User login
Reduced-Dose Vaccines Protect Patients With HIV Against Mpox
The smallpox vaccine effectively induces immunity against mpox virus infection (formerly simian smallpox) in patients with human immunodeficiency virus (HIV) infection, although patients with lymphocyte counts below 500 cells/mm3 require booster doses, according to data from a study published in the Journal of Medical Virology.
The data come from the prospective observational study conducted by researchers at the Infection Biology Laboratory of the Department of Medicine and Life Sciences at Pompeu Fabra University and the HIV Unit of the Hospital del Mar Medical Research Institute in Barcelona, Spain. The investigators analyzed T-cell responses induced by vaccination with JYNNEOS.
Despite the substantial decrease in the reporting frequency of mpox cases from the global peak in August 2022 (30,894 cases) to 804 monthly cases in the last six months of 2023, mpox continues to circulate, and there is no specific vaccine. The JYNNEOS vaccine, with protective cross-reactivity against orthopoxviruses, is approved by the US Food and Drug Administration and the European Medicines Agency for the prevention of smallpox and mpox in adults at high risk for infection.
During the 2022 outbreak in the United States and Europe, vaccine shortages led to the emergency use authorization of a lower intradermal dose. This strategy was aimed at increasing vaccine supply up to fivefold.
Further clinical trials are needed to evaluate responses to JYNNEOS vaccination and compare different administration routes in patients with HIV infection. Protecting this population against mpox is a priority because people with high viral loads or loCD4+ T-lymphocyte counts are especially susceptible to severe disease.
Vaccination Responses
The study assessed the immune response to the JYNNEOS vaccine in patients with HIV who were receiving antiretroviral therapy as outpatients at the Infectious Diseases Unit of Hospital del Mar in Barcelona, Spain. Participants had viral loads controlled by antiretroviral therapy and CD4+ T-lymphocyte counts ≤ 500/mm3 (loCD4 group) or ≥ 500/mm3 (hiCD4 group) in blood. Vaccine responses were compared with those of vaccinated controls without the disease. The study included cases that received the standard subcutaneous vaccine (before August 2022) or the emergency dose-saving intradermal vaccine after its approval in August 2022.
The results demonstrated that the intradermal dose-saving vaccination route is preferable to the subcutaneous route and that patients in the loCD4 group may require at least one booster to generate an efficient response of specific T cells for mpox, wrote the authors.
“This study has two relevant points,” study author Robert Güerri-Fernandez, MD, PhD, head of infectious diseases at the Hospital del Mar Medical Research Institute, told this news organization. “In the subgroup of patients with HIV with effective treatment but without an immune response (ie, loCD4), the vaccine response is worse than in people who have recovered immunity or do not have HIV. Therefore, they need a booster dose.
“The second point is that the intradermal route with one-fifth of the standard subcutaneous dose has a better immune response than the standard subcutaneous route.” He added that it was a good strategy to save doses and be able to vaccinate many more people when vaccine shortages occurred.
“A general conclusion cannot be drawn,” he said. “It needs to be validated with many more subjects, of course, but in some way, it reinforced our confidence in the strategy of health authorities to promote intradermal vaccination. There we had evidence that the patients we were vaccinating intradermally were responding well.”
In Spain, although there is no shortage of vaccines today, they continue to be administered intradermally with a fractionated dose equivalent to one fifth of a standard dose, said Dr. Güerri-Fernandez.
However, in his opinion, observations regarding the two administration routes signal a need for further research. The main message should be that for patients with HIV infection who do not have an immune response, the vaccine response is incomplete, and they need booster doses as well as monitoring of the vaccine immune response, said Dr. Güerri-Fernandez.
More Studies Required
The research, which prospectively collected data and blood samples from patients with HIV who received the JYNNEOS vaccine, is small and included only 24 patients with HIV infection, with seven hospital workers who also received the vaccine and seven unvaccinated individuals as controls. “I am one of the control subjects of the study, and intradermal vaccination is not especially pleasant,” said Dr. Güerri-Fernandez. “It is a very innervated area, and the moment of introducing the liquid is uncomfortable. But it is perfectly bearable.”
Outpatient HIV-infected patients from the Infectious Diseases Unit of Hospital del Mar on antiretroviral therapy and with undetectable viral loads were grouped according to their CD4+ T-lymphocyte counts. Those with CD4+ T-lymphocyte counts ≤ 500/mm3 required at least one booster vaccine to exhibit efficient virus-specific T-lymphocyte responses. The magnitude of the T-cell response after this booster correlated directly with the CD4+ T-lymphocyte count of those vaccinated.
For Argentine infectious disease specialist Julián García, MD, clinical researcher at the Huésped Foundation in Buenos Aires, Argentina, who did not participate in the study, it is always productive to know that T-cell responses develop in patients with HIV infection, with CD4+ T-lymphocyte counts > and < 500/mm3, through an intradermal administration route.
Dr. García emphasized that the most novel aspect is that the JYNNEOS vaccine induces a specific T-cell response in patients with HIV infection that increases with higher CD4+ T-lymphocyte levels. However, he noted that the number of patients was less than 10 in most study groups, and the control group had only intradermal administration, which limits the interpretation of the results. “It will be necessary to verify this in studies with larger groups with control groups from all routes and with a correlate of protection.”
Dr. García referred to this latter point as a significant source of uncertainty. “The study is fundamentally based on the cellular response, but nowadays, there is no immune correlate of real-life protection.” He concluded that the study builds knowledge, which is essential for a vaccine that began to be used for mpox and the effectiveness of which is based on estimates.
Dr. Güerri-Fernandez and Dr. Garcia declared no relevant financial conflicts of interest.
This story was translated from the Medscape Spanish edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The smallpox vaccine effectively induces immunity against mpox virus infection (formerly simian smallpox) in patients with human immunodeficiency virus (HIV) infection, although patients with lymphocyte counts below 500 cells/mm3 require booster doses, according to data from a study published in the Journal of Medical Virology.
The data come from the prospective observational study conducted by researchers at the Infection Biology Laboratory of the Department of Medicine and Life Sciences at Pompeu Fabra University and the HIV Unit of the Hospital del Mar Medical Research Institute in Barcelona, Spain. The investigators analyzed T-cell responses induced by vaccination with JYNNEOS.
Despite the substantial decrease in the reporting frequency of mpox cases from the global peak in August 2022 (30,894 cases) to 804 monthly cases in the last six months of 2023, mpox continues to circulate, and there is no specific vaccine. The JYNNEOS vaccine, with protective cross-reactivity against orthopoxviruses, is approved by the US Food and Drug Administration and the European Medicines Agency for the prevention of smallpox and mpox in adults at high risk for infection.
During the 2022 outbreak in the United States and Europe, vaccine shortages led to the emergency use authorization of a lower intradermal dose. This strategy was aimed at increasing vaccine supply up to fivefold.
Further clinical trials are needed to evaluate responses to JYNNEOS vaccination and compare different administration routes in patients with HIV infection. Protecting this population against mpox is a priority because people with high viral loads or loCD4+ T-lymphocyte counts are especially susceptible to severe disease.
Vaccination Responses
The study assessed the immune response to the JYNNEOS vaccine in patients with HIV who were receiving antiretroviral therapy as outpatients at the Infectious Diseases Unit of Hospital del Mar in Barcelona, Spain. Participants had viral loads controlled by antiretroviral therapy and CD4+ T-lymphocyte counts ≤ 500/mm3 (loCD4 group) or ≥ 500/mm3 (hiCD4 group) in blood. Vaccine responses were compared with those of vaccinated controls without the disease. The study included cases that received the standard subcutaneous vaccine (before August 2022) or the emergency dose-saving intradermal vaccine after its approval in August 2022.
The results demonstrated that the intradermal dose-saving vaccination route is preferable to the subcutaneous route and that patients in the loCD4 group may require at least one booster to generate an efficient response of specific T cells for mpox, wrote the authors.
“This study has two relevant points,” study author Robert Güerri-Fernandez, MD, PhD, head of infectious diseases at the Hospital del Mar Medical Research Institute, told this news organization. “In the subgroup of patients with HIV with effective treatment but without an immune response (ie, loCD4), the vaccine response is worse than in people who have recovered immunity or do not have HIV. Therefore, they need a booster dose.
“The second point is that the intradermal route with one-fifth of the standard subcutaneous dose has a better immune response than the standard subcutaneous route.” He added that it was a good strategy to save doses and be able to vaccinate many more people when vaccine shortages occurred.
“A general conclusion cannot be drawn,” he said. “It needs to be validated with many more subjects, of course, but in some way, it reinforced our confidence in the strategy of health authorities to promote intradermal vaccination. There we had evidence that the patients we were vaccinating intradermally were responding well.”
In Spain, although there is no shortage of vaccines today, they continue to be administered intradermally with a fractionated dose equivalent to one fifth of a standard dose, said Dr. Güerri-Fernandez.
However, in his opinion, observations regarding the two administration routes signal a need for further research. The main message should be that for patients with HIV infection who do not have an immune response, the vaccine response is incomplete, and they need booster doses as well as monitoring of the vaccine immune response, said Dr. Güerri-Fernandez.
More Studies Required
The research, which prospectively collected data and blood samples from patients with HIV who received the JYNNEOS vaccine, is small and included only 24 patients with HIV infection, with seven hospital workers who also received the vaccine and seven unvaccinated individuals as controls. “I am one of the control subjects of the study, and intradermal vaccination is not especially pleasant,” said Dr. Güerri-Fernandez. “It is a very innervated area, and the moment of introducing the liquid is uncomfortable. But it is perfectly bearable.”
Outpatient HIV-infected patients from the Infectious Diseases Unit of Hospital del Mar on antiretroviral therapy and with undetectable viral loads were grouped according to their CD4+ T-lymphocyte counts. Those with CD4+ T-lymphocyte counts ≤ 500/mm3 required at least one booster vaccine to exhibit efficient virus-specific T-lymphocyte responses. The magnitude of the T-cell response after this booster correlated directly with the CD4+ T-lymphocyte count of those vaccinated.
For Argentine infectious disease specialist Julián García, MD, clinical researcher at the Huésped Foundation in Buenos Aires, Argentina, who did not participate in the study, it is always productive to know that T-cell responses develop in patients with HIV infection, with CD4+ T-lymphocyte counts > and < 500/mm3, through an intradermal administration route.
Dr. García emphasized that the most novel aspect is that the JYNNEOS vaccine induces a specific T-cell response in patients with HIV infection that increases with higher CD4+ T-lymphocyte levels. However, he noted that the number of patients was less than 10 in most study groups, and the control group had only intradermal administration, which limits the interpretation of the results. “It will be necessary to verify this in studies with larger groups with control groups from all routes and with a correlate of protection.”
Dr. García referred to this latter point as a significant source of uncertainty. “The study is fundamentally based on the cellular response, but nowadays, there is no immune correlate of real-life protection.” He concluded that the study builds knowledge, which is essential for a vaccine that began to be used for mpox and the effectiveness of which is based on estimates.
Dr. Güerri-Fernandez and Dr. Garcia declared no relevant financial conflicts of interest.
This story was translated from the Medscape Spanish edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The smallpox vaccine effectively induces immunity against mpox virus infection (formerly simian smallpox) in patients with human immunodeficiency virus (HIV) infection, although patients with lymphocyte counts below 500 cells/mm3 require booster doses, according to data from a study published in the Journal of Medical Virology.
The data come from the prospective observational study conducted by researchers at the Infection Biology Laboratory of the Department of Medicine and Life Sciences at Pompeu Fabra University and the HIV Unit of the Hospital del Mar Medical Research Institute in Barcelona, Spain. The investigators analyzed T-cell responses induced by vaccination with JYNNEOS.
Despite the substantial decrease in the reporting frequency of mpox cases from the global peak in August 2022 (30,894 cases) to 804 monthly cases in the last six months of 2023, mpox continues to circulate, and there is no specific vaccine. The JYNNEOS vaccine, with protective cross-reactivity against orthopoxviruses, is approved by the US Food and Drug Administration and the European Medicines Agency for the prevention of smallpox and mpox in adults at high risk for infection.
During the 2022 outbreak in the United States and Europe, vaccine shortages led to the emergency use authorization of a lower intradermal dose. This strategy was aimed at increasing vaccine supply up to fivefold.
Further clinical trials are needed to evaluate responses to JYNNEOS vaccination and compare different administration routes in patients with HIV infection. Protecting this population against mpox is a priority because people with high viral loads or loCD4+ T-lymphocyte counts are especially susceptible to severe disease.
Vaccination Responses
The study assessed the immune response to the JYNNEOS vaccine in patients with HIV who were receiving antiretroviral therapy as outpatients at the Infectious Diseases Unit of Hospital del Mar in Barcelona, Spain. Participants had viral loads controlled by antiretroviral therapy and CD4+ T-lymphocyte counts ≤ 500/mm3 (loCD4 group) or ≥ 500/mm3 (hiCD4 group) in blood. Vaccine responses were compared with those of vaccinated controls without the disease. The study included cases that received the standard subcutaneous vaccine (before August 2022) or the emergency dose-saving intradermal vaccine after its approval in August 2022.
The results demonstrated that the intradermal dose-saving vaccination route is preferable to the subcutaneous route and that patients in the loCD4 group may require at least one booster to generate an efficient response of specific T cells for mpox, wrote the authors.
“This study has two relevant points,” study author Robert Güerri-Fernandez, MD, PhD, head of infectious diseases at the Hospital del Mar Medical Research Institute, told this news organization. “In the subgroup of patients with HIV with effective treatment but without an immune response (ie, loCD4), the vaccine response is worse than in people who have recovered immunity or do not have HIV. Therefore, they need a booster dose.
“The second point is that the intradermal route with one-fifth of the standard subcutaneous dose has a better immune response than the standard subcutaneous route.” He added that it was a good strategy to save doses and be able to vaccinate many more people when vaccine shortages occurred.
“A general conclusion cannot be drawn,” he said. “It needs to be validated with many more subjects, of course, but in some way, it reinforced our confidence in the strategy of health authorities to promote intradermal vaccination. There we had evidence that the patients we were vaccinating intradermally were responding well.”
In Spain, although there is no shortage of vaccines today, they continue to be administered intradermally with a fractionated dose equivalent to one fifth of a standard dose, said Dr. Güerri-Fernandez.
However, in his opinion, observations regarding the two administration routes signal a need for further research. The main message should be that for patients with HIV infection who do not have an immune response, the vaccine response is incomplete, and they need booster doses as well as monitoring of the vaccine immune response, said Dr. Güerri-Fernandez.
More Studies Required
The research, which prospectively collected data and blood samples from patients with HIV who received the JYNNEOS vaccine, is small and included only 24 patients with HIV infection, with seven hospital workers who also received the vaccine and seven unvaccinated individuals as controls. “I am one of the control subjects of the study, and intradermal vaccination is not especially pleasant,” said Dr. Güerri-Fernandez. “It is a very innervated area, and the moment of introducing the liquid is uncomfortable. But it is perfectly bearable.”
Outpatient HIV-infected patients from the Infectious Diseases Unit of Hospital del Mar on antiretroviral therapy and with undetectable viral loads were grouped according to their CD4+ T-lymphocyte counts. Those with CD4+ T-lymphocyte counts ≤ 500/mm3 required at least one booster vaccine to exhibit efficient virus-specific T-lymphocyte responses. The magnitude of the T-cell response after this booster correlated directly with the CD4+ T-lymphocyte count of those vaccinated.
For Argentine infectious disease specialist Julián García, MD, clinical researcher at the Huésped Foundation in Buenos Aires, Argentina, who did not participate in the study, it is always productive to know that T-cell responses develop in patients with HIV infection, with CD4+ T-lymphocyte counts > and < 500/mm3, through an intradermal administration route.
Dr. García emphasized that the most novel aspect is that the JYNNEOS vaccine induces a specific T-cell response in patients with HIV infection that increases with higher CD4+ T-lymphocyte levels. However, he noted that the number of patients was less than 10 in most study groups, and the control group had only intradermal administration, which limits the interpretation of the results. “It will be necessary to verify this in studies with larger groups with control groups from all routes and with a correlate of protection.”
Dr. García referred to this latter point as a significant source of uncertainty. “The study is fundamentally based on the cellular response, but nowadays, there is no immune correlate of real-life protection.” He concluded that the study builds knowledge, which is essential for a vaccine that began to be used for mpox and the effectiveness of which is based on estimates.
Dr. Güerri-Fernandez and Dr. Garcia declared no relevant financial conflicts of interest.
This story was translated from the Medscape Spanish edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Curbing Antibiotic Use Works
, revealed the fourth joint interagency antimicrobial consumption and resistance analysis report.
The report was published by the European Centre for Disease Prevention and Control, the European Food Safety Authority, and the European Medicines Agency. Its findings were derived from an integrated analysis of the potential relationship between antimicrobial consumption (AMC) by humans and animals and the occurrence of antimicrobial resistance (AMR) using data collected between 2019 and 2021.
A Real Threat
AMR poses a significant threat to public and animal health, causing more than 35,000 deaths annually in the European Union (EU) and the European Economic Area. It also imposes a substantial economic burden on European healthcare systems, amounting to approximately €11.7 billion per year.
To address this challenge, the Council of the European Union recommended concerted and sustained efforts to achieve a 20% reduction in AMC in humans (compared with 2019 levels) and a 50% reduction in food-producing animals (compared with 2018 levels) by 2030. These targets are outlined in the European Commission’s Farm to Fork strategy.
It Really Works
Analysis of the trends of AMC and AMR in Escherichia coli from humans and food-producing animals, conducted for the first time, revealed that the susceptibility of E coli to antimicrobials in humans and animals increases with an overall decrease in the consumption of antibiotics.
Concurrent trends in AMC and AMR from 2014 to 2021 were also assessed. AMC in both human and animal sectors, measured in mg/kg of estimated biomass, was compared at country and European levels. In 2021, human AMC totaled 125.0 mg/kg of biomass, while food-producing animals registered 92.6 mg/kg of biomass.
Over the 2014-2021 period, total AMC in food-producing animals decreased by 44%, while in humans, it remained relatively stable. The consumption of certain antimicrobials was positively associated with resistance to those substances in bacteria from both humans and food-producing animals.
The report also highlighted that E coli resistance is linked in humans to the use of carbapenems, third- and fourth-generation cephalosporins, and quinolones and in food-producing animals to the administration of quinolones, polymyxins, aminopenicillins, and tetracyclines. Further, a connection exists between bacterial resistance in humans and food-producing animals, particularly for bacterial species such as Campylobacter jejuni and C coli.
The findings suggest that measures to reduce AMC in both food-producing animals and humans have been effective in many countries. However, reinforcing these measures is crucial to maintain and further advance reductions in AMC.
More Work
Aligned with the European Commission’s One Health holistic and coordinated approach to managing the human and veterinary sectors together, the European agencies advocate for:
- Sustained efforts to combat AMR at national, EU, and global levels.
- Coordinated surveillance of antibiotic use and AMR in both human and animal sectors.
- Continued research in the field of AMR.
The statistical code used to conduct these analyses was made publicly available in order to support further research analyses.
A version of this article appeared on Medscape.com.
, revealed the fourth joint interagency antimicrobial consumption and resistance analysis report.
The report was published by the European Centre for Disease Prevention and Control, the European Food Safety Authority, and the European Medicines Agency. Its findings were derived from an integrated analysis of the potential relationship between antimicrobial consumption (AMC) by humans and animals and the occurrence of antimicrobial resistance (AMR) using data collected between 2019 and 2021.
A Real Threat
AMR poses a significant threat to public and animal health, causing more than 35,000 deaths annually in the European Union (EU) and the European Economic Area. It also imposes a substantial economic burden on European healthcare systems, amounting to approximately €11.7 billion per year.
To address this challenge, the Council of the European Union recommended concerted and sustained efforts to achieve a 20% reduction in AMC in humans (compared with 2019 levels) and a 50% reduction in food-producing animals (compared with 2018 levels) by 2030. These targets are outlined in the European Commission’s Farm to Fork strategy.
It Really Works
Analysis of the trends of AMC and AMR in Escherichia coli from humans and food-producing animals, conducted for the first time, revealed that the susceptibility of E coli to antimicrobials in humans and animals increases with an overall decrease in the consumption of antibiotics.
Concurrent trends in AMC and AMR from 2014 to 2021 were also assessed. AMC in both human and animal sectors, measured in mg/kg of estimated biomass, was compared at country and European levels. In 2021, human AMC totaled 125.0 mg/kg of biomass, while food-producing animals registered 92.6 mg/kg of biomass.
Over the 2014-2021 period, total AMC in food-producing animals decreased by 44%, while in humans, it remained relatively stable. The consumption of certain antimicrobials was positively associated with resistance to those substances in bacteria from both humans and food-producing animals.
The report also highlighted that E coli resistance is linked in humans to the use of carbapenems, third- and fourth-generation cephalosporins, and quinolones and in food-producing animals to the administration of quinolones, polymyxins, aminopenicillins, and tetracyclines. Further, a connection exists between bacterial resistance in humans and food-producing animals, particularly for bacterial species such as Campylobacter jejuni and C coli.
The findings suggest that measures to reduce AMC in both food-producing animals and humans have been effective in many countries. However, reinforcing these measures is crucial to maintain and further advance reductions in AMC.
More Work
Aligned with the European Commission’s One Health holistic and coordinated approach to managing the human and veterinary sectors together, the European agencies advocate for:
- Sustained efforts to combat AMR at national, EU, and global levels.
- Coordinated surveillance of antibiotic use and AMR in both human and animal sectors.
- Continued research in the field of AMR.
The statistical code used to conduct these analyses was made publicly available in order to support further research analyses.
A version of this article appeared on Medscape.com.
, revealed the fourth joint interagency antimicrobial consumption and resistance analysis report.
The report was published by the European Centre for Disease Prevention and Control, the European Food Safety Authority, and the European Medicines Agency. Its findings were derived from an integrated analysis of the potential relationship between antimicrobial consumption (AMC) by humans and animals and the occurrence of antimicrobial resistance (AMR) using data collected between 2019 and 2021.
A Real Threat
AMR poses a significant threat to public and animal health, causing more than 35,000 deaths annually in the European Union (EU) and the European Economic Area. It also imposes a substantial economic burden on European healthcare systems, amounting to approximately €11.7 billion per year.
To address this challenge, the Council of the European Union recommended concerted and sustained efforts to achieve a 20% reduction in AMC in humans (compared with 2019 levels) and a 50% reduction in food-producing animals (compared with 2018 levels) by 2030. These targets are outlined in the European Commission’s Farm to Fork strategy.
It Really Works
Analysis of the trends of AMC and AMR in Escherichia coli from humans and food-producing animals, conducted for the first time, revealed that the susceptibility of E coli to antimicrobials in humans and animals increases with an overall decrease in the consumption of antibiotics.
Concurrent trends in AMC and AMR from 2014 to 2021 were also assessed. AMC in both human and animal sectors, measured in mg/kg of estimated biomass, was compared at country and European levels. In 2021, human AMC totaled 125.0 mg/kg of biomass, while food-producing animals registered 92.6 mg/kg of biomass.
Over the 2014-2021 period, total AMC in food-producing animals decreased by 44%, while in humans, it remained relatively stable. The consumption of certain antimicrobials was positively associated with resistance to those substances in bacteria from both humans and food-producing animals.
The report also highlighted that E coli resistance is linked in humans to the use of carbapenems, third- and fourth-generation cephalosporins, and quinolones and in food-producing animals to the administration of quinolones, polymyxins, aminopenicillins, and tetracyclines. Further, a connection exists between bacterial resistance in humans and food-producing animals, particularly for bacterial species such as Campylobacter jejuni and C coli.
The findings suggest that measures to reduce AMC in both food-producing animals and humans have been effective in many countries. However, reinforcing these measures is crucial to maintain and further advance reductions in AMC.
More Work
Aligned with the European Commission’s One Health holistic and coordinated approach to managing the human and veterinary sectors together, the European agencies advocate for:
- Sustained efforts to combat AMR at national, EU, and global levels.
- Coordinated surveillance of antibiotic use and AMR in both human and animal sectors.
- Continued research in the field of AMR.
The statistical code used to conduct these analyses was made publicly available in order to support further research analyses.
A version of this article appeared on Medscape.com.
Study IDs Immune Abnormality Possibly Causing Long COVID
Swiss scientists have identified immune system abnormalities in patients with long COVID that might open the door to new diagnostic tests and treatments.
The researchers found that a group of proteins in the blood that are part of the body’s immune response called the “complement system” are not working properly in patients with long COVID.
Blood samples turned up important differences between those who recovered from COVID and those who did not. These differences might be used as biomarkers to diagnose long COVID and might even point the way to new treatments for the condition, the researchers said.
By testing for 6500 blood proteins in about 300 patients, the Swiss researchers found that dysfunctional complement system proteins could possibly explain fatigue and “smoldering inflammation,” said Onur Boyman, MD, a professor of immunology from University Hospital Zurich in Zurich, Switzerland.
Long COVID has been linked to hundreds of symptoms including brain fog, chronic fatigue, pain, and digestive issues. Various factors drive the condition and likely work with one another other, said David Putrino, PhD, from the Icahn School of Medicine at Mount Sinai in New York City. The Swiss study is useful because “we’re trying to best understand how we can explain all of this far-reaching pathobiology,” he said.
Testing Across Continents
Dr. Boyman’s team collected blood samples from people with COVID in Europe and New York and tracked them. They compared those who developed long COVID with those who did not. One protein that was most unique to patients with long COVID is a blood complement that activates the immune system, Dr. Boyman said. But in people with long COVID, the immune response stays activated after the virus is gone. He described the response as “smoldering inflammation” in multiple organs, including the lungs and the gastrointestinal system.
The complement system also plays a role in clearing the body of dead cells. If the cells “lie around too much,” they can trigger an immune response, he said.
That may explain exercise intolerance in people with long COVID, Dr. Boyman said. Some people with long COVID have inflammation in the epithelium — the inner layer of their blood vessels. This would make it harder for the circulatory systems to recover from exercise, Dr. Boyman said.
“We think this regulated complement system is actually quite a central piece of the puzzle,” he said.
The Microclot Connection
The findings also support past research linking blood clots to long COVID. He suggested that clinicians and researchers consider testing drugs that regulate or inhibit the complementary system as a treatment of long COVID. Dr. Boyman said they are currently used for rare immune diseases.
Resia Pretorius, PhD, a professor of physiological sciences at Stellenbosch University in Stellenbosch, South Africa, said scientists studying the role of microclots in patients with long COVID often see complementary proteins inside the clots, so it has already been associated with long COVID. But she likened this clotting process to a garbage can that “just rolls along and collects everything that gets in its way. I think they are actively driving inflammation and disease.”
One factor complicating long COVID diagnosis and treatment is that it is a complex condition that involves multiple organ systems. That’s why the latest research suggests an underlying driver for the multiple symptoms of long COVID, Dr. Putrino said.
“Not every person has every symptom; not every person has every organ system affected,” Dr. Putrino said. “Whatever is happening is decided across the whole body.”
Research Offers New Direction
The Swiss paper contributes to the effort to identify systemic issues contributing to long COVID. It gives researchers one more thing to test for and link to specific, long COVID symptoms, opening the door to new treatments, Dr. Putrino said.
He doesn’t think the study supports treating the complement dysfunction if researchers don’t know what’s driving it. It may be complicated by the body’s failure to clear the virus completely, he said.
Dr. Pretorius recommended doctors test patients with long COVID for specific symptoms that may be treated using existing therapies. “If you think your patient had vascular pathology, you can test for it,” she said.
Some patients have found certain supplements and over-the-counter products helpful, she said. Among them: Coenzyme Q 10 and clot-busters such as streptokinase and Nattokinase (though she noted some doctors may not be comfortable with supplements).
“It’s the only thing we have until we’ve got trials,” she said.
Dr. Putrino said more research is needed to identify potential root causes and symptoms. A common refrain, but the only thing that will lead to specific treatments.
A version of this article appeared on Medscape.com.
Swiss scientists have identified immune system abnormalities in patients with long COVID that might open the door to new diagnostic tests and treatments.
The researchers found that a group of proteins in the blood that are part of the body’s immune response called the “complement system” are not working properly in patients with long COVID.
Blood samples turned up important differences between those who recovered from COVID and those who did not. These differences might be used as biomarkers to diagnose long COVID and might even point the way to new treatments for the condition, the researchers said.
By testing for 6500 blood proteins in about 300 patients, the Swiss researchers found that dysfunctional complement system proteins could possibly explain fatigue and “smoldering inflammation,” said Onur Boyman, MD, a professor of immunology from University Hospital Zurich in Zurich, Switzerland.
Long COVID has been linked to hundreds of symptoms including brain fog, chronic fatigue, pain, and digestive issues. Various factors drive the condition and likely work with one another other, said David Putrino, PhD, from the Icahn School of Medicine at Mount Sinai in New York City. The Swiss study is useful because “we’re trying to best understand how we can explain all of this far-reaching pathobiology,” he said.
Testing Across Continents
Dr. Boyman’s team collected blood samples from people with COVID in Europe and New York and tracked them. They compared those who developed long COVID with those who did not. One protein that was most unique to patients with long COVID is a blood complement that activates the immune system, Dr. Boyman said. But in people with long COVID, the immune response stays activated after the virus is gone. He described the response as “smoldering inflammation” in multiple organs, including the lungs and the gastrointestinal system.
The complement system also plays a role in clearing the body of dead cells. If the cells “lie around too much,” they can trigger an immune response, he said.
That may explain exercise intolerance in people with long COVID, Dr. Boyman said. Some people with long COVID have inflammation in the epithelium — the inner layer of their blood vessels. This would make it harder for the circulatory systems to recover from exercise, Dr. Boyman said.
“We think this regulated complement system is actually quite a central piece of the puzzle,” he said.
The Microclot Connection
The findings also support past research linking blood clots to long COVID. He suggested that clinicians and researchers consider testing drugs that regulate or inhibit the complementary system as a treatment of long COVID. Dr. Boyman said they are currently used for rare immune diseases.
Resia Pretorius, PhD, a professor of physiological sciences at Stellenbosch University in Stellenbosch, South Africa, said scientists studying the role of microclots in patients with long COVID often see complementary proteins inside the clots, so it has already been associated with long COVID. But she likened this clotting process to a garbage can that “just rolls along and collects everything that gets in its way. I think they are actively driving inflammation and disease.”
One factor complicating long COVID diagnosis and treatment is that it is a complex condition that involves multiple organ systems. That’s why the latest research suggests an underlying driver for the multiple symptoms of long COVID, Dr. Putrino said.
“Not every person has every symptom; not every person has every organ system affected,” Dr. Putrino said. “Whatever is happening is decided across the whole body.”
Research Offers New Direction
The Swiss paper contributes to the effort to identify systemic issues contributing to long COVID. It gives researchers one more thing to test for and link to specific, long COVID symptoms, opening the door to new treatments, Dr. Putrino said.
He doesn’t think the study supports treating the complement dysfunction if researchers don’t know what’s driving it. It may be complicated by the body’s failure to clear the virus completely, he said.
Dr. Pretorius recommended doctors test patients with long COVID for specific symptoms that may be treated using existing therapies. “If you think your patient had vascular pathology, you can test for it,” she said.
Some patients have found certain supplements and over-the-counter products helpful, she said. Among them: Coenzyme Q 10 and clot-busters such as streptokinase and Nattokinase (though she noted some doctors may not be comfortable with supplements).
“It’s the only thing we have until we’ve got trials,” she said.
Dr. Putrino said more research is needed to identify potential root causes and symptoms. A common refrain, but the only thing that will lead to specific treatments.
A version of this article appeared on Medscape.com.
Swiss scientists have identified immune system abnormalities in patients with long COVID that might open the door to new diagnostic tests and treatments.
The researchers found that a group of proteins in the blood that are part of the body’s immune response called the “complement system” are not working properly in patients with long COVID.
Blood samples turned up important differences between those who recovered from COVID and those who did not. These differences might be used as biomarkers to diagnose long COVID and might even point the way to new treatments for the condition, the researchers said.
By testing for 6500 blood proteins in about 300 patients, the Swiss researchers found that dysfunctional complement system proteins could possibly explain fatigue and “smoldering inflammation,” said Onur Boyman, MD, a professor of immunology from University Hospital Zurich in Zurich, Switzerland.
Long COVID has been linked to hundreds of symptoms including brain fog, chronic fatigue, pain, and digestive issues. Various factors drive the condition and likely work with one another other, said David Putrino, PhD, from the Icahn School of Medicine at Mount Sinai in New York City. The Swiss study is useful because “we’re trying to best understand how we can explain all of this far-reaching pathobiology,” he said.
Testing Across Continents
Dr. Boyman’s team collected blood samples from people with COVID in Europe and New York and tracked them. They compared those who developed long COVID with those who did not. One protein that was most unique to patients with long COVID is a blood complement that activates the immune system, Dr. Boyman said. But in people with long COVID, the immune response stays activated after the virus is gone. He described the response as “smoldering inflammation” in multiple organs, including the lungs and the gastrointestinal system.
The complement system also plays a role in clearing the body of dead cells. If the cells “lie around too much,” they can trigger an immune response, he said.
That may explain exercise intolerance in people with long COVID, Dr. Boyman said. Some people with long COVID have inflammation in the epithelium — the inner layer of their blood vessels. This would make it harder for the circulatory systems to recover from exercise, Dr. Boyman said.
“We think this regulated complement system is actually quite a central piece of the puzzle,” he said.
The Microclot Connection
The findings also support past research linking blood clots to long COVID. He suggested that clinicians and researchers consider testing drugs that regulate or inhibit the complementary system as a treatment of long COVID. Dr. Boyman said they are currently used for rare immune diseases.
Resia Pretorius, PhD, a professor of physiological sciences at Stellenbosch University in Stellenbosch, South Africa, said scientists studying the role of microclots in patients with long COVID often see complementary proteins inside the clots, so it has already been associated with long COVID. But she likened this clotting process to a garbage can that “just rolls along and collects everything that gets in its way. I think they are actively driving inflammation and disease.”
One factor complicating long COVID diagnosis and treatment is that it is a complex condition that involves multiple organ systems. That’s why the latest research suggests an underlying driver for the multiple symptoms of long COVID, Dr. Putrino said.
“Not every person has every symptom; not every person has every organ system affected,” Dr. Putrino said. “Whatever is happening is decided across the whole body.”
Research Offers New Direction
The Swiss paper contributes to the effort to identify systemic issues contributing to long COVID. It gives researchers one more thing to test for and link to specific, long COVID symptoms, opening the door to new treatments, Dr. Putrino said.
He doesn’t think the study supports treating the complement dysfunction if researchers don’t know what’s driving it. It may be complicated by the body’s failure to clear the virus completely, he said.
Dr. Pretorius recommended doctors test patients with long COVID for specific symptoms that may be treated using existing therapies. “If you think your patient had vascular pathology, you can test for it,” she said.
Some patients have found certain supplements and over-the-counter products helpful, she said. Among them: Coenzyme Q 10 and clot-busters such as streptokinase and Nattokinase (though she noted some doctors may not be comfortable with supplements).
“It’s the only thing we have until we’ve got trials,” she said.
Dr. Putrino said more research is needed to identify potential root causes and symptoms. A common refrain, but the only thing that will lead to specific treatments.
A version of this article appeared on Medscape.com.
Herpes Zoster and Varicella Encephalitis Following the Recombinant Zoster Vaccine
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
Practice Points
- Patients with chronic lymphocytic leukemia (CLL) are at risk for herpes zoster reactivation even with vaccination due to a decreased immune response. These patients may have an aberrant response due to immune cell dysregulation.
- It is important to increase monitoring of CLL patients for signs of viral reactivation and shift the focus to providing antiviral therapy quickly if herpes zoster symptoms occur.
Rapidly Progressive Necrotizing Myositis Mimicking Pyoderma Gangrenosum
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
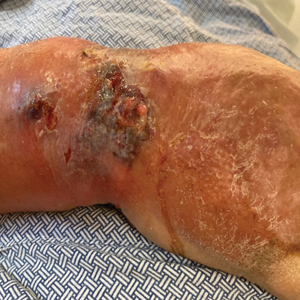
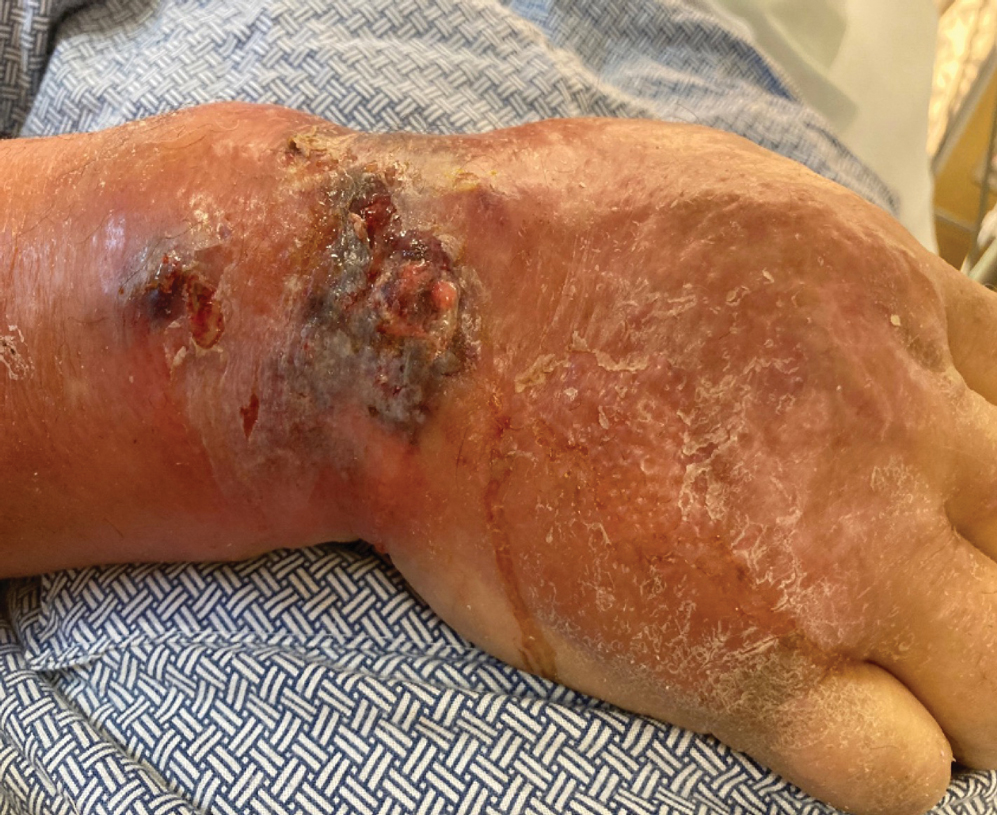
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.
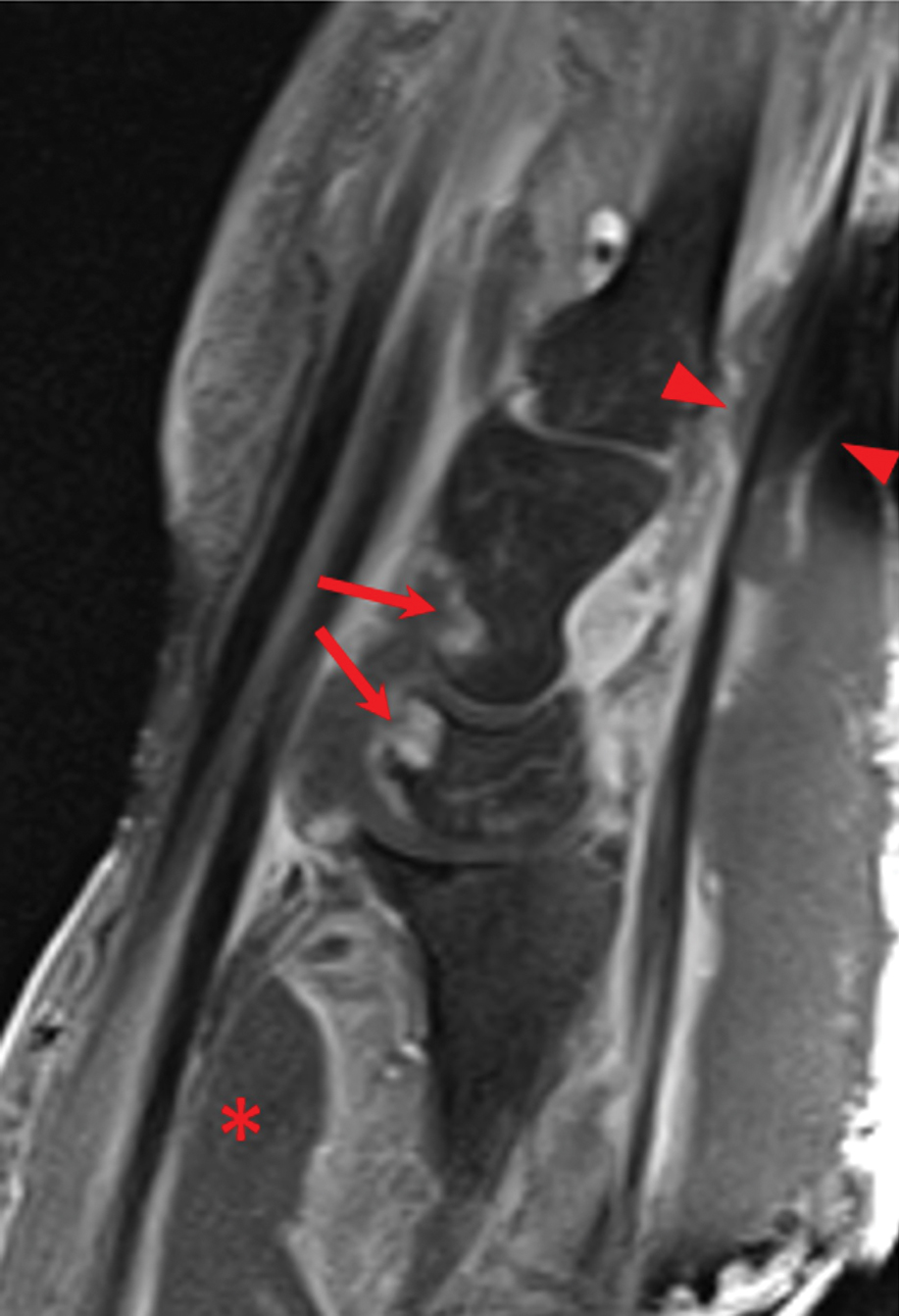
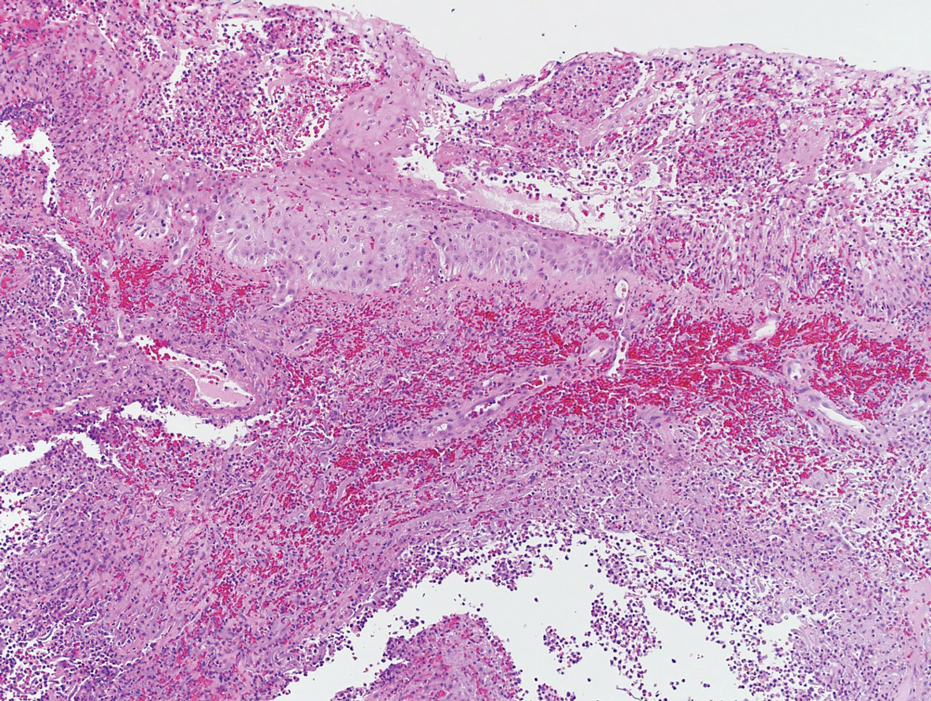
Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).
Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.
Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
Practice Points
- The accurate diagnosis of necrotizing myositis (NM) requires a multimodal approach with complete clinical, histological, and radiographic correlation.
- Necrotizing myositis can manifest as violaceous erythematous plaques, bullae, blisters, or vesicles with petechiae, marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane.
- The differential diagnosis of NM includes pyoderma gangrenosum.
Dermatologic Reactions Following COVID-19 Vaccination: A Case Series
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).
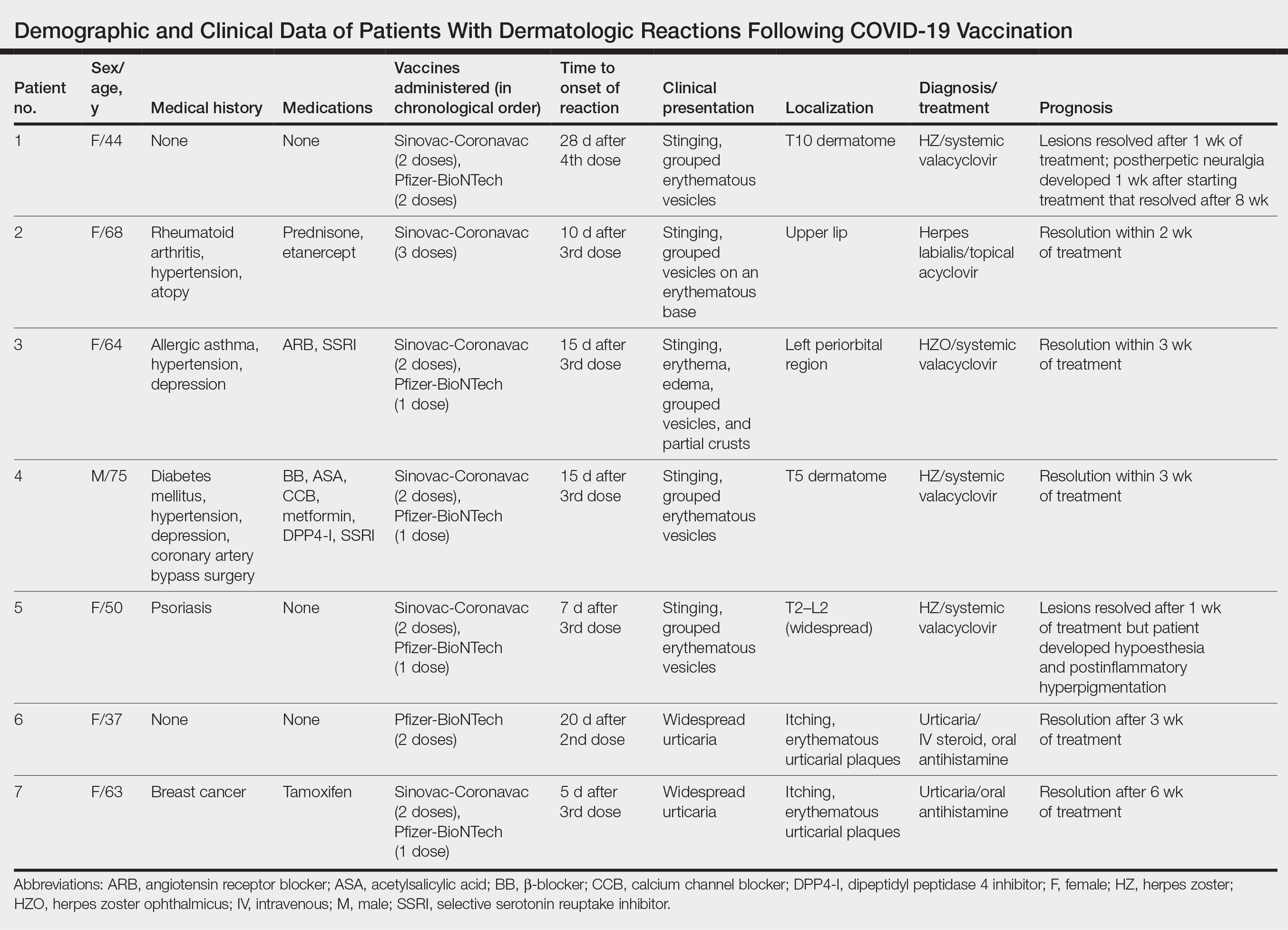
Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Practice Points
- Cutaneous reactions have been reported following COVID-19 vaccination.
- Herpes infections and urticarial reactions can be associated with COVID-19 vaccination, regardless of the delay in onset between the injection and symptom development.
The Daycare Petri Dish
I can’t remember where I heard it. Maybe I made it up myself. But, one definition of a family is a group of folks with whom you share your genes and germs. In that same vein, one could define daycare as a group of germ-sharing children. Of course that’s news to almost no one. Parents who decide, or are forced, to send their children to daycare expect that those children will get more colds, “stomach flu,” and ear infections than the children who spend their days in isolation at home. Everyone from the pediatrician to the little old lady next door has warned parents of the inevitable reality of daycare. Of course there are upsides that parents can cling to, including increased socialization and the hope that getting sick young will build a more robust immunity in the long run.
However, there has been little research exploring the nuances of the germ sharing that we all know is happening in these and other social settings.
A team of evolutionary biologists at Harvard is working to better define the “social microbiome” and its “role in individuals’ susceptibility to, and resilience against, both communicable and noncommunicable diseases.” These researchers point out that while we harbor our own unique collection of microbes, we share those with the microbiomes of the people with whom we interact socially. They report that studies by other investigators have shown that residents of a household share a significant proportion of their gastrointestinal flora. There are other studies that have shown that villages can be identified by their own social biome. There are few social settings more intimately involved in microbe sharing than daycares. I have a friends who calls them “petri dishes.”
The biologists point out that antibiotic-resistant microbes can become part of an individual’s microbiome and can be shared with other individuals in their social group, who can then go on and share them in a different social environment. Imagine there is one popular physician in a community whose sense of antibiotic stewardship is, shall we say, somewhat lacking. By inappropriately prescribing antibiotics to a child or two in a daycare, he may be altering the social biome in that daycare, which could then jeopardize the health of all the children and eventually their own home-based social biomes, that may include an immune deficient individual.
The researchers also remind us that different cultures and countries may have different antibiotic usage patterns. Does this mean I am taking a risk by traveling in these “culture-dependent transmission landscapes”? Am I more likely to encounter an antibiotic-resistant microbe when I am visiting a country whose healthcare providers are less prudent prescribers?
However, as these evolutionary biologists point out, not all shared microbes are bad. There is some evidence in animals that individuals can share microbes that have been found to “increase resilience against colitis or improve their responsiveness to cancer therapy.” If a microbe can contribute to a disease that was once considered to be “noncommunicable,” we may need to redefine “communicable” in the light this more nuanced view of the social biome.
It became standard practice during the COVID pandemic to test dormitory and community sewage water to determine the level of infection. Can sewage water be used as a proxy for a social biome? If a parent is lucky enough to have a choice of daycares, would knowing each facility’s biome, as reflected in an analysis of its sewage effluent, help him or her decide? Should a daycare ask for a stool sample from each child before accepting him or her? Seems like this would raise some privacy issues, not to mention the logistical messiness of the process. As we learn more about social biomes, can we imagine a time when a daycare or country or region might proudly advertise itself as having the healthiest spectrum of microbes in its sewage system?
Communal living certainly has its benefits, not just for children but also adults as we realize how loneliness is eating its way into our society. However,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I can’t remember where I heard it. Maybe I made it up myself. But, one definition of a family is a group of folks with whom you share your genes and germs. In that same vein, one could define daycare as a group of germ-sharing children. Of course that’s news to almost no one. Parents who decide, or are forced, to send their children to daycare expect that those children will get more colds, “stomach flu,” and ear infections than the children who spend their days in isolation at home. Everyone from the pediatrician to the little old lady next door has warned parents of the inevitable reality of daycare. Of course there are upsides that parents can cling to, including increased socialization and the hope that getting sick young will build a more robust immunity in the long run.
However, there has been little research exploring the nuances of the germ sharing that we all know is happening in these and other social settings.
A team of evolutionary biologists at Harvard is working to better define the “social microbiome” and its “role in individuals’ susceptibility to, and resilience against, both communicable and noncommunicable diseases.” These researchers point out that while we harbor our own unique collection of microbes, we share those with the microbiomes of the people with whom we interact socially. They report that studies by other investigators have shown that residents of a household share a significant proportion of their gastrointestinal flora. There are other studies that have shown that villages can be identified by their own social biome. There are few social settings more intimately involved in microbe sharing than daycares. I have a friends who calls them “petri dishes.”
The biologists point out that antibiotic-resistant microbes can become part of an individual’s microbiome and can be shared with other individuals in their social group, who can then go on and share them in a different social environment. Imagine there is one popular physician in a community whose sense of antibiotic stewardship is, shall we say, somewhat lacking. By inappropriately prescribing antibiotics to a child or two in a daycare, he may be altering the social biome in that daycare, which could then jeopardize the health of all the children and eventually their own home-based social biomes, that may include an immune deficient individual.
The researchers also remind us that different cultures and countries may have different antibiotic usage patterns. Does this mean I am taking a risk by traveling in these “culture-dependent transmission landscapes”? Am I more likely to encounter an antibiotic-resistant microbe when I am visiting a country whose healthcare providers are less prudent prescribers?
However, as these evolutionary biologists point out, not all shared microbes are bad. There is some evidence in animals that individuals can share microbes that have been found to “increase resilience against colitis or improve their responsiveness to cancer therapy.” If a microbe can contribute to a disease that was once considered to be “noncommunicable,” we may need to redefine “communicable” in the light this more nuanced view of the social biome.
It became standard practice during the COVID pandemic to test dormitory and community sewage water to determine the level of infection. Can sewage water be used as a proxy for a social biome? If a parent is lucky enough to have a choice of daycares, would knowing each facility’s biome, as reflected in an analysis of its sewage effluent, help him or her decide? Should a daycare ask for a stool sample from each child before accepting him or her? Seems like this would raise some privacy issues, not to mention the logistical messiness of the process. As we learn more about social biomes, can we imagine a time when a daycare or country or region might proudly advertise itself as having the healthiest spectrum of microbes in its sewage system?
Communal living certainly has its benefits, not just for children but also adults as we realize how loneliness is eating its way into our society. However,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I can’t remember where I heard it. Maybe I made it up myself. But, one definition of a family is a group of folks with whom you share your genes and germs. In that same vein, one could define daycare as a group of germ-sharing children. Of course that’s news to almost no one. Parents who decide, or are forced, to send their children to daycare expect that those children will get more colds, “stomach flu,” and ear infections than the children who spend their days in isolation at home. Everyone from the pediatrician to the little old lady next door has warned parents of the inevitable reality of daycare. Of course there are upsides that parents can cling to, including increased socialization and the hope that getting sick young will build a more robust immunity in the long run.
However, there has been little research exploring the nuances of the germ sharing that we all know is happening in these and other social settings.
A team of evolutionary biologists at Harvard is working to better define the “social microbiome” and its “role in individuals’ susceptibility to, and resilience against, both communicable and noncommunicable diseases.” These researchers point out that while we harbor our own unique collection of microbes, we share those with the microbiomes of the people with whom we interact socially. They report that studies by other investigators have shown that residents of a household share a significant proportion of their gastrointestinal flora. There are other studies that have shown that villages can be identified by their own social biome. There are few social settings more intimately involved in microbe sharing than daycares. I have a friends who calls them “petri dishes.”
The biologists point out that antibiotic-resistant microbes can become part of an individual’s microbiome and can be shared with other individuals in their social group, who can then go on and share them in a different social environment. Imagine there is one popular physician in a community whose sense of antibiotic stewardship is, shall we say, somewhat lacking. By inappropriately prescribing antibiotics to a child or two in a daycare, he may be altering the social biome in that daycare, which could then jeopardize the health of all the children and eventually their own home-based social biomes, that may include an immune deficient individual.
The researchers also remind us that different cultures and countries may have different antibiotic usage patterns. Does this mean I am taking a risk by traveling in these “culture-dependent transmission landscapes”? Am I more likely to encounter an antibiotic-resistant microbe when I am visiting a country whose healthcare providers are less prudent prescribers?
However, as these evolutionary biologists point out, not all shared microbes are bad. There is some evidence in animals that individuals can share microbes that have been found to “increase resilience against colitis or improve their responsiveness to cancer therapy.” If a microbe can contribute to a disease that was once considered to be “noncommunicable,” we may need to redefine “communicable” in the light this more nuanced view of the social biome.
It became standard practice during the COVID pandemic to test dormitory and community sewage water to determine the level of infection. Can sewage water be used as a proxy for a social biome? If a parent is lucky enough to have a choice of daycares, would knowing each facility’s biome, as reflected in an analysis of its sewage effluent, help him or her decide? Should a daycare ask for a stool sample from each child before accepting him or her? Seems like this would raise some privacy issues, not to mention the logistical messiness of the process. As we learn more about social biomes, can we imagine a time when a daycare or country or region might proudly advertise itself as having the healthiest spectrum of microbes in its sewage system?
Communal living certainly has its benefits, not just for children but also adults as we realize how loneliness is eating its way into our society. However,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
What Skin Manifestations Are Associated With Pediatric IBD?
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
Long-Term Follow-Up Emphasizes HPV Vaccination Importance
This transcript has been edited for clarity.
I want to briefly discuss a critically important topic that cannot be overly emphasized. It is the relevance, the importance, the benefits, and the outcome of HPV vaccination.
The paper I’m referring to was published in Pediatrics in October 2023. It’s titled, “Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety.”
Let me emphasize that we’re talking about a 10-year follow-up. In this particular paper and analysis, 301 boys — I emphasize boys — were included and 971 girls at 40 different sites in 13 countries, who received the 9-valent vaccine, which includes HPV 16, 18, and seven other types.
Most importantly, there was not a single case. Not one. Let me repeat this: There was not a single case of high-grade intraepithelial neoplasia, or worse, or condyloma in either males or females. There was not a single case in over 1000 individuals with a follow-up of more than 10 years.
It is difficult to overstate the magnitude of the benefit associated with HPV vaccination for our children and young adults on their risk of developing highly relevant, life-changing, potentially deadly cancers.
For those of you who are interested in this topic — which should include almost all of you, if not all of you — I encourage you to read this very important follow-up paper, again, demonstrating the simple, overwhelming magnitude of the benefit of HPV vaccination. I thank you for your attention.
Dr. Markman is a professor in the department of medical oncology and therapeutics research, City of Hope, Duarte, California, and president of medicine and science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline; AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly discuss a critically important topic that cannot be overly emphasized. It is the relevance, the importance, the benefits, and the outcome of HPV vaccination.
The paper I’m referring to was published in Pediatrics in October 2023. It’s titled, “Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety.”
Let me emphasize that we’re talking about a 10-year follow-up. In this particular paper and analysis, 301 boys — I emphasize boys — were included and 971 girls at 40 different sites in 13 countries, who received the 9-valent vaccine, which includes HPV 16, 18, and seven other types.
Most importantly, there was not a single case. Not one. Let me repeat this: There was not a single case of high-grade intraepithelial neoplasia, or worse, or condyloma in either males or females. There was not a single case in over 1000 individuals with a follow-up of more than 10 years.
It is difficult to overstate the magnitude of the benefit associated with HPV vaccination for our children and young adults on their risk of developing highly relevant, life-changing, potentially deadly cancers.
For those of you who are interested in this topic — which should include almost all of you, if not all of you — I encourage you to read this very important follow-up paper, again, demonstrating the simple, overwhelming magnitude of the benefit of HPV vaccination. I thank you for your attention.
Dr. Markman is a professor in the department of medical oncology and therapeutics research, City of Hope, Duarte, California, and president of medicine and science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline; AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly discuss a critically important topic that cannot be overly emphasized. It is the relevance, the importance, the benefits, and the outcome of HPV vaccination.
The paper I’m referring to was published in Pediatrics in October 2023. It’s titled, “Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety.”
Let me emphasize that we’re talking about a 10-year follow-up. In this particular paper and analysis, 301 boys — I emphasize boys — were included and 971 girls at 40 different sites in 13 countries, who received the 9-valent vaccine, which includes HPV 16, 18, and seven other types.
Most importantly, there was not a single case. Not one. Let me repeat this: There was not a single case of high-grade intraepithelial neoplasia, or worse, or condyloma in either males or females. There was not a single case in over 1000 individuals with a follow-up of more than 10 years.
It is difficult to overstate the magnitude of the benefit associated with HPV vaccination for our children and young adults on their risk of developing highly relevant, life-changing, potentially deadly cancers.
For those of you who are interested in this topic — which should include almost all of you, if not all of you — I encourage you to read this very important follow-up paper, again, demonstrating the simple, overwhelming magnitude of the benefit of HPV vaccination. I thank you for your attention.
Dr. Markman is a professor in the department of medical oncology and therapeutics research, City of Hope, Duarte, California, and president of medicine and science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline; AstraZeneca.
A version of this article appeared on Medscape.com.
Postinfectious Cough: Are Treatments Ever Warranted?
Lingering postinfectious cough has been a concern across Canada this winter. , according to an overview published on February 12 in the Canadian Medical Association Journal
“It’s something a lot of patients are worried about: That lingering cough after a common cold or flu,” lead author Kevin Liang, MD, of the Department of Family Medicine at The University of British Columbia in Vancouver, British Columbia, Canada, told this news organization. He added that some studies show that as much as a quarter of adult patients have this complaint.
Dr. Liang and his colleagues emphasized that the diagnosis of postinfectious cough is one of exclusion. It relies on the absence of concerning physical examination findings and other “subacute cough mimics” such as asthma, chronic obstructive pulmonary disease (COPD), gastroesophageal reflux disease, or use of angiotensin-converting enzyme inhibitors.
“Pertussis should be considered in patients with a paroxysmal cough, post-tussive vomiting, and inspiratory whoop,” they added. Coughs that persist beyond 8 weeks warrant further workup such as a pulmonary function test to rule out asthma or COPD. Coughs accompanied by hemoptysis, systemic symptoms, dysphagia, excessive dyspnea, or hoarseness also warrant further workup, they added. And patients with a history of smoking or recurrent pneumonia should be followed more closely.
In the absence of red flags, Dr. Liang and coauthors advised that there is no evidence supporting pharmacologic treatment, “which is associated with harms,” such as medication adverse effects, cost, strain on the medical supply chain, and the fact that pressurized metered-dose inhalers emit powerful greenhouse gases. “A lot of patients come in looking for solutions, but really, all the evidence says the over-the-counter cough syrup just doesn’t work. Or I see clinicians prescribing inhalers or different medication that can cost hundreds of dollars, and their efficacy, at least from the literature, shows that there’s really no improvement. Time and patience are the two keys to solving this,” Dr. Liang told this news organization.
Moreover, there is a distinct absence of guidelines on this topic. The College of Family Physicians of Canada’s recent literature review cited limited data supporting a trial of inhaled corticosteroids, a bronchodilator such as ipratropium-salbutamol, or an intranasal steroid if postnasal drip is suspected. However, “there’s a high risk of bias in the study they cite from using the short-acting bronchodilators, and what it ultimately says is that in most cases, this is self-resolving by around the 20-day mark,” said Dr. Liang. “Our advice is just to err on the side of caution and just provide that information piece to the patient.”
‘Significant Nuance’
Imran Satia, MD, assistant professor of respirology at McMaster University in Hamilton, Ontario, Canada, agreed that “most people who get a viral or bacterial upper or lower respiratory tract infection will get better with time, and there is very little evidence that giving steroids, antibiotics, or cough suppressants is better than waiting it out.” There is “significant nuance” in how to manage this situation, however.
“In some patients with underlying lung disease like asthma or COPD, increasing the frequency of regular inhaled steroids, bronchodilators, oral steroids, antibiotics, and chest imaging with breathing tests may be clinically warranted, and many physicians will do this,” he told this news organization. “In some patients with refractory chronic cough, there is no underlying identifiable disease, despite completing the necessary investigations. Or coughing persists despite trials of treatment for lung diseases, nasal diseases, and stomach reflux disease. This is commonly described as cough hypersensitivity syndrome, for which therapies targeting the neuronal pathways that control coughing are needed.”
Physicians should occasionally consider trying a temporary course of a short-acting bronchodilator inhaler, said Nicholas Vozoris, MD, assistant professor and clinician investigator in respirology at the University of Toronto, Toronto, Ontario, Canada. “I think that would be a reasonable first step in a case of really bad postinfectious cough,” he told this news organization. “But in general, drug treatments are not indicated.”
Environmental Concerns
Yet some things should raise clinicians’ suspicion of more complex issues.
“A pattern of recurrent colds or bronchitis with protracted coughing afterward raises strong suspicion for asthma, which can present as repeated, prolonged respiratory exacerbations,” he said. “Unless asthma is treated with appropriate inhaler therapy on a regular basis, it will unlikely come under control.”
Dr. Vozoris added that the environmental concerns over the use of metered dose inhalers (MDIs) are minimal compared with the other sources of pollution and the risks for undertreatment. “The authors are overplaying the environmental impact of MDI, in my opinion,” he said. “Physicians already have to deal with the challenging issue of suboptimal patient adherence to inhalers, and I fear that such comments may further drive that up. Furthermore, there is also an environmental footprint with not using inhalers, as patients can then experience suboptimally controlled lung disease as a result — and then present to the ER and get admitted to hospital for exacerbations of disease, where more resources and medications are used up.”
“In addition, in patients who are immunocompromised, protracted coughing after what was thought to be a cold may be associated with an “atypical” respiratory infection, such as tuberculosis, that will require special medical treatment,” Dr. Vozoris concluded.
No funding for the review of postinfectious cough was reported. Dr. Liang and Dr. Vozoris disclosed no competing interests. Dr. Satia reported receiving funding from the ERS Respire 3 Fellowship Award, BMA James Trust Award, North-West Lung Centre Charity (Manchester), NIHR CRF Manchester, Merck MSD, AstraZeneca, and GSK. Dr. Satia also has received consulting fees from Merck MSD, Genentech, and Respiplus; as well as speaker fees from AstraZeneca, GSK, Merck MSD, Sanofi-Regeneron. Satia has served on the following task force committees: Chronic Cough (ERS), Asthma Diagnosis and Management (ERS), NEUROCOUGH (ERS CRC), and the CTS Chronic Cough working group.
A version of this article appeared on Medscape.com.
Lingering postinfectious cough has been a concern across Canada this winter. , according to an overview published on February 12 in the Canadian Medical Association Journal
“It’s something a lot of patients are worried about: That lingering cough after a common cold or flu,” lead author Kevin Liang, MD, of the Department of Family Medicine at The University of British Columbia in Vancouver, British Columbia, Canada, told this news organization. He added that some studies show that as much as a quarter of adult patients have this complaint.
Dr. Liang and his colleagues emphasized that the diagnosis of postinfectious cough is one of exclusion. It relies on the absence of concerning physical examination findings and other “subacute cough mimics” such as asthma, chronic obstructive pulmonary disease (COPD), gastroesophageal reflux disease, or use of angiotensin-converting enzyme inhibitors.
“Pertussis should be considered in patients with a paroxysmal cough, post-tussive vomiting, and inspiratory whoop,” they added. Coughs that persist beyond 8 weeks warrant further workup such as a pulmonary function test to rule out asthma or COPD. Coughs accompanied by hemoptysis, systemic symptoms, dysphagia, excessive dyspnea, or hoarseness also warrant further workup, they added. And patients with a history of smoking or recurrent pneumonia should be followed more closely.
In the absence of red flags, Dr. Liang and coauthors advised that there is no evidence supporting pharmacologic treatment, “which is associated with harms,” such as medication adverse effects, cost, strain on the medical supply chain, and the fact that pressurized metered-dose inhalers emit powerful greenhouse gases. “A lot of patients come in looking for solutions, but really, all the evidence says the over-the-counter cough syrup just doesn’t work. Or I see clinicians prescribing inhalers or different medication that can cost hundreds of dollars, and their efficacy, at least from the literature, shows that there’s really no improvement. Time and patience are the two keys to solving this,” Dr. Liang told this news organization.
Moreover, there is a distinct absence of guidelines on this topic. The College of Family Physicians of Canada’s recent literature review cited limited data supporting a trial of inhaled corticosteroids, a bronchodilator such as ipratropium-salbutamol, or an intranasal steroid if postnasal drip is suspected. However, “there’s a high risk of bias in the study they cite from using the short-acting bronchodilators, and what it ultimately says is that in most cases, this is self-resolving by around the 20-day mark,” said Dr. Liang. “Our advice is just to err on the side of caution and just provide that information piece to the patient.”
‘Significant Nuance’
Imran Satia, MD, assistant professor of respirology at McMaster University in Hamilton, Ontario, Canada, agreed that “most people who get a viral or bacterial upper or lower respiratory tract infection will get better with time, and there is very little evidence that giving steroids, antibiotics, or cough suppressants is better than waiting it out.” There is “significant nuance” in how to manage this situation, however.
“In some patients with underlying lung disease like asthma or COPD, increasing the frequency of regular inhaled steroids, bronchodilators, oral steroids, antibiotics, and chest imaging with breathing tests may be clinically warranted, and many physicians will do this,” he told this news organization. “In some patients with refractory chronic cough, there is no underlying identifiable disease, despite completing the necessary investigations. Or coughing persists despite trials of treatment for lung diseases, nasal diseases, and stomach reflux disease. This is commonly described as cough hypersensitivity syndrome, for which therapies targeting the neuronal pathways that control coughing are needed.”
Physicians should occasionally consider trying a temporary course of a short-acting bronchodilator inhaler, said Nicholas Vozoris, MD, assistant professor and clinician investigator in respirology at the University of Toronto, Toronto, Ontario, Canada. “I think that would be a reasonable first step in a case of really bad postinfectious cough,” he told this news organization. “But in general, drug treatments are not indicated.”
Environmental Concerns
Yet some things should raise clinicians’ suspicion of more complex issues.
“A pattern of recurrent colds or bronchitis with protracted coughing afterward raises strong suspicion for asthma, which can present as repeated, prolonged respiratory exacerbations,” he said. “Unless asthma is treated with appropriate inhaler therapy on a regular basis, it will unlikely come under control.”
Dr. Vozoris added that the environmental concerns over the use of metered dose inhalers (MDIs) are minimal compared with the other sources of pollution and the risks for undertreatment. “The authors are overplaying the environmental impact of MDI, in my opinion,” he said. “Physicians already have to deal with the challenging issue of suboptimal patient adherence to inhalers, and I fear that such comments may further drive that up. Furthermore, there is also an environmental footprint with not using inhalers, as patients can then experience suboptimally controlled lung disease as a result — and then present to the ER and get admitted to hospital for exacerbations of disease, where more resources and medications are used up.”
“In addition, in patients who are immunocompromised, protracted coughing after what was thought to be a cold may be associated with an “atypical” respiratory infection, such as tuberculosis, that will require special medical treatment,” Dr. Vozoris concluded.
No funding for the review of postinfectious cough was reported. Dr. Liang and Dr. Vozoris disclosed no competing interests. Dr. Satia reported receiving funding from the ERS Respire 3 Fellowship Award, BMA James Trust Award, North-West Lung Centre Charity (Manchester), NIHR CRF Manchester, Merck MSD, AstraZeneca, and GSK. Dr. Satia also has received consulting fees from Merck MSD, Genentech, and Respiplus; as well as speaker fees from AstraZeneca, GSK, Merck MSD, Sanofi-Regeneron. Satia has served on the following task force committees: Chronic Cough (ERS), Asthma Diagnosis and Management (ERS), NEUROCOUGH (ERS CRC), and the CTS Chronic Cough working group.
A version of this article appeared on Medscape.com.
Lingering postinfectious cough has been a concern across Canada this winter. , according to an overview published on February 12 in the Canadian Medical Association Journal
“It’s something a lot of patients are worried about: That lingering cough after a common cold or flu,” lead author Kevin Liang, MD, of the Department of Family Medicine at The University of British Columbia in Vancouver, British Columbia, Canada, told this news organization. He added that some studies show that as much as a quarter of adult patients have this complaint.
Dr. Liang and his colleagues emphasized that the diagnosis of postinfectious cough is one of exclusion. It relies on the absence of concerning physical examination findings and other “subacute cough mimics” such as asthma, chronic obstructive pulmonary disease (COPD), gastroesophageal reflux disease, or use of angiotensin-converting enzyme inhibitors.
“Pertussis should be considered in patients with a paroxysmal cough, post-tussive vomiting, and inspiratory whoop,” they added. Coughs that persist beyond 8 weeks warrant further workup such as a pulmonary function test to rule out asthma or COPD. Coughs accompanied by hemoptysis, systemic symptoms, dysphagia, excessive dyspnea, or hoarseness also warrant further workup, they added. And patients with a history of smoking or recurrent pneumonia should be followed more closely.
In the absence of red flags, Dr. Liang and coauthors advised that there is no evidence supporting pharmacologic treatment, “which is associated with harms,” such as medication adverse effects, cost, strain on the medical supply chain, and the fact that pressurized metered-dose inhalers emit powerful greenhouse gases. “A lot of patients come in looking for solutions, but really, all the evidence says the over-the-counter cough syrup just doesn’t work. Or I see clinicians prescribing inhalers or different medication that can cost hundreds of dollars, and their efficacy, at least from the literature, shows that there’s really no improvement. Time and patience are the two keys to solving this,” Dr. Liang told this news organization.
Moreover, there is a distinct absence of guidelines on this topic. The College of Family Physicians of Canada’s recent literature review cited limited data supporting a trial of inhaled corticosteroids, a bronchodilator such as ipratropium-salbutamol, or an intranasal steroid if postnasal drip is suspected. However, “there’s a high risk of bias in the study they cite from using the short-acting bronchodilators, and what it ultimately says is that in most cases, this is self-resolving by around the 20-day mark,” said Dr. Liang. “Our advice is just to err on the side of caution and just provide that information piece to the patient.”
‘Significant Nuance’
Imran Satia, MD, assistant professor of respirology at McMaster University in Hamilton, Ontario, Canada, agreed that “most people who get a viral or bacterial upper or lower respiratory tract infection will get better with time, and there is very little evidence that giving steroids, antibiotics, or cough suppressants is better than waiting it out.” There is “significant nuance” in how to manage this situation, however.
“In some patients with underlying lung disease like asthma or COPD, increasing the frequency of regular inhaled steroids, bronchodilators, oral steroids, antibiotics, and chest imaging with breathing tests may be clinically warranted, and many physicians will do this,” he told this news organization. “In some patients with refractory chronic cough, there is no underlying identifiable disease, despite completing the necessary investigations. Or coughing persists despite trials of treatment for lung diseases, nasal diseases, and stomach reflux disease. This is commonly described as cough hypersensitivity syndrome, for which therapies targeting the neuronal pathways that control coughing are needed.”
Physicians should occasionally consider trying a temporary course of a short-acting bronchodilator inhaler, said Nicholas Vozoris, MD, assistant professor and clinician investigator in respirology at the University of Toronto, Toronto, Ontario, Canada. “I think that would be a reasonable first step in a case of really bad postinfectious cough,” he told this news organization. “But in general, drug treatments are not indicated.”
Environmental Concerns
Yet some things should raise clinicians’ suspicion of more complex issues.
“A pattern of recurrent colds or bronchitis with protracted coughing afterward raises strong suspicion for asthma, which can present as repeated, prolonged respiratory exacerbations,” he said. “Unless asthma is treated with appropriate inhaler therapy on a regular basis, it will unlikely come under control.”
Dr. Vozoris added that the environmental concerns over the use of metered dose inhalers (MDIs) are minimal compared with the other sources of pollution and the risks for undertreatment. “The authors are overplaying the environmental impact of MDI, in my opinion,” he said. “Physicians already have to deal with the challenging issue of suboptimal patient adherence to inhalers, and I fear that such comments may further drive that up. Furthermore, there is also an environmental footprint with not using inhalers, as patients can then experience suboptimally controlled lung disease as a result — and then present to the ER and get admitted to hospital for exacerbations of disease, where more resources and medications are used up.”
“In addition, in patients who are immunocompromised, protracted coughing after what was thought to be a cold may be associated with an “atypical” respiratory infection, such as tuberculosis, that will require special medical treatment,” Dr. Vozoris concluded.
No funding for the review of postinfectious cough was reported. Dr. Liang and Dr. Vozoris disclosed no competing interests. Dr. Satia reported receiving funding from the ERS Respire 3 Fellowship Award, BMA James Trust Award, North-West Lung Centre Charity (Manchester), NIHR CRF Manchester, Merck MSD, AstraZeneca, and GSK. Dr. Satia also has received consulting fees from Merck MSD, Genentech, and Respiplus; as well as speaker fees from AstraZeneca, GSK, Merck MSD, Sanofi-Regeneron. Satia has served on the following task force committees: Chronic Cough (ERS), Asthma Diagnosis and Management (ERS), NEUROCOUGH (ERS CRC), and the CTS Chronic Cough working group.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL