User login
Military Health Care at a Crossroads
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
HHS drug importation proposals aim to address high costs
The Department of Health & Human Services is taking the first steps in allowing drugs to be imported into the United States.
HHS proposes to offer two different pathways for importation: One allowing states to design programs to import certain drugs directly from Canada and another allowing manufacturers to obtain a new National Drug Code (NDC) number to import their own Food and Drug Administration–approved products manufactured outside of the United States.
“The importation proposals we are rolling out ... are a historic step forward in efforts to bring down drug prices and out-of-pocket costs,” HHS Secretary Alex Azar said during a Dec. 17, 2019, press conference. “New pathways for importation can move us toward a more open and competitive marketplace that supplies American patients with safe, effective, affordable prescription drugs.”
The proposals were made public on Dec. 18, the day the House Rules committee was scheduled to vote on impeaching President Trump.
He emphasized that these proposals “are both important steps in advancing the FDA’s safe-importation action plan, [which] aims to insure that importation is done in a way that prioritizes safety and includes elements to help insure importation does not put patients or the U.S. drug supply chain at risk.”
The pathway for states to import drugs from Canada will be proposed through the federal regulatory process. The notice of proposed rulemaking, which implements authority for FDA regulation of importation granted in the Medicare Modernization Act of 2003, will outline a process by which states, potentially working with wholesalers and/or pharmacies, will submit proposals for FDA review and approval on how they would implement an importation program.
Only certain drugs would be eligible for importation from Canada under this proposal. The drugs would need to be approved in Canada and, except for Canadian labeling, need to meet the conditions of an FDA-approved new drug application or abbreviated new drug application.
Controlled substances, biologics, intravenously injected drugs, drugs with a risk evaluation and management strategy, and drugs injected into the spinal column or eye would be excluded from importation.
Drugs coming in from Canada would be relabeled with U.S.-approved labels and would be subject to testing to ensure they are authentic, not degraded, and compliant with U.S. standards.
States would be required to show that importing drugs poses no additional risk in public health and safety and it would result in the reduction of costs, according to information provided by HHS.
Many of the most expensive drugs, as well as insulins, would not be eligible for importation under this pathway, Mr. Azar acknowledged, adding that “I would envision that as we demonstrate the safety as well as the cost savings from this pathway, [this could serve as] a pilot and a proof of concept that Congress could then look to and potentially take up for more complex molecules that involve cold-chain storage and more complex distribution channels.”
The proposed regulations do not offer any estimates on how much savings could be achieved. He said that there is no way to estimate which states might develop importation plans and how those plans might work.
The second proposed pathway would involve FDA guidance to manufacturers allowing them to import their own FDA-approved products manufactured abroad. Under this proposal, there would be no restriction on which type or kind of FDA-approved product to be imported.
“The FDA has become aware that manufacturers of some brand-name drugs want to offer their drugs at lower costs in the U.S. market but, due to certain challenges in the private market, are not readily [able] to do so without obtaining a different national drug code for their drugs,” Adm. Brett Giroir, MD, HHS assistant secretary for health, said during the press conference.
Obtaining a separate NDC for imported drugs could address the challenges, particularly those posed by the incentives to raise list prices and offer higher rebates to pharmacy benefit managers, Mr. Azar said.
The draft guidance outlines procedures manufacturers could follow to get that NDC for those products and how manufacturers can demonstrate that these products meet U.S. regulatory standards. Products imported in this pathway could be made available to patients in hospitals, physician offices, and pharmacies. Generic drugs are not part of this guidance, but the proposed guidance asked for feedback on whether a similar approach is needed for generic products.
“This would potentially allow for the sale of these drugs at lower prices than currently offered to American consumers, giving drugmakers new flexibility to reduce list prices,” Mr. Azar said.
The proposed regulation on state-level importation will have a 75-day comment period from the day it is published in the Federal Register, and Mr. Azar said that the FDA is committing resources to getting the comments analyzed and reflected in the final rule.
“We will be moving as quickly as we possibly can,” Mr. Azar said, adding that the FDA guidance to manufacturers may move more quickly through its approval process because it is not a formal rule.
The Department of Health & Human Services is taking the first steps in allowing drugs to be imported into the United States.
HHS proposes to offer two different pathways for importation: One allowing states to design programs to import certain drugs directly from Canada and another allowing manufacturers to obtain a new National Drug Code (NDC) number to import their own Food and Drug Administration–approved products manufactured outside of the United States.
“The importation proposals we are rolling out ... are a historic step forward in efforts to bring down drug prices and out-of-pocket costs,” HHS Secretary Alex Azar said during a Dec. 17, 2019, press conference. “New pathways for importation can move us toward a more open and competitive marketplace that supplies American patients with safe, effective, affordable prescription drugs.”
The proposals were made public on Dec. 18, the day the House Rules committee was scheduled to vote on impeaching President Trump.
He emphasized that these proposals “are both important steps in advancing the FDA’s safe-importation action plan, [which] aims to insure that importation is done in a way that prioritizes safety and includes elements to help insure importation does not put patients or the U.S. drug supply chain at risk.”
The pathway for states to import drugs from Canada will be proposed through the federal regulatory process. The notice of proposed rulemaking, which implements authority for FDA regulation of importation granted in the Medicare Modernization Act of 2003, will outline a process by which states, potentially working with wholesalers and/or pharmacies, will submit proposals for FDA review and approval on how they would implement an importation program.
Only certain drugs would be eligible for importation from Canada under this proposal. The drugs would need to be approved in Canada and, except for Canadian labeling, need to meet the conditions of an FDA-approved new drug application or abbreviated new drug application.
Controlled substances, biologics, intravenously injected drugs, drugs with a risk evaluation and management strategy, and drugs injected into the spinal column or eye would be excluded from importation.
Drugs coming in from Canada would be relabeled with U.S.-approved labels and would be subject to testing to ensure they are authentic, not degraded, and compliant with U.S. standards.
States would be required to show that importing drugs poses no additional risk in public health and safety and it would result in the reduction of costs, according to information provided by HHS.
Many of the most expensive drugs, as well as insulins, would not be eligible for importation under this pathway, Mr. Azar acknowledged, adding that “I would envision that as we demonstrate the safety as well as the cost savings from this pathway, [this could serve as] a pilot and a proof of concept that Congress could then look to and potentially take up for more complex molecules that involve cold-chain storage and more complex distribution channels.”
The proposed regulations do not offer any estimates on how much savings could be achieved. He said that there is no way to estimate which states might develop importation plans and how those plans might work.
The second proposed pathway would involve FDA guidance to manufacturers allowing them to import their own FDA-approved products manufactured abroad. Under this proposal, there would be no restriction on which type or kind of FDA-approved product to be imported.
“The FDA has become aware that manufacturers of some brand-name drugs want to offer their drugs at lower costs in the U.S. market but, due to certain challenges in the private market, are not readily [able] to do so without obtaining a different national drug code for their drugs,” Adm. Brett Giroir, MD, HHS assistant secretary for health, said during the press conference.
Obtaining a separate NDC for imported drugs could address the challenges, particularly those posed by the incentives to raise list prices and offer higher rebates to pharmacy benefit managers, Mr. Azar said.
The draft guidance outlines procedures manufacturers could follow to get that NDC for those products and how manufacturers can demonstrate that these products meet U.S. regulatory standards. Products imported in this pathway could be made available to patients in hospitals, physician offices, and pharmacies. Generic drugs are not part of this guidance, but the proposed guidance asked for feedback on whether a similar approach is needed for generic products.
“This would potentially allow for the sale of these drugs at lower prices than currently offered to American consumers, giving drugmakers new flexibility to reduce list prices,” Mr. Azar said.
The proposed regulation on state-level importation will have a 75-day comment period from the day it is published in the Federal Register, and Mr. Azar said that the FDA is committing resources to getting the comments analyzed and reflected in the final rule.
“We will be moving as quickly as we possibly can,” Mr. Azar said, adding that the FDA guidance to manufacturers may move more quickly through its approval process because it is not a formal rule.
The Department of Health & Human Services is taking the first steps in allowing drugs to be imported into the United States.
HHS proposes to offer two different pathways for importation: One allowing states to design programs to import certain drugs directly from Canada and another allowing manufacturers to obtain a new National Drug Code (NDC) number to import their own Food and Drug Administration–approved products manufactured outside of the United States.
“The importation proposals we are rolling out ... are a historic step forward in efforts to bring down drug prices and out-of-pocket costs,” HHS Secretary Alex Azar said during a Dec. 17, 2019, press conference. “New pathways for importation can move us toward a more open and competitive marketplace that supplies American patients with safe, effective, affordable prescription drugs.”
The proposals were made public on Dec. 18, the day the House Rules committee was scheduled to vote on impeaching President Trump.
He emphasized that these proposals “are both important steps in advancing the FDA’s safe-importation action plan, [which] aims to insure that importation is done in a way that prioritizes safety and includes elements to help insure importation does not put patients or the U.S. drug supply chain at risk.”
The pathway for states to import drugs from Canada will be proposed through the federal regulatory process. The notice of proposed rulemaking, which implements authority for FDA regulation of importation granted in the Medicare Modernization Act of 2003, will outline a process by which states, potentially working with wholesalers and/or pharmacies, will submit proposals for FDA review and approval on how they would implement an importation program.
Only certain drugs would be eligible for importation from Canada under this proposal. The drugs would need to be approved in Canada and, except for Canadian labeling, need to meet the conditions of an FDA-approved new drug application or abbreviated new drug application.
Controlled substances, biologics, intravenously injected drugs, drugs with a risk evaluation and management strategy, and drugs injected into the spinal column or eye would be excluded from importation.
Drugs coming in from Canada would be relabeled with U.S.-approved labels and would be subject to testing to ensure they are authentic, not degraded, and compliant with U.S. standards.
States would be required to show that importing drugs poses no additional risk in public health and safety and it would result in the reduction of costs, according to information provided by HHS.
Many of the most expensive drugs, as well as insulins, would not be eligible for importation under this pathway, Mr. Azar acknowledged, adding that “I would envision that as we demonstrate the safety as well as the cost savings from this pathway, [this could serve as] a pilot and a proof of concept that Congress could then look to and potentially take up for more complex molecules that involve cold-chain storage and more complex distribution channels.”
The proposed regulations do not offer any estimates on how much savings could be achieved. He said that there is no way to estimate which states might develop importation plans and how those plans might work.
The second proposed pathway would involve FDA guidance to manufacturers allowing them to import their own FDA-approved products manufactured abroad. Under this proposal, there would be no restriction on which type or kind of FDA-approved product to be imported.
“The FDA has become aware that manufacturers of some brand-name drugs want to offer their drugs at lower costs in the U.S. market but, due to certain challenges in the private market, are not readily [able] to do so without obtaining a different national drug code for their drugs,” Adm. Brett Giroir, MD, HHS assistant secretary for health, said during the press conference.
Obtaining a separate NDC for imported drugs could address the challenges, particularly those posed by the incentives to raise list prices and offer higher rebates to pharmacy benefit managers, Mr. Azar said.
The draft guidance outlines procedures manufacturers could follow to get that NDC for those products and how manufacturers can demonstrate that these products meet U.S. regulatory standards. Products imported in this pathway could be made available to patients in hospitals, physician offices, and pharmacies. Generic drugs are not part of this guidance, but the proposed guidance asked for feedback on whether a similar approach is needed for generic products.
“This would potentially allow for the sale of these drugs at lower prices than currently offered to American consumers, giving drugmakers new flexibility to reduce list prices,” Mr. Azar said.
The proposed regulation on state-level importation will have a 75-day comment period from the day it is published in the Federal Register, and Mr. Azar said that the FDA is committing resources to getting the comments analyzed and reflected in the final rule.
“We will be moving as quickly as we possibly can,” Mr. Azar said, adding that the FDA guidance to manufacturers may move more quickly through its approval process because it is not a formal rule.
Culture Change Needed Around Addiction Treatment in the Military
More work is needed to address how addiction is handled both within the military and for veterans after they serve. Removing the stigma around addiction treatment is key to addressing the issue, Anthony Dekker, DO, Northern Arizona VA Healthcare System, said at the AMSUS annual meeting on December 4, 2019.
“When we are talking about treating addiction in the military and the VA, those are 2 different issues,” Dr. Dekker said. “In the VA, it should be understood this is a service that needs to be provided. In the military, it is highly dependent on command. Some commands are in favor of treatment. Some commands are in favor of separation.”
But that separation comes with a cost. Dr. Dekker noted that the military spends about $200,000 to get a person from recruitment to an E-5 pay grade and about $400,000 to an O-5 pay grade. That can be a huge investment loss considering the cost of treatment for someone who is suffering from a use disorder.
“Treatment is going to cost about $44,000,” he said. “That will be treatment in a residential center. That was a person who is serious enough to come into a residential center, follow up with a partial hospital program and continue with ongoing [treatment] that would last a year.”
He touted some of the successes addiction treatment programs have had, recalling data from 2008-2009 from 110 military active-duty service members treated across 5 residential treatment centers in the Washington, DC, area. Within a year, 91% had separated from the military either because of a command decision before treatment or because of loss of recover.
“We know what works in addiction,” he said, noting that program changes that involved using a combination of medication-assisted treatments with regular substance use screenings and medical practitioner follow-up has helped to reduce the rate of lost recovery to 12%. He also noted that in 2013-2014, 41 active military members who received treatment in the same centers were able to be redeployed to Afghanistan and had no relapses during that deployment.
“We try to take the stigma away,” Dr. Dekker said. “So if you have leadership who has a stigma against addiction treatment, you are going to have a steep incline to work against, whereas if you have leadership that were, I would say, endowed with a different sense of knowledge and experience,” there is a much greater chance for helping both military service members and veterans alike.
He called on the US Department of Veterans Affairs (VA) to look more closely at how it is prescribing opioids. While acknowledging the opioid addiction epidemic, he noted that simply cutting back on prescribing may not be the right solution because it is having a ripple effect and causing other problems, namely that although the VA has written 60% fewer opioid prescriptions from 2012 to 2019, overdose rates have doubled as military members and veterans are seeking opioids from other sources outside of a controlled, safer medical environment.
It can be especially problematic for those who have a legitimate medical need for opioids but have a disqualifying event that causes the pain medication to be cut off.
“We need to have a different answer to this because termination of opioids because a patient is positive for marijuana or even positive for cocaine doesn’t mean you take services away. You ramp services up,” Dr. Dekker said. “If I have a patient who has a chronic pain syndrome, the only thing that is going to push him out of my system is if they threaten my staff.…The loss from recovery is not a reason to lose treatment. That is another concept that needs to be addressed and we need to really look at that.”
He also called on VA leadership to be more encouraging in prescribing buprenorphine, noting that many doctors and nurses have waivers to prescribe it, but there is a lot of reluctance to do so, even though there is a lot of success with that treatment.
More work is needed to address how addiction is handled both within the military and for veterans after they serve. Removing the stigma around addiction treatment is key to addressing the issue, Anthony Dekker, DO, Northern Arizona VA Healthcare System, said at the AMSUS annual meeting on December 4, 2019.
“When we are talking about treating addiction in the military and the VA, those are 2 different issues,” Dr. Dekker said. “In the VA, it should be understood this is a service that needs to be provided. In the military, it is highly dependent on command. Some commands are in favor of treatment. Some commands are in favor of separation.”
But that separation comes with a cost. Dr. Dekker noted that the military spends about $200,000 to get a person from recruitment to an E-5 pay grade and about $400,000 to an O-5 pay grade. That can be a huge investment loss considering the cost of treatment for someone who is suffering from a use disorder.
“Treatment is going to cost about $44,000,” he said. “That will be treatment in a residential center. That was a person who is serious enough to come into a residential center, follow up with a partial hospital program and continue with ongoing [treatment] that would last a year.”
He touted some of the successes addiction treatment programs have had, recalling data from 2008-2009 from 110 military active-duty service members treated across 5 residential treatment centers in the Washington, DC, area. Within a year, 91% had separated from the military either because of a command decision before treatment or because of loss of recover.
“We know what works in addiction,” he said, noting that program changes that involved using a combination of medication-assisted treatments with regular substance use screenings and medical practitioner follow-up has helped to reduce the rate of lost recovery to 12%. He also noted that in 2013-2014, 41 active military members who received treatment in the same centers were able to be redeployed to Afghanistan and had no relapses during that deployment.
“We try to take the stigma away,” Dr. Dekker said. “So if you have leadership who has a stigma against addiction treatment, you are going to have a steep incline to work against, whereas if you have leadership that were, I would say, endowed with a different sense of knowledge and experience,” there is a much greater chance for helping both military service members and veterans alike.
He called on the US Department of Veterans Affairs (VA) to look more closely at how it is prescribing opioids. While acknowledging the opioid addiction epidemic, he noted that simply cutting back on prescribing may not be the right solution because it is having a ripple effect and causing other problems, namely that although the VA has written 60% fewer opioid prescriptions from 2012 to 2019, overdose rates have doubled as military members and veterans are seeking opioids from other sources outside of a controlled, safer medical environment.
It can be especially problematic for those who have a legitimate medical need for opioids but have a disqualifying event that causes the pain medication to be cut off.
“We need to have a different answer to this because termination of opioids because a patient is positive for marijuana or even positive for cocaine doesn’t mean you take services away. You ramp services up,” Dr. Dekker said. “If I have a patient who has a chronic pain syndrome, the only thing that is going to push him out of my system is if they threaten my staff.…The loss from recovery is not a reason to lose treatment. That is another concept that needs to be addressed and we need to really look at that.”
He also called on VA leadership to be more encouraging in prescribing buprenorphine, noting that many doctors and nurses have waivers to prescribe it, but there is a lot of reluctance to do so, even though there is a lot of success with that treatment.
More work is needed to address how addiction is handled both within the military and for veterans after they serve. Removing the stigma around addiction treatment is key to addressing the issue, Anthony Dekker, DO, Northern Arizona VA Healthcare System, said at the AMSUS annual meeting on December 4, 2019.
“When we are talking about treating addiction in the military and the VA, those are 2 different issues,” Dr. Dekker said. “In the VA, it should be understood this is a service that needs to be provided. In the military, it is highly dependent on command. Some commands are in favor of treatment. Some commands are in favor of separation.”
But that separation comes with a cost. Dr. Dekker noted that the military spends about $200,000 to get a person from recruitment to an E-5 pay grade and about $400,000 to an O-5 pay grade. That can be a huge investment loss considering the cost of treatment for someone who is suffering from a use disorder.
“Treatment is going to cost about $44,000,” he said. “That will be treatment in a residential center. That was a person who is serious enough to come into a residential center, follow up with a partial hospital program and continue with ongoing [treatment] that would last a year.”
He touted some of the successes addiction treatment programs have had, recalling data from 2008-2009 from 110 military active-duty service members treated across 5 residential treatment centers in the Washington, DC, area. Within a year, 91% had separated from the military either because of a command decision before treatment or because of loss of recover.
“We know what works in addiction,” he said, noting that program changes that involved using a combination of medication-assisted treatments with regular substance use screenings and medical practitioner follow-up has helped to reduce the rate of lost recovery to 12%. He also noted that in 2013-2014, 41 active military members who received treatment in the same centers were able to be redeployed to Afghanistan and had no relapses during that deployment.
“We try to take the stigma away,” Dr. Dekker said. “So if you have leadership who has a stigma against addiction treatment, you are going to have a steep incline to work against, whereas if you have leadership that were, I would say, endowed with a different sense of knowledge and experience,” there is a much greater chance for helping both military service members and veterans alike.
He called on the US Department of Veterans Affairs (VA) to look more closely at how it is prescribing opioids. While acknowledging the opioid addiction epidemic, he noted that simply cutting back on prescribing may not be the right solution because it is having a ripple effect and causing other problems, namely that although the VA has written 60% fewer opioid prescriptions from 2012 to 2019, overdose rates have doubled as military members and veterans are seeking opioids from other sources outside of a controlled, safer medical environment.
It can be especially problematic for those who have a legitimate medical need for opioids but have a disqualifying event that causes the pain medication to be cut off.
“We need to have a different answer to this because termination of opioids because a patient is positive for marijuana or even positive for cocaine doesn’t mean you take services away. You ramp services up,” Dr. Dekker said. “If I have a patient who has a chronic pain syndrome, the only thing that is going to push him out of my system is if they threaten my staff.…The loss from recovery is not a reason to lose treatment. That is another concept that needs to be addressed and we need to really look at that.”
He also called on VA leadership to be more encouraging in prescribing buprenorphine, noting that many doctors and nurses have waivers to prescribe it, but there is a lot of reluctance to do so, even though there is a lot of success with that treatment.
Leaders Outline DHA “Market” Transition
One theme emerged from multiple military healthy system (MHS) leaders at the recent AMSUS annual conference: Significant change is coming to the MHS, and military health care providers can either embrace and shape that change or somebody else will. “If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs; Defense Health Agency (DHA), and the Uniformed Service University for the Health Sciences (USU) told the audience.
The “historic” changes are underway, as the DHA has already begun to take over control of many military treatment facilities (MTFs) that were formerly operated by the separate services. In the next step of the transition, nearly 250 individual MTFs will be combined—along with TRICARE providers—in 21 geographically based “markets” in order to streamline management and avoid redundancies.
The exact details of the changes in store have not been released. McCaffrey noted that US Department of Defense (DoD) leadership will submit the “framework” for their assessment of the MHS to Congress “very soon, and at that point we will begin the hard work of detailed implementation of the results of that assessment and recommendations from the department.” Changes are expected to continue through fiscal year 2021, and some sources have estimated that as many as 18,000 jobs could be eliminated in the process.
Although Congress drove these changes in the National Defense Authorization Act of 2017, MHS leaders insist they are determining how to make the transformation without hurting medical readiness. “We the senior leadership of the MHS must continue to work together to shape our system to meet the challenges of the new environment,” McCaffrey insisted.
It seems as though all elements of the MHS are on the table. One report has suggested that the USU budget could be cut by a third. “Given the USU’s track record of excellence, we were alarmed to learn that the department is considering cuts as high as 30% to the university’s budget for research, development, testing, and evaluation, and 34% to university operations and maintenance... includ[ing] the cancellation of a $445 million military construction project and closure of the USU medical school,” US senators Chris Van Hollen (D-MD), Ben Cardin (D-MD.), Jack Reed (D-RI), and Congressman Jamie Raskin (D-MD) wrote in a November 21, 2019, letter to US Department of Defense Secretary Mark Esper. “These cuts, even if only partially implemented or scaled back, will adversely impact the enterprise across recruitment, retention, access to research funding, and severely impact medical readiness at a time when demand is increasing.”
The medical readiness of military health care providers remains one of the thorniest challenges revolving around the DHA transition. “As an infantryman, from my perspective if you can't maintain effectiveness on the trauma side than it is not worth getting more efficient,” argued LTG (Ret) Jeffrey S. Buchannon, who formerly served as senior commander of Fort Sam Houston, which includes Brooke Army Medical Center, the military’s only level 1 trauma center and 1 of only 2 trauma centers in San Antonio. “We need the home game in order to prepare for the away game,”
In its review, DoD is looking at how the MTFs support inpatient and/or outpatient services to maintain medical force readiness. “We need to identify those areas where we can expand capacity at MTFs that offer potential for sustaining the skills and knowledge of our members,” said McCaffrey. “But we also must examine those areas where facilities do not offer now and likely will not be able to offer in the future a platform for maximizing capabilities to support medical readiness. In those situations, we must be open to right sizing MTF services and capabilities so as to ensure that we are using finite resources most efficiently while not compromising our ability to meet the mission.”
“Our military healthy system is the envy of the world. Any great power competitor would trade its health care and battlefield medicine capabilities for the system you have built,” McCaffrey said. “But just as America’s combat supremacy is not guaranteed nor is the supremacy of the MHS.” The US faces new global security challenges, McCaffrey argued, and “we must adapt and evolve if we are to successfully meet these challenges.”
One theme emerged from multiple military healthy system (MHS) leaders at the recent AMSUS annual conference: Significant change is coming to the MHS, and military health care providers can either embrace and shape that change or somebody else will. “If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs; Defense Health Agency (DHA), and the Uniformed Service University for the Health Sciences (USU) told the audience.
The “historic” changes are underway, as the DHA has already begun to take over control of many military treatment facilities (MTFs) that were formerly operated by the separate services. In the next step of the transition, nearly 250 individual MTFs will be combined—along with TRICARE providers—in 21 geographically based “markets” in order to streamline management and avoid redundancies.
The exact details of the changes in store have not been released. McCaffrey noted that US Department of Defense (DoD) leadership will submit the “framework” for their assessment of the MHS to Congress “very soon, and at that point we will begin the hard work of detailed implementation of the results of that assessment and recommendations from the department.” Changes are expected to continue through fiscal year 2021, and some sources have estimated that as many as 18,000 jobs could be eliminated in the process.
Although Congress drove these changes in the National Defense Authorization Act of 2017, MHS leaders insist they are determining how to make the transformation without hurting medical readiness. “We the senior leadership of the MHS must continue to work together to shape our system to meet the challenges of the new environment,” McCaffrey insisted.
It seems as though all elements of the MHS are on the table. One report has suggested that the USU budget could be cut by a third. “Given the USU’s track record of excellence, we were alarmed to learn that the department is considering cuts as high as 30% to the university’s budget for research, development, testing, and evaluation, and 34% to university operations and maintenance... includ[ing] the cancellation of a $445 million military construction project and closure of the USU medical school,” US senators Chris Van Hollen (D-MD), Ben Cardin (D-MD.), Jack Reed (D-RI), and Congressman Jamie Raskin (D-MD) wrote in a November 21, 2019, letter to US Department of Defense Secretary Mark Esper. “These cuts, even if only partially implemented or scaled back, will adversely impact the enterprise across recruitment, retention, access to research funding, and severely impact medical readiness at a time when demand is increasing.”
The medical readiness of military health care providers remains one of the thorniest challenges revolving around the DHA transition. “As an infantryman, from my perspective if you can't maintain effectiveness on the trauma side than it is not worth getting more efficient,” argued LTG (Ret) Jeffrey S. Buchannon, who formerly served as senior commander of Fort Sam Houston, which includes Brooke Army Medical Center, the military’s only level 1 trauma center and 1 of only 2 trauma centers in San Antonio. “We need the home game in order to prepare for the away game,”
In its review, DoD is looking at how the MTFs support inpatient and/or outpatient services to maintain medical force readiness. “We need to identify those areas where we can expand capacity at MTFs that offer potential for sustaining the skills and knowledge of our members,” said McCaffrey. “But we also must examine those areas where facilities do not offer now and likely will not be able to offer in the future a platform for maximizing capabilities to support medical readiness. In those situations, we must be open to right sizing MTF services and capabilities so as to ensure that we are using finite resources most efficiently while not compromising our ability to meet the mission.”
“Our military healthy system is the envy of the world. Any great power competitor would trade its health care and battlefield medicine capabilities for the system you have built,” McCaffrey said. “But just as America’s combat supremacy is not guaranteed nor is the supremacy of the MHS.” The US faces new global security challenges, McCaffrey argued, and “we must adapt and evolve if we are to successfully meet these challenges.”
One theme emerged from multiple military healthy system (MHS) leaders at the recent AMSUS annual conference: Significant change is coming to the MHS, and military health care providers can either embrace and shape that change or somebody else will. “If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs; Defense Health Agency (DHA), and the Uniformed Service University for the Health Sciences (USU) told the audience.
The “historic” changes are underway, as the DHA has already begun to take over control of many military treatment facilities (MTFs) that were formerly operated by the separate services. In the next step of the transition, nearly 250 individual MTFs will be combined—along with TRICARE providers—in 21 geographically based “markets” in order to streamline management and avoid redundancies.
The exact details of the changes in store have not been released. McCaffrey noted that US Department of Defense (DoD) leadership will submit the “framework” for their assessment of the MHS to Congress “very soon, and at that point we will begin the hard work of detailed implementation of the results of that assessment and recommendations from the department.” Changes are expected to continue through fiscal year 2021, and some sources have estimated that as many as 18,000 jobs could be eliminated in the process.
Although Congress drove these changes in the National Defense Authorization Act of 2017, MHS leaders insist they are determining how to make the transformation without hurting medical readiness. “We the senior leadership of the MHS must continue to work together to shape our system to meet the challenges of the new environment,” McCaffrey insisted.
It seems as though all elements of the MHS are on the table. One report has suggested that the USU budget could be cut by a third. “Given the USU’s track record of excellence, we were alarmed to learn that the department is considering cuts as high as 30% to the university’s budget for research, development, testing, and evaluation, and 34% to university operations and maintenance... includ[ing] the cancellation of a $445 million military construction project and closure of the USU medical school,” US senators Chris Van Hollen (D-MD), Ben Cardin (D-MD.), Jack Reed (D-RI), and Congressman Jamie Raskin (D-MD) wrote in a November 21, 2019, letter to US Department of Defense Secretary Mark Esper. “These cuts, even if only partially implemented or scaled back, will adversely impact the enterprise across recruitment, retention, access to research funding, and severely impact medical readiness at a time when demand is increasing.”
The medical readiness of military health care providers remains one of the thorniest challenges revolving around the DHA transition. “As an infantryman, from my perspective if you can't maintain effectiveness on the trauma side than it is not worth getting more efficient,” argued LTG (Ret) Jeffrey S. Buchannon, who formerly served as senior commander of Fort Sam Houston, which includes Brooke Army Medical Center, the military’s only level 1 trauma center and 1 of only 2 trauma centers in San Antonio. “We need the home game in order to prepare for the away game,”
In its review, DoD is looking at how the MTFs support inpatient and/or outpatient services to maintain medical force readiness. “We need to identify those areas where we can expand capacity at MTFs that offer potential for sustaining the skills and knowledge of our members,” said McCaffrey. “But we also must examine those areas where facilities do not offer now and likely will not be able to offer in the future a platform for maximizing capabilities to support medical readiness. In those situations, we must be open to right sizing MTF services and capabilities so as to ensure that we are using finite resources most efficiently while not compromising our ability to meet the mission.”
“Our military healthy system is the envy of the world. Any great power competitor would trade its health care and battlefield medicine capabilities for the system you have built,” McCaffrey said. “But just as America’s combat supremacy is not guaranteed nor is the supremacy of the MHS.” The US faces new global security challenges, McCaffrey argued, and “we must adapt and evolve if we are to successfully meet these challenges.”
PHS Message to Military Health Providers: Join Us
NATIONAL HARBOR, MD—As the Military Health System is undergoing significant structural and eventually manpower changes, ADM Brett P. Giroir, MD, the Assistant Secretary for Health in the US Department of Health and Human Services (HHS) and the US Food and Drug Administration acting commissioner, had one message: Come and join us. “Recruitment and retention are our top priorities,” ADM Giroir told the largely military health care provider audience, “If there is downsizing of any of the military health [system], we want you. If you touch health in any way…we need great people who are committed to our national goals in the Commissioned Corps.”
Not long ago, the US Public Health Service (PHS) was facing its own pressures to either reduce its workforce or to eliminate the PHS Commissioned Corps altogether. “The Corps’ mission assignments and functions have not evolved in step with the public health needs of the nation,” argued the fiscal year 2019 Office of Management and Budget, Budget of The U.S. Government. “It is time for that to change. HHS is committed to providing the best public health services and emergency response at the lowest cost and is undertaking a comprehensive look at how the Corps is structured.”
In response, PHS has undertaken a top-to-bottom audit and reevaluation of its mission, ADM Giroir noted, with the goal of defining the role for the PHS in the 21st century and beyond. As a result, the PHS recently completed the development of a modernization plan. The plan entails specifically managing the force to meet mission requirements, developing and training a Ready Reserve force, enhancing training and professional development for the Commissioned Corps, and updating and improving PHS systems and processes.
As a part of the modernization plan, ADM Giroir outlined projected growth plans for the Corps: an increase from the 6,400 regular Corps officers in FY 2018 to 7,725 by FY 2024 with an additional 2,500 Ready Reserve officers, to “minimally meet the mission requirements as we understand it,” ADM Giroir noted.
According to ADM Giroir, the goals for the Ready Reserve are an essential component in the PHS mission to meet any regional, national, or global public health emergency. The Ready Reserve would be a well-trained public health force that would be ready to deploy quickly. Whereas in the past, PHS officer deployments and specialties were tailored to the needs of the agencies in which they are embedded, this force would be more aligned with the needs for a rapid public health emergency response and would include specialized providers. In that context health care providers with military rapid response training would be highly valued.
Although the PHS has outlined its modernization plan, no budget has been allocated for it. Moreover, as ADM Giroir, has noted, PHS still remains dependent on the budgets of the embedding agencies to pay for the Commissioned Corps. “Right now our force structure is really determined by what federal agencies need,” he noted.
Currently, there are bills pending in both the House of Representatives and the Senate to codify the modernization effort.
NATIONAL HARBOR, MD—As the Military Health System is undergoing significant structural and eventually manpower changes, ADM Brett P. Giroir, MD, the Assistant Secretary for Health in the US Department of Health and Human Services (HHS) and the US Food and Drug Administration acting commissioner, had one message: Come and join us. “Recruitment and retention are our top priorities,” ADM Giroir told the largely military health care provider audience, “If there is downsizing of any of the military health [system], we want you. If you touch health in any way…we need great people who are committed to our national goals in the Commissioned Corps.”
Not long ago, the US Public Health Service (PHS) was facing its own pressures to either reduce its workforce or to eliminate the PHS Commissioned Corps altogether. “The Corps’ mission assignments and functions have not evolved in step with the public health needs of the nation,” argued the fiscal year 2019 Office of Management and Budget, Budget of The U.S. Government. “It is time for that to change. HHS is committed to providing the best public health services and emergency response at the lowest cost and is undertaking a comprehensive look at how the Corps is structured.”
In response, PHS has undertaken a top-to-bottom audit and reevaluation of its mission, ADM Giroir noted, with the goal of defining the role for the PHS in the 21st century and beyond. As a result, the PHS recently completed the development of a modernization plan. The plan entails specifically managing the force to meet mission requirements, developing and training a Ready Reserve force, enhancing training and professional development for the Commissioned Corps, and updating and improving PHS systems and processes.
As a part of the modernization plan, ADM Giroir outlined projected growth plans for the Corps: an increase from the 6,400 regular Corps officers in FY 2018 to 7,725 by FY 2024 with an additional 2,500 Ready Reserve officers, to “minimally meet the mission requirements as we understand it,” ADM Giroir noted.
According to ADM Giroir, the goals for the Ready Reserve are an essential component in the PHS mission to meet any regional, national, or global public health emergency. The Ready Reserve would be a well-trained public health force that would be ready to deploy quickly. Whereas in the past, PHS officer deployments and specialties were tailored to the needs of the agencies in which they are embedded, this force would be more aligned with the needs for a rapid public health emergency response and would include specialized providers. In that context health care providers with military rapid response training would be highly valued.
Although the PHS has outlined its modernization plan, no budget has been allocated for it. Moreover, as ADM Giroir, has noted, PHS still remains dependent on the budgets of the embedding agencies to pay for the Commissioned Corps. “Right now our force structure is really determined by what federal agencies need,” he noted.
Currently, there are bills pending in both the House of Representatives and the Senate to codify the modernization effort.
NATIONAL HARBOR, MD—As the Military Health System is undergoing significant structural and eventually manpower changes, ADM Brett P. Giroir, MD, the Assistant Secretary for Health in the US Department of Health and Human Services (HHS) and the US Food and Drug Administration acting commissioner, had one message: Come and join us. “Recruitment and retention are our top priorities,” ADM Giroir told the largely military health care provider audience, “If there is downsizing of any of the military health [system], we want you. If you touch health in any way…we need great people who are committed to our national goals in the Commissioned Corps.”
Not long ago, the US Public Health Service (PHS) was facing its own pressures to either reduce its workforce or to eliminate the PHS Commissioned Corps altogether. “The Corps’ mission assignments and functions have not evolved in step with the public health needs of the nation,” argued the fiscal year 2019 Office of Management and Budget, Budget of The U.S. Government. “It is time for that to change. HHS is committed to providing the best public health services and emergency response at the lowest cost and is undertaking a comprehensive look at how the Corps is structured.”
In response, PHS has undertaken a top-to-bottom audit and reevaluation of its mission, ADM Giroir noted, with the goal of defining the role for the PHS in the 21st century and beyond. As a result, the PHS recently completed the development of a modernization plan. The plan entails specifically managing the force to meet mission requirements, developing and training a Ready Reserve force, enhancing training and professional development for the Commissioned Corps, and updating and improving PHS systems and processes.
As a part of the modernization plan, ADM Giroir outlined projected growth plans for the Corps: an increase from the 6,400 regular Corps officers in FY 2018 to 7,725 by FY 2024 with an additional 2,500 Ready Reserve officers, to “minimally meet the mission requirements as we understand it,” ADM Giroir noted.
According to ADM Giroir, the goals for the Ready Reserve are an essential component in the PHS mission to meet any regional, national, or global public health emergency. The Ready Reserve would be a well-trained public health force that would be ready to deploy quickly. Whereas in the past, PHS officer deployments and specialties were tailored to the needs of the agencies in which they are embedded, this force would be more aligned with the needs for a rapid public health emergency response and would include specialized providers. In that context health care providers with military rapid response training would be highly valued.
Although the PHS has outlined its modernization plan, no budget has been allocated for it. Moreover, as ADM Giroir, has noted, PHS still remains dependent on the budgets of the embedding agencies to pay for the Commissioned Corps. “Right now our force structure is really determined by what federal agencies need,” he noted.
Currently, there are bills pending in both the House of Representatives and the Senate to codify the modernization effort.
Millennials in Medicine: Cross-Trained Physicians Not Valued in Medical Marketplace
Millennials, defined as those born between 1981 and 1996, currently comprise 15% of all active physicians in the US.1,2 A recent survey found that nearly 4 of 5 US millennial physicians have a desire for cross-sectional work in areas beyond patient care, such as academic research, health care consulting, entrepreneurship, and health care administration.3
For employers and educators, a better understanding of these preferences, through consideration of the unique education and skill set of the millennial physician workforce, may lead to more effective recruitment of young physicians and improved health systems, avoiding a mismatch between health care provider skills and available jobs that can be costly for both employers and employees.4
This article describes how US millennial physicians are choosing to cross-train (obtaining multiple degrees and/or completing combined medical residency training) throughout undergraduate, medical, and graduate medical education. We also outline ways in which the current physician marketplace may not match the skills of this population and suggest some ways that health care organizations could capitalize on this trend toward more cross-trained personnel in order to effectively recruit and retain the next generation of physicians.
Millennial Education
Undergraduates
The number of interdisciplinary undergraduate majors increased by almost 250% from 1975 to 2000.5 In 2010, nearly 20% of US college students graduated with 2 majors, representing a 70% increase in double majors between 2001 and 2011.6,7 One emerging category of interdisciplinary majors in US colleges is health humanities programs, which have quadrupled since 2000.8
Medical school applicants and matriculants reflect this trend. Whereas in 1994, only 19% of applicants to medical school held nonscience degrees, about one-third of applicants now hold such degrees.9,10 We have found no aggregated data on double majors entering US medical schools, but public class profiles suggest that medical school matriculants mirror their undergraduate counterparts in their tendency to hold double majors. In 2016, for example, 15% of the incoming class at the University of Michigan Medical School was composed of double majors, increasing to over 25% in 2017.11
Medical Students
Early dual-degree programs in undergraduate medical training were reserved for MD/PhD programs.12 Most US MD/PhD programs (90 out of 151) now offer doctorates in social sciences, humanities, or other nontraditional fields of graduate medical study, reflecting a shift in interests of those seeking dual-degree training in undergraduate medical education.13 While only 3 MD/PhD programs in the 1970s included trainees in the social sciences, 17 such programs exist today.14
Interest in dual-degree programs offering master’s level study has also increased over the past decade. In 2017, 87 medical schools offered programs for students to pursue a master of public health (MPH) and 41 offered master of science degrees in various fields, up from 52 and 37 institutions, respectively in 2006.15 The number of schools offering combined training in nonscience fields has also grown, with 63 institutions now offering a master of business administration (MBA), nearly double the number offered in 2006.15 At some institutions more than 20% of students are earning a master’s degree or doctorate in addition to their MD degree.16
Residents
The authors found no documentation of US residency training programs, outside of those in the specialty of preventive medicine, providing trainees with formal opportunities to obtain an MBA or MPH prior to 2001.17 However, of the 510 internal medicine residency programs listed on the American Medical Association residency and fellowship database (freida.ama-assn.org), 45 identified as having established a pathway for residents to pursue an MBA, MPH, or PhD during residency.18
Over the past 20 years, combined residency programs have increased 49% (from 128 to 191), which is triple the 16% rate (1,350 to 1,562) of increase in programs in internal medicine, pediatrics, family medicine, psychiatry, and emergency medicine.19,20 A 2009 moratorium on the creation of new combined residency programs in psychiatry and neurology was lifted in 2016and is likely to increase the rate of total combined programs.21
The Table shows the number of categorical and combined residency programs available in 1996 and in 2016. Over 2 decades, 17 new specialty combinations became available for residency training. While there were no combined training programs within these 17 new combinations in 1996,there were 66 programs with these combinations in 2016.19,20
Although surgical specialties are notably absent from the list of combined residency options, likely due to the duration of surgical training, some surgical training programs do offer pathways that culminate in combined degrees,22 and a high number of surgery program directors agree that residents should receive formal training in business and practice management.23
The Medical Job Market
Although today’s young physicians are cross-trained in multiple disciplines, the current job market may not directly match these skill sets. Of the 7,235 jobs listed by the New England Journal of Medicine (NEJM) career center (www.nejmcareercenter.org/jobs), only 54 were targeted at those with combined training, the majority of which were aimed at those trained in internal medicine/pediatrics. Of the combined specialties in the Table, formal positions were listed for only 6.24 A search of nearly 1,500 federal medical positions on USAJOBS (www.usajobs.gov) found only 4 jobs that combined specialties, all restricted to internal medicine/pediatrics.25 When searching for jobs containing the terms MBA, MPH, and public health there were only 8 such positions on NEJM and 7 on USAJOBS.24,25 Although the totality of the medical marketplace may not be best encompassed by these sources, the authors believe NEJM and USAJOBS are somewhat representative of the opportunities for physicians in the US.
Medical jobs tailored to cross-trained physicians do not appear to have kept pace with the numbers of such specialists currently in medical school and residency training. Though millennials are cross-training in increasing numbers, we surmise that they are not doing so as a direct result of the job market.
Future Medicine
Regardless of the mismatch between cross-trained physicians and the current job market, millennials may be well suited for future health systems. In 2001, the National Academies of Sciences, Engineering and Medicine (NASEM) called for increasing interdisciplinary training and improving cross-functional team performance as a major goal for health care providers in twenty-first century health systems.26 NASEM also recommended that academic medical centers develop medical leaders who can manage systems changes required to enhance health, a proposal supported by the fact that hospitals with medically trained CEOs outperform others.27,28
Public Health 3.0, a federal initiative to improve and integrate public health efforts, also emphasizes cross-disciplinary teams and cross-sector partnerships,29 while the Centers for Medicare and Medicaid Services (CMS) has incentivized the development of interprofessional health care teams.30 While cross-training does not automatically connote interdisciplinary training, we believe that cross-training may reveal or develop an interdisciplinary mind-set that may support and embrace interdisciplinary performance. Finally, the US Department of Health and Human Services’ (HHS) Strategic Goals emphasize integrated care for vulnerable populations, something that cross-trained physicians may be especially poised to accomplish.31
A Path Forward
The education, training, and priorities of young physicians demonstrates career interests that diverge from mainstream, traditional options. Data provided herein describe the increasing rates at which millennial physicians are cross-training and have suggested that the current marketplace may not match the interests of this population. The ultimate question is where such cross-trained physicians fit into today’s (or tomorrow’s) health system?
It may be easiest to deploy cross-trained physicians in their respective clinical departments (eg, having a physician trained in internal medicine and pediatrics perform clinical duties in both a medicine department and a pediatrics department). But < 40% of dual-boarded physicians practice both specialties in which they’re trained, so other opportunities should be pursued.32,33 One strategy may be to embrace the promise of interdisciplinary care, as supported by Public Health 3.0 and NASEM.26,29 Our evidence may demonstrate that the interdisciplinary mind-set may be more readily evident in the millennial generation, and that this mind-set may improve interdisciplinary care.
As health is impacted both by direct clinical care as well as programs designed to address population health, cross-trained physicians may be better equipped to integrate aspects of clinical care spanning a variety of clinical fields as well as orchestrating programs designed to improve health at the population level. This mind-set may be best captured by organizations willing to adapt their medical positions to emphasize multidisciplinary training, skills, and capabilities. For example, a physician trained in internal medicine and psychiatry may have the unique training and skill-set to establish an integrated behavioral health clinic that crosses boundaries between traditional departments, emphasizing the whole health of the clinic’s population and not simply focusing on providing services of a particular specialty. Hiring cross-trained physicians throughout such a clinic may benefit the operations of the clinic and improve not only the services provided, but ultimately, the health of that clinic’s patients. By embracing cross-trained physicians, health care organizations and educators may better meet the needs of their employees, likely resulting in a more cost-effective investment for employers, employees, and the health system as a whole.4 Additionally, patient health may also improve.
There is evidence that cross-trained physicians are already likely to hold leadership positions compared with their categorically-trained counterparts, and this may reflect the benefits of an interdisciplinary mind-set.33 Perhaps a cross-trained physician is more likely to see beyond standard, specialty-based institutional barriers and develop processes and programs designed for overall patient benefit. Leadership is a skill that many millennials clearly wish to enhance throughout their career.34 Recruiting cross-trained physicians for leadership positions may reveal synergies between such training and an ability to lead health care organizations into the future.
Many millennial physicians are bringing a new set of skills into the medical marketplace. Health organizations should identify ways to recruit for these skills and deploy them within their systems in order to have more dedicated, engaged employees, more effective health systems, and ultimately, healthier patients.
Acknowledgments
Data from this analysis were presented at the 10th Consortium of Universities for Global Health conference in 2019.35
1. Dimock M. Defining generations: where millennials end and generation Z begins. http://www.pewresearch.org/fact-tank/2018/03/01/defining-generations-where-millennials-end-and-post-millennials-begin/. Published January 17, 2019. Accessed November 7, 2019.
2. IHS Inc. The complexities of physician supply and demand: projections from 2014 to 2025. Final report. https://www.modernhealthcare.com/assets/pdf/CH10888123.pdf. Published April 5, 2016. Accessed November 7, 2019.
3. Miller RN. Millennial physicians sound off on state of medicine today. https://wire.ama-assn.org/life-career/millennial-physicians-sound-state-medicine-today. Published March 27, 2017. Accessed November 7, 2019.
4. World Economic Forum. Matching skills and labour market needs: building social partnerships for better skills and better jobs. http://www3.weforum.org/docs/GAC/2014/WEF_GAC_Employment_MatchingSkillsLabourMarket_Report_2014.pdf. Published January 2014. Accessed November 7, 2019.
5. Brint SG, Turk-Bicakci L, Proctor K, Murphy SP. Expanding the social frame of knowledge: interdisciplinary, degree-granting fields in American Colleges and Universities, 1975–2000. Rev High Ed. 2009;32(2):155-183.
6. National Science Foundation. National survey of college graduates. https://www.nsf.gov/statistics/srvygrads. Updated February 2019. Accessed November 7, 2019.
7. Simon CC. Major decisions. New York Times. November 2, 2012. http://www.nytimes.com/2012/11/04/education/edlife/choosing-one-college-major-out-of-hundreds.html. Accessed November 7, 2019.
8. Berry SL, Erin GL, Therese J. Health humanities baccalaureate programs in the United States. http://www.hiram.edu/wp-content/uploads/2017/09/HHBP2017.pdf. Published September 2017. Accessed November 7, 2019.
9. Sorensen NE, Jackson JR. Science majors and nonscience majors entering medical school: acceptance rates and academic performance. NACADA J. 1997;17(1):32-41.
10. Association of American Medical Colleges. Table A-17: MCAT and GPAs for applicants and matriculants to U.S. medical schools by primary undergraduate major, 2019-2020. https://www.aamc.org/download/321496/data/factstablea17.pdf. Published October 16, 2019. Accessed November 7, 2019.
11. University of Michigan Medical School. Many paths, one destination: medical school welcomes its 170th class of medical students. https://medicine.umich.edu/medschool/news/many-paths-one-destination-medical-school-welcomes-its-170th-class-medical-students. Updated July 29, 2016. Accessed November 7, 2019.
12. Harding CV, Akabas MH, Andersen OS. History and outcomes of 50 years of physician-scientist training in medical scientist training programs. Acad Med. 2017; 92(10):1390-1398.
13. Association of American Medical Colleges. MD-PhD in “social sciences or humanities” and “other non-traditional fields of graduate study” - by school. https://students-residents.aamc.org/choosing-medical-career/careers-medical-research/md-phd-dual-degree-training/non-basic-science-phd-training-school/. Accessed November 8, 2019.
14. Holmes SM, Karlin J, Stonington SD, Gottheil DL. The first nationwide survey of MD-PhDs in the social sciences and humanities: training patterns and career choices. BMC Med Educ. 2017;17(1):60.
15. Association of American Medical Colleges Combined degrees and early acceptance programs. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/combined-degrees-and-early-acceptance-programs. Accessed November 8, 2019.
16. Tufts University School of Medicine. 2023 class profile. http://medicine.tufts.edu/Education/MD-Programs/Doctor-of-Medicine/Class-Profile. Published 2015. Accessed November 8, 2019.
17. Zweifler J, Evan R. Development of a residency/MPH program. Family Med. 2001;33(6):453-458.
18. American Medical Association. The AMA residency and fellowship database. http://freida.ama-assn.org/Freida. Accessed November 7, 2019.
19. National Resident Matching Program. NRMP data. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata1996.pdf. Published March 1996. Accessed November 7, 2019.
20. Brotherton SE, Etzel SI. Graduate medical education, 2016-2017. JAMA. 2017;318(23):2368-2387.
21. American Board of Psychiatry and Neurology. Update for psychiatry GME programs on combined training program accreditation/approval February 2012. https://www.umassmed.edu/globalassets/neuropsychiatry/files/combined-program-letter.pdf. Accessed November 7, 2019.
22. Massachusetts General Hospital. Surgical residency program. https://www.massgeneral.org/surgery/education/residency.aspx?id=77. Accessed November 7, 2019.
23. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
24. New England Journal of Medicine. NEJM CareerCenter. http://www.nejmcareercenter.org. Accessed November 7, 2019.
25. US Office of Personnel Management. USAJOBS. https://www.usajobs.gov. Accessed November 7, 2019.
26. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published March 2001. Accessed November 7, 2019.
27. Kohn LT, ed; Committee on the Roles of Academic Health Centers in the 21st Century; Institute of Medicine of the National Academies. Academic Health Centers: Leading Change in the 21st Century. National Academy Press: Washington, DC; 2004.
28. Goodall AH. Physician-leaders and hospital performance: is there an association? http://ftp.iza.org/dp5830.pdf. Published July 2011. Accessed November 7, 2019.
29. US Department of Health and Human Services, Office of the Assistant Secretary for Health. Public health 3.0: a call to action to create a 21st century public health infrastructure. https://www.healthypeople.gov/sites/default/files/Public-Health-3.0-White-Paper.pdf. Accessed November 7, 2019.
30. Centers for Medicare and Medicaid Services. Health care innovation awards round one project profiles. http://innovation.cms.gov/files/x/hcia-project-profiles.pdf. Updated December 2013. Accessed November 7, 2019.
31. US Department of Health and Human Services. Strategic Objective 1.3: Improve Americans’ access to healthcare and expand choices of care and service options. https://www.hhs.gov/about/strategic-plan/strategic-goal-1/index.html#obj_1_3. Updated March 18, 2019. Accessed November 7, 2019.
32. Kessler CS, Stallings LA, Gonzalez AA, Templeman TA. Combined residency training in emergency medicine and internal medicine: an update on career outcomes and job satisfaction. Acad Emerg Med. 2009;16(9):894-899.
33. Summergrad P, Silberman E, Price LL. Practice and career outcomes of double-boarded psychiatrists. Psychosomatics. 2011;52(6):537-543.
34. Rigoni B, Adkins A. What millennials want from a new job. Harvard Business Rev. May 11, 2016. https://hbr.org/2016/05/what-millennials-want-from-a-new-job. Accessed November 7, 2019.
35. Jung P, Smith C. Medical millennials: a mismatch between training preferences and employment opportunities. Lancet Glob Health. 2019;7(suppl 1):S38.
Millennials, defined as those born between 1981 and 1996, currently comprise 15% of all active physicians in the US.1,2 A recent survey found that nearly 4 of 5 US millennial physicians have a desire for cross-sectional work in areas beyond patient care, such as academic research, health care consulting, entrepreneurship, and health care administration.3
For employers and educators, a better understanding of these preferences, through consideration of the unique education and skill set of the millennial physician workforce, may lead to more effective recruitment of young physicians and improved health systems, avoiding a mismatch between health care provider skills and available jobs that can be costly for both employers and employees.4
This article describes how US millennial physicians are choosing to cross-train (obtaining multiple degrees and/or completing combined medical residency training) throughout undergraduate, medical, and graduate medical education. We also outline ways in which the current physician marketplace may not match the skills of this population and suggest some ways that health care organizations could capitalize on this trend toward more cross-trained personnel in order to effectively recruit and retain the next generation of physicians.
Millennial Education
Undergraduates
The number of interdisciplinary undergraduate majors increased by almost 250% from 1975 to 2000.5 In 2010, nearly 20% of US college students graduated with 2 majors, representing a 70% increase in double majors between 2001 and 2011.6,7 One emerging category of interdisciplinary majors in US colleges is health humanities programs, which have quadrupled since 2000.8
Medical school applicants and matriculants reflect this trend. Whereas in 1994, only 19% of applicants to medical school held nonscience degrees, about one-third of applicants now hold such degrees.9,10 We have found no aggregated data on double majors entering US medical schools, but public class profiles suggest that medical school matriculants mirror their undergraduate counterparts in their tendency to hold double majors. In 2016, for example, 15% of the incoming class at the University of Michigan Medical School was composed of double majors, increasing to over 25% in 2017.11
Medical Students
Early dual-degree programs in undergraduate medical training were reserved for MD/PhD programs.12 Most US MD/PhD programs (90 out of 151) now offer doctorates in social sciences, humanities, or other nontraditional fields of graduate medical study, reflecting a shift in interests of those seeking dual-degree training in undergraduate medical education.13 While only 3 MD/PhD programs in the 1970s included trainees in the social sciences, 17 such programs exist today.14
Interest in dual-degree programs offering master’s level study has also increased over the past decade. In 2017, 87 medical schools offered programs for students to pursue a master of public health (MPH) and 41 offered master of science degrees in various fields, up from 52 and 37 institutions, respectively in 2006.15 The number of schools offering combined training in nonscience fields has also grown, with 63 institutions now offering a master of business administration (MBA), nearly double the number offered in 2006.15 At some institutions more than 20% of students are earning a master’s degree or doctorate in addition to their MD degree.16
Residents
The authors found no documentation of US residency training programs, outside of those in the specialty of preventive medicine, providing trainees with formal opportunities to obtain an MBA or MPH prior to 2001.17 However, of the 510 internal medicine residency programs listed on the American Medical Association residency and fellowship database (freida.ama-assn.org), 45 identified as having established a pathway for residents to pursue an MBA, MPH, or PhD during residency.18
Over the past 20 years, combined residency programs have increased 49% (from 128 to 191), which is triple the 16% rate (1,350 to 1,562) of increase in programs in internal medicine, pediatrics, family medicine, psychiatry, and emergency medicine.19,20 A 2009 moratorium on the creation of new combined residency programs in psychiatry and neurology was lifted in 2016and is likely to increase the rate of total combined programs.21
The Table shows the number of categorical and combined residency programs available in 1996 and in 2016. Over 2 decades, 17 new specialty combinations became available for residency training. While there were no combined training programs within these 17 new combinations in 1996,there were 66 programs with these combinations in 2016.19,20
Although surgical specialties are notably absent from the list of combined residency options, likely due to the duration of surgical training, some surgical training programs do offer pathways that culminate in combined degrees,22 and a high number of surgery program directors agree that residents should receive formal training in business and practice management.23
The Medical Job Market
Although today’s young physicians are cross-trained in multiple disciplines, the current job market may not directly match these skill sets. Of the 7,235 jobs listed by the New England Journal of Medicine (NEJM) career center (www.nejmcareercenter.org/jobs), only 54 were targeted at those with combined training, the majority of which were aimed at those trained in internal medicine/pediatrics. Of the combined specialties in the Table, formal positions were listed for only 6.24 A search of nearly 1,500 federal medical positions on USAJOBS (www.usajobs.gov) found only 4 jobs that combined specialties, all restricted to internal medicine/pediatrics.25 When searching for jobs containing the terms MBA, MPH, and public health there were only 8 such positions on NEJM and 7 on USAJOBS.24,25 Although the totality of the medical marketplace may not be best encompassed by these sources, the authors believe NEJM and USAJOBS are somewhat representative of the opportunities for physicians in the US.
Medical jobs tailored to cross-trained physicians do not appear to have kept pace with the numbers of such specialists currently in medical school and residency training. Though millennials are cross-training in increasing numbers, we surmise that they are not doing so as a direct result of the job market.
Future Medicine
Regardless of the mismatch between cross-trained physicians and the current job market, millennials may be well suited for future health systems. In 2001, the National Academies of Sciences, Engineering and Medicine (NASEM) called for increasing interdisciplinary training and improving cross-functional team performance as a major goal for health care providers in twenty-first century health systems.26 NASEM also recommended that academic medical centers develop medical leaders who can manage systems changes required to enhance health, a proposal supported by the fact that hospitals with medically trained CEOs outperform others.27,28
Public Health 3.0, a federal initiative to improve and integrate public health efforts, also emphasizes cross-disciplinary teams and cross-sector partnerships,29 while the Centers for Medicare and Medicaid Services (CMS) has incentivized the development of interprofessional health care teams.30 While cross-training does not automatically connote interdisciplinary training, we believe that cross-training may reveal or develop an interdisciplinary mind-set that may support and embrace interdisciplinary performance. Finally, the US Department of Health and Human Services’ (HHS) Strategic Goals emphasize integrated care for vulnerable populations, something that cross-trained physicians may be especially poised to accomplish.31
A Path Forward
The education, training, and priorities of young physicians demonstrates career interests that diverge from mainstream, traditional options. Data provided herein describe the increasing rates at which millennial physicians are cross-training and have suggested that the current marketplace may not match the interests of this population. The ultimate question is where such cross-trained physicians fit into today’s (or tomorrow’s) health system?
It may be easiest to deploy cross-trained physicians in their respective clinical departments (eg, having a physician trained in internal medicine and pediatrics perform clinical duties in both a medicine department and a pediatrics department). But < 40% of dual-boarded physicians practice both specialties in which they’re trained, so other opportunities should be pursued.32,33 One strategy may be to embrace the promise of interdisciplinary care, as supported by Public Health 3.0 and NASEM.26,29 Our evidence may demonstrate that the interdisciplinary mind-set may be more readily evident in the millennial generation, and that this mind-set may improve interdisciplinary care.
As health is impacted both by direct clinical care as well as programs designed to address population health, cross-trained physicians may be better equipped to integrate aspects of clinical care spanning a variety of clinical fields as well as orchestrating programs designed to improve health at the population level. This mind-set may be best captured by organizations willing to adapt their medical positions to emphasize multidisciplinary training, skills, and capabilities. For example, a physician trained in internal medicine and psychiatry may have the unique training and skill-set to establish an integrated behavioral health clinic that crosses boundaries between traditional departments, emphasizing the whole health of the clinic’s population and not simply focusing on providing services of a particular specialty. Hiring cross-trained physicians throughout such a clinic may benefit the operations of the clinic and improve not only the services provided, but ultimately, the health of that clinic’s patients. By embracing cross-trained physicians, health care organizations and educators may better meet the needs of their employees, likely resulting in a more cost-effective investment for employers, employees, and the health system as a whole.4 Additionally, patient health may also improve.
There is evidence that cross-trained physicians are already likely to hold leadership positions compared with their categorically-trained counterparts, and this may reflect the benefits of an interdisciplinary mind-set.33 Perhaps a cross-trained physician is more likely to see beyond standard, specialty-based institutional barriers and develop processes and programs designed for overall patient benefit. Leadership is a skill that many millennials clearly wish to enhance throughout their career.34 Recruiting cross-trained physicians for leadership positions may reveal synergies between such training and an ability to lead health care organizations into the future.
Many millennial physicians are bringing a new set of skills into the medical marketplace. Health organizations should identify ways to recruit for these skills and deploy them within their systems in order to have more dedicated, engaged employees, more effective health systems, and ultimately, healthier patients.
Acknowledgments
Data from this analysis were presented at the 10th Consortium of Universities for Global Health conference in 2019.35
Millennials, defined as those born between 1981 and 1996, currently comprise 15% of all active physicians in the US.1,2 A recent survey found that nearly 4 of 5 US millennial physicians have a desire for cross-sectional work in areas beyond patient care, such as academic research, health care consulting, entrepreneurship, and health care administration.3
For employers and educators, a better understanding of these preferences, through consideration of the unique education and skill set of the millennial physician workforce, may lead to more effective recruitment of young physicians and improved health systems, avoiding a mismatch between health care provider skills and available jobs that can be costly for both employers and employees.4
This article describes how US millennial physicians are choosing to cross-train (obtaining multiple degrees and/or completing combined medical residency training) throughout undergraduate, medical, and graduate medical education. We also outline ways in which the current physician marketplace may not match the skills of this population and suggest some ways that health care organizations could capitalize on this trend toward more cross-trained personnel in order to effectively recruit and retain the next generation of physicians.
Millennial Education
Undergraduates
The number of interdisciplinary undergraduate majors increased by almost 250% from 1975 to 2000.5 In 2010, nearly 20% of US college students graduated with 2 majors, representing a 70% increase in double majors between 2001 and 2011.6,7 One emerging category of interdisciplinary majors in US colleges is health humanities programs, which have quadrupled since 2000.8
Medical school applicants and matriculants reflect this trend. Whereas in 1994, only 19% of applicants to medical school held nonscience degrees, about one-third of applicants now hold such degrees.9,10 We have found no aggregated data on double majors entering US medical schools, but public class profiles suggest that medical school matriculants mirror their undergraduate counterparts in their tendency to hold double majors. In 2016, for example, 15% of the incoming class at the University of Michigan Medical School was composed of double majors, increasing to over 25% in 2017.11
Medical Students
Early dual-degree programs in undergraduate medical training were reserved for MD/PhD programs.12 Most US MD/PhD programs (90 out of 151) now offer doctorates in social sciences, humanities, or other nontraditional fields of graduate medical study, reflecting a shift in interests of those seeking dual-degree training in undergraduate medical education.13 While only 3 MD/PhD programs in the 1970s included trainees in the social sciences, 17 such programs exist today.14
Interest in dual-degree programs offering master’s level study has also increased over the past decade. In 2017, 87 medical schools offered programs for students to pursue a master of public health (MPH) and 41 offered master of science degrees in various fields, up from 52 and 37 institutions, respectively in 2006.15 The number of schools offering combined training in nonscience fields has also grown, with 63 institutions now offering a master of business administration (MBA), nearly double the number offered in 2006.15 At some institutions more than 20% of students are earning a master’s degree or doctorate in addition to their MD degree.16
Residents
The authors found no documentation of US residency training programs, outside of those in the specialty of preventive medicine, providing trainees with formal opportunities to obtain an MBA or MPH prior to 2001.17 However, of the 510 internal medicine residency programs listed on the American Medical Association residency and fellowship database (freida.ama-assn.org), 45 identified as having established a pathway for residents to pursue an MBA, MPH, or PhD during residency.18
Over the past 20 years, combined residency programs have increased 49% (from 128 to 191), which is triple the 16% rate (1,350 to 1,562) of increase in programs in internal medicine, pediatrics, family medicine, psychiatry, and emergency medicine.19,20 A 2009 moratorium on the creation of new combined residency programs in psychiatry and neurology was lifted in 2016and is likely to increase the rate of total combined programs.21
The Table shows the number of categorical and combined residency programs available in 1996 and in 2016. Over 2 decades, 17 new specialty combinations became available for residency training. While there were no combined training programs within these 17 new combinations in 1996,there were 66 programs with these combinations in 2016.19,20
Although surgical specialties are notably absent from the list of combined residency options, likely due to the duration of surgical training, some surgical training programs do offer pathways that culminate in combined degrees,22 and a high number of surgery program directors agree that residents should receive formal training in business and practice management.23
The Medical Job Market
Although today’s young physicians are cross-trained in multiple disciplines, the current job market may not directly match these skill sets. Of the 7,235 jobs listed by the New England Journal of Medicine (NEJM) career center (www.nejmcareercenter.org/jobs), only 54 were targeted at those with combined training, the majority of which were aimed at those trained in internal medicine/pediatrics. Of the combined specialties in the Table, formal positions were listed for only 6.24 A search of nearly 1,500 federal medical positions on USAJOBS (www.usajobs.gov) found only 4 jobs that combined specialties, all restricted to internal medicine/pediatrics.25 When searching for jobs containing the terms MBA, MPH, and public health there were only 8 such positions on NEJM and 7 on USAJOBS.24,25 Although the totality of the medical marketplace may not be best encompassed by these sources, the authors believe NEJM and USAJOBS are somewhat representative of the opportunities for physicians in the US.
Medical jobs tailored to cross-trained physicians do not appear to have kept pace with the numbers of such specialists currently in medical school and residency training. Though millennials are cross-training in increasing numbers, we surmise that they are not doing so as a direct result of the job market.
Future Medicine
Regardless of the mismatch between cross-trained physicians and the current job market, millennials may be well suited for future health systems. In 2001, the National Academies of Sciences, Engineering and Medicine (NASEM) called for increasing interdisciplinary training and improving cross-functional team performance as a major goal for health care providers in twenty-first century health systems.26 NASEM also recommended that academic medical centers develop medical leaders who can manage systems changes required to enhance health, a proposal supported by the fact that hospitals with medically trained CEOs outperform others.27,28
Public Health 3.0, a federal initiative to improve and integrate public health efforts, also emphasizes cross-disciplinary teams and cross-sector partnerships,29 while the Centers for Medicare and Medicaid Services (CMS) has incentivized the development of interprofessional health care teams.30 While cross-training does not automatically connote interdisciplinary training, we believe that cross-training may reveal or develop an interdisciplinary mind-set that may support and embrace interdisciplinary performance. Finally, the US Department of Health and Human Services’ (HHS) Strategic Goals emphasize integrated care for vulnerable populations, something that cross-trained physicians may be especially poised to accomplish.31
A Path Forward
The education, training, and priorities of young physicians demonstrates career interests that diverge from mainstream, traditional options. Data provided herein describe the increasing rates at which millennial physicians are cross-training and have suggested that the current marketplace may not match the interests of this population. The ultimate question is where such cross-trained physicians fit into today’s (or tomorrow’s) health system?
It may be easiest to deploy cross-trained physicians in their respective clinical departments (eg, having a physician trained in internal medicine and pediatrics perform clinical duties in both a medicine department and a pediatrics department). But < 40% of dual-boarded physicians practice both specialties in which they’re trained, so other opportunities should be pursued.32,33 One strategy may be to embrace the promise of interdisciplinary care, as supported by Public Health 3.0 and NASEM.26,29 Our evidence may demonstrate that the interdisciplinary mind-set may be more readily evident in the millennial generation, and that this mind-set may improve interdisciplinary care.
As health is impacted both by direct clinical care as well as programs designed to address population health, cross-trained physicians may be better equipped to integrate aspects of clinical care spanning a variety of clinical fields as well as orchestrating programs designed to improve health at the population level. This mind-set may be best captured by organizations willing to adapt their medical positions to emphasize multidisciplinary training, skills, and capabilities. For example, a physician trained in internal medicine and psychiatry may have the unique training and skill-set to establish an integrated behavioral health clinic that crosses boundaries between traditional departments, emphasizing the whole health of the clinic’s population and not simply focusing on providing services of a particular specialty. Hiring cross-trained physicians throughout such a clinic may benefit the operations of the clinic and improve not only the services provided, but ultimately, the health of that clinic’s patients. By embracing cross-trained physicians, health care organizations and educators may better meet the needs of their employees, likely resulting in a more cost-effective investment for employers, employees, and the health system as a whole.4 Additionally, patient health may also improve.
There is evidence that cross-trained physicians are already likely to hold leadership positions compared with their categorically-trained counterparts, and this may reflect the benefits of an interdisciplinary mind-set.33 Perhaps a cross-trained physician is more likely to see beyond standard, specialty-based institutional barriers and develop processes and programs designed for overall patient benefit. Leadership is a skill that many millennials clearly wish to enhance throughout their career.34 Recruiting cross-trained physicians for leadership positions may reveal synergies between such training and an ability to lead health care organizations into the future.
Many millennial physicians are bringing a new set of skills into the medical marketplace. Health organizations should identify ways to recruit for these skills and deploy them within their systems in order to have more dedicated, engaged employees, more effective health systems, and ultimately, healthier patients.
Acknowledgments
Data from this analysis were presented at the 10th Consortium of Universities for Global Health conference in 2019.35
1. Dimock M. Defining generations: where millennials end and generation Z begins. http://www.pewresearch.org/fact-tank/2018/03/01/defining-generations-where-millennials-end-and-post-millennials-begin/. Published January 17, 2019. Accessed November 7, 2019.
2. IHS Inc. The complexities of physician supply and demand: projections from 2014 to 2025. Final report. https://www.modernhealthcare.com/assets/pdf/CH10888123.pdf. Published April 5, 2016. Accessed November 7, 2019.
3. Miller RN. Millennial physicians sound off on state of medicine today. https://wire.ama-assn.org/life-career/millennial-physicians-sound-state-medicine-today. Published March 27, 2017. Accessed November 7, 2019.
4. World Economic Forum. Matching skills and labour market needs: building social partnerships for better skills and better jobs. http://www3.weforum.org/docs/GAC/2014/WEF_GAC_Employment_MatchingSkillsLabourMarket_Report_2014.pdf. Published January 2014. Accessed November 7, 2019.
5. Brint SG, Turk-Bicakci L, Proctor K, Murphy SP. Expanding the social frame of knowledge: interdisciplinary, degree-granting fields in American Colleges and Universities, 1975–2000. Rev High Ed. 2009;32(2):155-183.
6. National Science Foundation. National survey of college graduates. https://www.nsf.gov/statistics/srvygrads. Updated February 2019. Accessed November 7, 2019.
7. Simon CC. Major decisions. New York Times. November 2, 2012. http://www.nytimes.com/2012/11/04/education/edlife/choosing-one-college-major-out-of-hundreds.html. Accessed November 7, 2019.
8. Berry SL, Erin GL, Therese J. Health humanities baccalaureate programs in the United States. http://www.hiram.edu/wp-content/uploads/2017/09/HHBP2017.pdf. Published September 2017. Accessed November 7, 2019.
9. Sorensen NE, Jackson JR. Science majors and nonscience majors entering medical school: acceptance rates and academic performance. NACADA J. 1997;17(1):32-41.
10. Association of American Medical Colleges. Table A-17: MCAT and GPAs for applicants and matriculants to U.S. medical schools by primary undergraduate major, 2019-2020. https://www.aamc.org/download/321496/data/factstablea17.pdf. Published October 16, 2019. Accessed November 7, 2019.
11. University of Michigan Medical School. Many paths, one destination: medical school welcomes its 170th class of medical students. https://medicine.umich.edu/medschool/news/many-paths-one-destination-medical-school-welcomes-its-170th-class-medical-students. Updated July 29, 2016. Accessed November 7, 2019.
12. Harding CV, Akabas MH, Andersen OS. History and outcomes of 50 years of physician-scientist training in medical scientist training programs. Acad Med. 2017; 92(10):1390-1398.
13. Association of American Medical Colleges. MD-PhD in “social sciences or humanities” and “other non-traditional fields of graduate study” - by school. https://students-residents.aamc.org/choosing-medical-career/careers-medical-research/md-phd-dual-degree-training/non-basic-science-phd-training-school/. Accessed November 8, 2019.
14. Holmes SM, Karlin J, Stonington SD, Gottheil DL. The first nationwide survey of MD-PhDs in the social sciences and humanities: training patterns and career choices. BMC Med Educ. 2017;17(1):60.
15. Association of American Medical Colleges Combined degrees and early acceptance programs. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/combined-degrees-and-early-acceptance-programs. Accessed November 8, 2019.
16. Tufts University School of Medicine. 2023 class profile. http://medicine.tufts.edu/Education/MD-Programs/Doctor-of-Medicine/Class-Profile. Published 2015. Accessed November 8, 2019.
17. Zweifler J, Evan R. Development of a residency/MPH program. Family Med. 2001;33(6):453-458.
18. American Medical Association. The AMA residency and fellowship database. http://freida.ama-assn.org/Freida. Accessed November 7, 2019.
19. National Resident Matching Program. NRMP data. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata1996.pdf. Published March 1996. Accessed November 7, 2019.
20. Brotherton SE, Etzel SI. Graduate medical education, 2016-2017. JAMA. 2017;318(23):2368-2387.
21. American Board of Psychiatry and Neurology. Update for psychiatry GME programs on combined training program accreditation/approval February 2012. https://www.umassmed.edu/globalassets/neuropsychiatry/files/combined-program-letter.pdf. Accessed November 7, 2019.
22. Massachusetts General Hospital. Surgical residency program. https://www.massgeneral.org/surgery/education/residency.aspx?id=77. Accessed November 7, 2019.
23. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
24. New England Journal of Medicine. NEJM CareerCenter. http://www.nejmcareercenter.org. Accessed November 7, 2019.
25. US Office of Personnel Management. USAJOBS. https://www.usajobs.gov. Accessed November 7, 2019.
26. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published March 2001. Accessed November 7, 2019.
27. Kohn LT, ed; Committee on the Roles of Academic Health Centers in the 21st Century; Institute of Medicine of the National Academies. Academic Health Centers: Leading Change in the 21st Century. National Academy Press: Washington, DC; 2004.
28. Goodall AH. Physician-leaders and hospital performance: is there an association? http://ftp.iza.org/dp5830.pdf. Published July 2011. Accessed November 7, 2019.
29. US Department of Health and Human Services, Office of the Assistant Secretary for Health. Public health 3.0: a call to action to create a 21st century public health infrastructure. https://www.healthypeople.gov/sites/default/files/Public-Health-3.0-White-Paper.pdf. Accessed November 7, 2019.
30. Centers for Medicare and Medicaid Services. Health care innovation awards round one project profiles. http://innovation.cms.gov/files/x/hcia-project-profiles.pdf. Updated December 2013. Accessed November 7, 2019.
31. US Department of Health and Human Services. Strategic Objective 1.3: Improve Americans’ access to healthcare and expand choices of care and service options. https://www.hhs.gov/about/strategic-plan/strategic-goal-1/index.html#obj_1_3. Updated March 18, 2019. Accessed November 7, 2019.
32. Kessler CS, Stallings LA, Gonzalez AA, Templeman TA. Combined residency training in emergency medicine and internal medicine: an update on career outcomes and job satisfaction. Acad Emerg Med. 2009;16(9):894-899.
33. Summergrad P, Silberman E, Price LL. Practice and career outcomes of double-boarded psychiatrists. Psychosomatics. 2011;52(6):537-543.
34. Rigoni B, Adkins A. What millennials want from a new job. Harvard Business Rev. May 11, 2016. https://hbr.org/2016/05/what-millennials-want-from-a-new-job. Accessed November 7, 2019.
35. Jung P, Smith C. Medical millennials: a mismatch between training preferences and employment opportunities. Lancet Glob Health. 2019;7(suppl 1):S38.
1. Dimock M. Defining generations: where millennials end and generation Z begins. http://www.pewresearch.org/fact-tank/2018/03/01/defining-generations-where-millennials-end-and-post-millennials-begin/. Published January 17, 2019. Accessed November 7, 2019.
2. IHS Inc. The complexities of physician supply and demand: projections from 2014 to 2025. Final report. https://www.modernhealthcare.com/assets/pdf/CH10888123.pdf. Published April 5, 2016. Accessed November 7, 2019.
3. Miller RN. Millennial physicians sound off on state of medicine today. https://wire.ama-assn.org/life-career/millennial-physicians-sound-state-medicine-today. Published March 27, 2017. Accessed November 7, 2019.
4. World Economic Forum. Matching skills and labour market needs: building social partnerships for better skills and better jobs. http://www3.weforum.org/docs/GAC/2014/WEF_GAC_Employment_MatchingSkillsLabourMarket_Report_2014.pdf. Published January 2014. Accessed November 7, 2019.
5. Brint SG, Turk-Bicakci L, Proctor K, Murphy SP. Expanding the social frame of knowledge: interdisciplinary, degree-granting fields in American Colleges and Universities, 1975–2000. Rev High Ed. 2009;32(2):155-183.
6. National Science Foundation. National survey of college graduates. https://www.nsf.gov/statistics/srvygrads. Updated February 2019. Accessed November 7, 2019.
7. Simon CC. Major decisions. New York Times. November 2, 2012. http://www.nytimes.com/2012/11/04/education/edlife/choosing-one-college-major-out-of-hundreds.html. Accessed November 7, 2019.
8. Berry SL, Erin GL, Therese J. Health humanities baccalaureate programs in the United States. http://www.hiram.edu/wp-content/uploads/2017/09/HHBP2017.pdf. Published September 2017. Accessed November 7, 2019.
9. Sorensen NE, Jackson JR. Science majors and nonscience majors entering medical school: acceptance rates and academic performance. NACADA J. 1997;17(1):32-41.
10. Association of American Medical Colleges. Table A-17: MCAT and GPAs for applicants and matriculants to U.S. medical schools by primary undergraduate major, 2019-2020. https://www.aamc.org/download/321496/data/factstablea17.pdf. Published October 16, 2019. Accessed November 7, 2019.
11. University of Michigan Medical School. Many paths, one destination: medical school welcomes its 170th class of medical students. https://medicine.umich.edu/medschool/news/many-paths-one-destination-medical-school-welcomes-its-170th-class-medical-students. Updated July 29, 2016. Accessed November 7, 2019.
12. Harding CV, Akabas MH, Andersen OS. History and outcomes of 50 years of physician-scientist training in medical scientist training programs. Acad Med. 2017; 92(10):1390-1398.
13. Association of American Medical Colleges. MD-PhD in “social sciences or humanities” and “other non-traditional fields of graduate study” - by school. https://students-residents.aamc.org/choosing-medical-career/careers-medical-research/md-phd-dual-degree-training/non-basic-science-phd-training-school/. Accessed November 8, 2019.
14. Holmes SM, Karlin J, Stonington SD, Gottheil DL. The first nationwide survey of MD-PhDs in the social sciences and humanities: training patterns and career choices. BMC Med Educ. 2017;17(1):60.
15. Association of American Medical Colleges Combined degrees and early acceptance programs. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/combined-degrees-and-early-acceptance-programs. Accessed November 8, 2019.
16. Tufts University School of Medicine. 2023 class profile. http://medicine.tufts.edu/Education/MD-Programs/Doctor-of-Medicine/Class-Profile. Published 2015. Accessed November 8, 2019.
17. Zweifler J, Evan R. Development of a residency/MPH program. Family Med. 2001;33(6):453-458.
18. American Medical Association. The AMA residency and fellowship database. http://freida.ama-assn.org/Freida. Accessed November 7, 2019.
19. National Resident Matching Program. NRMP data. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata1996.pdf. Published March 1996. Accessed November 7, 2019.
20. Brotherton SE, Etzel SI. Graduate medical education, 2016-2017. JAMA. 2017;318(23):2368-2387.
21. American Board of Psychiatry and Neurology. Update for psychiatry GME programs on combined training program accreditation/approval February 2012. https://www.umassmed.edu/globalassets/neuropsychiatry/files/combined-program-letter.pdf. Accessed November 7, 2019.
22. Massachusetts General Hospital. Surgical residency program. https://www.massgeneral.org/surgery/education/residency.aspx?id=77. Accessed November 7, 2019.
23. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
24. New England Journal of Medicine. NEJM CareerCenter. http://www.nejmcareercenter.org. Accessed November 7, 2019.
25. US Office of Personnel Management. USAJOBS. https://www.usajobs.gov. Accessed November 7, 2019.
26. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published March 2001. Accessed November 7, 2019.
27. Kohn LT, ed; Committee on the Roles of Academic Health Centers in the 21st Century; Institute of Medicine of the National Academies. Academic Health Centers: Leading Change in the 21st Century. National Academy Press: Washington, DC; 2004.
28. Goodall AH. Physician-leaders and hospital performance: is there an association? http://ftp.iza.org/dp5830.pdf. Published July 2011. Accessed November 7, 2019.
29. US Department of Health and Human Services, Office of the Assistant Secretary for Health. Public health 3.0: a call to action to create a 21st century public health infrastructure. https://www.healthypeople.gov/sites/default/files/Public-Health-3.0-White-Paper.pdf. Accessed November 7, 2019.
30. Centers for Medicare and Medicaid Services. Health care innovation awards round one project profiles. http://innovation.cms.gov/files/x/hcia-project-profiles.pdf. Updated December 2013. Accessed November 7, 2019.
31. US Department of Health and Human Services. Strategic Objective 1.3: Improve Americans’ access to healthcare and expand choices of care and service options. https://www.hhs.gov/about/strategic-plan/strategic-goal-1/index.html#obj_1_3. Updated March 18, 2019. Accessed November 7, 2019.
32. Kessler CS, Stallings LA, Gonzalez AA, Templeman TA. Combined residency training in emergency medicine and internal medicine: an update on career outcomes and job satisfaction. Acad Emerg Med. 2009;16(9):894-899.
33. Summergrad P, Silberman E, Price LL. Practice and career outcomes of double-boarded psychiatrists. Psychosomatics. 2011;52(6):537-543.
34. Rigoni B, Adkins A. What millennials want from a new job. Harvard Business Rev. May 11, 2016. https://hbr.org/2016/05/what-millennials-want-from-a-new-job. Accessed November 7, 2019.
35. Jung P, Smith C. Medical millennials: a mismatch between training preferences and employment opportunities. Lancet Glob Health. 2019;7(suppl 1):S38.
Understanding Principles of High Reliability Organizations Through the Eyes of VIONE, A Clinical Program to Improve Patient Safety by Deprescribing Potentially Inappropriate Medications and Reducing Polypharmacy
High reliability organizations (HROs) incorporate continuous process improvement through leadership commitment to create a safety culture that works toward creating a zero-harm environment.1 The Veterans Health Administration (VHA) has set transformational goals for becoming an HRO. In this article, we describe VIONE, an expanding medication deprescribing clinical program, which exemplifies the translation of HRO principles into health care system models. Both VIONE and HRO are globally relevant.
Reducing medication errors and related adverse drug events are important for achieving zero harm. Preventable medical errors rank behind heart disease and cancer as the third leading cause of death in the US.2 The simultaneous use of multiple medications can lead to dangerous drug interactions, adverse outcomes, and challenges with adherence. When a person is taking multiple medicines, known as polypharmacy, it is more likely that some are potentially inappropriate medications (PIM). Current literature highlights the prevalence and dangers of polypharmacy, which ranks among the top 10 common causes of death in the US, as well as suggestions to address preventable adverse outcomes from polypharmacy and PIM.3-5
Deprescribing of PIM frequently results in better disease management with improved health outcomes and quality of life.4 Many health care settings lack standardized approaches or set expectations to proactively deprescribe PIM. There has been insufficient emphasis on how to make decisions for deprescribing medications when therapeutic benefits are not clear and/or when the adverse effects may outweigh the therapeutic benefits.5
It is imperative to provide practice guidance for deprescribing nonessential medications along with systems-based infrastructure to enable integrated and effective assessments during opportune moments in the health care continuum. Multimodal approaches that include education, risk stratification, population health management interventions, research and resource allocation can help transform organizational culture in health care facilities toward HRO models of care, aiming at zero harm to patients.
The practical lessons learned from VIONE implementation science experiences on various scales and under diverse circumstances, cumulative wisdom from hindsight, foresight and critical insights gathered during nationwide spread of VIONE over the past 3 years continues to propel us toward the desirable direction and core concepts of an HRO.
The VIONE program facilitates practical, real-time interventions that could be tailored to various health care settings, organizational needs, and available resources. VIONE implements an electronic Computerized Patient Record System (CPRS) tool to enable planned cessation of nonessential medications that are potentially harmful, inappropriate, not indicated, or not necessary. The VIONE tool supports systematic, individualized assessment and adjustment through 5 filters (Figure 1). It prompts providers to assign 1 of these filters intuitively and objectively. VIONE combines clinical evidence for best practices, an interprofessional team approach, patient engagement, adapted use of existing medical records systems, and HRO principles for effective implementation.
As a tool to support safer prescribing practices, VIONE aligns closely with HRO principles (Table 1) and core pillars (Table 2).6-8 A zero-harm safety culture necessitates that medications be used for correct reasons, over a correct duration of time, and following a correct schedule while monitoring for adverse outcomes. However, reality generally falls significantly short of this for a myriad of reasons, such as compromised health literacy, functional limitations, affordability, communication gaps, patients seen by multiple providers, and an accumulation of prescriptions due to comorbidities, symptom progression, and management of adverse effects. Through a sharpened focus on both precision medicine and competent prescription management, VIONE is a viable opportunity for investing in the zero-harm philosophy that is integral to an HRO.
Design and Implementation
Initially launched in 2016 in a 15-bed inpatient, subacute rehabilitation unit within a VHA tertiary care facility, VIONE has been sustained and gradually expanded to 38 other VHA facility programs (Figure 2). Recognizing the potential value if adopted into widespread use, VIONE was a Gold Status winner in the VHA Under Secretary for Health Shark Tank-style competition in 2017 and was selected by the VHA Diffusion of Excellence as an innovation worthy of scale and spread through national dissemination.9 A toolkit for VIONE implementation, patient and provider brochures, VIONE vignette, and National Dialog template also have been created.10
Implementing VIONE in a new facility requires an actively engaged core team committed to patient safety and reduction of polypharmacy and PIM, interest and availability to lead project implementation strategies, along with meaningful local organizational support. The current structure for VIONE spread is as follows:
- Interested VHA participants review information and contact [email protected].
- The VIONE team orients implementing champions, mainly pharmacists, physicians, nurse practitioners, and physician assistants at a facility program level, offering guidance and available resources.
- Clinical Application Coordinators at Central Arkansas VA Healthcare System and participating facilities collaborate to add deprescribing menu options in CPRS and install the VIONE Polypharmacy Reminder Dialog template.
- Through close and ongoing collaborations, medical providers and clinical pharmacists proceed with deprescribing, aiming at planned cessation of nonessential and PIM, using the mnemonic prompt of VIONE. Vital and Important medications are continued and consolidated while a methodical plan is developed to deprescribe any medications that could lead to more harm than benefit and qualify based on the filters of Optional, Not indicated, and Every medicine has a diagnosis/reason. They select the proper discontinuation reasons in the CPRS medication menu (Figure 3) and document the rationale in the progress notes. It is highly encouraged that the collaborating pharmacists and health care providers add each other as cosigners and communicate effectively. Clinical pharmacy specialists also use the VIONE Polypharmacy Reminder Dialog Template (RDT) to document complete medication reviews with veterans to include deprescribing rationale and document shared decision making.
- A VIONE national dashboard captures deprescribing data in real time and automates reporting with daily updates that are readily accessible to all implementing facilities. Minimum data captured include the number of unique veterans impacted, number of medications deprescribed, cumulative cost avoidance to date, and number of prescriptions deprescribed per veteran. The dashboard facilitates real-time use of individual patient data and has also been designed to capture data from VHA administrative data portals and Corporate Data Warehouse.
Results
As of October 31, 2019, the assessment of polypharmacy using the VIONE tool across VHA sites has benefited > 60,000 unique veterans, of whom 49.2% were in urban areas, 47.7% in rural areas, and 3.1% in highly rural areas. Elderly male veterans comprised a clear majority. More than 128,000 medications have been deprescribed. The top classes of medications deprescribed are antihypertensives, over-the-counter medications, and antidiabetic medications. An annualized cost avoidance of > $4.0 million has been achieved. Cost avoidance is the cost of medications that otherwise would have continued to be filled and paid for by the VHA if they had not been deprescribed, projected for a maximum of 365 days. The calculation methodology can be summarized as follows:
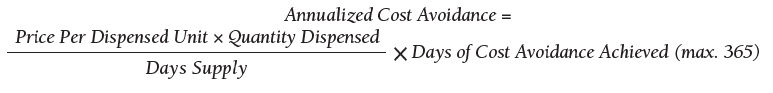
The calculations reported in Table 3 and Figure 4 are conservative and include only chronic outpatient prescriptions and do not account for medications deprescribed in inpatient units, nursing home, community living centers, or domiciliary populations. Data tracked separately from inpatient and community living center patient populations indicated an additional 25,536 deprescribed medications, across 28 VA facilities, impacting 7,076 veterans with an average 2.15 medications deprescribed per veteran. The additional achieved cost avoidance was $370,272 (based on $14.50 average cost per prescription). Medications restarted within 30 days of deprescribing are not included in these calculations.
The cost avoidance calculation further excludes the effects of VIONE implementation on many other types of interventions. These interventions include, but are not limited to, changing from aggressive care to end of life, comfort care when strongly indicated; reduced emergency department visits or invasive diagnostic and therapeutic approaches, when not indicated; medical supplies, antimicrobial preparations; labor costs related to packaging, mailing, and administering prescriptions; reduced/prevented clinical waste; reduced decompensation of systemic illnesses and subsequent health care needs precipitated by iatrogenic disturbances and prolonged convalescence; and overall changes to prescribing practices through purposeful and targeted interactions with colleagues across various disciplines and various hierarchical levels.
Discussion
The VIONE clinical program exemplifies the translation of HRO principles into health care system practices. VIONE offers a systematic approach to improve medication management with an emphasis on deprescribing nonessential medications across various health care settings, facilitating VHA efforts toward zero harm. It demonstrates close alignment with the key building blocks of an HRO. Effective VIONE incorporation into an organizational culture reflects leadership commitment to safety and reliability in their vision and actions. By empowering staff to proactively reduce inappropriate medications and thereby prevent patient harm, VIONE contributes to enhancing an enterprise-wide culture of safety, with fewer errors and greater reliability. As a standardized decision support tool for the ongoing practice of assessment and planned cessation of potentially inappropriate medications, VIONE illustrates how continuous process improvement can be a part of staff-engaged, veteran-centered, highly reliable care. The standardization of the VIONE tool promotes achievement and sustainment of desired HRO principles and practices within health care delivery systems.
Conclusions
The VIONE program was launched not as a cost savings or research program but as a practical, real-time bedside or ambulatory care intervention to improve patient safety. Its value is reflected in the overwhelming response from scholarly and well-engaged colleagues expressing serious interests in expanding collaborations and tailoring efforts to add more depth and breadth to VIONE related efforts.
Acknowledgments
The authors express their gratitude to Central Arkansas VA Healthcare System leadership, Clinical Applications Coordinators, and colleagues for their unconditional support, to the Diffusion of Excellence programs at US Department of Veterans Affairs Central Office for their endorsement, and to the many VHA participants who renew our optimism and energy as we continue this exciting journey. We also thank Bridget B. Kelly for her assistance in writing and editing of the manuscript.
1. Chassin MR, Jerod ML. High-reliability health care: getting there from here. The Joint Commission. Milbank Q. 2013;91(3):459-490.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017;4:170167.
4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
5. Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med. 2016;176(4):482-483.
6. US Department of Veterans Affairs. High reliability. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx. [Nonpublic source, not verified.]
7. Gordon S, Mendenhall P, O’Connor BB. Beyond the Checklist: What Else Health Care Can Learn from Aviation Teamwork and Safety. Ithaca, NY: Cornell University Press; 2013.
8. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
9. US Department of Veterans Affairs. Diffusion of Excellence. https://www.va.gov/HEALTHCAREEXCELLENCE/diffusion-of-excellence/. Updated August 10, 2018. Accessed June 26, 2019.
10. US Department of Veterans Affairs. VIONE program toolkit. https://www.vapulse.net/docs/DOC-259375. [Nonpublic source, not verified.]
High reliability organizations (HROs) incorporate continuous process improvement through leadership commitment to create a safety culture that works toward creating a zero-harm environment.1 The Veterans Health Administration (VHA) has set transformational goals for becoming an HRO. In this article, we describe VIONE, an expanding medication deprescribing clinical program, which exemplifies the translation of HRO principles into health care system models. Both VIONE and HRO are globally relevant.
Reducing medication errors and related adverse drug events are important for achieving zero harm. Preventable medical errors rank behind heart disease and cancer as the third leading cause of death in the US.2 The simultaneous use of multiple medications can lead to dangerous drug interactions, adverse outcomes, and challenges with adherence. When a person is taking multiple medicines, known as polypharmacy, it is more likely that some are potentially inappropriate medications (PIM). Current literature highlights the prevalence and dangers of polypharmacy, which ranks among the top 10 common causes of death in the US, as well as suggestions to address preventable adverse outcomes from polypharmacy and PIM.3-5
Deprescribing of PIM frequently results in better disease management with improved health outcomes and quality of life.4 Many health care settings lack standardized approaches or set expectations to proactively deprescribe PIM. There has been insufficient emphasis on how to make decisions for deprescribing medications when therapeutic benefits are not clear and/or when the adverse effects may outweigh the therapeutic benefits.5
It is imperative to provide practice guidance for deprescribing nonessential medications along with systems-based infrastructure to enable integrated and effective assessments during opportune moments in the health care continuum. Multimodal approaches that include education, risk stratification, population health management interventions, research and resource allocation can help transform organizational culture in health care facilities toward HRO models of care, aiming at zero harm to patients.
The practical lessons learned from VIONE implementation science experiences on various scales and under diverse circumstances, cumulative wisdom from hindsight, foresight and critical insights gathered during nationwide spread of VIONE over the past 3 years continues to propel us toward the desirable direction and core concepts of an HRO.
The VIONE program facilitates practical, real-time interventions that could be tailored to various health care settings, organizational needs, and available resources. VIONE implements an electronic Computerized Patient Record System (CPRS) tool to enable planned cessation of nonessential medications that are potentially harmful, inappropriate, not indicated, or not necessary. The VIONE tool supports systematic, individualized assessment and adjustment through 5 filters (Figure 1). It prompts providers to assign 1 of these filters intuitively and objectively. VIONE combines clinical evidence for best practices, an interprofessional team approach, patient engagement, adapted use of existing medical records systems, and HRO principles for effective implementation.
As a tool to support safer prescribing practices, VIONE aligns closely with HRO principles (Table 1) and core pillars (Table 2).6-8 A zero-harm safety culture necessitates that medications be used for correct reasons, over a correct duration of time, and following a correct schedule while monitoring for adverse outcomes. However, reality generally falls significantly short of this for a myriad of reasons, such as compromised health literacy, functional limitations, affordability, communication gaps, patients seen by multiple providers, and an accumulation of prescriptions due to comorbidities, symptom progression, and management of adverse effects. Through a sharpened focus on both precision medicine and competent prescription management, VIONE is a viable opportunity for investing in the zero-harm philosophy that is integral to an HRO.
Design and Implementation
Initially launched in 2016 in a 15-bed inpatient, subacute rehabilitation unit within a VHA tertiary care facility, VIONE has been sustained and gradually expanded to 38 other VHA facility programs (Figure 2). Recognizing the potential value if adopted into widespread use, VIONE was a Gold Status winner in the VHA Under Secretary for Health Shark Tank-style competition in 2017 and was selected by the VHA Diffusion of Excellence as an innovation worthy of scale and spread through national dissemination.9 A toolkit for VIONE implementation, patient and provider brochures, VIONE vignette, and National Dialog template also have been created.10
Implementing VIONE in a new facility requires an actively engaged core team committed to patient safety and reduction of polypharmacy and PIM, interest and availability to lead project implementation strategies, along with meaningful local organizational support. The current structure for VIONE spread is as follows:
- Interested VHA participants review information and contact [email protected].
- The VIONE team orients implementing champions, mainly pharmacists, physicians, nurse practitioners, and physician assistants at a facility program level, offering guidance and available resources.
- Clinical Application Coordinators at Central Arkansas VA Healthcare System and participating facilities collaborate to add deprescribing menu options in CPRS and install the VIONE Polypharmacy Reminder Dialog template.
- Through close and ongoing collaborations, medical providers and clinical pharmacists proceed with deprescribing, aiming at planned cessation of nonessential and PIM, using the mnemonic prompt of VIONE. Vital and Important medications are continued and consolidated while a methodical plan is developed to deprescribe any medications that could lead to more harm than benefit and qualify based on the filters of Optional, Not indicated, and Every medicine has a diagnosis/reason. They select the proper discontinuation reasons in the CPRS medication menu (Figure 3) and document the rationale in the progress notes. It is highly encouraged that the collaborating pharmacists and health care providers add each other as cosigners and communicate effectively. Clinical pharmacy specialists also use the VIONE Polypharmacy Reminder Dialog Template (RDT) to document complete medication reviews with veterans to include deprescribing rationale and document shared decision making.
- A VIONE national dashboard captures deprescribing data in real time and automates reporting with daily updates that are readily accessible to all implementing facilities. Minimum data captured include the number of unique veterans impacted, number of medications deprescribed, cumulative cost avoidance to date, and number of prescriptions deprescribed per veteran. The dashboard facilitates real-time use of individual patient data and has also been designed to capture data from VHA administrative data portals and Corporate Data Warehouse.
Results
As of October 31, 2019, the assessment of polypharmacy using the VIONE tool across VHA sites has benefited > 60,000 unique veterans, of whom 49.2% were in urban areas, 47.7% in rural areas, and 3.1% in highly rural areas. Elderly male veterans comprised a clear majority. More than 128,000 medications have been deprescribed. The top classes of medications deprescribed are antihypertensives, over-the-counter medications, and antidiabetic medications. An annualized cost avoidance of > $4.0 million has been achieved. Cost avoidance is the cost of medications that otherwise would have continued to be filled and paid for by the VHA if they had not been deprescribed, projected for a maximum of 365 days. The calculation methodology can be summarized as follows:
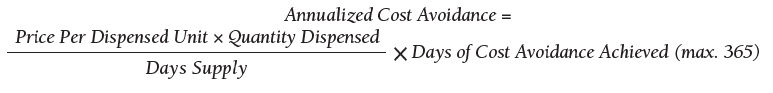
The calculations reported in Table 3 and Figure 4 are conservative and include only chronic outpatient prescriptions and do not account for medications deprescribed in inpatient units, nursing home, community living centers, or domiciliary populations. Data tracked separately from inpatient and community living center patient populations indicated an additional 25,536 deprescribed medications, across 28 VA facilities, impacting 7,076 veterans with an average 2.15 medications deprescribed per veteran. The additional achieved cost avoidance was $370,272 (based on $14.50 average cost per prescription). Medications restarted within 30 days of deprescribing are not included in these calculations.
The cost avoidance calculation further excludes the effects of VIONE implementation on many other types of interventions. These interventions include, but are not limited to, changing from aggressive care to end of life, comfort care when strongly indicated; reduced emergency department visits or invasive diagnostic and therapeutic approaches, when not indicated; medical supplies, antimicrobial preparations; labor costs related to packaging, mailing, and administering prescriptions; reduced/prevented clinical waste; reduced decompensation of systemic illnesses and subsequent health care needs precipitated by iatrogenic disturbances and prolonged convalescence; and overall changes to prescribing practices through purposeful and targeted interactions with colleagues across various disciplines and various hierarchical levels.
Discussion
The VIONE clinical program exemplifies the translation of HRO principles into health care system practices. VIONE offers a systematic approach to improve medication management with an emphasis on deprescribing nonessential medications across various health care settings, facilitating VHA efforts toward zero harm. It demonstrates close alignment with the key building blocks of an HRO. Effective VIONE incorporation into an organizational culture reflects leadership commitment to safety and reliability in their vision and actions. By empowering staff to proactively reduce inappropriate medications and thereby prevent patient harm, VIONE contributes to enhancing an enterprise-wide culture of safety, with fewer errors and greater reliability. As a standardized decision support tool for the ongoing practice of assessment and planned cessation of potentially inappropriate medications, VIONE illustrates how continuous process improvement can be a part of staff-engaged, veteran-centered, highly reliable care. The standardization of the VIONE tool promotes achievement and sustainment of desired HRO principles and practices within health care delivery systems.
Conclusions
The VIONE program was launched not as a cost savings or research program but as a practical, real-time bedside or ambulatory care intervention to improve patient safety. Its value is reflected in the overwhelming response from scholarly and well-engaged colleagues expressing serious interests in expanding collaborations and tailoring efforts to add more depth and breadth to VIONE related efforts.
Acknowledgments
The authors express their gratitude to Central Arkansas VA Healthcare System leadership, Clinical Applications Coordinators, and colleagues for their unconditional support, to the Diffusion of Excellence programs at US Department of Veterans Affairs Central Office for their endorsement, and to the many VHA participants who renew our optimism and energy as we continue this exciting journey. We also thank Bridget B. Kelly for her assistance in writing and editing of the manuscript.
High reliability organizations (HROs) incorporate continuous process improvement through leadership commitment to create a safety culture that works toward creating a zero-harm environment.1 The Veterans Health Administration (VHA) has set transformational goals for becoming an HRO. In this article, we describe VIONE, an expanding medication deprescribing clinical program, which exemplifies the translation of HRO principles into health care system models. Both VIONE and HRO are globally relevant.
Reducing medication errors and related adverse drug events are important for achieving zero harm. Preventable medical errors rank behind heart disease and cancer as the third leading cause of death in the US.2 The simultaneous use of multiple medications can lead to dangerous drug interactions, adverse outcomes, and challenges with adherence. When a person is taking multiple medicines, known as polypharmacy, it is more likely that some are potentially inappropriate medications (PIM). Current literature highlights the prevalence and dangers of polypharmacy, which ranks among the top 10 common causes of death in the US, as well as suggestions to address preventable adverse outcomes from polypharmacy and PIM.3-5
Deprescribing of PIM frequently results in better disease management with improved health outcomes and quality of life.4 Many health care settings lack standardized approaches or set expectations to proactively deprescribe PIM. There has been insufficient emphasis on how to make decisions for deprescribing medications when therapeutic benefits are not clear and/or when the adverse effects may outweigh the therapeutic benefits.5
It is imperative to provide practice guidance for deprescribing nonessential medications along with systems-based infrastructure to enable integrated and effective assessments during opportune moments in the health care continuum. Multimodal approaches that include education, risk stratification, population health management interventions, research and resource allocation can help transform organizational culture in health care facilities toward HRO models of care, aiming at zero harm to patients.
The practical lessons learned from VIONE implementation science experiences on various scales and under diverse circumstances, cumulative wisdom from hindsight, foresight and critical insights gathered during nationwide spread of VIONE over the past 3 years continues to propel us toward the desirable direction and core concepts of an HRO.
The VIONE program facilitates practical, real-time interventions that could be tailored to various health care settings, organizational needs, and available resources. VIONE implements an electronic Computerized Patient Record System (CPRS) tool to enable planned cessation of nonessential medications that are potentially harmful, inappropriate, not indicated, or not necessary. The VIONE tool supports systematic, individualized assessment and adjustment through 5 filters (Figure 1). It prompts providers to assign 1 of these filters intuitively and objectively. VIONE combines clinical evidence for best practices, an interprofessional team approach, patient engagement, adapted use of existing medical records systems, and HRO principles for effective implementation.
As a tool to support safer prescribing practices, VIONE aligns closely with HRO principles (Table 1) and core pillars (Table 2).6-8 A zero-harm safety culture necessitates that medications be used for correct reasons, over a correct duration of time, and following a correct schedule while monitoring for adverse outcomes. However, reality generally falls significantly short of this for a myriad of reasons, such as compromised health literacy, functional limitations, affordability, communication gaps, patients seen by multiple providers, and an accumulation of prescriptions due to comorbidities, symptom progression, and management of adverse effects. Through a sharpened focus on both precision medicine and competent prescription management, VIONE is a viable opportunity for investing in the zero-harm philosophy that is integral to an HRO.
Design and Implementation
Initially launched in 2016 in a 15-bed inpatient, subacute rehabilitation unit within a VHA tertiary care facility, VIONE has been sustained and gradually expanded to 38 other VHA facility programs (Figure 2). Recognizing the potential value if adopted into widespread use, VIONE was a Gold Status winner in the VHA Under Secretary for Health Shark Tank-style competition in 2017 and was selected by the VHA Diffusion of Excellence as an innovation worthy of scale and spread through national dissemination.9 A toolkit for VIONE implementation, patient and provider brochures, VIONE vignette, and National Dialog template also have been created.10
Implementing VIONE in a new facility requires an actively engaged core team committed to patient safety and reduction of polypharmacy and PIM, interest and availability to lead project implementation strategies, along with meaningful local organizational support. The current structure for VIONE spread is as follows:
- Interested VHA participants review information and contact [email protected].
- The VIONE team orients implementing champions, mainly pharmacists, physicians, nurse practitioners, and physician assistants at a facility program level, offering guidance and available resources.
- Clinical Application Coordinators at Central Arkansas VA Healthcare System and participating facilities collaborate to add deprescribing menu options in CPRS and install the VIONE Polypharmacy Reminder Dialog template.
- Through close and ongoing collaborations, medical providers and clinical pharmacists proceed with deprescribing, aiming at planned cessation of nonessential and PIM, using the mnemonic prompt of VIONE. Vital and Important medications are continued and consolidated while a methodical plan is developed to deprescribe any medications that could lead to more harm than benefit and qualify based on the filters of Optional, Not indicated, and Every medicine has a diagnosis/reason. They select the proper discontinuation reasons in the CPRS medication menu (Figure 3) and document the rationale in the progress notes. It is highly encouraged that the collaborating pharmacists and health care providers add each other as cosigners and communicate effectively. Clinical pharmacy specialists also use the VIONE Polypharmacy Reminder Dialog Template (RDT) to document complete medication reviews with veterans to include deprescribing rationale and document shared decision making.
- A VIONE national dashboard captures deprescribing data in real time and automates reporting with daily updates that are readily accessible to all implementing facilities. Minimum data captured include the number of unique veterans impacted, number of medications deprescribed, cumulative cost avoidance to date, and number of prescriptions deprescribed per veteran. The dashboard facilitates real-time use of individual patient data and has also been designed to capture data from VHA administrative data portals and Corporate Data Warehouse.
Results
As of October 31, 2019, the assessment of polypharmacy using the VIONE tool across VHA sites has benefited > 60,000 unique veterans, of whom 49.2% were in urban areas, 47.7% in rural areas, and 3.1% in highly rural areas. Elderly male veterans comprised a clear majority. More than 128,000 medications have been deprescribed. The top classes of medications deprescribed are antihypertensives, over-the-counter medications, and antidiabetic medications. An annualized cost avoidance of > $4.0 million has been achieved. Cost avoidance is the cost of medications that otherwise would have continued to be filled and paid for by the VHA if they had not been deprescribed, projected for a maximum of 365 days. The calculation methodology can be summarized as follows:
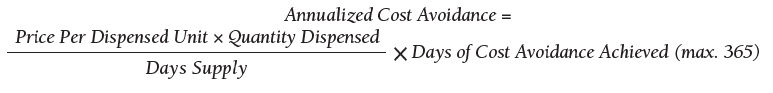
The calculations reported in Table 3 and Figure 4 are conservative and include only chronic outpatient prescriptions and do not account for medications deprescribed in inpatient units, nursing home, community living centers, or domiciliary populations. Data tracked separately from inpatient and community living center patient populations indicated an additional 25,536 deprescribed medications, across 28 VA facilities, impacting 7,076 veterans with an average 2.15 medications deprescribed per veteran. The additional achieved cost avoidance was $370,272 (based on $14.50 average cost per prescription). Medications restarted within 30 days of deprescribing are not included in these calculations.
The cost avoidance calculation further excludes the effects of VIONE implementation on many other types of interventions. These interventions include, but are not limited to, changing from aggressive care to end of life, comfort care when strongly indicated; reduced emergency department visits or invasive diagnostic and therapeutic approaches, when not indicated; medical supplies, antimicrobial preparations; labor costs related to packaging, mailing, and administering prescriptions; reduced/prevented clinical waste; reduced decompensation of systemic illnesses and subsequent health care needs precipitated by iatrogenic disturbances and prolonged convalescence; and overall changes to prescribing practices through purposeful and targeted interactions with colleagues across various disciplines and various hierarchical levels.
Discussion
The VIONE clinical program exemplifies the translation of HRO principles into health care system practices. VIONE offers a systematic approach to improve medication management with an emphasis on deprescribing nonessential medications across various health care settings, facilitating VHA efforts toward zero harm. It demonstrates close alignment with the key building blocks of an HRO. Effective VIONE incorporation into an organizational culture reflects leadership commitment to safety and reliability in their vision and actions. By empowering staff to proactively reduce inappropriate medications and thereby prevent patient harm, VIONE contributes to enhancing an enterprise-wide culture of safety, with fewer errors and greater reliability. As a standardized decision support tool for the ongoing practice of assessment and planned cessation of potentially inappropriate medications, VIONE illustrates how continuous process improvement can be a part of staff-engaged, veteran-centered, highly reliable care. The standardization of the VIONE tool promotes achievement and sustainment of desired HRO principles and practices within health care delivery systems.
Conclusions
The VIONE program was launched not as a cost savings or research program but as a practical, real-time bedside or ambulatory care intervention to improve patient safety. Its value is reflected in the overwhelming response from scholarly and well-engaged colleagues expressing serious interests in expanding collaborations and tailoring efforts to add more depth and breadth to VIONE related efforts.
Acknowledgments
The authors express their gratitude to Central Arkansas VA Healthcare System leadership, Clinical Applications Coordinators, and colleagues for their unconditional support, to the Diffusion of Excellence programs at US Department of Veterans Affairs Central Office for their endorsement, and to the many VHA participants who renew our optimism and energy as we continue this exciting journey. We also thank Bridget B. Kelly for her assistance in writing and editing of the manuscript.
1. Chassin MR, Jerod ML. High-reliability health care: getting there from here. The Joint Commission. Milbank Q. 2013;91(3):459-490.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017;4:170167.
4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
5. Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med. 2016;176(4):482-483.
6. US Department of Veterans Affairs. High reliability. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx. [Nonpublic source, not verified.]
7. Gordon S, Mendenhall P, O’Connor BB. Beyond the Checklist: What Else Health Care Can Learn from Aviation Teamwork and Safety. Ithaca, NY: Cornell University Press; 2013.
8. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
9. US Department of Veterans Affairs. Diffusion of Excellence. https://www.va.gov/HEALTHCAREEXCELLENCE/diffusion-of-excellence/. Updated August 10, 2018. Accessed June 26, 2019.
10. US Department of Veterans Affairs. VIONE program toolkit. https://www.vapulse.net/docs/DOC-259375. [Nonpublic source, not verified.]
1. Chassin MR, Jerod ML. High-reliability health care: getting there from here. The Joint Commission. Milbank Q. 2013;91(3):459-490.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017;4:170167.
4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
5. Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med. 2016;176(4):482-483.
6. US Department of Veterans Affairs. High reliability. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx. [Nonpublic source, not verified.]
7. Gordon S, Mendenhall P, O’Connor BB. Beyond the Checklist: What Else Health Care Can Learn from Aviation Teamwork and Safety. Ithaca, NY: Cornell University Press; 2013.
8. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
9. US Department of Veterans Affairs. Diffusion of Excellence. https://www.va.gov/HEALTHCAREEXCELLENCE/diffusion-of-excellence/. Updated August 10, 2018. Accessed June 26, 2019.
10. US Department of Veterans Affairs. VIONE program toolkit. https://www.vapulse.net/docs/DOC-259375. [Nonpublic source, not verified.]
VA Ketamine Controversies
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
Improving Veteran Care With the Mission Act
NATIONAL HARBOR, MD–The US Department of Veterans Affairs (VA) is in the midst of a significant change in the way it will deliver care to veterans. Agency officials remain optimistic that the change will be for the better, and early indications are positive.
The change is being driven by the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (Mission) Act of 2018, a bill that opens health services options for veterans and integrates VA-administered care and care from community-based providers.
“This is change that is enhancing their experience in the system, and this is enhancing their options and the quality of the options in the system,” Jennifer MacDonald, MD, chief consultant to the principal deputy undersecretary for health at the VA, said during a December 3 session at the AMSUS 2019 annual meeting. “We need also for our workforce to understand how important they are to us across this degree of change.”
Dr. MacDonald highlighted integration with community-based care, including a community urgent care provision that allows veterans to access urgent care facilities and receive care without the need for prior authorization.
“The important piece about that is that we are also looking at the way this care has been accessed,” she said. “By and large, what we have seen from the data is that veterans are indeed seeking community urgent care at a site close to home. This may be CVS or Walgreens. It may be a stand-alone urgent care with a bit more functionality than those Minute Clinics tend to have. We are seeing veterans typically access care through those sites for those minor concerns and illnesses.”
However, she noted that this type of access does not alter the role the VA plays in administration of health care services.
“We are seeing them come back to VA for the majority of their care and for their core care–when there are serious issues, when insulin needs to be adjusted for diabetes, when there are heart disease medications that need to be refilled–we are seeing veterans not seek out urgent care, but come to us, and that is exactly what we want,” she said. “We want the continuity of care to continue and we want to help guide people to the right care, right place, right time.”
Dr. MacDonald also highlighted the expansion of a program that provides a stipend to caregivers that allows veterans to avoid institutionalization and remain within the community under that caregiver’s (a family or friend) supervision. This will expand by year’s end to Vietnam War-era veterans and within 2 years, to veterans that fall between the Vietnam War-era and the September 11, 2001, terrorist attacks.
“We wanted to do this equitably across all eras of veterans,” she said. “This now gives us that opportunity.”
Telehealth also plays a key role.
“For the first time ever, VA now has what we term ‘anywhere-to-anywhere’ telehealth under the Mission Act, an enormous opportunity for us,” she said. “Since we stretch … from New York City to Guam, we need the opportunity to provide care where it may be difficult to recruit and retain providers wherever veterans choose to live,” she said. “We believe that we should be able to meet people where they are regardless of where they choose to live. That’s an aspirational vision, but it is one we believe is exceptionally important and indeed we are moving toward that.”
These are just the beginning; the full implementation of the act goes out to 2034.
According to Dr. MacDonald, the agency is working hard to engage both veterans and the workforce to keep tabs on how the implementation is going.
“It’s a fundamental change in the day-to-day business that they’ve been doing, sometimes for years, and so extremely important across this change is that we have set up processes and now a joint operations center and a number of forums to hear directly from our front line and make sure that their issues are our issues in central office, in DC here, and that they feel heard and that they know that when they have needs, those needs are actioned,” she said.
The VA, under the Mission Act, is also working hard to engage health care providers in the community, including making VA training to community partners, including training on opioid use, suicide prevent and military culture.
However, all these change are for naught if the veterans are not on board. But so far, Dr. MacDonald said the early feedback is very positive.
She cited a VFW survey that asked a question about the Mission Act changes so far and whether they would recommend the VA to other veterans. Ninety percent of the respondents answered they would.
“That’s our marker that we are getting somewhere with these changes and the way we do business,” she said. “That is what we want to see continue to increase.”
NATIONAL HARBOR, MD–The US Department of Veterans Affairs (VA) is in the midst of a significant change in the way it will deliver care to veterans. Agency officials remain optimistic that the change will be for the better, and early indications are positive.
The change is being driven by the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (Mission) Act of 2018, a bill that opens health services options for veterans and integrates VA-administered care and care from community-based providers.
“This is change that is enhancing their experience in the system, and this is enhancing their options and the quality of the options in the system,” Jennifer MacDonald, MD, chief consultant to the principal deputy undersecretary for health at the VA, said during a December 3 session at the AMSUS 2019 annual meeting. “We need also for our workforce to understand how important they are to us across this degree of change.”
Dr. MacDonald highlighted integration with community-based care, including a community urgent care provision that allows veterans to access urgent care facilities and receive care without the need for prior authorization.
“The important piece about that is that we are also looking at the way this care has been accessed,” she said. “By and large, what we have seen from the data is that veterans are indeed seeking community urgent care at a site close to home. This may be CVS or Walgreens. It may be a stand-alone urgent care with a bit more functionality than those Minute Clinics tend to have. We are seeing veterans typically access care through those sites for those minor concerns and illnesses.”
However, she noted that this type of access does not alter the role the VA plays in administration of health care services.
“We are seeing them come back to VA for the majority of their care and for their core care–when there are serious issues, when insulin needs to be adjusted for diabetes, when there are heart disease medications that need to be refilled–we are seeing veterans not seek out urgent care, but come to us, and that is exactly what we want,” she said. “We want the continuity of care to continue and we want to help guide people to the right care, right place, right time.”
Dr. MacDonald also highlighted the expansion of a program that provides a stipend to caregivers that allows veterans to avoid institutionalization and remain within the community under that caregiver’s (a family or friend) supervision. This will expand by year’s end to Vietnam War-era veterans and within 2 years, to veterans that fall between the Vietnam War-era and the September 11, 2001, terrorist attacks.
“We wanted to do this equitably across all eras of veterans,” she said. “This now gives us that opportunity.”
Telehealth also plays a key role.
“For the first time ever, VA now has what we term ‘anywhere-to-anywhere’ telehealth under the Mission Act, an enormous opportunity for us,” she said. “Since we stretch … from New York City to Guam, we need the opportunity to provide care where it may be difficult to recruit and retain providers wherever veterans choose to live,” she said. “We believe that we should be able to meet people where they are regardless of where they choose to live. That’s an aspirational vision, but it is one we believe is exceptionally important and indeed we are moving toward that.”
These are just the beginning; the full implementation of the act goes out to 2034.
According to Dr. MacDonald, the agency is working hard to engage both veterans and the workforce to keep tabs on how the implementation is going.
“It’s a fundamental change in the day-to-day business that they’ve been doing, sometimes for years, and so extremely important across this change is that we have set up processes and now a joint operations center and a number of forums to hear directly from our front line and make sure that their issues are our issues in central office, in DC here, and that they feel heard and that they know that when they have needs, those needs are actioned,” she said.
The VA, under the Mission Act, is also working hard to engage health care providers in the community, including making VA training to community partners, including training on opioid use, suicide prevent and military culture.
However, all these change are for naught if the veterans are not on board. But so far, Dr. MacDonald said the early feedback is very positive.
She cited a VFW survey that asked a question about the Mission Act changes so far and whether they would recommend the VA to other veterans. Ninety percent of the respondents answered they would.
“That’s our marker that we are getting somewhere with these changes and the way we do business,” she said. “That is what we want to see continue to increase.”
NATIONAL HARBOR, MD–The US Department of Veterans Affairs (VA) is in the midst of a significant change in the way it will deliver care to veterans. Agency officials remain optimistic that the change will be for the better, and early indications are positive.
The change is being driven by the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (Mission) Act of 2018, a bill that opens health services options for veterans and integrates VA-administered care and care from community-based providers.
“This is change that is enhancing their experience in the system, and this is enhancing their options and the quality of the options in the system,” Jennifer MacDonald, MD, chief consultant to the principal deputy undersecretary for health at the VA, said during a December 3 session at the AMSUS 2019 annual meeting. “We need also for our workforce to understand how important they are to us across this degree of change.”
Dr. MacDonald highlighted integration with community-based care, including a community urgent care provision that allows veterans to access urgent care facilities and receive care without the need for prior authorization.
“The important piece about that is that we are also looking at the way this care has been accessed,” she said. “By and large, what we have seen from the data is that veterans are indeed seeking community urgent care at a site close to home. This may be CVS or Walgreens. It may be a stand-alone urgent care with a bit more functionality than those Minute Clinics tend to have. We are seeing veterans typically access care through those sites for those minor concerns and illnesses.”
However, she noted that this type of access does not alter the role the VA plays in administration of health care services.
“We are seeing them come back to VA for the majority of their care and for their core care–when there are serious issues, when insulin needs to be adjusted for diabetes, when there are heart disease medications that need to be refilled–we are seeing veterans not seek out urgent care, but come to us, and that is exactly what we want,” she said. “We want the continuity of care to continue and we want to help guide people to the right care, right place, right time.”
Dr. MacDonald also highlighted the expansion of a program that provides a stipend to caregivers that allows veterans to avoid institutionalization and remain within the community under that caregiver’s (a family or friend) supervision. This will expand by year’s end to Vietnam War-era veterans and within 2 years, to veterans that fall between the Vietnam War-era and the September 11, 2001, terrorist attacks.
“We wanted to do this equitably across all eras of veterans,” she said. “This now gives us that opportunity.”
Telehealth also plays a key role.
“For the first time ever, VA now has what we term ‘anywhere-to-anywhere’ telehealth under the Mission Act, an enormous opportunity for us,” she said. “Since we stretch … from New York City to Guam, we need the opportunity to provide care where it may be difficult to recruit and retain providers wherever veterans choose to live,” she said. “We believe that we should be able to meet people where they are regardless of where they choose to live. That’s an aspirational vision, but it is one we believe is exceptionally important and indeed we are moving toward that.”
These are just the beginning; the full implementation of the act goes out to 2034.
According to Dr. MacDonald, the agency is working hard to engage both veterans and the workforce to keep tabs on how the implementation is going.
“It’s a fundamental change in the day-to-day business that they’ve been doing, sometimes for years, and so extremely important across this change is that we have set up processes and now a joint operations center and a number of forums to hear directly from our front line and make sure that their issues are our issues in central office, in DC here, and that they feel heard and that they know that when they have needs, those needs are actioned,” she said.
The VA, under the Mission Act, is also working hard to engage health care providers in the community, including making VA training to community partners, including training on opioid use, suicide prevent and military culture.
However, all these change are for naught if the veterans are not on board. But so far, Dr. MacDonald said the early feedback is very positive.
She cited a VFW survey that asked a question about the Mission Act changes so far and whether they would recommend the VA to other veterans. Ninety percent of the respondents answered they would.
“That’s our marker that we are getting somewhere with these changes and the way we do business,” she said. “That is what we want to see continue to increase.”
OTC hormonal contraception: An important goal in the fight for reproductive justice
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.