User login
Silicone Arthroplasty After Ankylosis of Proximal Interphalangeal Joints in Rheumatoid Arthritis: A Case Report
Rheumatoid arthritis (RA) commonly affects the hand and fingers, most often at the metacarpophalangeal and proximal interphalangeal (PIP) joints. Synovitis, tendon ruptures, Boutonnière and swan-neck deformities, and joint destruction often occur. Bony ankylosis is not commonly described yet frequently occurs in patients with RA.1
Implant arthroplasty is an established treatment for arthritis of the hand and fingers. Indications for its use include RA, osteoarthritis, and posttraumatic arthritis. Most patients treated with implant arthroplasty can expect pain relief and 40° to 65° of PIP joint motion.2,3 Silicone arthroplasty historically has been used for pain relief but not for restoration of motion in an ankylosed joint. To our knowledge, there are no reports of using implant arthroplasty in the treatment of spontaneous ankylosis in RA. Contraindications for this procedure would include infection, irreparable flexor or extensor apparatus, and severe medical comorbidities.
In this article, we report a case of PIP joint autofusion treated with silicone PIP arthroplasty in a patient with RA. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
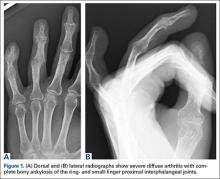
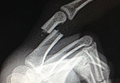
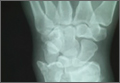
A 56-year-old woman who had had RA for more than 20 years underwent left carpometacarpal arthroplasty and thumb reconstruction. She subsequently presented with complaints of progressively worsening functioning of the left ring and small fingers. On initial evaluation, her PIP joints were fused in about 15° of flexion. Radiographs (Figures 1A, 1B) showed severe diffuse arthritis of the hands and complete bony ankylosis of the ring- and small-finger PIP joints with radial deviation of the ring-finger middle phalanx. The patient had minimal pain but wanted improved hand motion and opted for takedown of the ankylosis with silicone PIP joint arthroplasty.
Radial dorsal incisions were made over the PIP joints of the ring and small fingers. As is not the case with arthroplasty for routine PIP joint arthritis, presence of bony ankylosis made identification of the native PIP joint more difficult. The transverse retinacular ligament was identified and opened, and the collateral ligament, which was not ankylosed, was dissected off the proximal phalanx. These landmarks were useful in locating the PIP joint, and proper positioning was confirmed with fluoroscopy. The ankylosed joint space was opened with an osteotome, and about 8 to 10 mm of bone was resected to create space for the instrumentation. As the amount of scarring within the flexor tendon sheath was not significant, restoration of motion did not require extensive tenolysis. The extensor mechanism was slightly contracted, but the bony resection allowed flexion to be restored. The distal portion of the proximal phalanx was then resected. The proximal and middle phalanges were reamed, and a silicone prosthesis was placed with the finger held straight. The collateral ligament was repaired back to the proximal phalanx with 4-0 polydioxanone sutures placed through a bone tunnel created with a Kirschner wire. The skin was closed with 4-0 nylon, and a postoperative splint was applied.
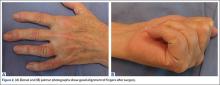
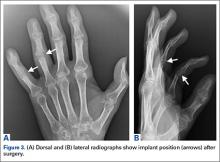
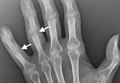
The initial postoperative course was unremarkable. The patient was immobilized in 10° of PIP joint flexion for 10 days, and therapy was initiated after the splint was removed. Twenty-four months after surgery, the patient was pain-free and had 60° of active PIP joint flexion, with extensor lag of only 10°. Clinically, alignment of the fingers was satisfactory; there was mild persistent radial deviation of 10° to 15° (Figures 2A, 2B). Radiographs showed good positioning of the implants (Figures 3A, 3B) and no sign of coronal instability. The patient was satisfied with her improved functioning and returned to employment as a hospital clerk, working full-time.
Discussion
RA of the hand and fingers can be painful and disabling. Although there are several treatment options for many of the most common manifestations, options are limited for bony ankylosis of the finger joints. The patient described in this case report had minimal pain, but the loss of motion of the PIP joints in her ring and small fingers created difficulties for her at work. She wanted surgery that would improve the functioning of her fingers. PIP joint arthroplasty traditionally has been the treatment of choice for PIP joint arthritis. In 1985, Swanson and colleagues2 reported on more than 400 silicone PIP arthroplasties performed over 16 years. Mean range of motion (ROM) was between 45° and 60°, with 70% of patients having ROM of more than 40°. Pain relief was complete in 98% of cases. Complications included implant fracture (5%) and recurrent or new deformities (6.5%). A 10.9% revision rate was noted at minimum 1-year follow-up. Recent implants made of improved biomaterials hold promise, but longer term follow-up is still needed.
Silicone arthroplasty has also been used as an effective treatment for non-RA of the PIP joint. Bales and colleagues4 reviewed long-term results of silicone arthroplasty for PIP joint osteoarthritis in 22 patients. At a mean of 10 years, mean QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score was 17, mean visual analog scale score for pain was 0.4, and implant survivorship was 90%. Despite unchanged ROM and considerable implant deformation or fracture, patients’ pain relief and satisfaction were consistent.
Hage and colleagues5 reviewed long-term results of silicone PIP arthroplasty for posttraumatic arthritis in 14 patients. Most of the patients were satisfied: Although they had notable rotational deformity, alignment deviation, and loss of pinch strength and ROM, they were pain-free. The authors concluded that silicone arthroplasty should be used for posttraumatic arthrosis cases in which associated adhesions may be corrected with simple tenolysis, and even in these cases the objective results may not be as good as the subjective outcome.
Kaye6 used radiographs to determine the incidence of bony ankylosis in 203 patients with RA. Hand and wrist radiographs of 48 (23.6%) of these patients showed ankylosis, and 34 of the 48 patients had 2 or more joints fused. On a questionnaire, patients with ankylosis indicated more difficulty with activities of daily living and more limited activity. The authors concluded that radiographic bony ankylosis was a relatively common feature of RA and a marker of disease that was clinically, radiographically, and functionally more severe.
The patient described in this case report had a satisfactory result after PIP joint arthroplasty. At 2-year follow-up, she remained pain-free, and her previously ankylosed PIP joint had an arc of motion of 10° to 60°. Most patients with bony ankylosis of PIP joints present with minimal pain and do not seek surgical treatment. However, patients with ankylosis that limits functioning or activities of daily living may wish to pursue intervention that could be restorative. PIP joint arthroplasty may be effective in improving motion in patients with bony ankylosis of the finger joints.
1. Kaye JJ, Callahan LF, Nance EP Jr, Brooks R, Pincus T. Bony ankylosis in rheumatoid arthritis. Associations with longer duration and greater severity of disease. Invest Radiol. 1987;22(4):303-309.
2. Swanson AB, Maupin BK, Gajjar NV, Swanson GD. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J Hand Surg Am. 1985;10(6 pt 1):796-805.
3. Rizzo M, Beckenbaugh RD. Proximal interphalangeal joint arthroplasty. J Am Acad Orthop Surg. 2007;15(3):189-197.
4. Bales J, Wall L, Stern PJ. Long-term results of Swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J Hand Surg Am. 2014;39(3):455-461.
5. Hage J, Yoe E, Zering J, de Groot P. Proximal interphalangeal joint silicone arthroplasty for posttraumatic arthritis. J Hand Surg Am. 1999;24(1):73-77.
6. Kaye JJ. Radiographic assessment of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21(2):395-406.
Rheumatoid arthritis (RA) commonly affects the hand and fingers, most often at the metacarpophalangeal and proximal interphalangeal (PIP) joints. Synovitis, tendon ruptures, Boutonnière and swan-neck deformities, and joint destruction often occur. Bony ankylosis is not commonly described yet frequently occurs in patients with RA.1
Implant arthroplasty is an established treatment for arthritis of the hand and fingers. Indications for its use include RA, osteoarthritis, and posttraumatic arthritis. Most patients treated with implant arthroplasty can expect pain relief and 40° to 65° of PIP joint motion.2,3 Silicone arthroplasty historically has been used for pain relief but not for restoration of motion in an ankylosed joint. To our knowledge, there are no reports of using implant arthroplasty in the treatment of spontaneous ankylosis in RA. Contraindications for this procedure would include infection, irreparable flexor or extensor apparatus, and severe medical comorbidities.
In this article, we report a case of PIP joint autofusion treated with silicone PIP arthroplasty in a patient with RA. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman who had had RA for more than 20 years underwent left carpometacarpal arthroplasty and thumb reconstruction. She subsequently presented with complaints of progressively worsening functioning of the left ring and small fingers. On initial evaluation, her PIP joints were fused in about 15° of flexion. Radiographs (Figures 1A, 1B) showed severe diffuse arthritis of the hands and complete bony ankylosis of the ring- and small-finger PIP joints with radial deviation of the ring-finger middle phalanx. The patient had minimal pain but wanted improved hand motion and opted for takedown of the ankylosis with silicone PIP joint arthroplasty.
Radial dorsal incisions were made over the PIP joints of the ring and small fingers. As is not the case with arthroplasty for routine PIP joint arthritis, presence of bony ankylosis made identification of the native PIP joint more difficult. The transverse retinacular ligament was identified and opened, and the collateral ligament, which was not ankylosed, was dissected off the proximal phalanx. These landmarks were useful in locating the PIP joint, and proper positioning was confirmed with fluoroscopy. The ankylosed joint space was opened with an osteotome, and about 8 to 10 mm of bone was resected to create space for the instrumentation. As the amount of scarring within the flexor tendon sheath was not significant, restoration of motion did not require extensive tenolysis. The extensor mechanism was slightly contracted, but the bony resection allowed flexion to be restored. The distal portion of the proximal phalanx was then resected. The proximal and middle phalanges were reamed, and a silicone prosthesis was placed with the finger held straight. The collateral ligament was repaired back to the proximal phalanx with 4-0 polydioxanone sutures placed through a bone tunnel created with a Kirschner wire. The skin was closed with 4-0 nylon, and a postoperative splint was applied.
The initial postoperative course was unremarkable. The patient was immobilized in 10° of PIP joint flexion for 10 days, and therapy was initiated after the splint was removed. Twenty-four months after surgery, the patient was pain-free and had 60° of active PIP joint flexion, with extensor lag of only 10°. Clinically, alignment of the fingers was satisfactory; there was mild persistent radial deviation of 10° to 15° (Figures 2A, 2B). Radiographs showed good positioning of the implants (Figures 3A, 3B) and no sign of coronal instability. The patient was satisfied with her improved functioning and returned to employment as a hospital clerk, working full-time.
Discussion
RA of the hand and fingers can be painful and disabling. Although there are several treatment options for many of the most common manifestations, options are limited for bony ankylosis of the finger joints. The patient described in this case report had minimal pain, but the loss of motion of the PIP joints in her ring and small fingers created difficulties for her at work. She wanted surgery that would improve the functioning of her fingers. PIP joint arthroplasty traditionally has been the treatment of choice for PIP joint arthritis. In 1985, Swanson and colleagues2 reported on more than 400 silicone PIP arthroplasties performed over 16 years. Mean range of motion (ROM) was between 45° and 60°, with 70% of patients having ROM of more than 40°. Pain relief was complete in 98% of cases. Complications included implant fracture (5%) and recurrent or new deformities (6.5%). A 10.9% revision rate was noted at minimum 1-year follow-up. Recent implants made of improved biomaterials hold promise, but longer term follow-up is still needed.
Silicone arthroplasty has also been used as an effective treatment for non-RA of the PIP joint. Bales and colleagues4 reviewed long-term results of silicone arthroplasty for PIP joint osteoarthritis in 22 patients. At a mean of 10 years, mean QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score was 17, mean visual analog scale score for pain was 0.4, and implant survivorship was 90%. Despite unchanged ROM and considerable implant deformation or fracture, patients’ pain relief and satisfaction were consistent.
Hage and colleagues5 reviewed long-term results of silicone PIP arthroplasty for posttraumatic arthritis in 14 patients. Most of the patients were satisfied: Although they had notable rotational deformity, alignment deviation, and loss of pinch strength and ROM, they were pain-free. The authors concluded that silicone arthroplasty should be used for posttraumatic arthrosis cases in which associated adhesions may be corrected with simple tenolysis, and even in these cases the objective results may not be as good as the subjective outcome.
Kaye6 used radiographs to determine the incidence of bony ankylosis in 203 patients with RA. Hand and wrist radiographs of 48 (23.6%) of these patients showed ankylosis, and 34 of the 48 patients had 2 or more joints fused. On a questionnaire, patients with ankylosis indicated more difficulty with activities of daily living and more limited activity. The authors concluded that radiographic bony ankylosis was a relatively common feature of RA and a marker of disease that was clinically, radiographically, and functionally more severe.
The patient described in this case report had a satisfactory result after PIP joint arthroplasty. At 2-year follow-up, she remained pain-free, and her previously ankylosed PIP joint had an arc of motion of 10° to 60°. Most patients with bony ankylosis of PIP joints present with minimal pain and do not seek surgical treatment. However, patients with ankylosis that limits functioning or activities of daily living may wish to pursue intervention that could be restorative. PIP joint arthroplasty may be effective in improving motion in patients with bony ankylosis of the finger joints.
Rheumatoid arthritis (RA) commonly affects the hand and fingers, most often at the metacarpophalangeal and proximal interphalangeal (PIP) joints. Synovitis, tendon ruptures, Boutonnière and swan-neck deformities, and joint destruction often occur. Bony ankylosis is not commonly described yet frequently occurs in patients with RA.1
Implant arthroplasty is an established treatment for arthritis of the hand and fingers. Indications for its use include RA, osteoarthritis, and posttraumatic arthritis. Most patients treated with implant arthroplasty can expect pain relief and 40° to 65° of PIP joint motion.2,3 Silicone arthroplasty historically has been used for pain relief but not for restoration of motion in an ankylosed joint. To our knowledge, there are no reports of using implant arthroplasty in the treatment of spontaneous ankylosis in RA. Contraindications for this procedure would include infection, irreparable flexor or extensor apparatus, and severe medical comorbidities.
In this article, we report a case of PIP joint autofusion treated with silicone PIP arthroplasty in a patient with RA. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman who had had RA for more than 20 years underwent left carpometacarpal arthroplasty and thumb reconstruction. She subsequently presented with complaints of progressively worsening functioning of the left ring and small fingers. On initial evaluation, her PIP joints were fused in about 15° of flexion. Radiographs (Figures 1A, 1B) showed severe diffuse arthritis of the hands and complete bony ankylosis of the ring- and small-finger PIP joints with radial deviation of the ring-finger middle phalanx. The patient had minimal pain but wanted improved hand motion and opted for takedown of the ankylosis with silicone PIP joint arthroplasty.
Radial dorsal incisions were made over the PIP joints of the ring and small fingers. As is not the case with arthroplasty for routine PIP joint arthritis, presence of bony ankylosis made identification of the native PIP joint more difficult. The transverse retinacular ligament was identified and opened, and the collateral ligament, which was not ankylosed, was dissected off the proximal phalanx. These landmarks were useful in locating the PIP joint, and proper positioning was confirmed with fluoroscopy. The ankylosed joint space was opened with an osteotome, and about 8 to 10 mm of bone was resected to create space for the instrumentation. As the amount of scarring within the flexor tendon sheath was not significant, restoration of motion did not require extensive tenolysis. The extensor mechanism was slightly contracted, but the bony resection allowed flexion to be restored. The distal portion of the proximal phalanx was then resected. The proximal and middle phalanges were reamed, and a silicone prosthesis was placed with the finger held straight. The collateral ligament was repaired back to the proximal phalanx with 4-0 polydioxanone sutures placed through a bone tunnel created with a Kirschner wire. The skin was closed with 4-0 nylon, and a postoperative splint was applied.
The initial postoperative course was unremarkable. The patient was immobilized in 10° of PIP joint flexion for 10 days, and therapy was initiated after the splint was removed. Twenty-four months after surgery, the patient was pain-free and had 60° of active PIP joint flexion, with extensor lag of only 10°. Clinically, alignment of the fingers was satisfactory; there was mild persistent radial deviation of 10° to 15° (Figures 2A, 2B). Radiographs showed good positioning of the implants (Figures 3A, 3B) and no sign of coronal instability. The patient was satisfied with her improved functioning and returned to employment as a hospital clerk, working full-time.
Discussion
RA of the hand and fingers can be painful and disabling. Although there are several treatment options for many of the most common manifestations, options are limited for bony ankylosis of the finger joints. The patient described in this case report had minimal pain, but the loss of motion of the PIP joints in her ring and small fingers created difficulties for her at work. She wanted surgery that would improve the functioning of her fingers. PIP joint arthroplasty traditionally has been the treatment of choice for PIP joint arthritis. In 1985, Swanson and colleagues2 reported on more than 400 silicone PIP arthroplasties performed over 16 years. Mean range of motion (ROM) was between 45° and 60°, with 70% of patients having ROM of more than 40°. Pain relief was complete in 98% of cases. Complications included implant fracture (5%) and recurrent or new deformities (6.5%). A 10.9% revision rate was noted at minimum 1-year follow-up. Recent implants made of improved biomaterials hold promise, but longer term follow-up is still needed.
Silicone arthroplasty has also been used as an effective treatment for non-RA of the PIP joint. Bales and colleagues4 reviewed long-term results of silicone arthroplasty for PIP joint osteoarthritis in 22 patients. At a mean of 10 years, mean QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score was 17, mean visual analog scale score for pain was 0.4, and implant survivorship was 90%. Despite unchanged ROM and considerable implant deformation or fracture, patients’ pain relief and satisfaction were consistent.
Hage and colleagues5 reviewed long-term results of silicone PIP arthroplasty for posttraumatic arthritis in 14 patients. Most of the patients were satisfied: Although they had notable rotational deformity, alignment deviation, and loss of pinch strength and ROM, they were pain-free. The authors concluded that silicone arthroplasty should be used for posttraumatic arthrosis cases in which associated adhesions may be corrected with simple tenolysis, and even in these cases the objective results may not be as good as the subjective outcome.
Kaye6 used radiographs to determine the incidence of bony ankylosis in 203 patients with RA. Hand and wrist radiographs of 48 (23.6%) of these patients showed ankylosis, and 34 of the 48 patients had 2 or more joints fused. On a questionnaire, patients with ankylosis indicated more difficulty with activities of daily living and more limited activity. The authors concluded that radiographic bony ankylosis was a relatively common feature of RA and a marker of disease that was clinically, radiographically, and functionally more severe.
The patient described in this case report had a satisfactory result after PIP joint arthroplasty. At 2-year follow-up, she remained pain-free, and her previously ankylosed PIP joint had an arc of motion of 10° to 60°. Most patients with bony ankylosis of PIP joints present with minimal pain and do not seek surgical treatment. However, patients with ankylosis that limits functioning or activities of daily living may wish to pursue intervention that could be restorative. PIP joint arthroplasty may be effective in improving motion in patients with bony ankylosis of the finger joints.
1. Kaye JJ, Callahan LF, Nance EP Jr, Brooks R, Pincus T. Bony ankylosis in rheumatoid arthritis. Associations with longer duration and greater severity of disease. Invest Radiol. 1987;22(4):303-309.
2. Swanson AB, Maupin BK, Gajjar NV, Swanson GD. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J Hand Surg Am. 1985;10(6 pt 1):796-805.
3. Rizzo M, Beckenbaugh RD. Proximal interphalangeal joint arthroplasty. J Am Acad Orthop Surg. 2007;15(3):189-197.
4. Bales J, Wall L, Stern PJ. Long-term results of Swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J Hand Surg Am. 2014;39(3):455-461.
5. Hage J, Yoe E, Zering J, de Groot P. Proximal interphalangeal joint silicone arthroplasty for posttraumatic arthritis. J Hand Surg Am. 1999;24(1):73-77.
6. Kaye JJ. Radiographic assessment of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21(2):395-406.
1. Kaye JJ, Callahan LF, Nance EP Jr, Brooks R, Pincus T. Bony ankylosis in rheumatoid arthritis. Associations with longer duration and greater severity of disease. Invest Radiol. 1987;22(4):303-309.
2. Swanson AB, Maupin BK, Gajjar NV, Swanson GD. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J Hand Surg Am. 1985;10(6 pt 1):796-805.
3. Rizzo M, Beckenbaugh RD. Proximal interphalangeal joint arthroplasty. J Am Acad Orthop Surg. 2007;15(3):189-197.
4. Bales J, Wall L, Stern PJ. Long-term results of Swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J Hand Surg Am. 2014;39(3):455-461.
5. Hage J, Yoe E, Zering J, de Groot P. Proximal interphalangeal joint silicone arthroplasty for posttraumatic arthritis. J Hand Surg Am. 1999;24(1):73-77.
6. Kaye JJ. Radiographic assessment of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21(2):395-406.
Carpal tunnel syndrome: Guidelines rate evidence for diagnosis, treatment
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.
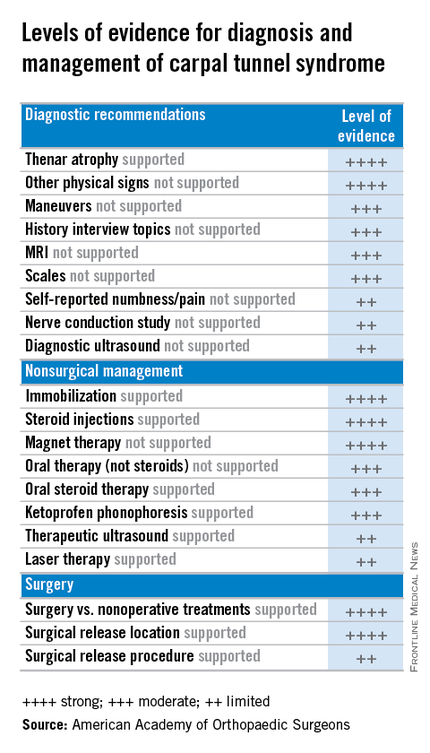
Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.

Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.

Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
Partial Flexor Tendon Laceration Assessment: Interobserver and Intraobserver Reliability
How to manage complete flexor tendon lacerations in the hand is well documented and a subject of relative agreement among authors. However, treatment of partial flexor tendon lacerations is controversial and lacking clear consensus in the literature. Managing these injuries can be challenging, as clinicians must weigh the diminished tensile strength in the injured tendon and the potential for later complications (eg, entrapment, triggering, rupture) against the negative effects of tenorrhaphy.1 Several studies have found impaired tendon gliding on the basis of bulk and inflammatory reaction secondary to suture material within the flexor sheath as well as decreased tendon strength after tenorrhaphy.2-6 This finding led the investigators to recommend nonsurgical management for partial lacerations up to as much as 95% of the cross-sectional area (CSA) of the tendon. According to a survey by McCarthy and colleagues,7 45% of 591 members of the American Society for Surgery of the Hand (ASSH) indicated they would perform tenorrhaphy for a laceration that involved more than 50% of the tendon.
However, accurate assessment of partial-thickness flexor tendon lacerations is difficult owing to the subjectivity of evaluation. In the survey just mentioned,7 the majority of surgeons used the naked eye to make assessments, and only 14% used other means, such as a ruler, a pair of calipers, or loupe magnification. In addition, flexor tendon injuries are often evaluated under less than ideal circumstances—a dirty or bloody field, poor lighting, an uncomfortable patient.
We conducted a study to determine the interobserver and intraobserver reliability of surgeons assessing the percentage of CSA injured in partially lacerated digital flexor tendons. We hypothesized that participants’ accuracy and agreement would be poor.
Materials and Methods
Eight 1-cm transverse, volar skin incisions were made over the midportions of the middle and proximal phalanges of the index, middle, ring, and small fingers of a fresh-frozen human cadaver hand (Figure 1). The tendon sheaths were incised, and the flexor digitorum profundus tendons to each digit were delivered through the wound. With use of a method described previously by Manning and colleagues,8 the tendon was then placed over a flat metal post to be used as a cutting board, and the proposed laceration site was marked with ink. Under loupe magnification, a No. 15 blade was used to create a partial transverse, volar-to-dorsal laceration in each tendon.8 The goal was to create lacerations of about 30%, 50%, and 70% of the total CSA of the tendon. The tendons were then returned to the wound, and visibility of the marked laceration within the wound was ensured. A similar exercise was performed at the level of the proximal palmar crease. Four flexor digitorum superficialis tendons were exposed through 1-cm transverse incisions, and partial lacerations were made in the volar substance of the tendons. The tendons were then returned to the wound, resulting in 12 partially lacerated tendons (8 flexor digitorum profundus, 4 flexor digitorum superficialis).
Six orthopedic surgery residents (2 postgraduate year 1 [PGY-1], 2 PGY-3, 2 PGY-5) and 4 fellowship-trained hand surgeons participated in our study. Each was asked to evaluate the tendons and determine the percentage of total CSA lacerated. Loupe magnification and measuring tools were not permitted, but participants were allowed to handle the tendons. In addition, they were asked if they would perform tenorrhaphy on the injured tendons, given only the amount of injury. The participants repeated this exercise 4 weeks later.
After all measurements were made, a longitudinal incision was made down each of the digits, and the flexor tendons were exposed within the flexor sheath. The transverse incisions in the palm were connected to expose the flexor digitorum superficialis tendons. Under an operating microscope, a pair of digital microcalipers (Kobalt 0.5-ft Metric and SAE Caliper; Figure 2) accurate to 0.01 mm was used to measure the external width (a) and height (b + bˈ) of the tendons just proximal to the lacerations. Measurements were made with the caliper blades just touching the edges of the lacerated tendon, thus minimizing deformation of the tendon. Other measurements made at the laceration site were width of the remaining tendon (c) and height of the remaining tendon (bˈ). CSA of the tendon was calculated assuming a regular ellipsoid shape and using the equation:
Area = 1/2π(b+b')
The area of the tendon injured was determined by calculating the area under a parabola and using the equation:
Area = 2/3c[(b+b')-b']
Last, the percentage of total CSA lacerated was calculated using the equation:
Area (total area)
Statistical analysis was performed to determine accuracy and interobserver and intraobserver reliability. Paired t tests were used in the assessment of accuracy to determine if there were differences between estimated and calibrated measurements.
Results
The 10 participants’ estimates differed significantly (P < .0006) from the calibrated measurements, as did residents’ estimates (P < .0025) and fellowship-trained hand surgeons’ estimates (P < .0002). Estimates were scored 1 to 5 on the basis of proximity to calibrated measurements (Table 1). Thus, more accurate estimates received lower scores. Individual estimates were then scored and stratified into groups for comparison. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. These results are listed in Table 2. Once overall and grouped accuracy was analyzed, κ statistics were calculated to compare interobserver and intraobserver reliability. Overall interobserver agreement was poor for both initial readings (κ = 0.16) and secondary readings (κ = 0.16), indicating poor strength of agreement between individuals both initially and secondarily. Table 3 presents the κ interpretations. There was moderate overall intraobserver agreement (45.83%), indicating participants’ secondary estimates agreed with their primary estimates 46% of the time. Fellowship-trained hand surgeons and first-year residents had the highest intraobserver agreement (50.0%). These results are listed in Table 4.
Discussion
Accurate assessment of partial flexor tendon lacerations is difficult and subjective. There is no standardized method for determining the extent of injury, regardless of whether the evaluation is performed in an emergency department or in the operating room. As McCarthy and colleagues7 noted in their survey of ASSH members, naked eye assessment was by far the most popular means of estimating percentage injured in partial lacerations, and only 10% of the survey respondents used intraoperative measuring devices. Our study showed that participants agreed with one another less than 50% of the time when evaluating injuries without the aid of measuring devices. In addition, interobserver agreement in this study was about 50%, highlighting the difficulty in making an accurate and reproducible assessment.
In a study of canine flexor tendons, McCarthy and colleagues9 found calipers are inaccurate as well and do not provide a reliable means of assessing partial flexor tendon lacerations. They compared caliper measurements with laser micrometer measurements, and the differences averaged 29.3%. They suggested that methods for calculating loss of CSA and for creating precise lacerations must be developed in order to evaluate treatments. One such method is the “tenotome,” devised by Hitchcock and colleagues10: A device with standard scalpel blades is used to make uniform lacerations in tendons by leaving a constant area of the tendon intact, regardless of the size or shape of the original tendon. Measurements made with calipers or rulers assume the tendon has a regular ellipsoid shape, but in reality the shape is a double-ellipse, particularly within the flexor sheath.
Dobyns and colleagues11 observed that changes in CSA size can be related to changes in the size of the bundle pattern of the tendon. They found that, on average, the radial bundle comprised about 60% of the total CSA of the tendon. This finding was clarified by Grewal and colleagues.12 Using histologic sections of tendons plus photomicrographs, they determined that, in zone II of the index and small fingers, the ulnar bundle had an area consistently larger than 50% and the radial bundle less than 50% of the total tendon area. In the ring and middle fingers, the areas of both bundles were almost 50% of the total tendon area. The authors suggested that, using this bundle pattern theory of injury, surgeons could more accurately evaluate the extent of injury with the naked eye.
One of the questions that prompted our study is how reliable is the information a surgeon receives regarding a partial flexor tendon injury evaluated by someone else in another setting. What is done with this information is another question. The scenario can be considered in 2 settings: emergency department and operating room.
Given the poor accuracy and interobserver agreement found in our study, along with the inaccuracy of caliper and ruler measurements, it seems decisions to perform tenorrhaphy based on reported percentages lacerated are unreliable. Our results showed that the ability to accurately assess partial tendon injuries does not improve with surgeon experience, as fellowship-trained hand surgeons were not statistically more accurate or consistent than residents. To this effect, one institution treats all its partial flexor tendon lacerations with wound inspection and irrigation in the emergency department, under digital block and after neurovascular injury has been excluded.8 If the patient is able to actively flex and extend the digit without triggering, then the wound is closed without closing the tendon sheath, a dorsal blocking splint is applied, and motion is begun early, 48 hours later, regardless of laceration severity.
Once the decision has been made to go to the operating room and the injury is being evaluated, what should be done with the information from the measurement, whether made with loupe magnification, calipers, rulers, or the naked eye? Surgeons must weigh the risks for triggering, entrapment, and rupture of untreated partial tendon lacerations1 with the added bulk and potential for adhesions, along with the tensile strength reduction that accompanies tendon repair. Both Reynolds and colleagues13 and Ollinger and colleagues14 found tensile strength significantly diminished in sutured tendons. Ollinger and colleagues14 showed a decrease in tendon gliding after surgical exposure and tenorrhaphy for partial tendon lacerations. Reynolds and colleagues13 concluded that surgical repair leads to poorer results than nonsurgical treatment.
Clinical studies have demonstrated excellent results with nonintervention, and in vivo and in vitro studies have indicated that early motion can be initiated in partial lacerations of up to 95% of total CSA. Wray and Weeks6 treated 26 patients with partial lacerations varying from 25% to 95% of total CSA and noted 1 incidence of trigger finger (which resolved) and no late ruptures. They advocated treatment with early motion and excision or repair of beveled partial lacerations with simple sutures. Stahl and colleagues2 reported comparable outcomes in children with partial lacerations up to 75% of total CSA treated with and without surgery and noted no complications in either group. In a biomechanical study, Hariharan and colleagues4 found lacerations up to 75% can withstand forces associated with active unresisted mobilization.
Conversely, how many patients or surgeons want to return to the operating room to fix a late rupture when it could have been repaired in the primary setting? Schlenker and colleagues,1 reporting on a late flexor pollicus tendon rupture that required tendon grafting, recommended exploration and primary repair of all partial flexor tendon lacerations. Often, it is difficult to determine whether surgical repair is necessary to ensure the best outcome for the patient.
Our study results showed that, in the evaluation of flexor tendon lacerations, both accuracy and interobserver agreement were poor among residents and fellowship-trained hand surgeons, and intraobserver agreement was moderate. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. Our results highlight the difficulty in making accurate assessments of flexor tendon lacerations owing to the subjectivity of evaluation, which appear not to improve with surgeon experience.
1. Schlenker JD, Lister GD, Kleinert HE. Three complications of untreated partial laceration of flexor tendon—entrapment, rupture, and triggering. J Hand Surg Am. 1981;6(4):392-398.
2. Stahl S, Kaufman T, Bialik V. Partial lacerations of flexor tendons in children. Primary repair versus conservative treatment. J Hand Surg Br. 1997;22(3):377-380.
3. Al-Qattan MM. Conservative management of zone II partial flexor tendon lacerations greater than half the width of the tendon. J Hand Surg Am. 2000;25(6):1118-1121.
4. Hariharan JS, Diao E, Soejima O, Lotz JC. Partial lacerations of human digital flexor tendons: a biomechanical analysis. J Hand Surg Am. 1997;22(6):1011-1015.
5. Bishop AT, Cooney WP 3rd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26(4):301-312.
6. Wray RC Jr, Weeks PM. Treatment of partial tendon lacerations. Hand. 1980;12(2):163-166.
7. McCarthy DM, Boardman ND 3rd, Tramaglini DM, Sotereanos DG, Herndon JH. Clinical management of partially lacerated digital flexor tendons: a survey of hand surgeons. J Hand Surg Am. 1995;20(2):273-275.
8. Manning DW, Spiguel AR, Mass DP. Biomechanical analysis of partial flexor tendon lacerations in zone II of human cadavers. J Hand Surg Am. 2010;35(1):11-18.
9. McCarthy DM, Tramaglini DM, Chan SS, Schmidt CC, Sotereanos DG, Herndon JH. Effect of partial laceration on the structural properties of the canine FDP tendon: an in vitro study. J Hand Surg Am. 1995;20(5):795-800.
10. Hitchcock TF, Candel AG, Light TR, Blevens AD. New technique for producing uniform partial lacerations of tendons. J Orthop Res. 1989;7(3):451-455.
11. Dobyns RC, Cooney WC, Wood MB. Effect of partial lacerations on canine flexor tendons. Minn Med. 1982;65(1):27-32.
12. Grewal R, Sotereanos DG, Rao U, Herndon JH, Woo SL. Bundle pattern of the flexor digitorum profundus tendon in zone II of the hand: a quantitative assessment of the size of a laceration. J Hand Surg Am. 1996;21(6):978-983.
13. Reynolds B, Wray RC Jr, Weeks PM. Should an incompletely severed tendon be sutured? Plast Reconstr Surg. 1976;57(1):36-38.
14. Ollinger H, Wray RC Jr, Weeks PM. Effects of suture on tensile strength gain of partially and completely severed tendons. Surg Forum. 1975;26:63-64.
How to manage complete flexor tendon lacerations in the hand is well documented and a subject of relative agreement among authors. However, treatment of partial flexor tendon lacerations is controversial and lacking clear consensus in the literature. Managing these injuries can be challenging, as clinicians must weigh the diminished tensile strength in the injured tendon and the potential for later complications (eg, entrapment, triggering, rupture) against the negative effects of tenorrhaphy.1 Several studies have found impaired tendon gliding on the basis of bulk and inflammatory reaction secondary to suture material within the flexor sheath as well as decreased tendon strength after tenorrhaphy.2-6 This finding led the investigators to recommend nonsurgical management for partial lacerations up to as much as 95% of the cross-sectional area (CSA) of the tendon. According to a survey by McCarthy and colleagues,7 45% of 591 members of the American Society for Surgery of the Hand (ASSH) indicated they would perform tenorrhaphy for a laceration that involved more than 50% of the tendon.
However, accurate assessment of partial-thickness flexor tendon lacerations is difficult owing to the subjectivity of evaluation. In the survey just mentioned,7 the majority of surgeons used the naked eye to make assessments, and only 14% used other means, such as a ruler, a pair of calipers, or loupe magnification. In addition, flexor tendon injuries are often evaluated under less than ideal circumstances—a dirty or bloody field, poor lighting, an uncomfortable patient.
We conducted a study to determine the interobserver and intraobserver reliability of surgeons assessing the percentage of CSA injured in partially lacerated digital flexor tendons. We hypothesized that participants’ accuracy and agreement would be poor.
Materials and Methods
Eight 1-cm transverse, volar skin incisions were made over the midportions of the middle and proximal phalanges of the index, middle, ring, and small fingers of a fresh-frozen human cadaver hand (Figure 1). The tendon sheaths were incised, and the flexor digitorum profundus tendons to each digit were delivered through the wound. With use of a method described previously by Manning and colleagues,8 the tendon was then placed over a flat metal post to be used as a cutting board, and the proposed laceration site was marked with ink. Under loupe magnification, a No. 15 blade was used to create a partial transverse, volar-to-dorsal laceration in each tendon.8 The goal was to create lacerations of about 30%, 50%, and 70% of the total CSA of the tendon. The tendons were then returned to the wound, and visibility of the marked laceration within the wound was ensured. A similar exercise was performed at the level of the proximal palmar crease. Four flexor digitorum superficialis tendons were exposed through 1-cm transverse incisions, and partial lacerations were made in the volar substance of the tendons. The tendons were then returned to the wound, resulting in 12 partially lacerated tendons (8 flexor digitorum profundus, 4 flexor digitorum superficialis).
Six orthopedic surgery residents (2 postgraduate year 1 [PGY-1], 2 PGY-3, 2 PGY-5) and 4 fellowship-trained hand surgeons participated in our study. Each was asked to evaluate the tendons and determine the percentage of total CSA lacerated. Loupe magnification and measuring tools were not permitted, but participants were allowed to handle the tendons. In addition, they were asked if they would perform tenorrhaphy on the injured tendons, given only the amount of injury. The participants repeated this exercise 4 weeks later.
After all measurements were made, a longitudinal incision was made down each of the digits, and the flexor tendons were exposed within the flexor sheath. The transverse incisions in the palm were connected to expose the flexor digitorum superficialis tendons. Under an operating microscope, a pair of digital microcalipers (Kobalt 0.5-ft Metric and SAE Caliper; Figure 2) accurate to 0.01 mm was used to measure the external width (a) and height (b + bˈ) of the tendons just proximal to the lacerations. Measurements were made with the caliper blades just touching the edges of the lacerated tendon, thus minimizing deformation of the tendon. Other measurements made at the laceration site were width of the remaining tendon (c) and height of the remaining tendon (bˈ). CSA of the tendon was calculated assuming a regular ellipsoid shape and using the equation:
Area = 1/2π(b+b')
The area of the tendon injured was determined by calculating the area under a parabola and using the equation:
Area = 2/3c[(b+b')-b']
Last, the percentage of total CSA lacerated was calculated using the equation:
Area (total area)
Statistical analysis was performed to determine accuracy and interobserver and intraobserver reliability. Paired t tests were used in the assessment of accuracy to determine if there were differences between estimated and calibrated measurements.
Results
The 10 participants’ estimates differed significantly (P < .0006) from the calibrated measurements, as did residents’ estimates (P < .0025) and fellowship-trained hand surgeons’ estimates (P < .0002). Estimates were scored 1 to 5 on the basis of proximity to calibrated measurements (Table 1). Thus, more accurate estimates received lower scores. Individual estimates were then scored and stratified into groups for comparison. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. These results are listed in Table 2. Once overall and grouped accuracy was analyzed, κ statistics were calculated to compare interobserver and intraobserver reliability. Overall interobserver agreement was poor for both initial readings (κ = 0.16) and secondary readings (κ = 0.16), indicating poor strength of agreement between individuals both initially and secondarily. Table 3 presents the κ interpretations. There was moderate overall intraobserver agreement (45.83%), indicating participants’ secondary estimates agreed with their primary estimates 46% of the time. Fellowship-trained hand surgeons and first-year residents had the highest intraobserver agreement (50.0%). These results are listed in Table 4.
Discussion
Accurate assessment of partial flexor tendon lacerations is difficult and subjective. There is no standardized method for determining the extent of injury, regardless of whether the evaluation is performed in an emergency department or in the operating room. As McCarthy and colleagues7 noted in their survey of ASSH members, naked eye assessment was by far the most popular means of estimating percentage injured in partial lacerations, and only 10% of the survey respondents used intraoperative measuring devices. Our study showed that participants agreed with one another less than 50% of the time when evaluating injuries without the aid of measuring devices. In addition, interobserver agreement in this study was about 50%, highlighting the difficulty in making an accurate and reproducible assessment.
In a study of canine flexor tendons, McCarthy and colleagues9 found calipers are inaccurate as well and do not provide a reliable means of assessing partial flexor tendon lacerations. They compared caliper measurements with laser micrometer measurements, and the differences averaged 29.3%. They suggested that methods for calculating loss of CSA and for creating precise lacerations must be developed in order to evaluate treatments. One such method is the “tenotome,” devised by Hitchcock and colleagues10: A device with standard scalpel blades is used to make uniform lacerations in tendons by leaving a constant area of the tendon intact, regardless of the size or shape of the original tendon. Measurements made with calipers or rulers assume the tendon has a regular ellipsoid shape, but in reality the shape is a double-ellipse, particularly within the flexor sheath.
Dobyns and colleagues11 observed that changes in CSA size can be related to changes in the size of the bundle pattern of the tendon. They found that, on average, the radial bundle comprised about 60% of the total CSA of the tendon. This finding was clarified by Grewal and colleagues.12 Using histologic sections of tendons plus photomicrographs, they determined that, in zone II of the index and small fingers, the ulnar bundle had an area consistently larger than 50% and the radial bundle less than 50% of the total tendon area. In the ring and middle fingers, the areas of both bundles were almost 50% of the total tendon area. The authors suggested that, using this bundle pattern theory of injury, surgeons could more accurately evaluate the extent of injury with the naked eye.
One of the questions that prompted our study is how reliable is the information a surgeon receives regarding a partial flexor tendon injury evaluated by someone else in another setting. What is done with this information is another question. The scenario can be considered in 2 settings: emergency department and operating room.
Given the poor accuracy and interobserver agreement found in our study, along with the inaccuracy of caliper and ruler measurements, it seems decisions to perform tenorrhaphy based on reported percentages lacerated are unreliable. Our results showed that the ability to accurately assess partial tendon injuries does not improve with surgeon experience, as fellowship-trained hand surgeons were not statistically more accurate or consistent than residents. To this effect, one institution treats all its partial flexor tendon lacerations with wound inspection and irrigation in the emergency department, under digital block and after neurovascular injury has been excluded.8 If the patient is able to actively flex and extend the digit without triggering, then the wound is closed without closing the tendon sheath, a dorsal blocking splint is applied, and motion is begun early, 48 hours later, regardless of laceration severity.
Once the decision has been made to go to the operating room and the injury is being evaluated, what should be done with the information from the measurement, whether made with loupe magnification, calipers, rulers, or the naked eye? Surgeons must weigh the risks for triggering, entrapment, and rupture of untreated partial tendon lacerations1 with the added bulk and potential for adhesions, along with the tensile strength reduction that accompanies tendon repair. Both Reynolds and colleagues13 and Ollinger and colleagues14 found tensile strength significantly diminished in sutured tendons. Ollinger and colleagues14 showed a decrease in tendon gliding after surgical exposure and tenorrhaphy for partial tendon lacerations. Reynolds and colleagues13 concluded that surgical repair leads to poorer results than nonsurgical treatment.
Clinical studies have demonstrated excellent results with nonintervention, and in vivo and in vitro studies have indicated that early motion can be initiated in partial lacerations of up to 95% of total CSA. Wray and Weeks6 treated 26 patients with partial lacerations varying from 25% to 95% of total CSA and noted 1 incidence of trigger finger (which resolved) and no late ruptures. They advocated treatment with early motion and excision or repair of beveled partial lacerations with simple sutures. Stahl and colleagues2 reported comparable outcomes in children with partial lacerations up to 75% of total CSA treated with and without surgery and noted no complications in either group. In a biomechanical study, Hariharan and colleagues4 found lacerations up to 75% can withstand forces associated with active unresisted mobilization.
Conversely, how many patients or surgeons want to return to the operating room to fix a late rupture when it could have been repaired in the primary setting? Schlenker and colleagues,1 reporting on a late flexor pollicus tendon rupture that required tendon grafting, recommended exploration and primary repair of all partial flexor tendon lacerations. Often, it is difficult to determine whether surgical repair is necessary to ensure the best outcome for the patient.
Our study results showed that, in the evaluation of flexor tendon lacerations, both accuracy and interobserver agreement were poor among residents and fellowship-trained hand surgeons, and intraobserver agreement was moderate. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. Our results highlight the difficulty in making accurate assessments of flexor tendon lacerations owing to the subjectivity of evaluation, which appear not to improve with surgeon experience.
How to manage complete flexor tendon lacerations in the hand is well documented and a subject of relative agreement among authors. However, treatment of partial flexor tendon lacerations is controversial and lacking clear consensus in the literature. Managing these injuries can be challenging, as clinicians must weigh the diminished tensile strength in the injured tendon and the potential for later complications (eg, entrapment, triggering, rupture) against the negative effects of tenorrhaphy.1 Several studies have found impaired tendon gliding on the basis of bulk and inflammatory reaction secondary to suture material within the flexor sheath as well as decreased tendon strength after tenorrhaphy.2-6 This finding led the investigators to recommend nonsurgical management for partial lacerations up to as much as 95% of the cross-sectional area (CSA) of the tendon. According to a survey by McCarthy and colleagues,7 45% of 591 members of the American Society for Surgery of the Hand (ASSH) indicated they would perform tenorrhaphy for a laceration that involved more than 50% of the tendon.
However, accurate assessment of partial-thickness flexor tendon lacerations is difficult owing to the subjectivity of evaluation. In the survey just mentioned,7 the majority of surgeons used the naked eye to make assessments, and only 14% used other means, such as a ruler, a pair of calipers, or loupe magnification. In addition, flexor tendon injuries are often evaluated under less than ideal circumstances—a dirty or bloody field, poor lighting, an uncomfortable patient.
We conducted a study to determine the interobserver and intraobserver reliability of surgeons assessing the percentage of CSA injured in partially lacerated digital flexor tendons. We hypothesized that participants’ accuracy and agreement would be poor.
Materials and Methods
Eight 1-cm transverse, volar skin incisions were made over the midportions of the middle and proximal phalanges of the index, middle, ring, and small fingers of a fresh-frozen human cadaver hand (Figure 1). The tendon sheaths were incised, and the flexor digitorum profundus tendons to each digit were delivered through the wound. With use of a method described previously by Manning and colleagues,8 the tendon was then placed over a flat metal post to be used as a cutting board, and the proposed laceration site was marked with ink. Under loupe magnification, a No. 15 blade was used to create a partial transverse, volar-to-dorsal laceration in each tendon.8 The goal was to create lacerations of about 30%, 50%, and 70% of the total CSA of the tendon. The tendons were then returned to the wound, and visibility of the marked laceration within the wound was ensured. A similar exercise was performed at the level of the proximal palmar crease. Four flexor digitorum superficialis tendons were exposed through 1-cm transverse incisions, and partial lacerations were made in the volar substance of the tendons. The tendons were then returned to the wound, resulting in 12 partially lacerated tendons (8 flexor digitorum profundus, 4 flexor digitorum superficialis).
Six orthopedic surgery residents (2 postgraduate year 1 [PGY-1], 2 PGY-3, 2 PGY-5) and 4 fellowship-trained hand surgeons participated in our study. Each was asked to evaluate the tendons and determine the percentage of total CSA lacerated. Loupe magnification and measuring tools were not permitted, but participants were allowed to handle the tendons. In addition, they were asked if they would perform tenorrhaphy on the injured tendons, given only the amount of injury. The participants repeated this exercise 4 weeks later.
After all measurements were made, a longitudinal incision was made down each of the digits, and the flexor tendons were exposed within the flexor sheath. The transverse incisions in the palm were connected to expose the flexor digitorum superficialis tendons. Under an operating microscope, a pair of digital microcalipers (Kobalt 0.5-ft Metric and SAE Caliper; Figure 2) accurate to 0.01 mm was used to measure the external width (a) and height (b + bˈ) of the tendons just proximal to the lacerations. Measurements were made with the caliper blades just touching the edges of the lacerated tendon, thus minimizing deformation of the tendon. Other measurements made at the laceration site were width of the remaining tendon (c) and height of the remaining tendon (bˈ). CSA of the tendon was calculated assuming a regular ellipsoid shape and using the equation:
Area = 1/2π(b+b')
The area of the tendon injured was determined by calculating the area under a parabola and using the equation:
Area = 2/3c[(b+b')-b']
Last, the percentage of total CSA lacerated was calculated using the equation:
Area (total area)
Statistical analysis was performed to determine accuracy and interobserver and intraobserver reliability. Paired t tests were used in the assessment of accuracy to determine if there were differences between estimated and calibrated measurements.
Results
The 10 participants’ estimates differed significantly (P < .0006) from the calibrated measurements, as did residents’ estimates (P < .0025) and fellowship-trained hand surgeons’ estimates (P < .0002). Estimates were scored 1 to 5 on the basis of proximity to calibrated measurements (Table 1). Thus, more accurate estimates received lower scores. Individual estimates were then scored and stratified into groups for comparison. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. These results are listed in Table 2. Once overall and grouped accuracy was analyzed, κ statistics were calculated to compare interobserver and intraobserver reliability. Overall interobserver agreement was poor for both initial readings (κ = 0.16) and secondary readings (κ = 0.16), indicating poor strength of agreement between individuals both initially and secondarily. Table 3 presents the κ interpretations. There was moderate overall intraobserver agreement (45.83%), indicating participants’ secondary estimates agreed with their primary estimates 46% of the time. Fellowship-trained hand surgeons and first-year residents had the highest intraobserver agreement (50.0%). These results are listed in Table 4.
Discussion
Accurate assessment of partial flexor tendon lacerations is difficult and subjective. There is no standardized method for determining the extent of injury, regardless of whether the evaluation is performed in an emergency department or in the operating room. As McCarthy and colleagues7 noted in their survey of ASSH members, naked eye assessment was by far the most popular means of estimating percentage injured in partial lacerations, and only 10% of the survey respondents used intraoperative measuring devices. Our study showed that participants agreed with one another less than 50% of the time when evaluating injuries without the aid of measuring devices. In addition, interobserver agreement in this study was about 50%, highlighting the difficulty in making an accurate and reproducible assessment.
In a study of canine flexor tendons, McCarthy and colleagues9 found calipers are inaccurate as well and do not provide a reliable means of assessing partial flexor tendon lacerations. They compared caliper measurements with laser micrometer measurements, and the differences averaged 29.3%. They suggested that methods for calculating loss of CSA and for creating precise lacerations must be developed in order to evaluate treatments. One such method is the “tenotome,” devised by Hitchcock and colleagues10: A device with standard scalpel blades is used to make uniform lacerations in tendons by leaving a constant area of the tendon intact, regardless of the size or shape of the original tendon. Measurements made with calipers or rulers assume the tendon has a regular ellipsoid shape, but in reality the shape is a double-ellipse, particularly within the flexor sheath.
Dobyns and colleagues11 observed that changes in CSA size can be related to changes in the size of the bundle pattern of the tendon. They found that, on average, the radial bundle comprised about 60% of the total CSA of the tendon. This finding was clarified by Grewal and colleagues.12 Using histologic sections of tendons plus photomicrographs, they determined that, in zone II of the index and small fingers, the ulnar bundle had an area consistently larger than 50% and the radial bundle less than 50% of the total tendon area. In the ring and middle fingers, the areas of both bundles were almost 50% of the total tendon area. The authors suggested that, using this bundle pattern theory of injury, surgeons could more accurately evaluate the extent of injury with the naked eye.
One of the questions that prompted our study is how reliable is the information a surgeon receives regarding a partial flexor tendon injury evaluated by someone else in another setting. What is done with this information is another question. The scenario can be considered in 2 settings: emergency department and operating room.
Given the poor accuracy and interobserver agreement found in our study, along with the inaccuracy of caliper and ruler measurements, it seems decisions to perform tenorrhaphy based on reported percentages lacerated are unreliable. Our results showed that the ability to accurately assess partial tendon injuries does not improve with surgeon experience, as fellowship-trained hand surgeons were not statistically more accurate or consistent than residents. To this effect, one institution treats all its partial flexor tendon lacerations with wound inspection and irrigation in the emergency department, under digital block and after neurovascular injury has been excluded.8 If the patient is able to actively flex and extend the digit without triggering, then the wound is closed without closing the tendon sheath, a dorsal blocking splint is applied, and motion is begun early, 48 hours later, regardless of laceration severity.
Once the decision has been made to go to the operating room and the injury is being evaluated, what should be done with the information from the measurement, whether made with loupe magnification, calipers, rulers, or the naked eye? Surgeons must weigh the risks for triggering, entrapment, and rupture of untreated partial tendon lacerations1 with the added bulk and potential for adhesions, along with the tensile strength reduction that accompanies tendon repair. Both Reynolds and colleagues13 and Ollinger and colleagues14 found tensile strength significantly diminished in sutured tendons. Ollinger and colleagues14 showed a decrease in tendon gliding after surgical exposure and tenorrhaphy for partial tendon lacerations. Reynolds and colleagues13 concluded that surgical repair leads to poorer results than nonsurgical treatment.
Clinical studies have demonstrated excellent results with nonintervention, and in vivo and in vitro studies have indicated that early motion can be initiated in partial lacerations of up to 95% of total CSA. Wray and Weeks6 treated 26 patients with partial lacerations varying from 25% to 95% of total CSA and noted 1 incidence of trigger finger (which resolved) and no late ruptures. They advocated treatment with early motion and excision or repair of beveled partial lacerations with simple sutures. Stahl and colleagues2 reported comparable outcomes in children with partial lacerations up to 75% of total CSA treated with and without surgery and noted no complications in either group. In a biomechanical study, Hariharan and colleagues4 found lacerations up to 75% can withstand forces associated with active unresisted mobilization.
Conversely, how many patients or surgeons want to return to the operating room to fix a late rupture when it could have been repaired in the primary setting? Schlenker and colleagues,1 reporting on a late flexor pollicus tendon rupture that required tendon grafting, recommended exploration and primary repair of all partial flexor tendon lacerations. Often, it is difficult to determine whether surgical repair is necessary to ensure the best outcome for the patient.
Our study results showed that, in the evaluation of flexor tendon lacerations, both accuracy and interobserver agreement were poor among residents and fellowship-trained hand surgeons, and intraobserver agreement was moderate. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. Our results highlight the difficulty in making accurate assessments of flexor tendon lacerations owing to the subjectivity of evaluation, which appear not to improve with surgeon experience.
1. Schlenker JD, Lister GD, Kleinert HE. Three complications of untreated partial laceration of flexor tendon—entrapment, rupture, and triggering. J Hand Surg Am. 1981;6(4):392-398.
2. Stahl S, Kaufman T, Bialik V. Partial lacerations of flexor tendons in children. Primary repair versus conservative treatment. J Hand Surg Br. 1997;22(3):377-380.
3. Al-Qattan MM. Conservative management of zone II partial flexor tendon lacerations greater than half the width of the tendon. J Hand Surg Am. 2000;25(6):1118-1121.
4. Hariharan JS, Diao E, Soejima O, Lotz JC. Partial lacerations of human digital flexor tendons: a biomechanical analysis. J Hand Surg Am. 1997;22(6):1011-1015.
5. Bishop AT, Cooney WP 3rd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26(4):301-312.
6. Wray RC Jr, Weeks PM. Treatment of partial tendon lacerations. Hand. 1980;12(2):163-166.
7. McCarthy DM, Boardman ND 3rd, Tramaglini DM, Sotereanos DG, Herndon JH. Clinical management of partially lacerated digital flexor tendons: a survey of hand surgeons. J Hand Surg Am. 1995;20(2):273-275.
8. Manning DW, Spiguel AR, Mass DP. Biomechanical analysis of partial flexor tendon lacerations in zone II of human cadavers. J Hand Surg Am. 2010;35(1):11-18.
9. McCarthy DM, Tramaglini DM, Chan SS, Schmidt CC, Sotereanos DG, Herndon JH. Effect of partial laceration on the structural properties of the canine FDP tendon: an in vitro study. J Hand Surg Am. 1995;20(5):795-800.
10. Hitchcock TF, Candel AG, Light TR, Blevens AD. New technique for producing uniform partial lacerations of tendons. J Orthop Res. 1989;7(3):451-455.
11. Dobyns RC, Cooney WC, Wood MB. Effect of partial lacerations on canine flexor tendons. Minn Med. 1982;65(1):27-32.
12. Grewal R, Sotereanos DG, Rao U, Herndon JH, Woo SL. Bundle pattern of the flexor digitorum profundus tendon in zone II of the hand: a quantitative assessment of the size of a laceration. J Hand Surg Am. 1996;21(6):978-983.
13. Reynolds B, Wray RC Jr, Weeks PM. Should an incompletely severed tendon be sutured? Plast Reconstr Surg. 1976;57(1):36-38.
14. Ollinger H, Wray RC Jr, Weeks PM. Effects of suture on tensile strength gain of partially and completely severed tendons. Surg Forum. 1975;26:63-64.
1. Schlenker JD, Lister GD, Kleinert HE. Three complications of untreated partial laceration of flexor tendon—entrapment, rupture, and triggering. J Hand Surg Am. 1981;6(4):392-398.
2. Stahl S, Kaufman T, Bialik V. Partial lacerations of flexor tendons in children. Primary repair versus conservative treatment. J Hand Surg Br. 1997;22(3):377-380.
3. Al-Qattan MM. Conservative management of zone II partial flexor tendon lacerations greater than half the width of the tendon. J Hand Surg Am. 2000;25(6):1118-1121.
4. Hariharan JS, Diao E, Soejima O, Lotz JC. Partial lacerations of human digital flexor tendons: a biomechanical analysis. J Hand Surg Am. 1997;22(6):1011-1015.
5. Bishop AT, Cooney WP 3rd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26(4):301-312.
6. Wray RC Jr, Weeks PM. Treatment of partial tendon lacerations. Hand. 1980;12(2):163-166.
7. McCarthy DM, Boardman ND 3rd, Tramaglini DM, Sotereanos DG, Herndon JH. Clinical management of partially lacerated digital flexor tendons: a survey of hand surgeons. J Hand Surg Am. 1995;20(2):273-275.
8. Manning DW, Spiguel AR, Mass DP. Biomechanical analysis of partial flexor tendon lacerations in zone II of human cadavers. J Hand Surg Am. 2010;35(1):11-18.
9. McCarthy DM, Tramaglini DM, Chan SS, Schmidt CC, Sotereanos DG, Herndon JH. Effect of partial laceration on the structural properties of the canine FDP tendon: an in vitro study. J Hand Surg Am. 1995;20(5):795-800.
10. Hitchcock TF, Candel AG, Light TR, Blevens AD. New technique for producing uniform partial lacerations of tendons. J Orthop Res. 1989;7(3):451-455.
11. Dobyns RC, Cooney WC, Wood MB. Effect of partial lacerations on canine flexor tendons. Minn Med. 1982;65(1):27-32.
12. Grewal R, Sotereanos DG, Rao U, Herndon JH, Woo SL. Bundle pattern of the flexor digitorum profundus tendon in zone II of the hand: a quantitative assessment of the size of a laceration. J Hand Surg Am. 1996;21(6):978-983.
13. Reynolds B, Wray RC Jr, Weeks PM. Should an incompletely severed tendon be sutured? Plast Reconstr Surg. 1976;57(1):36-38.
14. Ollinger H, Wray RC Jr, Weeks PM. Effects of suture on tensile strength gain of partially and completely severed tendons. Surg Forum. 1975;26:63-64.
Hand Blisters in Major League Baseball Pitchers: Current Concepts and Management
Friction blisters result from repetitive friction and strain forces that develop between the skin and various objects. Blisters form in areas where the stratum corneum and stratum granulosum are sufficiently robust (Figure), such as the palmar and plantar surfaces of the hand and feet. Thus, these layers are capable of transmitting the surface forces to the underlying layer, the stratum spinosum. In areas without strong stratum corneum and stratum granulosum layers, an abrasion forms instead.1
It has been shown that the transmitted frictional forces disrupt the stratum spinosum, with the blister roof being composed of the 2 upper epidermal layers as well as prickle cells of the traumatically disrupted stratum spinosum. The basal cell layer typically shows little damage and the dermal-epidermal junction remains intact.1
Early experimental studies in humans using repeatedly cycled probes demonstrated the pathologic sequence of events in blister formation. First, there is slight exfoliation of the stratum corneum layer, accompanied by a reddened area in the zone of rubbing (erythroderma). This is followed by a pale, narrow demarcation, which forms around the reddened region. Subsequently, this pale area fills in toward the center to occupy the entire affected area, which becomes the blister lesion.1,2
Hydrostatic pressure then causes blister fluid to accumulate within 1 to 2 hours following the trauma. Compared to plasma, the blister fluid has a lower protein level and similar electrolyte content.3,4 Cells in the blister cavity continue to degrade for about 4 hours following the injury, with resumption of cellular activity beginning at 6 hours. Mitotic activity is increased after 24 to 30 hours, and at 48 hours, a new granular layer is present. By 120 hours post-injury, a new stratum corneum is formed.5,6
A number of factors have been found to affect blister formation. Frictional force magnitude and number of cycles play the most obvious role. There is an inverse relationship between the two: as the frictional force increases, fewer cycles are required for blister formation.7 This is likely the reason blisters occur most commonly in areas where the fingertips are in contact with a seam, as opposed to the smoother surface of a baseball.
Many authors have examined moisture’s effect on frictional forces, and found that very dry and very wet skin produce low frictional forces, whereas moist skin produces the highest frictional force.1,2,8-10 In the case of dry skin, this is thought to be due to exfoliation and sloughing of cells from the stratum corneum, which produces a dry lubrication similar to graphite. Very wet skin has a fluid layer that lubricates the 2 surfaces. In the case of moist skin, however, it is hypothesized that surface tension impedes the movement of squamous cells, increasing the frictional forces.2,9 This moist environment is most commonly produced by sweating.
Other factors include skin temperature, which, when elevated, mildly predisposes the skin to blister formation. Some studies have shown temperatures as high as 50°C in rubbing experiments2,7,8,11; however, it should be noted that friction blisters do not resemble second-degree burns, either histologically or clinically.12,13
Blisters in Baseball Pitchers
Blisters in baseball pitchers are a well-known and frequently publicized problem; however, there is a paucity of literature describing the incidence or treatment of such blisters.14,15 The digital pulp experiences frictional forces from the baseball stiches as well as from the distal margin of the nail plate during release of the ball. Forces are transmitted to the ball predominately through the thumb, index, and long fingers. While the thumb acts mainly as a post, the index and long fingers impart the “action” on the ball. Not surprisingly, blisters form most commonly on these 2 fingers. While relatively small in size and significance, the impact of such a blister on a pitcher’s ability to maintain the fine control of his pitches cannot be overlooked. Biomechanical studies have shown that maximum gripping strength is attained when the fingers grasp a dynamometer handle at the level of the distal interphalangeal joint.16 Contact pressure mapping during gripping of a cylindrical object has shown that phalanges 2, 3, and 4 experience the highest forces in gripping and pulling activities.17 No study has specifically addressed phalangeal pressure generation in pitchers, however.
Blister Prevention
Blister prevention and treatment methods in baseball pitchers are steeped in folklore and tradition. Methods for drying out blisters and hardening calluses have included the use of pickle juice, urine, bags of rice, and superglue.14,15 Superglue, surgical glue, or any other foreign substance is not allowed during a game on the finger or hands of pitchers by Major League Baseball rules. Other anecdotal options include the use of compounded medicines that are marketed as creams and sprays designed to toughen skin.
As with other injuries, it is important to recognize any predisposing factors and ways to avoid them. Dampness and temperature (>104°F) have been identified as chief factors that substantially increase the friction coefficient and increase blister incidence.18 While temperature and perspiration are impossible to avoid during competition, steps can be taken to keep the pitcher’s hand dry on the mound as well as between innings, such as a rosin bag, a dry towel, and a rice bucket.
Maintaining fingernail length plays an important role in preventing blister formation. The nail can both protect the adjacent skin by decreasing the frictional force on the skin as well as lead to the development of blisters on the other fingers by repetitive abrasion. Nail length and contour need to be tailored to each pitcher specifically. The length of the nail can protect the finger pulp by minimally “elevating” the ball off of the finger itself. However, too long of a nail may come at the cost of abrading the abutting finger as the spin is imparted onto the ball. The shape of the nail is generally kept well contoured to avoid any sharp edges, which can act as local irritants. In the instance of soft, cracked, or torn nails, some pitchers have used acrylic nails. Maintaining proper fingernail shape and length is an essential preventive measure that requires regular use of clippers and emery boards.
Callus care is also paramount in preventing blister formation. It is believed that development of a callus is inevitable with repetitive throwing and likely protective of the underlying skin. The size and shape of the callus, like that of the nail, needs to be carefully monitored. A callus that becomes overly prominent can lead to increased friction with a baseball seam. This can lead to blister development. A small, smooth callus without edges or loose borders is the goal. The free edges of a callus can be trimmed with clean clippers. Contouring is best performed with careful use of an emery board.
Treatment of Finger Blisters
Blister management is determined by the size of the blister as well as the integrity of the overlying callus. Small blisters with intact skin coverage can be sterilely drained with a needle or a No. 11 blade.6,19-21 This allows apposition of the skin layers and quicker healing. The free edge of the blister can then be repaired with surgical glue. In these instances, a starting pitcher may be required to miss a start to allow further healing. In most cases, there is no need to place the player on the disabled list (DL).
Larger blisters, or those that traumatically open, represent a more concerning issue. The loose layers of skin can be removed, and the raw bed can then be treated with antibiotic ointment for the first 2 to 3 days. Subsequently, benzoin tincture, a commonly used paste of benzoin and alum, can be utilized to toughen the raw skin. Bulky dressings can be applied early in treatment but should then be discouraged, as the underlying skin softens due to the presence of moisture. These instances generally lead to lost time on the field. It is not uncommon that the pitcher requires placement on the 15-day DL.
Summary
Blisters on the fingertips of professional baseball players can lead to significant pain and decreased performance. Prevention of blister formation represents the goal of the player and the medical staff. Skin and nail care requires daily evaluation. When blisters do form, appropriate management can minimize lost time.
1. Sulzberger MB, Cortese TA, Fishman L, Wiley HS. Studies on blisters produced by friction. I. Results of linear rubbing and twisting technics. J Invest Dermatol. 1966;47(5):456-465.
2. Naylor PFD. Experimental friction blisters. Brit J Dermatol. 1955;67(10):327-342.
3. Cortese TA, Mitchell W, Sulzberger MB. Studies on blisters produced by friction. II. The blister fluid. J Invest Dermatol. 1968;50(1):47-53.
4. Schmidt P. Quantification of specific proteins in blister fluid. J Invest Dermatol. 1970;55(4):244-248.
5. Epstein WL, Fukuyama K, Cortese TA. Autographic study of friction blisters. RNA, DNA, and protein synthesis. Arch Dermatol. 1969;99(1):94-106.
6. Cortese TA Jr, Fukuyama K, Epstein W, Sulzberger MB. Treatment of friction blisters. An experimental study. Arch Dermatol. 1968;97(6):717-721.
7. Comaish JS. Epidermal fatigue as a cause of friction blisters. Lancet. 1973;1(7794):81-83.
8. Akers WA, Sulzberger MB. The friction blister. Mil Med. 1972;137(1):l-7.
9. Highley DR, Coomey M, DenBeste M, Wolfman LJ. Frictional properties of skin. J Invest Dermatol. 1977;69(3):303-305.
10. Nacht S, Close J, Yeung D, et al. Skin friction coefficient: changes induced by skin hydration and emollient application and correlation with perceived skin feel. J Soc Cosmet Chern. 1981;32:55-65.
11. Griffin TB, Corqese TA, Layton LL, et al. Inverse time and temperature relationship in experimental friction blisters. J Invest Dermatol. 1969;52:391.
12. Shupp JW, Nazabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31(6):849-873.
13. Knapik JJ, Reynolds KL, Duplantis KL, Jones BH. Friction blisters: pathophysiology, prevention and treatment. Sports Med. 1995;20(3):136-147.
14. Sielski M. C.J. Wilson on pitching-hand care. The Wall Street Journal. October 30, 2010. Available at: http://blogs.wsj.com/dailyfix/2010/10/30/cj-wilson-on-caring-for-his-pitching-hand Accessed January 10, 2016.
15. Trezza J. Blisters are normal part of pitching for Lynn. St. Louis Post-Dispatch. July 4, 2014. Available at: http://www.stltoday.com/sports/baseball/professional/blisters-are-normal-part-of-pitching-for-lynn/article_9743c6f9-14b2-5c50-83b4-fa6a3c34cb83.html Accessed January 10, 2016.
16. Kaufmann RA, Kozin SH, Mirarchi A, Holland B, Porter S. Biomechanical analysis of flexor digitorum profundus and superficialis in grip-strenth generation. Am J Orthop. 2007;36(9):E128-E132.
17. Nicholas JW, Corvese RJ, Woolley C, Armstrong TJ. Quantification of hand grasp force using a pressure mapping system. Work. 2012;41(Suppl 1):605-612.
18. Knapik JJ, Reynolds KL. Risk factors for foot blisters during road marching: tobacco use, ethnicity, foot type, previous illness and others. Mil Med. 1999;164(2):92-97.
19. Emer J, Sivek R, Marciniak B. Sports Dermatology: Part 1 of 2. Traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthet Dermatol. 2015;8(4):31-43.
20. De Luca JF, Adams BB, Yosipovitch G. Skin manifestations of athletes competing in the summer olympics: what a sports medicine physician should know. Sports Med. 2012;42(5):399-413.
21. Helm TN, Bergfeld WF. Sports dermatology. Clin Dermatol. 199;16(1):159-165.
Friction blisters result from repetitive friction and strain forces that develop between the skin and various objects. Blisters form in areas where the stratum corneum and stratum granulosum are sufficiently robust (Figure), such as the palmar and plantar surfaces of the hand and feet. Thus, these layers are capable of transmitting the surface forces to the underlying layer, the stratum spinosum. In areas without strong stratum corneum and stratum granulosum layers, an abrasion forms instead.1
It has been shown that the transmitted frictional forces disrupt the stratum spinosum, with the blister roof being composed of the 2 upper epidermal layers as well as prickle cells of the traumatically disrupted stratum spinosum. The basal cell layer typically shows little damage and the dermal-epidermal junction remains intact.1
Early experimental studies in humans using repeatedly cycled probes demonstrated the pathologic sequence of events in blister formation. First, there is slight exfoliation of the stratum corneum layer, accompanied by a reddened area in the zone of rubbing (erythroderma). This is followed by a pale, narrow demarcation, which forms around the reddened region. Subsequently, this pale area fills in toward the center to occupy the entire affected area, which becomes the blister lesion.1,2
Hydrostatic pressure then causes blister fluid to accumulate within 1 to 2 hours following the trauma. Compared to plasma, the blister fluid has a lower protein level and similar electrolyte content.3,4 Cells in the blister cavity continue to degrade for about 4 hours following the injury, with resumption of cellular activity beginning at 6 hours. Mitotic activity is increased after 24 to 30 hours, and at 48 hours, a new granular layer is present. By 120 hours post-injury, a new stratum corneum is formed.5,6
A number of factors have been found to affect blister formation. Frictional force magnitude and number of cycles play the most obvious role. There is an inverse relationship between the two: as the frictional force increases, fewer cycles are required for blister formation.7 This is likely the reason blisters occur most commonly in areas where the fingertips are in contact with a seam, as opposed to the smoother surface of a baseball.
Many authors have examined moisture’s effect on frictional forces, and found that very dry and very wet skin produce low frictional forces, whereas moist skin produces the highest frictional force.1,2,8-10 In the case of dry skin, this is thought to be due to exfoliation and sloughing of cells from the stratum corneum, which produces a dry lubrication similar to graphite. Very wet skin has a fluid layer that lubricates the 2 surfaces. In the case of moist skin, however, it is hypothesized that surface tension impedes the movement of squamous cells, increasing the frictional forces.2,9 This moist environment is most commonly produced by sweating.
Other factors include skin temperature, which, when elevated, mildly predisposes the skin to blister formation. Some studies have shown temperatures as high as 50°C in rubbing experiments2,7,8,11; however, it should be noted that friction blisters do not resemble second-degree burns, either histologically or clinically.12,13
Blisters in Baseball Pitchers
Blisters in baseball pitchers are a well-known and frequently publicized problem; however, there is a paucity of literature describing the incidence or treatment of such blisters.14,15 The digital pulp experiences frictional forces from the baseball stiches as well as from the distal margin of the nail plate during release of the ball. Forces are transmitted to the ball predominately through the thumb, index, and long fingers. While the thumb acts mainly as a post, the index and long fingers impart the “action” on the ball. Not surprisingly, blisters form most commonly on these 2 fingers. While relatively small in size and significance, the impact of such a blister on a pitcher’s ability to maintain the fine control of his pitches cannot be overlooked. Biomechanical studies have shown that maximum gripping strength is attained when the fingers grasp a dynamometer handle at the level of the distal interphalangeal joint.16 Contact pressure mapping during gripping of a cylindrical object has shown that phalanges 2, 3, and 4 experience the highest forces in gripping and pulling activities.17 No study has specifically addressed phalangeal pressure generation in pitchers, however.
Blister Prevention
Blister prevention and treatment methods in baseball pitchers are steeped in folklore and tradition. Methods for drying out blisters and hardening calluses have included the use of pickle juice, urine, bags of rice, and superglue.14,15 Superglue, surgical glue, or any other foreign substance is not allowed during a game on the finger or hands of pitchers by Major League Baseball rules. Other anecdotal options include the use of compounded medicines that are marketed as creams and sprays designed to toughen skin.
As with other injuries, it is important to recognize any predisposing factors and ways to avoid them. Dampness and temperature (>104°F) have been identified as chief factors that substantially increase the friction coefficient and increase blister incidence.18 While temperature and perspiration are impossible to avoid during competition, steps can be taken to keep the pitcher’s hand dry on the mound as well as between innings, such as a rosin bag, a dry towel, and a rice bucket.
Maintaining fingernail length plays an important role in preventing blister formation. The nail can both protect the adjacent skin by decreasing the frictional force on the skin as well as lead to the development of blisters on the other fingers by repetitive abrasion. Nail length and contour need to be tailored to each pitcher specifically. The length of the nail can protect the finger pulp by minimally “elevating” the ball off of the finger itself. However, too long of a nail may come at the cost of abrading the abutting finger as the spin is imparted onto the ball. The shape of the nail is generally kept well contoured to avoid any sharp edges, which can act as local irritants. In the instance of soft, cracked, or torn nails, some pitchers have used acrylic nails. Maintaining proper fingernail shape and length is an essential preventive measure that requires regular use of clippers and emery boards.
Callus care is also paramount in preventing blister formation. It is believed that development of a callus is inevitable with repetitive throwing and likely protective of the underlying skin. The size and shape of the callus, like that of the nail, needs to be carefully monitored. A callus that becomes overly prominent can lead to increased friction with a baseball seam. This can lead to blister development. A small, smooth callus without edges or loose borders is the goal. The free edges of a callus can be trimmed with clean clippers. Contouring is best performed with careful use of an emery board.
Treatment of Finger Blisters
Blister management is determined by the size of the blister as well as the integrity of the overlying callus. Small blisters with intact skin coverage can be sterilely drained with a needle or a No. 11 blade.6,19-21 This allows apposition of the skin layers and quicker healing. The free edge of the blister can then be repaired with surgical glue. In these instances, a starting pitcher may be required to miss a start to allow further healing. In most cases, there is no need to place the player on the disabled list (DL).
Larger blisters, or those that traumatically open, represent a more concerning issue. The loose layers of skin can be removed, and the raw bed can then be treated with antibiotic ointment for the first 2 to 3 days. Subsequently, benzoin tincture, a commonly used paste of benzoin and alum, can be utilized to toughen the raw skin. Bulky dressings can be applied early in treatment but should then be discouraged, as the underlying skin softens due to the presence of moisture. These instances generally lead to lost time on the field. It is not uncommon that the pitcher requires placement on the 15-day DL.
Summary
Blisters on the fingertips of professional baseball players can lead to significant pain and decreased performance. Prevention of blister formation represents the goal of the player and the medical staff. Skin and nail care requires daily evaluation. When blisters do form, appropriate management can minimize lost time.
Friction blisters result from repetitive friction and strain forces that develop between the skin and various objects. Blisters form in areas where the stratum corneum and stratum granulosum are sufficiently robust (Figure), such as the palmar and plantar surfaces of the hand and feet. Thus, these layers are capable of transmitting the surface forces to the underlying layer, the stratum spinosum. In areas without strong stratum corneum and stratum granulosum layers, an abrasion forms instead.1
It has been shown that the transmitted frictional forces disrupt the stratum spinosum, with the blister roof being composed of the 2 upper epidermal layers as well as prickle cells of the traumatically disrupted stratum spinosum. The basal cell layer typically shows little damage and the dermal-epidermal junction remains intact.1
Early experimental studies in humans using repeatedly cycled probes demonstrated the pathologic sequence of events in blister formation. First, there is slight exfoliation of the stratum corneum layer, accompanied by a reddened area in the zone of rubbing (erythroderma). This is followed by a pale, narrow demarcation, which forms around the reddened region. Subsequently, this pale area fills in toward the center to occupy the entire affected area, which becomes the blister lesion.1,2
Hydrostatic pressure then causes blister fluid to accumulate within 1 to 2 hours following the trauma. Compared to plasma, the blister fluid has a lower protein level and similar electrolyte content.3,4 Cells in the blister cavity continue to degrade for about 4 hours following the injury, with resumption of cellular activity beginning at 6 hours. Mitotic activity is increased after 24 to 30 hours, and at 48 hours, a new granular layer is present. By 120 hours post-injury, a new stratum corneum is formed.5,6
A number of factors have been found to affect blister formation. Frictional force magnitude and number of cycles play the most obvious role. There is an inverse relationship between the two: as the frictional force increases, fewer cycles are required for blister formation.7 This is likely the reason blisters occur most commonly in areas where the fingertips are in contact with a seam, as opposed to the smoother surface of a baseball.
Many authors have examined moisture’s effect on frictional forces, and found that very dry and very wet skin produce low frictional forces, whereas moist skin produces the highest frictional force.1,2,8-10 In the case of dry skin, this is thought to be due to exfoliation and sloughing of cells from the stratum corneum, which produces a dry lubrication similar to graphite. Very wet skin has a fluid layer that lubricates the 2 surfaces. In the case of moist skin, however, it is hypothesized that surface tension impedes the movement of squamous cells, increasing the frictional forces.2,9 This moist environment is most commonly produced by sweating.
Other factors include skin temperature, which, when elevated, mildly predisposes the skin to blister formation. Some studies have shown temperatures as high as 50°C in rubbing experiments2,7,8,11; however, it should be noted that friction blisters do not resemble second-degree burns, either histologically or clinically.12,13
Blisters in Baseball Pitchers
Blisters in baseball pitchers are a well-known and frequently publicized problem; however, there is a paucity of literature describing the incidence or treatment of such blisters.14,15 The digital pulp experiences frictional forces from the baseball stiches as well as from the distal margin of the nail plate during release of the ball. Forces are transmitted to the ball predominately through the thumb, index, and long fingers. While the thumb acts mainly as a post, the index and long fingers impart the “action” on the ball. Not surprisingly, blisters form most commonly on these 2 fingers. While relatively small in size and significance, the impact of such a blister on a pitcher’s ability to maintain the fine control of his pitches cannot be overlooked. Biomechanical studies have shown that maximum gripping strength is attained when the fingers grasp a dynamometer handle at the level of the distal interphalangeal joint.16 Contact pressure mapping during gripping of a cylindrical object has shown that phalanges 2, 3, and 4 experience the highest forces in gripping and pulling activities.17 No study has specifically addressed phalangeal pressure generation in pitchers, however.
Blister Prevention
Blister prevention and treatment methods in baseball pitchers are steeped in folklore and tradition. Methods for drying out blisters and hardening calluses have included the use of pickle juice, urine, bags of rice, and superglue.14,15 Superglue, surgical glue, or any other foreign substance is not allowed during a game on the finger or hands of pitchers by Major League Baseball rules. Other anecdotal options include the use of compounded medicines that are marketed as creams and sprays designed to toughen skin.
As with other injuries, it is important to recognize any predisposing factors and ways to avoid them. Dampness and temperature (>104°F) have been identified as chief factors that substantially increase the friction coefficient and increase blister incidence.18 While temperature and perspiration are impossible to avoid during competition, steps can be taken to keep the pitcher’s hand dry on the mound as well as between innings, such as a rosin bag, a dry towel, and a rice bucket.
Maintaining fingernail length plays an important role in preventing blister formation. The nail can both protect the adjacent skin by decreasing the frictional force on the skin as well as lead to the development of blisters on the other fingers by repetitive abrasion. Nail length and contour need to be tailored to each pitcher specifically. The length of the nail can protect the finger pulp by minimally “elevating” the ball off of the finger itself. However, too long of a nail may come at the cost of abrading the abutting finger as the spin is imparted onto the ball. The shape of the nail is generally kept well contoured to avoid any sharp edges, which can act as local irritants. In the instance of soft, cracked, or torn nails, some pitchers have used acrylic nails. Maintaining proper fingernail shape and length is an essential preventive measure that requires regular use of clippers and emery boards.
Callus care is also paramount in preventing blister formation. It is believed that development of a callus is inevitable with repetitive throwing and likely protective of the underlying skin. The size and shape of the callus, like that of the nail, needs to be carefully monitored. A callus that becomes overly prominent can lead to increased friction with a baseball seam. This can lead to blister development. A small, smooth callus without edges or loose borders is the goal. The free edges of a callus can be trimmed with clean clippers. Contouring is best performed with careful use of an emery board.
Treatment of Finger Blisters
Blister management is determined by the size of the blister as well as the integrity of the overlying callus. Small blisters with intact skin coverage can be sterilely drained with a needle or a No. 11 blade.6,19-21 This allows apposition of the skin layers and quicker healing. The free edge of the blister can then be repaired with surgical glue. In these instances, a starting pitcher may be required to miss a start to allow further healing. In most cases, there is no need to place the player on the disabled list (DL).
Larger blisters, or those that traumatically open, represent a more concerning issue. The loose layers of skin can be removed, and the raw bed can then be treated with antibiotic ointment for the first 2 to 3 days. Subsequently, benzoin tincture, a commonly used paste of benzoin and alum, can be utilized to toughen the raw skin. Bulky dressings can be applied early in treatment but should then be discouraged, as the underlying skin softens due to the presence of moisture. These instances generally lead to lost time on the field. It is not uncommon that the pitcher requires placement on the 15-day DL.
Summary
Blisters on the fingertips of professional baseball players can lead to significant pain and decreased performance. Prevention of blister formation represents the goal of the player and the medical staff. Skin and nail care requires daily evaluation. When blisters do form, appropriate management can minimize lost time.
1. Sulzberger MB, Cortese TA, Fishman L, Wiley HS. Studies on blisters produced by friction. I. Results of linear rubbing and twisting technics. J Invest Dermatol. 1966;47(5):456-465.
2. Naylor PFD. Experimental friction blisters. Brit J Dermatol. 1955;67(10):327-342.
3. Cortese TA, Mitchell W, Sulzberger MB. Studies on blisters produced by friction. II. The blister fluid. J Invest Dermatol. 1968;50(1):47-53.
4. Schmidt P. Quantification of specific proteins in blister fluid. J Invest Dermatol. 1970;55(4):244-248.
5. Epstein WL, Fukuyama K, Cortese TA. Autographic study of friction blisters. RNA, DNA, and protein synthesis. Arch Dermatol. 1969;99(1):94-106.
6. Cortese TA Jr, Fukuyama K, Epstein W, Sulzberger MB. Treatment of friction blisters. An experimental study. Arch Dermatol. 1968;97(6):717-721.
7. Comaish JS. Epidermal fatigue as a cause of friction blisters. Lancet. 1973;1(7794):81-83.
8. Akers WA, Sulzberger MB. The friction blister. Mil Med. 1972;137(1):l-7.
9. Highley DR, Coomey M, DenBeste M, Wolfman LJ. Frictional properties of skin. J Invest Dermatol. 1977;69(3):303-305.
10. Nacht S, Close J, Yeung D, et al. Skin friction coefficient: changes induced by skin hydration and emollient application and correlation with perceived skin feel. J Soc Cosmet Chern. 1981;32:55-65.
11. Griffin TB, Corqese TA, Layton LL, et al. Inverse time and temperature relationship in experimental friction blisters. J Invest Dermatol. 1969;52:391.
12. Shupp JW, Nazabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31(6):849-873.
13. Knapik JJ, Reynolds KL, Duplantis KL, Jones BH. Friction blisters: pathophysiology, prevention and treatment. Sports Med. 1995;20(3):136-147.
14. Sielski M. C.J. Wilson on pitching-hand care. The Wall Street Journal. October 30, 2010. Available at: http://blogs.wsj.com/dailyfix/2010/10/30/cj-wilson-on-caring-for-his-pitching-hand Accessed January 10, 2016.
15. Trezza J. Blisters are normal part of pitching for Lynn. St. Louis Post-Dispatch. July 4, 2014. Available at: http://www.stltoday.com/sports/baseball/professional/blisters-are-normal-part-of-pitching-for-lynn/article_9743c6f9-14b2-5c50-83b4-fa6a3c34cb83.html Accessed January 10, 2016.
16. Kaufmann RA, Kozin SH, Mirarchi A, Holland B, Porter S. Biomechanical analysis of flexor digitorum profundus and superficialis in grip-strenth generation. Am J Orthop. 2007;36(9):E128-E132.
17. Nicholas JW, Corvese RJ, Woolley C, Armstrong TJ. Quantification of hand grasp force using a pressure mapping system. Work. 2012;41(Suppl 1):605-612.
18. Knapik JJ, Reynolds KL. Risk factors for foot blisters during road marching: tobacco use, ethnicity, foot type, previous illness and others. Mil Med. 1999;164(2):92-97.
19. Emer J, Sivek R, Marciniak B. Sports Dermatology: Part 1 of 2. Traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthet Dermatol. 2015;8(4):31-43.
20. De Luca JF, Adams BB, Yosipovitch G. Skin manifestations of athletes competing in the summer olympics: what a sports medicine physician should know. Sports Med. 2012;42(5):399-413.
21. Helm TN, Bergfeld WF. Sports dermatology. Clin Dermatol. 199;16(1):159-165.
1. Sulzberger MB, Cortese TA, Fishman L, Wiley HS. Studies on blisters produced by friction. I. Results of linear rubbing and twisting technics. J Invest Dermatol. 1966;47(5):456-465.
2. Naylor PFD. Experimental friction blisters. Brit J Dermatol. 1955;67(10):327-342.
3. Cortese TA, Mitchell W, Sulzberger MB. Studies on blisters produced by friction. II. The blister fluid. J Invest Dermatol. 1968;50(1):47-53.
4. Schmidt P. Quantification of specific proteins in blister fluid. J Invest Dermatol. 1970;55(4):244-248.
5. Epstein WL, Fukuyama K, Cortese TA. Autographic study of friction blisters. RNA, DNA, and protein synthesis. Arch Dermatol. 1969;99(1):94-106.
6. Cortese TA Jr, Fukuyama K, Epstein W, Sulzberger MB. Treatment of friction blisters. An experimental study. Arch Dermatol. 1968;97(6):717-721.
7. Comaish JS. Epidermal fatigue as a cause of friction blisters. Lancet. 1973;1(7794):81-83.
8. Akers WA, Sulzberger MB. The friction blister. Mil Med. 1972;137(1):l-7.
9. Highley DR, Coomey M, DenBeste M, Wolfman LJ. Frictional properties of skin. J Invest Dermatol. 1977;69(3):303-305.
10. Nacht S, Close J, Yeung D, et al. Skin friction coefficient: changes induced by skin hydration and emollient application and correlation with perceived skin feel. J Soc Cosmet Chern. 1981;32:55-65.
11. Griffin TB, Corqese TA, Layton LL, et al. Inverse time and temperature relationship in experimental friction blisters. J Invest Dermatol. 1969;52:391.
12. Shupp JW, Nazabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31(6):849-873.
13. Knapik JJ, Reynolds KL, Duplantis KL, Jones BH. Friction blisters: pathophysiology, prevention and treatment. Sports Med. 1995;20(3):136-147.
14. Sielski M. C.J. Wilson on pitching-hand care. The Wall Street Journal. October 30, 2010. Available at: http://blogs.wsj.com/dailyfix/2010/10/30/cj-wilson-on-caring-for-his-pitching-hand Accessed January 10, 2016.
15. Trezza J. Blisters are normal part of pitching for Lynn. St. Louis Post-Dispatch. July 4, 2014. Available at: http://www.stltoday.com/sports/baseball/professional/blisters-are-normal-part-of-pitching-for-lynn/article_9743c6f9-14b2-5c50-83b4-fa6a3c34cb83.html Accessed January 10, 2016.
16. Kaufmann RA, Kozin SH, Mirarchi A, Holland B, Porter S. Biomechanical analysis of flexor digitorum profundus and superficialis in grip-strenth generation. Am J Orthop. 2007;36(9):E128-E132.
17. Nicholas JW, Corvese RJ, Woolley C, Armstrong TJ. Quantification of hand grasp force using a pressure mapping system. Work. 2012;41(Suppl 1):605-612.
18. Knapik JJ, Reynolds KL. Risk factors for foot blisters during road marching: tobacco use, ethnicity, foot type, previous illness and others. Mil Med. 1999;164(2):92-97.
19. Emer J, Sivek R, Marciniak B. Sports Dermatology: Part 1 of 2. Traumatic or mechanical injuries, inflammatory conditions, and exacerbations of pre-existing conditions. J Clin Aesthet Dermatol. 2015;8(4):31-43.
20. De Luca JF, Adams BB, Yosipovitch G. Skin manifestations of athletes competing in the summer olympics: what a sports medicine physician should know. Sports Med. 2012;42(5):399-413.
21. Helm TN, Bergfeld WF. Sports dermatology. Clin Dermatol. 199;16(1):159-165.
Distal Ulna Fracture With Delayed Ulnar Nerve Palsy in a Baseball Player
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
Definitive Fixation of Hand and Wrist Fractures in the Emergency Department
A mentor—now in his 60s—related his experiences as a resident. On call as a second-year resident, he would often be alone at a busy trauma center with no backup. When a case came in, he would quickly read about it in the library, then manage it in the emergency department (ED) if possible, or, if necessary, take the patient to the operating room (OR).
In the era of improved patient care, increased supervision, and decreased autonomy, this is not the reality anymore.1 In theory, more reliable patient care is the result; however, the pendulum may have swung too far.
There are a number of injuries that are amenable to definitive fixation in the ED, but not as limited an array of injuries as we have perhaps grown accustomed to. Hand injuries are among the most common orthopedic injuries seen in the ED, with fractures of the metacarpals and phalanges constituting nearly one-half of all hand injuries.2 The authors recently attended an excellent instructional course lecture on “The Lost and Found Art of Percutaneous Pinning in the Hand and Wrist” at the annual conference of the American Academy of Orthopaedic Surgeons.3 The presenters itemized a comprehensive list of fractures and simple dislocations of the hand, which could be simply, safely, effectively, and definitively managed through percutaneous pinning techniques. A significant number of unstable fractures of the phalanges and metacarpals can be treated in the ED under mini–C-arm fluoroscopy without an admission and trip to the OR.3,4 Most phalangeal and metacarpal fractures are nondisplaced or minimally displaced and stable, and can often be handled with a combination of closed reduction, buddy-taping, and splinting.5 The indications for percutaneous versus internal fixation depend on a number of factors, including bone quality, degree of comminution, quality of the soft-tissue envelope, articular involvement, acuity of presentation, and goals for motion.6,7
Many simple injury patterns involving unstable fractures or dislocations may be definitively managed in the ED with percutaneous pinning (eg, injuries that are unstable with closed reduction alone but that do not necessitate soft-tissue dissection). These include but are not limited to bony mallet injuries, unstable transverse or oblique fractures or fracture-dislocations of the phalanges and metacarpals, carpometacarpal fracture- dislocations, and underlying fractures that need protection of nail-bed repairs, soft-tissue flaps, or extensor tendon injuries (Figures 1, 2).7,8 The techniques for specific fracture types are beyond the scope of this article but are readily available.5,6
There are certain situations that undoubtedly warrant surgery in the OR, such as neurovascular injury necessitating microvascular repair, flexor tendon laceration, severely comminuted or segmental fractures, irreducible dislocations, and fractures with severe soft-tissue injury or contamination not amenable to primary irrigation, débridement, and closure at bedside.4,7,8
You might ask, “Why would one treat an operative injury in the ED and not formally in the OR?,” and we submit that there are a number of reasons.
First, and most important, with increasing health care costs and decreasing reimbursements, physicians are faced with providing safe but economical care. Percutaneous Kirschner wire (K-wire) fixation is dramatically more cost-effective when performed in the ED than in the OR. The cost of a procedure performed in either setting is similarly dependent on a variety of factors, generally including complexity of the patient or procedure, costs of supplies and pharmacologic agents, fixed versus variable overhead costs, and the professional fees of providers and ancillary personnel.9,10
While the patient is not charged per hour in the ED, it is estimated that ORs in the United States cost, on average, $62 per minute, ranging from as low as $22 to as high as $133 per minute.9 Additionally, the number of personnel involved in running an OR exceeds those for a similar procedure performed in the ED, considering (at a minimum) the orthopedic surgeon, anesthesiologist, scrub and radiology technicians, and nursing personnel required before, during, and after an operation.
While analgesia and procedural sedation can be performed similarly in either setting, it is our experience that patients are managed much more often in the ED with local anesthesia under direct care of only the orthopedic provider, whereas intravenous sedation and general anesthesia are far more commonly implemented in the OR. There are exceptions for pediatric patients or those who are unable to tolerate the procedure under only local anesthesia. Local anesthesia or even intravenous conscious sedation entails less risk as well as lower associated drug costs.11
The difference in risk is especially true for sicker patients undergoing minimally invasive procedures.11 Although administration of adequate procedural analgesia grows increasingly difficult the more proximal the injury, the hand and the fingers are easily and reliably anesthetized with well-placed wrist or digital blocks, with infrequent complications.12 Application of a lidocaine/bupivacaine mixture provides up to 6 to 8 hours of analgesia. A small tourniquet alternative, such as the finger of a sterile glove or phlebotomy tourniquet, applied to the base of the finger or the wrist additionally provides a relatively bloodless field and effectively acts as a Bier block.
Percutaneous pins are much more forgiving than rigid internal fixation. If the initial placement of a pin is unsatisfactory, the pin can be reinserted at little cost.12 Conversely, it may not be possible to reposition a misplaced screw or screw with inadequate purchase and still maintain adequate fixation. While percutaneous pin fixation is not as rigid as screw fixation, the degree of stability provided is adequate for the small forces affecting the hand in most cases. Accordingly, there is a very low incidence of fibrous union or nonunion.13,14 With an increasing appreciation of soft-tissue handling over the past few decades, another significant advantage of K-wire fixation is the obviation of soft-tissue dissection, preserving the biology to maximize healing and minimize adverse sequelae.12 Percutaneous fixation has been shown to achieve functional outcomes comparable to open reduction with internal fixation of operative phalangeal and metacarpal fractures, without soft-tissue disruption, scarring, or implant irritation, and with minimal risk of infection.3,13,15,16 Ultimate range of motion after percutaneous fixation is comparable, if not superior, to that of internal fixation, despite the initial advantage of rigid internal fixation secondary to decreased scarring and lack of indwelling hardware.16,17
While the risk of infection, perhaps the primary concern with percutaneous fixation, has been cited as high as 7%, osteomyelitis is exceedingly rare (<0.5%).3,13,14 Furthermore, pins are often left in place for 3 to 6 weeks, and infection has been found to occur most often at a mean of 10 weeks.7,13 Infection can also be mitigated by intelligent pin placement, relief of residual tension, and splint immobilization.4,15 Pin loosening has similarly been reported in up to 4% of cases in large retrospective studies, occurring at an average of 8 weeks, by which time most pins would have been extricated.13 Other complications related to impaling adjacent neurovascular or tendinous structures have also been cited but are rare.13 A 12-month prospective study of 75 patients specifically evaluating the outcomes after closed reduction with percutaneous fixation of unstable hand fractures in the ED reported only 6 complications at final follow-up.4 Complications were all minor, with no cases of nonunion, delayed union, malunion, pin-tract infection, pyarthrosis, or cellulitis, even in the setting of open fractures. Three patients required revision in the OR for pin migration, initial malreduction, and bone loss in the setting of comminution, respectively. The authors credited their low complication rate to supplementary immobilization.
In conclusion, many unstable simple fractures and dislocations of the hand and wrist can be safely and effectively treated in the ED. While it may seem daunting for a junior resident who is unfamiliar with percutaneous techniques, the authors advocate learning from a more senior mentor. The only additional training required is an understanding of how to apply this skill set in a different setting.
1. Levine WN, Spang RC 3rd. ACGME duty hour requirements: perceptions and impact on resident training and patient care. J Am Acad Orthop Surg. 2014;22(9):535-544.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Catalano LW 3rd, Glickel SZ, Strauch RJ, Barron AO. The lost and found art of percutaneous pinning in the hand and wrist. Instructional Course Lectures. Annual Meeting of the American Academy of Orthopaedic Surgeons; March 24, 2015; Las Vegas, NV.
4. Starker I, Eaton RG. Kirschner wire placement in the emergency room. Is there a risk? J Hand Surg Br. 1995;20(4):535-538.
5. Meals C, Meals R. Hand fractures: a review of current treatment strategies. J Hand Surg Am. 2013;38(5):1021-1031.
6. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595.
7. Klein DM, Belsole RJ. Percutaneous treatment of carpal, metacarpal, and phalangeal injuries. Clin Orthop Relat Res. 2000;(375):116-125.
8. Bernstein ML, Chung KC. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048.
9. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
10. Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
11. Bodenham AR, Howell SJ. General anesthesia vs local anaesthesia: an ongoing story. Br J Anaesth. 2009;103(6):785-789.
12. Stern PJ. Management of fractures of the hand over the last 25 years. J Hand Surg Am. 2000;25(5):817-823.
13. Botte MJ, Davis JL, Rose BA, et al. Complications of smooth pin fixation of fractures and dislocations in the hand and wrist. Clin Orthop Relat Res. 1992;(276):194-201.
14. Wray RC Jr, Glunk R. Treatment of delayed union, nonunion, and malunion of the phalanges of the hand. Ann Plast Surg. 1989;22(1):14-18.
15. Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am. 2011;36(4):610-616.
16. Stem PJ, Wieser MJ, Reilly DG. Complications of plate fixation in the hand skeleton. Clin Orthop Relat Res. 1987;(214):59-65.
17. Page SM, Stern PJ. Complications and range of motion following plate fixation of metacarpal and phalangeal fractures. J Hand Surg Am. 1998;23(5):827-832.
A mentor—now in his 60s—related his experiences as a resident. On call as a second-year resident, he would often be alone at a busy trauma center with no backup. When a case came in, he would quickly read about it in the library, then manage it in the emergency department (ED) if possible, or, if necessary, take the patient to the operating room (OR).
In the era of improved patient care, increased supervision, and decreased autonomy, this is not the reality anymore.1 In theory, more reliable patient care is the result; however, the pendulum may have swung too far.
There are a number of injuries that are amenable to definitive fixation in the ED, but not as limited an array of injuries as we have perhaps grown accustomed to. Hand injuries are among the most common orthopedic injuries seen in the ED, with fractures of the metacarpals and phalanges constituting nearly one-half of all hand injuries.2 The authors recently attended an excellent instructional course lecture on “The Lost and Found Art of Percutaneous Pinning in the Hand and Wrist” at the annual conference of the American Academy of Orthopaedic Surgeons.3 The presenters itemized a comprehensive list of fractures and simple dislocations of the hand, which could be simply, safely, effectively, and definitively managed through percutaneous pinning techniques. A significant number of unstable fractures of the phalanges and metacarpals can be treated in the ED under mini–C-arm fluoroscopy without an admission and trip to the OR.3,4 Most phalangeal and metacarpal fractures are nondisplaced or minimally displaced and stable, and can often be handled with a combination of closed reduction, buddy-taping, and splinting.5 The indications for percutaneous versus internal fixation depend on a number of factors, including bone quality, degree of comminution, quality of the soft-tissue envelope, articular involvement, acuity of presentation, and goals for motion.6,7
Many simple injury patterns involving unstable fractures or dislocations may be definitively managed in the ED with percutaneous pinning (eg, injuries that are unstable with closed reduction alone but that do not necessitate soft-tissue dissection). These include but are not limited to bony mallet injuries, unstable transverse or oblique fractures or fracture-dislocations of the phalanges and metacarpals, carpometacarpal fracture- dislocations, and underlying fractures that need protection of nail-bed repairs, soft-tissue flaps, or extensor tendon injuries (Figures 1, 2).7,8 The techniques for specific fracture types are beyond the scope of this article but are readily available.5,6
There are certain situations that undoubtedly warrant surgery in the OR, such as neurovascular injury necessitating microvascular repair, flexor tendon laceration, severely comminuted or segmental fractures, irreducible dislocations, and fractures with severe soft-tissue injury or contamination not amenable to primary irrigation, débridement, and closure at bedside.4,7,8
You might ask, “Why would one treat an operative injury in the ED and not formally in the OR?,” and we submit that there are a number of reasons.
First, and most important, with increasing health care costs and decreasing reimbursements, physicians are faced with providing safe but economical care. Percutaneous Kirschner wire (K-wire) fixation is dramatically more cost-effective when performed in the ED than in the OR. The cost of a procedure performed in either setting is similarly dependent on a variety of factors, generally including complexity of the patient or procedure, costs of supplies and pharmacologic agents, fixed versus variable overhead costs, and the professional fees of providers and ancillary personnel.9,10
While the patient is not charged per hour in the ED, it is estimated that ORs in the United States cost, on average, $62 per minute, ranging from as low as $22 to as high as $133 per minute.9 Additionally, the number of personnel involved in running an OR exceeds those for a similar procedure performed in the ED, considering (at a minimum) the orthopedic surgeon, anesthesiologist, scrub and radiology technicians, and nursing personnel required before, during, and after an operation.
While analgesia and procedural sedation can be performed similarly in either setting, it is our experience that patients are managed much more often in the ED with local anesthesia under direct care of only the orthopedic provider, whereas intravenous sedation and general anesthesia are far more commonly implemented in the OR. There are exceptions for pediatric patients or those who are unable to tolerate the procedure under only local anesthesia. Local anesthesia or even intravenous conscious sedation entails less risk as well as lower associated drug costs.11
The difference in risk is especially true for sicker patients undergoing minimally invasive procedures.11 Although administration of adequate procedural analgesia grows increasingly difficult the more proximal the injury, the hand and the fingers are easily and reliably anesthetized with well-placed wrist or digital blocks, with infrequent complications.12 Application of a lidocaine/bupivacaine mixture provides up to 6 to 8 hours of analgesia. A small tourniquet alternative, such as the finger of a sterile glove or phlebotomy tourniquet, applied to the base of the finger or the wrist additionally provides a relatively bloodless field and effectively acts as a Bier block.
Percutaneous pins are much more forgiving than rigid internal fixation. If the initial placement of a pin is unsatisfactory, the pin can be reinserted at little cost.12 Conversely, it may not be possible to reposition a misplaced screw or screw with inadequate purchase and still maintain adequate fixation. While percutaneous pin fixation is not as rigid as screw fixation, the degree of stability provided is adequate for the small forces affecting the hand in most cases. Accordingly, there is a very low incidence of fibrous union or nonunion.13,14 With an increasing appreciation of soft-tissue handling over the past few decades, another significant advantage of K-wire fixation is the obviation of soft-tissue dissection, preserving the biology to maximize healing and minimize adverse sequelae.12 Percutaneous fixation has been shown to achieve functional outcomes comparable to open reduction with internal fixation of operative phalangeal and metacarpal fractures, without soft-tissue disruption, scarring, or implant irritation, and with minimal risk of infection.3,13,15,16 Ultimate range of motion after percutaneous fixation is comparable, if not superior, to that of internal fixation, despite the initial advantage of rigid internal fixation secondary to decreased scarring and lack of indwelling hardware.16,17
While the risk of infection, perhaps the primary concern with percutaneous fixation, has been cited as high as 7%, osteomyelitis is exceedingly rare (<0.5%).3,13,14 Furthermore, pins are often left in place for 3 to 6 weeks, and infection has been found to occur most often at a mean of 10 weeks.7,13 Infection can also be mitigated by intelligent pin placement, relief of residual tension, and splint immobilization.4,15 Pin loosening has similarly been reported in up to 4% of cases in large retrospective studies, occurring at an average of 8 weeks, by which time most pins would have been extricated.13 Other complications related to impaling adjacent neurovascular or tendinous structures have also been cited but are rare.13 A 12-month prospective study of 75 patients specifically evaluating the outcomes after closed reduction with percutaneous fixation of unstable hand fractures in the ED reported only 6 complications at final follow-up.4 Complications were all minor, with no cases of nonunion, delayed union, malunion, pin-tract infection, pyarthrosis, or cellulitis, even in the setting of open fractures. Three patients required revision in the OR for pin migration, initial malreduction, and bone loss in the setting of comminution, respectively. The authors credited their low complication rate to supplementary immobilization.
In conclusion, many unstable simple fractures and dislocations of the hand and wrist can be safely and effectively treated in the ED. While it may seem daunting for a junior resident who is unfamiliar with percutaneous techniques, the authors advocate learning from a more senior mentor. The only additional training required is an understanding of how to apply this skill set in a different setting.
A mentor—now in his 60s—related his experiences as a resident. On call as a second-year resident, he would often be alone at a busy trauma center with no backup. When a case came in, he would quickly read about it in the library, then manage it in the emergency department (ED) if possible, or, if necessary, take the patient to the operating room (OR).
In the era of improved patient care, increased supervision, and decreased autonomy, this is not the reality anymore.1 In theory, more reliable patient care is the result; however, the pendulum may have swung too far.
There are a number of injuries that are amenable to definitive fixation in the ED, but not as limited an array of injuries as we have perhaps grown accustomed to. Hand injuries are among the most common orthopedic injuries seen in the ED, with fractures of the metacarpals and phalanges constituting nearly one-half of all hand injuries.2 The authors recently attended an excellent instructional course lecture on “The Lost and Found Art of Percutaneous Pinning in the Hand and Wrist” at the annual conference of the American Academy of Orthopaedic Surgeons.3 The presenters itemized a comprehensive list of fractures and simple dislocations of the hand, which could be simply, safely, effectively, and definitively managed through percutaneous pinning techniques. A significant number of unstable fractures of the phalanges and metacarpals can be treated in the ED under mini–C-arm fluoroscopy without an admission and trip to the OR.3,4 Most phalangeal and metacarpal fractures are nondisplaced or minimally displaced and stable, and can often be handled with a combination of closed reduction, buddy-taping, and splinting.5 The indications for percutaneous versus internal fixation depend on a number of factors, including bone quality, degree of comminution, quality of the soft-tissue envelope, articular involvement, acuity of presentation, and goals for motion.6,7
Many simple injury patterns involving unstable fractures or dislocations may be definitively managed in the ED with percutaneous pinning (eg, injuries that are unstable with closed reduction alone but that do not necessitate soft-tissue dissection). These include but are not limited to bony mallet injuries, unstable transverse or oblique fractures or fracture-dislocations of the phalanges and metacarpals, carpometacarpal fracture- dislocations, and underlying fractures that need protection of nail-bed repairs, soft-tissue flaps, or extensor tendon injuries (Figures 1, 2).7,8 The techniques for specific fracture types are beyond the scope of this article but are readily available.5,6
There are certain situations that undoubtedly warrant surgery in the OR, such as neurovascular injury necessitating microvascular repair, flexor tendon laceration, severely comminuted or segmental fractures, irreducible dislocations, and fractures with severe soft-tissue injury or contamination not amenable to primary irrigation, débridement, and closure at bedside.4,7,8
You might ask, “Why would one treat an operative injury in the ED and not formally in the OR?,” and we submit that there are a number of reasons.
First, and most important, with increasing health care costs and decreasing reimbursements, physicians are faced with providing safe but economical care. Percutaneous Kirschner wire (K-wire) fixation is dramatically more cost-effective when performed in the ED than in the OR. The cost of a procedure performed in either setting is similarly dependent on a variety of factors, generally including complexity of the patient or procedure, costs of supplies and pharmacologic agents, fixed versus variable overhead costs, and the professional fees of providers and ancillary personnel.9,10
While the patient is not charged per hour in the ED, it is estimated that ORs in the United States cost, on average, $62 per minute, ranging from as low as $22 to as high as $133 per minute.9 Additionally, the number of personnel involved in running an OR exceeds those for a similar procedure performed in the ED, considering (at a minimum) the orthopedic surgeon, anesthesiologist, scrub and radiology technicians, and nursing personnel required before, during, and after an operation.
While analgesia and procedural sedation can be performed similarly in either setting, it is our experience that patients are managed much more often in the ED with local anesthesia under direct care of only the orthopedic provider, whereas intravenous sedation and general anesthesia are far more commonly implemented in the OR. There are exceptions for pediatric patients or those who are unable to tolerate the procedure under only local anesthesia. Local anesthesia or even intravenous conscious sedation entails less risk as well as lower associated drug costs.11
The difference in risk is especially true for sicker patients undergoing minimally invasive procedures.11 Although administration of adequate procedural analgesia grows increasingly difficult the more proximal the injury, the hand and the fingers are easily and reliably anesthetized with well-placed wrist or digital blocks, with infrequent complications.12 Application of a lidocaine/bupivacaine mixture provides up to 6 to 8 hours of analgesia. A small tourniquet alternative, such as the finger of a sterile glove or phlebotomy tourniquet, applied to the base of the finger or the wrist additionally provides a relatively bloodless field and effectively acts as a Bier block.
Percutaneous pins are much more forgiving than rigid internal fixation. If the initial placement of a pin is unsatisfactory, the pin can be reinserted at little cost.12 Conversely, it may not be possible to reposition a misplaced screw or screw with inadequate purchase and still maintain adequate fixation. While percutaneous pin fixation is not as rigid as screw fixation, the degree of stability provided is adequate for the small forces affecting the hand in most cases. Accordingly, there is a very low incidence of fibrous union or nonunion.13,14 With an increasing appreciation of soft-tissue handling over the past few decades, another significant advantage of K-wire fixation is the obviation of soft-tissue dissection, preserving the biology to maximize healing and minimize adverse sequelae.12 Percutaneous fixation has been shown to achieve functional outcomes comparable to open reduction with internal fixation of operative phalangeal and metacarpal fractures, without soft-tissue disruption, scarring, or implant irritation, and with minimal risk of infection.3,13,15,16 Ultimate range of motion after percutaneous fixation is comparable, if not superior, to that of internal fixation, despite the initial advantage of rigid internal fixation secondary to decreased scarring and lack of indwelling hardware.16,17
While the risk of infection, perhaps the primary concern with percutaneous fixation, has been cited as high as 7%, osteomyelitis is exceedingly rare (<0.5%).3,13,14 Furthermore, pins are often left in place for 3 to 6 weeks, and infection has been found to occur most often at a mean of 10 weeks.7,13 Infection can also be mitigated by intelligent pin placement, relief of residual tension, and splint immobilization.4,15 Pin loosening has similarly been reported in up to 4% of cases in large retrospective studies, occurring at an average of 8 weeks, by which time most pins would have been extricated.13 Other complications related to impaling adjacent neurovascular or tendinous structures have also been cited but are rare.13 A 12-month prospective study of 75 patients specifically evaluating the outcomes after closed reduction with percutaneous fixation of unstable hand fractures in the ED reported only 6 complications at final follow-up.4 Complications were all minor, with no cases of nonunion, delayed union, malunion, pin-tract infection, pyarthrosis, or cellulitis, even in the setting of open fractures. Three patients required revision in the OR for pin migration, initial malreduction, and bone loss in the setting of comminution, respectively. The authors credited their low complication rate to supplementary immobilization.
In conclusion, many unstable simple fractures and dislocations of the hand and wrist can be safely and effectively treated in the ED. While it may seem daunting for a junior resident who is unfamiliar with percutaneous techniques, the authors advocate learning from a more senior mentor. The only additional training required is an understanding of how to apply this skill set in a different setting.
1. Levine WN, Spang RC 3rd. ACGME duty hour requirements: perceptions and impact on resident training and patient care. J Am Acad Orthop Surg. 2014;22(9):535-544.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Catalano LW 3rd, Glickel SZ, Strauch RJ, Barron AO. The lost and found art of percutaneous pinning in the hand and wrist. Instructional Course Lectures. Annual Meeting of the American Academy of Orthopaedic Surgeons; March 24, 2015; Las Vegas, NV.
4. Starker I, Eaton RG. Kirschner wire placement in the emergency room. Is there a risk? J Hand Surg Br. 1995;20(4):535-538.
5. Meals C, Meals R. Hand fractures: a review of current treatment strategies. J Hand Surg Am. 2013;38(5):1021-1031.
6. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595.
7. Klein DM, Belsole RJ. Percutaneous treatment of carpal, metacarpal, and phalangeal injuries. Clin Orthop Relat Res. 2000;(375):116-125.
8. Bernstein ML, Chung KC. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048.
9. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
10. Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
11. Bodenham AR, Howell SJ. General anesthesia vs local anaesthesia: an ongoing story. Br J Anaesth. 2009;103(6):785-789.
12. Stern PJ. Management of fractures of the hand over the last 25 years. J Hand Surg Am. 2000;25(5):817-823.
13. Botte MJ, Davis JL, Rose BA, et al. Complications of smooth pin fixation of fractures and dislocations in the hand and wrist. Clin Orthop Relat Res. 1992;(276):194-201.
14. Wray RC Jr, Glunk R. Treatment of delayed union, nonunion, and malunion of the phalanges of the hand. Ann Plast Surg. 1989;22(1):14-18.
15. Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am. 2011;36(4):610-616.
16. Stem PJ, Wieser MJ, Reilly DG. Complications of plate fixation in the hand skeleton. Clin Orthop Relat Res. 1987;(214):59-65.
17. Page SM, Stern PJ. Complications and range of motion following plate fixation of metacarpal and phalangeal fractures. J Hand Surg Am. 1998;23(5):827-832.
1. Levine WN, Spang RC 3rd. ACGME duty hour requirements: perceptions and impact on resident training and patient care. J Am Acad Orthop Surg. 2014;22(9):535-544.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Catalano LW 3rd, Glickel SZ, Strauch RJ, Barron AO. The lost and found art of percutaneous pinning in the hand and wrist. Instructional Course Lectures. Annual Meeting of the American Academy of Orthopaedic Surgeons; March 24, 2015; Las Vegas, NV.
4. Starker I, Eaton RG. Kirschner wire placement in the emergency room. Is there a risk? J Hand Surg Br. 1995;20(4):535-538.
5. Meals C, Meals R. Hand fractures: a review of current treatment strategies. J Hand Surg Am. 2013;38(5):1021-1031.
6. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595.
7. Klein DM, Belsole RJ. Percutaneous treatment of carpal, metacarpal, and phalangeal injuries. Clin Orthop Relat Res. 2000;(375):116-125.
8. Bernstein ML, Chung KC. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048.
9. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
10. Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
11. Bodenham AR, Howell SJ. General anesthesia vs local anaesthesia: an ongoing story. Br J Anaesth. 2009;103(6):785-789.
12. Stern PJ. Management of fractures of the hand over the last 25 years. J Hand Surg Am. 2000;25(5):817-823.
13. Botte MJ, Davis JL, Rose BA, et al. Complications of smooth pin fixation of fractures and dislocations in the hand and wrist. Clin Orthop Relat Res. 1992;(276):194-201.
14. Wray RC Jr, Glunk R. Treatment of delayed union, nonunion, and malunion of the phalanges of the hand. Ann Plast Surg. 1989;22(1):14-18.
15. Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am. 2011;36(4):610-616.
16. Stem PJ, Wieser MJ, Reilly DG. Complications of plate fixation in the hand skeleton. Clin Orthop Relat Res. 1987;(214):59-65.
17. Page SM, Stern PJ. Complications and range of motion following plate fixation of metacarpal and phalangeal fractures. J Hand Surg Am. 1998;23(5):827-832.
Concomitant Ulnar Styloid Fracture and Distal Radius Fracture Portend Poorer Outcome
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
Necrotizing Fasciitis Caused by Cryptococcus gattii
Necrotizing fasciitis (NF) is a severe, rapidly spreading soft-tissue infection with high morbidity and mortality. Bacteriology in NF may be varied, and the etiology is often polymicrobial. It is important to consider the potential for fungal involvement despite its rarity. Cryptococcal NF has been reported in immunocompromised patients, with Cryptococcus neoformans being the most common offending organism.1-4
C neoformans is a basidiomycotic yeast that was previously considered a homogenous species.5,6 From the antigenic properties of its polysaccharide capsule, 3 main variants were described: C neoformans var. grubii, C neoformans var. neoformans, and C neoformans var. gattii. Subsequently, C neoformans var. gattii was found to be genetically and biochemically different from C neoformans. This discovery led to the distinction of C neoformans var. gattii as a separate species and it being renamed C gattii.6
C gattii was first recognized on Vancouver Island in 2001.7 Although C gattii is predominantly restricted to tropical and subtropical climates, its true epidemiology has been limited by diagnostic methods. C gattii can be diagnosed with laboratory culture media such as birdseed agars and L-canavanine-glycine-bromothymol (CGB) agar.6 However, most reports of Cryptococcus NF do not specify the culture media used to isolate Cryptococcus. In addition to culture media, molecular genotyping studies also allow for confirmation of the diagnosis of C gattii and have the added benefit of enabling identification of the molecular genotype. Nonetheless, in many clinical microbiology laboratories, Cryptococcus is not identified to the species level, much less to the molecular genotype.7 Given these diagnostic limitations and the fact that C gattii was only recently identified as a separate species, it is possible that any pre-2006 cases of NF attributed to C neoformans could in fact have been caused by C gattii.
In this article, we review the literature and report a case of NF of the hand that was caused by C gattii in a patient with diabetes. To our knowledge, this is the first reported case of NF caused by C gattii. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old man was admitted with a 1-week history of swelling and pain in the dorsum of the left hand. He had been sitting in an outdoor eatery in Singapore when an insect bit the hand over the dorsum. Two days later, he consulted his family physician, who began treatment with oral amoxicillin/clavulanic acid. After 4 days of treatment, there was clinical progression of increased swelling and pain in the hand. Six days after initial injury, the patient presented to the department of orthopedic surgery.
Physical examination revealed diffuse, brawny, nonfluctuant swelling over the entire dorsum of the left hand (Figure 1). There was a 1×1-cm ruptured blister with some nonpurulent discharge just distal to the wrist joint. Neurovascular status and the extensor mechanism of the fingers were intact. The wrist joint had full range of motion. There was no fever.
Laboratory testing revealed an elevated white blood cell count (16.6×109/L), a C-reactive protein (CRP) level of 237 nmol/L, a random blood glucose level of 12.6 mmol/L, and a LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score of 7.8
Given the severe swelling, intravenous amoxicillin/clavulanic acid was started. The patient received a total of 3 doses before operative débridement of the left hand. Operative findings were NF of the hand, grayish necrotic fascia, and foul-smelling “dishwater” fluid. A single specimen of fascia from the surgical site was sent for examination. Histopathologic examination of formalin-fixed, paraffin-embedded tissue revealed necrotizing suppurative inflammation with fungal organisms present (Figures 2, 3).
Tissue cultures were obtained during surgery. The organism grew as scanty, small, wet-looking colonies on sheep blood agar after 48 hours of incubation. Microscopy revealed an oval yeast. The organism was identified and reported as C gattii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Biotyper 2.0.1 software; Bruker Daltonics), with a score of 1.914.9 All other intraoperative cultures for aerobic and anaerobic bacteria were negative. Molecular genotyping was performed with polymerase chain reaction assay to identify the molecular subtype.10C gattii genotype VGII was isolated. A cryptococcal serum antigen assay was positive at 1:256.
A series of tests was performed to screen for disseminated disease. Blood cultures were negative for fungus. Chest radiography and computed tomography of the brain did not show any pulmonary or cerebral involvement. Cerebrospinal fluid was not available for examination, as the patient declined lumbar puncture. Blood tests included a negative result for human immunodeficiency virus (HIV). The patient was found to have previously undiagnosed diabetes mellitus (hemoglobin A1c, 7.9%). T-cell counts and ratios were normal.
The patient was started on intravenous amphotericin B 60 mg/d and flucytosine 500 mg every 6 hours for 3 weeks. Oral fluconazole 400 mg every morning was also given (intended duration, 6 mo). Given that diabetes was newly diagnosed, the patient was treated with metformin; his capillary blood glucose level remained stable during his inpatient stay.
Four débridements of the dorsal hand wound were performed—the first on day of admission and the other 3 on hospitalization days 3, 7, and 18 (Figure 4). Subsequent wound resurfacing with a split skin graft harvested from the forearm was performed on hospitalization day 22. After surgery, the hand was dressed with a bulky cotton dressing. Five days after the patient was discharged, during review in the outpatient clinic, the skin graft was noted to be taking well. The patient did not attend postoperative physical therapy. He was maintained on metformin and given a follow-up clinic appointment for his diabetes. Four months after surgery, the wound was completely healed, and normal functional use of the hand recovered.
Discussion
NF is a severe soft-tissue infection with potential for rapid progression. Surgical débridement should be performed urgently to reduce the chance of morbidity and mortality.11 The initial classification by Giuliano and colleagues12 was based on bacteriology and included type I (anaerobic species in combination with a facultative species) and type II (monomicrobial usually involving group A β-hemolytic Streptococcus). This classification was modified by Morgan13 to include gram-negative organisms as well as fungal organisms (Table 1).
Fungal NF is rare, with Candida, Apophysomyces, and Cryptococcus described in the literature.1,14,15 Fungal infections tend to occur in immunocompromised patients; risk factors are steroid immunosuppression, poorly controlled diabetes, and peripheral vascular disease.16 Some zygomycetes may also affect immunocompetent patients.15
C gattii is an encapsulated yeast organism that is genetically and biochemically distinct from C neoformans. It is endemic to tropical parts of Africa and Australia. Its main environmental sources are eucalyptus trees (Eucalyptus camaldulensis, Eucalyptus tereticornis) and decaying hollows in living trees.17 In addition, there have been reports of isolation of C gattii from insect frass,18 which would make infection by an insect bite a possible transmission route. Worldwide distribution of this pathogen has increased recently, with outbreaks noted on Vancouver Island and in areas in Canada and the northwest United States.7
The true incidence of NF secondary to C gattii is difficult to determine. C gattii was only recently identified as a separate species, and pre-2006 cases of NF attributed to C neoformans may instead have been caused by C gattii. Misidentification has been compounded by the fact that the tests required for accurate diagnosis of C gattii infection may not be readily available in many clinical microbiology laboratories. Cryptococcus can be identified with various methods, including direct microscopy, culturing of tissue or fluid samples, and measurement of cryptococcal serum antigen. However, tests such as specific culture media, mass spectrometry, and molecular typing studies are required to determine cryptococcal species. L-canavanine-glycine-bromothymol blue (CGB) agar is a medium that is often used to differentiate C gattii from C neoformans because of the ability of C gattii to produce a blue appearance.6 Modern techniques, such as MALDI-TOF MS, have also been used to successfully distinguish between C gattii and C neoformans.9 MALDI-TOF MS identifies species on the basis of characteristic protein spectra extracted from whole cells. Using commercial and supplemental reference libraries, the system compares signal matches in the reference spectrum with Cryptococcus entries in the library—allowing rapid and accurate identification of cryptococcal species. However, this diagnostic method is limited by availability of adequate Cryptococcus entries in the reference library and by the high cost of acquiring the machine.
Serotyping is based on the antigenic property of the capsule and was once used to differentiate C neoformans into its 3 main varieties: var. neoformans, var. grubii, var. gattii. However, when it was realized that the antigenic property of the strain can be unstable and that there are hybrids containing more than 1 serotype, serotyping was abandoned as a species-differentiation test.6 The current gold standard for species differentiation is molecular genotyping. Molecular genotyping studies can confirm the diagnosis of C gattii infection and allow differentiation of C gattii into its 4 main molecular types: VGI, VGII, VGIII, VGIV. Using methods such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis, molecular typing allows for specific epidemiology charting of C gattii genotypes.7
Although the transmission route for cryptococcal infection is mainly respiratory, direct inoculation has been reported as well.19 Cutaneous lesions, which occur in 5% to 20% of cryptococcal infections, often present in the head and neck.2,20,21 Primary cutaneous infections from cryptococcosis are rare, and cutaneous manifestations are often a sign of disseminated disease. Disseminated disease is defined as the involvement of 2 or more noncontiguous sites or evidence of high fungal burden based on cryptococcal antigen titer of more than 1:512.12 It is important to exclude disseminated disease in all cases of cryptococcosis, as it may be fatal.20 The neural and pulmonary systems should be screened.22 Cellulitis from cryptococcosis is almost always limited to immunocompromised patients, though there are reports of crytococcal cutaneous disease in immunocompetent patients.3,15 Interestingly, though C neoformans often affects immunocompromised patients, the emerging pathogen of C gattii affects immunocompetent patients.7,17,23 Our patient’s undiagnosed diabetes may have been a risk factor for cryptococcal infection. His cryptococcal antigen titer was 1:256, with no evidence of other sites of involvement. We therefore believe this to be a rare case of direct inoculation secondary to an insect bite.
The literature includes 12 reported cases of NF secondary to Cryptococcus (Table 2), all C neoformans. Of these cases, 9 involved immunosuppression, and most of these patients were on long-term steroid treatment after organ transplantation. The most common infection site was the lower extremity. These cases of cryptococcal NF show that immunosuppression, and long-term steroid use in particular, is an important risk factor. The mortality rate for these reviewed cases was 41.6% (5/12). According to the literature, the mortality rates for patients with cryptococcal soft-tissue infections24 and posttransplant patients with cryptococcal NF21 were 37.5% and 60%, respectively. We believe the mortality rate in our reviewed cases likely was confounded by the fact that most of the patients were posttransplant patients on long-term immunosuppression.
Of the 12 patients, 5 had primary cutaneous disease. There seems to be no relationship between outcome and dissemination of disease. In addition, there is a paucity of literature on the effect of disseminated disease and cryptococcal soft-tissue infections. Therefore, no firm conclusions can be drawn regarding the effects of disseminated disease on severity of cryptococcal soft-tissue infection.
Treatment of cryptococcal NF involves a combination of surgical débridement and long-term antifungal therapy. Surgical débridement of NF includes delineating the extent of infection with complete surgical excision of the affected tissue.25 The aims of surgery should be to remove all unhealthy tissue, identify the offending organism, and plan for resurfacing or reconstruction of the afflicted extremity. Intraoperative-tissue histology should be performed to confirm the diagnosis of NF. Histology can be used to demonstrate cryptococcal infection. The diagnosis of cryptococcal infection can be aided with fungal cultures, and therefore we recommend that tissue cultures be sent not only for routine aerobic/anaerobic bacteria but also for mycobacteria and fungal organisms. Laboratory tests that aid in diagnosis include serum cryptococcal antigen titer.
The current treatment recommendation for cryptococcal disease in patients who are not HIV-positive or transplant hosts is amphotericin B deoxycholate 0.7 to 1.0 mg/kg/d plus flucytosine 100 mg/kg/d for at least 4 weeks.22 The regimen period may be shortened to 14 days for patients at low risk of treatment failure. Fluconazole should be given as maintenance therapy (200 mg/d) for 6 to 12 months. There is no compelling evidence for immunoglobulin therapy for cryptococcal disease.22
Conclusion
NF caused by Cryptococcus is rare. A high level of suspicion, and intraoperative specimens for histology and fungal microscopy and culture, can help in establishing the diagnosis. Molecular genotyping remains the diagnostic method of choice for NF secondary to Cryptococcus. Effective treatment consists of aggressive surgical débridement and antifungal therapy.
1. Marcus JR, Hussong JW, Gonzalez C, Dumanian GA. Risk factors in necrotizing fasciitis: a case involving Cryptococcus neoformans. Ann Plast Surg. 1998;40(1):80-83.
2. Huang KC, Tu YK, Lee KF, Huang TJ, Wen-Wei Hsu R. Disseminated cryptococcosis presented as necrotizing fasciitis of a limb. J Trauma. 2007;63(2):E44-E46.
3. Capoor MR, Khanna G, Malhotra R. Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med Mycol. 2008;46:269-273.
4. Adachi M, Tsurata D, Imanishi H, Ishii M, Kobayashi H. Necrotizing fasciitis caused by Cryptococcus neoformans in a patient with pemphigus vegetans. Clin Exp Dermatol. 2009;34(8):e751-e753.
5. Enache-Angoulvant A, Chandenier J, Symoens F, et al. Molecular identification of Cryptococcus neoformans serotypes. J Clin Microbiol. 2007;45(4):1261-1265.
6. Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006;6(4):657-687.
7. Datta K, Bartlett KH, Baer R, et al; Cryptococcus gattii Working Group of the Pacific Northwest. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185-1191.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. McTaggart LR, Lei E, Richardson SE, Hoang L, Fothergill A, Zhang SX. Rapid identification of Cryptococcus neoformans and Cryptococcus gattii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):3050-3053.
10. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189-195.
11. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460.
12. Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52-57.
13. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249-257.
14. Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plastic Surg. 2013;70(6):739-741.
15. Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Modern Pathol. 2006;19(9):1221-1226.
16. Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8(3):151-158.
17. Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155-168.
18. Kidd SE, Sorrell TC, Meyer W. Isolation of two molecular types of Cryptococcus neoformans var. gattii from insect frass. Med Mycol. 2003;41(2):171-176.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36(3):337-347.
20. Basaran O, Emiroglu R, Arikan U, Karakayali H, Haberal M. Cryptococcal necrotizing fasciitis with multiple sites of involvement in the lower extremities. Dermatol Surg. 2003;29(11):1158-1160.
21. Baer S, Baddley JW, Gnann JW, Pappas PG. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl Infect Dis. 2009;11(4):353-358.
22. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291-322.
23. Chan M, Lye D, Win MK, Chow A, Barkham T. Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int J Infect Dis. 2014;26:110-115.
24. Gave AA, Torres R, Kaplan L. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg Infect. 2004;5(3):309-313.
25. Wong CH, Yam AK, Tan AB, Song C. Approach to debridement in necrotizing fasciitis. Am J Surg. 2008;196(3):e19-e24.
26. Bégon E, Bachmeyer C, Thibault M, et al. Necrotizing fasciitis due to Cryptococcus neoformans in a diabetic patient with chronic renal insufficiency. Clin Exp Dermatol. 2009;34(8):935-936.
27. Doorenbos-Bot AC, Hooymans JM, Blanksma LJ. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc Ophthalmol. 1990;75(3-4):315-320.
28. Yoneda T, Itami Y, Hirayama A, Saka T, Yoshida K, Fujimoto K. Cryptococcal necrotizing fasciitis in a patient after renal transplantation—a case report. Transplant Proc. 2014;46(2):620-622.
Necrotizing fasciitis (NF) is a severe, rapidly spreading soft-tissue infection with high morbidity and mortality. Bacteriology in NF may be varied, and the etiology is often polymicrobial. It is important to consider the potential for fungal involvement despite its rarity. Cryptococcal NF has been reported in immunocompromised patients, with Cryptococcus neoformans being the most common offending organism.1-4
C neoformans is a basidiomycotic yeast that was previously considered a homogenous species.5,6 From the antigenic properties of its polysaccharide capsule, 3 main variants were described: C neoformans var. grubii, C neoformans var. neoformans, and C neoformans var. gattii. Subsequently, C neoformans var. gattii was found to be genetically and biochemically different from C neoformans. This discovery led to the distinction of C neoformans var. gattii as a separate species and it being renamed C gattii.6
C gattii was first recognized on Vancouver Island in 2001.7 Although C gattii is predominantly restricted to tropical and subtropical climates, its true epidemiology has been limited by diagnostic methods. C gattii can be diagnosed with laboratory culture media such as birdseed agars and L-canavanine-glycine-bromothymol (CGB) agar.6 However, most reports of Cryptococcus NF do not specify the culture media used to isolate Cryptococcus. In addition to culture media, molecular genotyping studies also allow for confirmation of the diagnosis of C gattii and have the added benefit of enabling identification of the molecular genotype. Nonetheless, in many clinical microbiology laboratories, Cryptococcus is not identified to the species level, much less to the molecular genotype.7 Given these diagnostic limitations and the fact that C gattii was only recently identified as a separate species, it is possible that any pre-2006 cases of NF attributed to C neoformans could in fact have been caused by C gattii.
In this article, we review the literature and report a case of NF of the hand that was caused by C gattii in a patient with diabetes. To our knowledge, this is the first reported case of NF caused by C gattii. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old man was admitted with a 1-week history of swelling and pain in the dorsum of the left hand. He had been sitting in an outdoor eatery in Singapore when an insect bit the hand over the dorsum. Two days later, he consulted his family physician, who began treatment with oral amoxicillin/clavulanic acid. After 4 days of treatment, there was clinical progression of increased swelling and pain in the hand. Six days after initial injury, the patient presented to the department of orthopedic surgery.
Physical examination revealed diffuse, brawny, nonfluctuant swelling over the entire dorsum of the left hand (Figure 1). There was a 1×1-cm ruptured blister with some nonpurulent discharge just distal to the wrist joint. Neurovascular status and the extensor mechanism of the fingers were intact. The wrist joint had full range of motion. There was no fever.
Laboratory testing revealed an elevated white blood cell count (16.6×109/L), a C-reactive protein (CRP) level of 237 nmol/L, a random blood glucose level of 12.6 mmol/L, and a LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score of 7.8
Given the severe swelling, intravenous amoxicillin/clavulanic acid was started. The patient received a total of 3 doses before operative débridement of the left hand. Operative findings were NF of the hand, grayish necrotic fascia, and foul-smelling “dishwater” fluid. A single specimen of fascia from the surgical site was sent for examination. Histopathologic examination of formalin-fixed, paraffin-embedded tissue revealed necrotizing suppurative inflammation with fungal organisms present (Figures 2, 3).
Tissue cultures were obtained during surgery. The organism grew as scanty, small, wet-looking colonies on sheep blood agar after 48 hours of incubation. Microscopy revealed an oval yeast. The organism was identified and reported as C gattii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Biotyper 2.0.1 software; Bruker Daltonics), with a score of 1.914.9 All other intraoperative cultures for aerobic and anaerobic bacteria were negative. Molecular genotyping was performed with polymerase chain reaction assay to identify the molecular subtype.10C gattii genotype VGII was isolated. A cryptococcal serum antigen assay was positive at 1:256.
A series of tests was performed to screen for disseminated disease. Blood cultures were negative for fungus. Chest radiography and computed tomography of the brain did not show any pulmonary or cerebral involvement. Cerebrospinal fluid was not available for examination, as the patient declined lumbar puncture. Blood tests included a negative result for human immunodeficiency virus (HIV). The patient was found to have previously undiagnosed diabetes mellitus (hemoglobin A1c, 7.9%). T-cell counts and ratios were normal.
The patient was started on intravenous amphotericin B 60 mg/d and flucytosine 500 mg every 6 hours for 3 weeks. Oral fluconazole 400 mg every morning was also given (intended duration, 6 mo). Given that diabetes was newly diagnosed, the patient was treated with metformin; his capillary blood glucose level remained stable during his inpatient stay.
Four débridements of the dorsal hand wound were performed—the first on day of admission and the other 3 on hospitalization days 3, 7, and 18 (Figure 4). Subsequent wound resurfacing with a split skin graft harvested from the forearm was performed on hospitalization day 22. After surgery, the hand was dressed with a bulky cotton dressing. Five days after the patient was discharged, during review in the outpatient clinic, the skin graft was noted to be taking well. The patient did not attend postoperative physical therapy. He was maintained on metformin and given a follow-up clinic appointment for his diabetes. Four months after surgery, the wound was completely healed, and normal functional use of the hand recovered.
Discussion
NF is a severe soft-tissue infection with potential for rapid progression. Surgical débridement should be performed urgently to reduce the chance of morbidity and mortality.11 The initial classification by Giuliano and colleagues12 was based on bacteriology and included type I (anaerobic species in combination with a facultative species) and type II (monomicrobial usually involving group A β-hemolytic Streptococcus). This classification was modified by Morgan13 to include gram-negative organisms as well as fungal organisms (Table 1).
Fungal NF is rare, with Candida, Apophysomyces, and Cryptococcus described in the literature.1,14,15 Fungal infections tend to occur in immunocompromised patients; risk factors are steroid immunosuppression, poorly controlled diabetes, and peripheral vascular disease.16 Some zygomycetes may also affect immunocompetent patients.15
C gattii is an encapsulated yeast organism that is genetically and biochemically distinct from C neoformans. It is endemic to tropical parts of Africa and Australia. Its main environmental sources are eucalyptus trees (Eucalyptus camaldulensis, Eucalyptus tereticornis) and decaying hollows in living trees.17 In addition, there have been reports of isolation of C gattii from insect frass,18 which would make infection by an insect bite a possible transmission route. Worldwide distribution of this pathogen has increased recently, with outbreaks noted on Vancouver Island and in areas in Canada and the northwest United States.7
The true incidence of NF secondary to C gattii is difficult to determine. C gattii was only recently identified as a separate species, and pre-2006 cases of NF attributed to C neoformans may instead have been caused by C gattii. Misidentification has been compounded by the fact that the tests required for accurate diagnosis of C gattii infection may not be readily available in many clinical microbiology laboratories. Cryptococcus can be identified with various methods, including direct microscopy, culturing of tissue or fluid samples, and measurement of cryptococcal serum antigen. However, tests such as specific culture media, mass spectrometry, and molecular typing studies are required to determine cryptococcal species. L-canavanine-glycine-bromothymol blue (CGB) agar is a medium that is often used to differentiate C gattii from C neoformans because of the ability of C gattii to produce a blue appearance.6 Modern techniques, such as MALDI-TOF MS, have also been used to successfully distinguish between C gattii and C neoformans.9 MALDI-TOF MS identifies species on the basis of characteristic protein spectra extracted from whole cells. Using commercial and supplemental reference libraries, the system compares signal matches in the reference spectrum with Cryptococcus entries in the library—allowing rapid and accurate identification of cryptococcal species. However, this diagnostic method is limited by availability of adequate Cryptococcus entries in the reference library and by the high cost of acquiring the machine.
Serotyping is based on the antigenic property of the capsule and was once used to differentiate C neoformans into its 3 main varieties: var. neoformans, var. grubii, var. gattii. However, when it was realized that the antigenic property of the strain can be unstable and that there are hybrids containing more than 1 serotype, serotyping was abandoned as a species-differentiation test.6 The current gold standard for species differentiation is molecular genotyping. Molecular genotyping studies can confirm the diagnosis of C gattii infection and allow differentiation of C gattii into its 4 main molecular types: VGI, VGII, VGIII, VGIV. Using methods such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis, molecular typing allows for specific epidemiology charting of C gattii genotypes.7
Although the transmission route for cryptococcal infection is mainly respiratory, direct inoculation has been reported as well.19 Cutaneous lesions, which occur in 5% to 20% of cryptococcal infections, often present in the head and neck.2,20,21 Primary cutaneous infections from cryptococcosis are rare, and cutaneous manifestations are often a sign of disseminated disease. Disseminated disease is defined as the involvement of 2 or more noncontiguous sites or evidence of high fungal burden based on cryptococcal antigen titer of more than 1:512.12 It is important to exclude disseminated disease in all cases of cryptococcosis, as it may be fatal.20 The neural and pulmonary systems should be screened.22 Cellulitis from cryptococcosis is almost always limited to immunocompromised patients, though there are reports of crytococcal cutaneous disease in immunocompetent patients.3,15 Interestingly, though C neoformans often affects immunocompromised patients, the emerging pathogen of C gattii affects immunocompetent patients.7,17,23 Our patient’s undiagnosed diabetes may have been a risk factor for cryptococcal infection. His cryptococcal antigen titer was 1:256, with no evidence of other sites of involvement. We therefore believe this to be a rare case of direct inoculation secondary to an insect bite.
The literature includes 12 reported cases of NF secondary to Cryptococcus (Table 2), all C neoformans. Of these cases, 9 involved immunosuppression, and most of these patients were on long-term steroid treatment after organ transplantation. The most common infection site was the lower extremity. These cases of cryptococcal NF show that immunosuppression, and long-term steroid use in particular, is an important risk factor. The mortality rate for these reviewed cases was 41.6% (5/12). According to the literature, the mortality rates for patients with cryptococcal soft-tissue infections24 and posttransplant patients with cryptococcal NF21 were 37.5% and 60%, respectively. We believe the mortality rate in our reviewed cases likely was confounded by the fact that most of the patients were posttransplant patients on long-term immunosuppression.
Of the 12 patients, 5 had primary cutaneous disease. There seems to be no relationship between outcome and dissemination of disease. In addition, there is a paucity of literature on the effect of disseminated disease and cryptococcal soft-tissue infections. Therefore, no firm conclusions can be drawn regarding the effects of disseminated disease on severity of cryptococcal soft-tissue infection.
Treatment of cryptococcal NF involves a combination of surgical débridement and long-term antifungal therapy. Surgical débridement of NF includes delineating the extent of infection with complete surgical excision of the affected tissue.25 The aims of surgery should be to remove all unhealthy tissue, identify the offending organism, and plan for resurfacing or reconstruction of the afflicted extremity. Intraoperative-tissue histology should be performed to confirm the diagnosis of NF. Histology can be used to demonstrate cryptococcal infection. The diagnosis of cryptococcal infection can be aided with fungal cultures, and therefore we recommend that tissue cultures be sent not only for routine aerobic/anaerobic bacteria but also for mycobacteria and fungal organisms. Laboratory tests that aid in diagnosis include serum cryptococcal antigen titer.
The current treatment recommendation for cryptococcal disease in patients who are not HIV-positive or transplant hosts is amphotericin B deoxycholate 0.7 to 1.0 mg/kg/d plus flucytosine 100 mg/kg/d for at least 4 weeks.22 The regimen period may be shortened to 14 days for patients at low risk of treatment failure. Fluconazole should be given as maintenance therapy (200 mg/d) for 6 to 12 months. There is no compelling evidence for immunoglobulin therapy for cryptococcal disease.22
Conclusion
NF caused by Cryptococcus is rare. A high level of suspicion, and intraoperative specimens for histology and fungal microscopy and culture, can help in establishing the diagnosis. Molecular genotyping remains the diagnostic method of choice for NF secondary to Cryptococcus. Effective treatment consists of aggressive surgical débridement and antifungal therapy.
Necrotizing fasciitis (NF) is a severe, rapidly spreading soft-tissue infection with high morbidity and mortality. Bacteriology in NF may be varied, and the etiology is often polymicrobial. It is important to consider the potential for fungal involvement despite its rarity. Cryptococcal NF has been reported in immunocompromised patients, with Cryptococcus neoformans being the most common offending organism.1-4
C neoformans is a basidiomycotic yeast that was previously considered a homogenous species.5,6 From the antigenic properties of its polysaccharide capsule, 3 main variants were described: C neoformans var. grubii, C neoformans var. neoformans, and C neoformans var. gattii. Subsequently, C neoformans var. gattii was found to be genetically and biochemically different from C neoformans. This discovery led to the distinction of C neoformans var. gattii as a separate species and it being renamed C gattii.6
C gattii was first recognized on Vancouver Island in 2001.7 Although C gattii is predominantly restricted to tropical and subtropical climates, its true epidemiology has been limited by diagnostic methods. C gattii can be diagnosed with laboratory culture media such as birdseed agars and L-canavanine-glycine-bromothymol (CGB) agar.6 However, most reports of Cryptococcus NF do not specify the culture media used to isolate Cryptococcus. In addition to culture media, molecular genotyping studies also allow for confirmation of the diagnosis of C gattii and have the added benefit of enabling identification of the molecular genotype. Nonetheless, in many clinical microbiology laboratories, Cryptococcus is not identified to the species level, much less to the molecular genotype.7 Given these diagnostic limitations and the fact that C gattii was only recently identified as a separate species, it is possible that any pre-2006 cases of NF attributed to C neoformans could in fact have been caused by C gattii.
In this article, we review the literature and report a case of NF of the hand that was caused by C gattii in a patient with diabetes. To our knowledge, this is the first reported case of NF caused by C gattii. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old man was admitted with a 1-week history of swelling and pain in the dorsum of the left hand. He had been sitting in an outdoor eatery in Singapore when an insect bit the hand over the dorsum. Two days later, he consulted his family physician, who began treatment with oral amoxicillin/clavulanic acid. After 4 days of treatment, there was clinical progression of increased swelling and pain in the hand. Six days after initial injury, the patient presented to the department of orthopedic surgery.
Physical examination revealed diffuse, brawny, nonfluctuant swelling over the entire dorsum of the left hand (Figure 1). There was a 1×1-cm ruptured blister with some nonpurulent discharge just distal to the wrist joint. Neurovascular status and the extensor mechanism of the fingers were intact. The wrist joint had full range of motion. There was no fever.
Laboratory testing revealed an elevated white blood cell count (16.6×109/L), a C-reactive protein (CRP) level of 237 nmol/L, a random blood glucose level of 12.6 mmol/L, and a LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score of 7.8
Given the severe swelling, intravenous amoxicillin/clavulanic acid was started. The patient received a total of 3 doses before operative débridement of the left hand. Operative findings were NF of the hand, grayish necrotic fascia, and foul-smelling “dishwater” fluid. A single specimen of fascia from the surgical site was sent for examination. Histopathologic examination of formalin-fixed, paraffin-embedded tissue revealed necrotizing suppurative inflammation with fungal organisms present (Figures 2, 3).
Tissue cultures were obtained during surgery. The organism grew as scanty, small, wet-looking colonies on sheep blood agar after 48 hours of incubation. Microscopy revealed an oval yeast. The organism was identified and reported as C gattii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Biotyper 2.0.1 software; Bruker Daltonics), with a score of 1.914.9 All other intraoperative cultures for aerobic and anaerobic bacteria were negative. Molecular genotyping was performed with polymerase chain reaction assay to identify the molecular subtype.10C gattii genotype VGII was isolated. A cryptococcal serum antigen assay was positive at 1:256.
A series of tests was performed to screen for disseminated disease. Blood cultures were negative for fungus. Chest radiography and computed tomography of the brain did not show any pulmonary or cerebral involvement. Cerebrospinal fluid was not available for examination, as the patient declined lumbar puncture. Blood tests included a negative result for human immunodeficiency virus (HIV). The patient was found to have previously undiagnosed diabetes mellitus (hemoglobin A1c, 7.9%). T-cell counts and ratios were normal.
The patient was started on intravenous amphotericin B 60 mg/d and flucytosine 500 mg every 6 hours for 3 weeks. Oral fluconazole 400 mg every morning was also given (intended duration, 6 mo). Given that diabetes was newly diagnosed, the patient was treated with metformin; his capillary blood glucose level remained stable during his inpatient stay.
Four débridements of the dorsal hand wound were performed—the first on day of admission and the other 3 on hospitalization days 3, 7, and 18 (Figure 4). Subsequent wound resurfacing with a split skin graft harvested from the forearm was performed on hospitalization day 22. After surgery, the hand was dressed with a bulky cotton dressing. Five days after the patient was discharged, during review in the outpatient clinic, the skin graft was noted to be taking well. The patient did not attend postoperative physical therapy. He was maintained on metformin and given a follow-up clinic appointment for his diabetes. Four months after surgery, the wound was completely healed, and normal functional use of the hand recovered.
Discussion
NF is a severe soft-tissue infection with potential for rapid progression. Surgical débridement should be performed urgently to reduce the chance of morbidity and mortality.11 The initial classification by Giuliano and colleagues12 was based on bacteriology and included type I (anaerobic species in combination with a facultative species) and type II (monomicrobial usually involving group A β-hemolytic Streptococcus). This classification was modified by Morgan13 to include gram-negative organisms as well as fungal organisms (Table 1).
Fungal NF is rare, with Candida, Apophysomyces, and Cryptococcus described in the literature.1,14,15 Fungal infections tend to occur in immunocompromised patients; risk factors are steroid immunosuppression, poorly controlled diabetes, and peripheral vascular disease.16 Some zygomycetes may also affect immunocompetent patients.15
C gattii is an encapsulated yeast organism that is genetically and biochemically distinct from C neoformans. It is endemic to tropical parts of Africa and Australia. Its main environmental sources are eucalyptus trees (Eucalyptus camaldulensis, Eucalyptus tereticornis) and decaying hollows in living trees.17 In addition, there have been reports of isolation of C gattii from insect frass,18 which would make infection by an insect bite a possible transmission route. Worldwide distribution of this pathogen has increased recently, with outbreaks noted on Vancouver Island and in areas in Canada and the northwest United States.7
The true incidence of NF secondary to C gattii is difficult to determine. C gattii was only recently identified as a separate species, and pre-2006 cases of NF attributed to C neoformans may instead have been caused by C gattii. Misidentification has been compounded by the fact that the tests required for accurate diagnosis of C gattii infection may not be readily available in many clinical microbiology laboratories. Cryptococcus can be identified with various methods, including direct microscopy, culturing of tissue or fluid samples, and measurement of cryptococcal serum antigen. However, tests such as specific culture media, mass spectrometry, and molecular typing studies are required to determine cryptococcal species. L-canavanine-glycine-bromothymol blue (CGB) agar is a medium that is often used to differentiate C gattii from C neoformans because of the ability of C gattii to produce a blue appearance.6 Modern techniques, such as MALDI-TOF MS, have also been used to successfully distinguish between C gattii and C neoformans.9 MALDI-TOF MS identifies species on the basis of characteristic protein spectra extracted from whole cells. Using commercial and supplemental reference libraries, the system compares signal matches in the reference spectrum with Cryptococcus entries in the library—allowing rapid and accurate identification of cryptococcal species. However, this diagnostic method is limited by availability of adequate Cryptococcus entries in the reference library and by the high cost of acquiring the machine.
Serotyping is based on the antigenic property of the capsule and was once used to differentiate C neoformans into its 3 main varieties: var. neoformans, var. grubii, var. gattii. However, when it was realized that the antigenic property of the strain can be unstable and that there are hybrids containing more than 1 serotype, serotyping was abandoned as a species-differentiation test.6 The current gold standard for species differentiation is molecular genotyping. Molecular genotyping studies can confirm the diagnosis of C gattii infection and allow differentiation of C gattii into its 4 main molecular types: VGI, VGII, VGIII, VGIV. Using methods such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis, molecular typing allows for specific epidemiology charting of C gattii genotypes.7
Although the transmission route for cryptococcal infection is mainly respiratory, direct inoculation has been reported as well.19 Cutaneous lesions, which occur in 5% to 20% of cryptococcal infections, often present in the head and neck.2,20,21 Primary cutaneous infections from cryptococcosis are rare, and cutaneous manifestations are often a sign of disseminated disease. Disseminated disease is defined as the involvement of 2 or more noncontiguous sites or evidence of high fungal burden based on cryptococcal antigen titer of more than 1:512.12 It is important to exclude disseminated disease in all cases of cryptococcosis, as it may be fatal.20 The neural and pulmonary systems should be screened.22 Cellulitis from cryptococcosis is almost always limited to immunocompromised patients, though there are reports of crytococcal cutaneous disease in immunocompetent patients.3,15 Interestingly, though C neoformans often affects immunocompromised patients, the emerging pathogen of C gattii affects immunocompetent patients.7,17,23 Our patient’s undiagnosed diabetes may have been a risk factor for cryptococcal infection. His cryptococcal antigen titer was 1:256, with no evidence of other sites of involvement. We therefore believe this to be a rare case of direct inoculation secondary to an insect bite.
The literature includes 12 reported cases of NF secondary to Cryptococcus (Table 2), all C neoformans. Of these cases, 9 involved immunosuppression, and most of these patients were on long-term steroid treatment after organ transplantation. The most common infection site was the lower extremity. These cases of cryptococcal NF show that immunosuppression, and long-term steroid use in particular, is an important risk factor. The mortality rate for these reviewed cases was 41.6% (5/12). According to the literature, the mortality rates for patients with cryptococcal soft-tissue infections24 and posttransplant patients with cryptococcal NF21 were 37.5% and 60%, respectively. We believe the mortality rate in our reviewed cases likely was confounded by the fact that most of the patients were posttransplant patients on long-term immunosuppression.
Of the 12 patients, 5 had primary cutaneous disease. There seems to be no relationship between outcome and dissemination of disease. In addition, there is a paucity of literature on the effect of disseminated disease and cryptococcal soft-tissue infections. Therefore, no firm conclusions can be drawn regarding the effects of disseminated disease on severity of cryptococcal soft-tissue infection.
Treatment of cryptococcal NF involves a combination of surgical débridement and long-term antifungal therapy. Surgical débridement of NF includes delineating the extent of infection with complete surgical excision of the affected tissue.25 The aims of surgery should be to remove all unhealthy tissue, identify the offending organism, and plan for resurfacing or reconstruction of the afflicted extremity. Intraoperative-tissue histology should be performed to confirm the diagnosis of NF. Histology can be used to demonstrate cryptococcal infection. The diagnosis of cryptococcal infection can be aided with fungal cultures, and therefore we recommend that tissue cultures be sent not only for routine aerobic/anaerobic bacteria but also for mycobacteria and fungal organisms. Laboratory tests that aid in diagnosis include serum cryptococcal antigen titer.
The current treatment recommendation for cryptococcal disease in patients who are not HIV-positive or transplant hosts is amphotericin B deoxycholate 0.7 to 1.0 mg/kg/d plus flucytosine 100 mg/kg/d for at least 4 weeks.22 The regimen period may be shortened to 14 days for patients at low risk of treatment failure. Fluconazole should be given as maintenance therapy (200 mg/d) for 6 to 12 months. There is no compelling evidence for immunoglobulin therapy for cryptococcal disease.22
Conclusion
NF caused by Cryptococcus is rare. A high level of suspicion, and intraoperative specimens for histology and fungal microscopy and culture, can help in establishing the diagnosis. Molecular genotyping remains the diagnostic method of choice for NF secondary to Cryptococcus. Effective treatment consists of aggressive surgical débridement and antifungal therapy.
1. Marcus JR, Hussong JW, Gonzalez C, Dumanian GA. Risk factors in necrotizing fasciitis: a case involving Cryptococcus neoformans. Ann Plast Surg. 1998;40(1):80-83.
2. Huang KC, Tu YK, Lee KF, Huang TJ, Wen-Wei Hsu R. Disseminated cryptococcosis presented as necrotizing fasciitis of a limb. J Trauma. 2007;63(2):E44-E46.
3. Capoor MR, Khanna G, Malhotra R. Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med Mycol. 2008;46:269-273.
4. Adachi M, Tsurata D, Imanishi H, Ishii M, Kobayashi H. Necrotizing fasciitis caused by Cryptococcus neoformans in a patient with pemphigus vegetans. Clin Exp Dermatol. 2009;34(8):e751-e753.
5. Enache-Angoulvant A, Chandenier J, Symoens F, et al. Molecular identification of Cryptococcus neoformans serotypes. J Clin Microbiol. 2007;45(4):1261-1265.
6. Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006;6(4):657-687.
7. Datta K, Bartlett KH, Baer R, et al; Cryptococcus gattii Working Group of the Pacific Northwest. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185-1191.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. McTaggart LR, Lei E, Richardson SE, Hoang L, Fothergill A, Zhang SX. Rapid identification of Cryptococcus neoformans and Cryptococcus gattii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):3050-3053.
10. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189-195.
11. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460.
12. Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52-57.
13. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249-257.
14. Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plastic Surg. 2013;70(6):739-741.
15. Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Modern Pathol. 2006;19(9):1221-1226.
16. Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8(3):151-158.
17. Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155-168.
18. Kidd SE, Sorrell TC, Meyer W. Isolation of two molecular types of Cryptococcus neoformans var. gattii from insect frass. Med Mycol. 2003;41(2):171-176.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36(3):337-347.
20. Basaran O, Emiroglu R, Arikan U, Karakayali H, Haberal M. Cryptococcal necrotizing fasciitis with multiple sites of involvement in the lower extremities. Dermatol Surg. 2003;29(11):1158-1160.
21. Baer S, Baddley JW, Gnann JW, Pappas PG. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl Infect Dis. 2009;11(4):353-358.
22. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291-322.
23. Chan M, Lye D, Win MK, Chow A, Barkham T. Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int J Infect Dis. 2014;26:110-115.
24. Gave AA, Torres R, Kaplan L. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg Infect. 2004;5(3):309-313.
25. Wong CH, Yam AK, Tan AB, Song C. Approach to debridement in necrotizing fasciitis. Am J Surg. 2008;196(3):e19-e24.
26. Bégon E, Bachmeyer C, Thibault M, et al. Necrotizing fasciitis due to Cryptococcus neoformans in a diabetic patient with chronic renal insufficiency. Clin Exp Dermatol. 2009;34(8):935-936.
27. Doorenbos-Bot AC, Hooymans JM, Blanksma LJ. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc Ophthalmol. 1990;75(3-4):315-320.
28. Yoneda T, Itami Y, Hirayama A, Saka T, Yoshida K, Fujimoto K. Cryptococcal necrotizing fasciitis in a patient after renal transplantation—a case report. Transplant Proc. 2014;46(2):620-622.
1. Marcus JR, Hussong JW, Gonzalez C, Dumanian GA. Risk factors in necrotizing fasciitis: a case involving Cryptococcus neoformans. Ann Plast Surg. 1998;40(1):80-83.
2. Huang KC, Tu YK, Lee KF, Huang TJ, Wen-Wei Hsu R. Disseminated cryptococcosis presented as necrotizing fasciitis of a limb. J Trauma. 2007;63(2):E44-E46.
3. Capoor MR, Khanna G, Malhotra R. Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med Mycol. 2008;46:269-273.
4. Adachi M, Tsurata D, Imanishi H, Ishii M, Kobayashi H. Necrotizing fasciitis caused by Cryptococcus neoformans in a patient with pemphigus vegetans. Clin Exp Dermatol. 2009;34(8):e751-e753.
5. Enache-Angoulvant A, Chandenier J, Symoens F, et al. Molecular identification of Cryptococcus neoformans serotypes. J Clin Microbiol. 2007;45(4):1261-1265.
6. Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006;6(4):657-687.
7. Datta K, Bartlett KH, Baer R, et al; Cryptococcus gattii Working Group of the Pacific Northwest. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185-1191.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. McTaggart LR, Lei E, Richardson SE, Hoang L, Fothergill A, Zhang SX. Rapid identification of Cryptococcus neoformans and Cryptococcus gattii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):3050-3053.
10. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189-195.
11. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460.
12. Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52-57.
13. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249-257.
14. Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plastic Surg. 2013;70(6):739-741.
15. Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Modern Pathol. 2006;19(9):1221-1226.
16. Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8(3):151-158.
17. Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155-168.
18. Kidd SE, Sorrell TC, Meyer W. Isolation of two molecular types of Cryptococcus neoformans var. gattii from insect frass. Med Mycol. 2003;41(2):171-176.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36(3):337-347.
20. Basaran O, Emiroglu R, Arikan U, Karakayali H, Haberal M. Cryptococcal necrotizing fasciitis with multiple sites of involvement in the lower extremities. Dermatol Surg. 2003;29(11):1158-1160.
21. Baer S, Baddley JW, Gnann JW, Pappas PG. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl Infect Dis. 2009;11(4):353-358.
22. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291-322.
23. Chan M, Lye D, Win MK, Chow A, Barkham T. Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int J Infect Dis. 2014;26:110-115.
24. Gave AA, Torres R, Kaplan L. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg Infect. 2004;5(3):309-313.
25. Wong CH, Yam AK, Tan AB, Song C. Approach to debridement in necrotizing fasciitis. Am J Surg. 2008;196(3):e19-e24.
26. Bégon E, Bachmeyer C, Thibault M, et al. Necrotizing fasciitis due to Cryptococcus neoformans in a diabetic patient with chronic renal insufficiency. Clin Exp Dermatol. 2009;34(8):935-936.
27. Doorenbos-Bot AC, Hooymans JM, Blanksma LJ. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc Ophthalmol. 1990;75(3-4):315-320.
28. Yoneda T, Itami Y, Hirayama A, Saka T, Yoshida K, Fujimoto K. Cryptococcal necrotizing fasciitis in a patient after renal transplantation—a case report. Transplant Proc. 2014;46(2):620-622.
Prevalence of Low Bone Mineral Density in Younger Versus Older Women With Distal Radius Fractures
Many organizations and work groups have issued recommendations regarding which patients should undergo bone densitometry. In 2004, the US Surgeon General recommended bone mineral density (BMD) evaluation for all women over age 65 years and for women and men with fragility fractures.1 The Centers for Medicare & Medicaid Services recommended BMD assessment for estrogen-deficient patients, for patients with vertebral abnormalities or hyperparathyroidism, and for patients receiving either steroid therapy or osteoporosis medications approved by the US Food and Drug Administration.2 The US Preventive Services Task Force and the National Osteoporosis Foundation each recommended screening for all women age 65 years or older and for postmenopausal women (age, 60-64 years) at high risk.3,4 The International Society for Clinical Densitometry (ISCD) recommended screening for all women age 65 years or older, all men age 70 years or older, and high-risk women under age 65 years.5
These current recommendations for BMD evaluation focus on women over age 65 years. More recent studies of postmenopausal women with distal radius fractures (DRFs) have found that both younger women (age, 45-65 years) and older women (age, ≥65 years) can have lower BMD and increased risk for hip and spine fracture.6,7 The authors of those studies recommended that all postmenopausal women with DRFs be evaluated for low BMD and that fracture prevention treatment be initiated. Earnshaw and colleagues8 and Oyen and colleagues9 found that men and women (age, ≥50 years) with DRFs had low BMD and elevated 10-year fracture rates. They concluded that BMD should be evaluated and treated in all DRF patients age 50 years or older. Other studies have shown low BMD in the contralateral distal radius of patients of all ages who presented with Colles fractures.10,11 These 2 studies did not measure spine or hip BMD.
The literature on BMD of younger women with DRFs is limited, relying solely on data collected for the contralateral distal radius.10,11 The ISCD recommended measuring both hip and spine BMD in premenopausal women. They also stated that z scores, not t scores, should be used for premenopausal women.5 The causes of low BMD in women over age 55 years are primarily nutritional deficiency and normal aging.1 In younger females, low BMD results from secondary causes, such as diet, medications, medical conditions, and endocrine disorders. When the secondary cause of low BMD can be identified and treated, osteoporosis can be stopped and even reversed in younger patients.12-14 Low BMD is more amenable to treatment in younger patients than in postmenopausal women. Younger patients with low BMD carry a higher lifetime fracture risk because they have more years of life with low BMD; therefore, early identification and treatment have a more significant impact on fracture prevention in these patients.
In the present study, we determined the prevalence of osteoporosis and osteopenia in younger women (age, 35-50 years) with DRFs and compared BMD measurements from younger women (age, 35-50 years) and older women (age, >50 years) with DRFs. The main goal was to determine which patients should be referred for bone densitometry and subsequent treatment.
Patients and Methods
This study received institutional review board approval. During a 5-year period (January 2005–August 2010), we prospectively collected dual-energy x-ray absorptiometry (DXA) scans for 128 women (age, >35 years) who presented with DRFs to our level I trauma center. Age ranged from 35 to 86 years. Data on mechanism of injury, treatment, and body mass index (BMI) were collected. The 128 patients were divided into a younger group (47 women; age range, 35-50 years; mean age, 44 years) and an older group (81 women; age, ≥51 years; mean age, 61 years). Mean BMI was 29.3 in the younger group and 28.8 in the older group (P = .88) (Table).
BMD was measured with a General Electric Lunar Prodigy Advance scanner that was tested annually for accuracy and precision. BMD of hips and lumbar spines was measured with a 76-Kv x-ray source. All DXA scans were analyzed by the same physician. BMD was omitted in cases of patients with a history of lumbar spine or hip fracture.
Two-sample Student t test was used to compare the 2 groups’ data. When multiple groups were being compared, analysis of variance was used. Spearman rank-order test was used to calculate a correlation coefficient for evaluation of the relationships between age and BMD.
Results
Mean lumbar spine (L1–L4) BMD was 1.12 in the younger group and 1.063 in the older group (P = .02); t scores were –0.63 and –1.132, respectively (P = .02); and mean z scores were –0.69 and –0.61, respectively (P = .81). Mean femoral neck BMD was 0.91 in the younger group and 0.80 in the older group (P < .05); t scores were –0.87 and –1.65, respectively (P < .01), and mean femoral neck z scores were –0.69 and –0.67, respectively (P = .92).
To further analyze BMD of specific age groups, we divided patients by decade: 35-39, 40-49, 50-59, 60-69, 70-79, 80-89 years. Among all 6 decades, there were no statistically significant differences between hip z scores (P = .83) (Figure 1). Spearman rank-order correlation test showed a moderate inverse correlation between age and femoral neck BMD (R = –0.42) and t score (R = –0.43). There was a weak correlation between increasing age and decreasing spine BMD, t score, and z score (Rs = –0.27, –0.31, 0.03). There was no correlation between age and femoral neck z score (R = –0.04).
According to the WHO classification system, 11 (23%) of the 47 women in the younger group were osteopenic, and 8 (17%) were osteoporotic, based on spine BMD. Hip BMD values indicated that 20 patients (43%) were osteopenic, and 3 (6%) were osteoporotic. One patient in the younger group had a hip z score of less than –2, and 14 patients (39%) had a hip z score between –2 and –1. Six patients (18%) had a spine z score of less than –2, and 6 patients (18%) had a spine z score between –2 and –1. Of the 81 older patients, 22 (27%) were osteopenic, and 21 (26%) were osteoporotic, according to spine measurements. The femoral neck data indicated that 39 (48%) of the older patients were osteopenic, and 22 (27%) were osteoporotic.
In both groups, mechanisms of injury were identified. Of the 47 younger patients, 26 fell from standing, 7 fell from a height of more than 6 feet, and 14 were injured in motor vehicle collisions (MVCs). Of the 81 older patients, 2 sustained a direct blow, 64 fell from standing, 4 fell from a height of more than 6 feet, and 11 were injured in MVCs. The differences in z scores based on mechanism of injury were not statistically significant (P = .22) (Figure 2).
Discussion
Several studies have shown that older women with DRFs have low BMD in the spine and femoral neck.8,9 These studies focused on older women who sustained low-energy fractures caused by a fall from a standing height. Studies of younger women with DRFs focused on BMD of the contralateral distal radius, not the spine or femoral neck.10,11 Those study groups also had low BMD. Findings from a multitude of studies have established that patients who are older than 50 years when they sustain distal radius fragility fractures should be referred for bone densitometry studies, and there is increasing evidence that younger patients with fragility fractures should undergo this evaluation as well.
The present study was designed to expand the range of patients and mechanisms of injury. Women in this study were 35 years or older. In addition to collecting data from patients injured in a fall from standing, we examined the medical records of women injured in MVCs, in falls from heights of more than 6 feet, and from direct trauma to the wrist. We measured the BMD of the spine and femoral neck and of the contralateral distal radius.
For this discussion, several key points should be made about BMD evaluation in younger versus older women. Most organizations caution against using spine BMD in older women. The ISCD, however, recommended measuring both hip and spine BMD; whereas BMD can be falsely elevated by spine osteoarthritis in older patients, spine BMD measurements are accurate in younger patients not affected by osteoarthritis. The ISCD also stipulated that z scores should be used in examining BMD in younger patients. The z score is a value of how many standard deviations BMD differs from a matched population of the same age, sex, ethnicity, and weight. The t score, which is useful in evaluating older patients, compares a patient’s BMD with that of an average 30-year-old.12
According to the WHO classification system (intended for older women), osteopenia is indicated by a t score between –1.0 and –2.5, and osteoporosis is indicated by a t score of less than –2.5. In the present study, about 43% of the younger patients (age, 35-50 years) with DRFs were osteopenic, and 6% of these patients were osteoporotic. In concert with previous studies,9 48% of our older women (age, >50 years) with DRFs were osteopenic, and 27% were osteoporotic. The difference in mean spinal z scores between the younger and older groups was not statistically significant (P = .81).
As mentioned, when examining BMD of younger patients, it is imperative to use spine z scores. About 18% of our younger patients had a z score of less than –2, and 18% had a z score between –2 and –1. In our comparison of patients from 5 different age decades (range, 35-79 years), there was no statistically significant difference in z scores (P = .83). In addition, there was no correlation between increasing age and decreasing z score (R = –0.04).
Secondary causes of osteoporosis have been documented in 30% of premenopausal women and 55% of men with vertebral fractures.13-15 Primary osteoporosis results from the normal aging process; secondary osteoporosis results from reversible causes, including medications, gastrointestinal disorders, renal disease, endocrine disorders, and sedentary lifestyle.15,16 When a secondary cause of osteoporosis is identified, treatment can be initiated to increase BMD. As younger patients can reverse bone loss and even increase BMD, it is important to identify reversible causes of osteopenia and osteoporosis in this age group. It is well documented that both younger and older patients with DRFs are at increased risk for subsequent fractures.6 Preventing further bone loss at a younger age may drastically decrease lifetime fracture risk.12,17
Most previous studies of BMD in women were limited to patients with DRFs caused by a low-energy mechanism or by a fall from standing. Current recommendations for BMD testing focus on postmenopausal women who have sustained a fragility or low-energy DRF. When an osteoporotic or osteopenic patient’s distal radius is subjected to a high-energy force, a fracture is likely. Therefore, we expanded our study to include high-energy mechanisms of injury. Our analysis of BMD in patients with DRFs sustained in MVCs indicated that 12% of this group were osteoporotic, and 44% were osteopenic. Forty-three percent of our younger patients with a DRF fractured in a MVC were osteopenic, and 6% were osteoporotic. Among 4 mechanisms of injury for DRFs, there was no statistically significant difference in z scores (P = .22) (Figure 2). This provides evidence that a significant portion of patients with DRFs from both high- and low-energy mechanisms are osteoporotic or osteopenic. Patients with DRFs sustained in MVCs or in falls from heights of more than 6 feet should be referred for BMD evaluation.
Conclusion
A significant proportion of younger patients with DRFs are osteopenic or osteoporotic (43% and 6%, respectively), and their z scores are comparable to those of older patients with DRFs. There was no statistically significant difference in BMD z scores between younger and older patients and no difference in mechanisms of injury. This is evidence that younger patients with DRFs caused by a high- or low-energy mechanism of injury should undergo both DXA scan and BMD evaluation. If osteoporosis or osteopenia can be diagnosed at an earlier age, and if these patients can be properly treated, subsequent fractures could be prevented. The present study provides evidence supporting a simplification of the current recommendations for BMD evaluation: All women with DRFs should undergo bone densitometry.
1. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. http://www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf. Accessed November 3, 2015.
2. Bone mass measurement (bone density). Medicare website. https://www.medicare.gov/coverage/bone-density.html. Accessed November 3, 2015.
3. Final update summary: osteoporosis: screening. US Preventive Services Task Force website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening. Updated July 2015. Accessed November 3, 2015.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. http://nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed November 3, 2015.
5. Khan AA, Bachrach L, Brown JP, et al. Canadian Panel of International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64.
6. Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008;19(5):607-613.
7. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3(3):127-132.
8. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60.
9. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radius fractures: a case–control study. J Bone Joint Surg Am. 2011;93(4):348-356.
10. Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85(3):423-425.
11. Wigderowitz CA, Rowley DI, Mole PA, Paterson CR, Abel EW. Bone mineral density of the radius in patients with Colles’ fracture. J Bone Joint Surg Br. 2000;82(1):87-89.
12. Khan A, Syed Z. Bone mineral density assessment in premenopausal women. Womens Health. 2006;2(4):639-645.
13. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
14. Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120-128.
15. Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;7(3):589-601.
16. Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643-645.
17. Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123(6):452-460.
Many organizations and work groups have issued recommendations regarding which patients should undergo bone densitometry. In 2004, the US Surgeon General recommended bone mineral density (BMD) evaluation for all women over age 65 years and for women and men with fragility fractures.1 The Centers for Medicare & Medicaid Services recommended BMD assessment for estrogen-deficient patients, for patients with vertebral abnormalities or hyperparathyroidism, and for patients receiving either steroid therapy or osteoporosis medications approved by the US Food and Drug Administration.2 The US Preventive Services Task Force and the National Osteoporosis Foundation each recommended screening for all women age 65 years or older and for postmenopausal women (age, 60-64 years) at high risk.3,4 The International Society for Clinical Densitometry (ISCD) recommended screening for all women age 65 years or older, all men age 70 years or older, and high-risk women under age 65 years.5
These current recommendations for BMD evaluation focus on women over age 65 years. More recent studies of postmenopausal women with distal radius fractures (DRFs) have found that both younger women (age, 45-65 years) and older women (age, ≥65 years) can have lower BMD and increased risk for hip and spine fracture.6,7 The authors of those studies recommended that all postmenopausal women with DRFs be evaluated for low BMD and that fracture prevention treatment be initiated. Earnshaw and colleagues8 and Oyen and colleagues9 found that men and women (age, ≥50 years) with DRFs had low BMD and elevated 10-year fracture rates. They concluded that BMD should be evaluated and treated in all DRF patients age 50 years or older. Other studies have shown low BMD in the contralateral distal radius of patients of all ages who presented with Colles fractures.10,11 These 2 studies did not measure spine or hip BMD.
The literature on BMD of younger women with DRFs is limited, relying solely on data collected for the contralateral distal radius.10,11 The ISCD recommended measuring both hip and spine BMD in premenopausal women. They also stated that z scores, not t scores, should be used for premenopausal women.5 The causes of low BMD in women over age 55 years are primarily nutritional deficiency and normal aging.1 In younger females, low BMD results from secondary causes, such as diet, medications, medical conditions, and endocrine disorders. When the secondary cause of low BMD can be identified and treated, osteoporosis can be stopped and even reversed in younger patients.12-14 Low BMD is more amenable to treatment in younger patients than in postmenopausal women. Younger patients with low BMD carry a higher lifetime fracture risk because they have more years of life with low BMD; therefore, early identification and treatment have a more significant impact on fracture prevention in these patients.
In the present study, we determined the prevalence of osteoporosis and osteopenia in younger women (age, 35-50 years) with DRFs and compared BMD measurements from younger women (age, 35-50 years) and older women (age, >50 years) with DRFs. The main goal was to determine which patients should be referred for bone densitometry and subsequent treatment.
Patients and Methods
This study received institutional review board approval. During a 5-year period (January 2005–August 2010), we prospectively collected dual-energy x-ray absorptiometry (DXA) scans for 128 women (age, >35 years) who presented with DRFs to our level I trauma center. Age ranged from 35 to 86 years. Data on mechanism of injury, treatment, and body mass index (BMI) were collected. The 128 patients were divided into a younger group (47 women; age range, 35-50 years; mean age, 44 years) and an older group (81 women; age, ≥51 years; mean age, 61 years). Mean BMI was 29.3 in the younger group and 28.8 in the older group (P = .88) (Table).
BMD was measured with a General Electric Lunar Prodigy Advance scanner that was tested annually for accuracy and precision. BMD of hips and lumbar spines was measured with a 76-Kv x-ray source. All DXA scans were analyzed by the same physician. BMD was omitted in cases of patients with a history of lumbar spine or hip fracture.
Two-sample Student t test was used to compare the 2 groups’ data. When multiple groups were being compared, analysis of variance was used. Spearman rank-order test was used to calculate a correlation coefficient for evaluation of the relationships between age and BMD.
Results
Mean lumbar spine (L1–L4) BMD was 1.12 in the younger group and 1.063 in the older group (P = .02); t scores were –0.63 and –1.132, respectively (P = .02); and mean z scores were –0.69 and –0.61, respectively (P = .81). Mean femoral neck BMD was 0.91 in the younger group and 0.80 in the older group (P < .05); t scores were –0.87 and –1.65, respectively (P < .01), and mean femoral neck z scores were –0.69 and –0.67, respectively (P = .92).
To further analyze BMD of specific age groups, we divided patients by decade: 35-39, 40-49, 50-59, 60-69, 70-79, 80-89 years. Among all 6 decades, there were no statistically significant differences between hip z scores (P = .83) (Figure 1). Spearman rank-order correlation test showed a moderate inverse correlation between age and femoral neck BMD (R = –0.42) and t score (R = –0.43). There was a weak correlation between increasing age and decreasing spine BMD, t score, and z score (Rs = –0.27, –0.31, 0.03). There was no correlation between age and femoral neck z score (R = –0.04).
According to the WHO classification system, 11 (23%) of the 47 women in the younger group were osteopenic, and 8 (17%) were osteoporotic, based on spine BMD. Hip BMD values indicated that 20 patients (43%) were osteopenic, and 3 (6%) were osteoporotic. One patient in the younger group had a hip z score of less than –2, and 14 patients (39%) had a hip z score between –2 and –1. Six patients (18%) had a spine z score of less than –2, and 6 patients (18%) had a spine z score between –2 and –1. Of the 81 older patients, 22 (27%) were osteopenic, and 21 (26%) were osteoporotic, according to spine measurements. The femoral neck data indicated that 39 (48%) of the older patients were osteopenic, and 22 (27%) were osteoporotic.
In both groups, mechanisms of injury were identified. Of the 47 younger patients, 26 fell from standing, 7 fell from a height of more than 6 feet, and 14 were injured in motor vehicle collisions (MVCs). Of the 81 older patients, 2 sustained a direct blow, 64 fell from standing, 4 fell from a height of more than 6 feet, and 11 were injured in MVCs. The differences in z scores based on mechanism of injury were not statistically significant (P = .22) (Figure 2).
Discussion
Several studies have shown that older women with DRFs have low BMD in the spine and femoral neck.8,9 These studies focused on older women who sustained low-energy fractures caused by a fall from a standing height. Studies of younger women with DRFs focused on BMD of the contralateral distal radius, not the spine or femoral neck.10,11 Those study groups also had low BMD. Findings from a multitude of studies have established that patients who are older than 50 years when they sustain distal radius fragility fractures should be referred for bone densitometry studies, and there is increasing evidence that younger patients with fragility fractures should undergo this evaluation as well.
The present study was designed to expand the range of patients and mechanisms of injury. Women in this study were 35 years or older. In addition to collecting data from patients injured in a fall from standing, we examined the medical records of women injured in MVCs, in falls from heights of more than 6 feet, and from direct trauma to the wrist. We measured the BMD of the spine and femoral neck and of the contralateral distal radius.
For this discussion, several key points should be made about BMD evaluation in younger versus older women. Most organizations caution against using spine BMD in older women. The ISCD, however, recommended measuring both hip and spine BMD; whereas BMD can be falsely elevated by spine osteoarthritis in older patients, spine BMD measurements are accurate in younger patients not affected by osteoarthritis. The ISCD also stipulated that z scores should be used in examining BMD in younger patients. The z score is a value of how many standard deviations BMD differs from a matched population of the same age, sex, ethnicity, and weight. The t score, which is useful in evaluating older patients, compares a patient’s BMD with that of an average 30-year-old.12
According to the WHO classification system (intended for older women), osteopenia is indicated by a t score between –1.0 and –2.5, and osteoporosis is indicated by a t score of less than –2.5. In the present study, about 43% of the younger patients (age, 35-50 years) with DRFs were osteopenic, and 6% of these patients were osteoporotic. In concert with previous studies,9 48% of our older women (age, >50 years) with DRFs were osteopenic, and 27% were osteoporotic. The difference in mean spinal z scores between the younger and older groups was not statistically significant (P = .81).
As mentioned, when examining BMD of younger patients, it is imperative to use spine z scores. About 18% of our younger patients had a z score of less than –2, and 18% had a z score between –2 and –1. In our comparison of patients from 5 different age decades (range, 35-79 years), there was no statistically significant difference in z scores (P = .83). In addition, there was no correlation between increasing age and decreasing z score (R = –0.04).
Secondary causes of osteoporosis have been documented in 30% of premenopausal women and 55% of men with vertebral fractures.13-15 Primary osteoporosis results from the normal aging process; secondary osteoporosis results from reversible causes, including medications, gastrointestinal disorders, renal disease, endocrine disorders, and sedentary lifestyle.15,16 When a secondary cause of osteoporosis is identified, treatment can be initiated to increase BMD. As younger patients can reverse bone loss and even increase BMD, it is important to identify reversible causes of osteopenia and osteoporosis in this age group. It is well documented that both younger and older patients with DRFs are at increased risk for subsequent fractures.6 Preventing further bone loss at a younger age may drastically decrease lifetime fracture risk.12,17
Most previous studies of BMD in women were limited to patients with DRFs caused by a low-energy mechanism or by a fall from standing. Current recommendations for BMD testing focus on postmenopausal women who have sustained a fragility or low-energy DRF. When an osteoporotic or osteopenic patient’s distal radius is subjected to a high-energy force, a fracture is likely. Therefore, we expanded our study to include high-energy mechanisms of injury. Our analysis of BMD in patients with DRFs sustained in MVCs indicated that 12% of this group were osteoporotic, and 44% were osteopenic. Forty-three percent of our younger patients with a DRF fractured in a MVC were osteopenic, and 6% were osteoporotic. Among 4 mechanisms of injury for DRFs, there was no statistically significant difference in z scores (P = .22) (Figure 2). This provides evidence that a significant portion of patients with DRFs from both high- and low-energy mechanisms are osteoporotic or osteopenic. Patients with DRFs sustained in MVCs or in falls from heights of more than 6 feet should be referred for BMD evaluation.
Conclusion
A significant proportion of younger patients with DRFs are osteopenic or osteoporotic (43% and 6%, respectively), and their z scores are comparable to those of older patients with DRFs. There was no statistically significant difference in BMD z scores between younger and older patients and no difference in mechanisms of injury. This is evidence that younger patients with DRFs caused by a high- or low-energy mechanism of injury should undergo both DXA scan and BMD evaluation. If osteoporosis or osteopenia can be diagnosed at an earlier age, and if these patients can be properly treated, subsequent fractures could be prevented. The present study provides evidence supporting a simplification of the current recommendations for BMD evaluation: All women with DRFs should undergo bone densitometry.
Many organizations and work groups have issued recommendations regarding which patients should undergo bone densitometry. In 2004, the US Surgeon General recommended bone mineral density (BMD) evaluation for all women over age 65 years and for women and men with fragility fractures.1 The Centers for Medicare & Medicaid Services recommended BMD assessment for estrogen-deficient patients, for patients with vertebral abnormalities or hyperparathyroidism, and for patients receiving either steroid therapy or osteoporosis medications approved by the US Food and Drug Administration.2 The US Preventive Services Task Force and the National Osteoporosis Foundation each recommended screening for all women age 65 years or older and for postmenopausal women (age, 60-64 years) at high risk.3,4 The International Society for Clinical Densitometry (ISCD) recommended screening for all women age 65 years or older, all men age 70 years or older, and high-risk women under age 65 years.5
These current recommendations for BMD evaluation focus on women over age 65 years. More recent studies of postmenopausal women with distal radius fractures (DRFs) have found that both younger women (age, 45-65 years) and older women (age, ≥65 years) can have lower BMD and increased risk for hip and spine fracture.6,7 The authors of those studies recommended that all postmenopausal women with DRFs be evaluated for low BMD and that fracture prevention treatment be initiated. Earnshaw and colleagues8 and Oyen and colleagues9 found that men and women (age, ≥50 years) with DRFs had low BMD and elevated 10-year fracture rates. They concluded that BMD should be evaluated and treated in all DRF patients age 50 years or older. Other studies have shown low BMD in the contralateral distal radius of patients of all ages who presented with Colles fractures.10,11 These 2 studies did not measure spine or hip BMD.
The literature on BMD of younger women with DRFs is limited, relying solely on data collected for the contralateral distal radius.10,11 The ISCD recommended measuring both hip and spine BMD in premenopausal women. They also stated that z scores, not t scores, should be used for premenopausal women.5 The causes of low BMD in women over age 55 years are primarily nutritional deficiency and normal aging.1 In younger females, low BMD results from secondary causes, such as diet, medications, medical conditions, and endocrine disorders. When the secondary cause of low BMD can be identified and treated, osteoporosis can be stopped and even reversed in younger patients.12-14 Low BMD is more amenable to treatment in younger patients than in postmenopausal women. Younger patients with low BMD carry a higher lifetime fracture risk because they have more years of life with low BMD; therefore, early identification and treatment have a more significant impact on fracture prevention in these patients.
In the present study, we determined the prevalence of osteoporosis and osteopenia in younger women (age, 35-50 years) with DRFs and compared BMD measurements from younger women (age, 35-50 years) and older women (age, >50 years) with DRFs. The main goal was to determine which patients should be referred for bone densitometry and subsequent treatment.
Patients and Methods
This study received institutional review board approval. During a 5-year period (January 2005–August 2010), we prospectively collected dual-energy x-ray absorptiometry (DXA) scans for 128 women (age, >35 years) who presented with DRFs to our level I trauma center. Age ranged from 35 to 86 years. Data on mechanism of injury, treatment, and body mass index (BMI) were collected. The 128 patients were divided into a younger group (47 women; age range, 35-50 years; mean age, 44 years) and an older group (81 women; age, ≥51 years; mean age, 61 years). Mean BMI was 29.3 in the younger group and 28.8 in the older group (P = .88) (Table).
BMD was measured with a General Electric Lunar Prodigy Advance scanner that was tested annually for accuracy and precision. BMD of hips and lumbar spines was measured with a 76-Kv x-ray source. All DXA scans were analyzed by the same physician. BMD was omitted in cases of patients with a history of lumbar spine or hip fracture.
Two-sample Student t test was used to compare the 2 groups’ data. When multiple groups were being compared, analysis of variance was used. Spearman rank-order test was used to calculate a correlation coefficient for evaluation of the relationships between age and BMD.
Results
Mean lumbar spine (L1–L4) BMD was 1.12 in the younger group and 1.063 in the older group (P = .02); t scores were –0.63 and –1.132, respectively (P = .02); and mean z scores were –0.69 and –0.61, respectively (P = .81). Mean femoral neck BMD was 0.91 in the younger group and 0.80 in the older group (P < .05); t scores were –0.87 and –1.65, respectively (P < .01), and mean femoral neck z scores were –0.69 and –0.67, respectively (P = .92).
To further analyze BMD of specific age groups, we divided patients by decade: 35-39, 40-49, 50-59, 60-69, 70-79, 80-89 years. Among all 6 decades, there were no statistically significant differences between hip z scores (P = .83) (Figure 1). Spearman rank-order correlation test showed a moderate inverse correlation between age and femoral neck BMD (R = –0.42) and t score (R = –0.43). There was a weak correlation between increasing age and decreasing spine BMD, t score, and z score (Rs = –0.27, –0.31, 0.03). There was no correlation between age and femoral neck z score (R = –0.04).
According to the WHO classification system, 11 (23%) of the 47 women in the younger group were osteopenic, and 8 (17%) were osteoporotic, based on spine BMD. Hip BMD values indicated that 20 patients (43%) were osteopenic, and 3 (6%) were osteoporotic. One patient in the younger group had a hip z score of less than –2, and 14 patients (39%) had a hip z score between –2 and –1. Six patients (18%) had a spine z score of less than –2, and 6 patients (18%) had a spine z score between –2 and –1. Of the 81 older patients, 22 (27%) were osteopenic, and 21 (26%) were osteoporotic, according to spine measurements. The femoral neck data indicated that 39 (48%) of the older patients were osteopenic, and 22 (27%) were osteoporotic.
In both groups, mechanisms of injury were identified. Of the 47 younger patients, 26 fell from standing, 7 fell from a height of more than 6 feet, and 14 were injured in motor vehicle collisions (MVCs). Of the 81 older patients, 2 sustained a direct blow, 64 fell from standing, 4 fell from a height of more than 6 feet, and 11 were injured in MVCs. The differences in z scores based on mechanism of injury were not statistically significant (P = .22) (Figure 2).
Discussion
Several studies have shown that older women with DRFs have low BMD in the spine and femoral neck.8,9 These studies focused on older women who sustained low-energy fractures caused by a fall from a standing height. Studies of younger women with DRFs focused on BMD of the contralateral distal radius, not the spine or femoral neck.10,11 Those study groups also had low BMD. Findings from a multitude of studies have established that patients who are older than 50 years when they sustain distal radius fragility fractures should be referred for bone densitometry studies, and there is increasing evidence that younger patients with fragility fractures should undergo this evaluation as well.
The present study was designed to expand the range of patients and mechanisms of injury. Women in this study were 35 years or older. In addition to collecting data from patients injured in a fall from standing, we examined the medical records of women injured in MVCs, in falls from heights of more than 6 feet, and from direct trauma to the wrist. We measured the BMD of the spine and femoral neck and of the contralateral distal radius.
For this discussion, several key points should be made about BMD evaluation in younger versus older women. Most organizations caution against using spine BMD in older women. The ISCD, however, recommended measuring both hip and spine BMD; whereas BMD can be falsely elevated by spine osteoarthritis in older patients, spine BMD measurements are accurate in younger patients not affected by osteoarthritis. The ISCD also stipulated that z scores should be used in examining BMD in younger patients. The z score is a value of how many standard deviations BMD differs from a matched population of the same age, sex, ethnicity, and weight. The t score, which is useful in evaluating older patients, compares a patient’s BMD with that of an average 30-year-old.12
According to the WHO classification system (intended for older women), osteopenia is indicated by a t score between –1.0 and –2.5, and osteoporosis is indicated by a t score of less than –2.5. In the present study, about 43% of the younger patients (age, 35-50 years) with DRFs were osteopenic, and 6% of these patients were osteoporotic. In concert with previous studies,9 48% of our older women (age, >50 years) with DRFs were osteopenic, and 27% were osteoporotic. The difference in mean spinal z scores between the younger and older groups was not statistically significant (P = .81).
As mentioned, when examining BMD of younger patients, it is imperative to use spine z scores. About 18% of our younger patients had a z score of less than –2, and 18% had a z score between –2 and –1. In our comparison of patients from 5 different age decades (range, 35-79 years), there was no statistically significant difference in z scores (P = .83). In addition, there was no correlation between increasing age and decreasing z score (R = –0.04).
Secondary causes of osteoporosis have been documented in 30% of premenopausal women and 55% of men with vertebral fractures.13-15 Primary osteoporosis results from the normal aging process; secondary osteoporosis results from reversible causes, including medications, gastrointestinal disorders, renal disease, endocrine disorders, and sedentary lifestyle.15,16 When a secondary cause of osteoporosis is identified, treatment can be initiated to increase BMD. As younger patients can reverse bone loss and even increase BMD, it is important to identify reversible causes of osteopenia and osteoporosis in this age group. It is well documented that both younger and older patients with DRFs are at increased risk for subsequent fractures.6 Preventing further bone loss at a younger age may drastically decrease lifetime fracture risk.12,17
Most previous studies of BMD in women were limited to patients with DRFs caused by a low-energy mechanism or by a fall from standing. Current recommendations for BMD testing focus on postmenopausal women who have sustained a fragility or low-energy DRF. When an osteoporotic or osteopenic patient’s distal radius is subjected to a high-energy force, a fracture is likely. Therefore, we expanded our study to include high-energy mechanisms of injury. Our analysis of BMD in patients with DRFs sustained in MVCs indicated that 12% of this group were osteoporotic, and 44% were osteopenic. Forty-three percent of our younger patients with a DRF fractured in a MVC were osteopenic, and 6% were osteoporotic. Among 4 mechanisms of injury for DRFs, there was no statistically significant difference in z scores (P = .22) (Figure 2). This provides evidence that a significant portion of patients with DRFs from both high- and low-energy mechanisms are osteoporotic or osteopenic. Patients with DRFs sustained in MVCs or in falls from heights of more than 6 feet should be referred for BMD evaluation.
Conclusion
A significant proportion of younger patients with DRFs are osteopenic or osteoporotic (43% and 6%, respectively), and their z scores are comparable to those of older patients with DRFs. There was no statistically significant difference in BMD z scores between younger and older patients and no difference in mechanisms of injury. This is evidence that younger patients with DRFs caused by a high- or low-energy mechanism of injury should undergo both DXA scan and BMD evaluation. If osteoporosis or osteopenia can be diagnosed at an earlier age, and if these patients can be properly treated, subsequent fractures could be prevented. The present study provides evidence supporting a simplification of the current recommendations for BMD evaluation: All women with DRFs should undergo bone densitometry.
1. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. http://www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf. Accessed November 3, 2015.
2. Bone mass measurement (bone density). Medicare website. https://www.medicare.gov/coverage/bone-density.html. Accessed November 3, 2015.
3. Final update summary: osteoporosis: screening. US Preventive Services Task Force website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening. Updated July 2015. Accessed November 3, 2015.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. http://nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed November 3, 2015.
5. Khan AA, Bachrach L, Brown JP, et al. Canadian Panel of International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64.
6. Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008;19(5):607-613.
7. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3(3):127-132.
8. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60.
9. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radius fractures: a case–control study. J Bone Joint Surg Am. 2011;93(4):348-356.
10. Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85(3):423-425.
11. Wigderowitz CA, Rowley DI, Mole PA, Paterson CR, Abel EW. Bone mineral density of the radius in patients with Colles’ fracture. J Bone Joint Surg Br. 2000;82(1):87-89.
12. Khan A, Syed Z. Bone mineral density assessment in premenopausal women. Womens Health. 2006;2(4):639-645.
13. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
14. Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120-128.
15. Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;7(3):589-601.
16. Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643-645.
17. Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123(6):452-460.
1. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. http://www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf. Accessed November 3, 2015.
2. Bone mass measurement (bone density). Medicare website. https://www.medicare.gov/coverage/bone-density.html. Accessed November 3, 2015.
3. Final update summary: osteoporosis: screening. US Preventive Services Task Force website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening. Updated July 2015. Accessed November 3, 2015.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. http://nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed November 3, 2015.
5. Khan AA, Bachrach L, Brown JP, et al. Canadian Panel of International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64.
6. Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008;19(5):607-613.
7. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3(3):127-132.
8. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60.
9. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radius fractures: a case–control study. J Bone Joint Surg Am. 2011;93(4):348-356.
10. Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85(3):423-425.
11. Wigderowitz CA, Rowley DI, Mole PA, Paterson CR, Abel EW. Bone mineral density of the radius in patients with Colles’ fracture. J Bone Joint Surg Br. 2000;82(1):87-89.
12. Khan A, Syed Z. Bone mineral density assessment in premenopausal women. Womens Health. 2006;2(4):639-645.
13. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
14. Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120-128.
15. Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;7(3):589-601.
16. Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643-645.
17. Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123(6):452-460.
Lipoma of the Tendon Sheath in the Fourth Extensor Compartment of the Hand
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.