User login
CarePostRoe.com: Study seeks to document poor quality medical care due to new abortion bans
In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
Risk of expulsion low after early postpartum IUD placement
Intrauterine device (IUD) placement at 2-4 weeks postpartum was noninferior to placement at 6-8 weeks postpartum for complete expulsion, and carried only a slightly higher risk of partial expulsion. A randomized study of expulsion rates reports the risk of expulsion at these points may help patients and clinicians make informed choices about the timing of IUD insertion, wrote the study authors, led by Sarah H. Averbach, MD, MAS, an obstetrician-gynecologist at the University of California, San Diego. “We found that the risk of complete IUD expulsion was low at 2% after early IUD placement 2-4 weeks after delivery, and was noninferior to interval placement 6-8 weeks after delivery at 0%,” Dr. Averbach said in an interview.
Although the risks of partial expulsion and malposition were modestly greater after early placement, “the possibility of a small increase in the risk of IUD expulsion or malposition with early IUD placement should be weighed against the risk of undesired pregnancy and short-interval pregnancy by delaying placement.”
The timing of IUD placement in the postpartum period should be guided by patients’ goals and preferences, she added. The early postpartum period 2-4 weeks after birth has the advantage of convenience since it coincides with early-postpartum or well-baby visits. The absolute risk differences observed between early and interval placement were small for both complete or partial expulsion at 3.8%, and the rate for complete expulsion after early placement was much lower than historical expulsion rates for immediate postpartum placement within in few days of delivery.
Last year, a large study showed an increase in expulsion risk with IUD insertion within 3 days of delivery. Current guidelines, however, support immediate insertion as a safe practice.
The study
Enrolling 404 participants from diverse settings during the period of 2018 to July 2021, researchers for the noninferiority trial randomly assigned 203 to early IUD placement 14-28 days postpartum and 201 to standard-interval placement at 42-56 days. Patients had a mean age of 29.9 years, 11.4% were Black, 56.4% were White, and 43.3% were Hispanic (some Hispanic participants self-identified as White and some as Black). By 6 months postpartum, 73% of the cohort had received an IUD and completed 6-months of follow-up, while 13% had never received an IUD and 14% were lost to follow-up. Complete expulsion rates were 3 of 149, or 2.0% (95% confidence interval [CI], 0.4-5.8) in the early group and 0 of 145, or 0% (95% CI, 0.0-2.5) in the standard group, for a between-group difference of 2.0 percentage points (95% CI, −0.5 to 5.7, P = .04). Two women chose to replace their IUDs.
Partial expulsion occurred in 14, or 9.4% (95% CI, 5.2-15.3) of patients in the early group and 11, or 7.6% (95% CI, 3.9-13.2) in the standard-interval group, for a between-group difference of 1.8 (95% CI, −4.8 to 8.6) percentage points (P = .22).
The small absolute increase in risk of partial expulsion in the early arm did not meet the prespecified criterion for noninferiority of 6%. Three pelvic infections occurred in the early placement arm.
There were 42 IUD removals: 25 in the early placement group and 17 in the standard interval group. Thirteen participants had their IUDs removed for symptoms such as cramping and bothersome vaginal bleeding.
No perforations were identified in either group at 6 months, suggesting that the rate of uterine perforations is low when IUDs are placed in the early and standard-interval postpartum periods. IUD use at 6 months remained comparable between arms: 69.5% in the early group vs. 67.2% in the standard-interval group.
Commenting on the trial but not involved in it, Maureen K. Baldwin, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland, said it provides further data on the prevalence of expulsion and malposition after placements using ultrasonography as needed. While two failures occurred with asymptomatic malposition, she added, “It should be noted that IUD position can change as a result of pregnancy, so it was not determined that malposition occurred prior to contraceptive failure.”
According to Dr. Baldwin, one strategy to reduce concerns is to use transvaginal ultrasonography at a later time or in the presence of unusual symptoms.
Overall, the study establishes that postpartum placement is an option equivalent to standard timing and it should be incorporated into patient preferences, she said. “Pain may be lowest at early placement compared to other timings, particularly for those who had vaginal birth.”
The study was supported by the Society of Family Planning research fund and the National Institutes of Health - National Institute of Child Health and Human Development. Dr. Averbach reported personal fees from Bayer Pharmaceuticals for advice on postpartum IUD placement as well as grants from the NIH outside of the submitted work. Dr. Baldwin disclosed no potential conflicts of interest with regard to her comments.
Intrauterine device (IUD) placement at 2-4 weeks postpartum was noninferior to placement at 6-8 weeks postpartum for complete expulsion, and carried only a slightly higher risk of partial expulsion. A randomized study of expulsion rates reports the risk of expulsion at these points may help patients and clinicians make informed choices about the timing of IUD insertion, wrote the study authors, led by Sarah H. Averbach, MD, MAS, an obstetrician-gynecologist at the University of California, San Diego. “We found that the risk of complete IUD expulsion was low at 2% after early IUD placement 2-4 weeks after delivery, and was noninferior to interval placement 6-8 weeks after delivery at 0%,” Dr. Averbach said in an interview.
Although the risks of partial expulsion and malposition were modestly greater after early placement, “the possibility of a small increase in the risk of IUD expulsion or malposition with early IUD placement should be weighed against the risk of undesired pregnancy and short-interval pregnancy by delaying placement.”
The timing of IUD placement in the postpartum period should be guided by patients’ goals and preferences, she added. The early postpartum period 2-4 weeks after birth has the advantage of convenience since it coincides with early-postpartum or well-baby visits. The absolute risk differences observed between early and interval placement were small for both complete or partial expulsion at 3.8%, and the rate for complete expulsion after early placement was much lower than historical expulsion rates for immediate postpartum placement within in few days of delivery.
Last year, a large study showed an increase in expulsion risk with IUD insertion within 3 days of delivery. Current guidelines, however, support immediate insertion as a safe practice.
The study
Enrolling 404 participants from diverse settings during the period of 2018 to July 2021, researchers for the noninferiority trial randomly assigned 203 to early IUD placement 14-28 days postpartum and 201 to standard-interval placement at 42-56 days. Patients had a mean age of 29.9 years, 11.4% were Black, 56.4% were White, and 43.3% were Hispanic (some Hispanic participants self-identified as White and some as Black). By 6 months postpartum, 73% of the cohort had received an IUD and completed 6-months of follow-up, while 13% had never received an IUD and 14% were lost to follow-up. Complete expulsion rates were 3 of 149, or 2.0% (95% confidence interval [CI], 0.4-5.8) in the early group and 0 of 145, or 0% (95% CI, 0.0-2.5) in the standard group, for a between-group difference of 2.0 percentage points (95% CI, −0.5 to 5.7, P = .04). Two women chose to replace their IUDs.
Partial expulsion occurred in 14, or 9.4% (95% CI, 5.2-15.3) of patients in the early group and 11, or 7.6% (95% CI, 3.9-13.2) in the standard-interval group, for a between-group difference of 1.8 (95% CI, −4.8 to 8.6) percentage points (P = .22).
The small absolute increase in risk of partial expulsion in the early arm did not meet the prespecified criterion for noninferiority of 6%. Three pelvic infections occurred in the early placement arm.
There were 42 IUD removals: 25 in the early placement group and 17 in the standard interval group. Thirteen participants had their IUDs removed for symptoms such as cramping and bothersome vaginal bleeding.
No perforations were identified in either group at 6 months, suggesting that the rate of uterine perforations is low when IUDs are placed in the early and standard-interval postpartum periods. IUD use at 6 months remained comparable between arms: 69.5% in the early group vs. 67.2% in the standard-interval group.
Commenting on the trial but not involved in it, Maureen K. Baldwin, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland, said it provides further data on the prevalence of expulsion and malposition after placements using ultrasonography as needed. While two failures occurred with asymptomatic malposition, she added, “It should be noted that IUD position can change as a result of pregnancy, so it was not determined that malposition occurred prior to contraceptive failure.”
According to Dr. Baldwin, one strategy to reduce concerns is to use transvaginal ultrasonography at a later time or in the presence of unusual symptoms.
Overall, the study establishes that postpartum placement is an option equivalent to standard timing and it should be incorporated into patient preferences, she said. “Pain may be lowest at early placement compared to other timings, particularly for those who had vaginal birth.”
The study was supported by the Society of Family Planning research fund and the National Institutes of Health - National Institute of Child Health and Human Development. Dr. Averbach reported personal fees from Bayer Pharmaceuticals for advice on postpartum IUD placement as well as grants from the NIH outside of the submitted work. Dr. Baldwin disclosed no potential conflicts of interest with regard to her comments.
Intrauterine device (IUD) placement at 2-4 weeks postpartum was noninferior to placement at 6-8 weeks postpartum for complete expulsion, and carried only a slightly higher risk of partial expulsion. A randomized study of expulsion rates reports the risk of expulsion at these points may help patients and clinicians make informed choices about the timing of IUD insertion, wrote the study authors, led by Sarah H. Averbach, MD, MAS, an obstetrician-gynecologist at the University of California, San Diego. “We found that the risk of complete IUD expulsion was low at 2% after early IUD placement 2-4 weeks after delivery, and was noninferior to interval placement 6-8 weeks after delivery at 0%,” Dr. Averbach said in an interview.
Although the risks of partial expulsion and malposition were modestly greater after early placement, “the possibility of a small increase in the risk of IUD expulsion or malposition with early IUD placement should be weighed against the risk of undesired pregnancy and short-interval pregnancy by delaying placement.”
The timing of IUD placement in the postpartum period should be guided by patients’ goals and preferences, she added. The early postpartum period 2-4 weeks after birth has the advantage of convenience since it coincides with early-postpartum or well-baby visits. The absolute risk differences observed between early and interval placement were small for both complete or partial expulsion at 3.8%, and the rate for complete expulsion after early placement was much lower than historical expulsion rates for immediate postpartum placement within in few days of delivery.
Last year, a large study showed an increase in expulsion risk with IUD insertion within 3 days of delivery. Current guidelines, however, support immediate insertion as a safe practice.
The study
Enrolling 404 participants from diverse settings during the period of 2018 to July 2021, researchers for the noninferiority trial randomly assigned 203 to early IUD placement 14-28 days postpartum and 201 to standard-interval placement at 42-56 days. Patients had a mean age of 29.9 years, 11.4% were Black, 56.4% were White, and 43.3% were Hispanic (some Hispanic participants self-identified as White and some as Black). By 6 months postpartum, 73% of the cohort had received an IUD and completed 6-months of follow-up, while 13% had never received an IUD and 14% were lost to follow-up. Complete expulsion rates were 3 of 149, or 2.0% (95% confidence interval [CI], 0.4-5.8) in the early group and 0 of 145, or 0% (95% CI, 0.0-2.5) in the standard group, for a between-group difference of 2.0 percentage points (95% CI, −0.5 to 5.7, P = .04). Two women chose to replace their IUDs.
Partial expulsion occurred in 14, or 9.4% (95% CI, 5.2-15.3) of patients in the early group and 11, or 7.6% (95% CI, 3.9-13.2) in the standard-interval group, for a between-group difference of 1.8 (95% CI, −4.8 to 8.6) percentage points (P = .22).
The small absolute increase in risk of partial expulsion in the early arm did not meet the prespecified criterion for noninferiority of 6%. Three pelvic infections occurred in the early placement arm.
There were 42 IUD removals: 25 in the early placement group and 17 in the standard interval group. Thirteen participants had their IUDs removed for symptoms such as cramping and bothersome vaginal bleeding.
No perforations were identified in either group at 6 months, suggesting that the rate of uterine perforations is low when IUDs are placed in the early and standard-interval postpartum periods. IUD use at 6 months remained comparable between arms: 69.5% in the early group vs. 67.2% in the standard-interval group.
Commenting on the trial but not involved in it, Maureen K. Baldwin, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland, said it provides further data on the prevalence of expulsion and malposition after placements using ultrasonography as needed. While two failures occurred with asymptomatic malposition, she added, “It should be noted that IUD position can change as a result of pregnancy, so it was not determined that malposition occurred prior to contraceptive failure.”
According to Dr. Baldwin, one strategy to reduce concerns is to use transvaginal ultrasonography at a later time or in the presence of unusual symptoms.
Overall, the study establishes that postpartum placement is an option equivalent to standard timing and it should be incorporated into patient preferences, she said. “Pain may be lowest at early placement compared to other timings, particularly for those who had vaginal birth.”
The study was supported by the Society of Family Planning research fund and the National Institutes of Health - National Institute of Child Health and Human Development. Dr. Averbach reported personal fees from Bayer Pharmaceuticals for advice on postpartum IUD placement as well as grants from the NIH outside of the submitted work. Dr. Baldwin disclosed no potential conflicts of interest with regard to her comments.
FROM JAMA
Ectopic pregnancy risk and levonorgestrel-releasing IUDs
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
FROM JAMA
Iron deficiency and anemia in patients with heavy menstrual bleeding: Mechanisms and management
Recurrent episodic blood loss from normal menstruation is not expected to result in anemia. But without treatment, chronic heavy periods will progress through the stages of low iron stores to iron deficiency and then to anemia. When iron storage levels are low, the bone marrow’s blood cell factory cannot keep up with continued losses. Every patient with heavy menstrual bleeding (HMB) or prolonged menstrual episodes should be tested and treated for iron deficiency and anemia.1,2
Particular attention should be paid to assessment of iron storage levels with serum ferritin, recognizing that low iron levels progress to anemia once the storage is depleted. Recovery from anemia is much slower in individuals with iron deficiency, so assessment for iron storage also should be included in preoperative assessments and following a diagnosis of acute blood loss anemia.
The mechanics of erythropoiesis, hemoglobin, and oxygen transport
Red blood cells (erythrocytes) have a short life cycle and require constant replacement. Erythrocytes are generated on demand in erythropoiesis by a hormonal signaling process, regardless of whether sufficient components are available.3 Hemoglobin, the main intracellular component of erythrocytes, is comprised of 4 globin chains, which each contain 1 iron atom bound to a heme molecule. After erythrocytes are assembled, they are sent out into circulation for approximately 120 days. A hemoglobin level measures the oxygen-carrying capacity of erythrocytes, and anemia is defined as hemoglobin less than 12 g/dL.
Unless erythrocytes are lost from bleeding, they are decommissioned—that is, the heme molecule is metabolized into bilirubin and excreted, and the iron atoms are recycled back to the bone marrow or to storage.4 Ferritin is the storage molecule that binds to iron, a glycoprotein with numerous subunits around a core that can contain about 4,000 iron atoms. Most ferritin is intracellular, but a small proportion is present in serum, where it can be measured.
Serum ferritin is a good marker for the iron supply in healthy individuals because it has high correlation to iron in the bone marrow and correlates to total intracellular storage unless there is inflammation, when mobilization to serum increases. The ferritin level at which the iron supply is deficient to meet demand, defined as iron deficiency, is hotly debated and ranges from less than 15 to 50 ng/mL in menstruating individuals, with higher thresholds based on onset of erythropoiesis signaling and the lower threshold being the World Health Organization recommendation.5-7 When iron atoms are in short supply, erythrocytes still are generated but they have lower amounts of intracellular hemoglobin, which makes them thinner, smaller, and paler—and less effective at oxygen transport.
CASE Patient seeks treatment for HMB-associated symptoms
A 17-year-old patient presents with HMB, fatigue, and difficulty with concentration. She reports that her periods have been regular and lasting 7 days since menarche at age 13. While they are manageable, they seem to be getting heavier, soaking pads in 2 to 3 hours. The patient reports that she would like to start treatment for her progressively heavy bleeding and prefers lighter scheduled bleeding; she currently does not desire contraception. The patient has no family history of bleeding problems and self-reports no personal history of epistaxis or bleeding with tooth extraction or tonsillectomy. Laboratory tests confirm iron deficiency with a hemoglobin level of 12.5 g/dL (reference range, 12.0–17.5 g/dL) and a serum ferritin level of 8 ng/mL (reference range, 50–420 ng/mL). Results from a coagulopathy panel are normal, as are von Willebrand factor levels.
Untreated iron deficiency will progress to anemia
This patient has iron deficiency without anemia, which warrants significant attention in HMB because without treatment it eventually will progress to anemia. The prevalence of iron deficiency, which makes up half of all causes of anemia, is at least double that of iron deficiency anemia.3
Adult bodies usually contain about 3 to 4 g of iron, with two-thirds in erythrocytes as hemoglobin.8 Approximately 40 to 60 mg of iron is recycled daily, 1 to 2 mg/day is lost from sloughed cells and sweat, and at least 1 mg/day is lost during normal menstruation. These losses are balanced with gastrointestinal uptake of 1 to 2 mg/day until bleeding exceeds about 10 mL/day. In this 17-year-old patient, iron stores have likely been on a progressive decline since menarche.
For normally menstruating individuals to maintain iron homeostasis, the daily dietary iron requirement is 18 mg/day. Iron requirements also increase during periods of illness or inflammation due to hormonal signaling in the iron absorption and transport pathway, in athletes due to sweating, foot strike hemolysis and bruising, and during growth spurts.9
Continue to: Managing iron deficiency and anemia...
Managing iron deficiency and anemia
Management of iron deficiency and iron deficiency anemia in the setting of HMB includes:
- workup for the etiology of the abnormal uterine bleeding (TABLE)
- reducing the source of blood loss, and
- iron supplementation to correct the iron deficiency state.
In most cases, workup, reduction, and repletion can occur simultaneously. The goal is not always complete cessation of menstrual bleeding; even short-term therapy can allow time to replenish iron storage. Use a shared decision-making process to assess what is important to the patient, and provide information about relative amounts of bleeding cessation that can be expected with various therapies.10
Treatment options
Medical treatments to decrease menstrual iron losses are recommended prior to proceeding with surgical interventions.11 Hormonal treatments are the most consistently recommended, with many guidelines citing the 52-mg levonorgestrel-releasing intrauterine device (LNG IUD) as first-line treatment due to its substantial reduction in the amount of bleeding, HMB treatment indication approved by the US Food and Drug Administration (FDA), and evidence of success in those with HMB.12
Any progestin or combined hormonal medication with estrogen and a progestin will result in an approximately 60% to 90% bleeding reduction, thus providing many effective options for blood loss while considering patient preferences for bleeding pattern, route of administration, and concomitant benefits. While only 1 oral product (estradiol valerate/dienogest) is FDA approved for managementof HMB, use of any of the commercially available contraceptive products will provide substantial benefit.11,13
Nonhormonal options, such as antifibrinolytics and nonsteroidal anti-inflammatory drugs (NSAIDs), tend to be listed as second-line therapies or for those who want to avoid hormonal medications. Antifibrinolytics, such as tranexamic acid, require frequent dosing of large pills and result in approximately 40% blood loss reduction, but they are a very successful and well-tolerated method for those seeking on-demand therapy.14 NSAIDs may result in a slight bleeding reduction, but they are far less effective than other therapies.15 Antifibrinolytics have a theoretical risk of thrombosis and a contraindication to use with hormonal contraceptives; therefore, concomitant use with estrogen-containing medications is reserved for patients with refractory heavy bleeding or for heavy bleeding days during the hormone-free interval, when benefits likely outweigh potential risk.16,17
Guidelines for medical management of acute HMB typically cite 3 small comparative studies with high-dose regimens of parenteral conjugated estrogen, combined ethinyl estradiol and progestin, or oral medroxyprogesterone acetate.18,19 Dosing recommendations for the oral medications include a loading dose followed by a taper regimen that is poorly tolerated and for which there is no evidence of superior effectiveness over the standard dose.20,21 In most cases, initiation of the preferred long-term hormonal medication plan will reduce bleeding significantly within 2 to 3 days. Many clinicians who commonly treat acute HMB prescribe norethindrone acetate 5 mg daily (up to 3 times daily, if needed) for effective and safe menstrual suppression.22
Iron replenishment: Dosing frequency, dietary iron, and multivitamins
Iron repletion is usually via the oral route unless surgery is imminent, anemia is severe, or the oral route is not tolerated or effective.23 Oral iron has substantial adverse effects that limit tolerance, including nausea, epigastric pain, diarrhea, and constipation. Fortunately, evidence supports lower oral iron doses than previously used.4
Iron homeostasis is controlled by the peptide hormone hepcidin, produced by the liver, which controls mobilization of iron from the gut and spleen and aids iron absorption from the diet and supplements.24 Hepcidin levels decrease in response to high circulating levels of iron, so the ideal iron repletion dose in iron-deficient nonanemic women was determined by assessing the dose response of hepcidin. Researchers compared iron 60 mg daily for 14 days versus every other day for 28 days and found that iron absorption was greater in the every-other-day group (21.8% vs 16.3%).25 They concluded that changing iron administration to 60 mg or more in a single dose every other day is most efficient in those with iron deficiency without anemia. Since study participants did not have anemia, research is pending on whether different strategies (such as daily dosing) are more effective for more severe cases. The bottom line is that conventional high-dose divided daily oral iron administration results in reduced iron bioavailability compared with alternate-day dosing.
Increasing dietary iron is insufficient to treat low iron storage, iron deficiency, and iron deficiency anemia. Likewise, multivitamins, which contain very little elemental iron, are not recommended for repletion. Any iron salt with 60 to 120 mg of elemental iron can be used (for examples, ferrous sulfate, ferrous gluconate).25 Once ingested, stomach and pancreatic acids release elemental iron from its bound form. For that reason, absorption may be improved by administering iron at least 1 hour before a meal and avoiding antacids, including milk. Meat proteins and ascorbic acid help maintain the soluble ferrous form and also aid absorption. Tea, coffee, and tannins prevent absorption when polyphenol compounds form an insoluble complex with iron (see box at end of article). Gastrointestinal adverse effects can be minimized by decreasing the dose and taking after meals, although with reduced efficacy.
Intravenous iron treatment raises hemoglobin levels significantly faster than oral administration but is limited by cost and availability, so it is reserved for individuals with a hemoglobin level less than 9 g/dL, prior gastrointestinal or bariatric surgery, imminent surgery, and intolerance, poor adherence, or nonresponse to oral iron therapy. Several approved formulations are available, all with equivalent effectiveness and similar safety profiles. Lower-dose formulations (such as iron sucrose) may require several infusions, but higher-dose intravenous iron products (ferric carboxymaltose, low-molecular weight iron dextran, etc) have a stable carbohydrate shell that inhibits free iron release and improves safety, allowing a single administration.26
Common adverse effects of intravenous iron treatment include a metallic taste and headache during administration. More serious adverse effects, such as hypotension, arthralgia, malaise, and nausea, are usually self-limited. With mild infusion reactions (1 in 200), the infusion can be stopped until symptoms improve and can be resumed at a slower rate.27
Continue to: The role of blood transfusion...
The role of blood transfusion
Blood transfusion is expensive and potentially hazardous, so its use is limited to treatment of acute blood loss or severe anemia.
A one-time red blood cell transfusion does not impact diagnostic criteria to assess for iron deficiency with ferritin, and it does not improve underlying iron deficiency.28 Patients with acute blood loss anemia superimposed on chronic blood loss should be screened and treated for iron deficiency even after receiving a transfusion.
Since ferritin levels can rise significantly as an acute phase reactant, even following a hemorrhage, iron deficiency during inflammation is defined as ferritin less than 70 ng/mL.
The potential for iron overload
Since iron is never metabolized or excreted, it is possible to have iron overload following accidental overdose, transfusion dependency, and disorders of iron transport, such as hemochromatosis and thalassemia.
While a low ferritin level always indicates iron deficiency, high ferritin levels can be an acute phase reactant. Ferritin levels greater than 150 ng/mL in healthy menstruating individuals and greater than 500 ng/mL in unhealthy individuals should raise concern for excess iron and should prompt discontinuation of iron intake or workup for conditions at risk for overload.5
Oral iron supplements should be stored away from small children, who are at particular risk of toxicity.
How long to treat?
Treatment duration depends on the individual’s degree of iron deficiency, whether anemia is present, and the amount of ongoing blood loss. The main treatment goal is normalization and maintenance of serum ferritin.
Successful treatment should be confirmed with a complete blood count and ferritin level. Hemoglobin levels improve 2 g/dL after 3 weeks of oral iron therapy, but repletion may take 4 to 6 months.23,29 The American College of Obstetricians and Gynecologists recommends 3 to 6 months of continued iron therapy after resolution of HMB.19
In a comparative study of treatment for HMB with the 52-mg LNG IUD versus hysterectomy, hemoglobin levels increased in both treatment groups but stayed lower in those with initial anemia.8 Ferritin levels normalized only after 5 years and were still lower in individuals with initial anemia.
Increase in hemoglobin is faster after intravenous iron administration but is equivalent to oral therapy by 12 weeks. If management to reduce menstrual losses is discontinued, periodic or maintenance iron repletion will be necessary.
CASE Management plan initiated
This 17-year-old patient with iron deficiency resulting from HMB requests management to reduce menstrual iron losses with a preference for predictable menses. We have already completed a basic workup, which could also include assessment for hypermobility with a Beighton score, as connective tissue disorders also are associated with HMB.30 We discuss the options of cyclic hormonal therapy, antifibrinolytic treatment, and an LNG IUD. The patient is concerned about adherence and wants to avoid unscheduled bleeding, so she opts for a trial of tranexamic acid 1,300 mg 3 times daily for 5 days during menses. This regimen results in a 50% reduction in bleeding amount, which the patient finds satisfactory. Iron repletion with oral ferrous sulfate 325 mg (containing 65 mg of elemental iron) is administered on alternating days with vitamin C taken 1 hour prior to dinner. Repeat laboratory test results at 3 weeks show improvement to a hemoglobin level of 14.2 g/dL and a ferritin level of 12 ng/mL. By 3 months, her ferritin levels are greater than 30 ng/mL and oral iron is administered only during menses.
Summing up
Chronic HMB results in a progressive net loss of iron and eventual anemia. Screening with complete blood count and ferritin and early treatment of low iron storage when ferritin is less than 30 ng/mL will help avoid symptoms. Any amount of reduction of menstrual blood loss can be beneficial, allowing a variety of effective hormonal and nonhormonal treatment options. ●
- Take 60 to 120 mg elemental iron every other day.
- To help with absorption:
—Take 1 hour before a meal, but not with coffee, tea, tannins, antacids, or milk
—Take with vitamin C or other acidic fruit juice
- Recheck complete blood count and ferritin in 2 to 3 weeks to confirm initial response.
- Continue treatment for up to 3 to 6 months until ferritin levels are greater than 30 to 50 ng/mL.
- Munro MG, Mast AE, Powers JM, et al. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol. 2023;S00029378(23)00024-8.
- Tsakiridis I, Giouleka S, Koutsouki G, et al. Investigation and management of abnormal uterine bleeding in reproductive aged women: a descriptive review of national and international recommendations. Eur J Contracept Reprod Health Care. 2022;27:504-517.
- Camaschella C. Iron deficiency. Blood. 2019;133:30-39.
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272.
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. April 21, 2020. Accessed February 17, 2023. https://www.who.int/publications/i/item/9789240000124
- Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8: e572-e582.
- Galetti V, Stoffel NU, Sieber C, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. 2021;39:101052.
- Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67.
- Brittenham GM. Short-term periods of strenuous physical activity lower iron absorption. Am J Clin Nutr. 2021;113:261-262.
- Chen M, Lindley A, Kimport K, et al. An in-depth analysis of the use of shared decision making in contraceptive counseling. Contraception. 2019;99:187-191.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
- Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30:1-13.
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;4:CD000249.
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;9:CD000400.
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract T hromb Haemost. 2021;5:e12546.
- Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plann Reprod Health Care. 2010;36:117-122.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 785: screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. Obstet Gynecol. 2019;134:e71-e83.
- Haamid F, Sass AE, Dietrich JE. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335-340.
- Roth LP, Haley KM, Baldwin MK. A retrospective comparison of time to cessation of acute heavy menstrual bleeding in adolescents following two dose regimens of combined oral hormonal therapy. J Pediatr Adolesc Gynecol. 2022;35:294-298.
- Huguelet PS, Buyers EM, Lange-Liss JH, et al. Treatment of acute abnormal uterine bleeding in adolescents: what are providers doing in various specialties? J Pediatr Adolesc Gynecol. 2016;29:286-291.
- Elstrott B, Khan L, Olson S, et al. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104:153-161.
- Pagani A, Nai A, Silvestri L, et al. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
- Stoffel NU, von Siebenthal HK, Moretti D, et al. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Dave CV, Brittenham GM, Carson JL, et al. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175:656-664.
- Froissart A, Rossi B, Ranque B, et al; SiMFI Group. Effect of a red blood cell transfusion on biological markers used to determine the cause of anemia: a prospective study. Am J Med. 2018;131:319-322.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2022;blood.2022018521.
- Borzutzky C, Jaffray J. Diagnosis and management of heavy menstrual bleeding and bleeding disorders in adolescents. JAMA Pediatr. 2020;174:186-194.
Recurrent episodic blood loss from normal menstruation is not expected to result in anemia. But without treatment, chronic heavy periods will progress through the stages of low iron stores to iron deficiency and then to anemia. When iron storage levels are low, the bone marrow’s blood cell factory cannot keep up with continued losses. Every patient with heavy menstrual bleeding (HMB) or prolonged menstrual episodes should be tested and treated for iron deficiency and anemia.1,2
Particular attention should be paid to assessment of iron storage levels with serum ferritin, recognizing that low iron levels progress to anemia once the storage is depleted. Recovery from anemia is much slower in individuals with iron deficiency, so assessment for iron storage also should be included in preoperative assessments and following a diagnosis of acute blood loss anemia.
The mechanics of erythropoiesis, hemoglobin, and oxygen transport
Red blood cells (erythrocytes) have a short life cycle and require constant replacement. Erythrocytes are generated on demand in erythropoiesis by a hormonal signaling process, regardless of whether sufficient components are available.3 Hemoglobin, the main intracellular component of erythrocytes, is comprised of 4 globin chains, which each contain 1 iron atom bound to a heme molecule. After erythrocytes are assembled, they are sent out into circulation for approximately 120 days. A hemoglobin level measures the oxygen-carrying capacity of erythrocytes, and anemia is defined as hemoglobin less than 12 g/dL.
Unless erythrocytes are lost from bleeding, they are decommissioned—that is, the heme molecule is metabolized into bilirubin and excreted, and the iron atoms are recycled back to the bone marrow or to storage.4 Ferritin is the storage molecule that binds to iron, a glycoprotein with numerous subunits around a core that can contain about 4,000 iron atoms. Most ferritin is intracellular, but a small proportion is present in serum, where it can be measured.
Serum ferritin is a good marker for the iron supply in healthy individuals because it has high correlation to iron in the bone marrow and correlates to total intracellular storage unless there is inflammation, when mobilization to serum increases. The ferritin level at which the iron supply is deficient to meet demand, defined as iron deficiency, is hotly debated and ranges from less than 15 to 50 ng/mL in menstruating individuals, with higher thresholds based on onset of erythropoiesis signaling and the lower threshold being the World Health Organization recommendation.5-7 When iron atoms are in short supply, erythrocytes still are generated but they have lower amounts of intracellular hemoglobin, which makes them thinner, smaller, and paler—and less effective at oxygen transport.

CASE Patient seeks treatment for HMB-associated symptoms
A 17-year-old patient presents with HMB, fatigue, and difficulty with concentration. She reports that her periods have been regular and lasting 7 days since menarche at age 13. While they are manageable, they seem to be getting heavier, soaking pads in 2 to 3 hours. The patient reports that she would like to start treatment for her progressively heavy bleeding and prefers lighter scheduled bleeding; she currently does not desire contraception. The patient has no family history of bleeding problems and self-reports no personal history of epistaxis or bleeding with tooth extraction or tonsillectomy. Laboratory tests confirm iron deficiency with a hemoglobin level of 12.5 g/dL (reference range, 12.0–17.5 g/dL) and a serum ferritin level of 8 ng/mL (reference range, 50–420 ng/mL). Results from a coagulopathy panel are normal, as are von Willebrand factor levels.
Untreated iron deficiency will progress to anemia
This patient has iron deficiency without anemia, which warrants significant attention in HMB because without treatment it eventually will progress to anemia. The prevalence of iron deficiency, which makes up half of all causes of anemia, is at least double that of iron deficiency anemia.3
Adult bodies usually contain about 3 to 4 g of iron, with two-thirds in erythrocytes as hemoglobin.8 Approximately 40 to 60 mg of iron is recycled daily, 1 to 2 mg/day is lost from sloughed cells and sweat, and at least 1 mg/day is lost during normal menstruation. These losses are balanced with gastrointestinal uptake of 1 to 2 mg/day until bleeding exceeds about 10 mL/day. In this 17-year-old patient, iron stores have likely been on a progressive decline since menarche.
For normally menstruating individuals to maintain iron homeostasis, the daily dietary iron requirement is 18 mg/day. Iron requirements also increase during periods of illness or inflammation due to hormonal signaling in the iron absorption and transport pathway, in athletes due to sweating, foot strike hemolysis and bruising, and during growth spurts.9
Continue to: Managing iron deficiency and anemia...
Managing iron deficiency and anemia
Management of iron deficiency and iron deficiency anemia in the setting of HMB includes:
- workup for the etiology of the abnormal uterine bleeding (TABLE)
- reducing the source of blood loss, and
- iron supplementation to correct the iron deficiency state.
In most cases, workup, reduction, and repletion can occur simultaneously. The goal is not always complete cessation of menstrual bleeding; even short-term therapy can allow time to replenish iron storage. Use a shared decision-making process to assess what is important to the patient, and provide information about relative amounts of bleeding cessation that can be expected with various therapies.10

Treatment options
Medical treatments to decrease menstrual iron losses are recommended prior to proceeding with surgical interventions.11 Hormonal treatments are the most consistently recommended, with many guidelines citing the 52-mg levonorgestrel-releasing intrauterine device (LNG IUD) as first-line treatment due to its substantial reduction in the amount of bleeding, HMB treatment indication approved by the US Food and Drug Administration (FDA), and evidence of success in those with HMB.12
Any progestin or combined hormonal medication with estrogen and a progestin will result in an approximately 60% to 90% bleeding reduction, thus providing many effective options for blood loss while considering patient preferences for bleeding pattern, route of administration, and concomitant benefits. While only 1 oral product (estradiol valerate/dienogest) is FDA approved for managementof HMB, use of any of the commercially available contraceptive products will provide substantial benefit.11,13
Nonhormonal options, such as antifibrinolytics and nonsteroidal anti-inflammatory drugs (NSAIDs), tend to be listed as second-line therapies or for those who want to avoid hormonal medications. Antifibrinolytics, such as tranexamic acid, require frequent dosing of large pills and result in approximately 40% blood loss reduction, but they are a very successful and well-tolerated method for those seeking on-demand therapy.14 NSAIDs may result in a slight bleeding reduction, but they are far less effective than other therapies.15 Antifibrinolytics have a theoretical risk of thrombosis and a contraindication to use with hormonal contraceptives; therefore, concomitant use with estrogen-containing medications is reserved for patients with refractory heavy bleeding or for heavy bleeding days during the hormone-free interval, when benefits likely outweigh potential risk.16,17
Guidelines for medical management of acute HMB typically cite 3 small comparative studies with high-dose regimens of parenteral conjugated estrogen, combined ethinyl estradiol and progestin, or oral medroxyprogesterone acetate.18,19 Dosing recommendations for the oral medications include a loading dose followed by a taper regimen that is poorly tolerated and for which there is no evidence of superior effectiveness over the standard dose.20,21 In most cases, initiation of the preferred long-term hormonal medication plan will reduce bleeding significantly within 2 to 3 days. Many clinicians who commonly treat acute HMB prescribe norethindrone acetate 5 mg daily (up to 3 times daily, if needed) for effective and safe menstrual suppression.22
Iron replenishment: Dosing frequency, dietary iron, and multivitamins
Iron repletion is usually via the oral route unless surgery is imminent, anemia is severe, or the oral route is not tolerated or effective.23 Oral iron has substantial adverse effects that limit tolerance, including nausea, epigastric pain, diarrhea, and constipation. Fortunately, evidence supports lower oral iron doses than previously used.4
Iron homeostasis is controlled by the peptide hormone hepcidin, produced by the liver, which controls mobilization of iron from the gut and spleen and aids iron absorption from the diet and supplements.24 Hepcidin levels decrease in response to high circulating levels of iron, so the ideal iron repletion dose in iron-deficient nonanemic women was determined by assessing the dose response of hepcidin. Researchers compared iron 60 mg daily for 14 days versus every other day for 28 days and found that iron absorption was greater in the every-other-day group (21.8% vs 16.3%).25 They concluded that changing iron administration to 60 mg or more in a single dose every other day is most efficient in those with iron deficiency without anemia. Since study participants did not have anemia, research is pending on whether different strategies (such as daily dosing) are more effective for more severe cases. The bottom line is that conventional high-dose divided daily oral iron administration results in reduced iron bioavailability compared with alternate-day dosing.
Increasing dietary iron is insufficient to treat low iron storage, iron deficiency, and iron deficiency anemia. Likewise, multivitamins, which contain very little elemental iron, are not recommended for repletion. Any iron salt with 60 to 120 mg of elemental iron can be used (for examples, ferrous sulfate, ferrous gluconate).25 Once ingested, stomach and pancreatic acids release elemental iron from its bound form. For that reason, absorption may be improved by administering iron at least 1 hour before a meal and avoiding antacids, including milk. Meat proteins and ascorbic acid help maintain the soluble ferrous form and also aid absorption. Tea, coffee, and tannins prevent absorption when polyphenol compounds form an insoluble complex with iron (see box at end of article). Gastrointestinal adverse effects can be minimized by decreasing the dose and taking after meals, although with reduced efficacy.
Intravenous iron treatment raises hemoglobin levels significantly faster than oral administration but is limited by cost and availability, so it is reserved for individuals with a hemoglobin level less than 9 g/dL, prior gastrointestinal or bariatric surgery, imminent surgery, and intolerance, poor adherence, or nonresponse to oral iron therapy. Several approved formulations are available, all with equivalent effectiveness and similar safety profiles. Lower-dose formulations (such as iron sucrose) may require several infusions, but higher-dose intravenous iron products (ferric carboxymaltose, low-molecular weight iron dextran, etc) have a stable carbohydrate shell that inhibits free iron release and improves safety, allowing a single administration.26
Common adverse effects of intravenous iron treatment include a metallic taste and headache during administration. More serious adverse effects, such as hypotension, arthralgia, malaise, and nausea, are usually self-limited. With mild infusion reactions (1 in 200), the infusion can be stopped until symptoms improve and can be resumed at a slower rate.27
Continue to: The role of blood transfusion...
The role of blood transfusion
Blood transfusion is expensive and potentially hazardous, so its use is limited to treatment of acute blood loss or severe anemia.
A one-time red blood cell transfusion does not impact diagnostic criteria to assess for iron deficiency with ferritin, and it does not improve underlying iron deficiency.28 Patients with acute blood loss anemia superimposed on chronic blood loss should be screened and treated for iron deficiency even after receiving a transfusion.
Since ferritin levels can rise significantly as an acute phase reactant, even following a hemorrhage, iron deficiency during inflammation is defined as ferritin less than 70 ng/mL.
The potential for iron overload
Since iron is never metabolized or excreted, it is possible to have iron overload following accidental overdose, transfusion dependency, and disorders of iron transport, such as hemochromatosis and thalassemia.
While a low ferritin level always indicates iron deficiency, high ferritin levels can be an acute phase reactant. Ferritin levels greater than 150 ng/mL in healthy menstruating individuals and greater than 500 ng/mL in unhealthy individuals should raise concern for excess iron and should prompt discontinuation of iron intake or workup for conditions at risk for overload.5
Oral iron supplements should be stored away from small children, who are at particular risk of toxicity.
How long to treat?
Treatment duration depends on the individual’s degree of iron deficiency, whether anemia is present, and the amount of ongoing blood loss. The main treatment goal is normalization and maintenance of serum ferritin.
Successful treatment should be confirmed with a complete blood count and ferritin level. Hemoglobin levels improve 2 g/dL after 3 weeks of oral iron therapy, but repletion may take 4 to 6 months.23,29 The American College of Obstetricians and Gynecologists recommends 3 to 6 months of continued iron therapy after resolution of HMB.19
In a comparative study of treatment for HMB with the 52-mg LNG IUD versus hysterectomy, hemoglobin levels increased in both treatment groups but stayed lower in those with initial anemia.8 Ferritin levels normalized only after 5 years and were still lower in individuals with initial anemia.
Increase in hemoglobin is faster after intravenous iron administration but is equivalent to oral therapy by 12 weeks. If management to reduce menstrual losses is discontinued, periodic or maintenance iron repletion will be necessary.
CASE Management plan initiated
This 17-year-old patient with iron deficiency resulting from HMB requests management to reduce menstrual iron losses with a preference for predictable menses. We have already completed a basic workup, which could also include assessment for hypermobility with a Beighton score, as connective tissue disorders also are associated with HMB.30 We discuss the options of cyclic hormonal therapy, antifibrinolytic treatment, and an LNG IUD. The patient is concerned about adherence and wants to avoid unscheduled bleeding, so she opts for a trial of tranexamic acid 1,300 mg 3 times daily for 5 days during menses. This regimen results in a 50% reduction in bleeding amount, which the patient finds satisfactory. Iron repletion with oral ferrous sulfate 325 mg (containing 65 mg of elemental iron) is administered on alternating days with vitamin C taken 1 hour prior to dinner. Repeat laboratory test results at 3 weeks show improvement to a hemoglobin level of 14.2 g/dL and a ferritin level of 12 ng/mL. By 3 months, her ferritin levels are greater than 30 ng/mL and oral iron is administered only during menses.
Summing up
Chronic HMB results in a progressive net loss of iron and eventual anemia. Screening with complete blood count and ferritin and early treatment of low iron storage when ferritin is less than 30 ng/mL will help avoid symptoms. Any amount of reduction of menstrual blood loss can be beneficial, allowing a variety of effective hormonal and nonhormonal treatment options. ●
- Take 60 to 120 mg elemental iron every other day.
- To help with absorption:
—Take 1 hour before a meal, but not with coffee, tea, tannins, antacids, or milk
—Take with vitamin C or other acidic fruit juice
- Recheck complete blood count and ferritin in 2 to 3 weeks to confirm initial response.
- Continue treatment for up to 3 to 6 months until ferritin levels are greater than 30 to 50 ng/mL.
Recurrent episodic blood loss from normal menstruation is not expected to result in anemia. But without treatment, chronic heavy periods will progress through the stages of low iron stores to iron deficiency and then to anemia. When iron storage levels are low, the bone marrow’s blood cell factory cannot keep up with continued losses. Every patient with heavy menstrual bleeding (HMB) or prolonged menstrual episodes should be tested and treated for iron deficiency and anemia.1,2
Particular attention should be paid to assessment of iron storage levels with serum ferritin, recognizing that low iron levels progress to anemia once the storage is depleted. Recovery from anemia is much slower in individuals with iron deficiency, so assessment for iron storage also should be included in preoperative assessments and following a diagnosis of acute blood loss anemia.
The mechanics of erythropoiesis, hemoglobin, and oxygen transport
Red blood cells (erythrocytes) have a short life cycle and require constant replacement. Erythrocytes are generated on demand in erythropoiesis by a hormonal signaling process, regardless of whether sufficient components are available.3 Hemoglobin, the main intracellular component of erythrocytes, is comprised of 4 globin chains, which each contain 1 iron atom bound to a heme molecule. After erythrocytes are assembled, they are sent out into circulation for approximately 120 days. A hemoglobin level measures the oxygen-carrying capacity of erythrocytes, and anemia is defined as hemoglobin less than 12 g/dL.
Unless erythrocytes are lost from bleeding, they are decommissioned—that is, the heme molecule is metabolized into bilirubin and excreted, and the iron atoms are recycled back to the bone marrow or to storage.4 Ferritin is the storage molecule that binds to iron, a glycoprotein with numerous subunits around a core that can contain about 4,000 iron atoms. Most ferritin is intracellular, but a small proportion is present in serum, where it can be measured.
Serum ferritin is a good marker for the iron supply in healthy individuals because it has high correlation to iron in the bone marrow and correlates to total intracellular storage unless there is inflammation, when mobilization to serum increases. The ferritin level at which the iron supply is deficient to meet demand, defined as iron deficiency, is hotly debated and ranges from less than 15 to 50 ng/mL in menstruating individuals, with higher thresholds based on onset of erythropoiesis signaling and the lower threshold being the World Health Organization recommendation.5-7 When iron atoms are in short supply, erythrocytes still are generated but they have lower amounts of intracellular hemoglobin, which makes them thinner, smaller, and paler—and less effective at oxygen transport.

CASE Patient seeks treatment for HMB-associated symptoms
A 17-year-old patient presents with HMB, fatigue, and difficulty with concentration. She reports that her periods have been regular and lasting 7 days since menarche at age 13. While they are manageable, they seem to be getting heavier, soaking pads in 2 to 3 hours. The patient reports that she would like to start treatment for her progressively heavy bleeding and prefers lighter scheduled bleeding; she currently does not desire contraception. The patient has no family history of bleeding problems and self-reports no personal history of epistaxis or bleeding with tooth extraction or tonsillectomy. Laboratory tests confirm iron deficiency with a hemoglobin level of 12.5 g/dL (reference range, 12.0–17.5 g/dL) and a serum ferritin level of 8 ng/mL (reference range, 50–420 ng/mL). Results from a coagulopathy panel are normal, as are von Willebrand factor levels.
Untreated iron deficiency will progress to anemia
This patient has iron deficiency without anemia, which warrants significant attention in HMB because without treatment it eventually will progress to anemia. The prevalence of iron deficiency, which makes up half of all causes of anemia, is at least double that of iron deficiency anemia.3
Adult bodies usually contain about 3 to 4 g of iron, with two-thirds in erythrocytes as hemoglobin.8 Approximately 40 to 60 mg of iron is recycled daily, 1 to 2 mg/day is lost from sloughed cells and sweat, and at least 1 mg/day is lost during normal menstruation. These losses are balanced with gastrointestinal uptake of 1 to 2 mg/day until bleeding exceeds about 10 mL/day. In this 17-year-old patient, iron stores have likely been on a progressive decline since menarche.
For normally menstruating individuals to maintain iron homeostasis, the daily dietary iron requirement is 18 mg/day. Iron requirements also increase during periods of illness or inflammation due to hormonal signaling in the iron absorption and transport pathway, in athletes due to sweating, foot strike hemolysis and bruising, and during growth spurts.9
Continue to: Managing iron deficiency and anemia...
Managing iron deficiency and anemia
Management of iron deficiency and iron deficiency anemia in the setting of HMB includes:
- workup for the etiology of the abnormal uterine bleeding (TABLE)
- reducing the source of blood loss, and
- iron supplementation to correct the iron deficiency state.
In most cases, workup, reduction, and repletion can occur simultaneously. The goal is not always complete cessation of menstrual bleeding; even short-term therapy can allow time to replenish iron storage. Use a shared decision-making process to assess what is important to the patient, and provide information about relative amounts of bleeding cessation that can be expected with various therapies.10

Treatment options
Medical treatments to decrease menstrual iron losses are recommended prior to proceeding with surgical interventions.11 Hormonal treatments are the most consistently recommended, with many guidelines citing the 52-mg levonorgestrel-releasing intrauterine device (LNG IUD) as first-line treatment due to its substantial reduction in the amount of bleeding, HMB treatment indication approved by the US Food and Drug Administration (FDA), and evidence of success in those with HMB.12
Any progestin or combined hormonal medication with estrogen and a progestin will result in an approximately 60% to 90% bleeding reduction, thus providing many effective options for blood loss while considering patient preferences for bleeding pattern, route of administration, and concomitant benefits. While only 1 oral product (estradiol valerate/dienogest) is FDA approved for managementof HMB, use of any of the commercially available contraceptive products will provide substantial benefit.11,13
Nonhormonal options, such as antifibrinolytics and nonsteroidal anti-inflammatory drugs (NSAIDs), tend to be listed as second-line therapies or for those who want to avoid hormonal medications. Antifibrinolytics, such as tranexamic acid, require frequent dosing of large pills and result in approximately 40% blood loss reduction, but they are a very successful and well-tolerated method for those seeking on-demand therapy.14 NSAIDs may result in a slight bleeding reduction, but they are far less effective than other therapies.15 Antifibrinolytics have a theoretical risk of thrombosis and a contraindication to use with hormonal contraceptives; therefore, concomitant use with estrogen-containing medications is reserved for patients with refractory heavy bleeding or for heavy bleeding days during the hormone-free interval, when benefits likely outweigh potential risk.16,17
Guidelines for medical management of acute HMB typically cite 3 small comparative studies with high-dose regimens of parenteral conjugated estrogen, combined ethinyl estradiol and progestin, or oral medroxyprogesterone acetate.18,19 Dosing recommendations for the oral medications include a loading dose followed by a taper regimen that is poorly tolerated and for which there is no evidence of superior effectiveness over the standard dose.20,21 In most cases, initiation of the preferred long-term hormonal medication plan will reduce bleeding significantly within 2 to 3 days. Many clinicians who commonly treat acute HMB prescribe norethindrone acetate 5 mg daily (up to 3 times daily, if needed) for effective and safe menstrual suppression.22
Iron replenishment: Dosing frequency, dietary iron, and multivitamins
Iron repletion is usually via the oral route unless surgery is imminent, anemia is severe, or the oral route is not tolerated or effective.23 Oral iron has substantial adverse effects that limit tolerance, including nausea, epigastric pain, diarrhea, and constipation. Fortunately, evidence supports lower oral iron doses than previously used.4
Iron homeostasis is controlled by the peptide hormone hepcidin, produced by the liver, which controls mobilization of iron from the gut and spleen and aids iron absorption from the diet and supplements.24 Hepcidin levels decrease in response to high circulating levels of iron, so the ideal iron repletion dose in iron-deficient nonanemic women was determined by assessing the dose response of hepcidin. Researchers compared iron 60 mg daily for 14 days versus every other day for 28 days and found that iron absorption was greater in the every-other-day group (21.8% vs 16.3%).25 They concluded that changing iron administration to 60 mg or more in a single dose every other day is most efficient in those with iron deficiency without anemia. Since study participants did not have anemia, research is pending on whether different strategies (such as daily dosing) are more effective for more severe cases. The bottom line is that conventional high-dose divided daily oral iron administration results in reduced iron bioavailability compared with alternate-day dosing.
Increasing dietary iron is insufficient to treat low iron storage, iron deficiency, and iron deficiency anemia. Likewise, multivitamins, which contain very little elemental iron, are not recommended for repletion. Any iron salt with 60 to 120 mg of elemental iron can be used (for examples, ferrous sulfate, ferrous gluconate).25 Once ingested, stomach and pancreatic acids release elemental iron from its bound form. For that reason, absorption may be improved by administering iron at least 1 hour before a meal and avoiding antacids, including milk. Meat proteins and ascorbic acid help maintain the soluble ferrous form and also aid absorption. Tea, coffee, and tannins prevent absorption when polyphenol compounds form an insoluble complex with iron (see box at end of article). Gastrointestinal adverse effects can be minimized by decreasing the dose and taking after meals, although with reduced efficacy.
Intravenous iron treatment raises hemoglobin levels significantly faster than oral administration but is limited by cost and availability, so it is reserved for individuals with a hemoglobin level less than 9 g/dL, prior gastrointestinal or bariatric surgery, imminent surgery, and intolerance, poor adherence, or nonresponse to oral iron therapy. Several approved formulations are available, all with equivalent effectiveness and similar safety profiles. Lower-dose formulations (such as iron sucrose) may require several infusions, but higher-dose intravenous iron products (ferric carboxymaltose, low-molecular weight iron dextran, etc) have a stable carbohydrate shell that inhibits free iron release and improves safety, allowing a single administration.26
Common adverse effects of intravenous iron treatment include a metallic taste and headache during administration. More serious adverse effects, such as hypotension, arthralgia, malaise, and nausea, are usually self-limited. With mild infusion reactions (1 in 200), the infusion can be stopped until symptoms improve and can be resumed at a slower rate.27
Continue to: The role of blood transfusion...
The role of blood transfusion
Blood transfusion is expensive and potentially hazardous, so its use is limited to treatment of acute blood loss or severe anemia.
A one-time red blood cell transfusion does not impact diagnostic criteria to assess for iron deficiency with ferritin, and it does not improve underlying iron deficiency.28 Patients with acute blood loss anemia superimposed on chronic blood loss should be screened and treated for iron deficiency even after receiving a transfusion.
Since ferritin levels can rise significantly as an acute phase reactant, even following a hemorrhage, iron deficiency during inflammation is defined as ferritin less than 70 ng/mL.
The potential for iron overload
Since iron is never metabolized or excreted, it is possible to have iron overload following accidental overdose, transfusion dependency, and disorders of iron transport, such as hemochromatosis and thalassemia.
While a low ferritin level always indicates iron deficiency, high ferritin levels can be an acute phase reactant. Ferritin levels greater than 150 ng/mL in healthy menstruating individuals and greater than 500 ng/mL in unhealthy individuals should raise concern for excess iron and should prompt discontinuation of iron intake or workup for conditions at risk for overload.5
Oral iron supplements should be stored away from small children, who are at particular risk of toxicity.
How long to treat?
Treatment duration depends on the individual’s degree of iron deficiency, whether anemia is present, and the amount of ongoing blood loss. The main treatment goal is normalization and maintenance of serum ferritin.
Successful treatment should be confirmed with a complete blood count and ferritin level. Hemoglobin levels improve 2 g/dL after 3 weeks of oral iron therapy, but repletion may take 4 to 6 months.23,29 The American College of Obstetricians and Gynecologists recommends 3 to 6 months of continued iron therapy after resolution of HMB.19
In a comparative study of treatment for HMB with the 52-mg LNG IUD versus hysterectomy, hemoglobin levels increased in both treatment groups but stayed lower in those with initial anemia.8 Ferritin levels normalized only after 5 years and were still lower in individuals with initial anemia.
Increase in hemoglobin is faster after intravenous iron administration but is equivalent to oral therapy by 12 weeks. If management to reduce menstrual losses is discontinued, periodic or maintenance iron repletion will be necessary.
CASE Management plan initiated
This 17-year-old patient with iron deficiency resulting from HMB requests management to reduce menstrual iron losses with a preference for predictable menses. We have already completed a basic workup, which could also include assessment for hypermobility with a Beighton score, as connective tissue disorders also are associated with HMB.30 We discuss the options of cyclic hormonal therapy, antifibrinolytic treatment, and an LNG IUD. The patient is concerned about adherence and wants to avoid unscheduled bleeding, so she opts for a trial of tranexamic acid 1,300 mg 3 times daily for 5 days during menses. This regimen results in a 50% reduction in bleeding amount, which the patient finds satisfactory. Iron repletion with oral ferrous sulfate 325 mg (containing 65 mg of elemental iron) is administered on alternating days with vitamin C taken 1 hour prior to dinner. Repeat laboratory test results at 3 weeks show improvement to a hemoglobin level of 14.2 g/dL and a ferritin level of 12 ng/mL. By 3 months, her ferritin levels are greater than 30 ng/mL and oral iron is administered only during menses.
Summing up
Chronic HMB results in a progressive net loss of iron and eventual anemia. Screening with complete blood count and ferritin and early treatment of low iron storage when ferritin is less than 30 ng/mL will help avoid symptoms. Any amount of reduction of menstrual blood loss can be beneficial, allowing a variety of effective hormonal and nonhormonal treatment options. ●
- Take 60 to 120 mg elemental iron every other day.
- To help with absorption:
—Take 1 hour before a meal, but not with coffee, tea, tannins, antacids, or milk
—Take with vitamin C or other acidic fruit juice
- Recheck complete blood count and ferritin in 2 to 3 weeks to confirm initial response.
- Continue treatment for up to 3 to 6 months until ferritin levels are greater than 30 to 50 ng/mL.
- Munro MG, Mast AE, Powers JM, et al. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol. 2023;S00029378(23)00024-8.
- Tsakiridis I, Giouleka S, Koutsouki G, et al. Investigation and management of abnormal uterine bleeding in reproductive aged women: a descriptive review of national and international recommendations. Eur J Contracept Reprod Health Care. 2022;27:504-517.
- Camaschella C. Iron deficiency. Blood. 2019;133:30-39.
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272.
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. April 21, 2020. Accessed February 17, 2023. https://www.who.int/publications/i/item/9789240000124
- Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8: e572-e582.
- Galetti V, Stoffel NU, Sieber C, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. 2021;39:101052.
- Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67.
- Brittenham GM. Short-term periods of strenuous physical activity lower iron absorption. Am J Clin Nutr. 2021;113:261-262.
- Chen M, Lindley A, Kimport K, et al. An in-depth analysis of the use of shared decision making in contraceptive counseling. Contraception. 2019;99:187-191.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
- Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30:1-13.
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;4:CD000249.
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;9:CD000400.
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract T hromb Haemost. 2021;5:e12546.
- Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plann Reprod Health Care. 2010;36:117-122.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 785: screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. Obstet Gynecol. 2019;134:e71-e83.
- Haamid F, Sass AE, Dietrich JE. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335-340.
- Roth LP, Haley KM, Baldwin MK. A retrospective comparison of time to cessation of acute heavy menstrual bleeding in adolescents following two dose regimens of combined oral hormonal therapy. J Pediatr Adolesc Gynecol. 2022;35:294-298.
- Huguelet PS, Buyers EM, Lange-Liss JH, et al. Treatment of acute abnormal uterine bleeding in adolescents: what are providers doing in various specialties? J Pediatr Adolesc Gynecol. 2016;29:286-291.
- Elstrott B, Khan L, Olson S, et al. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104:153-161.
- Pagani A, Nai A, Silvestri L, et al. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
- Stoffel NU, von Siebenthal HK, Moretti D, et al. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Dave CV, Brittenham GM, Carson JL, et al. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175:656-664.
- Froissart A, Rossi B, Ranque B, et al; SiMFI Group. Effect of a red blood cell transfusion on biological markers used to determine the cause of anemia: a prospective study. Am J Med. 2018;131:319-322.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2022;blood.2022018521.
- Borzutzky C, Jaffray J. Diagnosis and management of heavy menstrual bleeding and bleeding disorders in adolescents. JAMA Pediatr. 2020;174:186-194.
- Munro MG, Mast AE, Powers JM, et al. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol. 2023;S00029378(23)00024-8.
- Tsakiridis I, Giouleka S, Koutsouki G, et al. Investigation and management of abnormal uterine bleeding in reproductive aged women: a descriptive review of national and international recommendations. Eur J Contracept Reprod Health Care. 2022;27:504-517.
- Camaschella C. Iron deficiency. Blood. 2019;133:30-39.
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272.
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. April 21, 2020. Accessed February 17, 2023. https://www.who.int/publications/i/item/9789240000124
- Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8: e572-e582.
- Galetti V, Stoffel NU, Sieber C, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. 2021;39:101052.
- Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67.
- Brittenham GM. Short-term periods of strenuous physical activity lower iron absorption. Am J Clin Nutr. 2021;113:261-262.
- Chen M, Lindley A, Kimport K, et al. An in-depth analysis of the use of shared decision making in contraceptive counseling. Contraception. 2019;99:187-191.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
- Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30:1-13.
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;4:CD000249.
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;9:CD000400.
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract T hromb Haemost. 2021;5:e12546.
- Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plann Reprod Health Care. 2010;36:117-122.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 785: screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. Obstet Gynecol. 2019;134:e71-e83.
- Haamid F, Sass AE, Dietrich JE. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335-340.
- Roth LP, Haley KM, Baldwin MK. A retrospective comparison of time to cessation of acute heavy menstrual bleeding in adolescents following two dose regimens of combined oral hormonal therapy. J Pediatr Adolesc Gynecol. 2022;35:294-298.
- Huguelet PS, Buyers EM, Lange-Liss JH, et al. Treatment of acute abnormal uterine bleeding in adolescents: what are providers doing in various specialties? J Pediatr Adolesc Gynecol. 2016;29:286-291.
- Elstrott B, Khan L, Olson S, et al. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104:153-161.
- Pagani A, Nai A, Silvestri L, et al. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
- Stoffel NU, von Siebenthal HK, Moretti D, et al. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Dave CV, Brittenham GM, Carson JL, et al. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175:656-664.
- Froissart A, Rossi B, Ranque B, et al; SiMFI Group. Effect of a red blood cell transfusion on biological markers used to determine the cause of anemia: a prospective study. Am J Med. 2018;131:319-322.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2022;blood.2022018521.
- Borzutzky C, Jaffray J. Diagnosis and management of heavy menstrual bleeding and bleeding disorders in adolescents. JAMA Pediatr. 2020;174:186-194.
Prepare for endometriosis excision surgery with a multidisciplinary approach
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Ninety-four women allege a Utah doctor sexually assaulted them. Here’s why a judge threw out their case
This article was produced for ProPublica’s Local Reporting Network in partnership with The Salt Lake Tribune.
At 19 years old and about to be married, Stephanie Mateer went to an ob.gyn. within walking distance of her student housing near Brigham Young University in Provo, Utah.
She wanted to start using birth control, and she was looking for guidance about having sex for the first time on her 2008 wedding night.
Ms. Mateer was shocked, she said, when David Broadbent, MD, reached under her gown to grab and squeeze her breasts, started a vaginal exam without warning, then followed it with an extremely painful examination of her rectum.
She felt disgusted and violated, but doubt also crept in. She told herself she must have misinterpreted his actions, or that she should have known that he would do a rectal exam. Raised as a member of The Church of Jesus Christ of Latter-day Saints, she said she was taught to defer to men in leadership.
“I viewed him as being a man in authority,” Ms. Mateer said. “He’s a doctor.”
It was years, she said, before she learned that her experience was in a sharp contrast to the conduct called for in professional standards, including that doctors use only their fingertips during a breast exam and communicate clearly what they are doing in advance, to gain the consent of their patients. Eventually, she gave her experience another name: sexual assault.
Utah judges, however, have called it health care.
And that legal distinction means Utahns like Ms. Mateer who decide to sue a health care provider for alleged sexual abuse are treated more harshly by the court system than plaintiffs who say they were harmed in other settings.
The chance to go to civil court for damages is an important option for survivors, experts say. While a criminal conviction can provide a sense of justice, winning a lawsuit can help victims pay for the therapy and additional support they need to heal after trauma.
Ms. Mateer laid out her allegations in a lawsuit that she and 93 other women filed against Dr. Broadbent last year. But they quickly learned they would be treated differently than other sexual assault survivors.
Filing their case, which alleged the Utah County doctor sexually assaulted them over the span of his 47-year career, was an empowering moment, Ms. Mateer said. But a judge threw out the lawsuit without even considering the merits, determining that because their alleged assailant is a doctor, the case must be governed by medical malpractice rules rather than those that apply to cases of sexual assault.
Under Utah’s rules of medical malpractice, claims made by victims who allege a health care worker sexually assaulted them are literally worth less than lawsuits brought by someone who was assaulted in other settings – even if a jury rules in their favor, a judge is required to limit how much money they receive. And they must meet a shorter filing deadline.
“It’s just crazy that a doctor can sexually assault women and then be protected by the white coat,” Ms. Mateer said. “It’s just a really scary precedent to be calling sexual assault ‘health care.’ ”
Because of the judge’s ruling that leaves them with a shorter window in which to file, some of Dr. Broadbent’s accusers stand to lose their chance to sue. Others were already past that deadline but had hoped to take advantage of an exception that allows plaintiffs to sue if they can prove that the person who harmed them had covered up the wrongdoing and if they discovered they had been hurt within the previous year.
As a group, the women are appealing the ruling to the Utah Supreme Court, which has agreed to hear the case. This decision will set a precedent for future sexual assault victims in Utah.
Dr. Broadbent’s attorney, Chris Nelson, declined an interview request but wrote in an email: “We believe that the allegations against Dr. Broadbent are without merit and will present our case in court. Given that this is an active legal matter, we will not be sharing any details outside the courtroom.”
States have varying legal definitions of medical malpractice, but it’s generally described as treatment that falls short of accepted standards of care. That includes mistakes, such as a surgeon leaving a piece of gauze inside a patient.
The Utah Supreme Court has ruled that a teenage boy was receiving health care when he was allowed to climb a steep, snow-dusted rock outcrop as part of wilderness therapy. When he broke his leg, he could only sue for medical malpractice, so the case faced shorter filing deadlines and lower monetary caps. Similarly, the court has ruled that a boy harmed by another child while in foster care was also bound by medical malpractice law.
Despite these state Supreme Court rulings, Utah legislators have so far not moved to narrow the wording of the malpractice act.
The lawsuit against Dr. Broadbent – and the questions it raises about the broadness of Utah’s medical malpractice laws – comes during a national reckoning with how sexual assault survivors are treated by the law. Legislators in several states have been rewriting laws to give sexual assault victims more time to sue their attackers, in response to the growing cultural understanding of the impact of trauma and the barriers to reporting. Even in Utah, those who were sexually abused as children now have no deadline to file suits against their abusers.
That isn’t true for sexual abuse in a medical setting, where cases must be filed within 2 years of the assault.
These higher hurdles should not exist in Utah, said state Sen. Mike K. McKell, a Utah County Republican who works as a personal injury attorney. He is trying to change state law to ensure that sexual assault lawsuits do not fall under Utah’s Health Care Malpractice Act, a law designed to cover negligence and poor care, not necessarily deliberate actions like an assault.
“Sexual assault, to me, is not medical care. Period,” he said. “It’s sad that we need to clarify that sexual assault is not medical care. But trying to tie sexual assault to a medical malpractice [filing deadline] – it’s just wrong.”
‘Your husband is a lucky man’
Ms. Mateer had gone to Dr. Broadbent in 2008 for a premarital exam, a uniquely Utah visit often scheduled by young women who are members of The Church of Jesus Christ of Latter-day Saints.
Leaders of the faith, which is predominant in Utah, focus on chastity when speaking to young, unmarried people about sex, and public schools have typically focused on abstinence-based sex education. So for some, these visits are the first place they learn about sexual health.
Young women who get premarital exams are typically given a birth control prescription, but the appointments can include care that’s less common for healthy women in other states – such as doctors giving them vaginal dilators to stretch their tissues before their wedding nights.
That’s what Ms. Mateer was expecting when she visited Dr. Broadbent’s office. The ob.gyn. had been practicing for decades in his Provo clinic nestled between student housing apartments across the street from Brigham Young University, which is owned by The Church of Jesus Christ of Latter-day Saints.
So Ms. Mateer was “just totally taken aback,” she said, by the painful examination and by Dr. Broadbent snapping off his gloves after the exam and saying, “Your husband is a lucky man.”
She repeated that remark in her legal filing, along with the doctor’s advice for her: If she bled during intercourse, “just do what the Boy Scouts do and apply pressure.”
“The whole thing was like I’m some object for my husband to enjoy and let him do whatever he wants,” Ms. Mateer said. “It was just very violating and not a great way to start my sexual relationship with my new husband, with these ideas in mind.”
Ms. Mateer thought back to that visit over the years, particularly when she went to other ob.gyns. for health care. Her subsequent doctors, she said, never performed a rectal exam and always explained to her what they were doing and how it would feel, and asked for her consent.
She thought about Dr. Broadbent again in 2017, as the #MeToo movement gained momentum, and looked him up online. Ms. Mateer found reviews from other women who described Dr. Broadbent doing rough examinations without warning that left them feeling the same way she had years before.
Then in December 2021, she spoke out on “Mormon Stories,” a podcast where people who have left or have questioned their Latter-day Saint faith share their life stories. In the episode, she described the painful way he examined her, how it left her feeling traumatized, and her discovery of the reviews that echoed her experience.
“He’s on University Avenue, in Provo, giving these exams to who knows how many naive Mormon 18-year-old, 19-year-old girls who are getting married. … They are naive and they don’t know what to expect,” she said on the podcast. “His name is Dr. David Broadbent.”
After the podcast aired, Ms. Mateer was flooded with messages from women who heard the episode and reached out to tell her that Dr. Broadbent had harmed them, too.
Ms. Mateer and three other women decided to sue the ob.gyn., and in the following weeks and months, 90 additional women joined the lawsuit they filed in Provo. Many of the women allege Dr. Broadbent inappropriately touched their breasts, vaginas and rectums, hurting them, without warning or explanation. Some said he used his bare hand – instead of using a speculum or gloves – during exams. One alleged that she saw he had an erection while he was touching her.
Dr. Broadbent’s actions were not medically necessary, the women allege, and were instead “performed for no other reason than his own sexual gratification.”
The lawsuit also named as defendants two hospitals where Dr. Broadbent had delivered babies and where some of the women allege they were assaulted. The suit accused hospital administrators of knowing about Dr. Broadbent’s inappropriate behavior and doing nothing about it.
After he was sued, the ob.gyn. quickly lost his privileges at the hospitals where he worked. Dr. Broadbent, now 75, has also voluntarily put his medical license in Utah on hold while police investigate 29 reports of sexual assault made against him.
Prosecutors are still considering whether to criminally prosecute Dr. Broadbent. Provo police forwarded more than a dozen reports to the Utah County attorney’s office in November, which are still being reviewed by a local prosecutor.
A spokesperson for Intermountain Health, the nonprofit health system that owns Utah Valley Hospital, where some of the women in the suit were treated, did not respond to specific questions. The spokesperson emphasized in an email that Dr. Broadbent was an “independent physician” who was not employed by Utah Valley Hospital, adding that most of the alleged incidents took place at Dr. Broadbent’s medical office.
A representative for MountainStar Healthcare, another hospital chain named as a defendant, denied knowledge of any allegations of inappropriate conduct reported to its hospital and also emphasized that Dr. Broadbent worked independently, not as an employee.
“Our position since this lawsuit was filed has been that we were inappropriately named in this suit,” said Brittany Glas, the communications director for MountainStar.
Debating whether sexual abuse is health care
For the women who sued Dr. Broadbent, their case boiled down to a key question: Were the sexual assaults they say they experienced part of their health care? There was a lot hanging on the answer.
If their case was considered medical malpractice, they would be limited in how much money they could receive in damages for their pain and suffering. If a jury awarded them millions of dollars, a judge would be required by law to cut that down to $450,000. There’s no cap on these monetary awards for victims sexually assaulted in other settings.
They would also be required to go before a panel, which includes a doctor, a lawyer and a community member, that decides whether their claims have merit. This step, aimed at resolving disputes out of court, does not block anyone from suing afterward. But it does add cost and delay, and for sexual assault victims who’ve gone through this step, it has been another time they were required to describe their experiences and hope they were believed.
The shorter, 2-year filing deadline for medical malpractice cases can also be a particular challenge for those who have been sexually abused because research shows that it’s common to delay reporting such assaults.
Nationwide, these kinds of malpractice reforms were adopted in the 1970s amid concerns – largely driven by insurance companies – that the cost of health care was rising because of frivolous lawsuits and “runaway juries” doling out multimillion-dollar payouts.
Restricting the size of malpractice awards and imposing other limits, many argued, were effective ways to balance compensating injured patients with protecting everyone’s access to health care.
State laws are generally silent on whether sexual assault lawsuits should be covered by malpractice laws, leaving courts to grapple with that question and leading to different conclusions across the country. The Tribune and ProPublica identified at least six cases in which state appellate judges sharply distinguished between assault and health care in considering whether malpractice laws should apply to sexual assault–related cases.
An appellate court in Wisconsin, for example, ruled in 1993 that a physician having an erection and groping a patient was a purposeful harm, not medical malpractice.
Florida’s law is similar to Utah’s, defining allegations “arising” out of medical care as malpractice. While an earlier ruling did treat sexual assault in a health care setting as medical malpractice, appellate rulings in the last decade have moved away from that interpretation. In 2005, an appellate court affirmed a lower-court ruling that when a dentist “stopped providing dental treatment to the victim and began sexually assaulting her, his professional services ended.”
Similarly, a federal judge in Iowa in 1995 weighed in on the meaning of “arising” out of health care: “Rape is not patient care activity,” he wrote.
But Utah’s malpractice law is so broad that judges have been interpreting it as covering any act performed by a health care provider during medical care. The law was passed in 1976 and is popular with doctors and other health care providers, who have lobbied to keep it in place – and who use it to get lawsuits dismissed.
One precedent-setting case in Utah shows the law’s power to safeguard health care providers and was an important test of how Utah defines medical malpractice. Jacob Scott sued WinGate Wilderness Therapy after the teen broke his leg in 2015 when a hiking guide from the center allowed him to climb up and down a steep outcrop in Utah’s red rock desert.
His parents are both lawyers, and after they found that Utah had a 4-year deadline for filing a personal injury lawsuit, court records said, they decided to prioritize “getting Jacob better” for the first 2 years after the accident. But when Mr. Scott’s suit was filed, WinGate argued it was too late – based on the shorter, 2-year deadline for medical malpractice claims.
Mr. Scott’s attorneys scoffed. “Interacting with nature,” his attorneys argued, “is not health care even under the broadest interpretation of … the Utah Health Care Malpractice Act.”
A judge disagreed and threw out Mr. Scott’s case. The Utah Supreme Court unanimously upheld that ruling in 2021.
“We agree with WinGate,” the justices wrote, “that it was acting as a ‘health care provider’ and providing ‘health care’ when Jacob was hiking and rock climbing.”
Last summer, the women who had sued Dr. Broadbent and the two hospitals watched online as lawyers debated whether the abuse they allegedly suffered was health care.
At the hearing, attorneys for Dr. Broadbent and the hospitals argued that the women should have pursued a medical malpractice case, which required them to first notify Dr. Broadbent and the hospitals that they wanted to sue. They also argued to Judge Robert Lunnen that the case couldn’t move forward because the women hadn’t gone before a prelitigation panel.
Attorneys for Dr. Broadbent and the hospitals argued, one after the other, that the painful and traumatic exams the women described arose out of health care treatments.
“Accepting the allegations of the complaint as true – as we must for purposes of this proceeding – we have to assume that [Broadbent] did something that was medically unnecessary, medically inappropriate,” argued David Jordan, a lawyer for Intermountain Health.
“But it doesn’t change the fact that it’s an act performed to a patient, during the patient’s treatment,” he said. “Because that’s what the patient is doing in the doctor’s office. They’re there for treatment.”
The attorney team for the women pushed back. Terry Rooney argued that if Dr. Broadbent’s actions fell under medical malpractice laws, many women would be knocked out of the case because of the age of their claims, and those who remained would be limited in the amount of money in damages they could receive.
“That’s really what this is about,” he argued. “And so it’s troubling – quite frankly it’s shocking to me – that we’re debating heavily the question of whether sexual abuse is health care.”
The judge mulled the issue for months. Judge Lunnen wrote in a September ruling that if the allegations were true, Dr. Broadbent’s treatment of his patients was “insensitive, disrespectful and degrading.”
But Utah law is clear, he said. Malpractice law covers any act or treatment performed by any health care provider during the patient’s medical care. The women had all been seeking health care, Judge Lunnen wrote, and Dr. Broadbent was providing that when the alleged assaults happened.
Their lawsuit was dismissed.
‘I felt defeated’
Brooke, another plaintiff who alleges Dr. Broadbent groped her, remembers feeling sick on the June day she watched the attorneys arguing. She asked to be identified by only her first name for this story.
She alleges Dr. Broadbent violated her in December 2008 while she was hospitalized after experiencing complications with her first pregnancy.
The nearest hospital to her rural town didn’t have a special unit to take care of premature babies, and her doctors feared she might need to deliver her son 6 weeks early. So Brooke had been rushed by ambulance over a mountain pass in a snowstorm to Utah Valley Hospital.
Brooke and her husband were terrified, she said, when they arrived at the Provo hospital. Dr. Broadbent happened to be the doctor on call. With Brooke’s husband and brother-in-law in the room, Dr. Broadbent examined her late that evening, she said, listening to her chest with a stethoscope.
The doctor then suddenly grabbed her breasts, she recalled – his movements causing her hospital gown to fall to expose her chest. She recounted this experience in her lawsuit, saying it was nothing like the breast exams she has had since.
“It was really traumatizing,” she said. “I was mortified. My husband and brother-in-law – we just didn’t say anything about it because it was so uncomfortable.”
Brooke voiced concerns to the nurse manager, and she was assigned a new doctor.
She gave birth to a healthy baby a little more than a month later, at the hospital near her home.
Hearing the judge’s ruling 14 years later, Brooke felt the decision revealed how Utah’s laws are broken.
“I was frustrated,” she said, “and I felt defeated. … I thought justice is not on our side with this.”
If the Utah Supreme Court rules that these alleged sexual assaults should legally be considered health care, the women will likely refile their claims as a medical malpractice lawsuit, said their attorney, Adam Sorensen. But it would be a challenge to keep all 94 women in the case, he said, due to the shorter filing window. Only two women in the lawsuit allege that they were harmed within the last 2 years.
The legal team for the women would have to convince a judge that their claims should still be allowed because they only recently discovered they were harmed. But based on previous rulings, Mr. Sorensen believes the women will have a better chance to win that argument if the civil suit remained a sexual assault case.
Regardless of what happens in their legal case, the decision by Brooke and the other women to come forward could help change state law for victims who come after them.
Recently, Mr. McKell, the state senator, introduced legislation to clarify that civil lawsuits alleging sexual assault by a health care worker do not fall under Utah’s Health Care Malpractice Act.
“I don’t think it’s a close call. Sexual assault is not medical care,” he said. “I know we’ve got some bizarre rulings that have come down through our courts in Utah.”
Both an association of Utah trial lawyers and the Utah Medical Association, which lobbies on behalf of the state’s physicians, support this reform.
“We support the fact that sexual assault should not be part of health care medical malpractice,” said Michelle McOmber, the CEO for the Utah Medical Association. “Sexual assault should be sexual assault, regardless of where it happens or who’s doing it. Sexual assault should be in that category, which is separate from actual health care. Because it’s not health care.”
MountainStar doesn’t have a position on the bill, Ms. Glas said. “If the laws were to change via new legislation and/or interpretation by the courts, we would abide by and comply with those new laws.”
But lawmakers are running out of time. With only a short time left in Utah’s legislative session, state senate and house leaders have so far prioritized passing new laws banning gender-affirming health care for transgender youths and creating a controversial school voucher program that will provide taxpayer funds for students to attend private school.
Utah lawmakers were also expected to consider a dramatic change for other sexual assault victims: a bill that would remove filing deadlines for civil lawsuits brought by people abused as adults. But that bill stalled before it could be debated.
Brooke had been eager to share her story, she said, in hopes it would help the first four women who’d come forward bolster their lawsuit against Dr. Broadbent. She later joined the case as a plaintiff. She read in their lawsuit about one woman who complained about him to the same hospital 7 years before she did, and about another woman who said Dr. Broadbent similarly molested her 2 days after Brooke had expressed her own concern.
“That bothered me so much,” she said. “It didn’t have to happen to all these women.”
Brooke doubts she’ll get vindication in a courtroom. Justice for her, she suspects, won’t come in the form of a legal ruling or a settlement against the doctor she says hurt her years ago.
Instead, she said, “maybe justice looks like changing the laws for future women.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
This article was produced for ProPublica’s Local Reporting Network in partnership with The Salt Lake Tribune.
At 19 years old and about to be married, Stephanie Mateer went to an ob.gyn. within walking distance of her student housing near Brigham Young University in Provo, Utah.
She wanted to start using birth control, and she was looking for guidance about having sex for the first time on her 2008 wedding night.
Ms. Mateer was shocked, she said, when David Broadbent, MD, reached under her gown to grab and squeeze her breasts, started a vaginal exam without warning, then followed it with an extremely painful examination of her rectum.
She felt disgusted and violated, but doubt also crept in. She told herself she must have misinterpreted his actions, or that she should have known that he would do a rectal exam. Raised as a member of The Church of Jesus Christ of Latter-day Saints, she said she was taught to defer to men in leadership.
“I viewed him as being a man in authority,” Ms. Mateer said. “He’s a doctor.”
It was years, she said, before she learned that her experience was in a sharp contrast to the conduct called for in professional standards, including that doctors use only their fingertips during a breast exam and communicate clearly what they are doing in advance, to gain the consent of their patients. Eventually, she gave her experience another name: sexual assault.
Utah judges, however, have called it health care.
And that legal distinction means Utahns like Ms. Mateer who decide to sue a health care provider for alleged sexual abuse are treated more harshly by the court system than plaintiffs who say they were harmed in other settings.
The chance to go to civil court for damages is an important option for survivors, experts say. While a criminal conviction can provide a sense of justice, winning a lawsuit can help victims pay for the therapy and additional support they need to heal after trauma.
Ms. Mateer laid out her allegations in a lawsuit that she and 93 other women filed against Dr. Broadbent last year. But they quickly learned they would be treated differently than other sexual assault survivors.
Filing their case, which alleged the Utah County doctor sexually assaulted them over the span of his 47-year career, was an empowering moment, Ms. Mateer said. But a judge threw out the lawsuit without even considering the merits, determining that because their alleged assailant is a doctor, the case must be governed by medical malpractice rules rather than those that apply to cases of sexual assault.
Under Utah’s rules of medical malpractice, claims made by victims who allege a health care worker sexually assaulted them are literally worth less than lawsuits brought by someone who was assaulted in other settings – even if a jury rules in their favor, a judge is required to limit how much money they receive. And they must meet a shorter filing deadline.
“It’s just crazy that a doctor can sexually assault women and then be protected by the white coat,” Ms. Mateer said. “It’s just a really scary precedent to be calling sexual assault ‘health care.’ ”
Because of the judge’s ruling that leaves them with a shorter window in which to file, some of Dr. Broadbent’s accusers stand to lose their chance to sue. Others were already past that deadline but had hoped to take advantage of an exception that allows plaintiffs to sue if they can prove that the person who harmed them had covered up the wrongdoing and if they discovered they had been hurt within the previous year.
As a group, the women are appealing the ruling to the Utah Supreme Court, which has agreed to hear the case. This decision will set a precedent for future sexual assault victims in Utah.
Dr. Broadbent’s attorney, Chris Nelson, declined an interview request but wrote in an email: “We believe that the allegations against Dr. Broadbent are without merit and will present our case in court. Given that this is an active legal matter, we will not be sharing any details outside the courtroom.”
States have varying legal definitions of medical malpractice, but it’s generally described as treatment that falls short of accepted standards of care. That includes mistakes, such as a surgeon leaving a piece of gauze inside a patient.
The Utah Supreme Court has ruled that a teenage boy was receiving health care when he was allowed to climb a steep, snow-dusted rock outcrop as part of wilderness therapy. When he broke his leg, he could only sue for medical malpractice, so the case faced shorter filing deadlines and lower monetary caps. Similarly, the court has ruled that a boy harmed by another child while in foster care was also bound by medical malpractice law.
Despite these state Supreme Court rulings, Utah legislators have so far not moved to narrow the wording of the malpractice act.
The lawsuit against Dr. Broadbent – and the questions it raises about the broadness of Utah’s medical malpractice laws – comes during a national reckoning with how sexual assault survivors are treated by the law. Legislators in several states have been rewriting laws to give sexual assault victims more time to sue their attackers, in response to the growing cultural understanding of the impact of trauma and the barriers to reporting. Even in Utah, those who were sexually abused as children now have no deadline to file suits against their abusers.
That isn’t true for sexual abuse in a medical setting, where cases must be filed within 2 years of the assault.
These higher hurdles should not exist in Utah, said state Sen. Mike K. McKell, a Utah County Republican who works as a personal injury attorney. He is trying to change state law to ensure that sexual assault lawsuits do not fall under Utah’s Health Care Malpractice Act, a law designed to cover negligence and poor care, not necessarily deliberate actions like an assault.
“Sexual assault, to me, is not medical care. Period,” he said. “It’s sad that we need to clarify that sexual assault is not medical care. But trying to tie sexual assault to a medical malpractice [filing deadline] – it’s just wrong.”
‘Your husband is a lucky man’
Ms. Mateer had gone to Dr. Broadbent in 2008 for a premarital exam, a uniquely Utah visit often scheduled by young women who are members of The Church of Jesus Christ of Latter-day Saints.
Leaders of the faith, which is predominant in Utah, focus on chastity when speaking to young, unmarried people about sex, and public schools have typically focused on abstinence-based sex education. So for some, these visits are the first place they learn about sexual health.
Young women who get premarital exams are typically given a birth control prescription, but the appointments can include care that’s less common for healthy women in other states – such as doctors giving them vaginal dilators to stretch their tissues before their wedding nights.
That’s what Ms. Mateer was expecting when she visited Dr. Broadbent’s office. The ob.gyn. had been practicing for decades in his Provo clinic nestled between student housing apartments across the street from Brigham Young University, which is owned by The Church of Jesus Christ of Latter-day Saints.
So Ms. Mateer was “just totally taken aback,” she said, by the painful examination and by Dr. Broadbent snapping off his gloves after the exam and saying, “Your husband is a lucky man.”
She repeated that remark in her legal filing, along with the doctor’s advice for her: If she bled during intercourse, “just do what the Boy Scouts do and apply pressure.”
“The whole thing was like I’m some object for my husband to enjoy and let him do whatever he wants,” Ms. Mateer said. “It was just very violating and not a great way to start my sexual relationship with my new husband, with these ideas in mind.”
Ms. Mateer thought back to that visit over the years, particularly when she went to other ob.gyns. for health care. Her subsequent doctors, she said, never performed a rectal exam and always explained to her what they were doing and how it would feel, and asked for her consent.
She thought about Dr. Broadbent again in 2017, as the #MeToo movement gained momentum, and looked him up online. Ms. Mateer found reviews from other women who described Dr. Broadbent doing rough examinations without warning that left them feeling the same way she had years before.
Then in December 2021, she spoke out on “Mormon Stories,” a podcast where people who have left or have questioned their Latter-day Saint faith share their life stories. In the episode, she described the painful way he examined her, how it left her feeling traumatized, and her discovery of the reviews that echoed her experience.
“He’s on University Avenue, in Provo, giving these exams to who knows how many naive Mormon 18-year-old, 19-year-old girls who are getting married. … They are naive and they don’t know what to expect,” she said on the podcast. “His name is Dr. David Broadbent.”
After the podcast aired, Ms. Mateer was flooded with messages from women who heard the episode and reached out to tell her that Dr. Broadbent had harmed them, too.
Ms. Mateer and three other women decided to sue the ob.gyn., and in the following weeks and months, 90 additional women joined the lawsuit they filed in Provo. Many of the women allege Dr. Broadbent inappropriately touched their breasts, vaginas and rectums, hurting them, without warning or explanation. Some said he used his bare hand – instead of using a speculum or gloves – during exams. One alleged that she saw he had an erection while he was touching her.
Dr. Broadbent’s actions were not medically necessary, the women allege, and were instead “performed for no other reason than his own sexual gratification.”
The lawsuit also named as defendants two hospitals where Dr. Broadbent had delivered babies and where some of the women allege they were assaulted. The suit accused hospital administrators of knowing about Dr. Broadbent’s inappropriate behavior and doing nothing about it.
After he was sued, the ob.gyn. quickly lost his privileges at the hospitals where he worked. Dr. Broadbent, now 75, has also voluntarily put his medical license in Utah on hold while police investigate 29 reports of sexual assault made against him.
Prosecutors are still considering whether to criminally prosecute Dr. Broadbent. Provo police forwarded more than a dozen reports to the Utah County attorney’s office in November, which are still being reviewed by a local prosecutor.
A spokesperson for Intermountain Health, the nonprofit health system that owns Utah Valley Hospital, where some of the women in the suit were treated, did not respond to specific questions. The spokesperson emphasized in an email that Dr. Broadbent was an “independent physician” who was not employed by Utah Valley Hospital, adding that most of the alleged incidents took place at Dr. Broadbent’s medical office.
A representative for MountainStar Healthcare, another hospital chain named as a defendant, denied knowledge of any allegations of inappropriate conduct reported to its hospital and also emphasized that Dr. Broadbent worked independently, not as an employee.
“Our position since this lawsuit was filed has been that we were inappropriately named in this suit,” said Brittany Glas, the communications director for MountainStar.
Debating whether sexual abuse is health care
For the women who sued Dr. Broadbent, their case boiled down to a key question: Were the sexual assaults they say they experienced part of their health care? There was a lot hanging on the answer.
If their case was considered medical malpractice, they would be limited in how much money they could receive in damages for their pain and suffering. If a jury awarded them millions of dollars, a judge would be required by law to cut that down to $450,000. There’s no cap on these monetary awards for victims sexually assaulted in other settings.
They would also be required to go before a panel, which includes a doctor, a lawyer and a community member, that decides whether their claims have merit. This step, aimed at resolving disputes out of court, does not block anyone from suing afterward. But it does add cost and delay, and for sexual assault victims who’ve gone through this step, it has been another time they were required to describe their experiences and hope they were believed.
The shorter, 2-year filing deadline for medical malpractice cases can also be a particular challenge for those who have been sexually abused because research shows that it’s common to delay reporting such assaults.
Nationwide, these kinds of malpractice reforms were adopted in the 1970s amid concerns – largely driven by insurance companies – that the cost of health care was rising because of frivolous lawsuits and “runaway juries” doling out multimillion-dollar payouts.
Restricting the size of malpractice awards and imposing other limits, many argued, were effective ways to balance compensating injured patients with protecting everyone’s access to health care.
State laws are generally silent on whether sexual assault lawsuits should be covered by malpractice laws, leaving courts to grapple with that question and leading to different conclusions across the country. The Tribune and ProPublica identified at least six cases in which state appellate judges sharply distinguished between assault and health care in considering whether malpractice laws should apply to sexual assault–related cases.
An appellate court in Wisconsin, for example, ruled in 1993 that a physician having an erection and groping a patient was a purposeful harm, not medical malpractice.
Florida’s law is similar to Utah’s, defining allegations “arising” out of medical care as malpractice. While an earlier ruling did treat sexual assault in a health care setting as medical malpractice, appellate rulings in the last decade have moved away from that interpretation. In 2005, an appellate court affirmed a lower-court ruling that when a dentist “stopped providing dental treatment to the victim and began sexually assaulting her, his professional services ended.”
Similarly, a federal judge in Iowa in 1995 weighed in on the meaning of “arising” out of health care: “Rape is not patient care activity,” he wrote.
But Utah’s malpractice law is so broad that judges have been interpreting it as covering any act performed by a health care provider during medical care. The law was passed in 1976 and is popular with doctors and other health care providers, who have lobbied to keep it in place – and who use it to get lawsuits dismissed.
One precedent-setting case in Utah shows the law’s power to safeguard health care providers and was an important test of how Utah defines medical malpractice. Jacob Scott sued WinGate Wilderness Therapy after the teen broke his leg in 2015 when a hiking guide from the center allowed him to climb up and down a steep outcrop in Utah’s red rock desert.
His parents are both lawyers, and after they found that Utah had a 4-year deadline for filing a personal injury lawsuit, court records said, they decided to prioritize “getting Jacob better” for the first 2 years after the accident. But when Mr. Scott’s suit was filed, WinGate argued it was too late – based on the shorter, 2-year deadline for medical malpractice claims.
Mr. Scott’s attorneys scoffed. “Interacting with nature,” his attorneys argued, “is not health care even under the broadest interpretation of … the Utah Health Care Malpractice Act.”
A judge disagreed and threw out Mr. Scott’s case. The Utah Supreme Court unanimously upheld that ruling in 2021.
“We agree with WinGate,” the justices wrote, “that it was acting as a ‘health care provider’ and providing ‘health care’ when Jacob was hiking and rock climbing.”
Last summer, the women who had sued Dr. Broadbent and the two hospitals watched online as lawyers debated whether the abuse they allegedly suffered was health care.
At the hearing, attorneys for Dr. Broadbent and the hospitals argued that the women should have pursued a medical malpractice case, which required them to first notify Dr. Broadbent and the hospitals that they wanted to sue. They also argued to Judge Robert Lunnen that the case couldn’t move forward because the women hadn’t gone before a prelitigation panel.
Attorneys for Dr. Broadbent and the hospitals argued, one after the other, that the painful and traumatic exams the women described arose out of health care treatments.
“Accepting the allegations of the complaint as true – as we must for purposes of this proceeding – we have to assume that [Broadbent] did something that was medically unnecessary, medically inappropriate,” argued David Jordan, a lawyer for Intermountain Health.
“But it doesn’t change the fact that it’s an act performed to a patient, during the patient’s treatment,” he said. “Because that’s what the patient is doing in the doctor’s office. They’re there for treatment.”
The attorney team for the women pushed back. Terry Rooney argued that if Dr. Broadbent’s actions fell under medical malpractice laws, many women would be knocked out of the case because of the age of their claims, and those who remained would be limited in the amount of money in damages they could receive.
“That’s really what this is about,” he argued. “And so it’s troubling – quite frankly it’s shocking to me – that we’re debating heavily the question of whether sexual abuse is health care.”
The judge mulled the issue for months. Judge Lunnen wrote in a September ruling that if the allegations were true, Dr. Broadbent’s treatment of his patients was “insensitive, disrespectful and degrading.”
But Utah law is clear, he said. Malpractice law covers any act or treatment performed by any health care provider during the patient’s medical care. The women had all been seeking health care, Judge Lunnen wrote, and Dr. Broadbent was providing that when the alleged assaults happened.
Their lawsuit was dismissed.
‘I felt defeated’
Brooke, another plaintiff who alleges Dr. Broadbent groped her, remembers feeling sick on the June day she watched the attorneys arguing. She asked to be identified by only her first name for this story.
She alleges Dr. Broadbent violated her in December 2008 while she was hospitalized after experiencing complications with her first pregnancy.
The nearest hospital to her rural town didn’t have a special unit to take care of premature babies, and her doctors feared she might need to deliver her son 6 weeks early. So Brooke had been rushed by ambulance over a mountain pass in a snowstorm to Utah Valley Hospital.
Brooke and her husband were terrified, she said, when they arrived at the Provo hospital. Dr. Broadbent happened to be the doctor on call. With Brooke’s husband and brother-in-law in the room, Dr. Broadbent examined her late that evening, she said, listening to her chest with a stethoscope.
The doctor then suddenly grabbed her breasts, she recalled – his movements causing her hospital gown to fall to expose her chest. She recounted this experience in her lawsuit, saying it was nothing like the breast exams she has had since.
“It was really traumatizing,” she said. “I was mortified. My husband and brother-in-law – we just didn’t say anything about it because it was so uncomfortable.”
Brooke voiced concerns to the nurse manager, and she was assigned a new doctor.
She gave birth to a healthy baby a little more than a month later, at the hospital near her home.
Hearing the judge’s ruling 14 years later, Brooke felt the decision revealed how Utah’s laws are broken.
“I was frustrated,” she said, “and I felt defeated. … I thought justice is not on our side with this.”
If the Utah Supreme Court rules that these alleged sexual assaults should legally be considered health care, the women will likely refile their claims as a medical malpractice lawsuit, said their attorney, Adam Sorensen. But it would be a challenge to keep all 94 women in the case, he said, due to the shorter filing window. Only two women in the lawsuit allege that they were harmed within the last 2 years.
The legal team for the women would have to convince a judge that their claims should still be allowed because they only recently discovered they were harmed. But based on previous rulings, Mr. Sorensen believes the women will have a better chance to win that argument if the civil suit remained a sexual assault case.
Regardless of what happens in their legal case, the decision by Brooke and the other women to come forward could help change state law for victims who come after them.
Recently, Mr. McKell, the state senator, introduced legislation to clarify that civil lawsuits alleging sexual assault by a health care worker do not fall under Utah’s Health Care Malpractice Act.
“I don’t think it’s a close call. Sexual assault is not medical care,” he said. “I know we’ve got some bizarre rulings that have come down through our courts in Utah.”
Both an association of Utah trial lawyers and the Utah Medical Association, which lobbies on behalf of the state’s physicians, support this reform.
“We support the fact that sexual assault should not be part of health care medical malpractice,” said Michelle McOmber, the CEO for the Utah Medical Association. “Sexual assault should be sexual assault, regardless of where it happens or who’s doing it. Sexual assault should be in that category, which is separate from actual health care. Because it’s not health care.”
MountainStar doesn’t have a position on the bill, Ms. Glas said. “If the laws were to change via new legislation and/or interpretation by the courts, we would abide by and comply with those new laws.”
But lawmakers are running out of time. With only a short time left in Utah’s legislative session, state senate and house leaders have so far prioritized passing new laws banning gender-affirming health care for transgender youths and creating a controversial school voucher program that will provide taxpayer funds for students to attend private school.
Utah lawmakers were also expected to consider a dramatic change for other sexual assault victims: a bill that would remove filing deadlines for civil lawsuits brought by people abused as adults. But that bill stalled before it could be debated.
Brooke had been eager to share her story, she said, in hopes it would help the first four women who’d come forward bolster their lawsuit against Dr. Broadbent. She later joined the case as a plaintiff. She read in their lawsuit about one woman who complained about him to the same hospital 7 years before she did, and about another woman who said Dr. Broadbent similarly molested her 2 days after Brooke had expressed her own concern.
“That bothered me so much,” she said. “It didn’t have to happen to all these women.”
Brooke doubts she’ll get vindication in a courtroom. Justice for her, she suspects, won’t come in the form of a legal ruling or a settlement against the doctor she says hurt her years ago.
Instead, she said, “maybe justice looks like changing the laws for future women.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
This article was produced for ProPublica’s Local Reporting Network in partnership with The Salt Lake Tribune.
At 19 years old and about to be married, Stephanie Mateer went to an ob.gyn. within walking distance of her student housing near Brigham Young University in Provo, Utah.
She wanted to start using birth control, and she was looking for guidance about having sex for the first time on her 2008 wedding night.
Ms. Mateer was shocked, she said, when David Broadbent, MD, reached under her gown to grab and squeeze her breasts, started a vaginal exam without warning, then followed it with an extremely painful examination of her rectum.
She felt disgusted and violated, but doubt also crept in. She told herself she must have misinterpreted his actions, or that she should have known that he would do a rectal exam. Raised as a member of The Church of Jesus Christ of Latter-day Saints, she said she was taught to defer to men in leadership.
“I viewed him as being a man in authority,” Ms. Mateer said. “He’s a doctor.”
It was years, she said, before she learned that her experience was in a sharp contrast to the conduct called for in professional standards, including that doctors use only their fingertips during a breast exam and communicate clearly what they are doing in advance, to gain the consent of their patients. Eventually, she gave her experience another name: sexual assault.
Utah judges, however, have called it health care.
And that legal distinction means Utahns like Ms. Mateer who decide to sue a health care provider for alleged sexual abuse are treated more harshly by the court system than plaintiffs who say they were harmed in other settings.
The chance to go to civil court for damages is an important option for survivors, experts say. While a criminal conviction can provide a sense of justice, winning a lawsuit can help victims pay for the therapy and additional support they need to heal after trauma.
Ms. Mateer laid out her allegations in a lawsuit that she and 93 other women filed against Dr. Broadbent last year. But they quickly learned they would be treated differently than other sexual assault survivors.
Filing their case, which alleged the Utah County doctor sexually assaulted them over the span of his 47-year career, was an empowering moment, Ms. Mateer said. But a judge threw out the lawsuit without even considering the merits, determining that because their alleged assailant is a doctor, the case must be governed by medical malpractice rules rather than those that apply to cases of sexual assault.
Under Utah’s rules of medical malpractice, claims made by victims who allege a health care worker sexually assaulted them are literally worth less than lawsuits brought by someone who was assaulted in other settings – even if a jury rules in their favor, a judge is required to limit how much money they receive. And they must meet a shorter filing deadline.
“It’s just crazy that a doctor can sexually assault women and then be protected by the white coat,” Ms. Mateer said. “It’s just a really scary precedent to be calling sexual assault ‘health care.’ ”
Because of the judge’s ruling that leaves them with a shorter window in which to file, some of Dr. Broadbent’s accusers stand to lose their chance to sue. Others were already past that deadline but had hoped to take advantage of an exception that allows plaintiffs to sue if they can prove that the person who harmed them had covered up the wrongdoing and if they discovered they had been hurt within the previous year.
As a group, the women are appealing the ruling to the Utah Supreme Court, which has agreed to hear the case. This decision will set a precedent for future sexual assault victims in Utah.
Dr. Broadbent’s attorney, Chris Nelson, declined an interview request but wrote in an email: “We believe that the allegations against Dr. Broadbent are without merit and will present our case in court. Given that this is an active legal matter, we will not be sharing any details outside the courtroom.”
States have varying legal definitions of medical malpractice, but it’s generally described as treatment that falls short of accepted standards of care. That includes mistakes, such as a surgeon leaving a piece of gauze inside a patient.
The Utah Supreme Court has ruled that a teenage boy was receiving health care when he was allowed to climb a steep, snow-dusted rock outcrop as part of wilderness therapy. When he broke his leg, he could only sue for medical malpractice, so the case faced shorter filing deadlines and lower monetary caps. Similarly, the court has ruled that a boy harmed by another child while in foster care was also bound by medical malpractice law.
Despite these state Supreme Court rulings, Utah legislators have so far not moved to narrow the wording of the malpractice act.
The lawsuit against Dr. Broadbent – and the questions it raises about the broadness of Utah’s medical malpractice laws – comes during a national reckoning with how sexual assault survivors are treated by the law. Legislators in several states have been rewriting laws to give sexual assault victims more time to sue their attackers, in response to the growing cultural understanding of the impact of trauma and the barriers to reporting. Even in Utah, those who were sexually abused as children now have no deadline to file suits against their abusers.
That isn’t true for sexual abuse in a medical setting, where cases must be filed within 2 years of the assault.
These higher hurdles should not exist in Utah, said state Sen. Mike K. McKell, a Utah County Republican who works as a personal injury attorney. He is trying to change state law to ensure that sexual assault lawsuits do not fall under Utah’s Health Care Malpractice Act, a law designed to cover negligence and poor care, not necessarily deliberate actions like an assault.
“Sexual assault, to me, is not medical care. Period,” he said. “It’s sad that we need to clarify that sexual assault is not medical care. But trying to tie sexual assault to a medical malpractice [filing deadline] – it’s just wrong.”
‘Your husband is a lucky man’
Ms. Mateer had gone to Dr. Broadbent in 2008 for a premarital exam, a uniquely Utah visit often scheduled by young women who are members of The Church of Jesus Christ of Latter-day Saints.
Leaders of the faith, which is predominant in Utah, focus on chastity when speaking to young, unmarried people about sex, and public schools have typically focused on abstinence-based sex education. So for some, these visits are the first place they learn about sexual health.
Young women who get premarital exams are typically given a birth control prescription, but the appointments can include care that’s less common for healthy women in other states – such as doctors giving them vaginal dilators to stretch their tissues before their wedding nights.
That’s what Ms. Mateer was expecting when she visited Dr. Broadbent’s office. The ob.gyn. had been practicing for decades in his Provo clinic nestled between student housing apartments across the street from Brigham Young University, which is owned by The Church of Jesus Christ of Latter-day Saints.
So Ms. Mateer was “just totally taken aback,” she said, by the painful examination and by Dr. Broadbent snapping off his gloves after the exam and saying, “Your husband is a lucky man.”
She repeated that remark in her legal filing, along with the doctor’s advice for her: If she bled during intercourse, “just do what the Boy Scouts do and apply pressure.”
“The whole thing was like I’m some object for my husband to enjoy and let him do whatever he wants,” Ms. Mateer said. “It was just very violating and not a great way to start my sexual relationship with my new husband, with these ideas in mind.”
Ms. Mateer thought back to that visit over the years, particularly when she went to other ob.gyns. for health care. Her subsequent doctors, she said, never performed a rectal exam and always explained to her what they were doing and how it would feel, and asked for her consent.
She thought about Dr. Broadbent again in 2017, as the #MeToo movement gained momentum, and looked him up online. Ms. Mateer found reviews from other women who described Dr. Broadbent doing rough examinations without warning that left them feeling the same way she had years before.
Then in December 2021, she spoke out on “Mormon Stories,” a podcast where people who have left or have questioned their Latter-day Saint faith share their life stories. In the episode, she described the painful way he examined her, how it left her feeling traumatized, and her discovery of the reviews that echoed her experience.
“He’s on University Avenue, in Provo, giving these exams to who knows how many naive Mormon 18-year-old, 19-year-old girls who are getting married. … They are naive and they don’t know what to expect,” she said on the podcast. “His name is Dr. David Broadbent.”
After the podcast aired, Ms. Mateer was flooded with messages from women who heard the episode and reached out to tell her that Dr. Broadbent had harmed them, too.
Ms. Mateer and three other women decided to sue the ob.gyn., and in the following weeks and months, 90 additional women joined the lawsuit they filed in Provo. Many of the women allege Dr. Broadbent inappropriately touched their breasts, vaginas and rectums, hurting them, without warning or explanation. Some said he used his bare hand – instead of using a speculum or gloves – during exams. One alleged that she saw he had an erection while he was touching her.
Dr. Broadbent’s actions were not medically necessary, the women allege, and were instead “performed for no other reason than his own sexual gratification.”
The lawsuit also named as defendants two hospitals where Dr. Broadbent had delivered babies and where some of the women allege they were assaulted. The suit accused hospital administrators of knowing about Dr. Broadbent’s inappropriate behavior and doing nothing about it.
After he was sued, the ob.gyn. quickly lost his privileges at the hospitals where he worked. Dr. Broadbent, now 75, has also voluntarily put his medical license in Utah on hold while police investigate 29 reports of sexual assault made against him.
Prosecutors are still considering whether to criminally prosecute Dr. Broadbent. Provo police forwarded more than a dozen reports to the Utah County attorney’s office in November, which are still being reviewed by a local prosecutor.
A spokesperson for Intermountain Health, the nonprofit health system that owns Utah Valley Hospital, where some of the women in the suit were treated, did not respond to specific questions. The spokesperson emphasized in an email that Dr. Broadbent was an “independent physician” who was not employed by Utah Valley Hospital, adding that most of the alleged incidents took place at Dr. Broadbent’s medical office.
A representative for MountainStar Healthcare, another hospital chain named as a defendant, denied knowledge of any allegations of inappropriate conduct reported to its hospital and also emphasized that Dr. Broadbent worked independently, not as an employee.
“Our position since this lawsuit was filed has been that we were inappropriately named in this suit,” said Brittany Glas, the communications director for MountainStar.
Debating whether sexual abuse is health care
For the women who sued Dr. Broadbent, their case boiled down to a key question: Were the sexual assaults they say they experienced part of their health care? There was a lot hanging on the answer.
If their case was considered medical malpractice, they would be limited in how much money they could receive in damages for their pain and suffering. If a jury awarded them millions of dollars, a judge would be required by law to cut that down to $450,000. There’s no cap on these monetary awards for victims sexually assaulted in other settings.
They would also be required to go before a panel, which includes a doctor, a lawyer and a community member, that decides whether their claims have merit. This step, aimed at resolving disputes out of court, does not block anyone from suing afterward. But it does add cost and delay, and for sexual assault victims who’ve gone through this step, it has been another time they were required to describe their experiences and hope they were believed.
The shorter, 2-year filing deadline for medical malpractice cases can also be a particular challenge for those who have been sexually abused because research shows that it’s common to delay reporting such assaults.
Nationwide, these kinds of malpractice reforms were adopted in the 1970s amid concerns – largely driven by insurance companies – that the cost of health care was rising because of frivolous lawsuits and “runaway juries” doling out multimillion-dollar payouts.
Restricting the size of malpractice awards and imposing other limits, many argued, were effective ways to balance compensating injured patients with protecting everyone’s access to health care.
State laws are generally silent on whether sexual assault lawsuits should be covered by malpractice laws, leaving courts to grapple with that question and leading to different conclusions across the country. The Tribune and ProPublica identified at least six cases in which state appellate judges sharply distinguished between assault and health care in considering whether malpractice laws should apply to sexual assault–related cases.
An appellate court in Wisconsin, for example, ruled in 1993 that a physician having an erection and groping a patient was a purposeful harm, not medical malpractice.
Florida’s law is similar to Utah’s, defining allegations “arising” out of medical care as malpractice. While an earlier ruling did treat sexual assault in a health care setting as medical malpractice, appellate rulings in the last decade have moved away from that interpretation. In 2005, an appellate court affirmed a lower-court ruling that when a dentist “stopped providing dental treatment to the victim and began sexually assaulting her, his professional services ended.”
Similarly, a federal judge in Iowa in 1995 weighed in on the meaning of “arising” out of health care: “Rape is not patient care activity,” he wrote.
But Utah’s malpractice law is so broad that judges have been interpreting it as covering any act performed by a health care provider during medical care. The law was passed in 1976 and is popular with doctors and other health care providers, who have lobbied to keep it in place – and who use it to get lawsuits dismissed.
One precedent-setting case in Utah shows the law’s power to safeguard health care providers and was an important test of how Utah defines medical malpractice. Jacob Scott sued WinGate Wilderness Therapy after the teen broke his leg in 2015 when a hiking guide from the center allowed him to climb up and down a steep outcrop in Utah’s red rock desert.
His parents are both lawyers, and after they found that Utah had a 4-year deadline for filing a personal injury lawsuit, court records said, they decided to prioritize “getting Jacob better” for the first 2 years after the accident. But when Mr. Scott’s suit was filed, WinGate argued it was too late – based on the shorter, 2-year deadline for medical malpractice claims.
Mr. Scott’s attorneys scoffed. “Interacting with nature,” his attorneys argued, “is not health care even under the broadest interpretation of … the Utah Health Care Malpractice Act.”
A judge disagreed and threw out Mr. Scott’s case. The Utah Supreme Court unanimously upheld that ruling in 2021.
“We agree with WinGate,” the justices wrote, “that it was acting as a ‘health care provider’ and providing ‘health care’ when Jacob was hiking and rock climbing.”
Last summer, the women who had sued Dr. Broadbent and the two hospitals watched online as lawyers debated whether the abuse they allegedly suffered was health care.
At the hearing, attorneys for Dr. Broadbent and the hospitals argued that the women should have pursued a medical malpractice case, which required them to first notify Dr. Broadbent and the hospitals that they wanted to sue. They also argued to Judge Robert Lunnen that the case couldn’t move forward because the women hadn’t gone before a prelitigation panel.
Attorneys for Dr. Broadbent and the hospitals argued, one after the other, that the painful and traumatic exams the women described arose out of health care treatments.
“Accepting the allegations of the complaint as true – as we must for purposes of this proceeding – we have to assume that [Broadbent] did something that was medically unnecessary, medically inappropriate,” argued David Jordan, a lawyer for Intermountain Health.
“But it doesn’t change the fact that it’s an act performed to a patient, during the patient’s treatment,” he said. “Because that’s what the patient is doing in the doctor’s office. They’re there for treatment.”
The attorney team for the women pushed back. Terry Rooney argued that if Dr. Broadbent’s actions fell under medical malpractice laws, many women would be knocked out of the case because of the age of their claims, and those who remained would be limited in the amount of money in damages they could receive.
“That’s really what this is about,” he argued. “And so it’s troubling – quite frankly it’s shocking to me – that we’re debating heavily the question of whether sexual abuse is health care.”
The judge mulled the issue for months. Judge Lunnen wrote in a September ruling that if the allegations were true, Dr. Broadbent’s treatment of his patients was “insensitive, disrespectful and degrading.”
But Utah law is clear, he said. Malpractice law covers any act or treatment performed by any health care provider during the patient’s medical care. The women had all been seeking health care, Judge Lunnen wrote, and Dr. Broadbent was providing that when the alleged assaults happened.
Their lawsuit was dismissed.
‘I felt defeated’
Brooke, another plaintiff who alleges Dr. Broadbent groped her, remembers feeling sick on the June day she watched the attorneys arguing. She asked to be identified by only her first name for this story.
She alleges Dr. Broadbent violated her in December 2008 while she was hospitalized after experiencing complications with her first pregnancy.
The nearest hospital to her rural town didn’t have a special unit to take care of premature babies, and her doctors feared she might need to deliver her son 6 weeks early. So Brooke had been rushed by ambulance over a mountain pass in a snowstorm to Utah Valley Hospital.
Brooke and her husband were terrified, she said, when they arrived at the Provo hospital. Dr. Broadbent happened to be the doctor on call. With Brooke’s husband and brother-in-law in the room, Dr. Broadbent examined her late that evening, she said, listening to her chest with a stethoscope.
The doctor then suddenly grabbed her breasts, she recalled – his movements causing her hospital gown to fall to expose her chest. She recounted this experience in her lawsuit, saying it was nothing like the breast exams she has had since.
“It was really traumatizing,” she said. “I was mortified. My husband and brother-in-law – we just didn’t say anything about it because it was so uncomfortable.”
Brooke voiced concerns to the nurse manager, and she was assigned a new doctor.
She gave birth to a healthy baby a little more than a month later, at the hospital near her home.
Hearing the judge’s ruling 14 years later, Brooke felt the decision revealed how Utah’s laws are broken.
“I was frustrated,” she said, “and I felt defeated. … I thought justice is not on our side with this.”
If the Utah Supreme Court rules that these alleged sexual assaults should legally be considered health care, the women will likely refile their claims as a medical malpractice lawsuit, said their attorney, Adam Sorensen. But it would be a challenge to keep all 94 women in the case, he said, due to the shorter filing window. Only two women in the lawsuit allege that they were harmed within the last 2 years.
The legal team for the women would have to convince a judge that their claims should still be allowed because they only recently discovered they were harmed. But based on previous rulings, Mr. Sorensen believes the women will have a better chance to win that argument if the civil suit remained a sexual assault case.
Regardless of what happens in their legal case, the decision by Brooke and the other women to come forward could help change state law for victims who come after them.
Recently, Mr. McKell, the state senator, introduced legislation to clarify that civil lawsuits alleging sexual assault by a health care worker do not fall under Utah’s Health Care Malpractice Act.
“I don’t think it’s a close call. Sexual assault is not medical care,” he said. “I know we’ve got some bizarre rulings that have come down through our courts in Utah.”
Both an association of Utah trial lawyers and the Utah Medical Association, which lobbies on behalf of the state’s physicians, support this reform.
“We support the fact that sexual assault should not be part of health care medical malpractice,” said Michelle McOmber, the CEO for the Utah Medical Association. “Sexual assault should be sexual assault, regardless of where it happens or who’s doing it. Sexual assault should be in that category, which is separate from actual health care. Because it’s not health care.”
MountainStar doesn’t have a position on the bill, Ms. Glas said. “If the laws were to change via new legislation and/or interpretation by the courts, we would abide by and comply with those new laws.”
But lawmakers are running out of time. With only a short time left in Utah’s legislative session, state senate and house leaders have so far prioritized passing new laws banning gender-affirming health care for transgender youths and creating a controversial school voucher program that will provide taxpayer funds for students to attend private school.
Utah lawmakers were also expected to consider a dramatic change for other sexual assault victims: a bill that would remove filing deadlines for civil lawsuits brought by people abused as adults. But that bill stalled before it could be debated.
Brooke had been eager to share her story, she said, in hopes it would help the first four women who’d come forward bolster their lawsuit against Dr. Broadbent. She later joined the case as a plaintiff. She read in their lawsuit about one woman who complained about him to the same hospital 7 years before she did, and about another woman who said Dr. Broadbent similarly molested her 2 days after Brooke had expressed her own concern.
“That bothered me so much,” she said. “It didn’t have to happen to all these women.”
Brooke doubts she’ll get vindication in a courtroom. Justice for her, she suspects, won’t come in the form of a legal ruling or a settlement against the doctor she says hurt her years ago.
Instead, she said, “maybe justice looks like changing the laws for future women.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
Trends in HPV vaccination among adults aged 27 to 45 years
In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?
In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?

In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?

Is there still a role for tubal surgery in the modern world of IVF?
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.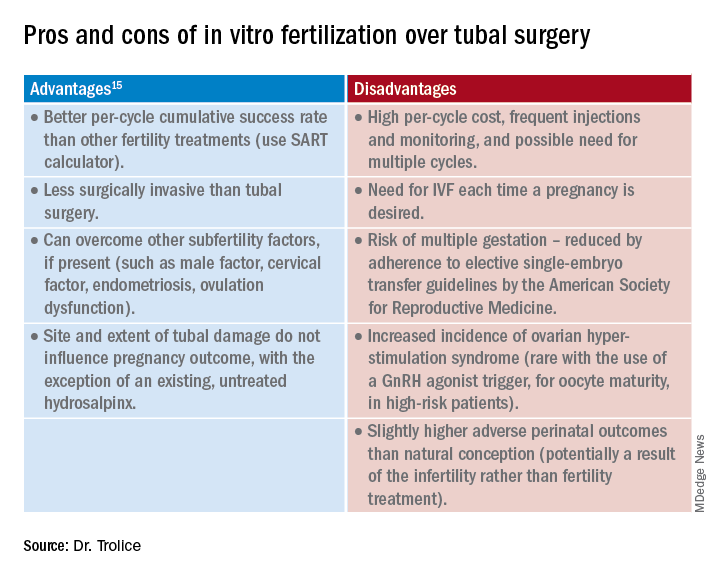
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
‘Forever chemicals’ up type 2 diabetes risk in midlife White women
Middle-aged White women who had higher levels of some breakdown products of phthalates – a class of endocrine disrupting chemicals (EDCs), or “forever chemicals,” that act as plasticizers – had a significantly greater risk of developing type 2 diabetes over a 6-year period compared with other similar women.
However, this association was not seen among Black or Asian middle-aged women.
These findings from the Study of Women’s Health Across the Nation – Multipollutant Study (SWAN-MPS), by Mia Q. Peng, PhD, MPH, and colleagues, have been published online in the Journal of Clinical Endocrinology & Metabolism.
“Overall, our study has added some evidence to support the potential diabetogenic effects of phthalates, but it also highlights that much is still unknown about the metabolic effects of these chemicals,” the group noted.
“The apparent racial/ethnic differences in the associations between phthalates and incident diabetes should be investigated in future studies,” they cautioned.
Recruiting younger participants and observing them longer, they suggested, “will also help us understand the effects of phthalates on different stages of the diabetogenic process, including whether body fat gain is an important mediator.”
Phthalates are all around us
Low-molecular-weight phthalates are frequently added to personal care products, such as fragrance, nail polish, and some feminine hygiene products, as solvents, plasticizers, and fixatives, the researchers explained.
And high-molecular-weight phthalates are frequently added to polyvinyl chloride plastic products, such as plastic food packaging, clothing, and vinyl flooring, as plasticizers.
Phthalates have been hypothesized to contribute to the development of diabetes, but longitudinal evidence in humans was limited.
“Given widespread exposure to phthalates and the enormous costs of diabetes to individuals and societies, ongoing investments in the research on phthalates’ metabolic effects are warranted,” the researchers concluded.
Racial differences in phthalates and incident diabetes
“A new finding is that we observed some phthalates are associated with a higher risk of diabetes development, especially in White women [that] were not seen in Black or Asian women,” senior author Sung Kyun Park, ScD, MPH, of the University of Michigan, Ann Arbor, told this news organization.
“We were surprised to see the racial/ethnic differences,” added Dr. Peng, formerly of the University of Michigan and now at Lifecourse Epidemiology of Adiposity and Diabetes Center, University of Colorado Anschutz Medical Campus.
A possible explanation is that “compared to White women, Black women develop diabetes at a younger age and are exposed to higher levels of several phthalates,” and this study excluded women who already had diabetes by midlife, she noted.
“Although our study was conducted in a cohort of women,” Dr. Park stressed, “we hope that our findings are not interpreted that only women should be concerned of phthalates. Our findings add to the current literature that phthalates may be a potential risk factor for type 2 diabetes.
“Certain phthalates are prohibited in children’s toys and child care articles,” Dr. Peng noted, as explained by the U.S. Consumer Product Safety Commission. In addition, a bill has been introduced in Congress to ban phthalates in food contact substances.
“If phthalates are removed from plastics and other consumer products,” she cautioned, “we do have to be careful in the process to avoid replacing them with some other potentially harmful chemicals.”
A well-known example of this type of “regrettable substitution,” Dr. Park added, “is ‘BPA-free’ plastics that replaced bisphenol A with other bisphenols such as bisphenol-F (BPF) or bisphenol-S (BPS). The product has a label of ‘BPA-free’, but those replaced chemicals turned out to be equally toxic. Science is slow to determine if a new chemical introduced to the market is safe and can replace a regulated chemical.”
And studies have shown that a diet rich in meat, fat, and ultraprocessed foods is associated with increased exposures to some phthalates, especially when the foods are obtained away from home, such as fast foods, Dr. Peng observed. In addition, some phthalates are added to personal care products such as fragrance.
“As a first step,” she said, “I think reducing consumption of ultraprocessed foods packaged in plastics may help reduce phthalate exposure.”
A 2020 report from the Endocrine Society and the International Pollutants Elimination Network (IPEN), titled, “Plastics, EDCs, and Health,” summarizes research on bisphenol A, per- and polyfluoroalkyl substances (PFAS), phthalates, and other EDCs that leach from plastics. The Endocrine Society website also has a link to a 2-page summary.
Levels of 12 phthalate metabolites
Previously, the researchers reported how another class of “forever chemicals,” PFAS, were associated with risk of hypertension in a 17-year follow-up of middle-aged women in the SWAN study.
In the current study, they analyzed data from 1,308 women in SWAN-MPS who had been recruited at five study sites (Oakland, Calif; Los Angeles; Detroit; Pittsburgh; and Boston).
The women were between ages 42 and 52 years in 1996-1997 and self-identified as White, Black, Chinese, or Japanese.
They did not have diabetes in 1999-2000 and had sufficient urine samples for phthalate assessment then and midway through a 6-year follow-up.
The women were a median age of 49 years in 1999-2000. About half were White, 20% were Black, 13% were Chinese, and 15% were Japanese.
Researchers analyzed levels of 12 metabolites, chosen because their parent phthalates have been widely used in industry and commerce, and exposure to these phthalates is a national biomonitoring priority.
The measured phthalates were:
Three metabolites of low-molecular-weight phthalates:
- mono-ethyl phthalate (MEP)
- mono-n-butyl phthalate (MnBP)
- mono-isobutyl phthalate (MiBP)
Four metabolites of the high-molecular-weight phthalate di(2-ethylhexyl) phthalate (DEHP), which is of particular public health interest:
- mono(2-ethylhexyl) phthalate (MEHP)
- mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP)
- mono(2-ethyl-5-oxohexyl) phthalate (MEOHP)
- mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)
Five metabolites of other high-molecular-weight phthalates:
- monobenzyl phthalate (MBzP)
- monoisononyl phthalate (MiNP)
- mono-carboxyoctyl phthalate (MCOP)
- mono-carboxy-isononyl phthalate (MCNP)
- mono(3-carboxypropyl) phthalate (MCPP)
The researchers excluded MiNP from all analyses because it was detected in less than 1% of urine samples.
The different phthalate metabolites were detected in 84.8% of samples (MEHP) to 100% of samples (MnBP and MECPP).
Women who were younger, Black, current smokers, or obese generally had higher concentrations of phthalate metabolites.
Over 6 years, 61 women developed diabetes (an incidence rate of 8.1 per 1000 person-years).
Compared with other women, those with incident diabetes had significantly higher concentrations of all phthalate metabolites except DEHP metabolites and MCPP.
Phthalates were not associated with incident diabetes in Black or Asian women.
However, among White women, each doubling of the concentrations of MiBP, MBzP, MCOP, MCNP, and MCCP was associated with a 30% to 63% higher incidence of diabetes (HR 1.30 for MCNP; HR 1.63 for MiBP).
The SWAN study was supported by the National Institutes of Health, Department of Health & Human Services, National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and SWAN Repository. The current study was supported by the National Center for Research Resources, National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. Dr. Peng was supported by an Interdisciplinary Research Training on Health and Aging grant from the NIA. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Middle-aged White women who had higher levels of some breakdown products of phthalates – a class of endocrine disrupting chemicals (EDCs), or “forever chemicals,” that act as plasticizers – had a significantly greater risk of developing type 2 diabetes over a 6-year period compared with other similar women.
However, this association was not seen among Black or Asian middle-aged women.
These findings from the Study of Women’s Health Across the Nation – Multipollutant Study (SWAN-MPS), by Mia Q. Peng, PhD, MPH, and colleagues, have been published online in the Journal of Clinical Endocrinology & Metabolism.
“Overall, our study has added some evidence to support the potential diabetogenic effects of phthalates, but it also highlights that much is still unknown about the metabolic effects of these chemicals,” the group noted.
“The apparent racial/ethnic differences in the associations between phthalates and incident diabetes should be investigated in future studies,” they cautioned.
Recruiting younger participants and observing them longer, they suggested, “will also help us understand the effects of phthalates on different stages of the diabetogenic process, including whether body fat gain is an important mediator.”
Phthalates are all around us
Low-molecular-weight phthalates are frequently added to personal care products, such as fragrance, nail polish, and some feminine hygiene products, as solvents, plasticizers, and fixatives, the researchers explained.
And high-molecular-weight phthalates are frequently added to polyvinyl chloride plastic products, such as plastic food packaging, clothing, and vinyl flooring, as plasticizers.
Phthalates have been hypothesized to contribute to the development of diabetes, but longitudinal evidence in humans was limited.
“Given widespread exposure to phthalates and the enormous costs of diabetes to individuals and societies, ongoing investments in the research on phthalates’ metabolic effects are warranted,” the researchers concluded.
Racial differences in phthalates and incident diabetes
“A new finding is that we observed some phthalates are associated with a higher risk of diabetes development, especially in White women [that] were not seen in Black or Asian women,” senior author Sung Kyun Park, ScD, MPH, of the University of Michigan, Ann Arbor, told this news organization.
“We were surprised to see the racial/ethnic differences,” added Dr. Peng, formerly of the University of Michigan and now at Lifecourse Epidemiology of Adiposity and Diabetes Center, University of Colorado Anschutz Medical Campus.
A possible explanation is that “compared to White women, Black women develop diabetes at a younger age and are exposed to higher levels of several phthalates,” and this study excluded women who already had diabetes by midlife, she noted.
“Although our study was conducted in a cohort of women,” Dr. Park stressed, “we hope that our findings are not interpreted that only women should be concerned of phthalates. Our findings add to the current literature that phthalates may be a potential risk factor for type 2 diabetes.
“Certain phthalates are prohibited in children’s toys and child care articles,” Dr. Peng noted, as explained by the U.S. Consumer Product Safety Commission. In addition, a bill has been introduced in Congress to ban phthalates in food contact substances.
“If phthalates are removed from plastics and other consumer products,” she cautioned, “we do have to be careful in the process to avoid replacing them with some other potentially harmful chemicals.”
A well-known example of this type of “regrettable substitution,” Dr. Park added, “is ‘BPA-free’ plastics that replaced bisphenol A with other bisphenols such as bisphenol-F (BPF) or bisphenol-S (BPS). The product has a label of ‘BPA-free’, but those replaced chemicals turned out to be equally toxic. Science is slow to determine if a new chemical introduced to the market is safe and can replace a regulated chemical.”
And studies have shown that a diet rich in meat, fat, and ultraprocessed foods is associated with increased exposures to some phthalates, especially when the foods are obtained away from home, such as fast foods, Dr. Peng observed. In addition, some phthalates are added to personal care products such as fragrance.
“As a first step,” she said, “I think reducing consumption of ultraprocessed foods packaged in plastics may help reduce phthalate exposure.”
A 2020 report from the Endocrine Society and the International Pollutants Elimination Network (IPEN), titled, “Plastics, EDCs, and Health,” summarizes research on bisphenol A, per- and polyfluoroalkyl substances (PFAS), phthalates, and other EDCs that leach from plastics. The Endocrine Society website also has a link to a 2-page summary.
Levels of 12 phthalate metabolites
Previously, the researchers reported how another class of “forever chemicals,” PFAS, were associated with risk of hypertension in a 17-year follow-up of middle-aged women in the SWAN study.
In the current study, they analyzed data from 1,308 women in SWAN-MPS who had been recruited at five study sites (Oakland, Calif; Los Angeles; Detroit; Pittsburgh; and Boston).
The women were between ages 42 and 52 years in 1996-1997 and self-identified as White, Black, Chinese, or Japanese.
They did not have diabetes in 1999-2000 and had sufficient urine samples for phthalate assessment then and midway through a 6-year follow-up.
The women were a median age of 49 years in 1999-2000. About half were White, 20% were Black, 13% were Chinese, and 15% were Japanese.
Researchers analyzed levels of 12 metabolites, chosen because their parent phthalates have been widely used in industry and commerce, and exposure to these phthalates is a national biomonitoring priority.
The measured phthalates were:
Three metabolites of low-molecular-weight phthalates:
- mono-ethyl phthalate (MEP)
- mono-n-butyl phthalate (MnBP)
- mono-isobutyl phthalate (MiBP)
Four metabolites of the high-molecular-weight phthalate di(2-ethylhexyl) phthalate (DEHP), which is of particular public health interest:
- mono(2-ethylhexyl) phthalate (MEHP)
- mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP)
- mono(2-ethyl-5-oxohexyl) phthalate (MEOHP)
- mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)
Five metabolites of other high-molecular-weight phthalates:
- monobenzyl phthalate (MBzP)
- monoisononyl phthalate (MiNP)
- mono-carboxyoctyl phthalate (MCOP)
- mono-carboxy-isononyl phthalate (MCNP)
- mono(3-carboxypropyl) phthalate (MCPP)
The researchers excluded MiNP from all analyses because it was detected in less than 1% of urine samples.
The different phthalate metabolites were detected in 84.8% of samples (MEHP) to 100% of samples (MnBP and MECPP).
Women who were younger, Black, current smokers, or obese generally had higher concentrations of phthalate metabolites.
Over 6 years, 61 women developed diabetes (an incidence rate of 8.1 per 1000 person-years).
Compared with other women, those with incident diabetes had significantly higher concentrations of all phthalate metabolites except DEHP metabolites and MCPP.
Phthalates were not associated with incident diabetes in Black or Asian women.
However, among White women, each doubling of the concentrations of MiBP, MBzP, MCOP, MCNP, and MCCP was associated with a 30% to 63% higher incidence of diabetes (HR 1.30 for MCNP; HR 1.63 for MiBP).
The SWAN study was supported by the National Institutes of Health, Department of Health & Human Services, National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and SWAN Repository. The current study was supported by the National Center for Research Resources, National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. Dr. Peng was supported by an Interdisciplinary Research Training on Health and Aging grant from the NIA. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Middle-aged White women who had higher levels of some breakdown products of phthalates – a class of endocrine disrupting chemicals (EDCs), or “forever chemicals,” that act as plasticizers – had a significantly greater risk of developing type 2 diabetes over a 6-year period compared with other similar women.
However, this association was not seen among Black or Asian middle-aged women.
These findings from the Study of Women’s Health Across the Nation – Multipollutant Study (SWAN-MPS), by Mia Q. Peng, PhD, MPH, and colleagues, have been published online in the Journal of Clinical Endocrinology & Metabolism.
“Overall, our study has added some evidence to support the potential diabetogenic effects of phthalates, but it also highlights that much is still unknown about the metabolic effects of these chemicals,” the group noted.
“The apparent racial/ethnic differences in the associations between phthalates and incident diabetes should be investigated in future studies,” they cautioned.
Recruiting younger participants and observing them longer, they suggested, “will also help us understand the effects of phthalates on different stages of the diabetogenic process, including whether body fat gain is an important mediator.”
Phthalates are all around us
Low-molecular-weight phthalates are frequently added to personal care products, such as fragrance, nail polish, and some feminine hygiene products, as solvents, plasticizers, and fixatives, the researchers explained.
And high-molecular-weight phthalates are frequently added to polyvinyl chloride plastic products, such as plastic food packaging, clothing, and vinyl flooring, as plasticizers.
Phthalates have been hypothesized to contribute to the development of diabetes, but longitudinal evidence in humans was limited.
“Given widespread exposure to phthalates and the enormous costs of diabetes to individuals and societies, ongoing investments in the research on phthalates’ metabolic effects are warranted,” the researchers concluded.
Racial differences in phthalates and incident diabetes
“A new finding is that we observed some phthalates are associated with a higher risk of diabetes development, especially in White women [that] were not seen in Black or Asian women,” senior author Sung Kyun Park, ScD, MPH, of the University of Michigan, Ann Arbor, told this news organization.
“We were surprised to see the racial/ethnic differences,” added Dr. Peng, formerly of the University of Michigan and now at Lifecourse Epidemiology of Adiposity and Diabetes Center, University of Colorado Anschutz Medical Campus.
A possible explanation is that “compared to White women, Black women develop diabetes at a younger age and are exposed to higher levels of several phthalates,” and this study excluded women who already had diabetes by midlife, she noted.
“Although our study was conducted in a cohort of women,” Dr. Park stressed, “we hope that our findings are not interpreted that only women should be concerned of phthalates. Our findings add to the current literature that phthalates may be a potential risk factor for type 2 diabetes.
“Certain phthalates are prohibited in children’s toys and child care articles,” Dr. Peng noted, as explained by the U.S. Consumer Product Safety Commission. In addition, a bill has been introduced in Congress to ban phthalates in food contact substances.
“If phthalates are removed from plastics and other consumer products,” she cautioned, “we do have to be careful in the process to avoid replacing them with some other potentially harmful chemicals.”
A well-known example of this type of “regrettable substitution,” Dr. Park added, “is ‘BPA-free’ plastics that replaced bisphenol A with other bisphenols such as bisphenol-F (BPF) or bisphenol-S (BPS). The product has a label of ‘BPA-free’, but those replaced chemicals turned out to be equally toxic. Science is slow to determine if a new chemical introduced to the market is safe and can replace a regulated chemical.”
And studies have shown that a diet rich in meat, fat, and ultraprocessed foods is associated with increased exposures to some phthalates, especially when the foods are obtained away from home, such as fast foods, Dr. Peng observed. In addition, some phthalates are added to personal care products such as fragrance.
“As a first step,” she said, “I think reducing consumption of ultraprocessed foods packaged in plastics may help reduce phthalate exposure.”
A 2020 report from the Endocrine Society and the International Pollutants Elimination Network (IPEN), titled, “Plastics, EDCs, and Health,” summarizes research on bisphenol A, per- and polyfluoroalkyl substances (PFAS), phthalates, and other EDCs that leach from plastics. The Endocrine Society website also has a link to a 2-page summary.
Levels of 12 phthalate metabolites
Previously, the researchers reported how another class of “forever chemicals,” PFAS, were associated with risk of hypertension in a 17-year follow-up of middle-aged women in the SWAN study.
In the current study, they analyzed data from 1,308 women in SWAN-MPS who had been recruited at five study sites (Oakland, Calif; Los Angeles; Detroit; Pittsburgh; and Boston).
The women were between ages 42 and 52 years in 1996-1997 and self-identified as White, Black, Chinese, or Japanese.
They did not have diabetes in 1999-2000 and had sufficient urine samples for phthalate assessment then and midway through a 6-year follow-up.
The women were a median age of 49 years in 1999-2000. About half were White, 20% were Black, 13% were Chinese, and 15% were Japanese.
Researchers analyzed levels of 12 metabolites, chosen because their parent phthalates have been widely used in industry and commerce, and exposure to these phthalates is a national biomonitoring priority.
The measured phthalates were:
Three metabolites of low-molecular-weight phthalates:
- mono-ethyl phthalate (MEP)
- mono-n-butyl phthalate (MnBP)
- mono-isobutyl phthalate (MiBP)
Four metabolites of the high-molecular-weight phthalate di(2-ethylhexyl) phthalate (DEHP), which is of particular public health interest:
- mono(2-ethylhexyl) phthalate (MEHP)
- mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP)
- mono(2-ethyl-5-oxohexyl) phthalate (MEOHP)
- mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)
Five metabolites of other high-molecular-weight phthalates:
- monobenzyl phthalate (MBzP)
- monoisononyl phthalate (MiNP)
- mono-carboxyoctyl phthalate (MCOP)
- mono-carboxy-isononyl phthalate (MCNP)
- mono(3-carboxypropyl) phthalate (MCPP)
The researchers excluded MiNP from all analyses because it was detected in less than 1% of urine samples.
The different phthalate metabolites were detected in 84.8% of samples (MEHP) to 100% of samples (MnBP and MECPP).
Women who were younger, Black, current smokers, or obese generally had higher concentrations of phthalate metabolites.
Over 6 years, 61 women developed diabetes (an incidence rate of 8.1 per 1000 person-years).
Compared with other women, those with incident diabetes had significantly higher concentrations of all phthalate metabolites except DEHP metabolites and MCPP.
Phthalates were not associated with incident diabetes in Black or Asian women.
However, among White women, each doubling of the concentrations of MiBP, MBzP, MCOP, MCNP, and MCCP was associated with a 30% to 63% higher incidence of diabetes (HR 1.30 for MCNP; HR 1.63 for MiBP).
The SWAN study was supported by the National Institutes of Health, Department of Health & Human Services, National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and SWAN Repository. The current study was supported by the National Center for Research Resources, National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. Dr. Peng was supported by an Interdisciplinary Research Training on Health and Aging grant from the NIA. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Scientists create ‘vagina on a chip’: What to know
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
FROM MICROBIOME