User login
WCD: Topical tofacitinib hits marks in atopic dermatitis
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
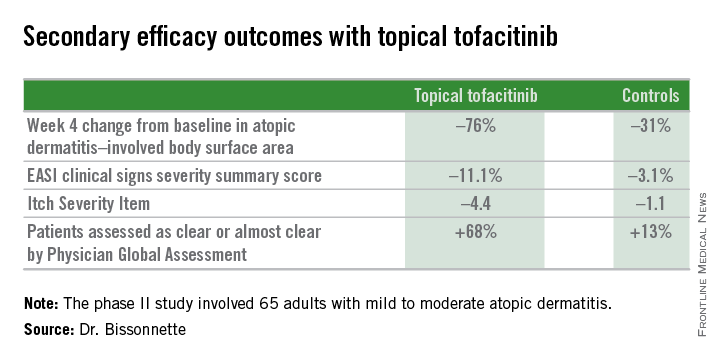
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
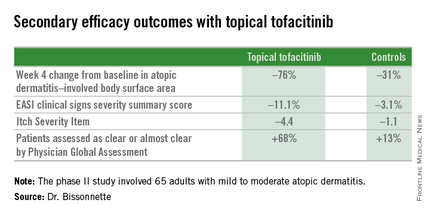
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

AT WCD 2015
Key clinical point: Topical tofacitinib, a Janus kinase inhibitor, may provide a novel safe and effective therapy for atopic dermatitis.
Major finding: After 4 weeks of topical tofacitinib, atopic dermatitis patients experienced a mean 82% reduction in their Eczema Area and Severity Index total score, compared with a 30% decrease in vehicle-treated controls.
Data source: A five-center, randomized, double-blind, prospective, vehicle-controlled phase II study involving 65 adults with atopic dermatitis.
Disclosures: Dr. Robert Bissonnette disclosed ties with Pfizer – the study sponsor – and more than a dozen other pharmaceutical companies.
Elderly-onset atopic dermatitis is on the rise
VANCOUVER – Atopic dermatitis arising de novo in patients in their 60s or older with no history of the disease poses a diagnostic challenge, and a low threshold for biopsy is warranted, Dr. Thomas Bieber said at the World Congress of Dermatology.
“The diagnosis is not very easy, and if you are not sure what you are facing, I urge you to take biopsies in order to exclude cutaneous T-cell lymphoma before treating the patient with any kind of active compound,” cautioned Dr. Bieber, professor and chair of the department of dermatology and allergy at the University of Bonn (Germany).
Very-late-onset atopic dermatitis and cutaneous T-cell lymphoma (CTCL) may look quite similar clinically. There is but a single exception: Primary CTCL usually doesn’t itch, while pruritus is a prominent feature of atopic dermatitis arising in seniors, he added.
New-onset atopic dermatitis at an advanced age is increasing in prevalence, as is true of atopic dermatitis across the rest of the age spectrum. Dr. Bieber said that statistic is certainly borne out in his own clinical practice, where with the graying of the population he is seeing more cases.
He credited Dr. Ryoji Tanei of Tokyo Metropolitan Geriatric Hospital with doing pioneering work in bringing this particular variant of atopic dermatitis to wider attention (J. Clin. Med. 2015;4:979-97). Roughly 30% of patients with atopic dermatitis in their 60s or older report they never had the disease before. Another 20% had atopic dermatitis in childhood, while it arose in early adulthood in the rest.
Atopic dermatitis arising de novo in seniors is a special form of the disease that characteristically involves the face, neck, and trunk while sparing the flexural areas which are so prominently involved in younger patients. The eczema is often erythrodermic. Older men are affected threefold more often than women.
The disorder is characterized by extraordinarily high serum IgE levels: a mean of 8,000 IU in one series reported by Dr. Tanei.
This very-late-onset form of atopic dermatitis tends not to fade away over time. Dr. Tanei has reported that many affected patients die with the inflammatory skin disease, never outgrowing it.
Very-late-onset atopic dermatitis is often resistant to topical therapies; repeated courses of oral corticosteroids may be required.
The differential diagnosis is quite different than in children, where genetic immunodeficiency syndromes are a real concern. While CTCL is the biggie in the differential diagnosis of very-late-onset atopic dermatitis, other conditions that need to be considered include psoriasis, contact dermatitis, pityriasis rubra pilaris, and pityriasis rosea.
Dr. Bieber is a consultant to and recipient of research grants from numerous pharmaceutical companies having an interest in dermatology.
VANCOUVER – Atopic dermatitis arising de novo in patients in their 60s or older with no history of the disease poses a diagnostic challenge, and a low threshold for biopsy is warranted, Dr. Thomas Bieber said at the World Congress of Dermatology.
“The diagnosis is not very easy, and if you are not sure what you are facing, I urge you to take biopsies in order to exclude cutaneous T-cell lymphoma before treating the patient with any kind of active compound,” cautioned Dr. Bieber, professor and chair of the department of dermatology and allergy at the University of Bonn (Germany).
Very-late-onset atopic dermatitis and cutaneous T-cell lymphoma (CTCL) may look quite similar clinically. There is but a single exception: Primary CTCL usually doesn’t itch, while pruritus is a prominent feature of atopic dermatitis arising in seniors, he added.
New-onset atopic dermatitis at an advanced age is increasing in prevalence, as is true of atopic dermatitis across the rest of the age spectrum. Dr. Bieber said that statistic is certainly borne out in his own clinical practice, where with the graying of the population he is seeing more cases.
He credited Dr. Ryoji Tanei of Tokyo Metropolitan Geriatric Hospital with doing pioneering work in bringing this particular variant of atopic dermatitis to wider attention (J. Clin. Med. 2015;4:979-97). Roughly 30% of patients with atopic dermatitis in their 60s or older report they never had the disease before. Another 20% had atopic dermatitis in childhood, while it arose in early adulthood in the rest.
Atopic dermatitis arising de novo in seniors is a special form of the disease that characteristically involves the face, neck, and trunk while sparing the flexural areas which are so prominently involved in younger patients. The eczema is often erythrodermic. Older men are affected threefold more often than women.
The disorder is characterized by extraordinarily high serum IgE levels: a mean of 8,000 IU in one series reported by Dr. Tanei.
This very-late-onset form of atopic dermatitis tends not to fade away over time. Dr. Tanei has reported that many affected patients die with the inflammatory skin disease, never outgrowing it.
Very-late-onset atopic dermatitis is often resistant to topical therapies; repeated courses of oral corticosteroids may be required.
The differential diagnosis is quite different than in children, where genetic immunodeficiency syndromes are a real concern. While CTCL is the biggie in the differential diagnosis of very-late-onset atopic dermatitis, other conditions that need to be considered include psoriasis, contact dermatitis, pityriasis rubra pilaris, and pityriasis rosea.
Dr. Bieber is a consultant to and recipient of research grants from numerous pharmaceutical companies having an interest in dermatology.
VANCOUVER – Atopic dermatitis arising de novo in patients in their 60s or older with no history of the disease poses a diagnostic challenge, and a low threshold for biopsy is warranted, Dr. Thomas Bieber said at the World Congress of Dermatology.
“The diagnosis is not very easy, and if you are not sure what you are facing, I urge you to take biopsies in order to exclude cutaneous T-cell lymphoma before treating the patient with any kind of active compound,” cautioned Dr. Bieber, professor and chair of the department of dermatology and allergy at the University of Bonn (Germany).
Very-late-onset atopic dermatitis and cutaneous T-cell lymphoma (CTCL) may look quite similar clinically. There is but a single exception: Primary CTCL usually doesn’t itch, while pruritus is a prominent feature of atopic dermatitis arising in seniors, he added.
New-onset atopic dermatitis at an advanced age is increasing in prevalence, as is true of atopic dermatitis across the rest of the age spectrum. Dr. Bieber said that statistic is certainly borne out in his own clinical practice, where with the graying of the population he is seeing more cases.
He credited Dr. Ryoji Tanei of Tokyo Metropolitan Geriatric Hospital with doing pioneering work in bringing this particular variant of atopic dermatitis to wider attention (J. Clin. Med. 2015;4:979-97). Roughly 30% of patients with atopic dermatitis in their 60s or older report they never had the disease before. Another 20% had atopic dermatitis in childhood, while it arose in early adulthood in the rest.
Atopic dermatitis arising de novo in seniors is a special form of the disease that characteristically involves the face, neck, and trunk while sparing the flexural areas which are so prominently involved in younger patients. The eczema is often erythrodermic. Older men are affected threefold more often than women.
The disorder is characterized by extraordinarily high serum IgE levels: a mean of 8,000 IU in one series reported by Dr. Tanei.
This very-late-onset form of atopic dermatitis tends not to fade away over time. Dr. Tanei has reported that many affected patients die with the inflammatory skin disease, never outgrowing it.
Very-late-onset atopic dermatitis is often resistant to topical therapies; repeated courses of oral corticosteroids may be required.
The differential diagnosis is quite different than in children, where genetic immunodeficiency syndromes are a real concern. While CTCL is the biggie in the differential diagnosis of very-late-onset atopic dermatitis, other conditions that need to be considered include psoriasis, contact dermatitis, pityriasis rubra pilaris, and pityriasis rosea.
Dr. Bieber is a consultant to and recipient of research grants from numerous pharmaceutical companies having an interest in dermatology.
EXPERT ANALYSIS FROM WCD 2015
DDW: Budesonide improves dysphagia, histology, and endoscopic findings in EoE
WASHINGTON – Treatment with an oral formulation of budesonide was associated with significant improvements in dysphagia and esophageal eosinophil counts in adolescents and adults with eosinophilic esophagitis, in a study that is also the first to use a recently validated scoring instrument to evaluate medical therapy for this disorder in a randomized trial.
Results of the multicenter, double-blind, randomized study comparing treatment with an oral budesonide suspension to placebo for 12 weeks in almost 100 patients with eosinophilic esophagitis (EoE) were reported in two separate presentations at the annual Digestive Disease Week. One of the investigators, Dr. Evan Dellon of the Center for Gastrointestinal Biology and Disease, at the University of North Carolina, Chapel Hill, said that treatment with this “mucoadherent” formulation of the topical steroid was associated with significant improvements in dysphagia symptoms, as measured with the Dysphagia Symptom Questionnaire (DSQ), and a histologic response rate of 39%, vs. 3% among those on placebo.
“The study not only adds to the evidence that topical budesonide is effective for inducing histologic response in subjects with active EoE, but [also] shows for the first time that symptoms of dysphagia, as measured with a validated symptom instrument, improve concordantly with the histologic and endoscopic findings,” Dr. Dellon said in an interview after the meeting. “Moreover, this study shows that a topical steroid formulation designed specifically for EoE, rather than an asthma formulation that is adapted for esophageal use, will likely be a beneficial and potentially preferred clinical treatment option.”
Another study investigator, Dr. Ikuo Hirano, professor of medicine and director of the gastroenterology and hepatology fellowship program at Northwestern University, Chicago, reported that treatment was also associated with significant improvements in the EoE Endoscopic Reference Score, EREFS, which was designed to classify and grade the severity of five major endoscopic features of EoE: edema, rings, exudates, furrows, and stricture formation. This was the first study to use this validated endoscopic scoring instrument in a randomized controlled trial of a medical therapy in patients with EoE, he said at the meeting.
The study was conducted between 2012 and 2014 at 25 U.S. sites, in patients aged 11-40 years with EoE. Baseline demographic and endoscopic characteristics were similar in the two groups. Their mean age was 21-22 years (41% of those on placebo and 35% of those on budesonide were younger than age 18 years) and 69% were male; most patients had edema, all had dysphagia, and 39%-41% had heartburn. Patients were excluded if they had esophageal stricture on screening endoscopy that did not allow passage of a standard adult diagnostic endoscope.
Patients were randomized to treatment with budesonide suspension, at a dose of 2 mg twice a day (51 patients) or a placebo suspension (42 patients). The primary outcomes were a change in the DSQ score from baseline, and the proportion of patients with a histologic response, defined as at least 6 eosinophils per high-power field (eos/hpf) from all biopsies. The final analysis included 87 patients.
At baseline, the mean peak eosinophil counts were 156/hpf among those on budesonide and 130/hpf among those on placebo; after treatment, the mean peak counts dropped to 39/hpf among those on budesonide (a 65% reduction) and to 113/hpf among those on placebo (a 10% reduction), a statistically significant difference (P < .05), said Dr. Dellon, also with the department of medicine at UNC.
From a mean of about 29 in both groups at baseline, DSQ scores dropped by a mean of 14.3 among the treated patients, vs. 7.5 among those on placebo, which was a statistically significant difference (P = .0096). The other primary endpoint, the histologic response rate, was 39% among treated patients, vs. 3% among those on placebo, also a significant difference (P < .0001).
Adverse events were not different between the two groups, and growth velocity among those under age 18 years and cortisol levels were not different between the two groups, he added. There was one case of esophageal candidiasis in a patient on budesonide.
During his presentation, Dr. Hirano said that there were also significant improvements in EREFS scores from baseline in the proximal and distal esophagus among those treated with budesonide, but not among those on placebo. Based on EREFS scores, oral budesonide “resulted in significant improvement in endoscopic features of edema, exudate, rings, [and] furrows, compared to placebo,” but there was no significant change in strictures, another component of the EREFS, in either the treatment or placebo groups. However, patients with high-grade strictures were not enrolled in the study, he added.
After treatment, proximal, distal, and total EREFS scores correlated with peak eosinophil counts, a highly statistically significant finding.
Dr. Hirano said that the primary endpoints used in most EoE clinical trials to date have focused mostly on assessments of symptoms and histopathology, which have limitations. “Symptoms are difficult to quantify and often intermittent [and] they may improve as a result of changes in eating behavior and food avoidance,” he said. Patient-reported outcome instruments have been recently validated, “but have questionable utility in clinical practice” and histology “has shown limited correlation between degree of esophageal eosinophilia and symptom severity [and does not] assess for modeling, an important determinant of overall disease complications,” he added.
The utility of endoscopy in EoE includes the features that are present in vast majority of patients with EoE, and provides a gross assessment of overall disease activity, “both in terms of inflammatory and fibrostenotic features,” he said.
“Endoscopic outcomes are now emerging as clinically relevant endpoints of therapy of trials of eosinophilic esophagitis that supports and complements” symptom and histologic assessments, Dr. Hirano commented, adding that more studies are need to determine the “relative importance of these individual endoscopic features as well as the appropriate utilization of endoscopic parameters in disease management.”
The study was funded by Meritage Pharma, recently acquired by the Shire group of companies. All authors received research funds to conduct the study, and Dr. Hirano disclosed having worked as a consultant for Meritage. Dr. Dellon’s disclosures included receiving grant and research support from Meritage. Shire is developing the oral budesonide suspension formulation as a treatment for adolescents and adults with EoE.
WASHINGTON – Treatment with an oral formulation of budesonide was associated with significant improvements in dysphagia and esophageal eosinophil counts in adolescents and adults with eosinophilic esophagitis, in a study that is also the first to use a recently validated scoring instrument to evaluate medical therapy for this disorder in a randomized trial.
Results of the multicenter, double-blind, randomized study comparing treatment with an oral budesonide suspension to placebo for 12 weeks in almost 100 patients with eosinophilic esophagitis (EoE) were reported in two separate presentations at the annual Digestive Disease Week. One of the investigators, Dr. Evan Dellon of the Center for Gastrointestinal Biology and Disease, at the University of North Carolina, Chapel Hill, said that treatment with this “mucoadherent” formulation of the topical steroid was associated with significant improvements in dysphagia symptoms, as measured with the Dysphagia Symptom Questionnaire (DSQ), and a histologic response rate of 39%, vs. 3% among those on placebo.
“The study not only adds to the evidence that topical budesonide is effective for inducing histologic response in subjects with active EoE, but [also] shows for the first time that symptoms of dysphagia, as measured with a validated symptom instrument, improve concordantly with the histologic and endoscopic findings,” Dr. Dellon said in an interview after the meeting. “Moreover, this study shows that a topical steroid formulation designed specifically for EoE, rather than an asthma formulation that is adapted for esophageal use, will likely be a beneficial and potentially preferred clinical treatment option.”
Another study investigator, Dr. Ikuo Hirano, professor of medicine and director of the gastroenterology and hepatology fellowship program at Northwestern University, Chicago, reported that treatment was also associated with significant improvements in the EoE Endoscopic Reference Score, EREFS, which was designed to classify and grade the severity of five major endoscopic features of EoE: edema, rings, exudates, furrows, and stricture formation. This was the first study to use this validated endoscopic scoring instrument in a randomized controlled trial of a medical therapy in patients with EoE, he said at the meeting.
The study was conducted between 2012 and 2014 at 25 U.S. sites, in patients aged 11-40 years with EoE. Baseline demographic and endoscopic characteristics were similar in the two groups. Their mean age was 21-22 years (41% of those on placebo and 35% of those on budesonide were younger than age 18 years) and 69% were male; most patients had edema, all had dysphagia, and 39%-41% had heartburn. Patients were excluded if they had esophageal stricture on screening endoscopy that did not allow passage of a standard adult diagnostic endoscope.
Patients were randomized to treatment with budesonide suspension, at a dose of 2 mg twice a day (51 patients) or a placebo suspension (42 patients). The primary outcomes were a change in the DSQ score from baseline, and the proportion of patients with a histologic response, defined as at least 6 eosinophils per high-power field (eos/hpf) from all biopsies. The final analysis included 87 patients.
At baseline, the mean peak eosinophil counts were 156/hpf among those on budesonide and 130/hpf among those on placebo; after treatment, the mean peak counts dropped to 39/hpf among those on budesonide (a 65% reduction) and to 113/hpf among those on placebo (a 10% reduction), a statistically significant difference (P < .05), said Dr. Dellon, also with the department of medicine at UNC.
From a mean of about 29 in both groups at baseline, DSQ scores dropped by a mean of 14.3 among the treated patients, vs. 7.5 among those on placebo, which was a statistically significant difference (P = .0096). The other primary endpoint, the histologic response rate, was 39% among treated patients, vs. 3% among those on placebo, also a significant difference (P < .0001).
Adverse events were not different between the two groups, and growth velocity among those under age 18 years and cortisol levels were not different between the two groups, he added. There was one case of esophageal candidiasis in a patient on budesonide.
During his presentation, Dr. Hirano said that there were also significant improvements in EREFS scores from baseline in the proximal and distal esophagus among those treated with budesonide, but not among those on placebo. Based on EREFS scores, oral budesonide “resulted in significant improvement in endoscopic features of edema, exudate, rings, [and] furrows, compared to placebo,” but there was no significant change in strictures, another component of the EREFS, in either the treatment or placebo groups. However, patients with high-grade strictures were not enrolled in the study, he added.
After treatment, proximal, distal, and total EREFS scores correlated with peak eosinophil counts, a highly statistically significant finding.
Dr. Hirano said that the primary endpoints used in most EoE clinical trials to date have focused mostly on assessments of symptoms and histopathology, which have limitations. “Symptoms are difficult to quantify and often intermittent [and] they may improve as a result of changes in eating behavior and food avoidance,” he said. Patient-reported outcome instruments have been recently validated, “but have questionable utility in clinical practice” and histology “has shown limited correlation between degree of esophageal eosinophilia and symptom severity [and does not] assess for modeling, an important determinant of overall disease complications,” he added.
The utility of endoscopy in EoE includes the features that are present in vast majority of patients with EoE, and provides a gross assessment of overall disease activity, “both in terms of inflammatory and fibrostenotic features,” he said.
“Endoscopic outcomes are now emerging as clinically relevant endpoints of therapy of trials of eosinophilic esophagitis that supports and complements” symptom and histologic assessments, Dr. Hirano commented, adding that more studies are need to determine the “relative importance of these individual endoscopic features as well as the appropriate utilization of endoscopic parameters in disease management.”
The study was funded by Meritage Pharma, recently acquired by the Shire group of companies. All authors received research funds to conduct the study, and Dr. Hirano disclosed having worked as a consultant for Meritage. Dr. Dellon’s disclosures included receiving grant and research support from Meritage. Shire is developing the oral budesonide suspension formulation as a treatment for adolescents and adults with EoE.
WASHINGTON – Treatment with an oral formulation of budesonide was associated with significant improvements in dysphagia and esophageal eosinophil counts in adolescents and adults with eosinophilic esophagitis, in a study that is also the first to use a recently validated scoring instrument to evaluate medical therapy for this disorder in a randomized trial.
Results of the multicenter, double-blind, randomized study comparing treatment with an oral budesonide suspension to placebo for 12 weeks in almost 100 patients with eosinophilic esophagitis (EoE) were reported in two separate presentations at the annual Digestive Disease Week. One of the investigators, Dr. Evan Dellon of the Center for Gastrointestinal Biology and Disease, at the University of North Carolina, Chapel Hill, said that treatment with this “mucoadherent” formulation of the topical steroid was associated with significant improvements in dysphagia symptoms, as measured with the Dysphagia Symptom Questionnaire (DSQ), and a histologic response rate of 39%, vs. 3% among those on placebo.
“The study not only adds to the evidence that topical budesonide is effective for inducing histologic response in subjects with active EoE, but [also] shows for the first time that symptoms of dysphagia, as measured with a validated symptom instrument, improve concordantly with the histologic and endoscopic findings,” Dr. Dellon said in an interview after the meeting. “Moreover, this study shows that a topical steroid formulation designed specifically for EoE, rather than an asthma formulation that is adapted for esophageal use, will likely be a beneficial and potentially preferred clinical treatment option.”
Another study investigator, Dr. Ikuo Hirano, professor of medicine and director of the gastroenterology and hepatology fellowship program at Northwestern University, Chicago, reported that treatment was also associated with significant improvements in the EoE Endoscopic Reference Score, EREFS, which was designed to classify and grade the severity of five major endoscopic features of EoE: edema, rings, exudates, furrows, and stricture formation. This was the first study to use this validated endoscopic scoring instrument in a randomized controlled trial of a medical therapy in patients with EoE, he said at the meeting.
The study was conducted between 2012 and 2014 at 25 U.S. sites, in patients aged 11-40 years with EoE. Baseline demographic and endoscopic characteristics were similar in the two groups. Their mean age was 21-22 years (41% of those on placebo and 35% of those on budesonide were younger than age 18 years) and 69% were male; most patients had edema, all had dysphagia, and 39%-41% had heartburn. Patients were excluded if they had esophageal stricture on screening endoscopy that did not allow passage of a standard adult diagnostic endoscope.
Patients were randomized to treatment with budesonide suspension, at a dose of 2 mg twice a day (51 patients) or a placebo suspension (42 patients). The primary outcomes were a change in the DSQ score from baseline, and the proportion of patients with a histologic response, defined as at least 6 eosinophils per high-power field (eos/hpf) from all biopsies. The final analysis included 87 patients.
At baseline, the mean peak eosinophil counts were 156/hpf among those on budesonide and 130/hpf among those on placebo; after treatment, the mean peak counts dropped to 39/hpf among those on budesonide (a 65% reduction) and to 113/hpf among those on placebo (a 10% reduction), a statistically significant difference (P < .05), said Dr. Dellon, also with the department of medicine at UNC.
From a mean of about 29 in both groups at baseline, DSQ scores dropped by a mean of 14.3 among the treated patients, vs. 7.5 among those on placebo, which was a statistically significant difference (P = .0096). The other primary endpoint, the histologic response rate, was 39% among treated patients, vs. 3% among those on placebo, also a significant difference (P < .0001).
Adverse events were not different between the two groups, and growth velocity among those under age 18 years and cortisol levels were not different between the two groups, he added. There was one case of esophageal candidiasis in a patient on budesonide.
During his presentation, Dr. Hirano said that there were also significant improvements in EREFS scores from baseline in the proximal and distal esophagus among those treated with budesonide, but not among those on placebo. Based on EREFS scores, oral budesonide “resulted in significant improvement in endoscopic features of edema, exudate, rings, [and] furrows, compared to placebo,” but there was no significant change in strictures, another component of the EREFS, in either the treatment or placebo groups. However, patients with high-grade strictures were not enrolled in the study, he added.
After treatment, proximal, distal, and total EREFS scores correlated with peak eosinophil counts, a highly statistically significant finding.
Dr. Hirano said that the primary endpoints used in most EoE clinical trials to date have focused mostly on assessments of symptoms and histopathology, which have limitations. “Symptoms are difficult to quantify and often intermittent [and] they may improve as a result of changes in eating behavior and food avoidance,” he said. Patient-reported outcome instruments have been recently validated, “but have questionable utility in clinical practice” and histology “has shown limited correlation between degree of esophageal eosinophilia and symptom severity [and does not] assess for modeling, an important determinant of overall disease complications,” he added.
The utility of endoscopy in EoE includes the features that are present in vast majority of patients with EoE, and provides a gross assessment of overall disease activity, “both in terms of inflammatory and fibrostenotic features,” he said.
“Endoscopic outcomes are now emerging as clinically relevant endpoints of therapy of trials of eosinophilic esophagitis that supports and complements” symptom and histologic assessments, Dr. Hirano commented, adding that more studies are need to determine the “relative importance of these individual endoscopic features as well as the appropriate utilization of endoscopic parameters in disease management.”
The study was funded by Meritage Pharma, recently acquired by the Shire group of companies. All authors received research funds to conduct the study, and Dr. Hirano disclosed having worked as a consultant for Meritage. Dr. Dellon’s disclosures included receiving grant and research support from Meritage. Shire is developing the oral budesonide suspension formulation as a treatment for adolescents and adults with EoE.
AT DDW 2015
Key clinical point: A mucoadherent oral formulation of budesonide shows promise as an effective treatment for eosinophilic esophagitis (EoE), with a favorable safety profile.
Major finding: Beneficial effects of oral budesonide in a study of adolescents and adults with EoE included a histologic response rate of 39% and significantly improved dysphagia symptoms.
Data source: A randomized, double-blind multicenter U.S. study evaluated the healing effects and response to of oral budesonide vs. placebo in 93 patients with EoE, aged 11-40 years.
Disclosures: The study was funded by Meritage Pharma, recently acquired by the Shire group of companies. All authors received research funds to conduct the study. Dr. Dellon’s disclosures included receiving grant and research support from Meritage. Dr. Hirano disclosed having worked as a consultant for Meritage.
VIDEO: Assessment tool rapidly screens lupus cognition
ROME – The Montreal Cognitive Assessment provides a quick and easy-to-use screening tool to identify patients with systemic lupus erythematosus with cognitive impairment, Dr. Zahi Touma reported in a poster at the European Congress of Rheumatology.
In a consecutive series of 78 patients screened with the Montreal Cognitive Assessment (MoCA), the free, single-page test, which can be administered in about 10 minutes, showed a sensitivity of 69% and specificity of 68%, compared with the current standard, the Hopkins Verbal Learning Test-Revised, said Dr. Touma, a rheumatologist at the University of Toronto.
Other easy-to-use and quick screening tools, such as the Mini-Mental State Examination, had substantially worse performance in the study. He and his associates found a sensitivity of 21% and specificity of 91% using the Mini-Mental State exam. For screening, higher sensitivity is desirable so that fewer patients with potential cognitive impairment are missed, he noted.
“Ease of use and time needed for assessment as well as appropriate psychometric properties make the MoCA the preferential screening test for cognitive impairment in patients with SLE,” Dr. Touma said in his poster.
Cognitive impairment is very common among patients with systemic lupus erythematosus (SLE). In this study, Dr Touma found a 47% prevalence using MoCA. Cognitive impairment, however, often goes unidentified in SLE patients, likely because of lack of awareness among rheumatologists as well as the absence of a quick and easily administered screening tool, he said in a video interview.
Dr. Touma said he hopes that the apparent efficacy of an easy-to-use screening tool like MoCA will help boost appreciation for the high prevalence of cognitive impairment in SLE patients. He suggested that clinicians screen for cognitive impairment as soon as SLE is diagnosed and that they perform follow-up screening during subsequent patient encounters with SLE patients who initially present without cognitive impairment.
Dr. Touma had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
ROME – The Montreal Cognitive Assessment provides a quick and easy-to-use screening tool to identify patients with systemic lupus erythematosus with cognitive impairment, Dr. Zahi Touma reported in a poster at the European Congress of Rheumatology.
In a consecutive series of 78 patients screened with the Montreal Cognitive Assessment (MoCA), the free, single-page test, which can be administered in about 10 minutes, showed a sensitivity of 69% and specificity of 68%, compared with the current standard, the Hopkins Verbal Learning Test-Revised, said Dr. Touma, a rheumatologist at the University of Toronto.
Other easy-to-use and quick screening tools, such as the Mini-Mental State Examination, had substantially worse performance in the study. He and his associates found a sensitivity of 21% and specificity of 91% using the Mini-Mental State exam. For screening, higher sensitivity is desirable so that fewer patients with potential cognitive impairment are missed, he noted.
“Ease of use and time needed for assessment as well as appropriate psychometric properties make the MoCA the preferential screening test for cognitive impairment in patients with SLE,” Dr. Touma said in his poster.
Cognitive impairment is very common among patients with systemic lupus erythematosus (SLE). In this study, Dr Touma found a 47% prevalence using MoCA. Cognitive impairment, however, often goes unidentified in SLE patients, likely because of lack of awareness among rheumatologists as well as the absence of a quick and easily administered screening tool, he said in a video interview.
Dr. Touma said he hopes that the apparent efficacy of an easy-to-use screening tool like MoCA will help boost appreciation for the high prevalence of cognitive impairment in SLE patients. He suggested that clinicians screen for cognitive impairment as soon as SLE is diagnosed and that they perform follow-up screening during subsequent patient encounters with SLE patients who initially present without cognitive impairment.
Dr. Touma had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
ROME – The Montreal Cognitive Assessment provides a quick and easy-to-use screening tool to identify patients with systemic lupus erythematosus with cognitive impairment, Dr. Zahi Touma reported in a poster at the European Congress of Rheumatology.
In a consecutive series of 78 patients screened with the Montreal Cognitive Assessment (MoCA), the free, single-page test, which can be administered in about 10 minutes, showed a sensitivity of 69% and specificity of 68%, compared with the current standard, the Hopkins Verbal Learning Test-Revised, said Dr. Touma, a rheumatologist at the University of Toronto.
Other easy-to-use and quick screening tools, such as the Mini-Mental State Examination, had substantially worse performance in the study. He and his associates found a sensitivity of 21% and specificity of 91% using the Mini-Mental State exam. For screening, higher sensitivity is desirable so that fewer patients with potential cognitive impairment are missed, he noted.
“Ease of use and time needed for assessment as well as appropriate psychometric properties make the MoCA the preferential screening test for cognitive impairment in patients with SLE,” Dr. Touma said in his poster.
Cognitive impairment is very common among patients with systemic lupus erythematosus (SLE). In this study, Dr Touma found a 47% prevalence using MoCA. Cognitive impairment, however, often goes unidentified in SLE patients, likely because of lack of awareness among rheumatologists as well as the absence of a quick and easily administered screening tool, he said in a video interview.
Dr. Touma said he hopes that the apparent efficacy of an easy-to-use screening tool like MoCA will help boost appreciation for the high prevalence of cognitive impairment in SLE patients. He suggested that clinicians screen for cognitive impairment as soon as SLE is diagnosed and that they perform follow-up screening during subsequent patient encounters with SLE patients who initially present without cognitive impairment.
Dr. Touma had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE EULAR 2015 CONGRESS
FDA Panel Gives Nod to Mepolizumab for Severe Asthma in Adults
GAITHERSBURG, MD. – The biologic agent mepolizumab was unanimously recommended for approval as a treatment for severe asthma in adults at a June 4 meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
All 14 members of the advisory panel agreed that the efficacy data provide substantial evidence of a clinically meaningful benefit of mepolizumab for the treatment of severe asthma in adults; 13 of the 14 members agreed that the data adequately demonstrated safety in adults. However, only four panel members recommended approval for adolescents aged 12-17 years, with the majority of panel members citing concerns that the low number of adolescents studied to date did not allow safety to be adequately evaluated in a younger population, especially for a medication that would be taken for many years – perhaps for a lifetime.
If approved by the FDA, the biologic agent would be available as a once-monthly treatment that is injected subcutaneously by a health care professional. Mepolizumab would be marketed by Glaxo Smith Kline under the trade name Nucala. Mepolizumab is a first-in-class humanized monoclonal antibody that targets interleukin-5, a glycoprotein cytokine that mediates production of eosinophils. Elevation of eosinophils in blood and tissue is associated with an increase in cytokines and other inflammatory molecules that can trigger or exacerbate airway inflammation in asthma. One other monoclonal antibody, the anti-IgE biologic omulizimab (Xolair), has been approved to treat asthma. Glaxo Smith Kline brought mepolizumab to the FDA for use as an add-on therapy for the small subset of asthma patients whose disease remains uncontrolled despite the optimal use of inhaled corticosteroids and additional therapies such as leukotriene inhibitors or theophylline. This population experiences more frequent asthma exacerbations, has more emergency department visits and hospitalizations, and uses higher doses of oral corticosteroids. Approximately 60% of those with severe asthma have marked eosinophilia.Panel members uniformly cited the efficacy data for adults with severe asthma; several panelists also remarked on the importance of developing more steroid-sparing alternatives for this population.
The panel endorsed neither efficacy nor safety for those aged 12-17 years, with 9 of the 14 panelists voting not to endorse efficacy and 13 members voting not to endorse safety findings. Mepolizumab’s efficacy, many panelists said, was not clearly established from the data presented, which drew from small numbers of adolescents enrolled in the studies.
Dr. David Au, acting director of Health Services Research and Development at Seattle’s VA Puget Sound Health Care system, observed that “adolescents are not small adults – their lungs continue to mature over time.” Many panelists, however, also called for ongoing study, noting the significant unmet need for steroid alternatives in the adolescent population.
Several panelists advocated postmarketing surveillance for long-term use, with particular attention to those with parasitic disease, to monitoring any sign of malignancy, and to tracking opportunistic diseases such as herpes zoster.
An early clinical trial of mepolizumab, conducted in 1999, failed to show benefit for an undifferentiated population of patients with moderate to severe asthma. However, independent research later identified marked eosinophilia as a factor associated with more frequent asthma exacerbations and a series of clinical trials begun in 2011 targeted patients with severe asthma and eosinophilic inflammation. A global program was initiated, with 12% of patients overall coming from the United States.
Pivotal phase 2b/3, double blind, placebo-controlled clinical trials included a dose-ranging study tracking asthma exacerbations enrolling 616 patients for 52 weeks.
The recommended dose of 100 mg subcutaneously every 4 weeks, as well as a 75-mg IV dose, was used for an additional 576 patients for 32 weeks, with the primary outcome measure being the number of asthma exacerbations. A final 24-week study of 135 patients with severe asthma measured the reduction in oral corticosteroid use, compared with placebo, as well as the number of asthma exacerbations. In all of the studies, patients’ asthma treatment was optimized according to standard of care before adding mepolizumab.
In each study and in pooled data, mepolizumab approximately halved the number of asthma exacerbations for study participants when compared with those using placebo. A 24-week corticosteroid-sparing study showed significant reduction in oral corticosteroid use, without loss of asthma control, for the mepolizumab group. Prespecified subgroup analyses were hampered because of low participation numbers for African Americans and adolescents, and because confidence intervals for these subgroups often ranged over 1, limiting interpretation of benefit results for these groups. The overall safety profile was good, with adverse event rates similar in the treatment and placebo arms. Headache and injection-site reactions were the most commonly reported adverse events but were similar between treatment and placebo arms. No episodes of anaphylaxis were reported, and neutralizing antibodies developed in one patient total across all studies. Ongoing open-label studies continue.The FDA’s independent biostatistical analysis showed clear evidence of efficacy for mepolizumab, with demonstrated consistent, statistically significant decreases of about one exacerbation per year, according to the agency.In its analysis, the agency observed a positive association between higher eosinophil count and mepolizumab treatment effect, meaning that those with higher eosinophil counts saw a greater benefit from mepolizumab, as measured by a reduction in exacerbations.
The number of deaths was balanced across treatment arms, though a larger number of respiratory-related deaths than expected was seen overall. This higher number of deaths may reflect the severity of asthma in the study population. The respiratory-related serious adverse events, according to the FDA, favor treatment over placebo.
No treatment-related cardiovascular risks were identified.
The FDA usually follows the recommendations of its advisory panels. The FDA panelists reported no relevant conflicts of interest.
GAITHERSBURG, MD. – The biologic agent mepolizumab was unanimously recommended for approval as a treatment for severe asthma in adults at a June 4 meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
All 14 members of the advisory panel agreed that the efficacy data provide substantial evidence of a clinically meaningful benefit of mepolizumab for the treatment of severe asthma in adults; 13 of the 14 members agreed that the data adequately demonstrated safety in adults. However, only four panel members recommended approval for adolescents aged 12-17 years, with the majority of panel members citing concerns that the low number of adolescents studied to date did not allow safety to be adequately evaluated in a younger population, especially for a medication that would be taken for many years – perhaps for a lifetime.
If approved by the FDA, the biologic agent would be available as a once-monthly treatment that is injected subcutaneously by a health care professional. Mepolizumab would be marketed by Glaxo Smith Kline under the trade name Nucala. Mepolizumab is a first-in-class humanized monoclonal antibody that targets interleukin-5, a glycoprotein cytokine that mediates production of eosinophils. Elevation of eosinophils in blood and tissue is associated with an increase in cytokines and other inflammatory molecules that can trigger or exacerbate airway inflammation in asthma. One other monoclonal antibody, the anti-IgE biologic omulizimab (Xolair), has been approved to treat asthma. Glaxo Smith Kline brought mepolizumab to the FDA for use as an add-on therapy for the small subset of asthma patients whose disease remains uncontrolled despite the optimal use of inhaled corticosteroids and additional therapies such as leukotriene inhibitors or theophylline. This population experiences more frequent asthma exacerbations, has more emergency department visits and hospitalizations, and uses higher doses of oral corticosteroids. Approximately 60% of those with severe asthma have marked eosinophilia.Panel members uniformly cited the efficacy data for adults with severe asthma; several panelists also remarked on the importance of developing more steroid-sparing alternatives for this population.
The panel endorsed neither efficacy nor safety for those aged 12-17 years, with 9 of the 14 panelists voting not to endorse efficacy and 13 members voting not to endorse safety findings. Mepolizumab’s efficacy, many panelists said, was not clearly established from the data presented, which drew from small numbers of adolescents enrolled in the studies.
Dr. David Au, acting director of Health Services Research and Development at Seattle’s VA Puget Sound Health Care system, observed that “adolescents are not small adults – their lungs continue to mature over time.” Many panelists, however, also called for ongoing study, noting the significant unmet need for steroid alternatives in the adolescent population.
Several panelists advocated postmarketing surveillance for long-term use, with particular attention to those with parasitic disease, to monitoring any sign of malignancy, and to tracking opportunistic diseases such as herpes zoster.
An early clinical trial of mepolizumab, conducted in 1999, failed to show benefit for an undifferentiated population of patients with moderate to severe asthma. However, independent research later identified marked eosinophilia as a factor associated with more frequent asthma exacerbations and a series of clinical trials begun in 2011 targeted patients with severe asthma and eosinophilic inflammation. A global program was initiated, with 12% of patients overall coming from the United States.
Pivotal phase 2b/3, double blind, placebo-controlled clinical trials included a dose-ranging study tracking asthma exacerbations enrolling 616 patients for 52 weeks.
The recommended dose of 100 mg subcutaneously every 4 weeks, as well as a 75-mg IV dose, was used for an additional 576 patients for 32 weeks, with the primary outcome measure being the number of asthma exacerbations. A final 24-week study of 135 patients with severe asthma measured the reduction in oral corticosteroid use, compared with placebo, as well as the number of asthma exacerbations. In all of the studies, patients’ asthma treatment was optimized according to standard of care before adding mepolizumab.
In each study and in pooled data, mepolizumab approximately halved the number of asthma exacerbations for study participants when compared with those using placebo. A 24-week corticosteroid-sparing study showed significant reduction in oral corticosteroid use, without loss of asthma control, for the mepolizumab group. Prespecified subgroup analyses were hampered because of low participation numbers for African Americans and adolescents, and because confidence intervals for these subgroups often ranged over 1, limiting interpretation of benefit results for these groups. The overall safety profile was good, with adverse event rates similar in the treatment and placebo arms. Headache and injection-site reactions were the most commonly reported adverse events but were similar between treatment and placebo arms. No episodes of anaphylaxis were reported, and neutralizing antibodies developed in one patient total across all studies. Ongoing open-label studies continue.The FDA’s independent biostatistical analysis showed clear evidence of efficacy for mepolizumab, with demonstrated consistent, statistically significant decreases of about one exacerbation per year, according to the agency.In its analysis, the agency observed a positive association between higher eosinophil count and mepolizumab treatment effect, meaning that those with higher eosinophil counts saw a greater benefit from mepolizumab, as measured by a reduction in exacerbations.
The number of deaths was balanced across treatment arms, though a larger number of respiratory-related deaths than expected was seen overall. This higher number of deaths may reflect the severity of asthma in the study population. The respiratory-related serious adverse events, according to the FDA, favor treatment over placebo.
No treatment-related cardiovascular risks were identified.
The FDA usually follows the recommendations of its advisory panels. The FDA panelists reported no relevant conflicts of interest.
GAITHERSBURG, MD. – The biologic agent mepolizumab was unanimously recommended for approval as a treatment for severe asthma in adults at a June 4 meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
All 14 members of the advisory panel agreed that the efficacy data provide substantial evidence of a clinically meaningful benefit of mepolizumab for the treatment of severe asthma in adults; 13 of the 14 members agreed that the data adequately demonstrated safety in adults. However, only four panel members recommended approval for adolescents aged 12-17 years, with the majority of panel members citing concerns that the low number of adolescents studied to date did not allow safety to be adequately evaluated in a younger population, especially for a medication that would be taken for many years – perhaps for a lifetime.
If approved by the FDA, the biologic agent would be available as a once-monthly treatment that is injected subcutaneously by a health care professional. Mepolizumab would be marketed by Glaxo Smith Kline under the trade name Nucala. Mepolizumab is a first-in-class humanized monoclonal antibody that targets interleukin-5, a glycoprotein cytokine that mediates production of eosinophils. Elevation of eosinophils in blood and tissue is associated with an increase in cytokines and other inflammatory molecules that can trigger or exacerbate airway inflammation in asthma. One other monoclonal antibody, the anti-IgE biologic omulizimab (Xolair), has been approved to treat asthma. Glaxo Smith Kline brought mepolizumab to the FDA for use as an add-on therapy for the small subset of asthma patients whose disease remains uncontrolled despite the optimal use of inhaled corticosteroids and additional therapies such as leukotriene inhibitors or theophylline. This population experiences more frequent asthma exacerbations, has more emergency department visits and hospitalizations, and uses higher doses of oral corticosteroids. Approximately 60% of those with severe asthma have marked eosinophilia.Panel members uniformly cited the efficacy data for adults with severe asthma; several panelists also remarked on the importance of developing more steroid-sparing alternatives for this population.
The panel endorsed neither efficacy nor safety for those aged 12-17 years, with 9 of the 14 panelists voting not to endorse efficacy and 13 members voting not to endorse safety findings. Mepolizumab’s efficacy, many panelists said, was not clearly established from the data presented, which drew from small numbers of adolescents enrolled in the studies.
Dr. David Au, acting director of Health Services Research and Development at Seattle’s VA Puget Sound Health Care system, observed that “adolescents are not small adults – their lungs continue to mature over time.” Many panelists, however, also called for ongoing study, noting the significant unmet need for steroid alternatives in the adolescent population.
Several panelists advocated postmarketing surveillance for long-term use, with particular attention to those with parasitic disease, to monitoring any sign of malignancy, and to tracking opportunistic diseases such as herpes zoster.
An early clinical trial of mepolizumab, conducted in 1999, failed to show benefit for an undifferentiated population of patients with moderate to severe asthma. However, independent research later identified marked eosinophilia as a factor associated with more frequent asthma exacerbations and a series of clinical trials begun in 2011 targeted patients with severe asthma and eosinophilic inflammation. A global program was initiated, with 12% of patients overall coming from the United States.
Pivotal phase 2b/3, double blind, placebo-controlled clinical trials included a dose-ranging study tracking asthma exacerbations enrolling 616 patients for 52 weeks.
The recommended dose of 100 mg subcutaneously every 4 weeks, as well as a 75-mg IV dose, was used for an additional 576 patients for 32 weeks, with the primary outcome measure being the number of asthma exacerbations. A final 24-week study of 135 patients with severe asthma measured the reduction in oral corticosteroid use, compared with placebo, as well as the number of asthma exacerbations. In all of the studies, patients’ asthma treatment was optimized according to standard of care before adding mepolizumab.
In each study and in pooled data, mepolizumab approximately halved the number of asthma exacerbations for study participants when compared with those using placebo. A 24-week corticosteroid-sparing study showed significant reduction in oral corticosteroid use, without loss of asthma control, for the mepolizumab group. Prespecified subgroup analyses were hampered because of low participation numbers for African Americans and adolescents, and because confidence intervals for these subgroups often ranged over 1, limiting interpretation of benefit results for these groups. The overall safety profile was good, with adverse event rates similar in the treatment and placebo arms. Headache and injection-site reactions were the most commonly reported adverse events but were similar between treatment and placebo arms. No episodes of anaphylaxis were reported, and neutralizing antibodies developed in one patient total across all studies. Ongoing open-label studies continue.The FDA’s independent biostatistical analysis showed clear evidence of efficacy for mepolizumab, with demonstrated consistent, statistically significant decreases of about one exacerbation per year, according to the agency.In its analysis, the agency observed a positive association between higher eosinophil count and mepolizumab treatment effect, meaning that those with higher eosinophil counts saw a greater benefit from mepolizumab, as measured by a reduction in exacerbations.
The number of deaths was balanced across treatment arms, though a larger number of respiratory-related deaths than expected was seen overall. This higher number of deaths may reflect the severity of asthma in the study population. The respiratory-related serious adverse events, according to the FDA, favor treatment over placebo.
No treatment-related cardiovascular risks were identified.
The FDA usually follows the recommendations of its advisory panels. The FDA panelists reported no relevant conflicts of interest.
AT AN FDA ADVISORY COMMITTEE MEETING
FDA panel gives nod to mepolizumab for severe asthma in adults
GAITHERSBURG, MD. – The biologic agent mepolizumab was unanimously recommended for approval as a treatment for severe asthma in adults at a June 4 meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
All 14 members of the advisory panel agreed that the efficacy data provide substantial evidence of a clinically meaningful benefit of mepolizumab for the treatment of severe asthma in adults; 13 of the 14 members agreed that the data adequately demonstrated safety in adults. However, only four panel members recommended approval for adolescents aged 12-17 years, with the majority of panel members citing concerns that the low number of adolescents studied to date did not allow safety to be adequately evaluated in a younger population, especially for a medication that would be taken for many years – perhaps for a lifetime.
If approved by the FDA, the biologic agent would be available as a once-monthly treatment that is injected subcutaneously by a health care professional. Mepolizumab would be marketed by Glaxo Smith Kline under the trade name Nucala. Mepolizumab is a first-in-class humanized monoclonal antibody that targets interleukin-5, a glycoprotein cytokine that mediates production of eosinophils. Elevation of eosinophils in blood and tissue is associated with an increase in cytokines and other inflammatory molecules that can trigger or exacerbate airway inflammation in asthma. One other monoclonal antibody, the anti-IgE biologic omulizimab (Xolair), has been approved to treat asthma. Glaxo Smith Kline brought mepolizumab to the FDA for use as an add-on therapy for the small subset of asthma patients whose disease remains uncontrolled despite the optimal use of inhaled corticosteroids and additional therapies such as leukotriene inhibitors or theophylline. This population experiences more frequent asthma exacerbations, has more emergency department visits and hospitalizations, and uses higher doses of oral corticosteroids. Approximately 60% of those with severe asthma have marked eosinophilia.Panel members uniformly cited the efficacy data for adults with severe asthma; several panelists also remarked on the importance of developing more steroid-sparing alternatives for this population.
The panel endorsed neither efficacy nor safety for those aged 12-17 years, with 9 of the 14 panelists voting not to endorse efficacy and 13 members voting not to endorse safety findings. Mepolizumab’s efficacy, many panelists said, was not clearly established from the data presented, which drew from small numbers of adolescents enrolled in the studies.
Dr. David Au, acting director of Health Services Research and Development at Seattle’s VA Puget Sound Health Care system, observed that “adolescents are not small adults – their lungs continue to mature over time.” Many panelists, however, also called for ongoing study, noting the significant unmet need for steroid alternatives in the adolescent population.
Several panelists advocated postmarketing surveillance for long-term use, with particular attention to those with parasitic disease, to monitoring any sign of malignancy, and to tracking opportunistic diseases such as herpes zoster.
An early clinical trial of mepolizumab, conducted in 1999, failed to show benefit for an undifferentiated population of patients with moderate to severe asthma. However, independent research later identified marked eosinophilia as a factor associated with more frequent asthma exacerbations and a series of clinical trials begun in 2011 targeted patients with severe asthma and eosinophilic inflammation. A global program was initiated, with 12% of patients overall coming from the United States.
Pivotal phase 2b/3, double blind, placebo-controlled clinical trials included a dose-ranging study tracking asthma exacerbations enrolling 616 patients for 52 weeks.
The recommended dose of 100 mg subcutaneously every 4 weeks, as well as a 75-mg IV dose, was used for an additional 576 patients for 32 weeks, with the primary outcome measure being the number of asthma exacerbations. A final 24-week study of 135 patients with severe asthma measured the reduction in oral corticosteroid use, compared with placebo, as well as the number of asthma exacerbations. In all of the studies, patients’ asthma treatment was optimized according to standard of care before adding mepolizumab.
In each study and in pooled data, mepolizumab approximately halved the number of asthma exacerbations for study participants when compared with those using placebo. A 24-week corticosteroid-sparing study showed significant reduction in oral corticosteroid use, without loss of asthma control, for the mepolizumab group. Prespecified subgroup analyses were hampered because of low participation numbers for African Americans and adolescents, and because confidence intervals for these subgroups often ranged over 1, limiting interpretation of benefit results for these groups. The overall safety profile was good, with adverse event rates similar in the treatment and placebo arms. Headache and injection-site reactions were the most commonly reported adverse events but were similar between treatment and placebo arms. No episodes of anaphylaxis were reported, and neutralizing antibodies developed in one patient total across all studies. Ongoing open-label studies continue.The FDA’s independent biostatistical analysis showed clear evidence of efficacy for mepolizumab, with demonstrated consistent, statistically significant decreases of about one exacerbation per year, according to the agency.In its analysis, the agency observed a positive association between higher eosinophil count and mepolizumab treatment effect, meaning that those with higher eosinophil counts saw a greater benefit from mepolizumab, as measured by a reduction in exacerbations.
The number of deaths was balanced across treatment arms, though a larger number of respiratory-related deaths than expected was seen overall. This higher number of deaths may reflect the severity of asthma in the study population. The respiratory-related serious adverse events, according to the FDA, favor treatment over placebo.
No treatment-related cardiovascular risks were identified.
The FDA usually follows the recommendations of its advisory panels. The FDA panelists reported no relevant conflicts of interest.
GAITHERSBURG, MD. – The biologic agent mepolizumab was unanimously recommended for approval as a treatment for severe asthma in adults at a June 4 meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
All 14 members of the advisory panel agreed that the efficacy data provide substantial evidence of a clinically meaningful benefit of mepolizumab for the treatment of severe asthma in adults; 13 of the 14 members agreed that the data adequately demonstrated safety in adults. However, only four panel members recommended approval for adolescents aged 12-17 years, with the majority of panel members citing concerns that the low number of adolescents studied to date did not allow safety to be adequately evaluated in a younger population, especially for a medication that would be taken for many years – perhaps for a lifetime.
If approved by the FDA, the biologic agent would be available as a once-monthly treatment that is injected subcutaneously by a health care professional. Mepolizumab would be marketed by Glaxo Smith Kline under the trade name Nucala. Mepolizumab is a first-in-class humanized monoclonal antibody that targets interleukin-5, a glycoprotein cytokine that mediates production of eosinophils. Elevation of eosinophils in blood and tissue is associated with an increase in cytokines and other inflammatory molecules that can trigger or exacerbate airway inflammation in asthma. One other monoclonal antibody, the anti-IgE biologic omulizimab (Xolair), has been approved to treat asthma. Glaxo Smith Kline brought mepolizumab to the FDA for use as an add-on therapy for the small subset of asthma patients whose disease remains uncontrolled despite the optimal use of inhaled corticosteroids and additional therapies such as leukotriene inhibitors or theophylline. This population experiences more frequent asthma exacerbations, has more emergency department visits and hospitalizations, and uses higher doses of oral corticosteroids. Approximately 60% of those with severe asthma have marked eosinophilia.Panel members uniformly cited the efficacy data for adults with severe asthma; several panelists also remarked on the importance of developing more steroid-sparing alternatives for this population.
The panel endorsed neither efficacy nor safety for those aged 12-17 years, with 9 of the 14 panelists voting not to endorse efficacy and 13 members voting not to endorse safety findings. Mepolizumab’s efficacy, many panelists said, was not clearly established from the data presented, which drew from small numbers of adolescents enrolled in the studies.
Dr. David Au, acting director of Health Services Research and Development at Seattle’s VA Puget Sound Health Care system, observed that “adolescents are not small adults – their lungs continue to mature over time.” Many panelists, however, also called for ongoing study, noting the significant unmet need for steroid alternatives in the adolescent population.
Several panelists advocated postmarketing surveillance for long-term use, with particular attention to those with parasitic disease, to monitoring any sign of malignancy, and to tracking opportunistic diseases such as herpes zoster.
An early clinical trial of mepolizumab, conducted in 1999, failed to show benefit for an undifferentiated population of patients with moderate to severe asthma. However, independent research later identified marked eosinophilia as a factor associated with more frequent asthma exacerbations and a series of clinical trials begun in 2011 targeted patients with severe asthma and eosinophilic inflammation. A global program was initiated, with 12% of patients overall coming from the United States.
Pivotal phase 2b/3, double blind, placebo-controlled clinical trials included a dose-ranging study tracking asthma exacerbations enrolling 616 patients for 52 weeks.
The recommended dose of 100 mg subcutaneously every 4 weeks, as well as a 75-mg IV dose, was used for an additional 576 patients for 32 weeks, with the primary outcome measure being the number of asthma exacerbations. A final 24-week study of 135 patients with severe asthma measured the reduction in oral corticosteroid use, compared with placebo, as well as the number of asthma exacerbations. In all of the studies, patients’ asthma treatment was optimized according to standard of care before adding mepolizumab.
In each study and in pooled data, mepolizumab approximately halved the number of asthma exacerbations for study participants when compared with those using placebo. A 24-week corticosteroid-sparing study showed significant reduction in oral corticosteroid use, without loss of asthma control, for the mepolizumab group. Prespecified subgroup analyses were hampered because of low participation numbers for African Americans and adolescents, and because confidence intervals for these subgroups often ranged over 1, limiting interpretation of benefit results for these groups. The overall safety profile was good, with adverse event rates similar in the treatment and placebo arms. Headache and injection-site reactions were the most commonly reported adverse events but were similar between treatment and placebo arms. No episodes of anaphylaxis were reported, and neutralizing antibodies developed in one patient total across all studies. Ongoing open-label studies continue.The FDA’s independent biostatistical analysis showed clear evidence of efficacy for mepolizumab, with demonstrated consistent, statistically significant decreases of about one exacerbation per year, according to the agency.In its analysis, the agency observed a positive association between higher eosinophil count and mepolizumab treatment effect, meaning that those with higher eosinophil counts saw a greater benefit from mepolizumab, as measured by a reduction in exacerbations.
The number of deaths was balanced across treatment arms, though a larger number of respiratory-related deaths than expected was seen overall. This higher number of deaths may reflect the severity of asthma in the study population. The respiratory-related serious adverse events, according to the FDA, favor treatment over placebo.
No treatment-related cardiovascular risks were identified.
The FDA usually follows the recommendations of its advisory panels. The FDA panelists reported no relevant conflicts of interest.
GAITHERSBURG, MD. – The biologic agent mepolizumab was unanimously recommended for approval as a treatment for severe asthma in adults at a June 4 meeting of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
All 14 members of the advisory panel agreed that the efficacy data provide substantial evidence of a clinically meaningful benefit of mepolizumab for the treatment of severe asthma in adults; 13 of the 14 members agreed that the data adequately demonstrated safety in adults. However, only four panel members recommended approval for adolescents aged 12-17 years, with the majority of panel members citing concerns that the low number of adolescents studied to date did not allow safety to be adequately evaluated in a younger population, especially for a medication that would be taken for many years – perhaps for a lifetime.
If approved by the FDA, the biologic agent would be available as a once-monthly treatment that is injected subcutaneously by a health care professional. Mepolizumab would be marketed by Glaxo Smith Kline under the trade name Nucala. Mepolizumab is a first-in-class humanized monoclonal antibody that targets interleukin-5, a glycoprotein cytokine that mediates production of eosinophils. Elevation of eosinophils in blood and tissue is associated with an increase in cytokines and other inflammatory molecules that can trigger or exacerbate airway inflammation in asthma. One other monoclonal antibody, the anti-IgE biologic omulizimab (Xolair), has been approved to treat asthma. Glaxo Smith Kline brought mepolizumab to the FDA for use as an add-on therapy for the small subset of asthma patients whose disease remains uncontrolled despite the optimal use of inhaled corticosteroids and additional therapies such as leukotriene inhibitors or theophylline. This population experiences more frequent asthma exacerbations, has more emergency department visits and hospitalizations, and uses higher doses of oral corticosteroids. Approximately 60% of those with severe asthma have marked eosinophilia.Panel members uniformly cited the efficacy data for adults with severe asthma; several panelists also remarked on the importance of developing more steroid-sparing alternatives for this population.
The panel endorsed neither efficacy nor safety for those aged 12-17 years, with 9 of the 14 panelists voting not to endorse efficacy and 13 members voting not to endorse safety findings. Mepolizumab’s efficacy, many panelists said, was not clearly established from the data presented, which drew from small numbers of adolescents enrolled in the studies.
Dr. David Au, acting director of Health Services Research and Development at Seattle’s VA Puget Sound Health Care system, observed that “adolescents are not small adults – their lungs continue to mature over time.” Many panelists, however, also called for ongoing study, noting the significant unmet need for steroid alternatives in the adolescent population.
Several panelists advocated postmarketing surveillance for long-term use, with particular attention to those with parasitic disease, to monitoring any sign of malignancy, and to tracking opportunistic diseases such as herpes zoster.
An early clinical trial of mepolizumab, conducted in 1999, failed to show benefit for an undifferentiated population of patients with moderate to severe asthma. However, independent research later identified marked eosinophilia as a factor associated with more frequent asthma exacerbations and a series of clinical trials begun in 2011 targeted patients with severe asthma and eosinophilic inflammation. A global program was initiated, with 12% of patients overall coming from the United States.
Pivotal phase 2b/3, double blind, placebo-controlled clinical trials included a dose-ranging study tracking asthma exacerbations enrolling 616 patients for 52 weeks.
The recommended dose of 100 mg subcutaneously every 4 weeks, as well as a 75-mg IV dose, was used for an additional 576 patients for 32 weeks, with the primary outcome measure being the number of asthma exacerbations. A final 24-week study of 135 patients with severe asthma measured the reduction in oral corticosteroid use, compared with placebo, as well as the number of asthma exacerbations. In all of the studies, patients’ asthma treatment was optimized according to standard of care before adding mepolizumab.
In each study and in pooled data, mepolizumab approximately halved the number of asthma exacerbations for study participants when compared with those using placebo. A 24-week corticosteroid-sparing study showed significant reduction in oral corticosteroid use, without loss of asthma control, for the mepolizumab group. Prespecified subgroup analyses were hampered because of low participation numbers for African Americans and adolescents, and because confidence intervals for these subgroups often ranged over 1, limiting interpretation of benefit results for these groups. The overall safety profile was good, with adverse event rates similar in the treatment and placebo arms. Headache and injection-site reactions were the most commonly reported adverse events but were similar between treatment and placebo arms. No episodes of anaphylaxis were reported, and neutralizing antibodies developed in one patient total across all studies. Ongoing open-label studies continue.The FDA’s independent biostatistical analysis showed clear evidence of efficacy for mepolizumab, with demonstrated consistent, statistically significant decreases of about one exacerbation per year, according to the agency.In its analysis, the agency observed a positive association between higher eosinophil count and mepolizumab treatment effect, meaning that those with higher eosinophil counts saw a greater benefit from mepolizumab, as measured by a reduction in exacerbations.
The number of deaths was balanced across treatment arms, though a larger number of respiratory-related deaths than expected was seen overall. This higher number of deaths may reflect the severity of asthma in the study population. The respiratory-related serious adverse events, according to the FDA, favor treatment over placebo.
No treatment-related cardiovascular risks were identified.
The FDA usually follows the recommendations of its advisory panels. The FDA panelists reported no relevant conflicts of interest.
AT AN FDA ADVISORY COMMITTEE MEETING
Mepolizumab reduces asthma exacerbations more in the elderly
DENVER – The rate of asthma exacerbations was reduced more among elderly patients than among younger patients treated with mepolizumab vs. placebo as part of the Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma (MENSA) trial, according to a post hoc analysis of the trial data.
The analysis also demonstrated that mepolizumab improved quality of life, compared with standard care, in both older and younger patients, although little differentiation was seen with respect to asthma control, Dr. Hector Ortega reported in a poster at an international conference of the American Thoracic Society.
A 76% greater reduction in clinically significant exacerbations was seen in 54 mepolizumab-treated patients aged 65 years and older in the trial, compared with 26 in that age group who received placebo (mean exacerbation rate per year, 0.92 vs. 1.65); a 44% greater reduction was seen in 331 mepolizumab-treated patients under age 65 years, compared with 165 in that age group who received placebo (mean exacerbation rate per year, 0.42 vs. 1.78), said Dr. Ortega, medical director at GlaxoSmithKline, Research Triangle Park, N.C.
The adjusted mean difference vs. placebo in change in St. George’s Respiratory Questionnaire scores from baseline to 32 weeks was -4.5 in the older patients, and -7.3 in the younger patients, and the adjusted mean difference vs. placebo in change in Asthma Control Questionnaire scores from baseline to 32 weeks was -0.1 and -0.5 in the older and younger patients, respectively, he noted.
The older patients, as expected, suffered more frequently from comorbidities, and this may have been accentuated by the chronic use of inhaled and oral corticosteroids in the older patients.
However, comorbidities had no impact on the response to treatment, and the safety profile of mepolizumab was similar to placebo in both age groups, he said.
Patients in the multicenter, randomized, placebo-controlled MENSA trial received high dose inhaled corticosteroids plus at least one additional controller, had a history of frequent exacerbations and a predefined eosinophilic threshold, and were randomized to receive add-on therapy with either 75 mg of intravenous mepolizumab, 100 mg subcutaneous mepolizumab, or corresponding placebo every 4 weeks for 32 weeks. In the primary planned analysis, the responses to the two doses of mepolizumab were similar, but efficacy in older patients had not been previously reported.
The MENSA trial and post hoc analysis were funded by GSK. Dr. Ortega is employed by GSK.
DENVER – The rate of asthma exacerbations was reduced more among elderly patients than among younger patients treated with mepolizumab vs. placebo as part of the Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma (MENSA) trial, according to a post hoc analysis of the trial data.
The analysis also demonstrated that mepolizumab improved quality of life, compared with standard care, in both older and younger patients, although little differentiation was seen with respect to asthma control, Dr. Hector Ortega reported in a poster at an international conference of the American Thoracic Society.
A 76% greater reduction in clinically significant exacerbations was seen in 54 mepolizumab-treated patients aged 65 years and older in the trial, compared with 26 in that age group who received placebo (mean exacerbation rate per year, 0.92 vs. 1.65); a 44% greater reduction was seen in 331 mepolizumab-treated patients under age 65 years, compared with 165 in that age group who received placebo (mean exacerbation rate per year, 0.42 vs. 1.78), said Dr. Ortega, medical director at GlaxoSmithKline, Research Triangle Park, N.C.
The adjusted mean difference vs. placebo in change in St. George’s Respiratory Questionnaire scores from baseline to 32 weeks was -4.5 in the older patients, and -7.3 in the younger patients, and the adjusted mean difference vs. placebo in change in Asthma Control Questionnaire scores from baseline to 32 weeks was -0.1 and -0.5 in the older and younger patients, respectively, he noted.
The older patients, as expected, suffered more frequently from comorbidities, and this may have been accentuated by the chronic use of inhaled and oral corticosteroids in the older patients.
However, comorbidities had no impact on the response to treatment, and the safety profile of mepolizumab was similar to placebo in both age groups, he said.
Patients in the multicenter, randomized, placebo-controlled MENSA trial received high dose inhaled corticosteroids plus at least one additional controller, had a history of frequent exacerbations and a predefined eosinophilic threshold, and were randomized to receive add-on therapy with either 75 mg of intravenous mepolizumab, 100 mg subcutaneous mepolizumab, or corresponding placebo every 4 weeks for 32 weeks. In the primary planned analysis, the responses to the two doses of mepolizumab were similar, but efficacy in older patients had not been previously reported.
The MENSA trial and post hoc analysis were funded by GSK. Dr. Ortega is employed by GSK.
DENVER – The rate of asthma exacerbations was reduced more among elderly patients than among younger patients treated with mepolizumab vs. placebo as part of the Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma (MENSA) trial, according to a post hoc analysis of the trial data.
The analysis also demonstrated that mepolizumab improved quality of life, compared with standard care, in both older and younger patients, although little differentiation was seen with respect to asthma control, Dr. Hector Ortega reported in a poster at an international conference of the American Thoracic Society.
A 76% greater reduction in clinically significant exacerbations was seen in 54 mepolizumab-treated patients aged 65 years and older in the trial, compared with 26 in that age group who received placebo (mean exacerbation rate per year, 0.92 vs. 1.65); a 44% greater reduction was seen in 331 mepolizumab-treated patients under age 65 years, compared with 165 in that age group who received placebo (mean exacerbation rate per year, 0.42 vs. 1.78), said Dr. Ortega, medical director at GlaxoSmithKline, Research Triangle Park, N.C.
The adjusted mean difference vs. placebo in change in St. George’s Respiratory Questionnaire scores from baseline to 32 weeks was -4.5 in the older patients, and -7.3 in the younger patients, and the adjusted mean difference vs. placebo in change in Asthma Control Questionnaire scores from baseline to 32 weeks was -0.1 and -0.5 in the older and younger patients, respectively, he noted.
The older patients, as expected, suffered more frequently from comorbidities, and this may have been accentuated by the chronic use of inhaled and oral corticosteroids in the older patients.
However, comorbidities had no impact on the response to treatment, and the safety profile of mepolizumab was similar to placebo in both age groups, he said.
Patients in the multicenter, randomized, placebo-controlled MENSA trial received high dose inhaled corticosteroids plus at least one additional controller, had a history of frequent exacerbations and a predefined eosinophilic threshold, and were randomized to receive add-on therapy with either 75 mg of intravenous mepolizumab, 100 mg subcutaneous mepolizumab, or corresponding placebo every 4 weeks for 32 weeks. In the primary planned analysis, the responses to the two doses of mepolizumab were similar, but efficacy in older patients had not been previously reported.
The MENSA trial and post hoc analysis were funded by GSK. Dr. Ortega is employed by GSK.
AT ATS 2015
Key clinical point: The rate of asthma exacerbations was reduced more among elderly patients than among younger patients treated with mepolizumab vs. placebo.
Major finding: The mean reduction in the exacerbation rate with mepolizumab vs. placebo was 76% for those aged 65 and older vs. 44% in those under age 65.
Data source: A post hoc analysis of data from 576 patients in the multicenter, randomized, placebo-controlled MENSA trial.
Disclosures: The trial and post hoc analysis were funded by GSK. Dr. Ortega is employed by GSK.
ACIP Recommends MenB Vaccination for At-risk Groups
The Advisory Committee on Immunization Practices (ACIP) recommends the serogroup B meningitis (MenB) vaccine for individuals at increased risk for meningococcal disease aged 10 years or older, according to a report published in the June 12 Morbidity and Mortality Weekly Report.
The guideline recommends the MenB vaccine for persons with persistent complement component deficiencies, persons with anatomic or functional asplenia, microbiologists regularly exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.
The recommendations were revised following recent Food and Drug Administration approval of two MenB vaccines: Trumenba, approved in October 2014, and Bexsero, approved in January 2015. Both MenB vaccines are approved for use in people aged 10-25 years, but are not currently recommended for routine use in first-year college students living in residence halls, in adolescents, or in military recruits.
MenB vaccine should be administered as either a three-dose series of Trumenba or a two-dose series of Bexsero. The same vaccine product should be used for all doses, according to ACIP.
“Recommendations for broader use of MenB vaccines in adolescents and college students will be considered separately by the ACIP,” the report said.
Read more in this week’s MMWR.
The Advisory Committee on Immunization Practices (ACIP) recommends the serogroup B meningitis (MenB) vaccine for individuals at increased risk for meningococcal disease aged 10 years or older, according to a report published in the June 12 Morbidity and Mortality Weekly Report.
The guideline recommends the MenB vaccine for persons with persistent complement component deficiencies, persons with anatomic or functional asplenia, microbiologists regularly exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.
The recommendations were revised following recent Food and Drug Administration approval of two MenB vaccines: Trumenba, approved in October 2014, and Bexsero, approved in January 2015. Both MenB vaccines are approved for use in people aged 10-25 years, but are not currently recommended for routine use in first-year college students living in residence halls, in adolescents, or in military recruits.
MenB vaccine should be administered as either a three-dose series of Trumenba or a two-dose series of Bexsero. The same vaccine product should be used for all doses, according to ACIP.
“Recommendations for broader use of MenB vaccines in adolescents and college students will be considered separately by the ACIP,” the report said.
Read more in this week’s MMWR.
The Advisory Committee on Immunization Practices (ACIP) recommends the serogroup B meningitis (MenB) vaccine for individuals at increased risk for meningococcal disease aged 10 years or older, according to a report published in the June 12 Morbidity and Mortality Weekly Report.
The guideline recommends the MenB vaccine for persons with persistent complement component deficiencies, persons with anatomic or functional asplenia, microbiologists regularly exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.
The recommendations were revised following recent Food and Drug Administration approval of two MenB vaccines: Trumenba, approved in October 2014, and Bexsero, approved in January 2015. Both MenB vaccines are approved for use in people aged 10-25 years, but are not currently recommended for routine use in first-year college students living in residence halls, in adolescents, or in military recruits.
MenB vaccine should be administered as either a three-dose series of Trumenba or a two-dose series of Bexsero. The same vaccine product should be used for all doses, according to ACIP.
“Recommendations for broader use of MenB vaccines in adolescents and college students will be considered separately by the ACIP,” the report said.
Read more in this week’s MMWR.
Single HPV Vaccine Dose Appears as Effective as Full Course
One or two doses of the bivalent human papillomavirus vaccine provide a level of protection against cervical HPV infection similar to that of the full three-dose schedule, according to post hoc analysis from two phase III, double-blind, randomized controlled trials with 4 years follow-up.
Data from the Costa Rica Vaccine Trial and PATRICIA trials of the HPV-16/18 vaccine, tested in 24,055 women, showed vaccine efficacy against HPV-16/18 infection for one dose was 85.7%, two doses, 76%, and three doses, 77%, according to a paper published online June 10 in Lancet Oncology.
The analysis also showed that vaccine efficacy was significantly improved in women who received two doses when the second dose was received 6 months after the first, rather than 1 month (Lancet Oncology 2015, June 10 [doi: dx.doi.org/10.1016/ S1470-2045(15)70199-3]). “If one-dose HPV vaccine administration provides strong protection against HPV-16/18 for the long term, this approach might be what is necessary to overcome the barriers prohibiting vaccine uptake in many world regions,” wrote Aimée R Kreimer, Ph.D., of the National Cancer Institute, Bethesda, Md., and her associates. “These data strongly argue for a direct assessment of one-dose efficacy of the HPV-16/18 vaccine.”
Because these results “were replicated in a cohort of women naive to HPV-16/18 infection at the time of vaccination” these results are “probably relevant to girls in the preferred age range for HPV vaccination (i.e., 11-12 years),” they said..
The study and included trials were supported by the U.S. National Cancer Institute, National Institutes of Health Office of Research on Women’s Health, and Ministry of Health of Costa Rica (CVT) and GlaxoSmithKline Biologicals SA (PATRICIA). Some authors disclosed ties with GlaxoSmithKline.
If human papillomavirus vaccines could be delivered as one dose, while retaining their efficacy against the most oncogenic HPV types 16 and 18, we could substantially decrease the global burden of cervical cancer, particularly in resource-poor settings where an annual vaccination program might be difficult to sustain.
Importantly, strong vaccine protection irrespective of dose also was shown for persistent infection, a recommended outcome measure for assessment of protection in prophylactic HPV vaccine trials.
Dr. Julia M.L. Brotherton is from the National HPV Vaccination Program Register, Melbourne. These comments were excerpted from an editorial (Lancet Oncology 2015, June 10 [http://dx.doi.org/10.1016/S1470-2045(15)70253-6]). She declared research grants from bioCSL/Merck.
If human papillomavirus vaccines could be delivered as one dose, while retaining their efficacy against the most oncogenic HPV types 16 and 18, we could substantially decrease the global burden of cervical cancer, particularly in resource-poor settings where an annual vaccination program might be difficult to sustain.
Importantly, strong vaccine protection irrespective of dose also was shown for persistent infection, a recommended outcome measure for assessment of protection in prophylactic HPV vaccine trials.
Dr. Julia M.L. Brotherton is from the National HPV Vaccination Program Register, Melbourne. These comments were excerpted from an editorial (Lancet Oncology 2015, June 10 [http://dx.doi.org/10.1016/S1470-2045(15)70253-6]). She declared research grants from bioCSL/Merck.
If human papillomavirus vaccines could be delivered as one dose, while retaining their efficacy against the most oncogenic HPV types 16 and 18, we could substantially decrease the global burden of cervical cancer, particularly in resource-poor settings where an annual vaccination program might be difficult to sustain.
Importantly, strong vaccine protection irrespective of dose also was shown for persistent infection, a recommended outcome measure for assessment of protection in prophylactic HPV vaccine trials.
Dr. Julia M.L. Brotherton is from the National HPV Vaccination Program Register, Melbourne. These comments were excerpted from an editorial (Lancet Oncology 2015, June 10 [http://dx.doi.org/10.1016/S1470-2045(15)70253-6]). She declared research grants from bioCSL/Merck.
One or two doses of the bivalent human papillomavirus vaccine provide a level of protection against cervical HPV infection similar to that of the full three-dose schedule, according to post hoc analysis from two phase III, double-blind, randomized controlled trials with 4 years follow-up.
Data from the Costa Rica Vaccine Trial and PATRICIA trials of the HPV-16/18 vaccine, tested in 24,055 women, showed vaccine efficacy against HPV-16/18 infection for one dose was 85.7%, two doses, 76%, and three doses, 77%, according to a paper published online June 10 in Lancet Oncology.
The analysis also showed that vaccine efficacy was significantly improved in women who received two doses when the second dose was received 6 months after the first, rather than 1 month (Lancet Oncology 2015, June 10 [doi: dx.doi.org/10.1016/ S1470-2045(15)70199-3]). “If one-dose HPV vaccine administration provides strong protection against HPV-16/18 for the long term, this approach might be what is necessary to overcome the barriers prohibiting vaccine uptake in many world regions,” wrote Aimée R Kreimer, Ph.D., of the National Cancer Institute, Bethesda, Md., and her associates. “These data strongly argue for a direct assessment of one-dose efficacy of the HPV-16/18 vaccine.”
Because these results “were replicated in a cohort of women naive to HPV-16/18 infection at the time of vaccination” these results are “probably relevant to girls in the preferred age range for HPV vaccination (i.e., 11-12 years),” they said..
The study and included trials were supported by the U.S. National Cancer Institute, National Institutes of Health Office of Research on Women’s Health, and Ministry of Health of Costa Rica (CVT) and GlaxoSmithKline Biologicals SA (PATRICIA). Some authors disclosed ties with GlaxoSmithKline.
One or two doses of the bivalent human papillomavirus vaccine provide a level of protection against cervical HPV infection similar to that of the full three-dose schedule, according to post hoc analysis from two phase III, double-blind, randomized controlled trials with 4 years follow-up.
Data from the Costa Rica Vaccine Trial and PATRICIA trials of the HPV-16/18 vaccine, tested in 24,055 women, showed vaccine efficacy against HPV-16/18 infection for one dose was 85.7%, two doses, 76%, and three doses, 77%, according to a paper published online June 10 in Lancet Oncology.
The analysis also showed that vaccine efficacy was significantly improved in women who received two doses when the second dose was received 6 months after the first, rather than 1 month (Lancet Oncology 2015, June 10 [doi: dx.doi.org/10.1016/ S1470-2045(15)70199-3]). “If one-dose HPV vaccine administration provides strong protection against HPV-16/18 for the long term, this approach might be what is necessary to overcome the barriers prohibiting vaccine uptake in many world regions,” wrote Aimée R Kreimer, Ph.D., of the National Cancer Institute, Bethesda, Md., and her associates. “These data strongly argue for a direct assessment of one-dose efficacy of the HPV-16/18 vaccine.”
Because these results “were replicated in a cohort of women naive to HPV-16/18 infection at the time of vaccination” these results are “probably relevant to girls in the preferred age range for HPV vaccination (i.e., 11-12 years),” they said..
The study and included trials were supported by the U.S. National Cancer Institute, National Institutes of Health Office of Research on Women’s Health, and Ministry of Health of Costa Rica (CVT) and GlaxoSmithKline Biologicals SA (PATRICIA). Some authors disclosed ties with GlaxoSmithKline.
FROM LANCET ONCOLOGY
Majority of eczema appears after childhood
Though atopic dermatitis is often regarded as infrequent in adulthood, only 40% of study subjects with current eczema reported onset of the disease in childhood, with most beginning after adulthood, according to data published in the Journal of the European Academy of Dermatology and Venereology.
In a national multicenter, population-based study, Dr. Giancarlo Pesce of the University of Verona (Italy) and his associates administered the GEIRD (Gene-Environment Interactions in Respiratory Diseases) screening questionnaire to 10,464 randomly selected adults aged 20-44 years and gathered data on the frequency and prevalence of doctor-diagnosed eczema, asthma, and hay fever, as well as sociodemographic characteristics and environmental determinants such as smoking habits, traffic, and local pollution levels.
The prevalence of current eczema was 8.1%; the prevalence of eczema with asthma and/or hay fever (EAH), which was adopted as a proxy of atopic dermatitis, was 3.4%. About 60% of the subjects with current eczema reported onset of the disease in adulthood. After adjusting for confounders, the risk of having eczema and EAH was 57% higher in women than in men, and the risk for EAH was 86% higher in women than in men.
Read the full article here: J. Eur. Acad. Dermatol. Venereol. 2015;29:1180-7 (doi: 10.1111/jdv.12784).
Though atopic dermatitis is often regarded as infrequent in adulthood, only 40% of study subjects with current eczema reported onset of the disease in childhood, with most beginning after adulthood, according to data published in the Journal of the European Academy of Dermatology and Venereology.
In a national multicenter, population-based study, Dr. Giancarlo Pesce of the University of Verona (Italy) and his associates administered the GEIRD (Gene-Environment Interactions in Respiratory Diseases) screening questionnaire to 10,464 randomly selected adults aged 20-44 years and gathered data on the frequency and prevalence of doctor-diagnosed eczema, asthma, and hay fever, as well as sociodemographic characteristics and environmental determinants such as smoking habits, traffic, and local pollution levels.
The prevalence of current eczema was 8.1%; the prevalence of eczema with asthma and/or hay fever (EAH), which was adopted as a proxy of atopic dermatitis, was 3.4%. About 60% of the subjects with current eczema reported onset of the disease in adulthood. After adjusting for confounders, the risk of having eczema and EAH was 57% higher in women than in men, and the risk for EAH was 86% higher in women than in men.
Read the full article here: J. Eur. Acad. Dermatol. Venereol. 2015;29:1180-7 (doi: 10.1111/jdv.12784).
Though atopic dermatitis is often regarded as infrequent in adulthood, only 40% of study subjects with current eczema reported onset of the disease in childhood, with most beginning after adulthood, according to data published in the Journal of the European Academy of Dermatology and Venereology.
In a national multicenter, population-based study, Dr. Giancarlo Pesce of the University of Verona (Italy) and his associates administered the GEIRD (Gene-Environment Interactions in Respiratory Diseases) screening questionnaire to 10,464 randomly selected adults aged 20-44 years and gathered data on the frequency and prevalence of doctor-diagnosed eczema, asthma, and hay fever, as well as sociodemographic characteristics and environmental determinants such as smoking habits, traffic, and local pollution levels.
The prevalence of current eczema was 8.1%; the prevalence of eczema with asthma and/or hay fever (EAH), which was adopted as a proxy of atopic dermatitis, was 3.4%. About 60% of the subjects with current eczema reported onset of the disease in adulthood. After adjusting for confounders, the risk of having eczema and EAH was 57% higher in women than in men, and the risk for EAH was 86% higher in women than in men.
Read the full article here: J. Eur. Acad. Dermatol. Venereol. 2015;29:1180-7 (doi: 10.1111/jdv.12784).