User login
Ultrasound renal denervation drops BP in patients on triple therapy
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
FROM ACC 2021
ACC 21 looks to repeat success despite pandemic headwinds
The American College of Cardiology pulled off an impressive all-virtual meeting in March 2020, less than 3 weeks after canceling its in-person event and just 2 weeks after COVID-19 was declared a national emergency.
Optimistic plans for the annual scientific sessions of the American College of Cardiology (ACC 2021) to be a March hybrid affair in Atlanta pivoted not once, but twice, as the pandemic evolved, with the date pushed back 2 full months, to May 15-17, and the format revised to fully virtual.
“While this meeting is being delivered virtually, I think you’ll see there have been benefits in the time to plan and also the lessons that ACC has learned in virtual education over the past year. This has come together to really create a robust educational and scientific agenda,” ACC 2021 chair Pamela B. Morris, MD, said in a press conference focused on the upcoming meeting.
Over the 3 days, there will be more than 200 education sessions, 10 guideline-specific sessions, and 11 learning pathways that include core areas, but also special topics, such as COVID-19 and the emerging cardio-obstetrics subspecialty.
The meeting will be delivered through a new virtual education program built to optimize real-time interaction between faculty members and attendees, she said. A dedicated portal on the platform will allow attendees to interact virtually, for example, with presenters of the nearly 3,000 ePosters and 420 moderated posters.
For those suffering from Zoom fatigue, the increasingly popular Heart2Heart stage talks have also been converted to podcasts, which cover topics like gender equity in cardiology, the evolving role of advanced practice professionals, and “one of my favorites: art as a tool for healing,” said Dr. Morris, from the Medical University of South Carolina, Charleston. “Those sessions are really not to be missed.”
Reconnecting is an underlying theme of the meeting but the great divider will not be ignored. COVID-19 will be the focus of two 90-minute Intensive Sessions on Saturday, May 15, the first kicking off at 10:30 a.m. ET, with the Bishop Keynote lecture on bringing health equity to the frontline of cardiovascular care, followed by lessons learned during the pandemic, how to conduct clinical trials, and vaccine development.
The second session, set for 12:15 p.m., continues the “silver linings” theme, with case presentations on advances in telehealth, myocardial involvement, and thrombosis in COVID. For those wanting more, 18 abstracts are on tap in a 2-hour Spotlight on Special Topics session beginning at 2:30 p.m.
Asked about the pandemic’s effect on bringing science to fruition this past year, Dr. Morris said there’s no question it’s slowed some of the progress the cardiology community had made but, like clinical practice, “we’ve also surmounted many of those obstacles.”
“I think research has rebounded,” she said. “Just in terms of the number of abstracts and the quality of abstracts that were submitted this year, I don’t think there’s any question that we are right on par with previous years.”
Indeed, 5,258 abstracts from 76 countries were submitted, with more than 3,400 chosen for oral and poster presentation, including 25 late-breaking clinical trials to be presented in five sessions.
The late-breaking presentations and discussions will be prerecorded but speakers and panelists have been invited to be present during the streaming to answer live any questions that may arise in the chat box, ACC 2021 vice chair Douglas Drachman, MD, Massachusetts General Hospital, Boston, said in an interview.
Late-breaking clinical trials
The Joint ACC/JACC Late-Breaking Clinical Trials I (Saturday, May 15, 9:00 a.m.–-10:00 a.m.) kicks off with PARADISE-MI, the first head-to-head comparison of an angiotensin receptor neprilysin inhibitor (ARNI) and an ACE inhibitor in patients with reduced ejection fractions (EFs) after MI but no history of heart failure (HF), studying 200 mg sacubitril/valsartan (Entresto) versus 5 mg of ramipril, both twice daily, in 5,669 patients.
Sacubitril/valsartan was initially approved for HF with reduced EF and added a new indication to treat some HF patients with preserved EF. Novartis, however, recently told investors that although numerical trends consistently favored the ARNI over the ACE inhibitor ramipril, the phase 3 study failed to meet the primary endpoint for efficacy superiority of reducing the risk for cardiovascular (CV) death and HF events after an acute MI.
Second up is ADAPTABLE, which looks to close a surprising evidence gap over whether 81 mg or 325 mg daily is the optimal dose of the ubiquitously prescribed aspirin for secondary prevention in high-risk patients with established atherosclerotic CV disease.
The open-label, randomized study will look at efficacy and major bleeding over roughly 4 years in 15,000 patients within PCORnet, the National Patient-centered Clinical Research Network, a partnership of clinical research, health plan research, and patient-powered networks created to streamline patient-reported outcomes research.
“This study will not only give important clinical information for us, practically speaking, whether we should prescribe lower- or higher-dose aspirin, but it may also serve as a template for future pragmatic clinical trial design in the real world,” Dr. Drachman said during the press conference.
Up next is the 4,812-patient Canadian LAAOS III, the largest trial to examine the efficacy of left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation (AFib) already undergoing cardiac surgery. The primary outcome is the first occurrence of stroke or systemic arterial embolism over an average follow-up of 4 years.
Percutaneous closure of the left atrial appendage (LAA) has been shown to reduce stroke in AFib patients at high-risk of bleeding on systemic anticoagulation. But these devices can be expensive and studies haven’t included patients who also have valvular heart disease, a group that actually comprises more than half of patients undergoing cardiac surgery who also have AFib, he noted.
At the same time, surgical LAA closure studies have been small and have had very mixed results. “There isn’t a large-scale rigorous assessment out there for these patients undergoing surgery, so I think this is going to be fascinating to see,” Dr. Drachman said.
The session closes with ATLANTIS, which looks to shed some light on the role of anticoagulation therapy in patients after transcatheter aortic valve replacement (TAVR or TAVI). POPular TAVI, presented at ACC 2020, showed aspirin alone was the preferred antithrombotic therapy over aspirin plus clopidogrel (Plavix) in patients not on oral anticoagulants, but the optimal anticoagulation regimen remains unsettled.
The French open-label, 1,510-patient ATLANTIS trial examined whether the novel oral anticoagulant apixaban (Eliquis) is superior in preventing CV events after TAVR, compared with antiplatelet therapy in patients without an indication for anticoagulation and compared with vitamin K antagonists in those receiving anticoagulants.
An ATLANTIS 4D CT substudy of valve thrombosis is also slated for Saturday’s Featured Clinical Research 1 session at 12:15 p.m. to 1:45 p.m..
Sunday LBCTs
Dr. Drachman highlighted a series of other late-breaking studies, including the global DARE-19 trial testing the diabetes and HF drug dapagliflozin (Farxiga) given with local standard-of-care therapy for 30 days in hospitalized COVID-19 patients with CV, metabolic, or renal risk factors.
Although sodium-glucose cotransporter-2 inhibitors have been white-hot of late, top-line results reported last month show dapagliflozin failed to achieve statistical significance for the primary endpoints of reducing organ dysfunction and all-cause mortality and for improving recovery. Details will be presented in the Joint ACC/JAMA Late-Breaking Clinical Trials II (Sunday, May 16, 8:00 a.m.-9:30 a.m.).
Two trials, FLOWER-MI and RADIANCE-HTN TRIO, were singled out in the Joint ACC/New England Journal of Medicine Late-Breaking Clinical Trials III (Sunday, May 16, 10:45 a.m.-12:00 p.m.). FLOWER-MI examines whether fractional flow reserve (FFR) is better than angiography to guide complete multivessel revascularization in ST-elevation MI patients with at least 50% stenosis in at least one nonculprit lesion requiring percutaneous coronary intervention (PCI). Recent studies have shown the superiority of FFR-guided PCI for nonculprit lesions, compared with culprit lesion treatment-only, but this is the first time FFR- and angiography-guided PCI have been compared in STEMI patients.
RADIANCE-HTN TRIO already tipped its hand, with top-line results reported in late 2020 showing that the trial met its primary efficacy endpoint of greater reduction in daytime blood pressure over 2 months with the Paradise endovascular ultrasound renal denervation system, compared with a sham procedure, in 136 patients with resistant hypertension, importantly, after being given a single pill containing a calcium channel blocker, angiotensin II receptor blocker, and diuretic.
Renal denervation for hypertension has been making something of a comeback, with the 2018 RADIANCE-HTN SOLO reporting better ambulatory blood pressure control with the Paradise system than with a sham procedure in the absence of antihypertensive agents. The device has been granted breakthrough device designation from the Food and Drug Administration for the treatment of hypertensive patients who are unable to sufficiently respond to or are intolerant of antihypertensive therapy.
Monday LBCTs
In the Late-Breaking Clinical Trials IV session (Monday, May 17, 8 a.m.–9:30 a.m.), Drachman called out a secondary analysis from GALATIC-HF looking at the impact of EF on the therapeutic effect of omecamtiv mecarbil. In last year’s primary analysis, the selective cardiac myosin activator produced a modest but significant reduction in HF events or CV death in 8,232 patients with HF and an EF of 35% or less.
Rounding out the list is the Canadian CAPITAL CHILL study of moderate versus mild therapeutic hypothermia in out-of-hospital cardiac arrest, to be presented in the final Late-Breaking Clinical Trials V session (Monday, May 17, 10:45 a.m.–12:00 p.m.).
The double-blind trial sought to determine whether neurologic outcomes at 6 months are improved by targeting a core temperature of 31 ˚C versus 34 ˚C after the return of spontaneous circulation in comatose survivors of out-of-hospital cardiac arrest.
“For me, I think this could really change practice and has personal relevance from experience with cardiac arrest survivors that I’ve known and care for very deeply,” Dr. Drachman said in an interview. “I think that there’s a lot of opportunity here as well.”
Asked what other trials have the potential to change practice, Dr. Drachman said FLOWER-MI holds particular interest because it looks at how to manage patients with STEMI with multiple lesions at the point of care.
“We’ve gained a lot of clarity from several other prior clinical trials, but this will help to answer the question in a slightly different way of saying: can you eyeball it, can you look at the angiogram and say whether or not that other, nonculprit lesion ought to be treated in the same hospitalization or should you really be using a pressure wire,” he said. “For me as an interventionalist, this is really important because when you finish up doing an intervention on a patient it might be the middle of the night and the patient may be more or less stable, but you’ve already exposed them to the risk of a procedure, should you then move on and do another aspect of the procedure to interrogate with a pressure wire a remaining narrowing? I think that’s very important; that’ll help me make decisions on a day-to-day basis.”
Dr. Drachman also cited RADIANCE-HTN TRIO because it employs an endovascular technique to control blood pressure in patients with hypertension, specifically those resistant to multiple drugs.
During the press conference, Dr. Morris, a preventive cardiologist, put her money on the ADAPTABLE study of aspirin dosing, reiterating that the unique trial design could inform future research, and on Sunday’s 8:45 a.m. late-breaking post hoc analysis from the STRENGTH trial that looks to pick up where the controversy over omega-3 fatty acid preparations left off at last year’s American Heart Association meeting.
A lack of benefit on CV event rates reported with Epanova, a high-dose combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid, led to a contentious debate over how to reconcile STRENGTH with the findings from REDUCE-IT, which showed a 25% relative risk reduction in major CV events with the EPA product icosapent ethyl (Vascepa).
STRENGTH investigator Steven Nissen, MD, Cleveland Clinic, and REDUCE-IT investigator and session panelist Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, will share the virtual stage at ACC 2021, but Dr. Morris said the “good news” is both researchers know one another very well and “will really be focusing on no political issues, just the omega-3 fatty levels in the bloodstream and what does that mean in either trial.
“This is not designed to be a debate, point counterpoint,” she added.
For that, as all cardiologists and journalists know, there will be the wild and woolly #CardioTwitter sphere.
A version of this article first appeared on Medscape.com.
The American College of Cardiology pulled off an impressive all-virtual meeting in March 2020, less than 3 weeks after canceling its in-person event and just 2 weeks after COVID-19 was declared a national emergency.
Optimistic plans for the annual scientific sessions of the American College of Cardiology (ACC 2021) to be a March hybrid affair in Atlanta pivoted not once, but twice, as the pandemic evolved, with the date pushed back 2 full months, to May 15-17, and the format revised to fully virtual.
“While this meeting is being delivered virtually, I think you’ll see there have been benefits in the time to plan and also the lessons that ACC has learned in virtual education over the past year. This has come together to really create a robust educational and scientific agenda,” ACC 2021 chair Pamela B. Morris, MD, said in a press conference focused on the upcoming meeting.
Over the 3 days, there will be more than 200 education sessions, 10 guideline-specific sessions, and 11 learning pathways that include core areas, but also special topics, such as COVID-19 and the emerging cardio-obstetrics subspecialty.
The meeting will be delivered through a new virtual education program built to optimize real-time interaction between faculty members and attendees, she said. A dedicated portal on the platform will allow attendees to interact virtually, for example, with presenters of the nearly 3,000 ePosters and 420 moderated posters.
For those suffering from Zoom fatigue, the increasingly popular Heart2Heart stage talks have also been converted to podcasts, which cover topics like gender equity in cardiology, the evolving role of advanced practice professionals, and “one of my favorites: art as a tool for healing,” said Dr. Morris, from the Medical University of South Carolina, Charleston. “Those sessions are really not to be missed.”
Reconnecting is an underlying theme of the meeting but the great divider will not be ignored. COVID-19 will be the focus of two 90-minute Intensive Sessions on Saturday, May 15, the first kicking off at 10:30 a.m. ET, with the Bishop Keynote lecture on bringing health equity to the frontline of cardiovascular care, followed by lessons learned during the pandemic, how to conduct clinical trials, and vaccine development.
The second session, set for 12:15 p.m., continues the “silver linings” theme, with case presentations on advances in telehealth, myocardial involvement, and thrombosis in COVID. For those wanting more, 18 abstracts are on tap in a 2-hour Spotlight on Special Topics session beginning at 2:30 p.m.
Asked about the pandemic’s effect on bringing science to fruition this past year, Dr. Morris said there’s no question it’s slowed some of the progress the cardiology community had made but, like clinical practice, “we’ve also surmounted many of those obstacles.”
“I think research has rebounded,” she said. “Just in terms of the number of abstracts and the quality of abstracts that were submitted this year, I don’t think there’s any question that we are right on par with previous years.”
Indeed, 5,258 abstracts from 76 countries were submitted, with more than 3,400 chosen for oral and poster presentation, including 25 late-breaking clinical trials to be presented in five sessions.
The late-breaking presentations and discussions will be prerecorded but speakers and panelists have been invited to be present during the streaming to answer live any questions that may arise in the chat box, ACC 2021 vice chair Douglas Drachman, MD, Massachusetts General Hospital, Boston, said in an interview.
Late-breaking clinical trials
The Joint ACC/JACC Late-Breaking Clinical Trials I (Saturday, May 15, 9:00 a.m.–-10:00 a.m.) kicks off with PARADISE-MI, the first head-to-head comparison of an angiotensin receptor neprilysin inhibitor (ARNI) and an ACE inhibitor in patients with reduced ejection fractions (EFs) after MI but no history of heart failure (HF), studying 200 mg sacubitril/valsartan (Entresto) versus 5 mg of ramipril, both twice daily, in 5,669 patients.
Sacubitril/valsartan was initially approved for HF with reduced EF and added a new indication to treat some HF patients with preserved EF. Novartis, however, recently told investors that although numerical trends consistently favored the ARNI over the ACE inhibitor ramipril, the phase 3 study failed to meet the primary endpoint for efficacy superiority of reducing the risk for cardiovascular (CV) death and HF events after an acute MI.
Second up is ADAPTABLE, which looks to close a surprising evidence gap over whether 81 mg or 325 mg daily is the optimal dose of the ubiquitously prescribed aspirin for secondary prevention in high-risk patients with established atherosclerotic CV disease.
The open-label, randomized study will look at efficacy and major bleeding over roughly 4 years in 15,000 patients within PCORnet, the National Patient-centered Clinical Research Network, a partnership of clinical research, health plan research, and patient-powered networks created to streamline patient-reported outcomes research.
“This study will not only give important clinical information for us, practically speaking, whether we should prescribe lower- or higher-dose aspirin, but it may also serve as a template for future pragmatic clinical trial design in the real world,” Dr. Drachman said during the press conference.
Up next is the 4,812-patient Canadian LAAOS III, the largest trial to examine the efficacy of left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation (AFib) already undergoing cardiac surgery. The primary outcome is the first occurrence of stroke or systemic arterial embolism over an average follow-up of 4 years.
Percutaneous closure of the left atrial appendage (LAA) has been shown to reduce stroke in AFib patients at high-risk of bleeding on systemic anticoagulation. But these devices can be expensive and studies haven’t included patients who also have valvular heart disease, a group that actually comprises more than half of patients undergoing cardiac surgery who also have AFib, he noted.
At the same time, surgical LAA closure studies have been small and have had very mixed results. “There isn’t a large-scale rigorous assessment out there for these patients undergoing surgery, so I think this is going to be fascinating to see,” Dr. Drachman said.
The session closes with ATLANTIS, which looks to shed some light on the role of anticoagulation therapy in patients after transcatheter aortic valve replacement (TAVR or TAVI). POPular TAVI, presented at ACC 2020, showed aspirin alone was the preferred antithrombotic therapy over aspirin plus clopidogrel (Plavix) in patients not on oral anticoagulants, but the optimal anticoagulation regimen remains unsettled.
The French open-label, 1,510-patient ATLANTIS trial examined whether the novel oral anticoagulant apixaban (Eliquis) is superior in preventing CV events after TAVR, compared with antiplatelet therapy in patients without an indication for anticoagulation and compared with vitamin K antagonists in those receiving anticoagulants.
An ATLANTIS 4D CT substudy of valve thrombosis is also slated for Saturday’s Featured Clinical Research 1 session at 12:15 p.m. to 1:45 p.m..
Sunday LBCTs
Dr. Drachman highlighted a series of other late-breaking studies, including the global DARE-19 trial testing the diabetes and HF drug dapagliflozin (Farxiga) given with local standard-of-care therapy for 30 days in hospitalized COVID-19 patients with CV, metabolic, or renal risk factors.
Although sodium-glucose cotransporter-2 inhibitors have been white-hot of late, top-line results reported last month show dapagliflozin failed to achieve statistical significance for the primary endpoints of reducing organ dysfunction and all-cause mortality and for improving recovery. Details will be presented in the Joint ACC/JAMA Late-Breaking Clinical Trials II (Sunday, May 16, 8:00 a.m.-9:30 a.m.).
Two trials, FLOWER-MI and RADIANCE-HTN TRIO, were singled out in the Joint ACC/New England Journal of Medicine Late-Breaking Clinical Trials III (Sunday, May 16, 10:45 a.m.-12:00 p.m.). FLOWER-MI examines whether fractional flow reserve (FFR) is better than angiography to guide complete multivessel revascularization in ST-elevation MI patients with at least 50% stenosis in at least one nonculprit lesion requiring percutaneous coronary intervention (PCI). Recent studies have shown the superiority of FFR-guided PCI for nonculprit lesions, compared with culprit lesion treatment-only, but this is the first time FFR- and angiography-guided PCI have been compared in STEMI patients.
RADIANCE-HTN TRIO already tipped its hand, with top-line results reported in late 2020 showing that the trial met its primary efficacy endpoint of greater reduction in daytime blood pressure over 2 months with the Paradise endovascular ultrasound renal denervation system, compared with a sham procedure, in 136 patients with resistant hypertension, importantly, after being given a single pill containing a calcium channel blocker, angiotensin II receptor blocker, and diuretic.
Renal denervation for hypertension has been making something of a comeback, with the 2018 RADIANCE-HTN SOLO reporting better ambulatory blood pressure control with the Paradise system than with a sham procedure in the absence of antihypertensive agents. The device has been granted breakthrough device designation from the Food and Drug Administration for the treatment of hypertensive patients who are unable to sufficiently respond to or are intolerant of antihypertensive therapy.
Monday LBCTs
In the Late-Breaking Clinical Trials IV session (Monday, May 17, 8 a.m.–9:30 a.m.), Drachman called out a secondary analysis from GALATIC-HF looking at the impact of EF on the therapeutic effect of omecamtiv mecarbil. In last year’s primary analysis, the selective cardiac myosin activator produced a modest but significant reduction in HF events or CV death in 8,232 patients with HF and an EF of 35% or less.
Rounding out the list is the Canadian CAPITAL CHILL study of moderate versus mild therapeutic hypothermia in out-of-hospital cardiac arrest, to be presented in the final Late-Breaking Clinical Trials V session (Monday, May 17, 10:45 a.m.–12:00 p.m.).
The double-blind trial sought to determine whether neurologic outcomes at 6 months are improved by targeting a core temperature of 31 ˚C versus 34 ˚C after the return of spontaneous circulation in comatose survivors of out-of-hospital cardiac arrest.
“For me, I think this could really change practice and has personal relevance from experience with cardiac arrest survivors that I’ve known and care for very deeply,” Dr. Drachman said in an interview. “I think that there’s a lot of opportunity here as well.”
Asked what other trials have the potential to change practice, Dr. Drachman said FLOWER-MI holds particular interest because it looks at how to manage patients with STEMI with multiple lesions at the point of care.
“We’ve gained a lot of clarity from several other prior clinical trials, but this will help to answer the question in a slightly different way of saying: can you eyeball it, can you look at the angiogram and say whether or not that other, nonculprit lesion ought to be treated in the same hospitalization or should you really be using a pressure wire,” he said. “For me as an interventionalist, this is really important because when you finish up doing an intervention on a patient it might be the middle of the night and the patient may be more or less stable, but you’ve already exposed them to the risk of a procedure, should you then move on and do another aspect of the procedure to interrogate with a pressure wire a remaining narrowing? I think that’s very important; that’ll help me make decisions on a day-to-day basis.”
Dr. Drachman also cited RADIANCE-HTN TRIO because it employs an endovascular technique to control blood pressure in patients with hypertension, specifically those resistant to multiple drugs.
During the press conference, Dr. Morris, a preventive cardiologist, put her money on the ADAPTABLE study of aspirin dosing, reiterating that the unique trial design could inform future research, and on Sunday’s 8:45 a.m. late-breaking post hoc analysis from the STRENGTH trial that looks to pick up where the controversy over omega-3 fatty acid preparations left off at last year’s American Heart Association meeting.
A lack of benefit on CV event rates reported with Epanova, a high-dose combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid, led to a contentious debate over how to reconcile STRENGTH with the findings from REDUCE-IT, which showed a 25% relative risk reduction in major CV events with the EPA product icosapent ethyl (Vascepa).
STRENGTH investigator Steven Nissen, MD, Cleveland Clinic, and REDUCE-IT investigator and session panelist Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, will share the virtual stage at ACC 2021, but Dr. Morris said the “good news” is both researchers know one another very well and “will really be focusing on no political issues, just the omega-3 fatty levels in the bloodstream and what does that mean in either trial.
“This is not designed to be a debate, point counterpoint,” she added.
For that, as all cardiologists and journalists know, there will be the wild and woolly #CardioTwitter sphere.
A version of this article first appeared on Medscape.com.
The American College of Cardiology pulled off an impressive all-virtual meeting in March 2020, less than 3 weeks after canceling its in-person event and just 2 weeks after COVID-19 was declared a national emergency.
Optimistic plans for the annual scientific sessions of the American College of Cardiology (ACC 2021) to be a March hybrid affair in Atlanta pivoted not once, but twice, as the pandemic evolved, with the date pushed back 2 full months, to May 15-17, and the format revised to fully virtual.
“While this meeting is being delivered virtually, I think you’ll see there have been benefits in the time to plan and also the lessons that ACC has learned in virtual education over the past year. This has come together to really create a robust educational and scientific agenda,” ACC 2021 chair Pamela B. Morris, MD, said in a press conference focused on the upcoming meeting.
Over the 3 days, there will be more than 200 education sessions, 10 guideline-specific sessions, and 11 learning pathways that include core areas, but also special topics, such as COVID-19 and the emerging cardio-obstetrics subspecialty.
The meeting will be delivered through a new virtual education program built to optimize real-time interaction between faculty members and attendees, she said. A dedicated portal on the platform will allow attendees to interact virtually, for example, with presenters of the nearly 3,000 ePosters and 420 moderated posters.
For those suffering from Zoom fatigue, the increasingly popular Heart2Heart stage talks have also been converted to podcasts, which cover topics like gender equity in cardiology, the evolving role of advanced practice professionals, and “one of my favorites: art as a tool for healing,” said Dr. Morris, from the Medical University of South Carolina, Charleston. “Those sessions are really not to be missed.”
Reconnecting is an underlying theme of the meeting but the great divider will not be ignored. COVID-19 will be the focus of two 90-minute Intensive Sessions on Saturday, May 15, the first kicking off at 10:30 a.m. ET, with the Bishop Keynote lecture on bringing health equity to the frontline of cardiovascular care, followed by lessons learned during the pandemic, how to conduct clinical trials, and vaccine development.
The second session, set for 12:15 p.m., continues the “silver linings” theme, with case presentations on advances in telehealth, myocardial involvement, and thrombosis in COVID. For those wanting more, 18 abstracts are on tap in a 2-hour Spotlight on Special Topics session beginning at 2:30 p.m.
Asked about the pandemic’s effect on bringing science to fruition this past year, Dr. Morris said there’s no question it’s slowed some of the progress the cardiology community had made but, like clinical practice, “we’ve also surmounted many of those obstacles.”
“I think research has rebounded,” she said. “Just in terms of the number of abstracts and the quality of abstracts that were submitted this year, I don’t think there’s any question that we are right on par with previous years.”
Indeed, 5,258 abstracts from 76 countries were submitted, with more than 3,400 chosen for oral and poster presentation, including 25 late-breaking clinical trials to be presented in five sessions.
The late-breaking presentations and discussions will be prerecorded but speakers and panelists have been invited to be present during the streaming to answer live any questions that may arise in the chat box, ACC 2021 vice chair Douglas Drachman, MD, Massachusetts General Hospital, Boston, said in an interview.
Late-breaking clinical trials
The Joint ACC/JACC Late-Breaking Clinical Trials I (Saturday, May 15, 9:00 a.m.–-10:00 a.m.) kicks off with PARADISE-MI, the first head-to-head comparison of an angiotensin receptor neprilysin inhibitor (ARNI) and an ACE inhibitor in patients with reduced ejection fractions (EFs) after MI but no history of heart failure (HF), studying 200 mg sacubitril/valsartan (Entresto) versus 5 mg of ramipril, both twice daily, in 5,669 patients.
Sacubitril/valsartan was initially approved for HF with reduced EF and added a new indication to treat some HF patients with preserved EF. Novartis, however, recently told investors that although numerical trends consistently favored the ARNI over the ACE inhibitor ramipril, the phase 3 study failed to meet the primary endpoint for efficacy superiority of reducing the risk for cardiovascular (CV) death and HF events after an acute MI.
Second up is ADAPTABLE, which looks to close a surprising evidence gap over whether 81 mg or 325 mg daily is the optimal dose of the ubiquitously prescribed aspirin for secondary prevention in high-risk patients with established atherosclerotic CV disease.
The open-label, randomized study will look at efficacy and major bleeding over roughly 4 years in 15,000 patients within PCORnet, the National Patient-centered Clinical Research Network, a partnership of clinical research, health plan research, and patient-powered networks created to streamline patient-reported outcomes research.
“This study will not only give important clinical information for us, practically speaking, whether we should prescribe lower- or higher-dose aspirin, but it may also serve as a template for future pragmatic clinical trial design in the real world,” Dr. Drachman said during the press conference.
Up next is the 4,812-patient Canadian LAAOS III, the largest trial to examine the efficacy of left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation (AFib) already undergoing cardiac surgery. The primary outcome is the first occurrence of stroke or systemic arterial embolism over an average follow-up of 4 years.
Percutaneous closure of the left atrial appendage (LAA) has been shown to reduce stroke in AFib patients at high-risk of bleeding on systemic anticoagulation. But these devices can be expensive and studies haven’t included patients who also have valvular heart disease, a group that actually comprises more than half of patients undergoing cardiac surgery who also have AFib, he noted.
At the same time, surgical LAA closure studies have been small and have had very mixed results. “There isn’t a large-scale rigorous assessment out there for these patients undergoing surgery, so I think this is going to be fascinating to see,” Dr. Drachman said.
The session closes with ATLANTIS, which looks to shed some light on the role of anticoagulation therapy in patients after transcatheter aortic valve replacement (TAVR or TAVI). POPular TAVI, presented at ACC 2020, showed aspirin alone was the preferred antithrombotic therapy over aspirin plus clopidogrel (Plavix) in patients not on oral anticoagulants, but the optimal anticoagulation regimen remains unsettled.
The French open-label, 1,510-patient ATLANTIS trial examined whether the novel oral anticoagulant apixaban (Eliquis) is superior in preventing CV events after TAVR, compared with antiplatelet therapy in patients without an indication for anticoagulation and compared with vitamin K antagonists in those receiving anticoagulants.
An ATLANTIS 4D CT substudy of valve thrombosis is also slated for Saturday’s Featured Clinical Research 1 session at 12:15 p.m. to 1:45 p.m..
Sunday LBCTs
Dr. Drachman highlighted a series of other late-breaking studies, including the global DARE-19 trial testing the diabetes and HF drug dapagliflozin (Farxiga) given with local standard-of-care therapy for 30 days in hospitalized COVID-19 patients with CV, metabolic, or renal risk factors.
Although sodium-glucose cotransporter-2 inhibitors have been white-hot of late, top-line results reported last month show dapagliflozin failed to achieve statistical significance for the primary endpoints of reducing organ dysfunction and all-cause mortality and for improving recovery. Details will be presented in the Joint ACC/JAMA Late-Breaking Clinical Trials II (Sunday, May 16, 8:00 a.m.-9:30 a.m.).
Two trials, FLOWER-MI and RADIANCE-HTN TRIO, were singled out in the Joint ACC/New England Journal of Medicine Late-Breaking Clinical Trials III (Sunday, May 16, 10:45 a.m.-12:00 p.m.). FLOWER-MI examines whether fractional flow reserve (FFR) is better than angiography to guide complete multivessel revascularization in ST-elevation MI patients with at least 50% stenosis in at least one nonculprit lesion requiring percutaneous coronary intervention (PCI). Recent studies have shown the superiority of FFR-guided PCI for nonculprit lesions, compared with culprit lesion treatment-only, but this is the first time FFR- and angiography-guided PCI have been compared in STEMI patients.
RADIANCE-HTN TRIO already tipped its hand, with top-line results reported in late 2020 showing that the trial met its primary efficacy endpoint of greater reduction in daytime blood pressure over 2 months with the Paradise endovascular ultrasound renal denervation system, compared with a sham procedure, in 136 patients with resistant hypertension, importantly, after being given a single pill containing a calcium channel blocker, angiotensin II receptor blocker, and diuretic.
Renal denervation for hypertension has been making something of a comeback, with the 2018 RADIANCE-HTN SOLO reporting better ambulatory blood pressure control with the Paradise system than with a sham procedure in the absence of antihypertensive agents. The device has been granted breakthrough device designation from the Food and Drug Administration for the treatment of hypertensive patients who are unable to sufficiently respond to or are intolerant of antihypertensive therapy.
Monday LBCTs
In the Late-Breaking Clinical Trials IV session (Monday, May 17, 8 a.m.–9:30 a.m.), Drachman called out a secondary analysis from GALATIC-HF looking at the impact of EF on the therapeutic effect of omecamtiv mecarbil. In last year’s primary analysis, the selective cardiac myosin activator produced a modest but significant reduction in HF events or CV death in 8,232 patients with HF and an EF of 35% or less.
Rounding out the list is the Canadian CAPITAL CHILL study of moderate versus mild therapeutic hypothermia in out-of-hospital cardiac arrest, to be presented in the final Late-Breaking Clinical Trials V session (Monday, May 17, 10:45 a.m.–12:00 p.m.).
The double-blind trial sought to determine whether neurologic outcomes at 6 months are improved by targeting a core temperature of 31 ˚C versus 34 ˚C after the return of spontaneous circulation in comatose survivors of out-of-hospital cardiac arrest.
“For me, I think this could really change practice and has personal relevance from experience with cardiac arrest survivors that I’ve known and care for very deeply,” Dr. Drachman said in an interview. “I think that there’s a lot of opportunity here as well.”
Asked what other trials have the potential to change practice, Dr. Drachman said FLOWER-MI holds particular interest because it looks at how to manage patients with STEMI with multiple lesions at the point of care.
“We’ve gained a lot of clarity from several other prior clinical trials, but this will help to answer the question in a slightly different way of saying: can you eyeball it, can you look at the angiogram and say whether or not that other, nonculprit lesion ought to be treated in the same hospitalization or should you really be using a pressure wire,” he said. “For me as an interventionalist, this is really important because when you finish up doing an intervention on a patient it might be the middle of the night and the patient may be more or less stable, but you’ve already exposed them to the risk of a procedure, should you then move on and do another aspect of the procedure to interrogate with a pressure wire a remaining narrowing? I think that’s very important; that’ll help me make decisions on a day-to-day basis.”
Dr. Drachman also cited RADIANCE-HTN TRIO because it employs an endovascular technique to control blood pressure in patients with hypertension, specifically those resistant to multiple drugs.
During the press conference, Dr. Morris, a preventive cardiologist, put her money on the ADAPTABLE study of aspirin dosing, reiterating that the unique trial design could inform future research, and on Sunday’s 8:45 a.m. late-breaking post hoc analysis from the STRENGTH trial that looks to pick up where the controversy over omega-3 fatty acid preparations left off at last year’s American Heart Association meeting.
A lack of benefit on CV event rates reported with Epanova, a high-dose combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid, led to a contentious debate over how to reconcile STRENGTH with the findings from REDUCE-IT, which showed a 25% relative risk reduction in major CV events with the EPA product icosapent ethyl (Vascepa).
STRENGTH investigator Steven Nissen, MD, Cleveland Clinic, and REDUCE-IT investigator and session panelist Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, will share the virtual stage at ACC 2021, but Dr. Morris said the “good news” is both researchers know one another very well and “will really be focusing on no political issues, just the omega-3 fatty levels in the bloodstream and what does that mean in either trial.
“This is not designed to be a debate, point counterpoint,” she added.
For that, as all cardiologists and journalists know, there will be the wild and woolly #CardioTwitter sphere.
A version of this article first appeared on Medscape.com.
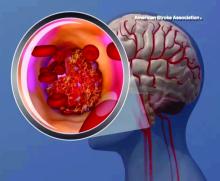
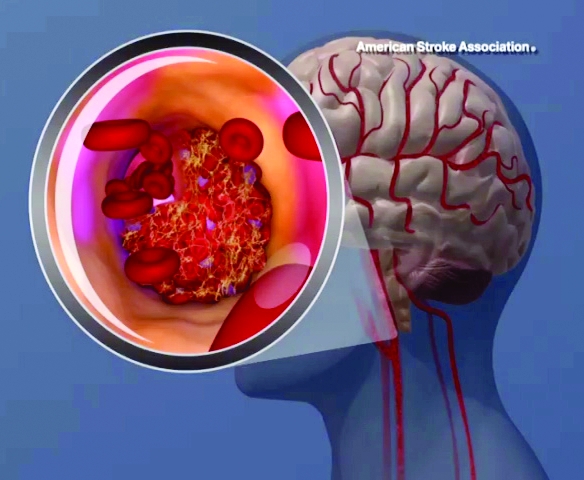
High teen BMI linked to stroke risk in young adulthood
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
FROM STROKE
Coffee intake may be driven by cardiovascular symptoms
An examination of coffee consumption habits of almost 400,000 people suggests that those habits are largely driven by a person’s cardiovascular health.
Data from a large population database showed that people with essential hypertension, angina, or cardiac arrhythmias drank less coffee than people who had none of these conditions. When they did drink coffee, it tended to be decaffeinated.
The investigators, led by Elina Hyppönen, PhD, director of the Australian Centre for Precision Health at the University of South Australia, Adelaide, say that this predilection for avoiding coffee, which is known to produce jitteriness and heart palpitations, is based on genetics.
“If your body is telling you not to drink that extra cup of coffee, there’s likely a reason why,” Dr. Hyppönen said in an interview.
The study was published online in the American Journal of Clinical Nutrition.
“People drink coffee as a pick-me-up when they’re feeling tired, or because it tastes good, or simply because it’s part of their daily routine, but what we don’t recognize is that people subconsciously self-regulate safe levels of caffeine based on how high their blood pressure is, and this is likely a result of a protective genetic mechanism, [meaning] that someone who drinks a lot of coffee is likely more genetically tolerant of caffeine, as compared to someone who drinks very little,” Dr. Hyppönen said.
“In addition, we’ve known from past research that when people feel unwell, they tend to drink less coffee. This type of phenomenon, where disease drives behavior, is called reverse causality,” Dr. Hyppönen said.
For this analysis, she and her team used information on 390,435 individuals of European ancestry from the UK Biobank, a large epidemiologic database. Habitual coffee consumption was self-reported, and systolic and diastolic blood pressure and heart rate were measured at baseline. Cardiovascular symptoms at baseline were gleaned from hospital diagnoses, primary care records, and/or self report, the authors note.
To look at the relationship of systolic BP, diastolic BP, and heart rate with coffee consumption, they used a strategy called Mendelian randomization, which allows genetic information such as variants reflecting higher blood pressures and heart rate to be used to provide evidence for a causal association.
Results showed that participants with essential hypertension, angina, or arrhythmia were “all more likely to drink less caffeinated coffee and to be nonhabitual or decaffeinated coffee drinkers compared with those who did not report related symptoms,” the authors write.
Those with higher systolic and diastolic BP based on their genetics tended to drink less caffeinated coffee at baseline, “with consistent genetic evidence to support a causal explanation across all methods,” they noted.
They also found that those people who have a higher resting heart rate due to their genes were more likely to choose decaffeinated coffee.
“These results have two major implications,” Dr. Hyppönen said. “Firstly, they show that our bodies can regulate behavior in ways that we may not realize, and that if something does not feel good to us, there is a likely to be a reason why.”
“Second, our results show that our health status in part regulates the amount of coffee we drink. This is important, because when disease drives behavior, it can lead to misleading health associations in observational studies, and indeed, create a false impression for health benefits if the group of people who do not drink coffee also includes more people who are unwell,” she said.
For now, doctors can tell their patients that this study provides an explanation as to why research on the health effects of habitual coffee consumption has been conflicting, Dr. Hyppönen said.
“Our study also highlights the uncertainty that underlies the claimed health benefits of coffee, but at the same time, it gives a positive message about the ability of our body to regulate our level of coffee consumption in a way that helps us avoid adverse effects.”
“The most common symptoms of excessive coffee consumption are palpitations and rapid heartbeat, also known as tachycardia,” Nieca Goldberg, MD, medical director of the NYU Women’s Heart Program at NYU Langone Health, said in an interview.
“This study was designed to see if cardiac symptoms affect coffee consumption, and it showed that people with hypertension, angina, history of arrhythmias, and poor health tend to be decaffeinated coffee drinkers or no coffee drinkers,” Dr. Goldberg said.
“People naturally alter their coffee intake base on their blood pressure and symptoms of palpitations and/or rapid heart rate,” she said.
The results also suggest that, “we cannot infer health benefit or harm based on the available coffee studies,” Dr. Goldberg added.
The study was funded by the National Health and Medical Research Council, Australia. Dr. Hyppönen and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An examination of coffee consumption habits of almost 400,000 people suggests that those habits are largely driven by a person’s cardiovascular health.
Data from a large population database showed that people with essential hypertension, angina, or cardiac arrhythmias drank less coffee than people who had none of these conditions. When they did drink coffee, it tended to be decaffeinated.
The investigators, led by Elina Hyppönen, PhD, director of the Australian Centre for Precision Health at the University of South Australia, Adelaide, say that this predilection for avoiding coffee, which is known to produce jitteriness and heart palpitations, is based on genetics.
“If your body is telling you not to drink that extra cup of coffee, there’s likely a reason why,” Dr. Hyppönen said in an interview.
The study was published online in the American Journal of Clinical Nutrition.
“People drink coffee as a pick-me-up when they’re feeling tired, or because it tastes good, or simply because it’s part of their daily routine, but what we don’t recognize is that people subconsciously self-regulate safe levels of caffeine based on how high their blood pressure is, and this is likely a result of a protective genetic mechanism, [meaning] that someone who drinks a lot of coffee is likely more genetically tolerant of caffeine, as compared to someone who drinks very little,” Dr. Hyppönen said.
“In addition, we’ve known from past research that when people feel unwell, they tend to drink less coffee. This type of phenomenon, where disease drives behavior, is called reverse causality,” Dr. Hyppönen said.
For this analysis, she and her team used information on 390,435 individuals of European ancestry from the UK Biobank, a large epidemiologic database. Habitual coffee consumption was self-reported, and systolic and diastolic blood pressure and heart rate were measured at baseline. Cardiovascular symptoms at baseline were gleaned from hospital diagnoses, primary care records, and/or self report, the authors note.
To look at the relationship of systolic BP, diastolic BP, and heart rate with coffee consumption, they used a strategy called Mendelian randomization, which allows genetic information such as variants reflecting higher blood pressures and heart rate to be used to provide evidence for a causal association.
Results showed that participants with essential hypertension, angina, or arrhythmia were “all more likely to drink less caffeinated coffee and to be nonhabitual or decaffeinated coffee drinkers compared with those who did not report related symptoms,” the authors write.
Those with higher systolic and diastolic BP based on their genetics tended to drink less caffeinated coffee at baseline, “with consistent genetic evidence to support a causal explanation across all methods,” they noted.
They also found that those people who have a higher resting heart rate due to their genes were more likely to choose decaffeinated coffee.
“These results have two major implications,” Dr. Hyppönen said. “Firstly, they show that our bodies can regulate behavior in ways that we may not realize, and that if something does not feel good to us, there is a likely to be a reason why.”
“Second, our results show that our health status in part regulates the amount of coffee we drink. This is important, because when disease drives behavior, it can lead to misleading health associations in observational studies, and indeed, create a false impression for health benefits if the group of people who do not drink coffee also includes more people who are unwell,” she said.
For now, doctors can tell their patients that this study provides an explanation as to why research on the health effects of habitual coffee consumption has been conflicting, Dr. Hyppönen said.
“Our study also highlights the uncertainty that underlies the claimed health benefits of coffee, but at the same time, it gives a positive message about the ability of our body to regulate our level of coffee consumption in a way that helps us avoid adverse effects.”
“The most common symptoms of excessive coffee consumption are palpitations and rapid heartbeat, also known as tachycardia,” Nieca Goldberg, MD, medical director of the NYU Women’s Heart Program at NYU Langone Health, said in an interview.
“This study was designed to see if cardiac symptoms affect coffee consumption, and it showed that people with hypertension, angina, history of arrhythmias, and poor health tend to be decaffeinated coffee drinkers or no coffee drinkers,” Dr. Goldberg said.
“People naturally alter their coffee intake base on their blood pressure and symptoms of palpitations and/or rapid heart rate,” she said.
The results also suggest that, “we cannot infer health benefit or harm based on the available coffee studies,” Dr. Goldberg added.
The study was funded by the National Health and Medical Research Council, Australia. Dr. Hyppönen and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An examination of coffee consumption habits of almost 400,000 people suggests that those habits are largely driven by a person’s cardiovascular health.
Data from a large population database showed that people with essential hypertension, angina, or cardiac arrhythmias drank less coffee than people who had none of these conditions. When they did drink coffee, it tended to be decaffeinated.
The investigators, led by Elina Hyppönen, PhD, director of the Australian Centre for Precision Health at the University of South Australia, Adelaide, say that this predilection for avoiding coffee, which is known to produce jitteriness and heart palpitations, is based on genetics.
“If your body is telling you not to drink that extra cup of coffee, there’s likely a reason why,” Dr. Hyppönen said in an interview.
The study was published online in the American Journal of Clinical Nutrition.
“People drink coffee as a pick-me-up when they’re feeling tired, or because it tastes good, or simply because it’s part of their daily routine, but what we don’t recognize is that people subconsciously self-regulate safe levels of caffeine based on how high their blood pressure is, and this is likely a result of a protective genetic mechanism, [meaning] that someone who drinks a lot of coffee is likely more genetically tolerant of caffeine, as compared to someone who drinks very little,” Dr. Hyppönen said.
“In addition, we’ve known from past research that when people feel unwell, they tend to drink less coffee. This type of phenomenon, where disease drives behavior, is called reverse causality,” Dr. Hyppönen said.
For this analysis, she and her team used information on 390,435 individuals of European ancestry from the UK Biobank, a large epidemiologic database. Habitual coffee consumption was self-reported, and systolic and diastolic blood pressure and heart rate were measured at baseline. Cardiovascular symptoms at baseline were gleaned from hospital diagnoses, primary care records, and/or self report, the authors note.
To look at the relationship of systolic BP, diastolic BP, and heart rate with coffee consumption, they used a strategy called Mendelian randomization, which allows genetic information such as variants reflecting higher blood pressures and heart rate to be used to provide evidence for a causal association.
Results showed that participants with essential hypertension, angina, or arrhythmia were “all more likely to drink less caffeinated coffee and to be nonhabitual or decaffeinated coffee drinkers compared with those who did not report related symptoms,” the authors write.
Those with higher systolic and diastolic BP based on their genetics tended to drink less caffeinated coffee at baseline, “with consistent genetic evidence to support a causal explanation across all methods,” they noted.
They also found that those people who have a higher resting heart rate due to their genes were more likely to choose decaffeinated coffee.
“These results have two major implications,” Dr. Hyppönen said. “Firstly, they show that our bodies can regulate behavior in ways that we may not realize, and that if something does not feel good to us, there is a likely to be a reason why.”
“Second, our results show that our health status in part regulates the amount of coffee we drink. This is important, because when disease drives behavior, it can lead to misleading health associations in observational studies, and indeed, create a false impression for health benefits if the group of people who do not drink coffee also includes more people who are unwell,” she said.
For now, doctors can tell their patients that this study provides an explanation as to why research on the health effects of habitual coffee consumption has been conflicting, Dr. Hyppönen said.
“Our study also highlights the uncertainty that underlies the claimed health benefits of coffee, but at the same time, it gives a positive message about the ability of our body to regulate our level of coffee consumption in a way that helps us avoid adverse effects.”
“The most common symptoms of excessive coffee consumption are palpitations and rapid heartbeat, also known as tachycardia,” Nieca Goldberg, MD, medical director of the NYU Women’s Heart Program at NYU Langone Health, said in an interview.
“This study was designed to see if cardiac symptoms affect coffee consumption, and it showed that people with hypertension, angina, history of arrhythmias, and poor health tend to be decaffeinated coffee drinkers or no coffee drinkers,” Dr. Goldberg said.
“People naturally alter their coffee intake base on their blood pressure and symptoms of palpitations and/or rapid heart rate,” she said.
The results also suggest that, “we cannot infer health benefit or harm based on the available coffee studies,” Dr. Goldberg added.
The study was funded by the National Health and Medical Research Council, Australia. Dr. Hyppönen and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nutritional support may be lifesaving in heart failure
Personalized nutritional support for adults hospitalized with chronic heart failure and deemed to be at high nutritional risk reduced the risk of death or adverse cardiovascular events, compared with standard hospital food, new research indicates.
The Swiss EFFORT trial focused on patients with chronic heart failure and high risk of malnutrition defined by low body mass index, weight loss, and low food intake upon hospital admission.
“This high-risk group of chronic heart failure patients showed a significant improvement in mortality over 30 and 180 days, as well as other clinical outcomes, when individualized nutritional support interventions were offered to patients,” Philipp Schuetz, MD, MPH, Kantonsspital Aarau, Switzerland, said in an interview.
“While monitoring the nutritional status should be done also in outpatient settings by [general practitioners], malnutrition screening upon hospital admission may help to identify high-risk patients with high risk for nutritional status deterioration during the hospital stay who will benefit from nutritional assessment and treatment,” said Dr. Schuetz.
The study was published online May 3 in the Journal of the American College of Cardiology.
It’s not all about salt
The findings are based on a prespecified secondary analysis of outcomes in 645 patients (median age, 78.8 years, 52% men) hospitalized with chronic heart failure who participated in the open-label EFFORT study.
One-third of patients were hospitalized for acute decompensated heart failure and two-thirds had chronic heart failure and other acute medical illnesses requiring hospitalization.
All patients were at risk of malnutrition based on a Nutritional Risk Screening (NRS) score of 3 points or higher. They were randomly allocated 1:1 to individualized nutritional support to reach energy, protein, and micronutrient goals or usual hospital food (control group).
By 30 days, 27 of 321 patients (8.4%) receiving nutritional support had died compared with 48 of 324 patients (14.8%) in the control group (adjusted odds ratio [OR]: 0.44; 95% confidence interval, 0.26-0.75; P = .002)
Patients with high nutritional risk (NRS >4 points) showed the most benefit from nutritional support.
Compared with patients with moderate nutritional risk scores (NRS score 3-4), those with high nutritional risk (NRS >4) had a highly significant 65% increased mortality risk over 180 days.
The individual component of the NRS with the strongest association with mortality was low food intake in the week before hospitalization.
Patients who received nutritional support in the hospital also had a lower risk for major cardiovascular events at 30 days (17.4% vs. 26.9%; OR, 0.50; 95% CI, 0.34-0.75; P = .001).
“Historically, cardiologists and internists caring for patients with heart failure have mainly focused on salt-restrictive diets to reduce blood volume and thus optimize heart function. Yet, reduction of salt intake has not been shown to effectively improve clinical outcome but may, on the contrary, increase the risk of malnutrition as low-salt diets are often not tasty,” Dr. Schuetz said.
“Our data suggest that we should move our focus away from salt-restrictive diets to high-protein diets to cover individual nutritional goals in this high-risk group of patients, which includes screening, assessment, and nutritional support by dietitians,” Dr. Schuetz said.
In a linked editorial, Sheldon Gottlieb, MD, Johns Hopkins University, Baltimore, said there has been “relatively little attention” paid to the role of diet in heart failure other than recommending reduced salt intake.
In fact, in the 2021 American College of Cardiology expert consensus recommendations for optimizing heart failure treatment, roughly five words are devoted to diet and exercise and there is no mention of nutrition assessment by a dietitian, he points out.
“This study adds another tile to the still-fragmentary mosaic picture of the patient with heart failure at nutritional risk who might benefit from nutritional support,” Dr. Dr. Gottlieb wrote.
“ ‘Good medical care’ dictates that all hospitalized patients deserve to have a standardized nutritional assessment; the challenge remains: how to determine which patient with heart failure at nutritional risk will benefit by medical nutrition therapy,” Dr. Gottlieb said.
The Swiss National Science Foundation and the Research Council of the Kantonsspital Aarau provided funding for the trial. Dr. Schuetz’s institution has previously received unrestricted grant money unrelated to this project from Nestle Health Science and Abbott Nutrition. Dr. Gottlieb owns a federal trademark for the “Greens, Beans, and Leans” diet, and has a pending federal trademark for “FLOATS”: flax + oats cereal.
A version of this article first appeared on Medscape.com.
Personalized nutritional support for adults hospitalized with chronic heart failure and deemed to be at high nutritional risk reduced the risk of death or adverse cardiovascular events, compared with standard hospital food, new research indicates.
The Swiss EFFORT trial focused on patients with chronic heart failure and high risk of malnutrition defined by low body mass index, weight loss, and low food intake upon hospital admission.
“This high-risk group of chronic heart failure patients showed a significant improvement in mortality over 30 and 180 days, as well as other clinical outcomes, when individualized nutritional support interventions were offered to patients,” Philipp Schuetz, MD, MPH, Kantonsspital Aarau, Switzerland, said in an interview.
“While monitoring the nutritional status should be done also in outpatient settings by [general practitioners], malnutrition screening upon hospital admission may help to identify high-risk patients with high risk for nutritional status deterioration during the hospital stay who will benefit from nutritional assessment and treatment,” said Dr. Schuetz.
The study was published online May 3 in the Journal of the American College of Cardiology.
It’s not all about salt
The findings are based on a prespecified secondary analysis of outcomes in 645 patients (median age, 78.8 years, 52% men) hospitalized with chronic heart failure who participated in the open-label EFFORT study.
One-third of patients were hospitalized for acute decompensated heart failure and two-thirds had chronic heart failure and other acute medical illnesses requiring hospitalization.
All patients were at risk of malnutrition based on a Nutritional Risk Screening (NRS) score of 3 points or higher. They were randomly allocated 1:1 to individualized nutritional support to reach energy, protein, and micronutrient goals or usual hospital food (control group).
By 30 days, 27 of 321 patients (8.4%) receiving nutritional support had died compared with 48 of 324 patients (14.8%) in the control group (adjusted odds ratio [OR]: 0.44; 95% confidence interval, 0.26-0.75; P = .002)
Patients with high nutritional risk (NRS >4 points) showed the most benefit from nutritional support.
Compared with patients with moderate nutritional risk scores (NRS score 3-4), those with high nutritional risk (NRS >4) had a highly significant 65% increased mortality risk over 180 days.
The individual component of the NRS with the strongest association with mortality was low food intake in the week before hospitalization.
Patients who received nutritional support in the hospital also had a lower risk for major cardiovascular events at 30 days (17.4% vs. 26.9%; OR, 0.50; 95% CI, 0.34-0.75; P = .001).
“Historically, cardiologists and internists caring for patients with heart failure have mainly focused on salt-restrictive diets to reduce blood volume and thus optimize heart function. Yet, reduction of salt intake has not been shown to effectively improve clinical outcome but may, on the contrary, increase the risk of malnutrition as low-salt diets are often not tasty,” Dr. Schuetz said.
“Our data suggest that we should move our focus away from salt-restrictive diets to high-protein diets to cover individual nutritional goals in this high-risk group of patients, which includes screening, assessment, and nutritional support by dietitians,” Dr. Schuetz said.
In a linked editorial, Sheldon Gottlieb, MD, Johns Hopkins University, Baltimore, said there has been “relatively little attention” paid to the role of diet in heart failure other than recommending reduced salt intake.
In fact, in the 2021 American College of Cardiology expert consensus recommendations for optimizing heart failure treatment, roughly five words are devoted to diet and exercise and there is no mention of nutrition assessment by a dietitian, he points out.
“This study adds another tile to the still-fragmentary mosaic picture of the patient with heart failure at nutritional risk who might benefit from nutritional support,” Dr. Dr. Gottlieb wrote.
“ ‘Good medical care’ dictates that all hospitalized patients deserve to have a standardized nutritional assessment; the challenge remains: how to determine which patient with heart failure at nutritional risk will benefit by medical nutrition therapy,” Dr. Gottlieb said.
The Swiss National Science Foundation and the Research Council of the Kantonsspital Aarau provided funding for the trial. Dr. Schuetz’s institution has previously received unrestricted grant money unrelated to this project from Nestle Health Science and Abbott Nutrition. Dr. Gottlieb owns a federal trademark for the “Greens, Beans, and Leans” diet, and has a pending federal trademark for “FLOATS”: flax + oats cereal.
A version of this article first appeared on Medscape.com.
Personalized nutritional support for adults hospitalized with chronic heart failure and deemed to be at high nutritional risk reduced the risk of death or adverse cardiovascular events, compared with standard hospital food, new research indicates.
The Swiss EFFORT trial focused on patients with chronic heart failure and high risk of malnutrition defined by low body mass index, weight loss, and low food intake upon hospital admission.
“This high-risk group of chronic heart failure patients showed a significant improvement in mortality over 30 and 180 days, as well as other clinical outcomes, when individualized nutritional support interventions were offered to patients,” Philipp Schuetz, MD, MPH, Kantonsspital Aarau, Switzerland, said in an interview.
“While monitoring the nutritional status should be done also in outpatient settings by [general practitioners], malnutrition screening upon hospital admission may help to identify high-risk patients with high risk for nutritional status deterioration during the hospital stay who will benefit from nutritional assessment and treatment,” said Dr. Schuetz.
The study was published online May 3 in the Journal of the American College of Cardiology.
It’s not all about salt
The findings are based on a prespecified secondary analysis of outcomes in 645 patients (median age, 78.8 years, 52% men) hospitalized with chronic heart failure who participated in the open-label EFFORT study.
One-third of patients were hospitalized for acute decompensated heart failure and two-thirds had chronic heart failure and other acute medical illnesses requiring hospitalization.
All patients were at risk of malnutrition based on a Nutritional Risk Screening (NRS) score of 3 points or higher. They were randomly allocated 1:1 to individualized nutritional support to reach energy, protein, and micronutrient goals or usual hospital food (control group).
By 30 days, 27 of 321 patients (8.4%) receiving nutritional support had died compared with 48 of 324 patients (14.8%) in the control group (adjusted odds ratio [OR]: 0.44; 95% confidence interval, 0.26-0.75; P = .002)
Patients with high nutritional risk (NRS >4 points) showed the most benefit from nutritional support.
Compared with patients with moderate nutritional risk scores (NRS score 3-4), those with high nutritional risk (NRS >4) had a highly significant 65% increased mortality risk over 180 days.
The individual component of the NRS with the strongest association with mortality was low food intake in the week before hospitalization.
Patients who received nutritional support in the hospital also had a lower risk for major cardiovascular events at 30 days (17.4% vs. 26.9%; OR, 0.50; 95% CI, 0.34-0.75; P = .001).
“Historically, cardiologists and internists caring for patients with heart failure have mainly focused on salt-restrictive diets to reduce blood volume and thus optimize heart function. Yet, reduction of salt intake has not been shown to effectively improve clinical outcome but may, on the contrary, increase the risk of malnutrition as low-salt diets are often not tasty,” Dr. Schuetz said.
“Our data suggest that we should move our focus away from salt-restrictive diets to high-protein diets to cover individual nutritional goals in this high-risk group of patients, which includes screening, assessment, and nutritional support by dietitians,” Dr. Schuetz said.
In a linked editorial, Sheldon Gottlieb, MD, Johns Hopkins University, Baltimore, said there has been “relatively little attention” paid to the role of diet in heart failure other than recommending reduced salt intake.
In fact, in the 2021 American College of Cardiology expert consensus recommendations for optimizing heart failure treatment, roughly five words are devoted to diet and exercise and there is no mention of nutrition assessment by a dietitian, he points out.
“This study adds another tile to the still-fragmentary mosaic picture of the patient with heart failure at nutritional risk who might benefit from nutritional support,” Dr. Dr. Gottlieb wrote.
“ ‘Good medical care’ dictates that all hospitalized patients deserve to have a standardized nutritional assessment; the challenge remains: how to determine which patient with heart failure at nutritional risk will benefit by medical nutrition therapy,” Dr. Gottlieb said.
The Swiss National Science Foundation and the Research Council of the Kantonsspital Aarau provided funding for the trial. Dr. Schuetz’s institution has previously received unrestricted grant money unrelated to this project from Nestle Health Science and Abbott Nutrition. Dr. Gottlieb owns a federal trademark for the “Greens, Beans, and Leans” diet, and has a pending federal trademark for “FLOATS”: flax + oats cereal.
A version of this article first appeared on Medscape.com.
Hypertension worsened by commonly used prescription meds
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
FROM ACC 2021
Increasing salt intake proves beneficial in POTS
For patients with postural tachycardia syndrome (POTS), dietary sodium intake can be increased more confidently, suggests the first study to yield solid evidence to support this treatment strategy.
The results showed that high dietary sodium intake can lower plasma norepinephrine levels and ameliorate standing and orthostatic tachycardia for patients with POTS.
“These results suggest that increasing dietary salt is a good rationale for treatment of this condition, and this study gives reassurance we are doing the right thing for POTS patients by increasing their sodium intake,” senior author Satish R. Raj, MD, said in an interview.
The study, with lead author Emily M. Garland, PhD, was published online April 26, 2021, in the Journal of the American College of Cardiology.
Dr. Raj, who is professor of cardiac science at the University of Calgary (Alta.), explained that POTS includes a spectrum of disorders that affect the automatic nervous system, which regulates heart rate and blood pressure.
“It is a disorder of orthostatic intolerance – patients feel better when they lie down. It differs from orthostatic hypotension in that, when a POTS patient stands up, the blood pressure does not necessarily drop, but the heart rate increases excessively.”
Although it is normal for the heart rate to increase somewhat on standing, among patients with POTS, the heart rate increases excessively. The condition is defined as an orthostatic heart rate increase of at least 30 beats/min (or 40 beats/min among individuals aged 12-19 years ) in the absence of orthostatic hypotension.
The disorder is characterized by a range of symptoms, including lightheadedness, shortness of breath, palpitations, and exertional intolerance, that are worse when in an upright position. Patients also experience chronic fatigue and perceived cognitive impairment, Dr. Raj noted.
The typical demographic for POTS is young women; the condition often starts during the teenage years.
Patients often have low blood volume, so one approach to treatment is to increase the intake of salt and water so as to increase blood volume.
“This is one of the mainstays of treatment, but it has never really been properly studied,” Dr. Raj commented. Increasing salt intake “is an unusual message from a cardiologist, and there have been concerns that we are making recommendations against traditional advice, so we urgently need evidence to support this recommendation.”
The current crossover study enrolled 14 patients with POTS and 13 healthy control persons who, over a period of 6 days, underwent treatment with a low-sodium diet (10 mEq sodium per day) or a high-sodium diet (300 mEq sodium per day).
Supine and standing heart rate, blood pressure, serum aldosterone level, plasma renin activity, blood volume, and plasma norepinephrine and epinephrine levels were measured.
Results showed that, among the POTS patients, the high-sodium diet reduced upright heart rate and the change in heart rate on standing, compared with the low-sodium diet.
Heart rate increased by 46 beats/min with the high-sodium diet versus 60 beats/min with the low-sodium diet.
Total blood volume and plasma volume increased, and standing norepinephrine levels decreased with the high-sodium diet, compared with the low-sodium diet.
However, upright heart rate, change in heart rate, and upright norepinephrine levels remained higher among POTS patients than among control persons receiving the high-sodium diet.
There was a nonsignificant trend for a lower symptom burden score among the POTS patients who received the high-sodium diet in comparison with those taking the low-sodium diet. Scores for mental confusion, palpitations, lightheadedness, and headache trending downward on the high-sodium diet.
“We found that high levels of dietary salt did what we hoped, with increased blood volume and reduced norepinephrine levels on standing and reduced excessive increase in heart rate. While it didn’t completely normalize heart rate, this was reduced significantly,” Dr. Raj said.
Another observation from the study was that the increased salt intake seemed to be beneficial across the whole spectrum of patients.
“There are some patients who have very high levels of sympathetic activation, and there have been anecdotal reports that increasing salt may not work so well in this group,” he said. “In this study, we didn’t differentiate, but average norepinephrine levels were very high, and many patients would be considered to be hyperadrenergic. Our results suggest this treatment will help these patients too.”
He noted that sodium intake was increased in this study just through diet. “We had a special metabolic kitchen. In clinical practice, we advise patients to add regular table salt to their food, and we only use salt tablets when they cannot tolerate so much salt in their diet.”
Recognizing that there may be concerns about hypertension with long-term use of such a treatment, Dr. Raj said there were no signs of an increase in blood pressure in this study. “But this should be considered a short-term therapy for the time being, and patients need to be reassessed every few years as their physiology changes.”
The authors estimated that POTS affects up to 1% of the population. Because there is no diagnostic code for the condition at present, all incidence data are estimates.
Dr. Raj pointed out that potentially a lot of people are affected, but there is little recognition of the condition among patients and physicians.
“Many family doctors are unaware of POTS,” he noted. “Patients often have to research their symptoms themselves and inform their doctor of the condition. Many patients wait years and often see many different doctors before getting a correct diagnosis.”
He explained that patients with POTS are often diagnosed as having a psychiatric illness. “They are mainly young women with palpitations, heart pounding, shakiness, which is often labeled as anxiety.”
Dr. Raj urged clinicians to consider POTS if patients have symptoms that are worse when standing up. The diagnosis is confirmed if their blood pressure doesn’t fall when standing up but their heart rate increases by at least 30 beats/min.
He noted that not enough specialists treat this condition, so family doctors need to be able to diagnose and initiate treatment. If more aggressive treatment is required, patients can be referred to a specialist.
“One of the problems is that this condition pans across different medical specialties. No one field owns it, so it tends to get ignored. But there are clinicians who are interested in POTS, and the key is finding one of these,” he said.
“We have finally established that this high-sodium diet works as treatment for POTS,” he concluded. “We have been using it for some time, but now we have evidence for its use across the whole spectrum of patients.”
In an accompanying editorial (J Am Coll Cardiol. 2021 May 4;77[17]:2185-2186), Blair P. Grubb, MD, University of Toledo (Ohio) Medical Center, wrote that this “superb study by Garland et al. helps better establish our understanding of the pathophysiologic process taking place in POTS while at the same time providing good evidence for the augmentation of dietary sodium as one of the cornerstones of treatment.”
He added that the field needs more such studies “in our quest to better understand POTS and to elaborate therapeutic modalities to help those suffering from this debilitating illness.”
The study was supported in part by the National Heart, Lung, and Blood Institute; the National Center for Advancing Translational Sciences; and the Vanderbilt Hormone and Analytical Services Core. Dr. Raj has served as a consultant for Lundbeck NA and Theravance; has served as chair of the data safety and monitoring board for Arena Pharmaceuticals and as Cardiac Arrhythmia Network of Canada network investigator; and has served on the medical advisory board of Dysautonomia International and PoTS UK, both without financial compensation.
A version of this article first appeared on Medscape.com.
For patients with postural tachycardia syndrome (POTS), dietary sodium intake can be increased more confidently, suggests the first study to yield solid evidence to support this treatment strategy.
The results showed that high dietary sodium intake can lower plasma norepinephrine levels and ameliorate standing and orthostatic tachycardia for patients with POTS.
“These results suggest that increasing dietary salt is a good rationale for treatment of this condition, and this study gives reassurance we are doing the right thing for POTS patients by increasing their sodium intake,” senior author Satish R. Raj, MD, said in an interview.
The study, with lead author Emily M. Garland, PhD, was published online April 26, 2021, in the Journal of the American College of Cardiology.
Dr. Raj, who is professor of cardiac science at the University of Calgary (Alta.), explained that POTS includes a spectrum of disorders that affect the automatic nervous system, which regulates heart rate and blood pressure.
“It is a disorder of orthostatic intolerance – patients feel better when they lie down. It differs from orthostatic hypotension in that, when a POTS patient stands up, the blood pressure does not necessarily drop, but the heart rate increases excessively.”
Although it is normal for the heart rate to increase somewhat on standing, among patients with POTS, the heart rate increases excessively. The condition is defined as an orthostatic heart rate increase of at least 30 beats/min (or 40 beats/min among individuals aged 12-19 years ) in the absence of orthostatic hypotension.
The disorder is characterized by a range of symptoms, including lightheadedness, shortness of breath, palpitations, and exertional intolerance, that are worse when in an upright position. Patients also experience chronic fatigue and perceived cognitive impairment, Dr. Raj noted.
The typical demographic for POTS is young women; the condition often starts during the teenage years.
Patients often have low blood volume, so one approach to treatment is to increase the intake of salt and water so as to increase blood volume.
“This is one of the mainstays of treatment, but it has never really been properly studied,” Dr. Raj commented. Increasing salt intake “is an unusual message from a cardiologist, and there have been concerns that we are making recommendations against traditional advice, so we urgently need evidence to support this recommendation.”
The current crossover study enrolled 14 patients with POTS and 13 healthy control persons who, over a period of 6 days, underwent treatment with a low-sodium diet (10 mEq sodium per day) or a high-sodium diet (300 mEq sodium per day).
Supine and standing heart rate, blood pressure, serum aldosterone level, plasma renin activity, blood volume, and plasma norepinephrine and epinephrine levels were measured.
Results showed that, among the POTS patients, the high-sodium diet reduced upright heart rate and the change in heart rate on standing, compared with the low-sodium diet.
Heart rate increased by 46 beats/min with the high-sodium diet versus 60 beats/min with the low-sodium diet.
Total blood volume and plasma volume increased, and standing norepinephrine levels decreased with the high-sodium diet, compared with the low-sodium diet.
However, upright heart rate, change in heart rate, and upright norepinephrine levels remained higher among POTS patients than among control persons receiving the high-sodium diet.
There was a nonsignificant trend for a lower symptom burden score among the POTS patients who received the high-sodium diet in comparison with those taking the low-sodium diet. Scores for mental confusion, palpitations, lightheadedness, and headache trending downward on the high-sodium diet.
“We found that high levels of dietary salt did what we hoped, with increased blood volume and reduced norepinephrine levels on standing and reduced excessive increase in heart rate. While it didn’t completely normalize heart rate, this was reduced significantly,” Dr. Raj said.
Another observation from the study was that the increased salt intake seemed to be beneficial across the whole spectrum of patients.
“There are some patients who have very high levels of sympathetic activation, and there have been anecdotal reports that increasing salt may not work so well in this group,” he said. “In this study, we didn’t differentiate, but average norepinephrine levels were very high, and many patients would be considered to be hyperadrenergic. Our results suggest this treatment will help these patients too.”
He noted that sodium intake was increased in this study just through diet. “We had a special metabolic kitchen. In clinical practice, we advise patients to add regular table salt to their food, and we only use salt tablets when they cannot tolerate so much salt in their diet.”
Recognizing that there may be concerns about hypertension with long-term use of such a treatment, Dr. Raj said there were no signs of an increase in blood pressure in this study. “But this should be considered a short-term therapy for the time being, and patients need to be reassessed every few years as their physiology changes.”
The authors estimated that POTS affects up to 1% of the population. Because there is no diagnostic code for the condition at present, all incidence data are estimates.
Dr. Raj pointed out that potentially a lot of people are affected, but there is little recognition of the condition among patients and physicians.
“Many family doctors are unaware of POTS,” he noted. “Patients often have to research their symptoms themselves and inform their doctor of the condition. Many patients wait years and often see many different doctors before getting a correct diagnosis.”
He explained that patients with POTS are often diagnosed as having a psychiatric illness. “They are mainly young women with palpitations, heart pounding, shakiness, which is often labeled as anxiety.”
Dr. Raj urged clinicians to consider POTS if patients have symptoms that are worse when standing up. The diagnosis is confirmed if their blood pressure doesn’t fall when standing up but their heart rate increases by at least 30 beats/min.
He noted that not enough specialists treat this condition, so family doctors need to be able to diagnose and initiate treatment. If more aggressive treatment is required, patients can be referred to a specialist.
“One of the problems is that this condition pans across different medical specialties. No one field owns it, so it tends to get ignored. But there are clinicians who are interested in POTS, and the key is finding one of these,” he said.
“We have finally established that this high-sodium diet works as treatment for POTS,” he concluded. “We have been using it for some time, but now we have evidence for its use across the whole spectrum of patients.”
In an accompanying editorial (J Am Coll Cardiol. 2021 May 4;77[17]:2185-2186), Blair P. Grubb, MD, University of Toledo (Ohio) Medical Center, wrote that this “superb study by Garland et al. helps better establish our understanding of the pathophysiologic process taking place in POTS while at the same time providing good evidence for the augmentation of dietary sodium as one of the cornerstones of treatment.”
He added that the field needs more such studies “in our quest to better understand POTS and to elaborate therapeutic modalities to help those suffering from this debilitating illness.”
The study was supported in part by the National Heart, Lung, and Blood Institute; the National Center for Advancing Translational Sciences; and the Vanderbilt Hormone and Analytical Services Core. Dr. Raj has served as a consultant for Lundbeck NA and Theravance; has served as chair of the data safety and monitoring board for Arena Pharmaceuticals and as Cardiac Arrhythmia Network of Canada network investigator; and has served on the medical advisory board of Dysautonomia International and PoTS UK, both without financial compensation.
A version of this article first appeared on Medscape.com.
For patients with postural tachycardia syndrome (POTS), dietary sodium intake can be increased more confidently, suggests the first study to yield solid evidence to support this treatment strategy.
The results showed that high dietary sodium intake can lower plasma norepinephrine levels and ameliorate standing and orthostatic tachycardia for patients with POTS.
“These results suggest that increasing dietary salt is a good rationale for treatment of this condition, and this study gives reassurance we are doing the right thing for POTS patients by increasing their sodium intake,” senior author Satish R. Raj, MD, said in an interview.
The study, with lead author Emily M. Garland, PhD, was published online April 26, 2021, in the Journal of the American College of Cardiology.
Dr. Raj, who is professor of cardiac science at the University of Calgary (Alta.), explained that POTS includes a spectrum of disorders that affect the automatic nervous system, which regulates heart rate and blood pressure.
“It is a disorder of orthostatic intolerance – patients feel better when they lie down. It differs from orthostatic hypotension in that, when a POTS patient stands up, the blood pressure does not necessarily drop, but the heart rate increases excessively.”
Although it is normal for the heart rate to increase somewhat on standing, among patients with POTS, the heart rate increases excessively. The condition is defined as an orthostatic heart rate increase of at least 30 beats/min (or 40 beats/min among individuals aged 12-19 years ) in the absence of orthostatic hypotension.
The disorder is characterized by a range of symptoms, including lightheadedness, shortness of breath, palpitations, and exertional intolerance, that are worse when in an upright position. Patients also experience chronic fatigue and perceived cognitive impairment, Dr. Raj noted.
The typical demographic for POTS is young women; the condition often starts during the teenage years.
Patients often have low blood volume, so one approach to treatment is to increase the intake of salt and water so as to increase blood volume.
“This is one of the mainstays of treatment, but it has never really been properly studied,” Dr. Raj commented. Increasing salt intake “is an unusual message from a cardiologist, and there have been concerns that we are making recommendations against traditional advice, so we urgently need evidence to support this recommendation.”
The current crossover study enrolled 14 patients with POTS and 13 healthy control persons who, over a period of 6 days, underwent treatment with a low-sodium diet (10 mEq sodium per day) or a high-sodium diet (300 mEq sodium per day).
Supine and standing heart rate, blood pressure, serum aldosterone level, plasma renin activity, blood volume, and plasma norepinephrine and epinephrine levels were measured.
Results showed that, among the POTS patients, the high-sodium diet reduced upright heart rate and the change in heart rate on standing, compared with the low-sodium diet.
Heart rate increased by 46 beats/min with the high-sodium diet versus 60 beats/min with the low-sodium diet.
Total blood volume and plasma volume increased, and standing norepinephrine levels decreased with the high-sodium diet, compared with the low-sodium diet.
However, upright heart rate, change in heart rate, and upright norepinephrine levels remained higher among POTS patients than among control persons receiving the high-sodium diet.
There was a nonsignificant trend for a lower symptom burden score among the POTS patients who received the high-sodium diet in comparison with those taking the low-sodium diet. Scores for mental confusion, palpitations, lightheadedness, and headache trending downward on the high-sodium diet.
“We found that high levels of dietary salt did what we hoped, with increased blood volume and reduced norepinephrine levels on standing and reduced excessive increase in heart rate. While it didn’t completely normalize heart rate, this was reduced significantly,” Dr. Raj said.
Another observation from the study was that the increased salt intake seemed to be beneficial across the whole spectrum of patients.
“There are some patients who have very high levels of sympathetic activation, and there have been anecdotal reports that increasing salt may not work so well in this group,” he said. “In this study, we didn’t differentiate, but average norepinephrine levels were very high, and many patients would be considered to be hyperadrenergic. Our results suggest this treatment will help these patients too.”
He noted that sodium intake was increased in this study just through diet. “We had a special metabolic kitchen. In clinical practice, we advise patients to add regular table salt to their food, and we only use salt tablets when they cannot tolerate so much salt in their diet.”
Recognizing that there may be concerns about hypertension with long-term use of such a treatment, Dr. Raj said there were no signs of an increase in blood pressure in this study. “But this should be considered a short-term therapy for the time being, and patients need to be reassessed every few years as their physiology changes.”
The authors estimated that POTS affects up to 1% of the population. Because there is no diagnostic code for the condition at present, all incidence data are estimates.
Dr. Raj pointed out that potentially a lot of people are affected, but there is little recognition of the condition among patients and physicians.
“Many family doctors are unaware of POTS,” he noted. “Patients often have to research their symptoms themselves and inform their doctor of the condition. Many patients wait years and often see many different doctors before getting a correct diagnosis.”
He explained that patients with POTS are often diagnosed as having a psychiatric illness. “They are mainly young women with palpitations, heart pounding, shakiness, which is often labeled as anxiety.”
Dr. Raj urged clinicians to consider POTS if patients have symptoms that are worse when standing up. The diagnosis is confirmed if their blood pressure doesn’t fall when standing up but their heart rate increases by at least 30 beats/min.
He noted that not enough specialists treat this condition, so family doctors need to be able to diagnose and initiate treatment. If more aggressive treatment is required, patients can be referred to a specialist.
“One of the problems is that this condition pans across different medical specialties. No one field owns it, so it tends to get ignored. But there are clinicians who are interested in POTS, and the key is finding one of these,” he said.
“We have finally established that this high-sodium diet works as treatment for POTS,” he concluded. “We have been using it for some time, but now we have evidence for its use across the whole spectrum of patients.”
In an accompanying editorial (J Am Coll Cardiol. 2021 May 4;77[17]:2185-2186), Blair P. Grubb, MD, University of Toledo (Ohio) Medical Center, wrote that this “superb study by Garland et al. helps better establish our understanding of the pathophysiologic process taking place in POTS while at the same time providing good evidence for the augmentation of dietary sodium as one of the cornerstones of treatment.”
He added that the field needs more such studies “in our quest to better understand POTS and to elaborate therapeutic modalities to help those suffering from this debilitating illness.”
The study was supported in part by the National Heart, Lung, and Blood Institute; the National Center for Advancing Translational Sciences; and the Vanderbilt Hormone and Analytical Services Core. Dr. Raj has served as a consultant for Lundbeck NA and Theravance; has served as chair of the data safety and monitoring board for Arena Pharmaceuticals and as Cardiac Arrhythmia Network of Canada network investigator; and has served on the medical advisory board of Dysautonomia International and PoTS UK, both without financial compensation.
A version of this article first appeared on Medscape.com.
AHA issues new advice on managing stage 1 hypertension
Clinicians should consider the use of medication for adults with untreated stage 1 hypertension (130-139/80-89 mm Hg) whose 10-year risk for atherosclerotic cardiovascular disease is <10% and who fail to meet the blood pressure goal of <130/80 mm Hg after 6 months of guideline-based lifestyle therapy, the American Heart Association (AHA) advises in new scientific statement.
The statement was published online April 29 in Hypertension.
The recommendation complements the 2017 American College of Cardiology/American Heart Association Blood Pressure Management Guidelines, which do not fully address how to manage untreated stage 1 hypertension, the AHA says.
“There are no treatment recommendations in current guidelines for patients who are at relatively low short-term risk of heart disease when blood pressure does not drop below 130 mm Hg after six months of recommended lifestyle changes. This statement fills that gap,” Daniel W. Jones, MD, chair of the statement writing group and a past president of the AHA, said in a news release.
If after 6 months with lifestyle changes, blood pressure does not improve, lifestyle therapy should be continued and “clinicians should consider adding medications to control blood pressure,” said Dr. Jones, professor and dean emeritus, University of Mississippi, Jackson.
Healthy lifestyle changes to lower blood pressure include achieving ideal body weight, exercising (30 min of moderate to vigorous physical activity on most days, if possible), limiting dietary sodium, enhancing potassium intake, and following the Dietary Approaches to Stop Hypertension (DASH) diet, which is plentiful in fruits and vegetables with low-fat dairy products and reduced saturated fat and total fat. In addition, patients should be advised to limit alcohol intake and to not smoke.
The writing group acknowledges that these goals can be hard to achieve and maintain over time.
“It is very hard in America and most industrialized countries to limit sodium sufficiently to lower blood pressure, and it is difficult for all of us to maintain a healthy weight in what I refer to as a toxic food environment,” Dr. Jones said.
“We want clinicians to advise patients to take healthy lifestyle changes seriously and do their best. We certainly prefer to achieve blood pressure goals without adding medication; however, successfully treating high blood pressure does extend both years and quality of life,” said Dr. Jones.
The AHA statement also addresses cases in which adults were found to have hypertension during adolescence or childhood and were prescribed antihypertensive drug therapy.
In this patient population, clinicians should consider the original indications for starting antihypertensive drug treatment and the need to continue the medication and lifestyle therapy as young adults, the AHA advises.
“In young adults with stage 1 hypertension who are not controlled with lifestyle therapy, special consideration should be given to use of antihypertensive medication in individuals with a family history of premature CVD, a history of hypertension during pregnancy, or a personal history of premature birth,” the AHA states.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Hypertension; the Council on the Kidney in Cardiovascular Disease; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular Radiology and Intervention; the Council on Lifelong Congenital Heart Disease and Heart Health in the Young; and the Stroke Council.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should consider the use of medication for adults with untreated stage 1 hypertension (130-139/80-89 mm Hg) whose 10-year risk for atherosclerotic cardiovascular disease is <10% and who fail to meet the blood pressure goal of <130/80 mm Hg after 6 months of guideline-based lifestyle therapy, the American Heart Association (AHA) advises in new scientific statement.
The statement was published online April 29 in Hypertension.
The recommendation complements the 2017 American College of Cardiology/American Heart Association Blood Pressure Management Guidelines, which do not fully address how to manage untreated stage 1 hypertension, the AHA says.
“There are no treatment recommendations in current guidelines for patients who are at relatively low short-term risk of heart disease when blood pressure does not drop below 130 mm Hg after six months of recommended lifestyle changes. This statement fills that gap,” Daniel W. Jones, MD, chair of the statement writing group and a past president of the AHA, said in a news release.
If after 6 months with lifestyle changes, blood pressure does not improve, lifestyle therapy should be continued and “clinicians should consider adding medications to control blood pressure,” said Dr. Jones, professor and dean emeritus, University of Mississippi, Jackson.
Healthy lifestyle changes to lower blood pressure include achieving ideal body weight, exercising (30 min of moderate to vigorous physical activity on most days, if possible), limiting dietary sodium, enhancing potassium intake, and following the Dietary Approaches to Stop Hypertension (DASH) diet, which is plentiful in fruits and vegetables with low-fat dairy products and reduced saturated fat and total fat. In addition, patients should be advised to limit alcohol intake and to not smoke.
The writing group acknowledges that these goals can be hard to achieve and maintain over time.
“It is very hard in America and most industrialized countries to limit sodium sufficiently to lower blood pressure, and it is difficult for all of us to maintain a healthy weight in what I refer to as a toxic food environment,” Dr. Jones said.
“We want clinicians to advise patients to take healthy lifestyle changes seriously and do their best. We certainly prefer to achieve blood pressure goals without adding medication; however, successfully treating high blood pressure does extend both years and quality of life,” said Dr. Jones.
The AHA statement also addresses cases in which adults were found to have hypertension during adolescence or childhood and were prescribed antihypertensive drug therapy.
In this patient population, clinicians should consider the original indications for starting antihypertensive drug treatment and the need to continue the medication and lifestyle therapy as young adults, the AHA advises.
“In young adults with stage 1 hypertension who are not controlled with lifestyle therapy, special consideration should be given to use of antihypertensive medication in individuals with a family history of premature CVD, a history of hypertension during pregnancy, or a personal history of premature birth,” the AHA states.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Hypertension; the Council on the Kidney in Cardiovascular Disease; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular Radiology and Intervention; the Council on Lifelong Congenital Heart Disease and Heart Health in the Young; and the Stroke Council.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should consider the use of medication for adults with untreated stage 1 hypertension (130-139/80-89 mm Hg) whose 10-year risk for atherosclerotic cardiovascular disease is <10% and who fail to meet the blood pressure goal of <130/80 mm Hg after 6 months of guideline-based lifestyle therapy, the American Heart Association (AHA) advises in new scientific statement.
The statement was published online April 29 in Hypertension.
The recommendation complements the 2017 American College of Cardiology/American Heart Association Blood Pressure Management Guidelines, which do not fully address how to manage untreated stage 1 hypertension, the AHA says.
“There are no treatment recommendations in current guidelines for patients who are at relatively low short-term risk of heart disease when blood pressure does not drop below 130 mm Hg after six months of recommended lifestyle changes. This statement fills that gap,” Daniel W. Jones, MD, chair of the statement writing group and a past president of the AHA, said in a news release.
If after 6 months with lifestyle changes, blood pressure does not improve, lifestyle therapy should be continued and “clinicians should consider adding medications to control blood pressure,” said Dr. Jones, professor and dean emeritus, University of Mississippi, Jackson.
Healthy lifestyle changes to lower blood pressure include achieving ideal body weight, exercising (30 min of moderate to vigorous physical activity on most days, if possible), limiting dietary sodium, enhancing potassium intake, and following the Dietary Approaches to Stop Hypertension (DASH) diet, which is plentiful in fruits and vegetables with low-fat dairy products and reduced saturated fat and total fat. In addition, patients should be advised to limit alcohol intake and to not smoke.
The writing group acknowledges that these goals can be hard to achieve and maintain over time.
“It is very hard in America and most industrialized countries to limit sodium sufficiently to lower blood pressure, and it is difficult for all of us to maintain a healthy weight in what I refer to as a toxic food environment,” Dr. Jones said.
“We want clinicians to advise patients to take healthy lifestyle changes seriously and do their best. We certainly prefer to achieve blood pressure goals without adding medication; however, successfully treating high blood pressure does extend both years and quality of life,” said Dr. Jones.
The AHA statement also addresses cases in which adults were found to have hypertension during adolescence or childhood and were prescribed antihypertensive drug therapy.
In this patient population, clinicians should consider the original indications for starting antihypertensive drug treatment and the need to continue the medication and lifestyle therapy as young adults, the AHA advises.
“In young adults with stage 1 hypertension who are not controlled with lifestyle therapy, special consideration should be given to use of antihypertensive medication in individuals with a family history of premature CVD, a history of hypertension during pregnancy, or a personal history of premature birth,” the AHA states.
The scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Hypertension; the Council on the Kidney in Cardiovascular Disease; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular Radiology and Intervention; the Council on Lifelong Congenital Heart Disease and Heart Health in the Young; and the Stroke Council.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
USPSTF reaffirms advice to screen all adults for hypertension
The U.S. Preventive Services Task Force continues to recommend that clinicians screen all adults aged 18 years and older for high blood pressure and that they confirm a diagnosis of hypertension with blood pressure measurements taken outside the office before starting treatment.
This grade A recommendation is consistent with the 2015 recommendation from the task force.
Hypertension affects approximately 45% of adults in the United States and is a major contributing risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease.
Using a reaffirmation deliberation process, the USPSTF concluded with high certainty that there was “substantial net benefit” from screening adults for hypertension in clinical office settings.
The reaffirmation recommendation clarifies that initial screening should be performed with office-based blood pressure measurement.
The task force found “convincing” evidence that screening for and treatment of hypertension detected in clinical office settings substantially reduces cardiovascular events and have few major harms.
To confirm a diagnosis of hypertension outside the office before starting treatment, ambulatory blood pressure monitoring or home blood pressure monitoring is recommended. Blood pressure measurements should be taken at the brachial artery with a validated and accurate device in a seated position after 5 minutes of rest.
Although evidence regarding optimal screening intervals is limited, the task force says “reasonable” options include screening for hypertension every year for adults aged 40 years or older and for adults who are at increased risk for hypertension, such as Black persons, persons with high-normal blood pressure, or those who are overweight or obese.
Screening less frequently (every 3-5 years) is appropriate for adults aged 18-39 years who are not at increased risk for hypertension and who have received a prior blood pressure reading that was in the normal range, said the task force, led by Alex Krist, MD, MPH, Virginia Commonwealth University, Richmond.
The recommendation and supporting evidence report were published online April 27, 2021, in JAMA.
‘Screening is just the first step’
In a JAMA editorial, Marwah Abdalla, MD, MPH, Columbia University Irving Medical Center, New York, and coauthors said the COVID-19 pandemic has demonstrated that “rapid and significant innovation in science, health care, and society is possible. Implementing the latest USPSTF recommendations will require widespread changes to how the health care system and other entities screen for hypertension.
“Yet screening is just the first step in a long road to controlling hypertension. Medicine and society need to implement a variety of interventions proven to be effective in controlling blood pressure at scale,” the editorialists said.
“Additionally, these efforts need to consider how to achieve success for all people. This will require working to address the roots of structural racism and reduce the racial disparities that increase hypertension-related morbidity and mortality for vulnerable populations,” they added.
“These changes will take innovation in how care delivery is provided at both the individual and population levels – lessons the health care system and society learned are achievable through the response to the COVID-19 pandemic,” Dr. Abdalla and colleagues concluded.
The USPSTF and Dr. Abdalla reported no relevant financial relationships. One editorialist reported receiving personal fees from Livongo and Cerner and grants from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force continues to recommend that clinicians screen all adults aged 18 years and older for high blood pressure and that they confirm a diagnosis of hypertension with blood pressure measurements taken outside the office before starting treatment.
This grade A recommendation is consistent with the 2015 recommendation from the task force.
Hypertension affects approximately 45% of adults in the United States and is a major contributing risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease.
Using a reaffirmation deliberation process, the USPSTF concluded with high certainty that there was “substantial net benefit” from screening adults for hypertension in clinical office settings.
The reaffirmation recommendation clarifies that initial screening should be performed with office-based blood pressure measurement.
The task force found “convincing” evidence that screening for and treatment of hypertension detected in clinical office settings substantially reduces cardiovascular events and have few major harms.
To confirm a diagnosis of hypertension outside the office before starting treatment, ambulatory blood pressure monitoring or home blood pressure monitoring is recommended. Blood pressure measurements should be taken at the brachial artery with a validated and accurate device in a seated position after 5 minutes of rest.
Although evidence regarding optimal screening intervals is limited, the task force says “reasonable” options include screening for hypertension every year for adults aged 40 years or older and for adults who are at increased risk for hypertension, such as Black persons, persons with high-normal blood pressure, or those who are overweight or obese.
Screening less frequently (every 3-5 years) is appropriate for adults aged 18-39 years who are not at increased risk for hypertension and who have received a prior blood pressure reading that was in the normal range, said the task force, led by Alex Krist, MD, MPH, Virginia Commonwealth University, Richmond.
The recommendation and supporting evidence report were published online April 27, 2021, in JAMA.
‘Screening is just the first step’
In a JAMA editorial, Marwah Abdalla, MD, MPH, Columbia University Irving Medical Center, New York, and coauthors said the COVID-19 pandemic has demonstrated that “rapid and significant innovation in science, health care, and society is possible. Implementing the latest USPSTF recommendations will require widespread changes to how the health care system and other entities screen for hypertension.
“Yet screening is just the first step in a long road to controlling hypertension. Medicine and society need to implement a variety of interventions proven to be effective in controlling blood pressure at scale,” the editorialists said.
“Additionally, these efforts need to consider how to achieve success for all people. This will require working to address the roots of structural racism and reduce the racial disparities that increase hypertension-related morbidity and mortality for vulnerable populations,” they added.
“These changes will take innovation in how care delivery is provided at both the individual and population levels – lessons the health care system and society learned are achievable through the response to the COVID-19 pandemic,” Dr. Abdalla and colleagues concluded.
The USPSTF and Dr. Abdalla reported no relevant financial relationships. One editorialist reported receiving personal fees from Livongo and Cerner and grants from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force continues to recommend that clinicians screen all adults aged 18 years and older for high blood pressure and that they confirm a diagnosis of hypertension with blood pressure measurements taken outside the office before starting treatment.
This grade A recommendation is consistent with the 2015 recommendation from the task force.
Hypertension affects approximately 45% of adults in the United States and is a major contributing risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease.
Using a reaffirmation deliberation process, the USPSTF concluded with high certainty that there was “substantial net benefit” from screening adults for hypertension in clinical office settings.
The reaffirmation recommendation clarifies that initial screening should be performed with office-based blood pressure measurement.
The task force found “convincing” evidence that screening for and treatment of hypertension detected in clinical office settings substantially reduces cardiovascular events and have few major harms.
To confirm a diagnosis of hypertension outside the office before starting treatment, ambulatory blood pressure monitoring or home blood pressure monitoring is recommended. Blood pressure measurements should be taken at the brachial artery with a validated and accurate device in a seated position after 5 minutes of rest.
Although evidence regarding optimal screening intervals is limited, the task force says “reasonable” options include screening for hypertension every year for adults aged 40 years or older and for adults who are at increased risk for hypertension, such as Black persons, persons with high-normal blood pressure, or those who are overweight or obese.
Screening less frequently (every 3-5 years) is appropriate for adults aged 18-39 years who are not at increased risk for hypertension and who have received a prior blood pressure reading that was in the normal range, said the task force, led by Alex Krist, MD, MPH, Virginia Commonwealth University, Richmond.
The recommendation and supporting evidence report were published online April 27, 2021, in JAMA.
‘Screening is just the first step’
In a JAMA editorial, Marwah Abdalla, MD, MPH, Columbia University Irving Medical Center, New York, and coauthors said the COVID-19 pandemic has demonstrated that “rapid and significant innovation in science, health care, and society is possible. Implementing the latest USPSTF recommendations will require widespread changes to how the health care system and other entities screen for hypertension.
“Yet screening is just the first step in a long road to controlling hypertension. Medicine and society need to implement a variety of interventions proven to be effective in controlling blood pressure at scale,” the editorialists said.
“Additionally, these efforts need to consider how to achieve success for all people. This will require working to address the roots of structural racism and reduce the racial disparities that increase hypertension-related morbidity and mortality for vulnerable populations,” they added.
“These changes will take innovation in how care delivery is provided at both the individual and population levels – lessons the health care system and society learned are achievable through the response to the COVID-19 pandemic,” Dr. Abdalla and colleagues concluded.
The USPSTF and Dr. Abdalla reported no relevant financial relationships. One editorialist reported receiving personal fees from Livongo and Cerner and grants from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Transgender hormone therapy linked to blood pressure changes
Transgender people treated with gender-affirming hormone therapy show distinctive changes in blood pressure that begin soon after treatment initiation and do not subside over years of treatment, according to the largest and longest observational study to date to look at the issue.
“Many physicians may not be aware of the changes to blood pressure in trans patients who start hormone therapy,” senior author Michael S. Irwig, MD, director of transgender medicine at Beth Israel Deaconess Medical Center in Boston, told this news organization.
“The take-away message for physicians is to monitor blood pressure both before and after starting hormone therapy in transgender patients, as over a third of transgender individuals had stage 1 hypertension before starting hormone therapy, and many had their blood pressure increase after starting hormone therapy.”
Mean blood pressure increases in transgender males, decreases in females
In the study, published in Hypertension, Katherine Banks, MD, George Washington University, Washington, and colleagues, followed 470 transgender adult patients for up to 5 years.
The mean systolic blood pressure levels in transgender female patients (male at birth) significantly decreased compared with baseline within a few months of them starting gender-affirming hormone treatment.
Conversely, the systolic blood pressure levels in transgender males (females at birth) who were treated with testosterone increased over the same period.
There were no significant changes in the groups in terms of diastolic blood pressure, consistent with other studies.
“Our study is the first to describe the time course of the blood pressure effects of gender-affirming hormone therapy and to compare the rates of elevated blood pressure and stage 1 and stage 2 hypertension using blood pressure readings from gender-diverse individuals pre- and post–gender-affirming hormone therapy,” the authors note.
Gender-affirming hormone therapy – which has been prescribed to transgender patients for more than 25 years – typically involves a combination of estrogen and an anti-androgen for males transitioning to female, while the therapy for those transitioning to male generally only involves testosterone.
The therapy has previously been linked to various cardiac effects, with evidence showing transgender men have as much as a 5-times greater risk of heart attack versus cisgender women, the authors note.
Although the American Heart Association issued a 2020 Scientific Statement addressing the cardiovascular disease risk, evidence on the effects specifically on blood pressure in transgender patients has been inconsistent.
For the new study, Dr. Banks and colleagues enrolled 247 transgender females and 223 transgender males who were treated between 2007 and 2015 at two medical centers in Washington, D.C. Of the individuals, who had a mean age of 27.8, about 27% were non-White and 16% were Latinx.
They had blood pressure measurements taken at baseline and at follow-up clinical visits for up to 57 months following the initiation of gender-affirming hormone therapy.
Over the follow-up period, the transgender females had decreases in mean systolic blood pressure of 4.0 mm Hg within 2 to 4 months of starting hormone therapy (P < .0001) and mean declines of 6.0 mm Hg were further observed at 11 to 21 months compared with baseline.
In transgender males, the mean systolic blood pressure increased by 2.6 mm Hg at 2 to 4 months (P = .02), and by 2.9 mm Hg at 11 to 21 months after starting therapy.
Furthermore, “although the average increase in systolic blood pressure was 2.6 mm Hg in transgender men within 2 to 4 months, some patients had much higher increases,” Dr. Irwig noted.
As many as 40% of transgender men had stage 1 hypertension after 11 to 21 months of hormone therapy.
The blood pressure changes in transgender males and females were observed across all three racial ethnic groups of Whites, Blacks, and Latinx, and the changes remained consistent throughout the entire follow-up period of approximately 5 years while hormone therapy was continued.
In addition to the changes after therapy initiation, the researchers note that more than one-third of individuals in both groups had stage 1 hypertension even before starting hormone therapy.
The findings are a concern in light of “clear evidence linking hypertension and higher blood pressure with cardiovascular events such as stroke and heart attacks,” Dr. Irwig said.
Protective effects for transgender females?
Transgender females showed as much as a 47% decrease in the prevalence of stage 2 hypertension, from 19% to 10%, within 2 to 4 months of treatment with gender-affirming hormone therapy (P = .001), and the rate declined further to 8% at 11 to 21 months, suggesting a protective effect of the treatment.
“The rate of stage 2 hypertension did drop in transgender feminine individuals, which could be protective and lower their risk for cardiovascular events,” Dr. Irwig said.
“This was not a surprise, as lowering testosterone and the use of spironolactone can lower blood pressure,” he noted.
Exceptions in both groups
Of note, a sizable proportion of patients had blood pressure changes that were in fact the opposite of the patterns seen in the majority of their gender group.
Specifically, while 42% to 53% of the transgender females had systolic blood pressure readings of at least 5 mm Hg lower than their baseline readings, up to 32% had increases of at least 5 mm Hg compared to baseline readings.
Likewise, whereas 41% to 59% of transgender males had increases of at least 5 mm Hg compared with baseline, up to 35% had levels that were at least 5 mm Hg lower than baseline.
“It was a surprise that over a quarter of individuals had changes opposite to the mean changes,” Dr. Irwig said.
The differing blood pressure changes underscore that “more research is needed to determine which formulations of estrogen, testosterone, and antiandrogens are optimal regarding blood pressure and cardiovascular health, especially in older individuals,” the authors note.
Gender-affirming hormone therapy formulations differ
Various formulations for gender-affirming hormone regimens are available, including oral, transdermal, sublingual, and intramuscular preparations.
In the study, 77% to 91% of transgender males were on intramuscular testosterone injections, with the rest on transdermal formulations, and 92% of transgender female patients were started on oral estradiol, with mean doses generally increasing over time.
The study’s results are consistent with evidence from other studies, with 7 of 8 involving transgender males showing mean increases in systolic blood pressure ranging from 1 to 14 mm Hg.
Previous research supports cardiovascular risk
As reported by this news organization, other emerging research on cardiovascular risks to transgender people include a recent study showing more than 10% of transgender males were found to have hematocrit levels that could put them at risk for blood clots.
And further research on transgender youth also shows concerning elevations in lipids and other cardiovascular risks.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender people treated with gender-affirming hormone therapy show distinctive changes in blood pressure that begin soon after treatment initiation and do not subside over years of treatment, according to the largest and longest observational study to date to look at the issue.
“Many physicians may not be aware of the changes to blood pressure in trans patients who start hormone therapy,” senior author Michael S. Irwig, MD, director of transgender medicine at Beth Israel Deaconess Medical Center in Boston, told this news organization.
“The take-away message for physicians is to monitor blood pressure both before and after starting hormone therapy in transgender patients, as over a third of transgender individuals had stage 1 hypertension before starting hormone therapy, and many had their blood pressure increase after starting hormone therapy.”
Mean blood pressure increases in transgender males, decreases in females
In the study, published in Hypertension, Katherine Banks, MD, George Washington University, Washington, and colleagues, followed 470 transgender adult patients for up to 5 years.
The mean systolic blood pressure levels in transgender female patients (male at birth) significantly decreased compared with baseline within a few months of them starting gender-affirming hormone treatment.
Conversely, the systolic blood pressure levels in transgender males (females at birth) who were treated with testosterone increased over the same period.
There were no significant changes in the groups in terms of diastolic blood pressure, consistent with other studies.
“Our study is the first to describe the time course of the blood pressure effects of gender-affirming hormone therapy and to compare the rates of elevated blood pressure and stage 1 and stage 2 hypertension using blood pressure readings from gender-diverse individuals pre- and post–gender-affirming hormone therapy,” the authors note.
Gender-affirming hormone therapy – which has been prescribed to transgender patients for more than 25 years – typically involves a combination of estrogen and an anti-androgen for males transitioning to female, while the therapy for those transitioning to male generally only involves testosterone.
The therapy has previously been linked to various cardiac effects, with evidence showing transgender men have as much as a 5-times greater risk of heart attack versus cisgender women, the authors note.
Although the American Heart Association issued a 2020 Scientific Statement addressing the cardiovascular disease risk, evidence on the effects specifically on blood pressure in transgender patients has been inconsistent.
For the new study, Dr. Banks and colleagues enrolled 247 transgender females and 223 transgender males who were treated between 2007 and 2015 at two medical centers in Washington, D.C. Of the individuals, who had a mean age of 27.8, about 27% were non-White and 16% were Latinx.
They had blood pressure measurements taken at baseline and at follow-up clinical visits for up to 57 months following the initiation of gender-affirming hormone therapy.
Over the follow-up period, the transgender females had decreases in mean systolic blood pressure of 4.0 mm Hg within 2 to 4 months of starting hormone therapy (P < .0001) and mean declines of 6.0 mm Hg were further observed at 11 to 21 months compared with baseline.
In transgender males, the mean systolic blood pressure increased by 2.6 mm Hg at 2 to 4 months (P = .02), and by 2.9 mm Hg at 11 to 21 months after starting therapy.
Furthermore, “although the average increase in systolic blood pressure was 2.6 mm Hg in transgender men within 2 to 4 months, some patients had much higher increases,” Dr. Irwig noted.
As many as 40% of transgender men had stage 1 hypertension after 11 to 21 months of hormone therapy.
The blood pressure changes in transgender males and females were observed across all three racial ethnic groups of Whites, Blacks, and Latinx, and the changes remained consistent throughout the entire follow-up period of approximately 5 years while hormone therapy was continued.
In addition to the changes after therapy initiation, the researchers note that more than one-third of individuals in both groups had stage 1 hypertension even before starting hormone therapy.
The findings are a concern in light of “clear evidence linking hypertension and higher blood pressure with cardiovascular events such as stroke and heart attacks,” Dr. Irwig said.
Protective effects for transgender females?
Transgender females showed as much as a 47% decrease in the prevalence of stage 2 hypertension, from 19% to 10%, within 2 to 4 months of treatment with gender-affirming hormone therapy (P = .001), and the rate declined further to 8% at 11 to 21 months, suggesting a protective effect of the treatment.
“The rate of stage 2 hypertension did drop in transgender feminine individuals, which could be protective and lower their risk for cardiovascular events,” Dr. Irwig said.
“This was not a surprise, as lowering testosterone and the use of spironolactone can lower blood pressure,” he noted.
Exceptions in both groups
Of note, a sizable proportion of patients had blood pressure changes that were in fact the opposite of the patterns seen in the majority of their gender group.
Specifically, while 42% to 53% of the transgender females had systolic blood pressure readings of at least 5 mm Hg lower than their baseline readings, up to 32% had increases of at least 5 mm Hg compared to baseline readings.
Likewise, whereas 41% to 59% of transgender males had increases of at least 5 mm Hg compared with baseline, up to 35% had levels that were at least 5 mm Hg lower than baseline.
“It was a surprise that over a quarter of individuals had changes opposite to the mean changes,” Dr. Irwig said.
The differing blood pressure changes underscore that “more research is needed to determine which formulations of estrogen, testosterone, and antiandrogens are optimal regarding blood pressure and cardiovascular health, especially in older individuals,” the authors note.
Gender-affirming hormone therapy formulations differ
Various formulations for gender-affirming hormone regimens are available, including oral, transdermal, sublingual, and intramuscular preparations.
In the study, 77% to 91% of transgender males were on intramuscular testosterone injections, with the rest on transdermal formulations, and 92% of transgender female patients were started on oral estradiol, with mean doses generally increasing over time.
The study’s results are consistent with evidence from other studies, with 7 of 8 involving transgender males showing mean increases in systolic blood pressure ranging from 1 to 14 mm Hg.
Previous research supports cardiovascular risk
As reported by this news organization, other emerging research on cardiovascular risks to transgender people include a recent study showing more than 10% of transgender males were found to have hematocrit levels that could put them at risk for blood clots.
And further research on transgender youth also shows concerning elevations in lipids and other cardiovascular risks.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender people treated with gender-affirming hormone therapy show distinctive changes in blood pressure that begin soon after treatment initiation and do not subside over years of treatment, according to the largest and longest observational study to date to look at the issue.
“Many physicians may not be aware of the changes to blood pressure in trans patients who start hormone therapy,” senior author Michael S. Irwig, MD, director of transgender medicine at Beth Israel Deaconess Medical Center in Boston, told this news organization.
“The take-away message for physicians is to monitor blood pressure both before and after starting hormone therapy in transgender patients, as over a third of transgender individuals had stage 1 hypertension before starting hormone therapy, and many had their blood pressure increase after starting hormone therapy.”
Mean blood pressure increases in transgender males, decreases in females
In the study, published in Hypertension, Katherine Banks, MD, George Washington University, Washington, and colleagues, followed 470 transgender adult patients for up to 5 years.
The mean systolic blood pressure levels in transgender female patients (male at birth) significantly decreased compared with baseline within a few months of them starting gender-affirming hormone treatment.
Conversely, the systolic blood pressure levels in transgender males (females at birth) who were treated with testosterone increased over the same period.
There were no significant changes in the groups in terms of diastolic blood pressure, consistent with other studies.
“Our study is the first to describe the time course of the blood pressure effects of gender-affirming hormone therapy and to compare the rates of elevated blood pressure and stage 1 and stage 2 hypertension using blood pressure readings from gender-diverse individuals pre- and post–gender-affirming hormone therapy,” the authors note.
Gender-affirming hormone therapy – which has been prescribed to transgender patients for more than 25 years – typically involves a combination of estrogen and an anti-androgen for males transitioning to female, while the therapy for those transitioning to male generally only involves testosterone.
The therapy has previously been linked to various cardiac effects, with evidence showing transgender men have as much as a 5-times greater risk of heart attack versus cisgender women, the authors note.
Although the American Heart Association issued a 2020 Scientific Statement addressing the cardiovascular disease risk, evidence on the effects specifically on blood pressure in transgender patients has been inconsistent.
For the new study, Dr. Banks and colleagues enrolled 247 transgender females and 223 transgender males who were treated between 2007 and 2015 at two medical centers in Washington, D.C. Of the individuals, who had a mean age of 27.8, about 27% were non-White and 16% were Latinx.
They had blood pressure measurements taken at baseline and at follow-up clinical visits for up to 57 months following the initiation of gender-affirming hormone therapy.
Over the follow-up period, the transgender females had decreases in mean systolic blood pressure of 4.0 mm Hg within 2 to 4 months of starting hormone therapy (P < .0001) and mean declines of 6.0 mm Hg were further observed at 11 to 21 months compared with baseline.
In transgender males, the mean systolic blood pressure increased by 2.6 mm Hg at 2 to 4 months (P = .02), and by 2.9 mm Hg at 11 to 21 months after starting therapy.
Furthermore, “although the average increase in systolic blood pressure was 2.6 mm Hg in transgender men within 2 to 4 months, some patients had much higher increases,” Dr. Irwig noted.
As many as 40% of transgender men had stage 1 hypertension after 11 to 21 months of hormone therapy.
The blood pressure changes in transgender males and females were observed across all three racial ethnic groups of Whites, Blacks, and Latinx, and the changes remained consistent throughout the entire follow-up period of approximately 5 years while hormone therapy was continued.
In addition to the changes after therapy initiation, the researchers note that more than one-third of individuals in both groups had stage 1 hypertension even before starting hormone therapy.
The findings are a concern in light of “clear evidence linking hypertension and higher blood pressure with cardiovascular events such as stroke and heart attacks,” Dr. Irwig said.
Protective effects for transgender females?
Transgender females showed as much as a 47% decrease in the prevalence of stage 2 hypertension, from 19% to 10%, within 2 to 4 months of treatment with gender-affirming hormone therapy (P = .001), and the rate declined further to 8% at 11 to 21 months, suggesting a protective effect of the treatment.
“The rate of stage 2 hypertension did drop in transgender feminine individuals, which could be protective and lower their risk for cardiovascular events,” Dr. Irwig said.
“This was not a surprise, as lowering testosterone and the use of spironolactone can lower blood pressure,” he noted.
Exceptions in both groups
Of note, a sizable proportion of patients had blood pressure changes that were in fact the opposite of the patterns seen in the majority of their gender group.
Specifically, while 42% to 53% of the transgender females had systolic blood pressure readings of at least 5 mm Hg lower than their baseline readings, up to 32% had increases of at least 5 mm Hg compared to baseline readings.
Likewise, whereas 41% to 59% of transgender males had increases of at least 5 mm Hg compared with baseline, up to 35% had levels that were at least 5 mm Hg lower than baseline.
“It was a surprise that over a quarter of individuals had changes opposite to the mean changes,” Dr. Irwig said.
The differing blood pressure changes underscore that “more research is needed to determine which formulations of estrogen, testosterone, and antiandrogens are optimal regarding blood pressure and cardiovascular health, especially in older individuals,” the authors note.
Gender-affirming hormone therapy formulations differ
Various formulations for gender-affirming hormone regimens are available, including oral, transdermal, sublingual, and intramuscular preparations.
In the study, 77% to 91% of transgender males were on intramuscular testosterone injections, with the rest on transdermal formulations, and 92% of transgender female patients were started on oral estradiol, with mean doses generally increasing over time.
The study’s results are consistent with evidence from other studies, with 7 of 8 involving transgender males showing mean increases in systolic blood pressure ranging from 1 to 14 mm Hg.
Previous research supports cardiovascular risk
As reported by this news organization, other emerging research on cardiovascular risks to transgender people include a recent study showing more than 10% of transgender males were found to have hematocrit levels that could put them at risk for blood clots.
And further research on transgender youth also shows concerning elevations in lipids and other cardiovascular risks.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.