User login
Lipoma of the Tendon Sheath in the Fourth Extensor Compartment of the Hand
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
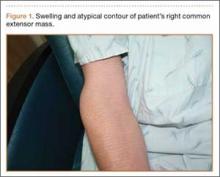
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
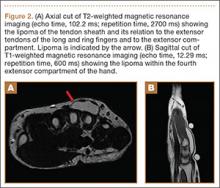
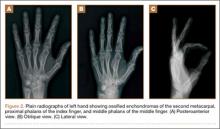
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
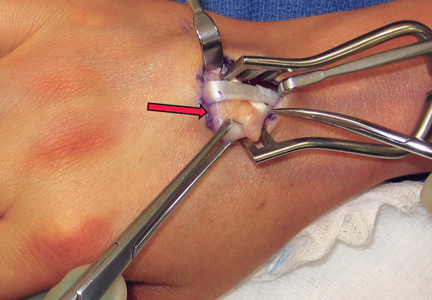
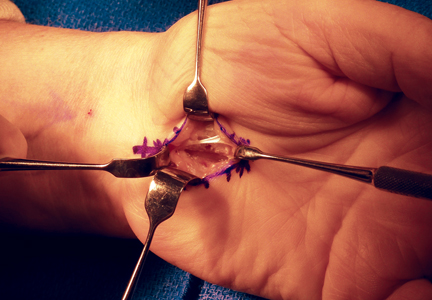
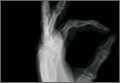
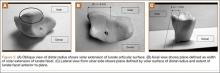
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
Acute Multiple Flexor Tendon Injury and Carpal Tunnel Syndrome After Open Distal Radius Fracture
The literature on extensor tendon rupture and even chronic flexor tendon rupture after volar plating and distal radius fracture malunion is ubiquitous. However, acute and subacute flexor tendon ruptures caused by distal radius fractures have been reported only in limited case reports. These rare injuries may involve multiple tendons and are associated with high-energy mechanisms. This case report details the involvement of multiple flexor tendon injuries associated with a Gustilo-Anderson type II distal radius fracture and the development of acute carpal tunnel syndrome (CTS) after a motor vehicle collision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
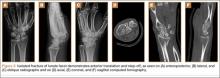
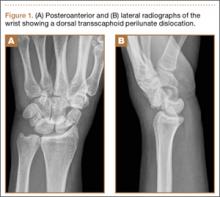
The patient is a 46-year-old woman who was involved in a motor vehicle collision. She was triaged as a trauma patient via Advanced Trauma Life Support protocol, and treated with antibiotic and tetanus prophylaxis. Radiographs showed an open, comminuted, displaced intra-articular distal radius fracture on the right side (Figures 1A, 1B). The fracture was closed reduced and splinted in the emergency department (Figures 2A, 2B). On initial examination, the patient had diffuse paresthesias in the digits that were most pronounced in the median nerve distribution. Motor examination was limited secondary to pain; however, she demonstrated gentle flexion and extension of the digits. The hand was well perfused, and a palpable radial pulse was present.
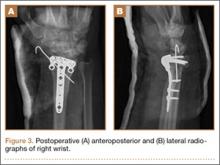
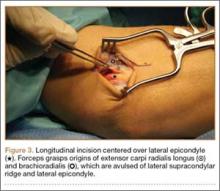
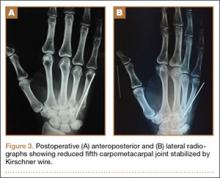
After clearance was obtained, she was taken urgently to the operating room. The wound was volar and transverse, approximately 2 cm in length, and approximately 4 cm proximal to the wrist crease. The wound was extended proximally and distally for a standard volar (Henry) approach. The flexor carpi radialis tendon was found to be partially lacerated, comprising 60% of the tendon. The fracture was readily identified because the deep fascia and the pronator quadratus were disrupted. No deep tendon lacerations were identified. The median nerve was found to be in continuity. After satisfactory débridement of the fracture and the wound, reduction and fixation was achieved with a volar locking plate and a single Kirschner wire. The flexor carpi radialis tendon was repaired with a modified Kessler stitch and epitenon repair. The wound was closed primarily in layers (Figures 3A, 3B).
The patient’s immediate postoperative neurologic examination was compromised secondary to the patient having a supraclavicular nerve block for anesthesia. Regional anesthesia was chosen because the patient’s pulmonologist recommended avoiding general anesthesia owing to her history of severe asthma that frequently required corticosteroid treatment. Once the block wore off, she complained of persistent paresthesias in all digits but most pronounced in the median nerve distribution. She was able to flex the interphalangeal joint to the index finger but could not flex the interphalangeal joint to the thumb. Over the course of the night, she was also noted to have worsening pain out of proportion to her injury.
As the paresthesias became denser in the median nerve distribution, she was diagnosed with acute CTS and was taken urgently back to the operating room under general anesthesia. After releasing the carpal tunnel through a separate incision, the original wound was reopened and explored. The median nerve was again visualized and found to be in continuity. All 4 tendons to both the flexor digitorum superficialis and flexor digitorum profundus were identified. The flexor pollicis longus (FPL) was not visualized in the wound. The distal portion of the FPL was retracted in the thumb tendon sheath and retrieved blindly with a tendon passer. The proximal portion was retracted to the mid-forearm. The laceration occurred distal to the musculotendinous junction. The tendon was repaired with a modified Kessler stitch as well as a box suture, resulting in 4 core strands across the tendon. The hand and the wrist were splinted in a thumb spica cast, and the patient was started on a modified Duran protocol 1 week after surgery. Median nerve function improved postoperatively.
Discussion
The rupture of the extensor pollicis longus tendon in nondisplaced distal radius fractures is not uncommon, but occurs in fewer than 5% of nondisplaced distal radius fractures.1 Although less common, chronic complications with flexor tendon rupture after distal radius fracture are well described.1-6 Flexor tendon rupture after distal radius malunion or volar plating is a known complication and is thought to be the result of attritional tendon wear because the flexors rub against protruding bone or plate;3,4,7 however, the initial tendon injury may play a role in those tendons that rupture more quickly.3 When secondary to volar plating, the rupture typically occurs within 1 year of injury,7 but, in both plating and malunion, it has been characterized as a late complication up to 10 years and even 20 years after injury.3,4 Similar to other reports, this rupture was encountered during a volar wrist approach. It has been suggested that, as the incidence of volar plating rises, more acute flexor tendon injuries may be diagnosed because of anatomic exposure,2 but this has not been reported in the literature.
Acute and subacute flexor tendon ruptures are rarely reported in the literature. To our knowledge, there are only 2 other reports of acute flexor tendon rupture2,5 after a distal radius fracture, neither of which involved the FPL. These cases, which involved ruptures of the flexor digitorum superficialis and flexor carpi radialis, were thought to be the result of tendon laceration by a volar bone spike. There is also one report of subacute FPL and flexor digitorum profundus rupture approximately 4 weeks after closed reduction of a distal radius fracture.6 Although sparse, the literature regarding flexor tendon rupture and distal radius fractures suggests that involvement of the flexor digitorum superficialis and the flexor digitorum profundus tendons is most common and that the rupture typically occurs in 1 to 4 months.1
We report a rare case of 2 acute flexor tendon lacerations after a Gustilo-Anderson type II open distal radius fracture, likely caused by the volar spike of bone that created the open injury. This case also was complicated by the development of acute CTS.
To our knowledge, despite a rate of acute CTS reported as high as 5.4% in operatively treated distal radius fractures, there are no established associations between acute CTS and flexor tendon rupture in the setting of distal radius fracture.8,9 In a 2008 retrospective case–control study by Dyer and colleagues,8 fracture translation is the most important risk factor for the development of acute CTS associated with fracture of the distal radius. Although not statistically significant, ipsilateral upper extremity trauma, higher-energy injuries, younger age, and male sex were also associated with the development of acute CTS. Open injuries occurred in only 3 of 50 cases of acute CTS.8
In agreement with published reports, the probability and the timing of tendon rupture are likely related to the severity of the deforming forces applied during the initial insult rather than the resultant stresses.1 Clinicians should have a high suspicion of acute CTS and possible tendon injuries after a high-energy injury with a significantly displaced open distal radius fracture and median nerve paresthesias. A thoughtful and complete preoperative examination of the flexor tendons may prevent the need for reoperation. Concerns for flexor injury and acute CTS should be elevated with the observation of a disrupted pronator. For patients with a volarly displaced fragment after fracture reduction, this concern should be even more elevated.9 Preoperative median nerve symptoms in the setting of the severely displaced fracture should necessitate an acute carpal tunnel release. If 1 flexor tendon is injured, the surgeon should remember that multiple flexor tendons may be involved. We recommend that any injured tendons be repaired primarily, if possible, and the patient started on appropriate rehabilitation.
1. Ashall G. Flexor pollicis longus rupture after fracture of the distal radius. Injury. 1991;22(2):153-155.
2. Dimatteo L, Wolf JM. Flexor carpi radialis tendon rupture as a complication of a closed distal radius fracture: a case report. J Hand Surg Am. 2007;32(6):818-820.
3. Kato N, Nemoto K, Arino H, Ichikawa T, Fujikawa K. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg. 2002;36(4):245-248.
4. Monda MK, Ellis A, Karmani S. Late rupture of flexor pollicis longus tendon 10 years after volar buttress plate fixation of a distal radius fracture: a case report. Acta Orthop Belg. 2010;76(4):549-551.
5. Southmayd WW, Millender LH, Nalebuff EA. Rupture of the flexor tendons of the index finger after Colles’ fracture. Case report. J Bone Joint Surg Am. 1975;57(4):562-563.
6. Wong FY, Pho RW. Median nerve compression, with tendon ruptures, after Colles’ fracture. J Hand Surg Br. 1984;9(2):139-141.
7. Woon CYL, Lee JYL, Ng SW, Teoh LC. Late rupture of flexor pollicis longus tendon after volar distal radius plating: a case report and review of the literature. Inj Extra. 2007;38(7):235-238.
8. Dyer G, Lozano-Calderon S, Gannon C, Baratz M, Ring D. Predictors of acute carpal tunnel syndrome associated with fracture of the distal radius. J Hand Surg Am. 2008;33(8):1309-1313.
9. Paley D, McMurtry RY. Median nerve compression by volarly displaced fragments of the distal radius. Clin Orthop Relat Res. 1987;(215):139-147.
The literature on extensor tendon rupture and even chronic flexor tendon rupture after volar plating and distal radius fracture malunion is ubiquitous. However, acute and subacute flexor tendon ruptures caused by distal radius fractures have been reported only in limited case reports. These rare injuries may involve multiple tendons and are associated with high-energy mechanisms. This case report details the involvement of multiple flexor tendon injuries associated with a Gustilo-Anderson type II distal radius fracture and the development of acute carpal tunnel syndrome (CTS) after a motor vehicle collision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient is a 46-year-old woman who was involved in a motor vehicle collision. She was triaged as a trauma patient via Advanced Trauma Life Support protocol, and treated with antibiotic and tetanus prophylaxis. Radiographs showed an open, comminuted, displaced intra-articular distal radius fracture on the right side (Figures 1A, 1B). The fracture was closed reduced and splinted in the emergency department (Figures 2A, 2B). On initial examination, the patient had diffuse paresthesias in the digits that were most pronounced in the median nerve distribution. Motor examination was limited secondary to pain; however, she demonstrated gentle flexion and extension of the digits. The hand was well perfused, and a palpable radial pulse was present.
After clearance was obtained, she was taken urgently to the operating room. The wound was volar and transverse, approximately 2 cm in length, and approximately 4 cm proximal to the wrist crease. The wound was extended proximally and distally for a standard volar (Henry) approach. The flexor carpi radialis tendon was found to be partially lacerated, comprising 60% of the tendon. The fracture was readily identified because the deep fascia and the pronator quadratus were disrupted. No deep tendon lacerations were identified. The median nerve was found to be in continuity. After satisfactory débridement of the fracture and the wound, reduction and fixation was achieved with a volar locking plate and a single Kirschner wire. The flexor carpi radialis tendon was repaired with a modified Kessler stitch and epitenon repair. The wound was closed primarily in layers (Figures 3A, 3B).
The patient’s immediate postoperative neurologic examination was compromised secondary to the patient having a supraclavicular nerve block for anesthesia. Regional anesthesia was chosen because the patient’s pulmonologist recommended avoiding general anesthesia owing to her history of severe asthma that frequently required corticosteroid treatment. Once the block wore off, she complained of persistent paresthesias in all digits but most pronounced in the median nerve distribution. She was able to flex the interphalangeal joint to the index finger but could not flex the interphalangeal joint to the thumb. Over the course of the night, she was also noted to have worsening pain out of proportion to her injury.
As the paresthesias became denser in the median nerve distribution, she was diagnosed with acute CTS and was taken urgently back to the operating room under general anesthesia. After releasing the carpal tunnel through a separate incision, the original wound was reopened and explored. The median nerve was again visualized and found to be in continuity. All 4 tendons to both the flexor digitorum superficialis and flexor digitorum profundus were identified. The flexor pollicis longus (FPL) was not visualized in the wound. The distal portion of the FPL was retracted in the thumb tendon sheath and retrieved blindly with a tendon passer. The proximal portion was retracted to the mid-forearm. The laceration occurred distal to the musculotendinous junction. The tendon was repaired with a modified Kessler stitch as well as a box suture, resulting in 4 core strands across the tendon. The hand and the wrist were splinted in a thumb spica cast, and the patient was started on a modified Duran protocol 1 week after surgery. Median nerve function improved postoperatively.
Discussion
The rupture of the extensor pollicis longus tendon in nondisplaced distal radius fractures is not uncommon, but occurs in fewer than 5% of nondisplaced distal radius fractures.1 Although less common, chronic complications with flexor tendon rupture after distal radius fracture are well described.1-6 Flexor tendon rupture after distal radius malunion or volar plating is a known complication and is thought to be the result of attritional tendon wear because the flexors rub against protruding bone or plate;3,4,7 however, the initial tendon injury may play a role in those tendons that rupture more quickly.3 When secondary to volar plating, the rupture typically occurs within 1 year of injury,7 but, in both plating and malunion, it has been characterized as a late complication up to 10 years and even 20 years after injury.3,4 Similar to other reports, this rupture was encountered during a volar wrist approach. It has been suggested that, as the incidence of volar plating rises, more acute flexor tendon injuries may be diagnosed because of anatomic exposure,2 but this has not been reported in the literature.
Acute and subacute flexor tendon ruptures are rarely reported in the literature. To our knowledge, there are only 2 other reports of acute flexor tendon rupture2,5 after a distal radius fracture, neither of which involved the FPL. These cases, which involved ruptures of the flexor digitorum superficialis and flexor carpi radialis, were thought to be the result of tendon laceration by a volar bone spike. There is also one report of subacute FPL and flexor digitorum profundus rupture approximately 4 weeks after closed reduction of a distal radius fracture.6 Although sparse, the literature regarding flexor tendon rupture and distal radius fractures suggests that involvement of the flexor digitorum superficialis and the flexor digitorum profundus tendons is most common and that the rupture typically occurs in 1 to 4 months.1
We report a rare case of 2 acute flexor tendon lacerations after a Gustilo-Anderson type II open distal radius fracture, likely caused by the volar spike of bone that created the open injury. This case also was complicated by the development of acute CTS.
To our knowledge, despite a rate of acute CTS reported as high as 5.4% in operatively treated distal radius fractures, there are no established associations between acute CTS and flexor tendon rupture in the setting of distal radius fracture.8,9 In a 2008 retrospective case–control study by Dyer and colleagues,8 fracture translation is the most important risk factor for the development of acute CTS associated with fracture of the distal radius. Although not statistically significant, ipsilateral upper extremity trauma, higher-energy injuries, younger age, and male sex were also associated with the development of acute CTS. Open injuries occurred in only 3 of 50 cases of acute CTS.8
In agreement with published reports, the probability and the timing of tendon rupture are likely related to the severity of the deforming forces applied during the initial insult rather than the resultant stresses.1 Clinicians should have a high suspicion of acute CTS and possible tendon injuries after a high-energy injury with a significantly displaced open distal radius fracture and median nerve paresthesias. A thoughtful and complete preoperative examination of the flexor tendons may prevent the need for reoperation. Concerns for flexor injury and acute CTS should be elevated with the observation of a disrupted pronator. For patients with a volarly displaced fragment after fracture reduction, this concern should be even more elevated.9 Preoperative median nerve symptoms in the setting of the severely displaced fracture should necessitate an acute carpal tunnel release. If 1 flexor tendon is injured, the surgeon should remember that multiple flexor tendons may be involved. We recommend that any injured tendons be repaired primarily, if possible, and the patient started on appropriate rehabilitation.
The literature on extensor tendon rupture and even chronic flexor tendon rupture after volar plating and distal radius fracture malunion is ubiquitous. However, acute and subacute flexor tendon ruptures caused by distal radius fractures have been reported only in limited case reports. These rare injuries may involve multiple tendons and are associated with high-energy mechanisms. This case report details the involvement of multiple flexor tendon injuries associated with a Gustilo-Anderson type II distal radius fracture and the development of acute carpal tunnel syndrome (CTS) after a motor vehicle collision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient is a 46-year-old woman who was involved in a motor vehicle collision. She was triaged as a trauma patient via Advanced Trauma Life Support protocol, and treated with antibiotic and tetanus prophylaxis. Radiographs showed an open, comminuted, displaced intra-articular distal radius fracture on the right side (Figures 1A, 1B). The fracture was closed reduced and splinted in the emergency department (Figures 2A, 2B). On initial examination, the patient had diffuse paresthesias in the digits that were most pronounced in the median nerve distribution. Motor examination was limited secondary to pain; however, she demonstrated gentle flexion and extension of the digits. The hand was well perfused, and a palpable radial pulse was present.
After clearance was obtained, she was taken urgently to the operating room. The wound was volar and transverse, approximately 2 cm in length, and approximately 4 cm proximal to the wrist crease. The wound was extended proximally and distally for a standard volar (Henry) approach. The flexor carpi radialis tendon was found to be partially lacerated, comprising 60% of the tendon. The fracture was readily identified because the deep fascia and the pronator quadratus were disrupted. No deep tendon lacerations were identified. The median nerve was found to be in continuity. After satisfactory débridement of the fracture and the wound, reduction and fixation was achieved with a volar locking plate and a single Kirschner wire. The flexor carpi radialis tendon was repaired with a modified Kessler stitch and epitenon repair. The wound was closed primarily in layers (Figures 3A, 3B).
The patient’s immediate postoperative neurologic examination was compromised secondary to the patient having a supraclavicular nerve block for anesthesia. Regional anesthesia was chosen because the patient’s pulmonologist recommended avoiding general anesthesia owing to her history of severe asthma that frequently required corticosteroid treatment. Once the block wore off, she complained of persistent paresthesias in all digits but most pronounced in the median nerve distribution. She was able to flex the interphalangeal joint to the index finger but could not flex the interphalangeal joint to the thumb. Over the course of the night, she was also noted to have worsening pain out of proportion to her injury.
As the paresthesias became denser in the median nerve distribution, she was diagnosed with acute CTS and was taken urgently back to the operating room under general anesthesia. After releasing the carpal tunnel through a separate incision, the original wound was reopened and explored. The median nerve was again visualized and found to be in continuity. All 4 tendons to both the flexor digitorum superficialis and flexor digitorum profundus were identified. The flexor pollicis longus (FPL) was not visualized in the wound. The distal portion of the FPL was retracted in the thumb tendon sheath and retrieved blindly with a tendon passer. The proximal portion was retracted to the mid-forearm. The laceration occurred distal to the musculotendinous junction. The tendon was repaired with a modified Kessler stitch as well as a box suture, resulting in 4 core strands across the tendon. The hand and the wrist were splinted in a thumb spica cast, and the patient was started on a modified Duran protocol 1 week after surgery. Median nerve function improved postoperatively.
Discussion
The rupture of the extensor pollicis longus tendon in nondisplaced distal radius fractures is not uncommon, but occurs in fewer than 5% of nondisplaced distal radius fractures.1 Although less common, chronic complications with flexor tendon rupture after distal radius fracture are well described.1-6 Flexor tendon rupture after distal radius malunion or volar plating is a known complication and is thought to be the result of attritional tendon wear because the flexors rub against protruding bone or plate;3,4,7 however, the initial tendon injury may play a role in those tendons that rupture more quickly.3 When secondary to volar plating, the rupture typically occurs within 1 year of injury,7 but, in both plating and malunion, it has been characterized as a late complication up to 10 years and even 20 years after injury.3,4 Similar to other reports, this rupture was encountered during a volar wrist approach. It has been suggested that, as the incidence of volar plating rises, more acute flexor tendon injuries may be diagnosed because of anatomic exposure,2 but this has not been reported in the literature.
Acute and subacute flexor tendon ruptures are rarely reported in the literature. To our knowledge, there are only 2 other reports of acute flexor tendon rupture2,5 after a distal radius fracture, neither of which involved the FPL. These cases, which involved ruptures of the flexor digitorum superficialis and flexor carpi radialis, were thought to be the result of tendon laceration by a volar bone spike. There is also one report of subacute FPL and flexor digitorum profundus rupture approximately 4 weeks after closed reduction of a distal radius fracture.6 Although sparse, the literature regarding flexor tendon rupture and distal radius fractures suggests that involvement of the flexor digitorum superficialis and the flexor digitorum profundus tendons is most common and that the rupture typically occurs in 1 to 4 months.1
We report a rare case of 2 acute flexor tendon lacerations after a Gustilo-Anderson type II open distal radius fracture, likely caused by the volar spike of bone that created the open injury. This case also was complicated by the development of acute CTS.
To our knowledge, despite a rate of acute CTS reported as high as 5.4% in operatively treated distal radius fractures, there are no established associations between acute CTS and flexor tendon rupture in the setting of distal radius fracture.8,9 In a 2008 retrospective case–control study by Dyer and colleagues,8 fracture translation is the most important risk factor for the development of acute CTS associated with fracture of the distal radius. Although not statistically significant, ipsilateral upper extremity trauma, higher-energy injuries, younger age, and male sex were also associated with the development of acute CTS. Open injuries occurred in only 3 of 50 cases of acute CTS.8
In agreement with published reports, the probability and the timing of tendon rupture are likely related to the severity of the deforming forces applied during the initial insult rather than the resultant stresses.1 Clinicians should have a high suspicion of acute CTS and possible tendon injuries after a high-energy injury with a significantly displaced open distal radius fracture and median nerve paresthesias. A thoughtful and complete preoperative examination of the flexor tendons may prevent the need for reoperation. Concerns for flexor injury and acute CTS should be elevated with the observation of a disrupted pronator. For patients with a volarly displaced fragment after fracture reduction, this concern should be even more elevated.9 Preoperative median nerve symptoms in the setting of the severely displaced fracture should necessitate an acute carpal tunnel release. If 1 flexor tendon is injured, the surgeon should remember that multiple flexor tendons may be involved. We recommend that any injured tendons be repaired primarily, if possible, and the patient started on appropriate rehabilitation.
1. Ashall G. Flexor pollicis longus rupture after fracture of the distal radius. Injury. 1991;22(2):153-155.
2. Dimatteo L, Wolf JM. Flexor carpi radialis tendon rupture as a complication of a closed distal radius fracture: a case report. J Hand Surg Am. 2007;32(6):818-820.
3. Kato N, Nemoto K, Arino H, Ichikawa T, Fujikawa K. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg. 2002;36(4):245-248.
4. Monda MK, Ellis A, Karmani S. Late rupture of flexor pollicis longus tendon 10 years after volar buttress plate fixation of a distal radius fracture: a case report. Acta Orthop Belg. 2010;76(4):549-551.
5. Southmayd WW, Millender LH, Nalebuff EA. Rupture of the flexor tendons of the index finger after Colles’ fracture. Case report. J Bone Joint Surg Am. 1975;57(4):562-563.
6. Wong FY, Pho RW. Median nerve compression, with tendon ruptures, after Colles’ fracture. J Hand Surg Br. 1984;9(2):139-141.
7. Woon CYL, Lee JYL, Ng SW, Teoh LC. Late rupture of flexor pollicis longus tendon after volar distal radius plating: a case report and review of the literature. Inj Extra. 2007;38(7):235-238.
8. Dyer G, Lozano-Calderon S, Gannon C, Baratz M, Ring D. Predictors of acute carpal tunnel syndrome associated with fracture of the distal radius. J Hand Surg Am. 2008;33(8):1309-1313.
9. Paley D, McMurtry RY. Median nerve compression by volarly displaced fragments of the distal radius. Clin Orthop Relat Res. 1987;(215):139-147.
1. Ashall G. Flexor pollicis longus rupture after fracture of the distal radius. Injury. 1991;22(2):153-155.
2. Dimatteo L, Wolf JM. Flexor carpi radialis tendon rupture as a complication of a closed distal radius fracture: a case report. J Hand Surg Am. 2007;32(6):818-820.
3. Kato N, Nemoto K, Arino H, Ichikawa T, Fujikawa K. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg. 2002;36(4):245-248.
4. Monda MK, Ellis A, Karmani S. Late rupture of flexor pollicis longus tendon 10 years after volar buttress plate fixation of a distal radius fracture: a case report. Acta Orthop Belg. 2010;76(4):549-551.
5. Southmayd WW, Millender LH, Nalebuff EA. Rupture of the flexor tendons of the index finger after Colles’ fracture. Case report. J Bone Joint Surg Am. 1975;57(4):562-563.
6. Wong FY, Pho RW. Median nerve compression, with tendon ruptures, after Colles’ fracture. J Hand Surg Br. 1984;9(2):139-141.
7. Woon CYL, Lee JYL, Ng SW, Teoh LC. Late rupture of flexor pollicis longus tendon after volar distal radius plating: a case report and review of the literature. Inj Extra. 2007;38(7):235-238.
8. Dyer G, Lozano-Calderon S, Gannon C, Baratz M, Ring D. Predictors of acute carpal tunnel syndrome associated with fracture of the distal radius. J Hand Surg Am. 2008;33(8):1309-1313.
9. Paley D, McMurtry RY. Median nerve compression by volarly displaced fragments of the distal radius. Clin Orthop Relat Res. 1987;(215):139-147.
Collagenase Enzymatic Fasciotomy for Dupuytren Contracture in Patients on Chronic Immunosuppression
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
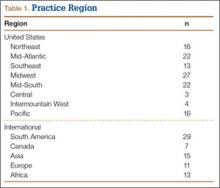
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
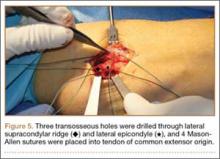
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
Open Carpal Tunnel Release With Use of a Nasal Turbinate Speculum
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
Isolated Avulsion of Extensor Carpi Radialis Longus and Brachioradialis Origins: A Case Report and Surgical Repair Technique
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
Isolated Radiopalmar Dislocation of Fifth Carpometacarpal Joint: A Rare Presentation
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
Nonoperative Management of Multiple Hand Enchondromas in Ollier Disease With Progressive Ossification
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
Fingertip Amputation Treatment: A Survey Study
Finger injuries are common, representing an estimated 3 million emergency department visits per year in the United States, with 44% of these diagnosed as lacerations.1 Amputations of the finger (partial and complete) in non-work-related accidents alone are estimated at 30,000 per year.1 The fingertip is a highly specialized structure that contributes to precision function of the hand through tactile feedback and fine motor control as well as hand aesthetics. An injury can compromise a variety of fingertip structures, including the distal phalanx, which provides length and structural support; the fingernail, germinal matrix, and sterile matrix, which protect the fingertip and function as tools; and the volar skin pad, which is important for sensation and fine motor activity.
There is considerable debate regarding optimal management of fingertip amputations, and to date there have been no prospective, randomly controlled trials to guide treatment.2 Injury characteristics, amputation levels, and patient priorities all contribute to management decisions. Treatment goals are to maintain length when possible; to provide stable, supple, and sensate skin coverage; to ensure the nail plate regrows without complication; and to maintain normal overall finger shape and cosmesis. In addition, a simple, cost-effective treatment with short recovery time and no donor-site morbidity is desired.
Treatment recommendations are wide-ranging, and evidence-based literature is sparse. About 30 years ago, 2 retrospective comparative studies found no difference in outcomes between simpler treatments (primary closure, secondary wound healing) and various operative strategies.3,4 Since then, most of the scientific studies have been retrospective noncomparative case series, all reporting good to excellent results.5-17 Investigators generally implied superior results of a studied procedure over those of more conservative treatments. Recommended treatments include secondary wound healing, simple flaps, staged flaps, pedicle flaps, allograft and autograft coverage, composite grafting, and replantation, for all levels of fingertip injury.
Given our surgical advances, improved techniques, and accumulating experience, we may have expected better outcomes with newer and more complex reconstructive efforts. Unfortunately, in a recent review of 53 fingertip injuries treated with a reconstructive procedure, bone shortening with closure, or secondary healing, Wang and colleagues18 found no discernible differences in outcomes at 4.5-year follow-up. They questioned whether complex reconstructive procedures are worth the time, expense, and risk. In the absence of prospective, comparative studies, surgeons must rely on anecdotal evidence (including predominantly level IV evidence), training bias, previous experience, and the prevailing common wisdom.
Toward that end, we became interested in identifying treatment preferences for fingertip amputations. We conducted a study to better understand how surgeon and patient factors influence the treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. We hypothesized that hand surgeons’ treatment preferences would be varied and influenced by surgeon and patient demographics.
Materials and Methods
An online multiple-choice survey was created and powered by Constant Contact. The survey consisted of 6 surgeon demographic questions; 5 treatment preference questions regarding patient age, sex, occupation, and germinal matrix management; and 5 clinical scenarios based on Allen levels 2, 3 (with and without exposed distal phalanx), and 4 and volar oblique middle-finger amputations. The Allen classification designates level 2 injuries as those involving only the distal pulp and nail.19 Level 3 injuries also involve the terminal distal phalanx, and level 4 injuries extend to the lunula. The survey questions are listed in the Appendix. For the clinical scenario questions, treatment choices included wound care, skeletal shortening and closure, composite graft, autograft, allograft, V-Y/Kutler flap, advancement flap, thenar flap, cross-finger flap, pedicle and homodigital flap, replantation, and other.
An email invitation was sent to members of the American Association for Hand Surgery (AAHS). The survey was also submitted to personal contacts of international hand societies named on the AAHS website to expand the international response. A reminder email was sent 1 week after the original invitation. The survey was closed 5 weeks later, and the responses were analyzed with all non-US hand surgeons grouped collectively as an international group, compared with the US group. Institutional review board approval was not needed for this survey study.
Statistics
A generalized linear regression model was used to implement logistic regression with random effects for question and respondent. This approach accounts for multiple observations from the same respondent, assuming that both respondent and question are random samples from a larger population. The model estimated the probability that a given surgical approach (eg, skeletal shortening, wound care) would be selected, based on the predictors of the US versus international respondent, time in practice, practice type, and whether the fingertip was available. The model returned adjusted odds ratios (ORs) for each predictor, controlling for all the others. By convention, P < .05 was considered significant. No attempt was made to prune the model of nonsignificant factors. Analyses were performed using the lme4 package on the R statistical platform (R Foundation for Statistical Computing).
Results
One hundred ninety-eight responses were recorded. Of the 1054 AAHS members invited to take the survey, 174 (US, international) responded (17% response rate). One hundred twenty-three responses and 62% of the total were generated from US hand surgeons. Fifty-eight percent of US responses were from the Mid-South, Midwest, or Mid-Atlantic region. Fifty-seven percent of international responses were from Brazil and Europe. Respondents’ demographic data are listed in Tables 1 and 2.
Responses to the 5 clinical scenarios showed a wide variation in treatment preferences. The top 6 preferred treatment selections for an acute, clean long-finger amputation in a healthy 40-year-old office worker are shown in Figures 1 to 5. When surgeons who preferred replant were asked what they would do if the amputated part was not available, they indicated flap coverage more often than less complex treatments, such as skeletal shortening/primary closure or wound care.
There were statistically significant differences in treatment preferences between US and international hand surgeons when controlling for all other demographic variables. Adjusted ORs and their confidence intervals (CIs) for the aggregate clinical scenarios are presented in a forest plot in Figure 6. Figure 4 shows that US surgeons were more likely to choose wound care (OR, 3.6; P < .0004) and less likely to attempt a replant (OR, 0.01; P < .0001). US surgeons were also less likely to use a pedicle or homodigital island flap when the amputated fingertip was both available (OR, 0.04; P = .039) and unavailable (OR, 0.47; Ps = .029).
Among all respondents and across all clinical scenarios, skeletal shortening with closure was favored among hand surgeons in practice less than 5 years compared with those in practice longer (OR, 2.11; 95% CI, 1.36-3.25; P = .0008). Similarly, surgeons with more than 30 years of experience were the least likely to favor wound care (OR, 0.2; 95% CI, 0.09-0.93; P = .037). Compared with orthopedic surgeons, plastic surgeons opted for wound care less often (OR, 0.44; 95% CI, 0.23-0.98; P = .018) and appeared to prefer replantation, but the difference was not statistically significant (OR, 8.86; 95% CI, 0.99-79.61; P = .054).
Replantation was less often chosen by private practice versus full-time academic surgeons (OR, 0.09; 95% CI, 0.01-0.91; P = .041.) Part-time academics were no more or less likely to perform replantation than full-time academics were (OR, 0.52; 95% CI, 0.05-5.41; P = .58). Of the 59 respondents who performed more than 10 microvascular cases a year, 18 (31%) chose replant for Allen level 4 amputations. In comparison, 9 (20%) of the 45 respondents who performed fewer than 3 microvascular cases a year chose replant for amputations at this level. Amount of time working with fellows did not affect treatment preferences.
Patient demographics (age, sex, occupation) also played a role in treatment decisions (Table 3). The most significant factors appeared to be age and occupation. Regarding age, 41% of respondents chose more complex procedures for patients younger than 15, and 62% chose less complex procedures for patients older than 70 years. Regarding occupation, 61% chose more complex procedures for professional musicians, and 60% chose less complex procedures for manual laborers. Sex did not influence clinical decisions for 78% of respondents. There was also substantial variation in both the indications for germinal matrix ablation and the frequency of sterile matrix transplant (Table 3).
Discussion
Although there is a variety of treatment options and published treatment guidelines for distal fingertip amputations, few comparative studies support use of one treatment over another. In our experience, treatment decisions are based mainly on injury parameters, but surgeon preference and patient factors (age, sex, occupation) can also influence care. Our goal in this study was to better understand how surgeon and patient factors influence treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. Our survey results showed lack of consensus among hand surgeons and highlighted several trends.
As expected, we found a wide range of treatment preferences for each clinical scenario queried, ranging from more simple treatments (eg, wound care) to more complex ones (eg, replantation). With patient parameters (age, profession, finger, acuity, injury type, tissue preservation, smoking status) standardized in the clinical scenarios, the treatment differences noted should reflect surgeon preference. However, other patient factors (eg, cultural differences, religious beliefs, surgeon setting, practice pattern, resource availability) that were not included in the clinical scenarios could also affect treatment preferences.
One particularly interesting finding was that international hand surgeons were 6.8 times more likely to replant a distal fingertip amputation. One possible explanation for this variation is the influence of cultural differences. For example, in East Asian countries, there can be a cultural stigma associated with loss of a fingertip, and therefore more of a desire on the part of the patient to restore the original finger.20,21 In addition, the international respondents were biased toward academic practices—which could skew the treatment preference toward replantation, as we found that academic surgeons were more inclined to replantation.
Our finding that replantation was more commonly preferred by academic versus private practice surgeons may suggest a training bias, an affinity for more complex or interesting procedures, or access to hospital equipment and staff, including residents and fellows, not usually found at smaller community hospitals, where private practice surgeons are more commonly based. Jazayeri and colleagues22 found that institutions specializing in microsurgery often produced better outcomes than nonspecializing institutions. Therefore, it is not surprising that private practice hand surgeons may less often opt to replant a distal fingertip amputation. It is also not surprising that plastic surgeons are more inclined to perform a replantation or flap coverage, as their training is more microsurgery-intensive and their practice more focused on aesthetics compared with the other specialists.
Distal fingertip replantation is accepted by most as technically demanding, but it seems that the additional effort and resources would be justified if the procedure provided a superior outcome. However, other factors, such as cost of treatment and length of recovery, should also be considered. Average replantation cost has been estimated to range from $7500 to $14,000, compared with $2800 for non-replantation-related care, and median stay is about 4 days longer for replantation-related care.23,24 These estimates do not include indirect costs, such as for postoperative rehabilitation, which is likely longer and more expensive, even in distal fingertip replantation. These disparities may not justify the outcome (of having a complete fingertip) if more conservative treatments yield similar results.17,18 In addition, there is the expected failure rate of limb replantation surgery. In analysis of the overall societal costs and benefits of larger upper extremity limb replantation, the loss of invested resources sustained with failed limb replantation may be outweighed by the benefit of another patient having a successful outcome. In the case of fingertip replantation, however, does the undefined benefit of the successful patient outcome outweigh the investment of resources lost in cases of replantation failure? Understandably, there is a need for more robust clinical outcome and cost-comparative evidence to better inform decisions regarding distal fingertip amputation.
We found that wound care and skeletal shortening with primary closure (particularly with Allen level 3 injuries) were preferred more by surgeons within the first 5 years of practice. This finding seems to imply a lack of experience or confidence on the part of younger surgeons performing more complex procedures, such as flap coverage. Conversely, this finding may indicate a shift in treatment principle based on recent literature suggesting equivalent outcomes with simpler procedures.17,18 Although our survey study did not provide an option for treatment combinations or staged procedures, several respondents wrote in that skeletal shortening supplemented with various types of autografts and allografts would be their preferred treatment.
Patient factors also play a significant role in clinical decisions. Age and profession seem to be important determinants, with more than 50% of respondents, on average, changing their treatment recommendation based on these 2 factors. A majority of respondents would perform a less involved procedure for a manual laborer, suggesting a quicker return to work is prioritized over a perceived improved clinical outcome. Interestingly, for patients younger than 15 years, the preference was divided, with 41% of surgeons opting for a more complex procedure. This suggests the importance of restoring anatomy in a younger patient, or the perceived decreased risk or failure rate with more involved treatment. Twenty percent preferred a less complex procedure in a younger patient, perhaps relying on the patient’s developmental potential for a good outcome or suggesting a concern for patient intolerance or compliance with complex surgery.
Nail plate regrowth can be a problem with fingertip amputations. Nail deformity is highly correlated with injury level, with amputations proximal to the lunula more likely to cause nail plate deformity.25,26 Jebson and colleagues27 recommended germinal matrix ablation for amputations proximal to the lunula. We found respondents often performed ablations for other indications, including injured or minimal remaining sterile matrix and lack of bony support for the sterile matrix. Forty-six percent of respondents had never performed sterile matrix transplant, which could indicate that they were unfamiliar with the technique or had donor-site concerns, or that postinjury nail deformities are uncommon, well tolerated, or treated along with other procedures, such as germinal matrix ablation.
Several weaknesses of this study must be highlighted. First, our response rate was smaller than desired. Although this work incorporated a large number of surgeon responses, nearly 200, the response rate was only 17%. In addition, although number of responses was likely adequate to show the diversity of opinion, the preferences and trends reported might not be representative of all hand surgeons. We could not perform a nonresponder analysis because of a lack of specific demographic data for the AAHS and international hand society members. However, AAHS has an approximate 50/50 mix of plastic and orthopedic surgeons, similar to our responder demographic, suggesting our smaller subset of responses might be representative of the whole. According to AAHS, a majority of its members are “academic” hand surgeons, so our results might not adequately reflect the preferences of community hand surgeons and ultimately might overstate the frequency of more complex treatments. Last, our international response was limited to a few countries. A larger, more broadly distributed response would provide a better understanding of regional preferences, which could shed light on the importance of cultural differences.
Variations in patient insurance status were not queried in this survey but might also affect treatment decisions. More involved, costly, and highly reimbursing procedures might be deemed reasonable options for a small perceived clinical benefit for insured patients.
When multiple digits or the thumb is injured, or there are other concomitant injuries, surgeons may alter their choice of intervention. In mangled extremities, preservation of salvageable functional units takes precedence over aesthetics and likely affects choice of treatment for the amputated fingertips. Similarly, multiple fingertip amputations, even if all at the same level, may be differently regarded than a solitary injury.
Conclusion
For distal fingertip amputations, there is little evidence supporting one approach over another. Without level I comparative data guiding treatment, anecdotal evidence and surgeon personal preferences likely contribute to the large variation noted in this survey. Our study results showed the disparity of fingertip treatment preferences among a cross section of US and international hand surgeons. More important, results underscored the need for a well-designed comparative study to determine the most effective treatments for distal fingertip amputations.
1. Conn JM, Annest JL, Ryan GW, Budnitz DS. Non-work-related finger amputations in the United States, 2001-2002. Ann Emerg Med. 2005;45(6):630-635.
2. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am. 2008;33(8):1417-1419.
3. Söderberg T, Nyström Å, Hallmans G, Hultén J. Treatment of fingertip amputations with bone exposure. A comparative study between surgical and conservative treatment methods. Scand J Plast Reconstr Surg. 1983;17(2):147-152.
4. Braun M, Horton RC, Snelling CF. Fingertip amputation: review of 100 digits. Can J Surg. 1985;28(1):72-75.
5. Sammut D. Fingertip injuries. A review of indications and methods of management. Curr Orthop. 2002;16:271-285.
6. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993;18(4):416-422.
7. Atasoy E, Ioakimidis E, Kasdan ML, Kutz JE, Kleinert HE. Reconstruction of the amputated fingertip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am. 1970;52(5):921-926.
8. Kutler W. A new method for finger tip amputation. J Am Med Assoc. 1947;133(1):29-30.
9. Takeishi M, Shinoda A, Sugiyama A, Ui K. Innervated reverse dorsal digital island flap for fingertip reconstruction. J Hand Surg Am. 2006;31(7):1094-1099.
10. Tuncali D, Barutcu AY, Gokrem S, Terzioglu A, Aslan G. The hatchet flap for reconstruction of fingertip amputations. Plast Reconstr Surg. 2006;117(6):1933-1939.
11. Teoh LC, Tay SC, Yong FC, Tan SH, Khoo DB. Heterodigital arterialized flaps for large finger wounds: results and indications. Plast Reconstr Surg. 2003;111(6):1905-1913.
12. Nishikawa H, Smith PJ. The recovery of sensation and function after cross-finger flaps for fingertip injury. J Hand Surg Br. 1992;17(1):102-107.
13. Rinker B. Fingertip reconstruction with the laterally based thenar flap: indications and long-term functional results. Hand. 2006;1(1):2-8.
14. Jung MS, Lim YK, Hong YT, Kim HN. Treatment of fingertip amputation in adults by palmar pocketing of the amputated part. Arch Plast Surg. 2012;39(4):404-410.
15. Venkatramani H, Sabapathy SR. Fingertip replantation: technical considerations and outcome analysis of 24 consecutive fingertip replantations. Indian J Plast Surg. 2011;44(2):237-245.
16. Chen SY, Wang CH, Fu JP, Chang SC, Chen SG. Composite grafting for traumatic fingertip amputation in adults: technique reinforcement and experience in 31 digits. J Trauma. 2011;70(1):148-153.
17. van den Berg WB, Vergeer RA, van der Sluis CK, Ten Duis HJ, Werker PM. Comparison of three types of treatment modalities on the outcome of fingertip injuries. J Trauma Acute Care Surg. 2012;72(6):1681-1687.
18. Wang K, Sears ED, Shauver MJ, Chung KC. A systematic review of outcomes of revision amputation treatment for fingertip amputations. Hand. 2013;8(2):139-145.
19. Allen MJ. Conservative management of finger tip injuries in adults. Hand. 1980;12(3):257-265.
20. Chen CT, Wei FC, Chen HC, Chuang CC, Chen HT, Hsu WM. Distal phalanx replantation. Microsurgery. 1994;15(1):77-82.
21. Kim WK, Lim JH, Han SK. Fingertip replantations: clinical evaluation of 135 digits. Plast Reconstr Surg. 1996;98(3):470-476.
22. Jazayeri L, Klausner JQ, Chang J. Distal digital replantation. Plast Reconstr Surg. 2013;132(5):1207-1217.
23. Hattori Y, Doi K, Sakamoto S, Yamasaki H, Wahegaonkar A, Addosooki A. Fingertip replantation. J Hand Surg Am. 2007;32(4):548-555.
24. Goldner RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital replantation at the level of the distal interphalangeal joint and the distal phalanx. J Hand Surg Am. 1989;14(2 pt 1):214-220.
25. Nishi G, Shibata Y, Tago K, Kubota M, Suzuki M. Nail regeneration in digits replanted after amputation through the distal phalanx. J Hand Surg Am. 1996;21(2):229-233.
26. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985;10(2):211-218.
27. Jebson PJ, Louis DS, Bagg M. Amputations. In: Wolfe SW, Pederson WC, Hotchkiss RN, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2010:1885-1927.
Finger injuries are common, representing an estimated 3 million emergency department visits per year in the United States, with 44% of these diagnosed as lacerations.1 Amputations of the finger (partial and complete) in non-work-related accidents alone are estimated at 30,000 per year.1 The fingertip is a highly specialized structure that contributes to precision function of the hand through tactile feedback and fine motor control as well as hand aesthetics. An injury can compromise a variety of fingertip structures, including the distal phalanx, which provides length and structural support; the fingernail, germinal matrix, and sterile matrix, which protect the fingertip and function as tools; and the volar skin pad, which is important for sensation and fine motor activity.
There is considerable debate regarding optimal management of fingertip amputations, and to date there have been no prospective, randomly controlled trials to guide treatment.2 Injury characteristics, amputation levels, and patient priorities all contribute to management decisions. Treatment goals are to maintain length when possible; to provide stable, supple, and sensate skin coverage; to ensure the nail plate regrows without complication; and to maintain normal overall finger shape and cosmesis. In addition, a simple, cost-effective treatment with short recovery time and no donor-site morbidity is desired.
Treatment recommendations are wide-ranging, and evidence-based literature is sparse. About 30 years ago, 2 retrospective comparative studies found no difference in outcomes between simpler treatments (primary closure, secondary wound healing) and various operative strategies.3,4 Since then, most of the scientific studies have been retrospective noncomparative case series, all reporting good to excellent results.5-17 Investigators generally implied superior results of a studied procedure over those of more conservative treatments. Recommended treatments include secondary wound healing, simple flaps, staged flaps, pedicle flaps, allograft and autograft coverage, composite grafting, and replantation, for all levels of fingertip injury.
Given our surgical advances, improved techniques, and accumulating experience, we may have expected better outcomes with newer and more complex reconstructive efforts. Unfortunately, in a recent review of 53 fingertip injuries treated with a reconstructive procedure, bone shortening with closure, or secondary healing, Wang and colleagues18 found no discernible differences in outcomes at 4.5-year follow-up. They questioned whether complex reconstructive procedures are worth the time, expense, and risk. In the absence of prospective, comparative studies, surgeons must rely on anecdotal evidence (including predominantly level IV evidence), training bias, previous experience, and the prevailing common wisdom.
Toward that end, we became interested in identifying treatment preferences for fingertip amputations. We conducted a study to better understand how surgeon and patient factors influence the treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. We hypothesized that hand surgeons’ treatment preferences would be varied and influenced by surgeon and patient demographics.
Materials and Methods
An online multiple-choice survey was created and powered by Constant Contact. The survey consisted of 6 surgeon demographic questions; 5 treatment preference questions regarding patient age, sex, occupation, and germinal matrix management; and 5 clinical scenarios based on Allen levels 2, 3 (with and without exposed distal phalanx), and 4 and volar oblique middle-finger amputations. The Allen classification designates level 2 injuries as those involving only the distal pulp and nail.19 Level 3 injuries also involve the terminal distal phalanx, and level 4 injuries extend to the lunula. The survey questions are listed in the Appendix. For the clinical scenario questions, treatment choices included wound care, skeletal shortening and closure, composite graft, autograft, allograft, V-Y/Kutler flap, advancement flap, thenar flap, cross-finger flap, pedicle and homodigital flap, replantation, and other.
An email invitation was sent to members of the American Association for Hand Surgery (AAHS). The survey was also submitted to personal contacts of international hand societies named on the AAHS website to expand the international response. A reminder email was sent 1 week after the original invitation. The survey was closed 5 weeks later, and the responses were analyzed with all non-US hand surgeons grouped collectively as an international group, compared with the US group. Institutional review board approval was not needed for this survey study.
Statistics
A generalized linear regression model was used to implement logistic regression with random effects for question and respondent. This approach accounts for multiple observations from the same respondent, assuming that both respondent and question are random samples from a larger population. The model estimated the probability that a given surgical approach (eg, skeletal shortening, wound care) would be selected, based on the predictors of the US versus international respondent, time in practice, practice type, and whether the fingertip was available. The model returned adjusted odds ratios (ORs) for each predictor, controlling for all the others. By convention, P < .05 was considered significant. No attempt was made to prune the model of nonsignificant factors. Analyses were performed using the lme4 package on the R statistical platform (R Foundation for Statistical Computing).
Results
One hundred ninety-eight responses were recorded. Of the 1054 AAHS members invited to take the survey, 174 (US, international) responded (17% response rate). One hundred twenty-three responses and 62% of the total were generated from US hand surgeons. Fifty-eight percent of US responses were from the Mid-South, Midwest, or Mid-Atlantic region. Fifty-seven percent of international responses were from Brazil and Europe. Respondents’ demographic data are listed in Tables 1 and 2.
Responses to the 5 clinical scenarios showed a wide variation in treatment preferences. The top 6 preferred treatment selections for an acute, clean long-finger amputation in a healthy 40-year-old office worker are shown in Figures 1 to 5. When surgeons who preferred replant were asked what they would do if the amputated part was not available, they indicated flap coverage more often than less complex treatments, such as skeletal shortening/primary closure or wound care.
There were statistically significant differences in treatment preferences between US and international hand surgeons when controlling for all other demographic variables. Adjusted ORs and their confidence intervals (CIs) for the aggregate clinical scenarios are presented in a forest plot in Figure 6. Figure 4 shows that US surgeons were more likely to choose wound care (OR, 3.6; P < .0004) and less likely to attempt a replant (OR, 0.01; P < .0001). US surgeons were also less likely to use a pedicle or homodigital island flap when the amputated fingertip was both available (OR, 0.04; P = .039) and unavailable (OR, 0.47; Ps = .029).
Among all respondents and across all clinical scenarios, skeletal shortening with closure was favored among hand surgeons in practice less than 5 years compared with those in practice longer (OR, 2.11; 95% CI, 1.36-3.25; P = .0008). Similarly, surgeons with more than 30 years of experience were the least likely to favor wound care (OR, 0.2; 95% CI, 0.09-0.93; P = .037). Compared with orthopedic surgeons, plastic surgeons opted for wound care less often (OR, 0.44; 95% CI, 0.23-0.98; P = .018) and appeared to prefer replantation, but the difference was not statistically significant (OR, 8.86; 95% CI, 0.99-79.61; P = .054).
Replantation was less often chosen by private practice versus full-time academic surgeons (OR, 0.09; 95% CI, 0.01-0.91; P = .041.) Part-time academics were no more or less likely to perform replantation than full-time academics were (OR, 0.52; 95% CI, 0.05-5.41; P = .58). Of the 59 respondents who performed more than 10 microvascular cases a year, 18 (31%) chose replant for Allen level 4 amputations. In comparison, 9 (20%) of the 45 respondents who performed fewer than 3 microvascular cases a year chose replant for amputations at this level. Amount of time working with fellows did not affect treatment preferences.
Patient demographics (age, sex, occupation) also played a role in treatment decisions (Table 3). The most significant factors appeared to be age and occupation. Regarding age, 41% of respondents chose more complex procedures for patients younger than 15, and 62% chose less complex procedures for patients older than 70 years. Regarding occupation, 61% chose more complex procedures for professional musicians, and 60% chose less complex procedures for manual laborers. Sex did not influence clinical decisions for 78% of respondents. There was also substantial variation in both the indications for germinal matrix ablation and the frequency of sterile matrix transplant (Table 3).
Discussion
Although there is a variety of treatment options and published treatment guidelines for distal fingertip amputations, few comparative studies support use of one treatment over another. In our experience, treatment decisions are based mainly on injury parameters, but surgeon preference and patient factors (age, sex, occupation) can also influence care. Our goal in this study was to better understand how surgeon and patient factors influence treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. Our survey results showed lack of consensus among hand surgeons and highlighted several trends.
As expected, we found a wide range of treatment preferences for each clinical scenario queried, ranging from more simple treatments (eg, wound care) to more complex ones (eg, replantation). With patient parameters (age, profession, finger, acuity, injury type, tissue preservation, smoking status) standardized in the clinical scenarios, the treatment differences noted should reflect surgeon preference. However, other patient factors (eg, cultural differences, religious beliefs, surgeon setting, practice pattern, resource availability) that were not included in the clinical scenarios could also affect treatment preferences.
One particularly interesting finding was that international hand surgeons were 6.8 times more likely to replant a distal fingertip amputation. One possible explanation for this variation is the influence of cultural differences. For example, in East Asian countries, there can be a cultural stigma associated with loss of a fingertip, and therefore more of a desire on the part of the patient to restore the original finger.20,21 In addition, the international respondents were biased toward academic practices—which could skew the treatment preference toward replantation, as we found that academic surgeons were more inclined to replantation.
Our finding that replantation was more commonly preferred by academic versus private practice surgeons may suggest a training bias, an affinity for more complex or interesting procedures, or access to hospital equipment and staff, including residents and fellows, not usually found at smaller community hospitals, where private practice surgeons are more commonly based. Jazayeri and colleagues22 found that institutions specializing in microsurgery often produced better outcomes than nonspecializing institutions. Therefore, it is not surprising that private practice hand surgeons may less often opt to replant a distal fingertip amputation. It is also not surprising that plastic surgeons are more inclined to perform a replantation or flap coverage, as their training is more microsurgery-intensive and their practice more focused on aesthetics compared with the other specialists.
Distal fingertip replantation is accepted by most as technically demanding, but it seems that the additional effort and resources would be justified if the procedure provided a superior outcome. However, other factors, such as cost of treatment and length of recovery, should also be considered. Average replantation cost has been estimated to range from $7500 to $14,000, compared with $2800 for non-replantation-related care, and median stay is about 4 days longer for replantation-related care.23,24 These estimates do not include indirect costs, such as for postoperative rehabilitation, which is likely longer and more expensive, even in distal fingertip replantation. These disparities may not justify the outcome (of having a complete fingertip) if more conservative treatments yield similar results.17,18 In addition, there is the expected failure rate of limb replantation surgery. In analysis of the overall societal costs and benefits of larger upper extremity limb replantation, the loss of invested resources sustained with failed limb replantation may be outweighed by the benefit of another patient having a successful outcome. In the case of fingertip replantation, however, does the undefined benefit of the successful patient outcome outweigh the investment of resources lost in cases of replantation failure? Understandably, there is a need for more robust clinical outcome and cost-comparative evidence to better inform decisions regarding distal fingertip amputation.
We found that wound care and skeletal shortening with primary closure (particularly with Allen level 3 injuries) were preferred more by surgeons within the first 5 years of practice. This finding seems to imply a lack of experience or confidence on the part of younger surgeons performing more complex procedures, such as flap coverage. Conversely, this finding may indicate a shift in treatment principle based on recent literature suggesting equivalent outcomes with simpler procedures.17,18 Although our survey study did not provide an option for treatment combinations or staged procedures, several respondents wrote in that skeletal shortening supplemented with various types of autografts and allografts would be their preferred treatment.
Patient factors also play a significant role in clinical decisions. Age and profession seem to be important determinants, with more than 50% of respondents, on average, changing their treatment recommendation based on these 2 factors. A majority of respondents would perform a less involved procedure for a manual laborer, suggesting a quicker return to work is prioritized over a perceived improved clinical outcome. Interestingly, for patients younger than 15 years, the preference was divided, with 41% of surgeons opting for a more complex procedure. This suggests the importance of restoring anatomy in a younger patient, or the perceived decreased risk or failure rate with more involved treatment. Twenty percent preferred a less complex procedure in a younger patient, perhaps relying on the patient’s developmental potential for a good outcome or suggesting a concern for patient intolerance or compliance with complex surgery.
Nail plate regrowth can be a problem with fingertip amputations. Nail deformity is highly correlated with injury level, with amputations proximal to the lunula more likely to cause nail plate deformity.25,26 Jebson and colleagues27 recommended germinal matrix ablation for amputations proximal to the lunula. We found respondents often performed ablations for other indications, including injured or minimal remaining sterile matrix and lack of bony support for the sterile matrix. Forty-six percent of respondents had never performed sterile matrix transplant, which could indicate that they were unfamiliar with the technique or had donor-site concerns, or that postinjury nail deformities are uncommon, well tolerated, or treated along with other procedures, such as germinal matrix ablation.
Several weaknesses of this study must be highlighted. First, our response rate was smaller than desired. Although this work incorporated a large number of surgeon responses, nearly 200, the response rate was only 17%. In addition, although number of responses was likely adequate to show the diversity of opinion, the preferences and trends reported might not be representative of all hand surgeons. We could not perform a nonresponder analysis because of a lack of specific demographic data for the AAHS and international hand society members. However, AAHS has an approximate 50/50 mix of plastic and orthopedic surgeons, similar to our responder demographic, suggesting our smaller subset of responses might be representative of the whole. According to AAHS, a majority of its members are “academic” hand surgeons, so our results might not adequately reflect the preferences of community hand surgeons and ultimately might overstate the frequency of more complex treatments. Last, our international response was limited to a few countries. A larger, more broadly distributed response would provide a better understanding of regional preferences, which could shed light on the importance of cultural differences.
Variations in patient insurance status were not queried in this survey but might also affect treatment decisions. More involved, costly, and highly reimbursing procedures might be deemed reasonable options for a small perceived clinical benefit for insured patients.
When multiple digits or the thumb is injured, or there are other concomitant injuries, surgeons may alter their choice of intervention. In mangled extremities, preservation of salvageable functional units takes precedence over aesthetics and likely affects choice of treatment for the amputated fingertips. Similarly, multiple fingertip amputations, even if all at the same level, may be differently regarded than a solitary injury.
Conclusion
For distal fingertip amputations, there is little evidence supporting one approach over another. Without level I comparative data guiding treatment, anecdotal evidence and surgeon personal preferences likely contribute to the large variation noted in this survey. Our study results showed the disparity of fingertip treatment preferences among a cross section of US and international hand surgeons. More important, results underscored the need for a well-designed comparative study to determine the most effective treatments for distal fingertip amputations.
Finger injuries are common, representing an estimated 3 million emergency department visits per year in the United States, with 44% of these diagnosed as lacerations.1 Amputations of the finger (partial and complete) in non-work-related accidents alone are estimated at 30,000 per year.1 The fingertip is a highly specialized structure that contributes to precision function of the hand through tactile feedback and fine motor control as well as hand aesthetics. An injury can compromise a variety of fingertip structures, including the distal phalanx, which provides length and structural support; the fingernail, germinal matrix, and sterile matrix, which protect the fingertip and function as tools; and the volar skin pad, which is important for sensation and fine motor activity.
There is considerable debate regarding optimal management of fingertip amputations, and to date there have been no prospective, randomly controlled trials to guide treatment.2 Injury characteristics, amputation levels, and patient priorities all contribute to management decisions. Treatment goals are to maintain length when possible; to provide stable, supple, and sensate skin coverage; to ensure the nail plate regrows without complication; and to maintain normal overall finger shape and cosmesis. In addition, a simple, cost-effective treatment with short recovery time and no donor-site morbidity is desired.
Treatment recommendations are wide-ranging, and evidence-based literature is sparse. About 30 years ago, 2 retrospective comparative studies found no difference in outcomes between simpler treatments (primary closure, secondary wound healing) and various operative strategies.3,4 Since then, most of the scientific studies have been retrospective noncomparative case series, all reporting good to excellent results.5-17 Investigators generally implied superior results of a studied procedure over those of more conservative treatments. Recommended treatments include secondary wound healing, simple flaps, staged flaps, pedicle flaps, allograft and autograft coverage, composite grafting, and replantation, for all levels of fingertip injury.
Given our surgical advances, improved techniques, and accumulating experience, we may have expected better outcomes with newer and more complex reconstructive efforts. Unfortunately, in a recent review of 53 fingertip injuries treated with a reconstructive procedure, bone shortening with closure, or secondary healing, Wang and colleagues18 found no discernible differences in outcomes at 4.5-year follow-up. They questioned whether complex reconstructive procedures are worth the time, expense, and risk. In the absence of prospective, comparative studies, surgeons must rely on anecdotal evidence (including predominantly level IV evidence), training bias, previous experience, and the prevailing common wisdom.
Toward that end, we became interested in identifying treatment preferences for fingertip amputations. We conducted a study to better understand how surgeon and patient factors influence the treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. We hypothesized that hand surgeons’ treatment preferences would be varied and influenced by surgeon and patient demographics.
Materials and Methods
An online multiple-choice survey was created and powered by Constant Contact. The survey consisted of 6 surgeon demographic questions; 5 treatment preference questions regarding patient age, sex, occupation, and germinal matrix management; and 5 clinical scenarios based on Allen levels 2, 3 (with and without exposed distal phalanx), and 4 and volar oblique middle-finger amputations. The Allen classification designates level 2 injuries as those involving only the distal pulp and nail.19 Level 3 injuries also involve the terminal distal phalanx, and level 4 injuries extend to the lunula. The survey questions are listed in the Appendix. For the clinical scenario questions, treatment choices included wound care, skeletal shortening and closure, composite graft, autograft, allograft, V-Y/Kutler flap, advancement flap, thenar flap, cross-finger flap, pedicle and homodigital flap, replantation, and other.
An email invitation was sent to members of the American Association for Hand Surgery (AAHS). The survey was also submitted to personal contacts of international hand societies named on the AAHS website to expand the international response. A reminder email was sent 1 week after the original invitation. The survey was closed 5 weeks later, and the responses were analyzed with all non-US hand surgeons grouped collectively as an international group, compared with the US group. Institutional review board approval was not needed for this survey study.
Statistics
A generalized linear regression model was used to implement logistic regression with random effects for question and respondent. This approach accounts for multiple observations from the same respondent, assuming that both respondent and question are random samples from a larger population. The model estimated the probability that a given surgical approach (eg, skeletal shortening, wound care) would be selected, based on the predictors of the US versus international respondent, time in practice, practice type, and whether the fingertip was available. The model returned adjusted odds ratios (ORs) for each predictor, controlling for all the others. By convention, P < .05 was considered significant. No attempt was made to prune the model of nonsignificant factors. Analyses were performed using the lme4 package on the R statistical platform (R Foundation for Statistical Computing).
Results
One hundred ninety-eight responses were recorded. Of the 1054 AAHS members invited to take the survey, 174 (US, international) responded (17% response rate). One hundred twenty-three responses and 62% of the total were generated from US hand surgeons. Fifty-eight percent of US responses were from the Mid-South, Midwest, or Mid-Atlantic region. Fifty-seven percent of international responses were from Brazil and Europe. Respondents’ demographic data are listed in Tables 1 and 2.
Responses to the 5 clinical scenarios showed a wide variation in treatment preferences. The top 6 preferred treatment selections for an acute, clean long-finger amputation in a healthy 40-year-old office worker are shown in Figures 1 to 5. When surgeons who preferred replant were asked what they would do if the amputated part was not available, they indicated flap coverage more often than less complex treatments, such as skeletal shortening/primary closure or wound care.
There were statistically significant differences in treatment preferences between US and international hand surgeons when controlling for all other demographic variables. Adjusted ORs and their confidence intervals (CIs) for the aggregate clinical scenarios are presented in a forest plot in Figure 6. Figure 4 shows that US surgeons were more likely to choose wound care (OR, 3.6; P < .0004) and less likely to attempt a replant (OR, 0.01; P < .0001). US surgeons were also less likely to use a pedicle or homodigital island flap when the amputated fingertip was both available (OR, 0.04; P = .039) and unavailable (OR, 0.47; Ps = .029).
Among all respondents and across all clinical scenarios, skeletal shortening with closure was favored among hand surgeons in practice less than 5 years compared with those in practice longer (OR, 2.11; 95% CI, 1.36-3.25; P = .0008). Similarly, surgeons with more than 30 years of experience were the least likely to favor wound care (OR, 0.2; 95% CI, 0.09-0.93; P = .037). Compared with orthopedic surgeons, plastic surgeons opted for wound care less often (OR, 0.44; 95% CI, 0.23-0.98; P = .018) and appeared to prefer replantation, but the difference was not statistically significant (OR, 8.86; 95% CI, 0.99-79.61; P = .054).
Replantation was less often chosen by private practice versus full-time academic surgeons (OR, 0.09; 95% CI, 0.01-0.91; P = .041.) Part-time academics were no more or less likely to perform replantation than full-time academics were (OR, 0.52; 95% CI, 0.05-5.41; P = .58). Of the 59 respondents who performed more than 10 microvascular cases a year, 18 (31%) chose replant for Allen level 4 amputations. In comparison, 9 (20%) of the 45 respondents who performed fewer than 3 microvascular cases a year chose replant for amputations at this level. Amount of time working with fellows did not affect treatment preferences.
Patient demographics (age, sex, occupation) also played a role in treatment decisions (Table 3). The most significant factors appeared to be age and occupation. Regarding age, 41% of respondents chose more complex procedures for patients younger than 15, and 62% chose less complex procedures for patients older than 70 years. Regarding occupation, 61% chose more complex procedures for professional musicians, and 60% chose less complex procedures for manual laborers. Sex did not influence clinical decisions for 78% of respondents. There was also substantial variation in both the indications for germinal matrix ablation and the frequency of sterile matrix transplant (Table 3).
Discussion
Although there is a variety of treatment options and published treatment guidelines for distal fingertip amputations, few comparative studies support use of one treatment over another. In our experience, treatment decisions are based mainly on injury parameters, but surgeon preference and patient factors (age, sex, occupation) can also influence care. Our goal in this study was to better understand how surgeon and patient factors influence treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. Our survey results showed lack of consensus among hand surgeons and highlighted several trends.
As expected, we found a wide range of treatment preferences for each clinical scenario queried, ranging from more simple treatments (eg, wound care) to more complex ones (eg, replantation). With patient parameters (age, profession, finger, acuity, injury type, tissue preservation, smoking status) standardized in the clinical scenarios, the treatment differences noted should reflect surgeon preference. However, other patient factors (eg, cultural differences, religious beliefs, surgeon setting, practice pattern, resource availability) that were not included in the clinical scenarios could also affect treatment preferences.
One particularly interesting finding was that international hand surgeons were 6.8 times more likely to replant a distal fingertip amputation. One possible explanation for this variation is the influence of cultural differences. For example, in East Asian countries, there can be a cultural stigma associated with loss of a fingertip, and therefore more of a desire on the part of the patient to restore the original finger.20,21 In addition, the international respondents were biased toward academic practices—which could skew the treatment preference toward replantation, as we found that academic surgeons were more inclined to replantation.
Our finding that replantation was more commonly preferred by academic versus private practice surgeons may suggest a training bias, an affinity for more complex or interesting procedures, or access to hospital equipment and staff, including residents and fellows, not usually found at smaller community hospitals, where private practice surgeons are more commonly based. Jazayeri and colleagues22 found that institutions specializing in microsurgery often produced better outcomes than nonspecializing institutions. Therefore, it is not surprising that private practice hand surgeons may less often opt to replant a distal fingertip amputation. It is also not surprising that plastic surgeons are more inclined to perform a replantation or flap coverage, as their training is more microsurgery-intensive and their practice more focused on aesthetics compared with the other specialists.
Distal fingertip replantation is accepted by most as technically demanding, but it seems that the additional effort and resources would be justified if the procedure provided a superior outcome. However, other factors, such as cost of treatment and length of recovery, should also be considered. Average replantation cost has been estimated to range from $7500 to $14,000, compared with $2800 for non-replantation-related care, and median stay is about 4 days longer for replantation-related care.23,24 These estimates do not include indirect costs, such as for postoperative rehabilitation, which is likely longer and more expensive, even in distal fingertip replantation. These disparities may not justify the outcome (of having a complete fingertip) if more conservative treatments yield similar results.17,18 In addition, there is the expected failure rate of limb replantation surgery. In analysis of the overall societal costs and benefits of larger upper extremity limb replantation, the loss of invested resources sustained with failed limb replantation may be outweighed by the benefit of another patient having a successful outcome. In the case of fingertip replantation, however, does the undefined benefit of the successful patient outcome outweigh the investment of resources lost in cases of replantation failure? Understandably, there is a need for more robust clinical outcome and cost-comparative evidence to better inform decisions regarding distal fingertip amputation.
We found that wound care and skeletal shortening with primary closure (particularly with Allen level 3 injuries) were preferred more by surgeons within the first 5 years of practice. This finding seems to imply a lack of experience or confidence on the part of younger surgeons performing more complex procedures, such as flap coverage. Conversely, this finding may indicate a shift in treatment principle based on recent literature suggesting equivalent outcomes with simpler procedures.17,18 Although our survey study did not provide an option for treatment combinations or staged procedures, several respondents wrote in that skeletal shortening supplemented with various types of autografts and allografts would be their preferred treatment.
Patient factors also play a significant role in clinical decisions. Age and profession seem to be important determinants, with more than 50% of respondents, on average, changing their treatment recommendation based on these 2 factors. A majority of respondents would perform a less involved procedure for a manual laborer, suggesting a quicker return to work is prioritized over a perceived improved clinical outcome. Interestingly, for patients younger than 15 years, the preference was divided, with 41% of surgeons opting for a more complex procedure. This suggests the importance of restoring anatomy in a younger patient, or the perceived decreased risk or failure rate with more involved treatment. Twenty percent preferred a less complex procedure in a younger patient, perhaps relying on the patient’s developmental potential for a good outcome or suggesting a concern for patient intolerance or compliance with complex surgery.
Nail plate regrowth can be a problem with fingertip amputations. Nail deformity is highly correlated with injury level, with amputations proximal to the lunula more likely to cause nail plate deformity.25,26 Jebson and colleagues27 recommended germinal matrix ablation for amputations proximal to the lunula. We found respondents often performed ablations for other indications, including injured or minimal remaining sterile matrix and lack of bony support for the sterile matrix. Forty-six percent of respondents had never performed sterile matrix transplant, which could indicate that they were unfamiliar with the technique or had donor-site concerns, or that postinjury nail deformities are uncommon, well tolerated, or treated along with other procedures, such as germinal matrix ablation.
Several weaknesses of this study must be highlighted. First, our response rate was smaller than desired. Although this work incorporated a large number of surgeon responses, nearly 200, the response rate was only 17%. In addition, although number of responses was likely adequate to show the diversity of opinion, the preferences and trends reported might not be representative of all hand surgeons. We could not perform a nonresponder analysis because of a lack of specific demographic data for the AAHS and international hand society members. However, AAHS has an approximate 50/50 mix of plastic and orthopedic surgeons, similar to our responder demographic, suggesting our smaller subset of responses might be representative of the whole. According to AAHS, a majority of its members are “academic” hand surgeons, so our results might not adequately reflect the preferences of community hand surgeons and ultimately might overstate the frequency of more complex treatments. Last, our international response was limited to a few countries. A larger, more broadly distributed response would provide a better understanding of regional preferences, which could shed light on the importance of cultural differences.
Variations in patient insurance status were not queried in this survey but might also affect treatment decisions. More involved, costly, and highly reimbursing procedures might be deemed reasonable options for a small perceived clinical benefit for insured patients.
When multiple digits or the thumb is injured, or there are other concomitant injuries, surgeons may alter their choice of intervention. In mangled extremities, preservation of salvageable functional units takes precedence over aesthetics and likely affects choice of treatment for the amputated fingertips. Similarly, multiple fingertip amputations, even if all at the same level, may be differently regarded than a solitary injury.
Conclusion
For distal fingertip amputations, there is little evidence supporting one approach over another. Without level I comparative data guiding treatment, anecdotal evidence and surgeon personal preferences likely contribute to the large variation noted in this survey. Our study results showed the disparity of fingertip treatment preferences among a cross section of US and international hand surgeons. More important, results underscored the need for a well-designed comparative study to determine the most effective treatments for distal fingertip amputations.
1. Conn JM, Annest JL, Ryan GW, Budnitz DS. Non-work-related finger amputations in the United States, 2001-2002. Ann Emerg Med. 2005;45(6):630-635.
2. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am. 2008;33(8):1417-1419.
3. Söderberg T, Nyström Å, Hallmans G, Hultén J. Treatment of fingertip amputations with bone exposure. A comparative study between surgical and conservative treatment methods. Scand J Plast Reconstr Surg. 1983;17(2):147-152.
4. Braun M, Horton RC, Snelling CF. Fingertip amputation: review of 100 digits. Can J Surg. 1985;28(1):72-75.
5. Sammut D. Fingertip injuries. A review of indications and methods of management. Curr Orthop. 2002;16:271-285.
6. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993;18(4):416-422.
7. Atasoy E, Ioakimidis E, Kasdan ML, Kutz JE, Kleinert HE. Reconstruction of the amputated fingertip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am. 1970;52(5):921-926.
8. Kutler W. A new method for finger tip amputation. J Am Med Assoc. 1947;133(1):29-30.
9. Takeishi M, Shinoda A, Sugiyama A, Ui K. Innervated reverse dorsal digital island flap for fingertip reconstruction. J Hand Surg Am. 2006;31(7):1094-1099.
10. Tuncali D, Barutcu AY, Gokrem S, Terzioglu A, Aslan G. The hatchet flap for reconstruction of fingertip amputations. Plast Reconstr Surg. 2006;117(6):1933-1939.
11. Teoh LC, Tay SC, Yong FC, Tan SH, Khoo DB. Heterodigital arterialized flaps for large finger wounds: results and indications. Plast Reconstr Surg. 2003;111(6):1905-1913.
12. Nishikawa H, Smith PJ. The recovery of sensation and function after cross-finger flaps for fingertip injury. J Hand Surg Br. 1992;17(1):102-107.
13. Rinker B. Fingertip reconstruction with the laterally based thenar flap: indications and long-term functional results. Hand. 2006;1(1):2-8.
14. Jung MS, Lim YK, Hong YT, Kim HN. Treatment of fingertip amputation in adults by palmar pocketing of the amputated part. Arch Plast Surg. 2012;39(4):404-410.
15. Venkatramani H, Sabapathy SR. Fingertip replantation: technical considerations and outcome analysis of 24 consecutive fingertip replantations. Indian J Plast Surg. 2011;44(2):237-245.
16. Chen SY, Wang CH, Fu JP, Chang SC, Chen SG. Composite grafting for traumatic fingertip amputation in adults: technique reinforcement and experience in 31 digits. J Trauma. 2011;70(1):148-153.
17. van den Berg WB, Vergeer RA, van der Sluis CK, Ten Duis HJ, Werker PM. Comparison of three types of treatment modalities on the outcome of fingertip injuries. J Trauma Acute Care Surg. 2012;72(6):1681-1687.
18. Wang K, Sears ED, Shauver MJ, Chung KC. A systematic review of outcomes of revision amputation treatment for fingertip amputations. Hand. 2013;8(2):139-145.
19. Allen MJ. Conservative management of finger tip injuries in adults. Hand. 1980;12(3):257-265.
20. Chen CT, Wei FC, Chen HC, Chuang CC, Chen HT, Hsu WM. Distal phalanx replantation. Microsurgery. 1994;15(1):77-82.
21. Kim WK, Lim JH, Han SK. Fingertip replantations: clinical evaluation of 135 digits. Plast Reconstr Surg. 1996;98(3):470-476.
22. Jazayeri L, Klausner JQ, Chang J. Distal digital replantation. Plast Reconstr Surg. 2013;132(5):1207-1217.
23. Hattori Y, Doi K, Sakamoto S, Yamasaki H, Wahegaonkar A, Addosooki A. Fingertip replantation. J Hand Surg Am. 2007;32(4):548-555.
24. Goldner RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital replantation at the level of the distal interphalangeal joint and the distal phalanx. J Hand Surg Am. 1989;14(2 pt 1):214-220.
25. Nishi G, Shibata Y, Tago K, Kubota M, Suzuki M. Nail regeneration in digits replanted after amputation through the distal phalanx. J Hand Surg Am. 1996;21(2):229-233.
26. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985;10(2):211-218.
27. Jebson PJ, Louis DS, Bagg M. Amputations. In: Wolfe SW, Pederson WC, Hotchkiss RN, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2010:1885-1927.
1. Conn JM, Annest JL, Ryan GW, Budnitz DS. Non-work-related finger amputations in the United States, 2001-2002. Ann Emerg Med. 2005;45(6):630-635.
2. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am. 2008;33(8):1417-1419.
3. Söderberg T, Nyström Å, Hallmans G, Hultén J. Treatment of fingertip amputations with bone exposure. A comparative study between surgical and conservative treatment methods. Scand J Plast Reconstr Surg. 1983;17(2):147-152.
4. Braun M, Horton RC, Snelling CF. Fingertip amputation: review of 100 digits. Can J Surg. 1985;28(1):72-75.
5. Sammut D. Fingertip injuries. A review of indications and methods of management. Curr Orthop. 2002;16:271-285.
6. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993;18(4):416-422.
7. Atasoy E, Ioakimidis E, Kasdan ML, Kutz JE, Kleinert HE. Reconstruction of the amputated fingertip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am. 1970;52(5):921-926.
8. Kutler W. A new method for finger tip amputation. J Am Med Assoc. 1947;133(1):29-30.
9. Takeishi M, Shinoda A, Sugiyama A, Ui K. Innervated reverse dorsal digital island flap for fingertip reconstruction. J Hand Surg Am. 2006;31(7):1094-1099.
10. Tuncali D, Barutcu AY, Gokrem S, Terzioglu A, Aslan G. The hatchet flap for reconstruction of fingertip amputations. Plast Reconstr Surg. 2006;117(6):1933-1939.
11. Teoh LC, Tay SC, Yong FC, Tan SH, Khoo DB. Heterodigital arterialized flaps for large finger wounds: results and indications. Plast Reconstr Surg. 2003;111(6):1905-1913.
12. Nishikawa H, Smith PJ. The recovery of sensation and function after cross-finger flaps for fingertip injury. J Hand Surg Br. 1992;17(1):102-107.
13. Rinker B. Fingertip reconstruction with the laterally based thenar flap: indications and long-term functional results. Hand. 2006;1(1):2-8.
14. Jung MS, Lim YK, Hong YT, Kim HN. Treatment of fingertip amputation in adults by palmar pocketing of the amputated part. Arch Plast Surg. 2012;39(4):404-410.
15. Venkatramani H, Sabapathy SR. Fingertip replantation: technical considerations and outcome analysis of 24 consecutive fingertip replantations. Indian J Plast Surg. 2011;44(2):237-245.
16. Chen SY, Wang CH, Fu JP, Chang SC, Chen SG. Composite grafting for traumatic fingertip amputation in adults: technique reinforcement and experience in 31 digits. J Trauma. 2011;70(1):148-153.
17. van den Berg WB, Vergeer RA, van der Sluis CK, Ten Duis HJ, Werker PM. Comparison of three types of treatment modalities on the outcome of fingertip injuries. J Trauma Acute Care Surg. 2012;72(6):1681-1687.
18. Wang K, Sears ED, Shauver MJ, Chung KC. A systematic review of outcomes of revision amputation treatment for fingertip amputations. Hand. 2013;8(2):139-145.
19. Allen MJ. Conservative management of finger tip injuries in adults. Hand. 1980;12(3):257-265.
20. Chen CT, Wei FC, Chen HC, Chuang CC, Chen HT, Hsu WM. Distal phalanx replantation. Microsurgery. 1994;15(1):77-82.
21. Kim WK, Lim JH, Han SK. Fingertip replantations: clinical evaluation of 135 digits. Plast Reconstr Surg. 1996;98(3):470-476.
22. Jazayeri L, Klausner JQ, Chang J. Distal digital replantation. Plast Reconstr Surg. 2013;132(5):1207-1217.
23. Hattori Y, Doi K, Sakamoto S, Yamasaki H, Wahegaonkar A, Addosooki A. Fingertip replantation. J Hand Surg Am. 2007;32(4):548-555.
24. Goldner RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital replantation at the level of the distal interphalangeal joint and the distal phalanx. J Hand Surg Am. 1989;14(2 pt 1):214-220.
25. Nishi G, Shibata Y, Tago K, Kubota M, Suzuki M. Nail regeneration in digits replanted after amputation through the distal phalanx. J Hand Surg Am. 1996;21(2):229-233.
26. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985;10(2):211-218.
27. Jebson PJ, Louis DS, Bagg M. Amputations. In: Wolfe SW, Pederson WC, Hotchkiss RN, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2010:1885-1927.
Sustentaculum Lunatum: Appreciation of the Palmar Lunate Facet in Management of Complex Intra-Articular Fractures of the Distal Radius
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
Perilunate Injuries
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.