User login
Staff education cuts psychotropic drug use in long-term care
The effect of the intervention was transient, possibly because of high staff turnover, according to the investigators in the new randomized, controlled trial.
The findings were presented by Ulla Aalto, MD, PhD, during a session at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
There was a significant reduction in the use of psychotropic agents at 6 months in long-term care wards where the nursing staff had undergone a short training session on drug therapy for older patients, but there was no improvement in wards that were randomly assigned to serve as controls, Dr. Aalto, from Helsinki Hospital, reported during the session.
“Future research would be investigating how we could maintain the positive effects that were gained at 6 months but not seen any more at 1 year, and how to implement the good practice in nursing homes by this kind of staff training,” she said.
Heavy drug use
Psychotropic medications are widely used in long-term care settings, but their indiscriminate use or use of the wrong drug for the wrong patient can be harmful. Inappropriate drug use in long-term care settings is also associated with higher costs, Dr. Aalto said.
To see whether a staff-training intervention could reduce drugs use and lower costs, the investigators conducted a randomized clinical trial in assisted living facilities in Helsinki in 2011, with a total of 227 patients 65 years and older.
Long-term care wards were randomly assigned to either an intervention for nursing staff consisting of two 4-hour sessions on good drug-therapy practice for older adults, or to serve as controls (10 wards in each group).
Drug use and costs were monitored at both 6 and 12 months after randomization. Psychotropic drugs included antipsychotics, antidepressants, anxiolytics, and hypnotics as classified by the World Health Organization. For the purposes of comparison, actual doses were counted and converted into relative proportions of defined daily doses.
The baseline characteristics of patients in each group were generally similar, with a mean age of around 83 years. In each study arm, nearly two-thirds of patients were on at least one psychotropic drug, and of this group, a third had been prescribed 2 or more psychotropic agents.
Nearly half of the patients were on at least one antipsychotic agent and/or antidepressant.
Short-term benefit
As noted before, in the wards randomized to staff training, there was a significant reduction in use of all psychotropics from baseline at 6 months after randomization (P = .045), but there was no change among the control wards.
By 12 months, however, the differences between the intervention and control arms narrowed, and drug use in the intervention arm was no longer significantly lower over baseline.
Drugs costs significantly decreased in the intervention group at 6 months (P = .027) and were numerically but not statistically lower over baseline at 12 months.
In contrast, drug costs in the control arm were numerically (but not statistically) higher at both 6 and 12 months of follow-up.
Annual drug costs in the intervention group decreased by mean of 12.3 euros ($14.22) whereas costs in the control group increased by a mean of 20.6 euros ($23.81).
“This quite light and feasible intervention succeeded in reducing overall defined daily doses of psychotropics in the short term,” Dr. Aalto said.
The waning of the intervention’s effect on drug use and costs may be caused partly by the high employee turnover rate in long-term care facilities and to the dilution effect, she said, referring to a form of judgment bias in which people tend to devalue diagnostic information when other, nondiagnostic information is also available.
Randomized design
In the question-and-answer session following her presentation, audience member Jesper Ryg, MD, PhD from Odense (Denmark) University Hospital and the University of Southern Denmark, also in Odense, commented: “It’s a great study, doing a [randomized, controlled trial] on deprescribing, we need more of those.”
“But what we know now is that a lot of studies show it is possible to deprescribe and get less drugs, but do we have any clinical data? Does this deprescribing lead to less falls, did it lead to lower mortality?” he asked.
Dr. Aalto replied that, in an earlier report from this study, investigators showed that harmful medication use was reduced and negative outcomes were reduced.
Another audience member asked why nursing staff were the target of the intervention, given that physicians do the actual drug prescribing.
Dr. Aalto responded: “It is the physician of course who prescribes, but in nursing homes and long-term care, nursing staff is there all the time, and the physicians are kind of consultants who just come there once in a while, so it’s important that the nurses also know about these harmful medications and can bring them to the doctor when he or she arrives there.”
Dr. Aalto and Dr. Ryg had no disclosures.
The effect of the intervention was transient, possibly because of high staff turnover, according to the investigators in the new randomized, controlled trial.
The findings were presented by Ulla Aalto, MD, PhD, during a session at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
There was a significant reduction in the use of psychotropic agents at 6 months in long-term care wards where the nursing staff had undergone a short training session on drug therapy for older patients, but there was no improvement in wards that were randomly assigned to serve as controls, Dr. Aalto, from Helsinki Hospital, reported during the session.
“Future research would be investigating how we could maintain the positive effects that were gained at 6 months but not seen any more at 1 year, and how to implement the good practice in nursing homes by this kind of staff training,” she said.
Heavy drug use
Psychotropic medications are widely used in long-term care settings, but their indiscriminate use or use of the wrong drug for the wrong patient can be harmful. Inappropriate drug use in long-term care settings is also associated with higher costs, Dr. Aalto said.
To see whether a staff-training intervention could reduce drugs use and lower costs, the investigators conducted a randomized clinical trial in assisted living facilities in Helsinki in 2011, with a total of 227 patients 65 years and older.
Long-term care wards were randomly assigned to either an intervention for nursing staff consisting of two 4-hour sessions on good drug-therapy practice for older adults, or to serve as controls (10 wards in each group).
Drug use and costs were monitored at both 6 and 12 months after randomization. Psychotropic drugs included antipsychotics, antidepressants, anxiolytics, and hypnotics as classified by the World Health Organization. For the purposes of comparison, actual doses were counted and converted into relative proportions of defined daily doses.
The baseline characteristics of patients in each group were generally similar, with a mean age of around 83 years. In each study arm, nearly two-thirds of patients were on at least one psychotropic drug, and of this group, a third had been prescribed 2 or more psychotropic agents.
Nearly half of the patients were on at least one antipsychotic agent and/or antidepressant.
Short-term benefit
As noted before, in the wards randomized to staff training, there was a significant reduction in use of all psychotropics from baseline at 6 months after randomization (P = .045), but there was no change among the control wards.
By 12 months, however, the differences between the intervention and control arms narrowed, and drug use in the intervention arm was no longer significantly lower over baseline.
Drugs costs significantly decreased in the intervention group at 6 months (P = .027) and were numerically but not statistically lower over baseline at 12 months.
In contrast, drug costs in the control arm were numerically (but not statistically) higher at both 6 and 12 months of follow-up.
Annual drug costs in the intervention group decreased by mean of 12.3 euros ($14.22) whereas costs in the control group increased by a mean of 20.6 euros ($23.81).
“This quite light and feasible intervention succeeded in reducing overall defined daily doses of psychotropics in the short term,” Dr. Aalto said.
The waning of the intervention’s effect on drug use and costs may be caused partly by the high employee turnover rate in long-term care facilities and to the dilution effect, she said, referring to a form of judgment bias in which people tend to devalue diagnostic information when other, nondiagnostic information is also available.
Randomized design
In the question-and-answer session following her presentation, audience member Jesper Ryg, MD, PhD from Odense (Denmark) University Hospital and the University of Southern Denmark, also in Odense, commented: “It’s a great study, doing a [randomized, controlled trial] on deprescribing, we need more of those.”
“But what we know now is that a lot of studies show it is possible to deprescribe and get less drugs, but do we have any clinical data? Does this deprescribing lead to less falls, did it lead to lower mortality?” he asked.
Dr. Aalto replied that, in an earlier report from this study, investigators showed that harmful medication use was reduced and negative outcomes were reduced.
Another audience member asked why nursing staff were the target of the intervention, given that physicians do the actual drug prescribing.
Dr. Aalto responded: “It is the physician of course who prescribes, but in nursing homes and long-term care, nursing staff is there all the time, and the physicians are kind of consultants who just come there once in a while, so it’s important that the nurses also know about these harmful medications and can bring them to the doctor when he or she arrives there.”
Dr. Aalto and Dr. Ryg had no disclosures.
The effect of the intervention was transient, possibly because of high staff turnover, according to the investigators in the new randomized, controlled trial.
The findings were presented by Ulla Aalto, MD, PhD, during a session at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
There was a significant reduction in the use of psychotropic agents at 6 months in long-term care wards where the nursing staff had undergone a short training session on drug therapy for older patients, but there was no improvement in wards that were randomly assigned to serve as controls, Dr. Aalto, from Helsinki Hospital, reported during the session.
“Future research would be investigating how we could maintain the positive effects that were gained at 6 months but not seen any more at 1 year, and how to implement the good practice in nursing homes by this kind of staff training,” she said.
Heavy drug use
Psychotropic medications are widely used in long-term care settings, but their indiscriminate use or use of the wrong drug for the wrong patient can be harmful. Inappropriate drug use in long-term care settings is also associated with higher costs, Dr. Aalto said.
To see whether a staff-training intervention could reduce drugs use and lower costs, the investigators conducted a randomized clinical trial in assisted living facilities in Helsinki in 2011, with a total of 227 patients 65 years and older.
Long-term care wards were randomly assigned to either an intervention for nursing staff consisting of two 4-hour sessions on good drug-therapy practice for older adults, or to serve as controls (10 wards in each group).
Drug use and costs were monitored at both 6 and 12 months after randomization. Psychotropic drugs included antipsychotics, antidepressants, anxiolytics, and hypnotics as classified by the World Health Organization. For the purposes of comparison, actual doses were counted and converted into relative proportions of defined daily doses.
The baseline characteristics of patients in each group were generally similar, with a mean age of around 83 years. In each study arm, nearly two-thirds of patients were on at least one psychotropic drug, and of this group, a third had been prescribed 2 or more psychotropic agents.
Nearly half of the patients were on at least one antipsychotic agent and/or antidepressant.
Short-term benefit
As noted before, in the wards randomized to staff training, there was a significant reduction in use of all psychotropics from baseline at 6 months after randomization (P = .045), but there was no change among the control wards.
By 12 months, however, the differences between the intervention and control arms narrowed, and drug use in the intervention arm was no longer significantly lower over baseline.
Drugs costs significantly decreased in the intervention group at 6 months (P = .027) and were numerically but not statistically lower over baseline at 12 months.
In contrast, drug costs in the control arm were numerically (but not statistically) higher at both 6 and 12 months of follow-up.
Annual drug costs in the intervention group decreased by mean of 12.3 euros ($14.22) whereas costs in the control group increased by a mean of 20.6 euros ($23.81).
“This quite light and feasible intervention succeeded in reducing overall defined daily doses of psychotropics in the short term,” Dr. Aalto said.
The waning of the intervention’s effect on drug use and costs may be caused partly by the high employee turnover rate in long-term care facilities and to the dilution effect, she said, referring to a form of judgment bias in which people tend to devalue diagnostic information when other, nondiagnostic information is also available.
Randomized design
In the question-and-answer session following her presentation, audience member Jesper Ryg, MD, PhD from Odense (Denmark) University Hospital and the University of Southern Denmark, also in Odense, commented: “It’s a great study, doing a [randomized, controlled trial] on deprescribing, we need more of those.”
“But what we know now is that a lot of studies show it is possible to deprescribe and get less drugs, but do we have any clinical data? Does this deprescribing lead to less falls, did it lead to lower mortality?” he asked.
Dr. Aalto replied that, in an earlier report from this study, investigators showed that harmful medication use was reduced and negative outcomes were reduced.
Another audience member asked why nursing staff were the target of the intervention, given that physicians do the actual drug prescribing.
Dr. Aalto responded: “It is the physician of course who prescribes, but in nursing homes and long-term care, nursing staff is there all the time, and the physicians are kind of consultants who just come there once in a while, so it’s important that the nurses also know about these harmful medications and can bring them to the doctor when he or she arrives there.”
Dr. Aalto and Dr. Ryg had no disclosures.
FROM EUGMS 2021
MIND diet preserves cognition, new data show
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pelvic floor dysfunction imaging: New guidelines provide recommendations
New consensus guidelines from a multispecialty working group of the Pelvic Floor Disorders Consortium (PFDC) clear up inconsistencies in the use of magnetic resonance defecography (MRD) and provide universal recommendations on MRD technique, interpretation, reporting, and other factors.
“The consensus language used to describe pelvic floor disorders is critical, so as to allow the various experts who treat these patients [to] communicate and collaborate effectively with each other,” coauthor Liliana Bordeianou, MD, MPH, an associate professor of surgery at Harvard Medical School and chair of the Massachusetts General Hospital Colorectal and Pelvic Floor Centers, told this news organization.
“These diseases do not choose an arbitrary side in the pelvis,” she noted. “Instead, these diseases affect the entire pelvis and require a multidisciplinary and collaborative solution.”
MRD is a key component in that solution, providing dynamic evaluation of pelvic floor function and visualization of the complex interaction in pelvic compartments among patients with defecatory pelvic floor disorders, such as vaginal or uterine prolapse, constipation, incontinence, or other pelvic floor dysfunctions.
However, a key shortcoming has been a lack of consistency in nomenclature and the reporting of MRD findings among institutions and subspecialties.
Clinicians may wind up using different definitions for the same condition and different thresholds for grading severity, resulting in inconsistent communication not only between clinicians across institutions but even within the same institution, the report notes.
To address the situation, radiologists with the Pelvic Floor Dysfunction Disease Focused Panel of the Society of Abdominal Radiology (SAR) published recommendations on MRD protocol and technique in April.
However, even with that guidance, there has been significant variability in the interpretation and utilization of MRD findings among specialties outside of radiology.
The new report was therefore developed to include input from the broad variety of specialists involved in the treatment of patients with pelvic floor disorders, including colorectal surgeons, urogynecologists, urologists, gynecologists, gastroenterologists, radiologists, physiotherapists, and other advanced care practitioners.
“The goal of this effort was to create a universal set of recommendations and language for MRD technique, interpretation, and reporting that can be utilized and carry the same significance across disciplines,” write the authors of the report, published in the American Journal of Roentgenology.
One key area addressed in the report is a recommendation that MRD can be performed in either the upright or supine position, which has been a topic of inconsistency, said Brooke Gurland, MD, medical director of the Pelvic Health Center at Stanford University, California, a co-author on the consensus statement.
“Supine versus upright position was a source of debate, but ultimately there was a consensus that supine position was acceptable,” she told said in an interview.
Regarding positioning, the recommendations conclude that “given the variable results from different studies, consortium members agreed that it is acceptable to perform MRD in the supine position when upright MRD is not available.”
“Importantly, consortium experts stressed that it is very important that this imaging be performed after proper patient education on the purpose of the examination,” they note.
Other recommendations delve into contrast medium considerations, such as the recommendation that MRD does not require the routine use of vaginal contrast medium for adequate imaging of pathology.
And guidance on the technique and grading of relevant pathology include a recommendation to use the pubococcygeal line (PCL) as a point of reference to quantify the prolapse of organs in all compartments of the pelvic floor.
“There is an increasing appreciation that most patients with pelvic organ prolapse experience dual or even triple compartment pathology, making it important to describe the observations in all three compartments to ensure the mobilization of the appropriate team of experts to treat the patient,” the authors note.
The consensus report features an interpretative template providing synopses of the recommendations, which can be adjusted and modified according to additional radiologic information, as well as individualized patient information or clinician preferences.
However, “the suggested verbiage and steps should be advocated as the minimum requirements when performing and interpreting MRD in patients with evacuation disorders of the pelvic floor,” the authors note.
Dr. Gurland added that, in addition to providing benefits in the present utilization of MRD, the clearer guidelines should help advance its use to improve patient care in the future.
“Standardizing imaging techniques, reporting, and language is critical to improving our understanding and then developing therapies for pelvic floor disorders,” she said.
“In the future, correlating MRD with surgical outcomes and identifying modifiable risk factors will improve patient care.”
In addition to being published in the AJR, the report was published concurrently in the journals Diseases of the Colon & Rectum, International Urogynecology Journal, and Female Pelvic Medicine and Reconstructive Surgery.
The authors of the guidelines have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New consensus guidelines from a multispecialty working group of the Pelvic Floor Disorders Consortium (PFDC) clear up inconsistencies in the use of magnetic resonance defecography (MRD) and provide universal recommendations on MRD technique, interpretation, reporting, and other factors.
“The consensus language used to describe pelvic floor disorders is critical, so as to allow the various experts who treat these patients [to] communicate and collaborate effectively with each other,” coauthor Liliana Bordeianou, MD, MPH, an associate professor of surgery at Harvard Medical School and chair of the Massachusetts General Hospital Colorectal and Pelvic Floor Centers, told this news organization.
“These diseases do not choose an arbitrary side in the pelvis,” she noted. “Instead, these diseases affect the entire pelvis and require a multidisciplinary and collaborative solution.”
MRD is a key component in that solution, providing dynamic evaluation of pelvic floor function and visualization of the complex interaction in pelvic compartments among patients with defecatory pelvic floor disorders, such as vaginal or uterine prolapse, constipation, incontinence, or other pelvic floor dysfunctions.
However, a key shortcoming has been a lack of consistency in nomenclature and the reporting of MRD findings among institutions and subspecialties.
Clinicians may wind up using different definitions for the same condition and different thresholds for grading severity, resulting in inconsistent communication not only between clinicians across institutions but even within the same institution, the report notes.
To address the situation, radiologists with the Pelvic Floor Dysfunction Disease Focused Panel of the Society of Abdominal Radiology (SAR) published recommendations on MRD protocol and technique in April.
However, even with that guidance, there has been significant variability in the interpretation and utilization of MRD findings among specialties outside of radiology.
The new report was therefore developed to include input from the broad variety of specialists involved in the treatment of patients with pelvic floor disorders, including colorectal surgeons, urogynecologists, urologists, gynecologists, gastroenterologists, radiologists, physiotherapists, and other advanced care practitioners.
“The goal of this effort was to create a universal set of recommendations and language for MRD technique, interpretation, and reporting that can be utilized and carry the same significance across disciplines,” write the authors of the report, published in the American Journal of Roentgenology.
One key area addressed in the report is a recommendation that MRD can be performed in either the upright or supine position, which has been a topic of inconsistency, said Brooke Gurland, MD, medical director of the Pelvic Health Center at Stanford University, California, a co-author on the consensus statement.
“Supine versus upright position was a source of debate, but ultimately there was a consensus that supine position was acceptable,” she told said in an interview.
Regarding positioning, the recommendations conclude that “given the variable results from different studies, consortium members agreed that it is acceptable to perform MRD in the supine position when upright MRD is not available.”
“Importantly, consortium experts stressed that it is very important that this imaging be performed after proper patient education on the purpose of the examination,” they note.
Other recommendations delve into contrast medium considerations, such as the recommendation that MRD does not require the routine use of vaginal contrast medium for adequate imaging of pathology.
And guidance on the technique and grading of relevant pathology include a recommendation to use the pubococcygeal line (PCL) as a point of reference to quantify the prolapse of organs in all compartments of the pelvic floor.
“There is an increasing appreciation that most patients with pelvic organ prolapse experience dual or even triple compartment pathology, making it important to describe the observations in all three compartments to ensure the mobilization of the appropriate team of experts to treat the patient,” the authors note.
The consensus report features an interpretative template providing synopses of the recommendations, which can be adjusted and modified according to additional radiologic information, as well as individualized patient information or clinician preferences.
However, “the suggested verbiage and steps should be advocated as the minimum requirements when performing and interpreting MRD in patients with evacuation disorders of the pelvic floor,” the authors note.
Dr. Gurland added that, in addition to providing benefits in the present utilization of MRD, the clearer guidelines should help advance its use to improve patient care in the future.
“Standardizing imaging techniques, reporting, and language is critical to improving our understanding and then developing therapies for pelvic floor disorders,” she said.
“In the future, correlating MRD with surgical outcomes and identifying modifiable risk factors will improve patient care.”
In addition to being published in the AJR, the report was published concurrently in the journals Diseases of the Colon & Rectum, International Urogynecology Journal, and Female Pelvic Medicine and Reconstructive Surgery.
The authors of the guidelines have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New consensus guidelines from a multispecialty working group of the Pelvic Floor Disorders Consortium (PFDC) clear up inconsistencies in the use of magnetic resonance defecography (MRD) and provide universal recommendations on MRD technique, interpretation, reporting, and other factors.
“The consensus language used to describe pelvic floor disorders is critical, so as to allow the various experts who treat these patients [to] communicate and collaborate effectively with each other,” coauthor Liliana Bordeianou, MD, MPH, an associate professor of surgery at Harvard Medical School and chair of the Massachusetts General Hospital Colorectal and Pelvic Floor Centers, told this news organization.
“These diseases do not choose an arbitrary side in the pelvis,” she noted. “Instead, these diseases affect the entire pelvis and require a multidisciplinary and collaborative solution.”
MRD is a key component in that solution, providing dynamic evaluation of pelvic floor function and visualization of the complex interaction in pelvic compartments among patients with defecatory pelvic floor disorders, such as vaginal or uterine prolapse, constipation, incontinence, or other pelvic floor dysfunctions.
However, a key shortcoming has been a lack of consistency in nomenclature and the reporting of MRD findings among institutions and subspecialties.
Clinicians may wind up using different definitions for the same condition and different thresholds for grading severity, resulting in inconsistent communication not only between clinicians across institutions but even within the same institution, the report notes.
To address the situation, radiologists with the Pelvic Floor Dysfunction Disease Focused Panel of the Society of Abdominal Radiology (SAR) published recommendations on MRD protocol and technique in April.
However, even with that guidance, there has been significant variability in the interpretation and utilization of MRD findings among specialties outside of radiology.
The new report was therefore developed to include input from the broad variety of specialists involved in the treatment of patients with pelvic floor disorders, including colorectal surgeons, urogynecologists, urologists, gynecologists, gastroenterologists, radiologists, physiotherapists, and other advanced care practitioners.
“The goal of this effort was to create a universal set of recommendations and language for MRD technique, interpretation, and reporting that can be utilized and carry the same significance across disciplines,” write the authors of the report, published in the American Journal of Roentgenology.
One key area addressed in the report is a recommendation that MRD can be performed in either the upright or supine position, which has been a topic of inconsistency, said Brooke Gurland, MD, medical director of the Pelvic Health Center at Stanford University, California, a co-author on the consensus statement.
“Supine versus upright position was a source of debate, but ultimately there was a consensus that supine position was acceptable,” she told said in an interview.
Regarding positioning, the recommendations conclude that “given the variable results from different studies, consortium members agreed that it is acceptable to perform MRD in the supine position when upright MRD is not available.”
“Importantly, consortium experts stressed that it is very important that this imaging be performed after proper patient education on the purpose of the examination,” they note.
Other recommendations delve into contrast medium considerations, such as the recommendation that MRD does not require the routine use of vaginal contrast medium for adequate imaging of pathology.
And guidance on the technique and grading of relevant pathology include a recommendation to use the pubococcygeal line (PCL) as a point of reference to quantify the prolapse of organs in all compartments of the pelvic floor.
“There is an increasing appreciation that most patients with pelvic organ prolapse experience dual or even triple compartment pathology, making it important to describe the observations in all three compartments to ensure the mobilization of the appropriate team of experts to treat the patient,” the authors note.
The consensus report features an interpretative template providing synopses of the recommendations, which can be adjusted and modified according to additional radiologic information, as well as individualized patient information or clinician preferences.
However, “the suggested verbiage and steps should be advocated as the minimum requirements when performing and interpreting MRD in patients with evacuation disorders of the pelvic floor,” the authors note.
Dr. Gurland added that, in addition to providing benefits in the present utilization of MRD, the clearer guidelines should help advance its use to improve patient care in the future.
“Standardizing imaging techniques, reporting, and language is critical to improving our understanding and then developing therapies for pelvic floor disorders,” she said.
“In the future, correlating MRD with surgical outcomes and identifying modifiable risk factors will improve patient care.”
In addition to being published in the AJR, the report was published concurrently in the journals Diseases of the Colon & Rectum, International Urogynecology Journal, and Female Pelvic Medicine and Reconstructive Surgery.
The authors of the guidelines have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Improving Physicians’ Bowel Documentation on Geriatric Wards
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
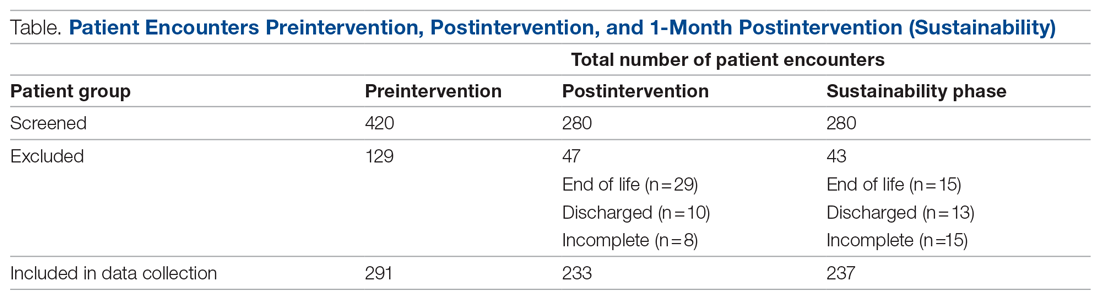
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
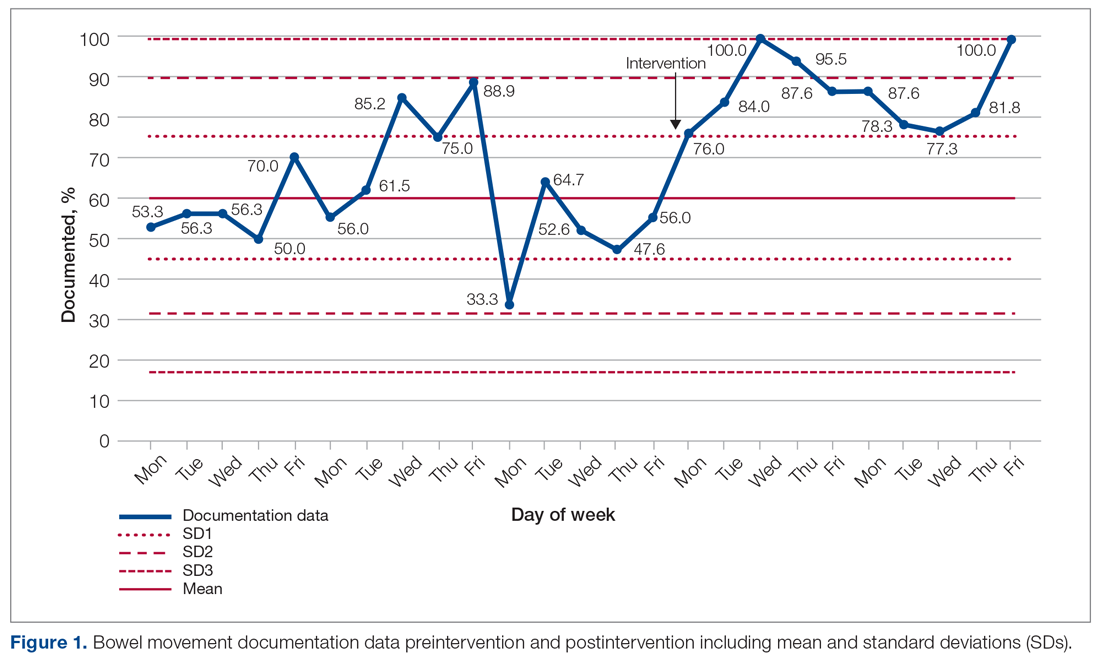
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
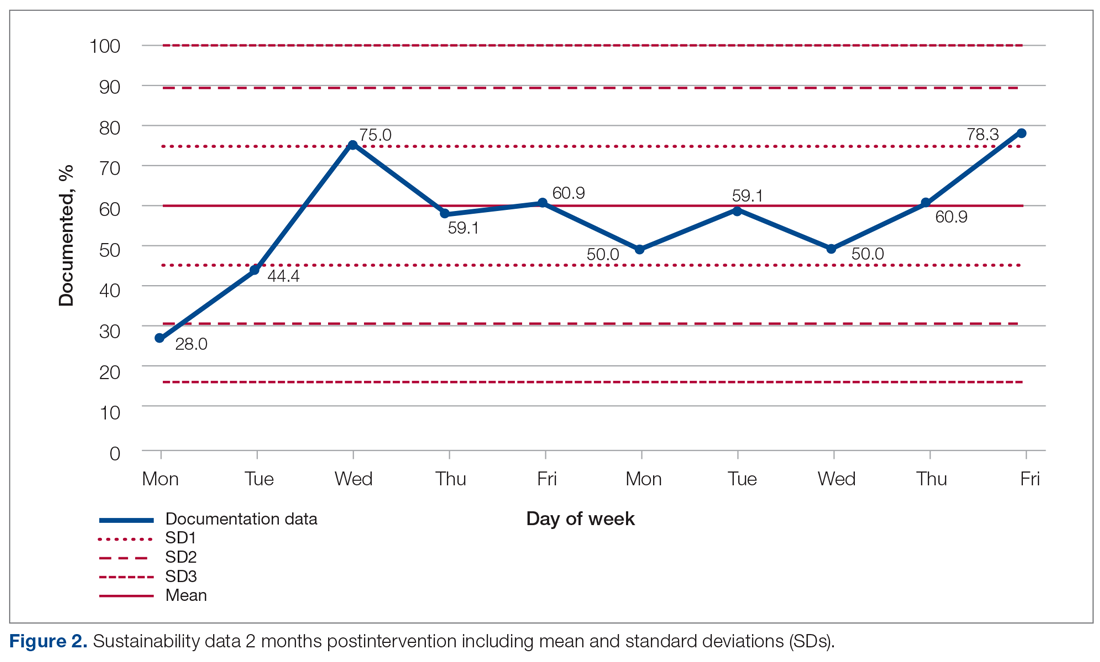
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; [email protected].
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).

The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.

The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.

Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; [email protected].
Financial disclosures: None.
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).

The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.

The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.

Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; [email protected].
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
Differences in Care by Race in Older Nursing Home Residents With Dementia
Study Overview
Objective. To examine differences in care, specifically hospitalization towards the end of life, among nursing home residents with dementia who were Black compared with those who were White.
Design. Population based cohort study in the US. The study included all decedents with Alzheimer’s disease or related dementia (ADRD) who resided in a nursing home from 2014 to 2017. Decedents from nursing homes were identified by death within 1 day of an identified nursing home stay or within 8 days of a hospital transfer from nursing home. Data were obtained from Minimum Data Set 3.0 (MDS) which contains clinical data from all Medicaid or Medicare certified nursing homes, and from the Medicare Beneficiary Summary File (MBSF) and Medicare Provider and Analysis and Review (MedPAR) which contains hospitalization events for all Medicare Beneficiaries. These files were linked to identify nursing home residents with ADRD who were hospitalized at the end of life. ADRD diagnosis was identified from the chronic condition list from the MBSF and from MDS diagnosis list.
Setting and participants. The study included 665 033 residents from 14 595 nursing homes who died during the study period. Resident race was categorized as White or Black based on the MBSF. Severe cognitive impairment was identified using the MDS that categorized residents as severe or not using the Brief Interview for Mental Status and the Cognitive Performance Scale. The mean (SD) age of the study population was 86.7 (9.2) years for White residents and 82.6 (11.1) years for Black residents. Of the participants, 68.8% and 61.2% were female for Black and White residents, respectively. Approximately 23.4% of White and 32.5% of Black residents had severe cognitive impairment. For nursing home characteristics, 71.5% of the 14 595 nursing homes represented were for profit; average bedside was 109.5 (57.0) and occupancy rate was on average 81.2% (14.3%).
Main outcome measures. The study outcome measure was any hospitalization within 30 days prior to death. The outcome was selected as an indicator of quality of care because as older adults living with ADRD experience progressive worsening of cognitive symptoms, at the end of life when dementia is severe, advance care planning and communication with health care proxies and surrogates often result in coordinated care that avoids acute hospitalizations, which are often burdensome to both patient and family and may yield poorer quality of life.
Main results. The study found that approximately 29.5% of White decedents and 40.7% of Black decedents were hospitalized towards the end of life. Nursing homes with a higher proportion of Black residents were more likely to have residents hospitalized towards the end of life with 35% of residents hospitalized in the highest quartile (27% Black) compared with 17% hospitalized for nursing homes in the lowest quartile (0% Black).After adjusting for covariates, Black residents were 7.9% more likely to be hospitalized in the last 30 days of life compared with White residents. Blacks with severe cognitive impairment has elevated risk of hospitalization by 4.9% when compared with White residents. After accounting for nursing home facility–level characteristics, nursing homes with a low proportion of Black residents had a 5.2% higher risk of hospitalizations compared with nursing homes with no Black residents, and nursing homes with a higher percentage of Black residents had a 13.3% higher risk of hospitalization compared with nursing homes with no Black residents.
Conclusion. Race is associated with care disparities in older nursing home residents with dementia. This study suggests that hospitalization towards the end of life as a quality of care marker differs across nursing homes, and nursing homes with a higher proportion of Black residents were more likely to be hospitalized. This suggests that these nursing homes may have fewer resources and delivered poorer quality of care, and that disparities in health systems or institutions contribute to differences in quality of care for this vulnerable group.
Commentary
Disparities of health status, health care, and affordability across race and ethnicity have persisted throughout the past 20 years.1 There is further evidence to support systemic differences that can contribute to differences in health outcomes.2 Although changes in health care policy such as the Affordable Care Act have expanded health care coverage, and instituted changes that aims to improve health care quality and reduce disparities, it is clear that factors contributing to disparities in care are structural and perhaps systemic. The latest evidence comes in this study that examines racial disparities in health care quality in one of the most vulnerable populations—older adults with Alzheimer’s disease and dementia. The finding that Black nursing home residents, when compared with White residents, often has higher risk of hospitalization at the end of life, even among those with severe dementia where better coordinated care, clear goals of care and perhaps instituting palliative care would result in lower rate of hospitalization. The disparities were observed across nursing homes as well, where nursing homes with higher proportion of Black residents appear to have lower quality of care.
These findings are consistent with prior work that has examined differences in Black and White population on uptake of palliative care, discussion, and the documentation of advance care planning.3 Factors that may contribute to these differences include mistrust of the health care system among minorities, and not being connected to adequate health care resources. Family members and surrogate health care decision makers may consider receiving more aggressive care as advocating for better health care for their family members.4 These differences may contribute to the differences in hospitalization rates among residents within the same nursing home; however, the differences between nursing homes even after accounting for individual differences may indicate more widespread systemic differences that is associated with race. Policy changes that will address these differences are needed to level these differences so that quality care can be delivered regardless of race.5 For this vulnerable population with a terminal illness, approaches to enhance uptake of palliative approaches and care delivery for dementia patients at terminal stage are needed and understanding and targeting factors that contribute to low uptake of these approaches will enhance end of life care. Understanding the differences in resources and systems of care in nursing homes and perhaps how palliative care is integrated in these settings will be important to address care disparities that occurs across nursing homes.
Applications for Clinical Practice
Clinicians who take care of this population of older adults with advanced dementia should be aware of the potential for racial disparities that may lead to differences in the quality of care. The underlying reasons for these differences could be targeted so that older adults in all racial groups may have equal access to quality care including palliative approaches that avoid aggressive care for terminal illnesses across settings that may yield better care and quality of life. Policy makers and health systems leaders need to consider the current realities with racial disparities that policies need to address these differences so that they may not continue to persist in our systems of care.
Financial disclosures: None.
1. Mahajan S, Caraballo C, Lu Y, et al. Trends in Differences in Health Status and Health Care Access and Affordability by Race and Ethnicity in the United States, 1999-2018. JAMA. 2021;326(7):637-648. doi:10.1001/jama.2021.9907
2. Gill TM, Zang EX, Murphy TE, et al. Association Between Neighborhood Disadvantage and Functional Well-being in Community-Living Older Persons. [published online ahead of print, 2021 Aug 23]. JAMA Intern Med. doi:10.1001/jamainternmed.2021.4260
3. Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. Am J Hosp Palliat Care. 2021;38(6):688-718. doi:10.1177/1049909120966585
4. Siler S, Arora K, Doyon K, Fischer SM. Spirituality and the Illness Experience: Perspectives of African American Older Adults. Am J Hosp Palliat Care. 2021;38(6):618-625. doi:10.1177/1049909120988280
5. Council on Ethical and Judicial Affairs. Black-white disparities in health care. JAMA. 1990;263(17):2344-2346. doi:10.1001/jama.1990.03440170066038
Study Overview
Objective. To examine differences in care, specifically hospitalization towards the end of life, among nursing home residents with dementia who were Black compared with those who were White.
Design. Population based cohort study in the US. The study included all decedents with Alzheimer’s disease or related dementia (ADRD) who resided in a nursing home from 2014 to 2017. Decedents from nursing homes were identified by death within 1 day of an identified nursing home stay or within 8 days of a hospital transfer from nursing home. Data were obtained from Minimum Data Set 3.0 (MDS) which contains clinical data from all Medicaid or Medicare certified nursing homes, and from the Medicare Beneficiary Summary File (MBSF) and Medicare Provider and Analysis and Review (MedPAR) which contains hospitalization events for all Medicare Beneficiaries. These files were linked to identify nursing home residents with ADRD who were hospitalized at the end of life. ADRD diagnosis was identified from the chronic condition list from the MBSF and from MDS diagnosis list.
Setting and participants. The study included 665 033 residents from 14 595 nursing homes who died during the study period. Resident race was categorized as White or Black based on the MBSF. Severe cognitive impairment was identified using the MDS that categorized residents as severe or not using the Brief Interview for Mental Status and the Cognitive Performance Scale. The mean (SD) age of the study population was 86.7 (9.2) years for White residents and 82.6 (11.1) years for Black residents. Of the participants, 68.8% and 61.2% were female for Black and White residents, respectively. Approximately 23.4% of White and 32.5% of Black residents had severe cognitive impairment. For nursing home characteristics, 71.5% of the 14 595 nursing homes represented were for profit; average bedside was 109.5 (57.0) and occupancy rate was on average 81.2% (14.3%).
Main outcome measures. The study outcome measure was any hospitalization within 30 days prior to death. The outcome was selected as an indicator of quality of care because as older adults living with ADRD experience progressive worsening of cognitive symptoms, at the end of life when dementia is severe, advance care planning and communication with health care proxies and surrogates often result in coordinated care that avoids acute hospitalizations, which are often burdensome to both patient and family and may yield poorer quality of life.
Main results. The study found that approximately 29.5% of White decedents and 40.7% of Black decedents were hospitalized towards the end of life. Nursing homes with a higher proportion of Black residents were more likely to have residents hospitalized towards the end of life with 35% of residents hospitalized in the highest quartile (27% Black) compared with 17% hospitalized for nursing homes in the lowest quartile (0% Black).After adjusting for covariates, Black residents were 7.9% more likely to be hospitalized in the last 30 days of life compared with White residents. Blacks with severe cognitive impairment has elevated risk of hospitalization by 4.9% when compared with White residents. After accounting for nursing home facility–level characteristics, nursing homes with a low proportion of Black residents had a 5.2% higher risk of hospitalizations compared with nursing homes with no Black residents, and nursing homes with a higher percentage of Black residents had a 13.3% higher risk of hospitalization compared with nursing homes with no Black residents.
Conclusion. Race is associated with care disparities in older nursing home residents with dementia. This study suggests that hospitalization towards the end of life as a quality of care marker differs across nursing homes, and nursing homes with a higher proportion of Black residents were more likely to be hospitalized. This suggests that these nursing homes may have fewer resources and delivered poorer quality of care, and that disparities in health systems or institutions contribute to differences in quality of care for this vulnerable group.
Commentary
Disparities of health status, health care, and affordability across race and ethnicity have persisted throughout the past 20 years.1 There is further evidence to support systemic differences that can contribute to differences in health outcomes.2 Although changes in health care policy such as the Affordable Care Act have expanded health care coverage, and instituted changes that aims to improve health care quality and reduce disparities, it is clear that factors contributing to disparities in care are structural and perhaps systemic. The latest evidence comes in this study that examines racial disparities in health care quality in one of the most vulnerable populations—older adults with Alzheimer’s disease and dementia. The finding that Black nursing home residents, when compared with White residents, often has higher risk of hospitalization at the end of life, even among those with severe dementia where better coordinated care, clear goals of care and perhaps instituting palliative care would result in lower rate of hospitalization. The disparities were observed across nursing homes as well, where nursing homes with higher proportion of Black residents appear to have lower quality of care.
These findings are consistent with prior work that has examined differences in Black and White population on uptake of palliative care, discussion, and the documentation of advance care planning.3 Factors that may contribute to these differences include mistrust of the health care system among minorities, and not being connected to adequate health care resources. Family members and surrogate health care decision makers may consider receiving more aggressive care as advocating for better health care for their family members.4 These differences may contribute to the differences in hospitalization rates among residents within the same nursing home; however, the differences between nursing homes even after accounting for individual differences may indicate more widespread systemic differences that is associated with race. Policy changes that will address these differences are needed to level these differences so that quality care can be delivered regardless of race.5 For this vulnerable population with a terminal illness, approaches to enhance uptake of palliative approaches and care delivery for dementia patients at terminal stage are needed and understanding and targeting factors that contribute to low uptake of these approaches will enhance end of life care. Understanding the differences in resources and systems of care in nursing homes and perhaps how palliative care is integrated in these settings will be important to address care disparities that occurs across nursing homes.
Applications for Clinical Practice
Clinicians who take care of this population of older adults with advanced dementia should be aware of the potential for racial disparities that may lead to differences in the quality of care. The underlying reasons for these differences could be targeted so that older adults in all racial groups may have equal access to quality care including palliative approaches that avoid aggressive care for terminal illnesses across settings that may yield better care and quality of life. Policy makers and health systems leaders need to consider the current realities with racial disparities that policies need to address these differences so that they may not continue to persist in our systems of care.
Financial disclosures: None.
Study Overview
Objective. To examine differences in care, specifically hospitalization towards the end of life, among nursing home residents with dementia who were Black compared with those who were White.
Design. Population based cohort study in the US. The study included all decedents with Alzheimer’s disease or related dementia (ADRD) who resided in a nursing home from 2014 to 2017. Decedents from nursing homes were identified by death within 1 day of an identified nursing home stay or within 8 days of a hospital transfer from nursing home. Data were obtained from Minimum Data Set 3.0 (MDS) which contains clinical data from all Medicaid or Medicare certified nursing homes, and from the Medicare Beneficiary Summary File (MBSF) and Medicare Provider and Analysis and Review (MedPAR) which contains hospitalization events for all Medicare Beneficiaries. These files were linked to identify nursing home residents with ADRD who were hospitalized at the end of life. ADRD diagnosis was identified from the chronic condition list from the MBSF and from MDS diagnosis list.
Setting and participants. The study included 665 033 residents from 14 595 nursing homes who died during the study period. Resident race was categorized as White or Black based on the MBSF. Severe cognitive impairment was identified using the MDS that categorized residents as severe or not using the Brief Interview for Mental Status and the Cognitive Performance Scale. The mean (SD) age of the study population was 86.7 (9.2) years for White residents and 82.6 (11.1) years for Black residents. Of the participants, 68.8% and 61.2% were female for Black and White residents, respectively. Approximately 23.4% of White and 32.5% of Black residents had severe cognitive impairment. For nursing home characteristics, 71.5% of the 14 595 nursing homes represented were for profit; average bedside was 109.5 (57.0) and occupancy rate was on average 81.2% (14.3%).
Main outcome measures. The study outcome measure was any hospitalization within 30 days prior to death. The outcome was selected as an indicator of quality of care because as older adults living with ADRD experience progressive worsening of cognitive symptoms, at the end of life when dementia is severe, advance care planning and communication with health care proxies and surrogates often result in coordinated care that avoids acute hospitalizations, which are often burdensome to both patient and family and may yield poorer quality of life.
Main results. The study found that approximately 29.5% of White decedents and 40.7% of Black decedents were hospitalized towards the end of life. Nursing homes with a higher proportion of Black residents were more likely to have residents hospitalized towards the end of life with 35% of residents hospitalized in the highest quartile (27% Black) compared with 17% hospitalized for nursing homes in the lowest quartile (0% Black).After adjusting for covariates, Black residents were 7.9% more likely to be hospitalized in the last 30 days of life compared with White residents. Blacks with severe cognitive impairment has elevated risk of hospitalization by 4.9% when compared with White residents. After accounting for nursing home facility–level characteristics, nursing homes with a low proportion of Black residents had a 5.2% higher risk of hospitalizations compared with nursing homes with no Black residents, and nursing homes with a higher percentage of Black residents had a 13.3% higher risk of hospitalization compared with nursing homes with no Black residents.
Conclusion. Race is associated with care disparities in older nursing home residents with dementia. This study suggests that hospitalization towards the end of life as a quality of care marker differs across nursing homes, and nursing homes with a higher proportion of Black residents were more likely to be hospitalized. This suggests that these nursing homes may have fewer resources and delivered poorer quality of care, and that disparities in health systems or institutions contribute to differences in quality of care for this vulnerable group.
Commentary
Disparities of health status, health care, and affordability across race and ethnicity have persisted throughout the past 20 years.1 There is further evidence to support systemic differences that can contribute to differences in health outcomes.2 Although changes in health care policy such as the Affordable Care Act have expanded health care coverage, and instituted changes that aims to improve health care quality and reduce disparities, it is clear that factors contributing to disparities in care are structural and perhaps systemic. The latest evidence comes in this study that examines racial disparities in health care quality in one of the most vulnerable populations—older adults with Alzheimer’s disease and dementia. The finding that Black nursing home residents, when compared with White residents, often has higher risk of hospitalization at the end of life, even among those with severe dementia where better coordinated care, clear goals of care and perhaps instituting palliative care would result in lower rate of hospitalization. The disparities were observed across nursing homes as well, where nursing homes with higher proportion of Black residents appear to have lower quality of care.
These findings are consistent with prior work that has examined differences in Black and White population on uptake of palliative care, discussion, and the documentation of advance care planning.3 Factors that may contribute to these differences include mistrust of the health care system among minorities, and not being connected to adequate health care resources. Family members and surrogate health care decision makers may consider receiving more aggressive care as advocating for better health care for their family members.4 These differences may contribute to the differences in hospitalization rates among residents within the same nursing home; however, the differences between nursing homes even after accounting for individual differences may indicate more widespread systemic differences that is associated with race. Policy changes that will address these differences are needed to level these differences so that quality care can be delivered regardless of race.5 For this vulnerable population with a terminal illness, approaches to enhance uptake of palliative approaches and care delivery for dementia patients at terminal stage are needed and understanding and targeting factors that contribute to low uptake of these approaches will enhance end of life care. Understanding the differences in resources and systems of care in nursing homes and perhaps how palliative care is integrated in these settings will be important to address care disparities that occurs across nursing homes.
Applications for Clinical Practice
Clinicians who take care of this population of older adults with advanced dementia should be aware of the potential for racial disparities that may lead to differences in the quality of care. The underlying reasons for these differences could be targeted so that older adults in all racial groups may have equal access to quality care including palliative approaches that avoid aggressive care for terminal illnesses across settings that may yield better care and quality of life. Policy makers and health systems leaders need to consider the current realities with racial disparities that policies need to address these differences so that they may not continue to persist in our systems of care.
Financial disclosures: None.
1. Mahajan S, Caraballo C, Lu Y, et al. Trends in Differences in Health Status and Health Care Access and Affordability by Race and Ethnicity in the United States, 1999-2018. JAMA. 2021;326(7):637-648. doi:10.1001/jama.2021.9907
2. Gill TM, Zang EX, Murphy TE, et al. Association Between Neighborhood Disadvantage and Functional Well-being in Community-Living Older Persons. [published online ahead of print, 2021 Aug 23]. JAMA Intern Med. doi:10.1001/jamainternmed.2021.4260
3. Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. Am J Hosp Palliat Care. 2021;38(6):688-718. doi:10.1177/1049909120966585
4. Siler S, Arora K, Doyon K, Fischer SM. Spirituality and the Illness Experience: Perspectives of African American Older Adults. Am J Hosp Palliat Care. 2021;38(6):618-625. doi:10.1177/1049909120988280
5. Council on Ethical and Judicial Affairs. Black-white disparities in health care. JAMA. 1990;263(17):2344-2346. doi:10.1001/jama.1990.03440170066038
1. Mahajan S, Caraballo C, Lu Y, et al. Trends in Differences in Health Status and Health Care Access and Affordability by Race and Ethnicity in the United States, 1999-2018. JAMA. 2021;326(7):637-648. doi:10.1001/jama.2021.9907
2. Gill TM, Zang EX, Murphy TE, et al. Association Between Neighborhood Disadvantage and Functional Well-being in Community-Living Older Persons. [published online ahead of print, 2021 Aug 23]. JAMA Intern Med. doi:10.1001/jamainternmed.2021.4260
3. Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. Am J Hosp Palliat Care. 2021;38(6):688-718. doi:10.1177/1049909120966585
4. Siler S, Arora K, Doyon K, Fischer SM. Spirituality and the Illness Experience: Perspectives of African American Older Adults. Am J Hosp Palliat Care. 2021;38(6):618-625. doi:10.1177/1049909120988280
5. Council on Ethical and Judicial Affairs. Black-white disparities in health care. JAMA. 1990;263(17):2344-2346. doi:10.1001/jama.1990.03440170066038
Preoperative Advance Care Planning for Older Adults Undergoing High-Risk Surgery: An Essential but Underutilized Aspect of Clinical Care
Study Overview
Objective. The objectives of this study were to (1) quantify the frequency of preoperative advance care planning (ACP) discussion and documentation for older adults undergoing major surgery in a national sample, and (2) characterize how surgical patients and their family members considered ACP after postoperative complications.
Design. A secondary analysis of data from a multisite randomized clinical trial testing the effects of a question prompt list intervention (a Question Problem List [QPL] brochure with 11 questions) given to patients aged 60 years or older undergoing high-risk surgery on preoperative communication with their surgeons.
Setting and participants. This multisite randomized controlled trial involved 5 study sites that encompassed distinct US geographic areas, including University of Wisconsin Hospital and Clinics (UWHC), Madison; the University of California, San Francisco, Medical Center (UCSF); Oregon Health & Science University (OHSU), Portland; the University Hospital of Rutgers New Jersey Medical School (Rutgers), Newark; and the Brigham and Women’s Hospital (BWH), Boston, Massachusetts. The study enrolled 40 surgeons who routinely performed high-risk oncological or vascular surgery via purposeful sampling; patients aged 60 years or older with at least 1 comorbidity and an oncological or vascular problem that were treatable with high-risk surgery; and 1 invited family member per enrolled patient to participate in open-ended interviews postsurgery. High-risk surgery was defined as an operation that has a 30-day in-hospital mortality rate greater than or equal to 1%. Data were collected from June 1, 2016, to November 30, 2018.
Main outcome measures. The frequency of preoperative discussions and documentation of ACP was determined. For patients who had major surgery, any mention of ACP (ie, mention of advance directive [AD], health care power of attorney, or preference for limitations of life-sustaining treatments) by the surgeon, patient or family member during the audio recorded, transcribed, and coded preoperative consultation was counted. The presence of a written AD in the medical record at the time of the initial consultation, filed between the consultation and the date of surgery, or added postoperatively, was recorded using a standardized abstraction form. Postoperative treatments administered and complications experienced within 6 weeks after surgery were recorded. Open-ended interviews with patients who experienced significant postoperative complications (eg, prolonged hospitalization > 8 days, intensive care unit stay > 3 days) and their family members were conducted 6 weeks after surgery. Information ascertained during interviews focused on treatment decisions, postoperative experiences, and interpersonal relationships among patients, families, and clinicians. Transcripts of these interviews were then subjected to qualitative content analysis.
Main results. A total of 446 patients were enrolled in the primary study. Of these patients, 213 (122 men [57%]; 91 women [43%]; mean [SD] age, 72 [7] years) underwent major surgery. Only 13 (6.1%) of those who had major surgery had any discussion related to ACP in the preoperative consultation. In this cohort, 141 (66%) patients did not have an AD on file before undergoing major surgery. The presence of AD was not associated with age (60-69 years, 26 [31%]; 70-79 years, 31 [33%]; ≥ 80 years, 15 [42%]; P = .55), number of comorbidities (1, 35 [32%]; 2, 18 [33%]; ≥ 3, 19 [40%]; P = .62), or type of procedure (oncological, 53 [32%]; vascular, 19 [42%]; P = .22). Moreover, there was no difference in preoperative communication about ACP or documentation of an AD for patients who were mailed a QPL brochure compared to those who received usual care (intervention, 38 [35%]; usual care, 34 [33%]; P = .77). Rates of AD documentation were associated with individual study sites with BWH and UWHC having higher rates of documentation (20 [50%] and 27 [44%], respectively) compared to OHSU, UCSF, or Rutgers (7 [17%], 17 [35%], and 1 [5%], respectively). Analysis from the interviews indicated that patients and families felt unprepared for serious surgical complications and had varied interpretations of ACP. Patients with complications were enthusiastic about ACP but did not think it was important to discuss their preferences for life-sustaining treatments with their surgeon preoperatively.
Conclusion. Although surgeons and patients report that they believe ACP is important, preoperative discussion of patient preferences rarely occurs. This study found that the frequency of ACP discussions or AD documentations among older patients undergoing high-risk oncologic or vascular surgery was low. Interventions that are aimed to increase rates of preoperative ACP discussions should be implemented to help prepare patients and their families for difficult decisions in the setting of serious surgical complications and could help decrease postoperative conflicts that result from unclear patient care goals.
Commentary
Surgeons and patients approach surgical interventions with optimistic outlooks while simultaneously preparing for unintended adverse outcomes. For patients, preoperative ACP discussions ease the burden on their families and ensure their wishes and care goals are communicated. For surgeons, these discussions inform them how best to support the values of the patient. Therefore, it is unsurprising that preoperative ACP is viewed favorably by both groups. Given the consensus that ACP is important in the care of older adults undergoing high-risk surgery, one would assume that preoperative ACP discussion is a standard of practice among surgeons and their aging patients. However, in a secondary analysis of a randomized control trial testing a patient-mediated intervention to improve preoperative communication, Kalbfell et al1 showed that ACP discussions rarely take place prior to major surgery in older adults. This finding highlights the significant discrepancy between the belief that ACP is important, and the actual rate that it is practiced, in older patients undergoing high-risk surgery. This discordance is highly concerning because it suggests that surgeons who provide care to a very vulnerable subset of older patients may overlook an essential aspect of preoperative care and therefore lack a thorough and thoughtful understanding of the patient’s care goals. In practice, this omission can pose significant challenges associated with the surgeon and family’s decisions to use postoperative life-sustaining interventions or to manage unforeseen complications should a patient become unable to make medical decisions.
The barriers to conducting successful ACP discussions between surgeons and patients are multifactorial. Kalbfell et al1 highlighted several of these barriers, including lack of patient efficacy, physician attitudes, and institutional values in older adults who require major surgeries. The inadequacy of patient efficacy in preoperative ACP is illustrated by findings from the primary, multisite trial of QPL intervention conducted by Schwarze et al. Interestingly, the authors found that patients who did not receive QPL brochure had no ACP discussions, and that QPL implementation did not significantly improve discussion rates despite its intent to encourage these discussions.2 Possible explanations for this lack of engagement might be a lack of health literacy or patient efficacy in the study population. Qualitative data from the current study provided further evidence to support these explanations. For instance, some patients provided limited or incomplete information about their wishes for health care management while others felt it was unnecessary to have ACP discussions unless complications arose.1 However, the latter example counters the purpose of ACP which is to enable patients to make plans about future health care and not reactive to a medical complication or emergency.
Surgeons bear a large responsibility in providing treatments that are consistent with the care goals of the patient. Thus, surgeons play a crucial role in engaging, guiding, and facilitating ACP discussions with patients. This role is even more critical when patients are unable or unwilling to initiate care goal discussions. Physician attitudes towards ACP, therefore, greatly influence the effectiveness of these discussions. In a study of self-administered surveys by vascular, neurologic, and cardiothoracic surgeons, greater than 90% of respondents viewed postoperative life-supporting therapy as necessary, and 54% would decline to operate on patients with an AD limiting life-supporting therapy.3 Moreover, the same study showed that 52% of respondents reported discussing AD before surgery, a figure that exceeded the actual rates at which ACP discussions occur in many other studies. In the current study, Kalbfell et al1 also found that surgeons viewed ACP discussions largely in the context of AD creation and declined to investigate the full scope of patient preferences. These findings, when combined with other studies that indicate an incomplete understanding of ACP in some surgeons, suggest that not all physicians are able or willing to navigate these sometimes lengthy and difficult conversations with patients. This gap in practice provides opportunities for training in surgical specialties that center on optimizing preoperative ACP discussions to meet the care needs of older patients.
Institutional value and culture are important factors that impact physician behavior and the practice of ACP discussion. In the current study, the authors reported that the majority of ACP discussions were held by a minority of surgeons and that different institutions and study sites had vastly different rates of ACP documentation.1 These results are further supported by findings of large variations between physicians and hospitals in ACP reporting in hospitalized frail older adults.4 These variations in practices at different institutions suggest that it is possible to improve rates of preoperative ACP discussion. Reasons for these differences need to be further investigated in order to identify strategies, resources, or trainings required by medical institutions to support surgeons to carry out ACP discussions with patients undergoing high-risk surgeries.
The study conducted by Kalbfell et al1 has several strengths. For example, it included Spanish-speaking patients and the use of a Spanish version of the QPL intervention to account for cultural differences. The study also included multiple surgical specialties and institutions and captured a large and national sample, thus making its findings more generalizable. However, the lack of data on the duration of preoperative consultation visits in patients who completed ACP discussions poses a limitation to this study. This is relevant because surgeon availability to engage in lengthy ACP discussions may be limited due to busy clinical schedules. Additional data on the duration of preoperative visits inclusive of a thoughtfully conducted ACP discussion could help to modify clinical workflow to facilitate its uptake in surgical practices.
Applications for Clinical Practice
The findings from the current study indicate that patients and surgeons agree that preoperative ACP discussions are beneficial to the clinical care of older adults before high-risk surgeries. However, these important conversations do not occur frequently. Surgeons and health care institutions need to identify strategies to initiate, facilitate, and optimize productive preoperative ACP discussions to provide patient-centered care in vulnerable older surgical patients.
Financial disclosures: None.
1. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
2. Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a Question Prompt List Intervention for Older Patients Considering Major Surgery: A Multisite Randomized Clinical Trial. JAMA Surg. 2020;155(1):6-13. doi:10.1001/jamasurg.2019.3778
3. Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surgery. 2012;255(3):418-423. doi:10.1097/SLA.0b013e31823b6782
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10:164-174. doi:10.1136/bmjspcare-2019-002093
Study Overview
Objective. The objectives of this study were to (1) quantify the frequency of preoperative advance care planning (ACP) discussion and documentation for older adults undergoing major surgery in a national sample, and (2) characterize how surgical patients and their family members considered ACP after postoperative complications.
Design. A secondary analysis of data from a multisite randomized clinical trial testing the effects of a question prompt list intervention (a Question Problem List [QPL] brochure with 11 questions) given to patients aged 60 years or older undergoing high-risk surgery on preoperative communication with their surgeons.
Setting and participants. This multisite randomized controlled trial involved 5 study sites that encompassed distinct US geographic areas, including University of Wisconsin Hospital and Clinics (UWHC), Madison; the University of California, San Francisco, Medical Center (UCSF); Oregon Health & Science University (OHSU), Portland; the University Hospital of Rutgers New Jersey Medical School (Rutgers), Newark; and the Brigham and Women’s Hospital (BWH), Boston, Massachusetts. The study enrolled 40 surgeons who routinely performed high-risk oncological or vascular surgery via purposeful sampling; patients aged 60 years or older with at least 1 comorbidity and an oncological or vascular problem that were treatable with high-risk surgery; and 1 invited family member per enrolled patient to participate in open-ended interviews postsurgery. High-risk surgery was defined as an operation that has a 30-day in-hospital mortality rate greater than or equal to 1%. Data were collected from June 1, 2016, to November 30, 2018.
Main outcome measures. The frequency of preoperative discussions and documentation of ACP was determined. For patients who had major surgery, any mention of ACP (ie, mention of advance directive [AD], health care power of attorney, or preference for limitations of life-sustaining treatments) by the surgeon, patient or family member during the audio recorded, transcribed, and coded preoperative consultation was counted. The presence of a written AD in the medical record at the time of the initial consultation, filed between the consultation and the date of surgery, or added postoperatively, was recorded using a standardized abstraction form. Postoperative treatments administered and complications experienced within 6 weeks after surgery were recorded. Open-ended interviews with patients who experienced significant postoperative complications (eg, prolonged hospitalization > 8 days, intensive care unit stay > 3 days) and their family members were conducted 6 weeks after surgery. Information ascertained during interviews focused on treatment decisions, postoperative experiences, and interpersonal relationships among patients, families, and clinicians. Transcripts of these interviews were then subjected to qualitative content analysis.
Main results. A total of 446 patients were enrolled in the primary study. Of these patients, 213 (122 men [57%]; 91 women [43%]; mean [SD] age, 72 [7] years) underwent major surgery. Only 13 (6.1%) of those who had major surgery had any discussion related to ACP in the preoperative consultation. In this cohort, 141 (66%) patients did not have an AD on file before undergoing major surgery. The presence of AD was not associated with age (60-69 years, 26 [31%]; 70-79 years, 31 [33%]; ≥ 80 years, 15 [42%]; P = .55), number of comorbidities (1, 35 [32%]; 2, 18 [33%]; ≥ 3, 19 [40%]; P = .62), or type of procedure (oncological, 53 [32%]; vascular, 19 [42%]; P = .22). Moreover, there was no difference in preoperative communication about ACP or documentation of an AD for patients who were mailed a QPL brochure compared to those who received usual care (intervention, 38 [35%]; usual care, 34 [33%]; P = .77). Rates of AD documentation were associated with individual study sites with BWH and UWHC having higher rates of documentation (20 [50%] and 27 [44%], respectively) compared to OHSU, UCSF, or Rutgers (7 [17%], 17 [35%], and 1 [5%], respectively). Analysis from the interviews indicated that patients and families felt unprepared for serious surgical complications and had varied interpretations of ACP. Patients with complications were enthusiastic about ACP but did not think it was important to discuss their preferences for life-sustaining treatments with their surgeon preoperatively.
Conclusion. Although surgeons and patients report that they believe ACP is important, preoperative discussion of patient preferences rarely occurs. This study found that the frequency of ACP discussions or AD documentations among older patients undergoing high-risk oncologic or vascular surgery was low. Interventions that are aimed to increase rates of preoperative ACP discussions should be implemented to help prepare patients and their families for difficult decisions in the setting of serious surgical complications and could help decrease postoperative conflicts that result from unclear patient care goals.
Commentary
Surgeons and patients approach surgical interventions with optimistic outlooks while simultaneously preparing for unintended adverse outcomes. For patients, preoperative ACP discussions ease the burden on their families and ensure their wishes and care goals are communicated. For surgeons, these discussions inform them how best to support the values of the patient. Therefore, it is unsurprising that preoperative ACP is viewed favorably by both groups. Given the consensus that ACP is important in the care of older adults undergoing high-risk surgery, one would assume that preoperative ACP discussion is a standard of practice among surgeons and their aging patients. However, in a secondary analysis of a randomized control trial testing a patient-mediated intervention to improve preoperative communication, Kalbfell et al1 showed that ACP discussions rarely take place prior to major surgery in older adults. This finding highlights the significant discrepancy between the belief that ACP is important, and the actual rate that it is practiced, in older patients undergoing high-risk surgery. This discordance is highly concerning because it suggests that surgeons who provide care to a very vulnerable subset of older patients may overlook an essential aspect of preoperative care and therefore lack a thorough and thoughtful understanding of the patient’s care goals. In practice, this omission can pose significant challenges associated with the surgeon and family’s decisions to use postoperative life-sustaining interventions or to manage unforeseen complications should a patient become unable to make medical decisions.
The barriers to conducting successful ACP discussions between surgeons and patients are multifactorial. Kalbfell et al1 highlighted several of these barriers, including lack of patient efficacy, physician attitudes, and institutional values in older adults who require major surgeries. The inadequacy of patient efficacy in preoperative ACP is illustrated by findings from the primary, multisite trial of QPL intervention conducted by Schwarze et al. Interestingly, the authors found that patients who did not receive QPL brochure had no ACP discussions, and that QPL implementation did not significantly improve discussion rates despite its intent to encourage these discussions.2 Possible explanations for this lack of engagement might be a lack of health literacy or patient efficacy in the study population. Qualitative data from the current study provided further evidence to support these explanations. For instance, some patients provided limited or incomplete information about their wishes for health care management while others felt it was unnecessary to have ACP discussions unless complications arose.1 However, the latter example counters the purpose of ACP which is to enable patients to make plans about future health care and not reactive to a medical complication or emergency.
Surgeons bear a large responsibility in providing treatments that are consistent with the care goals of the patient. Thus, surgeons play a crucial role in engaging, guiding, and facilitating ACP discussions with patients. This role is even more critical when patients are unable or unwilling to initiate care goal discussions. Physician attitudes towards ACP, therefore, greatly influence the effectiveness of these discussions. In a study of self-administered surveys by vascular, neurologic, and cardiothoracic surgeons, greater than 90% of respondents viewed postoperative life-supporting therapy as necessary, and 54% would decline to operate on patients with an AD limiting life-supporting therapy.3 Moreover, the same study showed that 52% of respondents reported discussing AD before surgery, a figure that exceeded the actual rates at which ACP discussions occur in many other studies. In the current study, Kalbfell et al1 also found that surgeons viewed ACP discussions largely in the context of AD creation and declined to investigate the full scope of patient preferences. These findings, when combined with other studies that indicate an incomplete understanding of ACP in some surgeons, suggest that not all physicians are able or willing to navigate these sometimes lengthy and difficult conversations with patients. This gap in practice provides opportunities for training in surgical specialties that center on optimizing preoperative ACP discussions to meet the care needs of older patients.
Institutional value and culture are important factors that impact physician behavior and the practice of ACP discussion. In the current study, the authors reported that the majority of ACP discussions were held by a minority of surgeons and that different institutions and study sites had vastly different rates of ACP documentation.1 These results are further supported by findings of large variations between physicians and hospitals in ACP reporting in hospitalized frail older adults.4 These variations in practices at different institutions suggest that it is possible to improve rates of preoperative ACP discussion. Reasons for these differences need to be further investigated in order to identify strategies, resources, or trainings required by medical institutions to support surgeons to carry out ACP discussions with patients undergoing high-risk surgeries.
The study conducted by Kalbfell et al1 has several strengths. For example, it included Spanish-speaking patients and the use of a Spanish version of the QPL intervention to account for cultural differences. The study also included multiple surgical specialties and institutions and captured a large and national sample, thus making its findings more generalizable. However, the lack of data on the duration of preoperative consultation visits in patients who completed ACP discussions poses a limitation to this study. This is relevant because surgeon availability to engage in lengthy ACP discussions may be limited due to busy clinical schedules. Additional data on the duration of preoperative visits inclusive of a thoughtfully conducted ACP discussion could help to modify clinical workflow to facilitate its uptake in surgical practices.
Applications for Clinical Practice
The findings from the current study indicate that patients and surgeons agree that preoperative ACP discussions are beneficial to the clinical care of older adults before high-risk surgeries. However, these important conversations do not occur frequently. Surgeons and health care institutions need to identify strategies to initiate, facilitate, and optimize productive preoperative ACP discussions to provide patient-centered care in vulnerable older surgical patients.
Financial disclosures: None.
Study Overview
Objective. The objectives of this study were to (1) quantify the frequency of preoperative advance care planning (ACP) discussion and documentation for older adults undergoing major surgery in a national sample, and (2) characterize how surgical patients and their family members considered ACP after postoperative complications.
Design. A secondary analysis of data from a multisite randomized clinical trial testing the effects of a question prompt list intervention (a Question Problem List [QPL] brochure with 11 questions) given to patients aged 60 years or older undergoing high-risk surgery on preoperative communication with their surgeons.
Setting and participants. This multisite randomized controlled trial involved 5 study sites that encompassed distinct US geographic areas, including University of Wisconsin Hospital and Clinics (UWHC), Madison; the University of California, San Francisco, Medical Center (UCSF); Oregon Health & Science University (OHSU), Portland; the University Hospital of Rutgers New Jersey Medical School (Rutgers), Newark; and the Brigham and Women’s Hospital (BWH), Boston, Massachusetts. The study enrolled 40 surgeons who routinely performed high-risk oncological or vascular surgery via purposeful sampling; patients aged 60 years or older with at least 1 comorbidity and an oncological or vascular problem that were treatable with high-risk surgery; and 1 invited family member per enrolled patient to participate in open-ended interviews postsurgery. High-risk surgery was defined as an operation that has a 30-day in-hospital mortality rate greater than or equal to 1%. Data were collected from June 1, 2016, to November 30, 2018.
Main outcome measures. The frequency of preoperative discussions and documentation of ACP was determined. For patients who had major surgery, any mention of ACP (ie, mention of advance directive [AD], health care power of attorney, or preference for limitations of life-sustaining treatments) by the surgeon, patient or family member during the audio recorded, transcribed, and coded preoperative consultation was counted. The presence of a written AD in the medical record at the time of the initial consultation, filed between the consultation and the date of surgery, or added postoperatively, was recorded using a standardized abstraction form. Postoperative treatments administered and complications experienced within 6 weeks after surgery were recorded. Open-ended interviews with patients who experienced significant postoperative complications (eg, prolonged hospitalization > 8 days, intensive care unit stay > 3 days) and their family members were conducted 6 weeks after surgery. Information ascertained during interviews focused on treatment decisions, postoperative experiences, and interpersonal relationships among patients, families, and clinicians. Transcripts of these interviews were then subjected to qualitative content analysis.
Main results. A total of 446 patients were enrolled in the primary study. Of these patients, 213 (122 men [57%]; 91 women [43%]; mean [SD] age, 72 [7] years) underwent major surgery. Only 13 (6.1%) of those who had major surgery had any discussion related to ACP in the preoperative consultation. In this cohort, 141 (66%) patients did not have an AD on file before undergoing major surgery. The presence of AD was not associated with age (60-69 years, 26 [31%]; 70-79 years, 31 [33%]; ≥ 80 years, 15 [42%]; P = .55), number of comorbidities (1, 35 [32%]; 2, 18 [33%]; ≥ 3, 19 [40%]; P = .62), or type of procedure (oncological, 53 [32%]; vascular, 19 [42%]; P = .22). Moreover, there was no difference in preoperative communication about ACP or documentation of an AD for patients who were mailed a QPL brochure compared to those who received usual care (intervention, 38 [35%]; usual care, 34 [33%]; P = .77). Rates of AD documentation were associated with individual study sites with BWH and UWHC having higher rates of documentation (20 [50%] and 27 [44%], respectively) compared to OHSU, UCSF, or Rutgers (7 [17%], 17 [35%], and 1 [5%], respectively). Analysis from the interviews indicated that patients and families felt unprepared for serious surgical complications and had varied interpretations of ACP. Patients with complications were enthusiastic about ACP but did not think it was important to discuss their preferences for life-sustaining treatments with their surgeon preoperatively.
Conclusion. Although surgeons and patients report that they believe ACP is important, preoperative discussion of patient preferences rarely occurs. This study found that the frequency of ACP discussions or AD documentations among older patients undergoing high-risk oncologic or vascular surgery was low. Interventions that are aimed to increase rates of preoperative ACP discussions should be implemented to help prepare patients and their families for difficult decisions in the setting of serious surgical complications and could help decrease postoperative conflicts that result from unclear patient care goals.
Commentary
Surgeons and patients approach surgical interventions with optimistic outlooks while simultaneously preparing for unintended adverse outcomes. For patients, preoperative ACP discussions ease the burden on their families and ensure their wishes and care goals are communicated. For surgeons, these discussions inform them how best to support the values of the patient. Therefore, it is unsurprising that preoperative ACP is viewed favorably by both groups. Given the consensus that ACP is important in the care of older adults undergoing high-risk surgery, one would assume that preoperative ACP discussion is a standard of practice among surgeons and their aging patients. However, in a secondary analysis of a randomized control trial testing a patient-mediated intervention to improve preoperative communication, Kalbfell et al1 showed that ACP discussions rarely take place prior to major surgery in older adults. This finding highlights the significant discrepancy between the belief that ACP is important, and the actual rate that it is practiced, in older patients undergoing high-risk surgery. This discordance is highly concerning because it suggests that surgeons who provide care to a very vulnerable subset of older patients may overlook an essential aspect of preoperative care and therefore lack a thorough and thoughtful understanding of the patient’s care goals. In practice, this omission can pose significant challenges associated with the surgeon and family’s decisions to use postoperative life-sustaining interventions or to manage unforeseen complications should a patient become unable to make medical decisions.
The barriers to conducting successful ACP discussions between surgeons and patients are multifactorial. Kalbfell et al1 highlighted several of these barriers, including lack of patient efficacy, physician attitudes, and institutional values in older adults who require major surgeries. The inadequacy of patient efficacy in preoperative ACP is illustrated by findings from the primary, multisite trial of QPL intervention conducted by Schwarze et al. Interestingly, the authors found that patients who did not receive QPL brochure had no ACP discussions, and that QPL implementation did not significantly improve discussion rates despite its intent to encourage these discussions.2 Possible explanations for this lack of engagement might be a lack of health literacy or patient efficacy in the study population. Qualitative data from the current study provided further evidence to support these explanations. For instance, some patients provided limited or incomplete information about their wishes for health care management while others felt it was unnecessary to have ACP discussions unless complications arose.1 However, the latter example counters the purpose of ACP which is to enable patients to make plans about future health care and not reactive to a medical complication or emergency.
Surgeons bear a large responsibility in providing treatments that are consistent with the care goals of the patient. Thus, surgeons play a crucial role in engaging, guiding, and facilitating ACP discussions with patients. This role is even more critical when patients are unable or unwilling to initiate care goal discussions. Physician attitudes towards ACP, therefore, greatly influence the effectiveness of these discussions. In a study of self-administered surveys by vascular, neurologic, and cardiothoracic surgeons, greater than 90% of respondents viewed postoperative life-supporting therapy as necessary, and 54% would decline to operate on patients with an AD limiting life-supporting therapy.3 Moreover, the same study showed that 52% of respondents reported discussing AD before surgery, a figure that exceeded the actual rates at which ACP discussions occur in many other studies. In the current study, Kalbfell et al1 also found that surgeons viewed ACP discussions largely in the context of AD creation and declined to investigate the full scope of patient preferences. These findings, when combined with other studies that indicate an incomplete understanding of ACP in some surgeons, suggest that not all physicians are able or willing to navigate these sometimes lengthy and difficult conversations with patients. This gap in practice provides opportunities for training in surgical specialties that center on optimizing preoperative ACP discussions to meet the care needs of older patients.
Institutional value and culture are important factors that impact physician behavior and the practice of ACP discussion. In the current study, the authors reported that the majority of ACP discussions were held by a minority of surgeons and that different institutions and study sites had vastly different rates of ACP documentation.1 These results are further supported by findings of large variations between physicians and hospitals in ACP reporting in hospitalized frail older adults.4 These variations in practices at different institutions suggest that it is possible to improve rates of preoperative ACP discussion. Reasons for these differences need to be further investigated in order to identify strategies, resources, or trainings required by medical institutions to support surgeons to carry out ACP discussions with patients undergoing high-risk surgeries.
The study conducted by Kalbfell et al1 has several strengths. For example, it included Spanish-speaking patients and the use of a Spanish version of the QPL intervention to account for cultural differences. The study also included multiple surgical specialties and institutions and captured a large and national sample, thus making its findings more generalizable. However, the lack of data on the duration of preoperative consultation visits in patients who completed ACP discussions poses a limitation to this study. This is relevant because surgeon availability to engage in lengthy ACP discussions may be limited due to busy clinical schedules. Additional data on the duration of preoperative visits inclusive of a thoughtfully conducted ACP discussion could help to modify clinical workflow to facilitate its uptake in surgical practices.
Applications for Clinical Practice
The findings from the current study indicate that patients and surgeons agree that preoperative ACP discussions are beneficial to the clinical care of older adults before high-risk surgeries. However, these important conversations do not occur frequently. Surgeons and health care institutions need to identify strategies to initiate, facilitate, and optimize productive preoperative ACP discussions to provide patient-centered care in vulnerable older surgical patients.
Financial disclosures: None.
1. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
2. Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a Question Prompt List Intervention for Older Patients Considering Major Surgery: A Multisite Randomized Clinical Trial. JAMA Surg. 2020;155(1):6-13. doi:10.1001/jamasurg.2019.3778
3. Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surgery. 2012;255(3):418-423. doi:10.1097/SLA.0b013e31823b6782
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10:164-174. doi:10.1136/bmjspcare-2019-002093
1. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
2. Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a Question Prompt List Intervention for Older Patients Considering Major Surgery: A Multisite Randomized Clinical Trial. JAMA Surg. 2020;155(1):6-13. doi:10.1001/jamasurg.2019.3778
3. Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surgery. 2012;255(3):418-423. doi:10.1097/SLA.0b013e31823b6782
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10:164-174. doi:10.1136/bmjspcare-2019-002093
Should Geriatric Veterans Get Immunotherapy?
Patients in their 90s with cancer tolerated immunotherapy well with few serious adverse effects, and they lived for an average of 1.6 years after treatment, a small new study within the US Department of Veterans Affairs (VA) health system reports.
Only 6.3% of 48 patients who were treated with immune checkpoint inhibitors experienced the most severe types of side effects – grade III/IV events – and a total of 27% had any adverse effects, according to the report, which was presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and inperson in Denver Colorado, September 24 to September 26, 2021.
“Our project should help give confidence to oncologists treating the elderly,” said Andrew Joseph Benefield, MD, a hematology/oncology fellow at Wake Forest Baptist Medical Center, in an interview. “Immunotherapy can be given safely and likely effectively in select individuals over the age of 90 with good performance status.”
Benefield and colleagues launched their study to gain insight into a little-studied area: How does cancer treatment affects nonagenarians? “I think many oncologists have been in a situation where they encounter an individual over the age of 90 years who has a good performance status, and they've wondered if immunotherapy would be helpful and safe, particularly given our knowledge of waning immune strength as people age,” he said.
The researchers retrospectively tracked patients with cancer who were at least 90 years old from 2016 to 2017 and were treated with immune checkpoint inhibitors. Most were fit or fairly fit with Eastern Cooperative Oncology Group (ECOG) physical performance scales of 0 or 1 (n = 26), and nearly all had cancer in stage IV (n = 42). Melanoma was the most common type of cancer (n = 19), followed by non-small-cell lung cancer (n = 15). Patients were treated with an average of 12.2 cycles.
“In general, we saw that treatment was well-tolerated,” Dr. Benefield said. “We also noted that a trend toward better long-term survival outcomes in individuals with very good performance status at the start of treatment. We hope to parse this out more as we add more data to our data-set, as the numbers are still too small for confident direct comparison.”
Dr. Benefield said he has treated a limited number of patients in their 90s who were highly physical fit for their age and “very eager” to be treated. “They wanted to do anything they could to maintain their lifestyle,” he said. “In my experience, aggressive supportive care and close monitoring for developing toxicities has been most helpful.”
The researchers don’t know the causes of death of many of the patients, and it’s not clear how they fared in their final days. Still, Dr. Benefield said, “extending someone's life by more than 1 year with relatively low risk of adverse effects is reasonable.”
Oncologist Melisa Wong, MD, MAS, of the University of California, San Francisco, reviewed the study and said in an interview that it “a valuable description of outcomes for nonagenarians receiving immunotherapy in the VA healthcare system.” As she noted, “many other studies of immunotherapy among older adults focus on patients aged 65 or 70 and older while very few focus on octogenarians or nonagenarians.”
The findings suggest that “it is important to move beyond chronological age and assess patients’ physiologic age through a geriatric assessment,” she said. “Geriatric assessment-derived risk scores have been shown to predict chemotherapy toxicity for older adults and research to develop similar tools for immunotherapy are ongoing.”
However, she cautioned that older patients may become suffer so much from the most common side effect of immunotherapy -- fatigue – that “their independence is at stake.”
“Some of these patient choose to stop immunotherapy because the side effects aren’t worth it anymore,” she said. “The challenge for oncologists is not knowing in advance which patients will fall into each of these categories.”
She added that her geriatric oncology research focuses on improving risk stratification for older adults, such as those who are at least 70 with lung adenocarcinoma.
Oncologist Grant R. Williams, MD, MSPH, director of the Cancer & Aging Program at the University of Alabama at Birmingham, agreed in an interview that comprehensive geriatric assessments are important to guide treatment in the oldest adults. “In addition, it is important to elicit the goals of treatment as well,” he said. “For older adults that are fit or at least pre-frail and desire aggressive treatment, immunotherapy is a very reasonable approach, particularly when patients are closely monitored for side effects.”
No study funding is reported. The authors report no disclosures. Dr. Wong discloses an immediate family member is an employee and stock holder of Genentech. Dr. Williams has no disclosures.
Patients in their 90s with cancer tolerated immunotherapy well with few serious adverse effects, and they lived for an average of 1.6 years after treatment, a small new study within the US Department of Veterans Affairs (VA) health system reports.
Only 6.3% of 48 patients who were treated with immune checkpoint inhibitors experienced the most severe types of side effects – grade III/IV events – and a total of 27% had any adverse effects, according to the report, which was presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and inperson in Denver Colorado, September 24 to September 26, 2021.
“Our project should help give confidence to oncologists treating the elderly,” said Andrew Joseph Benefield, MD, a hematology/oncology fellow at Wake Forest Baptist Medical Center, in an interview. “Immunotherapy can be given safely and likely effectively in select individuals over the age of 90 with good performance status.”
Benefield and colleagues launched their study to gain insight into a little-studied area: How does cancer treatment affects nonagenarians? “I think many oncologists have been in a situation where they encounter an individual over the age of 90 years who has a good performance status, and they've wondered if immunotherapy would be helpful and safe, particularly given our knowledge of waning immune strength as people age,” he said.
The researchers retrospectively tracked patients with cancer who were at least 90 years old from 2016 to 2017 and were treated with immune checkpoint inhibitors. Most were fit or fairly fit with Eastern Cooperative Oncology Group (ECOG) physical performance scales of 0 or 1 (n = 26), and nearly all had cancer in stage IV (n = 42). Melanoma was the most common type of cancer (n = 19), followed by non-small-cell lung cancer (n = 15). Patients were treated with an average of 12.2 cycles.
“In general, we saw that treatment was well-tolerated,” Dr. Benefield said. “We also noted that a trend toward better long-term survival outcomes in individuals with very good performance status at the start of treatment. We hope to parse this out more as we add more data to our data-set, as the numbers are still too small for confident direct comparison.”
Dr. Benefield said he has treated a limited number of patients in their 90s who were highly physical fit for their age and “very eager” to be treated. “They wanted to do anything they could to maintain their lifestyle,” he said. “In my experience, aggressive supportive care and close monitoring for developing toxicities has been most helpful.”
The researchers don’t know the causes of death of many of the patients, and it’s not clear how they fared in their final days. Still, Dr. Benefield said, “extending someone's life by more than 1 year with relatively low risk of adverse effects is reasonable.”
Oncologist Melisa Wong, MD, MAS, of the University of California, San Francisco, reviewed the study and said in an interview that it “a valuable description of outcomes for nonagenarians receiving immunotherapy in the VA healthcare system.” As she noted, “many other studies of immunotherapy among older adults focus on patients aged 65 or 70 and older while very few focus on octogenarians or nonagenarians.”
The findings suggest that “it is important to move beyond chronological age and assess patients’ physiologic age through a geriatric assessment,” she said. “Geriatric assessment-derived risk scores have been shown to predict chemotherapy toxicity for older adults and research to develop similar tools for immunotherapy are ongoing.”
However, she cautioned that older patients may become suffer so much from the most common side effect of immunotherapy -- fatigue – that “their independence is at stake.”
“Some of these patient choose to stop immunotherapy because the side effects aren’t worth it anymore,” she said. “The challenge for oncologists is not knowing in advance which patients will fall into each of these categories.”
She added that her geriatric oncology research focuses on improving risk stratification for older adults, such as those who are at least 70 with lung adenocarcinoma.
Oncologist Grant R. Williams, MD, MSPH, director of the Cancer & Aging Program at the University of Alabama at Birmingham, agreed in an interview that comprehensive geriatric assessments are important to guide treatment in the oldest adults. “In addition, it is important to elicit the goals of treatment as well,” he said. “For older adults that are fit or at least pre-frail and desire aggressive treatment, immunotherapy is a very reasonable approach, particularly when patients are closely monitored for side effects.”
No study funding is reported. The authors report no disclosures. Dr. Wong discloses an immediate family member is an employee and stock holder of Genentech. Dr. Williams has no disclosures.
Patients in their 90s with cancer tolerated immunotherapy well with few serious adverse effects, and they lived for an average of 1.6 years after treatment, a small new study within the US Department of Veterans Affairs (VA) health system reports.
Only 6.3% of 48 patients who were treated with immune checkpoint inhibitors experienced the most severe types of side effects – grade III/IV events – and a total of 27% had any adverse effects, according to the report, which was presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and inperson in Denver Colorado, September 24 to September 26, 2021.
“Our project should help give confidence to oncologists treating the elderly,” said Andrew Joseph Benefield, MD, a hematology/oncology fellow at Wake Forest Baptist Medical Center, in an interview. “Immunotherapy can be given safely and likely effectively in select individuals over the age of 90 with good performance status.”
Benefield and colleagues launched their study to gain insight into a little-studied area: How does cancer treatment affects nonagenarians? “I think many oncologists have been in a situation where they encounter an individual over the age of 90 years who has a good performance status, and they've wondered if immunotherapy would be helpful and safe, particularly given our knowledge of waning immune strength as people age,” he said.
The researchers retrospectively tracked patients with cancer who were at least 90 years old from 2016 to 2017 and were treated with immune checkpoint inhibitors. Most were fit or fairly fit with Eastern Cooperative Oncology Group (ECOG) physical performance scales of 0 or 1 (n = 26), and nearly all had cancer in stage IV (n = 42). Melanoma was the most common type of cancer (n = 19), followed by non-small-cell lung cancer (n = 15). Patients were treated with an average of 12.2 cycles.
“In general, we saw that treatment was well-tolerated,” Dr. Benefield said. “We also noted that a trend toward better long-term survival outcomes in individuals with very good performance status at the start of treatment. We hope to parse this out more as we add more data to our data-set, as the numbers are still too small for confident direct comparison.”
Dr. Benefield said he has treated a limited number of patients in their 90s who were highly physical fit for their age and “very eager” to be treated. “They wanted to do anything they could to maintain their lifestyle,” he said. “In my experience, aggressive supportive care and close monitoring for developing toxicities has been most helpful.”
The researchers don’t know the causes of death of many of the patients, and it’s not clear how they fared in their final days. Still, Dr. Benefield said, “extending someone's life by more than 1 year with relatively low risk of adverse effects is reasonable.”
Oncologist Melisa Wong, MD, MAS, of the University of California, San Francisco, reviewed the study and said in an interview that it “a valuable description of outcomes for nonagenarians receiving immunotherapy in the VA healthcare system.” As she noted, “many other studies of immunotherapy among older adults focus on patients aged 65 or 70 and older while very few focus on octogenarians or nonagenarians.”
The findings suggest that “it is important to move beyond chronological age and assess patients’ physiologic age through a geriatric assessment,” she said. “Geriatric assessment-derived risk scores have been shown to predict chemotherapy toxicity for older adults and research to develop similar tools for immunotherapy are ongoing.”
However, she cautioned that older patients may become suffer so much from the most common side effect of immunotherapy -- fatigue – that “their independence is at stake.”
“Some of these patient choose to stop immunotherapy because the side effects aren’t worth it anymore,” she said. “The challenge for oncologists is not knowing in advance which patients will fall into each of these categories.”
She added that her geriatric oncology research focuses on improving risk stratification for older adults, such as those who are at least 70 with lung adenocarcinoma.
Oncologist Grant R. Williams, MD, MSPH, director of the Cancer & Aging Program at the University of Alabama at Birmingham, agreed in an interview that comprehensive geriatric assessments are important to guide treatment in the oldest adults. “In addition, it is important to elicit the goals of treatment as well,” he said. “For older adults that are fit or at least pre-frail and desire aggressive treatment, immunotherapy is a very reasonable approach, particularly when patients are closely monitored for side effects.”
No study funding is reported. The authors report no disclosures. Dr. Wong discloses an immediate family member is an employee and stock holder of Genentech. Dr. Williams has no disclosures.
U.S. seniors’ pandemic care worst among wealthy nations: Survey
Older adults in the United States – particularly among Black and Latino/Hispanic populations – experienced worse access to health care for chronic conditions during the pandemic than older adults in 10 other wealthy countries, according to findings from The Commonwealth Fund’s 2021 International Health Policy Survey of Older Adults released today.
David Blumenthal, MD, president of The Commonwealth Fund, said during a press briefing that surveying the senior population in the United States is particularly insightful because it is the only group with the universal coverage of Medicare, which offers a more direct comparison with other countries’ universal health care coverage.
More than one-third (37%) of older U.S. adults with multiple chronic conditions reported pandemic-related disruptions in their care – higher than rates in Canada, the Netherlands, and U.K. In Germany, only 11% had canceled or postponed appointments.
The survey was conducted between March and June 2021 and included responses from 18,477 adults age 65 and older in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and U.K., and U.S. adults age 60 and older.
Among older adults who need help with daily activities, those in the United States, Canada, U.K., and Australia were the most likely to say they did not receive needed services from professionals or family members.
In the United States, 23% of people who said they needed help with activities such as housework, meal preparation, and medication management experienced a disruption in care because services were canceled or very limited during the pandemic. For comparison, only 8% of seniors in Germany and 11% of seniors in the Netherlands did not receive help with basic daily activities.
Many U.S. seniors used up savings
“Nearly one in five older adults report that they used up their savings or lost their main source of income because of the pandemic. We see much lower rates in other countries like Germany, Switzerland, the Netherlands, and Sweden,” Reginald D. Williams, vice president for international health policy and practice innovations at The Commonwealth Fund, said during a briefing.
Older U.S. adults reported economic difficulties related to the pandemic at a rate of up to six times that of other countries, he said.
The differences by race were stark. While 19% of U.S. seniors overall experienced financial hardships related to the pandemic, 32% of Black seniors and 39% of Latino/Hispanic seniors in the United States experienced hardships. Germany had the lowest rate, at 3% overall.
“As the COVID-19 pandemic in the United States continues to evolve,” Mr. Williams said, “finding ways to reduce care barriers – affordability and connecting adults to usual sources of primary care, enhancing access to economic supports and social services – can help narrow the gaps.”
Dr. Blumenthal said that even though “Medicare is a critical lifeline,” it has flaws.
“Medicare plans have significant gaps that leave beneficiaries vulnerable to sizable out-of-pocket expenses,” he said.
Placing caps on out-of-pocket costs and covering more health services, such as dental, vision, and hearing care, could help make the population less vulnerable, Dr. Blumenthal said. “The chronic lack of security facing U.S. seniors, especially those who are Black or Hispanic, is exacerbating the pandemic’s devastating toll,” he added.
Dr. Blumenthal and Mr. Williams have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults in the United States – particularly among Black and Latino/Hispanic populations – experienced worse access to health care for chronic conditions during the pandemic than older adults in 10 other wealthy countries, according to findings from The Commonwealth Fund’s 2021 International Health Policy Survey of Older Adults released today.
David Blumenthal, MD, president of The Commonwealth Fund, said during a press briefing that surveying the senior population in the United States is particularly insightful because it is the only group with the universal coverage of Medicare, which offers a more direct comparison with other countries’ universal health care coverage.
More than one-third (37%) of older U.S. adults with multiple chronic conditions reported pandemic-related disruptions in their care – higher than rates in Canada, the Netherlands, and U.K. In Germany, only 11% had canceled or postponed appointments.
The survey was conducted between March and June 2021 and included responses from 18,477 adults age 65 and older in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and U.K., and U.S. adults age 60 and older.
Among older adults who need help with daily activities, those in the United States, Canada, U.K., and Australia were the most likely to say they did not receive needed services from professionals or family members.
In the United States, 23% of people who said they needed help with activities such as housework, meal preparation, and medication management experienced a disruption in care because services were canceled or very limited during the pandemic. For comparison, only 8% of seniors in Germany and 11% of seniors in the Netherlands did not receive help with basic daily activities.
Many U.S. seniors used up savings
“Nearly one in five older adults report that they used up their savings or lost their main source of income because of the pandemic. We see much lower rates in other countries like Germany, Switzerland, the Netherlands, and Sweden,” Reginald D. Williams, vice president for international health policy and practice innovations at The Commonwealth Fund, said during a briefing.
Older U.S. adults reported economic difficulties related to the pandemic at a rate of up to six times that of other countries, he said.
The differences by race were stark. While 19% of U.S. seniors overall experienced financial hardships related to the pandemic, 32% of Black seniors and 39% of Latino/Hispanic seniors in the United States experienced hardships. Germany had the lowest rate, at 3% overall.
“As the COVID-19 pandemic in the United States continues to evolve,” Mr. Williams said, “finding ways to reduce care barriers – affordability and connecting adults to usual sources of primary care, enhancing access to economic supports and social services – can help narrow the gaps.”
Dr. Blumenthal said that even though “Medicare is a critical lifeline,” it has flaws.
“Medicare plans have significant gaps that leave beneficiaries vulnerable to sizable out-of-pocket expenses,” he said.
Placing caps on out-of-pocket costs and covering more health services, such as dental, vision, and hearing care, could help make the population less vulnerable, Dr. Blumenthal said. “The chronic lack of security facing U.S. seniors, especially those who are Black or Hispanic, is exacerbating the pandemic’s devastating toll,” he added.
Dr. Blumenthal and Mr. Williams have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults in the United States – particularly among Black and Latino/Hispanic populations – experienced worse access to health care for chronic conditions during the pandemic than older adults in 10 other wealthy countries, according to findings from The Commonwealth Fund’s 2021 International Health Policy Survey of Older Adults released today.
David Blumenthal, MD, president of The Commonwealth Fund, said during a press briefing that surveying the senior population in the United States is particularly insightful because it is the only group with the universal coverage of Medicare, which offers a more direct comparison with other countries’ universal health care coverage.
More than one-third (37%) of older U.S. adults with multiple chronic conditions reported pandemic-related disruptions in their care – higher than rates in Canada, the Netherlands, and U.K. In Germany, only 11% had canceled or postponed appointments.
The survey was conducted between March and June 2021 and included responses from 18,477 adults age 65 and older in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and U.K., and U.S. adults age 60 and older.
Among older adults who need help with daily activities, those in the United States, Canada, U.K., and Australia were the most likely to say they did not receive needed services from professionals or family members.
In the United States, 23% of people who said they needed help with activities such as housework, meal preparation, and medication management experienced a disruption in care because services were canceled or very limited during the pandemic. For comparison, only 8% of seniors in Germany and 11% of seniors in the Netherlands did not receive help with basic daily activities.
Many U.S. seniors used up savings
“Nearly one in five older adults report that they used up their savings or lost their main source of income because of the pandemic. We see much lower rates in other countries like Germany, Switzerland, the Netherlands, and Sweden,” Reginald D. Williams, vice president for international health policy and practice innovations at The Commonwealth Fund, said during a briefing.
Older U.S. adults reported economic difficulties related to the pandemic at a rate of up to six times that of other countries, he said.
The differences by race were stark. While 19% of U.S. seniors overall experienced financial hardships related to the pandemic, 32% of Black seniors and 39% of Latino/Hispanic seniors in the United States experienced hardships. Germany had the lowest rate, at 3% overall.
“As the COVID-19 pandemic in the United States continues to evolve,” Mr. Williams said, “finding ways to reduce care barriers – affordability and connecting adults to usual sources of primary care, enhancing access to economic supports and social services – can help narrow the gaps.”
Dr. Blumenthal said that even though “Medicare is a critical lifeline,” it has flaws.
“Medicare plans have significant gaps that leave beneficiaries vulnerable to sizable out-of-pocket expenses,” he said.
Placing caps on out-of-pocket costs and covering more health services, such as dental, vision, and hearing care, could help make the population less vulnerable, Dr. Blumenthal said. “The chronic lack of security facing U.S. seniors, especially those who are Black or Hispanic, is exacerbating the pandemic’s devastating toll,” he added.
Dr. Blumenthal and Mr. Williams have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADHD a new risk factor for Alzheimer’s?
results from a large, multigenerational study show.
“The findings suggest there are common genetic and/or environmental contributions to the association between ADHD and dementia,” study investigator Zheng Chang, PhD, from the department of medical epidemiology and biostatistics at Karolinska Institute, Stockholm, said in a statement.
“There have been few studies previously on the link between ADHD and dementia, all with limited sample size,” Dr. Chang said in an interview.
“This is the first study to look at ADHD and dementia within extended families. It’s a large population-based study including over 2 million individuals and their over 5 million biological relatives,” he noted.
The study was published online Sept. 9, 2021, in the journal Alzheimer’s & Dementia.
Shared familial risk
The researchers identified roughly 2.1 million people born in Sweden between 1980 and 2001. Overall, 3.2% of the cohort had a diagnosis of ADHD.
Using national registries, they linked these individuals to more than 5 million of their biological relatives including parents, grandparents, uncles, and aunts and determined which of these relatives developed dementia over time.
In adjusted analyses, parents of individuals with ADHD had 34% higher risk for any dementia than parents of those without ADHD (hazard ratio, 1.34; 95% CI, 1.11-1.63).
The risk for AD, the most common type of dementia, was 55% higher in parents of individuals with ADHD (HR, 1.55; 95% CI, 1.26-1.89).
Individuals with ADHD were more likely to have parents with early-onset dementia rather than late-onset dementia. However, the absolute risk for dementia was low for the parent cohort: Only 0.17% of the parents were diagnosed with dementia during follow-up.
The association between ADHD and dementia was not as strong for second-degree relatives of individuals with ADHD. For example, grandparents of individuals with ADHD had a 10% increased risk for dementia, compared with grandparents of individuals without ADHD.
The finding of attenuated associations with decreasing genetic relatedness (parents > grandparents and uncles/aunts), points to shared familial risk between ADHD and AD, the researchers said.
There could be “undiscovered genetic variants that contribute to either traits or family-wide environmental risk factors, such as socioeconomic status, that may have an impact on the association,” Dr. Chang said in the news release.
“There are no direct clinical implications from this study, but research like this could lead to further research with goals for improved detection, prevention, and treatment,” he said in an interview.
More questions than answers
Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association that the way different brain diseases are linked “is a question the Alzheimer’s Association is often asked, and it is a part of our funding portfolio to get that question answered.”
This study looking at ADHD and dementia is “intriguing,” Dr. Snyder said, “because, right now, there is limited information available. That said, this is an association study; it shows that two things are somehow connected. Because of how the study was conducted, it does not – and cannot – prove causation,” Dr. Snyder said. “But it is interesting all the same. More research is needed to uncover specifically why and how these two diseases are related. That might eventually give us insight into how to manage risk or even improve treatment.”
The study was supported by grants from the Swedish Council for Health, Working Life and Welfare, the Swedish Research Council, the Swedish Brain Foundation, the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie, the Fredrik & Ingrid Thurings Stiftelse, and the Karolinska Institutet Research Foundation. Dr. Chang and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results from a large, multigenerational study show.
“The findings suggest there are common genetic and/or environmental contributions to the association between ADHD and dementia,” study investigator Zheng Chang, PhD, from the department of medical epidemiology and biostatistics at Karolinska Institute, Stockholm, said in a statement.
“There have been few studies previously on the link between ADHD and dementia, all with limited sample size,” Dr. Chang said in an interview.
“This is the first study to look at ADHD and dementia within extended families. It’s a large population-based study including over 2 million individuals and their over 5 million biological relatives,” he noted.
The study was published online Sept. 9, 2021, in the journal Alzheimer’s & Dementia.
Shared familial risk
The researchers identified roughly 2.1 million people born in Sweden between 1980 and 2001. Overall, 3.2% of the cohort had a diagnosis of ADHD.
Using national registries, they linked these individuals to more than 5 million of their biological relatives including parents, grandparents, uncles, and aunts and determined which of these relatives developed dementia over time.
In adjusted analyses, parents of individuals with ADHD had 34% higher risk for any dementia than parents of those without ADHD (hazard ratio, 1.34; 95% CI, 1.11-1.63).
The risk for AD, the most common type of dementia, was 55% higher in parents of individuals with ADHD (HR, 1.55; 95% CI, 1.26-1.89).
Individuals with ADHD were more likely to have parents with early-onset dementia rather than late-onset dementia. However, the absolute risk for dementia was low for the parent cohort: Only 0.17% of the parents were diagnosed with dementia during follow-up.
The association between ADHD and dementia was not as strong for second-degree relatives of individuals with ADHD. For example, grandparents of individuals with ADHD had a 10% increased risk for dementia, compared with grandparents of individuals without ADHD.
The finding of attenuated associations with decreasing genetic relatedness (parents > grandparents and uncles/aunts), points to shared familial risk between ADHD and AD, the researchers said.
There could be “undiscovered genetic variants that contribute to either traits or family-wide environmental risk factors, such as socioeconomic status, that may have an impact on the association,” Dr. Chang said in the news release.
“There are no direct clinical implications from this study, but research like this could lead to further research with goals for improved detection, prevention, and treatment,” he said in an interview.
More questions than answers
Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association that the way different brain diseases are linked “is a question the Alzheimer’s Association is often asked, and it is a part of our funding portfolio to get that question answered.”
This study looking at ADHD and dementia is “intriguing,” Dr. Snyder said, “because, right now, there is limited information available. That said, this is an association study; it shows that two things are somehow connected. Because of how the study was conducted, it does not – and cannot – prove causation,” Dr. Snyder said. “But it is interesting all the same. More research is needed to uncover specifically why and how these two diseases are related. That might eventually give us insight into how to manage risk or even improve treatment.”
The study was supported by grants from the Swedish Council for Health, Working Life and Welfare, the Swedish Research Council, the Swedish Brain Foundation, the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie, the Fredrik & Ingrid Thurings Stiftelse, and the Karolinska Institutet Research Foundation. Dr. Chang and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results from a large, multigenerational study show.
“The findings suggest there are common genetic and/or environmental contributions to the association between ADHD and dementia,” study investigator Zheng Chang, PhD, from the department of medical epidemiology and biostatistics at Karolinska Institute, Stockholm, said in a statement.
“There have been few studies previously on the link between ADHD and dementia, all with limited sample size,” Dr. Chang said in an interview.
“This is the first study to look at ADHD and dementia within extended families. It’s a large population-based study including over 2 million individuals and their over 5 million biological relatives,” he noted.
The study was published online Sept. 9, 2021, in the journal Alzheimer’s & Dementia.
Shared familial risk
The researchers identified roughly 2.1 million people born in Sweden between 1980 and 2001. Overall, 3.2% of the cohort had a diagnosis of ADHD.
Using national registries, they linked these individuals to more than 5 million of their biological relatives including parents, grandparents, uncles, and aunts and determined which of these relatives developed dementia over time.
In adjusted analyses, parents of individuals with ADHD had 34% higher risk for any dementia than parents of those without ADHD (hazard ratio, 1.34; 95% CI, 1.11-1.63).
The risk for AD, the most common type of dementia, was 55% higher in parents of individuals with ADHD (HR, 1.55; 95% CI, 1.26-1.89).
Individuals with ADHD were more likely to have parents with early-onset dementia rather than late-onset dementia. However, the absolute risk for dementia was low for the parent cohort: Only 0.17% of the parents were diagnosed with dementia during follow-up.
The association between ADHD and dementia was not as strong for second-degree relatives of individuals with ADHD. For example, grandparents of individuals with ADHD had a 10% increased risk for dementia, compared with grandparents of individuals without ADHD.
The finding of attenuated associations with decreasing genetic relatedness (parents > grandparents and uncles/aunts), points to shared familial risk between ADHD and AD, the researchers said.
There could be “undiscovered genetic variants that contribute to either traits or family-wide environmental risk factors, such as socioeconomic status, that may have an impact on the association,” Dr. Chang said in the news release.
“There are no direct clinical implications from this study, but research like this could lead to further research with goals for improved detection, prevention, and treatment,” he said in an interview.
More questions than answers
Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association that the way different brain diseases are linked “is a question the Alzheimer’s Association is often asked, and it is a part of our funding portfolio to get that question answered.”
This study looking at ADHD and dementia is “intriguing,” Dr. Snyder said, “because, right now, there is limited information available. That said, this is an association study; it shows that two things are somehow connected. Because of how the study was conducted, it does not – and cannot – prove causation,” Dr. Snyder said. “But it is interesting all the same. More research is needed to uncover specifically why and how these two diseases are related. That might eventually give us insight into how to manage risk or even improve treatment.”
The study was supported by grants from the Swedish Council for Health, Working Life and Welfare, the Swedish Research Council, the Swedish Brain Foundation, the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie, the Fredrik & Ingrid Thurings Stiftelse, and the Karolinska Institutet Research Foundation. Dr. Chang and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Number of global deaths by suicide increased over 30 years
The overall global number of deaths by suicide increased by almost 20,000 during the past 30 years, new research shows.
The increase occurred despite a significant decrease in age-specific suicide rates from 1990 through 2019, according to data from the Global Burden of Disease Study 2019.
Population growth, population aging, and changes in population age structure may explain the increase in number of suicide deaths, the investigators note.
“As suicide rates are highest among the elderly (70 years or above) for both genders in almost all regions of the world, the rapidly aging population globally will pose huge challenges for the reduction in the number of suicide deaths in the future,” write the researchers, led by Paul Siu Fai Yip, PhD, of the HKJC Center for Suicide Research and Prevention, University of Hong Kong, China.
The findings were published online Aug. 16 in Injury Prevention.
Global public health concern
Around the world, approximately 800,000 individuals die by suicide each year, while many others attempt suicide. Yet suicide has not received the same level of attention as other global public health concerns, such as HIV/AIDS and cancer, the investigators write.
They examined data from the Global Burden of Disease Study 2019 to assess how demographic and epidemiologic factors contributed to the number of suicide deaths during the past 30 years.
The researchers also analyzed relationships between population growth, population age structure, income level, and gender- and age-specific suicide rates.
The Global Burden of Disease Study 2019 includes information from 204 countries about 369 diseases and injuries by age and gender. The dataset also includes population estimates for each year by location, age group, and gender.
In their analysis, the investigators looked at changes in suicide rates and the number of suicide deaths from 1990 to 2019 by gender and age group in the four income level regions defined by the World Bank. These categories include low-income, lower-middle–income, upper-middle–income, and high-income regions.
Number of deaths versus suicide rates
The number of deaths was 738,799 in 1990 and 758,696 in 2019.
The largest increase in deaths occurred in the lower-middle–income region, where the number of suicide deaths increased by 72,550 (from 232,340 to 304,890).
Population growth (300,942; 1,512.5%) was the major contributor to the overall increase in total number of suicide deaths. The second largest contributor was population age structure (189,512; 952.4%).
However, the effects of these factors were offset to a large extent by the effect of reduction in overall suicide rates (−470,556; −2,364.9%).
Interestingly, the overall suicide rate per 100,000 population decreased from 13.8 in 1990 to 9.8 in 2019.
The upper-middle–income region had the largest decline (−6.25 per 100,000), and the high-income region had the smallest decline (−1.77 per 100,000). Suicide rates also decreased in lower-middle–income (−2.51 per 100,000) and low-income regions (−1.96 per 100,000).
Reasons for the declines across all regions “have yet to be determined,” write the investigators. International efforts coordinated by the United Nations and World Health Organization likely contributed to these declines, they add.
‘Imbalance of resources’
The overall reduction in suicide rate of −4.01 per 100,000 “was mainly due” to reduction in age-specific suicide rates (−6.09; 152%), the researchers report.
This effect was partly offset, however, by the effect of the changing population age structure (2.08; −52%). In the high-income–level region, for example, the reduction in age-specific suicide rate (−3.83; 216.3%) was greater than the increase resulting from the change in population age structure (2.06; −116.3%).
“The overall contribution of population age structure mainly came from the 45-64 (565.2%) and 65+ (528.7%) age groups,” the investigators write. “This effect was observed in middle-income– as well as high-income–level regions, reflecting the global effect of population aging.”
They add that world populations will “experience pronounced and historically unprecedented aging in the coming decades” because of increasing life expectancy and declining fertility.
Men, but not women, had a notable increase in total number of suicide deaths. The significant effect of male population growth (177,128; 890.2% vs. 123,814; 622.3% for women) and male population age structure (120,186; 604.0% vs. 69,325; 348.4%) were the main factors that explained this increase, the investigators note.
However, from 1990 to 2019, the overall suicide rate per 100,000 men decreased from 16.6 to 13.5 (–3.09). The decline in overall suicide rate was even greater for women, from 11.0 to 6.1 (–4.91).
This finding was particularly notable in the upper-middle–income region (–8.12 women vs. –4.37 men per 100,000).
“This study highlighted the considerable imbalance of the resources in carrying out suicide prevention work, especially in low-income and middle-income countries,” the investigators write.
“It is time to revisit this situation to ensure that sufficient resources can be redeployed globally to meet the future challenges,” they add.
The study was funded by a Humanities and Social Sciences Prestigious Fellowship, which Dr. Yip received. He declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The overall global number of deaths by suicide increased by almost 20,000 during the past 30 years, new research shows.
The increase occurred despite a significant decrease in age-specific suicide rates from 1990 through 2019, according to data from the Global Burden of Disease Study 2019.
Population growth, population aging, and changes in population age structure may explain the increase in number of suicide deaths, the investigators note.
“As suicide rates are highest among the elderly (70 years or above) for both genders in almost all regions of the world, the rapidly aging population globally will pose huge challenges for the reduction in the number of suicide deaths in the future,” write the researchers, led by Paul Siu Fai Yip, PhD, of the HKJC Center for Suicide Research and Prevention, University of Hong Kong, China.
The findings were published online Aug. 16 in Injury Prevention.
Global public health concern
Around the world, approximately 800,000 individuals die by suicide each year, while many others attempt suicide. Yet suicide has not received the same level of attention as other global public health concerns, such as HIV/AIDS and cancer, the investigators write.
They examined data from the Global Burden of Disease Study 2019 to assess how demographic and epidemiologic factors contributed to the number of suicide deaths during the past 30 years.
The researchers also analyzed relationships between population growth, population age structure, income level, and gender- and age-specific suicide rates.
The Global Burden of Disease Study 2019 includes information from 204 countries about 369 diseases and injuries by age and gender. The dataset also includes population estimates for each year by location, age group, and gender.
In their analysis, the investigators looked at changes in suicide rates and the number of suicide deaths from 1990 to 2019 by gender and age group in the four income level regions defined by the World Bank. These categories include low-income, lower-middle–income, upper-middle–income, and high-income regions.
Number of deaths versus suicide rates
The number of deaths was 738,799 in 1990 and 758,696 in 2019.
The largest increase in deaths occurred in the lower-middle–income region, where the number of suicide deaths increased by 72,550 (from 232,340 to 304,890).
Population growth (300,942; 1,512.5%) was the major contributor to the overall increase in total number of suicide deaths. The second largest contributor was population age structure (189,512; 952.4%).
However, the effects of these factors were offset to a large extent by the effect of reduction in overall suicide rates (−470,556; −2,364.9%).
Interestingly, the overall suicide rate per 100,000 population decreased from 13.8 in 1990 to 9.8 in 2019.
The upper-middle–income region had the largest decline (−6.25 per 100,000), and the high-income region had the smallest decline (−1.77 per 100,000). Suicide rates also decreased in lower-middle–income (−2.51 per 100,000) and low-income regions (−1.96 per 100,000).
Reasons for the declines across all regions “have yet to be determined,” write the investigators. International efforts coordinated by the United Nations and World Health Organization likely contributed to these declines, they add.
‘Imbalance of resources’
The overall reduction in suicide rate of −4.01 per 100,000 “was mainly due” to reduction in age-specific suicide rates (−6.09; 152%), the researchers report.
This effect was partly offset, however, by the effect of the changing population age structure (2.08; −52%). In the high-income–level region, for example, the reduction in age-specific suicide rate (−3.83; 216.3%) was greater than the increase resulting from the change in population age structure (2.06; −116.3%).
“The overall contribution of population age structure mainly came from the 45-64 (565.2%) and 65+ (528.7%) age groups,” the investigators write. “This effect was observed in middle-income– as well as high-income–level regions, reflecting the global effect of population aging.”
They add that world populations will “experience pronounced and historically unprecedented aging in the coming decades” because of increasing life expectancy and declining fertility.
Men, but not women, had a notable increase in total number of suicide deaths. The significant effect of male population growth (177,128; 890.2% vs. 123,814; 622.3% for women) and male population age structure (120,186; 604.0% vs. 69,325; 348.4%) were the main factors that explained this increase, the investigators note.
However, from 1990 to 2019, the overall suicide rate per 100,000 men decreased from 16.6 to 13.5 (–3.09). The decline in overall suicide rate was even greater for women, from 11.0 to 6.1 (–4.91).
This finding was particularly notable in the upper-middle–income region (–8.12 women vs. –4.37 men per 100,000).
“This study highlighted the considerable imbalance of the resources in carrying out suicide prevention work, especially in low-income and middle-income countries,” the investigators write.
“It is time to revisit this situation to ensure that sufficient resources can be redeployed globally to meet the future challenges,” they add.
The study was funded by a Humanities and Social Sciences Prestigious Fellowship, which Dr. Yip received. He declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The overall global number of deaths by suicide increased by almost 20,000 during the past 30 years, new research shows.
The increase occurred despite a significant decrease in age-specific suicide rates from 1990 through 2019, according to data from the Global Burden of Disease Study 2019.
Population growth, population aging, and changes in population age structure may explain the increase in number of suicide deaths, the investigators note.
“As suicide rates are highest among the elderly (70 years or above) for both genders in almost all regions of the world, the rapidly aging population globally will pose huge challenges for the reduction in the number of suicide deaths in the future,” write the researchers, led by Paul Siu Fai Yip, PhD, of the HKJC Center for Suicide Research and Prevention, University of Hong Kong, China.
The findings were published online Aug. 16 in Injury Prevention.
Global public health concern
Around the world, approximately 800,000 individuals die by suicide each year, while many others attempt suicide. Yet suicide has not received the same level of attention as other global public health concerns, such as HIV/AIDS and cancer, the investigators write.
They examined data from the Global Burden of Disease Study 2019 to assess how demographic and epidemiologic factors contributed to the number of suicide deaths during the past 30 years.
The researchers also analyzed relationships between population growth, population age structure, income level, and gender- and age-specific suicide rates.
The Global Burden of Disease Study 2019 includes information from 204 countries about 369 diseases and injuries by age and gender. The dataset also includes population estimates for each year by location, age group, and gender.
In their analysis, the investigators looked at changes in suicide rates and the number of suicide deaths from 1990 to 2019 by gender and age group in the four income level regions defined by the World Bank. These categories include low-income, lower-middle–income, upper-middle–income, and high-income regions.
Number of deaths versus suicide rates
The number of deaths was 738,799 in 1990 and 758,696 in 2019.
The largest increase in deaths occurred in the lower-middle–income region, where the number of suicide deaths increased by 72,550 (from 232,340 to 304,890).
Population growth (300,942; 1,512.5%) was the major contributor to the overall increase in total number of suicide deaths. The second largest contributor was population age structure (189,512; 952.4%).
However, the effects of these factors were offset to a large extent by the effect of reduction in overall suicide rates (−470,556; −2,364.9%).
Interestingly, the overall suicide rate per 100,000 population decreased from 13.8 in 1990 to 9.8 in 2019.
The upper-middle–income region had the largest decline (−6.25 per 100,000), and the high-income region had the smallest decline (−1.77 per 100,000). Suicide rates also decreased in lower-middle–income (−2.51 per 100,000) and low-income regions (−1.96 per 100,000).
Reasons for the declines across all regions “have yet to be determined,” write the investigators. International efforts coordinated by the United Nations and World Health Organization likely contributed to these declines, they add.
‘Imbalance of resources’
The overall reduction in suicide rate of −4.01 per 100,000 “was mainly due” to reduction in age-specific suicide rates (−6.09; 152%), the researchers report.
This effect was partly offset, however, by the effect of the changing population age structure (2.08; −52%). In the high-income–level region, for example, the reduction in age-specific suicide rate (−3.83; 216.3%) was greater than the increase resulting from the change in population age structure (2.06; −116.3%).
“The overall contribution of population age structure mainly came from the 45-64 (565.2%) and 65+ (528.7%) age groups,” the investigators write. “This effect was observed in middle-income– as well as high-income–level regions, reflecting the global effect of population aging.”
They add that world populations will “experience pronounced and historically unprecedented aging in the coming decades” because of increasing life expectancy and declining fertility.
Men, but not women, had a notable increase in total number of suicide deaths. The significant effect of male population growth (177,128; 890.2% vs. 123,814; 622.3% for women) and male population age structure (120,186; 604.0% vs. 69,325; 348.4%) were the main factors that explained this increase, the investigators note.
However, from 1990 to 2019, the overall suicide rate per 100,000 men decreased from 16.6 to 13.5 (–3.09). The decline in overall suicide rate was even greater for women, from 11.0 to 6.1 (–4.91).
This finding was particularly notable in the upper-middle–income region (–8.12 women vs. –4.37 men per 100,000).
“This study highlighted the considerable imbalance of the resources in carrying out suicide prevention work, especially in low-income and middle-income countries,” the investigators write.
“It is time to revisit this situation to ensure that sufficient resources can be redeployed globally to meet the future challenges,” they add.
The study was funded by a Humanities and Social Sciences Prestigious Fellowship, which Dr. Yip received. He declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.

