User login
Liver transplant outcomes improving for U.S. patients with HIV/HCV
While liver transplant outcomes were historically poor in people coinfected with HIV and hepatitis C virus (HCV), they have improved significantly in the era of direct-acting antiviral (DAA) therapy, a recent analysis of U.S. organ transplant data showed.
The availability of highly potent DAA therapy should change how transplant specialists view patients coinfected with HIV/HCV who need a liver transplant, according to researcher Jennifer Wang, MD, chief gastroenterology fellow at the University of Chicago, who presented the results of the analysis at the annual Digestive Disease Week® (DDW). Cumulative graft survival rates since the introduction of DAAs are comparable between transplant recipients with HIV/HCV coinfection and recipients who are both HIV and HCV negative, according to the study.
“Having hepatitis C no longer confers worse patient survival in the DAA era, and this is the main takeaway from our study,” Dr. Wang said.
The study also showed that the number of liver transplants among HIV-infected patients has increased over the past 4-5 years. However, the absolute number remains low at 64 cases in 2019, or less than 1% of all liver transplants that year, and only about one-third of those HIV-positive recipients had HCV coinfection, according to Dr. Wang.
Moreover, relatively few centers are performing liver transplants for patients who are HIV/HCV coinfected, and there is significant geographic variation in where the procedures are done, she said in her presentation.
Reassuring data that should prompt referral
Taken together, these results should offer reassurance to transplant centers that patients coinfected with HIV/HCV are no longer at increased risk for poor outcomes after transplantation, said Christine M. Durand, MD, associate professor of medicine at Johns Hopkins University, Baltimore.
“The additional call for action should be beyond the transplantation community to ensure that referrals for liver transplant are where they should be,” Dr. Durand said in an interview.
“With a number of only 64 transplants a year, we’re not doing enough, and there are more patients that could benefit from liver transplants,” added Dr. Durand, who is principal investigator of HOPE in Action, a prospective, multicenter, clinical trial evaluating the safety and survival outcomes of HIV-positive deceased donor liver transplants in HIV-positive recipients.
Impact of the HOPE Act
Liver transplantation for HIV-positive patients has increased since the signing of the HIV Organ Policy Equity (HOPE) Act in 2013, according to Dr. Wang.
The HOPE act expanded the donor pool to include HIV-positive deceased donors, which not only increased the donor supply overall, but specifically helped HIV-positive individuals, who experience a higher rate of waiting-list mortality, according to a review on the topic authored by Dr. Durand and coauthors.
However, some transplant centers may be reluctant to do liver transplants in HIV-positive patients coinfected with HCV. That’s because, in previous studies that were conducted before the DAA era, outcomes after liver transplant in HIV/HCV-coinfected patients were inferior to those in patients with HIV but no HCV infection, Dr. Wang said.
Accordingly, Dr. Wang and colleagues analyzed Organ Procurement and Transplantation Network (OPTN) data on adult patients who underwent liver transplants between 2008 and 2019 to see if the introduction of DAAs had leveled the playing field for those with HCV coinfection.
Progress in a still-underserved population
The practice of liver transplant in the HIV population has been increasing since the HOPE Act, according to Dr. Wang.
Overall, out of 70,125 liver transplant recipients over the 2008-2019 period, 416 (0.6%) were HIV infected, the data show.
In 2014, 28 liver transplants (0.5%) were performed in HIV-infected individuals, which increased to 64 transplants (0.8%) in 2019, data show. Of those 64 HIV-positive liver transplant recipients in 2019, 23 (35.9%) were coinfected with HCV.
Graft survival has greatly improved, from a 3-year survival of only 58% in patients transplanted before the availability of DAAs to 82% in the DAA era, a difference that was statistically significant, Dr. Wang said.
In the DAA era, there was no significant difference in graft failure outcomes when comparing HIV/HCV-coinfected recipients with uninfected recipients, she added.
The largest proportion of liver transplantations in HIV/HCV-coinfected recipients have been done in OPTN Region 9 (New York), both in the pre- and post-DAA eras, according to Dr. Wang. Several regions have very low numbers or have performed no liver transplants in HIV/HCV-coinfected patients in either era.
“The number of transplant centers participating in liver transplant for coinfected patients is still quite low, so this is a very underserved patient population,” Dr. Wang said.
Dr. Wang provided no financial disclosures related to the research. Dr. Durand receives grants to the institution from Abbvie and GlaxoSmithKline and she receives honoraria from Gilead Sciences for serving on a grant review committee.
While liver transplant outcomes were historically poor in people coinfected with HIV and hepatitis C virus (HCV), they have improved significantly in the era of direct-acting antiviral (DAA) therapy, a recent analysis of U.S. organ transplant data showed.
The availability of highly potent DAA therapy should change how transplant specialists view patients coinfected with HIV/HCV who need a liver transplant, according to researcher Jennifer Wang, MD, chief gastroenterology fellow at the University of Chicago, who presented the results of the analysis at the annual Digestive Disease Week® (DDW). Cumulative graft survival rates since the introduction of DAAs are comparable between transplant recipients with HIV/HCV coinfection and recipients who are both HIV and HCV negative, according to the study.
“Having hepatitis C no longer confers worse patient survival in the DAA era, and this is the main takeaway from our study,” Dr. Wang said.
The study also showed that the number of liver transplants among HIV-infected patients has increased over the past 4-5 years. However, the absolute number remains low at 64 cases in 2019, or less than 1% of all liver transplants that year, and only about one-third of those HIV-positive recipients had HCV coinfection, according to Dr. Wang.
Moreover, relatively few centers are performing liver transplants for patients who are HIV/HCV coinfected, and there is significant geographic variation in where the procedures are done, she said in her presentation.
Reassuring data that should prompt referral
Taken together, these results should offer reassurance to transplant centers that patients coinfected with HIV/HCV are no longer at increased risk for poor outcomes after transplantation, said Christine M. Durand, MD, associate professor of medicine at Johns Hopkins University, Baltimore.
“The additional call for action should be beyond the transplantation community to ensure that referrals for liver transplant are where they should be,” Dr. Durand said in an interview.
“With a number of only 64 transplants a year, we’re not doing enough, and there are more patients that could benefit from liver transplants,” added Dr. Durand, who is principal investigator of HOPE in Action, a prospective, multicenter, clinical trial evaluating the safety and survival outcomes of HIV-positive deceased donor liver transplants in HIV-positive recipients.
Impact of the HOPE Act
Liver transplantation for HIV-positive patients has increased since the signing of the HIV Organ Policy Equity (HOPE) Act in 2013, according to Dr. Wang.
The HOPE act expanded the donor pool to include HIV-positive deceased donors, which not only increased the donor supply overall, but specifically helped HIV-positive individuals, who experience a higher rate of waiting-list mortality, according to a review on the topic authored by Dr. Durand and coauthors.
However, some transplant centers may be reluctant to do liver transplants in HIV-positive patients coinfected with HCV. That’s because, in previous studies that were conducted before the DAA era, outcomes after liver transplant in HIV/HCV-coinfected patients were inferior to those in patients with HIV but no HCV infection, Dr. Wang said.
Accordingly, Dr. Wang and colleagues analyzed Organ Procurement and Transplantation Network (OPTN) data on adult patients who underwent liver transplants between 2008 and 2019 to see if the introduction of DAAs had leveled the playing field for those with HCV coinfection.
Progress in a still-underserved population
The practice of liver transplant in the HIV population has been increasing since the HOPE Act, according to Dr. Wang.
Overall, out of 70,125 liver transplant recipients over the 2008-2019 period, 416 (0.6%) were HIV infected, the data show.
In 2014, 28 liver transplants (0.5%) were performed in HIV-infected individuals, which increased to 64 transplants (0.8%) in 2019, data show. Of those 64 HIV-positive liver transplant recipients in 2019, 23 (35.9%) were coinfected with HCV.
Graft survival has greatly improved, from a 3-year survival of only 58% in patients transplanted before the availability of DAAs to 82% in the DAA era, a difference that was statistically significant, Dr. Wang said.
In the DAA era, there was no significant difference in graft failure outcomes when comparing HIV/HCV-coinfected recipients with uninfected recipients, she added.
The largest proportion of liver transplantations in HIV/HCV-coinfected recipients have been done in OPTN Region 9 (New York), both in the pre- and post-DAA eras, according to Dr. Wang. Several regions have very low numbers or have performed no liver transplants in HIV/HCV-coinfected patients in either era.
“The number of transplant centers participating in liver transplant for coinfected patients is still quite low, so this is a very underserved patient population,” Dr. Wang said.
Dr. Wang provided no financial disclosures related to the research. Dr. Durand receives grants to the institution from Abbvie and GlaxoSmithKline and she receives honoraria from Gilead Sciences for serving on a grant review committee.
While liver transplant outcomes were historically poor in people coinfected with HIV and hepatitis C virus (HCV), they have improved significantly in the era of direct-acting antiviral (DAA) therapy, a recent analysis of U.S. organ transplant data showed.
The availability of highly potent DAA therapy should change how transplant specialists view patients coinfected with HIV/HCV who need a liver transplant, according to researcher Jennifer Wang, MD, chief gastroenterology fellow at the University of Chicago, who presented the results of the analysis at the annual Digestive Disease Week® (DDW). Cumulative graft survival rates since the introduction of DAAs are comparable between transplant recipients with HIV/HCV coinfection and recipients who are both HIV and HCV negative, according to the study.
“Having hepatitis C no longer confers worse patient survival in the DAA era, and this is the main takeaway from our study,” Dr. Wang said.
The study also showed that the number of liver transplants among HIV-infected patients has increased over the past 4-5 years. However, the absolute number remains low at 64 cases in 2019, or less than 1% of all liver transplants that year, and only about one-third of those HIV-positive recipients had HCV coinfection, according to Dr. Wang.
Moreover, relatively few centers are performing liver transplants for patients who are HIV/HCV coinfected, and there is significant geographic variation in where the procedures are done, she said in her presentation.
Reassuring data that should prompt referral
Taken together, these results should offer reassurance to transplant centers that patients coinfected with HIV/HCV are no longer at increased risk for poor outcomes after transplantation, said Christine M. Durand, MD, associate professor of medicine at Johns Hopkins University, Baltimore.
“The additional call for action should be beyond the transplantation community to ensure that referrals for liver transplant are where they should be,” Dr. Durand said in an interview.
“With a number of only 64 transplants a year, we’re not doing enough, and there are more patients that could benefit from liver transplants,” added Dr. Durand, who is principal investigator of HOPE in Action, a prospective, multicenter, clinical trial evaluating the safety and survival outcomes of HIV-positive deceased donor liver transplants in HIV-positive recipients.
Impact of the HOPE Act
Liver transplantation for HIV-positive patients has increased since the signing of the HIV Organ Policy Equity (HOPE) Act in 2013, according to Dr. Wang.
The HOPE act expanded the donor pool to include HIV-positive deceased donors, which not only increased the donor supply overall, but specifically helped HIV-positive individuals, who experience a higher rate of waiting-list mortality, according to a review on the topic authored by Dr. Durand and coauthors.
However, some transplant centers may be reluctant to do liver transplants in HIV-positive patients coinfected with HCV. That’s because, in previous studies that were conducted before the DAA era, outcomes after liver transplant in HIV/HCV-coinfected patients were inferior to those in patients with HIV but no HCV infection, Dr. Wang said.
Accordingly, Dr. Wang and colleagues analyzed Organ Procurement and Transplantation Network (OPTN) data on adult patients who underwent liver transplants between 2008 and 2019 to see if the introduction of DAAs had leveled the playing field for those with HCV coinfection.
Progress in a still-underserved population
The practice of liver transplant in the HIV population has been increasing since the HOPE Act, according to Dr. Wang.
Overall, out of 70,125 liver transplant recipients over the 2008-2019 period, 416 (0.6%) were HIV infected, the data show.
In 2014, 28 liver transplants (0.5%) were performed in HIV-infected individuals, which increased to 64 transplants (0.8%) in 2019, data show. Of those 64 HIV-positive liver transplant recipients in 2019, 23 (35.9%) were coinfected with HCV.
Graft survival has greatly improved, from a 3-year survival of only 58% in patients transplanted before the availability of DAAs to 82% in the DAA era, a difference that was statistically significant, Dr. Wang said.
In the DAA era, there was no significant difference in graft failure outcomes when comparing HIV/HCV-coinfected recipients with uninfected recipients, she added.
The largest proportion of liver transplantations in HIV/HCV-coinfected recipients have been done in OPTN Region 9 (New York), both in the pre- and post-DAA eras, according to Dr. Wang. Several regions have very low numbers or have performed no liver transplants in HIV/HCV-coinfected patients in either era.
“The number of transplant centers participating in liver transplant for coinfected patients is still quite low, so this is a very underserved patient population,” Dr. Wang said.
Dr. Wang provided no financial disclosures related to the research. Dr. Durand receives grants to the institution from Abbvie and GlaxoSmithKline and she receives honoraria from Gilead Sciences for serving on a grant review committee.
FROM DDW 2021
Reducing False-Positive Results With Fourth-Generation HIV Testing at a Veterans Affairs Medical Center
Ever since the first clinical reports of patients with AIDS in 1981, there have been improvements both in the knowledge base of the pathogenesis of HIV in causing AIDS as well as a progressive refinement in the test methodologies used to diagnose this illness.1-3 Given that there are both public health and clinical benefits in earlier diagnosis and treatment of patients with available antiretroviral therapies, universal screening with opt-out consent has been a standard of practice recommendation by the Centers of Disease Control and Prevention (CDC) since 2006; universal screening with opt-out consent also has been recommended by the US Preventative Task Force and has been widely implemented.4-7
HIV Screening
While HIV screening assays have evolved to be accurate with very high sensitivities and specificities, false-positive results are a significant issue both currently and historically.8-16 The use of an HIV assay on a low prevalence population predictably reduces the positive predictive value (PPV) of even an otherwise accurate assay.8-23 In light of this, laboratory HIV testing algorithms include confirmatory testing to increase the likelihood that the correct diagnosis is being rendered.
The fourth-generation assay has been shown to be more sensitive and specific compared with that of the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 Due to these improvements, in the general population, increased sensitivity/specificity with a reduction in both false positives and false negatives have been reported.
It has been observed in the nonveteran population that switching from the older third-generation to a more sensitive and specific fourth-generation HIV screening assay has reduced the false-positive screening rate.18,19,22 For instance, Muthukumar and colleagues demonstrated a false-positive rate of only 2 out of 99 (2%) tested specimens for the fourth-generation ARCHITECT HIV Ag/Ab Combo assay vs 9 out of 99 tested specimens (9%) for the third-generation ADVIA Centaur HIV 1/O/2 Enhanced assay.18 In addition, it has been noted that fourth-generation HIV screening assays can reduce the window period by detecting HIV infection sooner after initial acute infection.19 Mitchell and colleagues demonstrated even highly specific fourth-generation HIV assays with specificities estimated at 99.7% can have PPVs as low as 25.0% if used in a population of low HIV prevalence (such as a 0.1% prevalence population).19 However, the veteran population has been documented to differ significantly on a number of population variables, including severity of disease and susceptibility to infections, and as a result extrapolation of these data from the general population may be limited.24-26 To our knowledge, this article represents the first study directly examining the reduction in false-positive results with the switch to a fourth-generation HIV generation assay from a third-generation assay for the veteran patient population at a regional US Department of Veterans Affairs (VA) medical center (VAMC).8,11
Methods
Quality assurance documents on test volume were retrospectively reviewed to obtain the number of HIV screening tests that were performed by the laboratory at the Corporal Michael J. Crescenz VAMC (CMJCVAMC) in Philadelphia, Pennsylvania, between March 1, 2016 and February 28, 2017, prior to implementation of the fourth-generation assay. The study also include results from the first year of use of the fourth-generation assay (March 1, 2017 to February 28, 2018). In addition, paper quality assurance records of all positive screening results during those periods were reviewed and manually counted for the abstract presentation of these data.
For assurance of accuracy, a search of all HIV testing assays using Veterans Health Information Systems and Technology Architecture and FileMan also was performed, and the results were compared to records in the Computerized Patient Record System (CPRS). Any discrepancies in the numbers of test results generated by both searches were investigated, and data for the manuscript were derived from records associating tests with particular patients. Only results from patient samples were considered for the electronic search. Quality samples that did not correspond to a true patient as identified in CPRS or same time patient sample duplicates were excluded from the calculations. Basic demographic data (age, ethnicity, and gender) were obtained from this FileMan search. The third-generation assay was the Ortho-Clinical Diagnostics Vitros, and the fourth-generation assay was the Abbott Architect.
To interpret the true HIV result of each sample with a reactive or positive screening result, the CDC laboratory HIV testing algorithm was followed and reviewed with a clinical pathologist or microbiologist director.12,13 All specimens interpreted as HIV positive by the pathologist or microbiologist director were discussed with the clinical health care provider at the time of the test with results added to CPRS after all testing was complete and discussions had taken place. All initially reactive specimens (confirmed with retesting in duplicate on the screening platform with at least 1 repeat reactive result) were further tested with the Bio-Rad Geenius HIV 1/2 Supplemental Assay, which screens for both HIV-1 and HIV-2 antibodies. Specimens with reactive results by this supplemental assay were interpreted as positive for HIV based on the CDC laboratory HIV testing algorithm. Specimens with negative or indeterminant results by the supplemental assay then underwent HIV-1 nucleic acid testing (NAT) using the Roche Diagnostics COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0. Specimens with viral load detected on NAT were positive for HIV infection, while specimens with viral load not detected on NAT testing were interpreted as negative for HIV-1 infection. Although there were no HIV-2 positive or indeterminant specimens during the study period, HIV-2 reactivity also would have been interpreted per the CDC laboratory HIV testing algorithm. Specimens with inadequate volume to complete all testing steps would be interpreted as indeterminant for HIV with request for additional specimen to complete testing. All testing platforms used for HIV testing in the laboratory had been properly validated prior to use.
The number of false positives and indeterminant results was tabulated in Microsoft Excel by month throughout the study period alongside the total number of HIV screening tests performed. Statistical analyses to verify statistical significance was performed by 1-tailed homoscedastic t test calculation using Excel.
Results
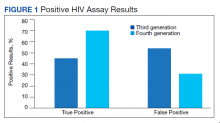
From March 1, 2016 to February 28, 2017, 7,516 specimens were screened for HIV, using the third-generation assay, and 52 specimens tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 24 tests were true positive and 28 were false positives with a PPV of 46% (24/52) (Figure 1).
From March 1, 2017 to February 28, 2018, 7,802 specimens were screened for HIV using a fourth-generation assay and 23 tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 16 were true positive and 7 were false positives with a PPV of 70% (16/23).
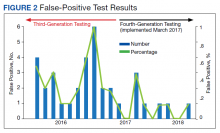
The fourth-generation assay was more specific when compared with the third-generation assay (0.09% vs 0.37%, respectively) with a 75.7% decrease in the false-positivity rate after the implementation of fourth-generation testing. The decreased number of false-positive test results per month with the fourth-generation test implementation was statistically significant (P = .002). The mean (SD) number of false-positive test results for the third-generation assay was 2.3 (1.7) per month, while the fourth-generation assay only had a mean (SD) of 0.58 (0.9) false positives monthly. The decrease in the percentage of false positives per month with the implementation of the fourth-generation assay also was statistically significant (P = .002) (Figure 2).
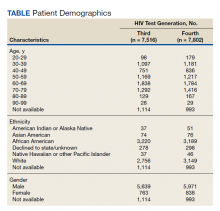
For population-based reference of the tested population at CMJCVAMC, there was a FileMan search for basic demographic data of patients for the HIV specimens screened by the third- or fourth-generation test (Table). For the population tested by the third-generation assay, 1,114 out of the 7,516 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For 6,402 of 7,516 patients tested by the third-generation assay with demographic information, the age ranged from 25 to 97 years with a mean of 57 years. This population of 6,402 was 88% male (n = 5,639), 50% African American (n = 3,220) and 43% White (n = 2,756). For the population tested by the fourth-generation assay, 993 of 7,802 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For the 6,809 of 7,802 patients tested by the fourth-generation assay with demographic information, the age ranged from 24 to 97 years with a mean age of 56 years. This population was 88% male (n = 5,971), 47% African American (n = 3,189), and 46% White (n = 3,149).
Discussion
Current practice guidelines from the CDC and the US Preventive Services Task Force recommend universal screening of the population for HIV infection.5,6 As the general population to be screened would normally have a low prevalence of HIV infection, the risk of a false positive on the initial screen is significant.17 Indeed, the CMJCVAMC experience has been that with the third-generation screening assay, the number of false-positive test results outnumbered the number of true-positive test results. Even with the fourth-generation assay, approximately one-third of the results were false positives. These results are similar to those observed in studies involving nonveteran populations in which the implementation of a fourth-generation screening assay led to significantly fewer false-positive results.18
For laboratories that do not follows CDC testing algorithm guidelines, each false-positive screening result represents a potential opportunity for a HIV misdiagnosis.Even in laboratories with proper procedures in place, false-positive results have consequences for the patients and for the cost-effectiveness of laboratory operations.9-11,18 As per CDC HIV testing guidelines, all positive screening results should be retested, which leads to additional use of technologist time and reagents. After this additional testing is performed and reviewed appropriately, only then can an appropriate final laboratory diagnosis be rendered that meets the standard of laboratory care.
Cost Savings
As observed at CMJCVAMC, the use of a fourth-generation assay with increased sensitivity/specificity led to a reduction in these false-positive results, which improved laboratory efficiency and avoided wasted resources for confirmatory tests.11,18 Cost savings at CMJCVAMC from the implementation of the fourth-generation assay would include technologist time and reagent cost. Generalizable technologist time costs at any institution would include the time needed to perform the confirmatory HIV-1/HIV-2 antibody differentiation assay (slightly less than 1 hour at CMJCVAMC per specimen) and the time needed to perform the viral load assay (about 6 hours to run a batch of 24 tests at CMJCVAMC). We calculated that confirmatory testing cost $184.51 per test at CMJCVAMC. Replacing the third-generation assay with the more sensitive and specific fourth-generation test saved an estimated $3,875 annually. This cost savings does not even consider savings in the pathologist/director’s time for reviewing HIV results after the completion of the algorithm or the clinician/patient costs or anxiety while waiting for results of the confirmatory sequence of tests.
As diagnosis of HIV can have a significant psychological impact on the patient, it is important to ensure the diagnosis conveyed is correct.27 The provision of an HIV diagnosis to a patient has been described as a traumatic stressor capable of causing psychological harm; this harm should ideally be avoided if the HIV diagnosis is not accurate. There can be a temptation, when presented with a positive or reactive screening test that is known to come from an instrument or assay with a very high sensitivity and specificity, to present this result as a diagnosis to the patient. However, a false diagnosis from a false-positive screen would not only be harmful, but given the low prevalence of the disease in the screened population, would happen fairly frequently; in some settings the number of false positives may actually outnumber the number of true positive test results.
Better screening assays with greater specificity (even fractions of a percentage, given that specificities are already > 99%) would help reduce the number of false positives and reduce the number of potential enticements to convey an incorrect diagnosis. Therefore, by adding an additional layer of safety through greater specificity, the fourth-generation assay implementation helped improve the diagnostic safety of the laboratory and reduced the significant error risk to the clinician who would ultimately bear responsibility for conveying the HIV diagnoses to the patient. Given the increased prevalence of psychological and physical ailments in veterans, it may be even more important to ensure the diagnosis is correct to avoid increased psychological harm.27,28
Veteran Population
For the general population, the fourth-generation assay has been shown to be more sensitive and specific when compared with the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 However, the veteran population that receives VA medical care differs significantly from the nonveteran general population. Compared with nonveterans, veterans tend to have generally poorer health status, more comorbid conditions, and greater need to use medical resources.24-26 In addition, veterans also may differ in sociodemographic status, race, ethnicity, and gender.24-26
VA research in the veteran population is unique, and veterans who use VA health care services are an even more highly selected subpopulation.26 Conclusions made from studies of the general population may not always be applicable to the veteran population treated by VA health care services due to these population differences. Therefore, specific studies tailored to this special veteran population in the specific VA health care setting are essential to ensure that the results of the general population truly and definitively apply to the veteran population.
While the false-positive risk is most closely associated with testing in a population of low prevalence, it also should be noted that false-positive screening results also can occur in high-risk individuals, such as an individual on preexposure prophylaxis (PrEP) for continuous behavior that places the individual at high risk of HIV acquisition.8,29 The false-positive result in these cases can lead to a conundrum for the clinician, and the differential diagnosis should consider both detection of very early infection as well as false positive. Interventions could include either stopping PrEP and treating for presumed early primary infection with HIV or continuing the PrEP. These interventions all have the potential to impact the patient whether through the production of resistant HIV virus due to the inadvertent provision of an inadequate treatment regimen, increased risk of infection if taken off PrEP as the patient may likely continue the behavior regardless, or the risks carried by the administration of additional antiretroviral therapies for the complete empiric therapy. Cases of an individual on PrEP who had a false-positive HIV screening test has been reported previously both within and outside the veteran population.8 Better screening tests with greater sensitivity/specificity can only help in guiding better patient care.
Limitations
This quality assurance study was limited to retrospectively identifying the improvement in the false-positive rate on the transition from the third-generation to the more advanced fourth-generation HIV screen. False-positive screen cases could be easily picked up on review of the confirmatory testing per the CDC laboratory HIV testing algorithm.12,13 This study also was a retrospective review of clinically ordered and indicated testing; as a result, without confirmatory testing performed on all negative screen cases, a false-negative rate would not be calculable.
This study also was restricted to only the population being treated in a VA health care setting. This population is known to be different from the general population.24-26
Conclusions
The switch to a fourth-generation assay resulted in a significant reduction in false-positive test results for veteran patients at CMJCVAMC. This reduction in false-positive screening not only reduced laboratory workload due to the necessary confirmatory testing and subsequent review, but also saved costs for technologist’s time and reagents. While this reduction in false-positive results has been documented in nonveteran populations, this is the first study specifically on a veteran population treated at a VAMC.8,11,18 This study confirms previously documented findings of improvement in the false-positive rate of HIV screening tests with the change from third-generation to fourth-generation assay for a veteran population.24
1. Feinberg MB. Changing the natural history of HIV disease. Lancet. 1996;348(9022):239-246. doi:10.1016/s0140-6736(96)06231-9.
2. Alexander TS. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol. 2016;23(4):249-253. Published 2016 Apr 4. doi:10.1128/CVI.00053-16
3. Mortimer PP, Parry JV, Mortimer JY. Which anti-HTLV III/LAV assays for screening and confirmatory testing?. Lancet. 1985;2(8460):873-877. doi:10.1016/s0140-6736(85)90136-9
4. Holmberg SD, Palella FJ Jr, Lichtenstein KA, Havlir DV. The case for earlier treatment of HIV infection [published correction appears in Clin Infect Dis. 2004 Dec 15;39(12):1869]. Clin Infect Dis. 2004;39(11):1699-1704. doi:10.1086/425743
5. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(23):2326-2336. doi:10.1001/jama.2019.6587
6. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-CE4.
7. Bayer R, Philbin M, Remien RH. The end of written informed consent for HIV testing: not with a bang but a whimper. Am J Public Health. 2017;107(8):1259-1265. doi:10.2105/AJPH.2017.303819
8. Petersen J, Jhala D. Its not HIV! The pitfall of unconfirmed positive HIV screening assays. Abstract presented at: Annual Meeting Pennsylvania Association of Pathologists; April 14, 2018.
9. Wood RW, Dunphy C, Okita K, Swenson P. Two “HIV-infected” persons not really infected. Arch Intern Med. 2003;163(15):1857-1859. doi:10.1001/archinte.163.15.1857
10. Permpalung N, Ungprasert P, Chongnarungsin D, Okoli A, Hyman CL. A diagnostic blind spot: acute infectious mononucleosis or acute retroviral syndrome. Am J Med. 2013;126(9):e5-e6. doi:10.1016/j.amjmed.2013.03.017
11. Dalal S, Petersen J, Luta D, Jhala D. Third- to fourth-generation HIV testing: reduction in false-positive results with the new way of testing, the Corporal Michael J. Crescenz Veteran Affairs Medical Center (CMCVAMC) Experience. Am J Clin Pathol.2018;150(suppl 1):S70-S71. doi:10.1093/ajcp/aqy093.172
12. Centers for Disease Control and Prevention. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Published June 27, 2014. Accessed April 14, 2021. doi:10.15620/cdc.23447
13. Centers for Disease Control and Prevention. 2018 quick reference guide: recommended laboratory HIV testing algorithm for serum or plasma specimens. Updated January 2018. Accessed April 14, 202. https://stacks.cdc.gov/view/cdc/50872
14. Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(suppl 1):S17-S22. doi:10.1016/j.jcv.2011.09.011
15. Morton A. When lab tests lie … heterophile antibodies. Aust Fam Physician. 2014;43(6):391-393.
16. Spencer DV, Nolte FS, Zhu Y. Heterophilic antibody interference causing false-positive rapid human immunodeficiency virus antibody testing. Clin Chim Acta. 2009;399(1-2):121-122. doi:10.1016/j.cca.2008.09.030
17. Kim S, Lee JH, Choi JY, Kim JM, Kim HS. False-positive rate of a “fourth-generation” HIV antigen/antibody combination assay in an area of low HIV prevalence. Clin Vaccine Immunol. 2010;17(10):1642-1644. doi:10.1128/CVI.00258-10
18. Muthukumar A, Alatoom A, Burns S, et al. Comparison of 4th-generation HIV antigen/antibody combination assay with 3rd-generation HIV antibody assays for the occurrence of false-positive and false-negative results. Lab Med. 2015;46(2):84-e29. doi:10.1309/LMM3X37NSWUCMVRS
19. Mitchell EO, Stewart G, Bajzik O, Ferret M, Bentsen C, Shriver MK. Performance comparison of the 4th generation Bio-Rad Laboratories GS HIV Combo Ag/Ab EIA on the EVOLIS™ automated system versus Abbott ARCHITECT HIV Ag/Ab Combo, Ortho Anti-HIV 1+2 EIA on Vitros ECi and Siemens HIV-1/O/2 enhanced on Advia Centaur. J Clin Virol. 2013;58(suppl 1):e79-e84. doi:10.1016/j.jcv.2013.08.009
20. Dubravac T, Gahan TF, Pentella MA. Use of the Abbott Architect HIV antigen/antibody assay in a low incidence population. J Clin Virol. 2013;58(suppl 1):e76-e78. doi:10.1016/j.jcv.2013.10.020
21. Montesinos I, Eykmans J, Delforge ML. Evaluation of the Bio-Rad Geenius HIV-1/2 test as a confirmatory assay. J Clin Virol. 2014;60(4):399-401. doi:10.1016/j.jcv.2014.04.025
22. van Binsbergen J, Siebelink A, Jacobs A, et al. Improved performance of seroconversion with a 4th generation HIV antigen/antibody assay. J Virol Methods. 1999;82(1):77-84. doi:10.1016/s0166-0934(99)00086-5
23. CLSI. User Protocol for Evaluation of Qualitative Test Performance: Approved Guideline. Second ed. EP12-A2. CLSI; 2008:1-46.
24. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
25. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
26. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x
27. Nightingale VR, Sher TG, Hansen NB. The impact of receiving an HIV diagnosis and cognitive processing on psychological distress and posttraumatic growth. J Trauma Stress. 2010;23(4):452-460. doi:10.1002/jts.20554
28. Spelman JF, Hunt SC, Seal KH, Burgo-Black AL. Post deployment care for returning combat veterans. J Gen Intern Med. 2012;27(9):1200-1209. doi:10.1007/s11606-012-2061-1
29. Ndase P, Celum C, Kidoguchi L, et al. Frequency of false positive rapid HIV serologic tests in African men and women receiving PrEP for HIV prevention: implications for programmatic roll-out of biomedical interventions. PLoS One. 2015;10(4):e0123005. Published 2015 Apr 17. doi:10.1371/journal.pone.0123005
Ever since the first clinical reports of patients with AIDS in 1981, there have been improvements both in the knowledge base of the pathogenesis of HIV in causing AIDS as well as a progressive refinement in the test methodologies used to diagnose this illness.1-3 Given that there are both public health and clinical benefits in earlier diagnosis and treatment of patients with available antiretroviral therapies, universal screening with opt-out consent has been a standard of practice recommendation by the Centers of Disease Control and Prevention (CDC) since 2006; universal screening with opt-out consent also has been recommended by the US Preventative Task Force and has been widely implemented.4-7
HIV Screening
While HIV screening assays have evolved to be accurate with very high sensitivities and specificities, false-positive results are a significant issue both currently and historically.8-16 The use of an HIV assay on a low prevalence population predictably reduces the positive predictive value (PPV) of even an otherwise accurate assay.8-23 In light of this, laboratory HIV testing algorithms include confirmatory testing to increase the likelihood that the correct diagnosis is being rendered.
The fourth-generation assay has been shown to be more sensitive and specific compared with that of the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 Due to these improvements, in the general population, increased sensitivity/specificity with a reduction in both false positives and false negatives have been reported.
It has been observed in the nonveteran population that switching from the older third-generation to a more sensitive and specific fourth-generation HIV screening assay has reduced the false-positive screening rate.18,19,22 For instance, Muthukumar and colleagues demonstrated a false-positive rate of only 2 out of 99 (2%) tested specimens for the fourth-generation ARCHITECT HIV Ag/Ab Combo assay vs 9 out of 99 tested specimens (9%) for the third-generation ADVIA Centaur HIV 1/O/2 Enhanced assay.18 In addition, it has been noted that fourth-generation HIV screening assays can reduce the window period by detecting HIV infection sooner after initial acute infection.19 Mitchell and colleagues demonstrated even highly specific fourth-generation HIV assays with specificities estimated at 99.7% can have PPVs as low as 25.0% if used in a population of low HIV prevalence (such as a 0.1% prevalence population).19 However, the veteran population has been documented to differ significantly on a number of population variables, including severity of disease and susceptibility to infections, and as a result extrapolation of these data from the general population may be limited.24-26 To our knowledge, this article represents the first study directly examining the reduction in false-positive results with the switch to a fourth-generation HIV generation assay from a third-generation assay for the veteran patient population at a regional US Department of Veterans Affairs (VA) medical center (VAMC).8,11
Methods
Quality assurance documents on test volume were retrospectively reviewed to obtain the number of HIV screening tests that were performed by the laboratory at the Corporal Michael J. Crescenz VAMC (CMJCVAMC) in Philadelphia, Pennsylvania, between March 1, 2016 and February 28, 2017, prior to implementation of the fourth-generation assay. The study also include results from the first year of use of the fourth-generation assay (March 1, 2017 to February 28, 2018). In addition, paper quality assurance records of all positive screening results during those periods were reviewed and manually counted for the abstract presentation of these data.
For assurance of accuracy, a search of all HIV testing assays using Veterans Health Information Systems and Technology Architecture and FileMan also was performed, and the results were compared to records in the Computerized Patient Record System (CPRS). Any discrepancies in the numbers of test results generated by both searches were investigated, and data for the manuscript were derived from records associating tests with particular patients. Only results from patient samples were considered for the electronic search. Quality samples that did not correspond to a true patient as identified in CPRS or same time patient sample duplicates were excluded from the calculations. Basic demographic data (age, ethnicity, and gender) were obtained from this FileMan search. The third-generation assay was the Ortho-Clinical Diagnostics Vitros, and the fourth-generation assay was the Abbott Architect.
To interpret the true HIV result of each sample with a reactive or positive screening result, the CDC laboratory HIV testing algorithm was followed and reviewed with a clinical pathologist or microbiologist director.12,13 All specimens interpreted as HIV positive by the pathologist or microbiologist director were discussed with the clinical health care provider at the time of the test with results added to CPRS after all testing was complete and discussions had taken place. All initially reactive specimens (confirmed with retesting in duplicate on the screening platform with at least 1 repeat reactive result) were further tested with the Bio-Rad Geenius HIV 1/2 Supplemental Assay, which screens for both HIV-1 and HIV-2 antibodies. Specimens with reactive results by this supplemental assay were interpreted as positive for HIV based on the CDC laboratory HIV testing algorithm. Specimens with negative or indeterminant results by the supplemental assay then underwent HIV-1 nucleic acid testing (NAT) using the Roche Diagnostics COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0. Specimens with viral load detected on NAT were positive for HIV infection, while specimens with viral load not detected on NAT testing were interpreted as negative for HIV-1 infection. Although there were no HIV-2 positive or indeterminant specimens during the study period, HIV-2 reactivity also would have been interpreted per the CDC laboratory HIV testing algorithm. Specimens with inadequate volume to complete all testing steps would be interpreted as indeterminant for HIV with request for additional specimen to complete testing. All testing platforms used for HIV testing in the laboratory had been properly validated prior to use.
The number of false positives and indeterminant results was tabulated in Microsoft Excel by month throughout the study period alongside the total number of HIV screening tests performed. Statistical analyses to verify statistical significance was performed by 1-tailed homoscedastic t test calculation using Excel.
Results
From March 1, 2016 to February 28, 2017, 7,516 specimens were screened for HIV, using the third-generation assay, and 52 specimens tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 24 tests were true positive and 28 were false positives with a PPV of 46% (24/52) (Figure 1).
From March 1, 2017 to February 28, 2018, 7,802 specimens were screened for HIV using a fourth-generation assay and 23 tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 16 were true positive and 7 were false positives with a PPV of 70% (16/23).
The fourth-generation assay was more specific when compared with the third-generation assay (0.09% vs 0.37%, respectively) with a 75.7% decrease in the false-positivity rate after the implementation of fourth-generation testing. The decreased number of false-positive test results per month with the fourth-generation test implementation was statistically significant (P = .002). The mean (SD) number of false-positive test results for the third-generation assay was 2.3 (1.7) per month, while the fourth-generation assay only had a mean (SD) of 0.58 (0.9) false positives monthly. The decrease in the percentage of false positives per month with the implementation of the fourth-generation assay also was statistically significant (P = .002) (Figure 2).
For population-based reference of the tested population at CMJCVAMC, there was a FileMan search for basic demographic data of patients for the HIV specimens screened by the third- or fourth-generation test (Table). For the population tested by the third-generation assay, 1,114 out of the 7,516 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For 6,402 of 7,516 patients tested by the third-generation assay with demographic information, the age ranged from 25 to 97 years with a mean of 57 years. This population of 6,402 was 88% male (n = 5,639), 50% African American (n = 3,220) and 43% White (n = 2,756). For the population tested by the fourth-generation assay, 993 of 7,802 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For the 6,809 of 7,802 patients tested by the fourth-generation assay with demographic information, the age ranged from 24 to 97 years with a mean age of 56 years. This population was 88% male (n = 5,971), 47% African American (n = 3,189), and 46% White (n = 3,149).
Discussion
Current practice guidelines from the CDC and the US Preventive Services Task Force recommend universal screening of the population for HIV infection.5,6 As the general population to be screened would normally have a low prevalence of HIV infection, the risk of a false positive on the initial screen is significant.17 Indeed, the CMJCVAMC experience has been that with the third-generation screening assay, the number of false-positive test results outnumbered the number of true-positive test results. Even with the fourth-generation assay, approximately one-third of the results were false positives. These results are similar to those observed in studies involving nonveteran populations in which the implementation of a fourth-generation screening assay led to significantly fewer false-positive results.18
For laboratories that do not follows CDC testing algorithm guidelines, each false-positive screening result represents a potential opportunity for a HIV misdiagnosis.Even in laboratories with proper procedures in place, false-positive results have consequences for the patients and for the cost-effectiveness of laboratory operations.9-11,18 As per CDC HIV testing guidelines, all positive screening results should be retested, which leads to additional use of technologist time and reagents. After this additional testing is performed and reviewed appropriately, only then can an appropriate final laboratory diagnosis be rendered that meets the standard of laboratory care.
Cost Savings
As observed at CMJCVAMC, the use of a fourth-generation assay with increased sensitivity/specificity led to a reduction in these false-positive results, which improved laboratory efficiency and avoided wasted resources for confirmatory tests.11,18 Cost savings at CMJCVAMC from the implementation of the fourth-generation assay would include technologist time and reagent cost. Generalizable technologist time costs at any institution would include the time needed to perform the confirmatory HIV-1/HIV-2 antibody differentiation assay (slightly less than 1 hour at CMJCVAMC per specimen) and the time needed to perform the viral load assay (about 6 hours to run a batch of 24 tests at CMJCVAMC). We calculated that confirmatory testing cost $184.51 per test at CMJCVAMC. Replacing the third-generation assay with the more sensitive and specific fourth-generation test saved an estimated $3,875 annually. This cost savings does not even consider savings in the pathologist/director’s time for reviewing HIV results after the completion of the algorithm or the clinician/patient costs or anxiety while waiting for results of the confirmatory sequence of tests.
As diagnosis of HIV can have a significant psychological impact on the patient, it is important to ensure the diagnosis conveyed is correct.27 The provision of an HIV diagnosis to a patient has been described as a traumatic stressor capable of causing psychological harm; this harm should ideally be avoided if the HIV diagnosis is not accurate. There can be a temptation, when presented with a positive or reactive screening test that is known to come from an instrument or assay with a very high sensitivity and specificity, to present this result as a diagnosis to the patient. However, a false diagnosis from a false-positive screen would not only be harmful, but given the low prevalence of the disease in the screened population, would happen fairly frequently; in some settings the number of false positives may actually outnumber the number of true positive test results.
Better screening assays with greater specificity (even fractions of a percentage, given that specificities are already > 99%) would help reduce the number of false positives and reduce the number of potential enticements to convey an incorrect diagnosis. Therefore, by adding an additional layer of safety through greater specificity, the fourth-generation assay implementation helped improve the diagnostic safety of the laboratory and reduced the significant error risk to the clinician who would ultimately bear responsibility for conveying the HIV diagnoses to the patient. Given the increased prevalence of psychological and physical ailments in veterans, it may be even more important to ensure the diagnosis is correct to avoid increased psychological harm.27,28
Veteran Population
For the general population, the fourth-generation assay has been shown to be more sensitive and specific when compared with the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 However, the veteran population that receives VA medical care differs significantly from the nonveteran general population. Compared with nonveterans, veterans tend to have generally poorer health status, more comorbid conditions, and greater need to use medical resources.24-26 In addition, veterans also may differ in sociodemographic status, race, ethnicity, and gender.24-26
VA research in the veteran population is unique, and veterans who use VA health care services are an even more highly selected subpopulation.26 Conclusions made from studies of the general population may not always be applicable to the veteran population treated by VA health care services due to these population differences. Therefore, specific studies tailored to this special veteran population in the specific VA health care setting are essential to ensure that the results of the general population truly and definitively apply to the veteran population.
While the false-positive risk is most closely associated with testing in a population of low prevalence, it also should be noted that false-positive screening results also can occur in high-risk individuals, such as an individual on preexposure prophylaxis (PrEP) for continuous behavior that places the individual at high risk of HIV acquisition.8,29 The false-positive result in these cases can lead to a conundrum for the clinician, and the differential diagnosis should consider both detection of very early infection as well as false positive. Interventions could include either stopping PrEP and treating for presumed early primary infection with HIV or continuing the PrEP. These interventions all have the potential to impact the patient whether through the production of resistant HIV virus due to the inadvertent provision of an inadequate treatment regimen, increased risk of infection if taken off PrEP as the patient may likely continue the behavior regardless, or the risks carried by the administration of additional antiretroviral therapies for the complete empiric therapy. Cases of an individual on PrEP who had a false-positive HIV screening test has been reported previously both within and outside the veteran population.8 Better screening tests with greater sensitivity/specificity can only help in guiding better patient care.
Limitations
This quality assurance study was limited to retrospectively identifying the improvement in the false-positive rate on the transition from the third-generation to the more advanced fourth-generation HIV screen. False-positive screen cases could be easily picked up on review of the confirmatory testing per the CDC laboratory HIV testing algorithm.12,13 This study also was a retrospective review of clinically ordered and indicated testing; as a result, without confirmatory testing performed on all negative screen cases, a false-negative rate would not be calculable.
This study also was restricted to only the population being treated in a VA health care setting. This population is known to be different from the general population.24-26
Conclusions
The switch to a fourth-generation assay resulted in a significant reduction in false-positive test results for veteran patients at CMJCVAMC. This reduction in false-positive screening not only reduced laboratory workload due to the necessary confirmatory testing and subsequent review, but also saved costs for technologist’s time and reagents. While this reduction in false-positive results has been documented in nonveteran populations, this is the first study specifically on a veteran population treated at a VAMC.8,11,18 This study confirms previously documented findings of improvement in the false-positive rate of HIV screening tests with the change from third-generation to fourth-generation assay for a veteran population.24
Ever since the first clinical reports of patients with AIDS in 1981, there have been improvements both in the knowledge base of the pathogenesis of HIV in causing AIDS as well as a progressive refinement in the test methodologies used to diagnose this illness.1-3 Given that there are both public health and clinical benefits in earlier diagnosis and treatment of patients with available antiretroviral therapies, universal screening with opt-out consent has been a standard of practice recommendation by the Centers of Disease Control and Prevention (CDC) since 2006; universal screening with opt-out consent also has been recommended by the US Preventative Task Force and has been widely implemented.4-7
HIV Screening
While HIV screening assays have evolved to be accurate with very high sensitivities and specificities, false-positive results are a significant issue both currently and historically.8-16 The use of an HIV assay on a low prevalence population predictably reduces the positive predictive value (PPV) of even an otherwise accurate assay.8-23 In light of this, laboratory HIV testing algorithms include confirmatory testing to increase the likelihood that the correct diagnosis is being rendered.
The fourth-generation assay has been shown to be more sensitive and specific compared with that of the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 Due to these improvements, in the general population, increased sensitivity/specificity with a reduction in both false positives and false negatives have been reported.
It has been observed in the nonveteran population that switching from the older third-generation to a more sensitive and specific fourth-generation HIV screening assay has reduced the false-positive screening rate.18,19,22 For instance, Muthukumar and colleagues demonstrated a false-positive rate of only 2 out of 99 (2%) tested specimens for the fourth-generation ARCHITECT HIV Ag/Ab Combo assay vs 9 out of 99 tested specimens (9%) for the third-generation ADVIA Centaur HIV 1/O/2 Enhanced assay.18 In addition, it has been noted that fourth-generation HIV screening assays can reduce the window period by detecting HIV infection sooner after initial acute infection.19 Mitchell and colleagues demonstrated even highly specific fourth-generation HIV assays with specificities estimated at 99.7% can have PPVs as low as 25.0% if used in a population of low HIV prevalence (such as a 0.1% prevalence population).19 However, the veteran population has been documented to differ significantly on a number of population variables, including severity of disease and susceptibility to infections, and as a result extrapolation of these data from the general population may be limited.24-26 To our knowledge, this article represents the first study directly examining the reduction in false-positive results with the switch to a fourth-generation HIV generation assay from a third-generation assay for the veteran patient population at a regional US Department of Veterans Affairs (VA) medical center (VAMC).8,11
Methods
Quality assurance documents on test volume were retrospectively reviewed to obtain the number of HIV screening tests that were performed by the laboratory at the Corporal Michael J. Crescenz VAMC (CMJCVAMC) in Philadelphia, Pennsylvania, between March 1, 2016 and February 28, 2017, prior to implementation of the fourth-generation assay. The study also include results from the first year of use of the fourth-generation assay (March 1, 2017 to February 28, 2018). In addition, paper quality assurance records of all positive screening results during those periods were reviewed and manually counted for the abstract presentation of these data.
For assurance of accuracy, a search of all HIV testing assays using Veterans Health Information Systems and Technology Architecture and FileMan also was performed, and the results were compared to records in the Computerized Patient Record System (CPRS). Any discrepancies in the numbers of test results generated by both searches were investigated, and data for the manuscript were derived from records associating tests with particular patients. Only results from patient samples were considered for the electronic search. Quality samples that did not correspond to a true patient as identified in CPRS or same time patient sample duplicates were excluded from the calculations. Basic demographic data (age, ethnicity, and gender) were obtained from this FileMan search. The third-generation assay was the Ortho-Clinical Diagnostics Vitros, and the fourth-generation assay was the Abbott Architect.
To interpret the true HIV result of each sample with a reactive or positive screening result, the CDC laboratory HIV testing algorithm was followed and reviewed with a clinical pathologist or microbiologist director.12,13 All specimens interpreted as HIV positive by the pathologist or microbiologist director were discussed with the clinical health care provider at the time of the test with results added to CPRS after all testing was complete and discussions had taken place. All initially reactive specimens (confirmed with retesting in duplicate on the screening platform with at least 1 repeat reactive result) were further tested with the Bio-Rad Geenius HIV 1/2 Supplemental Assay, which screens for both HIV-1 and HIV-2 antibodies. Specimens with reactive results by this supplemental assay were interpreted as positive for HIV based on the CDC laboratory HIV testing algorithm. Specimens with negative or indeterminant results by the supplemental assay then underwent HIV-1 nucleic acid testing (NAT) using the Roche Diagnostics COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0. Specimens with viral load detected on NAT were positive for HIV infection, while specimens with viral load not detected on NAT testing were interpreted as negative for HIV-1 infection. Although there were no HIV-2 positive or indeterminant specimens during the study period, HIV-2 reactivity also would have been interpreted per the CDC laboratory HIV testing algorithm. Specimens with inadequate volume to complete all testing steps would be interpreted as indeterminant for HIV with request for additional specimen to complete testing. All testing platforms used for HIV testing in the laboratory had been properly validated prior to use.
The number of false positives and indeterminant results was tabulated in Microsoft Excel by month throughout the study period alongside the total number of HIV screening tests performed. Statistical analyses to verify statistical significance was performed by 1-tailed homoscedastic t test calculation using Excel.
Results
From March 1, 2016 to February 28, 2017, 7,516 specimens were screened for HIV, using the third-generation assay, and 52 specimens tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 24 tests were true positive and 28 were false positives with a PPV of 46% (24/52) (Figure 1).
From March 1, 2017 to February 28, 2018, 7,802 specimens were screened for HIV using a fourth-generation assay and 23 tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 16 were true positive and 7 were false positives with a PPV of 70% (16/23).
The fourth-generation assay was more specific when compared with the third-generation assay (0.09% vs 0.37%, respectively) with a 75.7% decrease in the false-positivity rate after the implementation of fourth-generation testing. The decreased number of false-positive test results per month with the fourth-generation test implementation was statistically significant (P = .002). The mean (SD) number of false-positive test results for the third-generation assay was 2.3 (1.7) per month, while the fourth-generation assay only had a mean (SD) of 0.58 (0.9) false positives monthly. The decrease in the percentage of false positives per month with the implementation of the fourth-generation assay also was statistically significant (P = .002) (Figure 2).
For population-based reference of the tested population at CMJCVAMC, there was a FileMan search for basic demographic data of patients for the HIV specimens screened by the third- or fourth-generation test (Table). For the population tested by the third-generation assay, 1,114 out of the 7,516 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For 6,402 of 7,516 patients tested by the third-generation assay with demographic information, the age ranged from 25 to 97 years with a mean of 57 years. This population of 6,402 was 88% male (n = 5,639), 50% African American (n = 3,220) and 43% White (n = 2,756). For the population tested by the fourth-generation assay, 993 of 7,802 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For the 6,809 of 7,802 patients tested by the fourth-generation assay with demographic information, the age ranged from 24 to 97 years with a mean age of 56 years. This population was 88% male (n = 5,971), 47% African American (n = 3,189), and 46% White (n = 3,149).
Discussion
Current practice guidelines from the CDC and the US Preventive Services Task Force recommend universal screening of the population for HIV infection.5,6 As the general population to be screened would normally have a low prevalence of HIV infection, the risk of a false positive on the initial screen is significant.17 Indeed, the CMJCVAMC experience has been that with the third-generation screening assay, the number of false-positive test results outnumbered the number of true-positive test results. Even with the fourth-generation assay, approximately one-third of the results were false positives. These results are similar to those observed in studies involving nonveteran populations in which the implementation of a fourth-generation screening assay led to significantly fewer false-positive results.18
For laboratories that do not follows CDC testing algorithm guidelines, each false-positive screening result represents a potential opportunity for a HIV misdiagnosis.Even in laboratories with proper procedures in place, false-positive results have consequences for the patients and for the cost-effectiveness of laboratory operations.9-11,18 As per CDC HIV testing guidelines, all positive screening results should be retested, which leads to additional use of technologist time and reagents. After this additional testing is performed and reviewed appropriately, only then can an appropriate final laboratory diagnosis be rendered that meets the standard of laboratory care.
Cost Savings
As observed at CMJCVAMC, the use of a fourth-generation assay with increased sensitivity/specificity led to a reduction in these false-positive results, which improved laboratory efficiency and avoided wasted resources for confirmatory tests.11,18 Cost savings at CMJCVAMC from the implementation of the fourth-generation assay would include technologist time and reagent cost. Generalizable technologist time costs at any institution would include the time needed to perform the confirmatory HIV-1/HIV-2 antibody differentiation assay (slightly less than 1 hour at CMJCVAMC per specimen) and the time needed to perform the viral load assay (about 6 hours to run a batch of 24 tests at CMJCVAMC). We calculated that confirmatory testing cost $184.51 per test at CMJCVAMC. Replacing the third-generation assay with the more sensitive and specific fourth-generation test saved an estimated $3,875 annually. This cost savings does not even consider savings in the pathologist/director’s time for reviewing HIV results after the completion of the algorithm or the clinician/patient costs or anxiety while waiting for results of the confirmatory sequence of tests.
As diagnosis of HIV can have a significant psychological impact on the patient, it is important to ensure the diagnosis conveyed is correct.27 The provision of an HIV diagnosis to a patient has been described as a traumatic stressor capable of causing psychological harm; this harm should ideally be avoided if the HIV diagnosis is not accurate. There can be a temptation, when presented with a positive or reactive screening test that is known to come from an instrument or assay with a very high sensitivity and specificity, to present this result as a diagnosis to the patient. However, a false diagnosis from a false-positive screen would not only be harmful, but given the low prevalence of the disease in the screened population, would happen fairly frequently; in some settings the number of false positives may actually outnumber the number of true positive test results.
Better screening assays with greater specificity (even fractions of a percentage, given that specificities are already > 99%) would help reduce the number of false positives and reduce the number of potential enticements to convey an incorrect diagnosis. Therefore, by adding an additional layer of safety through greater specificity, the fourth-generation assay implementation helped improve the diagnostic safety of the laboratory and reduced the significant error risk to the clinician who would ultimately bear responsibility for conveying the HIV diagnoses to the patient. Given the increased prevalence of psychological and physical ailments in veterans, it may be even more important to ensure the diagnosis is correct to avoid increased psychological harm.27,28
Veteran Population
For the general population, the fourth-generation assay has been shown to be more sensitive and specific when compared with the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 However, the veteran population that receives VA medical care differs significantly from the nonveteran general population. Compared with nonveterans, veterans tend to have generally poorer health status, more comorbid conditions, and greater need to use medical resources.24-26 In addition, veterans also may differ in sociodemographic status, race, ethnicity, and gender.24-26
VA research in the veteran population is unique, and veterans who use VA health care services are an even more highly selected subpopulation.26 Conclusions made from studies of the general population may not always be applicable to the veteran population treated by VA health care services due to these population differences. Therefore, specific studies tailored to this special veteran population in the specific VA health care setting are essential to ensure that the results of the general population truly and definitively apply to the veteran population.
While the false-positive risk is most closely associated with testing in a population of low prevalence, it also should be noted that false-positive screening results also can occur in high-risk individuals, such as an individual on preexposure prophylaxis (PrEP) for continuous behavior that places the individual at high risk of HIV acquisition.8,29 The false-positive result in these cases can lead to a conundrum for the clinician, and the differential diagnosis should consider both detection of very early infection as well as false positive. Interventions could include either stopping PrEP and treating for presumed early primary infection with HIV or continuing the PrEP. These interventions all have the potential to impact the patient whether through the production of resistant HIV virus due to the inadvertent provision of an inadequate treatment regimen, increased risk of infection if taken off PrEP as the patient may likely continue the behavior regardless, or the risks carried by the administration of additional antiretroviral therapies for the complete empiric therapy. Cases of an individual on PrEP who had a false-positive HIV screening test has been reported previously both within and outside the veteran population.8 Better screening tests with greater sensitivity/specificity can only help in guiding better patient care.
Limitations
This quality assurance study was limited to retrospectively identifying the improvement in the false-positive rate on the transition from the third-generation to the more advanced fourth-generation HIV screen. False-positive screen cases could be easily picked up on review of the confirmatory testing per the CDC laboratory HIV testing algorithm.12,13 This study also was a retrospective review of clinically ordered and indicated testing; as a result, without confirmatory testing performed on all negative screen cases, a false-negative rate would not be calculable.
This study also was restricted to only the population being treated in a VA health care setting. This population is known to be different from the general population.24-26
Conclusions
The switch to a fourth-generation assay resulted in a significant reduction in false-positive test results for veteran patients at CMJCVAMC. This reduction in false-positive screening not only reduced laboratory workload due to the necessary confirmatory testing and subsequent review, but also saved costs for technologist’s time and reagents. While this reduction in false-positive results has been documented in nonveteran populations, this is the first study specifically on a veteran population treated at a VAMC.8,11,18 This study confirms previously documented findings of improvement in the false-positive rate of HIV screening tests with the change from third-generation to fourth-generation assay for a veteran population.24
1. Feinberg MB. Changing the natural history of HIV disease. Lancet. 1996;348(9022):239-246. doi:10.1016/s0140-6736(96)06231-9.
2. Alexander TS. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol. 2016;23(4):249-253. Published 2016 Apr 4. doi:10.1128/CVI.00053-16
3. Mortimer PP, Parry JV, Mortimer JY. Which anti-HTLV III/LAV assays for screening and confirmatory testing?. Lancet. 1985;2(8460):873-877. doi:10.1016/s0140-6736(85)90136-9
4. Holmberg SD, Palella FJ Jr, Lichtenstein KA, Havlir DV. The case for earlier treatment of HIV infection [published correction appears in Clin Infect Dis. 2004 Dec 15;39(12):1869]. Clin Infect Dis. 2004;39(11):1699-1704. doi:10.1086/425743
5. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(23):2326-2336. doi:10.1001/jama.2019.6587
6. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-CE4.
7. Bayer R, Philbin M, Remien RH. The end of written informed consent for HIV testing: not with a bang but a whimper. Am J Public Health. 2017;107(8):1259-1265. doi:10.2105/AJPH.2017.303819
8. Petersen J, Jhala D. Its not HIV! The pitfall of unconfirmed positive HIV screening assays. Abstract presented at: Annual Meeting Pennsylvania Association of Pathologists; April 14, 2018.
9. Wood RW, Dunphy C, Okita K, Swenson P. Two “HIV-infected” persons not really infected. Arch Intern Med. 2003;163(15):1857-1859. doi:10.1001/archinte.163.15.1857
10. Permpalung N, Ungprasert P, Chongnarungsin D, Okoli A, Hyman CL. A diagnostic blind spot: acute infectious mononucleosis or acute retroviral syndrome. Am J Med. 2013;126(9):e5-e6. doi:10.1016/j.amjmed.2013.03.017
11. Dalal S, Petersen J, Luta D, Jhala D. Third- to fourth-generation HIV testing: reduction in false-positive results with the new way of testing, the Corporal Michael J. Crescenz Veteran Affairs Medical Center (CMCVAMC) Experience. Am J Clin Pathol.2018;150(suppl 1):S70-S71. doi:10.1093/ajcp/aqy093.172
12. Centers for Disease Control and Prevention. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Published June 27, 2014. Accessed April 14, 2021. doi:10.15620/cdc.23447
13. Centers for Disease Control and Prevention. 2018 quick reference guide: recommended laboratory HIV testing algorithm for serum or plasma specimens. Updated January 2018. Accessed April 14, 202. https://stacks.cdc.gov/view/cdc/50872
14. Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(suppl 1):S17-S22. doi:10.1016/j.jcv.2011.09.011
15. Morton A. When lab tests lie … heterophile antibodies. Aust Fam Physician. 2014;43(6):391-393.
16. Spencer DV, Nolte FS, Zhu Y. Heterophilic antibody interference causing false-positive rapid human immunodeficiency virus antibody testing. Clin Chim Acta. 2009;399(1-2):121-122. doi:10.1016/j.cca.2008.09.030
17. Kim S, Lee JH, Choi JY, Kim JM, Kim HS. False-positive rate of a “fourth-generation” HIV antigen/antibody combination assay in an area of low HIV prevalence. Clin Vaccine Immunol. 2010;17(10):1642-1644. doi:10.1128/CVI.00258-10
18. Muthukumar A, Alatoom A, Burns S, et al. Comparison of 4th-generation HIV antigen/antibody combination assay with 3rd-generation HIV antibody assays for the occurrence of false-positive and false-negative results. Lab Med. 2015;46(2):84-e29. doi:10.1309/LMM3X37NSWUCMVRS
19. Mitchell EO, Stewart G, Bajzik O, Ferret M, Bentsen C, Shriver MK. Performance comparison of the 4th generation Bio-Rad Laboratories GS HIV Combo Ag/Ab EIA on the EVOLIS™ automated system versus Abbott ARCHITECT HIV Ag/Ab Combo, Ortho Anti-HIV 1+2 EIA on Vitros ECi and Siemens HIV-1/O/2 enhanced on Advia Centaur. J Clin Virol. 2013;58(suppl 1):e79-e84. doi:10.1016/j.jcv.2013.08.009
20. Dubravac T, Gahan TF, Pentella MA. Use of the Abbott Architect HIV antigen/antibody assay in a low incidence population. J Clin Virol. 2013;58(suppl 1):e76-e78. doi:10.1016/j.jcv.2013.10.020
21. Montesinos I, Eykmans J, Delforge ML. Evaluation of the Bio-Rad Geenius HIV-1/2 test as a confirmatory assay. J Clin Virol. 2014;60(4):399-401. doi:10.1016/j.jcv.2014.04.025
22. van Binsbergen J, Siebelink A, Jacobs A, et al. Improved performance of seroconversion with a 4th generation HIV antigen/antibody assay. J Virol Methods. 1999;82(1):77-84. doi:10.1016/s0166-0934(99)00086-5
23. CLSI. User Protocol for Evaluation of Qualitative Test Performance: Approved Guideline. Second ed. EP12-A2. CLSI; 2008:1-46.
24. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
25. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
26. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x
27. Nightingale VR, Sher TG, Hansen NB. The impact of receiving an HIV diagnosis and cognitive processing on psychological distress and posttraumatic growth. J Trauma Stress. 2010;23(4):452-460. doi:10.1002/jts.20554
28. Spelman JF, Hunt SC, Seal KH, Burgo-Black AL. Post deployment care for returning combat veterans. J Gen Intern Med. 2012;27(9):1200-1209. doi:10.1007/s11606-012-2061-1
29. Ndase P, Celum C, Kidoguchi L, et al. Frequency of false positive rapid HIV serologic tests in African men and women receiving PrEP for HIV prevention: implications for programmatic roll-out of biomedical interventions. PLoS One. 2015;10(4):e0123005. Published 2015 Apr 17. doi:10.1371/journal.pone.0123005
1. Feinberg MB. Changing the natural history of HIV disease. Lancet. 1996;348(9022):239-246. doi:10.1016/s0140-6736(96)06231-9.
2. Alexander TS. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol. 2016;23(4):249-253. Published 2016 Apr 4. doi:10.1128/CVI.00053-16
3. Mortimer PP, Parry JV, Mortimer JY. Which anti-HTLV III/LAV assays for screening and confirmatory testing?. Lancet. 1985;2(8460):873-877. doi:10.1016/s0140-6736(85)90136-9
4. Holmberg SD, Palella FJ Jr, Lichtenstein KA, Havlir DV. The case for earlier treatment of HIV infection [published correction appears in Clin Infect Dis. 2004 Dec 15;39(12):1869]. Clin Infect Dis. 2004;39(11):1699-1704. doi:10.1086/425743
5. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(23):2326-2336. doi:10.1001/jama.2019.6587
6. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-CE4.
7. Bayer R, Philbin M, Remien RH. The end of written informed consent for HIV testing: not with a bang but a whimper. Am J Public Health. 2017;107(8):1259-1265. doi:10.2105/AJPH.2017.303819
8. Petersen J, Jhala D. Its not HIV! The pitfall of unconfirmed positive HIV screening assays. Abstract presented at: Annual Meeting Pennsylvania Association of Pathologists; April 14, 2018.
9. Wood RW, Dunphy C, Okita K, Swenson P. Two “HIV-infected” persons not really infected. Arch Intern Med. 2003;163(15):1857-1859. doi:10.1001/archinte.163.15.1857
10. Permpalung N, Ungprasert P, Chongnarungsin D, Okoli A, Hyman CL. A diagnostic blind spot: acute infectious mononucleosis or acute retroviral syndrome. Am J Med. 2013;126(9):e5-e6. doi:10.1016/j.amjmed.2013.03.017
11. Dalal S, Petersen J, Luta D, Jhala D. Third- to fourth-generation HIV testing: reduction in false-positive results with the new way of testing, the Corporal Michael J. Crescenz Veteran Affairs Medical Center (CMCVAMC) Experience. Am J Clin Pathol.2018;150(suppl 1):S70-S71. doi:10.1093/ajcp/aqy093.172
12. Centers for Disease Control and Prevention. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Published June 27, 2014. Accessed April 14, 2021. doi:10.15620/cdc.23447
13. Centers for Disease Control and Prevention. 2018 quick reference guide: recommended laboratory HIV testing algorithm for serum or plasma specimens. Updated January 2018. Accessed April 14, 202. https://stacks.cdc.gov/view/cdc/50872
14. Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(suppl 1):S17-S22. doi:10.1016/j.jcv.2011.09.011
15. Morton A. When lab tests lie … heterophile antibodies. Aust Fam Physician. 2014;43(6):391-393.
16. Spencer DV, Nolte FS, Zhu Y. Heterophilic antibody interference causing false-positive rapid human immunodeficiency virus antibody testing. Clin Chim Acta. 2009;399(1-2):121-122. doi:10.1016/j.cca.2008.09.030
17. Kim S, Lee JH, Choi JY, Kim JM, Kim HS. False-positive rate of a “fourth-generation” HIV antigen/antibody combination assay in an area of low HIV prevalence. Clin Vaccine Immunol. 2010;17(10):1642-1644. doi:10.1128/CVI.00258-10
18. Muthukumar A, Alatoom A, Burns S, et al. Comparison of 4th-generation HIV antigen/antibody combination assay with 3rd-generation HIV antibody assays for the occurrence of false-positive and false-negative results. Lab Med. 2015;46(2):84-e29. doi:10.1309/LMM3X37NSWUCMVRS
19. Mitchell EO, Stewart G, Bajzik O, Ferret M, Bentsen C, Shriver MK. Performance comparison of the 4th generation Bio-Rad Laboratories GS HIV Combo Ag/Ab EIA on the EVOLIS™ automated system versus Abbott ARCHITECT HIV Ag/Ab Combo, Ortho Anti-HIV 1+2 EIA on Vitros ECi and Siemens HIV-1/O/2 enhanced on Advia Centaur. J Clin Virol. 2013;58(suppl 1):e79-e84. doi:10.1016/j.jcv.2013.08.009
20. Dubravac T, Gahan TF, Pentella MA. Use of the Abbott Architect HIV antigen/antibody assay in a low incidence population. J Clin Virol. 2013;58(suppl 1):e76-e78. doi:10.1016/j.jcv.2013.10.020
21. Montesinos I, Eykmans J, Delforge ML. Evaluation of the Bio-Rad Geenius HIV-1/2 test as a confirmatory assay. J Clin Virol. 2014;60(4):399-401. doi:10.1016/j.jcv.2014.04.025
22. van Binsbergen J, Siebelink A, Jacobs A, et al. Improved performance of seroconversion with a 4th generation HIV antigen/antibody assay. J Virol Methods. 1999;82(1):77-84. doi:10.1016/s0166-0934(99)00086-5
23. CLSI. User Protocol for Evaluation of Qualitative Test Performance: Approved Guideline. Second ed. EP12-A2. CLSI; 2008:1-46.
24. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
25. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
26. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x
27. Nightingale VR, Sher TG, Hansen NB. The impact of receiving an HIV diagnosis and cognitive processing on psychological distress and posttraumatic growth. J Trauma Stress. 2010;23(4):452-460. doi:10.1002/jts.20554
28. Spelman JF, Hunt SC, Seal KH, Burgo-Black AL. Post deployment care for returning combat veterans. J Gen Intern Med. 2012;27(9):1200-1209. doi:10.1007/s11606-012-2061-1
29. Ndase P, Celum C, Kidoguchi L, et al. Frequency of false positive rapid HIV serologic tests in African men and women receiving PrEP for HIV prevention: implications for programmatic roll-out of biomedical interventions. PLoS One. 2015;10(4):e0123005. Published 2015 Apr 17. doi:10.1371/journal.pone.0123005
Success in LGBTQ+ medicine requires awareness of risk
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
FROM INTERNAL MEDICINE 2021
HIV patients show accelerated aging related to altered sleep
Accelerated brain aging among HIV-infected adults might be caused in part by altered deep sleep patterns, new research suggests.
Using a measure known as the brain age index (BAI) – a machine-learning model that measures deviations in brain activity during sleep relative to healthy individuals – investigators identified 34 sleep electroencephalogram features that were significantly altered by HIV infection. The most notable of these was the decline in slow-wave activity during non-REM sleep, which has been previously associated with MRI markers of brain aging in healthy adults.
“One of the functions of slow-wave sleep appears to be its association with the glymphatic system, which clears [metabolic] waste products and supports memory consolidation,” study coauthor Brandon Westover, MD, PhD, associate professor of neurology at Massachusetts General Hospital/Harvard Medical School, Boston, said in an interview. “It’s also believed to be associated with an accelerated risk for dementia and other cognitive issues.”
Previous work conducted at Johns Hopkins and other institutions confirm Dr. Westerson’s hypothesis. Charlene Gamaldo, MD, medical director of Johns Hopkins Sleep Disorders Center in Baltimore, pointed to other study findings in patients with neurodegenerative disease that have shown a link between predominant habitual sleep positions and dementia, potentially driven by inefficient glymphatic transport. Dr. Gamaldo was not involved in the current study.
Threefold acceleration vs. healthy volunteers
“We’ve been grappling with whether people with HIV on ART experience accelerated aging or accentuated aging,” coauthor Shibani Mukerji, MD, PhD, associate director of the neuroinfectious diseases unit at Massachusetts General, said in an interview. “We have yet to have biomarkers to address this question, and most of the tools are limited to invasive or expensive diagnostics. “In general, sleep and its influence on health have been understudied in the HIV population.”
To address this question, the researchers retrospectively examined a Massachusetts General Hospital database of diagnostic sleep study participants from 2008 to 2018, identifying 3,155 healthy, HIV-negative control subjects and 43 HIV-positive participants. Thirty-four (79%) of the HIV-positive participants were men, 30 (70%) were White, and 38 (93%) were virally suppressed at the time of their sleep study. Four patients were taking efavirenz, 13 were taking an integrase strand transfer inhibitor, and all were adherent to antiretroviral therapy (ART) at the time of their sleep study.
None of the HIV-positive participants had a history of secondary brain infection or brain tumor, although one patient had recovered fully from a previous HIV-associated encephalitis.
The study findings, which were published online March 30, 2021, in Sleep, first showed that HIV-positive participants had an average BAI of 3.19 years (standard error of the mean,1.43 years), compared with the control participants, who had an average BAI of –0.16 (SEM, 0.18 years).
These findings held after adjustment for potential confounders (age, sex, race, tobacco use disorder, and alcohol use disorder), yielding a total effect of HIV on BAI of 3.35 years (P < .01).
“Despite being well controlled on ART, HIV-positive individuals who had participated in the sleep studies still had elevated brain age,” said Dr. Westover. “We didn’t have enough information to determine the pathways by which HIV increases the BAI, but chronic inflammation appears to be an important factor.”
The findings also demonstrated that comorbidities accounted for roughly a quarter of the effect of HIV on BAI. However, the lack of statistical significance (in part because of the limited sample size) precluded the ability to determine if treating or preventing them might influence the degree to which HIV affects BAI and, in turn, cognitive decline.
HIV, sleep EEG, and brain aging
To estimate the effect of HIV on specific EEG features, the investigators again evaluated the total effect, this time replacing BAI with individual sleep EEG as the primary outcome. Among the 34 EEG features significantly altered by HIV, none were observed in the wake state and three were altered in REM (each associated with reduced delta band power). The rest were distributed in non-REM sleep, most notably in the deepest phase, corresponding to relative reductions in delta wave power.
The study findings build on the investigators’ previous research, which demonstrated an association between greater mean BAI and dementia, psychotic disorders, and anxiety/mood disorders in HIV-negative subjects, all of which correlated to attenuated slow-wave sleep.
More research is needed to determine if BAI, as it relates to sleep EEG, can effectively track the risk for cognitive decline among HIV-positive people, and if certain confounders might attenuate or accelerate this risk.
“While our team has not specifically looked at BAI, the findings in this study seem perfectly in line with what we have found with our own research,” Dr. Gamaldo said in an interview. “Not only have we observed a robust association between minimal cognitive deficits and patients’ sleep complaints (despite being virally controlled), but also, the potential value of measuring the architectural sleep features by ambulatory EEG to identify HIV patients’ vulnerability to cognitive decline.”
“BAI is a physiologic, easily repeatable measurement that can be used to track if an intervention is having a good effect,” Dr. Westover said.
Dr. Mukerji concurred, adding that “having a tool that can be used in resource-challenged settings and also be incorporated into longitudinal studies in a patient population with substantial age-related comorbidities, like HIV, would be really helpful.”
Dr. Westover and Dr. Mukerji disclosed no relevant financial relationships. Dr. Gamaldo is a consultant for Jazz Pharmaceuticals, and has received author royalties from UpToDate and honoraria from Medscape CME for content contribution.
A version of this article first appeared on Medscape.com.
Accelerated brain aging among HIV-infected adults might be caused in part by altered deep sleep patterns, new research suggests.
Using a measure known as the brain age index (BAI) – a machine-learning model that measures deviations in brain activity during sleep relative to healthy individuals – investigators identified 34 sleep electroencephalogram features that were significantly altered by HIV infection. The most notable of these was the decline in slow-wave activity during non-REM sleep, which has been previously associated with MRI markers of brain aging in healthy adults.
“One of the functions of slow-wave sleep appears to be its association with the glymphatic system, which clears [metabolic] waste products and supports memory consolidation,” study coauthor Brandon Westover, MD, PhD, associate professor of neurology at Massachusetts General Hospital/Harvard Medical School, Boston, said in an interview. “It’s also believed to be associated with an accelerated risk for dementia and other cognitive issues.”
Previous work conducted at Johns Hopkins and other institutions confirm Dr. Westerson’s hypothesis. Charlene Gamaldo, MD, medical director of Johns Hopkins Sleep Disorders Center in Baltimore, pointed to other study findings in patients with neurodegenerative disease that have shown a link between predominant habitual sleep positions and dementia, potentially driven by inefficient glymphatic transport. Dr. Gamaldo was not involved in the current study.
Threefold acceleration vs. healthy volunteers
“We’ve been grappling with whether people with HIV on ART experience accelerated aging or accentuated aging,” coauthor Shibani Mukerji, MD, PhD, associate director of the neuroinfectious diseases unit at Massachusetts General, said in an interview. “We have yet to have biomarkers to address this question, and most of the tools are limited to invasive or expensive diagnostics. “In general, sleep and its influence on health have been understudied in the HIV population.”
To address this question, the researchers retrospectively examined a Massachusetts General Hospital database of diagnostic sleep study participants from 2008 to 2018, identifying 3,155 healthy, HIV-negative control subjects and 43 HIV-positive participants. Thirty-four (79%) of the HIV-positive participants were men, 30 (70%) were White, and 38 (93%) were virally suppressed at the time of their sleep study. Four patients were taking efavirenz, 13 were taking an integrase strand transfer inhibitor, and all were adherent to antiretroviral therapy (ART) at the time of their sleep study.
None of the HIV-positive participants had a history of secondary brain infection or brain tumor, although one patient had recovered fully from a previous HIV-associated encephalitis.
The study findings, which were published online March 30, 2021, in Sleep, first showed that HIV-positive participants had an average BAI of 3.19 years (standard error of the mean,1.43 years), compared with the control participants, who had an average BAI of –0.16 (SEM, 0.18 years).
These findings held after adjustment for potential confounders (age, sex, race, tobacco use disorder, and alcohol use disorder), yielding a total effect of HIV on BAI of 3.35 years (P < .01).
“Despite being well controlled on ART, HIV-positive individuals who had participated in the sleep studies still had elevated brain age,” said Dr. Westover. “We didn’t have enough information to determine the pathways by which HIV increases the BAI, but chronic inflammation appears to be an important factor.”
The findings also demonstrated that comorbidities accounted for roughly a quarter of the effect of HIV on BAI. However, the lack of statistical significance (in part because of the limited sample size) precluded the ability to determine if treating or preventing them might influence the degree to which HIV affects BAI and, in turn, cognitive decline.
HIV, sleep EEG, and brain aging
To estimate the effect of HIV on specific EEG features, the investigators again evaluated the total effect, this time replacing BAI with individual sleep EEG as the primary outcome. Among the 34 EEG features significantly altered by HIV, none were observed in the wake state and three were altered in REM (each associated with reduced delta band power). The rest were distributed in non-REM sleep, most notably in the deepest phase, corresponding to relative reductions in delta wave power.
The study findings build on the investigators’ previous research, which demonstrated an association between greater mean BAI and dementia, psychotic disorders, and anxiety/mood disorders in HIV-negative subjects, all of which correlated to attenuated slow-wave sleep.
More research is needed to determine if BAI, as it relates to sleep EEG, can effectively track the risk for cognitive decline among HIV-positive people, and if certain confounders might attenuate or accelerate this risk.
“While our team has not specifically looked at BAI, the findings in this study seem perfectly in line with what we have found with our own research,” Dr. Gamaldo said in an interview. “Not only have we observed a robust association between minimal cognitive deficits and patients’ sleep complaints (despite being virally controlled), but also, the potential value of measuring the architectural sleep features by ambulatory EEG to identify HIV patients’ vulnerability to cognitive decline.”
“BAI is a physiologic, easily repeatable measurement that can be used to track if an intervention is having a good effect,” Dr. Westover said.
Dr. Mukerji concurred, adding that “having a tool that can be used in resource-challenged settings and also be incorporated into longitudinal studies in a patient population with substantial age-related comorbidities, like HIV, would be really helpful.”
Dr. Westover and Dr. Mukerji disclosed no relevant financial relationships. Dr. Gamaldo is a consultant for Jazz Pharmaceuticals, and has received author royalties from UpToDate and honoraria from Medscape CME for content contribution.
A version of this article first appeared on Medscape.com.
Accelerated brain aging among HIV-infected adults might be caused in part by altered deep sleep patterns, new research suggests.
Using a measure known as the brain age index (BAI) – a machine-learning model that measures deviations in brain activity during sleep relative to healthy individuals – investigators identified 34 sleep electroencephalogram features that were significantly altered by HIV infection. The most notable of these was the decline in slow-wave activity during non-REM sleep, which has been previously associated with MRI markers of brain aging in healthy adults.
“One of the functions of slow-wave sleep appears to be its association with the glymphatic system, which clears [metabolic] waste products and supports memory consolidation,” study coauthor Brandon Westover, MD, PhD, associate professor of neurology at Massachusetts General Hospital/Harvard Medical School, Boston, said in an interview. “It’s also believed to be associated with an accelerated risk for dementia and other cognitive issues.”
Previous work conducted at Johns Hopkins and other institutions confirm Dr. Westerson’s hypothesis. Charlene Gamaldo, MD, medical director of Johns Hopkins Sleep Disorders Center in Baltimore, pointed to other study findings in patients with neurodegenerative disease that have shown a link between predominant habitual sleep positions and dementia, potentially driven by inefficient glymphatic transport. Dr. Gamaldo was not involved in the current study.
Threefold acceleration vs. healthy volunteers
“We’ve been grappling with whether people with HIV on ART experience accelerated aging or accentuated aging,” coauthor Shibani Mukerji, MD, PhD, associate director of the neuroinfectious diseases unit at Massachusetts General, said in an interview. “We have yet to have biomarkers to address this question, and most of the tools are limited to invasive or expensive diagnostics. “In general, sleep and its influence on health have been understudied in the HIV population.”
To address this question, the researchers retrospectively examined a Massachusetts General Hospital database of diagnostic sleep study participants from 2008 to 2018, identifying 3,155 healthy, HIV-negative control subjects and 43 HIV-positive participants. Thirty-four (79%) of the HIV-positive participants were men, 30 (70%) were White, and 38 (93%) were virally suppressed at the time of their sleep study. Four patients were taking efavirenz, 13 were taking an integrase strand transfer inhibitor, and all were adherent to antiretroviral therapy (ART) at the time of their sleep study.
None of the HIV-positive participants had a history of secondary brain infection or brain tumor, although one patient had recovered fully from a previous HIV-associated encephalitis.
The study findings, which were published online March 30, 2021, in Sleep, first showed that HIV-positive participants had an average BAI of 3.19 years (standard error of the mean,1.43 years), compared with the control participants, who had an average BAI of –0.16 (SEM, 0.18 years).
These findings held after adjustment for potential confounders (age, sex, race, tobacco use disorder, and alcohol use disorder), yielding a total effect of HIV on BAI of 3.35 years (P < .01).
“Despite being well controlled on ART, HIV-positive individuals who had participated in the sleep studies still had elevated brain age,” said Dr. Westover. “We didn’t have enough information to determine the pathways by which HIV increases the BAI, but chronic inflammation appears to be an important factor.”
The findings also demonstrated that comorbidities accounted for roughly a quarter of the effect of HIV on BAI. However, the lack of statistical significance (in part because of the limited sample size) precluded the ability to determine if treating or preventing them might influence the degree to which HIV affects BAI and, in turn, cognitive decline.
HIV, sleep EEG, and brain aging
To estimate the effect of HIV on specific EEG features, the investigators again evaluated the total effect, this time replacing BAI with individual sleep EEG as the primary outcome. Among the 34 EEG features significantly altered by HIV, none were observed in the wake state and three were altered in REM (each associated with reduced delta band power). The rest were distributed in non-REM sleep, most notably in the deepest phase, corresponding to relative reductions in delta wave power.
The study findings build on the investigators’ previous research, which demonstrated an association between greater mean BAI and dementia, psychotic disorders, and anxiety/mood disorders in HIV-negative subjects, all of which correlated to attenuated slow-wave sleep.
More research is needed to determine if BAI, as it relates to sleep EEG, can effectively track the risk for cognitive decline among HIV-positive people, and if certain confounders might attenuate or accelerate this risk.
“While our team has not specifically looked at BAI, the findings in this study seem perfectly in line with what we have found with our own research,” Dr. Gamaldo said in an interview. “Not only have we observed a robust association between minimal cognitive deficits and patients’ sleep complaints (despite being virally controlled), but also, the potential value of measuring the architectural sleep features by ambulatory EEG to identify HIV patients’ vulnerability to cognitive decline.”
“BAI is a physiologic, easily repeatable measurement that can be used to track if an intervention is having a good effect,” Dr. Westover said.
Dr. Mukerji concurred, adding that “having a tool that can be used in resource-challenged settings and also be incorporated into longitudinal studies in a patient population with substantial age-related comorbidities, like HIV, would be really helpful.”
Dr. Westover and Dr. Mukerji disclosed no relevant financial relationships. Dr. Gamaldo is a consultant for Jazz Pharmaceuticals, and has received author royalties from UpToDate and honoraria from Medscape CME for content contribution.
A version of this article first appeared on Medscape.com.
Overshadowed by COVID, HIV Epidemic Rages On
How many epidemics can one country contain? Nearly all of our national attention has been focused on the COVID-19 epidemic but, lest we forget, there’s still another one to be reckoned with: the HIV epidemic is still going strong. In fact, it’s gathering strength, in part, because of the economic and health devastation wrought by COVID.
Over nearly 4 decades, the epidemiology of HIV has changed, according to The Lancet’s HIV in the USA series. Current data, the report says, “illustrate an epidemic defined by stark health inequities that largely fall along lines of disadvantages in economic opportunity and social capital.” Moreover, the US, the authors say, “continues to lag behind other G-7 nations when it comes to controlling its HIV epidemic and is the only high-income country among the top 10 countries most affected by HIV.”
The 6-paper series’ release comes 2 years after the launch of the US Department of Health and Human Services’ (HHS) announcement of its goal to reduce HIV transmissions by at least 90% by 2030.
The authors analyzed publicly available HIV surveillance and census data to describe current prevalence and new HIV diagnoses by region, race, ethnicity, and age, as well as trends in those categories over time. They also reviewed literature to “explore the reasons” for the distribution of cases and important disparities in prevalence. Among other things, the researchers found “pronounced” racial, sexual, and gender disparities, “substantial” gaps in domestic program funding, and a “patchwork healthcare system” that limited access to treatment and prevention services.
Although when it began, the HIV epidemic was focused largely on the bicoastal big cities, mainly New York and San Francisco, in recent years the South has been hit particularly hard, with 52% of new HIV transmissions in 2018, despite representing only 37% of the US population. Six Southern states (Florida, Georgia, Louisiana, Maryland, Mississippi, and Tennessee) and the District of Columbia had the highest annual HIV diagnosis rates between 2010 and 2018, likely reflecting the higher burden of infection among black residents: In 2018, 38% of all new HIV diagnoses among men who have sex with men (MSM) were in the black population, and 63% of those were in the South.
The South’s HIV problem is intensified by disparities, the report says, that are probably driven by the restricted expansion of Medicaid, health care provider shortages, low health literacy, and stigma. The South also has the lowest number of pre-exposure prophylaxis (PrEP) users per new HIV diagnosis, in part because of the longer distances to PrEP services relative to other regions. More than half of MSM who live at least 60 minutes away from PrEP services live in the South. While HIV in the rural South largely is due to sexual transmission, the researchers note, the largest clusters of the concurrent opioid epidemic have been detected in rural and periurban counties of West Virginia and Indiana.
Identifying HIV transmission clusters and outbreaks has traditionally been challenging for several reasons, the researchers say, including delays between infection and diagnosis, mobility of populations, and limitations in tracing sex and drug partners. They suggest that analysis of molecular data can help overcome some of those barriers, making it possible to identify clusters of ongoing HIV transmission.
The report also recommends other approaches to better understand and respond to ongoing HIV infections, such as mapping and data visualization, telemedicine, and automated data systems to facilitate linkage to care. However, the authors add, gaps in data and data systems remain that prevent full understanding of some key impacts of the epidemic. But any interventions to promote HIV prevention and treatment adherence, the authors suggested, should take a multifaceted approach and address the whole individual.
Chris Beyrer, MD, MPH, investigator at the Johns Hopkins Bloomberg School of Public Health, Baltimore, and a lead author on the series, says, “We have incredible tools to prevent and treat HIV, but people may not fully utilize them if they are facing personal or structural issues that pose more immediate hardship, like substance use and mental health disorders. You may struggle to take a daily medication if you are facing food insecurity or cannot find affordable treatment for your substance use disorder.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and now also Chief Medical Advisor to President Biden, says, “To end the HIV epidemic, we must continue to develop and deploy novel HIV treatment and prevention strategies suited to the different needs and preferences of diverse populations disproportionately affected by HIV. It is also essential that HIV health services continue during the COVID-19 pandemic.”
How many epidemics can one country contain? Nearly all of our national attention has been focused on the COVID-19 epidemic but, lest we forget, there’s still another one to be reckoned with: the HIV epidemic is still going strong. In fact, it’s gathering strength, in part, because of the economic and health devastation wrought by COVID.
Over nearly 4 decades, the epidemiology of HIV has changed, according to The Lancet’s HIV in the USA series. Current data, the report says, “illustrate an epidemic defined by stark health inequities that largely fall along lines of disadvantages in economic opportunity and social capital.” Moreover, the US, the authors say, “continues to lag behind other G-7 nations when it comes to controlling its HIV epidemic and is the only high-income country among the top 10 countries most affected by HIV.”
The 6-paper series’ release comes 2 years after the launch of the US Department of Health and Human Services’ (HHS) announcement of its goal to reduce HIV transmissions by at least 90% by 2030.
The authors analyzed publicly available HIV surveillance and census data to describe current prevalence and new HIV diagnoses by region, race, ethnicity, and age, as well as trends in those categories over time. They also reviewed literature to “explore the reasons” for the distribution of cases and important disparities in prevalence. Among other things, the researchers found “pronounced” racial, sexual, and gender disparities, “substantial” gaps in domestic program funding, and a “patchwork healthcare system” that limited access to treatment and prevention services.
Although when it began, the HIV epidemic was focused largely on the bicoastal big cities, mainly New York and San Francisco, in recent years the South has been hit particularly hard, with 52% of new HIV transmissions in 2018, despite representing only 37% of the US population. Six Southern states (Florida, Georgia, Louisiana, Maryland, Mississippi, and Tennessee) and the District of Columbia had the highest annual HIV diagnosis rates between 2010 and 2018, likely reflecting the higher burden of infection among black residents: In 2018, 38% of all new HIV diagnoses among men who have sex with men (MSM) were in the black population, and 63% of those were in the South.
The South’s HIV problem is intensified by disparities, the report says, that are probably driven by the restricted expansion of Medicaid, health care provider shortages, low health literacy, and stigma. The South also has the lowest number of pre-exposure prophylaxis (PrEP) users per new HIV diagnosis, in part because of the longer distances to PrEP services relative to other regions. More than half of MSM who live at least 60 minutes away from PrEP services live in the South. While HIV in the rural South largely is due to sexual transmission, the researchers note, the largest clusters of the concurrent opioid epidemic have been detected in rural and periurban counties of West Virginia and Indiana.
Identifying HIV transmission clusters and outbreaks has traditionally been challenging for several reasons, the researchers say, including delays between infection and diagnosis, mobility of populations, and limitations in tracing sex and drug partners. They suggest that analysis of molecular data can help overcome some of those barriers, making it possible to identify clusters of ongoing HIV transmission.
The report also recommends other approaches to better understand and respond to ongoing HIV infections, such as mapping and data visualization, telemedicine, and automated data systems to facilitate linkage to care. However, the authors add, gaps in data and data systems remain that prevent full understanding of some key impacts of the epidemic. But any interventions to promote HIV prevention and treatment adherence, the authors suggested, should take a multifaceted approach and address the whole individual.
Chris Beyrer, MD, MPH, investigator at the Johns Hopkins Bloomberg School of Public Health, Baltimore, and a lead author on the series, says, “We have incredible tools to prevent and treat HIV, but people may not fully utilize them if they are facing personal or structural issues that pose more immediate hardship, like substance use and mental health disorders. You may struggle to take a daily medication if you are facing food insecurity or cannot find affordable treatment for your substance use disorder.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and now also Chief Medical Advisor to President Biden, says, “To end the HIV epidemic, we must continue to develop and deploy novel HIV treatment and prevention strategies suited to the different needs and preferences of diverse populations disproportionately affected by HIV. It is also essential that HIV health services continue during the COVID-19 pandemic.”
How many epidemics can one country contain? Nearly all of our national attention has been focused on the COVID-19 epidemic but, lest we forget, there’s still another one to be reckoned with: the HIV epidemic is still going strong. In fact, it’s gathering strength, in part, because of the economic and health devastation wrought by COVID.
Over nearly 4 decades, the epidemiology of HIV has changed, according to The Lancet’s HIV in the USA series. Current data, the report says, “illustrate an epidemic defined by stark health inequities that largely fall along lines of disadvantages in economic opportunity and social capital.” Moreover, the US, the authors say, “continues to lag behind other G-7 nations when it comes to controlling its HIV epidemic and is the only high-income country among the top 10 countries most affected by HIV.”
The 6-paper series’ release comes 2 years after the launch of the US Department of Health and Human Services’ (HHS) announcement of its goal to reduce HIV transmissions by at least 90% by 2030.
The authors analyzed publicly available HIV surveillance and census data to describe current prevalence and new HIV diagnoses by region, race, ethnicity, and age, as well as trends in those categories over time. They also reviewed literature to “explore the reasons” for the distribution of cases and important disparities in prevalence. Among other things, the researchers found “pronounced” racial, sexual, and gender disparities, “substantial” gaps in domestic program funding, and a “patchwork healthcare system” that limited access to treatment and prevention services.
Although when it began, the HIV epidemic was focused largely on the bicoastal big cities, mainly New York and San Francisco, in recent years the South has been hit particularly hard, with 52% of new HIV transmissions in 2018, despite representing only 37% of the US population. Six Southern states (Florida, Georgia, Louisiana, Maryland, Mississippi, and Tennessee) and the District of Columbia had the highest annual HIV diagnosis rates between 2010 and 2018, likely reflecting the higher burden of infection among black residents: In 2018, 38% of all new HIV diagnoses among men who have sex with men (MSM) were in the black population, and 63% of those were in the South.
The South’s HIV problem is intensified by disparities, the report says, that are probably driven by the restricted expansion of Medicaid, health care provider shortages, low health literacy, and stigma. The South also has the lowest number of pre-exposure prophylaxis (PrEP) users per new HIV diagnosis, in part because of the longer distances to PrEP services relative to other regions. More than half of MSM who live at least 60 minutes away from PrEP services live in the South. While HIV in the rural South largely is due to sexual transmission, the researchers note, the largest clusters of the concurrent opioid epidemic have been detected in rural and periurban counties of West Virginia and Indiana.
Identifying HIV transmission clusters and outbreaks has traditionally been challenging for several reasons, the researchers say, including delays between infection and diagnosis, mobility of populations, and limitations in tracing sex and drug partners. They suggest that analysis of molecular data can help overcome some of those barriers, making it possible to identify clusters of ongoing HIV transmission.
The report also recommends other approaches to better understand and respond to ongoing HIV infections, such as mapping and data visualization, telemedicine, and automated data systems to facilitate linkage to care. However, the authors add, gaps in data and data systems remain that prevent full understanding of some key impacts of the epidemic. But any interventions to promote HIV prevention and treatment adherence, the authors suggested, should take a multifaceted approach and address the whole individual.
Chris Beyrer, MD, MPH, investigator at the Johns Hopkins Bloomberg School of Public Health, Baltimore, and a lead author on the series, says, “We have incredible tools to prevent and treat HIV, but people may not fully utilize them if they are facing personal or structural issues that pose more immediate hardship, like substance use and mental health disorders. You may struggle to take a daily medication if you are facing food insecurity or cannot find affordable treatment for your substance use disorder.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and now also Chief Medical Advisor to President Biden, says, “To end the HIV epidemic, we must continue to develop and deploy novel HIV treatment and prevention strategies suited to the different needs and preferences of diverse populations disproportionately affected by HIV. It is also essential that HIV health services continue during the COVID-19 pandemic.”
Doxorubicin-pomalidomide combo shows promise for Kaposi sarcoma
Liposomal doxorubicin (Dox) plus pomalidomide (Pom) was safe and active in heavily pretreated patients with Kaposi sarcoma, according to results from a phase 1/2 trial.
“The results of our phase 1/2 study suggest pomalidomide and liposomal doxorubicin is safe with evidence of activity among patients with Kaposi sarcoma,” said investigator Ramya Ramaswami, MBBS, MPH, of the HIV & AIDS malignancy branch at the National Cancer Institute. The results were presented at the Conference on Retroviruses and Opportunistic Infections.
The researchers evaluated the safety and tolerability of Pom/Dox in two groups of patients with Kaposi sarcoma: group 1 included patients with Kaposi sarcoma alone and group 2 included patients with Kaposi sarcoma–associated herpesvirus and concurrent multicentric Castleman disease (KSHV-MCD) and KSHV inflammatory cytokine syndrome (KICS).
“Kaposi sarcoma can be challenging to treat when it co-occurs with KSHV-MCD or KICS, resulting in high mortality rates,” Dr. Ramaswami explained.
Study participants received IV liposomal Dox at 20 mg/m2 on day 1 of a 28-day cycle, in addition to oral Pom once daily on days 1-21 at three escalating dose levels (2 mg, 3 mg, or 4 mg, respectively) using a standard 3 + 3 design until plateau of response, progression, dose-limiting toxicities (DLTs) or patient preference. Some eligibility criteria differed between groups 1 and 2. Participants in group 1 were required to be on antiretroviral therapy for at least 1 month and had a performance status of 2 or less, while those in group 2 had a performance status of 3 or less and could be antiretroviral therapy naive.
All participants received oral aspirin thromboprophylaxis (81 mg daily) and could have received prior Kaposi sarcoma therapy.
With respect to outcomes, Kaposi sarcoma responses were assessed using the modified AIDS Clinical Trial Group criteria and KICS and KSHV-MCD responses were evaluated using an NCI clinical benefit criteria.
Results
Overall, 34 cisgender men were enrolled in the study: 21 (62%) in group 1 and 13 (38%) in group 2. All participants had severe (T1) Kaposi sarcoma; 32 (94%) participants were HIV-infected and 22 (65%) had prior chemotherapy for Kaposi sarcoma.
While the HIV viral load was largely controlled in both groups, the CD4 count differed, with median CD4 counts of 286 and 92 cells/mcL in groups 1 and 2, respectively.
With respect to safety, no DLTs were observed in group 1. As a result, 12 participants were treated at the maximum tolerated dose (MTD) of 4 mg of Pom. However, two DLTs (grade 3 rash and pharyngeal edema) were observed in group 2 at the 3 mg dose level.
A median of six cycles were administered for all participants and the most common grade 3/4 toxicity was neutropenia; nine patients with grade 3 neutropenia required dose reduction and three patients had febrile neutropenia requiring hospitalization. Other Pom-related adverse events were rash, constipation, and fatigue.
Among evaluable participants receiving two or more cycles, 17 (81%) patients in group 1 had a response (95% confidence interval, 58-95%; 16 partial response and 1 complete response) and 5 (50%) patients in group 2 had a response (95% CI, 19-81%; 4 PR and 1 CR).
“Our waterfall plots indicated that the vast majority of patients in group 1 had a positive change in nodular lesions at baseline,” Dr. Ramaswami said. “Participants in group 2 showed some decrease in nodular lesions, but this was usually temporary.”
Among seven participants with KICS responses, four participants (57%) experienced a CR or PR in symptoms and lab abnormalities associated with KICS; three of six participants (50%) with KSHV-MCD responses experienced a PR as per response criteria.
“While activity was noted, the combination was less well tolerated in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS,” Dr. Ramaswami said.
During a live discussion, Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles, asked Dr. Ramaswami about the use of liposomal doxorubicin alone in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS.
While there is currently no data on the use of doxorubicin alone in this population, Dr. Ramaswami noted that she was more confident administering Pom/Dox combination therapy for these patients.
Dr. Ramaswami disclosed financial relationships with the National Cancer Institute, Celgene/Bristol-Myers Squibb, EMD Serono, Merck, CTI Biopharma, and Janssen. The study was funded by a cooperative research and drug development agreement between the National Cancer Institute and Celgene/BMS, EMD Serono, Merck, CTI Biopharma, and Janssen.
Liposomal doxorubicin (Dox) plus pomalidomide (Pom) was safe and active in heavily pretreated patients with Kaposi sarcoma, according to results from a phase 1/2 trial.
“The results of our phase 1/2 study suggest pomalidomide and liposomal doxorubicin is safe with evidence of activity among patients with Kaposi sarcoma,” said investigator Ramya Ramaswami, MBBS, MPH, of the HIV & AIDS malignancy branch at the National Cancer Institute. The results were presented at the Conference on Retroviruses and Opportunistic Infections.
The researchers evaluated the safety and tolerability of Pom/Dox in two groups of patients with Kaposi sarcoma: group 1 included patients with Kaposi sarcoma alone and group 2 included patients with Kaposi sarcoma–associated herpesvirus and concurrent multicentric Castleman disease (KSHV-MCD) and KSHV inflammatory cytokine syndrome (KICS).
“Kaposi sarcoma can be challenging to treat when it co-occurs with KSHV-MCD or KICS, resulting in high mortality rates,” Dr. Ramaswami explained.
Study participants received IV liposomal Dox at 20 mg/m2 on day 1 of a 28-day cycle, in addition to oral Pom once daily on days 1-21 at three escalating dose levels (2 mg, 3 mg, or 4 mg, respectively) using a standard 3 + 3 design until plateau of response, progression, dose-limiting toxicities (DLTs) or patient preference. Some eligibility criteria differed between groups 1 and 2. Participants in group 1 were required to be on antiretroviral therapy for at least 1 month and had a performance status of 2 or less, while those in group 2 had a performance status of 3 or less and could be antiretroviral therapy naive.
All participants received oral aspirin thromboprophylaxis (81 mg daily) and could have received prior Kaposi sarcoma therapy.
With respect to outcomes, Kaposi sarcoma responses were assessed using the modified AIDS Clinical Trial Group criteria and KICS and KSHV-MCD responses were evaluated using an NCI clinical benefit criteria.
Results
Overall, 34 cisgender men were enrolled in the study: 21 (62%) in group 1 and 13 (38%) in group 2. All participants had severe (T1) Kaposi sarcoma; 32 (94%) participants were HIV-infected and 22 (65%) had prior chemotherapy for Kaposi sarcoma.
While the HIV viral load was largely controlled in both groups, the CD4 count differed, with median CD4 counts of 286 and 92 cells/mcL in groups 1 and 2, respectively.
With respect to safety, no DLTs were observed in group 1. As a result, 12 participants were treated at the maximum tolerated dose (MTD) of 4 mg of Pom. However, two DLTs (grade 3 rash and pharyngeal edema) were observed in group 2 at the 3 mg dose level.
A median of six cycles were administered for all participants and the most common grade 3/4 toxicity was neutropenia; nine patients with grade 3 neutropenia required dose reduction and three patients had febrile neutropenia requiring hospitalization. Other Pom-related adverse events were rash, constipation, and fatigue.
Among evaluable participants receiving two or more cycles, 17 (81%) patients in group 1 had a response (95% confidence interval, 58-95%; 16 partial response and 1 complete response) and 5 (50%) patients in group 2 had a response (95% CI, 19-81%; 4 PR and 1 CR).
“Our waterfall plots indicated that the vast majority of patients in group 1 had a positive change in nodular lesions at baseline,” Dr. Ramaswami said. “Participants in group 2 showed some decrease in nodular lesions, but this was usually temporary.”
Among seven participants with KICS responses, four participants (57%) experienced a CR or PR in symptoms and lab abnormalities associated with KICS; three of six participants (50%) with KSHV-MCD responses experienced a PR as per response criteria.
“While activity was noted, the combination was less well tolerated in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS,” Dr. Ramaswami said.
During a live discussion, Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles, asked Dr. Ramaswami about the use of liposomal doxorubicin alone in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS.
While there is currently no data on the use of doxorubicin alone in this population, Dr. Ramaswami noted that she was more confident administering Pom/Dox combination therapy for these patients.
Dr. Ramaswami disclosed financial relationships with the National Cancer Institute, Celgene/Bristol-Myers Squibb, EMD Serono, Merck, CTI Biopharma, and Janssen. The study was funded by a cooperative research and drug development agreement between the National Cancer Institute and Celgene/BMS, EMD Serono, Merck, CTI Biopharma, and Janssen.
Liposomal doxorubicin (Dox) plus pomalidomide (Pom) was safe and active in heavily pretreated patients with Kaposi sarcoma, according to results from a phase 1/2 trial.
“The results of our phase 1/2 study suggest pomalidomide and liposomal doxorubicin is safe with evidence of activity among patients with Kaposi sarcoma,” said investigator Ramya Ramaswami, MBBS, MPH, of the HIV & AIDS malignancy branch at the National Cancer Institute. The results were presented at the Conference on Retroviruses and Opportunistic Infections.
The researchers evaluated the safety and tolerability of Pom/Dox in two groups of patients with Kaposi sarcoma: group 1 included patients with Kaposi sarcoma alone and group 2 included patients with Kaposi sarcoma–associated herpesvirus and concurrent multicentric Castleman disease (KSHV-MCD) and KSHV inflammatory cytokine syndrome (KICS).
“Kaposi sarcoma can be challenging to treat when it co-occurs with KSHV-MCD or KICS, resulting in high mortality rates,” Dr. Ramaswami explained.
Study participants received IV liposomal Dox at 20 mg/m2 on day 1 of a 28-day cycle, in addition to oral Pom once daily on days 1-21 at three escalating dose levels (2 mg, 3 mg, or 4 mg, respectively) using a standard 3 + 3 design until plateau of response, progression, dose-limiting toxicities (DLTs) or patient preference. Some eligibility criteria differed between groups 1 and 2. Participants in group 1 were required to be on antiretroviral therapy for at least 1 month and had a performance status of 2 or less, while those in group 2 had a performance status of 3 or less and could be antiretroviral therapy naive.
All participants received oral aspirin thromboprophylaxis (81 mg daily) and could have received prior Kaposi sarcoma therapy.
With respect to outcomes, Kaposi sarcoma responses were assessed using the modified AIDS Clinical Trial Group criteria and KICS and KSHV-MCD responses were evaluated using an NCI clinical benefit criteria.
Results
Overall, 34 cisgender men were enrolled in the study: 21 (62%) in group 1 and 13 (38%) in group 2. All participants had severe (T1) Kaposi sarcoma; 32 (94%) participants were HIV-infected and 22 (65%) had prior chemotherapy for Kaposi sarcoma.
While the HIV viral load was largely controlled in both groups, the CD4 count differed, with median CD4 counts of 286 and 92 cells/mcL in groups 1 and 2, respectively.
With respect to safety, no DLTs were observed in group 1. As a result, 12 participants were treated at the maximum tolerated dose (MTD) of 4 mg of Pom. However, two DLTs (grade 3 rash and pharyngeal edema) were observed in group 2 at the 3 mg dose level.
A median of six cycles were administered for all participants and the most common grade 3/4 toxicity was neutropenia; nine patients with grade 3 neutropenia required dose reduction and three patients had febrile neutropenia requiring hospitalization. Other Pom-related adverse events were rash, constipation, and fatigue.
Among evaluable participants receiving two or more cycles, 17 (81%) patients in group 1 had a response (95% confidence interval, 58-95%; 16 partial response and 1 complete response) and 5 (50%) patients in group 2 had a response (95% CI, 19-81%; 4 PR and 1 CR).
“Our waterfall plots indicated that the vast majority of patients in group 1 had a positive change in nodular lesions at baseline,” Dr. Ramaswami said. “Participants in group 2 showed some decrease in nodular lesions, but this was usually temporary.”
Among seven participants with KICS responses, four participants (57%) experienced a CR or PR in symptoms and lab abnormalities associated with KICS; three of six participants (50%) with KSHV-MCD responses experienced a PR as per response criteria.
“While activity was noted, the combination was less well tolerated in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS,” Dr. Ramaswami said.
During a live discussion, Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles, asked Dr. Ramaswami about the use of liposomal doxorubicin alone in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS.
While there is currently no data on the use of doxorubicin alone in this population, Dr. Ramaswami noted that she was more confident administering Pom/Dox combination therapy for these patients.
Dr. Ramaswami disclosed financial relationships with the National Cancer Institute, Celgene/Bristol-Myers Squibb, EMD Serono, Merck, CTI Biopharma, and Janssen. The study was funded by a cooperative research and drug development agreement between the National Cancer Institute and Celgene/BMS, EMD Serono, Merck, CTI Biopharma, and Janssen.
FROM CROI 2021
Vaginal pH may predict CIN 2 progression in HIV-positive women
Elevated vaginal pH at the time of cervical intraepithelial neoplasia 2 diagnosis may be a useful marker of CIN 2 persistence/progression, as well as the rate of persistence/progression in HIV-positive women, new research suggests.
“We analyzed data from the Women’s Interagency HIV Study [WIHS], an observational, longitudinal cohort of women with and without HIV to determine factors that may influence CIN 2 natural history,” said Kate Michel, PhD, MPH, of Georgetown University, Washington. She presented the results at the Conference on Retroviruses and Opportunistic Infections.
As previous data have shown a high incidence of CIN 2 progression among women with HIV, the researchers evaluated the role of human papillomavirus (HPV) type, local immune response, and markers of the cervicovaginal microbiome on the risk of CIN 2 persistence/progression.
Within the cohort, follow-up visits occur every 6 months, and clinical data is collected via questionnaires, physical and gynecologic exams, and biological samples. As no specific treatment is offered in the WIHS, treatment for cervical abnormalities is abstracted from medical records.
In the present study, Dr. Michel and colleagues selected up to four banked cervicovaginal lavage (CVL) samples per woman, with the first sample selected 6-12 months prior to CIN 2 diagnosis, the second at CIN 2 diagnosis, the third between CIN 2 diagnosis and outcome, and the fourth at the outcome visit.
The investigators performed HPV typing and muiltiplex immune mediator testing on each CVL sample. Lab results from WIHS core testing were also extracted, including plasma CD4+ T-cell count and HIV viral load, as well as vaginal pH and Nugent’s score.
Study outcomes included persistence/progression and regression, defined as a subsequent CIN 2 or CIN 3 diagnosis and subsequent CIN 1 or normal diagnosis, respectively. Logistic regression models were used to determine CIN 2 regression versus persistence/progression.
Results
A total of 337 samples were obtained and 94 women were included in the analysis. Key demographic and behavioral factor were similar at CIN 2 diagnosis.
The majority of participants were African American (53.2%) and on antiretroviral therapy (66.0%). The most prevalent high-risk types were HPV-58 (18.4%) and HPV-16 (17.5%).
After a median 12.5 years of follow-up, 33 participants (35.1%) with incident CIN 2 had a subsequent CIN 2/CIN 3 diagnosis and those who regressed had a higher CD4 T-cell count at CIN 2 diagnosis (P = .02).
Each subsequent high-risk HPV type identified at the pre–CIN 2 visit was associated with higher odds of CIN2 persistence/progression (odds ratio, 2.27; 95% confidence interval, 1.15-4.50).
Bacterial vaginosis (adjusted OR, 5.08; 95% CI, 1.30-19.94) and vaginal pH (aOR, 2.27; 95% CI, 1.15-4.50) at the CIN 2 diagnosis visit were each associated with increased odds of CIN 2 persistence/progression.
Vaginal pH greater than 4.5 at CIN 2 diagnosis was also associated with unadjusted time to CIN 2 persistence/progression (log rank P = .002) and an increased rate of CIN 2 persistence/progression (adjusted hazard ratio, 3.37; 95% CI, 1.26-8.99).
Furthermore, among participants who did not receive CIN 2 treatment, vaginal pH remained associated with greater odds of CIN 2 persistence/progression (OR, 2.46; 95% CI, 1.19-5.13). Cervicovaginal immune mediator levels were not associated with CIN 2 persistence/progression.
“The most striking finding from this work was that vaginal pH was associated with higher odds of, quicker time to, and increased hazard of CIN 2 persistence/progression,” Dr. Michel said. “We postulate this effect is mediated by the cervical microbiome, but more work is needed to establish the exact mechanism.”
“It would be interesting to test whether this association might be explained by different vaginal cleaning techniques, such as douching,” said moderator Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles.
“We’re currently working on an analysis of cervicovaginal bacterial species to explore the microbiome in more detail,” Dr. Michel concluded.
Dr. Michel disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Georgetown-Howard Universities Center for Clinical and Translational Science.
Elevated vaginal pH at the time of cervical intraepithelial neoplasia 2 diagnosis may be a useful marker of CIN 2 persistence/progression, as well as the rate of persistence/progression in HIV-positive women, new research suggests.
“We analyzed data from the Women’s Interagency HIV Study [WIHS], an observational, longitudinal cohort of women with and without HIV to determine factors that may influence CIN 2 natural history,” said Kate Michel, PhD, MPH, of Georgetown University, Washington. She presented the results at the Conference on Retroviruses and Opportunistic Infections.
As previous data have shown a high incidence of CIN 2 progression among women with HIV, the researchers evaluated the role of human papillomavirus (HPV) type, local immune response, and markers of the cervicovaginal microbiome on the risk of CIN 2 persistence/progression.
Within the cohort, follow-up visits occur every 6 months, and clinical data is collected via questionnaires, physical and gynecologic exams, and biological samples. As no specific treatment is offered in the WIHS, treatment for cervical abnormalities is abstracted from medical records.
In the present study, Dr. Michel and colleagues selected up to four banked cervicovaginal lavage (CVL) samples per woman, with the first sample selected 6-12 months prior to CIN 2 diagnosis, the second at CIN 2 diagnosis, the third between CIN 2 diagnosis and outcome, and the fourth at the outcome visit.
The investigators performed HPV typing and muiltiplex immune mediator testing on each CVL sample. Lab results from WIHS core testing were also extracted, including plasma CD4+ T-cell count and HIV viral load, as well as vaginal pH and Nugent’s score.
Study outcomes included persistence/progression and regression, defined as a subsequent CIN 2 or CIN 3 diagnosis and subsequent CIN 1 or normal diagnosis, respectively. Logistic regression models were used to determine CIN 2 regression versus persistence/progression.
Results
A total of 337 samples were obtained and 94 women were included in the analysis. Key demographic and behavioral factor were similar at CIN 2 diagnosis.
The majority of participants were African American (53.2%) and on antiretroviral therapy (66.0%). The most prevalent high-risk types were HPV-58 (18.4%) and HPV-16 (17.5%).
After a median 12.5 years of follow-up, 33 participants (35.1%) with incident CIN 2 had a subsequent CIN 2/CIN 3 diagnosis and those who regressed had a higher CD4 T-cell count at CIN 2 diagnosis (P = .02).
Each subsequent high-risk HPV type identified at the pre–CIN 2 visit was associated with higher odds of CIN2 persistence/progression (odds ratio, 2.27; 95% confidence interval, 1.15-4.50).
Bacterial vaginosis (adjusted OR, 5.08; 95% CI, 1.30-19.94) and vaginal pH (aOR, 2.27; 95% CI, 1.15-4.50) at the CIN 2 diagnosis visit were each associated with increased odds of CIN 2 persistence/progression.
Vaginal pH greater than 4.5 at CIN 2 diagnosis was also associated with unadjusted time to CIN 2 persistence/progression (log rank P = .002) and an increased rate of CIN 2 persistence/progression (adjusted hazard ratio, 3.37; 95% CI, 1.26-8.99).
Furthermore, among participants who did not receive CIN 2 treatment, vaginal pH remained associated with greater odds of CIN 2 persistence/progression (OR, 2.46; 95% CI, 1.19-5.13). Cervicovaginal immune mediator levels were not associated with CIN 2 persistence/progression.
“The most striking finding from this work was that vaginal pH was associated with higher odds of, quicker time to, and increased hazard of CIN 2 persistence/progression,” Dr. Michel said. “We postulate this effect is mediated by the cervical microbiome, but more work is needed to establish the exact mechanism.”
“It would be interesting to test whether this association might be explained by different vaginal cleaning techniques, such as douching,” said moderator Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles.
“We’re currently working on an analysis of cervicovaginal bacterial species to explore the microbiome in more detail,” Dr. Michel concluded.
Dr. Michel disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Georgetown-Howard Universities Center for Clinical and Translational Science.
Elevated vaginal pH at the time of cervical intraepithelial neoplasia 2 diagnosis may be a useful marker of CIN 2 persistence/progression, as well as the rate of persistence/progression in HIV-positive women, new research suggests.
“We analyzed data from the Women’s Interagency HIV Study [WIHS], an observational, longitudinal cohort of women with and without HIV to determine factors that may influence CIN 2 natural history,” said Kate Michel, PhD, MPH, of Georgetown University, Washington. She presented the results at the Conference on Retroviruses and Opportunistic Infections.
As previous data have shown a high incidence of CIN 2 progression among women with HIV, the researchers evaluated the role of human papillomavirus (HPV) type, local immune response, and markers of the cervicovaginal microbiome on the risk of CIN 2 persistence/progression.
Within the cohort, follow-up visits occur every 6 months, and clinical data is collected via questionnaires, physical and gynecologic exams, and biological samples. As no specific treatment is offered in the WIHS, treatment for cervical abnormalities is abstracted from medical records.
In the present study, Dr. Michel and colleagues selected up to four banked cervicovaginal lavage (CVL) samples per woman, with the first sample selected 6-12 months prior to CIN 2 diagnosis, the second at CIN 2 diagnosis, the third between CIN 2 diagnosis and outcome, and the fourth at the outcome visit.
The investigators performed HPV typing and muiltiplex immune mediator testing on each CVL sample. Lab results from WIHS core testing were also extracted, including plasma CD4+ T-cell count and HIV viral load, as well as vaginal pH and Nugent’s score.
Study outcomes included persistence/progression and regression, defined as a subsequent CIN 2 or CIN 3 diagnosis and subsequent CIN 1 or normal diagnosis, respectively. Logistic regression models were used to determine CIN 2 regression versus persistence/progression.
Results
A total of 337 samples were obtained and 94 women were included in the analysis. Key demographic and behavioral factor were similar at CIN 2 diagnosis.
The majority of participants were African American (53.2%) and on antiretroviral therapy (66.0%). The most prevalent high-risk types were HPV-58 (18.4%) and HPV-16 (17.5%).
After a median 12.5 years of follow-up, 33 participants (35.1%) with incident CIN 2 had a subsequent CIN 2/CIN 3 diagnosis and those who regressed had a higher CD4 T-cell count at CIN 2 diagnosis (P = .02).
Each subsequent high-risk HPV type identified at the pre–CIN 2 visit was associated with higher odds of CIN2 persistence/progression (odds ratio, 2.27; 95% confidence interval, 1.15-4.50).
Bacterial vaginosis (adjusted OR, 5.08; 95% CI, 1.30-19.94) and vaginal pH (aOR, 2.27; 95% CI, 1.15-4.50) at the CIN 2 diagnosis visit were each associated with increased odds of CIN 2 persistence/progression.
Vaginal pH greater than 4.5 at CIN 2 diagnosis was also associated with unadjusted time to CIN 2 persistence/progression (log rank P = .002) and an increased rate of CIN 2 persistence/progression (adjusted hazard ratio, 3.37; 95% CI, 1.26-8.99).
Furthermore, among participants who did not receive CIN 2 treatment, vaginal pH remained associated with greater odds of CIN 2 persistence/progression (OR, 2.46; 95% CI, 1.19-5.13). Cervicovaginal immune mediator levels were not associated with CIN 2 persistence/progression.
“The most striking finding from this work was that vaginal pH was associated with higher odds of, quicker time to, and increased hazard of CIN 2 persistence/progression,” Dr. Michel said. “We postulate this effect is mediated by the cervical microbiome, but more work is needed to establish the exact mechanism.”
“It would be interesting to test whether this association might be explained by different vaginal cleaning techniques, such as douching,” said moderator Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles.
“We’re currently working on an analysis of cervicovaginal bacterial species to explore the microbiome in more detail,” Dr. Michel concluded.
Dr. Michel disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Georgetown-Howard Universities Center for Clinical and Translational Science.
FROM CROI 2021
The vanguard of HIV care: Don’t forget this screening
In response, clinical care is continually adapting to the dramatically altered natural history of disease.
Today, the cutting edge of clinical care overlaps with primary care. The clinical vanguard addresses the medical vulnerabilities of patients with HIV, seeking to eliminate preventable morbidity and premature death. Among this clinical vanguard is the screening for and prevention of anal cancer. With the increased longevity of people living with HIV and the nearly universal exposure to human papillomavirus (HPV), there is now potential for progression to mucosal cellular dysplasia and eventual malignancy.
We know that prevention is possible because of the example of cervical cancer, the etiology of which is exposure to oncogenic serotypes of HPV (16 and 18 are most common). Screenings for cervical cancer (regular clinical examinations and Pap smears) and treatments to eliminate high-grade dysplasia have decreased the incidence rate by over 50% since the 1970s. Vaccination against HPV has been available since 2006 and offers the prospect of preventing HPV-associated malignancies, including head and neck cancer, in future decades.
However, rates of anal cancer are increasing. The CDC estimates that about 4,700 new cases of HPV-associated anal cancers are diagnosed in women and about 2,300 are diagnosed in men each year in the United States. Anal cancer rates in individuals with HIV have increased in the era of effective antiretrovirals and greater longevity. The highest rates, at 95 per 100,000, are in HIV-positive men who have sex with men. Very similar rates were noted in a more recent study that found increased risk with advancing age and in those with an AIDS diagnosis.
All patients with HIV should be screened
The New York State AIDS Institute Clinical Guidelines Program recommends screening for anal dysplasia in all patients with HIV. A proactive approach similar to cervical cancer screening is appropriate and includes measures easily implemented by all clinicians.
- History: Assess for rectal symptoms, anal pain, discharge, and lumps.
- Physical exam: Assess for presence of perianal lesions; perform a thorough digital rectal exam.
- Anal Pap test for anal cytology: Insert a Dacron swab moistened with tap water about 3 inches into the anal canal, applying pressure to lateral anal walls and rotating the swab. Then remove and place the swab into liquid cytology solution, shake vigorously for a full 30 seconds, and assess for any dysplasia (high-grade squamous intraepithelial lesion, low-grade intraepithelial lesion, atypical squamous cells of undetermined significance), which would warrant further evaluation by high-resolution anoscopy (HRA).
High-resolution anoscopy
HRA for anal dysplasia corresponds to colposcopy for cervical dysplasia. The ability to treat and eliminate high-risk precursor lesions interrupts the progression to malignancy. The efficacy of this strategy is being evaluated in a National Institutes of Health prospective trial called the Anchor Study. The epidemiology of HPV; the clinical horror of witnessing the painful, preventable deaths of young patients with well-controlled HIV caused by anal cancer; and the example of controlling cervical cancer have motivated my practice to assure comprehensive care for our patients.
Unfortunately, establishing HRA in one’s practice is challenging. Barriers to practice include the expense of required equipment and the absence of consensus on specific products. In addition, hands-on precepting to ease newcomers to competence is not generally available. Considerable skill is required for complete visualization of the anal transformative zone in the folds of the anal canal, and recognizing high-risk lesions requires study and accumulated experience. The International Anal Neoplasia Society is a useful resource that also offers a training course. We are invited to train ourselves, and to rely on the eventual feedback of biopsy results and the forbearance of our early patients.
The expanding scope of our medical practices must shift to meet the evolving needs of the growing population of virologically suppressed patients who are living longer. HIV care involves curing life-threatening opportunistic infections, encouraging antiretroviral adherence, and providing comprehensive care – which now includes preventing anal cancer.
A version of this article first appeared on Medscape.com.
In response, clinical care is continually adapting to the dramatically altered natural history of disease.
Today, the cutting edge of clinical care overlaps with primary care. The clinical vanguard addresses the medical vulnerabilities of patients with HIV, seeking to eliminate preventable morbidity and premature death. Among this clinical vanguard is the screening for and prevention of anal cancer. With the increased longevity of people living with HIV and the nearly universal exposure to human papillomavirus (HPV), there is now potential for progression to mucosal cellular dysplasia and eventual malignancy.
We know that prevention is possible because of the example of cervical cancer, the etiology of which is exposure to oncogenic serotypes of HPV (16 and 18 are most common). Screenings for cervical cancer (regular clinical examinations and Pap smears) and treatments to eliminate high-grade dysplasia have decreased the incidence rate by over 50% since the 1970s. Vaccination against HPV has been available since 2006 and offers the prospect of preventing HPV-associated malignancies, including head and neck cancer, in future decades.
However, rates of anal cancer are increasing. The CDC estimates that about 4,700 new cases of HPV-associated anal cancers are diagnosed in women and about 2,300 are diagnosed in men each year in the United States. Anal cancer rates in individuals with HIV have increased in the era of effective antiretrovirals and greater longevity. The highest rates, at 95 per 100,000, are in HIV-positive men who have sex with men. Very similar rates were noted in a more recent study that found increased risk with advancing age and in those with an AIDS diagnosis.
All patients with HIV should be screened
The New York State AIDS Institute Clinical Guidelines Program recommends screening for anal dysplasia in all patients with HIV. A proactive approach similar to cervical cancer screening is appropriate and includes measures easily implemented by all clinicians.
- History: Assess for rectal symptoms, anal pain, discharge, and lumps.
- Physical exam: Assess for presence of perianal lesions; perform a thorough digital rectal exam.
- Anal Pap test for anal cytology: Insert a Dacron swab moistened with tap water about 3 inches into the anal canal, applying pressure to lateral anal walls and rotating the swab. Then remove and place the swab into liquid cytology solution, shake vigorously for a full 30 seconds, and assess for any dysplasia (high-grade squamous intraepithelial lesion, low-grade intraepithelial lesion, atypical squamous cells of undetermined significance), which would warrant further evaluation by high-resolution anoscopy (HRA).
High-resolution anoscopy
HRA for anal dysplasia corresponds to colposcopy for cervical dysplasia. The ability to treat and eliminate high-risk precursor lesions interrupts the progression to malignancy. The efficacy of this strategy is being evaluated in a National Institutes of Health prospective trial called the Anchor Study. The epidemiology of HPV; the clinical horror of witnessing the painful, preventable deaths of young patients with well-controlled HIV caused by anal cancer; and the example of controlling cervical cancer have motivated my practice to assure comprehensive care for our patients.
Unfortunately, establishing HRA in one’s practice is challenging. Barriers to practice include the expense of required equipment and the absence of consensus on specific products. In addition, hands-on precepting to ease newcomers to competence is not generally available. Considerable skill is required for complete visualization of the anal transformative zone in the folds of the anal canal, and recognizing high-risk lesions requires study and accumulated experience. The International Anal Neoplasia Society is a useful resource that also offers a training course. We are invited to train ourselves, and to rely on the eventual feedback of biopsy results and the forbearance of our early patients.
The expanding scope of our medical practices must shift to meet the evolving needs of the growing population of virologically suppressed patients who are living longer. HIV care involves curing life-threatening opportunistic infections, encouraging antiretroviral adherence, and providing comprehensive care – which now includes preventing anal cancer.
A version of this article first appeared on Medscape.com.
In response, clinical care is continually adapting to the dramatically altered natural history of disease.
Today, the cutting edge of clinical care overlaps with primary care. The clinical vanguard addresses the medical vulnerabilities of patients with HIV, seeking to eliminate preventable morbidity and premature death. Among this clinical vanguard is the screening for and prevention of anal cancer. With the increased longevity of people living with HIV and the nearly universal exposure to human papillomavirus (HPV), there is now potential for progression to mucosal cellular dysplasia and eventual malignancy.
We know that prevention is possible because of the example of cervical cancer, the etiology of which is exposure to oncogenic serotypes of HPV (16 and 18 are most common). Screenings for cervical cancer (regular clinical examinations and Pap smears) and treatments to eliminate high-grade dysplasia have decreased the incidence rate by over 50% since the 1970s. Vaccination against HPV has been available since 2006 and offers the prospect of preventing HPV-associated malignancies, including head and neck cancer, in future decades.
However, rates of anal cancer are increasing. The CDC estimates that about 4,700 new cases of HPV-associated anal cancers are diagnosed in women and about 2,300 are diagnosed in men each year in the United States. Anal cancer rates in individuals with HIV have increased in the era of effective antiretrovirals and greater longevity. The highest rates, at 95 per 100,000, are in HIV-positive men who have sex with men. Very similar rates were noted in a more recent study that found increased risk with advancing age and in those with an AIDS diagnosis.
All patients with HIV should be screened
The New York State AIDS Institute Clinical Guidelines Program recommends screening for anal dysplasia in all patients with HIV. A proactive approach similar to cervical cancer screening is appropriate and includes measures easily implemented by all clinicians.
- History: Assess for rectal symptoms, anal pain, discharge, and lumps.
- Physical exam: Assess for presence of perianal lesions; perform a thorough digital rectal exam.
- Anal Pap test for anal cytology: Insert a Dacron swab moistened with tap water about 3 inches into the anal canal, applying pressure to lateral anal walls and rotating the swab. Then remove and place the swab into liquid cytology solution, shake vigorously for a full 30 seconds, and assess for any dysplasia (high-grade squamous intraepithelial lesion, low-grade intraepithelial lesion, atypical squamous cells of undetermined significance), which would warrant further evaluation by high-resolution anoscopy (HRA).
High-resolution anoscopy
HRA for anal dysplasia corresponds to colposcopy for cervical dysplasia. The ability to treat and eliminate high-risk precursor lesions interrupts the progression to malignancy. The efficacy of this strategy is being evaluated in a National Institutes of Health prospective trial called the Anchor Study. The epidemiology of HPV; the clinical horror of witnessing the painful, preventable deaths of young patients with well-controlled HIV caused by anal cancer; and the example of controlling cervical cancer have motivated my practice to assure comprehensive care for our patients.
Unfortunately, establishing HRA in one’s practice is challenging. Barriers to practice include the expense of required equipment and the absence of consensus on specific products. In addition, hands-on precepting to ease newcomers to competence is not generally available. Considerable skill is required for complete visualization of the anal transformative zone in the folds of the anal canal, and recognizing high-risk lesions requires study and accumulated experience. The International Anal Neoplasia Society is a useful resource that also offers a training course. We are invited to train ourselves, and to rely on the eventual feedback of biopsy results and the forbearance of our early patients.
The expanding scope of our medical practices must shift to meet the evolving needs of the growing population of virologically suppressed patients who are living longer. HIV care involves curing life-threatening opportunistic infections, encouraging antiretroviral adherence, and providing comprehensive care – which now includes preventing anal cancer.
A version of this article first appeared on Medscape.com.
HBV viremia linked to HCC risk in HIV/HBV coinfection
Any level of hepatitis B virus (HBV) viremia was associated with increased hepatocellular carcinoma (HCC) risk in adults with HIV/HBV coinfection, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (Abstract 136).
“Chronic HBV coinfection is common among people with HIV, but the determinants of HBV-associated HCC are not well characterized,” said presenter H. Nina Kim MD, MSc, of the University of Washington, Seattle. “We sought to identify factors that contribute to HCC development in persons with HIV/HBV coinfection to guide early detection and prevention measures.”
The researchers conducted a longitudinal cohort study within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite cohorts throughout the United States and Canada; 22 cohorts from NA-ACCORD were included in the analysis.
Potential HIV and HBV risk factors were examined, including viremia and CD4 percentage, as well as HBV DNA levels. Traditional risk factors for liver disease progression, including age, sex, and heavy alcohol use, were also assessed.
Eligible patients were 18 years of age or older who were followed for at least 6 months, had evidence of chronic HBV, and had HIV RNA or CD4+ cell measurement during this period. Persons with prevalent HCC at baseline were excluded.
The primary outcome was first occurrence of HCC, which was adjudicated by medical chart review and/or cancer registry. Multivariable Cox regression was used to determine adjusted hazard ratios of risk factors.
Results
Among 9,383 HIV/HBV-coinfected individuals identified, 8,354 (89%) were included in the analysis. The median age of participants was 43 years and 93.1% were male. Heavy alcohol use (35.3%) and chronic hepatitis C virus (HCV) coinfection (21.6%) were common among participants.
Among 8,354 eligible participants, 115 developed HCC over a median 6.9 years of follow-up (incidence rate, 1.8 events per 1,000 person-years; 95% confidence interval [CI], 1.5-2.1).
Independent risk factors for HCC were chronic HCV coinfection (adjusted hazard ratio [aHR], 1.60 [95% confidence interval, 1.07-2.39]), age 40 years and older (aHR, 2.14 [1.36-3.37]), and heavy alcohol use (aHR, 1.51 [1.03-2.21]); however, time-updated CD4+ percentage less than 14% (aHR, 1.03 [0.56-1.90]) and time-updated HIV RNA level over 500 copies/mL (aHR, 0.88 [0.55-1.41]) were not associated with HCC risk.
In a second model, among 3,054 patients who had HBV DNA measured, the risk of HCC was higher with HBV DNA levels greater than 200 IU/mL (aHR, 2.70 [1.23-5.93]), and the risk was particularly elevated at levels greater than 200,000 IU/mL (aHR, 4.34 [1.72-10.94]).
The researchers also found that the risk of HCC was significantly lower in patients with HBV DNA suppression less than 200 IU/mL receiving HBV-active ART for 1 year or more (aHR, 0.42 [0.24-0.73]). In addition, a dose-response relationship was observed between the duration of suppression and this protective effect.
Dr. Nina Kim acknowledged that a key limitation of the study was inconsistent monitoring of HBV DNA level while patients were on treatment. Furthermore, given the demographics of the cohort, these results may not be generalizable outside of North America.
“Our study was the first to show that any level of HBV viremia using 200 as a threshold of detection was associated with HCC risk in a large regionally diverse cohort of adults outside of Asia,” Dr. Kim said. “To gain maximal protective benefit from antiviral therapy for HCC prevention, sustained and ideally uninterrupted suppression of HBV may be necessary over years.”
“HIV/HBV coinfected patients can take much longer than a year to achieve less than 200 copies on HBV DNA due to their baseline levels, but we still don’t know if HBV therapy intensification could hasten this process,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
Dr. Kim disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the National Cancer Institute.
Any level of hepatitis B virus (HBV) viremia was associated with increased hepatocellular carcinoma (HCC) risk in adults with HIV/HBV coinfection, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (Abstract 136).
“Chronic HBV coinfection is common among people with HIV, but the determinants of HBV-associated HCC are not well characterized,” said presenter H. Nina Kim MD, MSc, of the University of Washington, Seattle. “We sought to identify factors that contribute to HCC development in persons with HIV/HBV coinfection to guide early detection and prevention measures.”
The researchers conducted a longitudinal cohort study within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite cohorts throughout the United States and Canada; 22 cohorts from NA-ACCORD were included in the analysis.
Potential HIV and HBV risk factors were examined, including viremia and CD4 percentage, as well as HBV DNA levels. Traditional risk factors for liver disease progression, including age, sex, and heavy alcohol use, were also assessed.
Eligible patients were 18 years of age or older who were followed for at least 6 months, had evidence of chronic HBV, and had HIV RNA or CD4+ cell measurement during this period. Persons with prevalent HCC at baseline were excluded.
The primary outcome was first occurrence of HCC, which was adjudicated by medical chart review and/or cancer registry. Multivariable Cox regression was used to determine adjusted hazard ratios of risk factors.
Results
Among 9,383 HIV/HBV-coinfected individuals identified, 8,354 (89%) were included in the analysis. The median age of participants was 43 years and 93.1% were male. Heavy alcohol use (35.3%) and chronic hepatitis C virus (HCV) coinfection (21.6%) were common among participants.
Among 8,354 eligible participants, 115 developed HCC over a median 6.9 years of follow-up (incidence rate, 1.8 events per 1,000 person-years; 95% confidence interval [CI], 1.5-2.1).
Independent risk factors for HCC were chronic HCV coinfection (adjusted hazard ratio [aHR], 1.60 [95% confidence interval, 1.07-2.39]), age 40 years and older (aHR, 2.14 [1.36-3.37]), and heavy alcohol use (aHR, 1.51 [1.03-2.21]); however, time-updated CD4+ percentage less than 14% (aHR, 1.03 [0.56-1.90]) and time-updated HIV RNA level over 500 copies/mL (aHR, 0.88 [0.55-1.41]) were not associated with HCC risk.
In a second model, among 3,054 patients who had HBV DNA measured, the risk of HCC was higher with HBV DNA levels greater than 200 IU/mL (aHR, 2.70 [1.23-5.93]), and the risk was particularly elevated at levels greater than 200,000 IU/mL (aHR, 4.34 [1.72-10.94]).
The researchers also found that the risk of HCC was significantly lower in patients with HBV DNA suppression less than 200 IU/mL receiving HBV-active ART for 1 year or more (aHR, 0.42 [0.24-0.73]). In addition, a dose-response relationship was observed between the duration of suppression and this protective effect.
Dr. Nina Kim acknowledged that a key limitation of the study was inconsistent monitoring of HBV DNA level while patients were on treatment. Furthermore, given the demographics of the cohort, these results may not be generalizable outside of North America.
“Our study was the first to show that any level of HBV viremia using 200 as a threshold of detection was associated with HCC risk in a large regionally diverse cohort of adults outside of Asia,” Dr. Kim said. “To gain maximal protective benefit from antiviral therapy for HCC prevention, sustained and ideally uninterrupted suppression of HBV may be necessary over years.”
“HIV/HBV coinfected patients can take much longer than a year to achieve less than 200 copies on HBV DNA due to their baseline levels, but we still don’t know if HBV therapy intensification could hasten this process,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
Dr. Kim disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the National Cancer Institute.
Any level of hepatitis B virus (HBV) viremia was associated with increased hepatocellular carcinoma (HCC) risk in adults with HIV/HBV coinfection, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (Abstract 136).
“Chronic HBV coinfection is common among people with HIV, but the determinants of HBV-associated HCC are not well characterized,” said presenter H. Nina Kim MD, MSc, of the University of Washington, Seattle. “We sought to identify factors that contribute to HCC development in persons with HIV/HBV coinfection to guide early detection and prevention measures.”
The researchers conducted a longitudinal cohort study within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite cohorts throughout the United States and Canada; 22 cohorts from NA-ACCORD were included in the analysis.
Potential HIV and HBV risk factors were examined, including viremia and CD4 percentage, as well as HBV DNA levels. Traditional risk factors for liver disease progression, including age, sex, and heavy alcohol use, were also assessed.
Eligible patients were 18 years of age or older who were followed for at least 6 months, had evidence of chronic HBV, and had HIV RNA or CD4+ cell measurement during this period. Persons with prevalent HCC at baseline were excluded.
The primary outcome was first occurrence of HCC, which was adjudicated by medical chart review and/or cancer registry. Multivariable Cox regression was used to determine adjusted hazard ratios of risk factors.
Results
Among 9,383 HIV/HBV-coinfected individuals identified, 8,354 (89%) were included in the analysis. The median age of participants was 43 years and 93.1% were male. Heavy alcohol use (35.3%) and chronic hepatitis C virus (HCV) coinfection (21.6%) were common among participants.
Among 8,354 eligible participants, 115 developed HCC over a median 6.9 years of follow-up (incidence rate, 1.8 events per 1,000 person-years; 95% confidence interval [CI], 1.5-2.1).
Independent risk factors for HCC were chronic HCV coinfection (adjusted hazard ratio [aHR], 1.60 [95% confidence interval, 1.07-2.39]), age 40 years and older (aHR, 2.14 [1.36-3.37]), and heavy alcohol use (aHR, 1.51 [1.03-2.21]); however, time-updated CD4+ percentage less than 14% (aHR, 1.03 [0.56-1.90]) and time-updated HIV RNA level over 500 copies/mL (aHR, 0.88 [0.55-1.41]) were not associated with HCC risk.
In a second model, among 3,054 patients who had HBV DNA measured, the risk of HCC was higher with HBV DNA levels greater than 200 IU/mL (aHR, 2.70 [1.23-5.93]), and the risk was particularly elevated at levels greater than 200,000 IU/mL (aHR, 4.34 [1.72-10.94]).
The researchers also found that the risk of HCC was significantly lower in patients with HBV DNA suppression less than 200 IU/mL receiving HBV-active ART for 1 year or more (aHR, 0.42 [0.24-0.73]). In addition, a dose-response relationship was observed between the duration of suppression and this protective effect.
Dr. Nina Kim acknowledged that a key limitation of the study was inconsistent monitoring of HBV DNA level while patients were on treatment. Furthermore, given the demographics of the cohort, these results may not be generalizable outside of North America.
“Our study was the first to show that any level of HBV viremia using 200 as a threshold of detection was associated with HCC risk in a large regionally diverse cohort of adults outside of Asia,” Dr. Kim said. “To gain maximal protective benefit from antiviral therapy for HCC prevention, sustained and ideally uninterrupted suppression of HBV may be necessary over years.”
“HIV/HBV coinfected patients can take much longer than a year to achieve less than 200 copies on HBV DNA due to their baseline levels, but we still don’t know if HBV therapy intensification could hasten this process,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
Dr. Kim disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the National Cancer Institute.
FROM CROI 2021
Infectious diseases ‘giant’ John Bartlett: His ‘impact will endure’
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.