User login
Pediatric dermatology update: New research offers insight into psoriasis, alopecia
LAS VEGAS – Recent research is offering new insights into psoriasis and alopecia in the pediatric population, a dermatologist told colleagues, and it’s time to be on the lookout for psoriasis linked to treatment with tumor necrosis factor (TNF) inhibitors.
Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics, at the University of California, San Diego, offered these tips and comments in a presentation at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar:
Psoriasis
It’s a brand new day for adult psoriasis sufferers, but it seems to be only a brand new morning for their pediatric counterparts. “Kids and teenagers were left behind in the biologic revolution,” Dr. Eichenfield said. “Only two systemic biologics have been approved for psoriasis in children.” They are ustekinumab (Stelara), approved by the Food and Drug Administration for treating psoriasis in children aged 12 years and older, and etanercept, approved for aged 4 years and older.
The good news, he said, is that “our new biologic agents are now being studied in children.”
Research is also providing new insight into pediatric psoriasis, said Dr. Eichenfield, who is also chief of pediatric and adolescent dermatology at Rady Children’s Hospital in San Diego. It’s now clear that “there’s a lot more facial involvement, and a high involvement of scalp and nail,” he noted.
It’s also clear, he said, that inflammation begins early in pediatric psoriasis. That raises the question of whether it’s a good idea to launch aggressive treatment to stop the “psoriatic march” toward cardiovascular and other medical problems down the line, he commented.
“Keep an open mind to getting aggressive in therapy,” he advised, although he acknowledged that “it’s hard to get beyond the two biologics, and only one is approved for children under 12.”
Dr. Eichenfield advised colleagues to keep an eye out for TNF inhibitor–induced psoriasis. “We’re seeing it pretty regularly,” he said, commonly in children who are treated with TNF inhibitors for rheumatoid arthritis or Crohn’s disease.
The lesions “look like dermatitis but are very psoriasiform,” he said, and research suggests this can appear after a single dose or after as many as 63 months of treatment. Topical and light therapy can be helpful. But if those treatments do not help, he said, it’s time to consider changing the biologic that the patient is taking. “Is the biologic adequately controlling their underlying disease? If not, you can help find one that would be great for their underlying disease and clear up their psoriasis.”
Alopecia
Pediatric alopecia “is a problem I see pretty regularly in practice,” Dr. Eichenfield said. When he sees patients with alopecia, he says that, “‘if your child doesn’t have 50% hair loss, you’re in the good group. It will generally heal up and never come back again.’ ”
He referred to a recent study, where investigators at the Children’s Hospital of Philadelphia retrospectively studied 125 children under age 4 years who were diagnosed with alopecia areata and followed for 2 years. Over time, those children with over 50% of hair loss initially were more likely to have worsening Severity of Alopecia Tool (SALT) scores over the follow-up period. But a high proportion of those with mild alopecia initially continued to have mild alopecia at follow-up (Pediatr Dermatol. 2019 Aug 29. doi: 10.1111/pde.13990).
Dr. Eichenfield noted that the study found that 41% of the patients also had atopic dermatitis.
He also highlighted two other recent studies on pediatric alopecia: One found that while vitamin D levels were low in a majority of children with alopecia in the study, the proportion who had a deficiency was similar to the proportion in a larger pediatric population, at about 22% in both groups (J Am Acad Dermatol. 2018 Sep;79(3):e43-e44). Supplementation doesn’t seem to help. “It’s not important to test levels,” he said.
Another study examined whether it’s a good idea to test patients for celiac disease in children with alopecia (Pediatr Dermatol. 2018 Jul;35[4]:535-8). Some parents may ask this question, but the answer, he said, is generally no.
What’s next? “We were hoping oral and topical JAK inhibitors would work well” in this population, Dr. Eichenfield said, but study findings haven’t been promising.
Still, oral tofacitinib (Xeljanz) showed some “pretty impressive” success in a recent study in four children, he noted. Based on the results, the authors wrote that “we suggest that, after proper counseling regarding the risks, including severe infection and malignancy, the use of tofacitinib may be considered for preadolescent children with AA [alopecia areata] who are experiencing psychosocial impairment” (J Am Acad Dermatol. 2019 Feb;80[2]:568-70).
In general, Dr. Eichenfield said, research on pediatric alopecia “will be secondary, especially with JAK inhibitors because of the risk of side effects. But [children will] probably tolerate them better than adults do because they have fewer medical problems.”
Meanwhile, he added, controversy continues to swirl around how to treat children over age 10 years who have lost 50% or more of their hair. “I’ve seen hundreds of kids with alopecia areata,” he said, “and I can’t predict what the course may be.”
Dr. Eichenfield reports multiple relationships (consultant or investigator) with various pharmaceutical companies, including Abbvie, Allergan, Lilly, Novartis, and others. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Recent research is offering new insights into psoriasis and alopecia in the pediatric population, a dermatologist told colleagues, and it’s time to be on the lookout for psoriasis linked to treatment with tumor necrosis factor (TNF) inhibitors.
Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics, at the University of California, San Diego, offered these tips and comments in a presentation at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar:
Psoriasis
It’s a brand new day for adult psoriasis sufferers, but it seems to be only a brand new morning for their pediatric counterparts. “Kids and teenagers were left behind in the biologic revolution,” Dr. Eichenfield said. “Only two systemic biologics have been approved for psoriasis in children.” They are ustekinumab (Stelara), approved by the Food and Drug Administration for treating psoriasis in children aged 12 years and older, and etanercept, approved for aged 4 years and older.
The good news, he said, is that “our new biologic agents are now being studied in children.”
Research is also providing new insight into pediatric psoriasis, said Dr. Eichenfield, who is also chief of pediatric and adolescent dermatology at Rady Children’s Hospital in San Diego. It’s now clear that “there’s a lot more facial involvement, and a high involvement of scalp and nail,” he noted.
It’s also clear, he said, that inflammation begins early in pediatric psoriasis. That raises the question of whether it’s a good idea to launch aggressive treatment to stop the “psoriatic march” toward cardiovascular and other medical problems down the line, he commented.
“Keep an open mind to getting aggressive in therapy,” he advised, although he acknowledged that “it’s hard to get beyond the two biologics, and only one is approved for children under 12.”
Dr. Eichenfield advised colleagues to keep an eye out for TNF inhibitor–induced psoriasis. “We’re seeing it pretty regularly,” he said, commonly in children who are treated with TNF inhibitors for rheumatoid arthritis or Crohn’s disease.
The lesions “look like dermatitis but are very psoriasiform,” he said, and research suggests this can appear after a single dose or after as many as 63 months of treatment. Topical and light therapy can be helpful. But if those treatments do not help, he said, it’s time to consider changing the biologic that the patient is taking. “Is the biologic adequately controlling their underlying disease? If not, you can help find one that would be great for their underlying disease and clear up their psoriasis.”
Alopecia
Pediatric alopecia “is a problem I see pretty regularly in practice,” Dr. Eichenfield said. When he sees patients with alopecia, he says that, “‘if your child doesn’t have 50% hair loss, you’re in the good group. It will generally heal up and never come back again.’ ”
He referred to a recent study, where investigators at the Children’s Hospital of Philadelphia retrospectively studied 125 children under age 4 years who were diagnosed with alopecia areata and followed for 2 years. Over time, those children with over 50% of hair loss initially were more likely to have worsening Severity of Alopecia Tool (SALT) scores over the follow-up period. But a high proportion of those with mild alopecia initially continued to have mild alopecia at follow-up (Pediatr Dermatol. 2019 Aug 29. doi: 10.1111/pde.13990).
Dr. Eichenfield noted that the study found that 41% of the patients also had atopic dermatitis.
He also highlighted two other recent studies on pediatric alopecia: One found that while vitamin D levels were low in a majority of children with alopecia in the study, the proportion who had a deficiency was similar to the proportion in a larger pediatric population, at about 22% in both groups (J Am Acad Dermatol. 2018 Sep;79(3):e43-e44). Supplementation doesn’t seem to help. “It’s not important to test levels,” he said.
Another study examined whether it’s a good idea to test patients for celiac disease in children with alopecia (Pediatr Dermatol. 2018 Jul;35[4]:535-8). Some parents may ask this question, but the answer, he said, is generally no.
What’s next? “We were hoping oral and topical JAK inhibitors would work well” in this population, Dr. Eichenfield said, but study findings haven’t been promising.
Still, oral tofacitinib (Xeljanz) showed some “pretty impressive” success in a recent study in four children, he noted. Based on the results, the authors wrote that “we suggest that, after proper counseling regarding the risks, including severe infection and malignancy, the use of tofacitinib may be considered for preadolescent children with AA [alopecia areata] who are experiencing psychosocial impairment” (J Am Acad Dermatol. 2019 Feb;80[2]:568-70).
In general, Dr. Eichenfield said, research on pediatric alopecia “will be secondary, especially with JAK inhibitors because of the risk of side effects. But [children will] probably tolerate them better than adults do because they have fewer medical problems.”
Meanwhile, he added, controversy continues to swirl around how to treat children over age 10 years who have lost 50% or more of their hair. “I’ve seen hundreds of kids with alopecia areata,” he said, “and I can’t predict what the course may be.”
Dr. Eichenfield reports multiple relationships (consultant or investigator) with various pharmaceutical companies, including Abbvie, Allergan, Lilly, Novartis, and others. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Recent research is offering new insights into psoriasis and alopecia in the pediatric population, a dermatologist told colleagues, and it’s time to be on the lookout for psoriasis linked to treatment with tumor necrosis factor (TNF) inhibitors.
Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics, at the University of California, San Diego, offered these tips and comments in a presentation at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar:
Psoriasis
It’s a brand new day for adult psoriasis sufferers, but it seems to be only a brand new morning for their pediatric counterparts. “Kids and teenagers were left behind in the biologic revolution,” Dr. Eichenfield said. “Only two systemic biologics have been approved for psoriasis in children.” They are ustekinumab (Stelara), approved by the Food and Drug Administration for treating psoriasis in children aged 12 years and older, and etanercept, approved for aged 4 years and older.
The good news, he said, is that “our new biologic agents are now being studied in children.”
Research is also providing new insight into pediatric psoriasis, said Dr. Eichenfield, who is also chief of pediatric and adolescent dermatology at Rady Children’s Hospital in San Diego. It’s now clear that “there’s a lot more facial involvement, and a high involvement of scalp and nail,” he noted.
It’s also clear, he said, that inflammation begins early in pediatric psoriasis. That raises the question of whether it’s a good idea to launch aggressive treatment to stop the “psoriatic march” toward cardiovascular and other medical problems down the line, he commented.
“Keep an open mind to getting aggressive in therapy,” he advised, although he acknowledged that “it’s hard to get beyond the two biologics, and only one is approved for children under 12.”
Dr. Eichenfield advised colleagues to keep an eye out for TNF inhibitor–induced psoriasis. “We’re seeing it pretty regularly,” he said, commonly in children who are treated with TNF inhibitors for rheumatoid arthritis or Crohn’s disease.
The lesions “look like dermatitis but are very psoriasiform,” he said, and research suggests this can appear after a single dose or after as many as 63 months of treatment. Topical and light therapy can be helpful. But if those treatments do not help, he said, it’s time to consider changing the biologic that the patient is taking. “Is the biologic adequately controlling their underlying disease? If not, you can help find one that would be great for their underlying disease and clear up their psoriasis.”
Alopecia
Pediatric alopecia “is a problem I see pretty regularly in practice,” Dr. Eichenfield said. When he sees patients with alopecia, he says that, “‘if your child doesn’t have 50% hair loss, you’re in the good group. It will generally heal up and never come back again.’ ”
He referred to a recent study, where investigators at the Children’s Hospital of Philadelphia retrospectively studied 125 children under age 4 years who were diagnosed with alopecia areata and followed for 2 years. Over time, those children with over 50% of hair loss initially were more likely to have worsening Severity of Alopecia Tool (SALT) scores over the follow-up period. But a high proportion of those with mild alopecia initially continued to have mild alopecia at follow-up (Pediatr Dermatol. 2019 Aug 29. doi: 10.1111/pde.13990).
Dr. Eichenfield noted that the study found that 41% of the patients also had atopic dermatitis.
He also highlighted two other recent studies on pediatric alopecia: One found that while vitamin D levels were low in a majority of children with alopecia in the study, the proportion who had a deficiency was similar to the proportion in a larger pediatric population, at about 22% in both groups (J Am Acad Dermatol. 2018 Sep;79(3):e43-e44). Supplementation doesn’t seem to help. “It’s not important to test levels,” he said.
Another study examined whether it’s a good idea to test patients for celiac disease in children with alopecia (Pediatr Dermatol. 2018 Jul;35[4]:535-8). Some parents may ask this question, but the answer, he said, is generally no.
What’s next? “We were hoping oral and topical JAK inhibitors would work well” in this population, Dr. Eichenfield said, but study findings haven’t been promising.
Still, oral tofacitinib (Xeljanz) showed some “pretty impressive” success in a recent study in four children, he noted. Based on the results, the authors wrote that “we suggest that, after proper counseling regarding the risks, including severe infection and malignancy, the use of tofacitinib may be considered for preadolescent children with AA [alopecia areata] who are experiencing psychosocial impairment” (J Am Acad Dermatol. 2019 Feb;80[2]:568-70).
In general, Dr. Eichenfield said, research on pediatric alopecia “will be secondary, especially with JAK inhibitors because of the risk of side effects. But [children will] probably tolerate them better than adults do because they have fewer medical problems.”
Meanwhile, he added, controversy continues to swirl around how to treat children over age 10 years who have lost 50% or more of their hair. “I’ve seen hundreds of kids with alopecia areata,” he said, “and I can’t predict what the course may be.”
Dr. Eichenfield reports multiple relationships (consultant or investigator) with various pharmaceutical companies, including Abbvie, Allergan, Lilly, Novartis, and others. SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
FDA still concerned about biotin affecting troponin tests
The
However, not all troponin tests are affected, according to the update. “Since the FDA’s safety communication on this topic in 2017, some lab test developers have been successful at mitigating the biotin interference of their assays, but others have not yet addressed it,” according to the new communication, issued in early November.
Also known as vitamin B7 and appearing in many dietary supplements, including prenatal multivitamins and supplements for hair, skin, and nail growth, biotin can lead to falsely low results on some troponin tests, especially at high levels. The worry is that biotin interference could therefore lead to missed diagnoses. The FDA has provided a list of those tests that have not taken biotin’s effects into account, titled “Biotin Interference with Troponin Lab Tests – Assays Subject to Biotin Interference.”
The daily recommended allowance for biotin, according to the communication, is about 0.3 mg, but it isn’t always clear how much is actually included in supplements – some can contain 20 mg or even as much as 100 mg per pill of biotin. The communication includes recommendations for patients, health care professionals, laboratory personnel, and lab test manufacturers and developers.
The full safety communication can be found on the FDA website, and problems with tests can be reported via the FDA’s MedWatch Voluntary Reporting Form.
The
However, not all troponin tests are affected, according to the update. “Since the FDA’s safety communication on this topic in 2017, some lab test developers have been successful at mitigating the biotin interference of their assays, but others have not yet addressed it,” according to the new communication, issued in early November.
Also known as vitamin B7 and appearing in many dietary supplements, including prenatal multivitamins and supplements for hair, skin, and nail growth, biotin can lead to falsely low results on some troponin tests, especially at high levels. The worry is that biotin interference could therefore lead to missed diagnoses. The FDA has provided a list of those tests that have not taken biotin’s effects into account, titled “Biotin Interference with Troponin Lab Tests – Assays Subject to Biotin Interference.”
The daily recommended allowance for biotin, according to the communication, is about 0.3 mg, but it isn’t always clear how much is actually included in supplements – some can contain 20 mg or even as much as 100 mg per pill of biotin. The communication includes recommendations for patients, health care professionals, laboratory personnel, and lab test manufacturers and developers.
The full safety communication can be found on the FDA website, and problems with tests can be reported via the FDA’s MedWatch Voluntary Reporting Form.
The
However, not all troponin tests are affected, according to the update. “Since the FDA’s safety communication on this topic in 2017, some lab test developers have been successful at mitigating the biotin interference of their assays, but others have not yet addressed it,” according to the new communication, issued in early November.
Also known as vitamin B7 and appearing in many dietary supplements, including prenatal multivitamins and supplements for hair, skin, and nail growth, biotin can lead to falsely low results on some troponin tests, especially at high levels. The worry is that biotin interference could therefore lead to missed diagnoses. The FDA has provided a list of those tests that have not taken biotin’s effects into account, titled “Biotin Interference with Troponin Lab Tests – Assays Subject to Biotin Interference.”
The daily recommended allowance for biotin, according to the communication, is about 0.3 mg, but it isn’t always clear how much is actually included in supplements – some can contain 20 mg or even as much as 100 mg per pill of biotin. The communication includes recommendations for patients, health care professionals, laboratory personnel, and lab test manufacturers and developers.
The full safety communication can be found on the FDA website, and problems with tests can be reported via the FDA’s MedWatch Voluntary Reporting Form.
Expert reviews strategies for diagnosing, treating onychomycosis
LAS VEGAS – The way Neal Bhatia, MD, sees it, there is no such thing as a classical presentation of onychomycosis.
“This is where proving your diagnosis is half the battle, even though we are sometimes using empiric therapy in suspected cases,” he said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Prove the diagnosis and get the extra tests necessary.”
According to Dr. Bhatia, director of clinical dermatology research at San Diego-based Therapeutics Clinical Research, . With onychomycosis, the ultimate treatment goal from the standpoint of clinicians is no more fungus, he noted, while the desired endpoint from the standpoint of some patients is normal-looking nails.
“Endpoint failures in a research trial are not the same as what we tell patients in the clinic,” Dr. Bhatia said. “If you have a patient using something topical for 52 weeks and they see two-thirds of their nail improve, you’re not going to say, ‘Stop; you’re a failure now.’ You tell them, ‘Keep going and we’ll keep watching.’ But in the research world, it’s very different when you look at all of the different endpoints that have to be met at the finish line. It’s very important to measure success based on what that patient’s experiencing.”
According to Dr. Bhatia, diagnosing onychomycosis by visual assessment has a sensitivity of 77% and a specificity of 47%, while KOH has a sensitivity between 67% and 93% and specificity between 38% and 78%. PCR, meanwhile, “is quick, but it’s expensive and it has a high false-positive rate. So, in those difficult patients who aren’t responding [to treatment], maybe we can’t just trust our eyes alone [to make a diagnosis].”
Tests such as a KOH stain have low sensitivity, with high costs of more sensitive tests such as the periodic acid–Schiff (PAS) stain. In a recent study, researchers conducted a retrospective cohort analysis of 600 patients with toenail clippings sent for PAS stain during January 2000–December 2013 (J Am Acad Dermatol. 2015 May; 72(5):AB116). They reviewed records to determine which PAS stains were performed to confirm probable clinical diagnosis of onychomycosis.
The researchers found that 30% of toenail clippings were sent for confirmatory PAS staining by dermatologists, compared with 37% by podiatrists and 34% by other clinicians. Of these tests, 75% ordered by dermatologists were positive for fungus, compared with 81% ordered by podiatrists, and 66% ordered by other physicians. “The positive predictive value of clinical suspicion for true onychomycosis was high, and the findings question whether or not a confirmatory test is really necessary,” Dr. Bhatia said.
Preventative strategies to control recurrence of onychomycosis include using maintenance regimens of the recommended antifungal agent, discarding old shoes, alternating wearing different pairs of shoes, periodically disinfecting shoes, washing feet regularly, and alerting the physician to the first sign of infection.
In an effort to investigate strategies to minimize recurrence of onychomycosis, Dr. Bhatia and colleagues evaluated 73 patients over the course of 7 years who were taking either terbinafine or itraconazole (J Drugs Dermatol. 2016;15[3]:279-82). Thirty-six months later, the overall mean recurrence rate among patients was 14%. Prognostic factors influencing recurrence included patient’s family history; lifestyle; underlying physiology (presence of a very thick nail, extensive involvement of the entire nail unit, lateral nail disease and yellow spikes); physical trauma, especially in the elderly; concomitant disease, such as peripheral artery disease and/or diabetes; immunocompromised or immunosuppressed patients, and the presence of tinea pedis.
Based on their analysis, they recommended the following strategies to prevent or limit recurrence: prophylactic use of a topical antifungal twice-weekly for 2-3 years; periodic application of a topical antifungal to plantar surface and/or interdigital spaces; treatment of any coexisting tinea pedis; treating immediate family members; footwear and sock decontamination with antifungal powder; ultraviolet light or ozone; avoidance of activities known to risk spread of disease, such as communal swimming pools.
Dr. Bhatia concluded his presentation by noting that the ideal treatment for onychomycosis would not pose a systemic risk to the liver, heart, or other organs; would not require monitoring of labs; would not require debridement; and would not interact with other drugs. It would also penetrate the nail plate – especially the diseased nail – and would be quick drying.
SDEF and this news organization are owned by the same parent company.
Dr. Bhatia disclosed having affiliations with Actavis, Allergan, Anacor, Aqua, Bayer, Biofrontera, BiopharmX, Cipher, Dermira, Dusa, Exeltis, Ferndale, Foamix, Galderma, Intraderm, ISDIN, LaRoche-Posay, Leo, Novan, Novartis, PharmaDerm, Promius, Regeneron, Sanofi, Sun Pharma, and Valeant.
LAS VEGAS – The way Neal Bhatia, MD, sees it, there is no such thing as a classical presentation of onychomycosis.
“This is where proving your diagnosis is half the battle, even though we are sometimes using empiric therapy in suspected cases,” he said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Prove the diagnosis and get the extra tests necessary.”
According to Dr. Bhatia, director of clinical dermatology research at San Diego-based Therapeutics Clinical Research, . With onychomycosis, the ultimate treatment goal from the standpoint of clinicians is no more fungus, he noted, while the desired endpoint from the standpoint of some patients is normal-looking nails.
“Endpoint failures in a research trial are not the same as what we tell patients in the clinic,” Dr. Bhatia said. “If you have a patient using something topical for 52 weeks and they see two-thirds of their nail improve, you’re not going to say, ‘Stop; you’re a failure now.’ You tell them, ‘Keep going and we’ll keep watching.’ But in the research world, it’s very different when you look at all of the different endpoints that have to be met at the finish line. It’s very important to measure success based on what that patient’s experiencing.”
According to Dr. Bhatia, diagnosing onychomycosis by visual assessment has a sensitivity of 77% and a specificity of 47%, while KOH has a sensitivity between 67% and 93% and specificity between 38% and 78%. PCR, meanwhile, “is quick, but it’s expensive and it has a high false-positive rate. So, in those difficult patients who aren’t responding [to treatment], maybe we can’t just trust our eyes alone [to make a diagnosis].”
Tests such as a KOH stain have low sensitivity, with high costs of more sensitive tests such as the periodic acid–Schiff (PAS) stain. In a recent study, researchers conducted a retrospective cohort analysis of 600 patients with toenail clippings sent for PAS stain during January 2000–December 2013 (J Am Acad Dermatol. 2015 May; 72(5):AB116). They reviewed records to determine which PAS stains were performed to confirm probable clinical diagnosis of onychomycosis.
The researchers found that 30% of toenail clippings were sent for confirmatory PAS staining by dermatologists, compared with 37% by podiatrists and 34% by other clinicians. Of these tests, 75% ordered by dermatologists were positive for fungus, compared with 81% ordered by podiatrists, and 66% ordered by other physicians. “The positive predictive value of clinical suspicion for true onychomycosis was high, and the findings question whether or not a confirmatory test is really necessary,” Dr. Bhatia said.
Preventative strategies to control recurrence of onychomycosis include using maintenance regimens of the recommended antifungal agent, discarding old shoes, alternating wearing different pairs of shoes, periodically disinfecting shoes, washing feet regularly, and alerting the physician to the first sign of infection.
In an effort to investigate strategies to minimize recurrence of onychomycosis, Dr. Bhatia and colleagues evaluated 73 patients over the course of 7 years who were taking either terbinafine or itraconazole (J Drugs Dermatol. 2016;15[3]:279-82). Thirty-six months later, the overall mean recurrence rate among patients was 14%. Prognostic factors influencing recurrence included patient’s family history; lifestyle; underlying physiology (presence of a very thick nail, extensive involvement of the entire nail unit, lateral nail disease and yellow spikes); physical trauma, especially in the elderly; concomitant disease, such as peripheral artery disease and/or diabetes; immunocompromised or immunosuppressed patients, and the presence of tinea pedis.
Based on their analysis, they recommended the following strategies to prevent or limit recurrence: prophylactic use of a topical antifungal twice-weekly for 2-3 years; periodic application of a topical antifungal to plantar surface and/or interdigital spaces; treatment of any coexisting tinea pedis; treating immediate family members; footwear and sock decontamination with antifungal powder; ultraviolet light or ozone; avoidance of activities known to risk spread of disease, such as communal swimming pools.
Dr. Bhatia concluded his presentation by noting that the ideal treatment for onychomycosis would not pose a systemic risk to the liver, heart, or other organs; would not require monitoring of labs; would not require debridement; and would not interact with other drugs. It would also penetrate the nail plate – especially the diseased nail – and would be quick drying.
SDEF and this news organization are owned by the same parent company.
Dr. Bhatia disclosed having affiliations with Actavis, Allergan, Anacor, Aqua, Bayer, Biofrontera, BiopharmX, Cipher, Dermira, Dusa, Exeltis, Ferndale, Foamix, Galderma, Intraderm, ISDIN, LaRoche-Posay, Leo, Novan, Novartis, PharmaDerm, Promius, Regeneron, Sanofi, Sun Pharma, and Valeant.
LAS VEGAS – The way Neal Bhatia, MD, sees it, there is no such thing as a classical presentation of onychomycosis.
“This is where proving your diagnosis is half the battle, even though we are sometimes using empiric therapy in suspected cases,” he said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Prove the diagnosis and get the extra tests necessary.”
According to Dr. Bhatia, director of clinical dermatology research at San Diego-based Therapeutics Clinical Research, . With onychomycosis, the ultimate treatment goal from the standpoint of clinicians is no more fungus, he noted, while the desired endpoint from the standpoint of some patients is normal-looking nails.
“Endpoint failures in a research trial are not the same as what we tell patients in the clinic,” Dr. Bhatia said. “If you have a patient using something topical for 52 weeks and they see two-thirds of their nail improve, you’re not going to say, ‘Stop; you’re a failure now.’ You tell them, ‘Keep going and we’ll keep watching.’ But in the research world, it’s very different when you look at all of the different endpoints that have to be met at the finish line. It’s very important to measure success based on what that patient’s experiencing.”
According to Dr. Bhatia, diagnosing onychomycosis by visual assessment has a sensitivity of 77% and a specificity of 47%, while KOH has a sensitivity between 67% and 93% and specificity between 38% and 78%. PCR, meanwhile, “is quick, but it’s expensive and it has a high false-positive rate. So, in those difficult patients who aren’t responding [to treatment], maybe we can’t just trust our eyes alone [to make a diagnosis].”
Tests such as a KOH stain have low sensitivity, with high costs of more sensitive tests such as the periodic acid–Schiff (PAS) stain. In a recent study, researchers conducted a retrospective cohort analysis of 600 patients with toenail clippings sent for PAS stain during January 2000–December 2013 (J Am Acad Dermatol. 2015 May; 72(5):AB116). They reviewed records to determine which PAS stains were performed to confirm probable clinical diagnosis of onychomycosis.
The researchers found that 30% of toenail clippings were sent for confirmatory PAS staining by dermatologists, compared with 37% by podiatrists and 34% by other clinicians. Of these tests, 75% ordered by dermatologists were positive for fungus, compared with 81% ordered by podiatrists, and 66% ordered by other physicians. “The positive predictive value of clinical suspicion for true onychomycosis was high, and the findings question whether or not a confirmatory test is really necessary,” Dr. Bhatia said.
Preventative strategies to control recurrence of onychomycosis include using maintenance regimens of the recommended antifungal agent, discarding old shoes, alternating wearing different pairs of shoes, periodically disinfecting shoes, washing feet regularly, and alerting the physician to the first sign of infection.
In an effort to investigate strategies to minimize recurrence of onychomycosis, Dr. Bhatia and colleagues evaluated 73 patients over the course of 7 years who were taking either terbinafine or itraconazole (J Drugs Dermatol. 2016;15[3]:279-82). Thirty-six months later, the overall mean recurrence rate among patients was 14%. Prognostic factors influencing recurrence included patient’s family history; lifestyle; underlying physiology (presence of a very thick nail, extensive involvement of the entire nail unit, lateral nail disease and yellow spikes); physical trauma, especially in the elderly; concomitant disease, such as peripheral artery disease and/or diabetes; immunocompromised or immunosuppressed patients, and the presence of tinea pedis.
Based on their analysis, they recommended the following strategies to prevent or limit recurrence: prophylactic use of a topical antifungal twice-weekly for 2-3 years; periodic application of a topical antifungal to plantar surface and/or interdigital spaces; treatment of any coexisting tinea pedis; treating immediate family members; footwear and sock decontamination with antifungal powder; ultraviolet light or ozone; avoidance of activities known to risk spread of disease, such as communal swimming pools.
Dr. Bhatia concluded his presentation by noting that the ideal treatment for onychomycosis would not pose a systemic risk to the liver, heart, or other organs; would not require monitoring of labs; would not require debridement; and would not interact with other drugs. It would also penetrate the nail plate – especially the diseased nail – and would be quick drying.
SDEF and this news organization are owned by the same parent company.
Dr. Bhatia disclosed having affiliations with Actavis, Allergan, Anacor, Aqua, Bayer, Biofrontera, BiopharmX, Cipher, Dermira, Dusa, Exeltis, Ferndale, Foamix, Galderma, Intraderm, ISDIN, LaRoche-Posay, Leo, Novan, Novartis, PharmaDerm, Promius, Regeneron, Sanofi, Sun Pharma, and Valeant.
AT THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
Oral JAK1/2 inhibitor promising in alopecia areata
MADRID – In a phase 2, dose-ranging study, 78% of patients with long-standing moderate or severe alopecia areata rated their condition as “much improved” or “very much improved” after 24 weeks on the top dose of an investigational oral selective Janus kinase 1 and 2 (JAK1/2) inhibitor, compared with 21% of placebo-treated controls, James V. Cassella, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The primary study endpoint – at least a 50% reduction in the Severity of Alopecia Tool (SALT) score between baseline and 24 weeks – was achieved in 58% of patients on the JAK1/2 inhibitor (known for now as CTP-543) at 12 mg twice a day, 47% of those on CTP-543 at 8 mg twice a day, 21% on 4 mg twice a day, and in 9% of controls on placebo in the double-blind randomized trial. The 4-mg twice-daily dosing will not move on to phase 3 studies because of its demonstrated lack of efficacy, according to Dr. Cassella, chief development officer at Concert Pharmaceuticals, the study sponsor.
The study included 149 adults whose current episode of alopecia areata was of 3-6 years’ duration, with an average lifetime 15-year history of active disease. Their average baseline score on the 0-100 SALT scale was in the upper 80s, indicative of 80% or greater hair loss.
A SALT 75 response, meaning at least a 75% reduction in SALT score from baseline, was achieved in a dose-dependent fashion: In 42% of patients at the top dose of CTP-543, 29% of those on 8 mg twice a day, 14% with 4 mg twice a day, and in 7% of controls. An even more rigorous SALT 90 response was attained in 36%, 16%, 2%, and no controls, respectively.
The 12-mg twice-daily dosing produced faster onset and greater magnitude of response than did the 8-mg twice-daily dosing, but this dose-ranging study is not the final word on that score, according to Dr. Cassella.
“Week 24 is not a magic number,” he said. “The slope of the efficacy line for 8 mg [twice a day] looked like it was still going up at week 24, and the 12-mg BID curve hadn’t completely plateaued. Those are things we will consider for the future in terms of long-term trials.”
Changes in the eyebrows and lashes weren’t formally assessed in this study, although they will be in future trials. Anecdotally, however, patients with alopecia areata at those sites typically experienced complete or nearly complete regrowth in response to the higher doses of CTP-543, he said.
Safety-wise, there was no trend for increased adverse events with increasing doses of CTP-543. The observed treatment side effects were those typical of JAK inhibitors as a class effect, mainly headache, nasopharyngitis, upper respiratory infections, and new-onset acne. In terms of hematologic findings of special interest, there was one case of reversible neutropenia in the control group and another in the 8-mg twice-daily group, which resolved upon temporary suspension of treatment.
“Nothing surprising to us, and nothing very serious,” Dr. Cassella said.
Most patients in the 12-mg twice daily group have enrolled in an ongoing long-term extension study. In addition, two phase 2 studies are ongoing, with a focus on once-daily versus twice-daily dosing at 8 mg or 12 mg. Phase 3 studies are in the planning stage, he added.
The phase 2 dose-ranging study was sponsored by Concert Pharmaceuticals.
CTP-543 is one of an array of oral JAK inhibitors now in the developmental pipeline for alopecia areata, a severe, psychosocially devastating disease for which at present there is no Food and Drug Administration–approved therapy.
MADRID – In a phase 2, dose-ranging study, 78% of patients with long-standing moderate or severe alopecia areata rated their condition as “much improved” or “very much improved” after 24 weeks on the top dose of an investigational oral selective Janus kinase 1 and 2 (JAK1/2) inhibitor, compared with 21% of placebo-treated controls, James V. Cassella, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The primary study endpoint – at least a 50% reduction in the Severity of Alopecia Tool (SALT) score between baseline and 24 weeks – was achieved in 58% of patients on the JAK1/2 inhibitor (known for now as CTP-543) at 12 mg twice a day, 47% of those on CTP-543 at 8 mg twice a day, 21% on 4 mg twice a day, and in 9% of controls on placebo in the double-blind randomized trial. The 4-mg twice-daily dosing will not move on to phase 3 studies because of its demonstrated lack of efficacy, according to Dr. Cassella, chief development officer at Concert Pharmaceuticals, the study sponsor.
The study included 149 adults whose current episode of alopecia areata was of 3-6 years’ duration, with an average lifetime 15-year history of active disease. Their average baseline score on the 0-100 SALT scale was in the upper 80s, indicative of 80% or greater hair loss.
A SALT 75 response, meaning at least a 75% reduction in SALT score from baseline, was achieved in a dose-dependent fashion: In 42% of patients at the top dose of CTP-543, 29% of those on 8 mg twice a day, 14% with 4 mg twice a day, and in 7% of controls. An even more rigorous SALT 90 response was attained in 36%, 16%, 2%, and no controls, respectively.
The 12-mg twice-daily dosing produced faster onset and greater magnitude of response than did the 8-mg twice-daily dosing, but this dose-ranging study is not the final word on that score, according to Dr. Cassella.
“Week 24 is not a magic number,” he said. “The slope of the efficacy line for 8 mg [twice a day] looked like it was still going up at week 24, and the 12-mg BID curve hadn’t completely plateaued. Those are things we will consider for the future in terms of long-term trials.”
Changes in the eyebrows and lashes weren’t formally assessed in this study, although they will be in future trials. Anecdotally, however, patients with alopecia areata at those sites typically experienced complete or nearly complete regrowth in response to the higher doses of CTP-543, he said.
Safety-wise, there was no trend for increased adverse events with increasing doses of CTP-543. The observed treatment side effects were those typical of JAK inhibitors as a class effect, mainly headache, nasopharyngitis, upper respiratory infections, and new-onset acne. In terms of hematologic findings of special interest, there was one case of reversible neutropenia in the control group and another in the 8-mg twice-daily group, which resolved upon temporary suspension of treatment.
“Nothing surprising to us, and nothing very serious,” Dr. Cassella said.
Most patients in the 12-mg twice daily group have enrolled in an ongoing long-term extension study. In addition, two phase 2 studies are ongoing, with a focus on once-daily versus twice-daily dosing at 8 mg or 12 mg. Phase 3 studies are in the planning stage, he added.
The phase 2 dose-ranging study was sponsored by Concert Pharmaceuticals.
CTP-543 is one of an array of oral JAK inhibitors now in the developmental pipeline for alopecia areata, a severe, psychosocially devastating disease for which at present there is no Food and Drug Administration–approved therapy.
MADRID – In a phase 2, dose-ranging study, 78% of patients with long-standing moderate or severe alopecia areata rated their condition as “much improved” or “very much improved” after 24 weeks on the top dose of an investigational oral selective Janus kinase 1 and 2 (JAK1/2) inhibitor, compared with 21% of placebo-treated controls, James V. Cassella, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The primary study endpoint – at least a 50% reduction in the Severity of Alopecia Tool (SALT) score between baseline and 24 weeks – was achieved in 58% of patients on the JAK1/2 inhibitor (known for now as CTP-543) at 12 mg twice a day, 47% of those on CTP-543 at 8 mg twice a day, 21% on 4 mg twice a day, and in 9% of controls on placebo in the double-blind randomized trial. The 4-mg twice-daily dosing will not move on to phase 3 studies because of its demonstrated lack of efficacy, according to Dr. Cassella, chief development officer at Concert Pharmaceuticals, the study sponsor.
The study included 149 adults whose current episode of alopecia areata was of 3-6 years’ duration, with an average lifetime 15-year history of active disease. Their average baseline score on the 0-100 SALT scale was in the upper 80s, indicative of 80% or greater hair loss.
A SALT 75 response, meaning at least a 75% reduction in SALT score from baseline, was achieved in a dose-dependent fashion: In 42% of patients at the top dose of CTP-543, 29% of those on 8 mg twice a day, 14% with 4 mg twice a day, and in 7% of controls. An even more rigorous SALT 90 response was attained in 36%, 16%, 2%, and no controls, respectively.
The 12-mg twice-daily dosing produced faster onset and greater magnitude of response than did the 8-mg twice-daily dosing, but this dose-ranging study is not the final word on that score, according to Dr. Cassella.
“Week 24 is not a magic number,” he said. “The slope of the efficacy line for 8 mg [twice a day] looked like it was still going up at week 24, and the 12-mg BID curve hadn’t completely plateaued. Those are things we will consider for the future in terms of long-term trials.”
Changes in the eyebrows and lashes weren’t formally assessed in this study, although they will be in future trials. Anecdotally, however, patients with alopecia areata at those sites typically experienced complete or nearly complete regrowth in response to the higher doses of CTP-543, he said.
Safety-wise, there was no trend for increased adverse events with increasing doses of CTP-543. The observed treatment side effects were those typical of JAK inhibitors as a class effect, mainly headache, nasopharyngitis, upper respiratory infections, and new-onset acne. In terms of hematologic findings of special interest, there was one case of reversible neutropenia in the control group and another in the 8-mg twice-daily group, which resolved upon temporary suspension of treatment.
“Nothing surprising to us, and nothing very serious,” Dr. Cassella said.
Most patients in the 12-mg twice daily group have enrolled in an ongoing long-term extension study. In addition, two phase 2 studies are ongoing, with a focus on once-daily versus twice-daily dosing at 8 mg or 12 mg. Phase 3 studies are in the planning stage, he added.
The phase 2 dose-ranging study was sponsored by Concert Pharmaceuticals.
CTP-543 is one of an array of oral JAK inhibitors now in the developmental pipeline for alopecia areata, a severe, psychosocially devastating disease for which at present there is no Food and Drug Administration–approved therapy.
REPORTING FROM THE EADV CONGRESS
Central centrifugal cicatricial alopecia called epidemic in skin of color
NEW YORK – For unclear reasons, the prevalence of , creating an urgent need for early diagnosis and treatment, according to an expert who described this phenomenon at the Skin of Color Update 2019.
“CCCA has reached epidemic proportions,” contended Susan Taylor, MD, director of diversity, department of dermatology, Penn Medicine, Philadelphia.
Published data place prevalence rates of CCCA somewhere in the range of 3% to 6% among black women, but Dr. Taylor reported that she believes the condition is underdiagnosed. “I am seeing far more patients now than I was 30 years ago,” she maintained.
Others participating in the Skin of Color Update 2019 agreed. Heather Woolery-Lloyd, MD, director of ethnic skin care, department of dermatology and cutaneous surgery, University of Miami, also called the rising incidence of CCCA “an epidemic.” She, like Dr. Taylor, emphasized the critical importance of early diagnosis and treatment.
“I tell patients that if we can prevent hair loss over the next 10 years, this is a treatment success,” Dr. Woolery-Lloyd said. Although hair regrowth can be achieved in a minority of patients with treatments such as minoxidil, “the first goal is to prevent hair loss.”
Upon diagnosis, Dr. Woolery-Lloyd recommends treatment with intralesional triamcinolone and topical steroids immediately, adding other agents, such as oral antibiotics, if needed. Even in cases where CCCA has been identified before hair loss is visible, Dr. Woolery-Lloyd advised immediate therapy. Given that CCCA is a disease of reversible hair loss, she said, “do not take a wait-and-see approach.”
One potential obstacle for early diagnosis of CCCA, shared by other types of alopecia that are common in skin of color, is the failure of many clinicians to employ a standardized diagnosis in this patient population.
“If you do not have tightly coiled hair, it does not mean you cannot understand tightly coiled hair, but you have to learn, and you have to let patients know that you understand and have experience,” said Dr. Taylor, emphasizing the role of reassuring patients so they can be confident in the clinical recommendations.
Part of this reassurance will be derived from “interacting with patients in a culturally competent manner,” Dr. Taylor said. Clinicians must develop comfort and confidence in physically examining the hair and scalp, in asking patients to remove weaves or braids for a thorough inspection, and in avoiding comments that might be misinterpreted. Among these, she advised tactful questions about shampooing to avoid any implication that the clinician is implying inadequate hygiene.
When CCCA is suspected, a “biopsy is important” even in circumstances when the diagnosis seems straightforward. For one reason, a substantial proportion of patients may have a concomitant diagnosis. Dr. Taylor cited data from one study in which nearly 20% of CCCA patients also had traction alopecia and more than 10% had androgenic alopecia. Other coexisting problems identified on biopsy, including infection or seborrheic dermatitis, can help clinicians tailor a more effective intervention.
The initial signs of CCCA are typically hair breakage in the vertex of the scalp, which then expands in a central centrifugal pattern, according to Dr. Taylor. Although not all patients have signs of inflammation, such as itching and pustules, inhibition of inflammation represents the first line of therapy.
Relative to hair in the white population, the hair of black individuals grows more slowly and is more fragile, with greater amounts of breakage, said Dr. Taylor, citing published studies that support these differences. To improve early diagnosis of CCCA, understanding the hair in the black population is the first step for spotting problems in routine physical examinations. It may be this lack of familiarity that is contributing to underdiagnosis of CCCA.
“Almost 70% of African-American patients feel that physicians do not understand their hair,” Dr. Taylor said. “Let’s begin to change that.”
Cautioning that it is too often assumed that hairstyles and hair care, such as relaxants or hot combs, are the source of hair loss in black women, Dr. Taylor advised not to jump to conclusions. As an example, she described a case where weaves, braids, and other strategies were employed to mask alopecia after CCCA developed, not before.
“CCCA is the most important cause of scarring alopecia in African-American women,” Dr. Taylor said. Reiterating that hair loss can be prevented or modified with treatment, she added that this places the emphasis on first obtaining an accurate diagnosis.
Dr. Taylor has a financial relationship with Biersdorf; Dr. Woolery-Lloyd has financial relationships with Allergan, Galderma, Ortho Diagnostics, Pfizer, and Somabella Laboratories.
NEW YORK – For unclear reasons, the prevalence of , creating an urgent need for early diagnosis and treatment, according to an expert who described this phenomenon at the Skin of Color Update 2019.
“CCCA has reached epidemic proportions,” contended Susan Taylor, MD, director of diversity, department of dermatology, Penn Medicine, Philadelphia.
Published data place prevalence rates of CCCA somewhere in the range of 3% to 6% among black women, but Dr. Taylor reported that she believes the condition is underdiagnosed. “I am seeing far more patients now than I was 30 years ago,” she maintained.
Others participating in the Skin of Color Update 2019 agreed. Heather Woolery-Lloyd, MD, director of ethnic skin care, department of dermatology and cutaneous surgery, University of Miami, also called the rising incidence of CCCA “an epidemic.” She, like Dr. Taylor, emphasized the critical importance of early diagnosis and treatment.
“I tell patients that if we can prevent hair loss over the next 10 years, this is a treatment success,” Dr. Woolery-Lloyd said. Although hair regrowth can be achieved in a minority of patients with treatments such as minoxidil, “the first goal is to prevent hair loss.”
Upon diagnosis, Dr. Woolery-Lloyd recommends treatment with intralesional triamcinolone and topical steroids immediately, adding other agents, such as oral antibiotics, if needed. Even in cases where CCCA has been identified before hair loss is visible, Dr. Woolery-Lloyd advised immediate therapy. Given that CCCA is a disease of reversible hair loss, she said, “do not take a wait-and-see approach.”
One potential obstacle for early diagnosis of CCCA, shared by other types of alopecia that are common in skin of color, is the failure of many clinicians to employ a standardized diagnosis in this patient population.
“If you do not have tightly coiled hair, it does not mean you cannot understand tightly coiled hair, but you have to learn, and you have to let patients know that you understand and have experience,” said Dr. Taylor, emphasizing the role of reassuring patients so they can be confident in the clinical recommendations.
Part of this reassurance will be derived from “interacting with patients in a culturally competent manner,” Dr. Taylor said. Clinicians must develop comfort and confidence in physically examining the hair and scalp, in asking patients to remove weaves or braids for a thorough inspection, and in avoiding comments that might be misinterpreted. Among these, she advised tactful questions about shampooing to avoid any implication that the clinician is implying inadequate hygiene.
When CCCA is suspected, a “biopsy is important” even in circumstances when the diagnosis seems straightforward. For one reason, a substantial proportion of patients may have a concomitant diagnosis. Dr. Taylor cited data from one study in which nearly 20% of CCCA patients also had traction alopecia and more than 10% had androgenic alopecia. Other coexisting problems identified on biopsy, including infection or seborrheic dermatitis, can help clinicians tailor a more effective intervention.
The initial signs of CCCA are typically hair breakage in the vertex of the scalp, which then expands in a central centrifugal pattern, according to Dr. Taylor. Although not all patients have signs of inflammation, such as itching and pustules, inhibition of inflammation represents the first line of therapy.
Relative to hair in the white population, the hair of black individuals grows more slowly and is more fragile, with greater amounts of breakage, said Dr. Taylor, citing published studies that support these differences. To improve early diagnosis of CCCA, understanding the hair in the black population is the first step for spotting problems in routine physical examinations. It may be this lack of familiarity that is contributing to underdiagnosis of CCCA.
“Almost 70% of African-American patients feel that physicians do not understand their hair,” Dr. Taylor said. “Let’s begin to change that.”
Cautioning that it is too often assumed that hairstyles and hair care, such as relaxants or hot combs, are the source of hair loss in black women, Dr. Taylor advised not to jump to conclusions. As an example, she described a case where weaves, braids, and other strategies were employed to mask alopecia after CCCA developed, not before.
“CCCA is the most important cause of scarring alopecia in African-American women,” Dr. Taylor said. Reiterating that hair loss can be prevented or modified with treatment, she added that this places the emphasis on first obtaining an accurate diagnosis.
Dr. Taylor has a financial relationship with Biersdorf; Dr. Woolery-Lloyd has financial relationships with Allergan, Galderma, Ortho Diagnostics, Pfizer, and Somabella Laboratories.
NEW YORK – For unclear reasons, the prevalence of , creating an urgent need for early diagnosis and treatment, according to an expert who described this phenomenon at the Skin of Color Update 2019.
“CCCA has reached epidemic proportions,” contended Susan Taylor, MD, director of diversity, department of dermatology, Penn Medicine, Philadelphia.
Published data place prevalence rates of CCCA somewhere in the range of 3% to 6% among black women, but Dr. Taylor reported that she believes the condition is underdiagnosed. “I am seeing far more patients now than I was 30 years ago,” she maintained.
Others participating in the Skin of Color Update 2019 agreed. Heather Woolery-Lloyd, MD, director of ethnic skin care, department of dermatology and cutaneous surgery, University of Miami, also called the rising incidence of CCCA “an epidemic.” She, like Dr. Taylor, emphasized the critical importance of early diagnosis and treatment.
“I tell patients that if we can prevent hair loss over the next 10 years, this is a treatment success,” Dr. Woolery-Lloyd said. Although hair regrowth can be achieved in a minority of patients with treatments such as minoxidil, “the first goal is to prevent hair loss.”
Upon diagnosis, Dr. Woolery-Lloyd recommends treatment with intralesional triamcinolone and topical steroids immediately, adding other agents, such as oral antibiotics, if needed. Even in cases where CCCA has been identified before hair loss is visible, Dr. Woolery-Lloyd advised immediate therapy. Given that CCCA is a disease of reversible hair loss, she said, “do not take a wait-and-see approach.”
One potential obstacle for early diagnosis of CCCA, shared by other types of alopecia that are common in skin of color, is the failure of many clinicians to employ a standardized diagnosis in this patient population.
“If you do not have tightly coiled hair, it does not mean you cannot understand tightly coiled hair, but you have to learn, and you have to let patients know that you understand and have experience,” said Dr. Taylor, emphasizing the role of reassuring patients so they can be confident in the clinical recommendations.
Part of this reassurance will be derived from “interacting with patients in a culturally competent manner,” Dr. Taylor said. Clinicians must develop comfort and confidence in physically examining the hair and scalp, in asking patients to remove weaves or braids for a thorough inspection, and in avoiding comments that might be misinterpreted. Among these, she advised tactful questions about shampooing to avoid any implication that the clinician is implying inadequate hygiene.
When CCCA is suspected, a “biopsy is important” even in circumstances when the diagnosis seems straightforward. For one reason, a substantial proportion of patients may have a concomitant diagnosis. Dr. Taylor cited data from one study in which nearly 20% of CCCA patients also had traction alopecia and more than 10% had androgenic alopecia. Other coexisting problems identified on biopsy, including infection or seborrheic dermatitis, can help clinicians tailor a more effective intervention.
The initial signs of CCCA are typically hair breakage in the vertex of the scalp, which then expands in a central centrifugal pattern, according to Dr. Taylor. Although not all patients have signs of inflammation, such as itching and pustules, inhibition of inflammation represents the first line of therapy.
Relative to hair in the white population, the hair of black individuals grows more slowly and is more fragile, with greater amounts of breakage, said Dr. Taylor, citing published studies that support these differences. To improve early diagnosis of CCCA, understanding the hair in the black population is the first step for spotting problems in routine physical examinations. It may be this lack of familiarity that is contributing to underdiagnosis of CCCA.
“Almost 70% of African-American patients feel that physicians do not understand their hair,” Dr. Taylor said. “Let’s begin to change that.”
Cautioning that it is too often assumed that hairstyles and hair care, such as relaxants or hot combs, are the source of hair loss in black women, Dr. Taylor advised not to jump to conclusions. As an example, she described a case where weaves, braids, and other strategies were employed to mask alopecia after CCCA developed, not before.
“CCCA is the most important cause of scarring alopecia in African-American women,” Dr. Taylor said. Reiterating that hair loss can be prevented or modified with treatment, she added that this places the emphasis on first obtaining an accurate diagnosis.
Dr. Taylor has a financial relationship with Biersdorf; Dr. Woolery-Lloyd has financial relationships with Allergan, Galderma, Ortho Diagnostics, Pfizer, and Somabella Laboratories.
REPORTING FROM SOC 2019
The Role of Vitamins and Supplements on Skin Appearance
As the largest and most exposed organ in the body, the skin experiences trauma from both extrinsic and intrinsic aging factors, resulting in loss of elasticity, increased laxity, wrinkling, and rough-textured appearance.1 Chronologically aged skin appears dry, thin, and finely wrinkled; photoaged skin appears leathery with coarse wrinkles and uneven pigmentation.2 In recent years, numerous systemic nutrients have been proposed to improve skin appearance. This article reviews the efficacy of these vitamins and supplements.
Carotenoids
Carotenoids are a group of lipophilic molecules derived from vitamin A.3,4 Ingestion of carotenoids may play a role in photoprotection against UV radiation (UVR) by acting as acceptors of reactive oxygen species.4-6 Stahl et al7 investigated lycopene’s usefulness in protection against UVR-induced erythema. Over 10 weeks, 9 volunteers received 40 g of tomato paste containing 16 mg daily of lycopene while 10 controls received placebo. A solar simulator was used to induce erythema of the skin at weeks 0, 4, and 10. At week 10, erythema formation was 40% lower in the lycopene group compared to controls (P=.02).7
In another study assessing the photoprotective effects of a novel nutritional and phytonutrient blend of carotenoids, 36 women with Fitzpatrick skin types I and II were treated for 8 weeks.8 Presupplementation, UVR-induced erythema, and skin carotenoid concentrations were determined along with facial skin attributes and characteristics. Results showed protection against UVR-induced skin damage, with reductions in erythema at 3 minimal erythema doses (MEDs)(P=.01). Additionally, significant improvements were noted in facial skin elasticity, radiance, and overall appearance (all P<.05).8
In 2013, Meinke et al9 conducted an 8-week, double-blind, placebo-controlled study on 24 volunteers whose diets were supplemented with moderate amounts of carotenoids, including lutein, beta-carotene, and lycopene. Utilizing novel techniques to measure the skin’s ability to scavenge free radicals, they discovered that dietary carotenoids provided notable protection against stress-induced radical formation and increased baseline radical scavenging activity of the skin by 34%. The authors concluded that dietary supplementation could avoid premature skin aging.9
Vitamins C and E
Vitamin C is an essential vitamin that must be obtained through dietary sources.10 It functions as a free radical scavenger and is a necessary cofactor for the synthesis and stabilization of collagen.
A study evaluated the effect of UVR-induced oxidative stress and the association with vitamin C supplementation among 20 white patients with Fitzpatrick skin types II or III.11 The volunteers were treated with UVR on two 1-cm sites on the buttock. Six punch biopsies of these sites and 2 control biopsies from nonexposed skin were taken. Volunteers took vitamin C supplements (500 mg) for 8 weeks, and the exposure and biopsy were repeated. Researchers concluded that supplementation with vitamin C had no effect on the MED, with identical concentrations at baseline and after 8 weeks of supplementation. Additionally, there was no evidence that vitamin C affects UVR-induced oxidative stress.11
In 2007, Cosgrove et al12 conducted a study to assess the associations between nutrient intake and skin aging in more than 4000 women aged 40 to 74 years. Higher dietary vitamin C intakes were associated with a significantly lower likelihood of senile xerosis and wrinkled appearance (P<.009).12
Vitamin E is a lipid-soluble, membrane-bound vitamin, and its most active form is α-tocopherol.11,13 Vitamin E functions as an antioxidant and protects cellular membranes from lipid peroxidation by free radicals.13-15 Once oxidized, vitamin E can be regenerated to its reduced form by vitamin C.11 Their synergistic effects on skin protection have been studied extensively. A double-blind, placebo-controlled study of 10 patients compared 2 g of vitamin C combined with 1000 IU of vitamin E vs placebo.16 The patients’ skin reaction before and after 8 days of treatment were assessed by determination of MED and the cutaneous blood flow of skin irradiated with UV light. Results showed that the median MED of those taking vitamins increased from 80 to 96.5 mJ/cm2 (P<.01) and decreased for the placebo group. Investigators concluded that the combination of vitamins C and E reduces the sunburn reaction and leads to a reduction in the sequelae of UV-induced skin damage.16 A prospective, randomized, placebo-controlled study by Fuchs and Kern17 replicated these findings, also concluding that combinations of vitamins C and E provide improved photoprotective effects than either vitamin alone.
Vitamin D
Vitamin D is a fat-soluble vitamin obtained through dietary intake and exposure to UV light.3,18,19 Precursors of vitamin D require interaction with UV light for conversion into active forms. The highest concentrations of 7-dehydrocholesterol are found in keratinocytes in the basal cell and spinous cell layers of the skin where they are protected from UV light by melanin. As such, individuals with higher melanin content in their skin require more exposure to UV light to produce the same levels of vitamin D as those with less melanin,20 leading to a high rate of vitamin D deficiency in dark-skinned individuals. Because of their prodifferentiating and antiproliferative effects, vitamin D analogs have been very effective in the treatment of psoriasis.20,21 Vitamin D deficiency also has been implicated in the pathogenesis of vitiligo. A systematic review and meta-analysis conducted in 2016 found that a significant relationship existed between low 25-hydroxyvitamin D levels and vitiligo (P<.01), but no causal relationship could be established.22
A 2017 double-blind, placebo-controlled study performed by Scott et al23 aimed to elucidate the relationship between vitamin D concentrations and sunburn. Twenty adults received either placebo or high-dose vitamin D3 (200,000 IU) 1 hour after experimental sunburn induced by an erythemogenic dose of UVR. Investigators measured participants’ concentrations of the proinflammatory mediators tumor necrosis factor α and nitric oxide synthase via skin biopsy 48 hours later. Patients in the experimental group were found to have significantly reduced expression of both tumor necrosis factor α (P=.04) and nitric oxide synthase (P=.02). Additionally, participants with significantly higher vitamin D3 levels following supplementation (P=.007) demonstrated increased skin expression of the anti-inflammatory marker arginase-1 (P=.005) as well as a persistent reduction in skin redness (P=.02). Investigators concluded that vitamin D plays a large role in skin homeostasis and implicated vitamin D’s upregulation of arginase-1 as a potent mechanism of its anti-inflammatory effects.23
Collagen
As humans age, the density of collagen in the dermis decreases, leading to sagging and wrinkling of skin.24 Oral supplementation of collagen has been examined for its dermatologic benefits, primarily increasing the thickness and density of collagen in the dermal layer. In 2014, Proksch et al25 performed a double-blind, placebo-controlled trial in which 69 women were randomized to receive 2.5 or 5 g of collagen peptides or placebo for 8 weeks. Both treatment groups demonstrated improvements in skin elasticity as well as improved skin moisture and decreased skin evaporation; however, changes in the latter 2 qualities failed to reach statistical significance.25
The results of this study were replicated by Asserin et al.26 One hundred six female patients were randomly assigned to receive 10 g of collagen peptides or placebo daily for 8 weeks. The collagen group demonstrated significantly improved skin hydration (P=.003) and increased density of collagen in the dermis (P=.007) relative to placebo.26
In another randomized, double-blind, placebo-controlled study, 71 women consumed a 20-mL beverage containing either 3000 mg of collagen peptides or placebo for 12 weeks.27 Participants in the treatment group demonstrated significant decreases in periorbital wrinkles (P<.05) and enhanced facial skin moisture (P<.001) and elasticity (P<.001) after 12 weeks. Researchers concluded that oral supplementation with collagen peptides holds promise as a natural supplement to provide cutaneous antiaging properties.27
Ceramides
Ceramides are lipids composed of a sphingoid base conjugated to a fatty acid and serve as the main component of the stratum corneum of the skin. Ceramides are crucial for the maintenance of skin barrier integrity and for preventing transepidermal water loss.28 In a 3-month study of 51 women with dry skin, Guillou et al29 showed that a ceramide wheat extract capsule significantly increased corneometry measurements of skin hydration on the arms (P<.001) and the legs (P=.012) compared to placebo.
Mixed Supplements
The discovery that nutritional contents can affect skin appearance has energized the development of combination supplements containing multiple vitamins and micronutrients. Imedeen is a biomarine complex and antioxidant supplement with several different formulations, including Prime Renewal, Time Perfection, and Derma One (Pfizer Inc). The ingredients include a combination of a biomarine complex (blend of fish proteins and polysaccharides), lycopene, grape seed extract, vitamin C, vitamin E, and zinc. Several trials have been conducted to assess the efficacy of the supplements on improving the appearance of photodamaged and aged skin (Table).
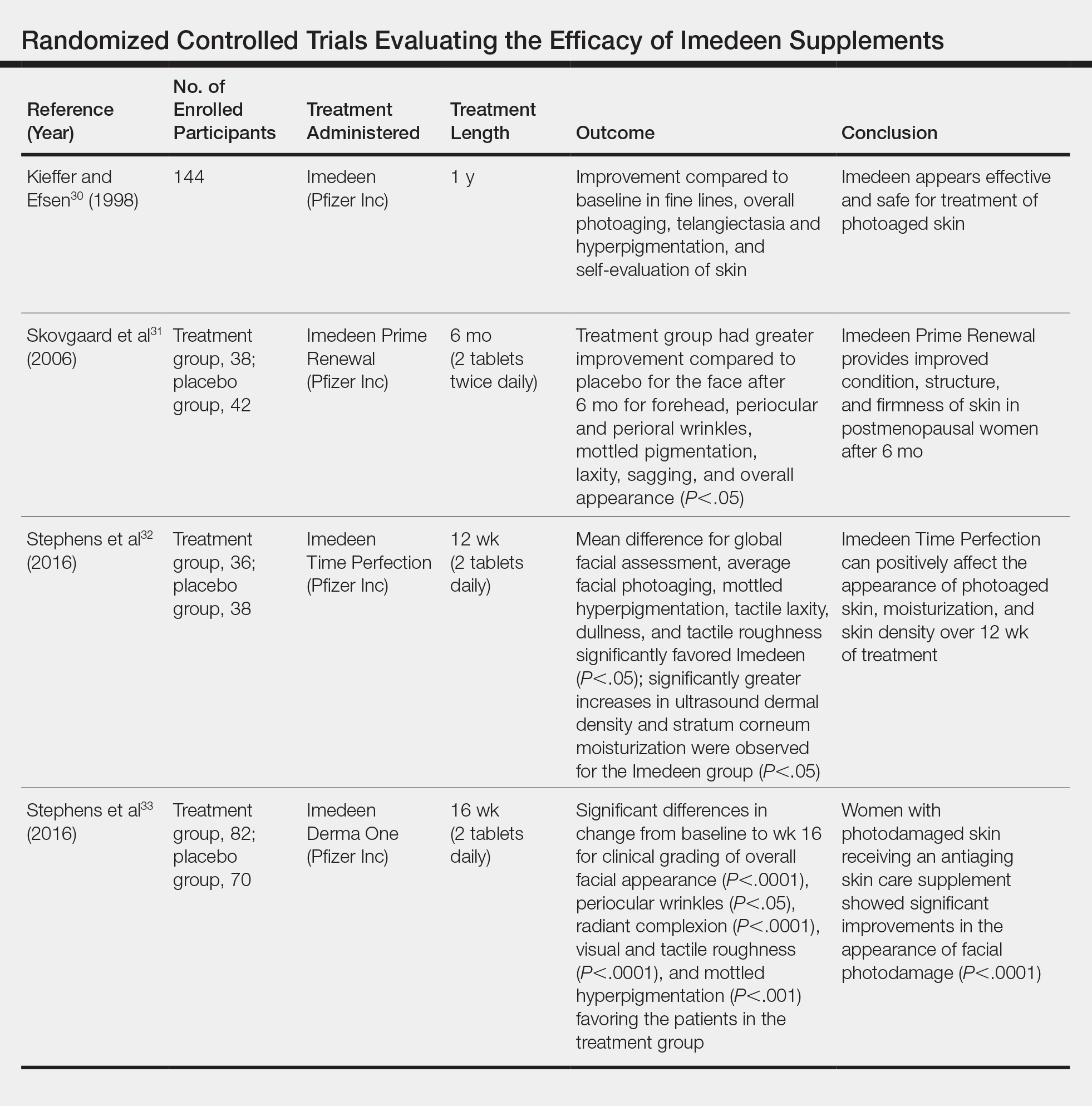
A placebo-controlled, randomized study of 144 participants conducted by Kieffer and Efsen30 assessed the efficacy of Imedeen supplements over 12 months. The trial included a 3-month placebo-controlled study and 9-month uncontrolled continuation. Imedeen’s efficacy was measured using clinical evaluation, transepidermal water loss, self-evaluation, and photograph evaluation. After 1 year of treatment, improvement occurred in photograph evaluation of fine lines, overall photoaging, telangiectasia and hyperpigmentation, and self-evaluation of skin condition.30 Additional double-blind, placebo-controlled, randomized studies assessing the efficacy of Imedeen have shown increased dermal and epidermal thickness, improvement of stratum corneum moisturization, and improved overall facial complexion.31-33
Several combined supplements containing collagen peptide as the main ingredient have been created for use in skin care. Collagen is found in the extracellular matrix of the dermis and is responsible for the resiliency and strength of skin.34,35 Damage to the dermis can occur with prolonged UV light exposure and is seen histologically as disorganized collagen fibrils and grossly as wrinkles and photoaged skin.35,36
A study assessed the effect of BioCell Collagen (BioCell Technology, LLC), a supplement containing type II collagen, on skin aging.37 Twenty-six women underwent baseline visual assessments of their skin before taking 2 tablets of the supplement daily. Twelve weeks of supplementation led to significant reduction in global lines and wrinkles (13.2%; P=.028) as well as skin dryness and scaling (76%; P=.002). Assessment of collagen content at 6 weeks revealed a significant increase from baseline (6.3%; P=.002), though the difference after 12 weeks was not significant (3.5%; P=.134). The authors concluded that although preliminary data suggested that BioCell Collagen may reduce visible signs of aging, a controlled study was necessary to verify this finding.37
A single-blind, case-controlled study assessed a similar supplement, Celergen, that contained marine collagen peptides.38 Forty-one adults took 2 capsules each day for 60 days. Assessment of their skin physiology was conducted at the enrollment visit, 2 months later, and after the treatment period ended. Skin elasticity, transepidermal water loss, epidermal and dermal thickness, and density were measured. Investigators found that Celergen administration significantly enhanced skin elasticity and sebum production (P<.0001) but did not influence cutaneous moisture. The dermal thickness and homogenous distribution of collagen fibers were enhanced in 11 patients while properties of the epidermis remained unchanged. The study determined that supplementation remarkably improved skin elasticity, sebum production, and dermal ultrasonic markers.38
A double-blind, randomized, placebo-controlled study assessed a collagen- and antioxidant-containing supplement, Gold Collagen Forte, on skin properties.39 The treatment and placebo groups each consisted of 60 patients who consumed 1 bottle (50 mL) of the product each day for 90 days. Patients completed a self-assessment of their skin regarding photoaging, focusing on the crow’s-feet area and nasolabial folds, while skin elasticity was assessed with the SkinLab USB elasticity module. Results showed a significant increase in skin elasticity (+7.5%; P≤.001). Self-assessment results showed improvements in both the treatment and placebo groups, and investigators concluded that Gold Collagen Forte may have photoprotective effects and help improve skin health.39
Safety
Although trials have demonstrated vitamin supplementation to be safe and effective for skin enhancement, it is important to consider potential vitamin toxicities. High doses of vitamin C supplementation have been shown to cause damage via lipid peroxidation.40 In a study assessing if high levels of beta-carotene and vitamin E were associated with a lower risk for lung cancer, data showed that these supplements may actually have harmful effects.40,41 Additionally, consumption of high-dose dietary supplements has been associated with an increased risk for severe medical events, including disability and death among adolescents and young adults.42
Conclusion
Numerous trials have indicated that the use of systemic vitamins can have beneficial effects on the protection and appearance of skin. Photodamage from UV light–induced erythema can be decreased by carotenoids and vitamins C and E. Similarly, supplements that combine multiple nutrients with collagen have been shown to improve the appearance of aging skin by decreasing the prominence of wrinkles. Given the growing number of products and advertisements that exist in the supplement marketplace, it is crucial for clinicians to ground their recommendations to patients in the scientific data of robust studies.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729-738.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370.
- Draelos ZD. Nutrition and enhancing youthful-appearing skin. Clin Dermatol. 2010;28:400-408.
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51-54.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Anstey AV. Systemic photoprotection with alpha-tocopherol (vitamin E) and beta-carotene. Clin Exp Dermatol. 2002;27:170-176.
- Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449-1451.
- Wood SM, Mastaloudis AF, Hester SN, et al. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J Cosmet Dermatol. 2017;16:491-499.
- Meinke MC, Friedrich A, Tscherch K, et al. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013;84:365-373.
- Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27:469-474.
- McArdle F, Rhodes LE, Parslew R, et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic Biol Med. 2002;33:1355-1362.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86:1225-1231.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667.
- Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298-307.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725-731.
- Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998;38:45-48.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006-1012.
- Shahriari M, Kerr PE, Slade K, et al. Vitamin D and the skin. Clin Dermatol. 2010;28:663-668.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54:383-392.
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(suppl 4):11-15.
- Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30:137-145.
- Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2016;32:181-190.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-1868.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291-301.
- Koizumi S, Inoue N, Shimizu M, et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int J Peptide Res Ther. 2018;24:397-402.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103059.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33:138-143.
- Kieffer ME, Efsen J. Imedeen in the treatment of photoaged skin: an efficacy and safety trial over 12 months. J Eur Acad Dermatol Venereol. 1998;11:129-136.
- Skovgaard GR, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60:1201-1206.
- Stephens TJ, Sigler ML, Herndon JH Jr, et al. A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin. Clin Cosmet Investig Dermatol. 2016;9:63-70.
- Stephens TJ, Sigler ML, Hino PD, et al. A randomized, double-blind, placebo-controlled clinical trial evaluating an oral anti-aging skin care supplement for treating photodamaged skin. J Clin Aesthet Dermatol. 2016;9:25-32.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Kang MC, Yumnam S, Kim SY. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19113551.
- Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267-273.
- De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410.
- Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30:146-158.
- Hamishehkar H, Ranjdoost F, Asgharian P, et al. Vitamins, are they safe? Adv Pharm Bull. 2016;6:467-477.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029-1035.
- Or F, Yongjoo K, Simms J, et al. Taking stock of dietary supplements’ harmful effects on children, adolescents, and young adults [published online June 3, 2019]. J Adolesc Health. S1054-139X(19)30163-6. doi:10.1016/j.jadohealth.2019.03.005.
As the largest and most exposed organ in the body, the skin experiences trauma from both extrinsic and intrinsic aging factors, resulting in loss of elasticity, increased laxity, wrinkling, and rough-textured appearance.1 Chronologically aged skin appears dry, thin, and finely wrinkled; photoaged skin appears leathery with coarse wrinkles and uneven pigmentation.2 In recent years, numerous systemic nutrients have been proposed to improve skin appearance. This article reviews the efficacy of these vitamins and supplements.
Carotenoids
Carotenoids are a group of lipophilic molecules derived from vitamin A.3,4 Ingestion of carotenoids may play a role in photoprotection against UV radiation (UVR) by acting as acceptors of reactive oxygen species.4-6 Stahl et al7 investigated lycopene’s usefulness in protection against UVR-induced erythema. Over 10 weeks, 9 volunteers received 40 g of tomato paste containing 16 mg daily of lycopene while 10 controls received placebo. A solar simulator was used to induce erythema of the skin at weeks 0, 4, and 10. At week 10, erythema formation was 40% lower in the lycopene group compared to controls (P=.02).7
In another study assessing the photoprotective effects of a novel nutritional and phytonutrient blend of carotenoids, 36 women with Fitzpatrick skin types I and II were treated for 8 weeks.8 Presupplementation, UVR-induced erythema, and skin carotenoid concentrations were determined along with facial skin attributes and characteristics. Results showed protection against UVR-induced skin damage, with reductions in erythema at 3 minimal erythema doses (MEDs)(P=.01). Additionally, significant improvements were noted in facial skin elasticity, radiance, and overall appearance (all P<.05).8
In 2013, Meinke et al9 conducted an 8-week, double-blind, placebo-controlled study on 24 volunteers whose diets were supplemented with moderate amounts of carotenoids, including lutein, beta-carotene, and lycopene. Utilizing novel techniques to measure the skin’s ability to scavenge free radicals, they discovered that dietary carotenoids provided notable protection against stress-induced radical formation and increased baseline radical scavenging activity of the skin by 34%. The authors concluded that dietary supplementation could avoid premature skin aging.9
Vitamins C and E
Vitamin C is an essential vitamin that must be obtained through dietary sources.10 It functions as a free radical scavenger and is a necessary cofactor for the synthesis and stabilization of collagen.
A study evaluated the effect of UVR-induced oxidative stress and the association with vitamin C supplementation among 20 white patients with Fitzpatrick skin types II or III.11 The volunteers were treated with UVR on two 1-cm sites on the buttock. Six punch biopsies of these sites and 2 control biopsies from nonexposed skin were taken. Volunteers took vitamin C supplements (500 mg) for 8 weeks, and the exposure and biopsy were repeated. Researchers concluded that supplementation with vitamin C had no effect on the MED, with identical concentrations at baseline and after 8 weeks of supplementation. Additionally, there was no evidence that vitamin C affects UVR-induced oxidative stress.11
In 2007, Cosgrove et al12 conducted a study to assess the associations between nutrient intake and skin aging in more than 4000 women aged 40 to 74 years. Higher dietary vitamin C intakes were associated with a significantly lower likelihood of senile xerosis and wrinkled appearance (P<.009).12
Vitamin E is a lipid-soluble, membrane-bound vitamin, and its most active form is α-tocopherol.11,13 Vitamin E functions as an antioxidant and protects cellular membranes from lipid peroxidation by free radicals.13-15 Once oxidized, vitamin E can be regenerated to its reduced form by vitamin C.11 Their synergistic effects on skin protection have been studied extensively. A double-blind, placebo-controlled study of 10 patients compared 2 g of vitamin C combined with 1000 IU of vitamin E vs placebo.16 The patients’ skin reaction before and after 8 days of treatment were assessed by determination of MED and the cutaneous blood flow of skin irradiated with UV light. Results showed that the median MED of those taking vitamins increased from 80 to 96.5 mJ/cm2 (P<.01) and decreased for the placebo group. Investigators concluded that the combination of vitamins C and E reduces the sunburn reaction and leads to a reduction in the sequelae of UV-induced skin damage.16 A prospective, randomized, placebo-controlled study by Fuchs and Kern17 replicated these findings, also concluding that combinations of vitamins C and E provide improved photoprotective effects than either vitamin alone.
Vitamin D
Vitamin D is a fat-soluble vitamin obtained through dietary intake and exposure to UV light.3,18,19 Precursors of vitamin D require interaction with UV light for conversion into active forms. The highest concentrations of 7-dehydrocholesterol are found in keratinocytes in the basal cell and spinous cell layers of the skin where they are protected from UV light by melanin. As such, individuals with higher melanin content in their skin require more exposure to UV light to produce the same levels of vitamin D as those with less melanin,20 leading to a high rate of vitamin D deficiency in dark-skinned individuals. Because of their prodifferentiating and antiproliferative effects, vitamin D analogs have been very effective in the treatment of psoriasis.20,21 Vitamin D deficiency also has been implicated in the pathogenesis of vitiligo. A systematic review and meta-analysis conducted in 2016 found that a significant relationship existed between low 25-hydroxyvitamin D levels and vitiligo (P<.01), but no causal relationship could be established.22
A 2017 double-blind, placebo-controlled study performed by Scott et al23 aimed to elucidate the relationship between vitamin D concentrations and sunburn. Twenty adults received either placebo or high-dose vitamin D3 (200,000 IU) 1 hour after experimental sunburn induced by an erythemogenic dose of UVR. Investigators measured participants’ concentrations of the proinflammatory mediators tumor necrosis factor α and nitric oxide synthase via skin biopsy 48 hours later. Patients in the experimental group were found to have significantly reduced expression of both tumor necrosis factor α (P=.04) and nitric oxide synthase (P=.02). Additionally, participants with significantly higher vitamin D3 levels following supplementation (P=.007) demonstrated increased skin expression of the anti-inflammatory marker arginase-1 (P=.005) as well as a persistent reduction in skin redness (P=.02). Investigators concluded that vitamin D plays a large role in skin homeostasis and implicated vitamin D’s upregulation of arginase-1 as a potent mechanism of its anti-inflammatory effects.23
Collagen
As humans age, the density of collagen in the dermis decreases, leading to sagging and wrinkling of skin.24 Oral supplementation of collagen has been examined for its dermatologic benefits, primarily increasing the thickness and density of collagen in the dermal layer. In 2014, Proksch et al25 performed a double-blind, placebo-controlled trial in which 69 women were randomized to receive 2.5 or 5 g of collagen peptides or placebo for 8 weeks. Both treatment groups demonstrated improvements in skin elasticity as well as improved skin moisture and decreased skin evaporation; however, changes in the latter 2 qualities failed to reach statistical significance.25
The results of this study were replicated by Asserin et al.26 One hundred six female patients were randomly assigned to receive 10 g of collagen peptides or placebo daily for 8 weeks. The collagen group demonstrated significantly improved skin hydration (P=.003) and increased density of collagen in the dermis (P=.007) relative to placebo.26
In another randomized, double-blind, placebo-controlled study, 71 women consumed a 20-mL beverage containing either 3000 mg of collagen peptides or placebo for 12 weeks.27 Participants in the treatment group demonstrated significant decreases in periorbital wrinkles (P<.05) and enhanced facial skin moisture (P<.001) and elasticity (P<.001) after 12 weeks. Researchers concluded that oral supplementation with collagen peptides holds promise as a natural supplement to provide cutaneous antiaging properties.27
Ceramides
Ceramides are lipids composed of a sphingoid base conjugated to a fatty acid and serve as the main component of the stratum corneum of the skin. Ceramides are crucial for the maintenance of skin barrier integrity and for preventing transepidermal water loss.28 In a 3-month study of 51 women with dry skin, Guillou et al29 showed that a ceramide wheat extract capsule significantly increased corneometry measurements of skin hydration on the arms (P<.001) and the legs (P=.012) compared to placebo.
Mixed Supplements
The discovery that nutritional contents can affect skin appearance has energized the development of combination supplements containing multiple vitamins and micronutrients. Imedeen is a biomarine complex and antioxidant supplement with several different formulations, including Prime Renewal, Time Perfection, and Derma One (Pfizer Inc). The ingredients include a combination of a biomarine complex (blend of fish proteins and polysaccharides), lycopene, grape seed extract, vitamin C, vitamin E, and zinc. Several trials have been conducted to assess the efficacy of the supplements on improving the appearance of photodamaged and aged skin (Table).

A placebo-controlled, randomized study of 144 participants conducted by Kieffer and Efsen30 assessed the efficacy of Imedeen supplements over 12 months. The trial included a 3-month placebo-controlled study and 9-month uncontrolled continuation. Imedeen’s efficacy was measured using clinical evaluation, transepidermal water loss, self-evaluation, and photograph evaluation. After 1 year of treatment, improvement occurred in photograph evaluation of fine lines, overall photoaging, telangiectasia and hyperpigmentation, and self-evaluation of skin condition.30 Additional double-blind, placebo-controlled, randomized studies assessing the efficacy of Imedeen have shown increased dermal and epidermal thickness, improvement of stratum corneum moisturization, and improved overall facial complexion.31-33
Several combined supplements containing collagen peptide as the main ingredient have been created for use in skin care. Collagen is found in the extracellular matrix of the dermis and is responsible for the resiliency and strength of skin.34,35 Damage to the dermis can occur with prolonged UV light exposure and is seen histologically as disorganized collagen fibrils and grossly as wrinkles and photoaged skin.35,36
A study assessed the effect of BioCell Collagen (BioCell Technology, LLC), a supplement containing type II collagen, on skin aging.37 Twenty-six women underwent baseline visual assessments of their skin before taking 2 tablets of the supplement daily. Twelve weeks of supplementation led to significant reduction in global lines and wrinkles (13.2%; P=.028) as well as skin dryness and scaling (76%; P=.002). Assessment of collagen content at 6 weeks revealed a significant increase from baseline (6.3%; P=.002), though the difference after 12 weeks was not significant (3.5%; P=.134). The authors concluded that although preliminary data suggested that BioCell Collagen may reduce visible signs of aging, a controlled study was necessary to verify this finding.37
A single-blind, case-controlled study assessed a similar supplement, Celergen, that contained marine collagen peptides.38 Forty-one adults took 2 capsules each day for 60 days. Assessment of their skin physiology was conducted at the enrollment visit, 2 months later, and after the treatment period ended. Skin elasticity, transepidermal water loss, epidermal and dermal thickness, and density were measured. Investigators found that Celergen administration significantly enhanced skin elasticity and sebum production (P<.0001) but did not influence cutaneous moisture. The dermal thickness and homogenous distribution of collagen fibers were enhanced in 11 patients while properties of the epidermis remained unchanged. The study determined that supplementation remarkably improved skin elasticity, sebum production, and dermal ultrasonic markers.38
A double-blind, randomized, placebo-controlled study assessed a collagen- and antioxidant-containing supplement, Gold Collagen Forte, on skin properties.39 The treatment and placebo groups each consisted of 60 patients who consumed 1 bottle (50 mL) of the product each day for 90 days. Patients completed a self-assessment of their skin regarding photoaging, focusing on the crow’s-feet area and nasolabial folds, while skin elasticity was assessed with the SkinLab USB elasticity module. Results showed a significant increase in skin elasticity (+7.5%; P≤.001). Self-assessment results showed improvements in both the treatment and placebo groups, and investigators concluded that Gold Collagen Forte may have photoprotective effects and help improve skin health.39
Safety
Although trials have demonstrated vitamin supplementation to be safe and effective for skin enhancement, it is important to consider potential vitamin toxicities. High doses of vitamin C supplementation have been shown to cause damage via lipid peroxidation.40 In a study assessing if high levels of beta-carotene and vitamin E were associated with a lower risk for lung cancer, data showed that these supplements may actually have harmful effects.40,41 Additionally, consumption of high-dose dietary supplements has been associated with an increased risk for severe medical events, including disability and death among adolescents and young adults.42
Conclusion
Numerous trials have indicated that the use of systemic vitamins can have beneficial effects on the protection and appearance of skin. Photodamage from UV light–induced erythema can be decreased by carotenoids and vitamins C and E. Similarly, supplements that combine multiple nutrients with collagen have been shown to improve the appearance of aging skin by decreasing the prominence of wrinkles. Given the growing number of products and advertisements that exist in the supplement marketplace, it is crucial for clinicians to ground their recommendations to patients in the scientific data of robust studies.
As the largest and most exposed organ in the body, the skin experiences trauma from both extrinsic and intrinsic aging factors, resulting in loss of elasticity, increased laxity, wrinkling, and rough-textured appearance.1 Chronologically aged skin appears dry, thin, and finely wrinkled; photoaged skin appears leathery with coarse wrinkles and uneven pigmentation.2 In recent years, numerous systemic nutrients have been proposed to improve skin appearance. This article reviews the efficacy of these vitamins and supplements.
Carotenoids
Carotenoids are a group of lipophilic molecules derived from vitamin A.3,4 Ingestion of carotenoids may play a role in photoprotection against UV radiation (UVR) by acting as acceptors of reactive oxygen species.4-6 Stahl et al7 investigated lycopene’s usefulness in protection against UVR-induced erythema. Over 10 weeks, 9 volunteers received 40 g of tomato paste containing 16 mg daily of lycopene while 10 controls received placebo. A solar simulator was used to induce erythema of the skin at weeks 0, 4, and 10. At week 10, erythema formation was 40% lower in the lycopene group compared to controls (P=.02).7
In another study assessing the photoprotective effects of a novel nutritional and phytonutrient blend of carotenoids, 36 women with Fitzpatrick skin types I and II were treated for 8 weeks.8 Presupplementation, UVR-induced erythema, and skin carotenoid concentrations were determined along with facial skin attributes and characteristics. Results showed protection against UVR-induced skin damage, with reductions in erythema at 3 minimal erythema doses (MEDs)(P=.01). Additionally, significant improvements were noted in facial skin elasticity, radiance, and overall appearance (all P<.05).8
In 2013, Meinke et al9 conducted an 8-week, double-blind, placebo-controlled study on 24 volunteers whose diets were supplemented with moderate amounts of carotenoids, including lutein, beta-carotene, and lycopene. Utilizing novel techniques to measure the skin’s ability to scavenge free radicals, they discovered that dietary carotenoids provided notable protection against stress-induced radical formation and increased baseline radical scavenging activity of the skin by 34%. The authors concluded that dietary supplementation could avoid premature skin aging.9
Vitamins C and E
Vitamin C is an essential vitamin that must be obtained through dietary sources.10 It functions as a free radical scavenger and is a necessary cofactor for the synthesis and stabilization of collagen.
A study evaluated the effect of UVR-induced oxidative stress and the association with vitamin C supplementation among 20 white patients with Fitzpatrick skin types II or III.11 The volunteers were treated with UVR on two 1-cm sites on the buttock. Six punch biopsies of these sites and 2 control biopsies from nonexposed skin were taken. Volunteers took vitamin C supplements (500 mg) for 8 weeks, and the exposure and biopsy were repeated. Researchers concluded that supplementation with vitamin C had no effect on the MED, with identical concentrations at baseline and after 8 weeks of supplementation. Additionally, there was no evidence that vitamin C affects UVR-induced oxidative stress.11
In 2007, Cosgrove et al12 conducted a study to assess the associations between nutrient intake and skin aging in more than 4000 women aged 40 to 74 years. Higher dietary vitamin C intakes were associated with a significantly lower likelihood of senile xerosis and wrinkled appearance (P<.009).12
Vitamin E is a lipid-soluble, membrane-bound vitamin, and its most active form is α-tocopherol.11,13 Vitamin E functions as an antioxidant and protects cellular membranes from lipid peroxidation by free radicals.13-15 Once oxidized, vitamin E can be regenerated to its reduced form by vitamin C.11 Their synergistic effects on skin protection have been studied extensively. A double-blind, placebo-controlled study of 10 patients compared 2 g of vitamin C combined with 1000 IU of vitamin E vs placebo.16 The patients’ skin reaction before and after 8 days of treatment were assessed by determination of MED and the cutaneous blood flow of skin irradiated with UV light. Results showed that the median MED of those taking vitamins increased from 80 to 96.5 mJ/cm2 (P<.01) and decreased for the placebo group. Investigators concluded that the combination of vitamins C and E reduces the sunburn reaction and leads to a reduction in the sequelae of UV-induced skin damage.16 A prospective, randomized, placebo-controlled study by Fuchs and Kern17 replicated these findings, also concluding that combinations of vitamins C and E provide improved photoprotective effects than either vitamin alone.
Vitamin D
Vitamin D is a fat-soluble vitamin obtained through dietary intake and exposure to UV light.3,18,19 Precursors of vitamin D require interaction with UV light for conversion into active forms. The highest concentrations of 7-dehydrocholesterol are found in keratinocytes in the basal cell and spinous cell layers of the skin where they are protected from UV light by melanin. As such, individuals with higher melanin content in their skin require more exposure to UV light to produce the same levels of vitamin D as those with less melanin,20 leading to a high rate of vitamin D deficiency in dark-skinned individuals. Because of their prodifferentiating and antiproliferative effects, vitamin D analogs have been very effective in the treatment of psoriasis.20,21 Vitamin D deficiency also has been implicated in the pathogenesis of vitiligo. A systematic review and meta-analysis conducted in 2016 found that a significant relationship existed between low 25-hydroxyvitamin D levels and vitiligo (P<.01), but no causal relationship could be established.22
A 2017 double-blind, placebo-controlled study performed by Scott et al23 aimed to elucidate the relationship between vitamin D concentrations and sunburn. Twenty adults received either placebo or high-dose vitamin D3 (200,000 IU) 1 hour after experimental sunburn induced by an erythemogenic dose of UVR. Investigators measured participants’ concentrations of the proinflammatory mediators tumor necrosis factor α and nitric oxide synthase via skin biopsy 48 hours later. Patients in the experimental group were found to have significantly reduced expression of both tumor necrosis factor α (P=.04) and nitric oxide synthase (P=.02). Additionally, participants with significantly higher vitamin D3 levels following supplementation (P=.007) demonstrated increased skin expression of the anti-inflammatory marker arginase-1 (P=.005) as well as a persistent reduction in skin redness (P=.02). Investigators concluded that vitamin D plays a large role in skin homeostasis and implicated vitamin D’s upregulation of arginase-1 as a potent mechanism of its anti-inflammatory effects.23
Collagen
As humans age, the density of collagen in the dermis decreases, leading to sagging and wrinkling of skin.24 Oral supplementation of collagen has been examined for its dermatologic benefits, primarily increasing the thickness and density of collagen in the dermal layer. In 2014, Proksch et al25 performed a double-blind, placebo-controlled trial in which 69 women were randomized to receive 2.5 or 5 g of collagen peptides or placebo for 8 weeks. Both treatment groups demonstrated improvements in skin elasticity as well as improved skin moisture and decreased skin evaporation; however, changes in the latter 2 qualities failed to reach statistical significance.25
The results of this study were replicated by Asserin et al.26 One hundred six female patients were randomly assigned to receive 10 g of collagen peptides or placebo daily for 8 weeks. The collagen group demonstrated significantly improved skin hydration (P=.003) and increased density of collagen in the dermis (P=.007) relative to placebo.26
In another randomized, double-blind, placebo-controlled study, 71 women consumed a 20-mL beverage containing either 3000 mg of collagen peptides or placebo for 12 weeks.27 Participants in the treatment group demonstrated significant decreases in periorbital wrinkles (P<.05) and enhanced facial skin moisture (P<.001) and elasticity (P<.001) after 12 weeks. Researchers concluded that oral supplementation with collagen peptides holds promise as a natural supplement to provide cutaneous antiaging properties.27
Ceramides
Ceramides are lipids composed of a sphingoid base conjugated to a fatty acid and serve as the main component of the stratum corneum of the skin. Ceramides are crucial for the maintenance of skin barrier integrity and for preventing transepidermal water loss.28 In a 3-month study of 51 women with dry skin, Guillou et al29 showed that a ceramide wheat extract capsule significantly increased corneometry measurements of skin hydration on the arms (P<.001) and the legs (P=.012) compared to placebo.
Mixed Supplements
The discovery that nutritional contents can affect skin appearance has energized the development of combination supplements containing multiple vitamins and micronutrients. Imedeen is a biomarine complex and antioxidant supplement with several different formulations, including Prime Renewal, Time Perfection, and Derma One (Pfizer Inc). The ingredients include a combination of a biomarine complex (blend of fish proteins and polysaccharides), lycopene, grape seed extract, vitamin C, vitamin E, and zinc. Several trials have been conducted to assess the efficacy of the supplements on improving the appearance of photodamaged and aged skin (Table).

A placebo-controlled, randomized study of 144 participants conducted by Kieffer and Efsen30 assessed the efficacy of Imedeen supplements over 12 months. The trial included a 3-month placebo-controlled study and 9-month uncontrolled continuation. Imedeen’s efficacy was measured using clinical evaluation, transepidermal water loss, self-evaluation, and photograph evaluation. After 1 year of treatment, improvement occurred in photograph evaluation of fine lines, overall photoaging, telangiectasia and hyperpigmentation, and self-evaluation of skin condition.30 Additional double-blind, placebo-controlled, randomized studies assessing the efficacy of Imedeen have shown increased dermal and epidermal thickness, improvement of stratum corneum moisturization, and improved overall facial complexion.31-33
Several combined supplements containing collagen peptide as the main ingredient have been created for use in skin care. Collagen is found in the extracellular matrix of the dermis and is responsible for the resiliency and strength of skin.34,35 Damage to the dermis can occur with prolonged UV light exposure and is seen histologically as disorganized collagen fibrils and grossly as wrinkles and photoaged skin.35,36
A study assessed the effect of BioCell Collagen (BioCell Technology, LLC), a supplement containing type II collagen, on skin aging.37 Twenty-six women underwent baseline visual assessments of their skin before taking 2 tablets of the supplement daily. Twelve weeks of supplementation led to significant reduction in global lines and wrinkles (13.2%; P=.028) as well as skin dryness and scaling (76%; P=.002). Assessment of collagen content at 6 weeks revealed a significant increase from baseline (6.3%; P=.002), though the difference after 12 weeks was not significant (3.5%; P=.134). The authors concluded that although preliminary data suggested that BioCell Collagen may reduce visible signs of aging, a controlled study was necessary to verify this finding.37
A single-blind, case-controlled study assessed a similar supplement, Celergen, that contained marine collagen peptides.38 Forty-one adults took 2 capsules each day for 60 days. Assessment of their skin physiology was conducted at the enrollment visit, 2 months later, and after the treatment period ended. Skin elasticity, transepidermal water loss, epidermal and dermal thickness, and density were measured. Investigators found that Celergen administration significantly enhanced skin elasticity and sebum production (P<.0001) but did not influence cutaneous moisture. The dermal thickness and homogenous distribution of collagen fibers were enhanced in 11 patients while properties of the epidermis remained unchanged. The study determined that supplementation remarkably improved skin elasticity, sebum production, and dermal ultrasonic markers.38
A double-blind, randomized, placebo-controlled study assessed a collagen- and antioxidant-containing supplement, Gold Collagen Forte, on skin properties.39 The treatment and placebo groups each consisted of 60 patients who consumed 1 bottle (50 mL) of the product each day for 90 days. Patients completed a self-assessment of their skin regarding photoaging, focusing on the crow’s-feet area and nasolabial folds, while skin elasticity was assessed with the SkinLab USB elasticity module. Results showed a significant increase in skin elasticity (+7.5%; P≤.001). Self-assessment results showed improvements in both the treatment and placebo groups, and investigators concluded that Gold Collagen Forte may have photoprotective effects and help improve skin health.39
Safety
Although trials have demonstrated vitamin supplementation to be safe and effective for skin enhancement, it is important to consider potential vitamin toxicities. High doses of vitamin C supplementation have been shown to cause damage via lipid peroxidation.40 In a study assessing if high levels of beta-carotene and vitamin E were associated with a lower risk for lung cancer, data showed that these supplements may actually have harmful effects.40,41 Additionally, consumption of high-dose dietary supplements has been associated with an increased risk for severe medical events, including disability and death among adolescents and young adults.42
Conclusion
Numerous trials have indicated that the use of systemic vitamins can have beneficial effects on the protection and appearance of skin. Photodamage from UV light–induced erythema can be decreased by carotenoids and vitamins C and E. Similarly, supplements that combine multiple nutrients with collagen have been shown to improve the appearance of aging skin by decreasing the prominence of wrinkles. Given the growing number of products and advertisements that exist in the supplement marketplace, it is crucial for clinicians to ground their recommendations to patients in the scientific data of robust studies.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729-738.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370.
- Draelos ZD. Nutrition and enhancing youthful-appearing skin. Clin Dermatol. 2010;28:400-408.
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51-54.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Anstey AV. Systemic photoprotection with alpha-tocopherol (vitamin E) and beta-carotene. Clin Exp Dermatol. 2002;27:170-176.
- Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449-1451.
- Wood SM, Mastaloudis AF, Hester SN, et al. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J Cosmet Dermatol. 2017;16:491-499.
- Meinke MC, Friedrich A, Tscherch K, et al. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013;84:365-373.
- Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27:469-474.
- McArdle F, Rhodes LE, Parslew R, et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic Biol Med. 2002;33:1355-1362.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86:1225-1231.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667.
- Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298-307.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725-731.
- Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998;38:45-48.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006-1012.
- Shahriari M, Kerr PE, Slade K, et al. Vitamin D and the skin. Clin Dermatol. 2010;28:663-668.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54:383-392.
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(suppl 4):11-15.
- Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30:137-145.
- Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2016;32:181-190.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-1868.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291-301.
- Koizumi S, Inoue N, Shimizu M, et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int J Peptide Res Ther. 2018;24:397-402.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103059.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33:138-143.
- Kieffer ME, Efsen J. Imedeen in the treatment of photoaged skin: an efficacy and safety trial over 12 months. J Eur Acad Dermatol Venereol. 1998;11:129-136.
- Skovgaard GR, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60:1201-1206.
- Stephens TJ, Sigler ML, Herndon JH Jr, et al. A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin. Clin Cosmet Investig Dermatol. 2016;9:63-70.
- Stephens TJ, Sigler ML, Hino PD, et al. A randomized, double-blind, placebo-controlled clinical trial evaluating an oral anti-aging skin care supplement for treating photodamaged skin. J Clin Aesthet Dermatol. 2016;9:25-32.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Kang MC, Yumnam S, Kim SY. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19113551.
- Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267-273.
- De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410.
- Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30:146-158.
- Hamishehkar H, Ranjdoost F, Asgharian P, et al. Vitamins, are they safe? Adv Pharm Bull. 2016;6:467-477.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029-1035.
- Or F, Yongjoo K, Simms J, et al. Taking stock of dietary supplements’ harmful effects on children, adolescents, and young adults [published online June 3, 2019]. J Adolesc Health. S1054-139X(19)30163-6. doi:10.1016/j.jadohealth.2019.03.005.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729-738.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370.
- Draelos ZD. Nutrition and enhancing youthful-appearing skin. Clin Dermatol. 2010;28:400-408.
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51-54.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Anstey AV. Systemic photoprotection with alpha-tocopherol (vitamin E) and beta-carotene. Clin Exp Dermatol. 2002;27:170-176.
- Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449-1451.
- Wood SM, Mastaloudis AF, Hester SN, et al. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J Cosmet Dermatol. 2017;16:491-499.
- Meinke MC, Friedrich A, Tscherch K, et al. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013;84:365-373.
- Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27:469-474.
- McArdle F, Rhodes LE, Parslew R, et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic Biol Med. 2002;33:1355-1362.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86:1225-1231.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667.
- Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298-307.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725-731.
- Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998;38:45-48.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006-1012.
- Shahriari M, Kerr PE, Slade K, et al. Vitamin D and the skin. Clin Dermatol. 2010;28:663-668.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54:383-392.
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(suppl 4):11-15.
- Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30:137-145.
- Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2016;32:181-190.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-1868.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291-301.
- Koizumi S, Inoue N, Shimizu M, et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int J Peptide Res Ther. 2018;24:397-402.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103059.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33:138-143.
- Kieffer ME, Efsen J. Imedeen in the treatment of photoaged skin: an efficacy and safety trial over 12 months. J Eur Acad Dermatol Venereol. 1998;11:129-136.
- Skovgaard GR, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60:1201-1206.
- Stephens TJ, Sigler ML, Herndon JH Jr, et al. A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin. Clin Cosmet Investig Dermatol. 2016;9:63-70.
- Stephens TJ, Sigler ML, Hino PD, et al. A randomized, double-blind, placebo-controlled clinical trial evaluating an oral anti-aging skin care supplement for treating photodamaged skin. J Clin Aesthet Dermatol. 2016;9:25-32.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Kang MC, Yumnam S, Kim SY. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19113551.
- Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267-273.
- De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410.
- Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30:146-158.
- Hamishehkar H, Ranjdoost F, Asgharian P, et al. Vitamins, are they safe? Adv Pharm Bull. 2016;6:467-477.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029-1035.
- Or F, Yongjoo K, Simms J, et al. Taking stock of dietary supplements’ harmful effects on children, adolescents, and young adults [published online June 3, 2019]. J Adolesc Health. S1054-139X(19)30163-6. doi:10.1016/j.jadohealth.2019.03.005.
Practice Points
- Multiple vitamins and supplements have demonstrated evidence in improving skin appearance.
- Carotenoids, along with vitamins C and E, have been shown to protect skin from UV-induced photodamage, while supplements containing collagen decrease the appearance of wrinkles.
Barber’s Sinus Between the Toes of a Female Hairdresser
To the Editor:
Barber’s sinus, or interdigital pilonidal sinus, is an occupational dermatosis with a pathognomonic clinical picture. Nearly all reports of barber’s sinus in the literature have involved the hands of male barbers and hairdressers. We present an uncommon case of barber’s sinus between the toes of a female hairdresser. If left untreated, potential complications of barber’s sinus include abscess formation, cellulitis, lymphangitis, and osteomyelitis. Clinicians should advise patients with an occupational risk of barber’s sinus to wear protective footwear and maintain hygiene in the interdigital spaces.
A 23-year-old female hairdresser was referred to our outpatient dermatology clinic by general surgery for evaluation of an asymptomatic interdigital toe lesion of several months’ duration. She was otherwise healthy. Physical examination revealed a 3-mm sinus in the interdigital web space between the fourth and fifth digits of the left foot, creating a partial fistula terminating in an umbilicated pink papule on the dorsal aspect of the interdigital space (Figure). While at work, the patient reported that she usually wore open-toed flip-flops. A diagnosis of barber’s sinus was made clinically. She returned for follow-up to the referring surgeon within 2 months and was offered surgical debridement, but the patient declined treatment, instead opting to wait and monitor for any potential complications. The lesion showed no change in clinical appearance and remained asymptomatic.
Barber’s sinus is caused by sharp fragments of clipped hair that penetrate the fragile interdigital skin and cause a foreign-body reaction. Males are almost exclusively contributory to the reported cases of barber’s sinus in the literature.1,2
The clinical picture of barber’s sinus is pathognomonic, as demonstrated in our case. Other potential diagnoses to consider include atypical mycobacterial infection, deep fungal infection, other foreign-body granuloma, and erosio interdigitalis blastomycetica. Although thorough removal of embedded hair fragments may be curative, most cases require surgical excision, often by curette, and subsequent skin closure. Pathology shows a foreign-body granulomatous reaction to hair fragments. If left untreated, potential complications of barber’s sinus include abscess formation, cellulitis, lymphangitis, and osteomyelitis. This lesion is preventable by maintaining hygiene of the interdigital spaces, use of barrier creams, and wearing protective footwear.3,4
- Efthimiadis C, Kosmidis C, Anthimidis G, et al. Barber’s hair sinus in a female hairdresser: uncommon manifestation of an occupational disease: a case report. Cases J. 2008;1:214.
- O’Neill AC, Purcell EM, Regan PJ. Interdigital pilonidal sinus of the foot [published online May 31, 2009]. Foot (Edinb). 2009;19:227-228.
- Schröder CM, Merk HF, Frank J. Barber’s hair sinus in a female hairdresser: uncommon manifestation of an occupational dermatosis. J Eur Acad Dermatol Venereol. 2006;20:209-211.
- Joseph HL, Gifford H. Barber’s interdigital pilonidal sinus: the incidence, pathology, and pathogenesis. AMA Arch Derm Syphilol. 1954;70:616-624.
To the Editor:
Barber’s sinus, or interdigital pilonidal sinus, is an occupational dermatosis with a pathognomonic clinical picture. Nearly all reports of barber’s sinus in the literature have involved the hands of male barbers and hairdressers. We present an uncommon case of barber’s sinus between the toes of a female hairdresser. If left untreated, potential complications of barber’s sinus include abscess formation, cellulitis, lymphangitis, and osteomyelitis. Clinicians should advise patients with an occupational risk of barber’s sinus to wear protective footwear and maintain hygiene in the interdigital spaces.
A 23-year-old female hairdresser was referred to our outpatient dermatology clinic by general surgery for evaluation of an asymptomatic interdigital toe lesion of several months’ duration. She was otherwise healthy. Physical examination revealed a 3-mm sinus in the interdigital web space between the fourth and fifth digits of the left foot, creating a partial fistula terminating in an umbilicated pink papule on the dorsal aspect of the interdigital space (Figure). While at work, the patient reported that she usually wore open-toed flip-flops. A diagnosis of barber’s sinus was made clinically. She returned for follow-up to the referring surgeon within 2 months and was offered surgical debridement, but the patient declined treatment, instead opting to wait and monitor for any potential complications. The lesion showed no change in clinical appearance and remained asymptomatic.

Barber’s sinus is caused by sharp fragments of clipped hair that penetrate the fragile interdigital skin and cause a foreign-body reaction. Males are almost exclusively contributory to the reported cases of barber’s sinus in the literature.1,2
The clinical picture of barber’s sinus is pathognomonic, as demonstrated in our case. Other potential diagnoses to consider include atypical mycobacterial infection, deep fungal infection, other foreign-body granuloma, and erosio interdigitalis blastomycetica. Although thorough removal of embedded hair fragments may be curative, most cases require surgical excision, often by curette, and subsequent skin closure. Pathology shows a foreign-body granulomatous reaction to hair fragments. If left untreated, potential complications of barber’s sinus include abscess formation, cellulitis, lymphangitis, and osteomyelitis. This lesion is preventable by maintaining hygiene of the interdigital spaces, use of barrier creams, and wearing protective footwear.3,4
To the Editor:
Barber’s sinus, or interdigital pilonidal sinus, is an occupational dermatosis with a pathognomonic clinical picture. Nearly all reports of barber’s sinus in the literature have involved the hands of male barbers and hairdressers. We present an uncommon case of barber’s sinus between the toes of a female hairdresser. If left untreated, potential complications of barber’s sinus include abscess formation, cellulitis, lymphangitis, and osteomyelitis. Clinicians should advise patients with an occupational risk of barber’s sinus to wear protective footwear and maintain hygiene in the interdigital spaces.
A 23-year-old female hairdresser was referred to our outpatient dermatology clinic by general surgery for evaluation of an asymptomatic interdigital toe lesion of several months’ duration. She was otherwise healthy. Physical examination revealed a 3-mm sinus in the interdigital web space between the fourth and fifth digits of the left foot, creating a partial fistula terminating in an umbilicated pink papule on the dorsal aspect of the interdigital space (Figure). While at work, the patient reported that she usually wore open-toed flip-flops. A diagnosis of barber’s sinus was made clinically. She returned for follow-up to the referring surgeon within 2 months and was offered surgical debridement, but the patient declined treatment, instead opting to wait and monitor for any potential complications. The lesion showed no change in clinical appearance and remained asymptomatic.

Barber’s sinus is caused by sharp fragments of clipped hair that penetrate the fragile interdigital skin and cause a foreign-body reaction. Males are almost exclusively contributory to the reported cases of barber’s sinus in the literature.1,2
The clinical picture of barber’s sinus is pathognomonic, as demonstrated in our case. Other potential diagnoses to consider include atypical mycobacterial infection, deep fungal infection, other foreign-body granuloma, and erosio interdigitalis blastomycetica. Although thorough removal of embedded hair fragments may be curative, most cases require surgical excision, often by curette, and subsequent skin closure. Pathology shows a foreign-body granulomatous reaction to hair fragments. If left untreated, potential complications of barber’s sinus include abscess formation, cellulitis, lymphangitis, and osteomyelitis. This lesion is preventable by maintaining hygiene of the interdigital spaces, use of barrier creams, and wearing protective footwear.3,4
- Efthimiadis C, Kosmidis C, Anthimidis G, et al. Barber’s hair sinus in a female hairdresser: uncommon manifestation of an occupational disease: a case report. Cases J. 2008;1:214.
- O’Neill AC, Purcell EM, Regan PJ. Interdigital pilonidal sinus of the foot [published online May 31, 2009]. Foot (Edinb). 2009;19:227-228.
- Schröder CM, Merk HF, Frank J. Barber’s hair sinus in a female hairdresser: uncommon manifestation of an occupational dermatosis. J Eur Acad Dermatol Venereol. 2006;20:209-211.
- Joseph HL, Gifford H. Barber’s interdigital pilonidal sinus: the incidence, pathology, and pathogenesis. AMA Arch Derm Syphilol. 1954;70:616-624.
- Efthimiadis C, Kosmidis C, Anthimidis G, et al. Barber’s hair sinus in a female hairdresser: uncommon manifestation of an occupational disease: a case report. Cases J. 2008;1:214.
- O’Neill AC, Purcell EM, Regan PJ. Interdigital pilonidal sinus of the foot [published online May 31, 2009]. Foot (Edinb). 2009;19:227-228.
- Schröder CM, Merk HF, Frank J. Barber’s hair sinus in a female hairdresser: uncommon manifestation of an occupational dermatosis. J Eur Acad Dermatol Venereol. 2006;20:209-211.
- Joseph HL, Gifford H. Barber’s interdigital pilonidal sinus: the incidence, pathology, and pathogenesis. AMA Arch Derm Syphilol. 1954;70:616-624.
Practice Points
- This case illustrates a disease in which a medical history and simple clinical examination can lead to the diagnosis.
- Patients may value a diagnosis without treatment. A patient with barber’s sinus may be satisfied with watchful waiting.
Review looks at natural course of alopecia areata in young children
, according to a retrospective chart review of 125 children.
Almost 90% of the children presented between aged 2 and 4 years, compared with 11.9% between ages 1 and 2 years, and 1.6% aged under 1 year, “in keeping with the existing literature,” the study authors reported in Pediatric Dermatology. “A high percentage of patients continued to have mild, patchy alopecia at their follow‐up visits,” they added.
Epidemiologic studies of children with alopecia areata are few and have not focused on the youngest patients, said Sneha Rangu, of the section of dermatology at Children’s Hospital of Philadelphia, and coauthors. They performed a retrospective chart review of 125 patients, who initially presented at the hospital with alopecia areata between Jan. 1, 2016, and June 1, 2018, when they were younger than 4 years. Patients who received systemic therapy or topical JAK inhibitors for alopecia were excluded. Severity was measured with the Severity of Alopecia Tool (SALT) score, to monitor progression of hair loss, analyzing scores at the initial presentation, at 3-6 months, at 1 year, and at 2 or more years.
Almost 70% were female, which the authors said was similar to other studies that have found alopecia areata is more prevalent in females; and 86.6% were between ages 2 and 4 years when they first presented. The initial diagnosis was alopecia areata in 72.0%, alopecia totalis in 8.8%, and alopecia universalis in 19.2%. Of the 41 boys, 39% had alopecia totalis or alopecia universalis, as did 22% of the girls, which suggested that boys presenting under aged 4 years were more likely to have more severe disease, or that “guardians of boys are more likely to present for therapy when disease is more severe,” the authors wrote.
About 40% of the children presented with a history of atopic dermatitis, and 4% had an autoimmune disease (vitiligo, celiac disease, or type 1 diabetes). Twenty-eight percent of patients had a family history of alopecia areata, 27.2% had a family history of other autoimmune diseases, and 32% had a family history of hypothyroidism.
At the first visit, 57.6% had patch‐stage alopecia and SALT scores in the mild range (0%‐24% hair loss), which was present in a high proportion of these patients at follow-up: 49.4% at 3-6 months, 39.5% at 1 year, and 42.9% at two or more years.
At the first visit, 28% had high SALT scores (50%-100% hair loss), increasing to 36% at 3-6 months, 41.8% at 1 year, and 46.4% at 2 or more years. They calculated that for those with more than 50% hair loss at the initial presentation, the likelihood of being in a high category of hair loss, as measured by increasing SALT scores, was significantly higher at 1 year (odds ratio, 1.85, P =.033) and at 2 or more years (OR, 2.29, P = .038).
“While there is a likelihood of increasing disease severity, those with higher severity at initial presentation are likely to stay severe after one or 2 years,” the authors noted.
They concluded that their results add to the understanding of the epidemiology of alopecia areata in children “and perhaps can provide clinicians and families with a better sense of prognosis for progression in the youngest patients presenting with alopecia areata.”
They said the retrospective design and small sample size were among the study’s limitations. They had no conflicts of interest to disclose.
SOURCE: Rangu S et al. Pediatr Dermatol. 2019 Aug 29. doi: 10.1111/pde.13990.
, according to a retrospective chart review of 125 children.
Almost 90% of the children presented between aged 2 and 4 years, compared with 11.9% between ages 1 and 2 years, and 1.6% aged under 1 year, “in keeping with the existing literature,” the study authors reported in Pediatric Dermatology. “A high percentage of patients continued to have mild, patchy alopecia at their follow‐up visits,” they added.
Epidemiologic studies of children with alopecia areata are few and have not focused on the youngest patients, said Sneha Rangu, of the section of dermatology at Children’s Hospital of Philadelphia, and coauthors. They performed a retrospective chart review of 125 patients, who initially presented at the hospital with alopecia areata between Jan. 1, 2016, and June 1, 2018, when they were younger than 4 years. Patients who received systemic therapy or topical JAK inhibitors for alopecia were excluded. Severity was measured with the Severity of Alopecia Tool (SALT) score, to monitor progression of hair loss, analyzing scores at the initial presentation, at 3-6 months, at 1 year, and at 2 or more years.
Almost 70% were female, which the authors said was similar to other studies that have found alopecia areata is more prevalent in females; and 86.6% were between ages 2 and 4 years when they first presented. The initial diagnosis was alopecia areata in 72.0%, alopecia totalis in 8.8%, and alopecia universalis in 19.2%. Of the 41 boys, 39% had alopecia totalis or alopecia universalis, as did 22% of the girls, which suggested that boys presenting under aged 4 years were more likely to have more severe disease, or that “guardians of boys are more likely to present for therapy when disease is more severe,” the authors wrote.
About 40% of the children presented with a history of atopic dermatitis, and 4% had an autoimmune disease (vitiligo, celiac disease, or type 1 diabetes). Twenty-eight percent of patients had a family history of alopecia areata, 27.2% had a family history of other autoimmune diseases, and 32% had a family history of hypothyroidism.
At the first visit, 57.6% had patch‐stage alopecia and SALT scores in the mild range (0%‐24% hair loss), which was present in a high proportion of these patients at follow-up: 49.4% at 3-6 months, 39.5% at 1 year, and 42.9% at two or more years.
At the first visit, 28% had high SALT scores (50%-100% hair loss), increasing to 36% at 3-6 months, 41.8% at 1 year, and 46.4% at 2 or more years. They calculated that for those with more than 50% hair loss at the initial presentation, the likelihood of being in a high category of hair loss, as measured by increasing SALT scores, was significantly higher at 1 year (odds ratio, 1.85, P =.033) and at 2 or more years (OR, 2.29, P = .038).
“While there is a likelihood of increasing disease severity, those with higher severity at initial presentation are likely to stay severe after one or 2 years,” the authors noted.
They concluded that their results add to the understanding of the epidemiology of alopecia areata in children “and perhaps can provide clinicians and families with a better sense of prognosis for progression in the youngest patients presenting with alopecia areata.”
They said the retrospective design and small sample size were among the study’s limitations. They had no conflicts of interest to disclose.
SOURCE: Rangu S et al. Pediatr Dermatol. 2019 Aug 29. doi: 10.1111/pde.13990.
, according to a retrospective chart review of 125 children.
Almost 90% of the children presented between aged 2 and 4 years, compared with 11.9% between ages 1 and 2 years, and 1.6% aged under 1 year, “in keeping with the existing literature,” the study authors reported in Pediatric Dermatology. “A high percentage of patients continued to have mild, patchy alopecia at their follow‐up visits,” they added.
Epidemiologic studies of children with alopecia areata are few and have not focused on the youngest patients, said Sneha Rangu, of the section of dermatology at Children’s Hospital of Philadelphia, and coauthors. They performed a retrospective chart review of 125 patients, who initially presented at the hospital with alopecia areata between Jan. 1, 2016, and June 1, 2018, when they were younger than 4 years. Patients who received systemic therapy or topical JAK inhibitors for alopecia were excluded. Severity was measured with the Severity of Alopecia Tool (SALT) score, to monitor progression of hair loss, analyzing scores at the initial presentation, at 3-6 months, at 1 year, and at 2 or more years.
Almost 70% were female, which the authors said was similar to other studies that have found alopecia areata is more prevalent in females; and 86.6% were between ages 2 and 4 years when they first presented. The initial diagnosis was alopecia areata in 72.0%, alopecia totalis in 8.8%, and alopecia universalis in 19.2%. Of the 41 boys, 39% had alopecia totalis or alopecia universalis, as did 22% of the girls, which suggested that boys presenting under aged 4 years were more likely to have more severe disease, or that “guardians of boys are more likely to present for therapy when disease is more severe,” the authors wrote.
About 40% of the children presented with a history of atopic dermatitis, and 4% had an autoimmune disease (vitiligo, celiac disease, or type 1 diabetes). Twenty-eight percent of patients had a family history of alopecia areata, 27.2% had a family history of other autoimmune diseases, and 32% had a family history of hypothyroidism.
At the first visit, 57.6% had patch‐stage alopecia and SALT scores in the mild range (0%‐24% hair loss), which was present in a high proportion of these patients at follow-up: 49.4% at 3-6 months, 39.5% at 1 year, and 42.9% at two or more years.
At the first visit, 28% had high SALT scores (50%-100% hair loss), increasing to 36% at 3-6 months, 41.8% at 1 year, and 46.4% at 2 or more years. They calculated that for those with more than 50% hair loss at the initial presentation, the likelihood of being in a high category of hair loss, as measured by increasing SALT scores, was significantly higher at 1 year (odds ratio, 1.85, P =.033) and at 2 or more years (OR, 2.29, P = .038).
“While there is a likelihood of increasing disease severity, those with higher severity at initial presentation are likely to stay severe after one or 2 years,” the authors noted.
They concluded that their results add to the understanding of the epidemiology of alopecia areata in children “and perhaps can provide clinicians and families with a better sense of prognosis for progression in the youngest patients presenting with alopecia areata.”
They said the retrospective design and small sample size were among the study’s limitations. They had no conflicts of interest to disclose.
SOURCE: Rangu S et al. Pediatr Dermatol. 2019 Aug 29. doi: 10.1111/pde.13990.
FROM PEDIATRIC DERMATOLOGY
Meta-analysis finds platelet-rich plasma may improve hair growth
Treatment with intradermal injections of autologous compared with placebo, according to a meta-analysis of seven studies.
In the report of the results, published in the Journal of Cosmetic Dermatology, Gezim Dervishi, from University Hospital Cologne (Germany), and coauthors noted that autologous PRP contains cytokines and growth factors – including platelet‐derived growth factor, vascular endothelial growth factor, and insulinlike growth factor–1 – that are believed to play a role in new hair growth. While the evidence to support the clinical efficacy of this approach is currently limited and PRP is not approved for hair-loss treatment, it is being used in clinical trials and off label for hair restoration in Europe and the United States.
The authors reviewed 13 randomized, controlled trials involving 343 people with pattern hair loss randomized to PRP control treatment, or placebo in a simple-parallel or half-head design, and assessed at 3-6 months after starting treatment. The studies were conducted in countries that included the United States, Brazil, Spain, and Italy.
The meta-analysis included seven studies that used change in hair density as the primary outcome. Six of these studies compared PRP with placebo and one compared PRP plus minoxidil or finasteride versus placebo plus minoxidil or finasteride. Five evaluated outcomes 6 months after the intervention, and two studies evaluated outcomes 3 months afterwards.
Of these seven studies, five reported statistically significant increases in hair density in favor of PRP over placebo, while the remaining two did not find any significant treatment effect. In the seven studies, the pooled mean difference in increasing hair density between treatment and placebo was 30.35 (95% confidence interval, 1.77-58.93; P less than .00001).
The 13 studies involved applications of platelet-rich plasma one to eight times over treatment periods of 1-5 months. Three studies compared PRP in conjunction with either minoxidil, finasteride, or polydeoxyribonucleotide, compared with those same therapies without PRP, and 10 studies compared PRP with either normal saline or distilled water. Five studies were judged to be at high risk of bias for at least one item, but none of these studies were included in the meta-analysis.
None of the studies identified any significant short-term treatment-related adverse effects, but the authors suggested that future studies look at potential long-term adverse effects of treatment.
“We recommend further [randomized, controlled trials] that should ensure adequate random sequence generation, adequate allocation concealment, blinding of performance and detection, and that should prevent incomplete data and selective reporting,” they wrote.
Funding and conflicts of interest statements were not available.
SOURCE: Dervishi G et al. J Cosmet Dermatol. 2019 Aug 12. doi: 10.1111/jocd.13113.
Treatment with intradermal injections of autologous compared with placebo, according to a meta-analysis of seven studies.
In the report of the results, published in the Journal of Cosmetic Dermatology, Gezim Dervishi, from University Hospital Cologne (Germany), and coauthors noted that autologous PRP contains cytokines and growth factors – including platelet‐derived growth factor, vascular endothelial growth factor, and insulinlike growth factor–1 – that are believed to play a role in new hair growth. While the evidence to support the clinical efficacy of this approach is currently limited and PRP is not approved for hair-loss treatment, it is being used in clinical trials and off label for hair restoration in Europe and the United States.
The authors reviewed 13 randomized, controlled trials involving 343 people with pattern hair loss randomized to PRP control treatment, or placebo in a simple-parallel or half-head design, and assessed at 3-6 months after starting treatment. The studies were conducted in countries that included the United States, Brazil, Spain, and Italy.
The meta-analysis included seven studies that used change in hair density as the primary outcome. Six of these studies compared PRP with placebo and one compared PRP plus minoxidil or finasteride versus placebo plus minoxidil or finasteride. Five evaluated outcomes 6 months after the intervention, and two studies evaluated outcomes 3 months afterwards.
Of these seven studies, five reported statistically significant increases in hair density in favor of PRP over placebo, while the remaining two did not find any significant treatment effect. In the seven studies, the pooled mean difference in increasing hair density between treatment and placebo was 30.35 (95% confidence interval, 1.77-58.93; P less than .00001).
The 13 studies involved applications of platelet-rich plasma one to eight times over treatment periods of 1-5 months. Three studies compared PRP in conjunction with either minoxidil, finasteride, or polydeoxyribonucleotide, compared with those same therapies without PRP, and 10 studies compared PRP with either normal saline or distilled water. Five studies were judged to be at high risk of bias for at least one item, but none of these studies were included in the meta-analysis.
None of the studies identified any significant short-term treatment-related adverse effects, but the authors suggested that future studies look at potential long-term adverse effects of treatment.
“We recommend further [randomized, controlled trials] that should ensure adequate random sequence generation, adequate allocation concealment, blinding of performance and detection, and that should prevent incomplete data and selective reporting,” they wrote.
Funding and conflicts of interest statements were not available.
SOURCE: Dervishi G et al. J Cosmet Dermatol. 2019 Aug 12. doi: 10.1111/jocd.13113.
Treatment with intradermal injections of autologous compared with placebo, according to a meta-analysis of seven studies.
In the report of the results, published in the Journal of Cosmetic Dermatology, Gezim Dervishi, from University Hospital Cologne (Germany), and coauthors noted that autologous PRP contains cytokines and growth factors – including platelet‐derived growth factor, vascular endothelial growth factor, and insulinlike growth factor–1 – that are believed to play a role in new hair growth. While the evidence to support the clinical efficacy of this approach is currently limited and PRP is not approved for hair-loss treatment, it is being used in clinical trials and off label for hair restoration in Europe and the United States.
The authors reviewed 13 randomized, controlled trials involving 343 people with pattern hair loss randomized to PRP control treatment, or placebo in a simple-parallel or half-head design, and assessed at 3-6 months after starting treatment. The studies were conducted in countries that included the United States, Brazil, Spain, and Italy.
The meta-analysis included seven studies that used change in hair density as the primary outcome. Six of these studies compared PRP with placebo and one compared PRP plus minoxidil or finasteride versus placebo plus minoxidil or finasteride. Five evaluated outcomes 6 months after the intervention, and two studies evaluated outcomes 3 months afterwards.
Of these seven studies, five reported statistically significant increases in hair density in favor of PRP over placebo, while the remaining two did not find any significant treatment effect. In the seven studies, the pooled mean difference in increasing hair density between treatment and placebo was 30.35 (95% confidence interval, 1.77-58.93; P less than .00001).
The 13 studies involved applications of platelet-rich plasma one to eight times over treatment periods of 1-5 months. Three studies compared PRP in conjunction with either minoxidil, finasteride, or polydeoxyribonucleotide, compared with those same therapies without PRP, and 10 studies compared PRP with either normal saline or distilled water. Five studies were judged to be at high risk of bias for at least one item, but none of these studies were included in the meta-analysis.
None of the studies identified any significant short-term treatment-related adverse effects, but the authors suggested that future studies look at potential long-term adverse effects of treatment.
“We recommend further [randomized, controlled trials] that should ensure adequate random sequence generation, adequate allocation concealment, blinding of performance and detection, and that should prevent incomplete data and selective reporting,” they wrote.
Funding and conflicts of interest statements were not available.
SOURCE: Dervishi G et al. J Cosmet Dermatol. 2019 Aug 12. doi: 10.1111/jocd.13113.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Comment on “Analysis of Nail-Related Content of the Basic Dermatology Curriculum”
To the Editor:
In the April 2019 Cutis article by John and Lipner,1 the authors critiqued the American Academy of Dermatology Basic Dermatology Curriculum (BDC) for not providing an adequate scaffolding of nail findings on which dermatology residents can build their knowledge base; however, that criticism belies a misunderstanding of the BDC’s purpose. It was carefully designed to address the needs of undifferentiated medical students and primary care learners based on needs assessments from practicing primary care physicians and experienced dermatology educators.2,3 Given the limited amount of time to teach, a basic curriculum must focus on the most high-yield items. The BDC work group developed goals and objectives based on needs assessments for primary care practice with 38 core dermatology diagnoses, including 3 diagnoses with important nail findings: onychomycosis, melanoma, and psoriasis. Much repetition is built into the BDC, and the same diagnosis is used in multiple cases in different modules to encourage retention of information. Therefore, “analysis of nail-related content” should focus on diagnoses rather than cases, and for each diagnosis, note whether the nail findings are a pertinent negative or pertinent positive. In cases of the other 35 diagnoses covered in the BDC, nail findings are omitted for space because they are not relevant (eg, in cases of seborrheic dermatitis or rosacea). Normal nail findings are not pertinent negatives for most diagnoses in the BDC, except in cases with diagnoses for which psoriasis is in the differential, such as nummular dermatitis or pityriasis rosea.
Furthermore, a true analysis of the needs of medical students and primary care learners with regard to nail findings would begin with a needs assessment of the most common nail conditions evaluated in the primary care and urgent care settings. Ingrown nails, paronychia, onychomycosis, and subungual hematomas and other nail traumas are the most common nail conditions encountered in primary care and urgent care,4-10 but John and Lipner1 failed to perform analysis or needs assessment based on the incidence of nail diagnoses in these settings.
Other sources for medical students and primary care residents include excellent introductions to nail findings. The newly revised skin chapter of Bates’ Guide to Physical Examination and History Taking11 includes updated photographs of common nail findings and discusses the importance of examining nails in the full-body skin examination. Additionally, Clinical Dermatology: A Color Guide to Diagnosis and Therapy,12Lookingbill and Marks’ Principles of Dermatology,13 and The Color Atlas and Synopsis of Family Medicine14 cover nail disease beautifully for medical students and primary care learners. The BDC was never meant to supplant these bountiful resources.
The authors referred to lack of confidence in nail diagnoses among dermatology residents,1 which is a very real problem that must be addressed by dermatology residency programs. The BDC is not the proper vehicle for training dermatology residents about these conditions; that is the responsibility and challenge of our dermatology residency programs. The authors also suggested teaching how to perform nail biopsies in the BDC.1 It not reasonable to expect that our primary care colleagues will be performing nail biopsies. A more appropriate level of expectation is that they would know when to refer patients to dermatology; for example, they should know that a pigmented streak on a single nail that is expanding is an indication for referral to a dermatologist.
If the authors or others were to propose an additional nail module to the BDC work group, they would need to include an analysis of the literature regarding the incidence of nail disease seen in primary care and urgent care settings rather than the nail conditions seen by referral bias experienced by consulting dermatologists. The analysis would be worth considering and worthy of the goodwill engendered by the creation of the BDC in the first place.
Sincerely,
Patrick E. McCleskey, MD
From the Department of Dermatology, Kaiser Permanente Oakland Medical Center, California.
Dr. McCleskey previously served as Chair of the American Academy of Dermatology Basic Dermatology Curriculum Work Group (2013-2017) .
Correspondence: Patrick E. McCleskey, MD, 3701 Broadway, 4th Floor, Oakland, CA 94611 ([email protected]).
References
1. John JJ, Lipner SR. Analysis of nail-related content in the basic dermatology curriculum. Cutis. 2019;103:214-216.
2. Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
3. McCleskey PE, Gilson RT, Devillez R. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
4. Vierhoeven EWM, Kraaimaat FW, van Wheel C, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med. 2008;6:349-354.
5. Fleisher AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by non-dermatologists. Am J Manag Care. 2000;6;1149-1156.
6. Akbas A, Kilinc F, Yakut HI, et al. Nail disorders in children, a clinical study. Our Dermatol Online. 2016;7:149-154.
7. Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1262.
8. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol. 2011;147:118-120.
9. Wang E, Lim BL, Than KY. Dermatological conditions presenting at an emergency department in Singapore. Singapore Med J. 2009;50:881-884.
10. Lai-Kwon J, Weiland TJ, Chong AH, et al. Which dermatological conditions present to an emergency department in Australia? Emerg Med Int. 2014;2014:463026.
11. McCleskey PE. The skin, hair, and nails. In: Bickley L, ed. Bates’ Guide to Physical Examination and History Taking. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017:173-214.
12. Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:960-985.
13. Marks JG, Miller JJ. Nail disorders. In: Marks JG, Miller JJ, eds. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019:277-282.
14. Mayeaux EJ Jr, Williams J. Hair and nail conditions. In: Usatine RP, Smith MA, Mayeaux EJ Jr, et al. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill Education; 2019.
Author Response
I thank Dr. McCleskey for his interest in our article. Although I acknowledge that the Basic Dermatology Curriculum (BDC) serves as an introduction to dermatology for medical students and primary care physicians, I disagree that the current curriculum should be limited to only 3 diagnoses with important nail findings—onychomycosis, melanoma, and psoriasis—and exclude other common and potentially fatal nail diseases.
To characterize the overall nail burden of ambulatory care visits in the United States, data from the National Ambulatory Medical Care Survey from 2007 to 2016 were analyzed and there were more than 20 million outpatient visits for nail concerns during this period; furthermore, although many patients were seen by dermatologists, a considerable number were seen by pediatricians and general practitioners (Lipner SR, Hancock J, Fleischer AB Jr; unpublished data; July 2019). These findings underscore the importance of educating medical students and primary care physicians on the diagnosis and appropriate referral of patients with nail diseases.
Some limited information on nail unit melanomas is included in the BDC, but it is essential that medical students and general practitioners be educated about early diagnosis of squamous cell carcinomas and melanomas of the nail unit, which may help avoid unnecessary amputations and decrease mortality.1 Unfortunately, the vast majority of nail unit melanomas are diagnosed at stage II or later, which has been partially attributed to clinical knowledge gaps in the understanding of nail disease.2
Several studies have shown that many physicians fail to examine their patients’ nails during physical examinations, either due to concealment with nail polish or lack of clinical awareness. In a survey-based study analyzing patients’ awareness of longitudinal melanonychia and worrisome signs of nail unit melanoma, only 12% of patients (43/363) stated that their dermatologist or internist specifically asked them about nail changes.3 Furthermore, in another survey-based study of nail examinations at a free cancer screening by the American Academy of Dermatology, more than half of female participants (47/87 [54%]) stated that they were wearing nail polish at the time of screening.4,5 Therefore, examinations of the nails were not performed as part of the total-body skin examination.
In summary, nail diseases are an important concern in clinical practice with aesthetic and functional consequences. There is a strong need to emphasize the importance of nail examinations for diagnostic purposes and to incorporate more expansive nail-related content into the BDC, which can result in longer and more functional lives for our patients.
Sincerely,
Shari R. Lipner, MD, PhD
From the Department of Dermatology, Weill Cornell Medicine, New York, New York.
The author reports no conflict of interest.
References
1. Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713.
2. Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2:156-161.
4. Ko D, Lipner SR. A survey-based study on nail examinations at an American Academy of Dermatology free skin cancer screening. J Am Acad Dermatol. 2018;79:975-978.
5. Ko D, Lipner SR. Comment on: “The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985-2014.” J Am Acad Dermatol. 2019;80:e23.
In response to a recent analysis of nail-related content in the Basic Dermatology Curriculum (BDC), the author suggests the BDC is not the proper vehicle for training dermatology residents about nail conditions and proposes alternative sources for mastering this material.
To the Editor:
In the April 2019 Cutis article by John and Lipner,1 the authors critiqued the American Academy of Dermatology Basic Dermatology Curriculum (BDC) for not providing an adequate scaffolding of nail findings on which dermatology residents can build their knowledge base; however, that criticism belies a misunderstanding of the BDC’s purpose. It was carefully designed to address the needs of undifferentiated medical students and primary care learners based on needs assessments from practicing primary care physicians and experienced dermatology educators.2,3 Given the limited amount of time to teach, a basic curriculum must focus on the most high-yield items. The BDC work group developed goals and objectives based on needs assessments for primary care practice with 38 core dermatology diagnoses, including 3 diagnoses with important nail findings: onychomycosis, melanoma, and psoriasis. Much repetition is built into the BDC, and the same diagnosis is used in multiple cases in different modules to encourage retention of information. Therefore, “analysis of nail-related content” should focus on diagnoses rather than cases, and for each diagnosis, note whether the nail findings are a pertinent negative or pertinent positive. In cases of the other 35 diagnoses covered in the BDC, nail findings are omitted for space because they are not relevant (eg, in cases of seborrheic dermatitis or rosacea). Normal nail findings are not pertinent negatives for most diagnoses in the BDC, except in cases with diagnoses for which psoriasis is in the differential, such as nummular dermatitis or pityriasis rosea.
Furthermore, a true analysis of the needs of medical students and primary care learners with regard to nail findings would begin with a needs assessment of the most common nail conditions evaluated in the primary care and urgent care settings. Ingrown nails, paronychia, onychomycosis, and subungual hematomas and other nail traumas are the most common nail conditions encountered in primary care and urgent care,4-10 but John and Lipner1 failed to perform analysis or needs assessment based on the incidence of nail diagnoses in these settings.
Other sources for medical students and primary care residents include excellent introductions to nail findings. The newly revised skin chapter of Bates’ Guide to Physical Examination and History Taking11 includes updated photographs of common nail findings and discusses the importance of examining nails in the full-body skin examination. Additionally, Clinical Dermatology: A Color Guide to Diagnosis and Therapy,12Lookingbill and Marks’ Principles of Dermatology,13 and The Color Atlas and Synopsis of Family Medicine14 cover nail disease beautifully for medical students and primary care learners. The BDC was never meant to supplant these bountiful resources.
The authors referred to lack of confidence in nail diagnoses among dermatology residents,1 which is a very real problem that must be addressed by dermatology residency programs. The BDC is not the proper vehicle for training dermatology residents about these conditions; that is the responsibility and challenge of our dermatology residency programs. The authors also suggested teaching how to perform nail biopsies in the BDC.1 It not reasonable to expect that our primary care colleagues will be performing nail biopsies. A more appropriate level of expectation is that they would know when to refer patients to dermatology; for example, they should know that a pigmented streak on a single nail that is expanding is an indication for referral to a dermatologist.
If the authors or others were to propose an additional nail module to the BDC work group, they would need to include an analysis of the literature regarding the incidence of nail disease seen in primary care and urgent care settings rather than the nail conditions seen by referral bias experienced by consulting dermatologists. The analysis would be worth considering and worthy of the goodwill engendered by the creation of the BDC in the first place.
Sincerely,
Patrick E. McCleskey, MD
From the Department of Dermatology, Kaiser Permanente Oakland Medical Center, California.
Dr. McCleskey previously served as Chair of the American Academy of Dermatology Basic Dermatology Curriculum Work Group (2013-2017) .
Correspondence: Patrick E. McCleskey, MD, 3701 Broadway, 4th Floor, Oakland, CA 94611 ([email protected]).
References
1. John JJ, Lipner SR. Analysis of nail-related content in the basic dermatology curriculum. Cutis. 2019;103:214-216.
2. Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
3. McCleskey PE, Gilson RT, Devillez R. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
4. Vierhoeven EWM, Kraaimaat FW, van Wheel C, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med. 2008;6:349-354.
5. Fleisher AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by non-dermatologists. Am J Manag Care. 2000;6;1149-1156.
6. Akbas A, Kilinc F, Yakut HI, et al. Nail disorders in children, a clinical study. Our Dermatol Online. 2016;7:149-154.
7. Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1262.
8. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol. 2011;147:118-120.
9. Wang E, Lim BL, Than KY. Dermatological conditions presenting at an emergency department in Singapore. Singapore Med J. 2009;50:881-884.
10. Lai-Kwon J, Weiland TJ, Chong AH, et al. Which dermatological conditions present to an emergency department in Australia? Emerg Med Int. 2014;2014:463026.
11. McCleskey PE. The skin, hair, and nails. In: Bickley L, ed. Bates’ Guide to Physical Examination and History Taking. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017:173-214.
12. Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:960-985.
13. Marks JG, Miller JJ. Nail disorders. In: Marks JG, Miller JJ, eds. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019:277-282.
14. Mayeaux EJ Jr, Williams J. Hair and nail conditions. In: Usatine RP, Smith MA, Mayeaux EJ Jr, et al. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill Education; 2019.
Author Response
I thank Dr. McCleskey for his interest in our article. Although I acknowledge that the Basic Dermatology Curriculum (BDC) serves as an introduction to dermatology for medical students and primary care physicians, I disagree that the current curriculum should be limited to only 3 diagnoses with important nail findings—onychomycosis, melanoma, and psoriasis—and exclude other common and potentially fatal nail diseases.
To characterize the overall nail burden of ambulatory care visits in the United States, data from the National Ambulatory Medical Care Survey from 2007 to 2016 were analyzed and there were more than 20 million outpatient visits for nail concerns during this period; furthermore, although many patients were seen by dermatologists, a considerable number were seen by pediatricians and general practitioners (Lipner SR, Hancock J, Fleischer AB Jr; unpublished data; July 2019). These findings underscore the importance of educating medical students and primary care physicians on the diagnosis and appropriate referral of patients with nail diseases.
Some limited information on nail unit melanomas is included in the BDC, but it is essential that medical students and general practitioners be educated about early diagnosis of squamous cell carcinomas and melanomas of the nail unit, which may help avoid unnecessary amputations and decrease mortality.1 Unfortunately, the vast majority of nail unit melanomas are diagnosed at stage II or later, which has been partially attributed to clinical knowledge gaps in the understanding of nail disease.2
Several studies have shown that many physicians fail to examine their patients’ nails during physical examinations, either due to concealment with nail polish or lack of clinical awareness. In a survey-based study analyzing patients’ awareness of longitudinal melanonychia and worrisome signs of nail unit melanoma, only 12% of patients (43/363) stated that their dermatologist or internist specifically asked them about nail changes.3 Furthermore, in another survey-based study of nail examinations at a free cancer screening by the American Academy of Dermatology, more than half of female participants (47/87 [54%]) stated that they were wearing nail polish at the time of screening.4,5 Therefore, examinations of the nails were not performed as part of the total-body skin examination.
In summary, nail diseases are an important concern in clinical practice with aesthetic and functional consequences. There is a strong need to emphasize the importance of nail examinations for diagnostic purposes and to incorporate more expansive nail-related content into the BDC, which can result in longer and more functional lives for our patients.
Sincerely,
Shari R. Lipner, MD, PhD
From the Department of Dermatology, Weill Cornell Medicine, New York, New York.
The author reports no conflict of interest.
References
1. Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713.
2. Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2:156-161.
4. Ko D, Lipner SR. A survey-based study on nail examinations at an American Academy of Dermatology free skin cancer screening. J Am Acad Dermatol. 2018;79:975-978.
5. Ko D, Lipner SR. Comment on: “The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985-2014.” J Am Acad Dermatol. 2019;80:e23.
To the Editor:
In the April 2019 Cutis article by John and Lipner,1 the authors critiqued the American Academy of Dermatology Basic Dermatology Curriculum (BDC) for not providing an adequate scaffolding of nail findings on which dermatology residents can build their knowledge base; however, that criticism belies a misunderstanding of the BDC’s purpose. It was carefully designed to address the needs of undifferentiated medical students and primary care learners based on needs assessments from practicing primary care physicians and experienced dermatology educators.2,3 Given the limited amount of time to teach, a basic curriculum must focus on the most high-yield items. The BDC work group developed goals and objectives based on needs assessments for primary care practice with 38 core dermatology diagnoses, including 3 diagnoses with important nail findings: onychomycosis, melanoma, and psoriasis. Much repetition is built into the BDC, and the same diagnosis is used in multiple cases in different modules to encourage retention of information. Therefore, “analysis of nail-related content” should focus on diagnoses rather than cases, and for each diagnosis, note whether the nail findings are a pertinent negative or pertinent positive. In cases of the other 35 diagnoses covered in the BDC, nail findings are omitted for space because they are not relevant (eg, in cases of seborrheic dermatitis or rosacea). Normal nail findings are not pertinent negatives for most diagnoses in the BDC, except in cases with diagnoses for which psoriasis is in the differential, such as nummular dermatitis or pityriasis rosea.
Furthermore, a true analysis of the needs of medical students and primary care learners with regard to nail findings would begin with a needs assessment of the most common nail conditions evaluated in the primary care and urgent care settings. Ingrown nails, paronychia, onychomycosis, and subungual hematomas and other nail traumas are the most common nail conditions encountered in primary care and urgent care,4-10 but John and Lipner1 failed to perform analysis or needs assessment based on the incidence of nail diagnoses in these settings.
Other sources for medical students and primary care residents include excellent introductions to nail findings. The newly revised skin chapter of Bates’ Guide to Physical Examination and History Taking11 includes updated photographs of common nail findings and discusses the importance of examining nails in the full-body skin examination. Additionally, Clinical Dermatology: A Color Guide to Diagnosis and Therapy,12Lookingbill and Marks’ Principles of Dermatology,13 and The Color Atlas and Synopsis of Family Medicine14 cover nail disease beautifully for medical students and primary care learners. The BDC was never meant to supplant these bountiful resources.
The authors referred to lack of confidence in nail diagnoses among dermatology residents,1 which is a very real problem that must be addressed by dermatology residency programs. The BDC is not the proper vehicle for training dermatology residents about these conditions; that is the responsibility and challenge of our dermatology residency programs. The authors also suggested teaching how to perform nail biopsies in the BDC.1 It not reasonable to expect that our primary care colleagues will be performing nail biopsies. A more appropriate level of expectation is that they would know when to refer patients to dermatology; for example, they should know that a pigmented streak on a single nail that is expanding is an indication for referral to a dermatologist.
If the authors or others were to propose an additional nail module to the BDC work group, they would need to include an analysis of the literature regarding the incidence of nail disease seen in primary care and urgent care settings rather than the nail conditions seen by referral bias experienced by consulting dermatologists. The analysis would be worth considering and worthy of the goodwill engendered by the creation of the BDC in the first place.
Sincerely,
Patrick E. McCleskey, MD
From the Department of Dermatology, Kaiser Permanente Oakland Medical Center, California.
Dr. McCleskey previously served as Chair of the American Academy of Dermatology Basic Dermatology Curriculum Work Group (2013-2017) .
Correspondence: Patrick E. McCleskey, MD, 3701 Broadway, 4th Floor, Oakland, CA 94611 ([email protected]).
References
1. John JJ, Lipner SR. Analysis of nail-related content in the basic dermatology curriculum. Cutis. 2019;103:214-216.
2. Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
3. McCleskey PE, Gilson RT, Devillez R. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
4. Vierhoeven EWM, Kraaimaat FW, van Wheel C, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med. 2008;6:349-354.
5. Fleisher AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by non-dermatologists. Am J Manag Care. 2000;6;1149-1156.
6. Akbas A, Kilinc F, Yakut HI, et al. Nail disorders in children, a clinical study. Our Dermatol Online. 2016;7:149-154.
7. Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1262.
8. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol. 2011;147:118-120.
9. Wang E, Lim BL, Than KY. Dermatological conditions presenting at an emergency department in Singapore. Singapore Med J. 2009;50:881-884.
10. Lai-Kwon J, Weiland TJ, Chong AH, et al. Which dermatological conditions present to an emergency department in Australia? Emerg Med Int. 2014;2014:463026.
11. McCleskey PE. The skin, hair, and nails. In: Bickley L, ed. Bates’ Guide to Physical Examination and History Taking. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017:173-214.
12. Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:960-985.
13. Marks JG, Miller JJ. Nail disorders. In: Marks JG, Miller JJ, eds. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019:277-282.
14. Mayeaux EJ Jr, Williams J. Hair and nail conditions. In: Usatine RP, Smith MA, Mayeaux EJ Jr, et al. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill Education; 2019.
Author Response
I thank Dr. McCleskey for his interest in our article. Although I acknowledge that the Basic Dermatology Curriculum (BDC) serves as an introduction to dermatology for medical students and primary care physicians, I disagree that the current curriculum should be limited to only 3 diagnoses with important nail findings—onychomycosis, melanoma, and psoriasis—and exclude other common and potentially fatal nail diseases.
To characterize the overall nail burden of ambulatory care visits in the United States, data from the National Ambulatory Medical Care Survey from 2007 to 2016 were analyzed and there were more than 20 million outpatient visits for nail concerns during this period; furthermore, although many patients were seen by dermatologists, a considerable number were seen by pediatricians and general practitioners (Lipner SR, Hancock J, Fleischer AB Jr; unpublished data; July 2019). These findings underscore the importance of educating medical students and primary care physicians on the diagnosis and appropriate referral of patients with nail diseases.
Some limited information on nail unit melanomas is included in the BDC, but it is essential that medical students and general practitioners be educated about early diagnosis of squamous cell carcinomas and melanomas of the nail unit, which may help avoid unnecessary amputations and decrease mortality.1 Unfortunately, the vast majority of nail unit melanomas are diagnosed at stage II or later, which has been partially attributed to clinical knowledge gaps in the understanding of nail disease.2
Several studies have shown that many physicians fail to examine their patients’ nails during physical examinations, either due to concealment with nail polish or lack of clinical awareness. In a survey-based study analyzing patients’ awareness of longitudinal melanonychia and worrisome signs of nail unit melanoma, only 12% of patients (43/363) stated that their dermatologist or internist specifically asked them about nail changes.3 Furthermore, in another survey-based study of nail examinations at a free cancer screening by the American Academy of Dermatology, more than half of female participants (47/87 [54%]) stated that they were wearing nail polish at the time of screening.4,5 Therefore, examinations of the nails were not performed as part of the total-body skin examination.
In summary, nail diseases are an important concern in clinical practice with aesthetic and functional consequences. There is a strong need to emphasize the importance of nail examinations for diagnostic purposes and to incorporate more expansive nail-related content into the BDC, which can result in longer and more functional lives for our patients.
Sincerely,
Shari R. Lipner, MD, PhD
From the Department of Dermatology, Weill Cornell Medicine, New York, New York.
The author reports no conflict of interest.
References
1. Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713.
2. Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2:156-161.
4. Ko D, Lipner SR. A survey-based study on nail examinations at an American Academy of Dermatology free skin cancer screening. J Am Acad Dermatol. 2018;79:975-978.
5. Ko D, Lipner SR. Comment on: “The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985-2014.” J Am Acad Dermatol. 2019;80:e23.
In response to a recent analysis of nail-related content in the Basic Dermatology Curriculum (BDC), the author suggests the BDC is not the proper vehicle for training dermatology residents about nail conditions and proposes alternative sources for mastering this material.
In response to a recent analysis of nail-related content in the Basic Dermatology Curriculum (BDC), the author suggests the BDC is not the proper vehicle for training dermatology residents about nail conditions and proposes alternative sources for mastering this material.