User login
Clinical trials: More to learn than the results
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
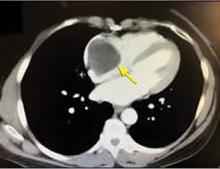
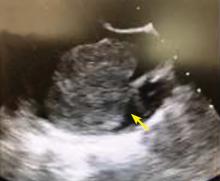
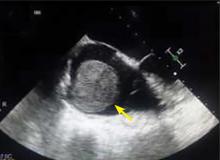
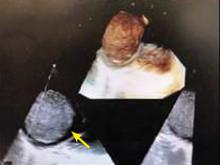
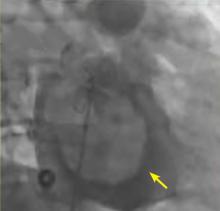
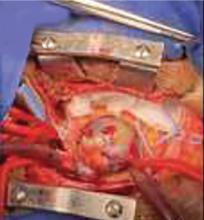
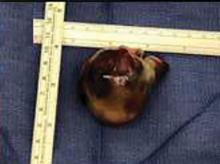
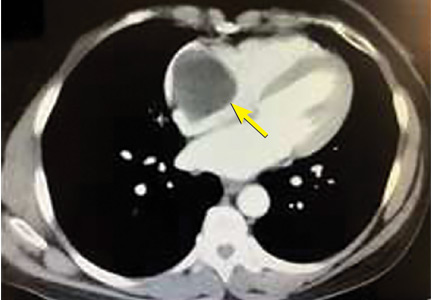
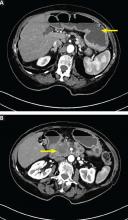
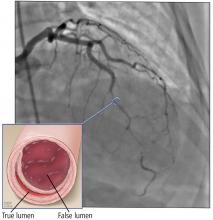
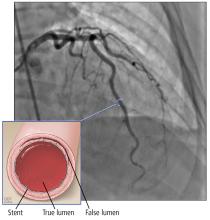
A right atrial mass
PRIMARY HEART TUMORS ARE RARE
The most common neoplasms that metastasize to the heart are malignant melanoma, lymphoma, leukemia, breast, and lung cancers. The layers of the heart affected by malignant neoplasms in order of frequency from highest to lowest are the pericardium, epicardium, myocardium, and endocardium.3
MYXOMA: A PRIMARY CARDIAC TUMOR
The most common type of primary cardiac tumor is myxoma. Most—75% to 80%—occur in the left atrium, while 15% to 20% occur in the right atrium.5 Right atrial myxomas are usually found in the intraatrial septum at the border of the fossa ovalis.6 Myxomas can occur at any age, but are most common in women between the third and sixth decades.2
The cause of atrial myxomas is currently unknown. Most cases are sporadic. However, 10% are familial, with an autosomal-dominant pattern.7
The clinical symptoms of right atrial myxoma depend on the tumor’s size, location, and mobility and on the patient’s physical activity and body position.4 Common presenting symptoms include shortness of breath, pulmonary edema, cough, hemoptysis, and fatigue. Thirty percent of patients present with constitutional symptoms.4
Auscultation may reveal a characteristic “tumor plop” early in diastole.4,7 About 35% of patients have laboratory abnormalities such as elevations in erythrocyte sedimentation rate, C-reactive protein, and globulin levels and anemia. Our patient did not.
Embolization occurs in about 10% of cases of right-sided myxoma and can result in pulmonary artery embolism or a stroke. Pulmonary artery embolism occurs with myxoma embolization to the lungs. Strokes can occur in patients who have a patent foramen ovale or atrial septal defect, through which embolism to the systemic arterial circulation can occur.
The primary treatment for myxoma is complete resection of the tumor and its base with wide safety margins. This is particularly important to prevent recurrence of the myxoma and need for repeat operations, with their risk of surgical complications.9
- Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 2000; 13(12):1080–1083. pmid:11119275
- Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: thrombus or myxoma? J Cardiovasc Ultrasound 2010; 18(4):154–156. doi:10.4250/jcu.2010.18.4.154
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128(16):1790–1794. doi:10.1161/CIRCULATIONAHA.112.000790
- Aggarwal SK, Barik R, Sarma TC, et al. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J 2007; 154(6):1102–1107. doi:10.1016/j.ahj.2007.07.032
- Diaz A, Di Salvo C, Lawrence D, Hayward M. Left atrial and right ventricular myxoma: an uncommon presentation of a rare tumour. Interact Cardiovasc Thorac Surg 2011; 12(4):622–623. doi:10.1510/icvts.2010.255661
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24):1610–1617. doi:10.1056/NEJM199512143332407
- Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol 2011; 57(21):e371. doi:10.1016/j.jacc.2010.09.085
- Kassab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: unusual and isolated manifestation of myxoma of the left atrium. J Med Liban 1999; 47(4):246–250. French. pmid:10641454
- Guhathakurta S, Riordan JP. Surgical treatment of right atrial myxoma. Tex Heart Inst J 2000; 27(1):61–63. pmid:10830633
PRIMARY HEART TUMORS ARE RARE
The most common neoplasms that metastasize to the heart are malignant melanoma, lymphoma, leukemia, breast, and lung cancers. The layers of the heart affected by malignant neoplasms in order of frequency from highest to lowest are the pericardium, epicardium, myocardium, and endocardium.3
MYXOMA: A PRIMARY CARDIAC TUMOR
The most common type of primary cardiac tumor is myxoma. Most—75% to 80%—occur in the left atrium, while 15% to 20% occur in the right atrium.5 Right atrial myxomas are usually found in the intraatrial septum at the border of the fossa ovalis.6 Myxomas can occur at any age, but are most common in women between the third and sixth decades.2
The cause of atrial myxomas is currently unknown. Most cases are sporadic. However, 10% are familial, with an autosomal-dominant pattern.7
The clinical symptoms of right atrial myxoma depend on the tumor’s size, location, and mobility and on the patient’s physical activity and body position.4 Common presenting symptoms include shortness of breath, pulmonary edema, cough, hemoptysis, and fatigue. Thirty percent of patients present with constitutional symptoms.4
Auscultation may reveal a characteristic “tumor plop” early in diastole.4,7 About 35% of patients have laboratory abnormalities such as elevations in erythrocyte sedimentation rate, C-reactive protein, and globulin levels and anemia. Our patient did not.
Embolization occurs in about 10% of cases of right-sided myxoma and can result in pulmonary artery embolism or a stroke. Pulmonary artery embolism occurs with myxoma embolization to the lungs. Strokes can occur in patients who have a patent foramen ovale or atrial septal defect, through which embolism to the systemic arterial circulation can occur.
The primary treatment for myxoma is complete resection of the tumor and its base with wide safety margins. This is particularly important to prevent recurrence of the myxoma and need for repeat operations, with their risk of surgical complications.9
PRIMARY HEART TUMORS ARE RARE
The most common neoplasms that metastasize to the heart are malignant melanoma, lymphoma, leukemia, breast, and lung cancers. The layers of the heart affected by malignant neoplasms in order of frequency from highest to lowest are the pericardium, epicardium, myocardium, and endocardium.3
MYXOMA: A PRIMARY CARDIAC TUMOR
The most common type of primary cardiac tumor is myxoma. Most—75% to 80%—occur in the left atrium, while 15% to 20% occur in the right atrium.5 Right atrial myxomas are usually found in the intraatrial septum at the border of the fossa ovalis.6 Myxomas can occur at any age, but are most common in women between the third and sixth decades.2
The cause of atrial myxomas is currently unknown. Most cases are sporadic. However, 10% are familial, with an autosomal-dominant pattern.7
The clinical symptoms of right atrial myxoma depend on the tumor’s size, location, and mobility and on the patient’s physical activity and body position.4 Common presenting symptoms include shortness of breath, pulmonary edema, cough, hemoptysis, and fatigue. Thirty percent of patients present with constitutional symptoms.4
Auscultation may reveal a characteristic “tumor plop” early in diastole.4,7 About 35% of patients have laboratory abnormalities such as elevations in erythrocyte sedimentation rate, C-reactive protein, and globulin levels and anemia. Our patient did not.
Embolization occurs in about 10% of cases of right-sided myxoma and can result in pulmonary artery embolism or a stroke. Pulmonary artery embolism occurs with myxoma embolization to the lungs. Strokes can occur in patients who have a patent foramen ovale or atrial septal defect, through which embolism to the systemic arterial circulation can occur.
The primary treatment for myxoma is complete resection of the tumor and its base with wide safety margins. This is particularly important to prevent recurrence of the myxoma and need for repeat operations, with their risk of surgical complications.9
- Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 2000; 13(12):1080–1083. pmid:11119275
- Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: thrombus or myxoma? J Cardiovasc Ultrasound 2010; 18(4):154–156. doi:10.4250/jcu.2010.18.4.154
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128(16):1790–1794. doi:10.1161/CIRCULATIONAHA.112.000790
- Aggarwal SK, Barik R, Sarma TC, et al. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J 2007; 154(6):1102–1107. doi:10.1016/j.ahj.2007.07.032
- Diaz A, Di Salvo C, Lawrence D, Hayward M. Left atrial and right ventricular myxoma: an uncommon presentation of a rare tumour. Interact Cardiovasc Thorac Surg 2011; 12(4):622–623. doi:10.1510/icvts.2010.255661
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24):1610–1617. doi:10.1056/NEJM199512143332407
- Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol 2011; 57(21):e371. doi:10.1016/j.jacc.2010.09.085
- Kassab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: unusual and isolated manifestation of myxoma of the left atrium. J Med Liban 1999; 47(4):246–250. French. pmid:10641454
- Guhathakurta S, Riordan JP. Surgical treatment of right atrial myxoma. Tex Heart Inst J 2000; 27(1):61–63. pmid:10830633
- Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 2000; 13(12):1080–1083. pmid:11119275
- Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: thrombus or myxoma? J Cardiovasc Ultrasound 2010; 18(4):154–156. doi:10.4250/jcu.2010.18.4.154
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128(16):1790–1794. doi:10.1161/CIRCULATIONAHA.112.000790
- Aggarwal SK, Barik R, Sarma TC, et al. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J 2007; 154(6):1102–1107. doi:10.1016/j.ahj.2007.07.032
- Diaz A, Di Salvo C, Lawrence D, Hayward M. Left atrial and right ventricular myxoma: an uncommon presentation of a rare tumour. Interact Cardiovasc Thorac Surg 2011; 12(4):622–623. doi:10.1510/icvts.2010.255661
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24):1610–1617. doi:10.1056/NEJM199512143332407
- Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol 2011; 57(21):e371. doi:10.1016/j.jacc.2010.09.085
- Kassab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: unusual and isolated manifestation of myxoma of the left atrium. J Med Liban 1999; 47(4):246–250. French. pmid:10641454
- Guhathakurta S, Riordan JP. Surgical treatment of right atrial myxoma. Tex Heart Inst J 2000; 27(1):61–63. pmid:10830633
Imaging remission decried as ticket to RA overtreatment
MADRID – Defining remission in patients with rheumatoid arthritis depends on their clinical status, not on the presence or absence of inflammatory signals on ultrasound or MRI, many rheumatologists now agree.
The strong consensus that’s formed against using imaging as a criterion for RA remission was apparent at the European Congress of Rheumatology during presentation of a pending update to the EULAR recommendations for managing RA, as well as in at least two separate, invited lectures.
“Imaging is out,” proclaimed Josef S. Smolen, MD, as he spoke at the congress about the pending RA management revisions. This condemnation of imaging by ultrasound or MRI as an unsafe and misleading target for RA treatment by Dr. Smolen, professor of medicine at the Medical University of Vienna, was perhaps the most forceful statement he made while presenting the draft revision of EULAR’s RA recommendations.
The case for using ultrasound or MR to find inflammatory signatures in joints that can function as treatment targets collapsed earlier in 2019 with publication of results from IMAGINE-RA (An MRI-guided Treatment Strategy to Prevent Disease Progression in Patients With Rheumatoid Arthritis), a multicenter Danish study that randomized 200 RA patients in remission to either a conventional, disease activity–guided treatment target (in this case the DAS28-CRP [Disease Activity Score in 28 joints plus C-reactive protein]), or a treatment target that included the conventional clinical target plus treating to eliminate any bone marrow edema visualized by MRI. After 24 months of treatment, the prevalence of clinical remission and MRI remission was about the same in both arms, with no statistically significant differences. But serious adverse events in 6 patients managed by their clinical assessment compared favorably against 17 among those managed to an imaging remission endpoint, a difference that strongly hinted at dangerous overtreatment of the imaging-guided patients (JAMA. 2019 Feb 5;321[5]:461-72).
The failure of MRI assessment of inflammation to improve RA treatment in IMAGINE-RA came against the backdrop of two 2016 reports that documented the same limitation when using ultrasound to detect joint inflammation and guide treatment in RA patients. The TaSER (Targeting Synovitis in Early Rheumatoid Arthritis) study randomized 111 patients with newly diagnosed RA or undifferentiated arthritis to conventional disease activity assessment, DAS28–erythrocyte sedimentation rate, or to that plus assessment by musculoskeletal ultrasound, and found no difference in clinical or imaging outcomes (Ann Rheum Dis. 2016 Jun;75[6]:1043-50). The second report, ARCTIC (Aiming for Remission in Rheumatoid Arthritis), randomized 238 RA patients to either a tight RA control strategy based on DAS alone or based on DAS plus serial examination of joints with ultrasound. The results showed that, after 16-24 months on treatment, the two strategies produced no significant difference in the rates of sustained RA remission with no radiographic damage or swollen joints detected (BMJ. 2016 Aug 16;354:i4205).
The results from these three studies have shown that “not all inflammation seen by ultrasound or MR is pathological,” and that “no imaging technique or biomarker has shown superiority to clinical assessment as a treat-to-target” goal, Sofia Ramiro, MD, said in a talk at the congress during which she reviewed this evidence.
“Treat-to-target that takes imaging into account is high risk because it exposes patients to overtreatment, which has costs in the broad sense, safety included,” said Dr. Ramiro, a rheumatologist at Leiden (the Netherlands) University Medical Center. “I think that systematically evaluating a patient’s joint with imaging won’t have additional value, and is the wrong approach.”
A similar assessment came from Stefan Siebert, MD, during a separate lecture during the congress. He highlighted that use of ultrasound or MRI to guide treatment in these three studies consistently led to substantially higher rates of treatment escalation, treatment with biologics, and in two of the three studies a notable increase in serious adverse events. Treatment with a biologic drug was roughly twice as frequent in the imaging-guided arms of TaSER and ARCTIC, compared with the control arms in those studies, and in IMAGINE-RA, the use of a biologic drug occurred more than 20 times more often in the imaging arms, he noted. And in both TaSER and IMAGINE-RA the rate of serious adverse events was more than doubled in the imaging arms, compared with the controls.
“Just identifying inflammation [in a joint] is not enough to make a diagnosis. Inflammation is normal process, and finding it does not identify a pathological state,” noted Dr. Siebert, a rheumatologist at the University of Glasgow. “Imaging leads to overdiagnosis and overtreatment when physicians use imaging inappropriately,” he concluded.
Dr. Smolen has been a consultant to several drug companies. Dr. Ramiro has been a consultant to or speaker on behalf of AbbVie, Eli Lilly, Merck, Novartis, and Sanofi, and she has received research funding from Merck. Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB.
MADRID – Defining remission in patients with rheumatoid arthritis depends on their clinical status, not on the presence or absence of inflammatory signals on ultrasound or MRI, many rheumatologists now agree.
The strong consensus that’s formed against using imaging as a criterion for RA remission was apparent at the European Congress of Rheumatology during presentation of a pending update to the EULAR recommendations for managing RA, as well as in at least two separate, invited lectures.
“Imaging is out,” proclaimed Josef S. Smolen, MD, as he spoke at the congress about the pending RA management revisions. This condemnation of imaging by ultrasound or MRI as an unsafe and misleading target for RA treatment by Dr. Smolen, professor of medicine at the Medical University of Vienna, was perhaps the most forceful statement he made while presenting the draft revision of EULAR’s RA recommendations.
The case for using ultrasound or MR to find inflammatory signatures in joints that can function as treatment targets collapsed earlier in 2019 with publication of results from IMAGINE-RA (An MRI-guided Treatment Strategy to Prevent Disease Progression in Patients With Rheumatoid Arthritis), a multicenter Danish study that randomized 200 RA patients in remission to either a conventional, disease activity–guided treatment target (in this case the DAS28-CRP [Disease Activity Score in 28 joints plus C-reactive protein]), or a treatment target that included the conventional clinical target plus treating to eliminate any bone marrow edema visualized by MRI. After 24 months of treatment, the prevalence of clinical remission and MRI remission was about the same in both arms, with no statistically significant differences. But serious adverse events in 6 patients managed by their clinical assessment compared favorably against 17 among those managed to an imaging remission endpoint, a difference that strongly hinted at dangerous overtreatment of the imaging-guided patients (JAMA. 2019 Feb 5;321[5]:461-72).
The failure of MRI assessment of inflammation to improve RA treatment in IMAGINE-RA came against the backdrop of two 2016 reports that documented the same limitation when using ultrasound to detect joint inflammation and guide treatment in RA patients. The TaSER (Targeting Synovitis in Early Rheumatoid Arthritis) study randomized 111 patients with newly diagnosed RA or undifferentiated arthritis to conventional disease activity assessment, DAS28–erythrocyte sedimentation rate, or to that plus assessment by musculoskeletal ultrasound, and found no difference in clinical or imaging outcomes (Ann Rheum Dis. 2016 Jun;75[6]:1043-50). The second report, ARCTIC (Aiming for Remission in Rheumatoid Arthritis), randomized 238 RA patients to either a tight RA control strategy based on DAS alone or based on DAS plus serial examination of joints with ultrasound. The results showed that, after 16-24 months on treatment, the two strategies produced no significant difference in the rates of sustained RA remission with no radiographic damage or swollen joints detected (BMJ. 2016 Aug 16;354:i4205).
The results from these three studies have shown that “not all inflammation seen by ultrasound or MR is pathological,” and that “no imaging technique or biomarker has shown superiority to clinical assessment as a treat-to-target” goal, Sofia Ramiro, MD, said in a talk at the congress during which she reviewed this evidence.
“Treat-to-target that takes imaging into account is high risk because it exposes patients to overtreatment, which has costs in the broad sense, safety included,” said Dr. Ramiro, a rheumatologist at Leiden (the Netherlands) University Medical Center. “I think that systematically evaluating a patient’s joint with imaging won’t have additional value, and is the wrong approach.”
A similar assessment came from Stefan Siebert, MD, during a separate lecture during the congress. He highlighted that use of ultrasound or MRI to guide treatment in these three studies consistently led to substantially higher rates of treatment escalation, treatment with biologics, and in two of the three studies a notable increase in serious adverse events. Treatment with a biologic drug was roughly twice as frequent in the imaging-guided arms of TaSER and ARCTIC, compared with the control arms in those studies, and in IMAGINE-RA, the use of a biologic drug occurred more than 20 times more often in the imaging arms, he noted. And in both TaSER and IMAGINE-RA the rate of serious adverse events was more than doubled in the imaging arms, compared with the controls.
“Just identifying inflammation [in a joint] is not enough to make a diagnosis. Inflammation is normal process, and finding it does not identify a pathological state,” noted Dr. Siebert, a rheumatologist at the University of Glasgow. “Imaging leads to overdiagnosis and overtreatment when physicians use imaging inappropriately,” he concluded.
Dr. Smolen has been a consultant to several drug companies. Dr. Ramiro has been a consultant to or speaker on behalf of AbbVie, Eli Lilly, Merck, Novartis, and Sanofi, and she has received research funding from Merck. Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB.
MADRID – Defining remission in patients with rheumatoid arthritis depends on their clinical status, not on the presence or absence of inflammatory signals on ultrasound or MRI, many rheumatologists now agree.
The strong consensus that’s formed against using imaging as a criterion for RA remission was apparent at the European Congress of Rheumatology during presentation of a pending update to the EULAR recommendations for managing RA, as well as in at least two separate, invited lectures.
“Imaging is out,” proclaimed Josef S. Smolen, MD, as he spoke at the congress about the pending RA management revisions. This condemnation of imaging by ultrasound or MRI as an unsafe and misleading target for RA treatment by Dr. Smolen, professor of medicine at the Medical University of Vienna, was perhaps the most forceful statement he made while presenting the draft revision of EULAR’s RA recommendations.
The case for using ultrasound or MR to find inflammatory signatures in joints that can function as treatment targets collapsed earlier in 2019 with publication of results from IMAGINE-RA (An MRI-guided Treatment Strategy to Prevent Disease Progression in Patients With Rheumatoid Arthritis), a multicenter Danish study that randomized 200 RA patients in remission to either a conventional, disease activity–guided treatment target (in this case the DAS28-CRP [Disease Activity Score in 28 joints plus C-reactive protein]), or a treatment target that included the conventional clinical target plus treating to eliminate any bone marrow edema visualized by MRI. After 24 months of treatment, the prevalence of clinical remission and MRI remission was about the same in both arms, with no statistically significant differences. But serious adverse events in 6 patients managed by their clinical assessment compared favorably against 17 among those managed to an imaging remission endpoint, a difference that strongly hinted at dangerous overtreatment of the imaging-guided patients (JAMA. 2019 Feb 5;321[5]:461-72).
The failure of MRI assessment of inflammation to improve RA treatment in IMAGINE-RA came against the backdrop of two 2016 reports that documented the same limitation when using ultrasound to detect joint inflammation and guide treatment in RA patients. The TaSER (Targeting Synovitis in Early Rheumatoid Arthritis) study randomized 111 patients with newly diagnosed RA or undifferentiated arthritis to conventional disease activity assessment, DAS28–erythrocyte sedimentation rate, or to that plus assessment by musculoskeletal ultrasound, and found no difference in clinical or imaging outcomes (Ann Rheum Dis. 2016 Jun;75[6]:1043-50). The second report, ARCTIC (Aiming for Remission in Rheumatoid Arthritis), randomized 238 RA patients to either a tight RA control strategy based on DAS alone or based on DAS plus serial examination of joints with ultrasound. The results showed that, after 16-24 months on treatment, the two strategies produced no significant difference in the rates of sustained RA remission with no radiographic damage or swollen joints detected (BMJ. 2016 Aug 16;354:i4205).
The results from these three studies have shown that “not all inflammation seen by ultrasound or MR is pathological,” and that “no imaging technique or biomarker has shown superiority to clinical assessment as a treat-to-target” goal, Sofia Ramiro, MD, said in a talk at the congress during which she reviewed this evidence.
“Treat-to-target that takes imaging into account is high risk because it exposes patients to overtreatment, which has costs in the broad sense, safety included,” said Dr. Ramiro, a rheumatologist at Leiden (the Netherlands) University Medical Center. “I think that systematically evaluating a patient’s joint with imaging won’t have additional value, and is the wrong approach.”
A similar assessment came from Stefan Siebert, MD, during a separate lecture during the congress. He highlighted that use of ultrasound or MRI to guide treatment in these three studies consistently led to substantially higher rates of treatment escalation, treatment with biologics, and in two of the three studies a notable increase in serious adverse events. Treatment with a biologic drug was roughly twice as frequent in the imaging-guided arms of TaSER and ARCTIC, compared with the control arms in those studies, and in IMAGINE-RA, the use of a biologic drug occurred more than 20 times more often in the imaging arms, he noted. And in both TaSER and IMAGINE-RA the rate of serious adverse events was more than doubled in the imaging arms, compared with the controls.
“Just identifying inflammation [in a joint] is not enough to make a diagnosis. Inflammation is normal process, and finding it does not identify a pathological state,” noted Dr. Siebert, a rheumatologist at the University of Glasgow. “Imaging leads to overdiagnosis and overtreatment when physicians use imaging inappropriately,” he concluded.
Dr. Smolen has been a consultant to several drug companies. Dr. Ramiro has been a consultant to or speaker on behalf of AbbVie, Eli Lilly, Merck, Novartis, and Sanofi, and she has received research funding from Merck. Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB.
REPORTING FROM EULAR 2019 CONGRESS
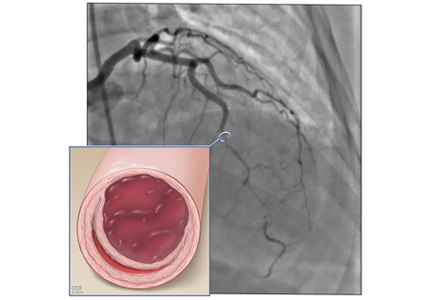
MRI-guided revascularization noninferior to FFR
Myocardial perfusion cardiovascular MRI is as good as invasive angiography and measurement of fractional flow reserve to guide revascularization in patients with angina, research suggests.
In the New England Journal of Medicine, researchers present the outcomes of an unblinded, multicenter, clinical effectiveness trial comparing the two revascularization strategies in 918 patients who had typical angina and either two or more cardiovascular risk factors or a positive exercise treadmill test.
In the fractional flow reserve method, revascularization was recommended in all vessels with an FFR of 0.8 or less. In the MRI-guided method, all patients underwent myocardial perfusion cardiovascular MRI, and patients with clinically significant inducible ischemia then underwent invasive angiography, and revascularization if required.
Significantly fewer patients in the cardiovascular MRI group underwent index revascularization, compared with the fractional flow reserve group (36% vs. 45% respectively; P = .005), and only 48% in the cardiovascular MRI group underwent invasive angiography, compared with 97% of patients in the fractional flow reserve arm.
However, there was no significant difference between the two groups in the incidence of major cardiac adverse events after 1 year, signifying that the MRI approach met the criteria for noninferiority.
There was also no significant difference between the two groups in the percentage of patients who were free from angina after 12 months (49.2% in the MRI group and 43.8% in the FFR group).
“Current guidelines on the management of the care of patients with suspected coronary artery disease separate diagnostic strategies from therapeutic strategies owing to a lack of evidence comparing combined diagnostic and therapeutic pathways,” wrote Eike Nagel, MD, of the Goethe University Frankfurt Institute for Experimental and Translational Cardiovascular Imaging and coauthors. “The MR-INFORM trial closes this knowledge gap by comparing two frequently used, well-defined, standardized, and validated clinical management strategies.”
However, they pointed out that one limitation of their study was the lack of a third group of patients who received medical therapy without planned revascularization. They also noted that the incidence of the primary outcome of major adverse cardiac events was lower than expected at 1 year.
The study was supported by the Guy’s and St. Thomas’ Biomedical Research Centre of the National Institute for Health Research. Three authors declared support from study supporters related to the study, three declared grants, personal fees, and other support from the private sector unrelated to the study. No other conflicts of interest were declared.
SOURCE: Nagel E et al. New Engl J Med. 2019;380:2418-28. doi: 10.1056/NEJMoa1716734.
Myocardial perfusion cardiovascular MRI is as good as invasive angiography and measurement of fractional flow reserve to guide revascularization in patients with angina, research suggests.
In the New England Journal of Medicine, researchers present the outcomes of an unblinded, multicenter, clinical effectiveness trial comparing the two revascularization strategies in 918 patients who had typical angina and either two or more cardiovascular risk factors or a positive exercise treadmill test.
In the fractional flow reserve method, revascularization was recommended in all vessels with an FFR of 0.8 or less. In the MRI-guided method, all patients underwent myocardial perfusion cardiovascular MRI, and patients with clinically significant inducible ischemia then underwent invasive angiography, and revascularization if required.
Significantly fewer patients in the cardiovascular MRI group underwent index revascularization, compared with the fractional flow reserve group (36% vs. 45% respectively; P = .005), and only 48% in the cardiovascular MRI group underwent invasive angiography, compared with 97% of patients in the fractional flow reserve arm.
However, there was no significant difference between the two groups in the incidence of major cardiac adverse events after 1 year, signifying that the MRI approach met the criteria for noninferiority.
There was also no significant difference between the two groups in the percentage of patients who were free from angina after 12 months (49.2% in the MRI group and 43.8% in the FFR group).
“Current guidelines on the management of the care of patients with suspected coronary artery disease separate diagnostic strategies from therapeutic strategies owing to a lack of evidence comparing combined diagnostic and therapeutic pathways,” wrote Eike Nagel, MD, of the Goethe University Frankfurt Institute for Experimental and Translational Cardiovascular Imaging and coauthors. “The MR-INFORM trial closes this knowledge gap by comparing two frequently used, well-defined, standardized, and validated clinical management strategies.”
However, they pointed out that one limitation of their study was the lack of a third group of patients who received medical therapy without planned revascularization. They also noted that the incidence of the primary outcome of major adverse cardiac events was lower than expected at 1 year.
The study was supported by the Guy’s and St. Thomas’ Biomedical Research Centre of the National Institute for Health Research. Three authors declared support from study supporters related to the study, three declared grants, personal fees, and other support from the private sector unrelated to the study. No other conflicts of interest were declared.
SOURCE: Nagel E et al. New Engl J Med. 2019;380:2418-28. doi: 10.1056/NEJMoa1716734.
Myocardial perfusion cardiovascular MRI is as good as invasive angiography and measurement of fractional flow reserve to guide revascularization in patients with angina, research suggests.
In the New England Journal of Medicine, researchers present the outcomes of an unblinded, multicenter, clinical effectiveness trial comparing the two revascularization strategies in 918 patients who had typical angina and either two or more cardiovascular risk factors or a positive exercise treadmill test.
In the fractional flow reserve method, revascularization was recommended in all vessels with an FFR of 0.8 or less. In the MRI-guided method, all patients underwent myocardial perfusion cardiovascular MRI, and patients with clinically significant inducible ischemia then underwent invasive angiography, and revascularization if required.
Significantly fewer patients in the cardiovascular MRI group underwent index revascularization, compared with the fractional flow reserve group (36% vs. 45% respectively; P = .005), and only 48% in the cardiovascular MRI group underwent invasive angiography, compared with 97% of patients in the fractional flow reserve arm.
However, there was no significant difference between the two groups in the incidence of major cardiac adverse events after 1 year, signifying that the MRI approach met the criteria for noninferiority.
There was also no significant difference between the two groups in the percentage of patients who were free from angina after 12 months (49.2% in the MRI group and 43.8% in the FFR group).
“Current guidelines on the management of the care of patients with suspected coronary artery disease separate diagnostic strategies from therapeutic strategies owing to a lack of evidence comparing combined diagnostic and therapeutic pathways,” wrote Eike Nagel, MD, of the Goethe University Frankfurt Institute for Experimental and Translational Cardiovascular Imaging and coauthors. “The MR-INFORM trial closes this knowledge gap by comparing two frequently used, well-defined, standardized, and validated clinical management strategies.”
However, they pointed out that one limitation of their study was the lack of a third group of patients who received medical therapy without planned revascularization. They also noted that the incidence of the primary outcome of major adverse cardiac events was lower than expected at 1 year.
The study was supported by the Guy’s and St. Thomas’ Biomedical Research Centre of the National Institute for Health Research. Three authors declared support from study supporters related to the study, three declared grants, personal fees, and other support from the private sector unrelated to the study. No other conflicts of interest were declared.
SOURCE: Nagel E et al. New Engl J Med. 2019;380:2418-28. doi: 10.1056/NEJMoa1716734.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: .
Major finding: The incidence of major cardiac adverse events similar at 1 year with cardiovascular MRI and invasive angiography.
Study details: MR-INFORM, an unblinded, multicenter, clinical effectiveness trial in 918 patients with angina.
Disclosures: The study was supported by the Guy’s and St. Thomas’ Biomedical Research Centre of the National Institute for Health Research. Three authors declared support from study supporters related to the study, three declared grants, personal fees and other support from the private sector unrelated to the study. No other conflicts of interest were declared.
Source: Nagel E et al. New Engl J Med. 2019;380:2418-28. doi: 10.1056/NEJMoa1716734.
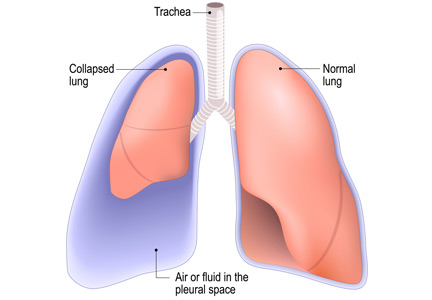
Is chest radiography routinely needed after thoracentesis?
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
Significant increase in low-attenuation coronary plaques found in lupus
SAN FRANCISCO – according to an investigation from Johns Hopkins University, Baltimore.
All of the 102 lupus patients in the coronary artery CT angiography study also had positive plaque remodeling, meaning that at least one low-attenuation plaque was growing into the lumen wall, not the lumen itself, which makes them difficult to detect on standard imaging. Low-attenuation plaques were defined in the study as a plaque larger than 1 mm2 with a radiodensity below 30 Hounsfield units.
Low-attenuation plaques are inherently unstable; they’re fatty, necrotic, and have a high risk of rupturing; their presence in the lumen wall is especially worrisome. In the general population, they sometimes regress, scarring down over time and no longer posing a threat. That didn’t happen in the 30 lupus patients who had follow-up CT angiographies, some 9 years after their first.
The team conducted the study to help understand why cardiovascular disease is so common in lupus, and the leading cause of death. Hopkins investigators have shown previously that statins have no effect on the risk or plaque occurrence and progression, and the cardiovascular risk doesn’t always seem to correlate with disease control. For those and other reasons, the current thinking at Hopkins is that cardiovascular disease in lupus is somehow different than in the general population, said George Stojan, MD, an assistant professor of rheumatology at the school and codirector of the Hopkins Lupus Center.
The goal is “to figure out exactly what to look for when we assess the risk; I don’t think we understand that at this point. We assume patients with lupus behave exactly like patients who don’t have lupus, but they obviously don’t. They do not respond to statins. They have a higher risk no matter what you do for them, even when their disease activity is low, and how much plaque they have over time doesn’t really correlate with disease activity,” he said at an international conference on systemic lupus erythematosus.
“Once we understand” the mechanism, “then we can try to [alter] it. Maybe we can look at new drugs, like the PCSK9 inhibitors which have shown a lot of promise in the general population.” At this point, however, “we don’t really know how to intervene,” Dr. Stojan said.
In the meantime, positive remodeling and low-attenuation, noncalcified plaques (LANCPs) might be something to look for when assessing systemic lupus erythematosus cardiovascular risk. “A simple coronary calcium score, something that all doctors do,” is not enough in lupus, nor is simply checking for lumen obstruction. Also, it’s important not to be misled by an overall reduction in noncalcified plaques. “Low-attenuation, noncalcified plaques don’t [regress] over time in lupus, and they are the ones that lead to cardiovascular events,” he said.
The CT angiography findings were compared with findings in 100 healthy controls who had two CT angiograms in a University of California, Los Angeles, cohort. Overall, there was a mean of 458 LANCPs among lupus patients, versus 42 among controls, a more than 900% difference (P less than .001).
Women with lupus aged under 44 years had a mean of 63 LANCPs; none were detected in healthy women under 44 years. Among women aged 45-59 years, there was a mean of 451 LANCPs in the lupus group versus 53 in the control arm. The findings were highly statistically significant, and almost statistically significant for women 60 years or older, 695 lesions among lupus patients versus 22 (P = .0576).
There were only nine men with lupus in the study, but the findings were similar versus male controls.
While mean LANCP volume regressed over time in the control group (mean, –6.90 mm3; P = .0002), a mean regression of –13.56 mm3 in the lupus group was not statistically significant (P = .4570).
Both controls and lupus patients had a positive remodeling index. It progressed in the lupus group over time, and regressed in controls, but the findings were not statistically significant.
“Statins did nothing for the lupus patients. They didn’t affect progress of coronary plaques at all. We still treat patients because theoretically we don’t have anything better, but we know that they don’t really work in this population,” Dr. Stojan said
The work is funded by the National Institutes of Health. Dr. Stojan didn’t report any relevant disclosures.
SOURCE: Stojan G et al. Lupus Sci Med. 2019;6[suppl 1]:A200, Abstract 274.
SAN FRANCISCO – according to an investigation from Johns Hopkins University, Baltimore.
All of the 102 lupus patients in the coronary artery CT angiography study also had positive plaque remodeling, meaning that at least one low-attenuation plaque was growing into the lumen wall, not the lumen itself, which makes them difficult to detect on standard imaging. Low-attenuation plaques were defined in the study as a plaque larger than 1 mm2 with a radiodensity below 30 Hounsfield units.
Low-attenuation plaques are inherently unstable; they’re fatty, necrotic, and have a high risk of rupturing; their presence in the lumen wall is especially worrisome. In the general population, they sometimes regress, scarring down over time and no longer posing a threat. That didn’t happen in the 30 lupus patients who had follow-up CT angiographies, some 9 years after their first.
The team conducted the study to help understand why cardiovascular disease is so common in lupus, and the leading cause of death. Hopkins investigators have shown previously that statins have no effect on the risk or plaque occurrence and progression, and the cardiovascular risk doesn’t always seem to correlate with disease control. For those and other reasons, the current thinking at Hopkins is that cardiovascular disease in lupus is somehow different than in the general population, said George Stojan, MD, an assistant professor of rheumatology at the school and codirector of the Hopkins Lupus Center.
The goal is “to figure out exactly what to look for when we assess the risk; I don’t think we understand that at this point. We assume patients with lupus behave exactly like patients who don’t have lupus, but they obviously don’t. They do not respond to statins. They have a higher risk no matter what you do for them, even when their disease activity is low, and how much plaque they have over time doesn’t really correlate with disease activity,” he said at an international conference on systemic lupus erythematosus.
“Once we understand” the mechanism, “then we can try to [alter] it. Maybe we can look at new drugs, like the PCSK9 inhibitors which have shown a lot of promise in the general population.” At this point, however, “we don’t really know how to intervene,” Dr. Stojan said.
In the meantime, positive remodeling and low-attenuation, noncalcified plaques (LANCPs) might be something to look for when assessing systemic lupus erythematosus cardiovascular risk. “A simple coronary calcium score, something that all doctors do,” is not enough in lupus, nor is simply checking for lumen obstruction. Also, it’s important not to be misled by an overall reduction in noncalcified plaques. “Low-attenuation, noncalcified plaques don’t [regress] over time in lupus, and they are the ones that lead to cardiovascular events,” he said.
The CT angiography findings were compared with findings in 100 healthy controls who had two CT angiograms in a University of California, Los Angeles, cohort. Overall, there was a mean of 458 LANCPs among lupus patients, versus 42 among controls, a more than 900% difference (P less than .001).
Women with lupus aged under 44 years had a mean of 63 LANCPs; none were detected in healthy women under 44 years. Among women aged 45-59 years, there was a mean of 451 LANCPs in the lupus group versus 53 in the control arm. The findings were highly statistically significant, and almost statistically significant for women 60 years or older, 695 lesions among lupus patients versus 22 (P = .0576).
There were only nine men with lupus in the study, but the findings were similar versus male controls.
While mean LANCP volume regressed over time in the control group (mean, –6.90 mm3; P = .0002), a mean regression of –13.56 mm3 in the lupus group was not statistically significant (P = .4570).
Both controls and lupus patients had a positive remodeling index. It progressed in the lupus group over time, and regressed in controls, but the findings were not statistically significant.
“Statins did nothing for the lupus patients. They didn’t affect progress of coronary plaques at all. We still treat patients because theoretically we don’t have anything better, but we know that they don’t really work in this population,” Dr. Stojan said
The work is funded by the National Institutes of Health. Dr. Stojan didn’t report any relevant disclosures.
SOURCE: Stojan G et al. Lupus Sci Med. 2019;6[suppl 1]:A200, Abstract 274.
SAN FRANCISCO – according to an investigation from Johns Hopkins University, Baltimore.
All of the 102 lupus patients in the coronary artery CT angiography study also had positive plaque remodeling, meaning that at least one low-attenuation plaque was growing into the lumen wall, not the lumen itself, which makes them difficult to detect on standard imaging. Low-attenuation plaques were defined in the study as a plaque larger than 1 mm2 with a radiodensity below 30 Hounsfield units.
Low-attenuation plaques are inherently unstable; they’re fatty, necrotic, and have a high risk of rupturing; their presence in the lumen wall is especially worrisome. In the general population, they sometimes regress, scarring down over time and no longer posing a threat. That didn’t happen in the 30 lupus patients who had follow-up CT angiographies, some 9 years after their first.
The team conducted the study to help understand why cardiovascular disease is so common in lupus, and the leading cause of death. Hopkins investigators have shown previously that statins have no effect on the risk or plaque occurrence and progression, and the cardiovascular risk doesn’t always seem to correlate with disease control. For those and other reasons, the current thinking at Hopkins is that cardiovascular disease in lupus is somehow different than in the general population, said George Stojan, MD, an assistant professor of rheumatology at the school and codirector of the Hopkins Lupus Center.
The goal is “to figure out exactly what to look for when we assess the risk; I don’t think we understand that at this point. We assume patients with lupus behave exactly like patients who don’t have lupus, but they obviously don’t. They do not respond to statins. They have a higher risk no matter what you do for them, even when their disease activity is low, and how much plaque they have over time doesn’t really correlate with disease activity,” he said at an international conference on systemic lupus erythematosus.
“Once we understand” the mechanism, “then we can try to [alter] it. Maybe we can look at new drugs, like the PCSK9 inhibitors which have shown a lot of promise in the general population.” At this point, however, “we don’t really know how to intervene,” Dr. Stojan said.
In the meantime, positive remodeling and low-attenuation, noncalcified plaques (LANCPs) might be something to look for when assessing systemic lupus erythematosus cardiovascular risk. “A simple coronary calcium score, something that all doctors do,” is not enough in lupus, nor is simply checking for lumen obstruction. Also, it’s important not to be misled by an overall reduction in noncalcified plaques. “Low-attenuation, noncalcified plaques don’t [regress] over time in lupus, and they are the ones that lead to cardiovascular events,” he said.
The CT angiography findings were compared with findings in 100 healthy controls who had two CT angiograms in a University of California, Los Angeles, cohort. Overall, there was a mean of 458 LANCPs among lupus patients, versus 42 among controls, a more than 900% difference (P less than .001).
Women with lupus aged under 44 years had a mean of 63 LANCPs; none were detected in healthy women under 44 years. Among women aged 45-59 years, there was a mean of 451 LANCPs in the lupus group versus 53 in the control arm. The findings were highly statistically significant, and almost statistically significant for women 60 years or older, 695 lesions among lupus patients versus 22 (P = .0576).
There were only nine men with lupus in the study, but the findings were similar versus male controls.
While mean LANCP volume regressed over time in the control group (mean, –6.90 mm3; P = .0002), a mean regression of –13.56 mm3 in the lupus group was not statistically significant (P = .4570).
Both controls and lupus patients had a positive remodeling index. It progressed in the lupus group over time, and regressed in controls, but the findings were not statistically significant.
“Statins did nothing for the lupus patients. They didn’t affect progress of coronary plaques at all. We still treat patients because theoretically we don’t have anything better, but we know that they don’t really work in this population,” Dr. Stojan said
The work is funded by the National Institutes of Health. Dr. Stojan didn’t report any relevant disclosures.
SOURCE: Stojan G et al. Lupus Sci Med. 2019;6[suppl 1]:A200, Abstract 274.
REPORTING FROM LUPUS 2019
Key clinical point: Positive remodeling and low-attenuation, noncalcified plaques might be something to look for when assessing systemic lupus erythematosus cardiovascular risk.
Major finding: There was a mean of 458 low-attenuation, noncalcified plaques among lupus patients versus 42 among controls, a more than 900% difference (P less than .001)
Study details: Coronary CT angiography in 102 lupus patients and 100 healthy controls
Disclosures: The National Institutes of Health funded the work. The lead investigator didn’t report any relevant disclosures.
Source: Stojan G et al. Lupus Sci Med. 2019;6[suppl 1]:A200, Abstract 274.
Gastric outlet obstruction: A red flag, potentially manageable
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
KEY POINTS
- Causes of gastric outlet obstruction fall into 2 categories: benign and malignant. The cause should be presumed to be malignant until proven otherwise.
- Peptic ulcer disease, a benign cause, used to account for most cases of gastric outlet obstruction. It is still common but has declined in frequency with the development of acid-suppressing drugs.
- Gastric cancer used to be the most common malignant cause but has declined in frequency in Western countries with treatment for Helicobacter pylori infection. Now, pancreatic cancer predominates.
- Endoscopic stenting is an effective, minimally invasive treatment for patients with malignant gastric outlet obstruction and poor prognosis, allowing resumption of oral intake and improving quality of life.
Noninvasive FFRCT called ADVANCE in chest pain assessment
NEW ORLEANS – Fractional flow reserve derived noninvasively from coronary CT angiography showed clinical merit as a practical tool for evaluation of chest pain at 1 year of follow-up in the ADVANCE registry, Manesh R. Patel, MD, reported at the annual meeting of the American College of Cardiology.
In ADVANCE, a fractional flow reserve value greater than 0.80 derived from CT angiography, or FFRCT, was associated with a significantly lower rate of cardiovascular death or MI at 1 year than in patients with an FFRCT of 0.80 or lower, according to Dr. Patel, professor of medicine and chief of the division of cardiology at Duke University, Durham, N.C.
“The lower rates of revascularization and clinical events in patients with FFRCT who were managed conservatively provide reassurance regarding this clinical strategy if you were to put it into your practice,” he observed.
ADVANCE is in an international, real-world, prospective registry of more than 5,000 patients in Europe, Japan, and North America. All had clinically suspected ischemic coronary artery disease (CAD). They also had at least 30% atherosclerosis documented on coronary CT angiography as a trigger for noninvasive assessment of FFR calculated from computational fluid dynamics. The idea behind FFRCT is that by combining the anatomic information provided by CT angiography with the physiological, functional data from FFR, the result is a better guide to need for revascularization of true obstructive CAD than with conventional invasive coronary angiography alone. Indeed, FFRCT could eventually prove to be a cost-effective gatekeeper to the cardiac catheterization laboratory by cutting down on high rates of invasive coronary angiography for nonactionable CAD.
That point was suggested by the previously reported 90-day outcomes of the ADVANCE registry, the cardiologist explained. Participating physicians first classified patients and made a revascularization/no-revascularization management plan on the basis of the core laboratory CT angiography results alone. But when they received the FFRCT results, they reclassified patients and changed the management plan in 67% of cases. That’s because the prevalence of nonobstructive CAD was 44% in patients with an FFRCT greater than 0.80 in all coronary arteries, compared with just 14% in those with an FFRCT of 0.80 or less. As a result, 72% of patients with an FFRCT of 0.80 or less underwent revascularization, while the vast majority of patients with an FFRCT greater than 0.80 were initially managed conservatively (Eur Heart J. 2018 Nov 1;39[41]:3701-11).
The 1-year outcomes from ADVANCE as presented by Dr. Patel showed low rates of major adverse cardiovascular events overall. Of note, the composite endpoint of cardiovascular death or MI occurred significantly more often in patients with an FFRCT of 0.80 or less, by a margin of 0.8% versus 0.2%, for a 320% increased relative risk. The patients with a FFRCT greater than 0.80 continued to have a much lower revascularization rate from 90 days through 1 year: 5.8% versus 38.4% in the lower-FFRCT group. And 93% of patients placed on medical therapy alone after receiving their FFRCT results remained on medical therapy without revascularization or a major adverse cardiovascular event at 1 year.
Discussant Matthew J. Budoff, MD, commented that it’s time to move beyond observational studies and conduct randomized trials of an FFRCT-based screening strategy in patients with clinical suspicion of obstructive CAD.
“We want to understand the enormous advantages of having FFR-like data before we take patients to the cath lab. And I do think that adding physiology to the anatomy is going to be the approach that we’re going to be predominantly using in the future,” said Dr. Budoff, professor of medicine at the University of California, Los Angeles.
Dr. Patel noted that the ongoing, randomized, 2,100-patient PRECISE study is directed at determining in a more definitive way the clinical and cost-effectiveness of an FFRCT strategy.
The ADVANCE registry is funded by HeartFlow. Dr. Patel reported receiving research grants from that company and several others, as well as the National Institutes of Health. He serves on advisory boards for Bayer, Janssen, and Amgen.
Simultaneous with Dr. Patel’s presentation at ACC 2019, the 1-year ADVANCE registry results were published online (JACC Cardiovasc Imag. 2019 Mar 17. doi: 10.1016/j.jcmg.2019.03.003).
NEW ORLEANS – Fractional flow reserve derived noninvasively from coronary CT angiography showed clinical merit as a practical tool for evaluation of chest pain at 1 year of follow-up in the ADVANCE registry, Manesh R. Patel, MD, reported at the annual meeting of the American College of Cardiology.
In ADVANCE, a fractional flow reserve value greater than 0.80 derived from CT angiography, or FFRCT, was associated with a significantly lower rate of cardiovascular death or MI at 1 year than in patients with an FFRCT of 0.80 or lower, according to Dr. Patel, professor of medicine and chief of the division of cardiology at Duke University, Durham, N.C.
“The lower rates of revascularization and clinical events in patients with FFRCT who were managed conservatively provide reassurance regarding this clinical strategy if you were to put it into your practice,” he observed.
ADVANCE is in an international, real-world, prospective registry of more than 5,000 patients in Europe, Japan, and North America. All had clinically suspected ischemic coronary artery disease (CAD). They also had at least 30% atherosclerosis documented on coronary CT angiography as a trigger for noninvasive assessment of FFR calculated from computational fluid dynamics. The idea behind FFRCT is that by combining the anatomic information provided by CT angiography with the physiological, functional data from FFR, the result is a better guide to need for revascularization of true obstructive CAD than with conventional invasive coronary angiography alone. Indeed, FFRCT could eventually prove to be a cost-effective gatekeeper to the cardiac catheterization laboratory by cutting down on high rates of invasive coronary angiography for nonactionable CAD.
That point was suggested by the previously reported 90-day outcomes of the ADVANCE registry, the cardiologist explained. Participating physicians first classified patients and made a revascularization/no-revascularization management plan on the basis of the core laboratory CT angiography results alone. But when they received the FFRCT results, they reclassified patients and changed the management plan in 67% of cases. That’s because the prevalence of nonobstructive CAD was 44% in patients with an FFRCT greater than 0.80 in all coronary arteries, compared with just 14% in those with an FFRCT of 0.80 or less. As a result, 72% of patients with an FFRCT of 0.80 or less underwent revascularization, while the vast majority of patients with an FFRCT greater than 0.80 were initially managed conservatively (Eur Heart J. 2018 Nov 1;39[41]:3701-11).
The 1-year outcomes from ADVANCE as presented by Dr. Patel showed low rates of major adverse cardiovascular events overall. Of note, the composite endpoint of cardiovascular death or MI occurred significantly more often in patients with an FFRCT of 0.80 or less, by a margin of 0.8% versus 0.2%, for a 320% increased relative risk. The patients with a FFRCT greater than 0.80 continued to have a much lower revascularization rate from 90 days through 1 year: 5.8% versus 38.4% in the lower-FFRCT group. And 93% of patients placed on medical therapy alone after receiving their FFRCT results remained on medical therapy without revascularization or a major adverse cardiovascular event at 1 year.
Discussant Matthew J. Budoff, MD, commented that it’s time to move beyond observational studies and conduct randomized trials of an FFRCT-based screening strategy in patients with clinical suspicion of obstructive CAD.
“We want to understand the enormous advantages of having FFR-like data before we take patients to the cath lab. And I do think that adding physiology to the anatomy is going to be the approach that we’re going to be predominantly using in the future,” said Dr. Budoff, professor of medicine at the University of California, Los Angeles.
Dr. Patel noted that the ongoing, randomized, 2,100-patient PRECISE study is directed at determining in a more definitive way the clinical and cost-effectiveness of an FFRCT strategy.
The ADVANCE registry is funded by HeartFlow. Dr. Patel reported receiving research grants from that company and several others, as well as the National Institutes of Health. He serves on advisory boards for Bayer, Janssen, and Amgen.
Simultaneous with Dr. Patel’s presentation at ACC 2019, the 1-year ADVANCE registry results were published online (JACC Cardiovasc Imag. 2019 Mar 17. doi: 10.1016/j.jcmg.2019.03.003).
NEW ORLEANS – Fractional flow reserve derived noninvasively from coronary CT angiography showed clinical merit as a practical tool for evaluation of chest pain at 1 year of follow-up in the ADVANCE registry, Manesh R. Patel, MD, reported at the annual meeting of the American College of Cardiology.
In ADVANCE, a fractional flow reserve value greater than 0.80 derived from CT angiography, or FFRCT, was associated with a significantly lower rate of cardiovascular death or MI at 1 year than in patients with an FFRCT of 0.80 or lower, according to Dr. Patel, professor of medicine and chief of the division of cardiology at Duke University, Durham, N.C.
“The lower rates of revascularization and clinical events in patients with FFRCT who were managed conservatively provide reassurance regarding this clinical strategy if you were to put it into your practice,” he observed.
ADVANCE is in an international, real-world, prospective registry of more than 5,000 patients in Europe, Japan, and North America. All had clinically suspected ischemic coronary artery disease (CAD). They also had at least 30% atherosclerosis documented on coronary CT angiography as a trigger for noninvasive assessment of FFR calculated from computational fluid dynamics. The idea behind FFRCT is that by combining the anatomic information provided by CT angiography with the physiological, functional data from FFR, the result is a better guide to need for revascularization of true obstructive CAD than with conventional invasive coronary angiography alone. Indeed, FFRCT could eventually prove to be a cost-effective gatekeeper to the cardiac catheterization laboratory by cutting down on high rates of invasive coronary angiography for nonactionable CAD.
That point was suggested by the previously reported 90-day outcomes of the ADVANCE registry, the cardiologist explained. Participating physicians first classified patients and made a revascularization/no-revascularization management plan on the basis of the core laboratory CT angiography results alone. But when they received the FFRCT results, they reclassified patients and changed the management plan in 67% of cases. That’s because the prevalence of nonobstructive CAD was 44% in patients with an FFRCT greater than 0.80 in all coronary arteries, compared with just 14% in those with an FFRCT of 0.80 or less. As a result, 72% of patients with an FFRCT of 0.80 or less underwent revascularization, while the vast majority of patients with an FFRCT greater than 0.80 were initially managed conservatively (Eur Heart J. 2018 Nov 1;39[41]:3701-11).
The 1-year outcomes from ADVANCE as presented by Dr. Patel showed low rates of major adverse cardiovascular events overall. Of note, the composite endpoint of cardiovascular death or MI occurred significantly more often in patients with an FFRCT of 0.80 or less, by a margin of 0.8% versus 0.2%, for a 320% increased relative risk. The patients with a FFRCT greater than 0.80 continued to have a much lower revascularization rate from 90 days through 1 year: 5.8% versus 38.4% in the lower-FFRCT group. And 93% of patients placed on medical therapy alone after receiving their FFRCT results remained on medical therapy without revascularization or a major adverse cardiovascular event at 1 year.
Discussant Matthew J. Budoff, MD, commented that it’s time to move beyond observational studies and conduct randomized trials of an FFRCT-based screening strategy in patients with clinical suspicion of obstructive CAD.
“We want to understand the enormous advantages of having FFR-like data before we take patients to the cath lab. And I do think that adding physiology to the anatomy is going to be the approach that we’re going to be predominantly using in the future,” said Dr. Budoff, professor of medicine at the University of California, Los Angeles.
Dr. Patel noted that the ongoing, randomized, 2,100-patient PRECISE study is directed at determining in a more definitive way the clinical and cost-effectiveness of an FFRCT strategy.
The ADVANCE registry is funded by HeartFlow. Dr. Patel reported receiving research grants from that company and several others, as well as the National Institutes of Health. He serves on advisory boards for Bayer, Janssen, and Amgen.
Simultaneous with Dr. Patel’s presentation at ACC 2019, the 1-year ADVANCE registry results were published online (JACC Cardiovasc Imag. 2019 Mar 17. doi: 10.1016/j.jcmg.2019.03.003).
REPORTING FROM ACC 19
Prevalence and outcomes of incidental imaging findings
Background: As frequency of imaging studies increases, and those studies become more advanced, incidental findings on imaging are a growing concern. Incidentalomas can lead to anxiety for patients, increased testing, and possible interventions such as biopsies. Current literature does not provide adequate guidance for providers to discuss the risks of incidentalomas with patients, nor are there clear methods described to manage incidentalomas when discovered.
Study design: This study was an umbrella review of systematic reviews and meta-analyses. Authors conduced their own meta-analyses using data from pooled sources.
Setting: MEDLINE and EMBASE were searched, which resulted in 20 unique systematic reviews analyzed, 15 of which provided incidence data and 18 included outcome data.
Synopsis: To assess prevalence of incidentalomas, the authors conducted nine meta-analyses, with a median number of 14,409 patients. Each analysis was created based on the imaging modality used and the area of the body where the incidental finding occurred. They examined the outcomes specific to incidentalomas within those organs. Their analysis showed that CT of the chest had the highest prevalence of incidentalomas (45%; 95% confidence interval, 36%-55%). Incidental findings in the breast had the highest rates of malignancy (42%; 95% CI, 31%-54%). Noncancerous outcomes described included disc degeneration on MRIs of the spine, aneurysms in brain imaging, and subclinical Cushing’s syndrome. There was significant heterogeneity in all the meta-analyses conducted.
Limitations included variations in how primary study authors defined a positive result and in imaging protocols. Although the authors of this study used primary data extracted from the individual studies in the systematic reviews, they did not analyze the primary studies for inclusion based on methods.
Bottom line: This study provides guidance to clinicians regarding counseling patients on the risks of incidentalomas and how to manage those incidental findings.
Citation: O’Sullivan JW et al. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. 2018 Jun 18. doi: 10.1136/bmj.k2387.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: As frequency of imaging studies increases, and those studies become more advanced, incidental findings on imaging are a growing concern. Incidentalomas can lead to anxiety for patients, increased testing, and possible interventions such as biopsies. Current literature does not provide adequate guidance for providers to discuss the risks of incidentalomas with patients, nor are there clear methods described to manage incidentalomas when discovered.
Study design: This study was an umbrella review of systematic reviews and meta-analyses. Authors conduced their own meta-analyses using data from pooled sources.
Setting: MEDLINE and EMBASE were searched, which resulted in 20 unique systematic reviews analyzed, 15 of which provided incidence data and 18 included outcome data.
Synopsis: To assess prevalence of incidentalomas, the authors conducted nine meta-analyses, with a median number of 14,409 patients. Each analysis was created based on the imaging modality used and the area of the body where the incidental finding occurred. They examined the outcomes specific to incidentalomas within those organs. Their analysis showed that CT of the chest had the highest prevalence of incidentalomas (45%; 95% confidence interval, 36%-55%). Incidental findings in the breast had the highest rates of malignancy (42%; 95% CI, 31%-54%). Noncancerous outcomes described included disc degeneration on MRIs of the spine, aneurysms in brain imaging, and subclinical Cushing’s syndrome. There was significant heterogeneity in all the meta-analyses conducted.
Limitations included variations in how primary study authors defined a positive result and in imaging protocols. Although the authors of this study used primary data extracted from the individual studies in the systematic reviews, they did not analyze the primary studies for inclusion based on methods.
Bottom line: This study provides guidance to clinicians regarding counseling patients on the risks of incidentalomas and how to manage those incidental findings.
Citation: O’Sullivan JW et al. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. 2018 Jun 18. doi: 10.1136/bmj.k2387.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: As frequency of imaging studies increases, and those studies become more advanced, incidental findings on imaging are a growing concern. Incidentalomas can lead to anxiety for patients, increased testing, and possible interventions such as biopsies. Current literature does not provide adequate guidance for providers to discuss the risks of incidentalomas with patients, nor are there clear methods described to manage incidentalomas when discovered.
Study design: This study was an umbrella review of systematic reviews and meta-analyses. Authors conduced their own meta-analyses using data from pooled sources.
Setting: MEDLINE and EMBASE were searched, which resulted in 20 unique systematic reviews analyzed, 15 of which provided incidence data and 18 included outcome data.
Synopsis: To assess prevalence of incidentalomas, the authors conducted nine meta-analyses, with a median number of 14,409 patients. Each analysis was created based on the imaging modality used and the area of the body where the incidental finding occurred. They examined the outcomes specific to incidentalomas within those organs. Their analysis showed that CT of the chest had the highest prevalence of incidentalomas (45%; 95% confidence interval, 36%-55%). Incidental findings in the breast had the highest rates of malignancy (42%; 95% CI, 31%-54%). Noncancerous outcomes described included disc degeneration on MRIs of the spine, aneurysms in brain imaging, and subclinical Cushing’s syndrome. There was significant heterogeneity in all the meta-analyses conducted.
Limitations included variations in how primary study authors defined a positive result and in imaging protocols. Although the authors of this study used primary data extracted from the individual studies in the systematic reviews, they did not analyze the primary studies for inclusion based on methods.
Bottom line: This study provides guidance to clinicians regarding counseling patients on the risks of incidentalomas and how to manage those incidental findings.
Citation: O’Sullivan JW et al. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. 2018 Jun 18. doi: 10.1136/bmj.k2387.
Dr. Witt is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Spontaneous coronary artery dissection: An often unrecognized cause of acute coronary syndrome
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
KEY POINTS
- SCAD often presents with symptoms of acute coronary syndrome but can be asymptomatic or cause sudden death.
- Management is generally conservative, but a left main or severe proximal 2-vessel dissection, hemodynamic instability, or ongoing ischemic symptoms may warrant revascularization.
- All patients with SCAD should be screened for other vascular problems, especially fibromuscular dysplasia.
- Long-term aspirin therapy and 1 year of clopidogrel are recommended after an episode of SCAD.