User login
What’s the best way to sort out red nail discoloration?
NEW YORK – Nail discoloration, in all its variety, has a wide differential. And while that differential narrows when a patient presents with concerns about nails with red discoloration, there’s still a long list of diagnoses to consider.
During a nail-focused session at the American Academy of Dermatology summer meeting, Shari Lipner, MD, PhD, took attendees through a presentation-based approach that gets to the root etiology of erythronychia and guides diagnosis and treatment options.
“However, regardless of the etiology, erythronychia shares a common pathogenesis,” said Dr. Lipner, a dermatologist at New York–Presbyterian Hospital and Weil Cornell Medicine. The process begins in the distal nail matrix, resulting in a thin long strip of ventral nail becoming discolored, with the nail bed filling in the concavity. The engorged nail bed also makes the affected nail unit prone to splinter hemorrhages, and the thinned, transparent nail makes the erythema more visible, she explained.
Polydactylous longitudinal erythronychia
For erythronychia affecting several nails, onychotillomania is among the possible causes. This condition “often goes hand in hand with onychophagia,” and trichotillomania, skin-picking, or other self-mutilating disorders may also be present, she said. In both the adult and pediatric population, onychotillomania can accompany psychiatric disorders, including depression and obsessive-compulsive disorder, and may be associated with suicidal ideation, she added.
When onychotillomania is the cause, erythronychia may be accompanied by paronychia, and patients will often have a shortened nail bed and an atrophic nail plate. Dorsal pterygium may also be present.
Dermoscopy can provide some clues that onychotillomania is the culprit, said Dr. Lipner, citing a study that looked at dermoscopic images of 36 cases, which found scales in 94%, absence of the nail plate in 83%, and characteristic wavy lines in 69% (J Am Acad Dermatol. 2018 Oct;79[4]:702-5). Other frequent dermoscopy findings included hemorrhages (64%), crusts (61%), nail bed pigmentation (47%), and speckled dots (39%).
Lichen planus can also affect the nails, with erythronychia among its manifestations, she noted. Though lichen planus is thought of as a disease of middle or older age, usually affecting those aged 50-70 years, “15% of those affected are less than 20 years old,” she said.
The erythronychia of lichen planus is often accompanied by longitudinal riding, splitting, and atrophy of the nail plate, she said. Pterygium can also be present, representing a scar in the nail matrix. Dermatopathology will reveal a patchy, bandlike lichenoid infiltrate, with variable sawtooth hypergranulosis and hyperplasia.
“There’s not much evidence about how to treat lichen planus of the nails,” noted Dr. Lipner. Options can include intralesional corticosteroid injections at the nail matrix, topical corticosteroids, oral methotrexate, and retinoids.
Darier disease, an inherited condition caused by mutations in ATP2A2, has both skin and nail manifestations. Characteristic skin signs include hyperkeratotic papules, cobblestone papules, and palmar pits, she said. When nails are affected – as they are in up to 95% of Darier disease patients – they can have a characteristic “candy cane” appearance, with bands of longitudinal erythronychia alternating with normal-colored nail. The nails can also have V-shaped notching, she added.
Patients with systemic lupus erythematosus can also have longitudinal erythronychia; here, dermoscopy will show the characteristic prominent capillary loops in the proximal nail folds, she said.
Foreign substances such as nail polish and dyes, when they’re the source of erythronychia in one or several nails, can usually be wiped off with alcohol or acetone; also, “the proximal margin of discoloration will follow the same pattern as the nail fold,” said Dr. Lipner.
Localized longitudinal erythronychia
When the red discoloration is limited to a single nail, the differential shifts, said Dr. Lipner. With a hematoma, there’s often a history of trauma, and dermoscopy will show characteristic globules and streaks.
The first clue that erythronychia caused by onychopapilloma can be seen at the lunula: There will often be a comet- or pencil-shaped point to the discoloration in that region. But, she said, “the key is to do dermoscopy at the free edge of the nail plate. In 100 percent of cases, there will be a subungual hyperkeratotic papule” that will solidify the diagnosis.
Histopathology of onychopapillomas – a relatively recently recognized entity – will show nail bed and distal matrix acanthosis, with the distal nail bed showing matrix metaplasia, Dr. Lipner said.
Glomus tumors arise from cells of the glomus body, a specialized vascular apparatus that is involved in temperature regulation. “These structures are abundant in the digits and the subungual region,” said Dr. Lipner, which means that glomus tumors – a benign lesion – may develop subungually. Glomus tumors can be idiopathic, but she added, “think neurofibromatosis type 1 if you see multiple glomus tumors.”
Glomus tumors will present with longitudinal erythronychia, with a distal split of the nail sometimes accompanying the discoloration. A round bluish to gray nodule can also be seen. A thorough history and exam can help solidify the diagnosis, said Dr. Lipner; there’s a characteristic triad of point tenderness with the application of pressure to the nail, pain, and cold hypersensitivity.
Classically, the point tenderness is assessed by Love’s test, which elicits severe pain when the subungual tumor is pressed with a small object like the end of paper clip or a ballpoint pen. Application of a tourniquet to the arm after elevation, termed Hildreth’s test, should alleviate subungual glomus tumor pain, and release of the tourniquet causes an abrupt and marked recurrence of pain. Immersing the patient’s affected hand in cold water also increases glomus tumor pain.
“Imaging can be quite helpful” to confirm a glomus tumor diagnosis, said Dr. Lipner. “X-ray is cheap, and you can see erosions in 50% of patients.” However, she added, doppler ultrasound and magnetic resonance imaging are more sensitive, detecting tumors as small as 2 mm.
“Biopsy with histopathology is the gold standard in making the diagnosis” of glomus tumor, though, she noted. Pathologic examination will show monomorphic cells with small caliber vascular channels.
Malignancy is actually uncommon with erythronychia, though it’s always in the differential diagnosis, she said.
In one case series examining subungual squamous cell carcinomas, common findings included onycholysis and localized hyperkeratosis, along with longitudinal erythronychia. “This may be subtle; splinter hemorrhages can also be present,” noted Dr. Lipner, adding, “If there are any symptoms, or an evolving band, a biopsy is indicated.”
“Even with erythronychia, the presentation can be quite variable,” she said. Nail unit melanoma can count erythronychia among the presenting signs. Clues that should raise suspicion for melanoma can include a wide band and prominent onycholysis, especially distal onycholysis and splintering. Again, she said, “biopsy with histopathology is the gold standard in making the diagnosis”; any symptoms or evidence of an evolving band should trigger a biopsy.
Dr. Lipner had no conflicts of interest relevant to her presentation.
SOURCE: Lipner S. Summer AAD 2019, Presentation F035.
NEW YORK – Nail discoloration, in all its variety, has a wide differential. And while that differential narrows when a patient presents with concerns about nails with red discoloration, there’s still a long list of diagnoses to consider.
During a nail-focused session at the American Academy of Dermatology summer meeting, Shari Lipner, MD, PhD, took attendees through a presentation-based approach that gets to the root etiology of erythronychia and guides diagnosis and treatment options.
“However, regardless of the etiology, erythronychia shares a common pathogenesis,” said Dr. Lipner, a dermatologist at New York–Presbyterian Hospital and Weil Cornell Medicine. The process begins in the distal nail matrix, resulting in a thin long strip of ventral nail becoming discolored, with the nail bed filling in the concavity. The engorged nail bed also makes the affected nail unit prone to splinter hemorrhages, and the thinned, transparent nail makes the erythema more visible, she explained.
Polydactylous longitudinal erythronychia
For erythronychia affecting several nails, onychotillomania is among the possible causes. This condition “often goes hand in hand with onychophagia,” and trichotillomania, skin-picking, or other self-mutilating disorders may also be present, she said. In both the adult and pediatric population, onychotillomania can accompany psychiatric disorders, including depression and obsessive-compulsive disorder, and may be associated with suicidal ideation, she added.
When onychotillomania is the cause, erythronychia may be accompanied by paronychia, and patients will often have a shortened nail bed and an atrophic nail plate. Dorsal pterygium may also be present.
Dermoscopy can provide some clues that onychotillomania is the culprit, said Dr. Lipner, citing a study that looked at dermoscopic images of 36 cases, which found scales in 94%, absence of the nail plate in 83%, and characteristic wavy lines in 69% (J Am Acad Dermatol. 2018 Oct;79[4]:702-5). Other frequent dermoscopy findings included hemorrhages (64%), crusts (61%), nail bed pigmentation (47%), and speckled dots (39%).
Lichen planus can also affect the nails, with erythronychia among its manifestations, she noted. Though lichen planus is thought of as a disease of middle or older age, usually affecting those aged 50-70 years, “15% of those affected are less than 20 years old,” she said.
The erythronychia of lichen planus is often accompanied by longitudinal riding, splitting, and atrophy of the nail plate, she said. Pterygium can also be present, representing a scar in the nail matrix. Dermatopathology will reveal a patchy, bandlike lichenoid infiltrate, with variable sawtooth hypergranulosis and hyperplasia.
“There’s not much evidence about how to treat lichen planus of the nails,” noted Dr. Lipner. Options can include intralesional corticosteroid injections at the nail matrix, topical corticosteroids, oral methotrexate, and retinoids.
Darier disease, an inherited condition caused by mutations in ATP2A2, has both skin and nail manifestations. Characteristic skin signs include hyperkeratotic papules, cobblestone papules, and palmar pits, she said. When nails are affected – as they are in up to 95% of Darier disease patients – they can have a characteristic “candy cane” appearance, with bands of longitudinal erythronychia alternating with normal-colored nail. The nails can also have V-shaped notching, she added.
Patients with systemic lupus erythematosus can also have longitudinal erythronychia; here, dermoscopy will show the characteristic prominent capillary loops in the proximal nail folds, she said.
Foreign substances such as nail polish and dyes, when they’re the source of erythronychia in one or several nails, can usually be wiped off with alcohol or acetone; also, “the proximal margin of discoloration will follow the same pattern as the nail fold,” said Dr. Lipner.
Localized longitudinal erythronychia
When the red discoloration is limited to a single nail, the differential shifts, said Dr. Lipner. With a hematoma, there’s often a history of trauma, and dermoscopy will show characteristic globules and streaks.
The first clue that erythronychia caused by onychopapilloma can be seen at the lunula: There will often be a comet- or pencil-shaped point to the discoloration in that region. But, she said, “the key is to do dermoscopy at the free edge of the nail plate. In 100 percent of cases, there will be a subungual hyperkeratotic papule” that will solidify the diagnosis.
Histopathology of onychopapillomas – a relatively recently recognized entity – will show nail bed and distal matrix acanthosis, with the distal nail bed showing matrix metaplasia, Dr. Lipner said.
Glomus tumors arise from cells of the glomus body, a specialized vascular apparatus that is involved in temperature regulation. “These structures are abundant in the digits and the subungual region,” said Dr. Lipner, which means that glomus tumors – a benign lesion – may develop subungually. Glomus tumors can be idiopathic, but she added, “think neurofibromatosis type 1 if you see multiple glomus tumors.”
Glomus tumors will present with longitudinal erythronychia, with a distal split of the nail sometimes accompanying the discoloration. A round bluish to gray nodule can also be seen. A thorough history and exam can help solidify the diagnosis, said Dr. Lipner; there’s a characteristic triad of point tenderness with the application of pressure to the nail, pain, and cold hypersensitivity.
Classically, the point tenderness is assessed by Love’s test, which elicits severe pain when the subungual tumor is pressed with a small object like the end of paper clip or a ballpoint pen. Application of a tourniquet to the arm after elevation, termed Hildreth’s test, should alleviate subungual glomus tumor pain, and release of the tourniquet causes an abrupt and marked recurrence of pain. Immersing the patient’s affected hand in cold water also increases glomus tumor pain.
“Imaging can be quite helpful” to confirm a glomus tumor diagnosis, said Dr. Lipner. “X-ray is cheap, and you can see erosions in 50% of patients.” However, she added, doppler ultrasound and magnetic resonance imaging are more sensitive, detecting tumors as small as 2 mm.
“Biopsy with histopathology is the gold standard in making the diagnosis” of glomus tumor, though, she noted. Pathologic examination will show monomorphic cells with small caliber vascular channels.
Malignancy is actually uncommon with erythronychia, though it’s always in the differential diagnosis, she said.
In one case series examining subungual squamous cell carcinomas, common findings included onycholysis and localized hyperkeratosis, along with longitudinal erythronychia. “This may be subtle; splinter hemorrhages can also be present,” noted Dr. Lipner, adding, “If there are any symptoms, or an evolving band, a biopsy is indicated.”
“Even with erythronychia, the presentation can be quite variable,” she said. Nail unit melanoma can count erythronychia among the presenting signs. Clues that should raise suspicion for melanoma can include a wide band and prominent onycholysis, especially distal onycholysis and splintering. Again, she said, “biopsy with histopathology is the gold standard in making the diagnosis”; any symptoms or evidence of an evolving band should trigger a biopsy.
Dr. Lipner had no conflicts of interest relevant to her presentation.
SOURCE: Lipner S. Summer AAD 2019, Presentation F035.
NEW YORK – Nail discoloration, in all its variety, has a wide differential. And while that differential narrows when a patient presents with concerns about nails with red discoloration, there’s still a long list of diagnoses to consider.
During a nail-focused session at the American Academy of Dermatology summer meeting, Shari Lipner, MD, PhD, took attendees through a presentation-based approach that gets to the root etiology of erythronychia and guides diagnosis and treatment options.
“However, regardless of the etiology, erythronychia shares a common pathogenesis,” said Dr. Lipner, a dermatologist at New York–Presbyterian Hospital and Weil Cornell Medicine. The process begins in the distal nail matrix, resulting in a thin long strip of ventral nail becoming discolored, with the nail bed filling in the concavity. The engorged nail bed also makes the affected nail unit prone to splinter hemorrhages, and the thinned, transparent nail makes the erythema more visible, she explained.
Polydactylous longitudinal erythronychia
For erythronychia affecting several nails, onychotillomania is among the possible causes. This condition “often goes hand in hand with onychophagia,” and trichotillomania, skin-picking, or other self-mutilating disorders may also be present, she said. In both the adult and pediatric population, onychotillomania can accompany psychiatric disorders, including depression and obsessive-compulsive disorder, and may be associated with suicidal ideation, she added.
When onychotillomania is the cause, erythronychia may be accompanied by paronychia, and patients will often have a shortened nail bed and an atrophic nail plate. Dorsal pterygium may also be present.
Dermoscopy can provide some clues that onychotillomania is the culprit, said Dr. Lipner, citing a study that looked at dermoscopic images of 36 cases, which found scales in 94%, absence of the nail plate in 83%, and characteristic wavy lines in 69% (J Am Acad Dermatol. 2018 Oct;79[4]:702-5). Other frequent dermoscopy findings included hemorrhages (64%), crusts (61%), nail bed pigmentation (47%), and speckled dots (39%).
Lichen planus can also affect the nails, with erythronychia among its manifestations, she noted. Though lichen planus is thought of as a disease of middle or older age, usually affecting those aged 50-70 years, “15% of those affected are less than 20 years old,” she said.
The erythronychia of lichen planus is often accompanied by longitudinal riding, splitting, and atrophy of the nail plate, she said. Pterygium can also be present, representing a scar in the nail matrix. Dermatopathology will reveal a patchy, bandlike lichenoid infiltrate, with variable sawtooth hypergranulosis and hyperplasia.
“There’s not much evidence about how to treat lichen planus of the nails,” noted Dr. Lipner. Options can include intralesional corticosteroid injections at the nail matrix, topical corticosteroids, oral methotrexate, and retinoids.
Darier disease, an inherited condition caused by mutations in ATP2A2, has both skin and nail manifestations. Characteristic skin signs include hyperkeratotic papules, cobblestone papules, and palmar pits, she said. When nails are affected – as they are in up to 95% of Darier disease patients – they can have a characteristic “candy cane” appearance, with bands of longitudinal erythronychia alternating with normal-colored nail. The nails can also have V-shaped notching, she added.
Patients with systemic lupus erythematosus can also have longitudinal erythronychia; here, dermoscopy will show the characteristic prominent capillary loops in the proximal nail folds, she said.
Foreign substances such as nail polish and dyes, when they’re the source of erythronychia in one or several nails, can usually be wiped off with alcohol or acetone; also, “the proximal margin of discoloration will follow the same pattern as the nail fold,” said Dr. Lipner.
Localized longitudinal erythronychia
When the red discoloration is limited to a single nail, the differential shifts, said Dr. Lipner. With a hematoma, there’s often a history of trauma, and dermoscopy will show characteristic globules and streaks.
The first clue that erythronychia caused by onychopapilloma can be seen at the lunula: There will often be a comet- or pencil-shaped point to the discoloration in that region. But, she said, “the key is to do dermoscopy at the free edge of the nail plate. In 100 percent of cases, there will be a subungual hyperkeratotic papule” that will solidify the diagnosis.
Histopathology of onychopapillomas – a relatively recently recognized entity – will show nail bed and distal matrix acanthosis, with the distal nail bed showing matrix metaplasia, Dr. Lipner said.
Glomus tumors arise from cells of the glomus body, a specialized vascular apparatus that is involved in temperature regulation. “These structures are abundant in the digits and the subungual region,” said Dr. Lipner, which means that glomus tumors – a benign lesion – may develop subungually. Glomus tumors can be idiopathic, but she added, “think neurofibromatosis type 1 if you see multiple glomus tumors.”
Glomus tumors will present with longitudinal erythronychia, with a distal split of the nail sometimes accompanying the discoloration. A round bluish to gray nodule can also be seen. A thorough history and exam can help solidify the diagnosis, said Dr. Lipner; there’s a characteristic triad of point tenderness with the application of pressure to the nail, pain, and cold hypersensitivity.
Classically, the point tenderness is assessed by Love’s test, which elicits severe pain when the subungual tumor is pressed with a small object like the end of paper clip or a ballpoint pen. Application of a tourniquet to the arm after elevation, termed Hildreth’s test, should alleviate subungual glomus tumor pain, and release of the tourniquet causes an abrupt and marked recurrence of pain. Immersing the patient’s affected hand in cold water also increases glomus tumor pain.
“Imaging can be quite helpful” to confirm a glomus tumor diagnosis, said Dr. Lipner. “X-ray is cheap, and you can see erosions in 50% of patients.” However, she added, doppler ultrasound and magnetic resonance imaging are more sensitive, detecting tumors as small as 2 mm.
“Biopsy with histopathology is the gold standard in making the diagnosis” of glomus tumor, though, she noted. Pathologic examination will show monomorphic cells with small caliber vascular channels.
Malignancy is actually uncommon with erythronychia, though it’s always in the differential diagnosis, she said.
In one case series examining subungual squamous cell carcinomas, common findings included onycholysis and localized hyperkeratosis, along with longitudinal erythronychia. “This may be subtle; splinter hemorrhages can also be present,” noted Dr. Lipner, adding, “If there are any symptoms, or an evolving band, a biopsy is indicated.”
“Even with erythronychia, the presentation can be quite variable,” she said. Nail unit melanoma can count erythronychia among the presenting signs. Clues that should raise suspicion for melanoma can include a wide band and prominent onycholysis, especially distal onycholysis and splintering. Again, she said, “biopsy with histopathology is the gold standard in making the diagnosis”; any symptoms or evidence of an evolving band should trigger a biopsy.
Dr. Lipner had no conflicts of interest relevant to her presentation.
SOURCE: Lipner S. Summer AAD 2019, Presentation F035.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Can sunscreens cause frontal fibrosing alopecia? The jury’s out
NEW YORK – Is there an association between sunscreen use and frontal fibrosing alopecia? Perhaps, but causation is far from well established, according to Henry Lim, MD.
“Frontal fibrosing alopecia is becoming more common – definitely,” said Dr. Lim, speaking in an exclusive interview at the American Academy of Dermatology summer meeting. “Those of us who see patients would see, on average, probably a few patients a week with new-onset frontal fibrosing alopecia.”
Dr. Lim, program director of dermatology research and the photomedicine fellowship at Henry Ford Hospital, Detroit, said in a presentation at the meeting that, in the medical literature, just four surveys have been identified that examined the potential association of UV filters in sunscreen preparations with the risk for frontal fibrosing alopecia (FFA).
The first survey-based study questioned 205 women – 105 of whom had FFA – about sunscreen use, finding increased risk for FFA when sunscreen use was reported at least twice a week (Br J Dermatol. 2016 Oct;175:762-7). The second study, by contrast, looked at men only, examining 17 patients with FFA and 73 control participants. This study found increased frequency of FFA in patients who reported use of sunscreen or sunscreen-containing moisturizers (Br J Dermatol. 2011 Jul;177:260-1).
The final two survey-based studies each also found increased FFA frequency in patients using sunscreens, said Dr. Lim. The first of these was the largest among the FFA studies, surveying 308 patients, 19 of whom were men, with FFA and 347 control participants (Clin Exp Dermatol. 2019 Jun;44:404-10). The other study, involving women only, compared 130 patients with FFA and 130 control participants (Br J Dermatol. 2019 Apr;180:943-4).
Several factors need to be taken into consideration when evaluating the available data, said Dr. Lim. “With any type of retrospective study, there are some limitations, recall bias being one. No. 2, the studies were not designed to look at which sunscreens’ ingredient is causing the frontal fibrosing alopecia, so all we can say is that there is an association of sunscreen with frontal fibrosing alopecia without knowing which active ingredient is involved.”
Also, “there is no conclusion that we can draw in terms of causation,” he said. Data are insufficient for this kind of inference.
Titanium dioxide, a mineral filter used in some sunscreen preparations, has also been implicated in FFA. Elemental titanium has been detected in the hair shafts of some patients with alopecia, said Dr. Lim, noting a case report and another small study. In the smaller study, 20 patients were included. Of them, 16 were FFA-affected women, 4 were unaffected women, and 1 was an unaffected man. Here, however, titanium was found in the hair shaft of all participants save the one unaffected man (Br J Dermatol. 2019 Jul;181:216-7).
However, he warned, “the studies are still early. The numbers are still relatively small that have been examined.” Furthermore, the controls, meaning individuals who did not have FFA in the study, also had titanium element in their hair shaft. All of this means that it’s too early to draw firm conclusions about whether there’s a causal relationship between titanium-containing preparations and FFA.
In questioning after the session, an audience member pointed out that sunscreens are not the sole source of titanium among topical preparations; many cosmetics and hair products also use titanium.
Dr. Lim reported financial relationships with Eli Lilly, Estee Lauder, Ferndale Laboratories, Incyte Corporation, ISDIN, Pierre Fabre Dermatologie, and Unigen.
NEW YORK – Is there an association between sunscreen use and frontal fibrosing alopecia? Perhaps, but causation is far from well established, according to Henry Lim, MD.
“Frontal fibrosing alopecia is becoming more common – definitely,” said Dr. Lim, speaking in an exclusive interview at the American Academy of Dermatology summer meeting. “Those of us who see patients would see, on average, probably a few patients a week with new-onset frontal fibrosing alopecia.”
Dr. Lim, program director of dermatology research and the photomedicine fellowship at Henry Ford Hospital, Detroit, said in a presentation at the meeting that, in the medical literature, just four surveys have been identified that examined the potential association of UV filters in sunscreen preparations with the risk for frontal fibrosing alopecia (FFA).
The first survey-based study questioned 205 women – 105 of whom had FFA – about sunscreen use, finding increased risk for FFA when sunscreen use was reported at least twice a week (Br J Dermatol. 2016 Oct;175:762-7). The second study, by contrast, looked at men only, examining 17 patients with FFA and 73 control participants. This study found increased frequency of FFA in patients who reported use of sunscreen or sunscreen-containing moisturizers (Br J Dermatol. 2011 Jul;177:260-1).
The final two survey-based studies each also found increased FFA frequency in patients using sunscreens, said Dr. Lim. The first of these was the largest among the FFA studies, surveying 308 patients, 19 of whom were men, with FFA and 347 control participants (Clin Exp Dermatol. 2019 Jun;44:404-10). The other study, involving women only, compared 130 patients with FFA and 130 control participants (Br J Dermatol. 2019 Apr;180:943-4).
Several factors need to be taken into consideration when evaluating the available data, said Dr. Lim. “With any type of retrospective study, there are some limitations, recall bias being one. No. 2, the studies were not designed to look at which sunscreens’ ingredient is causing the frontal fibrosing alopecia, so all we can say is that there is an association of sunscreen with frontal fibrosing alopecia without knowing which active ingredient is involved.”
Also, “there is no conclusion that we can draw in terms of causation,” he said. Data are insufficient for this kind of inference.
Titanium dioxide, a mineral filter used in some sunscreen preparations, has also been implicated in FFA. Elemental titanium has been detected in the hair shafts of some patients with alopecia, said Dr. Lim, noting a case report and another small study. In the smaller study, 20 patients were included. Of them, 16 were FFA-affected women, 4 were unaffected women, and 1 was an unaffected man. Here, however, titanium was found in the hair shaft of all participants save the one unaffected man (Br J Dermatol. 2019 Jul;181:216-7).
However, he warned, “the studies are still early. The numbers are still relatively small that have been examined.” Furthermore, the controls, meaning individuals who did not have FFA in the study, also had titanium element in their hair shaft. All of this means that it’s too early to draw firm conclusions about whether there’s a causal relationship between titanium-containing preparations and FFA.
In questioning after the session, an audience member pointed out that sunscreens are not the sole source of titanium among topical preparations; many cosmetics and hair products also use titanium.
Dr. Lim reported financial relationships with Eli Lilly, Estee Lauder, Ferndale Laboratories, Incyte Corporation, ISDIN, Pierre Fabre Dermatologie, and Unigen.
NEW YORK – Is there an association between sunscreen use and frontal fibrosing alopecia? Perhaps, but causation is far from well established, according to Henry Lim, MD.
“Frontal fibrosing alopecia is becoming more common – definitely,” said Dr. Lim, speaking in an exclusive interview at the American Academy of Dermatology summer meeting. “Those of us who see patients would see, on average, probably a few patients a week with new-onset frontal fibrosing alopecia.”
Dr. Lim, program director of dermatology research and the photomedicine fellowship at Henry Ford Hospital, Detroit, said in a presentation at the meeting that, in the medical literature, just four surveys have been identified that examined the potential association of UV filters in sunscreen preparations with the risk for frontal fibrosing alopecia (FFA).
The first survey-based study questioned 205 women – 105 of whom had FFA – about sunscreen use, finding increased risk for FFA when sunscreen use was reported at least twice a week (Br J Dermatol. 2016 Oct;175:762-7). The second study, by contrast, looked at men only, examining 17 patients with FFA and 73 control participants. This study found increased frequency of FFA in patients who reported use of sunscreen or sunscreen-containing moisturizers (Br J Dermatol. 2011 Jul;177:260-1).
The final two survey-based studies each also found increased FFA frequency in patients using sunscreens, said Dr. Lim. The first of these was the largest among the FFA studies, surveying 308 patients, 19 of whom were men, with FFA and 347 control participants (Clin Exp Dermatol. 2019 Jun;44:404-10). The other study, involving women only, compared 130 patients with FFA and 130 control participants (Br J Dermatol. 2019 Apr;180:943-4).
Several factors need to be taken into consideration when evaluating the available data, said Dr. Lim. “With any type of retrospective study, there are some limitations, recall bias being one. No. 2, the studies were not designed to look at which sunscreens’ ingredient is causing the frontal fibrosing alopecia, so all we can say is that there is an association of sunscreen with frontal fibrosing alopecia without knowing which active ingredient is involved.”
Also, “there is no conclusion that we can draw in terms of causation,” he said. Data are insufficient for this kind of inference.
Titanium dioxide, a mineral filter used in some sunscreen preparations, has also been implicated in FFA. Elemental titanium has been detected in the hair shafts of some patients with alopecia, said Dr. Lim, noting a case report and another small study. In the smaller study, 20 patients were included. Of them, 16 were FFA-affected women, 4 were unaffected women, and 1 was an unaffected man. Here, however, titanium was found in the hair shaft of all participants save the one unaffected man (Br J Dermatol. 2019 Jul;181:216-7).
However, he warned, “the studies are still early. The numbers are still relatively small that have been examined.” Furthermore, the controls, meaning individuals who did not have FFA in the study, also had titanium element in their hair shaft. All of this means that it’s too early to draw firm conclusions about whether there’s a causal relationship between titanium-containing preparations and FFA.
In questioning after the session, an audience member pointed out that sunscreens are not the sole source of titanium among topical preparations; many cosmetics and hair products also use titanium.
Dr. Lim reported financial relationships with Eli Lilly, Estee Lauder, Ferndale Laboratories, Incyte Corporation, ISDIN, Pierre Fabre Dermatologie, and Unigen.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Alopecia areata: Study finds racial disparities
, according to a new study involving registry data for more than 11,000 individuals.
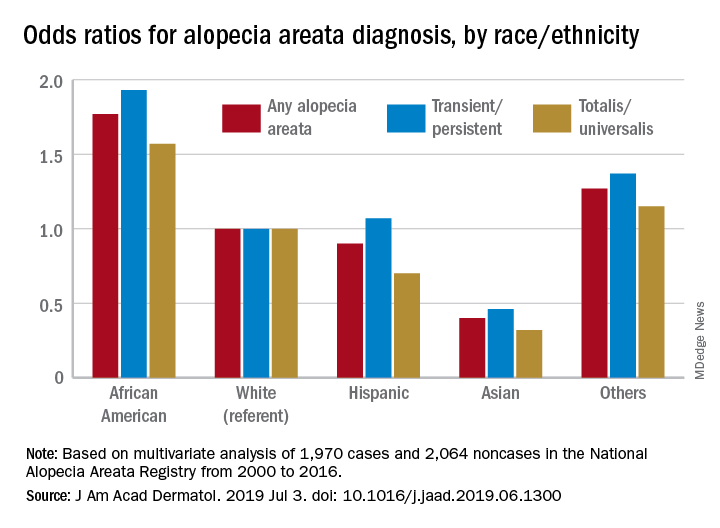
These new findings “raise a different perspective from the conventional view that AA does not differ by race/ethnicity,” said Hemin Lee, MD, MPH, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston, and associates.
Multivariate-adjusted odds ratios for ever-diagnosis of AA were 1.77 for African Americans and 0.4 for Asians when whites were the referent group. Hispanics/Latinos were similar to whites, with an odds ratio of 0.9, and the group of other races/ethnicities (including American Indians and Pacific Islanders) was higher at 1.27, the investigators noted. The report is in the Journal of the American Academy of Dermatology.
The odds played out in a similar fashion when broken down by AA subtype. With whites as the referent at 1.0, blacks were most likely to have been diagnosed with AA transient/persistent at 1.93 and Asians were lowest at 0.46. For AA totalis/universalis, odds ratios were 1.57 for blacks and 0.32 for Asians, they said, based on 2000-2016 data from the National Alopecia Areata Registry.
“An intricate interplay between genetic and environmental factors may account for the racial differences. Pathogenesis of AA is at times linked with autoimmunity by its strong association with HLA class II alleles,” Dr. Lee and associates wrote.
The study involved 11,404 participants from the registry: 9,340 had reported at least one episode of AA and 2,064 had no history of lifetime alopecia. The multivariate analysis was based on the same group of noncases but a subgroup of 1,970 AA patients who had been enrolled in the registry “through academic institutions after dermatologist-confirmed diagnosis,” they said.
There was no funding source to report. One of Dr. Lee’s associates has received honoraria from Abbvie, Amgen, the Centers for Disease Control and Prevention, Janssen, Merck, Novartis, Pfizer, and Amgen, which have been donated to charity, and is an investigator for Sanofi/Regeneron with no financial compensation. All other authors have no conflicts of interest.
SOURCE: Lee H et al. J Am Acad Dermatol. 2019 Jul 3. doi: 10.1016/j.jaad.2019.06.1300.
, according to a new study involving registry data for more than 11,000 individuals.

These new findings “raise a different perspective from the conventional view that AA does not differ by race/ethnicity,” said Hemin Lee, MD, MPH, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston, and associates.
Multivariate-adjusted odds ratios for ever-diagnosis of AA were 1.77 for African Americans and 0.4 for Asians when whites were the referent group. Hispanics/Latinos were similar to whites, with an odds ratio of 0.9, and the group of other races/ethnicities (including American Indians and Pacific Islanders) was higher at 1.27, the investigators noted. The report is in the Journal of the American Academy of Dermatology.
The odds played out in a similar fashion when broken down by AA subtype. With whites as the referent at 1.0, blacks were most likely to have been diagnosed with AA transient/persistent at 1.93 and Asians were lowest at 0.46. For AA totalis/universalis, odds ratios were 1.57 for blacks and 0.32 for Asians, they said, based on 2000-2016 data from the National Alopecia Areata Registry.
“An intricate interplay between genetic and environmental factors may account for the racial differences. Pathogenesis of AA is at times linked with autoimmunity by its strong association with HLA class II alleles,” Dr. Lee and associates wrote.
The study involved 11,404 participants from the registry: 9,340 had reported at least one episode of AA and 2,064 had no history of lifetime alopecia. The multivariate analysis was based on the same group of noncases but a subgroup of 1,970 AA patients who had been enrolled in the registry “through academic institutions after dermatologist-confirmed diagnosis,” they said.
There was no funding source to report. One of Dr. Lee’s associates has received honoraria from Abbvie, Amgen, the Centers for Disease Control and Prevention, Janssen, Merck, Novartis, Pfizer, and Amgen, which have been donated to charity, and is an investigator for Sanofi/Regeneron with no financial compensation. All other authors have no conflicts of interest.
SOURCE: Lee H et al. J Am Acad Dermatol. 2019 Jul 3. doi: 10.1016/j.jaad.2019.06.1300.
, according to a new study involving registry data for more than 11,000 individuals.

These new findings “raise a different perspective from the conventional view that AA does not differ by race/ethnicity,” said Hemin Lee, MD, MPH, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston, and associates.
Multivariate-adjusted odds ratios for ever-diagnosis of AA were 1.77 for African Americans and 0.4 for Asians when whites were the referent group. Hispanics/Latinos were similar to whites, with an odds ratio of 0.9, and the group of other races/ethnicities (including American Indians and Pacific Islanders) was higher at 1.27, the investigators noted. The report is in the Journal of the American Academy of Dermatology.
The odds played out in a similar fashion when broken down by AA subtype. With whites as the referent at 1.0, blacks were most likely to have been diagnosed with AA transient/persistent at 1.93 and Asians were lowest at 0.46. For AA totalis/universalis, odds ratios were 1.57 for blacks and 0.32 for Asians, they said, based on 2000-2016 data from the National Alopecia Areata Registry.
“An intricate interplay between genetic and environmental factors may account for the racial differences. Pathogenesis of AA is at times linked with autoimmunity by its strong association with HLA class II alleles,” Dr. Lee and associates wrote.
The study involved 11,404 participants from the registry: 9,340 had reported at least one episode of AA and 2,064 had no history of lifetime alopecia. The multivariate analysis was based on the same group of noncases but a subgroup of 1,970 AA patients who had been enrolled in the registry “through academic institutions after dermatologist-confirmed diagnosis,” they said.
There was no funding source to report. One of Dr. Lee’s associates has received honoraria from Abbvie, Amgen, the Centers for Disease Control and Prevention, Janssen, Merck, Novartis, Pfizer, and Amgen, which have been donated to charity, and is an investigator for Sanofi/Regeneron with no financial compensation. All other authors have no conflicts of interest.
SOURCE: Lee H et al. J Am Acad Dermatol. 2019 Jul 3. doi: 10.1016/j.jaad.2019.06.1300.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
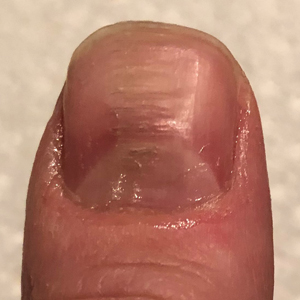
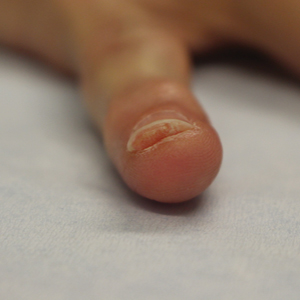
Clinical Pearl: Benzethonium Chloride for Habit-Tic Nail Deformity
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).

Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).

Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).

Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
Nonsurgical Hair Restoration Treatment
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).
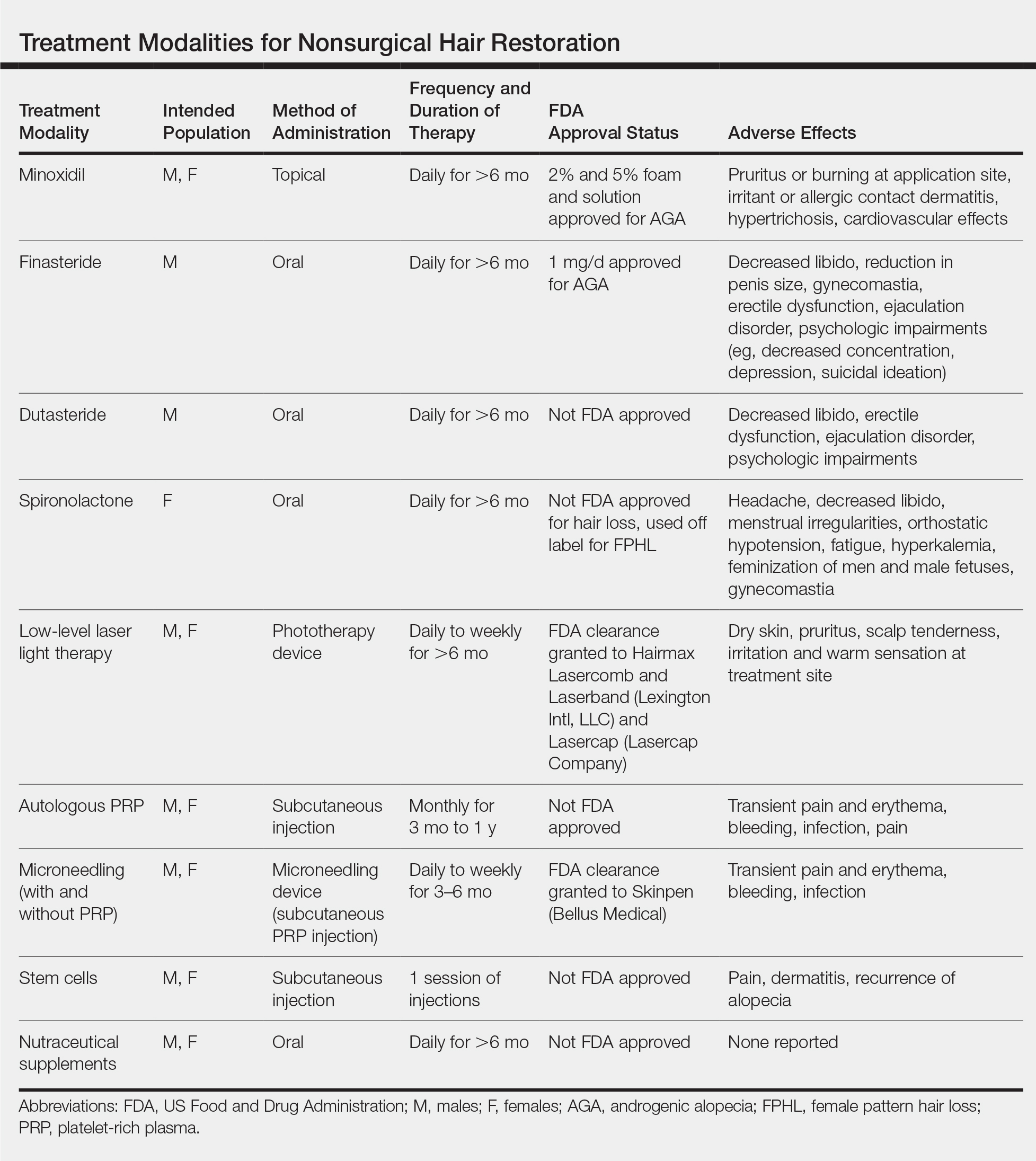
Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Practice Points
- Hair loss is a common phenomenon in both men and women and can seriously impact psychosocial functioning.
- There are numerous US Food and Drug Administration–approved and off-label nonsurgical treatment options for alopecia. Dermatologists should be well versed in these treatment modalities and the associated sideeffect profiles to select the appropriate therapy for each patient.
JAK inhibitors are the ‘near future’ of alopecia areata treatment
MILAN – Janus kinase (JAK) inhibitors have clear science supporting their use in alopecia areata, and an increasing number of positive studies demonstrate their efficacy in regrowing hair, Brett King, MD, PhD, said at the World Congress of Dermatology.
Although not yet specifically approved for , said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn.
“JAK inhibitors are very much within our reach for the treatment of severe alopecia areata,” Dr. King said in an oral presentation on therapeutic advances for alopecia. “We need to follow the science,” he added. “We would not be here telling a story about JAK inhibitors and these other agents without very bright scientists, so we really have to applaud the people who made this the focus of their research.”
JAK science
The science supporting JAK inhibitors can be traced back to a 2014 report by Angela M. Christiano, PhD,, Raphael Clynes, of Columbia University, New York, and others showing that alopecia areata is driven by cytotoxic T lymphocytes, and is reversed by inhibition of the JAK/STAT pathway in mouse models of disease (Nat Med. 2014 Sep;20[9]:1043-9). Those investigators also reported near-complete regrowth of hair in three patients who received oral ruxolitinib, an inhibitor of JAK1 and JAK2, hinting at the potential clinical importance of this targeted approach.
As Dr. King explained, secretion of interleukin (IL)-15 from the hair follicle endothelial cell activates CD8+NKG2D+ T cells leading to secretion of interferon (IFN)-gamma, which has a receptor on the hair follicle epithelial cell, activating that cell to secrete more IL-15.
“IL-15 and IFN-gamma both signal through the JAK/STAT pathway,” he said. “There are over 50 cytokines that signal through the JAK/STAT pathway, including IFN-gamma and IL-15, and on binding their receptor at the cell surface, they pass the baton, if you will, to the JAK enzymes, of which there are 4 members – JAK1, 2, 3 and tyrosine kinase 2. These enzymes subsequently pass the baton to STAT, and STAT translocates to the nucleus, where transcription occurs and disease happens. So we have an opportunity then with a small molecule JAK inhibitor to mediate disease, such that if we give this person a JAK inhibitor, they should regrow hair.”
JAK data
A number of studies of JAK inhibitors support that science, including an open-label study of 66 patients treated with the JAK1/3 inhibitor tofacitinib twice daily (JCI Insight. 2016 Sep 22;1[15]:e89776). About one-third experienced a 50% or greater improvement from baseline, as measured by the severity of alopecia tool (SALT) score over 3 months of treatment, with adverse events limited to grade 1-2 infections, according to the authors, which included Dr. King.
Around the same time, results of an open-label study with ruxolitinib, a JAK1/2 inhibitor, were published showing that 9 of 12 patients had complete or near complete scalp hair regrowth over 6 months of treatment, he said.
In a subsequent retrospective study of 90 patients treated with tofacitinib, about 66%-70% of patients experienced regrowth of hair, depending on the dose received. However, that study also showed that hair regrowth was unlikely in patients with complete or near complete scalp hair loss for 10 years or more, Dr. King said. An additional study showed that tofacitinib may be effective in adolescents as in adults, or even more effective, he added, while another found that low-dose ruxolitinib was as effective as higher dose ruxolitinib for the treatment of severe alopecia areata.
News earlier in 2019 surrounded the results of two randomized, double-blind placebo controlled trials, reported at the annual American Academy of Dermatology meeting in Washington, DC, showing efficacy for investigational oral JAK-targeted agents, a JAK 1/2 inhibitor (CTP-543), and a TYK2/JAK1 inhibitor (PF-06700841) and a JAK3 inhibitor (PF-06651600).
“I think this really is the near future of alopecia areata treatment,” Dr. King said.
No success yet for topical JAKs
One area where JAK inhibitors have not shined yet is in topical formulations. In a pilot study of tofacitinib 2% ointment, only 1 of 10 patients had significant scalp hair growth, while a study of topical ruxolitinib was stopped early and results have not yet been reported, according to Dr. King. “As dermatologists, we’re always interested in topical therapy for skin disease, but I’m not sure that alopecia areata is a disease for which topical JAK inhibitors will be effective,” he said.
Dr. King reported disclosures related to Aclaris Therapeutics, Concert Pharmaceuticals, Dermavant Sciences, Eli Lilly, Pfizer, Regeneron, and Sanofi Genzyme.
MILAN – Janus kinase (JAK) inhibitors have clear science supporting their use in alopecia areata, and an increasing number of positive studies demonstrate their efficacy in regrowing hair, Brett King, MD, PhD, said at the World Congress of Dermatology.
Although not yet specifically approved for , said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn.
“JAK inhibitors are very much within our reach for the treatment of severe alopecia areata,” Dr. King said in an oral presentation on therapeutic advances for alopecia. “We need to follow the science,” he added. “We would not be here telling a story about JAK inhibitors and these other agents without very bright scientists, so we really have to applaud the people who made this the focus of their research.”
JAK science
The science supporting JAK inhibitors can be traced back to a 2014 report by Angela M. Christiano, PhD,, Raphael Clynes, of Columbia University, New York, and others showing that alopecia areata is driven by cytotoxic T lymphocytes, and is reversed by inhibition of the JAK/STAT pathway in mouse models of disease (Nat Med. 2014 Sep;20[9]:1043-9). Those investigators also reported near-complete regrowth of hair in three patients who received oral ruxolitinib, an inhibitor of JAK1 and JAK2, hinting at the potential clinical importance of this targeted approach.
As Dr. King explained, secretion of interleukin (IL)-15 from the hair follicle endothelial cell activates CD8+NKG2D+ T cells leading to secretion of interferon (IFN)-gamma, which has a receptor on the hair follicle epithelial cell, activating that cell to secrete more IL-15.
“IL-15 and IFN-gamma both signal through the JAK/STAT pathway,” he said. “There are over 50 cytokines that signal through the JAK/STAT pathway, including IFN-gamma and IL-15, and on binding their receptor at the cell surface, they pass the baton, if you will, to the JAK enzymes, of which there are 4 members – JAK1, 2, 3 and tyrosine kinase 2. These enzymes subsequently pass the baton to STAT, and STAT translocates to the nucleus, where transcription occurs and disease happens. So we have an opportunity then with a small molecule JAK inhibitor to mediate disease, such that if we give this person a JAK inhibitor, they should regrow hair.”
JAK data
A number of studies of JAK inhibitors support that science, including an open-label study of 66 patients treated with the JAK1/3 inhibitor tofacitinib twice daily (JCI Insight. 2016 Sep 22;1[15]:e89776). About one-third experienced a 50% or greater improvement from baseline, as measured by the severity of alopecia tool (SALT) score over 3 months of treatment, with adverse events limited to grade 1-2 infections, according to the authors, which included Dr. King.
Around the same time, results of an open-label study with ruxolitinib, a JAK1/2 inhibitor, were published showing that 9 of 12 patients had complete or near complete scalp hair regrowth over 6 months of treatment, he said.
In a subsequent retrospective study of 90 patients treated with tofacitinib, about 66%-70% of patients experienced regrowth of hair, depending on the dose received. However, that study also showed that hair regrowth was unlikely in patients with complete or near complete scalp hair loss for 10 years or more, Dr. King said. An additional study showed that tofacitinib may be effective in adolescents as in adults, or even more effective, he added, while another found that low-dose ruxolitinib was as effective as higher dose ruxolitinib for the treatment of severe alopecia areata.
News earlier in 2019 surrounded the results of two randomized, double-blind placebo controlled trials, reported at the annual American Academy of Dermatology meeting in Washington, DC, showing efficacy for investigational oral JAK-targeted agents, a JAK 1/2 inhibitor (CTP-543), and a TYK2/JAK1 inhibitor (PF-06700841) and a JAK3 inhibitor (PF-06651600).
“I think this really is the near future of alopecia areata treatment,” Dr. King said.
No success yet for topical JAKs
One area where JAK inhibitors have not shined yet is in topical formulations. In a pilot study of tofacitinib 2% ointment, only 1 of 10 patients had significant scalp hair growth, while a study of topical ruxolitinib was stopped early and results have not yet been reported, according to Dr. King. “As dermatologists, we’re always interested in topical therapy for skin disease, but I’m not sure that alopecia areata is a disease for which topical JAK inhibitors will be effective,” he said.
Dr. King reported disclosures related to Aclaris Therapeutics, Concert Pharmaceuticals, Dermavant Sciences, Eli Lilly, Pfizer, Regeneron, and Sanofi Genzyme.
MILAN – Janus kinase (JAK) inhibitors have clear science supporting their use in alopecia areata, and an increasing number of positive studies demonstrate their efficacy in regrowing hair, Brett King, MD, PhD, said at the World Congress of Dermatology.
Although not yet specifically approved for , said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn.
“JAK inhibitors are very much within our reach for the treatment of severe alopecia areata,” Dr. King said in an oral presentation on therapeutic advances for alopecia. “We need to follow the science,” he added. “We would not be here telling a story about JAK inhibitors and these other agents without very bright scientists, so we really have to applaud the people who made this the focus of their research.”
JAK science
The science supporting JAK inhibitors can be traced back to a 2014 report by Angela M. Christiano, PhD,, Raphael Clynes, of Columbia University, New York, and others showing that alopecia areata is driven by cytotoxic T lymphocytes, and is reversed by inhibition of the JAK/STAT pathway in mouse models of disease (Nat Med. 2014 Sep;20[9]:1043-9). Those investigators also reported near-complete regrowth of hair in three patients who received oral ruxolitinib, an inhibitor of JAK1 and JAK2, hinting at the potential clinical importance of this targeted approach.
As Dr. King explained, secretion of interleukin (IL)-15 from the hair follicle endothelial cell activates CD8+NKG2D+ T cells leading to secretion of interferon (IFN)-gamma, which has a receptor on the hair follicle epithelial cell, activating that cell to secrete more IL-15.
“IL-15 and IFN-gamma both signal through the JAK/STAT pathway,” he said. “There are over 50 cytokines that signal through the JAK/STAT pathway, including IFN-gamma and IL-15, and on binding their receptor at the cell surface, they pass the baton, if you will, to the JAK enzymes, of which there are 4 members – JAK1, 2, 3 and tyrosine kinase 2. These enzymes subsequently pass the baton to STAT, and STAT translocates to the nucleus, where transcription occurs and disease happens. So we have an opportunity then with a small molecule JAK inhibitor to mediate disease, such that if we give this person a JAK inhibitor, they should regrow hair.”
JAK data
A number of studies of JAK inhibitors support that science, including an open-label study of 66 patients treated with the JAK1/3 inhibitor tofacitinib twice daily (JCI Insight. 2016 Sep 22;1[15]:e89776). About one-third experienced a 50% or greater improvement from baseline, as measured by the severity of alopecia tool (SALT) score over 3 months of treatment, with adverse events limited to grade 1-2 infections, according to the authors, which included Dr. King.
Around the same time, results of an open-label study with ruxolitinib, a JAK1/2 inhibitor, were published showing that 9 of 12 patients had complete or near complete scalp hair regrowth over 6 months of treatment, he said.
In a subsequent retrospective study of 90 patients treated with tofacitinib, about 66%-70% of patients experienced regrowth of hair, depending on the dose received. However, that study also showed that hair regrowth was unlikely in patients with complete or near complete scalp hair loss for 10 years or more, Dr. King said. An additional study showed that tofacitinib may be effective in adolescents as in adults, or even more effective, he added, while another found that low-dose ruxolitinib was as effective as higher dose ruxolitinib for the treatment of severe alopecia areata.
News earlier in 2019 surrounded the results of two randomized, double-blind placebo controlled trials, reported at the annual American Academy of Dermatology meeting in Washington, DC, showing efficacy for investigational oral JAK-targeted agents, a JAK 1/2 inhibitor (CTP-543), and a TYK2/JAK1 inhibitor (PF-06700841) and a JAK3 inhibitor (PF-06651600).
“I think this really is the near future of alopecia areata treatment,” Dr. King said.
No success yet for topical JAKs
One area where JAK inhibitors have not shined yet is in topical formulations. In a pilot study of tofacitinib 2% ointment, only 1 of 10 patients had significant scalp hair growth, while a study of topical ruxolitinib was stopped early and results have not yet been reported, according to Dr. King. “As dermatologists, we’re always interested in topical therapy for skin disease, but I’m not sure that alopecia areata is a disease for which topical JAK inhibitors will be effective,” he said.
Dr. King reported disclosures related to Aclaris Therapeutics, Concert Pharmaceuticals, Dermavant Sciences, Eli Lilly, Pfizer, Regeneron, and Sanofi Genzyme.
EXPERT ANALYSIS FROM WCD2019
Are nutritional supplements important in the treatment of female pattern hair loss?
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Acquired Digital Fibrokeratoma Presenting as a Painless Nodule on the Right Fifth Fingernail
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
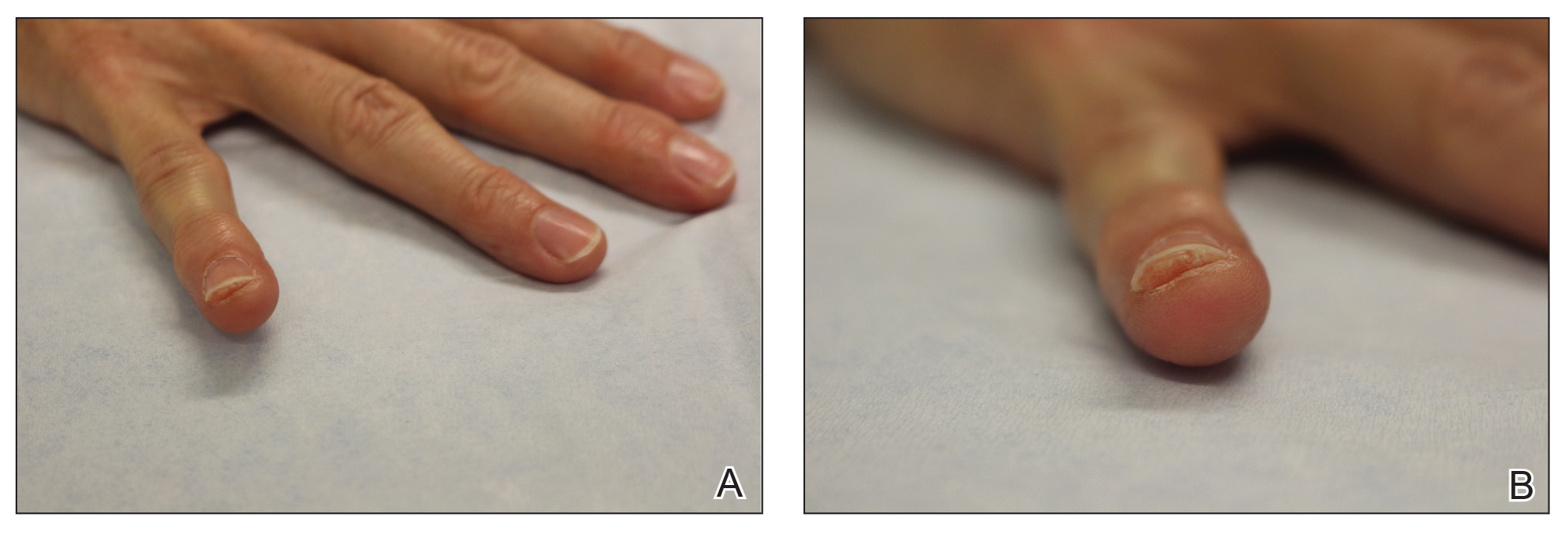
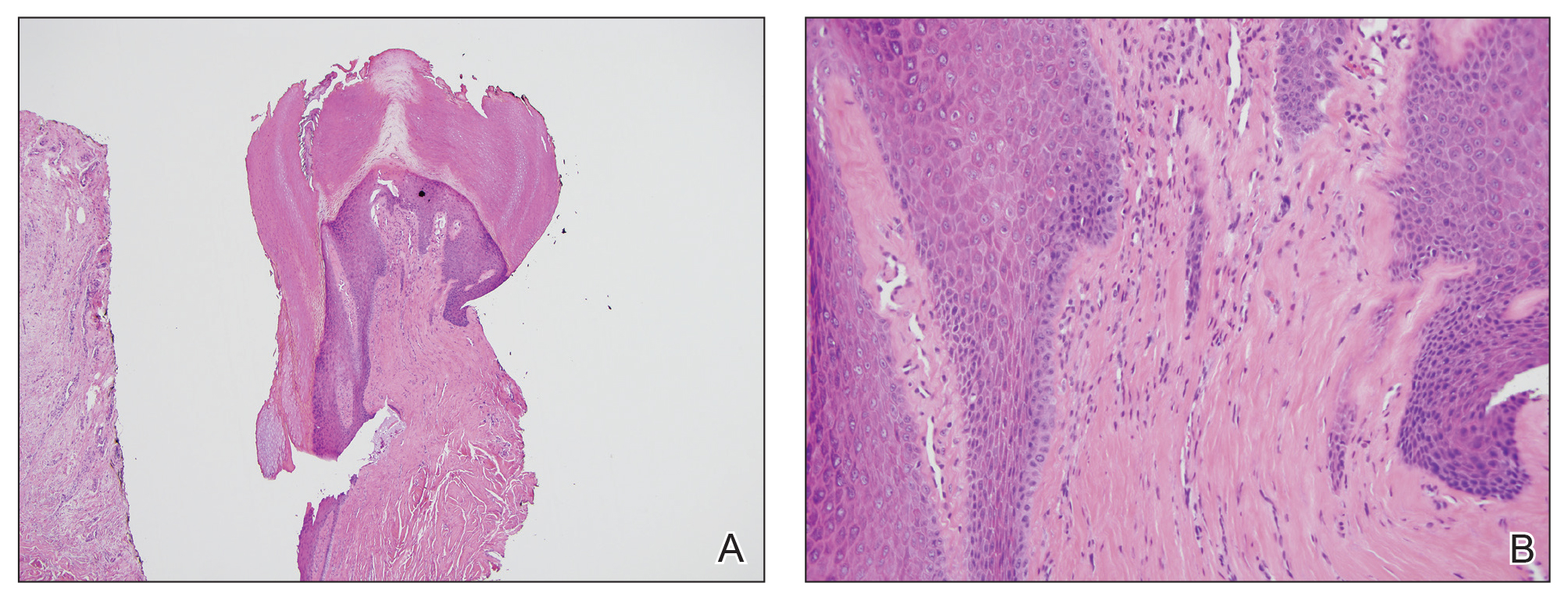
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.


Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.


Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
Practice Points
- Acquired digital fibrokeratoma is a benign tumor of the nail bed and periungual area.
- Histopathology shows epidermal acanthosis and hyperkeratosis, and collagen bundles are arranged in a vertical orientation to the long axis of the epidermis.
- Acquired digital fibrokeratoma should be considered in the differential diagnosis of flesh-colored papules on the nail unit associated with longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.
Treatment Consideration for US Military Members With Skin Disease
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4
Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4

Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4

Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
Practice Points
- Certain conditions and treatments are incompatible with military service and may result in separation.
- Dermatologists must consider a patient’s profession when choosing a treatment modality.
Trends in Nail Services May Cause Dermatitis: Not Your Mother’s Nail Polish
In 2017, consumers spent an average of $8.53 billion on nail services.1 This booming industry is set to grow to more than $15.5 billion by 2024.2 Nail polishes and other nail cosmetic trends can present new exposures for consumers, including chemicals that can elicit allergic contact dermatitis. In this article, we discuss new nail trends and their associated allergens, the acrylates.
Tosylamide/Formaldehyde Resin
Traditionally, the most widely recognized nail polish allergen has been tosylamide/formaldehyde resin (TSFR). However, there now are many touted TSFR-free nail polishes on the market, and the rate of positive reactions to this chemical has been declining in recent years. The North American Contact Dermatitis Group reported a positive reaction rate of 1.3% from 2005 through 2006,3 and rates decreased to 0.9% from 2015 through 2016.4 An Australian study demonstrated a similar reduction in positive reaction rates to nail polish chemicals, with only 0.7% of patients reacting to TSFR from 2014 to 2016 and 0% in 2017. It is theorized that this reduction occurred from replacing TSFR in traditional nail polishes with other chemicals such as polyester resins and cellulose acetate butyrate.5
Acrylate-Based Nail Treatments
Consumers recently have been gravitating toward acrylate-based nail treatments vs traditional nail polishes for a variety of reasons. Often referred to as gels, dips, or shellac, acrylate-based nail treatments represent a hot new trend in nail cosmetics. These manicures are resistant to chipping and scratches, creating a like-new look that lasts for weeks after application. The long-lasting nature of acrylate-based nail polishes has made them wildly popular with consumers.
Traditional acrylic nails consist of a powder polymer mixed with a liquid monomer, which polymerizes when a catalyst is added.6 The procedure is time consuming and can take up to 2 hours for application. In contrast, the newer gel manicure can be completed faster and includes application of acrylate-based nail polish, including a base coat, 2 coats of color, and a top coat. Exposure to either a light-emitting diode (30–60 seconds) or UVA (2 minutes) lamp is necessary after each coat is applied for polymerization (Figure 1).6 This long-lasting, semipermanent manicure typically is what patients are referring to when they say they have “gel nails.”
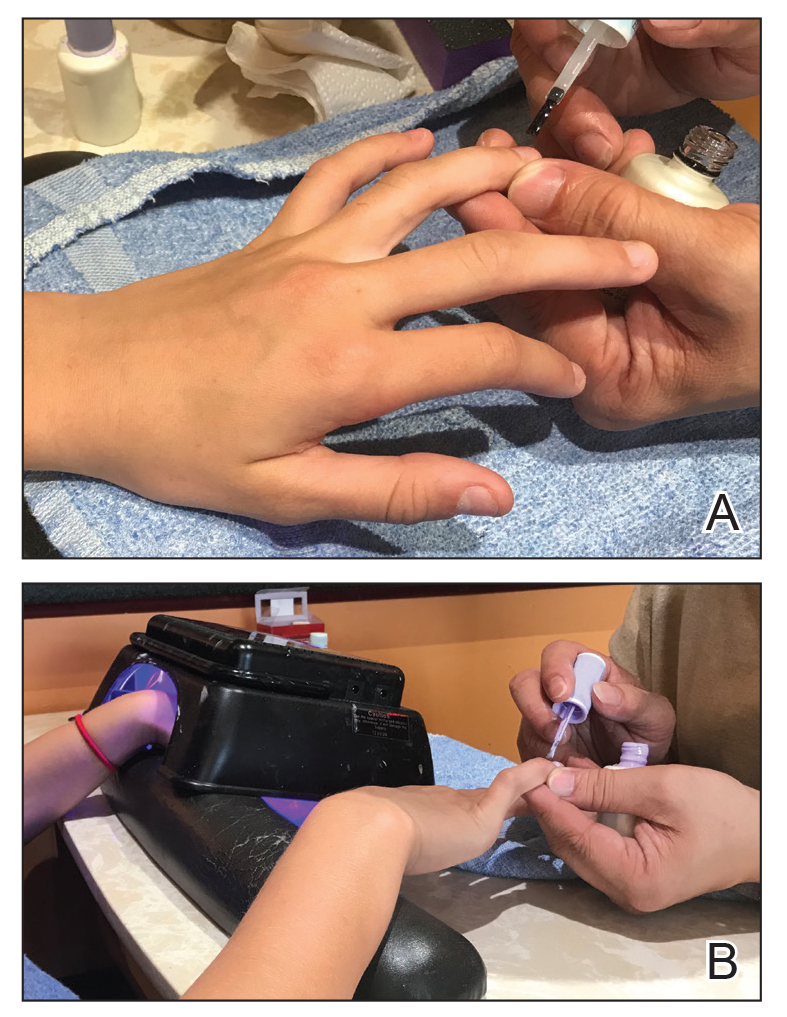
Gel dipping powders (referred to as dips) are another long-lasting acrylate-based nail treatment. This type of polish uses ethyl cyanoacrylate, a slightly different acrylate (yes, that IS super glue). After the nail is prepared, a base polish is applied to three-quarters of the nail and it is dipped into a natural color dip powder. The base polish is then applied to the entire nail, followed by a dip into the polish color of choice. This process is completed twice, followed by shaping and application of a top coat (Figure 2).
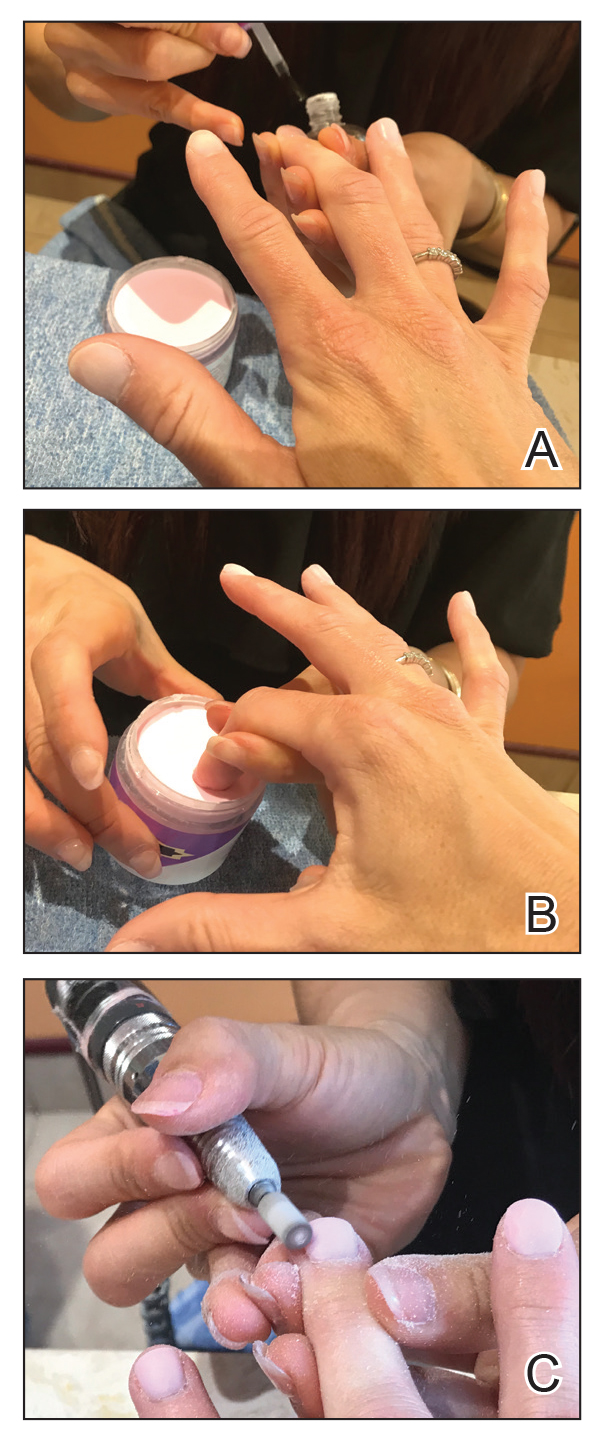
base coat. B, Application of dip powder to gel polish. Note the entire
distal finger and nail are dipped into the powder. C, Shaping of the
nail after the second coat of color is applied.
Finally, there are nail wraps, which are similar to stickers placed over or extending the nail plate. The wraps can be made from linen, silk, vinyl, or other material. Ethyl cyanoacrylate and isopropyl-2-cyanoacrylates have been identified in nail wrap adhesive.7 The heated product is directly applied to the prepared nail, and the excess wrap is filed off. Additional nail polish and a top coat usually are applied to finish the nail. Many of these products are available for in-salon use as well as online purchase and home application by consumers.
Acrylate Allergy
Patients who are allergic to acrylates can present with different patterns of dermatitis. Although the majority of patients present with dermatitis on the hands, fingers, or wrists, up to 10% may only have facial and neck dermatitis.8 Less commonly, the abdomen and thighs can be involved.6,8 Nail technicians most commonly present with pulpitis with cutaneous fissures.8 Other symptoms can include subungual hyperkeratosis, onycholysis, and nail dystrophy. Paresthesia, urticaria, and upper respiratory tract symptoms can occur but are less common.6,8
Acrylate allergy typically is the result of sensitization to the acrylate monomers. In theory, gel nail acrylate materials are polymerized following exposure to a light-emitting diode or UVA lamp; however, there likely is some incomplete polymerization, which can increase the risk for development of allergy. Allergen exposure can occur due to incorrect application of the light source; inadvertent monomer exposure, which occurs when nail technicians wipe extra acrylate off of a client’s finger(s); or inadvertent application of acrylate monomers to objects in the nail technician’s work environment.6,8
Several acrylate nail allergens have been reported. Many studies have identified 2-hydroxyethyl methacrylate (HEMA) as the most common nail acrylate allergen.8,9 At least one study identified 2-hydroxypropyl methacrylate as the most common, with HEMA in second place.6 Other reported acrylate allergens have included ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, methyl methacrylate, ethyl cyanoacrylate, 1,4-butanediol diacrylate, hydroxypropyl acrylate, and 2-hydroxyethyl acrylate.8,9
The American Contact Dermatitis Society Core Allergen Series and the North American Contact Dermatitis Group screening series currently include HEMA, methyl methacrylate, ethyl acrylate, ethyl cyanoacrylate, and TSFR.4,10 Of note, acrylates are not included in the thin-layer rapid use epicutaneous (T.R.U.E.) patch test (SmartPractice), so they will be missed if this series is used.11 In the setting of suspected nail acrylate allergy, some authors recommend initial screening with HEMA and ethyl cyanoacrylate, with extended acrylate testing if both are negative.8
Upon patch testing with an acrylate series, patients frequently react to 2 or more acrylates and the reactions can be strong (++) or extreme (+++), which may represent cosensitization or cross-sensitization.8 The likelihood of cross-reactivity between acrylates is not clear, though it has been postulated that it is theoretically possible.6
An important pearl for patch testers using the chamber method is proper storage of acrylate allergens and assembly of trays prior to patch testing. Similar to all haptens, manufacturers recommend that acrylates should be stored in a refrigerator, but some authors suggest that acrylates should be stored in the freezer.12 Acrylates are volatile chemicals and rapidly degrade when exposed to air. A methyl methacrylate preparation loaded into an inert quadrate (IQ) chamber and stored at room temperature showed a nearly undetectable amount of any residual methyl methacrylate 24 hours later. Refrigeration of allergens in chambers slowed but did not stop eventual degradation, with nearly all acrylate preparations reaching an undetectable level of allergen by day 8.13 Acrylates, along with other volatile allergens, should only be loaded into chambers immediately prior to placement on the patient.
Allergy Prevention
Prevention of nail acrylate allergy among consumers is simple: avoid contact with the offending allergen. Acrylate spillover (ie, applying the acrylate onto the skin) and direct contact with objects and working surfaces contaminated with acrylate-based nail products should be avoided.8 Avoidance is more complicated for nail technicians, but it is thought that nitrile gloves allow for the best dexterity and allergen avoidance when acrylate exposure is brief.14 Allowable exposure times with nitrile gloves may be 15 to 30 minutes. After this times passes, a glove change is required to avoid exposure.14 Wearing nitrile gloves for longer than 15 to 30 minutes will result in cutaneous exposure and risk for dermatitis in sensitized patients. If longer wear is desired, one option includes cutting the fingertips off of Silver Shield/4H gloves (Honeywell Safety Products USA, Inc), applying them to the distal fingers, and wearing a standard nitrile glove over top, known as the finger stall technique.6 In one study, this technique was recommended to nail technicians with acrylate allergy. A telephone survey conducted 4 to 43 months later confirmed that 36% (8/22) of participants were using the technique without symptoms. In this same study, 73% (16/22) had continued working as nail technicians.6
Acrylates are used for other medical purposes, including dental procedures, orthopedic procedures, surgical glues, wound dressings, and contact and intraocular lenses. They also have additional cosmetic applications, including eyelash and hair extensions.8 Therefore, it is vital that patients disclose any history of acrylate allergy to both their medical and cosmetic providers.
Our Final Interpretation
Acrylate allergy has become increasingly common, and long-lasting nail treatments often are the culprit. Whether through gels, dips, or shellac, repeated exposure to acrylates through nail treatments can increase the risk for allergy. The T.R.U.E. test alone will not make the diagnosis, as acrylates are not present in this patch test system. It is important to remind your allergic patients that acrylates are present in other compounds used for medical and cosmetic purposes. Avoidance is key, and for allergic patients who love to bedazzle their nails, we suggest less-permanent, acrylate-free nail polishes as alternatives.
- 2017-2018 industry statistics highlights. Nails Magazine. http://files.nailsmag.com/handouts/nabb2017-18stats-lr.pdf. Accessed May 17, 2019.
- Nail polish market size worth $15.55 billion by 2024. Grand View Research website. https://www.grandviewresearch.com/press-release/global-nail-polish-market. Published October 2017. Accessed May 17, 2019.
- Zug KA, Warshaw EM, Fowler JF, et al. Patch-test results of the North American Contact Dermatitis Group 2005-2006. Dermatitis. 2009;20:149-160.
- DeKoven J, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Lee S, Maor D, Palmer A, et al. Declining prevalence of allergic contact dermatitis caused by tosylamide/formaldehyde in nail polish. Contact Dermatitis. 2018;79:184-185.
- Gatica-Ortega ME, Pastor-Nieto MA, Mercader-García P, et al. Allergic contact dermatitis caused by (meth)acrylates in long-lasting nail polish: are we facing a new epidemic in the beauty industry? Contact Dermatitis. 2017;7:360-366.
- Fitzgerald DA, Bhaggoe R, English JS. Contact sensitivity to cyanoacrylate nail-adhesive with dermatitis at remote sites. Contact Dermatitis. 1995;32:175-176.
- Goncalo M, Pinho A, Agner T et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2017;78:254-260.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study [published online January 14, 2019]. Contact Dermatitis. doi:10.1111/cod.13216.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2017 update. Dermatitis. 2017;28:141-143.
- T.R.U.E. TEST ready-to-use patch test panels. Smart Practice website. https://www.smartpractice.com/shop/wa/category?cn=T.R.U.E.-TEST%C2%AE-Ready-to-Use-Patch-Test-Panels&id=508222&m=SPA. Accessed May 17, 2019.
- Good AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methylacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Goon A, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Morgado F, Batista M, Gonçalo M. Short exposures and glove protection against (meth)acrylates in nail beauticians—thoughts on a rising concern [published online January 17, 2019]. Contact Dermatitis. doi:10.1111/cod.13222.
In 2017, consumers spent an average of $8.53 billion on nail services.1 This booming industry is set to grow to more than $15.5 billion by 2024.2 Nail polishes and other nail cosmetic trends can present new exposures for consumers, including chemicals that can elicit allergic contact dermatitis. In this article, we discuss new nail trends and their associated allergens, the acrylates.
Tosylamide/Formaldehyde Resin
Traditionally, the most widely recognized nail polish allergen has been tosylamide/formaldehyde resin (TSFR). However, there now are many touted TSFR-free nail polishes on the market, and the rate of positive reactions to this chemical has been declining in recent years. The North American Contact Dermatitis Group reported a positive reaction rate of 1.3% from 2005 through 2006,3 and rates decreased to 0.9% from 2015 through 2016.4 An Australian study demonstrated a similar reduction in positive reaction rates to nail polish chemicals, with only 0.7% of patients reacting to TSFR from 2014 to 2016 and 0% in 2017. It is theorized that this reduction occurred from replacing TSFR in traditional nail polishes with other chemicals such as polyester resins and cellulose acetate butyrate.5
Acrylate-Based Nail Treatments
Consumers recently have been gravitating toward acrylate-based nail treatments vs traditional nail polishes for a variety of reasons. Often referred to as gels, dips, or shellac, acrylate-based nail treatments represent a hot new trend in nail cosmetics. These manicures are resistant to chipping and scratches, creating a like-new look that lasts for weeks after application. The long-lasting nature of acrylate-based nail polishes has made them wildly popular with consumers.
Traditional acrylic nails consist of a powder polymer mixed with a liquid monomer, which polymerizes when a catalyst is added.6 The procedure is time consuming and can take up to 2 hours for application. In contrast, the newer gel manicure can be completed faster and includes application of acrylate-based nail polish, including a base coat, 2 coats of color, and a top coat. Exposure to either a light-emitting diode (30–60 seconds) or UVA (2 minutes) lamp is necessary after each coat is applied for polymerization (Figure 1).6 This long-lasting, semipermanent manicure typically is what patients are referring to when they say they have “gel nails.”

Gel dipping powders (referred to as dips) are another long-lasting acrylate-based nail treatment. This type of polish uses ethyl cyanoacrylate, a slightly different acrylate (yes, that IS super glue). After the nail is prepared, a base polish is applied to three-quarters of the nail and it is dipped into a natural color dip powder. The base polish is then applied to the entire nail, followed by a dip into the polish color of choice. This process is completed twice, followed by shaping and application of a top coat (Figure 2).

base coat. B, Application of dip powder to gel polish. Note the entire
distal finger and nail are dipped into the powder. C, Shaping of the
nail after the second coat of color is applied.
Finally, there are nail wraps, which are similar to stickers placed over or extending the nail plate. The wraps can be made from linen, silk, vinyl, or other material. Ethyl cyanoacrylate and isopropyl-2-cyanoacrylates have been identified in nail wrap adhesive.7 The heated product is directly applied to the prepared nail, and the excess wrap is filed off. Additional nail polish and a top coat usually are applied to finish the nail. Many of these products are available for in-salon use as well as online purchase and home application by consumers.
Acrylate Allergy
Patients who are allergic to acrylates can present with different patterns of dermatitis. Although the majority of patients present with dermatitis on the hands, fingers, or wrists, up to 10% may only have facial and neck dermatitis.8 Less commonly, the abdomen and thighs can be involved.6,8 Nail technicians most commonly present with pulpitis with cutaneous fissures.8 Other symptoms can include subungual hyperkeratosis, onycholysis, and nail dystrophy. Paresthesia, urticaria, and upper respiratory tract symptoms can occur but are less common.6,8
Acrylate allergy typically is the result of sensitization to the acrylate monomers. In theory, gel nail acrylate materials are polymerized following exposure to a light-emitting diode or UVA lamp; however, there likely is some incomplete polymerization, which can increase the risk for development of allergy. Allergen exposure can occur due to incorrect application of the light source; inadvertent monomer exposure, which occurs when nail technicians wipe extra acrylate off of a client’s finger(s); or inadvertent application of acrylate monomers to objects in the nail technician’s work environment.6,8
Several acrylate nail allergens have been reported. Many studies have identified 2-hydroxyethyl methacrylate (HEMA) as the most common nail acrylate allergen.8,9 At least one study identified 2-hydroxypropyl methacrylate as the most common, with HEMA in second place.6 Other reported acrylate allergens have included ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, methyl methacrylate, ethyl cyanoacrylate, 1,4-butanediol diacrylate, hydroxypropyl acrylate, and 2-hydroxyethyl acrylate.8,9
The American Contact Dermatitis Society Core Allergen Series and the North American Contact Dermatitis Group screening series currently include HEMA, methyl methacrylate, ethyl acrylate, ethyl cyanoacrylate, and TSFR.4,10 Of note, acrylates are not included in the thin-layer rapid use epicutaneous (T.R.U.E.) patch test (SmartPractice), so they will be missed if this series is used.11 In the setting of suspected nail acrylate allergy, some authors recommend initial screening with HEMA and ethyl cyanoacrylate, with extended acrylate testing if both are negative.8
Upon patch testing with an acrylate series, patients frequently react to 2 or more acrylates and the reactions can be strong (++) or extreme (+++), which may represent cosensitization or cross-sensitization.8 The likelihood of cross-reactivity between acrylates is not clear, though it has been postulated that it is theoretically possible.6
An important pearl for patch testers using the chamber method is proper storage of acrylate allergens and assembly of trays prior to patch testing. Similar to all haptens, manufacturers recommend that acrylates should be stored in a refrigerator, but some authors suggest that acrylates should be stored in the freezer.12 Acrylates are volatile chemicals and rapidly degrade when exposed to air. A methyl methacrylate preparation loaded into an inert quadrate (IQ) chamber and stored at room temperature showed a nearly undetectable amount of any residual methyl methacrylate 24 hours later. Refrigeration of allergens in chambers slowed but did not stop eventual degradation, with nearly all acrylate preparations reaching an undetectable level of allergen by day 8.13 Acrylates, along with other volatile allergens, should only be loaded into chambers immediately prior to placement on the patient.
Allergy Prevention
Prevention of nail acrylate allergy among consumers is simple: avoid contact with the offending allergen. Acrylate spillover (ie, applying the acrylate onto the skin) and direct contact with objects and working surfaces contaminated with acrylate-based nail products should be avoided.8 Avoidance is more complicated for nail technicians, but it is thought that nitrile gloves allow for the best dexterity and allergen avoidance when acrylate exposure is brief.14 Allowable exposure times with nitrile gloves may be 15 to 30 minutes. After this times passes, a glove change is required to avoid exposure.14 Wearing nitrile gloves for longer than 15 to 30 minutes will result in cutaneous exposure and risk for dermatitis in sensitized patients. If longer wear is desired, one option includes cutting the fingertips off of Silver Shield/4H gloves (Honeywell Safety Products USA, Inc), applying them to the distal fingers, and wearing a standard nitrile glove over top, known as the finger stall technique.6 In one study, this technique was recommended to nail technicians with acrylate allergy. A telephone survey conducted 4 to 43 months later confirmed that 36% (8/22) of participants were using the technique without symptoms. In this same study, 73% (16/22) had continued working as nail technicians.6
Acrylates are used for other medical purposes, including dental procedures, orthopedic procedures, surgical glues, wound dressings, and contact and intraocular lenses. They also have additional cosmetic applications, including eyelash and hair extensions.8 Therefore, it is vital that patients disclose any history of acrylate allergy to both their medical and cosmetic providers.
Our Final Interpretation
Acrylate allergy has become increasingly common, and long-lasting nail treatments often are the culprit. Whether through gels, dips, or shellac, repeated exposure to acrylates through nail treatments can increase the risk for allergy. The T.R.U.E. test alone will not make the diagnosis, as acrylates are not present in this patch test system. It is important to remind your allergic patients that acrylates are present in other compounds used for medical and cosmetic purposes. Avoidance is key, and for allergic patients who love to bedazzle their nails, we suggest less-permanent, acrylate-free nail polishes as alternatives.
In 2017, consumers spent an average of $8.53 billion on nail services.1 This booming industry is set to grow to more than $15.5 billion by 2024.2 Nail polishes and other nail cosmetic trends can present new exposures for consumers, including chemicals that can elicit allergic contact dermatitis. In this article, we discuss new nail trends and their associated allergens, the acrylates.
Tosylamide/Formaldehyde Resin
Traditionally, the most widely recognized nail polish allergen has been tosylamide/formaldehyde resin (TSFR). However, there now are many touted TSFR-free nail polishes on the market, and the rate of positive reactions to this chemical has been declining in recent years. The North American Contact Dermatitis Group reported a positive reaction rate of 1.3% from 2005 through 2006,3 and rates decreased to 0.9% from 2015 through 2016.4 An Australian study demonstrated a similar reduction in positive reaction rates to nail polish chemicals, with only 0.7% of patients reacting to TSFR from 2014 to 2016 and 0% in 2017. It is theorized that this reduction occurred from replacing TSFR in traditional nail polishes with other chemicals such as polyester resins and cellulose acetate butyrate.5
Acrylate-Based Nail Treatments
Consumers recently have been gravitating toward acrylate-based nail treatments vs traditional nail polishes for a variety of reasons. Often referred to as gels, dips, or shellac, acrylate-based nail treatments represent a hot new trend in nail cosmetics. These manicures are resistant to chipping and scratches, creating a like-new look that lasts for weeks after application. The long-lasting nature of acrylate-based nail polishes has made them wildly popular with consumers.
Traditional acrylic nails consist of a powder polymer mixed with a liquid monomer, which polymerizes when a catalyst is added.6 The procedure is time consuming and can take up to 2 hours for application. In contrast, the newer gel manicure can be completed faster and includes application of acrylate-based nail polish, including a base coat, 2 coats of color, and a top coat. Exposure to either a light-emitting diode (30–60 seconds) or UVA (2 minutes) lamp is necessary after each coat is applied for polymerization (Figure 1).6 This long-lasting, semipermanent manicure typically is what patients are referring to when they say they have “gel nails.”

Gel dipping powders (referred to as dips) are another long-lasting acrylate-based nail treatment. This type of polish uses ethyl cyanoacrylate, a slightly different acrylate (yes, that IS super glue). After the nail is prepared, a base polish is applied to three-quarters of the nail and it is dipped into a natural color dip powder. The base polish is then applied to the entire nail, followed by a dip into the polish color of choice. This process is completed twice, followed by shaping and application of a top coat (Figure 2).

base coat. B, Application of dip powder to gel polish. Note the entire
distal finger and nail are dipped into the powder. C, Shaping of the
nail after the second coat of color is applied.
Finally, there are nail wraps, which are similar to stickers placed over or extending the nail plate. The wraps can be made from linen, silk, vinyl, or other material. Ethyl cyanoacrylate and isopropyl-2-cyanoacrylates have been identified in nail wrap adhesive.7 The heated product is directly applied to the prepared nail, and the excess wrap is filed off. Additional nail polish and a top coat usually are applied to finish the nail. Many of these products are available for in-salon use as well as online purchase and home application by consumers.
Acrylate Allergy
Patients who are allergic to acrylates can present with different patterns of dermatitis. Although the majority of patients present with dermatitis on the hands, fingers, or wrists, up to 10% may only have facial and neck dermatitis.8 Less commonly, the abdomen and thighs can be involved.6,8 Nail technicians most commonly present with pulpitis with cutaneous fissures.8 Other symptoms can include subungual hyperkeratosis, onycholysis, and nail dystrophy. Paresthesia, urticaria, and upper respiratory tract symptoms can occur but are less common.6,8
Acrylate allergy typically is the result of sensitization to the acrylate monomers. In theory, gel nail acrylate materials are polymerized following exposure to a light-emitting diode or UVA lamp; however, there likely is some incomplete polymerization, which can increase the risk for development of allergy. Allergen exposure can occur due to incorrect application of the light source; inadvertent monomer exposure, which occurs when nail technicians wipe extra acrylate off of a client’s finger(s); or inadvertent application of acrylate monomers to objects in the nail technician’s work environment.6,8
Several acrylate nail allergens have been reported. Many studies have identified 2-hydroxyethyl methacrylate (HEMA) as the most common nail acrylate allergen.8,9 At least one study identified 2-hydroxypropyl methacrylate as the most common, with HEMA in second place.6 Other reported acrylate allergens have included ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, methyl methacrylate, ethyl cyanoacrylate, 1,4-butanediol diacrylate, hydroxypropyl acrylate, and 2-hydroxyethyl acrylate.8,9
The American Contact Dermatitis Society Core Allergen Series and the North American Contact Dermatitis Group screening series currently include HEMA, methyl methacrylate, ethyl acrylate, ethyl cyanoacrylate, and TSFR.4,10 Of note, acrylates are not included in the thin-layer rapid use epicutaneous (T.R.U.E.) patch test (SmartPractice), so they will be missed if this series is used.11 In the setting of suspected nail acrylate allergy, some authors recommend initial screening with HEMA and ethyl cyanoacrylate, with extended acrylate testing if both are negative.8
Upon patch testing with an acrylate series, patients frequently react to 2 or more acrylates and the reactions can be strong (++) or extreme (+++), which may represent cosensitization or cross-sensitization.8 The likelihood of cross-reactivity between acrylates is not clear, though it has been postulated that it is theoretically possible.6
An important pearl for patch testers using the chamber method is proper storage of acrylate allergens and assembly of trays prior to patch testing. Similar to all haptens, manufacturers recommend that acrylates should be stored in a refrigerator, but some authors suggest that acrylates should be stored in the freezer.12 Acrylates are volatile chemicals and rapidly degrade when exposed to air. A methyl methacrylate preparation loaded into an inert quadrate (IQ) chamber and stored at room temperature showed a nearly undetectable amount of any residual methyl methacrylate 24 hours later. Refrigeration of allergens in chambers slowed but did not stop eventual degradation, with nearly all acrylate preparations reaching an undetectable level of allergen by day 8.13 Acrylates, along with other volatile allergens, should only be loaded into chambers immediately prior to placement on the patient.
Allergy Prevention
Prevention of nail acrylate allergy among consumers is simple: avoid contact with the offending allergen. Acrylate spillover (ie, applying the acrylate onto the skin) and direct contact with objects and working surfaces contaminated with acrylate-based nail products should be avoided.8 Avoidance is more complicated for nail technicians, but it is thought that nitrile gloves allow for the best dexterity and allergen avoidance when acrylate exposure is brief.14 Allowable exposure times with nitrile gloves may be 15 to 30 minutes. After this times passes, a glove change is required to avoid exposure.14 Wearing nitrile gloves for longer than 15 to 30 minutes will result in cutaneous exposure and risk for dermatitis in sensitized patients. If longer wear is desired, one option includes cutting the fingertips off of Silver Shield/4H gloves (Honeywell Safety Products USA, Inc), applying them to the distal fingers, and wearing a standard nitrile glove over top, known as the finger stall technique.6 In one study, this technique was recommended to nail technicians with acrylate allergy. A telephone survey conducted 4 to 43 months later confirmed that 36% (8/22) of participants were using the technique without symptoms. In this same study, 73% (16/22) had continued working as nail technicians.6
Acrylates are used for other medical purposes, including dental procedures, orthopedic procedures, surgical glues, wound dressings, and contact and intraocular lenses. They also have additional cosmetic applications, including eyelash and hair extensions.8 Therefore, it is vital that patients disclose any history of acrylate allergy to both their medical and cosmetic providers.
Our Final Interpretation
Acrylate allergy has become increasingly common, and long-lasting nail treatments often are the culprit. Whether through gels, dips, or shellac, repeated exposure to acrylates through nail treatments can increase the risk for allergy. The T.R.U.E. test alone will not make the diagnosis, as acrylates are not present in this patch test system. It is important to remind your allergic patients that acrylates are present in other compounds used for medical and cosmetic purposes. Avoidance is key, and for allergic patients who love to bedazzle their nails, we suggest less-permanent, acrylate-free nail polishes as alternatives.
- 2017-2018 industry statistics highlights. Nails Magazine. http://files.nailsmag.com/handouts/nabb2017-18stats-lr.pdf. Accessed May 17, 2019.
- Nail polish market size worth $15.55 billion by 2024. Grand View Research website. https://www.grandviewresearch.com/press-release/global-nail-polish-market. Published October 2017. Accessed May 17, 2019.
- Zug KA, Warshaw EM, Fowler JF, et al. Patch-test results of the North American Contact Dermatitis Group 2005-2006. Dermatitis. 2009;20:149-160.
- DeKoven J, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Lee S, Maor D, Palmer A, et al. Declining prevalence of allergic contact dermatitis caused by tosylamide/formaldehyde in nail polish. Contact Dermatitis. 2018;79:184-185.
- Gatica-Ortega ME, Pastor-Nieto MA, Mercader-García P, et al. Allergic contact dermatitis caused by (meth)acrylates in long-lasting nail polish: are we facing a new epidemic in the beauty industry? Contact Dermatitis. 2017;7:360-366.
- Fitzgerald DA, Bhaggoe R, English JS. Contact sensitivity to cyanoacrylate nail-adhesive with dermatitis at remote sites. Contact Dermatitis. 1995;32:175-176.
- Goncalo M, Pinho A, Agner T et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2017;78:254-260.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study [published online January 14, 2019]. Contact Dermatitis. doi:10.1111/cod.13216.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2017 update. Dermatitis. 2017;28:141-143.
- T.R.U.E. TEST ready-to-use patch test panels. Smart Practice website. https://www.smartpractice.com/shop/wa/category?cn=T.R.U.E.-TEST%C2%AE-Ready-to-Use-Patch-Test-Panels&id=508222&m=SPA. Accessed May 17, 2019.
- Good AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methylacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Goon A, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Morgado F, Batista M, Gonçalo M. Short exposures and glove protection against (meth)acrylates in nail beauticians—thoughts on a rising concern [published online January 17, 2019]. Contact Dermatitis. doi:10.1111/cod.13222.
- 2017-2018 industry statistics highlights. Nails Magazine. http://files.nailsmag.com/handouts/nabb2017-18stats-lr.pdf. Accessed May 17, 2019.
- Nail polish market size worth $15.55 billion by 2024. Grand View Research website. https://www.grandviewresearch.com/press-release/global-nail-polish-market. Published October 2017. Accessed May 17, 2019.
- Zug KA, Warshaw EM, Fowler JF, et al. Patch-test results of the North American Contact Dermatitis Group 2005-2006. Dermatitis. 2009;20:149-160.
- DeKoven J, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Lee S, Maor D, Palmer A, et al. Declining prevalence of allergic contact dermatitis caused by tosylamide/formaldehyde in nail polish. Contact Dermatitis. 2018;79:184-185.
- Gatica-Ortega ME, Pastor-Nieto MA, Mercader-García P, et al. Allergic contact dermatitis caused by (meth)acrylates in long-lasting nail polish: are we facing a new epidemic in the beauty industry? Contact Dermatitis. 2017;7:360-366.
- Fitzgerald DA, Bhaggoe R, English JS. Contact sensitivity to cyanoacrylate nail-adhesive with dermatitis at remote sites. Contact Dermatitis. 1995;32:175-176.
- Goncalo M, Pinho A, Agner T et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2017;78:254-260.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study [published online January 14, 2019]. Contact Dermatitis. doi:10.1111/cod.13216.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2017 update. Dermatitis. 2017;28:141-143.
- T.R.U.E. TEST ready-to-use patch test panels. Smart Practice website. https://www.smartpractice.com/shop/wa/category?cn=T.R.U.E.-TEST%C2%AE-Ready-to-Use-Patch-Test-Panels&id=508222&m=SPA. Accessed May 17, 2019.
- Good AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methylacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Goon A, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Morgado F, Batista M, Gonçalo M. Short exposures and glove protection against (meth)acrylates in nail beauticians—thoughts on a rising concern [published online January 17, 2019]. Contact Dermatitis. doi:10.1111/cod.13222.
Practice Points
- Changing trends in nail services mean new exposures for consumers. Traditional nail polish has been replaced by semipermanent nail polish, which contains acrylates.
- Acrylates are a common cause of allergic contact dermatitis from nail polish. Acrylates can be found in gel, dip, and shellac nail polishes, among others.
- Patch testing with 2-hydroxyethyl methacrylate and ethyl cyanoacrylate can screen many patients for allergy due to nail services.