User login
Patterns of malignancies in patients with HIV-AIDS: a single institution observational study
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
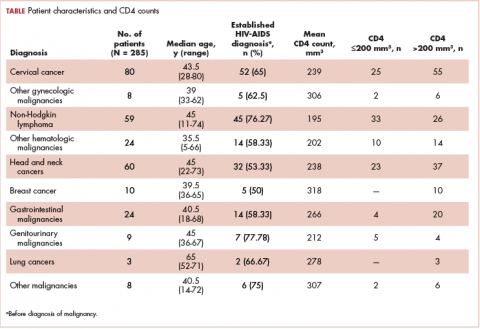
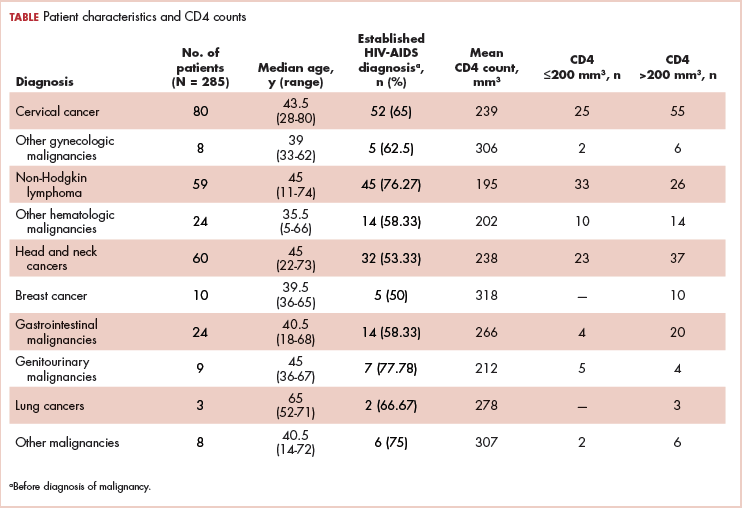
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
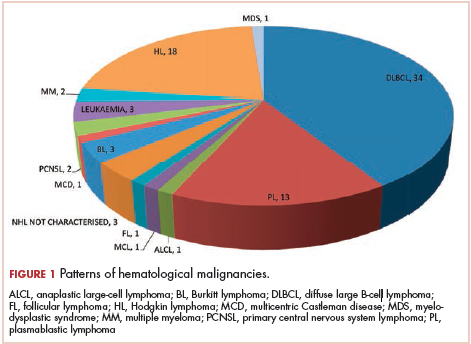
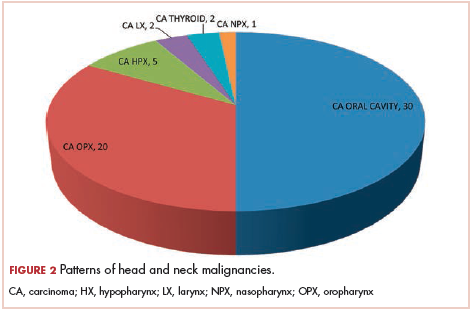
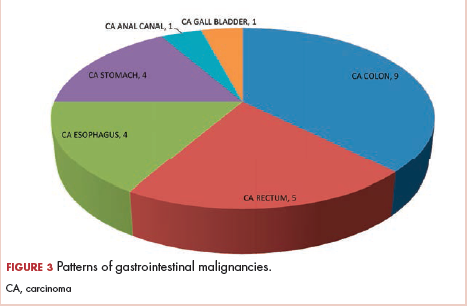
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
Immunotherapy may hold the key to defeating virally associated cancers
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
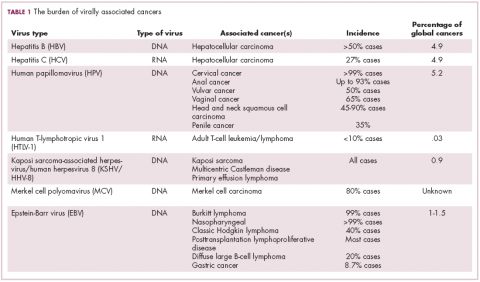
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
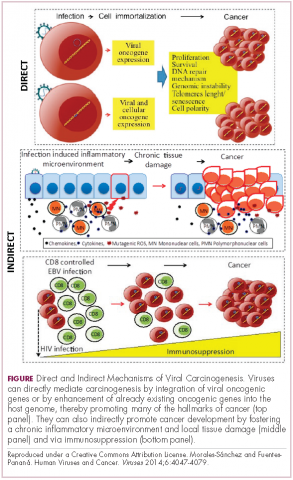
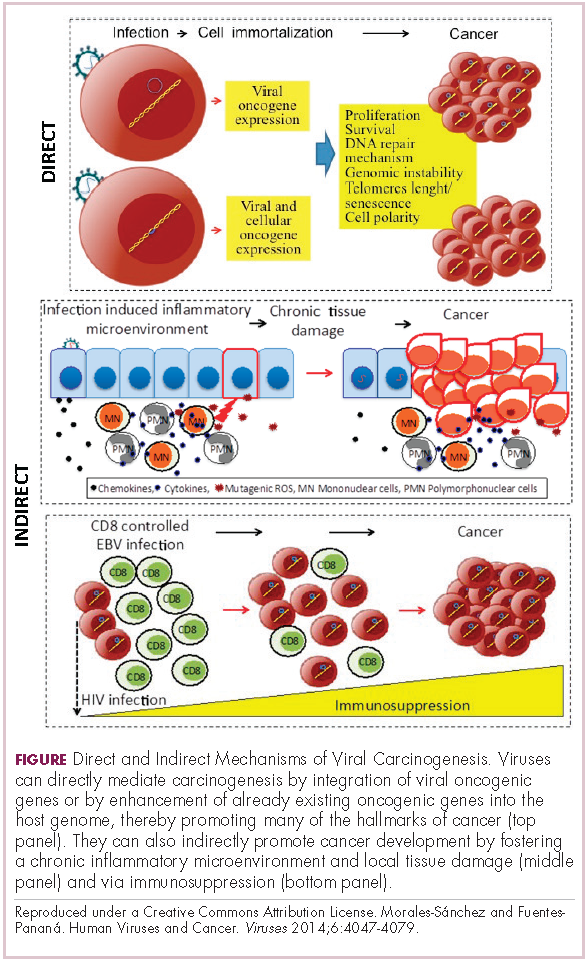
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
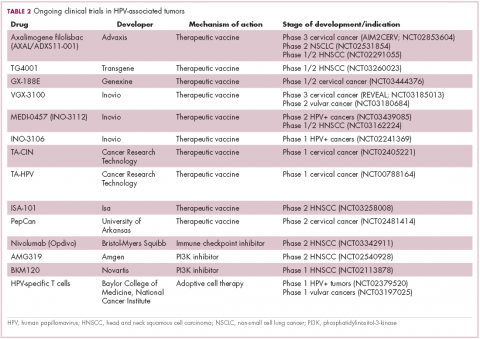
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
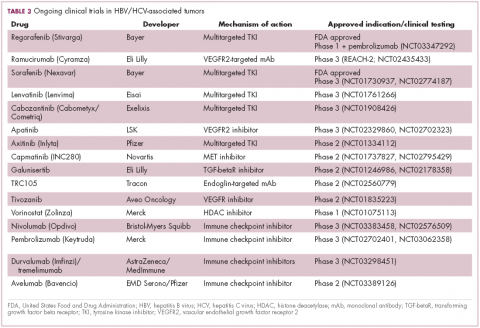
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
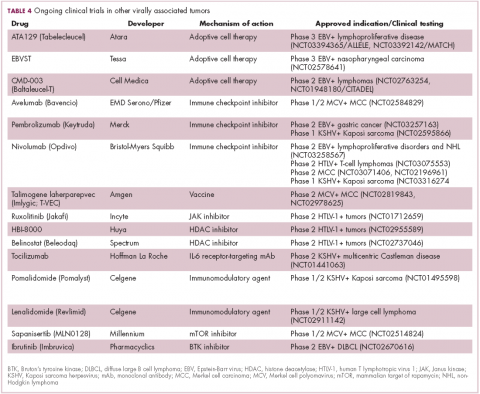
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
MIS for cervical cancer: Is it not for anyone or not for everyone?
Shock waves moved through the gynecologic oncology world on Oct. 31, 2018, when the New England Journal of Medicine published two papers on survival outcomes for women undergoing surgery for early stage cervical cancer.
The first was a randomized controlled trial of laparotomy and minimally invasive surgery (MIS) for radical hysterectomy called the LACC trial.1 In the multicenter, international trial of 631 women, the primary objective was disease-specific survival (cervical cancer–related deaths) and was powered to detect noninferiority of the MIS approach when compared with laparotomy. The trial was closed early when investigators noted a lower than expected rate of 3-year, disease-free survival (91% vs. 97%) from cervical cancer in the MIS group, which was made up of 84% laparoscopic and 16% robotic approaches, versus laparotomy. There were 19 deaths in the MIS group observed versus three in the laparotomy group. The conclusions of the trial were that MIS surgery is associated with inferior cervical cancer survival.
In the second study, authors analyzed data from large U.S. databases – the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Program – to collect all-cause mortality for patients with early-stage cervical cancer who had undergone radical hysterectomy during 2010-2013.2 Among 2,461 observed results, 1,225 had undergone MIS surgery with the majority (79.8%) via robotic-assistance. Women undergoing MIS approaches had smaller, lower grade tumors; were more likely to be white, privately insured, and of a higher income; and had surgery later in the cohort and by nonacademic centers. The researchers adjusted for risk factors with an analytic process called propensity-score weighting, which matched the groups more closely in an attempt to minimize confounders. They identified higher all-cause mortality among women who were treated with an MIS approach, compared with those treated with laparotomy (hazard ratio, 1.65). They also observed a significant decline in the survival from cervical cancer annually that corresponded to the uptake of MIS radical hysterectomies.
In the wake of these publications, many concluded that gynecologic oncologists should no longer offer a minimally invasive approach for radical hysterectomy. Certainly level I evidence published in a highly influential journal is compelling, and the consistency in findings over two studies adds further weight to the results. However, was this the correct conclusion to draw from these results? Surgeons who had been performing MIS radical hysterectomies for many years with favorable outcomes are challenging this and are raising questions about external generalizability and whether these findings were driven by the surgery itself or by the surgeon.
The studies’ authors proposed hypotheses for their results that implicate the surgical route rather than the surgeon; however, these seem ad hoc and not well supported by data, including the authors’ own data. The first was the hypothesis that cervical tumors were being disrupted and disseminated through the use of uterine manipulators in MIS approaches. However, cervical cancers are fairly routinely “disrupted” by preoperative cone biopsies, loop electrosurgical excision procedures (LEEP), and sharp biopsies, which are arguably more invasive than placement of a manipulator. Uterine manipulators routinely are used in endometrial cancer surgeries, in which the manipulator is embedded within the tumor, without an associated negative survival effect in randomized trials.3 Additionally, not all surgeons utilize manipulators for radical hysterectomies, and these studies did not measure or report on their use; therefore, it is impossible to know whether, and by what magnitude, manipulators played a role. Finally, if uterine manipulators are the explanation for inferior survival, surely the recommendation should be to discourage their use, rather than abandon the MIS approach all together.
The other explanation offered was exposure of the tumor to CO2 gas. This seems an even less plausible explanation because CO2 gas is routinely used in MIS cancer surgeries for endometrial, prostate, gastric, and colorectal surgeries and is used as insufflation for malignant interventional endoscopies without a significant deleterious effect. Additionally, the cervix is not exposed to CO2 until colpotomy at the procedure’s end – and only briefly. The in vitro studies implicating a negative effect of simulated CO2 pneumoperitoneum are neither compelling nor consistent.4,5
I would like to propose another hypothesis for the results: surgical proficiency. Surgery, unlike medical interventions, is not a simple variable that is dichotomous – performed or not. Surgeons do not randomly select operative approaches for patients. We select surgical approaches based on patients’ circumstances and surgeon factors, including our own mastery of the various techniques. and any surgeon recognizes this if he or she has observed more than one surgeon or has attempted a procedure via different routes. While some procedures, such as extrafascial hysterectomy for endometrial cancer, are relatively straightforward and surgeon capabilities are more equitable across different approaches, cervical cancer surgery is quite different.
Early-stage cervical cancer primarily exerts radial growth into the cervical stroma and parametria. Curative surgical excision requires broadly negative margins through this tissue, a so called “radical hysterectomy.” The radicality of hysterectomy has been categorized in stages, acknowledging that different sized lesions require different volumes of parametrial resection to achieve adequate clearance from the tumor.6 In doing so, the surgeon must skeletonize and mobilize the distal ureters, cardinal ligament webs, and uterosacral ligaments. These structures are in close proximity to major vascular and neural structures. Hence, the radical hysterectomy is, without dispute, a technically challenging procedure.
Minimally invasive surgery further handicaps the surgeon by eliminating manual contact with tissue, and relying on complex instrumentation, electrosurgical modalities, and loss of haptics. The learning curve for MIS radical hysterectomy is further attenuated by their relative infrequency. Therefore, it makes sense that, when the MIS approach is randomly assigned to surgeons (such as in the LACC trial) or broadly and independently applied (as in the retrospective series), one might see variations in skill, quality, and outcomes, including oncologic outcomes.
The retrospective study by Melamed et al. acknowledged that surgeon skill and volume may contribute to their findings but stated that, because of the nature of their source data, they were unable to explain why they observed their results. The LACC trial attempted to overcome the issue of surgeon skill by ensuring all surgeons were from high-volume sites and had videos reviewed of their cases. However, the videos were chosen by the surgeons themselves and not available for audit in the study’s supplemental material. The LACC trial was conducted over a 9-year period across 33 sites and enrolled a total of 631 subjects. This equates to an enrollment of approximately two patients per site per year and either reflects extremely low-volume sites or highly selective patient enrollment. If the latter, what was different about the unenrolled patients and what was the preferred chosen route of surgery for them?
All 34 recurrences occurred in patients from just 14 of the 33 sites in the LACC trial. That means that less than half of the sites contributed to all of the recurrences. The authors provided no details on the specific sites, surgeons, or accrual rates in their manuscript or supplemental materials. Therefore, readers are unable to know what was different about those sites; whether they contributed the most patients and, therefore, the most recurrences; or whether they were low-volume sites with lower quality.
While margin status, positive or negative, was reported, there was no data captured regarding volume of resected parametrial tissue, or relative distance from tumor to margin, both of which might provide the reader with a better appraisal of surgeon proficiency and consistency in radicality of the two approaches. The incidence of locoregional (pelvic) recurrences were higher in the MIS arm, which is expected if there were inadequate margins around the laparoscopically resected tumors.
Finally, the authors of the LACC trial observed equivalent rates of postoperative complications between the laparotomy and MIS groups. The main virtue for MIS approaches is the reduction in perioperative morbidity. To observe no perioperative morbidity benefit in the MIS group is a red flag suggesting that these surgeons may not have achieved proficiency with the MIS approach.
Despite these arguments, the results of these studies should be taken seriously. Clearly, it is apparent that preservation of oncologic outcomes is not guaranteed with MIS radical hysterectomy, and it should not be the chosen approach for all patients and all surgeons. However, rather than entirely abandoning this less morbid approach, I would argue that it is a call to arms for gynecologic oncologists to self-evaluate. We should know our own data with respect to case volumes, perioperative complications, and cancer-related recurrence and death.
Perhaps MIS radical hysterectomies should be consolidated among high-volume surgeons with demonstrated good outcomes? Just as has been done for rectal cancer surgery with positive effect, we should establish accredited centers of excellence.7 We also need to improve the training of surgeons in novel, difficult techniques, as well as enhance the sophistication of MIS equipment such as improved instrumentation, haptics, and vision-guided surgery (for example, real-time intraoperative assessment of the tumor margins).
Let’s not take a wholesale step backwards to the surgical approaches of a 100 years ago just because they are more straightforward. Let’s do a better job of advancing the quality of what we do for our patients in the future.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
2. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923.
3. J Clin Oncol. 2012 Mar 1;30(7):695-700.
4. Med Sci Monit. 2014 Dec 1;20:2497-503.
5. Surg Endosc. 2006 Oct;20(10):1556-9.
6. Gynecol Oncol. 2011 Aug;122(2):264-8.
7. Surgery. 2016 Mar;159(3):736-48.
Shock waves moved through the gynecologic oncology world on Oct. 31, 2018, when the New England Journal of Medicine published two papers on survival outcomes for women undergoing surgery for early stage cervical cancer.
The first was a randomized controlled trial of laparotomy and minimally invasive surgery (MIS) for radical hysterectomy called the LACC trial.1 In the multicenter, international trial of 631 women, the primary objective was disease-specific survival (cervical cancer–related deaths) and was powered to detect noninferiority of the MIS approach when compared with laparotomy. The trial was closed early when investigators noted a lower than expected rate of 3-year, disease-free survival (91% vs. 97%) from cervical cancer in the MIS group, which was made up of 84% laparoscopic and 16% robotic approaches, versus laparotomy. There were 19 deaths in the MIS group observed versus three in the laparotomy group. The conclusions of the trial were that MIS surgery is associated with inferior cervical cancer survival.
In the second study, authors analyzed data from large U.S. databases – the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Program – to collect all-cause mortality for patients with early-stage cervical cancer who had undergone radical hysterectomy during 2010-2013.2 Among 2,461 observed results, 1,225 had undergone MIS surgery with the majority (79.8%) via robotic-assistance. Women undergoing MIS approaches had smaller, lower grade tumors; were more likely to be white, privately insured, and of a higher income; and had surgery later in the cohort and by nonacademic centers. The researchers adjusted for risk factors with an analytic process called propensity-score weighting, which matched the groups more closely in an attempt to minimize confounders. They identified higher all-cause mortality among women who were treated with an MIS approach, compared with those treated with laparotomy (hazard ratio, 1.65). They also observed a significant decline in the survival from cervical cancer annually that corresponded to the uptake of MIS radical hysterectomies.
In the wake of these publications, many concluded that gynecologic oncologists should no longer offer a minimally invasive approach for radical hysterectomy. Certainly level I evidence published in a highly influential journal is compelling, and the consistency in findings over two studies adds further weight to the results. However, was this the correct conclusion to draw from these results? Surgeons who had been performing MIS radical hysterectomies for many years with favorable outcomes are challenging this and are raising questions about external generalizability and whether these findings were driven by the surgery itself or by the surgeon.
The studies’ authors proposed hypotheses for their results that implicate the surgical route rather than the surgeon; however, these seem ad hoc and not well supported by data, including the authors’ own data. The first was the hypothesis that cervical tumors were being disrupted and disseminated through the use of uterine manipulators in MIS approaches. However, cervical cancers are fairly routinely “disrupted” by preoperative cone biopsies, loop electrosurgical excision procedures (LEEP), and sharp biopsies, which are arguably more invasive than placement of a manipulator. Uterine manipulators routinely are used in endometrial cancer surgeries, in which the manipulator is embedded within the tumor, without an associated negative survival effect in randomized trials.3 Additionally, not all surgeons utilize manipulators for radical hysterectomies, and these studies did not measure or report on their use; therefore, it is impossible to know whether, and by what magnitude, manipulators played a role. Finally, if uterine manipulators are the explanation for inferior survival, surely the recommendation should be to discourage their use, rather than abandon the MIS approach all together.
The other explanation offered was exposure of the tumor to CO2 gas. This seems an even less plausible explanation because CO2 gas is routinely used in MIS cancer surgeries for endometrial, prostate, gastric, and colorectal surgeries and is used as insufflation for malignant interventional endoscopies without a significant deleterious effect. Additionally, the cervix is not exposed to CO2 until colpotomy at the procedure’s end – and only briefly. The in vitro studies implicating a negative effect of simulated CO2 pneumoperitoneum are neither compelling nor consistent.4,5
I would like to propose another hypothesis for the results: surgical proficiency. Surgery, unlike medical interventions, is not a simple variable that is dichotomous – performed or not. Surgeons do not randomly select operative approaches for patients. We select surgical approaches based on patients’ circumstances and surgeon factors, including our own mastery of the various techniques. and any surgeon recognizes this if he or she has observed more than one surgeon or has attempted a procedure via different routes. While some procedures, such as extrafascial hysterectomy for endometrial cancer, are relatively straightforward and surgeon capabilities are more equitable across different approaches, cervical cancer surgery is quite different.
Early-stage cervical cancer primarily exerts radial growth into the cervical stroma and parametria. Curative surgical excision requires broadly negative margins through this tissue, a so called “radical hysterectomy.” The radicality of hysterectomy has been categorized in stages, acknowledging that different sized lesions require different volumes of parametrial resection to achieve adequate clearance from the tumor.6 In doing so, the surgeon must skeletonize and mobilize the distal ureters, cardinal ligament webs, and uterosacral ligaments. These structures are in close proximity to major vascular and neural structures. Hence, the radical hysterectomy is, without dispute, a technically challenging procedure.
Minimally invasive surgery further handicaps the surgeon by eliminating manual contact with tissue, and relying on complex instrumentation, electrosurgical modalities, and loss of haptics. The learning curve for MIS radical hysterectomy is further attenuated by their relative infrequency. Therefore, it makes sense that, when the MIS approach is randomly assigned to surgeons (such as in the LACC trial) or broadly and independently applied (as in the retrospective series), one might see variations in skill, quality, and outcomes, including oncologic outcomes.
The retrospective study by Melamed et al. acknowledged that surgeon skill and volume may contribute to their findings but stated that, because of the nature of their source data, they were unable to explain why they observed their results. The LACC trial attempted to overcome the issue of surgeon skill by ensuring all surgeons were from high-volume sites and had videos reviewed of their cases. However, the videos were chosen by the surgeons themselves and not available for audit in the study’s supplemental material. The LACC trial was conducted over a 9-year period across 33 sites and enrolled a total of 631 subjects. This equates to an enrollment of approximately two patients per site per year and either reflects extremely low-volume sites or highly selective patient enrollment. If the latter, what was different about the unenrolled patients and what was the preferred chosen route of surgery for them?
All 34 recurrences occurred in patients from just 14 of the 33 sites in the LACC trial. That means that less than half of the sites contributed to all of the recurrences. The authors provided no details on the specific sites, surgeons, or accrual rates in their manuscript or supplemental materials. Therefore, readers are unable to know what was different about those sites; whether they contributed the most patients and, therefore, the most recurrences; or whether they were low-volume sites with lower quality.
While margin status, positive or negative, was reported, there was no data captured regarding volume of resected parametrial tissue, or relative distance from tumor to margin, both of which might provide the reader with a better appraisal of surgeon proficiency and consistency in radicality of the two approaches. The incidence of locoregional (pelvic) recurrences were higher in the MIS arm, which is expected if there were inadequate margins around the laparoscopically resected tumors.
Finally, the authors of the LACC trial observed equivalent rates of postoperative complications between the laparotomy and MIS groups. The main virtue for MIS approaches is the reduction in perioperative morbidity. To observe no perioperative morbidity benefit in the MIS group is a red flag suggesting that these surgeons may not have achieved proficiency with the MIS approach.
Despite these arguments, the results of these studies should be taken seriously. Clearly, it is apparent that preservation of oncologic outcomes is not guaranteed with MIS radical hysterectomy, and it should not be the chosen approach for all patients and all surgeons. However, rather than entirely abandoning this less morbid approach, I would argue that it is a call to arms for gynecologic oncologists to self-evaluate. We should know our own data with respect to case volumes, perioperative complications, and cancer-related recurrence and death.
Perhaps MIS radical hysterectomies should be consolidated among high-volume surgeons with demonstrated good outcomes? Just as has been done for rectal cancer surgery with positive effect, we should establish accredited centers of excellence.7 We also need to improve the training of surgeons in novel, difficult techniques, as well as enhance the sophistication of MIS equipment such as improved instrumentation, haptics, and vision-guided surgery (for example, real-time intraoperative assessment of the tumor margins).
Let’s not take a wholesale step backwards to the surgical approaches of a 100 years ago just because they are more straightforward. Let’s do a better job of advancing the quality of what we do for our patients in the future.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
2. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923.
3. J Clin Oncol. 2012 Mar 1;30(7):695-700.
4. Med Sci Monit. 2014 Dec 1;20:2497-503.
5. Surg Endosc. 2006 Oct;20(10):1556-9.
6. Gynecol Oncol. 2011 Aug;122(2):264-8.
7. Surgery. 2016 Mar;159(3):736-48.
Shock waves moved through the gynecologic oncology world on Oct. 31, 2018, when the New England Journal of Medicine published two papers on survival outcomes for women undergoing surgery for early stage cervical cancer.
The first was a randomized controlled trial of laparotomy and minimally invasive surgery (MIS) for radical hysterectomy called the LACC trial.1 In the multicenter, international trial of 631 women, the primary objective was disease-specific survival (cervical cancer–related deaths) and was powered to detect noninferiority of the MIS approach when compared with laparotomy. The trial was closed early when investigators noted a lower than expected rate of 3-year, disease-free survival (91% vs. 97%) from cervical cancer in the MIS group, which was made up of 84% laparoscopic and 16% robotic approaches, versus laparotomy. There were 19 deaths in the MIS group observed versus three in the laparotomy group. The conclusions of the trial were that MIS surgery is associated with inferior cervical cancer survival.
In the second study, authors analyzed data from large U.S. databases – the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Program – to collect all-cause mortality for patients with early-stage cervical cancer who had undergone radical hysterectomy during 2010-2013.2 Among 2,461 observed results, 1,225 had undergone MIS surgery with the majority (79.8%) via robotic-assistance. Women undergoing MIS approaches had smaller, lower grade tumors; were more likely to be white, privately insured, and of a higher income; and had surgery later in the cohort and by nonacademic centers. The researchers adjusted for risk factors with an analytic process called propensity-score weighting, which matched the groups more closely in an attempt to minimize confounders. They identified higher all-cause mortality among women who were treated with an MIS approach, compared with those treated with laparotomy (hazard ratio, 1.65). They also observed a significant decline in the survival from cervical cancer annually that corresponded to the uptake of MIS radical hysterectomies.
In the wake of these publications, many concluded that gynecologic oncologists should no longer offer a minimally invasive approach for radical hysterectomy. Certainly level I evidence published in a highly influential journal is compelling, and the consistency in findings over two studies adds further weight to the results. However, was this the correct conclusion to draw from these results? Surgeons who had been performing MIS radical hysterectomies for many years with favorable outcomes are challenging this and are raising questions about external generalizability and whether these findings were driven by the surgery itself or by the surgeon.
The studies’ authors proposed hypotheses for their results that implicate the surgical route rather than the surgeon; however, these seem ad hoc and not well supported by data, including the authors’ own data. The first was the hypothesis that cervical tumors were being disrupted and disseminated through the use of uterine manipulators in MIS approaches. However, cervical cancers are fairly routinely “disrupted” by preoperative cone biopsies, loop electrosurgical excision procedures (LEEP), and sharp biopsies, which are arguably more invasive than placement of a manipulator. Uterine manipulators routinely are used in endometrial cancer surgeries, in which the manipulator is embedded within the tumor, without an associated negative survival effect in randomized trials.3 Additionally, not all surgeons utilize manipulators for radical hysterectomies, and these studies did not measure or report on their use; therefore, it is impossible to know whether, and by what magnitude, manipulators played a role. Finally, if uterine manipulators are the explanation for inferior survival, surely the recommendation should be to discourage their use, rather than abandon the MIS approach all together.
The other explanation offered was exposure of the tumor to CO2 gas. This seems an even less plausible explanation because CO2 gas is routinely used in MIS cancer surgeries for endometrial, prostate, gastric, and colorectal surgeries and is used as insufflation for malignant interventional endoscopies without a significant deleterious effect. Additionally, the cervix is not exposed to CO2 until colpotomy at the procedure’s end – and only briefly. The in vitro studies implicating a negative effect of simulated CO2 pneumoperitoneum are neither compelling nor consistent.4,5
I would like to propose another hypothesis for the results: surgical proficiency. Surgery, unlike medical interventions, is not a simple variable that is dichotomous – performed or not. Surgeons do not randomly select operative approaches for patients. We select surgical approaches based on patients’ circumstances and surgeon factors, including our own mastery of the various techniques. and any surgeon recognizes this if he or she has observed more than one surgeon or has attempted a procedure via different routes. While some procedures, such as extrafascial hysterectomy for endometrial cancer, are relatively straightforward and surgeon capabilities are more equitable across different approaches, cervical cancer surgery is quite different.
Early-stage cervical cancer primarily exerts radial growth into the cervical stroma and parametria. Curative surgical excision requires broadly negative margins through this tissue, a so called “radical hysterectomy.” The radicality of hysterectomy has been categorized in stages, acknowledging that different sized lesions require different volumes of parametrial resection to achieve adequate clearance from the tumor.6 In doing so, the surgeon must skeletonize and mobilize the distal ureters, cardinal ligament webs, and uterosacral ligaments. These structures are in close proximity to major vascular and neural structures. Hence, the radical hysterectomy is, without dispute, a technically challenging procedure.
Minimally invasive surgery further handicaps the surgeon by eliminating manual contact with tissue, and relying on complex instrumentation, electrosurgical modalities, and loss of haptics. The learning curve for MIS radical hysterectomy is further attenuated by their relative infrequency. Therefore, it makes sense that, when the MIS approach is randomly assigned to surgeons (such as in the LACC trial) or broadly and independently applied (as in the retrospective series), one might see variations in skill, quality, and outcomes, including oncologic outcomes.
The retrospective study by Melamed et al. acknowledged that surgeon skill and volume may contribute to their findings but stated that, because of the nature of their source data, they were unable to explain why they observed their results. The LACC trial attempted to overcome the issue of surgeon skill by ensuring all surgeons were from high-volume sites and had videos reviewed of their cases. However, the videos were chosen by the surgeons themselves and not available for audit in the study’s supplemental material. The LACC trial was conducted over a 9-year period across 33 sites and enrolled a total of 631 subjects. This equates to an enrollment of approximately two patients per site per year and either reflects extremely low-volume sites or highly selective patient enrollment. If the latter, what was different about the unenrolled patients and what was the preferred chosen route of surgery for them?
All 34 recurrences occurred in patients from just 14 of the 33 sites in the LACC trial. That means that less than half of the sites contributed to all of the recurrences. The authors provided no details on the specific sites, surgeons, or accrual rates in their manuscript or supplemental materials. Therefore, readers are unable to know what was different about those sites; whether they contributed the most patients and, therefore, the most recurrences; or whether they were low-volume sites with lower quality.
While margin status, positive or negative, was reported, there was no data captured regarding volume of resected parametrial tissue, or relative distance from tumor to margin, both of which might provide the reader with a better appraisal of surgeon proficiency and consistency in radicality of the two approaches. The incidence of locoregional (pelvic) recurrences were higher in the MIS arm, which is expected if there were inadequate margins around the laparoscopically resected tumors.
Finally, the authors of the LACC trial observed equivalent rates of postoperative complications between the laparotomy and MIS groups. The main virtue for MIS approaches is the reduction in perioperative morbidity. To observe no perioperative morbidity benefit in the MIS group is a red flag suggesting that these surgeons may not have achieved proficiency with the MIS approach.
Despite these arguments, the results of these studies should be taken seriously. Clearly, it is apparent that preservation of oncologic outcomes is not guaranteed with MIS radical hysterectomy, and it should not be the chosen approach for all patients and all surgeons. However, rather than entirely abandoning this less morbid approach, I would argue that it is a call to arms for gynecologic oncologists to self-evaluate. We should know our own data with respect to case volumes, perioperative complications, and cancer-related recurrence and death.
Perhaps MIS radical hysterectomies should be consolidated among high-volume surgeons with demonstrated good outcomes? Just as has been done for rectal cancer surgery with positive effect, we should establish accredited centers of excellence.7 We also need to improve the training of surgeons in novel, difficult techniques, as well as enhance the sophistication of MIS equipment such as improved instrumentation, haptics, and vision-guided surgery (for example, real-time intraoperative assessment of the tumor margins).
Let’s not take a wholesale step backwards to the surgical approaches of a 100 years ago just because they are more straightforward. Let’s do a better job of advancing the quality of what we do for our patients in the future.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
2. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923.
3. J Clin Oncol. 2012 Mar 1;30(7):695-700.
4. Med Sci Monit. 2014 Dec 1;20:2497-503.
5. Surg Endosc. 2006 Oct;20(10):1556-9.
6. Gynecol Oncol. 2011 Aug;122(2):264-8.
7. Surgery. 2016 Mar;159(3):736-48.
Circulating tumor DNA identified by fragment size
Circulating tumor DNA could be effectively isolated from plasma by focusing on a particular range of fragment sizes, which paves the way for noninvasive genomic analysis of tumor DNA, new research suggests.
In a study of 344 plasma samples from 200 patients with 18 cancer types and 65 samples from healthy controls, DNA fragment length could be used to distinguish circulating tumor DNA (ctDNA) from other cell-free DNA (cfDNA), investigators reported in Science Translational Medicine.
“We hypothesized that we could improve the sensitivity for noninvasive cancer genomics by selective sequencing of ctDNA fragments and by leveraging differences in the biology that determine DNA fragmentation,” wrote Florent Mouliere, PhD, from the Cancer Research UK Cambridge Institute, and coauthors.
Cell-free plasma fragments are often cleaved at around 167 base pairs in length and differences in length between circulating fetal and maternal DNA are already used for noninvasive prenatal diagnosis. However, the authors said that only a few studies, with conflicting results, have looked at the size distribution of tumor-derived cfDNA.
The study used two approaches to determining the size profile of mutant ctDNA. The first looked at tumor and nontumor cfDNA in mice with human ovarian cancer xenografts and the second approach used deep sequencing in 19 cancer patients. This revealed that tumor-derived cfDNA was most commonly found in fragments between 90-150 base pairs or 250-320 base pairs in size.
The researchers also noted that mutant circulating tumor DNA was generally more fragmented than nonmutant cfDNA and that patients with untreated advanced cancer showed consistently shorter lengths of mutant DNA.
The next question was whether size selection and other biological properties – such as somatic alterations – of the cfDNA could be used to enhance detection of ctDNA via machine learning technology.
Two models, designed to distinguish between healthy and cancerous samples, were developed using 153 samples, then validated on two datasets of 94 and 83 samples.
One of these models correctly classified cancerous samples in 94% of samples from patients with cancers known to have high levels of ctDNA – colorectal, cholangiocarcinoma, ovarian, breast, and melanoma – and in 65% of samples from low-ctDNA cancers – pancreatic, renal, and glioma.
Another model focused just on fragmentation patterns and was still able to distinguish cancer samples from those of healthy controls, although with slightly reduced area under the curve.
“Our results indicate that exploiting fundamental properties of cfDNA with fragment-specific analyses can allow more sensitive evaluation of ctDNA,” the authors wrote. “We identified features that could determine the presence and amount of ctDNA in plasma samples, without a prior knowledge of somatic aberrations.”
The authors pointed out that size selection of DNA fragments was relatively simple and cheap, and was also compatible with other genome-wide and targeted genomic analyses, “greatly increasing the potential value and utility of liquid biopsies as well as the cost-effectiveness of cfDNA sequencing.”
However, they cautioned that their catalogue had focused solely on double-stranded DNA and was subject to potential biases from the DNA extraction and sequencing methods they used in the study. They also commented that other biological effects could help refine the analysis of ctDNA.
“Other bodily fluids [urine, cerebrospinal fluid, and saliva], different nucleic acids and structures, altered mechanisms of release into circulation, or sample processing methods could exhibit varying fragment size signatures and could offer additional exploitable biological patterns for selective sequencing,” they wrote.
The study was supported by the University of Cambridge, Cancer Research UK, and the Engineering and Physical Sciences Research Council. Research supporting the study was also funded by the European Research Council, the National Institute for Health Research Cambridge, National Cancer Research Network, Cambridge Experimental Cancer Medicine Centre, Hutchison Whampoa, Target Ovarian Cancer, the Medical Research Council, and AstraZeneca. Three authors are cofounders, shareholders, and officers/consultants in a company specializing in ctDNA analysis. One author declared research funding and advisory board fees from private industry. Seven authors are listed on related patents.
SOURCE: Mouliere F et al. Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aat4921.
Cell-free DNA analysis has tremendous diagnostic potential and so is a very active area of research. In this study, researchers were able to identify five variables and develop models for the detection of cancer following analysis of circulating tumor DNA. One of these models based on DNA fragmentation pattern performed very well, and so fragment length analyses could develop into a general test for the presence of cancer.
However confirmation of these findings in large, multicenter clinical trials is still needed. There is also the problem that size selection can result in a loss of circulating tumor DNA for analysis or may introduce biases. We also need to understand the mechanisms underpinning the different fragment size patterns seen in the study. But this study still substantially extends the potential of cell-free, DNA-based diagnostic tests.
Ellen Heitzer, PhD, and Michael R. Speicher, MD, are from the Medical University of Graz (Austria). These comments are taken from an accompanying editorial (Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aav3873). Both authors declared research funding from Servier and Dr. Heitzer declared laboratory research funding from Freenome and PreAnalytiX.
Cell-free DNA analysis has tremendous diagnostic potential and so is a very active area of research. In this study, researchers were able to identify five variables and develop models for the detection of cancer following analysis of circulating tumor DNA. One of these models based on DNA fragmentation pattern performed very well, and so fragment length analyses could develop into a general test for the presence of cancer.
However confirmation of these findings in large, multicenter clinical trials is still needed. There is also the problem that size selection can result in a loss of circulating tumor DNA for analysis or may introduce biases. We also need to understand the mechanisms underpinning the different fragment size patterns seen in the study. But this study still substantially extends the potential of cell-free, DNA-based diagnostic tests.
Ellen Heitzer, PhD, and Michael R. Speicher, MD, are from the Medical University of Graz (Austria). These comments are taken from an accompanying editorial (Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aav3873). Both authors declared research funding from Servier and Dr. Heitzer declared laboratory research funding from Freenome and PreAnalytiX.
Cell-free DNA analysis has tremendous diagnostic potential and so is a very active area of research. In this study, researchers were able to identify five variables and develop models for the detection of cancer following analysis of circulating tumor DNA. One of these models based on DNA fragmentation pattern performed very well, and so fragment length analyses could develop into a general test for the presence of cancer.
However confirmation of these findings in large, multicenter clinical trials is still needed. There is also the problem that size selection can result in a loss of circulating tumor DNA for analysis or may introduce biases. We also need to understand the mechanisms underpinning the different fragment size patterns seen in the study. But this study still substantially extends the potential of cell-free, DNA-based diagnostic tests.
Ellen Heitzer, PhD, and Michael R. Speicher, MD, are from the Medical University of Graz (Austria). These comments are taken from an accompanying editorial (Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aav3873). Both authors declared research funding from Servier and Dr. Heitzer declared laboratory research funding from Freenome and PreAnalytiX.
Circulating tumor DNA could be effectively isolated from plasma by focusing on a particular range of fragment sizes, which paves the way for noninvasive genomic analysis of tumor DNA, new research suggests.
In a study of 344 plasma samples from 200 patients with 18 cancer types and 65 samples from healthy controls, DNA fragment length could be used to distinguish circulating tumor DNA (ctDNA) from other cell-free DNA (cfDNA), investigators reported in Science Translational Medicine.
“We hypothesized that we could improve the sensitivity for noninvasive cancer genomics by selective sequencing of ctDNA fragments and by leveraging differences in the biology that determine DNA fragmentation,” wrote Florent Mouliere, PhD, from the Cancer Research UK Cambridge Institute, and coauthors.
Cell-free plasma fragments are often cleaved at around 167 base pairs in length and differences in length between circulating fetal and maternal DNA are already used for noninvasive prenatal diagnosis. However, the authors said that only a few studies, with conflicting results, have looked at the size distribution of tumor-derived cfDNA.
The study used two approaches to determining the size profile of mutant ctDNA. The first looked at tumor and nontumor cfDNA in mice with human ovarian cancer xenografts and the second approach used deep sequencing in 19 cancer patients. This revealed that tumor-derived cfDNA was most commonly found in fragments between 90-150 base pairs or 250-320 base pairs in size.
The researchers also noted that mutant circulating tumor DNA was generally more fragmented than nonmutant cfDNA and that patients with untreated advanced cancer showed consistently shorter lengths of mutant DNA.
The next question was whether size selection and other biological properties – such as somatic alterations – of the cfDNA could be used to enhance detection of ctDNA via machine learning technology.
Two models, designed to distinguish between healthy and cancerous samples, were developed using 153 samples, then validated on two datasets of 94 and 83 samples.
One of these models correctly classified cancerous samples in 94% of samples from patients with cancers known to have high levels of ctDNA – colorectal, cholangiocarcinoma, ovarian, breast, and melanoma – and in 65% of samples from low-ctDNA cancers – pancreatic, renal, and glioma.
Another model focused just on fragmentation patterns and was still able to distinguish cancer samples from those of healthy controls, although with slightly reduced area under the curve.
“Our results indicate that exploiting fundamental properties of cfDNA with fragment-specific analyses can allow more sensitive evaluation of ctDNA,” the authors wrote. “We identified features that could determine the presence and amount of ctDNA in plasma samples, without a prior knowledge of somatic aberrations.”
The authors pointed out that size selection of DNA fragments was relatively simple and cheap, and was also compatible with other genome-wide and targeted genomic analyses, “greatly increasing the potential value and utility of liquid biopsies as well as the cost-effectiveness of cfDNA sequencing.”
However, they cautioned that their catalogue had focused solely on double-stranded DNA and was subject to potential biases from the DNA extraction and sequencing methods they used in the study. They also commented that other biological effects could help refine the analysis of ctDNA.
“Other bodily fluids [urine, cerebrospinal fluid, and saliva], different nucleic acids and structures, altered mechanisms of release into circulation, or sample processing methods could exhibit varying fragment size signatures and could offer additional exploitable biological patterns for selective sequencing,” they wrote.
The study was supported by the University of Cambridge, Cancer Research UK, and the Engineering and Physical Sciences Research Council. Research supporting the study was also funded by the European Research Council, the National Institute for Health Research Cambridge, National Cancer Research Network, Cambridge Experimental Cancer Medicine Centre, Hutchison Whampoa, Target Ovarian Cancer, the Medical Research Council, and AstraZeneca. Three authors are cofounders, shareholders, and officers/consultants in a company specializing in ctDNA analysis. One author declared research funding and advisory board fees from private industry. Seven authors are listed on related patents.
SOURCE: Mouliere F et al. Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aat4921.
Circulating tumor DNA could be effectively isolated from plasma by focusing on a particular range of fragment sizes, which paves the way for noninvasive genomic analysis of tumor DNA, new research suggests.
In a study of 344 plasma samples from 200 patients with 18 cancer types and 65 samples from healthy controls, DNA fragment length could be used to distinguish circulating tumor DNA (ctDNA) from other cell-free DNA (cfDNA), investigators reported in Science Translational Medicine.
“We hypothesized that we could improve the sensitivity for noninvasive cancer genomics by selective sequencing of ctDNA fragments and by leveraging differences in the biology that determine DNA fragmentation,” wrote Florent Mouliere, PhD, from the Cancer Research UK Cambridge Institute, and coauthors.
Cell-free plasma fragments are often cleaved at around 167 base pairs in length and differences in length between circulating fetal and maternal DNA are already used for noninvasive prenatal diagnosis. However, the authors said that only a few studies, with conflicting results, have looked at the size distribution of tumor-derived cfDNA.
The study used two approaches to determining the size profile of mutant ctDNA. The first looked at tumor and nontumor cfDNA in mice with human ovarian cancer xenografts and the second approach used deep sequencing in 19 cancer patients. This revealed that tumor-derived cfDNA was most commonly found in fragments between 90-150 base pairs or 250-320 base pairs in size.
The researchers also noted that mutant circulating tumor DNA was generally more fragmented than nonmutant cfDNA and that patients with untreated advanced cancer showed consistently shorter lengths of mutant DNA.
The next question was whether size selection and other biological properties – such as somatic alterations – of the cfDNA could be used to enhance detection of ctDNA via machine learning technology.
Two models, designed to distinguish between healthy and cancerous samples, were developed using 153 samples, then validated on two datasets of 94 and 83 samples.
One of these models correctly classified cancerous samples in 94% of samples from patients with cancers known to have high levels of ctDNA – colorectal, cholangiocarcinoma, ovarian, breast, and melanoma – and in 65% of samples from low-ctDNA cancers – pancreatic, renal, and glioma.
Another model focused just on fragmentation patterns and was still able to distinguish cancer samples from those of healthy controls, although with slightly reduced area under the curve.
“Our results indicate that exploiting fundamental properties of cfDNA with fragment-specific analyses can allow more sensitive evaluation of ctDNA,” the authors wrote. “We identified features that could determine the presence and amount of ctDNA in plasma samples, without a prior knowledge of somatic aberrations.”
The authors pointed out that size selection of DNA fragments was relatively simple and cheap, and was also compatible with other genome-wide and targeted genomic analyses, “greatly increasing the potential value and utility of liquid biopsies as well as the cost-effectiveness of cfDNA sequencing.”
However, they cautioned that their catalogue had focused solely on double-stranded DNA and was subject to potential biases from the DNA extraction and sequencing methods they used in the study. They also commented that other biological effects could help refine the analysis of ctDNA.
“Other bodily fluids [urine, cerebrospinal fluid, and saliva], different nucleic acids and structures, altered mechanisms of release into circulation, or sample processing methods could exhibit varying fragment size signatures and could offer additional exploitable biological patterns for selective sequencing,” they wrote.
The study was supported by the University of Cambridge, Cancer Research UK, and the Engineering and Physical Sciences Research Council. Research supporting the study was also funded by the European Research Council, the National Institute for Health Research Cambridge, National Cancer Research Network, Cambridge Experimental Cancer Medicine Centre, Hutchison Whampoa, Target Ovarian Cancer, the Medical Research Council, and AstraZeneca. Three authors are cofounders, shareholders, and officers/consultants in a company specializing in ctDNA analysis. One author declared research funding and advisory board fees from private industry. Seven authors are listed on related patents.
SOURCE: Mouliere F et al. Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aat4921.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: The size of cell-free DNA could be used to single out circulating tumor DNA.
Major finding: Circulating tumor DNA fragments are more commonly found in the 90-150 base pair range.
Study details: A study of 344 plasma samples from 200 patients with 18 cancer types and 65 samples from healthy controls.
Disclosures: The study was supported by the University of Cambridge, Cancer Research UK, and the Engineering and Physical Sciences Research Council. Research supporting the study was also funded by the European Research Council, the National Institute for Health Research Cambridge, National Cancer Research Network, Cambridge Experimental Cancer Medicine Centre, Hutchison Whampoa, Target Ovarian Cancer, the Medical Research Council, and AstraZeneca. Three authors are cofounders, shareholders, and officers/consultants in a company specializing in circulating tumor DNA analysis. One author declared research funding and advisory board fees from private industry. Seven authors are listed on related patents.
Source: Mouliere F et al. Sci Transl Med. 2018 Nov 7. doi: 10.1126/scitranslmed.aat4921.
Cervical cancer survival higher with open surgery in LACC trial
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Cervical cancer recurrence and survival rates were worse with minimally invasive vs. open surgery in a prospective study.
Major finding: Disease-free survival at 4.5 years was 86% with minimally invasive vs. 96.5% with open surgery.
Study details: The phase 3 LACC trial of more than 600 women with cervical cancer, and a population based study of nearly 2,500 women with cervical cancer.
Disclosures: Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani is a member of the OB.GYN. News editorial board, but reported having no other relevant disclosures.
Source: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
‘Woeful lack of awareness’ leads to delayed diagnoses for women with ovarian cancer
Lack of knowledge about ovarian cancer prevents many women from seeking medical attention, which delays diagnosis and treatment and may prove increasingly dangerous as incidence rises by an expected 55% over the next 2 decades, according to the World Ovarian Cancer Coalition.
Data from the coalition’s 2018 survey of women with ovarian cancer show that 18% had never heard of the disease before their diagnosis and 51% had heard of it but did not know anything about it. The Every Women Study survey, completed by 1,531 women in 44 countries, also reveals that nine out of ten had experienced symptoms prior to diagnosis but fewer than half saw a physician within a month of noticing those symptoms, the coalition said.
“This study, for the first time, provides powerful evidence of the challenges faced by women diagnosed with ovarian cancer across the world and sets an agenda for global change. We were especially shocked by the widespread, woeful lack of awareness of ovarian cancer,” Annwen Jones, coalition vice-chair and cochair of the study, said in a separate written statement.
Results varied considerably by country, and only 10 countries provided enough responses to allow comparisons: Australia (120), Brazil (52), Canada (167), Germany (141), Hungary (58), Italy (92), Japan (250), Spain (70), the United Kingdom (176), and the United States (248).
Among those comparisons, women in Germany (5.5 weeks) and Spain (7.9 weeks) were the first to visit a physician after first experiencing symptoms, while those in Italy (15.2 weeks) and the United States (12.9 weeks) were last. The United States was also longest in time from first visit to diagnosis (23.6 weeks), and Japan was the shortest (11 weeks). Despite that world-longest time to diagnosis, however, over 69% of U.S. women said that their care around the time of diagnosis was very good, which was higher than any other country, the coalition reported.
Surgery statistics were closer among countries, with an average of 94% of all women undergoing surgery to treat their ovarian cancer. The United States, at 98.3%, was second to Spain’s 98.5%, and Hungary was the largest outlier on the low side at 59%. Over 87% of all women reported having chemotherapy to treat or control their cancer, and 9.8% of women said that they had received intraperitoneal chemotherapy. In the United States, 22.5% of women received intraperitoneal therapy, compared with 0.7% for the United Kingdom.
“No one country has all the answers, and whilst there is still an urgent need for a step-change in the level of investment in research for better treatments and tools for early diagnosis, there are significant opportunities to improve survival and quality of life for women in the immediate and short term to make a series of marginal gains if these variations are addressed by individual countries,” the coalition said in the report.
Lack of knowledge about ovarian cancer prevents many women from seeking medical attention, which delays diagnosis and treatment and may prove increasingly dangerous as incidence rises by an expected 55% over the next 2 decades, according to the World Ovarian Cancer Coalition.

Data from the coalition’s 2018 survey of women with ovarian cancer show that 18% had never heard of the disease before their diagnosis and 51% had heard of it but did not know anything about it. The Every Women Study survey, completed by 1,531 women in 44 countries, also reveals that nine out of ten had experienced symptoms prior to diagnosis but fewer than half saw a physician within a month of noticing those symptoms, the coalition said.
“This study, for the first time, provides powerful evidence of the challenges faced by women diagnosed with ovarian cancer across the world and sets an agenda for global change. We were especially shocked by the widespread, woeful lack of awareness of ovarian cancer,” Annwen Jones, coalition vice-chair and cochair of the study, said in a separate written statement.
Results varied considerably by country, and only 10 countries provided enough responses to allow comparisons: Australia (120), Brazil (52), Canada (167), Germany (141), Hungary (58), Italy (92), Japan (250), Spain (70), the United Kingdom (176), and the United States (248).
Among those comparisons, women in Germany (5.5 weeks) and Spain (7.9 weeks) were the first to visit a physician after first experiencing symptoms, while those in Italy (15.2 weeks) and the United States (12.9 weeks) were last. The United States was also longest in time from first visit to diagnosis (23.6 weeks), and Japan was the shortest (11 weeks). Despite that world-longest time to diagnosis, however, over 69% of U.S. women said that their care around the time of diagnosis was very good, which was higher than any other country, the coalition reported.
Surgery statistics were closer among countries, with an average of 94% of all women undergoing surgery to treat their ovarian cancer. The United States, at 98.3%, was second to Spain’s 98.5%, and Hungary was the largest outlier on the low side at 59%. Over 87% of all women reported having chemotherapy to treat or control their cancer, and 9.8% of women said that they had received intraperitoneal chemotherapy. In the United States, 22.5% of women received intraperitoneal therapy, compared with 0.7% for the United Kingdom.
“No one country has all the answers, and whilst there is still an urgent need for a step-change in the level of investment in research for better treatments and tools for early diagnosis, there are significant opportunities to improve survival and quality of life for women in the immediate and short term to make a series of marginal gains if these variations are addressed by individual countries,” the coalition said in the report.
Lack of knowledge about ovarian cancer prevents many women from seeking medical attention, which delays diagnosis and treatment and may prove increasingly dangerous as incidence rises by an expected 55% over the next 2 decades, according to the World Ovarian Cancer Coalition.

Data from the coalition’s 2018 survey of women with ovarian cancer show that 18% had never heard of the disease before their diagnosis and 51% had heard of it but did not know anything about it. The Every Women Study survey, completed by 1,531 women in 44 countries, also reveals that nine out of ten had experienced symptoms prior to diagnosis but fewer than half saw a physician within a month of noticing those symptoms, the coalition said.
“This study, for the first time, provides powerful evidence of the challenges faced by women diagnosed with ovarian cancer across the world and sets an agenda for global change. We were especially shocked by the widespread, woeful lack of awareness of ovarian cancer,” Annwen Jones, coalition vice-chair and cochair of the study, said in a separate written statement.
Results varied considerably by country, and only 10 countries provided enough responses to allow comparisons: Australia (120), Brazil (52), Canada (167), Germany (141), Hungary (58), Italy (92), Japan (250), Spain (70), the United Kingdom (176), and the United States (248).
Among those comparisons, women in Germany (5.5 weeks) and Spain (7.9 weeks) were the first to visit a physician after first experiencing symptoms, while those in Italy (15.2 weeks) and the United States (12.9 weeks) were last. The United States was also longest in time from first visit to diagnosis (23.6 weeks), and Japan was the shortest (11 weeks). Despite that world-longest time to diagnosis, however, over 69% of U.S. women said that their care around the time of diagnosis was very good, which was higher than any other country, the coalition reported.
Surgery statistics were closer among countries, with an average of 94% of all women undergoing surgery to treat their ovarian cancer. The United States, at 98.3%, was second to Spain’s 98.5%, and Hungary was the largest outlier on the low side at 59%. Over 87% of all women reported having chemotherapy to treat or control their cancer, and 9.8% of women said that they had received intraperitoneal chemotherapy. In the United States, 22.5% of women received intraperitoneal therapy, compared with 0.7% for the United Kingdom.
“No one country has all the answers, and whilst there is still an urgent need for a step-change in the level of investment in research for better treatments and tools for early diagnosis, there are significant opportunities to improve survival and quality of life for women in the immediate and short term to make a series of marginal gains if these variations are addressed by individual countries,” the coalition said in the report.
VTE risk after gynecologic surgery lower with laparoscopic procedures
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Laparoscopic gynecologic surgery is associated with a lower risk of postoperative VTE than laparotomy.
Major finding: Laparoscopic hysterectomy was associated with a 78% lower incidence of postoperative VTE than laparotomy.
Study details: Retrospective cohort study of 37,485 patients who underwent 43,751 gynecologic surgical procedures
Disclosures: No conflicts of interest were declared.
Source: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
Intervention may improve genetic testing for HBOC
Researchers from general obstetrics and gynecology (ob.gyn.) practices in New York and Connecticut have shown that (HBOC).
Genetic screening and testing can reduce the morbidity and mortality from breast, ovarian, and endometrial cancers through prevention and early detection. Mark S. DeFrancesco, MD, of Westwood Women’s Health, Waterbury, Conn., and his colleagues reported that, in spite of the American College of Obstetricians and Gynecologists’ recommendation for ob.gyns. to regularly screen, counsel, and refer accordingly for HBOC (Obstet Gynecol. 2015;125:153843), the “incorporation of hereditary cancer risk assessment and testing remains underutilized in the [ob.gyn.] setting.” The authors have addressed this issue in their own practice with promising results and important caveats (Obstet Gynecol 2018;132:1121-9).
The intervention included a process evaluation, improvements to patient work flow, and training of providers by genetic counselors and engineering personnel from the testing laboratory (Myriad Genetics), which provided support for the study. Patients in the study completed a family history questionnaire and, those meeting National Comprehensive Cancer Center Network criteria for genetic testing, were given pretest counseling and offered testing on the same day or referral for testing within 2 weeks.
Of the 3,811 women who completed the questionnaire, 24% (906) met NCCN criteria, 90% of whom were offered testing. However, only 52% (165) of patients who agreed to testing underwent genetic evaluation. This included 70% of patients who were offered same-day testing and 35% of patients who were offered a referral appointment for testing.
Conversations about HBOC and genetic testing can be complicated and may not be a patient’s initial priority. The authors should be commended for identifying the vast majority of high-risk patients. However, only half of patients meeting criteria completed testing and 10% who should have been offered testing were not – numbers still well below target.
Incorporation of family history questionnaires should become commonplace in the generalist’s office and optimizing EHRs may be an opportunity for rapid risk interpretation. As the success of same-day genetic testing was striking, opportunities for partnerships with insurance companies and private laboratories are likely needed to make this a more feasible option. Lastly, assessing women’s knowledge and attitudes around genetic testing could help to more specifically address barriers to testing in future interventions.
Improving genetic screening and testing completion rates requires a coordinated effort. Using tools and applications to optimize convenience (same-day testing, telemedicine) and simplification (electronic screening platforms), we can strive to appropriately detect all women at high risk for hereditary breast and ovarian cancers.
Michelle Lightfoot is a gynecologic oncology fellow at the Ohio State University in Columbus. She has no relevant financial disclosures. Email her at [email protected].
Researchers from general obstetrics and gynecology (ob.gyn.) practices in New York and Connecticut have shown that (HBOC).
Genetic screening and testing can reduce the morbidity and mortality from breast, ovarian, and endometrial cancers through prevention and early detection. Mark S. DeFrancesco, MD, of Westwood Women’s Health, Waterbury, Conn., and his colleagues reported that, in spite of the American College of Obstetricians and Gynecologists’ recommendation for ob.gyns. to regularly screen, counsel, and refer accordingly for HBOC (Obstet Gynecol. 2015;125:153843), the “incorporation of hereditary cancer risk assessment and testing remains underutilized in the [ob.gyn.] setting.” The authors have addressed this issue in their own practice with promising results and important caveats (Obstet Gynecol 2018;132:1121-9).
The intervention included a process evaluation, improvements to patient work flow, and training of providers by genetic counselors and engineering personnel from the testing laboratory (Myriad Genetics), which provided support for the study. Patients in the study completed a family history questionnaire and, those meeting National Comprehensive Cancer Center Network criteria for genetic testing, were given pretest counseling and offered testing on the same day or referral for testing within 2 weeks.
Of the 3,811 women who completed the questionnaire, 24% (906) met NCCN criteria, 90% of whom were offered testing. However, only 52% (165) of patients who agreed to testing underwent genetic evaluation. This included 70% of patients who were offered same-day testing and 35% of patients who were offered a referral appointment for testing.
Conversations about HBOC and genetic testing can be complicated and may not be a patient’s initial priority. The authors should be commended for identifying the vast majority of high-risk patients. However, only half of patients meeting criteria completed testing and 10% who should have been offered testing were not – numbers still well below target.
Incorporation of family history questionnaires should become commonplace in the generalist’s office and optimizing EHRs may be an opportunity for rapid risk interpretation. As the success of same-day genetic testing was striking, opportunities for partnerships with insurance companies and private laboratories are likely needed to make this a more feasible option. Lastly, assessing women’s knowledge and attitudes around genetic testing could help to more specifically address barriers to testing in future interventions.
Improving genetic screening and testing completion rates requires a coordinated effort. Using tools and applications to optimize convenience (same-day testing, telemedicine) and simplification (electronic screening platforms), we can strive to appropriately detect all women at high risk for hereditary breast and ovarian cancers.
Michelle Lightfoot is a gynecologic oncology fellow at the Ohio State University in Columbus. She has no relevant financial disclosures. Email her at [email protected].
Researchers from general obstetrics and gynecology (ob.gyn.) practices in New York and Connecticut have shown that (HBOC).
Genetic screening and testing can reduce the morbidity and mortality from breast, ovarian, and endometrial cancers through prevention and early detection. Mark S. DeFrancesco, MD, of Westwood Women’s Health, Waterbury, Conn., and his colleagues reported that, in spite of the American College of Obstetricians and Gynecologists’ recommendation for ob.gyns. to regularly screen, counsel, and refer accordingly for HBOC (Obstet Gynecol. 2015;125:153843), the “incorporation of hereditary cancer risk assessment and testing remains underutilized in the [ob.gyn.] setting.” The authors have addressed this issue in their own practice with promising results and important caveats (Obstet Gynecol 2018;132:1121-9).
The intervention included a process evaluation, improvements to patient work flow, and training of providers by genetic counselors and engineering personnel from the testing laboratory (Myriad Genetics), which provided support for the study. Patients in the study completed a family history questionnaire and, those meeting National Comprehensive Cancer Center Network criteria for genetic testing, were given pretest counseling and offered testing on the same day or referral for testing within 2 weeks.
Of the 3,811 women who completed the questionnaire, 24% (906) met NCCN criteria, 90% of whom were offered testing. However, only 52% (165) of patients who agreed to testing underwent genetic evaluation. This included 70% of patients who were offered same-day testing and 35% of patients who were offered a referral appointment for testing.
Conversations about HBOC and genetic testing can be complicated and may not be a patient’s initial priority. The authors should be commended for identifying the vast majority of high-risk patients. However, only half of patients meeting criteria completed testing and 10% who should have been offered testing were not – numbers still well below target.
Incorporation of family history questionnaires should become commonplace in the generalist’s office and optimizing EHRs may be an opportunity for rapid risk interpretation. As the success of same-day genetic testing was striking, opportunities for partnerships with insurance companies and private laboratories are likely needed to make this a more feasible option. Lastly, assessing women’s knowledge and attitudes around genetic testing could help to more specifically address barriers to testing in future interventions.
Improving genetic screening and testing completion rates requires a coordinated effort. Using tools and applications to optimize convenience (same-day testing, telemedicine) and simplification (electronic screening platforms), we can strive to appropriately detect all women at high risk for hereditary breast and ovarian cancers.
Michelle Lightfoot is a gynecologic oncology fellow at the Ohio State University in Columbus. She has no relevant financial disclosures. Email her at [email protected].
VTE risk may be high throughout disease course in uterine serous carcinoma
Uterine serous carcinoma patients may have a high risk of venous thromboembolism (VTE), not just in the postoperative period, but throughout the natural history of the disease, results of a retrospective analysis suggest.
Most patients developed VTE either before staging surgery or more than 6 months postoperatively, according to results of the analysis reported in Obstetrics & Gynecology.
Nearly one-third (31%) of the women developed VTE while receiving chemotherapy, reported Gregory M. Gressel, MD, a gynecologic oncology fellow at Montefiore Medical Center, New York, and his coinvestigators.
The risk was highest in women with cardiovascular disease, hypertension, and stage III and IV disease, they said.
“Although this is a retrospective study, it generates the hypothesis that venous thromboembolism prophylaxis may be beneficial in women with active uterine serous carcinoma, at least while receiving treatment such as neoadjuvant or adjuvant chemotherapy,” Dr. Gressel and his coauthors noted.
Historically, clinical practice guidelines have focused on risk stratification in the perioperative period due to the strong association between cancer-related VTE and surgery, the authors wrote.
To better assess the timing and risk factors associated with clot development, Dr. Gressel and his colleagues abstracted clinical data from the medical records of 413 patients with uterine serous carcinoma between 1999 and 2016 at one center in New York, about half of whom identified as black and one-quarter as Hispanic.
Eighty-four percent of the patients were diagnosed with VTE before or after the 6-week postoperative window when thromboprophylaxis typically is recommended, and 31% developed clots during chemotherapy, the investigators reported. The median time to clot development was 7.2 months after diagnosis, and, after excluding patients who developed clots preoperatively or during chemotherapy, the investigators found the median time from surgery to VTE was 13.2 months.
Patients with stage III and IV disease were, respectively, 2.6 and 4 times more likely to develop thrombosis, compared with patients with stage I disease. Conversely, age, body mass index, and race were not associated with VTE diagnosis.
Patients who developed VTE on chemotherapy had a median Khorana score of 1, which corresponds to an intermediate risk of VTE, the investigators said, adding that pharmacologic prophylaxis is recommended only in patients with scores of 3 or higher.
“Ours is not the first report to posit that currently available venous thromboembolism risk stratification tools are of limited utility in gynecologic oncology patients,” said Dr. Gressel and his coauthors.
However, larger prospective studies are needed, not only to look at the utility of Khorana scoring in this high-risk histologic subtype, they said, but also to test their hypothesis that VTE prophylaxis may be beneficial during chemotherapy or other active treatment.
Dr. Gressel and his colleagues reported no conflicts of interest. The study was supported by the National Institutes of Health and a grant from the National Center for Advancing Translational Science.
SOURCE: Gressel GM et al. Obstet Gynecol. 2018 Oct 5;132:1130-6.
It has been reported in previous literature that the risk of developing VTE is typically within 48 hours of surgical staging, and guidelines have focused on risk factors to identify patients needing extended thromboprophylaxis after surgery. Therefore, Gressel et al. set forth to identify risk factors and timing of development of VTE in women with uterine serous carcinoma.
The study retrospectively evaluated 413 women with uterine serous cancer from 1999 to 2016. Of these, 70 women (about 17%) were diagnosed with a VTE. There was not a statistically significant association between age, body mass index, race, or surgical approach with risk of VTE. However, stage and/or having two or more medical comorbidities were significant. Compared with stage I patients, stage III and IV patients had a 2.6- and fourfold increase in risk, respectively. Not surprisingly, hypertension and cardiovascular disease (CVD) were independently associated with risk of VTE development; odds ratio 2.97 and 1.87, respectively. Interestingly, in the women diagnosed with VTE, 84% were outside the 6-week postoperative period and 31% occurred during chemotherapy treatment. The median time to develop VTE was 13.2 months and occurred sooner in women with advanced stage.
In this study, the majority of patients developed a VTE outside of the standard 28-day thromboprophylaxis window. This finding suggests that there is a subset of patients who would benefit from longer duration of thromboprophylaxis. Perhaps patients with hypertension, CVD, and/or advanced stage malignancy would benefit from thromboprophylaxis throughout their treatment.
Based on the data presented, at this time, we cannot recommend treating patients with the above risk factors during the entirety of their care. Although further studies and even a nomogram may help determine who is likely to benefit from prolonged prophylaxis, it is unknown if doing so would result in significant decreased morbidity or increased adverse events (e.g. bleeding, thrombocytopenia). However, this should remain a topic for further evaluation and providers should remain vigilant for prevention and diagnosis of VTE in at-risk patients by adhering to validated risk assessment tools and clinical evaluation.
Antonio Castaneda, MD, is a gynecologic oncology fellow at The Ohio State University in Columbus. He said he had no relevant financial disclosures.
It has been reported in previous literature that the risk of developing VTE is typically within 48 hours of surgical staging, and guidelines have focused on risk factors to identify patients needing extended thromboprophylaxis after surgery. Therefore, Gressel et al. set forth to identify risk factors and timing of development of VTE in women with uterine serous carcinoma.
The study retrospectively evaluated 413 women with uterine serous cancer from 1999 to 2016. Of these, 70 women (about 17%) were diagnosed with a VTE. There was not a statistically significant association between age, body mass index, race, or surgical approach with risk of VTE. However, stage and/or having two or more medical comorbidities were significant. Compared with stage I patients, stage III and IV patients had a 2.6- and fourfold increase in risk, respectively. Not surprisingly, hypertension and cardiovascular disease (CVD) were independently associated with risk of VTE development; odds ratio 2.97 and 1.87, respectively. Interestingly, in the women diagnosed with VTE, 84% were outside the 6-week postoperative period and 31% occurred during chemotherapy treatment. The median time to develop VTE was 13.2 months and occurred sooner in women with advanced stage.
In this study, the majority of patients developed a VTE outside of the standard 28-day thromboprophylaxis window. This finding suggests that there is a subset of patients who would benefit from longer duration of thromboprophylaxis. Perhaps patients with hypertension, CVD, and/or advanced stage malignancy would benefit from thromboprophylaxis throughout their treatment.
Based on the data presented, at this time, we cannot recommend treating patients with the above risk factors during the entirety of their care. Although further studies and even a nomogram may help determine who is likely to benefit from prolonged prophylaxis, it is unknown if doing so would result in significant decreased morbidity or increased adverse events (e.g. bleeding, thrombocytopenia). However, this should remain a topic for further evaluation and providers should remain vigilant for prevention and diagnosis of VTE in at-risk patients by adhering to validated risk assessment tools and clinical evaluation.
Antonio Castaneda, MD, is a gynecologic oncology fellow at The Ohio State University in Columbus. He said he had no relevant financial disclosures.
It has been reported in previous literature that the risk of developing VTE is typically within 48 hours of surgical staging, and guidelines have focused on risk factors to identify patients needing extended thromboprophylaxis after surgery. Therefore, Gressel et al. set forth to identify risk factors and timing of development of VTE in women with uterine serous carcinoma.
The study retrospectively evaluated 413 women with uterine serous cancer from 1999 to 2016. Of these, 70 women (about 17%) were diagnosed with a VTE. There was not a statistically significant association between age, body mass index, race, or surgical approach with risk of VTE. However, stage and/or having two or more medical comorbidities were significant. Compared with stage I patients, stage III and IV patients had a 2.6- and fourfold increase in risk, respectively. Not surprisingly, hypertension and cardiovascular disease (CVD) were independently associated with risk of VTE development; odds ratio 2.97 and 1.87, respectively. Interestingly, in the women diagnosed with VTE, 84% were outside the 6-week postoperative period and 31% occurred during chemotherapy treatment. The median time to develop VTE was 13.2 months and occurred sooner in women with advanced stage.
In this study, the majority of patients developed a VTE outside of the standard 28-day thromboprophylaxis window. This finding suggests that there is a subset of patients who would benefit from longer duration of thromboprophylaxis. Perhaps patients with hypertension, CVD, and/or advanced stage malignancy would benefit from thromboprophylaxis throughout their treatment.
Based on the data presented, at this time, we cannot recommend treating patients with the above risk factors during the entirety of their care. Although further studies and even a nomogram may help determine who is likely to benefit from prolonged prophylaxis, it is unknown if doing so would result in significant decreased morbidity or increased adverse events (e.g. bleeding, thrombocytopenia). However, this should remain a topic for further evaluation and providers should remain vigilant for prevention and diagnosis of VTE in at-risk patients by adhering to validated risk assessment tools and clinical evaluation.
Antonio Castaneda, MD, is a gynecologic oncology fellow at The Ohio State University in Columbus. He said he had no relevant financial disclosures.
Uterine serous carcinoma patients may have a high risk of venous thromboembolism (VTE), not just in the postoperative period, but throughout the natural history of the disease, results of a retrospective analysis suggest.
Most patients developed VTE either before staging surgery or more than 6 months postoperatively, according to results of the analysis reported in Obstetrics & Gynecology.
Nearly one-third (31%) of the women developed VTE while receiving chemotherapy, reported Gregory M. Gressel, MD, a gynecologic oncology fellow at Montefiore Medical Center, New York, and his coinvestigators.
The risk was highest in women with cardiovascular disease, hypertension, and stage III and IV disease, they said.
“Although this is a retrospective study, it generates the hypothesis that venous thromboembolism prophylaxis may be beneficial in women with active uterine serous carcinoma, at least while receiving treatment such as neoadjuvant or adjuvant chemotherapy,” Dr. Gressel and his coauthors noted.
Historically, clinical practice guidelines have focused on risk stratification in the perioperative period due to the strong association between cancer-related VTE and surgery, the authors wrote.
To better assess the timing and risk factors associated with clot development, Dr. Gressel and his colleagues abstracted clinical data from the medical records of 413 patients with uterine serous carcinoma between 1999 and 2016 at one center in New York, about half of whom identified as black and one-quarter as Hispanic.
Eighty-four percent of the patients were diagnosed with VTE before or after the 6-week postoperative window when thromboprophylaxis typically is recommended, and 31% developed clots during chemotherapy, the investigators reported. The median time to clot development was 7.2 months after diagnosis, and, after excluding patients who developed clots preoperatively or during chemotherapy, the investigators found the median time from surgery to VTE was 13.2 months.
Patients with stage III and IV disease were, respectively, 2.6 and 4 times more likely to develop thrombosis, compared with patients with stage I disease. Conversely, age, body mass index, and race were not associated with VTE diagnosis.
Patients who developed VTE on chemotherapy had a median Khorana score of 1, which corresponds to an intermediate risk of VTE, the investigators said, adding that pharmacologic prophylaxis is recommended only in patients with scores of 3 or higher.
“Ours is not the first report to posit that currently available venous thromboembolism risk stratification tools are of limited utility in gynecologic oncology patients,” said Dr. Gressel and his coauthors.
However, larger prospective studies are needed, not only to look at the utility of Khorana scoring in this high-risk histologic subtype, they said, but also to test their hypothesis that VTE prophylaxis may be beneficial during chemotherapy or other active treatment.
Dr. Gressel and his colleagues reported no conflicts of interest. The study was supported by the National Institutes of Health and a grant from the National Center for Advancing Translational Science.
SOURCE: Gressel GM et al. Obstet Gynecol. 2018 Oct 5;132:1130-6.
Uterine serous carcinoma patients may have a high risk of venous thromboembolism (VTE), not just in the postoperative period, but throughout the natural history of the disease, results of a retrospective analysis suggest.
Most patients developed VTE either before staging surgery or more than 6 months postoperatively, according to results of the analysis reported in Obstetrics & Gynecology.
Nearly one-third (31%) of the women developed VTE while receiving chemotherapy, reported Gregory M. Gressel, MD, a gynecologic oncology fellow at Montefiore Medical Center, New York, and his coinvestigators.
The risk was highest in women with cardiovascular disease, hypertension, and stage III and IV disease, they said.
“Although this is a retrospective study, it generates the hypothesis that venous thromboembolism prophylaxis may be beneficial in women with active uterine serous carcinoma, at least while receiving treatment such as neoadjuvant or adjuvant chemotherapy,” Dr. Gressel and his coauthors noted.
Historically, clinical practice guidelines have focused on risk stratification in the perioperative period due to the strong association between cancer-related VTE and surgery, the authors wrote.
To better assess the timing and risk factors associated with clot development, Dr. Gressel and his colleagues abstracted clinical data from the medical records of 413 patients with uterine serous carcinoma between 1999 and 2016 at one center in New York, about half of whom identified as black and one-quarter as Hispanic.
Eighty-four percent of the patients were diagnosed with VTE before or after the 6-week postoperative window when thromboprophylaxis typically is recommended, and 31% developed clots during chemotherapy, the investigators reported. The median time to clot development was 7.2 months after diagnosis, and, after excluding patients who developed clots preoperatively or during chemotherapy, the investigators found the median time from surgery to VTE was 13.2 months.
Patients with stage III and IV disease were, respectively, 2.6 and 4 times more likely to develop thrombosis, compared with patients with stage I disease. Conversely, age, body mass index, and race were not associated with VTE diagnosis.
Patients who developed VTE on chemotherapy had a median Khorana score of 1, which corresponds to an intermediate risk of VTE, the investigators said, adding that pharmacologic prophylaxis is recommended only in patients with scores of 3 or higher.
“Ours is not the first report to posit that currently available venous thromboembolism risk stratification tools are of limited utility in gynecologic oncology patients,” said Dr. Gressel and his coauthors.
However, larger prospective studies are needed, not only to look at the utility of Khorana scoring in this high-risk histologic subtype, they said, but also to test their hypothesis that VTE prophylaxis may be beneficial during chemotherapy or other active treatment.
Dr. Gressel and his colleagues reported no conflicts of interest. The study was supported by the National Institutes of Health and a grant from the National Center for Advancing Translational Science.
SOURCE: Gressel GM et al. Obstet Gynecol. 2018 Oct 5;132:1130-6.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Eighty-four percent of VTEs were diagnosed before or after the 6-week postoperative window, and 31% developed during chemotherapy.
Study details: Retrospective study of 431 women diagnosed with uterine serous carcinoma between 1999 and 2016 at one center in New York.
Disclosures: Dr. Gressel and his coauthors reported no conflicts of interest. The study was supported by the National Institutes of Health and a grant from the National Center for Advancing Translational Science.
Source: Gressel GM et al. Obstet Gynecol. 2018 Oct 5;132:1130-6.
Surgical quality: How do we measure something so difficult to define?
Quality in medicine is a peculiar thing. It is clearly apparent, and yet, can be very difficult to measure and quantify. Surgery, a performance art of sorts, can be even more challenging to qualify or rate. However, as a means to elevate the quality of care for all patients, hospital systems and care providers have aggressively made attempts to do so. This is a noble objective.
In September 2018, the Committee of Gynecologic Practice of the American College of Obstetricians and Gynecologists released ACOG Committee Opinion Number 750, titled, “Perioperative Pathways: Enhanced Recovery After Surgery.”1
The goals of this committee opinion were to advocate for gynecologic surgeons using the “ERAS” pathways in their perioperative care as part of an evidenced-based approach to quality improvement. ERAS pathways have been previously discussed in this column and feature bundled perioperative pathways that incorporate various concepts such as avoidance of prolonged preoperative fasting, early postoperative feeding, multimodal analgesia (with an avoidance of opiates), and inclusion of antibiotic and antiembolic prophylaxis, among other elements.
What was alarming upon closer review of this ACOG Committee Opinion was its omission of the randomized controlled trial by Dickson et al., the only randomized trial published in gynecologic surgery evaluating ERAS pathways.2 This trial compared the length of stay for patients receiving laparotomy for gynecologic cancer surgery who received perioperative care according the ERAS pathway versus those who received standard perioperative care. They found no difference in length of stay – the primary outcome – between the two groups, an impressive 3 days for both. The secondary outcome of postoperative pain was improved for the ERAS group for some of the time points. It was likely that the excellent outcomes in both groups resulted from a Hawthorne effect in which the behavior of study participants is influenced by the fact that they were being observed, in addition to the fact that the physicians involved in the study already were practicing high quality care as part of their “standard” regimen. It simply may be that the act of trying to improve quality is what improves outcomes, not a specific pathway. As senior author, Dr. Peter A. Argenta, explained to me, many of the ERAS elements are “simply good medicine.”
ERAS pathways are an example of process measures of quality. They include elements of care or processes in the delivery of care that are thought to be associated with improved outcomes. Prescription of antibiotics or venous thromboembolism (VTE) prophylaxis are other examples of process measures thought to be associated with improved surgical quality. Rather than rating surgeons’ outcomes (surgical site infection), surgeons are rated on their compliance with a process (the rate of appropriate perioperative antibiotic prescription). However, high compliance with these processes is not automatically associated with improved observed outcomes. For example, hospitals that meet the definition of high quality by virtue of structural measures (such as procedural volume and use of hospital-level quality initiatives) are associated with worse risk-adjusted VTE rates despite demonstrating higher adherence to VTE prophylaxis.3 This is felt to be a function of surveillance bias and the fact that these same hospitals have better capabilities to capture events as part of a feedback mechanism built into their quality initiatives.
What ERAS has favorably done for surgical care is to shine a glaring light on and challenge the unnecessary, old-fashioned, and non–patient-centric interventions that were considered dogma by many. For example, minimizing preoperative fasting is most certainly a patient-friendly adjustment that should absolutely be embraced, regardless of whether or not it speeds up time to discharge. Multimodal approaches to analgesia consistently have been shown to preserve or improve postoperative pain levels with a focus on minimizing opiate use, once again a noble and patient-centered objective.
However, all too many surgical quality interventions focus on their ability to reduce postoperative length of stay. Length of stay is an important driver of health care cost, and an indirect measure of perioperative complications; however, it is not a patient-centered outcome. So long as patients recover from their surgery quickly with respect to pain and function, the location of that recovery (home versus hospital) is less of a focus for most patients. In addition, in the pursuit of shorter hospital stays and less perioperative morbidity, we may encourage practices with unintentional adverse patient-centered outcomes. For example, to preserve a surgeon’s quality metrics, patients who are at high risk for complications may not be offered surgery at all. Long-term ovarian cancer outcomes, such as survival, can be negatively impacted when surgeons opt for less morbid, less radical surgical approaches which have favorable short-term morbidity such as surgical complications and readmissions.4
Ultimately we are most likely to see improvement in quality with a complex, nuanced approach to metrics, not simplistic interventions or pathways. We should recognize interventions that are consistently associated with better outcomes such as high procedural volume, consolidating less common procedures to fewer surgeons, data ascertainment, and reporting data to surgeons.5 Physicians need to take ownership and involvement in the quality metrics that are created to assess the care we provide. Hospital administrators may not fully understand the confounders, such as comorbidities, that contribute to outcomes, which can lead to mischaracterization, cause unfair comparisons between surgeons, or create unintentional incentives that are not patient-centered.6
We all need to understand the epidemiologic science behind evidence-based medicine and to be sophisticated in our ability to review and appraise data so that we can be sensible in what interventions we promote as supported by good evidence. If we fail to correctly identify and characterize what is truly good quality, if we miss the point of what is driving outcomes, or overstate the value of certain interventions, we miss the opportunity to intervene in ways that actually do make a meaningful difference.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Obstet Gynecol 2018;132:e120-e30.
2. Obstet Gynecol. 2017 Feb;129(2):355-62.
3. JAMA. 2013 Oct 9;310(14):1482-9.
4. Gynecol Oncol. 2017 Dec;147(3):607-11.
5. J Am Coll Surg. 2004 Apr;198(4):626-32.
6. Gynecol Oncol. 2018 Oct;151(1):141-4.
Quality in medicine is a peculiar thing. It is clearly apparent, and yet, can be very difficult to measure and quantify. Surgery, a performance art of sorts, can be even more challenging to qualify or rate. However, as a means to elevate the quality of care for all patients, hospital systems and care providers have aggressively made attempts to do so. This is a noble objective.
In September 2018, the Committee of Gynecologic Practice of the American College of Obstetricians and Gynecologists released ACOG Committee Opinion Number 750, titled, “Perioperative Pathways: Enhanced Recovery After Surgery.”1
The goals of this committee opinion were to advocate for gynecologic surgeons using the “ERAS” pathways in their perioperative care as part of an evidenced-based approach to quality improvement. ERAS pathways have been previously discussed in this column and feature bundled perioperative pathways that incorporate various concepts such as avoidance of prolonged preoperative fasting, early postoperative feeding, multimodal analgesia (with an avoidance of opiates), and inclusion of antibiotic and antiembolic prophylaxis, among other elements.
What was alarming upon closer review of this ACOG Committee Opinion was its omission of the randomized controlled trial by Dickson et al., the only randomized trial published in gynecologic surgery evaluating ERAS pathways.2 This trial compared the length of stay for patients receiving laparotomy for gynecologic cancer surgery who received perioperative care according the ERAS pathway versus those who received standard perioperative care. They found no difference in length of stay – the primary outcome – between the two groups, an impressive 3 days for both. The secondary outcome of postoperative pain was improved for the ERAS group for some of the time points. It was likely that the excellent outcomes in both groups resulted from a Hawthorne effect in which the behavior of study participants is influenced by the fact that they were being observed, in addition to the fact that the physicians involved in the study already were practicing high quality care as part of their “standard” regimen. It simply may be that the act of trying to improve quality is what improves outcomes, not a specific pathway. As senior author, Dr. Peter A. Argenta, explained to me, many of the ERAS elements are “simply good medicine.”
ERAS pathways are an example of process measures of quality. They include elements of care or processes in the delivery of care that are thought to be associated with improved outcomes. Prescription of antibiotics or venous thromboembolism (VTE) prophylaxis are other examples of process measures thought to be associated with improved surgical quality. Rather than rating surgeons’ outcomes (surgical site infection), surgeons are rated on their compliance with a process (the rate of appropriate perioperative antibiotic prescription). However, high compliance with these processes is not automatically associated with improved observed outcomes. For example, hospitals that meet the definition of high quality by virtue of structural measures (such as procedural volume and use of hospital-level quality initiatives) are associated with worse risk-adjusted VTE rates despite demonstrating higher adherence to VTE prophylaxis.3 This is felt to be a function of surveillance bias and the fact that these same hospitals have better capabilities to capture events as part of a feedback mechanism built into their quality initiatives.
What ERAS has favorably done for surgical care is to shine a glaring light on and challenge the unnecessary, old-fashioned, and non–patient-centric interventions that were considered dogma by many. For example, minimizing preoperative fasting is most certainly a patient-friendly adjustment that should absolutely be embraced, regardless of whether or not it speeds up time to discharge. Multimodal approaches to analgesia consistently have been shown to preserve or improve postoperative pain levels with a focus on minimizing opiate use, once again a noble and patient-centered objective.
However, all too many surgical quality interventions focus on their ability to reduce postoperative length of stay. Length of stay is an important driver of health care cost, and an indirect measure of perioperative complications; however, it is not a patient-centered outcome. So long as patients recover from their surgery quickly with respect to pain and function, the location of that recovery (home versus hospital) is less of a focus for most patients. In addition, in the pursuit of shorter hospital stays and less perioperative morbidity, we may encourage practices with unintentional adverse patient-centered outcomes. For example, to preserve a surgeon’s quality metrics, patients who are at high risk for complications may not be offered surgery at all. Long-term ovarian cancer outcomes, such as survival, can be negatively impacted when surgeons opt for less morbid, less radical surgical approaches which have favorable short-term morbidity such as surgical complications and readmissions.4
Ultimately we are most likely to see improvement in quality with a complex, nuanced approach to metrics, not simplistic interventions or pathways. We should recognize interventions that are consistently associated with better outcomes such as high procedural volume, consolidating less common procedures to fewer surgeons, data ascertainment, and reporting data to surgeons.5 Physicians need to take ownership and involvement in the quality metrics that are created to assess the care we provide. Hospital administrators may not fully understand the confounders, such as comorbidities, that contribute to outcomes, which can lead to mischaracterization, cause unfair comparisons between surgeons, or create unintentional incentives that are not patient-centered.6
We all need to understand the epidemiologic science behind evidence-based medicine and to be sophisticated in our ability to review and appraise data so that we can be sensible in what interventions we promote as supported by good evidence. If we fail to correctly identify and characterize what is truly good quality, if we miss the point of what is driving outcomes, or overstate the value of certain interventions, we miss the opportunity to intervene in ways that actually do make a meaningful difference.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Obstet Gynecol 2018;132:e120-e30.
2. Obstet Gynecol. 2017 Feb;129(2):355-62.
3. JAMA. 2013 Oct 9;310(14):1482-9.
4. Gynecol Oncol. 2017 Dec;147(3):607-11.
5. J Am Coll Surg. 2004 Apr;198(4):626-32.
6. Gynecol Oncol. 2018 Oct;151(1):141-4.
Quality in medicine is a peculiar thing. It is clearly apparent, and yet, can be very difficult to measure and quantify. Surgery, a performance art of sorts, can be even more challenging to qualify or rate. However, as a means to elevate the quality of care for all patients, hospital systems and care providers have aggressively made attempts to do so. This is a noble objective.
In September 2018, the Committee of Gynecologic Practice of the American College of Obstetricians and Gynecologists released ACOG Committee Opinion Number 750, titled, “Perioperative Pathways: Enhanced Recovery After Surgery.”1
The goals of this committee opinion were to advocate for gynecologic surgeons using the “ERAS” pathways in their perioperative care as part of an evidenced-based approach to quality improvement. ERAS pathways have been previously discussed in this column and feature bundled perioperative pathways that incorporate various concepts such as avoidance of prolonged preoperative fasting, early postoperative feeding, multimodal analgesia (with an avoidance of opiates), and inclusion of antibiotic and antiembolic prophylaxis, among other elements.
What was alarming upon closer review of this ACOG Committee Opinion was its omission of the randomized controlled trial by Dickson et al., the only randomized trial published in gynecologic surgery evaluating ERAS pathways.2 This trial compared the length of stay for patients receiving laparotomy for gynecologic cancer surgery who received perioperative care according the ERAS pathway versus those who received standard perioperative care. They found no difference in length of stay – the primary outcome – between the two groups, an impressive 3 days for both. The secondary outcome of postoperative pain was improved for the ERAS group for some of the time points. It was likely that the excellent outcomes in both groups resulted from a Hawthorne effect in which the behavior of study participants is influenced by the fact that they were being observed, in addition to the fact that the physicians involved in the study already were practicing high quality care as part of their “standard” regimen. It simply may be that the act of trying to improve quality is what improves outcomes, not a specific pathway. As senior author, Dr. Peter A. Argenta, explained to me, many of the ERAS elements are “simply good medicine.”
ERAS pathways are an example of process measures of quality. They include elements of care or processes in the delivery of care that are thought to be associated with improved outcomes. Prescription of antibiotics or venous thromboembolism (VTE) prophylaxis are other examples of process measures thought to be associated with improved surgical quality. Rather than rating surgeons’ outcomes (surgical site infection), surgeons are rated on their compliance with a process (the rate of appropriate perioperative antibiotic prescription). However, high compliance with these processes is not automatically associated with improved observed outcomes. For example, hospitals that meet the definition of high quality by virtue of structural measures (such as procedural volume and use of hospital-level quality initiatives) are associated with worse risk-adjusted VTE rates despite demonstrating higher adherence to VTE prophylaxis.3 This is felt to be a function of surveillance bias and the fact that these same hospitals have better capabilities to capture events as part of a feedback mechanism built into their quality initiatives.
What ERAS has favorably done for surgical care is to shine a glaring light on and challenge the unnecessary, old-fashioned, and non–patient-centric interventions that were considered dogma by many. For example, minimizing preoperative fasting is most certainly a patient-friendly adjustment that should absolutely be embraced, regardless of whether or not it speeds up time to discharge. Multimodal approaches to analgesia consistently have been shown to preserve or improve postoperative pain levels with a focus on minimizing opiate use, once again a noble and patient-centered objective.
However, all too many surgical quality interventions focus on their ability to reduce postoperative length of stay. Length of stay is an important driver of health care cost, and an indirect measure of perioperative complications; however, it is not a patient-centered outcome. So long as patients recover from their surgery quickly with respect to pain and function, the location of that recovery (home versus hospital) is less of a focus for most patients. In addition, in the pursuit of shorter hospital stays and less perioperative morbidity, we may encourage practices with unintentional adverse patient-centered outcomes. For example, to preserve a surgeon’s quality metrics, patients who are at high risk for complications may not be offered surgery at all. Long-term ovarian cancer outcomes, such as survival, can be negatively impacted when surgeons opt for less morbid, less radical surgical approaches which have favorable short-term morbidity such as surgical complications and readmissions.4
Ultimately we are most likely to see improvement in quality with a complex, nuanced approach to metrics, not simplistic interventions or pathways. We should recognize interventions that are consistently associated with better outcomes such as high procedural volume, consolidating less common procedures to fewer surgeons, data ascertainment, and reporting data to surgeons.5 Physicians need to take ownership and involvement in the quality metrics that are created to assess the care we provide. Hospital administrators may not fully understand the confounders, such as comorbidities, that contribute to outcomes, which can lead to mischaracterization, cause unfair comparisons between surgeons, or create unintentional incentives that are not patient-centered.6
We all need to understand the epidemiologic science behind evidence-based medicine and to be sophisticated in our ability to review and appraise data so that we can be sensible in what interventions we promote as supported by good evidence. If we fail to correctly identify and characterize what is truly good quality, if we miss the point of what is driving outcomes, or overstate the value of certain interventions, we miss the opportunity to intervene in ways that actually do make a meaningful difference.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Obstet Gynecol 2018;132:e120-e30.
2. Obstet Gynecol. 2017 Feb;129(2):355-62.
3. JAMA. 2013 Oct 9;310(14):1482-9.
4. Gynecol Oncol. 2017 Dec;147(3):607-11.
5. J Am Coll Surg. 2004 Apr;198(4):626-32.
6. Gynecol Oncol. 2018 Oct;151(1):141-4.