User login
Deprescribing: Nicolas Badre
In this episode of the MDedge Psychcast, Dr. Lorenzo Norris speaks with Dr. Nicolas Badre about ways to approach reducing dosages or discontinuing medications that are not beneficial. Dr. Badre, who has written about “deprescribing,” is a forensic psychiatrist who holds teaching positions at the University of California San Diego, and the University of San Diego.
Subscribe here:
Amazon
Apple Podcasts
Google Podcasts
Spotify
In this episode of the MDedge Psychcast, Dr. Lorenzo Norris speaks with Dr. Nicolas Badre about ways to approach reducing dosages or discontinuing medications that are not beneficial. Dr. Badre, who has written about “deprescribing,” is a forensic psychiatrist who holds teaching positions at the University of California San Diego, and the University of San Diego.
Subscribe here:
Amazon
Apple Podcasts
Google Podcasts
Spotify
In this episode of the MDedge Psychcast, Dr. Lorenzo Norris speaks with Dr. Nicolas Badre about ways to approach reducing dosages or discontinuing medications that are not beneficial. Dr. Badre, who has written about “deprescribing,” is a forensic psychiatrist who holds teaching positions at the University of California San Diego, and the University of San Diego.
Subscribe here:
Amazon
Apple Podcasts
Google Podcasts
Spotify
Large survey reveals that few MS patients have long-term care insurance
DALLAS – A number of sociodemographic factors may influence health and disability insurance access by individuals with multiple sclerosis, including employment, age, gender, disease duration, marital status, and ethnicity, results from a large survey suggest.
“The last similar work was conducted over 10 years ago and so much has happened in the meantime, including the Great Recession and the introduction of the Affordable Care Act, that offers protection for health care but not for other important types of insurance (short- and long-term disability, long-term care, and life),” lead study author Sarah Planchon, PhD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “MS is one of the most costly chronic diseases today. That is not only because of the cost of disease-modifying therapies but also because of lost employment and income. We wanted to better understand the insurance landscape so that we could in turn educate patients and professionals about the protection these insurances offer and advise them on how to obtain these policies.”
In an effort to evaluate factors that affect insurance access in MS, Dr. Planchon, a project scientist at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic in Ohio, and her colleagues used the North American Research Committee on MS (NARCOMS), iConquerMS, and the National Multiple Sclerosis Society to survey 2,507 individuals with the disease regarding insurance, demographic, health, disability, and employment status. They used covariate-adjusted nominal logistic regression to estimate odds ratios for the likelihood of having or not having a type of insurance. The majority of respondents (83%) were female, their mean age was 54 years, 91% were white, 65% were currently married, and their mean disease duration at the time of the survey was 16 years. In addition, 43% were employed full/part-time, and 29% were not employed or retired because of disability. Nearly all respondents (96%) reported having health insurance, while 59% had life insurance, 29% had private long-term disability insurance, 18% had short-term disability insurance, and 10% had long-term care insurance.
The researchers found that employment status had the greatest impact on insurance coverage. Of those with health insurance, 33% were employed full-time, compared with 89% of those with short-term disability insurance, 42% of those with private long-term disability insurance, 44% of those with long-term care insurance, and 41% of those with life insurance. Logistic regression analyses indicated that respondents employed part time were significantly more likely to have short-term disability insurance if they were currently married (odds ratio, 4.4). Short-term disability insurance was significantly more likely among fully employed patients with disease duration of 5-10 years vs. more than 20 years (OR, 2.0). Private long-term disability insurance was significantly associated with female gender (OR, 1.6), age 50-59 years vs. younger than 40 (OR, 1.6), full-time vs. part-time employment (OR, 2.3), and shorter disease duration (ORs, 1.4-1.6 for 6-10, 11-15, and 16-20 years’ duration). Long-term care insurance was associated with older age (ORs, 2.5 and 4.3 for those aged 50-59 and 60-65 vs. younger than 40), and having excellent or good general health status vs. fair or poor (OR, 1.8). Life insurance was associated with non-Hispanic ethnicity (OR, 1.6), full-time vs. part-time employment (OR, 2.4), older age (ORs, 1.6-1.7 for ages 40-49 and 50-59 vs. younger than 40), and marital status (currently/previously married, ORs, 1.6-2.6). Considering the high rate of survey respondents with health insurance, covariate-adjusted modeling was not applicable.
“The number of people with MS who do not have long-term care insurance was surprisingly high,” Dr. Planchon said. “Although the improved treatment climate recently may decrease the long-term disability levels, we do not yet know this with certainty. A large number of people with MS are likely to need long-term care in the future, which often is a significant financial burden to families.” The findings suggest that clinical care teams “need to initiate early discussions of possible long-term needs with their patients,” she continued. “Incorporation of social work teams, who are familiar with the needs of people with MS and insurance options available to them, within MS specialty practices will bolster the comprehensive care of patients and their families.”
She acknowledged certain limitations of the study, including the low proportion of respondents who were Hispanic/Latino and African American (about 4% each). “The insurance landscape may differ in these groups compared to the majority Caucasian population who responded to this survey,” Dr. Planchon said.
The National Multiple Sclerosis Society funded the study. Dr. Planchon reported having no relevant financial disclosures.
SOURCE: Planchon S et al. ACTRIMS Forum 2019, Abstract P295.
DALLAS – A number of sociodemographic factors may influence health and disability insurance access by individuals with multiple sclerosis, including employment, age, gender, disease duration, marital status, and ethnicity, results from a large survey suggest.
“The last similar work was conducted over 10 years ago and so much has happened in the meantime, including the Great Recession and the introduction of the Affordable Care Act, that offers protection for health care but not for other important types of insurance (short- and long-term disability, long-term care, and life),” lead study author Sarah Planchon, PhD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “MS is one of the most costly chronic diseases today. That is not only because of the cost of disease-modifying therapies but also because of lost employment and income. We wanted to better understand the insurance landscape so that we could in turn educate patients and professionals about the protection these insurances offer and advise them on how to obtain these policies.”
In an effort to evaluate factors that affect insurance access in MS, Dr. Planchon, a project scientist at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic in Ohio, and her colleagues used the North American Research Committee on MS (NARCOMS), iConquerMS, and the National Multiple Sclerosis Society to survey 2,507 individuals with the disease regarding insurance, demographic, health, disability, and employment status. They used covariate-adjusted nominal logistic regression to estimate odds ratios for the likelihood of having or not having a type of insurance. The majority of respondents (83%) were female, their mean age was 54 years, 91% were white, 65% were currently married, and their mean disease duration at the time of the survey was 16 years. In addition, 43% were employed full/part-time, and 29% were not employed or retired because of disability. Nearly all respondents (96%) reported having health insurance, while 59% had life insurance, 29% had private long-term disability insurance, 18% had short-term disability insurance, and 10% had long-term care insurance.
The researchers found that employment status had the greatest impact on insurance coverage. Of those with health insurance, 33% were employed full-time, compared with 89% of those with short-term disability insurance, 42% of those with private long-term disability insurance, 44% of those with long-term care insurance, and 41% of those with life insurance. Logistic regression analyses indicated that respondents employed part time were significantly more likely to have short-term disability insurance if they were currently married (odds ratio, 4.4). Short-term disability insurance was significantly more likely among fully employed patients with disease duration of 5-10 years vs. more than 20 years (OR, 2.0). Private long-term disability insurance was significantly associated with female gender (OR, 1.6), age 50-59 years vs. younger than 40 (OR, 1.6), full-time vs. part-time employment (OR, 2.3), and shorter disease duration (ORs, 1.4-1.6 for 6-10, 11-15, and 16-20 years’ duration). Long-term care insurance was associated with older age (ORs, 2.5 and 4.3 for those aged 50-59 and 60-65 vs. younger than 40), and having excellent or good general health status vs. fair or poor (OR, 1.8). Life insurance was associated with non-Hispanic ethnicity (OR, 1.6), full-time vs. part-time employment (OR, 2.4), older age (ORs, 1.6-1.7 for ages 40-49 and 50-59 vs. younger than 40), and marital status (currently/previously married, ORs, 1.6-2.6). Considering the high rate of survey respondents with health insurance, covariate-adjusted modeling was not applicable.
“The number of people with MS who do not have long-term care insurance was surprisingly high,” Dr. Planchon said. “Although the improved treatment climate recently may decrease the long-term disability levels, we do not yet know this with certainty. A large number of people with MS are likely to need long-term care in the future, which often is a significant financial burden to families.” The findings suggest that clinical care teams “need to initiate early discussions of possible long-term needs with their patients,” she continued. “Incorporation of social work teams, who are familiar with the needs of people with MS and insurance options available to them, within MS specialty practices will bolster the comprehensive care of patients and their families.”
She acknowledged certain limitations of the study, including the low proportion of respondents who were Hispanic/Latino and African American (about 4% each). “The insurance landscape may differ in these groups compared to the majority Caucasian population who responded to this survey,” Dr. Planchon said.
The National Multiple Sclerosis Society funded the study. Dr. Planchon reported having no relevant financial disclosures.
SOURCE: Planchon S et al. ACTRIMS Forum 2019, Abstract P295.
DALLAS – A number of sociodemographic factors may influence health and disability insurance access by individuals with multiple sclerosis, including employment, age, gender, disease duration, marital status, and ethnicity, results from a large survey suggest.
“The last similar work was conducted over 10 years ago and so much has happened in the meantime, including the Great Recession and the introduction of the Affordable Care Act, that offers protection for health care but not for other important types of insurance (short- and long-term disability, long-term care, and life),” lead study author Sarah Planchon, PhD, said in an interview in advance of the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “MS is one of the most costly chronic diseases today. That is not only because of the cost of disease-modifying therapies but also because of lost employment and income. We wanted to better understand the insurance landscape so that we could in turn educate patients and professionals about the protection these insurances offer and advise them on how to obtain these policies.”
In an effort to evaluate factors that affect insurance access in MS, Dr. Planchon, a project scientist at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic in Ohio, and her colleagues used the North American Research Committee on MS (NARCOMS), iConquerMS, and the National Multiple Sclerosis Society to survey 2,507 individuals with the disease regarding insurance, demographic, health, disability, and employment status. They used covariate-adjusted nominal logistic regression to estimate odds ratios for the likelihood of having or not having a type of insurance. The majority of respondents (83%) were female, their mean age was 54 years, 91% were white, 65% were currently married, and their mean disease duration at the time of the survey was 16 years. In addition, 43% were employed full/part-time, and 29% were not employed or retired because of disability. Nearly all respondents (96%) reported having health insurance, while 59% had life insurance, 29% had private long-term disability insurance, 18% had short-term disability insurance, and 10% had long-term care insurance.
The researchers found that employment status had the greatest impact on insurance coverage. Of those with health insurance, 33% were employed full-time, compared with 89% of those with short-term disability insurance, 42% of those with private long-term disability insurance, 44% of those with long-term care insurance, and 41% of those with life insurance. Logistic regression analyses indicated that respondents employed part time were significantly more likely to have short-term disability insurance if they were currently married (odds ratio, 4.4). Short-term disability insurance was significantly more likely among fully employed patients with disease duration of 5-10 years vs. more than 20 years (OR, 2.0). Private long-term disability insurance was significantly associated with female gender (OR, 1.6), age 50-59 years vs. younger than 40 (OR, 1.6), full-time vs. part-time employment (OR, 2.3), and shorter disease duration (ORs, 1.4-1.6 for 6-10, 11-15, and 16-20 years’ duration). Long-term care insurance was associated with older age (ORs, 2.5 and 4.3 for those aged 50-59 and 60-65 vs. younger than 40), and having excellent or good general health status vs. fair or poor (OR, 1.8). Life insurance was associated with non-Hispanic ethnicity (OR, 1.6), full-time vs. part-time employment (OR, 2.4), older age (ORs, 1.6-1.7 for ages 40-49 and 50-59 vs. younger than 40), and marital status (currently/previously married, ORs, 1.6-2.6). Considering the high rate of survey respondents with health insurance, covariate-adjusted modeling was not applicable.
“The number of people with MS who do not have long-term care insurance was surprisingly high,” Dr. Planchon said. “Although the improved treatment climate recently may decrease the long-term disability levels, we do not yet know this with certainty. A large number of people with MS are likely to need long-term care in the future, which often is a significant financial burden to families.” The findings suggest that clinical care teams “need to initiate early discussions of possible long-term needs with their patients,” she continued. “Incorporation of social work teams, who are familiar with the needs of people with MS and insurance options available to them, within MS specialty practices will bolster the comprehensive care of patients and their families.”
She acknowledged certain limitations of the study, including the low proportion of respondents who were Hispanic/Latino and African American (about 4% each). “The insurance landscape may differ in these groups compared to the majority Caucasian population who responded to this survey,” Dr. Planchon said.
The National Multiple Sclerosis Society funded the study. Dr. Planchon reported having no relevant financial disclosures.
SOURCE: Planchon S et al. ACTRIMS Forum 2019, Abstract P295.
REPORTING FROM ACTRIMS FORUM 2019
Big pharma says it can’t drop drug list prices alone
Top pharmaceutical executives expressed willingness to lower the list prices of their drugs, but only if there were cooperation among all sectors to reform how drugs get from manufacturer to patient.

That theme was common in the testimony of seven pharmaceutical executives before the Senate Finance Committee during a Feb. 26 hearing.
“We are in a system that used to be fit for purpose and really drove enormous savings over the last few years but it is no longer fit for purpose,” Pascal Soriot, executive director and CEO of AstraZeneca, testified before the committee. “It’s one of those situations where nobody in the system can do anything, can fix it by themselves.”
The problem, the executives agreed, is the financial structure of drug delivery that ties list prices and their associated rebates to formulary placement.
“If you went back a few years ago, when we negotiated to get our drugs on formulary, our goal was to have the lowest copay by patients,” Kenneth Frazier, chairman and CEO of Merck, testified before the committee. “Today, the goal is to pay into the supply chain the biggest rebate. That actually puts the patient at a disadvantage since they are the only ones that are paying a portion of the list price. The list price is actually working against the patient.”
When asked why the list prices of prescription drugs are so high, Olivier Brandicourt, MD, CEO of Sanofi, said, “We are trying to get formulary position with those high list price-high rebate. It’s a preferred position. Unfortunately that preferred position doesn’t automatically ensure affordability.”
Mr. Frazier added that if a manufacturer brings a product “with a low list price in this system, you get punished financially and you get no uptake because everyone in the supply chain makes money as a result of a higher list price.”
Executives noted that when accounting for financial incentives such as rebates, discounts, and coupons, net prices for pharmaceuticals have actually come down even as list prices are on the rise to accommodate competition on formulary placement.
But that is obscured at the pharmacy counter, where patients are paying higher and higher out-of-pocket costs because more often than not, payment is tied to the list price of the drug, not the net price after all rebates and other discounts have been taken into consideration.
This is a particular problem in Medicare Part D, said AbbVie Chairman and CEO Richard Gonzalez.
“Due to the structure of the Part D benefit design, patients are charged out-of-pocket costs on a medicine’s list price which does not reflect the market-based rebates that Medicare receives,” he testified.
Despite acknowledging that this is a problem, the executives gathered were hesitant to commit to simply lowering the list prices, or anything for that matter.
The closest the panel came to a commitment to lowering the list prices of their drugs was to do so if all rebates went away in both the public and private sector.
But beyond that, the pharma executives continued to assign responsibility for high out-of-pocket drug costs to other players in the health care system, adding that the only way to change the situation would be to have everyone come to the table simultaneously.
“I understand the dissatisfaction with our industry,” Mr. Frazier said. “I understand why patients are frustrated because they need these medicines and they can’t afford them. I would pledge to do everything that we could, but I would urge you to recognize that the system itself is complex and it is interdependent and no one company can unilaterally lower list prices without running into financial and operating disadvantages that make it impossible to do that. But if we all bring the parties together around the table with the goal of doing what’s best for the patient, I think we can some up with a system that works for all Americans.”
Ultimately, the panel suggested, legislation is going to be required to change the system.
Top pharmaceutical executives expressed willingness to lower the list prices of their drugs, but only if there were cooperation among all sectors to reform how drugs get from manufacturer to patient.

That theme was common in the testimony of seven pharmaceutical executives before the Senate Finance Committee during a Feb. 26 hearing.
“We are in a system that used to be fit for purpose and really drove enormous savings over the last few years but it is no longer fit for purpose,” Pascal Soriot, executive director and CEO of AstraZeneca, testified before the committee. “It’s one of those situations where nobody in the system can do anything, can fix it by themselves.”
The problem, the executives agreed, is the financial structure of drug delivery that ties list prices and their associated rebates to formulary placement.
“If you went back a few years ago, when we negotiated to get our drugs on formulary, our goal was to have the lowest copay by patients,” Kenneth Frazier, chairman and CEO of Merck, testified before the committee. “Today, the goal is to pay into the supply chain the biggest rebate. That actually puts the patient at a disadvantage since they are the only ones that are paying a portion of the list price. The list price is actually working against the patient.”
When asked why the list prices of prescription drugs are so high, Olivier Brandicourt, MD, CEO of Sanofi, said, “We are trying to get formulary position with those high list price-high rebate. It’s a preferred position. Unfortunately that preferred position doesn’t automatically ensure affordability.”
Mr. Frazier added that if a manufacturer brings a product “with a low list price in this system, you get punished financially and you get no uptake because everyone in the supply chain makes money as a result of a higher list price.”
Executives noted that when accounting for financial incentives such as rebates, discounts, and coupons, net prices for pharmaceuticals have actually come down even as list prices are on the rise to accommodate competition on formulary placement.
But that is obscured at the pharmacy counter, where patients are paying higher and higher out-of-pocket costs because more often than not, payment is tied to the list price of the drug, not the net price after all rebates and other discounts have been taken into consideration.
This is a particular problem in Medicare Part D, said AbbVie Chairman and CEO Richard Gonzalez.
“Due to the structure of the Part D benefit design, patients are charged out-of-pocket costs on a medicine’s list price which does not reflect the market-based rebates that Medicare receives,” he testified.
Despite acknowledging that this is a problem, the executives gathered were hesitant to commit to simply lowering the list prices, or anything for that matter.
The closest the panel came to a commitment to lowering the list prices of their drugs was to do so if all rebates went away in both the public and private sector.
But beyond that, the pharma executives continued to assign responsibility for high out-of-pocket drug costs to other players in the health care system, adding that the only way to change the situation would be to have everyone come to the table simultaneously.
“I understand the dissatisfaction with our industry,” Mr. Frazier said. “I understand why patients are frustrated because they need these medicines and they can’t afford them. I would pledge to do everything that we could, but I would urge you to recognize that the system itself is complex and it is interdependent and no one company can unilaterally lower list prices without running into financial and operating disadvantages that make it impossible to do that. But if we all bring the parties together around the table with the goal of doing what’s best for the patient, I think we can some up with a system that works for all Americans.”
Ultimately, the panel suggested, legislation is going to be required to change the system.
Top pharmaceutical executives expressed willingness to lower the list prices of their drugs, but only if there were cooperation among all sectors to reform how drugs get from manufacturer to patient.

That theme was common in the testimony of seven pharmaceutical executives before the Senate Finance Committee during a Feb. 26 hearing.
“We are in a system that used to be fit for purpose and really drove enormous savings over the last few years but it is no longer fit for purpose,” Pascal Soriot, executive director and CEO of AstraZeneca, testified before the committee. “It’s one of those situations where nobody in the system can do anything, can fix it by themselves.”
The problem, the executives agreed, is the financial structure of drug delivery that ties list prices and their associated rebates to formulary placement.
“If you went back a few years ago, when we negotiated to get our drugs on formulary, our goal was to have the lowest copay by patients,” Kenneth Frazier, chairman and CEO of Merck, testified before the committee. “Today, the goal is to pay into the supply chain the biggest rebate. That actually puts the patient at a disadvantage since they are the only ones that are paying a portion of the list price. The list price is actually working against the patient.”
When asked why the list prices of prescription drugs are so high, Olivier Brandicourt, MD, CEO of Sanofi, said, “We are trying to get formulary position with those high list price-high rebate. It’s a preferred position. Unfortunately that preferred position doesn’t automatically ensure affordability.”
Mr. Frazier added that if a manufacturer brings a product “with a low list price in this system, you get punished financially and you get no uptake because everyone in the supply chain makes money as a result of a higher list price.”
Executives noted that when accounting for financial incentives such as rebates, discounts, and coupons, net prices for pharmaceuticals have actually come down even as list prices are on the rise to accommodate competition on formulary placement.
But that is obscured at the pharmacy counter, where patients are paying higher and higher out-of-pocket costs because more often than not, payment is tied to the list price of the drug, not the net price after all rebates and other discounts have been taken into consideration.
This is a particular problem in Medicare Part D, said AbbVie Chairman and CEO Richard Gonzalez.
“Due to the structure of the Part D benefit design, patients are charged out-of-pocket costs on a medicine’s list price which does not reflect the market-based rebates that Medicare receives,” he testified.
Despite acknowledging that this is a problem, the executives gathered were hesitant to commit to simply lowering the list prices, or anything for that matter.
The closest the panel came to a commitment to lowering the list prices of their drugs was to do so if all rebates went away in both the public and private sector.
But beyond that, the pharma executives continued to assign responsibility for high out-of-pocket drug costs to other players in the health care system, adding that the only way to change the situation would be to have everyone come to the table simultaneously.
“I understand the dissatisfaction with our industry,” Mr. Frazier said. “I understand why patients are frustrated because they need these medicines and they can’t afford them. I would pledge to do everything that we could, but I would urge you to recognize that the system itself is complex and it is interdependent and no one company can unilaterally lower list prices without running into financial and operating disadvantages that make it impossible to do that. But if we all bring the parties together around the table with the goal of doing what’s best for the patient, I think we can some up with a system that works for all Americans.”
Ultimately, the panel suggested, legislation is going to be required to change the system.
REPORTING FROM SENATE FINANCE COMMITTEE HEARING
What does 'Medicare for all' mean?
Only about a fifth of Americans correctly identified the description of a Medicare-for-all system in a recent national tracking poll.
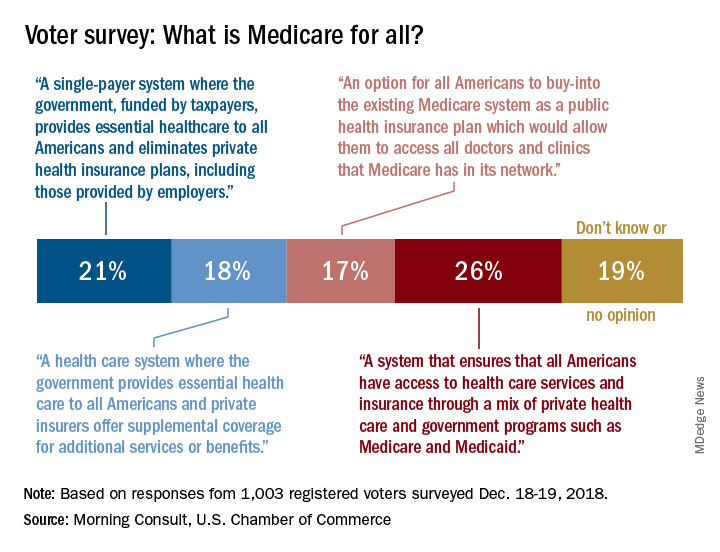
Four descriptions of a Medicare-for-all health care system were provided, and only 21% of respondents correctly selected “a single-payer system where the government, funded by taxpayers, provides essential health care to all Americans and eliminates private health insurance plans, including those provided by employers,” according to the tracking poll from the U.S. Chamber of Commerce and digital media company Morning Consult.
The most common selection – chosen by 26% of the 1,003 registered voters who answered the question (about half of all the respondents) – involved “a system that ensures that all Americans have access to health care services and insurance through a mix of private health care and government programs such as Medicare and Medicaid.”
The other choices covered a federal system with available private supplemental coverage and another with the option of buying in to the existing Medicare system, the report said. Another 19% of respondents to the survey, which was conducted Dec. 18-19, said that they didn’t know or had no opinion.
Questions covering other areas of possible future legislation, which were answered by all of the 2,000 respondents, showed strong support for protection against surprise hospital bills (90%), reforming the Affordable Care Act (73%), and protecting the Affordable Care Act (63%), the U.S. Chamber and Morning Consult reported. The survey’s margin of error was plus or minus two percentage points.
Only about a fifth of Americans correctly identified the description of a Medicare-for-all system in a recent national tracking poll.

Four descriptions of a Medicare-for-all health care system were provided, and only 21% of respondents correctly selected “a single-payer system where the government, funded by taxpayers, provides essential health care to all Americans and eliminates private health insurance plans, including those provided by employers,” according to the tracking poll from the U.S. Chamber of Commerce and digital media company Morning Consult.
The most common selection – chosen by 26% of the 1,003 registered voters who answered the question (about half of all the respondents) – involved “a system that ensures that all Americans have access to health care services and insurance through a mix of private health care and government programs such as Medicare and Medicaid.”
The other choices covered a federal system with available private supplemental coverage and another with the option of buying in to the existing Medicare system, the report said. Another 19% of respondents to the survey, which was conducted Dec. 18-19, said that they didn’t know or had no opinion.
Questions covering other areas of possible future legislation, which were answered by all of the 2,000 respondents, showed strong support for protection against surprise hospital bills (90%), reforming the Affordable Care Act (73%), and protecting the Affordable Care Act (63%), the U.S. Chamber and Morning Consult reported. The survey’s margin of error was plus or minus two percentage points.
Only about a fifth of Americans correctly identified the description of a Medicare-for-all system in a recent national tracking poll.

Four descriptions of a Medicare-for-all health care system were provided, and only 21% of respondents correctly selected “a single-payer system where the government, funded by taxpayers, provides essential health care to all Americans and eliminates private health insurance plans, including those provided by employers,” according to the tracking poll from the U.S. Chamber of Commerce and digital media company Morning Consult.
The most common selection – chosen by 26% of the 1,003 registered voters who answered the question (about half of all the respondents) – involved “a system that ensures that all Americans have access to health care services and insurance through a mix of private health care and government programs such as Medicare and Medicaid.”
The other choices covered a federal system with available private supplemental coverage and another with the option of buying in to the existing Medicare system, the report said. Another 19% of respondents to the survey, which was conducted Dec. 18-19, said that they didn’t know or had no opinion.
Questions covering other areas of possible future legislation, which were answered by all of the 2,000 respondents, showed strong support for protection against surprise hospital bills (90%), reforming the Affordable Care Act (73%), and protecting the Affordable Care Act (63%), the U.S. Chamber and Morning Consult reported. The survey’s margin of error was plus or minus two percentage points.
Medicine grapples with COI reporting
Conflict of interest (COI) reporting has moved center stage again in recent months, with some medical journals, professional societies, cancer centers, and academic medical institutions reviewing policies and practices in the wake of a highly publicized disclosure failure last fall at Memorial Sloan Kettering Cancer Center (MSK).
And in some settings, oncologists and other physician researchers are being encouraged to check what the federal Open Payments database says about their payments from industry.
The spotlight is on the field of cancer research and treatment, where MSK’s chief medical officer, José Baselga, MD, PhD, resigned in September 2018 after the New York Times and ProPublica reported that he’d failed to disclose millions of dollars of industry payments and ownership interests in the majority of journal articles he wrote or cowrote over a 4-year period.
COI disclosure issues have a broad reach, however, and the policy reviews, debates, and hashing out of responsibilities that are now taking place likely will have implications for all of medicine.
Among the questions: Who enforces disclosure rules and how should cases of incomplete or inconsistent disclosure be handled? How can COI declarations be made easier for researchers? Should disclosure be based on self-reported relevancy, or more comprehensive in nature?
Such questions are being debated nationally. On Feb. 12, 2019, leaders from academia, journals, and medical societies came together in Washington, D.C., at the offices of the Association of American Medical Colleges (AAMC) for a closed-door meeting focused on COI disclosures. MSK, the Journal of the American Medical Association (JAMA), the American Society of Clinical Oncology (ASCO), and the Council of Medical Specialty Societies led the charge as cosponsors of the meeting.
“We’ve been dealing with disclosure issues in a siloed way in exchanges [between journal editors and authors, for instance, or between speakers and CME providers]. And academic institutions have their own robust disclosure mechanisms that they use internally,” said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the AAMC. “There’s a growing understanding that these conversations need to be happening across these different sectors.”
Pleas for accuracy
At academic medical institutions, conflicts of interest are identified and then managed; it’s common for researchers’ COI management plans to include requirements for disclosure in all presentations and publications.
Journals and professional medical societies require authors and speakers to submit disclosure forms of varying lengths and with differing questions about relationships with industry, often based on the notion of relevancy to the subject at hand. Disclosure forms are reviewed, but editors and other reviewers rely largely – if not entirely – on the honor system.
Dr. Baselga’s disclosure lapses and his subsequent resignation have rattled leaders in each of these settings. Researchers at MSK were instructed to review their COI disclosures and submit corrections when necessary, and in December 2018 the hospital was reportedly evaluating its process for reviewing conflicts of interest, according to reports in the New York Times and ProPublica. (MSK did not respond to requests for comment about actions taken.)
The Dana Farber Cancer Institute in Boston similarly has “been reminding faculty and other researchers” of their disclosure responsibilities and is conducting a review of “all our policies in this area,” a spokeswoman said. And at Fred Hutchinson Cancer Research Center in Seattle, a spokesman said they have established an internal task force to review individual and institutional COI policies to ensure that COIs are “appropriately managed while also enabling research collaborations that bring scientific advances to our patients.”
Other centers contacted for this article, such as the Cleveland Clinic Cancer Center and the Mayo Clinic Cancer Center, said that they have no new reviews ongoing and no plans to change policies at this time.
The heightened attention to disclosure has, in turn, shone a spotlight on increasingly complex physician-industry relationships and on the Open Payments website run by the Centers for Medicare and Medicaid Services. Open Payments is a disclosure program and database that tracks payments made to physicians and teaching hospitals by drug and device companies.
Journalists, including those who reported on Dr. Baselga’s disclosures, have searched the public database for industry payment data. So have other researchers who have studied financial disclosure statements; a study reported in JAMA Oncology last year, for instance, concluded through the use of Open Payments data that about one-third of authors of cancer drug trial reports did not completely disclose payments from trial sponsors (JAMA Oncol. 2018;4[10]:1426-8
In a column published in December 2018 in AAMC News, AAMC President and CEO Darrell G. Kirch, MD, wrote that failures to disclose can raise questions about the integrity of research, whether or not there is an actual conflict. He advised institutions to “encourage faculty to review the information posted about them on the Open Payments website” of the CMS to “ensure it is accurate and consistent with disclosures related to all their professional responsibilities.”
ASCO issues similar advice, encouraging authors and CME speakers and participants of other ASCO activities to double-check their disclosures against other sources, including “publicly reported interactions with companies that may have been inadvertently omitted.”
In the world of journals, the New England Journal of Medicine (NEJM) began asking authors at the end of 2018 to “certify that they have reconciled their disclosures” with the Open Payments database, said Jennifer Zeis, a spokeswoman forthe journal.
Time may tell how well such requests work. When the Institute of Medicine (now called the National Academy of Medicine) called on Congress in 2009 to create a national program requiring pharmaceutical, device, and biotechnology companies to publicly report their payments, it envisioned universities, journals, and others using the program to verify disclosures made to them. But the resulting Open Payments database has limitations – for instance, it doesn’t include payments from companies without FDA-approved products, it is not necessarily up to date, and its payment categories do not necessarily match categories of disclosure.
“Some entries in the Open Payments database need further explanation,” said Ms. Zeis of NEJM. “Some authors, for example, have said that the database does not fully and accurately explain that the funds were disbursed not to them personally, but to their academic institutions.” While the database provides transparency, it also “needs context that’s not currently provided,” she said.
Mistakes in the database can also be “very hard to challenge,” said Clifford A. Hudis, MD, CEO of the American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO).
All in all, he said, “there’s really no timely, comprehensive, and fully reliable source of information with which to verify an individual’s disclosures.”
Policies and practices are also under review at the American Association for Cancer Research (AACR) and the American Society of Hematology (ASH).
The AACR has appointed a panel of experts, including physicians, basic scientists, a patient advocate, and others to conduct “a comprehensive review of [its] disclosure policies and to explore whether any current policies need to be revised,” said Rachel Salis-Silverman, director of public relations. It also will convene a session on COI disclosures at its 2019 annual meeting at the end of March, she said.
ASH, which publishes the journal Blood, is exploring possible changes in its “internal processes with regards to ASH publications,” said Matt Gertzog, deputy executive director of the group. COI disclosure is “more of a journey than a destination,” he said. “We are continuously reflecting on and refining our processes.”
Moving away from ‘relevancy’
Physicians and others who have relationships with industry have long complained about a patchwork of disclosure requirements, and significant efforts have been made in the last decade to standardize forms and practices. However, the current system is still “a little bit of a Tower of Babel,” said Dr. Hudis of ASCO. “Every day, physicians have to complete from scratch similar, but not identical, disclosure forms that ask similar, but not identical, questions.”
A disclosable compensation amount “might be first dollar, or it might be over $10,000. [Time periods] might cover 1 year, or 3 years. ... Stock ownership might be dollar value, or a percentage of shares,” he explained. “If you want a system that would make it hard to be compliant and easy to mess up, that’s what we have.”
To standardize the COI disclosure process for all Society-related publications and activities – including CME, JCO, and practice guidelines – ASCO moved about 5 years ago to a system of general disclosure, asking physicians and others to disclose all financial interests and industry relationships rather than what they deem relevant.
The thinking was that general disclosure “would be easier for disclosers, and nobody would ever be accused of hiding anything,” Dr. Hudis said.
“We’d [also] recognized,” he explained, “that there was a risk to the relevancy approach in that it put the judgment for the potential conflict in the hands of the potentially conflicted, while others might have a different point of view about what is or isn’t relevant.”
Those concerned about general disclosure worry that it may “obscure [for the reader or listener] what’s really important or the most meaningful,” he said.
Some physicians have expressed in interviews for this article, moreover, the concern that too many disclosures – too long a list of financial relationships – will be viewed negatively. This is something ASCO aims to guard against as it strives to achieve full transparency, Dr. Hudis said. “If one were to suggest that engagement itself is automatically a negative, then you’re starting to put negative pressure on compliance with disclosure.”
And full disclosure matters, he said. “We have to err in the direction of believing that disclosure is good,” he said, “even if we can’t prove it has clear and measurable impact. That is why our goal is to make full disclosure easier. What potential conflicts are acceptable, or not, is an important but entirely separate matter.”
Howard Bauchner, MD, editor in chief of JAMA and the JAMA Network, frames the pros and cons of general and relevant disclosure similarly, and emphasizes that the relationships of authors with industry – particularly with private equity start-up companies – has changed dramatically over the past decade. Editors have “talked about complete versus [more narrowly] relevant disclosures at length,” he said, and have been moving overall “toward more complete disclosure where the reader can make a decision on their own.”
Other journals also are taking this approach. In 2009, in an effort to reduce variability in reporting processes and formats, the International Committee of Medical Journal Editors (ICMJE) developed a uniform electronic disclosure form that asks about financial relationships and interactions with any entity that could be considered “broadly relevant” to the submitted work. The group updated the form in December 2018.
As an example, the form reads, an article about testing an epidermal growth factor receptor (EGFR) antagonist in lung cancer requires the reporting of “all associations with entities pursuing diagnostic or therapeutic strategies in cancer in general, not just in the area of EGFR or lung cancer.” JAMA, NEJM, and The Lancet are among those journals that embrace the ICMJE’s policies and use its form.
To simplify its own disclosure process, JAMA and the network’s journals ended the practice in January 2019 of requiring both the ICMJE form and JAMA’s own separate disclosure form. The journals now use a single electronic form that includes questions from the ICMJE form. And to promote more consistent and complete reporting, the electronic form contains prompts that ask authors each time they answer “no” to one of four specific questions about potential COI whether they are certain of their answers and whether their answers are consistent with other disclosures they recently made.
While relevancy statements “create struggles for authors,” about two-thirds of the disclosure inaccuracies reported by readers and verified by JAMA’s editors (most often through editor-author discussions) involve a complete lack of disclosure rather than questions of relevancy, Dr. Bauchner noted. (JAMA and the network’s journals receive about 30,000 disclosure forms each year.)
The AAMC, in the meantime, has developed a central web-based repository for disclosures called Convey. Physicians and others can maintain secure records of financial interests in the repository, and these records can then be disclosed directly to any journal or organization that uses the system. The tool – born from discussions that followed the 2009 IOM report on COI – is “intended to facilitate more complete, more accurate, and more consistent” disclosures,” said Ms. Pierce of the AAMC. It is now live and in its early stages of use; NEJM assisted in its development and has been one of its pilot testers.
Enforcement questions
Some experts believe that institutions should maintain public databases of disclosures and/or that disclosure requirements should be better enforced in-house.
“There often are no clear guidelines in institutions about how to respond to people who are negligent in how they’re managing their disclosures,” said Jeffrey R. Botkin, MD, MPH, professor of pediatrics and associate vice president for research at the University of Utah, Salt Lake City, who has served on a variety of ethics committees and is an elected member of the Hastings Center. Dr. Botkin proposed in a Viewpoint published last October in JAMA that failure to disclose significant COIs should be considered research misconduct (JAMA 2018;320[22]:2307-8).
At the University of Utah, “we’re getting better at saying, ‘show us that you’ve disclosed,’ ” he said. “In some cases we’ll do spot checks of journal articles to make sure [researchers have] followed through with their disclosures.”
John Abramson, MD, a lecturer in the department of health care policy at Harvard Medical School, Boston, contends that incomplete declarations of COI have been shown to correlate with reporting of manufacturer-friendly research results. Journals should have “zero tolerance” standards for incomplete or inaccurate COI declarations and should, among other things, “inform academic institutions of breaches of integrity.”
At JAMA, which in 2017 published a theme issue on COI and COI declarations, editors have been discussing whether they will contact an author’s institution “if there’s a pattern involved [with disclosure problems] or if there’s a lack of declaration of multiple COIs,” Dr. Bauchner said.
Conflict of interest (COI) reporting has moved center stage again in recent months, with some medical journals, professional societies, cancer centers, and academic medical institutions reviewing policies and practices in the wake of a highly publicized disclosure failure last fall at Memorial Sloan Kettering Cancer Center (MSK).
And in some settings, oncologists and other physician researchers are being encouraged to check what the federal Open Payments database says about their payments from industry.
The spotlight is on the field of cancer research and treatment, where MSK’s chief medical officer, José Baselga, MD, PhD, resigned in September 2018 after the New York Times and ProPublica reported that he’d failed to disclose millions of dollars of industry payments and ownership interests in the majority of journal articles he wrote or cowrote over a 4-year period.
COI disclosure issues have a broad reach, however, and the policy reviews, debates, and hashing out of responsibilities that are now taking place likely will have implications for all of medicine.
Among the questions: Who enforces disclosure rules and how should cases of incomplete or inconsistent disclosure be handled? How can COI declarations be made easier for researchers? Should disclosure be based on self-reported relevancy, or more comprehensive in nature?
Such questions are being debated nationally. On Feb. 12, 2019, leaders from academia, journals, and medical societies came together in Washington, D.C., at the offices of the Association of American Medical Colleges (AAMC) for a closed-door meeting focused on COI disclosures. MSK, the Journal of the American Medical Association (JAMA), the American Society of Clinical Oncology (ASCO), and the Council of Medical Specialty Societies led the charge as cosponsors of the meeting.
“We’ve been dealing with disclosure issues in a siloed way in exchanges [between journal editors and authors, for instance, or between speakers and CME providers]. And academic institutions have their own robust disclosure mechanisms that they use internally,” said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the AAMC. “There’s a growing understanding that these conversations need to be happening across these different sectors.”
Pleas for accuracy
At academic medical institutions, conflicts of interest are identified and then managed; it’s common for researchers’ COI management plans to include requirements for disclosure in all presentations and publications.
Journals and professional medical societies require authors and speakers to submit disclosure forms of varying lengths and with differing questions about relationships with industry, often based on the notion of relevancy to the subject at hand. Disclosure forms are reviewed, but editors and other reviewers rely largely – if not entirely – on the honor system.
Dr. Baselga’s disclosure lapses and his subsequent resignation have rattled leaders in each of these settings. Researchers at MSK were instructed to review their COI disclosures and submit corrections when necessary, and in December 2018 the hospital was reportedly evaluating its process for reviewing conflicts of interest, according to reports in the New York Times and ProPublica. (MSK did not respond to requests for comment about actions taken.)
The Dana Farber Cancer Institute in Boston similarly has “been reminding faculty and other researchers” of their disclosure responsibilities and is conducting a review of “all our policies in this area,” a spokeswoman said. And at Fred Hutchinson Cancer Research Center in Seattle, a spokesman said they have established an internal task force to review individual and institutional COI policies to ensure that COIs are “appropriately managed while also enabling research collaborations that bring scientific advances to our patients.”
Other centers contacted for this article, such as the Cleveland Clinic Cancer Center and the Mayo Clinic Cancer Center, said that they have no new reviews ongoing and no plans to change policies at this time.
The heightened attention to disclosure has, in turn, shone a spotlight on increasingly complex physician-industry relationships and on the Open Payments website run by the Centers for Medicare and Medicaid Services. Open Payments is a disclosure program and database that tracks payments made to physicians and teaching hospitals by drug and device companies.
Journalists, including those who reported on Dr. Baselga’s disclosures, have searched the public database for industry payment data. So have other researchers who have studied financial disclosure statements; a study reported in JAMA Oncology last year, for instance, concluded through the use of Open Payments data that about one-third of authors of cancer drug trial reports did not completely disclose payments from trial sponsors (JAMA Oncol. 2018;4[10]:1426-8
In a column published in December 2018 in AAMC News, AAMC President and CEO Darrell G. Kirch, MD, wrote that failures to disclose can raise questions about the integrity of research, whether or not there is an actual conflict. He advised institutions to “encourage faculty to review the information posted about them on the Open Payments website” of the CMS to “ensure it is accurate and consistent with disclosures related to all their professional responsibilities.”
ASCO issues similar advice, encouraging authors and CME speakers and participants of other ASCO activities to double-check their disclosures against other sources, including “publicly reported interactions with companies that may have been inadvertently omitted.”
In the world of journals, the New England Journal of Medicine (NEJM) began asking authors at the end of 2018 to “certify that they have reconciled their disclosures” with the Open Payments database, said Jennifer Zeis, a spokeswoman forthe journal.
Time may tell how well such requests work. When the Institute of Medicine (now called the National Academy of Medicine) called on Congress in 2009 to create a national program requiring pharmaceutical, device, and biotechnology companies to publicly report their payments, it envisioned universities, journals, and others using the program to verify disclosures made to them. But the resulting Open Payments database has limitations – for instance, it doesn’t include payments from companies without FDA-approved products, it is not necessarily up to date, and its payment categories do not necessarily match categories of disclosure.
“Some entries in the Open Payments database need further explanation,” said Ms. Zeis of NEJM. “Some authors, for example, have said that the database does not fully and accurately explain that the funds were disbursed not to them personally, but to their academic institutions.” While the database provides transparency, it also “needs context that’s not currently provided,” she said.
Mistakes in the database can also be “very hard to challenge,” said Clifford A. Hudis, MD, CEO of the American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO).
All in all, he said, “there’s really no timely, comprehensive, and fully reliable source of information with which to verify an individual’s disclosures.”
Policies and practices are also under review at the American Association for Cancer Research (AACR) and the American Society of Hematology (ASH).
The AACR has appointed a panel of experts, including physicians, basic scientists, a patient advocate, and others to conduct “a comprehensive review of [its] disclosure policies and to explore whether any current policies need to be revised,” said Rachel Salis-Silverman, director of public relations. It also will convene a session on COI disclosures at its 2019 annual meeting at the end of March, she said.
ASH, which publishes the journal Blood, is exploring possible changes in its “internal processes with regards to ASH publications,” said Matt Gertzog, deputy executive director of the group. COI disclosure is “more of a journey than a destination,” he said. “We are continuously reflecting on and refining our processes.”
Moving away from ‘relevancy’
Physicians and others who have relationships with industry have long complained about a patchwork of disclosure requirements, and significant efforts have been made in the last decade to standardize forms and practices. However, the current system is still “a little bit of a Tower of Babel,” said Dr. Hudis of ASCO. “Every day, physicians have to complete from scratch similar, but not identical, disclosure forms that ask similar, but not identical, questions.”
A disclosable compensation amount “might be first dollar, or it might be over $10,000. [Time periods] might cover 1 year, or 3 years. ... Stock ownership might be dollar value, or a percentage of shares,” he explained. “If you want a system that would make it hard to be compliant and easy to mess up, that’s what we have.”
To standardize the COI disclosure process for all Society-related publications and activities – including CME, JCO, and practice guidelines – ASCO moved about 5 years ago to a system of general disclosure, asking physicians and others to disclose all financial interests and industry relationships rather than what they deem relevant.
The thinking was that general disclosure “would be easier for disclosers, and nobody would ever be accused of hiding anything,” Dr. Hudis said.
“We’d [also] recognized,” he explained, “that there was a risk to the relevancy approach in that it put the judgment for the potential conflict in the hands of the potentially conflicted, while others might have a different point of view about what is or isn’t relevant.”
Those concerned about general disclosure worry that it may “obscure [for the reader or listener] what’s really important or the most meaningful,” he said.
Some physicians have expressed in interviews for this article, moreover, the concern that too many disclosures – too long a list of financial relationships – will be viewed negatively. This is something ASCO aims to guard against as it strives to achieve full transparency, Dr. Hudis said. “If one were to suggest that engagement itself is automatically a negative, then you’re starting to put negative pressure on compliance with disclosure.”
And full disclosure matters, he said. “We have to err in the direction of believing that disclosure is good,” he said, “even if we can’t prove it has clear and measurable impact. That is why our goal is to make full disclosure easier. What potential conflicts are acceptable, or not, is an important but entirely separate matter.”
Howard Bauchner, MD, editor in chief of JAMA and the JAMA Network, frames the pros and cons of general and relevant disclosure similarly, and emphasizes that the relationships of authors with industry – particularly with private equity start-up companies – has changed dramatically over the past decade. Editors have “talked about complete versus [more narrowly] relevant disclosures at length,” he said, and have been moving overall “toward more complete disclosure where the reader can make a decision on their own.”
Other journals also are taking this approach. In 2009, in an effort to reduce variability in reporting processes and formats, the International Committee of Medical Journal Editors (ICMJE) developed a uniform electronic disclosure form that asks about financial relationships and interactions with any entity that could be considered “broadly relevant” to the submitted work. The group updated the form in December 2018.
As an example, the form reads, an article about testing an epidermal growth factor receptor (EGFR) antagonist in lung cancer requires the reporting of “all associations with entities pursuing diagnostic or therapeutic strategies in cancer in general, not just in the area of EGFR or lung cancer.” JAMA, NEJM, and The Lancet are among those journals that embrace the ICMJE’s policies and use its form.
To simplify its own disclosure process, JAMA and the network’s journals ended the practice in January 2019 of requiring both the ICMJE form and JAMA’s own separate disclosure form. The journals now use a single electronic form that includes questions from the ICMJE form. And to promote more consistent and complete reporting, the electronic form contains prompts that ask authors each time they answer “no” to one of four specific questions about potential COI whether they are certain of their answers and whether their answers are consistent with other disclosures they recently made.
While relevancy statements “create struggles for authors,” about two-thirds of the disclosure inaccuracies reported by readers and verified by JAMA’s editors (most often through editor-author discussions) involve a complete lack of disclosure rather than questions of relevancy, Dr. Bauchner noted. (JAMA and the network’s journals receive about 30,000 disclosure forms each year.)
The AAMC, in the meantime, has developed a central web-based repository for disclosures called Convey. Physicians and others can maintain secure records of financial interests in the repository, and these records can then be disclosed directly to any journal or organization that uses the system. The tool – born from discussions that followed the 2009 IOM report on COI – is “intended to facilitate more complete, more accurate, and more consistent” disclosures,” said Ms. Pierce of the AAMC. It is now live and in its early stages of use; NEJM assisted in its development and has been one of its pilot testers.
Enforcement questions
Some experts believe that institutions should maintain public databases of disclosures and/or that disclosure requirements should be better enforced in-house.
“There often are no clear guidelines in institutions about how to respond to people who are negligent in how they’re managing their disclosures,” said Jeffrey R. Botkin, MD, MPH, professor of pediatrics and associate vice president for research at the University of Utah, Salt Lake City, who has served on a variety of ethics committees and is an elected member of the Hastings Center. Dr. Botkin proposed in a Viewpoint published last October in JAMA that failure to disclose significant COIs should be considered research misconduct (JAMA 2018;320[22]:2307-8).
At the University of Utah, “we’re getting better at saying, ‘show us that you’ve disclosed,’ ” he said. “In some cases we’ll do spot checks of journal articles to make sure [researchers have] followed through with their disclosures.”
John Abramson, MD, a lecturer in the department of health care policy at Harvard Medical School, Boston, contends that incomplete declarations of COI have been shown to correlate with reporting of manufacturer-friendly research results. Journals should have “zero tolerance” standards for incomplete or inaccurate COI declarations and should, among other things, “inform academic institutions of breaches of integrity.”
At JAMA, which in 2017 published a theme issue on COI and COI declarations, editors have been discussing whether they will contact an author’s institution “if there’s a pattern involved [with disclosure problems] or if there’s a lack of declaration of multiple COIs,” Dr. Bauchner said.
Conflict of interest (COI) reporting has moved center stage again in recent months, with some medical journals, professional societies, cancer centers, and academic medical institutions reviewing policies and practices in the wake of a highly publicized disclosure failure last fall at Memorial Sloan Kettering Cancer Center (MSK).
And in some settings, oncologists and other physician researchers are being encouraged to check what the federal Open Payments database says about their payments from industry.
The spotlight is on the field of cancer research and treatment, where MSK’s chief medical officer, José Baselga, MD, PhD, resigned in September 2018 after the New York Times and ProPublica reported that he’d failed to disclose millions of dollars of industry payments and ownership interests in the majority of journal articles he wrote or cowrote over a 4-year period.
COI disclosure issues have a broad reach, however, and the policy reviews, debates, and hashing out of responsibilities that are now taking place likely will have implications for all of medicine.
Among the questions: Who enforces disclosure rules and how should cases of incomplete or inconsistent disclosure be handled? How can COI declarations be made easier for researchers? Should disclosure be based on self-reported relevancy, or more comprehensive in nature?
Such questions are being debated nationally. On Feb. 12, 2019, leaders from academia, journals, and medical societies came together in Washington, D.C., at the offices of the Association of American Medical Colleges (AAMC) for a closed-door meeting focused on COI disclosures. MSK, the Journal of the American Medical Association (JAMA), the American Society of Clinical Oncology (ASCO), and the Council of Medical Specialty Societies led the charge as cosponsors of the meeting.
“We’ve been dealing with disclosure issues in a siloed way in exchanges [between journal editors and authors, for instance, or between speakers and CME providers]. And academic institutions have their own robust disclosure mechanisms that they use internally,” said Heather Pierce, JD, MPH, senior director of science policy and regulatory counsel for the AAMC. “There’s a growing understanding that these conversations need to be happening across these different sectors.”
Pleas for accuracy
At academic medical institutions, conflicts of interest are identified and then managed; it’s common for researchers’ COI management plans to include requirements for disclosure in all presentations and publications.
Journals and professional medical societies require authors and speakers to submit disclosure forms of varying lengths and with differing questions about relationships with industry, often based on the notion of relevancy to the subject at hand. Disclosure forms are reviewed, but editors and other reviewers rely largely – if not entirely – on the honor system.
Dr. Baselga’s disclosure lapses and his subsequent resignation have rattled leaders in each of these settings. Researchers at MSK were instructed to review their COI disclosures and submit corrections when necessary, and in December 2018 the hospital was reportedly evaluating its process for reviewing conflicts of interest, according to reports in the New York Times and ProPublica. (MSK did not respond to requests for comment about actions taken.)
The Dana Farber Cancer Institute in Boston similarly has “been reminding faculty and other researchers” of their disclosure responsibilities and is conducting a review of “all our policies in this area,” a spokeswoman said. And at Fred Hutchinson Cancer Research Center in Seattle, a spokesman said they have established an internal task force to review individual and institutional COI policies to ensure that COIs are “appropriately managed while also enabling research collaborations that bring scientific advances to our patients.”
Other centers contacted for this article, such as the Cleveland Clinic Cancer Center and the Mayo Clinic Cancer Center, said that they have no new reviews ongoing and no plans to change policies at this time.
The heightened attention to disclosure has, in turn, shone a spotlight on increasingly complex physician-industry relationships and on the Open Payments website run by the Centers for Medicare and Medicaid Services. Open Payments is a disclosure program and database that tracks payments made to physicians and teaching hospitals by drug and device companies.
Journalists, including those who reported on Dr. Baselga’s disclosures, have searched the public database for industry payment data. So have other researchers who have studied financial disclosure statements; a study reported in JAMA Oncology last year, for instance, concluded through the use of Open Payments data that about one-third of authors of cancer drug trial reports did not completely disclose payments from trial sponsors (JAMA Oncol. 2018;4[10]:1426-8
In a column published in December 2018 in AAMC News, AAMC President and CEO Darrell G. Kirch, MD, wrote that failures to disclose can raise questions about the integrity of research, whether or not there is an actual conflict. He advised institutions to “encourage faculty to review the information posted about them on the Open Payments website” of the CMS to “ensure it is accurate and consistent with disclosures related to all their professional responsibilities.”
ASCO issues similar advice, encouraging authors and CME speakers and participants of other ASCO activities to double-check their disclosures against other sources, including “publicly reported interactions with companies that may have been inadvertently omitted.”
In the world of journals, the New England Journal of Medicine (NEJM) began asking authors at the end of 2018 to “certify that they have reconciled their disclosures” with the Open Payments database, said Jennifer Zeis, a spokeswoman forthe journal.
Time may tell how well such requests work. When the Institute of Medicine (now called the National Academy of Medicine) called on Congress in 2009 to create a national program requiring pharmaceutical, device, and biotechnology companies to publicly report their payments, it envisioned universities, journals, and others using the program to verify disclosures made to them. But the resulting Open Payments database has limitations – for instance, it doesn’t include payments from companies without FDA-approved products, it is not necessarily up to date, and its payment categories do not necessarily match categories of disclosure.
“Some entries in the Open Payments database need further explanation,” said Ms. Zeis of NEJM. “Some authors, for example, have said that the database does not fully and accurately explain that the funds were disbursed not to them personally, but to their academic institutions.” While the database provides transparency, it also “needs context that’s not currently provided,” she said.
Mistakes in the database can also be “very hard to challenge,” said Clifford A. Hudis, MD, CEO of the American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO).
All in all, he said, “there’s really no timely, comprehensive, and fully reliable source of information with which to verify an individual’s disclosures.”
Policies and practices are also under review at the American Association for Cancer Research (AACR) and the American Society of Hematology (ASH).
The AACR has appointed a panel of experts, including physicians, basic scientists, a patient advocate, and others to conduct “a comprehensive review of [its] disclosure policies and to explore whether any current policies need to be revised,” said Rachel Salis-Silverman, director of public relations. It also will convene a session on COI disclosures at its 2019 annual meeting at the end of March, she said.
ASH, which publishes the journal Blood, is exploring possible changes in its “internal processes with regards to ASH publications,” said Matt Gertzog, deputy executive director of the group. COI disclosure is “more of a journey than a destination,” he said. “We are continuously reflecting on and refining our processes.”
Moving away from ‘relevancy’
Physicians and others who have relationships with industry have long complained about a patchwork of disclosure requirements, and significant efforts have been made in the last decade to standardize forms and practices. However, the current system is still “a little bit of a Tower of Babel,” said Dr. Hudis of ASCO. “Every day, physicians have to complete from scratch similar, but not identical, disclosure forms that ask similar, but not identical, questions.”
A disclosable compensation amount “might be first dollar, or it might be over $10,000. [Time periods] might cover 1 year, or 3 years. ... Stock ownership might be dollar value, or a percentage of shares,” he explained. “If you want a system that would make it hard to be compliant and easy to mess up, that’s what we have.”
To standardize the COI disclosure process for all Society-related publications and activities – including CME, JCO, and practice guidelines – ASCO moved about 5 years ago to a system of general disclosure, asking physicians and others to disclose all financial interests and industry relationships rather than what they deem relevant.
The thinking was that general disclosure “would be easier for disclosers, and nobody would ever be accused of hiding anything,” Dr. Hudis said.
“We’d [also] recognized,” he explained, “that there was a risk to the relevancy approach in that it put the judgment for the potential conflict in the hands of the potentially conflicted, while others might have a different point of view about what is or isn’t relevant.”
Those concerned about general disclosure worry that it may “obscure [for the reader or listener] what’s really important or the most meaningful,” he said.
Some physicians have expressed in interviews for this article, moreover, the concern that too many disclosures – too long a list of financial relationships – will be viewed negatively. This is something ASCO aims to guard against as it strives to achieve full transparency, Dr. Hudis said. “If one were to suggest that engagement itself is automatically a negative, then you’re starting to put negative pressure on compliance with disclosure.”
And full disclosure matters, he said. “We have to err in the direction of believing that disclosure is good,” he said, “even if we can’t prove it has clear and measurable impact. That is why our goal is to make full disclosure easier. What potential conflicts are acceptable, or not, is an important but entirely separate matter.”
Howard Bauchner, MD, editor in chief of JAMA and the JAMA Network, frames the pros and cons of general and relevant disclosure similarly, and emphasizes that the relationships of authors with industry – particularly with private equity start-up companies – has changed dramatically over the past decade. Editors have “talked about complete versus [more narrowly] relevant disclosures at length,” he said, and have been moving overall “toward more complete disclosure where the reader can make a decision on their own.”
Other journals also are taking this approach. In 2009, in an effort to reduce variability in reporting processes and formats, the International Committee of Medical Journal Editors (ICMJE) developed a uniform electronic disclosure form that asks about financial relationships and interactions with any entity that could be considered “broadly relevant” to the submitted work. The group updated the form in December 2018.
As an example, the form reads, an article about testing an epidermal growth factor receptor (EGFR) antagonist in lung cancer requires the reporting of “all associations with entities pursuing diagnostic or therapeutic strategies in cancer in general, not just in the area of EGFR or lung cancer.” JAMA, NEJM, and The Lancet are among those journals that embrace the ICMJE’s policies and use its form.
To simplify its own disclosure process, JAMA and the network’s journals ended the practice in January 2019 of requiring both the ICMJE form and JAMA’s own separate disclosure form. The journals now use a single electronic form that includes questions from the ICMJE form. And to promote more consistent and complete reporting, the electronic form contains prompts that ask authors each time they answer “no” to one of four specific questions about potential COI whether they are certain of their answers and whether their answers are consistent with other disclosures they recently made.
While relevancy statements “create struggles for authors,” about two-thirds of the disclosure inaccuracies reported by readers and verified by JAMA’s editors (most often through editor-author discussions) involve a complete lack of disclosure rather than questions of relevancy, Dr. Bauchner noted. (JAMA and the network’s journals receive about 30,000 disclosure forms each year.)
The AAMC, in the meantime, has developed a central web-based repository for disclosures called Convey. Physicians and others can maintain secure records of financial interests in the repository, and these records can then be disclosed directly to any journal or organization that uses the system. The tool – born from discussions that followed the 2009 IOM report on COI – is “intended to facilitate more complete, more accurate, and more consistent” disclosures,” said Ms. Pierce of the AAMC. It is now live and in its early stages of use; NEJM assisted in its development and has been one of its pilot testers.
Enforcement questions
Some experts believe that institutions should maintain public databases of disclosures and/or that disclosure requirements should be better enforced in-house.
“There often are no clear guidelines in institutions about how to respond to people who are negligent in how they’re managing their disclosures,” said Jeffrey R. Botkin, MD, MPH, professor of pediatrics and associate vice president for research at the University of Utah, Salt Lake City, who has served on a variety of ethics committees and is an elected member of the Hastings Center. Dr. Botkin proposed in a Viewpoint published last October in JAMA that failure to disclose significant COIs should be considered research misconduct (JAMA 2018;320[22]:2307-8).
At the University of Utah, “we’re getting better at saying, ‘show us that you’ve disclosed,’ ” he said. “In some cases we’ll do spot checks of journal articles to make sure [researchers have] followed through with their disclosures.”
John Abramson, MD, a lecturer in the department of health care policy at Harvard Medical School, Boston, contends that incomplete declarations of COI have been shown to correlate with reporting of manufacturer-friendly research results. Journals should have “zero tolerance” standards for incomplete or inaccurate COI declarations and should, among other things, “inform academic institutions of breaches of integrity.”
At JAMA, which in 2017 published a theme issue on COI and COI declarations, editors have been discussing whether they will contact an author’s institution “if there’s a pattern involved [with disclosure problems] or if there’s a lack of declaration of multiple COIs,” Dr. Bauchner said.
Physician PAC dollars support candidates against gun regulation
Despite many physician professional organizations endorsing policies that support firearm regulation, more of their political donations go to candidates who oppose those policies, according to a study of political action committee (PAC) campaign contributions during the 2016 election cycle.
“Our analysis indicates that most of the largest physician organizations’ PACs contribute more to candidates whose stances on firearm policy are in direct opposition to evidence-based firearm policies and to their organization’s stances,” wrote lead author Jeremiah D. Schuur, MD, of Brown University, Providence, R.I., and his coauthors.
The study was published in JAMA Network Open.
This retrospective, cross sectional study examined contributions from the 25 largest physician organization–affiliated PACs during the 2016 election cycle and compared them to federal candidate support for firearm regulation.
Support for regulation was measured by voting history on U.S. House and Senate legislation proposing firearm background checks and their rating from the National Rifle Association Political Victory Fund (NRA-PVF).
Health care professional–related PACs in general contributed $23.7 million during the 2016 election cycle; 57% of that sum ($13.6 million) came from the 25 largest physician-affiliated PACs.
Of the 29 Senate incumbents running for reelection who voted on S.A. 4750, an amendment that would have expanded background checks, those who voted against it (n = 21) received $500,000 more in contributions than did those who voted for it (n = 8).
The findings were similar with H.R. 1217, a bill in the House of Representatives to expand background checks; the PACs contributed $2,878,675 more to candidates who did not cosponsor it (n = 227) than to cosponsors (n = 166).
In regard to ratings from the NRA-PVF, the 25 PACs gave $5.6 million to candidates with an A rating and $4.1 million to candidates with a rating other than A.
But the trend was somewhat different when it came to the 2015 call to action on firearm-related injury and death, endorsed by several physician groups. Among the nine PACs with affiliated organizations that had endorsed the call to action, eight contributed to more candidates who did not support firearm safety policies. But after adjustment for political factors, those nine PACs had a lower likelihood of donating to NRA-PVF A-rated candidates, compared with nonendorsing PACs (odds ratio, 0.76; 95% confidence interval, 0.58-0.99; P = .04).
“Although endorsement of firearm safety policies may reflect a small difference in political giving, it does not mean that a physicians’ organization has elevated firearm policy to the level of a contribution criteria for the PAC,” the researchers wrote.
The researchers noted that it is “unlikely that physician organization–affiliated PACs contribute to candidates because they are opposed to firearm regulation.” Rather, they said, these PACs consider a number of factors, including a candidates’ stance on malpractice reform, physician payment policies, and the Patient Protection and Affordable Care Act, as well as their chance of winning.
The authors reported having no conflicts of interest.
SOURCE: Schuur JD et al. JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7831.
Though national membership organizations have finally taken a lead in advocating for firearm safety, this study from Schuur et al. illustrates the disconnect between physician PACs and the physicians themselves, according to Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, of the University of Michigan, Ann Arbor.
The study comes in the wake of the NRA admonishing physicians to “stay in their lane,” which was met by a very vocal response via social and mass media. “Health care professionals demonstrated that, contrary to the NRA position, they have an undeniably central role and authority in addressing this public health problem through the direct care that they provide to patients and their families, prevention-based research, and advocacy for policy-level changes that make patients safer,” they wrote.
The coauthors noted the parallels to the American Medical Association previously calling for tobacco regulation while financially supporting politicians who felt otherwise. It’s a comparison that is meant as a cautionary tale; as more focus is placed on this particular issue, “medical PACs must consider the increasing physician voice on the need to address firearm-associated morbidity and mortality in the policy arena to reduce their experience with this issue in emergency bays, operating rooms, and clinics.”
Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, are with the University of Michigan, Ann Arbor. They reported having no conflicts of interest. Their comments are adapted from an accompanying editorial (JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7823 ).
Though national membership organizations have finally taken a lead in advocating for firearm safety, this study from Schuur et al. illustrates the disconnect between physician PACs and the physicians themselves, according to Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, of the University of Michigan, Ann Arbor.
The study comes in the wake of the NRA admonishing physicians to “stay in their lane,” which was met by a very vocal response via social and mass media. “Health care professionals demonstrated that, contrary to the NRA position, they have an undeniably central role and authority in addressing this public health problem through the direct care that they provide to patients and their families, prevention-based research, and advocacy for policy-level changes that make patients safer,” they wrote.
The coauthors noted the parallels to the American Medical Association previously calling for tobacco regulation while financially supporting politicians who felt otherwise. It’s a comparison that is meant as a cautionary tale; as more focus is placed on this particular issue, “medical PACs must consider the increasing physician voice on the need to address firearm-associated morbidity and mortality in the policy arena to reduce their experience with this issue in emergency bays, operating rooms, and clinics.”
Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, are with the University of Michigan, Ann Arbor. They reported having no conflicts of interest. Their comments are adapted from an accompanying editorial (JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7823 ).
Though national membership organizations have finally taken a lead in advocating for firearm safety, this study from Schuur et al. illustrates the disconnect between physician PACs and the physicians themselves, according to Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, of the University of Michigan, Ann Arbor.
The study comes in the wake of the NRA admonishing physicians to “stay in their lane,” which was met by a very vocal response via social and mass media. “Health care professionals demonstrated that, contrary to the NRA position, they have an undeniably central role and authority in addressing this public health problem through the direct care that they provide to patients and their families, prevention-based research, and advocacy for policy-level changes that make patients safer,” they wrote.
The coauthors noted the parallels to the American Medical Association previously calling for tobacco regulation while financially supporting politicians who felt otherwise. It’s a comparison that is meant as a cautionary tale; as more focus is placed on this particular issue, “medical PACs must consider the increasing physician voice on the need to address firearm-associated morbidity and mortality in the policy arena to reduce their experience with this issue in emergency bays, operating rooms, and clinics.”
Rebecca M. Cunningham, MD, Marc A. Zimmerman, PhD, and Patrick M. Carter, MD, are with the University of Michigan, Ann Arbor. They reported having no conflicts of interest. Their comments are adapted from an accompanying editorial (JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7823 ).
Despite many physician professional organizations endorsing policies that support firearm regulation, more of their political donations go to candidates who oppose those policies, according to a study of political action committee (PAC) campaign contributions during the 2016 election cycle.
“Our analysis indicates that most of the largest physician organizations’ PACs contribute more to candidates whose stances on firearm policy are in direct opposition to evidence-based firearm policies and to their organization’s stances,” wrote lead author Jeremiah D. Schuur, MD, of Brown University, Providence, R.I., and his coauthors.
The study was published in JAMA Network Open.
This retrospective, cross sectional study examined contributions from the 25 largest physician organization–affiliated PACs during the 2016 election cycle and compared them to federal candidate support for firearm regulation.
Support for regulation was measured by voting history on U.S. House and Senate legislation proposing firearm background checks and their rating from the National Rifle Association Political Victory Fund (NRA-PVF).
Health care professional–related PACs in general contributed $23.7 million during the 2016 election cycle; 57% of that sum ($13.6 million) came from the 25 largest physician-affiliated PACs.
Of the 29 Senate incumbents running for reelection who voted on S.A. 4750, an amendment that would have expanded background checks, those who voted against it (n = 21) received $500,000 more in contributions than did those who voted for it (n = 8).
The findings were similar with H.R. 1217, a bill in the House of Representatives to expand background checks; the PACs contributed $2,878,675 more to candidates who did not cosponsor it (n = 227) than to cosponsors (n = 166).
In regard to ratings from the NRA-PVF, the 25 PACs gave $5.6 million to candidates with an A rating and $4.1 million to candidates with a rating other than A.
But the trend was somewhat different when it came to the 2015 call to action on firearm-related injury and death, endorsed by several physician groups. Among the nine PACs with affiliated organizations that had endorsed the call to action, eight contributed to more candidates who did not support firearm safety policies. But after adjustment for political factors, those nine PACs had a lower likelihood of donating to NRA-PVF A-rated candidates, compared with nonendorsing PACs (odds ratio, 0.76; 95% confidence interval, 0.58-0.99; P = .04).
“Although endorsement of firearm safety policies may reflect a small difference in political giving, it does not mean that a physicians’ organization has elevated firearm policy to the level of a contribution criteria for the PAC,” the researchers wrote.
The researchers noted that it is “unlikely that physician organization–affiliated PACs contribute to candidates because they are opposed to firearm regulation.” Rather, they said, these PACs consider a number of factors, including a candidates’ stance on malpractice reform, physician payment policies, and the Patient Protection and Affordable Care Act, as well as their chance of winning.
The authors reported having no conflicts of interest.
SOURCE: Schuur JD et al. JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7831.
Despite many physician professional organizations endorsing policies that support firearm regulation, more of their political donations go to candidates who oppose those policies, according to a study of political action committee (PAC) campaign contributions during the 2016 election cycle.
“Our analysis indicates that most of the largest physician organizations’ PACs contribute more to candidates whose stances on firearm policy are in direct opposition to evidence-based firearm policies and to their organization’s stances,” wrote lead author Jeremiah D. Schuur, MD, of Brown University, Providence, R.I., and his coauthors.
The study was published in JAMA Network Open.
This retrospective, cross sectional study examined contributions from the 25 largest physician organization–affiliated PACs during the 2016 election cycle and compared them to federal candidate support for firearm regulation.
Support for regulation was measured by voting history on U.S. House and Senate legislation proposing firearm background checks and their rating from the National Rifle Association Political Victory Fund (NRA-PVF).
Health care professional–related PACs in general contributed $23.7 million during the 2016 election cycle; 57% of that sum ($13.6 million) came from the 25 largest physician-affiliated PACs.
Of the 29 Senate incumbents running for reelection who voted on S.A. 4750, an amendment that would have expanded background checks, those who voted against it (n = 21) received $500,000 more in contributions than did those who voted for it (n = 8).
The findings were similar with H.R. 1217, a bill in the House of Representatives to expand background checks; the PACs contributed $2,878,675 more to candidates who did not cosponsor it (n = 227) than to cosponsors (n = 166).
In regard to ratings from the NRA-PVF, the 25 PACs gave $5.6 million to candidates with an A rating and $4.1 million to candidates with a rating other than A.
But the trend was somewhat different when it came to the 2015 call to action on firearm-related injury and death, endorsed by several physician groups. Among the nine PACs with affiliated organizations that had endorsed the call to action, eight contributed to more candidates who did not support firearm safety policies. But after adjustment for political factors, those nine PACs had a lower likelihood of donating to NRA-PVF A-rated candidates, compared with nonendorsing PACs (odds ratio, 0.76; 95% confidence interval, 0.58-0.99; P = .04).
“Although endorsement of firearm safety policies may reflect a small difference in political giving, it does not mean that a physicians’ organization has elevated firearm policy to the level of a contribution criteria for the PAC,” the researchers wrote.
The researchers noted that it is “unlikely that physician organization–affiliated PACs contribute to candidates because they are opposed to firearm regulation.” Rather, they said, these PACs consider a number of factors, including a candidates’ stance on malpractice reform, physician payment policies, and the Patient Protection and Affordable Care Act, as well as their chance of winning.
The authors reported having no conflicts of interest.
SOURCE: Schuur JD et al. JAMA Netw Open. 2019 Feb 22. doi: 10.1001/jamanetworkopen.2018.7831.
FROM JAMA NETWORK OPEN
Health spending: Boomers will spike costs, but growing uninsured will soften their impact
Spending on health care is projected to rise at a faster-than-average rate throughout the next decade, according to the Office of the Actuary at the Centers for Medicare & Medicaid Services.
“Overall, national health spending is projected to grow at 5.5% per year, on average, for 2018-27,” wrote Andrea Sisko, economist in the Office of the Actuary, and colleagues (Health Aff. 2019 Feb 20. doi: 10.1377/hlthaff.2018.05499). “This is faster than the average growth rate experienced following the last recession (3.9% for 2008-2013) and the more recent period inclusive of the Affordable Care Act’s major coverage expansions (5.3% for 2014-16).”
Medicare is projected to see the fastest growth in spending at 7.4% per year “as the shift of the Baby Boom generation into the program continues to result in robust growth in enrollment,” according to the authors.
Private payers should see a corollary slower growth in spending (4.8% per year) over the same period, while Medicaid spending is projected at 5.5% per year.
Faster growth in Medicare spending is expected to come from higher spending on prescription drugs and hospital services, as well as higher fee-for-service payment updates.
Spending increases are projected to be mitigated somewhat by the end of the ACA penalty for not having insurance – which is projected to add 1.3 million people this year to the ranks of the uninsured, according to the report.
Half of the overall growth in health care spending is attributable to rising prices in personal health care prices, on average, Ms. Sisko and colleagues wrote. “Growth in use and intensity is expected to account for just under one-third of the average annual personal health care spending growth, with population growth and the changing age-sex mix of the population accounting for the remainder.”
For those with private insurance, out-of-pocket spending is projected to accelerate to a 3.6% growth rate in 2018 from 2.6% in 2017 “a rate that is consistent with faster income growth as well as with the higher average deductibles for employer-based private health insurance enrollees in 2018 compared to 2017,” the authors note.
“Growth in out-of-pocket spending, which is also primarily influenced by economic factors, is expected to be similar to that of private health insurance spending in 2020-27, at 5%,” they add.
Prescription drug spending also is expected to grow.
“Following growth of just 0.4% in 2017, prescription drug spending is expected to have grown 3.3% in 2018 but still be among the slowest-growing health care sectors,” according to the authors. “Higher utilization growth is anticipated, compared to the relatively low growth in 2016 and 2017, partially driven by an increase in the number of new drug introductions.”
Growth in prescription drug spending is expected to accelerate further to 4.6% in 2019, based on growth in utilization and a “modest increase in drug price growth.”
Starting in 2020, that growth rate is projected to increase, on average, by 6.1% per year, based on the expectation that employers and insurers will lower barriers to maintenance medications for chronic conditions.
In 2019, growth in spending for physician clinical services is projected to accelerate to 5.4% from 4.9% in 2018.
“An acceleration in Medicaid spending growth is the primary factor contributing to the trend, which is in part associated with program’s expansion by additional states,” the authors note.
From 2020 to 2027, growth in spending on physician and clinical services is expected to average 5.4% per year, driven in part by price growth for these services.
“Underlying this acceleration are projected rising costs related to the provision of care,” the report said. “In particular, wages are expected to increase as a result of the supply of physicians not being able to meet expected increases in demand for care connected with the aging population. Furthermore, some of the productivity gains that have been achieved through the use of lower-cost providers as a substitute for physician care within physician practices may be less pronounced in the future, because of limitations such as licensing restrictions on the scope of care that may be provided by nonphysician providers.”
SOURCE: Sisko A et al. Health Aff. 2019 Feb 20. doi: 10.1377/hlthaff.2018.05499.
Spending on health care is projected to rise at a faster-than-average rate throughout the next decade, according to the Office of the Actuary at the Centers for Medicare & Medicaid Services.
“Overall, national health spending is projected to grow at 5.5% per year, on average, for 2018-27,” wrote Andrea Sisko, economist in the Office of the Actuary, and colleagues (Health Aff. 2019 Feb 20. doi: 10.1377/hlthaff.2018.05499). “This is faster than the average growth rate experienced following the last recession (3.9% for 2008-2013) and the more recent period inclusive of the Affordable Care Act’s major coverage expansions (5.3% for 2014-16).”
Medicare is projected to see the fastest growth in spending at 7.4% per year “as the shift of the Baby Boom generation into the program continues to result in robust growth in enrollment,” according to the authors.
Private payers should see a corollary slower growth in spending (4.8% per year) over the same period, while Medicaid spending is projected at 5.5% per year.
Faster growth in Medicare spending is expected to come from higher spending on prescription drugs and hospital services, as well as higher fee-for-service payment updates.
Spending increases are projected to be mitigated somewhat by the end of the ACA penalty for not having insurance – which is projected to add 1.3 million people this year to the ranks of the uninsured, according to the report.
Half of the overall growth in health care spending is attributable to rising prices in personal health care prices, on average, Ms. Sisko and colleagues wrote. “Growth in use and intensity is expected to account for just under one-third of the average annual personal health care spending growth, with population growth and the changing age-sex mix of the population accounting for the remainder.”
For those with private insurance, out-of-pocket spending is projected to accelerate to a 3.6% growth rate in 2018 from 2.6% in 2017 “a rate that is consistent with faster income growth as well as with the higher average deductibles for employer-based private health insurance enrollees in 2018 compared to 2017,” the authors note.
“Growth in out-of-pocket spending, which is also primarily influenced by economic factors, is expected to be similar to that of private health insurance spending in 2020-27, at 5%,” they add.
Prescription drug spending also is expected to grow.
“Following growth of just 0.4% in 2017, prescription drug spending is expected to have grown 3.3% in 2018 but still be among the slowest-growing health care sectors,” according to the authors. “Higher utilization growth is anticipated, compared to the relatively low growth in 2016 and 2017, partially driven by an increase in the number of new drug introductions.”
Growth in prescription drug spending is expected to accelerate further to 4.6% in 2019, based on growth in utilization and a “modest increase in drug price growth.”
Starting in 2020, that growth rate is projected to increase, on average, by 6.1% per year, based on the expectation that employers and insurers will lower barriers to maintenance medications for chronic conditions.
In 2019, growth in spending for physician clinical services is projected to accelerate to 5.4% from 4.9% in 2018.
“An acceleration in Medicaid spending growth is the primary factor contributing to the trend, which is in part associated with program’s expansion by additional states,” the authors note.
From 2020 to 2027, growth in spending on physician and clinical services is expected to average 5.4% per year, driven in part by price growth for these services.
“Underlying this acceleration are projected rising costs related to the provision of care,” the report said. “In particular, wages are expected to increase as a result of the supply of physicians not being able to meet expected increases in demand for care connected with the aging population. Furthermore, some of the productivity gains that have been achieved through the use of lower-cost providers as a substitute for physician care within physician practices may be less pronounced in the future, because of limitations such as licensing restrictions on the scope of care that may be provided by nonphysician providers.”
SOURCE: Sisko A et al. Health Aff. 2019 Feb 20. doi: 10.1377/hlthaff.2018.05499.
Spending on health care is projected to rise at a faster-than-average rate throughout the next decade, according to the Office of the Actuary at the Centers for Medicare & Medicaid Services.
“Overall, national health spending is projected to grow at 5.5% per year, on average, for 2018-27,” wrote Andrea Sisko, economist in the Office of the Actuary, and colleagues (Health Aff. 2019 Feb 20. doi: 10.1377/hlthaff.2018.05499). “This is faster than the average growth rate experienced following the last recession (3.9% for 2008-2013) and the more recent period inclusive of the Affordable Care Act’s major coverage expansions (5.3% for 2014-16).”
Medicare is projected to see the fastest growth in spending at 7.4% per year “as the shift of the Baby Boom generation into the program continues to result in robust growth in enrollment,” according to the authors.
Private payers should see a corollary slower growth in spending (4.8% per year) over the same period, while Medicaid spending is projected at 5.5% per year.
Faster growth in Medicare spending is expected to come from higher spending on prescription drugs and hospital services, as well as higher fee-for-service payment updates.
Spending increases are projected to be mitigated somewhat by the end of the ACA penalty for not having insurance – which is projected to add 1.3 million people this year to the ranks of the uninsured, according to the report.
Half of the overall growth in health care spending is attributable to rising prices in personal health care prices, on average, Ms. Sisko and colleagues wrote. “Growth in use and intensity is expected to account for just under one-third of the average annual personal health care spending growth, with population growth and the changing age-sex mix of the population accounting for the remainder.”
For those with private insurance, out-of-pocket spending is projected to accelerate to a 3.6% growth rate in 2018 from 2.6% in 2017 “a rate that is consistent with faster income growth as well as with the higher average deductibles for employer-based private health insurance enrollees in 2018 compared to 2017,” the authors note.
“Growth in out-of-pocket spending, which is also primarily influenced by economic factors, is expected to be similar to that of private health insurance spending in 2020-27, at 5%,” they add.
Prescription drug spending also is expected to grow.
“Following growth of just 0.4% in 2017, prescription drug spending is expected to have grown 3.3% in 2018 but still be among the slowest-growing health care sectors,” according to the authors. “Higher utilization growth is anticipated, compared to the relatively low growth in 2016 and 2017, partially driven by an increase in the number of new drug introductions.”
Growth in prescription drug spending is expected to accelerate further to 4.6% in 2019, based on growth in utilization and a “modest increase in drug price growth.”
Starting in 2020, that growth rate is projected to increase, on average, by 6.1% per year, based on the expectation that employers and insurers will lower barriers to maintenance medications for chronic conditions.
In 2019, growth in spending for physician clinical services is projected to accelerate to 5.4% from 4.9% in 2018.
“An acceleration in Medicaid spending growth is the primary factor contributing to the trend, which is in part associated with program’s expansion by additional states,” the authors note.
From 2020 to 2027, growth in spending on physician and clinical services is expected to average 5.4% per year, driven in part by price growth for these services.
“Underlying this acceleration are projected rising costs related to the provision of care,” the report said. “In particular, wages are expected to increase as a result of the supply of physicians not being able to meet expected increases in demand for care connected with the aging population. Furthermore, some of the productivity gains that have been achieved through the use of lower-cost providers as a substitute for physician care within physician practices may be less pronounced in the future, because of limitations such as licensing restrictions on the scope of care that may be provided by nonphysician providers.”
SOURCE: Sisko A et al. Health Aff. 2019 Feb 20. doi: 10.1377/hlthaff.2018.05499.
FROM HEALTH AFFAIRS
McAneny: Transparency needed for meaningful talk on drug pricing
WASHINGTON – As the rising cost of prescription drugs continues to garner heightened scrutiny from the federal government, one thing is missing from the conversation that would make any solution more effective, according to American Medical Association President Barbara L. McAneny, MD.
“We would like to see transparency from end to end in this pipeline because that is the way we will have the ability to look for savings,” she said in an interview at a national advocacy conference sponsored by the American Medical Association.
“I think we don’t have the information we need to make rational decisions,” she said. “I think the first thing we need to do is to understand the entire pipeline from the basic research that results in a drug’s clinical trials that results in a new drug to the pharmacy benefit managers and all of the ways that they have increased the cost of the drugs all the way to when the patient actually gets it.”
In particular, Dr. McAneny targeted the need for transparency in the role and financial impact pharmacy benefit managers have on the cost of prescription drugs.
“We were told at our state advocacy conference, which we held in January, by an expert who studied pharmacy benefit managers, that 42% of the cost of any drug is attributable to the profits of pharmacy benefit managers,” she said. “To me, that makes me wonder what value do they add that is worth 42% of these exorbitant costs and if they are not adding value, why do we have them in this process?”
She also called on the pharmaceutical manufacturers to be more forthcoming with their financial information regarding marketing and advertising, but she stressed that it needs to be done in a way that does not hinder future development of life-saving therapies.
“We do not want to stifle innovation.” Dr. McAneny said. “As a cancer doctor, I have seen diseases that I used to treat with morphine and sympathy now be diseases that I can treat, where I can restore people to good quality of life and buy them additional years of life because of these drugs. They are amazing and I don’t want to do without them and I want more of them. ... We want to support that research. That is probably worth a lot of the price tag. But how much of that goes to direct-to-consumer advertising? How much of that is the advertising budget? Where can we cut some of this out of the system so that we get the innovation, but we get innovation that all of our patients can afford?”
Another issue that is looming for physicians is a payment rate freeze from 2020-2025 under the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program, which was created under the Medicare Access and CHIP Reauthorization Act (MACRA).
“A 5-year freeze when my expenses do not freeze is a terrifying thing and could be a practice-ending expense for a lot of practices,” Dr. McAneny warned. “I will point out that independent practices, if they sell to a hospital, the cost of care immediately doubles. The amount that is paid for it, not the cost of delivering it. So that is not a great solution. But we also have this increasing practice expense and a flat rate is not going to help practices survive. So I am very concerned.”
Related to the QPP, Dr. McAneny also wants to see the Centers for Medicare & Medicaid Services do more with physician-developed alternative payment models. The Physician-Focused Payment Model Technical Advisory Committee (PTAC) continues to review and evaluate submissions, but to date, the CMS has yet to implement any of the committee’s recommendations.
“I read all of the PTAC submissions that went to CMS. Some of them I thought were a little on the weak side, but there are a lot of them that I thought were really good ideas. I do not know why CMS did not approve them and fund them and let them be tried,” she said, although she did offer an opinion on why the agency has yet to act on any of them.
“I read their paper saying why they didn’t approve [the recommended physician-developed alternative payment models]. It seemed to me they are waiting for one silver bullet that will fix all of health care. I don’t think a silver bullet is going to fix health care. It’s not an issue. It’s a complicated set of issues. You will not have a quick fix.”
WASHINGTON – As the rising cost of prescription drugs continues to garner heightened scrutiny from the federal government, one thing is missing from the conversation that would make any solution more effective, according to American Medical Association President Barbara L. McAneny, MD.
“We would like to see transparency from end to end in this pipeline because that is the way we will have the ability to look for savings,” she said in an interview at a national advocacy conference sponsored by the American Medical Association.
“I think we don’t have the information we need to make rational decisions,” she said. “I think the first thing we need to do is to understand the entire pipeline from the basic research that results in a drug’s clinical trials that results in a new drug to the pharmacy benefit managers and all of the ways that they have increased the cost of the drugs all the way to when the patient actually gets it.”
In particular, Dr. McAneny targeted the need for transparency in the role and financial impact pharmacy benefit managers have on the cost of prescription drugs.
“We were told at our state advocacy conference, which we held in January, by an expert who studied pharmacy benefit managers, that 42% of the cost of any drug is attributable to the profits of pharmacy benefit managers,” she said. “To me, that makes me wonder what value do they add that is worth 42% of these exorbitant costs and if they are not adding value, why do we have them in this process?”
She also called on the pharmaceutical manufacturers to be more forthcoming with their financial information regarding marketing and advertising, but she stressed that it needs to be done in a way that does not hinder future development of life-saving therapies.
“We do not want to stifle innovation.” Dr. McAneny said. “As a cancer doctor, I have seen diseases that I used to treat with morphine and sympathy now be diseases that I can treat, where I can restore people to good quality of life and buy them additional years of life because of these drugs. They are amazing and I don’t want to do without them and I want more of them. ... We want to support that research. That is probably worth a lot of the price tag. But how much of that goes to direct-to-consumer advertising? How much of that is the advertising budget? Where can we cut some of this out of the system so that we get the innovation, but we get innovation that all of our patients can afford?”
Another issue that is looming for physicians is a payment rate freeze from 2020-2025 under the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program, which was created under the Medicare Access and CHIP Reauthorization Act (MACRA).
“A 5-year freeze when my expenses do not freeze is a terrifying thing and could be a practice-ending expense for a lot of practices,” Dr. McAneny warned. “I will point out that independent practices, if they sell to a hospital, the cost of care immediately doubles. The amount that is paid for it, not the cost of delivering it. So that is not a great solution. But we also have this increasing practice expense and a flat rate is not going to help practices survive. So I am very concerned.”
Related to the QPP, Dr. McAneny also wants to see the Centers for Medicare & Medicaid Services do more with physician-developed alternative payment models. The Physician-Focused Payment Model Technical Advisory Committee (PTAC) continues to review and evaluate submissions, but to date, the CMS has yet to implement any of the committee’s recommendations.
“I read all of the PTAC submissions that went to CMS. Some of them I thought were a little on the weak side, but there are a lot of them that I thought were really good ideas. I do not know why CMS did not approve them and fund them and let them be tried,” she said, although she did offer an opinion on why the agency has yet to act on any of them.
“I read their paper saying why they didn’t approve [the recommended physician-developed alternative payment models]. It seemed to me they are waiting for one silver bullet that will fix all of health care. I don’t think a silver bullet is going to fix health care. It’s not an issue. It’s a complicated set of issues. You will not have a quick fix.”
WASHINGTON – As the rising cost of prescription drugs continues to garner heightened scrutiny from the federal government, one thing is missing from the conversation that would make any solution more effective, according to American Medical Association President Barbara L. McAneny, MD.
“We would like to see transparency from end to end in this pipeline because that is the way we will have the ability to look for savings,” she said in an interview at a national advocacy conference sponsored by the American Medical Association.
“I think we don’t have the information we need to make rational decisions,” she said. “I think the first thing we need to do is to understand the entire pipeline from the basic research that results in a drug’s clinical trials that results in a new drug to the pharmacy benefit managers and all of the ways that they have increased the cost of the drugs all the way to when the patient actually gets it.”
In particular, Dr. McAneny targeted the need for transparency in the role and financial impact pharmacy benefit managers have on the cost of prescription drugs.
“We were told at our state advocacy conference, which we held in January, by an expert who studied pharmacy benefit managers, that 42% of the cost of any drug is attributable to the profits of pharmacy benefit managers,” she said. “To me, that makes me wonder what value do they add that is worth 42% of these exorbitant costs and if they are not adding value, why do we have them in this process?”
She also called on the pharmaceutical manufacturers to be more forthcoming with their financial information regarding marketing and advertising, but she stressed that it needs to be done in a way that does not hinder future development of life-saving therapies.
“We do not want to stifle innovation.” Dr. McAneny said. “As a cancer doctor, I have seen diseases that I used to treat with morphine and sympathy now be diseases that I can treat, where I can restore people to good quality of life and buy them additional years of life because of these drugs. They are amazing and I don’t want to do without them and I want more of them. ... We want to support that research. That is probably worth a lot of the price tag. But how much of that goes to direct-to-consumer advertising? How much of that is the advertising budget? Where can we cut some of this out of the system so that we get the innovation, but we get innovation that all of our patients can afford?”
Another issue that is looming for physicians is a payment rate freeze from 2020-2025 under the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program, which was created under the Medicare Access and CHIP Reauthorization Act (MACRA).
“A 5-year freeze when my expenses do not freeze is a terrifying thing and could be a practice-ending expense for a lot of practices,” Dr. McAneny warned. “I will point out that independent practices, if they sell to a hospital, the cost of care immediately doubles. The amount that is paid for it, not the cost of delivering it. So that is not a great solution. But we also have this increasing practice expense and a flat rate is not going to help practices survive. So I am very concerned.”
Related to the QPP, Dr. McAneny also wants to see the Centers for Medicare & Medicaid Services do more with physician-developed alternative payment models. The Physician-Focused Payment Model Technical Advisory Committee (PTAC) continues to review and evaluate submissions, but to date, the CMS has yet to implement any of the committee’s recommendations.
“I read all of the PTAC submissions that went to CMS. Some of them I thought were a little on the weak side, but there are a lot of them that I thought were really good ideas. I do not know why CMS did not approve them and fund them and let them be tried,” she said, although she did offer an opinion on why the agency has yet to act on any of them.
“I read their paper saying why they didn’t approve [the recommended physician-developed alternative payment models]. It seemed to me they are waiting for one silver bullet that will fix all of health care. I don’t think a silver bullet is going to fix health care. It’s not an issue. It’s a complicated set of issues. You will not have a quick fix.”
REPORTING FROM THE AMA NATIONAL ADVOCACY CONFERENCE
A prescription for medicine’s gender inequality
A small study identifies skin microbiome changes after UV exposure. Lower prices drive OTC insulin sales at Walmart. And early intensive treatment of multiple sclerosis may benefit patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A small study identifies skin microbiome changes after UV exposure. Lower prices drive OTC insulin sales at Walmart. And early intensive treatment of multiple sclerosis may benefit patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A small study identifies skin microbiome changes after UV exposure. Lower prices drive OTC insulin sales at Walmart. And early intensive treatment of multiple sclerosis may benefit patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Possible mechanism for fluoroquinolone-induced aortopathy uncovered
A new study finds that patients taking fluoroquinolone antibiotics may be at higher risk of aortopathy in part because of human aortic myofibroblast–mediated extracellular matrix (ECM) dysregulation.
“Emerging evidence supports pharmacologic-associated aortopathy in patients receiving fluoroquinolone [FQ] antibiotics,” said first author David G. Guzzardi, PhD, and his colleagues, citing previous research showing that, “compared with patients receiving amoxicillin antibiotics, those receiving FQ have a 66% higher risk of aneurysm or dissection within a 2-month period after commencing FQ use.”
Based upon such data, the Food and Drug Administration issued a December 2018 warning about the increased risk of ruptures or tears in the aorta with fluoroquinolone antibiotics in certain patients, updating their May 2017 warning regarding “disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” upon exposure to this class of antibiotics. Earlier in 2018, the FDA had reinforced their safety information about serious and potentially fatal low blood sugar levels and mental health side effects with fluoroquinolone antibiotics.
Dr. Guzzardi and his colleagues at the University of Calgary (Alta.) performed a study to attempt to determine the possible cellular mechanisms for the observed aortopathy. In their study published in the Journal of Thoracic and Cardiovascular Surgery, Dr. Guzzardi and his colleagues isolated human aortic myofibroblasts from nine patients with aortopathy who were undergoing elective ascending aortic resection.
Following exposure of cells to FQ, the researchers assessed secreted matrix metalloproteinases relative to tissue inhibitors of matrix metalloproteinases (TIMPs). In addition, they examined ECM degradation by using a three-dimensional gelatin-fluorescein isothiocyanate fluorescence microgel assay. Aortic cellular collagen type I expression following FQ exposure was determined by immunoblotting and immunofluorescent staining. Dr. Guzzardi and his colleagues also looked at cell apoptosis, necrosis, and metabolic viability using two versions of vital staining.
They found that FQ exposure significantly decreased aortic cell TIMP-1 (P less than .004) and TIMP-2 (P less than .0004) protein expression, compared with controls, and the ratio of matrix metalloproteinase 9/TIMP-2 was increased (P less than .01). This suggests an increased capacity for ECM degradation after FQ exposure, according to the researchers.
In addition, FQ exposure attenuated collagen type I expression as assessed by immunoblotting (P less than .002) and immunofluorescence (P less than .02).
“FQ induces human aortic myofibroblast–mediated ECM dysregulation by decreasing TIMP expression and preventing compensatory collagen deposition. These data provide novel insights into the mechanisms that may underlie the clinical association of FQ exposure and increased risk of acute aortic events in the community. Our data suggest cautious use of FQ in selected patient populations with preexistent aortopathy and connective tissue disorders,” the researchers concluded.
In an accompanying editorial, while warning that these are preliminary observations based upon a small number of patients with aortopathy, Ari A. Mennander, MD, PhD, of Tampere (Finland) University, wrote that, “for the first time, the wild theory of fluoroquinolone-associated aortopathy has a molecular hint that is based on collagen degeneration and progression of aortic disease. ... This theory is in line with previous observations revealing antifibrotic activity and decreased collagen-1 protein expression with fluoroquinolones. The enigmatic puzzle of the progression of some aortic events may alarmingly be iatrogenic, and the clinician may wisely consider a prudent use of fluoroquinolones in patients with aortic dilatation.”
The authors and commentators reported that they had no commercial conflicts to disclose.
SOURCE: Guzzardi DG et al. J Thorac Cardiovasc Surg. 2019;157:109-19.
The issue of fluoroquinolones is certainly of concern. I wonder how many of my patients who have suffered a ruptured aneurysm were on one of these drugs? In the last few years, Cipro (ciprofloxacin) and Levaquin (levofloxacin) were commonly used by our family practice and internal medicine colleagues for almost all outpatient infections. It was so common that even my wife, who is not a physician, would request Cipro whenever she had a sneeze. We would also bring large bottles of Cipro every time we went traveling to some exotic destination, reassuring ourselves that the only “runs” we would have would be in the airport trying to catch a flight. A cousin of mine, prescribed Levaquin by a well-meaning physician while he was cruising the Nile River, ruptured his Achilles tendon.
Russell H. Samson, MD, FACS, DFSVS , is a clinical professor of surgery at Florida State University, Sarasota, a senior surgeon at Sarasota Vascular Specialists, and President of the Mote Vascular Foundation.
The issue of fluoroquinolones is certainly of concern. I wonder how many of my patients who have suffered a ruptured aneurysm were on one of these drugs? In the last few years, Cipro (ciprofloxacin) and Levaquin (levofloxacin) were commonly used by our family practice and internal medicine colleagues for almost all outpatient infections. It was so common that even my wife, who is not a physician, would request Cipro whenever she had a sneeze. We would also bring large bottles of Cipro every time we went traveling to some exotic destination, reassuring ourselves that the only “runs” we would have would be in the airport trying to catch a flight. A cousin of mine, prescribed Levaquin by a well-meaning physician while he was cruising the Nile River, ruptured his Achilles tendon.
Russell H. Samson, MD, FACS, DFSVS , is a clinical professor of surgery at Florida State University, Sarasota, a senior surgeon at Sarasota Vascular Specialists, and President of the Mote Vascular Foundation.
The issue of fluoroquinolones is certainly of concern. I wonder how many of my patients who have suffered a ruptured aneurysm were on one of these drugs? In the last few years, Cipro (ciprofloxacin) and Levaquin (levofloxacin) were commonly used by our family practice and internal medicine colleagues for almost all outpatient infections. It was so common that even my wife, who is not a physician, would request Cipro whenever she had a sneeze. We would also bring large bottles of Cipro every time we went traveling to some exotic destination, reassuring ourselves that the only “runs” we would have would be in the airport trying to catch a flight. A cousin of mine, prescribed Levaquin by a well-meaning physician while he was cruising the Nile River, ruptured his Achilles tendon.
Russell H. Samson, MD, FACS, DFSVS , is a clinical professor of surgery at Florida State University, Sarasota, a senior surgeon at Sarasota Vascular Specialists, and President of the Mote Vascular Foundation.
A new study finds that patients taking fluoroquinolone antibiotics may be at higher risk of aortopathy in part because of human aortic myofibroblast–mediated extracellular matrix (ECM) dysregulation.
“Emerging evidence supports pharmacologic-associated aortopathy in patients receiving fluoroquinolone [FQ] antibiotics,” said first author David G. Guzzardi, PhD, and his colleagues, citing previous research showing that, “compared with patients receiving amoxicillin antibiotics, those receiving FQ have a 66% higher risk of aneurysm or dissection within a 2-month period after commencing FQ use.”
Based upon such data, the Food and Drug Administration issued a December 2018 warning about the increased risk of ruptures or tears in the aorta with fluoroquinolone antibiotics in certain patients, updating their May 2017 warning regarding “disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” upon exposure to this class of antibiotics. Earlier in 2018, the FDA had reinforced their safety information about serious and potentially fatal low blood sugar levels and mental health side effects with fluoroquinolone antibiotics.
Dr. Guzzardi and his colleagues at the University of Calgary (Alta.) performed a study to attempt to determine the possible cellular mechanisms for the observed aortopathy. In their study published in the Journal of Thoracic and Cardiovascular Surgery, Dr. Guzzardi and his colleagues isolated human aortic myofibroblasts from nine patients with aortopathy who were undergoing elective ascending aortic resection.
Following exposure of cells to FQ, the researchers assessed secreted matrix metalloproteinases relative to tissue inhibitors of matrix metalloproteinases (TIMPs). In addition, they examined ECM degradation by using a three-dimensional gelatin-fluorescein isothiocyanate fluorescence microgel assay. Aortic cellular collagen type I expression following FQ exposure was determined by immunoblotting and immunofluorescent staining. Dr. Guzzardi and his colleagues also looked at cell apoptosis, necrosis, and metabolic viability using two versions of vital staining.
They found that FQ exposure significantly decreased aortic cell TIMP-1 (P less than .004) and TIMP-2 (P less than .0004) protein expression, compared with controls, and the ratio of matrix metalloproteinase 9/TIMP-2 was increased (P less than .01). This suggests an increased capacity for ECM degradation after FQ exposure, according to the researchers.
In addition, FQ exposure attenuated collagen type I expression as assessed by immunoblotting (P less than .002) and immunofluorescence (P less than .02).
“FQ induces human aortic myofibroblast–mediated ECM dysregulation by decreasing TIMP expression and preventing compensatory collagen deposition. These data provide novel insights into the mechanisms that may underlie the clinical association of FQ exposure and increased risk of acute aortic events in the community. Our data suggest cautious use of FQ in selected patient populations with preexistent aortopathy and connective tissue disorders,” the researchers concluded.
In an accompanying editorial, while warning that these are preliminary observations based upon a small number of patients with aortopathy, Ari A. Mennander, MD, PhD, of Tampere (Finland) University, wrote that, “for the first time, the wild theory of fluoroquinolone-associated aortopathy has a molecular hint that is based on collagen degeneration and progression of aortic disease. ... This theory is in line with previous observations revealing antifibrotic activity and decreased collagen-1 protein expression with fluoroquinolones. The enigmatic puzzle of the progression of some aortic events may alarmingly be iatrogenic, and the clinician may wisely consider a prudent use of fluoroquinolones in patients with aortic dilatation.”
The authors and commentators reported that they had no commercial conflicts to disclose.
SOURCE: Guzzardi DG et al. J Thorac Cardiovasc Surg. 2019;157:109-19.
A new study finds that patients taking fluoroquinolone antibiotics may be at higher risk of aortopathy in part because of human aortic myofibroblast–mediated extracellular matrix (ECM) dysregulation.
“Emerging evidence supports pharmacologic-associated aortopathy in patients receiving fluoroquinolone [FQ] antibiotics,” said first author David G. Guzzardi, PhD, and his colleagues, citing previous research showing that, “compared with patients receiving amoxicillin antibiotics, those receiving FQ have a 66% higher risk of aneurysm or dissection within a 2-month period after commencing FQ use.”
Based upon such data, the Food and Drug Administration issued a December 2018 warning about the increased risk of ruptures or tears in the aorta with fluoroquinolone antibiotics in certain patients, updating their May 2017 warning regarding “disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” upon exposure to this class of antibiotics. Earlier in 2018, the FDA had reinforced their safety information about serious and potentially fatal low blood sugar levels and mental health side effects with fluoroquinolone antibiotics.
Dr. Guzzardi and his colleagues at the University of Calgary (Alta.) performed a study to attempt to determine the possible cellular mechanisms for the observed aortopathy. In their study published in the Journal of Thoracic and Cardiovascular Surgery, Dr. Guzzardi and his colleagues isolated human aortic myofibroblasts from nine patients with aortopathy who were undergoing elective ascending aortic resection.
Following exposure of cells to FQ, the researchers assessed secreted matrix metalloproteinases relative to tissue inhibitors of matrix metalloproteinases (TIMPs). In addition, they examined ECM degradation by using a three-dimensional gelatin-fluorescein isothiocyanate fluorescence microgel assay. Aortic cellular collagen type I expression following FQ exposure was determined by immunoblotting and immunofluorescent staining. Dr. Guzzardi and his colleagues also looked at cell apoptosis, necrosis, and metabolic viability using two versions of vital staining.
They found that FQ exposure significantly decreased aortic cell TIMP-1 (P less than .004) and TIMP-2 (P less than .0004) protein expression, compared with controls, and the ratio of matrix metalloproteinase 9/TIMP-2 was increased (P less than .01). This suggests an increased capacity for ECM degradation after FQ exposure, according to the researchers.
In addition, FQ exposure attenuated collagen type I expression as assessed by immunoblotting (P less than .002) and immunofluorescence (P less than .02).
“FQ induces human aortic myofibroblast–mediated ECM dysregulation by decreasing TIMP expression and preventing compensatory collagen deposition. These data provide novel insights into the mechanisms that may underlie the clinical association of FQ exposure and increased risk of acute aortic events in the community. Our data suggest cautious use of FQ in selected patient populations with preexistent aortopathy and connective tissue disorders,” the researchers concluded.
In an accompanying editorial, while warning that these are preliminary observations based upon a small number of patients with aortopathy, Ari A. Mennander, MD, PhD, of Tampere (Finland) University, wrote that, “for the first time, the wild theory of fluoroquinolone-associated aortopathy has a molecular hint that is based on collagen degeneration and progression of aortic disease. ... This theory is in line with previous observations revealing antifibrotic activity and decreased collagen-1 protein expression with fluoroquinolones. The enigmatic puzzle of the progression of some aortic events may alarmingly be iatrogenic, and the clinician may wisely consider a prudent use of fluoroquinolones in patients with aortic dilatation.”
The authors and commentators reported that they had no commercial conflicts to disclose.
SOURCE: Guzzardi DG et al. J Thorac Cardiovasc Surg. 2019;157:109-19.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY