User login
Epacadostat plus pembrolizumab shows promise in advanced solid tumors
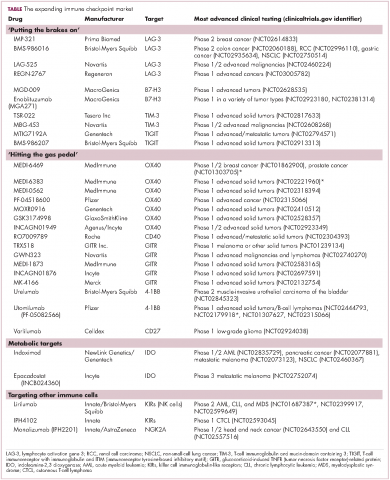
Epacadostat, a highly selective oral inhibitor of the indoleamine 2,3-dioxygenase 1 (IDO1) enzyme, was well tolerated when combined with pembrolizumab and demonstrated encouraging antitumor activity in multiple types of advanced solid tumors, according to the results of a phase l/ll trial.
Tumors may evade immunosurveillance through upregulation of the IDO1 enzyme, and thus there is a great interest in developing combination therapies that can target various immune evasion pathways to improve therapeutic response and outcomes. In this study, the authors evaluated the investigational agent epacadostat combined with pembrolizumab in 62 patients with advanced solid tumors.
In the dose escalation phase, patents received increasing doses of oral epacadostat (25, 50, 100, or 300 mg) twice per day plus intravenous pembrolizumab 2 mg/kg or 200 mg every 3 weeks. During the safety expansion, epacadostat at 50, 100, or 300 mg was given twice per day, plus pembrolizumab 200 mg every 3 weeks. The maximum tolerated dose of epacadostat in combination with pembrolizumab was not reached.
Objective responses (per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1) occurred in 12 (55%) of 22 patients with melanoma and in patients with non–small-cell lung cancer, renal cell carcinoma, endometrial adenocarcinoma, urothelial carcinoma, and squamous cell carcinoma of the head and neck, reported Tara C. Mitchell, MD, of the Abramson Cancer Center, University of Pennsylvania, Philadelphia, and her colleagues. The report is in the Journal of Clinical Oncology.
The authors observed that there was antitumor activity at all epacadostat doses and in several tumor types. A complete response was achieved by 8 patients (treatment naive melanoma [5 patients] and previously treated for advanced/ metastatic melanoma, endometrial adenocarcinoma [EA], or urothelial carcinoma [UC] [1 patient each]), while 17 patients achieved a partial response (treatment-naive melanoma [6 patients], non–small cell lung cancer [NSCLC] [5 patients], renal cell carcinoma [RCC] and UC [2 patients each], and EA and squamous cell carcinoma of the head and neck [1 patient each]).
Most patients (n = 52, 84%) experienced treatment-related adverse events (TRAEs), the most frequently observed being fatigue (36%), rash (36%), arthralgia (24%), pruritus (23%), and nausea (21%). Grade 3/4 TRAEs occurred in 24% of patients, and 7 patients (11%) discontinued their treatment because of TRAEs. There were no deaths associated with TRAEs.
“The safety profile observed with epacadostat plus pembrolizumab compares favorably with studies of other combination immunotherapies,” wrote Dr. Mitchell and her colleagues. “Although not powered to evaluate efficacy, the phase I portion of this study showed that epacadostat plus pembrolizumab had encouraging and durable antitumor activity,” they said.
SOURCE: Mitchell TC et al. J Clin Oncol. 2018 Sep 28. doi: 10.1200/JCO.2018.78.9602.
Epacadostat, a highly selective oral inhibitor of the indoleamine 2,3-dioxygenase 1 (IDO1) enzyme, was well tolerated when combined with pembrolizumab and demonstrated encouraging antitumor activity in multiple types of advanced solid tumors, according to the results of a phase l/ll trial.
Tumors may evade immunosurveillance through upregulation of the IDO1 enzyme, and thus there is a great interest in developing combination therapies that can target various immune evasion pathways to improve therapeutic response and outcomes. In this study, the authors evaluated the investigational agent epacadostat combined with pembrolizumab in 62 patients with advanced solid tumors.
In the dose escalation phase, patents received increasing doses of oral epacadostat (25, 50, 100, or 300 mg) twice per day plus intravenous pembrolizumab 2 mg/kg or 200 mg every 3 weeks. During the safety expansion, epacadostat at 50, 100, or 300 mg was given twice per day, plus pembrolizumab 200 mg every 3 weeks. The maximum tolerated dose of epacadostat in combination with pembrolizumab was not reached.
Objective responses (per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1) occurred in 12 (55%) of 22 patients with melanoma and in patients with non–small-cell lung cancer, renal cell carcinoma, endometrial adenocarcinoma, urothelial carcinoma, and squamous cell carcinoma of the head and neck, reported Tara C. Mitchell, MD, of the Abramson Cancer Center, University of Pennsylvania, Philadelphia, and her colleagues. The report is in the Journal of Clinical Oncology.
The authors observed that there was antitumor activity at all epacadostat doses and in several tumor types. A complete response was achieved by 8 patients (treatment naive melanoma [5 patients] and previously treated for advanced/ metastatic melanoma, endometrial adenocarcinoma [EA], or urothelial carcinoma [UC] [1 patient each]), while 17 patients achieved a partial response (treatment-naive melanoma [6 patients], non–small cell lung cancer [NSCLC] [5 patients], renal cell carcinoma [RCC] and UC [2 patients each], and EA and squamous cell carcinoma of the head and neck [1 patient each]).
Most patients (n = 52, 84%) experienced treatment-related adverse events (TRAEs), the most frequently observed being fatigue (36%), rash (36%), arthralgia (24%), pruritus (23%), and nausea (21%). Grade 3/4 TRAEs occurred in 24% of patients, and 7 patients (11%) discontinued their treatment because of TRAEs. There were no deaths associated with TRAEs.
“The safety profile observed with epacadostat plus pembrolizumab compares favorably with studies of other combination immunotherapies,” wrote Dr. Mitchell and her colleagues. “Although not powered to evaluate efficacy, the phase I portion of this study showed that epacadostat plus pembrolizumab had encouraging and durable antitumor activity,” they said.
SOURCE: Mitchell TC et al. J Clin Oncol. 2018 Sep 28. doi: 10.1200/JCO.2018.78.9602.
Epacadostat, a highly selective oral inhibitor of the indoleamine 2,3-dioxygenase 1 (IDO1) enzyme, was well tolerated when combined with pembrolizumab and demonstrated encouraging antitumor activity in multiple types of advanced solid tumors, according to the results of a phase l/ll trial.
Tumors may evade immunosurveillance through upregulation of the IDO1 enzyme, and thus there is a great interest in developing combination therapies that can target various immune evasion pathways to improve therapeutic response and outcomes. In this study, the authors evaluated the investigational agent epacadostat combined with pembrolizumab in 62 patients with advanced solid tumors.
In the dose escalation phase, patents received increasing doses of oral epacadostat (25, 50, 100, or 300 mg) twice per day plus intravenous pembrolizumab 2 mg/kg or 200 mg every 3 weeks. During the safety expansion, epacadostat at 50, 100, or 300 mg was given twice per day, plus pembrolizumab 200 mg every 3 weeks. The maximum tolerated dose of epacadostat in combination with pembrolizumab was not reached.
Objective responses (per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1) occurred in 12 (55%) of 22 patients with melanoma and in patients with non–small-cell lung cancer, renal cell carcinoma, endometrial adenocarcinoma, urothelial carcinoma, and squamous cell carcinoma of the head and neck, reported Tara C. Mitchell, MD, of the Abramson Cancer Center, University of Pennsylvania, Philadelphia, and her colleagues. The report is in the Journal of Clinical Oncology.
The authors observed that there was antitumor activity at all epacadostat doses and in several tumor types. A complete response was achieved by 8 patients (treatment naive melanoma [5 patients] and previously treated for advanced/ metastatic melanoma, endometrial adenocarcinoma [EA], or urothelial carcinoma [UC] [1 patient each]), while 17 patients achieved a partial response (treatment-naive melanoma [6 patients], non–small cell lung cancer [NSCLC] [5 patients], renal cell carcinoma [RCC] and UC [2 patients each], and EA and squamous cell carcinoma of the head and neck [1 patient each]).
Most patients (n = 52, 84%) experienced treatment-related adverse events (TRAEs), the most frequently observed being fatigue (36%), rash (36%), arthralgia (24%), pruritus (23%), and nausea (21%). Grade 3/4 TRAEs occurred in 24% of patients, and 7 patients (11%) discontinued their treatment because of TRAEs. There were no deaths associated with TRAEs.
“The safety profile observed with epacadostat plus pembrolizumab compares favorably with studies of other combination immunotherapies,” wrote Dr. Mitchell and her colleagues. “Although not powered to evaluate efficacy, the phase I portion of this study showed that epacadostat plus pembrolizumab had encouraging and durable antitumor activity,” they said.
SOURCE: Mitchell TC et al. J Clin Oncol. 2018 Sep 28. doi: 10.1200/JCO.2018.78.9602.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Epacadostat plus pembrolizumab showed antitumor activity and tolerability in patients with advanced solid tumors.
Major finding: Among 62 patients, 25 achieved an objective response.
Study details: Phase l/ll clinical trial of 62 patients with advanced solid tumors.
Disclosures: Incyte and Merck funded the study. All of the authors have disclosed relationships with industry, including the study sponsor.
Source: Mitchell TC et al. J Clin Oncol. 2018 Sep 28. doi: 10.1200/JCO.2018.78.9602.
First combo trial of mTOR/BRAF inhibition shows potential
A combination of the mammalian target of rapamycin (mTOR) inhibitor everolimus and the BRAF inhibitor vemurafenib appears safe for patients with advanced, BRAF-mutated, solid tumors that had progressed on BRAF and/or MEK therapy, investigators reported.
The combination provided partial responses in a variety of tumor types, and half of the patients achieved stable disease, reported Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center in Houston and his coauthors.
“Activation of alternative parallel signaling pathways such as the PI3K–mTOR pathway have been hypothesized to contribute to primary and acquired resistance to BRAF-targeted therapy,” the authors wrote in JCO Precision Oncology. Preclinical studies have supported this hypothesis, which led to the present study; it is the first to evaluate a combination of a BRAF inhibitor and an mTOR inhibitor.
The open-label, phase 1 trial included 20 patients with BRAF-mutated, advanced cancer that had progressed on BRAF and/or MEK therapy. Solid tumor types included melanoma (n = 7), glioma (n = 5), thyroid cancer (n = 4), appendiceal carcinoma (n = 1), colorectal cancer (n = 1), non–small cell lung cancer (NSCLC; n =1), and cancer of unknown primary (n = 1). More than half of the patients had already been treated with surgery, clinical trial therapy, radiation therapy, or chemotherapy. The median adult age was 64 years; two pediatric patients were aged 10 and 13 years.
Dose-escalation revealed a maximum-tolerated dose of everolimus 5 mg orally once a day and vemurafenib 720 mg orally twice a day. Across doses, the most common grade 3 adverse events were fatigue (20%) and rash (15%), followed distantly by anemia, thrombocytopenia, hyperglycemia, or hypertriglyceridemia, which occurred in one patient each.
Responses were evaluated in 18 patients. Of these, 22% had partial responses and 50% achieved stable disease. Partial responses occurred in patients with pleomorphic xanthoastrocytoma, optic nerve glioma, melanoma, and NSCLC.
“Our trial demonstrates that the combination of vemurafenib and everolimus can be tolerated in patients with advanced malignancies,” the authors concluded. “Our trial also demonstrates that the addition of an mTOR inhibitor to everolimus treatment is able to overcome resistance to BRAF and/or MEK inhibition in a subset of patients with BRAF-mutant advanced cancers.”
The authors reported affiliations with Baxter, Bayer, Novartis, Roche, Trovagene, and others.
SOURCE: Subbiah V et al. JCO Prec Oncol. 2018 Sep 13. doi: 10.1200/PO.18.00189.
A combination of the mammalian target of rapamycin (mTOR) inhibitor everolimus and the BRAF inhibitor vemurafenib appears safe for patients with advanced, BRAF-mutated, solid tumors that had progressed on BRAF and/or MEK therapy, investigators reported.
The combination provided partial responses in a variety of tumor types, and half of the patients achieved stable disease, reported Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center in Houston and his coauthors.
“Activation of alternative parallel signaling pathways such as the PI3K–mTOR pathway have been hypothesized to contribute to primary and acquired resistance to BRAF-targeted therapy,” the authors wrote in JCO Precision Oncology. Preclinical studies have supported this hypothesis, which led to the present study; it is the first to evaluate a combination of a BRAF inhibitor and an mTOR inhibitor.
The open-label, phase 1 trial included 20 patients with BRAF-mutated, advanced cancer that had progressed on BRAF and/or MEK therapy. Solid tumor types included melanoma (n = 7), glioma (n = 5), thyroid cancer (n = 4), appendiceal carcinoma (n = 1), colorectal cancer (n = 1), non–small cell lung cancer (NSCLC; n =1), and cancer of unknown primary (n = 1). More than half of the patients had already been treated with surgery, clinical trial therapy, radiation therapy, or chemotherapy. The median adult age was 64 years; two pediatric patients were aged 10 and 13 years.
Dose-escalation revealed a maximum-tolerated dose of everolimus 5 mg orally once a day and vemurafenib 720 mg orally twice a day. Across doses, the most common grade 3 adverse events were fatigue (20%) and rash (15%), followed distantly by anemia, thrombocytopenia, hyperglycemia, or hypertriglyceridemia, which occurred in one patient each.
Responses were evaluated in 18 patients. Of these, 22% had partial responses and 50% achieved stable disease. Partial responses occurred in patients with pleomorphic xanthoastrocytoma, optic nerve glioma, melanoma, and NSCLC.
“Our trial demonstrates that the combination of vemurafenib and everolimus can be tolerated in patients with advanced malignancies,” the authors concluded. “Our trial also demonstrates that the addition of an mTOR inhibitor to everolimus treatment is able to overcome resistance to BRAF and/or MEK inhibition in a subset of patients with BRAF-mutant advanced cancers.”
The authors reported affiliations with Baxter, Bayer, Novartis, Roche, Trovagene, and others.
SOURCE: Subbiah V et al. JCO Prec Oncol. 2018 Sep 13. doi: 10.1200/PO.18.00189.
A combination of the mammalian target of rapamycin (mTOR) inhibitor everolimus and the BRAF inhibitor vemurafenib appears safe for patients with advanced, BRAF-mutated, solid tumors that had progressed on BRAF and/or MEK therapy, investigators reported.
The combination provided partial responses in a variety of tumor types, and half of the patients achieved stable disease, reported Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center in Houston and his coauthors.
“Activation of alternative parallel signaling pathways such as the PI3K–mTOR pathway have been hypothesized to contribute to primary and acquired resistance to BRAF-targeted therapy,” the authors wrote in JCO Precision Oncology. Preclinical studies have supported this hypothesis, which led to the present study; it is the first to evaluate a combination of a BRAF inhibitor and an mTOR inhibitor.
The open-label, phase 1 trial included 20 patients with BRAF-mutated, advanced cancer that had progressed on BRAF and/or MEK therapy. Solid tumor types included melanoma (n = 7), glioma (n = 5), thyroid cancer (n = 4), appendiceal carcinoma (n = 1), colorectal cancer (n = 1), non–small cell lung cancer (NSCLC; n =1), and cancer of unknown primary (n = 1). More than half of the patients had already been treated with surgery, clinical trial therapy, radiation therapy, or chemotherapy. The median adult age was 64 years; two pediatric patients were aged 10 and 13 years.
Dose-escalation revealed a maximum-tolerated dose of everolimus 5 mg orally once a day and vemurafenib 720 mg orally twice a day. Across doses, the most common grade 3 adverse events were fatigue (20%) and rash (15%), followed distantly by anemia, thrombocytopenia, hyperglycemia, or hypertriglyceridemia, which occurred in one patient each.
Responses were evaluated in 18 patients. Of these, 22% had partial responses and 50% achieved stable disease. Partial responses occurred in patients with pleomorphic xanthoastrocytoma, optic nerve glioma, melanoma, and NSCLC.
“Our trial demonstrates that the combination of vemurafenib and everolimus can be tolerated in patients with advanced malignancies,” the authors concluded. “Our trial also demonstrates that the addition of an mTOR inhibitor to everolimus treatment is able to overcome resistance to BRAF and/or MEK inhibition in a subset of patients with BRAF-mutant advanced cancers.”
The authors reported affiliations with Baxter, Bayer, Novartis, Roche, Trovagene, and others.
SOURCE: Subbiah V et al. JCO Prec Oncol. 2018 Sep 13. doi: 10.1200/PO.18.00189.
FROM JCO PRECISION ONCOLOGY
Key clinical point: A combination of the mTOR inhibitor everolimus and the BRAF inhibitor vemurafenib is safe and effective against some treatment-refractory, BRAF-mutated solid tumors.
Major finding: Twenty-two percent (22%) of patients had a partial response to therapy.
Study details: A phase I, dose-escalation trial involving 20 patients with advanced, BRAF-mutated cancer that had progressed on MEK and/or BRAF inhibitor therapy.
Disclosures: The authors reported affiliations with Bayer, Baxter, Novartis, Roche, Trovagene, and others.
Source: Subbiah V et al. JCO Prec Oncol. 2018 Sep 13. doi: 10.1200/PO.18.00189.
Repurposing Itraconazole as a Molecularl Targeted Agent for Esophageal Cancer
Background: Esophageal cancer continues to affect US veterans as the risk factors for esophageal adenocarcinoma and squamous cell carcinoma are highly prevalent in this patient population. While localized esophageal cancer can be cured with a tri-modality approach that includes neoadjuvant chemoradiation followed by esophagectomy, only those patients who achieve a pathologic complete remission to neoadjuvant chemoradiation have a 50% five-year overall survival. Those who do not achieve a pathologic complete remission or those with metastatic disease have a worse prognosis. Thus, there is a need to develop novel molecularly targeted agents for the treatment of esophageal
cancer. We have found that the Hedgehog signaling pathway, required for normal esophageal embryogenesis but silenced in the adult esophagus, is reactivated in both histologic subtypes of esophageal cancer.
Results: Using immunohistochemistry for the pathway ligand Sonic hedgehog or in situ hybridization for either Sonic hedgehog or the pathway target gene Gli1 on esophageal cancer tissue microarrays, we found that 206/346 (60%) cases were Hedgehog pathway active while normal squamous esophagus was negative. The anti-fungal agent itraconazole has previously been shown to inhibit Hedgehog signaling, and we were able to inhibit cell proliferation (cell number), Hedgehog pathway activity (quantitative real-time PCR), and VEGFR2 phosphorylation (Western blot) in vitro in OE33 esophageal adenocarcinoma cells. In a novel intraperitoneal xenograft model of liver metastases, itraconazole significantly improved overall survival in mice injected intraperitoneally with OE33 cells.
Conclusions: Based on these results we are conducting a phase 0 clinical trial administering itraconazole 300 mg po bid for 14-17 days to patients with localized esophageal cancer before neoadjuvant chemoradiation. To date, we have treated 6 patients with itraconazole and demonstrated inhibition of Hedgehog signaling by quantitative real-time PCR. It is hoped that results from this early phase trial may lead to further study and development of itraconazole as a molecularly targeted agent for esophageal cancer.
Background: Esophageal cancer continues to affect US veterans as the risk factors for esophageal adenocarcinoma and squamous cell carcinoma are highly prevalent in this patient population. While localized esophageal cancer can be cured with a tri-modality approach that includes neoadjuvant chemoradiation followed by esophagectomy, only those patients who achieve a pathologic complete remission to neoadjuvant chemoradiation have a 50% five-year overall survival. Those who do not achieve a pathologic complete remission or those with metastatic disease have a worse prognosis. Thus, there is a need to develop novel molecularly targeted agents for the treatment of esophageal
cancer. We have found that the Hedgehog signaling pathway, required for normal esophageal embryogenesis but silenced in the adult esophagus, is reactivated in both histologic subtypes of esophageal cancer.
Results: Using immunohistochemistry for the pathway ligand Sonic hedgehog or in situ hybridization for either Sonic hedgehog or the pathway target gene Gli1 on esophageal cancer tissue microarrays, we found that 206/346 (60%) cases were Hedgehog pathway active while normal squamous esophagus was negative. The anti-fungal agent itraconazole has previously been shown to inhibit Hedgehog signaling, and we were able to inhibit cell proliferation (cell number), Hedgehog pathway activity (quantitative real-time PCR), and VEGFR2 phosphorylation (Western blot) in vitro in OE33 esophageal adenocarcinoma cells. In a novel intraperitoneal xenograft model of liver metastases, itraconazole significantly improved overall survival in mice injected intraperitoneally with OE33 cells.
Conclusions: Based on these results we are conducting a phase 0 clinical trial administering itraconazole 300 mg po bid for 14-17 days to patients with localized esophageal cancer before neoadjuvant chemoradiation. To date, we have treated 6 patients with itraconazole and demonstrated inhibition of Hedgehog signaling by quantitative real-time PCR. It is hoped that results from this early phase trial may lead to further study and development of itraconazole as a molecularly targeted agent for esophageal cancer.
Background: Esophageal cancer continues to affect US veterans as the risk factors for esophageal adenocarcinoma and squamous cell carcinoma are highly prevalent in this patient population. While localized esophageal cancer can be cured with a tri-modality approach that includes neoadjuvant chemoradiation followed by esophagectomy, only those patients who achieve a pathologic complete remission to neoadjuvant chemoradiation have a 50% five-year overall survival. Those who do not achieve a pathologic complete remission or those with metastatic disease have a worse prognosis. Thus, there is a need to develop novel molecularly targeted agents for the treatment of esophageal
cancer. We have found that the Hedgehog signaling pathway, required for normal esophageal embryogenesis but silenced in the adult esophagus, is reactivated in both histologic subtypes of esophageal cancer.
Results: Using immunohistochemistry for the pathway ligand Sonic hedgehog or in situ hybridization for either Sonic hedgehog or the pathway target gene Gli1 on esophageal cancer tissue microarrays, we found that 206/346 (60%) cases were Hedgehog pathway active while normal squamous esophagus was negative. The anti-fungal agent itraconazole has previously been shown to inhibit Hedgehog signaling, and we were able to inhibit cell proliferation (cell number), Hedgehog pathway activity (quantitative real-time PCR), and VEGFR2 phosphorylation (Western blot) in vitro in OE33 esophageal adenocarcinoma cells. In a novel intraperitoneal xenograft model of liver metastases, itraconazole significantly improved overall survival in mice injected intraperitoneally with OE33 cells.
Conclusions: Based on these results we are conducting a phase 0 clinical trial administering itraconazole 300 mg po bid for 14-17 days to patients with localized esophageal cancer before neoadjuvant chemoradiation. To date, we have treated 6 patients with itraconazole and demonstrated inhibition of Hedgehog signaling by quantitative real-time PCR. It is hoped that results from this early phase trial may lead to further study and development of itraconazole as a molecularly targeted agent for esophageal cancer.
Patterns of HPV Testing Positivity, Smoking and Clinical Presentation Among Veterans With Oropharyngeal Cancer: A National Veterans Affairs Study
Purpose/Rationale: To examine HPV testing, positivity, smoking status, and clinical presentation in a national sample of veterans with oropharyngeal cancer.
Background: HPV positivity confers a favorable prognosis in patients with oropharyngeal cancer. Some data suggest that smoking may influence prognosis in HPV+ patients. However, much of our understanding of HPV+ disease originates from single institution academic centers, which may not be representative of the veteran population with respect to smoking status and other features. Multiple de-escalation trials have recently been designed for HPV+ patients, and it is important to understand disease epidemiology in veterans to determine whether the findings will be generalizable to them.
Methods: We used the Veterans Affairs Central Cancer Registry to identify patients diagnosed with oropharyngeal cancer from 2010-2015. We quantified the frequency of HPV testing and positivity over time. We calculated smoking prevalence by HPV status. We then performed logistic regression to investigate associations between smoking status and presentation with T3/N2c or higher stage disease.
Results: For the 5,231 evaluable patients, rates of HPV testing increased from 20% in 2010 to 53% in 2015. Among the patients tested in 2015: 64% were high risk HPV+, 4% low risk HPV+, and 32% HPV−. Few patients were never smokers regardless of HPV status: 11% HPV− and 18% HPV+. Greater than one third (37%) of HPV+ and half (57%) of HPV− patients were current smokers. Current smoking was associated with an increased risk of presentation with American Joint Committee on Cancer 7 stage T3, N2c, or higher disease for both HPV+ (OR 1.48, P = .019) and HPV− patients (OR 2.14, P = .002).
Conclusions/Implications: HPV testing is increasing within the VA. Among tested patients, HPV positivity rates are comparable to that of the overall population. However, compared to a number of published trials that have established treatment outcomes in HPV+ patients, a larger proportion of veterans are current smokers. We found that smoking is associated with an increased risk of advanced primary tumor or nodal stage in veterans, regardless of HPV status. Efforts should be undertaken to increase HPV testing among veterans with oropharyngeal cancer in order to more reliably establish prognosis and understand the impact of smoking.
Purpose/Rationale: To examine HPV testing, positivity, smoking status, and clinical presentation in a national sample of veterans with oropharyngeal cancer.
Background: HPV positivity confers a favorable prognosis in patients with oropharyngeal cancer. Some data suggest that smoking may influence prognosis in HPV+ patients. However, much of our understanding of HPV+ disease originates from single institution academic centers, which may not be representative of the veteran population with respect to smoking status and other features. Multiple de-escalation trials have recently been designed for HPV+ patients, and it is important to understand disease epidemiology in veterans to determine whether the findings will be generalizable to them.
Methods: We used the Veterans Affairs Central Cancer Registry to identify patients diagnosed with oropharyngeal cancer from 2010-2015. We quantified the frequency of HPV testing and positivity over time. We calculated smoking prevalence by HPV status. We then performed logistic regression to investigate associations between smoking status and presentation with T3/N2c or higher stage disease.
Results: For the 5,231 evaluable patients, rates of HPV testing increased from 20% in 2010 to 53% in 2015. Among the patients tested in 2015: 64% were high risk HPV+, 4% low risk HPV+, and 32% HPV−. Few patients were never smokers regardless of HPV status: 11% HPV− and 18% HPV+. Greater than one third (37%) of HPV+ and half (57%) of HPV− patients were current smokers. Current smoking was associated with an increased risk of presentation with American Joint Committee on Cancer 7 stage T3, N2c, or higher disease for both HPV+ (OR 1.48, P = .019) and HPV− patients (OR 2.14, P = .002).
Conclusions/Implications: HPV testing is increasing within the VA. Among tested patients, HPV positivity rates are comparable to that of the overall population. However, compared to a number of published trials that have established treatment outcomes in HPV+ patients, a larger proportion of veterans are current smokers. We found that smoking is associated with an increased risk of advanced primary tumor or nodal stage in veterans, regardless of HPV status. Efforts should be undertaken to increase HPV testing among veterans with oropharyngeal cancer in order to more reliably establish prognosis and understand the impact of smoking.
Purpose/Rationale: To examine HPV testing, positivity, smoking status, and clinical presentation in a national sample of veterans with oropharyngeal cancer.
Background: HPV positivity confers a favorable prognosis in patients with oropharyngeal cancer. Some data suggest that smoking may influence prognosis in HPV+ patients. However, much of our understanding of HPV+ disease originates from single institution academic centers, which may not be representative of the veteran population with respect to smoking status and other features. Multiple de-escalation trials have recently been designed for HPV+ patients, and it is important to understand disease epidemiology in veterans to determine whether the findings will be generalizable to them.
Methods: We used the Veterans Affairs Central Cancer Registry to identify patients diagnosed with oropharyngeal cancer from 2010-2015. We quantified the frequency of HPV testing and positivity over time. We calculated smoking prevalence by HPV status. We then performed logistic regression to investigate associations between smoking status and presentation with T3/N2c or higher stage disease.
Results: For the 5,231 evaluable patients, rates of HPV testing increased from 20% in 2010 to 53% in 2015. Among the patients tested in 2015: 64% were high risk HPV+, 4% low risk HPV+, and 32% HPV−. Few patients were never smokers regardless of HPV status: 11% HPV− and 18% HPV+. Greater than one third (37%) of HPV+ and half (57%) of HPV− patients were current smokers. Current smoking was associated with an increased risk of presentation with American Joint Committee on Cancer 7 stage T3, N2c, or higher disease for both HPV+ (OR 1.48, P = .019) and HPV− patients (OR 2.14, P = .002).
Conclusions/Implications: HPV testing is increasing within the VA. Among tested patients, HPV positivity rates are comparable to that of the overall population. However, compared to a number of published trials that have established treatment outcomes in HPV+ patients, a larger proportion of veterans are current smokers. We found that smoking is associated with an increased risk of advanced primary tumor or nodal stage in veterans, regardless of HPV status. Efforts should be undertaken to increase HPV testing among veterans with oropharyngeal cancer in order to more reliably establish prognosis and understand the impact of smoking.
A Nurse Navigation Model to Improve Coordination and Timeliness of Care in Esophageal Cancer
Purpose: Describe how case management by a nurse navigator has positively impacted the coordination and timeliness of care for Veterans newly diagnosed with esophageal cancer.
Background: Veterans with esophageal cancer have a complicated work-up and treatment course, involving many providers and ancillary services to support them through multi-modality treatment. Veterans are at high-risk for experiencing delays in care due to the complex coordination required.
Methods: A Cancer Care Navigation Team (CCNT) RN is dedicated to the case management of all newly diagnosed esophageal cancer cases. The RN completes an intake to identify barriers to completing timely work-up and accessing treatment and identifies education deficits related to their new suspicion or diagnosis. The RN provides an overview of the work-up process, identifies the providers involved in caring for the patient, and describes the ancillary supportive services, such as a specialized dietician and social worker. The RN educates the Veteran on possible treatment modalities and lodging options for treatment. The RN involves caregivers in education and treatment planning. Advance directives and release of information are completed to facilitate care planning and communication. The RN accompanies the Veteran to all treatment planning appointments with thoracic surgery, medical oncology, radiation oncology, and attends esophageal tumor board when the case is discussed. The RN facilitates communication and collaboration between providers and ancillary services as needed. The RN stays in close contact with the Veteran and caregivers throughout treatment to keep updated on the plan of care, provide support, and proactively identify barriers. The relationship developed by the RN with the patient and caregivers allows for ongoing discussions related to goals of care and, when necessary, end-of-life support.
Results: In the two years a CCNT RN has been case managing esophageal cancer cases, there has been an increase in patients verbalizing satisfaction with the education they receive about their disease, improved clustering of appointments and reduction of travel, and improved
coordination between VA and non-VA treating facilities. The multidisciplinary team has expressed increased satisfaction with the management of these cases. Next steps include formalizing a multidisciplinary clinic for esophageal cancer and finalizing esophageal cancer-specific educational materials.
Purpose: Describe how case management by a nurse navigator has positively impacted the coordination and timeliness of care for Veterans newly diagnosed with esophageal cancer.
Background: Veterans with esophageal cancer have a complicated work-up and treatment course, involving many providers and ancillary services to support them through multi-modality treatment. Veterans are at high-risk for experiencing delays in care due to the complex coordination required.
Methods: A Cancer Care Navigation Team (CCNT) RN is dedicated to the case management of all newly diagnosed esophageal cancer cases. The RN completes an intake to identify barriers to completing timely work-up and accessing treatment and identifies education deficits related to their new suspicion or diagnosis. The RN provides an overview of the work-up process, identifies the providers involved in caring for the patient, and describes the ancillary supportive services, such as a specialized dietician and social worker. The RN educates the Veteran on possible treatment modalities and lodging options for treatment. The RN involves caregivers in education and treatment planning. Advance directives and release of information are completed to facilitate care planning and communication. The RN accompanies the Veteran to all treatment planning appointments with thoracic surgery, medical oncology, radiation oncology, and attends esophageal tumor board when the case is discussed. The RN facilitates communication and collaboration between providers and ancillary services as needed. The RN stays in close contact with the Veteran and caregivers throughout treatment to keep updated on the plan of care, provide support, and proactively identify barriers. The relationship developed by the RN with the patient and caregivers allows for ongoing discussions related to goals of care and, when necessary, end-of-life support.
Results: In the two years a CCNT RN has been case managing esophageal cancer cases, there has been an increase in patients verbalizing satisfaction with the education they receive about their disease, improved clustering of appointments and reduction of travel, and improved
coordination between VA and non-VA treating facilities. The multidisciplinary team has expressed increased satisfaction with the management of these cases. Next steps include formalizing a multidisciplinary clinic for esophageal cancer and finalizing esophageal cancer-specific educational materials.
Purpose: Describe how case management by a nurse navigator has positively impacted the coordination and timeliness of care for Veterans newly diagnosed with esophageal cancer.
Background: Veterans with esophageal cancer have a complicated work-up and treatment course, involving many providers and ancillary services to support them through multi-modality treatment. Veterans are at high-risk for experiencing delays in care due to the complex coordination required.
Methods: A Cancer Care Navigation Team (CCNT) RN is dedicated to the case management of all newly diagnosed esophageal cancer cases. The RN completes an intake to identify barriers to completing timely work-up and accessing treatment and identifies education deficits related to their new suspicion or diagnosis. The RN provides an overview of the work-up process, identifies the providers involved in caring for the patient, and describes the ancillary supportive services, such as a specialized dietician and social worker. The RN educates the Veteran on possible treatment modalities and lodging options for treatment. The RN involves caregivers in education and treatment planning. Advance directives and release of information are completed to facilitate care planning and communication. The RN accompanies the Veteran to all treatment planning appointments with thoracic surgery, medical oncology, radiation oncology, and attends esophageal tumor board when the case is discussed. The RN facilitates communication and collaboration between providers and ancillary services as needed. The RN stays in close contact with the Veteran and caregivers throughout treatment to keep updated on the plan of care, provide support, and proactively identify barriers. The relationship developed by the RN with the patient and caregivers allows for ongoing discussions related to goals of care and, when necessary, end-of-life support.
Results: In the two years a CCNT RN has been case managing esophageal cancer cases, there has been an increase in patients verbalizing satisfaction with the education they receive about their disease, improved clustering of appointments and reduction of travel, and improved
coordination between VA and non-VA treating facilities. The multidisciplinary team has expressed increased satisfaction with the management of these cases. Next steps include formalizing a multidisciplinary clinic for esophageal cancer and finalizing esophageal cancer-specific educational materials.
Extramedullary plasmacytoma of the thyroid, refractory to radiation therapy and treated with bortezomib
Plasma cell neoplasms involving tissues other than the bone marrow are known as extramedullary plasmacytoma (EMP).1 EMPs mostly involve the head and neck region.2 Solitary EMP involving only the thyroid gland is very rare.3,4 Because of the limited knowledge about this condition and its rarity, its management can be challenging and is often extrapolated from plasma cell myeloma.5,6 In general, surgery or radiation are considered as front-line therapy.3,5 EMPs usually respond well to radiotherapy with almost complete remission. No definite guidelines outlining the treatment of radio-resistant EMP of the thyroid have yet been published. Data supporting the use of chemotherapy is particularly limited.4,7,8
Here, we describe the case of a 53-year-old woman with a long history of thyroiditis who presented with rapidly worsening symptomatic thyroid enlargement. She was diagnosed with EMP of the thyroid gland that was not amenable to surgery and was refractory to radiotherapy but responded to adjuvant chemotherapy with bortezomib. This report highlights 2 unique aspects of this condition: it focuses on a rare case of EMP and, as far as we know, it reports for the first time on EMP that was resistant to radiotherapy. It also highlights the need for guidelines for the treatment of EMPs.
Case presentation and summary
A 53-year-old woman presented to the emergency department with complaints of difficulty swallowing, hoarseness, and neck pain during the previous 1 month. She had a known history of Hashimoto’s thyroiditis, and an ultrasound scan of her neck 6 years previously had demonstrated diffuse thyromegaly without discrete nodules. On presentation, the patient’s vitals were stable, and a neck examination revealed a firm and enlarged thyroid without any cervical adenopathy. Laboratory investigations revealed a normal complete blood count and comprehensive metabolic panel. She had an elevated thyroid-stimulating hormone level of 13.40 mIU/L (reference range, 0.47-4.68 mIU/L) and normal thyroxine level of 4.5 pmol/L (reference range, 4.5-12.0 pmol/L). A computerized tomography (CT) scan of the neck revealed an enlarged thyroid gland (right lobe length, 10.3 cm; isthmus, 2 cm; left lobe, 8 cm) with a focal area of increased echogenicity in the midpole of the left lobe measuring 9.5 mm × 5.5 mm. The patient was discharged to home with pain medications, and urgent follow-up with an otolaryngologist was arranged. A flexible laryngoscopy was done in the otolaryngology clinic, which revealed retropharyngeal bulging that correlated with the thyromegaly evident on the CT scan.
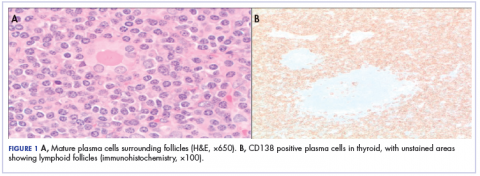
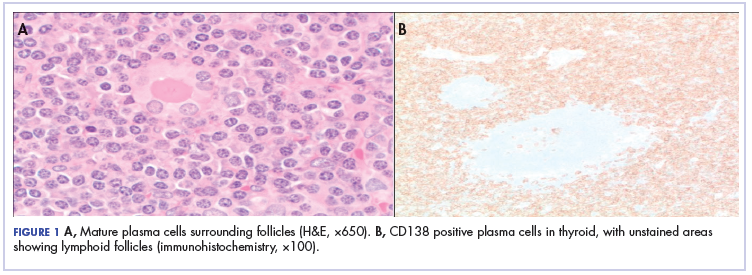
Because of the patient’s significant symptoms, we decided to proceed with surgery with a clinical diagnosis of likely thyroiditis. A left subtotal thyroidectomy with extension to the superior mediastinum was performed, but a right thyroidectomy could not be done safely. On gross examination, a well-capsulated left lobe with a tan-white, lobulated, soft cut surface was seen. Microscopic examination revealed replacement of thyroid parenchyma with sheets of mature-appearing plasma cells with eccentric round nuclei, abundant eosinophilic cytoplasm without atypia, and few scattered thyroid follicles with lymphoepithelial lesions (Figure 1A). Immunohistochemistry confirmed plasma cells with expression of CD138 (Figure 1B).
Fluorescence in situ hybridization (FISH) showed that the neoplastic plasma cells contained monotypic kappa immunoglobulin light chain messenger RNA. Clonal immunoglobulin gene rearrangement was detected on polymerase chain reaction. A diagnosis of plasmacytoma of the thyroid gland in a background of thyroiditis was made on the basis of the aforementioned observations.
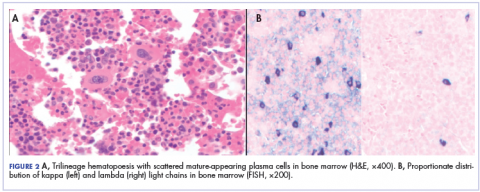
After that diagnosis, we performed an extensive work-up for plasma cell myeloma. Bone marrow biopsy showed normal maturing trilineage hematopoiesis with scattered mature-appearing plasma cells Figure 2A. Flow cytometry showed a rare (0.2%) population of polytypic plasma cells and was confirmed by CD138 immunohistochemistry. FISH showed proportionate distribution (2-5:1) of kappa and lambda light chains in plasma cells (Figure 2B).
Serum protein electrophoresis showed normal levels of serum proteins with no M spike. Serum total protein was 7.9 g/dL, albumin 5.0 g/dL, α1-globulin 0.3 g/dL, α2-globulin 0.8 g/dL, β-globulin 0.7 g/dL, and γ-globulin 1.6 g/dL, with an albumin–globulin ratio of 1.47. Calcium and β2-microglobulin were also in the normal ranges. Serum-free kappa light chain was found to be elevated (20.9 mg/L; reference range, 3.3-19.4 mg/L). The immunoglobulin G level was also elevated at 3,104 mg/dL (reference range, 700-1,600 mg/dL).
A positron-emission tomographic (PET) scan done 1 month after the surgery showed no other sites of disease except the thyroid. No lytic bone lesions were present. The patient was treated with 50.4 Gy of radiation by external beam radiotherapy to the thyroid in 28 fractions as definitive therapy. Despite treatment with surgery and radiation, she continued to have pain around the neck, and a repeat PET scan 3 months after completion of radiation showed persistent uptake in the thyroid. Because of her refractoriness to radiotherapy, she was started on systemic therapy with a weekly regimen of bortezomib and dexamethasone for 9 cycles. Her symptoms began to resolve, and a repeat PET scan done after completion of chemotherapy showed no evidence of uptake, suggesting adequate response to chemotherapy, and chemotherapy was therefore stopped. The patient was scheduled a regular follow-up in 3 months. Because of continued local symptoms in this follow-up period, the decision was made to perform surgical gland removal, and she underwent completion of
Discussion
Plasma cells are well-differentiated B-lymphocytes that secrete antibodies and provide protective immunity to the human body.9 Plasma cell neoplasms are clonal proliferation of plasma cells, producing monoclonal immunoglobulins. They are of the following different types: plasma cell myeloma, monoclonal gammopathy of unknown significance, immunoglobulin deposition disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, and plasmacytomas, which are divided into 2 types – solitary plasmacytoma of the bone, and extramedullary plasmacytoma (EMP).10 EMP is a rare condition and encompasses 3% to 5% of all plasma cell neoplasms, depending on the study.1,2,5 It is more common in men than in women (2.6:1, respectively), with equal incidence among black and white patients. Median age at diagnosis is 62 years, and it is more common among those aged 40 to 70 years.2,11 The most common sites of occurrence are the respiratory tract, the mouth, and the pharynx, but other sites such as the eyes, brain, skin, and lymph nodes may also be involved.2
EMP involving the thyroid gland is a very rare occurrence, but plasma cell myeloma has been shown to secondarily involve the thyroid.4 Similar to our report, EMP of the thyroid in the setting of thyroiditis has been reported by other authors.3,4 The incidence of EMP occurring in the thyroid varies according to different authors. Wiltshaw found 7 cases involving the thyroid out of 272 cases of EMP.1 Galieni and colleagues reported only 1 case that involved the thyroid out of 46 described cases of EMP.12
El- Siemińska and colleagues showed that levels of interleukin (IL)-6 are elevated in thyroiditis.13 IL-6 promotes monoclonal as well as polyclonal proliferation of plasma cells. Kovalchuk and colleagues showed an increase in EMP in IL-6 transgenic mice, suggesting a pathophysiologic explanation.14
The diagnostic requirements of EMP include the following: histology showing monoclonal plasma cell infiltration in tissue; bone marrow biopsy with normal plasma cell aspirate and biopsy (plasma cells, <5%); no lytic lesions on skeletal survey; no anemia, renal impairment, or hypercalcemia; and absent or low serum M protein.12
Our case fulfilled those criteria.
The treatment options for EMP include surgery, radiotherapy, or a combined approach including both. Usually, EMPs are very sensitive to radiotherapy, and complete remission can be achieved by radiotherapy alone in 80% to 100% of cases.6,11,15 Surgery is considered if the tumor is diffuse or is causing symptoms secondary to pressure on surrounding structures. A combined approach is recommended in cases with incomplete surgical margin or lymph node involvement.5,6
There is limited evidence about and experience with the use of chemotherapy in the treatment of EMP. It has been recommended that chemotherapy be considered in patients with refractory or relapsed disease using the same regimen used in plasma cell myeloma.5 Katodritou and colleagues have reported using bortezomib and dexamethasone without surgery in a solitary gastric plasmacytoma to avoid the toxicity of gastrointestinal irradiation.7 Wei and colleagues treated a patient with EMP in the pancreas with bortezomib and achieved a near-complete remission.8 To our knowledge, there is no documented literature about the treatment of EMP of the thyroid with chemotherapy. In our patient, despite the treatment with surgery and radiation, there was persistent uptake on the PET scan, so we treated her with bortezomib and dexamethasone. Because there is an 11% to 30% risk of progression to multiple myeloma, long-term follow-up is recommended in EMP.11
Conclusions
Solitary EMP of the thyroid gland is a rare condition. Plasma cell myeloma must be ruled out to make a diagnosis. Data on the incidence of EMP and its clinicopathological features are sparse, and literature describing proper guidelines on treatment is limited. It can be treated with radiotherapy, surgery, or a combined approach. There is limited data on the role of chemotherapy; our case adds to the available literature on using myeloma-based therapy in refractory disease and, to our knowledge, is the only case report using this in the literature on cases of EMP of the thyroid. Regular follow-up, even after the disease is in remission, is necessary because of the high risk of progression to plasma cell myeloma.
1. Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore). 1976;55(3):217-238.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86-94.
3. Kovacs CS, Mant MJ, Nguyen GK, Ginsberg J. Plasma cell lesions of the thyroid: report of a case of solitary plasmacytoma and a review of the literature. Thyroid. 1994;4(1):65-71.
4. Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13(2):119-123.
5. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London, United Kingdom: British Committee for Standards in Haematology; 2009.
6. Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. 2005;373-376.
7. Katodritou E, Kartsios C, Gastari V, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32(2):339-341.
8. Wei JY, Tong HY, Zhu WF, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2009;8(3):329-331.
9. Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R, Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014;85(1):15-24.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008.
11. Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305-2314.
12. Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47-51.
13. Siemińska L, Wojciechowska C, Kos-Kudła B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61(1):112-116.
14. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci US
15. Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211-215.
Plasma cell neoplasms involving tissues other than the bone marrow are known as extramedullary plasmacytoma (EMP).1 EMPs mostly involve the head and neck region.2 Solitary EMP involving only the thyroid gland is very rare.3,4 Because of the limited knowledge about this condition and its rarity, its management can be challenging and is often extrapolated from plasma cell myeloma.5,6 In general, surgery or radiation are considered as front-line therapy.3,5 EMPs usually respond well to radiotherapy with almost complete remission. No definite guidelines outlining the treatment of radio-resistant EMP of the thyroid have yet been published. Data supporting the use of chemotherapy is particularly limited.4,7,8
Here, we describe the case of a 53-year-old woman with a long history of thyroiditis who presented with rapidly worsening symptomatic thyroid enlargement. She was diagnosed with EMP of the thyroid gland that was not amenable to surgery and was refractory to radiotherapy but responded to adjuvant chemotherapy with bortezomib. This report highlights 2 unique aspects of this condition: it focuses on a rare case of EMP and, as far as we know, it reports for the first time on EMP that was resistant to radiotherapy. It also highlights the need for guidelines for the treatment of EMPs.
Case presentation and summary
A 53-year-old woman presented to the emergency department with complaints of difficulty swallowing, hoarseness, and neck pain during the previous 1 month. She had a known history of Hashimoto’s thyroiditis, and an ultrasound scan of her neck 6 years previously had demonstrated diffuse thyromegaly without discrete nodules. On presentation, the patient’s vitals were stable, and a neck examination revealed a firm and enlarged thyroid without any cervical adenopathy. Laboratory investigations revealed a normal complete blood count and comprehensive metabolic panel. She had an elevated thyroid-stimulating hormone level of 13.40 mIU/L (reference range, 0.47-4.68 mIU/L) and normal thyroxine level of 4.5 pmol/L (reference range, 4.5-12.0 pmol/L). A computerized tomography (CT) scan of the neck revealed an enlarged thyroid gland (right lobe length, 10.3 cm; isthmus, 2 cm; left lobe, 8 cm) with a focal area of increased echogenicity in the midpole of the left lobe measuring 9.5 mm × 5.5 mm. The patient was discharged to home with pain medications, and urgent follow-up with an otolaryngologist was arranged. A flexible laryngoscopy was done in the otolaryngology clinic, which revealed retropharyngeal bulging that correlated with the thyromegaly evident on the CT scan.
Because of the patient’s significant symptoms, we decided to proceed with surgery with a clinical diagnosis of likely thyroiditis. A left subtotal thyroidectomy with extension to the superior mediastinum was performed, but a right thyroidectomy could not be done safely. On gross examination, a well-capsulated left lobe with a tan-white, lobulated, soft cut surface was seen. Microscopic examination revealed replacement of thyroid parenchyma with sheets of mature-appearing plasma cells with eccentric round nuclei, abundant eosinophilic cytoplasm without atypia, and few scattered thyroid follicles with lymphoepithelial lesions (Figure 1A). Immunohistochemistry confirmed plasma cells with expression of CD138 (Figure 1B).
Fluorescence in situ hybridization (FISH) showed that the neoplastic plasma cells contained monotypic kappa immunoglobulin light chain messenger RNA. Clonal immunoglobulin gene rearrangement was detected on polymerase chain reaction. A diagnosis of plasmacytoma of the thyroid gland in a background of thyroiditis was made on the basis of the aforementioned observations.
After that diagnosis, we performed an extensive work-up for plasma cell myeloma. Bone marrow biopsy showed normal maturing trilineage hematopoiesis with scattered mature-appearing plasma cells Figure 2A. Flow cytometry showed a rare (0.2%) population of polytypic plasma cells and was confirmed by CD138 immunohistochemistry. FISH showed proportionate distribution (2-5:1) of kappa and lambda light chains in plasma cells (Figure 2B).
Serum protein electrophoresis showed normal levels of serum proteins with no M spike. Serum total protein was 7.9 g/dL, albumin 5.0 g/dL, α1-globulin 0.3 g/dL, α2-globulin 0.8 g/dL, β-globulin 0.7 g/dL, and γ-globulin 1.6 g/dL, with an albumin–globulin ratio of 1.47. Calcium and β2-microglobulin were also in the normal ranges. Serum-free kappa light chain was found to be elevated (20.9 mg/L; reference range, 3.3-19.4 mg/L). The immunoglobulin G level was also elevated at 3,104 mg/dL (reference range, 700-1,600 mg/dL).
A positron-emission tomographic (PET) scan done 1 month after the surgery showed no other sites of disease except the thyroid. No lytic bone lesions were present. The patient was treated with 50.4 Gy of radiation by external beam radiotherapy to the thyroid in 28 fractions as definitive therapy. Despite treatment with surgery and radiation, she continued to have pain around the neck, and a repeat PET scan 3 months after completion of radiation showed persistent uptake in the thyroid. Because of her refractoriness to radiotherapy, she was started on systemic therapy with a weekly regimen of bortezomib and dexamethasone for 9 cycles. Her symptoms began to resolve, and a repeat PET scan done after completion of chemotherapy showed no evidence of uptake, suggesting adequate response to chemotherapy, and chemotherapy was therefore stopped. The patient was scheduled a regular follow-up in 3 months. Because of continued local symptoms in this follow-up period, the decision was made to perform surgical gland removal, and she underwent completion of
Discussion
Plasma cells are well-differentiated B-lymphocytes that secrete antibodies and provide protective immunity to the human body.9 Plasma cell neoplasms are clonal proliferation of plasma cells, producing monoclonal immunoglobulins. They are of the following different types: plasma cell myeloma, monoclonal gammopathy of unknown significance, immunoglobulin deposition disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, and plasmacytomas, which are divided into 2 types – solitary plasmacytoma of the bone, and extramedullary plasmacytoma (EMP).10 EMP is a rare condition and encompasses 3% to 5% of all plasma cell neoplasms, depending on the study.1,2,5 It is more common in men than in women (2.6:1, respectively), with equal incidence among black and white patients. Median age at diagnosis is 62 years, and it is more common among those aged 40 to 70 years.2,11 The most common sites of occurrence are the respiratory tract, the mouth, and the pharynx, but other sites such as the eyes, brain, skin, and lymph nodes may also be involved.2
EMP involving the thyroid gland is a very rare occurrence, but plasma cell myeloma has been shown to secondarily involve the thyroid.4 Similar to our report, EMP of the thyroid in the setting of thyroiditis has been reported by other authors.3,4 The incidence of EMP occurring in the thyroid varies according to different authors. Wiltshaw found 7 cases involving the thyroid out of 272 cases of EMP.1 Galieni and colleagues reported only 1 case that involved the thyroid out of 46 described cases of EMP.12
El- Siemińska and colleagues showed that levels of interleukin (IL)-6 are elevated in thyroiditis.13 IL-6 promotes monoclonal as well as polyclonal proliferation of plasma cells. Kovalchuk and colleagues showed an increase in EMP in IL-6 transgenic mice, suggesting a pathophysiologic explanation.14
The diagnostic requirements of EMP include the following: histology showing monoclonal plasma cell infiltration in tissue; bone marrow biopsy with normal plasma cell aspirate and biopsy (plasma cells, <5%); no lytic lesions on skeletal survey; no anemia, renal impairment, or hypercalcemia; and absent or low serum M protein.12
Our case fulfilled those criteria.
The treatment options for EMP include surgery, radiotherapy, or a combined approach including both. Usually, EMPs are very sensitive to radiotherapy, and complete remission can be achieved by radiotherapy alone in 80% to 100% of cases.6,11,15 Surgery is considered if the tumor is diffuse or is causing symptoms secondary to pressure on surrounding structures. A combined approach is recommended in cases with incomplete surgical margin or lymph node involvement.5,6
There is limited evidence about and experience with the use of chemotherapy in the treatment of EMP. It has been recommended that chemotherapy be considered in patients with refractory or relapsed disease using the same regimen used in plasma cell myeloma.5 Katodritou and colleagues have reported using bortezomib and dexamethasone without surgery in a solitary gastric plasmacytoma to avoid the toxicity of gastrointestinal irradiation.7 Wei and colleagues treated a patient with EMP in the pancreas with bortezomib and achieved a near-complete remission.8 To our knowledge, there is no documented literature about the treatment of EMP of the thyroid with chemotherapy. In our patient, despite the treatment with surgery and radiation, there was persistent uptake on the PET scan, so we treated her with bortezomib and dexamethasone. Because there is an 11% to 30% risk of progression to multiple myeloma, long-term follow-up is recommended in EMP.11
Conclusions
Solitary EMP of the thyroid gland is a rare condition. Plasma cell myeloma must be ruled out to make a diagnosis. Data on the incidence of EMP and its clinicopathological features are sparse, and literature describing proper guidelines on treatment is limited. It can be treated with radiotherapy, surgery, or a combined approach. There is limited data on the role of chemotherapy; our case adds to the available literature on using myeloma-based therapy in refractory disease and, to our knowledge, is the only case report using this in the literature on cases of EMP of the thyroid. Regular follow-up, even after the disease is in remission, is necessary because of the high risk of progression to plasma cell myeloma.
Plasma cell neoplasms involving tissues other than the bone marrow are known as extramedullary plasmacytoma (EMP).1 EMPs mostly involve the head and neck region.2 Solitary EMP involving only the thyroid gland is very rare.3,4 Because of the limited knowledge about this condition and its rarity, its management can be challenging and is often extrapolated from plasma cell myeloma.5,6 In general, surgery or radiation are considered as front-line therapy.3,5 EMPs usually respond well to radiotherapy with almost complete remission. No definite guidelines outlining the treatment of radio-resistant EMP of the thyroid have yet been published. Data supporting the use of chemotherapy is particularly limited.4,7,8
Here, we describe the case of a 53-year-old woman with a long history of thyroiditis who presented with rapidly worsening symptomatic thyroid enlargement. She was diagnosed with EMP of the thyroid gland that was not amenable to surgery and was refractory to radiotherapy but responded to adjuvant chemotherapy with bortezomib. This report highlights 2 unique aspects of this condition: it focuses on a rare case of EMP and, as far as we know, it reports for the first time on EMP that was resistant to radiotherapy. It also highlights the need for guidelines for the treatment of EMPs.
Case presentation and summary
A 53-year-old woman presented to the emergency department with complaints of difficulty swallowing, hoarseness, and neck pain during the previous 1 month. She had a known history of Hashimoto’s thyroiditis, and an ultrasound scan of her neck 6 years previously had demonstrated diffuse thyromegaly without discrete nodules. On presentation, the patient’s vitals were stable, and a neck examination revealed a firm and enlarged thyroid without any cervical adenopathy. Laboratory investigations revealed a normal complete blood count and comprehensive metabolic panel. She had an elevated thyroid-stimulating hormone level of 13.40 mIU/L (reference range, 0.47-4.68 mIU/L) and normal thyroxine level of 4.5 pmol/L (reference range, 4.5-12.0 pmol/L). A computerized tomography (CT) scan of the neck revealed an enlarged thyroid gland (right lobe length, 10.3 cm; isthmus, 2 cm; left lobe, 8 cm) with a focal area of increased echogenicity in the midpole of the left lobe measuring 9.5 mm × 5.5 mm. The patient was discharged to home with pain medications, and urgent follow-up with an otolaryngologist was arranged. A flexible laryngoscopy was done in the otolaryngology clinic, which revealed retropharyngeal bulging that correlated with the thyromegaly evident on the CT scan.
Because of the patient’s significant symptoms, we decided to proceed with surgery with a clinical diagnosis of likely thyroiditis. A left subtotal thyroidectomy with extension to the superior mediastinum was performed, but a right thyroidectomy could not be done safely. On gross examination, a well-capsulated left lobe with a tan-white, lobulated, soft cut surface was seen. Microscopic examination revealed replacement of thyroid parenchyma with sheets of mature-appearing plasma cells with eccentric round nuclei, abundant eosinophilic cytoplasm without atypia, and few scattered thyroid follicles with lymphoepithelial lesions (Figure 1A). Immunohistochemistry confirmed plasma cells with expression of CD138 (Figure 1B).
Fluorescence in situ hybridization (FISH) showed that the neoplastic plasma cells contained monotypic kappa immunoglobulin light chain messenger RNA. Clonal immunoglobulin gene rearrangement was detected on polymerase chain reaction. A diagnosis of plasmacytoma of the thyroid gland in a background of thyroiditis was made on the basis of the aforementioned observations.
After that diagnosis, we performed an extensive work-up for plasma cell myeloma. Bone marrow biopsy showed normal maturing trilineage hematopoiesis with scattered mature-appearing plasma cells Figure 2A. Flow cytometry showed a rare (0.2%) population of polytypic plasma cells and was confirmed by CD138 immunohistochemistry. FISH showed proportionate distribution (2-5:1) of kappa and lambda light chains in plasma cells (Figure 2B).
Serum protein electrophoresis showed normal levels of serum proteins with no M spike. Serum total protein was 7.9 g/dL, albumin 5.0 g/dL, α1-globulin 0.3 g/dL, α2-globulin 0.8 g/dL, β-globulin 0.7 g/dL, and γ-globulin 1.6 g/dL, with an albumin–globulin ratio of 1.47. Calcium and β2-microglobulin were also in the normal ranges. Serum-free kappa light chain was found to be elevated (20.9 mg/L; reference range, 3.3-19.4 mg/L). The immunoglobulin G level was also elevated at 3,104 mg/dL (reference range, 700-1,600 mg/dL).
A positron-emission tomographic (PET) scan done 1 month after the surgery showed no other sites of disease except the thyroid. No lytic bone lesions were present. The patient was treated with 50.4 Gy of radiation by external beam radiotherapy to the thyroid in 28 fractions as definitive therapy. Despite treatment with surgery and radiation, she continued to have pain around the neck, and a repeat PET scan 3 months after completion of radiation showed persistent uptake in the thyroid. Because of her refractoriness to radiotherapy, she was started on systemic therapy with a weekly regimen of bortezomib and dexamethasone for 9 cycles. Her symptoms began to resolve, and a repeat PET scan done after completion of chemotherapy showed no evidence of uptake, suggesting adequate response to chemotherapy, and chemotherapy was therefore stopped. The patient was scheduled a regular follow-up in 3 months. Because of continued local symptoms in this follow-up period, the decision was made to perform surgical gland removal, and she underwent completion of
Discussion
Plasma cells are well-differentiated B-lymphocytes that secrete antibodies and provide protective immunity to the human body.9 Plasma cell neoplasms are clonal proliferation of plasma cells, producing monoclonal immunoglobulins. They are of the following different types: plasma cell myeloma, monoclonal gammopathy of unknown significance, immunoglobulin deposition disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, and plasmacytomas, which are divided into 2 types – solitary plasmacytoma of the bone, and extramedullary plasmacytoma (EMP).10 EMP is a rare condition and encompasses 3% to 5% of all plasma cell neoplasms, depending on the study.1,2,5 It is more common in men than in women (2.6:1, respectively), with equal incidence among black and white patients. Median age at diagnosis is 62 years, and it is more common among those aged 40 to 70 years.2,11 The most common sites of occurrence are the respiratory tract, the mouth, and the pharynx, but other sites such as the eyes, brain, skin, and lymph nodes may also be involved.2
EMP involving the thyroid gland is a very rare occurrence, but plasma cell myeloma has been shown to secondarily involve the thyroid.4 Similar to our report, EMP of the thyroid in the setting of thyroiditis has been reported by other authors.3,4 The incidence of EMP occurring in the thyroid varies according to different authors. Wiltshaw found 7 cases involving the thyroid out of 272 cases of EMP.1 Galieni and colleagues reported only 1 case that involved the thyroid out of 46 described cases of EMP.12
El- Siemińska and colleagues showed that levels of interleukin (IL)-6 are elevated in thyroiditis.13 IL-6 promotes monoclonal as well as polyclonal proliferation of plasma cells. Kovalchuk and colleagues showed an increase in EMP in IL-6 transgenic mice, suggesting a pathophysiologic explanation.14
The diagnostic requirements of EMP include the following: histology showing monoclonal plasma cell infiltration in tissue; bone marrow biopsy with normal plasma cell aspirate and biopsy (plasma cells, <5%); no lytic lesions on skeletal survey; no anemia, renal impairment, or hypercalcemia; and absent or low serum M protein.12
Our case fulfilled those criteria.
The treatment options for EMP include surgery, radiotherapy, or a combined approach including both. Usually, EMPs are very sensitive to radiotherapy, and complete remission can be achieved by radiotherapy alone in 80% to 100% of cases.6,11,15 Surgery is considered if the tumor is diffuse or is causing symptoms secondary to pressure on surrounding structures. A combined approach is recommended in cases with incomplete surgical margin or lymph node involvement.5,6
There is limited evidence about and experience with the use of chemotherapy in the treatment of EMP. It has been recommended that chemotherapy be considered in patients with refractory or relapsed disease using the same regimen used in plasma cell myeloma.5 Katodritou and colleagues have reported using bortezomib and dexamethasone without surgery in a solitary gastric plasmacytoma to avoid the toxicity of gastrointestinal irradiation.7 Wei and colleagues treated a patient with EMP in the pancreas with bortezomib and achieved a near-complete remission.8 To our knowledge, there is no documented literature about the treatment of EMP of the thyroid with chemotherapy. In our patient, despite the treatment with surgery and radiation, there was persistent uptake on the PET scan, so we treated her with bortezomib and dexamethasone. Because there is an 11% to 30% risk of progression to multiple myeloma, long-term follow-up is recommended in EMP.11
Conclusions
Solitary EMP of the thyroid gland is a rare condition. Plasma cell myeloma must be ruled out to make a diagnosis. Data on the incidence of EMP and its clinicopathological features are sparse, and literature describing proper guidelines on treatment is limited. It can be treated with radiotherapy, surgery, or a combined approach. There is limited data on the role of chemotherapy; our case adds to the available literature on using myeloma-based therapy in refractory disease and, to our knowledge, is the only case report using this in the literature on cases of EMP of the thyroid. Regular follow-up, even after the disease is in remission, is necessary because of the high risk of progression to plasma cell myeloma.
1. Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore). 1976;55(3):217-238.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86-94.
3. Kovacs CS, Mant MJ, Nguyen GK, Ginsberg J. Plasma cell lesions of the thyroid: report of a case of solitary plasmacytoma and a review of the literature. Thyroid. 1994;4(1):65-71.
4. Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13(2):119-123.
5. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London, United Kingdom: British Committee for Standards in Haematology; 2009.
6. Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. 2005;373-376.
7. Katodritou E, Kartsios C, Gastari V, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32(2):339-341.
8. Wei JY, Tong HY, Zhu WF, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2009;8(3):329-331.
9. Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R, Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014;85(1):15-24.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008.
11. Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305-2314.
12. Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47-51.
13. Siemińska L, Wojciechowska C, Kos-Kudła B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61(1):112-116.
14. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci US
15. Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211-215.
1. Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore). 1976;55(3):217-238.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86-94.
3. Kovacs CS, Mant MJ, Nguyen GK, Ginsberg J. Plasma cell lesions of the thyroid: report of a case of solitary plasmacytoma and a review of the literature. Thyroid. 1994;4(1):65-71.
4. Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13(2):119-123.
5. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London, United Kingdom: British Committee for Standards in Haematology; 2009.
6. Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. 2005;373-376.
7. Katodritou E, Kartsios C, Gastari V, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32(2):339-341.
8. Wei JY, Tong HY, Zhu WF, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2009;8(3):329-331.
9. Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R, Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014;85(1):15-24.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008.
11. Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305-2314.
12. Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47-51.
13. Siemińska L, Wojciechowska C, Kos-Kudła B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61(1):112-116.
14. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci US
15. Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211-215.
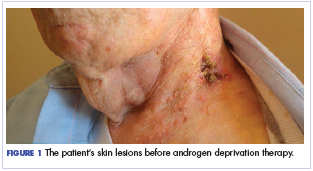
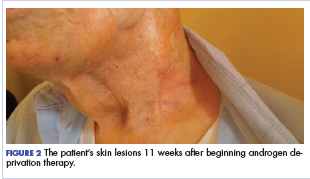
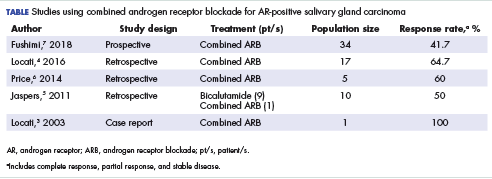
Salivary ductal adenocarcinoma with complete response to androgen blockade
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
Meeting the potential of immunotherapy: new targets provide rational combinations
The relationship between the immune system and tumors is complex and dynamic, and for immunotherapy to reach its full potential it will likely need to attack on multiple fronts. Here, we discuss some of the latest and most promising developments in the immuno-oncology field designed to build on the successes and address limitations.
The anti-tumor immune response
Cancer is a disease of genomic instability, whereby genetic alterations ranging from a single nucleotide to the whole chromosome level frequently occur. Although cancers derive from a patient’s own tissues, these genetic differences can mark the cancer cell as non-self, triggering an immune response to eliminate these cells.
The first hints of this anti-tumor immunity date back more than a century and a half and sparked the concept of mobilizing the immune system to treat patients.1-3 Although early pioneers achieved little progress in this regard, their efforts provided invaluable insights into the complex and dynamic relationship between a tumor and the immune system that are now translating into real clinical successes.
We now understand that the immune system has a dual role in both restraining and promoting cancer development and have translated this understanding into the theory of cancer immunoediting. Immunoediting has three stages: elimination, wherein the tumor is seemingly destroyed by the innate and adaptive immune response; equilibrium, in which cancer cells that were able to escape elimination are selected for growth; and escape, whereby these resistant cancer cells overwhelm the immune system and develop into a symptomatic lesion.4,5
Immuno-oncologists have also described the cancer immunity cycle to capture the steps that are required for an effective anti-tumor immune response and defects in this cycle form the basis of the most common mechanisms used by cancer cells to subvert the anti-tumor immune response. Much like the cancer hallmarks did for molecularly targeted cancer drugs, the cancer immunity cycle serves as the intellectual framework for cancer immunotherapy.6,7
Exploiting nature’s weapon of mass destruction
Initially, attempts at immunotherapy focused on boosting the immune response using adjuvants and cytokines. The characterization of subtle differences between tumor cells and normal cells led to the development of vaccines and cell-based therapies that exploited these tumor-associated antigens (TAAs).1-6
Despite the approval of a therapeutic vaccine, sipuleucel-T, in 2010 for the treatment of metastatic prostate cancer, in general the success of vaccines has been limited. Marketing authorization for sipuleucel-T was recently withdrawn in Europe, and although it is still available in the United States, it is not widely used because of issues with production and administration. Other vaccines, such as GVAX, which looked particularly promising in early-stage clinical trials, failed to show clinical efficacy in subsequent testing.8,9
Cell-based therapies, such as adoptive cellular therapy (ACT), in which immune cells are removed from the host, primed to attack cancer cells, and then reinfused back into the patient, have focused on T cells because they are the major effectors of the adaptive immune response. Clinical success with the most common approach, tumor-infiltrating lymphocyte (TIL)
Two key techniques have been developed (Figure 1). T-cell receptor (TCR) therapy involves genetically modifying the receptor on the surface of T cells that is responsible for recognizing antigens bound to major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs). The TCR can be altered to recognize a specific TAA or modified to improve its antigen recognition and binding capabilities. This type of therapy is limited by the fact that the TCRs need to be genetically matched to the patient’s immune type.
Releasing the brakes
To ensure that it is only activated at the appropriate time and not in response to the antigens expressed on the surface of the host’s own tissues or harmless materials, the immune system has developed numerous mechanisms for immunological tolerance. Cancer cells are able to exploit these mechanisms to allow them to evade the anti-tumor immune response. One of the main ways in which they do this is by manipulating the signaling pathways involved in T-cell activation, which play a vital role in tolerance.12
To become fully activated, T cells require a primary signal generated by an interaction between the TCR and the antigen-MHC complex on the surface of an APC, followed by secondary costimulatory signals generated by a range of different receptors present on the T-cell surface binding to their ligands on the APC.
If the second signal is inhibitory rather than stimulatory, then the T cell is deactivated instead of becoming activated. Two key coinhibitory receptors are programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) and tumor cells are able to overcome the anti-tumor immune response in part by expressing the ligands that bind these receptors to dampen the activity of tumor-infiltrating T cells and induce tolerance.13
The development of inhibitors of CTLA-4 and PD-1 and their respective ligands has driven some of the most dramatic successes with cancer immunotherapy, particularly with PD-1-targeting drugs which have fewer side effects. Targeting of this pathway has resulted in durable responses, revolutionizing the treatment of metastatic melanoma, with recently published long-term survival data for pembrolizumab showing that 40% of patients were alive 3 years after initiating treatment and, in a separate study, 34% of nivolumab-treated patients were still alive after 5 years.14,15 More recently, PD-1 inhibitors have been slowly expanding into a range of other cancer types and 4 immune checkpoint inhibitors are now approved by the United States Food and Drug Administration (FDA): ipilimumab (Yervoy), nivolumab (Opdivo), pembrolizumab (Keytruda) and atezolizumab (Tecentriq).
Six years on from the first approval in this drug class and an extensive network of coinhibitory receptors has been uncovered – so-called immune checkpoints – many of which are now also serving as therapeutic targets (Table, Figure 2).16 Lymphocyte activation gene 3 (LAG-3) is a member of the immunoglobulin superfamily of receptors that is expressed on a number of different types of immune cell. In addition to negatively regulating cytotoxic T-cell activation like PD-1 and CTLA-4, it is also thought to regulate the immunosuppressive functions of regulatory T cells and the maturation and activation of dendritic cells. T-cell immunoglobulin and mucin domain-containing 3 (TIM-3) is found on the surface of helper and cytotoxic T cells and regulates T-cell inhibition as well as macrophage activation. Inhibitors of both proteins have been developed that are being evaluated in phase 1 or 2 clinical trials in a variety of tumor types.17
Indeed, although T cells have commanded the most attention, there is growing appreciation of the potential for targeting other types of immune cell that play a role in the anti-tumor immune response or in fostering an immunosuppressive microenvironment. NK cells have been a particular focus, since they represent the body’s first line of immune defense and they appear to have analogous inhibitory and activating receptors expressed on their surface that regulate their cytotoxic activity.
The best-defined NK cell receptors are the killer cell immunoglobulin-like receptors (KIRs) that bind to the MHC class I proteins found on the surface of all cells that distinguish them as ‘self’ or ‘non-self’. KIRs can be either activating or inhibitory, depending upon their structure and the ligands to which they bind.19 To date, 2 antibodies targeting inhibitory KIRs have been developed. Though there has been some disappointment with these drugs, most recently a phase 2 trial of lirilumab in elderly patients with acute myeloid leukemia, which missed its primary endpoint, they continue to be evaluated in clinical trials.20
The inhibitory immune checkpoint field has also expanded to include molecules that regulate T-cell activity in other ways. Most prominently, this includes enzymes like indoleamine-2,3 dioxygenase (IDO), which is involved in the metabolism of the essential amino acid tryptophan. IDO-induced depletion of tryptophan and generation of tryptophan metabolites is toxic to cytotoxic T cells, and IDO is also thought to directly activate regulatory T cells, thus the net effect of IDO is immunosuppression. Two IDO inhibitors are currently being developed.21
Stepping on the gas
Despite their unprecedented success, immune checkpoint inhibitors are not effective in all patients or in all tumor types. Their efficacy is limited in large part by the requirement for a pre-existing anti-tumor immune response. If there are no T cells within the tumor microenvironment then releasing the brakes on the immune system won’t help.
More recently, researchers have returned to the idea of stimulating an anti-tumor immune response, this time by targeting the other side of the immune checkpoint coin, the costimulatory molecules. These drugs could prove more effective as they aren’t reliant on a pre-existing anti-tumor immune response. A number of agonist antibodies designed to target these receptors have now been developed and are undergoing clinical evaluation.22
Furthest along in development are those targeting OX40, a costimulatory molecule that is upregulated on the surface of T cells once they have been fully activated by the TCR signal and an initial costimulatory signal. OX40 is thought to be involved in a more long-term immune response and in the formation of a memory response. A mouse monoclonal antibody had a potent immune-stimulating effect accompanied by the regression of at least 1 metastatic lesion in 30% of patients treated in a phase 1 clinical trial, but was limited by the generation of anti-mouse antibodies. 7 OX40 agonists are now in clinical development, 6 fully human monoclonal antibodies and 1 OX40 ligand-Fc fusion protein, MEDI-6383.23
Combinations are key
Many researchers are now reaching the conclusion that combination therapy is likely to be key in expanding the scope of immunotherapy into currently unresponsive patient populations. Investigating rational combinations is already becoming a burgeoning area of the immuno-oncology field, with a variety of different strategies being tested.
Now the question becomes what are the optimal combinations and the timing and sequencing of combination therapy is likely to be a paramount consideration. Developing combinations that have distinct mechanisms of action or target multiple steps in the cancer immunity cycle offers the greatest potential for therapeutic synergy since this is most likely to address potential mechanisms of resistance by blocking other paths to immune evasion for cancer cells (Figure 3).
Given the expanding network of immune-checkpoint inhibitors and agonists, the focal point of combination therapy has been combining immune checkpoint-targeting drugs with different mechanisms of action, including those that would simultaneously release the brakes and step on the gas pedal. The vast majority of ongoing clinical trials of approved checkpoint inhibitors and the drugs in development listed in the table are combination trials.
These efforts yielded the first FDA-approved combination immunotherapy regimen in 2015; nivolumab and ipilimumab for the treatment of metastatic melanoma. Approval was based on the demonstration of improved ORR, prolonged response duration, and improved progression-free survival among 142 patients treated with the combination, compared to either drug alone.24
The results of a phase 1/2 trial evaluating the combination of a 4-1BB receptor agonist urelumab with nivolumab in hematologic malignancies and solid tumors found the combination to be safe and particularly effective in patients with advanced/metastatic melanoma, with an ORR of 50%.25 Nivolumab was also combined with the CD27 agonist varlilumab in a phase 1/2 clinical trial of patients with solid tumors, for which data was also recently released. Among 46 patients enrolled, primarily those with colorectal and ovarian cancer the combination had an acceptable safety profile and favorable changes in intratumoral immune biomarkers were observed. The phase 2 portion of the trial is ongoing.26
Meanwhile, Incyte’s IDO inhibitor epacadostat has recently been making waves in combination with pembrolizumab in patients with advanced solid tumors. It demonstrated particularly promising clinical activity in patients with metastatic melanoma, with an overall response rate (ORR) of 57%, including 2 complete responses (CRs), prompting initiation of a phase 3 trial of this combination (NCT02752074).27
1. Adams JL, Smothers J, Srinivasan R, et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Disc. 2015;14:603-622.
2. D’Errico G, Machado HL, Sainz Jr B. A current perspective on cancer immune therapy: step-by-step approach to constructing the magic bullet. Clin Trans Med. 2017;6:3.
3. Farkona S, Diamandis EP, Blaustig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
4. Meiliana A, Dewi NM, Wijaya A. Cancer immunotherapy: a review. Indones Biomed J. 2016;8(1):1-20.
5. Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumor microenvironment. Nat Rev Clin Oncol. 2016;13:143-158.
6. de Charette M, Marabelle A, Houot R. Turning tumor cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur J Cancer 2016;68:134-147.
7. Chen DS and Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013;39:1-10.
8. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-489.
9. Le DT, Wang-Gillam A, Picozzi V Jr, et al. A phase 2, randomized trial of GVAX Pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. Presented at: the ASCO Gastrointestinal Cancers Symposium; January 16-18, 2014; San Francisco, CA. Abstract 177.
10. Sharpe M and Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
11. Perica K, Varela JC, Oelke M, et al. Adoptive T Cell Immunotherapy for Cancer. Ram Mai Med J. 2015;6(1):e0004.
12. Xing Y and Hogquist KA. T-Cell Tolerance: Central and Peripheral. Cold Spring Harb Perspect Biol. 2012;4:a006957.
13. Buchbinder EI and Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98-106.
14. Robert C, Ribas A, Hamid O, et al. 3-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. 2016(suppl;abstr 9503).
15. Hodi SF, Kluger HM, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Presented at the 2016 AACR Annual Meeting; April 16-20; New Orleans, LA. Abstract CT001.
16. Bakdash G, Sittig SP, van Dijk T, et al. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front Immunol. 2013;4(53):1-18.
17. Sheridan C. Immuno-oncology moves beyond PD-1. Nat Biotechnol. 2015;33(7):673-675.
18. Blake SJ, Dougall WC, Miles JJ, et al. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin Cancer Res. 2016;22(21):5183-5188.
19. Carotta S. Targeting NK cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol. 2016;7:152.
20. Innate Pharma Web site. Innate Pharma Announces Top-Line Results from EFFIKIR Trial Evaluating the Efficacy of Lirilumab as a Single Agent in Elderly Patients with Acute Myeloid Leukemia. http://www.innate-pharma.com/en/news-events/press-releases/innate-pharma-announces-top-line-results-effikir-trial-evaluating-efficacy-lirilumab-single-agent-elderly-patients-acute-myeloid-leukemia. Last updated February 6, 2017. Accessed online February 22, 2017.
21. Sheridan C. IDO inhibitors move center stage in immuno-oncology. Nat Biotechnol. 2015;33(4):321-322.
22. Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640-655.
23. Linch SN, McNamara MJ, Redmond WL. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34.
24. U.S. Food and Drug Administration Web site. Nivolumab in combination with ipilimumab. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm465274.htm. Last updated October 1, 2015. Accessed online February 22, 2017.
25. Massarelli E. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. Presented at the 31st Annual Meeting of the Society for the Immunotherapy of Cancer; November 9-13, 2016; National Harbor, MD. Abstract 239.
26. Sanborn RE, Pishvain MJ, Callahan MK, et al. Phase I results from the combination of an immune-activating anti-CD27 antibody (varlilumab) in combination with PD-1 blockade (nivolumab): activation across multiple immune pathways without untoward immune-related adverse events. Clin Cancer Res. 2016;76(14):suppl. Abstract CT023.
27. Gangadhar T, Hamid O, Smith D.C, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: updated phase 1 results from ECHO-202/KEYNOTE-037. Ann Oncol. 2016;27(6):379-400.
The relationship between the immune system and tumors is complex and dynamic, and for immunotherapy to reach its full potential it will likely need to attack on multiple fronts. Here, we discuss some of the latest and most promising developments in the immuno-oncology field designed to build on the successes and address limitations.
The anti-tumor immune response
Cancer is a disease of genomic instability, whereby genetic alterations ranging from a single nucleotide to the whole chromosome level frequently occur. Although cancers derive from a patient’s own tissues, these genetic differences can mark the cancer cell as non-self, triggering an immune response to eliminate these cells.
The first hints of this anti-tumor immunity date back more than a century and a half and sparked the concept of mobilizing the immune system to treat patients.1-3 Although early pioneers achieved little progress in this regard, their efforts provided invaluable insights into the complex and dynamic relationship between a tumor and the immune system that are now translating into real clinical successes.
We now understand that the immune system has a dual role in both restraining and promoting cancer development and have translated this understanding into the theory of cancer immunoediting. Immunoediting has three stages: elimination, wherein the tumor is seemingly destroyed by the innate and adaptive immune response; equilibrium, in which cancer cells that were able to escape elimination are selected for growth; and escape, whereby these resistant cancer cells overwhelm the immune system and develop into a symptomatic lesion.4,5
Immuno-oncologists have also described the cancer immunity cycle to capture the steps that are required for an effective anti-tumor immune response and defects in this cycle form the basis of the most common mechanisms used by cancer cells to subvert the anti-tumor immune response. Much like the cancer hallmarks did for molecularly targeted cancer drugs, the cancer immunity cycle serves as the intellectual framework for cancer immunotherapy.6,7
Exploiting nature’s weapon of mass destruction
Initially, attempts at immunotherapy focused on boosting the immune response using adjuvants and cytokines. The characterization of subtle differences between tumor cells and normal cells led to the development of vaccines and cell-based therapies that exploited these tumor-associated antigens (TAAs).1-6
Despite the approval of a therapeutic vaccine, sipuleucel-T, in 2010 for the treatment of metastatic prostate cancer, in general the success of vaccines has been limited. Marketing authorization for sipuleucel-T was recently withdrawn in Europe, and although it is still available in the United States, it is not widely used because of issues with production and administration. Other vaccines, such as GVAX, which looked particularly promising in early-stage clinical trials, failed to show clinical efficacy in subsequent testing.8,9
Cell-based therapies, such as adoptive cellular therapy (ACT), in which immune cells are removed from the host, primed to attack cancer cells, and then reinfused back into the patient, have focused on T cells because they are the major effectors of the adaptive immune response. Clinical success with the most common approach, tumor-infiltrating lymphocyte (TIL)
Two key techniques have been developed (Figure 1). T-cell receptor (TCR) therapy involves genetically modifying the receptor on the surface of T cells that is responsible for recognizing antigens bound to major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs). The TCR can be altered to recognize a specific TAA or modified to improve its antigen recognition and binding capabilities. This type of therapy is limited by the fact that the TCRs need to be genetically matched to the patient’s immune type.
Releasing the brakes
To ensure that it is only activated at the appropriate time and not in response to the antigens expressed on the surface of the host’s own tissues or harmless materials, the immune system has developed numerous mechanisms for immunological tolerance. Cancer cells are able to exploit these mechanisms to allow them to evade the anti-tumor immune response. One of the main ways in which they do this is by manipulating the signaling pathways involved in T-cell activation, which play a vital role in tolerance.12
To become fully activated, T cells require a primary signal generated by an interaction between the TCR and the antigen-MHC complex on the surface of an APC, followed by secondary costimulatory signals generated by a range of different receptors present on the T-cell surface binding to their ligands on the APC.
If the second signal is inhibitory rather than stimulatory, then the T cell is deactivated instead of becoming activated. Two key coinhibitory receptors are programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) and tumor cells are able to overcome the anti-tumor immune response in part by expressing the ligands that bind these receptors to dampen the activity of tumor-infiltrating T cells and induce tolerance.13
The development of inhibitors of CTLA-4 and PD-1 and their respective ligands has driven some of the most dramatic successes with cancer immunotherapy, particularly with PD-1-targeting drugs which have fewer side effects. Targeting of this pathway has resulted in durable responses, revolutionizing the treatment of metastatic melanoma, with recently published long-term survival data for pembrolizumab showing that 40% of patients were alive 3 years after initiating treatment and, in a separate study, 34% of nivolumab-treated patients were still alive after 5 years.14,15 More recently, PD-1 inhibitors have been slowly expanding into a range of other cancer types and 4 immune checkpoint inhibitors are now approved by the United States Food and Drug Administration (FDA): ipilimumab (Yervoy), nivolumab (Opdivo), pembrolizumab (Keytruda) and atezolizumab (Tecentriq).
Six years on from the first approval in this drug class and an extensive network of coinhibitory receptors has been uncovered – so-called immune checkpoints – many of which are now also serving as therapeutic targets (Table, Figure 2).16 Lymphocyte activation gene 3 (LAG-3) is a member of the immunoglobulin superfamily of receptors that is expressed on a number of different types of immune cell. In addition to negatively regulating cytotoxic T-cell activation like PD-1 and CTLA-4, it is also thought to regulate the immunosuppressive functions of regulatory T cells and the maturation and activation of dendritic cells. T-cell immunoglobulin and mucin domain-containing 3 (TIM-3) is found on the surface of helper and cytotoxic T cells and regulates T-cell inhibition as well as macrophage activation. Inhibitors of both proteins have been developed that are being evaluated in phase 1 or 2 clinical trials in a variety of tumor types.17
Indeed, although T cells have commanded the most attention, there is growing appreciation of the potential for targeting other types of immune cell that play a role in the anti-tumor immune response or in fostering an immunosuppressive microenvironment. NK cells have been a particular focus, since they represent the body’s first line of immune defense and they appear to have analogous inhibitory and activating receptors expressed on their surface that regulate their cytotoxic activity.
The best-defined NK cell receptors are the killer cell immunoglobulin-like receptors (KIRs) that bind to the MHC class I proteins found on the surface of all cells that distinguish them as ‘self’ or ‘non-self’. KIRs can be either activating or inhibitory, depending upon their structure and the ligands to which they bind.19 To date, 2 antibodies targeting inhibitory KIRs have been developed. Though there has been some disappointment with these drugs, most recently a phase 2 trial of lirilumab in elderly patients with acute myeloid leukemia, which missed its primary endpoint, they continue to be evaluated in clinical trials.20
The inhibitory immune checkpoint field has also expanded to include molecules that regulate T-cell activity in other ways. Most prominently, this includes enzymes like indoleamine-2,3 dioxygenase (IDO), which is involved in the metabolism of the essential amino acid tryptophan. IDO-induced depletion of tryptophan and generation of tryptophan metabolites is toxic to cytotoxic T cells, and IDO is also thought to directly activate regulatory T cells, thus the net effect of IDO is immunosuppression. Two IDO inhibitors are currently being developed.21
Stepping on the gas
Despite their unprecedented success, immune checkpoint inhibitors are not effective in all patients or in all tumor types. Their efficacy is limited in large part by the requirement for a pre-existing anti-tumor immune response. If there are no T cells within the tumor microenvironment then releasing the brakes on the immune system won’t help.
More recently, researchers have returned to the idea of stimulating an anti-tumor immune response, this time by targeting the other side of the immune checkpoint coin, the costimulatory molecules. These drugs could prove more effective as they aren’t reliant on a pre-existing anti-tumor immune response. A number of agonist antibodies designed to target these receptors have now been developed and are undergoing clinical evaluation.22
Furthest along in development are those targeting OX40, a costimulatory molecule that is upregulated on the surface of T cells once they have been fully activated by the TCR signal and an initial costimulatory signal. OX40 is thought to be involved in a more long-term immune response and in the formation of a memory response. A mouse monoclonal antibody had a potent immune-stimulating effect accompanied by the regression of at least 1 metastatic lesion in 30% of patients treated in a phase 1 clinical trial, but was limited by the generation of anti-mouse antibodies. 7 OX40 agonists are now in clinical development, 6 fully human monoclonal antibodies and 1 OX40 ligand-Fc fusion protein, MEDI-6383.23
Combinations are key
Many researchers are now reaching the conclusion that combination therapy is likely to be key in expanding the scope of immunotherapy into currently unresponsive patient populations. Investigating rational combinations is already becoming a burgeoning area of the immuno-oncology field, with a variety of different strategies being tested.
Now the question becomes what are the optimal combinations and the timing and sequencing of combination therapy is likely to be a paramount consideration. Developing combinations that have distinct mechanisms of action or target multiple steps in the cancer immunity cycle offers the greatest potential for therapeutic synergy since this is most likely to address potential mechanisms of resistance by blocking other paths to immune evasion for cancer cells (Figure 3).
Given the expanding network of immune-checkpoint inhibitors and agonists, the focal point of combination therapy has been combining immune checkpoint-targeting drugs with different mechanisms of action, including those that would simultaneously release the brakes and step on the gas pedal. The vast majority of ongoing clinical trials of approved checkpoint inhibitors and the drugs in development listed in the table are combination trials.
These efforts yielded the first FDA-approved combination immunotherapy regimen in 2015; nivolumab and ipilimumab for the treatment of metastatic melanoma. Approval was based on the demonstration of improved ORR, prolonged response duration, and improved progression-free survival among 142 patients treated with the combination, compared to either drug alone.24
The results of a phase 1/2 trial evaluating the combination of a 4-1BB receptor agonist urelumab with nivolumab in hematologic malignancies and solid tumors found the combination to be safe and particularly effective in patients with advanced/metastatic melanoma, with an ORR of 50%.25 Nivolumab was also combined with the CD27 agonist varlilumab in a phase 1/2 clinical trial of patients with solid tumors, for which data was also recently released. Among 46 patients enrolled, primarily those with colorectal and ovarian cancer the combination had an acceptable safety profile and favorable changes in intratumoral immune biomarkers were observed. The phase 2 portion of the trial is ongoing.26
Meanwhile, Incyte’s IDO inhibitor epacadostat has recently been making waves in combination with pembrolizumab in patients with advanced solid tumors. It demonstrated particularly promising clinical activity in patients with metastatic melanoma, with an overall response rate (ORR) of 57%, including 2 complete responses (CRs), prompting initiation of a phase 3 trial of this combination (NCT02752074).27
The relationship between the immune system and tumors is complex and dynamic, and for immunotherapy to reach its full potential it will likely need to attack on multiple fronts. Here, we discuss some of the latest and most promising developments in the immuno-oncology field designed to build on the successes and address limitations.
The anti-tumor immune response
Cancer is a disease of genomic instability, whereby genetic alterations ranging from a single nucleotide to the whole chromosome level frequently occur. Although cancers derive from a patient’s own tissues, these genetic differences can mark the cancer cell as non-self, triggering an immune response to eliminate these cells.
The first hints of this anti-tumor immunity date back more than a century and a half and sparked the concept of mobilizing the immune system to treat patients.1-3 Although early pioneers achieved little progress in this regard, their efforts provided invaluable insights into the complex and dynamic relationship between a tumor and the immune system that are now translating into real clinical successes.
We now understand that the immune system has a dual role in both restraining and promoting cancer development and have translated this understanding into the theory of cancer immunoediting. Immunoediting has three stages: elimination, wherein the tumor is seemingly destroyed by the innate and adaptive immune response; equilibrium, in which cancer cells that were able to escape elimination are selected for growth; and escape, whereby these resistant cancer cells overwhelm the immune system and develop into a symptomatic lesion.4,5
Immuno-oncologists have also described the cancer immunity cycle to capture the steps that are required for an effective anti-tumor immune response and defects in this cycle form the basis of the most common mechanisms used by cancer cells to subvert the anti-tumor immune response. Much like the cancer hallmarks did for molecularly targeted cancer drugs, the cancer immunity cycle serves as the intellectual framework for cancer immunotherapy.6,7
Exploiting nature’s weapon of mass destruction
Initially, attempts at immunotherapy focused on boosting the immune response using adjuvants and cytokines. The characterization of subtle differences between tumor cells and normal cells led to the development of vaccines and cell-based therapies that exploited these tumor-associated antigens (TAAs).1-6
Despite the approval of a therapeutic vaccine, sipuleucel-T, in 2010 for the treatment of metastatic prostate cancer, in general the success of vaccines has been limited. Marketing authorization for sipuleucel-T was recently withdrawn in Europe, and although it is still available in the United States, it is not widely used because of issues with production and administration. Other vaccines, such as GVAX, which looked particularly promising in early-stage clinical trials, failed to show clinical efficacy in subsequent testing.8,9
Cell-based therapies, such as adoptive cellular therapy (ACT), in which immune cells are removed from the host, primed to attack cancer cells, and then reinfused back into the patient, have focused on T cells because they are the major effectors of the adaptive immune response. Clinical success with the most common approach, tumor-infiltrating lymphocyte (TIL)
Two key techniques have been developed (Figure 1). T-cell receptor (TCR) therapy involves genetically modifying the receptor on the surface of T cells that is responsible for recognizing antigens bound to major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs). The TCR can be altered to recognize a specific TAA or modified to improve its antigen recognition and binding capabilities. This type of therapy is limited by the fact that the TCRs need to be genetically matched to the patient’s immune type.
Releasing the brakes
To ensure that it is only activated at the appropriate time and not in response to the antigens expressed on the surface of the host’s own tissues or harmless materials, the immune system has developed numerous mechanisms for immunological tolerance. Cancer cells are able to exploit these mechanisms to allow them to evade the anti-tumor immune response. One of the main ways in which they do this is by manipulating the signaling pathways involved in T-cell activation, which play a vital role in tolerance.12
To become fully activated, T cells require a primary signal generated by an interaction between the TCR and the antigen-MHC complex on the surface of an APC, followed by secondary costimulatory signals generated by a range of different receptors present on the T-cell surface binding to their ligands on the APC.
If the second signal is inhibitory rather than stimulatory, then the T cell is deactivated instead of becoming activated. Two key coinhibitory receptors are programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) and tumor cells are able to overcome the anti-tumor immune response in part by expressing the ligands that bind these receptors to dampen the activity of tumor-infiltrating T cells and induce tolerance.13
The development of inhibitors of CTLA-4 and PD-1 and their respective ligands has driven some of the most dramatic successes with cancer immunotherapy, particularly with PD-1-targeting drugs which have fewer side effects. Targeting of this pathway has resulted in durable responses, revolutionizing the treatment of metastatic melanoma, with recently published long-term survival data for pembrolizumab showing that 40% of patients were alive 3 years after initiating treatment and, in a separate study, 34% of nivolumab-treated patients were still alive after 5 years.14,15 More recently, PD-1 inhibitors have been slowly expanding into a range of other cancer types and 4 immune checkpoint inhibitors are now approved by the United States Food and Drug Administration (FDA): ipilimumab (Yervoy), nivolumab (Opdivo), pembrolizumab (Keytruda) and atezolizumab (Tecentriq).
Six years on from the first approval in this drug class and an extensive network of coinhibitory receptors has been uncovered – so-called immune checkpoints – many of which are now also serving as therapeutic targets (Table, Figure 2).16 Lymphocyte activation gene 3 (LAG-3) is a member of the immunoglobulin superfamily of receptors that is expressed on a number of different types of immune cell. In addition to negatively regulating cytotoxic T-cell activation like PD-1 and CTLA-4, it is also thought to regulate the immunosuppressive functions of regulatory T cells and the maturation and activation of dendritic cells. T-cell immunoglobulin and mucin domain-containing 3 (TIM-3) is found on the surface of helper and cytotoxic T cells and regulates T-cell inhibition as well as macrophage activation. Inhibitors of both proteins have been developed that are being evaluated in phase 1 or 2 clinical trials in a variety of tumor types.17
Indeed, although T cells have commanded the most attention, there is growing appreciation of the potential for targeting other types of immune cell that play a role in the anti-tumor immune response or in fostering an immunosuppressive microenvironment. NK cells have been a particular focus, since they represent the body’s first line of immune defense and they appear to have analogous inhibitory and activating receptors expressed on their surface that regulate their cytotoxic activity.
The best-defined NK cell receptors are the killer cell immunoglobulin-like receptors (KIRs) that bind to the MHC class I proteins found on the surface of all cells that distinguish them as ‘self’ or ‘non-self’. KIRs can be either activating or inhibitory, depending upon their structure and the ligands to which they bind.19 To date, 2 antibodies targeting inhibitory KIRs have been developed. Though there has been some disappointment with these drugs, most recently a phase 2 trial of lirilumab in elderly patients with acute myeloid leukemia, which missed its primary endpoint, they continue to be evaluated in clinical trials.20
The inhibitory immune checkpoint field has also expanded to include molecules that regulate T-cell activity in other ways. Most prominently, this includes enzymes like indoleamine-2,3 dioxygenase (IDO), which is involved in the metabolism of the essential amino acid tryptophan. IDO-induced depletion of tryptophan and generation of tryptophan metabolites is toxic to cytotoxic T cells, and IDO is also thought to directly activate regulatory T cells, thus the net effect of IDO is immunosuppression. Two IDO inhibitors are currently being developed.21
Stepping on the gas
Despite their unprecedented success, immune checkpoint inhibitors are not effective in all patients or in all tumor types. Their efficacy is limited in large part by the requirement for a pre-existing anti-tumor immune response. If there are no T cells within the tumor microenvironment then releasing the brakes on the immune system won’t help.
More recently, researchers have returned to the idea of stimulating an anti-tumor immune response, this time by targeting the other side of the immune checkpoint coin, the costimulatory molecules. These drugs could prove more effective as they aren’t reliant on a pre-existing anti-tumor immune response. A number of agonist antibodies designed to target these receptors have now been developed and are undergoing clinical evaluation.22
Furthest along in development are those targeting OX40, a costimulatory molecule that is upregulated on the surface of T cells once they have been fully activated by the TCR signal and an initial costimulatory signal. OX40 is thought to be involved in a more long-term immune response and in the formation of a memory response. A mouse monoclonal antibody had a potent immune-stimulating effect accompanied by the regression of at least 1 metastatic lesion in 30% of patients treated in a phase 1 clinical trial, but was limited by the generation of anti-mouse antibodies. 7 OX40 agonists are now in clinical development, 6 fully human monoclonal antibodies and 1 OX40 ligand-Fc fusion protein, MEDI-6383.23
Combinations are key
Many researchers are now reaching the conclusion that combination therapy is likely to be key in expanding the scope of immunotherapy into currently unresponsive patient populations. Investigating rational combinations is already becoming a burgeoning area of the immuno-oncology field, with a variety of different strategies being tested.
Now the question becomes what are the optimal combinations and the timing and sequencing of combination therapy is likely to be a paramount consideration. Developing combinations that have distinct mechanisms of action or target multiple steps in the cancer immunity cycle offers the greatest potential for therapeutic synergy since this is most likely to address potential mechanisms of resistance by blocking other paths to immune evasion for cancer cells (Figure 3).
Given the expanding network of immune-checkpoint inhibitors and agonists, the focal point of combination therapy has been combining immune checkpoint-targeting drugs with different mechanisms of action, including those that would simultaneously release the brakes and step on the gas pedal. The vast majority of ongoing clinical trials of approved checkpoint inhibitors and the drugs in development listed in the table are combination trials.
These efforts yielded the first FDA-approved combination immunotherapy regimen in 2015; nivolumab and ipilimumab for the treatment of metastatic melanoma. Approval was based on the demonstration of improved ORR, prolonged response duration, and improved progression-free survival among 142 patients treated with the combination, compared to either drug alone.24
The results of a phase 1/2 trial evaluating the combination of a 4-1BB receptor agonist urelumab with nivolumab in hematologic malignancies and solid tumors found the combination to be safe and particularly effective in patients with advanced/metastatic melanoma, with an ORR of 50%.25 Nivolumab was also combined with the CD27 agonist varlilumab in a phase 1/2 clinical trial of patients with solid tumors, for which data was also recently released. Among 46 patients enrolled, primarily those with colorectal and ovarian cancer the combination had an acceptable safety profile and favorable changes in intratumoral immune biomarkers were observed. The phase 2 portion of the trial is ongoing.26
Meanwhile, Incyte’s IDO inhibitor epacadostat has recently been making waves in combination with pembrolizumab in patients with advanced solid tumors. It demonstrated particularly promising clinical activity in patients with metastatic melanoma, with an overall response rate (ORR) of 57%, including 2 complete responses (CRs), prompting initiation of a phase 3 trial of this combination (NCT02752074).27
1. Adams JL, Smothers J, Srinivasan R, et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Disc. 2015;14:603-622.
2. D’Errico G, Machado HL, Sainz Jr B. A current perspective on cancer immune therapy: step-by-step approach to constructing the magic bullet. Clin Trans Med. 2017;6:3.
3. Farkona S, Diamandis EP, Blaustig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
4. Meiliana A, Dewi NM, Wijaya A. Cancer immunotherapy: a review. Indones Biomed J. 2016;8(1):1-20.
5. Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumor microenvironment. Nat Rev Clin Oncol. 2016;13:143-158.
6. de Charette M, Marabelle A, Houot R. Turning tumor cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur J Cancer 2016;68:134-147.
7. Chen DS and Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013;39:1-10.
8. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-489.
9. Le DT, Wang-Gillam A, Picozzi V Jr, et al. A phase 2, randomized trial of GVAX Pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. Presented at: the ASCO Gastrointestinal Cancers Symposium; January 16-18, 2014; San Francisco, CA. Abstract 177.
10. Sharpe M and Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
11. Perica K, Varela JC, Oelke M, et al. Adoptive T Cell Immunotherapy for Cancer. Ram Mai Med J. 2015;6(1):e0004.
12. Xing Y and Hogquist KA. T-Cell Tolerance: Central and Peripheral. Cold Spring Harb Perspect Biol. 2012;4:a006957.
13. Buchbinder EI and Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98-106.
14. Robert C, Ribas A, Hamid O, et al. 3-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. 2016(suppl;abstr 9503).
15. Hodi SF, Kluger HM, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Presented at the 2016 AACR Annual Meeting; April 16-20; New Orleans, LA. Abstract CT001.
16. Bakdash G, Sittig SP, van Dijk T, et al. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front Immunol. 2013;4(53):1-18.
17. Sheridan C. Immuno-oncology moves beyond PD-1. Nat Biotechnol. 2015;33(7):673-675.
18. Blake SJ, Dougall WC, Miles JJ, et al. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin Cancer Res. 2016;22(21):5183-5188.
19. Carotta S. Targeting NK cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol. 2016;7:152.
20. Innate Pharma Web site. Innate Pharma Announces Top-Line Results from EFFIKIR Trial Evaluating the Efficacy of Lirilumab as a Single Agent in Elderly Patients with Acute Myeloid Leukemia. http://www.innate-pharma.com/en/news-events/press-releases/innate-pharma-announces-top-line-results-effikir-trial-evaluating-efficacy-lirilumab-single-agent-elderly-patients-acute-myeloid-leukemia. Last updated February 6, 2017. Accessed online February 22, 2017.
21. Sheridan C. IDO inhibitors move center stage in immuno-oncology. Nat Biotechnol. 2015;33(4):321-322.
22. Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640-655.
23. Linch SN, McNamara MJ, Redmond WL. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34.
24. U.S. Food and Drug Administration Web site. Nivolumab in combination with ipilimumab. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm465274.htm. Last updated October 1, 2015. Accessed online February 22, 2017.
25. Massarelli E. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. Presented at the 31st Annual Meeting of the Society for the Immunotherapy of Cancer; November 9-13, 2016; National Harbor, MD. Abstract 239.
26. Sanborn RE, Pishvain MJ, Callahan MK, et al. Phase I results from the combination of an immune-activating anti-CD27 antibody (varlilumab) in combination with PD-1 blockade (nivolumab): activation across multiple immune pathways without untoward immune-related adverse events. Clin Cancer Res. 2016;76(14):suppl. Abstract CT023.
27. Gangadhar T, Hamid O, Smith D.C, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: updated phase 1 results from ECHO-202/KEYNOTE-037. Ann Oncol. 2016;27(6):379-400.
1. Adams JL, Smothers J, Srinivasan R, et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Disc. 2015;14:603-622.
2. D’Errico G, Machado HL, Sainz Jr B. A current perspective on cancer immune therapy: step-by-step approach to constructing the magic bullet. Clin Trans Med. 2017;6:3.
3. Farkona S, Diamandis EP, Blaustig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
4. Meiliana A, Dewi NM, Wijaya A. Cancer immunotherapy: a review. Indones Biomed J. 2016;8(1):1-20.
5. Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumor microenvironment. Nat Rev Clin Oncol. 2016;13:143-158.
6. de Charette M, Marabelle A, Houot R. Turning tumor cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur J Cancer 2016;68:134-147.
7. Chen DS and Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013;39:1-10.
8. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-489.
9. Le DT, Wang-Gillam A, Picozzi V Jr, et al. A phase 2, randomized trial of GVAX Pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. Presented at: the ASCO Gastrointestinal Cancers Symposium; January 16-18, 2014; San Francisco, CA. Abstract 177.
10. Sharpe M and Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
11. Perica K, Varela JC, Oelke M, et al. Adoptive T Cell Immunotherapy for Cancer. Ram Mai Med J. 2015;6(1):e0004.
12. Xing Y and Hogquist KA. T-Cell Tolerance: Central and Peripheral. Cold Spring Harb Perspect Biol. 2012;4:a006957.
13. Buchbinder EI and Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98-106.
14. Robert C, Ribas A, Hamid O, et al. 3-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. 2016(suppl;abstr 9503).
15. Hodi SF, Kluger HM, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a phase I trial. Presented at the 2016 AACR Annual Meeting; April 16-20; New Orleans, LA. Abstract CT001.
16. Bakdash G, Sittig SP, van Dijk T, et al. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front Immunol. 2013;4(53):1-18.
17. Sheridan C. Immuno-oncology moves beyond PD-1. Nat Biotechnol. 2015;33(7):673-675.
18. Blake SJ, Dougall WC, Miles JJ, et al. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin Cancer Res. 2016;22(21):5183-5188.
19. Carotta S. Targeting NK cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol. 2016;7:152.
20. Innate Pharma Web site. Innate Pharma Announces Top-Line Results from EFFIKIR Trial Evaluating the Efficacy of Lirilumab as a Single Agent in Elderly Patients with Acute Myeloid Leukemia. http://www.innate-pharma.com/en/news-events/press-releases/innate-pharma-announces-top-line-results-effikir-trial-evaluating-efficacy-lirilumab-single-agent-elderly-patients-acute-myeloid-leukemia. Last updated February 6, 2017. Accessed online February 22, 2017.
21. Sheridan C. IDO inhibitors move center stage in immuno-oncology. Nat Biotechnol. 2015;33(4):321-322.
22. Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640-655.
23. Linch SN, McNamara MJ, Redmond WL. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34.
24. U.S. Food and Drug Administration Web site. Nivolumab in combination with ipilimumab. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm465274.htm. Last updated October 1, 2015. Accessed online February 22, 2017.
25. Massarelli E. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. Presented at the 31st Annual Meeting of the Society for the Immunotherapy of Cancer; November 9-13, 2016; National Harbor, MD. Abstract 239.
26. Sanborn RE, Pishvain MJ, Callahan MK, et al. Phase I results from the combination of an immune-activating anti-CD27 antibody (varlilumab) in combination with PD-1 blockade (nivolumab): activation across multiple immune pathways without untoward immune-related adverse events. Clin Cancer Res. 2016;76(14):suppl. Abstract CT023.
27. Gangadhar T, Hamid O, Smith D.C, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: updated phase 1 results from ECHO-202/KEYNOTE-037. Ann Oncol. 2016;27(6):379-400.
Systems Automation for Cancer Surveillance: A Lean Six Sigma Project for Tracking Care of Patients With Head and Neck Cancer (FULL)
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
The American Cancer Society estimates that there were 1.68 million newly diagnosed cases of cancer in the U.S. in 2016, with an associated 595,690 deaths.1 Of this number, about 3% was attributable to head and neck cancer (HNC), with 48,330 new cases and 9,570 deaths in 2016. Cancer is among the leading causes of death worldwide, and veterans have a prevalence of HNC nearly twice that of the general population.2 The number of people living with and beyond a cancer diagnosis in the U.S. has risen to an estimated 15.5 million survivors.
Head and neck cancer comprises several subsites, including the oral cavity (lips, buccal mucosa, anterior tongue, floor of mouth, hard palate, and gingiva), the pharynx (nasopharynx, oropharynx, and hypopharynx), the larynx (supraglottis, glottis, and subglottis), the nasal cavity, paranasal sinuses, and the saliva glands.3 The economic burden for HNC treatment was estimated at $3.64 billion in 2010.4
Treatment is based on primary site and staging, and staging is according to the tumor node metastasis system of the American Joint Committee on Cancer.5 In general, lower stages (in situ, stages I and II) are treated with single modalities of organ-sparing surgery or radiation, whereas higher stages (stages III and IV) are treated with multiple modalities, which may include radiation combined with chemotherapy or surgery before or after radiation/chemotherapy.
Survival rate after treatment varies by primary site, cancer stage at diagnosis, histopathologic cell type, viral association, tobacco use, chemical exposure, and treatment modality; survival ranges from 24% to 90% at 5 years based on these variables.6 There is not yet a reliable blood test or other biochemical marker for recurrence, and serial radiologic examinations are expensive and expose the survivor to large amounts of additional ionizing radiation.7,8 Surveillance for recurrence after treatment consists primarily of physical examination and reported symptoms, which may be difficult for the primary care provider (PCP) to perform and distinguish from treatment sequelae.9,10 Thus, HNC survivors are followed in the ear, nose, and throat (ENT) otolaryngology clinic on a decreasing frequency schedule based on risk of relapse, second primaries, treatment sequelae, and toxicities (every 1-3 months in year 1, 2-6 months in year 2, 4-8 months in years 3-5, and every 12 months after 5 years) according to the National Comprehensive Cancer Network (NCCN) guidelines.11
Adherence with posttreatment surveillance in HNC recently was associated with length of survival; however, this observation at a single tertiary academic center was discordant with earlier published reports.12-15 About 80% to 90% of all postcurative intent treatment recurrences and second primary cancers occur within the first 4 years, with a better functional outcome if the recurrence is surgically salvageable or amenable to adjuvant radiation or combined radiation and chemotherapy.16,17 Nonadherence is generally associated with worse clinical and acute care utilization outcomes.18
Problem
At the Raymond G. Murphy VAMC, a tertiary care center in Albuquerque, New Mexico, there was a propensity of veteran HNC patients who missed scheduled surveillance appointments or were lost to follow-up. An informal review of several VA ENT departments revealed similar issues without any consistent method to solve the problem. In an effort to recapture these patients, in 2011 an ENT registered nurse (RN) was added to the team as cancer care coordinator (CCC). After several weeks of chart review of clinic records, it was determined that 31% of HNC patients had missed 1 or more ongoing surveillance appointments, either by patient no-show, clinic cancellations that failed to reschedule patients, or patient cancellation without rescheduling. The CCC was tasked with recapturing these lost patients, returning them to regular follow-up per NCCN guidelines, and tracking new cancer patients as they were diagnosed and progressed through treatment and surveillance. As there had been no one previously in this role in the ENT clinic, there was no guidance about how to proceed.
The mechanism in place for rescheduling no-show patients at that time consisted of a mailed postcard reminder sent by a medical support assistant who requested that the veteran contact the clinic to reschedule. Veterans reported that these reminders often appeared in their mail mingled with so-called junk mail and were discarded without reading. The CCC spent several more weeks examining clinic records in the computerized patient record system (CPRS), looking for patients with cancer in the 5-year surveillance period, and compiling a database of survivors and newly diagnosed patients. This database was compiled initially on paper and then converted to a spreadsheet. Patients who had missed appointments were contacted by the CCC and rescheduled, which resulted in a 100% recovery rate.
Unfortunately, although the manual tracking process was successful, it was laborious and time consuming. Weekly and sometimes daily examination of CPRS clinic records for new patients and survivor adherence was followed by tedious data entry into the spreadsheet. The manual tracking system was deemed suboptimal and a Lean Six Sigma process improvement project was initiated. The project goal was to produce a dashboard database tool that was patient centered to improve the quality of cancer care to veterans.
Methods
Lean Six Sigma is a combination of 2 improvement processes and is embraced by large business and government entities with the goal of improving efficiencies, reducing waste, decreasing errors, and generating cost savings.19 The first improvement process, Six Sigma, is a statistical concept with the goal of producing no more than 3.4 defects per million opportunities.20Using specific tools, Six Sigma identifies the cause of the problem to help develop effective solutions. Six Sigma also helps uncover defects and problems by using a standardized and systematic method for each process improvement project in a sequence of steps known as DMAIC (Define, Measure, Analyze, Improve, and Control) to ensure a defect-free product at a rate of 99.99966%. Define, the first step, contains a written statement defining the problem and the goals; Measure scrutinizes the current baseline of the project in measureable data to identify possible contributing factors; Analyze uses data and tools to understand the cause-and-effect relationships in the process; Improve uses creative developments and changes that lead to process improvements; and Control takes measures to ensure the improvements are implemented, reliable, and constant.
Although slightly different but complementary, Lean focuses on streamlining improvement processes by identifying and eliminating waste that has little or no value to the customer. The 8 most common forms of waste are identified through the mnemonic DOWNTIME (Defects, Overproduction, Waiting, Not utilizing human talent, Transportation, Inventory excess, Motion excess, and Excess processing).21 When both Lean and Six Sigma are used together, the synergistic effects have a powerful impact on the complete quality improvement process and yield consistent reliability. The combined process then includes several methodologic tools for systems redesign, including root-cause analysis, defining waste barriers, measuring current and expected performance, analyzing the data collected, improving the target process, and controlling the improvements. Though already existing and used within the VA system, Lean Six Sigma training was included as a mandatory component of new employee orientation in a memo issued in August 2015 from the assistant secretary for human resources and administration (VA access-only memo VAIQ 7595924).
Root-cause analysis was accomplished using the “5 Why” technique adapted into Lean and Six Sigma from the Toyota Motor Corporation. For example, the question “Why do patients miss appointments?” was asked 5 different ways, and it was determined that many patients lacked transportation, some were not able to reschedule at the time they called to cancel their appointment, those with multiple same-day appointments at the tertiary medical center were not able to wait to schedule a follow-up appointment for fear of missing or being late to their next appointment, and others were placed on recall lists with appointment reminders that failed to accomplish the purpose of self-scheduling by veterans. Thus, the common denominator and answer to the question “why” was that there was no tracking system in place to identify and reschedule missed follow-ups, and before employing a dedicated coordinator, no one accountable for the process (Figure 1).
Wasteful barriers to efficiency were examined with particular attention to the rescheduling process. Rescheduling produced immediate duplication of work for scheduling staff and increased wait time for future appointments. There was potential for additional health care expenses related to costs of late and progressive salvage treatment or for less-than-timely correction of HNC treatment sequelae, such as scarring, lymphedema, or dysphagia. Ear, nose, and throat providers were concerned about missing occult recurrence or residual cancer.
In 2013, the Lean Six Sigma process was used again to critique efforts by the CCC to identify and track HNC patients. One suggestion was to automate the process, and the Information Resource Management (IRM) office was contacted via work order to explore options for mining CPRS data. Working with a committed health information analyst, further discussion was aimed at pulling in additional data that would simultaneously track required posttreatment laboratory results and imaging. It was decided that a secure dashboard format would provide greater utility than would an online report that the CCC had to request and generate daily.
Integrated technologist Stephen Few defines a data dashboard as “… a visual display of the most important information needed to achieve one or more objectives; consolidated and arranged on a single screen so the information can be monitored at a glance.”22 The Head & Neck Cancer Tracking Dashboard (HNC Dashboard), designed by the IRM analyst, queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with HNC by examining outpatient visit and inpatient discharge International Classification of Disease (ICD) codes entered by providers when coding encounter notes in CPRS. It also adds those with a HNC diagnosis in the VistA problem list and the HNC pathology department Systematized Nomenclature of Medicine (SNOMED) codes (Figure 2).
The automated ENT cancer tracking dashboard prototype debuted in 2014, but several months of trial and error took place to reanalyze ICD codes and narrow the list. The dashboard underwent multiple tests to ensure accuracy. Identified patients are presented using an interactive report hosted on a secure SharePoint (Redmond,WA) site, which reduced the risk of a data breach as access requires multi-authenticated user identification from a VA computer.
Another characteristic of the dashboard’s format is the ability to add custom features as needed. Several features now included in the dashboard are location of residence, diagnosis date, ICD code, date captured in the tracking system, most recent ENT clinic visit, future scheduled ENT clinic appointment, date of last thyroid stimulating hormone (TSH) laboratory test, and date of last position emission tomography scan. In addition, cancellations, no-shows, and patients overdue for TSH testing are highlighted in bold. Highlighted fields alert the CCC to reschedule patients in a timely manner and can alert providers to order needed follow-up tests and procedures.
Among the merits of the ENT cancer tracking dashboard is ease of use. The CCC uses a simple ABC acronym to describe utilization:
- A—added: The CCC daily edits new patients added to the dashboard with a HNC diagnosis. Several times recently the CCC saw a new diagnosis before the provider had been notified by pathology of biopsy results (Figure 3).
- B—browse: The dashboard format allows for rapid perusal of critical information at a glance (Figure 4). Recent labs and imaging can be discussed with providers immediately or at weekly ENT team cancer update meetings. Notification to clinicians can be rapid if the results show suspicion for residual/recurrent disease, a second primary site, metastasis, or there is need to notify the patient’s primary care provider to treat elevated TSH levels (hypothyroidism incidence after head and neck radiation is reportedly as high as 44%, with most patients being asymptomatic or simply fatigued).10,23
- C—check: Appointments are checked for those in the future, cancelled without rescheduling, or no-show dates. Empty fields under the “Next ENT Appointment” header alert the CCC to reschedule a follow-up appointment within NCCN guidelines. Alerting providers to upcoming surveillance appointments allows timely coordination with other care providers and departments, including speech pathology, nutrition, audiology, and social work. The “ENT Recall Date” has a unique time-sensitive feature and will visually display a bold type font when ready to be scheduled for a physical appointment (Figure 5).
Results
The cancer dashboard has demonstrated its success by supporting consistent and reliable monthly data. Results recorded over a 24-month period (from January 1, 2015 through December 31, 2016) showed that the electronic tracker identified 101 new HNC patients. During this period, 1,067 HNC patients were scheduled for follow-up appointments for cancer surveillance. Of these, the authors found that 112 HNC patients had missed their appointments due to calling and cancelling or not showing up as scheduled; resulting in a no-show status. This yielded an appointment nonadherence rate of 10%. The authors also found that 73 (7%) HNC patients did not have an elected scheduled appointment to return to the clinic for continued cancer surveillance. This number comprises all HNC patients whose appointments were cancelled by clinic cancellation, self-cancellation, no-show appointments, or those who left the clinic without scheduling a subsequent follow-up appointment. The electronic tracker identified 100% of these patients as missing and needing a future appointment. These patients may have otherwise been lost through manual tracking.
Implementation and utilization of a robust automated dashboard format HNC patient tracking system has been rewarding for the ENT department. The CCC has saved an estimated 600 to 800 hours per year of chart review and data entry. Although a time study was never conducted to measure the work process of this task, it is reasonable to conclude based on the following multiple manual step-by-step processes that the CCC had to perform frequently were now performed within the dashboard: reviewing consults for HNC diagnosis, recording new patient profile data on the spreadsheet; reviewing VA hospital pathology reports for new HNC diagnoses, reviewing the clinic schedule to track patient appointment adherence, updating and recording recent appointment activity, and reviewing the electronic medical records daily for recommended treatment plan and follow-up.
A side-by-side comparison of the functional features of tracking both manually and with automation showed that automation outnumbers the function of manual tracking by 36% and offers improved efficiency (Table). This has allowed time for the CCC to participate in simultaneous HNC care initiatives, including facilitating interfacility telehealth referrals for complex cancer surgery, scheduling and monitoring rural cancer surveillance telehealth appointments, and development of an ENT Survivorship Care Plan. These programs optimize time and workflow, reduce waste, reduce expenditures related to costly treatment modalities associated with advanced stages of malignancy, and improve the veteran experience. Further benefits to the veteran HNC patient population include increased self-efficacy and awareness for disease management through continuity of care, reduced cost associated with travel expense, and reduced potential copays due to additional medical care related to advanced stages of recurrent or residual disease.
In-house development of the HNC tracking dashboard has contributed to further cost savings for the VA. Specialized third-party acquired software can cost thousands of dollars for purchase and implementation and often includes ongoing fees for use. The Sustain and Spread concept of Lean Six Sigma is proven by a 100% recapture rate of HNC patients in the ENT clinic that potentially would have been lost to follow-up. The success in Spreading this innovation forward has resulted in adoption by other VAMCs for current use and implementation. After sharing information regarding the dashboard at 2 national conferences via presentations and poster, other VAMCs in neighboring states have requested the software and initiated custom versions. Because of this success and further demand, dashboard use is currently under consideration by the VA for nationwide availability.
Conclusion
Deficiencies in tracking cancer patients in the VA system exist in part due to little or no sophisticated electronic tracking systems that could perform multiple task functions to identify new cancer patients, the type of cancer, when appointments are missed, and notification when the required labs and procedures are completed. Often, the CCC is dependent on the arduous task of inputting of data to keep him/her up-to-date with patient care and coordination in a timely manner. As new VA policies attempts to perfect and streamline the scheduling process by way of providers placing “return to clinic” orders for patient follow-up care, there remains a potential risk of those patients not getting scheduled without a vigilant tracking process in place to monitor and ensure that all patients are scheduled.
The dashboard has proved to be an easy to use and vital tool in tracking HNC patients by the CCC. It will continue to assist in the identification of new HNC patients, provide ready access to patient information and follow-up care, and help facilitate CCC and provider communication on a daily basis, thereby meeting the goal of a patient-centered product that proves to improve the quality of cancer care of veterans.
Acknowledgment
The authors thank Mr. Dominic B. Ruiz, Visual Information Specialist, at the Raymond Murphy VAMC, who created images in high resolution for this article.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner , Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Patil RD, Meinzen-Derr JK, Hendricks BL, Patil YJ. Improving access and timelines of care for veterans with head and neck squamous cell carcinoma: a multidisciplinary team’s approach. Laryngoscope. 2016;126(3):627-631.
3. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882.
4. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010.
6. Cancer.net. Head and neck cancer: statistics. http://www.cancer.net/cancer-types/head-and-neck-cancer/Statistics. Updated September 2016. Accessed April 12, 2017.
7. Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074.
8. Cheung PK, Chin RY, Eslick GD. Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg. 2016;154(3):421-432.
9. Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. http://www .uptodate .com/contents/overview-of-approach-to-long-term-survivors-of-head-and-neck-cancer. Updated October 26, 2016. Accessed April 12, 2017.
10. Manikantan K, Khode S, Dwivedi RC, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev. 2009;35(8):744-753.
11. National Comprehensive Cancer Network. NCCN Clinical practice guidelines in onclology:head and neck cancers(2.2017).2017. Updated May 8, 2017. https://www.nccn.org/professionals/physician_gls/f_/pdf/head-and-neck.pdf. Accessed July 18, 2017.
12. Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Schnayder Y. The impact of compliance in post treatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(6):519-525.
13. Merkx MA, van Gulick JJ, Marres HA, et al. Effectiveness of routine follow-up of patients treated for T1-2N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006:28(1):1-7.
14. Ritoe SC, de Vegt F, Scheike IM, et al. Effect of routine follow-up after treatment for laryngeal cancer on life expectancy and mortality: results of a Markov model analysis. Cancer. 2007;109(2):239-247.
15. Agrawal A, Hammond TH, Young GS, Avon AL, Ozer E, Schuller DE. Factors affecting long-term survival in patients with recurrent head and neck cancer may help define the role of post-treatment surveillance. Laryngoscope. 2009;119(11):2135-2140.
16. Roland NJ, Bradley PJ. The role of surgery in the palliation of head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):101-108.
17. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382-387.
18. Hwang AS, Atlas SJ, Cronin P, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
19. Healthcare Daily Online. VA healthcare system adopts lean six sigma. http://www.healthcaredailyonline.com/news/va-lean-six-sigma-in-healthcare. Updated December 7, 2015. Accessed April 12, 2017.
20. Gygi C, Williams B. Six Sigma for Dummies. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2012.
21. Kavanagh S, Krings D. The 8 sources of waste and how to eliminate them: improving performance with LEAN management techniques. http://www.gfoa.org/sites/default /files/GFR_DEC_11_18.pdf. Updated December, 2011. Accessed April 14, 2017.
22. Few S. What is a dashboard? In: Wheeler C, ed. Information Dashboard Design: The Effective Visual Communication of Data. 1st ed. Sebastopol, CA: O’Reilly Media; 2006:34.
23. Murthy V, Narang K, Ghosh-Laskar S, Gupta T, Budrukkar A, Agrawal JP. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck. 2014;36(11):1573-1780.
HPV positivity associated with good esophageal adenocarcinoma outcomes
Patients with Barrett high-grade dysplasia or esophageal adenocarcinoma who are positive for human papillomavirus (HPV) infection have significantly better outcomes than patients with the same diseases who are negative for HPV, investigators report.
Among patients with Barrett high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC), mean disease-free survival (DFS) was 40.3 months for HPV-positive patients, compared with 24.1 months for HPV-negative patients (P = .003). Mean overall survival was also significantly better in HPV-positive patients, at 43.7 months versus 29.8 months (P =.009) respectively, reported Shanmugarajah Rajendra, MD, from Bankstown-Lincombe Hospital in Sydney and colleagues.
“If these findings of a favorable prognosis of HPV-positive HGD and EAC are confirmed in larger cohorts with more advanced disease, it presents an opportunity for treatment de-escalation in the hope of reducing toxic effects without deleteriously affecting survival,” they wrote in JAMA Network Open.
The findings support those of earlier studies suggesting that HPV infection is associated with better prognosis among patients with other cancers of the head and neck. For example, a retrospective analysis of data from two clinical trials reported in 2014 found that, 2 years after a diagnosis of recurrent oropharyngeal cancer, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients (P less than .001).
To determine whether there was a similar association between HPV infection and better prognosis of Barrett HGD or EAC, Dr. Rajendra and associates conducted a retrospective case-control study of 142 patients with HGD or EAC treated at secondary or tertiary referral centers in Australia. The patients, all of whom were white, included 126 men. The mean age was 66 years, and in all, 37 patients were positive for HPV.
As noted before, both DFS and overall survival were significantly better for HPV-infected patients, with mean differences of 16.2 months and 13.9 months, respectively. HPV-positive patients also had lower rates of progression or recurrence (24.3% vs. 58.1%; P less than .001), distant metastases (8.1% vs. 27.6%; P = .02), and death from EAC (13.5% vs. 36.2%; P = .02).
In multivariate analysis, superior DFS was associated with HPV positivity, (hazard ratio, 0.39; P = .02), biologically active virus (HR, 0.36; P = .02), E6 and E7 messenger RNA (HR, 0.36; P = .04), and with high p16 expression (HR, 0.49; P = .02).
The study was supported by the South Western Sydney Clinical School; the University of New South Wales, Sydney; and the Oesophageal Cancer Research Fund. Dr. Rajendra reported grants from the University of New South Wales and the Oesophageal Cancer Research Fund during the conduct of the study. No other disclosures were reported.
SOURCE: Rajendra S et al. JAMA Network Open. 2018 Aug 3. doi:10.1001/jamanetworkopen.2018.1054.
The study by Rajendra et al highlights the potential role of human papillomavirus (HPV) status in the prognosis of esophageal adenocarcinoma (EAC). However, the use of HPV as a predictive marker for treatment remains unproven, and many questions abound. Important considerations for studies of de-escalation of treatment in HPV-positive EAC include how best to select patients for less intensive treatment: Should trials be restricted to nonsmoking patients with better prognosis pathology characteristics, including lower T stage and lack of lymph node involvement? What is the best method to assess HPV status in a cost-effective and easily available assay for broad international use? Should there be a de-escalation of chemotherapy, radiation, or both? Is there potentially a role for de-escalation of surgery? Are these trials that patients would consider participation in given the lethality of these cancers?
Finally, the presence of a vaccine for HPV may affect the incidence of cervical, oropharyngeal, and other cancers. If HPV is an important risk factor for EAC, we may see a reduction in the rates of this highly lethal cancer over time. These benefits may take some time to bear out and are highly dependent on vaccination rates. Nonetheless, primary prevention of HPV infection may result in a significant reduction in the global burden of this disease. In the meantime, larger-scale studies of the role of HPV in the pathogenesis of EAC are warranted, particularly before moving toward trials of less intensive therapy. While the results of this small cohort study are impressive, they are preliminary, and the study requires confirmation in a larger, prospective trial.
Sukhbinder Dhesy-Thind, MD, FRCPC is from McMaster University, Hamilton, Ontario. Her remarks are excerpted from an editorial accompanying the study. She reported personal fees from Teva Canada Innovation and Novartis Pharmaceuticals Canada outside the submitted work.
The study by Rajendra et al highlights the potential role of human papillomavirus (HPV) status in the prognosis of esophageal adenocarcinoma (EAC). However, the use of HPV as a predictive marker for treatment remains unproven, and many questions abound. Important considerations for studies of de-escalation of treatment in HPV-positive EAC include how best to select patients for less intensive treatment: Should trials be restricted to nonsmoking patients with better prognosis pathology characteristics, including lower T stage and lack of lymph node involvement? What is the best method to assess HPV status in a cost-effective and easily available assay for broad international use? Should there be a de-escalation of chemotherapy, radiation, or both? Is there potentially a role for de-escalation of surgery? Are these trials that patients would consider participation in given the lethality of these cancers?
Finally, the presence of a vaccine for HPV may affect the incidence of cervical, oropharyngeal, and other cancers. If HPV is an important risk factor for EAC, we may see a reduction in the rates of this highly lethal cancer over time. These benefits may take some time to bear out and are highly dependent on vaccination rates. Nonetheless, primary prevention of HPV infection may result in a significant reduction in the global burden of this disease. In the meantime, larger-scale studies of the role of HPV in the pathogenesis of EAC are warranted, particularly before moving toward trials of less intensive therapy. While the results of this small cohort study are impressive, they are preliminary, and the study requires confirmation in a larger, prospective trial.
Sukhbinder Dhesy-Thind, MD, FRCPC is from McMaster University, Hamilton, Ontario. Her remarks are excerpted from an editorial accompanying the study. She reported personal fees from Teva Canada Innovation and Novartis Pharmaceuticals Canada outside the submitted work.
The study by Rajendra et al highlights the potential role of human papillomavirus (HPV) status in the prognosis of esophageal adenocarcinoma (EAC). However, the use of HPV as a predictive marker for treatment remains unproven, and many questions abound. Important considerations for studies of de-escalation of treatment in HPV-positive EAC include how best to select patients for less intensive treatment: Should trials be restricted to nonsmoking patients with better prognosis pathology characteristics, including lower T stage and lack of lymph node involvement? What is the best method to assess HPV status in a cost-effective and easily available assay for broad international use? Should there be a de-escalation of chemotherapy, radiation, or both? Is there potentially a role for de-escalation of surgery? Are these trials that patients would consider participation in given the lethality of these cancers?
Finally, the presence of a vaccine for HPV may affect the incidence of cervical, oropharyngeal, and other cancers. If HPV is an important risk factor for EAC, we may see a reduction in the rates of this highly lethal cancer over time. These benefits may take some time to bear out and are highly dependent on vaccination rates. Nonetheless, primary prevention of HPV infection may result in a significant reduction in the global burden of this disease. In the meantime, larger-scale studies of the role of HPV in the pathogenesis of EAC are warranted, particularly before moving toward trials of less intensive therapy. While the results of this small cohort study are impressive, they are preliminary, and the study requires confirmation in a larger, prospective trial.
Sukhbinder Dhesy-Thind, MD, FRCPC is from McMaster University, Hamilton, Ontario. Her remarks are excerpted from an editorial accompanying the study. She reported personal fees from Teva Canada Innovation and Novartis Pharmaceuticals Canada outside the submitted work.
Patients with Barrett high-grade dysplasia or esophageal adenocarcinoma who are positive for human papillomavirus (HPV) infection have significantly better outcomes than patients with the same diseases who are negative for HPV, investigators report.
Among patients with Barrett high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC), mean disease-free survival (DFS) was 40.3 months for HPV-positive patients, compared with 24.1 months for HPV-negative patients (P = .003). Mean overall survival was also significantly better in HPV-positive patients, at 43.7 months versus 29.8 months (P =.009) respectively, reported Shanmugarajah Rajendra, MD, from Bankstown-Lincombe Hospital in Sydney and colleagues.
“If these findings of a favorable prognosis of HPV-positive HGD and EAC are confirmed in larger cohorts with more advanced disease, it presents an opportunity for treatment de-escalation in the hope of reducing toxic effects without deleteriously affecting survival,” they wrote in JAMA Network Open.
The findings support those of earlier studies suggesting that HPV infection is associated with better prognosis among patients with other cancers of the head and neck. For example, a retrospective analysis of data from two clinical trials reported in 2014 found that, 2 years after a diagnosis of recurrent oropharyngeal cancer, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients (P less than .001).
To determine whether there was a similar association between HPV infection and better prognosis of Barrett HGD or EAC, Dr. Rajendra and associates conducted a retrospective case-control study of 142 patients with HGD or EAC treated at secondary or tertiary referral centers in Australia. The patients, all of whom were white, included 126 men. The mean age was 66 years, and in all, 37 patients were positive for HPV.
As noted before, both DFS and overall survival were significantly better for HPV-infected patients, with mean differences of 16.2 months and 13.9 months, respectively. HPV-positive patients also had lower rates of progression or recurrence (24.3% vs. 58.1%; P less than .001), distant metastases (8.1% vs. 27.6%; P = .02), and death from EAC (13.5% vs. 36.2%; P = .02).
In multivariate analysis, superior DFS was associated with HPV positivity, (hazard ratio, 0.39; P = .02), biologically active virus (HR, 0.36; P = .02), E6 and E7 messenger RNA (HR, 0.36; P = .04), and with high p16 expression (HR, 0.49; P = .02).
The study was supported by the South Western Sydney Clinical School; the University of New South Wales, Sydney; and the Oesophageal Cancer Research Fund. Dr. Rajendra reported grants from the University of New South Wales and the Oesophageal Cancer Research Fund during the conduct of the study. No other disclosures were reported.
SOURCE: Rajendra S et al. JAMA Network Open. 2018 Aug 3. doi:10.1001/jamanetworkopen.2018.1054.
Patients with Barrett high-grade dysplasia or esophageal adenocarcinoma who are positive for human papillomavirus (HPV) infection have significantly better outcomes than patients with the same diseases who are negative for HPV, investigators report.
Among patients with Barrett high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC), mean disease-free survival (DFS) was 40.3 months for HPV-positive patients, compared with 24.1 months for HPV-negative patients (P = .003). Mean overall survival was also significantly better in HPV-positive patients, at 43.7 months versus 29.8 months (P =.009) respectively, reported Shanmugarajah Rajendra, MD, from Bankstown-Lincombe Hospital in Sydney and colleagues.
“If these findings of a favorable prognosis of HPV-positive HGD and EAC are confirmed in larger cohorts with more advanced disease, it presents an opportunity for treatment de-escalation in the hope of reducing toxic effects without deleteriously affecting survival,” they wrote in JAMA Network Open.
The findings support those of earlier studies suggesting that HPV infection is associated with better prognosis among patients with other cancers of the head and neck. For example, a retrospective analysis of data from two clinical trials reported in 2014 found that, 2 years after a diagnosis of recurrent oropharyngeal cancer, 54.6% of HPV-positive patients were alive, compared with 27.6% of HPV-negative patients (P less than .001).
To determine whether there was a similar association between HPV infection and better prognosis of Barrett HGD or EAC, Dr. Rajendra and associates conducted a retrospective case-control study of 142 patients with HGD or EAC treated at secondary or tertiary referral centers in Australia. The patients, all of whom were white, included 126 men. The mean age was 66 years, and in all, 37 patients were positive for HPV.
As noted before, both DFS and overall survival were significantly better for HPV-infected patients, with mean differences of 16.2 months and 13.9 months, respectively. HPV-positive patients also had lower rates of progression or recurrence (24.3% vs. 58.1%; P less than .001), distant metastases (8.1% vs. 27.6%; P = .02), and death from EAC (13.5% vs. 36.2%; P = .02).
In multivariate analysis, superior DFS was associated with HPV positivity, (hazard ratio, 0.39; P = .02), biologically active virus (HR, 0.36; P = .02), E6 and E7 messenger RNA (HR, 0.36; P = .04), and with high p16 expression (HR, 0.49; P = .02).
The study was supported by the South Western Sydney Clinical School; the University of New South Wales, Sydney; and the Oesophageal Cancer Research Fund. Dr. Rajendra reported grants from the University of New South Wales and the Oesophageal Cancer Research Fund during the conduct of the study. No other disclosures were reported.
SOURCE: Rajendra S et al. JAMA Network Open. 2018 Aug 3. doi:10.1001/jamanetworkopen.2018.1054.
FROM JAMA NETWORK OPEN
Key clinical point: Human papillomavirus infection is associated with better outcomes for patients with esophageal adenocarcinoma and other head and neck cancers.
Major finding: Mean disease-free survival was 40.3 months for HPV-positive patients versus 24.1 months for HPV-negative patients (P = .003).
Study details: A retrospective case-control study of 142 patients with Barrett high-grade dysplasia or esophageal adenocarcinoma.
Disclosures: The study was supported by the South Western Sydney Clinical School; the University of New South Wales, Sydney; and the Oesophageal Cancer Research Fund. Dr. Rajendra reported grants from University of New South Wales and the Oesophageal Cancer Research Fund during the conduct of the study. No other disclosures were reported.
Source: Rajendra S et al. JAMA Network Open. 2018 Aug 3. doi: 10.1001/jamanetworkopen.2018.1054.