User login
FDA clears 5-minute test for early dementia
The U.S. Food and Drug Administration has given marketing clearance to CognICA, an artificial intelligence–powered integrated cognitive assessment for the early detection of dementia.
Developed by Cognetivity Neurosciences, CognICA is a 5-minute, computerized cognitive assessment that is completed using an iPad. The test offers several advantages over traditional pen-and-paper–based cognitive tests, the company said in a news release.
“These include its high sensitivity to early-stage cognitive impairment, avoidance of cultural or educational bias, and absence of learning effect upon repeat testing,” the company notes.
Because the test runs on a computer, it can support remote, self-administered testing at scale and is geared toward seamless integration with existing electronic health record systems, they add.
According to the latest Alzheimer’s Disease Facts and Figures, published by the Alzheimer’s Association, more than 6 million Americans are now living with Alzheimer’s disease. That number is projected to increase to 12.7 million by 2050.
“We’re excited about the opportunity to revolutionize the way cognitive impairment is assessed and managed in the U.S. and make a positive impact on the health and wellbeing of millions of Americans,” Sina Habibi, PhD, cofounder and CEO of Cognetivity, said in the news release.
The test has already received European regulatory approval as a CE-marked medical device and has been deployed in both primary and specialist clinical care in the U.K.’s National Health Service.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has given marketing clearance to CognICA, an artificial intelligence–powered integrated cognitive assessment for the early detection of dementia.
Developed by Cognetivity Neurosciences, CognICA is a 5-minute, computerized cognitive assessment that is completed using an iPad. The test offers several advantages over traditional pen-and-paper–based cognitive tests, the company said in a news release.
“These include its high sensitivity to early-stage cognitive impairment, avoidance of cultural or educational bias, and absence of learning effect upon repeat testing,” the company notes.
Because the test runs on a computer, it can support remote, self-administered testing at scale and is geared toward seamless integration with existing electronic health record systems, they add.
According to the latest Alzheimer’s Disease Facts and Figures, published by the Alzheimer’s Association, more than 6 million Americans are now living with Alzheimer’s disease. That number is projected to increase to 12.7 million by 2050.
“We’re excited about the opportunity to revolutionize the way cognitive impairment is assessed and managed in the U.S. and make a positive impact on the health and wellbeing of millions of Americans,” Sina Habibi, PhD, cofounder and CEO of Cognetivity, said in the news release.
The test has already received European regulatory approval as a CE-marked medical device and has been deployed in both primary and specialist clinical care in the U.K.’s National Health Service.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has given marketing clearance to CognICA, an artificial intelligence–powered integrated cognitive assessment for the early detection of dementia.
Developed by Cognetivity Neurosciences, CognICA is a 5-minute, computerized cognitive assessment that is completed using an iPad. The test offers several advantages over traditional pen-and-paper–based cognitive tests, the company said in a news release.
“These include its high sensitivity to early-stage cognitive impairment, avoidance of cultural or educational bias, and absence of learning effect upon repeat testing,” the company notes.
Because the test runs on a computer, it can support remote, self-administered testing at scale and is geared toward seamless integration with existing electronic health record systems, they add.
According to the latest Alzheimer’s Disease Facts and Figures, published by the Alzheimer’s Association, more than 6 million Americans are now living with Alzheimer’s disease. That number is projected to increase to 12.7 million by 2050.
“We’re excited about the opportunity to revolutionize the way cognitive impairment is assessed and managed in the U.S. and make a positive impact on the health and wellbeing of millions of Americans,” Sina Habibi, PhD, cofounder and CEO of Cognetivity, said in the news release.
The test has already received European regulatory approval as a CE-marked medical device and has been deployed in both primary and specialist clinical care in the U.K.’s National Health Service.
A version of this article first appeared on Medscape.com.
Guidelines for dementia and age-related cognitive changes
It is estimated that by the year 2060, 13.9 million Americans over the age of 65 will be diagnosed with dementia. Few good treatments are currently available.
Earlier this year, the American Psychological Association (APA) Task Force issued clinical guidelines “for the Evaluation of Dementia and Age-Related Cognitive Change.” While these 16 guidelines are aimed at psychologists, primary care doctors are often the first ones to evaluate a patient who may have dementia. As a family physician, I find having these guidelines especially helpful.
Neuropsychiatric testing and defining severity and type
This new guidance places emphasis on neuropsychiatric testing and defining the severity and type of dementia present.
Over the past 2 decades, diagnoses of mild neurocognitive disorders have increased, and this, in part, is due to diagnosing these problems earlier and with greater precision. It is also important to know that biomarkers are being increasingly researched, and it is imperative that we stay current with this research.
Cognitive decline may also occur with the coexistence of other mental health disorders, such as depression, so it is important that we screen for these as well. This is often difficult given the behavioral changes that can arise in dementia, but, as primary care doctors, we must differentiate these to treat our patients appropriately.
Informed consent
Informed consent can become an issue with patients with dementia. It must be assessed whether the patient has the capacity to make an informed decision and can competently communicate that decision.
The diagnosis of dementia alone does not preclude a patient from giving informed consent. A patient’s mental capacity must be determined, and if they are not capable of making an informed decision, the person legally responsible for giving informed consent on behalf of the patient must be identified.
Patients with dementia often have other medical comorbidities and take several medications. It is imperative to keep accurate medical records and medication lists. Sometimes, patients with dementia cannot provide this information. If that is the case, every attempt should be made to obtain records from every possible source.
Cultural competence
The guidelines also stress that there may be cultural differences when applying neuropsychiatric tests. It is our duty to maintain cultural competence and understand these differences. We all need to work to ensure we control our biases, and it is suggested that we review relevant evidence-based literature.
While ageism is common in our society, it shouldn’t be in our practices. For these reasons, outreach in at-risk populations is very important.
Pertinent data
The guidelines also suggest obtaining all possible information in our evaluation, especially when the patient is unable to give it to us.
Often, as primary care physicians, we refer these patients to other providers, and we should be providing all pertinent data to those we are referring these patients to. If all information is not available at the time of evaluation, follow-up visits should be scheduled.
If possible, family members should be present at the time of visit. They often provide valuable information regarding the extent and progression of the decline. Also, they know how the patient is functioning in the home setting and how much assistance they need with activities of daily living.
Caretaker support
Another important factor to consider is caretaker burnout. Caretakers are often under a lot of stress and have high rates of depression. It is important to provide them with education and support, as well as resources that may be available to them. For some, accepting the diagnosis that their loved one has dementia may be a struggle.
As doctors treating dementia patients, we need to know the resources that are available to assist dementia patients and their families. There are many local organizations that can help.
Also, research into dementia is ongoing and we need to stay current. The diagnosis of dementia should be made as early as possible using appropriate screening tools. The sooner the diagnosis is made, the quicker interventions can be started and the family members, as well as the patient, can come to accept the diagnosis.
As the population ages, we can expect the demands of dementia to rise as well. Primary care doctors are in a unique position to diagnose dementia once it starts to appear.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
It is estimated that by the year 2060, 13.9 million Americans over the age of 65 will be diagnosed with dementia. Few good treatments are currently available.
Earlier this year, the American Psychological Association (APA) Task Force issued clinical guidelines “for the Evaluation of Dementia and Age-Related Cognitive Change.” While these 16 guidelines are aimed at psychologists, primary care doctors are often the first ones to evaluate a patient who may have dementia. As a family physician, I find having these guidelines especially helpful.
Neuropsychiatric testing and defining severity and type
This new guidance places emphasis on neuropsychiatric testing and defining the severity and type of dementia present.
Over the past 2 decades, diagnoses of mild neurocognitive disorders have increased, and this, in part, is due to diagnosing these problems earlier and with greater precision. It is also important to know that biomarkers are being increasingly researched, and it is imperative that we stay current with this research.
Cognitive decline may also occur with the coexistence of other mental health disorders, such as depression, so it is important that we screen for these as well. This is often difficult given the behavioral changes that can arise in dementia, but, as primary care doctors, we must differentiate these to treat our patients appropriately.
Informed consent
Informed consent can become an issue with patients with dementia. It must be assessed whether the patient has the capacity to make an informed decision and can competently communicate that decision.
The diagnosis of dementia alone does not preclude a patient from giving informed consent. A patient’s mental capacity must be determined, and if they are not capable of making an informed decision, the person legally responsible for giving informed consent on behalf of the patient must be identified.
Patients with dementia often have other medical comorbidities and take several medications. It is imperative to keep accurate medical records and medication lists. Sometimes, patients with dementia cannot provide this information. If that is the case, every attempt should be made to obtain records from every possible source.
Cultural competence
The guidelines also stress that there may be cultural differences when applying neuropsychiatric tests. It is our duty to maintain cultural competence and understand these differences. We all need to work to ensure we control our biases, and it is suggested that we review relevant evidence-based literature.
While ageism is common in our society, it shouldn’t be in our practices. For these reasons, outreach in at-risk populations is very important.
Pertinent data
The guidelines also suggest obtaining all possible information in our evaluation, especially when the patient is unable to give it to us.
Often, as primary care physicians, we refer these patients to other providers, and we should be providing all pertinent data to those we are referring these patients to. If all information is not available at the time of evaluation, follow-up visits should be scheduled.
If possible, family members should be present at the time of visit. They often provide valuable information regarding the extent and progression of the decline. Also, they know how the patient is functioning in the home setting and how much assistance they need with activities of daily living.
Caretaker support
Another important factor to consider is caretaker burnout. Caretakers are often under a lot of stress and have high rates of depression. It is important to provide them with education and support, as well as resources that may be available to them. For some, accepting the diagnosis that their loved one has dementia may be a struggle.
As doctors treating dementia patients, we need to know the resources that are available to assist dementia patients and their families. There are many local organizations that can help.
Also, research into dementia is ongoing and we need to stay current. The diagnosis of dementia should be made as early as possible using appropriate screening tools. The sooner the diagnosis is made, the quicker interventions can be started and the family members, as well as the patient, can come to accept the diagnosis.
As the population ages, we can expect the demands of dementia to rise as well. Primary care doctors are in a unique position to diagnose dementia once it starts to appear.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
It is estimated that by the year 2060, 13.9 million Americans over the age of 65 will be diagnosed with dementia. Few good treatments are currently available.
Earlier this year, the American Psychological Association (APA) Task Force issued clinical guidelines “for the Evaluation of Dementia and Age-Related Cognitive Change.” While these 16 guidelines are aimed at psychologists, primary care doctors are often the first ones to evaluate a patient who may have dementia. As a family physician, I find having these guidelines especially helpful.
Neuropsychiatric testing and defining severity and type
This new guidance places emphasis on neuropsychiatric testing and defining the severity and type of dementia present.
Over the past 2 decades, diagnoses of mild neurocognitive disorders have increased, and this, in part, is due to diagnosing these problems earlier and with greater precision. It is also important to know that biomarkers are being increasingly researched, and it is imperative that we stay current with this research.
Cognitive decline may also occur with the coexistence of other mental health disorders, such as depression, so it is important that we screen for these as well. This is often difficult given the behavioral changes that can arise in dementia, but, as primary care doctors, we must differentiate these to treat our patients appropriately.
Informed consent
Informed consent can become an issue with patients with dementia. It must be assessed whether the patient has the capacity to make an informed decision and can competently communicate that decision.
The diagnosis of dementia alone does not preclude a patient from giving informed consent. A patient’s mental capacity must be determined, and if they are not capable of making an informed decision, the person legally responsible for giving informed consent on behalf of the patient must be identified.
Patients with dementia often have other medical comorbidities and take several medications. It is imperative to keep accurate medical records and medication lists. Sometimes, patients with dementia cannot provide this information. If that is the case, every attempt should be made to obtain records from every possible source.
Cultural competence
The guidelines also stress that there may be cultural differences when applying neuropsychiatric tests. It is our duty to maintain cultural competence and understand these differences. We all need to work to ensure we control our biases, and it is suggested that we review relevant evidence-based literature.
While ageism is common in our society, it shouldn’t be in our practices. For these reasons, outreach in at-risk populations is very important.
Pertinent data
The guidelines also suggest obtaining all possible information in our evaluation, especially when the patient is unable to give it to us.
Often, as primary care physicians, we refer these patients to other providers, and we should be providing all pertinent data to those we are referring these patients to. If all information is not available at the time of evaluation, follow-up visits should be scheduled.
If possible, family members should be present at the time of visit. They often provide valuable information regarding the extent and progression of the decline. Also, they know how the patient is functioning in the home setting and how much assistance they need with activities of daily living.
Caretaker support
Another important factor to consider is caretaker burnout. Caretakers are often under a lot of stress and have high rates of depression. It is important to provide them with education and support, as well as resources that may be available to them. For some, accepting the diagnosis that their loved one has dementia may be a struggle.
As doctors treating dementia patients, we need to know the resources that are available to assist dementia patients and their families. There are many local organizations that can help.
Also, research into dementia is ongoing and we need to stay current. The diagnosis of dementia should be made as early as possible using appropriate screening tools. The sooner the diagnosis is made, the quicker interventions can be started and the family members, as well as the patient, can come to accept the diagnosis.
As the population ages, we can expect the demands of dementia to rise as well. Primary care doctors are in a unique position to diagnose dementia once it starts to appear.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
Let’s talk about healthy aging (but where to begin?)
This month’s cover story, “A 4-pronged approach to foster healthy aging in older adults,” by Wilson and colleagues (page 376) provides a wealth of information about aspects of healthy aging that we should consider when we see our older patients. After reading this manuscript, I was a bit overwhelmed by the amount of information presented and, more so, by the thought of attempting to incorporate into my practice all of the possible screenings and interventions available to help older adults improve and maintain their health.
There is no debate about the importance of issues such as diet, exercise and mobility, mental health and cognition, vision and hearing, and strong social connections for maintaining health as we age. The difficulty comes in deciding how to spend our limited time with older patients during office encounters. Most older adults have several chronic diseases that need our attention, and they often have various medications that need to be monitored for effectiveness and safety, which can be time consuming. And, of course, we need to take time to screen for cardiovascular risk and cancer, too. Where to start?
Dr. Wilson’s solution makes sense to me: Take advantage of the annual wellness visit to discuss diet, exercise, mental health, vision and hearing, and social relationships. I am not so sure, however, if using formal screening instruments is the best way to do this, especially since there is no strong research that demonstrates improved patient-relevant outcomes using screening instruments, except, perhaps, for periodically screening for anxiety and depression.
It may be as effective to use what I will call the “chat technique.” Start with open-ended questions and statements, such as: “How are things going for you?” “Tell me about your family.” “What do you do for physical activity?” and “How has your mood been lately?”
An excellent complement to the chat technique is the goal-setting approach championed by geriatrician and family physician Jim Mold.1 His premise is that health itself is not the most important goal for most people, but rather a means to an end. That end is very specific to every person. An elderly, frail woman’s main life goal, for example, might be to remain in her own home for as long as possible or to live long enough to attend her great-grandson’s wedding.
Goal setting provides an excellent context for true shared decision-making. I agree with Dr. Wilson’s closing statement:
“As family physicians, it is important to capitalize on longitudinal relationships with patients and begin educating younger patients using this multidimensional framework to promote living ‘a productive and meaningful life’at any age.”
1. Mold, JW. Goal-Oriented Medical Care: Helping Patients Achieve Their Personal Health Goals. Full Court Press; 2017.
This month’s cover story, “A 4-pronged approach to foster healthy aging in older adults,” by Wilson and colleagues (page 376) provides a wealth of information about aspects of healthy aging that we should consider when we see our older patients. After reading this manuscript, I was a bit overwhelmed by the amount of information presented and, more so, by the thought of attempting to incorporate into my practice all of the possible screenings and interventions available to help older adults improve and maintain their health.
There is no debate about the importance of issues such as diet, exercise and mobility, mental health and cognition, vision and hearing, and strong social connections for maintaining health as we age. The difficulty comes in deciding how to spend our limited time with older patients during office encounters. Most older adults have several chronic diseases that need our attention, and they often have various medications that need to be monitored for effectiveness and safety, which can be time consuming. And, of course, we need to take time to screen for cardiovascular risk and cancer, too. Where to start?
Dr. Wilson’s solution makes sense to me: Take advantage of the annual wellness visit to discuss diet, exercise, mental health, vision and hearing, and social relationships. I am not so sure, however, if using formal screening instruments is the best way to do this, especially since there is no strong research that demonstrates improved patient-relevant outcomes using screening instruments, except, perhaps, for periodically screening for anxiety and depression.
It may be as effective to use what I will call the “chat technique.” Start with open-ended questions and statements, such as: “How are things going for you?” “Tell me about your family.” “What do you do for physical activity?” and “How has your mood been lately?”
An excellent complement to the chat technique is the goal-setting approach championed by geriatrician and family physician Jim Mold.1 His premise is that health itself is not the most important goal for most people, but rather a means to an end. That end is very specific to every person. An elderly, frail woman’s main life goal, for example, might be to remain in her own home for as long as possible or to live long enough to attend her great-grandson’s wedding.
Goal setting provides an excellent context for true shared decision-making. I agree with Dr. Wilson’s closing statement:
“As family physicians, it is important to capitalize on longitudinal relationships with patients and begin educating younger patients using this multidimensional framework to promote living ‘a productive and meaningful life’at any age.”
This month’s cover story, “A 4-pronged approach to foster healthy aging in older adults,” by Wilson and colleagues (page 376) provides a wealth of information about aspects of healthy aging that we should consider when we see our older patients. After reading this manuscript, I was a bit overwhelmed by the amount of information presented and, more so, by the thought of attempting to incorporate into my practice all of the possible screenings and interventions available to help older adults improve and maintain their health.
There is no debate about the importance of issues such as diet, exercise and mobility, mental health and cognition, vision and hearing, and strong social connections for maintaining health as we age. The difficulty comes in deciding how to spend our limited time with older patients during office encounters. Most older adults have several chronic diseases that need our attention, and they often have various medications that need to be monitored for effectiveness and safety, which can be time consuming. And, of course, we need to take time to screen for cardiovascular risk and cancer, too. Where to start?
Dr. Wilson’s solution makes sense to me: Take advantage of the annual wellness visit to discuss diet, exercise, mental health, vision and hearing, and social relationships. I am not so sure, however, if using formal screening instruments is the best way to do this, especially since there is no strong research that demonstrates improved patient-relevant outcomes using screening instruments, except, perhaps, for periodically screening for anxiety and depression.
It may be as effective to use what I will call the “chat technique.” Start with open-ended questions and statements, such as: “How are things going for you?” “Tell me about your family.” “What do you do for physical activity?” and “How has your mood been lately?”
An excellent complement to the chat technique is the goal-setting approach championed by geriatrician and family physician Jim Mold.1 His premise is that health itself is not the most important goal for most people, but rather a means to an end. That end is very specific to every person. An elderly, frail woman’s main life goal, for example, might be to remain in her own home for as long as possible or to live long enough to attend her great-grandson’s wedding.
Goal setting provides an excellent context for true shared decision-making. I agree with Dr. Wilson’s closing statement:
“As family physicians, it is important to capitalize on longitudinal relationships with patients and begin educating younger patients using this multidimensional framework to promote living ‘a productive and meaningful life’at any age.”
1. Mold, JW. Goal-Oriented Medical Care: Helping Patients Achieve Their Personal Health Goals. Full Court Press; 2017.
1. Mold, JW. Goal-Oriented Medical Care: Helping Patients Achieve Their Personal Health Goals. Full Court Press; 2017.
Bone risk: Is time since menopause a better predictor than age?
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
FDA proposes new rule for over-the-counter hearing aids
The action comes nearly 5 years after Congress passed a law to allow over-the-counter sales for people with mild to moderate hearing loss.
Those with severe hearing loss or people under 18 years old would still need to see a doctor or specialist for a hearing device.
In the United States, access to hearing aids can be difficult and expensive. Usually, patients have to go see their health care providers for a prescription. Then, they go to an audiologist, or a hearing aid specialist, to get the devices fitted.
With the proposed rule, patients could skip both of those steps and buy hearing aids in retail stores or online. This would make the process easier and more cost-friendly, as well increase access to specialists for many Americans who don’t have it.
“This allows us to put hearing devices more in reach of communities that have often been left out. Communities of color and the underserved typically and traditionally lacked access to hearing aids,” Xavier Becerra, secretary of the U.S. Department of Health and Human Services, said at a news briefing.
The FDA says it’s unclear exactly when the new products will be in stores, but finalizing the ruling is a top priority.
For new products, the ruling is expected to go into effect 60 days after it is finalized. Current products would have 180 days to make changes, according to the FDA.
The American Academy of Audiology said in a statement that it is reviewing the proposed rules and will provide comments to the FDA. But in July, Angela Shoup, PhD, a professor at the School of Behavioral and Brain Sciences at the University of Texas at Dallas, wrote to Mr. Becerra with concerns about over-the-counter sales of hearing aids.
“While we certainly support efforts to lower costs and improve access to hearing aids, we have grave concerns about the oversimplification of hearing loss and treatment in the advancement of OTC devices,” she wrote.
“It is through involvement of an audiologist that consumers will achieve the best possible outcomes with OTC hearing aids and avoid the risks of under- or untreated hearing loss,” Dr. Shoup said.
This new category would apply to certain air conduction hearing aids, which are worn inside of the ear and improve hearing by boosting sound into the ear canal.
The FDA is proposing labeling requirements for the hearing devices, including warnings, age restrictions, and information on severe hearing loss and other medical conditions that would prompt patients to seek treatment from their doctors.
The FDA said that it would closely monitor the marketplace to make sure companies advertising hearing loss products follow federal regulations.
There are a number of reasons for hearing loss, including exposure to extremely loud noises, aging, and various medical conditions. Approximately 38 million Americans 18 years old and older report having hearing trouble, said Janet Woodcock, MD, acting commissioner of the FDA.
About 20% of people who could benefit from hearing aids are using them, with barriers to access being a major factor, she added.
“Hearing loss can have a profound impact on daily communication, social interaction, and overall health and quality of life for millions of Americans,” Dr. Woodcock said.
The FDA has updated its guidance on hearing devices and personal sound amplification products.
Personal sound amplification products (PSAPs) are nonmedical devices designed to amplify sounds for people with normal hearing and are usually used for activities such as bird-watching and hunting.
Amplification devices are not regulated by the FDA.
A version of this article first appeared on WebMD.com.
The action comes nearly 5 years after Congress passed a law to allow over-the-counter sales for people with mild to moderate hearing loss.
Those with severe hearing loss or people under 18 years old would still need to see a doctor or specialist for a hearing device.
In the United States, access to hearing aids can be difficult and expensive. Usually, patients have to go see their health care providers for a prescription. Then, they go to an audiologist, or a hearing aid specialist, to get the devices fitted.
With the proposed rule, patients could skip both of those steps and buy hearing aids in retail stores or online. This would make the process easier and more cost-friendly, as well increase access to specialists for many Americans who don’t have it.
“This allows us to put hearing devices more in reach of communities that have often been left out. Communities of color and the underserved typically and traditionally lacked access to hearing aids,” Xavier Becerra, secretary of the U.S. Department of Health and Human Services, said at a news briefing.
The FDA says it’s unclear exactly when the new products will be in stores, but finalizing the ruling is a top priority.
For new products, the ruling is expected to go into effect 60 days after it is finalized. Current products would have 180 days to make changes, according to the FDA.
The American Academy of Audiology said in a statement that it is reviewing the proposed rules and will provide comments to the FDA. But in July, Angela Shoup, PhD, a professor at the School of Behavioral and Brain Sciences at the University of Texas at Dallas, wrote to Mr. Becerra with concerns about over-the-counter sales of hearing aids.
“While we certainly support efforts to lower costs and improve access to hearing aids, we have grave concerns about the oversimplification of hearing loss and treatment in the advancement of OTC devices,” she wrote.
“It is through involvement of an audiologist that consumers will achieve the best possible outcomes with OTC hearing aids and avoid the risks of under- or untreated hearing loss,” Dr. Shoup said.
This new category would apply to certain air conduction hearing aids, which are worn inside of the ear and improve hearing by boosting sound into the ear canal.
The FDA is proposing labeling requirements for the hearing devices, including warnings, age restrictions, and information on severe hearing loss and other medical conditions that would prompt patients to seek treatment from their doctors.
The FDA said that it would closely monitor the marketplace to make sure companies advertising hearing loss products follow federal regulations.
There are a number of reasons for hearing loss, including exposure to extremely loud noises, aging, and various medical conditions. Approximately 38 million Americans 18 years old and older report having hearing trouble, said Janet Woodcock, MD, acting commissioner of the FDA.
About 20% of people who could benefit from hearing aids are using them, with barriers to access being a major factor, she added.
“Hearing loss can have a profound impact on daily communication, social interaction, and overall health and quality of life for millions of Americans,” Dr. Woodcock said.
The FDA has updated its guidance on hearing devices and personal sound amplification products.
Personal sound amplification products (PSAPs) are nonmedical devices designed to amplify sounds for people with normal hearing and are usually used for activities such as bird-watching and hunting.
Amplification devices are not regulated by the FDA.
A version of this article first appeared on WebMD.com.
The action comes nearly 5 years after Congress passed a law to allow over-the-counter sales for people with mild to moderate hearing loss.
Those with severe hearing loss or people under 18 years old would still need to see a doctor or specialist for a hearing device.
In the United States, access to hearing aids can be difficult and expensive. Usually, patients have to go see their health care providers for a prescription. Then, they go to an audiologist, or a hearing aid specialist, to get the devices fitted.
With the proposed rule, patients could skip both of those steps and buy hearing aids in retail stores or online. This would make the process easier and more cost-friendly, as well increase access to specialists for many Americans who don’t have it.
“This allows us to put hearing devices more in reach of communities that have often been left out. Communities of color and the underserved typically and traditionally lacked access to hearing aids,” Xavier Becerra, secretary of the U.S. Department of Health and Human Services, said at a news briefing.
The FDA says it’s unclear exactly when the new products will be in stores, but finalizing the ruling is a top priority.
For new products, the ruling is expected to go into effect 60 days after it is finalized. Current products would have 180 days to make changes, according to the FDA.
The American Academy of Audiology said in a statement that it is reviewing the proposed rules and will provide comments to the FDA. But in July, Angela Shoup, PhD, a professor at the School of Behavioral and Brain Sciences at the University of Texas at Dallas, wrote to Mr. Becerra with concerns about over-the-counter sales of hearing aids.
“While we certainly support efforts to lower costs and improve access to hearing aids, we have grave concerns about the oversimplification of hearing loss and treatment in the advancement of OTC devices,” she wrote.
“It is through involvement of an audiologist that consumers will achieve the best possible outcomes with OTC hearing aids and avoid the risks of under- or untreated hearing loss,” Dr. Shoup said.
This new category would apply to certain air conduction hearing aids, which are worn inside of the ear and improve hearing by boosting sound into the ear canal.
The FDA is proposing labeling requirements for the hearing devices, including warnings, age restrictions, and information on severe hearing loss and other medical conditions that would prompt patients to seek treatment from their doctors.
The FDA said that it would closely monitor the marketplace to make sure companies advertising hearing loss products follow federal regulations.
There are a number of reasons for hearing loss, including exposure to extremely loud noises, aging, and various medical conditions. Approximately 38 million Americans 18 years old and older report having hearing trouble, said Janet Woodcock, MD, acting commissioner of the FDA.
About 20% of people who could benefit from hearing aids are using them, with barriers to access being a major factor, she added.
“Hearing loss can have a profound impact on daily communication, social interaction, and overall health and quality of life for millions of Americans,” Dr. Woodcock said.
The FDA has updated its guidance on hearing devices and personal sound amplification products.
Personal sound amplification products (PSAPs) are nonmedical devices designed to amplify sounds for people with normal hearing and are usually used for activities such as bird-watching and hunting.
Amplification devices are not regulated by the FDA.
A version of this article first appeared on WebMD.com.
A 4-pronged approach to foster healthy aging in older adults
Our approach to caring for the growing number of community-dwelling US adults ages ≥ 65 years has shifted. Although we continue to manage disease and disability, there is an increasing emphasis on the promotion of healthy aging by optimizing health care needs and quality of life (QOL).
The American Geriatric Society (AGS) uses the term “healthy aging” to reflect a dedication to improving the health, independence, and QOL of older people.1 The World Health Organization (WHO) defines healthy aging as “the process of developing and maintaining the functional ability that enables well-being in older age.”2 Functional ability encompasses capabilities that align with a person’s values, including meeting basic needs; learning, growing, and making independent decisions; being mobile; building and maintaining healthy relationships; and contributing to society.2 Similarly, the US Department of Health and Human Services has adopted a multidimensional approach to support people in creating “a productive and meaningful life” as they grow older.3
Numerous theoretical models have emerged from research on aging as a multidimensional construct, as evidenced by a 2016 citation analysis that identified 1755 articles written between 1902 and 2015 relating to “successful aging.”4 The analysis revealed 609 definitions operationalized by researchers’ measurement tools (mostly focused on physical function and other health metrics) and 1146 descriptions created by older adults, many emphasizing psychosocial strategies and cultural factors as key to successful aging.4

One approach that is likely to be useful for family physicians is the Age-Friendly Health System. This is an initiative of The John A. Hartford Foundation and the Institute for Healthcare Improvement that uses a multidisciplinary approach to create environments that foster inclusivity and address the needs of older people.5 Following this guidance, primary care providers use evidence-informed strategies that promote safety and address what matters most to older adults and their family caregivers.
The Age-Friendly Health System, as well as AGS and WHO, recognize that there are multiple aspects to well-being as one grows older. By using focused, evidence-based screening, assessments, and interventions, family physicians can best support aging patients in living their most fulfilling lives.
Here we present a review of evidence-based strategies that promote safety and address what matters most to older adults and their family caregivers using a 4-pronged framework, in the style of the Age-Friendly Health System model. However, the literature on healthy aging includes important messages about patient context and lifelong health behaviors, which we capture in an expanded set of thematic guidance. As such, we encourage family physicians to approach healthy aging as follows: (1) monitor health (screening and prevention), (2) promote mobility (physical function), (3) manage mentation (emotional health and cognitive function), and (4) encourage maintenance of social connections (social networks and QOL).
Monitoring health
Leverage Medicare annual wellness visits. A systematic approach is needed to prevent frailty and functional decline, and thus increase the QOL of older adults. To do this, it is important to focus on health promotion and disease prevention, while addressing existing ailments. One method is to leverage the Medicare annual wellness visit (AWV), which provides an opportunity to assess current health status as well as discuss behavior-change and risk-reduction strategies with patients.
Continue to: Although AWVs...
Although AWVs are an opportunity to improve patient outcomes, we are not taking full advantage of them.6 While AWVs have gained traction since their introduction in 2011, usage rates among ethnoracial minority groups has lagged behind.6 A 2018 cohort study examined reasons for disparate utilization rates among individuals ages ≥ 66 years (N = 14,687).7 Researchers found that differences in utilization between ethnoracial groups were explained by socioeconomic factors. Lower education and lower income, as well as rural living, were associated with lower rates of AWV completion.7 In addition, having a usual, nonemergent place to obtain medical care served as a powerful predictor of AWV utilization for all groups.7
Strategies to increase AWV completion rates among all eligible adults include increasing staff awareness of health literacy challenges and ensuring communication strategies are inclusive by providing printed materials in multiple languages, Braille, or larger typefaces; using accessible vocabulary that does not include medical jargon; and making medical interpreters accessible. In addition, training clinicians about unconscious bias and cultural humility can help foster empathy and awareness of differences in health beliefs and behaviors within diverse patient populations.
A 2019 scoping review of 11 studies (N > 60 million) focused on outcomes from Medicare AWVs for patients ages ≥ 65 years.8 This included uptake of preventive services, such as vaccinations or cancer screenings; advice, education, or referrals offered during the AWV; medication use; and hospitalization rates. Overall findings showed that older adults who received a Medicare AWV were more likely to receive referrals for preventive screenings and follow-through on these recommendations compared with those who did not undergo an AWV.8
Completion rates for vaccines, while remaining low overall, were higher among those who completed an AWV. Additionally, these studies showed improved completion of screenings for breast cancer, bone density, and colon cancer. Several studies in the scoping review supported the use of AWVs as an effective means by which to offer health education and advice related to health promotion and risk reduction, such as diet and lifestyle modifications.8 Little evidence exists on long-term outcomes related to AWV completion.8
Utilize shared decision-making to determine whether preventive screening makes sense for your patient. Although cancer remains the second leading cause of death among Americans ages ≥ 65 years,9 clear screening guidelines for this age group remain elusive.10 Physicians and patients often are reluctant to stop cancer screening despite lower life expectancy and fewer potential benefits of diagnosis in this population.9 Some recent studies reinforce the heterogeneity of the older adult population and further underscore the importance of individual-level decision-making.11-14 It is important to let older adult patients and their caregivers know about the potential risks of screening tests, especially the possibility that incidental findings may lead to unwarranted additional care or monitoring.9
Continue to: Avoid these screening conversation missteps
Avoid these screening conversation missteps. A 2017 qualitative study asked 40 community-dwelling older adults (mean age = 76 years) about their preferences for discussing screening cessation with their physicians.13 Three themes emerged.First, they were open to stopping their screenings, especially when suggested by a trusted physician. Second, health status and physical function made sense as decision points, but life expectancy did not. Finally, lengthy discussions with expanded details about risks and benefits were not appreciated, especially if coupled with comments on the limited benefits for those nearing the end of life. When discussing life expectancy, patients preferred phrasing that focused on how the screening was unnecessary because it would not help them live longer.13
Ensure that your message is understood—and culturally relevant. Recent studies on lower health literacy among older adults15,16 and ethnic and racial minorities17-21—as revealed in the 2003 National Assessment of Adult Literacy22—might offer clues to patient receptivity to discussions about preventive screening and other health decisions.
One study found a significant correlation between higher self-rated health literacy and higher engagement in health behaviors such as mammography screening, moderate physical activity, and tobacco avoidance.16 Perceptions of personal control over health status, as well as perceived social standing, also correlated with health literacy score levels.16 Another study concluded that lower health literacy combined with lower self-efficacy, cultural beliefs about health topics (eg, diet and exercise), and distrust in the health care system contributed to lower rates of preventive care utilization among ethnocultural minority older adults in Canada, the United Kingdom, the United States, and Australia.18
Ensuring that easy-to-understand information is equitably shared with older adults of all races and ethnicities is critical. A 2018 study showed that distrust of the health system and cultural issues contributed to the lower incidence of colorectal cancer screenings in Hispanic and Asian American patients ages 50 to 75 years.21 Patients whose physicians engaged in “health literate practices” (eg, offering clear explanations of diagnostic plans and asking patients to describe what they understood) were more likely to obtain recommended breast and colorectal cancer screenings.20 In particular, researchers found that non-Hispanic Blacks were nearly twice as likely to follow through on colorectal cancer screening if their physicians engaged in health literate practices.20 In addition, receiving clear instructions from physicians increased the odds of completing breast cancer screening among Hispanic and non-Hispanic White women.20
Overall, screening information and recommendations should be standardized for all patients. This is particularly important in light of research that found that older non-Hispanic Black patients were less likely than their non-Hispanic White counterparts to receive information from their physicians about colorectal cancer screening.20
Continue to: Mobility
Mobility
Encourage physical activity. Frequent exercise and other forms of physical activity are associated with healthy aging, as shown in a 2017 systematic review and meta-analysis of 23 studies (N = 174,114).23 Despite considerable heterogeneity between studies in how researchers defined healthy aging and physical activity, they found that adults who incorporate regular movement and exercise into daily life are likely to continue to benefit from it into older age.23 In addition, a 2016 secondary analysis of data from the InCHIANTI longitudinal aging study concluded that adults ages ≥ 65 years (N = 1149) who had maintained higher physical activity levels throughout adulthood had less physical function decline and reduced rates of mobility disability and premature death compared with those who reported being less active.24
Preserve gait speed (and bolster health) with these activities. Walking speed, or gait, measured on a level surface has been used as a predictor for various aspects of well-being in older age, such as daily function, mobility, independence, falls, mortality, and hospitalization risk.25 Reduced gait speed is also one of the key indicators of functional impairment in older adults.
A 2015 systematic review sought to determine which type of exercise intervention (resistance, coordination, or multimodal training) would be most effective in preserving gait speed in healthy older adults (N = 2495; mean age = 74.2 years).25 While the 42 included studies were deemed to be fairly low quality, the review revealed (with large effect size [0.84]) that a number of exercise modalities might stave off loss of gait speed in older adults. Patients in the resistance training group had the greatest improvement in gait speed (0.11 m/s), followed by those in the coordination training group (0.09 m/s) and the multimodal training group (0.05 m/s).25
Finally, muscle mass and strength offer a measure of physical performance and functionality. A 2020 systematic review of 83 studies (N = 108,428) showed that low muscle mass and strength, reduced handgrip strength, and lower physical performance were predictive of reduced capacities in activities of daily living and instrumental activities of daily living.26 It is important to counsel adults to remain active throughout their lives and to include resistance training to maintain muscle mass and strength to preserve their motor function, mobility, independence, and QOL.
Use 1 of these scales to identify frailty. Frailty is a distinct clinical syndrome, in which an individual has low reserves and is highly vulnerable to internal and external stressors. It affects many community-dwelling older adults. Within the literature, there has been ongoing discussion regarding the definition of frailty27 (TABLE 128-31).
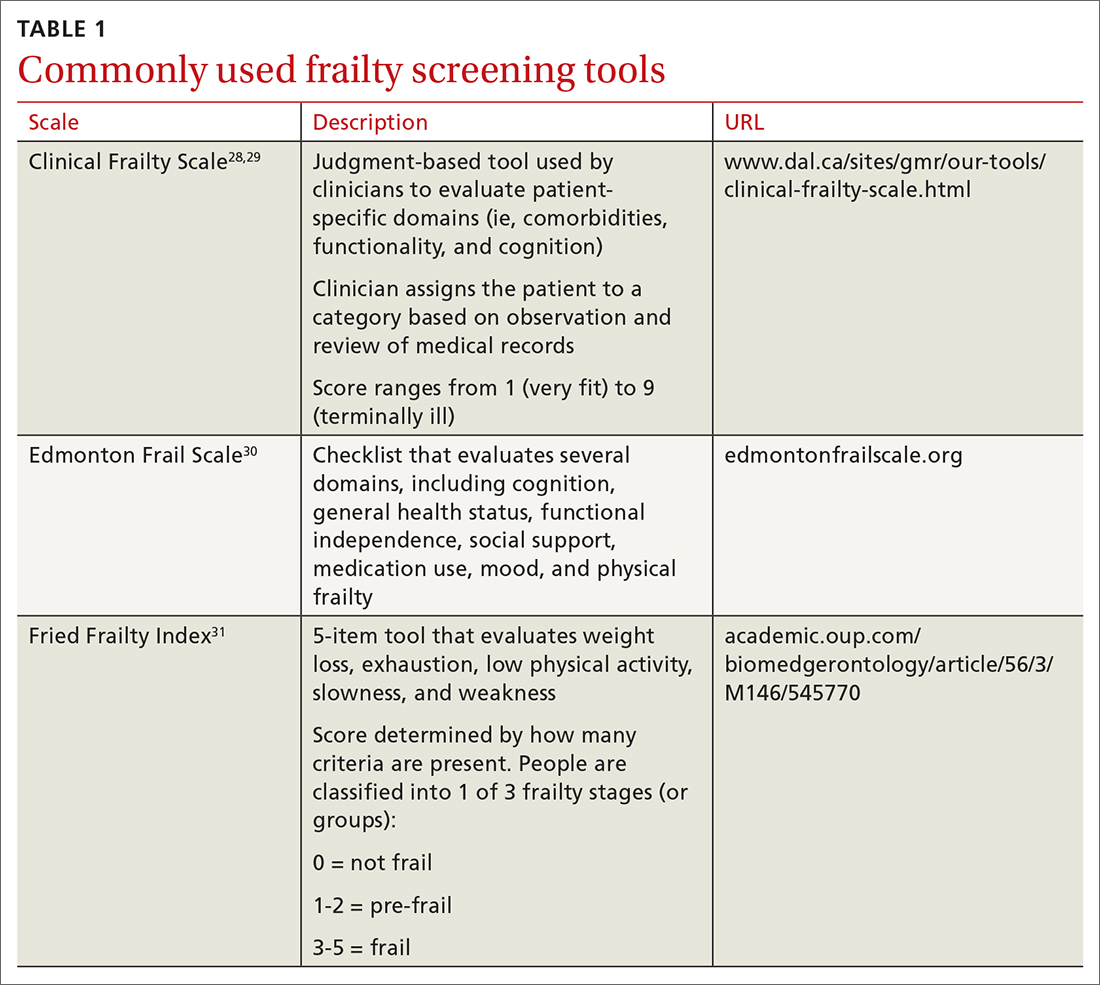
Continue to: The Fried Frailty Index...
The Fried Frailty Index defines frailty as a purely physical condition; patients need to exhibit 3 of 5 components (ie, weight loss, exhaustion, weakness, slowness, and low physical activity) to be deemed frail.31 The Edmonton Frail Scale is commonly used in geriatric assessments and counts impairments across several domains including physical activity, mood, cognition, and incontinence.30,32,33 Physicians need to complete a training course prior to its use. Finally, the definition of frailty used by Rockwood et al28, 29 was used to develop the Clinical Frailty Scale, which relies on broader criteria that include social and psychological elements in addition to physical elements.The Clinical Frailty Scale uses clinician judgment to evaluate patient-specific domains (eg, comorbidities, functionality, and cognition) and to generate a score ranging from 1 (very fit) to 9 (terminally ill).29 This scale is accessible and easy to implement. As a result, use of this scale has increased during the COVID-19 pandemic. All definitions include a pre-frail state, indicating the dynamic nature of frailty over time.
It is important to identify pre-frail and frail older adults using 1 of these screening tools. Interventions to reverse frailty that can be initiated in the primary care setting include identifying treatable medical conditions, assessing medication appropriateness, providing nutritional advice, and developing an exercise plan.34
Conduct a nutritional assessment; consider this diet. Studies show that nutritional status can predict physical function and frailty risk in older adults. A 2017 systematic review of 19 studies (N = 22,270) of frail adults ages ≥ 65 years found associations related to specific dietary constructs (ie, micronutrients, macronutrients, antioxidants, overall diet quality, and timing of consumption).35 Plant-based diets with higher levels of micronutrients, such as vitamins C and E and beta-carotene, or diets with more protein or macronutrients, regardless of source foods, all resulted in inverse associations with frailty syndrome.35
A 2017 study showed that physical exercise and maintaining good nutritional status may be effective for preventing frailty in community-dwelling pre-frail older individuals.36 A 2019 study showed that a combination of muscle strength training and protein supplementation was the most effective intervention to delay or reverse frailty and was easiest to implement in primary care.37 A 2020 meta-analysis of 31 studies (N = 4794) addressing frailty among primary care patients > 60 years showed that interventions using predominantly resistance-based exercise and nutrition supplementation improved frailty status over the control.38 Researchers also found that a comprehensive geriatric assessment or exercise—alone or in combination with nutrition education—reduced physical frailty.
Mentation
Screen and treat cognitive impairments. Cognitive function and autonomy in decision-making are important factors in healthy aging. Aspects of mental health (eg, depression and anxiety), sensory impairment (eg, visual and auditory impairment), and mentation issues (eg, delirium, dementia, and related conditions), as well as diet, physical exercise, and mobility, can all impede cognitive functionality. The long-term effects of depression, anxiety,39 sensory deficits,40 mobility,41 diet,42 and, ultimately, aging may impact Alzheimer disease (AD). The risk of an AD diagnosis increases with age.39
Continue to: A 2018 prospective cohort study...
A 2018 prospective cohort study using data from the National Alzheimer’s Coordinating Center followed individuals (N = 12,053) who were cognitively asymptomatic at their initial visits to determine who developed clinical signs of AD.39 Survival analysis showed several psychosocial factors—anxiety, sleep disturbances, and depressive episodes of any type (occurring within the past 2 years, clinician verified, lifetime report)—were significantly associated with an eventual AD diagnosis and increased the risk of AD.39 More research is needed to verify the impact of early intervention for these conditions on neurodegenerative disease; however, screening and treating psychosocial factors such as anxiety and depression should be maintained.
Researchers evaluated the impact of a dual sensory impairment (DSI) on dementia risk using data from 2051 participants in the Ginkgo Evaluation of Memory Study.40 Hearing and visual impairments (defined as DSI when these conditions coexist) or visual impairment alone were significantly associated with increased risk of dementia in older adults. The researchers reported that DSI was significantly associated with a higher risk of all-cause dementia (hazard ratio [HR] = 1.86; 95% CI, 1.25-2.76) and AD (HR = 2.12; 95% CI, 1.34-3.36).40 Visual impairment alone was associated with an increased risk of all-cause dementia (HR = 1.32; 95% CI, 1.02-1.71).40 These results suggest that screening of DSI or visual impairment earlier in the patient’s lifespan may identify those at high risk of dementia in older adulthood.
The American Academy of Ophthalmology recommends patients with healthy eyes be screened once during their 20s and twice in their 30s; a full examination is recommended by age 40. For patients ages ≥ 65 years, it is recommended that eye examinations occur every 1 to 2 years.43
Diet and mobility play a big role in cognition. Diet43 and exercise41,42,44 are believed to have an impact on mentation, and recent studies show memory and global cognition could be malleable later in life. A 2015 meta-analysis of 490 treatment arms of 24 randomized controlled studies showed improvement in global cognition with consumption of a Mediterranean diet plus olive oil (effect size [ES] standardized mean difference [SMD] = 0.22; 95% CI, 0.16-0.27) and tai chi exercises (ES SMD = 0.18; 95% CI, 0.06-0.29).42 The analysis also found improved memory among participants who consumed the Mediterranean diet/olive oil combination (ES SMD = 0.22; 95% CI, 0.12-0.32) and soy isoflavone supplements (ES SMD = 0.11; 95% CI, 0.04-0.17). Although the ESs are small, they are significant and offer promising evidence that individual choices related to nutrition or exercise may influence cognition and memory.
A 2018 systematic review found that all domains of cognition showed improvement with 45 to 60 minutes of moderate-to-vigorous physical exercise.44 Attention, executive function, memory, and working memory showed significant increases, whereas global cognition improvements were not statistically significant.44 A 2016 meta-analysis of 26 studies (N = 26,355) found a positive association between an objective mobility measure (gait, lower-extremity function, and balance) and cognitive function (global, executive function, memory, and processing speed) in older adults.41 These results highlight that diet, mobility, and physical exercise impact cognitive functioning.
Continue to: Maintaining social connections
Maintaining social connections
Social isolation and loneliness—compounded by a pandemic. The US Department of Health and Human Services notes that “community connections” are among the key factors required for healthy aging.3 Similarly, the WHO definition of healthy aging considers whether individuals can build and sustain relationships with other people and find ways to engender their personal values through these connections.2
As people age, their social connections often decrease due to the death of friends and family, shifts in living arrangements, loss of mobility or eyesight (and thus self-transport), and the onset or increased acuity of illness or chronic conditions.45 This has been exacerbated by the COVID-19 pandemic, which has spurred shelter-in-place and stay-at-home orders along with recommendations for physical distancing (also known as social distancing), especially for older adults who are at higher risk.46 Smith et al47 calls this the COVID-19 Social Connectivity Paradox, in which older adults limit their interactions with others to protect their physical health and reduce their risk of contracting the virus, but as a result they may undermine their psychosocial health by placing themselves at risk of social isolation and loneliness.47
The double threat. Social isolation and loneliness have been shown to negatively impact physical health and well-being, resulting in an increased risk of early death48-50; higher likelihood of specific diagnoses, including dementia and cardiovascular conditions48,50; and more frequent use of health care services.50 These concepts, while related, represent different mechanisms for negative health outcomes. Social isolation is an objective condition when one has a lack of opportunities for interaction with other people; loneliness refers to the emotional disconnect one feels when separated from others. Few studies have compared outcomes between these concepts, but in those that have, social isolation appears to be more strongly associated with early death.48-50
A 2013 observational study using data from the English Longitudinal Study on Aging found that both social isolation and loneliness were associated with increased mortality among men and women ages ≥ 52 years (N = 6500).48 However, when studied independently, loneliness was not found to be a significant risk factor. In contrast, social isolation significantly impacted mortality risk, even after adjusting for demographic factors and baseline health status.48 These findings are supported by a 2018 cohort study of individuals (N = 479,054) with a history of an acute cardiovascular event that concluded social isolation was a predictor of mortality, whereas loneliness was not.50
A large 2015 meta-analysis (70 studies, N = 3,407,134) of mortality causes among community-dwelling older adults (average age, 66) confirmed that both objective measures of isolation, as well as subjective measures (such as feelings of loneliness or living alone), have a significant predictive effect in longer-term studies. Each measure shows an approximately 30% increase in the likelihood of death after an average of 7 years.49
Continue to: Health care remains a connection point
Health care remains a connection point. Even when life course events and conditions (eg, death of loved ones, loss of transportation or financial resources, or disengagement from community activities) reduce social connections, most older adults engage in some way with the health care system. A 2020 consensus report by the National Academies of Sciences, Engineering, and Medicine suggests health care professionals capitalize on these connection points with adults ages ≥ 50 years by periodically screening for social isolation and loneliness, documenting social status updates in medical records, and piloting and evaluating interventions in the clinical setting.51
The report offered information about potential avenues for intervention by primary care professionals beyond screening, such as participating in research studies that investigate screening tools and multisystem interventions; social prescribing (linking patients to embedded social work services or community-based organizations); referring patients to support groups; initiating cognitive-based therapy or other behavioral health interventions; or recommending mindfulness practices.51 However, most of the cited intervention studies were not specific to primary care settings and contained poor-quality evidence related to efficacy.
Isolation creates a greater reliance on health services due to a lack of a social support system, while a feeling of emotional disconnection (loneliness) seems to be a barrier to accessing care. A 2017 cohort study linking data from the Health and Retirement Study and Medicare claims revealed that social isolation predicts higher annual health expenditures (> $1600 per beneficiary) driven by hospitalization and skilled nursing facility usage, along with greater mortality, whereas individuals who are lonely result in reduced costs (a reduction of $770 annually) due to lower usage of inpatient and outpatient services.52 Prioritizing interventions that identify and connect isolated older adults to social support, therefore, may increase survivability by ensuring they have access to resources and health care interventions when needed.
In addition, these findings underscore the importance of looking at quality—not just quantity—of older adults’ social connections. A number of validated screening tools exist for social isolation and loneliness (TABLE 253-59); however, concerns exist about assessing risk using a unidimensional tool for a complex concern,47 as well as identifying a problem without having evidence-based interventions to offer as solutions.47,51 Until future studies resolve these concerns, leveraging the physician-patient relationship to broach these difficult subjects may help normalize the issues and create safe spaces to identify individuals who are at risk.
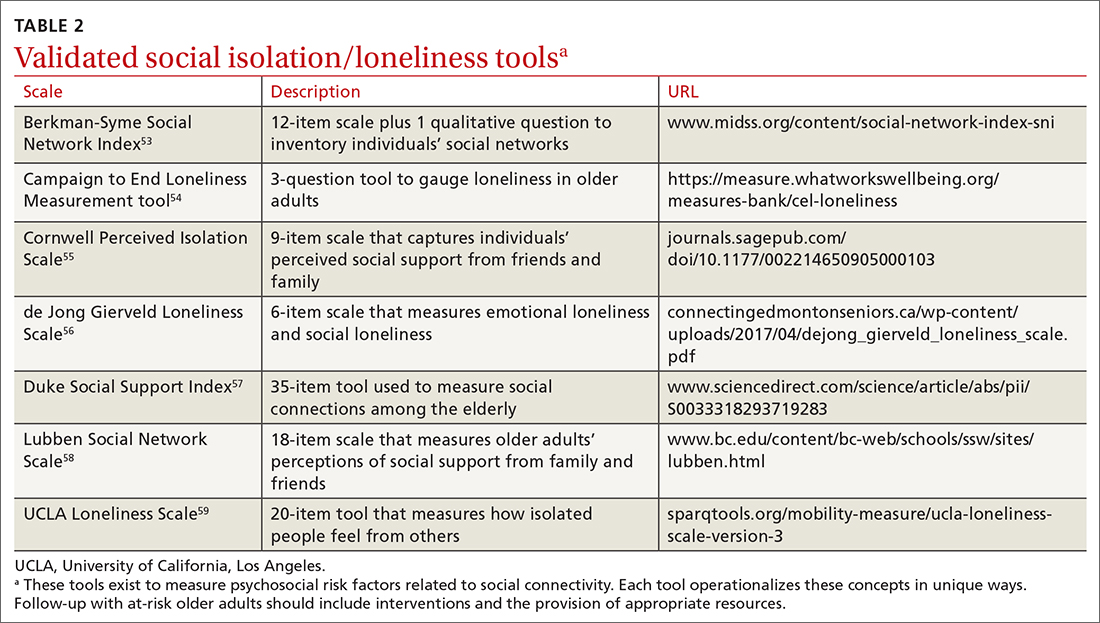
QOL is key to healthy aging. As Kusumastuti et al4 state, “successful ageing lies in the eyes of the beholder.” A 2019 systematic review of 48 qualitative studies revealed that community-dwelling older adults ages ≥ 50 years in 11 countries (N > 4175) perceive well-being by considering QOL within 9 domains: health perception, autonomy, role and activity, relationships, emotional comfort, attitude and adaptation, spirituality, financial security, and home and neighborhood.60 Researchers found that as engagement in any one of these domains declines, older adults may shift their definition of health toward their remaining abilities.60 This offers an explanation as to why patients might rate their health status much higher than their physicians do: older adults tend to have a more holistic concept of health.
Continue to: Take a multidimensional approach to healthy aging
Take a multidimensional approach to healthy aging
Although we have separately examined each of the 4 components of managing healthy aging in a community-dwelling adult, applying a multidimensional approach is most effective. Increasing use of the Medicare AWV provides an opportunity to assess patient health status, determine care preferences, and improve follow-through on preventive screening. It is also important to encourage older adults to engage in regular physical activity—especially muscle-strengthening exercises—and to discuss nutrition and caloric intake to prevent frailty and functional decline.
Assessing and treating vision and hearing impairments and mental health issues, including anxiety and depression, may guard against losses in cognition. When speaking with older adult patients about their social connections, consider asking not only about frequency of contact and access to resources such as food and transportation, but also about whether they are finding ways to bring their own values into those relationships to bolster their QOL. This guidance also may be useful for primary care practices and health care networks when planning future quality-improvement initiatives.
Additional research is needed to support the evidence base for aligning older adult preferences in health care interventions, such as preventive screenings. Also, clinical decision-making requires more clarity about the efficacy of specific diet and exercise interventions for older adults; the impact of early intervention for depression, anxiety, and sleep disorders on neurodegenerative disease; whether loneliness predicts mortality; and how health care delivery systems can be effective at building social connectivity.
For now, it is essential to recognize that initiating health education, screening, and prevention throughout the patient’s lifespan can promote healthy aging outcomes. As family physicians, it is important to capitalize on longitudinal relationships with patients and begin educating younger patients using this multidimensional framework to promote living “a productive and meaningful life”at any age.3
Lynn M. Wilson, DO, 707 Hamilton Street, 8th floor, Department of Family Medicine, Lehigh Valley Health Network, Allentown, PA 18101; [email protected]
1. Friedman S, Mulhausen P, Cleveland M, et al. Healthy aging: American Geriatrics Society white paper executive summary. J Am Geriatr Soc. 2018;67:17-20. doi: 10.1111/jgs.15644
2. World Health Organization. World report on ageing and health. 2015. Accessed June 29, 2020. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1
3. U.S. Department of Health & Human Services. Healthy aging. Accessed June 29, 2020. www.hhs.gov/aging/healthy-aging
4. Kusumastuti S, Derks MGM, Tellier S, et al. Successful ageing: a study of the literature using citation network analysis. Maturitas. 2016;93:4-12. doi: 10.1016/j.maturitas.2016.04.010
5. Institute for Healthcare Improvement. Age-friendly health systems: guide to using the 4Ms in the care of older adults [white paper]. 2020. Accessed June 29, 2020. www.ihi.org/Engage/Initiatives/Age-Friendly-Health-systems/Documents/IHIAgeFriendlyHealthSystems_GuidetoUsing4MsCare.pdf
6. Lind KE, Hildreth KL, Perraillon MC. Persistent disparities in Medicare’s annual wellness visit utilization. Med Care. 2019;57:984-989. doi: 10.1097/MLR.0000000000001229
7. Lind KE, Hildreth K, Lindrooth R, et al. Ethnoracial disparities in Medicare annual wellness visit utilization: evidence from a nationally representative database. Med Care. 2018;56:761-766. doi: 10.1097/MLR.0000000000000962
8. Simpson VL, Kovich M. Outcomes of primary care-based Medicare annual wellness visits with older adults: a scoping review. Geriatr Nurs. 2019;40:590-596. doi: 10.1016/j.gerinurse.2019.06.001
9. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1-77.
10. Salzman B, Beldowski K, de la Paz A. Cancer screening in older patients. Am Fam Physician. 2016;93:659-667.
11. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399-406. doi: 10.1001/jamainternmed.2016.9022
12. Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311:1336-1347. doi: 10.1001/jama.2014.2834
13. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177:1121-1128. doi: 10.1001/jamainternmed.2017.1778
14. Butterworth JE, Hays R, McDonagh ST, et al. Interventions for involving older patients with multi-morbidity in decision-making during primary care consultations. Cochrane Database Syst Rev. 2019;10:CD013124. doi: 10.1002/14651858.CD013124.pub2
15. Bostock S, Steptoe A. Association between low functional health literacy and mortality in older adults: longitudinal cohort study. BMJ. 2012;344:e1602. doi: 10.1136/bmj.e1602
16. Fernandez DM, Larson JL, Zikmund-Fisher BJ. Associations between health literacy and preventive health behaviors among older adults: findings from the health and retirement study. BMC Public Health. 2016;16:596. doi: 10.1186/s12889-016-3267-7
17. Weekes CV. African Americans and health literacy: a systematic review. ABNF J. 2012;23:76-80.
18. Mantwill S, Monestel-Umaña S, Schulz PJ. The relationship between health literacy and health disparities: a systematic review. PLoS One. 2015;10:e0145455. doi: 10.1371/journal.pone.0145455
19. Khan MM, Kobayashi K. Optimizing health promotion among ethnocultural minority older adults (EMOA). Int J Migration Health Soc Care. 2015;11:268-281. doi: 10.1108/IJMHSC-12-2014-0047
20. Kindratt TB, Dallo FJ, Allicock M, et al. The influence of patient-provider communication on cancer screenings differs among racial and ethnic groups. Prev Med Rep. 2020;18:101086. doi: 10.1016/j.pmedr.2020.101086
21. Hong Y-R, Tauscher J, Cardel M. Distrust in health care and cultural factors are associated with uptake of colorectal cancer screening in Hispanic and Asian Americans. Cancer. 2018;124:335-345. doi: 10.1002/cncr.31052
22. Kutner M, Greenberg E, Jin Y, et al. Literacy in everyday life: results from the 2003 National Assessment of Adult Literacy. NCES 2007-480. U.S. Department of Education, National Center for Education Statistics. April 2007. Accessed August 27, 2021. http://nces.ed.gov/Pubs2007/2007480_1.pdf
23. Daskalopoulou C, Stubbs B, Kralj C, et al. Physical activity and healthy ageing: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2017;38:6-17. doi: 10.1016/j.arr.2017.06.003
24. Stenholm S, Koster A, Valkeinen H, et al. Association of physical activity history with physical function and mortality in old age. J Gerontol A Biol Sci Med Sci. 2016;71:496-501. doi: 10.1093/gerona/glv111
25. Hortobágyi T, Lesinski M, Gäbler M, et al. Effects of three types of exercise interventions on healthy old adults’ gait speed: a systematic review and meta-analysis. Sports Med. 2015;45:1627‐1643. Published correction appears in Sports Med. 2016;46:453. doi: 10.1007/s40279-015-0371-2
26. Wang DXM, Yao J, Zirek Y, et al. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:3‐25. doi: 10.1002/jcsm.12502
27. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129-2138. doi: 10.1111/j.1532-5415.2011.03597.x
28. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. doi: 10.1503/cmaj.050051
29. Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20:393. doi: 10.1186/s12877-020-01801-7
30. Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526-529. doi: 10.1093/ageing/afl041
31. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
32. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Euro J Intern Med. 2016;31:3-10. doi: 10.1016/j.ejim.2016.03.007
33. Perna S, Francis MD, Bologna C, et al. Performance of Edmonton Frail Scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017;17:2. doi: 10.1186/s12877-016-0382-3
34. Chen CY, Gan P, How CH. Approach to frailty in the elderly in primary care and the community. Singapore Med J. 2018;59:338. doi: 10.11622/smedj.2018052
35. Lorenzo-López L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17:108. doi: 10.1186/s12877-017-0496-2
36. Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46:401-407. doi: 10.1093/ageing/afw242
37. Travers J, Romero-Ortuno R, Bailey J, et al. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69:e61-e69. doi: 10.3399/bjgp18X700241
38. Macdonald SHF, Travers J, Shé ÉN, et al. Primary care interventions to address physical frailty among community-dwelling adults aged 60 years or older: a meta-analysis. PLoS One. 2020;15:e0228821. doi: 10.1371/journal.pone.0228821
39. Burke SL, Cadet T, Alcide A, et al. Psychosocial risk factors and Alzheimer’s disease: the associative effect of depression, sleep disturbance, and anxiety. Aging Ment Health. 2018;22:1577-1584. doi: 10.1080/13607863.2017.1387760
40. Hwang PH, Longstreth WT Jr, Brenowitz WD, et al. Dual sensory impairment in older adults and risk of dementia from the GEM Study. Alzheimers Dement (Amst). 2020;12:e12054. doi: 10.1002/dad2.12054
41. Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164‐174. doi: 10.1016/j.gaitpost.2016.08.028
42. Lehert P, Villaseca P, Hogervorst E, et al. Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric. 2015;18:678-689. doi: 10.3109/13697137.2015.1078106
43. Turbert D. Eye exam and vision testing basics. American Academy of Ophthalmology Web site. January 14, 2021. Accessed March 5, 2021. www.aao.org/eye-health/tips-prevention/eye-exams-101
44. Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154-160. doi: 10.1136/bjsports-2016-096587
45. CDC. Percent of U.S. adults 55 and over with chronic conditions. November 6, 2015. Accessed April 29, 2021. www.cdc.gov/nchs/health_policy/adult_chronic_conditions.htm
46. National Council on Aging. COVID-driven isolation can be dangerous for older adults. March 31, 2021. Accessed April 29, 2021. www.ncoa.org/article/covid-driven-isolation-can-be-dangerous-for-older-adults
47. Smith ML, Steinman LE, Casey EA. Combatting social isolation among older adults in a time of physical distancing: the COVID-19 social connectivity paradox. Front Public Health. 2020;8:403. doi: 10.3389/fpubh.2020.00403
48. Steptoe A, Shankar A, Demakakos P, et al. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797-5801. doi: 10.1073/pnas.1219686110
49. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227-237. doi: 10.1177/1745691614568352
50. Hakulinen C, Pulkki-Råback L, Virtanen M, et al. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart. 2018;104:1536-1542. doi: 10.1136/heartjnl-2017-312663
51. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. The National Academies Press; 2020. doi: 10.17226/25663
52. Shaw JG, Farid M, Noel-Miller C, et al. Social isolation and Medicare spending: among older adults, objective isolation increases expenditures while loneliness does not. J Aging Health. 2017;29:1119-1143. doi: 10.1177/0898264317703559
53. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186-204. doi: 10.1093/oxfordjournals.aje.a112674
54. Campaign to End Loneliness. Measuring your impact on loneliness in later life. Accessed April 29, 2021. www.campaigntoendloneliness.org/wp-content/uploads/Loneliness-Measurement-Guidance1-1.pdf
55. Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50:31-48. doi: 10.1177/002214650905000103
56. Gierveld JDJ, Van Tilburg T. A 6-item scale for overall, emotional, and social loneliness: confirmatory tests on survey data. Res Aging. 2006;28:582-598. doi: 10.1177/0164027506289723
57. Koenig HG, Westlund RE, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34:61-69. doi: 10.1016/S0033-3182(93)71928-3
58. Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503-513. doi: 10.1093/geront/46.4.503
59. Russell DW. UCLA Loneliness Scale (version 3): reliability, validity, factor structure. J Pers Assess. 1996;66:20-40. doi: 10.1207/s15327752jpa6601_2
60. van Leeuwen KM, van Loon MS, van Nes FA, et al. What does quality of life mean to older adults? A thematic synthesis. PLoS One. 2019;14:e0213263. doi: 10.1371/journal.pone.0213263
Our approach to caring for the growing number of community-dwelling US adults ages ≥ 65 years has shifted. Although we continue to manage disease and disability, there is an increasing emphasis on the promotion of healthy aging by optimizing health care needs and quality of life (QOL).
The American Geriatric Society (AGS) uses the term “healthy aging” to reflect a dedication to improving the health, independence, and QOL of older people.1 The World Health Organization (WHO) defines healthy aging as “the process of developing and maintaining the functional ability that enables well-being in older age.”2 Functional ability encompasses capabilities that align with a person’s values, including meeting basic needs; learning, growing, and making independent decisions; being mobile; building and maintaining healthy relationships; and contributing to society.2 Similarly, the US Department of Health and Human Services has adopted a multidimensional approach to support people in creating “a productive and meaningful life” as they grow older.3
Numerous theoretical models have emerged from research on aging as a multidimensional construct, as evidenced by a 2016 citation analysis that identified 1755 articles written between 1902 and 2015 relating to “successful aging.”4 The analysis revealed 609 definitions operationalized by researchers’ measurement tools (mostly focused on physical function and other health metrics) and 1146 descriptions created by older adults, many emphasizing psychosocial strategies and cultural factors as key to successful aging.4

One approach that is likely to be useful for family physicians is the Age-Friendly Health System. This is an initiative of The John A. Hartford Foundation and the Institute for Healthcare Improvement that uses a multidisciplinary approach to create environments that foster inclusivity and address the needs of older people.5 Following this guidance, primary care providers use evidence-informed strategies that promote safety and address what matters most to older adults and their family caregivers.
The Age-Friendly Health System, as well as AGS and WHO, recognize that there are multiple aspects to well-being as one grows older. By using focused, evidence-based screening, assessments, and interventions, family physicians can best support aging patients in living their most fulfilling lives.
Here we present a review of evidence-based strategies that promote safety and address what matters most to older adults and their family caregivers using a 4-pronged framework, in the style of the Age-Friendly Health System model. However, the literature on healthy aging includes important messages about patient context and lifelong health behaviors, which we capture in an expanded set of thematic guidance. As such, we encourage family physicians to approach healthy aging as follows: (1) monitor health (screening and prevention), (2) promote mobility (physical function), (3) manage mentation (emotional health and cognitive function), and (4) encourage maintenance of social connections (social networks and QOL).
Monitoring health
Leverage Medicare annual wellness visits. A systematic approach is needed to prevent frailty and functional decline, and thus increase the QOL of older adults. To do this, it is important to focus on health promotion and disease prevention, while addressing existing ailments. One method is to leverage the Medicare annual wellness visit (AWV), which provides an opportunity to assess current health status as well as discuss behavior-change and risk-reduction strategies with patients.
Continue to: Although AWVs...
Although AWVs are an opportunity to improve patient outcomes, we are not taking full advantage of them.6 While AWVs have gained traction since their introduction in 2011, usage rates among ethnoracial minority groups has lagged behind.6 A 2018 cohort study examined reasons for disparate utilization rates among individuals ages ≥ 66 years (N = 14,687).7 Researchers found that differences in utilization between ethnoracial groups were explained by socioeconomic factors. Lower education and lower income, as well as rural living, were associated with lower rates of AWV completion.7 In addition, having a usual, nonemergent place to obtain medical care served as a powerful predictor of AWV utilization for all groups.7
Strategies to increase AWV completion rates among all eligible adults include increasing staff awareness of health literacy challenges and ensuring communication strategies are inclusive by providing printed materials in multiple languages, Braille, or larger typefaces; using accessible vocabulary that does not include medical jargon; and making medical interpreters accessible. In addition, training clinicians about unconscious bias and cultural humility can help foster empathy and awareness of differences in health beliefs and behaviors within diverse patient populations.
A 2019 scoping review of 11 studies (N > 60 million) focused on outcomes from Medicare AWVs for patients ages ≥ 65 years.8 This included uptake of preventive services, such as vaccinations or cancer screenings; advice, education, or referrals offered during the AWV; medication use; and hospitalization rates. Overall findings showed that older adults who received a Medicare AWV were more likely to receive referrals for preventive screenings and follow-through on these recommendations compared with those who did not undergo an AWV.8
Completion rates for vaccines, while remaining low overall, were higher among those who completed an AWV. Additionally, these studies showed improved completion of screenings for breast cancer, bone density, and colon cancer. Several studies in the scoping review supported the use of AWVs as an effective means by which to offer health education and advice related to health promotion and risk reduction, such as diet and lifestyle modifications.8 Little evidence exists on long-term outcomes related to AWV completion.8
Utilize shared decision-making to determine whether preventive screening makes sense for your patient. Although cancer remains the second leading cause of death among Americans ages ≥ 65 years,9 clear screening guidelines for this age group remain elusive.10 Physicians and patients often are reluctant to stop cancer screening despite lower life expectancy and fewer potential benefits of diagnosis in this population.9 Some recent studies reinforce the heterogeneity of the older adult population and further underscore the importance of individual-level decision-making.11-14 It is important to let older adult patients and their caregivers know about the potential risks of screening tests, especially the possibility that incidental findings may lead to unwarranted additional care or monitoring.9
Continue to: Avoid these screening conversation missteps
Avoid these screening conversation missteps. A 2017 qualitative study asked 40 community-dwelling older adults (mean age = 76 years) about their preferences for discussing screening cessation with their physicians.13 Three themes emerged.First, they were open to stopping their screenings, especially when suggested by a trusted physician. Second, health status and physical function made sense as decision points, but life expectancy did not. Finally, lengthy discussions with expanded details about risks and benefits were not appreciated, especially if coupled with comments on the limited benefits for those nearing the end of life. When discussing life expectancy, patients preferred phrasing that focused on how the screening was unnecessary because it would not help them live longer.13
Ensure that your message is understood—and culturally relevant. Recent studies on lower health literacy among older adults15,16 and ethnic and racial minorities17-21—as revealed in the 2003 National Assessment of Adult Literacy22—might offer clues to patient receptivity to discussions about preventive screening and other health decisions.
One study found a significant correlation between higher self-rated health literacy and higher engagement in health behaviors such as mammography screening, moderate physical activity, and tobacco avoidance.16 Perceptions of personal control over health status, as well as perceived social standing, also correlated with health literacy score levels.16 Another study concluded that lower health literacy combined with lower self-efficacy, cultural beliefs about health topics (eg, diet and exercise), and distrust in the health care system contributed to lower rates of preventive care utilization among ethnocultural minority older adults in Canada, the United Kingdom, the United States, and Australia.18
Ensuring that easy-to-understand information is equitably shared with older adults of all races and ethnicities is critical. A 2018 study showed that distrust of the health system and cultural issues contributed to the lower incidence of colorectal cancer screenings in Hispanic and Asian American patients ages 50 to 75 years.21 Patients whose physicians engaged in “health literate practices” (eg, offering clear explanations of diagnostic plans and asking patients to describe what they understood) were more likely to obtain recommended breast and colorectal cancer screenings.20 In particular, researchers found that non-Hispanic Blacks were nearly twice as likely to follow through on colorectal cancer screening if their physicians engaged in health literate practices.20 In addition, receiving clear instructions from physicians increased the odds of completing breast cancer screening among Hispanic and non-Hispanic White women.20
Overall, screening information and recommendations should be standardized for all patients. This is particularly important in light of research that found that older non-Hispanic Black patients were less likely than their non-Hispanic White counterparts to receive information from their physicians about colorectal cancer screening.20
Continue to: Mobility
Mobility
Encourage physical activity. Frequent exercise and other forms of physical activity are associated with healthy aging, as shown in a 2017 systematic review and meta-analysis of 23 studies (N = 174,114).23 Despite considerable heterogeneity between studies in how researchers defined healthy aging and physical activity, they found that adults who incorporate regular movement and exercise into daily life are likely to continue to benefit from it into older age.23 In addition, a 2016 secondary analysis of data from the InCHIANTI longitudinal aging study concluded that adults ages ≥ 65 years (N = 1149) who had maintained higher physical activity levels throughout adulthood had less physical function decline and reduced rates of mobility disability and premature death compared with those who reported being less active.24
Preserve gait speed (and bolster health) with these activities. Walking speed, or gait, measured on a level surface has been used as a predictor for various aspects of well-being in older age, such as daily function, mobility, independence, falls, mortality, and hospitalization risk.25 Reduced gait speed is also one of the key indicators of functional impairment in older adults.
A 2015 systematic review sought to determine which type of exercise intervention (resistance, coordination, or multimodal training) would be most effective in preserving gait speed in healthy older adults (N = 2495; mean age = 74.2 years).25 While the 42 included studies were deemed to be fairly low quality, the review revealed (with large effect size [0.84]) that a number of exercise modalities might stave off loss of gait speed in older adults. Patients in the resistance training group had the greatest improvement in gait speed (0.11 m/s), followed by those in the coordination training group (0.09 m/s) and the multimodal training group (0.05 m/s).25
Finally, muscle mass and strength offer a measure of physical performance and functionality. A 2020 systematic review of 83 studies (N = 108,428) showed that low muscle mass and strength, reduced handgrip strength, and lower physical performance were predictive of reduced capacities in activities of daily living and instrumental activities of daily living.26 It is important to counsel adults to remain active throughout their lives and to include resistance training to maintain muscle mass and strength to preserve their motor function, mobility, independence, and QOL.
Use 1 of these scales to identify frailty. Frailty is a distinct clinical syndrome, in which an individual has low reserves and is highly vulnerable to internal and external stressors. It affects many community-dwelling older adults. Within the literature, there has been ongoing discussion regarding the definition of frailty27 (TABLE 128-31).

Continue to: The Fried Frailty Index...
The Fried Frailty Index defines frailty as a purely physical condition; patients need to exhibit 3 of 5 components (ie, weight loss, exhaustion, weakness, slowness, and low physical activity) to be deemed frail.31 The Edmonton Frail Scale is commonly used in geriatric assessments and counts impairments across several domains including physical activity, mood, cognition, and incontinence.30,32,33 Physicians need to complete a training course prior to its use. Finally, the definition of frailty used by Rockwood et al28, 29 was used to develop the Clinical Frailty Scale, which relies on broader criteria that include social and psychological elements in addition to physical elements.The Clinical Frailty Scale uses clinician judgment to evaluate patient-specific domains (eg, comorbidities, functionality, and cognition) and to generate a score ranging from 1 (very fit) to 9 (terminally ill).29 This scale is accessible and easy to implement. As a result, use of this scale has increased during the COVID-19 pandemic. All definitions include a pre-frail state, indicating the dynamic nature of frailty over time.
It is important to identify pre-frail and frail older adults using 1 of these screening tools. Interventions to reverse frailty that can be initiated in the primary care setting include identifying treatable medical conditions, assessing medication appropriateness, providing nutritional advice, and developing an exercise plan.34
Conduct a nutritional assessment; consider this diet. Studies show that nutritional status can predict physical function and frailty risk in older adults. A 2017 systematic review of 19 studies (N = 22,270) of frail adults ages ≥ 65 years found associations related to specific dietary constructs (ie, micronutrients, macronutrients, antioxidants, overall diet quality, and timing of consumption).35 Plant-based diets with higher levels of micronutrients, such as vitamins C and E and beta-carotene, or diets with more protein or macronutrients, regardless of source foods, all resulted in inverse associations with frailty syndrome.35
A 2017 study showed that physical exercise and maintaining good nutritional status may be effective for preventing frailty in community-dwelling pre-frail older individuals.36 A 2019 study showed that a combination of muscle strength training and protein supplementation was the most effective intervention to delay or reverse frailty and was easiest to implement in primary care.37 A 2020 meta-analysis of 31 studies (N = 4794) addressing frailty among primary care patients > 60 years showed that interventions using predominantly resistance-based exercise and nutrition supplementation improved frailty status over the control.38 Researchers also found that a comprehensive geriatric assessment or exercise—alone or in combination with nutrition education—reduced physical frailty.
Mentation
Screen and treat cognitive impairments. Cognitive function and autonomy in decision-making are important factors in healthy aging. Aspects of mental health (eg, depression and anxiety), sensory impairment (eg, visual and auditory impairment), and mentation issues (eg, delirium, dementia, and related conditions), as well as diet, physical exercise, and mobility, can all impede cognitive functionality. The long-term effects of depression, anxiety,39 sensory deficits,40 mobility,41 diet,42 and, ultimately, aging may impact Alzheimer disease (AD). The risk of an AD diagnosis increases with age.39
Continue to: A 2018 prospective cohort study...
A 2018 prospective cohort study using data from the National Alzheimer’s Coordinating Center followed individuals (N = 12,053) who were cognitively asymptomatic at their initial visits to determine who developed clinical signs of AD.39 Survival analysis showed several psychosocial factors—anxiety, sleep disturbances, and depressive episodes of any type (occurring within the past 2 years, clinician verified, lifetime report)—were significantly associated with an eventual AD diagnosis and increased the risk of AD.39 More research is needed to verify the impact of early intervention for these conditions on neurodegenerative disease; however, screening and treating psychosocial factors such as anxiety and depression should be maintained.
Researchers evaluated the impact of a dual sensory impairment (DSI) on dementia risk using data from 2051 participants in the Ginkgo Evaluation of Memory Study.40 Hearing and visual impairments (defined as DSI when these conditions coexist) or visual impairment alone were significantly associated with increased risk of dementia in older adults. The researchers reported that DSI was significantly associated with a higher risk of all-cause dementia (hazard ratio [HR] = 1.86; 95% CI, 1.25-2.76) and AD (HR = 2.12; 95% CI, 1.34-3.36).40 Visual impairment alone was associated with an increased risk of all-cause dementia (HR = 1.32; 95% CI, 1.02-1.71).40 These results suggest that screening of DSI or visual impairment earlier in the patient’s lifespan may identify those at high risk of dementia in older adulthood.
The American Academy of Ophthalmology recommends patients with healthy eyes be screened once during their 20s and twice in their 30s; a full examination is recommended by age 40. For patients ages ≥ 65 years, it is recommended that eye examinations occur every 1 to 2 years.43
Diet and mobility play a big role in cognition. Diet43 and exercise41,42,44 are believed to have an impact on mentation, and recent studies show memory and global cognition could be malleable later in life. A 2015 meta-analysis of 490 treatment arms of 24 randomized controlled studies showed improvement in global cognition with consumption of a Mediterranean diet plus olive oil (effect size [ES] standardized mean difference [SMD] = 0.22; 95% CI, 0.16-0.27) and tai chi exercises (ES SMD = 0.18; 95% CI, 0.06-0.29).42 The analysis also found improved memory among participants who consumed the Mediterranean diet/olive oil combination (ES SMD = 0.22; 95% CI, 0.12-0.32) and soy isoflavone supplements (ES SMD = 0.11; 95% CI, 0.04-0.17). Although the ESs are small, they are significant and offer promising evidence that individual choices related to nutrition or exercise may influence cognition and memory.
A 2018 systematic review found that all domains of cognition showed improvement with 45 to 60 minutes of moderate-to-vigorous physical exercise.44 Attention, executive function, memory, and working memory showed significant increases, whereas global cognition improvements were not statistically significant.44 A 2016 meta-analysis of 26 studies (N = 26,355) found a positive association between an objective mobility measure (gait, lower-extremity function, and balance) and cognitive function (global, executive function, memory, and processing speed) in older adults.41 These results highlight that diet, mobility, and physical exercise impact cognitive functioning.
Continue to: Maintaining social connections
Maintaining social connections
Social isolation and loneliness—compounded by a pandemic. The US Department of Health and Human Services notes that “community connections” are among the key factors required for healthy aging.3 Similarly, the WHO definition of healthy aging considers whether individuals can build and sustain relationships with other people and find ways to engender their personal values through these connections.2
As people age, their social connections often decrease due to the death of friends and family, shifts in living arrangements, loss of mobility or eyesight (and thus self-transport), and the onset or increased acuity of illness or chronic conditions.45 This has been exacerbated by the COVID-19 pandemic, which has spurred shelter-in-place and stay-at-home orders along with recommendations for physical distancing (also known as social distancing), especially for older adults who are at higher risk.46 Smith et al47 calls this the COVID-19 Social Connectivity Paradox, in which older adults limit their interactions with others to protect their physical health and reduce their risk of contracting the virus, but as a result they may undermine their psychosocial health by placing themselves at risk of social isolation and loneliness.47
The double threat. Social isolation and loneliness have been shown to negatively impact physical health and well-being, resulting in an increased risk of early death48-50; higher likelihood of specific diagnoses, including dementia and cardiovascular conditions48,50; and more frequent use of health care services.50 These concepts, while related, represent different mechanisms for negative health outcomes. Social isolation is an objective condition when one has a lack of opportunities for interaction with other people; loneliness refers to the emotional disconnect one feels when separated from others. Few studies have compared outcomes between these concepts, but in those that have, social isolation appears to be more strongly associated with early death.48-50
A 2013 observational study using data from the English Longitudinal Study on Aging found that both social isolation and loneliness were associated with increased mortality among men and women ages ≥ 52 years (N = 6500).48 However, when studied independently, loneliness was not found to be a significant risk factor. In contrast, social isolation significantly impacted mortality risk, even after adjusting for demographic factors and baseline health status.48 These findings are supported by a 2018 cohort study of individuals (N = 479,054) with a history of an acute cardiovascular event that concluded social isolation was a predictor of mortality, whereas loneliness was not.50
A large 2015 meta-analysis (70 studies, N = 3,407,134) of mortality causes among community-dwelling older adults (average age, 66) confirmed that both objective measures of isolation, as well as subjective measures (such as feelings of loneliness or living alone), have a significant predictive effect in longer-term studies. Each measure shows an approximately 30% increase in the likelihood of death after an average of 7 years.49
Continue to: Health care remains a connection point
Health care remains a connection point. Even when life course events and conditions (eg, death of loved ones, loss of transportation or financial resources, or disengagement from community activities) reduce social connections, most older adults engage in some way with the health care system. A 2020 consensus report by the National Academies of Sciences, Engineering, and Medicine suggests health care professionals capitalize on these connection points with adults ages ≥ 50 years by periodically screening for social isolation and loneliness, documenting social status updates in medical records, and piloting and evaluating interventions in the clinical setting.51
The report offered information about potential avenues for intervention by primary care professionals beyond screening, such as participating in research studies that investigate screening tools and multisystem interventions; social prescribing (linking patients to embedded social work services or community-based organizations); referring patients to support groups; initiating cognitive-based therapy or other behavioral health interventions; or recommending mindfulness practices.51 However, most of the cited intervention studies were not specific to primary care settings and contained poor-quality evidence related to efficacy.
Isolation creates a greater reliance on health services due to a lack of a social support system, while a feeling of emotional disconnection (loneliness) seems to be a barrier to accessing care. A 2017 cohort study linking data from the Health and Retirement Study and Medicare claims revealed that social isolation predicts higher annual health expenditures (> $1600 per beneficiary) driven by hospitalization and skilled nursing facility usage, along with greater mortality, whereas individuals who are lonely result in reduced costs (a reduction of $770 annually) due to lower usage of inpatient and outpatient services.52 Prioritizing interventions that identify and connect isolated older adults to social support, therefore, may increase survivability by ensuring they have access to resources and health care interventions when needed.
In addition, these findings underscore the importance of looking at quality—not just quantity—of older adults’ social connections. A number of validated screening tools exist for social isolation and loneliness (TABLE 253-59); however, concerns exist about assessing risk using a unidimensional tool for a complex concern,47 as well as identifying a problem without having evidence-based interventions to offer as solutions.47,51 Until future studies resolve these concerns, leveraging the physician-patient relationship to broach these difficult subjects may help normalize the issues and create safe spaces to identify individuals who are at risk.

QOL is key to healthy aging. As Kusumastuti et al4 state, “successful ageing lies in the eyes of the beholder.” A 2019 systematic review of 48 qualitative studies revealed that community-dwelling older adults ages ≥ 50 years in 11 countries (N > 4175) perceive well-being by considering QOL within 9 domains: health perception, autonomy, role and activity, relationships, emotional comfort, attitude and adaptation, spirituality, financial security, and home and neighborhood.60 Researchers found that as engagement in any one of these domains declines, older adults may shift their definition of health toward their remaining abilities.60 This offers an explanation as to why patients might rate their health status much higher than their physicians do: older adults tend to have a more holistic concept of health.
Continue to: Take a multidimensional approach to healthy aging
Take a multidimensional approach to healthy aging
Although we have separately examined each of the 4 components of managing healthy aging in a community-dwelling adult, applying a multidimensional approach is most effective. Increasing use of the Medicare AWV provides an opportunity to assess patient health status, determine care preferences, and improve follow-through on preventive screening. It is also important to encourage older adults to engage in regular physical activity—especially muscle-strengthening exercises—and to discuss nutrition and caloric intake to prevent frailty and functional decline.
Assessing and treating vision and hearing impairments and mental health issues, including anxiety and depression, may guard against losses in cognition. When speaking with older adult patients about their social connections, consider asking not only about frequency of contact and access to resources such as food and transportation, but also about whether they are finding ways to bring their own values into those relationships to bolster their QOL. This guidance also may be useful for primary care practices and health care networks when planning future quality-improvement initiatives.
Additional research is needed to support the evidence base for aligning older adult preferences in health care interventions, such as preventive screenings. Also, clinical decision-making requires more clarity about the efficacy of specific diet and exercise interventions for older adults; the impact of early intervention for depression, anxiety, and sleep disorders on neurodegenerative disease; whether loneliness predicts mortality; and how health care delivery systems can be effective at building social connectivity.
For now, it is essential to recognize that initiating health education, screening, and prevention throughout the patient’s lifespan can promote healthy aging outcomes. As family physicians, it is important to capitalize on longitudinal relationships with patients and begin educating younger patients using this multidimensional framework to promote living “a productive and meaningful life”at any age.3
Lynn M. Wilson, DO, 707 Hamilton Street, 8th floor, Department of Family Medicine, Lehigh Valley Health Network, Allentown, PA 18101; [email protected]
Our approach to caring for the growing number of community-dwelling US adults ages ≥ 65 years has shifted. Although we continue to manage disease and disability, there is an increasing emphasis on the promotion of healthy aging by optimizing health care needs and quality of life (QOL).
The American Geriatric Society (AGS) uses the term “healthy aging” to reflect a dedication to improving the health, independence, and QOL of older people.1 The World Health Organization (WHO) defines healthy aging as “the process of developing and maintaining the functional ability that enables well-being in older age.”2 Functional ability encompasses capabilities that align with a person’s values, including meeting basic needs; learning, growing, and making independent decisions; being mobile; building and maintaining healthy relationships; and contributing to society.2 Similarly, the US Department of Health and Human Services has adopted a multidimensional approach to support people in creating “a productive and meaningful life” as they grow older.3
Numerous theoretical models have emerged from research on aging as a multidimensional construct, as evidenced by a 2016 citation analysis that identified 1755 articles written between 1902 and 2015 relating to “successful aging.”4 The analysis revealed 609 definitions operationalized by researchers’ measurement tools (mostly focused on physical function and other health metrics) and 1146 descriptions created by older adults, many emphasizing psychosocial strategies and cultural factors as key to successful aging.4

One approach that is likely to be useful for family physicians is the Age-Friendly Health System. This is an initiative of The John A. Hartford Foundation and the Institute for Healthcare Improvement that uses a multidisciplinary approach to create environments that foster inclusivity and address the needs of older people.5 Following this guidance, primary care providers use evidence-informed strategies that promote safety and address what matters most to older adults and their family caregivers.
The Age-Friendly Health System, as well as AGS and WHO, recognize that there are multiple aspects to well-being as one grows older. By using focused, evidence-based screening, assessments, and interventions, family physicians can best support aging patients in living their most fulfilling lives.
Here we present a review of evidence-based strategies that promote safety and address what matters most to older adults and their family caregivers using a 4-pronged framework, in the style of the Age-Friendly Health System model. However, the literature on healthy aging includes important messages about patient context and lifelong health behaviors, which we capture in an expanded set of thematic guidance. As such, we encourage family physicians to approach healthy aging as follows: (1) monitor health (screening and prevention), (2) promote mobility (physical function), (3) manage mentation (emotional health and cognitive function), and (4) encourage maintenance of social connections (social networks and QOL).
Monitoring health
Leverage Medicare annual wellness visits. A systematic approach is needed to prevent frailty and functional decline, and thus increase the QOL of older adults. To do this, it is important to focus on health promotion and disease prevention, while addressing existing ailments. One method is to leverage the Medicare annual wellness visit (AWV), which provides an opportunity to assess current health status as well as discuss behavior-change and risk-reduction strategies with patients.
Continue to: Although AWVs...
Although AWVs are an opportunity to improve patient outcomes, we are not taking full advantage of them.6 While AWVs have gained traction since their introduction in 2011, usage rates among ethnoracial minority groups has lagged behind.6 A 2018 cohort study examined reasons for disparate utilization rates among individuals ages ≥ 66 years (N = 14,687).7 Researchers found that differences in utilization between ethnoracial groups were explained by socioeconomic factors. Lower education and lower income, as well as rural living, were associated with lower rates of AWV completion.7 In addition, having a usual, nonemergent place to obtain medical care served as a powerful predictor of AWV utilization for all groups.7
Strategies to increase AWV completion rates among all eligible adults include increasing staff awareness of health literacy challenges and ensuring communication strategies are inclusive by providing printed materials in multiple languages, Braille, or larger typefaces; using accessible vocabulary that does not include medical jargon; and making medical interpreters accessible. In addition, training clinicians about unconscious bias and cultural humility can help foster empathy and awareness of differences in health beliefs and behaviors within diverse patient populations.
A 2019 scoping review of 11 studies (N > 60 million) focused on outcomes from Medicare AWVs for patients ages ≥ 65 years.8 This included uptake of preventive services, such as vaccinations or cancer screenings; advice, education, or referrals offered during the AWV; medication use; and hospitalization rates. Overall findings showed that older adults who received a Medicare AWV were more likely to receive referrals for preventive screenings and follow-through on these recommendations compared with those who did not undergo an AWV.8
Completion rates for vaccines, while remaining low overall, were higher among those who completed an AWV. Additionally, these studies showed improved completion of screenings for breast cancer, bone density, and colon cancer. Several studies in the scoping review supported the use of AWVs as an effective means by which to offer health education and advice related to health promotion and risk reduction, such as diet and lifestyle modifications.8 Little evidence exists on long-term outcomes related to AWV completion.8
Utilize shared decision-making to determine whether preventive screening makes sense for your patient. Although cancer remains the second leading cause of death among Americans ages ≥ 65 years,9 clear screening guidelines for this age group remain elusive.10 Physicians and patients often are reluctant to stop cancer screening despite lower life expectancy and fewer potential benefits of diagnosis in this population.9 Some recent studies reinforce the heterogeneity of the older adult population and further underscore the importance of individual-level decision-making.11-14 It is important to let older adult patients and their caregivers know about the potential risks of screening tests, especially the possibility that incidental findings may lead to unwarranted additional care or monitoring.9
Continue to: Avoid these screening conversation missteps
Avoid these screening conversation missteps. A 2017 qualitative study asked 40 community-dwelling older adults (mean age = 76 years) about their preferences for discussing screening cessation with their physicians.13 Three themes emerged.First, they were open to stopping their screenings, especially when suggested by a trusted physician. Second, health status and physical function made sense as decision points, but life expectancy did not. Finally, lengthy discussions with expanded details about risks and benefits were not appreciated, especially if coupled with comments on the limited benefits for those nearing the end of life. When discussing life expectancy, patients preferred phrasing that focused on how the screening was unnecessary because it would not help them live longer.13
Ensure that your message is understood—and culturally relevant. Recent studies on lower health literacy among older adults15,16 and ethnic and racial minorities17-21—as revealed in the 2003 National Assessment of Adult Literacy22—might offer clues to patient receptivity to discussions about preventive screening and other health decisions.
One study found a significant correlation between higher self-rated health literacy and higher engagement in health behaviors such as mammography screening, moderate physical activity, and tobacco avoidance.16 Perceptions of personal control over health status, as well as perceived social standing, also correlated with health literacy score levels.16 Another study concluded that lower health literacy combined with lower self-efficacy, cultural beliefs about health topics (eg, diet and exercise), and distrust in the health care system contributed to lower rates of preventive care utilization among ethnocultural minority older adults in Canada, the United Kingdom, the United States, and Australia.18
Ensuring that easy-to-understand information is equitably shared with older adults of all races and ethnicities is critical. A 2018 study showed that distrust of the health system and cultural issues contributed to the lower incidence of colorectal cancer screenings in Hispanic and Asian American patients ages 50 to 75 years.21 Patients whose physicians engaged in “health literate practices” (eg, offering clear explanations of diagnostic plans and asking patients to describe what they understood) were more likely to obtain recommended breast and colorectal cancer screenings.20 In particular, researchers found that non-Hispanic Blacks were nearly twice as likely to follow through on colorectal cancer screening if their physicians engaged in health literate practices.20 In addition, receiving clear instructions from physicians increased the odds of completing breast cancer screening among Hispanic and non-Hispanic White women.20
Overall, screening information and recommendations should be standardized for all patients. This is particularly important in light of research that found that older non-Hispanic Black patients were less likely than their non-Hispanic White counterparts to receive information from their physicians about colorectal cancer screening.20
Continue to: Mobility
Mobility
Encourage physical activity. Frequent exercise and other forms of physical activity are associated with healthy aging, as shown in a 2017 systematic review and meta-analysis of 23 studies (N = 174,114).23 Despite considerable heterogeneity between studies in how researchers defined healthy aging and physical activity, they found that adults who incorporate regular movement and exercise into daily life are likely to continue to benefit from it into older age.23 In addition, a 2016 secondary analysis of data from the InCHIANTI longitudinal aging study concluded that adults ages ≥ 65 years (N = 1149) who had maintained higher physical activity levels throughout adulthood had less physical function decline and reduced rates of mobility disability and premature death compared with those who reported being less active.24
Preserve gait speed (and bolster health) with these activities. Walking speed, or gait, measured on a level surface has been used as a predictor for various aspects of well-being in older age, such as daily function, mobility, independence, falls, mortality, and hospitalization risk.25 Reduced gait speed is also one of the key indicators of functional impairment in older adults.
A 2015 systematic review sought to determine which type of exercise intervention (resistance, coordination, or multimodal training) would be most effective in preserving gait speed in healthy older adults (N = 2495; mean age = 74.2 years).25 While the 42 included studies were deemed to be fairly low quality, the review revealed (with large effect size [0.84]) that a number of exercise modalities might stave off loss of gait speed in older adults. Patients in the resistance training group had the greatest improvement in gait speed (0.11 m/s), followed by those in the coordination training group (0.09 m/s) and the multimodal training group (0.05 m/s).25
Finally, muscle mass and strength offer a measure of physical performance and functionality. A 2020 systematic review of 83 studies (N = 108,428) showed that low muscle mass and strength, reduced handgrip strength, and lower physical performance were predictive of reduced capacities in activities of daily living and instrumental activities of daily living.26 It is important to counsel adults to remain active throughout their lives and to include resistance training to maintain muscle mass and strength to preserve their motor function, mobility, independence, and QOL.
Use 1 of these scales to identify frailty. Frailty is a distinct clinical syndrome, in which an individual has low reserves and is highly vulnerable to internal and external stressors. It affects many community-dwelling older adults. Within the literature, there has been ongoing discussion regarding the definition of frailty27 (TABLE 128-31).

Continue to: The Fried Frailty Index...
The Fried Frailty Index defines frailty as a purely physical condition; patients need to exhibit 3 of 5 components (ie, weight loss, exhaustion, weakness, slowness, and low physical activity) to be deemed frail.31 The Edmonton Frail Scale is commonly used in geriatric assessments and counts impairments across several domains including physical activity, mood, cognition, and incontinence.30,32,33 Physicians need to complete a training course prior to its use. Finally, the definition of frailty used by Rockwood et al28, 29 was used to develop the Clinical Frailty Scale, which relies on broader criteria that include social and psychological elements in addition to physical elements.The Clinical Frailty Scale uses clinician judgment to evaluate patient-specific domains (eg, comorbidities, functionality, and cognition) and to generate a score ranging from 1 (very fit) to 9 (terminally ill).29 This scale is accessible and easy to implement. As a result, use of this scale has increased during the COVID-19 pandemic. All definitions include a pre-frail state, indicating the dynamic nature of frailty over time.
It is important to identify pre-frail and frail older adults using 1 of these screening tools. Interventions to reverse frailty that can be initiated in the primary care setting include identifying treatable medical conditions, assessing medication appropriateness, providing nutritional advice, and developing an exercise plan.34
Conduct a nutritional assessment; consider this diet. Studies show that nutritional status can predict physical function and frailty risk in older adults. A 2017 systematic review of 19 studies (N = 22,270) of frail adults ages ≥ 65 years found associations related to specific dietary constructs (ie, micronutrients, macronutrients, antioxidants, overall diet quality, and timing of consumption).35 Plant-based diets with higher levels of micronutrients, such as vitamins C and E and beta-carotene, or diets with more protein or macronutrients, regardless of source foods, all resulted in inverse associations with frailty syndrome.35
A 2017 study showed that physical exercise and maintaining good nutritional status may be effective for preventing frailty in community-dwelling pre-frail older individuals.36 A 2019 study showed that a combination of muscle strength training and protein supplementation was the most effective intervention to delay or reverse frailty and was easiest to implement in primary care.37 A 2020 meta-analysis of 31 studies (N = 4794) addressing frailty among primary care patients > 60 years showed that interventions using predominantly resistance-based exercise and nutrition supplementation improved frailty status over the control.38 Researchers also found that a comprehensive geriatric assessment or exercise—alone or in combination with nutrition education—reduced physical frailty.
Mentation
Screen and treat cognitive impairments. Cognitive function and autonomy in decision-making are important factors in healthy aging. Aspects of mental health (eg, depression and anxiety), sensory impairment (eg, visual and auditory impairment), and mentation issues (eg, delirium, dementia, and related conditions), as well as diet, physical exercise, and mobility, can all impede cognitive functionality. The long-term effects of depression, anxiety,39 sensory deficits,40 mobility,41 diet,42 and, ultimately, aging may impact Alzheimer disease (AD). The risk of an AD diagnosis increases with age.39
Continue to: A 2018 prospective cohort study...
A 2018 prospective cohort study using data from the National Alzheimer’s Coordinating Center followed individuals (N = 12,053) who were cognitively asymptomatic at their initial visits to determine who developed clinical signs of AD.39 Survival analysis showed several psychosocial factors—anxiety, sleep disturbances, and depressive episodes of any type (occurring within the past 2 years, clinician verified, lifetime report)—were significantly associated with an eventual AD diagnosis and increased the risk of AD.39 More research is needed to verify the impact of early intervention for these conditions on neurodegenerative disease; however, screening and treating psychosocial factors such as anxiety and depression should be maintained.
Researchers evaluated the impact of a dual sensory impairment (DSI) on dementia risk using data from 2051 participants in the Ginkgo Evaluation of Memory Study.40 Hearing and visual impairments (defined as DSI when these conditions coexist) or visual impairment alone were significantly associated with increased risk of dementia in older adults. The researchers reported that DSI was significantly associated with a higher risk of all-cause dementia (hazard ratio [HR] = 1.86; 95% CI, 1.25-2.76) and AD (HR = 2.12; 95% CI, 1.34-3.36).40 Visual impairment alone was associated with an increased risk of all-cause dementia (HR = 1.32; 95% CI, 1.02-1.71).40 These results suggest that screening of DSI or visual impairment earlier in the patient’s lifespan may identify those at high risk of dementia in older adulthood.
The American Academy of Ophthalmology recommends patients with healthy eyes be screened once during their 20s and twice in their 30s; a full examination is recommended by age 40. For patients ages ≥ 65 years, it is recommended that eye examinations occur every 1 to 2 years.43
Diet and mobility play a big role in cognition. Diet43 and exercise41,42,44 are believed to have an impact on mentation, and recent studies show memory and global cognition could be malleable later in life. A 2015 meta-analysis of 490 treatment arms of 24 randomized controlled studies showed improvement in global cognition with consumption of a Mediterranean diet plus olive oil (effect size [ES] standardized mean difference [SMD] = 0.22; 95% CI, 0.16-0.27) and tai chi exercises (ES SMD = 0.18; 95% CI, 0.06-0.29).42 The analysis also found improved memory among participants who consumed the Mediterranean diet/olive oil combination (ES SMD = 0.22; 95% CI, 0.12-0.32) and soy isoflavone supplements (ES SMD = 0.11; 95% CI, 0.04-0.17). Although the ESs are small, they are significant and offer promising evidence that individual choices related to nutrition or exercise may influence cognition and memory.
A 2018 systematic review found that all domains of cognition showed improvement with 45 to 60 minutes of moderate-to-vigorous physical exercise.44 Attention, executive function, memory, and working memory showed significant increases, whereas global cognition improvements were not statistically significant.44 A 2016 meta-analysis of 26 studies (N = 26,355) found a positive association between an objective mobility measure (gait, lower-extremity function, and balance) and cognitive function (global, executive function, memory, and processing speed) in older adults.41 These results highlight that diet, mobility, and physical exercise impact cognitive functioning.
Continue to: Maintaining social connections
Maintaining social connections
Social isolation and loneliness—compounded by a pandemic. The US Department of Health and Human Services notes that “community connections” are among the key factors required for healthy aging.3 Similarly, the WHO definition of healthy aging considers whether individuals can build and sustain relationships with other people and find ways to engender their personal values through these connections.2
As people age, their social connections often decrease due to the death of friends and family, shifts in living arrangements, loss of mobility or eyesight (and thus self-transport), and the onset or increased acuity of illness or chronic conditions.45 This has been exacerbated by the COVID-19 pandemic, which has spurred shelter-in-place and stay-at-home orders along with recommendations for physical distancing (also known as social distancing), especially for older adults who are at higher risk.46 Smith et al47 calls this the COVID-19 Social Connectivity Paradox, in which older adults limit their interactions with others to protect their physical health and reduce their risk of contracting the virus, but as a result they may undermine their psychosocial health by placing themselves at risk of social isolation and loneliness.47
The double threat. Social isolation and loneliness have been shown to negatively impact physical health and well-being, resulting in an increased risk of early death48-50; higher likelihood of specific diagnoses, including dementia and cardiovascular conditions48,50; and more frequent use of health care services.50 These concepts, while related, represent different mechanisms for negative health outcomes. Social isolation is an objective condition when one has a lack of opportunities for interaction with other people; loneliness refers to the emotional disconnect one feels when separated from others. Few studies have compared outcomes between these concepts, but in those that have, social isolation appears to be more strongly associated with early death.48-50
A 2013 observational study using data from the English Longitudinal Study on Aging found that both social isolation and loneliness were associated with increased mortality among men and women ages ≥ 52 years (N = 6500).48 However, when studied independently, loneliness was not found to be a significant risk factor. In contrast, social isolation significantly impacted mortality risk, even after adjusting for demographic factors and baseline health status.48 These findings are supported by a 2018 cohort study of individuals (N = 479,054) with a history of an acute cardiovascular event that concluded social isolation was a predictor of mortality, whereas loneliness was not.50
A large 2015 meta-analysis (70 studies, N = 3,407,134) of mortality causes among community-dwelling older adults (average age, 66) confirmed that both objective measures of isolation, as well as subjective measures (such as feelings of loneliness or living alone), have a significant predictive effect in longer-term studies. Each measure shows an approximately 30% increase in the likelihood of death after an average of 7 years.49
Continue to: Health care remains a connection point
Health care remains a connection point. Even when life course events and conditions (eg, death of loved ones, loss of transportation or financial resources, or disengagement from community activities) reduce social connections, most older adults engage in some way with the health care system. A 2020 consensus report by the National Academies of Sciences, Engineering, and Medicine suggests health care professionals capitalize on these connection points with adults ages ≥ 50 years by periodically screening for social isolation and loneliness, documenting social status updates in medical records, and piloting and evaluating interventions in the clinical setting.51
The report offered information about potential avenues for intervention by primary care professionals beyond screening, such as participating in research studies that investigate screening tools and multisystem interventions; social prescribing (linking patients to embedded social work services or community-based organizations); referring patients to support groups; initiating cognitive-based therapy or other behavioral health interventions; or recommending mindfulness practices.51 However, most of the cited intervention studies were not specific to primary care settings and contained poor-quality evidence related to efficacy.
Isolation creates a greater reliance on health services due to a lack of a social support system, while a feeling of emotional disconnection (loneliness) seems to be a barrier to accessing care. A 2017 cohort study linking data from the Health and Retirement Study and Medicare claims revealed that social isolation predicts higher annual health expenditures (> $1600 per beneficiary) driven by hospitalization and skilled nursing facility usage, along with greater mortality, whereas individuals who are lonely result in reduced costs (a reduction of $770 annually) due to lower usage of inpatient and outpatient services.52 Prioritizing interventions that identify and connect isolated older adults to social support, therefore, may increase survivability by ensuring they have access to resources and health care interventions when needed.
In addition, these findings underscore the importance of looking at quality—not just quantity—of older adults’ social connections. A number of validated screening tools exist for social isolation and loneliness (TABLE 253-59); however, concerns exist about assessing risk using a unidimensional tool for a complex concern,47 as well as identifying a problem without having evidence-based interventions to offer as solutions.47,51 Until future studies resolve these concerns, leveraging the physician-patient relationship to broach these difficult subjects may help normalize the issues and create safe spaces to identify individuals who are at risk.

QOL is key to healthy aging. As Kusumastuti et al4 state, “successful ageing lies in the eyes of the beholder.” A 2019 systematic review of 48 qualitative studies revealed that community-dwelling older adults ages ≥ 50 years in 11 countries (N > 4175) perceive well-being by considering QOL within 9 domains: health perception, autonomy, role and activity, relationships, emotional comfort, attitude and adaptation, spirituality, financial security, and home and neighborhood.60 Researchers found that as engagement in any one of these domains declines, older adults may shift their definition of health toward their remaining abilities.60 This offers an explanation as to why patients might rate their health status much higher than their physicians do: older adults tend to have a more holistic concept of health.
Continue to: Take a multidimensional approach to healthy aging
Take a multidimensional approach to healthy aging
Although we have separately examined each of the 4 components of managing healthy aging in a community-dwelling adult, applying a multidimensional approach is most effective. Increasing use of the Medicare AWV provides an opportunity to assess patient health status, determine care preferences, and improve follow-through on preventive screening. It is also important to encourage older adults to engage in regular physical activity—especially muscle-strengthening exercises—and to discuss nutrition and caloric intake to prevent frailty and functional decline.
Assessing and treating vision and hearing impairments and mental health issues, including anxiety and depression, may guard against losses in cognition. When speaking with older adult patients about their social connections, consider asking not only about frequency of contact and access to resources such as food and transportation, but also about whether they are finding ways to bring their own values into those relationships to bolster their QOL. This guidance also may be useful for primary care practices and health care networks when planning future quality-improvement initiatives.
Additional research is needed to support the evidence base for aligning older adult preferences in health care interventions, such as preventive screenings. Also, clinical decision-making requires more clarity about the efficacy of specific diet and exercise interventions for older adults; the impact of early intervention for depression, anxiety, and sleep disorders on neurodegenerative disease; whether loneliness predicts mortality; and how health care delivery systems can be effective at building social connectivity.
For now, it is essential to recognize that initiating health education, screening, and prevention throughout the patient’s lifespan can promote healthy aging outcomes. As family physicians, it is important to capitalize on longitudinal relationships with patients and begin educating younger patients using this multidimensional framework to promote living “a productive and meaningful life”at any age.3
Lynn M. Wilson, DO, 707 Hamilton Street, 8th floor, Department of Family Medicine, Lehigh Valley Health Network, Allentown, PA 18101; [email protected]
1. Friedman S, Mulhausen P, Cleveland M, et al. Healthy aging: American Geriatrics Society white paper executive summary. J Am Geriatr Soc. 2018;67:17-20. doi: 10.1111/jgs.15644
2. World Health Organization. World report on ageing and health. 2015. Accessed June 29, 2020. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1
3. U.S. Department of Health & Human Services. Healthy aging. Accessed June 29, 2020. www.hhs.gov/aging/healthy-aging
4. Kusumastuti S, Derks MGM, Tellier S, et al. Successful ageing: a study of the literature using citation network analysis. Maturitas. 2016;93:4-12. doi: 10.1016/j.maturitas.2016.04.010
5. Institute for Healthcare Improvement. Age-friendly health systems: guide to using the 4Ms in the care of older adults [white paper]. 2020. Accessed June 29, 2020. www.ihi.org/Engage/Initiatives/Age-Friendly-Health-systems/Documents/IHIAgeFriendlyHealthSystems_GuidetoUsing4MsCare.pdf
6. Lind KE, Hildreth KL, Perraillon MC. Persistent disparities in Medicare’s annual wellness visit utilization. Med Care. 2019;57:984-989. doi: 10.1097/MLR.0000000000001229
7. Lind KE, Hildreth K, Lindrooth R, et al. Ethnoracial disparities in Medicare annual wellness visit utilization: evidence from a nationally representative database. Med Care. 2018;56:761-766. doi: 10.1097/MLR.0000000000000962
8. Simpson VL, Kovich M. Outcomes of primary care-based Medicare annual wellness visits with older adults: a scoping review. Geriatr Nurs. 2019;40:590-596. doi: 10.1016/j.gerinurse.2019.06.001
9. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1-77.
10. Salzman B, Beldowski K, de la Paz A. Cancer screening in older patients. Am Fam Physician. 2016;93:659-667.
11. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399-406. doi: 10.1001/jamainternmed.2016.9022
12. Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311:1336-1347. doi: 10.1001/jama.2014.2834
13. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177:1121-1128. doi: 10.1001/jamainternmed.2017.1778
14. Butterworth JE, Hays R, McDonagh ST, et al. Interventions for involving older patients with multi-morbidity in decision-making during primary care consultations. Cochrane Database Syst Rev. 2019;10:CD013124. doi: 10.1002/14651858.CD013124.pub2
15. Bostock S, Steptoe A. Association between low functional health literacy and mortality in older adults: longitudinal cohort study. BMJ. 2012;344:e1602. doi: 10.1136/bmj.e1602
16. Fernandez DM, Larson JL, Zikmund-Fisher BJ. Associations between health literacy and preventive health behaviors among older adults: findings from the health and retirement study. BMC Public Health. 2016;16:596. doi: 10.1186/s12889-016-3267-7
17. Weekes CV. African Americans and health literacy: a systematic review. ABNF J. 2012;23:76-80.
18. Mantwill S, Monestel-Umaña S, Schulz PJ. The relationship between health literacy and health disparities: a systematic review. PLoS One. 2015;10:e0145455. doi: 10.1371/journal.pone.0145455
19. Khan MM, Kobayashi K. Optimizing health promotion among ethnocultural minority older adults (EMOA). Int J Migration Health Soc Care. 2015;11:268-281. doi: 10.1108/IJMHSC-12-2014-0047
20. Kindratt TB, Dallo FJ, Allicock M, et al. The influence of patient-provider communication on cancer screenings differs among racial and ethnic groups. Prev Med Rep. 2020;18:101086. doi: 10.1016/j.pmedr.2020.101086
21. Hong Y-R, Tauscher J, Cardel M. Distrust in health care and cultural factors are associated with uptake of colorectal cancer screening in Hispanic and Asian Americans. Cancer. 2018;124:335-345. doi: 10.1002/cncr.31052
22. Kutner M, Greenberg E, Jin Y, et al. Literacy in everyday life: results from the 2003 National Assessment of Adult Literacy. NCES 2007-480. U.S. Department of Education, National Center for Education Statistics. April 2007. Accessed August 27, 2021. http://nces.ed.gov/Pubs2007/2007480_1.pdf
23. Daskalopoulou C, Stubbs B, Kralj C, et al. Physical activity and healthy ageing: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2017;38:6-17. doi: 10.1016/j.arr.2017.06.003
24. Stenholm S, Koster A, Valkeinen H, et al. Association of physical activity history with physical function and mortality in old age. J Gerontol A Biol Sci Med Sci. 2016;71:496-501. doi: 10.1093/gerona/glv111
25. Hortobágyi T, Lesinski M, Gäbler M, et al. Effects of three types of exercise interventions on healthy old adults’ gait speed: a systematic review and meta-analysis. Sports Med. 2015;45:1627‐1643. Published correction appears in Sports Med. 2016;46:453. doi: 10.1007/s40279-015-0371-2
26. Wang DXM, Yao J, Zirek Y, et al. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:3‐25. doi: 10.1002/jcsm.12502
27. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129-2138. doi: 10.1111/j.1532-5415.2011.03597.x
28. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. doi: 10.1503/cmaj.050051
29. Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20:393. doi: 10.1186/s12877-020-01801-7
30. Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526-529. doi: 10.1093/ageing/afl041
31. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
32. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Euro J Intern Med. 2016;31:3-10. doi: 10.1016/j.ejim.2016.03.007
33. Perna S, Francis MD, Bologna C, et al. Performance of Edmonton Frail Scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017;17:2. doi: 10.1186/s12877-016-0382-3
34. Chen CY, Gan P, How CH. Approach to frailty in the elderly in primary care and the community. Singapore Med J. 2018;59:338. doi: 10.11622/smedj.2018052
35. Lorenzo-López L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17:108. doi: 10.1186/s12877-017-0496-2
36. Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46:401-407. doi: 10.1093/ageing/afw242
37. Travers J, Romero-Ortuno R, Bailey J, et al. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69:e61-e69. doi: 10.3399/bjgp18X700241
38. Macdonald SHF, Travers J, Shé ÉN, et al. Primary care interventions to address physical frailty among community-dwelling adults aged 60 years or older: a meta-analysis. PLoS One. 2020;15:e0228821. doi: 10.1371/journal.pone.0228821
39. Burke SL, Cadet T, Alcide A, et al. Psychosocial risk factors and Alzheimer’s disease: the associative effect of depression, sleep disturbance, and anxiety. Aging Ment Health. 2018;22:1577-1584. doi: 10.1080/13607863.2017.1387760
40. Hwang PH, Longstreth WT Jr, Brenowitz WD, et al. Dual sensory impairment in older adults and risk of dementia from the GEM Study. Alzheimers Dement (Amst). 2020;12:e12054. doi: 10.1002/dad2.12054
41. Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164‐174. doi: 10.1016/j.gaitpost.2016.08.028
42. Lehert P, Villaseca P, Hogervorst E, et al. Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric. 2015;18:678-689. doi: 10.3109/13697137.2015.1078106
43. Turbert D. Eye exam and vision testing basics. American Academy of Ophthalmology Web site. January 14, 2021. Accessed March 5, 2021. www.aao.org/eye-health/tips-prevention/eye-exams-101
44. Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154-160. doi: 10.1136/bjsports-2016-096587
45. CDC. Percent of U.S. adults 55 and over with chronic conditions. November 6, 2015. Accessed April 29, 2021. www.cdc.gov/nchs/health_policy/adult_chronic_conditions.htm
46. National Council on Aging. COVID-driven isolation can be dangerous for older adults. March 31, 2021. Accessed April 29, 2021. www.ncoa.org/article/covid-driven-isolation-can-be-dangerous-for-older-adults
47. Smith ML, Steinman LE, Casey EA. Combatting social isolation among older adults in a time of physical distancing: the COVID-19 social connectivity paradox. Front Public Health. 2020;8:403. doi: 10.3389/fpubh.2020.00403
48. Steptoe A, Shankar A, Demakakos P, et al. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797-5801. doi: 10.1073/pnas.1219686110
49. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227-237. doi: 10.1177/1745691614568352
50. Hakulinen C, Pulkki-Råback L, Virtanen M, et al. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart. 2018;104:1536-1542. doi: 10.1136/heartjnl-2017-312663
51. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. The National Academies Press; 2020. doi: 10.17226/25663
52. Shaw JG, Farid M, Noel-Miller C, et al. Social isolation and Medicare spending: among older adults, objective isolation increases expenditures while loneliness does not. J Aging Health. 2017;29:1119-1143. doi: 10.1177/0898264317703559
53. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186-204. doi: 10.1093/oxfordjournals.aje.a112674
54. Campaign to End Loneliness. Measuring your impact on loneliness in later life. Accessed April 29, 2021. www.campaigntoendloneliness.org/wp-content/uploads/Loneliness-Measurement-Guidance1-1.pdf
55. Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50:31-48. doi: 10.1177/002214650905000103
56. Gierveld JDJ, Van Tilburg T. A 6-item scale for overall, emotional, and social loneliness: confirmatory tests on survey data. Res Aging. 2006;28:582-598. doi: 10.1177/0164027506289723
57. Koenig HG, Westlund RE, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34:61-69. doi: 10.1016/S0033-3182(93)71928-3
58. Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503-513. doi: 10.1093/geront/46.4.503
59. Russell DW. UCLA Loneliness Scale (version 3): reliability, validity, factor structure. J Pers Assess. 1996;66:20-40. doi: 10.1207/s15327752jpa6601_2
60. van Leeuwen KM, van Loon MS, van Nes FA, et al. What does quality of life mean to older adults? A thematic synthesis. PLoS One. 2019;14:e0213263. doi: 10.1371/journal.pone.0213263
1. Friedman S, Mulhausen P, Cleveland M, et al. Healthy aging: American Geriatrics Society white paper executive summary. J Am Geriatr Soc. 2018;67:17-20. doi: 10.1111/jgs.15644
2. World Health Organization. World report on ageing and health. 2015. Accessed June 29, 2020. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1
3. U.S. Department of Health & Human Services. Healthy aging. Accessed June 29, 2020. www.hhs.gov/aging/healthy-aging
4. Kusumastuti S, Derks MGM, Tellier S, et al. Successful ageing: a study of the literature using citation network analysis. Maturitas. 2016;93:4-12. doi: 10.1016/j.maturitas.2016.04.010
5. Institute for Healthcare Improvement. Age-friendly health systems: guide to using the 4Ms in the care of older adults [white paper]. 2020. Accessed June 29, 2020. www.ihi.org/Engage/Initiatives/Age-Friendly-Health-systems/Documents/IHIAgeFriendlyHealthSystems_GuidetoUsing4MsCare.pdf
6. Lind KE, Hildreth KL, Perraillon MC. Persistent disparities in Medicare’s annual wellness visit utilization. Med Care. 2019;57:984-989. doi: 10.1097/MLR.0000000000001229
7. Lind KE, Hildreth K, Lindrooth R, et al. Ethnoracial disparities in Medicare annual wellness visit utilization: evidence from a nationally representative database. Med Care. 2018;56:761-766. doi: 10.1097/MLR.0000000000000962
8. Simpson VL, Kovich M. Outcomes of primary care-based Medicare annual wellness visits with older adults: a scoping review. Geriatr Nurs. 2019;40:590-596. doi: 10.1016/j.gerinurse.2019.06.001
9. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1-77.
10. Salzman B, Beldowski K, de la Paz A. Cancer screening in older patients. Am Fam Physician. 2016;93:659-667.
11. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399-406. doi: 10.1001/jamainternmed.2016.9022
12. Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311:1336-1347. doi: 10.1001/jama.2014.2834
13. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177:1121-1128. doi: 10.1001/jamainternmed.2017.1778
14. Butterworth JE, Hays R, McDonagh ST, et al. Interventions for involving older patients with multi-morbidity in decision-making during primary care consultations. Cochrane Database Syst Rev. 2019;10:CD013124. doi: 10.1002/14651858.CD013124.pub2
15. Bostock S, Steptoe A. Association between low functional health literacy and mortality in older adults: longitudinal cohort study. BMJ. 2012;344:e1602. doi: 10.1136/bmj.e1602
16. Fernandez DM, Larson JL, Zikmund-Fisher BJ. Associations between health literacy and preventive health behaviors among older adults: findings from the health and retirement study. BMC Public Health. 2016;16:596. doi: 10.1186/s12889-016-3267-7
17. Weekes CV. African Americans and health literacy: a systematic review. ABNF J. 2012;23:76-80.
18. Mantwill S, Monestel-Umaña S, Schulz PJ. The relationship between health literacy and health disparities: a systematic review. PLoS One. 2015;10:e0145455. doi: 10.1371/journal.pone.0145455
19. Khan MM, Kobayashi K. Optimizing health promotion among ethnocultural minority older adults (EMOA). Int J Migration Health Soc Care. 2015;11:268-281. doi: 10.1108/IJMHSC-12-2014-0047
20. Kindratt TB, Dallo FJ, Allicock M, et al. The influence of patient-provider communication on cancer screenings differs among racial and ethnic groups. Prev Med Rep. 2020;18:101086. doi: 10.1016/j.pmedr.2020.101086
21. Hong Y-R, Tauscher J, Cardel M. Distrust in health care and cultural factors are associated with uptake of colorectal cancer screening in Hispanic and Asian Americans. Cancer. 2018;124:335-345. doi: 10.1002/cncr.31052
22. Kutner M, Greenberg E, Jin Y, et al. Literacy in everyday life: results from the 2003 National Assessment of Adult Literacy. NCES 2007-480. U.S. Department of Education, National Center for Education Statistics. April 2007. Accessed August 27, 2021. http://nces.ed.gov/Pubs2007/2007480_1.pdf
23. Daskalopoulou C, Stubbs B, Kralj C, et al. Physical activity and healthy ageing: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2017;38:6-17. doi: 10.1016/j.arr.2017.06.003
24. Stenholm S, Koster A, Valkeinen H, et al. Association of physical activity history with physical function and mortality in old age. J Gerontol A Biol Sci Med Sci. 2016;71:496-501. doi: 10.1093/gerona/glv111
25. Hortobágyi T, Lesinski M, Gäbler M, et al. Effects of three types of exercise interventions on healthy old adults’ gait speed: a systematic review and meta-analysis. Sports Med. 2015;45:1627‐1643. Published correction appears in Sports Med. 2016;46:453. doi: 10.1007/s40279-015-0371-2
26. Wang DXM, Yao J, Zirek Y, et al. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:3‐25. doi: 10.1002/jcsm.12502
27. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129-2138. doi: 10.1111/j.1532-5415.2011.03597.x
28. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. doi: 10.1503/cmaj.050051
29. Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20:393. doi: 10.1186/s12877-020-01801-7
30. Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526-529. doi: 10.1093/ageing/afl041
31. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
32. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Euro J Intern Med. 2016;31:3-10. doi: 10.1016/j.ejim.2016.03.007
33. Perna S, Francis MD, Bologna C, et al. Performance of Edmonton Frail Scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017;17:2. doi: 10.1186/s12877-016-0382-3
34. Chen CY, Gan P, How CH. Approach to frailty in the elderly in primary care and the community. Singapore Med J. 2018;59:338. doi: 10.11622/smedj.2018052
35. Lorenzo-López L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17:108. doi: 10.1186/s12877-017-0496-2
36. Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46:401-407. doi: 10.1093/ageing/afw242
37. Travers J, Romero-Ortuno R, Bailey J, et al. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69:e61-e69. doi: 10.3399/bjgp18X700241
38. Macdonald SHF, Travers J, Shé ÉN, et al. Primary care interventions to address physical frailty among community-dwelling adults aged 60 years or older: a meta-analysis. PLoS One. 2020;15:e0228821. doi: 10.1371/journal.pone.0228821
39. Burke SL, Cadet T, Alcide A, et al. Psychosocial risk factors and Alzheimer’s disease: the associative effect of depression, sleep disturbance, and anxiety. Aging Ment Health. 2018;22:1577-1584. doi: 10.1080/13607863.2017.1387760
40. Hwang PH, Longstreth WT Jr, Brenowitz WD, et al. Dual sensory impairment in older adults and risk of dementia from the GEM Study. Alzheimers Dement (Amst). 2020;12:e12054. doi: 10.1002/dad2.12054
41. Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164‐174. doi: 10.1016/j.gaitpost.2016.08.028
42. Lehert P, Villaseca P, Hogervorst E, et al. Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric. 2015;18:678-689. doi: 10.3109/13697137.2015.1078106
43. Turbert D. Eye exam and vision testing basics. American Academy of Ophthalmology Web site. January 14, 2021. Accessed March 5, 2021. www.aao.org/eye-health/tips-prevention/eye-exams-101
44. Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154-160. doi: 10.1136/bjsports-2016-096587
45. CDC. Percent of U.S. adults 55 and over with chronic conditions. November 6, 2015. Accessed April 29, 2021. www.cdc.gov/nchs/health_policy/adult_chronic_conditions.htm
46. National Council on Aging. COVID-driven isolation can be dangerous for older adults. March 31, 2021. Accessed April 29, 2021. www.ncoa.org/article/covid-driven-isolation-can-be-dangerous-for-older-adults
47. Smith ML, Steinman LE, Casey EA. Combatting social isolation among older adults in a time of physical distancing: the COVID-19 social connectivity paradox. Front Public Health. 2020;8:403. doi: 10.3389/fpubh.2020.00403
48. Steptoe A, Shankar A, Demakakos P, et al. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797-5801. doi: 10.1073/pnas.1219686110
49. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227-237. doi: 10.1177/1745691614568352
50. Hakulinen C, Pulkki-Råback L, Virtanen M, et al. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart. 2018;104:1536-1542. doi: 10.1136/heartjnl-2017-312663
51. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. The National Academies Press; 2020. doi: 10.17226/25663
52. Shaw JG, Farid M, Noel-Miller C, et al. Social isolation and Medicare spending: among older adults, objective isolation increases expenditures while loneliness does not. J Aging Health. 2017;29:1119-1143. doi: 10.1177/0898264317703559
53. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186-204. doi: 10.1093/oxfordjournals.aje.a112674
54. Campaign to End Loneliness. Measuring your impact on loneliness in later life. Accessed April 29, 2021. www.campaigntoendloneliness.org/wp-content/uploads/Loneliness-Measurement-Guidance1-1.pdf
55. Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50:31-48. doi: 10.1177/002214650905000103
56. Gierveld JDJ, Van Tilburg T. A 6-item scale for overall, emotional, and social loneliness: confirmatory tests on survey data. Res Aging. 2006;28:582-598. doi: 10.1177/0164027506289723
57. Koenig HG, Westlund RE, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34:61-69. doi: 10.1016/S0033-3182(93)71928-3
58. Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503-513. doi: 10.1093/geront/46.4.503
59. Russell DW. UCLA Loneliness Scale (version 3): reliability, validity, factor structure. J Pers Assess. 1996;66:20-40. doi: 10.1207/s15327752jpa6601_2
60. van Leeuwen KM, van Loon MS, van Nes FA, et al. What does quality of life mean to older adults? A thematic synthesis. PLoS One. 2019;14:e0213263. doi: 10.1371/journal.pone.0213263
PRACTICE RECOMMENDATIONS
› Prioritize annual wellness visits to improve patient follow-through on recommended services. B
› Encourage physical activity, especially musclestrengthening exercises, to prevent frailty and to mediate decline in the ability to perform activities of daily living. A
› Assess and treat older adults for visual and hearing impairments A , as well as anxiety, depression, and mobility impairments. C They are all associated with cognitive function.
› Ask patients about the frequency of their social interactions A and quality of their relationships B to determine their access to resources, such as food and transportation, as well as their perceptions about their quality of life.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sleep apnea has many faces
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
‘Fascinating’ link between Alzheimer’s and COVID-19
The findings could lead to new treatment targets to slow progression and severity of both diseases.
Investigators found that a single genetic variant in the oligoadenylate synthetase 1 (OAS1) gene increases the risk for AD and that related variants in the same gene increase the likelihood of severe COVID-19 outcomes.
“These findings may allow us to identify new drug targets to slow progression of both diseases and reduce their severity,” Dervis Salih, PhD, senior research associate, UK Dementia Research Institute, University College London, said in an interview.
“Our work also suggests new approaches to treat both diseases with the same drugs,” Dr. Salih added.
The study was published online Oct. 7 in Brain.
Shared genetic network
The OAS1 gene is expressed in microglia, a type of immune cell that makes up around 10% of all cells in the brain.
In earlier work, investigators found evidence suggesting a link between the OAS1 gene and AD, but the function of the gene in microglia was unknown.
To further investigate the gene’s link to AD, they sequenced genetic data from 2,547 people – half with AD, and half without.
The genotyping analysis confirmed that the single-nucleotide polymorphism (SNP) rs1131454 within OAS1 is significantly associated with AD.
Given that the same OAS1 locus has recently been linked with severe COVID-19 outcomes, the researchers investigated four variants on the OAS1 gene.
Results indicate that SNPs within OAS1 associated with AD also show linkage to SNP variants associated with critical illness in COVID-19.
The rs1131454 (risk allele A) and rs4766676 (risk allele T) are associated with AD, and rs10735079 (risk allele A) and rs6489867 (risk allele T) are associated with critical illness with COVID-19, the investigators reported. All of these risk alleles dampen expression of OAS1.
“This study also provides strong new evidence that interferon signaling by the innate immune system plays a substantial role in the progression of Alzheimer’s,” said Dr. Salih.
“Identifying this shared genetic network in innate immune cells will allow us with future work to identify new biomarkers to track disease progression and also predict disease risk better for both disorders,” he added.
‘Fascinating’ link
In a statement from the UK nonprofit organization, Science Media Center, Kenneth Baillie, MBChB, with the University of Edinburgh, said this study builds on a discovery he and his colleagues made last year that OAS1 variants are associated with severe COVID-19.
“In the ISARIC4C study, we recently found that this is probably due to a change in the way cell membranes detect viruses, but this mechanism doesn’t explain the fascinating association with Alzheimer’s disease reported in this new work,” Dr. Baillie said.
“It is often the case that the same gene can have different roles in different parts of the body. Importantly, it doesn’t mean that having COVID-19 has any effect on your risk of Alzheimer’s,” he added.
Also weighing in on the new study, Jonathan Schott, MD, professor of neurology, University College London, noted that dementia is the “main preexisting health condition associated with COVID-19 mortality, accounting for about one in four deaths from COVID-19 between March and June 2020.
“While some of this excessive mortality may relate to people with dementia being overrepresented in care homes, which were particularly hard hit by the pandemic, or due to general increased vulnerability to infections, there have been questions as to whether there are common factors that might increase susceptibility both to developing dementia and to dying from COVID-19,” Dr. Schott explained.
This “elegant paper” provides evidence for the latter, “suggesting a common genetic mechanism both for Alzheimer’s disease and for severe COVID-19 infection,” Dr. Schott said.
“The identification of a genetic risk factor and elucidation of inflammatory pathways through which it may increase risk has important implications for our understanding of both diseases, with potential implications for novel treatments,” he added.
The study was funded by the UK Dementia Research Institute. The authors have disclosed no relevant financial relationships. Dr. Schott serves as chief medical officer for Alzheimer’s Research UK and is clinical adviser to the UK Dementia Research Institute. Dr. Baillie has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings could lead to new treatment targets to slow progression and severity of both diseases.
Investigators found that a single genetic variant in the oligoadenylate synthetase 1 (OAS1) gene increases the risk for AD and that related variants in the same gene increase the likelihood of severe COVID-19 outcomes.
“These findings may allow us to identify new drug targets to slow progression of both diseases and reduce their severity,” Dervis Salih, PhD, senior research associate, UK Dementia Research Institute, University College London, said in an interview.
“Our work also suggests new approaches to treat both diseases with the same drugs,” Dr. Salih added.
The study was published online Oct. 7 in Brain.
Shared genetic network
The OAS1 gene is expressed in microglia, a type of immune cell that makes up around 10% of all cells in the brain.
In earlier work, investigators found evidence suggesting a link between the OAS1 gene and AD, but the function of the gene in microglia was unknown.
To further investigate the gene’s link to AD, they sequenced genetic data from 2,547 people – half with AD, and half without.
The genotyping analysis confirmed that the single-nucleotide polymorphism (SNP) rs1131454 within OAS1 is significantly associated with AD.
Given that the same OAS1 locus has recently been linked with severe COVID-19 outcomes, the researchers investigated four variants on the OAS1 gene.
Results indicate that SNPs within OAS1 associated with AD also show linkage to SNP variants associated with critical illness in COVID-19.
The rs1131454 (risk allele A) and rs4766676 (risk allele T) are associated with AD, and rs10735079 (risk allele A) and rs6489867 (risk allele T) are associated with critical illness with COVID-19, the investigators reported. All of these risk alleles dampen expression of OAS1.
“This study also provides strong new evidence that interferon signaling by the innate immune system plays a substantial role in the progression of Alzheimer’s,” said Dr. Salih.
“Identifying this shared genetic network in innate immune cells will allow us with future work to identify new biomarkers to track disease progression and also predict disease risk better for both disorders,” he added.
‘Fascinating’ link
In a statement from the UK nonprofit organization, Science Media Center, Kenneth Baillie, MBChB, with the University of Edinburgh, said this study builds on a discovery he and his colleagues made last year that OAS1 variants are associated with severe COVID-19.
“In the ISARIC4C study, we recently found that this is probably due to a change in the way cell membranes detect viruses, but this mechanism doesn’t explain the fascinating association with Alzheimer’s disease reported in this new work,” Dr. Baillie said.
“It is often the case that the same gene can have different roles in different parts of the body. Importantly, it doesn’t mean that having COVID-19 has any effect on your risk of Alzheimer’s,” he added.
Also weighing in on the new study, Jonathan Schott, MD, professor of neurology, University College London, noted that dementia is the “main preexisting health condition associated with COVID-19 mortality, accounting for about one in four deaths from COVID-19 between March and June 2020.
“While some of this excessive mortality may relate to people with dementia being overrepresented in care homes, which were particularly hard hit by the pandemic, or due to general increased vulnerability to infections, there have been questions as to whether there are common factors that might increase susceptibility both to developing dementia and to dying from COVID-19,” Dr. Schott explained.
This “elegant paper” provides evidence for the latter, “suggesting a common genetic mechanism both for Alzheimer’s disease and for severe COVID-19 infection,” Dr. Schott said.
“The identification of a genetic risk factor and elucidation of inflammatory pathways through which it may increase risk has important implications for our understanding of both diseases, with potential implications for novel treatments,” he added.
The study was funded by the UK Dementia Research Institute. The authors have disclosed no relevant financial relationships. Dr. Schott serves as chief medical officer for Alzheimer’s Research UK and is clinical adviser to the UK Dementia Research Institute. Dr. Baillie has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings could lead to new treatment targets to slow progression and severity of both diseases.
Investigators found that a single genetic variant in the oligoadenylate synthetase 1 (OAS1) gene increases the risk for AD and that related variants in the same gene increase the likelihood of severe COVID-19 outcomes.
“These findings may allow us to identify new drug targets to slow progression of both diseases and reduce their severity,” Dervis Salih, PhD, senior research associate, UK Dementia Research Institute, University College London, said in an interview.
“Our work also suggests new approaches to treat both diseases with the same drugs,” Dr. Salih added.
The study was published online Oct. 7 in Brain.
Shared genetic network
The OAS1 gene is expressed in microglia, a type of immune cell that makes up around 10% of all cells in the brain.
In earlier work, investigators found evidence suggesting a link between the OAS1 gene and AD, but the function of the gene in microglia was unknown.
To further investigate the gene’s link to AD, they sequenced genetic data from 2,547 people – half with AD, and half without.
The genotyping analysis confirmed that the single-nucleotide polymorphism (SNP) rs1131454 within OAS1 is significantly associated with AD.
Given that the same OAS1 locus has recently been linked with severe COVID-19 outcomes, the researchers investigated four variants on the OAS1 gene.
Results indicate that SNPs within OAS1 associated with AD also show linkage to SNP variants associated with critical illness in COVID-19.
The rs1131454 (risk allele A) and rs4766676 (risk allele T) are associated with AD, and rs10735079 (risk allele A) and rs6489867 (risk allele T) are associated with critical illness with COVID-19, the investigators reported. All of these risk alleles dampen expression of OAS1.
“This study also provides strong new evidence that interferon signaling by the innate immune system plays a substantial role in the progression of Alzheimer’s,” said Dr. Salih.
“Identifying this shared genetic network in innate immune cells will allow us with future work to identify new biomarkers to track disease progression and also predict disease risk better for both disorders,” he added.
‘Fascinating’ link
In a statement from the UK nonprofit organization, Science Media Center, Kenneth Baillie, MBChB, with the University of Edinburgh, said this study builds on a discovery he and his colleagues made last year that OAS1 variants are associated with severe COVID-19.
“In the ISARIC4C study, we recently found that this is probably due to a change in the way cell membranes detect viruses, but this mechanism doesn’t explain the fascinating association with Alzheimer’s disease reported in this new work,” Dr. Baillie said.
“It is often the case that the same gene can have different roles in different parts of the body. Importantly, it doesn’t mean that having COVID-19 has any effect on your risk of Alzheimer’s,” he added.
Also weighing in on the new study, Jonathan Schott, MD, professor of neurology, University College London, noted that dementia is the “main preexisting health condition associated with COVID-19 mortality, accounting for about one in four deaths from COVID-19 between March and June 2020.
“While some of this excessive mortality may relate to people with dementia being overrepresented in care homes, which were particularly hard hit by the pandemic, or due to general increased vulnerability to infections, there have been questions as to whether there are common factors that might increase susceptibility both to developing dementia and to dying from COVID-19,” Dr. Schott explained.
This “elegant paper” provides evidence for the latter, “suggesting a common genetic mechanism both for Alzheimer’s disease and for severe COVID-19 infection,” Dr. Schott said.
“The identification of a genetic risk factor and elucidation of inflammatory pathways through which it may increase risk has important implications for our understanding of both diseases, with potential implications for novel treatments,” he added.
The study was funded by the UK Dementia Research Institute. The authors have disclosed no relevant financial relationships. Dr. Schott serves as chief medical officer for Alzheimer’s Research UK and is clinical adviser to the UK Dementia Research Institute. Dr. Baillie has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
No short-term death risk in elderly after COVID-19 vaccines
and launched an investigation into the safety of the BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech).
Now, the results of that investigation and of a subsequent larger study of nursing home residents in Norway have shown no increased risk for short-term mortality following COVID-19 vaccination in the overall population of elderly patients. The new research also showed clear evidence of a survival benefit compared with the unvaccinated population, Anette Hylen Ranhoff, MD, PhD, said at the annual meeting of the European Geriatric Medicine Society, held in a hybrid format in Athens, Greece, and online.
“We found no evidence of increased short-term mortality among vaccinated older individuals, and particularly not among the nursing home patients,” said Dr. Ranhoff, a senior researcher at the Norwegian Institute of Public Health and professor at University of Bergen, Norway. “But we think that this [lower] mortality risk was most likely a sort of ‘healthy-vaccinee’ effect, which means that people who were a bit more healthy were vaccinated, and not those who were the very, very most frail.”
“We have more or less the same data in France about events, with very high rates of vaccination,” said session moderator Athanase Benetos MD, PhD, professor and chairman of geriatric medicine at the University Hospital of Nancy in France, who was not involved in the study.
“In my department, a month after the end of the vaccination and at the same time while the pandemic in the city was going up, we had a 90% decrease in mortality from COVID in the nursing homes,” he told Dr. Ranhoff.
Potential risks
Frail elderly patients were not included in clinical trials of COVID-19 vaccines, and although previous studies have shown a low incidence of local or systemic reactions to vaccination among older people, “we think that quite mild adverse events following vaccination could trigger and destabilize a frail person,” Dr. Ranhoff said.
As reported Jan. 15, 2021, in BMJ, investigation by the Norwegian Medicines Agency (NOMA) into 13 of the 23 reported cases concluded that common adverse reactions associated with mRNA vaccines could have contributed to the deaths of some of the frail elderly patients
Steinar Madsen, MD, NOMA medical director, told BMJ “we are not alarmed or worried about this, because these are very rare occurrences and they occurred in very frail patients with very serious disease.”
Health authorities investigate
In response to the report and at the request of the Norwegian Public Health Institute and NOMA, Dr. Ranhoff and colleagues investigated the first 100 deaths among nursing-home residents who received the vaccine. The team consisted of three geriatricians and an infectious disease specialist who sees patients in nursing homes.
They looked at each patient’s clinical course before and after vaccination, their health trajectory and life expectancy at the time of vaccination, new symptoms following vaccination, and the time from vaccination to new symptoms and to death.
In addition, the investigators evaluated Clinical Frailty Scale (CFS) scores for each patient. CFS scores range from 1 (very fit) to 9 (terminally ill, with a life expectancy of less than 6 months who are otherwise evidently frail).
The initial investigation found that among 95 evaluable patients, the association between vaccination and death was “probable” in 10, “possible” in 26, and “unlikely” in 59.
The mean time from vaccination to symptoms was 1.4 days in the probable cases, 2.5 days in the possible cases, and 4.7 days in the unlikely cases.
The mean time from vaccination to death was 3.1, 8.3, and 8.2 days, respectively.
In all three categories, the patients had mean CFS scores ranging from 7.6 to 7.9, putting them in the “severely frail” category, defined as people who are completely dependent for personal care but seem stable and not at high risk for dying.
“We have quite many nursing home residents in Norway, 35,000; more than 80% have dementia, and the mean age is 85 years. We know that approximately 45 people die every day in these nursing homes, and their mean age of death is 87.5 years,” Dr. Ranhoff said.
Population-wide study
Dr. Ranhoff and colleagues also looked more broadly into the question of potential vaccine-related mortality in the total population of older people in Norway from the day of vaccination to follow-up at 3 weeks.
They conducted a matched cohort study to investigate the relationship between the mRNA SARS-CoV-2 vaccine and overall death among persons aged 65 and older in the general population, and across four groups: patients receiving home-based care, long-term nursing home patients, short-term nursing home patients, and those not receiving health services.
The researchers identified a total of 967,786 residents of Norway aged 65 and over at the start of the country’s vaccination campaign at the end of December, 2020, and they matched vaccinated individuals with unvaccinated persons based on demographic, geographic, and clinical risk group factors.
Dr. Ranhoff showed Kaplan-Meier survival curves for the total population and for each of the health-service states. In all cases there was a clear survival benefit for vaccinated vs. unvaccinated patients. She did not, however, provide specific numbers or hazard ratios for the differences between vaccinated and unvaccinated individuals in each of the comparisons.
The study was supported by the Norwegian Institute of Public Health. Dr. Ranhoff and Dr. Benetos reported no conflicts of interest.
and launched an investigation into the safety of the BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech).
Now, the results of that investigation and of a subsequent larger study of nursing home residents in Norway have shown no increased risk for short-term mortality following COVID-19 vaccination in the overall population of elderly patients. The new research also showed clear evidence of a survival benefit compared with the unvaccinated population, Anette Hylen Ranhoff, MD, PhD, said at the annual meeting of the European Geriatric Medicine Society, held in a hybrid format in Athens, Greece, and online.
“We found no evidence of increased short-term mortality among vaccinated older individuals, and particularly not among the nursing home patients,” said Dr. Ranhoff, a senior researcher at the Norwegian Institute of Public Health and professor at University of Bergen, Norway. “But we think that this [lower] mortality risk was most likely a sort of ‘healthy-vaccinee’ effect, which means that people who were a bit more healthy were vaccinated, and not those who were the very, very most frail.”
“We have more or less the same data in France about events, with very high rates of vaccination,” said session moderator Athanase Benetos MD, PhD, professor and chairman of geriatric medicine at the University Hospital of Nancy in France, who was not involved in the study.
“In my department, a month after the end of the vaccination and at the same time while the pandemic in the city was going up, we had a 90% decrease in mortality from COVID in the nursing homes,” he told Dr. Ranhoff.
Potential risks
Frail elderly patients were not included in clinical trials of COVID-19 vaccines, and although previous studies have shown a low incidence of local or systemic reactions to vaccination among older people, “we think that quite mild adverse events following vaccination could trigger and destabilize a frail person,” Dr. Ranhoff said.
As reported Jan. 15, 2021, in BMJ, investigation by the Norwegian Medicines Agency (NOMA) into 13 of the 23 reported cases concluded that common adverse reactions associated with mRNA vaccines could have contributed to the deaths of some of the frail elderly patients
Steinar Madsen, MD, NOMA medical director, told BMJ “we are not alarmed or worried about this, because these are very rare occurrences and they occurred in very frail patients with very serious disease.”
Health authorities investigate
In response to the report and at the request of the Norwegian Public Health Institute and NOMA, Dr. Ranhoff and colleagues investigated the first 100 deaths among nursing-home residents who received the vaccine. The team consisted of three geriatricians and an infectious disease specialist who sees patients in nursing homes.
They looked at each patient’s clinical course before and after vaccination, their health trajectory and life expectancy at the time of vaccination, new symptoms following vaccination, and the time from vaccination to new symptoms and to death.
In addition, the investigators evaluated Clinical Frailty Scale (CFS) scores for each patient. CFS scores range from 1 (very fit) to 9 (terminally ill, with a life expectancy of less than 6 months who are otherwise evidently frail).
The initial investigation found that among 95 evaluable patients, the association between vaccination and death was “probable” in 10, “possible” in 26, and “unlikely” in 59.
The mean time from vaccination to symptoms was 1.4 days in the probable cases, 2.5 days in the possible cases, and 4.7 days in the unlikely cases.
The mean time from vaccination to death was 3.1, 8.3, and 8.2 days, respectively.
In all three categories, the patients had mean CFS scores ranging from 7.6 to 7.9, putting them in the “severely frail” category, defined as people who are completely dependent for personal care but seem stable and not at high risk for dying.
“We have quite many nursing home residents in Norway, 35,000; more than 80% have dementia, and the mean age is 85 years. We know that approximately 45 people die every day in these nursing homes, and their mean age of death is 87.5 years,” Dr. Ranhoff said.
Population-wide study
Dr. Ranhoff and colleagues also looked more broadly into the question of potential vaccine-related mortality in the total population of older people in Norway from the day of vaccination to follow-up at 3 weeks.
They conducted a matched cohort study to investigate the relationship between the mRNA SARS-CoV-2 vaccine and overall death among persons aged 65 and older in the general population, and across four groups: patients receiving home-based care, long-term nursing home patients, short-term nursing home patients, and those not receiving health services.
The researchers identified a total of 967,786 residents of Norway aged 65 and over at the start of the country’s vaccination campaign at the end of December, 2020, and they matched vaccinated individuals with unvaccinated persons based on demographic, geographic, and clinical risk group factors.
Dr. Ranhoff showed Kaplan-Meier survival curves for the total population and for each of the health-service states. In all cases there was a clear survival benefit for vaccinated vs. unvaccinated patients. She did not, however, provide specific numbers or hazard ratios for the differences between vaccinated and unvaccinated individuals in each of the comparisons.
The study was supported by the Norwegian Institute of Public Health. Dr. Ranhoff and Dr. Benetos reported no conflicts of interest.
and launched an investigation into the safety of the BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech).
Now, the results of that investigation and of a subsequent larger study of nursing home residents in Norway have shown no increased risk for short-term mortality following COVID-19 vaccination in the overall population of elderly patients. The new research also showed clear evidence of a survival benefit compared with the unvaccinated population, Anette Hylen Ranhoff, MD, PhD, said at the annual meeting of the European Geriatric Medicine Society, held in a hybrid format in Athens, Greece, and online.
“We found no evidence of increased short-term mortality among vaccinated older individuals, and particularly not among the nursing home patients,” said Dr. Ranhoff, a senior researcher at the Norwegian Institute of Public Health and professor at University of Bergen, Norway. “But we think that this [lower] mortality risk was most likely a sort of ‘healthy-vaccinee’ effect, which means that people who were a bit more healthy were vaccinated, and not those who were the very, very most frail.”
“We have more or less the same data in France about events, with very high rates of vaccination,” said session moderator Athanase Benetos MD, PhD, professor and chairman of geriatric medicine at the University Hospital of Nancy in France, who was not involved in the study.
“In my department, a month after the end of the vaccination and at the same time while the pandemic in the city was going up, we had a 90% decrease in mortality from COVID in the nursing homes,” he told Dr. Ranhoff.
Potential risks
Frail elderly patients were not included in clinical trials of COVID-19 vaccines, and although previous studies have shown a low incidence of local or systemic reactions to vaccination among older people, “we think that quite mild adverse events following vaccination could trigger and destabilize a frail person,” Dr. Ranhoff said.
As reported Jan. 15, 2021, in BMJ, investigation by the Norwegian Medicines Agency (NOMA) into 13 of the 23 reported cases concluded that common adverse reactions associated with mRNA vaccines could have contributed to the deaths of some of the frail elderly patients
Steinar Madsen, MD, NOMA medical director, told BMJ “we are not alarmed or worried about this, because these are very rare occurrences and they occurred in very frail patients with very serious disease.”
Health authorities investigate
In response to the report and at the request of the Norwegian Public Health Institute and NOMA, Dr. Ranhoff and colleagues investigated the first 100 deaths among nursing-home residents who received the vaccine. The team consisted of three geriatricians and an infectious disease specialist who sees patients in nursing homes.
They looked at each patient’s clinical course before and after vaccination, their health trajectory and life expectancy at the time of vaccination, new symptoms following vaccination, and the time from vaccination to new symptoms and to death.
In addition, the investigators evaluated Clinical Frailty Scale (CFS) scores for each patient. CFS scores range from 1 (very fit) to 9 (terminally ill, with a life expectancy of less than 6 months who are otherwise evidently frail).
The initial investigation found that among 95 evaluable patients, the association between vaccination and death was “probable” in 10, “possible” in 26, and “unlikely” in 59.
The mean time from vaccination to symptoms was 1.4 days in the probable cases, 2.5 days in the possible cases, and 4.7 days in the unlikely cases.
The mean time from vaccination to death was 3.1, 8.3, and 8.2 days, respectively.
In all three categories, the patients had mean CFS scores ranging from 7.6 to 7.9, putting them in the “severely frail” category, defined as people who are completely dependent for personal care but seem stable and not at high risk for dying.
“We have quite many nursing home residents in Norway, 35,000; more than 80% have dementia, and the mean age is 85 years. We know that approximately 45 people die every day in these nursing homes, and their mean age of death is 87.5 years,” Dr. Ranhoff said.
Population-wide study
Dr. Ranhoff and colleagues also looked more broadly into the question of potential vaccine-related mortality in the total population of older people in Norway from the day of vaccination to follow-up at 3 weeks.
They conducted a matched cohort study to investigate the relationship between the mRNA SARS-CoV-2 vaccine and overall death among persons aged 65 and older in the general population, and across four groups: patients receiving home-based care, long-term nursing home patients, short-term nursing home patients, and those not receiving health services.
The researchers identified a total of 967,786 residents of Norway aged 65 and over at the start of the country’s vaccination campaign at the end of December, 2020, and they matched vaccinated individuals with unvaccinated persons based on demographic, geographic, and clinical risk group factors.
Dr. Ranhoff showed Kaplan-Meier survival curves for the total population and for each of the health-service states. In all cases there was a clear survival benefit for vaccinated vs. unvaccinated patients. She did not, however, provide specific numbers or hazard ratios for the differences between vaccinated and unvaccinated individuals in each of the comparisons.
The study was supported by the Norwegian Institute of Public Health. Dr. Ranhoff and Dr. Benetos reported no conflicts of interest.
FROM EUGMS 2021
Omega-3s tame inflammation in elderly COVID-19 patients
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUGMS







