User login
Mini-invasive MV repair as safe, effective as sternotomy surgery but has advantages: UK Mini-Mitral Trial
Patients with degenerative mitral valve (MV) regurgitation that calls for surgery may, for the most part, safely choose either a standard procedure requiring a midline sternotomy or one performed through a minithoracotomy, suggests a randomized comparison of the two techniques.
Still, the minimally invasive approach showed some advantages in the study. Patients’ quality of recovery was about the same with both procedures at 12 weeks, but those who had the minimally invasive thoracoscopy-guided surgery had shown greater improvement 6 weeks earlier.
Also in the UK Mini Mitral Trial, hospital length of stay (LOS) was significantly shorter for patients who underwent the mini-thoracotomy procedure, and that group spent fewer days in the hospital over the following months.
But neither procedure had an edge in terms of postoperative clinical risk in the study. Rates of clinical events, such as death or hospitalization for heart failure (HHF), were about the same over 1 year.
Patients in this trial had been deemed suitable for either of the two surgeries, which were always performed by surgeons specially chosen by the steering committee for their experience and expertise.
This first randomized head-to-head comparison of the two approaches in such patients should make both patients and clinicians more confident about choosing the minimally invasive surgery for degenerative MV disease, said Enoch Akowuah, MD, Newcastle (England) University, United Kingdom.
Dr. Akowuah presented the UK Mini-Mitral Trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
A “main takeaway” for clinical practice from the trial would be that minithoracotomy MV repair “is as safe and effective as conventional sternotomy for degenerative mitral regurgitation,” said discussant Amy E. Simone, PA-C, following Dr. Akowuah’s presentation.
“I think this study is unique in that its focus is on delivering high-quality, cost-efficient care for mitral regurgitation, but also with an emphasis on patients’ goals and wishes,” said Ms. Simone, who directs the Marcus Heart Valve Center of the Piedmont Heart Institute, Atlanta.
Cardiac surgeon Thomas MacGillivray, MD, another discussant, agreed that the data presented from at least this study suggest neither the minithoracotomy nor sternotomy approach is better than the other. But he questioned whether that would hold true if applied to broader clinical practice.
Dr. MacGillivray, of MedStar Washington Hospital Center, Washington, observed that only 330 patients were randomly assigned among a total of 1,167 candidates for candidates for MV repair surgery.
Indeed, he noted, more than 200 declined and about 600 were declared ineligible for the study, “even though it had seemed as if all were appropriate for mitral valve repair. That could be viewed as a significant limitation in terms of scalability in the real world.”
Some of those patients weren’t randomly assigned because they ultimately were not considered appropriate for both procedures, and some expressed a preference for one or the other approach, Dr. Akowuah replied. Those were the most common reasons. Many others did not enter the study, he said, because their mitral regurgitation was functional, not degenerative.
The two randomization groups fared similarly for the primary endpoint reflecting recovery from surgery, so the trial was actually “negative,” Dr. Akowuah said in an interview. However, “I see it as very much a win for minithoracotomy. The outstanding questions for clinicians and patients have been about the clinical efficacy and safety of the technique. And we’ve shown in this trial that minithoracotomy is safe and effective.”
If the minithoracotomy procedure is available, he continued, “and it’s just as clinically effective and safe – and we weren’t sure that was the case until we did this trial – and the repair is almost as durable, then why have a sternotomy?”
The researchers assigned 330 patients with degenerative MV disease who were deemed suitable for either type of surgery to undergo the standard operation via sternotomy or the minithoracotomy procedure at 10 centers in the United Kingdom. The steering committee had hand-selected its 28 experienced surgeons, each of whom performed only one of the two surgeries consistently for the trial’s patients.
The technically more demanding minithoracotomy procedure took longer to perform by a mean of 44 minutes, it prolonged cross-clamp time by 11 minutes, and it required 30 minutes more cardiopulmonary bypass support, Dr. Akowuah reported.
The two patient groups showed no significant differences in the primary endpoint of physical function and ability to return to usual activity levels at 12 weeks, as assessed by scores on the 36-Item Short Form Survey and wrist-worn accelerometer monitoring. At 6 weeks, however, the mini-thoracotomy patients had shown a significant early but temporary advantage for those recovery measures.
The minithoracotomy group clearly fared better, however, on some secondary endpoints. For example, their median hospital LOS was 5 days, compared with 6 days for the sternotomy group (P = .003), and 33.1% of the mini-thoracotomy patients were discharged within 4 days of the surgery, compared with only 15.3% of patients who had the standard procedure (P < .001).
The minithoracotomy group also had marginally more days alive out of the hospital at both 30 days (23.6 days vs. 22.4 days in the sternotomy group) and 90 days (82.7 days and 80.5 days, respectively) after the surgery (P = .03 for both differences).
Safety outcomes at 12 weeks were similar, with no significant differences in rate of death, strokes, MI, or renal impairment, or in ICU length of stay or need for more than 48 hours of mechanical ventilation, Dr. Akowuah reported.
Safety outcomes at 1 year were also similar. Mortality by then was 2.4% for the minithoracotomy patients and 2.5% for the sternotomy group, nor were there significant differences in HHF rates or need for repeat MV surgical repair.
Dr. Akowuah said the patients will be followed for up to 5 years for the primary outcomes, echocardiographic changes, and clinical events.
The minithoracotomy surgery’s longer operative times and specialized equipment make it more a expensive procedure than the standard surgery, he said. “So we need to work out in a cost-effectiveness analysis whether that is offset by the benefits,” such as shorter hospital stays or perhaps fewer transfusions or readmissions.
The study was funded by the United Kingdom’s National Institute for Health and Care Research. Dr. Akowuah reported no relevant financial relationships with industry.
A version of this article first appeared on Medscape.com.
Patients with degenerative mitral valve (MV) regurgitation that calls for surgery may, for the most part, safely choose either a standard procedure requiring a midline sternotomy or one performed through a minithoracotomy, suggests a randomized comparison of the two techniques.
Still, the minimally invasive approach showed some advantages in the study. Patients’ quality of recovery was about the same with both procedures at 12 weeks, but those who had the minimally invasive thoracoscopy-guided surgery had shown greater improvement 6 weeks earlier.
Also in the UK Mini Mitral Trial, hospital length of stay (LOS) was significantly shorter for patients who underwent the mini-thoracotomy procedure, and that group spent fewer days in the hospital over the following months.
But neither procedure had an edge in terms of postoperative clinical risk in the study. Rates of clinical events, such as death or hospitalization for heart failure (HHF), were about the same over 1 year.
Patients in this trial had been deemed suitable for either of the two surgeries, which were always performed by surgeons specially chosen by the steering committee for their experience and expertise.
This first randomized head-to-head comparison of the two approaches in such patients should make both patients and clinicians more confident about choosing the minimally invasive surgery for degenerative MV disease, said Enoch Akowuah, MD, Newcastle (England) University, United Kingdom.
Dr. Akowuah presented the UK Mini-Mitral Trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
A “main takeaway” for clinical practice from the trial would be that minithoracotomy MV repair “is as safe and effective as conventional sternotomy for degenerative mitral regurgitation,” said discussant Amy E. Simone, PA-C, following Dr. Akowuah’s presentation.
“I think this study is unique in that its focus is on delivering high-quality, cost-efficient care for mitral regurgitation, but also with an emphasis on patients’ goals and wishes,” said Ms. Simone, who directs the Marcus Heart Valve Center of the Piedmont Heart Institute, Atlanta.
Cardiac surgeon Thomas MacGillivray, MD, another discussant, agreed that the data presented from at least this study suggest neither the minithoracotomy nor sternotomy approach is better than the other. But he questioned whether that would hold true if applied to broader clinical practice.
Dr. MacGillivray, of MedStar Washington Hospital Center, Washington, observed that only 330 patients were randomly assigned among a total of 1,167 candidates for candidates for MV repair surgery.
Indeed, he noted, more than 200 declined and about 600 were declared ineligible for the study, “even though it had seemed as if all were appropriate for mitral valve repair. That could be viewed as a significant limitation in terms of scalability in the real world.”
Some of those patients weren’t randomly assigned because they ultimately were not considered appropriate for both procedures, and some expressed a preference for one or the other approach, Dr. Akowuah replied. Those were the most common reasons. Many others did not enter the study, he said, because their mitral regurgitation was functional, not degenerative.
The two randomization groups fared similarly for the primary endpoint reflecting recovery from surgery, so the trial was actually “negative,” Dr. Akowuah said in an interview. However, “I see it as very much a win for minithoracotomy. The outstanding questions for clinicians and patients have been about the clinical efficacy and safety of the technique. And we’ve shown in this trial that minithoracotomy is safe and effective.”
If the minithoracotomy procedure is available, he continued, “and it’s just as clinically effective and safe – and we weren’t sure that was the case until we did this trial – and the repair is almost as durable, then why have a sternotomy?”
The researchers assigned 330 patients with degenerative MV disease who were deemed suitable for either type of surgery to undergo the standard operation via sternotomy or the minithoracotomy procedure at 10 centers in the United Kingdom. The steering committee had hand-selected its 28 experienced surgeons, each of whom performed only one of the two surgeries consistently for the trial’s patients.
The technically more demanding minithoracotomy procedure took longer to perform by a mean of 44 minutes, it prolonged cross-clamp time by 11 minutes, and it required 30 minutes more cardiopulmonary bypass support, Dr. Akowuah reported.
The two patient groups showed no significant differences in the primary endpoint of physical function and ability to return to usual activity levels at 12 weeks, as assessed by scores on the 36-Item Short Form Survey and wrist-worn accelerometer monitoring. At 6 weeks, however, the mini-thoracotomy patients had shown a significant early but temporary advantage for those recovery measures.
The minithoracotomy group clearly fared better, however, on some secondary endpoints. For example, their median hospital LOS was 5 days, compared with 6 days for the sternotomy group (P = .003), and 33.1% of the mini-thoracotomy patients were discharged within 4 days of the surgery, compared with only 15.3% of patients who had the standard procedure (P < .001).
The minithoracotomy group also had marginally more days alive out of the hospital at both 30 days (23.6 days vs. 22.4 days in the sternotomy group) and 90 days (82.7 days and 80.5 days, respectively) after the surgery (P = .03 for both differences).
Safety outcomes at 12 weeks were similar, with no significant differences in rate of death, strokes, MI, or renal impairment, or in ICU length of stay or need for more than 48 hours of mechanical ventilation, Dr. Akowuah reported.
Safety outcomes at 1 year were also similar. Mortality by then was 2.4% for the minithoracotomy patients and 2.5% for the sternotomy group, nor were there significant differences in HHF rates or need for repeat MV surgical repair.
Dr. Akowuah said the patients will be followed for up to 5 years for the primary outcomes, echocardiographic changes, and clinical events.
The minithoracotomy surgery’s longer operative times and specialized equipment make it more a expensive procedure than the standard surgery, he said. “So we need to work out in a cost-effectiveness analysis whether that is offset by the benefits,” such as shorter hospital stays or perhaps fewer transfusions or readmissions.
The study was funded by the United Kingdom’s National Institute for Health and Care Research. Dr. Akowuah reported no relevant financial relationships with industry.
A version of this article first appeared on Medscape.com.
Patients with degenerative mitral valve (MV) regurgitation that calls for surgery may, for the most part, safely choose either a standard procedure requiring a midline sternotomy or one performed through a minithoracotomy, suggests a randomized comparison of the two techniques.
Still, the minimally invasive approach showed some advantages in the study. Patients’ quality of recovery was about the same with both procedures at 12 weeks, but those who had the minimally invasive thoracoscopy-guided surgery had shown greater improvement 6 weeks earlier.
Also in the UK Mini Mitral Trial, hospital length of stay (LOS) was significantly shorter for patients who underwent the mini-thoracotomy procedure, and that group spent fewer days in the hospital over the following months.
But neither procedure had an edge in terms of postoperative clinical risk in the study. Rates of clinical events, such as death or hospitalization for heart failure (HHF), were about the same over 1 year.
Patients in this trial had been deemed suitable for either of the two surgeries, which were always performed by surgeons specially chosen by the steering committee for their experience and expertise.
This first randomized head-to-head comparison of the two approaches in such patients should make both patients and clinicians more confident about choosing the minimally invasive surgery for degenerative MV disease, said Enoch Akowuah, MD, Newcastle (England) University, United Kingdom.
Dr. Akowuah presented the UK Mini-Mitral Trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
A “main takeaway” for clinical practice from the trial would be that minithoracotomy MV repair “is as safe and effective as conventional sternotomy for degenerative mitral regurgitation,” said discussant Amy E. Simone, PA-C, following Dr. Akowuah’s presentation.
“I think this study is unique in that its focus is on delivering high-quality, cost-efficient care for mitral regurgitation, but also with an emphasis on patients’ goals and wishes,” said Ms. Simone, who directs the Marcus Heart Valve Center of the Piedmont Heart Institute, Atlanta.
Cardiac surgeon Thomas MacGillivray, MD, another discussant, agreed that the data presented from at least this study suggest neither the minithoracotomy nor sternotomy approach is better than the other. But he questioned whether that would hold true if applied to broader clinical practice.
Dr. MacGillivray, of MedStar Washington Hospital Center, Washington, observed that only 330 patients were randomly assigned among a total of 1,167 candidates for candidates for MV repair surgery.
Indeed, he noted, more than 200 declined and about 600 were declared ineligible for the study, “even though it had seemed as if all were appropriate for mitral valve repair. That could be viewed as a significant limitation in terms of scalability in the real world.”
Some of those patients weren’t randomly assigned because they ultimately were not considered appropriate for both procedures, and some expressed a preference for one or the other approach, Dr. Akowuah replied. Those were the most common reasons. Many others did not enter the study, he said, because their mitral regurgitation was functional, not degenerative.
The two randomization groups fared similarly for the primary endpoint reflecting recovery from surgery, so the trial was actually “negative,” Dr. Akowuah said in an interview. However, “I see it as very much a win for minithoracotomy. The outstanding questions for clinicians and patients have been about the clinical efficacy and safety of the technique. And we’ve shown in this trial that minithoracotomy is safe and effective.”
If the minithoracotomy procedure is available, he continued, “and it’s just as clinically effective and safe – and we weren’t sure that was the case until we did this trial – and the repair is almost as durable, then why have a sternotomy?”
The researchers assigned 330 patients with degenerative MV disease who were deemed suitable for either type of surgery to undergo the standard operation via sternotomy or the minithoracotomy procedure at 10 centers in the United Kingdom. The steering committee had hand-selected its 28 experienced surgeons, each of whom performed only one of the two surgeries consistently for the trial’s patients.
The technically more demanding minithoracotomy procedure took longer to perform by a mean of 44 minutes, it prolonged cross-clamp time by 11 minutes, and it required 30 minutes more cardiopulmonary bypass support, Dr. Akowuah reported.
The two patient groups showed no significant differences in the primary endpoint of physical function and ability to return to usual activity levels at 12 weeks, as assessed by scores on the 36-Item Short Form Survey and wrist-worn accelerometer monitoring. At 6 weeks, however, the mini-thoracotomy patients had shown a significant early but temporary advantage for those recovery measures.
The minithoracotomy group clearly fared better, however, on some secondary endpoints. For example, their median hospital LOS was 5 days, compared with 6 days for the sternotomy group (P = .003), and 33.1% of the mini-thoracotomy patients were discharged within 4 days of the surgery, compared with only 15.3% of patients who had the standard procedure (P < .001).
The minithoracotomy group also had marginally more days alive out of the hospital at both 30 days (23.6 days vs. 22.4 days in the sternotomy group) and 90 days (82.7 days and 80.5 days, respectively) after the surgery (P = .03 for both differences).
Safety outcomes at 12 weeks were similar, with no significant differences in rate of death, strokes, MI, or renal impairment, or in ICU length of stay or need for more than 48 hours of mechanical ventilation, Dr. Akowuah reported.
Safety outcomes at 1 year were also similar. Mortality by then was 2.4% for the minithoracotomy patients and 2.5% for the sternotomy group, nor were there significant differences in HHF rates or need for repeat MV surgical repair.
Dr. Akowuah said the patients will be followed for up to 5 years for the primary outcomes, echocardiographic changes, and clinical events.
The minithoracotomy surgery’s longer operative times and specialized equipment make it more a expensive procedure than the standard surgery, he said. “So we need to work out in a cost-effectiveness analysis whether that is offset by the benefits,” such as shorter hospital stays or perhaps fewer transfusions or readmissions.
The study was funded by the United Kingdom’s National Institute for Health and Care Research. Dr. Akowuah reported no relevant financial relationships with industry.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Causal AI quantifies CV risk, providing patient-specific goals
NEW ORLEANS – Causal artificial intelligence (AI) can translate polygenic scores (PGS) and other genetic information into risk reduction strategies for coronary artery disease (CAD) that is tailored for each individual patient, according to an analysis presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Tested for LDL cholesterol (LDL-C) and systolic blood pressure (SBP), causal AI explained how much each of these risk factors must improve at the level of each individual patient “to overcome overall inherited risk,” reported Brian Ference, MD, MPhil, director of translational therapeutics, University of Cambridge (England).
Unlike the “black box” risk assessments common to machine learning, which relies on disparate forms of information of often unknown relative significance, causal AI explains cause and effect. In the case of CAD, its ability to encode the biological causes means that it can “both predict outcomes and prescribe specific actions to change those outcomes,” Dr. Ference explained.
The concept is testable against observed biology using randomized evidence, which was the objective of the study Dr. Ference presented in the late-breaker session.
Causal AI trained on nearly 2 million patients
This study employed a causal AI platform trained on roughly 1.3 million participants in Mendelian randomization studies, as well as more than 500,000 participants in randomized clinical trials. The PGS estimate of inherited risk was constructed from almost 4.1 million variants from genomewide association studies.
To test the ability of causal AI to reveal how much LDL-C or SBP had to be reduced to overcome the inherited risk of CAD based on PGS, it was applied to 445,765 participants of European ancestry in the UK Biobank. The goal was to determine how much those with greater than average risk would need to lower their LDL-C or SBP to achieve average CAD risk.
When validated against observed rates of events, causal AI accurately characterized risk before estimating what reductions in LDL-C, SBP, or both would attenuate that risk.
Providing examples, Dr. Ference explained that a PGS in the 80th percentile can be overcome by lowering LDL-C by 14 mg/dL. Alternatively, the 80th percentile risk could also be overcome by simultaneously lowering LDL-C and SBP by 7 mg/dL and 2.5 mm Hg, respectively.
Required risk factor reductions increase with age because of the increased risk of the events. For example, while a 14.8 mg/dL reduction in LDL-C would be adequate to overcome risk defined by a PGS in the 80th percentile at age 35, reductions of 18.2 mg/dL, 28.9 mg/dL, and 42.6 mg/dL would be required, respectively, at ages 45, 55, and 65 years. The values climb similarly for SBP.
Family history of CAD adds an independent variable that further contributes to the ability of causal AI to estimate risk and the degree of risk factor attenuation to overcome the risk.
Even though family history is equivalent to having PGS above the 95th percentile, it is an independent and additive variable, according to Dr. Ference. As a result, inherited risk of CAD depends on both.
Still when family history is factored into the analysis, “causal AI accurately estimated the magnitude of lower LDL-C, SBP, or both needed to overcome overall inherited risk at all levels of higher or lower PGS,” he reported.
According to Dr. Ference, the value of causal AI is that it can generate very specific goals for each patient regarding modifiable risk factors. Causal effects of risk factors encoded in time units of exposure allow the patient and the clinician to understand the biology and the basis of the disease burden.
Treatments become understandable to patients
“Encoding biology creates algorithms that are deeply explainable because they reveal why a person is at risk, how to reduce that risk, and how much each person will benefit from specific actions to reduce risk,” Dr. Ference said.
A real-world, randomized trial to confirm that the information from causal AI can reduce the risk of CAD is expected to start in 2023, but Dr. Ference thinks that causal AI for managing CAD risk, independent of this planned trial, is essentially inevitable. PGS, which he thinks will be performed routinely in all individuals within 10 years, is only likely to improve. He foresees large advantages of this form of personalized medicine.
Ami Bhatt, MD, chief innovation officer for the American College of Cardiology, Washington, agreed, seeing a direct relationship between precision health as the pathway to improvements in population health.
By explaining risk factors in terms of mechanisms and specific goals to ameliorate these risks, it “engages our patients with agency,” said Dr. Bhatt. She suggested that the information provided by causal AI has the potential to empower patients while creating a collaborative approach with clinicians to CAD prevention.
With patient-specific information provided in the context of the disease biology, “you increase the sense of transparency,” Dr. Bhatt said.
She suggested this direction of research is wholly consistent with initiatives such as those from the World Health Organization to improve precision medicine as a step toward equipping patients to manage their own health.
Dr. Ference reported financial relationships with Amgen, AstraZeneca, CiVi Pharma, Daiichi Sankyo, DalCOR, Esperion, Eli Lilly, Ionis Pharmaceuticals, KrKA, Medicines Company, Merck, Mylan, Novo Nordisk, Novartis, and Sanofi, and Viatris. Dr. Bhatt reported no potential conflicts of interest.
NEW ORLEANS – Causal artificial intelligence (AI) can translate polygenic scores (PGS) and other genetic information into risk reduction strategies for coronary artery disease (CAD) that is tailored for each individual patient, according to an analysis presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Tested for LDL cholesterol (LDL-C) and systolic blood pressure (SBP), causal AI explained how much each of these risk factors must improve at the level of each individual patient “to overcome overall inherited risk,” reported Brian Ference, MD, MPhil, director of translational therapeutics, University of Cambridge (England).
Unlike the “black box” risk assessments common to machine learning, which relies on disparate forms of information of often unknown relative significance, causal AI explains cause and effect. In the case of CAD, its ability to encode the biological causes means that it can “both predict outcomes and prescribe specific actions to change those outcomes,” Dr. Ference explained.
The concept is testable against observed biology using randomized evidence, which was the objective of the study Dr. Ference presented in the late-breaker session.
Causal AI trained on nearly 2 million patients
This study employed a causal AI platform trained on roughly 1.3 million participants in Mendelian randomization studies, as well as more than 500,000 participants in randomized clinical trials. The PGS estimate of inherited risk was constructed from almost 4.1 million variants from genomewide association studies.
To test the ability of causal AI to reveal how much LDL-C or SBP had to be reduced to overcome the inherited risk of CAD based on PGS, it was applied to 445,765 participants of European ancestry in the UK Biobank. The goal was to determine how much those with greater than average risk would need to lower their LDL-C or SBP to achieve average CAD risk.
When validated against observed rates of events, causal AI accurately characterized risk before estimating what reductions in LDL-C, SBP, or both would attenuate that risk.
Providing examples, Dr. Ference explained that a PGS in the 80th percentile can be overcome by lowering LDL-C by 14 mg/dL. Alternatively, the 80th percentile risk could also be overcome by simultaneously lowering LDL-C and SBP by 7 mg/dL and 2.5 mm Hg, respectively.
Required risk factor reductions increase with age because of the increased risk of the events. For example, while a 14.8 mg/dL reduction in LDL-C would be adequate to overcome risk defined by a PGS in the 80th percentile at age 35, reductions of 18.2 mg/dL, 28.9 mg/dL, and 42.6 mg/dL would be required, respectively, at ages 45, 55, and 65 years. The values climb similarly for SBP.
Family history of CAD adds an independent variable that further contributes to the ability of causal AI to estimate risk and the degree of risk factor attenuation to overcome the risk.
Even though family history is equivalent to having PGS above the 95th percentile, it is an independent and additive variable, according to Dr. Ference. As a result, inherited risk of CAD depends on both.
Still when family history is factored into the analysis, “causal AI accurately estimated the magnitude of lower LDL-C, SBP, or both needed to overcome overall inherited risk at all levels of higher or lower PGS,” he reported.
According to Dr. Ference, the value of causal AI is that it can generate very specific goals for each patient regarding modifiable risk factors. Causal effects of risk factors encoded in time units of exposure allow the patient and the clinician to understand the biology and the basis of the disease burden.
Treatments become understandable to patients
“Encoding biology creates algorithms that are deeply explainable because they reveal why a person is at risk, how to reduce that risk, and how much each person will benefit from specific actions to reduce risk,” Dr. Ference said.
A real-world, randomized trial to confirm that the information from causal AI can reduce the risk of CAD is expected to start in 2023, but Dr. Ference thinks that causal AI for managing CAD risk, independent of this planned trial, is essentially inevitable. PGS, which he thinks will be performed routinely in all individuals within 10 years, is only likely to improve. He foresees large advantages of this form of personalized medicine.
Ami Bhatt, MD, chief innovation officer for the American College of Cardiology, Washington, agreed, seeing a direct relationship between precision health as the pathway to improvements in population health.
By explaining risk factors in terms of mechanisms and specific goals to ameliorate these risks, it “engages our patients with agency,” said Dr. Bhatt. She suggested that the information provided by causal AI has the potential to empower patients while creating a collaborative approach with clinicians to CAD prevention.
With patient-specific information provided in the context of the disease biology, “you increase the sense of transparency,” Dr. Bhatt said.
She suggested this direction of research is wholly consistent with initiatives such as those from the World Health Organization to improve precision medicine as a step toward equipping patients to manage their own health.
Dr. Ference reported financial relationships with Amgen, AstraZeneca, CiVi Pharma, Daiichi Sankyo, DalCOR, Esperion, Eli Lilly, Ionis Pharmaceuticals, KrKA, Medicines Company, Merck, Mylan, Novo Nordisk, Novartis, and Sanofi, and Viatris. Dr. Bhatt reported no potential conflicts of interest.
NEW ORLEANS – Causal artificial intelligence (AI) can translate polygenic scores (PGS) and other genetic information into risk reduction strategies for coronary artery disease (CAD) that is tailored for each individual patient, according to an analysis presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Tested for LDL cholesterol (LDL-C) and systolic blood pressure (SBP), causal AI explained how much each of these risk factors must improve at the level of each individual patient “to overcome overall inherited risk,” reported Brian Ference, MD, MPhil, director of translational therapeutics, University of Cambridge (England).
Unlike the “black box” risk assessments common to machine learning, which relies on disparate forms of information of often unknown relative significance, causal AI explains cause and effect. In the case of CAD, its ability to encode the biological causes means that it can “both predict outcomes and prescribe specific actions to change those outcomes,” Dr. Ference explained.
The concept is testable against observed biology using randomized evidence, which was the objective of the study Dr. Ference presented in the late-breaker session.
Causal AI trained on nearly 2 million patients
This study employed a causal AI platform trained on roughly 1.3 million participants in Mendelian randomization studies, as well as more than 500,000 participants in randomized clinical trials. The PGS estimate of inherited risk was constructed from almost 4.1 million variants from genomewide association studies.
To test the ability of causal AI to reveal how much LDL-C or SBP had to be reduced to overcome the inherited risk of CAD based on PGS, it was applied to 445,765 participants of European ancestry in the UK Biobank. The goal was to determine how much those with greater than average risk would need to lower their LDL-C or SBP to achieve average CAD risk.
When validated against observed rates of events, causal AI accurately characterized risk before estimating what reductions in LDL-C, SBP, or both would attenuate that risk.
Providing examples, Dr. Ference explained that a PGS in the 80th percentile can be overcome by lowering LDL-C by 14 mg/dL. Alternatively, the 80th percentile risk could also be overcome by simultaneously lowering LDL-C and SBP by 7 mg/dL and 2.5 mm Hg, respectively.
Required risk factor reductions increase with age because of the increased risk of the events. For example, while a 14.8 mg/dL reduction in LDL-C would be adequate to overcome risk defined by a PGS in the 80th percentile at age 35, reductions of 18.2 mg/dL, 28.9 mg/dL, and 42.6 mg/dL would be required, respectively, at ages 45, 55, and 65 years. The values climb similarly for SBP.
Family history of CAD adds an independent variable that further contributes to the ability of causal AI to estimate risk and the degree of risk factor attenuation to overcome the risk.
Even though family history is equivalent to having PGS above the 95th percentile, it is an independent and additive variable, according to Dr. Ference. As a result, inherited risk of CAD depends on both.
Still when family history is factored into the analysis, “causal AI accurately estimated the magnitude of lower LDL-C, SBP, or both needed to overcome overall inherited risk at all levels of higher or lower PGS,” he reported.
According to Dr. Ference, the value of causal AI is that it can generate very specific goals for each patient regarding modifiable risk factors. Causal effects of risk factors encoded in time units of exposure allow the patient and the clinician to understand the biology and the basis of the disease burden.
Treatments become understandable to patients
“Encoding biology creates algorithms that are deeply explainable because they reveal why a person is at risk, how to reduce that risk, and how much each person will benefit from specific actions to reduce risk,” Dr. Ference said.
A real-world, randomized trial to confirm that the information from causal AI can reduce the risk of CAD is expected to start in 2023, but Dr. Ference thinks that causal AI for managing CAD risk, independent of this planned trial, is essentially inevitable. PGS, which he thinks will be performed routinely in all individuals within 10 years, is only likely to improve. He foresees large advantages of this form of personalized medicine.
Ami Bhatt, MD, chief innovation officer for the American College of Cardiology, Washington, agreed, seeing a direct relationship between precision health as the pathway to improvements in population health.
By explaining risk factors in terms of mechanisms and specific goals to ameliorate these risks, it “engages our patients with agency,” said Dr. Bhatt. She suggested that the information provided by causal AI has the potential to empower patients while creating a collaborative approach with clinicians to CAD prevention.
With patient-specific information provided in the context of the disease biology, “you increase the sense of transparency,” Dr. Bhatt said.
She suggested this direction of research is wholly consistent with initiatives such as those from the World Health Organization to improve precision medicine as a step toward equipping patients to manage their own health.
Dr. Ference reported financial relationships with Amgen, AstraZeneca, CiVi Pharma, Daiichi Sankyo, DalCOR, Esperion, Eli Lilly, Ionis Pharmaceuticals, KrKA, Medicines Company, Merck, Mylan, Novo Nordisk, Novartis, and Sanofi, and Viatris. Dr. Bhatt reported no potential conflicts of interest.
AT ACC 2023
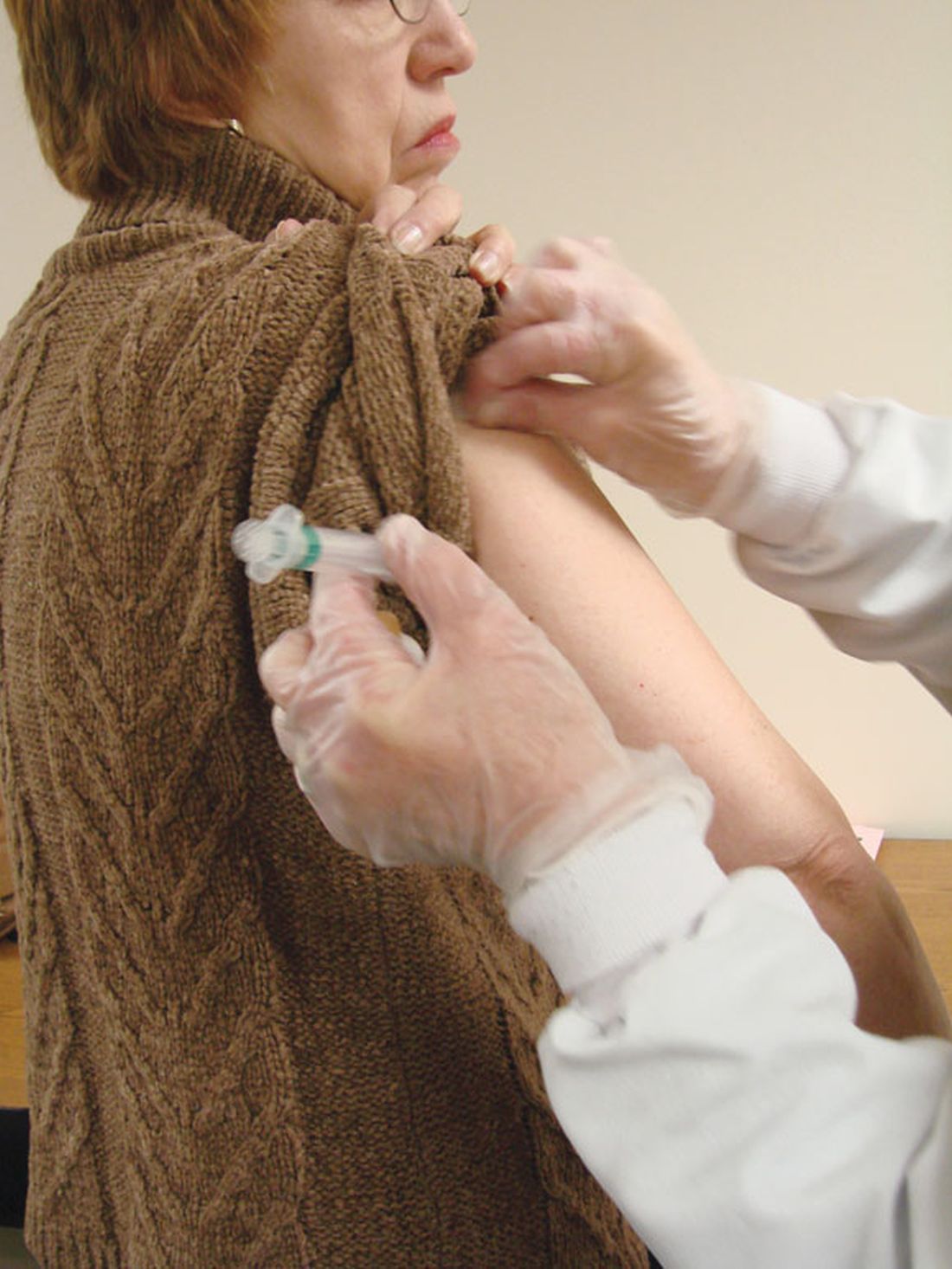
NUDGE-FLU: Electronic ‘nudges’ boost flu shot uptake in seniors
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
EHR alerts boosted MRA prescribing to patients with HFrEF
NEW ORLEANS – EHR-embedded alerts that a patient with heart failure with reduced ejection fraction (HFrEF) is a great candidate for treatment with a mineralocorticoid receptor antagonist (MRA) more than doubled prescribing of this “pillar” class for HFrEF, compared with control practices that used usual care and no alerts.
That’s according to results of BETTER CARE-HF, a single-center, randomized trial with more than 2,000 patients and involving 180 cardiologists.
“EHR-embedded tools cans be a rapid, low-cost, and high-impact method to increase prescription of life-saving therapies across large populations,” said Amrita Mukhopadhyay, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Her study targeted underprescribing of an MRA – spironolactone or eplerenone (Inspra) – because of its “vastly underprescribed” status in U.S. practice, where roughly two-thirds of patients with HFrEF do not receive an MRA despite clear recommendations from several medical groups that it is an essential part of treatment for most patients with HFrEF. Dr. Mukhopadhyay estimated that more comprehensive prescribing of MRAs to U.S. patients with HFrEF could prevent more than 20,000 deaths annually.
She also explained that the EHR-embedded alert was carefully devised, through interviews with cardiologists and pilot testing, to optimize the nudge so that it was less intrusive but effective for capturing attention and initiating action.
‘Clinically relevant, impressive results’
“This is a really important study, because despite overwhelming evidence for more than a decade favoring MRA use for patients with HFrEF there is an incredibly large treatment gap. MRAs can reduce all-cause death in people with HFrEF by 25%-30%, as well as reduce hospitalizations for heart failure, at a cost of less than $50 a year,” commented Gregg C. Fonarow, MD, interim chief of cardiology at the University of California, Los Angeles. The study showed “very clinically relevant, impressive results” for individualized, patient-specific alerts to prescribe an MRA and order the laboratory tests, particularly for serum potassium levels, needed to safely start the treatment, Dr. Fonarow said in an interview.
The BETTER CARE-HF study ran at more than 60 practices in the New York City region operated by the NYU Langone Health system, which sponsored the study. The trial randomized 180 cardiologists from these practices in a cluster format to one of three study arms: Sixty cardiologists received the EHR-embedded alerts for their relevant patients (755 patients) when the patient was in the physician’s office; another 60 cardiologists received a less tailored, monthly message that flagged all patients with HFrEF in a cardiologist’s practice who remained untreated candidates for MRA intervention (812 patients); and a third arm of 60 cardiologists and their HFrEF patients served as controls where the clinicians received no alert or message (644 patients).
The study included 2,211 patients with HFrEF and not on MRA treatment at baseline who were all identified as good candidates for starting treatment with the class, with no contraindications, no preexisting hyperkalemia, and no advanced-stage renal dysfunction.
The study’s primary outcome was the percentage of patients in each subgroup who received a new prescription for an MRA. This occurred in 29.6% of the patients whose physicians received an alert, in 15.6% of the patients whose physicians received a monthly message, and in 11.7% of patients in the control practices. Statistical analyses showed that the alerts led to a significant 2.53-fold increase in MRA prescribing, while the messages linked with a significant 67% increase in prescribing, compared with the control practices, reported Dr. Mukhopadhyay, a health services researcher at NYU Langone Health in New York. Simultaneously with her report, the results also appeared in the Journal of the American College of Cardiology.
The findings also showed that the alert and message had no significant impact on the prescribing of any other medication classes for HFrEF, compared with the controls. And the alert intervention had minimal adverse effects. While patients in the alert arm showed a significant, 45% relative increase in the incidence of hyperkalemia episodes, compared with control patients (because of a 4.5% absolute increase in hyperkalemia events), the rate of “significant” hyperkalemia with a value of at least 5.5 mmol/L, occurred in 5.0% of patients in the alert group and 5.1% of patients in the control arm.
Potassium testing poses another barrier
Even though the alerts substantially improved MRA prescribing, 70% of patients deemed MRA eligible in the alert subgroup still failed to receive a prescription. One additional barrier specific to MRA prescribing is the need it triggers for serial laboratory testing to monitor serum potassium levels. “Potassium testing generates additional work outside the index visit, which along with the risk for hyperkalemia exists as a barrier,” commented Lee R. Goldberg, MD, a heart failure specialist and professor at the University of Pennsylvania in Philadelphia. “This may be the next aspect to focus on to improve MRA uptake,” he said as a designated discussant for the report.
“It’s not enough to just prompt medication treatment. We also need to prompt appropriate laboratory testing,” noted Dr. Fonarow.
He also said that the approach tested by Dr. Mukhopadhyay could now be expanded to outpatient cardiologists. “The onus is on everyone involved in caring for patients with HFrEF failure to explain why maximum effort is not being made to deploy” all of the guideline-directed medical therapies for the disorder.
EHR alerts “are one way to bridge the prescribing gap, but we need multiple approaches so that all eligible patients receive guideline-directed medical therapy,” Dr. Fonarow said.
BETTER CARE-HF received no commercial funding, and Dr. Mukhopadhyay had no disclosures. Dr. Fonarow has been a consultant to AstraZeneca, Amgen, Cytokinetics, Lilly, Merck, Novartis, and Pfizer. Dr. Goldberg has received personal fees from Abbott, VisCardia, and Zoll/Respircardia.
NEW ORLEANS – EHR-embedded alerts that a patient with heart failure with reduced ejection fraction (HFrEF) is a great candidate for treatment with a mineralocorticoid receptor antagonist (MRA) more than doubled prescribing of this “pillar” class for HFrEF, compared with control practices that used usual care and no alerts.
That’s according to results of BETTER CARE-HF, a single-center, randomized trial with more than 2,000 patients and involving 180 cardiologists.
“EHR-embedded tools cans be a rapid, low-cost, and high-impact method to increase prescription of life-saving therapies across large populations,” said Amrita Mukhopadhyay, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Her study targeted underprescribing of an MRA – spironolactone or eplerenone (Inspra) – because of its “vastly underprescribed” status in U.S. practice, where roughly two-thirds of patients with HFrEF do not receive an MRA despite clear recommendations from several medical groups that it is an essential part of treatment for most patients with HFrEF. Dr. Mukhopadhyay estimated that more comprehensive prescribing of MRAs to U.S. patients with HFrEF could prevent more than 20,000 deaths annually.
She also explained that the EHR-embedded alert was carefully devised, through interviews with cardiologists and pilot testing, to optimize the nudge so that it was less intrusive but effective for capturing attention and initiating action.
‘Clinically relevant, impressive results’
“This is a really important study, because despite overwhelming evidence for more than a decade favoring MRA use for patients with HFrEF there is an incredibly large treatment gap. MRAs can reduce all-cause death in people with HFrEF by 25%-30%, as well as reduce hospitalizations for heart failure, at a cost of less than $50 a year,” commented Gregg C. Fonarow, MD, interim chief of cardiology at the University of California, Los Angeles. The study showed “very clinically relevant, impressive results” for individualized, patient-specific alerts to prescribe an MRA and order the laboratory tests, particularly for serum potassium levels, needed to safely start the treatment, Dr. Fonarow said in an interview.
The BETTER CARE-HF study ran at more than 60 practices in the New York City region operated by the NYU Langone Health system, which sponsored the study. The trial randomized 180 cardiologists from these practices in a cluster format to one of three study arms: Sixty cardiologists received the EHR-embedded alerts for their relevant patients (755 patients) when the patient was in the physician’s office; another 60 cardiologists received a less tailored, monthly message that flagged all patients with HFrEF in a cardiologist’s practice who remained untreated candidates for MRA intervention (812 patients); and a third arm of 60 cardiologists and their HFrEF patients served as controls where the clinicians received no alert or message (644 patients).
The study included 2,211 patients with HFrEF and not on MRA treatment at baseline who were all identified as good candidates for starting treatment with the class, with no contraindications, no preexisting hyperkalemia, and no advanced-stage renal dysfunction.
The study’s primary outcome was the percentage of patients in each subgroup who received a new prescription for an MRA. This occurred in 29.6% of the patients whose physicians received an alert, in 15.6% of the patients whose physicians received a monthly message, and in 11.7% of patients in the control practices. Statistical analyses showed that the alerts led to a significant 2.53-fold increase in MRA prescribing, while the messages linked with a significant 67% increase in prescribing, compared with the control practices, reported Dr. Mukhopadhyay, a health services researcher at NYU Langone Health in New York. Simultaneously with her report, the results also appeared in the Journal of the American College of Cardiology.
The findings also showed that the alert and message had no significant impact on the prescribing of any other medication classes for HFrEF, compared with the controls. And the alert intervention had minimal adverse effects. While patients in the alert arm showed a significant, 45% relative increase in the incidence of hyperkalemia episodes, compared with control patients (because of a 4.5% absolute increase in hyperkalemia events), the rate of “significant” hyperkalemia with a value of at least 5.5 mmol/L, occurred in 5.0% of patients in the alert group and 5.1% of patients in the control arm.
Potassium testing poses another barrier
Even though the alerts substantially improved MRA prescribing, 70% of patients deemed MRA eligible in the alert subgroup still failed to receive a prescription. One additional barrier specific to MRA prescribing is the need it triggers for serial laboratory testing to monitor serum potassium levels. “Potassium testing generates additional work outside the index visit, which along with the risk for hyperkalemia exists as a barrier,” commented Lee R. Goldberg, MD, a heart failure specialist and professor at the University of Pennsylvania in Philadelphia. “This may be the next aspect to focus on to improve MRA uptake,” he said as a designated discussant for the report.
“It’s not enough to just prompt medication treatment. We also need to prompt appropriate laboratory testing,” noted Dr. Fonarow.
He also said that the approach tested by Dr. Mukhopadhyay could now be expanded to outpatient cardiologists. “The onus is on everyone involved in caring for patients with HFrEF failure to explain why maximum effort is not being made to deploy” all of the guideline-directed medical therapies for the disorder.
EHR alerts “are one way to bridge the prescribing gap, but we need multiple approaches so that all eligible patients receive guideline-directed medical therapy,” Dr. Fonarow said.
BETTER CARE-HF received no commercial funding, and Dr. Mukhopadhyay had no disclosures. Dr. Fonarow has been a consultant to AstraZeneca, Amgen, Cytokinetics, Lilly, Merck, Novartis, and Pfizer. Dr. Goldberg has received personal fees from Abbott, VisCardia, and Zoll/Respircardia.
NEW ORLEANS – EHR-embedded alerts that a patient with heart failure with reduced ejection fraction (HFrEF) is a great candidate for treatment with a mineralocorticoid receptor antagonist (MRA) more than doubled prescribing of this “pillar” class for HFrEF, compared with control practices that used usual care and no alerts.
That’s according to results of BETTER CARE-HF, a single-center, randomized trial with more than 2,000 patients and involving 180 cardiologists.
“EHR-embedded tools cans be a rapid, low-cost, and high-impact method to increase prescription of life-saving therapies across large populations,” said Amrita Mukhopadhyay, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Her study targeted underprescribing of an MRA – spironolactone or eplerenone (Inspra) – because of its “vastly underprescribed” status in U.S. practice, where roughly two-thirds of patients with HFrEF do not receive an MRA despite clear recommendations from several medical groups that it is an essential part of treatment for most patients with HFrEF. Dr. Mukhopadhyay estimated that more comprehensive prescribing of MRAs to U.S. patients with HFrEF could prevent more than 20,000 deaths annually.
She also explained that the EHR-embedded alert was carefully devised, through interviews with cardiologists and pilot testing, to optimize the nudge so that it was less intrusive but effective for capturing attention and initiating action.
‘Clinically relevant, impressive results’
“This is a really important study, because despite overwhelming evidence for more than a decade favoring MRA use for patients with HFrEF there is an incredibly large treatment gap. MRAs can reduce all-cause death in people with HFrEF by 25%-30%, as well as reduce hospitalizations for heart failure, at a cost of less than $50 a year,” commented Gregg C. Fonarow, MD, interim chief of cardiology at the University of California, Los Angeles. The study showed “very clinically relevant, impressive results” for individualized, patient-specific alerts to prescribe an MRA and order the laboratory tests, particularly for serum potassium levels, needed to safely start the treatment, Dr. Fonarow said in an interview.
The BETTER CARE-HF study ran at more than 60 practices in the New York City region operated by the NYU Langone Health system, which sponsored the study. The trial randomized 180 cardiologists from these practices in a cluster format to one of three study arms: Sixty cardiologists received the EHR-embedded alerts for their relevant patients (755 patients) when the patient was in the physician’s office; another 60 cardiologists received a less tailored, monthly message that flagged all patients with HFrEF in a cardiologist’s practice who remained untreated candidates for MRA intervention (812 patients); and a third arm of 60 cardiologists and their HFrEF patients served as controls where the clinicians received no alert or message (644 patients).
The study included 2,211 patients with HFrEF and not on MRA treatment at baseline who were all identified as good candidates for starting treatment with the class, with no contraindications, no preexisting hyperkalemia, and no advanced-stage renal dysfunction.
The study’s primary outcome was the percentage of patients in each subgroup who received a new prescription for an MRA. This occurred in 29.6% of the patients whose physicians received an alert, in 15.6% of the patients whose physicians received a monthly message, and in 11.7% of patients in the control practices. Statistical analyses showed that the alerts led to a significant 2.53-fold increase in MRA prescribing, while the messages linked with a significant 67% increase in prescribing, compared with the control practices, reported Dr. Mukhopadhyay, a health services researcher at NYU Langone Health in New York. Simultaneously with her report, the results also appeared in the Journal of the American College of Cardiology.
The findings also showed that the alert and message had no significant impact on the prescribing of any other medication classes for HFrEF, compared with the controls. And the alert intervention had minimal adverse effects. While patients in the alert arm showed a significant, 45% relative increase in the incidence of hyperkalemia episodes, compared with control patients (because of a 4.5% absolute increase in hyperkalemia events), the rate of “significant” hyperkalemia with a value of at least 5.5 mmol/L, occurred in 5.0% of patients in the alert group and 5.1% of patients in the control arm.
Potassium testing poses another barrier
Even though the alerts substantially improved MRA prescribing, 70% of patients deemed MRA eligible in the alert subgroup still failed to receive a prescription. One additional barrier specific to MRA prescribing is the need it triggers for serial laboratory testing to monitor serum potassium levels. “Potassium testing generates additional work outside the index visit, which along with the risk for hyperkalemia exists as a barrier,” commented Lee R. Goldberg, MD, a heart failure specialist and professor at the University of Pennsylvania in Philadelphia. “This may be the next aspect to focus on to improve MRA uptake,” he said as a designated discussant for the report.
“It’s not enough to just prompt medication treatment. We also need to prompt appropriate laboratory testing,” noted Dr. Fonarow.
He also said that the approach tested by Dr. Mukhopadhyay could now be expanded to outpatient cardiologists. “The onus is on everyone involved in caring for patients with HFrEF failure to explain why maximum effort is not being made to deploy” all of the guideline-directed medical therapies for the disorder.
EHR alerts “are one way to bridge the prescribing gap, but we need multiple approaches so that all eligible patients receive guideline-directed medical therapy,” Dr. Fonarow said.
BETTER CARE-HF received no commercial funding, and Dr. Mukhopadhyay had no disclosures. Dr. Fonarow has been a consultant to AstraZeneca, Amgen, Cytokinetics, Lilly, Merck, Novartis, and Pfizer. Dr. Goldberg has received personal fees from Abbott, VisCardia, and Zoll/Respircardia.
AT ACC 2023
Viability-guided PCI doubted in stable severe CAD: REVIVED-BCIS2
There is no magical amount of viable ventricular myocardium that makes percutaneous coronary intervention (PCI) an effective addition to optimal medical therapy (OMT) in stable patients with coronary disease and poor ventricular function, suggests an analysis from a major trial.
The REVIVED-BCIS2 trial recently made waves when it showed no clinical advantage from adding PCI to OMT in stable patients with severe ischemic left ventricular (LV) dysfunction. All the patients had shown viable but dysfunctional myocardium that could potentially be revascularized.
But in a secondary analysis, extent of such hibernating heart muscle was not a good predictor of clinical outcomes, which in the trial meant death from any cause or hospitalization for heart failure (HHF).
Burden of myocardial scar tissue, however, turned out to be a potent predictor of clinical risk regardless of coronary disease severity or even LV ejection fraction (LVEF).
Because myocardial viability tracks poorly with outcomes in patients like those enrolled in the trial, as the new analysis suggests, conventional viability testing isn’t an effective guide for deciding who among them should get PCI, Divaka Perera, MD, said in an interview.
Dr. Perera, of King’s College London and the trial’s principal investigator, presented the REVIVED-BCIS2 secondary results at the joint scientific sessions of the American College of Cardiology and the World Heart Federation..
Viability testing for ischemia, he noted, is often used in practice to aid revascularization decisions. As the extent of myocardial viability can vary, it’s been asked – ever since the trial’s primary publication – whether there could be “a sweet spot or Goldilocks zone of viability that would allow prediction of which patients will do better with PCI compared to medical therapy,” Dr. Perera said. “The trial conclusively shows that is not the case.”
That the extent of hibernating myocardium, which is viable but dysfunctional, didn’t predict clinical outcomes or LV functional recovery “is disruptive of current practice and challenges a view that’s been held for decades.”
The trial’s 700 patients receiving OMT had been randomly assigned to undergo PCI or not, 347 and 353 patients, respectively. About 12% of the total were women.
About 70% of patients underwent baseline and follow-up myocardial viability testing using cardiac magnetic resonance (CMR) imaging with late gadolinium enhancement for estimation of scar burden; the remainder underwent dobutamine-stress echocardiography. All imaging assessments were conducted at independent core laboratories, Dr. Perera reported.
Extent of myocardial viability was defined three ways: volume of hibernating heart muscle, total volume of viable myocardium, and scar burden – all expressed as a percentage of total LV volume.
Every 10% increment in LV volume found to be hibernating related to a hazard ratio of 0.98 (95% confidence interval, 0.93-1.04; P = .56) for all-cause mortality or HHF at a median of 3.3 years. The analysis was adjusted for age, sex, diabetes, previous HHF, chronic renal failure, extent of CAD, type of viability testing, and baseline LVEF.
The adjusted HR for the same percentage increment in total viable myocardium was marginally significantly reduced at 0.93 (95% CI, 0.87-1.00; P = .048).
The correlation with scar burden was stronger. The adjusted composite-endpoint HR per 10% increment in scar burden was significantly increased at 1.18 (95% CI, 1.04-1.33; P = .009).
Extent of myocardial viability by tertiles, regardless of viability definition, did not highlight any group with a reduced risk for death or HHF, or group with better LV functional recovery, from OMT plus PCI, compared with OMT alone.
The findings appear to suggest that scar burden, but not extent of viability as it’s usually measured, may effectively guide PCI decisions in such patients, Dr. Perera said.
“I would say that viability testing as we understand it now, based on the paradigm of hibernating myocardium, is very useful,” he said, “but that is not the only information we can get from a viability test.”
Scar burden can also be determined from the same tests but isn’t typically looked at. “We’re actually collecting this information but not using it,” Dr. Perera said. “When we do, it is really powerfully predictive” of both clinical outcomes and LV functional recovery. “Yet scar burden is not in any of the guidelines for stratifying risk.”
REVIVED-BCIS2 was funded by the National Institute for Health and Care Research Health Technology Assessment Program. Dr. Perera had no disclosures.
A version of this article first appeared on Medscape.com.
There is no magical amount of viable ventricular myocardium that makes percutaneous coronary intervention (PCI) an effective addition to optimal medical therapy (OMT) in stable patients with coronary disease and poor ventricular function, suggests an analysis from a major trial.
The REVIVED-BCIS2 trial recently made waves when it showed no clinical advantage from adding PCI to OMT in stable patients with severe ischemic left ventricular (LV) dysfunction. All the patients had shown viable but dysfunctional myocardium that could potentially be revascularized.
But in a secondary analysis, extent of such hibernating heart muscle was not a good predictor of clinical outcomes, which in the trial meant death from any cause or hospitalization for heart failure (HHF).
Burden of myocardial scar tissue, however, turned out to be a potent predictor of clinical risk regardless of coronary disease severity or even LV ejection fraction (LVEF).
Because myocardial viability tracks poorly with outcomes in patients like those enrolled in the trial, as the new analysis suggests, conventional viability testing isn’t an effective guide for deciding who among them should get PCI, Divaka Perera, MD, said in an interview.
Dr. Perera, of King’s College London and the trial’s principal investigator, presented the REVIVED-BCIS2 secondary results at the joint scientific sessions of the American College of Cardiology and the World Heart Federation..
Viability testing for ischemia, he noted, is often used in practice to aid revascularization decisions. As the extent of myocardial viability can vary, it’s been asked – ever since the trial’s primary publication – whether there could be “a sweet spot or Goldilocks zone of viability that would allow prediction of which patients will do better with PCI compared to medical therapy,” Dr. Perera said. “The trial conclusively shows that is not the case.”
That the extent of hibernating myocardium, which is viable but dysfunctional, didn’t predict clinical outcomes or LV functional recovery “is disruptive of current practice and challenges a view that’s been held for decades.”
The trial’s 700 patients receiving OMT had been randomly assigned to undergo PCI or not, 347 and 353 patients, respectively. About 12% of the total were women.
About 70% of patients underwent baseline and follow-up myocardial viability testing using cardiac magnetic resonance (CMR) imaging with late gadolinium enhancement for estimation of scar burden; the remainder underwent dobutamine-stress echocardiography. All imaging assessments were conducted at independent core laboratories, Dr. Perera reported.
Extent of myocardial viability was defined three ways: volume of hibernating heart muscle, total volume of viable myocardium, and scar burden – all expressed as a percentage of total LV volume.
Every 10% increment in LV volume found to be hibernating related to a hazard ratio of 0.98 (95% confidence interval, 0.93-1.04; P = .56) for all-cause mortality or HHF at a median of 3.3 years. The analysis was adjusted for age, sex, diabetes, previous HHF, chronic renal failure, extent of CAD, type of viability testing, and baseline LVEF.
The adjusted HR for the same percentage increment in total viable myocardium was marginally significantly reduced at 0.93 (95% CI, 0.87-1.00; P = .048).
The correlation with scar burden was stronger. The adjusted composite-endpoint HR per 10% increment in scar burden was significantly increased at 1.18 (95% CI, 1.04-1.33; P = .009).
Extent of myocardial viability by tertiles, regardless of viability definition, did not highlight any group with a reduced risk for death or HHF, or group with better LV functional recovery, from OMT plus PCI, compared with OMT alone.
The findings appear to suggest that scar burden, but not extent of viability as it’s usually measured, may effectively guide PCI decisions in such patients, Dr. Perera said.
“I would say that viability testing as we understand it now, based on the paradigm of hibernating myocardium, is very useful,” he said, “but that is not the only information we can get from a viability test.”
Scar burden can also be determined from the same tests but isn’t typically looked at. “We’re actually collecting this information but not using it,” Dr. Perera said. “When we do, it is really powerfully predictive” of both clinical outcomes and LV functional recovery. “Yet scar burden is not in any of the guidelines for stratifying risk.”
REVIVED-BCIS2 was funded by the National Institute for Health and Care Research Health Technology Assessment Program. Dr. Perera had no disclosures.
A version of this article first appeared on Medscape.com.
There is no magical amount of viable ventricular myocardium that makes percutaneous coronary intervention (PCI) an effective addition to optimal medical therapy (OMT) in stable patients with coronary disease and poor ventricular function, suggests an analysis from a major trial.
The REVIVED-BCIS2 trial recently made waves when it showed no clinical advantage from adding PCI to OMT in stable patients with severe ischemic left ventricular (LV) dysfunction. All the patients had shown viable but dysfunctional myocardium that could potentially be revascularized.
But in a secondary analysis, extent of such hibernating heart muscle was not a good predictor of clinical outcomes, which in the trial meant death from any cause or hospitalization for heart failure (HHF).
Burden of myocardial scar tissue, however, turned out to be a potent predictor of clinical risk regardless of coronary disease severity or even LV ejection fraction (LVEF).
Because myocardial viability tracks poorly with outcomes in patients like those enrolled in the trial, as the new analysis suggests, conventional viability testing isn’t an effective guide for deciding who among them should get PCI, Divaka Perera, MD, said in an interview.
Dr. Perera, of King’s College London and the trial’s principal investigator, presented the REVIVED-BCIS2 secondary results at the joint scientific sessions of the American College of Cardiology and the World Heart Federation..
Viability testing for ischemia, he noted, is often used in practice to aid revascularization decisions. As the extent of myocardial viability can vary, it’s been asked – ever since the trial’s primary publication – whether there could be “a sweet spot or Goldilocks zone of viability that would allow prediction of which patients will do better with PCI compared to medical therapy,” Dr. Perera said. “The trial conclusively shows that is not the case.”
That the extent of hibernating myocardium, which is viable but dysfunctional, didn’t predict clinical outcomes or LV functional recovery “is disruptive of current practice and challenges a view that’s been held for decades.”
The trial’s 700 patients receiving OMT had been randomly assigned to undergo PCI or not, 347 and 353 patients, respectively. About 12% of the total were women.
About 70% of patients underwent baseline and follow-up myocardial viability testing using cardiac magnetic resonance (CMR) imaging with late gadolinium enhancement for estimation of scar burden; the remainder underwent dobutamine-stress echocardiography. All imaging assessments were conducted at independent core laboratories, Dr. Perera reported.
Extent of myocardial viability was defined three ways: volume of hibernating heart muscle, total volume of viable myocardium, and scar burden – all expressed as a percentage of total LV volume.
Every 10% increment in LV volume found to be hibernating related to a hazard ratio of 0.98 (95% confidence interval, 0.93-1.04; P = .56) for all-cause mortality or HHF at a median of 3.3 years. The analysis was adjusted for age, sex, diabetes, previous HHF, chronic renal failure, extent of CAD, type of viability testing, and baseline LVEF.
The adjusted HR for the same percentage increment in total viable myocardium was marginally significantly reduced at 0.93 (95% CI, 0.87-1.00; P = .048).
The correlation with scar burden was stronger. The adjusted composite-endpoint HR per 10% increment in scar burden was significantly increased at 1.18 (95% CI, 1.04-1.33; P = .009).
Extent of myocardial viability by tertiles, regardless of viability definition, did not highlight any group with a reduced risk for death or HHF, or group with better LV functional recovery, from OMT plus PCI, compared with OMT alone.
The findings appear to suggest that scar burden, but not extent of viability as it’s usually measured, may effectively guide PCI decisions in such patients, Dr. Perera said.
“I would say that viability testing as we understand it now, based on the paradigm of hibernating myocardium, is very useful,” he said, “but that is not the only information we can get from a viability test.”
Scar burden can also be determined from the same tests but isn’t typically looked at. “We’re actually collecting this information but not using it,” Dr. Perera said. “When we do, it is really powerfully predictive” of both clinical outcomes and LV functional recovery. “Yet scar burden is not in any of the guidelines for stratifying risk.”
REVIVED-BCIS2 was funded by the National Institute for Health and Care Research Health Technology Assessment Program. Dr. Perera had no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Atorvastatin cut anthracycline cardiac dysfunction in lymphoma
NEW ORLEANS – Atorvastatin treatment of patients with lymphoma undergoing treatment with an anthracycline significantly cut the incidence of incident cardiac dysfunction by about two-thirds during 12 months of treatment, in a multicenter, randomized trial with 300 enrolled patients.
“These data support the use of atorvastatin among patients with lymphoma being treated with anthracyclines where prevention of cardiac systolic dysfunction is important,” concluded Tomas G. Neilan, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
He highlighted that an important difference between the new study, STOP-CA, and a major prior study with a neutral effect published in 2022, was that STOP-CA “was powered for a major change” in cardiac function as the study’s primary outcome, a decline from baseline in left ventricular ejection fraction (LVEF) of at least 10% that also reduced ejection fraction to less than 55%.
“We can consider these medications [atorvastatin] for patients at higher risk for cardiac toxicity from anthracyclines, such as patients who receive a higher dose of an anthracycline, older patients, people with obesity, and women, commented Anita Deswal, MD, professor and chair of the department of cardiology at the University of Texas MD Anderson Cancer Center, Houston, who was not involved with the study.
A basis for an ‘important discussion’ with patients
“For patients receiving higher doses of anthracyclines, the STOP-CA trial says that whether to start a statin for cardiac protection is now an important discussion” for these patients to have with their treating clinicians. ”That was not the case before today,” commented Ronald M. Witteles, MD, a cardiologist and professor who specializes in cardio-oncology at Stanford (Calif.) University.
“For a patient being treated for lymphoma or for another cancer and treated with equal or higher anthracycline doses, such as patients with a sarcoma, this trial’s results at the very least warrant a discussion between physicians and patients to make the decision,” Dr. Witteles, who was not involved in the study, said in an interview. But he also cautioned that “whether an individual patient should take a statin in this scenario is still not a no-brainer. While the trial was positive, it was for an imaging rather than for a clinical endpoint.”
Experts noted that a similar study with the clinical endpoint of heart failure would require both many more randomized patients as well as much longer follow-up. STOP-CA was not powered for this endpoint. During its 12-month duration, a total of 11 patients developed heart failure, with no between group difference.
STOP-CA enrolled adults with lymphoma (Hodgkin or non-Hodgkin) and scheduled to undergo anthracycline treatment at eight U.S. centers and one in Canada, and excluded patients already on statin treatment or those for whom a statin was already indicated. Of the 300 enrolled patients, 286 had 12-month follow-up. Randomization assigned patients to receive either 40 mg daily of atorvastatin or placebo.
Their cumulative, median anthracycline dose was 300 mg/m2, which is typical for treating lymphoma, but higher than the typical dose use for patients with breast cancer. At baseline, average LVEF was 63%, and after 12 months this had declined to 59%. Forty-six of the 286 patients assessed after 12 months fulfilled the primary outcome of at least a 10–percentage point reduction from baseline in their LVEF and a decline in LVEF to less than 55%. Researchers used cardiac MR to assess LVEF at baseline, and in most patients at follow-up, but a minority of patients had their follow-up assessments by echocardiography because of logistical issues. Greater than 90% of patients were adherent to their assigned regimen.
Tripled incidence of cardiac dysfunction in placebo patients
The incidence of this outcome was 9% among the patients who received atorvastatin, and 22% among those on placebo, a significant difference. The calculated odds of the primary outcome was 2.9-fold more likely among the patients treated with placebo, compared with those who received atorvastatin, also a significant difference.
The study’s secondary outcome was patients who had at least a 5% drop from baseline in their LVEF and with a LVEF of less than 55% after 12 months. This outcome occurred in 13% of patients treated with atorvastatin and in 29% of those who received placebo, a significant difference.
The atorvastatin and placebo arms showed no significant differences in adverse events during the study, with roughly similar incidence rates for muscle pain, elevated liver enzymes, and renal failure. None of the enrolled patients developed myositis.
Atorvastatin treatment also produced an expected average 37% decline from baseline in levels of LDL cholesterol.
“This was a well-designed and important trial,” said Dr. Witteles. “Anthracyclines remain a mainstay of cancer therapies for a number of malignancies, such as lymphoma and sarcoma, and the cardiac side effects of development of cardiac dysfunction are unequivocally real.”
The importance of a clinically meaningful effect
The results especially contrast with the findings from the PREVENT study, published in 2022, which compared a daily, 40-mg atorvastatin treatment with placebo in 279 randomized patients with breast cancer and treated for 24 months. However, patients in PREVENT had a cumulative, median anthracycline dose of 240 mg/m2, and the study’s primary outcome was the average change from baseline in LVEF after 24 months of treatment, which was a reduction of 0.08 percentage points in the placebo arm, a nonsignificant difference.
In STOP-CA, the average change in LVEF from baseline was a 1–percentage point reduction in the placebo arm, compared with the atorvastatin-treated patients, a difference that was statistically significant, but “not clinically significant,” said Dr. Neilan, director of the cardio-oncology program at Massachusetts General Hospital, Boston. He cited the good fortune of the STOP-CA investigators when they received a recommendation from reviewers early on to design their study to track a clinically meaningful change in LVEF rather than just looking at the average overall change.
Dr. Deswal also noted that it is unlikely that future studies will examine the efficacy of a statin for preventing LVEF in patients across the range of cancers that are eligible for anthracycline treatment. As a result, she predicted that “we may have to extrapolate” the results from STOP-CA to patients with other cancer types.
STOP-CA received no commercial funding. Dr. Neilan has been a consultant for and received fees from Abbvie, Amgen, Bristol-Myers Squibb, CRC Oncology, Genentech, Roche, and Sanofi, and has received grant funding from AstraZeneca and Bristol Myers Squib. Dr. Deswal and Dr. Witteles had no relevant disclosures.
NEW ORLEANS – Atorvastatin treatment of patients with lymphoma undergoing treatment with an anthracycline significantly cut the incidence of incident cardiac dysfunction by about two-thirds during 12 months of treatment, in a multicenter, randomized trial with 300 enrolled patients.
“These data support the use of atorvastatin among patients with lymphoma being treated with anthracyclines where prevention of cardiac systolic dysfunction is important,” concluded Tomas G. Neilan, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
He highlighted that an important difference between the new study, STOP-CA, and a major prior study with a neutral effect published in 2022, was that STOP-CA “was powered for a major change” in cardiac function as the study’s primary outcome, a decline from baseline in left ventricular ejection fraction (LVEF) of at least 10% that also reduced ejection fraction to less than 55%.
“We can consider these medications [atorvastatin] for patients at higher risk for cardiac toxicity from anthracyclines, such as patients who receive a higher dose of an anthracycline, older patients, people with obesity, and women, commented Anita Deswal, MD, professor and chair of the department of cardiology at the University of Texas MD Anderson Cancer Center, Houston, who was not involved with the study.
A basis for an ‘important discussion’ with patients
“For patients receiving higher doses of anthracyclines, the STOP-CA trial says that whether to start a statin for cardiac protection is now an important discussion” for these patients to have with their treating clinicians. ”That was not the case before today,” commented Ronald M. Witteles, MD, a cardiologist and professor who specializes in cardio-oncology at Stanford (Calif.) University.
“For a patient being treated for lymphoma or for another cancer and treated with equal or higher anthracycline doses, such as patients with a sarcoma, this trial’s results at the very least warrant a discussion between physicians and patients to make the decision,” Dr. Witteles, who was not involved in the study, said in an interview. But he also cautioned that “whether an individual patient should take a statin in this scenario is still not a no-brainer. While the trial was positive, it was for an imaging rather than for a clinical endpoint.”
Experts noted that a similar study with the clinical endpoint of heart failure would require both many more randomized patients as well as much longer follow-up. STOP-CA was not powered for this endpoint. During its 12-month duration, a total of 11 patients developed heart failure, with no between group difference.
STOP-CA enrolled adults with lymphoma (Hodgkin or non-Hodgkin) and scheduled to undergo anthracycline treatment at eight U.S. centers and one in Canada, and excluded patients already on statin treatment or those for whom a statin was already indicated. Of the 300 enrolled patients, 286 had 12-month follow-up. Randomization assigned patients to receive either 40 mg daily of atorvastatin or placebo.
Their cumulative, median anthracycline dose was 300 mg/m2, which is typical for treating lymphoma, but higher than the typical dose use for patients with breast cancer. At baseline, average LVEF was 63%, and after 12 months this had declined to 59%. Forty-six of the 286 patients assessed after 12 months fulfilled the primary outcome of at least a 10–percentage point reduction from baseline in their LVEF and a decline in LVEF to less than 55%. Researchers used cardiac MR to assess LVEF at baseline, and in most patients at follow-up, but a minority of patients had their follow-up assessments by echocardiography because of logistical issues. Greater than 90% of patients were adherent to their assigned regimen.
Tripled incidence of cardiac dysfunction in placebo patients
The incidence of this outcome was 9% among the patients who received atorvastatin, and 22% among those on placebo, a significant difference. The calculated odds of the primary outcome was 2.9-fold more likely among the patients treated with placebo, compared with those who received atorvastatin, also a significant difference.
The study’s secondary outcome was patients who had at least a 5% drop from baseline in their LVEF and with a LVEF of less than 55% after 12 months. This outcome occurred in 13% of patients treated with atorvastatin and in 29% of those who received placebo, a significant difference.
The atorvastatin and placebo arms showed no significant differences in adverse events during the study, with roughly similar incidence rates for muscle pain, elevated liver enzymes, and renal failure. None of the enrolled patients developed myositis.
Atorvastatin treatment also produced an expected average 37% decline from baseline in levels of LDL cholesterol.
“This was a well-designed and important trial,” said Dr. Witteles. “Anthracyclines remain a mainstay of cancer therapies for a number of malignancies, such as lymphoma and sarcoma, and the cardiac side effects of development of cardiac dysfunction are unequivocally real.”
The importance of a clinically meaningful effect
The results especially contrast with the findings from the PREVENT study, published in 2022, which compared a daily, 40-mg atorvastatin treatment with placebo in 279 randomized patients with breast cancer and treated for 24 months. However, patients in PREVENT had a cumulative, median anthracycline dose of 240 mg/m2, and the study’s primary outcome was the average change from baseline in LVEF after 24 months of treatment, which was a reduction of 0.08 percentage points in the placebo arm, a nonsignificant difference.
In STOP-CA, the average change in LVEF from baseline was a 1–percentage point reduction in the placebo arm, compared with the atorvastatin-treated patients, a difference that was statistically significant, but “not clinically significant,” said Dr. Neilan, director of the cardio-oncology program at Massachusetts General Hospital, Boston. He cited the good fortune of the STOP-CA investigators when they received a recommendation from reviewers early on to design their study to track a clinically meaningful change in LVEF rather than just looking at the average overall change.
Dr. Deswal also noted that it is unlikely that future studies will examine the efficacy of a statin for preventing LVEF in patients across the range of cancers that are eligible for anthracycline treatment. As a result, she predicted that “we may have to extrapolate” the results from STOP-CA to patients with other cancer types.
STOP-CA received no commercial funding. Dr. Neilan has been a consultant for and received fees from Abbvie, Amgen, Bristol-Myers Squibb, CRC Oncology, Genentech, Roche, and Sanofi, and has received grant funding from AstraZeneca and Bristol Myers Squib. Dr. Deswal and Dr. Witteles had no relevant disclosures.
NEW ORLEANS – Atorvastatin treatment of patients with lymphoma undergoing treatment with an anthracycline significantly cut the incidence of incident cardiac dysfunction by about two-thirds during 12 months of treatment, in a multicenter, randomized trial with 300 enrolled patients.
“These data support the use of atorvastatin among patients with lymphoma being treated with anthracyclines where prevention of cardiac systolic dysfunction is important,” concluded Tomas G. Neilan, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
He highlighted that an important difference between the new study, STOP-CA, and a major prior study with a neutral effect published in 2022, was that STOP-CA “was powered for a major change” in cardiac function as the study’s primary outcome, a decline from baseline in left ventricular ejection fraction (LVEF) of at least 10% that also reduced ejection fraction to less than 55%.
“We can consider these medications [atorvastatin] for patients at higher risk for cardiac toxicity from anthracyclines, such as patients who receive a higher dose of an anthracycline, older patients, people with obesity, and women, commented Anita Deswal, MD, professor and chair of the department of cardiology at the University of Texas MD Anderson Cancer Center, Houston, who was not involved with the study.
A basis for an ‘important discussion’ with patients
“For patients receiving higher doses of anthracyclines, the STOP-CA trial says that whether to start a statin for cardiac protection is now an important discussion” for these patients to have with their treating clinicians. ”That was not the case before today,” commented Ronald M. Witteles, MD, a cardiologist and professor who specializes in cardio-oncology at Stanford (Calif.) University.
“For a patient being treated for lymphoma or for another cancer and treated with equal or higher anthracycline doses, such as patients with a sarcoma, this trial’s results at the very least warrant a discussion between physicians and patients to make the decision,” Dr. Witteles, who was not involved in the study, said in an interview. But he also cautioned that “whether an individual patient should take a statin in this scenario is still not a no-brainer. While the trial was positive, it was for an imaging rather than for a clinical endpoint.”
Experts noted that a similar study with the clinical endpoint of heart failure would require both many more randomized patients as well as much longer follow-up. STOP-CA was not powered for this endpoint. During its 12-month duration, a total of 11 patients developed heart failure, with no between group difference.
STOP-CA enrolled adults with lymphoma (Hodgkin or non-Hodgkin) and scheduled to undergo anthracycline treatment at eight U.S. centers and one in Canada, and excluded patients already on statin treatment or those for whom a statin was already indicated. Of the 300 enrolled patients, 286 had 12-month follow-up. Randomization assigned patients to receive either 40 mg daily of atorvastatin or placebo.
Their cumulative, median anthracycline dose was 300 mg/m2, which is typical for treating lymphoma, but higher than the typical dose use for patients with breast cancer. At baseline, average LVEF was 63%, and after 12 months this had declined to 59%. Forty-six of the 286 patients assessed after 12 months fulfilled the primary outcome of at least a 10–percentage point reduction from baseline in their LVEF and a decline in LVEF to less than 55%. Researchers used cardiac MR to assess LVEF at baseline, and in most patients at follow-up, but a minority of patients had their follow-up assessments by echocardiography because of logistical issues. Greater than 90% of patients were adherent to their assigned regimen.
Tripled incidence of cardiac dysfunction in placebo patients
The incidence of this outcome was 9% among the patients who received atorvastatin, and 22% among those on placebo, a significant difference. The calculated odds of the primary outcome was 2.9-fold more likely among the patients treated with placebo, compared with those who received atorvastatin, also a significant difference.
The study’s secondary outcome was patients who had at least a 5% drop from baseline in their LVEF and with a LVEF of less than 55% after 12 months. This outcome occurred in 13% of patients treated with atorvastatin and in 29% of those who received placebo, a significant difference.
The atorvastatin and placebo arms showed no significant differences in adverse events during the study, with roughly similar incidence rates for muscle pain, elevated liver enzymes, and renal failure. None of the enrolled patients developed myositis.
Atorvastatin treatment also produced an expected average 37% decline from baseline in levels of LDL cholesterol.
“This was a well-designed and important trial,” said Dr. Witteles. “Anthracyclines remain a mainstay of cancer therapies for a number of malignancies, such as lymphoma and sarcoma, and the cardiac side effects of development of cardiac dysfunction are unequivocally real.”
The importance of a clinically meaningful effect
The results especially contrast with the findings from the PREVENT study, published in 2022, which compared a daily, 40-mg atorvastatin treatment with placebo in 279 randomized patients with breast cancer and treated for 24 months. However, patients in PREVENT had a cumulative, median anthracycline dose of 240 mg/m2, and the study’s primary outcome was the average change from baseline in LVEF after 24 months of treatment, which was a reduction of 0.08 percentage points in the placebo arm, a nonsignificant difference.
In STOP-CA, the average change in LVEF from baseline was a 1–percentage point reduction in the placebo arm, compared with the atorvastatin-treated patients, a difference that was statistically significant, but “not clinically significant,” said Dr. Neilan, director of the cardio-oncology program at Massachusetts General Hospital, Boston. He cited the good fortune of the STOP-CA investigators when they received a recommendation from reviewers early on to design their study to track a clinically meaningful change in LVEF rather than just looking at the average overall change.
Dr. Deswal also noted that it is unlikely that future studies will examine the efficacy of a statin for preventing LVEF in patients across the range of cancers that are eligible for anthracycline treatment. As a result, she predicted that “we may have to extrapolate” the results from STOP-CA to patients with other cancer types.
STOP-CA received no commercial funding. Dr. Neilan has been a consultant for and received fees from Abbvie, Amgen, Bristol-Myers Squibb, CRC Oncology, Genentech, Roche, and Sanofi, and has received grant funding from AstraZeneca and Bristol Myers Squib. Dr. Deswal and Dr. Witteles had no relevant disclosures.
AT ACC 2023
Transcatheter tricuspid valve repair effective and safe for regurgitation
NEW ORLEANS – In the first pivotal randomized, controlled trial of a transcatheter device for the repair of severe tricuspid regurgitation, a large reduction in valve dysfunction was associated with substantial improvement in quality of life (QOL) persisting out of 1 year of follow-up, according to results of the TRILUMINATE trial.
Based on the low procedural risks of the repair, the principal investigator, Paul Sorajja, MD, called the results “very clinically meaningful” as he presented the results at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Conducted at 65 centers in the United States, Canada, and North America, TRILUMINATE evaluated a transcatheter end-to-end (TEER) repair performed with the TriClip G4 Delivery System (Abbott). The study included two cohorts, both of which will be followed for 5 years. One included patients with very severe tricuspid regurgitation enrolled in a single arm. Data on this cohort is expected later in 2023.
In the randomized portion of the study, 350 patients enrolled with severe tricuspid regurgitation underwent TEER with a clipping device and then were followed on the guideline-directed therapy (GDMT) for heart failure they were receiving at baseline. The control group was managed on GDMT alone.
The primary composite endpoint at 1 year was a composite of death from any cause and/or tricuspid valve surgery, hospitalization for heart failure, and quality of life as measured with the Kansas City Cardiomyopathy questionnaire (KCCQ).
Benefit driven by quality of life
For the primary endpoint, the win ratio, a statistical calculation of those who did relative to those who did not benefit, was 1.48, signifying a 48% advantage (P = .02). This was driven almost entirely by the KCCQ endpoint. There was no significant difference death and/or tricuspid valve surgery, which occurred in about 10% of both groups (P = .75) or heart failure hospitalization, which was occurred in slightly more patients randomized to repair (14.9% vs. 12.1%; P = .41).
For KCCQ, the mean increase at 1 year was 12.3 points in the repair group versus 0.6 points (P < .001) in the control group. With an increase of 5-10 points typically considered to be clinically meaningful, the advantage of repair over GDMT at the threshold of 15 points or greater was highly statistically significant (49.7% vs. 26.4%; P < .0001).
This advantage was attributed to control of regurgitation. The proportion achieving moderate or less regurgitation sustained at 1 year was 87% in the repair group versus 4.8% in the GDMT group (P < .0001).
When assessed independent of treatment, KCCQ benefits at 1 year increased in a stepwise fashion as severity of regurgitation was reduced, climbing from 2 points if there was no improvement to 6 points with one grade in improvement and then to 18 points with at least a two-grade improvement.
For regurgitation, “the repair was extremely effective,” said Dr. Sorajja of Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital, Minneapolis. He added that the degree of regurgitation control in the TRILUMINATE trial “is the highest ever reported.” With previous trials with other transcatheter devices in development, the improvement so far has been on the order of 70%-80%.
For enrollment in TRILUMINATE, patients were required to have at least an intermediate risk of morbidity or mortality from tricuspid valve surgery. Exclusion criteria included a left ventricular ejection fraction (LVEF) less than 20% and severe pulmonary hypertension.
More than 70% of patients had the highest (torrential) or second highest (massive) category of regurgitation on a five-level scale by echocardiography. Almost all the remaining were at the third level (severe).
Of those enrolled, the average age was roughly 78 years. About 55% were women. Nearly 60% were in New York Heart Association class III or IV heart failure and most had significant comorbidities, including hypertension (> 80%), atrial fibrillation (about 90%), and renal disease (35%). Patients with diabetes (16%), chronic obstructive pulmonary disease (10%), and liver disease (7.5%) were represented in lower numbers.
Surgery is not necessarily an option
All enrolled patients were considered to be at intermediate or greater risk for mortality with surgical replacement of the tricuspid valve, but Dr. Sorajja pointed out that surgery, which involves valve replacement, is not necessarily an alternative to valve repair. Even in fit patients, the high morbidity, mortality, and extended hospital stay associated with surgical valve replacement makes this procedure unattractive.
In this trial, most patients who underwent the transcatheter procedure were discharged within a day. The safety was excellent, Dr. Sorajja said. Only three patients (1.7%) had a major adverse event. This included two cases of new-onset renal failure and one cardiovascular death. There were no cases of endocarditis requiring surgery or any other type of nonelective cardiovascular surgery, including for any device-related issue.
In the sick population enrolled, Dr. Sorajja characterized the number of adverse events over 1 year as “very low.”
These results are important, according to Kendra Grubb, MD, surgical director of the Structural Heart and Valve Center, Emory University, Atlanta. While she expressed surprise that there was no signal of benefit on hard endpoints at 1 year, she emphasized that “these patients feel terrible,” and they are frustrating to manage because surgery is often contraindicated or impractical.
“Finally, we have something for this group,” she said, noting that the mortality from valve replacement surgery even among patients who are fit enough for surgery to be considered is about 10%.
Ajay Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, was more circumspect. He agreed that the improvement in QOL was encouraging, but cautioned that QOL is a particularly soft outcome in a nonrandomized trial in which patients may feel better just knowing that there regurgitation has been controlled. He found the lack of benefit on hard outcomes not just surprising but “disappointing.”
Still, he agreed the improvement in QOL is potentially meaningful for a procedure that appears to be relatively safe.
Dr. Sorajja reported financial relationships with Boston Scientific, Edwards Lifesciences, Foldax. 4C Medical, Gore Medtronic, Phillips, Siemens, Shifamed, Vdyne, xDot, and Abbott Structural, which provided funding for this trial. Dr. Grubb reported financial relationships with Abbott Vascular, Ancora Heart, Bioventrix, Boston Scientific, Edwards Lifesciences, 4C Medical, JenaValve, and Medtronic. Dr. Kirtane reported financial relationships with Abbott Vascular, Amgen, Boston Scientific, Chiesi, Medtronic, Opsens, Phillips, ReCor, Regeneron, and Zoll.
NEW ORLEANS – In the first pivotal randomized, controlled trial of a transcatheter device for the repair of severe tricuspid regurgitation, a large reduction in valve dysfunction was associated with substantial improvement in quality of life (QOL) persisting out of 1 year of follow-up, according to results of the TRILUMINATE trial.
Based on the low procedural risks of the repair, the principal investigator, Paul Sorajja, MD, called the results “very clinically meaningful” as he presented the results at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Conducted at 65 centers in the United States, Canada, and North America, TRILUMINATE evaluated a transcatheter end-to-end (TEER) repair performed with the TriClip G4 Delivery System (Abbott). The study included two cohorts, both of which will be followed for 5 years. One included patients with very severe tricuspid regurgitation enrolled in a single arm. Data on this cohort is expected later in 2023.
In the randomized portion of the study, 350 patients enrolled with severe tricuspid regurgitation underwent TEER with a clipping device and then were followed on the guideline-directed therapy (GDMT) for heart failure they were receiving at baseline. The control group was managed on GDMT alone.
The primary composite endpoint at 1 year was a composite of death from any cause and/or tricuspid valve surgery, hospitalization for heart failure, and quality of life as measured with the Kansas City Cardiomyopathy questionnaire (KCCQ).
Benefit driven by quality of life
For the primary endpoint, the win ratio, a statistical calculation of those who did relative to those who did not benefit, was 1.48, signifying a 48% advantage (P = .02). This was driven almost entirely by the KCCQ endpoint. There was no significant difference death and/or tricuspid valve surgery, which occurred in about 10% of both groups (P = .75) or heart failure hospitalization, which was occurred in slightly more patients randomized to repair (14.9% vs. 12.1%; P = .41).
For KCCQ, the mean increase at 1 year was 12.3 points in the repair group versus 0.6 points (P < .001) in the control group. With an increase of 5-10 points typically considered to be clinically meaningful, the advantage of repair over GDMT at the threshold of 15 points or greater was highly statistically significant (49.7% vs. 26.4%; P < .0001).
This advantage was attributed to control of regurgitation. The proportion achieving moderate or less regurgitation sustained at 1 year was 87% in the repair group versus 4.8% in the GDMT group (P < .0001).
When assessed independent of treatment, KCCQ benefits at 1 year increased in a stepwise fashion as severity of regurgitation was reduced, climbing from 2 points if there was no improvement to 6 points with one grade in improvement and then to 18 points with at least a two-grade improvement.
For regurgitation, “the repair was extremely effective,” said Dr. Sorajja of Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital, Minneapolis. He added that the degree of regurgitation control in the TRILUMINATE trial “is the highest ever reported.” With previous trials with other transcatheter devices in development, the improvement so far has been on the order of 70%-80%.
For enrollment in TRILUMINATE, patients were required to have at least an intermediate risk of morbidity or mortality from tricuspid valve surgery. Exclusion criteria included a left ventricular ejection fraction (LVEF) less than 20% and severe pulmonary hypertension.
More than 70% of patients had the highest (torrential) or second highest (massive) category of regurgitation on a five-level scale by echocardiography. Almost all the remaining were at the third level (severe).
Of those enrolled, the average age was roughly 78 years. About 55% were women. Nearly 60% were in New York Heart Association class III or IV heart failure and most had significant comorbidities, including hypertension (> 80%), atrial fibrillation (about 90%), and renal disease (35%). Patients with diabetes (16%), chronic obstructive pulmonary disease (10%), and liver disease (7.5%) were represented in lower numbers.
Surgery is not necessarily an option
All enrolled patients were considered to be at intermediate or greater risk for mortality with surgical replacement of the tricuspid valve, but Dr. Sorajja pointed out that surgery, which involves valve replacement, is not necessarily an alternative to valve repair. Even in fit patients, the high morbidity, mortality, and extended hospital stay associated with surgical valve replacement makes this procedure unattractive.
In this trial, most patients who underwent the transcatheter procedure were discharged within a day. The safety was excellent, Dr. Sorajja said. Only three patients (1.7%) had a major adverse event. This included two cases of new-onset renal failure and one cardiovascular death. There were no cases of endocarditis requiring surgery or any other type of nonelective cardiovascular surgery, including for any device-related issue.
In the sick population enrolled, Dr. Sorajja characterized the number of adverse events over 1 year as “very low.”
These results are important, according to Kendra Grubb, MD, surgical director of the Structural Heart and Valve Center, Emory University, Atlanta. While she expressed surprise that there was no signal of benefit on hard endpoints at 1 year, she emphasized that “these patients feel terrible,” and they are frustrating to manage because surgery is often contraindicated or impractical.
“Finally, we have something for this group,” she said, noting that the mortality from valve replacement surgery even among patients who are fit enough for surgery to be considered is about 10%.
Ajay Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, was more circumspect. He agreed that the improvement in QOL was encouraging, but cautioned that QOL is a particularly soft outcome in a nonrandomized trial in which patients may feel better just knowing that there regurgitation has been controlled. He found the lack of benefit on hard outcomes not just surprising but “disappointing.”
Still, he agreed the improvement in QOL is potentially meaningful for a procedure that appears to be relatively safe.
Dr. Sorajja reported financial relationships with Boston Scientific, Edwards Lifesciences, Foldax. 4C Medical, Gore Medtronic, Phillips, Siemens, Shifamed, Vdyne, xDot, and Abbott Structural, which provided funding for this trial. Dr. Grubb reported financial relationships with Abbott Vascular, Ancora Heart, Bioventrix, Boston Scientific, Edwards Lifesciences, 4C Medical, JenaValve, and Medtronic. Dr. Kirtane reported financial relationships with Abbott Vascular, Amgen, Boston Scientific, Chiesi, Medtronic, Opsens, Phillips, ReCor, Regeneron, and Zoll.
NEW ORLEANS – In the first pivotal randomized, controlled trial of a transcatheter device for the repair of severe tricuspid regurgitation, a large reduction in valve dysfunction was associated with substantial improvement in quality of life (QOL) persisting out of 1 year of follow-up, according to results of the TRILUMINATE trial.
Based on the low procedural risks of the repair, the principal investigator, Paul Sorajja, MD, called the results “very clinically meaningful” as he presented the results at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Conducted at 65 centers in the United States, Canada, and North America, TRILUMINATE evaluated a transcatheter end-to-end (TEER) repair performed with the TriClip G4 Delivery System (Abbott). The study included two cohorts, both of which will be followed for 5 years. One included patients with very severe tricuspid regurgitation enrolled in a single arm. Data on this cohort is expected later in 2023.
In the randomized portion of the study, 350 patients enrolled with severe tricuspid regurgitation underwent TEER with a clipping device and then were followed on the guideline-directed therapy (GDMT) for heart failure they were receiving at baseline. The control group was managed on GDMT alone.
The primary composite endpoint at 1 year was a composite of death from any cause and/or tricuspid valve surgery, hospitalization for heart failure, and quality of life as measured with the Kansas City Cardiomyopathy questionnaire (KCCQ).
Benefit driven by quality of life
For the primary endpoint, the win ratio, a statistical calculation of those who did relative to those who did not benefit, was 1.48, signifying a 48% advantage (P = .02). This was driven almost entirely by the KCCQ endpoint. There was no significant difference death and/or tricuspid valve surgery, which occurred in about 10% of both groups (P = .75) or heart failure hospitalization, which was occurred in slightly more patients randomized to repair (14.9% vs. 12.1%; P = .41).
For KCCQ, the mean increase at 1 year was 12.3 points in the repair group versus 0.6 points (P < .001) in the control group. With an increase of 5-10 points typically considered to be clinically meaningful, the advantage of repair over GDMT at the threshold of 15 points or greater was highly statistically significant (49.7% vs. 26.4%; P < .0001).
This advantage was attributed to control of regurgitation. The proportion achieving moderate or less regurgitation sustained at 1 year was 87% in the repair group versus 4.8% in the GDMT group (P < .0001).
When assessed independent of treatment, KCCQ benefits at 1 year increased in a stepwise fashion as severity of regurgitation was reduced, climbing from 2 points if there was no improvement to 6 points with one grade in improvement and then to 18 points with at least a two-grade improvement.
For regurgitation, “the repair was extremely effective,” said Dr. Sorajja of Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital, Minneapolis. He added that the degree of regurgitation control in the TRILUMINATE trial “is the highest ever reported.” With previous trials with other transcatheter devices in development, the improvement so far has been on the order of 70%-80%.
For enrollment in TRILUMINATE, patients were required to have at least an intermediate risk of morbidity or mortality from tricuspid valve surgery. Exclusion criteria included a left ventricular ejection fraction (LVEF) less than 20% and severe pulmonary hypertension.
More than 70% of patients had the highest (torrential) or second highest (massive) category of regurgitation on a five-level scale by echocardiography. Almost all the remaining were at the third level (severe).
Of those enrolled, the average age was roughly 78 years. About 55% were women. Nearly 60% were in New York Heart Association class III or IV heart failure and most had significant comorbidities, including hypertension (> 80%), atrial fibrillation (about 90%), and renal disease (35%). Patients with diabetes (16%), chronic obstructive pulmonary disease (10%), and liver disease (7.5%) were represented in lower numbers.
Surgery is not necessarily an option
All enrolled patients were considered to be at intermediate or greater risk for mortality with surgical replacement of the tricuspid valve, but Dr. Sorajja pointed out that surgery, which involves valve replacement, is not necessarily an alternative to valve repair. Even in fit patients, the high morbidity, mortality, and extended hospital stay associated with surgical valve replacement makes this procedure unattractive.
In this trial, most patients who underwent the transcatheter procedure were discharged within a day. The safety was excellent, Dr. Sorajja said. Only three patients (1.7%) had a major adverse event. This included two cases of new-onset renal failure and one cardiovascular death. There were no cases of endocarditis requiring surgery or any other type of nonelective cardiovascular surgery, including for any device-related issue.
In the sick population enrolled, Dr. Sorajja characterized the number of adverse events over 1 year as “very low.”
These results are important, according to Kendra Grubb, MD, surgical director of the Structural Heart and Valve Center, Emory University, Atlanta. While she expressed surprise that there was no signal of benefit on hard endpoints at 1 year, she emphasized that “these patients feel terrible,” and they are frustrating to manage because surgery is often contraindicated or impractical.
“Finally, we have something for this group,” she said, noting that the mortality from valve replacement surgery even among patients who are fit enough for surgery to be considered is about 10%.
Ajay Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, was more circumspect. He agreed that the improvement in QOL was encouraging, but cautioned that QOL is a particularly soft outcome in a nonrandomized trial in which patients may feel better just knowing that there regurgitation has been controlled. He found the lack of benefit on hard outcomes not just surprising but “disappointing.”
Still, he agreed the improvement in QOL is potentially meaningful for a procedure that appears to be relatively safe.
Dr. Sorajja reported financial relationships with Boston Scientific, Edwards Lifesciences, Foldax. 4C Medical, Gore Medtronic, Phillips, Siemens, Shifamed, Vdyne, xDot, and Abbott Structural, which provided funding for this trial. Dr. Grubb reported financial relationships with Abbott Vascular, Ancora Heart, Bioventrix, Boston Scientific, Edwards Lifesciences, 4C Medical, JenaValve, and Medtronic. Dr. Kirtane reported financial relationships with Abbott Vascular, Amgen, Boston Scientific, Chiesi, Medtronic, Opsens, Phillips, ReCor, Regeneron, and Zoll.
AT ACC 2023
500 more steps a day tied to 14% lower CVD risk in older adults
Older adults who added a quarter mile of steps to their day showed a reduction in risk of cardiovascular events by 14% within 4 years, according to a study in more than 400 individuals.
“Aging is such a dynamic process, but most studies of daily steps and step goals are conducted on younger populations,” lead author Erin E. Dooley, PhD, an epidemiologist at the University of Alabama at Birmingham, said in an interview.
The impact of more modest step goals in older adults has not been well studied, Dr. Dooley said.
The population in the current study ranged from 71 to 92 years, with an average age of 78 years. The older age and relatively short follow-up period show the importance of steps and physical activity in older adults, she said.
Dr. Dooley presented the study at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
She and her colleagues analyzed a subsample of participants in Atherosclerosis Risk in Communities (ARIC) study, an ongoing study conducted by the National Heart, Lung, and Blood Institute. The study population included 452 adults for whom step data were available at visit 6 of the ARIC study between 2016 and 2017. Participants wore an accelerometer on the waist for at least 10 hours a day for at least 3 days. The mean age of the participants was 78.4 years, 59% were women, and 20% were Black.
Outcomes were measured through December 2019 and included fatal and nonfatal cardiovascular disease (CVD) events of coronary heart disease, stroke, and heart failure.
Overall, each additional 500 steps per day was linked to a 14% reduction in risk of a CVD event (hazard ratio, 0.86; 95% confidence interval, 0.76-0.98). The mean step count was 3,447 steps per day, and 34 participants (7.5%) experienced a CVD event over 1,269 person-years of follow-up.
The cumulative risk of CVD was significantly higher (11.5%) in the quartile of adults with the lowest step count (defined as fewer than 2,077 steps per day), compared with 3.5% in those with the highest step count (defined as at least 4,453 steps per day).
In addition, adults in the highest quartile of steps had a 77% reduced risk of a proximal CVD (within 3.5 years) event over the study period (HR, 0.23).
Additional research is needed to explore whether increased steps prevent or delay CVD and whether low step counts may be a biomarker for underlying disease, the researchers noted in their abstract.
However, the results support the value of even a modest increase in activity to reduce CVD risk in older adults.
Small steps may get patients started
Dr. Dooley said she was surprised at the degree of benefits on heart health from 500 steps, and noted that the findings have clinical implications.
“Steps may be a more understandable metric for physical activity for patients than talking about moderate to vigorous intensity physical activity,” she said in an interview. “While we do not want to diminish the importance of higher intensity physical activity, encouraging small increases in the number of daily steps can also have great benefits for heart health.
“Steps are counted using a variety of devices and phones, so it may be helpful for patients to show clinicians their activity during well visits,” Dr. Dooley said. “Walking may also be more manageable for people as it is low impact. Achievable goals are also important. This study suggests that, for older adults, around 3,000 steps or more was associated with reduced CVD risk,” although the greatest benefits were seen with the most active group who averaged 4,500 or more steps per day.
More research is needed to show how steps may change over time, and how this relates to CVD and heart health,” she said. “At this time, we only had a single measure of physical activity.”
Study fills research gap for older adults
“Currently, the majority of the literature exploring a relationship between physical activity and the risk for developing cardiovascular disease has evaluated all adults together, not only those who are 70 year of age and older,” Monica C. Serra, PhD, of the University of Texas, San Antonio, said in an interview. “This study allows us to start to target specific cardiovascular recommendations for older adults.”.
“It is always exciting to see results from physical activity studies that continue to support prior evidence that even small amounts of physical activity are beneficial to cardiovascular health,” said Dr. Serra, who is also vice chair of the program committee for the meeting. “These results suggest that even if only small additions in physical activity are achievable, they may have cumulative benefits in reducing cardiovascular disease risk.” For clinicians, the results also provide targets that are easy for patients to understand, said Dr. Serra. Daily step counts allow clinicians to provide specific and measurable goals to help their older patients increase physical activity.
“Small additions in total daily step counts may have clinically meaningful benefits to heart health, so promoting their patients to make any slight changes that are able to be consistently incorporated into their schedule should be encouraged. This may be best monitored by encouraging the use of an activity tracker,” she said.
Although the current study adds to the literature with objective measures of physical activity utilizing accelerometers, these devices are not as sensitive at picking up activities such as bicycling or swimming, which may be more appropriate for some older adults with mobility limitations and chronic conditions, Dr. Serra said. Additional research is needed to assess the impact of other activities on CVD in the older population.
The meeting was sponsored by the American Heart Association. The study received no outside funding. Dr. Dooley and Dr. Serra had no financial conflicts to disclose.
Older adults who added a quarter mile of steps to their day showed a reduction in risk of cardiovascular events by 14% within 4 years, according to a study in more than 400 individuals.
“Aging is such a dynamic process, but most studies of daily steps and step goals are conducted on younger populations,” lead author Erin E. Dooley, PhD, an epidemiologist at the University of Alabama at Birmingham, said in an interview.
The impact of more modest step goals in older adults has not been well studied, Dr. Dooley said.
The population in the current study ranged from 71 to 92 years, with an average age of 78 years. The older age and relatively short follow-up period show the importance of steps and physical activity in older adults, she said.
Dr. Dooley presented the study at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
She and her colleagues analyzed a subsample of participants in Atherosclerosis Risk in Communities (ARIC) study, an ongoing study conducted by the National Heart, Lung, and Blood Institute. The study population included 452 adults for whom step data were available at visit 6 of the ARIC study between 2016 and 2017. Participants wore an accelerometer on the waist for at least 10 hours a day for at least 3 days. The mean age of the participants was 78.4 years, 59% were women, and 20% were Black.
Outcomes were measured through December 2019 and included fatal and nonfatal cardiovascular disease (CVD) events of coronary heart disease, stroke, and heart failure.
Overall, each additional 500 steps per day was linked to a 14% reduction in risk of a CVD event (hazard ratio, 0.86; 95% confidence interval, 0.76-0.98). The mean step count was 3,447 steps per day, and 34 participants (7.5%) experienced a CVD event over 1,269 person-years of follow-up.
The cumulative risk of CVD was significantly higher (11.5%) in the quartile of adults with the lowest step count (defined as fewer than 2,077 steps per day), compared with 3.5% in those with the highest step count (defined as at least 4,453 steps per day).
In addition, adults in the highest quartile of steps had a 77% reduced risk of a proximal CVD (within 3.5 years) event over the study period (HR, 0.23).
Additional research is needed to explore whether increased steps prevent or delay CVD and whether low step counts may be a biomarker for underlying disease, the researchers noted in their abstract.
However, the results support the value of even a modest increase in activity to reduce CVD risk in older adults.
Small steps may get patients started
Dr. Dooley said she was surprised at the degree of benefits on heart health from 500 steps, and noted that the findings have clinical implications.
“Steps may be a more understandable metric for physical activity for patients than talking about moderate to vigorous intensity physical activity,” she said in an interview. “While we do not want to diminish the importance of higher intensity physical activity, encouraging small increases in the number of daily steps can also have great benefits for heart health.
“Steps are counted using a variety of devices and phones, so it may be helpful for patients to show clinicians their activity during well visits,” Dr. Dooley said. “Walking may also be more manageable for people as it is low impact. Achievable goals are also important. This study suggests that, for older adults, around 3,000 steps or more was associated with reduced CVD risk,” although the greatest benefits were seen with the most active group who averaged 4,500 or more steps per day.
More research is needed to show how steps may change over time, and how this relates to CVD and heart health,” she said. “At this time, we only had a single measure of physical activity.”
Study fills research gap for older adults
“Currently, the majority of the literature exploring a relationship between physical activity and the risk for developing cardiovascular disease has evaluated all adults together, not only those who are 70 year of age and older,” Monica C. Serra, PhD, of the University of Texas, San Antonio, said in an interview. “This study allows us to start to target specific cardiovascular recommendations for older adults.”.
“It is always exciting to see results from physical activity studies that continue to support prior evidence that even small amounts of physical activity are beneficial to cardiovascular health,” said Dr. Serra, who is also vice chair of the program committee for the meeting. “These results suggest that even if only small additions in physical activity are achievable, they may have cumulative benefits in reducing cardiovascular disease risk.” For clinicians, the results also provide targets that are easy for patients to understand, said Dr. Serra. Daily step counts allow clinicians to provide specific and measurable goals to help their older patients increase physical activity.
“Small additions in total daily step counts may have clinically meaningful benefits to heart health, so promoting their patients to make any slight changes that are able to be consistently incorporated into their schedule should be encouraged. This may be best monitored by encouraging the use of an activity tracker,” she said.
Although the current study adds to the literature with objective measures of physical activity utilizing accelerometers, these devices are not as sensitive at picking up activities such as bicycling or swimming, which may be more appropriate for some older adults with mobility limitations and chronic conditions, Dr. Serra said. Additional research is needed to assess the impact of other activities on CVD in the older population.
The meeting was sponsored by the American Heart Association. The study received no outside funding. Dr. Dooley and Dr. Serra had no financial conflicts to disclose.
Older adults who added a quarter mile of steps to their day showed a reduction in risk of cardiovascular events by 14% within 4 years, according to a study in more than 400 individuals.
“Aging is such a dynamic process, but most studies of daily steps and step goals are conducted on younger populations,” lead author Erin E. Dooley, PhD, an epidemiologist at the University of Alabama at Birmingham, said in an interview.
The impact of more modest step goals in older adults has not been well studied, Dr. Dooley said.
The population in the current study ranged from 71 to 92 years, with an average age of 78 years. The older age and relatively short follow-up period show the importance of steps and physical activity in older adults, she said.
Dr. Dooley presented the study at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
She and her colleagues analyzed a subsample of participants in Atherosclerosis Risk in Communities (ARIC) study, an ongoing study conducted by the National Heart, Lung, and Blood Institute. The study population included 452 adults for whom step data were available at visit 6 of the ARIC study between 2016 and 2017. Participants wore an accelerometer on the waist for at least 10 hours a day for at least 3 days. The mean age of the participants was 78.4 years, 59% were women, and 20% were Black.
Outcomes were measured through December 2019 and included fatal and nonfatal cardiovascular disease (CVD) events of coronary heart disease, stroke, and heart failure.
Overall, each additional 500 steps per day was linked to a 14% reduction in risk of a CVD event (hazard ratio, 0.86; 95% confidence interval, 0.76-0.98). The mean step count was 3,447 steps per day, and 34 participants (7.5%) experienced a CVD event over 1,269 person-years of follow-up.
The cumulative risk of CVD was significantly higher (11.5%) in the quartile of adults with the lowest step count (defined as fewer than 2,077 steps per day), compared with 3.5% in those with the highest step count (defined as at least 4,453 steps per day).
In addition, adults in the highest quartile of steps had a 77% reduced risk of a proximal CVD (within 3.5 years) event over the study period (HR, 0.23).
Additional research is needed to explore whether increased steps prevent or delay CVD and whether low step counts may be a biomarker for underlying disease, the researchers noted in their abstract.
However, the results support the value of even a modest increase in activity to reduce CVD risk in older adults.
Small steps may get patients started
Dr. Dooley said she was surprised at the degree of benefits on heart health from 500 steps, and noted that the findings have clinical implications.
“Steps may be a more understandable metric for physical activity for patients than talking about moderate to vigorous intensity physical activity,” she said in an interview. “While we do not want to diminish the importance of higher intensity physical activity, encouraging small increases in the number of daily steps can also have great benefits for heart health.
“Steps are counted using a variety of devices and phones, so it may be helpful for patients to show clinicians their activity during well visits,” Dr. Dooley said. “Walking may also be more manageable for people as it is low impact. Achievable goals are also important. This study suggests that, for older adults, around 3,000 steps or more was associated with reduced CVD risk,” although the greatest benefits were seen with the most active group who averaged 4,500 or more steps per day.
More research is needed to show how steps may change over time, and how this relates to CVD and heart health,” she said. “At this time, we only had a single measure of physical activity.”
Study fills research gap for older adults
“Currently, the majority of the literature exploring a relationship between physical activity and the risk for developing cardiovascular disease has evaluated all adults together, not only those who are 70 year of age and older,” Monica C. Serra, PhD, of the University of Texas, San Antonio, said in an interview. “This study allows us to start to target specific cardiovascular recommendations for older adults.”.
“It is always exciting to see results from physical activity studies that continue to support prior evidence that even small amounts of physical activity are beneficial to cardiovascular health,” said Dr. Serra, who is also vice chair of the program committee for the meeting. “These results suggest that even if only small additions in physical activity are achievable, they may have cumulative benefits in reducing cardiovascular disease risk.” For clinicians, the results also provide targets that are easy for patients to understand, said Dr. Serra. Daily step counts allow clinicians to provide specific and measurable goals to help their older patients increase physical activity.
“Small additions in total daily step counts may have clinically meaningful benefits to heart health, so promoting their patients to make any slight changes that are able to be consistently incorporated into their schedule should be encouraged. This may be best monitored by encouraging the use of an activity tracker,” she said.
Although the current study adds to the literature with objective measures of physical activity utilizing accelerometers, these devices are not as sensitive at picking up activities such as bicycling or swimming, which may be more appropriate for some older adults with mobility limitations and chronic conditions, Dr. Serra said. Additional research is needed to assess the impact of other activities on CVD in the older population.
The meeting was sponsored by the American Heart Association. The study received no outside funding. Dr. Dooley and Dr. Serra had no financial conflicts to disclose.
FROM EPI/LIFESTYLE 2023
FDA declines approval for omecamtiv mecarbil in HFrEF
The Food and Drug Administration has declined to approve omecamtiv mecarbil (Cytokinetics) for treatment of adults with chronic heart failure with reduced ejection fraction (HFrEF), citing a lack of evidence on efficacy.
Omecamtiv mecarbil is a first-in-class, selective cardiac myosin activator designed to improve cardiac performance.
Last December, a panel of FDA advisers recommended against approval of omecamtiv mecarbil for chronic HFrEF, as reported by this news organization.
The FDA Cardiovascular and Renal Drugs Advisory Committee voted 8 to 3 (with no abstentions) that the benefits of omecamtiv mecarbil do not outweigh the risks for HFrEF. The drug had a PDUFA date of February 28.
The committee’s decision was based on results from the phase 3 GALACTIC-HF trial, which enrolled 8,256 patients with HFrEF who were at risk of hospitalization and death, despite standard-of-care therapy.
As previously reported by this news organization, omecamtiv mecarbil produced a positive result for the study’s primary endpoint, with a 2.1% absolute reduction in the combined rate of cardiovascular (CV) death, first HF hospitalization, or first urgent visit for HF, compared with placebo during a median follow-up of about 22 months.
This represented an 8% relative risk reduction and broke down as a 0.6% absolute drop in CV death, compared with placebo, a 0.7% cut in HF hospitalization, and a 0.8% drop in urgent outpatient HF visits.
In a complete response letter, the FDA said GALACTIC-HF is “not sufficiently persuasive to establish substantial evidence of effectiveness for reducing the risk of heart failure events and cardiovascular death” in adults with HFrEF, Cytokinetics shared in a news release.
Further, the FDA said results from an additional clinical trial of omecamtiv mecarbil are required to establish substantial evidence of effectiveness for the treatment of HFrEF, with benefits that outweigh the risks, Cytokinetics said.
The company said it will request a meeting with the FDA to gain a better understanding of what may be required to support potential approval of omecamtiv mecarbil. However, the company also said it has “no plans” to conduct an additional clinical trial of omecamtiv mecarbil.
Instead, the company said its focus remains on the development of aficamten, the next-in-class cardiac myosin inhibitor, currently the subject of SEQUOIA-HCM, a phase 3 clinical trial in patients with obstructive hypertrophic cardiomyopathy.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has declined to approve omecamtiv mecarbil (Cytokinetics) for treatment of adults with chronic heart failure with reduced ejection fraction (HFrEF), citing a lack of evidence on efficacy.
Omecamtiv mecarbil is a first-in-class, selective cardiac myosin activator designed to improve cardiac performance.
Last December, a panel of FDA advisers recommended against approval of omecamtiv mecarbil for chronic HFrEF, as reported by this news organization.
The FDA Cardiovascular and Renal Drugs Advisory Committee voted 8 to 3 (with no abstentions) that the benefits of omecamtiv mecarbil do not outweigh the risks for HFrEF. The drug had a PDUFA date of February 28.
The committee’s decision was based on results from the phase 3 GALACTIC-HF trial, which enrolled 8,256 patients with HFrEF who were at risk of hospitalization and death, despite standard-of-care therapy.
As previously reported by this news organization, omecamtiv mecarbil produced a positive result for the study’s primary endpoint, with a 2.1% absolute reduction in the combined rate of cardiovascular (CV) death, first HF hospitalization, or first urgent visit for HF, compared with placebo during a median follow-up of about 22 months.
This represented an 8% relative risk reduction and broke down as a 0.6% absolute drop in CV death, compared with placebo, a 0.7% cut in HF hospitalization, and a 0.8% drop in urgent outpatient HF visits.
In a complete response letter, the FDA said GALACTIC-HF is “not sufficiently persuasive to establish substantial evidence of effectiveness for reducing the risk of heart failure events and cardiovascular death” in adults with HFrEF, Cytokinetics shared in a news release.
Further, the FDA said results from an additional clinical trial of omecamtiv mecarbil are required to establish substantial evidence of effectiveness for the treatment of HFrEF, with benefits that outweigh the risks, Cytokinetics said.
The company said it will request a meeting with the FDA to gain a better understanding of what may be required to support potential approval of omecamtiv mecarbil. However, the company also said it has “no plans” to conduct an additional clinical trial of omecamtiv mecarbil.
Instead, the company said its focus remains on the development of aficamten, the next-in-class cardiac myosin inhibitor, currently the subject of SEQUOIA-HCM, a phase 3 clinical trial in patients with obstructive hypertrophic cardiomyopathy.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has declined to approve omecamtiv mecarbil (Cytokinetics) for treatment of adults with chronic heart failure with reduced ejection fraction (HFrEF), citing a lack of evidence on efficacy.
Omecamtiv mecarbil is a first-in-class, selective cardiac myosin activator designed to improve cardiac performance.
Last December, a panel of FDA advisers recommended against approval of omecamtiv mecarbil for chronic HFrEF, as reported by this news organization.
The FDA Cardiovascular and Renal Drugs Advisory Committee voted 8 to 3 (with no abstentions) that the benefits of omecamtiv mecarbil do not outweigh the risks for HFrEF. The drug had a PDUFA date of February 28.
The committee’s decision was based on results from the phase 3 GALACTIC-HF trial, which enrolled 8,256 patients with HFrEF who were at risk of hospitalization and death, despite standard-of-care therapy.
As previously reported by this news organization, omecamtiv mecarbil produced a positive result for the study’s primary endpoint, with a 2.1% absolute reduction in the combined rate of cardiovascular (CV) death, first HF hospitalization, or first urgent visit for HF, compared with placebo during a median follow-up of about 22 months.
This represented an 8% relative risk reduction and broke down as a 0.6% absolute drop in CV death, compared with placebo, a 0.7% cut in HF hospitalization, and a 0.8% drop in urgent outpatient HF visits.
In a complete response letter, the FDA said GALACTIC-HF is “not sufficiently persuasive to establish substantial evidence of effectiveness for reducing the risk of heart failure events and cardiovascular death” in adults with HFrEF, Cytokinetics shared in a news release.
Further, the FDA said results from an additional clinical trial of omecamtiv mecarbil are required to establish substantial evidence of effectiveness for the treatment of HFrEF, with benefits that outweigh the risks, Cytokinetics said.
The company said it will request a meeting with the FDA to gain a better understanding of what may be required to support potential approval of omecamtiv mecarbil. However, the company also said it has “no plans” to conduct an additional clinical trial of omecamtiv mecarbil.
Instead, the company said its focus remains on the development of aficamten, the next-in-class cardiac myosin inhibitor, currently the subject of SEQUOIA-HCM, a phase 3 clinical trial in patients with obstructive hypertrophic cardiomyopathy.
A version of this article first appeared on Medscape.com.
FDA warns of potential problems with Abbott Trifecta valves
There is a potential risk of early structural valve deterioration with the Abbott Trifecta valve and Trifecta valve with glide technology (Trifecta GT), the U.S. Food and Drug Administration says in a letter to health care professionals posted on its website.
Evidence in the literature suggests a higher cumulative incidence of early structural valve deterioration (SVD) and a lower freedom from reintervention due to SVD with the Trifecta valves, compared with other commercially available bovine pericardial valves, the FDA says.
The Trifecta and Trifecta GT valves are heart valve replacement devices intended to treat diseased, damaged, or malfunctioning native or prosthetic aortic heart valves, the letter notes. The first-generation Trifecta valve was approved in 2011 but is no longer marketed in the United States. The Trifecta GT valve was approved in 2016.
Medical device reports (MDRs) received by the FDA describe early SVD with Trifecta valves, with a peak time to SVD of 3 to 4 years post-implant. “Reported outcomes include surgical valve explant/replacement, transcatheter valve-in-valve intervention, and in some cases death,” the FDA notes.
In a letter to customers, Abbott says a “complaint analysis has shown that most cases of early SVD which occur within 5 years post-implant are characterized as a non-calcific leaflet tear, while most cases of late SVD which occur beyond 5 years post-implant are characterized as a fibrous-calcific SVD.”
The FDA recommends that health care providers take the following actions:
- Be aware of the potential risk of early SVD with Trifecta valves, and current patient management considerations, as communicated by Abbott.
- Discuss the risks and benefits of all available aortic valve treatment options with patients and caregivers as part of shared clinical decision-making prior to surgery.
- Read and carefully follow the Instructions for Use when implanting a Trifecta GT valve.
- Monitor patients who have undergone implantation with Trifecta valves for signs and symptoms of potential SVD.
- Instruct patients to seek medical attention with new onset of symptoms such as shortness of breath or fatigue.
- Ensure lifelong follow-up visits, conducted at least yearly, including transthoracic echocardiogram assessment of the valve beginning 1-year post-implant.
The FDA is working with Abbott to further evaluate the issue and develop additional patient management strategies, if needed. The FDA says it will continue to monitor the literature and reports of adverse events related to the issue.
Clinicians are encouraged to report any adverse events or quality problems with the Trifecta valves to their local Abbott representative or the customer service department at 1-800-544-1664.
Health care professionals can also report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
There is a potential risk of early structural valve deterioration with the Abbott Trifecta valve and Trifecta valve with glide technology (Trifecta GT), the U.S. Food and Drug Administration says in a letter to health care professionals posted on its website.
Evidence in the literature suggests a higher cumulative incidence of early structural valve deterioration (SVD) and a lower freedom from reintervention due to SVD with the Trifecta valves, compared with other commercially available bovine pericardial valves, the FDA says.
The Trifecta and Trifecta GT valves are heart valve replacement devices intended to treat diseased, damaged, or malfunctioning native or prosthetic aortic heart valves, the letter notes. The first-generation Trifecta valve was approved in 2011 but is no longer marketed in the United States. The Trifecta GT valve was approved in 2016.
Medical device reports (MDRs) received by the FDA describe early SVD with Trifecta valves, with a peak time to SVD of 3 to 4 years post-implant. “Reported outcomes include surgical valve explant/replacement, transcatheter valve-in-valve intervention, and in some cases death,” the FDA notes.
In a letter to customers, Abbott says a “complaint analysis has shown that most cases of early SVD which occur within 5 years post-implant are characterized as a non-calcific leaflet tear, while most cases of late SVD which occur beyond 5 years post-implant are characterized as a fibrous-calcific SVD.”
The FDA recommends that health care providers take the following actions:
- Be aware of the potential risk of early SVD with Trifecta valves, and current patient management considerations, as communicated by Abbott.
- Discuss the risks and benefits of all available aortic valve treatment options with patients and caregivers as part of shared clinical decision-making prior to surgery.
- Read and carefully follow the Instructions for Use when implanting a Trifecta GT valve.
- Monitor patients who have undergone implantation with Trifecta valves for signs and symptoms of potential SVD.
- Instruct patients to seek medical attention with new onset of symptoms such as shortness of breath or fatigue.
- Ensure lifelong follow-up visits, conducted at least yearly, including transthoracic echocardiogram assessment of the valve beginning 1-year post-implant.
The FDA is working with Abbott to further evaluate the issue and develop additional patient management strategies, if needed. The FDA says it will continue to monitor the literature and reports of adverse events related to the issue.
Clinicians are encouraged to report any adverse events or quality problems with the Trifecta valves to their local Abbott representative or the customer service department at 1-800-544-1664.
Health care professionals can also report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
There is a potential risk of early structural valve deterioration with the Abbott Trifecta valve and Trifecta valve with glide technology (Trifecta GT), the U.S. Food and Drug Administration says in a letter to health care professionals posted on its website.
Evidence in the literature suggests a higher cumulative incidence of early structural valve deterioration (SVD) and a lower freedom from reintervention due to SVD with the Trifecta valves, compared with other commercially available bovine pericardial valves, the FDA says.
The Trifecta and Trifecta GT valves are heart valve replacement devices intended to treat diseased, damaged, or malfunctioning native or prosthetic aortic heart valves, the letter notes. The first-generation Trifecta valve was approved in 2011 but is no longer marketed in the United States. The Trifecta GT valve was approved in 2016.
Medical device reports (MDRs) received by the FDA describe early SVD with Trifecta valves, with a peak time to SVD of 3 to 4 years post-implant. “Reported outcomes include surgical valve explant/replacement, transcatheter valve-in-valve intervention, and in some cases death,” the FDA notes.
In a letter to customers, Abbott says a “complaint analysis has shown that most cases of early SVD which occur within 5 years post-implant are characterized as a non-calcific leaflet tear, while most cases of late SVD which occur beyond 5 years post-implant are characterized as a fibrous-calcific SVD.”
The FDA recommends that health care providers take the following actions:
- Be aware of the potential risk of early SVD with Trifecta valves, and current patient management considerations, as communicated by Abbott.
- Discuss the risks and benefits of all available aortic valve treatment options with patients and caregivers as part of shared clinical decision-making prior to surgery.
- Read and carefully follow the Instructions for Use when implanting a Trifecta GT valve.
- Monitor patients who have undergone implantation with Trifecta valves for signs and symptoms of potential SVD.
- Instruct patients to seek medical attention with new onset of symptoms such as shortness of breath or fatigue.
- Ensure lifelong follow-up visits, conducted at least yearly, including transthoracic echocardiogram assessment of the valve beginning 1-year post-implant.
The FDA is working with Abbott to further evaluate the issue and develop additional patient management strategies, if needed. The FDA says it will continue to monitor the literature and reports of adverse events related to the issue.
Clinicians are encouraged to report any adverse events or quality problems with the Trifecta valves to their local Abbott representative or the customer service department at 1-800-544-1664.
Health care professionals can also report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.