User login
Bacterial vaginosis linked with persistent HPV infections
Montrouge, France – Four in five women will be infected by one or more human papillomavirus (HPV) strains during their lifetimes. For most of these women, the HPV will be cleared from the body, but 5% of them will develop precancerous lesions in the cervix.
At a press conference ahead of the 46th meeting of the French Colposcopy and Cervical and Vaginal Diseases Society, Julia Maruani, MD, a medical gynecologist in Marseille, France, took the opportunity to discuss the importance of vaginal flora and the need to treat cases of bacterial vaginosis.
Striking a balance
Essential for reducing the risk of sexually transmitted infections, a healthy vaginal flora is made up of millions of microorganisms, mainly lactobacilli, as well as other bacteria (Gardnerella vaginalis, Atopobium vaginae, Prevotella, streptococcus, gonococcus), HPV, and fungi.
Lactobacilli produce lactic acid, which reduces the vagina’s pH, as well as hydrogen peroxide, which is toxic to the other bacteria.
Different factors, such as alcohol, a diet rich in polyunsaturated fatty acids and sugar, and especially smoking, can lead to an imbalance of the bacteria in the vaginal flora and thus result in vaginosis. What occurs is an abnormal multiplication of different types of anaerobic bacteria that are normally present in much lower numbers. There is a relative reduction in lactobacilli, which results in an increased vaginal pH, a greater risk of contracting an STI, and reduced clearance of the HPV infection. “Women who smoke probably experience persistent HPV infections due to an imbalance in vaginal flora,” said Dr. Maruani.
Vaginosis and HPV
When there are fewer lactobacilli than there should be, these bacteria can no longer protect the vaginal mucosa, which is disrupted by other bacteria. “HPV then has access to the basal cells,” said Dr. Maruani, acknowledging that the relationship between bacterial vaginosis and persistent HPV infections has been the subject of numerous research studies over the past decade or so. “For years, I would see this same link in my patients. Those with persistent vaginosis were also the ones with persistent HPV. And I’m not the only one to notice this. Studies have also been carried out investigating this exact correlation,” she added.
These studies have shown that HPV infections persist in cases of vaginosis, resulting in the appearance of epithelial lesions. Additionally, the lesions are more severe when dysbiosis is more severe.
What about probiotics? Can they treat dysbiosis and an HPV infection at the same time? “Probiotics work very well for vaginosis, provided they are used for a long time. We know that they lessen HPV infections and low-grade lesions,” said Dr. Maruani, although no randomized studies support this conclusion. “It’s not a one size fits all. We aren’t about to treat patients with precancerous lesions with probiotics.” There are currently no data concerning the efficacy of probiotics on high-grade lesions. These days, Dr. Maruani has been thinking about a new issue: the benefit of diagnosing cases of asymptomatic vaginosis – because treating them would reduce the risk of persistent HPV infection.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Montrouge, France – Four in five women will be infected by one or more human papillomavirus (HPV) strains during their lifetimes. For most of these women, the HPV will be cleared from the body, but 5% of them will develop precancerous lesions in the cervix.
At a press conference ahead of the 46th meeting of the French Colposcopy and Cervical and Vaginal Diseases Society, Julia Maruani, MD, a medical gynecologist in Marseille, France, took the opportunity to discuss the importance of vaginal flora and the need to treat cases of bacterial vaginosis.
Striking a balance
Essential for reducing the risk of sexually transmitted infections, a healthy vaginal flora is made up of millions of microorganisms, mainly lactobacilli, as well as other bacteria (Gardnerella vaginalis, Atopobium vaginae, Prevotella, streptococcus, gonococcus), HPV, and fungi.
Lactobacilli produce lactic acid, which reduces the vagina’s pH, as well as hydrogen peroxide, which is toxic to the other bacteria.
Different factors, such as alcohol, a diet rich in polyunsaturated fatty acids and sugar, and especially smoking, can lead to an imbalance of the bacteria in the vaginal flora and thus result in vaginosis. What occurs is an abnormal multiplication of different types of anaerobic bacteria that are normally present in much lower numbers. There is a relative reduction in lactobacilli, which results in an increased vaginal pH, a greater risk of contracting an STI, and reduced clearance of the HPV infection. “Women who smoke probably experience persistent HPV infections due to an imbalance in vaginal flora,” said Dr. Maruani.
Vaginosis and HPV
When there are fewer lactobacilli than there should be, these bacteria can no longer protect the vaginal mucosa, which is disrupted by other bacteria. “HPV then has access to the basal cells,” said Dr. Maruani, acknowledging that the relationship between bacterial vaginosis and persistent HPV infections has been the subject of numerous research studies over the past decade or so. “For years, I would see this same link in my patients. Those with persistent vaginosis were also the ones with persistent HPV. And I’m not the only one to notice this. Studies have also been carried out investigating this exact correlation,” she added.
These studies have shown that HPV infections persist in cases of vaginosis, resulting in the appearance of epithelial lesions. Additionally, the lesions are more severe when dysbiosis is more severe.
What about probiotics? Can they treat dysbiosis and an HPV infection at the same time? “Probiotics work very well for vaginosis, provided they are used for a long time. We know that they lessen HPV infections and low-grade lesions,” said Dr. Maruani, although no randomized studies support this conclusion. “It’s not a one size fits all. We aren’t about to treat patients with precancerous lesions with probiotics.” There are currently no data concerning the efficacy of probiotics on high-grade lesions. These days, Dr. Maruani has been thinking about a new issue: the benefit of diagnosing cases of asymptomatic vaginosis – because treating them would reduce the risk of persistent HPV infection.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Montrouge, France – Four in five women will be infected by one or more human papillomavirus (HPV) strains during their lifetimes. For most of these women, the HPV will be cleared from the body, but 5% of them will develop precancerous lesions in the cervix.
At a press conference ahead of the 46th meeting of the French Colposcopy and Cervical and Vaginal Diseases Society, Julia Maruani, MD, a medical gynecologist in Marseille, France, took the opportunity to discuss the importance of vaginal flora and the need to treat cases of bacterial vaginosis.
Striking a balance
Essential for reducing the risk of sexually transmitted infections, a healthy vaginal flora is made up of millions of microorganisms, mainly lactobacilli, as well as other bacteria (Gardnerella vaginalis, Atopobium vaginae, Prevotella, streptococcus, gonococcus), HPV, and fungi.
Lactobacilli produce lactic acid, which reduces the vagina’s pH, as well as hydrogen peroxide, which is toxic to the other bacteria.
Different factors, such as alcohol, a diet rich in polyunsaturated fatty acids and sugar, and especially smoking, can lead to an imbalance of the bacteria in the vaginal flora and thus result in vaginosis. What occurs is an abnormal multiplication of different types of anaerobic bacteria that are normally present in much lower numbers. There is a relative reduction in lactobacilli, which results in an increased vaginal pH, a greater risk of contracting an STI, and reduced clearance of the HPV infection. “Women who smoke probably experience persistent HPV infections due to an imbalance in vaginal flora,” said Dr. Maruani.
Vaginosis and HPV
When there are fewer lactobacilli than there should be, these bacteria can no longer protect the vaginal mucosa, which is disrupted by other bacteria. “HPV then has access to the basal cells,” said Dr. Maruani, acknowledging that the relationship between bacterial vaginosis and persistent HPV infections has been the subject of numerous research studies over the past decade or so. “For years, I would see this same link in my patients. Those with persistent vaginosis were also the ones with persistent HPV. And I’m not the only one to notice this. Studies have also been carried out investigating this exact correlation,” she added.
These studies have shown that HPV infections persist in cases of vaginosis, resulting in the appearance of epithelial lesions. Additionally, the lesions are more severe when dysbiosis is more severe.
What about probiotics? Can they treat dysbiosis and an HPV infection at the same time? “Probiotics work very well for vaginosis, provided they are used for a long time. We know that they lessen HPV infections and low-grade lesions,” said Dr. Maruani, although no randomized studies support this conclusion. “It’s not a one size fits all. We aren’t about to treat patients with precancerous lesions with probiotics.” There are currently no data concerning the efficacy of probiotics on high-grade lesions. These days, Dr. Maruani has been thinking about a new issue: the benefit of diagnosing cases of asymptomatic vaginosis – because treating them would reduce the risk of persistent HPV infection.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Advances in fertility preservation: Q & A
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
35 years in service to you, our community of reproductive health care clinicians
The mission of OBG
OBG
We wish all our readers a wonderful New Year and the best health possible for our patients.
Arnold P. Advincula, MD
I serve on the executive board that oversees the Fellowships in Minimally Invasive Gynecologic Surgery (FMIGS), and in January 2023 will transition into the role of President. I bring to this leadership role nearly 25 years of surgical experience, both as a clinician educator and inventor. My goal during the next 2 years will be to move toward subspecialty recognition of Complex Gynecology.
Linda D. Bradley, MD
My passion is diagnostic and operative hysteroscopy, simple procedures that can both evaluate and treat intrauterine pathology. Recently, I was thrilled to coauthor an article on office hysteroscopy for Obstetrics & Gynecology (September 2022). I will have a chapter on operative hysteroscopy in the 2023 edition of TeLinde’s Textbook of Gynecology, and I am an author for the topic Office and Operative Hysteroscopy in UpToDate. Locally, I am known as the “foodie gynecologist”—I travel, take cooking classes, and I have more cookbooks than gynecology textbooks. Since Covid, I have embraced biking and just completed a riverboat biking cruise from Salamanca, Spain, to Lisbon, Portugal.
Amy L. Garcia, MD
I am fellowship trained as a minimally invasive gynecologic surgeon (MIGS) and have had a private surgical practice since 2005. I am involved with The American College of Obstetricians and Gynecologists (ACOG), AAGL, and international surgical education for office hysteroscopy and related practice management. I am passionate about working with start-up companies in the gynecologic medical device arena and innovation in gynecologic surgery.
Steven R. Goldstein, MD, NCMP, CCD
I just completed my term as President of the International Menopause Society. This culminated in the society’s 18th World Congress in Lisbon, attended by over 1,700 health care providers from 76 countries. I delivered the Pieter van Keep Memorial Lecture, named for one of the society’s founders who died prematurely of pancreatic cancer. I was further honored by receiving the society’s Distinguished Service Award. I am very proud to have previously received the NAMS Thomas B. Clarkson award for Outstanding Clinical and Basic Science Research in Menopause. I also have one foot in the gynecologic ultrasound world and was given the Joseph H. Holmes Pioneer Award and was the 2023 recipient of the William J. Fry Memorial Lecture Award, both from the American Institute of Ultrasound in Medicine, having written the second book ever on vaginal ultrasonography.
On a personal level, I love to play golf (in spite of my foot drop and 14 orthopedic surgeries). My season tickets show some diversity—the New York City Ballet and St. John’s basketball.
Cheryl B. Iglesia, MD
I am the 49th president of the Society of Gynecologic Surgeons, the 5th woman to hold this position, and the first of Filipino-American descent. I recognize that it is only through extraordinary mentorship and support from other giants in gynecology, like Drs. Andrew Kaunitz (fellow OBG
PS—In the spirit of continually learning, I want to add the Argentine tango to my dancing repertoire and go on an African safari; both are on my bucket list as the pandemic eases.
Andrew M. Kaunitz, MD, NCMP
Since starting with the University of Florida College of Medicine-Jacksonville in 1984, I have enjoyed caring for patients, training residents and medical students, and being involved with publications and research. My areas of focus are menopause, contraception, gyn ultrasound and evaluation/management of women with abnormal uterine bleeding. In 2020, I received the North American Menopause Society/Leon Speroff Outstanding Educator Award. In 2021, I received the ACOG Distinguished Service Award. I enjoy spending time with my family, neighborhood bicycling, and searching for sharks’ teeth at the beach.
Barbara Levy, MD
I have been privileged to serve on the OBG
Continue to: David G. Mutch, MD...
David G. Mutch, MD
I am ending my 6-year term as Chair of the National Cancer Institute’s (NCI) gynecologic cancer steering committee. That is the committee that vets all NCI-sponsored clinical trials in gynecologic oncology. I am on the International Federation of Gynecology and Obstetrics (FIGO) Cancer committee, Co-Chair of the American Joint Committee on Cancer gyn staging committee and on the Reproductive Scientist Development Program selection committee. I also am completing my term as Chair of the Foundation for Women’s Cancer; this is the C3, charitable arm, of the Society of Gynecologic Oncology. We have distributed more than $3.5 million to young investigators to help start their research careers in gynecologic oncology.
Errol R. Norwitz, MD, PhD, MBA
I am a physician-scientist with subspecialty training in high-risk obstetrics (maternal-fetal medicine). I was born and raised in Cape Town, South Africa, and I have trained/practiced in 5 countries on 3 continents. My research interests include the pathophysiology, prediction, prevention, and management of pregnancy complications, primarily preterm birth and preeclampsia. I am a member of the Board of Scientific Counselors of the National Institute of Child Health and Human Development. I am currently President & CEO of Newton-Wellesley Hospital, a comprehensive community-based academic medical center and a member of the Mass General Brigham health care system in Boston, Massachusetts.
Jaimey Pauli, MD
I am the Division Chief and Professor of Maternal-Fetal Medicine (MFM) at the Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center. I had exceptional mentoring throughout my medical career, particularly by a former member of the Editorial Board, Dr. John T. Repke. One of the biggest perks of my job is that our division provides full-scope MFM care. While I often serve as the more traditional MFM consultant and academic educator, I also provide longitudinal prenatal care and deliver many of my own patients, often through subsequent pregnancies. Serving as a member of the Editorial Board combines my passion for clinical obstetrical care with my talents (as a former English major) of reading, writing, and editing. I believe that the work we do provides accessible, evidence-based, and practical guidance for our colleagues so they can provide excellence in obstetrical care.
JoAnn Pinkerton, MD, NCMP
I am a Professor of Obstetrics and Gynecology and Division Chief of Midlife Health at the University of Virginia (UVA) Health. Passionate about menopause, I am an executive director emeritus of The North American Menopause Society (NAMS) and past-President of NAMS (2008-2009). Within the past few years, I have served as an expert advisor for the recent ACOG Clinical Practice Guidelines on Osteoporosis, the NAMS Position Statements on Hormone Therapy and Osteoporosis, and the Global Consensus on Menopause and Androgen Therapy. I received the 2022 South Atlantic Association of Obstetricians and Gynecologists Lifetime Achievement Award for my expertise and work in menopause and the NAMS 2020 Ann Voda Community Service Award for my biannual community educational symposiums. I remain active in research, currently the lead and UVA principal investigator for the Oasis 2 multicenter clinical trial, which is testing a neurokinin receptor antagonist as a nonhormone therapy for the relief of hot flashes. Serving on the OBG
Joseph S. Sanfilippo, MD, MBA
I feel honored and privileged to have received the Golden Apple Teaching Award from the Universityof Pittsburgh School of Medicine. I am also fortunate to be the recipient of the Faculty Educator of the Month Award for resident teaching. I have been named Top Doctor 20 years in a row. My current academic activities include, since 2007, Program Director for Reproductive Endocrinology & Infertility Fellowship at the University of Pittsburgh and Chair of the Mentor-Mentee Program at University of Pittsburgh Department of Obstetrics, Gynecology & Reproductive Sciences. I am Guest Editor for the medical malpractice section of the journal Clinical Obstetrics and Gynecology. Recently, I completed a patient-focused book, “Experts Guide to Fertility,” which will be published in May 2023 by J Hopkins University Publisher and is designed for patients going through infertility treatment. Regarding outside events, I enjoy climbing steep hills and riding far and wide on my “electric bike.” Highly recommend it!
James Simon, MD, CCD, IF, NCMP
It’s been an honor serving on the OBG
I asked our distinguished Board of Editors to identify the most important changes that they believe will occur over the next 5 years, influencing the practice of obstetrics and gynecology. Their expert predictions are summarized below.
Arnold Advincula, MD
As one of the world’s most experienced gynecologic robotic surgeons, the role of this technology will become even more refined over the next 2-5 years with the introduction of sophisticated image guidance, “smart molecules,” and artificial intelligence. All of this will transform both the patient and surgeon experience as well as impact how we train future surgeons.
Linda Bradley, MD
My hope is that a partnership with industry and hysteroscopy thought leaders will enable new developments/technology in performing hysteroscopic sterilization. Conquering the tubal ostia for sterilization in an office setting would profoundly improve contraceptive options for women. Conquering the tubal ostia is the last frontier in gynecology.
Amy Garcia, MD
I predict that new technologies will allow for a significant increase in the number of gynecologists who perform in-office hysteroscopy and that a paradigm shift will occur to replace blind biopsy with hysteroscopy-directed biopsy and evaluation of the uterine cavity.
Steven Goldstein, MD, NCMP, CCD
Among the most important changes in the next 5 years, in my opinion, will be in the arenas of precision medicine, genetic advancement, and artificial intelligence. In addition, unfortunately, there will be an even greater movement toward guidelines utilizing algorithms and clinical pathways. I leave you with the following quote:
“Neither evidence nor clinical judgement alone is sufficient. Evidence without judgement can be applied by a technician. Judgement without evidence can be applied by a friend. But the integration of evidence and judgement is what the healthcare provider does in order to dispense the best clinical care.” —Hertzel Gerstein, MD
Cheryl Iglesia, MD
Technology related to minimally invasive surgery will continue to change our practice, and I predict that surgery will be more centralized to high volume practices. Reimbursements for these procedures may remain a hot button issue, however. The materials used for pelvic reconstruction will be derived from autologous stem cells and advancements made in regenerative medicine.
Andrew Kaunitz, MD, NCMP
As use of contraceptive implants and intrauterine devices continues to grow, I anticipate the incidence of unintended pregnancies will continue to decline. As the novel gonadotropin-releasing hormone (GnRH) antagonists combined with estrogen-progestin add-back grow in use, I anticipate this will provide our patients with more nonsurgical options for managing abnormal uterine bleeding, including that associated with uterine fibroids.
Barbara Levy, MD
Quality will be redefined by patient-defined outcome measures that assess what matters to the people we serve. Real-world evidence will be incorporated to support those measures and provide data on patient outcomes in populations not studied in the randomized controlled trials on which we have created guidelines. This will help to refine guidelines and support more equitable and accessible care.
David Mutch, MD
Over the next 5 years, our expanding insights into the molecular biology of cancer will lead to targeted therapies that will yield better responses with less toxicity.
Errol R. Norwitz, MD, PhD, MBA
In the near future we will use predictive AI algorithms to: 1) identify patients at risk of adverse pregnancy events; 2) stratify patients into high-, average-, and low-risk; and 3) design a personalized obstetric care journey for each patient based on their individualized risk stratification with a view to improving safety and quality outcome metrics, addressing health care disparity, and lowering the cost of care.
Jaimey Pauli, MD
I predict (and fervently hope) that breakthroughs will occur in the prevention of two of the most devastating diseases to affect obstetric patients and their families—preterm birth and preeclampsia.
JoAnn Pinkerton, MD, NCMP
New nonhormone management therapies will be available to treat hot flashes and the genitourinary syndrome of menopause. These treatments will be especially welcomed by patients who cannot or choose not to take hormone therapy. We should not allow new technology to overshadow the patient. We must remember to treat the patient with the condition, not just the disease. Consider what is important to the individual woman, her quality of life, and her ability to function, and keep that in mind when deciding what therapy to suggest.
Joseph S. Sanfilippo, MD, MBA
Artificial intelligence will change the way we educate and provide patient care. Three-dimensional perspectives will cross a number of horizons, some of which include:
- advances in assisted reproductive technology (IVF), offering the next level of “in vitro maturation” of oocytes for patients heretofore unable to conceive. They can progress to having a baby with decreased ovarian reserve or in association with “life after cancer.”
- biogenic engineering and bioinformatics will allow correction of genetic defects in embryos prior to implantation
- the surgical arena will incorporate direct robotic initiated procedures and bring robotic surgery to the next level
- with regard to medical education, at all levels, virtual reality, computer-generated 3-dimensional imaging will provide innovative tools.
James Simon, MD, CCD, IF, NCMP
Medicine’s near-term future portends the realization of truly personalized medicine based upon one’s genetic predisposition to disease, and intentional genetic manipulation to mitigate it. Such advances are here already, simply pending regulatory and ethical approval. My concern going forward is that such individualization, and an algorithm-driven decision-making process will result in taking the personal out of personalized medicine. We humans are more than the collected downstream impact of our genes. In our quest for advances, let’s not forget the balance between nature (our genes) and nurture (environment). The risk of forgetting this aphorism, like the electronic health record, gives me heartburn, or worse, burnout!
The mission of OBG
OBG
We wish all our readers a wonderful New Year and the best health possible for our patients.
Arnold P. Advincula, MD
I serve on the executive board that oversees the Fellowships in Minimally Invasive Gynecologic Surgery (FMIGS), and in January 2023 will transition into the role of President. I bring to this leadership role nearly 25 years of surgical experience, both as a clinician educator and inventor. My goal during the next 2 years will be to move toward subspecialty recognition of Complex Gynecology.
Linda D. Bradley, MD
My passion is diagnostic and operative hysteroscopy, simple procedures that can both evaluate and treat intrauterine pathology. Recently, I was thrilled to coauthor an article on office hysteroscopy for Obstetrics & Gynecology (September 2022). I will have a chapter on operative hysteroscopy in the 2023 edition of TeLinde’s Textbook of Gynecology, and I am an author for the topic Office and Operative Hysteroscopy in UpToDate. Locally, I am known as the “foodie gynecologist”—I travel, take cooking classes, and I have more cookbooks than gynecology textbooks. Since Covid, I have embraced biking and just completed a riverboat biking cruise from Salamanca, Spain, to Lisbon, Portugal.
Amy L. Garcia, MD
I am fellowship trained as a minimally invasive gynecologic surgeon (MIGS) and have had a private surgical practice since 2005. I am involved with The American College of Obstetricians and Gynecologists (ACOG), AAGL, and international surgical education for office hysteroscopy and related practice management. I am passionate about working with start-up companies in the gynecologic medical device arena and innovation in gynecologic surgery.
Steven R. Goldstein, MD, NCMP, CCD
I just completed my term as President of the International Menopause Society. This culminated in the society’s 18th World Congress in Lisbon, attended by over 1,700 health care providers from 76 countries. I delivered the Pieter van Keep Memorial Lecture, named for one of the society’s founders who died prematurely of pancreatic cancer. I was further honored by receiving the society’s Distinguished Service Award. I am very proud to have previously received the NAMS Thomas B. Clarkson award for Outstanding Clinical and Basic Science Research in Menopause. I also have one foot in the gynecologic ultrasound world and was given the Joseph H. Holmes Pioneer Award and was the 2023 recipient of the William J. Fry Memorial Lecture Award, both from the American Institute of Ultrasound in Medicine, having written the second book ever on vaginal ultrasonography.
On a personal level, I love to play golf (in spite of my foot drop and 14 orthopedic surgeries). My season tickets show some diversity—the New York City Ballet and St. John’s basketball.
Cheryl B. Iglesia, MD
I am the 49th president of the Society of Gynecologic Surgeons, the 5th woman to hold this position, and the first of Filipino-American descent. I recognize that it is only through extraordinary mentorship and support from other giants in gynecology, like Drs. Andrew Kaunitz (fellow OBG
PS—In the spirit of continually learning, I want to add the Argentine tango to my dancing repertoire and go on an African safari; both are on my bucket list as the pandemic eases.
Andrew M. Kaunitz, MD, NCMP
Since starting with the University of Florida College of Medicine-Jacksonville in 1984, I have enjoyed caring for patients, training residents and medical students, and being involved with publications and research. My areas of focus are menopause, contraception, gyn ultrasound and evaluation/management of women with abnormal uterine bleeding. In 2020, I received the North American Menopause Society/Leon Speroff Outstanding Educator Award. In 2021, I received the ACOG Distinguished Service Award. I enjoy spending time with my family, neighborhood bicycling, and searching for sharks’ teeth at the beach.
Barbara Levy, MD
I have been privileged to serve on the OBG
Continue to: David G. Mutch, MD...
David G. Mutch, MD
I am ending my 6-year term as Chair of the National Cancer Institute’s (NCI) gynecologic cancer steering committee. That is the committee that vets all NCI-sponsored clinical trials in gynecologic oncology. I am on the International Federation of Gynecology and Obstetrics (FIGO) Cancer committee, Co-Chair of the American Joint Committee on Cancer gyn staging committee and on the Reproductive Scientist Development Program selection committee. I also am completing my term as Chair of the Foundation for Women’s Cancer; this is the C3, charitable arm, of the Society of Gynecologic Oncology. We have distributed more than $3.5 million to young investigators to help start their research careers in gynecologic oncology.
Errol R. Norwitz, MD, PhD, MBA
I am a physician-scientist with subspecialty training in high-risk obstetrics (maternal-fetal medicine). I was born and raised in Cape Town, South Africa, and I have trained/practiced in 5 countries on 3 continents. My research interests include the pathophysiology, prediction, prevention, and management of pregnancy complications, primarily preterm birth and preeclampsia. I am a member of the Board of Scientific Counselors of the National Institute of Child Health and Human Development. I am currently President & CEO of Newton-Wellesley Hospital, a comprehensive community-based academic medical center and a member of the Mass General Brigham health care system in Boston, Massachusetts.
Jaimey Pauli, MD
I am the Division Chief and Professor of Maternal-Fetal Medicine (MFM) at the Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center. I had exceptional mentoring throughout my medical career, particularly by a former member of the Editorial Board, Dr. John T. Repke. One of the biggest perks of my job is that our division provides full-scope MFM care. While I often serve as the more traditional MFM consultant and academic educator, I also provide longitudinal prenatal care and deliver many of my own patients, often through subsequent pregnancies. Serving as a member of the Editorial Board combines my passion for clinical obstetrical care with my talents (as a former English major) of reading, writing, and editing. I believe that the work we do provides accessible, evidence-based, and practical guidance for our colleagues so they can provide excellence in obstetrical care.
JoAnn Pinkerton, MD, NCMP
I am a Professor of Obstetrics and Gynecology and Division Chief of Midlife Health at the University of Virginia (UVA) Health. Passionate about menopause, I am an executive director emeritus of The North American Menopause Society (NAMS) and past-President of NAMS (2008-2009). Within the past few years, I have served as an expert advisor for the recent ACOG Clinical Practice Guidelines on Osteoporosis, the NAMS Position Statements on Hormone Therapy and Osteoporosis, and the Global Consensus on Menopause and Androgen Therapy. I received the 2022 South Atlantic Association of Obstetricians and Gynecologists Lifetime Achievement Award for my expertise and work in menopause and the NAMS 2020 Ann Voda Community Service Award for my biannual community educational symposiums. I remain active in research, currently the lead and UVA principal investigator for the Oasis 2 multicenter clinical trial, which is testing a neurokinin receptor antagonist as a nonhormone therapy for the relief of hot flashes. Serving on the OBG
Joseph S. Sanfilippo, MD, MBA
I feel honored and privileged to have received the Golden Apple Teaching Award from the Universityof Pittsburgh School of Medicine. I am also fortunate to be the recipient of the Faculty Educator of the Month Award for resident teaching. I have been named Top Doctor 20 years in a row. My current academic activities include, since 2007, Program Director for Reproductive Endocrinology & Infertility Fellowship at the University of Pittsburgh and Chair of the Mentor-Mentee Program at University of Pittsburgh Department of Obstetrics, Gynecology & Reproductive Sciences. I am Guest Editor for the medical malpractice section of the journal Clinical Obstetrics and Gynecology. Recently, I completed a patient-focused book, “Experts Guide to Fertility,” which will be published in May 2023 by J Hopkins University Publisher and is designed for patients going through infertility treatment. Regarding outside events, I enjoy climbing steep hills and riding far and wide on my “electric bike.” Highly recommend it!
James Simon, MD, CCD, IF, NCMP
It’s been an honor serving on the OBG
I asked our distinguished Board of Editors to identify the most important changes that they believe will occur over the next 5 years, influencing the practice of obstetrics and gynecology. Their expert predictions are summarized below.
Arnold Advincula, MD
As one of the world’s most experienced gynecologic robotic surgeons, the role of this technology will become even more refined over the next 2-5 years with the introduction of sophisticated image guidance, “smart molecules,” and artificial intelligence. All of this will transform both the patient and surgeon experience as well as impact how we train future surgeons.
Linda Bradley, MD
My hope is that a partnership with industry and hysteroscopy thought leaders will enable new developments/technology in performing hysteroscopic sterilization. Conquering the tubal ostia for sterilization in an office setting would profoundly improve contraceptive options for women. Conquering the tubal ostia is the last frontier in gynecology.
Amy Garcia, MD
I predict that new technologies will allow for a significant increase in the number of gynecologists who perform in-office hysteroscopy and that a paradigm shift will occur to replace blind biopsy with hysteroscopy-directed biopsy and evaluation of the uterine cavity.
Steven Goldstein, MD, NCMP, CCD
Among the most important changes in the next 5 years, in my opinion, will be in the arenas of precision medicine, genetic advancement, and artificial intelligence. In addition, unfortunately, there will be an even greater movement toward guidelines utilizing algorithms and clinical pathways. I leave you with the following quote:
“Neither evidence nor clinical judgement alone is sufficient. Evidence without judgement can be applied by a technician. Judgement without evidence can be applied by a friend. But the integration of evidence and judgement is what the healthcare provider does in order to dispense the best clinical care.” —Hertzel Gerstein, MD
Cheryl Iglesia, MD
Technology related to minimally invasive surgery will continue to change our practice, and I predict that surgery will be more centralized to high volume practices. Reimbursements for these procedures may remain a hot button issue, however. The materials used for pelvic reconstruction will be derived from autologous stem cells and advancements made in regenerative medicine.
Andrew Kaunitz, MD, NCMP
As use of contraceptive implants and intrauterine devices continues to grow, I anticipate the incidence of unintended pregnancies will continue to decline. As the novel gonadotropin-releasing hormone (GnRH) antagonists combined with estrogen-progestin add-back grow in use, I anticipate this will provide our patients with more nonsurgical options for managing abnormal uterine bleeding, including that associated with uterine fibroids.
Barbara Levy, MD
Quality will be redefined by patient-defined outcome measures that assess what matters to the people we serve. Real-world evidence will be incorporated to support those measures and provide data on patient outcomes in populations not studied in the randomized controlled trials on which we have created guidelines. This will help to refine guidelines and support more equitable and accessible care.
David Mutch, MD
Over the next 5 years, our expanding insights into the molecular biology of cancer will lead to targeted therapies that will yield better responses with less toxicity.
Errol R. Norwitz, MD, PhD, MBA
In the near future we will use predictive AI algorithms to: 1) identify patients at risk of adverse pregnancy events; 2) stratify patients into high-, average-, and low-risk; and 3) design a personalized obstetric care journey for each patient based on their individualized risk stratification with a view to improving safety and quality outcome metrics, addressing health care disparity, and lowering the cost of care.
Jaimey Pauli, MD
I predict (and fervently hope) that breakthroughs will occur in the prevention of two of the most devastating diseases to affect obstetric patients and their families—preterm birth and preeclampsia.
JoAnn Pinkerton, MD, NCMP
New nonhormone management therapies will be available to treat hot flashes and the genitourinary syndrome of menopause. These treatments will be especially welcomed by patients who cannot or choose not to take hormone therapy. We should not allow new technology to overshadow the patient. We must remember to treat the patient with the condition, not just the disease. Consider what is important to the individual woman, her quality of life, and her ability to function, and keep that in mind when deciding what therapy to suggest.
Joseph S. Sanfilippo, MD, MBA
Artificial intelligence will change the way we educate and provide patient care. Three-dimensional perspectives will cross a number of horizons, some of which include:
- advances in assisted reproductive technology (IVF), offering the next level of “in vitro maturation” of oocytes for patients heretofore unable to conceive. They can progress to having a baby with decreased ovarian reserve or in association with “life after cancer.”
- biogenic engineering and bioinformatics will allow correction of genetic defects in embryos prior to implantation
- the surgical arena will incorporate direct robotic initiated procedures and bring robotic surgery to the next level
- with regard to medical education, at all levels, virtual reality, computer-generated 3-dimensional imaging will provide innovative tools.
James Simon, MD, CCD, IF, NCMP
Medicine’s near-term future portends the realization of truly personalized medicine based upon one’s genetic predisposition to disease, and intentional genetic manipulation to mitigate it. Such advances are here already, simply pending regulatory and ethical approval. My concern going forward is that such individualization, and an algorithm-driven decision-making process will result in taking the personal out of personalized medicine. We humans are more than the collected downstream impact of our genes. In our quest for advances, let’s not forget the balance between nature (our genes) and nurture (environment). The risk of forgetting this aphorism, like the electronic health record, gives me heartburn, or worse, burnout!
The mission of OBG
OBG
We wish all our readers a wonderful New Year and the best health possible for our patients.
Arnold P. Advincula, MD
I serve on the executive board that oversees the Fellowships in Minimally Invasive Gynecologic Surgery (FMIGS), and in January 2023 will transition into the role of President. I bring to this leadership role nearly 25 years of surgical experience, both as a clinician educator and inventor. My goal during the next 2 years will be to move toward subspecialty recognition of Complex Gynecology.
Linda D. Bradley, MD
My passion is diagnostic and operative hysteroscopy, simple procedures that can both evaluate and treat intrauterine pathology. Recently, I was thrilled to coauthor an article on office hysteroscopy for Obstetrics & Gynecology (September 2022). I will have a chapter on operative hysteroscopy in the 2023 edition of TeLinde’s Textbook of Gynecology, and I am an author for the topic Office and Operative Hysteroscopy in UpToDate. Locally, I am known as the “foodie gynecologist”—I travel, take cooking classes, and I have more cookbooks than gynecology textbooks. Since Covid, I have embraced biking and just completed a riverboat biking cruise from Salamanca, Spain, to Lisbon, Portugal.
Amy L. Garcia, MD
I am fellowship trained as a minimally invasive gynecologic surgeon (MIGS) and have had a private surgical practice since 2005. I am involved with The American College of Obstetricians and Gynecologists (ACOG), AAGL, and international surgical education for office hysteroscopy and related practice management. I am passionate about working with start-up companies in the gynecologic medical device arena and innovation in gynecologic surgery.
Steven R. Goldstein, MD, NCMP, CCD
I just completed my term as President of the International Menopause Society. This culminated in the society’s 18th World Congress in Lisbon, attended by over 1,700 health care providers from 76 countries. I delivered the Pieter van Keep Memorial Lecture, named for one of the society’s founders who died prematurely of pancreatic cancer. I was further honored by receiving the society’s Distinguished Service Award. I am very proud to have previously received the NAMS Thomas B. Clarkson award for Outstanding Clinical and Basic Science Research in Menopause. I also have one foot in the gynecologic ultrasound world and was given the Joseph H. Holmes Pioneer Award and was the 2023 recipient of the William J. Fry Memorial Lecture Award, both from the American Institute of Ultrasound in Medicine, having written the second book ever on vaginal ultrasonography.
On a personal level, I love to play golf (in spite of my foot drop and 14 orthopedic surgeries). My season tickets show some diversity—the New York City Ballet and St. John’s basketball.
Cheryl B. Iglesia, MD
I am the 49th president of the Society of Gynecologic Surgeons, the 5th woman to hold this position, and the first of Filipino-American descent. I recognize that it is only through extraordinary mentorship and support from other giants in gynecology, like Drs. Andrew Kaunitz (fellow OBG
PS—In the spirit of continually learning, I want to add the Argentine tango to my dancing repertoire and go on an African safari; both are on my bucket list as the pandemic eases.
Andrew M. Kaunitz, MD, NCMP
Since starting with the University of Florida College of Medicine-Jacksonville in 1984, I have enjoyed caring for patients, training residents and medical students, and being involved with publications and research. My areas of focus are menopause, contraception, gyn ultrasound and evaluation/management of women with abnormal uterine bleeding. In 2020, I received the North American Menopause Society/Leon Speroff Outstanding Educator Award. In 2021, I received the ACOG Distinguished Service Award. I enjoy spending time with my family, neighborhood bicycling, and searching for sharks’ teeth at the beach.
Barbara Levy, MD
I have been privileged to serve on the OBG
Continue to: David G. Mutch, MD...
David G. Mutch, MD
I am ending my 6-year term as Chair of the National Cancer Institute’s (NCI) gynecologic cancer steering committee. That is the committee that vets all NCI-sponsored clinical trials in gynecologic oncology. I am on the International Federation of Gynecology and Obstetrics (FIGO) Cancer committee, Co-Chair of the American Joint Committee on Cancer gyn staging committee and on the Reproductive Scientist Development Program selection committee. I also am completing my term as Chair of the Foundation for Women’s Cancer; this is the C3, charitable arm, of the Society of Gynecologic Oncology. We have distributed more than $3.5 million to young investigators to help start their research careers in gynecologic oncology.
Errol R. Norwitz, MD, PhD, MBA
I am a physician-scientist with subspecialty training in high-risk obstetrics (maternal-fetal medicine). I was born and raised in Cape Town, South Africa, and I have trained/practiced in 5 countries on 3 continents. My research interests include the pathophysiology, prediction, prevention, and management of pregnancy complications, primarily preterm birth and preeclampsia. I am a member of the Board of Scientific Counselors of the National Institute of Child Health and Human Development. I am currently President & CEO of Newton-Wellesley Hospital, a comprehensive community-based academic medical center and a member of the Mass General Brigham health care system in Boston, Massachusetts.
Jaimey Pauli, MD
I am the Division Chief and Professor of Maternal-Fetal Medicine (MFM) at the Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center. I had exceptional mentoring throughout my medical career, particularly by a former member of the Editorial Board, Dr. John T. Repke. One of the biggest perks of my job is that our division provides full-scope MFM care. While I often serve as the more traditional MFM consultant and academic educator, I also provide longitudinal prenatal care and deliver many of my own patients, often through subsequent pregnancies. Serving as a member of the Editorial Board combines my passion for clinical obstetrical care with my talents (as a former English major) of reading, writing, and editing. I believe that the work we do provides accessible, evidence-based, and practical guidance for our colleagues so they can provide excellence in obstetrical care.
JoAnn Pinkerton, MD, NCMP
I am a Professor of Obstetrics and Gynecology and Division Chief of Midlife Health at the University of Virginia (UVA) Health. Passionate about menopause, I am an executive director emeritus of The North American Menopause Society (NAMS) and past-President of NAMS (2008-2009). Within the past few years, I have served as an expert advisor for the recent ACOG Clinical Practice Guidelines on Osteoporosis, the NAMS Position Statements on Hormone Therapy and Osteoporosis, and the Global Consensus on Menopause and Androgen Therapy. I received the 2022 South Atlantic Association of Obstetricians and Gynecologists Lifetime Achievement Award for my expertise and work in menopause and the NAMS 2020 Ann Voda Community Service Award for my biannual community educational symposiums. I remain active in research, currently the lead and UVA principal investigator for the Oasis 2 multicenter clinical trial, which is testing a neurokinin receptor antagonist as a nonhormone therapy for the relief of hot flashes. Serving on the OBG
Joseph S. Sanfilippo, MD, MBA
I feel honored and privileged to have received the Golden Apple Teaching Award from the Universityof Pittsburgh School of Medicine. I am also fortunate to be the recipient of the Faculty Educator of the Month Award for resident teaching. I have been named Top Doctor 20 years in a row. My current academic activities include, since 2007, Program Director for Reproductive Endocrinology & Infertility Fellowship at the University of Pittsburgh and Chair of the Mentor-Mentee Program at University of Pittsburgh Department of Obstetrics, Gynecology & Reproductive Sciences. I am Guest Editor for the medical malpractice section of the journal Clinical Obstetrics and Gynecology. Recently, I completed a patient-focused book, “Experts Guide to Fertility,” which will be published in May 2023 by J Hopkins University Publisher and is designed for patients going through infertility treatment. Regarding outside events, I enjoy climbing steep hills and riding far and wide on my “electric bike.” Highly recommend it!
James Simon, MD, CCD, IF, NCMP
It’s been an honor serving on the OBG
I asked our distinguished Board of Editors to identify the most important changes that they believe will occur over the next 5 years, influencing the practice of obstetrics and gynecology. Their expert predictions are summarized below.
Arnold Advincula, MD
As one of the world’s most experienced gynecologic robotic surgeons, the role of this technology will become even more refined over the next 2-5 years with the introduction of sophisticated image guidance, “smart molecules,” and artificial intelligence. All of this will transform both the patient and surgeon experience as well as impact how we train future surgeons.
Linda Bradley, MD
My hope is that a partnership with industry and hysteroscopy thought leaders will enable new developments/technology in performing hysteroscopic sterilization. Conquering the tubal ostia for sterilization in an office setting would profoundly improve contraceptive options for women. Conquering the tubal ostia is the last frontier in gynecology.
Amy Garcia, MD
I predict that new technologies will allow for a significant increase in the number of gynecologists who perform in-office hysteroscopy and that a paradigm shift will occur to replace blind biopsy with hysteroscopy-directed biopsy and evaluation of the uterine cavity.
Steven Goldstein, MD, NCMP, CCD
Among the most important changes in the next 5 years, in my opinion, will be in the arenas of precision medicine, genetic advancement, and artificial intelligence. In addition, unfortunately, there will be an even greater movement toward guidelines utilizing algorithms and clinical pathways. I leave you with the following quote:
“Neither evidence nor clinical judgement alone is sufficient. Evidence without judgement can be applied by a technician. Judgement without evidence can be applied by a friend. But the integration of evidence and judgement is what the healthcare provider does in order to dispense the best clinical care.” —Hertzel Gerstein, MD
Cheryl Iglesia, MD
Technology related to minimally invasive surgery will continue to change our practice, and I predict that surgery will be more centralized to high volume practices. Reimbursements for these procedures may remain a hot button issue, however. The materials used for pelvic reconstruction will be derived from autologous stem cells and advancements made in regenerative medicine.
Andrew Kaunitz, MD, NCMP
As use of contraceptive implants and intrauterine devices continues to grow, I anticipate the incidence of unintended pregnancies will continue to decline. As the novel gonadotropin-releasing hormone (GnRH) antagonists combined with estrogen-progestin add-back grow in use, I anticipate this will provide our patients with more nonsurgical options for managing abnormal uterine bleeding, including that associated with uterine fibroids.
Barbara Levy, MD
Quality will be redefined by patient-defined outcome measures that assess what matters to the people we serve. Real-world evidence will be incorporated to support those measures and provide data on patient outcomes in populations not studied in the randomized controlled trials on which we have created guidelines. This will help to refine guidelines and support more equitable and accessible care.
David Mutch, MD
Over the next 5 years, our expanding insights into the molecular biology of cancer will lead to targeted therapies that will yield better responses with less toxicity.
Errol R. Norwitz, MD, PhD, MBA
In the near future we will use predictive AI algorithms to: 1) identify patients at risk of adverse pregnancy events; 2) stratify patients into high-, average-, and low-risk; and 3) design a personalized obstetric care journey for each patient based on their individualized risk stratification with a view to improving safety and quality outcome metrics, addressing health care disparity, and lowering the cost of care.
Jaimey Pauli, MD
I predict (and fervently hope) that breakthroughs will occur in the prevention of two of the most devastating diseases to affect obstetric patients and their families—preterm birth and preeclampsia.
JoAnn Pinkerton, MD, NCMP
New nonhormone management therapies will be available to treat hot flashes and the genitourinary syndrome of menopause. These treatments will be especially welcomed by patients who cannot or choose not to take hormone therapy. We should not allow new technology to overshadow the patient. We must remember to treat the patient with the condition, not just the disease. Consider what is important to the individual woman, her quality of life, and her ability to function, and keep that in mind when deciding what therapy to suggest.
Joseph S. Sanfilippo, MD, MBA
Artificial intelligence will change the way we educate and provide patient care. Three-dimensional perspectives will cross a number of horizons, some of which include:
- advances in assisted reproductive technology (IVF), offering the next level of “in vitro maturation” of oocytes for patients heretofore unable to conceive. They can progress to having a baby with decreased ovarian reserve or in association with “life after cancer.”
- biogenic engineering and bioinformatics will allow correction of genetic defects in embryos prior to implantation
- the surgical arena will incorporate direct robotic initiated procedures and bring robotic surgery to the next level
- with regard to medical education, at all levels, virtual reality, computer-generated 3-dimensional imaging will provide innovative tools.
James Simon, MD, CCD, IF, NCMP
Medicine’s near-term future portends the realization of truly personalized medicine based upon one’s genetic predisposition to disease, and intentional genetic manipulation to mitigate it. Such advances are here already, simply pending regulatory and ethical approval. My concern going forward is that such individualization, and an algorithm-driven decision-making process will result in taking the personal out of personalized medicine. We humans are more than the collected downstream impact of our genes. In our quest for advances, let’s not forget the balance between nature (our genes) and nurture (environment). The risk of forgetting this aphorism, like the electronic health record, gives me heartburn, or worse, burnout!
Does fertility preservation in patients with breast cancer impact relapse rates and disease-specific mortality?

Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and disease-specific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001/jamaoncol.2022.3677.
EXPERT COMMENTARY
Breast cancer is the most diagnosed cancer among US women after skin cancer.1 As of the end of 2020, 7.8 million women were alive who were diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer. Given the wide reach of breast cancer and the increase in its distant stage by more than 4% per year in women of reproductive age (20–39 years), clinicians are urged to address fertility preservation due to reproductive compromise of gonadotoxic therapies and gonadectomy.2 To predict the risk of infertility following chemotherapy, a Cyclophosphamide Equivalent Dose (CED) calculator can be used. A CED of 4,000 mg/m2 has been associated with a significant risk of infertility.3
In 2012, the American Society for Reproductive Medicine removed the experimental label of oocyte cryopreservation then recently endorsed ovarian cryopreservation, thereby providing acceptable procedures for fertility preservation.4 Gonadotropin-releasing hormone agonist use during chemotherapy, which is used to protect the ovary in premenopausal women against the effects of chemotherapy, has been shown to have inconsistent findings and should not replace the established modalities of oocyte/embryo/ovarian tissue cryopreservation.2,5
Details of the study
While studies have been reassuring that ovarian stimulation for fertility preservation in women with breast cancer does not worsen the prognosis, findings are limited by short-term follow-up.6
The recent study by Marklund and colleagues presented an analysis of breast cancer relapse and mortality following fertility preservation with and without hormonal stimulation. In their prospective cohort study of 425 Swedish women who underwent fertility preservation, the authors categorized patients into 2 groups: oocyte and embryo cryopreservation by ovarian hormonal stimulation and ovarian tissue cryopreservation without hormonal stimulation. The control group included 850 women with breast cancer who did not undergo fertility preservation. The cohort and the control groups were matched on age, calendar period of diagnosis, and region. Three Swedish registers for breast cancer were used to obtain the study cohort, and for each participant, 2 breast cancer patients who were unexposed to fertility preservation were used for comparison. The primary outcome was mortality while the secondary outcome was any event of death due to breast cancer or relapse.
Results. A total of 1,275 women were studied at the time of breast cancer diagnosis. After stratification, which included age, parity at diagnosis, tumor size, number of lymph node metastases, and estrogen receptor status, disease-specific mortality was similar in all categories of women, that is, hormonal fertility preservation, nonhormonal fertility preservation, and controls. In the subcohort of 723 women, the adjusted rate of relapse and disease-specific mortality remained the same among all groups.
Study strengths and limitations
This study prompts several areas of criticism. The follow-up of breast cancer patients was only 5 years, adding to the limitations of short-term monitoring seen in prior studies. The authors also considered a delay in pregnancy attempts following breast cancer treatment of hormonally sensitive cancers of 5 to 10 years. However, the long-term safety of pregnancy following breast cancer has shown a statistically significantly superior disease-free survival (DFS) in patients who became pregnant less than 2 years from diagnosis and no difference in those who became pregnant 2 or more years from diagnosis.7
Only 58 women in the nonhormonal fertility preservation group (ovarian tissue cryopreservation) were studied, which may limit an adequate evaluation although it is not expected to negatively impact breast cancer prognosis. Another area of potential bias was the use of only a subcohort to assess relapse-free survival as opposed to the entire cohort that was used to assess mortality.
Strengths of this study include obligatory reporting to the registry and equal access to anticancer treatment and fertility preservation in Sweden. Ovarian stimulating drugs were examined, as letrozole is often used in breast cancer patients to maintain lower estradiol levels due to aromatase inhibition. Nevertheless, this study did not demonstrate a difference in mortality with or without letrozole use. ●
Marklund and colleagues’ findings revealed no increase of breast cancer relapse and mortality following fertility preservation with or without hormonal stimulation. They also propose a “healthy user effect” whereby a woman who feels healthy may choose to undergo fertility preservation, thereby biasing the outcome by having a better survival.8
Future studies with longer follow-up are needed to address the hormonal impact of fertility preservation, if any, on breast cancer DFS and mortality, as well as to evaluate subsequent pregnancy outcomes, stratified for medication treatment type via the CED calculator. To date, evidence continues to support fertility preservation options that use hormonal ovarian stimulation in breast cancer patients as apparently safe for, at least, up to 5 years of follow-up.
MARK P. TROLICE, MD
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. doi:10.3322/caac.21754.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;1;36:1994-2001. doi:10.1200/JCO.2018.78.1914.
- Fertility Preservation in Pittsburgh. CED calculator. Accessed November 14, 2022. https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi:10.1016/j.fertnstert.2019.09.013.
- Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health. 2019;13: 1179558119870163. doi:10.1177/1179558119870163.
- Beebeejaun Y, Athithan A, Copeland TP, et al. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198-207. doi:10.1016/j.fertnstert.2021.01.044.
- Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426-429. doi:10.1093/jnci/djx206.
- Marklund A, Lundberg FE, Eloranta S, et al. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7:86-91. doi:10.1001/ jamaoncol.2020.5957.

Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and disease-specific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001/jamaoncol.2022.3677.
EXPERT COMMENTARY
Breast cancer is the most diagnosed cancer among US women after skin cancer.1 As of the end of 2020, 7.8 million women were alive who were diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer. Given the wide reach of breast cancer and the increase in its distant stage by more than 4% per year in women of reproductive age (20–39 years), clinicians are urged to address fertility preservation due to reproductive compromise of gonadotoxic therapies and gonadectomy.2 To predict the risk of infertility following chemotherapy, a Cyclophosphamide Equivalent Dose (CED) calculator can be used. A CED of 4,000 mg/m2 has been associated with a significant risk of infertility.3
In 2012, the American Society for Reproductive Medicine removed the experimental label of oocyte cryopreservation then recently endorsed ovarian cryopreservation, thereby providing acceptable procedures for fertility preservation.4 Gonadotropin-releasing hormone agonist use during chemotherapy, which is used to protect the ovary in premenopausal women against the effects of chemotherapy, has been shown to have inconsistent findings and should not replace the established modalities of oocyte/embryo/ovarian tissue cryopreservation.2,5
Details of the study
While studies have been reassuring that ovarian stimulation for fertility preservation in women with breast cancer does not worsen the prognosis, findings are limited by short-term follow-up.6
The recent study by Marklund and colleagues presented an analysis of breast cancer relapse and mortality following fertility preservation with and without hormonal stimulation. In their prospective cohort study of 425 Swedish women who underwent fertility preservation, the authors categorized patients into 2 groups: oocyte and embryo cryopreservation by ovarian hormonal stimulation and ovarian tissue cryopreservation without hormonal stimulation. The control group included 850 women with breast cancer who did not undergo fertility preservation. The cohort and the control groups were matched on age, calendar period of diagnosis, and region. Three Swedish registers for breast cancer were used to obtain the study cohort, and for each participant, 2 breast cancer patients who were unexposed to fertility preservation were used for comparison. The primary outcome was mortality while the secondary outcome was any event of death due to breast cancer or relapse.
Results. A total of 1,275 women were studied at the time of breast cancer diagnosis. After stratification, which included age, parity at diagnosis, tumor size, number of lymph node metastases, and estrogen receptor status, disease-specific mortality was similar in all categories of women, that is, hormonal fertility preservation, nonhormonal fertility preservation, and controls. In the subcohort of 723 women, the adjusted rate of relapse and disease-specific mortality remained the same among all groups.
Study strengths and limitations
This study prompts several areas of criticism. The follow-up of breast cancer patients was only 5 years, adding to the limitations of short-term monitoring seen in prior studies. The authors also considered a delay in pregnancy attempts following breast cancer treatment of hormonally sensitive cancers of 5 to 10 years. However, the long-term safety of pregnancy following breast cancer has shown a statistically significantly superior disease-free survival (DFS) in patients who became pregnant less than 2 years from diagnosis and no difference in those who became pregnant 2 or more years from diagnosis.7
Only 58 women in the nonhormonal fertility preservation group (ovarian tissue cryopreservation) were studied, which may limit an adequate evaluation although it is not expected to negatively impact breast cancer prognosis. Another area of potential bias was the use of only a subcohort to assess relapse-free survival as opposed to the entire cohort that was used to assess mortality.
Strengths of this study include obligatory reporting to the registry and equal access to anticancer treatment and fertility preservation in Sweden. Ovarian stimulating drugs were examined, as letrozole is often used in breast cancer patients to maintain lower estradiol levels due to aromatase inhibition. Nevertheless, this study did not demonstrate a difference in mortality with or without letrozole use. ●
Marklund and colleagues’ findings revealed no increase of breast cancer relapse and mortality following fertility preservation with or without hormonal stimulation. They also propose a “healthy user effect” whereby a woman who feels healthy may choose to undergo fertility preservation, thereby biasing the outcome by having a better survival.8
Future studies with longer follow-up are needed to address the hormonal impact of fertility preservation, if any, on breast cancer DFS and mortality, as well as to evaluate subsequent pregnancy outcomes, stratified for medication treatment type via the CED calculator. To date, evidence continues to support fertility preservation options that use hormonal ovarian stimulation in breast cancer patients as apparently safe for, at least, up to 5 years of follow-up.
MARK P. TROLICE, MD

Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and disease-specific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001/jamaoncol.2022.3677.
EXPERT COMMENTARY
Breast cancer is the most diagnosed cancer among US women after skin cancer.1 As of the end of 2020, 7.8 million women were alive who were diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer. Given the wide reach of breast cancer and the increase in its distant stage by more than 4% per year in women of reproductive age (20–39 years), clinicians are urged to address fertility preservation due to reproductive compromise of gonadotoxic therapies and gonadectomy.2 To predict the risk of infertility following chemotherapy, a Cyclophosphamide Equivalent Dose (CED) calculator can be used. A CED of 4,000 mg/m2 has been associated with a significant risk of infertility.3
In 2012, the American Society for Reproductive Medicine removed the experimental label of oocyte cryopreservation then recently endorsed ovarian cryopreservation, thereby providing acceptable procedures for fertility preservation.4 Gonadotropin-releasing hormone agonist use during chemotherapy, which is used to protect the ovary in premenopausal women against the effects of chemotherapy, has been shown to have inconsistent findings and should not replace the established modalities of oocyte/embryo/ovarian tissue cryopreservation.2,5
Details of the study
While studies have been reassuring that ovarian stimulation for fertility preservation in women with breast cancer does not worsen the prognosis, findings are limited by short-term follow-up.6
The recent study by Marklund and colleagues presented an analysis of breast cancer relapse and mortality following fertility preservation with and without hormonal stimulation. In their prospective cohort study of 425 Swedish women who underwent fertility preservation, the authors categorized patients into 2 groups: oocyte and embryo cryopreservation by ovarian hormonal stimulation and ovarian tissue cryopreservation without hormonal stimulation. The control group included 850 women with breast cancer who did not undergo fertility preservation. The cohort and the control groups were matched on age, calendar period of diagnosis, and region. Three Swedish registers for breast cancer were used to obtain the study cohort, and for each participant, 2 breast cancer patients who were unexposed to fertility preservation were used for comparison. The primary outcome was mortality while the secondary outcome was any event of death due to breast cancer or relapse.
Results. A total of 1,275 women were studied at the time of breast cancer diagnosis. After stratification, which included age, parity at diagnosis, tumor size, number of lymph node metastases, and estrogen receptor status, disease-specific mortality was similar in all categories of women, that is, hormonal fertility preservation, nonhormonal fertility preservation, and controls. In the subcohort of 723 women, the adjusted rate of relapse and disease-specific mortality remained the same among all groups.
Study strengths and limitations
This study prompts several areas of criticism. The follow-up of breast cancer patients was only 5 years, adding to the limitations of short-term monitoring seen in prior studies. The authors also considered a delay in pregnancy attempts following breast cancer treatment of hormonally sensitive cancers of 5 to 10 years. However, the long-term safety of pregnancy following breast cancer has shown a statistically significantly superior disease-free survival (DFS) in patients who became pregnant less than 2 years from diagnosis and no difference in those who became pregnant 2 or more years from diagnosis.7
Only 58 women in the nonhormonal fertility preservation group (ovarian tissue cryopreservation) were studied, which may limit an adequate evaluation although it is not expected to negatively impact breast cancer prognosis. Another area of potential bias was the use of only a subcohort to assess relapse-free survival as opposed to the entire cohort that was used to assess mortality.
Strengths of this study include obligatory reporting to the registry and equal access to anticancer treatment and fertility preservation in Sweden. Ovarian stimulating drugs were examined, as letrozole is often used in breast cancer patients to maintain lower estradiol levels due to aromatase inhibition. Nevertheless, this study did not demonstrate a difference in mortality with or without letrozole use. ●
Marklund and colleagues’ findings revealed no increase of breast cancer relapse and mortality following fertility preservation with or without hormonal stimulation. They also propose a “healthy user effect” whereby a woman who feels healthy may choose to undergo fertility preservation, thereby biasing the outcome by having a better survival.8
Future studies with longer follow-up are needed to address the hormonal impact of fertility preservation, if any, on breast cancer DFS and mortality, as well as to evaluate subsequent pregnancy outcomes, stratified for medication treatment type via the CED calculator. To date, evidence continues to support fertility preservation options that use hormonal ovarian stimulation in breast cancer patients as apparently safe for, at least, up to 5 years of follow-up.
MARK P. TROLICE, MD
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. doi:10.3322/caac.21754.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;1;36:1994-2001. doi:10.1200/JCO.2018.78.1914.
- Fertility Preservation in Pittsburgh. CED calculator. Accessed November 14, 2022. https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi:10.1016/j.fertnstert.2019.09.013.
- Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health. 2019;13: 1179558119870163. doi:10.1177/1179558119870163.
- Beebeejaun Y, Athithan A, Copeland TP, et al. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198-207. doi:10.1016/j.fertnstert.2021.01.044.
- Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426-429. doi:10.1093/jnci/djx206.
- Marklund A, Lundberg FE, Eloranta S, et al. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7:86-91. doi:10.1001/ jamaoncol.2020.5957.
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. doi:10.3322/caac.21754.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;1;36:1994-2001. doi:10.1200/JCO.2018.78.1914.
- Fertility Preservation in Pittsburgh. CED calculator. Accessed November 14, 2022. https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi:10.1016/j.fertnstert.2019.09.013.
- Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health. 2019;13: 1179558119870163. doi:10.1177/1179558119870163.
- Beebeejaun Y, Athithan A, Copeland TP, et al. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198-207. doi:10.1016/j.fertnstert.2021.01.044.
- Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426-429. doi:10.1093/jnci/djx206.
- Marklund A, Lundberg FE, Eloranta S, et al. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7:86-91. doi:10.1001/ jamaoncol.2020.5957.
Simplify your approach to the diagnosis and treatment of PCOS
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).
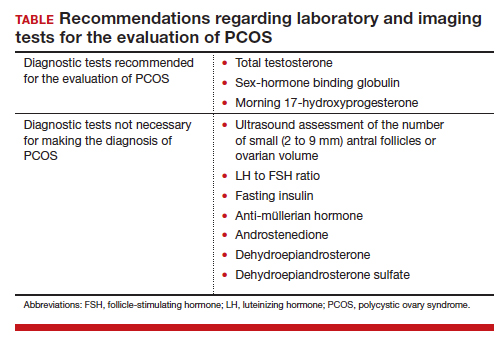
The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.
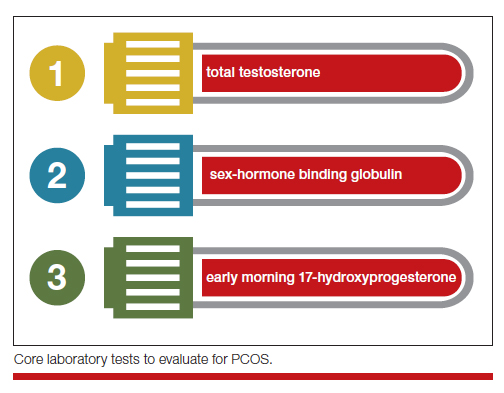
Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
A new use for dating apps: Chasing STDs
Heather Meador and Anna Herber-Downey use dating apps on the job – and their boss knows it.
Both are public health nurses employed by Linn County Public Health in eastern Iowa. They’ve learned that dating apps are the most efficient way to inform users that people they previously met on the sites may have exposed them to sexually transmitted infections.
A nationwide surge in STIs, also known as STDs – with reported cases of gonorrhea and syphilis increasing 10% and 7%, respectively, from 2019 to 2020, according to the Centers for Disease Control and Prevention – isn’t sparing Iowa. The duo has found that the telephone call, a traditional method of contact tracing, no longer works well.
“When I started 12 years ago, we called everyone,” said Ms. Meador, the county health department’s clinical branch supervisor. “It’s getting harder and harder to just call someone on the phone.”
Even texting is ineffective, they said. And people aren’t necessarily answering messages on Facebook. The dating apps are where they’re at.
Because many people are meeting sex partners online – via sites like Grindr or Snapchat, which are headquartered in West Hollywood and Santa Monica, Calif., respectively – contact tracers often don’t have much information to go on, just a screen name or a picture.
So, about a year ago, Ms. Meador and her colleagues got approval from their bosses at the local level to build profiles on the app, through which they can contact the sex partners of infected people.
Traditionally, contact tracers interview people infected with an STI about their recent encounters and then reach out to those partners to tell them about the potential exposure.
Linn County contact tracers use the apps throughout their workday. Grindr, in particular, relies on geolocation, showing users matches who are close by. So the tracers use the apps when they’re out and about, hoping to wander into the same neighborhoods as the person diagnosed with an STI. Sometimes users “tap” the contract tracers to see whether they’re interested – in dating, that is.
When the public health officials spot someone they’re looking for, they send a message asking for a call. It’s a successful method: Ms. Herber-Downey estimated they make an initial contact 75% of the time.
Linn County’s decision to move online comes as STI rates rise nationally, funding to fight them falls, and people adopt new technologies to meet people and seek fun. “STIs are increasing way faster than the funding we have,” said Leo Parker, director of prevention programs for the National Coalition of STD Directors, all while public health departments – many underfunded – are grappling with new behaviors.
“Social media companies have billions; we have tens of thousands,” said Jeffrey Klausner, MD, MPH, a University of Southern California, Los Angeles, public health professor, who previously served as San Francisco’s director of STD prevention and control services. That funding disparity means few public health departments have staff members who can go online. “It’s only really in major cities that they have anyone who’s tasked for that,” Dr. Klausner said.
Even when departments have enough employees to take on the challenge, institutional support can be lacking. Some public health officials question employees who log into the apps. Dr. Klausner once testified on behalf of a Ventura County, Calif., contact tracer who was fired for using sex sites for work.
But with people migrating online to meet partners, following them there makes sense. “We’re now in a digital age,” Mr. Parker said. Individuals might not be out, or might be questioning their identity, making online venues comfortable, anonymous spaces for romance – which, in turn, means people are harder to reach face-to-face, at least at first.
What’s more, online spaces like Grindr are effective public health tools beyond contact tracing. They can be useful ways to get the word out about public health concerns.
Mr. Parker and the Linn County officials said public service announcements on dating apps – advocating for condom use or sharing the business hours for sexual health clinics – do seem to lead people to services. “We do have individuals coming in, saying, ‘I saw you had free testing. I saw it on Grindr,’ ” Mr. Parker said.
Grindr, which touts itself as the biggest dating app focused on LGBTQ+ people, pushes out messages and information to its members, said Jack Harrison-Quintana, director of Grindr for Equality. That engagement intensified during a 2015 meningitis outbreak among LGBTQ+ communities in Chicago, for example.
During that outbreak, the app sent citywide messages about vaccination. Then Mr. Harrison-Quintana took advantage of the service’s design: Using the site’s geolocating capabilities, Grindr workers targeted messages to specific neighborhoods. “We could go in and really go block to block and say, ‘Is this where the cases are showing up?’ ” he said. If so, they sent more messages to that area.
That campaign encouraged further efforts from the app, which regularly sends public health messages about everything from COVID-19 to monkeypox to the platform’s base of roughly 11 million monthly users. Grindr also allows users to display their HIV status and indicate whether they’re vaccinated against COVID, monkeypox, and meningitis.
There are a couple of things Grindr won’t do, however. The company won’t allow public health departments to create institutional accounts. And it won’t allow automated notifications about STI exposures to be sent to users.
That’s due to privacy concerns, the company said, despite calls from public health advocates to deploy better messaging features. Grindr believes that a government presence on the app would be too intrusive and that even anonymous notifications would allow users to trace infections back to their source. (When asked about public health officials who join the site on their own, company spokesperson Patrick Lenihan said: “Individuals are free to say something like ‘I’m a public health professional – ask me about my work!’ in their profile and are free to discuss sexual and public health matters however they see fit.”)
Grindr’s position – however disappointing to some in the public health world – reflects a longtime balancing act attempted by the private sector, which aims to square government concerns with users’ privacy interests.
Dr. Klausner pointed to a 1999 syphilis outbreak in San Francisco as one of the first times he saw how those interests could be at odds. The outbreak was traced to an AOL chatroom. Based on his research, Dr. Klausner said it seemed as though people could go online and “get a sex partner faster than you can get a pizza delivered.”
But persuading New York–based Time Warner, eventually AOL’s corporate parent, to cooperate was time-intensive and tricky – gaining entrée into the chatroom required help from the New York attorney general’s office.
The online industry has advanced since then, Dr. Klausner said. He helped one service develop a system to send digital postcards to potentially exposed people. “Congratulations, you got syphilis,” the postcards read. “They were edgy postcards,” he said, although some options were less “snarky.”
Overall, however, the dating app world is still “bifurcated,” he said. For public health efforts, apps that appeal to LGBTQ+ users are generally more helpful than those that predominantly cater to heterosexual clients.
That’s due to the community’s history with sexual health, explained Jen Hecht, a leader of Building Healthy Online Communities, a public health group partnering with dating apps. “Folks in the queer community have – what – 30, 40 years of thinking about HIV?” she said.
Even though STIs affect everyone, “the norm and the expectation is not there” for straight-focused dating apps, she said. Indeed, neither Match Group nor Bumble – the corporations with the biggest apps focused on heterosexual dating, both based in Texas – responded to multiple requests for comment from KHN.
But users, at least so far, seem to appreciate the app-based interventions. Mr. Harrison-Quintana said Grindr has landed on a just-the-facts approach to conveying health information. He has never received any backlash, “which has been very nice.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Heather Meador and Anna Herber-Downey use dating apps on the job – and their boss knows it.
Both are public health nurses employed by Linn County Public Health in eastern Iowa. They’ve learned that dating apps are the most efficient way to inform users that people they previously met on the sites may have exposed them to sexually transmitted infections.
A nationwide surge in STIs, also known as STDs – with reported cases of gonorrhea and syphilis increasing 10% and 7%, respectively, from 2019 to 2020, according to the Centers for Disease Control and Prevention – isn’t sparing Iowa. The duo has found that the telephone call, a traditional method of contact tracing, no longer works well.
“When I started 12 years ago, we called everyone,” said Ms. Meador, the county health department’s clinical branch supervisor. “It’s getting harder and harder to just call someone on the phone.”
Even texting is ineffective, they said. And people aren’t necessarily answering messages on Facebook. The dating apps are where they’re at.
Because many people are meeting sex partners online – via sites like Grindr or Snapchat, which are headquartered in West Hollywood and Santa Monica, Calif., respectively – contact tracers often don’t have much information to go on, just a screen name or a picture.
So, about a year ago, Ms. Meador and her colleagues got approval from their bosses at the local level to build profiles on the app, through which they can contact the sex partners of infected people.
Traditionally, contact tracers interview people infected with an STI about their recent encounters and then reach out to those partners to tell them about the potential exposure.
Linn County contact tracers use the apps throughout their workday. Grindr, in particular, relies on geolocation, showing users matches who are close by. So the tracers use the apps when they’re out and about, hoping to wander into the same neighborhoods as the person diagnosed with an STI. Sometimes users “tap” the contract tracers to see whether they’re interested – in dating, that is.
When the public health officials spot someone they’re looking for, they send a message asking for a call. It’s a successful method: Ms. Herber-Downey estimated they make an initial contact 75% of the time.
Linn County’s decision to move online comes as STI rates rise nationally, funding to fight them falls, and people adopt new technologies to meet people and seek fun. “STIs are increasing way faster than the funding we have,” said Leo Parker, director of prevention programs for the National Coalition of STD Directors, all while public health departments – many underfunded – are grappling with new behaviors.
“Social media companies have billions; we have tens of thousands,” said Jeffrey Klausner, MD, MPH, a University of Southern California, Los Angeles, public health professor, who previously served as San Francisco’s director of STD prevention and control services. That funding disparity means few public health departments have staff members who can go online. “It’s only really in major cities that they have anyone who’s tasked for that,” Dr. Klausner said.
Even when departments have enough employees to take on the challenge, institutional support can be lacking. Some public health officials question employees who log into the apps. Dr. Klausner once testified on behalf of a Ventura County, Calif., contact tracer who was fired for using sex sites for work.
But with people migrating online to meet partners, following them there makes sense. “We’re now in a digital age,” Mr. Parker said. Individuals might not be out, or might be questioning their identity, making online venues comfortable, anonymous spaces for romance – which, in turn, means people are harder to reach face-to-face, at least at first.
What’s more, online spaces like Grindr are effective public health tools beyond contact tracing. They can be useful ways to get the word out about public health concerns.
Mr. Parker and the Linn County officials said public service announcements on dating apps – advocating for condom use or sharing the business hours for sexual health clinics – do seem to lead people to services. “We do have individuals coming in, saying, ‘I saw you had free testing. I saw it on Grindr,’ ” Mr. Parker said.
Grindr, which touts itself as the biggest dating app focused on LGBTQ+ people, pushes out messages and information to its members, said Jack Harrison-Quintana, director of Grindr for Equality. That engagement intensified during a 2015 meningitis outbreak among LGBTQ+ communities in Chicago, for example.
During that outbreak, the app sent citywide messages about vaccination. Then Mr. Harrison-Quintana took advantage of the service’s design: Using the site’s geolocating capabilities, Grindr workers targeted messages to specific neighborhoods. “We could go in and really go block to block and say, ‘Is this where the cases are showing up?’ ” he said. If so, they sent more messages to that area.
That campaign encouraged further efforts from the app, which regularly sends public health messages about everything from COVID-19 to monkeypox to the platform’s base of roughly 11 million monthly users. Grindr also allows users to display their HIV status and indicate whether they’re vaccinated against COVID, monkeypox, and meningitis.
There are a couple of things Grindr won’t do, however. The company won’t allow public health departments to create institutional accounts. And it won’t allow automated notifications about STI exposures to be sent to users.
That’s due to privacy concerns, the company said, despite calls from public health advocates to deploy better messaging features. Grindr believes that a government presence on the app would be too intrusive and that even anonymous notifications would allow users to trace infections back to their source. (When asked about public health officials who join the site on their own, company spokesperson Patrick Lenihan said: “Individuals are free to say something like ‘I’m a public health professional – ask me about my work!’ in their profile and are free to discuss sexual and public health matters however they see fit.”)
Grindr’s position – however disappointing to some in the public health world – reflects a longtime balancing act attempted by the private sector, which aims to square government concerns with users’ privacy interests.
Dr. Klausner pointed to a 1999 syphilis outbreak in San Francisco as one of the first times he saw how those interests could be at odds. The outbreak was traced to an AOL chatroom. Based on his research, Dr. Klausner said it seemed as though people could go online and “get a sex partner faster than you can get a pizza delivered.”
But persuading New York–based Time Warner, eventually AOL’s corporate parent, to cooperate was time-intensive and tricky – gaining entrée into the chatroom required help from the New York attorney general’s office.
The online industry has advanced since then, Dr. Klausner said. He helped one service develop a system to send digital postcards to potentially exposed people. “Congratulations, you got syphilis,” the postcards read. “They were edgy postcards,” he said, although some options were less “snarky.”
Overall, however, the dating app world is still “bifurcated,” he said. For public health efforts, apps that appeal to LGBTQ+ users are generally more helpful than those that predominantly cater to heterosexual clients.
That’s due to the community’s history with sexual health, explained Jen Hecht, a leader of Building Healthy Online Communities, a public health group partnering with dating apps. “Folks in the queer community have – what – 30, 40 years of thinking about HIV?” she said.
Even though STIs affect everyone, “the norm and the expectation is not there” for straight-focused dating apps, she said. Indeed, neither Match Group nor Bumble – the corporations with the biggest apps focused on heterosexual dating, both based in Texas – responded to multiple requests for comment from KHN.
But users, at least so far, seem to appreciate the app-based interventions. Mr. Harrison-Quintana said Grindr has landed on a just-the-facts approach to conveying health information. He has never received any backlash, “which has been very nice.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Heather Meador and Anna Herber-Downey use dating apps on the job – and their boss knows it.
Both are public health nurses employed by Linn County Public Health in eastern Iowa. They’ve learned that dating apps are the most efficient way to inform users that people they previously met on the sites may have exposed them to sexually transmitted infections.
A nationwide surge in STIs, also known as STDs – with reported cases of gonorrhea and syphilis increasing 10% and 7%, respectively, from 2019 to 2020, according to the Centers for Disease Control and Prevention – isn’t sparing Iowa. The duo has found that the telephone call, a traditional method of contact tracing, no longer works well.
“When I started 12 years ago, we called everyone,” said Ms. Meador, the county health department’s clinical branch supervisor. “It’s getting harder and harder to just call someone on the phone.”
Even texting is ineffective, they said. And people aren’t necessarily answering messages on Facebook. The dating apps are where they’re at.
Because many people are meeting sex partners online – via sites like Grindr or Snapchat, which are headquartered in West Hollywood and Santa Monica, Calif., respectively – contact tracers often don’t have much information to go on, just a screen name or a picture.
So, about a year ago, Ms. Meador and her colleagues got approval from their bosses at the local level to build profiles on the app, through which they can contact the sex partners of infected people.
Traditionally, contact tracers interview people infected with an STI about their recent encounters and then reach out to those partners to tell them about the potential exposure.
Linn County contact tracers use the apps throughout their workday. Grindr, in particular, relies on geolocation, showing users matches who are close by. So the tracers use the apps when they’re out and about, hoping to wander into the same neighborhoods as the person diagnosed with an STI. Sometimes users “tap” the contract tracers to see whether they’re interested – in dating, that is.
When the public health officials spot someone they’re looking for, they send a message asking for a call. It’s a successful method: Ms. Herber-Downey estimated they make an initial contact 75% of the time.
Linn County’s decision to move online comes as STI rates rise nationally, funding to fight them falls, and people adopt new technologies to meet people and seek fun. “STIs are increasing way faster than the funding we have,” said Leo Parker, director of prevention programs for the National Coalition of STD Directors, all while public health departments – many underfunded – are grappling with new behaviors.
“Social media companies have billions; we have tens of thousands,” said Jeffrey Klausner, MD, MPH, a University of Southern California, Los Angeles, public health professor, who previously served as San Francisco’s director of STD prevention and control services. That funding disparity means few public health departments have staff members who can go online. “It’s only really in major cities that they have anyone who’s tasked for that,” Dr. Klausner said.
Even when departments have enough employees to take on the challenge, institutional support can be lacking. Some public health officials question employees who log into the apps. Dr. Klausner once testified on behalf of a Ventura County, Calif., contact tracer who was fired for using sex sites for work.
But with people migrating online to meet partners, following them there makes sense. “We’re now in a digital age,” Mr. Parker said. Individuals might not be out, or might be questioning their identity, making online venues comfortable, anonymous spaces for romance – which, in turn, means people are harder to reach face-to-face, at least at first.
What’s more, online spaces like Grindr are effective public health tools beyond contact tracing. They can be useful ways to get the word out about public health concerns.
Mr. Parker and the Linn County officials said public service announcements on dating apps – advocating for condom use or sharing the business hours for sexual health clinics – do seem to lead people to services. “We do have individuals coming in, saying, ‘I saw you had free testing. I saw it on Grindr,’ ” Mr. Parker said.
Grindr, which touts itself as the biggest dating app focused on LGBTQ+ people, pushes out messages and information to its members, said Jack Harrison-Quintana, director of Grindr for Equality. That engagement intensified during a 2015 meningitis outbreak among LGBTQ+ communities in Chicago, for example.
During that outbreak, the app sent citywide messages about vaccination. Then Mr. Harrison-Quintana took advantage of the service’s design: Using the site’s geolocating capabilities, Grindr workers targeted messages to specific neighborhoods. “We could go in and really go block to block and say, ‘Is this where the cases are showing up?’ ” he said. If so, they sent more messages to that area.
That campaign encouraged further efforts from the app, which regularly sends public health messages about everything from COVID-19 to monkeypox to the platform’s base of roughly 11 million monthly users. Grindr also allows users to display their HIV status and indicate whether they’re vaccinated against COVID, monkeypox, and meningitis.
There are a couple of things Grindr won’t do, however. The company won’t allow public health departments to create institutional accounts. And it won’t allow automated notifications about STI exposures to be sent to users.
That’s due to privacy concerns, the company said, despite calls from public health advocates to deploy better messaging features. Grindr believes that a government presence on the app would be too intrusive and that even anonymous notifications would allow users to trace infections back to their source. (When asked about public health officials who join the site on their own, company spokesperson Patrick Lenihan said: “Individuals are free to say something like ‘I’m a public health professional – ask me about my work!’ in their profile and are free to discuss sexual and public health matters however they see fit.”)
Grindr’s position – however disappointing to some in the public health world – reflects a longtime balancing act attempted by the private sector, which aims to square government concerns with users’ privacy interests.
Dr. Klausner pointed to a 1999 syphilis outbreak in San Francisco as one of the first times he saw how those interests could be at odds. The outbreak was traced to an AOL chatroom. Based on his research, Dr. Klausner said it seemed as though people could go online and “get a sex partner faster than you can get a pizza delivered.”
But persuading New York–based Time Warner, eventually AOL’s corporate parent, to cooperate was time-intensive and tricky – gaining entrée into the chatroom required help from the New York attorney general’s office.
The online industry has advanced since then, Dr. Klausner said. He helped one service develop a system to send digital postcards to potentially exposed people. “Congratulations, you got syphilis,” the postcards read. “They were edgy postcards,” he said, although some options were less “snarky.”
Overall, however, the dating app world is still “bifurcated,” he said. For public health efforts, apps that appeal to LGBTQ+ users are generally more helpful than those that predominantly cater to heterosexual clients.
That’s due to the community’s history with sexual health, explained Jen Hecht, a leader of Building Healthy Online Communities, a public health group partnering with dating apps. “Folks in the queer community have – what – 30, 40 years of thinking about HIV?” she said.
Even though STIs affect everyone, “the norm and the expectation is not there” for straight-focused dating apps, she said. Indeed, neither Match Group nor Bumble – the corporations with the biggest apps focused on heterosexual dating, both based in Texas – responded to multiple requests for comment from KHN.
But users, at least so far, seem to appreciate the app-based interventions. Mr. Harrison-Quintana said Grindr has landed on a just-the-facts approach to conveying health information. He has never received any backlash, “which has been very nice.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The clitoris steps into the spotlight with major scientific discovery
The patients of Jill Krapf, MD, are often too embarrassed to tell her about discomfort in their clitoris.
“I ask all of my patients about clitoral pain, and it is often the first time they have ever been asked about this,” says Dr. Krapf, the associate director of the Center for Vulvovaginal Disorders, a private clinic in Washington and New York.
Dr. Krapf is an ob.gyn. who specializes in female sexual pain that involves the pelvis, vagina, and vulva.
Many of the conditions Dr. Krapf treats don’t have outward symptoms that appear abnormal, but internally, there are damaged or irritated nerves that can result in hypersensitivity, unwanted arousal, or pain.
“Most recent research indicates that even a herniated disk or tear in the spine can lead to clitoral or vulvar symptoms, just like sciatica pain that shoots down the leg is related to issues in the spine,” Dr. Krapf says.
Dr. Krapf was excited to read of a new discovery: Dr. Krapf and other doctors are hopeful that the attention to the clitoris will spark more interest and comprehensive education among people in their field. They also hope it will empower patients to seek medical help if they are having issues with their clitoris.
“Female sexual health has historically been underfunded, especially compared with male sexual health, like erectile dysfunction,” Dr. Krapf says. “Optimizing vulvar and vaginal health is not only necessary for sexual well-being.”
Blair Peters, MD, a plastic surgeon who specializes in gender-affirming care, led the study, which was presented at the Sexual Medicine Society of North America conference in October. Dr. Peters says he hopes that the new information decreases stigma that the clitoris is not worthy of the same medical attention that other organs of the body receive.
When the clitoris doesn’t properly function, there can be harm to a person’s physical and mental health. Paying attention to discomfort in the clitoris, and seeking medical attention, can help catch and prevent some urinary and vaginal infections.
“The fact that it took until 2022 for someone to do this work speaks to how little attention the clitoris has received,” says Dr. Peters, an assistant professor of surgery at the Oregon Health and Science University School of Medicine, Portland.
What’s inside?
Dr. Peters and his colleagues completed the study by taking clitoral nerve tissue from seven adult transgender men who had received gender-affirming genital surgery. The tissues were dyed and magnified 1,000 times under a microscope so the researchers could count nerve fibers.
Dr. Peters says the finding is important because many surgeries take place in the groin region – like hip replacements, episiotomies during childbirth, and pelvic mesh procedures – and the revived attention to the clitoris may help health care providers know where nerves are so that injuries from medical mistakes are prevented.
“Nerves are at risk of damage if it’s not understood where they are at all times,” he says.
Dr. Peters hopes the new finding will help create new surgical techniques for nerve repair and offer insight for gender-affirming phalloplasty, which is the surgical construction of a penis often for transmasculine people.
Ownership of the body part
When it comes to the clitoris, no one type of doctor has specialized in the sex organ.
Urologists, gynecologists, plastic surgeons, and sex therapists all address potential problems that can arise with the clitoris and its surrounding body parts. But specialists like Dr. Krapf are few and far between.
It wasn’t until 2005 that Australian urologist Helen O’Connell found that the clitoris is filled with erectile and non-erectile tissues that are often hidden in anatomy drawings by fat and bone. And it wasn’t until the early 2000s that researchers began delving in earnest into the anatomy of the clitoris and how it functions.
And a 2018 study showed that if more doctors examined the clitoris, they could identify issues like adhesions or infections in the area, most of which can be treated without surgery.
A body part built for pleasure
Randi Levinson, a sex, marriage, and family therapist in Los Angeles, sees patients who have less sensation in the clitoris or pain while having sex, many of whom have recently given birth or are going through menopause.
Women often become embarrassed when they can’t orgasm, or have less sensation in the clitoris, but tend to avoid seeking medical advice, she says. Normalizing discussions about women’s pleasure and the vast anatomy that supports it may help some of her patients.
“The more normal it is to talk about and explore women’s pleasure, the less shame women will have when getting help when they aren’t experiencing pleasure,” Ms. Levinson says. “I have many ... clients who experience pain and discomfort with sex [after pregnancy] and no longer feel pleasure and are concerned that something is wrong with them.”
A version of this article first appeared on WebMD.com.
The patients of Jill Krapf, MD, are often too embarrassed to tell her about discomfort in their clitoris.
“I ask all of my patients about clitoral pain, and it is often the first time they have ever been asked about this,” says Dr. Krapf, the associate director of the Center for Vulvovaginal Disorders, a private clinic in Washington and New York.
Dr. Krapf is an ob.gyn. who specializes in female sexual pain that involves the pelvis, vagina, and vulva.
Many of the conditions Dr. Krapf treats don’t have outward symptoms that appear abnormal, but internally, there are damaged or irritated nerves that can result in hypersensitivity, unwanted arousal, or pain.
“Most recent research indicates that even a herniated disk or tear in the spine can lead to clitoral or vulvar symptoms, just like sciatica pain that shoots down the leg is related to issues in the spine,” Dr. Krapf says.
Dr. Krapf was excited to read of a new discovery: Dr. Krapf and other doctors are hopeful that the attention to the clitoris will spark more interest and comprehensive education among people in their field. They also hope it will empower patients to seek medical help if they are having issues with their clitoris.
“Female sexual health has historically been underfunded, especially compared with male sexual health, like erectile dysfunction,” Dr. Krapf says. “Optimizing vulvar and vaginal health is not only necessary for sexual well-being.”
Blair Peters, MD, a plastic surgeon who specializes in gender-affirming care, led the study, which was presented at the Sexual Medicine Society of North America conference in October. Dr. Peters says he hopes that the new information decreases stigma that the clitoris is not worthy of the same medical attention that other organs of the body receive.
When the clitoris doesn’t properly function, there can be harm to a person’s physical and mental health. Paying attention to discomfort in the clitoris, and seeking medical attention, can help catch and prevent some urinary and vaginal infections.
“The fact that it took until 2022 for someone to do this work speaks to how little attention the clitoris has received,” says Dr. Peters, an assistant professor of surgery at the Oregon Health and Science University School of Medicine, Portland.
What’s inside?
Dr. Peters and his colleagues completed the study by taking clitoral nerve tissue from seven adult transgender men who had received gender-affirming genital surgery. The tissues were dyed and magnified 1,000 times under a microscope so the researchers could count nerve fibers.
Dr. Peters says the finding is important because many surgeries take place in the groin region – like hip replacements, episiotomies during childbirth, and pelvic mesh procedures – and the revived attention to the clitoris may help health care providers know where nerves are so that injuries from medical mistakes are prevented.
“Nerves are at risk of damage if it’s not understood where they are at all times,” he says.
Dr. Peters hopes the new finding will help create new surgical techniques for nerve repair and offer insight for gender-affirming phalloplasty, which is the surgical construction of a penis often for transmasculine people.
Ownership of the body part
When it comes to the clitoris, no one type of doctor has specialized in the sex organ.
Urologists, gynecologists, plastic surgeons, and sex therapists all address potential problems that can arise with the clitoris and its surrounding body parts. But specialists like Dr. Krapf are few and far between.
It wasn’t until 2005 that Australian urologist Helen O’Connell found that the clitoris is filled with erectile and non-erectile tissues that are often hidden in anatomy drawings by fat and bone. And it wasn’t until the early 2000s that researchers began delving in earnest into the anatomy of the clitoris and how it functions.
And a 2018 study showed that if more doctors examined the clitoris, they could identify issues like adhesions or infections in the area, most of which can be treated without surgery.
A body part built for pleasure
Randi Levinson, a sex, marriage, and family therapist in Los Angeles, sees patients who have less sensation in the clitoris or pain while having sex, many of whom have recently given birth or are going through menopause.
Women often become embarrassed when they can’t orgasm, or have less sensation in the clitoris, but tend to avoid seeking medical advice, she says. Normalizing discussions about women’s pleasure and the vast anatomy that supports it may help some of her patients.
“The more normal it is to talk about and explore women’s pleasure, the less shame women will have when getting help when they aren’t experiencing pleasure,” Ms. Levinson says. “I have many ... clients who experience pain and discomfort with sex [after pregnancy] and no longer feel pleasure and are concerned that something is wrong with them.”
A version of this article first appeared on WebMD.com.
The patients of Jill Krapf, MD, are often too embarrassed to tell her about discomfort in their clitoris.
“I ask all of my patients about clitoral pain, and it is often the first time they have ever been asked about this,” says Dr. Krapf, the associate director of the Center for Vulvovaginal Disorders, a private clinic in Washington and New York.
Dr. Krapf is an ob.gyn. who specializes in female sexual pain that involves the pelvis, vagina, and vulva.
Many of the conditions Dr. Krapf treats don’t have outward symptoms that appear abnormal, but internally, there are damaged or irritated nerves that can result in hypersensitivity, unwanted arousal, or pain.
“Most recent research indicates that even a herniated disk or tear in the spine can lead to clitoral or vulvar symptoms, just like sciatica pain that shoots down the leg is related to issues in the spine,” Dr. Krapf says.
Dr. Krapf was excited to read of a new discovery: Dr. Krapf and other doctors are hopeful that the attention to the clitoris will spark more interest and comprehensive education among people in their field. They also hope it will empower patients to seek medical help if they are having issues with their clitoris.
“Female sexual health has historically been underfunded, especially compared with male sexual health, like erectile dysfunction,” Dr. Krapf says. “Optimizing vulvar and vaginal health is not only necessary for sexual well-being.”
Blair Peters, MD, a plastic surgeon who specializes in gender-affirming care, led the study, which was presented at the Sexual Medicine Society of North America conference in October. Dr. Peters says he hopes that the new information decreases stigma that the clitoris is not worthy of the same medical attention that other organs of the body receive.
When the clitoris doesn’t properly function, there can be harm to a person’s physical and mental health. Paying attention to discomfort in the clitoris, and seeking medical attention, can help catch and prevent some urinary and vaginal infections.
“The fact that it took until 2022 for someone to do this work speaks to how little attention the clitoris has received,” says Dr. Peters, an assistant professor of surgery at the Oregon Health and Science University School of Medicine, Portland.
What’s inside?
Dr. Peters and his colleagues completed the study by taking clitoral nerve tissue from seven adult transgender men who had received gender-affirming genital surgery. The tissues were dyed and magnified 1,000 times under a microscope so the researchers could count nerve fibers.
Dr. Peters says the finding is important because many surgeries take place in the groin region – like hip replacements, episiotomies during childbirth, and pelvic mesh procedures – and the revived attention to the clitoris may help health care providers know where nerves are so that injuries from medical mistakes are prevented.
“Nerves are at risk of damage if it’s not understood where they are at all times,” he says.
Dr. Peters hopes the new finding will help create new surgical techniques for nerve repair and offer insight for gender-affirming phalloplasty, which is the surgical construction of a penis often for transmasculine people.
Ownership of the body part
When it comes to the clitoris, no one type of doctor has specialized in the sex organ.
Urologists, gynecologists, plastic surgeons, and sex therapists all address potential problems that can arise with the clitoris and its surrounding body parts. But specialists like Dr. Krapf are few and far between.
It wasn’t until 2005 that Australian urologist Helen O’Connell found that the clitoris is filled with erectile and non-erectile tissues that are often hidden in anatomy drawings by fat and bone. And it wasn’t until the early 2000s that researchers began delving in earnest into the anatomy of the clitoris and how it functions.
And a 2018 study showed that if more doctors examined the clitoris, they could identify issues like adhesions or infections in the area, most of which can be treated without surgery.
A body part built for pleasure
Randi Levinson, a sex, marriage, and family therapist in Los Angeles, sees patients who have less sensation in the clitoris or pain while having sex, many of whom have recently given birth or are going through menopause.
Women often become embarrassed when they can’t orgasm, or have less sensation in the clitoris, but tend to avoid seeking medical advice, she says. Normalizing discussions about women’s pleasure and the vast anatomy that supports it may help some of her patients.
“The more normal it is to talk about and explore women’s pleasure, the less shame women will have when getting help when they aren’t experiencing pleasure,” Ms. Levinson says. “I have many ... clients who experience pain and discomfort with sex [after pregnancy] and no longer feel pleasure and are concerned that something is wrong with them.”
A version of this article first appeared on WebMD.com.
Test strips ID fetal tissue in vaginal blood
A rapid test strip can accurately identify embryonic or fetal tissue in vaginal blood at the bedside, say authors of a paper published in Obstetrics and Gynecology.
The strip, called the ROM Plus test, can detect alpha-fetoprotein (AFP) and insulin-like growth factor–binding protein 1 (IGFBP-1) to identify the presence of the tissue, researchers say.
A positive test could help diagnose miscarriage and rule out ectopic pregnancy.
The ROM Plus test was originally created for diagnosing rupture of amniotic membranes and has been approved by the Food and Drug Administration. This study describes an off-label use of the test.
Lead author Michelle Volovsky, MD, of the department of obstetrics and gynecology at Maimonides Medical Center in Brooklyn, N.Y., said that in the current legal climate for abortion care, the test could have an additional use for women having vaginal bleeding.
“This test could be used as evidence to confirm that a miscarriage has occurred, and hence, a D&C (dilation and curettage) procedure in that case is not an induced abortion of a viable pregnancy,” Dr. Volovsky said.
Women in study
Three groups of reproductive-age women (totaling 90) were included in the study.
One was a negative control group consisting of nonpregnant women undergoing D&C or experiencing vaginal bleeding (n = 23). The positive control group of women had confirmed intrauterine pregnancy undergoing D&C (n = 31), and the third group was a study group of pregnant women with first-trimester bleeding (n = 36). Twelve women in the study group had confirmed ectopic pregnancies.
High sensitivity and specificity
Overall, 47 women had confirmed embryonic or fetal tissue in vaginal or uterine blood samples. The test strip was accurately positive in 45 of those 47 cases for a test sensitivity of 95.7%. The other 43 had confirmed absence of embryonic or fetal tissue in their vaginal or uterine blood samples.
The test had high specificity as well. “In the absence of embryonic or fetal tissue, such as vaginal blood sampled in cases of ectopic pregnancy, threatened or complete miscarriage, or nonpregnant individuals, the test strip had a specificity of 97.7% for obtaining a negative result,” the authors wrote.
The researchers noted that the high sensitivity and specificity were seen as all tests were performed in real-time, common clinical scenarios encountered in a high-volume, urban ob.gyn. unit.
First-trimester bleeding can be common
First-trimester bleeding occurs in 20%-40% of pregnancies and results in almost 500,000 emergency department visits in the United States every year, the authors wrote.
The most common causes include threatened miscarriage, but more serious etiologies include ectopic pregnancy.
Because criteria often are not met for ectopic pregnancy, many women are told they have a pregnancy of unknown location, which can lead to extensive and expensive follow-up.
“The current study was designed to offer a simple diagnostic alternative that does not require the use of an automated laboratory analyzer,” the authors wrote.
The test could be used by patients with confirmed intrauterine pregnancies at home – a highly desirable feature for people hesitant to come into medical offices or who live in remote areas, they noted.
Questions about test’s use
Lauren Thaxton, MD, assistant professor in the department of women’s health at the University of Texas at Austin, told this publication she sees the ease of use by patients at home as the biggest benefit of the test strips.
She said she’s not sure they would add much benefit otherwise because “we already have a pretty great way of identifying pregnancy tissue by floating the products of conception.”
She said the traditional “floating products” method is very inexpensive, involving a pan and strainer, and may be more comprehensive in that it can determine whether a miscarriage is finished.
She also wonders whether in the current legal climate of abortion laws, the test could be used not only to prove that an abortion didn’t happen but used as evidence the opposite way to criminalize abortion.
It’s unfortunate that we are evaluating new technology as ‘could this cause more harm than good?’ But I think it would be wrong not to recognize the long history of criminalization of abortion as well as the current reproductive health and policy climate,” Dr. Thaxton said.
Coauthors Amir Mor, MD, PhD, and Hugh Taylor, MD, hold U.S. patent rights related to the methods described in this article. Coauthor David Seifer, MD, received payment from the Women’s Integrated Network and Rutgers Medical School. In this article the authors describe off-label use of the ROM Plus test kit, produced by Laborie USA. This device was used to test vaginal blood for the presence of embryonic or fetal products. The other authors did not report any potential conflicts of interest. Dr. Thaxton reports no relevant financial relationships.
A rapid test strip can accurately identify embryonic or fetal tissue in vaginal blood at the bedside, say authors of a paper published in Obstetrics and Gynecology.
The strip, called the ROM Plus test, can detect alpha-fetoprotein (AFP) and insulin-like growth factor–binding protein 1 (IGFBP-1) to identify the presence of the tissue, researchers say.
A positive test could help diagnose miscarriage and rule out ectopic pregnancy.
The ROM Plus test was originally created for diagnosing rupture of amniotic membranes and has been approved by the Food and Drug Administration. This study describes an off-label use of the test.
Lead author Michelle Volovsky, MD, of the department of obstetrics and gynecology at Maimonides Medical Center in Brooklyn, N.Y., said that in the current legal climate for abortion care, the test could have an additional use for women having vaginal bleeding.
“This test could be used as evidence to confirm that a miscarriage has occurred, and hence, a D&C (dilation and curettage) procedure in that case is not an induced abortion of a viable pregnancy,” Dr. Volovsky said.
Women in study
Three groups of reproductive-age women (totaling 90) were included in the study.
One was a negative control group consisting of nonpregnant women undergoing D&C or experiencing vaginal bleeding (n = 23). The positive control group of women had confirmed intrauterine pregnancy undergoing D&C (n = 31), and the third group was a study group of pregnant women with first-trimester bleeding (n = 36). Twelve women in the study group had confirmed ectopic pregnancies.
High sensitivity and specificity
Overall, 47 women had confirmed embryonic or fetal tissue in vaginal or uterine blood samples. The test strip was accurately positive in 45 of those 47 cases for a test sensitivity of 95.7%. The other 43 had confirmed absence of embryonic or fetal tissue in their vaginal or uterine blood samples.
The test had high specificity as well. “In the absence of embryonic or fetal tissue, such as vaginal blood sampled in cases of ectopic pregnancy, threatened or complete miscarriage, or nonpregnant individuals, the test strip had a specificity of 97.7% for obtaining a negative result,” the authors wrote.
The researchers noted that the high sensitivity and specificity were seen as all tests were performed in real-time, common clinical scenarios encountered in a high-volume, urban ob.gyn. unit.
First-trimester bleeding can be common
First-trimester bleeding occurs in 20%-40% of pregnancies and results in almost 500,000 emergency department visits in the United States every year, the authors wrote.
The most common causes include threatened miscarriage, but more serious etiologies include ectopic pregnancy.
Because criteria often are not met for ectopic pregnancy, many women are told they have a pregnancy of unknown location, which can lead to extensive and expensive follow-up.
“The current study was designed to offer a simple diagnostic alternative that does not require the use of an automated laboratory analyzer,” the authors wrote.
The test could be used by patients with confirmed intrauterine pregnancies at home – a highly desirable feature for people hesitant to come into medical offices or who live in remote areas, they noted.
Questions about test’s use
Lauren Thaxton, MD, assistant professor in the department of women’s health at the University of Texas at Austin, told this publication she sees the ease of use by patients at home as the biggest benefit of the test strips.
She said she’s not sure they would add much benefit otherwise because “we already have a pretty great way of identifying pregnancy tissue by floating the products of conception.”
She said the traditional “floating products” method is very inexpensive, involving a pan and strainer, and may be more comprehensive in that it can determine whether a miscarriage is finished.
She also wonders whether in the current legal climate of abortion laws, the test could be used not only to prove that an abortion didn’t happen but used as evidence the opposite way to criminalize abortion.
It’s unfortunate that we are evaluating new technology as ‘could this cause more harm than good?’ But I think it would be wrong not to recognize the long history of criminalization of abortion as well as the current reproductive health and policy climate,” Dr. Thaxton said.
Coauthors Amir Mor, MD, PhD, and Hugh Taylor, MD, hold U.S. patent rights related to the methods described in this article. Coauthor David Seifer, MD, received payment from the Women’s Integrated Network and Rutgers Medical School. In this article the authors describe off-label use of the ROM Plus test kit, produced by Laborie USA. This device was used to test vaginal blood for the presence of embryonic or fetal products. The other authors did not report any potential conflicts of interest. Dr. Thaxton reports no relevant financial relationships.
A rapid test strip can accurately identify embryonic or fetal tissue in vaginal blood at the bedside, say authors of a paper published in Obstetrics and Gynecology.
The strip, called the ROM Plus test, can detect alpha-fetoprotein (AFP) and insulin-like growth factor–binding protein 1 (IGFBP-1) to identify the presence of the tissue, researchers say.
A positive test could help diagnose miscarriage and rule out ectopic pregnancy.
The ROM Plus test was originally created for diagnosing rupture of amniotic membranes and has been approved by the Food and Drug Administration. This study describes an off-label use of the test.
Lead author Michelle Volovsky, MD, of the department of obstetrics and gynecology at Maimonides Medical Center in Brooklyn, N.Y., said that in the current legal climate for abortion care, the test could have an additional use for women having vaginal bleeding.
“This test could be used as evidence to confirm that a miscarriage has occurred, and hence, a D&C (dilation and curettage) procedure in that case is not an induced abortion of a viable pregnancy,” Dr. Volovsky said.
Women in study
Three groups of reproductive-age women (totaling 90) were included in the study.
One was a negative control group consisting of nonpregnant women undergoing D&C or experiencing vaginal bleeding (n = 23). The positive control group of women had confirmed intrauterine pregnancy undergoing D&C (n = 31), and the third group was a study group of pregnant women with first-trimester bleeding (n = 36). Twelve women in the study group had confirmed ectopic pregnancies.
High sensitivity and specificity
Overall, 47 women had confirmed embryonic or fetal tissue in vaginal or uterine blood samples. The test strip was accurately positive in 45 of those 47 cases for a test sensitivity of 95.7%. The other 43 had confirmed absence of embryonic or fetal tissue in their vaginal or uterine blood samples.
The test had high specificity as well. “In the absence of embryonic or fetal tissue, such as vaginal blood sampled in cases of ectopic pregnancy, threatened or complete miscarriage, or nonpregnant individuals, the test strip had a specificity of 97.7% for obtaining a negative result,” the authors wrote.
The researchers noted that the high sensitivity and specificity were seen as all tests were performed in real-time, common clinical scenarios encountered in a high-volume, urban ob.gyn. unit.
First-trimester bleeding can be common
First-trimester bleeding occurs in 20%-40% of pregnancies and results in almost 500,000 emergency department visits in the United States every year, the authors wrote.
The most common causes include threatened miscarriage, but more serious etiologies include ectopic pregnancy.
Because criteria often are not met for ectopic pregnancy, many women are told they have a pregnancy of unknown location, which can lead to extensive and expensive follow-up.
“The current study was designed to offer a simple diagnostic alternative that does not require the use of an automated laboratory analyzer,” the authors wrote.
The test could be used by patients with confirmed intrauterine pregnancies at home – a highly desirable feature for people hesitant to come into medical offices or who live in remote areas, they noted.
Questions about test’s use
Lauren Thaxton, MD, assistant professor in the department of women’s health at the University of Texas at Austin, told this publication she sees the ease of use by patients at home as the biggest benefit of the test strips.
She said she’s not sure they would add much benefit otherwise because “we already have a pretty great way of identifying pregnancy tissue by floating the products of conception.”
She said the traditional “floating products” method is very inexpensive, involving a pan and strainer, and may be more comprehensive in that it can determine whether a miscarriage is finished.
She also wonders whether in the current legal climate of abortion laws, the test could be used not only to prove that an abortion didn’t happen but used as evidence the opposite way to criminalize abortion.
It’s unfortunate that we are evaluating new technology as ‘could this cause more harm than good?’ But I think it would be wrong not to recognize the long history of criminalization of abortion as well as the current reproductive health and policy climate,” Dr. Thaxton said.
Coauthors Amir Mor, MD, PhD, and Hugh Taylor, MD, hold U.S. patent rights related to the methods described in this article. Coauthor David Seifer, MD, received payment from the Women’s Integrated Network and Rutgers Medical School. In this article the authors describe off-label use of the ROM Plus test kit, produced by Laborie USA. This device was used to test vaginal blood for the presence of embryonic or fetal products. The other authors did not report any potential conflicts of interest. Dr. Thaxton reports no relevant financial relationships.
FROM OBSTETRICS AND GYNECOLOGY
Treating recurrent vulvovaginal candidiasis

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.
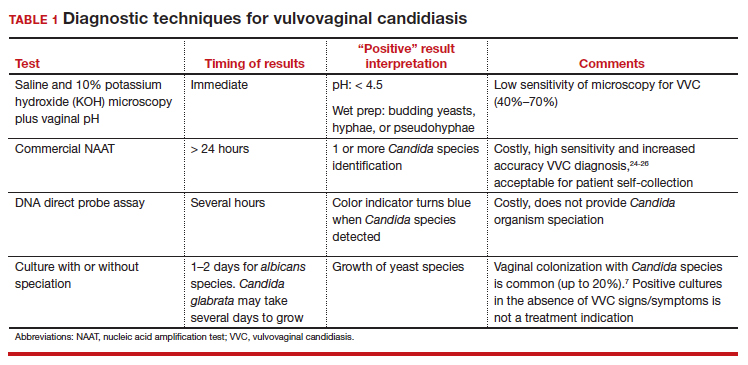
Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.

Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.

Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592
“Blind” endometrial sampling: A call to end the practice
Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.
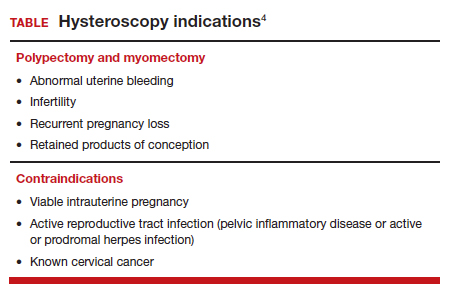
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.