arose, community care managers described themselves as “the liaison” between agencies and VHA HCPs who ordered the referrals. This is particularly the case if the agency needed help finding a VHA clinician or addressing differences in care delivery protocols.
Responding During Emergencies (n = 19)
During emergencies, VHA and agency staff described following their own organization’s protocols and communicating with each other only on a case-by-case basis rather than through formal or systematic channels and had little knowledge of their counterpart’s emergency protocols. Beyond patient care, there was no evidence of information sharing between VHA and agency staff. Regarding sharing information with their local community, an HBPC Program Director said, “it’s almost like the VHA had become siloed” and operated on its own without engaging with community health systems or emergency managers.
Beyond the guidance provided by state departments of public health, HHAs described collaborating with other agencies in their network and relying on their informal professional network to manage the volume of information and updates they followed during emergencies like the COVID-19 pandemic. One agency administrator did not frequently communicate with VHA partners during the pandemic but explained that the local public health department helped work through challenges. However, “we realized pretty quickly they were overloaded and there was only so much they could do.” The agency administrator turned to a “sister agency” and local hospitals, noting, “Wherever you have connections in the field or in the industry, you know you’re going to reach out to people for guidance on policies and… protocol.”
Opportunities for Extending VHA Resources to the Community (n = 16)
All VHA emergency managers were receptive to extending support to community-based HCPS and, in some cases, felt strongly that they were an essential part of veterans’ care networks. Emergency managers offered examples for how they supportedcommunity-based HCPs, such as helping those in the VAMC medical foster home program develop and evaluate emergency plans. Many said they had not explicitly considered HHAs before (Appendix).
Emergency managers also described how supporting community-based HCPs could be considered within the scope of the VHA role and mission, specifically the Fourth Mission. “I think that we should be making our best effort to make sure that we’re also providing that same level [of protection] to the people taking care of the veteran [as our VHA staff],” an emergency manager said. “It’s our responsibility to provide the best for the staff that are going into those homes to take care of that patient.”
In many cases, emergency managers had already developed practical tools that could be easily shared outside the VHA, including weather alerts, trainings, emergency plan templates, and lists of community resources and shelters (Figure 2). A number of these examples built on existing communication channels. One emergency manager said that the extension of resources could be an opportunity to decrease the perceived isolation of home health care workers through regular

Abbreviations: PPE, personal protective equipment; VA, US Department of Veterans Affairs.
On the agency side, participants noted that some HHAs could benefit more from support than others. While some agencies are well staffed and have good protocols and keep up to date, “There are smaller agencies, agencies that are starting up that may not have the resources to just disseminate all the information. Those are the agencies [that] could well benefit from the VHA,” an HBPC medical director explained. Agency administrators suggested several areas where they would welcome support, including a deeper understanding of available community resources and access to PPE for staff. Regarding informational resources, an administrator said, “Anytime we can get information, it’s good to have it come to you and not always have to go out searching for it.”
Barriers and Facilitators to Partnering With Community Agencies (n = 16)
A primary barrier regarding resource sharing was potential misalignment between each organization’s policies. HHAs followed state and federal public health guidelines, which sometimes differed from VHA policies. Given that agencies care for both VHA and non-VHA clients, questions also arose around how agencies would prioritize information from the VHA, if they were already receiving information from other sources. When asked about information sharing, both VHA staff and agencies agreed staff time to support any additional activities should be weighed against the value of the information gained.
Six participants also shared that education around emergency preparedness could be an opportunity to bridge gaps between VAMCs and their surrounding communities.
Two emergency managers noted the need to be sensitive in the way they engaged with partners, respecting and building on the work that agencies were already doing in this area to ensure VHA was seen as a trusted partner and resource rather than trying to impose new policies or rules on community-based HCPs. “I know that like all leadership in various organizations, there’s a little bit of bristling going on when other people try and tell them what to do,” an HBPC medical director said. “However, if it is established that as a sort of greater level like a state level or a federal level, that VHA can be a resource. I think that as long as that’s recognized by their own professional organizations within each state, then I think that that would be a tremendous advantage to many agencies.”
In terms of sharing physical resources, emergency managers raised concerns around potential liability, although they also acknowledged this issue was important enough to think about potential workarounds. As one emergency manager said, “I want to know that my PPE is not compromised in any way shape or form and that I am in charge of that PPE, so to rely upon going to a home and hoping that [the PPE] wasn’t compromised … would kind of make me a little uneasy.” This emergency manager suggested possible solutions, such as creating a sealed PPE package to give directly to an aide.
Discussion
As the prevalence of climate-related disasters increases, the need to ensure the safety and independence of older adults during emergencies grows more urgent. Health systems must think beyond the direct services they provide and consider the community resources upon which their patients rely. While relationships did not formally exist between VHA emergency managers and community home health HCPs in the sample analyzed in this article, there is precedent and interest in supporting contracted home health agencies caring for veterans in the community. Although not historically part of the VA Fourth Mission, creating a pipeline of support for contracted HHAs by leveraging existing relationships and resources can potentially strengthen its mission to protect older veterans in emergencies, help them age safely in place, and provide a model for health systems to collaborate with community-based HCPs around emergency planning and response (Figure 3).23

Existing research on the value of health care coalitions highlights the need for established and growing partnerships with a focus on ensuring they are value-added, which echoes concerns we heard in interviews.24 Investment in community partnerships not only includes sharing supplies but also relying on bidirectional support that can be a trusted form of timely information.1,25 The findings in this study exhibit strong communication practices within the VHA during periods of nonemergency and underscore the untapped value of the pre-existing relationship between VAMCs and their contracted HHAs as an area of potential growth for health care coalitions.
Sharing resources in a way that does not put new demands on partners contributes to the sustainability and value-added nature of coalitions. Examples include establishing new low-investment practices (ie, information sharing) that support capacity and compliance with existing requirements rather than create new responsibilities for either member of the coalition. The relationship between the VHA emergency managers and the VHA HBPC program can act as a guide. The emergency managers interviewed for this study are currently engaged with HBPC programs and therefore understand the needs of homebound older adults and their caregivers. Extending the information already available to the HBPC teams via existing channels strengthens workforce practices and increased security for the shared patient, even without direct relationships between emergency managers and agencies. It is important to understand the limitations of these practices, including concerns around conflicting federal and state mandates, legal concerns around the liability of sharing physical resources (such as PPE), and awareness that the objective is not for the VHA to increase burdens (eg, increasing compliance requirements) but rather to serve as a resource for a mutual population in a shared community.
Offering training and practical resources to HHA home health care workers can help them meet disaster preparedness requirements. This is particularly important considering the growing home care workforce shortages, a topic mentioned by all HBPC and community care participants interviewed for this study.26,27 Home health care workers report feeling underprepared and isolated while on the job in normal conditions, a sentiment exacerbated by the COVID-19 pandemic.3,10 Supporting these individuals may help them feel more prepared and connected to their work, improving stability and quality of care.
While these issues are priorities within the VHA, there is growing recognition at the state and federal level of the importance of including older adults and their HCPs in disaster preparedness and response.5,28 The US Department of Health and Human Services, for example, includes older adults and organizations that serve them on its National Advisory Committee on Seniors and Disasters. The Senate version of the 2023 reauthorization of the Pandemic and All-Hazards Preparedness and Response Act included specific provisions to support community-dwelling older adults and people with disabilities, incorporating funding for community organizations to support continuity of services and avoid institutionalization in an emergency.29 Other proposed legislation includes the Real Emergency Access for Aging and Disability Inclusion for Disasters Act, which would ensure the needs of older adults and people with disabilities are explicitly included in all phases of emergency planning and response.30
The VHA expansion of the its VEText program to include disaster response is an effort to more efficiently extend outreach to older and vulnerable patients who are veterans.31 Given these growing efforts, the VHA and other health systems have an opportunity to expand internal emergency preparedness efforts to ensure the health and safety of individuals living in the community.
Limitations
VISN 2 has been a target of terrorism and other disasters. In addition to the sites being initially recruited for their strong emergency management protocols, this context may have biased respondents who are favorable to extending their resources into the community. At the time of recruitment, contracted HHAs were still experiencing staff shortages due to the COVID-19 pandemic, which limited the ability of agency staff to participate in interviews. Additionally, while the comprehensive exploration of VISN 2 facilities allows for confidence of the organizational structures described, the qualitative research design and small study sample, the study findings cannot be immediately generalized to all VISNs.
Conclusions
Many older veterans increasingly rely on home health care workers to age safely. The VHA, as a large national health care system and leader in emergency preparedness, could play an important role in supporting home health care workers and ameliorating their sense of isolation during emergencies and natural disasters. Leveraging existing resources and relationships may be a low-cost, low-effort opportunity to build higher-level interventions that support the needs of patients. Future research and work in this field, including the authors’ ongoing work, will expand agency participation and engage agency staff in conceptualizing pilot projects to ensure they are viable and feasible for the field.

As large-scale natural disasters become more common, health care coalitions and the engagement of health systems with local, state, and federal public health departments have effectively bolstered communities’ resilience via collective sharing and distribution of resources.1 These resources may include supplies and the dissemination of emergency information, education, and training.2 The COVID-19 pandemic demonstrated that larger health care systems including hospital networks and nursing homes are better connected to health care coalition resources than smaller, independent systems, such as community home health agencies.3 This leaves some organizations on their own to meet requirements that maintain continuity of care and support their patients and staff throughout a natural disaster.
Home health care workers play important roles in the care of older adults.4 Older adults experience high levels of disability and comorbidities that put them at risk during emergencies; they often require support from paid, family, and neighborhood caregivers to live independently.5 More than 9.3 million US adults receive paid care from 2.6 million home health care workers (eg, home health aides and personal care assistants).6 Many of these individuals are hired through small independent home health agencies (HHAs), while others may work directly for an individual. When neighborhood resources and family caregiving are disrupted during emergencies, the critical services these workers administer become even more essential to ensuring continued access to medical care and social services.
The importance of these services was underscored by the Centers for Medicare and Medicaid Services 2017 inclusion of HHAs in federal emergency preparedness guidelines.7,8 The fractured and decentralized nature of the home health care industry means many HHAs struggle to maintain continuous care during emergencies and protect their staff. HHAs, and health care workers in the home, are often isolated, under-resourced, and disconnected from broader emergency planning efforts. Additionally, home care jobs are largely part-time, unstable, and low paying, making the workers themselves vulnerable during emergencies.3,9-13
This is a significant issue for the Veterans Health Administration (VHA), which annually purchases 10.5 million home health care worker visits for 150,000 veterans from community-based HHAs to enable those individuals to live independently. Figure 1 illustrates the existing structure of directly provided and contracted VHA services for community-dwelling veterans, highlighting the circle of care around the veteran.8,9 Home health care workers anchored health care teams during the COVID-19 pandemic, observing and reporting on patients’ well-being to family caregivers, primary care practitioners, and HHAs. They also provided critical emotional support and companionship to patients isolated from family and friends.9 These workers also exposed themselves and their families to considerable risk and often lacked the protection afforded by personal protective equipment (PPE) in accordance with infection prevention guidance.3,12

Abbreviations: HBPC, home based primary care; HHA, home health agency; VHA, Veterans Health Administration.
aAdapted with permission from Wyte-Lake and Franzosa.8,9
Through a combination of its national and local health care networks, the VHA has a robust and well-positioned emergency infrastructure to supportcommunity-dwelling older adults during disasters.14 This network is supported by the VHA Office of Emergency Management, which shares resources and guidance with local emergency managers at each facility as well as individual programs such as the VHA Home Based Primary Care (HBPC) program, which provides 38,000 seriously ill veterans with home medical visits.15 Working closely with their local and national hospital networks and emergency managers, individual VHA HBPC programs were able to maintain the safety of staff and continuity of care for patients enrolled in HBPC by rapidly administering COVID-19 vaccines to patients, caregivers, and staff, and providing emergency assistance during the 2017 hurricane season.16,17 These efforts were successful because HBPC practitioners and their patients, had access to a level of emergency-related information, resources, and technology that are often out of reach for individual community-based health care practitioners (HCPs). The US Department of Veterans Affairs (VA) also supports local communities through its Fourth Mission, which provides emergency resources to non-VHA health care facilities (ie, hospitals and nursing homes) during national emergencies and natural disasters.17 Although there has been an expansion in the definition of shared resources, such as extending behavioral health support to local communities, the VHA has not historically provided these resources to HHAs.14
This study examines opportunities to leverage VHA emergency management resources to support contracted HHAs and inform other large health system emergency planning efforts. The findings from the exploratory phase are described in this article. We interviewed VHA emergency managers, HBPC and VA staff who coordinate home health care worker services, as well as administrators at contracted HHAs within a Veterans Integrated Services Network (VISN). These findings will inform the second (single-site pilot study) and third (feasibility study) phases. Our intent was to (1) better understand the relationships between VA medical centers (VAMCs) and their contracted HHAs; (2) identify existing VHA emergency protocols to support community-dwelling older adults; and (3) determine opportunities to build on existing infrastructure and relationships to better support contracted HHAs and their staff in emergencies.
Methods
The 18 VISNs act as regional systems of care that are loosely connected to better meet local health needs and maximize access to care. This study was conducted at 6 of 9 VAMCs within VISN 2, the New York/New Jersey VHA Health Care Network.18 VAMCs that serve urban, rural, and mixed urban/rural catchment areas were included.
Each VAMC has an emergency management program led by an emergency manager, an HBPC program led by a program director and medical director, and a community care or purchased care office that has a liaison who manages contracted home health care worker services. The studyfocused on HBPC programs because they are most likely to interact with veterans’ home health care workers in the home and care for community-dwelling veterans during emergencies. Each VHA also contracts with a series of local HHAs that generally have a dedicated staff member who interfaces with the VHA liaison. Our goal was to interview ≥ 1 emergency manager, ≥ 1 HBPC team member, ≥ 1 community care staff person, and ≥ 1 contracted home health agency administrator at each site to gain multiple perspectives from the range of HCPs serving veterans in the community.
Recruitment and Data Collection
The 6 sites were selected in consultation with VISN 2 leadership for their strong HBPC and emergency management programs. To recruit respondents, we contacted VISN and VAMC leads and used our professional networks to identify a sample of multidisciplinary individuals who represent both community care and HBPC programs who were contacted via email.
Since each VAMC is organized differently, we utilized a snowball sampling approach to identify the appropriate contacts.19 At the completion of each interview, we asked the participant to suggest additional contacts and introduce us to any remaining stakeholders (eg, the emergency manager) at that site or colleagues at other VISN facilities. Because roles vary among VAMCs, we contacted the person who most closely resembled the identified role and asked them to direct us to a more appropriate contact, if necessary. We asked community care managers to identify 1 to 2 agencies serving the highest volume of patients who are veterans at their site and requested interviews with those liaisons. This resulted in the recruitment of key stakeholders from 4 teams across the 6 sites (Table).
A semistructured interview guide was jointly developed based on constructs of interest, including relationships within VAMCs and between VAMCs and HHAs; existing emergency protocols and experience during disasters; and suggestions and opportunities for supporting agencies during emergencies and potential barriers. Two researchers (TWL and EF) who were trained in qualitative methods jointly conducted interviews using the interview guide, with 1 researcher leading and another taking notes and asking clarifying questions.
Interviews were conducted virtually via Microsoft Teams with respondents at their work locations between September 2022 and January 2023. Interviews were audio recorded and transcribed and 2 authors (TWL and ESO) reviewed transcripts for accuracy. Interviews averaged 47 minutes in length (range, 20-59).
The study was reviewed and determined to be exempt by institutional review boards at the James J. Peters VAMC and Greater Los Angeles VAMC. We asked participants for verbal consent to participate and preserved their confidentiality.
Analysis
Data were analyzed via an inductive approach, which involves drawing salient themes rather than imposing preconceived theories.20 Three researchers (TWL, EF, and ES) listened to and discussed 2 staff interviews and tagged text with specific codes (eg, communication between the VHA and HHA, internal communication, and barriers to case fulfillment) so the team could selectively return to the interview text for deeper analysis, allowing for the development of a final codebook. The project team synthesized the findings to identify higher-level themes, drawing comparisons across and within the respondent groups, including within and between health care systems. Throughout the analysis, we maintained analytic memos, documented discussions, and engaged in analyst triangulation to ensure trustworthiness.21,22 To ensure the analysis accurately reflected the participants’ understanding, we held 2 virtual member-checking sessions with participants to share preliminary findings and conclusions and solicit feedback. Analysis was conducted using ATLAS.ti version 20.
Results
VHA-based participants described internal emergency management systems that are deployed during a disaster to support patients and staff. Agency participants described their own internal emergency management protocols. Respondents discussed how and when the 2 intersected, as well as opportunities for future mutual support. The analysis identified several themes: (1) relationships between VAMC teams; (2) relationships between VHA and HHAs; (3) VHA and agencies responses during emergencies; (4) receptivity and opportunities for extending VHA resources into the community; and (5) barriers and facilitators to deeper engagement.
Relationships Within VHA (n = 17)
Staff at all VHA sites described close relationships between the internal emergency management and HBPC teams. HBPC teams identified patients who were most at risk during emergencies to triage those with the highest medical needs (eg, patients dependent on home infusion, oxygen, or electronic medical devices) and worked alongside emergency managers to develop plans to continue care during an emergency. HBPC representatives were part of their facilities’ local emergency response committees. Due to this close collaboration, VHA emergency managers were familiar with the needs of homebound veterans and caregivers. “I invite our [HBPC] program manager to attend [committee] meetings and … they’re part of the EOC [emergency operations center]," an emergency manager said. “We work together and I’m constantly in contact with that individual, especially during natural disasters and so forth, to ensure that everybody’s prepared in the community.”
On the other hand, community caremanagers—who described frequent interactions with HBPC teams, largely around coordinating and managing non-VHA home care services—were less likely to have direct relationships with their facility emergency managers. For example, when asked if they had a relationship with their emergency manager, a community care manager admitted, “I [only] know who he is.” They also did not report having structured protocols for veteran outreach during emergencies, “because all those veterans who are receiving [home health care worker] services also belong to a primary care team,” and considered the outreach to be the responsibility of the primary care team and HHA.
Relationships Between the VHA and HHAs (n = 17)
Communication between VAMCs and contracted agencies primarily went through community care managers, who described established long-term relationships with agency administrators. Communication was commonly restricted to operational activities, such as processing referrals and occasional troubleshooting. According to a community care manager most communication is “why haven’t you signed my orders?” There was a general sense from participants that communication was promptly answered, problems were addressed, and professional collegiality existed between the agencies as patients were referred and placed for services. One community care manager reported meeting with agencies regularly, noting, “I talk to them pretty much daily.”
If problems arose, community care managers described themselves as “the liaison” between agencies and VHA HCPs who ordered the referrals. This is particularly the case if the agency needed help finding a VHA clinician or addressing differences in care delivery protocols.
Responding During Emergencies (n = 19)
During emergencies, VHA and agency staff described following their own organization’s protocols and communicating with each other only on a case-by-case basis rather than through formal or systematic channels and had little knowledge of their counterpart’s emergency protocols. Beyond patient care, there was no evidence of information sharing between VHA and agency staff. Regarding sharing information with their local community, an HBPC Program Director said, “it’s almost like the VHA had become siloed” and operated on its own without engaging with community health systems or emergency managers.
Beyond the guidance provided by state departments of public health, HHAs described collaborating with other agencies in their network and relying on their informal professional network to manage the volume of information and updates they followed during emergencies like the COVID-19 pandemic. One agency administrator did not frequently communicate with VHA partners during the pandemic but explained that the local public health department helped work through challenges. However, “we realized pretty quickly they were overloaded and there was only so much they could do.” The agency administrator turned to a “sister agency” and local hospitals, noting, “Wherever you have connections in the field or in the industry, you know you’re going to reach out to people for guidance on policies and… protocol.”
Opportunities for Extending VHA Resources to the Community (n = 16)
All VHA emergency managers were receptive to extending support to community-based HCPS and, in some cases, felt strongly that they were an essential part of veterans’ care networks. Emergency managers offered examples for how they supportedcommunity-based HCPs, such as helping those in the VAMC medical foster home program develop and evaluate emergency plans. Many said they had not explicitly considered HHAs before (Appendix).
Emergency managers also described how supporting community-based HCPs could be considered within the scope of the VHA role and mission, specifically the Fourth Mission. “I think that we should be making our best effort to make sure that we’re also providing that same level [of protection] to the people taking care of the veteran [as our VHA staff],” an emergency manager said. “It’s our responsibility to provide the best for the staff that are going into those homes to take care of that patient.”
In many cases, emergency managers had already developed practical tools that could be easily shared outside the VHA, including weather alerts, trainings, emergency plan templates, and lists of community resources and shelters (Figure 2). A number of these examples built on existing communication channels. One emergency manager said that the extension of resources could be an opportunity to decrease the perceived isolation of home health care workers through regular

Abbreviations: PPE, personal protective equipment; VA, US Department of Veterans Affairs.
On the agency side, participants noted that some HHAs could benefit more from support than others. While some agencies are well staffed and have good protocols and keep up to date, “There are smaller agencies, agencies that are starting up that may not have the resources to just disseminate all the information. Those are the agencies [that] could well benefit from the VHA,” an HBPC medical director explained. Agency administrators suggested several areas where they would welcome support, including a deeper understanding of available community resources and access to PPE for staff. Regarding informational resources, an administrator said, “Anytime we can get information, it’s good to have it come to you and not always have to go out searching for it.”
Barriers and Facilitators to Partnering With Community Agencies (n = 16)
A primary barrier regarding resource sharing was potential misalignment between each organization’s policies. HHAs followed state and federal public health guidelines, which sometimes differed from VHA policies. Given that agencies care for both VHA and non-VHA clients, questions also arose around how agencies would prioritize information from the VHA, if they were already receiving information from other sources. When asked about information sharing, both VHA staff and agencies agreed staff time to support any additional activities should be weighed against the value of the information gained.
Six participants also shared that education around emergency preparedness could be an opportunity to bridge gaps between VAMCs and their surrounding communities.
Two emergency managers noted the need to be sensitive in the way they engaged with partners, respecting and building on the work that agencies were already doing in this area to ensure VHA was seen as a trusted partner and resource rather than trying to impose new policies or rules on community-based HCPs. “I know that like all leadership in various organizations, there’s a little bit of bristling going on when other people try and tell them what to do,” an HBPC medical director said. “However, if it is established that as a sort of greater level like a state level or a federal level, that VHA can be a resource. I think that as long as that’s recognized by their own professional organizations within each state, then I think that that would be a tremendous advantage to many agencies.”
In terms of sharing physical resources, emergency managers raised concerns around potential liability, although they also acknowledged this issue was important enough to think about potential workarounds. As one emergency manager said, “I want to know that my PPE is not compromised in any way shape or form and that I am in charge of that PPE, so to rely upon going to a home and hoping that [the PPE] wasn’t compromised … would kind of make me a little uneasy.” This emergency manager suggested possible solutions, such as creating a sealed PPE package to give directly to an aide.
Discussion
As the prevalence of climate-related disasters increases, the need to ensure the safety and independence of older adults during emergencies grows more urgent. Health systems must think beyond the direct services they provide and consider the community resources upon which their patients rely. While relationships did not formally exist between VHA emergency managers and community home health HCPs in the sample analyzed in this article, there is precedent and interest in supporting contracted home health agencies caring for veterans in the community. Although not historically part of the VA Fourth Mission, creating a pipeline of support for contracted HHAs by leveraging existing relationships and resources can potentially strengthen its mission to protect older veterans in emergencies, help them age safely in place, and provide a model for health systems to collaborate with community-based HCPs around emergency planning and response (Figure 3).23

Existing research on the value of health care coalitions highlights the need for established and growing partnerships with a focus on ensuring they are value-added, which echoes concerns we heard in interviews.24 Investment in community partnerships not only includes sharing supplies but also relying on bidirectional support that can be a trusted form of timely information.1,25 The findings in this study exhibit strong communication practices within the VHA during periods of nonemergency and underscore the untapped value of the pre-existing relationship between VAMCs and their contracted HHAs as an area of potential growth for health care coalitions.
Sharing resources in a way that does not put new demands on partners contributes to the sustainability and value-added nature of coalitions. Examples include establishing new low-investment practices (ie, information sharing) that support capacity and compliance with existing requirements rather than create new responsibilities for either member of the coalition. The relationship between the VHA emergency managers and the VHA HBPC program can act as a guide. The emergency managers interviewed for this study are currently engaged with HBPC programs and therefore understand the needs of homebound older adults and their caregivers. Extending the information already available to the HBPC teams via existing channels strengthens workforce practices and increased security for the shared patient, even without direct relationships between emergency managers and agencies. It is important to understand the limitations of these practices, including concerns around conflicting federal and state mandates, legal concerns around the liability of sharing physical resources (such as PPE), and awareness that the objective is not for the VHA to increase burdens (eg, increasing compliance requirements) but rather to serve as a resource for a mutual population in a shared community.
Offering training and practical resources to HHA home health care workers can help them meet disaster preparedness requirements. This is particularly important considering the growing home care workforce shortages, a topic mentioned by all HBPC and community care participants interviewed for this study.26,27 Home health care workers report feeling underprepared and isolated while on the job in normal conditions, a sentiment exacerbated by the COVID-19 pandemic.3,10 Supporting these individuals may help them feel more prepared and connected to their work, improving stability and quality of care.
While these issues are priorities within the VHA, there is growing recognition at the state and federal level of the importance of including older adults and their HCPs in disaster preparedness and response.5,28 The US Department of Health and Human Services, for example, includes older adults and organizations that serve them on its National Advisory Committee on Seniors and Disasters. The Senate version of the 2023 reauthorization of the Pandemic and All-Hazards Preparedness and Response Act included specific provisions to support community-dwelling older adults and people with disabilities, incorporating funding for community organizations to support continuity of services and avoid institutionalization in an emergency.29 Other proposed legislation includes the Real Emergency Access for Aging and Disability Inclusion for Disasters Act, which would ensure the needs of older adults and people with disabilities are explicitly included in all phases of emergency planning and response.30
The VHA expansion of the its VEText program to include disaster response is an effort to more efficiently extend outreach to older and vulnerable patients who are veterans.31 Given these growing efforts, the VHA and other health systems have an opportunity to expand internal emergency preparedness efforts to ensure the health and safety of individuals living in the community.
Limitations
VISN 2 has been a target of terrorism and other disasters. In addition to the sites being initially recruited for their strong emergency management protocols, this context may have biased respondents who are favorable to extending their resources into the community. At the time of recruitment, contracted HHAs were still experiencing staff shortages due to the COVID-19 pandemic, which limited the ability of agency staff to participate in interviews. Additionally, while the comprehensive exploration of VISN 2 facilities allows for confidence of the organizational structures described, the qualitative research design and small study sample, the study findings cannot be immediately generalized to all VISNs.
Conclusions
Many older veterans increasingly rely on home health care workers to age safely. The VHA, as a large national health care system and leader in emergency preparedness, could play an important role in supporting home health care workers and ameliorating their sense of isolation during emergencies and natural disasters. Leveraging existing resources and relationships may be a low-cost, low-effort opportunity to build higher-level interventions that support the needs of patients. Future research and work in this field, including the authors’ ongoing work, will expand agency participation and engage agency staff in conceptualizing pilot projects to ensure they are viable and feasible for the field.

- Barnett DJ, Knieser L, Errett NA, Rosenblum AJ, Seshamani M, Kirsch TD. Reexamining health-care coalitions in light of COVID-19. Disaster Med public Health Prep. 2022;16(3):859-863. doi:10.1017/dmp.2020.431
- Wulff K, Donato D, Lurie N. What is health resilience and how can we build it? Annu Rev Public Health. 2015;36:361-374. doi:10.1146/annurev-publhealth-031914-122829
- Franzosa E, Wyte-Lake T, Tsui EK, Reckrey JM, Sterling MR. Essential but excluded: building disaster preparedness capacity for home health care workers and home care agencies. J Am Med Dir Assoc. 2022;23(12):1990-1996. doi:10.1016/j.jamda.2022.09.012
- Miner S, Masci L, Chimenti C, Rin N, Mann A, Noonan B. An outreach phone call project: using home health to reach isolated community dwelling adults during the COVID 19 lockdown. J Community Health. 2022;47(2):266-272. doi:10.1007/s10900-021-01044-6
- National Institute on Aging. Protecting older adults from the effects of natural disasters and extreme weather. October 18, 2022. Accessed August 19, 2024. https://www.nia.nih.gov/news/protecting-older-adults-effects-natural-disasters-and-extreme-weather
- PHI. Direct Care Workers in the United States: Key Facts. September 7, 2021. Accessed August 19, 2024. https://www.phinational.org/resource/direct-care-workers-in-the-united-states-key-facts-2/
- Centers for Medicare & Medicaid Services. Emergency Preparedness Rule. September 8, 2016. Updated September 6, 2023. Accessed August 19, 2024. https://www.cms.gov/medicare/health-safety-standards/quality-safety-oversight-emergency-preparedness/emergency-preparedness-rule
- Wyte-Lake T, Claver M, Tubbesing S, Davis D, Dobalian A. Development of a home health patient assessment tool for disaster planning. Gerontology. 2019;65(4):353-361. doi:10.1159/000494971
- Franzosa E, Judon KM, Gottesman EM, et al. Home health aides’ increased role in supporting older veterans and primary healthcare teams during COVID-19: a qualitative analysis. J Gen Intern Med. 2022;37(8):1830-1837. doi:10.1007/s11606-021-07271-w
- Franzosa E, Tsui EK, Baron S. “Who’s caring for us?”: understanding and addressing the effects of emotional labor on home health aides’ well-being. Gerontologist. 2019;59(6):1055-1064. doi:10.1093/geront/gny099
- Osakwe ZT, Osborne JC, Samuel T, et al. All alone: a qualitative study of home health aides’ experiences during the COVID-19 pandemic in New York. Am J Infect Control. 2021;49(11):1362-1368. doi:10.1016/j.ajic.2021.08.004
- Feldman PH, Russell D, Onorato N, et al. Ensuring the safety of the home health aide workforce and the continuation of essential patient care through sustainable pandemic preparedness. July 2022. Accessed August 19, 2024. https://www.vnshealth.org/wp-content/uploads/2022/08/Pandemic_Preparedness_IB_07_21_22.pdf
- Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Internal Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
- Wyte-Lake T, Schmitz S, Kornegay RJ, Acevedo F, Dobalian A. Three case studies of community behavioral health support from the US Department of Veterans Affairs after disasters. BMC Public Health. 2021;21(1):639. doi:10.1186/s12889-021-10650-x
- Beales JL, Edes T. Veteran’s affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-ix. doi:10.1016/j.cger.2008.11.002
- Wyte-Lake T, Manheim C, Gillespie SM, Dobalian A, Haverhals LM. COVID-19 vaccination in VA home based primary care: experience of interdisciplinary team members. J Am Med Dir Assoc. 2022;23(6):917-922. doi:10.1016/j.jamda.2022.03.014
- Wyte-Lake T, Schmitz S, Cosme Torres-Sabater R, Dobalian A. Case study of VA Caribbean Healthcare System’s community response to Hurricane Maria. J Emerg Manag. 2022;19(8):189-199. doi:10.5055/jem.0536
- US Department of Veterans Affairs. New York/New Jersey VA Health Care Network, VISN 2 Locations. Updated January 3, 2024. Accessed August 19, 2024. https://www.visn2.va.gov/visn2/facilities.asp
- Noy C. Sampling knowledge: the hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327-344. doi:10.1080/13645570701401305
- Ritchie J, Lewis J, Nicholls CM, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd ed. Sage; 2013.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol. 2005;52(2):250-260. doi:10.1037/0022-0167.52.2.250
- Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. doi:10.1111/j.1365-2648.2006.03727.x
- Schmitz S, Wyte-Lake T, Dobalian A. Facilitators and barriers to preparedness partnerships: a veterans affairs medical center perspective. Disaster Med Public Health Prep. 2018;12(4):431-436. doi:10.1017/dmp.2017.92
- Koch AE, Bohn J, Corvin JA, Seaberg J. Maturing into high-functioning health-care coalitions: a qualitative Nationwide study of emergency preparedness and response leadership. Disaster Med Public Health Prep. 2022;17:e111. doi:10.1017/dmp.2022.13
- Lin JS, Webber EM, Bean SI, Martin AM, Davies MC. Rapid evidence review: policy actions for the integration of public health and health care in the United States. Front Public Health. 2023;11:1098431. doi:10.3389/fpubh.2023.1098431
- Watts MOM, Burns A, Ammula M. Ongoing impacts of the pandemic on medicaid home & community-based services (HCBS) programs: findings from a 50-state survey. November 28, 2022. Accessed August 19, 2024. https://www.kff.org/medicaid/issue-brief/ongoing-impacts-of-the-pandemic-on-medicaid-home-community-based-services-hcbs-programs-findings-from-a-50-state-survey/
- Kreider AR, Werner RM. The home care workforce has not kept pace with growth in home and community-based services. Health Aff (Millwood). 2023;42(5):650-657. doi:10.1377/hlthaff.2022.01351
- FEMA introduces disaster preparedness guide for older adults. News release. FEMA. September 20, 2023. Accessed August 19, 2024. https://www.fema.gov/press-release/20230920/fema-introduces-disaster-preparedness-guide-older-adults
- Pandemic and All-Hazards Preparedness and Response Act, S 2333, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/senate-bill/2333/text
- REAADI for Disasters Act, HR 2371, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/house-bill/2371
- Wyte-Lake T, Brewster P, Hubert T, Gin J, Davis D, Dobalian A. VA’s experience building capability to conduct outreach to vulnerable patients during emergencies. Innov Aging. 2023;7(suppl 1):209. doi:10.1093/geroni/igad104.0690
- Barnett DJ, Knieser L, Errett NA, Rosenblum AJ, Seshamani M, Kirsch TD. Reexamining health-care coalitions in light of COVID-19. Disaster Med public Health Prep. 2022;16(3):859-863. doi:10.1017/dmp.2020.431
- Wulff K, Donato D, Lurie N. What is health resilience and how can we build it? Annu Rev Public Health. 2015;36:361-374. doi:10.1146/annurev-publhealth-031914-122829
- Franzosa E, Wyte-Lake T, Tsui EK, Reckrey JM, Sterling MR. Essential but excluded: building disaster preparedness capacity for home health care workers and home care agencies. J Am Med Dir Assoc. 2022;23(12):1990-1996. doi:10.1016/j.jamda.2022.09.012
- Miner S, Masci L, Chimenti C, Rin N, Mann A, Noonan B. An outreach phone call project: using home health to reach isolated community dwelling adults during the COVID 19 lockdown. J Community Health. 2022;47(2):266-272. doi:10.1007/s10900-021-01044-6
- National Institute on Aging. Protecting older adults from the effects of natural disasters and extreme weather. October 18, 2022. Accessed August 19, 2024. https://www.nia.nih.gov/news/protecting-older-adults-effects-natural-disasters-and-extreme-weather
- PHI. Direct Care Workers in the United States: Key Facts. September 7, 2021. Accessed August 19, 2024. https://www.phinational.org/resource/direct-care-workers-in-the-united-states-key-facts-2/
- Centers for Medicare & Medicaid Services. Emergency Preparedness Rule. September 8, 2016. Updated September 6, 2023. Accessed August 19, 2024. https://www.cms.gov/medicare/health-safety-standards/quality-safety-oversight-emergency-preparedness/emergency-preparedness-rule
- Wyte-Lake T, Claver M, Tubbesing S, Davis D, Dobalian A. Development of a home health patient assessment tool for disaster planning. Gerontology. 2019;65(4):353-361. doi:10.1159/000494971
- Franzosa E, Judon KM, Gottesman EM, et al. Home health aides’ increased role in supporting older veterans and primary healthcare teams during COVID-19: a qualitative analysis. J Gen Intern Med. 2022;37(8):1830-1837. doi:10.1007/s11606-021-07271-w
- Franzosa E, Tsui EK, Baron S. “Who’s caring for us?”: understanding and addressing the effects of emotional labor on home health aides’ well-being. Gerontologist. 2019;59(6):1055-1064. doi:10.1093/geront/gny099
- Osakwe ZT, Osborne JC, Samuel T, et al. All alone: a qualitative study of home health aides’ experiences during the COVID-19 pandemic in New York. Am J Infect Control. 2021;49(11):1362-1368. doi:10.1016/j.ajic.2021.08.004
- Feldman PH, Russell D, Onorato N, et al. Ensuring the safety of the home health aide workforce and the continuation of essential patient care through sustainable pandemic preparedness. July 2022. Accessed August 19, 2024. https://www.vnshealth.org/wp-content/uploads/2022/08/Pandemic_Preparedness_IB_07_21_22.pdf
- Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Internal Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
- Wyte-Lake T, Schmitz S, Kornegay RJ, Acevedo F, Dobalian A. Three case studies of community behavioral health support from the US Department of Veterans Affairs after disasters. BMC Public Health. 2021;21(1):639. doi:10.1186/s12889-021-10650-x
- Beales JL, Edes T. Veteran’s affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-ix. doi:10.1016/j.cger.2008.11.002
- Wyte-Lake T, Manheim C, Gillespie SM, Dobalian A, Haverhals LM. COVID-19 vaccination in VA home based primary care: experience of interdisciplinary team members. J Am Med Dir Assoc. 2022;23(6):917-922. doi:10.1016/j.jamda.2022.03.014
- Wyte-Lake T, Schmitz S, Cosme Torres-Sabater R, Dobalian A. Case study of VA Caribbean Healthcare System’s community response to Hurricane Maria. J Emerg Manag. 2022;19(8):189-199. doi:10.5055/jem.0536
- US Department of Veterans Affairs. New York/New Jersey VA Health Care Network, VISN 2 Locations. Updated January 3, 2024. Accessed August 19, 2024. https://www.visn2.va.gov/visn2/facilities.asp
- Noy C. Sampling knowledge: the hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327-344. doi:10.1080/13645570701401305
- Ritchie J, Lewis J, Nicholls CM, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd ed. Sage; 2013.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol. 2005;52(2):250-260. doi:10.1037/0022-0167.52.2.250
- Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. doi:10.1111/j.1365-2648.2006.03727.x
- Schmitz S, Wyte-Lake T, Dobalian A. Facilitators and barriers to preparedness partnerships: a veterans affairs medical center perspective. Disaster Med Public Health Prep. 2018;12(4):431-436. doi:10.1017/dmp.2017.92
- Koch AE, Bohn J, Corvin JA, Seaberg J. Maturing into high-functioning health-care coalitions: a qualitative Nationwide study of emergency preparedness and response leadership. Disaster Med Public Health Prep. 2022;17:e111. doi:10.1017/dmp.2022.13
- Lin JS, Webber EM, Bean SI, Martin AM, Davies MC. Rapid evidence review: policy actions for the integration of public health and health care in the United States. Front Public Health. 2023;11:1098431. doi:10.3389/fpubh.2023.1098431
- Watts MOM, Burns A, Ammula M. Ongoing impacts of the pandemic on medicaid home & community-based services (HCBS) programs: findings from a 50-state survey. November 28, 2022. Accessed August 19, 2024. https://www.kff.org/medicaid/issue-brief/ongoing-impacts-of-the-pandemic-on-medicaid-home-community-based-services-hcbs-programs-findings-from-a-50-state-survey/
- Kreider AR, Werner RM. The home care workforce has not kept pace with growth in home and community-based services. Health Aff (Millwood). 2023;42(5):650-657. doi:10.1377/hlthaff.2022.01351
- FEMA introduces disaster preparedness guide for older adults. News release. FEMA. September 20, 2023. Accessed August 19, 2024. https://www.fema.gov/press-release/20230920/fema-introduces-disaster-preparedness-guide-older-adults
- Pandemic and All-Hazards Preparedness and Response Act, S 2333, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/senate-bill/2333/text
- REAADI for Disasters Act, HR 2371, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/house-bill/2371
- Wyte-Lake T, Brewster P, Hubert T, Gin J, Davis D, Dobalian A. VA’s experience building capability to conduct outreach to vulnerable patients during emergencies. Innov Aging. 2023;7(suppl 1):209. doi:10.1093/geroni/igad104.0690
Managing Age-Related Muscle Loss in Primary Care
Scene 1: Exercise Medicine Clinic, Rio de Janeiro, Brazil I just finished one evaluation on physical fitness and health and looked at my schedule. My next patient would be a 65-year-old man. How fit will he be? Will he have evident age-related muscle loss? I gave myself a short break and my mind went to the late 1970s.
Once upon a time, the practice of medicine was based primarily on the skill of your physical examination, previous experiences, and your ability to reason logically and make solid deductions. In 1979, the stethoscope was part of my dress code. After one elective semester as a research fellow at the Ambrose Cardiorespiratory Unit at McMaster University Medical Centre, in Hamilton, Canada, where I was honored to witness the dawn of evidence-based medicine, I graduated from Federal University of Rio de Janeiro. I still remember being introduced to some promising novelties in cardiology, such as M-mode echocardiograms and myocardial scintigraphy. Radiology was primarily centered on x-rays, and lab testing was basic and poorly automatized.
Over the following decades, medical practice changed dramatically with the incorporation of new technologies. Recent advances in diagnostic tools, genetics, artificial intelligence, and sophisticated statistical analyses, along with well-collected scientific data, have molded how clinicians should think and work.
At the same time, clinical profiles also changed. Internists and primary care physicians are regularly managing patients who are, on average, older and have or are on the way to having potentially life-threatening chronic diseases, accompanied by poor lifestyle habits, and, highly important, often some degree of disability, frailty, and loss of independence. Many of them exhibit age-related muscle loss.
Scene 2: Exercise Medicine Clinic, Rio de Janeiro, Brazil
Conscious of the benefits of interrupting my sitting time with activity, I left my office and walked to meet my patient in the waiting room. I called his name and introduced myself. I watched how he listened and reacted to my speech, and how easy or hard it was for him to rise from the chair — readiness, velocity, and number of supports required: none, one, or two hands. I offered my own hand to him, and when we shook, I gauged the strength of his grip.
I invited him into my office and took note of his somatotype and body composition, and whether he had any central obesity. Of course, and I should by no means miss this chance, I carefully observed how he walked in — his gait, speed, balance, posture — how he pulled up the chair, and how he managed to lower himself into his seat. Before I even sat in my own chair, I asked him if he remembered what his body weight was 5 years ago and what it was today. Before we got started in earnest, I had already managed to collect several pieces of relevant information.
Exercise Physiology: Changing Landscape
Muscle activity depends on muscle mass and function, and peaks somewhere between ages 25 and 35 before declining. The drop is slow in the early stages but accelerates rapidly after age 60 or 65.
Two of the most relevant variables in muscle function are strength and power. As a product of force and velocity, muscle power could be a more crucial factor than strength for many daily activities that demand movement against gravity or inertia, such as placing carry-on baggage in the overhead bin of an airplane or rising from the floor or chair.
The association between muscle mass and muscle strength or power is moderate, and physiologic data have indicated that the decline of muscle power with aging is faster and larger than that of muscle strength.
The term “sarcopenia” has become definitively incorporated into the medical glossary. From the Greek (“sark” and “penia”), sarcopenia was defined as reduced muscle mass, but more recently it has encompassed muscle strength in its definition. However, a recent consensus paper from the Global Leadership Initiative in Sarcopenia, using a Delphi approach, rejected the inclusion of muscle power in the concept of sarcopenia. On the other hand, a long time ago, some authors coined and advocated the use the term “dynapenia” to more precisely reflect the reduced levels of muscle strength and power that often accompany aging.
The best available intervention to counteract age-related deterioration of muscle activity is resistance exercise. The types of resistance exercises vary widely — by number of sets and repetitions, intervals between sets, speed of execution of movement, and percentage of maximal weight/load.
We recently proposed that, after an evaluation to identify the main muscle variable requiring attention, the resistance exercise program should be individually tailored and prescribed according to the objective to counteract sarcopenia or dynapenia.
What is more important for autonomy and better daily living conditions in old and very old individuals: muscle mass, muscle strength, or muscle power? More likely the response is muscle power — in practical terms, dynapenia rather than sarcopenia. This short video presents practical tips for obtaining optimal results in fighting dynapenia. The first choice should be power training or high velocity–based training, emphasizing two to three sets of six to eight repetitions performed as fast as possible (on the concentric or shortening phase of muscle contraction) with relatively high loads.
Internists and primary care physicians are most likely satisfied with the information they obtain by simple observation, and already can superficially grade the magnitude of a patient’s age-related muscle loss and its consequences to daily living.
However, those who want more objective information on nonaerobic physical fitness can add one to three simple tests to their consultation: the sitting-rising test (SRT); the 10-second one-legged test (10sOLS test); and the Flexitest. Poor performance on each of these — and particularly all three — is strongly associated with an increased risk for premature death in middle-aged and older individuals. These tests require no extra equipment and can be performed rapidly, and interpreting the results takes only a few moments using published reference values.
Age-related muscle loss profoundly affects our ability to sit and rise from the floor, so if time is limited, the SRT is the best assessment, as it measures all nonaerobic components of physical fitness. For a quick interpretation, consider that SRT scores vary from 0 to 10, do not substantially differ by sex, and that a composite score equal to or greater than 8 will reflect a minimum age-adjusted percentile of 61, most likely indicating relevant age-related muscle loss is not yet occurring.
Dr. Araújo is Professor and Dean of Research and Education, Exercise Medicine Clinic (CLINIMEX), Rio de Janeiro, Brazil. He reported conflicts of interest with INBRAMED.
A version of this article first appeared on Medscape.com.
Scene 1: Exercise Medicine Clinic, Rio de Janeiro, Brazil I just finished one evaluation on physical fitness and health and looked at my schedule. My next patient would be a 65-year-old man. How fit will he be? Will he have evident age-related muscle loss? I gave myself a short break and my mind went to the late 1970s.
Once upon a time, the practice of medicine was based primarily on the skill of your physical examination, previous experiences, and your ability to reason logically and make solid deductions. In 1979, the stethoscope was part of my dress code. After one elective semester as a research fellow at the Ambrose Cardiorespiratory Unit at McMaster University Medical Centre, in Hamilton, Canada, where I was honored to witness the dawn of evidence-based medicine, I graduated from Federal University of Rio de Janeiro. I still remember being introduced to some promising novelties in cardiology, such as M-mode echocardiograms and myocardial scintigraphy. Radiology was primarily centered on x-rays, and lab testing was basic and poorly automatized.
Over the following decades, medical practice changed dramatically with the incorporation of new technologies. Recent advances in diagnostic tools, genetics, artificial intelligence, and sophisticated statistical analyses, along with well-collected scientific data, have molded how clinicians should think and work.
At the same time, clinical profiles also changed. Internists and primary care physicians are regularly managing patients who are, on average, older and have or are on the way to having potentially life-threatening chronic diseases, accompanied by poor lifestyle habits, and, highly important, often some degree of disability, frailty, and loss of independence. Many of them exhibit age-related muscle loss.
Scene 2: Exercise Medicine Clinic, Rio de Janeiro, Brazil
Conscious of the benefits of interrupting my sitting time with activity, I left my office and walked to meet my patient in the waiting room. I called his name and introduced myself. I watched how he listened and reacted to my speech, and how easy or hard it was for him to rise from the chair — readiness, velocity, and number of supports required: none, one, or two hands. I offered my own hand to him, and when we shook, I gauged the strength of his grip.
I invited him into my office and took note of his somatotype and body composition, and whether he had any central obesity. Of course, and I should by no means miss this chance, I carefully observed how he walked in — his gait, speed, balance, posture — how he pulled up the chair, and how he managed to lower himself into his seat. Before I even sat in my own chair, I asked him if he remembered what his body weight was 5 years ago and what it was today. Before we got started in earnest, I had already managed to collect several pieces of relevant information.
Exercise Physiology: Changing Landscape
Muscle activity depends on muscle mass and function, and peaks somewhere between ages 25 and 35 before declining. The drop is slow in the early stages but accelerates rapidly after age 60 or 65.
Two of the most relevant variables in muscle function are strength and power. As a product of force and velocity, muscle power could be a more crucial factor than strength for many daily activities that demand movement against gravity or inertia, such as placing carry-on baggage in the overhead bin of an airplane or rising from the floor or chair.
The association between muscle mass and muscle strength or power is moderate, and physiologic data have indicated that the decline of muscle power with aging is faster and larger than that of muscle strength.
The term “sarcopenia” has become definitively incorporated into the medical glossary. From the Greek (“sark” and “penia”), sarcopenia was defined as reduced muscle mass, but more recently it has encompassed muscle strength in its definition. However, a recent consensus paper from the Global Leadership Initiative in Sarcopenia, using a Delphi approach, rejected the inclusion of muscle power in the concept of sarcopenia. On the other hand, a long time ago, some authors coined and advocated the use the term “dynapenia” to more precisely reflect the reduced levels of muscle strength and power that often accompany aging.
The best available intervention to counteract age-related deterioration of muscle activity is resistance exercise. The types of resistance exercises vary widely — by number of sets and repetitions, intervals between sets, speed of execution of movement, and percentage of maximal weight/load.
We recently proposed that, after an evaluation to identify the main muscle variable requiring attention, the resistance exercise program should be individually tailored and prescribed according to the objective to counteract sarcopenia or dynapenia.
What is more important for autonomy and better daily living conditions in old and very old individuals: muscle mass, muscle strength, or muscle power? More likely the response is muscle power — in practical terms, dynapenia rather than sarcopenia. This short video presents practical tips for obtaining optimal results in fighting dynapenia. The first choice should be power training or high velocity–based training, emphasizing two to three sets of six to eight repetitions performed as fast as possible (on the concentric or shortening phase of muscle contraction) with relatively high loads.
Internists and primary care physicians are most likely satisfied with the information they obtain by simple observation, and already can superficially grade the magnitude of a patient’s age-related muscle loss and its consequences to daily living.
However, those who want more objective information on nonaerobic physical fitness can add one to three simple tests to their consultation: the sitting-rising test (SRT); the 10-second one-legged test (10sOLS test); and the Flexitest. Poor performance on each of these — and particularly all three — is strongly associated with an increased risk for premature death in middle-aged and older individuals. These tests require no extra equipment and can be performed rapidly, and interpreting the results takes only a few moments using published reference values.
Age-related muscle loss profoundly affects our ability to sit and rise from the floor, so if time is limited, the SRT is the best assessment, as it measures all nonaerobic components of physical fitness. For a quick interpretation, consider that SRT scores vary from 0 to 10, do not substantially differ by sex, and that a composite score equal to or greater than 8 will reflect a minimum age-adjusted percentile of 61, most likely indicating relevant age-related muscle loss is not yet occurring.
Dr. Araújo is Professor and Dean of Research and Education, Exercise Medicine Clinic (CLINIMEX), Rio de Janeiro, Brazil. He reported conflicts of interest with INBRAMED.
A version of this article first appeared on Medscape.com.
Scene 1: Exercise Medicine Clinic, Rio de Janeiro, Brazil I just finished one evaluation on physical fitness and health and looked at my schedule. My next patient would be a 65-year-old man. How fit will he be? Will he have evident age-related muscle loss? I gave myself a short break and my mind went to the late 1970s.
Once upon a time, the practice of medicine was based primarily on the skill of your physical examination, previous experiences, and your ability to reason logically and make solid deductions. In 1979, the stethoscope was part of my dress code. After one elective semester as a research fellow at the Ambrose Cardiorespiratory Unit at McMaster University Medical Centre, in Hamilton, Canada, where I was honored to witness the dawn of evidence-based medicine, I graduated from Federal University of Rio de Janeiro. I still remember being introduced to some promising novelties in cardiology, such as M-mode echocardiograms and myocardial scintigraphy. Radiology was primarily centered on x-rays, and lab testing was basic and poorly automatized.
Over the following decades, medical practice changed dramatically with the incorporation of new technologies. Recent advances in diagnostic tools, genetics, artificial intelligence, and sophisticated statistical analyses, along with well-collected scientific data, have molded how clinicians should think and work.
At the same time, clinical profiles also changed. Internists and primary care physicians are regularly managing patients who are, on average, older and have or are on the way to having potentially life-threatening chronic diseases, accompanied by poor lifestyle habits, and, highly important, often some degree of disability, frailty, and loss of independence. Many of them exhibit age-related muscle loss.
Scene 2: Exercise Medicine Clinic, Rio de Janeiro, Brazil
Conscious of the benefits of interrupting my sitting time with activity, I left my office and walked to meet my patient in the waiting room. I called his name and introduced myself. I watched how he listened and reacted to my speech, and how easy or hard it was for him to rise from the chair — readiness, velocity, and number of supports required: none, one, or two hands. I offered my own hand to him, and when we shook, I gauged the strength of his grip.
I invited him into my office and took note of his somatotype and body composition, and whether he had any central obesity. Of course, and I should by no means miss this chance, I carefully observed how he walked in — his gait, speed, balance, posture — how he pulled up the chair, and how he managed to lower himself into his seat. Before I even sat in my own chair, I asked him if he remembered what his body weight was 5 years ago and what it was today. Before we got started in earnest, I had already managed to collect several pieces of relevant information.
Exercise Physiology: Changing Landscape
Muscle activity depends on muscle mass and function, and peaks somewhere between ages 25 and 35 before declining. The drop is slow in the early stages but accelerates rapidly after age 60 or 65.
Two of the most relevant variables in muscle function are strength and power. As a product of force and velocity, muscle power could be a more crucial factor than strength for many daily activities that demand movement against gravity or inertia, such as placing carry-on baggage in the overhead bin of an airplane or rising from the floor or chair.
The association between muscle mass and muscle strength or power is moderate, and physiologic data have indicated that the decline of muscle power with aging is faster and larger than that of muscle strength.
The term “sarcopenia” has become definitively incorporated into the medical glossary. From the Greek (“sark” and “penia”), sarcopenia was defined as reduced muscle mass, but more recently it has encompassed muscle strength in its definition. However, a recent consensus paper from the Global Leadership Initiative in Sarcopenia, using a Delphi approach, rejected the inclusion of muscle power in the concept of sarcopenia. On the other hand, a long time ago, some authors coined and advocated the use the term “dynapenia” to more precisely reflect the reduced levels of muscle strength and power that often accompany aging.
The best available intervention to counteract age-related deterioration of muscle activity is resistance exercise. The types of resistance exercises vary widely — by number of sets and repetitions, intervals between sets, speed of execution of movement, and percentage of maximal weight/load.
We recently proposed that, after an evaluation to identify the main muscle variable requiring attention, the resistance exercise program should be individually tailored and prescribed according to the objective to counteract sarcopenia or dynapenia.
What is more important for autonomy and better daily living conditions in old and very old individuals: muscle mass, muscle strength, or muscle power? More likely the response is muscle power — in practical terms, dynapenia rather than sarcopenia. This short video presents practical tips for obtaining optimal results in fighting dynapenia. The first choice should be power training or high velocity–based training, emphasizing two to three sets of six to eight repetitions performed as fast as possible (on the concentric or shortening phase of muscle contraction) with relatively high loads.
Internists and primary care physicians are most likely satisfied with the information they obtain by simple observation, and already can superficially grade the magnitude of a patient’s age-related muscle loss and its consequences to daily living.
However, those who want more objective information on nonaerobic physical fitness can add one to three simple tests to their consultation: the sitting-rising test (SRT); the 10-second one-legged test (10sOLS test); and the Flexitest. Poor performance on each of these — and particularly all three — is strongly associated with an increased risk for premature death in middle-aged and older individuals. These tests require no extra equipment and can be performed rapidly, and interpreting the results takes only a few moments using published reference values.
Age-related muscle loss profoundly affects our ability to sit and rise from the floor, so if time is limited, the SRT is the best assessment, as it measures all nonaerobic components of physical fitness. For a quick interpretation, consider that SRT scores vary from 0 to 10, do not substantially differ by sex, and that a composite score equal to or greater than 8 will reflect a minimum age-adjusted percentile of 61, most likely indicating relevant age-related muscle loss is not yet occurring.
Dr. Araújo is Professor and Dean of Research and Education, Exercise Medicine Clinic (CLINIMEX), Rio de Janeiro, Brazil. He reported conflicts of interest with INBRAMED.
A version of this article first appeared on Medscape.com.
Myth of the Month: Are Thickened Liquids Helpful in Dysphagia Patients?
Case: An 80-year-old man with advanced Alzheimer’s disease is admitted to the hospital after a fall. He is noted to have coughing spells after drinking liquids. He has a swallowing study done which shows severe slowing of motility in the esophagus.
What do you recommend?
A. Feeding tube
B. Thickened liquids
C. Continue current diet
The correct answer for this patient is to allow them to continue their current diet. They do not need thickened liquids. A feeding tube would not be recommended.1
So are there any data supporting the widespread use of thickened liquid diets for patients with dysphagia and aspiration?
Multiple clinical guidelines for stroke recommend the use of thickened liquids despite minimal to no evidence of efficacy.2 It is a common practice to give patients with advanced dementia thickened liquids, especially in the hospital setting. Does this help?
Makhnevich and colleagues published a cohort study of Alzheimer’s disease patients with dysphagia admitted to the hospital over a 5-year period.3 Almost half of the cohort received a thickened liquid diet; these patients were matched with patients who received a thin liquid diet. There was no significant difference in hospital mortality between the groups that received thick liquids and thin liquids (hazard ratio, 0.92; P = .46). Patients receiving thickened liquids were less likely to be intubated but were more likely to have pulmonary infections.
A 2018 Cochrane review concluded that there was no consensus on immediate and long-term effects of modifying the consistency of fluid for swallowing difficulties in dementia because too few studies have been completed.4 So why is this important information or lack of information?
What is so bad about a thickened liquid diet?
Eric Widera, MD, shared in JAMA Internal Medicine his experience along with his hospice and palliative care team of drinking thickened liquids.5 He drank only thickened liquids for a 12-hour period. “The challenge was eye-opening. It was the first time I experienced the terrible taste and texture of thickened liquids,” he wrote. He shared some of the risks of thickened liquids: dehydration, poor oral intake, and decreased quality of life.
The bottom line is that there is scant evidence for the benefit of thickened liquids, especially for patients with advanced dementia and dysphagia, and giving thickened liquids is not a benign intervention, because of poor tolerability of the diet.
References
1. American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. J Am Geriatr Soc. 2014;62(8):1590-3.
2. McCurtin A et al. J Eval Clin Pract. 2020;26:1744-60.
3. Makhnevich A et al. JAMA Intern Med. 2024 Jul 1;184(7):778-85.
4. Flynn E et al. Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011077.
5. Widera E. JAMA Intern Med. 2024 Jul 1;184(7):786-7.
Case: An 80-year-old man with advanced Alzheimer’s disease is admitted to the hospital after a fall. He is noted to have coughing spells after drinking liquids. He has a swallowing study done which shows severe slowing of motility in the esophagus.
What do you recommend?
A. Feeding tube
B. Thickened liquids
C. Continue current diet
The correct answer for this patient is to allow them to continue their current diet. They do not need thickened liquids. A feeding tube would not be recommended.1
So are there any data supporting the widespread use of thickened liquid diets for patients with dysphagia and aspiration?
Multiple clinical guidelines for stroke recommend the use of thickened liquids despite minimal to no evidence of efficacy.2 It is a common practice to give patients with advanced dementia thickened liquids, especially in the hospital setting. Does this help?
Makhnevich and colleagues published a cohort study of Alzheimer’s disease patients with dysphagia admitted to the hospital over a 5-year period.3 Almost half of the cohort received a thickened liquid diet; these patients were matched with patients who received a thin liquid diet. There was no significant difference in hospital mortality between the groups that received thick liquids and thin liquids (hazard ratio, 0.92; P = .46). Patients receiving thickened liquids were less likely to be intubated but were more likely to have pulmonary infections.
A 2018 Cochrane review concluded that there was no consensus on immediate and long-term effects of modifying the consistency of fluid for swallowing difficulties in dementia because too few studies have been completed.4 So why is this important information or lack of information?
What is so bad about a thickened liquid diet?
Eric Widera, MD, shared in JAMA Internal Medicine his experience along with his hospice and palliative care team of drinking thickened liquids.5 He drank only thickened liquids for a 12-hour period. “The challenge was eye-opening. It was the first time I experienced the terrible taste and texture of thickened liquids,” he wrote. He shared some of the risks of thickened liquids: dehydration, poor oral intake, and decreased quality of life.
The bottom line is that there is scant evidence for the benefit of thickened liquids, especially for patients with advanced dementia and dysphagia, and giving thickened liquids is not a benign intervention, because of poor tolerability of the diet.
References
1. American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. J Am Geriatr Soc. 2014;62(8):1590-3.
2. McCurtin A et al. J Eval Clin Pract. 2020;26:1744-60.
3. Makhnevich A et al. JAMA Intern Med. 2024 Jul 1;184(7):778-85.
4. Flynn E et al. Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011077.
5. Widera E. JAMA Intern Med. 2024 Jul 1;184(7):786-7.
Case: An 80-year-old man with advanced Alzheimer’s disease is admitted to the hospital after a fall. He is noted to have coughing spells after drinking liquids. He has a swallowing study done which shows severe slowing of motility in the esophagus.
What do you recommend?
A. Feeding tube
B. Thickened liquids
C. Continue current diet
The correct answer for this patient is to allow them to continue their current diet. They do not need thickened liquids. A feeding tube would not be recommended.1
So are there any data supporting the widespread use of thickened liquid diets for patients with dysphagia and aspiration?
Multiple clinical guidelines for stroke recommend the use of thickened liquids despite minimal to no evidence of efficacy.2 It is a common practice to give patients with advanced dementia thickened liquids, especially in the hospital setting. Does this help?
Makhnevich and colleagues published a cohort study of Alzheimer’s disease patients with dysphagia admitted to the hospital over a 5-year period.3 Almost half of the cohort received a thickened liquid diet; these patients were matched with patients who received a thin liquid diet. There was no significant difference in hospital mortality between the groups that received thick liquids and thin liquids (hazard ratio, 0.92; P = .46). Patients receiving thickened liquids were less likely to be intubated but were more likely to have pulmonary infections.
A 2018 Cochrane review concluded that there was no consensus on immediate and long-term effects of modifying the consistency of fluid for swallowing difficulties in dementia because too few studies have been completed.4 So why is this important information or lack of information?
What is so bad about a thickened liquid diet?
Eric Widera, MD, shared in JAMA Internal Medicine his experience along with his hospice and palliative care team of drinking thickened liquids.5 He drank only thickened liquids for a 12-hour period. “The challenge was eye-opening. It was the first time I experienced the terrible taste and texture of thickened liquids,” he wrote. He shared some of the risks of thickened liquids: dehydration, poor oral intake, and decreased quality of life.
The bottom line is that there is scant evidence for the benefit of thickened liquids, especially for patients with advanced dementia and dysphagia, and giving thickened liquids is not a benign intervention, because of poor tolerability of the diet.
References
1. American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. J Am Geriatr Soc. 2014;62(8):1590-3.
2. McCurtin A et al. J Eval Clin Pract. 2020;26:1744-60.
3. Makhnevich A et al. JAMA Intern Med. 2024 Jul 1;184(7):778-85.
4. Flynn E et al. Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011077.
5. Widera E. JAMA Intern Med. 2024 Jul 1;184(7):786-7.
Innovative Biomaterial May Treat Common Vaginal Changes and Discomfort in Menopausal Women
A novel biomaterial developed by researchers at the University of California, San Diego, may help treat commonly overlooked menopausal vaginal changes and discomfort experienced by many women.
As many as 84% of menopausal women experience genitourinary syndrome of menopause, a condition that can cause vaginal dryness, irritation, and pain during intercourse and significantly affect quality of life. Current treatments, mainly estrogen creams, help with surface issues but don’t address deeper tissue problems.
Marianna Alperin, MD, and researchers at her lab created a gel-like material derived from pig vaginal tissue designed to mimic the natural environment of the vagina and stimulate the body’s own healing processes.
“We used porcine vaginal tissue that was minced, decellularized by detergent, lyophilized, milled into powder, and enzymatically digested,” said Alperin, professor and vice chair for translational research in the Department of Obstetrics, Gynecology, and Reproductive Sciences and professor of urology at the University of California, San Diego.
Using the vaginal extracellular matrix biomaterial on rats — which have vaginal tissue similar to that of humans — improved vaginal epithelial thickness and health of the vaginal lining.
Three days after administering the biomaterial, the treatment group exhibited a mean epithelial thickness of 32.37 ± 6.29 µm, compared with 19.00 ± 1.59 µm in the saline control group (P < .0001). Rats treated with vaginal extracellular matrix biomaterial also showed a mean smooth muscle layer thickness of 54.02 ± 10.56 µm, significantly thicker than the saline group’s 35.07 ± 7.80 µm (P < .05), the study found.
“While [the biomaterial] did not restore the epithelial thickness all the way to the level of the healthy, unperturbed animals, it certainly was superior to the other groups, especially at the higher dose,” she said.
It also enhanced the underlying muscle layer, something current treatments don’t typically achieve, the researchers noted.
Alperin’s research was awarded best overall paper at the American Urogynecologic Society’s PFD Week conference in Washington, DC.
The material seems to work by interacting with immune cells to carry the healing material deeper into the vaginal tissues, potentially explaining its widespread effects.
“It looked like the cells are trafficking the biomaterial into the deeper tissues, which is very exciting,” said Alperin, adding that unlike existing treatments, this new approach may improve both the surface layer and deeper tissues of the vagina.
Also, the benefits appeared to increase with higher doses of the material, they found.
While the study shows promise, Alperin acknowledged that further research is needed, particularly in comparing their treatment with topical estrogen.
“We are repeating the experiment with the dose adjusted to the volume of the rat vagina,” Alperin said.
A version of this article appeared on Medscape.com.
A novel biomaterial developed by researchers at the University of California, San Diego, may help treat commonly overlooked menopausal vaginal changes and discomfort experienced by many women.
As many as 84% of menopausal women experience genitourinary syndrome of menopause, a condition that can cause vaginal dryness, irritation, and pain during intercourse and significantly affect quality of life. Current treatments, mainly estrogen creams, help with surface issues but don’t address deeper tissue problems.
Marianna Alperin, MD, and researchers at her lab created a gel-like material derived from pig vaginal tissue designed to mimic the natural environment of the vagina and stimulate the body’s own healing processes.
“We used porcine vaginal tissue that was minced, decellularized by detergent, lyophilized, milled into powder, and enzymatically digested,” said Alperin, professor and vice chair for translational research in the Department of Obstetrics, Gynecology, and Reproductive Sciences and professor of urology at the University of California, San Diego.
Using the vaginal extracellular matrix biomaterial on rats — which have vaginal tissue similar to that of humans — improved vaginal epithelial thickness and health of the vaginal lining.
Three days after administering the biomaterial, the treatment group exhibited a mean epithelial thickness of 32.37 ± 6.29 µm, compared with 19.00 ± 1.59 µm in the saline control group (P < .0001). Rats treated with vaginal extracellular matrix biomaterial also showed a mean smooth muscle layer thickness of 54.02 ± 10.56 µm, significantly thicker than the saline group’s 35.07 ± 7.80 µm (P < .05), the study found.
“While [the biomaterial] did not restore the epithelial thickness all the way to the level of the healthy, unperturbed animals, it certainly was superior to the other groups, especially at the higher dose,” she said.
It also enhanced the underlying muscle layer, something current treatments don’t typically achieve, the researchers noted.
Alperin’s research was awarded best overall paper at the American Urogynecologic Society’s PFD Week conference in Washington, DC.
The material seems to work by interacting with immune cells to carry the healing material deeper into the vaginal tissues, potentially explaining its widespread effects.
“It looked like the cells are trafficking the biomaterial into the deeper tissues, which is very exciting,” said Alperin, adding that unlike existing treatments, this new approach may improve both the surface layer and deeper tissues of the vagina.
Also, the benefits appeared to increase with higher doses of the material, they found.
While the study shows promise, Alperin acknowledged that further research is needed, particularly in comparing their treatment with topical estrogen.
“We are repeating the experiment with the dose adjusted to the volume of the rat vagina,” Alperin said.
A version of this article appeared on Medscape.com.
A novel biomaterial developed by researchers at the University of California, San Diego, may help treat commonly overlooked menopausal vaginal changes and discomfort experienced by many women.
As many as 84% of menopausal women experience genitourinary syndrome of menopause, a condition that can cause vaginal dryness, irritation, and pain during intercourse and significantly affect quality of life. Current treatments, mainly estrogen creams, help with surface issues but don’t address deeper tissue problems.
Marianna Alperin, MD, and researchers at her lab created a gel-like material derived from pig vaginal tissue designed to mimic the natural environment of the vagina and stimulate the body’s own healing processes.
“We used porcine vaginal tissue that was minced, decellularized by detergent, lyophilized, milled into powder, and enzymatically digested,” said Alperin, professor and vice chair for translational research in the Department of Obstetrics, Gynecology, and Reproductive Sciences and professor of urology at the University of California, San Diego.
Using the vaginal extracellular matrix biomaterial on rats — which have vaginal tissue similar to that of humans — improved vaginal epithelial thickness and health of the vaginal lining.
Three days after administering the biomaterial, the treatment group exhibited a mean epithelial thickness of 32.37 ± 6.29 µm, compared with 19.00 ± 1.59 µm in the saline control group (P < .0001). Rats treated with vaginal extracellular matrix biomaterial also showed a mean smooth muscle layer thickness of 54.02 ± 10.56 µm, significantly thicker than the saline group’s 35.07 ± 7.80 µm (P < .05), the study found.
“While [the biomaterial] did not restore the epithelial thickness all the way to the level of the healthy, unperturbed animals, it certainly was superior to the other groups, especially at the higher dose,” she said.
It also enhanced the underlying muscle layer, something current treatments don’t typically achieve, the researchers noted.
Alperin’s research was awarded best overall paper at the American Urogynecologic Society’s PFD Week conference in Washington, DC.
The material seems to work by interacting with immune cells to carry the healing material deeper into the vaginal tissues, potentially explaining its widespread effects.
“It looked like the cells are trafficking the biomaterial into the deeper tissues, which is very exciting,” said Alperin, adding that unlike existing treatments, this new approach may improve both the surface layer and deeper tissues of the vagina.
Also, the benefits appeared to increase with higher doses of the material, they found.
While the study shows promise, Alperin acknowledged that further research is needed, particularly in comparing their treatment with topical estrogen.
“We are repeating the experiment with the dose adjusted to the volume of the rat vagina,” Alperin said.
A version of this article appeared on Medscape.com.
More Evidence Ties Semaglutide to Reduced Alzheimer’s Risk
Adults with type 2 diabetes who were prescribed the GLP-1 RA semaglutide had a significantly lower risk for Alzheimer’s disease compared with their peers who were prescribed any of seven other antidiabetic medications, including other types of GLP-1 receptor–targeting medications.
“These findings support further clinical trials to assess semaglutide’s potential in delaying or preventing Alzheimer’s disease,” wrote the investigators, led by Rong Xu, PhD, with Case Western Reserve School of Medicine, Cleveland, Ohio.
The study was published online on October 24 in Alzheimer’s & Dementia.
Real-World Data
Semaglutide has shown neuroprotective effects in animal models of neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. In animal models of Alzheimer’s disease, the drug reduced beta-amyloid deposition and improved spatial learning and memory, as well as glucose metabolism in the brain.
In a real-world analysis, Xu and colleagues used electronic health record data to identify 17,104 new users of semaglutide and 1,077,657 new users of seven other antidiabetic medications, including other GLP-1 RAs, insulin, metformin, dipeptidyl peptidase 4 inhibitors, sodium-glucose cotransporter 2 inhibitors, sulfonylurea, and thiazolidinedione.
Over 3 years, treatment with semaglutide was associated with significantly reduced risk of developing Alzheimer’s disease, most strongly compared with insulin (hazard ratio [HR], 0.33) and most weakly compared with other GLP-1 RAs (HR, 0.59).
Compared with the other medications, semaglutide was associated with a 40%-70% reduced risk for first-time diagnosis of Alzheimer’s disease in patients with type 2 diabetes, with similar reductions seen across obesity status and gender and age groups, the authors reported.
The findings align with recent evidence suggesting GLP-1 RAs may protect cognitive function.
For example, as previously reported, in the phase 2b ELAD clinical trial, adults with early-stage Alzheimer’s disease taking the GLP-1 RA liraglutide exhibited slower decline in memory and thinking and experienced less brain atrophy over 12 months compared with placebo.
Promising, but Preliminary
Reached for comment, Courtney Kloske, PhD, Alzheimer’s Association director of scientific engagement, noted that diabetes is a known risk factor for AD and managing diabetes with drugs such as semaglutide “could benefit brain health simply by managing diabetes.”
“However, we still need large clinical trials in representative populations to determine if semaglutide specifically lowers the risk of Alzheimer’s, so it is too early to recommend it for prevention,” Kloske said.
She noted that some research suggests that GLP-1 RAs “may help reduce inflammation and positively impact brain energy use. However, more research is needed to fully understand how these processes might contribute to preventing cognitive decline or Alzheimer’s,” Kloske cautioned.
The Alzheimer’s Association’s “Part the Cloud” initiative has invested more than $68 million to advance 65 clinical trials targeting a variety of compounds, including repurposed drugs that may address known and potential new aspects of the disease, Kloske said.
The study was supported by grants from the National Institute on Aging and the National Center for Advancing Translational Sciences. Xu and Kloske have no relevant conflicts.
A version of this article appeared on Medscape.com.
Adults with type 2 diabetes who were prescribed the GLP-1 RA semaglutide had a significantly lower risk for Alzheimer’s disease compared with their peers who were prescribed any of seven other antidiabetic medications, including other types of GLP-1 receptor–targeting medications.
“These findings support further clinical trials to assess semaglutide’s potential in delaying or preventing Alzheimer’s disease,” wrote the investigators, led by Rong Xu, PhD, with Case Western Reserve School of Medicine, Cleveland, Ohio.
The study was published online on October 24 in Alzheimer’s & Dementia.
Real-World Data
Semaglutide has shown neuroprotective effects in animal models of neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. In animal models of Alzheimer’s disease, the drug reduced beta-amyloid deposition and improved spatial learning and memory, as well as glucose metabolism in the brain.
In a real-world analysis, Xu and colleagues used electronic health record data to identify 17,104 new users of semaglutide and 1,077,657 new users of seven other antidiabetic medications, including other GLP-1 RAs, insulin, metformin, dipeptidyl peptidase 4 inhibitors, sodium-glucose cotransporter 2 inhibitors, sulfonylurea, and thiazolidinedione.
Over 3 years, treatment with semaglutide was associated with significantly reduced risk of developing Alzheimer’s disease, most strongly compared with insulin (hazard ratio [HR], 0.33) and most weakly compared with other GLP-1 RAs (HR, 0.59).
Compared with the other medications, semaglutide was associated with a 40%-70% reduced risk for first-time diagnosis of Alzheimer’s disease in patients with type 2 diabetes, with similar reductions seen across obesity status and gender and age groups, the authors reported.
The findings align with recent evidence suggesting GLP-1 RAs may protect cognitive function.
For example, as previously reported, in the phase 2b ELAD clinical trial, adults with early-stage Alzheimer’s disease taking the GLP-1 RA liraglutide exhibited slower decline in memory and thinking and experienced less brain atrophy over 12 months compared with placebo.
Promising, but Preliminary
Reached for comment, Courtney Kloske, PhD, Alzheimer’s Association director of scientific engagement, noted that diabetes is a known risk factor for AD and managing diabetes with drugs such as semaglutide “could benefit brain health simply by managing diabetes.”
“However, we still need large clinical trials in representative populations to determine if semaglutide specifically lowers the risk of Alzheimer’s, so it is too early to recommend it for prevention,” Kloske said.
She noted that some research suggests that GLP-1 RAs “may help reduce inflammation and positively impact brain energy use. However, more research is needed to fully understand how these processes might contribute to preventing cognitive decline or Alzheimer’s,” Kloske cautioned.
The Alzheimer’s Association’s “Part the Cloud” initiative has invested more than $68 million to advance 65 clinical trials targeting a variety of compounds, including repurposed drugs that may address known and potential new aspects of the disease, Kloske said.
The study was supported by grants from the National Institute on Aging and the National Center for Advancing Translational Sciences. Xu and Kloske have no relevant conflicts.
A version of this article appeared on Medscape.com.
Adults with type 2 diabetes who were prescribed the GLP-1 RA semaglutide had a significantly lower risk for Alzheimer’s disease compared with their peers who were prescribed any of seven other antidiabetic medications, including other types of GLP-1 receptor–targeting medications.
“These findings support further clinical trials to assess semaglutide’s potential in delaying or preventing Alzheimer’s disease,” wrote the investigators, led by Rong Xu, PhD, with Case Western Reserve School of Medicine, Cleveland, Ohio.
The study was published online on October 24 in Alzheimer’s & Dementia.
Real-World Data
Semaglutide has shown neuroprotective effects in animal models of neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. In animal models of Alzheimer’s disease, the drug reduced beta-amyloid deposition and improved spatial learning and memory, as well as glucose metabolism in the brain.
In a real-world analysis, Xu and colleagues used electronic health record data to identify 17,104 new users of semaglutide and 1,077,657 new users of seven other antidiabetic medications, including other GLP-1 RAs, insulin, metformin, dipeptidyl peptidase 4 inhibitors, sodium-glucose cotransporter 2 inhibitors, sulfonylurea, and thiazolidinedione.
Over 3 years, treatment with semaglutide was associated with significantly reduced risk of developing Alzheimer’s disease, most strongly compared with insulin (hazard ratio [HR], 0.33) and most weakly compared with other GLP-1 RAs (HR, 0.59).
Compared with the other medications, semaglutide was associated with a 40%-70% reduced risk for first-time diagnosis of Alzheimer’s disease in patients with type 2 diabetes, with similar reductions seen across obesity status and gender and age groups, the authors reported.
The findings align with recent evidence suggesting GLP-1 RAs may protect cognitive function.
For example, as previously reported, in the phase 2b ELAD clinical trial, adults with early-stage Alzheimer’s disease taking the GLP-1 RA liraglutide exhibited slower decline in memory and thinking and experienced less brain atrophy over 12 months compared with placebo.
Promising, but Preliminary
Reached for comment, Courtney Kloske, PhD, Alzheimer’s Association director of scientific engagement, noted that diabetes is a known risk factor for AD and managing diabetes with drugs such as semaglutide “could benefit brain health simply by managing diabetes.”
“However, we still need large clinical trials in representative populations to determine if semaglutide specifically lowers the risk of Alzheimer’s, so it is too early to recommend it for prevention,” Kloske said.
She noted that some research suggests that GLP-1 RAs “may help reduce inflammation and positively impact brain energy use. However, more research is needed to fully understand how these processes might contribute to preventing cognitive decline or Alzheimer’s,” Kloske cautioned.
The Alzheimer’s Association’s “Part the Cloud” initiative has invested more than $68 million to advance 65 clinical trials targeting a variety of compounds, including repurposed drugs that may address known and potential new aspects of the disease, Kloske said.
The study was supported by grants from the National Institute on Aging and the National Center for Advancing Translational Sciences. Xu and Kloske have no relevant conflicts.
A version of this article appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA

