User login
Trump seeks to cut NIH, CDC budgets, some Medicare spending
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
C. auris Infection: Rare, But Raising Concerns About Pan-Resistance
Candida auris (C. auris) infection was first detected in New York, in July 2016. As of June 2019, 801 patients have been identified in New York as having C auris—and of those, 3 had pan-resistant infection.
CDC researchers say C auris is “a globally emerging yeast.” Cases with resistance to all 3 classes of commonly prescribed antifungal drugs have been reported in multiple countries.
In New York, of the first 277 available clinical isolates, 276 were resistant to fluconazole and 170 were resistant to amphotericin B. None were resistant to echinocandins. Subsequent testing found 99.7% of 331 isolates from infected patients with susceptibilities were resistant to fluconazole, 63% were resistant to amphotericin B, and 4% were resistant to echinocandins. Three of the subsequent isolates were pan-resistant.
The first 2 of those 3 patients were > 50 years old and residents of long-term care facilities. Each had multiple medical conditions, including ventilator dependence and colonization with multidrug-resistant bacteria. Neither patient was known to have received antifungal medications before the diagnosis of C. auris infection, but both were treated with prolonged courses of echinocandins after the diagnosis. Cultures taken after echinocandin therapy showed resistance to fluconazole, amphotericin B, and echinocandins. Both patients died, but the role of C. auris in their deaths is unclear.
The researchers found no epidemiologic links between the 2 patients. They were residents at different health care facilities, neither had any known domestic or foreign travel. No pan-resistant isolates were identified among contacts or on environmental surfaces from their rooms or common equipment at the 3 facilities where they had been patients. Although C. auris was isolated from other patients, none was pan-resistant.
A retrospective review of all New York C. auris isolates turned up a third pan-resistant patient. The patient also was aged > 50 years old , had multiple comorbidities, and a prolonged hospital and long-term care stay. However, the patient received care at a third unique facility. This third patient, who died from underlying medical conditions, was also not known to have traveled recently, and had no known contact with the other 2 patients.
Isolates from all 3 patients were initially sensitive to echinocandins. Resistance was detected after treatment, indicating it emerged during treatment with the drugs. The researchers found no evidence of transmission.
Approximately 3 years after the beginning of the New York outbreak, the pan-resistant isolates still appear to be rare, the researchers say, but “their emergence is concerning.” They urge close monitoring for patients on antifungal treatment for C. auris, along with follow-up cultures and repeat susceptibility testing, especially in patients previously treated with echinocandins.
Candida auris (C. auris) infection was first detected in New York, in July 2016. As of June 2019, 801 patients have been identified in New York as having C auris—and of those, 3 had pan-resistant infection.
CDC researchers say C auris is “a globally emerging yeast.” Cases with resistance to all 3 classes of commonly prescribed antifungal drugs have been reported in multiple countries.
In New York, of the first 277 available clinical isolates, 276 were resistant to fluconazole and 170 were resistant to amphotericin B. None were resistant to echinocandins. Subsequent testing found 99.7% of 331 isolates from infected patients with susceptibilities were resistant to fluconazole, 63% were resistant to amphotericin B, and 4% were resistant to echinocandins. Three of the subsequent isolates were pan-resistant.
The first 2 of those 3 patients were > 50 years old and residents of long-term care facilities. Each had multiple medical conditions, including ventilator dependence and colonization with multidrug-resistant bacteria. Neither patient was known to have received antifungal medications before the diagnosis of C. auris infection, but both were treated with prolonged courses of echinocandins after the diagnosis. Cultures taken after echinocandin therapy showed resistance to fluconazole, amphotericin B, and echinocandins. Both patients died, but the role of C. auris in their deaths is unclear.
The researchers found no epidemiologic links between the 2 patients. They were residents at different health care facilities, neither had any known domestic or foreign travel. No pan-resistant isolates were identified among contacts or on environmental surfaces from their rooms or common equipment at the 3 facilities where they had been patients. Although C. auris was isolated from other patients, none was pan-resistant.
A retrospective review of all New York C. auris isolates turned up a third pan-resistant patient. The patient also was aged > 50 years old , had multiple comorbidities, and a prolonged hospital and long-term care stay. However, the patient received care at a third unique facility. This third patient, who died from underlying medical conditions, was also not known to have traveled recently, and had no known contact with the other 2 patients.
Isolates from all 3 patients were initially sensitive to echinocandins. Resistance was detected after treatment, indicating it emerged during treatment with the drugs. The researchers found no evidence of transmission.
Approximately 3 years after the beginning of the New York outbreak, the pan-resistant isolates still appear to be rare, the researchers say, but “their emergence is concerning.” They urge close monitoring for patients on antifungal treatment for C. auris, along with follow-up cultures and repeat susceptibility testing, especially in patients previously treated with echinocandins.
Candida auris (C. auris) infection was first detected in New York, in July 2016. As of June 2019, 801 patients have been identified in New York as having C auris—and of those, 3 had pan-resistant infection.
CDC researchers say C auris is “a globally emerging yeast.” Cases with resistance to all 3 classes of commonly prescribed antifungal drugs have been reported in multiple countries.
In New York, of the first 277 available clinical isolates, 276 were resistant to fluconazole and 170 were resistant to amphotericin B. None were resistant to echinocandins. Subsequent testing found 99.7% of 331 isolates from infected patients with susceptibilities were resistant to fluconazole, 63% were resistant to amphotericin B, and 4% were resistant to echinocandins. Three of the subsequent isolates were pan-resistant.
The first 2 of those 3 patients were > 50 years old and residents of long-term care facilities. Each had multiple medical conditions, including ventilator dependence and colonization with multidrug-resistant bacteria. Neither patient was known to have received antifungal medications before the diagnosis of C. auris infection, but both were treated with prolonged courses of echinocandins after the diagnosis. Cultures taken after echinocandin therapy showed resistance to fluconazole, amphotericin B, and echinocandins. Both patients died, but the role of C. auris in their deaths is unclear.
The researchers found no epidemiologic links between the 2 patients. They were residents at different health care facilities, neither had any known domestic or foreign travel. No pan-resistant isolates were identified among contacts or on environmental surfaces from their rooms or common equipment at the 3 facilities where they had been patients. Although C. auris was isolated from other patients, none was pan-resistant.
A retrospective review of all New York C. auris isolates turned up a third pan-resistant patient. The patient also was aged > 50 years old , had multiple comorbidities, and a prolonged hospital and long-term care stay. However, the patient received care at a third unique facility. This third patient, who died from underlying medical conditions, was also not known to have traveled recently, and had no known contact with the other 2 patients.
Isolates from all 3 patients were initially sensitive to echinocandins. Resistance was detected after treatment, indicating it emerged during treatment with the drugs. The researchers found no evidence of transmission.
Approximately 3 years after the beginning of the New York outbreak, the pan-resistant isolates still appear to be rare, the researchers say, but “their emergence is concerning.” They urge close monitoring for patients on antifungal treatment for C. auris, along with follow-up cultures and repeat susceptibility testing, especially in patients previously treated with echinocandins.
Study Warns of the Risk of Carbon Monoxide Poisoning in the Military
Carbon monoxide (CO)—colorless, odorless, tasteless and highly toxic—is one of the most common causes of unintentional poisoning deaths in the US. Researchers who described their analysis of CO-related incidents in the military for the Medical Surveillance Monthly Report say military activities, materials, and settings pose “unique and potentially lethal sources of significant CO exposure.”
They reported on episodes of CO poisoning among members of the US Armed Forces between 2009 and 2019 and expanded on reports that dated back to 2001. Their analysis included reserve members and nonservice member beneficiaries.
Over the 10 years, there were 1,288 confirmed/probable cases of CO poisoning among active component service members, 366 among reserve component service members, and 4,754 among nonservice member beneficiaries. The highest number of active-duty members with CO confirmed/probable poisoning were reported at Fort Carson, Colorado (60) and NMC San Diego, California (52).
Of the confirmed/probable cases among active-duty members, 613 were classified as having unintentional intent, 538 undetermined intent, and 136 self-harm intent. One was due to assault. Most of the cases were related to work in repair/engineering occupations. Although the majority of sources were “other or unspecified,” motor vehicle exhaust accounted for 17% of the confirmed cases and all of the probable cases. Similarly, in the reserve component and among nonservice member beneficiaries, vehicle exhaust was the second-most common source.
The researchers found that CO poisoning-related injuries/diagnoses in the military often involved a single exposure that affected multiple personnel. For example, 21 soldiers showed symptoms during a multi-day exercise at the Yukon Training Center.
Excessive CO exposure is “entirely preventable,” the researchers say. Primary medical care providers—including unit medics and emergency medical technicians—should be knowledgeable about and sensitive to the “diverse and nonspecific” early clinical manifestations of CO intoxication, such as dizziness, headache, malaise, fatigue, disorientation, nausea, and vomiting. High CO exposure can cause more pronounced and severe symptoms, including syncope, seizures, acute stroke-like syndromes, and coma.
It’s important to remember, the researchers add, that increased oxygen demand from muscular activity exacerbates the symptoms of CO exposure, but individuals at rest may experience no other symptoms before losing consciousness.
An editorial comment notes that the full impact of morbidity and mortality from CO poisoning is difficult to estimate. For one thing, because the symptoms can be so nonspecific, clinicians may not consider CO poisoning when patients present for care.
This study differs from previous ones in that it uses code data from both the Ninth and Tenth Revisions of the International Classification of Diseases. Such data, the editorial comment says, can be used at national and Military Health System–wide levels with relatively few resources, providing useful information on trends and risk factors that can be used in designing interventions
Carbon monoxide (CO)—colorless, odorless, tasteless and highly toxic—is one of the most common causes of unintentional poisoning deaths in the US. Researchers who described their analysis of CO-related incidents in the military for the Medical Surveillance Monthly Report say military activities, materials, and settings pose “unique and potentially lethal sources of significant CO exposure.”
They reported on episodes of CO poisoning among members of the US Armed Forces between 2009 and 2019 and expanded on reports that dated back to 2001. Their analysis included reserve members and nonservice member beneficiaries.
Over the 10 years, there were 1,288 confirmed/probable cases of CO poisoning among active component service members, 366 among reserve component service members, and 4,754 among nonservice member beneficiaries. The highest number of active-duty members with CO confirmed/probable poisoning were reported at Fort Carson, Colorado (60) and NMC San Diego, California (52).
Of the confirmed/probable cases among active-duty members, 613 were classified as having unintentional intent, 538 undetermined intent, and 136 self-harm intent. One was due to assault. Most of the cases were related to work in repair/engineering occupations. Although the majority of sources were “other or unspecified,” motor vehicle exhaust accounted for 17% of the confirmed cases and all of the probable cases. Similarly, in the reserve component and among nonservice member beneficiaries, vehicle exhaust was the second-most common source.
The researchers found that CO poisoning-related injuries/diagnoses in the military often involved a single exposure that affected multiple personnel. For example, 21 soldiers showed symptoms during a multi-day exercise at the Yukon Training Center.
Excessive CO exposure is “entirely preventable,” the researchers say. Primary medical care providers—including unit medics and emergency medical technicians—should be knowledgeable about and sensitive to the “diverse and nonspecific” early clinical manifestations of CO intoxication, such as dizziness, headache, malaise, fatigue, disorientation, nausea, and vomiting. High CO exposure can cause more pronounced and severe symptoms, including syncope, seizures, acute stroke-like syndromes, and coma.
It’s important to remember, the researchers add, that increased oxygen demand from muscular activity exacerbates the symptoms of CO exposure, but individuals at rest may experience no other symptoms before losing consciousness.
An editorial comment notes that the full impact of morbidity and mortality from CO poisoning is difficult to estimate. For one thing, because the symptoms can be so nonspecific, clinicians may not consider CO poisoning when patients present for care.
This study differs from previous ones in that it uses code data from both the Ninth and Tenth Revisions of the International Classification of Diseases. Such data, the editorial comment says, can be used at national and Military Health System–wide levels with relatively few resources, providing useful information on trends and risk factors that can be used in designing interventions
Carbon monoxide (CO)—colorless, odorless, tasteless and highly toxic—is one of the most common causes of unintentional poisoning deaths in the US. Researchers who described their analysis of CO-related incidents in the military for the Medical Surveillance Monthly Report say military activities, materials, and settings pose “unique and potentially lethal sources of significant CO exposure.”
They reported on episodes of CO poisoning among members of the US Armed Forces between 2009 and 2019 and expanded on reports that dated back to 2001. Their analysis included reserve members and nonservice member beneficiaries.
Over the 10 years, there were 1,288 confirmed/probable cases of CO poisoning among active component service members, 366 among reserve component service members, and 4,754 among nonservice member beneficiaries. The highest number of active-duty members with CO confirmed/probable poisoning were reported at Fort Carson, Colorado (60) and NMC San Diego, California (52).
Of the confirmed/probable cases among active-duty members, 613 were classified as having unintentional intent, 538 undetermined intent, and 136 self-harm intent. One was due to assault. Most of the cases were related to work in repair/engineering occupations. Although the majority of sources were “other or unspecified,” motor vehicle exhaust accounted for 17% of the confirmed cases and all of the probable cases. Similarly, in the reserve component and among nonservice member beneficiaries, vehicle exhaust was the second-most common source.
The researchers found that CO poisoning-related injuries/diagnoses in the military often involved a single exposure that affected multiple personnel. For example, 21 soldiers showed symptoms during a multi-day exercise at the Yukon Training Center.
Excessive CO exposure is “entirely preventable,” the researchers say. Primary medical care providers—including unit medics and emergency medical technicians—should be knowledgeable about and sensitive to the “diverse and nonspecific” early clinical manifestations of CO intoxication, such as dizziness, headache, malaise, fatigue, disorientation, nausea, and vomiting. High CO exposure can cause more pronounced and severe symptoms, including syncope, seizures, acute stroke-like syndromes, and coma.
It’s important to remember, the researchers add, that increased oxygen demand from muscular activity exacerbates the symptoms of CO exposure, but individuals at rest may experience no other symptoms before losing consciousness.
An editorial comment notes that the full impact of morbidity and mortality from CO poisoning is difficult to estimate. For one thing, because the symptoms can be so nonspecific, clinicians may not consider CO poisoning when patients present for care.
This study differs from previous ones in that it uses code data from both the Ninth and Tenth Revisions of the International Classification of Diseases. Such data, the editorial comment says, can be used at national and Military Health System–wide levels with relatively few resources, providing useful information on trends and risk factors that can be used in designing interventions
ObGyn malpractice liability risk: 2020 developments and probabilities
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
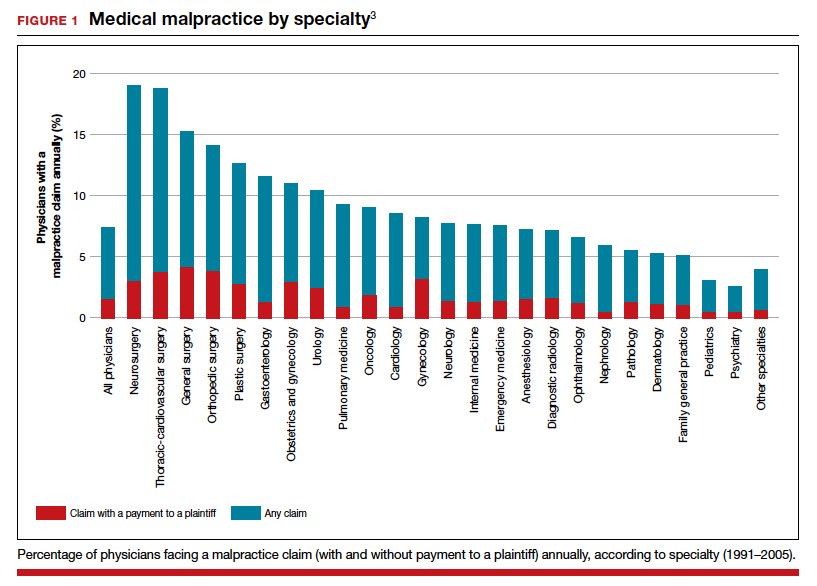
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)
Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4
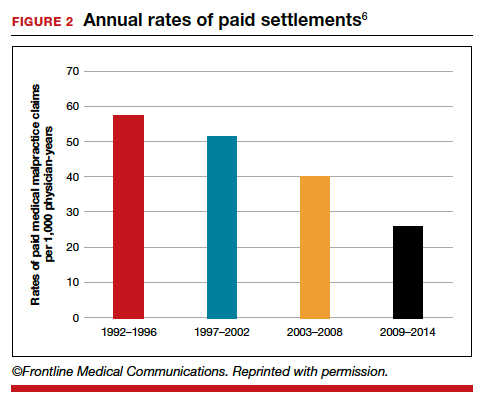
It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M
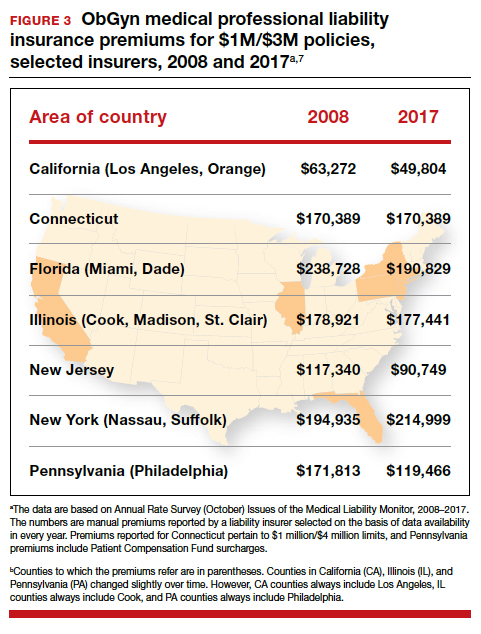
Why have malpractice payouts declined overall?
Have medical errors declined?
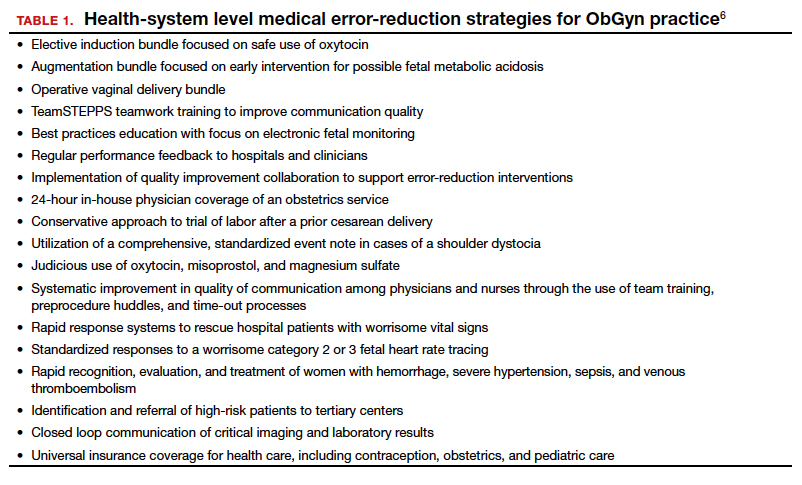
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.
The collective accountability factor
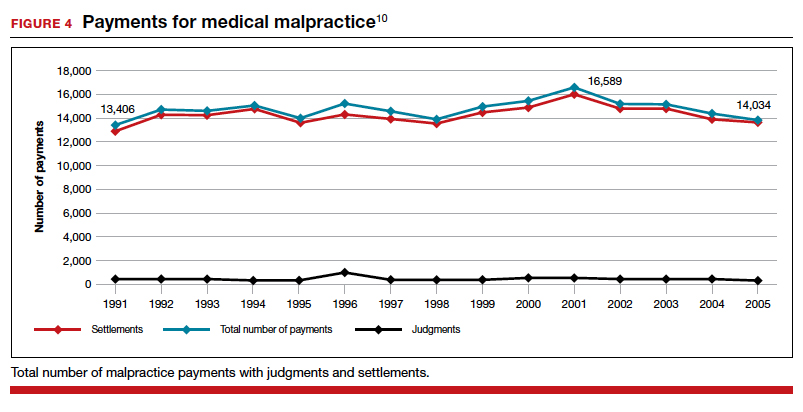
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.
Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
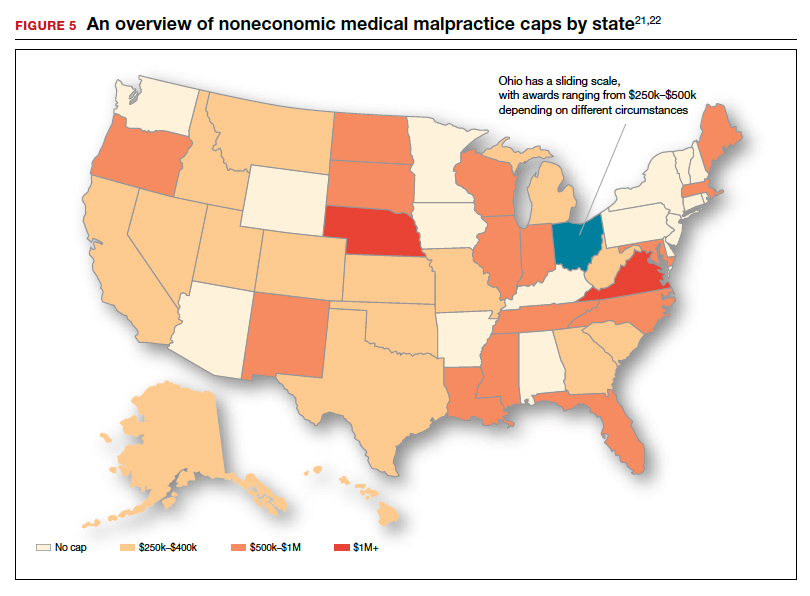
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.
Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
Bipartisan Bill to Help Reduce Veteran Suicides Readies for Senate Vote
In fiscal year 2010, the VA requested $62 million for suicide prevention outreach; in FY 2020, that leapt to $222 million. Yet despite the dramatic hike in funding, the rate of veteran suicides has remained basically unchanged: An estimated 20 veterans die by suicide every day.
Of those, roughly 14 were not receiving health care from the VA before their death. But a bipartisan bill introduced by US senators Mark Warner (D-VA) and John Boozman (R-AR) brings us “one step closer to making sure veterans get the services and resources they need.”
The senators say the alarming rate of veteran suicides points to “a significant need to empower the VA to work through community partners to expand outreach.” They cite national data indicating that there are > 50,000 organizations that provide suicide prevention services for veterans, yet “they are hard for veterans to find, access, apply for, and use.”
The IMPROVE (Incorporating Measurements and Providing Resources for Outreach to Veterans Everywhere) Well-Being for Veterans Act, introduced in 2019, creates a new grant program to enable the VA to conduct outreach through veteran-serving nonprofits in addition to state and local organizations. The funding would go to organizations with a proven track record of strong mental health and suicide prevention efforts among veterans, Warner says.
The bill supports coordination and planning of veteran mental health and suicide prevention services. Another goal is to provide tools to measure the effectiveness of the programs so the resources can be concentrated where they can do the most good. For example, Warner says, there are no shared tools to measure whether programs help improve mental resiliency and outlook, which can indicate reduced suicide risk.
On January 29, the Senate Veterans Affairs Committee included language from the bill as a provision in a comprehensive bill that expands veterans’ access to mental health services. The legislation unanimously passed the committee and now awaits consideration by the full Senate.
In fiscal year 2010, the VA requested $62 million for suicide prevention outreach; in FY 2020, that leapt to $222 million. Yet despite the dramatic hike in funding, the rate of veteran suicides has remained basically unchanged: An estimated 20 veterans die by suicide every day.
Of those, roughly 14 were not receiving health care from the VA before their death. But a bipartisan bill introduced by US senators Mark Warner (D-VA) and John Boozman (R-AR) brings us “one step closer to making sure veterans get the services and resources they need.”
The senators say the alarming rate of veteran suicides points to “a significant need to empower the VA to work through community partners to expand outreach.” They cite national data indicating that there are > 50,000 organizations that provide suicide prevention services for veterans, yet “they are hard for veterans to find, access, apply for, and use.”
The IMPROVE (Incorporating Measurements and Providing Resources for Outreach to Veterans Everywhere) Well-Being for Veterans Act, introduced in 2019, creates a new grant program to enable the VA to conduct outreach through veteran-serving nonprofits in addition to state and local organizations. The funding would go to organizations with a proven track record of strong mental health and suicide prevention efforts among veterans, Warner says.
The bill supports coordination and planning of veteran mental health and suicide prevention services. Another goal is to provide tools to measure the effectiveness of the programs so the resources can be concentrated where they can do the most good. For example, Warner says, there are no shared tools to measure whether programs help improve mental resiliency and outlook, which can indicate reduced suicide risk.
On January 29, the Senate Veterans Affairs Committee included language from the bill as a provision in a comprehensive bill that expands veterans’ access to mental health services. The legislation unanimously passed the committee and now awaits consideration by the full Senate.
In fiscal year 2010, the VA requested $62 million for suicide prevention outreach; in FY 2020, that leapt to $222 million. Yet despite the dramatic hike in funding, the rate of veteran suicides has remained basically unchanged: An estimated 20 veterans die by suicide every day.
Of those, roughly 14 were not receiving health care from the VA before their death. But a bipartisan bill introduced by US senators Mark Warner (D-VA) and John Boozman (R-AR) brings us “one step closer to making sure veterans get the services and resources they need.”
The senators say the alarming rate of veteran suicides points to “a significant need to empower the VA to work through community partners to expand outreach.” They cite national data indicating that there are > 50,000 organizations that provide suicide prevention services for veterans, yet “they are hard for veterans to find, access, apply for, and use.”
The IMPROVE (Incorporating Measurements and Providing Resources for Outreach to Veterans Everywhere) Well-Being for Veterans Act, introduced in 2019, creates a new grant program to enable the VA to conduct outreach through veteran-serving nonprofits in addition to state and local organizations. The funding would go to organizations with a proven track record of strong mental health and suicide prevention efforts among veterans, Warner says.
The bill supports coordination and planning of veteran mental health and suicide prevention services. Another goal is to provide tools to measure the effectiveness of the programs so the resources can be concentrated where they can do the most good. For example, Warner says, there are no shared tools to measure whether programs help improve mental resiliency and outlook, which can indicate reduced suicide risk.
On January 29, the Senate Veterans Affairs Committee included language from the bill as a provision in a comprehensive bill that expands veterans’ access to mental health services. The legislation unanimously passed the committee and now awaits consideration by the full Senate.
Families as Care Partners: Implementing the Better Together Initiative Across a Large Health System
From the Institute for Patient- and Family-Centered Care, Bethesda, MD (Ms. Dokken and Ms. Johnson), and Northwell Health, New Hyde Park, NY (Dr. Barden, Ms. Tuomey, and Ms. Giammarinaro).
Abstract
Objective: To describe the growth of Better Together: Partnering with Families, a campaign launched in 2014 to eliminate restrictive hospital visiting policies and to put in place policies that recognize families as partners in care, and to discuss the processes involved in implementing the initiative in a large, integrated health system.
Methods: Descriptive report.
Results: In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the Better Together campaign to emphasize the importance of family presence and participation to the quality, experience, safety, and outcomes of care. Since then, this initiative has expanded in both the United States and Canada. With support from 2 funders in the United States, special attention was focused on acute care hospitals across New York State. Nearly 50 hospitals participated in 2 separate but related projects. Fifteen of the hospitals are part of Northwell Health, New York State’s largest health system. Over a 10-month period, these hospitals made significant progress in changing policy, practice, and communication to support family presence.
Conclusion: The Better Together initiative was implemented across a health system with strong support from leadership and the involvement of patient and family advisors. An intervention offering structured training, coaching, and resources, like IPFCC’s Better Together initiative, can facilitate the change process.
Keywords: family presence; visiting policies; patient-centered care; family-centered care; patient experience.
The presence of families at the bedside of patients is often restricted by hospital visiting hours. Hospitals that maintain these restrictive policies cite concerns about negative impacts on security, infection control, privacy, and staff workload. But there are no data to support these concerns, and the experience of hospitals that have successfully changed policy and practice to welcome families demonstrates the potential positive impacts of less restrictive policies on patient care and outcomes.1 For example, hospitalization can lead to reduced cognitive function in elderly patients. Family members would recognize the changes and could provide valuable information to hospital staff, potentially improving outcomes.2
In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families.3 The campaign is is grounded in patient- and family- centered care, an approach to care that supports partnerships among health care providers, patients, and families, and, among other core principles, advocates that patients define their “families” and how they will participate in care and decision-making.
Emphasizing the importance of family presence and participation to quality and safety, the Better Together campaign seeks to eliminate restrictive visiting policies and calls upon hospitals to include families as members of the care team and to welcome them 24 hours a day, 7 days a week, according to patient preference. As part of the campaign, IPFCC developed an extensive toolkit of resources that is available to hospitals and other organizations at no cost. The resources include sample policies; profiles of hospitals that have implemented family presence policies; educational materials for staff, patients, and families; and a template for hospital websites. This article, a follow-up to an article published in the January 2015 issue of JCOM,1 discusses the growth of the Better Together initiative as well as the processes involved in implementing the initiative across a large health system.
Growth of the Initiative
Since its launch in 2014, the Better Together initiative has continued to expand in the United States and Canada. In Canada, under the leadership of the Canadian Foundation for Healthcare Improvement (CFHI), more than 50 organizations have made a commitment to the Better Together program and family presence.4 Utilizing and adapting IPFCC’s Toolkit, CFHI developed a change package of free resources for Canadian organizations.5 Some of the materials, including the Pocket Guide for Families (Manuel des Familles), were translated into French.6
With support from 2 funders in the United States, the United Hospital Fund and the New York State Health (NYSHealth) Foundation, through a subcontract with the New York Public Interest Research Group (NYPIRG), IPFCC has been able to focus on hospitals in New York City, including public hospitals, and, more broadly, acute care hospitals across New York State. Nearly 50 hospitals participated in these 2 separate but related projects.
Education and Support for New York City Hospitals
Supported by the United Hospital Fund, an 18-month project that focused specifically on New York City hospitals was completed in June 2017. The project began with a 1-day intensive training event with representatives of 21 hospitals. Eighteen of those hospitals were eligible to participate in follow-up consultation provided by IPFCC, and 14 participated in some kind of follow-up. NYC Health + Hospitals (H+H), the system of public hospitals in NYC, participated most fully in these activities.
The outcomes of the Better Together initiative in New York City are summarized in the report Sick, Scared, & Separated From Loved Ones,2 which is based on a pre/post review of hospital visitation/family presence policies and website communications. According to the report, hospitals that participated in the IPFCC training and consultation program performed better, as a group, with respect to improved policy and website scores on post review than those that did not. Of the 10 hospitals whose scores improved during the review period, 8 had participated in the IPFCC training and 1 hospital was part of a hospital network that did so. (Six of these hospitals are part of the H+H public hospital system.) Those 9 hospitals saw an average increase in scores of 4.9 points (out of a possible 11).
A Learning Community for Hospitals in New York State
With support from the NYSHealth Foundation, IPFCC again collaborated with NYPIRG and New Yorkers for Patient & Family Empowerment on a 2-year initiative, completed in November 2019, that involved 26 hospitals: 15 from Northwell Health, New York State’s largest health system, and 11 hospitals from health systems throughout the state (Greater Hudson Valley Health System, now Garnet Health; Mohawk Valley Health System; Rochester Regional Health; and University of Vermont Health Network). An update of the report Sick, Scared, & Separated From Loved Onescompared pre/post reviews of policies and website communications regarding hospital visitation/family presence.7 Its findings confirm that hospitals that participated in the Better Together Learning Community improved both their policy and website scores to a greater degree than hospitals that did not participate and that a planned intervention can help facilitate change.
During the survey period, 28 out of 40 hospitals’ website navigability scores improved. Of those, hospitals that did not participate in the Better Together Learning Community saw an average increase in scores of 1.2 points, out of a possible 11, while the participating hospitals saw an average increase of 2.7 points, with the top 5 largest increases in scores belonging to hospitals that participated in the Better Together Learning Community.7
The Northwell Health Experience
Northwell Health is a large integrated health care organization comprising more than 69,000 employees, 23 hospitals, and more than 750 medical practices, located geographically across New York State. Embracing patient- and family-centered care, Northwell is dedicated to improving the quality, experience, and safety of care for patients and their families. Welcoming and including patients, families, and care partners as members of the health care team has always been a core element of Northwell’s organizational goal of providing world-class patient care and experience.
Four years ago, the organization reorganized and formalized a system-wide Patient & Family Partnership Council (PFPC).8 Representatives on the PFPC include a Northwell patient experience leader and patient/family co-chair from local councils that have been established in nearly all 23 hospitals as well as service lines. Modeling partnership, the PFPC is grounded in listening to the “voice” of patients and families and promoting collaboration, with the goal of driving change across varied aspects and experiences of health care delivery.
Through the Office of Patient and Customer Experience (OPCE), a partnership with IPFCC and the Better Together Learning Community for Hospitals in New York State was initiated as a fundamental next step in Northwell’s journey to enhance system-wide family presence and participation. Results from Better Together’s Organizational Self-Assessment Tool and process identified opportunities to influence 3 distinct areas: policy/staff education, position descriptions/performance management, and website/signage. Over a 10-month period (September 2018 through June 2019), 15 Northwell hospitals implemened significant patient- and family-centered improvements through multifaceted shared work teams (SWT) that partnered around the common goal of supporting the patient and family experience (Figure). Northwell’s SWT structure allowed teams to meet individually on specific tasks, led by a dedicated staff member of the OPCE to ensure progress, support, and accountability. Six monthly coaching calls or report-out meetings were attended by participating teams, where feedback and recommendations shared by IPFCC were discussed in order to maintain momentum and results.
Policy/Staff Education
The policy/staff education SWT focused on appraising and updating existing policies to ensure alignment with key patient- and family-centered concepts and Better Together principles (Table 1). By establishing representation on the System Policy and Procedure Committee, OPCE enabled patients and families to have a voice at the decision-making table. OPCE leaders presented the ideology and scope of the transformation to this committee. After reviewing all system-wide policies, 4 were identified as key opportunities for revision. One overarching policy titled “Visitation Guidelines” was reviewed and updated to reflect Northwell’s mission of patient- and family-centered care, retiring the reference to “families” as “visitors” in definitions, incorporating language of inclusion and partnership, and citing other related policies. The policy was vetted through a multilayer process of review and stakeholder feedback and was ultimately approved at a system

Three additional related policies were also updated to reflect core principles of inclusion and partnership. These included system policies focused on discharge planning; identification of health care proxy, agent, support person and caregiver; and standards of behavior not conducive in a health care setting. As a result of this work, OPCE was invited to remain an active member of the System Policy and Procedure Committee, adding meaningful new perspectives to the clinical and administrative policy management process. Once policies were updated and approved, the SWT focused on educating leaders and teams. Using a diversified strategy, education was provided through various modes, including weekly system-wide internal communication channels, patient experience huddle messages, yearly mandatory topics training, and the incorporation of essential concepts in existing educational courses (classroom and e-learning modalities).
Position Descriptions/Performance Management
The position descriptions/performance management SWT focused its efforts on incorporating patient- and family-centered concepts and language into position descriptions and the performance appraisal process (Table 2). Due to the complex nature of this work, the process required collaboration from key subject matter experts in human resources, talent management, corporate compensation, and labor management. In 2019, Northwell began an initiative focused on streamlining and standardizing job titles, roles, and developmental pathways across the system. The overarching goal was to create system-wide consistency and standardization. The SWT was successful in advising the leaders overseeing this job architecture initiative on the importance of including language of patient- and family-centered care, like partnership and collaboration, and of highlighting the critical role of family members as part of the care team in subsequent documents.

Northwell has 6 behavioral expectations, standards to which all team members are held accountable: Patient/Customer Focus, Teamwork, Execution, Organizational Awareness, Enable Change, and Develop Self. As a result of the SWT’s work, Patient/Customer Focus was revised to include “families” as essential care partners, demonstrating Northwell’s ongoing commitment to honoring the role of families as members of the care team. It also ensures that all employees are aligned around this priority, as these expectations are utilized to support areas such as recognition and performance. Collaborating with talent management and organizational development, the SWT reviewed yearly performance management and new-hire evaluations. In doing so, they identified an opportunity to refresh the anchored qualitative rating scales to include behavioral demonstrations of patient- and family-centered care, collaboration, respect, and partnership with family members.
Website/Signage
Websites make an important first impression on patients and families looking for information to best prepare for a hospital experience. Therefore, the website/signage SWT worked to redesign hospital websites, enhance digital signage, and perform a baseline assessment of physical signage across facilities. Initial feedback on Northwell’s websites identified opportunities to include more patient- and family-centered, care-partner-infused language; improve navigation; and streamline click levels for easier access. Content for the websites was carefully crafted in collaboration with Northwell’s internal web team, utilizing IPFCC’s best practice standards as a framework and guide.
Next, a multidisciplinary website shared-governance team was established by the OPCE to ensure that key stakeholders were represented and had the opportunity to review and make recommendations for appropriate language and messaging about family presence and participation. This 13-person team was comprised of patient/family partners, patient-experience culture leaders, quality, compliance, human resources, policy, a chief nursing officer, a medical director, and representation from the Institute for Nursing. After careful review and consideration from Northwell’s family partners and teams, all participating hospital websites were enhanced as of June 2019 to include prominent 1-click access from homepages to information for “patients, families and visitors,” as well as “your care partners” information on the important role of families and care partners.
Along with refreshing websites, another step in Northwell’s work to strengthen messaging about family presence and participation was to partner and collaborate with the system’s digital web team as well as local facility councils to understand the capacity to adjust digital signage across facilities. Opportunities were found to make simple yet effective enhancements to the language and imagery of digital signage upon entry, creating a warmer and more welcoming first impression for patients and families. With patient and family partner feedback, the team designed digital signage with inclusive messaging and images that would circulate appropriately based on the facility. Signage specifically welcomes families and refers to them as members of patients’ care teams.
Northwell’s website/signage SWT also directed a 2-phase physical signage assessment to determine ongoing opportunities to alter signs in areas that particularly impact patients and families, such as emergency departments, main lobbies, cafeterias, surgical waiting areas, and intensive care units. Each hospital’s local PFPC did a “walk-about”9 to make enhancements to physical signage, such as removing paper and overcrowded signs, adjusting negative language, ensuring alignment with brand guidelines, and including language that welcomed families. As a result of the team’s efforts around signage, collaboration began with the health system’s signage committee to help standardize signage terminology to reflect family inclusiveness, and to implement the recommendation for a standardized signage shared-governance team to ensure accountability and a patient- and family-centered structure.
Sustainment
Since implementing Better Together, Northwell has been able to infuse a more patient- and family-centered emphasis into its overall patient experience message of “Every role, every person, every moment matters.” As a strategic tool aimed at encouraging leaders, clinicians, and staff to pause and reflect about the “heart” of their work, patient and family stories are now included at the beginning of meetings, forums, and team huddles. Elements of the initiative have been integrated in current Patient and Family Partnership sustainment plans at participating hospitals. Some highlights include continued integration of patient/family partners on committees and councils that impact areas such as way finding, signage, recruitment, new-hire orientation, and community outreach; focus on enhancing partner retention and development programs; and inclusion of patient- and family-centered care and Better Together principles in ongoing leadership meetings.
Factors Contributing to Success
Health care is a complex, regulated, and often bureaucratic world that can be very difficult for patients and families to navigate. The system’s partnership with the Better Together Learning Community for Hospitals in New York State enhanced its efforts to improve family presence and participation and created powerful synergy. The success of this partnership was based on a number of important factors:
A solid foundation of support, structure, and accountability. The OPCE initiated the IPFCC Better Together partnership and established a synergistic collaboration inclusive of leadership, frontline teams, multiple departments, and patient and family partners. As a major strategic component of Northwell’s mission to deliver high-quality, patient- and family-centered care, OPCE was instrumental in connecting key areas and stakeholders and mobilizing the recommendations coming from patients and families.
A visible commitment of leadership at all levels. Partnering with leadership across Northwell’s system required a delineated vision, clear purpose and ownership, and comprehensive implementation and sustainment strategies. The existing format of Northwell’s PFPC provided the structure and framework needed for engaged patient and family input; the OPCE motivated and organized key areas of involvement and led communication efforts across the organization. The IPFCC coaching calls provided the underlying guidance and accountability needed to sustain momentum. As leadership and frontline teams became aware of the vision, they understood the larger connection to the system’s purpose, which ultimately created a clear path for positive change.
Meaningful involvement and input of patient and family partners. Throughout this project, Northwell’s patient/family partners were involved through the PFPC and local councils. For example, patient/family partners attended every IPFCC coaching call; members had a central voice in every decision made within each SWT; and local PFPCs actively participated in physical signage “walk-abouts” across facilities, making key recommendations for improvement. This multifaceted, supportive collaboration created a rejuvenated and purposeful focus for all council members involved. Some of their reactions include, “…I am so happy to be able to help other families in crisis, so that they don’t have to be alone, like I was,” and “I feel how important the patient and family’s voice is … it’s truly a partnership between patients, families, and staff.”
Regular access to IPFCC as a best practice coach and expert resource. Throughout the 10-month process, IPFCC’s Better Together Learning Community for Hospitals in New York State provided ongoing learning interventions for members of the SWT; multiple and varied resources from the Better Together toolkit for adaptation; and opportunities to share and reinforce new, learned expertise with colleagues within the Northwell Health system and beyond through IPFCC’s free online learning community, PFCC.Connect.
Conclusion
Family presence and participation are important to the quality, experience, safety, and outcomes of care. IPFCC’s campaign, Better Together: Partnering with Families, encourages hospitals to change restrictive visiting policies and, instead, to welcome families and caregivers 24 hours a day.
Two projects within Better Together involving almost 50 acute care hospitals in New York State confirm that change in policy, practice, and communication is particularly effective when implemented with strong support from leadership. An intervention like the Better Together Learning Community, offering structured training, coaching, and resources, can facilitate the change process.
Corresponding author: IPFCC, Deborah L. Dokken, 6917 Arlington Rd., Ste. 309, Bethesda, MD 20814; [email protected].
Funding disclosures: None.
1. Dokken DL, Kaufman J, Johnson BJ et al. Changing hospital visiting policies: from families as “visitors” to families as partners. J Clin Outcomes Manag. 2015; 22:29-36.
2. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. third edition: A pathway to improvement in New York City. New York: NYPIRG: 2018. www.nypirg.org/pubs/201801/NYPIRG_SICK_SCARED_FINAL.pdf. Accessed December 12, 2019.
3. Institute for Patient- and Family-Centered Care. Better Together: Partnering with Families. www.ipfcc.org/bestpractices/better-together.html. Accessed December 12, 2019.
4. Canadian Foundation for Healthcare Improvement. Better Together. www.cfhi-fcass.ca/WhatWeDo/better-together. Accessed December 12, 2019.
5. Canadian Foundation for Healthcare Improvement. Better Together: A change package to support the adoption of family presence and participation in acute care hospitals and accelerate healthcare improvement. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/better-together-change-package.pdf?sfvrsn=9656d044_4. Accessed December 12, 2019.
6. Canadian Foundation for Healthcare Improvement. L’Objectif santé: main dans la main avec les familles. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/families-pocket-screen_fr.pdf. Accessed December 12, 2019.
7. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. fourth edition: A pathway to improvement in New York. New York: NYPIRG: 2019. www.nypirg.org/pubs/201911/Sick_Scared_Separated_2019_web_FINAL.pdf. Accessed December 12, 2019.
8. Northwell Health. Patient and Family Partnership Councils. www.northwell.edu/about/commitment-to-excellence/patient-and-customer-experience/care-delivery-hospitality. Accessed December 12, 2019.
9 . Institute for Patient- and Family-Centered Care. How to conduct a “walk-about” from the patient and family perspective. www.ipfcc.org/resources/How_To_Conduct_A_Walk-About.pdf. Accessed December 12, 2019.
From the Institute for Patient- and Family-Centered Care, Bethesda, MD (Ms. Dokken and Ms. Johnson), and Northwell Health, New Hyde Park, NY (Dr. Barden, Ms. Tuomey, and Ms. Giammarinaro).
Abstract
Objective: To describe the growth of Better Together: Partnering with Families, a campaign launched in 2014 to eliminate restrictive hospital visiting policies and to put in place policies that recognize families as partners in care, and to discuss the processes involved in implementing the initiative in a large, integrated health system.
Methods: Descriptive report.
Results: In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the Better Together campaign to emphasize the importance of family presence and participation to the quality, experience, safety, and outcomes of care. Since then, this initiative has expanded in both the United States and Canada. With support from 2 funders in the United States, special attention was focused on acute care hospitals across New York State. Nearly 50 hospitals participated in 2 separate but related projects. Fifteen of the hospitals are part of Northwell Health, New York State’s largest health system. Over a 10-month period, these hospitals made significant progress in changing policy, practice, and communication to support family presence.
Conclusion: The Better Together initiative was implemented across a health system with strong support from leadership and the involvement of patient and family advisors. An intervention offering structured training, coaching, and resources, like IPFCC’s Better Together initiative, can facilitate the change process.
Keywords: family presence; visiting policies; patient-centered care; family-centered care; patient experience.
The presence of families at the bedside of patients is often restricted by hospital visiting hours. Hospitals that maintain these restrictive policies cite concerns about negative impacts on security, infection control, privacy, and staff workload. But there are no data to support these concerns, and the experience of hospitals that have successfully changed policy and practice to welcome families demonstrates the potential positive impacts of less restrictive policies on patient care and outcomes.1 For example, hospitalization can lead to reduced cognitive function in elderly patients. Family members would recognize the changes and could provide valuable information to hospital staff, potentially improving outcomes.2
In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families.3 The campaign is is grounded in patient- and family- centered care, an approach to care that supports partnerships among health care providers, patients, and families, and, among other core principles, advocates that patients define their “families” and how they will participate in care and decision-making.
Emphasizing the importance of family presence and participation to quality and safety, the Better Together campaign seeks to eliminate restrictive visiting policies and calls upon hospitals to include families as members of the care team and to welcome them 24 hours a day, 7 days a week, according to patient preference. As part of the campaign, IPFCC developed an extensive toolkit of resources that is available to hospitals and other organizations at no cost. The resources include sample policies; profiles of hospitals that have implemented family presence policies; educational materials for staff, patients, and families; and a template for hospital websites. This article, a follow-up to an article published in the January 2015 issue of JCOM,1 discusses the growth of the Better Together initiative as well as the processes involved in implementing the initiative across a large health system.
Growth of the Initiative
Since its launch in 2014, the Better Together initiative has continued to expand in the United States and Canada. In Canada, under the leadership of the Canadian Foundation for Healthcare Improvement (CFHI), more than 50 organizations have made a commitment to the Better Together program and family presence.4 Utilizing and adapting IPFCC’s Toolkit, CFHI developed a change package of free resources for Canadian organizations.5 Some of the materials, including the Pocket Guide for Families (Manuel des Familles), were translated into French.6
With support from 2 funders in the United States, the United Hospital Fund and the New York State Health (NYSHealth) Foundation, through a subcontract with the New York Public Interest Research Group (NYPIRG), IPFCC has been able to focus on hospitals in New York City, including public hospitals, and, more broadly, acute care hospitals across New York State. Nearly 50 hospitals participated in these 2 separate but related projects.
Education and Support for New York City Hospitals
Supported by the United Hospital Fund, an 18-month project that focused specifically on New York City hospitals was completed in June 2017. The project began with a 1-day intensive training event with representatives of 21 hospitals. Eighteen of those hospitals were eligible to participate in follow-up consultation provided by IPFCC, and 14 participated in some kind of follow-up. NYC Health + Hospitals (H+H), the system of public hospitals in NYC, participated most fully in these activities.
The outcomes of the Better Together initiative in New York City are summarized in the report Sick, Scared, & Separated From Loved Ones,2 which is based on a pre/post review of hospital visitation/family presence policies and website communications. According to the report, hospitals that participated in the IPFCC training and consultation program performed better, as a group, with respect to improved policy and website scores on post review than those that did not. Of the 10 hospitals whose scores improved during the review period, 8 had participated in the IPFCC training and 1 hospital was part of a hospital network that did so. (Six of these hospitals are part of the H+H public hospital system.) Those 9 hospitals saw an average increase in scores of 4.9 points (out of a possible 11).
A Learning Community for Hospitals in New York State
With support from the NYSHealth Foundation, IPFCC again collaborated with NYPIRG and New Yorkers for Patient & Family Empowerment on a 2-year initiative, completed in November 2019, that involved 26 hospitals: 15 from Northwell Health, New York State’s largest health system, and 11 hospitals from health systems throughout the state (Greater Hudson Valley Health System, now Garnet Health; Mohawk Valley Health System; Rochester Regional Health; and University of Vermont Health Network). An update of the report Sick, Scared, & Separated From Loved Onescompared pre/post reviews of policies and website communications regarding hospital visitation/family presence.7 Its findings confirm that hospitals that participated in the Better Together Learning Community improved both their policy and website scores to a greater degree than hospitals that did not participate and that a planned intervention can help facilitate change.
During the survey period, 28 out of 40 hospitals’ website navigability scores improved. Of those, hospitals that did not participate in the Better Together Learning Community saw an average increase in scores of 1.2 points, out of a possible 11, while the participating hospitals saw an average increase of 2.7 points, with the top 5 largest increases in scores belonging to hospitals that participated in the Better Together Learning Community.7
The Northwell Health Experience
Northwell Health is a large integrated health care organization comprising more than 69,000 employees, 23 hospitals, and more than 750 medical practices, located geographically across New York State. Embracing patient- and family-centered care, Northwell is dedicated to improving the quality, experience, and safety of care for patients and their families. Welcoming and including patients, families, and care partners as members of the health care team has always been a core element of Northwell’s organizational goal of providing world-class patient care and experience.
Four years ago, the organization reorganized and formalized a system-wide Patient & Family Partnership Council (PFPC).8 Representatives on the PFPC include a Northwell patient experience leader and patient/family co-chair from local councils that have been established in nearly all 23 hospitals as well as service lines. Modeling partnership, the PFPC is grounded in listening to the “voice” of patients and families and promoting collaboration, with the goal of driving change across varied aspects and experiences of health care delivery.
Through the Office of Patient and Customer Experience (OPCE), a partnership with IPFCC and the Better Together Learning Community for Hospitals in New York State was initiated as a fundamental next step in Northwell’s journey to enhance system-wide family presence and participation. Results from Better Together’s Organizational Self-Assessment Tool and process identified opportunities to influence 3 distinct areas: policy/staff education, position descriptions/performance management, and website/signage. Over a 10-month period (September 2018 through June 2019), 15 Northwell hospitals implemened significant patient- and family-centered improvements through multifaceted shared work teams (SWT) that partnered around the common goal of supporting the patient and family experience (Figure). Northwell’s SWT structure allowed teams to meet individually on specific tasks, led by a dedicated staff member of the OPCE to ensure progress, support, and accountability. Six monthly coaching calls or report-out meetings were attended by participating teams, where feedback and recommendations shared by IPFCC were discussed in order to maintain momentum and results.

Policy/Staff Education
The policy/staff education SWT focused on appraising and updating existing policies to ensure alignment with key patient- and family-centered concepts and Better Together principles (Table 1). By establishing representation on the System Policy and Procedure Committee, OPCE enabled patients and families to have a voice at the decision-making table. OPCE leaders presented the ideology and scope of the transformation to this committee. After reviewing all system-wide policies, 4 were identified as key opportunities for revision. One overarching policy titled “Visitation Guidelines” was reviewed and updated to reflect Northwell’s mission of patient- and family-centered care, retiring the reference to “families” as “visitors” in definitions, incorporating language of inclusion and partnership, and citing other related policies. The policy was vetted through a multilayer process of review and stakeholder feedback and was ultimately approved at a system

Three additional related policies were also updated to reflect core principles of inclusion and partnership. These included system policies focused on discharge planning; identification of health care proxy, agent, support person and caregiver; and standards of behavior not conducive in a health care setting. As a result of this work, OPCE was invited to remain an active member of the System Policy and Procedure Committee, adding meaningful new perspectives to the clinical and administrative policy management process. Once policies were updated and approved, the SWT focused on educating leaders and teams. Using a diversified strategy, education was provided through various modes, including weekly system-wide internal communication channels, patient experience huddle messages, yearly mandatory topics training, and the incorporation of essential concepts in existing educational courses (classroom and e-learning modalities).
Position Descriptions/Performance Management
The position descriptions/performance management SWT focused its efforts on incorporating patient- and family-centered concepts and language into position descriptions and the performance appraisal process (Table 2). Due to the complex nature of this work, the process required collaboration from key subject matter experts in human resources, talent management, corporate compensation, and labor management. In 2019, Northwell began an initiative focused on streamlining and standardizing job titles, roles, and developmental pathways across the system. The overarching goal was to create system-wide consistency and standardization. The SWT was successful in advising the leaders overseeing this job architecture initiative on the importance of including language of patient- and family-centered care, like partnership and collaboration, and of highlighting the critical role of family members as part of the care team in subsequent documents.

Northwell has 6 behavioral expectations, standards to which all team members are held accountable: Patient/Customer Focus, Teamwork, Execution, Organizational Awareness, Enable Change, and Develop Self. As a result of the SWT’s work, Patient/Customer Focus was revised to include “families” as essential care partners, demonstrating Northwell’s ongoing commitment to honoring the role of families as members of the care team. It also ensures that all employees are aligned around this priority, as these expectations are utilized to support areas such as recognition and performance. Collaborating with talent management and organizational development, the SWT reviewed yearly performance management and new-hire evaluations. In doing so, they identified an opportunity to refresh the anchored qualitative rating scales to include behavioral demonstrations of patient- and family-centered care, collaboration, respect, and partnership with family members.
Website/Signage
Websites make an important first impression on patients and families looking for information to best prepare for a hospital experience. Therefore, the website/signage SWT worked to redesign hospital websites, enhance digital signage, and perform a baseline assessment of physical signage across facilities. Initial feedback on Northwell’s websites identified opportunities to include more patient- and family-centered, care-partner-infused language; improve navigation; and streamline click levels for easier access. Content for the websites was carefully crafted in collaboration with Northwell’s internal web team, utilizing IPFCC’s best practice standards as a framework and guide.
Next, a multidisciplinary website shared-governance team was established by the OPCE to ensure that key stakeholders were represented and had the opportunity to review and make recommendations for appropriate language and messaging about family presence and participation. This 13-person team was comprised of patient/family partners, patient-experience culture leaders, quality, compliance, human resources, policy, a chief nursing officer, a medical director, and representation from the Institute for Nursing. After careful review and consideration from Northwell’s family partners and teams, all participating hospital websites were enhanced as of June 2019 to include prominent 1-click access from homepages to information for “patients, families and visitors,” as well as “your care partners” information on the important role of families and care partners.
Along with refreshing websites, another step in Northwell’s work to strengthen messaging about family presence and participation was to partner and collaborate with the system’s digital web team as well as local facility councils to understand the capacity to adjust digital signage across facilities. Opportunities were found to make simple yet effective enhancements to the language and imagery of digital signage upon entry, creating a warmer and more welcoming first impression for patients and families. With patient and family partner feedback, the team designed digital signage with inclusive messaging and images that would circulate appropriately based on the facility. Signage specifically welcomes families and refers to them as members of patients’ care teams.
Northwell’s website/signage SWT also directed a 2-phase physical signage assessment to determine ongoing opportunities to alter signs in areas that particularly impact patients and families, such as emergency departments, main lobbies, cafeterias, surgical waiting areas, and intensive care units. Each hospital’s local PFPC did a “walk-about”9 to make enhancements to physical signage, such as removing paper and overcrowded signs, adjusting negative language, ensuring alignment with brand guidelines, and including language that welcomed families. As a result of the team’s efforts around signage, collaboration began with the health system’s signage committee to help standardize signage terminology to reflect family inclusiveness, and to implement the recommendation for a standardized signage shared-governance team to ensure accountability and a patient- and family-centered structure.
Sustainment
Since implementing Better Together, Northwell has been able to infuse a more patient- and family-centered emphasis into its overall patient experience message of “Every role, every person, every moment matters.” As a strategic tool aimed at encouraging leaders, clinicians, and staff to pause and reflect about the “heart” of their work, patient and family stories are now included at the beginning of meetings, forums, and team huddles. Elements of the initiative have been integrated in current Patient and Family Partnership sustainment plans at participating hospitals. Some highlights include continued integration of patient/family partners on committees and councils that impact areas such as way finding, signage, recruitment, new-hire orientation, and community outreach; focus on enhancing partner retention and development programs; and inclusion of patient- and family-centered care and Better Together principles in ongoing leadership meetings.
Factors Contributing to Success
Health care is a complex, regulated, and often bureaucratic world that can be very difficult for patients and families to navigate. The system’s partnership with the Better Together Learning Community for Hospitals in New York State enhanced its efforts to improve family presence and participation and created powerful synergy. The success of this partnership was based on a number of important factors:
A solid foundation of support, structure, and accountability. The OPCE initiated the IPFCC Better Together partnership and established a synergistic collaboration inclusive of leadership, frontline teams, multiple departments, and patient and family partners. As a major strategic component of Northwell’s mission to deliver high-quality, patient- and family-centered care, OPCE was instrumental in connecting key areas and stakeholders and mobilizing the recommendations coming from patients and families.
A visible commitment of leadership at all levels. Partnering with leadership across Northwell’s system required a delineated vision, clear purpose and ownership, and comprehensive implementation and sustainment strategies. The existing format of Northwell’s PFPC provided the structure and framework needed for engaged patient and family input; the OPCE motivated and organized key areas of involvement and led communication efforts across the organization. The IPFCC coaching calls provided the underlying guidance and accountability needed to sustain momentum. As leadership and frontline teams became aware of the vision, they understood the larger connection to the system’s purpose, which ultimately created a clear path for positive change.
Meaningful involvement and input of patient and family partners. Throughout this project, Northwell’s patient/family partners were involved through the PFPC and local councils. For example, patient/family partners attended every IPFCC coaching call; members had a central voice in every decision made within each SWT; and local PFPCs actively participated in physical signage “walk-abouts” across facilities, making key recommendations for improvement. This multifaceted, supportive collaboration created a rejuvenated and purposeful focus for all council members involved. Some of their reactions include, “…I am so happy to be able to help other families in crisis, so that they don’t have to be alone, like I was,” and “I feel how important the patient and family’s voice is … it’s truly a partnership between patients, families, and staff.”
Regular access to IPFCC as a best practice coach and expert resource. Throughout the 10-month process, IPFCC’s Better Together Learning Community for Hospitals in New York State provided ongoing learning interventions for members of the SWT; multiple and varied resources from the Better Together toolkit for adaptation; and opportunities to share and reinforce new, learned expertise with colleagues within the Northwell Health system and beyond through IPFCC’s free online learning community, PFCC.Connect.
Conclusion
Family presence and participation are important to the quality, experience, safety, and outcomes of care. IPFCC’s campaign, Better Together: Partnering with Families, encourages hospitals to change restrictive visiting policies and, instead, to welcome families and caregivers 24 hours a day.
Two projects within Better Together involving almost 50 acute care hospitals in New York State confirm that change in policy, practice, and communication is particularly effective when implemented with strong support from leadership. An intervention like the Better Together Learning Community, offering structured training, coaching, and resources, can facilitate the change process.
Corresponding author: IPFCC, Deborah L. Dokken, 6917 Arlington Rd., Ste. 309, Bethesda, MD 20814; [email protected].
Funding disclosures: None.
From the Institute for Patient- and Family-Centered Care, Bethesda, MD (Ms. Dokken and Ms. Johnson), and Northwell Health, New Hyde Park, NY (Dr. Barden, Ms. Tuomey, and Ms. Giammarinaro).
Abstract
Objective: To describe the growth of Better Together: Partnering with Families, a campaign launched in 2014 to eliminate restrictive hospital visiting policies and to put in place policies that recognize families as partners in care, and to discuss the processes involved in implementing the initiative in a large, integrated health system.
Methods: Descriptive report.
Results: In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the Better Together campaign to emphasize the importance of family presence and participation to the quality, experience, safety, and outcomes of care. Since then, this initiative has expanded in both the United States and Canada. With support from 2 funders in the United States, special attention was focused on acute care hospitals across New York State. Nearly 50 hospitals participated in 2 separate but related projects. Fifteen of the hospitals are part of Northwell Health, New York State’s largest health system. Over a 10-month period, these hospitals made significant progress in changing policy, practice, and communication to support family presence.
Conclusion: The Better Together initiative was implemented across a health system with strong support from leadership and the involvement of patient and family advisors. An intervention offering structured training, coaching, and resources, like IPFCC’s Better Together initiative, can facilitate the change process.
Keywords: family presence; visiting policies; patient-centered care; family-centered care; patient experience.
The presence of families at the bedside of patients is often restricted by hospital visiting hours. Hospitals that maintain these restrictive policies cite concerns about negative impacts on security, infection control, privacy, and staff workload. But there are no data to support these concerns, and the experience of hospitals that have successfully changed policy and practice to welcome families demonstrates the potential positive impacts of less restrictive policies on patient care and outcomes.1 For example, hospitalization can lead to reduced cognitive function in elderly patients. Family members would recognize the changes and could provide valuable information to hospital staff, potentially improving outcomes.2
In June 2014, the Institute for Patient- and Family-Centered Care (IPFCC) launched the campaign Better Together: Partnering with Families.3 The campaign is is grounded in patient- and family- centered care, an approach to care that supports partnerships among health care providers, patients, and families, and, among other core principles, advocates that patients define their “families” and how they will participate in care and decision-making.
Emphasizing the importance of family presence and participation to quality and safety, the Better Together campaign seeks to eliminate restrictive visiting policies and calls upon hospitals to include families as members of the care team and to welcome them 24 hours a day, 7 days a week, according to patient preference. As part of the campaign, IPFCC developed an extensive toolkit of resources that is available to hospitals and other organizations at no cost. The resources include sample policies; profiles of hospitals that have implemented family presence policies; educational materials for staff, patients, and families; and a template for hospital websites. This article, a follow-up to an article published in the January 2015 issue of JCOM,1 discusses the growth of the Better Together initiative as well as the processes involved in implementing the initiative across a large health system.
Growth of the Initiative
Since its launch in 2014, the Better Together initiative has continued to expand in the United States and Canada. In Canada, under the leadership of the Canadian Foundation for Healthcare Improvement (CFHI), more than 50 organizations have made a commitment to the Better Together program and family presence.4 Utilizing and adapting IPFCC’s Toolkit, CFHI developed a change package of free resources for Canadian organizations.5 Some of the materials, including the Pocket Guide for Families (Manuel des Familles), were translated into French.6
With support from 2 funders in the United States, the United Hospital Fund and the New York State Health (NYSHealth) Foundation, through a subcontract with the New York Public Interest Research Group (NYPIRG), IPFCC has been able to focus on hospitals in New York City, including public hospitals, and, more broadly, acute care hospitals across New York State. Nearly 50 hospitals participated in these 2 separate but related projects.
Education and Support for New York City Hospitals
Supported by the United Hospital Fund, an 18-month project that focused specifically on New York City hospitals was completed in June 2017. The project began with a 1-day intensive training event with representatives of 21 hospitals. Eighteen of those hospitals were eligible to participate in follow-up consultation provided by IPFCC, and 14 participated in some kind of follow-up. NYC Health + Hospitals (H+H), the system of public hospitals in NYC, participated most fully in these activities.
The outcomes of the Better Together initiative in New York City are summarized in the report Sick, Scared, & Separated From Loved Ones,2 which is based on a pre/post review of hospital visitation/family presence policies and website communications. According to the report, hospitals that participated in the IPFCC training and consultation program performed better, as a group, with respect to improved policy and website scores on post review than those that did not. Of the 10 hospitals whose scores improved during the review period, 8 had participated in the IPFCC training and 1 hospital was part of a hospital network that did so. (Six of these hospitals are part of the H+H public hospital system.) Those 9 hospitals saw an average increase in scores of 4.9 points (out of a possible 11).
A Learning Community for Hospitals in New York State
With support from the NYSHealth Foundation, IPFCC again collaborated with NYPIRG and New Yorkers for Patient & Family Empowerment on a 2-year initiative, completed in November 2019, that involved 26 hospitals: 15 from Northwell Health, New York State’s largest health system, and 11 hospitals from health systems throughout the state (Greater Hudson Valley Health System, now Garnet Health; Mohawk Valley Health System; Rochester Regional Health; and University of Vermont Health Network). An update of the report Sick, Scared, & Separated From Loved Onescompared pre/post reviews of policies and website communications regarding hospital visitation/family presence.7 Its findings confirm that hospitals that participated in the Better Together Learning Community improved both their policy and website scores to a greater degree than hospitals that did not participate and that a planned intervention can help facilitate change.
During the survey period, 28 out of 40 hospitals’ website navigability scores improved. Of those, hospitals that did not participate in the Better Together Learning Community saw an average increase in scores of 1.2 points, out of a possible 11, while the participating hospitals saw an average increase of 2.7 points, with the top 5 largest increases in scores belonging to hospitals that participated in the Better Together Learning Community.7
The Northwell Health Experience
Northwell Health is a large integrated health care organization comprising more than 69,000 employees, 23 hospitals, and more than 750 medical practices, located geographically across New York State. Embracing patient- and family-centered care, Northwell is dedicated to improving the quality, experience, and safety of care for patients and their families. Welcoming and including patients, families, and care partners as members of the health care team has always been a core element of Northwell’s organizational goal of providing world-class patient care and experience.
Four years ago, the organization reorganized and formalized a system-wide Patient & Family Partnership Council (PFPC).8 Representatives on the PFPC include a Northwell patient experience leader and patient/family co-chair from local councils that have been established in nearly all 23 hospitals as well as service lines. Modeling partnership, the PFPC is grounded in listening to the “voice” of patients and families and promoting collaboration, with the goal of driving change across varied aspects and experiences of health care delivery.
Through the Office of Patient and Customer Experience (OPCE), a partnership with IPFCC and the Better Together Learning Community for Hospitals in New York State was initiated as a fundamental next step in Northwell’s journey to enhance system-wide family presence and participation. Results from Better Together’s Organizational Self-Assessment Tool and process identified opportunities to influence 3 distinct areas: policy/staff education, position descriptions/performance management, and website/signage. Over a 10-month period (September 2018 through June 2019), 15 Northwell hospitals implemened significant patient- and family-centered improvements through multifaceted shared work teams (SWT) that partnered around the common goal of supporting the patient and family experience (Figure). Northwell’s SWT structure allowed teams to meet individually on specific tasks, led by a dedicated staff member of the OPCE to ensure progress, support, and accountability. Six monthly coaching calls or report-out meetings were attended by participating teams, where feedback and recommendations shared by IPFCC were discussed in order to maintain momentum and results.

Policy/Staff Education
The policy/staff education SWT focused on appraising and updating existing policies to ensure alignment with key patient- and family-centered concepts and Better Together principles (Table 1). By establishing representation on the System Policy and Procedure Committee, OPCE enabled patients and families to have a voice at the decision-making table. OPCE leaders presented the ideology and scope of the transformation to this committee. After reviewing all system-wide policies, 4 were identified as key opportunities for revision. One overarching policy titled “Visitation Guidelines” was reviewed and updated to reflect Northwell’s mission of patient- and family-centered care, retiring the reference to “families” as “visitors” in definitions, incorporating language of inclusion and partnership, and citing other related policies. The policy was vetted through a multilayer process of review and stakeholder feedback and was ultimately approved at a system

Three additional related policies were also updated to reflect core principles of inclusion and partnership. These included system policies focused on discharge planning; identification of health care proxy, agent, support person and caregiver; and standards of behavior not conducive in a health care setting. As a result of this work, OPCE was invited to remain an active member of the System Policy and Procedure Committee, adding meaningful new perspectives to the clinical and administrative policy management process. Once policies were updated and approved, the SWT focused on educating leaders and teams. Using a diversified strategy, education was provided through various modes, including weekly system-wide internal communication channels, patient experience huddle messages, yearly mandatory topics training, and the incorporation of essential concepts in existing educational courses (classroom and e-learning modalities).
Position Descriptions/Performance Management
The position descriptions/performance management SWT focused its efforts on incorporating patient- and family-centered concepts and language into position descriptions and the performance appraisal process (Table 2). Due to the complex nature of this work, the process required collaboration from key subject matter experts in human resources, talent management, corporate compensation, and labor management. In 2019, Northwell began an initiative focused on streamlining and standardizing job titles, roles, and developmental pathways across the system. The overarching goal was to create system-wide consistency and standardization. The SWT was successful in advising the leaders overseeing this job architecture initiative on the importance of including language of patient- and family-centered care, like partnership and collaboration, and of highlighting the critical role of family members as part of the care team in subsequent documents.

Northwell has 6 behavioral expectations, standards to which all team members are held accountable: Patient/Customer Focus, Teamwork, Execution, Organizational Awareness, Enable Change, and Develop Self. As a result of the SWT’s work, Patient/Customer Focus was revised to include “families” as essential care partners, demonstrating Northwell’s ongoing commitment to honoring the role of families as members of the care team. It also ensures that all employees are aligned around this priority, as these expectations are utilized to support areas such as recognition and performance. Collaborating with talent management and organizational development, the SWT reviewed yearly performance management and new-hire evaluations. In doing so, they identified an opportunity to refresh the anchored qualitative rating scales to include behavioral demonstrations of patient- and family-centered care, collaboration, respect, and partnership with family members.
Website/Signage
Websites make an important first impression on patients and families looking for information to best prepare for a hospital experience. Therefore, the website/signage SWT worked to redesign hospital websites, enhance digital signage, and perform a baseline assessment of physical signage across facilities. Initial feedback on Northwell’s websites identified opportunities to include more patient- and family-centered, care-partner-infused language; improve navigation; and streamline click levels for easier access. Content for the websites was carefully crafted in collaboration with Northwell’s internal web team, utilizing IPFCC’s best practice standards as a framework and guide.
Next, a multidisciplinary website shared-governance team was established by the OPCE to ensure that key stakeholders were represented and had the opportunity to review and make recommendations for appropriate language and messaging about family presence and participation. This 13-person team was comprised of patient/family partners, patient-experience culture leaders, quality, compliance, human resources, policy, a chief nursing officer, a medical director, and representation from the Institute for Nursing. After careful review and consideration from Northwell’s family partners and teams, all participating hospital websites were enhanced as of June 2019 to include prominent 1-click access from homepages to information for “patients, families and visitors,” as well as “your care partners” information on the important role of families and care partners.
Along with refreshing websites, another step in Northwell’s work to strengthen messaging about family presence and participation was to partner and collaborate with the system’s digital web team as well as local facility councils to understand the capacity to adjust digital signage across facilities. Opportunities were found to make simple yet effective enhancements to the language and imagery of digital signage upon entry, creating a warmer and more welcoming first impression for patients and families. With patient and family partner feedback, the team designed digital signage with inclusive messaging and images that would circulate appropriately based on the facility. Signage specifically welcomes families and refers to them as members of patients’ care teams.
Northwell’s website/signage SWT also directed a 2-phase physical signage assessment to determine ongoing opportunities to alter signs in areas that particularly impact patients and families, such as emergency departments, main lobbies, cafeterias, surgical waiting areas, and intensive care units. Each hospital’s local PFPC did a “walk-about”9 to make enhancements to physical signage, such as removing paper and overcrowded signs, adjusting negative language, ensuring alignment with brand guidelines, and including language that welcomed families. As a result of the team’s efforts around signage, collaboration began with the health system’s signage committee to help standardize signage terminology to reflect family inclusiveness, and to implement the recommendation for a standardized signage shared-governance team to ensure accountability and a patient- and family-centered structure.
Sustainment
Since implementing Better Together, Northwell has been able to infuse a more patient- and family-centered emphasis into its overall patient experience message of “Every role, every person, every moment matters.” As a strategic tool aimed at encouraging leaders, clinicians, and staff to pause and reflect about the “heart” of their work, patient and family stories are now included at the beginning of meetings, forums, and team huddles. Elements of the initiative have been integrated in current Patient and Family Partnership sustainment plans at participating hospitals. Some highlights include continued integration of patient/family partners on committees and councils that impact areas such as way finding, signage, recruitment, new-hire orientation, and community outreach; focus on enhancing partner retention and development programs; and inclusion of patient- and family-centered care and Better Together principles in ongoing leadership meetings.
Factors Contributing to Success
Health care is a complex, regulated, and often bureaucratic world that can be very difficult for patients and families to navigate. The system’s partnership with the Better Together Learning Community for Hospitals in New York State enhanced its efforts to improve family presence and participation and created powerful synergy. The success of this partnership was based on a number of important factors:
A solid foundation of support, structure, and accountability. The OPCE initiated the IPFCC Better Together partnership and established a synergistic collaboration inclusive of leadership, frontline teams, multiple departments, and patient and family partners. As a major strategic component of Northwell’s mission to deliver high-quality, patient- and family-centered care, OPCE was instrumental in connecting key areas and stakeholders and mobilizing the recommendations coming from patients and families.
A visible commitment of leadership at all levels. Partnering with leadership across Northwell’s system required a delineated vision, clear purpose and ownership, and comprehensive implementation and sustainment strategies. The existing format of Northwell’s PFPC provided the structure and framework needed for engaged patient and family input; the OPCE motivated and organized key areas of involvement and led communication efforts across the organization. The IPFCC coaching calls provided the underlying guidance and accountability needed to sustain momentum. As leadership and frontline teams became aware of the vision, they understood the larger connection to the system’s purpose, which ultimately created a clear path for positive change.
Meaningful involvement and input of patient and family partners. Throughout this project, Northwell’s patient/family partners were involved through the PFPC and local councils. For example, patient/family partners attended every IPFCC coaching call; members had a central voice in every decision made within each SWT; and local PFPCs actively participated in physical signage “walk-abouts” across facilities, making key recommendations for improvement. This multifaceted, supportive collaboration created a rejuvenated and purposeful focus for all council members involved. Some of their reactions include, “…I am so happy to be able to help other families in crisis, so that they don’t have to be alone, like I was,” and “I feel how important the patient and family’s voice is … it’s truly a partnership between patients, families, and staff.”
Regular access to IPFCC as a best practice coach and expert resource. Throughout the 10-month process, IPFCC’s Better Together Learning Community for Hospitals in New York State provided ongoing learning interventions for members of the SWT; multiple and varied resources from the Better Together toolkit for adaptation; and opportunities to share and reinforce new, learned expertise with colleagues within the Northwell Health system and beyond through IPFCC’s free online learning community, PFCC.Connect.
Conclusion
Family presence and participation are important to the quality, experience, safety, and outcomes of care. IPFCC’s campaign, Better Together: Partnering with Families, encourages hospitals to change restrictive visiting policies and, instead, to welcome families and caregivers 24 hours a day.
Two projects within Better Together involving almost 50 acute care hospitals in New York State confirm that change in policy, practice, and communication is particularly effective when implemented with strong support from leadership. An intervention like the Better Together Learning Community, offering structured training, coaching, and resources, can facilitate the change process.
Corresponding author: IPFCC, Deborah L. Dokken, 6917 Arlington Rd., Ste. 309, Bethesda, MD 20814; [email protected].
Funding disclosures: None.
1. Dokken DL, Kaufman J, Johnson BJ et al. Changing hospital visiting policies: from families as “visitors” to families as partners. J Clin Outcomes Manag. 2015; 22:29-36.
2. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. third edition: A pathway to improvement in New York City. New York: NYPIRG: 2018. www.nypirg.org/pubs/201801/NYPIRG_SICK_SCARED_FINAL.pdf. Accessed December 12, 2019.
3. Institute for Patient- and Family-Centered Care. Better Together: Partnering with Families. www.ipfcc.org/bestpractices/better-together.html. Accessed December 12, 2019.
4. Canadian Foundation for Healthcare Improvement. Better Together. www.cfhi-fcass.ca/WhatWeDo/better-together. Accessed December 12, 2019.
5. Canadian Foundation for Healthcare Improvement. Better Together: A change package to support the adoption of family presence and participation in acute care hospitals and accelerate healthcare improvement. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/better-together-change-package.pdf?sfvrsn=9656d044_4. Accessed December 12, 2019.
6. Canadian Foundation for Healthcare Improvement. L’Objectif santé: main dans la main avec les familles. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/families-pocket-screen_fr.pdf. Accessed December 12, 2019.
7. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. fourth edition: A pathway to improvement in New York. New York: NYPIRG: 2019. www.nypirg.org/pubs/201911/Sick_Scared_Separated_2019_web_FINAL.pdf. Accessed December 12, 2019.
8. Northwell Health. Patient and Family Partnership Councils. www.northwell.edu/about/commitment-to-excellence/patient-and-customer-experience/care-delivery-hospitality. Accessed December 12, 2019.
9 . Institute for Patient- and Family-Centered Care. How to conduct a “walk-about” from the patient and family perspective. www.ipfcc.org/resources/How_To_Conduct_A_Walk-About.pdf. Accessed December 12, 2019.
1. Dokken DL, Kaufman J, Johnson BJ et al. Changing hospital visiting policies: from families as “visitors” to families as partners. J Clin Outcomes Manag. 2015; 22:29-36.
2. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. third edition: A pathway to improvement in New York City. New York: NYPIRG: 2018. www.nypirg.org/pubs/201801/NYPIRG_SICK_SCARED_FINAL.pdf. Accessed December 12, 2019.
3. Institute for Patient- and Family-Centered Care. Better Together: Partnering with Families. www.ipfcc.org/bestpractices/better-together.html. Accessed December 12, 2019.
4. Canadian Foundation for Healthcare Improvement. Better Together. www.cfhi-fcass.ca/WhatWeDo/better-together. Accessed December 12, 2019.
5. Canadian Foundation for Healthcare Improvement. Better Together: A change package to support the adoption of family presence and participation in acute care hospitals and accelerate healthcare improvement. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/better-together-change-package.pdf?sfvrsn=9656d044_4. Accessed December 12, 2019.
6. Canadian Foundation for Healthcare Improvement. L’Objectif santé: main dans la main avec les familles. www.cfhi-fcass.ca/sf-docs/default-source/patient-engagement/families-pocket-screen_fr.pdf. Accessed December 12, 2019.
7. New York Public Interest Research Group and New Yorkers for Patient & Family Empowerment. Sick, scared and separated from loved ones. fourth edition: A pathway to improvement in New York. New York: NYPIRG: 2019. www.nypirg.org/pubs/201911/Sick_Scared_Separated_2019_web_FINAL.pdf. Accessed December 12, 2019.
8. Northwell Health. Patient and Family Partnership Councils. www.northwell.edu/about/commitment-to-excellence/patient-and-customer-experience/care-delivery-hospitality. Accessed December 12, 2019.
9 . Institute for Patient- and Family-Centered Care. How to conduct a “walk-about” from the patient and family perspective. www.ipfcc.org/resources/How_To_Conduct_A_Walk-About.pdf. Accessed December 12, 2019.
ATLAS Opens New Telehealth Site With Walmart
Groceries, maybe a new shirt, and now some veterans can fit in some shopping at their next health care visit. In a pilot project, the US Department of Veterans Affairs (VA) is partnering with Walmart to offer veterans easy access to health care at 5 sites.
The VA-led ATLAS (Accessing telehealth through local area stations) program is part of the VA Anywhere to Anywhere telehealth initiative, which aims to provide care to veterans no matter where they live. Other telehealth pilot sites are in Wisconsin, Michigan, and Iowa. In addition to Walmart, ATLAS sites are located at American Legion posts and Veterans of Foreign Wars (VFW) posts.
The local VA facility associated with the ATLAS site determines which clinical services the site offers. The health care services do not require hands-on exams. Clinical services may include, for instance, primary care, mental health counseling, clinical pharmacy, nutrition services, and social work. On-site attendants provide information, help the veterans get started, troubleshoot technical issues, and clean the space between appointments. Walmart donated equipment and space, where veterans can meet with a VA provider in a private room via video technology.
Last year, nearly 500,000 veterans logged > 1.3 million VA video telehealth encounters. It is the “way of the future,” says VA Secretary Robert Wilkie. “Veterans need the expansion of choice, and this partnership is vital to affording them convenient access to VA health care services where they live.”
Daryl Risinger, Chief Growth Officer for Walmart US Health and Wellness, is a veteran of the Air Force, and has a son and son-in-law serving. He says, “I know firsthand how important support and access is for our military, especially when it comes to health care. …This is another way we are helping our communities live better.”
For a veteran to attend an appointment at an ATLAS site, the site must be associated with the VA Medical Center where the veteran is enrolled. Family members who receive care through the VA can also visit ATLAS sites for select appointments.
Groceries, maybe a new shirt, and now some veterans can fit in some shopping at their next health care visit. In a pilot project, the US Department of Veterans Affairs (VA) is partnering with Walmart to offer veterans easy access to health care at 5 sites.
The VA-led ATLAS (Accessing telehealth through local area stations) program is part of the VA Anywhere to Anywhere telehealth initiative, which aims to provide care to veterans no matter where they live. Other telehealth pilot sites are in Wisconsin, Michigan, and Iowa. In addition to Walmart, ATLAS sites are located at American Legion posts and Veterans of Foreign Wars (VFW) posts.
The local VA facility associated with the ATLAS site determines which clinical services the site offers. The health care services do not require hands-on exams. Clinical services may include, for instance, primary care, mental health counseling, clinical pharmacy, nutrition services, and social work. On-site attendants provide information, help the veterans get started, troubleshoot technical issues, and clean the space between appointments. Walmart donated equipment and space, where veterans can meet with a VA provider in a private room via video technology.
Last year, nearly 500,000 veterans logged > 1.3 million VA video telehealth encounters. It is the “way of the future,” says VA Secretary Robert Wilkie. “Veterans need the expansion of choice, and this partnership is vital to affording them convenient access to VA health care services where they live.”
Daryl Risinger, Chief Growth Officer for Walmart US Health and Wellness, is a veteran of the Air Force, and has a son and son-in-law serving. He says, “I know firsthand how important support and access is for our military, especially when it comes to health care. …This is another way we are helping our communities live better.”
For a veteran to attend an appointment at an ATLAS site, the site must be associated with the VA Medical Center where the veteran is enrolled. Family members who receive care through the VA can also visit ATLAS sites for select appointments.
Groceries, maybe a new shirt, and now some veterans can fit in some shopping at their next health care visit. In a pilot project, the US Department of Veterans Affairs (VA) is partnering with Walmart to offer veterans easy access to health care at 5 sites.
The VA-led ATLAS (Accessing telehealth through local area stations) program is part of the VA Anywhere to Anywhere telehealth initiative, which aims to provide care to veterans no matter where they live. Other telehealth pilot sites are in Wisconsin, Michigan, and Iowa. In addition to Walmart, ATLAS sites are located at American Legion posts and Veterans of Foreign Wars (VFW) posts.
The local VA facility associated with the ATLAS site determines which clinical services the site offers. The health care services do not require hands-on exams. Clinical services may include, for instance, primary care, mental health counseling, clinical pharmacy, nutrition services, and social work. On-site attendants provide information, help the veterans get started, troubleshoot technical issues, and clean the space between appointments. Walmart donated equipment and space, where veterans can meet with a VA provider in a private room via video technology.
Last year, nearly 500,000 veterans logged > 1.3 million VA video telehealth encounters. It is the “way of the future,” says VA Secretary Robert Wilkie. “Veterans need the expansion of choice, and this partnership is vital to affording them convenient access to VA health care services where they live.”
Daryl Risinger, Chief Growth Officer for Walmart US Health and Wellness, is a veteran of the Air Force, and has a son and son-in-law serving. He says, “I know firsthand how important support and access is for our military, especially when it comes to health care. …This is another way we are helping our communities live better.”
For a veteran to attend an appointment at an ATLAS site, the site must be associated with the VA Medical Center where the veteran is enrolled. Family members who receive care through the VA can also visit ATLAS sites for select appointments.
Dermatology Continuing Certification Changes for the Better
Major changes in continuing board certification are occurring across medical specialties. On January 6, 2020, the American Board of Dermatology (ABD) launches its new web-based longitudinal assessment program called CertLink (https://abderm.mycertlink.org/).1 This new platform is designed to eventually replace the sit-down, high-stakes, once-every-10-year medical knowledge examination that dermatologists take to remain board certified. With this alternative, every participating dermatologist will receive a batch of 13 web-based questions every quarter that he/she may answer at a convenient time and place. Questions are answered one at a time or in batches, depending on the test taker’s preference, and can be completed on home or office computers (and eventually on smartphones). Participating in this type of testing does not require shutting down practice, traveling to a test center, or paying for expensive board review courses. CertLink is designed to be convenient, affordable, and relevant to an individual’s practice.
How did the ABD arrive at CertLink?
The ABD launched its original Maintenance of Certification (MOC) program in 2006. Since then, newly board-certified dermatologists, recertifying dermatologists with time-limited certificates, and time-unlimited dermatologists who volunteered to participate in MOC have experienced the dermatology MOC program. In its first 10 years, the program was met with very mixed reviews. The program was designed to assess and promote competence in a 4-part framework, including professionalism; commitment to lifelong learning and self-assessment; demonstration of knowledge, judgment, and skills; and improvement in medical practice. All 4 are areas of rational pursuit for medical professionals seeking to perform and maintain the highest quality patient care possible. But there were problems. First iterations are rarely perfect, and dermatology MOC was no exception.
At the onset, the ABD chose to oversee the MOC requirements and remained hands off in the delivery of education, relying instead on other organizations to fulfill the ABD’s requirements. Unfortunately, with limited educational offerings available, many diplomates paid notable registration fees for each qualifying MOC activity. Quality improvement activities were a relatively new experience for dermatologists and were time consuming. Required medical record reviews were onerous, often requiring more than 35 data points to be collected per medical record reviewed. The limited number and limited diversity of educational offerings also created circumstances in which the material covered was not maximally relevant to many participants. When paying to answer questions about patient populations or procedure types never encountered by the dermatologist who purchased the particular MOC activity, many asked the question “How does this make me a better doctor?” They were right to ask.
Cost, time commitment to participate in MOC, and relevance to practice were 3 key areas of concern for many dermatologists. In response to internal and external MOC feedback, in 2015 the ABD took a hard look at its 10-year experience with MOC. While contemplating its next strategies, the ABD temporarily put its component 4—practice improvement—requirements on hold. After much review, the ABD decided to take over a notable portion of the education delivery. Its goal was to provide education that would fulfill MOC requirements in a more affordable, relevant, quicker, and easier manner.
First, the ABD made the decision to assume a more notable role as educator, in part to offer qualifying activities at no additional cost to diplomates. By taking on the role as educator, 3 major changes resulted: the way ABD approached quality improvement activities, partnership to initiate a question-of-the-week self-assessment program, and initiation of a longitudinal assessment strategy that resulted in this month’s launch of CertLink.
The ABD revolutionized its quality improvement requirements with the launch of its practice improvement modules made available through its website.2 These modules utilize recently published clinical practice gaps in 5 dermatology subspecialty domains to fulfill the practice improvement requirements. Participants read a brief synopsis of the supporting literature explaining practice improvement recommendations found in the module. Next, they find 5 patients in their practice with the condition, medication, or process in question and review whether they provided the care supported by the best available evidence. No module requires more than 5 medical records to review, and no more than 3 questions are answered per medical record review. If review confirms that the care was appropriate, no further action is needed. If a care gap is identified, then participants implement changes and later remeasure practice to detect any change. This certification activity was incredibly popular with the thousands of diplomates who have participated thus far; more than 97% stated the modules were relevant to practice, 98% stated they would recommend the modules to fellow dermatologists, and nearly 25% reported the module helped to change their practice for the better (unpublished data, July 2019). Relevance had been restored.
The ABD worked closely with the American Academy of Dermatology (AAD) to develop new education for weekly self-assessment. The ABD created the content and delivered to the AAD the first year of material for what would become the most successful and popular dermatology CME activity in history: the AAD Question of the Week (QOW). Thousands of dermatologists are registered to receive the QOW, with very active weekly participation. Participants receive 1 self-assessment point and 0.25 CME credits for each attempted question, right or wrong. This quizzing tool also was educational, with explanation of right answers and wrong choices included. The average amount of time spent answering each question was approximately 40 seconds. American Academy of Dermatology members can participate in its QOW as a member benefit. Self-assessment is no longer a time- consuming or costly process.
The third major change was the ABD initiation of the longitudinal assessment strategy called CertLink, a web-based testing platform operated by the American Board of Medical Specialties. Longitudinal assessment differs from traditional certification and recertification assessment. It allows the test taker to answer the certification test questions over time instead of all at once. Longitudinal assessment not only provides a greater level of convenience to the test taker but also allows boards a more continuous set of touch points in the assessment of diplomates over the course of the continuing certification period.
What will be part of CertLink?
In addition to standard multiple-choice questions, there are many interesting elements to the CertLink program, such as article-based questions. At the beginning of each year, dermatologists select 8 articles from a list of those hosted by CertLink. These are recently published articles, chosen for their meaningfulness to practicing dermatologists. Each subsequent quarter, 2 of these articles are issued to the diplomate to read at his/her leisure. Once ready, participants launch and answer 2 questions about the key points of each article. The article-based questions were designed to help the practicing dermatologist stay up-to-date and relevant in personally chosen areas.
Diplomates are offered a chance to learn from any question that was missed, with explanations or resources provided to help them understand why the correct answer is correct. In this new learn-to-competence model, diplomates are not penalized the first time they answer a particular question incorrectly. Each is provided an opportunity to learn through the explanations given, and then in a future quarter, the dermatologist is given a second chance to answer a similarly themed question, with only that second chance counting toward his/her overall score.
Another unique aspect of CertLink is the allowance of time off from assessment. The ABD recognizes that life happens, and that intermittent time off from career-long assessment will be necessary to accommodate life events, including but not limited to maternity leave, other medical leave, or mental health breaks. Diplomates may take off up to 1 quarter of testing each year to accommodate such life events. Those who need extra time (beyond 1 quarter per year) would need to communicate directly with ABD to request. Those who continue to answer questions throughout the year will have their lowest-performing quarter dropped, to maximize fairness to all. Only the top 3 quarters of CertLink test performance will be counted each year when making certification status decisions. Those who take 1 quarter off will have their other 3 quarters counted toward their scoring.
How will CertLink measure performance?
At the onset of CertLink, there is no predetermined passing score. It will take a few years for the ABD psychometricians to determine an acceptable performance. Questions are written not to be tricky but rather to assess patient issues the dermatologist is likely to encounter in practice. Article-based questions are designed to assess the key points of important recent articles to advance the dermatologist’s practice.
Final Thoughts
In the end, the ABD approach to the new area of continuing certification centers on strategies to be relevant, inexpensive, and minimally disruptive to practice, and to teach to competence and advance practice by bringing forward articles that address key recent literature. We think it is a much better approach to dermatology continuing certification.
- ABD announces CertLink launch in 2020 [news release]. Newton, MA: American Board of Dermatology; 2019. https://www.abderm.org/public/announcements/certlink-2020.aspx. Accessed December 17, 2019.
- American Board of Dermatology. Focused practice improvement modules. https://www.abderm.org/diplomates/fulfilling-moc-requirements/abd-focused-pi-modules-for-moc.aspx. Accessed December 18, 2019.
Major changes in continuing board certification are occurring across medical specialties. On January 6, 2020, the American Board of Dermatology (ABD) launches its new web-based longitudinal assessment program called CertLink (https://abderm.mycertlink.org/).1 This new platform is designed to eventually replace the sit-down, high-stakes, once-every-10-year medical knowledge examination that dermatologists take to remain board certified. With this alternative, every participating dermatologist will receive a batch of 13 web-based questions every quarter that he/she may answer at a convenient time and place. Questions are answered one at a time or in batches, depending on the test taker’s preference, and can be completed on home or office computers (and eventually on smartphones). Participating in this type of testing does not require shutting down practice, traveling to a test center, or paying for expensive board review courses. CertLink is designed to be convenient, affordable, and relevant to an individual’s practice.
How did the ABD arrive at CertLink?
The ABD launched its original Maintenance of Certification (MOC) program in 2006. Since then, newly board-certified dermatologists, recertifying dermatologists with time-limited certificates, and time-unlimited dermatologists who volunteered to participate in MOC have experienced the dermatology MOC program. In its first 10 years, the program was met with very mixed reviews. The program was designed to assess and promote competence in a 4-part framework, including professionalism; commitment to lifelong learning and self-assessment; demonstration of knowledge, judgment, and skills; and improvement in medical practice. All 4 are areas of rational pursuit for medical professionals seeking to perform and maintain the highest quality patient care possible. But there were problems. First iterations are rarely perfect, and dermatology MOC was no exception.
At the onset, the ABD chose to oversee the MOC requirements and remained hands off in the delivery of education, relying instead on other organizations to fulfill the ABD’s requirements. Unfortunately, with limited educational offerings available, many diplomates paid notable registration fees for each qualifying MOC activity. Quality improvement activities were a relatively new experience for dermatologists and were time consuming. Required medical record reviews were onerous, often requiring more than 35 data points to be collected per medical record reviewed. The limited number and limited diversity of educational offerings also created circumstances in which the material covered was not maximally relevant to many participants. When paying to answer questions about patient populations or procedure types never encountered by the dermatologist who purchased the particular MOC activity, many asked the question “How does this make me a better doctor?” They were right to ask.
Cost, time commitment to participate in MOC, and relevance to practice were 3 key areas of concern for many dermatologists. In response to internal and external MOC feedback, in 2015 the ABD took a hard look at its 10-year experience with MOC. While contemplating its next strategies, the ABD temporarily put its component 4—practice improvement—requirements on hold. After much review, the ABD decided to take over a notable portion of the education delivery. Its goal was to provide education that would fulfill MOC requirements in a more affordable, relevant, quicker, and easier manner.
First, the ABD made the decision to assume a more notable role as educator, in part to offer qualifying activities at no additional cost to diplomates. By taking on the role as educator, 3 major changes resulted: the way ABD approached quality improvement activities, partnership to initiate a question-of-the-week self-assessment program, and initiation of a longitudinal assessment strategy that resulted in this month’s launch of CertLink.
The ABD revolutionized its quality improvement requirements with the launch of its practice improvement modules made available through its website.2 These modules utilize recently published clinical practice gaps in 5 dermatology subspecialty domains to fulfill the practice improvement requirements. Participants read a brief synopsis of the supporting literature explaining practice improvement recommendations found in the module. Next, they find 5 patients in their practice with the condition, medication, or process in question and review whether they provided the care supported by the best available evidence. No module requires more than 5 medical records to review, and no more than 3 questions are answered per medical record review. If review confirms that the care was appropriate, no further action is needed. If a care gap is identified, then participants implement changes and later remeasure practice to detect any change. This certification activity was incredibly popular with the thousands of diplomates who have participated thus far; more than 97% stated the modules were relevant to practice, 98% stated they would recommend the modules to fellow dermatologists, and nearly 25% reported the module helped to change their practice for the better (unpublished data, July 2019). Relevance had been restored.
The ABD worked closely with the American Academy of Dermatology (AAD) to develop new education for weekly self-assessment. The ABD created the content and delivered to the AAD the first year of material for what would become the most successful and popular dermatology CME activity in history: the AAD Question of the Week (QOW). Thousands of dermatologists are registered to receive the QOW, with very active weekly participation. Participants receive 1 self-assessment point and 0.25 CME credits for each attempted question, right or wrong. This quizzing tool also was educational, with explanation of right answers and wrong choices included. The average amount of time spent answering each question was approximately 40 seconds. American Academy of Dermatology members can participate in its QOW as a member benefit. Self-assessment is no longer a time- consuming or costly process.
The third major change was the ABD initiation of the longitudinal assessment strategy called CertLink, a web-based testing platform operated by the American Board of Medical Specialties. Longitudinal assessment differs from traditional certification and recertification assessment. It allows the test taker to answer the certification test questions over time instead of all at once. Longitudinal assessment not only provides a greater level of convenience to the test taker but also allows boards a more continuous set of touch points in the assessment of diplomates over the course of the continuing certification period.
What will be part of CertLink?
In addition to standard multiple-choice questions, there are many interesting elements to the CertLink program, such as article-based questions. At the beginning of each year, dermatologists select 8 articles from a list of those hosted by CertLink. These are recently published articles, chosen for their meaningfulness to practicing dermatologists. Each subsequent quarter, 2 of these articles are issued to the diplomate to read at his/her leisure. Once ready, participants launch and answer 2 questions about the key points of each article. The article-based questions were designed to help the practicing dermatologist stay up-to-date and relevant in personally chosen areas.
Diplomates are offered a chance to learn from any question that was missed, with explanations or resources provided to help them understand why the correct answer is correct. In this new learn-to-competence model, diplomates are not penalized the first time they answer a particular question incorrectly. Each is provided an opportunity to learn through the explanations given, and then in a future quarter, the dermatologist is given a second chance to answer a similarly themed question, with only that second chance counting toward his/her overall score.
Another unique aspect of CertLink is the allowance of time off from assessment. The ABD recognizes that life happens, and that intermittent time off from career-long assessment will be necessary to accommodate life events, including but not limited to maternity leave, other medical leave, or mental health breaks. Diplomates may take off up to 1 quarter of testing each year to accommodate such life events. Those who need extra time (beyond 1 quarter per year) would need to communicate directly with ABD to request. Those who continue to answer questions throughout the year will have their lowest-performing quarter dropped, to maximize fairness to all. Only the top 3 quarters of CertLink test performance will be counted each year when making certification status decisions. Those who take 1 quarter off will have their other 3 quarters counted toward their scoring.
How will CertLink measure performance?
At the onset of CertLink, there is no predetermined passing score. It will take a few years for the ABD psychometricians to determine an acceptable performance. Questions are written not to be tricky but rather to assess patient issues the dermatologist is likely to encounter in practice. Article-based questions are designed to assess the key points of important recent articles to advance the dermatologist’s practice.
Final Thoughts
In the end, the ABD approach to the new area of continuing certification centers on strategies to be relevant, inexpensive, and minimally disruptive to practice, and to teach to competence and advance practice by bringing forward articles that address key recent literature. We think it is a much better approach to dermatology continuing certification.
Major changes in continuing board certification are occurring across medical specialties. On January 6, 2020, the American Board of Dermatology (ABD) launches its new web-based longitudinal assessment program called CertLink (https://abderm.mycertlink.org/).1 This new platform is designed to eventually replace the sit-down, high-stakes, once-every-10-year medical knowledge examination that dermatologists take to remain board certified. With this alternative, every participating dermatologist will receive a batch of 13 web-based questions every quarter that he/she may answer at a convenient time and place. Questions are answered one at a time or in batches, depending on the test taker’s preference, and can be completed on home or office computers (and eventually on smartphones). Participating in this type of testing does not require shutting down practice, traveling to a test center, or paying for expensive board review courses. CertLink is designed to be convenient, affordable, and relevant to an individual’s practice.
How did the ABD arrive at CertLink?
The ABD launched its original Maintenance of Certification (MOC) program in 2006. Since then, newly board-certified dermatologists, recertifying dermatologists with time-limited certificates, and time-unlimited dermatologists who volunteered to participate in MOC have experienced the dermatology MOC program. In its first 10 years, the program was met with very mixed reviews. The program was designed to assess and promote competence in a 4-part framework, including professionalism; commitment to lifelong learning and self-assessment; demonstration of knowledge, judgment, and skills; and improvement in medical practice. All 4 are areas of rational pursuit for medical professionals seeking to perform and maintain the highest quality patient care possible. But there were problems. First iterations are rarely perfect, and dermatology MOC was no exception.
At the onset, the ABD chose to oversee the MOC requirements and remained hands off in the delivery of education, relying instead on other organizations to fulfill the ABD’s requirements. Unfortunately, with limited educational offerings available, many diplomates paid notable registration fees for each qualifying MOC activity. Quality improvement activities were a relatively new experience for dermatologists and were time consuming. Required medical record reviews were onerous, often requiring more than 35 data points to be collected per medical record reviewed. The limited number and limited diversity of educational offerings also created circumstances in which the material covered was not maximally relevant to many participants. When paying to answer questions about patient populations or procedure types never encountered by the dermatologist who purchased the particular MOC activity, many asked the question “How does this make me a better doctor?” They were right to ask.
Cost, time commitment to participate in MOC, and relevance to practice were 3 key areas of concern for many dermatologists. In response to internal and external MOC feedback, in 2015 the ABD took a hard look at its 10-year experience with MOC. While contemplating its next strategies, the ABD temporarily put its component 4—practice improvement—requirements on hold. After much review, the ABD decided to take over a notable portion of the education delivery. Its goal was to provide education that would fulfill MOC requirements in a more affordable, relevant, quicker, and easier manner.
First, the ABD made the decision to assume a more notable role as educator, in part to offer qualifying activities at no additional cost to diplomates. By taking on the role as educator, 3 major changes resulted: the way ABD approached quality improvement activities, partnership to initiate a question-of-the-week self-assessment program, and initiation of a longitudinal assessment strategy that resulted in this month’s launch of CertLink.
The ABD revolutionized its quality improvement requirements with the launch of its practice improvement modules made available through its website.2 These modules utilize recently published clinical practice gaps in 5 dermatology subspecialty domains to fulfill the practice improvement requirements. Participants read a brief synopsis of the supporting literature explaining practice improvement recommendations found in the module. Next, they find 5 patients in their practice with the condition, medication, or process in question and review whether they provided the care supported by the best available evidence. No module requires more than 5 medical records to review, and no more than 3 questions are answered per medical record review. If review confirms that the care was appropriate, no further action is needed. If a care gap is identified, then participants implement changes and later remeasure practice to detect any change. This certification activity was incredibly popular with the thousands of diplomates who have participated thus far; more than 97% stated the modules were relevant to practice, 98% stated they would recommend the modules to fellow dermatologists, and nearly 25% reported the module helped to change their practice for the better (unpublished data, July 2019). Relevance had been restored.
The ABD worked closely with the American Academy of Dermatology (AAD) to develop new education for weekly self-assessment. The ABD created the content and delivered to the AAD the first year of material for what would become the most successful and popular dermatology CME activity in history: the AAD Question of the Week (QOW). Thousands of dermatologists are registered to receive the QOW, with very active weekly participation. Participants receive 1 self-assessment point and 0.25 CME credits for each attempted question, right or wrong. This quizzing tool also was educational, with explanation of right answers and wrong choices included. The average amount of time spent answering each question was approximately 40 seconds. American Academy of Dermatology members can participate in its QOW as a member benefit. Self-assessment is no longer a time- consuming or costly process.
The third major change was the ABD initiation of the longitudinal assessment strategy called CertLink, a web-based testing platform operated by the American Board of Medical Specialties. Longitudinal assessment differs from traditional certification and recertification assessment. It allows the test taker to answer the certification test questions over time instead of all at once. Longitudinal assessment not only provides a greater level of convenience to the test taker but also allows boards a more continuous set of touch points in the assessment of diplomates over the course of the continuing certification period.
What will be part of CertLink?
In addition to standard multiple-choice questions, there are many interesting elements to the CertLink program, such as article-based questions. At the beginning of each year, dermatologists select 8 articles from a list of those hosted by CertLink. These are recently published articles, chosen for their meaningfulness to practicing dermatologists. Each subsequent quarter, 2 of these articles are issued to the diplomate to read at his/her leisure. Once ready, participants launch and answer 2 questions about the key points of each article. The article-based questions were designed to help the practicing dermatologist stay up-to-date and relevant in personally chosen areas.
Diplomates are offered a chance to learn from any question that was missed, with explanations or resources provided to help them understand why the correct answer is correct. In this new learn-to-competence model, diplomates are not penalized the first time they answer a particular question incorrectly. Each is provided an opportunity to learn through the explanations given, and then in a future quarter, the dermatologist is given a second chance to answer a similarly themed question, with only that second chance counting toward his/her overall score.
Another unique aspect of CertLink is the allowance of time off from assessment. The ABD recognizes that life happens, and that intermittent time off from career-long assessment will be necessary to accommodate life events, including but not limited to maternity leave, other medical leave, or mental health breaks. Diplomates may take off up to 1 quarter of testing each year to accommodate such life events. Those who need extra time (beyond 1 quarter per year) would need to communicate directly with ABD to request. Those who continue to answer questions throughout the year will have their lowest-performing quarter dropped, to maximize fairness to all. Only the top 3 quarters of CertLink test performance will be counted each year when making certification status decisions. Those who take 1 quarter off will have their other 3 quarters counted toward their scoring.
How will CertLink measure performance?
At the onset of CertLink, there is no predetermined passing score. It will take a few years for the ABD psychometricians to determine an acceptable performance. Questions are written not to be tricky but rather to assess patient issues the dermatologist is likely to encounter in practice. Article-based questions are designed to assess the key points of important recent articles to advance the dermatologist’s practice.
Final Thoughts
In the end, the ABD approach to the new area of continuing certification centers on strategies to be relevant, inexpensive, and minimally disruptive to practice, and to teach to competence and advance practice by bringing forward articles that address key recent literature. We think it is a much better approach to dermatology continuing certification.
- ABD announces CertLink launch in 2020 [news release]. Newton, MA: American Board of Dermatology; 2019. https://www.abderm.org/public/announcements/certlink-2020.aspx. Accessed December 17, 2019.
- American Board of Dermatology. Focused practice improvement modules. https://www.abderm.org/diplomates/fulfilling-moc-requirements/abd-focused-pi-modules-for-moc.aspx. Accessed December 18, 2019.
- ABD announces CertLink launch in 2020 [news release]. Newton, MA: American Board of Dermatology; 2019. https://www.abderm.org/public/announcements/certlink-2020.aspx. Accessed December 17, 2019.
- American Board of Dermatology. Focused practice improvement modules. https://www.abderm.org/diplomates/fulfilling-moc-requirements/abd-focused-pi-modules-for-moc.aspx. Accessed December 18, 2019.
Using Democratic Deliberation to Engage Veterans in Complex Policy Making for the Veterans Health Administration
Providing high-quality, patient-centered health care is a top priority for the US Department of Veterans Affairs (VA) Veteran Health Administration (VHA), whose core mission is to improve the health and well-being of US veterans. Thus, news of long wait times for medical appointments in the VHA sparked intense national attention and debate and led to changes in senior management and legislative action. 1 On August 8, 2014, President Bara c k Obama signed the Veterans Access, Choice, and Accountability Act of 2014, also known as the Choice Act, which provided an additional $16 billion in emergency spending over 3 years to improve veterans’ access to timely health care. 2 The Choice Act sought to develop an integrated health care network that allowed qualified VHA patients to receive specific health care services in their communities delivered by non-VHA health care providers (HCPs) but paid for by the VHA. The Choice Act also laid out explicit criteria for how to prioritize who would be eligible for VHA-purchased civilian care: (1) veterans who could not get timely appointments at a VHA medical facility within 30 days of referral; or (2) veterans who lived > 40 miles from the closest VHA medical facility.
VHA decision makers seeking to improve care delivery also need to weigh trade-offs between alternative approaches to providing rapid access. For instance, increasing access to non-VHA HCPs may not always decrease wait times and could result in loss of continuity, limited care coordination, limited ability to ensure and enforce high-quality standards at the VHA, and other challenges.3-6 Although the concerns and views of elected representatives, advocacy groups, and health system leaders are important, it is unknown whether these views and preferences align with those of veterans. Arguably, the range of views and concerns of informed veterans whose health is at stake should be particularly prominent in such policy decision making.
To identify the considerations that were most important to veterans regarding VHA policy around decreasing wait times, a study was designed to engage a group of veterans who were eligible for civilian care under the Choice Act. The study took place 1 year after the Choice Act was passed. Veterans were asked to focus on 2 related questions: First, how should funding be used for building VHA capacity (build) vs purchasing civilian care (buy)? Second, under what circumstances should civilian care be prioritized?
The aim of this paper is to describe democratic deliberation (DD), a specific method that engaged veteran patients in complex policy decisions around access to care. DD methods have been used increasingly in health care for developing policy guidance, setting priorities, providing advice on ethical dilemmas, weighing risk-benefit trade-offs, and determining decision-making authority.7-12 For example, DD helped guide national policy for mammography screening for breast cancer in New Zealand.13 The Agency for Healthcare Research and Quality has completed a systematic review and a large, randomized experiment on best practices for carrying out public deliberation.8,13,14 However, despite the potential value of this approach, there has been little use of deliberative methods within the VHA for the explicit purpose of informing veteran health care delivery.
This paper describes the experience engaging veterans by using DD methodology and informing VHA leadership about the results of those deliberations. The specific aims were to understand whether DD is an acceptable approach to use to engage patients in the medical services policy-making process within VHA and whether veterans are able to come to an informed consensus.
Methods
Engaging patients and incorporating their needs and concerns within the policy-making process may improve health system policies and make those policies more patient centered. Such engagement also could be a way to generate creative solutions. However, because health-system decisions often involve making difficult trade-offs, effectively obtaining patient population input on complex care delivery issues can be challenging.
Although surveys can provide intuitive, top-of-mind input from respondents, these opinions are generally not sufficient for resolving complex problems.15 Focus groups and interviews may produce results that are more in-depth than surveys, but these methods tend to elicit settled private preferences rather than opinions about what the community should do.16 DD, on the other hand, is designed to elicit deeply informed public opinions on complex, value-laden topics to develop recommendations and policies for a larger community.17 The goal is to find collective solutions to challenging social problems. DD achieves this by giving participants an opportunity to explore a topic in-depth, question experts, and engage peers in reason-based discussions.18,19 This method has its roots in political science and has been used over several decades to successfully inform policy making on a broad array of topics nationally and internationally—from health research ethics in the US to nuclear and energy policy in Japan.7,16,20,21 DD has been found to promote ownership of public programs and lend legitimacy to policy decisions, political institutions, and democracy itself.18
A single day (8 hours) DD session was convened, following a Citizens Jury model of deliberation, which brings veteran patients together to learn about a topic, ask questions of experts, deliberate with peers, and generate a “citizen’s report” that contains a set of recommendations (Table 1). An overview of the different models of DD and rationale for each can be found elsewhere.8,15
Recruitment Considerations
A purposively selected sample of civilian care-eligible veterans from a midwestern VHA health care system (1 medical center and 3 community-based outpatient clinics [CBOCs]) were invited to the DD session. The targeted number of participants was 30. Female veterans, who comprise only 7% of the local veteran population, were oversampled to account for their potentially different health care needs and to create balance between males and females in the session. Oversampling for other characteristics was not possible due to the relatively small sample size. Based on prior experience,7 it was assumed that 70% of willing participants would attend the session; therefore 34 veterans were invited and 24 attended. Each participant received a $200 incentive in appreciation for their substantial time commitment and to offset transportation costs.
Background Materials
A packet with educational materials (Flesch-Kincaid Grade Level of 10.5) was mailed to participants about 2 weeks before the DD session. Participants were asked to review prior to attending the session. These materials described the session (eg, purpose, organizers, importance) and provided factual information about the Choice Act (eg, eligibility, out-of-pocket costs, travel pay, prescription drug policies).
Session Overview
The session was structured to accomplish the following goals: (1) Elicit participants’ opinions about access to health care and reasons for those opinions; (2) Provide in-depth education about the Choice Act through presentations and discussions with topical experts; and (3) Elicit reasoning and recommendations on both the criteria by which participants prioritize candidates for civilian care and how participants would allocate additional funding to improve access (ie, by building VHA capacity to deliver more timely health care vs purchasing health care from civilian HCPs).
Participants were asked to fill out a survey on arrival in the morning and were assigned to 1 of 3 tables or small groups. Each table had a facilitator who had extensive experience in qualitative data collection methods and guided the dialogue using a scripted protocol that they helped develop and refine. The facilitation materials drew from and used previously published studies.22,23 Each facilitator audio recorded the sessions and took notes. Three experts presented during plenary education sessions. Presentations were designed to provide balanced factual information and included a veteran’s perspective. One presenter was a clinician on the project team, another was a local clinical leader responsible for making decisions about what services to provide via civilian care (buy) vs enhancing the local VHA health system’s ability to provide those services (build), and the third presenter was a veteran who was on the project team.
Education Session 1
The first plenary education session with expert presentations was conducted after each table completed an icebreaker exercise. The project team physician provided a brief history and description of the Choice Act to reinforce educational materials sent to participants prior to the session. The health system clinical leader described his decision process and principles and highlighted constraints placed on him by the Choice Act that were in place at the time of the DD session. He also described existing local and national programs to provide civilian care (eg, local fee-basis non-VHA care programs) and how these programs sought to achieve goals similar to the Choice Act. The veteran presenter focused on the importance of session participants providing candid insight and observations and emphasized that this session was a significant opportunity to “have their voices heard.”
Deliberation 1: What criteria should be used to prioritize patients for receiving civilian care paid for by the VHA? To elicit preferences on the central question of this deliberation, participants were presented with 8 real-world cases that were based on interviews conducted with Choice Act-eligible veterans (Table 2 and eAppendices A
Education Session 2
In the second plenary session, the project team physician provided information about health care access issues, both inside and outside of the VHA, particularly between urban and rural areas. He also discussed factors related to the insufficient capacity to meet growing demand that contributed to the VHA wait-time crisis. The veteran presenter shared reflections on health care access from a veteran’s perspective.
Deliberation 2: How should additional funding be divided between increasing the ability of the VHA to (1) provide care by VHA HCPs; and (2) pay for care from non-VHA civilian HCPs? Participants were presented the patient examples and Choice Act funding scenarios (the buy policy option) and contrasted that with a build policy option. Participants were explicitly encouraged to shift their perspectives from thinking about individual cases to considering policy-level decisions and the broader social good (Table 2).
Ensuring Robust Deliberations
If participants do not adequately grasp the complexities of the topic, a deliberation can fail. To facilitate nuanced reasoning, real-world concrete examples were developed as the starting point of each deliberation based on interviews with actual patients (deliberation 1) and actual policy proposals relevant to the funding allocation decisions within the Choice Act (deliberation 2).
A deliberation also can fail with self-silencing, where participants withhold opinions that differ from those articulated first or by more vocal members of the group.24 To combat self-silencing, highly experienced facilitators were used to ensure sharing from all participants and to support an open-minded, courteous, and reason-based environment for discourse. It was specified that the best solutions are achieved through reason-based and cordial disagreement and that success can be undermined when participants simply agree because it is easier or more comfortable.
A third way a deliberation can fail is if individuals do not adopt a group or system-level perspective. To counter this, facilitators reinforced at multiple points the importance of taking a broader social perspective rather than sharing only one’s personal preferences.
Finally, it is important to assess the quality of the deliberative process itself, to ensure that results are trustworthy.25 To assess the quality of the deliberative process, participants knowledge about key issues pre- and postdeliberation were assessed. Participants also were asked to rate the quality of the facilitators and how well they felt their voice was heard and respected, and facilitators made qualitative assessments about the extent to which participants were engaged in reason-based and collaborative discussion.
Data
Quantitative data were collected via pre- and postsession surveys. The surveys contained items related to knowledge about the Choice Act, expectations for the DD session, beliefs and opinions about the provision of health care for veterans, recommended funding allocations between build vs buy policy options, and general demographics. Qualitative data were collected through detailed notes taken by the 3 facilitators. Each table’s deliberations were audio recorded so that gaps in the notes could be filled.
The 3 facilitators, who were all experienced qualitative researchers, typed their written notes into a template immediately after the session. Two of the 3 facilitators led the analysis of the session notes. Findings within and across the 3 deliberation tables were developed using content and matrix analysis methods.26 Descriptive statistics were generated from survey responses and compared survey items pre- and postsession using paired t tests or χ2 tests for categorical responses.
Results
Thirty-three percent of individuals invited (n = 127) agreed to participate. Those who declined cited conflicts related to distance, transportation, work/school, medical appointments, family commitments, or were not interested. In all, 24 (69%) of the 35 veterans who accepted the invitation attended the deliberation session. Of the 11 who accepted but did not attend, 5 cancelled ahead of time because of conflicts (Figure). Most participants were male (70%), 48% were aged 61 to 75 years, 65% were white, 43% had some college education, 43% reported an annual income of between $25,000 and $40,000, and only 35% reported very good health (eAppendix D).
Deliberation 1
During the deliberation on the prioritization criteria, the concept of “condition severity” emerged as an important criterion for veterans. This criterion captured simultaneous consideration of both clinical necessity and burden on the veteran to obtain care. For example, participants felt that patients with a life-threatening illness should be prioritized for civilian care over patients who need preventative or primary care (clinical necessity) and that elderly patients with substantial difficulty traveling to VHA appointments should be prioritized over patients who can travel more easily (burden). The Choice Act regulations at the time of the DD session did not reflect this nuanced perspective, stipulating only that veterans must live > 40 miles from the nearest VHA medical facility.
One of the 3 groups did not prioritize the patient cases because some members felt that no veteran should be constrained from receiving civilian care if they desired it. Nonetheless, this group did agree with prioritizing the first 2 cases in Table 3. The other groups prioritized all 8 cases in generally similar ways.
Deliberation 2
No clear consensus emerged on the buy vs build question. A representative from each table presented their group’s positions, rationale, and recommendations after deliberations were completed. After hearing the range of positions, the groups then had another opportunity to deliberate based on what they heard from the other tables; no new recommendations or consensus emerged.
Participants who were in favor of allocating more funds toward the build policy offered a range of rationales, saying that it would (1) increase access for rural veterans by building CBOCs and deploying more mobile units that could bring outlets for health care closer to their home communities; (2) provide critical and unique medical expertise to address veteran-specific issues such as prosthetics, traumatic brain injury, posttraumatic stress disorder, spinal cord injury, and shrapnel wounds that are typically not available through civilian providers; (3) give VHA more oversight over the quality and cost of care, which is more challenging to do with civilian providers; and (4) Improve VHA infrastructure by, for example, upgrading technology and attracting the best clinicians and staff to support “our VHA.”
Participants who were in favor of allocating more funds toward the buy policy also offered a range of rationales, saying that it would (1) decrease patient burden by increasing access through community providers, decreasing wait time, and lessening personal cost and travel time; (2) allow more patients to receive civilian care, which was generally seen as beneficial by a few participants because of perceptions that the VHA provides lower quality care due to a shortage of VHA providers, run-down/older facilities, lack of technology, and poorer-quality VHA providers; and (3) provide an opportunity to divest of costly facilities and invest in other innovative approaches. Regarding this last reason, a few participants felt that the VHA is “gouged” when building medical centers that overrun budgets. They also were concerned that investing in facilities tied VHA to specific locations when current locations of veterans may change “25 years from now.”
Survey Results
Twenty-three of the 24 participants completed both pre- and postsession surveys. The majority of participants in the session felt people in the group respected their opinion (96%); felt that the facilitator did not try to influence the group with her own opinions (96%); indicated they understood the information enough to participate as much as they wanted (100%); and were hopeful that their reasoning and recommendations would help inform VHA policy makers (82%).
The surveys also provided an opportunity to examine the extent to which knowledge, attitudes, and opinions changed from before to after the deliberation. Even with the small sample, responses revealed a trend toward improved knowledge about key elements of the Choice Act and its goals. Further, there was a shift in some participants’ opinions about how patients should be prioritized to receive civilian care. For example, before the deliberation participants generally felt that all veterans should be able to receive civilian care, whereas postdeliberation this was not the case. Postdeliberation, most participants felt that primary care should not be a high priority for civilian care but continued to endorse prioritizing civilian care for specialty services like orthopedic or cardiology-related care. Finally, participants moved from more diverse recommendations regarding additional funds allocations, toward consensus after the deliberation around allocating funds to the build policy. Eight participants supported a build policy beforehand, whereas 16 supported this policy afterward.
Discussion
This study explored DD as a method for deeply engaging veterans in complex policy making to guide funding allocation and prioritization decisions related to the Choice Act, decisions that are still very relevant today within the context of the Mission Act and have substantial implications for how health care is delivered in the VHA. The Mission Act passed on June 6, 2018, with the goal of improving access to and the reliability of civilian or community care for eligible veterans.27 Decisions related to appropriating scarce funding to improve access to care is an emotional and value-laden topic that elicited strong and divergent opinions among the participants. Veterans were eager to have their voices heard and had strong expectations that VHA leadership would be briefed about their recommendations. The majority of participants were satisfied with the deliberation process, felt they understood the issues, and felt their opinions were respected. They expressed feelings of comradery and community throughout the process.
In this single deliberation session, the groups did not achieve a single, final consensus regarding how VHA funding should ultimately be allocated between buy and build policy options. Nonetheless, participants provided a rich array of recommendations and rationale for them. Session moderators observed rich, sophisticated, fair, and reason-based discussions on this complex topic. Participants left with a deeper knowledge and appreciation for the complex trade-offs and expressed strong rationales for both sides of the policy debate on build vs buy. In addition, the project yielded results of high interest to VHA policy makers.
This work was presented in multiple venues between 2015 to 2016, and to both local and national VHA leadership, including the local Executive Quality Leadership Boards, the VHA Central Office Committee on the Future State of VA Community Care, the VA Office of Patient Centered Care, and the National Veteran Experience Committee. Through these discussions and others, we saw great interest within the VHA system and high-level leaders to explore ways to include veterans’ voices in the policy-making process. This work was invaluable to our research team (eAppendix E
Many health system decisions regarding what care should be delivered (and how) involve making difficult, value-laden choices in the context of limited resources. DD methods can be used to target and obtain specific viewpoints from diverse populations, such as the informed perspectives of minority and underrepresented populations within the VHA.19 For example, female veterans were oversampled to ensure that the informed preferences of this population was obtained. Thus, DD methods could provide a valuable tool for health systems to elicit in-depth diverse patient input on high-profile policies that will have a substantial impact on the system’s patient population.
Limitations
One potential downside of DD is that, because of the resource-intensive nature of deliberation sessions, they are often conducted with relatively small groups.9 Viewpoints of those within these small samples who are willing to spend an entire day discussing a complex topic may not be representative of the larger patient community. However, the core goal of DD is diversity of opinions rather than representativeness.
A stratified random sampling strategy that oversampled for underrepresented and minority populations was used to help select a diverse group that represents the population on key characteristics and partially addresses concern about representativeness. Efforts to optimize participation rates, including providing monetary incentives, also are helpful and have led to high participation rates in past deliberations.7
Health system communication strategies that promote the importance of becoming involved in DD sessions also may be helpful in improving rates of recruitment. On particularly important topics where health system leaders feel a larger resource investment is justified, conducting larger scale deliberations with many small groups may obtain more generalizable evidence about what individual patients and groups of patients recommend.7 However, due to the inherent limitations of surveys and focus group approaches for obtaining informed views on complex topics, there are no clear systematic alternatives to the DD approach.
Conclusion
DD is an effective method to meaningfully engage patients in deep deliberations to guide complex policy making. Although design of deliberative sessions is resource-intensive, patient engagement efforts, such as those described in this paper, could be an important aspect of a well-functioning learning health system. Further research into alternative, streamlined methods that can also engage veterans more deeply is needed. DD also can be combined with other approaches to broaden and confirm findings, including focus groups, town hall meetings, or surveys.
Although this study did not provide consensus on how the VHA should allocate funds with respect to the Choice Act, it did provide insight into the importance and feasibility of engaging veterans in the policy-making process. As more policies aimed at improving veterans’ access to civilian care are created, such as the most recent Mission Act, policy makers should strongly consider using the DD method of obtaining informed veteran input into future policy decisions.
Acknowledgments
Funding was provided by the US Department of Veterans Affairs Office of Analytics and Business Intelligence (OABI) and the VA Quality Enhancement Research Initiative (QUERI). Dr. Caverly was supported in part by a VA Career Development Award (CDA 16-151). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). The authors thank the veterans who participated in this work. They also thank Caitlin Reardon and Natalya Wawrin for their assistance in organizing the deliberation session.
1. VA Office of the Inspector General. Veterans Health Administration. Interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix Health Care System. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed December 9, 2019.
2. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
3. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019; July 22. [Epub ahead of print.]
5. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among veterans: a nested case-control study. Ann Intern Med. 2019;170(7):433-442.
6. Meyer LJ, Clancy CM. Care fragmentation and prescription opioids. Ann Intern Med. 2019;170(7):497-498.
7. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
8. Street J, Duszynski K, Krawczyk S, Braunack-Mayer A. The use of citizens’ juries in health policy decision-making: a systematic review. Soc Sci Med. 2014;109:1-9.
9. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85(3):314-320.
10. Martin D, Abelson J, Singer P. Participation in health care priority-setting through the eyes of the participants. J Health Serv Res Pol. 2002;7(4):222-229.
11. Mort M, Finch T. Principles for telemedicine and telecare: the perspective of a citizens’ panel. J Telemed Telecare. 2005;11(suppl 1):66-68.
12. Kass N, Faden R, Fabi RE, et al. Alternative consent models for comparative effectiveness studies: views of patients from two institutions. AJOB Empir Bioeth. 2016;7(2):92-105.
13. Carman KL, Mallery C, Maurer M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Soc Sci Med. 2015;133:11-20.
14. Carman KL, Maurer M, Mangrum R, et al. Understanding an informed public’s views on the role of evidence in making health care decisions. Health Aff (Millwood). 2016;35(4):566-574.
15. Kim SYH, Wall IF, Stanczyk A, De Vries R. Assessing the public’s views in research ethics controversies: deliberative democracy and bioethics as natural allies, J Empir Res Hum Res Ethics. 2009;4(4):3-16.
16. Gastil J, Levine P, eds. The Deliberative Democracy Handbook: Strategies for Effective Civic Engagement in the Twenty-First Century. San Francisco, CA: Jossey-Bass; 2005.
17. Dryzek JS, Bächtiger A, Chambers S, et al. The crisis of democracy and the science of deliberation. Science. 2019;363(6432):1144-1146.
18. Blacksher E, Diebel A, Forest PG, Goold SD, Abelson J. What is public deliberation? Hastings Cent Rep. 2012;4(2):14-17.
19. Wang G, Gold M, Siegel J, et al. Deliberation: obtaining informed input from a diverse public. J Health Care Poor Underserved. 2015;26(1):223-242.
20. Simon RL, ed. The Blackwell Guide to Social and Political Philosophy. Malden, MA: Wiley-Blackwell; 2002.
21. Stanford University, Center for Deliberative Democracy. Deliberative polling on energy and environmental policy options in Japan. https://cdd.stanford.edu/2012/deliberative-polling-on-energy-and-environmental-policy-options-in-japan. Published August 12, 2012. Accessed December 9, 2019.
22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
23. Carman KL, Maurer M, Mallery C, et al. Community forum deliberative methods demonstration: evaluating effectiveness and eliciting public views on use of evidence. Final report. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/deliberative-methods_research-2013-1.pdf. Published November 2014. Accessed December 9, 2019.
24. Sunstein CR, Hastie R. Wiser: Getting Beyond Groupthink to Make Groups Smarter. Boston, MA: Harvard Business Review Press; 2014.
25. Damschroder LJ, Kim SY. Assessing the quality of democratic deliberation: a case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70(12):1896-1903.
26. Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed. Thousand Oaks: SAGE Publications, Inc; 1994.
27. US Department of Veterans Affairs. Veteran community care – general information. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/VHA-FS_MISSION-Act.pdf. Published September 9 2019. Accessed December 9, 2019.
Providing high-quality, patient-centered health care is a top priority for the US Department of Veterans Affairs (VA) Veteran Health Administration (VHA), whose core mission is to improve the health and well-being of US veterans. Thus, news of long wait times for medical appointments in the VHA sparked intense national attention and debate and led to changes in senior management and legislative action. 1 On August 8, 2014, President Bara c k Obama signed the Veterans Access, Choice, and Accountability Act of 2014, also known as the Choice Act, which provided an additional $16 billion in emergency spending over 3 years to improve veterans’ access to timely health care. 2 The Choice Act sought to develop an integrated health care network that allowed qualified VHA patients to receive specific health care services in their communities delivered by non-VHA health care providers (HCPs) but paid for by the VHA. The Choice Act also laid out explicit criteria for how to prioritize who would be eligible for VHA-purchased civilian care: (1) veterans who could not get timely appointments at a VHA medical facility within 30 days of referral; or (2) veterans who lived > 40 miles from the closest VHA medical facility.
VHA decision makers seeking to improve care delivery also need to weigh trade-offs between alternative approaches to providing rapid access. For instance, increasing access to non-VHA HCPs may not always decrease wait times and could result in loss of continuity, limited care coordination, limited ability to ensure and enforce high-quality standards at the VHA, and other challenges.3-6 Although the concerns and views of elected representatives, advocacy groups, and health system leaders are important, it is unknown whether these views and preferences align with those of veterans. Arguably, the range of views and concerns of informed veterans whose health is at stake should be particularly prominent in such policy decision making.
To identify the considerations that were most important to veterans regarding VHA policy around decreasing wait times, a study was designed to engage a group of veterans who were eligible for civilian care under the Choice Act. The study took place 1 year after the Choice Act was passed. Veterans were asked to focus on 2 related questions: First, how should funding be used for building VHA capacity (build) vs purchasing civilian care (buy)? Second, under what circumstances should civilian care be prioritized?
The aim of this paper is to describe democratic deliberation (DD), a specific method that engaged veteran patients in complex policy decisions around access to care. DD methods have been used increasingly in health care for developing policy guidance, setting priorities, providing advice on ethical dilemmas, weighing risk-benefit trade-offs, and determining decision-making authority.7-12 For example, DD helped guide national policy for mammography screening for breast cancer in New Zealand.13 The Agency for Healthcare Research and Quality has completed a systematic review and a large, randomized experiment on best practices for carrying out public deliberation.8,13,14 However, despite the potential value of this approach, there has been little use of deliberative methods within the VHA for the explicit purpose of informing veteran health care delivery.
This paper describes the experience engaging veterans by using DD methodology and informing VHA leadership about the results of those deliberations. The specific aims were to understand whether DD is an acceptable approach to use to engage patients in the medical services policy-making process within VHA and whether veterans are able to come to an informed consensus.
Methods
Engaging patients and incorporating their needs and concerns within the policy-making process may improve health system policies and make those policies more patient centered. Such engagement also could be a way to generate creative solutions. However, because health-system decisions often involve making difficult trade-offs, effectively obtaining patient population input on complex care delivery issues can be challenging.
Although surveys can provide intuitive, top-of-mind input from respondents, these opinions are generally not sufficient for resolving complex problems.15 Focus groups and interviews may produce results that are more in-depth than surveys, but these methods tend to elicit settled private preferences rather than opinions about what the community should do.16 DD, on the other hand, is designed to elicit deeply informed public opinions on complex, value-laden topics to develop recommendations and policies for a larger community.17 The goal is to find collective solutions to challenging social problems. DD achieves this by giving participants an opportunity to explore a topic in-depth, question experts, and engage peers in reason-based discussions.18,19 This method has its roots in political science and has been used over several decades to successfully inform policy making on a broad array of topics nationally and internationally—from health research ethics in the US to nuclear and energy policy in Japan.7,16,20,21 DD has been found to promote ownership of public programs and lend legitimacy to policy decisions, political institutions, and democracy itself.18
A single day (8 hours) DD session was convened, following a Citizens Jury model of deliberation, which brings veteran patients together to learn about a topic, ask questions of experts, deliberate with peers, and generate a “citizen’s report” that contains a set of recommendations (Table 1). An overview of the different models of DD and rationale for each can be found elsewhere.8,15
Recruitment Considerations
A purposively selected sample of civilian care-eligible veterans from a midwestern VHA health care system (1 medical center and 3 community-based outpatient clinics [CBOCs]) were invited to the DD session. The targeted number of participants was 30. Female veterans, who comprise only 7% of the local veteran population, were oversampled to account for their potentially different health care needs and to create balance between males and females in the session. Oversampling for other characteristics was not possible due to the relatively small sample size. Based on prior experience,7 it was assumed that 70% of willing participants would attend the session; therefore 34 veterans were invited and 24 attended. Each participant received a $200 incentive in appreciation for their substantial time commitment and to offset transportation costs.
Background Materials
A packet with educational materials (Flesch-Kincaid Grade Level of 10.5) was mailed to participants about 2 weeks before the DD session. Participants were asked to review prior to attending the session. These materials described the session (eg, purpose, organizers, importance) and provided factual information about the Choice Act (eg, eligibility, out-of-pocket costs, travel pay, prescription drug policies).
Session Overview
The session was structured to accomplish the following goals: (1) Elicit participants’ opinions about access to health care and reasons for those opinions; (2) Provide in-depth education about the Choice Act through presentations and discussions with topical experts; and (3) Elicit reasoning and recommendations on both the criteria by which participants prioritize candidates for civilian care and how participants would allocate additional funding to improve access (ie, by building VHA capacity to deliver more timely health care vs purchasing health care from civilian HCPs).
Participants were asked to fill out a survey on arrival in the morning and were assigned to 1 of 3 tables or small groups. Each table had a facilitator who had extensive experience in qualitative data collection methods and guided the dialogue using a scripted protocol that they helped develop and refine. The facilitation materials drew from and used previously published studies.22,23 Each facilitator audio recorded the sessions and took notes. Three experts presented during plenary education sessions. Presentations were designed to provide balanced factual information and included a veteran’s perspective. One presenter was a clinician on the project team, another was a local clinical leader responsible for making decisions about what services to provide via civilian care (buy) vs enhancing the local VHA health system’s ability to provide those services (build), and the third presenter was a veteran who was on the project team.
Education Session 1
The first plenary education session with expert presentations was conducted after each table completed an icebreaker exercise. The project team physician provided a brief history and description of the Choice Act to reinforce educational materials sent to participants prior to the session. The health system clinical leader described his decision process and principles and highlighted constraints placed on him by the Choice Act that were in place at the time of the DD session. He also described existing local and national programs to provide civilian care (eg, local fee-basis non-VHA care programs) and how these programs sought to achieve goals similar to the Choice Act. The veteran presenter focused on the importance of session participants providing candid insight and observations and emphasized that this session was a significant opportunity to “have their voices heard.”
Deliberation 1: What criteria should be used to prioritize patients for receiving civilian care paid for by the VHA? To elicit preferences on the central question of this deliberation, participants were presented with 8 real-world cases that were based on interviews conducted with Choice Act-eligible veterans (Table 2 and eAppendices A
Education Session 2
In the second plenary session, the project team physician provided information about health care access issues, both inside and outside of the VHA, particularly between urban and rural areas. He also discussed factors related to the insufficient capacity to meet growing demand that contributed to the VHA wait-time crisis. The veteran presenter shared reflections on health care access from a veteran’s perspective.
Deliberation 2: How should additional funding be divided between increasing the ability of the VHA to (1) provide care by VHA HCPs; and (2) pay for care from non-VHA civilian HCPs? Participants were presented the patient examples and Choice Act funding scenarios (the buy policy option) and contrasted that with a build policy option. Participants were explicitly encouraged to shift their perspectives from thinking about individual cases to considering policy-level decisions and the broader social good (Table 2).
Ensuring Robust Deliberations
If participants do not adequately grasp the complexities of the topic, a deliberation can fail. To facilitate nuanced reasoning, real-world concrete examples were developed as the starting point of each deliberation based on interviews with actual patients (deliberation 1) and actual policy proposals relevant to the funding allocation decisions within the Choice Act (deliberation 2).
A deliberation also can fail with self-silencing, where participants withhold opinions that differ from those articulated first or by more vocal members of the group.24 To combat self-silencing, highly experienced facilitators were used to ensure sharing from all participants and to support an open-minded, courteous, and reason-based environment for discourse. It was specified that the best solutions are achieved through reason-based and cordial disagreement and that success can be undermined when participants simply agree because it is easier or more comfortable.
A third way a deliberation can fail is if individuals do not adopt a group or system-level perspective. To counter this, facilitators reinforced at multiple points the importance of taking a broader social perspective rather than sharing only one’s personal preferences.
Finally, it is important to assess the quality of the deliberative process itself, to ensure that results are trustworthy.25 To assess the quality of the deliberative process, participants knowledge about key issues pre- and postdeliberation were assessed. Participants also were asked to rate the quality of the facilitators and how well they felt their voice was heard and respected, and facilitators made qualitative assessments about the extent to which participants were engaged in reason-based and collaborative discussion.
Data
Quantitative data were collected via pre- and postsession surveys. The surveys contained items related to knowledge about the Choice Act, expectations for the DD session, beliefs and opinions about the provision of health care for veterans, recommended funding allocations between build vs buy policy options, and general demographics. Qualitative data were collected through detailed notes taken by the 3 facilitators. Each table’s deliberations were audio recorded so that gaps in the notes could be filled.
The 3 facilitators, who were all experienced qualitative researchers, typed their written notes into a template immediately after the session. Two of the 3 facilitators led the analysis of the session notes. Findings within and across the 3 deliberation tables were developed using content and matrix analysis methods.26 Descriptive statistics were generated from survey responses and compared survey items pre- and postsession using paired t tests or χ2 tests for categorical responses.
Results
Thirty-three percent of individuals invited (n = 127) agreed to participate. Those who declined cited conflicts related to distance, transportation, work/school, medical appointments, family commitments, or were not interested. In all, 24 (69%) of the 35 veterans who accepted the invitation attended the deliberation session. Of the 11 who accepted but did not attend, 5 cancelled ahead of time because of conflicts (Figure). Most participants were male (70%), 48% were aged 61 to 75 years, 65% were white, 43% had some college education, 43% reported an annual income of between $25,000 and $40,000, and only 35% reported very good health (eAppendix D).
Deliberation 1
During the deliberation on the prioritization criteria, the concept of “condition severity” emerged as an important criterion for veterans. This criterion captured simultaneous consideration of both clinical necessity and burden on the veteran to obtain care. For example, participants felt that patients with a life-threatening illness should be prioritized for civilian care over patients who need preventative or primary care (clinical necessity) and that elderly patients with substantial difficulty traveling to VHA appointments should be prioritized over patients who can travel more easily (burden). The Choice Act regulations at the time of the DD session did not reflect this nuanced perspective, stipulating only that veterans must live > 40 miles from the nearest VHA medical facility.
One of the 3 groups did not prioritize the patient cases because some members felt that no veteran should be constrained from receiving civilian care if they desired it. Nonetheless, this group did agree with prioritizing the first 2 cases in Table 3. The other groups prioritized all 8 cases in generally similar ways.
Deliberation 2
No clear consensus emerged on the buy vs build question. A representative from each table presented their group’s positions, rationale, and recommendations after deliberations were completed. After hearing the range of positions, the groups then had another opportunity to deliberate based on what they heard from the other tables; no new recommendations or consensus emerged.
Participants who were in favor of allocating more funds toward the build policy offered a range of rationales, saying that it would (1) increase access for rural veterans by building CBOCs and deploying more mobile units that could bring outlets for health care closer to their home communities; (2) provide critical and unique medical expertise to address veteran-specific issues such as prosthetics, traumatic brain injury, posttraumatic stress disorder, spinal cord injury, and shrapnel wounds that are typically not available through civilian providers; (3) give VHA more oversight over the quality and cost of care, which is more challenging to do with civilian providers; and (4) Improve VHA infrastructure by, for example, upgrading technology and attracting the best clinicians and staff to support “our VHA.”
Participants who were in favor of allocating more funds toward the buy policy also offered a range of rationales, saying that it would (1) decrease patient burden by increasing access through community providers, decreasing wait time, and lessening personal cost and travel time; (2) allow more patients to receive civilian care, which was generally seen as beneficial by a few participants because of perceptions that the VHA provides lower quality care due to a shortage of VHA providers, run-down/older facilities, lack of technology, and poorer-quality VHA providers; and (3) provide an opportunity to divest of costly facilities and invest in other innovative approaches. Regarding this last reason, a few participants felt that the VHA is “gouged” when building medical centers that overrun budgets. They also were concerned that investing in facilities tied VHA to specific locations when current locations of veterans may change “25 years from now.”
Survey Results
Twenty-three of the 24 participants completed both pre- and postsession surveys. The majority of participants in the session felt people in the group respected their opinion (96%); felt that the facilitator did not try to influence the group with her own opinions (96%); indicated they understood the information enough to participate as much as they wanted (100%); and were hopeful that their reasoning and recommendations would help inform VHA policy makers (82%).
The surveys also provided an opportunity to examine the extent to which knowledge, attitudes, and opinions changed from before to after the deliberation. Even with the small sample, responses revealed a trend toward improved knowledge about key elements of the Choice Act and its goals. Further, there was a shift in some participants’ opinions about how patients should be prioritized to receive civilian care. For example, before the deliberation participants generally felt that all veterans should be able to receive civilian care, whereas postdeliberation this was not the case. Postdeliberation, most participants felt that primary care should not be a high priority for civilian care but continued to endorse prioritizing civilian care for specialty services like orthopedic or cardiology-related care. Finally, participants moved from more diverse recommendations regarding additional funds allocations, toward consensus after the deliberation around allocating funds to the build policy. Eight participants supported a build policy beforehand, whereas 16 supported this policy afterward.
Discussion
This study explored DD as a method for deeply engaging veterans in complex policy making to guide funding allocation and prioritization decisions related to the Choice Act, decisions that are still very relevant today within the context of the Mission Act and have substantial implications for how health care is delivered in the VHA. The Mission Act passed on June 6, 2018, with the goal of improving access to and the reliability of civilian or community care for eligible veterans.27 Decisions related to appropriating scarce funding to improve access to care is an emotional and value-laden topic that elicited strong and divergent opinions among the participants. Veterans were eager to have their voices heard and had strong expectations that VHA leadership would be briefed about their recommendations. The majority of participants were satisfied with the deliberation process, felt they understood the issues, and felt their opinions were respected. They expressed feelings of comradery and community throughout the process.
In this single deliberation session, the groups did not achieve a single, final consensus regarding how VHA funding should ultimately be allocated between buy and build policy options. Nonetheless, participants provided a rich array of recommendations and rationale for them. Session moderators observed rich, sophisticated, fair, and reason-based discussions on this complex topic. Participants left with a deeper knowledge and appreciation for the complex trade-offs and expressed strong rationales for both sides of the policy debate on build vs buy. In addition, the project yielded results of high interest to VHA policy makers.
This work was presented in multiple venues between 2015 to 2016, and to both local and national VHA leadership, including the local Executive Quality Leadership Boards, the VHA Central Office Committee on the Future State of VA Community Care, the VA Office of Patient Centered Care, and the National Veteran Experience Committee. Through these discussions and others, we saw great interest within the VHA system and high-level leaders to explore ways to include veterans’ voices in the policy-making process. This work was invaluable to our research team (eAppendix E
Many health system decisions regarding what care should be delivered (and how) involve making difficult, value-laden choices in the context of limited resources. DD methods can be used to target and obtain specific viewpoints from diverse populations, such as the informed perspectives of minority and underrepresented populations within the VHA.19 For example, female veterans were oversampled to ensure that the informed preferences of this population was obtained. Thus, DD methods could provide a valuable tool for health systems to elicit in-depth diverse patient input on high-profile policies that will have a substantial impact on the system’s patient population.
Limitations
One potential downside of DD is that, because of the resource-intensive nature of deliberation sessions, they are often conducted with relatively small groups.9 Viewpoints of those within these small samples who are willing to spend an entire day discussing a complex topic may not be representative of the larger patient community. However, the core goal of DD is diversity of opinions rather than representativeness.
A stratified random sampling strategy that oversampled for underrepresented and minority populations was used to help select a diverse group that represents the population on key characteristics and partially addresses concern about representativeness. Efforts to optimize participation rates, including providing monetary incentives, also are helpful and have led to high participation rates in past deliberations.7
Health system communication strategies that promote the importance of becoming involved in DD sessions also may be helpful in improving rates of recruitment. On particularly important topics where health system leaders feel a larger resource investment is justified, conducting larger scale deliberations with many small groups may obtain more generalizable evidence about what individual patients and groups of patients recommend.7 However, due to the inherent limitations of surveys and focus group approaches for obtaining informed views on complex topics, there are no clear systematic alternatives to the DD approach.
Conclusion
DD is an effective method to meaningfully engage patients in deep deliberations to guide complex policy making. Although design of deliberative sessions is resource-intensive, patient engagement efforts, such as those described in this paper, could be an important aspect of a well-functioning learning health system. Further research into alternative, streamlined methods that can also engage veterans more deeply is needed. DD also can be combined with other approaches to broaden and confirm findings, including focus groups, town hall meetings, or surveys.
Although this study did not provide consensus on how the VHA should allocate funds with respect to the Choice Act, it did provide insight into the importance and feasibility of engaging veterans in the policy-making process. As more policies aimed at improving veterans’ access to civilian care are created, such as the most recent Mission Act, policy makers should strongly consider using the DD method of obtaining informed veteran input into future policy decisions.
Acknowledgments
Funding was provided by the US Department of Veterans Affairs Office of Analytics and Business Intelligence (OABI) and the VA Quality Enhancement Research Initiative (QUERI). Dr. Caverly was supported in part by a VA Career Development Award (CDA 16-151). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). The authors thank the veterans who participated in this work. They also thank Caitlin Reardon and Natalya Wawrin for their assistance in organizing the deliberation session.
Providing high-quality, patient-centered health care is a top priority for the US Department of Veterans Affairs (VA) Veteran Health Administration (VHA), whose core mission is to improve the health and well-being of US veterans. Thus, news of long wait times for medical appointments in the VHA sparked intense national attention and debate and led to changes in senior management and legislative action. 1 On August 8, 2014, President Bara c k Obama signed the Veterans Access, Choice, and Accountability Act of 2014, also known as the Choice Act, which provided an additional $16 billion in emergency spending over 3 years to improve veterans’ access to timely health care. 2 The Choice Act sought to develop an integrated health care network that allowed qualified VHA patients to receive specific health care services in their communities delivered by non-VHA health care providers (HCPs) but paid for by the VHA. The Choice Act also laid out explicit criteria for how to prioritize who would be eligible for VHA-purchased civilian care: (1) veterans who could not get timely appointments at a VHA medical facility within 30 days of referral; or (2) veterans who lived > 40 miles from the closest VHA medical facility.
VHA decision makers seeking to improve care delivery also need to weigh trade-offs between alternative approaches to providing rapid access. For instance, increasing access to non-VHA HCPs may not always decrease wait times and could result in loss of continuity, limited care coordination, limited ability to ensure and enforce high-quality standards at the VHA, and other challenges.3-6 Although the concerns and views of elected representatives, advocacy groups, and health system leaders are important, it is unknown whether these views and preferences align with those of veterans. Arguably, the range of views and concerns of informed veterans whose health is at stake should be particularly prominent in such policy decision making.
To identify the considerations that were most important to veterans regarding VHA policy around decreasing wait times, a study was designed to engage a group of veterans who were eligible for civilian care under the Choice Act. The study took place 1 year after the Choice Act was passed. Veterans were asked to focus on 2 related questions: First, how should funding be used for building VHA capacity (build) vs purchasing civilian care (buy)? Second, under what circumstances should civilian care be prioritized?
The aim of this paper is to describe democratic deliberation (DD), a specific method that engaged veteran patients in complex policy decisions around access to care. DD methods have been used increasingly in health care for developing policy guidance, setting priorities, providing advice on ethical dilemmas, weighing risk-benefit trade-offs, and determining decision-making authority.7-12 For example, DD helped guide national policy for mammography screening for breast cancer in New Zealand.13 The Agency for Healthcare Research and Quality has completed a systematic review and a large, randomized experiment on best practices for carrying out public deliberation.8,13,14 However, despite the potential value of this approach, there has been little use of deliberative methods within the VHA for the explicit purpose of informing veteran health care delivery.
This paper describes the experience engaging veterans by using DD methodology and informing VHA leadership about the results of those deliberations. The specific aims were to understand whether DD is an acceptable approach to use to engage patients in the medical services policy-making process within VHA and whether veterans are able to come to an informed consensus.
Methods
Engaging patients and incorporating their needs and concerns within the policy-making process may improve health system policies and make those policies more patient centered. Such engagement also could be a way to generate creative solutions. However, because health-system decisions often involve making difficult trade-offs, effectively obtaining patient population input on complex care delivery issues can be challenging.
Although surveys can provide intuitive, top-of-mind input from respondents, these opinions are generally not sufficient for resolving complex problems.15 Focus groups and interviews may produce results that are more in-depth than surveys, but these methods tend to elicit settled private preferences rather than opinions about what the community should do.16 DD, on the other hand, is designed to elicit deeply informed public opinions on complex, value-laden topics to develop recommendations and policies for a larger community.17 The goal is to find collective solutions to challenging social problems. DD achieves this by giving participants an opportunity to explore a topic in-depth, question experts, and engage peers in reason-based discussions.18,19 This method has its roots in political science and has been used over several decades to successfully inform policy making on a broad array of topics nationally and internationally—from health research ethics in the US to nuclear and energy policy in Japan.7,16,20,21 DD has been found to promote ownership of public programs and lend legitimacy to policy decisions, political institutions, and democracy itself.18
A single day (8 hours) DD session was convened, following a Citizens Jury model of deliberation, which brings veteran patients together to learn about a topic, ask questions of experts, deliberate with peers, and generate a “citizen’s report” that contains a set of recommendations (Table 1). An overview of the different models of DD and rationale for each can be found elsewhere.8,15
Recruitment Considerations
A purposively selected sample of civilian care-eligible veterans from a midwestern VHA health care system (1 medical center and 3 community-based outpatient clinics [CBOCs]) were invited to the DD session. The targeted number of participants was 30. Female veterans, who comprise only 7% of the local veteran population, were oversampled to account for their potentially different health care needs and to create balance between males and females in the session. Oversampling for other characteristics was not possible due to the relatively small sample size. Based on prior experience,7 it was assumed that 70% of willing participants would attend the session; therefore 34 veterans were invited and 24 attended. Each participant received a $200 incentive in appreciation for their substantial time commitment and to offset transportation costs.
Background Materials
A packet with educational materials (Flesch-Kincaid Grade Level of 10.5) was mailed to participants about 2 weeks before the DD session. Participants were asked to review prior to attending the session. These materials described the session (eg, purpose, organizers, importance) and provided factual information about the Choice Act (eg, eligibility, out-of-pocket costs, travel pay, prescription drug policies).
Session Overview
The session was structured to accomplish the following goals: (1) Elicit participants’ opinions about access to health care and reasons for those opinions; (2) Provide in-depth education about the Choice Act through presentations and discussions with topical experts; and (3) Elicit reasoning and recommendations on both the criteria by which participants prioritize candidates for civilian care and how participants would allocate additional funding to improve access (ie, by building VHA capacity to deliver more timely health care vs purchasing health care from civilian HCPs).
Participants were asked to fill out a survey on arrival in the morning and were assigned to 1 of 3 tables or small groups. Each table had a facilitator who had extensive experience in qualitative data collection methods and guided the dialogue using a scripted protocol that they helped develop and refine. The facilitation materials drew from and used previously published studies.22,23 Each facilitator audio recorded the sessions and took notes. Three experts presented during plenary education sessions. Presentations were designed to provide balanced factual information and included a veteran’s perspective. One presenter was a clinician on the project team, another was a local clinical leader responsible for making decisions about what services to provide via civilian care (buy) vs enhancing the local VHA health system’s ability to provide those services (build), and the third presenter was a veteran who was on the project team.
Education Session 1
The first plenary education session with expert presentations was conducted after each table completed an icebreaker exercise. The project team physician provided a brief history and description of the Choice Act to reinforce educational materials sent to participants prior to the session. The health system clinical leader described his decision process and principles and highlighted constraints placed on him by the Choice Act that were in place at the time of the DD session. He also described existing local and national programs to provide civilian care (eg, local fee-basis non-VHA care programs) and how these programs sought to achieve goals similar to the Choice Act. The veteran presenter focused on the importance of session participants providing candid insight and observations and emphasized that this session was a significant opportunity to “have their voices heard.”
Deliberation 1: What criteria should be used to prioritize patients for receiving civilian care paid for by the VHA? To elicit preferences on the central question of this deliberation, participants were presented with 8 real-world cases that were based on interviews conducted with Choice Act-eligible veterans (Table 2 and eAppendices A
Education Session 2
In the second plenary session, the project team physician provided information about health care access issues, both inside and outside of the VHA, particularly between urban and rural areas. He also discussed factors related to the insufficient capacity to meet growing demand that contributed to the VHA wait-time crisis. The veteran presenter shared reflections on health care access from a veteran’s perspective.
Deliberation 2: How should additional funding be divided between increasing the ability of the VHA to (1) provide care by VHA HCPs; and (2) pay for care from non-VHA civilian HCPs? Participants were presented the patient examples and Choice Act funding scenarios (the buy policy option) and contrasted that with a build policy option. Participants were explicitly encouraged to shift their perspectives from thinking about individual cases to considering policy-level decisions and the broader social good (Table 2).
Ensuring Robust Deliberations
If participants do not adequately grasp the complexities of the topic, a deliberation can fail. To facilitate nuanced reasoning, real-world concrete examples were developed as the starting point of each deliberation based on interviews with actual patients (deliberation 1) and actual policy proposals relevant to the funding allocation decisions within the Choice Act (deliberation 2).
A deliberation also can fail with self-silencing, where participants withhold opinions that differ from those articulated first or by more vocal members of the group.24 To combat self-silencing, highly experienced facilitators were used to ensure sharing from all participants and to support an open-minded, courteous, and reason-based environment for discourse. It was specified that the best solutions are achieved through reason-based and cordial disagreement and that success can be undermined when participants simply agree because it is easier or more comfortable.
A third way a deliberation can fail is if individuals do not adopt a group or system-level perspective. To counter this, facilitators reinforced at multiple points the importance of taking a broader social perspective rather than sharing only one’s personal preferences.
Finally, it is important to assess the quality of the deliberative process itself, to ensure that results are trustworthy.25 To assess the quality of the deliberative process, participants knowledge about key issues pre- and postdeliberation were assessed. Participants also were asked to rate the quality of the facilitators and how well they felt their voice was heard and respected, and facilitators made qualitative assessments about the extent to which participants were engaged in reason-based and collaborative discussion.
Data
Quantitative data were collected via pre- and postsession surveys. The surveys contained items related to knowledge about the Choice Act, expectations for the DD session, beliefs and opinions about the provision of health care for veterans, recommended funding allocations between build vs buy policy options, and general demographics. Qualitative data were collected through detailed notes taken by the 3 facilitators. Each table’s deliberations were audio recorded so that gaps in the notes could be filled.
The 3 facilitators, who were all experienced qualitative researchers, typed their written notes into a template immediately after the session. Two of the 3 facilitators led the analysis of the session notes. Findings within and across the 3 deliberation tables were developed using content and matrix analysis methods.26 Descriptive statistics were generated from survey responses and compared survey items pre- and postsession using paired t tests or χ2 tests for categorical responses.
Results
Thirty-three percent of individuals invited (n = 127) agreed to participate. Those who declined cited conflicts related to distance, transportation, work/school, medical appointments, family commitments, or were not interested. In all, 24 (69%) of the 35 veterans who accepted the invitation attended the deliberation session. Of the 11 who accepted but did not attend, 5 cancelled ahead of time because of conflicts (Figure). Most participants were male (70%), 48% were aged 61 to 75 years, 65% were white, 43% had some college education, 43% reported an annual income of between $25,000 and $40,000, and only 35% reported very good health (eAppendix D).
Deliberation 1
During the deliberation on the prioritization criteria, the concept of “condition severity” emerged as an important criterion for veterans. This criterion captured simultaneous consideration of both clinical necessity and burden on the veteran to obtain care. For example, participants felt that patients with a life-threatening illness should be prioritized for civilian care over patients who need preventative or primary care (clinical necessity) and that elderly patients with substantial difficulty traveling to VHA appointments should be prioritized over patients who can travel more easily (burden). The Choice Act regulations at the time of the DD session did not reflect this nuanced perspective, stipulating only that veterans must live > 40 miles from the nearest VHA medical facility.
One of the 3 groups did not prioritize the patient cases because some members felt that no veteran should be constrained from receiving civilian care if they desired it. Nonetheless, this group did agree with prioritizing the first 2 cases in Table 3. The other groups prioritized all 8 cases in generally similar ways.
Deliberation 2
No clear consensus emerged on the buy vs build question. A representative from each table presented their group’s positions, rationale, and recommendations after deliberations were completed. After hearing the range of positions, the groups then had another opportunity to deliberate based on what they heard from the other tables; no new recommendations or consensus emerged.
Participants who were in favor of allocating more funds toward the build policy offered a range of rationales, saying that it would (1) increase access for rural veterans by building CBOCs and deploying more mobile units that could bring outlets for health care closer to their home communities; (2) provide critical and unique medical expertise to address veteran-specific issues such as prosthetics, traumatic brain injury, posttraumatic stress disorder, spinal cord injury, and shrapnel wounds that are typically not available through civilian providers; (3) give VHA more oversight over the quality and cost of care, which is more challenging to do with civilian providers; and (4) Improve VHA infrastructure by, for example, upgrading technology and attracting the best clinicians and staff to support “our VHA.”
Participants who were in favor of allocating more funds toward the buy policy also offered a range of rationales, saying that it would (1) decrease patient burden by increasing access through community providers, decreasing wait time, and lessening personal cost and travel time; (2) allow more patients to receive civilian care, which was generally seen as beneficial by a few participants because of perceptions that the VHA provides lower quality care due to a shortage of VHA providers, run-down/older facilities, lack of technology, and poorer-quality VHA providers; and (3) provide an opportunity to divest of costly facilities and invest in other innovative approaches. Regarding this last reason, a few participants felt that the VHA is “gouged” when building medical centers that overrun budgets. They also were concerned that investing in facilities tied VHA to specific locations when current locations of veterans may change “25 years from now.”
Survey Results
Twenty-three of the 24 participants completed both pre- and postsession surveys. The majority of participants in the session felt people in the group respected their opinion (96%); felt that the facilitator did not try to influence the group with her own opinions (96%); indicated they understood the information enough to participate as much as they wanted (100%); and were hopeful that their reasoning and recommendations would help inform VHA policy makers (82%).
The surveys also provided an opportunity to examine the extent to which knowledge, attitudes, and opinions changed from before to after the deliberation. Even with the small sample, responses revealed a trend toward improved knowledge about key elements of the Choice Act and its goals. Further, there was a shift in some participants’ opinions about how patients should be prioritized to receive civilian care. For example, before the deliberation participants generally felt that all veterans should be able to receive civilian care, whereas postdeliberation this was not the case. Postdeliberation, most participants felt that primary care should not be a high priority for civilian care but continued to endorse prioritizing civilian care for specialty services like orthopedic or cardiology-related care. Finally, participants moved from more diverse recommendations regarding additional funds allocations, toward consensus after the deliberation around allocating funds to the build policy. Eight participants supported a build policy beforehand, whereas 16 supported this policy afterward.
Discussion
This study explored DD as a method for deeply engaging veterans in complex policy making to guide funding allocation and prioritization decisions related to the Choice Act, decisions that are still very relevant today within the context of the Mission Act and have substantial implications for how health care is delivered in the VHA. The Mission Act passed on June 6, 2018, with the goal of improving access to and the reliability of civilian or community care for eligible veterans.27 Decisions related to appropriating scarce funding to improve access to care is an emotional and value-laden topic that elicited strong and divergent opinions among the participants. Veterans were eager to have their voices heard and had strong expectations that VHA leadership would be briefed about their recommendations. The majority of participants were satisfied with the deliberation process, felt they understood the issues, and felt their opinions were respected. They expressed feelings of comradery and community throughout the process.
In this single deliberation session, the groups did not achieve a single, final consensus regarding how VHA funding should ultimately be allocated between buy and build policy options. Nonetheless, participants provided a rich array of recommendations and rationale for them. Session moderators observed rich, sophisticated, fair, and reason-based discussions on this complex topic. Participants left with a deeper knowledge and appreciation for the complex trade-offs and expressed strong rationales for both sides of the policy debate on build vs buy. In addition, the project yielded results of high interest to VHA policy makers.
This work was presented in multiple venues between 2015 to 2016, and to both local and national VHA leadership, including the local Executive Quality Leadership Boards, the VHA Central Office Committee on the Future State of VA Community Care, the VA Office of Patient Centered Care, and the National Veteran Experience Committee. Through these discussions and others, we saw great interest within the VHA system and high-level leaders to explore ways to include veterans’ voices in the policy-making process. This work was invaluable to our research team (eAppendix E
Many health system decisions regarding what care should be delivered (and how) involve making difficult, value-laden choices in the context of limited resources. DD methods can be used to target and obtain specific viewpoints from diverse populations, such as the informed perspectives of minority and underrepresented populations within the VHA.19 For example, female veterans were oversampled to ensure that the informed preferences of this population was obtained. Thus, DD methods could provide a valuable tool for health systems to elicit in-depth diverse patient input on high-profile policies that will have a substantial impact on the system’s patient population.
Limitations
One potential downside of DD is that, because of the resource-intensive nature of deliberation sessions, they are often conducted with relatively small groups.9 Viewpoints of those within these small samples who are willing to spend an entire day discussing a complex topic may not be representative of the larger patient community. However, the core goal of DD is diversity of opinions rather than representativeness.
A stratified random sampling strategy that oversampled for underrepresented and minority populations was used to help select a diverse group that represents the population on key characteristics and partially addresses concern about representativeness. Efforts to optimize participation rates, including providing monetary incentives, also are helpful and have led to high participation rates in past deliberations.7
Health system communication strategies that promote the importance of becoming involved in DD sessions also may be helpful in improving rates of recruitment. On particularly important topics where health system leaders feel a larger resource investment is justified, conducting larger scale deliberations with many small groups may obtain more generalizable evidence about what individual patients and groups of patients recommend.7 However, due to the inherent limitations of surveys and focus group approaches for obtaining informed views on complex topics, there are no clear systematic alternatives to the DD approach.
Conclusion
DD is an effective method to meaningfully engage patients in deep deliberations to guide complex policy making. Although design of deliberative sessions is resource-intensive, patient engagement efforts, such as those described in this paper, could be an important aspect of a well-functioning learning health system. Further research into alternative, streamlined methods that can also engage veterans more deeply is needed. DD also can be combined with other approaches to broaden and confirm findings, including focus groups, town hall meetings, or surveys.
Although this study did not provide consensus on how the VHA should allocate funds with respect to the Choice Act, it did provide insight into the importance and feasibility of engaging veterans in the policy-making process. As more policies aimed at improving veterans’ access to civilian care are created, such as the most recent Mission Act, policy makers should strongly consider using the DD method of obtaining informed veteran input into future policy decisions.
Acknowledgments
Funding was provided by the US Department of Veterans Affairs Office of Analytics and Business Intelligence (OABI) and the VA Quality Enhancement Research Initiative (QUERI). Dr. Caverly was supported in part by a VA Career Development Award (CDA 16-151). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). The authors thank the veterans who participated in this work. They also thank Caitlin Reardon and Natalya Wawrin for their assistance in organizing the deliberation session.
1. VA Office of the Inspector General. Veterans Health Administration. Interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix Health Care System. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed December 9, 2019.
2. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
3. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019; July 22. [Epub ahead of print.]
5. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among veterans: a nested case-control study. Ann Intern Med. 2019;170(7):433-442.
6. Meyer LJ, Clancy CM. Care fragmentation and prescription opioids. Ann Intern Med. 2019;170(7):497-498.
7. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
8. Street J, Duszynski K, Krawczyk S, Braunack-Mayer A. The use of citizens’ juries in health policy decision-making: a systematic review. Soc Sci Med. 2014;109:1-9.
9. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85(3):314-320.
10. Martin D, Abelson J, Singer P. Participation in health care priority-setting through the eyes of the participants. J Health Serv Res Pol. 2002;7(4):222-229.
11. Mort M, Finch T. Principles for telemedicine and telecare: the perspective of a citizens’ panel. J Telemed Telecare. 2005;11(suppl 1):66-68.
12. Kass N, Faden R, Fabi RE, et al. Alternative consent models for comparative effectiveness studies: views of patients from two institutions. AJOB Empir Bioeth. 2016;7(2):92-105.
13. Carman KL, Mallery C, Maurer M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Soc Sci Med. 2015;133:11-20.
14. Carman KL, Maurer M, Mangrum R, et al. Understanding an informed public’s views on the role of evidence in making health care decisions. Health Aff (Millwood). 2016;35(4):566-574.
15. Kim SYH, Wall IF, Stanczyk A, De Vries R. Assessing the public’s views in research ethics controversies: deliberative democracy and bioethics as natural allies, J Empir Res Hum Res Ethics. 2009;4(4):3-16.
16. Gastil J, Levine P, eds. The Deliberative Democracy Handbook: Strategies for Effective Civic Engagement in the Twenty-First Century. San Francisco, CA: Jossey-Bass; 2005.
17. Dryzek JS, Bächtiger A, Chambers S, et al. The crisis of democracy and the science of deliberation. Science. 2019;363(6432):1144-1146.
18. Blacksher E, Diebel A, Forest PG, Goold SD, Abelson J. What is public deliberation? Hastings Cent Rep. 2012;4(2):14-17.
19. Wang G, Gold M, Siegel J, et al. Deliberation: obtaining informed input from a diverse public. J Health Care Poor Underserved. 2015;26(1):223-242.
20. Simon RL, ed. The Blackwell Guide to Social and Political Philosophy. Malden, MA: Wiley-Blackwell; 2002.
21. Stanford University, Center for Deliberative Democracy. Deliberative polling on energy and environmental policy options in Japan. https://cdd.stanford.edu/2012/deliberative-polling-on-energy-and-environmental-policy-options-in-japan. Published August 12, 2012. Accessed December 9, 2019.
22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
23. Carman KL, Maurer M, Mallery C, et al. Community forum deliberative methods demonstration: evaluating effectiveness and eliciting public views on use of evidence. Final report. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/deliberative-methods_research-2013-1.pdf. Published November 2014. Accessed December 9, 2019.
24. Sunstein CR, Hastie R. Wiser: Getting Beyond Groupthink to Make Groups Smarter. Boston, MA: Harvard Business Review Press; 2014.
25. Damschroder LJ, Kim SY. Assessing the quality of democratic deliberation: a case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70(12):1896-1903.
26. Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed. Thousand Oaks: SAGE Publications, Inc; 1994.
27. US Department of Veterans Affairs. Veteran community care – general information. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/VHA-FS_MISSION-Act.pdf. Published September 9 2019. Accessed December 9, 2019.
1. VA Office of the Inspector General. Veterans Health Administration. Interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix Health Care System. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed December 9, 2019.
2. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
3. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019; July 22. [Epub ahead of print.]
5. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among veterans: a nested case-control study. Ann Intern Med. 2019;170(7):433-442.
6. Meyer LJ, Clancy CM. Care fragmentation and prescription opioids. Ann Intern Med. 2019;170(7):497-498.
7. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
8. Street J, Duszynski K, Krawczyk S, Braunack-Mayer A. The use of citizens’ juries in health policy decision-making: a systematic review. Soc Sci Med. 2014;109:1-9.
9. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85(3):314-320.
10. Martin D, Abelson J, Singer P. Participation in health care priority-setting through the eyes of the participants. J Health Serv Res Pol. 2002;7(4):222-229.
11. Mort M, Finch T. Principles for telemedicine and telecare: the perspective of a citizens’ panel. J Telemed Telecare. 2005;11(suppl 1):66-68.
12. Kass N, Faden R, Fabi RE, et al. Alternative consent models for comparative effectiveness studies: views of patients from two institutions. AJOB Empir Bioeth. 2016;7(2):92-105.
13. Carman KL, Mallery C, Maurer M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Soc Sci Med. 2015;133:11-20.
14. Carman KL, Maurer M, Mangrum R, et al. Understanding an informed public’s views on the role of evidence in making health care decisions. Health Aff (Millwood). 2016;35(4):566-574.
15. Kim SYH, Wall IF, Stanczyk A, De Vries R. Assessing the public’s views in research ethics controversies: deliberative democracy and bioethics as natural allies, J Empir Res Hum Res Ethics. 2009;4(4):3-16.
16. Gastil J, Levine P, eds. The Deliberative Democracy Handbook: Strategies for Effective Civic Engagement in the Twenty-First Century. San Francisco, CA: Jossey-Bass; 2005.
17. Dryzek JS, Bächtiger A, Chambers S, et al. The crisis of democracy and the science of deliberation. Science. 2019;363(6432):1144-1146.
18. Blacksher E, Diebel A, Forest PG, Goold SD, Abelson J. What is public deliberation? Hastings Cent Rep. 2012;4(2):14-17.
19. Wang G, Gold M, Siegel J, et al. Deliberation: obtaining informed input from a diverse public. J Health Care Poor Underserved. 2015;26(1):223-242.
20. Simon RL, ed. The Blackwell Guide to Social and Political Philosophy. Malden, MA: Wiley-Blackwell; 2002.
21. Stanford University, Center for Deliberative Democracy. Deliberative polling on energy and environmental policy options in Japan. https://cdd.stanford.edu/2012/deliberative-polling-on-energy-and-environmental-policy-options-in-japan. Published August 12, 2012. Accessed December 9, 2019.
22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
23. Carman KL, Maurer M, Mallery C, et al. Community forum deliberative methods demonstration: evaluating effectiveness and eliciting public views on use of evidence. Final report. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/deliberative-methods_research-2013-1.pdf. Published November 2014. Accessed December 9, 2019.
24. Sunstein CR, Hastie R. Wiser: Getting Beyond Groupthink to Make Groups Smarter. Boston, MA: Harvard Business Review Press; 2014.
25. Damschroder LJ, Kim SY. Assessing the quality of democratic deliberation: a case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70(12):1896-1903.
26. Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed. Thousand Oaks: SAGE Publications, Inc; 1994.
27. US Department of Veterans Affairs. Veteran community care – general information. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/VHA-FS_MISSION-Act.pdf. Published September 9 2019. Accessed December 9, 2019.
Food Insecurity Among Veterans: Resources to Screen and Intervene
Nearly 1 in 8 households—and 1 in 6 households with children—experienced food insecurity in 2017, defined as limited or uncertain availability of nutritionally adequate and safe foods.1 Food insecurity is often even more pronounced among households with individuals with acute or chronic medical conditions.2-6 Moreover, food insecurity is independently associated with a range of adverse health outcomes, including poorer control of diabetes mellitus, hypertension, depression and other major psychiatric disorders, HIV, and chronic lung and kidney disease, as well as poorer overall health status.7-14 Food insecurity also has been associated with increased health care costs and acute care utilization as well as increased probability of delayed or missed care.15-19
The relationship between food insecurity and poor health outcomes is a complex and often cyclic phenomenon (Figure 1). Poor nutritional status is fueled by limited access to healthful foods as well as increased reliance on calorie-dense and nutrient-poor “junk” foods, which are less expensive and often more readily available in low-income neighborhoods.5,20-24 These compensatory dietary patterns place individuals at higher risk for developing cardiometabolic conditions and for poor control of these conditions.5,8,9,12,25,26 Additionally, the physiological and psychological stressors of food insecurity may precipitate depression and anxiety or worsen existing mental health conditions, resulting in feelings of overwhelm and decreased self-management capacity.5,8,27-31 Food insecurity has further been associated with poor sleep, declines in cognitive function, and increased falls, particularly among the frail and elderly.32-34
Individuals experiencing food insecurity often report having to make trade-offs between food and other necessities, such as paying rent or utilities. Additional strategies to stretch limited resources include cost-related underuse of medication and delays in needed medical care.4,17,31,35 In a nationally representative survey among adults with at least 1 chronic medical condition, 1 in 3 reported having to choose between food and medicine; 11% were unable to afford either.3 Furthermore, the inability to reliably adhere to medication regimens that need to be taken with food can result in potentially life-threatening hypoglycemia (as can lack of food regardless of medication use).5,26,36 In addition to the more obvious risks of glucose-lowering medications, such as insulin and long-acting sulfonylureas in patients experiencing food insecurity, many drugs commonly used among nondiabetic adults such as ACE-inhibitors, β blockers, quinolones, and salicylates can also precipitate hypoglycemia, and food insecurity has been associated with experiences of hypoglycemia even among individuals without diabetes mellitus.32,37 In one study the risk for hospital admissions for hypoglycemia among low-income populations increased by 27% at the end of the month when food budgets were more likely to be exhausted.38 Worsening health status and increased emergency department visits and hospitalizations may then result in lost wages and mounting medical bills, contributing to further financial strain and worsening food insecurity.
Prevalence and Importance of Food Insecurity Among US Veterans
Nearly 1.5 million veterans in the US are living below the federal poverty level (FPL).39 An additional 2.4 million veterans are living paycheck to paycheck at < 200% of the FPL.40 Veterans living in poverty are at even higher risk than nonveterans for food insecurity, homelessness, and other material hardship.41
Estimates of food insecurity among veterans vary widely, ranging from 6% to 24%—nearly twice that of the general US population.8,42-45 Higher rates of food insecurity have been reported among certain high-risk subgroups, including veterans who served in Iraq and Afghanistan (27%), female veterans (28%), homeless and formerly homeless veterans (49%), and veterans with serious mental illness (35%).6,32,43,46 Additional risk factors for food insecurity specific to veteran populations include younger age, having recently left active-duty military service, and lower final military paygrade.42,45-47 As in the general population, veteran food insecurity is associated with a range of adverse health outcomes, including poorer overall health status as well as increased probability of delayed or missed care.6,8,32,42-44,46
Even among veterans enrolled in federal food assistance programs, many still struggle to afford nutritionally adequate foods. As one example, in a study of mostly male homeless and formerly homeless veterans, O’Toole and colleagues found that nearly half of those reporting food insecurity were already receiving federal food assistance benefits, and 22% relied on emergency food resources.32 Of households served by Feeding America food pantries and meal programs, 20% have a member who has served in the US military.48
Federal Programs To Address Food Insecurity
There are several important federal food assistance programs designed to help alleviate food insecurity. The Supplemental Nutrition Assistance Program (SNAP, formerly the Food Stamp program) is the largest federal food assistance program and provides low-income Americans with cash benefits to purchase food. SNAP has been shown to substantially reduce food insecurity.7,49 The program also is associated with significant decreases in cost-related medication nonadherence as well as reductions in health care costs and both acute care and nursing home utilization.16,50-54 Although nearly 1.4 million veterans live in SNAP-enrolled households, 59% of eligible veterans are not enrolled.43,55 Closing this SNAP eligibility-enrollment gap, has been a focus of recent efforts to improve long-term food security among veterans. There also are several federal food assistance programs for households with children, including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and school meals programs. Among federal nutrition programs for seniors, the Older American’s Act contains designated funding to support nutrition services for older adults, including congregate meal programs in community settings like senior centers, places of worship, and housing communities, and home-delivered meals through programs like Meals on Wheels.56
VHA Response to Food Insecurity
The Veterans Health Administration (VHA) is the country’s largest integrated, federally funded health care system.57 In November 2015, congressional briefings on veteran food insecurity organized by the national non-profit organization MAZON: A Jewish Response to Hunger and hosted with bipartisan support were provided to the US House and Senate. As a result of these briefings, VHA chartered the national Ensuring Veteran Food Security Workgroup with a mandate to partner with governmental and nonprofit agencies to “focus on the issue of food insecurity, the identification of veterans at risk, the needed training of VHA staff and the coordination of resources and initiatives to support the veterans for whom we care.” Building off a pilot in US Department of Veterans Affairs (VA) Homeless Patient Aligned Care Teams (H-PACTs),32 VHA subsequently integrated a single-item food insecurity screening tool into the VA electronic health record (EHR) clinical reminder system (Figure 2). The clinical reminder, which was rolled out across VA medical centers nationally in October 2017, provides an alert to screen all noninstitutionalized veterans for food insecurity. To date, nearly 5 million veterans have been screened. When a veteran endorses food insecurity based on the initial screening question, a prompt appears to offer the veteran a referral to a social worker and/or dietitian. Positive screening results also should be communicated to the patient’s primary care provider. Depending on site-specific clinical flow, the reminders are typically completed in the outpatient setting either by nurses or medical assistants during intake or by providers as part of the clinical visit. However, any member of the health care team can complete the clinical reminder at any time. As of September 2019, approximately 74,000 veterans have been identified as food insecure.58
Addressing Food Insecurity
VHA has been a recognized leader in addressing homelessness and other social determinants of health through its integrated care and PACT delivery models.59-61 The food insecurity clinical reminder was designed to facilitate a tailored, interdisciplinary approach to identify and address food insecurity. Interdisciplinary care team members—including medical assistants, clinicians, social workers, registered dietitians, nurse care managers, occupational or physical therapists, and pharmacists—are uniquely positioned to identify veterans impacted by food insecurity, assess for associated clinical and/or social risk factors, and offer appropriate medical and nutrition interventions and resource referrals.
This interdisciplinary team-based model is essential given the range of potential drivers underlying veteran experiences of food insecurity and subsequent health outcomes. It is critically important for clinicians to review the medication list with veterans screening positive for food insecurity to assess for risk of hypoglycemia and/or cost-related nonadherence, make any necessary adjustments to therapeutic regimens, and assess for additional risk factors associated with food insecurity. Examples of tailored nutrition counseling that clinical dietitians may provide include meal preparation strategies for veterans who only have access to a microwave or hotplate, or recommendations for how veterans on medically restricted diets can best navigate food selection at soup kitchens or food pantries. Resource referrals provided by social workers or other care team members may include both emergency food resources to address immediate shortages (eg, food pantries, soup kitchens, or vouchers for free lunch) as well as resources focused on improving longer term food security (eg, federal food assistance programs or home delivered meal programs). Importantly, although providing a list of food resources may be helpful for some patients, such lists are often insufficient.62,63 Many patients require active assistance with program enrollment either onsite the day of their clinic visit or through connection with a partnering community-based organization that can, in turn, identify appropriate resources and facilitate program enrollment.63,64 Planned follow-up is also crucial to determine whether referrals are successful and to assess for ongoing need. Proposed roles for interdisciplinary care team members in addressing a positive food insecurity screen are outlined in Table 1.
VHA-Community Partnerships
In addition to services offered within VA, public and private sector partnerships can greatly enhance the range of resources available to food insecure veterans. Several VA facilities have developed formal community partnerships, such as the Veterans Pantry Pilot (VPP) program, a national partnership between Feeding America food banks and VA medical centers to establish onsite or mobile food pantries. There are currently 17 active Feeding America VPP sites, with a number of additional sites under development. Several of the VPP sites also include other “wraparound services,” such as SNAP application assistance.65,66
State Veterans Affairs offices67 and Veterans Service Organizations (VSOs)68 also can serve as valuable partners for connecting veterans with needed resources. VSOs offer a range of services, including assistancewith benefit claims, employment and housing assistance, emergency food assistance, and transportation to medical appointments. Some VSOs also have established local affiliations with Meals on Wheels focused on veteran outreach and providing hot meals for low-income, homebound, and disabled veterans.
Additional Resources
Although resources vary by regional setting, several key governmental and community-based food assistance programs are summarized in Table 2. Local community partners and online/phone-based directories, such as United Way’s 2-1-1 can help identify additional local resources. For older adults and individuals with disabilities, local Aging and Disability Resources Centers can provide information and assistance connecting to needed resources.69 Finally, there are a number of online resources available for clinicians interested in learning more about the impact of food insecurity on health and tools to use in the clinical setting (Table 3).
Conclusion
The VA has recognized food insecurity as a critical concern for the well-being of our nation’s veterans. Use of the EHR clinical reminder represents a crucial first step toward increasing provider awareness about veteran food insecurity and improving clinical efforts to address food insecurity once identified. Through the reminder, health care teams can connect veterans to needed resources and create both the individual and population-level data necessary to inform VHA and community efforts to address veteran food insecurity. Clinical reminder data are currently being used for local quality improvement efforts and have established the need nationally for formalized partnerships between VHA Social Work Services and Nutrition and Food Services to connect veterans with food and provide them with strategies to best use available food resources.
Moving forward, the Ensuring Veteran Food Security Workgroup continues to work with agencies and organizations across the country to improve food insecure veterans’ access to needed services. In addition to existing VA partnerships with Feeding America for the VPP, memorandums of understanding are currently underway to formalize partnerships with both the Food Research and Action Center (FRAC) and MAZON. Additional research is needed both to formally validate the current food insecurity clinical reminder screening question and to identify best practices and potential models for how to most effectively use VHA-community partnerships to address the unique needs of the veteran population.
Ensuring the food security of our nation’s veterans is essential to VA’s commitment to providing integrated, veteran-centered, whole person care. Toward that goal, VA health care teams are urged to use the clinical reminder and help connect food insecure veterans with relevant resources both within and outside of the VA health care system.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. http://www.ers.usda.gov/publications/pub-details/?pubid=90022. Published September 2018. Accessed December 9, 2019.
2. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257-265.
3. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303-310.e3.
4. Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610-624.
5. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6-9.
6. Narain K, Bean-Mayberry B, Washington DL, Canelo IA, Darling JE, Yano EM. Access to care and health outcomes among women veterans using veterans administration health care: association with food insufficiency. Womens Health Issues. 2018;28(3):267-272.
7. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34(11):1830-1839.
8. Wang EA, McGinnis KA, Goulet J, et al; Veterans Aging Cohort Study Project Team. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268.
9. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005-2012. PloS One. 2017;12(6):e0179172.
10. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304-310.
11. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093-3099.
12. Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233-238.
13. Banerjee T, Crews DC, Wesson DE, et al; CDC CKD Surveillance Team. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38-47.
14. Bruening M, Dinour LM, Chavez JBR. Food insecurity and emotional health in the USA: a systematic narrative review of longitudinal research. Public Health Nutr. 2017;20(17):3200-3208.
15. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011-2013. Health Serv Res. 2018;53(3):1600-1620.
16. Berkowitz SA, Seligman HK, Basu S. Impact of food insecurity and SNAP participation on healthcare utilization and expenditures. http://www.ukcpr.org/research/discussion-papers. Published 2017. Accessed December 9, 2019.
17. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71-77.
18. Garcia SP, Haddix A, Barnett K. Incremental health care costs associated with food insecurity and chronic conditions among older adults. Chronic Dis. 2018;15:180058.
19. Berkowitz SA, Seligman HK, Meigs JB, Basu S. Food insecurity, healthcare utilization, and high cost: a longitudinal cohort study. Am J Manag Care. 2018;24(9):399-404.
20. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74-81.
21. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643-660.
22. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117.
23. Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181-1188.
24. Lucan SC, Maroko AR, Seitchik JL, Yoon DH, Sperry LE, Schechter CB. Unexpected neighborhood sources of food and drink: implications for research and community health. Am J Prev Med. 2018;55(2):e29-e38.
25. Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245-250.
26. Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227-1233.
27. Siefert K, Heflin CM, Corcoran ME, Williams DR. Food insufficiency and physical and mental health in a longitudinal survey of welfare recipients. J Health Soc Behav. 2004;45(2):171-186.
28. Mangurian C, Sreshta N, Seligman H. Food insecurity among adults with severe mental illness. Psychiatr Serv. 2013;64(9):931-932.
29. Melchior M, Caspi A, Howard LM, et al. Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics. 2009;124(4):e564-e572.
30. Brostow DP, Gunzburger E, Abbate LM, Brenner LA, Thomas KS. Mental illness, not obesity status, is associated with food insecurity among the elderly in the health and retirement study. J Nutr Gerontol Geriatr. 2019;38(2):149-172.
31. Higashi RT, Craddock Lee SJ, Pezzia C, Quirk L, Leonard T, Pruitt SL. Family and social context contributes to the interplay of economic insecurity, food insecurity, and health. Ann Anthropol Pract. 2017;41(2):67-77.
32. O’Toole TP, Roberts CB, Johnson EE. Screening for food insecurity in six Veterans Administration clinics for the homeless, June-December 2015. Prev Chronic Dis. 2017;14:160375.
33. Feil DG, Pogach LM. Cognitive impairment is a major risk factor for serious hypoglycaemia; public health intervention is warranted. Evid Based Med. 2014;19(2):77.
34. Frith E, Loprinzi PD. Food insecurity and cognitive function in older adults: Brief report. Clin Nutr. 2018;37(5):1765-1768.
35. Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. Am J Public Health. 2015;105(10):e48-e59.
36. Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Vue MH, Setter SM. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177.
38. Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33(1):116-123.
39. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran poverty trends. https://www.va.gov/vetdata/docs/specialreports/veteran_poverty_trends.pdf. Published May 2015. Accessed December 9, 2019.
40. Robbins KG, Ravi A. Veterans living paycheck to paycheck are under threat during budget debates. https://www.americanprogress.org/issues/poverty/news/2017/09/19/439023/veterans-living-paycheck-paycheck-threat-budget-debates. Published September 19, 2017. Accessed December 9, 2019.
41. Wilmoth JM, London AS, Heflin CM. Economic well-being among older-adult households: variation by veteran and disability status. J Gerontol Soc Work. 2015;58(4):399-419.
42. Brostow DP, Gunzburger E, Thomas KS. Food insecurity among veterans: findings from the health and retirement study. J Nutr Health Aging. 2017;21(10):1358-1364.
43. Pooler J, Mian P, Srinivasan M, Miller Z. Veterans and food insecurity. https://www.impaqint.com/sites/default/files/issue-briefs/VeteransFoodInsecurity_IssueBrief_V1.3.pdf. Published November 2018. Accessed December 9, 2019.
44. Schure MB, Katon JG, Wong E, Liu C-F. Food and housing insecurity and health status among U.S. adults with and without prior military service. SSM Popul Health. 2016;29(2):244-248.
45. Miller DP, Larson MJ, Byrne T, DeVoe E. Food insecurity in veteran households: findings from nationally representative data. Public Health Nutr. 2016;19(10):1731-1740.
46. Widome R, Jensen A, Bangerter A, Fu SS. Food insecurity among veterans of the US wars in Iraq and Afghanistan. Public Health Nutr. 2015;18(5):844-849.
47. London AS, Heflin CM. Supplemental Nutrition Assistance Program (SNAP) use among active-duty military personnel, veterans, and reservists. Popul Res Policy Rev. 2015;34(6):805-826.
48. Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014. Natl rep prepared for Feeding America. https://www.feedingamerica.org/research/hunger-in-america. Published 2014. Accessed December 9, 2019.
49. Mabli J, Ohls J, Dragoset L, Castner L, Santos B. Measuring the Effect of Supplemental Nutrition Assistance Program (SNAP) Participation on Food Security. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2013.
50. Srinivasan M, Pooler JA. Cost-related medication nonadherence for older adults participating in SNAP, 2013–2015. Am J Public Health. 2017;108(2):224-230.
51. Heflin C, Hodges L, Mueser P. Supplemental Nutrition Assistance Progam benefits and emergency room visits for hypoglycaemia. Public Health Nutr. 2017;20(7):1314-1321.
52. Samuel LJ, Szanton SL, Cahill R, et al. Does the Supplemental Nutrition Assistance Program affect hospital utilization among older adults? The case of Maryland. Popul Health Manag. 2018;21(2):88-95.
53. Szanton SL, Samuel LJ, Cahill R, et al. Food assistance is associated with decreased nursing home admissions for Maryland’s dually eligible older adults. BMC Geriatr. 2017;17(1):162.
54. Carlson S, Keith-Jennings B. SNAP is linked with improved nutritional outcomes and lower health care costs. https://www.cbpp.org/research/food-assistance/snap-is-linked-with-improved-nutritional-outcomes-and-lower-health-care. Published January 17, 2018. Accessed December 10, 2019.
55. Keith-Jennings B, Cai L. SNAP helps almost 1.4 million low-income veterans, including thousands in every state. https://www.cbpp.org/research/food-assistance/snap-helps-almost-14-million-low-income-veterans-including-thousands-in. Updated November 8, 2018. Accessed December 10, 2019.
56. US Department of Health and Human Services. Older Americans Act nutrition programs. https://acl.gov/sites/default/files/news%202017-03/OAA-Nutrition_Programs_Fact_Sheet.pdf. Accessed December 10, 2019.
57. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed December 10, 2019.
58. US Department of Veterans Affairs. VA Corporate Data Warehouse.
59. Yano EM, Bair MJ, Carrasquillo O, Krein SL, Rubenstein LV. Patient aligned care teams (PACT): VA’s journey to implement patient-centered medical homes. J Gen Intern Med. 2014;29(suppl 2):S547-s549.
60. O’Toole TP, Pape L. Innovative efforts to address homelessness among veterans. N C Med J. 2015;76(5):311-314.
61. O’Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” Program. Prev Chronic Dis. 2016;13:150567.
62. Marpadga S, Fernandez A, Leung J, Tang A, Seligman H, Murphy EJ. Challenges and successes with food resource referrals for food-insecure patients with diabetes. Perm J. 2019;23.
63. Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22.
64. Martel ML, Klein LR, Hager KA, Cutts DB. Emergency department experience with novel electronic medical record order for referral to food resources. West J Emerg Med. 2018;19(2):232-237.
65. Going C, Cohen AJ, Bares M, Christensen M. Interdisciplinary approaches to addressing the food insecure veteran. Veterans Health Administration Employee Education System webinar; October 30, 2018.
66. Feeding America Announces New Partnership With U.S. Department Of Veterans Affairs. https://www.prnewswire.com/news-releases/feeding-america-announces-new-partnership-with-us-department-of-veterans-affairs-300481891.html. Published June 29, 2017. Accessed December 10, 2019.
67. US Department of Veterans Affairs. State Veterans Affairs offices. https://www.va.gov/statedva.htm. Updated March 20, 2019. Accessed December 10, 2019.
68. US Department of Veterans Affairs. Directory of veterans service organizations. https://www.va.gov/vso. Updated December 24, 2013. Accessed December 10, 2019.
69. ACL Administration for Community Living. Aging and disability resource centers. https://acl.gov/programs/aging-and-disability-networks/aging-and-disability-resource-centers. Updated December 13, 2017. Accessed December 10, 2019.
70. Nutrition and Obesity Policy Research and Evaluation Network (NOPREN). Clinical screening algorithms. https://nopren.org/resource/download-food-insecurity-screening-and-referral-algorithms-for-adults-patients-living-with-diabetes-and-pediatric-patients. Accessed December 10, 2019.
Nearly 1 in 8 households—and 1 in 6 households with children—experienced food insecurity in 2017, defined as limited or uncertain availability of nutritionally adequate and safe foods.1 Food insecurity is often even more pronounced among households with individuals with acute or chronic medical conditions.2-6 Moreover, food insecurity is independently associated with a range of adverse health outcomes, including poorer control of diabetes mellitus, hypertension, depression and other major psychiatric disorders, HIV, and chronic lung and kidney disease, as well as poorer overall health status.7-14 Food insecurity also has been associated with increased health care costs and acute care utilization as well as increased probability of delayed or missed care.15-19
The relationship between food insecurity and poor health outcomes is a complex and often cyclic phenomenon (Figure 1). Poor nutritional status is fueled by limited access to healthful foods as well as increased reliance on calorie-dense and nutrient-poor “junk” foods, which are less expensive and often more readily available in low-income neighborhoods.5,20-24 These compensatory dietary patterns place individuals at higher risk for developing cardiometabolic conditions and for poor control of these conditions.5,8,9,12,25,26 Additionally, the physiological and psychological stressors of food insecurity may precipitate depression and anxiety or worsen existing mental health conditions, resulting in feelings of overwhelm and decreased self-management capacity.5,8,27-31 Food insecurity has further been associated with poor sleep, declines in cognitive function, and increased falls, particularly among the frail and elderly.32-34
Individuals experiencing food insecurity often report having to make trade-offs between food and other necessities, such as paying rent or utilities. Additional strategies to stretch limited resources include cost-related underuse of medication and delays in needed medical care.4,17,31,35 In a nationally representative survey among adults with at least 1 chronic medical condition, 1 in 3 reported having to choose between food and medicine; 11% were unable to afford either.3 Furthermore, the inability to reliably adhere to medication regimens that need to be taken with food can result in potentially life-threatening hypoglycemia (as can lack of food regardless of medication use).5,26,36 In addition to the more obvious risks of glucose-lowering medications, such as insulin and long-acting sulfonylureas in patients experiencing food insecurity, many drugs commonly used among nondiabetic adults such as ACE-inhibitors, β blockers, quinolones, and salicylates can also precipitate hypoglycemia, and food insecurity has been associated with experiences of hypoglycemia even among individuals without diabetes mellitus.32,37 In one study the risk for hospital admissions for hypoglycemia among low-income populations increased by 27% at the end of the month when food budgets were more likely to be exhausted.38 Worsening health status and increased emergency department visits and hospitalizations may then result in lost wages and mounting medical bills, contributing to further financial strain and worsening food insecurity.
Prevalence and Importance of Food Insecurity Among US Veterans
Nearly 1.5 million veterans in the US are living below the federal poverty level (FPL).39 An additional 2.4 million veterans are living paycheck to paycheck at < 200% of the FPL.40 Veterans living in poverty are at even higher risk than nonveterans for food insecurity, homelessness, and other material hardship.41
Estimates of food insecurity among veterans vary widely, ranging from 6% to 24%—nearly twice that of the general US population.8,42-45 Higher rates of food insecurity have been reported among certain high-risk subgroups, including veterans who served in Iraq and Afghanistan (27%), female veterans (28%), homeless and formerly homeless veterans (49%), and veterans with serious mental illness (35%).6,32,43,46 Additional risk factors for food insecurity specific to veteran populations include younger age, having recently left active-duty military service, and lower final military paygrade.42,45-47 As in the general population, veteran food insecurity is associated with a range of adverse health outcomes, including poorer overall health status as well as increased probability of delayed or missed care.6,8,32,42-44,46
Even among veterans enrolled in federal food assistance programs, many still struggle to afford nutritionally adequate foods. As one example, in a study of mostly male homeless and formerly homeless veterans, O’Toole and colleagues found that nearly half of those reporting food insecurity were already receiving federal food assistance benefits, and 22% relied on emergency food resources.32 Of households served by Feeding America food pantries and meal programs, 20% have a member who has served in the US military.48
Federal Programs To Address Food Insecurity
There are several important federal food assistance programs designed to help alleviate food insecurity. The Supplemental Nutrition Assistance Program (SNAP, formerly the Food Stamp program) is the largest federal food assistance program and provides low-income Americans with cash benefits to purchase food. SNAP has been shown to substantially reduce food insecurity.7,49 The program also is associated with significant decreases in cost-related medication nonadherence as well as reductions in health care costs and both acute care and nursing home utilization.16,50-54 Although nearly 1.4 million veterans live in SNAP-enrolled households, 59% of eligible veterans are not enrolled.43,55 Closing this SNAP eligibility-enrollment gap, has been a focus of recent efforts to improve long-term food security among veterans. There also are several federal food assistance programs for households with children, including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and school meals programs. Among federal nutrition programs for seniors, the Older American’s Act contains designated funding to support nutrition services for older adults, including congregate meal programs in community settings like senior centers, places of worship, and housing communities, and home-delivered meals through programs like Meals on Wheels.56
VHA Response to Food Insecurity
The Veterans Health Administration (VHA) is the country’s largest integrated, federally funded health care system.57 In November 2015, congressional briefings on veteran food insecurity organized by the national non-profit organization MAZON: A Jewish Response to Hunger and hosted with bipartisan support were provided to the US House and Senate. As a result of these briefings, VHA chartered the national Ensuring Veteran Food Security Workgroup with a mandate to partner with governmental and nonprofit agencies to “focus on the issue of food insecurity, the identification of veterans at risk, the needed training of VHA staff and the coordination of resources and initiatives to support the veterans for whom we care.” Building off a pilot in US Department of Veterans Affairs (VA) Homeless Patient Aligned Care Teams (H-PACTs),32 VHA subsequently integrated a single-item food insecurity screening tool into the VA electronic health record (EHR) clinical reminder system (Figure 2). The clinical reminder, which was rolled out across VA medical centers nationally in October 2017, provides an alert to screen all noninstitutionalized veterans for food insecurity. To date, nearly 5 million veterans have been screened. When a veteran endorses food insecurity based on the initial screening question, a prompt appears to offer the veteran a referral to a social worker and/or dietitian. Positive screening results also should be communicated to the patient’s primary care provider. Depending on site-specific clinical flow, the reminders are typically completed in the outpatient setting either by nurses or medical assistants during intake or by providers as part of the clinical visit. However, any member of the health care team can complete the clinical reminder at any time. As of September 2019, approximately 74,000 veterans have been identified as food insecure.58
Addressing Food Insecurity
VHA has been a recognized leader in addressing homelessness and other social determinants of health through its integrated care and PACT delivery models.59-61 The food insecurity clinical reminder was designed to facilitate a tailored, interdisciplinary approach to identify and address food insecurity. Interdisciplinary care team members—including medical assistants, clinicians, social workers, registered dietitians, nurse care managers, occupational or physical therapists, and pharmacists—are uniquely positioned to identify veterans impacted by food insecurity, assess for associated clinical and/or social risk factors, and offer appropriate medical and nutrition interventions and resource referrals.
This interdisciplinary team-based model is essential given the range of potential drivers underlying veteran experiences of food insecurity and subsequent health outcomes. It is critically important for clinicians to review the medication list with veterans screening positive for food insecurity to assess for risk of hypoglycemia and/or cost-related nonadherence, make any necessary adjustments to therapeutic regimens, and assess for additional risk factors associated with food insecurity. Examples of tailored nutrition counseling that clinical dietitians may provide include meal preparation strategies for veterans who only have access to a microwave or hotplate, or recommendations for how veterans on medically restricted diets can best navigate food selection at soup kitchens or food pantries. Resource referrals provided by social workers or other care team members may include both emergency food resources to address immediate shortages (eg, food pantries, soup kitchens, or vouchers for free lunch) as well as resources focused on improving longer term food security (eg, federal food assistance programs or home delivered meal programs). Importantly, although providing a list of food resources may be helpful for some patients, such lists are often insufficient.62,63 Many patients require active assistance with program enrollment either onsite the day of their clinic visit or through connection with a partnering community-based organization that can, in turn, identify appropriate resources and facilitate program enrollment.63,64 Planned follow-up is also crucial to determine whether referrals are successful and to assess for ongoing need. Proposed roles for interdisciplinary care team members in addressing a positive food insecurity screen are outlined in Table 1.
VHA-Community Partnerships
In addition to services offered within VA, public and private sector partnerships can greatly enhance the range of resources available to food insecure veterans. Several VA facilities have developed formal community partnerships, such as the Veterans Pantry Pilot (VPP) program, a national partnership between Feeding America food banks and VA medical centers to establish onsite or mobile food pantries. There are currently 17 active Feeding America VPP sites, with a number of additional sites under development. Several of the VPP sites also include other “wraparound services,” such as SNAP application assistance.65,66
State Veterans Affairs offices67 and Veterans Service Organizations (VSOs)68 also can serve as valuable partners for connecting veterans with needed resources. VSOs offer a range of services, including assistancewith benefit claims, employment and housing assistance, emergency food assistance, and transportation to medical appointments. Some VSOs also have established local affiliations with Meals on Wheels focused on veteran outreach and providing hot meals for low-income, homebound, and disabled veterans.
Additional Resources
Although resources vary by regional setting, several key governmental and community-based food assistance programs are summarized in Table 2. Local community partners and online/phone-based directories, such as United Way’s 2-1-1 can help identify additional local resources. For older adults and individuals with disabilities, local Aging and Disability Resources Centers can provide information and assistance connecting to needed resources.69 Finally, there are a number of online resources available for clinicians interested in learning more about the impact of food insecurity on health and tools to use in the clinical setting (Table 3).
Conclusion
The VA has recognized food insecurity as a critical concern for the well-being of our nation’s veterans. Use of the EHR clinical reminder represents a crucial first step toward increasing provider awareness about veteran food insecurity and improving clinical efforts to address food insecurity once identified. Through the reminder, health care teams can connect veterans to needed resources and create both the individual and population-level data necessary to inform VHA and community efforts to address veteran food insecurity. Clinical reminder data are currently being used for local quality improvement efforts and have established the need nationally for formalized partnerships between VHA Social Work Services and Nutrition and Food Services to connect veterans with food and provide them with strategies to best use available food resources.
Moving forward, the Ensuring Veteran Food Security Workgroup continues to work with agencies and organizations across the country to improve food insecure veterans’ access to needed services. In addition to existing VA partnerships with Feeding America for the VPP, memorandums of understanding are currently underway to formalize partnerships with both the Food Research and Action Center (FRAC) and MAZON. Additional research is needed both to formally validate the current food insecurity clinical reminder screening question and to identify best practices and potential models for how to most effectively use VHA-community partnerships to address the unique needs of the veteran population.
Ensuring the food security of our nation’s veterans is essential to VA’s commitment to providing integrated, veteran-centered, whole person care. Toward that goal, VA health care teams are urged to use the clinical reminder and help connect food insecure veterans with relevant resources both within and outside of the VA health care system.
Nearly 1 in 8 households—and 1 in 6 households with children—experienced food insecurity in 2017, defined as limited or uncertain availability of nutritionally adequate and safe foods.1 Food insecurity is often even more pronounced among households with individuals with acute or chronic medical conditions.2-6 Moreover, food insecurity is independently associated with a range of adverse health outcomes, including poorer control of diabetes mellitus, hypertension, depression and other major psychiatric disorders, HIV, and chronic lung and kidney disease, as well as poorer overall health status.7-14 Food insecurity also has been associated with increased health care costs and acute care utilization as well as increased probability of delayed or missed care.15-19
The relationship between food insecurity and poor health outcomes is a complex and often cyclic phenomenon (Figure 1). Poor nutritional status is fueled by limited access to healthful foods as well as increased reliance on calorie-dense and nutrient-poor “junk” foods, which are less expensive and often more readily available in low-income neighborhoods.5,20-24 These compensatory dietary patterns place individuals at higher risk for developing cardiometabolic conditions and for poor control of these conditions.5,8,9,12,25,26 Additionally, the physiological and psychological stressors of food insecurity may precipitate depression and anxiety or worsen existing mental health conditions, resulting in feelings of overwhelm and decreased self-management capacity.5,8,27-31 Food insecurity has further been associated with poor sleep, declines in cognitive function, and increased falls, particularly among the frail and elderly.32-34
Individuals experiencing food insecurity often report having to make trade-offs between food and other necessities, such as paying rent or utilities. Additional strategies to stretch limited resources include cost-related underuse of medication and delays in needed medical care.4,17,31,35 In a nationally representative survey among adults with at least 1 chronic medical condition, 1 in 3 reported having to choose between food and medicine; 11% were unable to afford either.3 Furthermore, the inability to reliably adhere to medication regimens that need to be taken with food can result in potentially life-threatening hypoglycemia (as can lack of food regardless of medication use).5,26,36 In addition to the more obvious risks of glucose-lowering medications, such as insulin and long-acting sulfonylureas in patients experiencing food insecurity, many drugs commonly used among nondiabetic adults such as ACE-inhibitors, β blockers, quinolones, and salicylates can also precipitate hypoglycemia, and food insecurity has been associated with experiences of hypoglycemia even among individuals without diabetes mellitus.32,37 In one study the risk for hospital admissions for hypoglycemia among low-income populations increased by 27% at the end of the month when food budgets were more likely to be exhausted.38 Worsening health status and increased emergency department visits and hospitalizations may then result in lost wages and mounting medical bills, contributing to further financial strain and worsening food insecurity.
Prevalence and Importance of Food Insecurity Among US Veterans
Nearly 1.5 million veterans in the US are living below the federal poverty level (FPL).39 An additional 2.4 million veterans are living paycheck to paycheck at < 200% of the FPL.40 Veterans living in poverty are at even higher risk than nonveterans for food insecurity, homelessness, and other material hardship.41
Estimates of food insecurity among veterans vary widely, ranging from 6% to 24%—nearly twice that of the general US population.8,42-45 Higher rates of food insecurity have been reported among certain high-risk subgroups, including veterans who served in Iraq and Afghanistan (27%), female veterans (28%), homeless and formerly homeless veterans (49%), and veterans with serious mental illness (35%).6,32,43,46 Additional risk factors for food insecurity specific to veteran populations include younger age, having recently left active-duty military service, and lower final military paygrade.42,45-47 As in the general population, veteran food insecurity is associated with a range of adverse health outcomes, including poorer overall health status as well as increased probability of delayed or missed care.6,8,32,42-44,46
Even among veterans enrolled in federal food assistance programs, many still struggle to afford nutritionally adequate foods. As one example, in a study of mostly male homeless and formerly homeless veterans, O’Toole and colleagues found that nearly half of those reporting food insecurity were already receiving federal food assistance benefits, and 22% relied on emergency food resources.32 Of households served by Feeding America food pantries and meal programs, 20% have a member who has served in the US military.48
Federal Programs To Address Food Insecurity
There are several important federal food assistance programs designed to help alleviate food insecurity. The Supplemental Nutrition Assistance Program (SNAP, formerly the Food Stamp program) is the largest federal food assistance program and provides low-income Americans with cash benefits to purchase food. SNAP has been shown to substantially reduce food insecurity.7,49 The program also is associated with significant decreases in cost-related medication nonadherence as well as reductions in health care costs and both acute care and nursing home utilization.16,50-54 Although nearly 1.4 million veterans live in SNAP-enrolled households, 59% of eligible veterans are not enrolled.43,55 Closing this SNAP eligibility-enrollment gap, has been a focus of recent efforts to improve long-term food security among veterans. There also are several federal food assistance programs for households with children, including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and school meals programs. Among federal nutrition programs for seniors, the Older American’s Act contains designated funding to support nutrition services for older adults, including congregate meal programs in community settings like senior centers, places of worship, and housing communities, and home-delivered meals through programs like Meals on Wheels.56
VHA Response to Food Insecurity
The Veterans Health Administration (VHA) is the country’s largest integrated, federally funded health care system.57 In November 2015, congressional briefings on veteran food insecurity organized by the national non-profit organization MAZON: A Jewish Response to Hunger and hosted with bipartisan support were provided to the US House and Senate. As a result of these briefings, VHA chartered the national Ensuring Veteran Food Security Workgroup with a mandate to partner with governmental and nonprofit agencies to “focus on the issue of food insecurity, the identification of veterans at risk, the needed training of VHA staff and the coordination of resources and initiatives to support the veterans for whom we care.” Building off a pilot in US Department of Veterans Affairs (VA) Homeless Patient Aligned Care Teams (H-PACTs),32 VHA subsequently integrated a single-item food insecurity screening tool into the VA electronic health record (EHR) clinical reminder system (Figure 2). The clinical reminder, which was rolled out across VA medical centers nationally in October 2017, provides an alert to screen all noninstitutionalized veterans for food insecurity. To date, nearly 5 million veterans have been screened. When a veteran endorses food insecurity based on the initial screening question, a prompt appears to offer the veteran a referral to a social worker and/or dietitian. Positive screening results also should be communicated to the patient’s primary care provider. Depending on site-specific clinical flow, the reminders are typically completed in the outpatient setting either by nurses or medical assistants during intake or by providers as part of the clinical visit. However, any member of the health care team can complete the clinical reminder at any time. As of September 2019, approximately 74,000 veterans have been identified as food insecure.58
Addressing Food Insecurity
VHA has been a recognized leader in addressing homelessness and other social determinants of health through its integrated care and PACT delivery models.59-61 The food insecurity clinical reminder was designed to facilitate a tailored, interdisciplinary approach to identify and address food insecurity. Interdisciplinary care team members—including medical assistants, clinicians, social workers, registered dietitians, nurse care managers, occupational or physical therapists, and pharmacists—are uniquely positioned to identify veterans impacted by food insecurity, assess for associated clinical and/or social risk factors, and offer appropriate medical and nutrition interventions and resource referrals.
This interdisciplinary team-based model is essential given the range of potential drivers underlying veteran experiences of food insecurity and subsequent health outcomes. It is critically important for clinicians to review the medication list with veterans screening positive for food insecurity to assess for risk of hypoglycemia and/or cost-related nonadherence, make any necessary adjustments to therapeutic regimens, and assess for additional risk factors associated with food insecurity. Examples of tailored nutrition counseling that clinical dietitians may provide include meal preparation strategies for veterans who only have access to a microwave or hotplate, or recommendations for how veterans on medically restricted diets can best navigate food selection at soup kitchens or food pantries. Resource referrals provided by social workers or other care team members may include both emergency food resources to address immediate shortages (eg, food pantries, soup kitchens, or vouchers for free lunch) as well as resources focused on improving longer term food security (eg, federal food assistance programs or home delivered meal programs). Importantly, although providing a list of food resources may be helpful for some patients, such lists are often insufficient.62,63 Many patients require active assistance with program enrollment either onsite the day of their clinic visit or through connection with a partnering community-based organization that can, in turn, identify appropriate resources and facilitate program enrollment.63,64 Planned follow-up is also crucial to determine whether referrals are successful and to assess for ongoing need. Proposed roles for interdisciplinary care team members in addressing a positive food insecurity screen are outlined in Table 1.
VHA-Community Partnerships
In addition to services offered within VA, public and private sector partnerships can greatly enhance the range of resources available to food insecure veterans. Several VA facilities have developed formal community partnerships, such as the Veterans Pantry Pilot (VPP) program, a national partnership between Feeding America food banks and VA medical centers to establish onsite or mobile food pantries. There are currently 17 active Feeding America VPP sites, with a number of additional sites under development. Several of the VPP sites also include other “wraparound services,” such as SNAP application assistance.65,66
State Veterans Affairs offices67 and Veterans Service Organizations (VSOs)68 also can serve as valuable partners for connecting veterans with needed resources. VSOs offer a range of services, including assistancewith benefit claims, employment and housing assistance, emergency food assistance, and transportation to medical appointments. Some VSOs also have established local affiliations with Meals on Wheels focused on veteran outreach and providing hot meals for low-income, homebound, and disabled veterans.
Additional Resources
Although resources vary by regional setting, several key governmental and community-based food assistance programs are summarized in Table 2. Local community partners and online/phone-based directories, such as United Way’s 2-1-1 can help identify additional local resources. For older adults and individuals with disabilities, local Aging and Disability Resources Centers can provide information and assistance connecting to needed resources.69 Finally, there are a number of online resources available for clinicians interested in learning more about the impact of food insecurity on health and tools to use in the clinical setting (Table 3).
Conclusion
The VA has recognized food insecurity as a critical concern for the well-being of our nation’s veterans. Use of the EHR clinical reminder represents a crucial first step toward increasing provider awareness about veteran food insecurity and improving clinical efforts to address food insecurity once identified. Through the reminder, health care teams can connect veterans to needed resources and create both the individual and population-level data necessary to inform VHA and community efforts to address veteran food insecurity. Clinical reminder data are currently being used for local quality improvement efforts and have established the need nationally for formalized partnerships between VHA Social Work Services and Nutrition and Food Services to connect veterans with food and provide them with strategies to best use available food resources.
Moving forward, the Ensuring Veteran Food Security Workgroup continues to work with agencies and organizations across the country to improve food insecure veterans’ access to needed services. In addition to existing VA partnerships with Feeding America for the VPP, memorandums of understanding are currently underway to formalize partnerships with both the Food Research and Action Center (FRAC) and MAZON. Additional research is needed both to formally validate the current food insecurity clinical reminder screening question and to identify best practices and potential models for how to most effectively use VHA-community partnerships to address the unique needs of the veteran population.
Ensuring the food security of our nation’s veterans is essential to VA’s commitment to providing integrated, veteran-centered, whole person care. Toward that goal, VA health care teams are urged to use the clinical reminder and help connect food insecure veterans with relevant resources both within and outside of the VA health care system.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. http://www.ers.usda.gov/publications/pub-details/?pubid=90022. Published September 2018. Accessed December 9, 2019.
2. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257-265.
3. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303-310.e3.
4. Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610-624.
5. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6-9.
6. Narain K, Bean-Mayberry B, Washington DL, Canelo IA, Darling JE, Yano EM. Access to care and health outcomes among women veterans using veterans administration health care: association with food insufficiency. Womens Health Issues. 2018;28(3):267-272.
7. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34(11):1830-1839.
8. Wang EA, McGinnis KA, Goulet J, et al; Veterans Aging Cohort Study Project Team. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268.
9. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005-2012. PloS One. 2017;12(6):e0179172.
10. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304-310.
11. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093-3099.
12. Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233-238.
13. Banerjee T, Crews DC, Wesson DE, et al; CDC CKD Surveillance Team. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38-47.
14. Bruening M, Dinour LM, Chavez JBR. Food insecurity and emotional health in the USA: a systematic narrative review of longitudinal research. Public Health Nutr. 2017;20(17):3200-3208.
15. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011-2013. Health Serv Res. 2018;53(3):1600-1620.
16. Berkowitz SA, Seligman HK, Basu S. Impact of food insecurity and SNAP participation on healthcare utilization and expenditures. http://www.ukcpr.org/research/discussion-papers. Published 2017. Accessed December 9, 2019.
17. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71-77.
18. Garcia SP, Haddix A, Barnett K. Incremental health care costs associated with food insecurity and chronic conditions among older adults. Chronic Dis. 2018;15:180058.
19. Berkowitz SA, Seligman HK, Meigs JB, Basu S. Food insecurity, healthcare utilization, and high cost: a longitudinal cohort study. Am J Manag Care. 2018;24(9):399-404.
20. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74-81.
21. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643-660.
22. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117.
23. Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181-1188.
24. Lucan SC, Maroko AR, Seitchik JL, Yoon DH, Sperry LE, Schechter CB. Unexpected neighborhood sources of food and drink: implications for research and community health. Am J Prev Med. 2018;55(2):e29-e38.
25. Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245-250.
26. Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227-1233.
27. Siefert K, Heflin CM, Corcoran ME, Williams DR. Food insufficiency and physical and mental health in a longitudinal survey of welfare recipients. J Health Soc Behav. 2004;45(2):171-186.
28. Mangurian C, Sreshta N, Seligman H. Food insecurity among adults with severe mental illness. Psychiatr Serv. 2013;64(9):931-932.
29. Melchior M, Caspi A, Howard LM, et al. Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics. 2009;124(4):e564-e572.
30. Brostow DP, Gunzburger E, Abbate LM, Brenner LA, Thomas KS. Mental illness, not obesity status, is associated with food insecurity among the elderly in the health and retirement study. J Nutr Gerontol Geriatr. 2019;38(2):149-172.
31. Higashi RT, Craddock Lee SJ, Pezzia C, Quirk L, Leonard T, Pruitt SL. Family and social context contributes to the interplay of economic insecurity, food insecurity, and health. Ann Anthropol Pract. 2017;41(2):67-77.
32. O’Toole TP, Roberts CB, Johnson EE. Screening for food insecurity in six Veterans Administration clinics for the homeless, June-December 2015. Prev Chronic Dis. 2017;14:160375.
33. Feil DG, Pogach LM. Cognitive impairment is a major risk factor for serious hypoglycaemia; public health intervention is warranted. Evid Based Med. 2014;19(2):77.
34. Frith E, Loprinzi PD. Food insecurity and cognitive function in older adults: Brief report. Clin Nutr. 2018;37(5):1765-1768.
35. Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. Am J Public Health. 2015;105(10):e48-e59.
36. Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Vue MH, Setter SM. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177.
38. Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33(1):116-123.
39. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran poverty trends. https://www.va.gov/vetdata/docs/specialreports/veteran_poverty_trends.pdf. Published May 2015. Accessed December 9, 2019.
40. Robbins KG, Ravi A. Veterans living paycheck to paycheck are under threat during budget debates. https://www.americanprogress.org/issues/poverty/news/2017/09/19/439023/veterans-living-paycheck-paycheck-threat-budget-debates. Published September 19, 2017. Accessed December 9, 2019.
41. Wilmoth JM, London AS, Heflin CM. Economic well-being among older-adult households: variation by veteran and disability status. J Gerontol Soc Work. 2015;58(4):399-419.
42. Brostow DP, Gunzburger E, Thomas KS. Food insecurity among veterans: findings from the health and retirement study. J Nutr Health Aging. 2017;21(10):1358-1364.
43. Pooler J, Mian P, Srinivasan M, Miller Z. Veterans and food insecurity. https://www.impaqint.com/sites/default/files/issue-briefs/VeteransFoodInsecurity_IssueBrief_V1.3.pdf. Published November 2018. Accessed December 9, 2019.
44. Schure MB, Katon JG, Wong E, Liu C-F. Food and housing insecurity and health status among U.S. adults with and without prior military service. SSM Popul Health. 2016;29(2):244-248.
45. Miller DP, Larson MJ, Byrne T, DeVoe E. Food insecurity in veteran households: findings from nationally representative data. Public Health Nutr. 2016;19(10):1731-1740.
46. Widome R, Jensen A, Bangerter A, Fu SS. Food insecurity among veterans of the US wars in Iraq and Afghanistan. Public Health Nutr. 2015;18(5):844-849.
47. London AS, Heflin CM. Supplemental Nutrition Assistance Program (SNAP) use among active-duty military personnel, veterans, and reservists. Popul Res Policy Rev. 2015;34(6):805-826.
48. Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014. Natl rep prepared for Feeding America. https://www.feedingamerica.org/research/hunger-in-america. Published 2014. Accessed December 9, 2019.
49. Mabli J, Ohls J, Dragoset L, Castner L, Santos B. Measuring the Effect of Supplemental Nutrition Assistance Program (SNAP) Participation on Food Security. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2013.
50. Srinivasan M, Pooler JA. Cost-related medication nonadherence for older adults participating in SNAP, 2013–2015. Am J Public Health. 2017;108(2):224-230.
51. Heflin C, Hodges L, Mueser P. Supplemental Nutrition Assistance Progam benefits and emergency room visits for hypoglycaemia. Public Health Nutr. 2017;20(7):1314-1321.
52. Samuel LJ, Szanton SL, Cahill R, et al. Does the Supplemental Nutrition Assistance Program affect hospital utilization among older adults? The case of Maryland. Popul Health Manag. 2018;21(2):88-95.
53. Szanton SL, Samuel LJ, Cahill R, et al. Food assistance is associated with decreased nursing home admissions for Maryland’s dually eligible older adults. BMC Geriatr. 2017;17(1):162.
54. Carlson S, Keith-Jennings B. SNAP is linked with improved nutritional outcomes and lower health care costs. https://www.cbpp.org/research/food-assistance/snap-is-linked-with-improved-nutritional-outcomes-and-lower-health-care. Published January 17, 2018. Accessed December 10, 2019.
55. Keith-Jennings B, Cai L. SNAP helps almost 1.4 million low-income veterans, including thousands in every state. https://www.cbpp.org/research/food-assistance/snap-helps-almost-14-million-low-income-veterans-including-thousands-in. Updated November 8, 2018. Accessed December 10, 2019.
56. US Department of Health and Human Services. Older Americans Act nutrition programs. https://acl.gov/sites/default/files/news%202017-03/OAA-Nutrition_Programs_Fact_Sheet.pdf. Accessed December 10, 2019.
57. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed December 10, 2019.
58. US Department of Veterans Affairs. VA Corporate Data Warehouse.
59. Yano EM, Bair MJ, Carrasquillo O, Krein SL, Rubenstein LV. Patient aligned care teams (PACT): VA’s journey to implement patient-centered medical homes. J Gen Intern Med. 2014;29(suppl 2):S547-s549.
60. O’Toole TP, Pape L. Innovative efforts to address homelessness among veterans. N C Med J. 2015;76(5):311-314.
61. O’Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” Program. Prev Chronic Dis. 2016;13:150567.
62. Marpadga S, Fernandez A, Leung J, Tang A, Seligman H, Murphy EJ. Challenges and successes with food resource referrals for food-insecure patients with diabetes. Perm J. 2019;23.
63. Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22.
64. Martel ML, Klein LR, Hager KA, Cutts DB. Emergency department experience with novel electronic medical record order for referral to food resources. West J Emerg Med. 2018;19(2):232-237.
65. Going C, Cohen AJ, Bares M, Christensen M. Interdisciplinary approaches to addressing the food insecure veteran. Veterans Health Administration Employee Education System webinar; October 30, 2018.
66. Feeding America Announces New Partnership With U.S. Department Of Veterans Affairs. https://www.prnewswire.com/news-releases/feeding-america-announces-new-partnership-with-us-department-of-veterans-affairs-300481891.html. Published June 29, 2017. Accessed December 10, 2019.
67. US Department of Veterans Affairs. State Veterans Affairs offices. https://www.va.gov/statedva.htm. Updated March 20, 2019. Accessed December 10, 2019.
68. US Department of Veterans Affairs. Directory of veterans service organizations. https://www.va.gov/vso. Updated December 24, 2013. Accessed December 10, 2019.
69. ACL Administration for Community Living. Aging and disability resource centers. https://acl.gov/programs/aging-and-disability-networks/aging-and-disability-resource-centers. Updated December 13, 2017. Accessed December 10, 2019.
70. Nutrition and Obesity Policy Research and Evaluation Network (NOPREN). Clinical screening algorithms. https://nopren.org/resource/download-food-insecurity-screening-and-referral-algorithms-for-adults-patients-living-with-diabetes-and-pediatric-patients. Accessed December 10, 2019.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. http://www.ers.usda.gov/publications/pub-details/?pubid=90022. Published September 2018. Accessed December 9, 2019.
2. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257-265.
3. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303-310.e3.
4. Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610-624.
5. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6-9.
6. Narain K, Bean-Mayberry B, Washington DL, Canelo IA, Darling JE, Yano EM. Access to care and health outcomes among women veterans using veterans administration health care: association with food insufficiency. Womens Health Issues. 2018;28(3):267-272.
7. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34(11):1830-1839.
8. Wang EA, McGinnis KA, Goulet J, et al; Veterans Aging Cohort Study Project Team. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268.
9. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005-2012. PloS One. 2017;12(6):e0179172.
10. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304-310.
11. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093-3099.
12. Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233-238.
13. Banerjee T, Crews DC, Wesson DE, et al; CDC CKD Surveillance Team. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38-47.
14. Bruening M, Dinour LM, Chavez JBR. Food insecurity and emotional health in the USA: a systematic narrative review of longitudinal research. Public Health Nutr. 2017;20(17):3200-3208.
15. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011-2013. Health Serv Res. 2018;53(3):1600-1620.
16. Berkowitz SA, Seligman HK, Basu S. Impact of food insecurity and SNAP participation on healthcare utilization and expenditures. http://www.ukcpr.org/research/discussion-papers. Published 2017. Accessed December 9, 2019.
17. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71-77.
18. Garcia SP, Haddix A, Barnett K. Incremental health care costs associated with food insecurity and chronic conditions among older adults. Chronic Dis. 2018;15:180058.
19. Berkowitz SA, Seligman HK, Meigs JB, Basu S. Food insecurity, healthcare utilization, and high cost: a longitudinal cohort study. Am J Manag Care. 2018;24(9):399-404.
20. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74-81.
21. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643-660.
22. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117.
23. Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181-1188.
24. Lucan SC, Maroko AR, Seitchik JL, Yoon DH, Sperry LE, Schechter CB. Unexpected neighborhood sources of food and drink: implications for research and community health. Am J Prev Med. 2018;55(2):e29-e38.
25. Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245-250.
26. Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227-1233.
27. Siefert K, Heflin CM, Corcoran ME, Williams DR. Food insufficiency and physical and mental health in a longitudinal survey of welfare recipients. J Health Soc Behav. 2004;45(2):171-186.
28. Mangurian C, Sreshta N, Seligman H. Food insecurity among adults with severe mental illness. Psychiatr Serv. 2013;64(9):931-932.
29. Melchior M, Caspi A, Howard LM, et al. Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics. 2009;124(4):e564-e572.
30. Brostow DP, Gunzburger E, Abbate LM, Brenner LA, Thomas KS. Mental illness, not obesity status, is associated with food insecurity among the elderly in the health and retirement study. J Nutr Gerontol Geriatr. 2019;38(2):149-172.
31. Higashi RT, Craddock Lee SJ, Pezzia C, Quirk L, Leonard T, Pruitt SL. Family and social context contributes to the interplay of economic insecurity, food insecurity, and health. Ann Anthropol Pract. 2017;41(2):67-77.
32. O’Toole TP, Roberts CB, Johnson EE. Screening for food insecurity in six Veterans Administration clinics for the homeless, June-December 2015. Prev Chronic Dis. 2017;14:160375.
33. Feil DG, Pogach LM. Cognitive impairment is a major risk factor for serious hypoglycaemia; public health intervention is warranted. Evid Based Med. 2014;19(2):77.
34. Frith E, Loprinzi PD. Food insecurity and cognitive function in older adults: Brief report. Clin Nutr. 2018;37(5):1765-1768.
35. Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. Am J Public Health. 2015;105(10):e48-e59.
36. Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Vue MH, Setter SM. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177.
38. Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33(1):116-123.
39. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran poverty trends. https://www.va.gov/vetdata/docs/specialreports/veteran_poverty_trends.pdf. Published May 2015. Accessed December 9, 2019.
40. Robbins KG, Ravi A. Veterans living paycheck to paycheck are under threat during budget debates. https://www.americanprogress.org/issues/poverty/news/2017/09/19/439023/veterans-living-paycheck-paycheck-threat-budget-debates. Published September 19, 2017. Accessed December 9, 2019.
41. Wilmoth JM, London AS, Heflin CM. Economic well-being among older-adult households: variation by veteran and disability status. J Gerontol Soc Work. 2015;58(4):399-419.
42. Brostow DP, Gunzburger E, Thomas KS. Food insecurity among veterans: findings from the health and retirement study. J Nutr Health Aging. 2017;21(10):1358-1364.
43. Pooler J, Mian P, Srinivasan M, Miller Z. Veterans and food insecurity. https://www.impaqint.com/sites/default/files/issue-briefs/VeteransFoodInsecurity_IssueBrief_V1.3.pdf. Published November 2018. Accessed December 9, 2019.
44. Schure MB, Katon JG, Wong E, Liu C-F. Food and housing insecurity and health status among U.S. adults with and without prior military service. SSM Popul Health. 2016;29(2):244-248.
45. Miller DP, Larson MJ, Byrne T, DeVoe E. Food insecurity in veteran households: findings from nationally representative data. Public Health Nutr. 2016;19(10):1731-1740.
46. Widome R, Jensen A, Bangerter A, Fu SS. Food insecurity among veterans of the US wars in Iraq and Afghanistan. Public Health Nutr. 2015;18(5):844-849.
47. London AS, Heflin CM. Supplemental Nutrition Assistance Program (SNAP) use among active-duty military personnel, veterans, and reservists. Popul Res Policy Rev. 2015;34(6):805-826.
48. Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014. Natl rep prepared for Feeding America. https://www.feedingamerica.org/research/hunger-in-america. Published 2014. Accessed December 9, 2019.
49. Mabli J, Ohls J, Dragoset L, Castner L, Santos B. Measuring the Effect of Supplemental Nutrition Assistance Program (SNAP) Participation on Food Security. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2013.
50. Srinivasan M, Pooler JA. Cost-related medication nonadherence for older adults participating in SNAP, 2013–2015. Am J Public Health. 2017;108(2):224-230.
51. Heflin C, Hodges L, Mueser P. Supplemental Nutrition Assistance Progam benefits and emergency room visits for hypoglycaemia. Public Health Nutr. 2017;20(7):1314-1321.
52. Samuel LJ, Szanton SL, Cahill R, et al. Does the Supplemental Nutrition Assistance Program affect hospital utilization among older adults? The case of Maryland. Popul Health Manag. 2018;21(2):88-95.
53. Szanton SL, Samuel LJ, Cahill R, et al. Food assistance is associated with decreased nursing home admissions for Maryland’s dually eligible older adults. BMC Geriatr. 2017;17(1):162.
54. Carlson S, Keith-Jennings B. SNAP is linked with improved nutritional outcomes and lower health care costs. https://www.cbpp.org/research/food-assistance/snap-is-linked-with-improved-nutritional-outcomes-and-lower-health-care. Published January 17, 2018. Accessed December 10, 2019.
55. Keith-Jennings B, Cai L. SNAP helps almost 1.4 million low-income veterans, including thousands in every state. https://www.cbpp.org/research/food-assistance/snap-helps-almost-14-million-low-income-veterans-including-thousands-in. Updated November 8, 2018. Accessed December 10, 2019.
56. US Department of Health and Human Services. Older Americans Act nutrition programs. https://acl.gov/sites/default/files/news%202017-03/OAA-Nutrition_Programs_Fact_Sheet.pdf. Accessed December 10, 2019.
57. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed December 10, 2019.
58. US Department of Veterans Affairs. VA Corporate Data Warehouse.
59. Yano EM, Bair MJ, Carrasquillo O, Krein SL, Rubenstein LV. Patient aligned care teams (PACT): VA’s journey to implement patient-centered medical homes. J Gen Intern Med. 2014;29(suppl 2):S547-s549.
60. O’Toole TP, Pape L. Innovative efforts to address homelessness among veterans. N C Med J. 2015;76(5):311-314.
61. O’Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” Program. Prev Chronic Dis. 2016;13:150567.
62. Marpadga S, Fernandez A, Leung J, Tang A, Seligman H, Murphy EJ. Challenges and successes with food resource referrals for food-insecure patients with diabetes. Perm J. 2019;23.
63. Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22.
64. Martel ML, Klein LR, Hager KA, Cutts DB. Emergency department experience with novel electronic medical record order for referral to food resources. West J Emerg Med. 2018;19(2):232-237.
65. Going C, Cohen AJ, Bares M, Christensen M. Interdisciplinary approaches to addressing the food insecure veteran. Veterans Health Administration Employee Education System webinar; October 30, 2018.
66. Feeding America Announces New Partnership With U.S. Department Of Veterans Affairs. https://www.prnewswire.com/news-releases/feeding-america-announces-new-partnership-with-us-department-of-veterans-affairs-300481891.html. Published June 29, 2017. Accessed December 10, 2019.
67. US Department of Veterans Affairs. State Veterans Affairs offices. https://www.va.gov/statedva.htm. Updated March 20, 2019. Accessed December 10, 2019.
68. US Department of Veterans Affairs. Directory of veterans service organizations. https://www.va.gov/vso. Updated December 24, 2013. Accessed December 10, 2019.
69. ACL Administration for Community Living. Aging and disability resource centers. https://acl.gov/programs/aging-and-disability-networks/aging-and-disability-resource-centers. Updated December 13, 2017. Accessed December 10, 2019.
70. Nutrition and Obesity Policy Research and Evaluation Network (NOPREN). Clinical screening algorithms. https://nopren.org/resource/download-food-insecurity-screening-and-referral-algorithms-for-adults-patients-living-with-diabetes-and-pediatric-patients. Accessed December 10, 2019.