User login
Antimicrobial resistance linked to 1.2 million global deaths in 2019
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.
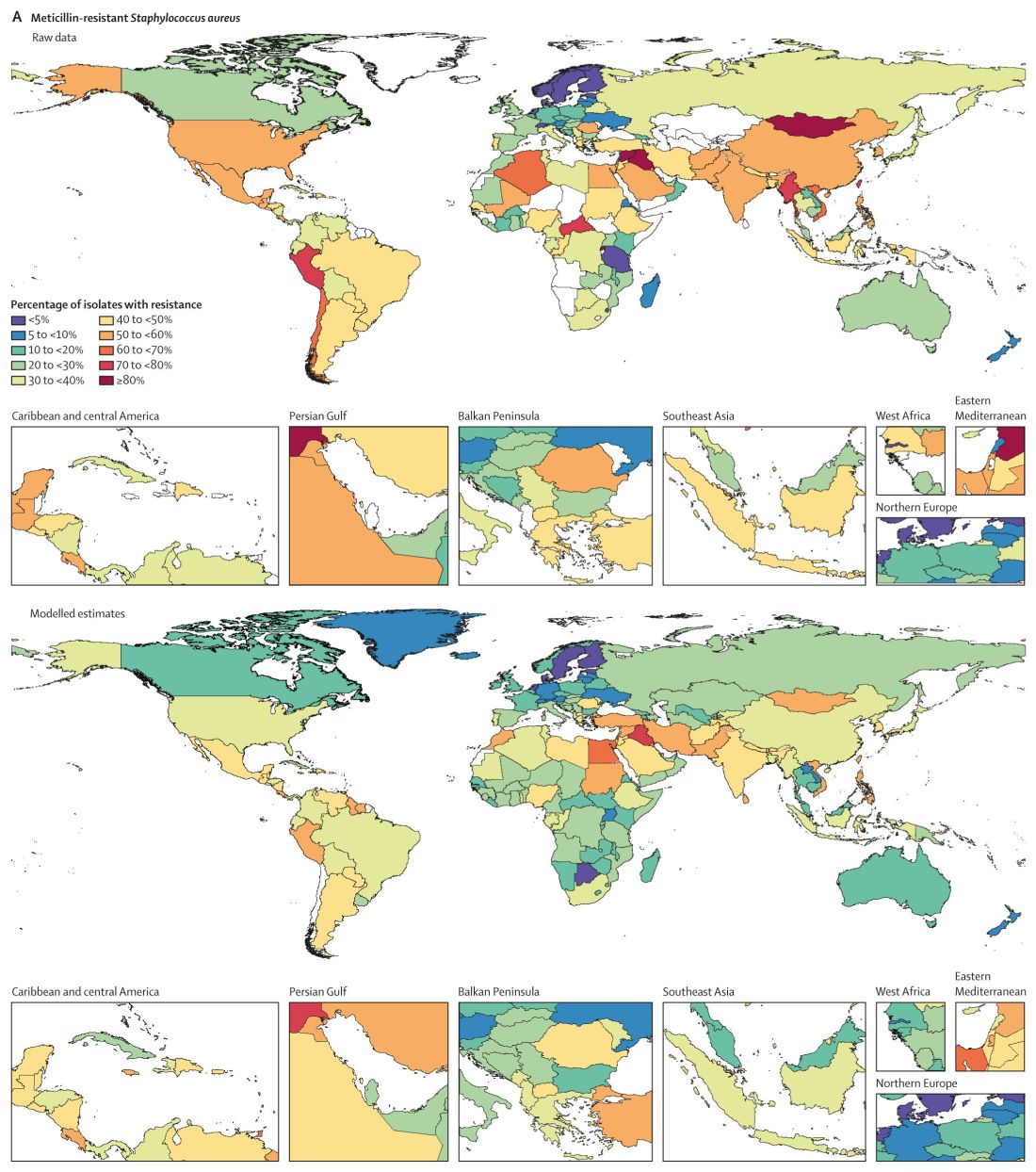
Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.

Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.

Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
Antibiotic choices for inpatients with SSTIs vary by race
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
FROM JAMA NETWORK OPEN
Severe outcomes increased in youth hospitalized after positive COVID-19 test
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM JAMA NETWORK OPEN
Pediatric community-acquired pneumonia: 5 days of antibiotics better than 10 days
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
Appendectomy or antibiotics? Large trial helps decision-making
The presence of mineralized stool, known as appendicolith, was associated with a nearly twofold increased risk of undergoing appendectomy within 30 days of initiating antibiotics, write David Flum, MD, of the University of Washington, Seattle, and coauthors in a paper published in JAMA Surgery on Jan. 12, 2021.
But the surprise was the lack of an association between appendectomy and factors often presumed to be consistent with more severe appendicitis.
Physicians have had their own ideas about what factors make a patient more likely to need an appendectomy after an initial round of treatment with antibiotics, such as a high white blood cell count or a perforation seen on CT scan, Dr. Flum said in an interview. But the research didn’t support some of these theories.
“This is why we do the studies,” Dr. Flum said. “Sometimes we find out that our hunches were wrong.”
Dr. Flum and coauthors measured the association between different patient factors and disease severity and the need for appendectomy following a course of antibiotics. They used adjusted odds ratios to describe these relationships while accounting for other differences.
An OR of 1.0 – or when the confidence interval around an OR crosses 1 – signals that there is no association between that factor and appendectomy. Positive ORs with confidence intervals that exclude 1.0 suggest the factor was associated with appendectomy.
The OR was 1.99 for the presence of appendicolith, a finding with a 95% confidence interval of 1.28-3.10. The OR was 1.53 (95% CI, 1.01-2.31) for female sex.
But the OR was 1.14 (95% CI, 0.66-1.98) for perforation, abscess, or fat stranding.
The OR was 1.09 (95% CI, 1.00-1.18) for radiographic finding of a larger appendix, as measured by diameter.
And the OR was 1.03 (95% CI, 0.98-1.09) for having a higher white blood cell count, as measured by a 1,000-cells/mcL increase.
Appy or not?
This paper draws from the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial (NCT02800785), for which top-line results were published in 2020 in the New England Journal of Medicine. In that paper, Dr. Flum and colleagues reported on results for 1,552 adults (414 with an appendicolith) who were evenly randomized to either antibiotics treatment or appendectomy. After 30 days, antibiotics were found to be noninferior to appendectomy, as reported by this news organization.
The federal Patient-Centered Outcomes Research Institute funded the CODA research. Dr. Flum said the National Institutes of Health had not appeared interested in funding a look at the different options available to patients experiencing appendicitis. Congress created PCORI as part of the Affordable Care Act of 2010, seeking to encourage researchers to study which treatments best serve patients through direct comparisons. Its support was critical for Dr. Flum and colleagues in seeking to help people weigh their options for treating appendicitis.
The CODA study “models what the patient’s experience is like, and this has not been the focus of NIH as much,” Dr. Flum said.
The CODA team has sought to make it easy for patients to consider what its findings and other research on appendicitis mean for them. They created an online decision-making tool, available at the aptly named http://www.appyornot.org/ website, which has videos in English and Spanish explaining patients’ options in simple terms. The website also asks questions about personal preferences, priorities, and resources to help them choose a treatment based on their individual situation.
Shift away from ‘paternalistic framing’
In the past, surgeons focused on the risk for patients from procedures, making the decisions for them about whether or not to proceed. There’s now a drive to shift away from this “paternalistic framing” toward shared decision-making, Dr. Flum said.
Surgeons need to have conversations with their patients about what’s happening in their lives as well as to assess their fears and concerns about treatment options, he said. These are aspects of patient care that were not covered in medical school or surgical training, but they lead to “less paternalistic” treatment. A patient’s decision about whether to choose surgery or antibiotics for appendicitis may hinge on factors such as insurance coverage, access to childcare, and the ability to miss days of work.
Dr. Flum said his fellow surgeons by and large have reacted well to the CODA team’s work.
“To their credit, the surgical community has embraced a healthy skepticism about the role of surgery,” Dr. Flum said.
The guidelines of the American College of Surgeons state that there is “high-quality evidence” that most patients with appendicitis can be managed with antibiotics instead of appendectomy (69% overall avoid appendectomy by 90 days, 75% of those without appendicolith, and 59% of those with appendicolith).
“Based on the surgeon’s judgment, patient preferences, and local resources (e.g., hospital staff, bed, and PPE supply availability) antibiotics are an acceptable first-line treatment, with appendectomy offered for those with worsening or recurrent symptoms,” the ACS guidelines say.
In an interview, Samir M. Fakhry, MD, vice president of HCA Center for Trauma and Acute Care Surgery Research in Nashville, Tenn., agreed with Dr. Flum about the shift taking place in medicine.
The CODA research, including the new paper in JAMA Surgery, makes it easier for physicians to work with patients and their families to reach decisions about how to treat appendicitis, Dr. Fakhry said.
These important discussions take time, he said, and patients must be allowed that time. Patients might feel misled, for example, if a surgeon pressed for appendectomy without explaining that a course of antibiotics may have served them well. Other patients may opt for surgery right away, especially in cases with appendicoliths, to avoid the potential for repeat episodes of medical care.
“You’ve got people who just want to get it done and over with. You’ve got people who want to avoid surgery no matter what,” Dr. Fakhry said. “It’s not just about the science and the data.”
This study was supported by a grant from PCORI. The authors reported having served as consultants or reviewers or have received fees for work outside of this paper from Stryker, Kerecis, Acera, Medline, Shriner’s Research Fund, UpToDate, and Tetraphase Pharmaceuticals Stryker.
A version of this article first appeared on Medscape.com.
The presence of mineralized stool, known as appendicolith, was associated with a nearly twofold increased risk of undergoing appendectomy within 30 days of initiating antibiotics, write David Flum, MD, of the University of Washington, Seattle, and coauthors in a paper published in JAMA Surgery on Jan. 12, 2021.
But the surprise was the lack of an association between appendectomy and factors often presumed to be consistent with more severe appendicitis.
Physicians have had their own ideas about what factors make a patient more likely to need an appendectomy after an initial round of treatment with antibiotics, such as a high white blood cell count or a perforation seen on CT scan, Dr. Flum said in an interview. But the research didn’t support some of these theories.
“This is why we do the studies,” Dr. Flum said. “Sometimes we find out that our hunches were wrong.”
Dr. Flum and coauthors measured the association between different patient factors and disease severity and the need for appendectomy following a course of antibiotics. They used adjusted odds ratios to describe these relationships while accounting for other differences.
An OR of 1.0 – or when the confidence interval around an OR crosses 1 – signals that there is no association between that factor and appendectomy. Positive ORs with confidence intervals that exclude 1.0 suggest the factor was associated with appendectomy.
The OR was 1.99 for the presence of appendicolith, a finding with a 95% confidence interval of 1.28-3.10. The OR was 1.53 (95% CI, 1.01-2.31) for female sex.
But the OR was 1.14 (95% CI, 0.66-1.98) for perforation, abscess, or fat stranding.
The OR was 1.09 (95% CI, 1.00-1.18) for radiographic finding of a larger appendix, as measured by diameter.
And the OR was 1.03 (95% CI, 0.98-1.09) for having a higher white blood cell count, as measured by a 1,000-cells/mcL increase.
Appy or not?
This paper draws from the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial (NCT02800785), for which top-line results were published in 2020 in the New England Journal of Medicine. In that paper, Dr. Flum and colleagues reported on results for 1,552 adults (414 with an appendicolith) who were evenly randomized to either antibiotics treatment or appendectomy. After 30 days, antibiotics were found to be noninferior to appendectomy, as reported by this news organization.
The federal Patient-Centered Outcomes Research Institute funded the CODA research. Dr. Flum said the National Institutes of Health had not appeared interested in funding a look at the different options available to patients experiencing appendicitis. Congress created PCORI as part of the Affordable Care Act of 2010, seeking to encourage researchers to study which treatments best serve patients through direct comparisons. Its support was critical for Dr. Flum and colleagues in seeking to help people weigh their options for treating appendicitis.
The CODA study “models what the patient’s experience is like, and this has not been the focus of NIH as much,” Dr. Flum said.
The CODA team has sought to make it easy for patients to consider what its findings and other research on appendicitis mean for them. They created an online decision-making tool, available at the aptly named http://www.appyornot.org/ website, which has videos in English and Spanish explaining patients’ options in simple terms. The website also asks questions about personal preferences, priorities, and resources to help them choose a treatment based on their individual situation.
Shift away from ‘paternalistic framing’
In the past, surgeons focused on the risk for patients from procedures, making the decisions for them about whether or not to proceed. There’s now a drive to shift away from this “paternalistic framing” toward shared decision-making, Dr. Flum said.
Surgeons need to have conversations with their patients about what’s happening in their lives as well as to assess their fears and concerns about treatment options, he said. These are aspects of patient care that were not covered in medical school or surgical training, but they lead to “less paternalistic” treatment. A patient’s decision about whether to choose surgery or antibiotics for appendicitis may hinge on factors such as insurance coverage, access to childcare, and the ability to miss days of work.
Dr. Flum said his fellow surgeons by and large have reacted well to the CODA team’s work.
“To their credit, the surgical community has embraced a healthy skepticism about the role of surgery,” Dr. Flum said.
The guidelines of the American College of Surgeons state that there is “high-quality evidence” that most patients with appendicitis can be managed with antibiotics instead of appendectomy (69% overall avoid appendectomy by 90 days, 75% of those without appendicolith, and 59% of those with appendicolith).
“Based on the surgeon’s judgment, patient preferences, and local resources (e.g., hospital staff, bed, and PPE supply availability) antibiotics are an acceptable first-line treatment, with appendectomy offered for those with worsening or recurrent symptoms,” the ACS guidelines say.
In an interview, Samir M. Fakhry, MD, vice president of HCA Center for Trauma and Acute Care Surgery Research in Nashville, Tenn., agreed with Dr. Flum about the shift taking place in medicine.
The CODA research, including the new paper in JAMA Surgery, makes it easier for physicians to work with patients and their families to reach decisions about how to treat appendicitis, Dr. Fakhry said.
These important discussions take time, he said, and patients must be allowed that time. Patients might feel misled, for example, if a surgeon pressed for appendectomy without explaining that a course of antibiotics may have served them well. Other patients may opt for surgery right away, especially in cases with appendicoliths, to avoid the potential for repeat episodes of medical care.
“You’ve got people who just want to get it done and over with. You’ve got people who want to avoid surgery no matter what,” Dr. Fakhry said. “It’s not just about the science and the data.”
This study was supported by a grant from PCORI. The authors reported having served as consultants or reviewers or have received fees for work outside of this paper from Stryker, Kerecis, Acera, Medline, Shriner’s Research Fund, UpToDate, and Tetraphase Pharmaceuticals Stryker.
A version of this article first appeared on Medscape.com.
The presence of mineralized stool, known as appendicolith, was associated with a nearly twofold increased risk of undergoing appendectomy within 30 days of initiating antibiotics, write David Flum, MD, of the University of Washington, Seattle, and coauthors in a paper published in JAMA Surgery on Jan. 12, 2021.
But the surprise was the lack of an association between appendectomy and factors often presumed to be consistent with more severe appendicitis.
Physicians have had their own ideas about what factors make a patient more likely to need an appendectomy after an initial round of treatment with antibiotics, such as a high white blood cell count or a perforation seen on CT scan, Dr. Flum said in an interview. But the research didn’t support some of these theories.
“This is why we do the studies,” Dr. Flum said. “Sometimes we find out that our hunches were wrong.”
Dr. Flum and coauthors measured the association between different patient factors and disease severity and the need for appendectomy following a course of antibiotics. They used adjusted odds ratios to describe these relationships while accounting for other differences.
An OR of 1.0 – or when the confidence interval around an OR crosses 1 – signals that there is no association between that factor and appendectomy. Positive ORs with confidence intervals that exclude 1.0 suggest the factor was associated with appendectomy.
The OR was 1.99 for the presence of appendicolith, a finding with a 95% confidence interval of 1.28-3.10. The OR was 1.53 (95% CI, 1.01-2.31) for female sex.
But the OR was 1.14 (95% CI, 0.66-1.98) for perforation, abscess, or fat stranding.
The OR was 1.09 (95% CI, 1.00-1.18) for radiographic finding of a larger appendix, as measured by diameter.
And the OR was 1.03 (95% CI, 0.98-1.09) for having a higher white blood cell count, as measured by a 1,000-cells/mcL increase.
Appy or not?
This paper draws from the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial (NCT02800785), for which top-line results were published in 2020 in the New England Journal of Medicine. In that paper, Dr. Flum and colleagues reported on results for 1,552 adults (414 with an appendicolith) who were evenly randomized to either antibiotics treatment or appendectomy. After 30 days, antibiotics were found to be noninferior to appendectomy, as reported by this news organization.
The federal Patient-Centered Outcomes Research Institute funded the CODA research. Dr. Flum said the National Institutes of Health had not appeared interested in funding a look at the different options available to patients experiencing appendicitis. Congress created PCORI as part of the Affordable Care Act of 2010, seeking to encourage researchers to study which treatments best serve patients through direct comparisons. Its support was critical for Dr. Flum and colleagues in seeking to help people weigh their options for treating appendicitis.
The CODA study “models what the patient’s experience is like, and this has not been the focus of NIH as much,” Dr. Flum said.
The CODA team has sought to make it easy for patients to consider what its findings and other research on appendicitis mean for them. They created an online decision-making tool, available at the aptly named http://www.appyornot.org/ website, which has videos in English and Spanish explaining patients’ options in simple terms. The website also asks questions about personal preferences, priorities, and resources to help them choose a treatment based on their individual situation.
Shift away from ‘paternalistic framing’
In the past, surgeons focused on the risk for patients from procedures, making the decisions for them about whether or not to proceed. There’s now a drive to shift away from this “paternalistic framing” toward shared decision-making, Dr. Flum said.
Surgeons need to have conversations with their patients about what’s happening in their lives as well as to assess their fears and concerns about treatment options, he said. These are aspects of patient care that were not covered in medical school or surgical training, but they lead to “less paternalistic” treatment. A patient’s decision about whether to choose surgery or antibiotics for appendicitis may hinge on factors such as insurance coverage, access to childcare, and the ability to miss days of work.
Dr. Flum said his fellow surgeons by and large have reacted well to the CODA team’s work.
“To their credit, the surgical community has embraced a healthy skepticism about the role of surgery,” Dr. Flum said.
The guidelines of the American College of Surgeons state that there is “high-quality evidence” that most patients with appendicitis can be managed with antibiotics instead of appendectomy (69% overall avoid appendectomy by 90 days, 75% of those without appendicolith, and 59% of those with appendicolith).
“Based on the surgeon’s judgment, patient preferences, and local resources (e.g., hospital staff, bed, and PPE supply availability) antibiotics are an acceptable first-line treatment, with appendectomy offered for those with worsening or recurrent symptoms,” the ACS guidelines say.
In an interview, Samir M. Fakhry, MD, vice president of HCA Center for Trauma and Acute Care Surgery Research in Nashville, Tenn., agreed with Dr. Flum about the shift taking place in medicine.
The CODA research, including the new paper in JAMA Surgery, makes it easier for physicians to work with patients and their families to reach decisions about how to treat appendicitis, Dr. Fakhry said.
These important discussions take time, he said, and patients must be allowed that time. Patients might feel misled, for example, if a surgeon pressed for appendectomy without explaining that a course of antibiotics may have served them well. Other patients may opt for surgery right away, especially in cases with appendicoliths, to avoid the potential for repeat episodes of medical care.
“You’ve got people who just want to get it done and over with. You’ve got people who want to avoid surgery no matter what,” Dr. Fakhry said. “It’s not just about the science and the data.”
This study was supported by a grant from PCORI. The authors reported having served as consultants or reviewers or have received fees for work outside of this paper from Stryker, Kerecis, Acera, Medline, Shriner’s Research Fund, UpToDate, and Tetraphase Pharmaceuticals Stryker.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Children and COVID: U.S. sees almost 1 million new cases
Another record week for COVID-19 brought almost 1 million new cases to the children of the United States, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
The pre-Omicron high for new cases in a week – 252,000 during the Delta surge of the late summer and early fall – has been surpassed each of the last 3 weeks and now stands at 981,000 (Jan. 7-13), according to the AAP/CHA weekly COVID-19 report. Over the 3-week stretch from Dec. 17 to Jan. 13, weekly cases increased by 394%.
Hospitalizations also climbed to new heights, as daily admissions reached 1.23 per 100,000 children on Jan. 14, an increase of 547% since Nov. 30, when the rate was 0.19 per 100,000. Before Omicron, the highest rate for children was 0.47 per 100,000, based on data from the Centers for Disease Control and Prevention.
The inpatient population count, meanwhile, has followed suit. On Jan. 16, there were 3,822 children hospitalized in pediatric inpatient beds with laboratory-confirmed COVID-19, which is 523% higher than the 613 children who were hospitalized on Nov. 14, according to the Department of Health & Human Services. In the last week, though, the population was up by just 10%.
The one thing that has not surged in the last few weeks is vaccination. Among children aged 5-11 years, the weekly count of those who have received at least one dose dropped by 34% over the last 5 weeks, falling from 527,000 for Dec.11-17 to 347,000 during Jan. 8-14, the CDC said on the COVID Data Tracker, which also noted that just 18.4% of this age group is fully vaccinated.
The situation was reversed in children aged 12-15, who were up by 36% over that same time, but their numbers were much smaller: 78,000 for the week of Dec. 11-17 and 106,000 for Jan. 8-14. Those aged 16-17 were up by just 4% over that 5-week span, the CDC data show.
Over the course of the entire pandemic, almost 9.5 million cases of COVID-19 in children have been reported, and children represent 17.8% of all cases reported in 49 states (excluding New York but including New York City), the District of Columbia, Puerto Rico, and Guam, the AAP and CHA said in their report.
Three states (Alabama, Nebraska, and Texas) stopped public reporting over the summer, but many states count individuals up to age 19 as children, and others (South Carolina, Tennessee, and West Virginia) go up to age 20, the AAP and CHA noted. The CDC, by comparison, puts the number of cases for those aged 0-17 at 8.3 million, but that estimate is based on only 51 million of the nearly 67 million U.S. cases as of Jan. 18.
Another record week for COVID-19 brought almost 1 million new cases to the children of the United States, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
The pre-Omicron high for new cases in a week – 252,000 during the Delta surge of the late summer and early fall – has been surpassed each of the last 3 weeks and now stands at 981,000 (Jan. 7-13), according to the AAP/CHA weekly COVID-19 report. Over the 3-week stretch from Dec. 17 to Jan. 13, weekly cases increased by 394%.
Hospitalizations also climbed to new heights, as daily admissions reached 1.23 per 100,000 children on Jan. 14, an increase of 547% since Nov. 30, when the rate was 0.19 per 100,000. Before Omicron, the highest rate for children was 0.47 per 100,000, based on data from the Centers for Disease Control and Prevention.
The inpatient population count, meanwhile, has followed suit. On Jan. 16, there were 3,822 children hospitalized in pediatric inpatient beds with laboratory-confirmed COVID-19, which is 523% higher than the 613 children who were hospitalized on Nov. 14, according to the Department of Health & Human Services. In the last week, though, the population was up by just 10%.
The one thing that has not surged in the last few weeks is vaccination. Among children aged 5-11 years, the weekly count of those who have received at least one dose dropped by 34% over the last 5 weeks, falling from 527,000 for Dec.11-17 to 347,000 during Jan. 8-14, the CDC said on the COVID Data Tracker, which also noted that just 18.4% of this age group is fully vaccinated.
The situation was reversed in children aged 12-15, who were up by 36% over that same time, but their numbers were much smaller: 78,000 for the week of Dec. 11-17 and 106,000 for Jan. 8-14. Those aged 16-17 were up by just 4% over that 5-week span, the CDC data show.
Over the course of the entire pandemic, almost 9.5 million cases of COVID-19 in children have been reported, and children represent 17.8% of all cases reported in 49 states (excluding New York but including New York City), the District of Columbia, Puerto Rico, and Guam, the AAP and CHA said in their report.
Three states (Alabama, Nebraska, and Texas) stopped public reporting over the summer, but many states count individuals up to age 19 as children, and others (South Carolina, Tennessee, and West Virginia) go up to age 20, the AAP and CHA noted. The CDC, by comparison, puts the number of cases for those aged 0-17 at 8.3 million, but that estimate is based on only 51 million of the nearly 67 million U.S. cases as of Jan. 18.
Another record week for COVID-19 brought almost 1 million new cases to the children of the United States, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
The pre-Omicron high for new cases in a week – 252,000 during the Delta surge of the late summer and early fall – has been surpassed each of the last 3 weeks and now stands at 981,000 (Jan. 7-13), according to the AAP/CHA weekly COVID-19 report. Over the 3-week stretch from Dec. 17 to Jan. 13, weekly cases increased by 394%.
Hospitalizations also climbed to new heights, as daily admissions reached 1.23 per 100,000 children on Jan. 14, an increase of 547% since Nov. 30, when the rate was 0.19 per 100,000. Before Omicron, the highest rate for children was 0.47 per 100,000, based on data from the Centers for Disease Control and Prevention.
The inpatient population count, meanwhile, has followed suit. On Jan. 16, there were 3,822 children hospitalized in pediatric inpatient beds with laboratory-confirmed COVID-19, which is 523% higher than the 613 children who were hospitalized on Nov. 14, according to the Department of Health & Human Services. In the last week, though, the population was up by just 10%.
The one thing that has not surged in the last few weeks is vaccination. Among children aged 5-11 years, the weekly count of those who have received at least one dose dropped by 34% over the last 5 weeks, falling from 527,000 for Dec.11-17 to 347,000 during Jan. 8-14, the CDC said on the COVID Data Tracker, which also noted that just 18.4% of this age group is fully vaccinated.
The situation was reversed in children aged 12-15, who were up by 36% over that same time, but their numbers were much smaller: 78,000 for the week of Dec. 11-17 and 106,000 for Jan. 8-14. Those aged 16-17 were up by just 4% over that 5-week span, the CDC data show.
Over the course of the entire pandemic, almost 9.5 million cases of COVID-19 in children have been reported, and children represent 17.8% of all cases reported in 49 states (excluding New York but including New York City), the District of Columbia, Puerto Rico, and Guam, the AAP and CHA said in their report.
Three states (Alabama, Nebraska, and Texas) stopped public reporting over the summer, but many states count individuals up to age 19 as children, and others (South Carolina, Tennessee, and West Virginia) go up to age 20, the AAP and CHA noted. The CDC, by comparison, puts the number of cases for those aged 0-17 at 8.3 million, but that estimate is based on only 51 million of the nearly 67 million U.S. cases as of Jan. 18.
Negative home COVID test no ‘free pass’ for kids, study finds
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
BMJ EVIDENCE-BASED MEDICINE
Antibiotics used in newborns despite low risk for sepsis
Antibiotics were administered to newborns at low risk for early-onset sepsis as frequently as to newborns with EOS risk factors, based on data from approximately 7,500 infants.
EOS remains a significant cause of morbidity and mortality, and predicting which newborns are at risk remains a challenge for neonatal care that often drives high rates of antibiotic use, Dustin D. Flannery, DO, of Children’s Hospital of Philadelphia and colleagues wrote.
Antibiotic exposures are associated with short- and long-term adverse effects in both preterm and term infants, which highlights the need for improved risk assessment in this population, the researchers said.
“A robust estimate of EOS risk in relation to delivery characteristics among infants of all gestational ages at birth could significantly contribute to newborn clinical management by identifying newborns unlikely to benefit from empirical antibiotic therapy,” they emphasized.
In a study published in Pediatrics, the researchers identified 7,540 infants born between Jan. 1, 2009, and Dec. 31, 2014, at two high-risk perinatal units in Philadelphia. Gestational age ranged from 22 to 43 weeks. Criteria for low risk of EOS were determined via an algorithm that included cesarean delivery (with or without labor or membrane rupture), and no antepartum concerns for intra-amniotic infection or nonreassuring fetal status.
A total of 6,428 infants did not meet the low-risk criteria; another 1,121 infants met the low-risk criteria. The primary outcome of EOS was defined as growth of a pathogen in at least 1 blood and/or cerebrospinal fluid culture obtained at 72 hours or less after birth. Overall, 41 infants who did not meet the low-risk criteria developed EOS; none of the infants who met the low-risk criteria developed EOS. Secondary outcomes included initiation of empirical antibiotics at 72 hours or less after birth and the duration of antibiotic use.
Although fewer low-risk infants received antibiotics, compared with infants with EOS (80.4% vs. 91.0%, P < .001), the duration of antibiotic use was not significantly different between the groups, with an adjusted difference of 0.6 hours.
Among infants who did not meet low-risk criteria, 157 were started on antibiotics for each case of EOS, the researchers noted in their discussion of the findings. “Because no cases of EOS were identified in the low-risk group, this proportion could not be calculated but suggests that antibiotic exposure in this group was disproportionately higher for incidence of EOS.”
The study findings were limited by several factors including the possible lack of generalizability to other centers and the use of data from a period before more refined EOS strategies, the researchers noted. Other limitations include the inability to assess the effect of lab results on antibiotic use, a lack of data on the exact indication for delivery, and potential misclassification bias.
Risk assessment tools should not be used alone, but should be used to inform clinical decision-making, the researchers emphasized. However, the results were strengthened by the inclusion of moderately preterm infants, who are rarely studied, and the clinical utility of the risk algorithm used in the study. “The implications of our study include potential adjustments to sepsis risk assessment in term infants, and confirmation and enhancement of previous studies that identify a subset of lower-risk preterm infants,” who may be spared empirical or prolonged antibiotic exposure, they concluded.
Data inform intelligent antibiotic use
“Early-onset sepsis is predominantly caused by exposure of the fetus or neonate to ascending maternal colonization or infection by gastrointestinal or genitourinary bacteria,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “Scenarios where there is limited neonatal exposure to these organisms would decrease the risk of development of EOS, therefore it is not surprising that delivery characteristics of low-risk deliveries as defined by investigators – the absence of labor, absence of intra-amniotic infection, rupture of membranes at time of delivery, and cesarean delivery – would have resulted in decreased likelihood of EOS.”
Inappropriate antibiotic use contributes to the development of resistant and more virulent strains of bacteria. A growing body of literature also suggests that early antibiotic usage in newborns may affect the neonatal gut microbiome, which is important for development of the neonatal immune system. Early alterations of the microbiome may have long-term implications,” Dr. Krishna said.
“Understanding the delivery characteristics that increase the risk of EOS are crucial to optimizing the use of antibiotics and thereby minimize potential harm to newborns,” she said. “Studies such as the current study are needed develop EOS prediction tools to improve antibiotic utilization.” More research is needed not only to adequately predict EOS, but to explore how antibiotics affect the neonatal microbiome, and how clinicians can circumvent potential adverse implications with antibiotic use to improve long-term health, Dr. Krishna concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Krishna had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Antibiotics were administered to newborns at low risk for early-onset sepsis as frequently as to newborns with EOS risk factors, based on data from approximately 7,500 infants.
EOS remains a significant cause of morbidity and mortality, and predicting which newborns are at risk remains a challenge for neonatal care that often drives high rates of antibiotic use, Dustin D. Flannery, DO, of Children’s Hospital of Philadelphia and colleagues wrote.
Antibiotic exposures are associated with short- and long-term adverse effects in both preterm and term infants, which highlights the need for improved risk assessment in this population, the researchers said.
“A robust estimate of EOS risk in relation to delivery characteristics among infants of all gestational ages at birth could significantly contribute to newborn clinical management by identifying newborns unlikely to benefit from empirical antibiotic therapy,” they emphasized.
In a study published in Pediatrics, the researchers identified 7,540 infants born between Jan. 1, 2009, and Dec. 31, 2014, at two high-risk perinatal units in Philadelphia. Gestational age ranged from 22 to 43 weeks. Criteria for low risk of EOS were determined via an algorithm that included cesarean delivery (with or without labor or membrane rupture), and no antepartum concerns for intra-amniotic infection or nonreassuring fetal status.
A total of 6,428 infants did not meet the low-risk criteria; another 1,121 infants met the low-risk criteria. The primary outcome of EOS was defined as growth of a pathogen in at least 1 blood and/or cerebrospinal fluid culture obtained at 72 hours or less after birth. Overall, 41 infants who did not meet the low-risk criteria developed EOS; none of the infants who met the low-risk criteria developed EOS. Secondary outcomes included initiation of empirical antibiotics at 72 hours or less after birth and the duration of antibiotic use.
Although fewer low-risk infants received antibiotics, compared with infants with EOS (80.4% vs. 91.0%, P < .001), the duration of antibiotic use was not significantly different between the groups, with an adjusted difference of 0.6 hours.
Among infants who did not meet low-risk criteria, 157 were started on antibiotics for each case of EOS, the researchers noted in their discussion of the findings. “Because no cases of EOS were identified in the low-risk group, this proportion could not be calculated but suggests that antibiotic exposure in this group was disproportionately higher for incidence of EOS.”
The study findings were limited by several factors including the possible lack of generalizability to other centers and the use of data from a period before more refined EOS strategies, the researchers noted. Other limitations include the inability to assess the effect of lab results on antibiotic use, a lack of data on the exact indication for delivery, and potential misclassification bias.
Risk assessment tools should not be used alone, but should be used to inform clinical decision-making, the researchers emphasized. However, the results were strengthened by the inclusion of moderately preterm infants, who are rarely studied, and the clinical utility of the risk algorithm used in the study. “The implications of our study include potential adjustments to sepsis risk assessment in term infants, and confirmation and enhancement of previous studies that identify a subset of lower-risk preterm infants,” who may be spared empirical or prolonged antibiotic exposure, they concluded.
Data inform intelligent antibiotic use
“Early-onset sepsis is predominantly caused by exposure of the fetus or neonate to ascending maternal colonization or infection by gastrointestinal or genitourinary bacteria,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “Scenarios where there is limited neonatal exposure to these organisms would decrease the risk of development of EOS, therefore it is not surprising that delivery characteristics of low-risk deliveries as defined by investigators – the absence of labor, absence of intra-amniotic infection, rupture of membranes at time of delivery, and cesarean delivery – would have resulted in decreased likelihood of EOS.”
Inappropriate antibiotic use contributes to the development of resistant and more virulent strains of bacteria. A growing body of literature also suggests that early antibiotic usage in newborns may affect the neonatal gut microbiome, which is important for development of the neonatal immune system. Early alterations of the microbiome may have long-term implications,” Dr. Krishna said.
“Understanding the delivery characteristics that increase the risk of EOS are crucial to optimizing the use of antibiotics and thereby minimize potential harm to newborns,” she said. “Studies such as the current study are needed develop EOS prediction tools to improve antibiotic utilization.” More research is needed not only to adequately predict EOS, but to explore how antibiotics affect the neonatal microbiome, and how clinicians can circumvent potential adverse implications with antibiotic use to improve long-term health, Dr. Krishna concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Krishna had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Antibiotics were administered to newborns at low risk for early-onset sepsis as frequently as to newborns with EOS risk factors, based on data from approximately 7,500 infants.
EOS remains a significant cause of morbidity and mortality, and predicting which newborns are at risk remains a challenge for neonatal care that often drives high rates of antibiotic use, Dustin D. Flannery, DO, of Children’s Hospital of Philadelphia and colleagues wrote.
Antibiotic exposures are associated with short- and long-term adverse effects in both preterm and term infants, which highlights the need for improved risk assessment in this population, the researchers said.
“A robust estimate of EOS risk in relation to delivery characteristics among infants of all gestational ages at birth could significantly contribute to newborn clinical management by identifying newborns unlikely to benefit from empirical antibiotic therapy,” they emphasized.
In a study published in Pediatrics, the researchers identified 7,540 infants born between Jan. 1, 2009, and Dec. 31, 2014, at two high-risk perinatal units in Philadelphia. Gestational age ranged from 22 to 43 weeks. Criteria for low risk of EOS were determined via an algorithm that included cesarean delivery (with or without labor or membrane rupture), and no antepartum concerns for intra-amniotic infection or nonreassuring fetal status.
A total of 6,428 infants did not meet the low-risk criteria; another 1,121 infants met the low-risk criteria. The primary outcome of EOS was defined as growth of a pathogen in at least 1 blood and/or cerebrospinal fluid culture obtained at 72 hours or less after birth. Overall, 41 infants who did not meet the low-risk criteria developed EOS; none of the infants who met the low-risk criteria developed EOS. Secondary outcomes included initiation of empirical antibiotics at 72 hours or less after birth and the duration of antibiotic use.
Although fewer low-risk infants received antibiotics, compared with infants with EOS (80.4% vs. 91.0%, P < .001), the duration of antibiotic use was not significantly different between the groups, with an adjusted difference of 0.6 hours.
Among infants who did not meet low-risk criteria, 157 were started on antibiotics for each case of EOS, the researchers noted in their discussion of the findings. “Because no cases of EOS were identified in the low-risk group, this proportion could not be calculated but suggests that antibiotic exposure in this group was disproportionately higher for incidence of EOS.”
The study findings were limited by several factors including the possible lack of generalizability to other centers and the use of data from a period before more refined EOS strategies, the researchers noted. Other limitations include the inability to assess the effect of lab results on antibiotic use, a lack of data on the exact indication for delivery, and potential misclassification bias.
Risk assessment tools should not be used alone, but should be used to inform clinical decision-making, the researchers emphasized. However, the results were strengthened by the inclusion of moderately preterm infants, who are rarely studied, and the clinical utility of the risk algorithm used in the study. “The implications of our study include potential adjustments to sepsis risk assessment in term infants, and confirmation and enhancement of previous studies that identify a subset of lower-risk preterm infants,” who may be spared empirical or prolonged antibiotic exposure, they concluded.
Data inform intelligent antibiotic use
“Early-onset sepsis is predominantly caused by exposure of the fetus or neonate to ascending maternal colonization or infection by gastrointestinal or genitourinary bacteria,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “Scenarios where there is limited neonatal exposure to these organisms would decrease the risk of development of EOS, therefore it is not surprising that delivery characteristics of low-risk deliveries as defined by investigators – the absence of labor, absence of intra-amniotic infection, rupture of membranes at time of delivery, and cesarean delivery – would have resulted in decreased likelihood of EOS.”
Inappropriate antibiotic use contributes to the development of resistant and more virulent strains of bacteria. A growing body of literature also suggests that early antibiotic usage in newborns may affect the neonatal gut microbiome, which is important for development of the neonatal immune system. Early alterations of the microbiome may have long-term implications,” Dr. Krishna said.
“Understanding the delivery characteristics that increase the risk of EOS are crucial to optimizing the use of antibiotics and thereby minimize potential harm to newborns,” she said. “Studies such as the current study are needed develop EOS prediction tools to improve antibiotic utilization.” More research is needed not only to adequately predict EOS, but to explore how antibiotics affect the neonatal microbiome, and how clinicians can circumvent potential adverse implications with antibiotic use to improve long-term health, Dr. Krishna concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Krishna had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
FROM PEDIATRICS
Epstein-Barr virus a likely leading cause of multiple sclerosis
This study is the first to provide compelling evidence of a causal link between EBV and MS, principal investigator Alberto Ascherio, MD, DrPH, professor of epidemiology, Harvard T. H. Chan School of Public Health, and professor of medicine, Harvard Medical School, Boston, told this news organization.
The “prevailing” view has been that MS is “an autoimmune disease of unknown etiology,” said Dr. Ascherio. “Now we know MS is a complication of a viral infection.” With this knowledge, he added, “we can redirect research” to find antiviral drugs to treat the disease.
The study was published online Jan. 13 in Science.
Unique dataset
A chronic disease of the central nervous system, MS involves an inflammatory attack on the myelin sheath and the axons it insulates. The disease affects 2.8 million people worldwide.
EBV is a human herpesvirus that can cause infectious mononucleosis. After infection, it persists in latent form in B-lymphocytes.
EBV is common and infects about 95% of adults. Most individuals are already infected with the virus by age 18 or 20 years, making it difficult to study uninfected populations, said Dr. Ascherio.
However, access to a “huge” database of more than 10 million active-duty U.S. service personnel made this possible, he said.
Service members are screened for HIV at the start of their service care and biennially thereafter. The investigators used stored blood samples to determine the relation between EBV infection and MS over a 20-year period from 1993 to 2013.
Researchers examined 801 MS case patients and 1,566 matched controls without MS. Most individuals were under 20 at the time of their first blood collection. Symptom onset for those who developed MS was a median of 10 years after the first sample was obtained.
Only one of the 801 MS case patients had no serologic evidence of EBV. This individual may have been infected with the virus after the last blood collection, failed to seroconvert in response to infection, or was misdiagnosed, the investigators note.
The hazard ratio for MS between EBV seroconversion versus persistent EBV seronegative was 32.4 (95% CI, 4.3-245.3; P < .001).
An MS vaccine?
MS risk was not increased after infection with cytomegalovirus, a herpesvirus that is transmitted through saliva, as is EBV.
Researchers measured serum concentrations of neurofilament light chain (sNflL), a biomarker of neuroaxonal degeneration, in samples from EBV-negative individuals at baseline. There were no signs of neuroaxonal degeneration before EBV seroconversion in subjects who later developed MS.
This indicates that “EBV infection preceded not only symptom onset but also the time of the first detectable pathological mechanisms underlying MS,” the investigators note.
The very magnitude of increased MS risk of MS observed EBV almost completely rules out confounding by known risk factors. Smoking and vitamin D deficiency double the risk, and genetic predisposition and childhood obesity also only raise the risks of MS to a “moderate” degree, said Dr. Ascherio.
It’s not clear why only some people infected with EBV go on to develop MS, he said.
The idea that reverse causation – that immune dysregulation during the preclinical phase of MS increases susceptibility to EBV infection – is unlikely, the investigators note. For instance, EBV seroconversion occurs before elevation of sNfL levels, an early marker of preclinical MS.
Since most MS cases appear to be caused by EBV, a suitable vaccine might thwart the disease. “A vaccine could, in theory, prevent infection and prevent MS,” said Dr. Ascherio, adding that there’s ongoing work to develop such a vaccine.
Another approach is to target the virus driving MS disease progression. Developing appropriate antivirals might treat and even cure MS, said Dr. Ascherio.
‘Compelling data’
In an accompanying commentary, William H. Robinson, MD, PhD, professor, Division of Immunology and Rheumatology, department of medicine, Stanford (Calif.) University, and a colleague said the study findings “provide compelling data that implicate EBV as the trigger for the development of MS.”
The mechanism or mechanisms by which EBV leads to MS “remain elusive,” the commentary authors write.
“Possibilities include molecular mimicry, through which EBV viral protein sequences mimic human myelin proteins and other CNS proteins and thereby induce autoimmunity against myelin and CNS antigens,” they note.
As other factors, including genetic susceptibility, are important to MS, EBV infection is likely necessary but not sufficient to trigger MS, said the commentary. “Infection with EBV is the initial pathogenic step in MS, but additional fuses must be ignited for the full pathophysiology.”
The commentary authors query whether there may be “new opportunities” for therapy with vaccines or antivirals. “Now that the initial trigger for MS has been identified, perhaps MS could be eradicated.”
In a statement from the Science Media Center, an independent venture promoting views from the scientific community, two other experts offered their take on the study.
Paul Farrell, PhD, professor of tumor virology, Imperial College London, said the paper “provides very clear confirmation of a causal role for EBV in most cases of MS.”
While there’s evidence that a vaccine can prevent the EBV disease infectious mononucleosis, no vaccine candidate has yet prevented the virus from infecting and establishing long-term persistence in people, noted Dr. Farrell.
“So, at this stage it is not clear whether a vaccine of the types currently being developed would be able to prevent the long-term effects of EBV in MS,” he said.
Daniel Davis, PhD, professor of immunology, University of Manchester, United Kingdom, commented that the value of this new discovery is not an immediate medical cure or treatment but is “a major step forward” in understanding MS.
The study “sets up new research working out the precise details of how this virus can sometimes lead to an autoimmune disease,” said Dr. Davis. “There is no shortage of ideas in how this might happen in principle and hopefully the correct details will emerge soon.”
The study received funding from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, National Multiple Sclerosis Society, the German Research Foundation, the National Institutes of Health, and the Howard Hughes Medical Institute. Dr. Ascherio reports no relevant financial relaitonships. Dr. Robinson is a coinventor on a patent application filed by Stanford University that includes antibodies to EBV. Dr. Farrell reports serving on an ad hoc review panel for GSK on EBV vaccines in 2019 as a one off. He has a current grant from MRC on EBV biology, including some EBV sequence variation, but the grant is not about MS. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This study is the first to provide compelling evidence of a causal link between EBV and MS, principal investigator Alberto Ascherio, MD, DrPH, professor of epidemiology, Harvard T. H. Chan School of Public Health, and professor of medicine, Harvard Medical School, Boston, told this news organization.
The “prevailing” view has been that MS is “an autoimmune disease of unknown etiology,” said Dr. Ascherio. “Now we know MS is a complication of a viral infection.” With this knowledge, he added, “we can redirect research” to find antiviral drugs to treat the disease.
The study was published online Jan. 13 in Science.
Unique dataset
A chronic disease of the central nervous system, MS involves an inflammatory attack on the myelin sheath and the axons it insulates. The disease affects 2.8 million people worldwide.
EBV is a human herpesvirus that can cause infectious mononucleosis. After infection, it persists in latent form in B-lymphocytes.
EBV is common and infects about 95% of adults. Most individuals are already infected with the virus by age 18 or 20 years, making it difficult to study uninfected populations, said Dr. Ascherio.
However, access to a “huge” database of more than 10 million active-duty U.S. service personnel made this possible, he said.
Service members are screened for HIV at the start of their service care and biennially thereafter. The investigators used stored blood samples to determine the relation between EBV infection and MS over a 20-year period from 1993 to 2013.
Researchers examined 801 MS case patients and 1,566 matched controls without MS. Most individuals were under 20 at the time of their first blood collection. Symptom onset for those who developed MS was a median of 10 years after the first sample was obtained.
Only one of the 801 MS case patients had no serologic evidence of EBV. This individual may have been infected with the virus after the last blood collection, failed to seroconvert in response to infection, or was misdiagnosed, the investigators note.
The hazard ratio for MS between EBV seroconversion versus persistent EBV seronegative was 32.4 (95% CI, 4.3-245.3; P < .001).
An MS vaccine?
MS risk was not increased after infection with cytomegalovirus, a herpesvirus that is transmitted through saliva, as is EBV.
Researchers measured serum concentrations of neurofilament light chain (sNflL), a biomarker of neuroaxonal degeneration, in samples from EBV-negative individuals at baseline. There were no signs of neuroaxonal degeneration before EBV seroconversion in subjects who later developed MS.
This indicates that “EBV infection preceded not only symptom onset but also the time of the first detectable pathological mechanisms underlying MS,” the investigators note.
The very magnitude of increased MS risk of MS observed EBV almost completely rules out confounding by known risk factors. Smoking and vitamin D deficiency double the risk, and genetic predisposition and childhood obesity also only raise the risks of MS to a “moderate” degree, said Dr. Ascherio.
It’s not clear why only some people infected with EBV go on to develop MS, he said.
The idea that reverse causation – that immune dysregulation during the preclinical phase of MS increases susceptibility to EBV infection – is unlikely, the investigators note. For instance, EBV seroconversion occurs before elevation of sNfL levels, an early marker of preclinical MS.
Since most MS cases appear to be caused by EBV, a suitable vaccine might thwart the disease. “A vaccine could, in theory, prevent infection and prevent MS,” said Dr. Ascherio, adding that there’s ongoing work to develop such a vaccine.
Another approach is to target the virus driving MS disease progression. Developing appropriate antivirals might treat and even cure MS, said Dr. Ascherio.
‘Compelling data’
In an accompanying commentary, William H. Robinson, MD, PhD, professor, Division of Immunology and Rheumatology, department of medicine, Stanford (Calif.) University, and a colleague said the study findings “provide compelling data that implicate EBV as the trigger for the development of MS.”
The mechanism or mechanisms by which EBV leads to MS “remain elusive,” the commentary authors write.
“Possibilities include molecular mimicry, through which EBV viral protein sequences mimic human myelin proteins and other CNS proteins and thereby induce autoimmunity against myelin and CNS antigens,” they note.
As other factors, including genetic susceptibility, are important to MS, EBV infection is likely necessary but not sufficient to trigger MS, said the commentary. “Infection with EBV is the initial pathogenic step in MS, but additional fuses must be ignited for the full pathophysiology.”
The commentary authors query whether there may be “new opportunities” for therapy with vaccines or antivirals. “Now that the initial trigger for MS has been identified, perhaps MS could be eradicated.”
In a statement from the Science Media Center, an independent venture promoting views from the scientific community, two other experts offered their take on the study.
Paul Farrell, PhD, professor of tumor virology, Imperial College London, said the paper “provides very clear confirmation of a causal role for EBV in most cases of MS.”
While there’s evidence that a vaccine can prevent the EBV disease infectious mononucleosis, no vaccine candidate has yet prevented the virus from infecting and establishing long-term persistence in people, noted Dr. Farrell.
“So, at this stage it is not clear whether a vaccine of the types currently being developed would be able to prevent the long-term effects of EBV in MS,” he said.
Daniel Davis, PhD, professor of immunology, University of Manchester, United Kingdom, commented that the value of this new discovery is not an immediate medical cure or treatment but is “a major step forward” in understanding MS.
The study “sets up new research working out the precise details of how this virus can sometimes lead to an autoimmune disease,” said Dr. Davis. “There is no shortage of ideas in how this might happen in principle and hopefully the correct details will emerge soon.”
The study received funding from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, National Multiple Sclerosis Society, the German Research Foundation, the National Institutes of Health, and the Howard Hughes Medical Institute. Dr. Ascherio reports no relevant financial relaitonships. Dr. Robinson is a coinventor on a patent application filed by Stanford University that includes antibodies to EBV. Dr. Farrell reports serving on an ad hoc review panel for GSK on EBV vaccines in 2019 as a one off. He has a current grant from MRC on EBV biology, including some EBV sequence variation, but the grant is not about MS. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This study is the first to provide compelling evidence of a causal link between EBV and MS, principal investigator Alberto Ascherio, MD, DrPH, professor of epidemiology, Harvard T. H. Chan School of Public Health, and professor of medicine, Harvard Medical School, Boston, told this news organization.
The “prevailing” view has been that MS is “an autoimmune disease of unknown etiology,” said Dr. Ascherio. “Now we know MS is a complication of a viral infection.” With this knowledge, he added, “we can redirect research” to find antiviral drugs to treat the disease.
The study was published online Jan. 13 in Science.
Unique dataset
A chronic disease of the central nervous system, MS involves an inflammatory attack on the myelin sheath and the axons it insulates. The disease affects 2.8 million people worldwide.
EBV is a human herpesvirus that can cause infectious mononucleosis. After infection, it persists in latent form in B-lymphocytes.
EBV is common and infects about 95% of adults. Most individuals are already infected with the virus by age 18 or 20 years, making it difficult to study uninfected populations, said Dr. Ascherio.
However, access to a “huge” database of more than 10 million active-duty U.S. service personnel made this possible, he said.
Service members are screened for HIV at the start of their service care and biennially thereafter. The investigators used stored blood samples to determine the relation between EBV infection and MS over a 20-year period from 1993 to 2013.
Researchers examined 801 MS case patients and 1,566 matched controls without MS. Most individuals were under 20 at the time of their first blood collection. Symptom onset for those who developed MS was a median of 10 years after the first sample was obtained.
Only one of the 801 MS case patients had no serologic evidence of EBV. This individual may have been infected with the virus after the last blood collection, failed to seroconvert in response to infection, or was misdiagnosed, the investigators note.
The hazard ratio for MS between EBV seroconversion versus persistent EBV seronegative was 32.4 (95% CI, 4.3-245.3; P < .001).
An MS vaccine?
MS risk was not increased after infection with cytomegalovirus, a herpesvirus that is transmitted through saliva, as is EBV.
Researchers measured serum concentrations of neurofilament light chain (sNflL), a biomarker of neuroaxonal degeneration, in samples from EBV-negative individuals at baseline. There were no signs of neuroaxonal degeneration before EBV seroconversion in subjects who later developed MS.
This indicates that “EBV infection preceded not only symptom onset but also the time of the first detectable pathological mechanisms underlying MS,” the investigators note.
The very magnitude of increased MS risk of MS observed EBV almost completely rules out confounding by known risk factors. Smoking and vitamin D deficiency double the risk, and genetic predisposition and childhood obesity also only raise the risks of MS to a “moderate” degree, said Dr. Ascherio.
It’s not clear why only some people infected with EBV go on to develop MS, he said.
The idea that reverse causation – that immune dysregulation during the preclinical phase of MS increases susceptibility to EBV infection – is unlikely, the investigators note. For instance, EBV seroconversion occurs before elevation of sNfL levels, an early marker of preclinical MS.
Since most MS cases appear to be caused by EBV, a suitable vaccine might thwart the disease. “A vaccine could, in theory, prevent infection and prevent MS,” said Dr. Ascherio, adding that there’s ongoing work to develop such a vaccine.
Another approach is to target the virus driving MS disease progression. Developing appropriate antivirals might treat and even cure MS, said Dr. Ascherio.
‘Compelling data’
In an accompanying commentary, William H. Robinson, MD, PhD, professor, Division of Immunology and Rheumatology, department of medicine, Stanford (Calif.) University, and a colleague said the study findings “provide compelling data that implicate EBV as the trigger for the development of MS.”
The mechanism or mechanisms by which EBV leads to MS “remain elusive,” the commentary authors write.
“Possibilities include molecular mimicry, through which EBV viral protein sequences mimic human myelin proteins and other CNS proteins and thereby induce autoimmunity against myelin and CNS antigens,” they note.
As other factors, including genetic susceptibility, are important to MS, EBV infection is likely necessary but not sufficient to trigger MS, said the commentary. “Infection with EBV is the initial pathogenic step in MS, but additional fuses must be ignited for the full pathophysiology.”
The commentary authors query whether there may be “new opportunities” for therapy with vaccines or antivirals. “Now that the initial trigger for MS has been identified, perhaps MS could be eradicated.”
In a statement from the Science Media Center, an independent venture promoting views from the scientific community, two other experts offered their take on the study.
Paul Farrell, PhD, professor of tumor virology, Imperial College London, said the paper “provides very clear confirmation of a causal role for EBV in most cases of MS.”
While there’s evidence that a vaccine can prevent the EBV disease infectious mononucleosis, no vaccine candidate has yet prevented the virus from infecting and establishing long-term persistence in people, noted Dr. Farrell.
“So, at this stage it is not clear whether a vaccine of the types currently being developed would be able to prevent the long-term effects of EBV in MS,” he said.
Daniel Davis, PhD, professor of immunology, University of Manchester, United Kingdom, commented that the value of this new discovery is not an immediate medical cure or treatment but is “a major step forward” in understanding MS.
The study “sets up new research working out the precise details of how this virus can sometimes lead to an autoimmune disease,” said Dr. Davis. “There is no shortage of ideas in how this might happen in principle and hopefully the correct details will emerge soon.”
The study received funding from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, National Multiple Sclerosis Society, the German Research Foundation, the National Institutes of Health, and the Howard Hughes Medical Institute. Dr. Ascherio reports no relevant financial relaitonships. Dr. Robinson is a coinventor on a patent application filed by Stanford University that includes antibodies to EBV. Dr. Farrell reports serving on an ad hoc review panel for GSK on EBV vaccines in 2019 as a one off. He has a current grant from MRC on EBV biology, including some EBV sequence variation, but the grant is not about MS. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCIENCE
Wilderness Medical Society issues clinical guidelines for tick-borne illness
The recently published “Clinical Practice Guidelines for the Prevention and Management of Tick-Borne Illness,” from the Wilderness Medical Society, are a good compilation of treatment suggestions but are not, in fact, new recommendations, lead author Benjamin Ho, MD, of Southern Wisconsin Emergency Associates in Janesville, acknowledged in an interview.
Dr. Ho emphasized that the focus of the report was on “practitioners who practice in resource-limited settings” and are “the group’s way of solidifying a ... standard of practice” for such physicians. Dr. Ho also said that, while “a lot of the recommendations aren’t well supported, the risk-benefit ratio, we believe, supports the recommendations.”
The article first reviewed the different types of ticks and their distribution in the United States, the specific pathogen associated with each, the disease it causes, and comments about seasonal variations in biting behavior. Another table outlines the most common clinical syndromes, typical lab findings, recommended diagnostic testing, and antibiotic treatments. A third section contains images of different types of ticks and photos of ticks in various life-cycle stages and different levels of engorgement.
The authors were careful to note: “Several tick species are able to carry multiple pathogens. In one study, nearly 25% of Ixodes were coinfected with some combination of the bacteria or parasites causing Lyme disease, anaplasmosis, or babesiosis. Although TBI [tick-borne illness] diagnosis is not the focus of this [clinical practice guideline], providers should be aware of high rates of coinfection; the presence of one TBI should in many instances prompt testing for others.”
In terms of recommendations for preventing TBIs, the authors challenge the suggestion of wearing light-colored clothing. For repellents, they recommend DEET, picaridin, and permethrin. And they also give instructions for laundering clothing and removing ticks.
One recommendation is controversial: that of providing single-dose doxycycline as prophylaxis against Lyme disease. Dr. Ho stresses that this was only for “high-risk” tick bites, defined as a tick bite from an identified Ixodes vector species in which the tick was attached for at least 36 hours and that occurred in an endemic area.
The recommendation for prophylactic doxycycline originated with an article by Robert Nadelman and colleagues in the New England Journal of Medicine and has been strongly challenged by ILADS (International Lyme and Associated Diseases Society) physicians, including Daniel Cameron, MD, and others.
Sam Donta, MD, a recent member of the Department of Health & Human Services Tick-borne Working Group and a member of the Infectious Disease Society of America, said in an interview: “The problem with the one-dose doxycycline is you may not begin to develop symptoms until 2 months later.” It might mask the early symptoms of Lyme. “My impression is that the doxycycline – even the single dose – might have abrogated the ability to see an immune response. The idea, though, if you’ve had a tick bite, is to do nothing and to wait for symptoms to develop. That becomes a little bit more complex. But even then, you could choose to follow the patient and see the patient in 2 weeks and then get blood testing.”
Dr. Donta added: “I think the screening test is inadequate. So you have to go directly to the Western blot. And you have to do both the IgM and IgG” and look for specific bands.
Dr. Donta emphasized that patients should be encouraged to save any ticks that were attached and that, if at all possible, ticks should be sent to a reference lab for testing before committing a patient to a course of antibiotics. There is no harm in that brief delay, he said, and most labs can identify an array of pathogens.
The Wilderness Society guidelines on TBIs provide a good overview for clinicians practicing in limited resource settings and mirror those from the IDSA.
Dr. Ho and Dr. Donta reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recently published “Clinical Practice Guidelines for the Prevention and Management of Tick-Borne Illness,” from the Wilderness Medical Society, are a good compilation of treatment suggestions but are not, in fact, new recommendations, lead author Benjamin Ho, MD, of Southern Wisconsin Emergency Associates in Janesville, acknowledged in an interview.
Dr. Ho emphasized that the focus of the report was on “practitioners who practice in resource-limited settings” and are “the group’s way of solidifying a ... standard of practice” for such physicians. Dr. Ho also said that, while “a lot of the recommendations aren’t well supported, the risk-benefit ratio, we believe, supports the recommendations.”
The article first reviewed the different types of ticks and their distribution in the United States, the specific pathogen associated with each, the disease it causes, and comments about seasonal variations in biting behavior. Another table outlines the most common clinical syndromes, typical lab findings, recommended diagnostic testing, and antibiotic treatments. A third section contains images of different types of ticks and photos of ticks in various life-cycle stages and different levels of engorgement.
The authors were careful to note: “Several tick species are able to carry multiple pathogens. In one study, nearly 25% of Ixodes were coinfected with some combination of the bacteria or parasites causing Lyme disease, anaplasmosis, or babesiosis. Although TBI [tick-borne illness] diagnosis is not the focus of this [clinical practice guideline], providers should be aware of high rates of coinfection; the presence of one TBI should in many instances prompt testing for others.”
In terms of recommendations for preventing TBIs, the authors challenge the suggestion of wearing light-colored clothing. For repellents, they recommend DEET, picaridin, and permethrin. And they also give instructions for laundering clothing and removing ticks.
One recommendation is controversial: that of providing single-dose doxycycline as prophylaxis against Lyme disease. Dr. Ho stresses that this was only for “high-risk” tick bites, defined as a tick bite from an identified Ixodes vector species in which the tick was attached for at least 36 hours and that occurred in an endemic area.
The recommendation for prophylactic doxycycline originated with an article by Robert Nadelman and colleagues in the New England Journal of Medicine and has been strongly challenged by ILADS (International Lyme and Associated Diseases Society) physicians, including Daniel Cameron, MD, and others.
Sam Donta, MD, a recent member of the Department of Health & Human Services Tick-borne Working Group and a member of the Infectious Disease Society of America, said in an interview: “The problem with the one-dose doxycycline is you may not begin to develop symptoms until 2 months later.” It might mask the early symptoms of Lyme. “My impression is that the doxycycline – even the single dose – might have abrogated the ability to see an immune response. The idea, though, if you’ve had a tick bite, is to do nothing and to wait for symptoms to develop. That becomes a little bit more complex. But even then, you could choose to follow the patient and see the patient in 2 weeks and then get blood testing.”
Dr. Donta added: “I think the screening test is inadequate. So you have to go directly to the Western blot. And you have to do both the IgM and IgG” and look for specific bands.
Dr. Donta emphasized that patients should be encouraged to save any ticks that were attached and that, if at all possible, ticks should be sent to a reference lab for testing before committing a patient to a course of antibiotics. There is no harm in that brief delay, he said, and most labs can identify an array of pathogens.
The Wilderness Society guidelines on TBIs provide a good overview for clinicians practicing in limited resource settings and mirror those from the IDSA.
Dr. Ho and Dr. Donta reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recently published “Clinical Practice Guidelines for the Prevention and Management of Tick-Borne Illness,” from the Wilderness Medical Society, are a good compilation of treatment suggestions but are not, in fact, new recommendations, lead author Benjamin Ho, MD, of Southern Wisconsin Emergency Associates in Janesville, acknowledged in an interview.
Dr. Ho emphasized that the focus of the report was on “practitioners who practice in resource-limited settings” and are “the group’s way of solidifying a ... standard of practice” for such physicians. Dr. Ho also said that, while “a lot of the recommendations aren’t well supported, the risk-benefit ratio, we believe, supports the recommendations.”
The article first reviewed the different types of ticks and their distribution in the United States, the specific pathogen associated with each, the disease it causes, and comments about seasonal variations in biting behavior. Another table outlines the most common clinical syndromes, typical lab findings, recommended diagnostic testing, and antibiotic treatments. A third section contains images of different types of ticks and photos of ticks in various life-cycle stages and different levels of engorgement.
The authors were careful to note: “Several tick species are able to carry multiple pathogens. In one study, nearly 25% of Ixodes were coinfected with some combination of the bacteria or parasites causing Lyme disease, anaplasmosis, or babesiosis. Although TBI [tick-borne illness] diagnosis is not the focus of this [clinical practice guideline], providers should be aware of high rates of coinfection; the presence of one TBI should in many instances prompt testing for others.”
In terms of recommendations for preventing TBIs, the authors challenge the suggestion of wearing light-colored clothing. For repellents, they recommend DEET, picaridin, and permethrin. And they also give instructions for laundering clothing and removing ticks.
One recommendation is controversial: that of providing single-dose doxycycline as prophylaxis against Lyme disease. Dr. Ho stresses that this was only for “high-risk” tick bites, defined as a tick bite from an identified Ixodes vector species in which the tick was attached for at least 36 hours and that occurred in an endemic area.
The recommendation for prophylactic doxycycline originated with an article by Robert Nadelman and colleagues in the New England Journal of Medicine and has been strongly challenged by ILADS (International Lyme and Associated Diseases Society) physicians, including Daniel Cameron, MD, and others.
Sam Donta, MD, a recent member of the Department of Health & Human Services Tick-borne Working Group and a member of the Infectious Disease Society of America, said in an interview: “The problem with the one-dose doxycycline is you may not begin to develop symptoms until 2 months later.” It might mask the early symptoms of Lyme. “My impression is that the doxycycline – even the single dose – might have abrogated the ability to see an immune response. The idea, though, if you’ve had a tick bite, is to do nothing and to wait for symptoms to develop. That becomes a little bit more complex. But even then, you could choose to follow the patient and see the patient in 2 weeks and then get blood testing.”
Dr. Donta added: “I think the screening test is inadequate. So you have to go directly to the Western blot. And you have to do both the IgM and IgG” and look for specific bands.
Dr. Donta emphasized that patients should be encouraged to save any ticks that were attached and that, if at all possible, ticks should be sent to a reference lab for testing before committing a patient to a course of antibiotics. There is no harm in that brief delay, he said, and most labs can identify an array of pathogens.
The Wilderness Society guidelines on TBIs provide a good overview for clinicians practicing in limited resource settings and mirror those from the IDSA.
Dr. Ho and Dr. Donta reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM WILDERNESS ENVIRONMENTAL MEDICINE


