User login
Remote cardio visits expand access for underserved during COVID
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six pregnancy complications flag later heart disease risk
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
The best exercises for BP control? European statement sorts it out
Recommendations for prescribing exercise to control high blood pressure have been put forward by various medical organizations and expert panels, but finding the bandwidth to craft personalized exercise training for their patients poses a challenge for clinicians.
Now, European cardiology societies have issued a consensus statement that offers an algorithm of sorts for developing personalized exercise programs as part of overall management approach for patients with or at risk of high BP.
The statement, published in the European Journal of Preventive Cardiology and issued by the European Association of Preventive Cardiology and the European Society of Cardiology Council on Hypertension, claims to be the first document to focus on personalized exercise for BP.
The statement draws on a systematic review, including meta-analyses, to produce guidance on how to lower BP in three specific types of patients: Those with hypertension (>140/90 mm Hg), high-normal blood pressure (130-139/85-89 mm Hg), and normal blood pressure (<130/84 mm Hg).
By making recommendations for these three specific groups, along with providing guidance for combined exercise – that is, blending aerobic exercise with resistance training (RT) – the consensus statement goes one step further than recommendations other organizations have issued, Matthew W. Martinez, MD, said in an interview.
“What it adds is an algorithmic approach, if you will,” said Dr. Martinez, a sports medicine cardiologist at Morristown (N.J.) Medical Center. “There are some recommendations to help the clinicians to decide what they’re going to offer individuals, but what’s a challenge for us when seeing patients is finding the time to deliver the message and explain how valuable nutrition and exercise are.”
Guidelines, updates, and statements that include the role of exercise in BP control have been issued by the European Society of Cardiology, American Heart Association, and American College of Sports Medicine (Med Sci Sports Exercise. 2019;51:1314-23).
The European consensus statement includes the expected range of BP lowering for each activity. For example, aerobic exercise for patients with hypertension should lead to a reduction from –4.9 to –12 mm Hg systolic and –3.4 to –5.8 mm Hg diastolic.
The consensus statement recommends the following exercise priorities based on a patient’s blood pressure:
- Hypertension: Aerobic training (AT) as a first-line exercise therapy; and low- to moderate-intensity RT – equally using dynamic and isometric RT – as second-line therapy. In non-White patients, dynamic RT should be considered as a first-line therapy. RT can be combined with aerobic exercise on an individual basis if the clinician determines either form of RT would provide a metabolic benefit.
- High-to-normal BP: Dynamic RT as a first-line exercise, which the systematic review determined led to greater BP reduction than that of aerobic training. “Isometric RT is likely to elicit similar if not superior BP-lowering effects as [dynamic RT], but the level of evidence is low and the available data are scarce,” wrote first author Henner Hanssen, MD, of the University of Basel, Switzerland, and coauthors. Combining dynamic resistance training with aerobic training “may be preferable” to dynamic RT alone in patients with a combination of cardiovascular risk factors.
- Normal BP: Isometric RT may be indicated as a first-line intervention in individuals with a family or gestational history or obese or overweight people currently with normal BP. This advice includes a caveat: “The number of studies is limited and the 95% confidence intervals are large,” Dr. Hanssen and coauthors noted. AT is also an option in these patients, with more high-quality meta-analyses than the recommendation for isometric RT. “Hence, the BP-lowering effects of [isometric RT] as compared to AT may be overestimated and both exercise modalities may have similar BP-lowering effects in individuals with normotension,” wrote the consensus statement authors.
They note that more research is needed to validate the BP-lowering effects of combined exercise.
The statement acknowledges the difficulty clinicians face in managing patients with high blood pressure. “From a socioeconomic health perspective, it is a major challenge to develop, promote, and implement individually tailored exercise programs for patients with hypertension under consideration of sustainable costs,” wrote Dr. Hanssen and coauthors.
Dr. Martinez noted that one strength of the consensus statement is that it addresses the impact exercise can have on vascular health and metabolic function. And, it points out existing knowledge gaps.
“Are we going to see greater applicability of this as we use IT health technology?” he asked. “Are wearables and telehealth going to help deliver this message more easily, more frequently? Is there work to be done in terms of differences in gender? Do men and women respond differently, and is there a different exercise prescription based on that as well as ethnicity? We well know there’s a different treatment for African Americans compared to other ethnic groups.”
The statement also raises the stakes for using exercise as part of a multifaceted, integrated approach to hypertension management, he said.
“It’s not enough to talk just about exercise or nutrition, or to just give an antihypertension medicine,” Dr. Martinez said. “Perhaps the sweet spot is in integrating an approach that includes all three.”
Consensus statement coauthor Antonio Coca, MD, reported financial relationships with Abbott, Berlin-Chemie, Biolab, Boehringer-Ingelheim, Ferrer, Menarini, Merck, Novartis and Sanofi-Aventis. Coauthor Maria Simonenko, MD, reported financial relationships with Novartis and Sanofi-Aventis. Linda Pescatello, PhD, is lead author of the American College of Sports Medicine 2019 statement. Dr. Hanssen and all other authors have no disclosures. Dr. Martinez has no relevant relationships to disclose.
Recommendations for prescribing exercise to control high blood pressure have been put forward by various medical organizations and expert panels, but finding the bandwidth to craft personalized exercise training for their patients poses a challenge for clinicians.
Now, European cardiology societies have issued a consensus statement that offers an algorithm of sorts for developing personalized exercise programs as part of overall management approach for patients with or at risk of high BP.
The statement, published in the European Journal of Preventive Cardiology and issued by the European Association of Preventive Cardiology and the European Society of Cardiology Council on Hypertension, claims to be the first document to focus on personalized exercise for BP.
The statement draws on a systematic review, including meta-analyses, to produce guidance on how to lower BP in three specific types of patients: Those with hypertension (>140/90 mm Hg), high-normal blood pressure (130-139/85-89 mm Hg), and normal blood pressure (<130/84 mm Hg).
By making recommendations for these three specific groups, along with providing guidance for combined exercise – that is, blending aerobic exercise with resistance training (RT) – the consensus statement goes one step further than recommendations other organizations have issued, Matthew W. Martinez, MD, said in an interview.
“What it adds is an algorithmic approach, if you will,” said Dr. Martinez, a sports medicine cardiologist at Morristown (N.J.) Medical Center. “There are some recommendations to help the clinicians to decide what they’re going to offer individuals, but what’s a challenge for us when seeing patients is finding the time to deliver the message and explain how valuable nutrition and exercise are.”
Guidelines, updates, and statements that include the role of exercise in BP control have been issued by the European Society of Cardiology, American Heart Association, and American College of Sports Medicine (Med Sci Sports Exercise. 2019;51:1314-23).
The European consensus statement includes the expected range of BP lowering for each activity. For example, aerobic exercise for patients with hypertension should lead to a reduction from –4.9 to –12 mm Hg systolic and –3.4 to –5.8 mm Hg diastolic.
The consensus statement recommends the following exercise priorities based on a patient’s blood pressure:
- Hypertension: Aerobic training (AT) as a first-line exercise therapy; and low- to moderate-intensity RT – equally using dynamic and isometric RT – as second-line therapy. In non-White patients, dynamic RT should be considered as a first-line therapy. RT can be combined with aerobic exercise on an individual basis if the clinician determines either form of RT would provide a metabolic benefit.
- High-to-normal BP: Dynamic RT as a first-line exercise, which the systematic review determined led to greater BP reduction than that of aerobic training. “Isometric RT is likely to elicit similar if not superior BP-lowering effects as [dynamic RT], but the level of evidence is low and the available data are scarce,” wrote first author Henner Hanssen, MD, of the University of Basel, Switzerland, and coauthors. Combining dynamic resistance training with aerobic training “may be preferable” to dynamic RT alone in patients with a combination of cardiovascular risk factors.
- Normal BP: Isometric RT may be indicated as a first-line intervention in individuals with a family or gestational history or obese or overweight people currently with normal BP. This advice includes a caveat: “The number of studies is limited and the 95% confidence intervals are large,” Dr. Hanssen and coauthors noted. AT is also an option in these patients, with more high-quality meta-analyses than the recommendation for isometric RT. “Hence, the BP-lowering effects of [isometric RT] as compared to AT may be overestimated and both exercise modalities may have similar BP-lowering effects in individuals with normotension,” wrote the consensus statement authors.
They note that more research is needed to validate the BP-lowering effects of combined exercise.
The statement acknowledges the difficulty clinicians face in managing patients with high blood pressure. “From a socioeconomic health perspective, it is a major challenge to develop, promote, and implement individually tailored exercise programs for patients with hypertension under consideration of sustainable costs,” wrote Dr. Hanssen and coauthors.
Dr. Martinez noted that one strength of the consensus statement is that it addresses the impact exercise can have on vascular health and metabolic function. And, it points out existing knowledge gaps.
“Are we going to see greater applicability of this as we use IT health technology?” he asked. “Are wearables and telehealth going to help deliver this message more easily, more frequently? Is there work to be done in terms of differences in gender? Do men and women respond differently, and is there a different exercise prescription based on that as well as ethnicity? We well know there’s a different treatment for African Americans compared to other ethnic groups.”
The statement also raises the stakes for using exercise as part of a multifaceted, integrated approach to hypertension management, he said.
“It’s not enough to talk just about exercise or nutrition, or to just give an antihypertension medicine,” Dr. Martinez said. “Perhaps the sweet spot is in integrating an approach that includes all three.”
Consensus statement coauthor Antonio Coca, MD, reported financial relationships with Abbott, Berlin-Chemie, Biolab, Boehringer-Ingelheim, Ferrer, Menarini, Merck, Novartis and Sanofi-Aventis. Coauthor Maria Simonenko, MD, reported financial relationships with Novartis and Sanofi-Aventis. Linda Pescatello, PhD, is lead author of the American College of Sports Medicine 2019 statement. Dr. Hanssen and all other authors have no disclosures. Dr. Martinez has no relevant relationships to disclose.
Recommendations for prescribing exercise to control high blood pressure have been put forward by various medical organizations and expert panels, but finding the bandwidth to craft personalized exercise training for their patients poses a challenge for clinicians.
Now, European cardiology societies have issued a consensus statement that offers an algorithm of sorts for developing personalized exercise programs as part of overall management approach for patients with or at risk of high BP.
The statement, published in the European Journal of Preventive Cardiology and issued by the European Association of Preventive Cardiology and the European Society of Cardiology Council on Hypertension, claims to be the first document to focus on personalized exercise for BP.
The statement draws on a systematic review, including meta-analyses, to produce guidance on how to lower BP in three specific types of patients: Those with hypertension (>140/90 mm Hg), high-normal blood pressure (130-139/85-89 mm Hg), and normal blood pressure (<130/84 mm Hg).
By making recommendations for these three specific groups, along with providing guidance for combined exercise – that is, blending aerobic exercise with resistance training (RT) – the consensus statement goes one step further than recommendations other organizations have issued, Matthew W. Martinez, MD, said in an interview.
“What it adds is an algorithmic approach, if you will,” said Dr. Martinez, a sports medicine cardiologist at Morristown (N.J.) Medical Center. “There are some recommendations to help the clinicians to decide what they’re going to offer individuals, but what’s a challenge for us when seeing patients is finding the time to deliver the message and explain how valuable nutrition and exercise are.”
Guidelines, updates, and statements that include the role of exercise in BP control have been issued by the European Society of Cardiology, American Heart Association, and American College of Sports Medicine (Med Sci Sports Exercise. 2019;51:1314-23).
The European consensus statement includes the expected range of BP lowering for each activity. For example, aerobic exercise for patients with hypertension should lead to a reduction from –4.9 to –12 mm Hg systolic and –3.4 to –5.8 mm Hg diastolic.
The consensus statement recommends the following exercise priorities based on a patient’s blood pressure:
- Hypertension: Aerobic training (AT) as a first-line exercise therapy; and low- to moderate-intensity RT – equally using dynamic and isometric RT – as second-line therapy. In non-White patients, dynamic RT should be considered as a first-line therapy. RT can be combined with aerobic exercise on an individual basis if the clinician determines either form of RT would provide a metabolic benefit.
- High-to-normal BP: Dynamic RT as a first-line exercise, which the systematic review determined led to greater BP reduction than that of aerobic training. “Isometric RT is likely to elicit similar if not superior BP-lowering effects as [dynamic RT], but the level of evidence is low and the available data are scarce,” wrote first author Henner Hanssen, MD, of the University of Basel, Switzerland, and coauthors. Combining dynamic resistance training with aerobic training “may be preferable” to dynamic RT alone in patients with a combination of cardiovascular risk factors.
- Normal BP: Isometric RT may be indicated as a first-line intervention in individuals with a family or gestational history or obese or overweight people currently with normal BP. This advice includes a caveat: “The number of studies is limited and the 95% confidence intervals are large,” Dr. Hanssen and coauthors noted. AT is also an option in these patients, with more high-quality meta-analyses than the recommendation for isometric RT. “Hence, the BP-lowering effects of [isometric RT] as compared to AT may be overestimated and both exercise modalities may have similar BP-lowering effects in individuals with normotension,” wrote the consensus statement authors.
They note that more research is needed to validate the BP-lowering effects of combined exercise.
The statement acknowledges the difficulty clinicians face in managing patients with high blood pressure. “From a socioeconomic health perspective, it is a major challenge to develop, promote, and implement individually tailored exercise programs for patients with hypertension under consideration of sustainable costs,” wrote Dr. Hanssen and coauthors.
Dr. Martinez noted that one strength of the consensus statement is that it addresses the impact exercise can have on vascular health and metabolic function. And, it points out existing knowledge gaps.
“Are we going to see greater applicability of this as we use IT health technology?” he asked. “Are wearables and telehealth going to help deliver this message more easily, more frequently? Is there work to be done in terms of differences in gender? Do men and women respond differently, and is there a different exercise prescription based on that as well as ethnicity? We well know there’s a different treatment for African Americans compared to other ethnic groups.”
The statement also raises the stakes for using exercise as part of a multifaceted, integrated approach to hypertension management, he said.
“It’s not enough to talk just about exercise or nutrition, or to just give an antihypertension medicine,” Dr. Martinez said. “Perhaps the sweet spot is in integrating an approach that includes all three.”
Consensus statement coauthor Antonio Coca, MD, reported financial relationships with Abbott, Berlin-Chemie, Biolab, Boehringer-Ingelheim, Ferrer, Menarini, Merck, Novartis and Sanofi-Aventis. Coauthor Maria Simonenko, MD, reported financial relationships with Novartis and Sanofi-Aventis. Linda Pescatello, PhD, is lead author of the American College of Sports Medicine 2019 statement. Dr. Hanssen and all other authors have no disclosures. Dr. Martinez has no relevant relationships to disclose.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Long-haul COVID-19 brings welcome attention to POTS
Before COVID-19, postural orthostatic tachycardia syndrome (POTS) was one of those diseases that many people, including physicians, dismissed.
“They thought it was just anxious, crazy young women,” said Pam R. Taub, MD, who runs the cardiac rehabilitation program at the University of California, San Diego.
The cryptic autonomic condition was estimated to affect 1-3 million Americans before the pandemic hit. Now case reports confirm that it is a manifestation of postacute sequelae of SARS-CoV-2 infection (PASC), or so-called long-haul COVID-19.
“I’m excited that this condition that has been so often the ugly stepchild of both cardiology and neurology is getting some attention,” said Dr. Taub. She said she is hopeful that the National Institutes of Health’s commitment to PASC research will benefit patients affected by the cardiovascular dysautonomia characterized by orthostatic intolerance in the absence of orthostatic hypotension.
Postinfection POTS is not exclusive to SARS-CoV-2. It has been reported after Lyme disease and Epstein-Barr virus infections, for example. One theory is that some of the antibodies generated against the virus cross react and damage the autonomic nervous system, which regulates heart rate and blood pressure, Dr. Taub explained.
It is not known whether COVID-19 is more likely to trigger POTS than are other infections or whether the rise in cases merely reflects the fact that more than 115 million people worldwide have been infected with the novel coronavirus.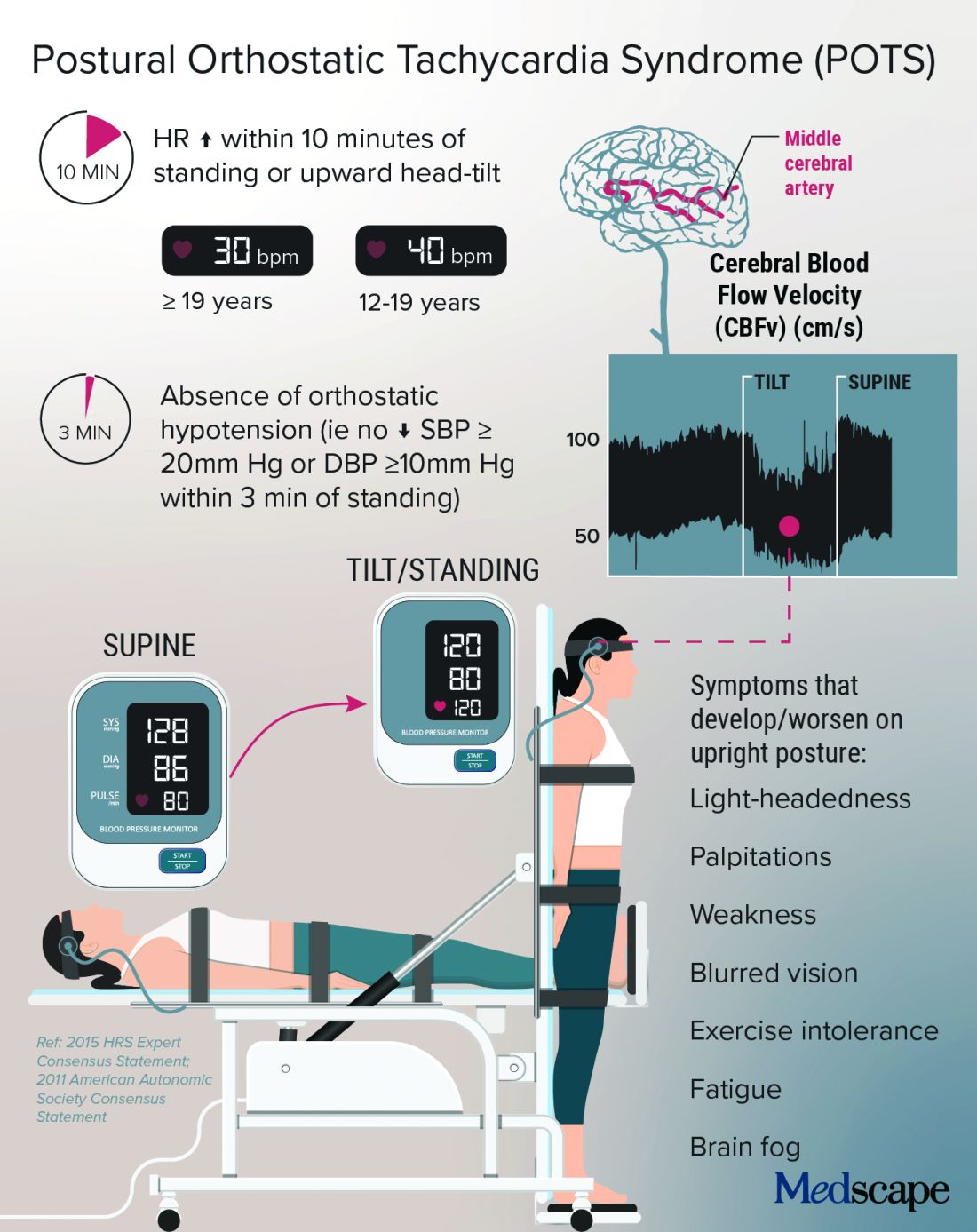
Low blood volume, dysregulation of the autonomic nervous system, and autoimmunity may all play a role in POTS, perhaps leading to distinct subtypes, according to a State of the Science document from the NIH; the National Heart, Lung, and Blood Institute; and the National Institute of Neurological Disorders and Stroke.
In Dr. Taub’s experience, “The truth is that patients actually have a mix of the subtypes.”
Kamal Shouman, MD, an autonomic neurologist at Mayo Clinic, Rochester, Minn., said in an interview that he has seen patients present with post–COVID-19 POTS in “all flavors,” including “neuropathic POTS, which is thought of as the classic postinfectious phenomenon.”
Why does it mostly affect athletic women?
The condition, which can be the result of dehydration or prolonged bed rest, leading to deconditioning, affects women disproportionately.
According to Manesh Patel, MD, if a patient with POTS who is not a young woman is presented on medical rounds, the response is, “Tell me again why you think this patient has POTS.”
Dr. Patel, chief of the division of cardiology at Duke University, Durham, N.C., has a theory for why many of the women who have POTS are athletes or are highly active: They likely have an underlying predisposition, compounded by a smaller body volume, leaving less margin for error. “If they decondition and lose 500 cc’s, it makes a bigger difference to them than, say, a 300-pound offensive lineman,” Dr. Patel explained.
That hypothesis makes sense to Dr. Taub, who added, “There are just some people metabolically that are more hyperadrenergic,” and it may be that “all their activity really helps tone down that sympathetic output,” but the infection affects these regulatory processes, and deconditioning disrupts things further.
Women also have more autoimmune disorders than do men. The driving force of the dysregulation of the autonomic nervous system is thought to be “immune mediated; we think it’s triggered by a response to a virus,” she said.
Dr. Shouman said the underlying susceptibility may predispose toward orthostatic intolerance. For example, patients will tell him, “Well, many years ago, I was prone to fainting.” He emphasized that POTS is not exclusive to women – he sees men with POTS, and one of the three recent case reports of post–COVID-19 POTS involved a 37-year-old man. So far, the male POTS patients that Dr. Patel has encountered have been deconditioned athletes.
Poor (wo)man’s tilt test and treatment options
POTS is typically diagnosed with a tilt test and transcranial Doppler. Dr. Taub described her “poor man’s tilt test” of asking the patient to lie down for 5-10 minutes and then having the patient stand up.
She likes the fact that transcranial Doppler helps validate the brain fog that patients report, which can be dismissed as “just your excuse for not wanting to work.” If blood perfusion to the brain is cut by 40%-50%, “how are you going to think clearly?” she said.
Dr. Shouman noted that overall volume expansion with salt water, compression garments, and a graduated exercise program play a major role in the rehabilitation of all POTS patients.
He likes to tailor treatments to the most likely underlying cause. But patients should first undergo a medical assessment by their internists to make sure there isn’t a primary lung or heart problem.
“Once the decision is made for them to be evaluated in the autonomic practice and [a] POTS diagnosis is made, I think it is very useful to determine what type of POTS,” he said.
With hyperadrenergic POTS, “you are looking at a standing norepinephrine level of over 600 pg/mL or so.” For these patients, drugs such as ivabradine or beta-blockers can help, he noted.
Dr. Taub recently conducted a small study that showed a benefit with the selective If channel blocker ivabradine for patients with hyperadrenergic POTS unrelated to COVID-19. She tends to favor ivabradine over beta-blockers because it lowers heart rate but not blood pressure. In addition, beta-blockers can exacerbate fatigue and brain fog.
A small crossover study will compare propranolol and ivabradine in POTS. For someone who is very hypovolemic, “you might try a salt tablet or a prescription drug like fludrocortisone,” Dr. Taub explained.
Another problem that patients with POTS experience is an inability to exercise because of orthostatic intolerance. Recumbent exercise targets deconditioning and can tamp down the hyperadrenergic effect. Dr. Shouman’s approach is to start gradually with swimming or the use of a recumbent bike or a rowing machine.
Dr. Taub recommends wearables to patients because POTS is “a very dynamic condition” that is easy to overmedicate or undermedicate. If it’s a good day, the patients are well hydrated, and the standing heart rate is only 80 bpm, she tells them they could titrate down their second dose of ivabradine, for example. The feedback from wearables also helps patients manage their exercise response.
For Dr. Shouman, wearables are not always as accurate as he would like. He tells his patients that it’s okay to use one as long as it doesn’t become a source of anxiety such that they’re constantly checking it.
POTS hope: A COVID-19 silver lining?
With increasing attention being paid to long-haul COVID-19, are there any concerns that POTS will get lost among the myriad symptoms connected to PASC?
Dr. Shouman cautioned, “Not all long COVID is POTS,” and said that clinicians at long-haul clinics should be able to recognize the different conditions “when POTS is suspected. I think it is useful for those providers to make the appropriate referral for POTS clinic autonomic assessment.”
He and his colleagues at Mayo have seen quite a few patients who have post–COVID-19 autonomic dysfunction, such as vasodepressor syncope, not just POTS. They plan to write about this soon.
“Of all the things I treat in cardiology, this is the most complex, because there’s so many different systems involved,” said Dr. Taub, who has seen patients recover fully from POTS. “There’s a spectrum, and there’s people that are definitely on one end of the spectrum where they have very severe diseases.”
For her, the important message is, “No matter where you are on the spectrum, there are things we can do to make your symptoms better.” And with grant funding for PASC research, “hopefully we will address the mechanisms of disease, and we’ll be able to cure this,” she said.
Dr. Patel has served as a consultant for Bayer, Janssen, AstraZeneca, and Heartflow and has received research grants from Bayer, Janssen, AstraZeneca, and the National Heart, Lung, and Blood Institute. Dr. Shouman reports no relevant financial relationships. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA.
A version of this article first appeared on Medscape.com.
Before COVID-19, postural orthostatic tachycardia syndrome (POTS) was one of those diseases that many people, including physicians, dismissed.
“They thought it was just anxious, crazy young women,” said Pam R. Taub, MD, who runs the cardiac rehabilitation program at the University of California, San Diego.
The cryptic autonomic condition was estimated to affect 1-3 million Americans before the pandemic hit. Now case reports confirm that it is a manifestation of postacute sequelae of SARS-CoV-2 infection (PASC), or so-called long-haul COVID-19.
“I’m excited that this condition that has been so often the ugly stepchild of both cardiology and neurology is getting some attention,” said Dr. Taub. She said she is hopeful that the National Institutes of Health’s commitment to PASC research will benefit patients affected by the cardiovascular dysautonomia characterized by orthostatic intolerance in the absence of orthostatic hypotension.
Postinfection POTS is not exclusive to SARS-CoV-2. It has been reported after Lyme disease and Epstein-Barr virus infections, for example. One theory is that some of the antibodies generated against the virus cross react and damage the autonomic nervous system, which regulates heart rate and blood pressure, Dr. Taub explained.
It is not known whether COVID-19 is more likely to trigger POTS than are other infections or whether the rise in cases merely reflects the fact that more than 115 million people worldwide have been infected with the novel coronavirus.
Low blood volume, dysregulation of the autonomic nervous system, and autoimmunity may all play a role in POTS, perhaps leading to distinct subtypes, according to a State of the Science document from the NIH; the National Heart, Lung, and Blood Institute; and the National Institute of Neurological Disorders and Stroke.
In Dr. Taub’s experience, “The truth is that patients actually have a mix of the subtypes.”
Kamal Shouman, MD, an autonomic neurologist at Mayo Clinic, Rochester, Minn., said in an interview that he has seen patients present with post–COVID-19 POTS in “all flavors,” including “neuropathic POTS, which is thought of as the classic postinfectious phenomenon.”
Why does it mostly affect athletic women?
The condition, which can be the result of dehydration or prolonged bed rest, leading to deconditioning, affects women disproportionately.
According to Manesh Patel, MD, if a patient with POTS who is not a young woman is presented on medical rounds, the response is, “Tell me again why you think this patient has POTS.”
Dr. Patel, chief of the division of cardiology at Duke University, Durham, N.C., has a theory for why many of the women who have POTS are athletes or are highly active: They likely have an underlying predisposition, compounded by a smaller body volume, leaving less margin for error. “If they decondition and lose 500 cc’s, it makes a bigger difference to them than, say, a 300-pound offensive lineman,” Dr. Patel explained.
That hypothesis makes sense to Dr. Taub, who added, “There are just some people metabolically that are more hyperadrenergic,” and it may be that “all their activity really helps tone down that sympathetic output,” but the infection affects these regulatory processes, and deconditioning disrupts things further.
Women also have more autoimmune disorders than do men. The driving force of the dysregulation of the autonomic nervous system is thought to be “immune mediated; we think it’s triggered by a response to a virus,” she said.
Dr. Shouman said the underlying susceptibility may predispose toward orthostatic intolerance. For example, patients will tell him, “Well, many years ago, I was prone to fainting.” He emphasized that POTS is not exclusive to women – he sees men with POTS, and one of the three recent case reports of post–COVID-19 POTS involved a 37-year-old man. So far, the male POTS patients that Dr. Patel has encountered have been deconditioned athletes.
Poor (wo)man’s tilt test and treatment options
POTS is typically diagnosed with a tilt test and transcranial Doppler. Dr. Taub described her “poor man’s tilt test” of asking the patient to lie down for 5-10 minutes and then having the patient stand up.
She likes the fact that transcranial Doppler helps validate the brain fog that patients report, which can be dismissed as “just your excuse for not wanting to work.” If blood perfusion to the brain is cut by 40%-50%, “how are you going to think clearly?” she said.
Dr. Shouman noted that overall volume expansion with salt water, compression garments, and a graduated exercise program play a major role in the rehabilitation of all POTS patients.
He likes to tailor treatments to the most likely underlying cause. But patients should first undergo a medical assessment by their internists to make sure there isn’t a primary lung or heart problem.
“Once the decision is made for them to be evaluated in the autonomic practice and [a] POTS diagnosis is made, I think it is very useful to determine what type of POTS,” he said.
With hyperadrenergic POTS, “you are looking at a standing norepinephrine level of over 600 pg/mL or so.” For these patients, drugs such as ivabradine or beta-blockers can help, he noted.
Dr. Taub recently conducted a small study that showed a benefit with the selective If channel blocker ivabradine for patients with hyperadrenergic POTS unrelated to COVID-19. She tends to favor ivabradine over beta-blockers because it lowers heart rate but not blood pressure. In addition, beta-blockers can exacerbate fatigue and brain fog.
A small crossover study will compare propranolol and ivabradine in POTS. For someone who is very hypovolemic, “you might try a salt tablet or a prescription drug like fludrocortisone,” Dr. Taub explained.
Another problem that patients with POTS experience is an inability to exercise because of orthostatic intolerance. Recumbent exercise targets deconditioning and can tamp down the hyperadrenergic effect. Dr. Shouman’s approach is to start gradually with swimming or the use of a recumbent bike or a rowing machine.
Dr. Taub recommends wearables to patients because POTS is “a very dynamic condition” that is easy to overmedicate or undermedicate. If it’s a good day, the patients are well hydrated, and the standing heart rate is only 80 bpm, she tells them they could titrate down their second dose of ivabradine, for example. The feedback from wearables also helps patients manage their exercise response.
For Dr. Shouman, wearables are not always as accurate as he would like. He tells his patients that it’s okay to use one as long as it doesn’t become a source of anxiety such that they’re constantly checking it.
POTS hope: A COVID-19 silver lining?
With increasing attention being paid to long-haul COVID-19, are there any concerns that POTS will get lost among the myriad symptoms connected to PASC?
Dr. Shouman cautioned, “Not all long COVID is POTS,” and said that clinicians at long-haul clinics should be able to recognize the different conditions “when POTS is suspected. I think it is useful for those providers to make the appropriate referral for POTS clinic autonomic assessment.”
He and his colleagues at Mayo have seen quite a few patients who have post–COVID-19 autonomic dysfunction, such as vasodepressor syncope, not just POTS. They plan to write about this soon.
“Of all the things I treat in cardiology, this is the most complex, because there’s so many different systems involved,” said Dr. Taub, who has seen patients recover fully from POTS. “There’s a spectrum, and there’s people that are definitely on one end of the spectrum where they have very severe diseases.”
For her, the important message is, “No matter where you are on the spectrum, there are things we can do to make your symptoms better.” And with grant funding for PASC research, “hopefully we will address the mechanisms of disease, and we’ll be able to cure this,” she said.
Dr. Patel has served as a consultant for Bayer, Janssen, AstraZeneca, and Heartflow and has received research grants from Bayer, Janssen, AstraZeneca, and the National Heart, Lung, and Blood Institute. Dr. Shouman reports no relevant financial relationships. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA.
A version of this article first appeared on Medscape.com.
Before COVID-19, postural orthostatic tachycardia syndrome (POTS) was one of those diseases that many people, including physicians, dismissed.
“They thought it was just anxious, crazy young women,” said Pam R. Taub, MD, who runs the cardiac rehabilitation program at the University of California, San Diego.
The cryptic autonomic condition was estimated to affect 1-3 million Americans before the pandemic hit. Now case reports confirm that it is a manifestation of postacute sequelae of SARS-CoV-2 infection (PASC), or so-called long-haul COVID-19.
“I’m excited that this condition that has been so often the ugly stepchild of both cardiology and neurology is getting some attention,” said Dr. Taub. She said she is hopeful that the National Institutes of Health’s commitment to PASC research will benefit patients affected by the cardiovascular dysautonomia characterized by orthostatic intolerance in the absence of orthostatic hypotension.
Postinfection POTS is not exclusive to SARS-CoV-2. It has been reported after Lyme disease and Epstein-Barr virus infections, for example. One theory is that some of the antibodies generated against the virus cross react and damage the autonomic nervous system, which regulates heart rate and blood pressure, Dr. Taub explained.
It is not known whether COVID-19 is more likely to trigger POTS than are other infections or whether the rise in cases merely reflects the fact that more than 115 million people worldwide have been infected with the novel coronavirus.
Low blood volume, dysregulation of the autonomic nervous system, and autoimmunity may all play a role in POTS, perhaps leading to distinct subtypes, according to a State of the Science document from the NIH; the National Heart, Lung, and Blood Institute; and the National Institute of Neurological Disorders and Stroke.
In Dr. Taub’s experience, “The truth is that patients actually have a mix of the subtypes.”
Kamal Shouman, MD, an autonomic neurologist at Mayo Clinic, Rochester, Minn., said in an interview that he has seen patients present with post–COVID-19 POTS in “all flavors,” including “neuropathic POTS, which is thought of as the classic postinfectious phenomenon.”
Why does it mostly affect athletic women?
The condition, which can be the result of dehydration or prolonged bed rest, leading to deconditioning, affects women disproportionately.
According to Manesh Patel, MD, if a patient with POTS who is not a young woman is presented on medical rounds, the response is, “Tell me again why you think this patient has POTS.”
Dr. Patel, chief of the division of cardiology at Duke University, Durham, N.C., has a theory for why many of the women who have POTS are athletes or are highly active: They likely have an underlying predisposition, compounded by a smaller body volume, leaving less margin for error. “If they decondition and lose 500 cc’s, it makes a bigger difference to them than, say, a 300-pound offensive lineman,” Dr. Patel explained.
That hypothesis makes sense to Dr. Taub, who added, “There are just some people metabolically that are more hyperadrenergic,” and it may be that “all their activity really helps tone down that sympathetic output,” but the infection affects these regulatory processes, and deconditioning disrupts things further.
Women also have more autoimmune disorders than do men. The driving force of the dysregulation of the autonomic nervous system is thought to be “immune mediated; we think it’s triggered by a response to a virus,” she said.
Dr. Shouman said the underlying susceptibility may predispose toward orthostatic intolerance. For example, patients will tell him, “Well, many years ago, I was prone to fainting.” He emphasized that POTS is not exclusive to women – he sees men with POTS, and one of the three recent case reports of post–COVID-19 POTS involved a 37-year-old man. So far, the male POTS patients that Dr. Patel has encountered have been deconditioned athletes.
Poor (wo)man’s tilt test and treatment options
POTS is typically diagnosed with a tilt test and transcranial Doppler. Dr. Taub described her “poor man’s tilt test” of asking the patient to lie down for 5-10 minutes and then having the patient stand up.
She likes the fact that transcranial Doppler helps validate the brain fog that patients report, which can be dismissed as “just your excuse for not wanting to work.” If blood perfusion to the brain is cut by 40%-50%, “how are you going to think clearly?” she said.
Dr. Shouman noted that overall volume expansion with salt water, compression garments, and a graduated exercise program play a major role in the rehabilitation of all POTS patients.
He likes to tailor treatments to the most likely underlying cause. But patients should first undergo a medical assessment by their internists to make sure there isn’t a primary lung or heart problem.
“Once the decision is made for them to be evaluated in the autonomic practice and [a] POTS diagnosis is made, I think it is very useful to determine what type of POTS,” he said.
With hyperadrenergic POTS, “you are looking at a standing norepinephrine level of over 600 pg/mL or so.” For these patients, drugs such as ivabradine or beta-blockers can help, he noted.
Dr. Taub recently conducted a small study that showed a benefit with the selective If channel blocker ivabradine for patients with hyperadrenergic POTS unrelated to COVID-19. She tends to favor ivabradine over beta-blockers because it lowers heart rate but not blood pressure. In addition, beta-blockers can exacerbate fatigue and brain fog.
A small crossover study will compare propranolol and ivabradine in POTS. For someone who is very hypovolemic, “you might try a salt tablet or a prescription drug like fludrocortisone,” Dr. Taub explained.
Another problem that patients with POTS experience is an inability to exercise because of orthostatic intolerance. Recumbent exercise targets deconditioning and can tamp down the hyperadrenergic effect. Dr. Shouman’s approach is to start gradually with swimming or the use of a recumbent bike or a rowing machine.
Dr. Taub recommends wearables to patients because POTS is “a very dynamic condition” that is easy to overmedicate or undermedicate. If it’s a good day, the patients are well hydrated, and the standing heart rate is only 80 bpm, she tells them they could titrate down their second dose of ivabradine, for example. The feedback from wearables also helps patients manage their exercise response.
For Dr. Shouman, wearables are not always as accurate as he would like. He tells his patients that it’s okay to use one as long as it doesn’t become a source of anxiety such that they’re constantly checking it.
POTS hope: A COVID-19 silver lining?
With increasing attention being paid to long-haul COVID-19, are there any concerns that POTS will get lost among the myriad symptoms connected to PASC?
Dr. Shouman cautioned, “Not all long COVID is POTS,” and said that clinicians at long-haul clinics should be able to recognize the different conditions “when POTS is suspected. I think it is useful for those providers to make the appropriate referral for POTS clinic autonomic assessment.”
He and his colleagues at Mayo have seen quite a few patients who have post–COVID-19 autonomic dysfunction, such as vasodepressor syncope, not just POTS. They plan to write about this soon.
“Of all the things I treat in cardiology, this is the most complex, because there’s so many different systems involved,” said Dr. Taub, who has seen patients recover fully from POTS. “There’s a spectrum, and there’s people that are definitely on one end of the spectrum where they have very severe diseases.”
For her, the important message is, “No matter where you are on the spectrum, there are things we can do to make your symptoms better.” And with grant funding for PASC research, “hopefully we will address the mechanisms of disease, and we’ll be able to cure this,” she said.
Dr. Patel has served as a consultant for Bayer, Janssen, AstraZeneca, and Heartflow and has received research grants from Bayer, Janssen, AstraZeneca, and the National Heart, Lung, and Blood Institute. Dr. Shouman reports no relevant financial relationships. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA.
A version of this article first appeared on Medscape.com.
Blood pressure meds tied to increased schizophrenia risk
ACE inhibitors may be associated with an increased risk for schizophrenia and may affect psychiatric symptoms, new research suggests.
Investigators found individuals who carry a genetic variant associated with lower levels of the ACE gene and protein have increased liability to schizophrenia, suggesting that drugs that lower ACE levels or activity may do the same.
“Our findings warrant further investigation into the role of ACE in schizophrenia and closer monitoring by clinicians of individuals, especially those with schizophrenia, who may be on medication that lower ACE activity, such as ACE inhibitors,” Sonia Shah, PhD, Institute for Biomedical Sciences, University of Queensland, Brisbane, Australia, said in an interview.
The study was published online March 10, 2021, in JAMA Psychiatry.
Antihypertensives and mental illness
Hypertension is common in patients with psychiatric disorders and observational studies have reported associations between antihypertensive medication and these disorders, although the findings have been mixed.
Dr. Shah and colleagues estimated the potential of different antihypertensive drug classes on schizophrenia, bipolar disorder, and major depressive disorder.
In a two-sample Mendelian randomization study, they evaluated ties between a single-nucleotide variant and drug-target gene expression derived from expression quantitative trait loci data in blood (sample 1) and the SNV disease association from published case-control, genomewide association studies (sample 2).
The analyses included 40,675 patients with schizophrenia and 64,643 controls; 20,352 with bipolar disorder and 31,358 controls; and 135,458 with major depressive disorder and 344,901 controls.
The major finding was that a one standard deviation–lower expression of the ACE gene in blood was associated with lower systolic blood pressure of 4.0 mm Hg (95% confidence interval, 2.7-5.3), but also an increased risk of schizophrenia (odds ratio, 1.75; 95% CI, 1.28-2.38).
Could ACE inhibitors worsen symptoms or trigger episodes?
In their article, the researchers noted that, in most patients, onset of schizophrenia occurs in late adolescence or early adult life, ruling out ACE inhibitor treatment as a potential causal factor for most cases.
“However, if lower ACE levels play a causal role for schizophrenia risk, it would be reasonable to hypothesize that further lowering of ACE activity in existing patients could worsen symptoms or trigger a new episode,” they wrote.
Dr. Shah emphasized that evidence from genetic analyses alone is “not sufficient to justify changes in prescription guidelines.”
“Patients should not stop taking these medications if they are effective at controlling their blood pressure and they don’t suffer any adverse effects. But it would be reasonable to encourage greater pharmacovigilance,” she said in an interview.
“One way in which we are hoping to follow up these findings,” said Dr. Shah, “is to access electronic health record data for millions of individuals to investigate if there is evidence of increased rates of psychotic episodes in individuals who use ACE inhibitors, compared to other classes of blood pressure–lowering medication.”
Caution warranted
Reached for comment, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Staten Island University Hospital in New York, noted that this is an “extremely complicated” study and urged caution in interpreting the results.
“Since most people develop schizophrenia earlier in life, before they usually develop problems with blood pressure, it’s not so much that these drugs might cause schizophrenia,” Dr. Sullivan said.
“But because of their effects on this particular gene, there’s a possibility that they might worsen symptoms or in somebody with borderline risk might cause them to develop symptoms later in life. This may apply to a relatively small number of people who develop symptoms of schizophrenia in their 40s and beyond,” he added.
That’s where “pharmacovigilance” comes into play, Dr. Sullivan said. “In other words, that they otherwise wouldn’t experience?”
Support for the study was provided by the National Health and Medical Research Council (Australia) and U.S. National Institute for Mental Health. Dr. Shah and Dr. Sullivan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ACE inhibitors may be associated with an increased risk for schizophrenia and may affect psychiatric symptoms, new research suggests.
Investigators found individuals who carry a genetic variant associated with lower levels of the ACE gene and protein have increased liability to schizophrenia, suggesting that drugs that lower ACE levels or activity may do the same.
“Our findings warrant further investigation into the role of ACE in schizophrenia and closer monitoring by clinicians of individuals, especially those with schizophrenia, who may be on medication that lower ACE activity, such as ACE inhibitors,” Sonia Shah, PhD, Institute for Biomedical Sciences, University of Queensland, Brisbane, Australia, said in an interview.
The study was published online March 10, 2021, in JAMA Psychiatry.
Antihypertensives and mental illness
Hypertension is common in patients with psychiatric disorders and observational studies have reported associations between antihypertensive medication and these disorders, although the findings have been mixed.
Dr. Shah and colleagues estimated the potential of different antihypertensive drug classes on schizophrenia, bipolar disorder, and major depressive disorder.
In a two-sample Mendelian randomization study, they evaluated ties between a single-nucleotide variant and drug-target gene expression derived from expression quantitative trait loci data in blood (sample 1) and the SNV disease association from published case-control, genomewide association studies (sample 2).
The analyses included 40,675 patients with schizophrenia and 64,643 controls; 20,352 with bipolar disorder and 31,358 controls; and 135,458 with major depressive disorder and 344,901 controls.
The major finding was that a one standard deviation–lower expression of the ACE gene in blood was associated with lower systolic blood pressure of 4.0 mm Hg (95% confidence interval, 2.7-5.3), but also an increased risk of schizophrenia (odds ratio, 1.75; 95% CI, 1.28-2.38).
Could ACE inhibitors worsen symptoms or trigger episodes?
In their article, the researchers noted that, in most patients, onset of schizophrenia occurs in late adolescence or early adult life, ruling out ACE inhibitor treatment as a potential causal factor for most cases.
“However, if lower ACE levels play a causal role for schizophrenia risk, it would be reasonable to hypothesize that further lowering of ACE activity in existing patients could worsen symptoms or trigger a new episode,” they wrote.
Dr. Shah emphasized that evidence from genetic analyses alone is “not sufficient to justify changes in prescription guidelines.”
“Patients should not stop taking these medications if they are effective at controlling their blood pressure and they don’t suffer any adverse effects. But it would be reasonable to encourage greater pharmacovigilance,” she said in an interview.
“One way in which we are hoping to follow up these findings,” said Dr. Shah, “is to access electronic health record data for millions of individuals to investigate if there is evidence of increased rates of psychotic episodes in individuals who use ACE inhibitors, compared to other classes of blood pressure–lowering medication.”
Caution warranted
Reached for comment, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Staten Island University Hospital in New York, noted that this is an “extremely complicated” study and urged caution in interpreting the results.
“Since most people develop schizophrenia earlier in life, before they usually develop problems with blood pressure, it’s not so much that these drugs might cause schizophrenia,” Dr. Sullivan said.
“But because of their effects on this particular gene, there’s a possibility that they might worsen symptoms or in somebody with borderline risk might cause them to develop symptoms later in life. This may apply to a relatively small number of people who develop symptoms of schizophrenia in their 40s and beyond,” he added.
That’s where “pharmacovigilance” comes into play, Dr. Sullivan said. “In other words, that they otherwise wouldn’t experience?”
Support for the study was provided by the National Health and Medical Research Council (Australia) and U.S. National Institute for Mental Health. Dr. Shah and Dr. Sullivan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ACE inhibitors may be associated with an increased risk for schizophrenia and may affect psychiatric symptoms, new research suggests.
Investigators found individuals who carry a genetic variant associated with lower levels of the ACE gene and protein have increased liability to schizophrenia, suggesting that drugs that lower ACE levels or activity may do the same.
“Our findings warrant further investigation into the role of ACE in schizophrenia and closer monitoring by clinicians of individuals, especially those with schizophrenia, who may be on medication that lower ACE activity, such as ACE inhibitors,” Sonia Shah, PhD, Institute for Biomedical Sciences, University of Queensland, Brisbane, Australia, said in an interview.
The study was published online March 10, 2021, in JAMA Psychiatry.
Antihypertensives and mental illness
Hypertension is common in patients with psychiatric disorders and observational studies have reported associations between antihypertensive medication and these disorders, although the findings have been mixed.
Dr. Shah and colleagues estimated the potential of different antihypertensive drug classes on schizophrenia, bipolar disorder, and major depressive disorder.
In a two-sample Mendelian randomization study, they evaluated ties between a single-nucleotide variant and drug-target gene expression derived from expression quantitative trait loci data in blood (sample 1) and the SNV disease association from published case-control, genomewide association studies (sample 2).
The analyses included 40,675 patients with schizophrenia and 64,643 controls; 20,352 with bipolar disorder and 31,358 controls; and 135,458 with major depressive disorder and 344,901 controls.
The major finding was that a one standard deviation–lower expression of the ACE gene in blood was associated with lower systolic blood pressure of 4.0 mm Hg (95% confidence interval, 2.7-5.3), but also an increased risk of schizophrenia (odds ratio, 1.75; 95% CI, 1.28-2.38).
Could ACE inhibitors worsen symptoms or trigger episodes?
In their article, the researchers noted that, in most patients, onset of schizophrenia occurs in late adolescence or early adult life, ruling out ACE inhibitor treatment as a potential causal factor for most cases.
“However, if lower ACE levels play a causal role for schizophrenia risk, it would be reasonable to hypothesize that further lowering of ACE activity in existing patients could worsen symptoms or trigger a new episode,” they wrote.
Dr. Shah emphasized that evidence from genetic analyses alone is “not sufficient to justify changes in prescription guidelines.”
“Patients should not stop taking these medications if they are effective at controlling their blood pressure and they don’t suffer any adverse effects. But it would be reasonable to encourage greater pharmacovigilance,” she said in an interview.
“One way in which we are hoping to follow up these findings,” said Dr. Shah, “is to access electronic health record data for millions of individuals to investigate if there is evidence of increased rates of psychotic episodes in individuals who use ACE inhibitors, compared to other classes of blood pressure–lowering medication.”
Caution warranted
Reached for comment, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Staten Island University Hospital in New York, noted that this is an “extremely complicated” study and urged caution in interpreting the results.
“Since most people develop schizophrenia earlier in life, before they usually develop problems with blood pressure, it’s not so much that these drugs might cause schizophrenia,” Dr. Sullivan said.
“But because of their effects on this particular gene, there’s a possibility that they might worsen symptoms or in somebody with borderline risk might cause them to develop symptoms later in life. This may apply to a relatively small number of people who develop symptoms of schizophrenia in their 40s and beyond,” he added.
That’s where “pharmacovigilance” comes into play, Dr. Sullivan said. “In other words, that they otherwise wouldn’t experience?”
Support for the study was provided by the National Health and Medical Research Council (Australia) and U.S. National Institute for Mental Health. Dr. Shah and Dr. Sullivan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Imaging alternative to AVS could boost detection of primary aldosteronism
A noninvasive imaging method for identifying whether the source of a patient’s primary aldosteronism is from unilateral or bilateral adrenal adenomas worked as well as the standard method, invasive adrenal vein sampling, in a head-to-head comparison with 143 patients.
This noninvasive alternative, which also does not require the substantial technical expertise that AVS demands, should make assessment of adenoma laterality in patients with primary aldosteronism (PA) much more widely available and accessible, predicted Dr. Wu, a researcher at Queen Mary University of London.
“It will allow more places to do this, and I think it will definitely allow more patients to be diagnosed” with PA from a unilateral source. AVS “is a real bottleneck,” she said. “We hope metomidate, or molecular imaging using other selective radiotracers, will enable many more patients to be diagnosed and appropriately managed.” Creating new diagnostic options for patients with PA and potentially increasing the number of these patients who are surgical candidates “is the aim of this study.”
Patients with PA develop a curable form of hypertension if their excess aldosterone can be neutralized with a mineralocorticoid receptor antagonist (MRA), or even more definitively by surgical removal of the adrenal aldosteronoma generating the hormonal excess as long as the adenoma is unilateral. Conventional imaging of the adrenals with CT or MRI has proven unreliable for identifying adrenal nodules noninvasively, which has made the invasive and technically challenging standard option of AVS the only game in town.
But some endocrinologists caution that the results from this one study do not suffice to make 11C-metomidate-based PET-CT imaging a widely used alternative.
‘This is a first step.’
“This study is a first step. It will take lots more data for endocrinologists to buy into a scan over AVS,” commented David A. D’Alessio, MD, professor and chief of the division of endocrinology and metabolism at Duke University in Durham, N.C.
But Dr. D’Alessio also acknowledged the clear benefits from a safe and effective alternative to AVS.
“A reliable, less invasive, and less technical means of lateralizing excess aldosterone production would increase the number of people [with a unilateral PA source] going to surgery. The reality is that, if you are not a patient at the Mayo Clinic . . .or the National Institutes of Health, then AVS is a bit of crap shoot” that is very operator and institution dependent for its accuracy, Dr. D’Alessio said in an interview.
Metomidate specifically binds to key enzymes of the adrenal corticosteroid biosynthetic pathway, making it a precise targeting agent for a radioactive tag as documented almost a decade ago. One limitation is that this radiotracer labeling of metomidate has a 20-minute half life, which means it must be produced on site, thereby making the technology out of reach for locations that can’t set up this capability.
MATCHing imaging against AVS
To test the clinical utility of metomidate-based PET-CT directly against AVS, Dr. Wu and her associates enrolled 143 adults with confirmed PA and hypertension at two centers in London and one in Cambridge, England. The MATCH study cohort averaged 53 years of age; two-thirds were men, 58% were White, and 30% were Black. Their median blood pressure was 147/91 mm Hg, and they were maintained on a median of two antihypertensive drugs.
The researchers assessed every patient with both the imaging method and AVS, performed in random order and blindly scored. They then began each patient on a 1-month regimen with an MRA (usually spironolactone but eplerenone [Inspra] was also an option) to test the responsiveness of each patient’s hypertension to this drug class and to gauge their likely response to adrenalectomy. After the MRA test, the researchers assessed the lateralization tests and determined that 78 patients were appropriate candidates for unilateral adrenalectomy while the remaining 65 patients were not and continued on the MRA regimen. They recommended surgery if patients were clear positives by AVS, by PET-CT imaging, or both.
The study had four primary outcomes to assess the ability of the two diagnostic methods to predict the success of surgery based on four increasingly stringent postsurgical criteria calculated in hierarchical sequence: Partial or complete biochemical success, complete biochemical success, partial or complete clinical success (partial meaning any significant reduction in blood pressure), or complete clinical success (systolic pressure reduced to less than 135 mm Hg). Only one of the 78 patients treated with surgery failed to achieve at least a partial biochemical response.
For each of the four metrics, 11C-metomidate PET-CT produced point estimates of diagnostic accuracy that consistently edged out AVS. While these advantages were not large enough to meet the prespecified threshold for proving superiority, they comfortably showed the noninferiority of this imaging method compared with AVS.
For example, the PET-CT method had 43.6% accuracy for predicting a clinical cure, compared with 39.7% accuracy for AVS. For complete biochemical cure, imaging had 68.8% accuracy, compared with 62.3% for AVS, Dr. Wu reported.
Another notable finding from the study was how strongly a robust blood pressure response to spironolactone predicted the clinical outcome from surgery. Patients whose systolic blood pressure fell below 135 mm Hg on MRA treatment had a nearly 18-fold higher rate of achieving a complete clinical cure following surgery compared with patients who did not have as dramatic a blood pressure response to MRA treatment.
Woefully low rates of PA assessment
But regardless of the success that PET-CT imaging has for identifying surgical candidates, the first step is to identify patients with PA, a diagnosis that’s woefully underperformed worldwide. One example: A separate report at ENDO 2021 retrospectively reviewed nearly 12,000 patients with hypertension and an indication of PA, such as treatment-resistant hypertension or early-onset hypertension, and managed at either of two university outpatient clinics in Michigan during 2010-2019. The report documented that 3% underwent PA assessment.
Diagnosis of patients with PA “is a major problem,” noted Dr. D’Alessio. “I think of PA as an underdiagnosed and undertreated condition, with a huge impact on morbidity and mortality. Any advance in this area is likely to be useful.” But, he added, “I’m dubious whether this [new imaging approach] will increase diagnosis of PA.” What’s needed is “getting more primary care physicians to do more screening” for PA among their patients with hypertension and a suggestion of a PA cause.
“Surgical cures are glamorous, but medical management is also very effective, and we have good, inexpensive drugs to do this,” the MRAs, Dr. D’Alessio said.
The study received no commercial funding. Dr. Wu and her coauthors had no disclosures. Dr. D’Alessio has been a speaker on behalf of Novo Nordisk, a consultant to Intarcia and Lilly, and has received research funding from Lilly and Merck.
A noninvasive imaging method for identifying whether the source of a patient’s primary aldosteronism is from unilateral or bilateral adrenal adenomas worked as well as the standard method, invasive adrenal vein sampling, in a head-to-head comparison with 143 patients.
This noninvasive alternative, which also does not require the substantial technical expertise that AVS demands, should make assessment of adenoma laterality in patients with primary aldosteronism (PA) much more widely available and accessible, predicted Dr. Wu, a researcher at Queen Mary University of London.
“It will allow more places to do this, and I think it will definitely allow more patients to be diagnosed” with PA from a unilateral source. AVS “is a real bottleneck,” she said. “We hope metomidate, or molecular imaging using other selective radiotracers, will enable many more patients to be diagnosed and appropriately managed.” Creating new diagnostic options for patients with PA and potentially increasing the number of these patients who are surgical candidates “is the aim of this study.”
Patients with PA develop a curable form of hypertension if their excess aldosterone can be neutralized with a mineralocorticoid receptor antagonist (MRA), or even more definitively by surgical removal of the adrenal aldosteronoma generating the hormonal excess as long as the adenoma is unilateral. Conventional imaging of the adrenals with CT or MRI has proven unreliable for identifying adrenal nodules noninvasively, which has made the invasive and technically challenging standard option of AVS the only game in town.
But some endocrinologists caution that the results from this one study do not suffice to make 11C-metomidate-based PET-CT imaging a widely used alternative.
‘This is a first step.’
“This study is a first step. It will take lots more data for endocrinologists to buy into a scan over AVS,” commented David A. D’Alessio, MD, professor and chief of the division of endocrinology and metabolism at Duke University in Durham, N.C.
But Dr. D’Alessio also acknowledged the clear benefits from a safe and effective alternative to AVS.
“A reliable, less invasive, and less technical means of lateralizing excess aldosterone production would increase the number of people [with a unilateral PA source] going to surgery. The reality is that, if you are not a patient at the Mayo Clinic . . .or the National Institutes of Health, then AVS is a bit of crap shoot” that is very operator and institution dependent for its accuracy, Dr. D’Alessio said in an interview.
Metomidate specifically binds to key enzymes of the adrenal corticosteroid biosynthetic pathway, making it a precise targeting agent for a radioactive tag as documented almost a decade ago. One limitation is that this radiotracer labeling of metomidate has a 20-minute half life, which means it must be produced on site, thereby making the technology out of reach for locations that can’t set up this capability.
MATCHing imaging against AVS
To test the clinical utility of metomidate-based PET-CT directly against AVS, Dr. Wu and her associates enrolled 143 adults with confirmed PA and hypertension at two centers in London and one in Cambridge, England. The MATCH study cohort averaged 53 years of age; two-thirds were men, 58% were White, and 30% were Black. Their median blood pressure was 147/91 mm Hg, and they were maintained on a median of two antihypertensive drugs.
The researchers assessed every patient with both the imaging method and AVS, performed in random order and blindly scored. They then began each patient on a 1-month regimen with an MRA (usually spironolactone but eplerenone [Inspra] was also an option) to test the responsiveness of each patient’s hypertension to this drug class and to gauge their likely response to adrenalectomy. After the MRA test, the researchers assessed the lateralization tests and determined that 78 patients were appropriate candidates for unilateral adrenalectomy while the remaining 65 patients were not and continued on the MRA regimen. They recommended surgery if patients were clear positives by AVS, by PET-CT imaging, or both.
The study had four primary outcomes to assess the ability of the two diagnostic methods to predict the success of surgery based on four increasingly stringent postsurgical criteria calculated in hierarchical sequence: Partial or complete biochemical success, complete biochemical success, partial or complete clinical success (partial meaning any significant reduction in blood pressure), or complete clinical success (systolic pressure reduced to less than 135 mm Hg). Only one of the 78 patients treated with surgery failed to achieve at least a partial biochemical response.
For each of the four metrics, 11C-metomidate PET-CT produced point estimates of diagnostic accuracy that consistently edged out AVS. While these advantages were not large enough to meet the prespecified threshold for proving superiority, they comfortably showed the noninferiority of this imaging method compared with AVS.
For example, the PET-CT method had 43.6% accuracy for predicting a clinical cure, compared with 39.7% accuracy for AVS. For complete biochemical cure, imaging had 68.8% accuracy, compared with 62.3% for AVS, Dr. Wu reported.
Another notable finding from the study was how strongly a robust blood pressure response to spironolactone predicted the clinical outcome from surgery. Patients whose systolic blood pressure fell below 135 mm Hg on MRA treatment had a nearly 18-fold higher rate of achieving a complete clinical cure following surgery compared with patients who did not have as dramatic a blood pressure response to MRA treatment.
Woefully low rates of PA assessment
But regardless of the success that PET-CT imaging has for identifying surgical candidates, the first step is to identify patients with PA, a diagnosis that’s woefully underperformed worldwide. One example: A separate report at ENDO 2021 retrospectively reviewed nearly 12,000 patients with hypertension and an indication of PA, such as treatment-resistant hypertension or early-onset hypertension, and managed at either of two university outpatient clinics in Michigan during 2010-2019. The report documented that 3% underwent PA assessment.
Diagnosis of patients with PA “is a major problem,” noted Dr. D’Alessio. “I think of PA as an underdiagnosed and undertreated condition, with a huge impact on morbidity and mortality. Any advance in this area is likely to be useful.” But, he added, “I’m dubious whether this [new imaging approach] will increase diagnosis of PA.” What’s needed is “getting more primary care physicians to do more screening” for PA among their patients with hypertension and a suggestion of a PA cause.
“Surgical cures are glamorous, but medical management is also very effective, and we have good, inexpensive drugs to do this,” the MRAs, Dr. D’Alessio said.
The study received no commercial funding. Dr. Wu and her coauthors had no disclosures. Dr. D’Alessio has been a speaker on behalf of Novo Nordisk, a consultant to Intarcia and Lilly, and has received research funding from Lilly and Merck.
A noninvasive imaging method for identifying whether the source of a patient’s primary aldosteronism is from unilateral or bilateral adrenal adenomas worked as well as the standard method, invasive adrenal vein sampling, in a head-to-head comparison with 143 patients.
This noninvasive alternative, which also does not require the substantial technical expertise that AVS demands, should make assessment of adenoma laterality in patients with primary aldosteronism (PA) much more widely available and accessible, predicted Dr. Wu, a researcher at Queen Mary University of London.
“It will allow more places to do this, and I think it will definitely allow more patients to be diagnosed” with PA from a unilateral source. AVS “is a real bottleneck,” she said. “We hope metomidate, or molecular imaging using other selective radiotracers, will enable many more patients to be diagnosed and appropriately managed.” Creating new diagnostic options for patients with PA and potentially increasing the number of these patients who are surgical candidates “is the aim of this study.”
Patients with PA develop a curable form of hypertension if their excess aldosterone can be neutralized with a mineralocorticoid receptor antagonist (MRA), or even more definitively by surgical removal of the adrenal aldosteronoma generating the hormonal excess as long as the adenoma is unilateral. Conventional imaging of the adrenals with CT or MRI has proven unreliable for identifying adrenal nodules noninvasively, which has made the invasive and technically challenging standard option of AVS the only game in town.
But some endocrinologists caution that the results from this one study do not suffice to make 11C-metomidate-based PET-CT imaging a widely used alternative.
‘This is a first step.’
“This study is a first step. It will take lots more data for endocrinologists to buy into a scan over AVS,” commented David A. D’Alessio, MD, professor and chief of the division of endocrinology and metabolism at Duke University in Durham, N.C.
But Dr. D’Alessio also acknowledged the clear benefits from a safe and effective alternative to AVS.
“A reliable, less invasive, and less technical means of lateralizing excess aldosterone production would increase the number of people [with a unilateral PA source] going to surgery. The reality is that, if you are not a patient at the Mayo Clinic . . .or the National Institutes of Health, then AVS is a bit of crap shoot” that is very operator and institution dependent for its accuracy, Dr. D’Alessio said in an interview.
Metomidate specifically binds to key enzymes of the adrenal corticosteroid biosynthetic pathway, making it a precise targeting agent for a radioactive tag as documented almost a decade ago. One limitation is that this radiotracer labeling of metomidate has a 20-minute half life, which means it must be produced on site, thereby making the technology out of reach for locations that can’t set up this capability.
MATCHing imaging against AVS
To test the clinical utility of metomidate-based PET-CT directly against AVS, Dr. Wu and her associates enrolled 143 adults with confirmed PA and hypertension at two centers in London and one in Cambridge, England. The MATCH study cohort averaged 53 years of age; two-thirds were men, 58% were White, and 30% were Black. Their median blood pressure was 147/91 mm Hg, and they were maintained on a median of two antihypertensive drugs.
The researchers assessed every patient with both the imaging method and AVS, performed in random order and blindly scored. They then began each patient on a 1-month regimen with an MRA (usually spironolactone but eplerenone [Inspra] was also an option) to test the responsiveness of each patient’s hypertension to this drug class and to gauge their likely response to adrenalectomy. After the MRA test, the researchers assessed the lateralization tests and determined that 78 patients were appropriate candidates for unilateral adrenalectomy while the remaining 65 patients were not and continued on the MRA regimen. They recommended surgery if patients were clear positives by AVS, by PET-CT imaging, or both.
The study had four primary outcomes to assess the ability of the two diagnostic methods to predict the success of surgery based on four increasingly stringent postsurgical criteria calculated in hierarchical sequence: Partial or complete biochemical success, complete biochemical success, partial or complete clinical success (partial meaning any significant reduction in blood pressure), or complete clinical success (systolic pressure reduced to less than 135 mm Hg). Only one of the 78 patients treated with surgery failed to achieve at least a partial biochemical response.
For each of the four metrics, 11C-metomidate PET-CT produced point estimates of diagnostic accuracy that consistently edged out AVS. While these advantages were not large enough to meet the prespecified threshold for proving superiority, they comfortably showed the noninferiority of this imaging method compared with AVS.
For example, the PET-CT method had 43.6% accuracy for predicting a clinical cure, compared with 39.7% accuracy for AVS. For complete biochemical cure, imaging had 68.8% accuracy, compared with 62.3% for AVS, Dr. Wu reported.
Another notable finding from the study was how strongly a robust blood pressure response to spironolactone predicted the clinical outcome from surgery. Patients whose systolic blood pressure fell below 135 mm Hg on MRA treatment had a nearly 18-fold higher rate of achieving a complete clinical cure following surgery compared with patients who did not have as dramatic a blood pressure response to MRA treatment.
Woefully low rates of PA assessment
But regardless of the success that PET-CT imaging has for identifying surgical candidates, the first step is to identify patients with PA, a diagnosis that’s woefully underperformed worldwide. One example: A separate report at ENDO 2021 retrospectively reviewed nearly 12,000 patients with hypertension and an indication of PA, such as treatment-resistant hypertension or early-onset hypertension, and managed at either of two university outpatient clinics in Michigan during 2010-2019. The report documented that 3% underwent PA assessment.
Diagnosis of patients with PA “is a major problem,” noted Dr. D’Alessio. “I think of PA as an underdiagnosed and undertreated condition, with a huge impact on morbidity and mortality. Any advance in this area is likely to be useful.” But, he added, “I’m dubious whether this [new imaging approach] will increase diagnosis of PA.” What’s needed is “getting more primary care physicians to do more screening” for PA among their patients with hypertension and a suggestion of a PA cause.
“Surgical cures are glamorous, but medical management is also very effective, and we have good, inexpensive drugs to do this,” the MRAs, Dr. D’Alessio said.
The study received no commercial funding. Dr. Wu and her coauthors had no disclosures. Dr. D’Alessio has been a speaker on behalf of Novo Nordisk, a consultant to Intarcia and Lilly, and has received research funding from Lilly and Merck.
FROM ENDO 2021
‘Major update’ of BP guidance for kidney disease; treat to 120 mm Hg
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
Vasodilatory medications found protective against rosacea
.
“Our initial hypothesis was that perhaps antihypertensive agents might be associated with worsening rosacea,” one of the study authors, Jennifer G. Powers, MD, associate professor of dermatology at the University of Iowa, Iowa City, said in an interview. “What we found was exactly the opposite – that in fact their presence in a medical chart correlated with lower rates of rosacea diagnoses, as defined by ICD 9/10 codes.”
According to the researchers, who published their findings in the Journal of the American Academy of Dermatology, cases of acute vasodilator-induced rosacea have been reported, but no long-term association has been established. “In fact, many widely used antihypertensive medications modulate peripheral vascular tone,” they wrote. “Therefore, chronic use in patients with hypertension may reduce damage to peripheral vessels, and thus decrease risk of rosacea.”
To determine the correlates between vasodilator use and risk of rosacea, Dr. Powers and colleagues identified 680 hypertensive patients being treated with vasodilators or a thiazide diuretic in whom rosacea developed within 5 years of initiating therapy between June 1, 2006, and April 31, 2019. Vasodilator therapies included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs). Patients on thiazide diuretics served as the control group. The researchers stratified the patients by age, gender, race, diabetes, chronic kidney disease, and coronary artery disease and calculated relative risk estimates comparing vasodilators with thiazides between strata.
Of the 680 patients, all but 40 were White; 127 were on thiazides, and the remaining 553 were on vasodilators. Overall, the researchers observed that use of vasodilators had a protective effect on the development of rosacea within 5 years, compared with thiazides (relative risk [RR], 0.56; P less than .0001). Specifically, the relative risk was 0.50 for ACE-inhibitors (P less than .0001); 0.69 for ARBs (P = .041); 0.55 for beta-blockers (P less than .0001); and 0.39 for CCBs (P less than .0001).
Dr. Powers and colleagues also observed significant inverse correlations in ACE-inhibitors, beta-blockers, and CCBs among White women aged 50 and older, but no significance was observed in non-White subgroups. The cohorts of patients with chronic kidney disease and coronary artery disease were too small for analysis.
“We were very surprised to find that many of the agents we think of as vasodilators might actually be beneficial for rosacea,” Dr. Powers said. “We would like to see these results reproduced in larger population studies. There are also potential questions about the mechanism at play. However, should these findings hold true, [it’s] all the more reason for our rosacea patients with hypertension to be managed well. They need not fear that those medications are worsening disease. Also, there might be new therapeutic options based on this data.”
The study received funding support from the National Center for Advancing Translational Sciences. The researchers reported having no financial disclosures.
One of Dr. Powers’ coauthors is her husband, Edward M. Powers, MD, a cardiology fellow at the University of Iowa. “We sometimes bounce ideas off one another and will talk about how systemic effects on the vasculature may impact skin disease,” she said, noting that they also published a report on statins and atopic dermatitis.
.
“Our initial hypothesis was that perhaps antihypertensive agents might be associated with worsening rosacea,” one of the study authors, Jennifer G. Powers, MD, associate professor of dermatology at the University of Iowa, Iowa City, said in an interview. “What we found was exactly the opposite – that in fact their presence in a medical chart correlated with lower rates of rosacea diagnoses, as defined by ICD 9/10 codes.”
According to the researchers, who published their findings in the Journal of the American Academy of Dermatology, cases of acute vasodilator-induced rosacea have been reported, but no long-term association has been established. “In fact, many widely used antihypertensive medications modulate peripheral vascular tone,” they wrote. “Therefore, chronic use in patients with hypertension may reduce damage to peripheral vessels, and thus decrease risk of rosacea.”
To determine the correlates between vasodilator use and risk of rosacea, Dr. Powers and colleagues identified 680 hypertensive patients being treated with vasodilators or a thiazide diuretic in whom rosacea developed within 5 years of initiating therapy between June 1, 2006, and April 31, 2019. Vasodilator therapies included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs). Patients on thiazide diuretics served as the control group. The researchers stratified the patients by age, gender, race, diabetes, chronic kidney disease, and coronary artery disease and calculated relative risk estimates comparing vasodilators with thiazides between strata.
Of the 680 patients, all but 40 were White; 127 were on thiazides, and the remaining 553 were on vasodilators. Overall, the researchers observed that use of vasodilators had a protective effect on the development of rosacea within 5 years, compared with thiazides (relative risk [RR], 0.56; P less than .0001). Specifically, the relative risk was 0.50 for ACE-inhibitors (P less than .0001); 0.69 for ARBs (P = .041); 0.55 for beta-blockers (P less than .0001); and 0.39 for CCBs (P less than .0001).
Dr. Powers and colleagues also observed significant inverse correlations in ACE-inhibitors, beta-blockers, and CCBs among White women aged 50 and older, but no significance was observed in non-White subgroups. The cohorts of patients with chronic kidney disease and coronary artery disease were too small for analysis.
“We were very surprised to find that many of the agents we think of as vasodilators might actually be beneficial for rosacea,” Dr. Powers said. “We would like to see these results reproduced in larger population studies. There are also potential questions about the mechanism at play. However, should these findings hold true, [it’s] all the more reason for our rosacea patients with hypertension to be managed well. They need not fear that those medications are worsening disease. Also, there might be new therapeutic options based on this data.”
The study received funding support from the National Center for Advancing Translational Sciences. The researchers reported having no financial disclosures.
One of Dr. Powers’ coauthors is her husband, Edward M. Powers, MD, a cardiology fellow at the University of Iowa. “We sometimes bounce ideas off one another and will talk about how systemic effects on the vasculature may impact skin disease,” she said, noting that they also published a report on statins and atopic dermatitis.
.
“Our initial hypothesis was that perhaps antihypertensive agents might be associated with worsening rosacea,” one of the study authors, Jennifer G. Powers, MD, associate professor of dermatology at the University of Iowa, Iowa City, said in an interview. “What we found was exactly the opposite – that in fact their presence in a medical chart correlated with lower rates of rosacea diagnoses, as defined by ICD 9/10 codes.”
According to the researchers, who published their findings in the Journal of the American Academy of Dermatology, cases of acute vasodilator-induced rosacea have been reported, but no long-term association has been established. “In fact, many widely used antihypertensive medications modulate peripheral vascular tone,” they wrote. “Therefore, chronic use in patients with hypertension may reduce damage to peripheral vessels, and thus decrease risk of rosacea.”
To determine the correlates between vasodilator use and risk of rosacea, Dr. Powers and colleagues identified 680 hypertensive patients being treated with vasodilators or a thiazide diuretic in whom rosacea developed within 5 years of initiating therapy between June 1, 2006, and April 31, 2019. Vasodilator therapies included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs). Patients on thiazide diuretics served as the control group. The researchers stratified the patients by age, gender, race, diabetes, chronic kidney disease, and coronary artery disease and calculated relative risk estimates comparing vasodilators with thiazides between strata.
Of the 680 patients, all but 40 were White; 127 were on thiazides, and the remaining 553 were on vasodilators. Overall, the researchers observed that use of vasodilators had a protective effect on the development of rosacea within 5 years, compared with thiazides (relative risk [RR], 0.56; P less than .0001). Specifically, the relative risk was 0.50 for ACE-inhibitors (P less than .0001); 0.69 for ARBs (P = .041); 0.55 for beta-blockers (P less than .0001); and 0.39 for CCBs (P less than .0001).
Dr. Powers and colleagues also observed significant inverse correlations in ACE-inhibitors, beta-blockers, and CCBs among White women aged 50 and older, but no significance was observed in non-White subgroups. The cohorts of patients with chronic kidney disease and coronary artery disease were too small for analysis.
“We were very surprised to find that many of the agents we think of as vasodilators might actually be beneficial for rosacea,” Dr. Powers said. “We would like to see these results reproduced in larger population studies. There are also potential questions about the mechanism at play. However, should these findings hold true, [it’s] all the more reason for our rosacea patients with hypertension to be managed well. They need not fear that those medications are worsening disease. Also, there might be new therapeutic options based on this data.”
The study received funding support from the National Center for Advancing Translational Sciences. The researchers reported having no financial disclosures.
One of Dr. Powers’ coauthors is her husband, Edward M. Powers, MD, a cardiology fellow at the University of Iowa. “We sometimes bounce ideas off one another and will talk about how systemic effects on the vasculature may impact skin disease,” she said, noting that they also published a report on statins and atopic dermatitis.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Heart failure redefined with new classifications, staging
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.
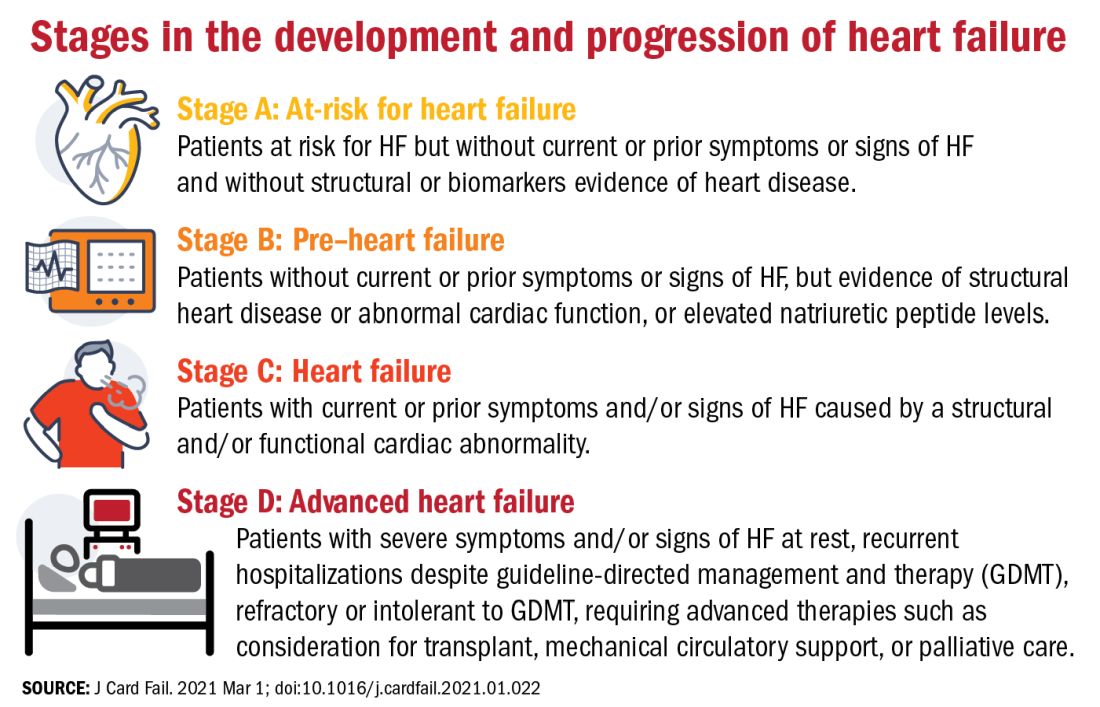
One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
FROM THE JOURNAL OF CARDIAC FAILURE
Ivabradine knocks down heart rate, symptoms in POTS
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.