User login
Your Guide to COVID Vaccines for 2024-2025
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
The updated COVID vaccines for 2024-2025 are officially here, designed to target the latest variants and offer robust protection — but getting Americans to roll up their sleeves could prove harder than ever. With COVID cases on the decline, many people feel the urgency has passed.
As of December 2, the CDC reports that COVID test positivity remains low, rising slightly to 4.5% for the week ending November 23, compared with 4.2% the previous week. That’s a far cry from the early days of 2022, when positivity rates soared above 30%. Emergency room visits for COVID now make up just 0.5%, and deaths are down to 0.8% of total weekly fatalities, compared to 1% the previous week.
This steady improvement in the numbers may explain why a recent Pew Research Center survey revealed that 6 in 10 US adults have no plans to get the updated vaccine this year.
As of December 2, according to the CDC, just 19.7% of the US adult population and 9.4% of children had gotten the updated vaccine. The age group most likely? Adults ages 65 and older, with 41.6% getting the updated shot.
Despite the good news about declining cases, our pandemic history suggests a pre-holiday increase is likely. On November 20, the CDC warned it expects levels of both COVID and RSV (respiratory syncytial virus) to rise in the coming weeks — the familiar post-Thanksgiving, pre-Christmas, and Hanukkah increase.
Here’s what to know about the 2024-2025 vaccines — what’s available, how the updated versions are tested, how well each protects you, side effects and other safety information, the best time to get them, and where.
What’s Available?
Three updated vaccines, which work two different ways, are authorized or licensed by the FDA for the 2024-2025 season:
Novavax. A protein subunit vaccine, Novavax is authorized for emergency use by the FDA in people ages 12 and older. The vaccine makes a protein that mimics the SARS-CoV-2 virus’ version of the spike protein and combines it with an adjuvant or “booster” to stimulate a protective immune response. This year’s version targets the JN.1 variant.
Pfizer/BioNTech. Its Comirnaty is a fully licensed vaccine for people ages 12 and older. Its mechanism of action is by messenger RNA (mRNA). It works by instructing cells to produce viral proteins, triggering an immune response. Pfizer’s COVID vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
Moderna. Its Spikevax is a fully licensed vaccine for people ages 12 and older. It is also an mRNA vaccine. Moderna’s COVID-19 vaccine is authorized for emergency use in children ages 6 months to 11 years. This year’s version targets KP.2.
How Effective Are They?
Before being approved for this year’s use, each company had to show its updated vaccine is effective against the currently circulating variants. For the 2 weeks ending November 23, KP.3.1.1 and XEC, from the Omicron lineage, made up the majority of cases, according to CDC data.
How do the vaccine makers know their updated vaccines are targeting the circulating variants? The companies use “pre-clinical” data, which means the updated versions have not yet been tested in people but in other ways, such as animal studies. But they do have to prove to the FDA that their updated vaccine can neutralize the circulating variants.
Companies continue to monitor their updated vaccines as new variants appear. Later in the season, there will be more specific information about how well each vaccine protects in people after tracking real-world data.
What About Side Effects?
The CDC lists comparable side effects for both mRNA and protein COVID vaccines, including pain and soreness from the needle, fatigue, headache, muscle pain joint pain, chills, fever, nausea, and vomiting.
Severe allergic reactions are rare, the CDC says, but cautions to be alert for low blood pressure, swelling of the lips, tongue, or throat, or difficulty breathing.
Which One Is Best?
“I consider the three currently available COVID vaccines — Pfizer, Moderna, and Novavax — interchangeable,’’ said Scott Roberts, MD, an infectious diseases specialist and assistant professor of medicine at Yale School of Medicine in New Haven, Connecticut. “There have not been head-to-head studies, and the initial vaccine studies for each were performed at different phases of the pandemic, so we do not have great data to guide which one is better than another.”
He does point out the different mechanisms of action, which may make a difference in people’s choice of vaccines. “So if someone has a reaction to one of them, they can switch to a different brand.”
Best Time to Get It?
“We have consistently seen COVID rates rise quite significantly in the winter season, especially around the holidays. So if anyone is on the fence and hasn’t gotten the updated vaccine yet, now is a great time to get it to maximize immunity for the holidays,” he said.
What’s next? In late October, the CDC recommended a second dose of the 2024-2025 vaccine 6 months after the first one for those age 65 and above and those 6 months old and older who are moderately or severely immunocompromised.
Now, while it’s tempting to think rates are down and will continue to drop steadily, Roberts reminds people that pandemic history suggests otherwise.
Coverage
Most people can get COVID-19 vaccines at no cost through their private health insurance, Medicaid, or Medicare. For the uninsured, there’s also the Vaccines for Children (VFC) program or access through state and local health departments and some health centers. Find details on the CDC website.
A version of this article first appeared on WebMD.
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
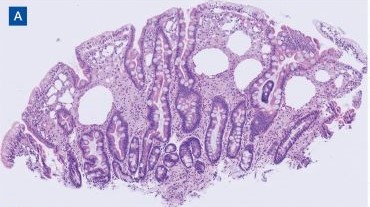
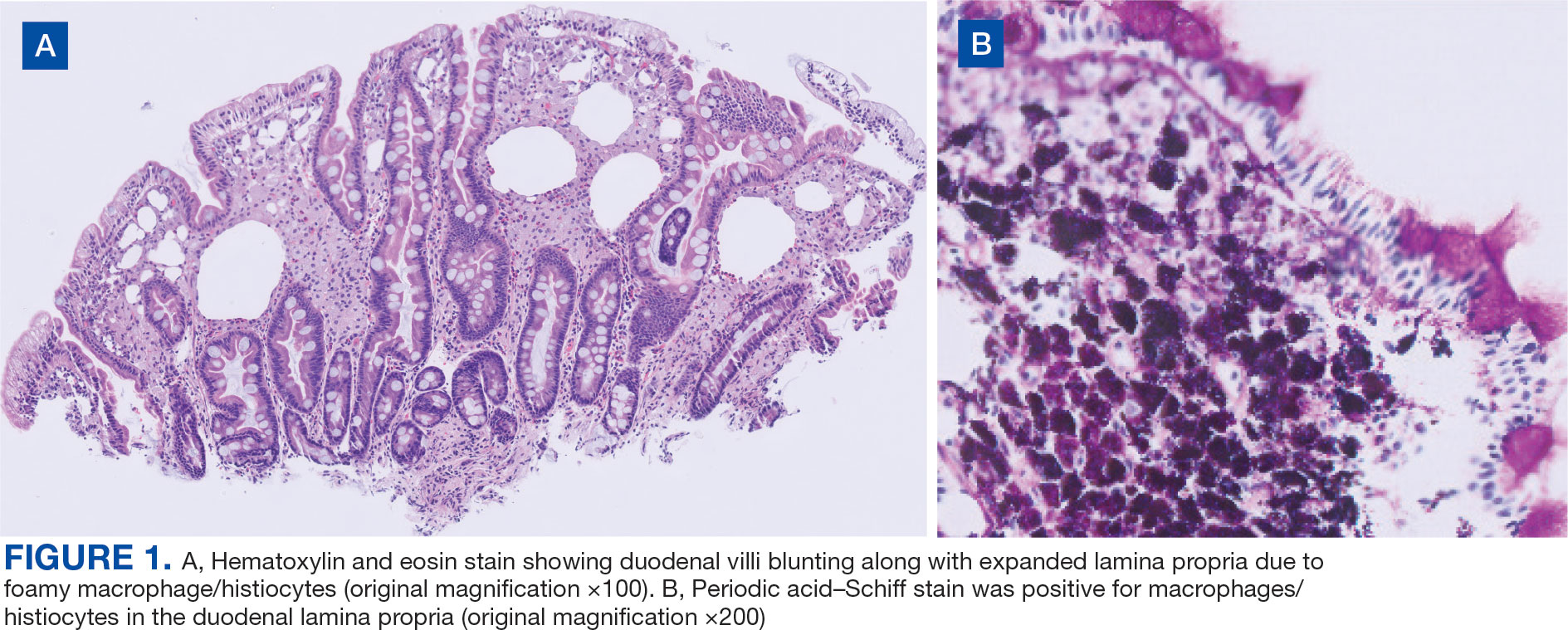
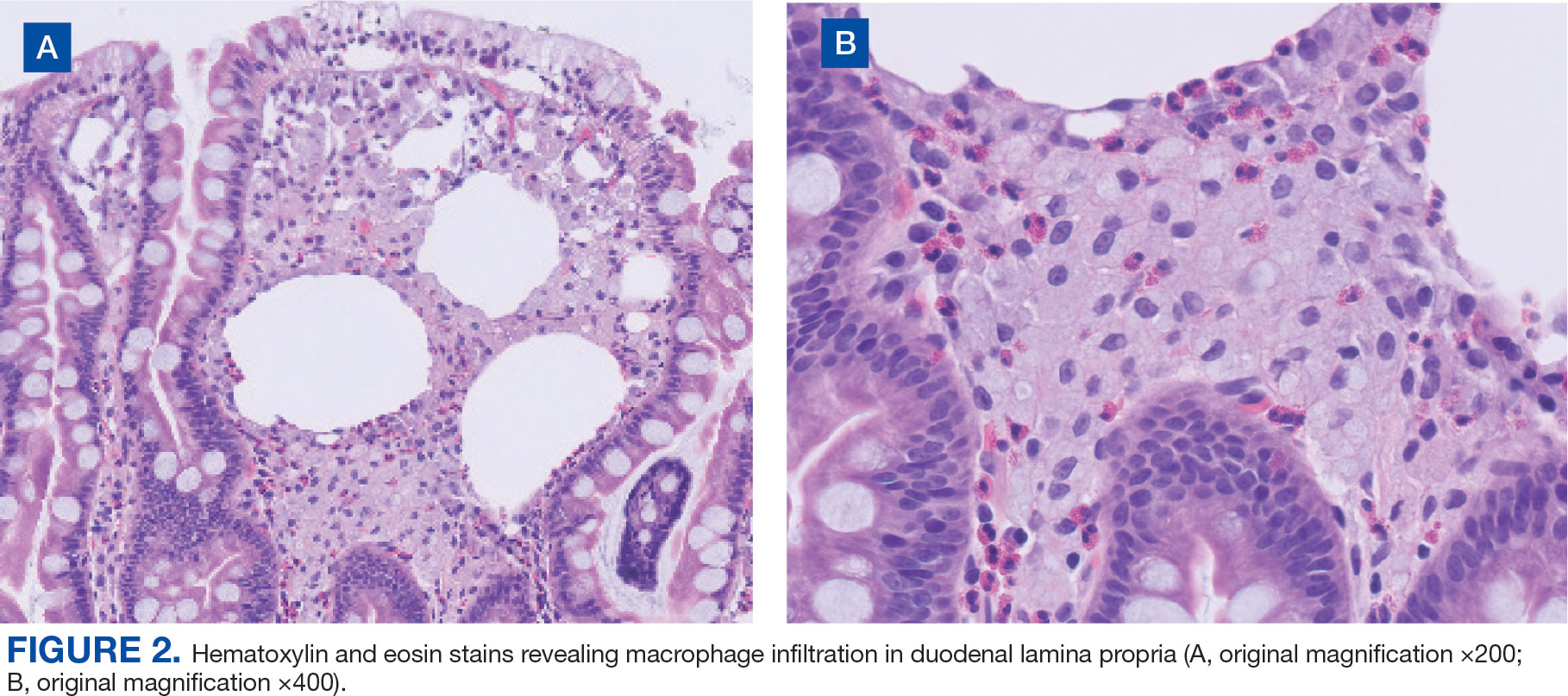
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.
We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.
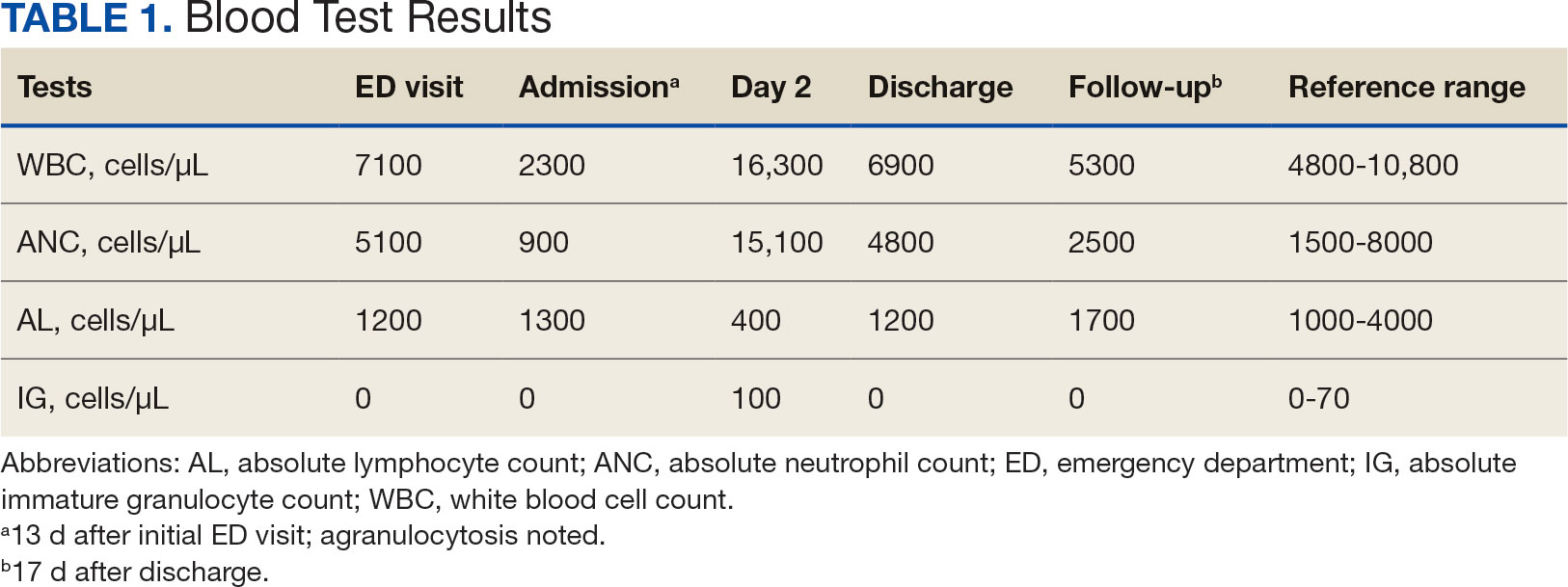
Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.

Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.

Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
The Multipronged Problem of Candida auris
The Multipronged Problem of Candida auris
Candida auris, a yeast-like fungus, is spreading globally, increasing the urgency for enhanced surveillance, new therapies, and more antimicrobial stewardship to combat its multidrug-resistant strains.
Since its discovery in 2009, C auris has been found in more than 50 countries across six continents, including Asia, Africa, and the Americas, according to the World Health Organization. In 2022, CDC reported 2377 clinical cases and 5754 screening cases of C auris in the United States.
In September, The Lancet Microbe reported on three C auris isolates from a Singapore hospital belonging to a new clade (clade six), “which is phenotypically and genotypically distinct” from the first five clades, the authors wrote. In June, Microbiology Spectrum published a study about two unusual C auris isolates from a Bangladesh NICU in 2021. They were also assigned to clade six “with potential for international transmission,” the study authors noted.
C auris has all the hallmarks of “critical pathogen,” as defined by the World Health Organization in 2022. It increases morbidity and mortality for affected patients, is difficult to eradicate in hospitals, and can be treatment resistant.
As a result, infectious disease specialists are raising more awareness and advocating for greater surveillance of C auris colonization and disease in the hospital setting for high-risk patients.
Arturo Casadevall, MD, PhD, MS, is one of them. “C auris could be a problem in your hospital as fungal diseases are getting worse every year,” said Casadevall, chair of Molecular Microbiology and Immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. The increasing number of cases “is incremental, but when [we] look at the data over years, it is a growing problem. We may see more of these cases in the coming years.”
Expediting Diagnoses
Symptoms of C auris disease vary and can cause invasive infections, such as bloodstream or intra-abdominal infections. This is why Casadevall encourages infectious disease specialists to “always consider fungal disease when you are approaching an individual. The diagnosis is sometimes delayed because you don’t look for it,” he said.
C auris can also be misidentified in the lab “when using traditional biochemical methods for yeast identification. Accurate identification of C auris requires use of sequencing or mass spectrometry,” according to CDC.
C auris is typically found on the skin of colonized patients and can enter the body through invasive devices, incisions, wounds, and during surgery. Mostly, immunosuppressed patients are at risk for serious fungal disease, Casadevall said.
Invasive fungal disease can be life-threatening for hospitalized patients. In one review of 37 studies from 2011 to 2021, researchers found that overall mortality rates for C auris infections ranged from 29% to 62%, with 30-day mortality rates between 23% and 67%, Medical Mycology reported. Patients typically had a median hospital stay of 46-68 days, sometimes extending up to 140 days. Late-onset complications included metastatic septic issues, according to the study.
Overcoming Treatment-Resistant Strains
A resilient yeast, C auris shows higher resistance to antifungal treatments compared to other Candida species, JAMA reported. Echinocandins are the first-line treatment for adults and children over 2 months old “and some of those therapies are already resistant,” said George Thompson, MD, professor of clinical medicine at the University of California Davis School of Medicine, Davis, California. The second line is liposomal amphotericin B (5 mg/kg daily), but it has toxicity problems, Thompson said.
New therapies sans toxicity are needed to treat C auris disease. Thompson, eg, served as the principal investigator in the ReSTORE trial to study a new therapy (rezafungin for injection). In March 2023, the US Food and Drug Administration approved the treatment for candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Thompson has observed that patients with C auris disease can present with “an infection in the urinary system with burning, pain, and bladder spasms. In the majority of cases of candida sepsis, the patients will have it in their blood stream with fever, chills, and sweats,” he said. The new treatment may clear the infection quickly, said Thompson, who noted results published in The Lancet.
Infection Prevention and Antimicrobial Stewardship
Institutions like University of Michigan Health (U-M Health) in Ann Arbor, Michigan, have increased measures to tackle the issue from different angles.
To address the broader issue of treatment-resistant fungal disease, U-M Health “has a robust antimicrobial stewardship program in place,” said Laraine Lynn Washer, MD, infectious disease physician.
The program includes oversight and restriction of various antifungals to avoid potential for overuse that could lead to increased risk for antifungal resistance. Use of echinocandins, for example, “requires prior approval by our antimicrobial stewardship team members,” said Washer, who is also Clinical Professor of Infectious Diseases and the Medical Director of Infection Prevention of Epidemiology at U-M Health.
Infection prevention measures entail screening hospitalized adult patients for risk factors for C auris, such as:
- Overnight international hospitalization
- Recent stay in a long-term acute care facility
- Recent stay in a ventilator skilled nursing facility.
“If a patient has these risk factors, we perform testing to assess for colonization (presence of C auris without infection) by obtaining skin swabs from the axilla and the groin and asking our lab to perform PCR to identify genetic elements of C auris,” Washer said. “Patients who are transferred directly from another hospital ICU to our ICU also undergo testing for colonization.”
If a patient is identified with C auris, hospitals ought to perform screening tests using cultures or PCR “on other patients who may have overlapped in time and space with the patient such as hospital roommates,” Washer explained.
Once in a hospital environment, the pathogen is hard to eradicate. C auris has a unique ability to be transmitted in the healthcare environment, is relatively heat tolerant, and is resistant to some common disinfectants, Washer added. The yeast can survive for over 2 weeks on plastic and months on skin, JAMA reported.
“Hospitals should partner with local and state level public health authorities in reporting cases of Candida auris and assist in any contact investigations as requested by public health authorities,” Washer advised.
Casadevall and Washer reported no conflicts of interest. Thompson has consulted and received research funding from Astellas, Basilea, Cidara, F2G, GSK, Melinta, Mundipharma, Pfizer, and Scynexis.
A version of this article appeared on Medscape.com.
Candida auris, a yeast-like fungus, is spreading globally, increasing the urgency for enhanced surveillance, new therapies, and more antimicrobial stewardship to combat its multidrug-resistant strains.
Since its discovery in 2009, C auris has been found in more than 50 countries across six continents, including Asia, Africa, and the Americas, according to the World Health Organization. In 2022, CDC reported 2377 clinical cases and 5754 screening cases of C auris in the United States.
In September, The Lancet Microbe reported on three C auris isolates from a Singapore hospital belonging to a new clade (clade six), “which is phenotypically and genotypically distinct” from the first five clades, the authors wrote. In June, Microbiology Spectrum published a study about two unusual C auris isolates from a Bangladesh NICU in 2021. They were also assigned to clade six “with potential for international transmission,” the study authors noted.
C auris has all the hallmarks of “critical pathogen,” as defined by the World Health Organization in 2022. It increases morbidity and mortality for affected patients, is difficult to eradicate in hospitals, and can be treatment resistant.
As a result, infectious disease specialists are raising more awareness and advocating for greater surveillance of C auris colonization and disease in the hospital setting for high-risk patients.
Arturo Casadevall, MD, PhD, MS, is one of them. “C auris could be a problem in your hospital as fungal diseases are getting worse every year,” said Casadevall, chair of Molecular Microbiology and Immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. The increasing number of cases “is incremental, but when [we] look at the data over years, it is a growing problem. We may see more of these cases in the coming years.”
Expediting Diagnoses
Symptoms of C auris disease vary and can cause invasive infections, such as bloodstream or intra-abdominal infections. This is why Casadevall encourages infectious disease specialists to “always consider fungal disease when you are approaching an individual. The diagnosis is sometimes delayed because you don’t look for it,” he said.
C auris can also be misidentified in the lab “when using traditional biochemical methods for yeast identification. Accurate identification of C auris requires use of sequencing or mass spectrometry,” according to CDC.
C auris is typically found on the skin of colonized patients and can enter the body through invasive devices, incisions, wounds, and during surgery. Mostly, immunosuppressed patients are at risk for serious fungal disease, Casadevall said.
Invasive fungal disease can be life-threatening for hospitalized patients. In one review of 37 studies from 2011 to 2021, researchers found that overall mortality rates for C auris infections ranged from 29% to 62%, with 30-day mortality rates between 23% and 67%, Medical Mycology reported. Patients typically had a median hospital stay of 46-68 days, sometimes extending up to 140 days. Late-onset complications included metastatic septic issues, according to the study.
Overcoming Treatment-Resistant Strains
A resilient yeast, C auris shows higher resistance to antifungal treatments compared to other Candida species, JAMA reported. Echinocandins are the first-line treatment for adults and children over 2 months old “and some of those therapies are already resistant,” said George Thompson, MD, professor of clinical medicine at the University of California Davis School of Medicine, Davis, California. The second line is liposomal amphotericin B (5 mg/kg daily), but it has toxicity problems, Thompson said.
New therapies sans toxicity are needed to treat C auris disease. Thompson, eg, served as the principal investigator in the ReSTORE trial to study a new therapy (rezafungin for injection). In March 2023, the US Food and Drug Administration approved the treatment for candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Thompson has observed that patients with C auris disease can present with “an infection in the urinary system with burning, pain, and bladder spasms. In the majority of cases of candida sepsis, the patients will have it in their blood stream with fever, chills, and sweats,” he said. The new treatment may clear the infection quickly, said Thompson, who noted results published in The Lancet.
Infection Prevention and Antimicrobial Stewardship
Institutions like University of Michigan Health (U-M Health) in Ann Arbor, Michigan, have increased measures to tackle the issue from different angles.
To address the broader issue of treatment-resistant fungal disease, U-M Health “has a robust antimicrobial stewardship program in place,” said Laraine Lynn Washer, MD, infectious disease physician.
The program includes oversight and restriction of various antifungals to avoid potential for overuse that could lead to increased risk for antifungal resistance. Use of echinocandins, for example, “requires prior approval by our antimicrobial stewardship team members,” said Washer, who is also Clinical Professor of Infectious Diseases and the Medical Director of Infection Prevention of Epidemiology at U-M Health.
Infection prevention measures entail screening hospitalized adult patients for risk factors for C auris, such as:
- Overnight international hospitalization
- Recent stay in a long-term acute care facility
- Recent stay in a ventilator skilled nursing facility.
“If a patient has these risk factors, we perform testing to assess for colonization (presence of C auris without infection) by obtaining skin swabs from the axilla and the groin and asking our lab to perform PCR to identify genetic elements of C auris,” Washer said. “Patients who are transferred directly from another hospital ICU to our ICU also undergo testing for colonization.”
If a patient is identified with C auris, hospitals ought to perform screening tests using cultures or PCR “on other patients who may have overlapped in time and space with the patient such as hospital roommates,” Washer explained.
Once in a hospital environment, the pathogen is hard to eradicate. C auris has a unique ability to be transmitted in the healthcare environment, is relatively heat tolerant, and is resistant to some common disinfectants, Washer added. The yeast can survive for over 2 weeks on plastic and months on skin, JAMA reported.
“Hospitals should partner with local and state level public health authorities in reporting cases of Candida auris and assist in any contact investigations as requested by public health authorities,” Washer advised.
Casadevall and Washer reported no conflicts of interest. Thompson has consulted and received research funding from Astellas, Basilea, Cidara, F2G, GSK, Melinta, Mundipharma, Pfizer, and Scynexis.
A version of this article appeared on Medscape.com.
Candida auris, a yeast-like fungus, is spreading globally, increasing the urgency for enhanced surveillance, new therapies, and more antimicrobial stewardship to combat its multidrug-resistant strains.
Since its discovery in 2009, C auris has been found in more than 50 countries across six continents, including Asia, Africa, and the Americas, according to the World Health Organization. In 2022, CDC reported 2377 clinical cases and 5754 screening cases of C auris in the United States.
In September, The Lancet Microbe reported on three C auris isolates from a Singapore hospital belonging to a new clade (clade six), “which is phenotypically and genotypically distinct” from the first five clades, the authors wrote. In June, Microbiology Spectrum published a study about two unusual C auris isolates from a Bangladesh NICU in 2021. They were also assigned to clade six “with potential for international transmission,” the study authors noted.
C auris has all the hallmarks of “critical pathogen,” as defined by the World Health Organization in 2022. It increases morbidity and mortality for affected patients, is difficult to eradicate in hospitals, and can be treatment resistant.
As a result, infectious disease specialists are raising more awareness and advocating for greater surveillance of C auris colonization and disease in the hospital setting for high-risk patients.
Arturo Casadevall, MD, PhD, MS, is one of them. “C auris could be a problem in your hospital as fungal diseases are getting worse every year,” said Casadevall, chair of Molecular Microbiology and Immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore. The increasing number of cases “is incremental, but when [we] look at the data over years, it is a growing problem. We may see more of these cases in the coming years.”
Expediting Diagnoses
Symptoms of C auris disease vary and can cause invasive infections, such as bloodstream or intra-abdominal infections. This is why Casadevall encourages infectious disease specialists to “always consider fungal disease when you are approaching an individual. The diagnosis is sometimes delayed because you don’t look for it,” he said.
C auris can also be misidentified in the lab “when using traditional biochemical methods for yeast identification. Accurate identification of C auris requires use of sequencing or mass spectrometry,” according to CDC.
C auris is typically found on the skin of colonized patients and can enter the body through invasive devices, incisions, wounds, and during surgery. Mostly, immunosuppressed patients are at risk for serious fungal disease, Casadevall said.
Invasive fungal disease can be life-threatening for hospitalized patients. In one review of 37 studies from 2011 to 2021, researchers found that overall mortality rates for C auris infections ranged from 29% to 62%, with 30-day mortality rates between 23% and 67%, Medical Mycology reported. Patients typically had a median hospital stay of 46-68 days, sometimes extending up to 140 days. Late-onset complications included metastatic septic issues, according to the study.
Overcoming Treatment-Resistant Strains
A resilient yeast, C auris shows higher resistance to antifungal treatments compared to other Candida species, JAMA reported. Echinocandins are the first-line treatment for adults and children over 2 months old “and some of those therapies are already resistant,” said George Thompson, MD, professor of clinical medicine at the University of California Davis School of Medicine, Davis, California. The second line is liposomal amphotericin B (5 mg/kg daily), but it has toxicity problems, Thompson said.
New therapies sans toxicity are needed to treat C auris disease. Thompson, eg, served as the principal investigator in the ReSTORE trial to study a new therapy (rezafungin for injection). In March 2023, the US Food and Drug Administration approved the treatment for candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
Thompson has observed that patients with C auris disease can present with “an infection in the urinary system with burning, pain, and bladder spasms. In the majority of cases of candida sepsis, the patients will have it in their blood stream with fever, chills, and sweats,” he said. The new treatment may clear the infection quickly, said Thompson, who noted results published in The Lancet.
Infection Prevention and Antimicrobial Stewardship
Institutions like University of Michigan Health (U-M Health) in Ann Arbor, Michigan, have increased measures to tackle the issue from different angles.
To address the broader issue of treatment-resistant fungal disease, U-M Health “has a robust antimicrobial stewardship program in place,” said Laraine Lynn Washer, MD, infectious disease physician.
The program includes oversight and restriction of various antifungals to avoid potential for overuse that could lead to increased risk for antifungal resistance. Use of echinocandins, for example, “requires prior approval by our antimicrobial stewardship team members,” said Washer, who is also Clinical Professor of Infectious Diseases and the Medical Director of Infection Prevention of Epidemiology at U-M Health.
Infection prevention measures entail screening hospitalized adult patients for risk factors for C auris, such as:
- Overnight international hospitalization
- Recent stay in a long-term acute care facility
- Recent stay in a ventilator skilled nursing facility.
“If a patient has these risk factors, we perform testing to assess for colonization (presence of C auris without infection) by obtaining skin swabs from the axilla and the groin and asking our lab to perform PCR to identify genetic elements of C auris,” Washer said. “Patients who are transferred directly from another hospital ICU to our ICU also undergo testing for colonization.”
If a patient is identified with C auris, hospitals ought to perform screening tests using cultures or PCR “on other patients who may have overlapped in time and space with the patient such as hospital roommates,” Washer explained.
Once in a hospital environment, the pathogen is hard to eradicate. C auris has a unique ability to be transmitted in the healthcare environment, is relatively heat tolerant, and is resistant to some common disinfectants, Washer added. The yeast can survive for over 2 weeks on plastic and months on skin, JAMA reported.
“Hospitals should partner with local and state level public health authorities in reporting cases of Candida auris and assist in any contact investigations as requested by public health authorities,” Washer advised.
Casadevall and Washer reported no conflicts of interest. Thompson has consulted and received research funding from Astellas, Basilea, Cidara, F2G, GSK, Melinta, Mundipharma, Pfizer, and Scynexis.
A version of this article appeared on Medscape.com.
The Multipronged Problem of Candida auris
The Multipronged Problem of Candida auris
H5N1 Avian Influenza Spreads Across North America
It’s been a while since I’ve discussed the H5N1 avian influenza clade 2.3.4.4b and its rapid spread in North America. I hope the facts prove me wrong, but many experts have been warning for some time that ideal conditions are forming for this virus, which for now only causes zoonoses, to pose a pandemic threat.
either because they work in other medical fields or think that the experience of the COVID-19 pandemic was a worst-case scenario that is unlikely to be repeated in the short term.
The Virus Has Flown to Hawaii
According to data from the Centers for Disease Control and Prevention in Atlanta, Georgia, the infection has now affected more than 500 cattle herds in 15 states. There are about 30 outbreaks reported in poultry, equally distributed between backyard and farm-raised birds, primarily located in California. Here alone, over 3 million birds have been affected.
Wild birds are believed to have transported the highly pathogenic virus via migration routes across the Pacific, introducing it to Hawaii for the first time. Just days after wastewater analysis detected the presence of H5N1 on the island of Oahu, home to the capital Honolulu, the first outbreak was promptly reported, killing at least a dozen ducks and geese in a backyard coop. Some of these birds had been taken in early November to the Mililani Pet Fair, a sort of domestic animal festival. Local authorities recommended that anyone who attended the fair, touched a duck or goose at the event, and developed symptoms including fever, cough, sore throat, and conjunctivitis, should isolate and seek medical advice.
Meanwhile, more than 50 farmers, animal handlers, or workers involved in the slaughter of cattle or poultry across seven states have been confirmed infected, presumably contracted at their workplace. The latest case, diagnosed recently in Oregon, presented with severe conjunctivitis and mild respiratory symptoms. More than half of these patients have been identified in recent weeks in California, where active surveillance measures have been implemented. However, there is strong suspicion that the actual number of people infected with mild symptoms in the rest of the country is much, much higher.
The Red Alert Lights Up in Canada
The level of concern was raised further with news of the first severe — indeed very severe — case of H5N1 avian influenza originating from the western edge of Canada. A teenager (gender not disclosed), previously healthy and without risk factors, was hospitalized with severe respiratory failure in the intensive care unit at British Columbia Children’s Hospital in Vancouver. The source of the infection is unknown, similar to only one other case in Missouri involving an adult already hospitalized for other reasons, which was identified by chance through influenza surveillance programs. We also know that the Canadian adolescent does not live on a farm and had no known contact with potentially infected animals. The only suspicions focus on the family dog, euthanized owing to unspecified health problems in the early days of the epidemiologic investigation. Although the dog tested negative for avian influenza, a necropsy will be conducted to rule out its involvement in the transmission chain.
An initial characterization of the virus has linked it to genotype D1.1, which is circulating among wild birds and poultry farms in Canada’s westernmost province, rather than the strain typical of dairy cows in the United States. The publication of the complete viral sequence over the past weekend has, for the first time, highlighted mutations that could enhance the virus’s ability to infect human cells.
How do we know this? From the highly contested “gain-of-function” studies, which artificially modify viruses to understand which genomic points require the most surveillance — those mutations that can make the infectious agent more virulent or more transmissible between people.
Under Special Surveillance for 20 Years
The influenza A (H5N1) avian virus is not new or previously unknown, like SARS-CoV-2, and this could (in theory) give us a slight advantage. We have known about it for decades, and it began infecting humans about 20 years ago, causing pneumonia with respiratory failure. It proved lethal in about half of the cases, but only in people who had close contact with infected poultry, primarily in Southeast Asia.
Hundreds of other human cases occurred worldwide, but always in low-income countries with poor hygiene conditions and where families lived in close contact with animals. This contributed to a false sense of security in Europe and North America, where the threat has been consistently underestimated. Despite an estimated fatality rate of around 50%, the media often labeled scientists’ warnings and health authorities’ efforts to remain prepared as false alarms, tainted by suspicions of catering to the interests of pharmaceutical companies.
Some people may recall the scandal involving Tamiflu, the Roche antiviral oseltamivir, that governments stockpiled when there were fears that the avian virus might acquire the ability to spread among humans. It was dubbed “a false antidote for a false pandemic,” referring to the potential avian pandemic and the 2009 H1N1 influenza pandemic, improperly called “swine flu,” and which turned out to be less severe than expected. There was talk of €2.64 billion being “wasted” to “please” the manufacturer. Although the Cochrane Collaboration made legitimate demands for rigor and transparency in conducting and publishing clinical trials, much of the public, and the journalists who wrote the stories, cared little about these technical aspects. The prevailing message was that stockpiling drugs (or vaccines) for a disease we don’t even know will occur is a waste of taxpayers’ money rather than a prudent preventive measure.
More Vulnerable Than Ever
If we were to ascribe strategic thinking to the virus, which it is not capable of, we might argue that it chose the ideal moment to conquer the world. It began circulating in the new clade in 2020, when experts and authorities were focused on the coronavirus. It spread from birds to marine mammals and finally to cattle, exploiting the public’s post-pandemic fatigue, as people no longer wanted to hear about infectious diseases and containment measures. It ultimately rode the wave of political polarization that irrationally equates prevention with supposed cowardice on the left, and recklessness with courageous freedom on the right.
The coincidence between the future appointments announced by the incoming Trump administration and the virus’s accelerated spread deserves attention from decision-makers and health professionals worldwide. The COVID-19 pandemic experience should have taught us that ignoring a threat doesn’t make it go away, if not in our health, then at least in our wallet. The economic repercussions of a virus circulating among animals crucial to our food chain and national economies should concern everyone, well before the threat crosses the ocean, because only then can we defend ourselves.
The proposed Secretary of Health and Human Services, Robert F. Kennedy, is a proponent of the supposed benefits of raw milk, which could serve as a potent vector for the virus. He is ideologically opposed to vaccinations. It’s hard to imagine he would utilize the H5N1 vaccine stockpiles held by the US government for a campaign starting at least with farmers, as was done prophylactically in Finland with products jointly procured by 15 European countries — a group the Italian government decided not to join.
If Kennedy indeed becomes responsible for US public health, it’s reasonable to fear that, in the name of freedom, he will try to delay as much as possible — even if necessary — the obligation to undergo testing and wear masks, not to mention more restrictive infection containment measures. It’s also unlikely he would support and promote the development of new mRNA products already under study, which would become indispensable if the disease begins to spread more easily among people, as well as animals. In such a case, traditional influenza vaccine cultivation methods using chicken eggs would prove too slow and quantitatively insufficient, especially if the virus continues to circulate among poultry.
In short, let’s keep our fingers crossed, but recognize that crossing our fingers might not be enough.
This story was translated from Univadis Italy using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
It’s been a while since I’ve discussed the H5N1 avian influenza clade 2.3.4.4b and its rapid spread in North America. I hope the facts prove me wrong, but many experts have been warning for some time that ideal conditions are forming for this virus, which for now only causes zoonoses, to pose a pandemic threat.
either because they work in other medical fields or think that the experience of the COVID-19 pandemic was a worst-case scenario that is unlikely to be repeated in the short term.
The Virus Has Flown to Hawaii
According to data from the Centers for Disease Control and Prevention in Atlanta, Georgia, the infection has now affected more than 500 cattle herds in 15 states. There are about 30 outbreaks reported in poultry, equally distributed between backyard and farm-raised birds, primarily located in California. Here alone, over 3 million birds have been affected.
Wild birds are believed to have transported the highly pathogenic virus via migration routes across the Pacific, introducing it to Hawaii for the first time. Just days after wastewater analysis detected the presence of H5N1 on the island of Oahu, home to the capital Honolulu, the first outbreak was promptly reported, killing at least a dozen ducks and geese in a backyard coop. Some of these birds had been taken in early November to the Mililani Pet Fair, a sort of domestic animal festival. Local authorities recommended that anyone who attended the fair, touched a duck or goose at the event, and developed symptoms including fever, cough, sore throat, and conjunctivitis, should isolate and seek medical advice.
Meanwhile, more than 50 farmers, animal handlers, or workers involved in the slaughter of cattle or poultry across seven states have been confirmed infected, presumably contracted at their workplace. The latest case, diagnosed recently in Oregon, presented with severe conjunctivitis and mild respiratory symptoms. More than half of these patients have been identified in recent weeks in California, where active surveillance measures have been implemented. However, there is strong suspicion that the actual number of people infected with mild symptoms in the rest of the country is much, much higher.
The Red Alert Lights Up in Canada
The level of concern was raised further with news of the first severe — indeed very severe — case of H5N1 avian influenza originating from the western edge of Canada. A teenager (gender not disclosed), previously healthy and without risk factors, was hospitalized with severe respiratory failure in the intensive care unit at British Columbia Children’s Hospital in Vancouver. The source of the infection is unknown, similar to only one other case in Missouri involving an adult already hospitalized for other reasons, which was identified by chance through influenza surveillance programs. We also know that the Canadian adolescent does not live on a farm and had no known contact with potentially infected animals. The only suspicions focus on the family dog, euthanized owing to unspecified health problems in the early days of the epidemiologic investigation. Although the dog tested negative for avian influenza, a necropsy will be conducted to rule out its involvement in the transmission chain.
An initial characterization of the virus has linked it to genotype D1.1, which is circulating among wild birds and poultry farms in Canada’s westernmost province, rather than the strain typical of dairy cows in the United States. The publication of the complete viral sequence over the past weekend has, for the first time, highlighted mutations that could enhance the virus’s ability to infect human cells.
How do we know this? From the highly contested “gain-of-function” studies, which artificially modify viruses to understand which genomic points require the most surveillance — those mutations that can make the infectious agent more virulent or more transmissible between people.
Under Special Surveillance for 20 Years
The influenza A (H5N1) avian virus is not new or previously unknown, like SARS-CoV-2, and this could (in theory) give us a slight advantage. We have known about it for decades, and it began infecting humans about 20 years ago, causing pneumonia with respiratory failure. It proved lethal in about half of the cases, but only in people who had close contact with infected poultry, primarily in Southeast Asia.
Hundreds of other human cases occurred worldwide, but always in low-income countries with poor hygiene conditions and where families lived in close contact with animals. This contributed to a false sense of security in Europe and North America, where the threat has been consistently underestimated. Despite an estimated fatality rate of around 50%, the media often labeled scientists’ warnings and health authorities’ efforts to remain prepared as false alarms, tainted by suspicions of catering to the interests of pharmaceutical companies.
Some people may recall the scandal involving Tamiflu, the Roche antiviral oseltamivir, that governments stockpiled when there were fears that the avian virus might acquire the ability to spread among humans. It was dubbed “a false antidote for a false pandemic,” referring to the potential avian pandemic and the 2009 H1N1 influenza pandemic, improperly called “swine flu,” and which turned out to be less severe than expected. There was talk of €2.64 billion being “wasted” to “please” the manufacturer. Although the Cochrane Collaboration made legitimate demands for rigor and transparency in conducting and publishing clinical trials, much of the public, and the journalists who wrote the stories, cared little about these technical aspects. The prevailing message was that stockpiling drugs (or vaccines) for a disease we don’t even know will occur is a waste of taxpayers’ money rather than a prudent preventive measure.
More Vulnerable Than Ever
If we were to ascribe strategic thinking to the virus, which it is not capable of, we might argue that it chose the ideal moment to conquer the world. It began circulating in the new clade in 2020, when experts and authorities were focused on the coronavirus. It spread from birds to marine mammals and finally to cattle, exploiting the public’s post-pandemic fatigue, as people no longer wanted to hear about infectious diseases and containment measures. It ultimately rode the wave of political polarization that irrationally equates prevention with supposed cowardice on the left, and recklessness with courageous freedom on the right.
The coincidence between the future appointments announced by the incoming Trump administration and the virus’s accelerated spread deserves attention from decision-makers and health professionals worldwide. The COVID-19 pandemic experience should have taught us that ignoring a threat doesn’t make it go away, if not in our health, then at least in our wallet. The economic repercussions of a virus circulating among animals crucial to our food chain and national economies should concern everyone, well before the threat crosses the ocean, because only then can we defend ourselves.
The proposed Secretary of Health and Human Services, Robert F. Kennedy, is a proponent of the supposed benefits of raw milk, which could serve as a potent vector for the virus. He is ideologically opposed to vaccinations. It’s hard to imagine he would utilize the H5N1 vaccine stockpiles held by the US government for a campaign starting at least with farmers, as was done prophylactically in Finland with products jointly procured by 15 European countries — a group the Italian government decided not to join.
If Kennedy indeed becomes responsible for US public health, it’s reasonable to fear that, in the name of freedom, he will try to delay as much as possible — even if necessary — the obligation to undergo testing and wear masks, not to mention more restrictive infection containment measures. It’s also unlikely he would support and promote the development of new mRNA products already under study, which would become indispensable if the disease begins to spread more easily among people, as well as animals. In such a case, traditional influenza vaccine cultivation methods using chicken eggs would prove too slow and quantitatively insufficient, especially if the virus continues to circulate among poultry.
In short, let’s keep our fingers crossed, but recognize that crossing our fingers might not be enough.
This story was translated from Univadis Italy using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
It’s been a while since I’ve discussed the H5N1 avian influenza clade 2.3.4.4b and its rapid spread in North America. I hope the facts prove me wrong, but many experts have been warning for some time that ideal conditions are forming for this virus, which for now only causes zoonoses, to pose a pandemic threat.
either because they work in other medical fields or think that the experience of the COVID-19 pandemic was a worst-case scenario that is unlikely to be repeated in the short term.
The Virus Has Flown to Hawaii
According to data from the Centers for Disease Control and Prevention in Atlanta, Georgia, the infection has now affected more than 500 cattle herds in 15 states. There are about 30 outbreaks reported in poultry, equally distributed between backyard and farm-raised birds, primarily located in California. Here alone, over 3 million birds have been affected.
Wild birds are believed to have transported the highly pathogenic virus via migration routes across the Pacific, introducing it to Hawaii for the first time. Just days after wastewater analysis detected the presence of H5N1 on the island of Oahu, home to the capital Honolulu, the first outbreak was promptly reported, killing at least a dozen ducks and geese in a backyard coop. Some of these birds had been taken in early November to the Mililani Pet Fair, a sort of domestic animal festival. Local authorities recommended that anyone who attended the fair, touched a duck or goose at the event, and developed symptoms including fever, cough, sore throat, and conjunctivitis, should isolate and seek medical advice.
Meanwhile, more than 50 farmers, animal handlers, or workers involved in the slaughter of cattle or poultry across seven states have been confirmed infected, presumably contracted at their workplace. The latest case, diagnosed recently in Oregon, presented with severe conjunctivitis and mild respiratory symptoms. More than half of these patients have been identified in recent weeks in California, where active surveillance measures have been implemented. However, there is strong suspicion that the actual number of people infected with mild symptoms in the rest of the country is much, much higher.
The Red Alert Lights Up in Canada
The level of concern was raised further with news of the first severe — indeed very severe — case of H5N1 avian influenza originating from the western edge of Canada. A teenager (gender not disclosed), previously healthy and without risk factors, was hospitalized with severe respiratory failure in the intensive care unit at British Columbia Children’s Hospital in Vancouver. The source of the infection is unknown, similar to only one other case in Missouri involving an adult already hospitalized for other reasons, which was identified by chance through influenza surveillance programs. We also know that the Canadian adolescent does not live on a farm and had no known contact with potentially infected animals. The only suspicions focus on the family dog, euthanized owing to unspecified health problems in the early days of the epidemiologic investigation. Although the dog tested negative for avian influenza, a necropsy will be conducted to rule out its involvement in the transmission chain.
An initial characterization of the virus has linked it to genotype D1.1, which is circulating among wild birds and poultry farms in Canada’s westernmost province, rather than the strain typical of dairy cows in the United States. The publication of the complete viral sequence over the past weekend has, for the first time, highlighted mutations that could enhance the virus’s ability to infect human cells.
How do we know this? From the highly contested “gain-of-function” studies, which artificially modify viruses to understand which genomic points require the most surveillance — those mutations that can make the infectious agent more virulent or more transmissible between people.
Under Special Surveillance for 20 Years
The influenza A (H5N1) avian virus is not new or previously unknown, like SARS-CoV-2, and this could (in theory) give us a slight advantage. We have known about it for decades, and it began infecting humans about 20 years ago, causing pneumonia with respiratory failure. It proved lethal in about half of the cases, but only in people who had close contact with infected poultry, primarily in Southeast Asia.
Hundreds of other human cases occurred worldwide, but always in low-income countries with poor hygiene conditions and where families lived in close contact with animals. This contributed to a false sense of security in Europe and North America, where the threat has been consistently underestimated. Despite an estimated fatality rate of around 50%, the media often labeled scientists’ warnings and health authorities’ efforts to remain prepared as false alarms, tainted by suspicions of catering to the interests of pharmaceutical companies.
Some people may recall the scandal involving Tamiflu, the Roche antiviral oseltamivir, that governments stockpiled when there were fears that the avian virus might acquire the ability to spread among humans. It was dubbed “a false antidote for a false pandemic,” referring to the potential avian pandemic and the 2009 H1N1 influenza pandemic, improperly called “swine flu,” and which turned out to be less severe than expected. There was talk of €2.64 billion being “wasted” to “please” the manufacturer. Although the Cochrane Collaboration made legitimate demands for rigor and transparency in conducting and publishing clinical trials, much of the public, and the journalists who wrote the stories, cared little about these technical aspects. The prevailing message was that stockpiling drugs (or vaccines) for a disease we don’t even know will occur is a waste of taxpayers’ money rather than a prudent preventive measure.
More Vulnerable Than Ever
If we were to ascribe strategic thinking to the virus, which it is not capable of, we might argue that it chose the ideal moment to conquer the world. It began circulating in the new clade in 2020, when experts and authorities were focused on the coronavirus. It spread from birds to marine mammals and finally to cattle, exploiting the public’s post-pandemic fatigue, as people no longer wanted to hear about infectious diseases and containment measures. It ultimately rode the wave of political polarization that irrationally equates prevention with supposed cowardice on the left, and recklessness with courageous freedom on the right.
The coincidence between the future appointments announced by the incoming Trump administration and the virus’s accelerated spread deserves attention from decision-makers and health professionals worldwide. The COVID-19 pandemic experience should have taught us that ignoring a threat doesn’t make it go away, if not in our health, then at least in our wallet. The economic repercussions of a virus circulating among animals crucial to our food chain and national economies should concern everyone, well before the threat crosses the ocean, because only then can we defend ourselves.
The proposed Secretary of Health and Human Services, Robert F. Kennedy, is a proponent of the supposed benefits of raw milk, which could serve as a potent vector for the virus. He is ideologically opposed to vaccinations. It’s hard to imagine he would utilize the H5N1 vaccine stockpiles held by the US government for a campaign starting at least with farmers, as was done prophylactically in Finland with products jointly procured by 15 European countries — a group the Italian government decided not to join.
If Kennedy indeed becomes responsible for US public health, it’s reasonable to fear that, in the name of freedom, he will try to delay as much as possible — even if necessary — the obligation to undergo testing and wear masks, not to mention more restrictive infection containment measures. It’s also unlikely he would support and promote the development of new mRNA products already under study, which would become indispensable if the disease begins to spread more easily among people, as well as animals. In such a case, traditional influenza vaccine cultivation methods using chicken eggs would prove too slow and quantitatively insufficient, especially if the virus continues to circulate among poultry.
In short, let’s keep our fingers crossed, but recognize that crossing our fingers might not be enough.
This story was translated from Univadis Italy using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pertussis Cases Spike in November
Six times as many cases of pertussis were reported in the United States for the week ending November 16, 2024, as the same week in 2023, according to new data from the Centers for Disease Control and Prevention (CDC).
Of the 434 cases reported for the week ending November 16, 2024, a majority (109) occurred in the East North Central region, mostly in Ohio (93). Another 70 cases occurred in the West North Central region, with 32 cases and 37 cases in Missouri and Nebraska, respectively.
None of the 75 cases in the Middle Atlantic region occurred in New Jersey or New York City; 38 were reported elsewhere in New York, and 37 in Pennsylvania. The South Atlantic region reported 55 cases, including 29 in Florida. The East South Central and West South Central regions reported 11 and 20 cases, respectively. The Mountain and Pacific regions reported 31 (20 in Arizona) and 47 (20 in Washington State) cases, respectively.
The CDC tracks pertussis cases through a national surveillance system, but many cases are likely unrecognized and unreported, according to the CDC.
Although vaccines for pertussis (whooping cough) provide protection, their effectiveness decreases over time, and the CDC expects rates to increase in vaccinated and unvaccinated populations as case levels rebound with the lifting of pandemic mitigation strategies such as masking and remote learning.
Recent CDC data reported by Medscape Medical News showed an association between lower vaccination rates and 2024’s uptick in pertussis cases.
A version of this article first appeared on Medscape.com.
Six times as many cases of pertussis were reported in the United States for the week ending November 16, 2024, as the same week in 2023, according to new data from the Centers for Disease Control and Prevention (CDC).
Of the 434 cases reported for the week ending November 16, 2024, a majority (109) occurred in the East North Central region, mostly in Ohio (93). Another 70 cases occurred in the West North Central region, with 32 cases and 37 cases in Missouri and Nebraska, respectively.
None of the 75 cases in the Middle Atlantic region occurred in New Jersey or New York City; 38 were reported elsewhere in New York, and 37 in Pennsylvania. The South Atlantic region reported 55 cases, including 29 in Florida. The East South Central and West South Central regions reported 11 and 20 cases, respectively. The Mountain and Pacific regions reported 31 (20 in Arizona) and 47 (20 in Washington State) cases, respectively.
The CDC tracks pertussis cases through a national surveillance system, but many cases are likely unrecognized and unreported, according to the CDC.
Although vaccines for pertussis (whooping cough) provide protection, their effectiveness decreases over time, and the CDC expects rates to increase in vaccinated and unvaccinated populations as case levels rebound with the lifting of pandemic mitigation strategies such as masking and remote learning.
Recent CDC data reported by Medscape Medical News showed an association between lower vaccination rates and 2024’s uptick in pertussis cases.
A version of this article first appeared on Medscape.com.
Six times as many cases of pertussis were reported in the United States for the week ending November 16, 2024, as the same week in 2023, according to new data from the Centers for Disease Control and Prevention (CDC).
Of the 434 cases reported for the week ending November 16, 2024, a majority (109) occurred in the East North Central region, mostly in Ohio (93). Another 70 cases occurred in the West North Central region, with 32 cases and 37 cases in Missouri and Nebraska, respectively.
None of the 75 cases in the Middle Atlantic region occurred in New Jersey or New York City; 38 were reported elsewhere in New York, and 37 in Pennsylvania. The South Atlantic region reported 55 cases, including 29 in Florida. The East South Central and West South Central regions reported 11 and 20 cases, respectively. The Mountain and Pacific regions reported 31 (20 in Arizona) and 47 (20 in Washington State) cases, respectively.
The CDC tracks pertussis cases through a national surveillance system, but many cases are likely unrecognized and unreported, according to the CDC.
Although vaccines for pertussis (whooping cough) provide protection, their effectiveness decreases over time, and the CDC expects rates to increase in vaccinated and unvaccinated populations as case levels rebound with the lifting of pandemic mitigation strategies such as masking and remote learning.
Recent CDC data reported by Medscape Medical News showed an association between lower vaccination rates and 2024’s uptick in pertussis cases.
A version of this article first appeared on Medscape.com.
Flu Vaccine Guards Household Contacts of Infected People
TOPLINE:
Vaccination lowers the risk of contracting the infection among household contacts.
METHODOLOGY:
- Researchers conducted a prospective cohort study of data between 2017 and 2020 to determine the estimated effectiveness of influenza vaccines in preventing secondary infections in household contacts.
- Overall, 699 people were primary contacts, or the first in a household to get infected (median age, 13 years; 54.5% women); there were 1581 household contacts (median age, 31 years; 52.7% women), and both groups were followed for 7 days.
- Participants collected daily symptom diaries and nasal swabs during the follow-up period.
- Participants also submitted their history of influenza vaccination; 50.1% of household contacts had received a shot at least 14 days before the first case of disease onset in the household.
- The risk for secondary infection and vaccine effectiveness in preventing infection among household contacts was estimated overall and by virus type, subtype, and lineage.
TAKEAWAY:
- Nearly half (48.2%) of primary cases were from children and teens between ages 5 and 17 years.
- Overall, 22% household contacts had laboratory-confirmed influenza during follow-up, of which 7% were asymptomatic.
- The overall risk for secondary infection among unvaccinated household contacts was 18.8%, with the highest risk observed among children younger than age 5 years (29.9%).
- The overall effectiveness of influenza vaccines in preventing laboratory-confirmed infections among household contacts was 21% (95% CI, 1.4%-36.7%).
- The vaccine demonstrated specific protection against influenza B infection (56.4%; 95% CI, 30.1%-72.8%), particularly among those between ages 5 and 17 years.
IN PRACTICE:
“Although complementary preventive strategies to prevent influenza in household settings may be considered, seasonal influenza vaccination is the primary strategy recommended for prevention of influenza illness and its complications,” the authors wrote.
SOURCE:
The study was led by Carlos G. Grijalva, MD, MPH, of Vanderbilt University Medical Center in Nashville, Tennessee, and was published online in JAMA Network Open.
LIMITATIONS:
The recruitment of infected individuals from clinical testing pools may have limited the generalizability of the risk for secondary infection in households in which the primary case had a milder or asymptomatic infection. The study was unable to assess the effectiveness of specific vaccine formulations, such as those receiving high doses. The stratification of estimates by influenza subtypes and lineages was challenging because of small cell sizes.
DISCLOSURES:
This study was supported by grants from the Centers for Disease Control and Prevention (CDC) and authors reported support from grants from the National Institute Of Allergy And Infectious Diseases. Some authors reported contracts, receiving personal fees and grants from the CDC and various pharmaceutical companies such as Merck and Sanofi.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Vaccination lowers the risk of contracting the infection among household contacts.
METHODOLOGY:
- Researchers conducted a prospective cohort study of data between 2017 and 2020 to determine the estimated effectiveness of influenza vaccines in preventing secondary infections in household contacts.
- Overall, 699 people were primary contacts, or the first in a household to get infected (median age, 13 years; 54.5% women); there were 1581 household contacts (median age, 31 years; 52.7% women), and both groups were followed for 7 days.
- Participants collected daily symptom diaries and nasal swabs during the follow-up period.
- Participants also submitted their history of influenza vaccination; 50.1% of household contacts had received a shot at least 14 days before the first case of disease onset in the household.
- The risk for secondary infection and vaccine effectiveness in preventing infection among household contacts was estimated overall and by virus type, subtype, and lineage.
TAKEAWAY:
- Nearly half (48.2%) of primary cases were from children and teens between ages 5 and 17 years.
- Overall, 22% household contacts had laboratory-confirmed influenza during follow-up, of which 7% were asymptomatic.
- The overall risk for secondary infection among unvaccinated household contacts was 18.8%, with the highest risk observed among children younger than age 5 years (29.9%).
- The overall effectiveness of influenza vaccines in preventing laboratory-confirmed infections among household contacts was 21% (95% CI, 1.4%-36.7%).
- The vaccine demonstrated specific protection against influenza B infection (56.4%; 95% CI, 30.1%-72.8%), particularly among those between ages 5 and 17 years.
IN PRACTICE:
“Although complementary preventive strategies to prevent influenza in household settings may be considered, seasonal influenza vaccination is the primary strategy recommended for prevention of influenza illness and its complications,” the authors wrote.
SOURCE:
The study was led by Carlos G. Grijalva, MD, MPH, of Vanderbilt University Medical Center in Nashville, Tennessee, and was published online in JAMA Network Open.
LIMITATIONS:
The recruitment of infected individuals from clinical testing pools may have limited the generalizability of the risk for secondary infection in households in which the primary case had a milder or asymptomatic infection. The study was unable to assess the effectiveness of specific vaccine formulations, such as those receiving high doses. The stratification of estimates by influenza subtypes and lineages was challenging because of small cell sizes.
DISCLOSURES:
This study was supported by grants from the Centers for Disease Control and Prevention (CDC) and authors reported support from grants from the National Institute Of Allergy And Infectious Diseases. Some authors reported contracts, receiving personal fees and grants from the CDC and various pharmaceutical companies such as Merck and Sanofi.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Vaccination lowers the risk of contracting the infection among household contacts.
METHODOLOGY:
- Researchers conducted a prospective cohort study of data between 2017 and 2020 to determine the estimated effectiveness of influenza vaccines in preventing secondary infections in household contacts.
- Overall, 699 people were primary contacts, or the first in a household to get infected (median age, 13 years; 54.5% women); there were 1581 household contacts (median age, 31 years; 52.7% women), and both groups were followed for 7 days.
- Participants collected daily symptom diaries and nasal swabs during the follow-up period.
- Participants also submitted their history of influenza vaccination; 50.1% of household contacts had received a shot at least 14 days before the first case of disease onset in the household.
- The risk for secondary infection and vaccine effectiveness in preventing infection among household contacts was estimated overall and by virus type, subtype, and lineage.
TAKEAWAY:
- Nearly half (48.2%) of primary cases were from children and teens between ages 5 and 17 years.
- Overall, 22% household contacts had laboratory-confirmed influenza during follow-up, of which 7% were asymptomatic.
- The overall risk for secondary infection among unvaccinated household contacts was 18.8%, with the highest risk observed among children younger than age 5 years (29.9%).
- The overall effectiveness of influenza vaccines in preventing laboratory-confirmed infections among household contacts was 21% (95% CI, 1.4%-36.7%).
- The vaccine demonstrated specific protection against influenza B infection (56.4%; 95% CI, 30.1%-72.8%), particularly among those between ages 5 and 17 years.
IN PRACTICE:
“Although complementary preventive strategies to prevent influenza in household settings may be considered, seasonal influenza vaccination is the primary strategy recommended for prevention of influenza illness and its complications,” the authors wrote.
SOURCE:
The study was led by Carlos G. Grijalva, MD, MPH, of Vanderbilt University Medical Center in Nashville, Tennessee, and was published online in JAMA Network Open.
LIMITATIONS:
The recruitment of infected individuals from clinical testing pools may have limited the generalizability of the risk for secondary infection in households in which the primary case had a milder or asymptomatic infection. The study was unable to assess the effectiveness of specific vaccine formulations, such as those receiving high doses. The stratification of estimates by influenza subtypes and lineages was challenging because of small cell sizes.
DISCLOSURES:
This study was supported by grants from the Centers for Disease Control and Prevention (CDC) and authors reported support from grants from the National Institute Of Allergy And Infectious Diseases. Some authors reported contracts, receiving personal fees and grants from the CDC and various pharmaceutical companies such as Merck and Sanofi.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
AMR Could Surpass Cancer as Leading Cause of Death by 2050
Antimicrobial resistance (AMR) is globally recognized as one of the greatest health threats of the 21st century, responsible for 1.27 million deaths annually. “According to the WHO, if no measures are taken promptly, AMR could lead to more deaths than cancer by 2050,” Arnaud Marchant, MD, PhD, director of the European Plotkin Institute for Vaccinology at Université libre de Bruxelles (EPIV-ULB), Anderlecht, Belgium, said in an interview with MediQuality, part of the Medscape Professional Network. “This is a huge problem, and vaccination could be part of the solution.”
EPIV-ULB marked the start of the World AMR Awareness Week (November 18-24) with an event highlighting the critical role of vaccination to counter the rise for resistant pathogens. During the event, MediQuality interviewed Marchant, along with several other experts in the field.
Antibiotics Losing Effectiveness
Marc Van Ranst, PhD, virologist at Rega Institute KU Leuven in Leuven, Belgium, echoed Marchant’s concerns. He noted that “an increasing number of bacteria are becoming resistant to more antibiotics.” “While antibiotics were once miracle drugs, they have now stopped — or almost stopped — working against certain bacteria. Although we are discovering more effective therapies, bacterial infections are increasingly likely to worsen due to AMR.”
Van Ranst issued a stark warning: “If this trend continues, it is entirely reasonable to predict that in 25 years, some antibiotics will become useless, certain bacterial infections will be much harder to treat, and deaths will outnumber those caused by cancer. It’s worth noting, however, that as cancer treatments improve, cancer-related deaths are expected to decline, further highlighting the growing burden of AMR-related fatalities.”
Viruses, Vaccines, and Resistance
Van Ranst emphasized that while AMR primarily involves bacteria, viral infections and vaccination against them also play a role in addressing the issue. “When vaccines prevent illness, they reduce the need for unnecessary antibiotic use. In the past, antibiotics were frequently prescribed for respiratory infections — typically caused by viruses — leading to misuse and heightened resistance. By preventing viral infections through vaccines, we reduce inappropriate antibiotic prescriptions and, subsequently, AMR.”
Strategic Areas of Focus
To maximize the impact of vaccination in combating AMR, Belgium must prioritize several strategic areas, according to EPIV-ULB. “Expanding vaccination coverage for recommended vaccines is crucial to effectively preventing the spread of resistant pathogens,” said Marchant.
“Innovation and development of new vaccines are also essential, including targeted research into vaccines for infections that are currently unavoidable through other means. Enhancing epidemiological surveillance through national data collection and analysis will further clarify the impact of vaccines on AMR and inform policy decisions.”
EPIV-ULB underscored the importance of educating the public and healthcare professionals. “Public awareness is essential to addressing vaccine hesitancy by providing clear information on the importance of prevention,” Marchant explained. “Healthcare professional training must also improve, encouraging preventive practices and judicious antibiotic use. Furthermore, additional research is necessary to fill data gaps and develop predictive models that can guide vaccine development in the future.”
Role of Vaccination
According to EPIV-ULB, Belgium needs a strengthened national strategy to address AMR effectively. “Complementary solutions are increasingly important as antimicrobials lose efficacy and treatments become more complex,” Marchant said. “Vaccination offers a proactive and effective preventive solution, directly and indirectly reducing the spread of resistant pathogens.”
Vaccines combat AMR through various mechanisms. “They prevent diseases such as pneumococcal pneumonia and meningitis, reducing the need for antibiotics to treat these infections,” Marchant explained. “Additionally, vaccination lowers inappropriate antibiotic use by preventing viral infections, reducing the risk of overprescribing antibiotics in cases where they are unnecessary. Lastly, herd immunity from vaccination slows the circulation of resistant pathogens, limiting their spread.”
Van Ranst urged healthcare professionals to prioritize vaccinating at-risk populations as identified by Belgium’s Superior Health Council. These include the elderly with underlying conditions and pregnant women, especially for influenza vaccines. University Hospitals Leuven in Belgium, also conducts annual vaccination campaigns for its staff, combining flu and COVID vaccines to increase uptake.
A Global Challenge
Marc Noppen, MD, PhD, director of University Hospital Brussels, Belgium, emphasized the complexity of AMR as a global issue. “The problem isn’t solely due to human antibiotic use; it also stems from veterinary medicine, plant breeding, and animal husbandry. This is a multifactorial, worldwide issue that requires public awareness. Improved vaccination strategies are one way to address AMR, particularly in this post-COVID era of heightened skepticism toward vaccines,” he explained.
Marie-Lise Verschelden from Pfizer highlighted the need for cooperation across the healthcare sector. “Belgium is fortunate to have a fantastic ecosystem of academics, clinicians, and industry experts. Collaboration, including government involvement, is critical to advancing our efforts. At Pfizer, we continue to develop new vaccines and technologies, and the COVID crisis has reinforced the critical role of vaccination in combating AMR. Through our vaccine portfolio and ongoing developments, we are well-positioned to contribute significantly to this global challenge.”
Elisabeth Van Damme from GSK reiterated that AMR is a global issue requiring joint efforts. “Existing vaccines are underutilized. Vaccination protects against certain infectious diseases, reducing the need for antibiotics. Antibiotics, in turn, are sometimes prescribed incorrectly, especially for viral infections they cannot treat. At GSK, we are already developing new vaccines to meet future needs.”
Vaccination remains a cornerstone in the fight against AMR. As pathogens grow increasingly resistant to antibiotics, coordinated efforts and innovative vaccine development are essential to mitigating this global health crisis.
This story was translated and adapted from MediQuality using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Antimicrobial resistance (AMR) is globally recognized as one of the greatest health threats of the 21st century, responsible for 1.27 million deaths annually. “According to the WHO, if no measures are taken promptly, AMR could lead to more deaths than cancer by 2050,” Arnaud Marchant, MD, PhD, director of the European Plotkin Institute for Vaccinology at Université libre de Bruxelles (EPIV-ULB), Anderlecht, Belgium, said in an interview with MediQuality, part of the Medscape Professional Network. “This is a huge problem, and vaccination could be part of the solution.”
EPIV-ULB marked the start of the World AMR Awareness Week (November 18-24) with an event highlighting the critical role of vaccination to counter the rise for resistant pathogens. During the event, MediQuality interviewed Marchant, along with several other experts in the field.
Antibiotics Losing Effectiveness
Marc Van Ranst, PhD, virologist at Rega Institute KU Leuven in Leuven, Belgium, echoed Marchant’s concerns. He noted that “an increasing number of bacteria are becoming resistant to more antibiotics.” “While antibiotics were once miracle drugs, they have now stopped — or almost stopped — working against certain bacteria. Although we are discovering more effective therapies, bacterial infections are increasingly likely to worsen due to AMR.”
Van Ranst issued a stark warning: “If this trend continues, it is entirely reasonable to predict that in 25 years, some antibiotics will become useless, certain bacterial infections will be much harder to treat, and deaths will outnumber those caused by cancer. It’s worth noting, however, that as cancer treatments improve, cancer-related deaths are expected to decline, further highlighting the growing burden of AMR-related fatalities.”
Viruses, Vaccines, and Resistance
Van Ranst emphasized that while AMR primarily involves bacteria, viral infections and vaccination against them also play a role in addressing the issue. “When vaccines prevent illness, they reduce the need for unnecessary antibiotic use. In the past, antibiotics were frequently prescribed for respiratory infections — typically caused by viruses — leading to misuse and heightened resistance. By preventing viral infections through vaccines, we reduce inappropriate antibiotic prescriptions and, subsequently, AMR.”
Strategic Areas of Focus
To maximize the impact of vaccination in combating AMR, Belgium must prioritize several strategic areas, according to EPIV-ULB. “Expanding vaccination coverage for recommended vaccines is crucial to effectively preventing the spread of resistant pathogens,” said Marchant.
“Innovation and development of new vaccines are also essential, including targeted research into vaccines for infections that are currently unavoidable through other means. Enhancing epidemiological surveillance through national data collection and analysis will further clarify the impact of vaccines on AMR and inform policy decisions.”
EPIV-ULB underscored the importance of educating the public and healthcare professionals. “Public awareness is essential to addressing vaccine hesitancy by providing clear information on the importance of prevention,” Marchant explained. “Healthcare professional training must also improve, encouraging preventive practices and judicious antibiotic use. Furthermore, additional research is necessary to fill data gaps and develop predictive models that can guide vaccine development in the future.”
Role of Vaccination
According to EPIV-ULB, Belgium needs a strengthened national strategy to address AMR effectively. “Complementary solutions are increasingly important as antimicrobials lose efficacy and treatments become more complex,” Marchant said. “Vaccination offers a proactive and effective preventive solution, directly and indirectly reducing the spread of resistant pathogens.”
Vaccines combat AMR through various mechanisms. “They prevent diseases such as pneumococcal pneumonia and meningitis, reducing the need for antibiotics to treat these infections,” Marchant explained. “Additionally, vaccination lowers inappropriate antibiotic use by preventing viral infections, reducing the risk of overprescribing antibiotics in cases where they are unnecessary. Lastly, herd immunity from vaccination slows the circulation of resistant pathogens, limiting their spread.”
Van Ranst urged healthcare professionals to prioritize vaccinating at-risk populations as identified by Belgium’s Superior Health Council. These include the elderly with underlying conditions and pregnant women, especially for influenza vaccines. University Hospitals Leuven in Belgium, also conducts annual vaccination campaigns for its staff, combining flu and COVID vaccines to increase uptake.
A Global Challenge
Marc Noppen, MD, PhD, director of University Hospital Brussels, Belgium, emphasized the complexity of AMR as a global issue. “The problem isn’t solely due to human antibiotic use; it also stems from veterinary medicine, plant breeding, and animal husbandry. This is a multifactorial, worldwide issue that requires public awareness. Improved vaccination strategies are one way to address AMR, particularly in this post-COVID era of heightened skepticism toward vaccines,” he explained.
Marie-Lise Verschelden from Pfizer highlighted the need for cooperation across the healthcare sector. “Belgium is fortunate to have a fantastic ecosystem of academics, clinicians, and industry experts. Collaboration, including government involvement, is critical to advancing our efforts. At Pfizer, we continue to develop new vaccines and technologies, and the COVID crisis has reinforced the critical role of vaccination in combating AMR. Through our vaccine portfolio and ongoing developments, we are well-positioned to contribute significantly to this global challenge.”
Elisabeth Van Damme from GSK reiterated that AMR is a global issue requiring joint efforts. “Existing vaccines are underutilized. Vaccination protects against certain infectious diseases, reducing the need for antibiotics. Antibiotics, in turn, are sometimes prescribed incorrectly, especially for viral infections they cannot treat. At GSK, we are already developing new vaccines to meet future needs.”
Vaccination remains a cornerstone in the fight against AMR. As pathogens grow increasingly resistant to antibiotics, coordinated efforts and innovative vaccine development are essential to mitigating this global health crisis.
This story was translated and adapted from MediQuality using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Antimicrobial resistance (AMR) is globally recognized as one of the greatest health threats of the 21st century, responsible for 1.27 million deaths annually. “According to the WHO, if no measures are taken promptly, AMR could lead to more deaths than cancer by 2050,” Arnaud Marchant, MD, PhD, director of the European Plotkin Institute for Vaccinology at Université libre de Bruxelles (EPIV-ULB), Anderlecht, Belgium, said in an interview with MediQuality, part of the Medscape Professional Network. “This is a huge problem, and vaccination could be part of the solution.”
EPIV-ULB marked the start of the World AMR Awareness Week (November 18-24) with an event highlighting the critical role of vaccination to counter the rise for resistant pathogens. During the event, MediQuality interviewed Marchant, along with several other experts in the field.
Antibiotics Losing Effectiveness
Marc Van Ranst, PhD, virologist at Rega Institute KU Leuven in Leuven, Belgium, echoed Marchant’s concerns. He noted that “an increasing number of bacteria are becoming resistant to more antibiotics.” “While antibiotics were once miracle drugs, they have now stopped — or almost stopped — working against certain bacteria. Although we are discovering more effective therapies, bacterial infections are increasingly likely to worsen due to AMR.”
Van Ranst issued a stark warning: “If this trend continues, it is entirely reasonable to predict that in 25 years, some antibiotics will become useless, certain bacterial infections will be much harder to treat, and deaths will outnumber those caused by cancer. It’s worth noting, however, that as cancer treatments improve, cancer-related deaths are expected to decline, further highlighting the growing burden of AMR-related fatalities.”
Viruses, Vaccines, and Resistance
Van Ranst emphasized that while AMR primarily involves bacteria, viral infections and vaccination against them also play a role in addressing the issue. “When vaccines prevent illness, they reduce the need for unnecessary antibiotic use. In the past, antibiotics were frequently prescribed for respiratory infections — typically caused by viruses — leading to misuse and heightened resistance. By preventing viral infections through vaccines, we reduce inappropriate antibiotic prescriptions and, subsequently, AMR.”
Strategic Areas of Focus
To maximize the impact of vaccination in combating AMR, Belgium must prioritize several strategic areas, according to EPIV-ULB. “Expanding vaccination coverage for recommended vaccines is crucial to effectively preventing the spread of resistant pathogens,” said Marchant.
“Innovation and development of new vaccines are also essential, including targeted research into vaccines for infections that are currently unavoidable through other means. Enhancing epidemiological surveillance through national data collection and analysis will further clarify the impact of vaccines on AMR and inform policy decisions.”
EPIV-ULB underscored the importance of educating the public and healthcare professionals. “Public awareness is essential to addressing vaccine hesitancy by providing clear information on the importance of prevention,” Marchant explained. “Healthcare professional training must also improve, encouraging preventive practices and judicious antibiotic use. Furthermore, additional research is necessary to fill data gaps and develop predictive models that can guide vaccine development in the future.”
Role of Vaccination
According to EPIV-ULB, Belgium needs a strengthened national strategy to address AMR effectively. “Complementary solutions are increasingly important as antimicrobials lose efficacy and treatments become more complex,” Marchant said. “Vaccination offers a proactive and effective preventive solution, directly and indirectly reducing the spread of resistant pathogens.”
Vaccines combat AMR through various mechanisms. “They prevent diseases such as pneumococcal pneumonia and meningitis, reducing the need for antibiotics to treat these infections,” Marchant explained. “Additionally, vaccination lowers inappropriate antibiotic use by preventing viral infections, reducing the risk of overprescribing antibiotics in cases where they are unnecessary. Lastly, herd immunity from vaccination slows the circulation of resistant pathogens, limiting their spread.”
Van Ranst urged healthcare professionals to prioritize vaccinating at-risk populations as identified by Belgium’s Superior Health Council. These include the elderly with underlying conditions and pregnant women, especially for influenza vaccines. University Hospitals Leuven in Belgium, also conducts annual vaccination campaigns for its staff, combining flu and COVID vaccines to increase uptake.
A Global Challenge
Marc Noppen, MD, PhD, director of University Hospital Brussels, Belgium, emphasized the complexity of AMR as a global issue. “The problem isn’t solely due to human antibiotic use; it also stems from veterinary medicine, plant breeding, and animal husbandry. This is a multifactorial, worldwide issue that requires public awareness. Improved vaccination strategies are one way to address AMR, particularly in this post-COVID era of heightened skepticism toward vaccines,” he explained.
Marie-Lise Verschelden from Pfizer highlighted the need for cooperation across the healthcare sector. “Belgium is fortunate to have a fantastic ecosystem of academics, clinicians, and industry experts. Collaboration, including government involvement, is critical to advancing our efforts. At Pfizer, we continue to develop new vaccines and technologies, and the COVID crisis has reinforced the critical role of vaccination in combating AMR. Through our vaccine portfolio and ongoing developments, we are well-positioned to contribute significantly to this global challenge.”
Elisabeth Van Damme from GSK reiterated that AMR is a global issue requiring joint efforts. “Existing vaccines are underutilized. Vaccination protects against certain infectious diseases, reducing the need for antibiotics. Antibiotics, in turn, are sometimes prescribed incorrectly, especially for viral infections they cannot treat. At GSK, we are already developing new vaccines to meet future needs.”
Vaccination remains a cornerstone in the fight against AMR. As pathogens grow increasingly resistant to antibiotics, coordinated efforts and innovative vaccine development are essential to mitigating this global health crisis.
This story was translated and adapted from MediQuality using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Treating Onychomycosis: Pearls from a Podiatrist
LAS VEGAS —
According to Tracey C. Vlahovic, DPM, a professor at the Samuel Merritt University College of Podiatric Medicine, Oakland, California, most cases of onychomycosis are caused by the dermatophytes Trichophyton rubrum and T mentagrophytes, although the cause can also be a mixed infection. “Dermatophytes are going to impact the nails first, and molds may come in and join the party later,” she said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference.
“The distal subungual onychomycosis (DSO) type is still the most common, but don’t forget that onychomycosis and nail psoriasis can happen at the same time. What we can’t lose sight of is that onychomycosis is a disease of the nail bed, which ultimately affects the nail plate; it’s not a disease of the nail plate first.”
Her diagnostic approach combines periodic acid-Schiff (PAS) staining with fungal culture “because I like to know the speciation,” she said. “PAS doesn’t give me the speciation; fungal cultures should. PCR can be expensive, but that can give me speciation.”
How Does This Happen?
Fungal DSO occurs because of exposure to a dermatophyte, which can be as simple as tinea pedis. “Perhaps it’s the environment in the shoe,” said Vlahovic, one of the authors of a textbook on onychomycosis. “That’s something I’m always concentrating on with the patient. What is your foot hygiene like? What’s your shoe and sock wear? What’s your level of physical activity? You can have trauma to the hyponychium, where the skin and the nail meet. Maybe they trim their nails too close to the skin, or maybe there’s another skin condition like psoriasis.”
The dermatophyte, she continued, enters and invades the nail at the hyponychium and uses the keratinase enzyme to digest keratin in the nail bed. Mild inflammation develops, and pH changes cause focal parakeratosis and subungual hyperkeratosis in the form of onycholysis and subungual debris. “Hyphae then invade the lamina of the nail plate, which causes brittle nails,” she said. “The compromised hyponychium creates a reservoir for molds and bacteria.”
Therapies approved by the Food and Drug Administration (FDA) for onychomycosis include the topical agents efinaconazole, tavaborole, and ciclopirox; the oral agents terbinafine and itraconazole; and laser therapy. Off-label, Vlahovic said that she sometimes uses oral fluconazole, pulsed dosing for terbinafine, and booster doses of terbinafine or any approved oral antifungal agent. Pulse dosing for itraconazole is FDA-approved for fingernails but not for toenails.
“We don’t have any oral antifungals that are approved for children, but we do have weight-based dosing,” she noted. Other off-label treatments for onychomycosis that patients may come across while browsing the internet but do not penetrate the nail plate, include products containing tolnaftate, tree oil, and undecylenic acid, “which is a very long-chain antifungal,” Vlahovic said. “It’s so huge that it can’t get through the nail plate. These products must get through the nail plate into the nail bed where the infection is.”
According to therapeutic recommendations for the treatment of toenail onychomycosis in the United States, published in 2021, terbinafine is the primary choice for oral treatment and efinaconazole 10% for topical treatment. There are no current treatment recommendations for pregnant or lactating patients. “I always defer to the obstetrician,” said Vlahovic, a coauthor of the recommendations. For pediatric patients, there are approved topical medications: Efinaconazole and tavaborole for ages 6 and up and ciclopirox for ages 12 years or older.
Treatment recommendations for adults vary based on clinical presentation and patient characteristics. Questions to consider: Are they older? Do they have diabetes? Are they able to reach their feet to apply medication? What other medications are they taking? Are there any kidney or liver issues that are cause for concern?
Another question to consider is whether they have concurrent nail psoriasis. “When I have those patients, I often treat the onychomycosis first and the nail psoriasis second,” she said.
Evidence for Lasers Weak
Though laser therapy is FDA approved for the temporary increase of clear nails in onychomycosis, Vlahovic is underwhelmed by the evidence of its use for onychomycosis. According to a systematic review of 261 studies, only 1 reported treatment success as 16.7%, and clinical cures ranged from 13% to 16%. “Many of the existing studies were so poorly done in terms of protocols; it was frustrating,” she said. “No study has reported complete cure. There’s a lack of standardization across laser companies and a lack of standardization across protocols.”
Before starting oral antifungal therapy, Vlahovic uses the Onychomycosis Severity Index to determine the number of nails involved and the proportion of nails that are affected. She also wants to know if the patient is taking any medication that might interfere with an oral antifungal and gets baseline liver function tests (LFTs) to document results in the chart. “You want to discuss the pros and cons of oral antifungal therapy, and you want to set realistic expectations,” she added. “These medications are not cosmetic products; they are meant to kill fungus. Sometimes patients lose sight of that.”
Vlahovic routinely offers pulse dosing of terbinafine, which is FDA approved at a dose of 250 mg/d for 90 days. Pulse dosing involves taking terbinafine 250 mg twice a day for 1 week, followed by a 3-week break. This cycle is repeated three or four times. A clinical trial found no significant difference in outcome between patients who received pulsed vs continuous terbinafine dosing for the treatment of dermatophyte onychomycosis.
What About Oral Antifungal Safety?
For patients who ask about the safety of oral antifungals, Vlahovic characterized them as “well tolerated and safe in an immunocompetent population.” In a meta-analysis of 122 studies of about 22,000 patients, the pooled risk for treatment discontinuation because of adverse events was 3.4% for terbinafine 250 mg/d and 4.21% for itraconazole 200 mg/d. The risk for liver injury requiring termination of treatment and the risk of having symptomatic elevation of LFTs were less than 2% for all regimens.
According to the best available published evidence, Vlahovic said, the onychomycosis recurrence rate ranges from 6% to 40%. “That’s a wild number. We really have no idea what the true recurrence rate is, and that’s a problem.”
Vlahovic disclosed having been a consultant to and an investigator for Ortho Dermatologics and Sagis Diagnostics.
A version of this article appeared on Medscape.com.
LAS VEGAS —
According to Tracey C. Vlahovic, DPM, a professor at the Samuel Merritt University College of Podiatric Medicine, Oakland, California, most cases of onychomycosis are caused by the dermatophytes Trichophyton rubrum and T mentagrophytes, although the cause can also be a mixed infection. “Dermatophytes are going to impact the nails first, and molds may come in and join the party later,” she said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference.
“The distal subungual onychomycosis (DSO) type is still the most common, but don’t forget that onychomycosis and nail psoriasis can happen at the same time. What we can’t lose sight of is that onychomycosis is a disease of the nail bed, which ultimately affects the nail plate; it’s not a disease of the nail plate first.”
Her diagnostic approach combines periodic acid-Schiff (PAS) staining with fungal culture “because I like to know the speciation,” she said. “PAS doesn’t give me the speciation; fungal cultures should. PCR can be expensive, but that can give me speciation.”
How Does This Happen?
Fungal DSO occurs because of exposure to a dermatophyte, which can be as simple as tinea pedis. “Perhaps it’s the environment in the shoe,” said Vlahovic, one of the authors of a textbook on onychomycosis. “That’s something I’m always concentrating on with the patient. What is your foot hygiene like? What’s your shoe and sock wear? What’s your level of physical activity? You can have trauma to the hyponychium, where the skin and the nail meet. Maybe they trim their nails too close to the skin, or maybe there’s another skin condition like psoriasis.”
The dermatophyte, she continued, enters and invades the nail at the hyponychium and uses the keratinase enzyme to digest keratin in the nail bed. Mild inflammation develops, and pH changes cause focal parakeratosis and subungual hyperkeratosis in the form of onycholysis and subungual debris. “Hyphae then invade the lamina of the nail plate, which causes brittle nails,” she said. “The compromised hyponychium creates a reservoir for molds and bacteria.”
Therapies approved by the Food and Drug Administration (FDA) for onychomycosis include the topical agents efinaconazole, tavaborole, and ciclopirox; the oral agents terbinafine and itraconazole; and laser therapy. Off-label, Vlahovic said that she sometimes uses oral fluconazole, pulsed dosing for terbinafine, and booster doses of terbinafine or any approved oral antifungal agent. Pulse dosing for itraconazole is FDA-approved for fingernails but not for toenails.
“We don’t have any oral antifungals that are approved for children, but we do have weight-based dosing,” she noted. Other off-label treatments for onychomycosis that patients may come across while browsing the internet but do not penetrate the nail plate, include products containing tolnaftate, tree oil, and undecylenic acid, “which is a very long-chain antifungal,” Vlahovic said. “It’s so huge that it can’t get through the nail plate. These products must get through the nail plate into the nail bed where the infection is.”
According to therapeutic recommendations for the treatment of toenail onychomycosis in the United States, published in 2021, terbinafine is the primary choice for oral treatment and efinaconazole 10% for topical treatment. There are no current treatment recommendations for pregnant or lactating patients. “I always defer to the obstetrician,” said Vlahovic, a coauthor of the recommendations. For pediatric patients, there are approved topical medications: Efinaconazole and tavaborole for ages 6 and up and ciclopirox for ages 12 years or older.
Treatment recommendations for adults vary based on clinical presentation and patient characteristics. Questions to consider: Are they older? Do they have diabetes? Are they able to reach their feet to apply medication? What other medications are they taking? Are there any kidney or liver issues that are cause for concern?
Another question to consider is whether they have concurrent nail psoriasis. “When I have those patients, I often treat the onychomycosis first and the nail psoriasis second,” she said.
Evidence for Lasers Weak
Though laser therapy is FDA approved for the temporary increase of clear nails in onychomycosis, Vlahovic is underwhelmed by the evidence of its use for onychomycosis. According to a systematic review of 261 studies, only 1 reported treatment success as 16.7%, and clinical cures ranged from 13% to 16%. “Many of the existing studies were so poorly done in terms of protocols; it was frustrating,” she said. “No study has reported complete cure. There’s a lack of standardization across laser companies and a lack of standardization across protocols.”
Before starting oral antifungal therapy, Vlahovic uses the Onychomycosis Severity Index to determine the number of nails involved and the proportion of nails that are affected. She also wants to know if the patient is taking any medication that might interfere with an oral antifungal and gets baseline liver function tests (LFTs) to document results in the chart. “You want to discuss the pros and cons of oral antifungal therapy, and you want to set realistic expectations,” she added. “These medications are not cosmetic products; they are meant to kill fungus. Sometimes patients lose sight of that.”
Vlahovic routinely offers pulse dosing of terbinafine, which is FDA approved at a dose of 250 mg/d for 90 days. Pulse dosing involves taking terbinafine 250 mg twice a day for 1 week, followed by a 3-week break. This cycle is repeated three or four times. A clinical trial found no significant difference in outcome between patients who received pulsed vs continuous terbinafine dosing for the treatment of dermatophyte onychomycosis.
What About Oral Antifungal Safety?
For patients who ask about the safety of oral antifungals, Vlahovic characterized them as “well tolerated and safe in an immunocompetent population.” In a meta-analysis of 122 studies of about 22,000 patients, the pooled risk for treatment discontinuation because of adverse events was 3.4% for terbinafine 250 mg/d and 4.21% for itraconazole 200 mg/d. The risk for liver injury requiring termination of treatment and the risk of having symptomatic elevation of LFTs were less than 2% for all regimens.
According to the best available published evidence, Vlahovic said, the onychomycosis recurrence rate ranges from 6% to 40%. “That’s a wild number. We really have no idea what the true recurrence rate is, and that’s a problem.”
Vlahovic disclosed having been a consultant to and an investigator for Ortho Dermatologics and Sagis Diagnostics.
A version of this article appeared on Medscape.com.
LAS VEGAS —
According to Tracey C. Vlahovic, DPM, a professor at the Samuel Merritt University College of Podiatric Medicine, Oakland, California, most cases of onychomycosis are caused by the dermatophytes Trichophyton rubrum and T mentagrophytes, although the cause can also be a mixed infection. “Dermatophytes are going to impact the nails first, and molds may come in and join the party later,” she said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference.
“The distal subungual onychomycosis (DSO) type is still the most common, but don’t forget that onychomycosis and nail psoriasis can happen at the same time. What we can’t lose sight of is that onychomycosis is a disease of the nail bed, which ultimately affects the nail plate; it’s not a disease of the nail plate first.”
Her diagnostic approach combines periodic acid-Schiff (PAS) staining with fungal culture “because I like to know the speciation,” she said. “PAS doesn’t give me the speciation; fungal cultures should. PCR can be expensive, but that can give me speciation.”
How Does This Happen?
Fungal DSO occurs because of exposure to a dermatophyte, which can be as simple as tinea pedis. “Perhaps it’s the environment in the shoe,” said Vlahovic, one of the authors of a textbook on onychomycosis. “That’s something I’m always concentrating on with the patient. What is your foot hygiene like? What’s your shoe and sock wear? What’s your level of physical activity? You can have trauma to the hyponychium, where the skin and the nail meet. Maybe they trim their nails too close to the skin, or maybe there’s another skin condition like psoriasis.”
The dermatophyte, she continued, enters and invades the nail at the hyponychium and uses the keratinase enzyme to digest keratin in the nail bed. Mild inflammation develops, and pH changes cause focal parakeratosis and subungual hyperkeratosis in the form of onycholysis and subungual debris. “Hyphae then invade the lamina of the nail plate, which causes brittle nails,” she said. “The compromised hyponychium creates a reservoir for molds and bacteria.”
Therapies approved by the Food and Drug Administration (FDA) for onychomycosis include the topical agents efinaconazole, tavaborole, and ciclopirox; the oral agents terbinafine and itraconazole; and laser therapy. Off-label, Vlahovic said that she sometimes uses oral fluconazole, pulsed dosing for terbinafine, and booster doses of terbinafine or any approved oral antifungal agent. Pulse dosing for itraconazole is FDA-approved for fingernails but not for toenails.
“We don’t have any oral antifungals that are approved for children, but we do have weight-based dosing,” she noted. Other off-label treatments for onychomycosis that patients may come across while browsing the internet but do not penetrate the nail plate, include products containing tolnaftate, tree oil, and undecylenic acid, “which is a very long-chain antifungal,” Vlahovic said. “It’s so huge that it can’t get through the nail plate. These products must get through the nail plate into the nail bed where the infection is.”
According to therapeutic recommendations for the treatment of toenail onychomycosis in the United States, published in 2021, terbinafine is the primary choice for oral treatment and efinaconazole 10% for topical treatment. There are no current treatment recommendations for pregnant or lactating patients. “I always defer to the obstetrician,” said Vlahovic, a coauthor of the recommendations. For pediatric patients, there are approved topical medications: Efinaconazole and tavaborole for ages 6 and up and ciclopirox for ages 12 years or older.
Treatment recommendations for adults vary based on clinical presentation and patient characteristics. Questions to consider: Are they older? Do they have diabetes? Are they able to reach their feet to apply medication? What other medications are they taking? Are there any kidney or liver issues that are cause for concern?
Another question to consider is whether they have concurrent nail psoriasis. “When I have those patients, I often treat the onychomycosis first and the nail psoriasis second,” she said.
Evidence for Lasers Weak
Though laser therapy is FDA approved for the temporary increase of clear nails in onychomycosis, Vlahovic is underwhelmed by the evidence of its use for onychomycosis. According to a systematic review of 261 studies, only 1 reported treatment success as 16.7%, and clinical cures ranged from 13% to 16%. “Many of the existing studies were so poorly done in terms of protocols; it was frustrating,” she said. “No study has reported complete cure. There’s a lack of standardization across laser companies and a lack of standardization across protocols.”
Before starting oral antifungal therapy, Vlahovic uses the Onychomycosis Severity Index to determine the number of nails involved and the proportion of nails that are affected. She also wants to know if the patient is taking any medication that might interfere with an oral antifungal and gets baseline liver function tests (LFTs) to document results in the chart. “You want to discuss the pros and cons of oral antifungal therapy, and you want to set realistic expectations,” she added. “These medications are not cosmetic products; they are meant to kill fungus. Sometimes patients lose sight of that.”
Vlahovic routinely offers pulse dosing of terbinafine, which is FDA approved at a dose of 250 mg/d for 90 days. Pulse dosing involves taking terbinafine 250 mg twice a day for 1 week, followed by a 3-week break. This cycle is repeated three or four times. A clinical trial found no significant difference in outcome between patients who received pulsed vs continuous terbinafine dosing for the treatment of dermatophyte onychomycosis.
What About Oral Antifungal Safety?
For patients who ask about the safety of oral antifungals, Vlahovic characterized them as “well tolerated and safe in an immunocompetent population.” In a meta-analysis of 122 studies of about 22,000 patients, the pooled risk for treatment discontinuation because of adverse events was 3.4% for terbinafine 250 mg/d and 4.21% for itraconazole 200 mg/d. The risk for liver injury requiring termination of treatment and the risk of having symptomatic elevation of LFTs were less than 2% for all regimens.
According to the best available published evidence, Vlahovic said, the onychomycosis recurrence rate ranges from 6% to 40%. “That’s a wild number. We really have no idea what the true recurrence rate is, and that’s a problem.”
Vlahovic disclosed having been a consultant to and an investigator for Ortho Dermatologics and Sagis Diagnostics.
A version of this article appeared on Medscape.com.
FROM SDPA 2024
Varicella Outbreaks: 2022-2024
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.
As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.

As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.
Practitioners providing care to children are familiar with the childhood immunization schedule and routinely administer varicella vaccine at the 12-month and 4- to 5-year visits. However, when is the last time most of us or any of the current trainees have seen a case?
Briefly, varicella is a highly contagious disease caused by varicella-zoster virus (VZV). It is characterized by a generalized pruritic erythematous rash in various stages of development beginning as macules, progressing to papules, and ultimately becoming vesicular lesions on an erythematous base (“dewdrop on a rose petal”) and resolves with crusting of the lesion (Figure 1). It has an incubation period of 10-21 days with symptoms usually developing within 14-16 days after exposure. The vesicular rash must be differentiated from enterovirus, Staphylococcus aureus, contact dermatitis, or insect bites, which initially may be difficult. Approximately 50% of children can have symptoms including fever, malaise, anorexia, headache, and occasionally, mild abdominal pain in the 24-48 hours prior to the appearance of rash. Lesions usually first appear on the scalp, face, or trunk in successive crops over several days. A person with varicella has lesions in various stages.
In a normal host, new vesicle formation usually stops within 4 days, and most lesions have fully crusted by day 6. VZV establishes latency in sensory ganglia and may reactivate years or decades later to cause herpes zoster (HZ). Most healthy children with varicella recover without sequelae so the disease is generally regarded as benign. However, varicella can lead to serious complications and deaths in healthy as well as immunocompromised persons.
Complications of Varicella: bacterial superinfection of skin lesions most often with Streptococcus pyogenes or S aureus manifested as cellulitis, myositis, or necrotizing fasciitis; neurologic complications include cerebellar ataxia and encephalitis with the latter seen most often in adults. Pneumonia occurs most often in adults, especially those infected during pregnancy. Another concern, infection during the first 20 weeks of pregnancy can lead to fetal death or severe birth defects, including limb hypoplasia, cutaneous scarring, ocular abnormalities, and central nervous system damage (congenital varicella syndrome).
The risk for development of severe disseminated disease was first noted in the 1960s as treatments for leukemia in children improved. They were surviving their cancer only to develop severe and often fatal varicella. Today it is recognized that development of disseminated disease is a risk for all infected persons with impaired T cell function, malignancies, HIV, or receiving immunosuppressive therapy.
Reye’s syndrome is rarely seen today since taking salicylates while infected with VZV was identified as a predisposing factor for development.
VZV is only found in humans and transmission is person to person or airborne. The secondary household attack rate is approximately 90%. In contrast, the secondary attack rates in classrooms may be as low as 12%-33%. Transmission rates in the tropics for unexplained reasons are also lower.
Vaccine History: Why do we rarely see this disease anymore? Varicella, a live attenuated vaccine, was developed in 1974 by Dr. Michiaki Takahashi. It remains the only vaccine directed against a herpes group virus. In 1979, the Collaborative Varicella Vaccine Study Group was established at the National Institutes of Health (NIH) and additional safety and efficacy trials were conducted in the United States initially in leukemic patients in remission and later in healthy children, which supported Takahashi’s data. Licensure of varicella vaccine was granted in 1995. That same year, due to continuing disease and societal burden, the United States was the first country to incorporate varicella into the routine childhood immunization schedule, which resulted in significant reductions in cases. To further improve control of varicella, in 2007 vaccine recommendations were revised and a routine two-dose schedule was implemented. The impact of varicella disease pre- and post-vaccine licensure is illustrated in Figure 2. Not listed, is that in the pre-vaccine era, there were approximately 44 cases of congenital varicella syndrome annually.

As of 2023 only 23% (45/195) of nations routinely administer this vaccine and 4% (8/195) have restricted recommendations. The remaining 73% of countries do not offer the vaccine, including all countries on the African continent, and Cuba, Guatemala, Haiti, Honduras, India, Jordan, Lebanon, Philippines, Portugal, and Venezuela to list a few.
Varicella Outbreak: In October 2022, New York City (NYC) identified a varicella outbreak primarily involving persons who recently migrated from Central and South America and lived in a shelter in NYC or residential facility (n = 105); the outbreak is ongoing. As of March 8, 2024, 873 cases (53%) were among children aged 4-18 years and 91.9% had no documentation of varicella vaccine at time of symptom onset. There were 28 hospitalizations, and no deaths reported. The most common sources of transmission were the residential facilities (41.3%) and importation or possible importation (39.4%). School transmission accounted for only 1.2% of cases.
Most migrants arrived from countries where varicella vaccination is not part of the routine childhood immunization schedule. Although most cases occurred in children, almost 30% occurred in adults. Many of the migrants arrived from tropical countries where susceptibility rates are also higher in adults. This outbreak is a reminder of the importance of limiting disease transmission by maintaining high vaccination rates. To curtail this outbreak, approximately 27,000 doses of varicella vaccine were administered to the arriving migrants. In addition, MMR, COVID-19, influenza, and all routine pediatric vaccines required for school entry were administered. Temporary closure of the residential facilities were required. Education was provided to residents regarding immunizations as well as assistance to help them establish a primary care home. Multiple agencies were mobilized to successfully coordinate these efforts.
Take Home Message
1. Each country has its own routine immunization schedule. It may not include all vaccines recommended in the US schedule. When questioned I’m frequently told that immunizations are up to date, only to review records and find they are not, especially when it is related to MMR. It is often administered at 9 months and/or MR or MM is administered depending on the country. As reported here, varicella is a routine vaccine in only 45 countries.
2.
3. Once an outbreak has been identified, the infrastructure to manage and contain it must already be established. In most instances there will be a need for a rapid and often large-scale effort involving multiple agencies including local health care providers.
4. Not all diseases are reportable. Only deaths by varicella are nationally notifiable. Otherwise, cases are reported voluntarily. As of November 2, 2024, there have been 5,157 cases of varicella reported, excluding any cases from NYC.
Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
CDC. Nationally Notifiable Infectious Diseases and Conditions, United States: Weekly Tables. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp.
Graham KA et al. Varicella Outbreak Among Recent Arrivals to New York City, 2022-2024. MMWR Morb Mortal Wkly Rep. 2024 May 30;73(21):478-483. doi: 10.15585/mmwr.mm7321a1.
Marin M et al. Health and Economic Impact of the United States Varicella Vaccination Program, 1996-2020. J Infect Dis. 2022 Oct 21;226(Suppl 4):S463-S469. doi: 10.1093/infdis/jiac271.
Varicella-Zoster Virus Infections in Kimberkin DW et al, eds. Red Book: 2024 Report of the Committee on Infectious Diseases, 33rd Edition. American Academy of Pediatrics, 2024:938-951. https://www.aap.org/Red-Book-2024-Report-of-the-Committee-on-Infectious-Diseases-33rd-Edition-Paperback?srsltid=AfmBOoqyF60rR9ZwQ5jA8AouNhtRRTyPLnc_r7HWw7JVYV8v33Hr2vQS.