User login
Active Comparator Trial Designs Used to Promote Development of Innovative New Medications
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Practice Points
- When evaluating a new treatment, it is important to consider not only whether it is effective but also whether it provides additional value compared to existing treatment options.
- Encouraging active comparator trials will provide clinicians and patients with important data to guide decision-making regarding the most appropriate treatment options.
Understanding the enduring power of caste
Isabel Wilkerson’s naming of the malady facilitates space for a shift in thinking.
America has been struggling to understand its racial dynamics since the arrival of enslaved Africans more than 400 years ago. Today, with much of the world more polarized than ever, and certainly in our United States, there is a need for something to shift us from our fear and survival paranoid schizoid (us-vs.-them) position to an integrated form if we are to come out of this unusual democratic and societal unrest whole.
Yet, we’ve never had the lexicon to adequately describe the sociopolitical dynamics rooted in race and racism and their power to shape the thinking of all who originate in this country and all who enter its self-made borders whether forcefully or voluntarily. Enter Isabel Wilkerson, a Pulitzer Prize–winning, former New York Times Chicago bureau chief, and author of “The Warmth of Other Suns: The Epic Story of America’s Great Migration” (New York: Random House, 2010) with her second book, “Caste: The Origins of Our Discontents” (New York: Random House, 2020).
Ms. Wilkerson quickly gets to work in an engaging storytelling style of weaving past to present with ideas she supports with letters from the past, historians’ impressions, research studies, and data. Her observations and research are bookended by the lead up to the 2016 presidential election and its aftermath on the one end, and the impending 2020 presidential election on the other. In her view, the reemergence of violence that has accelerated in the 21st century and the renewed commitment to promote white supremacy can be understood if we expand our view of race and racism to consider the enduring power of caste. For, in Ms. Wilkerson’s view, the fear of the 2042 U.S. census (which is predicted to reflect for the first time a non-White majority) is a driving force behind the dominant caste’s determination to maintain the status quo power dynamics in the United States.
In an effort to explain American’s racial hierarchy, Ms. Wilkerson explains the need for a new lexicon “that may sound like a foreign language,” but this is intentional on her part. She writes:
“To recalibrate how we see ourselves, I use language that may be more commonly associated with people in other cultures, to suggest a new way of understanding our hierarchy: Dominant caste, ruling majority, favored caste, or upper caste, instead of, or in addition to, white. Middle castes instead of, or in addition to, Asian or Latino. Subordinate caste, lowest caste, bottom caste, disfavored caste, historically stigmatized instead of African-American. Original, conquered, or indigenous peoples instead of, or in addition to, Native American. Marginalized people in addition to, or instead of, women of any race, or minorities of any kind.”
Early in the book Ms. Wilkerson anchors her argument in Rev. Dr. Martin Luther King Jr.’s sojourn to India. Rather than focus on the known history of Dr. King’s admiration of Mohandas Gandhi, Ms. Wilkerson directs our attention to Dr. King’s discovery of his connection to Dalits, those who had been considered “untouchables” until Bhimrao Ramji Ambedkar, the Indian economist, jurist, social reformer, and Dalit leader, fiercely and successfully advocated for a rebranding of his caste of origin; instead of “untouchables” they would be considered Dalits or “broken people.” Dr. King did not meet Mr. Ambedkar, who died 3 years before this journey, but Ms. Wilkerson writes that Dr. King acknowledged the kinship, “And he said unto himself, Yes, I am an untouchable, and every Negro in the United States is an untouchable.” The Dalits and Dr. King recognized in each other their shared positions as subordinates in a global caste system.
In answering the question about the difference between racism and casteism, Ms. Wilkerson writes:
“Because caste and race are interwoven in America, it can be hard to separate the two. ... Casteism is the investment in keeping the hierarchy as it is in order to maintain your own ranking, advantage, privilege, or to elevate yourself above others or to keep others beneath you.”
Reading “Caste: The Origins of Our Discontents” is akin to the experience of gaining relief after struggling for years with a chronic malady that has a fluctuating course: Under the surface is low-grade pain that is compartmentalized and often met with denial or gaslighting when symptoms and systems are reported to members of the dominant caste. Yet, when there are acute flare-ups and increasingly frequent deadly encounters, the defenses of denial are painfully revealed; structures are broken and sometimes burned down. This has been the clinical course of racism, particularly in the United States. In that vein, an early reaction while reading “Caste” might be comparable to hearing an interpretation that educates, clarifies, resonates, and lands perfectly on the right diagnosis at the right moment.
Approach proves clarifying
In conceptualizing the malady as one of caste, Ms. Wilkerson achieves several things simultaneously – she names the malady, thus providing a lexicon, describes its symptoms, and most importantly, in our opinion, shares some of the compelling data from her field studies. By focusing on India, Nazi Germany, and the United States, she describes how easily one system influences another in the global effort to maintain power among the privileged.
This is not a new way of conceptualizing racial hierarchy; however, what is truly persuasive is Ms. Wilkerson’s ability to weave her rigorous research, sociopolitical analysis, and cogent psychological insights and interpretations to explain the 400-year trajectory of racialized caste in the United States. She achieves this exigent task with beautiful prose that motivates the reader to return time and time again to learn gut-wrenching painful historical details. She summarizes truths that have been unearthed (again) about Germany, India, and, in particular, the United States during her research and travels around the world. In doing so, she provides vivid examples of racism layered on caste. Consider the following:
“The Nazis were impressed by the American custom of lynching its subordinate caste of African-Americans, having become aware of the ritual torture and mutilations that typically accompanied them. Hitler especially marveled at the American ‘knack for maintaining an air of robust innocence in the wake of mass death.’ ” Ms. Wilkerson informs us that Hitler sent emissaries to study America’s Jim Crow system and then imported some features to orchestrate the Holocaust in Nazi Germany.
and a corresponding sense of inadequacy in the presence of someone who is considered to be from a higher caste.
A painful account of interpersonal racism is captured as Ms. Wilkerson recounts her experience after a routine business flight from Chicago to Detroit. She details her difficulty leaving a rental car parking lot because she had become so disoriented after being profiled and accosted by Drug Enforcement Administration agents who had intercepted her in the airport terminal and followed her onto the airport shuttle bus as she attempted to reach her destination. She provides a description of “getting turned around in a parking lot that I had been to dozens of times, going in circles, not able to get out, not registering the signs to the exit, not seeing how to get to Interstate 94, when I knew full well how to get to I-94 after all the times I’d driven it. ... This was the thievery of caste, stealing the time and psychic resources of the marginalized, draining energy in an already uphill competition. They were not, like me, frozen and disoriented, trying to make sense of a public violation that seemed all the more menacing now that I could see it in full. The quiet mundanity of that terror has never left me, the scars outliving the cut.”
This account is consistent with the dissociative, disorienting dynamics of race-based trauma. Her experience is not uncommon and helps to explain the activism of those in the subordinate caste who have attained some measure of wealth, power, and influence, and are motivated to expend their resources (energy, time, fame, and/or wealth) to raise awareness about social and political injustices by calling out structural racism in medicine, protesting police use of force by taking a knee, boycotting sporting events, and even demanding that football stadiums be used as polling sites. At the end of the day, all of us who have “made it” know that when we leave our homes, our relegation to the subordinate caste determines how we are perceived and what landmines we must navigate to make it through the day and that determine whether we will make it home.
This tour de force work of art has the potential to be a game changer in the way that we think about racial polarization in the United States. It is hoped that this new language opens up a space that allows each of us to explore this hegemony while identifying our placement and actions we take to maintain it, for each of us undeniably has a position in this caste system.
Having this new lexicon summons to mind the reactions of patients who gain immediate relief from having their illnesses named. In the case of the U.S. malady that has gripped us all, Ms. Wilkerson reiterates the importance of naming the condition. She writes:
“Because, to truly understand America, we must open our eyes to the hidden work of a caste system that has gone unnamed but prevails among us to our collective detriment, to see that we have more in common with each other and with cultures that we might otherwise dismiss, and to summon the courage to consider that therein may lie the answers.”
The naming allows both doctor and patient to have greater insight, understanding its origins and course, as well as having hope that there is a remedy. Naming facilitates the space for a shift in thinking and implementation of treatment protocols, such as Nazi Germany’s “zero tolerance policy” of swastikas in comparison to the ongoing U.S. controversy about the display of Confederate symbols. At this point in history, we welcome a diagnosis that has the potential to shift us from these poles of dominant and subordinate, black and white, good and bad, toward integration and wholeness of the individual psyche and collective global community. This is similar to what Melanie Klein calls the depressive position. Ms. Wilkerson suggests, in relinquishing these polar splits, we increase our capacity to shift to a space where our psychic integration occurs and our inextricable interdependence and responsibility for one another are honored.
Dr. Dunlap is a psychiatrist and psychoanalyst, and clinical professor of psychiatry and behavioral sciences at George Washington University. She is interested in the management of “difference” – race, gender, ethnicity, and intersectionality – in dyadic relationships and group dynamics; and the impact of racism on interpersonal relationships in institutional structures. Dr. Dunlap practices in Washington and has no disclosures. Dr. Dennis is a clinical psychologist and psychoanalyst. Her interests are in gender and ethnic diversity, health equity, and supervision and training. Dr. Dennis practices in Washington and has no disclosures.
Isabel Wilkerson’s naming of the malady facilitates space for a shift in thinking.
Isabel Wilkerson’s naming of the malady facilitates space for a shift in thinking.
America has been struggling to understand its racial dynamics since the arrival of enslaved Africans more than 400 years ago. Today, with much of the world more polarized than ever, and certainly in our United States, there is a need for something to shift us from our fear and survival paranoid schizoid (us-vs.-them) position to an integrated form if we are to come out of this unusual democratic and societal unrest whole.
Yet, we’ve never had the lexicon to adequately describe the sociopolitical dynamics rooted in race and racism and their power to shape the thinking of all who originate in this country and all who enter its self-made borders whether forcefully or voluntarily. Enter Isabel Wilkerson, a Pulitzer Prize–winning, former New York Times Chicago bureau chief, and author of “The Warmth of Other Suns: The Epic Story of America’s Great Migration” (New York: Random House, 2010) with her second book, “Caste: The Origins of Our Discontents” (New York: Random House, 2020).
Ms. Wilkerson quickly gets to work in an engaging storytelling style of weaving past to present with ideas she supports with letters from the past, historians’ impressions, research studies, and data. Her observations and research are bookended by the lead up to the 2016 presidential election and its aftermath on the one end, and the impending 2020 presidential election on the other. In her view, the reemergence of violence that has accelerated in the 21st century and the renewed commitment to promote white supremacy can be understood if we expand our view of race and racism to consider the enduring power of caste. For, in Ms. Wilkerson’s view, the fear of the 2042 U.S. census (which is predicted to reflect for the first time a non-White majority) is a driving force behind the dominant caste’s determination to maintain the status quo power dynamics in the United States.
In an effort to explain American’s racial hierarchy, Ms. Wilkerson explains the need for a new lexicon “that may sound like a foreign language,” but this is intentional on her part. She writes:
“To recalibrate how we see ourselves, I use language that may be more commonly associated with people in other cultures, to suggest a new way of understanding our hierarchy: Dominant caste, ruling majority, favored caste, or upper caste, instead of, or in addition to, white. Middle castes instead of, or in addition to, Asian or Latino. Subordinate caste, lowest caste, bottom caste, disfavored caste, historically stigmatized instead of African-American. Original, conquered, or indigenous peoples instead of, or in addition to, Native American. Marginalized people in addition to, or instead of, women of any race, or minorities of any kind.”
Early in the book Ms. Wilkerson anchors her argument in Rev. Dr. Martin Luther King Jr.’s sojourn to India. Rather than focus on the known history of Dr. King’s admiration of Mohandas Gandhi, Ms. Wilkerson directs our attention to Dr. King’s discovery of his connection to Dalits, those who had been considered “untouchables” until Bhimrao Ramji Ambedkar, the Indian economist, jurist, social reformer, and Dalit leader, fiercely and successfully advocated for a rebranding of his caste of origin; instead of “untouchables” they would be considered Dalits or “broken people.” Dr. King did not meet Mr. Ambedkar, who died 3 years before this journey, but Ms. Wilkerson writes that Dr. King acknowledged the kinship, “And he said unto himself, Yes, I am an untouchable, and every Negro in the United States is an untouchable.” The Dalits and Dr. King recognized in each other their shared positions as subordinates in a global caste system.
In answering the question about the difference between racism and casteism, Ms. Wilkerson writes:
“Because caste and race are interwoven in America, it can be hard to separate the two. ... Casteism is the investment in keeping the hierarchy as it is in order to maintain your own ranking, advantage, privilege, or to elevate yourself above others or to keep others beneath you.”
Reading “Caste: The Origins of Our Discontents” is akin to the experience of gaining relief after struggling for years with a chronic malady that has a fluctuating course: Under the surface is low-grade pain that is compartmentalized and often met with denial or gaslighting when symptoms and systems are reported to members of the dominant caste. Yet, when there are acute flare-ups and increasingly frequent deadly encounters, the defenses of denial are painfully revealed; structures are broken and sometimes burned down. This has been the clinical course of racism, particularly in the United States. In that vein, an early reaction while reading “Caste” might be comparable to hearing an interpretation that educates, clarifies, resonates, and lands perfectly on the right diagnosis at the right moment.
Approach proves clarifying
In conceptualizing the malady as one of caste, Ms. Wilkerson achieves several things simultaneously – she names the malady, thus providing a lexicon, describes its symptoms, and most importantly, in our opinion, shares some of the compelling data from her field studies. By focusing on India, Nazi Germany, and the United States, she describes how easily one system influences another in the global effort to maintain power among the privileged.
This is not a new way of conceptualizing racial hierarchy; however, what is truly persuasive is Ms. Wilkerson’s ability to weave her rigorous research, sociopolitical analysis, and cogent psychological insights and interpretations to explain the 400-year trajectory of racialized caste in the United States. She achieves this exigent task with beautiful prose that motivates the reader to return time and time again to learn gut-wrenching painful historical details. She summarizes truths that have been unearthed (again) about Germany, India, and, in particular, the United States during her research and travels around the world. In doing so, she provides vivid examples of racism layered on caste. Consider the following:
“The Nazis were impressed by the American custom of lynching its subordinate caste of African-Americans, having become aware of the ritual torture and mutilations that typically accompanied them. Hitler especially marveled at the American ‘knack for maintaining an air of robust innocence in the wake of mass death.’ ” Ms. Wilkerson informs us that Hitler sent emissaries to study America’s Jim Crow system and then imported some features to orchestrate the Holocaust in Nazi Germany.
and a corresponding sense of inadequacy in the presence of someone who is considered to be from a higher caste.
A painful account of interpersonal racism is captured as Ms. Wilkerson recounts her experience after a routine business flight from Chicago to Detroit. She details her difficulty leaving a rental car parking lot because she had become so disoriented after being profiled and accosted by Drug Enforcement Administration agents who had intercepted her in the airport terminal and followed her onto the airport shuttle bus as she attempted to reach her destination. She provides a description of “getting turned around in a parking lot that I had been to dozens of times, going in circles, not able to get out, not registering the signs to the exit, not seeing how to get to Interstate 94, when I knew full well how to get to I-94 after all the times I’d driven it. ... This was the thievery of caste, stealing the time and psychic resources of the marginalized, draining energy in an already uphill competition. They were not, like me, frozen and disoriented, trying to make sense of a public violation that seemed all the more menacing now that I could see it in full. The quiet mundanity of that terror has never left me, the scars outliving the cut.”
This account is consistent with the dissociative, disorienting dynamics of race-based trauma. Her experience is not uncommon and helps to explain the activism of those in the subordinate caste who have attained some measure of wealth, power, and influence, and are motivated to expend their resources (energy, time, fame, and/or wealth) to raise awareness about social and political injustices by calling out structural racism in medicine, protesting police use of force by taking a knee, boycotting sporting events, and even demanding that football stadiums be used as polling sites. At the end of the day, all of us who have “made it” know that when we leave our homes, our relegation to the subordinate caste determines how we are perceived and what landmines we must navigate to make it through the day and that determine whether we will make it home.
This tour de force work of art has the potential to be a game changer in the way that we think about racial polarization in the United States. It is hoped that this new language opens up a space that allows each of us to explore this hegemony while identifying our placement and actions we take to maintain it, for each of us undeniably has a position in this caste system.
Having this new lexicon summons to mind the reactions of patients who gain immediate relief from having their illnesses named. In the case of the U.S. malady that has gripped us all, Ms. Wilkerson reiterates the importance of naming the condition. She writes:
“Because, to truly understand America, we must open our eyes to the hidden work of a caste system that has gone unnamed but prevails among us to our collective detriment, to see that we have more in common with each other and with cultures that we might otherwise dismiss, and to summon the courage to consider that therein may lie the answers.”
The naming allows both doctor and patient to have greater insight, understanding its origins and course, as well as having hope that there is a remedy. Naming facilitates the space for a shift in thinking and implementation of treatment protocols, such as Nazi Germany’s “zero tolerance policy” of swastikas in comparison to the ongoing U.S. controversy about the display of Confederate symbols. At this point in history, we welcome a diagnosis that has the potential to shift us from these poles of dominant and subordinate, black and white, good and bad, toward integration and wholeness of the individual psyche and collective global community. This is similar to what Melanie Klein calls the depressive position. Ms. Wilkerson suggests, in relinquishing these polar splits, we increase our capacity to shift to a space where our psychic integration occurs and our inextricable interdependence and responsibility for one another are honored.
Dr. Dunlap is a psychiatrist and psychoanalyst, and clinical professor of psychiatry and behavioral sciences at George Washington University. She is interested in the management of “difference” – race, gender, ethnicity, and intersectionality – in dyadic relationships and group dynamics; and the impact of racism on interpersonal relationships in institutional structures. Dr. Dunlap practices in Washington and has no disclosures. Dr. Dennis is a clinical psychologist and psychoanalyst. Her interests are in gender and ethnic diversity, health equity, and supervision and training. Dr. Dennis practices in Washington and has no disclosures.
America has been struggling to understand its racial dynamics since the arrival of enslaved Africans more than 400 years ago. Today, with much of the world more polarized than ever, and certainly in our United States, there is a need for something to shift us from our fear and survival paranoid schizoid (us-vs.-them) position to an integrated form if we are to come out of this unusual democratic and societal unrest whole.
Yet, we’ve never had the lexicon to adequately describe the sociopolitical dynamics rooted in race and racism and their power to shape the thinking of all who originate in this country and all who enter its self-made borders whether forcefully or voluntarily. Enter Isabel Wilkerson, a Pulitzer Prize–winning, former New York Times Chicago bureau chief, and author of “The Warmth of Other Suns: The Epic Story of America’s Great Migration” (New York: Random House, 2010) with her second book, “Caste: The Origins of Our Discontents” (New York: Random House, 2020).
Ms. Wilkerson quickly gets to work in an engaging storytelling style of weaving past to present with ideas she supports with letters from the past, historians’ impressions, research studies, and data. Her observations and research are bookended by the lead up to the 2016 presidential election and its aftermath on the one end, and the impending 2020 presidential election on the other. In her view, the reemergence of violence that has accelerated in the 21st century and the renewed commitment to promote white supremacy can be understood if we expand our view of race and racism to consider the enduring power of caste. For, in Ms. Wilkerson’s view, the fear of the 2042 U.S. census (which is predicted to reflect for the first time a non-White majority) is a driving force behind the dominant caste’s determination to maintain the status quo power dynamics in the United States.
In an effort to explain American’s racial hierarchy, Ms. Wilkerson explains the need for a new lexicon “that may sound like a foreign language,” but this is intentional on her part. She writes:
“To recalibrate how we see ourselves, I use language that may be more commonly associated with people in other cultures, to suggest a new way of understanding our hierarchy: Dominant caste, ruling majority, favored caste, or upper caste, instead of, or in addition to, white. Middle castes instead of, or in addition to, Asian or Latino. Subordinate caste, lowest caste, bottom caste, disfavored caste, historically stigmatized instead of African-American. Original, conquered, or indigenous peoples instead of, or in addition to, Native American. Marginalized people in addition to, or instead of, women of any race, or minorities of any kind.”
Early in the book Ms. Wilkerson anchors her argument in Rev. Dr. Martin Luther King Jr.’s sojourn to India. Rather than focus on the known history of Dr. King’s admiration of Mohandas Gandhi, Ms. Wilkerson directs our attention to Dr. King’s discovery of his connection to Dalits, those who had been considered “untouchables” until Bhimrao Ramji Ambedkar, the Indian economist, jurist, social reformer, and Dalit leader, fiercely and successfully advocated for a rebranding of his caste of origin; instead of “untouchables” they would be considered Dalits or “broken people.” Dr. King did not meet Mr. Ambedkar, who died 3 years before this journey, but Ms. Wilkerson writes that Dr. King acknowledged the kinship, “And he said unto himself, Yes, I am an untouchable, and every Negro in the United States is an untouchable.” The Dalits and Dr. King recognized in each other their shared positions as subordinates in a global caste system.
In answering the question about the difference between racism and casteism, Ms. Wilkerson writes:
“Because caste and race are interwoven in America, it can be hard to separate the two. ... Casteism is the investment in keeping the hierarchy as it is in order to maintain your own ranking, advantage, privilege, or to elevate yourself above others or to keep others beneath you.”
Reading “Caste: The Origins of Our Discontents” is akin to the experience of gaining relief after struggling for years with a chronic malady that has a fluctuating course: Under the surface is low-grade pain that is compartmentalized and often met with denial or gaslighting when symptoms and systems are reported to members of the dominant caste. Yet, when there are acute flare-ups and increasingly frequent deadly encounters, the defenses of denial are painfully revealed; structures are broken and sometimes burned down. This has been the clinical course of racism, particularly in the United States. In that vein, an early reaction while reading “Caste” might be comparable to hearing an interpretation that educates, clarifies, resonates, and lands perfectly on the right diagnosis at the right moment.
Approach proves clarifying
In conceptualizing the malady as one of caste, Ms. Wilkerson achieves several things simultaneously – she names the malady, thus providing a lexicon, describes its symptoms, and most importantly, in our opinion, shares some of the compelling data from her field studies. By focusing on India, Nazi Germany, and the United States, she describes how easily one system influences another in the global effort to maintain power among the privileged.
This is not a new way of conceptualizing racial hierarchy; however, what is truly persuasive is Ms. Wilkerson’s ability to weave her rigorous research, sociopolitical analysis, and cogent psychological insights and interpretations to explain the 400-year trajectory of racialized caste in the United States. She achieves this exigent task with beautiful prose that motivates the reader to return time and time again to learn gut-wrenching painful historical details. She summarizes truths that have been unearthed (again) about Germany, India, and, in particular, the United States during her research and travels around the world. In doing so, she provides vivid examples of racism layered on caste. Consider the following:
“The Nazis were impressed by the American custom of lynching its subordinate caste of African-Americans, having become aware of the ritual torture and mutilations that typically accompanied them. Hitler especially marveled at the American ‘knack for maintaining an air of robust innocence in the wake of mass death.’ ” Ms. Wilkerson informs us that Hitler sent emissaries to study America’s Jim Crow system and then imported some features to orchestrate the Holocaust in Nazi Germany.
and a corresponding sense of inadequacy in the presence of someone who is considered to be from a higher caste.
A painful account of interpersonal racism is captured as Ms. Wilkerson recounts her experience after a routine business flight from Chicago to Detroit. She details her difficulty leaving a rental car parking lot because she had become so disoriented after being profiled and accosted by Drug Enforcement Administration agents who had intercepted her in the airport terminal and followed her onto the airport shuttle bus as she attempted to reach her destination. She provides a description of “getting turned around in a parking lot that I had been to dozens of times, going in circles, not able to get out, not registering the signs to the exit, not seeing how to get to Interstate 94, when I knew full well how to get to I-94 after all the times I’d driven it. ... This was the thievery of caste, stealing the time and psychic resources of the marginalized, draining energy in an already uphill competition. They were not, like me, frozen and disoriented, trying to make sense of a public violation that seemed all the more menacing now that I could see it in full. The quiet mundanity of that terror has never left me, the scars outliving the cut.”
This account is consistent with the dissociative, disorienting dynamics of race-based trauma. Her experience is not uncommon and helps to explain the activism of those in the subordinate caste who have attained some measure of wealth, power, and influence, and are motivated to expend their resources (energy, time, fame, and/or wealth) to raise awareness about social and political injustices by calling out structural racism in medicine, protesting police use of force by taking a knee, boycotting sporting events, and even demanding that football stadiums be used as polling sites. At the end of the day, all of us who have “made it” know that when we leave our homes, our relegation to the subordinate caste determines how we are perceived and what landmines we must navigate to make it through the day and that determine whether we will make it home.
This tour de force work of art has the potential to be a game changer in the way that we think about racial polarization in the United States. It is hoped that this new language opens up a space that allows each of us to explore this hegemony while identifying our placement and actions we take to maintain it, for each of us undeniably has a position in this caste system.
Having this new lexicon summons to mind the reactions of patients who gain immediate relief from having their illnesses named. In the case of the U.S. malady that has gripped us all, Ms. Wilkerson reiterates the importance of naming the condition. She writes:
“Because, to truly understand America, we must open our eyes to the hidden work of a caste system that has gone unnamed but prevails among us to our collective detriment, to see that we have more in common with each other and with cultures that we might otherwise dismiss, and to summon the courage to consider that therein may lie the answers.”
The naming allows both doctor and patient to have greater insight, understanding its origins and course, as well as having hope that there is a remedy. Naming facilitates the space for a shift in thinking and implementation of treatment protocols, such as Nazi Germany’s “zero tolerance policy” of swastikas in comparison to the ongoing U.S. controversy about the display of Confederate symbols. At this point in history, we welcome a diagnosis that has the potential to shift us from these poles of dominant and subordinate, black and white, good and bad, toward integration and wholeness of the individual psyche and collective global community. This is similar to what Melanie Klein calls the depressive position. Ms. Wilkerson suggests, in relinquishing these polar splits, we increase our capacity to shift to a space where our psychic integration occurs and our inextricable interdependence and responsibility for one another are honored.
Dr. Dunlap is a psychiatrist and psychoanalyst, and clinical professor of psychiatry and behavioral sciences at George Washington University. She is interested in the management of “difference” – race, gender, ethnicity, and intersectionality – in dyadic relationships and group dynamics; and the impact of racism on interpersonal relationships in institutional structures. Dr. Dunlap practices in Washington and has no disclosures. Dr. Dennis is a clinical psychologist and psychoanalyst. Her interests are in gender and ethnic diversity, health equity, and supervision and training. Dr. Dennis practices in Washington and has no disclosures.
CDC playbook prepares states for rollout of COVID-19 vaccine if one is approved
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
A young physician hopes to buck the status quo in Congress
On March 3 of this year, Bryant Cameron Webb, MD, JD, won two-thirds of the vote in Virginia’s Democratic primary race. In November, he’ll compete against Republican Bob Good to represent the state’s 5th Congressional District. If he succeeds, he will become the first Black physician ever elected to a seat in Congress.
The political and social unrest across the United States in recent months has resulted in millions of people becoming more proactive: from sports arenas to the halls of Congress, the rally cry of Black Lives Matter has echoed like never before after the killing of George Floyd and Breonna Taylor at the hands of law enforcement. Dr. Webb, a practicing internist and professor at the University of Virginia, Charlottesville, is among many physicians joining the cause. If elected, he hopes to bring a unique perspective to Washington and advocate for racial equity to help combat systemic racist policies that result in health disparities.
“For me as a professor at UVA in both public health sciences and in medicine, I have a lot to bring to this moment,” he commented, “real expertise on issues that are critical to the nation. Beyond my passion for health and wellness, I have a passion for justice.”
Dr. Webb also believes that serving in Congress is a way to help his patients. “I balance the work of direct patient care and patient advocacy in different spaces,” said the Spotsylvania County native. “Working in Congress is patient advocacy to me. It’s where I can be at my highest use to the people I take care of. It is different from direct patient care. I think this [unique] background that I have is needed in Congress.”
Dr. Webb has never held an elected office before, and he’s looking to get elected in a district that voted for President Trump in the past election. He knows challenges lie ahead.
A calling
The field of medicine called for Dr. Webb at an early age. He credits his family doctor, a Black man, for inspiring him. “With six kids in our family, we saw the doctor frequently. Dr. Yarboro was a young Black man just a few years out of residency. My mom had supreme confidence in him, and he made us feel at ease. So I wanted to be a doctor ever since I was 5 or 6 years old.”
Dr. Webb earned a bachelor’s degree from the University of Virginia in 2005. He entered medical school at Wake Forest University, Winston-Salem, N.C., the following year. Following his third year of medical training, he heeded another calling: He took time off to attend law school. He enrolled in Loyola University of Chicago School of Law and earned his juris doctorate in 2012.
The move may seem an unexpected turn. But Dr. Webb feels his law degree enhances his work. “I think that it’s because I’m so steeped in the legal resources that folks need to navigate. I think I am able to provide better care. ... It’s a complement and helpful to me professionally, whether it’s fighting with an insurance company or with a prescription drug company.”
After law school, Dr. Webb finished his medical training at Wake Forest and moved north, where he completed an internal medicine residency at New York–Presbyterian Hospital. Then came yet another twist in Dr. Webb’s unconventional career path: in 2016, he was selected by President Obama as a White House fellow. He spent the next 2 years in Washington, where he worked on Mr. Obama’s My Brother’s Keeper Task Force, an initiative that addresses opportunity gaps faced by boys and young men of color.
Adeze Enekwechi, MD, president of Impaq and associate professor at the George Washington University, Washington, worked with Dr. Webb at the White House. “This is the place where he will have the most impact. We’ve been talking and writing about health equity ever since our time [there]. Not everybody can speak that language.
Why here? Why now?
Dr. Webb sees patients 2-3 days a week on alternating weeks and knows well the concerns of people who struggle with health. Now he’s ready to have those conversations on a larger platform. “As a Black physician, it’s about bringing that healer mindset to these problems. It’s not about just going there to brow beat people or add to that divisive nature in Congress. You acknowledge that the problems exist, and then bridge,” he said, hoping that bridging party divides can be a catalyst for healing.
Carla Boutin-Foster, MD, associate dean, office of diversity education and research at the State University of New York, Brooklyn, has mentored Dr. Webb since 2013. With his credentials, confidence, and persistence, she believes, he will be a great representative of the medical community in D.C. “You need someone who respects the Constitution. When policy needs to be developed, you need a healer, someone who understands the science of vaccines. This is something Cam has been groomed for. It’s something he has been living and practicing for years.”
The killing of George Floyd and the uprising that ensued has opened the dialogue about racial inequality in America. Health care is not immune to racial bias, and the effects are palatable. One survey conducted by the Larry A. Green Center, in collaboration with the Primary Care Collaborative and 3rd Conversation, found that more than 40% of clinicians say Mr. Floyd’s demise has become a topic of concern among patients of all demographics.
When it comes to racism, Dr. Webb understands that he plays a critical role in moving America forward. “We have so many voices that are powerful and important in the highest level of legislation. We have to use those voices to root out the injustices in our society, like in the Breonna Taylor case. We have to do so because that is how you achieve the American dream,” he said.
The social determinants of health – or “ZIP-code risk” – has been proven to influence health outcomes, yet few physicians screen for them during patient visits. For Dr. Webb, discussing things like housing security and interpersonal violence are critical to providing care.
One of Dr. Webb’s biggest supporters is his wife of 11 years, Leigh Ann Webb, MD, MBA, an emergency medicine physician and assistant professor of emergency medicine at UVA. “He is an effective leader and a consensus builder,” she said of her husband, with whom she has two children. “There has always been something very unique and special about him and the way he engages the world. We need more thoughtful, intelligent people like him to help our country move forward.”
In addition to being the director of health policy and equity at UVA this fall, Dr. Webb plans to teach a course at UVA centered around the social determinants of health called Place Matters. “The focus is on understanding how education and housing and food insecurity all come together to cause illness,” he said. “Health doesn’t happen in hospitals and clinics. It happens in the community.”
A version of this article originally appeared on Medscape.com.
On March 3 of this year, Bryant Cameron Webb, MD, JD, won two-thirds of the vote in Virginia’s Democratic primary race. In November, he’ll compete against Republican Bob Good to represent the state’s 5th Congressional District. If he succeeds, he will become the first Black physician ever elected to a seat in Congress.
The political and social unrest across the United States in recent months has resulted in millions of people becoming more proactive: from sports arenas to the halls of Congress, the rally cry of Black Lives Matter has echoed like never before after the killing of George Floyd and Breonna Taylor at the hands of law enforcement. Dr. Webb, a practicing internist and professor at the University of Virginia, Charlottesville, is among many physicians joining the cause. If elected, he hopes to bring a unique perspective to Washington and advocate for racial equity to help combat systemic racist policies that result in health disparities.
“For me as a professor at UVA in both public health sciences and in medicine, I have a lot to bring to this moment,” he commented, “real expertise on issues that are critical to the nation. Beyond my passion for health and wellness, I have a passion for justice.”
Dr. Webb also believes that serving in Congress is a way to help his patients. “I balance the work of direct patient care and patient advocacy in different spaces,” said the Spotsylvania County native. “Working in Congress is patient advocacy to me. It’s where I can be at my highest use to the people I take care of. It is different from direct patient care. I think this [unique] background that I have is needed in Congress.”
Dr. Webb has never held an elected office before, and he’s looking to get elected in a district that voted for President Trump in the past election. He knows challenges lie ahead.
A calling
The field of medicine called for Dr. Webb at an early age. He credits his family doctor, a Black man, for inspiring him. “With six kids in our family, we saw the doctor frequently. Dr. Yarboro was a young Black man just a few years out of residency. My mom had supreme confidence in him, and he made us feel at ease. So I wanted to be a doctor ever since I was 5 or 6 years old.”
Dr. Webb earned a bachelor’s degree from the University of Virginia in 2005. He entered medical school at Wake Forest University, Winston-Salem, N.C., the following year. Following his third year of medical training, he heeded another calling: He took time off to attend law school. He enrolled in Loyola University of Chicago School of Law and earned his juris doctorate in 2012.
The move may seem an unexpected turn. But Dr. Webb feels his law degree enhances his work. “I think that it’s because I’m so steeped in the legal resources that folks need to navigate. I think I am able to provide better care. ... It’s a complement and helpful to me professionally, whether it’s fighting with an insurance company or with a prescription drug company.”
After law school, Dr. Webb finished his medical training at Wake Forest and moved north, where he completed an internal medicine residency at New York–Presbyterian Hospital. Then came yet another twist in Dr. Webb’s unconventional career path: in 2016, he was selected by President Obama as a White House fellow. He spent the next 2 years in Washington, where he worked on Mr. Obama’s My Brother’s Keeper Task Force, an initiative that addresses opportunity gaps faced by boys and young men of color.
Adeze Enekwechi, MD, president of Impaq and associate professor at the George Washington University, Washington, worked with Dr. Webb at the White House. “This is the place where he will have the most impact. We’ve been talking and writing about health equity ever since our time [there]. Not everybody can speak that language.
Why here? Why now?
Dr. Webb sees patients 2-3 days a week on alternating weeks and knows well the concerns of people who struggle with health. Now he’s ready to have those conversations on a larger platform. “As a Black physician, it’s about bringing that healer mindset to these problems. It’s not about just going there to brow beat people or add to that divisive nature in Congress. You acknowledge that the problems exist, and then bridge,” he said, hoping that bridging party divides can be a catalyst for healing.
Carla Boutin-Foster, MD, associate dean, office of diversity education and research at the State University of New York, Brooklyn, has mentored Dr. Webb since 2013. With his credentials, confidence, and persistence, she believes, he will be a great representative of the medical community in D.C. “You need someone who respects the Constitution. When policy needs to be developed, you need a healer, someone who understands the science of vaccines. This is something Cam has been groomed for. It’s something he has been living and practicing for years.”
The killing of George Floyd and the uprising that ensued has opened the dialogue about racial inequality in America. Health care is not immune to racial bias, and the effects are palatable. One survey conducted by the Larry A. Green Center, in collaboration with the Primary Care Collaborative and 3rd Conversation, found that more than 40% of clinicians say Mr. Floyd’s demise has become a topic of concern among patients of all demographics.
When it comes to racism, Dr. Webb understands that he plays a critical role in moving America forward. “We have so many voices that are powerful and important in the highest level of legislation. We have to use those voices to root out the injustices in our society, like in the Breonna Taylor case. We have to do so because that is how you achieve the American dream,” he said.
The social determinants of health – or “ZIP-code risk” – has been proven to influence health outcomes, yet few physicians screen for them during patient visits. For Dr. Webb, discussing things like housing security and interpersonal violence are critical to providing care.
One of Dr. Webb’s biggest supporters is his wife of 11 years, Leigh Ann Webb, MD, MBA, an emergency medicine physician and assistant professor of emergency medicine at UVA. “He is an effective leader and a consensus builder,” she said of her husband, with whom she has two children. “There has always been something very unique and special about him and the way he engages the world. We need more thoughtful, intelligent people like him to help our country move forward.”
In addition to being the director of health policy and equity at UVA this fall, Dr. Webb plans to teach a course at UVA centered around the social determinants of health called Place Matters. “The focus is on understanding how education and housing and food insecurity all come together to cause illness,” he said. “Health doesn’t happen in hospitals and clinics. It happens in the community.”
A version of this article originally appeared on Medscape.com.
On March 3 of this year, Bryant Cameron Webb, MD, JD, won two-thirds of the vote in Virginia’s Democratic primary race. In November, he’ll compete against Republican Bob Good to represent the state’s 5th Congressional District. If he succeeds, he will become the first Black physician ever elected to a seat in Congress.
The political and social unrest across the United States in recent months has resulted in millions of people becoming more proactive: from sports arenas to the halls of Congress, the rally cry of Black Lives Matter has echoed like never before after the killing of George Floyd and Breonna Taylor at the hands of law enforcement. Dr. Webb, a practicing internist and professor at the University of Virginia, Charlottesville, is among many physicians joining the cause. If elected, he hopes to bring a unique perspective to Washington and advocate for racial equity to help combat systemic racist policies that result in health disparities.
“For me as a professor at UVA in both public health sciences and in medicine, I have a lot to bring to this moment,” he commented, “real expertise on issues that are critical to the nation. Beyond my passion for health and wellness, I have a passion for justice.”
Dr. Webb also believes that serving in Congress is a way to help his patients. “I balance the work of direct patient care and patient advocacy in different spaces,” said the Spotsylvania County native. “Working in Congress is patient advocacy to me. It’s where I can be at my highest use to the people I take care of. It is different from direct patient care. I think this [unique] background that I have is needed in Congress.”
Dr. Webb has never held an elected office before, and he’s looking to get elected in a district that voted for President Trump in the past election. He knows challenges lie ahead.
A calling
The field of medicine called for Dr. Webb at an early age. He credits his family doctor, a Black man, for inspiring him. “With six kids in our family, we saw the doctor frequently. Dr. Yarboro was a young Black man just a few years out of residency. My mom had supreme confidence in him, and he made us feel at ease. So I wanted to be a doctor ever since I was 5 or 6 years old.”
Dr. Webb earned a bachelor’s degree from the University of Virginia in 2005. He entered medical school at Wake Forest University, Winston-Salem, N.C., the following year. Following his third year of medical training, he heeded another calling: He took time off to attend law school. He enrolled in Loyola University of Chicago School of Law and earned his juris doctorate in 2012.
The move may seem an unexpected turn. But Dr. Webb feels his law degree enhances his work. “I think that it’s because I’m so steeped in the legal resources that folks need to navigate. I think I am able to provide better care. ... It’s a complement and helpful to me professionally, whether it’s fighting with an insurance company or with a prescription drug company.”
After law school, Dr. Webb finished his medical training at Wake Forest and moved north, where he completed an internal medicine residency at New York–Presbyterian Hospital. Then came yet another twist in Dr. Webb’s unconventional career path: in 2016, he was selected by President Obama as a White House fellow. He spent the next 2 years in Washington, where he worked on Mr. Obama’s My Brother’s Keeper Task Force, an initiative that addresses opportunity gaps faced by boys and young men of color.
Adeze Enekwechi, MD, president of Impaq and associate professor at the George Washington University, Washington, worked with Dr. Webb at the White House. “This is the place where he will have the most impact. We’ve been talking and writing about health equity ever since our time [there]. Not everybody can speak that language.
Why here? Why now?
Dr. Webb sees patients 2-3 days a week on alternating weeks and knows well the concerns of people who struggle with health. Now he’s ready to have those conversations on a larger platform. “As a Black physician, it’s about bringing that healer mindset to these problems. It’s not about just going there to brow beat people or add to that divisive nature in Congress. You acknowledge that the problems exist, and then bridge,” he said, hoping that bridging party divides can be a catalyst for healing.
Carla Boutin-Foster, MD, associate dean, office of diversity education and research at the State University of New York, Brooklyn, has mentored Dr. Webb since 2013. With his credentials, confidence, and persistence, she believes, he will be a great representative of the medical community in D.C. “You need someone who respects the Constitution. When policy needs to be developed, you need a healer, someone who understands the science of vaccines. This is something Cam has been groomed for. It’s something he has been living and practicing for years.”
The killing of George Floyd and the uprising that ensued has opened the dialogue about racial inequality in America. Health care is not immune to racial bias, and the effects are palatable. One survey conducted by the Larry A. Green Center, in collaboration with the Primary Care Collaborative and 3rd Conversation, found that more than 40% of clinicians say Mr. Floyd’s demise has become a topic of concern among patients of all demographics.
When it comes to racism, Dr. Webb understands that he plays a critical role in moving America forward. “We have so many voices that are powerful and important in the highest level of legislation. We have to use those voices to root out the injustices in our society, like in the Breonna Taylor case. We have to do so because that is how you achieve the American dream,” he said.
The social determinants of health – or “ZIP-code risk” – has been proven to influence health outcomes, yet few physicians screen for them during patient visits. For Dr. Webb, discussing things like housing security and interpersonal violence are critical to providing care.
One of Dr. Webb’s biggest supporters is his wife of 11 years, Leigh Ann Webb, MD, MBA, an emergency medicine physician and assistant professor of emergency medicine at UVA. “He is an effective leader and a consensus builder,” she said of her husband, with whom she has two children. “There has always been something very unique and special about him and the way he engages the world. We need more thoughtful, intelligent people like him to help our country move forward.”
In addition to being the director of health policy and equity at UVA this fall, Dr. Webb plans to teach a course at UVA centered around the social determinants of health called Place Matters. “The focus is on understanding how education and housing and food insecurity all come together to cause illness,” he said. “Health doesn’t happen in hospitals and clinics. It happens in the community.”
A version of this article originally appeared on Medscape.com.
Without Ginsburg, judicial threats to the ACA, reproductive rights heighten
On Feb. 27, 2018, I got an email from the Heritage Foundation that alerted me to a news conference that afternoon held by Republican attorneys general of Texas and other states. It was referred to only as a “discussion about the Affordable Care Act lawsuit.”
I sent the following note to my editor: “I’m off to the Hill anyway. I could stop by this. You never know what it might morph into.”
Few people took that case very seriously – barely a handful of reporters attended the news conference. But it has now “morphed into” the latest existential threat to the Affordable Care Act, scheduled for oral arguments at the Supreme Court a week after the general election in November. And with the death of Justice Ruth Bader Ginsburg on Friday, that case could well morph into the threat that brings down the law in its entirety.
Democrats are raising alarms about the future of the law without Ms. Ginsburg. House Speaker Nancy Pelosi, speaking on ABC’s “This Week” Sunday morning, said that part of the strategy by President Trump and Senate Republicans to quickly fill her seat was to help undermine the ACA.
“The president is rushing to make some kind of a decision because … Nov. 10 is when the arguments begin on the Affordable Care Act,” she said. “He doesn’t want to crush the virus. He wants to crush the Affordable Care Act.”
Ms. Ginsburg’s death could throw an already chaotic general election campaign during a pandemic into even more turmoil.
Let’s take them one at a time.
The ACA under fire – again
The GOP attorneys general argued in February 2018 that the Republican-sponsored tax cut bill Congress passed two months earlier had rendered the ACA unconstitutional by reducing to zero the ACA’s penalty for not having insurance. They based their argument on Chief Justice John Roberts’ 2012 conclusion that the ACA was valid, interpreting that penalty as a constitutionally appropriate tax.
Most legal scholars, including several who challenged the law before the Supreme Court in 2012 and again in 2015, find the argument that the entire law should fall to be unconvincing. “If courts invalidate an entire law merely because Congress eliminates or revises one part, as happened here, that may well inhibit necessary reform of federal legislation in the future by turning it into an ‘all or nothing’ proposition,” wrote a group of conservative and liberal law professors in a brief filed in the case.
Still, in December 2018, U.S. District Judge Reed O’Connor in Texas accepted the GOP argument and declared the law unconstitutional. In December 2019, a three-judge 5th Circuit appeals court panel in New Orleans agreed that without the penalty the requirement to buy insurance is unconstitutional. But it sent the case back to Mr. O’Connor to suggest that perhaps the entire law need not fall.
Not wanting to wait the months or years that reconsideration would take, Democratic attorneys general defending the ACA asked the Supreme Court to hear the case this year. (Democrats are defending the law in court because the Trump administration decided to support the GOP attorneys general’s case.) The court agreed to take the case but scheduled arguments for the week after the November election.
While the fate of the ACA was and is a live political issue, few legal observers were terribly worried about the legal outcome of the case, now known as Texas v. California, if only because the case seemed much weaker than the 2012 and 2015 cases in which Mr. Roberts joined the court’s four liberals. In the 2015 case, which challenged the validity of federal tax subsidies helping millions of Americans buy health insurance on the ACA’s marketplaces, both Mr. Roberts and now-retired Justice Anthony Kennedy voted to uphold the law.
But without Ms. Ginsburg, the case could wind up in a 4-4 tie, even if Mr. Roberts supports the law’s constitutionality. That could let the lower-court ruling stand, although it would not be binding on other courts outside of the 5th Circuit. The court could also put off the arguments or, if the Republican Senate replaces Ms. Ginsburg with another conservative justice before arguments are heard, Republicans could secure a 5-4 ruling against the law. Some court observers argue that Justice Brett Kavanaugh has not favored invalidating an entire statute if only part of it is flawed and might not approve overturning the ACA. Still, what started out as an effort to energize Republican voters for the 2018 midterms after Congress failed to “repeal and replace” the health law in 2017 could end up throwing the nation’s entire health system into chaos.
At least 20 million Americans – and likely many more who sought coverage since the start of the coronavirus pandemic — who buy insurance through the ACA marketplaces or have Medicaid through the law’s expansion could lose coverage right away. Many millions more would lose the law’s popular protections guaranteeing coverage for people with preexisting health conditions, including those who have had COVID-19.
Adult children under age 26 years would no longer be guaranteed the right to remain on their parents’ health plans, and Medicare patients would lose enhanced prescription drug coverage. Women would lose guaranteed access to birth control at no out-of-pocket cost.
But a sudden elimination would affect more than just health care consumers. Insurance companies, drug companies, hospitals, and doctors have all changed the way they do business because of incentives and penalties in the health law. If it’s struck down, many of the “rules of the road” would literally be wiped away, including billing and payment mechanisms.
A new Democratic president could not drop the lawsuit because the Trump administration is not the plaintiff (the GOP attorneys general are). But a Democratic Congress and president could in theory make the entire issue go away by reinstating the penalty for failure to have insurance, even at a minimal amount. However, as far as the health law goes, for now, nothing is a sure thing.
As Nicholas Bagley, a law professor at the University of Michigan, Ann Arbor, who specializes in health issues, tweeted: “Among other things, the Affordable Care Act now dangles from a thread.”
Reproductive rights
A woman’s right to abortion – and even to birth control – also has been hanging by a thread at the high court for more than a decade. This past term, Mr. Roberts joined the liberals to invalidate a Louisiana law that would have closed most of the state’s abortion clinics, but he made it clear it was not a vote for abortion rights. The Louisiana law was too similar to a Texas law the court (without his vote) struck down in 2016, Mr. Roberts argued.
Ms. Ginsburg had been a stalwart supporter of reproductive freedom for women. In her nearly 3 decades on the court, she always voted with backers of abortion rights and birth control and led the dissenters in 2007 when the court upheld a federal ban on a specific abortion procedure.
Adding a justice opposed to abortion to the bench – which is what Trump has promised his supporters – would almost certainly tilt the court in favor of far more dramatic restrictions on the procedure and possibly an overturn of the landmark 1973 ruling Roe v. Wade.
But not only is abortion on the line: The court in recent years has repeatedly ruled that employers with religious objections can refuse to provide contraception.
And waiting in the lower-court pipeline are cases involving federal funding of Planned Parenthood in both the Medicaid and federal family planning programs, and the ability of individual health workers to decline to participate in abortion and other procedures.
For Ms. Ginsburg, those issues came down to a clear question of a woman’s guarantee of equal status under the law.
“Women, it is now acknowledged, have the talent, capacity, and right ‘to participate equally in the economic and social life of the Nation,’ ” she wrote in her dissent in that 2007 abortion case. “Their ability to realize their full potential, the Court recognized, is intimately connected to ‘their ability to control their reproductive lives.’ ”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
On Feb. 27, 2018, I got an email from the Heritage Foundation that alerted me to a news conference that afternoon held by Republican attorneys general of Texas and other states. It was referred to only as a “discussion about the Affordable Care Act lawsuit.”
I sent the following note to my editor: “I’m off to the Hill anyway. I could stop by this. You never know what it might morph into.”
Few people took that case very seriously – barely a handful of reporters attended the news conference. But it has now “morphed into” the latest existential threat to the Affordable Care Act, scheduled for oral arguments at the Supreme Court a week after the general election in November. And with the death of Justice Ruth Bader Ginsburg on Friday, that case could well morph into the threat that brings down the law in its entirety.
Democrats are raising alarms about the future of the law without Ms. Ginsburg. House Speaker Nancy Pelosi, speaking on ABC’s “This Week” Sunday morning, said that part of the strategy by President Trump and Senate Republicans to quickly fill her seat was to help undermine the ACA.
“The president is rushing to make some kind of a decision because … Nov. 10 is when the arguments begin on the Affordable Care Act,” she said. “He doesn’t want to crush the virus. He wants to crush the Affordable Care Act.”
Ms. Ginsburg’s death could throw an already chaotic general election campaign during a pandemic into even more turmoil.
Let’s take them one at a time.
The ACA under fire – again
The GOP attorneys general argued in February 2018 that the Republican-sponsored tax cut bill Congress passed two months earlier had rendered the ACA unconstitutional by reducing to zero the ACA’s penalty for not having insurance. They based their argument on Chief Justice John Roberts’ 2012 conclusion that the ACA was valid, interpreting that penalty as a constitutionally appropriate tax.
Most legal scholars, including several who challenged the law before the Supreme Court in 2012 and again in 2015, find the argument that the entire law should fall to be unconvincing. “If courts invalidate an entire law merely because Congress eliminates or revises one part, as happened here, that may well inhibit necessary reform of federal legislation in the future by turning it into an ‘all or nothing’ proposition,” wrote a group of conservative and liberal law professors in a brief filed in the case.
Still, in December 2018, U.S. District Judge Reed O’Connor in Texas accepted the GOP argument and declared the law unconstitutional. In December 2019, a three-judge 5th Circuit appeals court panel in New Orleans agreed that without the penalty the requirement to buy insurance is unconstitutional. But it sent the case back to Mr. O’Connor to suggest that perhaps the entire law need not fall.
Not wanting to wait the months or years that reconsideration would take, Democratic attorneys general defending the ACA asked the Supreme Court to hear the case this year. (Democrats are defending the law in court because the Trump administration decided to support the GOP attorneys general’s case.) The court agreed to take the case but scheduled arguments for the week after the November election.
While the fate of the ACA was and is a live political issue, few legal observers were terribly worried about the legal outcome of the case, now known as Texas v. California, if only because the case seemed much weaker than the 2012 and 2015 cases in which Mr. Roberts joined the court’s four liberals. In the 2015 case, which challenged the validity of federal tax subsidies helping millions of Americans buy health insurance on the ACA’s marketplaces, both Mr. Roberts and now-retired Justice Anthony Kennedy voted to uphold the law.
But without Ms. Ginsburg, the case could wind up in a 4-4 tie, even if Mr. Roberts supports the law’s constitutionality. That could let the lower-court ruling stand, although it would not be binding on other courts outside of the 5th Circuit. The court could also put off the arguments or, if the Republican Senate replaces Ms. Ginsburg with another conservative justice before arguments are heard, Republicans could secure a 5-4 ruling against the law. Some court observers argue that Justice Brett Kavanaugh has not favored invalidating an entire statute if only part of it is flawed and might not approve overturning the ACA. Still, what started out as an effort to energize Republican voters for the 2018 midterms after Congress failed to “repeal and replace” the health law in 2017 could end up throwing the nation’s entire health system into chaos.
At least 20 million Americans – and likely many more who sought coverage since the start of the coronavirus pandemic — who buy insurance through the ACA marketplaces or have Medicaid through the law’s expansion could lose coverage right away. Many millions more would lose the law’s popular protections guaranteeing coverage for people with preexisting health conditions, including those who have had COVID-19.
Adult children under age 26 years would no longer be guaranteed the right to remain on their parents’ health plans, and Medicare patients would lose enhanced prescription drug coverage. Women would lose guaranteed access to birth control at no out-of-pocket cost.
But a sudden elimination would affect more than just health care consumers. Insurance companies, drug companies, hospitals, and doctors have all changed the way they do business because of incentives and penalties in the health law. If it’s struck down, many of the “rules of the road” would literally be wiped away, including billing and payment mechanisms.
A new Democratic president could not drop the lawsuit because the Trump administration is not the plaintiff (the GOP attorneys general are). But a Democratic Congress and president could in theory make the entire issue go away by reinstating the penalty for failure to have insurance, even at a minimal amount. However, as far as the health law goes, for now, nothing is a sure thing.
As Nicholas Bagley, a law professor at the University of Michigan, Ann Arbor, who specializes in health issues, tweeted: “Among other things, the Affordable Care Act now dangles from a thread.”
Reproductive rights
A woman’s right to abortion – and even to birth control – also has been hanging by a thread at the high court for more than a decade. This past term, Mr. Roberts joined the liberals to invalidate a Louisiana law that would have closed most of the state’s abortion clinics, but he made it clear it was not a vote for abortion rights. The Louisiana law was too similar to a Texas law the court (without his vote) struck down in 2016, Mr. Roberts argued.
Ms. Ginsburg had been a stalwart supporter of reproductive freedom for women. In her nearly 3 decades on the court, she always voted with backers of abortion rights and birth control and led the dissenters in 2007 when the court upheld a federal ban on a specific abortion procedure.
Adding a justice opposed to abortion to the bench – which is what Trump has promised his supporters – would almost certainly tilt the court in favor of far more dramatic restrictions on the procedure and possibly an overturn of the landmark 1973 ruling Roe v. Wade.
But not only is abortion on the line: The court in recent years has repeatedly ruled that employers with religious objections can refuse to provide contraception.
And waiting in the lower-court pipeline are cases involving federal funding of Planned Parenthood in both the Medicaid and federal family planning programs, and the ability of individual health workers to decline to participate in abortion and other procedures.
For Ms. Ginsburg, those issues came down to a clear question of a woman’s guarantee of equal status under the law.
“Women, it is now acknowledged, have the talent, capacity, and right ‘to participate equally in the economic and social life of the Nation,’ ” she wrote in her dissent in that 2007 abortion case. “Their ability to realize their full potential, the Court recognized, is intimately connected to ‘their ability to control their reproductive lives.’ ”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
On Feb. 27, 2018, I got an email from the Heritage Foundation that alerted me to a news conference that afternoon held by Republican attorneys general of Texas and other states. It was referred to only as a “discussion about the Affordable Care Act lawsuit.”
I sent the following note to my editor: “I’m off to the Hill anyway. I could stop by this. You never know what it might morph into.”
Few people took that case very seriously – barely a handful of reporters attended the news conference. But it has now “morphed into” the latest existential threat to the Affordable Care Act, scheduled for oral arguments at the Supreme Court a week after the general election in November. And with the death of Justice Ruth Bader Ginsburg on Friday, that case could well morph into the threat that brings down the law in its entirety.
Democrats are raising alarms about the future of the law without Ms. Ginsburg. House Speaker Nancy Pelosi, speaking on ABC’s “This Week” Sunday morning, said that part of the strategy by President Trump and Senate Republicans to quickly fill her seat was to help undermine the ACA.
“The president is rushing to make some kind of a decision because … Nov. 10 is when the arguments begin on the Affordable Care Act,” she said. “He doesn’t want to crush the virus. He wants to crush the Affordable Care Act.”
Ms. Ginsburg’s death could throw an already chaotic general election campaign during a pandemic into even more turmoil.
Let’s take them one at a time.
The ACA under fire – again
The GOP attorneys general argued in February 2018 that the Republican-sponsored tax cut bill Congress passed two months earlier had rendered the ACA unconstitutional by reducing to zero the ACA’s penalty for not having insurance. They based their argument on Chief Justice John Roberts’ 2012 conclusion that the ACA was valid, interpreting that penalty as a constitutionally appropriate tax.
Most legal scholars, including several who challenged the law before the Supreme Court in 2012 and again in 2015, find the argument that the entire law should fall to be unconvincing. “If courts invalidate an entire law merely because Congress eliminates or revises one part, as happened here, that may well inhibit necessary reform of federal legislation in the future by turning it into an ‘all or nothing’ proposition,” wrote a group of conservative and liberal law professors in a brief filed in the case.
Still, in December 2018, U.S. District Judge Reed O’Connor in Texas accepted the GOP argument and declared the law unconstitutional. In December 2019, a three-judge 5th Circuit appeals court panel in New Orleans agreed that without the penalty the requirement to buy insurance is unconstitutional. But it sent the case back to Mr. O’Connor to suggest that perhaps the entire law need not fall.
Not wanting to wait the months or years that reconsideration would take, Democratic attorneys general defending the ACA asked the Supreme Court to hear the case this year. (Democrats are defending the law in court because the Trump administration decided to support the GOP attorneys general’s case.) The court agreed to take the case but scheduled arguments for the week after the November election.
While the fate of the ACA was and is a live political issue, few legal observers were terribly worried about the legal outcome of the case, now known as Texas v. California, if only because the case seemed much weaker than the 2012 and 2015 cases in which Mr. Roberts joined the court’s four liberals. In the 2015 case, which challenged the validity of federal tax subsidies helping millions of Americans buy health insurance on the ACA’s marketplaces, both Mr. Roberts and now-retired Justice Anthony Kennedy voted to uphold the law.
But without Ms. Ginsburg, the case could wind up in a 4-4 tie, even if Mr. Roberts supports the law’s constitutionality. That could let the lower-court ruling stand, although it would not be binding on other courts outside of the 5th Circuit. The court could also put off the arguments or, if the Republican Senate replaces Ms. Ginsburg with another conservative justice before arguments are heard, Republicans could secure a 5-4 ruling against the law. Some court observers argue that Justice Brett Kavanaugh has not favored invalidating an entire statute if only part of it is flawed and might not approve overturning the ACA. Still, what started out as an effort to energize Republican voters for the 2018 midterms after Congress failed to “repeal and replace” the health law in 2017 could end up throwing the nation’s entire health system into chaos.
At least 20 million Americans – and likely many more who sought coverage since the start of the coronavirus pandemic — who buy insurance through the ACA marketplaces or have Medicaid through the law’s expansion could lose coverage right away. Many millions more would lose the law’s popular protections guaranteeing coverage for people with preexisting health conditions, including those who have had COVID-19.
Adult children under age 26 years would no longer be guaranteed the right to remain on their parents’ health plans, and Medicare patients would lose enhanced prescription drug coverage. Women would lose guaranteed access to birth control at no out-of-pocket cost.
But a sudden elimination would affect more than just health care consumers. Insurance companies, drug companies, hospitals, and doctors have all changed the way they do business because of incentives and penalties in the health law. If it’s struck down, many of the “rules of the road” would literally be wiped away, including billing and payment mechanisms.
A new Democratic president could not drop the lawsuit because the Trump administration is not the plaintiff (the GOP attorneys general are). But a Democratic Congress and president could in theory make the entire issue go away by reinstating the penalty for failure to have insurance, even at a minimal amount. However, as far as the health law goes, for now, nothing is a sure thing.
As Nicholas Bagley, a law professor at the University of Michigan, Ann Arbor, who specializes in health issues, tweeted: “Among other things, the Affordable Care Act now dangles from a thread.”
Reproductive rights
A woman’s right to abortion – and even to birth control – also has been hanging by a thread at the high court for more than a decade. This past term, Mr. Roberts joined the liberals to invalidate a Louisiana law that would have closed most of the state’s abortion clinics, but he made it clear it was not a vote for abortion rights. The Louisiana law was too similar to a Texas law the court (without his vote) struck down in 2016, Mr. Roberts argued.
Ms. Ginsburg had been a stalwart supporter of reproductive freedom for women. In her nearly 3 decades on the court, she always voted with backers of abortion rights and birth control and led the dissenters in 2007 when the court upheld a federal ban on a specific abortion procedure.
Adding a justice opposed to abortion to the bench – which is what Trump has promised his supporters – would almost certainly tilt the court in favor of far more dramatic restrictions on the procedure and possibly an overturn of the landmark 1973 ruling Roe v. Wade.
But not only is abortion on the line: The court in recent years has repeatedly ruled that employers with religious objections can refuse to provide contraception.
And waiting in the lower-court pipeline are cases involving federal funding of Planned Parenthood in both the Medicaid and federal family planning programs, and the ability of individual health workers to decline to participate in abortion and other procedures.
For Ms. Ginsburg, those issues came down to a clear question of a woman’s guarantee of equal status under the law.
“Women, it is now acknowledged, have the talent, capacity, and right ‘to participate equally in the economic and social life of the Nation,’ ” she wrote in her dissent in that 2007 abortion case. “Their ability to realize their full potential, the Court recognized, is intimately connected to ‘their ability to control their reproductive lives.’ ”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Major changes in Medicare billing are planned for January 2021: Some specialties fare better than others
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.
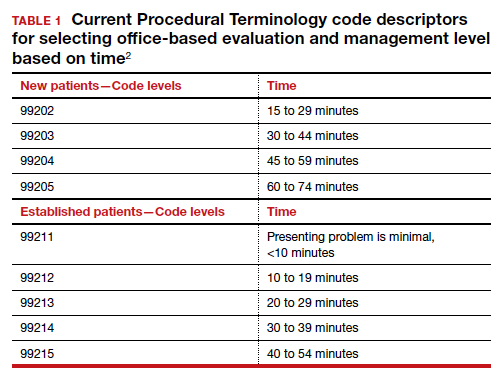
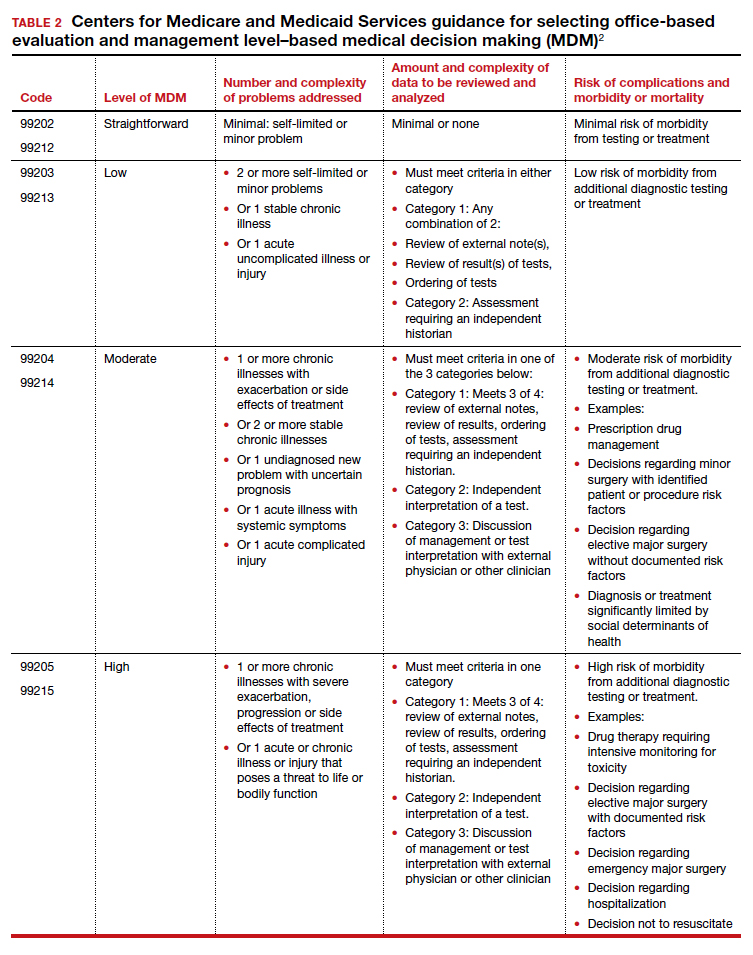
Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.
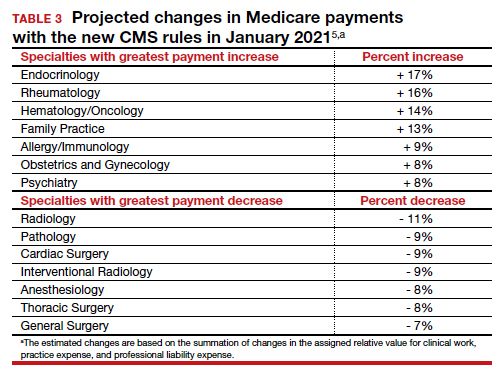
Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.
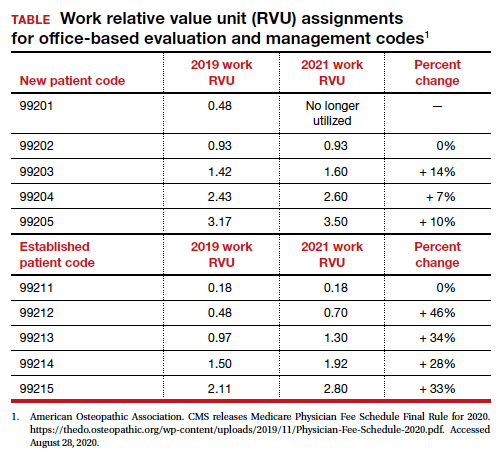
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
Lifting the restrictions on mifepristone during COVID-19: A step in the right direction
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
HHS plan to improve rural health focuses on better broadband, telehealth services
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
FDA expands remdesivir use for all COVID-19 hospitalized patients
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
Two PR employees at FDA fired after plasma therapy controversy
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.









