User login
Sex workers: High rates of HIV, low rates of treatment
The HIV pandemic among sex workers remains underaddressed and underresourced, with “glaring gaps” in comprehensive measures of HIV prevalence and incidence, and in prevention and treatment, according to the results of an updated literature review published in the Lancet.
Kate Shannon, PhD, director of the gender and sexual health initiative at the University of British Columbia, Vancouver, and her colleagues updated a 2013 literature search for the Lancet series on HIV and sex workers to include reports and manuscripts published from Jan. 1, 2006, to Sept. 6, 2017.
They found that In particular, 4 years after their previous Lancet series on HIV and sex workers, this updated analysis showed that the global HIV burden among female sex workers was still similar to the previously determined 11.8% and “unacceptably high” at 10.4%, (95% confidence interval, 9.5-11.5).
Although there has been some improvement in the assessment of HIV in transgender women since the previous analysis, according to Dr. Shannon and her colleagues, small sample sizes and conflation of transgender women and men who have sex with men (MSM) continue to limit the volume of transgender-specific HIV data, particularly in Africa.
Access to HIV prevention and treatment also remains a considerable problem for sex workers, according to the authors. In particular, “qualitative data in sub-Saharan Africa suggest that profound structural barriers of stigma and discrimination impede progress in the HIV care continuum,” with studies confirming that “successful HIV treatment trajectories are impeded by violence and displacement” because of policing, they wrote.
They pointed out that things may well become worse, with evidence-based progress on full decriminalization grounded in health and human rights – which was a key recommendation in their earlier Lancet Series – having stalled in all but South Africa. In fact, they reported that several countries had even rolled back rights further for sex workers.
“HIV prevention and treatment tools are available but, without comprehensive HIV epidemiology, a lack of denominators and failure to address structural determinants (including decriminalisation of sex work) means that progress in achieving health and rights for all sex workers will fall short,” the researchers concluded.
The authors reported that they had no competing interests.
SOURCE: Shannon K et al. Lancet. 2018 Aug 25;392:698-710.
The HIV pandemic among sex workers remains underaddressed and underresourced, with “glaring gaps” in comprehensive measures of HIV prevalence and incidence, and in prevention and treatment, according to the results of an updated literature review published in the Lancet.
Kate Shannon, PhD, director of the gender and sexual health initiative at the University of British Columbia, Vancouver, and her colleagues updated a 2013 literature search for the Lancet series on HIV and sex workers to include reports and manuscripts published from Jan. 1, 2006, to Sept. 6, 2017.
They found that In particular, 4 years after their previous Lancet series on HIV and sex workers, this updated analysis showed that the global HIV burden among female sex workers was still similar to the previously determined 11.8% and “unacceptably high” at 10.4%, (95% confidence interval, 9.5-11.5).
Although there has been some improvement in the assessment of HIV in transgender women since the previous analysis, according to Dr. Shannon and her colleagues, small sample sizes and conflation of transgender women and men who have sex with men (MSM) continue to limit the volume of transgender-specific HIV data, particularly in Africa.
Access to HIV prevention and treatment also remains a considerable problem for sex workers, according to the authors. In particular, “qualitative data in sub-Saharan Africa suggest that profound structural barriers of stigma and discrimination impede progress in the HIV care continuum,” with studies confirming that “successful HIV treatment trajectories are impeded by violence and displacement” because of policing, they wrote.
They pointed out that things may well become worse, with evidence-based progress on full decriminalization grounded in health and human rights – which was a key recommendation in their earlier Lancet Series – having stalled in all but South Africa. In fact, they reported that several countries had even rolled back rights further for sex workers.
“HIV prevention and treatment tools are available but, without comprehensive HIV epidemiology, a lack of denominators and failure to address structural determinants (including decriminalisation of sex work) means that progress in achieving health and rights for all sex workers will fall short,” the researchers concluded.
The authors reported that they had no competing interests.
SOURCE: Shannon K et al. Lancet. 2018 Aug 25;392:698-710.
The HIV pandemic among sex workers remains underaddressed and underresourced, with “glaring gaps” in comprehensive measures of HIV prevalence and incidence, and in prevention and treatment, according to the results of an updated literature review published in the Lancet.
Kate Shannon, PhD, director of the gender and sexual health initiative at the University of British Columbia, Vancouver, and her colleagues updated a 2013 literature search for the Lancet series on HIV and sex workers to include reports and manuscripts published from Jan. 1, 2006, to Sept. 6, 2017.
They found that In particular, 4 years after their previous Lancet series on HIV and sex workers, this updated analysis showed that the global HIV burden among female sex workers was still similar to the previously determined 11.8% and “unacceptably high” at 10.4%, (95% confidence interval, 9.5-11.5).
Although there has been some improvement in the assessment of HIV in transgender women since the previous analysis, according to Dr. Shannon and her colleagues, small sample sizes and conflation of transgender women and men who have sex with men (MSM) continue to limit the volume of transgender-specific HIV data, particularly in Africa.
Access to HIV prevention and treatment also remains a considerable problem for sex workers, according to the authors. In particular, “qualitative data in sub-Saharan Africa suggest that profound structural barriers of stigma and discrimination impede progress in the HIV care continuum,” with studies confirming that “successful HIV treatment trajectories are impeded by violence and displacement” because of policing, they wrote.
They pointed out that things may well become worse, with evidence-based progress on full decriminalization grounded in health and human rights – which was a key recommendation in their earlier Lancet Series – having stalled in all but South Africa. In fact, they reported that several countries had even rolled back rights further for sex workers.
“HIV prevention and treatment tools are available but, without comprehensive HIV epidemiology, a lack of denominators and failure to address structural determinants (including decriminalisation of sex work) means that progress in achieving health and rights for all sex workers will fall short,” the researchers concluded.
The authors reported that they had no competing interests.
SOURCE: Shannon K et al. Lancet. 2018 Aug 25;392:698-710.
FROM THE LANCET
Key clinical point: The HIV pandemic among sex workers remains underaddressed and underresourced, with “glaring gaps” in assessment, treatment.
Major finding: The global HIV burden among female sex workers shows that HIV prevalence was “unacceptably high” at 10.4%.
Study details: Researchers updated a 2013 literature review with reports published from Jan. 1, 2006, to Sept. 6, 2017.
Disclosures: The authors reported that they had no competing interests.
Source: Shannon K et al. Lancet. 2018 Aug 25;392:698-710.
Virus-specific T-cell infusion may resolve progressive multifocal leukoencephalopathy
, according to investigators from the University of Texas MD Anderson Cancer Center, Houston.
The infusion cleared JC virus from the cerebrospinal fluid (CSF) of two patients and reduced viral load in the third, reported lead author Muharrem Muftuoglu, MD, of MD Anderson’s department of stem cell transplantation and cellular therapy and colleagues. One of the patients completely recovered and returned to work.
“Several approaches for the treatment of PML, including the use of antiviral medications and mirtazapine, have been tested, with poor results,” the investigators wrote in the New England Journal of Medicine. Although virus-specific T-cell infusion is a novel approach to treating PML, this method has been used for other conditions.
“Several groups, including ours, have successfully used viral-specific T cells to treat BK virus infection after stem-cell transplantation,” the investigators wrote. “Because BK virus and JC virus are genetically similar to one another and share a number of immunogenic proteins with a substantial degree of sequence homology ... we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
This hypothesis proved accurate. The investigators infused three PML patients with “cryopreserved, third-party–produced, viral-specific T cells that had been designed for the treatment of patients with BK virus infection after stem-cell transplantation.” Each patient presented with a different condition and PML-precipitating therapy. The first patient was a 32-year-old woman with high-risk acute myeloid leukemia who had received a cord-blood transplantation, the second a 73-year-old woman with JAK2-positive myeloproliferative neoplasia on ruxolitinib (Jakafi) therapy, and the third a 35-year-old man with HIV who had received highly active antiretroviral therapy.
T-cell infusions cleared JC virus from the CSF of the woman with leukemia (three infusions) and the man with HIV (four infusions). These patients recovered to different degrees: The woman had full resolution of symptoms, while the man had slurred speech and walked with a cane. Treatment reduced JC viral load in the elderly woman with myeloproliferative neoplasia (two infusions), but she did not clear the virus and died about 8 months later.
No adverse events occurred, but two patients developed immune reconstitution inflammatory syndrome. This was likely caused by the T-cell infusion, since absolute T-cell counts remained steady and white matter enhancement was detected on MRI within 4 weeks of treatment. Still, the investigators were optimistic about future potential.
“Third-party–produced, ‘off-the-shelf,’ partially HLA-matched, BK virus–specific T cells may serve as therapy for PML,” the investigators concluded. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events associated with this treatment.”
The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
SOURCE: Muftuoglu M et al. N Engl J Med. 2018 Oct 11;379:1443-51
This article was updated 3/22/19.
, according to investigators from the University of Texas MD Anderson Cancer Center, Houston.
The infusion cleared JC virus from the cerebrospinal fluid (CSF) of two patients and reduced viral load in the third, reported lead author Muharrem Muftuoglu, MD, of MD Anderson’s department of stem cell transplantation and cellular therapy and colleagues. One of the patients completely recovered and returned to work.
“Several approaches for the treatment of PML, including the use of antiviral medications and mirtazapine, have been tested, with poor results,” the investigators wrote in the New England Journal of Medicine. Although virus-specific T-cell infusion is a novel approach to treating PML, this method has been used for other conditions.
“Several groups, including ours, have successfully used viral-specific T cells to treat BK virus infection after stem-cell transplantation,” the investigators wrote. “Because BK virus and JC virus are genetically similar to one another and share a number of immunogenic proteins with a substantial degree of sequence homology ... we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
This hypothesis proved accurate. The investigators infused three PML patients with “cryopreserved, third-party–produced, viral-specific T cells that had been designed for the treatment of patients with BK virus infection after stem-cell transplantation.” Each patient presented with a different condition and PML-precipitating therapy. The first patient was a 32-year-old woman with high-risk acute myeloid leukemia who had received a cord-blood transplantation, the second a 73-year-old woman with JAK2-positive myeloproliferative neoplasia on ruxolitinib (Jakafi) therapy, and the third a 35-year-old man with HIV who had received highly active antiretroviral therapy.
T-cell infusions cleared JC virus from the CSF of the woman with leukemia (three infusions) and the man with HIV (four infusions). These patients recovered to different degrees: The woman had full resolution of symptoms, while the man had slurred speech and walked with a cane. Treatment reduced JC viral load in the elderly woman with myeloproliferative neoplasia (two infusions), but she did not clear the virus and died about 8 months later.
No adverse events occurred, but two patients developed immune reconstitution inflammatory syndrome. This was likely caused by the T-cell infusion, since absolute T-cell counts remained steady and white matter enhancement was detected on MRI within 4 weeks of treatment. Still, the investigators were optimistic about future potential.
“Third-party–produced, ‘off-the-shelf,’ partially HLA-matched, BK virus–specific T cells may serve as therapy for PML,” the investigators concluded. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events associated with this treatment.”
The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
SOURCE: Muftuoglu M et al. N Engl J Med. 2018 Oct 11;379:1443-51
This article was updated 3/22/19.
, according to investigators from the University of Texas MD Anderson Cancer Center, Houston.
The infusion cleared JC virus from the cerebrospinal fluid (CSF) of two patients and reduced viral load in the third, reported lead author Muharrem Muftuoglu, MD, of MD Anderson’s department of stem cell transplantation and cellular therapy and colleagues. One of the patients completely recovered and returned to work.
“Several approaches for the treatment of PML, including the use of antiviral medications and mirtazapine, have been tested, with poor results,” the investigators wrote in the New England Journal of Medicine. Although virus-specific T-cell infusion is a novel approach to treating PML, this method has been used for other conditions.
“Several groups, including ours, have successfully used viral-specific T cells to treat BK virus infection after stem-cell transplantation,” the investigators wrote. “Because BK virus and JC virus are genetically similar to one another and share a number of immunogenic proteins with a substantial degree of sequence homology ... we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
This hypothesis proved accurate. The investigators infused three PML patients with “cryopreserved, third-party–produced, viral-specific T cells that had been designed for the treatment of patients with BK virus infection after stem-cell transplantation.” Each patient presented with a different condition and PML-precipitating therapy. The first patient was a 32-year-old woman with high-risk acute myeloid leukemia who had received a cord-blood transplantation, the second a 73-year-old woman with JAK2-positive myeloproliferative neoplasia on ruxolitinib (Jakafi) therapy, and the third a 35-year-old man with HIV who had received highly active antiretroviral therapy.
T-cell infusions cleared JC virus from the CSF of the woman with leukemia (three infusions) and the man with HIV (four infusions). These patients recovered to different degrees: The woman had full resolution of symptoms, while the man had slurred speech and walked with a cane. Treatment reduced JC viral load in the elderly woman with myeloproliferative neoplasia (two infusions), but she did not clear the virus and died about 8 months later.
No adverse events occurred, but two patients developed immune reconstitution inflammatory syndrome. This was likely caused by the T-cell infusion, since absolute T-cell counts remained steady and white matter enhancement was detected on MRI within 4 weeks of treatment. Still, the investigators were optimistic about future potential.
“Third-party–produced, ‘off-the-shelf,’ partially HLA-matched, BK virus–specific T cells may serve as therapy for PML,” the investigators concluded. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events associated with this treatment.”
The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
SOURCE: Muftuoglu M et al. N Engl J Med. 2018 Oct 11;379:1443-51
This article was updated 3/22/19.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Infusion of allogeneic BK virus-specific T cells may be an effective treatment for patients with PML.
Major finding: Two of three patients cleared JC virus from cerebrospinal fluid after infusion.
Study details: A case series involving three patients with PML.
Disclosures: The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
Source: Muftuoglu M et al. N Engl J Med. 2018 Oct 11;379:1443-51.
Automated algorithm improves HIV/HCV screening in the ED
More patients had newly diagnosed HIV and hepatitis C virus (HCV) infection during an automated-laboratory-order HIV/HCV screening algorithm than with a nurse-order HIV/HCV screening algorithm, according to the results of a retrospective before/after comparison study of the two electronic health record (EHR)–based protocols.
The results of nurse-order HIV/HCV screening in the 5-month period of March 1, 2016, through July 31, 2016, were compared to the subsequently adopted automated-laboratory-order system results from March 1, 2017, through July 31, 2017, according to Douglas A.E. White, MD, and his colleagues at Highland Hospital Emergency Department, Oakland, Calif.
Via the EHR, nurses were instructed to offer screening to all adults aged 18-75 years unless they were known to be HIV- or HCV-positive, unable to verbally consent (e.g., language barriers, intoxication), or medically unstable. Exclusion was at the discretion of the triage nurse. Using a drop-down menu, nurses could choose “accepts” or “declines” for HIV and HCV testing, according to patient response. Choosing “accepts” automatically ordered the test, according to the report (Ann Emerg Med. 2018 Oct;72[4]:438-48).
Automated-laboratory-order HIV/HCV screening was integrated into clinical care. With this protocol, the EHR-automated annual HIV/hepatitis C virus screening was performed on adult patients aged 18-75 years who had laboratory tests ordered. The EHR was configured to automatically order an HIV or HCV test for age-eligible patients who had any test ordered that required laboratory processing of whole blood (excluding point-of-care tests such as for lactate or glucose level) or a urine or urethral swab for chlamydia or gonorrhea testing, according to the researchers.
There were 20,975 and 19,887 unique, age-eligible patients during the nurse-order HIV/HCV virus screening algorithm and automated-laboratory-order HIV/HCV screening algorithm study periods, respectively. A total of 4,121 patients (19.6%) were screened for HIV and 2,968 (14.2%) patients were screened for HCV during the nurse-order period vs. 6,736 (33.9%) patients screened for HIV and 6,972 (35.1%) screened for HCV during the automated-laboratory-order period.
Overall, HIV screening increased from 19.6% to 33.9% and HCV screening, from 14.2% to 35.1% using the automated vs. the nurse-ordered EHR-based algorithm.
“An automated electronic health record algorithm that links nontargeted opt-out HIV and hepatitis C virus screening to physician laboratory ordering more effectively screens ED patients, provides results before discharge, minimizes repeated screening, and diagnoses more new infections than an algorithm that relies on nursing staff to offer screening. Because most EDs in the United States now use EHR systems, this model can be easily replicated and should be considered the standard for future programs,” the researchers concluded.
This work was supported, in part, by grant funding through the FOCUS program, Gilead Sciences, which also has provided funding to various of the authors of the study.
SOURCE: White DAE et al. Ann Emerg Med. 2018 Oct;72[4]:438-48.
More patients had newly diagnosed HIV and hepatitis C virus (HCV) infection during an automated-laboratory-order HIV/HCV screening algorithm than with a nurse-order HIV/HCV screening algorithm, according to the results of a retrospective before/after comparison study of the two electronic health record (EHR)–based protocols.
The results of nurse-order HIV/HCV screening in the 5-month period of March 1, 2016, through July 31, 2016, were compared to the subsequently adopted automated-laboratory-order system results from March 1, 2017, through July 31, 2017, according to Douglas A.E. White, MD, and his colleagues at Highland Hospital Emergency Department, Oakland, Calif.
Via the EHR, nurses were instructed to offer screening to all adults aged 18-75 years unless they were known to be HIV- or HCV-positive, unable to verbally consent (e.g., language barriers, intoxication), or medically unstable. Exclusion was at the discretion of the triage nurse. Using a drop-down menu, nurses could choose “accepts” or “declines” for HIV and HCV testing, according to patient response. Choosing “accepts” automatically ordered the test, according to the report (Ann Emerg Med. 2018 Oct;72[4]:438-48).
Automated-laboratory-order HIV/HCV screening was integrated into clinical care. With this protocol, the EHR-automated annual HIV/hepatitis C virus screening was performed on adult patients aged 18-75 years who had laboratory tests ordered. The EHR was configured to automatically order an HIV or HCV test for age-eligible patients who had any test ordered that required laboratory processing of whole blood (excluding point-of-care tests such as for lactate or glucose level) or a urine or urethral swab for chlamydia or gonorrhea testing, according to the researchers.
There were 20,975 and 19,887 unique, age-eligible patients during the nurse-order HIV/HCV virus screening algorithm and automated-laboratory-order HIV/HCV screening algorithm study periods, respectively. A total of 4,121 patients (19.6%) were screened for HIV and 2,968 (14.2%) patients were screened for HCV during the nurse-order period vs. 6,736 (33.9%) patients screened for HIV and 6,972 (35.1%) screened for HCV during the automated-laboratory-order period.
Overall, HIV screening increased from 19.6% to 33.9% and HCV screening, from 14.2% to 35.1% using the automated vs. the nurse-ordered EHR-based algorithm.
“An automated electronic health record algorithm that links nontargeted opt-out HIV and hepatitis C virus screening to physician laboratory ordering more effectively screens ED patients, provides results before discharge, minimizes repeated screening, and diagnoses more new infections than an algorithm that relies on nursing staff to offer screening. Because most EDs in the United States now use EHR systems, this model can be easily replicated and should be considered the standard for future programs,” the researchers concluded.
This work was supported, in part, by grant funding through the FOCUS program, Gilead Sciences, which also has provided funding to various of the authors of the study.
SOURCE: White DAE et al. Ann Emerg Med. 2018 Oct;72[4]:438-48.
More patients had newly diagnosed HIV and hepatitis C virus (HCV) infection during an automated-laboratory-order HIV/HCV screening algorithm than with a nurse-order HIV/HCV screening algorithm, according to the results of a retrospective before/after comparison study of the two electronic health record (EHR)–based protocols.
The results of nurse-order HIV/HCV screening in the 5-month period of March 1, 2016, through July 31, 2016, were compared to the subsequently adopted automated-laboratory-order system results from March 1, 2017, through July 31, 2017, according to Douglas A.E. White, MD, and his colleagues at Highland Hospital Emergency Department, Oakland, Calif.
Via the EHR, nurses were instructed to offer screening to all adults aged 18-75 years unless they were known to be HIV- or HCV-positive, unable to verbally consent (e.g., language barriers, intoxication), or medically unstable. Exclusion was at the discretion of the triage nurse. Using a drop-down menu, nurses could choose “accepts” or “declines” for HIV and HCV testing, according to patient response. Choosing “accepts” automatically ordered the test, according to the report (Ann Emerg Med. 2018 Oct;72[4]:438-48).
Automated-laboratory-order HIV/HCV screening was integrated into clinical care. With this protocol, the EHR-automated annual HIV/hepatitis C virus screening was performed on adult patients aged 18-75 years who had laboratory tests ordered. The EHR was configured to automatically order an HIV or HCV test for age-eligible patients who had any test ordered that required laboratory processing of whole blood (excluding point-of-care tests such as for lactate or glucose level) or a urine or urethral swab for chlamydia or gonorrhea testing, according to the researchers.
There were 20,975 and 19,887 unique, age-eligible patients during the nurse-order HIV/HCV virus screening algorithm and automated-laboratory-order HIV/HCV screening algorithm study periods, respectively. A total of 4,121 patients (19.6%) were screened for HIV and 2,968 (14.2%) patients were screened for HCV during the nurse-order period vs. 6,736 (33.9%) patients screened for HIV and 6,972 (35.1%) screened for HCV during the automated-laboratory-order period.
Overall, HIV screening increased from 19.6% to 33.9% and HCV screening, from 14.2% to 35.1% using the automated vs. the nurse-ordered EHR-based algorithm.
“An automated electronic health record algorithm that links nontargeted opt-out HIV and hepatitis C virus screening to physician laboratory ordering more effectively screens ED patients, provides results before discharge, minimizes repeated screening, and diagnoses more new infections than an algorithm that relies on nursing staff to offer screening. Because most EDs in the United States now use EHR systems, this model can be easily replicated and should be considered the standard for future programs,” the researchers concluded.
This work was supported, in part, by grant funding through the FOCUS program, Gilead Sciences, which also has provided funding to various of the authors of the study.
SOURCE: White DAE et al. Ann Emerg Med. 2018 Oct;72[4]:438-48.
FROM ANNALS OF EMERGENCY MEDICINE
Key clinical point:
Major finding: HIV screening increased from 19.6% to 33.9%; HCV screening, from 14.2% to 35.1%, with use of an automated vs. nurse-ordered EHR-based algorithm.
Study details: There were 20,975 and 19,887eligible patients assessed during the nurse-order HIV/HCV screening and the automated–laboratory-order screening periods, respectively.
Disclosures: This work was supported, in part, by grant funding through the FOCUS program, Gilead Sciences, which also has provided funding to various of the authors of the study.
Source: White DAE et al. Ann Emerg Med. 2018 Oct;72[4]:438-48.
In utero efavirenz, dolutegravir exposure linked to childhood neurologic problems
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
REPORTING FROM ID WEEK 2018
Transgender equality: U.S. physicians must lead the way
Physicians have a duty to uphold to all kinds of people we serve, and transgender people are just that: people.
According to the U.S. Transgender Survey of 2015, one-third of transgender individuals have experienced a negative reaction from a health care provider in the past year. About 40% have attempted suicide in their lifetime, nearly nine times the rate of the U.S. general population. HIV positivity in the transgender community is nearly five times the rate of the U.S. general population.
In many states across the United States, including Pennsylvania, there are no comprehensive nondiscrimination laws that protect members of the LGBTQ community from being denied housing or from being fired because of their sexual orientation or gender identity and expression. Members of the transgender community have experienced brutal, unfair judgment and have been denied fair opportunities.
There have been numerous cases where transgender individuals have been treated unfairly by private businesses and public institutions. These instances include people being physically assaulted, verbally harassed, or denied their basic rights.
The denial of these fundamental rights calls for change, and the responsibility of this shift toward equality falls upon a faction of some of the most important people in our society: American physicians.
These patients are at an already vulnerable time of their lives and often need support from those who are in the best position to provide it.
Esteemed medical organizations such as the American Medical Association have iterated their beliefs about the importance of equality in medical treatment several times, mentioning that their support for equal care is blind of gender, sexual orientation, and gender identity.
The AMA has developed numerous policies that support LGBTQ individuals. General policies developed include those on the Continued Support of Human Rights and Freedom, the Nondiscrimination Policy, and Civil Rights Restoration. Several additional physician- and patient-centered policies have also been developed to reinforce the AMA’s support.
As a doctor who can recognize the importance of this initiative, I think it is of utmost importance that physicians support, spearhead, and lead this movement – not as part of a political agenda, but for the purpose of providing aid to a community that has not been receiving the clinical or social acknowledgment it deserves.
Often, transgender patients look to their health care providers for counsel, support, and education when confused about government legislation, insurance policies, and benefits. Yet, many physicians find themselves to be either unaware of the answers or unable to help with current resources at hand when approached about this issue. That is the case despite the wide number of resources and articles that are available to educate physicians to support their patients.
In cases like these, it is imperative that transgender patients, as any other patient would, receive the guidance and support they need. It is a respected obligation to our valued profession that we are continuously learning – exploring, discovering, and seeing the future of treatment for the benefit of those we serve, especially for the growing needs of our transgender patients.
The dynamics of equal treatment for the transgender community require significant action of health care professionals, and it is the will and power of American physicians that will propel this movement toward victory. As a transgender Pennsylvanian and American, I am proud to serve my community, my state, and my nation as the secretary of health for the Commonwealth of Pennsylvania.
In addition to serving as Pennsylvania’s secretary of health, Dr. Levine is professor of pediatrics and psychiatry at Penn State University, Hershey.
Physicians have a duty to uphold to all kinds of people we serve, and transgender people are just that: people.
According to the U.S. Transgender Survey of 2015, one-third of transgender individuals have experienced a negative reaction from a health care provider in the past year. About 40% have attempted suicide in their lifetime, nearly nine times the rate of the U.S. general population. HIV positivity in the transgender community is nearly five times the rate of the U.S. general population.
In many states across the United States, including Pennsylvania, there are no comprehensive nondiscrimination laws that protect members of the LGBTQ community from being denied housing or from being fired because of their sexual orientation or gender identity and expression. Members of the transgender community have experienced brutal, unfair judgment and have been denied fair opportunities.
There have been numerous cases where transgender individuals have been treated unfairly by private businesses and public institutions. These instances include people being physically assaulted, verbally harassed, or denied their basic rights.
The denial of these fundamental rights calls for change, and the responsibility of this shift toward equality falls upon a faction of some of the most important people in our society: American physicians.
These patients are at an already vulnerable time of their lives and often need support from those who are in the best position to provide it.
Esteemed medical organizations such as the American Medical Association have iterated their beliefs about the importance of equality in medical treatment several times, mentioning that their support for equal care is blind of gender, sexual orientation, and gender identity.
The AMA has developed numerous policies that support LGBTQ individuals. General policies developed include those on the Continued Support of Human Rights and Freedom, the Nondiscrimination Policy, and Civil Rights Restoration. Several additional physician- and patient-centered policies have also been developed to reinforce the AMA’s support.
As a doctor who can recognize the importance of this initiative, I think it is of utmost importance that physicians support, spearhead, and lead this movement – not as part of a political agenda, but for the purpose of providing aid to a community that has not been receiving the clinical or social acknowledgment it deserves.
Often, transgender patients look to their health care providers for counsel, support, and education when confused about government legislation, insurance policies, and benefits. Yet, many physicians find themselves to be either unaware of the answers or unable to help with current resources at hand when approached about this issue. That is the case despite the wide number of resources and articles that are available to educate physicians to support their patients.
In cases like these, it is imperative that transgender patients, as any other patient would, receive the guidance and support they need. It is a respected obligation to our valued profession that we are continuously learning – exploring, discovering, and seeing the future of treatment for the benefit of those we serve, especially for the growing needs of our transgender patients.
The dynamics of equal treatment for the transgender community require significant action of health care professionals, and it is the will and power of American physicians that will propel this movement toward victory. As a transgender Pennsylvanian and American, I am proud to serve my community, my state, and my nation as the secretary of health for the Commonwealth of Pennsylvania.
In addition to serving as Pennsylvania’s secretary of health, Dr. Levine is professor of pediatrics and psychiatry at Penn State University, Hershey.
Physicians have a duty to uphold to all kinds of people we serve, and transgender people are just that: people.
According to the U.S. Transgender Survey of 2015, one-third of transgender individuals have experienced a negative reaction from a health care provider in the past year. About 40% have attempted suicide in their lifetime, nearly nine times the rate of the U.S. general population. HIV positivity in the transgender community is nearly five times the rate of the U.S. general population.
In many states across the United States, including Pennsylvania, there are no comprehensive nondiscrimination laws that protect members of the LGBTQ community from being denied housing or from being fired because of their sexual orientation or gender identity and expression. Members of the transgender community have experienced brutal, unfair judgment and have been denied fair opportunities.
There have been numerous cases where transgender individuals have been treated unfairly by private businesses and public institutions. These instances include people being physically assaulted, verbally harassed, or denied their basic rights.
The denial of these fundamental rights calls for change, and the responsibility of this shift toward equality falls upon a faction of some of the most important people in our society: American physicians.
These patients are at an already vulnerable time of their lives and often need support from those who are in the best position to provide it.
Esteemed medical organizations such as the American Medical Association have iterated their beliefs about the importance of equality in medical treatment several times, mentioning that their support for equal care is blind of gender, sexual orientation, and gender identity.
The AMA has developed numerous policies that support LGBTQ individuals. General policies developed include those on the Continued Support of Human Rights and Freedom, the Nondiscrimination Policy, and Civil Rights Restoration. Several additional physician- and patient-centered policies have also been developed to reinforce the AMA’s support.
As a doctor who can recognize the importance of this initiative, I think it is of utmost importance that physicians support, spearhead, and lead this movement – not as part of a political agenda, but for the purpose of providing aid to a community that has not been receiving the clinical or social acknowledgment it deserves.
Often, transgender patients look to their health care providers for counsel, support, and education when confused about government legislation, insurance policies, and benefits. Yet, many physicians find themselves to be either unaware of the answers or unable to help with current resources at hand when approached about this issue. That is the case despite the wide number of resources and articles that are available to educate physicians to support their patients.
In cases like these, it is imperative that transgender patients, as any other patient would, receive the guidance and support they need. It is a respected obligation to our valued profession that we are continuously learning – exploring, discovering, and seeing the future of treatment for the benefit of those we serve, especially for the growing needs of our transgender patients.
The dynamics of equal treatment for the transgender community require significant action of health care professionals, and it is the will and power of American physicians that will propel this movement toward victory. As a transgender Pennsylvanian and American, I am proud to serve my community, my state, and my nation as the secretary of health for the Commonwealth of Pennsylvania.
In addition to serving as Pennsylvania’s secretary of health, Dr. Levine is professor of pediatrics and psychiatry at Penn State University, Hershey.
Age, risk factors should guide chlamydia, gonorrhea screening of HIV-infected women
WASHINGTON – Among HIV-infected women, those aged 18-29 years had the highest rates of gonorrhea and chlamydia. These results suggest that screening for these sexually transmitted infections (STIs) should be based on age and risk in HIV-infected women, said Jodie Dionne-Odom, MD, of the University of Alabama at Birmingham.
Annual screening for gonorrhea and chlamydia is recommended for all sexually active adults with HIV, but prevalence varies by gender, age, and risk behavior, Dr. Dionne-Odom said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Dr. Odom and her colleagues calculated annual testing and positivity rates during 2007-2016 for chlamydia and gonorrhea among women engaged in HIV care in eight U.S. cities as part of the Centers for AIDS Research (CFAR) Clinical Networks and Integrated Clinical Services (CNICS) longitudinal cohort.
They assessed demographic data based on the most recent year the patient was in care and used validated surveys (AUDIT-C and ASSIST) to assess risk behaviors in the past 3-6 months. They collected information from 5,084 women and 158,745 HIV primary care and women’s health visits.
The median patient age was 47 years; 62.1% of the patients were black; 70% had CD4 counts greater than 350; and 73.6% had HIV viral loads of less than 500 copies/mL. In terms of reported risk, 60.6% of the women were sexually active, (85.5% of whom reported monogamy); 13.1% had problem alcohol use, and 11.6% had active drug use.
Sampling for gonorrhea and chlamydia were mostly from urogenital sites (86.6%), 6.6% were extragenital, and 6.8% were “other.” Nearly all (98.5%) of 23,492 chlamydia tests and 95.7% of 23,324 gonorrhea tests used nucleic acid amplification, Dr. Dionne-Odom said.
During the most recent year in care, 42.7% of women were tested for gonorrhea and chlamydia, and 3.4% were positive, with the annual positivity rates over the study ranging from 1.5% to 3.2% for chlamydia and 0.9% to 1.5% for gonorrhea. However, Dr. Dionne-Odom and her colleagues found that the prevalence of STIs was inversely related to patient age, with gonorrhea and chlamydia positivity in 2016 being 16% for chlamydia and 3.9% for gonorrhea among women aged 18-24 years, compared with 1.1% and 0.7%, respectively, for women older than 50 years.
“As with national data on women, HIV-infected women aged 18-29 years had the highest rates of gonorrhea and chlamydia. Our results show that targeted screening for chlamydia and gonorrhea in women with HIV based on age and risk is warranted,” Dr. Dionne-Odom concluded.
Dr. Dionne-Odom reported that she had no relevant disclosures.
WASHINGTON – Among HIV-infected women, those aged 18-29 years had the highest rates of gonorrhea and chlamydia. These results suggest that screening for these sexually transmitted infections (STIs) should be based on age and risk in HIV-infected women, said Jodie Dionne-Odom, MD, of the University of Alabama at Birmingham.
Annual screening for gonorrhea and chlamydia is recommended for all sexually active adults with HIV, but prevalence varies by gender, age, and risk behavior, Dr. Dionne-Odom said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Dr. Odom and her colleagues calculated annual testing and positivity rates during 2007-2016 for chlamydia and gonorrhea among women engaged in HIV care in eight U.S. cities as part of the Centers for AIDS Research (CFAR) Clinical Networks and Integrated Clinical Services (CNICS) longitudinal cohort.
They assessed demographic data based on the most recent year the patient was in care and used validated surveys (AUDIT-C and ASSIST) to assess risk behaviors in the past 3-6 months. They collected information from 5,084 women and 158,745 HIV primary care and women’s health visits.
The median patient age was 47 years; 62.1% of the patients were black; 70% had CD4 counts greater than 350; and 73.6% had HIV viral loads of less than 500 copies/mL. In terms of reported risk, 60.6% of the women were sexually active, (85.5% of whom reported monogamy); 13.1% had problem alcohol use, and 11.6% had active drug use.
Sampling for gonorrhea and chlamydia were mostly from urogenital sites (86.6%), 6.6% were extragenital, and 6.8% were “other.” Nearly all (98.5%) of 23,492 chlamydia tests and 95.7% of 23,324 gonorrhea tests used nucleic acid amplification, Dr. Dionne-Odom said.
During the most recent year in care, 42.7% of women were tested for gonorrhea and chlamydia, and 3.4% were positive, with the annual positivity rates over the study ranging from 1.5% to 3.2% for chlamydia and 0.9% to 1.5% for gonorrhea. However, Dr. Dionne-Odom and her colleagues found that the prevalence of STIs was inversely related to patient age, with gonorrhea and chlamydia positivity in 2016 being 16% for chlamydia and 3.9% for gonorrhea among women aged 18-24 years, compared with 1.1% and 0.7%, respectively, for women older than 50 years.
“As with national data on women, HIV-infected women aged 18-29 years had the highest rates of gonorrhea and chlamydia. Our results show that targeted screening for chlamydia and gonorrhea in women with HIV based on age and risk is warranted,” Dr. Dionne-Odom concluded.
Dr. Dionne-Odom reported that she had no relevant disclosures.
WASHINGTON – Among HIV-infected women, those aged 18-29 years had the highest rates of gonorrhea and chlamydia. These results suggest that screening for these sexually transmitted infections (STIs) should be based on age and risk in HIV-infected women, said Jodie Dionne-Odom, MD, of the University of Alabama at Birmingham.
Annual screening for gonorrhea and chlamydia is recommended for all sexually active adults with HIV, but prevalence varies by gender, age, and risk behavior, Dr. Dionne-Odom said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Dr. Odom and her colleagues calculated annual testing and positivity rates during 2007-2016 for chlamydia and gonorrhea among women engaged in HIV care in eight U.S. cities as part of the Centers for AIDS Research (CFAR) Clinical Networks and Integrated Clinical Services (CNICS) longitudinal cohort.
They assessed demographic data based on the most recent year the patient was in care and used validated surveys (AUDIT-C and ASSIST) to assess risk behaviors in the past 3-6 months. They collected information from 5,084 women and 158,745 HIV primary care and women’s health visits.
The median patient age was 47 years; 62.1% of the patients were black; 70% had CD4 counts greater than 350; and 73.6% had HIV viral loads of less than 500 copies/mL. In terms of reported risk, 60.6% of the women were sexually active, (85.5% of whom reported monogamy); 13.1% had problem alcohol use, and 11.6% had active drug use.
Sampling for gonorrhea and chlamydia were mostly from urogenital sites (86.6%), 6.6% were extragenital, and 6.8% were “other.” Nearly all (98.5%) of 23,492 chlamydia tests and 95.7% of 23,324 gonorrhea tests used nucleic acid amplification, Dr. Dionne-Odom said.
During the most recent year in care, 42.7% of women were tested for gonorrhea and chlamydia, and 3.4% were positive, with the annual positivity rates over the study ranging from 1.5% to 3.2% for chlamydia and 0.9% to 1.5% for gonorrhea. However, Dr. Dionne-Odom and her colleagues found that the prevalence of STIs was inversely related to patient age, with gonorrhea and chlamydia positivity in 2016 being 16% for chlamydia and 3.9% for gonorrhea among women aged 18-24 years, compared with 1.1% and 0.7%, respectively, for women older than 50 years.
“As with national data on women, HIV-infected women aged 18-29 years had the highest rates of gonorrhea and chlamydia. Our results show that targeted screening for chlamydia and gonorrhea in women with HIV based on age and risk is warranted,” Dr. Dionne-Odom concluded.
Dr. Dionne-Odom reported that she had no relevant disclosures.
REPORTING FROM THE 2018 STD PREVENTION CONFERENCE
Key clinical point: Targeted screening for chlamydia and gonorrhea in women with HIV based on age, risk is warranted.
Major finding: Chlamydia infections were seen in 16% and gonorrhea in 3.9% of HIV-infected women aged 18-24 years and in 1.1% and 0.7%, respectively, in women over age 50.
Study details: Data analysis of 5,084 women in 8 U.S. cities during 2007-2016.
Disclosures: Dr. Dionne-Odom reported that she had no relevant disclosures.
HIV patients getting younger ... and older
Men who have sex with men (MSM) were younger at HIV diagnosis in 2016 than in 2008, but those living with the disease were older, according to the Centers for Disease Control and Prevention.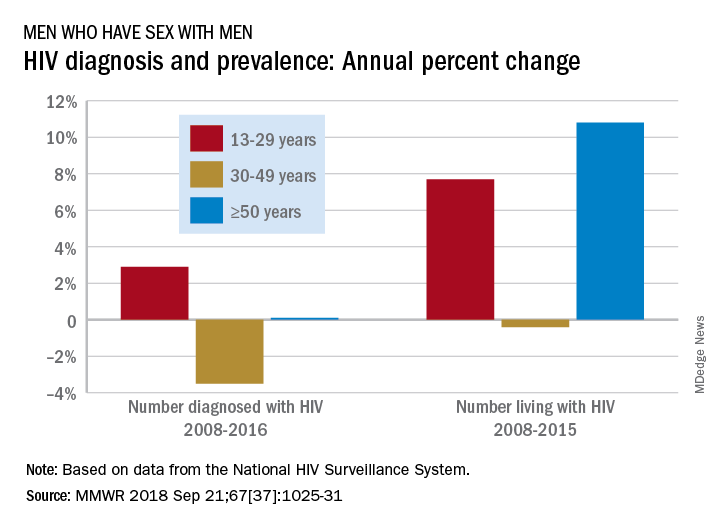
Among MSM aged 13-29 years, the number diagnosed with HIV increased by 2.9% per year from 2008 to 2016 but dropped 3.5% per year for those aged 30-49 and rose just 0.1% annually among those aged 50 and older. Over the period from 2008 to 2015, the number of MSM aged 50 and older who were living with HIV increased by 10.8% per year, compared with an annual percent change of 7.7% for those aged 13-29 and –0.4% for those aged 30-49, Andrew Mitsch, MPH, and his associates at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention reported in Morbidity and Mortality Weekly Report.
The size of the population of MSM living with HIV went from 384,000 in 2008 to 523,000 in 2016, with 13- to 29-year-olds going from 10.7% of the population to 13.3%, 30- to 49-year-olds dropping from 61% to 44%, and the 50-and-older group increasing from 28.3% to 42.7%, they said.
“The increase in annual diagnosis of HIV infections among younger MSM might reflect increased HIV testing, in addition to ongoing transmission,” they suggested, and increased prevalence among older men is probably the “result of increased survival associated with widespread use of antiretroviral therapy, surviving middle age, and advancing to the older group.”
The investigators also noted the persistence of racial/ethnic disparities over the course of the study. Among the three largest groups, whites had the smallest increase in new diagnoses for 13- to 29-year-olds and the largest decrease for 30- to 49-year-olds, and they were second to blacks in the less-than-or-equal-to-50-years-of-age group, according to data from the National HIV Surveillance System.
“Promotion of care and treatment by public health agencies and private sector partners to achieve viral suppression among MSM with diagnosed HIV infection will improve health outcomes and reduce transmission to others, particularly if prevention efforts are tailored to specific age groups,” the researchers wrote.
SOURCE: Mitsch A et al. MMWR 2018 Sep 21;67(37):1025-31.
Men who have sex with men (MSM) were younger at HIV diagnosis in 2016 than in 2008, but those living with the disease were older, according to the Centers for Disease Control and Prevention.
Among MSM aged 13-29 years, the number diagnosed with HIV increased by 2.9% per year from 2008 to 2016 but dropped 3.5% per year for those aged 30-49 and rose just 0.1% annually among those aged 50 and older. Over the period from 2008 to 2015, the number of MSM aged 50 and older who were living with HIV increased by 10.8% per year, compared with an annual percent change of 7.7% for those aged 13-29 and –0.4% for those aged 30-49, Andrew Mitsch, MPH, and his associates at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention reported in Morbidity and Mortality Weekly Report.
The size of the population of MSM living with HIV went from 384,000 in 2008 to 523,000 in 2016, with 13- to 29-year-olds going from 10.7% of the population to 13.3%, 30- to 49-year-olds dropping from 61% to 44%, and the 50-and-older group increasing from 28.3% to 42.7%, they said.
“The increase in annual diagnosis of HIV infections among younger MSM might reflect increased HIV testing, in addition to ongoing transmission,” they suggested, and increased prevalence among older men is probably the “result of increased survival associated with widespread use of antiretroviral therapy, surviving middle age, and advancing to the older group.”
The investigators also noted the persistence of racial/ethnic disparities over the course of the study. Among the three largest groups, whites had the smallest increase in new diagnoses for 13- to 29-year-olds and the largest decrease for 30- to 49-year-olds, and they were second to blacks in the less-than-or-equal-to-50-years-of-age group, according to data from the National HIV Surveillance System.
“Promotion of care and treatment by public health agencies and private sector partners to achieve viral suppression among MSM with diagnosed HIV infection will improve health outcomes and reduce transmission to others, particularly if prevention efforts are tailored to specific age groups,” the researchers wrote.
SOURCE: Mitsch A et al. MMWR 2018 Sep 21;67(37):1025-31.
Men who have sex with men (MSM) were younger at HIV diagnosis in 2016 than in 2008, but those living with the disease were older, according to the Centers for Disease Control and Prevention.
Among MSM aged 13-29 years, the number diagnosed with HIV increased by 2.9% per year from 2008 to 2016 but dropped 3.5% per year for those aged 30-49 and rose just 0.1% annually among those aged 50 and older. Over the period from 2008 to 2015, the number of MSM aged 50 and older who were living with HIV increased by 10.8% per year, compared with an annual percent change of 7.7% for those aged 13-29 and –0.4% for those aged 30-49, Andrew Mitsch, MPH, and his associates at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention reported in Morbidity and Mortality Weekly Report.
The size of the population of MSM living with HIV went from 384,000 in 2008 to 523,000 in 2016, with 13- to 29-year-olds going from 10.7% of the population to 13.3%, 30- to 49-year-olds dropping from 61% to 44%, and the 50-and-older group increasing from 28.3% to 42.7%, they said.
“The increase in annual diagnosis of HIV infections among younger MSM might reflect increased HIV testing, in addition to ongoing transmission,” they suggested, and increased prevalence among older men is probably the “result of increased survival associated with widespread use of antiretroviral therapy, surviving middle age, and advancing to the older group.”
The investigators also noted the persistence of racial/ethnic disparities over the course of the study. Among the three largest groups, whites had the smallest increase in new diagnoses for 13- to 29-year-olds and the largest decrease for 30- to 49-year-olds, and they were second to blacks in the less-than-or-equal-to-50-years-of-age group, according to data from the National HIV Surveillance System.
“Promotion of care and treatment by public health agencies and private sector partners to achieve viral suppression among MSM with diagnosed HIV infection will improve health outcomes and reduce transmission to others, particularly if prevention efforts are tailored to specific age groups,” the researchers wrote.
SOURCE: Mitsch A et al. MMWR 2018 Sep 21;67(37):1025-31.
FROM MMWR
Transgender health survey provides data on nearly 28,000 individuals
WASHINGTON – Respondents to the 2015 United States Transgender Survey (USTS) reported living with HIV at nearly five times the rate in the U.S. population. Reported HIV rates were even higher among transgender women, especially transgender women of color, according to Sandy James, JD, PhD, the lead author of the USTS and its former research director (2014-2017).
In addition, the survey results detailed high rates of physical and mental health issues, difficulties accessing health care, and negative experiences when receiving medical care.
“There [had been] a dearth of data available about trans people,” said Dr. James, and hard data are required to make any meaningful changes to health care systems, but “now we have numbers.”
The nationwide USTS was the largest survey ever to document the experiences of transgender adults in the United States, comprising 27,715 respondents from all 50 states, the District of Columbia, American Samoa, Guam, Puerto Rico, and U.S. military bases overseas.
The USTS provided a comprehensive examination of a wide range of life outcomes, including those related to health, employment, income, and education. This survey of transgender adults (18 years of age and older) was anonymous, was available in both English and Spanish, and was conducted in the summer of 2015 by the National Center for Transgender Equality.
The document details the stresses and dangers that transgender people face in their daily lives, including attempted suicide rates higher than the norm (40% having attempted suicide in their lifetime, nearly nine times the 4.6% rate in the U.S. population). Nearly 1 in 10 respondents were physically attacked in the past year because of being transgender, and nearly half (47%) of respondents reported having been sexually assaulted during their lifetime.
Respondents reported living with HIV (1.4%) at nearly five times the rate in the U.S. population (0.3%), with HIV rates higher among transgender women (3.4%), especially transgender women of color. Nearly one in five black transgender women were living with HIV, and Native American Indian and Latina women also reported higher rates of infection: 4.6% and 4.4%, respectively.
A total of 25% of respondents experienced a problem in the past year with their insurance related to being transgender, such as being denied coverage for care related to gender transition or being denied coverage for routine care because they were transgender.
In terms of the health care environment, 33% of those who saw a health care provider in the past year reported having at least one negative experience related to being transgender, with higher rates for people of color and people with disabilities. This included being refused treatment, being verbally harassed or physically or sexually assaulted, or having to teach the provider about transgender people to get appropriate care, according to the survey.
In addition, 23% of respondents reported that they did not see a doctor when they needed to in the past year because of fear of being mistreated as a transgender person, and 33% did not see a doctor when needed because they could not afford care.
“I urge you to go and find the survey and look at all of the results, it is really important,” Dr. James stated. He stressed the fact that the breakout reports, including the report on black respondents, the Latino/a response report (in both English and Spanish), and the other minority and individual state reports, can all provide a more detailed view of what is going on in the transgender community than anything previously available.
Dr. James reported having no disclosures.
SOURCE: James S. Sexually Transmitted Diseases 2018. 45 [Supplement 2] Session 5D. S289.
WASHINGTON – Respondents to the 2015 United States Transgender Survey (USTS) reported living with HIV at nearly five times the rate in the U.S. population. Reported HIV rates were even higher among transgender women, especially transgender women of color, according to Sandy James, JD, PhD, the lead author of the USTS and its former research director (2014-2017).
In addition, the survey results detailed high rates of physical and mental health issues, difficulties accessing health care, and negative experiences when receiving medical care.
“There [had been] a dearth of data available about trans people,” said Dr. James, and hard data are required to make any meaningful changes to health care systems, but “now we have numbers.”
The nationwide USTS was the largest survey ever to document the experiences of transgender adults in the United States, comprising 27,715 respondents from all 50 states, the District of Columbia, American Samoa, Guam, Puerto Rico, and U.S. military bases overseas.
The USTS provided a comprehensive examination of a wide range of life outcomes, including those related to health, employment, income, and education. This survey of transgender adults (18 years of age and older) was anonymous, was available in both English and Spanish, and was conducted in the summer of 2015 by the National Center for Transgender Equality.
The document details the stresses and dangers that transgender people face in their daily lives, including attempted suicide rates higher than the norm (40% having attempted suicide in their lifetime, nearly nine times the 4.6% rate in the U.S. population). Nearly 1 in 10 respondents were physically attacked in the past year because of being transgender, and nearly half (47%) of respondents reported having been sexually assaulted during their lifetime.
Respondents reported living with HIV (1.4%) at nearly five times the rate in the U.S. population (0.3%), with HIV rates higher among transgender women (3.4%), especially transgender women of color. Nearly one in five black transgender women were living with HIV, and Native American Indian and Latina women also reported higher rates of infection: 4.6% and 4.4%, respectively.
A total of 25% of respondents experienced a problem in the past year with their insurance related to being transgender, such as being denied coverage for care related to gender transition or being denied coverage for routine care because they were transgender.
In terms of the health care environment, 33% of those who saw a health care provider in the past year reported having at least one negative experience related to being transgender, with higher rates for people of color and people with disabilities. This included being refused treatment, being verbally harassed or physically or sexually assaulted, or having to teach the provider about transgender people to get appropriate care, according to the survey.
In addition, 23% of respondents reported that they did not see a doctor when they needed to in the past year because of fear of being mistreated as a transgender person, and 33% did not see a doctor when needed because they could not afford care.
“I urge you to go and find the survey and look at all of the results, it is really important,” Dr. James stated. He stressed the fact that the breakout reports, including the report on black respondents, the Latino/a response report (in both English and Spanish), and the other minority and individual state reports, can all provide a more detailed view of what is going on in the transgender community than anything previously available.
Dr. James reported having no disclosures.
SOURCE: James S. Sexually Transmitted Diseases 2018. 45 [Supplement 2] Session 5D. S289.
WASHINGTON – Respondents to the 2015 United States Transgender Survey (USTS) reported living with HIV at nearly five times the rate in the U.S. population. Reported HIV rates were even higher among transgender women, especially transgender women of color, according to Sandy James, JD, PhD, the lead author of the USTS and its former research director (2014-2017).
In addition, the survey results detailed high rates of physical and mental health issues, difficulties accessing health care, and negative experiences when receiving medical care.
“There [had been] a dearth of data available about trans people,” said Dr. James, and hard data are required to make any meaningful changes to health care systems, but “now we have numbers.”
The nationwide USTS was the largest survey ever to document the experiences of transgender adults in the United States, comprising 27,715 respondents from all 50 states, the District of Columbia, American Samoa, Guam, Puerto Rico, and U.S. military bases overseas.
The USTS provided a comprehensive examination of a wide range of life outcomes, including those related to health, employment, income, and education. This survey of transgender adults (18 years of age and older) was anonymous, was available in both English and Spanish, and was conducted in the summer of 2015 by the National Center for Transgender Equality.
The document details the stresses and dangers that transgender people face in their daily lives, including attempted suicide rates higher than the norm (40% having attempted suicide in their lifetime, nearly nine times the 4.6% rate in the U.S. population). Nearly 1 in 10 respondents were physically attacked in the past year because of being transgender, and nearly half (47%) of respondents reported having been sexually assaulted during their lifetime.
Respondents reported living with HIV (1.4%) at nearly five times the rate in the U.S. population (0.3%), with HIV rates higher among transgender women (3.4%), especially transgender women of color. Nearly one in five black transgender women were living with HIV, and Native American Indian and Latina women also reported higher rates of infection: 4.6% and 4.4%, respectively.
A total of 25% of respondents experienced a problem in the past year with their insurance related to being transgender, such as being denied coverage for care related to gender transition or being denied coverage for routine care because they were transgender.
In terms of the health care environment, 33% of those who saw a health care provider in the past year reported having at least one negative experience related to being transgender, with higher rates for people of color and people with disabilities. This included being refused treatment, being verbally harassed or physically or sexually assaulted, or having to teach the provider about transgender people to get appropriate care, according to the survey.
In addition, 23% of respondents reported that they did not see a doctor when they needed to in the past year because of fear of being mistreated as a transgender person, and 33% did not see a doctor when needed because they could not afford care.
“I urge you to go and find the survey and look at all of the results, it is really important,” Dr. James stated. He stressed the fact that the breakout reports, including the report on black respondents, the Latino/a response report (in both English and Spanish), and the other minority and individual state reports, can all provide a more detailed view of what is going on in the transgender community than anything previously available.
Dr. James reported having no disclosures.
SOURCE: James S. Sexually Transmitted Diseases 2018. 45 [Supplement 2] Session 5D. S289.
FROM THE STD PREVENTION CONFERENCE 2018
Key clinical point: The 2015 U.S. Transgender Survey provides more data on transgender life and health than ever previously available.
Major finding: Transgender respondents reported living with HIV at nearly five times the rate in the U.S. population.
Study details: Results from an anonymous, online survey of nearly 28,000 transgender individuals in the United States and its territories.
Disclosures: Dr. James reported having no disclosures.
Source: James S. Sexually Transmitted Diseases 2018. 45 [Supplement 2] Session 5D. S28.
UN aims to eradicate TB by 2030
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.
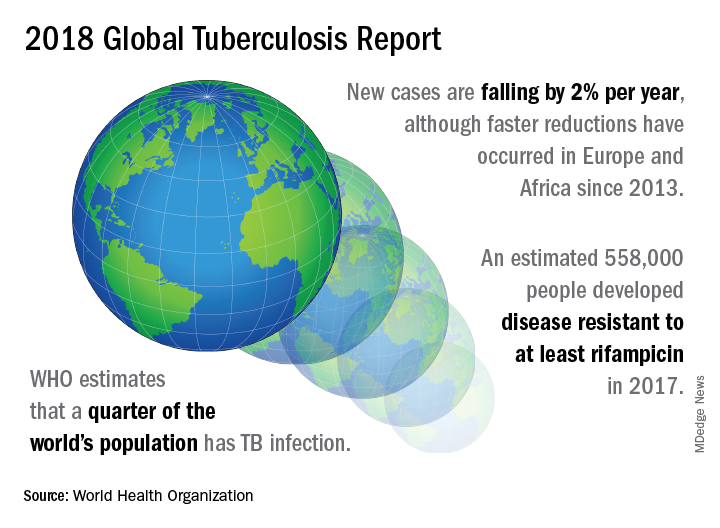
On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
FROM A WORLD HEALTH ORGANIZATION PRESS CONFERENCE
HIV intervention for drug users also benefits injection partners
A combination intervention that included standard of care antiretroviral therapy (ART), systems navigation, and psychosocial counseling showed success in HIV-infected people who inject drugs (PWID), according to the results of a randomized study in the Lancet.
The intervention patients showed an increase in both the use of ART and medication-assisted treatment (MAT) for drug use. In addition, they showed a reduced mortality, compared with standard-of-care controls. The study was carried out in one community site in the Ukraine and two health center sites in Vietnam.
A unique aspect of the study was that each HIV-positive PWID recruited one or more HIV-negative injection partners who were followed throughout the study to determine any change in their HIV status, according to a report by William C. Miller, MD, PhD, of the Ohio State University, Columbus, and his colleagues.
The study included 502 eligible and enrolled HIV-positive PWID along with 806 eligible and enrolled injection partners. The subjects comprised 85% men, with 65% of the participants between the ages of 30-39 years at time of enrollment. Patients were randomized to the intervention group (25%) or the standard of care–only group (75%).
At 1 year, self-reported ART use was higher among the index participants in the intervention group than in the standard of care group (probability ratio,1.7; 95% confidence interval, 1.4-1.9) and viral suppression also was higher with the intervention group than with standard of care (PR 1.7; 95% CI, 1.3-2.2). In addition, MAT use was higher with the intervention than with standard of care (PR, 1.7; 95% CI, 1.3-2.2). Seven HIV infections occurred during the study, all in the injection partners of the standard of care group, with none in the intervention group partners, but the study was not powered to determine if this was a significant difference.
Mortality was lower in the intervention group than in the standard of care group with 5.6 deaths/100 person-years (95% CI, 2.6-10.6) in the intervention group vs. 12.1 deaths/100 person-years (95% CI, 9.1-15.6) in the standard of care group (hazard ratio, 0.47; 95% CI 0.22-0.90). Similarly, mortality also was lower among injection partners in the intervention group than in the standard of care group (0.46 deaths/100 person-years; 95% CI, 0.01-2.6 vs. 2.6 deaths/100 person-years; 95% CI, 1.5-4.1, respectively (HR, 0.17; 95% CI, 0.01-0.84).
“This vanguard study provides evidence that a flexible, scalable intervention increases ART and MAT use and reduced mortality among PWID,” according to the authors. “The intervention might have reduced HIV incidence, but incidence was low in both groups of uninfected partners. This low incidence presents a challenge for any similar future trial assessing transmission and precludes a future randomized controlled trial,” they concluded.
The study was funded by the National Institutes of Health and the authors reported no conflicts of interest within the scope of the study.
SOURCE: Miller WC et al. Lancet 2018;392:747-59.
Although the study had unexpectedly low incidences of HIV infection in the standard of care population controls, which made it not powered to find a statistically significant difference in HIV incidence in the two groups, given the results, standard of care may be considered substandard in trials such as these, according to Don C. Des Jarlais, MD, and Huong Thi Duong, MD.
Because of this, “we propose that, when an incident case of HIV is identified, ART [antiretroviral therapy] and MAT [medication-assisted therapy] should be offered immediately, and all reasonable attempts should be made to identify potential sources of transmission and people who might have been infected within the person’s injecting and sexual networks,” in any future trials, “even if supplying ethically acceptable standard of care might make the study extremely expensive,” Dr. Des Jarlais and Dr. Duong wrote.
They pointed out that, because combined prevention and care have ended HIV epidemics among people who inject drugs (PWID) in high-income countries, the same should be the case for low- and middle-income settings, especially as MAT has been shown to be quite effective among these latter groups as well.
“Governments and community-based organizations should now unambiguously commit to the goal of using evidence-based interventions to end HIV epidemics among PWID globally,” Dr. Jarlais and Dr. Duong concluded.
Dr. Des Jarlais of the Icahn School of Medicine at Mount Sinai, N.Y., and Dr. Duong of Hai Phong (Vietnam) University of Medicine and Pharmacy made their comments in an accompanying editorial (Lancet 2018;392:714-6) .
Although the study had unexpectedly low incidences of HIV infection in the standard of care population controls, which made it not powered to find a statistically significant difference in HIV incidence in the two groups, given the results, standard of care may be considered substandard in trials such as these, according to Don C. Des Jarlais, MD, and Huong Thi Duong, MD.
Because of this, “we propose that, when an incident case of HIV is identified, ART [antiretroviral therapy] and MAT [medication-assisted therapy] should be offered immediately, and all reasonable attempts should be made to identify potential sources of transmission and people who might have been infected within the person’s injecting and sexual networks,” in any future trials, “even if supplying ethically acceptable standard of care might make the study extremely expensive,” Dr. Des Jarlais and Dr. Duong wrote.
They pointed out that, because combined prevention and care have ended HIV epidemics among people who inject drugs (PWID) in high-income countries, the same should be the case for low- and middle-income settings, especially as MAT has been shown to be quite effective among these latter groups as well.
“Governments and community-based organizations should now unambiguously commit to the goal of using evidence-based interventions to end HIV epidemics among PWID globally,” Dr. Jarlais and Dr. Duong concluded.
Dr. Des Jarlais of the Icahn School of Medicine at Mount Sinai, N.Y., and Dr. Duong of Hai Phong (Vietnam) University of Medicine and Pharmacy made their comments in an accompanying editorial (Lancet 2018;392:714-6) .
Although the study had unexpectedly low incidences of HIV infection in the standard of care population controls, which made it not powered to find a statistically significant difference in HIV incidence in the two groups, given the results, standard of care may be considered substandard in trials such as these, according to Don C. Des Jarlais, MD, and Huong Thi Duong, MD.
Because of this, “we propose that, when an incident case of HIV is identified, ART [antiretroviral therapy] and MAT [medication-assisted therapy] should be offered immediately, and all reasonable attempts should be made to identify potential sources of transmission and people who might have been infected within the person’s injecting and sexual networks,” in any future trials, “even if supplying ethically acceptable standard of care might make the study extremely expensive,” Dr. Des Jarlais and Dr. Duong wrote.
They pointed out that, because combined prevention and care have ended HIV epidemics among people who inject drugs (PWID) in high-income countries, the same should be the case for low- and middle-income settings, especially as MAT has been shown to be quite effective among these latter groups as well.
“Governments and community-based organizations should now unambiguously commit to the goal of using evidence-based interventions to end HIV epidemics among PWID globally,” Dr. Jarlais and Dr. Duong concluded.
Dr. Des Jarlais of the Icahn School of Medicine at Mount Sinai, N.Y., and Dr. Duong of Hai Phong (Vietnam) University of Medicine and Pharmacy made their comments in an accompanying editorial (Lancet 2018;392:714-6) .
A combination intervention that included standard of care antiretroviral therapy (ART), systems navigation, and psychosocial counseling showed success in HIV-infected people who inject drugs (PWID), according to the results of a randomized study in the Lancet.
The intervention patients showed an increase in both the use of ART and medication-assisted treatment (MAT) for drug use. In addition, they showed a reduced mortality, compared with standard-of-care controls. The study was carried out in one community site in the Ukraine and two health center sites in Vietnam.
A unique aspect of the study was that each HIV-positive PWID recruited one or more HIV-negative injection partners who were followed throughout the study to determine any change in their HIV status, according to a report by William C. Miller, MD, PhD, of the Ohio State University, Columbus, and his colleagues.
The study included 502 eligible and enrolled HIV-positive PWID along with 806 eligible and enrolled injection partners. The subjects comprised 85% men, with 65% of the participants between the ages of 30-39 years at time of enrollment. Patients were randomized to the intervention group (25%) or the standard of care–only group (75%).
At 1 year, self-reported ART use was higher among the index participants in the intervention group than in the standard of care group (probability ratio,1.7; 95% confidence interval, 1.4-1.9) and viral suppression also was higher with the intervention group than with standard of care (PR 1.7; 95% CI, 1.3-2.2). In addition, MAT use was higher with the intervention than with standard of care (PR, 1.7; 95% CI, 1.3-2.2). Seven HIV infections occurred during the study, all in the injection partners of the standard of care group, with none in the intervention group partners, but the study was not powered to determine if this was a significant difference.
Mortality was lower in the intervention group than in the standard of care group with 5.6 deaths/100 person-years (95% CI, 2.6-10.6) in the intervention group vs. 12.1 deaths/100 person-years (95% CI, 9.1-15.6) in the standard of care group (hazard ratio, 0.47; 95% CI 0.22-0.90). Similarly, mortality also was lower among injection partners in the intervention group than in the standard of care group (0.46 deaths/100 person-years; 95% CI, 0.01-2.6 vs. 2.6 deaths/100 person-years; 95% CI, 1.5-4.1, respectively (HR, 0.17; 95% CI, 0.01-0.84).
“This vanguard study provides evidence that a flexible, scalable intervention increases ART and MAT use and reduced mortality among PWID,” according to the authors. “The intervention might have reduced HIV incidence, but incidence was low in both groups of uninfected partners. This low incidence presents a challenge for any similar future trial assessing transmission and precludes a future randomized controlled trial,” they concluded.
The study was funded by the National Institutes of Health and the authors reported no conflicts of interest within the scope of the study.
SOURCE: Miller WC et al. Lancet 2018;392:747-59.
A combination intervention that included standard of care antiretroviral therapy (ART), systems navigation, and psychosocial counseling showed success in HIV-infected people who inject drugs (PWID), according to the results of a randomized study in the Lancet.
The intervention patients showed an increase in both the use of ART and medication-assisted treatment (MAT) for drug use. In addition, they showed a reduced mortality, compared with standard-of-care controls. The study was carried out in one community site in the Ukraine and two health center sites in Vietnam.
A unique aspect of the study was that each HIV-positive PWID recruited one or more HIV-negative injection partners who were followed throughout the study to determine any change in their HIV status, according to a report by William C. Miller, MD, PhD, of the Ohio State University, Columbus, and his colleagues.
The study included 502 eligible and enrolled HIV-positive PWID along with 806 eligible and enrolled injection partners. The subjects comprised 85% men, with 65% of the participants between the ages of 30-39 years at time of enrollment. Patients were randomized to the intervention group (25%) or the standard of care–only group (75%).
At 1 year, self-reported ART use was higher among the index participants in the intervention group than in the standard of care group (probability ratio,1.7; 95% confidence interval, 1.4-1.9) and viral suppression also was higher with the intervention group than with standard of care (PR 1.7; 95% CI, 1.3-2.2). In addition, MAT use was higher with the intervention than with standard of care (PR, 1.7; 95% CI, 1.3-2.2). Seven HIV infections occurred during the study, all in the injection partners of the standard of care group, with none in the intervention group partners, but the study was not powered to determine if this was a significant difference.
Mortality was lower in the intervention group than in the standard of care group with 5.6 deaths/100 person-years (95% CI, 2.6-10.6) in the intervention group vs. 12.1 deaths/100 person-years (95% CI, 9.1-15.6) in the standard of care group (hazard ratio, 0.47; 95% CI 0.22-0.90). Similarly, mortality also was lower among injection partners in the intervention group than in the standard of care group (0.46 deaths/100 person-years; 95% CI, 0.01-2.6 vs. 2.6 deaths/100 person-years; 95% CI, 1.5-4.1, respectively (HR, 0.17; 95% CI, 0.01-0.84).
“This vanguard study provides evidence that a flexible, scalable intervention increases ART and MAT use and reduced mortality among PWID,” according to the authors. “The intervention might have reduced HIV incidence, but incidence was low in both groups of uninfected partners. This low incidence presents a challenge for any similar future trial assessing transmission and precludes a future randomized controlled trial,” they concluded.
The study was funded by the National Institutes of Health and the authors reported no conflicts of interest within the scope of the study.
SOURCE: Miller WC et al. Lancet 2018;392:747-59.
FROM THE LANCET
Key clinical point: Mortality was lower in the intervention group and among their injection partners, compared with the standard of care group.
Major finding: Seven HIV infections occurred in injection partners of the standard-of-care group, with none in those of the intervention group, although this result was underpowered to detect significance.
Study details: Randomized, controlled vanguard study in 502 index HIV-infected participants and 806 uninfected injection partners in one Ukraine community site and two Vietnam health center sites.
Disclosures: The study was funded by the National Institutes of Health, and the authors reported no conflicts of interest within the scope of the study.
Source: Miller WC et al. Lancet 2018;392:747-59.