User login
Alopecia areata: Positive results reported for two investigational JAK inhibitors
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
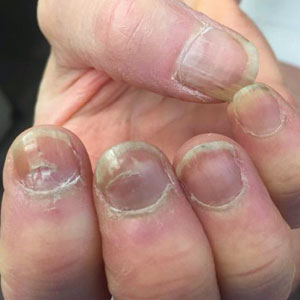
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
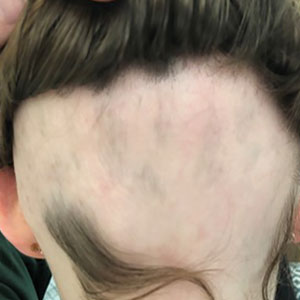
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Expert calls for thoughtful approach to curbing costs in dermatology
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
PORTLAND, ORE. – About 10 years ago when Arash Mostaghimi, MD, MPA, MPH, became an attending physician at Brigham and Women’s Hospital, Boston, he noticed that some of his dermatology colleagues checked the potassium levels religiously in their female patients taking spironolactone, while others never did.
“It led to this question: Dr. Mostaghimi, director of the dermatology inpatient service at Brigham and Women’s, said at the annual meeting of the Pacific Dermatologic Association.
To find out, he and his colleagues reviewed 1,802 serum potassium measurements in a study of healthy young women with no known health conditions who were taking spironolactone, published in 2015. They discovered that 13 of those tests suggested mild hyperkalemia, defined as a level greater than 5.0 mEq/L. Of these, six were rechecked and were normal; no action was taken in the other seven patients.
“This led us to conclude that we spent $78,000 at our institution on testing that did not appear to yield clinically significant information for these patients, and that routine potassium monitoring is unnecessary for most women taking spironolactone for acne,” he said. Their findings have been validated “in many cohorts of data,” he added.
The study serves as an example of efforts dermatologists can take to curb unnecessary costs within the field to be “appropriate stewards of resources,” he continued. “We have to think about the ratio of benefit over cost. It’s not just about the cost, it’s about what you’re getting for the amount of money that you’re spending. The idea of this is not restricting or not giving people medications or access to things that they need. The idea is to do it in a thoughtful way that works across the population.”
Value thresholds
Determining the value thresholds of a particular medicine or procedure is also essential to good dermatology practice. To illustrate, Dr. Mostaghimi cited a prospective cohort study that compared treatment patterns and clinical outcomes in 1,536 consecutive patients with nonmelanoma skin cancer (NMSC) with and without limited life expectancy. More than two-thirds of the NMSCs (69%) were treated surgically. After adjusting for tumor and patient characteristics, the researchers found that 43% of patients with low life expectancy died within 5 years, but not from NMSC.
“Does that mean we shouldn’t do surgery for NMSC patients with low life expectancy?” he asked. “Should we do it less? Should we let the patients decide? It’s complicated. As a society, we have to decide what’s worth doing and what’s not worth doing,” he said. “What about old diseases with new treatments, like alopecia areata? Is alopecia areata a cosmetic condition? Dermatologists and patients wouldn’t classify it that way, but many insurers do. How do you negotiate that?”
In 2013, the American Academy of Dermatology identified 10 evidence-based recommendations that can support conversations between patients and dermatologists about treatments, tests, and procedures that may not be necessary. One of the recommendations was not to prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.
“If a clinician thinks a patient has onychomycosis, he or she is usually right,” Dr. Mostaghimi said. “But what’s the added cost/benefit of performing a KOH followed by PAS testing if negative or performing a PAS test directly versus just treating the patient?”
In 2006, he and his colleagues published the results of a decision analysis to address these questions. They determined that the costs of testing to avoid one case of clinically apparent liver injury with terbinafine treatment was $18.2-$43.7 million for the KOH screening pathway and $37.6 to $90.2 million for the PAS testing pathway.
“Is that worth it?” he asked. “Would we get more value for spending the money elsewhere? In this case, the answer is most likely yes.”
Isotretinoin lab testing
Translating research into recommendations and standards of care is one way to help curb costs in dermatology. As an example, he cited lab monitoring for patients treated with isotretinoin for acne.
“There have been a number of papers over the years that have suggested that the number of labs we do is excessive, that the value that they provide is low, and that abnormal results do not impact our decision-making,” Dr. Mostaghimi said. “Do some patients on isotretinoin get mildly elevated [liver function tests] and hypertriglyceridemia? Yes, that happens. Does it matter? Nothing has demonstrated that it matters. Does it matter that an 18-year-old has high triglycerides for 6 months? Rarely, if ever.”
To promote a new approach, he and a panel of acne experts from five continents performed a Delphi consensus study. Based on their consensus, they proposed a simple approach: For “generally healthy patients without underlying abnormalities or preexisting conditions warranting further investigation,” check ALT and triglycerides prior to initiating isotretinoin. Then start isotretinoin.
“At the peak dose, recheck ALT and triglycerides – this might be at month 2,” Dr. Mostaghimi said. “Other people wait a little bit longer. No labs are required once treatment is complete. Of course, adjust this approach based on your assessment of the patient in front of you. None of these recommendations should replace your clinical judgment and intuition.”
He proposed a new paradigm where dermatologists can ask themselves three questions for every patient they see: Why is this intervention or test being done? Why is it being done in this patient? And why do it at that time? “If we think this way, we can identify some inconsistencies in our own thinking and opportunities for improvement,” he said.
Dr. Mostaghimi reported that he is a consultant to Pfizer, Concert, Lilly, and Bioniz. He is also an advisor to Him & Hers Cosmetics and Digital Diagnostics and is an associate editor for JAMA Dermatology.
AT PDA 2022
Uncombable hair syndrome: One gene, variants responsible for many cases
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Low-dose oral minoxidil for the treatment of alopecia
Other than oral finasteride, vitamins, and topicals, there has been little advancement in the treatment of AGA leaving many (including me) desperate for anything remotely new.
Oral minoxidil is a peripheral vasodilator approved by the Food and Drug Administration for use in patients with hypertensive disease taken at doses ranging between 10 mg to 40 mg daily. Animal studies have shown that minoxidil affects the hair growth cycle by shortening the telogen phase and prolonging the anagen phase.
Recent case studies have also shown growing evidence for the off-label use of low-dose oral minoxidil (LDOM) for treating different types of alopecia. Topical minoxidil is metabolized into its active metabolite minoxidil sulfate, by sulfotransferase enzymes located in the outer root sheath of hair follicles. The expression of sulfotransferase varies greatly in the scalp of different individuals, and this difference is directly correlated to the wide range of responses to minoxidil treatment. LDOM is, however, more widely effective because it requires decreased follicular enzymatic activity to form its active metabolite as compared with its topical form.
In a retrospective series by Beach and colleagues evaluating the efficacy and tolerability of LDOM for treating AGA, there was increased scalp hair growth in 33 of 51 patients (65%) and decreased hair shedding in 14 of the 51 patients (27%) with LDOM. Patients with nonscarring alopecia were most likely to show improvement. Side effects were dose dependent and infrequent. The most frequent adverse effects were hypertrichosis, lightheadedness, edema, and tachycardia. No life-threatening adverse effects were observed. Although there has been a recently reported case report of severe pericardial effusion, edema, and anasarca in a woman with frontal fibrosing alopecia treated with LDOM, life threatening side effects are rare.3
To compare the efficacy of topical versus oral minoxidil, Ramos and colleagues performed a 24-week prospective study of low-dose (1 mg/day) oral minoxidil, compared with topical 5% minoxidil, in the treatment of 52 women with female pattern hair loss. Blinded analysis of trichoscopic images were evaluated to compare the change in total hair density in a target area from baseline to week 24 by three dermatologists.
Results after 24 weeks of treatment showed an increase in total hair density (12%) among the women taking oral minoxidil, compared with 7.2% in women who applied topical minoxidil (P =.09).
In the armamentarium of hair-loss treatments, dermatologists have limited choices. LDOM can be used in patients with both scarring and nonscarring alopecia if monitored regularly. Treatment doses I recommend are 1.25-5 mg daily titrated up slowly in properly selected patients without contraindications and those who are not taking other vasodilators. Self-reported dizziness, edema, and headache are common and treatments for facial hypertrichosis in women are always discussed. Clinical efficacy can be evaluated after 10-12 months of therapy and concomitant spironolactone can be given to mitigate the side effect of hypertrichosis.Patient selection is crucial as patients with severe scarring alopecia and those with active inflammatory diseases of the scalp may not see similar results. Similar to other hair loss treatments, treatment courses of 10-12 months are often needed to see visible signs of hair growth.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. Dr. Talakoub had no relevant disclosures.
References
Beach RA et al. J Am Acad Dermatol. 2021 Mar;84(3):761-3.
Dlova et al. JAAD Case Reports. 2022 Oct;28:94-6.
Jimenez-Cauhe J et al. J Am Acad Dermatol. 2021 Jan;84(1):222-3.
Ramos PM et al. J Eur Acad Dermatol Venereol. 2020 Jan;34(1):e40-1.
Ramos PM et al. J Am Acad Dermatol. 2020 Jan;82(1):252-3.
Randolph M and Tosti A. J Am Acad Dermatol. 2021 Mar;84(3):737-46.
Vañó-Galván S et al. J Am Acad Dermatol. 2021 Jun;84(6):1644-51.
Other than oral finasteride, vitamins, and topicals, there has been little advancement in the treatment of AGA leaving many (including me) desperate for anything remotely new.
Oral minoxidil is a peripheral vasodilator approved by the Food and Drug Administration for use in patients with hypertensive disease taken at doses ranging between 10 mg to 40 mg daily. Animal studies have shown that minoxidil affects the hair growth cycle by shortening the telogen phase and prolonging the anagen phase.
Recent case studies have also shown growing evidence for the off-label use of low-dose oral minoxidil (LDOM) for treating different types of alopecia. Topical minoxidil is metabolized into its active metabolite minoxidil sulfate, by sulfotransferase enzymes located in the outer root sheath of hair follicles. The expression of sulfotransferase varies greatly in the scalp of different individuals, and this difference is directly correlated to the wide range of responses to minoxidil treatment. LDOM is, however, more widely effective because it requires decreased follicular enzymatic activity to form its active metabolite as compared with its topical form.
In a retrospective series by Beach and colleagues evaluating the efficacy and tolerability of LDOM for treating AGA, there was increased scalp hair growth in 33 of 51 patients (65%) and decreased hair shedding in 14 of the 51 patients (27%) with LDOM. Patients with nonscarring alopecia were most likely to show improvement. Side effects were dose dependent and infrequent. The most frequent adverse effects were hypertrichosis, lightheadedness, edema, and tachycardia. No life-threatening adverse effects were observed. Although there has been a recently reported case report of severe pericardial effusion, edema, and anasarca in a woman with frontal fibrosing alopecia treated with LDOM, life threatening side effects are rare.3
To compare the efficacy of topical versus oral minoxidil, Ramos and colleagues performed a 24-week prospective study of low-dose (1 mg/day) oral minoxidil, compared with topical 5% minoxidil, in the treatment of 52 women with female pattern hair loss. Blinded analysis of trichoscopic images were evaluated to compare the change in total hair density in a target area from baseline to week 24 by three dermatologists.
Results after 24 weeks of treatment showed an increase in total hair density (12%) among the women taking oral minoxidil, compared with 7.2% in women who applied topical minoxidil (P =.09).
In the armamentarium of hair-loss treatments, dermatologists have limited choices. LDOM can be used in patients with both scarring and nonscarring alopecia if monitored regularly. Treatment doses I recommend are 1.25-5 mg daily titrated up slowly in properly selected patients without contraindications and those who are not taking other vasodilators. Self-reported dizziness, edema, and headache are common and treatments for facial hypertrichosis in women are always discussed. Clinical efficacy can be evaluated after 10-12 months of therapy and concomitant spironolactone can be given to mitigate the side effect of hypertrichosis.Patient selection is crucial as patients with severe scarring alopecia and those with active inflammatory diseases of the scalp may not see similar results. Similar to other hair loss treatments, treatment courses of 10-12 months are often needed to see visible signs of hair growth.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. Dr. Talakoub had no relevant disclosures.
References
Beach RA et al. J Am Acad Dermatol. 2021 Mar;84(3):761-3.
Dlova et al. JAAD Case Reports. 2022 Oct;28:94-6.
Jimenez-Cauhe J et al. J Am Acad Dermatol. 2021 Jan;84(1):222-3.
Ramos PM et al. J Eur Acad Dermatol Venereol. 2020 Jan;34(1):e40-1.
Ramos PM et al. J Am Acad Dermatol. 2020 Jan;82(1):252-3.
Randolph M and Tosti A. J Am Acad Dermatol. 2021 Mar;84(3):737-46.
Vañó-Galván S et al. J Am Acad Dermatol. 2021 Jun;84(6):1644-51.
Other than oral finasteride, vitamins, and topicals, there has been little advancement in the treatment of AGA leaving many (including me) desperate for anything remotely new.
Oral minoxidil is a peripheral vasodilator approved by the Food and Drug Administration for use in patients with hypertensive disease taken at doses ranging between 10 mg to 40 mg daily. Animal studies have shown that minoxidil affects the hair growth cycle by shortening the telogen phase and prolonging the anagen phase.
Recent case studies have also shown growing evidence for the off-label use of low-dose oral minoxidil (LDOM) for treating different types of alopecia. Topical minoxidil is metabolized into its active metabolite minoxidil sulfate, by sulfotransferase enzymes located in the outer root sheath of hair follicles. The expression of sulfotransferase varies greatly in the scalp of different individuals, and this difference is directly correlated to the wide range of responses to minoxidil treatment. LDOM is, however, more widely effective because it requires decreased follicular enzymatic activity to form its active metabolite as compared with its topical form.
In a retrospective series by Beach and colleagues evaluating the efficacy and tolerability of LDOM for treating AGA, there was increased scalp hair growth in 33 of 51 patients (65%) and decreased hair shedding in 14 of the 51 patients (27%) with LDOM. Patients with nonscarring alopecia were most likely to show improvement. Side effects were dose dependent and infrequent. The most frequent adverse effects were hypertrichosis, lightheadedness, edema, and tachycardia. No life-threatening adverse effects were observed. Although there has been a recently reported case report of severe pericardial effusion, edema, and anasarca in a woman with frontal fibrosing alopecia treated with LDOM, life threatening side effects are rare.3
To compare the efficacy of topical versus oral minoxidil, Ramos and colleagues performed a 24-week prospective study of low-dose (1 mg/day) oral minoxidil, compared with topical 5% minoxidil, in the treatment of 52 women with female pattern hair loss. Blinded analysis of trichoscopic images were evaluated to compare the change in total hair density in a target area from baseline to week 24 by three dermatologists.
Results after 24 weeks of treatment showed an increase in total hair density (12%) among the women taking oral minoxidil, compared with 7.2% in women who applied topical minoxidil (P =.09).
In the armamentarium of hair-loss treatments, dermatologists have limited choices. LDOM can be used in patients with both scarring and nonscarring alopecia if monitored regularly. Treatment doses I recommend are 1.25-5 mg daily titrated up slowly in properly selected patients without contraindications and those who are not taking other vasodilators. Self-reported dizziness, edema, and headache are common and treatments for facial hypertrichosis in women are always discussed. Clinical efficacy can be evaluated after 10-12 months of therapy and concomitant spironolactone can be given to mitigate the side effect of hypertrichosis.Patient selection is crucial as patients with severe scarring alopecia and those with active inflammatory diseases of the scalp may not see similar results. Similar to other hair loss treatments, treatment courses of 10-12 months are often needed to see visible signs of hair growth.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. Dr. Talakoub had no relevant disclosures.
References
Beach RA et al. J Am Acad Dermatol. 2021 Mar;84(3):761-3.
Dlova et al. JAAD Case Reports. 2022 Oct;28:94-6.
Jimenez-Cauhe J et al. J Am Acad Dermatol. 2021 Jan;84(1):222-3.
Ramos PM et al. J Eur Acad Dermatol Venereol. 2020 Jan;34(1):e40-1.
Ramos PM et al. J Am Acad Dermatol. 2020 Jan;82(1):252-3.
Randolph M and Tosti A. J Am Acad Dermatol. 2021 Mar;84(3):737-46.
Vañó-Galván S et al. J Am Acad Dermatol. 2021 Jun;84(6):1644-51.
Expert shares tips on hair disorders and photoprotection for patients of color
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
AT PDA 2022
Transverse Leukonychia and Beau Lines Following COVID-19 Vaccination
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).
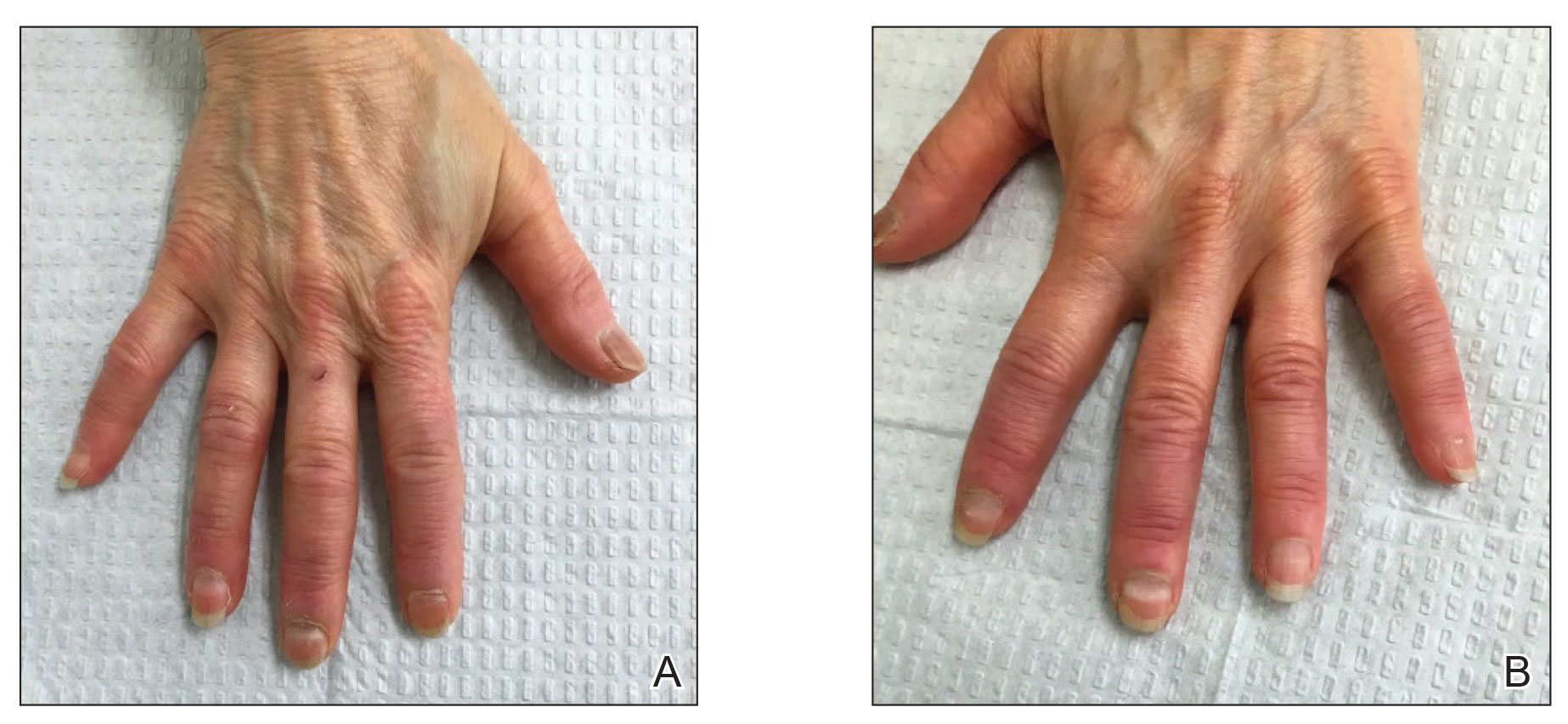
Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.
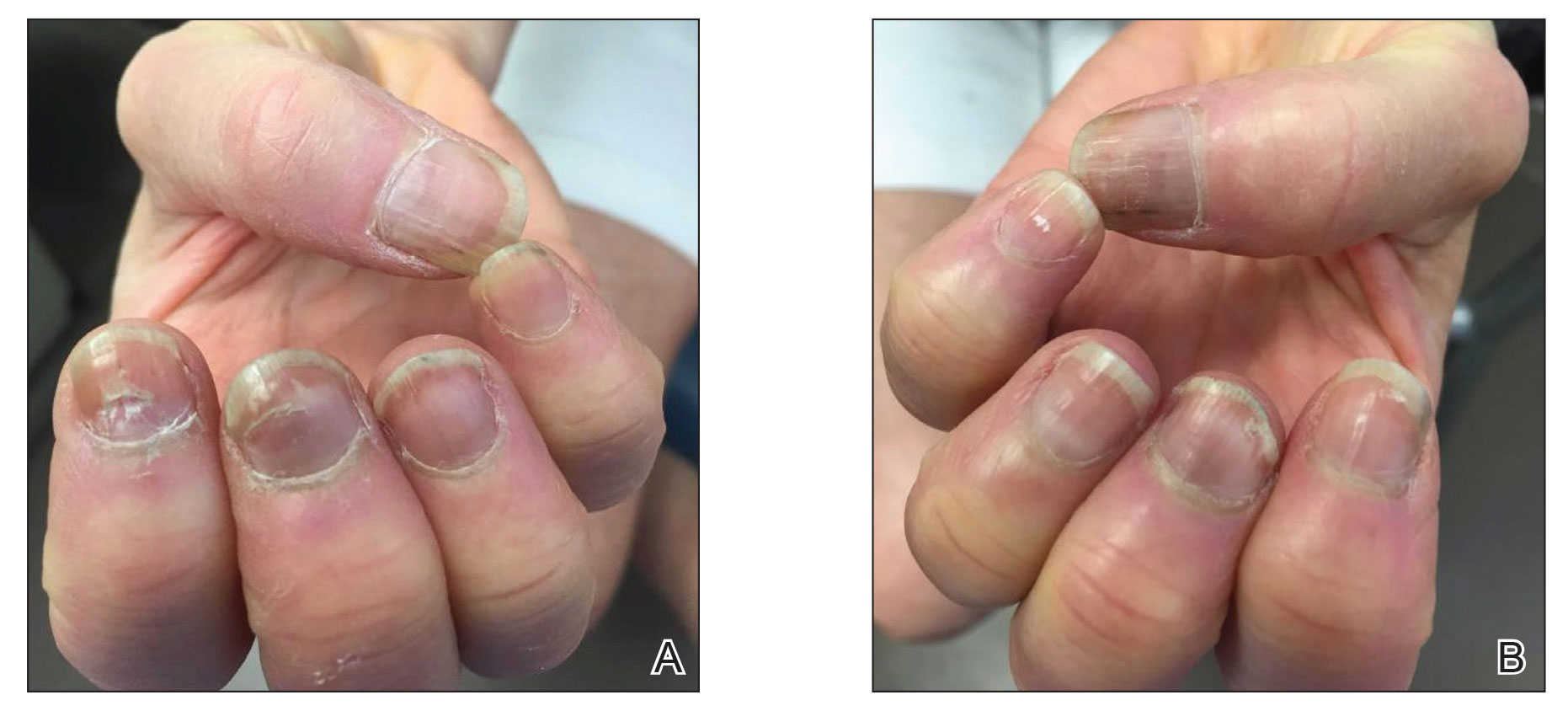
She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.
Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
Practice Points
- Given accelerated global vaccination efforts to control the COVID-19 pandemic, cases of nail changes associated with COVID-19 vaccines are expected.
- Nail abnormalities are a potential general, temporary, and self-limiting adverse effect of COVID-19 vaccines that should not discourage patients from getting vaccinated.
Review cautions against influencer-promoted hair-growth remedies
One day in 2020, Ronda S. Farah, MD, was spending some downtime from her dermatology practice scrolling through social media. When she opened TikTok, she came across something that piqued her interest: A popular content creator was promoting the supplement biotin as a way to grow hair. Dr. Farah was immediately alarmed, because not only was the evidence that biotin increases hair growth shoddy, but the FDA had also warned that biotin supplements may interfere with lab tests for troponin.
Dr. Farah was moved to action and made a brief TikTok stating that use of biotin does not result in hair growth for most patients, which quickly shot up to over half a million views. She was flooded with messages from influencers and people desperate for an answer to their hair growth questions.
From that point on, Dr. Farah was immersed in the world of hairfluencers, the social media personalities who promote hair care trends, which formed the basis of a review, published in the Journal of Cosmetic Dermatology that she conducted with her colleagues at the University of Minnesota, Minneapolis. .
They reviewed five treatments that represent some of the most frequently discussed hair-growth trends on social media: rosemary, onion juice, rice water, castor oil, and aloe vera. For each, they evaluated recommendations on how the treatments were applied, possible harmful effects to the user, claims that weren’t totally based on scientific evidence, and the theoretical mechanism of action. “Overall,” they concluded, “there is little to no literature supporting these social media trends for hair growth.”
Of the five, rosemary, applied to the scalp or hair, has perhaps the most significant research behind it, according to Dr. Farah and coauthors. Methods of applying rosemary described on social media included use of prepackaged oil, boiling fresh rosemary leaves, adding leaves to oils and spraying it on or massaging it on the scalp, applying it in the hair, or using it as a rinse. Dr. Farah noted that the literature supporting the use of rosemary for hair growth does not represent the most robust science; the studies had small sample sizes and used nonstandardized methods of measuring hair growth.
“It didn’t really meet rigorous, strong study methods that a board-certified dermatologist with their expertise would consider a really solid study,” she said.
For the remaining methods, there was little research to support their use for hair growth. A few, the authors pointed out, can cause scalp burns (aloe vera), damage to hair follicles (rice water), contact dermatitis (aloe vera, onion juice), and, in the case of castor oil, hair felting..
Dr. Farah thinks social media can be a great tool to reach patients, but that people should be wary of what kind of information they’re consuming “and need to be aware of who their hairfluencer is,” she said. And, as she and her coauthors wrote: “We call on dermatologists, as hair and scalp disease experts, to serve as authorities on ‘hairfluencer’ trends and appropriately counsel patients.”
The study was independently supported. Dr. Farah reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One day in 2020, Ronda S. Farah, MD, was spending some downtime from her dermatology practice scrolling through social media. When she opened TikTok, she came across something that piqued her interest: A popular content creator was promoting the supplement biotin as a way to grow hair. Dr. Farah was immediately alarmed, because not only was the evidence that biotin increases hair growth shoddy, but the FDA had also warned that biotin supplements may interfere with lab tests for troponin.
Dr. Farah was moved to action and made a brief TikTok stating that use of biotin does not result in hair growth for most patients, which quickly shot up to over half a million views. She was flooded with messages from influencers and people desperate for an answer to their hair growth questions.
From that point on, Dr. Farah was immersed in the world of hairfluencers, the social media personalities who promote hair care trends, which formed the basis of a review, published in the Journal of Cosmetic Dermatology that she conducted with her colleagues at the University of Minnesota, Minneapolis. .
They reviewed five treatments that represent some of the most frequently discussed hair-growth trends on social media: rosemary, onion juice, rice water, castor oil, and aloe vera. For each, they evaluated recommendations on how the treatments were applied, possible harmful effects to the user, claims that weren’t totally based on scientific evidence, and the theoretical mechanism of action. “Overall,” they concluded, “there is little to no literature supporting these social media trends for hair growth.”
Of the five, rosemary, applied to the scalp or hair, has perhaps the most significant research behind it, according to Dr. Farah and coauthors. Methods of applying rosemary described on social media included use of prepackaged oil, boiling fresh rosemary leaves, adding leaves to oils and spraying it on or massaging it on the scalp, applying it in the hair, or using it as a rinse. Dr. Farah noted that the literature supporting the use of rosemary for hair growth does not represent the most robust science; the studies had small sample sizes and used nonstandardized methods of measuring hair growth.
“It didn’t really meet rigorous, strong study methods that a board-certified dermatologist with their expertise would consider a really solid study,” she said.
For the remaining methods, there was little research to support their use for hair growth. A few, the authors pointed out, can cause scalp burns (aloe vera), damage to hair follicles (rice water), contact dermatitis (aloe vera, onion juice), and, in the case of castor oil, hair felting..
Dr. Farah thinks social media can be a great tool to reach patients, but that people should be wary of what kind of information they’re consuming “and need to be aware of who their hairfluencer is,” she said. And, as she and her coauthors wrote: “We call on dermatologists, as hair and scalp disease experts, to serve as authorities on ‘hairfluencer’ trends and appropriately counsel patients.”
The study was independently supported. Dr. Farah reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One day in 2020, Ronda S. Farah, MD, was spending some downtime from her dermatology practice scrolling through social media. When she opened TikTok, she came across something that piqued her interest: A popular content creator was promoting the supplement biotin as a way to grow hair. Dr. Farah was immediately alarmed, because not only was the evidence that biotin increases hair growth shoddy, but the FDA had also warned that biotin supplements may interfere with lab tests for troponin.
Dr. Farah was moved to action and made a brief TikTok stating that use of biotin does not result in hair growth for most patients, which quickly shot up to over half a million views. She was flooded with messages from influencers and people desperate for an answer to their hair growth questions.
From that point on, Dr. Farah was immersed in the world of hairfluencers, the social media personalities who promote hair care trends, which formed the basis of a review, published in the Journal of Cosmetic Dermatology that she conducted with her colleagues at the University of Minnesota, Minneapolis. .
They reviewed five treatments that represent some of the most frequently discussed hair-growth trends on social media: rosemary, onion juice, rice water, castor oil, and aloe vera. For each, they evaluated recommendations on how the treatments were applied, possible harmful effects to the user, claims that weren’t totally based on scientific evidence, and the theoretical mechanism of action. “Overall,” they concluded, “there is little to no literature supporting these social media trends for hair growth.”
Of the five, rosemary, applied to the scalp or hair, has perhaps the most significant research behind it, according to Dr. Farah and coauthors. Methods of applying rosemary described on social media included use of prepackaged oil, boiling fresh rosemary leaves, adding leaves to oils and spraying it on or massaging it on the scalp, applying it in the hair, or using it as a rinse. Dr. Farah noted that the literature supporting the use of rosemary for hair growth does not represent the most robust science; the studies had small sample sizes and used nonstandardized methods of measuring hair growth.
“It didn’t really meet rigorous, strong study methods that a board-certified dermatologist with their expertise would consider a really solid study,” she said.
For the remaining methods, there was little research to support their use for hair growth. A few, the authors pointed out, can cause scalp burns (aloe vera), damage to hair follicles (rice water), contact dermatitis (aloe vera, onion juice), and, in the case of castor oil, hair felting..
Dr. Farah thinks social media can be a great tool to reach patients, but that people should be wary of what kind of information they’re consuming “and need to be aware of who their hairfluencer is,” she said. And, as she and her coauthors wrote: “We call on dermatologists, as hair and scalp disease experts, to serve as authorities on ‘hairfluencer’ trends and appropriately counsel patients.”
The study was independently supported. Dr. Farah reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Sexual dysfunction, hair loss linked with long COVID
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Low-level light therapy cap shows subtle effects on CCCA
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Unique Treatment for Alopecia Areata Combining Epinephrine With an Intralesional Steroid
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.
Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.
Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.
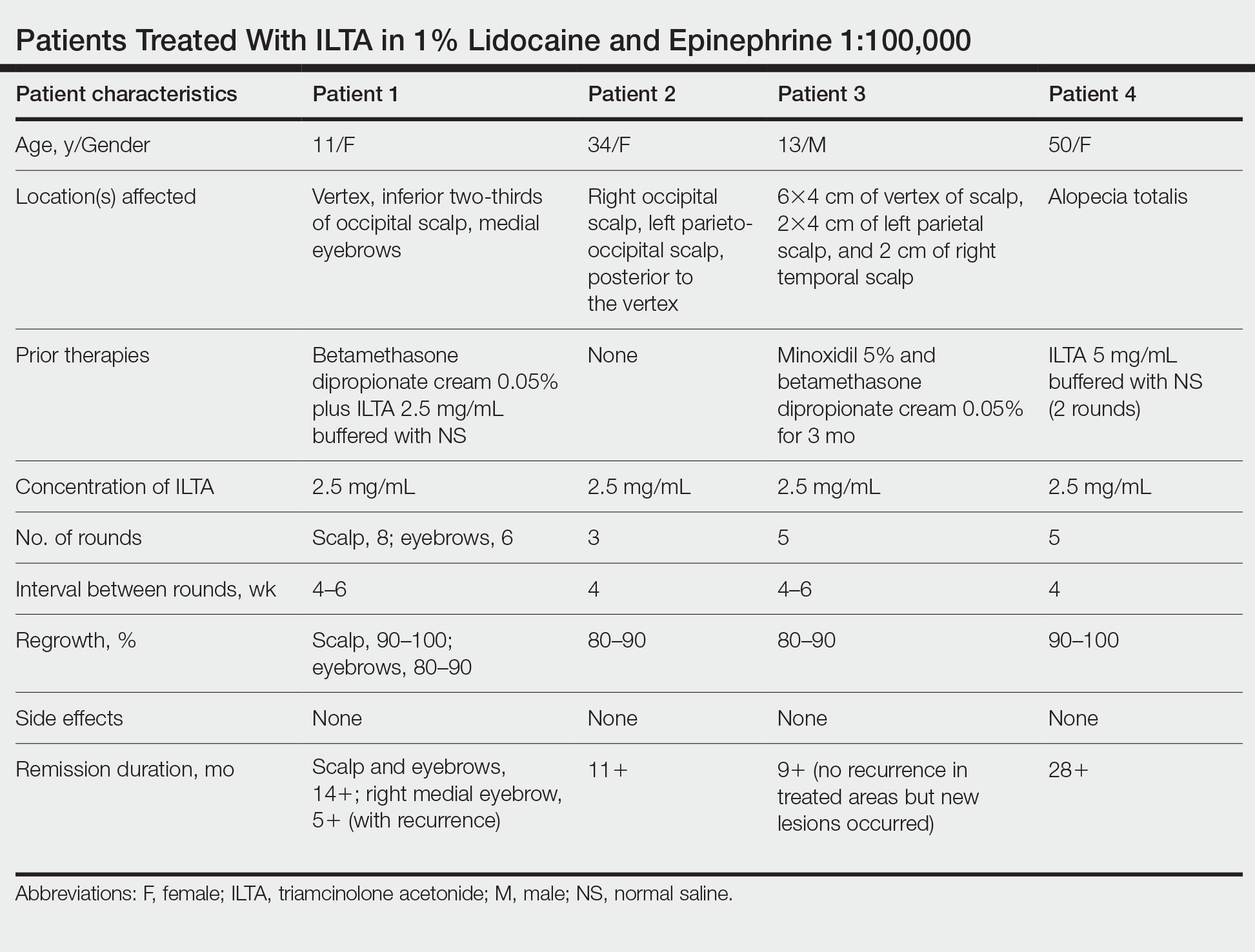
Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Practice Points
- Patients with alopecia areata that is refractory to first-line treatments may benefit from intralesional triamcinolone acetonide (ILTA) diluted to 2.5 mg/mL in 1% lidocaine and epinephrine 1:100,000 in place of normal saline.
- Local vasoconstriction due to epinephrine may potentiate ILTA effects and play an independent role.