User login
Cumulative blood pressure load: A better predictor of CV events?
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Should patients stand for office BP readings?
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2022
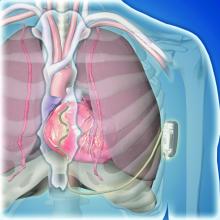
Extravascular ICD surpasses goals in pivotal trial
BARCELONA – A novel “extravascular” implantable cardioverter defibrillator (ICD) that uses substernally placed electrodes surpassed its prespecified efficacy and safety targets in the device’s pivotal trial with 299 patients who received an implant.
The results showed that the extravascular ICD “provides antitachycardia pacing and low energy defibrillation while avoiding the vascular space” for lead placement, Ian Crozier, MD, said at the annual congress of the European Society of Cardiology.
“The results are fantastic; they exceeded our expectations,” said Dr. Crozier in an interview, adding that he expects the new device to receive marketing approval from regulatory agencies based on the findings. “This will be the next generation of ICD going forward,” predicted Dr. Crozier, an electrophysiologist cardiologist at Christchurch (New Zealand) Hospital.
Moving beyond transvenous and subcutaneous ICDs
Traditional ICDs use transvenous leads, which can cause vascular injury, are prone to lead fracture over time, and can produce serious infections as well as other potential complications. The U.S. Food and Drug Administration first approved an alternative-design, subcutaneous ICD in 2012 that avoids the need for transvenous leads and the risks they pose. But subcutaneous ICDs have their own limitations: an inability to provide antitachycardia pacing or chronic pacing; a limited ability to provide bradycardia pacing; and an increased device size with shorter battery life, because of the high shock power needed for effective performance. These drawbacks have collectively hindered uptake, Dr. Crozier said.
This led to development of the extravascular ICD – 10 years in the making – which uses substernally placed leads that allow antitachycardia pacing and backup pacing in a device with the size of and the anticipated battery longevity of a transvenous ICD device, noted Dr. Crozier.
A 98.7% rate of arrhythmia termination at implant
The pivotal trial’s primary efficacy endpoint was successful defibrillation based on terminating an induced, sustained, shockable ventricular arrhythmia at the time of implantation. The rate was 98.7%, compared with a prespecified target of 88%. All patients had a class I or IIa indication for an ICD.
The primary safety endpoint was freedom from major system- or procedure-related complications at 6 months, which occurred at a rate of 92.6%, compared with the study’s prespecified target rate of 79%. Both targets were derived from the historical rates of ICDs with transvenous leads.
Simultaneously with Dr. Crozier’s report at the congress, the results also appeared online in the New England Journal of Medicine.
Although the pivotal study met both prespecified endpoints, the evidence has limitations that make it likely that regulatory bodies will seek additional data, commented Fred Kusumoto, MD, director of heart rhythm services for the Mayo Clinic in Jacksonville, Fla.
Short follow-up; questions remain
“Follow-up was relatively short, less than a year,” and “questions remain” about the extravascular ICD’s performance, Dr. Kusumoto said in an interview. “Inappropriate shocks occurred in nearly 10% of patients after 11 month follow-up,” he noted, and also cited the 29 patients who needed revisions including two cases with lead fractures.
“The extravascular lead strategy has an advantage over transvenous systems because of the lower risk for extraction or explant,” and it also provides the antitachycardia pacing that’s not available with subcutaneous ICDs, he granted. But in the new study, antitachycardia termination was delivered to only 10 patients and had “reasonable” effectiveness by resolving 70% of these episodes. “Wide adoption by clinicians will depend on results from larger studies with longer follow-up,” Dr. Kusumoto maintained. He also wanted to see confirmation of the ease of lead removal after longer periods of implantation.
Implantation ‘is not difficult’
The trial ran at 46 sites in 17 countries during September 2019 to October 2021. It enrolled patients with a class I or IIa indication for an ICD, excluding patients with a prior sternotomy or need for chronic pacing, and those unable to undergo defibrillation testing.
Clinicians attempted an implantation in 316 patients and had successful placement in 299 (314 had successful placement of their substernal leads), with 292 having a functional device after 6 months, and 284 completing their planned 6-month follow-up. The median procedure time was 66 minutes, including the time for defibrillation testing.
All of the cardiologists who did the implants had received a full day of training prior to performing the procedure. “This is not a difficult procedure, but it is not a region [the substernal space] that cardiologists are familiar working in,” noted Dr. Crozier, explaining the rationale behind a policy of required implantation training.
Twenty-five adverse events occurred in 23 patients. Eighteen of these events required a system revision, including nine lead dislodgments and five infections. The seven adverse events that did not require a revision included three wound-related episodes and three hospitalizations for inappropriate shock. No patients died, nor were there any cardiac injuries as result of the implant.
During average follow-up of 10.6 months, the implanted devices delivered antitachycardia pacing to 10 patients, successfully terminating 32 of 46 episodes (70%), a rate that Dr. Crozier called “very good, and very comparable to transvenous devices.” The devices also delivered 18 appropriate shocks that successfully converted all 18 episodes.
A 10% rate of inappropriate shocks
However, 29 patients (10% of the study cohort) received inappropriate shocks in 81 episodes, with a total of 118 inappropriate shocks delivered, including 34 episodes (42%) triggered by oversensing of a P wave.
“We fully acknowledge that the inappropriate shock rate is higher than what’s seen with transvenous ICDs, but the rate is comparable to what was seen in the early trials with subcutaneous ICDs,” said Dr. Crozier. “We have a number of strategies to reduce the inappropriate shock rate to what we’d expect with conventional devices,” such as making sure that P waves are not detected by the device at the time of implantation, using new algorithms to mitigate P wave sensing, and other programming changes, he added.
Two patients had lead fractures that Dr. Crozier attributed to atypical lead locations and that are likely avoidable in the future. He expressed optimism that the extravascular ICD will avoid the high lead fracture rate over time that remains a problem for ICDs with transvenous leads.
The study also followed a subgroup of 36 patients who underwent a prespecified protocol of chronic defibrillation testing that was successful in all 36.
Dr. Crozier conceded that the extravascular ICD cannot currently deliver chronic pacing, but he expressed optimism that this capability will be available in the future.
“This innovative [extravascular] ICD system would be particularly beneficial for patients with ventricular arrhythmias that can be reliably pace terminated and avoid a transvenous endocardial lead, but more information is required,” concluded Dr. Kusumoto.
The study was sponsored by Medtronic, the company that is developing the extravascular ICD. Dr. Crozier is a consultant to and has received research funding from Medtronic. Dr. Kusumoto had no disclosures.
BARCELONA – A novel “extravascular” implantable cardioverter defibrillator (ICD) that uses substernally placed electrodes surpassed its prespecified efficacy and safety targets in the device’s pivotal trial with 299 patients who received an implant.
The results showed that the extravascular ICD “provides antitachycardia pacing and low energy defibrillation while avoiding the vascular space” for lead placement, Ian Crozier, MD, said at the annual congress of the European Society of Cardiology.
“The results are fantastic; they exceeded our expectations,” said Dr. Crozier in an interview, adding that he expects the new device to receive marketing approval from regulatory agencies based on the findings. “This will be the next generation of ICD going forward,” predicted Dr. Crozier, an electrophysiologist cardiologist at Christchurch (New Zealand) Hospital.
Moving beyond transvenous and subcutaneous ICDs
Traditional ICDs use transvenous leads, which can cause vascular injury, are prone to lead fracture over time, and can produce serious infections as well as other potential complications. The U.S. Food and Drug Administration first approved an alternative-design, subcutaneous ICD in 2012 that avoids the need for transvenous leads and the risks they pose. But subcutaneous ICDs have their own limitations: an inability to provide antitachycardia pacing or chronic pacing; a limited ability to provide bradycardia pacing; and an increased device size with shorter battery life, because of the high shock power needed for effective performance. These drawbacks have collectively hindered uptake, Dr. Crozier said.
This led to development of the extravascular ICD – 10 years in the making – which uses substernally placed leads that allow antitachycardia pacing and backup pacing in a device with the size of and the anticipated battery longevity of a transvenous ICD device, noted Dr. Crozier.
A 98.7% rate of arrhythmia termination at implant
The pivotal trial’s primary efficacy endpoint was successful defibrillation based on terminating an induced, sustained, shockable ventricular arrhythmia at the time of implantation. The rate was 98.7%, compared with a prespecified target of 88%. All patients had a class I or IIa indication for an ICD.
The primary safety endpoint was freedom from major system- or procedure-related complications at 6 months, which occurred at a rate of 92.6%, compared with the study’s prespecified target rate of 79%. Both targets were derived from the historical rates of ICDs with transvenous leads.
Simultaneously with Dr. Crozier’s report at the congress, the results also appeared online in the New England Journal of Medicine.
Although the pivotal study met both prespecified endpoints, the evidence has limitations that make it likely that regulatory bodies will seek additional data, commented Fred Kusumoto, MD, director of heart rhythm services for the Mayo Clinic in Jacksonville, Fla.
Short follow-up; questions remain
“Follow-up was relatively short, less than a year,” and “questions remain” about the extravascular ICD’s performance, Dr. Kusumoto said in an interview. “Inappropriate shocks occurred in nearly 10% of patients after 11 month follow-up,” he noted, and also cited the 29 patients who needed revisions including two cases with lead fractures.
“The extravascular lead strategy has an advantage over transvenous systems because of the lower risk for extraction or explant,” and it also provides the antitachycardia pacing that’s not available with subcutaneous ICDs, he granted. But in the new study, antitachycardia termination was delivered to only 10 patients and had “reasonable” effectiveness by resolving 70% of these episodes. “Wide adoption by clinicians will depend on results from larger studies with longer follow-up,” Dr. Kusumoto maintained. He also wanted to see confirmation of the ease of lead removal after longer periods of implantation.
Implantation ‘is not difficult’
The trial ran at 46 sites in 17 countries during September 2019 to October 2021. It enrolled patients with a class I or IIa indication for an ICD, excluding patients with a prior sternotomy or need for chronic pacing, and those unable to undergo defibrillation testing.
Clinicians attempted an implantation in 316 patients and had successful placement in 299 (314 had successful placement of their substernal leads), with 292 having a functional device after 6 months, and 284 completing their planned 6-month follow-up. The median procedure time was 66 minutes, including the time for defibrillation testing.
All of the cardiologists who did the implants had received a full day of training prior to performing the procedure. “This is not a difficult procedure, but it is not a region [the substernal space] that cardiologists are familiar working in,” noted Dr. Crozier, explaining the rationale behind a policy of required implantation training.
Twenty-five adverse events occurred in 23 patients. Eighteen of these events required a system revision, including nine lead dislodgments and five infections. The seven adverse events that did not require a revision included three wound-related episodes and three hospitalizations for inappropriate shock. No patients died, nor were there any cardiac injuries as result of the implant.
During average follow-up of 10.6 months, the implanted devices delivered antitachycardia pacing to 10 patients, successfully terminating 32 of 46 episodes (70%), a rate that Dr. Crozier called “very good, and very comparable to transvenous devices.” The devices also delivered 18 appropriate shocks that successfully converted all 18 episodes.
A 10% rate of inappropriate shocks
However, 29 patients (10% of the study cohort) received inappropriate shocks in 81 episodes, with a total of 118 inappropriate shocks delivered, including 34 episodes (42%) triggered by oversensing of a P wave.
“We fully acknowledge that the inappropriate shock rate is higher than what’s seen with transvenous ICDs, but the rate is comparable to what was seen in the early trials with subcutaneous ICDs,” said Dr. Crozier. “We have a number of strategies to reduce the inappropriate shock rate to what we’d expect with conventional devices,” such as making sure that P waves are not detected by the device at the time of implantation, using new algorithms to mitigate P wave sensing, and other programming changes, he added.
Two patients had lead fractures that Dr. Crozier attributed to atypical lead locations and that are likely avoidable in the future. He expressed optimism that the extravascular ICD will avoid the high lead fracture rate over time that remains a problem for ICDs with transvenous leads.
The study also followed a subgroup of 36 patients who underwent a prespecified protocol of chronic defibrillation testing that was successful in all 36.
Dr. Crozier conceded that the extravascular ICD cannot currently deliver chronic pacing, but he expressed optimism that this capability will be available in the future.
“This innovative [extravascular] ICD system would be particularly beneficial for patients with ventricular arrhythmias that can be reliably pace terminated and avoid a transvenous endocardial lead, but more information is required,” concluded Dr. Kusumoto.
The study was sponsored by Medtronic, the company that is developing the extravascular ICD. Dr. Crozier is a consultant to and has received research funding from Medtronic. Dr. Kusumoto had no disclosures.
BARCELONA – A novel “extravascular” implantable cardioverter defibrillator (ICD) that uses substernally placed electrodes surpassed its prespecified efficacy and safety targets in the device’s pivotal trial with 299 patients who received an implant.
The results showed that the extravascular ICD “provides antitachycardia pacing and low energy defibrillation while avoiding the vascular space” for lead placement, Ian Crozier, MD, said at the annual congress of the European Society of Cardiology.
“The results are fantastic; they exceeded our expectations,” said Dr. Crozier in an interview, adding that he expects the new device to receive marketing approval from regulatory agencies based on the findings. “This will be the next generation of ICD going forward,” predicted Dr. Crozier, an electrophysiologist cardiologist at Christchurch (New Zealand) Hospital.
Moving beyond transvenous and subcutaneous ICDs
Traditional ICDs use transvenous leads, which can cause vascular injury, are prone to lead fracture over time, and can produce serious infections as well as other potential complications. The U.S. Food and Drug Administration first approved an alternative-design, subcutaneous ICD in 2012 that avoids the need for transvenous leads and the risks they pose. But subcutaneous ICDs have their own limitations: an inability to provide antitachycardia pacing or chronic pacing; a limited ability to provide bradycardia pacing; and an increased device size with shorter battery life, because of the high shock power needed for effective performance. These drawbacks have collectively hindered uptake, Dr. Crozier said.
This led to development of the extravascular ICD – 10 years in the making – which uses substernally placed leads that allow antitachycardia pacing and backup pacing in a device with the size of and the anticipated battery longevity of a transvenous ICD device, noted Dr. Crozier.
A 98.7% rate of arrhythmia termination at implant
The pivotal trial’s primary efficacy endpoint was successful defibrillation based on terminating an induced, sustained, shockable ventricular arrhythmia at the time of implantation. The rate was 98.7%, compared with a prespecified target of 88%. All patients had a class I or IIa indication for an ICD.
The primary safety endpoint was freedom from major system- or procedure-related complications at 6 months, which occurred at a rate of 92.6%, compared with the study’s prespecified target rate of 79%. Both targets were derived from the historical rates of ICDs with transvenous leads.
Simultaneously with Dr. Crozier’s report at the congress, the results also appeared online in the New England Journal of Medicine.
Although the pivotal study met both prespecified endpoints, the evidence has limitations that make it likely that regulatory bodies will seek additional data, commented Fred Kusumoto, MD, director of heart rhythm services for the Mayo Clinic in Jacksonville, Fla.
Short follow-up; questions remain
“Follow-up was relatively short, less than a year,” and “questions remain” about the extravascular ICD’s performance, Dr. Kusumoto said in an interview. “Inappropriate shocks occurred in nearly 10% of patients after 11 month follow-up,” he noted, and also cited the 29 patients who needed revisions including two cases with lead fractures.
“The extravascular lead strategy has an advantage over transvenous systems because of the lower risk for extraction or explant,” and it also provides the antitachycardia pacing that’s not available with subcutaneous ICDs, he granted. But in the new study, antitachycardia termination was delivered to only 10 patients and had “reasonable” effectiveness by resolving 70% of these episodes. “Wide adoption by clinicians will depend on results from larger studies with longer follow-up,” Dr. Kusumoto maintained. He also wanted to see confirmation of the ease of lead removal after longer periods of implantation.
Implantation ‘is not difficult’
The trial ran at 46 sites in 17 countries during September 2019 to October 2021. It enrolled patients with a class I or IIa indication for an ICD, excluding patients with a prior sternotomy or need for chronic pacing, and those unable to undergo defibrillation testing.
Clinicians attempted an implantation in 316 patients and had successful placement in 299 (314 had successful placement of their substernal leads), with 292 having a functional device after 6 months, and 284 completing their planned 6-month follow-up. The median procedure time was 66 minutes, including the time for defibrillation testing.
All of the cardiologists who did the implants had received a full day of training prior to performing the procedure. “This is not a difficult procedure, but it is not a region [the substernal space] that cardiologists are familiar working in,” noted Dr. Crozier, explaining the rationale behind a policy of required implantation training.
Twenty-five adverse events occurred in 23 patients. Eighteen of these events required a system revision, including nine lead dislodgments and five infections. The seven adverse events that did not require a revision included three wound-related episodes and three hospitalizations for inappropriate shock. No patients died, nor were there any cardiac injuries as result of the implant.
During average follow-up of 10.6 months, the implanted devices delivered antitachycardia pacing to 10 patients, successfully terminating 32 of 46 episodes (70%), a rate that Dr. Crozier called “very good, and very comparable to transvenous devices.” The devices also delivered 18 appropriate shocks that successfully converted all 18 episodes.
A 10% rate of inappropriate shocks
However, 29 patients (10% of the study cohort) received inappropriate shocks in 81 episodes, with a total of 118 inappropriate shocks delivered, including 34 episodes (42%) triggered by oversensing of a P wave.
“We fully acknowledge that the inappropriate shock rate is higher than what’s seen with transvenous ICDs, but the rate is comparable to what was seen in the early trials with subcutaneous ICDs,” said Dr. Crozier. “We have a number of strategies to reduce the inappropriate shock rate to what we’d expect with conventional devices,” such as making sure that P waves are not detected by the device at the time of implantation, using new algorithms to mitigate P wave sensing, and other programming changes, he added.
Two patients had lead fractures that Dr. Crozier attributed to atypical lead locations and that are likely avoidable in the future. He expressed optimism that the extravascular ICD will avoid the high lead fracture rate over time that remains a problem for ICDs with transvenous leads.
The study also followed a subgroup of 36 patients who underwent a prespecified protocol of chronic defibrillation testing that was successful in all 36.
Dr. Crozier conceded that the extravascular ICD cannot currently deliver chronic pacing, but he expressed optimism that this capability will be available in the future.
“This innovative [extravascular] ICD system would be particularly beneficial for patients with ventricular arrhythmias that can be reliably pace terminated and avoid a transvenous endocardial lead, but more information is required,” concluded Dr. Kusumoto.
The study was sponsored by Medtronic, the company that is developing the extravascular ICD. Dr. Crozier is a consultant to and has received research funding from Medtronic. Dr. Kusumoto had no disclosures.
AT ESC CONGRESS 2022
PARADISE-MI results obscured as post hoc analysis finds flaws
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
FROM ESC CONGRESS 2022
Lack of exercise linked to small heart, HFpEF
Chronic lack of exercise – dubbed “exercise deficiency” – is associated with cardiac atrophy, reduced cardiac output and chamber size, and diminished cardiorespiratory fitness (CRF) in a subgroup of patients with heart failure with preserved ejection fraction (HFpEF), researchers say.
Increasing the physical activity levels of these sedentary individuals could be an effective preventive strategy, particularly for those who are younger and middle-aged, they suggest.
Thinking of HFpEF as an exercise deficiency syndrome leading to a small heart “flies in the face of decades of cardiovascular teaching, because traditionally, we’ve thought of heart failure as the big floppy heart,” Andre La Gerche, MBBS, PhD, of the Baker Heart and Diabetes Institute, Melbourne, told this news organization.
“While it is true that some people with HFpEF have thick, stiff hearts, we propose that another subset has a normal heart, except it’s small because it’s been underexercised,” he said.
The article, published online as part of a Focus Seminar series in the Journal of the American College of Cardiology, has “gone viral on social media,” Jason C. Kovacic, MBBS, PhD, of the Victor Chang Cardiac Research Institute, Darlinghurst, Australia, told this news organization.
Dr. Kovacic is a JACC section editor and the coordinating and senior author of the series, which covers other issues surrounding physical activity, both in athletes and the general public.
‘Coin-dropping moment’
To support their hypothesis that HFpEF is an exercise deficiency in certain patients, Dr. La Gerche and colleagues conducted a literature review that highlights the following points:
- There is a strong association between physical activity and both CRF and heart function.
- Exercise deficiency is a major risk factor for HFpEF in a subset of patients.
- Increasing physical activity is associated with greater cardiac mass, stroke volumes, cardiac output, and peak oxygen consumption.
- Physical inactivity leads to loss of heart muscle, reduced output and chamber size, and less ability to improve cardiac performance with exercise.
- Aging results in a smaller, stiffer heart; however, this effect is mitigated by regular exercise.
- Individuals who are sedentary throughout life cannot attenuate age-related reductions in heart size and have increasing chamber stiffness.
“When we explain it, it’s like a coin-dropping moment, because it’s actually a really simple concept,” Dr. La Gerche said. “A small heart has a small stroke volume. A patient with a small heart with a maximal stroke volume of 60 mL can generate a cardiac output of 9 L/min at a heart rate of 150 beats/min during exercise – an output that just isn’t enough. It’s like trying to drive a truck with a 50cc motorbike engine.”
“Plus,” Dr. La Gerche added, “exercise deficiency also sets the stage for comorbidities such as obesity, diabetes, and high blood pressure, all of which can ultimately lead to HFpEF.”
Considering HFpEF as an exercise deficiency syndrome has two clinical implications, Dr. La Gerche said. “First, it helps us understand the condition and diagnose more cases. For example, I think practitioners will start to recognize that breathlessness in some of their patients is associated with a small heart.”
“Second,” he said, “if it’s an exercise deficiency syndrome, the treatment is exercise. For most people, that means exercising regularly before the age of 60 to prevent HFpEF, because studies have found that after the age of 60, the heart is a bit fixed and harder to remodel. That doesn’t mean you shouldn’t try after 60 or that you won’t get benefit. But the real sweet spot is in middle age and younger.”
The bigger picture
The JACC Focus Seminar series starts with an article that underscores the benefits of regular physical activity. “The key is getting our patients to meet the guidelines: 150 to 300 minutes of moderate intensity exercise per week, or 75 to 250 minutes of vigorous activity per week,” Dr. Kovacic emphasized.
“Yes, we can give a statin to lower cholesterol. Yes, we can give a blood pressure medication to lower blood pressure. But when you prescribe exercise, you impact patients’ blood pressure, their cholesterol, their weight, their sense of well-being,” he said. “It cuts across so many different aspects of people’s lives that it’s important to underscore the value of exercise to everybody.”
That includes physicians, he affirmed. “It behooves all physicians to be leading by example. I would encourage those who are overweight or aren’t exercising as much as they should be to make the time to be healthy and to exercise. If you don’t, then bad health will force you to make the time to deal with bad health issues.”
Other articles in the series deal with the athlete’s heart. Christopher Semsarian, MBBS, PhD, MPH, University of Sydney, and colleagues discuss emerging data on hypertrophic cardiomyopathy and other genetic cardiovascular diseases, with the conclusion that it is probably okay for more athletes with these conditions to participate in recreational and competitive sports than was previously thought – another paradigm shift, according to Dr. Kovacic.
The final article addresses some of the challenges and controversies related to the athlete’s heart, including whether extreme exercise is associated with vulnerability to atrial fibrillation and other arrhythmias, and the impact of gender on the cardiac response to exercise, which can’t be determined now because of a paucity of data on women in sports.
Overall, Dr. Kovacic said, the series makes for “compelling” reading that should encourage readers to embark on their own studies to add to the data and support exercise prescription across the board.
No commercial funding or relevant conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Chronic lack of exercise – dubbed “exercise deficiency” – is associated with cardiac atrophy, reduced cardiac output and chamber size, and diminished cardiorespiratory fitness (CRF) in a subgroup of patients with heart failure with preserved ejection fraction (HFpEF), researchers say.
Increasing the physical activity levels of these sedentary individuals could be an effective preventive strategy, particularly for those who are younger and middle-aged, they suggest.
Thinking of HFpEF as an exercise deficiency syndrome leading to a small heart “flies in the face of decades of cardiovascular teaching, because traditionally, we’ve thought of heart failure as the big floppy heart,” Andre La Gerche, MBBS, PhD, of the Baker Heart and Diabetes Institute, Melbourne, told this news organization.
“While it is true that some people with HFpEF have thick, stiff hearts, we propose that another subset has a normal heart, except it’s small because it’s been underexercised,” he said.
The article, published online as part of a Focus Seminar series in the Journal of the American College of Cardiology, has “gone viral on social media,” Jason C. Kovacic, MBBS, PhD, of the Victor Chang Cardiac Research Institute, Darlinghurst, Australia, told this news organization.
Dr. Kovacic is a JACC section editor and the coordinating and senior author of the series, which covers other issues surrounding physical activity, both in athletes and the general public.
‘Coin-dropping moment’
To support their hypothesis that HFpEF is an exercise deficiency in certain patients, Dr. La Gerche and colleagues conducted a literature review that highlights the following points:
- There is a strong association between physical activity and both CRF and heart function.
- Exercise deficiency is a major risk factor for HFpEF in a subset of patients.
- Increasing physical activity is associated with greater cardiac mass, stroke volumes, cardiac output, and peak oxygen consumption.
- Physical inactivity leads to loss of heart muscle, reduced output and chamber size, and less ability to improve cardiac performance with exercise.
- Aging results in a smaller, stiffer heart; however, this effect is mitigated by regular exercise.
- Individuals who are sedentary throughout life cannot attenuate age-related reductions in heart size and have increasing chamber stiffness.
“When we explain it, it’s like a coin-dropping moment, because it’s actually a really simple concept,” Dr. La Gerche said. “A small heart has a small stroke volume. A patient with a small heart with a maximal stroke volume of 60 mL can generate a cardiac output of 9 L/min at a heart rate of 150 beats/min during exercise – an output that just isn’t enough. It’s like trying to drive a truck with a 50cc motorbike engine.”
“Plus,” Dr. La Gerche added, “exercise deficiency also sets the stage for comorbidities such as obesity, diabetes, and high blood pressure, all of which can ultimately lead to HFpEF.”
Considering HFpEF as an exercise deficiency syndrome has two clinical implications, Dr. La Gerche said. “First, it helps us understand the condition and diagnose more cases. For example, I think practitioners will start to recognize that breathlessness in some of their patients is associated with a small heart.”
“Second,” he said, “if it’s an exercise deficiency syndrome, the treatment is exercise. For most people, that means exercising regularly before the age of 60 to prevent HFpEF, because studies have found that after the age of 60, the heart is a bit fixed and harder to remodel. That doesn’t mean you shouldn’t try after 60 or that you won’t get benefit. But the real sweet spot is in middle age and younger.”
The bigger picture
The JACC Focus Seminar series starts with an article that underscores the benefits of regular physical activity. “The key is getting our patients to meet the guidelines: 150 to 300 minutes of moderate intensity exercise per week, or 75 to 250 minutes of vigorous activity per week,” Dr. Kovacic emphasized.
“Yes, we can give a statin to lower cholesterol. Yes, we can give a blood pressure medication to lower blood pressure. But when you prescribe exercise, you impact patients’ blood pressure, their cholesterol, their weight, their sense of well-being,” he said. “It cuts across so many different aspects of people’s lives that it’s important to underscore the value of exercise to everybody.”
That includes physicians, he affirmed. “It behooves all physicians to be leading by example. I would encourage those who are overweight or aren’t exercising as much as they should be to make the time to be healthy and to exercise. If you don’t, then bad health will force you to make the time to deal with bad health issues.”
Other articles in the series deal with the athlete’s heart. Christopher Semsarian, MBBS, PhD, MPH, University of Sydney, and colleagues discuss emerging data on hypertrophic cardiomyopathy and other genetic cardiovascular diseases, with the conclusion that it is probably okay for more athletes with these conditions to participate in recreational and competitive sports than was previously thought – another paradigm shift, according to Dr. Kovacic.
The final article addresses some of the challenges and controversies related to the athlete’s heart, including whether extreme exercise is associated with vulnerability to atrial fibrillation and other arrhythmias, and the impact of gender on the cardiac response to exercise, which can’t be determined now because of a paucity of data on women in sports.
Overall, Dr. Kovacic said, the series makes for “compelling” reading that should encourage readers to embark on their own studies to add to the data and support exercise prescription across the board.
No commercial funding or relevant conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Chronic lack of exercise – dubbed “exercise deficiency” – is associated with cardiac atrophy, reduced cardiac output and chamber size, and diminished cardiorespiratory fitness (CRF) in a subgroup of patients with heart failure with preserved ejection fraction (HFpEF), researchers say.
Increasing the physical activity levels of these sedentary individuals could be an effective preventive strategy, particularly for those who are younger and middle-aged, they suggest.
Thinking of HFpEF as an exercise deficiency syndrome leading to a small heart “flies in the face of decades of cardiovascular teaching, because traditionally, we’ve thought of heart failure as the big floppy heart,” Andre La Gerche, MBBS, PhD, of the Baker Heart and Diabetes Institute, Melbourne, told this news organization.
“While it is true that some people with HFpEF have thick, stiff hearts, we propose that another subset has a normal heart, except it’s small because it’s been underexercised,” he said.
The article, published online as part of a Focus Seminar series in the Journal of the American College of Cardiology, has “gone viral on social media,” Jason C. Kovacic, MBBS, PhD, of the Victor Chang Cardiac Research Institute, Darlinghurst, Australia, told this news organization.
Dr. Kovacic is a JACC section editor and the coordinating and senior author of the series, which covers other issues surrounding physical activity, both in athletes and the general public.
‘Coin-dropping moment’
To support their hypothesis that HFpEF is an exercise deficiency in certain patients, Dr. La Gerche and colleagues conducted a literature review that highlights the following points:
- There is a strong association between physical activity and both CRF and heart function.
- Exercise deficiency is a major risk factor for HFpEF in a subset of patients.
- Increasing physical activity is associated with greater cardiac mass, stroke volumes, cardiac output, and peak oxygen consumption.
- Physical inactivity leads to loss of heart muscle, reduced output and chamber size, and less ability to improve cardiac performance with exercise.
- Aging results in a smaller, stiffer heart; however, this effect is mitigated by regular exercise.
- Individuals who are sedentary throughout life cannot attenuate age-related reductions in heart size and have increasing chamber stiffness.
“When we explain it, it’s like a coin-dropping moment, because it’s actually a really simple concept,” Dr. La Gerche said. “A small heart has a small stroke volume. A patient with a small heart with a maximal stroke volume of 60 mL can generate a cardiac output of 9 L/min at a heart rate of 150 beats/min during exercise – an output that just isn’t enough. It’s like trying to drive a truck with a 50cc motorbike engine.”
“Plus,” Dr. La Gerche added, “exercise deficiency also sets the stage for comorbidities such as obesity, diabetes, and high blood pressure, all of which can ultimately lead to HFpEF.”
Considering HFpEF as an exercise deficiency syndrome has two clinical implications, Dr. La Gerche said. “First, it helps us understand the condition and diagnose more cases. For example, I think practitioners will start to recognize that breathlessness in some of their patients is associated with a small heart.”
“Second,” he said, “if it’s an exercise deficiency syndrome, the treatment is exercise. For most people, that means exercising regularly before the age of 60 to prevent HFpEF, because studies have found that after the age of 60, the heart is a bit fixed and harder to remodel. That doesn’t mean you shouldn’t try after 60 or that you won’t get benefit. But the real sweet spot is in middle age and younger.”
The bigger picture
The JACC Focus Seminar series starts with an article that underscores the benefits of regular physical activity. “The key is getting our patients to meet the guidelines: 150 to 300 minutes of moderate intensity exercise per week, or 75 to 250 minutes of vigorous activity per week,” Dr. Kovacic emphasized.
“Yes, we can give a statin to lower cholesterol. Yes, we can give a blood pressure medication to lower blood pressure. But when you prescribe exercise, you impact patients’ blood pressure, their cholesterol, their weight, their sense of well-being,” he said. “It cuts across so many different aspects of people’s lives that it’s important to underscore the value of exercise to everybody.”
That includes physicians, he affirmed. “It behooves all physicians to be leading by example. I would encourage those who are overweight or aren’t exercising as much as they should be to make the time to be healthy and to exercise. If you don’t, then bad health will force you to make the time to deal with bad health issues.”
Other articles in the series deal with the athlete’s heart. Christopher Semsarian, MBBS, PhD, MPH, University of Sydney, and colleagues discuss emerging data on hypertrophic cardiomyopathy and other genetic cardiovascular diseases, with the conclusion that it is probably okay for more athletes with these conditions to participate in recreational and competitive sports than was previously thought – another paradigm shift, according to Dr. Kovacic.
The final article addresses some of the challenges and controversies related to the athlete’s heart, including whether extreme exercise is associated with vulnerability to atrial fibrillation and other arrhythmias, and the impact of gender on the cardiac response to exercise, which can’t be determined now because of a paucity of data on women in sports.
Overall, Dr. Kovacic said, the series makes for “compelling” reading that should encourage readers to embark on their own studies to add to the data and support exercise prescription across the board.
No commercial funding or relevant conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Patisiran benefits ATTR amyloidosis with cardiomyopathy: APOLLO-B
The RNA interference (RNAi) therapeutic, patisiran (Onpattro, Alnylam), showed a statistically significant and clinically meaningful benefit on functional capacity, as measured by the 6-minute walk test (6-MWT), compared with placebo, in the treatment of transthyretin-mediated amyloidosis with cardiomyopathy, in the APOLLO-B trial.
The study also met its first secondary endpoint, demonstrating a statistically significant and clinically meaningful benefit on health status and quality of life.
These positive results, their first formal presentation, were announced Sept. 8 at the 18th International Symposium on Amyloidosis. However, the company announced positive top-line results from the trial in early August.
Transthyretin-mediated (ATTR) amyloidosis is a rare, rapidly progressive, debilitating disease caused by misfolded transthyretin (TTR) proteins which accumulate as amyloid fibrils in multiple tissues including the nerves, heart, and gastrointestinal tract.
There are two different types of ATTR amyloidosis: hereditary ATTR (hATTR) amyloidosis, caused by a TTR gene variant, and wild-type ATTR (wtATTR) amyloidosis, which occurs without a TTR gene variant. hATTR amyloidosis affects approximately 50,000 people worldwide, whereas wtATTR amyloidosis is estimated to affect 200,000-300,000 people worldwide.
Patisiran is an intravenously administered RNAi therapeutic that is approved in the United States and Canada for the treatment of the polyneuropathy of hATTR amyloidosis in adults. It is also approved in the European Union, Switzerland, Brazil, and Japan for a similar indication. It is designed to target and silence TTR messenger RNA, thereby reducing the production of TTR protein before it is made. Reducing the pathogenic protein leads to a reduction in amyloid deposits in tissues.
“The results of the APOLLO-B phase 3 study are impressive, as I believe they underscore the potential for patisiran to provide a benefit on functional capacity and quality of life in patients living with ATTR amyloidosis with cardiomyopathy. Furthermore, these results were seen after only 12 months of treatment,” Mathew Maurer, MD, Arnold and Arlene Goldstein Professor of Cardiology at Columbia University Irving Medical Center, New York, said in an Alnylam press release.
“The cardiac manifestations associated with ATTR amyloidosis can have a devastating impact on patients’ lives and current treatment options are limited. With the rapidly progressive nature of the disease, there is a significant need for treatments like patisiran, which has the potential to be a new option for patients and physicians to treat the cardiomyopathy of ATTR amyloidosis,” Dr. Maurer added.
APOLLO-B is a phase 3, randomized, double-blind study evaluating the effects of patisiran on functional capacity and quality of life in patients with ATTR amyloidosis with cardiomyopathy. The study enrolled 360 adult patients with ATTR amyloidosis (hereditary or wild-type) with cardiomyopathy who were randomly assigned 1:1 to receive 0.3 mg/kg of patisiran or placebo intravenously administered every 3 weeks over a 12-month treatment period. After 12 months, all patients will receive patisiran in an open-label extension.
Results at 12 months, reported by Alnylam, found that the primary endpoint, the 6-MWT, showed a median change from baseline of –8.15 m for the patisiran group and –21.34 m for the placebo group, a significant difference favoring patisiran.
The first secondary endpoint was health status and quality of life, as measured by the Kansas City Cardiomyopathy Questionnaire Overall Summary score. This showed a mean change from baseline of +0.300 for the patisiran group and –3.408 for the placebo group, a significant difference favoring patisiran.
Secondary composite outcome endpoints did not achieve statistical significance.
A nonsignificant result (win ratio, 1.27; P = .0574) was found on the secondary composite endpoint of all-cause mortality, frequency of cardiovascular events, and change from baseline in 6-MWT over 12 months, compared with placebo.
The final two composite endpoints were not powered for statistical significance, given the sample size and short duration of the study – all-cause mortality and frequency of all-cause hospitalizations and urgent heart failure visits in patients not on tafamidis at baseline (hazard ratio, 0.997) and in the overall study population (HR, 0.883).
Patisiran achieved a rapid and sustained reduction in serum TTR levels, with a mean percent reduction from baseline in serum TTR reduction of 87% at month 12.
A beneficial effect on the exploratory endpoint, N-terminal of the prohormone brain natriuretic peptide, a measure of cardiac stress, was observed in the patisiran arm, with a 20% reduction in the adjusted geometric mean fold change from baseline, compared with placebo.
Patisiran also demonstrated an encouraging safety and tolerability profile, including no cardiac safety concerns relative to placebo, during the 12-month treatment period, Alnylam reported.
The majority of adverse events were mild or moderate in severity. Treatment emergent adverse events in the patisiran group included infusion-related reactions, arthralgia, and muscle spasms.
In the safety analysis, there were five deaths (2.8%) observed in patisiran-treated patients and eight deaths (4.5%) observed in the placebo group.
Pushkal Garg, MD, chief medical officer at Alnylam, said: “We believe these data validate the therapeutic hypothesis that TTR silencing by an RNAi therapeutic may be an effective approach to treating cardiomyopathy of both wild-type and hereditary ATTR amyloidosis.”
Alnylam plans to file a supplemental new drug application for patisiran as a potential treatment for ATTR amyloidosis with cardiomyopathy in the United States in late 2022.
A version of this article first appeared on Medscape.com.
The RNA interference (RNAi) therapeutic, patisiran (Onpattro, Alnylam), showed a statistically significant and clinically meaningful benefit on functional capacity, as measured by the 6-minute walk test (6-MWT), compared with placebo, in the treatment of transthyretin-mediated amyloidosis with cardiomyopathy, in the APOLLO-B trial.
The study also met its first secondary endpoint, demonstrating a statistically significant and clinically meaningful benefit on health status and quality of life.
These positive results, their first formal presentation, were announced Sept. 8 at the 18th International Symposium on Amyloidosis. However, the company announced positive top-line results from the trial in early August.
Transthyretin-mediated (ATTR) amyloidosis is a rare, rapidly progressive, debilitating disease caused by misfolded transthyretin (TTR) proteins which accumulate as amyloid fibrils in multiple tissues including the nerves, heart, and gastrointestinal tract.
There are two different types of ATTR amyloidosis: hereditary ATTR (hATTR) amyloidosis, caused by a TTR gene variant, and wild-type ATTR (wtATTR) amyloidosis, which occurs without a TTR gene variant. hATTR amyloidosis affects approximately 50,000 people worldwide, whereas wtATTR amyloidosis is estimated to affect 200,000-300,000 people worldwide.
Patisiran is an intravenously administered RNAi therapeutic that is approved in the United States and Canada for the treatment of the polyneuropathy of hATTR amyloidosis in adults. It is also approved in the European Union, Switzerland, Brazil, and Japan for a similar indication. It is designed to target and silence TTR messenger RNA, thereby reducing the production of TTR protein before it is made. Reducing the pathogenic protein leads to a reduction in amyloid deposits in tissues.
“The results of the APOLLO-B phase 3 study are impressive, as I believe they underscore the potential for patisiran to provide a benefit on functional capacity and quality of life in patients living with ATTR amyloidosis with cardiomyopathy. Furthermore, these results were seen after only 12 months of treatment,” Mathew Maurer, MD, Arnold and Arlene Goldstein Professor of Cardiology at Columbia University Irving Medical Center, New York, said in an Alnylam press release.
“The cardiac manifestations associated with ATTR amyloidosis can have a devastating impact on patients’ lives and current treatment options are limited. With the rapidly progressive nature of the disease, there is a significant need for treatments like patisiran, which has the potential to be a new option for patients and physicians to treat the cardiomyopathy of ATTR amyloidosis,” Dr. Maurer added.
APOLLO-B is a phase 3, randomized, double-blind study evaluating the effects of patisiran on functional capacity and quality of life in patients with ATTR amyloidosis with cardiomyopathy. The study enrolled 360 adult patients with ATTR amyloidosis (hereditary or wild-type) with cardiomyopathy who were randomly assigned 1:1 to receive 0.3 mg/kg of patisiran or placebo intravenously administered every 3 weeks over a 12-month treatment period. After 12 months, all patients will receive patisiran in an open-label extension.
Results at 12 months, reported by Alnylam, found that the primary endpoint, the 6-MWT, showed a median change from baseline of –8.15 m for the patisiran group and –21.34 m for the placebo group, a significant difference favoring patisiran.
The first secondary endpoint was health status and quality of life, as measured by the Kansas City Cardiomyopathy Questionnaire Overall Summary score. This showed a mean change from baseline of +0.300 for the patisiran group and –3.408 for the placebo group, a significant difference favoring patisiran.
Secondary composite outcome endpoints did not achieve statistical significance.
A nonsignificant result (win ratio, 1.27; P = .0574) was found on the secondary composite endpoint of all-cause mortality, frequency of cardiovascular events, and change from baseline in 6-MWT over 12 months, compared with placebo.
The final two composite endpoints were not powered for statistical significance, given the sample size and short duration of the study – all-cause mortality and frequency of all-cause hospitalizations and urgent heart failure visits in patients not on tafamidis at baseline (hazard ratio, 0.997) and in the overall study population (HR, 0.883).
Patisiran achieved a rapid and sustained reduction in serum TTR levels, with a mean percent reduction from baseline in serum TTR reduction of 87% at month 12.
A beneficial effect on the exploratory endpoint, N-terminal of the prohormone brain natriuretic peptide, a measure of cardiac stress, was observed in the patisiran arm, with a 20% reduction in the adjusted geometric mean fold change from baseline, compared with placebo.
Patisiran also demonstrated an encouraging safety and tolerability profile, including no cardiac safety concerns relative to placebo, during the 12-month treatment period, Alnylam reported.
The majority of adverse events were mild or moderate in severity. Treatment emergent adverse events in the patisiran group included infusion-related reactions, arthralgia, and muscle spasms.
In the safety analysis, there were five deaths (2.8%) observed in patisiran-treated patients and eight deaths (4.5%) observed in the placebo group.
Pushkal Garg, MD, chief medical officer at Alnylam, said: “We believe these data validate the therapeutic hypothesis that TTR silencing by an RNAi therapeutic may be an effective approach to treating cardiomyopathy of both wild-type and hereditary ATTR amyloidosis.”
Alnylam plans to file a supplemental new drug application for patisiran as a potential treatment for ATTR amyloidosis with cardiomyopathy in the United States in late 2022.
A version of this article first appeared on Medscape.com.
The RNA interference (RNAi) therapeutic, patisiran (Onpattro, Alnylam), showed a statistically significant and clinically meaningful benefit on functional capacity, as measured by the 6-minute walk test (6-MWT), compared with placebo, in the treatment of transthyretin-mediated amyloidosis with cardiomyopathy, in the APOLLO-B trial.
The study also met its first secondary endpoint, demonstrating a statistically significant and clinically meaningful benefit on health status and quality of life.
These positive results, their first formal presentation, were announced Sept. 8 at the 18th International Symposium on Amyloidosis. However, the company announced positive top-line results from the trial in early August.
Transthyretin-mediated (ATTR) amyloidosis is a rare, rapidly progressive, debilitating disease caused by misfolded transthyretin (TTR) proteins which accumulate as amyloid fibrils in multiple tissues including the nerves, heart, and gastrointestinal tract.
There are two different types of ATTR amyloidosis: hereditary ATTR (hATTR) amyloidosis, caused by a TTR gene variant, and wild-type ATTR (wtATTR) amyloidosis, which occurs without a TTR gene variant. hATTR amyloidosis affects approximately 50,000 people worldwide, whereas wtATTR amyloidosis is estimated to affect 200,000-300,000 people worldwide.
Patisiran is an intravenously administered RNAi therapeutic that is approved in the United States and Canada for the treatment of the polyneuropathy of hATTR amyloidosis in adults. It is also approved in the European Union, Switzerland, Brazil, and Japan for a similar indication. It is designed to target and silence TTR messenger RNA, thereby reducing the production of TTR protein before it is made. Reducing the pathogenic protein leads to a reduction in amyloid deposits in tissues.
“The results of the APOLLO-B phase 3 study are impressive, as I believe they underscore the potential for patisiran to provide a benefit on functional capacity and quality of life in patients living with ATTR amyloidosis with cardiomyopathy. Furthermore, these results were seen after only 12 months of treatment,” Mathew Maurer, MD, Arnold and Arlene Goldstein Professor of Cardiology at Columbia University Irving Medical Center, New York, said in an Alnylam press release.
“The cardiac manifestations associated with ATTR amyloidosis can have a devastating impact on patients’ lives and current treatment options are limited. With the rapidly progressive nature of the disease, there is a significant need for treatments like patisiran, which has the potential to be a new option for patients and physicians to treat the cardiomyopathy of ATTR amyloidosis,” Dr. Maurer added.
APOLLO-B is a phase 3, randomized, double-blind study evaluating the effects of patisiran on functional capacity and quality of life in patients with ATTR amyloidosis with cardiomyopathy. The study enrolled 360 adult patients with ATTR amyloidosis (hereditary or wild-type) with cardiomyopathy who were randomly assigned 1:1 to receive 0.3 mg/kg of patisiran or placebo intravenously administered every 3 weeks over a 12-month treatment period. After 12 months, all patients will receive patisiran in an open-label extension.
Results at 12 months, reported by Alnylam, found that the primary endpoint, the 6-MWT, showed a median change from baseline of –8.15 m for the patisiran group and –21.34 m for the placebo group, a significant difference favoring patisiran.
The first secondary endpoint was health status and quality of life, as measured by the Kansas City Cardiomyopathy Questionnaire Overall Summary score. This showed a mean change from baseline of +0.300 for the patisiran group and –3.408 for the placebo group, a significant difference favoring patisiran.
Secondary composite outcome endpoints did not achieve statistical significance.
A nonsignificant result (win ratio, 1.27; P = .0574) was found on the secondary composite endpoint of all-cause mortality, frequency of cardiovascular events, and change from baseline in 6-MWT over 12 months, compared with placebo.
The final two composite endpoints were not powered for statistical significance, given the sample size and short duration of the study – all-cause mortality and frequency of all-cause hospitalizations and urgent heart failure visits in patients not on tafamidis at baseline (hazard ratio, 0.997) and in the overall study population (HR, 0.883).
Patisiran achieved a rapid and sustained reduction in serum TTR levels, with a mean percent reduction from baseline in serum TTR reduction of 87% at month 12.
A beneficial effect on the exploratory endpoint, N-terminal of the prohormone brain natriuretic peptide, a measure of cardiac stress, was observed in the patisiran arm, with a 20% reduction in the adjusted geometric mean fold change from baseline, compared with placebo.
Patisiran also demonstrated an encouraging safety and tolerability profile, including no cardiac safety concerns relative to placebo, during the 12-month treatment period, Alnylam reported.
The majority of adverse events were mild or moderate in severity. Treatment emergent adverse events in the patisiran group included infusion-related reactions, arthralgia, and muscle spasms.
In the safety analysis, there were five deaths (2.8%) observed in patisiran-treated patients and eight deaths (4.5%) observed in the placebo group.
Pushkal Garg, MD, chief medical officer at Alnylam, said: “We believe these data validate the therapeutic hypothesis that TTR silencing by an RNAi therapeutic may be an effective approach to treating cardiomyopathy of both wild-type and hereditary ATTR amyloidosis.”
Alnylam plans to file a supplemental new drug application for patisiran as a potential treatment for ATTR amyloidosis with cardiomyopathy in the United States in late 2022.
A version of this article first appeared on Medscape.com.
FDA warns of clip lock malfunctions with MitraClip devices
The Food and Drug Administration is alerting health care providers about the potential for clip lock malfunctions with Abbott’s MitraClip’s delivery system.
“These events appear to occur in approximately 1.3% of MitraClip procedures and have been observed with all device models,” the FDA says in a letter posted on its website.
The MitraClip device was approved in 2013 for patients with symptomatic, degenerative mitral regurgitation (MR) deemed high risk for mitral-valve surgery.
In its own “urgent medical device correction letter” to providers, Abbott reports a recent increase in reports of the clips failing to “establish final arm angle (EFAA)” and of “clip opening while locked (COWL)” events.
During device preparation and prior to clip deployment, the operator intentionally attempts to open a locked clip to verify that the locking mechanism is engaged.
COWL describes when the clip arm angle increases postdeployment. “In these cases, users observe a slippage in the lock, resulting in an arm angle greater than 10 degrees from the angle observed at deployment,” which can be identified through fluoroscopy, Abbott says.
From February 2021 to January 2022, the EFAA failure rate was 0.51% and COWL rate 0.28%, increasing to 0.80% and 0.50%, respectively, from February 2022 to July 2022, according to the company.
Despite the increase in reports, the acute procedural success rate remains consistent with historical data, according to Abbott. “Further, EFAA failure or COWL most often results in no adverse patient outcomes. COWL may lead to less MR reduction, which is often treated with the use of one or more additional clips.”
Abbott says there is also a “low incidence” of required additional interventions. No immediate open surgical conversions have occurred as a result of EFAA/COWL events, whereas 0.53% of such events have resulted in nonurgent surgical conversions.
“In any case where significant residual MR is observed after clip deployment, a second clip should be considered and implanted in accordance with the IFU [instructions for use],” it advises.
Abbott says that a “change in the material properties of one of the clip locking components” has been identified as a contributing cause of EFAA/COWL events. It is working on producing new lots with updated manufacturing processing and raw material to mitigate the risk.
Certain use conditions can also contribute to EFAA/COWL events, and are referenced in the IFU, Appendix A, it notes.
The FDA is working with Abbott and recommends that health care providers do the following:
- Review the recall notice from Abbott for all MitraClip Clip Delivery Systems.
- Be aware of the potential for clip lock malfunctions before or after deployment with this device.
- Read and carefully follow the instructions for use and the recommendations provided in the recall notice to help minimize the chance of the clip failing to lock. These include recommendations about procedural steps for implant positioning, locking sequences, establishing clip arm angle, preparation for clip release, and avoiding excessive force and manipulation when unlocking the clip during device preparation and during the procedure.
Health care professionals can also report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is alerting health care providers about the potential for clip lock malfunctions with Abbott’s MitraClip’s delivery system.
“These events appear to occur in approximately 1.3% of MitraClip procedures and have been observed with all device models,” the FDA says in a letter posted on its website.
The MitraClip device was approved in 2013 for patients with symptomatic, degenerative mitral regurgitation (MR) deemed high risk for mitral-valve surgery.
In its own “urgent medical device correction letter” to providers, Abbott reports a recent increase in reports of the clips failing to “establish final arm angle (EFAA)” and of “clip opening while locked (COWL)” events.
During device preparation and prior to clip deployment, the operator intentionally attempts to open a locked clip to verify that the locking mechanism is engaged.
COWL describes when the clip arm angle increases postdeployment. “In these cases, users observe a slippage in the lock, resulting in an arm angle greater than 10 degrees from the angle observed at deployment,” which can be identified through fluoroscopy, Abbott says.
From February 2021 to January 2022, the EFAA failure rate was 0.51% and COWL rate 0.28%, increasing to 0.80% and 0.50%, respectively, from February 2022 to July 2022, according to the company.
Despite the increase in reports, the acute procedural success rate remains consistent with historical data, according to Abbott. “Further, EFAA failure or COWL most often results in no adverse patient outcomes. COWL may lead to less MR reduction, which is often treated with the use of one or more additional clips.”
Abbott says there is also a “low incidence” of required additional interventions. No immediate open surgical conversions have occurred as a result of EFAA/COWL events, whereas 0.53% of such events have resulted in nonurgent surgical conversions.
“In any case where significant residual MR is observed after clip deployment, a second clip should be considered and implanted in accordance with the IFU [instructions for use],” it advises.
Abbott says that a “change in the material properties of one of the clip locking components” has been identified as a contributing cause of EFAA/COWL events. It is working on producing new lots with updated manufacturing processing and raw material to mitigate the risk.
Certain use conditions can also contribute to EFAA/COWL events, and are referenced in the IFU, Appendix A, it notes.
The FDA is working with Abbott and recommends that health care providers do the following:
- Review the recall notice from Abbott for all MitraClip Clip Delivery Systems.
- Be aware of the potential for clip lock malfunctions before or after deployment with this device.
- Read and carefully follow the instructions for use and the recommendations provided in the recall notice to help minimize the chance of the clip failing to lock. These include recommendations about procedural steps for implant positioning, locking sequences, establishing clip arm angle, preparation for clip release, and avoiding excessive force and manipulation when unlocking the clip during device preparation and during the procedure.
Health care professionals can also report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is alerting health care providers about the potential for clip lock malfunctions with Abbott’s MitraClip’s delivery system.
“These events appear to occur in approximately 1.3% of MitraClip procedures and have been observed with all device models,” the FDA says in a letter posted on its website.
The MitraClip device was approved in 2013 for patients with symptomatic, degenerative mitral regurgitation (MR) deemed high risk for mitral-valve surgery.
In its own “urgent medical device correction letter” to providers, Abbott reports a recent increase in reports of the clips failing to “establish final arm angle (EFAA)” and of “clip opening while locked (COWL)” events.
During device preparation and prior to clip deployment, the operator intentionally attempts to open a locked clip to verify that the locking mechanism is engaged.
COWL describes when the clip arm angle increases postdeployment. “In these cases, users observe a slippage in the lock, resulting in an arm angle greater than 10 degrees from the angle observed at deployment,” which can be identified through fluoroscopy, Abbott says.
From February 2021 to January 2022, the EFAA failure rate was 0.51% and COWL rate 0.28%, increasing to 0.80% and 0.50%, respectively, from February 2022 to July 2022, according to the company.
Despite the increase in reports, the acute procedural success rate remains consistent with historical data, according to Abbott. “Further, EFAA failure or COWL most often results in no adverse patient outcomes. COWL may lead to less MR reduction, which is often treated with the use of one or more additional clips.”
Abbott says there is also a “low incidence” of required additional interventions. No immediate open surgical conversions have occurred as a result of EFAA/COWL events, whereas 0.53% of such events have resulted in nonurgent surgical conversions.
“In any case where significant residual MR is observed after clip deployment, a second clip should be considered and implanted in accordance with the IFU [instructions for use],” it advises.
Abbott says that a “change in the material properties of one of the clip locking components” has been identified as a contributing cause of EFAA/COWL events. It is working on producing new lots with updated manufacturing processing and raw material to mitigate the risk.
Certain use conditions can also contribute to EFAA/COWL events, and are referenced in the IFU, Appendix A, it notes.
The FDA is working with Abbott and recommends that health care providers do the following:
- Review the recall notice from Abbott for all MitraClip Clip Delivery Systems.
- Be aware of the potential for clip lock malfunctions before or after deployment with this device.
- Read and carefully follow the instructions for use and the recommendations provided in the recall notice to help minimize the chance of the clip failing to lock. These include recommendations about procedural steps for implant positioning, locking sequences, establishing clip arm angle, preparation for clip release, and avoiding excessive force and manipulation when unlocking the clip during device preparation and during the procedure.
Health care professionals can also report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Artificial sweeteners linked to higher CV event risk
Health concerns about the consumption of artificial sweeteners could be strengthened with the publication of a new study linking their intake to increased risk of heart disease and stroke events.
In this latest large-scale, prospective study of French adults, total artificial sweetener intake from all sources was associated with increased risk overall of cardiovascular and cerebrovascular disease.
The study was published online in the BMJ.
The current study differs from those done previously in that it includes artificial sweetener intake from both food and drinks, whereas previous studies have focused mainly on artificial sweetener content of beverages alone.
“Here we have quantified for the first time the global exposure to artificial sweeteners. This is not just beverages but includes the use of tabletop sweeteners, and other foods that include artificial sweeteners such as yogurts and desserts. This is the first time this information has been correlated to risk of heart disease,” senior author Mathilde Touvier, MD, Sorbonne Paris Nord University, told this news organization.
Just over half of the artificial sweetener intake in the study came from drinks, with the rest coming from tabletop sweeteners and foods.
“We included hard cardio- and cerebrovascular clinical endpoints such as a heart attack or stroke, and our results suggest that the amount of artificial sweetener in less than one can of soda could increase the risk of such events,” Dr. Touvier noted.
“This is an important and statistically significant association which shows robustness in all models after adjusting for many other possible confounding factors,” she said.
“There is now mounting evidence correlating artificial sweeteners to weight gain and heart disease,” she concluded. “My advice would be that we all need to try to limit sugar intake, but we should not consider artificial sweeteners as safe alternatives. Rather, we need to try to reduce our need for a sugary taste in our diet.”
But another leading researcher in the field urges caution in interpreting these results.
John Sievenpiper, MD, departments of nutritional sciences and medicine, University of Toronto, commented: “This paper shows the same relationship seen by many other large prospective cohorts which model the intake of artificial sweeteners as baseline or prevalent exposures.
“These observations are well recognized to be at high risk of residual confounding from behavior clustering and reverse causality in which being at risk for cardiovascular disease causes people to consume artificial sweeteners as a strategy to mitigate this risk as opposed to the other way around.”
Risk increased by 9%
The current study included 103,388 French adults from the NutriNet-Sante cohort, of whom 37.1% reported consumption of artificial sweeteners. The sweeteners assessed were mainly aspartame (58% of sweetener intake), acesulfame potassium (29%), and sucralose (10%), with the other 3% made up of various other sweeteners including cyclamates and saccharin.
Results showed that over an average 9 years of follow-up, artificial sweetener intake was associated with a 9% increased risk of cardiovascular or cerebrovascular events, including myocardial infarction, acute coronary syndrome, angioplasty, angina, stroke, or transient ischemic attack, with a hazard ratio of 1.09 (95% confidence interval, 1.01-1.18; P = .03).
The average intake of artificial sweeteners among those who reported consuming them was 42.46 mg/day, which corresponds to approximately one individual packet of tabletop sweetener or 100 mL of diet soda.
“We don’t have enough evidence to work out an amount of artificial sweetener that is harmful, but we did show a dose-effect association, with a higher risk of cardiovascular events with higher consumption,” Dr. Touvier said.
“Higher consumption in this study was a mean of 77 mg/day artificial sweetener, which is about 200 mL of soda – just a bit less than one standard can of soda,” she added.
The absolute incidence rate of cardiovascular or cerebrovascular events in higher consumers was 346 per 100,000 person-years vs. 314 per 100,000 person-years in nonconsumers.
Further analysis suggested that aspartame intake was particularly associated with increased risk of cerebrovascular events, while acesulfame potassium and sucralose were associated with increased coronary heart disease risk.
Study strengths
Dr. Touvier acknowledged that dietary studies, which generally rely on individuals self-reporting food and drink intake, are always hard to interpret. But she said this study used a more reliable method of dietary assessment, with repeated 24-hour dietary records, which were validated by interviews with a trained dietitian and against blood and urinary biomarkers.
And whereas residual confounding cannot be totally excluded, she pointed out that models were adjusted for a wide range of potential sociodemographic, anthropometric, dietary, and lifestyle confounders.
Dr. Touvier also noted that cases of cardiovascular disease in the first 2 years of follow-up were excluded to minimize the bias caused by individuals who maybe have switched to artificial sweeteners because of a cardiovascular issue.
“While this study has many strengths, it cannot on its own prove a causal relationship between artificial sweetener and increased cardiovascular risk,” she added. “We need health agencies to examine all the literature in the field. This is however another important piece of evidence.”
Dr. Touvier says that although observational studies have their issues, they will form the basis of the evidence on the effects of artificial sweeteners on health.
“Randomized studies in this area can only really look at short-term outcomes such as weight gain or biomarker changes. So, we will have to use observational studies together with experimental research to build the evidence. This is what happened with cigarette smoking and lung cancer. That link was not established by randomized trials, but by the accumulation of observational and experimental data.”
Different artificial sweeteners may be better?
Commenting on the study, Kim Williams Sr., MD, University of Louisville (Ky.), pointed out that this study included artificial sweeteners that increase insulin or decrease insulin sensitivity, and that insulin spikes increase obesity, insulin resistance, hypertension, and atherosclerosis.
“There are some safer artificial sweeteners that do not increase insulin much or at all, such as erythritol, yacon root/yacon syrup, stevia root, but they weren’t included in the analysis,” Dr. Williams added.
Dr. Sievenpiper explained that most studies on artificial sweeteners look at their consumption in isolation without considering how they compare to the intake of the sugars that they are intended to replace.
“The comparator matters as no food is consumed in a vacuum,” he said.
To address this, Dr. Sievenpiper and colleagues have recently published a systematic review and meta-analysis of the prospective cohort study evidence that shows if exposure to artificially sweetened beverages is modeled in substitution for sugar-sweetened beverages, then they are associated with less coronary heart disease, cardiovascular mortality, and all-cause mortality.
On the other hand, if exposure to artificially sweetened beverages is compared with water, then no difference in these outcomes was seen.
“These observations are more biologically plausible, robust, and reproducible and agree with the evidence for the effect of artificial sweeteners on intermediate risk factors in randomized trials,” Dr. Sievenpiper notes.
His group has also recently published a review of randomized studies showing that when compared with sugar-sweetened beverages, intake of artificially sweetened beverages was associated with small improvements in body weight and cardiometabolic risk factors without evidence of harm.
“I think the context provided by these studies is important, and taken together, the totality of the evidence suggests that artificial sweeteners are likely to be a useful tool in sugar reduction strategies,” Dr. Sievenpiper concludes.
The current study was funded by the European Research Council under the European Union’s Horizon 2020 research and innovation program, French National Cancer Institute, French Ministry of Health, IdEx Université de Paris Cité, Bettencourt-Schueller Foundation Research Prize 2021. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Health concerns about the consumption of artificial sweeteners could be strengthened with the publication of a new study linking their intake to increased risk of heart disease and stroke events.
In this latest large-scale, prospective study of French adults, total artificial sweetener intake from all sources was associated with increased risk overall of cardiovascular and cerebrovascular disease.
The study was published online in the BMJ.
The current study differs from those done previously in that it includes artificial sweetener intake from both food and drinks, whereas previous studies have focused mainly on artificial sweetener content of beverages alone.
“Here we have quantified for the first time the global exposure to artificial sweeteners. This is not just beverages but includes the use of tabletop sweeteners, and other foods that include artificial sweeteners such as yogurts and desserts. This is the first time this information has been correlated to risk of heart disease,” senior author Mathilde Touvier, MD, Sorbonne Paris Nord University, told this news organization.
Just over half of the artificial sweetener intake in the study came from drinks, with the rest coming from tabletop sweeteners and foods.
“We included hard cardio- and cerebrovascular clinical endpoints such as a heart attack or stroke, and our results suggest that the amount of artificial sweetener in less than one can of soda could increase the risk of such events,” Dr. Touvier noted.
“This is an important and statistically significant association which shows robustness in all models after adjusting for many other possible confounding factors,” she said.
“There is now mounting evidence correlating artificial sweeteners to weight gain and heart disease,” she concluded. “My advice would be that we all need to try to limit sugar intake, but we should not consider artificial sweeteners as safe alternatives. Rather, we need to try to reduce our need for a sugary taste in our diet.”
But another leading researcher in the field urges caution in interpreting these results.
John Sievenpiper, MD, departments of nutritional sciences and medicine, University of Toronto, commented: “This paper shows the same relationship seen by many other large prospective cohorts which model the intake of artificial sweeteners as baseline or prevalent exposures.
“These observations are well recognized to be at high risk of residual confounding from behavior clustering and reverse causality in which being at risk for cardiovascular disease causes people to consume artificial sweeteners as a strategy to mitigate this risk as opposed to the other way around.”
Risk increased by 9%
The current study included 103,388 French adults from the NutriNet-Sante cohort, of whom 37.1% reported consumption of artificial sweeteners. The sweeteners assessed were mainly aspartame (58% of sweetener intake), acesulfame potassium (29%), and sucralose (10%), with the other 3% made up of various other sweeteners including cyclamates and saccharin.
Results showed that over an average 9 years of follow-up, artificial sweetener intake was associated with a 9% increased risk of cardiovascular or cerebrovascular events, including myocardial infarction, acute coronary syndrome, angioplasty, angina, stroke, or transient ischemic attack, with a hazard ratio of 1.09 (95% confidence interval, 1.01-1.18; P = .03).
The average intake of artificial sweeteners among those who reported consuming them was 42.46 mg/day, which corresponds to approximately one individual packet of tabletop sweetener or 100 mL of diet soda.
“We don’t have enough evidence to work out an amount of artificial sweetener that is harmful, but we did show a dose-effect association, with a higher risk of cardiovascular events with higher consumption,” Dr. Touvier said.
“Higher consumption in this study was a mean of 77 mg/day artificial sweetener, which is about 200 mL of soda – just a bit less than one standard can of soda,” she added.
The absolute incidence rate of cardiovascular or cerebrovascular events in higher consumers was 346 per 100,000 person-years vs. 314 per 100,000 person-years in nonconsumers.
Further analysis suggested that aspartame intake was particularly associated with increased risk of cerebrovascular events, while acesulfame potassium and sucralose were associated with increased coronary heart disease risk.
Study strengths
Dr. Touvier acknowledged that dietary studies, which generally rely on individuals self-reporting food and drink intake, are always hard to interpret. But she said this study used a more reliable method of dietary assessment, with repeated 24-hour dietary records, which were validated by interviews with a trained dietitian and against blood and urinary biomarkers.
And whereas residual confounding cannot be totally excluded, she pointed out that models were adjusted for a wide range of potential sociodemographic, anthropometric, dietary, and lifestyle confounders.
Dr. Touvier also noted that cases of cardiovascular disease in the first 2 years of follow-up were excluded to minimize the bias caused by individuals who maybe have switched to artificial sweeteners because of a cardiovascular issue.
“While this study has many strengths, it cannot on its own prove a causal relationship between artificial sweetener and increased cardiovascular risk,” she added. “We need health agencies to examine all the literature in the field. This is however another important piece of evidence.”
Dr. Touvier says that although observational studies have their issues, they will form the basis of the evidence on the effects of artificial sweeteners on health.
“Randomized studies in this area can only really look at short-term outcomes such as weight gain or biomarker changes. So, we will have to use observational studies together with experimental research to build the evidence. This is what happened with cigarette smoking and lung cancer. That link was not established by randomized trials, but by the accumulation of observational and experimental data.”
Different artificial sweeteners may be better?
Commenting on the study, Kim Williams Sr., MD, University of Louisville (Ky.), pointed out that this study included artificial sweeteners that increase insulin or decrease insulin sensitivity, and that insulin spikes increase obesity, insulin resistance, hypertension, and atherosclerosis.
“There are some safer artificial sweeteners that do not increase insulin much or at all, such as erythritol, yacon root/yacon syrup, stevia root, but they weren’t included in the analysis,” Dr. Williams added.
Dr. Sievenpiper explained that most studies on artificial sweeteners look at their consumption in isolation without considering how they compare to the intake of the sugars that they are intended to replace.
“The comparator matters as no food is consumed in a vacuum,” he said.
To address this, Dr. Sievenpiper and colleagues have recently published a systematic review and meta-analysis of the prospective cohort study evidence that shows if exposure to artificially sweetened beverages is modeled in substitution for sugar-sweetened beverages, then they are associated with less coronary heart disease, cardiovascular mortality, and all-cause mortality.
On the other hand, if exposure to artificially sweetened beverages is compared with water, then no difference in these outcomes was seen.
“These observations are more biologically plausible, robust, and reproducible and agree with the evidence for the effect of artificial sweeteners on intermediate risk factors in randomized trials,” Dr. Sievenpiper notes.
His group has also recently published a review of randomized studies showing that when compared with sugar-sweetened beverages, intake of artificially sweetened beverages was associated with small improvements in body weight and cardiometabolic risk factors without evidence of harm.
“I think the context provided by these studies is important, and taken together, the totality of the evidence suggests that artificial sweeteners are likely to be a useful tool in sugar reduction strategies,” Dr. Sievenpiper concludes.
The current study was funded by the European Research Council under the European Union’s Horizon 2020 research and innovation program, French National Cancer Institute, French Ministry of Health, IdEx Université de Paris Cité, Bettencourt-Schueller Foundation Research Prize 2021. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Health concerns about the consumption of artificial sweeteners could be strengthened with the publication of a new study linking their intake to increased risk of heart disease and stroke events.
In this latest large-scale, prospective study of French adults, total artificial sweetener intake from all sources was associated with increased risk overall of cardiovascular and cerebrovascular disease.
The study was published online in the BMJ.
The current study differs from those done previously in that it includes artificial sweetener intake from both food and drinks, whereas previous studies have focused mainly on artificial sweetener content of beverages alone.
“Here we have quantified for the first time the global exposure to artificial sweeteners. This is not just beverages but includes the use of tabletop sweeteners, and other foods that include artificial sweeteners such as yogurts and desserts. This is the first time this information has been correlated to risk of heart disease,” senior author Mathilde Touvier, MD, Sorbonne Paris Nord University, told this news organization.
Just over half of the artificial sweetener intake in the study came from drinks, with the rest coming from tabletop sweeteners and foods.
“We included hard cardio- and cerebrovascular clinical endpoints such as a heart attack or stroke, and our results suggest that the amount of artificial sweetener in less than one can of soda could increase the risk of such events,” Dr. Touvier noted.
“This is an important and statistically significant association which shows robustness in all models after adjusting for many other possible confounding factors,” she said.
“There is now mounting evidence correlating artificial sweeteners to weight gain and heart disease,” she concluded. “My advice would be that we all need to try to limit sugar intake, but we should not consider artificial sweeteners as safe alternatives. Rather, we need to try to reduce our need for a sugary taste in our diet.”
But another leading researcher in the field urges caution in interpreting these results.
John Sievenpiper, MD, departments of nutritional sciences and medicine, University of Toronto, commented: “This paper shows the same relationship seen by many other large prospective cohorts which model the intake of artificial sweeteners as baseline or prevalent exposures.
“These observations are well recognized to be at high risk of residual confounding from behavior clustering and reverse causality in which being at risk for cardiovascular disease causes people to consume artificial sweeteners as a strategy to mitigate this risk as opposed to the other way around.”
Risk increased by 9%
The current study included 103,388 French adults from the NutriNet-Sante cohort, of whom 37.1% reported consumption of artificial sweeteners. The sweeteners assessed were mainly aspartame (58% of sweetener intake), acesulfame potassium (29%), and sucralose (10%), with the other 3% made up of various other sweeteners including cyclamates and saccharin.
Results showed that over an average 9 years of follow-up, artificial sweetener intake was associated with a 9% increased risk of cardiovascular or cerebrovascular events, including myocardial infarction, acute coronary syndrome, angioplasty, angina, stroke, or transient ischemic attack, with a hazard ratio of 1.09 (95% confidence interval, 1.01-1.18; P = .03).
The average intake of artificial sweeteners among those who reported consuming them was 42.46 mg/day, which corresponds to approximately one individual packet of tabletop sweetener or 100 mL of diet soda.
“We don’t have enough evidence to work out an amount of artificial sweetener that is harmful, but we did show a dose-effect association, with a higher risk of cardiovascular events with higher consumption,” Dr. Touvier said.
“Higher consumption in this study was a mean of 77 mg/day artificial sweetener, which is about 200 mL of soda – just a bit less than one standard can of soda,” she added.
The absolute incidence rate of cardiovascular or cerebrovascular events in higher consumers was 346 per 100,000 person-years vs. 314 per 100,000 person-years in nonconsumers.
Further analysis suggested that aspartame intake was particularly associated with increased risk of cerebrovascular events, while acesulfame potassium and sucralose were associated with increased coronary heart disease risk.
Study strengths
Dr. Touvier acknowledged that dietary studies, which generally rely on individuals self-reporting food and drink intake, are always hard to interpret. But she said this study used a more reliable method of dietary assessment, with repeated 24-hour dietary records, which were validated by interviews with a trained dietitian and against blood and urinary biomarkers.
And whereas residual confounding cannot be totally excluded, she pointed out that models were adjusted for a wide range of potential sociodemographic, anthropometric, dietary, and lifestyle confounders.
Dr. Touvier also noted that cases of cardiovascular disease in the first 2 years of follow-up were excluded to minimize the bias caused by individuals who maybe have switched to artificial sweeteners because of a cardiovascular issue.
“While this study has many strengths, it cannot on its own prove a causal relationship between artificial sweetener and increased cardiovascular risk,” she added. “We need health agencies to examine all the literature in the field. This is however another important piece of evidence.”
Dr. Touvier says that although observational studies have their issues, they will form the basis of the evidence on the effects of artificial sweeteners on health.
“Randomized studies in this area can only really look at short-term outcomes such as weight gain or biomarker changes. So, we will have to use observational studies together with experimental research to build the evidence. This is what happened with cigarette smoking and lung cancer. That link was not established by randomized trials, but by the accumulation of observational and experimental data.”
Different artificial sweeteners may be better?
Commenting on the study, Kim Williams Sr., MD, University of Louisville (Ky.), pointed out that this study included artificial sweeteners that increase insulin or decrease insulin sensitivity, and that insulin spikes increase obesity, insulin resistance, hypertension, and atherosclerosis.
“There are some safer artificial sweeteners that do not increase insulin much or at all, such as erythritol, yacon root/yacon syrup, stevia root, but they weren’t included in the analysis,” Dr. Williams added.
Dr. Sievenpiper explained that most studies on artificial sweeteners look at their consumption in isolation without considering how they compare to the intake of the sugars that they are intended to replace.
“The comparator matters as no food is consumed in a vacuum,” he said.
To address this, Dr. Sievenpiper and colleagues have recently published a systematic review and meta-analysis of the prospective cohort study evidence that shows if exposure to artificially sweetened beverages is modeled in substitution for sugar-sweetened beverages, then they are associated with less coronary heart disease, cardiovascular mortality, and all-cause mortality.
On the other hand, if exposure to artificially sweetened beverages is compared with water, then no difference in these outcomes was seen.
“These observations are more biologically plausible, robust, and reproducible and agree with the evidence for the effect of artificial sweeteners on intermediate risk factors in randomized trials,” Dr. Sievenpiper notes.
His group has also recently published a review of randomized studies showing that when compared with sugar-sweetened beverages, intake of artificially sweetened beverages was associated with small improvements in body weight and cardiometabolic risk factors without evidence of harm.
“I think the context provided by these studies is important, and taken together, the totality of the evidence suggests that artificial sweeteners are likely to be a useful tool in sugar reduction strategies,” Dr. Sievenpiper concludes.
The current study was funded by the European Research Council under the European Union’s Horizon 2020 research and innovation program, French National Cancer Institute, French Ministry of Health, IdEx Université de Paris Cité, Bettencourt-Schueller Foundation Research Prize 2021. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ
Low physical function tied to cardiac events in older adults
including coronary heart disease (CHD), stroke, and heart failure (HF) in older adults, according to new observational data from the Atherosclerosis Risk in Communities (ARIC) study.
“We found that physical function in older adults predicts future cardiovascular disease (CVD) beyond traditional heart disease risk factors, regardless of whether an individual has a history of cardiovascular disease,” senior author Kunihiro Matsushita, MD, PhD, division of cardiology, Johns Hopkins University, Baltimore, said in a news release.
The study was published online in the Journal of the American Heart Association.
Keeping fit with age
The researchers analyzed health data collected between 2011 and 2013 for 5,570 ARIC participants (mean age, 75 years; 58% women, 22% Black persons). They assessed physical function using the Short Physical Performance Battery (SPPB), which measures walking speed, leg strength, and balance.
On the basis of the results, participants were categorized into three physical function groups: low (score, 0-6; 13% of the cohort), intermediate (score, 7-9; 30%) and high (score, 10-12; 57%).
During a median follow up of 7 years, there were 930 composite CVD events (386 CHD, 251 stroke, and 529 HF).
Adults with lower SPPB scores had a higher cumulative incidence of composite CVD outcomes.
The 5-year cumulative incidence of the composite CVD outcome in the low- and intermediate-SPPB categories was about three times (23.4%) and two times (15.3%) higher than in the high-SPPB category (8.6%), the researchers reported.
In addition, continuous SPPB scores showed significant associations with composite and individual CVD outcomes in all models. A 1-point lower SPPB score was associated with 6%-10% higher risk for CVD events after adjusting for potential confounders.
In the fully adjusted model, the risk for composite CVD outcomes was 47% higher (hazard ratio, 1.47; 95% confidence interval, 1.20-1.79) in those with low physical function and 25% higher in those with intermediate physical function (HR, 1.25; 95% CI, 1.07-1.46) compared with peers with high physical function.
For the individual outcomes, low physical function was associated with higher risk for stroke (HR, 1.81; 95% CI, 1.24-2.64) and HF (HR, 1.33; 95% CI, 1.02-1.73), whereas the association for CHD was not significant.
The associations were largely consistent across subgroups, including those with CVD at baseline.
The addition of SPPB scores significantly improved risk prediction of CVD events beyond traditional CVD risk factors in adults regardless of prior CVD history, suggesting that this tool may be useful for classifying CVD risk in older adults, the researchers said.
Meaningful impact on care?
“Our findings highlight the value of assessing the physical function level of older adults in clinical practice,” lead author Xiao Hu, MHS, with the department of epidemiology at Johns Hopkins, said in the news release. “In addition to heart health, older adults are at higher risk for falls and disability. The assessment of physical function may also inform the risk of these concerning conditions in older adults.”
Weighing in on the study, Jonathan Halperin, MD, cardiologist at Mount Sinai Heart and professor of medicine (cardiology) at the Icahn School of Medicine at Mount Sinai, both in New York, said that “It’s known that cardiorespiratory fitness is an important predictor of cardiovascular risk, but it is one of the few physiological risk factors that are subjectively queried but not objectively assessed in routine clinical practice.”
In this study, Dr. Halperin noted, the investigators found that a battery of physical performance assessments, including a walk test, chair standing, and balance testing, improved cardiovascular risk prediction.
Dr. Halperin cautioned, however, that “since even the short sequence of tests takes time to perform and interpret, and is not currently reimbursed under most health insurance policies, it is not clear whether the report will have a meaningful impact on patient care.”
This research was funded by the National Institutes of Health. Dr. Matsushita and Dr. Halperin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
including coronary heart disease (CHD), stroke, and heart failure (HF) in older adults, according to new observational data from the Atherosclerosis Risk in Communities (ARIC) study.
“We found that physical function in older adults predicts future cardiovascular disease (CVD) beyond traditional heart disease risk factors, regardless of whether an individual has a history of cardiovascular disease,” senior author Kunihiro Matsushita, MD, PhD, division of cardiology, Johns Hopkins University, Baltimore, said in a news release.
The study was published online in the Journal of the American Heart Association.
Keeping fit with age
The researchers analyzed health data collected between 2011 and 2013 for 5,570 ARIC participants (mean age, 75 years; 58% women, 22% Black persons). They assessed physical function using the Short Physical Performance Battery (SPPB), which measures walking speed, leg strength, and balance.
On the basis of the results, participants were categorized into three physical function groups: low (score, 0-6; 13% of the cohort), intermediate (score, 7-9; 30%) and high (score, 10-12; 57%).
During a median follow up of 7 years, there were 930 composite CVD events (386 CHD, 251 stroke, and 529 HF).
Adults with lower SPPB scores had a higher cumulative incidence of composite CVD outcomes.
The 5-year cumulative incidence of the composite CVD outcome in the low- and intermediate-SPPB categories was about three times (23.4%) and two times (15.3%) higher than in the high-SPPB category (8.6%), the researchers reported.
In addition, continuous SPPB scores showed significant associations with composite and individual CVD outcomes in all models. A 1-point lower SPPB score was associated with 6%-10% higher risk for CVD events after adjusting for potential confounders.
In the fully adjusted model, the risk for composite CVD outcomes was 47% higher (hazard ratio, 1.47; 95% confidence interval, 1.20-1.79) in those with low physical function and 25% higher in those with intermediate physical function (HR, 1.25; 95% CI, 1.07-1.46) compared with peers with high physical function.
For the individual outcomes, low physical function was associated with higher risk for stroke (HR, 1.81; 95% CI, 1.24-2.64) and HF (HR, 1.33; 95% CI, 1.02-1.73), whereas the association for CHD was not significant.
The associations were largely consistent across subgroups, including those with CVD at baseline.
The addition of SPPB scores significantly improved risk prediction of CVD events beyond traditional CVD risk factors in adults regardless of prior CVD history, suggesting that this tool may be useful for classifying CVD risk in older adults, the researchers said.
Meaningful impact on care?
“Our findings highlight the value of assessing the physical function level of older adults in clinical practice,” lead author Xiao Hu, MHS, with the department of epidemiology at Johns Hopkins, said in the news release. “In addition to heart health, older adults are at higher risk for falls and disability. The assessment of physical function may also inform the risk of these concerning conditions in older adults.”
Weighing in on the study, Jonathan Halperin, MD, cardiologist at Mount Sinai Heart and professor of medicine (cardiology) at the Icahn School of Medicine at Mount Sinai, both in New York, said that “It’s known that cardiorespiratory fitness is an important predictor of cardiovascular risk, but it is one of the few physiological risk factors that are subjectively queried but not objectively assessed in routine clinical practice.”
In this study, Dr. Halperin noted, the investigators found that a battery of physical performance assessments, including a walk test, chair standing, and balance testing, improved cardiovascular risk prediction.
Dr. Halperin cautioned, however, that “since even the short sequence of tests takes time to perform and interpret, and is not currently reimbursed under most health insurance policies, it is not clear whether the report will have a meaningful impact on patient care.”
This research was funded by the National Institutes of Health. Dr. Matsushita and Dr. Halperin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
including coronary heart disease (CHD), stroke, and heart failure (HF) in older adults, according to new observational data from the Atherosclerosis Risk in Communities (ARIC) study.
“We found that physical function in older adults predicts future cardiovascular disease (CVD) beyond traditional heart disease risk factors, regardless of whether an individual has a history of cardiovascular disease,” senior author Kunihiro Matsushita, MD, PhD, division of cardiology, Johns Hopkins University, Baltimore, said in a news release.
The study was published online in the Journal of the American Heart Association.
Keeping fit with age
The researchers analyzed health data collected between 2011 and 2013 for 5,570 ARIC participants (mean age, 75 years; 58% women, 22% Black persons). They assessed physical function using the Short Physical Performance Battery (SPPB), which measures walking speed, leg strength, and balance.
On the basis of the results, participants were categorized into three physical function groups: low (score, 0-6; 13% of the cohort), intermediate (score, 7-9; 30%) and high (score, 10-12; 57%).
During a median follow up of 7 years, there were 930 composite CVD events (386 CHD, 251 stroke, and 529 HF).
Adults with lower SPPB scores had a higher cumulative incidence of composite CVD outcomes.
The 5-year cumulative incidence of the composite CVD outcome in the low- and intermediate-SPPB categories was about three times (23.4%) and two times (15.3%) higher than in the high-SPPB category (8.6%), the researchers reported.
In addition, continuous SPPB scores showed significant associations with composite and individual CVD outcomes in all models. A 1-point lower SPPB score was associated with 6%-10% higher risk for CVD events after adjusting for potential confounders.
In the fully adjusted model, the risk for composite CVD outcomes was 47% higher (hazard ratio, 1.47; 95% confidence interval, 1.20-1.79) in those with low physical function and 25% higher in those with intermediate physical function (HR, 1.25; 95% CI, 1.07-1.46) compared with peers with high physical function.
For the individual outcomes, low physical function was associated with higher risk for stroke (HR, 1.81; 95% CI, 1.24-2.64) and HF (HR, 1.33; 95% CI, 1.02-1.73), whereas the association for CHD was not significant.
The associations were largely consistent across subgroups, including those with CVD at baseline.
The addition of SPPB scores significantly improved risk prediction of CVD events beyond traditional CVD risk factors in adults regardless of prior CVD history, suggesting that this tool may be useful for classifying CVD risk in older adults, the researchers said.
Meaningful impact on care?
“Our findings highlight the value of assessing the physical function level of older adults in clinical practice,” lead author Xiao Hu, MHS, with the department of epidemiology at Johns Hopkins, said in the news release. “In addition to heart health, older adults are at higher risk for falls and disability. The assessment of physical function may also inform the risk of these concerning conditions in older adults.”
Weighing in on the study, Jonathan Halperin, MD, cardiologist at Mount Sinai Heart and professor of medicine (cardiology) at the Icahn School of Medicine at Mount Sinai, both in New York, said that “It’s known that cardiorespiratory fitness is an important predictor of cardiovascular risk, but it is one of the few physiological risk factors that are subjectively queried but not objectively assessed in routine clinical practice.”
In this study, Dr. Halperin noted, the investigators found that a battery of physical performance assessments, including a walk test, chair standing, and balance testing, improved cardiovascular risk prediction.
Dr. Halperin cautioned, however, that “since even the short sequence of tests takes time to perform and interpret, and is not currently reimbursed under most health insurance policies, it is not clear whether the report will have a meaningful impact on patient care.”
This research was funded by the National Institutes of Health. Dr. Matsushita and Dr. Halperin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
ARBs, beta-blockers independently inhibit Marfan syndrome progression
Early start might delay surgery
Beta-blockers have long been recommended to prevent aortic dissection associated with Marfan syndrome despite limited evidence, but a new analysis also supports a benefit from angiotensin receptors blockers (ARBs) and further suggests that beta-blockers and ARBs exert independent effects.
For the endpoint of inhibition of growth of the aortic root, “there is no evidence of any interaction between the effects of ARBs with beta-blockers, and so we think that the treatment effects are likely to be additive,” reported Alex Pitcher, BMBCh, DPhil, Oxford (England) University Hospitals, NHS Trust.
Based on these data, Dr. Pitcher recommended considering ARBs and beta-blockers together soon after the diagnosis of Marfan syndrome. This includes young children.
“We think that medical treatments can delay surgery and dissection substantially if given for a number of years,” he added.
In this study, undertaken by the Marfan Treatment Trialists (MTT) collaboration, data were available from 1,442 Marfan syndrome patients participating in seven treatment trials. The primary outcome was aortic root enlargement, a predictor of life-threatening aortic dissection and rupture. Rather than a meta-analysis of the pooled data, the meta-analysis was conducted with individual patient data that involved collaboration with the original trialists.
Four of the studies with 746 patients compared ARBs to placebo or a control medication. A second group of three trials with 766 patients compared ARBs to beta-blockers.
From the two sets of data, a calculation of the effect of beta-blockers was indirectly estimated.
ARBs slow annualized aortic growth rate significantly
In the first set of trials, the analysis showed a significantly slower annualized aortic root growth rate for those treated with ARBs relative to controls (0.07 vs. 0.13), producing a statistically significant absolute difference (0.7%; P = .01) in favor of the ARB.
“In other words, the rate of growth was nearly double in the control arm,” Dr. Pitcher said.
In the three trials comparing ARBs to beta-blockers, the annualized growth rate among those taking an ARB was similar (0.8%) to that seen in the previous set of controlled trials. This rate of annualized growth was not significantly different from the 0.11% annualized rate of growth in patients receiving beta-blockers. When an analysis of the impact of beta-blockers was conducted by indirectly evaluating the change in growth relative to controls, the estimated impact was an annualized growth rate of 0.9% (P = .042).
A second set of data provided the basis for suggesting that the effects of beta ARBs and beta-blockers are independent and potentially additive.
“We were able to look at subgroups of patients in the ARB trials that were broken down by whether they were or were not on beta-blockers at baseline, and so by doing able to estimate independent effects,” Dr. Pitcher said. The lack of any interactions led Dr. Pitcher to conclude that benefits are likely additive.
Of patients genotyped in the ARB studies, more than 80% had the FBN1 pathogenic variant of Marfan syndrome. When the data were analyzed by subgroups, including age or blood pressure, there were no differences in treatment effect except for those with the FBN1 mutation in whom the benefit of ARB therapy was greater relative to those without.
As FBN1 is one of the most common genetic signatures of Marfan syndrome, the “greater effect of ARBs in this group makes it more plausible that the effect is real,” Dr. Pitcher said.
Results could change treatment guidelines
Current guidelines recommend beta-blockers in Marfan syndrome prior to a dilatation size of 4.5 to 5 cm when surgery is indicated, according to Dr. Pitcher, but he said these data might change guidelines. While reinforcing the benefit of beta-blockers, this analysis suggests ARBs should also be considered, possibly in combination with beta-blockers.
“What I hope this meta-analysis does is add substantially to the certainty with which physicians can discuss treatments with patients.”
As for the mechanism, it is reasonable to speculate the antihypertensive effect of both medications is relevant, but each has plausible independent activities that might contribute to modifying aortic growth, according to Roland R.J. van Kimmenade, MD, PhD, a specialist in aortic diseases and heart failure at Raboud University Medical Center, Nijmegan, the Netherlands.
Citing several studies, he suggested that the benefit of beta-blockers could also stem from their ability to reduce heart rate and aortic stiffness while ARBs are likely to inhibit the interaction between the renin-angiotensin system (RAS) and TGF-beta pathway. Each of these might participate in risk of aortic root growth, according to Dr. van Kimmenade, who was invited by ESC to discuss this study.
On the basis of these data as well as past studies, he agreed that the combination of beta-blockers and ARBs might not just be additive but “even a little bit synergistic.”
While Dr. Pitcher suggested that the evidence supports starting both beta-blockers and ARBs soon after the diagnosis, Dr. van Kimmenade said, “I don’t like using beta-blockers in young patients, but ARBs are now shown to be an excellent alternative.”
Ultimately, “the prescription pencil will not replace the surgical knife” in a disease that is likely to eventually require surgery to prevent life-threatening events, according to Dr. van Kimmenade, but he agreed that these data provide more certainty about the value of beta-blockers and ARBs for slowing progression.
Dr. Pitcher reports no potential conflicts of interest. Dr. van Kimmenade has financial relationships with Bayer and Novartis.
Early start might delay surgery
Early start might delay surgery
Beta-blockers have long been recommended to prevent aortic dissection associated with Marfan syndrome despite limited evidence, but a new analysis also supports a benefit from angiotensin receptors blockers (ARBs) and further suggests that beta-blockers and ARBs exert independent effects.
For the endpoint of inhibition of growth of the aortic root, “there is no evidence of any interaction between the effects of ARBs with beta-blockers, and so we think that the treatment effects are likely to be additive,” reported Alex Pitcher, BMBCh, DPhil, Oxford (England) University Hospitals, NHS Trust.
Based on these data, Dr. Pitcher recommended considering ARBs and beta-blockers together soon after the diagnosis of Marfan syndrome. This includes young children.
“We think that medical treatments can delay surgery and dissection substantially if given for a number of years,” he added.
In this study, undertaken by the Marfan Treatment Trialists (MTT) collaboration, data were available from 1,442 Marfan syndrome patients participating in seven treatment trials. The primary outcome was aortic root enlargement, a predictor of life-threatening aortic dissection and rupture. Rather than a meta-analysis of the pooled data, the meta-analysis was conducted with individual patient data that involved collaboration with the original trialists.
Four of the studies with 746 patients compared ARBs to placebo or a control medication. A second group of three trials with 766 patients compared ARBs to beta-blockers.
From the two sets of data, a calculation of the effect of beta-blockers was indirectly estimated.
ARBs slow annualized aortic growth rate significantly
In the first set of trials, the analysis showed a significantly slower annualized aortic root growth rate for those treated with ARBs relative to controls (0.07 vs. 0.13), producing a statistically significant absolute difference (0.7%; P = .01) in favor of the ARB.
“In other words, the rate of growth was nearly double in the control arm,” Dr. Pitcher said.
In the three trials comparing ARBs to beta-blockers, the annualized growth rate among those taking an ARB was similar (0.8%) to that seen in the previous set of controlled trials. This rate of annualized growth was not significantly different from the 0.11% annualized rate of growth in patients receiving beta-blockers. When an analysis of the impact of beta-blockers was conducted by indirectly evaluating the change in growth relative to controls, the estimated impact was an annualized growth rate of 0.9% (P = .042).
A second set of data provided the basis for suggesting that the effects of beta ARBs and beta-blockers are independent and potentially additive.
“We were able to look at subgroups of patients in the ARB trials that were broken down by whether they were or were not on beta-blockers at baseline, and so by doing able to estimate independent effects,” Dr. Pitcher said. The lack of any interactions led Dr. Pitcher to conclude that benefits are likely additive.
Of patients genotyped in the ARB studies, more than 80% had the FBN1 pathogenic variant of Marfan syndrome. When the data were analyzed by subgroups, including age or blood pressure, there were no differences in treatment effect except for those with the FBN1 mutation in whom the benefit of ARB therapy was greater relative to those without.
As FBN1 is one of the most common genetic signatures of Marfan syndrome, the “greater effect of ARBs in this group makes it more plausible that the effect is real,” Dr. Pitcher said.
Results could change treatment guidelines
Current guidelines recommend beta-blockers in Marfan syndrome prior to a dilatation size of 4.5 to 5 cm when surgery is indicated, according to Dr. Pitcher, but he said these data might change guidelines. While reinforcing the benefit of beta-blockers, this analysis suggests ARBs should also be considered, possibly in combination with beta-blockers.
“What I hope this meta-analysis does is add substantially to the certainty with which physicians can discuss treatments with patients.”
As for the mechanism, it is reasonable to speculate the antihypertensive effect of both medications is relevant, but each has plausible independent activities that might contribute to modifying aortic growth, according to Roland R.J. van Kimmenade, MD, PhD, a specialist in aortic diseases and heart failure at Raboud University Medical Center, Nijmegan, the Netherlands.
Citing several studies, he suggested that the benefit of beta-blockers could also stem from their ability to reduce heart rate and aortic stiffness while ARBs are likely to inhibit the interaction between the renin-angiotensin system (RAS) and TGF-beta pathway. Each of these might participate in risk of aortic root growth, according to Dr. van Kimmenade, who was invited by ESC to discuss this study.
On the basis of these data as well as past studies, he agreed that the combination of beta-blockers and ARBs might not just be additive but “even a little bit synergistic.”
While Dr. Pitcher suggested that the evidence supports starting both beta-blockers and ARBs soon after the diagnosis, Dr. van Kimmenade said, “I don’t like using beta-blockers in young patients, but ARBs are now shown to be an excellent alternative.”
Ultimately, “the prescription pencil will not replace the surgical knife” in a disease that is likely to eventually require surgery to prevent life-threatening events, according to Dr. van Kimmenade, but he agreed that these data provide more certainty about the value of beta-blockers and ARBs for slowing progression.
Dr. Pitcher reports no potential conflicts of interest. Dr. van Kimmenade has financial relationships with Bayer and Novartis.
Beta-blockers have long been recommended to prevent aortic dissection associated with Marfan syndrome despite limited evidence, but a new analysis also supports a benefit from angiotensin receptors blockers (ARBs) and further suggests that beta-blockers and ARBs exert independent effects.
For the endpoint of inhibition of growth of the aortic root, “there is no evidence of any interaction between the effects of ARBs with beta-blockers, and so we think that the treatment effects are likely to be additive,” reported Alex Pitcher, BMBCh, DPhil, Oxford (England) University Hospitals, NHS Trust.
Based on these data, Dr. Pitcher recommended considering ARBs and beta-blockers together soon after the diagnosis of Marfan syndrome. This includes young children.
“We think that medical treatments can delay surgery and dissection substantially if given for a number of years,” he added.
In this study, undertaken by the Marfan Treatment Trialists (MTT) collaboration, data were available from 1,442 Marfan syndrome patients participating in seven treatment trials. The primary outcome was aortic root enlargement, a predictor of life-threatening aortic dissection and rupture. Rather than a meta-analysis of the pooled data, the meta-analysis was conducted with individual patient data that involved collaboration with the original trialists.
Four of the studies with 746 patients compared ARBs to placebo or a control medication. A second group of three trials with 766 patients compared ARBs to beta-blockers.
From the two sets of data, a calculation of the effect of beta-blockers was indirectly estimated.
ARBs slow annualized aortic growth rate significantly
In the first set of trials, the analysis showed a significantly slower annualized aortic root growth rate for those treated with ARBs relative to controls (0.07 vs. 0.13), producing a statistically significant absolute difference (0.7%; P = .01) in favor of the ARB.
“In other words, the rate of growth was nearly double in the control arm,” Dr. Pitcher said.
In the three trials comparing ARBs to beta-blockers, the annualized growth rate among those taking an ARB was similar (0.8%) to that seen in the previous set of controlled trials. This rate of annualized growth was not significantly different from the 0.11% annualized rate of growth in patients receiving beta-blockers. When an analysis of the impact of beta-blockers was conducted by indirectly evaluating the change in growth relative to controls, the estimated impact was an annualized growth rate of 0.9% (P = .042).
A second set of data provided the basis for suggesting that the effects of beta ARBs and beta-blockers are independent and potentially additive.
“We were able to look at subgroups of patients in the ARB trials that were broken down by whether they were or were not on beta-blockers at baseline, and so by doing able to estimate independent effects,” Dr. Pitcher said. The lack of any interactions led Dr. Pitcher to conclude that benefits are likely additive.
Of patients genotyped in the ARB studies, more than 80% had the FBN1 pathogenic variant of Marfan syndrome. When the data were analyzed by subgroups, including age or blood pressure, there were no differences in treatment effect except for those with the FBN1 mutation in whom the benefit of ARB therapy was greater relative to those without.
As FBN1 is one of the most common genetic signatures of Marfan syndrome, the “greater effect of ARBs in this group makes it more plausible that the effect is real,” Dr. Pitcher said.
Results could change treatment guidelines
Current guidelines recommend beta-blockers in Marfan syndrome prior to a dilatation size of 4.5 to 5 cm when surgery is indicated, according to Dr. Pitcher, but he said these data might change guidelines. While reinforcing the benefit of beta-blockers, this analysis suggests ARBs should also be considered, possibly in combination with beta-blockers.
“What I hope this meta-analysis does is add substantially to the certainty with which physicians can discuss treatments with patients.”
As for the mechanism, it is reasonable to speculate the antihypertensive effect of both medications is relevant, but each has plausible independent activities that might contribute to modifying aortic growth, according to Roland R.J. van Kimmenade, MD, PhD, a specialist in aortic diseases and heart failure at Raboud University Medical Center, Nijmegan, the Netherlands.
Citing several studies, he suggested that the benefit of beta-blockers could also stem from their ability to reduce heart rate and aortic stiffness while ARBs are likely to inhibit the interaction between the renin-angiotensin system (RAS) and TGF-beta pathway. Each of these might participate in risk of aortic root growth, according to Dr. van Kimmenade, who was invited by ESC to discuss this study.
On the basis of these data as well as past studies, he agreed that the combination of beta-blockers and ARBs might not just be additive but “even a little bit synergistic.”
While Dr. Pitcher suggested that the evidence supports starting both beta-blockers and ARBs soon after the diagnosis, Dr. van Kimmenade said, “I don’t like using beta-blockers in young patients, but ARBs are now shown to be an excellent alternative.”
Ultimately, “the prescription pencil will not replace the surgical knife” in a disease that is likely to eventually require surgery to prevent life-threatening events, according to Dr. van Kimmenade, but he agreed that these data provide more certainty about the value of beta-blockers and ARBs for slowing progression.
Dr. Pitcher reports no potential conflicts of interest. Dr. van Kimmenade has financial relationships with Bayer and Novartis.
FROM ESC CONGRESS 2022